Abstract
Mastocytosis is a hematologic neoplasm characterized by expansion and focal accumulation of neoplastic mast cells (MC) in diverse organs, including the skin, bone marrow (BM), spleen, liver, and gastrointestinal tract. The World Health Organization classification divides the disease into prognostically distinct variants of cutaneous mastocytosis (CM) and systemic mastocytosis (SM). Although this classification remains valid, recent developments in the field and the advent of new diagnostic and prognostic parameters created a need to update and refine definitions and diagnostic criteria in MC neoplasms. In addition, MC activation syndromes (MCAS) and genetic features predisposing to SM and MCAS have been identified. To discuss these developments and refinements in the classification, we organized a Working Conference comprised of experts from Europe and the United States in August 2020. This article reports on outcomes from this conference. Of particular note, we propose adjustments in the classification of CM and SM, refinements in diagnostic criteria of SM variants, including smoldering SM and BM mastocytosis (BMM), and updated criteria for MCAS and other conditions involving MC. CD30 expression in MC now qualifies as a minor SM criterion, and BMM is now defined by SM criteria, absence of skin lesions and absence of B- and C-findings. A basal serum tryptase level exceeding 20 ng/mL remains a minor SM criterion, with recognition that hereditary alpha-tryptasemia and various myeloid neoplasms may also cause elevations in tryptase. Our updated proposal will support diagnostic evaluations and prognostication in daily practice and the conduct of clinical trials in MC disorders.
Introduction
Mastocytosis is a hematologic neoplasm defined by expansion and accumulation of neoplastic mast cells (MC) in the skin and/or in internal organs, such as the bone marrow (BM), spleen, lymph nodes, liver, and gastrointestinal tract.1–5 The classification of the World Health Organization (WHO) delineates mastocytosis into cutaneous mastocytosis (CM), systemic mastocytosis (SM) and MC sarcoma (MCS) (Supplemental Digital Content, Table S1, http://links.lww.com/HS/A201).6–10 Based on disease-specific features, SM is further divided into indolent SM (ISM), smoldering SM (SSM), aggressive SM (ASM), SM with an associated hematopoietic neoplasm (SM-AHN), and MC leukemia (MCL).6–10 MCS is a rare, localized, aggressive MC tumor that usually progresses to MCL within a short time.11–14 The prognosis in advanced SM (ASM, SM-AHN, MCL) and MCS is unfavorable (Supplemental Digital Content, Table S1, http://links.lww.com/HS/A201). Without successful therapy, the estimated median survival time in these patients is less than 3 years.
In a vast majority of all patients with SM, the disease-driving KIT mutation D816V is expressed in neoplastic cells.15–19 In some SM patients, including those with well-differentiated (WD) MC morphology, other or no KIT mutations are detected, and in true MCS, neoplastic cells usually lack KIT D816V.12–14,18 In childhood CM, several different KIT mutations have been described, including KIT D816V.18,20
Patients with MC disorders frequently suffer from mediator-related symptoms.21–26 Depending on genetic variables, comorbidities, and efficacy of prophylactic therapy, the symptoms may be mild, severe, or even life-threatening.21–26 However, there are also patients with CM or SM who do not develop any mediator-related symptoms over years. In those with severe recurrent symptoms (anaphylaxis), serum tryptase levels usually increase substantially above the individual’s baseline during an attack, and a MC activation syndrome (MCAS) may be diagnosed.24,26–29 A genetic variable that may influence the frequency and severity of mediator-induced symptoms in SM is hereditary alpha-tryptasemia (HαT), a recently described autosomal dominant trait defined by an increased copy number of the TPSAB1 gene encoding alpha tryptase.30–33 Most HαT carriers present with an elevated basal serum tryptase level. Patients with SM who carry HαT may suffer from recurrent severe episodes of anaphylaxis and thereby qualify as a MCAS, especially when a concomitant allergy is present.29,33 Indeed, IgE-dependent allergies are relevant comorbidities in SM.21,22,25,29 Another relevant condition associated with SM is osteopathy which may manifest as osteosclerosis, osteopenia, osteoporosis, or osteolysis with the potential for pathologic fractures.34–38
With regard to survival and progression, prognostically relevant pathologies are concomitant myeloid neoplasms (AHN), including chronic myelomonocytic leukemia, myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPN), MDS/MPN overlap-neoplasms, chronic eosinophilic leukemia, and acute myeloid leukemia (AML).39–43 In these patients, neoplastic cells (MC, AHN cells, or both) may display chromosomal abnormalities and additional somatic defects, including mutations in ASXL1, SRSF2, TET2, JAK2, RUNX1, or RAS.43–51 The prognosis in these patients is unfavorable and is usually dictated by the aggressiveness of the AHN.
Over the past 15 years, a number of additional prognostic variables have been identified and validated in adult patients with SM. Moreover, multiparametric scoring systems have been established through which overall and progression-free survival can be predicted.52–56 In addition, a number of novel treatment concepts have been established in the past 2 decades, including KIT D816V-targeting tyrosine kinase inhibitors, allogeneic hematopoietic stem cell transplantation (SCT), immunotherapies, and IgE-targeting approaches.26,57–67 These treatments have greatly improved prognosis, survival, and the quality of life in patients.26,57–67 Therefore, it is of utmost importance to establish the correct diagnosis and to base treatment decisions on disease- and patient-related parameters, following the principles of personalized medicine.5,10,24,26,29,66
To provide contemporary standards, it is also important to adjust disease-related criteria to new developments. In fact, although the diagnostic criteria and classification defined between 2001 and 2017 are still valid, recent developments in the field and the advent of new markers have created a need to refine and update diagnostic criteria for MC disorders.
To address these issues, a Working Conference was organized in 2020. The current article provides a summary of discussions and outcomes of this conference.
Historical overview: criteria and classification of mastocytosis 2000–2020
Between 1991 and 2000, criteria to diagnose CM and SM were discussed, validated, and prepared in a series of clinical studies, workshops, and conferences.5,6,68 The resulting diagnostic criteria and classification of CM and SM were presented in the Year 2000 Working Conference and were adopted by the WHO in 2001.6,7 The WHO classification of MC disorders was subsequently refined in 20088 and 2017.9,10 To assist the WHO, our Europe (EU)/US consensus group organized Working Conferences in 2000,6 2005,69 2010,24 2012,70 2015,5,10 and 2020 (Supplemental Digital Content, Table S2, http://links.lww.com/HS/A201). Moreover, competence networks have been established in the EU and the United States, namely the European Competence Network on Mastocytosis71 and the American Initiative in Mast Cell Diseases,72 with the shared goals of improving patient management, to fostering research, and supporting the development of diagnostic criteria and standards of care in MC disorders.
Working conference, consensus discussion, and preparation of consensus statements
The year 2020 Working Conference on MC disorders was organized from August 30 to September 1, 2020. The related consensus discussions took place from February 2020 to January 2021. Because of the coronavirus (SARS-CoV-2) pandemic, the Working Conference was organized as a hybrid (combined on-site and web-based) meeting. The consensus-forming procedure and the development of consensus statements are described in the supplement. As in 2010,24 we also invited patients and their representatives to support the consensus group by formulating and forwarding important open issues, questions, and suggestions to the scientific community (Supplemental Digital Content, Table S3, http://links.lww.com/HS/A201). Details are described in the Supplemental Digital Content, http://links.lww.com/HS/A201.
CM and skin involvement in SM: proposed modifications
In the diagnostic algorithm, a first essential step is to define whether the patient is suffering from CM or SM.5–10,73 It is important to note that most patients with CM are children, whereas SM is usually diagnosed in adulthood.5–10,73 Still, most adult patients with SM present with skin lesions. In contrast to children, adults always undergo a complete staging, including a BM investigation (histology and aspirate), to confirm or exclude SM by applying SM criteria.5–10,69 In contrast, a BM examination is usually not recommended in children, unless clear signs for an advanced SM or another hematologic neoplasm are found.69,73
In children, CM is defined by typical skin lesions, a positive Darier’s sign, and the absence of clinical signs of systemic involvement (Supplemental Digital Content, Table S4, http://links.lww.com/HS/A201).69,73 In adults, CM is defined by typical skin lesions, the Darier’s sign, and/or a positive skin histology and absence of criteria sufficient to diagnose SM in staging examinations.69,73 An important point is that the discrimination between CM and SM in adults is of prognostic significance as patients with CM exhibit better progression-free survival.74 Another important point is that in patients with CM, systemic involvement with clonal MC (eg, 1 or 2 minor SM criteria detected) is not sufficient for the diagnosis of SM unless the full spectrum of SM criteria is fulfilled.5–10 In adults with skin lesions who did not (yet) undergo a complete staging with BM analyses, the provisional diagnosis of “mastocytosis in the skin” (MIS) is appropriate (Supplemental Digital Content, Table S4, http://links.lww.com/HS/A201).69,73,74 In children, the provisional diagnosis of MIS does not apply unless (i) serum tryptase levels exceed 100 ng/mL and/or (ii) clear signs for a systemic hematologic disease (eg, unexplained splenomegaly) are found and (iii) no BM studies were performed (Supplemental Digital Content, Table S4, http://links.lww.com/HS/A201).69,75 Otherwise, the diagnosis in children is CM.69
Once diagnosed, CM should be subclassified into maculopapular CM (MPCM), diffuse CM, and cutaneous mastocytoma.5–10,69,73 Diagnostic criteria for these variants are shown in Table 1. Most children with CM and almost all adults with CM exhibit MPCM. In many children, cutaneous lesions disappear spontaneously before or during adolescence. Two distinct forms of childhood MPCM have been recognized: a variant characterized by monomorphic small-sized lesions, and a second form defined by polymorphic (often larger) lesions.73,76 Only the monomorphic form is also found in adults, suggesting that only this variant is likely to persist into adulthood, whereas polymorphic lesions usually disappear which is in line with clinical observations.73,74,76 Therefore, childhood MPCM is further divided (subclassified) into the monomorphic form and polymorphic form (Table 1).
