Abstract
Five new C2-symmetric chiral ligands of 2,5-bis(imidazolinyl)thiophene (L1–L3) and 2,5-bis(oxazolinyl)thiophene (L4 and L5) were synthesized from thiophene-2,5-dicarboxylic acid (1) with enantiopure amino alcohols (4a–c) in excellent optical purity and chemical yield. The utility of these new chiral ligands for Friedel–Crafts asymmetric alkylation was explored. Subsequently, the optimized tridentate ligand L5 and Cu(OTf)2 catalyst (15 mol%) in toluene for 48 h promoted Friedel–Crafts asymmetric alkylation in moderate to good yields (up to 76%) and with good enantioselectivity (up to 81% ee). The bis(oxazolinyl)thiophene ligands were more potent than bis(imidazolinyl)thiophene analogues for the asymmetric induction of the Friedel–Crafts asymmetric alkylation.
Keywords: bis-oxazoline, bis-imidazoline, thiophene, indoles, β-nitroolefins, asymmetric catalysis, Friedel−Crafts alkylation
1. Introduction
Metal-catalyzed asymmetric transformation has become one of the most desirable strategies in advanced synthetic chemistry to access a variety of enantiopure organic molecules [1,2,3,4,5,6,7,8]. The optically active system can be achieved by means of various methodologies, such as chiral ligands assisted organocatalysis [9,10,11] and enzyme-catalyzed asymmetric conversion [12,13,14]. In addition, more advanced and refined approaches have been introduced effectively, such as stereo-convergent [15,16,17] and stereo-divergent synthesis [18,19,20,21] in order to acquire innumerable chiral frameworks.
Chiral ligand–Lewis acid metal complex-catalyzed asymmetric Friedel–Crafts alkylation reactions play a pivotal role in synthetic organic chemistry for the construction of new C–C bonds [22,23,24,25,26]. During the past few years, several chiral bidendate ligands have been developed and used in the Lewis acid metal-catalyzed asymmetric Friedel–Crafts alkylation reaction of indole with various substrates, including α,β-unsaturated-R-ketoesters (R = alkyl, aryl) [27,28], R-hydroxy enones (R = alkyl, aryl) [29,30], alkylidene malonates [31,32,33], acyl phosphonates [34,35], acyl heterocyclic compounds [36,37,38], N-sulfonyl aldimines catalyzed by Schiff base complexes of Cu(II)-chiral amino alcohol [39], α-trifluoromethylated β-nitrostyrenes catalyzed by chiral BINOL metal phosphate [40], nitroolefins catalyzed by oxazoline-imidazoline-Zn(II) [41], bis(oxazolinyl)-Cu(II) [42] and 2,5-bis(oxazolinyl)thiophenes-Cu(II) complexes [43]. Very recently, Tanaka et al. have documented homochiral metal–organic framework-catalyzed enantioselective Friedel–Crafts alkylation of N,N-dialkylanilines with trans-β-nitrostyrene [44]. That being said, very few examples of chiral metal–box-bis(oxazoline)/bis(imidazoline) complex-catalyzed enantioselective Friedel–Crafts alkylation of indole with nitroolefins have been documented to date [41,45,46,47].
In recent years, the application of nitroolefins as electrophiles has also been gaining notable interest among pharmacists due to the activation functionality of the nitro groups, which facilitate easy conversion to other useful functional groups to achieve numerous eye-catching chemical entities [48,49]. Furthermore, optically active Friedel–Crafts-alkylated product of indole with nitroolefins can also serve as an antecedent for the preparation of various drug molecules such as physostigmine [50,51], which acts as a clinically active anticholinergic drug [52], Recently, some examples of nitroalkenes have also been reported as Michael acceptors in metal-catalyzed asymmetric reaction due to the presence of strong electron-withdrawing nitro-groups [48,53,54] e.g., rhodium-catalyzed additions of boronic acids to nitroalkenes [55], copper-catalyzed dialkylzinc additions to nitroalkenes [56,57], conjugated reductions of nitroalkenes [58] and the organo-catalyzed additions of 1,3-dicarbonyl compounds to nitroalkenes [59,60].
Moreover, to date, most of the research work has been done with the main family of chiral ligands predominantly belonging to di-phosphine, diamine, di-ol, etc., i.e., phosphorous-, nitrogen- and oxygen-containing substrate. Very little research has been done in the recent past on developing chiral ligands based on sulfur-containing compounds. Therefore, researchers are highly interested in developing new chiral ligands based on a sulfur-containing moiety due to their high coordination ability to the most of the transition metals [61]. The sulfur atom is also considered as a soft atom that can bind strongly to soft metals, in particular copper metal Cu(II). In addition, sulfur-containing ligands are poor π-acceptors and poor σ-donors as compared to phosphine ligands, resulting in strong metal–sulfur bond strength. However, sulfur-containing ligand precursors are easily available, having extra advantages such as easy storage due to their higher tolerance to air as compared to phosphine-containing ligands, which makes them highly stable [61].
Recently, chiral ligand–Lewis acid-catalyzed asymmetric induction of indole with prochiral β-nitroolefin has become one of the most significant and successful pathways for accessing highly functionalized optically pure building blocks. Our research group has reported a new catalytic system based on the Cu(II) metal/chiral thiophene-2,5-bis(β-amino alcohol) ligands for an asymmetric Henry reaction of nitromethane with aromatic aldehyde with excellent ee (up to 94.6%) and chemical yield (up to 99%) [62]. In continuation of our research program, therefore, the design and synthesis of novel chiral 2,5-bis(imidazolinyl)thiophene and 2,5-bis(oxazolinyl)thiophene box-type ligands and their applications in various asymmetric catalyses remains a remarkable and interesting research topic to organic chemists. However, chiral ligands based on 2,5-bis(imidazolinyl)thiophene and 2,5-bis(oxazolinyl)thiophene framework could also be advantageous for several asymmetric transformations other than Friedel–Crafts alkylation reactions, such as asymmetric Henry reactions [63,64], Diels–Alder reactions [65,66], enantioselective additions of diethylzinc to acyclic enones [67,68,69], asymmetric allylic substitutions [70,71] and asymmetric cyclopropanation [72,73] reactions, etc. Keeping in mind the wide range of chiral applications of 2,5-bis(imidazolinyl)thiophene and 2,5-bis(oxazolinyl)thiophene box-type ligands and the diverse functionality of nitroolefins, we have decided to focus on this particular research field.
In this research article, we report the synthesis of novel chiral ligands thiophene-2,5-2,5-bis(imidazolinyl)thiophene (L1–L3) and thiophene-2,5-bis(oxazolinyl)thiophene (L4 and L5) and their applications in Lewis acid metal-catalyzed asymmetric Friedel–Crafts alkylations of indole with electron-deficient prochiral β-nitroolefins.
Figure 1 shows some of the previously reported potent ligand structures used for asymmetric Friedel–Crafts alkylation reactions of indole with β-nitrostyrenes [41,42,62,74,75,76,77,78].
Figure 1.
Previously reported potent ligand structures for asymmetric FC reaction.
2. Results and Discussion
2.1. Synthesis of chiral 2,5-bis(imidazolinyl)thiophene (L1–L3) and 2,5-bis(oxazolinyl)thiophene (L4 and L5)
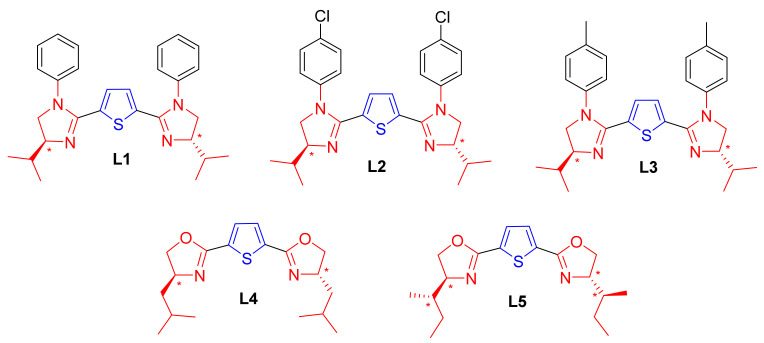
Two set of C2-symmetric 2,5-bis(imidazolinyl)thiophene (L1–L3) and 2,5-bis(oxazolinyl)thiophene (L4 and L5) ligands, based on thiophene framework, were synthesized from readily available and cheap thiophene-2,5-dicarboxlyic acid (1) and chiral amino alcohols (3a–c) using well-known procedures reported in the literature [79] in five steps, as shown in Scheme 1. At the very outset, thiophene-2,5-dicarboxlyic acid (1) was treated with thionylchloride (SOCl2) in the presence of a catalytic amount of N,N-dimethylformamide (DMF 2-3 drops) under reflux for 24 h, leading to the formation of acid chloride (2) in quantitative yields (crude), which was then allowed to react with three different amino alcohols (3a–c) in the presence of excess triethylamine (TEA) in dichloromethane (CH2Cl2) to produce thiophene-2,5-dicarboxamide alcohol derivatives (4a–c) with overall excellent isolated yield (75–97%). Thiophene-2,5-dicarboxamide alcohol (4a) was then refluxed in thionylchloride (SOCl2) for 24 h to afford crude thiophene-2,5-dicarboxamide dichloride (5a), which served as an intermediate for the synthesis of our target ligands L1–L3, while thiophene-2,5-dicarboxamide alcohol (4b–c) was chosen as the precursor for the synthesis of ligands L4 and L5 (Figure 2).
Scheme 1.
2,5-bis(imidazolinyl)thiophene (L1–L3) and 2,5-bis(oxazolinyl) thiophene (L4 and L5). Reaction conditions: (I) SOCl2 (8 mL/g), cat. DMF, 24 h, reflux; (II) i. CH2Cl2, TEA (5 eq.), −10 °C; ii. Amino alcohol (3a–c) (2.1 eq.); (III) 4a, SOCl2 (8.8 mL/g), refluxed, 24 h; (IV) i. 5a, Et2O, TEA (12.0 eq.), 0 °C; ii. 2.5 eq. R1NH2 (6a–c), 0 °C, then r.t., 12 h; (V) NaOH (15% aq. soln., 15 mL/g), r.t, 24 h; (VI) 4b–4c, Tosylchloride (1.25 eq.), DMAP (cat. 0.1 eq.), TEA (4.0 eq.), CH2Cl2, r.t, 48 h, N2.
Figure 2.
C2-symmetric 2,5-bis(imidazolinyl)thiophene (L1–L3) and ligands 2,5-bis(oxazolinyl)thiophene (L4 and L5) tested for the Friedel–Crafts alkylation reaction of indoles with trans-β-nitrostyrene derivatives.
Ligands (L1–L3) were synthesized using the intermediate thiophene-2,5-dicarboxamide dichloride 5a (2.92 mmol) by the reaction of three different aromatic amines 6a–c (2.5 mmol) (aniline 6a, p-chloroaniline 6b, p-toludine 6c) in the presence of excessive triethylamine (12 eq.) to form a corresponding thiophene-2,5-dicarboxamide intermediate (7a–c), which underwent a ring closure reaction upon treatment with 15% aqueous sodium hydroxide (NaOH) solution to form crude thiophene-2,5-bis(imidazolinyl)thiophene ligands (L1–L3). Then, the ligands were further purified by column chromatography by eluting with EtOAc/petroleum ether/Et3N (v:v:v = 75:24:1) to afford pure ligands L1–L3 (Scheme 1). The isolated yields of the ligands were found to be in the range of 35–40%.
Under inert condition, ligands (L4 and L5), were prepared from thiophene-2,5-dicarboxamide alcohol (4b and 4c) by ring closure reaction upon being treated with tosylchoride (1.25 eq.) and triethylamine (4.0 eq.) in the presence of a catalytic amount of DMAP (cat. 0.1eq.) in dichloromethane (CH2Cl2) after 48 h of stirring at room temperature. The ligands were then purified by column chromatography, using 95% CH2Cl2/CH3OH as an eluent to afford pure ligands L4 and L5 (Scheme 1) with 60% and 55% isolated yield, respectively. The formations of the compound thiophene-2,5-dicarboxamide alcohol (3a) and all the ligands (L1–L5) were confirmed and characterized by NMR and mass spectroscopy analysis.
2.2. Application of Chiral Ligand (L1–L5)
2.2.1. Catalytic asymmetric Friedel–Crafts Alkylation of Indoles with Trans-β-nitrostyrene Derivatives; Optimization of Various Reaction Parameters
As soon as we had in our hand optically pure ligands L1–L5, we decided to carry out the catalytic activity in an asymmetric Friedel–Crafts alkylation reaction between indoles 8a–d and nitrostyrene derivatives 9a–h. Indole (8a) and p-fluoronitrostyrene (9a) have been chosen as a model substrate for the reaction parameters optimization. In order to identify the best ligands for the asymmetric catalysis, initially, the Friedel–Crafts alkylation reaction of indole (8a) and p-fluoronitrostyrene (9a) was performed with the screened chiral bis(imidazoline) and bis(oxazoline) ligands L1–L5 (15 mol%) and Cu(OTf)2 (15 mol%) as metal sources in toluene at room temperature for 48 h, and the subsequent findings are documented in Table 1. It is evident from the results summarized in Table 1, entries 1–5, that the thiophene-2,5-bis(oxazoline) ligand L5 performed very well under the above-mentioned reaction conditions and afforded Friedel–Crafts alkylation adduct 10a at 66% chemical yield with 75% enantiomeric excess (ee) (Table 1; entry 5), while ligand L4 yielded 70% chemical yield with 45% ee (Table 1; entry 4). Although the ligands L1–L3 furnished better chemical yields (78, 75 and 70%, respectively), only trace enantiomeric excess (ee) (3–5%) was achieved (Table 1, entries 1-3). In order to improve the chemical yield, the reaction was repeated with ligand L5, and reaction time was extended up to 72 h, but no significant changes were observed (Table 1; entry 6). Aiming to improve the chemical yield as well as enantioselectivity output of the reaction, a set of trials was conducted by variation of the loading of catalyst L5:Cu(OTf)2 at 5, 10 and 20 mol%. The results showed that regardless of the % catalyst loading, the chemical yield was lower (20%, 46% and 65%, respectively) and did not result in any significant changes for the enantioselectivity (65%, 71% and 74% ee) (Table 1, entries 7–9). The influences of the solvent effects were also studied; Friedel–Crafts alkylation reactions of indole (8a) and p-fluoronitrostyrene (9a) were also performed using a ligand–metal ratio of 15 mol% of L5:Cu(OTf)2 at room temperature in several solvents, such as tetrahydrofuran, methanol, acetonitrile, dichloromethane, n-hexane and ethylacetate, within various time frames (84–96 h) (Table 1, entries 10–15), where dichloromethane was found to be the best solvent for chemical yield improvement but with no enantioselectivity (Table 1; entry 13), whereas no product formation took place in n-hexane and ethylacetate (Table 1, entries 14 and 15), although in THF, moderate yield (48%) and enantioselectivity (55%) were observed (Table 1; entry 10). From the above preliminary findings, it is obvious that a 15 mol% ligand–metal ratio [15 mol% L5:Cu(OTf)2] in toluene at room temperature in 48 h was the optimum set of reaction conditions to afford the final C–C bond formation adduct. Interestingly, it is clear from the preliminary results that oxazolinyl-based ligands are more potent than imidazolinyl-based ones; more interestingly, the substitution at the oxazolinyl moiety showed to also be critical for the asymmetric induction. Further investigation for better understanding is highly recommended.
