Abstract
Crohn’s Disease (CD) is a chronic inflammatory disorder in which up to 50% of patients develop fistula within 20 years after the initial diagnosis, and half of these patients suffer perianal fistulizing disease. The etiopathogenesis of CD-related perianal fistula is still unclear, and its phenotypical and molecular characteristics are even more indefinite. A better understanding would be crucial to develop targeted and more effective therapeutic strategies. At present, the most accredited theory for the formation of CD-related fistula identifies the epithelial-to-mesenchymal transition (EMT) as the driving force. It has been well recognized that CD carries an increased risk of malignancy, particularly mucinous adenocarcinoma is often associated with long-standing fistula in CD patients. Despite the availability of multiple treatment options, perianal fistulizing CD represents a therapeutic challenge and is associated with an important impact on patients’ quality of life. To date, the most effective management is multidisciplinary with the cooperation of gastroenterologists, surgeons, radiologists, and nutritionists and the best recommended treatment is a combination of medical and surgical approaches.
Keywords: Crohn-associated fistula, mucinous adenocarcinoma, epithelial-to-mesenchymal transition
1. Common Features of Fistulae in Crohn’s Disease
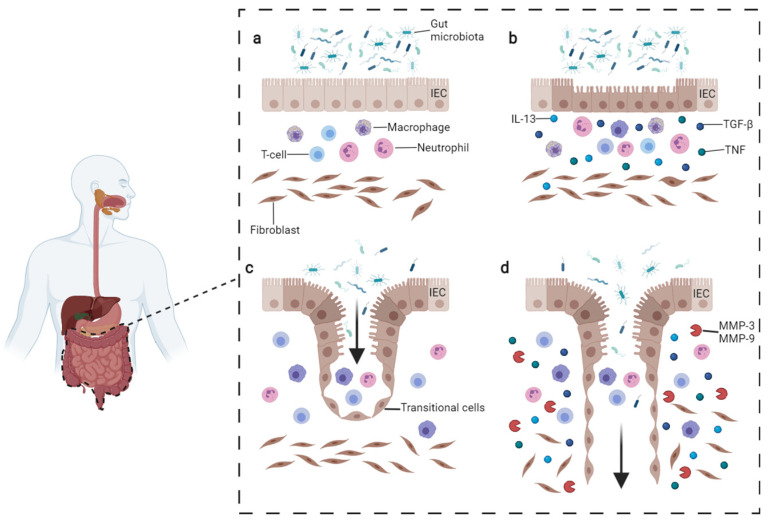
Crohn’s Disease (CD) is a chronic inflammation in which the disorder could affect any region of the gastrointestinal tract from the mouth to the anus (but with typically higher incidence on ileum and colon), and together with Ulcerative Colitis (UC) are stand for inflammatory bowel diseases (IBD). Although the precise beginning remains unknown, the starting point of CD is believed to result from the interplay between genetic susceptibility, environmental factors, and their interactions with gut microbiota [1,2]. Moreover, the interaction between these factors and the patient’s immune system is as well considered crucial for the pathogenesis of the disease (Figure 1a,b).
Figure 1.
Pathogenesis of CD fistula. (a) Normal condition persisting in the gut. (b) In CD, the intestinal lesions can be triggered by multiple factors such as inflammatory cells reacting to microbiota or intestinal pathogens. The inflammatory infiltrate includes T-cells, B-cells, and macrophages which produce TNFα. Fibroblasts, staying underneath, produce TGF-β and IL-13. These cytokines trigger EMT and tissue remodeling. (c) During EMT, intestinal epithelial cells (IECs) lose their adhesion properties, downregulating β-catenin and E-cadherin proteins. IECs start migrating underneath in order to repair the lesion and become transitional cells (TCs). (d) TCs produce IL-13 which induces other cells to undergo EMT, penetrating deeper in lower layers. The process is facilitated by the production of metalloproteinases (MMPs) which degrade the extracellular matrix. Image adapted from Panes et al. [15] and drawn with BioRender.com (accessed on 23 November 2021).
The clinical presentation of CD depends substantially on the location and severity of the inflammation. Symptoms and signs can be heterogeneous, ranging from anorexia, abdominal pain, and fatigue, to more severe conditions as rectal bleeding, bloody diarrhea, and development of perianal lesions such as ulcerations and fistulas [3]. About 50% of CD patients are reported to develop perianal fistula in no more than 20 years after the initial diagnosis, and half of these patients suffer perianal fistulizing disease [2]. Although CD treatment is constantly improved by new therapeutic tools, the optimization of existent therapies and the adoption of a multidisciplinary approach to fistula management remains a clinical challenge [4,5]. This is mainly due to a minority of patients achieving remission and sometimes due to the lack of availability of a few specific therapies and feasibility of surgical techniques [6].
CD-related fistula can be distinguished by the presence of a central fissure that invaginates deeply toward the mucosal barrier of the gut, surrounded by neutrophils, lymphocytes, and granulation tissue. The fence of the fistula canal is densely populated by T-cells CD45RO positive, macrophages CD68 positive, and B-cell CD20 positive.
Morphologically, the fistula is a tract between two surfaces lined by epithelial cells and loaded with debris, erythrocytes, and since it is a consequence of chronic inflammation—inflammatory infiltrates and fibrotic cells [7]. A reduced epithelial regeneration [7], together with weak migration capability of colonic lamina propria fibroblasts (CLPF) are responsible for the wound healing failure in CD-fistulizing/penetrating phenotype [8].
In order to restore the integrity of the intestinal epithelial barrier, the defect in CLFP migration is compensated by intestinal epithelial cells (IECs) converting into transitional cells (TCs), which gain mesenchymal myofibroblast-like features through a mechanism called epithelial-to-mesenchymal transition (EMT) [9] (Figure 1c,d). In this complex program, IECs lose cell polarity and adherence while acquiring invasive and migratory properties (see below).
Another important factor possibly contributing to the pathogenesis of CD-related fistula is the gut microbiota. The hypothesis is indirectly supported by the fact that antibiotic therapy might be beneficial for treating fistulizing CD. A study carried out during GEMINI II clinical trial revealed that patients treated with Vedolizumab and antibiotic therapy had a three-times higher chance of fistula closure than those on placebo [10]. Moreover, Fecal Microbiota Transplantation (FMT) seems to have a role in maintaining Crohn’s disease remission [11]. Haac and colleagues [12] highlighted a difference in the gut microbiota of patients with CD-fistula phenotype in comparison to other CD phenotypes. An abundance of opportunistic pathogens like Achromobacter, Corynebacterium, Actinomyces, and Fusobacterium was found in samples obtained from CD patients. Noticeably, Fusobacterium nucleatum was recently associated with colorectal cancer subtypes in several studies [13,14]. This evidence underlines the significant role that microorganisms, either pathogens or commensals, could play in the onset and/or maintenance of perianal fistulizing CD.