Table 1.
Proposed Classification and Criteria of CM.
| Variant and Subvariant(s) | Abbreviation | Features/Criteria |
|---|---|---|
| Maculopapular cutaneous mastocytosis | MPCM | Positive Darier’s signa |
| Typical pigmented skin lesions | ||
| Urticaria pigmentosa | UP | Positive histologyb |
| KIT mutation in lesional skin | ||
| Monomorphic variant | MPCM-m | Monomorphic skin lesionsc |
| Polymorphic variant | MPCM-p | Polymorphic skin lesionsc |
| No signs/criteria of SMd | ||
| Diffuse cutaneous mastocytosis | DCM | Positive Darier’s signa |
| Diffuse involvement of the entire skin | ||
| Positive histologyb | ||
| Criteria for SM not fulfilledd | ||
| Cutaneous mastocytoma | Positive Darier’s signa | |
| Positive histologyb | ||
| Isolated mastocytoma | One single lesion | |
| Multilocalized mastocytomas | Two or 3 lesions | |
| No signs/criteria of SMd |
aWhereas the Darier´s sign and typical skin lesions serve as major diagnostic criteria (in both the monomorphic and polymorphic variant), a positive histology and the presence of an activating KIT mutation serve as minor diagnostic criteria. In the case of atypical lesions or a negative Darier’s sign, the diagnosis of mastocytosis can still be established provided that minor criteria are fulfilled. In young children with typical mastocytoma, testing for the Darier’s sign is often avoided because of the risk to provoke systemic reactions. Testing for the Darier’s sign should always be done gently and only when needed for diagnosis.
bHistologic examination includes standard stains and immunohistochemistry using antibodies against tryptase and KIT regardless of the variant (monomorphic or polymorphic). The numbers of KIT+/tryptase+ mast cells is usually elevated in lesional skin in patients with mastocytosis and skin involvement.
cThe monomorphic variant is found in children and adults. When found in children, the likelihood that the lesions will persist into adulthood is high. Polymorphic skin lesions are detected in childhood MPCM but usually not in adults with CM or systemic mastocytosis. When detected in children, the likelihood that the polymorphic skin lesions will spontaneously disappear at or shortly after puberty (in adolescence) is high.
dIn all adult patients, SM has to be excluded by staging investigations including bone marrow studies. In children, bone marrow studies are only performed when clinical signs and symptoms and/or laboratory findings are indicative of an advanced hematologic disease.
CM = cutaneous mastocytosis; SM = systemic mastocytosis.
Refinements of major and minor SM criteria
In general, major and minor diagnostic criteria and the resulting definition of SM remain unchanged compared with previous proposals.5–10 The diagnosis of SM can be established when at least 1 major and 1 minor or 3 minor SM criteria are fulfilled (Table 2).5–10 The major criterion is the multifocal infiltrate of MC forming compact aggregates of at least 15 MC in the BM or another extracutaneous organ system.5–10 Sometimes, MC infiltrates may be distorted or even masked by AHN cell infiltrates. In these patients, the diagnosis SM (SM-AHN) can sometimes only be established after successful cytoreduction.43,77 Therefore, we recommend that all SM criteria are applied again in all patients with a KIT D816V-mutated myeloid neoplasm after (successful) cytoreductive therapy.
Table 2.
Proposed Refined Major and Minor SM Criteria.
| Major criterion: | Multifocal dense infiltrates of mast cells (≥15 mast cells in aggregates) in bone marrow biopsies and/or in sections of other extracutaneous organ(s) |
| Minor criteria: | a. ≥25% of all mast cells are atypical cells (type I or type II) on bone marrow smears or are spindle-shaped in mast cell infiltrates detected in sections of bone marrow or other extracutanous organsa |
| b. KIT-activating KIT point mutation(s) at codon 816 or in other critical regions of KITb in bone marrow or another extracutaneous organ | |
| c. Mast cells in bone marrow, blood, or another extracutaneous organ express one or more of: CD2 and/or CD25 and/or CD30c | |
| d. Baseline serum tryptase concentration >20 ng/mL (in the case of an unrelated myeloid neoplasm, an elevated tryptase does not count as an SM criterion. In the case of a known HαT, the tryptase level should be adjustedd | |
| If at least 1 major and 1 minor or 3 minor criteria are fulfilled → the diagnosis is SM |
aIn tissue sections, an abnormal mast cell morphology counts in both a compact infiltrate and a diffuse (or mixed diffuse + compact) mast cell infiltrate. However, the spindle-shaped form does not count as an SM criterion when mast cells are lining vascular cells, fat cells, nerve cells, or the endosteal-lining cell layer. In the bone marrow smear, an atypical morphology of mast cells does not count as SM criterion when mast cells are located in or adjacent to bone marrow particles. Morphologic criteria of atypical mast cells have been described previously.6
bAny type of KIT mutation counts as minor SM criterion when published solid evidence for its transforming behavior is available. A list of such KIT mutations (including variants in KIT codons 417, 501–509, 522, 557–560, 642, 654, 799, 816, 820, 822) is provided in Supplemental Digital Content, Table S6, http://links.lww.com/HS/A201 (KIT-activating mutations are labeled in bold).
cAll 3 markers fulfill this minor SM criterion when expression in mast cells can be confirmed by either flow cytometry or by immunohistochemistry or by both techniques.
dAlthough the optimal way of adjustment may still need to be defined, one way is to divide the basal tryptase level by 1 plus the extra copy numbers of the alpha tryptase gene. Example, when the tryptase level is 30 and 2 extra copies of the alpha tryptase gene are found in a patient with HαT, the HαT-corrected tryptase level is 10 (30/3 = 10) and thus is not a minor SM criterion.
HαT = hereditary alpha-tryptasemia; SM = systemic mastocytosis.
The abnormal morphology of MC (atypical spindle-shaped cells with hypogranulated cytoplasm and oval nucleus) is the first minor SM criterion.5–10 At least 25% of all MC must exhibit these morphologic features in BM smears or BM sections to qualify as a minor SM criterion.5–10 Even if MC form only diffuse infiltrates without compact aggregates in BM sections, the abnormal MC morphology (≥25%) counts as a minor SM criterion (Table 2). However, the spindle-shaped morphology criterion does not include MC that are adjacent to (lining) blood vessels, endosteal surfaces, nerve cells, or fat cells.
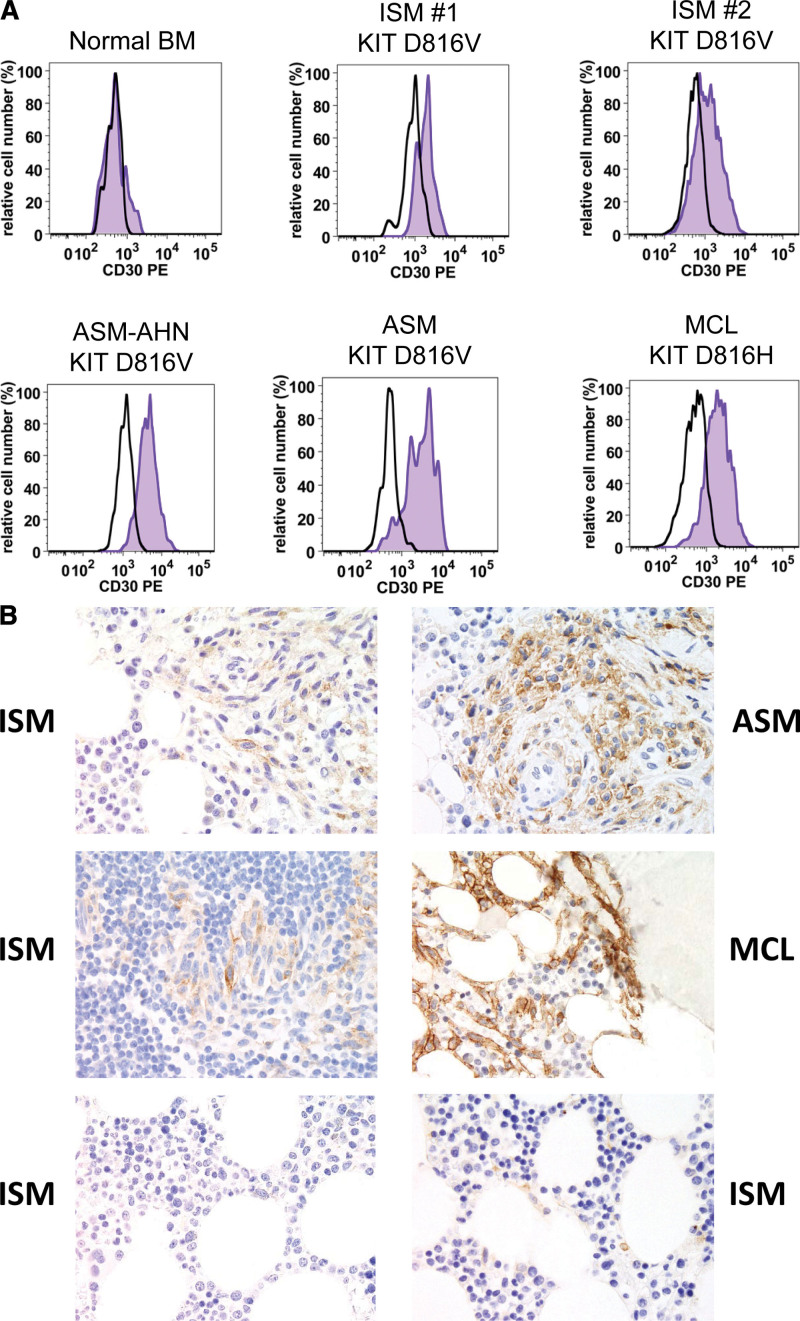
Recent data suggest that CD30 expression in MC is strongly associated with SM but is not found in other myeloid neoplasms.78–81 CD30 is detectable in neoplastic MC in a majority of patients with ISM and advanced SM by flow cytometry and immunohistochemistry (Figure 1; Supplemental Digital Content, Table S5, http://links.lww.com/HS/A201).78–81 Even in patients with a WD morphology, where MC often lack CD2 and/or CD25, neoplastic MC usually display CD30.80 Therefore, CD30 is proposed as a new addition to the existing minor SM criterion. The refined definition of this minor criterion is: MC express one or more of the 3 aberrantly expressed antigens: CD2, CD25, and CD30 (Table 2). It is important to note that all 3 markers may be detected by flow cytometry and/or immunohistochemistry. Initial data had reported a rough correlation between strong cytoplasmic expression of CD30 and advanced SM.78 However, subsequent validation did not show a clear-cut delineation.79–81 Therefore, our faculty concludes that CD30 does not qualify as a grading marker in SM. Another question was whether CD2 should be replaced by CD30. However, although CD2 is less frequently and less abundantly expressed in neoplastic MC compared with CD25, CD2 was not eliminated as criterion because of its specificity in SM, whereas CD25 is rarely detected also in MC in reactive states.