Table 1.
Friedel–Crafts alkylation reaction of indole (8a) with p-fluoronitrostyrene (9a) as model substrate; reaction optimization (ligands, solvents and time).

| Entry [a] | Ligands | L:Cu(OTf)2 [1:1] | Solvents | Time [h] | Yield (%) [b] | ee (%) [c,d] |
|---|---|---|---|---|---|---|
| 1. | L1 | 15 mol% | Toluene | 48 | 78 | 5 |
| 2. | L2 | 15 mol% | Toluene | 48 | 75 | 3 |
| 3. | L3 | 15 mol% | Toluene | 48 | 77 | 3 |
| 4. | L4 | 15 mol% | Toluene | 48 | 70 | 45 |
| 5. | L5/, | 15 mol% | Toluene | 48 | 66 | 75 |
| 6. | L5 | 15 mol% | Toluene | 72 | 68 | 74 |
| 7. | L5 | 5 mol% | Toluene | 48 | 20 | 65 |
| 8. | L5 | 10 mol% | Toluene | 48 | 46 | 71 |
| 9. | L5 | 20 mol% | Toluene | 48 | 65 | 74 |
| 10. | L5 | 15 mol% | THF | 48 | 55 | 50 |
| 11. | L5 | 15 mol% | MeOH | 72 | 30 | 5 |
| 12. | L5 | 15 mol% | ACN | 96 | 10 | 4 |
| 13. | L5 | 15 mol% | DCM | 72 | 80 | 0 |
| 14. | L5 | 15 mol% | Hexane | 72 | - | - |
| 15. | L5 | 15 mol% | EA | 96 | traces | - |
[a] All the reactions were conducted on a 0.2 mmol scale; [b] isolated yields after column purification; [c] the enantiomeric excess (ee) was measured by chiral HPLC using a Daicel OD-H column (25 cm × 4.6 mm × 5 μm); [d] the absolute configuration was assigned as (S) comparing the retention time and sign of optical rotation reported in the literature [74].
Next, another two factors were also investigated, namely metal salts and temperature effects. Therefore, a Friedel–Crafts alkylation of indole (8a) with p-fluoronitrostyrene (9a) was carried out using 15 mol% of ligand L5 with the combination of several metal triflates, such as Zn(OTf)2, Mg(OTf)2, Er(OTf)2 and Yb(OTf)2, and metal chlorides such as FeCl3 and PdCl2, in toluene at 25 °C, and the results are summarized in Table 2. It was observed from the metal screening that Zn(OTf)2, FeCl3 and PdCl2 yielded product 10a with excellent to good chemical yields (97%, 80% and 70%, respectively), while the enantioselectivity remains negligible (Table 2, entries 1, 5 and 6). Two attempts were carried out at low (0 °C) and high (70 °C) temperature for 92 h and 24 h, respectively, and henceforth, 42% and 70% chemical yields with 76% and 65% enantioselectivity were observed (Table 2, entries 7 and 8). The results showed no significant changes for either the chemical yield or the enantioselectivity (Table 2, entry 8). From the overall findings, a catalyst generated in situ from ligand L5 and Lewis acid Cu(OTf)2 in toluene was found to be the optimum reaction condition for the asymmetric Friedel–Crafts alkylation of indole (8a) and p-fluoronitrostyrene (9a).
Table 2.
Friedel–Crafts arylation of indole (8a) with p-fluoronitrostyrene (9a) as model substrate reaction optimization (temperature and metals salts).

| Entry [a] | Metals Salts (15 mol%) | Time [h] | Temp [°C] | Yield (%) [b] | ee (%) [c,d] |
|---|---|---|---|---|---|
| 1. | Zn(OTf)2 | 48 | 25 | 97 | 10 |
| 2. | Mg(OTf)2 | 72 | 25 | - | - |
| 3. | Er(OTf)2 | 72 | 25 | 40 | 2 |
| 4. | Yb(OTf)2 | 72 | 25 | 47 | 0 |
| 5. | FeCl3 | 24 | 25 | 80 | 2 |
| 6. | PdCl2 | 24 | 25 | 70 | 0 |
| 7. | Cu(OTf)2 | 92 | 0 | 42 | 76 |
| 8. | Cu(OTf)2 | 24 | 70 | 66 | 65 |
[a] All the reactions were conducted on a 0.2 mmol scale; [b] isolated yields after column purification; [c] the enantiomeric excess (ee) was measured by chiral HPLC using a Daicel OD-H column (25 cm × 4.6 mm × 5 μm); [d] the absolute configuration was assigned as (S) comparing the retention time and sign of optical rotation reported in the literature [74].
2.2.2. Substrate Scope
To illustrate the generality, 20 examples of asymmetric Friedel–Crafts alkylation reactions have been carried out using indoles 8a–d with various nitroolefins (9a–h) under the optimized reaction conditions, i.e., 15 mol% L5:Cu(OTf)2 in toluene at 25 °C for 48 h, and the results are shown in Table 3. After the observing the results, it seems that substrates 9a–h reacted with indole 8a moderately and yielded chiral products 10a–f in the range of 40–67% yields with 64–80% enantioselectivity. Substrates 9a, 9b, 9d, 9e and 9h performed fairly well, yielding corresponding FC products 10a, 10b, 10d, 10e and 10h with 67, 64, 66, 58 and 60% yields and good enantiomeric excess (ee) at 74, 80, 69, 70 and 64% ee, respectively (Table 3, entries 1, 2, 4, 5 and 8). While substrates 9c, 9f and 9g furnished the corresponding Friedel–Crafts alkylated products 10c, 10f and 10g with poor chemical yields (40, 48 and 52%, respectively) because of the steric hindrance of the substrate, the enantioselectivity remained good (75, 71 and 71%, respectively) (Table 3, entries 3, 6 and 7). When substrate 9a–h was allowed to react with 5-bromoindole (8b) under the optimized conditions, poor yields were observed (10i–p, 35–55%) with good enantioselectivity (60–81% ee) (Table 3, entries 9–16). A Friedel–Crafts reaction of 5-fluoro indole with β-nitrostyrene 9g furnished a moderate yield (57%) with good enantioselectivity (66% ee) as compared to the reaction with the more hindered 9h, which produced poor yield (45%) as well as poor enantioselectivity (21% ee) (Table 3, entries 17 and 18). We further performed the Friedel–Crafts reaction with N-ethyl-protected indole and β-nitrostyrene 9a and 9d, which produced good yields (73 and 76%) with poor enantiomeric excess (35 and 27%) (Table 3, entries 19 and 20). Interestingly, when the asymmetric Friedel–Crafts alkylation of indole 8a with nitrostyrene 9a was performed at a large scale (10-fold), both the yield (76%) and enantioselectivity (77% ee) were improved (Table 3, entry 1).
Table 3.
Substrate scope by reaction of indole derivatives (8a–d) with substituted nitrostyrene (9a–h) under optimized reaction condition.

| Entry [a] | R1 (9a–h) | R2 | R3 | 10a–i | Yields (%) [b] | ee (%) [c] | R/S | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1. | 4-F-C6H4 | H | H | 10a | 67 76[LS] | 74 77[LS] | (S) [d] | [74] |
| 2. | 3-Br-C6H4 | H | H | 10b | 64 | 80 | (S) [d] | [74,76] |
| 3. | 4-CF3-C6H4 | H | H | 10c | 40 | 75 | (S) [d] | [75] |
| 4. | 4-CH3O-C6H4 | H | H | 10d | 66 | 69 | (S) [d] | [74] |
| 5. | 2-NO2-C6H4 | H | H | 10e | 58 | 70 | (R) [d] | [80] |
| 6. | 2,4-Cl2-C6H3 | H | H | 10f | 48 | 71 | (R) [d] | [74] |
| 7. | 2-thienyl | H | H | 10g | 52 | 71 | (S) [e] | [42] |
| 8. | 2,6-Cl2-C6H3 | H | H | 10h | 60 | 64 | (R) [e] | [41] |
| 9. | 4-F-C6H4 | Br | H | 10i | 55 | 77 | (S) [e] | |
| 10. | 3-Br-C6H4 | Br | H | 10j | 46 | 81 | (S) [e] | |
| 11. | 4-CF3-C6H4 | Br | H | 10k | 35 | 79 | (S) [e] | |
| 12. | 4-CH3O-C6H4 | Br | H | 10l | 39 | 63 | (S) [d] | [81] |
| 13. | 2-NO2-C6H4 | Br | H | 10m | 42 | 78 | (R) [e] | |
| 14. | 2,4-Cl2-C6H3 | Br | H | 10n | 37 | 75 | (R) [e] | |
| 15. | 2-thienyl | Br | H | 10o | 47 | 72 | (S) [e] | [42] |
| 16. | 2,6-Cl2-C6H3 | Br | H | 10p | 52 | 60 | (R) [e] | |
| 17. | 2-thienyl | F | H | 10q | 57 | 66 | (S) [e] | |
| 18. | 2,6-Cl2-C6H3 | F | H | 10r | 45 | 21 | (R) [e] | |
| 19. | 4-F-C6H4 | H | Et | 10s | 73 | 35 | (S) [e] | |
| 20. | 4-CH3O-C6H4 | H | Et | 10t | 76 | 27 | (S) [e] | [82] |
[a] All the reactions were conducted on a 0.2 mmol scale; [b] isolated yields after column purification; [c] the ee values were determined by chiral HPLC using a Daicel OD-H column (25 cm × 4.6 mm × 5 μm) [74]; [d] the absolute configuration was determined as (S) or (R) comparing their retention time and sign of optical rotation reported in the literature; [e] the absolute configuration was assigned as (S) or (R) assuming uniform reaction mechanism and comparing with retention time and sign of optical rotation; [LS] large-scale reaction yield and enantiomeric excess (ee).
Finally, to examine another nitrostyrene system for the Friedel–Crafts arylation, two nitrostyrene (9i and 9j)-based indole scaffold were synthesized and characterized. The synthesized indole-based nitrostyrenes 9i and 9j were used as substrates for the asymmetric Friedel–Crafts arylation using our optimized method, but they unfortunately did not succeed in affording the final desired chiral FC products 10u and 10v, as shown in Scheme 2. The requisite final compounds either did not occur or decomposed.
Scheme 2.
Friedel–Crafts arylation of indole (8b) with nitrostyrene-based indole scaffold (9i and 9j).
In Figure 3, the proposed cycle of the catalytic mechanism has been shown, where in the intermediates (II) and (III), it has been clearly shown that the addition of an incoming nucleophilic group from the Si face is more favorable than the Re face since the latter is a more sterically hindered face as compared to former.
Figure 3.
Proposed mechanism: L5:Cu(OTf)2-catalyzed Friedel–Craft alkylation of indole with β-nitroolefin catalytic cycle.
In case of Friedel–Craft product with indole, the retention time of the S enantiomer was found to be lesser than the R enantiomer in the chiral HPLC analysis using Daicel OD-H chiral column and n-hexane/iso-propanol system in the reported literature, while for FC products with 5-bromoindole it was found to be vice versa. Therefore, the absolute configuration of the synthesized chiral FC products 10a–d, 10g, 10i–l, 10o, 10q, 10s and 10t was assigned as S, while 10e, 10f, 10h, 10m, 10n, 10p and 10r were assigned as R by comparing their retention time and optical rotation values found in reported literature, assuming that the reaction took place via uniform mechanistic pathway (Table 3) [41,74].
3. Materials and Methods
3.1. General
Reagents obtained from commercial suppliers were used without further purification. Preparation of bis(imidazoline) and bis(oxazoline) ligands was performed in dried glassware flasks under a static pressure of nitrogen. Solvents were dried prior to use following standard procedures. Reactions were monitored by thin layer chromatography using Merck silica gel 60 Kieselgel F254 TLC (Merck, Kenilworth, NJ, USA), and column chromatography was performed on silica gel 100–200 (40–63 µm, ASTM) from Merck using the indicated solvents. 1H and 13C-NMR spectra were recorded in CDCl3 and DMSO-d6 on a Jeol Spectrometer (Jeol, Tokyo, Japan) (400 MHz and 500 MHz). The chemical shifts are reported in ppm. All the racemic products were freshly prepared as per the method reported in the literature [83]. Infrared spectra were recorded on a Thermo Scientific Nicolet iS10 FT-IR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Enantiomeric ratios were determined by analytical chiral HPLC analysis on a Shimadzu LC-20A (Shimadzu, Kyoto, Japan) Prominence instrument with a chiral stationary phase using Daicel OD-H columns (Chiral Technologies Europe, Illkirch-Graffenstaden, France) and 70–75% n-hexane/iso-propanol as eluents (Supplementary Materials). Optical rotations were obtained with a PerkinElmer 343 Polarimeter (PerkinElmer, Waltham, MA, USA). Melting points (m.p.) were recorded on a Thomas-Hoover capillary melting point apparatus (Thomas-Hoover, Texas City, USA) and were not corrected. Mass spectrometric analysis was done using ESI mode on an Agilent Technologies 6410-triple quad LC/MS instrument (Agilent, Santa Clara, CA, USA). Elemental analyses were performed on Perkin-Elmer PE 2400 CHN Elemental Analyzer with autosampler, CHN mode. X-ray diffraction data were collected on a Rigaku Oxford Diffraction Supernova diffractometer and processed with CrysAlisPro software v. 1.171.41.93a (Rigaku Oxford Diffraction, Yarnton, UK, 2020) using Cu K_ radiation”.
3.2. General Procedure (GP1) for the Preparation of Bis(hydroxyamides) 4a–c
GP1: A 100-mL round bottom flask was charged with thiophene-2,5-dicarboxlyic acid (1) (0.5 mg, 2.9 mmol) and SOCl2 (7 mL). A catalytic amount of DMF (3 drops) was added, and the reaction was reflux for 24 h under inert atmosphere. The reaction was then cooled, and excess SOCl2 was removed under reduced pressure to give the corresponding crude acid chloride (2). The crude acid chloride 2 (2.9 mmol) solution in CH2Cl2 (10 mL) was then slowly added to a pre-stirred solution of amino alcohol 3a–c (6.9 mmol, 2.1 eq.) and triethylamine (2 mL, 5 eq.) in CH2Cl2 (35 mL) at −10 °C. The reaction was then stirred at ambient temperature for 24 h. After reaction completion, the solvents were removed and the residue was poured into water (55 mL). Upon standing at room temperature for 4 h, solid product was precipitated out, which was then collected by filtration and purified by column chromatography using 100–200 mesh silica gel and CH2Cl2/MeOH (95:5) as an eluent to afford pure products 4a–c.