2. Signature Differences Distinguish CD-Related Fistulae from Idiopathic Ones
Although there are several recognized risk factors for Crohn’s disease [1], no environmental factors have been associated with the etiopathogenesis of CD-related fistula yet. In fact, CD-related fistula appears mostly related to genetic, microbiological, and immunological components [16]. Furthermore, its phenotypical and molecular characteristics are not well-characterized.
Few comparative studies have allowed to identify markers able to distinguish CD fistulae from non-inflammatory bowel disease ones (also known as idiopathic or crypto-glandular).
The crypto-glandular type was first classified, albeit inconclusively, by Parks in 1961 [17] on the basis of histological evidence of infection in anal glands.
For a long time, it was thought that the persistence of the fistula depended upon inflammation, the presence of bacterial endotoxins perpetuating an inflammatory response within the lumen even when bacteria are destroyed [18], and upon the presence of bacterial pro-inflammatory peptidoglycan (PG) which may stimulate the secretion of interleukin-1β (IL-1β) [19].
The first evaluations on specific inflammatory characteristics of the fistulae were carried out by immunohistochemistry (IHC). CD fistulae diverge considerably from non-CD fistulae concerning their inflammatory composition, except for the lining epithelium. CD fistulae present high levels of infiltrated T cells CD45RO positive in the interior wall, dense infiltration of B cells CD20 positive surrounding lined up by a small group of macrophages CD68 positive. The second type has an intense infiltration of CD68 positive macrophages enclosed by CD45RO positive T lymphocytes [8].
Metalloproteases (MMP) and tissue inhibitors of metalloproteinases (TIMPs), which contribute to fistula formation through extracellular matrix degradation, did not show any difference between the two types of fistulae, with exception of MMP-3 and 9 that appear to be upregulated in idiopathic fistula [20].
Abundant expression of IL-1β, IL-8, IL-12p40 cytokines, and tumor necrosis factor (TNF)-α were found in idiopathic anal fistulae [21]. However, TNF-α was also significantly up-regulated in the serum of CD-related perianal fistula patients [22].
Only a subgroup of both fistula types showed a layer with easily recognizable epithelial cells which have tight junctions and a basal membrane. Of interest, the “non-epithelialized” CD fistulae were covered by a fine layer of myofibroblast-like “transitional cells” (TC) with gap junctions [7]. Their epithelial origin was strongly suggested by the cytokeratins (CK) 8 and 20 positivity, as well as vimentin negativity. In the transitional zone between the epithelium and the TC, β6-Integrin, and TGF-β had the highest staining intensities [10], as well as the expression of succinate receptor (SUCNR1) which seems to be correlated with the expression of Integrin Subunit Beta 6 (ITGB6), SNAIL, SLUG, and Vimentin [23]. These evidences suggested and supported again the involvement of EMT process in fistula formation [9].
Data on inflammation and EMT were confirmed also in non-CD fistulae through real-time PCR (RT-PCR) and Western blot analysis beside IHC. The inflammation was evaluated by the expression of IL-8 and IL-1β that were respectively more expressed in the proximal part than in the distal one and vice versa for the second marker [24,25]. The expression of markers such as TGF-β, Vimentin, ZEB1, SNAIL which were highly expressed in both proximal and distal parts, with mild E-cadherin reduction, were used for EMT evaluation [25].
Antimicrobial peptides, that are controlled in response to a bacterial antigens provocation or the presence of inflammatory cytokines, specifically hBD2 and hBD3 together with RNase7 and psoriasin, resulted to be elevated in the distal area of non-CD fistulae [24].
Furthermore, flow cytometry analysis (FACS) on curettage and tissue biopsy of CD-related fistulae do not present differences in the ratio of CD161+, IL-17+IFN-γ−, IL-17−IFN-γ+ and IL-17+IFN-γ+ cells between CD4+ or CD8+ T cells. CD161+ T lymphocytes were instead more expressed [26].
Proteomic analysis was performed to evaluate potential dissimilarity in cytokines and phosphoprotein concentration. The phosphorylation status of 28 Receptor Tyrosine Kinases (RTKs) and 11 signaling nodes in addition to 30 cytokines and chemokines was quantified. The two types of fistulae do not substantially differ in their protein expression pattern, even though the panel of cytokines and phosphoproteins analyzed was huge [27].
Recently, by metabolomic analysis, CD perianal fistula tissue has shown metabolic variations compared to idiopathic fistula tissue. Using two analytical platforms such as ultra-high-performance liquid chromatography system and a mass spectrometry detector coupled with chromatography, a broad coverage of the metabolome was achieved. Investigated markers occurred in pathways involved in many metabolisms such as amino acids, carnitine, phospholipids, lysine degradation, sphingolipids, glycerophospholipids, and purines. In CD fistula, dimethylarginine and decanoyl-l-carnitine were identified as the most significant predictors, whereas decanoy-l-carnitine was found to be decreased and positively correlated with idiopathic fistula. Lipid profiling revealed hexosylceramide and diglyceride, which belong to glycosphingolipids and diacylglycerol lipid classes, were found to be increased in CD fistulae with respect to idiopathic ones. A different class of compounds lipid-like called acylcarnitines, is implicated in lipid and fatty acid metabolism as well as cell signaling, playing a role in maintaining membrane integrity, and possibly in fistula persistence. Some of these acylcarnitines (e.g., carnitine precursor deoxycarnitine, lysine) are also thought to be determinant in EMT [28], but how this could be linked to specific metabolic changes remains unclear. It is plausible that metabolic changes accompanying EMT precede some of the more obvious signals of mesenchymal transition and indeed actively contribute to the activation of these indicators [29].
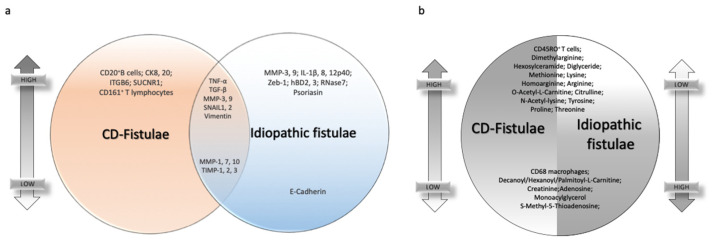
All described signatures are schematized in Figure 2.
Figure 2.