Figure 1.
Expression of CD30 in neoplastic mast cells in systemic mastocytosis. (A), Flow cytometric detection of CD30 on neoplastic MC in patients with SM. BM cells were obtained from a control patient (no known BM disease; upper left image), patients with indolent SM (ISM: upper middle and right panels), and patients with advanced SM, namely 1 with ASM with an AHN (ASM-AHN: lower left image), 1 with ASM (lower middle histogram), and 1 with MCL by multicolor flow cytometry on a FACSCanto (Becton Dickinson). MC were identified as CD117++/CD45+/CD34− cells and stained with a phycoerythrin-labeled monoclonal antibody against CD30 (BerH8 from BD Biosciences; blue histograms). The isotype-matched control antibody (black open histogram) is also shown. (B), Immunohistochemical detection of CD30 in neoplastic MC. BM biopsy sections from patients with ISM, ASM, and MCL (as indicated) were stained with a monoclonal antibody against CD30 (Ber-H2 from Dako, Glostrup, Denmark) by an indirect immunoperoxidase staining technique as reported.81 The 2 images at the bottom show the nonaffected BM in 2 patients with ISM (control). Images were prepared using an Olympus DP21 camera connected to an Olympus BX50 microscope equipped with 60×/0.90 UPlan-Apo objective lens (Olympus, Hamburg, Germany) and processed with Adobe Photoshop CS2 software version 9.0 (Adobe Systems, San Jose, CA) as described.81 All patients gave written informed consent before BM samples were obtained and analyzed. AHN = associated hematologic neoplasm; ASM = aggressive SM; BM = bone marrow; ISM = MC = mast cells; MCL = MC leukemia; SM = systemic mastocytosis.
A number of different KIT variants can be detected in SM.18 The most prevalent mutation is D816V. This mutation is found in around 90% of all adult patients with nonadvanced SM. However, other KIT-activating and thus disease-driving mutations may also be present, especially in cases with advanced SM.18 Therefore, any KIT mutation that is known to cause ligand-independent activation of KIT should count as a minor SM criterion. A list of all relevant KIT mutations is provided in Supplemental Digital Content, Table S6, http://links.lww.com/HS/A201.
The basal serum level of tryptase is elevated in most SM patients.82–85 Therefore, a clearly elevated basal serum tryptase is a minor SM criterion.5–10 The consensus threshold is 20 ng/mL. However, apart from SM, a number of other conditions and pathologies are associated with an elevated tryptase level. Therefore, the following restrictions are proposed: (1) only the basal serum tryptase level (measured in a symptom-free interval) can qualify as a minor SM criterion; (2) the basal serum tryptase level does not qualify as a SM criterion when an AHN is also diagnosed (AHN cells may produce tryptase), and (3) in patients with known HαT, the basal tryptase level should be corrected for the presence of HαT. One suggested approach discussed in the conference was to correct for HαT by dividing the basal tryptase level by one plus the number of extra alpha tryptase gene-copies. Details are described in the Supplemental Digital Content, http://links.lww.com/HS/A201.
In patients with unknown TPSAB1 status, the old definition of this SM criterion should apply. It is worth noting that testing for TPSAB1 copy numbers is not yet available in all centers.
Proposed criteria for BM mastocytosis and separation from ISM and SSM
The separation between BM mastocytosis (BMM) and other forms of SM is of crucial importance for several reasons. One is that the absence of skin lesions is often observed in advanced SM.5–10 Second, recent data suggest that patients with BMM with low disease burden (defined as no B-findings and a tryptase level <125 ng/mL) have a better prognosis than patients with typical ISM, SSM, and “BMM” with higher MC burden (high-risk BMM).86 Therefore, we propose that BMM be defined as a separate SM variant where no B-finding is detectable and the basal tryptase level is below 125 ng/mL (ie, exclusion of high-risk BMM patients). As soon as one B-finding is detected and/or serum tryptase levels exceed 125 ng/mL, the patient should be diagnosed as ISM without skin lesions but not as BMM. The same holds true when dense infiltrates of atypical MC (major SM criterion) are detected in an extramedullary organ which also changes the diagnosis to ISM. Table 3 shows adjusted diagnostic criteria proposed for BMM, typical ISM (with or without skin lesions), and SSM. Since patients with advanced SM often present without skin lesions, it is of crucial importance to exclude the presence of C-findings and MCL in all patients with BMM (Table 3).
Table 3.
Proposed Revised Criteria for BMM, Typical ISM, ISM Without Skin Involvement, and SSM.
| Variant | Criteria |
|---|---|
| BMM | SM criteria fulfilled |
| No skin lesions | |
| No B-finding(s) | |
| No C-finding(s) | |
| Basal serum tryptase <125 ng/mL | |
| No dense SM infiltrates in an extramedullary organ | |
| No signs/criteria for MCL | |
| No signs/criteria for an AHN | |
| (Typical) ISM | SM criteria fulfilled |
| Typical skin lesions | |
| No or one B-finding | |
| No C-finding | |
| No signs/criteria for MCL | |
| No signs/criteria for an AHN | |
| ISM without skin lesions | SM criteria fulfilled |
| No skin lesions | |
| No or one B-findinga and/or: | |
| Basal serum tryptase ≥125 ng/mL and/or: | |
| Dense SM infiltrates in an extramedullary organ | |
| No C-finding | |
| No signs/criteria for MCL | |
| No signs/criteria for an AHN | |
| SSM | SM criteria fulfilled |
| Two or 3 B-findings | |
| No C-finding | |
| No signs/criteria for MCL | |
| No signs/criteria for an AHNb |
aSerum tryptase levels may exceed 200 ng/mL (if no other B-finding is detected) or below 200 ng/mL (in which case 1 B-finding may be detected).
bAdditional mutations in other (driver) genes, such as TET2, may be detected by next generation sequencing. However, when new gene variants occur or the variant allele frequency increases over time, a re-examination of the bone marrow is required to exclude SM-AHN.
AHN = associated hematologic neoplasm; BMM = bone marrow mastocytosis; ISM = indolent SM; MCL = mast cell leukemia; SM = systemic mastocytosis; SSM = smoldering SM.
Other typical features of BMM are a relatively high prevalence of severe IgE-mediated allergies to bee and/or wasp venom (often with MCAS) and osteoporosis.21,22,86–88 In most cases, the KIT D816V allele burden in the blood and MC infiltration grade in the BM are low. Sometimes, a WD MC morphology is detected. Regarding progression to advanced SM, the prognosis of BMM is favorable.86
Proposed modifications in B-findings and C-findings
B-findings are indicative of a high MC burden, expansion of SM in various organ-systems, and involvement of multiple myeloid lineages, without organ damage.5–10 By contrast, C-findings are indicative of SM-induced organ damage.5–10,89,90 It is important to note that organ damage caused by an AHN or by other etiologies (such as infection or therapy-induced) does not count as a C-finding. In addition, it is important to note that the causative impact of the local SM infiltrate (aggressive growth pattern) should be demonstrated by biopsy whenever possible to document the presence of a C-finding.5–10,90,91 In patients with SM-AHN, it may sometimes be difficult to define the relative impact of the SM infiltrate and that of the AHN, especially when both disease components present as advanced malignancies (eg, ASM-AML). In these cases, both disorders may cause marked organ damage (eg, cytopenia).