3.2.1. N2,N5-Bis((S)-1-Hydroxy-3-methylbutan-2-yl)thiophene-2,5-dicarboxamide (4a)
Following GP1, thiophene-2,5-dicarboxlyic acid chloride (2) and (S)-2-amino-3-methylbutan-1-ol (3a) reacted to produce 2,5-dicarboxamide alcohol (4a) as white solid (0.74 g, 75%); m.p. 199–201 °C; (c 0.20, CH3OH); IR (KBr, cm−1): 3350, 3086, 3071, 2956, 2870, 2496, 1627, 1543, 1515, 1464, 1033, 743; 1H-NMR (400 MHz, DMSO-d6): δ(ppm) = 8.11 (d, J = 8.9 Hz, 2H, NH), 7.82 (s, 2H, Ar–H), 4.63 (t, J = 5.8 Hz, 2H, NHCH), 3.74 (p, J = 7.0, 6.4 Hz, CH2OH), 3.56–3.45 (m, 4H, CH2OH), 1.91 (dp, J = 13.3, 6.2 Hz, 2H, CH(CH3)2), 0.88 (dd, J = 11.5, 6.7 Hz, 12H, CH(CH3)2); 13C-NMR (101 MHz, DMSO-d6) δ(ppm) = 160.8, 143.5, 128.1, 61.2, 56.9, 28.6, 19.6, 18.7; LC/MS (ESI): found 342.2 [M + H]+, C16H26N2O4S requires 342.16; anal. calcd. for C16H26N2O4S: C, 56.12; H, 7.65; N, 8.18; found: C, 55.88; H, 7.72; N, 8.06.
3.2.2. N2,N5-Bis((S)-1-Hydroxy-4-methylpentan-2-yl)thiophene-2,5-dicarboxamide (4b)
Following GP1, thiophene-2,5-dicarboxlyic acid chloride (2) and (S)-2-amino-4-methylpentan-1-ol (3b) reacted to produce 2,5-dicarboxamide alcohol (4b) as white solid (1.02 g, 95%); m.p. 208–210 °C; (c 0.11, CH3OH); IR (KBr, cm-1): 3351, 3087, 2958, 2871, 2605, 2498, 1627, 1545, 1517, 1469, 1033, 745; 1H-NMR (500 MHz, DMSO-d6): δ(ppm) = 8.19 (d, J = 8.7 Hz, 2H, NH), 7.77 (s, 2H, Ar–H), 4.74 (s, 2H, NHCH), 4.04–3.91 (m, 2H, CH2OH), 3.41 (dt, J = 11.0, 5.7 Hz, 2H, CH2OH), 3.05 (q, J = 7.3 Hz, 2H, CH2OH), 1.66–1.54 (m, 2H, CH(CH3)2), 1.48–1.40 (m, 2H, CHCH2(a)), 1.38–1.33 (m, 2H, CHCH2(b)), 0.88 (d, J = 6.6 Hz, 6H, CH(CH3)2), 0.86 (d, J = 6.6 Hz, 6H, CH(CH3)2). 13C-NMR (126 MHz, DMSO-d6): δ(ppm) = 160.5, 143.4, 128.0, 63.8, 49.7, 45.4, 24.4, 23.3, 21.9; LC/MS (ESI): found 371.2 [M + H]+, C18H30N2O4S requires 370.19; anal. calcd. for C18H30N2O4S: C, 58.35; H, 8.16; N, 7.56; found: C, 58.33; H, 8.18; N, 7.55.
3.2.3. N2,N5-Bis((2S,3R)-1-Hydroxy-3-methylpentan-2-yl)thiophene-2,5-dicarboxamide (4c)
Following GP1, thiophene-2,5-dicarboxlyic acid chloride (2) and (2S,3R)-2-amino-3-methylpentan-1-ol (3c) reacted to produce 2,5-dicarboxamide alcohol (4c) as white solid (1.04 g, 97%); m.p. 233–234 °C; (c 0.10, CH3OH); IR (KBr, cm-1): 3352, 3086, 2956, 2870, 2609, 2493, 1625, 1544, 1516, 1465, 1030, 744; 1H-NMR (500 MHz, DMSO-d6): δ(ppm) = 8.09 (d, J = 8.9 Hz, 2H, NH), 7.76 (s, 2H, Ar–H), 4.53 (s, 2H, CH2OH), 3.78–3.70 (m, 2H, NHCH), 3.53–3.42 (m, 4H, CH2OH), 1.68–1.59 (m, 2H, CHCH3), 1.47–1.37 (m, 2H, CH2CH3), 1.12–1.01 (m, 2H, CH2CH3), 0.83 (d, J = 6.9 Hz, 6H, CHCH3), 0.80 (t, J = 7.4 Hz, 6H, CH2CH3); 13C-NMR (126 MHz, DMSO-d6): δ(ppm) = 160.7, 143.4, 128.0, 60.9, 55.7, 35.1, 25.1, 15.5, 11.2; LC/MS (ESI): found 399.3 [M + H]+, C20H34N2O4S requires 398.22; anal. calcd. for C18H30N2O4S: C, 58.35; H, 8.16; N, 7.56; found: C, 58.17; H, 8.26; N, 7.44.
3.3. General Procedure (GP2) for the Preparation of Thiophene-2,5-bis-imidazoline Chiral Ligands (L1–L3)
GP2: Thiophene-2,5-dicarboxamide alcohol (4a) (1.0 g, 2.92 mmol) in SOC12 (8.76 mL) was refluxed for 24 h. After removal of SOCl2, ice-water was added to the residue and the product was extracted with CH2Cl2 (3 × 25 mL). The combined extracts were washed with brine and dried over anhydrous Na2SO4. The organics were evaporated to give the crude thiophene-2,5-dicarboxamid dichloride (5a). The crude dichloride (5a) was then dissolved in dry diethyl ether (20 mL) and the insoluble impurities were filtered out. To this solution, dry triethylamine (4.9 mL, 35.0 mmol, 12.0 eq.) was added, followed by arylamine (6a–c) (2.5 eq.). After stirring for 12 h at room temperature, 15% NaOH (15 mL) was added and stirred for another 24 h. The aqueous portion was extracted with dichloromethane (3 × 20 mL) and then washed with brine. The combined organics were dried over anhydrous Na2SO4 and concentrated under reduced pressure to afford crude thiophene-2,5-bis(imidazolinyl)thiophene ligands (L1–L3). The pure ligands (L1–L3) were isolated by column chromatography, using the combination of ethylacetate/petroleumether/Et3N (v:v:v = 75:24:1) as an eluent.
3.3.1. 2,5-Bis((S)-4-IsoPropyl-1-phenyl-4,5-dihydro-1H-imidazol-2-yl)thiophene (L1)
Thiophene-2,5-dicarboxamide alcohol 4a (1.0 g, 2.92 mmol) and aniline 6a (0.68 g, 7.3 mmol) were reacted according to GP2 and afforded yellow-colored ligand L1 (yield 533 mg, 40%); (c 0.106, EtOH); 1H-NMR (400 MHz, DMSO-d6): δ(ppm) = 7.27 (t, J = 7.7 Hz, 4H, Ar–H), 7.10 (t, J = 7.3 Hz, 2H, Ar–H), 6.97 (d, J = 8.1 Hz, 4H, Ar–H), 6.55 (s, 2H, Ar–H), 4.00–3.86 (m, 4H, NCH2), 3.51 (t, J = 8.1 Hz, 2H, NCH), 1.72 (p, J = 6.6 Hz, 2H, CHCH3), 0.94 (d, J = 7.3 Hz, 6H, CHCH3(a)), 0.86 (d, J = 7.3 Hz, 6H, CHCH3(b)); 13C-NMR (101 MHz, DMSO-d6): δ(ppm) = 154.5, 143.1, 135.2, 129.1, 128.6 124.7, 124.2, 70.13, 59.8, 57.5, 32.7, 18.6; LC/MS (ESI): found 457.2 [M + H]+, C28H32N4S requires 456.65; anal. calcd. for C28H32N4S: C, 73.65; H, 7.06; N, 12.27; found: C, 73.60; H, 7.04; N, 12.25.
3.3.2. 2,5-Bis((S)-1-(4-Chlorophenyl)-4-isopropyl-4,5-dihydro-1H-imidazol-2-yl)thiophene (L2)
Thiophene-2,5-dicarboxamide alcohol (4a) (1.0 g, 2.92 mmol) and 4-chloroaniline (6b) (0.93 g, 7.3 mmol) were reacted according to GP2 and afforded yellow-colored ligand L2 (yield 583 mg, 38%); (c 0.07, CH2Cl2); 1H-NMR (400 MHz, DMSO-d6): δ(ppm) = 7.32 (d, J = 8.8 Hz, 4H, Ar–H), 6.97 (d, J = 8.8 Hz, 4H, Ar–H), 6.68 (s, 2H, Ar–H), 4.00–3.90 (m, 4H, NCH2), 3.54 (t, J = 7.3 Hz, 2H, NCH), 1.73 (h, J = 6.6 Hz, 2H, CHCH3), 0.93 (d, J = 6.6 Hz, 6H, CHCH3(a)), 0.85 (d, J = 6.6 Hz, 6H, CHCH3(b)); 13C NMR (101 MHz, DMSO-d6) δ(ppm) = 154.0, 141.7, 134.8, 129.1, 128.6, 125.5, 125.3, 70.0, 57.1, 54.9, 32.6, 18.7; LC/MS (ESI): found 525.2 [M + H]+, for C28H30Cl2N4S requires 524.16; anal. calcd. for C28H30Cl2N4S: C, 63.99; H, 5.75; N, 10.66; found: C, 63.87; H, 5.72; N, 10.61.
3.3.3. 2,5-Bis((S)-4-IsoPropyl-1-(p-tolyl)-4,5-dihydro-1H-imidazol-2-yl)thiophene (L3)
Thiophene-2,5-dicarboxamide alcohol (4a) (1.0 g, 2.92 mmol) and p-toluidine 6c (0.78 g, 7.3 mmol) were reacted according to GP2 and afforded yellow-colored ligand L3 (yield 538 mg, 38%); (c 0.05, EtOH); 1H-NMR (400 MHz, DMSO-d6): δ(ppm) = 7.08 (d, J = 8.1 Hz, 4H, Ar–H), 6.89 (d, J = 8.1 Hz, 4H, Ar–H), 6.52 (s, 2H, Ar–H), 3.93–3.86 (m, 4H, NCH2), 3.47–3.41 (m, 2H, NCH), 2.24 (s, 6H, PhCH3), 1.73 (q, J = 6.6 Hz, 2H, CHCH3), 0.94 (d, J = 7.3 Hz, 6H, CHCH3(a)), 0.85 (d, J = 6.6 Hz, 6H, CHCH3(b)); 13C NMR (101 MHz, DMSO-d6) δ(ppm) 154.8, 140.7, 135.2, 134.3, 129.6, 128.6, 124.5, 70.1, 57.8, 32.8, 20.5, 18.7, 18.1; LC/MS (ESI): found 485.3 [M + H]+, for C30H36N4S requires 484.27; anal. calcd. for C30H36N4S: C, 74.34; H, 7.49; N, 11.56; found: C, 74.30; H, 7.48; N, 11.52.
3.4. General Procedure (GP3) for the Synthesis of Thiophene-2,5-bis-oxazoline Chiral Ligands (L4 and L5)
GP3: Thiophene-2,5-dicarboxamide alcohol (4b–c) (2.92 mmol) was added to the solution of CH2Cl2 (60 mL) and triethylamine (4.0 eq., 1.18 g, 11.7 mmol). Catalytic amounts of DMAP (36 mg, 0.1 eq.) and p-tosylchoride (695 mg, 3.65 mmol, 1.25 eq.) were added, and the mixture was stirred at 0 °C to r.t. for 48 h. After completion of the reaction, saturated aqueous ammonium chloride solution (100 mL) was added and stirred for another 10 min at room temperature. The organic layer was extracted with CH2Cl2 (3 × 25 mL) and washed with saturated aqueous NaHCO3 solution (50 mL). The combined organic layers were dried over anhydrous Na2SO4, and the solvent was evaporated in vacuum to afford crude ligands (L4 and L5), which was purified by column chromatography (5% CH2Cl2/CH3OH) to afford pure thiophene-2,5-bis(oxazolinyl)thiophene ligands (L4 and L5).
3.4.1. 2,5-Bis((S)-4-isoButyl-4,5-dihydrooxazol-2-yl)thiophene (L4)
Following the GP2, thiophene-2,5-dicarboxamide (4b) (1.08 g, 2.92 mmol) underwent direct ring closure reaction to afford ligand L4 as white solid (yield 586 mg, 60%); m.p. 48–50 °C; (c 0.093, CH3OH); IR (KBr, cm-1): 3104, 2953, 2920, 2870, 2847, 1647, 1533, 1251, 1051, 1019, 944, 829; 1H-NMR (500 MHz, DMSO-d6): δ(ppm) = 7.53 (s, 2H, Ar–H), 4.54 (dd, J = 9.3, 8.0 Hz, 2H, OCH2(a)), 4.31–4.23 (m, 2H, NCH), 3.97 (t, J = 8.2 Hz, 2H, OCH2(b)), 1.76 (dt, J = 13.5, 6.7 Hz, 2H, CH(CH3)2), 1.52 (dt, J = 13.9, 7.0 Hz, 2H, CHCH2(a)), 1.35 (dt, J = 13.5, 7.2 Hz, 2H, CHCH2(b)), 0.93 (d, J = 3.9 Hz, 6H, CH(CH3)2), 0.91 (d, J = 3.7 Hz, 6H, CH(CH3)2); 13C-NMR (126 MHz, DMSO-d6): δ(ppm) = 157.1, 133.3, 130.5, 73.3, 64.9, 44.7, 25.0, 22.7, 22.5; LC/MS (ESI): found 335.2 [M + H]+, C18H26N2O2S requires 334.17; anal. calcd. for C18H26N2O2S: C, 64.64; H, 7.84; N, 8.38; found: C, 64.62; H, 7.86; N, 8.34.
