Abstract
The Saccharomyces cerevisiae p21-activated kinases, Ste20p and Cla4p, have individual functions but appear to share an essential function(s) as well because a strain lacking both kinases is inviable. To learn more about the shared function, we sought new mutations that were lethal in the absence of CLA4. This approach led to the identification of at least 10 complementation groups designated NCS (need CLA4 to survive). As for ste20 cla4-75 mutants, most ncs cla4-75 double mutants were defective for septin localization during budding. One group, NCS1/RRD1 (YIL153w), did not confer this defect, however, and we investigated its function further. ncs1Δ cla4Δ cells arrested with elongated buds and short mitotic spindles. The morphological defects and lethality were suppressed by mutations that abrogate the cell cycle morphogenetic checkpoint, CDC28Y19F or swe1Δ. The connection to the cell cycle may be direct, as we detected a Cla4p-Cdc28p complex. NCS1 encodes a protein with significant similarity to a mammalian phosphotyrosyl phosphatase activator (PTPA) regulatory subunit for type 2A protein phosphatases (PP2As). Genetic and biochemical evidence suggested that the phosphatase Sit4p is a target for Ncs1p. First, CLA4 and SIT4 were synthetically lethal. Second, Ncs1p and its yeast paralog, Noh1p (Rrd2p), bound to the catalytic domain of Sit4p in vitro, and Ncs1p could be immunoprecipitated with Sit4p but not with another PP2A (Pph21p) from yeast cell extracts. Strains lacking both NCS1 and NOH1 were inviable and arrested as unbudded cells, implying that PTPA function is required for proper G1 progression.
The yeast Saccharomyces cerevisiae contains three related protein kinases that are members of the PAK (p21-activated kinase) family, proteins that interact with, and presumably are regulated by, Cdc42p, a p21 GTPase required to establish polarity of the actin cytoskeleton (9, 17). The roles of these PAKs are seemingly quite different. In haploid cells, Ste20p participates in at least three signal transduction pathways: the pheromone response pathway (22, 23, 37), the invasive growth pathway (42), and the HOG pathway (35). Cla4p plays a role, albeit a poorly understood one, in the budding process (2, 7, 15); no specific function has yet been established for Cla4p. The third PAK, Skm1p, is expressed only in meiotic cells (29). Surprisingly, given their apparent distinct functions, loss of both STE20 and CLA4 is lethal (7), suggesting that these two protein kinases share an essential function(s). To investigate this essential function, especially as it relates to STE20, we carried out a screen for mutants that are lethal in a cla4Δ mutant background. This effort has identified 10 complementation groups (NCS, for need CLA4 to survive). Here we report on one of these groups, which encodes a regulatory subunit for type 2A protein phosphatases (PP2As).
There are five known members of the yeast PP2A family (54). PP2As are multimeric enzymes capable of catalyzing the hydrolysis of phosphate groups from phosphoseryl, phosphothreonyl, and phosphotyrosyl moieties. The catalytic (C) subunits are encoded by PPH21, PPH22, PPH3, SIT4, and PPG1 and share as much as 86% sequence similarity at the amino acid level (54). Enzymatic activity, substrate specificity, and subcellular localization are modulated through the interaction of the C subunit with an army of regulatory subunits to form a trimeric holoenzyme. Tpd3p is thought to be the A subunit, based on homology to the mammalian counterpart and on the ability to interact physically with at least three of the yeast PP2A C subunits (8, 34). However, the formation of a Tpd3p-PP2A dimeric core complex has not been demonstrated. In mammals, the dimeric core complex interacts with one of several B-type regulatory subunits, B (PR55), B′ (PR61), and B" (PR72) (32). In yeast, only the B (Cdc55p) (14) and B′ (Rts1p) (48, 57) subunits have been identified. Finally, the activity of some PP2As can be altered by a different type of regulatory subunit, a phosphotyrosyl phosphatase activator (PTPA), which stimulates the phosphotyrosyl phosphatase activity of PP2A C subunits in vitro (5, 56). S. cerevisiae has two putative PTPA subunits, encoded by YIL153w and YPL152w (39). Given the single A subunit, two B-type subunits, five C subunits, and two PTPA subunits, 30 PP2A holoenzymes could in principle be present in the yeast cell. With such a wide array of possible holoenzymes, it is not surprising that the molecular and cellular mechanisms of PP2A function are poorly understood.
