Abstract
Background
During the COVID-19 pandemic, the University of Virginia adult cystic fibrosis (CF) center transitioned from in-person clinical encounters to a model that included interdisciplinary telemedicine. The pandemic presented an unprecedented opportunity to assess the impact of the interdisciplinary telemedicine model on clinical CF outcomes.
Research Question
What are the clinical outcomes of a care model that includes interdisciplinary telemedicine (IDC-TM) compared with in-person clinical care for patients with CF during the COVID-19 pandemic?
Study Design and Methods
Adults with CF were included. The prepandemic year was defined as March 17, 2019, through March 16, 2020, and the pandemic year (PY) was defined as March 17, 2020, through March 16, 2021. Patients were enrolled starting in the PY. Prepandemic data were gathered retrospectively. Telemedicine visits were defined as clinical encounters via secured video communication. Hybrid visits were in-person evaluations by physician, with in-clinic video communication by other team members. In-person visits were encounters with in-person providers only. All encounters included previsit screening. Outcomes were lung function, BMI, exacerbations, and antibiotic use. FEV1 percent predicted, exacerbations, and antibiotic use were adjusted for the effect of elexacaftor/tezacaftor/ivacaftor treatment.
Results
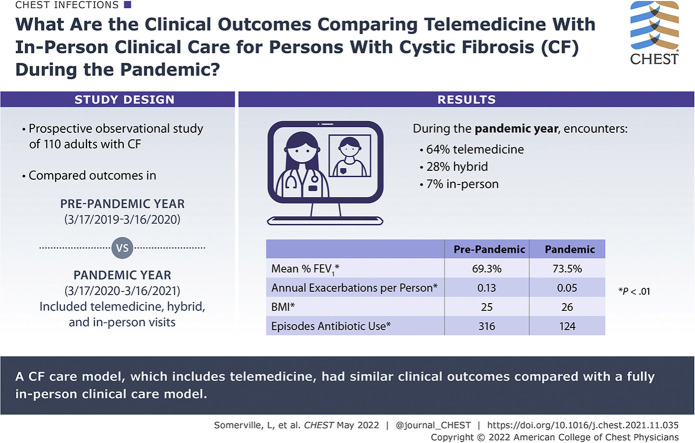
One hundred twenty-four patients participated. One hundred ten patients were analyzed (mean age, 35 years; range, 18-69 years). Ninety-five percent had access to telemedicine (n = 105). Telemedicine visits accounted for 64% of encounters (n = 260), hybrid visits with telemedicine support accounted for 28% of encounters (n = 114), and in-person visits accounted for 7% of encounters (n = 30). No difference in lung function or exacerbation rate during the PY was found. BMI increased from 25 to 26 kg/m2 (t100 = –4.72; P < .001). Antibiotic use decreased from 316 to 124 episodes (z = 8.81; P < .0001).
Interpretation
This CF care model, which includes IDC-TM, successfully monitored lung function and BMI, identified exacerbations, and followed guidelines-based care during the pandemic. A significant decrease in antibiotic use suggests that social mitigation strategies were protective.
Trial Registry
ClinicalTrials.gov; No.: NCT04402801; URL: www.clinicaltrials.gov.
Key Words: antibiotic use, BMI, clinical outcomes, COVID-19, cystic fibrosis, exacerbation rate, lung function, pandemic, telehealth, telemedicine
Abbreviations: CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator; ETI, elexacaftor/tezacaftor/ivacaftor; HS, home spirometry; IDC-TM, interdisciplinary telemedicine; PPY, prepandemic year; PVP, previsit planning; PY, pandemic year; RT, respiratory therapist; UVA, University of Virginia
Graphical Abstract
FOR EDITORIAL COMMENT, SEE PAGE 1127
Take-home Points.
Study Question: How does a clinical care model that includes interdisciplinary telemedicine (IDC-TM) compare with in-person clinical care for patients with cystic fibrosis (CF) during the COVID-19 pandemic, measured by primary outcomes of lung function, pulmonary exacerbations, maintenance of BMI, and use of antibiotics?
Results: A clinical care model for CF that includes IDC-TM was found to have similar clinical outcomes compared with a fully in-person clinical care model in terms of maintaining lung function and BMI and identifying CF pulmonary exacerbations during the COVID-19 global pandemic. This care model also was associated with decreased overall use of antibiotics.
Interpretation: Our CF care model that includes IDC-TM successfully monitored lung function, identified exacerbations, and followed guidelines-based care during the global COVID-19 pandemic. A significant decrease in antibiotic use suggests that social mitigation strategies were protective in adults with CF.
In the first 4 months of the COVID-19 pandemic, the use of telemedicine in the United States increased by 154%.1 The global pandemic presented an unprecedented opportunity to assess the impact of telemedicine on clinical outcomes in cystic fibrosis (CF). In recent years, CF survival has improved dramatically because of advances in therapeutics and widespread adoption of guideline-based interdisciplinary clinical care focused on early identification and treatment of CF pulmonary exacerbations.2 Telemedicine increases access to care for adults with CF living in regions remote to a CF specialty center, but routine use of telemedicine did not gain widespread traction until the COVID-19 pandemic.3 In March 2020, the Adult CF Clinical Care Team at the University of Virginia (UVA) rapidly transitioned from in-person clinical encounters to a CF care model that included interdisciplinary telemedicine (IDC-TM) using Health Insurance Portability and Accountability Act-compliant video communication.4 This prospective observational study set out to answer how a clinical care model that included IDC-TM compared with the classical CF clinical care model during the COVID-19 pandemic. Outcomes evaluated were lung function, rate of pulmonary exacerbation, maintenance of BMI, and use of antibiotics, as well as qualitative observations on the potentially protective effects of social mitigation in patients with CF during the COVID-19 pandemic on antibiotic use.5
Study Design and Methods
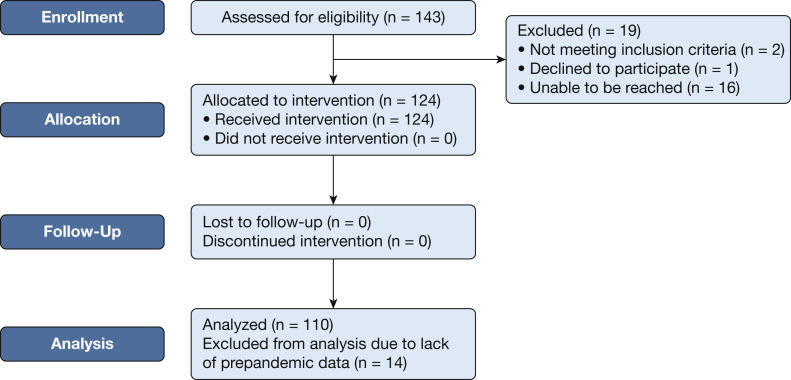
Adult patients with CF were enrolled starting in March 2020 (UVA Institutional Review Board for Health Sciences Research, Federal Wide Assurance 00006183, Identifier: 22327; ClinicalTrials.gov Identifier: NCT04402801). Patients with CF 18 years of age or older were included (Fig 1 ). Incarcerated patients and patients unable to provide informed consent were excluded. Patients provided informed consent to participate in a prospective, observational, cohort study monitoring real-world clinical outcomes using a CF care model that includes IDC-TM. Patients were invited by the UVA Adult CF Clinical Care Team as part of previsit planning (PVP) after the onset of the COVID-19 pandemic. The care model followed Cystic Fibrosis Foundation clinical care guidelines regarding frequency of clinical visits and spirometry once per quarter, with a comprehensive interdisciplinary evaluation at least annually.6 The pandemic year (PY) was defined as March 17, 2020, through March 16, 2021. The prepandemic year (PPY) was defined as March 17, 2019, through March 16, 2020. The primary outcome was stability of lung function; secondary outcomes were exacerbation rates, antibiotic use, and preservation of BMI. Data were collected by electronic medical record communications and chart review. Spirometry was measured in the laboratory or by handheld home spirometers, previously demonstrated to be valid and reproducible for spirometry analysis.7 Data were analyzed using GraphPad Prism version 9.0.0 for Windows software (GraphPad Software) and R version 4.1.0 software (R Foundation for Statistical Computing). Power analysis was not applicable.