3.4.2. 2,5-Bis((S)-4-((S)-sec-Butyl)-4,5-dihydrooxazol-2-yl)thiophene (L5)
Following the GP2, thiophene-2,5-dicarboxamide (4c) (1.08 g, 2.92 mmol) underwent direct ring closure reaction to afford ligand L5 as white solid (yield 537 mg, 55%); m.p.: 42–43 °C; (c 0.081, CH3OH); IR (KBr, cm−1): 3102, 2954, 2921, 2870, 2845, 1648, 1533, 1251, 1052, 1019, 942, 826; 1H-NMR (500 MHz, DMSO-d6): δ(ppm) = 7.53 (s, 2H, Ar–H), 4.47–4.41 (m, 2H, NCH), 4.18–4.11 (m, 4H, OCH2), 1.62–1.50 (m, 4H, CH2CH3), 1.20–1.11 (m, 2H, CHCH3), 0.90 (t, J = 7.3 Hz, 6H, CHCH3), 0.80 (d, J = 6.7 Hz, 6H, CH2CH3); 13C-NMR (126 MHz, DMSO-d6): δ(ppm) = 157.2, 133.2, 130.4, 70.9, 70.2, 38.6, 25.4, 14.4, 11.3; LC/MS (ESI): found 335.2 [M + H]+, C18H26N2O2S requires 334.17; anal. calcd. for C18H26N2O2S: C, 64.64; H, 7.84; N, 8.38; found: C, 64.60; H, 7.84; N, 8.38.
3.5. Synthesis of the β-nitrostyrene (9a–j)
All the β-nitrostyrenes (9a–j) were synthesized by using well-known methods reported in the literature [84]. An oven-dried round bottom flask (100 mL) was charged with aldehydes (10.0 mmol), nitromethane (3.70 g, 60.0 mmol), piperidine (85 mg, 1.0 mmol) and toluene as solvent (10 mL). Anhydrous FeCl3 (16.2 mg, 1.0 mmol) was then added to it. The reaction mixture was reflux gently for 4 h under dry condition, using guard tube. The completion of the reaction was confirmed by TLC, and the reaction mixture was cooled to room temperature. The excess solvent was removed under reduced pressure, and the residue was purified by silica gel (100–200 mesh) column chromatography to afford pure β-nitrostyrenes 9a–j as yellow solid product (yield 75–90%).
3.6. Synthesis of Racemic Friedal–Crafts Alkylated Product Race-(10a–t)
The racemic products were synthesized by using the reported method [41,83]. Indole derivatives (0.30 mmol), β-nitrostyrenes 9a–h (0.30 mmol), FeCl3 (10 mol%) and H2O (2 mL) were heated at 80 °C for the appropriate time (24 h). After the completion of the reaction, monitored by thin-layer chromatography (TLC), the product was extracted with ethyl acetate (2 × 20 mL). The combined organic layer was dried over anhydrous sodium sulfate, evaporated under reduced pressure and purified by silica gel (100–200 mesh) column chromatography using 15% ethylacetate/n-hexane as eluent to afford the pure racemic Friedal–Crafts alkylated product race-(10a–t) (yield 85 –90%).
3.7. General Procedure (GP4) for the Asymmetric Friedal–Crafts Alkylation of Indole to β-nitrostyrene (10a–t)
GP4: An oven-dried screw-capped vial (8 mL) was charged with ligand L5 (10 mg, 0.03 mmol, 15 mol%), Cu(OTf)2 (11 mg, 0.03 mmol, 15 mol %) and dry toluene (3 mL). The mixture was then stirred at reflux for 2 h. After cooling to room temperature, β-nitrostyrene 9a–h (0.2 mmol) and 4A° molecular sieves were added. Then, the mixture was stirred for another 30 min, followed by addition of indole 8a–d (0.2 mmol). The reaction was then left stirring for 48 h at room temperature. The solvent was removed under reduced pressure, and the crude product was isolated by flash column chromatography on silica gel with ethylacetate/n-hexane (2:8, v/v) as eluent to afford pure Friedel–Crafts product (10a–t) in 35–76% isolated yield with 21–81% enantiomeric excess (ee).
3.7.1. (S)-3-(1-(4-Fluorophenyl)-2-nitroethyl)-1H-indole (10a)
Indole 8a (24 mg, 0.2 mmol) and 4-floronitrostyrene (9a) (34 mg, 0.2 mmol) were reacted according to the GP4 to yield product 10a as colorless oil (isolated yield 38 mg, 67%). Enantiomeric excess (ee) was determined by chiral HPLC (Chiracel OD-H column) (70% n-hexane/i-PrOH, 1.0 mL/min; tmajor = 24.85 min; tminor = 30.28 min; λ = 254 nm); 74.3% ee; (c 0.10, CH3OH); [Lit. [74] (c 0.85, CH2Cl2)]; 1H-NMR (500 MHz, CDCl3): δ(ppm) = 8.13 (s, 1H, NH), 7.47–7.40 (m, 1H, Ar–H), 7.35 (s, 1H, Ar–H), 7.32–7.28 (m, 2H, Ar–H), 7.23 (ddd, J = 8.2, 7.0, 1.2 Hz, 1H, Ar–H), 7.11 (ddd, J = 8.1, 6.9, 1.0 Hz, 1H, Ar–H), 7.04–6.96 (m, 3H, Ar–H), 5.19 (t, J = 8.0 Hz, 1H, CH), 5.05 (dd, J = 12.5, 7.5 Hz, 1H, CH2(a)), 4.90 (dd, J = 12.5, 8.6 Hz, 1H, CH2(b)); 13C-NMR (126 MHz, CDCl3): δ(ppm) = 163.1 and 161.18 (C1-F, JC-F = 246.58 Hz), 136.6, 135.07 and 135.04 (C4-F, JC-F = 3.15 Hz), 129.50 and 129.44 (C3-F, JC-F = 7.94 Hz), 126.0, 122.9, 121.6, 120.1, 118.9, 115.99 and 115.82 (C2-F, JC-F = 21.67 Hz), 114.2, 111.6, 79.6, 41.0. All the analytical data are in accordance with the reported literature [42,74].
3.7.2. (S)-3-(1-(3-Bromophenyl)-2-nitroethyl)-1H-indole (10b)
Indole 8a (24 mg, 0.2 mmol) and 3-bromonitrostyrene (9b) (46 mg, 0.2 mmol) were reacted according to the GP4 to yield product 10b as colorless oil (isolated yield 44 mg, 64%). Enantiomeric excess (ee) was determined by chiral HPLC (Chiracel OD-H column) (70% n-hexane/i-PrOH, 1.0 mL/min; tmajor = 27.66 min; tminor = 36.16 min; λ = 254 nm); 79.5% ee; (c 0.104, CH3OH); [Lit. [74] (c 1.3, CH2Cl2]; 1H-NMR (500 MHz, CDCl3): δ(ppm) = 8.12 (s, 1H, NH), 7.49–7.32 (m, 4H, Ar–H), 7.28–7.08 (m, 4H, Ar–H), 6.98 (d, J = 2.5 Hz, 1H, Ar–H), 5.15 (t, J = 8.0 Hz, 1H, CH), 5.02 (dd, J = 12.8, 7.6 Hz, 1H, CH2(a)), 4.89 (dd, J = 12.0, 7.8 Hz, 1H, CH2(b)); 13C-NMR (126 MHz, CDCl3): δ(ppm) = 141.7, 136.5, 130.9, 130.9, 130.6, 126.6, 126.0, 123.1, 122.9, 121.7, 120.2, 118.8, 113.6, 111.6, 79.2, 41.2. All the analytical data are in accordance with the reported literature [74,76].
3.7.3. (S)-3-(2-Nitro-1-(4-(trifluoromethyl)phenyl)ethyl)-1H-indole (10c)
Indole 8a (24 mg, 0.2 mmol) and 4-trifluoromethynitrostyrene 9c (44 mg, 0.2 mmol) were reacted according to the GP4 to yield product 10c as colorless oil (isolated yield 27 mg, 40%). Enantiomeric excess (ee) was determined by chiral HPLC (Chiracel OD-H column) (70% n-hexane/i-PrOH, 1.0 mL/min; tmajor = 32.09 min; tminor = 39.93 min; λ = 254 nm); 75.4% ee; (c 0.05, CH3OH); [Lit. [75] (c 1.0, CHCl3]; 1H-NMR (500 MHz, CDCl3): δ(ppm) = 8.17 (s, 1H, NH), 7.59 (d, J = 8.1 Hz, 2H, Ar–H), 7.47 (d, J = 8.1 Hz, 2H, Ar–H), 7.42 (dq, J = 8.0, 1.0 Hz, 1H, Ar–H), 7.38 (dt, J = 8.3, 0.9 Hz, 1H, Ar–H), 7.23 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H, Ar–H), 7.13–7.08 (m, 1H, Ar–H), 7.03 (dd, J = 2.6, 0.9 Hz, 1H, Ar–H), 5.26 (t, J = 8.0 Hz, 1H, CH), 5.09 (dd, J = 12.8, 7.4 Hz, 1H, CH2(a)), 4.97 (dd, J = 12.7, 8.7 Hz, 1H, CH2(b)); 13C-NMR (126 MHz, CDCl3): δ(ppm) = 143.4, 136.6, 128.3, 126.11, 126.07, 126.04, 125.9, 123.1, 121.8, 120.3, 118.8, 113.6, 111.7, 79.1, 41.4. All the analytical data are in accordance with the reported literature [75].
3.7.4. (S)-3-(1-(4-Methoxyphenyl)-2-nitroethyl)-1H-indole (10d)
Indole 8a (24 mg, 0.2 mmol) and 4-methoxynitrostyrene 9d (36 mg, 0.2 mmol) were reacted according to the GP4 to yield product 10d as white solid (isolated yield 39 mg, 66%), m.p. 148–149 °C; Enantiomeric excess (ee) was determined by chiral HPLC (Chiracel OD-H column) (70% n-hexane/i-PrOH, 1.0 mL/min; tmajor = 26.24 min; tminor = 32.20 min; λ = 254 nm); 69.3% ee; (c 0.53, CH3OH) [Lit.[74] (c 1.1, CH2Cl2)]; 1H-NMR (500 MHz, CDCl3): δ(ppm) = 8.09 (s, 1H, NH), 7.44 (d, J = 8.0 Hz, 1H, Ar–H), 7.36 (dd, J = 8.2, 1.0 Hz, 1H, Ar–H), 7.29–7.23 (m, 2H, Ar–H), 7.20 (tt, J = 8.2, 1.2 Hz, 1H, Ar–H), 7.12–7.06 (m, 1H, Ar–H), 7.02 (dd, J = 2.5, 1.1 Hz, 1H, Ar–H), 6.91–6.81 (m, 2H, Ar–H), 5.14 (t, J = 8.0 Hz, 1H, CH), 5.05 (dd, J = 12.4, 7.5 Hz, 1H, CH2(a)), 4.90 (dd, J = 12.4, 8.5 Hz, 1H, CH2(b)), 3.78 (s, 3H, CH3); 13C-NMR (126 MHz, CDCl3): δ(ppm) = 159.0, 136.6, 131.3, 129.0, 126.2, 122.8, 121.6, 120.1, 119.1, 114.9, 114.4, 111.5, 79.9, 55.4, 41.0. All the analytical data are in accordance with the reported literature [42,74].
3.7.5. (R)-3-(2-Nitro-1-(2-nitrophenyl)ethyl)-1H-indole (10e)
Indole 8a (24 mg, 0.2 mmol) and 2-nitronitrostyrene 9e (39 mg, 0.2 mmol) were reacted according to the GP4 to yield product 10d as yellow oil (isolated yield 36 mg, 58%). Enantiomeric excess (ee) was determined by chiral HPLC (Chiracel OD-H column) (70% n-hexane/i-PrOH, 1.0 mL/min; tminor = 37.48 min; tmajor = 67.98 min; λ = 254 nm); 70.0% ee; (c 0.053, CH3OH); [Lit. [80] (c 0.7, CH2Cl2)]; 1H-NMR (500 MHz, CDCl3): δ(ppm) = 8.23 (s, 1H, NH), 7.90 (dd, J = 8.2, 1.4 Hz, 1H, Ar–H), 7.48 (td, J = 7.6, 1.4 Hz, 1H, Ar–H), 7.43 (dd, J = 7.9, 1.6 Hz, 1H, Ar–H), 7.39 (ddd, J = 8.5, 7.3, 1.6 Hz, 1H, Ar–H), 7.35–7.30 (m, 2H, Ar–H), 7.21–7.16 (m, 1H, Ar–H), 7.12 (d, J = 2.6 Hz, 1H, Ar–H), 7.07–7.02 (m, 1H, Ar–H), 5.88 (t, J = 7.7 Hz, 1H, CH), 5.12 (dd, J = 13.2, 7.1 Hz, 1H, CH2(a)), 5.07 (dd, J = 13.2, 8.3 Hz, 1H, CH2(b)); 13C-NMR (126 MHz, CDCl3): δ(ppm) = 149.7, 136.5, 133.8, 133.4, 130.1, 128.7, 126.0, 125.2, 123.0, 122.2, 120.3, 118.7, 112.8, 111.6, 78.2, 36.5. All the analytical data are in accordance with the reported literature [80].
3.7.6. (R)-3-(1-(2,4-Dichlorophenyl)-2-nitroethyl)-1H-indole (10f)
Indole 8a (24 mg, 0.2 mmol) and 2,4-dichloronitronitrostyrene 9f (44 mg, 0.2 mmol) were reacted according to the GP4 to yield product 10d as yellow oil (isolated yield 32 mg, 48%). Enantiomeric excess (ee) was determined by chiral HPLC (Chiracel OD-H column) (70% n-hexane/i-PrOH, 1.0 mL/min; tminor = 21.25 min; tmajor = 35.78 min; λ = 254 nm); 71.25% ee; (c 0.052, CH3OH); [Lit. [74] (c 0.8, CH2Cl2)]; 1H-NMR (500 MHz, CDCl3): δ(ppm) = 8.16 (s, 1H, NH), 7.47 (t, J = 1.3 Hz, 1H, Ar-H), 7.38 (ddt, J = 14.8, 8.2, 0.9 Hz, 2H, Ar–H), 7.24–7.20 (m, 1H, Ar–H), 7.14 (d, J = 1.2 Hz, 2H, Ar–H), 7.12–7.08 (m, 2H, Ar–H), 5.71–5.66 (m, 1H, CH), 4.99 (dd, J = 12.9, 8.7 Hz, 1H, CH2(a)), 4.93 (dd, J = 12.9, 7.0 Hz, 1H, CH2(b)); 13C-NMR (126 MHz, CDCl3): δ(ppm) = 136.6, 135.3, 134.7, 134.2, 130.1, 130.0, 127.8, 126.1, 123.1, 122.0, 120.3, 118.9, 113.0, 111.6, 77.6, 37.7. All the analytical data are in accordance with the reported literature [41,74].