Here we describe the identification and characterization of NCS1, which encodes a protein related to mammalian PTPA subunits. NCS1 (PTPA1) has previously been shown to play a role in lowering the mutagenesis rate in cells treated with known DNA mutagens (38) and is allelic to RRD1 (39). We find that NCS1 also plays a role at the G2/M transition. Cells lacking NCS1 and CLA4 arrest with grossly elongated buds, a phenotype that appears to be an exacerbation of the G2 delay observed in cells lacking CLA4. The abnormal morphology of cla4Δ and ncs1Δ cla4Δ strains and the lethality of ncs1Δ cla4Δ strains can be overcome by changing the tyrosyl residue at position 19 of Cdc28p to phenylalanine, mimicking the activated state of Cdc28p, or by deleting the Cdc28p regulatory kinase, SWE1. Yeast has a second PTPA homolog, encoded by YPL152w (also designated NOH1 [NCS1 homolog], PTPA2, and RRD2) (38, 39). Deletion of both NCS1 and NOH1 is lethal and results in the accumulation of unbudded, uninuclear cells, demonstrating that PTPA function is important for bud emergence. Both Ncs1p and Noh1p bind to the catalytic domain of Sit4p, a PP2A-like protein phosphatase that plays a role in the regulation of genes expressed late in the G1 phase of the cell cycle and also in bud emergence (11, 55). Thus, Ncs1p and Noh1p may regulate Sit4p activity and impinge on events that happen in late G1 and at the G2/M transition of the cell cycle.
MATERIALS AND METHODS
Microbiological techniques.
Yeast and bacterial strains were propagated by standard methods (47). Bacterial transformations, DNA preparations, and plasmid constructions were performed by standard methods (45). Yeast transformations were performed by the Li+ ion method (16). Samples for fluorescence-activated cell sorting (FACS) were prepared as described by Ma et al. (28) and assayed using a Becton Dickinson FACScan apparatus. Yeast extract-peptone-dextrose (YEPD) and synthetic medium supplemented with dextrose (SD) were prepared as described by Kaiser et al. (18). DNA-modifying enzymes were purchased from New England Biolabs, Inc. (Beverly, Mass.). Unless stated otherwise, all other reagents were purchased from Sigma Chemical Company (St. Louis, Mo.).
Yeast strains.
The yeast strains used in this study are listed in Table 1. All strains except Y3389 and SY3390 are congenic to the S288C genetic background, using YPH499 and YPH500 (51) as the initial parents. The ADE8 locus of YPH499 (and YPH500) was disrupted using the two-step approach by inserting pSL2534, which contains a disruption of the ADE8 open reading frame (ORF) marked by URA3, and then selecting for white, ade2-101 ade8Δ colonies on 5′-fluoroorotic acid (5′-FOA). The FUS1-lacZ reporter gene was inserted at the MFA2 locus using pSL1580 (13) to create SY3537 and SY3358. The his3-Δ200 and lys2-801 loci of SY3537 and SY3358 were repaired by a single-step gene replacement using the wild-type HIS3 and LYS2 genes, creating SY3359 and SY3357, respectively. Single-step gene disruptions were performed using DNA fragments generated either by PCR (1) or by digestion of the relevant plasmid (43).
TABLE 1.