Figure 1.
Study design and enrollment flowchart. All 143 adult patients with cystic fibrosis at the University of Virginia were considered for eligibility. Two were excluded because of inability to provide informed consent. One patient declined to participate. Sixteen were unable to be reached for consent. One hundred twenty-four patients were enrolled and participated in the telemedicine intervention. One hundred ten patients were analyzed; 14 of the participants were excluded from final analysis because of lack of retrospective prepandemic data.
Demographic Data
Demographic data including sex, age, ethnicity, mutation type, lung function, exacerbations per year, use of CFTR modulator therapy, microbiological data, status of CF-related diabetes and bone disease, and baseline BMI were obtained by chart review.
Clinical Encounters
Adult patients with CF at UVA were contacted by secured health system e-mail communication or by phone as part of routine PVP up to 1 week before scheduled appointments. With transition to the telemedicine intervention, PVP was adjusted to include screening questions to determine appropriateness for IDC-TM clinical care. Telemedicine eligibility was based on clinical stability, anticipated needs, and patient preferences, as well as access to required technology, including a Wi-Fi connection and a computer or smartphone with internet, video, and audio access. Additional equipment that was encouraged, but not required, included home spirometry (HS), a scale for weight, a pulse oximeter, and a BP cuff. Telemedicine visits were defined as any clinical encounter conducted entirely through secured video communication via the Health Insurance Portability and Accountability Act-compliant platform WebEx (Cisco Systems). Hybrid visits were visits in which the patient was seen in person in the clinic by a subset of the team with additional in-clinic telemedicine support. During these visits, additional team members communicate with the patient via secured webcast in the clinic room. Hybrid visits were recommended for patients who did not meet the prescreening criteria for telemedicine visits. In-person visits were clinical encounters in which the patient was seen only by in-person team members, typically the CF physician and one other team member. Telephone visits were conducted completely by phone when video communication was not possible.4
Lung Function
All patients who did not already own a home spirometer were provided one through the adult CF clinic at no cost to the patient. For telemedicine visits, the respiratory therapist (RT) provided education on HS by secured video communication. With each telemedicine encounter, the RT coached the patient through HS. Readings were sent by secured communication to the RT. The RT verified quality of HS and used the raw FEV1 to calculate Global Lung Initiative FEV1 percent predicted and difference compared with baseline. For hybrid and in-person visits, spirometry was performed in the clinic using the in-laboratory MedGraphics CPFS/D USB spirometer (Medical Graphics Corp.). Lung function analysis was performed for all patients who had at least one spirometry reading in both the PY and PPY and was adjusted for elexacaftor/tezacaftor/ivacaftor (ETI) use.
Exacerbation Rates
The electronic medical record was reviewed for all episodes of antimicrobial use. Exacerbation was defined as hospital admission, use of IV antimicrobials for treatment of CF, or both. All antibiotic use additionally included all filled prescriptions for any 14-day course of oral antibiotics, excluding antibiotics intended for non-CF care. Exacerbation rates are reported in annualized exacerbations per patient.
BMI
BMI was calculated from aggregate data during the PPY and PY. BMI was obtained from clinic weights and self-reported weights during telemedicine encounters using a scale at home. Patients who were pregnant at any time during the 2-year period were excluded from BMI analyses.
Statistical Analysis
Descriptive statistics were produced for all clinical measures. FEV1 percent predicted, exacerbations, antibiotic episodes, and BMI were collected as continuous variables. To analyze lung function, a series of linear mixed models were created that account for multiple measurements for each patient. One model was created to examine the effect of year alone, and a multivariate model was created to examine the effect of year after controlling for ETI use and other variables. Exacerbation rate was analyzed by developing a series of Poisson mixed models. Again, one model investigated year and a multivariate model was built to understand the effect of year after adjusting for ETI therapy and other variables. Two final Poisson mixed models were created to explain all antibiotic use: a univariate model for year and a multivariate model. For the multivariate models, full model statistics are reported along with estimated marginal means for any effect(s) of interest. Differences in BMI were analyzed using a paired two-tailed t test. Subgroup analyses were performed on BMI cohorts using paired two-tailed t tests that then were corrected for multiple testing using the Holm P value correction. The P value for significance was .05.
Results
Demographic Data
One hundred twenty-four of 143 patients were enrolled and participated in the telemedicine intervention. One hundred ten patients were included in final analysis; 14 were excluded because of lack of retrospective prepandemic data. None were lost to follow-up. Patients who were ineligible for the study, unable to be reached during the enrollment window, declined to participate in the study, or joined the clinic later in the year were given the option to use telemedicine care at their request; however, these patients were not included in data collection and outcomes analyses. The mean age at the start of the PY was 35 years (range, 18-69 years), with 59 women (54%) and 51 men (46%). Ninety-five percent of the enrolled patients were White and 5% were Black. No other ethnicities were identified in this cohort. Ninety percent had at least one del-F508 genetic mutation. Fifteen percent had advanced lung disease (FEV1 < 40%) at baseline. A total of 96 exacerbations occurred during the PPY (0.13/person/y) and 38 exacerbations occurred during the PY (0.05/person/y). CFTR modulators were taken by 93% of patients during the study period (n = 102). In the PPY, 50% were prescribed tezacaftor/ivacaftor, 6% were prescribed lumacaftor/ivacaftor, and 15% were prescribed ivacaftor. During the PPY, 88 patients (80%) began ETI therapy. During the PY, 2% continued to receive tezacaftor/ivacaftor, 2% continued to receive lumacaftor/ivacaftor, 4% continued to receive ivacaftor, and an additional 6 patients began ETI treatment (total, 85%). Colonization with Pseudomonas aeruginosa was identified in 60% during the PPY and in 47% during the PY, methicillin-sensitive Staphylococcus aureus was identified in 25% during the PPY and 22% during the PY, Stenotrophomonas maltophilia was identified in 18% during the PPY and 12% during the PY, Burkholderia cepacia complex was identified in 4% during the PPY and 3% during the PY, Aspergillus species were identified in 37% during the PPY and 13% during the PY, Achromobacter species were identified in 9% during the PPY and 6% during the PY, and nontuberculous mycobacterium species were identified in 21% during the PPY and 8% during the PY. Thirty-seven percent of patients had received a confirmed diagnosis of CF-related diabetes and 33% had received a confirmed diagnosis of CF bone disease. In both the PPY and PY, 97% had a BMI of > 18 kg/m2. Demographic data are summarized in Table 1 .