Our faculty also discussed whether new molecular and/or immunological parameters may qualify as indicators of a huge burden of MC and/or multilineage involvement and thus as B-findings. After a thorough discussion, our faculty agreed that a high variant allele frequency (VAF) of KIT D816V in aspirated BM cells or peripheral blood (PB) leukocytes (>10%) should qualify as indicator of a high burden of MC or a surrogate for multilineage involvement in SM and thus as B-finding (Table 4). In addition, multilineage involvement with KIT D816V as determined by quantitative polymerase chain reaction in sorted myeloid BM or PB leukocytes is indicative for (confirms the presence of) a B-finding and thus SSM. However, cell sorting is not routinely applied in daily practice in most centers. Finally, the presence of additional mutations (in other driver-genes such as SRSF2 or ASXL1) in SSM was discussed. However, although clearly being indicative of a huge burden of clonal cells, our faculty concluded that such additional mutations are more commonly expressed in AHN subclones and thus support the diagnosis SM-AHN rather than SSM. Whereas in the original definition, B-findings should not be accompanied by major blood count abnormalities, our faculty is of the opinion that marked leukocytosis (persistent neutrophilia, monocytosis, and/or eosinophilia) and thrombocytosis may well be detected and even count as indication of myeloproliferation and thus confirmation of a B-finding, unless leukocytosis/thrombocytosis are caused by a reactive process or the diagnostic criteria for an overt MPN or MDS/MPN are fulfilled—in which case, the diagnosis changes to SM-MPN. Refined B-findings are shown in Table 4.
Table 4.
Proposed Refined B-findings and C-findings.
| B-findings | C-findings (SM-induced Organ Damage) |
|---|---|
| High MC burden: | - |
| Infiltration grade (MC) in BM ≥30% in histology (IHC) and/or serum tryptase ≥200 ng/mLa and/or KIT D816V VAF ≥10% in BM or PB leukocytes | |
| Signs of myeloproliferation and/or myelodysplasiab: | Cytopenia/s: |
| Hypercellular BM with loss of fat cells and prominent myelopoiesis ± left shift and eosinophilia ± leukocytosis and eosinophilia and/or discrete signs of myelodysplasia (<10% neutrophils, erythrocytes, and megakaryocytes) | ANC < 1 × 109/L |
| Hb < 10 g/dL | |
| PLT < 100 × 109/L | |
| (one or more found) | |
| Organomegaly: | |
| Palpable hepatomegaly without ascites or other signs of organ damage or/and palpable splenomegaly without hypersplenism and without weight loss or/and lymphadenopathy palpable or visceral LN-enlargement found in ULS or CT (>2 cm) | Hepatopathy: |
| Ascites and elevated liver enzymesc ± hepatomegaly or cirrhotic liver ± portal hypertension | |
| Spleen: | |
| Palpable splenomegaly with hypersplenism ± weight loss ± hypalbuminemia | |
| GI tract: | |
| Malabsorption with hypoalbuminemia ± weight loss | |
| Bone: | |
| Large-sized osteolysis (≥2 cm) with pathologic fracture ± bone pain |
aIn the case of a known HαT, the basal serum tryptase level should be adjusted. Although the optimal way of adjustment still needs to be defined, one way is to divide the basal tryptase level by 1 plus the extra copy numbers of the alpha tryptase gene. Example, when the tryptase level is 300 and 2 extra copies of the alpha tryptase gene are found in a patient with HαT, the HαT-corrected tryptase level is 100 (300/3 = 100) and would thus not qualify as a B-finding.
bSigns of myeloproliferation and/or myelodysplasia must be discrete and stable (neither disappear nor progress) and must not reach diagnostic criteria of an MPN, MDS, or MPN/MDS in which case the diagnosis changes to SM-AHN. The presence of a myeloid AHN excludes B-findings and SSM by definition.
cAlkaline phosphatase levels are typically elevated in patients with advanced SM and SM-induced liver damage. In some of these patients, only elevated liver enzymes but no (clinically relevant) ascites is found.
AHN = associated hematologic neoplasm; ANC = absolute neutrophil count; BM = bone marrow; CT = computed tomography; GI = gastrointestinal; HαT = hereditary alpha-tryptasemia; Hb = hemoglobin; IHC = immunohistochemistry; LN = lymph node; MC = mast cells; MDS = myelodysplastic syndrome; MPN = myeloproliferative neoplasm; PB = peripheral blood; PLT = platelet count; SM = systemic mastocytosis; SSM = smoldering systemic mastocytosis; ULS = ultrasound; VAF = variant allele frequency.
Refinements of C-findings were also discussed in our Working Conference. First, weight loss was removed as an independent C-finding but is now added as a confirming feature to both malabsorption and splenomegaly. In fact, the presence of weight loss per se, although clinically relevant, is problematic for several reasons, one being that weight loss is difficult to define in patients with, for example, concomitant ascites. Therefore, when present, weight loss confirms the organ-damaging impact of SM-induced organopathy but is per se no longer required as a defining feature of a C-finding. The second change in C-findings relates to osteolyses. In fact, only huge osteolyses (>2 cm in diameter) with clear clinical impact (eg, fractures) and confirmed histology, count as a C-finding. Smaller osteolyses and osteoporosis, even when associated with (pathological) fractures, do not count as C-finding (Table 4). Finally, it should be noted that organomegaly without organ damage does not count as C-finding, even when organomegaly is associated with constitutional symptoms.
Updated criteria for SSM
In general, the basic definition for SSM should remain the same: when SM criteria are fulfilled and 2 or 3 B-findings can be documented, but no (not a single) C-finding(s), no signs/criteria for MCL and no signs/criteria for an AHN are found, the final diagnosis is SSM (Table 3).5–10 The updated B-findings described earlier should be applied in these patients. Since SSM may be associated with a less favorable prognosis compared with typical ISM or BMM,41,53 it is important and standard to follow these patients closely to detect signs of progression as early as possible.69 Re-evaluation and restaging should be performed when signs of advanced SM or AHN are detected.69,91 Useful follow-up parameters are the basal tryptase level, alkaline phosphatase, and KIT D816V VAF in PB.92–95 When these parameters change over time, C-findings occur, or blast cells or MC are detectable in differential counts, a re-examination of the BM is performed.5,69,91,96 The same holds true for patients in whom new molecular lesions or expansion of a mutated subclone is detectable by next generation sequencing (suspected AHN). Patients with SSM may progress to ASM, SM-AHN, or MCL.5,39,50,53,96,97
Criteria for ASM and ASM variants
The diagnosis ASM is based on SM criteria and visible signs of SM-induced organ damage (C-findings) as well as absence of signs/criteria indicative of MCL or AHN.5–10,97 At least 1 C-finding must be documented to call a condition ASM. MC on BM smears and PB smears must be below 20% (to exclude MCL).5–10,69,89 Based on established criteria, ASM can be divided into classical ASM (MC in BM smears <5%) and ASM in transformation to MCL (MC in BM smears 5%-19%) (Table 5).5,97 In addition, patients with ASM can be classified into pure ASM and ASM-AHN (Table 5). Finally, ASM can be split into primary ASM and secondary ASM following CM, BMM, ISM, or SSM (Table 5).5,97 All patients with ASM should have a close follow-up employing all clinical and laboratory parameters required to document progression and response to therapies. Indeed, despite therapy, patients with ASM may progress to MCL, ASM-AHN, or MCL-AHN.5–10,53–55,64
Table 5.
Refined Classification and Criteria for Advanced SM, Including SM-AHN, ASM, and MCL.
| Category | Subvariant | Defining Key Features (Criteria) |
|---|---|---|
| SM-AHN | According to SM variant: | |
| BMM-AHN | WHO criteria (consensus criteria) for SM variants | |
| ISM-AHN | ||
| SSM-AHNa | ||
| ASM-AHN | ||
| MCL-AHN | ||
| According to the AHN: | ||
| SM with myeloid AHN | WHO criteria for myeloid AHN type | |
| (SM-CMML, SM-AML, …) | ||
| SM with lymphoid AHN | WHO criteria for lymphoid AHN type | |
| (SM-ALL, SM-MM, …) | ||
| ASM | According to a previous MC neoplasm: | |
| Primary ASM | No previous SM known | |
| Secondary ASM | Previous BMM, ISM, SSM, … | |
| According to an AHN | ||
| ASM without AHN | ||
| ASM-AHN | WHO criteria for AHN | |
| According to signs of progression: | ||
| ASM | <5% MC in BM smears | |
| ASM in transformation | 5%–19% MC in BM smears | |
| (=ASM-T) | ||
| MCL | According to a previous MC neoplasm | |
| Primary MCL | No previous MC disease known | |
| Secondary MCL | Previous BMM, ISM, SSM, MCS, … | |
| According to an AHN | ||
| MCL without AHN | ||
| MCL-AHN | WHO criteria for AHN | |
| According to organ damage | ||
| Chronic MCL | No C-finding(s) | |
| Acute MCL | One or more C-finding(s) | |
| According to blood involvement | ||
| Aleukemic MCL | MC <10% of blood leukocytes | |
| Leukemic MCL | MC ≥10% of blood leukocytes |
aSSM-AHN is an extremely rare condition as signs of myeloproliferation and/or dysplasia will be regarded as sign of the (myeloid) AHN in almost all cases. However, SSM may still be diagnosed in a patient with AHN, for example, when the AHN is a lymphoid neoplasm (eg, SSM-CLL).