Yeast strains used
| Straina | Genotype |
|---|---|
| SY3357 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ |
| SY3358 | MATα leu2-Δ1 ura3-52 his3-Δ200 lys2-801 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ |
| SY3359 | MATα leu2-Δ1 ura3-52 lys2-801 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ |
| SY3360 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 |
| SY3361 | MATα leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 |
| SY3362 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 <pRS316ADE8CLA4> |
| SY3363 | MATα leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 <pRS316ADE8CLA4> |
| SY3364 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 ncs1Δ::LEU2 <pRS316ADE8CLA4> |
| SY3365 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 ncs1Δ::LEU2 <YCpHIS3cla4-75> |
| SY3366 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 ncs2-1 <pRS316ADE8CLA4> |
| SY3367 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 ncs3-1 <pRS316ADE8CLA4> |
| SY3368 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 ncs4-1 <pRS316ADE8CLA4> |
| SY3369 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 ncs5-1 <pRS316ADE8CLA4> |
| SY3370 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 ncs6-1 <pRS316ADE8CLA4> |
| SY3371 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 ncs7-1 <pRS316ADE8CLA4> |
| SY3372 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 ncs8-1 <pRS316ADE8CLA4> |
| SY3373 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 ncs10-1 <pRS316ADE8CLA4> |
| SY3376 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 ste20Δ::TRP1 <YCpHIS3cla4-75> |
| SY3377 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 cln1Δ::URA3 cln2Δ::LEU2 <YCpHIS3cla4-75> |
| SY3378 | MATα leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 ncs1Δ::HIS3 <pRS316ADE8CLA4> |
| SY3379 | MATa leu2-Δ1 ura3-52::cla4ΔPAK-MYC::URA3 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 ncs1Δ::LEU2 <YCpHIS3cla4-75> |
| SY3380 | MATa leu2-Δ1 ura3-52::cla4ΔPAK-MYC::URA3 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 <YCpHIS3cla4-75> |
| SY3382 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ ncs1Δ::LEU2 noh1Δ::HIS3 <pRS414ncs1-2> |
| SY3383 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ ncs1Δ::LEU2 noh1Δ::HIS3 <pRS414NCS1::3xMYC> |
| SY3384 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ ncs1Δ::LEU2 cla4Δ::HIS3 <pRS414ncs1-2> |
| SY3385 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ pph21Δ::LEU2 |
| SY3386 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ pph22Δ::URA3 |
| SY3387 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ ppg1Δ::HIS3 |
| SY3388 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ pph3Δ::HIS3 |
| SY3389b | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ sit4Δ::HIS3 |
| SY3390 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cdc28::CDC28Y19F::URA3 |
| SY3391 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 cdc28::CDC28Y19F::URA3 |
| SY3392 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 swe1::LEU2 |
| SY3393 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ mih1Δ::HIS3 |
| SY3394 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ ncs1Δ::HIS3 |
| SY3396 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ ncs1Δ::LEU2 |
| SY3397 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ mih1Δ::HIS3 ncs1Δ::LEU2 |
| SY3398 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 mih1Δ::HIS3 |
| SY3399 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ ssd1Δ::URA3 |
| SY3400 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cln3Δ::HIS3 |
| SY3403 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ ncs1Δ::HIS3 cla4Δ::TRP1 swe1::LEU2 |
| SY3404 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ ncs4-1 cla4Δ::TRP1 swe1::LEU2 |
| SY3408 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 <YCpHIS3cla4-75> |
| SY3409 | MATa leu2-Δ1 ura3-52::CLA4-MYC::URA3 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ |
| SY3413 | MATα leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ noh1Δ::HIS3 |
| SY3504 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 cln1::HIS3 cln2::HIS3 |
| SY3505 | MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ cla4Δ::TRP1 cln1::HIS3 cln2::HIS3 swe1::LEU2 |
| SY3537 | MATa leu2-Δ1 ura3-52 his3-Δ200 lys2-801 trp1-Δ63 ade8Δ ade2-101 mfa2-Δ1::FUS1-lacZ |
All are derivatives of YPH499 and YPH500 (S288C) (51).
In cases where alleles had to be crossed into the strain background, at least four backcrosses were performed.
Deoxyoligonucleotides (Keystone Laboratories, Camarillo, Calif.) were used to synthesize DNA fragments containing 40 bp of sequence 5′ to the ORF (as ascertained from the Saccharomyces genome database), a selectable marker, and 40 bp of sequence 3′ to the ORF by PCR. The pRS series of plasmids served as templates to provide the selectable marker DNA (51). The products of these reactions were used to make gene deletions through single-step gene replacements in SY3537, SY3357, SY3358, or SY3359. Plasmid-based single-step gene knockouts were performed using pEL46-2 (ste20::TRP1) (22), pSL2680 (cln1::URA3), pSL2681 (cln2::LEU2), AS3g (pph21::LEU2) (52), AS6g (pph22::URA3) (52), and DLB333 (swe1::LEU2) (50). pBB131 (CLA4-MYC) and pBB138 (cla4ΔPAK-MYC) (2) were inserted at the URA3 locus. Deletions were confirmed by PCR-based chromosome analysis and phenotypic analysis.
Plasmid construction.