Common and dissimilar signatures between CD- and idiopathic fistulae. (a) Venn diagram highlighting individual or common markers expression in CD-related fistulae and idiopathic ones, on the top if they are upregulated, on the bottom if they are downregulated. (b) Differential markers expression in CD-related fistulae versus idiopathic fistulae. On the right of the circle, the expression is lower on the top of the graph and higher on the bottom. Vice versa, on the left part of the circle, the expression of the same markers is higher on the top and lower on the bottom.
3. EMT Process in Fistula Formation
Even though the clinical relevance of perianal fistulizing CD is obvious due to its impact on patients’ life conditions, its etiopathogenesis is poorly understood. A better understanding would be crucial to develop targeted and more effective therapeutic strategies.
The most accredited theory for the formation of CD-related fistula identifies the epithelial-to-mesenchymal transition (EMT) as the driving force. A large number of EMT-associated events can be noted within and around the fistula tract. During EMT, epithelial cells lose their epithelial-specific features, such as apico-basal polarity and cell contacts, acquiring a mesenchymal cell shape, as well as enhanced motility and dissemination [30]. Consistently, epithelial markers (such as E-cadherin and Claudin-4) were found downregulated, while mesenchymal markers (such as alpha smooth muscle actin and vimentin) [10] resulted upregulated. Further evidences demonstrated a strong expression of SNAIL in the nuclei of TCs, but not in the fistula surrounding area, supporting the increased activity of EMT-related Transcription Factors (TFs) [31]. Around the CD-related fistula, also one other TF, such as SLUG, was found to be increased, but its distribution was different from that of SNAIL. SNAIL was detectable only in TCs, whilst SLUG was more pronounced in cells surrounding the fistula tract [31].
In a separate study, the same group demonstrated that TGF-β not only induces SNAI1 expression but also IL-13 at mRNA level in the fibroblasts of colonic lamina propria derived from fistulizing CD patients [32]. This expression was even validated at protein level in TCs covering the fistula tract, and in intestinal epithelial cells (IEC) of misshapen adjacent crypts. At the same time, IL-13 was scarcely detectable in specimens from active UC and basically absent in non-IBD mucosa controls. This observation was unexpected because, normally, IL-13 is expressed by immune cells [33], and correlates with fibrosis [34,35,36]. At the functional level, it has been proven that IL-13 causes an increased expression of genes associated with cell invasion into TCs [32], suggesting a synergistic step-by-step process whereby TGF-β induces EMT by causing epithelial disruption, possibly as part of regular wound healing during chronic intestinal inflammation, and IL-13 finally facilitate the EMT cells to penetrate into deeper tissue layers.
Along this migratory potential, the expression of Dickkpof-Homolog-1 (Dkk-1) [37,38] was also checked in fistulizing CD patients [39]. While Dkk-1 had weak expression in non-IBD controls, its expression was higher in the TCs layer and fibrotic area surrounding fistula, as well as in patients with active IBD [39].
Another study shows the increase of EMT phenomenon in patients with penetrating CD via an increased interaction between the Frizzled Class Receptor 4 (FDZ4) and the Wnt Family Member 2B (WNT2B). WNT2B induced the mRNA expression of cMYC, VIM, SNAI1, and SNAI2, but decreasing CDH1 one. In addition, WNT2B induced the phosphorylation of STAT3 in cells derived from intestinal crypts [40]. In patients with stricturing CD, the greater Wnt activation through cytoskeleton rearrangement and cell movement [10], might be also involved in the gain of EMT detection.
Ortiz-Masia and colleagues [23] demonstrated that in penetrating CD patients the SUCNR1 expression and succinate levels are increased in the fistula tract. More specifically, succinate is involved in the variation of Wnt ligands expression, activating the signaling cascade, and consequently provokes EMT.
In perianal fistulae, the accumulation of CD4+ CD161+ T-cells with a Th17, Th1, and both phenotypes have been described [23]. Other studies showed an altered balance between MMPs and their tissue inhibitors (TIMPs), resulting in aberrant extracellular matrix degradation [20]. Specifically, MMP-3 and 9 (expressed mostly in mononuclear cells and granulocytes respectively) were upregulated in the CD fistula area, while TIMP-1 to 3 expression was lower compared to normal colon tissue [21]. Differently, other types of MMPs such as MMP-1, MMP-2, MMP-7, and MMP-10 are downregulated or not expressed [20]. It is also important to mention that immunotherapy against MMP-9 seems to prevent intestinal fibrosis among CD complications [41].
Finally, in the last published work, patients with fistulizing CD showed more CD3+ CD8−- and this subset produced higher amounts of IL-13- and less CD3+ CD8+ T cells in blood compared to healthy donors. Particularly, around the fistula tract, CD4+ cells were highly present. Then, both cell subsets promoted the expression of EMT-related genes when co-cultured with HT-29 cells [42].
However, the knowledge of fistula pathogenesis and the precise contribution of the EMT process remain nowadays limited, and further studies are needed to better understand the process of fistula development.
4. Mucinous Adenocarcinoma as a Complication in CD Patients with Fistula
The risk of malignancy in CD patients is well recognized. Particularly, three types of cancers arise more frequently in patients with CD than in the general population: small bowel cancer, colorectal cancer, and mucinous carcinoma arising from perianal fistulae [43]. The mucinous adenocarcinoma (MA) represents approximately 2–3% of large bowel cancers [44], and even though is a rare disease, it is often associated with long-standing CD-fistula [45,46], with an increased incidence in the last 20 years [46]. Up to date, only a small number of MA arising from a chronic anorectal fistula have been described in the literature, especially in patients without CD, in case reports or small series [47]. The lack of a sufficient number of patients for trials makes it difficult to conduct functional studies, as well as to progress in diagnosis and treatment, surgical resection remaining the first choice.
To confirm the association between chronic perianal fistula and MA, Santos et al. [48] published a case report showing that a patient with persistent perianal fistulae developed as a para-rectal tumor. Then, the presence of MA was confirmed through histopathological biopsy.
Hugen et al. [49] listed the different causes of mucinous adenocarcinoma, such as genetic alterations, lifestyle variations, dietary changes, IBD, and radiotherapy, without including chronic anal fistula as a leading cause.
In 2018 [50], a group from India tried to explain the origin of MA from chronic anal fistula. In this study, ultrasound-guided biopsy and histopathology proved the development of MA in a patient with chronic fistula. Particularly, they highlighted that the persistence of the fistula from 4 to 10 years was consistently associated with the diagnosis.
Similarly, Diaz-Vico [51] reported three patients in whom chronic perianal fistula tissue gave rise to the development of a mucinous adenocarcinoma over time. The gold standard for the diagnosis remains histopathology, and it is characterized by the presence of extracellular mucinous lakes encompassed by well-differentiated dilated tortuous glands, nerves, and vessels.