AHN = associated hematologic neoplasm; ALL = acute lymphoblastic leukemia; AML = acute myeloid leukemia; ASM = aggressive SM; BMM = bone marrow mastocytosis; CMML = chronic myelomonocytic leukemia; ISM = indolent SM; MCL = mast cell leukemia; MCS = mast cell sarcoma; MM = multiple myeloma; SM = systemic mastocytosis; SSM = smoldering SM; WHO = World Health Organization.
SM-AHN and AHN variants
In accordance with previous proposals, any type of a hematologic neoplasm should qualify as an AHN, including myeloid neoplasms and (rarely) lymphoid neoplasms.5–10 An exception are lymphoid neoplasms detected in a separate organ system, for example, a stage 1 non-Hodgkin lymphoma (NHL) in a peripheral lymph node in a patient with BMM. In these exceptional cases, the NHL should not count as AHN. All AHN should be diagnosed, staged, and classified according to WHO criteria.5–10 Pre/subdiagnostic clonal conditions, such as clonal hematopoiesis with indeterminate potential, monoclonal gammopathy of undetermined significance or monoclonal B lymphocytosis, do not count as AHN.
MCL and MCL variants
The principle definition and diagnostic criteria for MCL defined by the WHO remain unchanged.5–10 The WHO classification also divides MCL into a classical (leukemic) form (MC ≥10% of all leukocytes in PB smears) and a more frequent, aleukemic variant (aleukemic MCL: MC <10% in PB smears).5–10,97 MCL can further be classified into primary MCL (no previous SM known) and secondary MCL following a previous (lower grade) SM (Table 5).97 In addition, MCL can be split into acute MCL (C-findings detectable) and chronic MCL where C-findings are not detectable (Table 5).5,97 Compared with acute MCL, patients with chronic MCL have a better prognosis and may respond to therapy with KIT-targeting drugs. However, many of these patients progress to acute MCL over time. Finally, MCL can be classified into pure MCL and MCL-AHN where the prognosis is particularly poor.96,97
An important differential diagnosis to MCL is myelomastocytic leukemia (MML).5–10,97–99 In these patients, SM criteria are not fulfilled and neoplastic MC (≥10% in BM or blood smears by definition) are derived from neoplastic stem cells of an underlying myeloid neoplasm.99 In a subset of these patients, KIT mutations outside of codon 816 may be found. Based on our updated SM criteria, some of these cases may be reclassified as true MCL over time.
MCS and extracutaneous mastocytoma
The definition and criteria of classical MCS are shown in Supplemental Digital Content, Table S7, http://links.lww.com/HS/A201. As per definition, MCS is a localized tumor consisting of more or less immature MC that expand rapidly and show an aggressive and often invasive (sarcoma-like) growth pattern.5–10 Any organ system may be affected and the disease can occur at any age.11–14 As per definition, SM criteria are not fulfilled.5–10 Apart from the classical MCS variant, a MCS-like progression of SM, including SM-AHN or MCL may be seen. In these patients, the primary diagnosis remains SM. Our faculty discussed whether in these cases, the term “secondary MCS” would be appropriate. However, to avoid confusion, the term “MCS-like progression” was selected as more appropriate. This decision is supported by the observation that MC in true MCS usually lack KIT mutations, whereas in most SM patients with “MCS-like progression,” KIT D816V is detected.11–14 Despite local and systemic therapy, the prognosis in MCS is unfavorable. Most patients progress to ASM or secondary MCL within short time.11–14 The only curative approach appears to be SCT.100
Extracutaneous mastocytoma is an extremely rare localized benign tumor consisting of mature MC without histopathological evidence of invasive growth or major cell atypia. So far, less than 10 well-documented cases have been described, most of them in the lung.101–103 Because of its rarity, extracutaneous mastocytoma was eliminated from the WHO classification in 2017. Since then, only 2 well-documented cases have been reported. Therefore, our faculty concluded that this very rare MC disease should not be reincorporated.
Impact of WD MC morphology
In some of the patients with SM, MC exhibit a rather mature morphology. In these cases, MC appear as round cells containing a round centralized nucleus and a well-granulated cytoplasm. These patients are often referred to as “well-differentiated SM.”104–107 However, a WD MC morphology can be detected (rarely) in almost all forms of SM and even in CM.104–107 Therefore, our faculty concluded that the WD morphology should be added as appendix to the diagnosis and WHO variant of mastocytosis. Indeed, the clinical course and prognosis of patients with WD mastocytosis depends on the WHO type of the disease. For example, patients with ISM with a WD morphology of MC would be classified as ISM-WDSM (ISMWDSM).
An important aspect is to recognize that in most patients with WDSM, KIT codon 816 mutations are not detected and neoplastic MC usually lack CD25 and CD2.105 However, other KIT mutations (like K509I or F522C) may be detected, and neoplastic MC often display CD30. Therefore, the diagnosis of WDSM should be based on SM criteria, including expression of CD30 and KIT-activating KIT mutations. An important aspect is that several KIT mutant forms detected in WDSM are sensitive to imatinib therapy.18,104,106,107
Diagnostic criteria and classification for MCAS and related disorders
Diagnostic criteria and a classification for MCAS have been proposed by the EU/USA consensus group.24,27–29 In addition, a diagnostic algorithm for patients with MCAS has been published.29,108 Our group is of the consensus opinion that these diagnostic criteria and standards should be followed in the evaluation and classification of cases with suspected MCAS. Diagnostic criteria for MCAS are shown in Supplemental Digital Content, Table S8, http://links.lww.com/HS/A201, and the consensus classification of MCAS is shown in Supplemental Digital Content, Table S9, http://links.lww.com/HS/A201. Based on the underlying condition, MCAS can be divided into primary (monoclonal) MCAS (=MMAS) where clonal MC (and usually SM or CM) are found, secondary MCAS, where an allergic disease or another reactive condition is present, and idiopathic MCAS, where neither clonal MC nor another underlying condition (allergy and other) are detected (Supplemental Digital Content, Table S9, http://links.lww.com/HS/A201).24,27–29,108 An important aspect is that MCAS can present as a mixed (clonal plus secondary) form where both SM and an underlying IgE-dependent allergy have been diagnosed.29 These patients are at high risk to develop fatal episodes of MCAS.
Another important consideration is that in some patients, local mono-organ or chronic MC activation may be found.29,108 However, it should be pointed out that it is often difficult or impossible to demonstrate the impact of MC in such conditions, and in many instances, other cell types (not MC) may be causative elicitors of clinical symptoms. The reality in these cases is that the terms “MC activation” or “MC involvement” are not justified from a scientific point of view.108
Global classification of MC disorders
In 2012, our consensus group proposed a global classification for all MC disorders.24 In the current project and conference, we discussed novel markers and concepts as well as predisposing conditions and pathologies that may contribute to the manifestation or progression of MC disorders. Table 6 shows an updated version of a proposed global classification of MC pathologies and disorders. Finally, our group discussed the relationship between this classification and the International Statistical Classification of Diseases and Related Health Problems (ICD-10) code. Supplemental Digital Content, Table S10, http://links.lww.com/HS/A201, shows an overview of all MC disorders, pathologies, and predisposing conditions and related ICD-10 codes, and Supplemental Digital Content, Table S11, http://links.lww.com/HS/A201, provides features of ICD-10–defined MC activation disorders.
Table 6.
Proposed Global Classification of Mast Cell Pathologies and Related Conditions.
| Disorder/Condition | Diagnostic Criteria/Defining Features |
|---|---|
| Mast cell hyperplasia | Reactive increase in normal mast cells |
| No evidence for clonal mast cells | |
| Criteria for CM/SM/MCS not fulfilled | |
| Mastocytosis | WHO criteria for CM, SM, or MCS fulfilled |
| MCASa | MCAS criteria fulfilled |
| ± Criteria for CM/SM/MCS fulfilled | |
| Other MCADa | Evidence for a clinically relevant, local or systemic mast cell activation but the criteria for MCAS are not fulfilleda |
| ± Criteria for CM/SM/MCS fulfilled | |
| MML | Advanced myeloid neoplasm diagnosed |
| based on WHO criteria (eg, AML) | |
| Increase in neoplastic mast cells ≥10% | |
| Criteria for CM/SM/MCS not fulfilled |
aMCAD can be divided into MCAS and other MCAD. The criteria to be used for concluding that there is evidence of clinically relevant, local, or systemic mast cell activation (disorder) in the absence of criteria that fulfill MCAS are discussed in the Supplemental Digital Content, Table S11, http://links.lww.com/HS/A201.
AML, acute myeloid leukemia; CM = cutaneous mastocytosis; MCAD = mast cell activation disorders; MCAS = mast cell activation syndromes; MCS = mast cell sarcoma; MML = myelomastocytic leukemia; SM = systemic mastocytosis; WHO = World Health Organization.
Standard approaches and diagnostic algorithms in daily practice
During the past 20 years, diagnostic markers, assays, and related diagnostic algorithms for the evaluation of patients with suspected MC disorders have been proposed.5–10,18,24,27–29,73,97,108 These standards remain valid and their updated versions should be applied in daily practice. An overview of updated standards and refined diagnostic algorithms proposed by our consensus group is provided in the Supplemental Digital Content, http://links.lww.com/HS/A201. The same holds true for prognostic markers and recently established prognostic scoring systems, including the international prognostic scoring system, the molecular-adjusted revised prognostic score, the Red Española de Mastocitosis score, and the global prognostic score.52–56 In addition, the Supplemental Digital Content, http://links.lww.com/HS/A201, provides a short overview of response criteria recommended in daily practice in MC disorders.