Plasmids used are listed in Table 2. pRS315ADE8 was constructed by inserting a 2.0-kb EcoRI/HindIII ADE8-containing fragment from pSL2535 (constructed by Charlie Boone) into a similarly digested pRS315 vector. A 4.0-kb NruI/SacI CLA4 genomic fragment from pATL3 (gift from Fred Cross) (2) was first made blunt using the Klenow fragment then inserted into SmaI-cut pRS315ADE8 to create pRS315ADE8CLA4. pRS316ADE8CLA4 was generated by inserting the 6.0-kb HindIII/XbaI fragment from pRS315ADE8CLA4 into a similarly cut pRS316 vector. CLA4 knockout plasmids pKScla4Δ::HIS3 and pKScla4Δ::TRP1 were constructed using homologous recombination (1, 27) by first replacing the ORF of a CLA4 gene, harbored in plasmid YEp13CLA4, with HIS3 or TRP1 (data not shown). The cla4 null allele was then amplified by PCR using deoxyoligonucleotides containing BamHI sites at their 5′ ends. The PCR product was inserted into the BamHI site of pKS (Stratagene). Plasmid YEpURA3ADH-SIT4-HA was created using homologous recombination to replace the LEU2 gene of YEpLEU2ADH-SIT4-HA (8) with URA3 (1, 27). Likewise, YCpHIS3cla4-75 was generated from YCpTRP1cla4-75 (7) by using homologous recombination to replace TRP1 with HIS3.
TABLE 2.
Plasmids used
| Plasmid name | Marker/yeast origin | Plasmid no. | Source or reference |
|---|---|---|---|
| pRS315ADE8 | LEU2, ADE8/CEN | pSL2672 | This study |
| pRS315ADE8CLA4 | LEU2, ADE8/CEN | pSL2673 | This study |
| pRS315ADE8cla4K549R | LEU2, ADE8/CEN | pSL2677 | Megan Keniry |
| pRS316ADE8CLA4 | URA3, ADE8/CEN | pSL2674 | This study |
| YCpTRP1cla4-75 | TRP1/CEN | 7 | |
| YCpHIS3cla4-75 | HIS3/CEN | pSL2679 | This study |
| pKScla4Δ::TRP1 | pSL2664 | This study | |
| pKScla4Δ::HIS3 | pSL2663 | This study | |
| pRS415NCS1-3xMYC | LEU2/CEN | pSL2665 | This study |
| pRS414NCS1-3xMYC | TRP1/CEN | pSL2670 | This study |
| pRS414ncs1-2 | TRP1/CEN | pSL2671 | This study |
| YEp24PPH21-HA | URA3/2μm | 8 | |
| YEpURA3ADH-SIT4-HA | URA3/2μm | pSL2667 | This study |
| YEp13NCS1 | LEU2/CEN | pSL2680 | This study |
| YEp13NCS1-3xMYC | LEU2/2μm | pSL2678 | This study |
| pGAL-3xHA-NCS1 | URA3/CEN | pSL2675 | This study |
| pGAL-3xHA-NOH1 | URA3/CEN | pSL2676 | This study |
| pMBP-SIT4 | pSL2666 | This study | |
| pMBP-SIT4(N) | pSL2669 | This study | |
| pMBP-SIT4(C) | pSL2668 | This study | |
| pRSQ306M | URA3 | pSL2682 | This study |
| pSF19(CDC28-HA) | TRP1 | 53 |
YEp13NCS1 was constructed by inserting a PCR product that encompassed 276 bp upstream and 135 bp downstream of the NCS1 putative start and termination codons. The deoxyoligonucleotides used to generate the approximately 1.6-kb fragment contained flanking BglII sites which permitted insertion into the BamHI site of YEp13. YEp13NCS1-3xMYC was created by the deoxyoligonucleotide-mediated method of Schneider et al. (46) and included the entire NCS1 ORF fused to the MYC triple-repeat sequence. To generate pRS415NCS1-3xMYC, an approximately 2.0-kb PCR fragment was amplified using YEp13NCS1-3xMYC as a template and the original NCS1 deoxyoligonucleotides as primers. The fragment was inserted into the BamHI site of pRS415. All plasmids were shown to complement the ncs1Δcla4Δ synthetic lethality. All fragments generated by PCR utilized a 5:1 mixture of TAQ (Promega, Madison, Wis.) and Vent (New England Biolabs) DNA polymerases.