3.7.7. (S)-3-(2-Nitro-1-(thiophen-2-yl)ethyl)-1H-indole (10g)
Indole 8a (24 mg, 0.2 mmol) and (E)-2-(2-nitrovinyl)thiophene 9g (31 mg, 0.2 mmol) were reacted according to the GP4 to yield product 10n as brown oil (isolated yield 28 mg, 52%). Enantiomeric excess (ee) was determined by chiral HPLC (Chiracel OD-H column) (75% n-hexane/i-PrOH, 1.0 mL/min; tminor = 28.57 min; tmajor = 32.38 min; λ = 254 nm); 71.3% ee; (c 0.037, CH3OH); 1H-NMR (500 MHz, CDCl3): δ(ppm) = 8.15 (s, 1H, NH), 7.53 (d, J = 8.0 Hz, 1H, Ar–H), 7.37 (d, J = 8.2 Hz, 1H, Ar–H), 7.25–7.21 (m, 1H, Ar–H), 7.20–7.18 (m, 1H, Ar–H), 7.15–7.10 (m, 1H, Ar–H), 7.09 (d, J = 2.58 Hz, 1H, Ar–H), 7.01–6.99 (m, 1H, Ar–H), 6.95 (dd, J = 5.1, 3.6 Hz, 1H, Ar–H), 5.47 (t, J = 7.9 Hz, 1H, CH), 5.08–4.96 (m, 2H, CH2); 13C-NMR (126 MHz, CDCl3): δ(ppm) = 143.07, 136.53, 127.08, 125.84, 125.38, 125.03, 122.88, 122.09, 120.20, 118.93, 114.15, 111.65, 80.13, 37.05. All the analytical data are in accordance with the reported literature [42].
3.7.8. (R)-3-(1-(2,6-Dichlorophenyl)-2-nitroethyl)-1H-indole (10h)
Indole 8a (24 mg, 0.2 mmol) and 2,6-dichloronitronitrostyrene 9h (44 mg, 0.2 mmol) were reacted according to the GP4 to yield product 10q as brown oil (isolated yield 40 mg, 60%). Enantiomeric excess (ee) was determined by chiral HPLC (Chiracel OD-H column) (75% n-hexane/i-PrOH, 1.0 mL/min; tminor = 11.59 min; tmajor = 13.27 min; λ = 254 nm); 64.1% ee; (c 0.031, CH3OH); 1H-NMR (500 MHz, CDCl3): δ(ppm) = 8.14 (s, 1H, NH), 7.42 (dq, J = 8.0, 0.9 Hz, 1H, Ar–H), 7.37–7.23 (m, 3H, Ar–H), 7.21–7.13 (m, 3H, Ar–H), 7.06 (ddd, J = 8.0, 7.1, 1.0 Hz, 1H, Ar–H), 6.21 (ddd, J = 8.4, 7.4, 1.2 Hz, 1H, CH), 5.43 (dd, J = 12.8, 7.4 Hz, 1H, CH2(a)), 5.36 (dd, J = 12.8, 8.0 Hz, 1H, CH2(b)); 13C-NMR (126 MHz, CDCl3): δ(ppm) = 136.17, 134.32, 130.47, 129.90, 129.48, 126.46, 122.71, 122.65, 120.17, 119.06, 111.63, 111.44, 76.44, 38.03. All the analytical data are in accordance with the reported literature [41].
3.7.9. (S)-5-Bromo-3-(1-(4-fluorophenyl)-2-nitroethyl)-1H-indole (10i)
5-bromoindole 8b (39 mg, 0.2 mmol) and 4-floronitrostyrene 9a (34 mg, 0.2 mmol) were reacted according to the GP4 to yield product 10g as yellow oil (isolated yield 40 mg, 55%). Enantiomeric excess (ee) was determined by chiral HPLC (Chiracel OD-H column) (70% n-hexane/i-PrOH, 1.0 mL/min; tminor = 9.58 min; tmajor = 14.14 min; λ = 254 nm); 77.2% ee; (c 0.051, CH3OH); IR (KBr): 3417, 1544, 1376, 1242, 1179, 1028, 743, 549, 524 cm-1; 1H-NMR (500 MHz, CDCl3): δ(ppm) = 8.28 (s, 1H, NH), 7.55 (d, J = 1.8 Hz, 1H, Ar–H), 7.34–7.29 (m, 3H, Ar–H), 7.25 (d, J = 8.6 Hz, 1H, Ar–H), 7.09–7.03 (m, 3H, Ar–H), 5.14 (t, J = 8.0 Hz, 1H, CH), 5.04 (dd, J = 12.6, 7.8 Hz, 1H, CH2(a)), 4.91 (dd, J = 12.5, 8.2 Hz, 1H, CH2(b)); 13C-NMR (126 MHz, CDCl3) δ(ppm) = 163.23 and 161.27 (C1-F, JC-F = 247.21 Hz), 135.2, 134.58 and 134.55 (C4-F, JC-F = 3.28 Hz), 129.44 and 129.37 (C3-F, JC-F = 8.19 Hz), 127.8, 125.9, 122.8, 121.5, 116.17 and 116.00 (C2-F, JC-F = 21.55 Hz), 113.9, 113.4, 113.1, 79.5, 40.7; LC/MS (ESI): found 363.02 [M+H]+, C16H12BrFN2O2 requires 362.01; anal. calcd. for C16H12BrFN2O2: C, 52.91; H, 3.33; N, 7.71; found: C, 53.01; H, 3.39; N, 7.65.
3.7.10. (S)-5-Bromo-3-(1-(3-bromophenyl)-2-nitroethyl)-1H-indole (10j)
5-bromoindole 8b (39 mg, 0.2 mmol) and 3-bromonitrostyrene 9b (46 mg, 0.2 mmol) were reacted according to the GP4 to yield product 10h as yellow oil (isolated yield 39 mg, 46%). Enantiomeric excess (ee) was determined by chiral HPLC (Chiracel OD-H column) (80% n-hexane/i-PrOH, 1.0 mL/min; tminor = 21.41 min; tmajor = 34.37 min; λ = 254 nm); 79.5% ee; (c 0.053, CH3OH); IR (KBr): 3401, 1538, 1378, 1009, 814, 745, 589, 535, 421 cm-1; 1H-NMR (500 MHz, CDCl3): δ(ppm) = 8.24 (s, 1H, NH), 7.52 (d, J = 1.9 Hz, 1H, Ar–H), 7.42–7.37 (m, 2H, Ar–H), 7.28–7.22 (m, 2H, Ar–H), 7.21–7.16 (m, 2H, Ar–H), 7.03 (dd, J = 2.6, 0.9 Hz, 1H, Ar–H), 5.07 (t, J = 8.0 Hz, 1H, CH), 4.97 (dd, J = 12.7, 8.0 Hz, 1H, CH2(a)), 4.86 (dd, J = 12.7, 8.0 Hz, 1H, CH2(b)); 13C-NMR (126 MHz, CDCl3): δ(ppm) = 141.2, 135.2, 131.1, 130.8, 130.7, 127.8, 126.5, 126.0, 123.2, 122.9, 121.3, 113.5, 113.3, 113.1, 79.2, 41.0; LC/MS (ESI): found 423.01 [M+H]+, C16H12Br2N2O2 requires 421.93; anal. calcd. for C16H12Br2N2O2: C, 45.31; H, 2.85; N, 6.61; found: C, 45.23; H, 2.96; N, 6.52.
3.7.11. (S)-5-Bromo-3-(2-nitro-1-(4-(trifluoromethyl)phenyl)ethyl)-1H-indole (10k)
5-bromoindole 8b (39 mg, 0.2 mmol) and 4-trifluoromethynitrostyrene 9c (44 mg, 0.2 mmol) were reacted according to the GP4 to yield product 10i as colorless oil (isolated yield 29 mg, 35%). Enantiomeric excess (ee) was determined by chiral HPLC (Chiracel OD-H column) (75% n-hexane/i-PrOH, 1.0 mL/min; tminor = 11.80 min; tmajor = 19.82 min; λ = 254 nm); 78.43% ee; (c 0.056, CH3OH); IR (KBr): 3418, 1537, 1371, 1247, 1103, 715, 519 cm-1; 1H-NMR (500 MHz, CDCl3): δ(ppm) = 8.22 (s, 1H, NH), 7.60 (d, J = 8.1 Hz, 2H, Ar–H), 7.53 (d, J = 1.8 Hz, 1H, Ar–H), 7.44 (d, J = 8.1 Hz, 2H, Ar–H), 7.30 (dd, J = 8.7, 1.9 Hz, 1H, Ar–H), 7.25 (d, J = 8.7 Hz, 1H, Ar–H), 7.07 (d, J = 2.6 Hz, 1H, Ar–H), 5.20 (t, J = 8.0 Hz, 1H, CH), 5.04 (dd, J = 12.8, 7.6 Hz, 1H, CH2(a)), 4.94 (dd, J = 12.8, 8.4 Hz, 1H, CH2(b)); 13C-NMR (126 MHz, CDCl3): δ(ppm) = 142.9, 135.2, 128.2, 127.7, 126.3, 126.23, 126.20, 126.1, 122.9, 121.4, 113.7, 113.3, 113.2, 79.0, 41.1; LC/MS (ESI): found 423.01 [M+H]+, C17H12BrF3N2O2 requires 421.93; anal. calcd. for C17H12BrF3N2O2: C, 49.42; H, 2.93; N, 6.78; found: C, 49.61; H, 3.07; N, 6.69.
3.7.12. (S)-5-Bromo-3-(1-(4-methoxyphenyl)-2-nitroethyl)-1H-indole (10l)
5-bromoindole 8b (39 mg, 0.2 mmol) and 4-methoxynitrostyrene 9d (36 mg, 0.2 mmol) were reacted according to the GP4 to yield product 10j as white solid (isolated yield 29 mg, 39%), m.p. 145–146 °C; Enantiomeric excess (ee) was determined by chiral HPLC (Chiracel OD-H column) (75% n-hexane/i-PrOH, 1.0 mL/min; tminor = 17.33 min; tmajor = 20.21 min; λ = 254 nm; 62.6% ee; (c 0.053, CH3OH); 1H-NMR (500 MHz, CDCl3): δ(ppm) = 8.13 (s, 1H, NH), 7.53 (d, J = 1.9 Hz, 1H, Ar–H), 7.26 (d, J = 3.4 Hz, 1H, Ar–H), 7.23–7.18 (m, 3H, Ar–H), 7.06 (dd, J = 2.6, 0.9 Hz, 1H, Ar–-H), 6.89–6.83 (m, 2H, Ar–H), 5.07 (t, J = 8.0 Hz, 1H, CH), 4.99 (dd, J = 12.3, 8.0 Hz, 1H, CH2(a)), 4.87 (dd, J = 12.3, 8.0 Hz, 1H, CH2(b)), 3.78 (s, 3H, CH3); 13C-NMR (126 MHz, CDCl3): δ(ppm) = 159.2, 135.3, 130.8, 128.9, 128.0, 125.8, 122.7, 121.7, 114.6, 114.5, 113.4, 112.9, 79.7, 55.4, 40.7. All the analytical data are in accordance with the reported literature [81].
3.7.13. (R)-5-Bromo-3-(2-nitro-1-(2-nitrophenyl)ethyl)-1H-indole (10m)
5-bromoindole 8b (39 mg, 0.2 mmol) and 2-nitronitrostyrene 9e (39 mg, 0.2 mmol) were reacted according to the GP4 to yield product 10k as yellow oil (isolated yield 33 mg, 42%). Enantiomeric excess (ee) was determined by chiral HPLC (Chiracel OD-H column) (75% n-hexane/i-PrOH, 1.0 mL/min; tminor = 25.65 min; tmajor = 28.75 min; λ = 254 nm); 77.69% ee; (c 0.07, CH3OH); IR (KBr): 3419, 1548, 1513, 1339, 723, 431 cm-1; 1H-NMR (500 MHz, CDCl3): δ(ppm) = 8.32 (s, 1H, NH), 7.92 (dd, J = 8.1, 1.4 Hz, 1H, Ar–H), 7.55–7.49 (m, 1H, Ar–H), 7.46–7.38 (m, 3H, Ar–H), 7.27–7.23 (m, 1H, Ar–H), 7.20 (d, J = 8.6 Hz, 1H, Ar–H), 7.13 (d, J = 2.6 Hz, 1H, Ar–H), 5.83 (t, J = 7.7 Hz, 1H, CH), 5.10 (dd, J = 13.3, 7.0 Hz, 1H, CH2(a)), 5.03 (dd, J = 13.3, 8.4 Hz, 1H, CH2(b)); 13C-NMR (126 MHz, CDCl3): δ(ppm) = 149.6, 135.2, 133.5, 133.4, 129.9, 129.0, 127.7, 126.1, 125.5, 123.5, 121.3, 113.6, 113.1, 112.3, 78.1, 36.4; LC/MS (ESI): found 390.02 [M+H]+, C16H12BrN3O4 requires 389.00; anal. calcd. for C16H12BrN3O4: C, 49.25; H, 3.10; N, 10.77; found: C, 49.33; H, 3.17; N, 10.84.
3.7.14. (R)-5-Bromo-3-(1-(2,4-dichlorophenyl)-2-nitroethyl)-1H-indole (10n)
5-bromoindole 8b (39 mg, 0.2 mmol) and 2,4-dichloronitronitrostyrene 9f (44 mg, 0.2 mmol) were reacted according to the GP4 to yield product 10l as brown oil (isolated yield 31 mg, 37%). Enantiomeric excess (ee) was determined by chiral HPLC (Chiracel OD-H column) (75% n-hexane/i-PrOH, 1.0 mL/min; tminor = 9.90 min; tmajor = 20.31 min; λ = 254 nm); 74.6% ee; (c 0.056, CH3OH); IR (KBr): 3417, 1542, 1456, 1348, 1098, 809, 742, 587 cm−1; 1H-NMR (500 MHz, CDCl3): δ(ppm) = 8.26 (s, 1H, NH), 7.48 (dd, J = 18.0, 2.0 Hz, 2H, Ar–H), 7.28–7.24 (m, 1H, Ar–H), 7.21 (dd, J = 8.6, 1.0 Hz, 1H, Ar–H), 7.14 (dd, J = 8.4, 2.1 Hz, 1H, Ar–H), 7.10 (dd, J = 2.6, 1.1 Hz, 1H, Ar–H), 7.07 (dd, J = 8.4, 1.1 Hz, 1H, Ar–H), 5.59 (t, J = 7.9 Hz, 1H, CH), 4.92 (d, J = 1.9 Hz, 1H, CH2(a)), 4.91 (d, J = 1.1 Hz, 1H, CH2(b)); 13C-NMR (126 MHz, CDCl3): δ(ppm) = 135.2, 134.8, 134.6, 134.4, 130.2, 129.8, 127.8, 126.1, 123.3, 121.4, 113.6, 113.1, 112.5, 77.4, 37.5; LC/MS (ESI): found 413.01 [M+H]+, C16H11BrCl2N2O2 requires 411.94; anal. calcd. for C16H11BrCl2N2O2: C, 46.41; H, 2.68; N, 6.77; found: C, 46.27; H, 2.57; N, 6.79.