Unmet needs and recommendations provided by patients
Within the frame of the current project, our faculty asked patients and patient groups from 12 countries/regions (global effort) to express their concerns, wishes, and recommendations to the scientific community. Among top issues were better education and knowledge of physicians, increased awareness, better/easier access to specialized centers, and development of improved criteria and better treatments (Supplemental Digital Content, Tables S12, http://links.lww.com/HS/A201, and S13, http://links.lww.com/HS/A201).
Concluding remarks and future perspectives
Based on new markers, tools and recent developments in the field, we propose refined diagnostic criteria for mastocytosis and its variants. Whereas the fundamental classification of the WHO remains unchanged, the updated diagnostic criteria address new disease-related genetic and immunological markers and parameters of MC activation. We also propose an updated global classification of MC disorders, including MCAS. Our refined criteria and classification should support clinicians in daily practice and the conduct of clinical trials.
Acknowledgments
We thank all patients, patient group representatives, and expert moderators who supported the patients and their representatives in formulating their top priorities and top suggestions to the scientific community in this project and conference. We also thank the team headed by PV for their support in the organization and conduct of this project and conference. Special thanks go to Irina Sadovnik, Daniela Berger, and Emir Hadzijusufovic for their technical assistance, helpful support, and helpful discussion. Finally, we thank Eugen Preuß and his team and Barbara Harrington and her team for their technical support.
Author Contributions
All authors contributed equally by actively participating in the Year 2020 Working Conference on Mast Cell Disorders (Vienna, August 30 to September 1, 2020) and by actively participating in preconference discussions (April 2020 until August 2020) and postconference discussions (September 2020 to March 2021). All authors contributed substantially by discussing and establishing refinements in the criteria and the classification of mast cell disorders.
Sources of funding
This study was supported by Austrian Science Funds (FWF), grants F4704-B20 and P32470-B (PV), the Swedish Research Council and the Swedish Cancer Society (GN), the Polish Ministry of Science and Higher Education – grant ST 02-0066/07/253 (ML), the Medical University of Gdansk, grant ST 02-0141/07/231 (MN) and NIAID Division of Intramural Research (MCC, JJL, and DDM).
Disclosures
PV: Advisory Board and Honoraria: Novartis, Blueprint Medicines, Deciphera; CA: Honoraria: Novartis, Deciphera, Patara; KH: Research Funding: Euroimmun, Thermo Fisher; Consultancy and Honoraria: Allergopharma, ALK-Abello, Blueprint, Deciphera, Leo Pharma, Menarini, Novartis, Pfizer, Takeda, Thermo Fisher; IA-T: Advisory Board and Honoraria: Novartis and Blueprint Medicines; KB: Advisory Board: Blueprint Medicines; OH: 1. Co-Founder and Stock Holder: AB Science, 2. Research Grant: AB Science, Novartis, Celgene, Lipomed; MN: Honoraria: ALK, Novartis; Support in Clinical Trials: AB Science, Novartis; JS 1. Advisory Board: Blueprint Medicines; 2. Honoraria: Novartis CU: Advisory Board and Honoraria: Blueprint Medicines, Novartis; KS: Advisory Board and Honoraria: Novartis; MJ: Honoraria and Research Grant: Novartis; FS: 1. Research Grants: Allakos, Blueprint Medicines, Celldex, Genentech, Novartis, 2. Advisory Board and Honoraria: Allakos, Blueprint Medicines, Novartis, Sanofi Uriach; RZ: Consultancy Honoraria: Novartis and Deciphera; ML: Consultancy Honoraria: Novartis; MC: Consultancy: Blueprint Medicines; DHR: Advisory Board and Honoraria: Novartis, Consultant and Honoraria: Blueprint Medicines, Celgene/BMS, Incyte WRS: 1. Research Grant: Phadia, 2. Advisory Board: Novartis, Deciphera, 3. Honoraria: Phadia, Novartis; MT: Honoraria: Novartis, Blueprint Medicines; TIG: Honoraria: Novartis, Blueprint Medicines, Celgene/BMS; HCK-N: Research Grant: Novartis; JG : 1. Research Grant (funds for administration of clinical trials): Novartis, Blueprint Medicines, Deciphera, 2. Advisory Board and Honoraria: Blueprint Medicines, Novartis, Deciphera, Cogent Biosciences, 3. Reimbursement of travel expenses: Novartis, Blueprint Medicines; LBS: 1. Virginia Commonwealth University receives royalties for the tryptase assay that are shared with LBS (ThermoFisher), 2. LBS Received grants as site PI from Novartis for their midostaurin and from Deciphera for their ripretinib in advanced mastocytosis clinical trials, and 3. Advisory Board: Deciphera and Blueprint Medicine; AR: 1. Honoraria: Novartis, Blueprint Medicines, 2. Advisory Board: Novartis, Deciphera, Blueprint Medicines; AO: Advisory Board: Novartis, Blueprint Medicines; H-PH: Advisory Board and Honoraria: Novartis, Deciphera, Blueprint Medicines; MA: 1. Research Grants: Blueprint Medicines, Deciphera, 2. Honoraria: Deciphera. All the other authors have no conflicts of interest to disclose.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1.Horny HP, Sotlar K, Valent P. Mastocytosis: state of the art. Pathobiology. 2007;74:121–132. [DOI] [PubMed] [Google Scholar]
- 2.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George TI, Horny HP. Systemic mastocytosis. Hematol Oncol Clin North Am. 2011;25:1067–1083, vii. [DOI] [PubMed] [Google Scholar]
- 4.Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373:163–172. [DOI] [PubMed] [Google Scholar]
- 5.Valent P, Akin C, Hartmann K, et al. Advances in the classification and treatment of mastocytosis: current status and outlook toward the future. Cancer Res. 2017;77:1261–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valent P, Horny HP, Escribano L, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25:603–625. [DOI] [PubMed] [Google Scholar]
- 7.Valent P, Horny H-P, Li CY, et al. Mastocytosis (mast cell disease). In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. World Health Organization (WHO) Classification of Tumours. Pathology & Genetics. Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press Lyon; 2001:291–302. [Google Scholar]
- 8.Horny HP, Akin C, Metcalfe DD, et al. Mastocytosis (mast cell disease). In: Swerdlow SH, Campo E, Harris NL, et al., eds. World Health Organization (WHO) Classification of Tumours. Pathology & Genetics. Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008;54–63. [Google Scholar]
- 9.Horny HP, Akin C, Arber D, et al. Mastocytosis. In: Swerdlow SH, Campo E, Harris NL, et al., eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2017:62–69. [Google Scholar]
- 10.Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129:1420–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horny HP, Parwaresch MR, Kaiserling E, et al. Mast cell sarcoma of the larynx. J Clin Pathol. 1986;39:596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chott A, Guenther P, Huebner A, et al. Morphologic and immunophenotypic properties of neoplastic cells in a case of mast cell sarcoma. Am J Surg Pathol. 2003;27:1013–1019. [DOI] [PubMed] [Google Scholar]
- 13.Georgin-Lavialle S, Aguilar C, Guieze R, et al. Mast cell sarcoma: a rare and aggressive entity–report of two cases and review of the literature. J Clin Oncol. 2013;31:e90–e97. [DOI] [PubMed] [Google Scholar]
- 14.Monnier J, Georgin-Lavialle S, Canioni D, et al. Mast cell sarcoma: new cases and literature review. Oncotarget. 2016;7:66299–66309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagata H, Worobec AS, Oh CK, et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci U S A. 1995;92:10560–10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longley BJ, Tyrrell L, Lu SZ, et al. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet. 1996;12:312–314. [DOI] [PubMed] [Google Scholar]
- 17.Fritsche-Polanz R, Jordan JH, Feix A, et al. Mutation analysis of C-KIT in patients with myelodysplastic syndromes without mastocytosis and cases of systemic mastocytosis. Br J Haematol. 2001;113:357–364. [DOI] [PubMed] [Google Scholar]
- 18.Arock M, Sotlar K, Akin C, et al. KIT mutation analysis in mast cell neoplasms: recommendations of the European Competence Network on Mastocytosis. Leukemia. 2015;29:1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Montero AC, Jara-Acevedo M, Teodosio C, et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: a prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108:2366–2372. [DOI] [PubMed] [Google Scholar]
- 20.Bodemer C, Hermine O, Palmérini F, et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol. 2010;130:804–815. [DOI] [PubMed] [Google Scholar]
- 21.Bonadonna P, Perbellini O, Passalacqua G, et al. Clonal mast cell disorders in patients with systemic reactions to Hymenoptera stings and increased serum tryptase levels. J Allergy Clin Immunol. 2009;123:680–686. [DOI] [PubMed] [Google Scholar]
- 22.Bonadonna P, Zanotti R, Müller U. Mastocytosis and insect venom allergy. Curr Opin Allergy Clin Immunol. 2010;10:347–353. [DOI] [PubMed] [Google Scholar]