Table 1.
Patient Characteristics
| Characteristic | Prepandemic Year (2019-2020) | Pandemic Year (2020-2021) |
|---|---|---|
| Sex | ||
| Female | 59 (54) | —a |
| Male | 51 (46) | —a |
| Age, y | ||
| 18-24 | 27 (25) | —a |
| 25-34 | 41 (37) | —a |
| 35-44 | 19 (17) | —a |
| 45-54 | 15 (14) | —a |
| 55+ | 8 (7) | —a |
| Ethnicity | ||
| White | 105 (95) | —a |
| Black | 5 (5) | —a |
| Hispanic | 0 | —a |
| Genetics | ||
| del F508 homozygous | 59 (54) | —a |
| del F508 heterozygous | 40 (36) | —a |
| Lung function, FEV1 % predicted | ||
| < 40% | 17 (15) | 15 (14) |
| 40%-69% | 30 (27) | 29 (26) |
| 70%-89% | 39 (35) | 35 (32) |
| > 90% | 24 (22) | 31 (28) |
| Pulmonary exacerbations per year | ||
| Total exacerbations | 96 | 38 |
| Annualized exacerbations per person | 0.13 | 0.05 |
| CFTR modulator use | ||
| Elexacaftor/tezacaftor/ivacaftor | 88 (80) | 94 (85) |
| Tezacaftor/ivacaftor | 55 (50) | 2 (2) |
| Lumacaftor/ivacaftor | 7 (6) | 2 (2) |
| Ivacaftor | 17 (15) | 4 (4) |
| Microbiology of colonizing species | ||
| P. aeruginosa | 66 (60) | 52 (47) |
| Methicillin-resistant S. aureus | 28 (25) | 25 (22) |
| S. maltophilia | 19 (18) | 13 (12) |
| B. cepacia complex | 5 (4) | 4 (3) |
| Aspergillus | 40 (37) | 14 (13) |
| Achromobacter | 10 (9) | 7 (6) |
| Nontuberculous mycobacterium | 23 (21) | 9 (8) |
| CF-related diabetes | ||
| Yes | 41 (37) | 45 (41) |
| Negative screening during calendar year | 38 (35) | 28 (25) |
| Not screened during calendar year | 31 (28) | 37 (34) |
| CF bone disease | ||
| Screened and normal | 43 (39) | 50 (45) |
| Osteopenia | 33 (30) | 40 (36) |
| Osteoporosis | 3 (3) | 5 (5) |
| Unknown | 31 (28) | 15 (14) |
| BMI, kg/m2 | ||
| ≤ 17 | 3 (3) | 3 (3) |
| 18-23 | 51 (46) | 38 (35) |
| ≥ 24 | 56 (51) | 69 (62) |
Data are presented as No. (%) or No. CF = cystic fibrosis; CFTR = cystic fibrosis transmembrane conductance regulator.
Data only collected for the baseline year for this characteristic.
Telemedicine Participation and Clinical Encounters
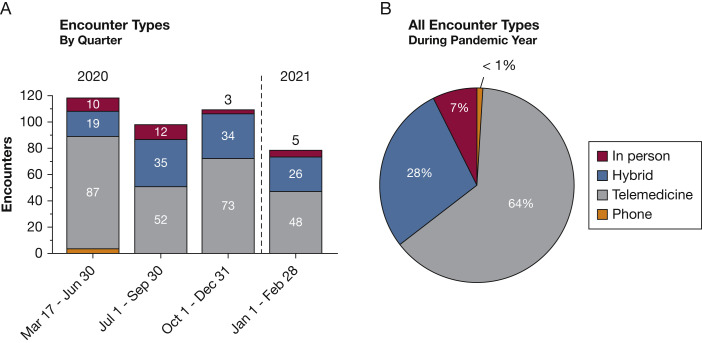
Ninety-five percent of analyzed patients had access to a telemedicine-compatible device (n = 105), whereas 5% had no telemedicine capability (n = 5). The patients with no telemedicine access were still eligible for other clinical encounter methods, including hybrid visits with telemedicine support. A total of 407 encounters were conducted during the PY, between March 17, 2020, and March 16, 2021. Telemedicine encounters accounted for 64% of all clinical visits (n = 260), whereas hybrid visits with telemedicine support accounted for 28% (n = 114) and entirely in-person visits accounted for 7% (n = 30) of all encounters. All fully in-person visits were urgent or sick appointments or were triggered as a follow-up to previous telemedicine encounters. Phone visits accounted for less than 1% of encounters (n = 3), and all occurred between March and April 2020 (Fig 2 ).
Figure 2.
A, Bar graph showing clinic encounters during the pandemic year (PY) by quarter. A total of 407 clinical encounters were conducted between March 17, 2020, and March 16, 2021. B, Pie graph showing encounter types as a percentage of all encounters for the PY. Telemedicine encounters made up most visits at 64% (n = 260). Hybrid visits that included in-clinic telemedicine support accounted for 28% (n = 114), whereas 7% were in-person visits (n = 30) and less than 1% were by phone (n = 3). All phone visits took place between March 17 and June 30, 2020.