3.7.15. (S)-5-Bromo-3-(2-nitro-1-(thiophen-2-yl)ethyl)-1H-indole (10o)
5-bromoindole 8b (39 mg, 0.2 mmol) and (E)-2-(2-nitrovinyl)thiophene 9g (31 mg, 0.2 mmol) were reacted according to the GP4 to yield product 10m as brown oil (isolated yield 33 mg, 47%). Enantiomeric excess (ee) was determined by chiral HPLC (Chiracel OD-H column) (75% n-hexane/i-PrOH, 1.0 mL/min; tminor = 12.18 min; tmajor = 20.57 min; λ = 254 nm); 72.0% ee; (c 0.081, CH3OH); 1H-NMR (500 MHz, CDCl3): δ(ppm) = 8.50 (s, 1H, NH), 7.62 (d, J = 2.0 Hz, 1H, Ar–H), 7.27 (dd, J = 8.7, 1.9 Hz, 1H, Ar–H), 7.22–7.18 (m, 2H, Ar–H), 7.10 (d, J = 2.6 Hz, 1H, Ar–H), 6.97–6.92 (m, 2H, Ar–H), 5.38 (t, J = 7.9 Hz, 1H, CH), 5.03–4.93 (m, 2H, CH2); 13C-NMR (126 MHz, CDCl3): δ(ppm) = 142.57, 135.16, 127.56, 127.18, 125.72, 125.46, 125.20, 123.31, 121.41, 113.62, 113.39, 113.16, 79.95, 36.80. All the analytical data are in accordance with the reported literature [42].
3.7.16. (R)-5-Bromo-3-(1-(2,6-dichlorophenyl)-2-nitroethyl)-1H-indole (10p)
5-Bromoindole 8b (39 mg, 0.2 mmol) and 2,6-dichloronitronitrostyrene 9h (44 mg, 0.2 mmol) were reacted according to the GP4 to yield product 10p as brown oil (isolated yield 43 mg, 52%). Enantiomeric excess (ee) was determined by chiral HPLC (Chiracel OD-H column) (75% n-hexane/i-PrOH, 1.0 mL/min; tminor = 10.29 min; tmajor = 11.29 min; λ = 254 nm); 60.1% ee; (c 0.034, CH3OH); IR (KBr): 3415, 1549, 1463, 1356, 1109, 822, 734, 605, 541, 424 cm−1; 1H-NMR (500 MHz, CDCl3): δ(ppm) = 8.85 (s, 1H, NH), 7.50 (s, 1H, Ar–H), 7.32 (s, 1H, Ar–H), 7.26 (s, 1H, Ar–H), 7.22–7.19 (m, 2H, Ar–H), 7.18–7.13 (m, 2H, Ar–H), 6.12 (td, J = 7.7, 1.2 Hz, 1H, CH), 5.39 (dd, J = 12.9, 7.7 Hz, 1H, CH2(a)), 5.29 (dd, J = 12.9, 7.8 Hz, 1H, CH2(b)); 13C-NMR (126 MHz, CDCl3): δ(ppm) = 134.86, 133.93, 130.06129.64, 128.19, 125.38, 124.10, 121.60, 114.17, 113.25, 112.94, 111.02, 76.27, 37.77; LC/MS (ESI): found 412.98 [M+H]+, C16H11BrCl2N2O2 requires 411.94; Anal. calcd. for C16H11BrCl2N2O2: C, 46.41; H, 2.68; N, 6.77; Found: C, 46.36; H, 2.74; N, 6.63.
3.7.17. (S)-5-Fluoro-3-(2-nitro-1-(thiophen-2-yl)ethyl)-1H-indole(10q)
5-Fluoroindole 8c (27 mg, 0.2 mmol) and (E)-2-(2-nitrovinyl)thiophene 9g (31 mg, 0.2 mmol) were reacted according to the GP4 to yield product 10o as brown oil (isolated yield 33 mg, 57%). Enantiomeric excess (ee) was determined by chiral HPLC (Chiracel OD-H column) (75% n-hexane/i-PrOH, 1.0 mL/min; tminor = 11.81 min; tmajor = 13.42 min; λ = 254 nm); 66.0% ee; (c 0.035, CH3OH); IR (KBr): 3417, 1547, 1469, 1343, 1205, 827, 731, 541 cm-1; 1H-NMR (500 MHz, CDCl3): δ(ppm) = 8.18 (s, 1H, NH), 7.40 (dd, J = 8.7, 5.2 Hz, 1H, Ar–H), 7.21 (dd, J = 5.1, 1.3 Hz, 1H, Ar–H), 7.13–7.08 (m, 1H, Ar–H), 7.04 (dd, J = 9.4, 2.3 Hz, 1H, Ar–H), 7.00–6.92 (m, 2H, Ar–H), 6.87 (td, J = 9.2, 2.3 Hz, 1H, Ar–H), 5.43 (t, J = 7.9 Hz, 1H, CH), 5.05–4.96 (m, 2H, CH2); 13C-NMR (126 MHz, CDCl3): δ(ppm) = 161.31 and 159.41 (C1-F, JC-F = 239.40 Hz), 142.84, 136.59, 136.49, 127.15, 125.46, 125.18, 122.48, 122.28 and 122.25 (C4-F, JC-F = 3.53 Hz), 119.84 and 119.76 (C3-F, JC-F = 10.04 Hz), 114.35, 109.22 and 109.02 (C2-F, JC-F = 23.94 Hz), 80.09, 36.99; LC/MS (ESI): found 291.10 [M+H]+, C14H11FN2O2S requires 290.05; Anal. calcd. for C14H11FN2O2S: C, 57.92; H, 3.82; N, 9.65; Found: C, 58.11; H, 3.93; N, 9.52.
3.7.18. (R)-3-(1-(2,6-Dichlorophenyl)-2-nitroethyl)-1H-indole (10r)
5-Fluoroindole 8c (27 mg, 0.2 mmol) and 2,6-dichloronitronitrostyrene 9h (44 mg, 0.2 mmol) were reacted according to the GP4 to yield product 10r as brown oil (isolated yield 32 mg, 45%). Enantiomeric excess (ee) was determined by chiral HPLC (Chiracel OD-H column) (75% n-hexane/i-PrOH, 1.0 mL/min; tminor = 7.99 min; tmajor = 10.29 min; λ = 254 nm); 24.3% ee; (c 0.029, CH3OH); IR (KBr): 3418, 1551, 1472, 1371, 1101, 819, 735 cm−1; 1H-NMR (500 MHz, CDCl3): δ(ppm) = 8.20 (s, 1H, NH), 7.43–7.22 (m, 3H, Ar–H), 7.18–7.12 (m, 2H, Ar–H), 7.02 (dd, J = 9.4, 2.3 Hz, 1H, Ar–H), 6.81 (ddd, J = 9.5, 8.8, 2.3 Hz, 1H, Ar–H), 6.17 (td, J = 7.6, 1.2 Hz, 1H, CH), 5.42 (dd, J = 12.8, 7.6 Hz, 1H, CH2(a)), 5.31 (dd, J = 12.9, 7.7 Hz, 1H, CH2(b)); 13C-NMR (126 MHz, CDCl3): δ(ppm) = 161.13 and 159.23 (C1-F, JC-F = 239.14 Hz), 136.22 and 136.12 (C5-F, JC-F = 10.34 Hz), 134.11, 129.61, 123.04, 122.94 and 122.91 (C4-F, JC-F = 3.65 Hz), 119.87 and 119.79 (C3-F, JC-F = 10.21 Hz), 111.85, 109.05 and 108.86 (C6-F, JC-F = 24.57 Hz), 97.85 and 97.64 (C2-F, JC-F = 25.96 Hz), 76.39, 37.92; LC/MS (ESI): found 353.10 [M+H]+, C16H11Cl2FN2O2 requires 352.01; anal. calcd. for C16H11Cl2FN2O2: C, 54.41; H, 3.14; N, 7.93; found: C, 54.58; H, 3.08; N, 8.03.
3.7.19. (S)-1-Ethyl-3-(1-(4-fluorophenyl)-2-nitroethyl)-1H-indole (10s)
1-Ethyl-1H-indole 8d (29 mg, 0.2 mmol) and 4-floronitrostyrene 9a (34 mg, 0.2 mmol) were reacted according to the GP4 to yield product 10s as yellow oil (isolated yield 46 mg, 73%). Enantiomeric excess (ee) was determined by chiral HPLC (Chiracel OD-H column) (70% n-hexane/i-PrOH, 1.0 mL/min; tminor = 16.38 min; tmajor = 34.92 min; λ = 254 nm); 35.2% ee; (c 0.022, CH3OH); IR (KBr): 3418, 1557, 1349, 1174, 739, 573 cm-1; 1H-NMR (500 MHz, CDCl3): δ(ppm) = 7.42 (d, J = 7.9 Hz, 1H, Ar–H), 7.36–7.29 (m, 3H, Ar–H), 7.25–7.21 (m, 1H, Ar–H), 7.08 (ddd, J = 8.0, 7.0, 1.0 Hz, 1H, Ar–H), 7.02 (t, J = 8.6 Hz, 2H, Ar–H), 6.92 (d, J = 0.9 Hz, 1H, Ar–H), 5.18 (dd, J = 8.7, 7.4 Hz, 1H, CH), 5.06 (dd, J = 12.5, 7.2 Hz, 1H, CH2(a)), 4.91 (dd, J = 12.5, 8.9 Hz, 1H, CH2(b)), 4.14 (q, J = 7.3 Hz, 2H, CH2), 1.45 (t, J = 7.3 Hz, 3H, CH3); 13C-NMR (126 MHz, CDCl3): δ(ppm) = 163.17 and 161.21 (C1-F, JC-F = 246.71 Hz), 136.50, 135.28 and 135.26 (C4-F, JC-F = 3.15 Hz), 129.52, 129.46 (C3-F, JC-F = 8.06 Hz), 126.66, 124.56, 122.34, 119.62, 119.14, 116.02, 115.85 (C2-F, JC-F = 21.55 Hz), 112.81, 109.79, 79.73, 41.19, 41.06, 15.55; LC/MS (ESI): found 313.10 [M+H]+, C18H17FN2O2 requires 312.13; anal. calcd. for C18H17FN2O2: C, 69.22; H, 5.49; N, 8.97; found: C, 69.34; H, 5.43; N, 8.85.
3.7.20. (S)-1-Ethyl-3-(1-(4-methoxyphenyl)-2-nitroethyl)-1H-indole (10t)
1-Ethyl-1H-indole 8d (29 mg, 0.2 mmol) and 4-methoxynitrostyrene 9d (36 mg, 0.2 mmol) were reacted according to the GP4 to yield product 10t as yellow oil (isolated yield 49 mg, 76%). Enantiomeric excess (ee) was determined by chiral HPLC (Chiracel OD-H column) (70% n-hexane/i-PrOH, 1.0 mL/min; tminor = 20.94 min; tmajor = 35.44 min; λ = 254 nm); 26.74% ee; (c 0.024, CH3OH); IR (KBr): 3417, 152, 1337, 1171, 741, 534 cm−1; 1H-NMR (500 MHz, CDCl3): δ(ppm) = 7.44 (d, J = 8.0 Hz, 1H, Ar–H), 7.32 (d, J = 8.3 Hz, 1H, Ar–H), 7.27–7.24 (m, 2H, Ar–H), 7.21 (t, J = 7.0 Hz, 1H, Ar–H), 7.06 (t, J = 7.5 Hz, 1H, Ar–H), 6.90 (s, 1H, Ar–H), 6.85 (d, J = 8.7 Hz, 2H, Ar–H), 5.13 (t, J = 8.0 Hz, 1H, CH), 5.03 (dd, J = 12.3, 7.3 Hz, 1H, CH2(a)), 4.89 (dd, J = 12.4, 8.8 Hz, 1H, CH2(b)), 4.12 (q, J = 7.3 Hz, 2H, CH2), 3.77 (s, 3H, CH3), 1.43 (t, J = 7.3 Hz, 3H, CH3); 13C-NMR (126 MHz, CDCl3): δ(ppm) = 158.99, 136.49, 131.51, 128.94, 126.82, 124.62, 122.18, 119.48, 119.28, 114.38, 113.33, 109.70, 55.37, 41.14, 41.06, 15.55; LC/MS (ESI): found 325.20 [M+H]+, C19H20N2O3 requires 324.15; anal. calcd. for C19H20N2O3: C, 70.35; H, 6.21; N, 8.64; found: C, 70.19; H, 6.13; N, 8.54.
4. Large-Scale Synthesis of (S)-3-(1-(4-Fluorophenyl)-2-nitroethyl)-1H-indole (10a)
An oven-dried 50-mL round bottom flask equipped with a condenser under nitrogen atmosphere was charged with ligand L5 (100 mg, 0.3 mmol, 15% mol), Cu(OTf)2 (110 mg, 0.3 mmol, 15 mol %) and dry toluene (20 mL). The mixture was then stirred at reflux for 2 h. After cooling to room temperature, 4-floronitrostyrene (9a) (334 mg, 2.0 mmol) and 4A° molecular sieves were added. Then, the mixture was stirred for another 30 min, followed by the addition of indole 8a (234 mg, 2.0 mmol). The reaction was then left stirring for 48 h at room temperature. The solvent was removed under reduced pressure, and the crude product was isolated by flash column chromatography on silica gel, eluting with ethylacetate/n-hexane (2:8, v/v) to afford a pure Friedel–Crafts product (10a) isolated yield of 76% (432 mg) with 77.2% enantiomeric excess (ee). Enantiomeric excess (ee) was determined by chiral HPLC (Chiracel OD-H column) (70% n-hexane/i-PrOH, 1.0 mL/min; tmajor = 25.09 min; tminor = 30.30 min; λ = 254 nm); 77.2% ee; 1H-NMR (500 MHz, CDCl3): δ(ppm) = 8.13 (s, 1H, NH), 7.47–7.40 (m, 1H, Ar–H), 7.35 (s, 1H, Ar–H), 7.32–7.28 (m, 2H, Ar–H), 7.23 (ddd, J = 8.2, 7.0, 1.2 Hz, 1H, Ar–H), 7.11 (ddd, J = 8.1, 6.9, 1.0 Hz, 1H, Ar–H), 7.04–6.96 (m, 3H, Ar–H), 5.19 (t, J = 8.0 Hz, 1H, CH), 5.05 (dd, J = 12.5, 7.5 Hz, 1H, CH2(a)), 4.90 (dd, J = 12.5, 8.6 Hz, 1H, CH2(b)); 13C-NMR (126 MHz, CDCl3): δ(ppm) = 163.1 and 161.18 (C1-F, JC-F = 246.58 Hz), 136.6, 135.07 and 135.04 (C4-F, JC-F = 3.15 Hz), 129.50 and 129.44 (C3-F, JC-F = 7.94 Hz), 126.0, 122.9, 121.6, 120.1, 118.9, 115.99 and 115.82 (C2-F, JC-F = 21.67 Hz), 114.2, 111.6, 79.6, 41.0.