Galactose-inducible triple-hemagglutinin (HA)-tagged yeast expression constructs pGAL-3xHA-NCS1 and pGAL-3xHA-NOH1 were created by generating blunt-ended ORFs by PCR using deoxyoligonucleotides specific for YIL153w and YPL152w (Research Genetics) and inserting the ORFs into p705-3 (10) that had been cut with HpaI. Both pGAL-3xHA-NCS1 and pGAL-3xHA-NOH1 could complement the synthetic lethality of an ncs1Δnoh1Δ yeast strain.
pmal-c2-SIT4 was created by PCR amplification of the SIT4 ORF from genomic DNA (SY3357), using deoxyoligonucleotides specific for the SIT4 ORF and containing flanking BamHI sites. The approximately 1.0-kb fragment was then inserted into the BamHI site of pmal-c2 (New England Biolabs). pmal-c2-SIT4(N) was constructed by partial digestion of pmal-c2-SIT4 using NcoI, which cuts once (bp 483) within the SIT4 ORF, filling in the 5′ overhang with the Klenow fragment of DNA polymerase I, and religating the newly formed blunt ends. pmal-c2-SIT4(C) was generated by partial digestion of pmal-c2-SIT4 using BglII followed by complete digestion using EcoRI. The free plasmid ends were made blunt using Klenow fragment, and the newly formed ends were religated.
Yeast mutagenesis and NCS screen.
Yeast cells were mutagenized by either of two methods. First, cells were spread onto YEP plates containing 8% dextrose (to improve red color development). Cells were radiated with UV light (75 μJ), which yielded 50% viability, and incubated at 30°C for 3 to 7 days. Cells were also mutagenized using the method and libraries of Burns et al. (4). The affected locus was identified essentially as described by Burns et al. (4) except that pRSQ306 (6) rather than YIp5 was inserted to retag and identify the locus.
Whole-cell extracts.
Yeast whole-cell extracts were prepared from mid-log-phase cultures (optical density at 600 nm [OD600] of 1.0 to 2.0). All manipulations were carried out at 4°C. Fifty-milliliter cultures were centrifuged, and the cells were suspended in lysis buffer (20 mM Tris-Cl [pH 7.5], 1 mM EDTA, 1× protease inhibitor cocktail [Boehringer Mannheim catalog no. 1697498], 1 mM phenylmethylsulfonyl fluoride, phosphatase inhibitors [25 mM sodium fluoride, 0.25 mM sodium orthovanadate, 15 mM sodium pyrophosphate, and 15 mM p-nitrophenyl phosphate]). In cases where detergent was required, the lysis buffer was supplemented with Triton X-100 to a final concentration of 0.5% (vol/vol). Yeast cells were broken by vortexing with glass beads. The extracts were centrifuged at 2,000 × g to remove the glass beads and unbroken cells.
For antiphosphotyrosine detection, Cdc28p was enriched from yeast whole-cell lysates using p13SUC1 protein-conjugated agarose. The precipitates were washed three times with lysis buffer, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% gel, and subjected to Western analysis. Cdc28p was detected using an antibody that recognizes the PSTAIRE amino acid sequence of Cdc28p (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.) at a dilution of 1/1,000. Cdc28p containing phosphotyrosine was detected using 4G18 (Upstate Biotechnology, Inc., Lake Placid, N.Y.) at a dilution of 1/1,000. Western blots were washed with 10 mM Tris-Cl (pH 7.5)–150 mM NaCl–0.2% Tween 20.
Protein concentrations were determined using the Bio-Rad (Hercules, Calif.) protein assay reagent as instructed by the manufacturer, with bovine serum albumin as a standard.
To prepare bacterial extracts, bacteria were centrifuged at 5,000 × g and resuspended in phosphate-buffered saline (50 mM phosphate, 150 mM NaCl [pH 7.0]) supplemented with 1 mM phenylmethylsulfonyl fluoride. Cells were broken by two passes through a French press apparatus, and the debris removed by centrifugation at 10,000 × g. Affinity chromatography using amylose resin (New England Biolabs) was performed in batch at 4°C with constant agitation for at least 1 h. After the incubation, the resin was washed three times with 10 bed volumes of phosphate-buffered saline.
In vitro binding assay.
Lysates were first cleared of particulate matter by centrifugation at 100,000 × g. Approximately 100 μg of total protein in a volume of 300 μl of lysis buffer was mixed with 50 μl (50% slurry) of maltose binding protein (MBP)–Sit4p-decorated amylose resin and incubated for 1 h at 4°C. After the incubation, the resin was washed four times with lysis buffer supplemented with 0.5% Triton X-100, resuspended in 50 μl of sample buffer, and subjected to SDS-PAGE (10% gel) (21); the proteins were detected by Western analysis.