- 23.Wimazal F, Geissler P, Shnawa P, et al. Severe life-threatening or disabling anaphylaxis in patients with systemic mastocytosis: a single-center experience. Int Arch Allergy Immunol. 2012;157:399–405. [DOI] [PubMed] [Google Scholar]
- 24.Valent P, Akin C, Arock M, et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 2012;157:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvarez-Twose I, Bonadonna P, Matito A, et al. Systemic mastocytosis as a risk factor for severe Hymenoptera sting-induced anaphylaxis. J Allergy Clin Immunol. 2013;131:614–615. [DOI] [PubMed] [Google Scholar]
- 26.Valent P, Akin C, Gleixner KV, et al. Multidisciplinary challenges in mastocytosis and how to address with personalized medicine approaches. Int J Mol Sci. 2019;20:2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akin C, Valent P, Metcalfe DD. Mast cell activation syndrome: proposed diagnostic criteria. J Allergy Clin Immunol. 2010;126:1099–104.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valent P. Mast cell activation syndromes: definition and classification. Allergy. 2013;68:417–424. [DOI] [PubMed] [Google Scholar]
- 29.Valent P, Akin C, Nedoszytko B, et al. Diagnosis, classification and management of mast cell activation syndromes (MCAS) in the era of personalized medicine. Int J Mol Sci. 2020;21:E9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyons JJ, Yu X, Hughes JD, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016;48:1564–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyons JJ. Hereditary alpha tryptasemia: genotyping and associated clinical features. Immunol Allergy Clin North Am. 2018;38:483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyons JJ, Chovanec J, O’Connell MP, et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase-encoding germline copy number at TPSAB1. J Allergy Clin Immunol. 2021;147:622–632. [DOI] [PubMed] [Google Scholar]
- 33.Greiner G, Sprinzl B, Górska A, et al. Hereditary α tryptasemia is a valid genetic biomarker for severe mediator-related symptoms in mastocytosis. Blood. 2021;137:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rabenhorst A, Christopeit B, Leja S, et al. Serum levels of bone cytokines are increased in indolent systemic mastocytosis associated with osteopenia or osteoporosis. J Allergy Clin Immunol. 2013;132:1234–1237. e7. [DOI] [PubMed] [Google Scholar]
- 35.Rossini M, Zanotti R, Viapiana O, et al. Bone involvement and osteoporosis in mastocytosis. Immunol Allergy Clin North Am. 2014;34:383–396. [DOI] [PubMed] [Google Scholar]
- 36.Rossini M, Zanotti R, Orsolini G, et al. Prevalence, pathogenesis, and treatment options for mastocytosis-related osteoporosis. Osteoporos Int. 2016;27:2411–2421. [DOI] [PubMed] [Google Scholar]
- 37.Degboé Y, Eischen M, Nigon D, et al. Prevalence and risk factors for fragility fracture in systemic mastocytosis. Bone. 2017;105:219–225. [DOI] [PubMed] [Google Scholar]
- 38.Riffel P, Jawhar M, Gawlik K, et al. Magnetic resonance imaging reveals distinct bone marrow patterns in indolent and advanced systemic mastocytosis. Ann Hematol. 2019;98:2693–2701. [DOI] [PubMed] [Google Scholar]
- 39.Sperr WR, Horny HP, Valent P. Spectrum of associated clonal hematologic non-mast cell lineage disorders occurring in patients with systemic mastocytosis. Int Arch Allergy Immunol. 2002;127:140–142. [DOI] [PubMed] [Google Scholar]
- 40.Horny HP, Sotlar K, Sperr WR, et al. Systemic mastocytosis with associated clonal haematological non-mast cell lineage diseases: a histopathological challenge. J Clin Pathol. 2004;57:604–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim KH, Tefferi A, Lasho TL, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113:5727–5736. [DOI] [PubMed] [Google Scholar]
- 42.Fritsche-Polanz R, Fritz M, Huber A, et al. High frequency of concomitant mastocytosis in patients with acute myeloid leukemia exhibiting the transforming KIT mutation D816V. Mol Oncol. 2010;4:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jawhar M, Döhner K, Kreil S, et al. KIT D816 mutated/CBF-negative acute myeloid leukemia: a poor-risk subtype associated with systemic mastocytosis. Leukemia. 2019;33:1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson TM, Maric I, Simakova O, et al. Clonal analysis of NRAS activating mutations in KIT-D816V systemic mastocytosis. Haematologica. 2011;96:459–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Traina F, Visconte V, Jankowska AM, et al. Single nucleotide polymorphism array lesions, TET2, DNMT3A, ASXL1 and CBL mutations are present in systemic mastocytosis. PLoS One. 2012;7:e43090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwaab J, Schnittger S, Sotlar K, et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood. 2013;122:2460–2466. [DOI] [PubMed] [Google Scholar]
- 47.Damaj G, Joris M, Chandesris O, et al. ASXL1 but not TET2 mutations adversely impact overall survival of patients suffering systemic mastocytosis with associated clonal hematologic non-mast-cell diseases. PLoS One. 2014;9:e85362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jawhar M, Schwaab J, Schnittger S, et al. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V(+) advanced systemic mastocytosis. Leukemia. 2016;30:136–143. [DOI] [PubMed] [Google Scholar]
- 49.Naumann N, Jawhar M, Schwaab J, et al. Incidence and prognostic impact of cytogenetic aberrations in patients with systemic mastocytosis. Genes Chromosomes Cancer. 2018;57:252–259. [DOI] [PubMed] [Google Scholar]
- 50.Kluin-Nelemans HC, Jawhar M, Reiter A, et al. Cytogenetic and molecular aberrations and worse outcome for male patients in systemic mastocytosis. Theranostics. 2021;11:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nedoszytko B, Arock M, Lyons JJ, et al. Clinical impact of inherited and acquired genetic variants in mastocytosis. Int J Mol Sci. 2021;22:E411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pardanani A, Lasho T, Elala Y, et al. Next-generation sequencing in systemic mastocytosis: derivation of a mutation-augmented clinical prognostic model for survival. Am J Hematol. 2016;91:888–893. [DOI] [PubMed] [Google Scholar]
- 53.Sperr WR, Kundi M, Alvarez-Twose I, et al. International prognostic scoring system for mastocytosis (IPSM): a retrospective cohort study. Lancet Haematol. 2019;6:e638–e649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jawhar M, Schwaab J, Álvarez-Twose I, et al. MARS: mutation-adjusted risk score for advanced systemic mastocytosis. J Clin Oncol. 2019;37:2846–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muñoz-González JI, Álvarez-Twose I, Jara-Acevedo M, et al. Proposed global prognostic score for systemic mastocytosis: a retrospective prognostic modelling study. Lancet Haematol. 2021;8:e194–e204. [DOI] [PubMed] [Google Scholar]
- 56.Muñoz-González JI, Álvarez-Twose I, Jara-Acevedo M, et al. Frequency and prognostic impact of KIT and other genetic variants in indolent systemic mastocytosis. Blood. 2019;134:456–468. [DOI] [PubMed] [Google Scholar]
- 57.Bonadonna P, Zanotti R, Caruso B, et al. Allergen specific immunotherapy is safe and effective in patients with systemic mastocytosis and Hymenoptera allergy. J Allergy Clin Immunol. 2008;121:256–257. [DOI] [PubMed] [Google Scholar]
- 58.González de Olano D, Alvarez-Twose I, Esteban-López MI, et al. Safety and effectiveness of immunotherapy in patients with indolent systemic mastocytosis presenting with Hymenoptera venom anaphylaxis. J Allergy Clin Immunol. 2008;121:519–526. [DOI] [PubMed] [Google Scholar]
- 59.Bonadonna P, Gonzalez-de-Olano D, Zanotti R, et al. Venom immunotherapy in patients with clonal mast cell disorders: efficacy, safety, and practical considerations. J Allergy Clin Immunol Pract. 2013;1:474–478. [DOI] [PubMed] [Google Scholar]
- 60.Niedoszytko M, Bonadonna P, Oude Elberink JN, et al. Epidemiology, diagnosis, and treatment of Hymenoptera venom allergy in mastocytosis patients. Immunol Allergy Clin North Am. 2014;34:365–381. [DOI] [PubMed] [Google Scholar]
- 61.Ustun C, Reiter A, Scott BL, et al. Hematopoietic stem-cell transplantation for advanced systemic mastocytosis. J Clin Oncol. 2014;32:3264–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alvarez-Twose I, Martínez-Barranco P, Gotlib J, et al. Complete response to gemtuzumab ozogamicin in a patient with refractory mast cell leukemia. Leukemia. 2016;30:1753–1756. [DOI] [PubMed] [Google Scholar]
- 63.Gotlib J, Kluin-Nelemans HC, George TI, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374:2530–2541. [DOI] [PubMed] [Google Scholar]
- 64.Valent P, Akin C, Hartmann K, et al. Midostaurin: a magic bullet that blocks mast cell expansion and activation. Ann Oncol. 2017;28:2367–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lemal R, Fouquet G, Terriou L, et al. Omalizumab therapy for mast cell-mediator symptoms in patients with ISM, CM, MMAS, and MCAS. J Allergy Clin Immunol Pract. 2019;7:2387–2395. e3. [DOI] [PubMed] [Google Scholar]
- 66.Reiter A, George TI, Gotlib J. New developments in diagnosis, prognostication, and treatment of advanced systemic mastocytosis. Blood. 2020;135:1365–1376. [DOI] [PubMed] [Google Scholar]