In 2015, a case report aimed to clarify the root of the mucinous adenocarcinoma associated with chronic perianal fistula in CD patients [52]. In this study, the mucosal biopsies collected from the lesions showed granulomatous tissue infiltrated by mucus-producing adenocarcinoma. The study supported the hypothesis that the constant mucosal regeneration inside CD-related fistula is a common pathogenetic mechanism for developing carcinoma. At the same time, immunosuppressants and anti-TNF agents may also facilitate the malignant transformation.
The research in this field has various limitations that could affect the accuracy of the results. Foremost, the number of affected patients is scarce, thus making it impossible to get sufficient data. Most investigations were based on small samples. Another important limitation is that the condition is difficult to diagnose until the onset of symptoms, and the very long latency makes the researcher bored or interrupt the study.
5. Therapeutic Approaches to CD-Related Fistulae
Perianal fistulizing CD is a particularly challenging form of CD, presenting in up to one-third of the patients [53]; in a tiny percentage of cases, it can be the only manifestation of the disease and may precede by several years intestinal manifestations of CD in up to 10% of the patients [54]. The presence of fistulae is often associated with an aggressive form of CD, with chronic course and disappointing rates of long-lasting remission [55].
As a consequence, patients commonly experience a negative impact on quality of life, including intimate and social relationships and a frequent need for hospital admissions and medical observations [6].
For these reasons, the scientific community is constantly searching for new therapeutic tools, with the aim to respond to this unmet clinical need.
Current guidelines recommend multidisciplinary management with the cooperation of gastroenterologists, surgeons, radiologists, and nutritionists, and suggest a combined medical and surgical approach as the most effective treatment for complex perianal fistulae in CD [56,57].
The establishment of biologic therapy has dramatically improved the efficacy of medical treatment of CD fistulae compared to the previous use of traditional immunomodulators, and, at present, anti-TNFα represent the therapy of choice in these patients [56,58]. The effectiveness of biologic therapy basically depends on the capability of these drugs to reduce tissue inflammation, which is the driving mechanism for fistulae development.
Medical therapy alone has demonstrated remission rates around 60%, and its combination with surgery improves reaction, recurrence rate, as well time to recurrence [59].
Anyway, immunosuppression by anti-TNF agents needs to evaluate the presence of abscesses (and the possibility to resolve them by drainage and antibiotics), due to their potential septic complications.
The use of anti-TNFα has also been studied as to its local/topic injections, with the aim to potentiate the efficacy on fistula healing [60,61,62]. Although several reports have shown promising results, this technique has not been standardized yet, therefore it can be intended as a supportive tool in case other approaches have failed or are not available.
The use of biologics other than anti-TNFα (ustekinumab and vedolizumab) is not currently recommended as first-line therapy and should be considered only in case of contraindications to anti-TNFα [56].
Surgical treatment of perianal fistula associated with CD has the aim to adjuvate medical treatment, favoring fistula healing without compromising fecal continence. Depending on the type and extension of the fistula, the surgical approach can vary from simple drainage to more complex techniques [57]. The most common intervention is the application of setons to drain collections and control pelvic sepsis. This procedure should always be combined with medical therapy [54,63].
The closure of the fistula tract can be attempted by using different techniques, either endoscopic or surgical, and materials including fibrine glue, plugs, and n-butyl-2-cyanoacrylate (Histoacryl). Among these, fibrin glue injection is the most common technique, with a good safety profile and limited costs, although penalized by limited efficacy [64]. The insertion of fistula plugs has also been tested; the procedure consists of the application of a bio-absorbable xenograft which should promote tissue regeneration and fistula closure. This technique has demonstrated a success rate equal to seton drainage [65], hence it is not recommended.
Considering endoscopic techniques, fistula closure can be achieved by clipping with either through-the-scope or over-the-scope clips [66]. Clipping is effective in fistula closure, being a safe and simple procedure in the hands of trained endoscopists.
The advancement flap is probably the most used among the surgical techniques. The procedure was first developed for treating cryptoglandular fistulae but is now routinely applied also in CD patients. Fistula healing is complete in about half of the patients [67].
Of comparable efficacy, ligation of the intersphincteric fistula tract (LIFT), which was also initially described in the treatment of cryptoglandular fistulae and has been then transferred to CD associated fistulae [54]. Consisting of the opening of the intersphincteric groove, dissection, and isolation of the fistulous tract, ligation of the tract with interrupted sutures and closure of the perianal wound LIFT is a demanding procedure and should always be performed by dedicated surgeons. Compared to advanced flap procedure, LIFT has demonstrated lower incontinence rates (7.8% versus 1.6%) [68].
In order to preserve the sphincter functionality, the video-assisted anal fistula treatment (VAAFT) consists of the video-assisted inspection of the fistula followed by a precise cauterization of the fistula tract from the external towards the internal margin, and closure of the internal opening. Although not yet routinely applied in CD fistula surgery, this technique appears promising, especially for the benefits of sphincteric preservation [69]. Similar to VAAFT, the fistula laser closure technique applies laser instead of electrocautery and is not performed under direct vision. This technique has similar efficacy to VAAFT but shorter learning curves [70].
Overall, surgery for CD fistulae should always be performed by expert operators in high-flow centers after adequate study of the clinical case. Local availability and expertise should guide the choice of the technique.
The limited success rate of combined medical and surgical therapy, although being slightly improved, has promoted the research of novel methods. One of the most promising is the injection in the fistula tract of mesenchymal stem cells (MSCs), aimed at tissue regeneration using a minimally invasive procedure [71].
MSCs are non-hematopoietic multipotent cells, which can be set apart from connective tissues, like adipose tissue, and from the bone marrow. These cells have been studied in fistula treatment due to their immunomodulatory, immunosuppressive, and regenerative properties [72]. Their use as treatment of CD fistulae has been described by Panés et al. in the ADMIRE trial [73] with promising results in terms of efficacy and safety, which paved the way to their entrance also in the last ECCO guidelines [57].
The progressive in-depth analysis on the pathogenetic mechanisms of CD fistulae has allowed hypothesizing new promising therapeutic tools, such as anti-MMP antibodies. Studies about anti-MMP drugs start from the assumption that MMP-9, a type IV collagenase, has a central role in tissue remodeling and is upregulated in crypt abscesses and around fistulae [74]. A study by Fontani et al. [75] showed in vitro that N-acetylcysteine and curcumin were able to downregulate MMP-3 in high oxidative state conditions, and specifically in TNFα stimulated cells, suggesting that such antioxidants may have a therapeutic use for the prevention and treatment of fistulae in the gut of CD patients.