Coimmunoprecipitation assays and Western blotting.
Yeast whole-cell extracts were normalized to a total protein concentration of 1 mg/ml. A 0.5-ml aliquot of the lysate was mixed with 20 μl of protein A-conjugated agarose (50% slurry) to remove material that binds nonspecifically to protein A-agarose. After removal of the agarose, the lysate was incubated for 1 h at 4°C with 20 μl of an anti-HA monoclonal antibody (12CA5) that had been cross-linked to protein A-agarose. The agarose beads were washed three times with lysis buffer and resuspended in 50 μl of sample buffer. One-third to one-half of the sample was analyzed by SDS-PAGE (10% gel). Proteins were transferred to nitrocellulose and detected using either anti-HA or anti-MYC monoclonal antibody. Horseradish peroxidase-conjugated goat anti-mouse secondary antibodies were used as instructed by the manufacturer (Bio-Rad). Signals were visualized by exposure to Kodak film (X-Omat AR) (typical exposure times were between 1 and 5 min).
Microscopy.
Yeast cells were grown in either YEPD or SD medium to a density of approximately 107 cells/ml. After centrifugation, the cell pellets were resuspended in either water or TE (10 mM Tris-Cl [pH 7.5], 1 mM EDTA) and visualized using a Zeiss Axioplan II photomicroscope with a 100× oil immersion objective. Antibodies were used at a dilution of 1/10 (α-Cdc3p; a generous gift from John Pringle) (19) or 1/20 (α-tubulinYOR1/34; a generous gift from John Chant) (36).
Generation of ncs1-2.
NCS1 mutants were generated essentially as described by Muhlrad et al. (33). Using deoxyoligonucleotides specific for the pRS415 polylinker, the NCS1 gene was amplified using pRS415NCS1-3xMYC as a template. The PCR mixtures contained 1× buffer (Promega), 2 mM MgCl2, 0.1 to 0.25 mM MnCl2, 1 μM each deoxyoligonucleotide, three deoxynucleoside triphosphates at 0.25 mM, and 1 deoxynucleoside triphosphate at 0.05 mM. The products of the reactions were subcloned into pRS414 using homologous recombination (27) in strain SY3414. The transformants were grown on medium supplemented with 5′-FOA to select for ncs1 mutants that could still complement the loss of NCS1 at 25°C. Temperature-sensitive alleles of NCS1 were identified from the 5′-FOAr subset of ncs1 mutants by their inability to complement the loss of NCS1 at 37°C.
RESULTS
Screen for mutations that are lethal in the absence of CLA4.
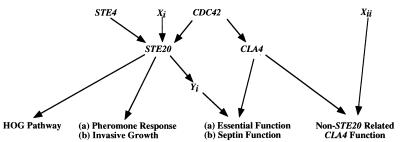
To identify potential activators or targets of the presumed STE20 essential function, we sought new mutations that are synthetically lethal with cla4Δ (Fig. 1). We used a variation of the red/white colony sectoring assay to identify synthetic lethal mutations (20). Two strains, SY3360 (MATa leu2-Δ1 ura3-52 his3-Δ200 trp1-Δ63 ade2-101 ade8Δ cla4Δ::TRP1) and SY3361 (MATα leu2-Δ1 ura3-52 lys2-801 trp1-Δ63 ade2-101 ade8Δ cla4Δ::TRP1), were transformed with pRS316ADE8CLA4, a low-copy-number plasmid, to create SY3362 (from SY3360) and SY3363 (from SY3361). ade2 ade8 colonies are white, whereas ade2 ADE8 colonies are red. Therefore, as a result of occasional plasmid loss under nonselective conditions, colonies of SY3362 and SY3363 are composed of red (plasmid-containing) and white (plasmid-free) pie-shaped sectors (sector+ phenotype). Occasional plasmid loss also allows SY3362 and SY3363 to sport mitotic segregants able to grow on medium supplemented with 5′-FOA, a toxin that kills cells expressing the URA3 gene. Therefore, mutations that result in the requirement for CLA4 (or ADE8) will yield red (sector−), 5′-FOAs colonies.
FIG. 1.