Lung Function
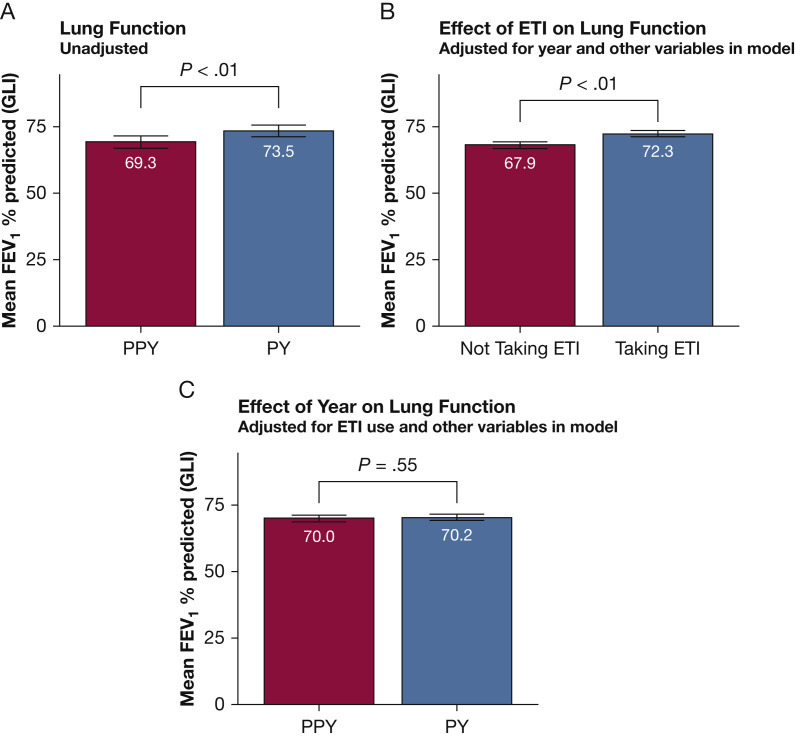
One hundred ten patients were included in the analyses of lung function. Using a linear mixed model to explain lung function using just the year and a random effect for each patient, the mean FEV1 % predicted in the PPY was 69.27% and increased by 4.28% during the PY (t 480 = 8.71; P < .01) (Fig 3 A). During the study period, 85% of patients (n = 94) began taking ETI; 94% of these began therapy in the PPY (n = 88). Within the subgroup of patients who began taking ETI in the PPY, 68% (n = 60) had at least one spirometry value after initiation of therapy, but before the start of the pandemic, allowing for determination of ETI effect on FEV1 independent of the pandemic. To this end, a linear mixed model was created with the 110 analyzed patients explaining FEV1 percent predicted using ETI use and controlling for the effects of time, sex, BMI, exacerbations, age group, year, baseline lung function cohort, the interaction between age group and year, and the interaction between baseline lung function cohort and year (Table 2 ). The effect of ETI on lung function after controlling for the effect of the pandemic and other variables was an increase in FEV1 % predicted of 4.31% (t 474 = 5.16; P < .01) (Fig 3B). Lung function adjusted for ETI use and other variables revealed no significant difference between the PPY and PY, with a difference in FEV1 % predicted of 1.30% in the PY (t 319 = 0.60; P = .55) (Fig 3C).
Figure 3.
A-C, Bar graphs showing changes in lung function during the PY. A, Without adjusting for ETI use and other variables, FEV1 percent predicted increased significantly from 69.3% in the PPY to 73.5% during the PY (P < .01). B, Determining the effect of triple combination cystic fibrosis transmembrane conductance regulator modulator therapy on lung function adjusting for year and other variables. Lung function improved by 4.4% because of therapy (P < .01). C, Adjusting for ETI use and other variables revealed no change in lung function from the PPY to PY (70.0 PPY vs 70.2 PY; P = .55). ETI = elexacaftor/tezacaftor/ivacaftor; PPY = prepandemic year; PY = pandemic year.
Table 2.
Multivariate Linear Mixed Model Explaining Lung Function in Adults With CF
| Term | Estimate | SE | df | t | P Value |
|---|---|---|---|---|---|
| Intercept | 32.35 | 3.90 | 145.28 | 8.29 | < .01 |
| Quarter of y | 0.15 | 0.27 | 418.88 | 0.56 | .57 |
| Male sex | –0.48 | 1.50 | 101.28 | –0.32 | .75 |
| BMI | 0.00 | 0.13 | 167.69 | 0.03 | .98 |
| Patient is taking ETI | 4.31 | 0.84 | 474.48 | 5.16 | < .01 |
| Age group, y | |||||
| 25-34 | –3.56 | 1.95 | 100.78 | –1.82 | .07 |
| 35-44 | –1.62 | 2.34 | 102.22 | –0.70 | .49 |
| 45-54 | 2.32 | 2.68 | 103.68 | 0.87 | .39 |
| 55+ | 2.57 | 3.25 | 104.78 | 0.79 | .43 |
| During PY | 1.30 | 2.15 | 319.33 | 0.60 | .55 |
| FEV1 % predicted | |||||
| 40-69 | 24.67 | 2.30 | 103.63 | 10.71 | < .01 |
| 70-89 | 49.68 | 2.32 | 106.84 | 21.40 | < .01 |
| > 90 | 66.31 | 2.66 | 106.87 | 24.92 | < .01 |
| All antibiotic episodes | 0.42 | 0.38 | 492.93 | 1.13 | .26 |
| Exacerbations | –1.26 | 0.62 | 491.14 | –2.04 | .04 |
| Age during PY, y | |||||
| 25-34 | –0.96 | 1.53 | 228.47 | –0.62 | .53 |
| 35-44 | 0.12 | 1.76 | 233.08 | 0.07 | .95 |
| 45-54 | –2.62 | 2.07 | 225.44 | –1.27 | .21 |
| 55+ | –1.77 | 2.39 | 225.30 | –0.74 | .46 |
| FEV1 % predicted during PY | |||||
| 40-69 | 0.61 | 1.77 | 226.98 | 0.34 | .73 |
| 70-89 | 0.65 | 1.82 | 234.66 | 0.36 | .72 |
| > 90 | –1.33 | 2.09 | 235.90 | –0.64 | .52 |
Change in lung function explained by ETI use and controlling for other variables. A multivariate linear mixed model was created with the 110 analyzed patients explaining lung function using ETI use and controlling for the effects of time, sex, BMI, exacerbations, age group, year, lung function cohort, and the interaction between age group and year and between lung function cohort and year. A random slope over time was included for each participant. The effect of ETI on lung function after controlling for the effect of the pandemic and other variables was an increase in FEV1 % predicted of 4.31% (t474 = 5.16; P < .01). Lung function adjusted for ETI use and other variables revealed no significant difference in lung function between the PPY and PY, with a difference in FEV1 % predicted of 1.30% during the PY (t319 = 0.60; P = .55). CF = cystic fibrosis; df = degrees of freedom; ETI = elexacaftor/tezacaftor/ivacaftor; PPY = prepandemic year; PY = pandemic year.