Another study by Goffin et al. [41] was conducted in vitro from human specimens and in mice xenograft, confirming in the patients affected by penetrating CD the upregulation of MMP-9, and showing in mice a protective effect of anti-MMP antibodies with respect to intestinal fibrosis. Albeit in literature only animal- and in vitro studies are available, the future application of such molecules could revolutionize the treatment of perianal fistulae.
The understanding of the crucial role of inflammation in fistula development has sustained the still limited but promising application of hyperbaric oxygen therapy as supportive treatment in patients affected by perianal CD [76]. The treatment consists of breathing 100% oxygen under increased atmospheric pressure, provoking tissue hyperoxygenation and oxidative stress which has been associated to stem cell mobilization and upregulation of growth factors and ultimately to anti-inflammatory effects. Considering its safety and limited costs if the equipment is available, this treatment appears a valid supportive method for patients with otherwise unsatisfactory healing [77].
6. Conclusions
Despite the availability of multiple treatment options, perianal fistulizing CD represents a therapeutic challenge and is associated with an important impact on patients’ quality of life. To date, the most effective management is multidisciplinary with the cooperation of gastroenterologists, surgeons, radiologists, and nutritionists and the best recommended treatment is a combination of medical and surgical approaches. In the last decades, the broadened knowledge about fistulae pathogenesis has allowed a surge of novel therapeutic tools: some are already applied, such as MSCs injection, some others show potentiality, such as the administration of anti-MMP antibodies. Overall, the best therapeutic option should be always tailored to the patient’s clinical condition. Based on the variable response to standard treatments, a new supportive tool should be considered.
Author Contributions
Writing—original draft preparation, F.R., L.G., A.d.C., F.G.; writing—review and editing, S.V., L.L., S.B., D.P.; visualization, F.R., S.V.; supervision, S.B., D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Piovani D., Danese S., Peyrin-Biroulet L., Nikolopoulos G.K., Lytras T., Bonovas S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-Analyses. Gastroenterology. 2019;157:647–659.e4. doi: 10.1053/j.gastro.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 2.Baumgart D.C., Sandborn W.J. Crohn’s disease. Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 3.Torres J., Mehandru S., Colombel J.F., Peyrin-Biroulet L. Crohn’s disease. Lancet. 2017;389:1741–1755. doi: 10.1016/S0140-6736(16)31711-1. [DOI] [PubMed] [Google Scholar]
- 4.Peyrin-Biroulet L., Harmsen W.S., Tremaine W.J., Zinsmeister A.R., Sandborn W.J., Loftus E.V. Cumulative Length of Bowel Resection in a Population-Based Cohort of Patients With Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2016;14:1439–1444. doi: 10.1016/j.cgh.2016.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thia K.T., Sandborn W.J., Harmsen W.S., Zinsmeister A.R., Loftus E.V. Risk Factors Associated with Progression to Intestinal Complications of Crohn’s Disease in a Population-Based Cohort. Gastroenterology. 2010;139:1147–1155. doi: 10.1053/j.gastro.2010.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adegbola S.O., Dibley L., Sahnan K., Wade T., Verjee A., Sawyer R., Mannick S., McCluskey D., Yassin N., Phillips R.K.S., et al. Burden of disease and adaptation to life in patients with Crohn’s perianal fistula: A qualitative exploration. Health Qual. Life Outcomes. 2020;18:370. doi: 10.1186/s12955-020-01622-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bataille F., Klebl F., Rümmele P., Schroeder J., Farkas S., Wild P.-J., Fürst A., Hofstädter F., Schölmerich J., Herfarth H., et al. Morphological characterisation of Crohn’s disease fistulae. Gut. 2004;53:1314–1321. doi: 10.1136/gut.2003.038208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meier J.K.H., Scharl M., Miller S.N., Brenmoehl J., Hausmann M., Kellermeier S., Schölmerich J., Rogler G. Specific differences in migratory function of myofibroblasts isolated from Crohn’s disease fistulae and strictures. Inflamm. Bowel Dis. 2011;17:202–212. doi: 10.1002/ibd.21344. [DOI] [PubMed] [Google Scholar]
- 9.Botaille F., Rohrmeier C., Bates R., Weber A., Reider F., Brenmoehl J., Strauch U., Farkas S., Fǔrst A., Hofstäáter F., et al. Evidence for a role of epithelial mesenchymal transition during pathogenesis of fistulae in Crohn’s disease. Inflamm. Bowel Dis. 2008;14:1514–1527. doi: 10.1002/ibd.20590. [DOI] [PubMed] [Google Scholar]
- 10.Feagan B.G., Schwartz D., Danese S., Rubin D.T., Lissoos T.W., Xu J., Lasch K. Efficacy of Vedolizumab in Fistulising Crohn’s Disease: Exploratory Analyses of Data from GEMINI 2. J. Crohn’s Colitis. 2018;12:621–626. doi: 10.1093/ecco-jcc/jjy019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sokol H., Landman C., Seksik P., Berard L., Montil M., Nion-Larmurier I., Bourrier A., Le Gall G., Lalande V., De Rougemont A., et al. Fecal microbiota transplantation to maintain remission in Crohn’s disease: A pilot randomized controlled study. Microbiome. 2020;8:12. doi: 10.1186/s40168-020-0792-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haac B.E., Palmateer N.C., Seaton M.E., Van Yperen R., Fraser C.M., Bafford A.C. A Distinct Gut Microbiota Exists within Crohn’s Disease–Related Perianal Fistulae. J. Surg. Res. 2019;242:118–128. doi: 10.1016/j.jss.2019.04.032. [DOI] [PubMed] [Google Scholar]