Schematic representation of the basis for the genetic screen used to isolate mutants that are synthetically lethal in the absence of CLA4. Xi (activator of STE20), Xii (component of a non-STE20-related pathway), and Yi (target of STE20) represent classes of mutants that are hypothesized to be recovered by the genetic screen.
Two independent techniques were used to generate mutations in NCS genes. First, strains SY3362 and SY3363 were mutated by UV irradiation, yielding 77 sector−, 5′-FOAs colonies for SY3362 and 28 sector−, 5′-FOAs colonies for SY3363 out of approximately 200,000 cells from each parent strain. Of the 77 mutants generated from SY3362, 61 were recessive, 14 were dominant, and 2 were apparently unable to mate with SY3363 (these mutants were later shown to carry a mutation at LYS2, an auxotrophic marker used in SY3362 for the selection of diploids when mated to SY3363). Mutation of SY3363 yielded 13 recessive and 10 dominant mutant strains. Mutations were organized into complementation groups either by mating the SY3362 mutants to the SY3363 mutants or by first backcrossing the SY3362 mutants to SY3363 to generate an α mating-type strain and then mating the newly constructed strain with the SY3362 mutants.
The second mutagenesis technique relied on transformation into SY3362 of linear DNA fragments isolated from a LEU2 transposon mutagenesis library (4). The procedure yielded only one mutant (out of approximately 30,000 transformants) in which (i) the LEU2 marker segregated 2:2 when backcrossed to the parental strain, demonstrating that the phenotype is due to an insertion at only one locus, and (ii) the LEU2 marker cosegregated with the mutant phenotype when crossed to SY3363. This gene is designated NCS1, and transposon-generated allele is designated ncs1Δ::LEU2. Since no alleles of NCS1 were found with the UV mutagenesis procedure, and only one allele by the insertion mutagenesis procedure, we assume that the screen has yet to be saturated.
As a first step to learn about NCS1 function, we examined the growth and morphology of ncs1Δ mutants. ncs1Δ single mutants grew at wild-type rates at all temperatures tested and had wild-type morphology. In addition, ncs1 mutants were not defective for any known STE20 function tested: they responded to pheromone, mated with wild-type efficiency, and underwent filamentous growth as assessed by agar invasion.
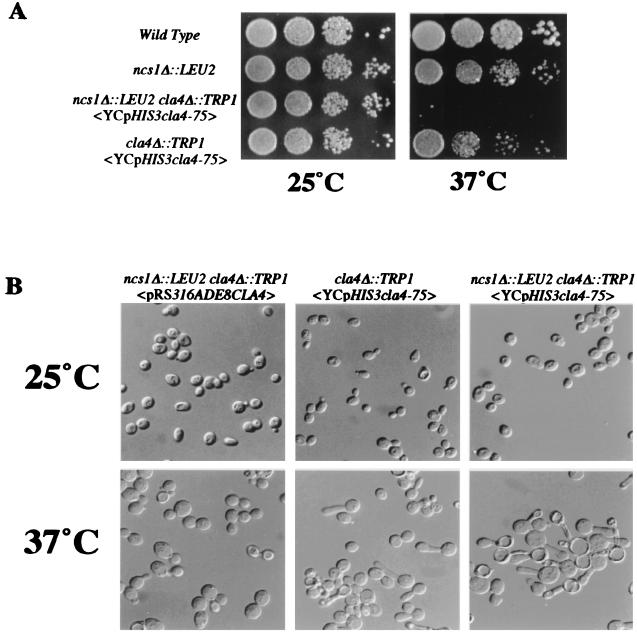
To investigate the phenotype of ncs1Δ::LEU2 cla4Δ cells (Fig. 2), we constructed a strain of that genotype carrying a plasmid-borne temperature-sensitive CLA4 allele, cla4-75. At the restrictive temperature, these cells arrested growth as cells containing a hyperpolarized bud, reminiscent of the cla4Δ and ste20Δ cla4Δ morphology. In the case of ste20Δ cla4Δ <YCpHIS3cla4-75> cells, the hyperpolarized morphology is accompanied by a second defect in bud growth. In wild-type cells, a pair of septin rings flank the mother/bud junction. New growth, for example, fusion of secretory vesicles with the plasma membrane, occurs on the daughter side of the septin rings. In ste20Δ cla4Δ <YCpHIS3cla4-75> double-mutant and cln1Δ cln2Δ cla4Δ <YCpHIS3cla4-75> triple-mutant cells, however, growth occurs on the mother side, and as a result the septin proteins are located along the length of or at the tip of the bud (Table 3; also see reference 7). ncs1Δ::LEU2 cla4Δ <YCpHIS3cla4-75> mutants did not exhibit this defect. Septin mislocalization was observed in only 3% of cells, comparable to what is seen in cla4Δ cells (Table 3). The absence of a septin localization defect is peculiar to ncs1Δ cla4Δ, as other ncs cla4Δ <YCpHIS3cla4-75> mutants tested exhibit the localization defect (data not shown). A septin localization defect also was not seen for cla4Δ ncs1-2 strains, which contain a temperature-sensitive NCS1 allele (4% septin mislocalization). Together, these data suggest either that NCS1 is involved in orchestrating only a subset of STE20 functions that are essential in a cla4Δ background or that loss of NCS1 reveals a separate, non-STE20-related function of CLA4.