Exacerbation Rates and Antibiotic Use
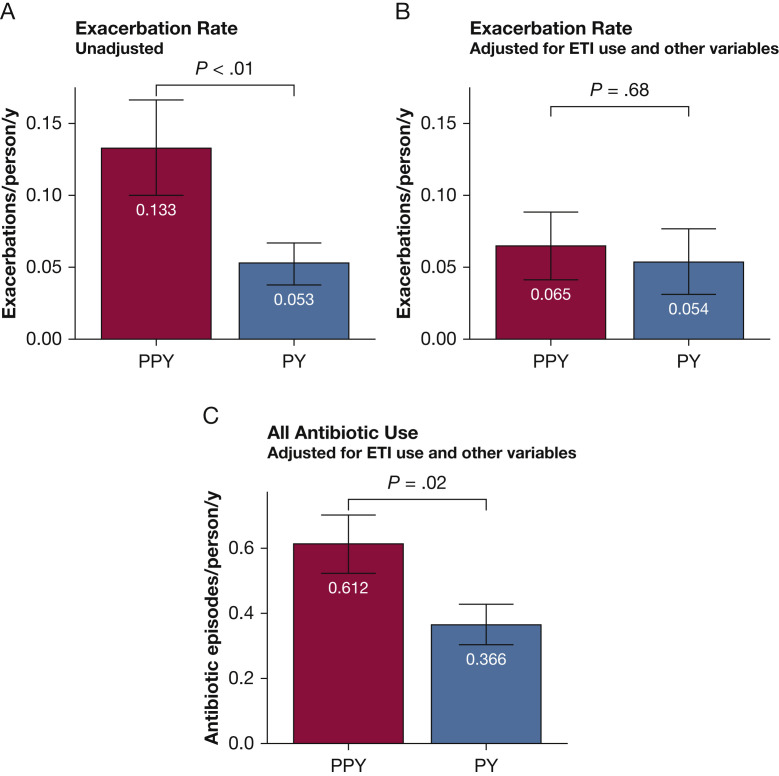
No patient deaths or lung transplantations occurred during the study period. Three exacerbations in the PY were the result of COVID-19. In the PPY, 14 exacerbations included treatment for confirmed or suspected influenza. No instances of confirmed or suspected influenza occurred in the PY. Based on a Poisson mixed model explaining the number of exacerbations by year and a random effect of patient, exacerbations decreased by a factor of 2.5 from a total of 96 during the PPY (0.13/person/y) to 38 during the PY (0.05/person/y; z = –4.83; P < .01). Using a model that adjusted for ETI use and other variables (Tables 3, 4 ), no significant difference was found in the exacerbation rate in the PPY and PY (0.065/person/y vs 0.054/person/y, respectively; z = 0.41; P = .68). Exacerbations differed by sex, with women showing 2.2 times as many exacerbations (0.088/person/y; sum = 96) as men (0.040/person/y; sum = 38; z = 2.18; P = .03) (Tables 3, 4). Overall use of antibiotics decreased by a factor of 2.5, from 316 episodes in the PPY (0.848/person/y) to 124 in the PY (0.333/person/y; z = 8.81; P < .0001). After adjusting for ETI use and other variables, this effect was preserved, although less pronounced, with 0.612/person/y in the PPY to 0.366/person/y in the PY (z = 2.31; P = .02) (Tables 5, 6 ). During the PY, 64% of all exacerbations were diagnosed during hybrid or in-person clinic encounters, whereas 36% were diagnosed during telemedicine clinic encounters (Fig 4 ).
Table 3.
Poisson Mixed Model Explaining Exacerbations in Adults With CF
| Term | Estimate | SE | z Ratio | P Value |
|---|---|---|---|---|
| Intercept | 0.22 | 0.91 | 0.24 | .81 |
| Quarter of y | –0.17 | 0.11 | –1.56 | .12 |
| Male sex | –0.78 | 0.36 | –2.18 | .03 |
| BMI | –0.02 | 0.03 | –0.53 | .60 |
| Patient is taking ETI | –0.43 | 0.33 | –1.29 | .20 |
| FEV1 % predicted | ||||
| 40-69 | –0.42 | 0.51 | –0.81 | .42 |
| 70-89 | –0.69 | 0.52 | –1.32 | .19 |
| > 90 | –1.97 | 0.68 | –2.91 | < .01 |
| During PY | –1.12 | 0.77 | –1.46 | .14 |
| Age group, y | ||||
| 25-34 | –0.31 | 0.42 | –0.73 | .46 |
| 35-44 | –1.16 | 0.57 | –2.05 | .04 |
| 45-54 | –1.52 | 0.66 | –2.30 | .02 |
| 55+ | –0.94 | 0.75 | –1.25 | .21 |
| FEV1 % predicted during PY | ||||
| 40-69 | 0.31 | 0.64 | 0.49 | .63 |
| 70-89 | 0.66 | 0.63 | 1.04 | .30 |
| > 90 | 1.49 | 0.79 | 1.88 | .06 |
| Age during PY, y | ||||
| 25-34 | 0.48 | 0.51 | 0.94 | .35 |
| 35-44 | –0.72 | 1.13 | –0.63 | .53 |
| 45-54 | 0.85 | 0.88 | 0.97 | .33 |
| 55+ | 0.97 | 0.81 | 1.20 | .23 |
Poisson mixed model explaining the number of exacerbations by year adjusted for ETI use, time, sex, BMI, year, lung function cohort, age group, and the interaction between year and lung function cohort and between age group and year. A random slope over time was included for each participant. No significant difference in exacerbation rate was found in the PPY or PY (0.065 /person/y vs 0.054/person/y, respectively; z = 0.41; P = .68). Moderate exacerbations differed by sex, with female patients experiencing 2.2 times as many exacerbations (0.088/person/y; sum = 96) as male patients (0.040/person/y; sum = 38; z = 2.18; P = .03). CF = cystic fibrosis; ETI = elexacaftor/tezacaftor/ivacaftor PPY = prepandemic year; PY = pandemic year.
Table 4.
Effect of Sex and Year on Exacerbation Rate
| Effect of Sex |
Effect of Year |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sex | Rate | SE | z Ratio | P Value | Year | Rate | SE | z Ratio | P Value |
| Female | 0.088 | 0.03 | 2.18 | .03 | PPY | 0.065 | 0.02 | 0.41 | .68 |
| Male | 0.040 | 0.02 | ... | ... | PY | 0.054 | 0.02 | ... | ... |
Table 5.