5. Conclusions
In summary, we have synthesized new C2-symmetric 2,5-bis(oxazolinyl)thiophene and 2,5-bis(imidazolinyl)thiophene ligands based on thiophene systems and successfully tested them in asymmetric Friedel–Crafts alkylation reactions of indole with trans β-nitroolefins. Our newly developed catalytic system (15 mol% of L5:Cu(OTf)2 in toluene at 25 °C) was found to be applicable in inducing chirality into nitroalkylated indoles with low to good yields (35–76%) and low to good enantioselectivity (21–81%) at room temperature. On the basis of the screening performed, this methodology could be an alternative tool for asymmetric Friedel–Crafts reactions using this catalytic system. The advantage of this catalytic system is that it is easy to prepare the chiral ligands from the widely accessible thiophene precursor, and the reaction can also be performed at room temperature as compared to other catalytic system carried out at lower temperatures. There is an ongoing research project to explore more utilities for these new chiral thiophene ligands and their applications in asymmetric transformation, and its outcome will be communicated soon in future.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project (RSP-2021/64), King Saud University, Riyadh, Saudi Arabia.
Supplementary Materials
Page S4–S35: 1H-NMR and 13C-NMR for compounds 4a–c, L1–L5 and 10a–t and chiral HPLC analysis for compound 10a-t.
Author Contributions
Conceptualization, A.M.A.-M. and A.B.; supervision, A.M.A.-M., A.B. and M.S.I.; methodology, A.S.A., M.S.I. and A.M.A.-M.; validation, M.S.I., A.S.A., S.A. and A.M.A.-M.; formal analysis, A.S.A., M.S.I., S.A. and M.H.; investigation, A.S.A., M.S.I. and S.A.; resources, A.M.A.-M. and A.B.; data curation, A.S.A., M.S.I., A.B. and M.H.; writing—original draft preparation, M.S.I., A.B. and A.S.A.; writing—review and editing, M.S.I., A.B., A.M.A.-M. and M.H.; visualization, A.B., M.S.I., S.A. and M.H.; project administration, A.M.A.-M. and A.B.; funding acquisition, A.M.A.-M. and A.B.; software, A.S.A., M.S.I., A.B. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
Researchers Supporting Project number (RSP-2021/64), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cai F., Pu X., Qi X., Lynch V., Radha A., Ready J.M. Chiral allene-containing phosphines in asymmetric catalysis. J. Am. Chem. Soc. 2011;133:18066–18069. doi: 10.1021/ja207748r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leeuwen P.W.v., Kamer P.C., Claver C., Pamies O., Dieguez M. Phosphite-containing ligands for asymmetric catalysis. Chem. Rev. 2011;111:2077–2118. doi: 10.1021/cr1002497. [DOI] [PubMed] [Google Scholar]
- 3.Fernández-Pérez H., Etayo P., Panossian A., Vidal-Ferran A. Phosphine− phosphinite and phosphine− phosphite ligands: Preparation and applications in asymmetric catalysis. Chem. Rev. 2011;111:2119–2176. doi: 10.1021/cr100244e. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee D., Buzas A.K., Besnard C.l., Kündig E.P. Chiral n-heterocyclic carbene gold complexes: Synthesis, properties, and application in asymmetric catalysis. Organometallics. 2012;31:8348–8354. doi: 10.1021/om300917m. [DOI] [Google Scholar]
- 5.Yoon M., Srirambalaji R., Kim K. Homochiral metal–organic frameworks for asymmetric heterogeneous catalysis. Chem. Rev. 2012;112:1196–1231. doi: 10.1021/cr2003147. [DOI] [PubMed] [Google Scholar]
- 6.Chen X., Lu Z. Recent advances in chiral imino-containing ligands for metal-catalyzed asymmetric transformations. Org. Biomol. Chem. 2017;15:2280–2306. doi: 10.1039/C6OB02817A. [DOI] [PubMed] [Google Scholar]
- 7.Pellissier H. Recent developments in enantioselective iron-catalyzed transformations. Coord. Chem. Rev. 2019;386:1–31. doi: 10.1016/j.ccr.2019.01.011. [DOI] [Google Scholar]
- 8.Barakat A., El-Faham A., Haukka M., Al-Majid A.M., Soliman S.M. S-triazine pincer ligands: Synthesis of their metal complexes, coordination behavior, and applications. Appl. Organomet. Chem. 2021;35:e6317. doi: 10.1002/aoc.6317. [DOI] [Google Scholar]
- 9.Kagan H.B., Gopalaiah K. Early history of asymmetric synthesis: Who are the scientists who set up the basic principles and the first experiments? New J. Chem. 2011;35:1933–1937. doi: 10.1039/c1nj20216b. [DOI] [Google Scholar]
- 10.Oliveira V.d.G., Cardoso M.F.d.C., Forezi L.d.S.M. Organocatalysis: A brief overview on its evolution and applications. Catalysts. 2018;8:605. doi: 10.3390/catal8120605. [DOI] [Google Scholar]
- 11.Pellissier H. Asymmetric organocatalysis. Tetrahedron. 2007;38:9267–9331. doi: 10.1016/j.tet.2007.06.024. [DOI] [Google Scholar]
- 12.Itoh T., Hanefeld U. Enzyme catalysis in organic synthesis. Green Chem. 2017;19:331–332. doi: 10.1039/C6GC90124G. [DOI] [Google Scholar]
- 13.Sheldon R.A., Brady D., Bode M.L. The hitchhiker’s guide to biocatalysis: Recent advances in the use of enzymes in organic synthesis. Chem. Sci. 2020;11:2587–2605. doi: 10.1039/C9SC05746C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pàmies O., Bäckvall J.-E. Combination of enzymes and metal catalysts. A powerful approach in asymmetric catalysis. Chem. Rev. 2003;103:3247–3262. doi: 10.1021/cr020029g. [DOI] [PubMed] [Google Scholar]
- 15.Choi J., Fu G.C. Catalytic asymmetric synthesis of secondary nitriles via stereoconvergent negishi arylations and alkenylations of racemic α-bromonitriles. J. Am. Chem. Soc. 2012;134:9102–9105. doi: 10.1021/ja303442q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalek M., Fu G.C. Phosphine-catalyzed doubly stereoconvergent γ-additions of racemic heterocycles to racemic allenoates: The catalytic enantioselective synthesis of protected α, α-disubstituted α-amino acid derivatives. J. Am. Chem. Soc. 2015;137:9438–9442. doi: 10.1021/jacs.5b05528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park J.K., Lackey H.H., Ondrusek B.A., McQuade D.T. Stereoconvergent synthesis of chiral allylboronates from an e/z mixture of allylic aryl ethers using a 6-NHC−Cu(I) catalyst. J. Am. Chem. Soc. 2011;133:2410–2413. doi: 10.1021/ja1112518. [DOI] [PubMed] [Google Scholar]
- 18.Li L., Chen Z., Zhang X., Jia Y. Divergent strategy in natural product total synthesis. Chem. Rev. 2018;118:3752–3832. doi: 10.1021/acs.chemrev.7b00653. [DOI] [PubMed] [Google Scholar]
- 19.Shimokawa J. Divergent strategy in natural product total synthesis. Tetrahedron Lett. 2014;55:6156–6162. doi: 10.1016/j.tetlet.2014.09.078. [DOI] [Google Scholar]
- 20.Krautwald S., Carreira E.M. Stereodivergence in asymmetric catalysis. J. Am. Chem. Soc. 2017;139:5627–5639. doi: 10.1021/jacs.6b13340. [DOI] [PubMed] [Google Scholar]
- 21.Pellissier H. Enantioselective vanadium-catalyzed transformations. An update. Coord. Chem. Rev. 2020;418:213395. doi: 10.1016/j.ccr.2020.213395. [DOI] [Google Scholar]
- 22.Roberts R.M., Khalaf A.A. Friedel–Crafts Alkylation Chemistry: A Century of Discovery. Volume 10 Marcel Dekker Incorporated; New York, NY, USA: 1984. [Google Scholar]
- 23.Olah G.A. Across Conventional Lines: Selected Papers of George a Olah Volume 1. World Scientific; Singapore: 2003. Friedel–Crafts and related reactions; pp. 109–118. [Google Scholar]
- 24.Bandini M., Melloni A., Umani-Ronchi A. New catalytic approaches in the stereoselective Friedel–Crafts alkylation reaction. Angew. Chem. Int. Ed. 2004;43:550–556. doi: 10.1002/anie.200301679. [DOI] [PubMed] [Google Scholar]
- 25.Bandini M., Eichholzer A., Umani-Ronchi A. An update on catalytic enantioselective alkylations of indoles. Mini Rev. Org. Chem. 2007;4:115–124. doi: 10.2174/157019307780599270. [DOI] [Google Scholar]
- 26.Bi X., Zhang Q., Gu Z. Transition-metal-catalyzed carbon-carbon bond activation in asymmetric synthesis. Chin. J. Chem. 2021;39:1397–1412. doi: 10.1002/cjoc.202000591. [DOI] [Google Scholar]
- 27.Barakat A., Islam M.S., Al Majid A.M., Al-Othman Z.A. Highly enantioselective Friedel–Crafts alkylation of indoles with α, β-unsaturated ketones with simple Cu(II)–oxazoline–imidazoline catalysts. Tetrahedron. 2013;69:5185–5192. doi: 10.1016/j.tet.2013.04.063. [DOI] [Google Scholar]
- 28.Liu L., Ma H., Xiao Y., Du F., Qin Z., Li N., Fu B. Highly enantioselective Friedel–Crafts alkylation of indoles and pyrrole with β, γ-unsaturated α-ketoesters catalyzed by heteroarylidene-tethered bis(oxazoline) copper complexes. Chem. Commun. 2012;48:9281–9283. doi: 10.1039/c2cc34803a. [DOI] [PubMed] [Google Scholar]
- 29.Palomo C., Oiarbide M., Kardak B.G., García J.M., Linden A. Highly enantioselective friedel−crafts alkylations of pyrroles and indoles with α ‘-hydroxy enones under Cu(II)-simple bis(oxazoline) catalysis. J. Am. Chem. Soc. 2005;127:4154–4155. doi: 10.1021/ja0423217. [DOI] [PubMed] [Google Scholar]
- 30.Bedekar A.V., Andersson P.G. A new class of bis-oxazoline ligands for the cu-catalysed asymmetric cyclopropanation of olefins. Tetrahedron Lett. 1996;37:4073–4076. doi: 10.1016/0040-4039(96)00736-8. [DOI] [Google Scholar]
- 31.Liu Y., Zhou X., Shang D., Liu X., Feng X. N, N′-dioxide–Scandium(III) complex catalyzed highly enantioselective Friedel–Crafts alkylation of indole to alkylidene malonates. Tetrahedron. 2010;66:1447–1457. doi: 10.1016/j.tet.2009.12.032. [DOI] [Google Scholar]
- 32.Chen H., Du F., Liu L., Li J., Zhao Q., Fu B. Malonate-type bis(oxazoline) ligands with sp2 hybridized bridge carbon: Synthesis and application in Friedel–Crafts alkylation and allylic alkylation. Tetrahedron. 2011;67:9602–9608. doi: 10.1016/j.tet.2011.09.106. [DOI] [Google Scholar]
- 33.Zhou J., Ye M.-C., Huang Z.-Z., Tang Y. Controllable enantioselective friedel−crafts reaction1 between indoles and alkylidene malonates catalyzed by pseudo-C3-symmetric trisoxazoline copper(II) complexes. J. Org. Chem. 2004;69:1309–1320. doi: 10.1021/jo035552p. [DOI] [PubMed] [Google Scholar]
- 34.Son S., Fu G.C. Nickel-catalyzed asymmetric negishi cross-couplings of secondary allylic chlorides with alkylzincs. J. Am. Chem. Soc. 2008;130:2756–2757. doi: 10.1021/ja800103z. [DOI] [PubMed] [Google Scholar]
- 35.Evans D.A., Scheidt K.A., Fandrick K.R., Lam H.W., Wu J. Enantioselective indole Friedel−Crafts alkylations catalyzed by bis (oxazolinyl) Pyridine−Scandium(III)triflate complexes. J. Am. Chem. Soc. 2003;125:10780–10781. doi: 10.1021/ja036985c. [DOI] [PubMed] [Google Scholar]
- 36.Zheng B., Wang M., Li Z., Bian Q., Mao J., Li S., Liu S., Wang M., Zhong J., Guo H. Asymmetric henry reaction catalyzed by a zn–amino alcohol system. Tetrahedron Asymmetry. 2011;22:1156–1160. doi: 10.1016/j.tetasy.2011.06.032. [DOI] [Google Scholar]
- 37.Zhu S.-F., Xu B., Wang G.-P., Zhou Q.-L. Well-defined binuclear chiral spiro copper catalysts for enantioselective n–h insertion. J. Am. Chem. Soc. 2012;134:436–442. doi: 10.1021/ja2084493. [DOI] [PubMed] [Google Scholar]
- 38.Lou S., Fu G.C. Nickel/bis(oxazoline)-catalyzed asymmetric kumada reactions of alkyl electrophiles: Cross-couplings of racemic α-bromoketones. J. Am. Chem. Soc. 2010;132:1264–1266. doi: 10.1021/ja909689t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumari P., Bera P.K., Noor-ul H.K., Kureshy R.I., Abdi S.H., Bajaj H.C. Asymmetric Friedel–Crafts addition of indoles to n-sulfonyl aldimines catalyzed by Cu(II) chiral amino alcohol based schiff base complexes. Catal. Sci. Technol. 2014;4:563–568. doi: 10.1039/C3CY00629H. [DOI] [Google Scholar]