- 67.Jendoubi F, Gaudenzio N, Gallini A, et al. Omalizumab in the treatment of adult patients with mastocytosis: a systematic review. Clin Exp Allergy. 2020;50:654–661. [DOI] [PubMed] [Google Scholar]
- 68.Valent P, Escribano L, Parwaresch RM, et al. Recent advances in mastocytosis research. Summary of the Vienna Mastocytosis Meeting 1998. Int Arch Allergy Immunol. 1999;120:1–7. [DOI] [PubMed] [Google Scholar]
- 69.Valent P, Akin C, Escribano L, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37:435–453. [DOI] [PubMed] [Google Scholar]
- 70.Akin C, Valent P. Diagnostic criteria and classification of mastocytosis in 2014. Immunol Allergy Clin North Am. 2014;34:207–218. [DOI] [PubMed] [Google Scholar]
- 71.Valent P, Arock M, Bonadonna P, et al. European Competence Network on Mastocytosis (ECNM): 10-year jubilee, update, and future perspectives. Wien Klin Wochenschr. 2012;124:807–814. [DOI] [PubMed] [Google Scholar]
- 72.Gotlib J, George T, Carter MC, et al. Proceedings from the inaugural American Initiative in Mast Cell Diseases (AIM) investigator conference. J Allergy Clin Immunol. 2021;147:2043–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol. 2016;137:35–45. [DOI] [PubMed] [Google Scholar]
- 74.Aberer E, Sperr WR, Bretterklieber A, et al. Clinical impact of skin lesions in mastocytosis: a multicenter study of the European Competence Network on Mastocytosis. J Invest Dermatol. 2021;141:1719–1727. [DOI] [PubMed] [Google Scholar]
- 75.Carter MC, Clayton ST, Komarow HD, et al. Assessment of clinical findings, tryptase levels, and bone marrow histopathology in the management of pediatric mastocytosis. J Allergy Clin Immunol. 2015;136:1673–1679. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wiechers T, Rabenhorst A, Schick T, et al. Large maculopapular cutaneous lesions are associated with favorable outcome in childhood-onset mastocytosis. J Allergy Clin Immunol. 2015;136:1581–1590. e3. [DOI] [PubMed] [Google Scholar]
- 77.Bernd HW, Sotlar K, Lorenzen J, et al. Acute myeloid leukaemia with t(8;21) associated with “occult” mastocytosis. Report of an unusual case and review of the literature. J Clin Pathol. 2004;57:324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sotlar K, Cerny-Reiterer S, Petat-Dutter K, et al. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod Pathol. 2011;24:585–595. [DOI] [PubMed] [Google Scholar]
- 79.Valent P, Sotlar K, Horny HP. Aberrant expression of CD30 in aggressive systemic mastocytosis and mast cell leukemia: a differential diagnosis to consider in aggressive hematopoietic CD30-positive neoplasms. Leuk Lymphoma. 2011;52:740–744. [DOI] [PubMed] [Google Scholar]
- 80.Morgado JM, Perbellini O, Johnson RC, et al. CD30 expression by bone marrow mast cells from different diagnostic variants of systemic mastocytosis. Histopathology. 2013;63:780–787. [DOI] [PubMed] [Google Scholar]
- 81.Blatt K, Cerny-Reiterer S, Schwaab J, et al. Identification of the Ki-1 antigen (CD30) as a novel therapeutic target in systemic mastocytosis. Blood. 2015;126:2832–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schwartz LB, Metcalfe DD, Miller JS, et al. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med. 1987;316:1622–1626. [DOI] [PubMed] [Google Scholar]
- 83.Schwartz LB, Irani AM. Serum tryptase and the laboratory diagnosis of systemic mastocytosis. Hematol Oncol Clin North Am. 2000;14:641–657. [DOI] [PubMed] [Google Scholar]
- 84.Sperr WR, Jordan JH, Fiegl M, et al. Serum tryptase levels in patients with mastocytosis: correlation with mast cell burden and implication for defining the category of disease. Int Arch Allergy Immunol. 2002;128:136–141. [DOI] [PubMed] [Google Scholar]
- 85.Valent P, Sperr WR, Sotlar K, et al. The serum tryptase test: an emerging robust biomarker in clinical hematology. Expert Rev Hematol. 2014;7:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zanotti R, Bonifacio M, Lucchini G, et al. Refined diagnostic criteria for bone marrow mastocytosis: a proposal of the European competence network on mastocytosis. Leukemia. In press. [DOI] [PubMed] [Google Scholar]
- 87.Alvarez-Twose I, Zanotti R, González-de-Olano D, et al. ; Spanish Network on Mastocytosis (REMA); Italian Network on Mastocytosis (RIMA). Nonaggressive systemic mastocytosis (SM) without skin lesions associated with insect-induced anaphylaxis shows unique features versus other indolent SM. J Allergy Clin Immunol. 2014;133:520–528. [DOI] [PubMed] [Google Scholar]
- 88.Zanotti R, Lombardo C, Passalacqua G, et al. Clonal mast cell disorders in patients with severe Hymenoptera venom allergy and normal serum tryptase levels. J Allergy Clin Immunol. 2015;136:135–139. [DOI] [PubMed] [Google Scholar]
- 89.Valent P, Akin C, Sperr WR, et al. Aggressive systemic mastocytosis and related mast cell disorders: current treatment options and proposed response criteria. Leuk Res. 2003;27:635–641. [DOI] [PubMed] [Google Scholar]
- 90.Valent P, Sperr WR, Akin C. How I treat patients with advanced systemic mastocytosis. Blood. 2010;116:5812–5817. [DOI] [PubMed] [Google Scholar]
- 91.Valent P, Escribano L, Broesby-Olsen S, et al. ; European Competence Network on Mastocytosis. Proposed diagnostic algorithm for patients with suspected mastocytosis: a proposal of the European Competence Network on Mastocytosis. Allergy. 2014;69:1267–1274. [DOI] [PubMed] [Google Scholar]
- 92.Hauswirth AW, Simonitsch-Klupp I, Uffmann M, et al. Response to therapy with interferon alpha-2b and prednisolone in aggressive systemic mastocytosis: report of five cases and review of the literature. Leuk Res. 2004;28:249–257. [DOI] [PubMed] [Google Scholar]
- 93.Erben P, Schwaab J, Metzgeroth G, et al. The KIT D816V expressed allele burden for diagnosis and disease monitoring of systemic mastocytosis. Ann Hematol. 2014;93:81–88. [DOI] [PubMed] [Google Scholar]
- 94.Hoermann G, Gleixner KV, Dinu GE, et al. The KIT D816V allele burden predicts survival in patients with mastocytosis and correlates with the WHO type of the disease. Allergy. 2014;69:810–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jawhar M, Schwaab J, Hausmann D, et al. Splenomegaly, elevated alkaline phosphatase and mutations in the SRSF2/ASXL1/RUNX1 gene panel are strong adverse prognostic markers in patients with systemic mastocytosis. Leukemia. 2016;30:2342–2350. [DOI] [PubMed] [Google Scholar]
- 96.Jawhar M, Schwaab J, Meggendorfer M, et al. The clinical and molecular diversity of mast cell leukemia with or without associated hematologic neoplasm. Haematologica. 2017;102:1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Valent P, Sotlar K, Sperr WR, et al. Refined diagnostic criteria and classification of mast cell leukemia (MCL) and myelomastocytic leukemia (MML): a consensus proposal. Ann Oncol. 2014;25:1691–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Horny HP, Sotlar K, Reiter A, et al. Myelomastocytic leukemia: histopathological features, diagnostic criteria and differential diagnosis. Expert Rev Hematol. 2014;7:431–437. [DOI] [PubMed] [Google Scholar]
- 99.Sperr WR, Drach J, Hauswirth AW, et al. Myelomastocytic leukemia: evidence for the origin of mast cells from the leukemic clone and eradication by allogeneic stem cell transplantation. Clin Cancer Res. 2005;11(19 pt 1):6787–6792. [DOI] [PubMed] [Google Scholar]
- 100.Kubasch AS, Franke GN, Aldaoud A, et al. Allogeneic hematopoietic stem cell transplantation in a rare case of tonsillar mast cell sarcoma. Front Oncol. 2020;10:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Charrette EE, Mariano AV, Laforet EG. Solitary mast cell “tumor” of lung. Its place in the spectrum of mast cell disease. Arch Intern Med. 1966;118:358–362. [PubMed] [Google Scholar]
- 102.Kudo H, Morinaga S, Shimosato Y, et al. Solitary mast cell tumor of the lung. Cancer. 1988;61:2089–2094. [DOI] [PubMed] [Google Scholar]
- 103.Ayadi L, Abid N, Makni S, et al. An unusual tumour of the lung. Pathologica. 2015;107:14–18. [PubMed] [Google Scholar]
- 104.Akin C, Fumo G, Yavuz AS, et al. A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood. 2004;103:3222–3225. [DOI] [PubMed] [Google Scholar]
- 105.Álvarez-Twose I, Jara-Acevedo M, Morgado JM, et al. Clinical, immunophenotypic, and molecular characteristics of well-differentiated systemic mastocytosis. J Allergy Clin Immunol. 2016;137:168–178. e1. [DOI] [PubMed] [Google Scholar]
- 106.Alvarez-Twose I, Matito A, Morgado JM, et al. Imatinib in systemic mastocytosis: a phase IV clinical trial in patients lacking exon 17 KIT mutations and review of the literature. Oncotarget. 2017;8:68950–68963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang L, Wang SA, Konoplev S, et al. Well-differentiated systemic mastocytosis showed excellent clinical response to imatinib in the absence of known molecular genetic abnormalities: a case report. Medicine (Baltimore). 2016;95:e4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Valent P, Akin C, Bonadonna P, et al. Proposed diagnostic algorithm for patients with suspected mast cell activation syndrome. J Allergy Clin Immunol Pract. 2019;7:1125–1133.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.