Poisson Mixed Model Explaining Antibiotic Episodes per Year in Adults With CF
| Term | Estimate | SE | z Ratio | P Value |
|---|---|---|---|---|
| Intercept | 0.21 | 0.45 | 0.48 | .63 |
| Quarter of y | –0.10 | 0.05 | –1.92 | .05 |
| Male sex | –0.39 | 0.16 | –2.38 | .02 |
| BMI | 0.01 | 0.01 | 0.52 | .60 |
| Patient is taking ETI | –0.09 | 0.17 | –0.51 | .61 |
| FEV1 % predicted | ||||
| 40-69 | –0.29 | 0.25 | –1.16 | .25 |
| 70-89 | –0.39 | 0.25 | –1.56 | .12 |
| > 90 | –0.74 | 0.30 | –2.48 | .01 |
| During PY | –0.82 | 0.40 | –2.08 | .04 |
| Age group, y | ||||
| 25-34 | 0.07 | 0.21 | 0.33 | .74 |
| 35-44 | –0.26 | 0.26 | –0.99 | .32 |
| 45-54 | –0.54 | 0.30 | –1.78 | .08 |
| 55+ | –0.56 | 0.38 | –1.47 | .14 |
| FEV1 % predicted during PY | ||||
| 40-69 | 0.02 | 0.33 | 0.06 | .95 |
| 70-89 | 0.20 | 0.33 | 0.62 | .54 |
| > 90 | 0.32 | 0.40 | 0.81 | .42 |
| Age group during PY | ||||
| 25-34 | 0.05 | 0.28 | 0.19 | .85 |
| 35-44 | –0.14 | 0.39 | –0.36 | .72 |
| 45-54 | 0.48 | 0.41 | 1.18 | .24 |
| 55+ | 0.47 | 0.49 | 0.95 | .34 |
Poisson mixed model explaining the number of antibiotic episodes per person per year adjusted for time, sex, BMI, and ETI use, year, lung function cohort, age group, and the interaction between lung function cohort and year and between age group and year. A random slope over time was included for each participant. After adjusting for ETI use and other variables, use of antibiotics decreased from 0.612/person/y during the PPY to 0.366/person/y during the PY (z = 2.31; P = .02). CF = cystic fibrosis; ETI = elexacaftor/tezacaftor/ivacaftor; PY = pandemic year.
Table 6.
Effect of Year on Annual Rate of Antibiotic Use per Person
| Year | Rate | SE | z Ratio | P Value |
|---|---|---|---|---|
| PPY | 0.612 | 0.089 | 2.31 | .02 |
| PY | 0.366 | 0.062 | ... | ... |
PPY = prepandemic year; PY = pandemic year.
Figure 4.
A-C, Bar graphs showing exacerbation rates. A, Exacerbation rate declined during the PY from 0.133 exacerbations/person/y to 0.053 exacerbations/person/y (P < .01). B, Exacerbation rates adjusted for ETI therapy and other variables demonstrated no significant difference in the PPY vs PY (0.065 vs 0.054 exacerbations/person/y; P = .68). C, All antibiotic use, including both IV and oral antibiotics, adjusted for ETI therapy and other variables, decreased from 0.612 occurrences/person/y during the PPY to 0.366 occurrences/person/y during in PY (P = .02). ETI = elexacaftor/tezacaftor/ivacaftor; PPY = prepandemic year; PY = pandemic year.
Preservation of BMI
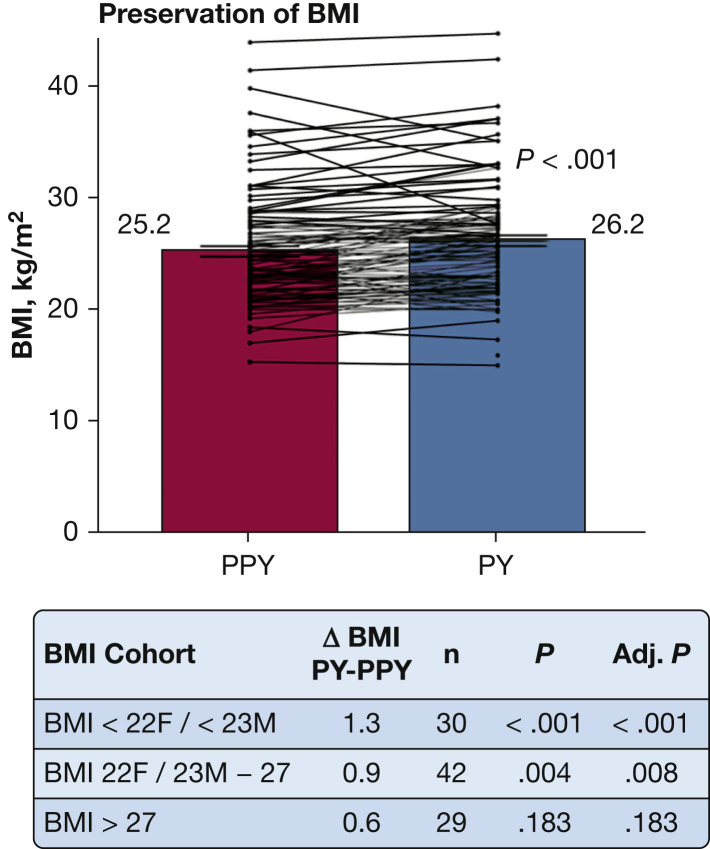
After excluding patients who were pregnant during the study period (n = 6) and patients who did not have BMI measurements in both the PPY and PY (n = 3), 101 patients were included in the BMI analyses (Fig 5 ). BMI was not adjusted for ETI because of lack of post-ETI data before the start of the pandemic. Overall, BMI increased during the PY, with mean BMI of 25.2 kg/m2 in the PPY and 26.2 kg/m2 in the PY (t 100 = –4.72; P < .001). Subgroup analysis by BMI cohort demonstrated that for patients whose BMI was less than their goal (BMI < 22 kg/m2 for women and < 23 kg/m2 for men) and for patients meeting their BMI goal (22-27 kg/m2 for women and 23-27 kg/m2 for men), a significant increase in BMI was observed during the pandemic period (t 29 = –4.57 [P < .001, adjusted] and t 41 = –3.03 [P = .008, adjusted], respectively). For patients with BMI > 27 kg/m2, no significant change was observed during the PY (t 28 = –1.37; P = .183, adjusted).
Figure 5.
Graph showing preservation of BMI during the PY. An increase in BMI was observed during the pandemic period, with mean BMI of 25.2 kg/m2 during the PPY and 26.2 kg/m2 during the PY (n = 101; P < .001). Six patients were excluded because of pregnancy and 1 because of missing data. BMI was not adjusted for elexacaftor/tezacaftor/ivacaftor (ETI) treatment because of lack of data before and after ETI treatment. Lines show each patient’s change. Subgroup analysis for BMI less than the patient’s goal (BMI < 22 kg/m2 for women or < 23 kg/m2 for men) and for BMI at goal (BMI of 22-27 kg/m2 for women and 23-27 kg/m2 for men) demonstrated significant improvement during the PY (P < .001 and P = .008, Adj., respectively). For BMI of more than the patient’s goal, no significant change in BMI was observed during the PY (P = .183, Adj.). Adj. = adjusted; F = female; M = male; PPY = prepandemic year; PY = pandemic year.