- 13.Bullman S., Pedamallu C.S., Sicinska E., Clancy T.E., Zhang X., Cai D., Neuberg D., Huang K., Guevara F., Nelson T., et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science. 2017;358:1443–1448. doi: 10.1126/science.aal5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu T., Guo F., Yu Y., Sun T., Ma D., Han J., Qian Y., Kryczek I., Sun D., Nagarsheth N., et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell. 2017;170:548–563.e16. doi: 10.1016/j.cell.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panés J., Rimola J. Perianal fistulizing Crohn’s disease: Pathogenesis, diagnosis and therapy. Nat. Rev. Gastroenterol. Hepatol. 2017;14:652–664. doi: 10.1038/nrgastro.2017.104. [DOI] [PubMed] [Google Scholar]
- 16.Tozer P.J., Whelan K., Phillips R.K.S., Hart A.L. Etiology of perianal Crohn’s disease: Role of genetic, microbiological, and immunological factors. Inflamm. Bowel Dis. 2009;15:1591–1598. doi: 10.1002/ibd.21026. [DOI] [PubMed] [Google Scholar]
- 17.Parks A.G. Pathogenesis and Treatment of Fistula-in-Ano. Br. Med. J. 1961;1:463–469. doi: 10.1136/bmj.1.5224.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yassin N.A., Bernardo D., Mallappa S. The presence of endotoxins in Crohn’s and idiopathic perianal fistula tracks. United Eur. Gastroenterol. J. 2013;1:A506. [Google Scholar]
- 19.van Onkelen R.S., Mitalas L.E., Gosselink M.P., van Belkum A., Laman J.D., Schouten W.R. Assessment of microbiota and peptidoglycan in perianal fistulas. Diagn. Microbiol. Infect. Dis. 2013;75:50–54. doi: 10.1016/j.diagmicrobio.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Kirkegaard T., Hansen A., Bruun E., Brynskov J. Expression and localisation of matrix metalloproteinases and their natural inhibitors in fistulae of patients with Crohn’s disease. Gut. 2004;53:701–709. doi: 10.1136/gut.2003.017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Onkelen R.S., Gosselink M.P., Van Meurs M., Melief M.J., Schouten W.R., Laman J.D. Pro-inflammatory cytokines in cryptoglandular anal fistulas. Tech. Coloproctol. 2016;20:619–625. doi: 10.1007/s10151-016-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plevy S.E., Landers C.J., Prehn J., Carramanzana N.M., Deem R.L., Shealy D., Targan S.R. A role for TNF-alpha and mucosal T helper-1 cytokines in the pathogenesis of Crohn’s disease. J. Immunol. 1997;159:6276–6282. [PubMed] [Google Scholar]
- 23.Ortiz-Masiá D., Gisbert-Ferrándiz L., Bauset C., Coll S., Mamie C., Scharl M., Esplugues J.V., Alós R., Navarro F., Cosín-Roger J., et al. Succinate Activates EMT in Intestinal Epithelial Cells through SUCNR1: A Novel Protagonist in Fistula Development. Cells. 2020;9:1104. doi: 10.3390/cells9051104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiehne K., Fincke A., Brunke G., Lange T., Fölsch U.R., Herzig K.-H. Antimicrobial peptides in chronic anal fistula epithelium. Scand. J. Gastroenterol. 2007;42:1063–1069. doi: 10.1080/00365520701320489. [DOI] [PubMed] [Google Scholar]
- 25.Ratto C., Litta F., Lucchetti D., Parello A., Boninsegna A., Arena V., Donisi L., Calapà F., Sgambato A. Immunopathological characterization of cryptoglandular anal fistula: A pilot study investigating its pathogenesis. Color. Dis. 2016;18:O436–O444. doi: 10.1111/codi.13527. [DOI] [PubMed] [Google Scholar]
- 26.Maggi L., Capone M., Giudici F., Santarlasci V., Querci V., Liotta F., Ficari F., Maggi E., Tonelli F., Annunziato F., et al. CD4+CD161+ T lymphocytes infiltrate crohn’s disease-associated perianal fistulas and are reduced by anti-TNF-α local therapy. Int. Arch. Allergy Immunol. 2013;161:81–86. doi: 10.1159/000343467. [DOI] [PubMed] [Google Scholar]
- 27.Haddow J.B., Musbahi O., Macdonald T.T., Knowles C.H. Comparison of cytokine and phosphoprotein profiles in idiopathic and Crohn’s disease-related perianal fistula. World J. Gastrointest. Pathophysiol. 2019;10:42–53. doi: 10.4291/wjgp.v10.i4.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adegbola S.O., Sarafian M., Sahnan K., Ding N.S., Faiz O.D., Warusavitarne J., Phillips R.K.S., Tozer P.J., Holmes E., Hart A.L. Differences in amino acid and lipid metabolism distinguish Crohn’s from idiopathic/cryptoglandular perianal fistulas by tissue metabonomic profiling and may offer clues to underlying pathogenesis. Eur. J. Gastroenterol. Hepatol. 2021;33:1469–1479. doi: 10.1097/MEG.0000000000001976. [DOI] [PubMed] [Google Scholar]
- 29.Jia D., Park J.H., Kaur H., Jung K.H., Yang S., Tripathi S., Galbraith M., Deng Y., Jolly M.K., Kaipparettu B.A., et al. Towards decoding the coupled decision-making of metabolism and epithelial-to-mesenchymal transition in cancer. Br. J. Cancer. 2021;124:1902–1911. doi: 10.1038/s41416-021-01385-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scharl M., Weber A., Fürst A., Farkas S., Jehle E., Pesch T., Kellermeier S., Fried M., Rogler G. Potential role for snail family transcription factors in the etiology of Crohn’s disease-associated fistulae. Inflamm. Bowel Dis. 2011;17:1907–1916. doi: 10.1002/ibd.21555. [DOI] [PubMed] [Google Scholar]
- 32.Scharl M., Frei S., Pesch T., Kellermeier S., Arikkat J., Frei P., Fried M., Weber A., Jehle E., Rühl A., et al. Interleukin-13 and transforming growth factor β synergise in the pathogenesis of human intestinal fistulae. Gut. 2013;62:63–72. doi: 10.1136/gutjnl-2011-300498. [DOI] [PubMed] [Google Scholar]
- 33.Wynn T.A. IL-13 Effector Functions. Annu. Rev. Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 34.Chiaramonte M.G., Donaldson D.D., Cheever A.W., Wynn T.A. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2–dominated inflammatory response. J. Clin. Investig. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu Z., Homer R.J., Wang Z., Chen Q., Geba G.P., Wang J., Zhang Y., Elias J.A. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J. Clin. Investig. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasegawa M., Fujimoto M., Kikuchi K., Takehara K. Elevated serum levels of interleukin 4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosis. J. Rheumatol. 1997;24:328–332. [PubMed] [Google Scholar]