- 40.Ibáñez I., Kaneko M., Kamei Y., Tsutsumi R., Yamanaka M., Akiyama T. Enantioselective Friedel–Crafts alkylation reaction of indoles with α-trifluoromethylated β-nitrostyrenes catalyzed by chiral binol metal phosphate. ACS Catal. 2019;9:6903–6909. doi: 10.1021/acscatal.9b01811. [DOI] [Google Scholar]
- 41.Islam M.S., Al Majid A.M., Al-Othman Z.A., Barakat A. Highly enantioselective Friedel–Crafts alkylation of indole with electron deficient trans-β-nitroalkenes using Zn(II)–oxazoline–imidazoline catalysts. Tetrahedron Asymmetry. 2014;25:245–251. doi: 10.1016/j.tetasy.2013.11.018. [DOI] [Google Scholar]
- 42.Singh P.K., Bisai A., Singh V.K. Enantioselective Friedel–Crafts alkylation of indoles with nitroalkenes catalyzed by a bis(oxazoline)–cu (ii) complex. Tetrahedron Lett. 2007;48:1127–1129. doi: 10.1016/j.tetlet.2006.12.081. [DOI] [Google Scholar]
- 43.Li W. Chiral bis(oxazolinyl) thiophenes for enantioselective Cu(II)-catalyzed Friedel–Crafts alkylation of indole derivatives with nitroalkenes. Catal. Lett. 2014;144:943–948. doi: 10.1007/s10562-014-1228-2. [DOI] [Google Scholar]
- 44.Tanaka K., Sakuragi K., Ozaki H., Takada Y. Highly enantioselective Friedel–Crafts alkylation of n, n-dialkylanilines with trans-β-nitrostyrene catalyzed by a homochiral metal–organic framework. Chem. Commun. 2018;54:6328–6331. doi: 10.1039/C8CC03447H. [DOI] [PubMed] [Google Scholar]
- 45.Li Z., He M., Xu D., Liu Z. Graphene materials-based energy acceptor systems and sensors. J. Photochem. Photobiol. C. Photochem. Rev. 2014;18:1–17. doi: 10.1016/j.jphotochemrev.2013.10.002. [DOI] [Google Scholar]
- 46.Gao J.-R., Wu H., Xiang B., Yu W.-B., Han L., Jia Y.-X. Highly enantioselective construction of trifluoromethylated all-carbon quaternary stereocenters via nickel-catalyzed Friedel–Crafts alkylation reaction. J. Am. Chem. Soc. 2013;135:2983–2986. doi: 10.1021/ja400650m. [DOI] [PubMed] [Google Scholar]
- 47.Chen J.-B., Jia Y.-X. Recent progress in transition-metal-catalyzed enantioselective indole functionalizations. Org. Biomol. Chem. 2017;15:3550–3567. doi: 10.1039/C7OB00413C. [DOI] [PubMed] [Google Scholar]
- 48.Ono N. The Nitro Group in Organic Synthesis. Volume 9 John Wiley & Sons; Hoboken, NJ, USA: 2003. [Google Scholar]
- 49.Aitken L.S., Arezki N.R., Dell’Isola A., Cobb A.J. Asymmetric organocatalysis and the nitro group functionality. Synthesis. 2013;45:2627–2648. [Google Scholar]
- 50.Robinson B. The Alkaloids: Chemistry and Physiology. Volume 13. Elsevier; Amsterdam, The Netherlands: 1971. Alkaloids of the calabar bean; pp. 213–226. [Google Scholar]
- 51.Takano S., Ogasawara K. The Alkaloids: Chemistry and Pharmacology. Volume 36. Elsevier; Amsterdam, The Netherlands: 1990. Alkaloids of the calabar bean; pp. 225–251. [Google Scholar]
- 52.Greig N.H., Pei X.F., Soncrant T.T., Ingram D.K., Brossi A. Phenserine and ring c hetero-analogues: Drug candidates for the treatment of alzheimer’s disease. Med. Res. Rev. 1995;15:3–31. doi: 10.1002/med.2610150103. [DOI] [PubMed] [Google Scholar]
- 53.Berner O.M., Tedeschi L., Enders D. Asymmetric michael additions to nitroalkenes. Eur. J. Org. Chem. 2002;2002:1877–1894. doi: 10.1002/1099-0690(200206)2002:12<1877::AID-EJOC1877>3.0.CO;2-U. [DOI] [Google Scholar]
- 54.Calderari G., Seebach D. Asymmetrische michael-additionen. Stereoselektive alkylierung chiraler, nicht racemischer enolate durch nitroolefine. Herstellung enantiomerenreiner γ-aminobuttersäure-und bernsteinsäure-derivate. Helv. Chim. Acta. 1985;68:1592–1604. doi: 10.1002/hlca.19850680611. [DOI] [Google Scholar]
- 55.Hayashi T., Senda T., Ogasawara M. Rhodium-catalyzed asymmetric conjugate addition of organoboronic acids to nitroalkenes. J. Am. Chem. Soc. 2000;122:10716–10717. doi: 10.1021/ja002805c. [DOI] [Google Scholar]
- 56.Choi H., Hua Z., Ojima I. Highly enantioselective copper-catalyzed conjugate addition of diethylzinc to nitroalkenes. Org. Lett. 2004;6:2689–2691. doi: 10.1021/ol0491282. [DOI] [PubMed] [Google Scholar]
- 57.Duursma A., Minnaard A.J., Feringa B.L. Highly enantioselective conjugate addition of dialkylzinc reagents to acyclic nitroalkenes: A catalytic route to β2-amino acids, aldehydes, and alcohols. J. Am. Chem. Soc. 2003;125:3700–3701. doi: 10.1021/ja029817d. [DOI] [PubMed] [Google Scholar]
- 58.Czekelius C., Carreira E.M. Catalytic enantioselective conjugate reduction of β, β-disubstituted nitroalkenes. Angew. Chem. Int. Ed. 2003;115:4941–4943. doi: 10.1002/ange.200352175. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe M., Ikagawa A., Wang H., Murata K., Ikariya T. Catalytic enantioselective michael addition of 1, 3-dicarbonyl compounds to nitroalkenes catalyzed by well-defined chiral ru amido complexes. J. Am. Chem. Soc. 2004;126:11148–11149. doi: 10.1021/ja046296g. [DOI] [PubMed] [Google Scholar]
- 60.Li H., Wang Y., Tang L., Deng L. Highly enantioselective conjugate addition of malonate and β-ketoester to nitroalkenes: Asymmetric c−c bond formation with new bifunctional organic catalysts based on cinchona alkaloids. J. Am. Chem. Soc. 2004;126:9906–9907. doi: 10.1021/ja047281l. [DOI] [PubMed] [Google Scholar]
- 61.Mellah M., Voituriez A., Schulz E. Chiral sulfur ligands for asymmetric catalysis. Chem. Rev. 2007;107:5133–5209. doi: 10.1021/cr068440h. [DOI] [PubMed] [Google Scholar]
- 62.Alammari A.S., Al-Majid A.M., Barakat A., Alshahrani S., Ali M., Islam M.S. Asymmetric henry reaction of nitromethane with substituted aldehydes catalyzed by novel in situ generated chiral bis(β-amino alcohol-cu(oac)2·h2o complex. Catalysts. 2021;11:1208. doi: 10.3390/catal11101208. [DOI] [Google Scholar]
- 63.Mao J., Nie X., Wang M., Wang Q., Zheng B., Bian Q., Zhong J. Catalytic asymmetric nitroaldol (henry) reactions with copper(ii)/cyclopropane-based bisoxazoline complexes. Tetrahedron Asymmetry. 2012;23:965–971. doi: 10.1016/j.tetasy.2012.06.014. [DOI] [Google Scholar]
- 64.Blay G., Climent E., Fernandez I., Hernández-Olmos V., Pedro J.R. Enantioselective henry reaction catalyzed with copper(II)–iminopyridine complexes. Tetrahedron Asymmetry. 2007;18:1603–1612. doi: 10.1016/j.tetasy.2007.06.023. [DOI] [Google Scholar]
- 65.Hao X.-Q., Xu Y.-X., Yang M.-J., Wang L., Niu J.-L., Gong J.-F., Song M.-P. A cationic NCN pincer Platinum(II) aquo complex with a bis(imidazolinyl) phenyl ligand: Studies toward its synthesis and asymmetric Friedel–Crafts alkylation of indoles with nitroalkenes. Organometallics. 2012;31:835–846. doi: 10.1021/om200714z. [DOI] [Google Scholar]
- 66.Wu L.-Y., Hao X.-Q., Xu Y.-X., Jia M.-Q., Wang Y.-N., Gong J.-F., Song M.-P. Chiral NCN pincer Pt(II) and Pd(II) complexes with 1,3-bis(2′-imidazolinyl) benzene: Synthesis via direct metalation, characterization, and catalytic activity in the friedel−crafts alkylation reaction. Organometallics. 2009;28:3369–3380. doi: 10.1021/om801005j. [DOI] [Google Scholar]
- 67.Schinnerl M., Seitz M., Kaiser A., Reiser O. New applications of bis(oxazoline) ligands in catalysis: Asymmetric 1,2 and 1,4 addition of znr2 to carbonyl compounds. Org. Lett. 2001;3:4259–4262. doi: 10.1021/ol016925g. [DOI] [PubMed] [Google Scholar]
- 68.Shintani R., Fu G.C. Copper-catalyzed enantioselective conjugate addition of diethylzinc to acyclic enones in the presence of planar-chiral phosphaferrocene-oxazoline ligands. Org. Lett. 2002;4:3699–3702. doi: 10.1021/ol026651c. [DOI] [PubMed] [Google Scholar]
- 69.Al Majid A.M., Islam M.S., Al-Othman Z.A., Al-Salhoob A.F. Enantioselective additions of diethylzinc to aldehydes catalyzed by titanate(IV) complex with chiral bidentate bis-amide ligands based on cyclopropane backbone. Arab. J. Chem. 2017;10:S964–S970. doi: 10.1016/j.arabjc.2012.12.036. [DOI] [Google Scholar]
- 70.Mei L.-y., Yuan Z.-l., Shi M. Chiral imidazoline–phosphine ligands for palladium-catalyzed asymmetric allylic substitutions. Organometallics. 2011;30:6466–6475. doi: 10.1021/om2008309. [DOI] [Google Scholar]
- 71.Dugal-Tessier J., Dake G.R., Gates D.P. Chiral phosphaalkene− oxazoline ligands for the palladium-catalyzed asymmetric allylic alkylation. Org. Lett. 2010;12:4667–4669. doi: 10.1021/ol1020652. [DOI] [PubMed] [Google Scholar]
- 72.Sawada T., Nakada M. Preparation of new chiral bisoxazoline ligands for the catalytic asymmetric intramolecular cyclopropanation of α-diazo-β-keto phenyl sulfone to afford a useful bicyclo [3.1.0] hexane derivative. Tetrahedron Asymmetry. 2012;23:350–356. doi: 10.1016/j.tetasy.2012.02.021. [DOI] [Google Scholar]
- 73.Burguete M.I., Fraile J.M., García J.I., García-Verdugo E., Herrerías C.I., Luis S.V., Mayoral J.A. Bis(oxazoline) copper complexes covalently bonded to insoluble support as catalysts in cyclopropanation reactions. J. Org. Chem. 2001;66:8893–8901. doi: 10.1021/jo0159338. [DOI] [PubMed] [Google Scholar]
- 74.Jia Y.-X., Zhu S.-F., Yang Y., Zhou Q.-L. Asymmetric friedel− crafts alkylations of indoles with nitroalkenes catalyzed by zn(ii)−bisoxazoline complexes. J. Org. Chem. 2006;71:75–80. doi: 10.1021/jo0516537. [DOI] [PubMed] [Google Scholar]
- 75.Itoh J., Fuchibe K., Akiyama T. Chiral phosphoric acid catalyzed enantioselective Friedel–Crafts alkylation of indoles with nitroalkenes: Cooperative effect of 3Å molecular sieves. Angew. Chem. Int. Ed. 2008;47:4016–4018. doi: 10.1002/anie.200800770. [DOI] [PubMed] [Google Scholar]
- 76.Ganesh M., Seidel D. Catalytic enantioselective additions of indoles to nitroalkenes. J. Am. Chem. Soc. 2008;130:16464–16465. doi: 10.1021/ja8063292. [DOI] [PubMed] [Google Scholar]
- 77.Liu H., Du D.M. Development of diphenylamine-linked bis(imidazoline) ligands and their application in asymmetric Friedel–Crafts alkylation of indole derivatives with nitroalkenes. Adv. Synth. Catal. 2010;352:1113–1118. doi: 10.1002/adsc.201000111. [DOI] [Google Scholar]
- 78.Buchcic A., Zawisza A., Leśniak S., Rachwalski M. Asymmetric Friedel–Crafts alkylation of indoles catalyzed by chiral aziridine-phosphines. Catalysts. 2020;10:971. doi: 10.3390/catal10090971. [DOI] [Google Scholar]
- 79.Gao M.-Z., Wang B., Liu H.-B., Xu Z.-L. Synthesis of chiral 2,5-bis(oxazolinyl)thiophenes and their application as chiral shift reagents for 1,1′-bi-2-naphthol. Chin. J. Chem. 2002;20:85–89. doi: 10.1002/cjoc.20020200117. [DOI] [Google Scholar]
- 80.Yuan Z.-L., Lei Z.-Y., Shi M. Binam and h8-binam-based chiral imines and Zn(OTf)2 catalyzed enantioselective Friedel–Crafts alkylation of indoles with nitroalkenes. Tetrahedron Asymmetry. 2008;19:1339–1346. doi: 10.1016/j.tetasy.2008.04.037. [DOI] [Google Scholar]
- 81.Meshram H.M., Kumar D.A., Reddy B.C. Simple and efficient Friedel–Crafts alkylation of 1h-indole with electron-deficient alkenes promoted by zinc acetate. Helv. Chim. Acta. 2009;92:1002–1006. doi: 10.1002/hlca.200800410. [DOI] [Google Scholar]
- 82.Yongcheng C., Yuanyuan C., Zhengfeng X., Wenping W. Friedel–Crafts reaction of indoles with nitroalkenes catalyzed by Yb(OTf)3. Chinese J. Org. Chem. 2011;31:1672–1677. [Google Scholar]
- 83.Liang L., Liu Q., Zhang J., Wang F., Yuan Y. Efficient iron-catalyzed michael addition of indole to nitroolefins under solvent-free conditions. Res. Chem. Intermed. 2013;39:1957–1962. doi: 10.1007/s11164-012-0728-1. [DOI] [Google Scholar]
- 84.Jalal S., Sarkar S., Bera K., Maiti S., Jana U. Synthesis of nitroalkenes involving a cooperative catalytic action of iron(III) and piperidine: A one-pot synthetic strategy to 3-alkylindoles, 2h-chromenes and n-arylpyrrole. Eur. J. Org. Chem. 2013;2013:4823–4828. doi: 10.1002/ejoc.201300172. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in Supplementary Materials.