Discussion
Rapid implementation of a CF clinical care model that included interdisciplinary telemedicine during the COVID-19 pandemic at the University of Virginia was associated with preservation of lung function and BMI, stability in the rate of pulmonary exacerbations, and decreased use of antibiotics. The introduction of ETI in October 2019 improved lung function and exacerbation rates, but introduced a major confounder to clinical outcome analysis during the PY. A significant improvement in lung function was observed initially in the PY, which we attribute to swift deployment of ETI therapy in the patient population.8 After adjusting for modulator therapy, we identified no significant change in lung function or exacerbation rates during the pandemic. Nonetheless, it is important to recognize that ETI use likely contributed to overall clinical stability in this patient population during the PY. The clinical outcomes during the pandemic suggest that the UVA clinical care model that includes IDC-TM with PVP and HS is comparable with the classical in-person CF care model used before the pandemic for monitoring lung function, BMI, and exacerbations during the COVID-19 pandemic. Moreover, this care model may offer significant advantages over the model used before the pandemic. One compelling finding is that the use of all antibiotics declined significantly, even after adjusting for modulator use. Although these findings suggest that social mitigation may play a protective role in CF, the use of ETI as a stabilizing therapy also is likely a factor. Combined, these observations suggest that, with appropriate PVP, adult CF clinical care with IDC-TM is an effective model for implementing guidelines-based CF care and that social mitigation strategies may decrease antibiotic overexposure.
An important consideration in the success of this model is the use of PVP and triage. PVP is not standardized, and practice varies by CF center. The UVA adult CF PVP process consists of communication with patients up to 1 week before scheduled clinical visits. Patients are contacted by phone or by secure health system messaging. Patients are encouraged to provide a patient-driven agenda and are asked about acute needs, including changes in insurance, refill requests, and health questions to screen for potential exacerbations. With the transition to telemedicine, PVP was adjusted to include prescreening questions to determine appropriateness for telemedicine as described above. We believe that appropriate PVP is a critical factor in the success of a care model that includes IDC-TM. In-person or hybrid visits triggered by PVP screening accounted for the diagnosis of nearly two-thirds of all exacerbations. The remainder of diagnosed exacerbations were missed during PVP screening and were identified during telemedicine clinic encounters with HS, indicating that PVP alone is not sufficient for identifying all exacerbations.
In the first PY, a total of three patients had COVID-19 (2%), significantly lower than the general US population estimated at 8%.9 Two were treated for exacerbation and recovered, whereas one experienced no pulmonary symptoms. The patient population also saw a dramatic reduction in influenza. In contrast to the previous year in which 14 exacerbations were the result of confirmed or suspected influenza, no cases of influenza occurred in the PY. This observation could be attributed to the protective role of social mitigation and widespread mask use during the PY, overestimation of influenza diagnosis and exposure in previous years, or both.
A modest increase in BMI occurred during the pandemic period. Subgroup analysis revealed that this effect was seen primarily in patients who were meeting the recommended BMI or below-recommended BMI. We attribute this primarily to the introduction of ETI therapy in the patient population, although quarantine-associated weight gain, which has been observed in the general population, also may have played a role.10 One limitation of this study is the use of home scales for self-reported weight measurements, which may not correlate with the scale used in the clinic. BMI was not calculated for patients who were unwilling or unable to measure weight at home using a scale. BMI analysis was not adjusted for ETI use because of a lack of data for all patients before and after ETI therapy. Patients whose BMI was more than their goal did not show a significant change in weight during the pandemic, suggesting that the overall increase in BMI may be attributed to ETI use, rather than lockdown-associated weight gain.
A significant strength of this study is the rapid implementation of telemedicine; no clinic days were canceled as a result of the COVID-19 pandemic because of transition to telemedicine, and adherence to guidelines-based recommendations for frequency of visits was maintained. Telemedicine use, either by telemedicine visits or in hybrid visits with in-clinic telemedicine support, accounted for 92% of all clinical encounters during the PY.
Another strength is our relatively large patient population for a single center with high buy-in for telemedicine and HS. Although 86% of the patients consented to data collection for this study, additional patients requested and used telemedicine care. This included patients who were unable to be reached during the enrollment window, patients who joined the clinic later in the year, and patients who declined to participate in data collection. More than 90% of the patient population used some form of telemedicine care during the year, although as a single-center study, it is difficult to draw conclusions on the generalizability of these results. Nonetheless, in the PPY, our center reported similar rates of exacerbation, lung function, and BMI compared with national data, suggesting external validity.11 One limitation of both in-person and IDC-TM clinical models is that accuracy for adherence to pulmonary clearance therapies and other medications may be underreported, especially after the widespread implementation of ETI, and may influence outcomes.
One advantage of this model compared with previously published work is our interdisciplinary approach to HS. Paynter et al12 recently published their investigation comparing twice-weekly HS with standard of care. This study highlighted the enormous potential of HS, but also the pitfalls and limitations of its implementation. Patients were provided in-person initial instruction on HS, but no maintenance instruction of spirometry technique over the course of the study, and were encouraged to provide HS readings twice per week. Over the course of the investigation, the authors observed that adherence to HS plummeted and accuracy of HS readings deteriorated compared with in-laboratory results. This study underscores the importance of technique and proper coaching by an RT, as well as acknowledgement of burnout on the part of the patient. One significant difference in our IDC-TM model is the use of one-on-one coaching by the RT to ensure accuracy of HS results, which were obtained under direct observation at the time of the clinical encounter.
To our knowledge, this is the first study to report on real-world clinical outcomes in CF using a clinical care model that incorporates previsit screening, interdisciplinary telemedicine, and quality-controlled HS. One challenge in CF, as well as many other chronic systemic diseases, is the need for interdisciplinary care and frequent disease-specific monitoring. Future direction includes a multicenter randomized controlled outcomes-based trial comparing a care model that includes IDC-TM with the classical in-person care model. The Early Intervention in Cystic Fibrosis Exacerbation study, a multicenter randomized controlled trial, previously compared the use of twice-weekly HS and self-reported symptom diaries with the usual care model.13 The authors concluded that regular, frequent monitoring of self-reported symptoms and HS increased detection of CF exacerbations; however, increased detection did not slow the decline in lung function over the 52-week period. Notably, the Early Intervention in Cystic Fibrosis Exacerbation study was completed before the introduction of ETI therapy, which has been shown to stabilize lung function and decrease exacerbations.14 An advantage to the care model described in our study is the near-seamless incorporation of guidelines-based care, specifically regarding frequency of clinical encounters, spirometry, and interdisciplinary evaluations. We anticipate that this model would have higher clinic retention and adherence in the long term compared with an Early Intervention in Cystic Fibrosis Exacerbation-style intervention.
Telemedicine broadly is associated with decreased travel costs, decreased time off from work or school, and high patient and provider satisfaction.15 With the widespread adoption of telehealth practices during the COVID-19 pandemic and the many advantages it offers, we anticipate that telemedicine is here to stay.16 However, the advantages of telemedicine cannot come at the cost of quality care. To the best of our knowledge, this study is the first to demonstrate longitudinal real-world clinical outcomes in patients with CF using an adult CF clinical care model that includes IDC-TM, appropriate previsit screening, and properly coached HS as an alternative to the classical in-person clinic model.