- 37.Aguilera O., Peña C., García J.M., Larriba M.J., Ordóñez-Morán P., Navarro D., Barbáchano A., de Silanes I.L., Ballestar E., Fraga M.F., et al. The Wnt antagonist DICKKOPF-1 gene is induced by 1α,25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis. 2007;28:1877–1884. doi: 10.1093/carcin/bgm094. [DOI] [PubMed] [Google Scholar]
- 38.González-Sancho J.M., Aguilera O., García J.M., Pendás-Franco N., Peña C., Cal S., De Herreros A.G., Bonilla F., Muñoz A. The Wnt antagonist DICKKOPF-1 gene is a downstream target of β-catenin/TCF and is downregulated in human colon cancer. Oncogene. 2005;24:1098–1103. doi: 10.1038/sj.onc.1208303. [DOI] [PubMed] [Google Scholar]
- 39.Frei S.M., Hemsley C., Pesch T., Lang S., Weber A., Jehle E., Rühl A., Fried M., Rogler G., Scharl M. The Role for Dickkopf-Homolog-1 in the Pathogenesis of Crohn’s Disease-Associated Fistulae. PLoS ONE. 2013;8:e78882. doi: 10.1371/journal.pone.0078882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ortiz-Masià D., Salvador P., Macias-Ceja D.C., Gisbert-Ferrándiz L., Esplugues J.V., Manyé J., Alós R., Navarro-Vicente F., Mamie C., Scharl M., et al. WNT2b Activates Epithelial-mesenchymal Transition Through FZD4: Relevance in Penetrating Crohńs Disease. J. Crohn’s Colitis. 2020;14:230–239. doi: 10.1093/ecco-jcc/jjz134. [DOI] [PubMed] [Google Scholar]
- 41.Goffin L., Fagagnini S., Vicari A., Mamie C., Melhem H., Weder B., Lutz C., Lang S., Scharl M., Rogler G., et al. Anti-MMP-9 Antibody: A promising therapeutic strategy for treatment of inflammatory bowel disease complications with fibrosis. Inflamm. Bowel Dis. 2016;22:2041–2057. doi: 10.1097/MIB.0000000000000863. [DOI] [PubMed] [Google Scholar]
- 42.Bruckner R.S., Spalinger M.R., Barnhoorn M.C., Feakins R., Fuerst A., Jehle E.C., Rickenbacher A., Turina M., Niechcial A., Lang S., et al. Contribution of CD3+CD8− and CD3+CD8+ T Cells to TNF-α Overexpression in Crohn Disease–Associated Perianal Fistulas and Induction of Epithelial-Mesenchymal Transition in HT-29 Cells. Inflamm. Bowel Dis. 2021;27:538–549. doi: 10.1093/ibd/izaa240. [DOI] [PubMed] [Google Scholar]
- 43.Bautista J.M., Marín-Jiménez I., Moreno L.H. A Perianal Mass in a Crohn’s Disease Patient. Gastroenterology. 2012;142:12–187. doi: 10.1053/j.gastro.2010.12.046. [DOI] [PubMed] [Google Scholar]
- 44.Yang B.-L., Shao W.-J., Sun G.-D., Chen Y.-Q., Huang J.-C. Perianal mucinous adenocarcinoma arising from chronic anorectal fistulae: A review from single institution. Int. J. Colorectal Dis. 2009;24:1001–1006. doi: 10.1007/s00384-009-0657-7. [DOI] [PubMed] [Google Scholar]
- 45.Ky A., Sohn N., Weinstein M.A., Korelitz B.I. Carcinoma arising in anorectal fistulas of Crohn’s disease. Dis. Colon Rectum. 1998;41:992–996. doi: 10.1007/BF02237388. [DOI] [PubMed] [Google Scholar]
- 46.Iesalnieks I., Gaertner W.B., Glaß H., Strauch U., Hipp M., Agha A., Schlitt H.J. Fistula-associated anal adenocarcinoma in Crohn’s disease. Inflamm. Bowel Dis. 2010;16:1643–1648. doi: 10.1002/ibd.21228. [DOI] [PubMed] [Google Scholar]
- 47.Smith R., Hicks D., Tomljanovich P.I., Lele S.B., Rajput A., Bullard Dunn K. Adenocarcinoma arising from chronic perianal Crohn’s disease: Case report and review of the literaturetle. Am. Surg. 2008;74:59–61. doi: 10.1177/000313480807400113. [DOI] [PubMed] [Google Scholar]
- 48.Santos M.D., Nogueira C., Lopes C. Mucinous Adenocarcinoma Arising in Chronic Perianal Fistula: Good Results with Neoadjuvant Chemoradiotherapy Followed by Surgery. Case Rep. Surg. 2014;2014:386150. doi: 10.1155/2014/386150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hugen N., van Beek J.J.P., De Wilt J.H.W., Nagtegaal I.D. Insight into Mucinous Colorectal Carcinoma: Clues from Etiology. Ann. Surg. Oncol. 2014;21:2963–2970. doi: 10.1245/s10434-014-3706-6. [DOI] [PubMed] [Google Scholar]
- 50.Prasad S.N., Razik A., Siddiqui F., Lal H. Mucinous adenocarcinoma arising from chronic perianal fistula mimicking horseshoe abscess. BMJ Case Rep. 2018;2018:bcr-2017. doi: 10.1136/bcr-2017-223063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Díaz-Vico T., Fernández-Martínez D., García-Gutiérrez C., Suárez-Sánchez A., Cifrián-Canales I., Mendoza-Pacas G.E., Sánchez-Farpón H., Truán-Alonso N. Mucinous adenocarcinoma arising from chronic perianal fistula-a multidisciplinary approach. J. Gastrointest. Oncol. 2019;10:589–596. doi: 10.21037/jgo.2019.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Papaconstantinou I., Mantzos D.S., Kondi-Pafiti A., Koutroubakis I.E. Anal adenocarcinoma complicating chronic Crohn’s disease. Int. J. Surg. Case Rep. 2015;10:201–203. doi: 10.1016/j.ijscr.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwartz D.A., Ghazi L.J., Regueiro M., Fichera A., Zoccali M., Ong E.M.W., Mortelé K.J. Guidelines for the multidisciplinary management of Crohn’s perianal fistulas: Summary statement. Inflamm. Bowel Dis. 2015;21:723–730. doi: 10.1097/MIB.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 54.Kotze P.G., Shen B., Lightner A., Yamamoto T., Spinelli A., Ghosh S., Panaccione R. Modern management of perianal fistulas in Crohn’s disease: Future directions. Gut. 2018;67:1181–1194. doi: 10.1136/gutjnl-2017-314918. [DOI] [PubMed] [Google Scholar]
- 55.van Koperen P.J., Safiruddin F., Bemelman W.A., Slors J.F.M. Outcome of surgical treatment for fistula in ano in Crohn’s disease. Br. J. Surg. 2009;96:675–679. doi: 10.1002/bjs.6608. [DOI] [PubMed] [Google Scholar]
- 56.Torres J., Bonovas S., Doherty G., Kucharzik T., Gisbert J.P., Raine T., Adamina M., Armuzzi A., Bachmann O., Bager P., et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn’s Colitis. 2020;14:4–22. doi: 10.1093/ecco-jcc/jjz180. [DOI] [PubMed] [Google Scholar]
- 57.Adamina M., Bonovas S., Raine T., Spinelli A., Warusavitarne J., Armuzzi A., Bachmann O., Bager P., Biancone L., Bokemeyer B., et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Surgical Treatment. J. Crohn’s Colitis. 2020;14:155–168. doi: 10.1093/ecco-jcc/jjz187. [DOI] [PubMed] [Google Scholar]
- 58.Sands B.E., Anderson F.H., Bernstein C.N., Chey W.Y., Feagan B.G., Fedorak R.N., Kamm M.A., Korzenik J.R., Lashner B.A., Onken J.E., et al. Infliximab Maintenance Therapy for Fistulizing Crohn’s Disease. N. Engl. J. Med. 2004;350:876–885. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 59.Regueiro M., Mardini H. Treatment of Perianal Fistulizing Crohn’s Disease with Infliximab Alone or as an Adjunct to Exam under Anesthesia with Seton Placement. Inflamm. Bowel Dis. 2003;9:98–103. doi: 10.1097/00054725-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Poggioli G., Laureti S., Pierangeli F., Rizzello F., Ugolini F., Gionchetti P., Campieri M. Local Injection of Infliximab for the Treatment of Perianal Crohn’s Disease. Dis. Colon Rectum. 2005;48:768–774. doi: 10.1007/s10350-004-0832-4. [DOI] [PubMed] [Google Scholar]
- 61.Tonelli F., Giudici F., Asteria C.R. Effectiveness and safety of local adalimumab injection in patients with fistulizing perianal crohn’s disease: A pilot study. Dis. Colon Rectum. 2012;55:870–875. doi: 10.1097/DCR.0b013e31825af532. [DOI] [PubMed] [Google Scholar]
- 62.Asteria C.R., Ficari F., Bagnoli S., Milla M., Tonelli F. Treatment of perianal fistulas in Crohn’s disease by local injection of antibody to TNF-α accounts for a favourable clinical response in selected cases: A pilot study. Scand. J. Gastroenterol. 2006;41:1064–1072. doi: 10.1080/00365520600609941. [DOI] [PubMed] [Google Scholar]
- 63.Sica G.S., Di Carlo S., Tema G., Montagnese F., Blanco G.D.V., Fiaschetti V., Maggi G., Biancone L. Treatment of peri-anal fistula in Crohn’s disease. World J. Gastroenterol. 2014;20:13205–13210. doi: 10.3748/wjg.v20.i37.13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Parades V., Far H.S., Etienney I., Zeitoun J.-D., Atienza P., Bauer P. Seton drainage and fibrin glue injection for complex anal fistulas. Color. Dis. 2010;12:459–463. doi: 10.1111/j.1463-1318.2009.01811.x. [DOI] [PubMed] [Google Scholar]
- 65.Senéjoux A., Siproudhis L., Abramowitz L., Munoz-Bongrand N., Desseaux K., Bouguen G., Bourreille A., Dewit O., Stefanescu C., Vernier G., et al. Fistula Plug in Fistulising Ano-Perineal Crohn’s Disease: A Randomised Controlled Trial. J. Crohn’s Colitis. 2016;10:141–148. doi: 10.1093/ecco-jcc/jjv162. [DOI] [PubMed] [Google Scholar]
- 66.Mennigen R., Laukötter M., Senninger N., Rijcken E. The OTSC® proctology clip system for the closure of refractory anal fistulas. Tech. Coloproctol. 2015;19:241–246. doi: 10.1007/s10151-015-1284-7. [DOI] [PubMed] [Google Scholar]
- 67.Tozer P.J., Burling D., Gupta A., Phillips R.K.S., Hart A.L. Review article: Medical, surgical and radiological management of perianal Crohn’s fistulas. Aliment. Pharmacol. Ther. 2011;33:5–22. doi: 10.1111/j.1365-2036.2010.04486.x. [DOI] [PubMed] [Google Scholar]
- 68.Stellingwerf M.E., van Praag E.M., Tozer P.J., Bemelman W.A., Buskens C.J. Systematic review and meta-analysis of endorectal advancement flap and ligation of the intersphincteric fistula tract for cryptoglandular and Crohn’s high perianal fistulas. BJS Open. 2019;3:231–241. doi: 10.1002/bjs5.50129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meinero P., Mori L. Video-assisted anal fistula treatment (VAAFT): A novel sphincter-saving procedure for treating complex anal fistulas. Tech. Coloproctol. 2011;15:417–422. doi: 10.1007/s10151-011-0769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilhelm A., Fiebig A., Krawczak M. Five years of experience with the FiLaCTM laser for fistula-in-ano management: Long-term follow-up from a single institution. Tech. Coloproctol. 2017;21:269–276. doi: 10.1007/s10151-017-1599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guadalajara H., García-Arranz M., Herreros M.D., Borycka-Kiciak K., Lightner A.L., García-Olmo D. Mesenchymal stem cells in perianal Crohn’s disease. Tech. Coloproctol. 2020;24:883–889. doi: 10.1007/s10151-020-02250-5. [DOI] [PubMed] [Google Scholar]
- 72.Jiang X.-X., Zhang Y., Liu B., Zhang S.-X., Wu Y., Yu X.-D., Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 73.Panés J., García-Olmo D., Van Assche G., Colombel J.F., Reinisch W., Baumgart D.C., Dignass A., Nachury M., Ferrante M., Kazemi-Shirazi L., et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: A phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281–1290. doi: 10.1016/S0140-6736(16)31203-X. [DOI] [PubMed] [Google Scholar]
- 74.Aguirre J.E., Beswick E.J., Grim C., Uribe G., Tafoya M., Palma G.C., Samedi V., McKee R., Villeger R., Fofanov Y., et al. Matrix metalloproteinases cleave membrane-bound PD-L1 on CD90+ (myo-)fibroblasts in Crohn’s disease and regulate Th1/Th17 cell responses. Int. Immunol. 2020;32:57–68. doi: 10.1093/intimm/dxz060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fontani F., Marcucci T., Picariello L., Tonelli F., Vincenzini M.T., Iantomasi T. Redox regulation of MMP-3/TIMP-1 ratio in intestinal myofibroblasts: Effect of N-acetylcysteine and curcumin. Exp. Cell Res. 2014;323:77–86. doi: 10.1016/j.yexcr.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 76.Dulai P.S., Gleeson M.W., Taylor D., Holubar S.D., Buckey J.C., Siegel C.A. Systematic review: The safety and efficacy of hyperbaric oxygen therapy for inflammatory bowel disease. Aliment. Pharmacol. Ther. 2014;39:1266–1275. doi: 10.1111/apt.12753. [DOI] [PubMed] [Google Scholar]
- 77.Lansdorp C.A., Gecse K.B., Buskens C.J., Löwenberg M., Stoker J., Bemelman W.A., D’Haens G.R.A.M., van Hulst R.A. Hyperbaric oxygen therapy for the treatment of perianal fistulas in 20 patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2021;53:587–597. doi: 10.1111/apt.16228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.