Interpretation
The authors previously demonstrated that implementation of telemedicine during the COVID-19 pandemic reduced patient and staff interactions and, by doing so, preserved personal protective equipment.17 Herein, we demonstrated that real-world clinical outcomes of a clinical care model with IDC-TM are similar to the classical clinical care model and that in the time of a pandemic, this model may offer significant advantages to in-person care. We conclude that at this large single center, the adult CF clinical care model that includes IDC-TM, appropriate previsit screening, and properly coached HS is a feasible alternative to the classical in-person clinic model used before the pandemic for maintaining lung function and BMI and identifying CF pulmonary exacerbations. Moreover, we observed a significant decrease in the overall use of antibiotics during the pandemic, suggesting that social mitigation plays a strong role in prevention of pulmonary exacerbations.
Acknowledgments
Author contributions: L. A. L. S. and D. P. A. accept official responsibility for the integrity of the manuscript, including ethics, data handling, reporting of results, and study conduct. All statements in this manuscript are true to the authors’ knowledge. L. A. L. S. is the primary author of original manuscript and coinvestigator and performed primary analysis and interpretation of data, visualization, project administration including methodology and investigation, and final approval of manuscript. R. P. L. performed project conceptualization, project administration including methodology, acquisition of data, and writing and revisions of intellectual content. M. H. C. performed project conceptualization, project administration including methodology, and acquisition of data. D. J. performed project conceptualization, project administration including methodology, acquisition of data, and analysis and interpretation of data. M. K. J. performed statistical consultation, mixed model statistical analyses, figure and table generation, and critical revision for intellectual content. H. M. B. performed project conceptualization, project administration including methodology, acquisition of data, and revisions for intellectual content. R. K. M. performed project conceptualization, project administration including methodology, and acquisition of data. E. R. S. performed project conceptualization, project administration, acquisition of data, and revisions for intellectual content. K. M. W. performed demographic data acquisition. L. S. G. performed project conceptualization, administration including methodology, and revisions for intellectual content. D. P. A. was the principal investigator and performed primary conceptualization, project administration including methodology, data acquisition and investigation, critical revision for intellectual content, and final approval of manuscript.
Financial/nonfinancial disclosures: None declared.
Othercontributions: The authors thank their patient partners Lauren Williamson and Jason Conyers for their input on creating the telemedicine clinic flow process, the UVA Telemedicine Group for technology support, and Rachel Turner, team administrator, for her dedication to our patients and team. The authors would like to thank Drs Daniel J. O’Hearn, MD, FCCP, and Imre Noth, MD, for their expertise and assistance throughout this study. The authors would also like to thank Dr Bruce Marshall, MD, the Cystic Fibrosis Foundation, and the Cystic Fibrosis Learning Network for their support for our center.
Footnotes
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
References
- 1.Koonin L.M., Hoots B., Tsang C.A., et al. Trends in the use of telehealth during the emergence of the COVID-19 pandemic—United States, January–March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(43):1595–1599. doi: 10.15585/mmwr.mm6943a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cystic Fibrosis Foundation . 2021. Understanding changes in life expectancy.https://cff.org/Research/Researcher-Resources/Patient-Registry/Understanding-Changes-in-Life-Expectancy/ Cystic Fibrosis Foundation website. [Google Scholar]
- 3.Wood J., Mulrennan S., Hill K., Cecins N., Morey S., Jenkins S. Telehealth clinics increase access to care for adults with cystic fibrosis living in rural and remote Western Australia. J Telemed Telecare. 2017;23(7):673–679. doi: 10.1177/1357633X16660646. [DOI] [PubMed] [Google Scholar]
- 4.Compton M., Soper M., Reilly B., et al. A feasibility study of urgent implementation of cystic fibrosis multidisciplinary telemedicine clinic in the face of COVID-19 pandemic: single-center experience. Telemed J E Health. 2020;26(8):978–984. doi: 10.1089/tmj.2020.0091. [DOI] [PubMed] [Google Scholar]
- 5.Al-Hasan A., Yim D., Khuntia J. Citizens’ adherence to COVID-19 mitigation recommendations by the government: a 3-country comparative evaluation using web-based cross-sectional survey data. J Med Internet Res. 2020;22(8) doi: 10.2196/20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cystic Fibrosis Foundation . 2021. Adult care clinical guidelines.https://www.cff.org/Care/Clinical-Care-Guidelines/Age-Specific-Clinical-Care-Guidelines/Adult-Care-Clinical-Care-Guidelines/ Cystic Fibrosis Foundation website. [Google Scholar]
- 7.Barr R.G., Stemple K.J., Mesia-Vela S., et al. Reproducibility and validity of a handheld spirometer. Respir Care. 2008;53(4):433–441. [PMC free article] [PubMed] [Google Scholar]
- 8.Heijerman H.G.M., McKone E.F., Downey D.G., et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial [published correction appears in Lancet. 2020;395(10238):1694] Lancet. 2019;394(10212):1940–1948. doi: 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention 2021. COVID data tracker. 2021. Centers for Disease Control and Prevention website. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100k
- 10.Zachary Z., Brianna F., Brianna L., et al. Self-quarantine and weight gain related risk factors during the COVID-19 pandemic. Obes Res Clin Pract. 2020;14(3):210–216. doi: 10.1016/j.orcp.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cystic Fibrosis Foundation . 2021. 2019 Patient registry annual data report.https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2019-Patient-Registry-Annual-Data-Report.pdf Cystic Fibrosis Foundation website. [Google Scholar]
- 12.Paynter A, Khan U, Heltshe SL, Goss CH, Lechtzin N, Hamblett NM. A comparison of clinic and home spirometry as longitudinal outcomes in cystic fibrosis [published online ahead of print August 30, 2021]. J Cyst Fibros. https://doi.org/10.1016/j.jcf.2021.08.013. [DOI] [PMC free article] [PubMed]
- 13.Lechtzin N., Mayer-Hamblett N., West N.E., et al. Home monitoring of patients with cystic fibrosis to identify and treat acute pulmonary exacerbations. eICE study results. Am J Respir Crit Care Med. 2017;196(9):1144–1151. doi: 10.1164/rccm.201610-2172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duckers J., Lesher B., Thorat T., et al. Real-world outcomes of ivacaftor treatment in people with cystic fibrosis: a systematic review. J Clin Med. 2021;10(7):1527. doi: 10.3390/jcm10071527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen M., Waller M., Pandya A., Portnoy J. A review of patient and provider satisfaction with telemedicine. Curr Allergy Asthma Rep. 2020;20(11):72. doi: 10.1007/s11882-020-00969-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Telehealth is here to stay. Nat Med. 2021;27(7):1121. doi: 10.1038/s41591-021-01447-x. [DOI] [PubMed] [Google Scholar]
- 17.List R., Compton M., Soper M., et al. Preserving multidisciplinary care model and patient safety during reopening of ambulatory cystic fibrosis clinic for nonurgent care: a hybrid telehealth model. Telemed J E Health. 2021;27(2):193–199. doi: 10.1089/tmj.2020.0247. [DOI] [PubMed] [Google Scholar]