FIG. 2.
Phenotypic analysis of ncs1Δ::LEU2 cla4Δ strains. (A) Loss of NCS1 is lethal in the absence of CLA4. Strains SY3357 (wild type), SY3364 (ncs1Δ::LEU2 cla4Δ::TRP1 <pRS316ADE8CLA4>), SY3365 (ncs1Δ::LEU2 cla4Δ::TRP1 <YCpHIS3cla4-75>), and SY3360 carrying the cla4-75 allele (cla4Δ::TRP1 <YCpHIS3cla4-75>) were grown in synthetic medium at 25°C to an OD600 of 1.0 to 2.0. A serial dilution (1/10) was performed starting with 10,000 cells. Cells were spotted onto prewarmed YEPD plates at 25 and 37°C and allowed to grow for 3 days. (B) Morphological examination of ncs1Δ::LEU2 cla4Δ::TRP1 strains. Strains SY3364, SY3365, and SY3360 (carrying YCpHIS3cla4-75) were grown in synthetic medium at 25°C and shifted to 37°C for 4 h.
TABLE 3.
Cdc3p mislocalization
| Strain | Genotype | % Mislocalization |
|---|---|---|
| SY3408 | MATa cla4Δ::TRP1 <YCpHIS3cla4-75> | 2 |
| SY3377 | MATa cln1::URA3 cln2::LEU2 cla4Δ::TRP1 <YCpHIS3cla4-75> | 65 |
| SY3365 | MATa ncs1Δ::LEU2 cla4Δ::TRP1 <YCpHIS3cla4-75> | 3 |
| SY3384 | MATa ncs1Δ::LEU2 cla4Δ::TRP1 <pRS414ncs1-2> | 4 |
| SY3376 | MATa ste20Δ::TRP1 cla4Δ::TRP1 <YCpHIS3cla4-75> | 45 |
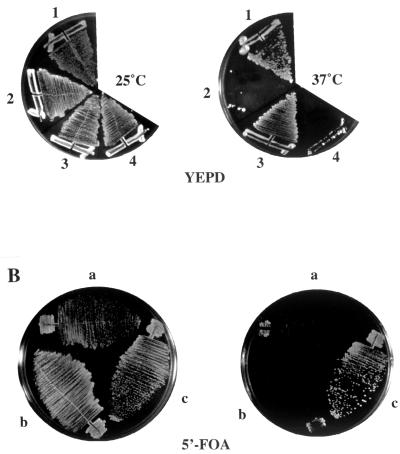
Cla4p has at least two biochemical activities: the ability to bind Cdc42p via the PAK domain, and protein kinase activity. To determine whether either activity is required for viability in an ncs1Δ::LEU2 cla4Δ mutant, we constructed two sets of ncs1Δ::LEU2 cla4Δ strains. The first set contained a plasmid-borne cla4-75 allele and also harbored another allele of CLA4 that encodes a version of CLA4 lacking the PAK domain (SY3380). This strain was temperature sensitive for growth (Fig. 3A), implying that the PAK domain mutant could not provide the CLA4 function that is essential in the ncs1Δ cell. The second set contained the URA3-marked plasmid, pRS316ADE8CLA4 (SY3378), and either an empty vector (pRS315ADE8), a plasmid encoding a kinase-dead version of CLA4 (pRS315ADE8cla4K549R), or wild-type CLA4 (pRS315ADE8CLA4). The ability to lose the URA3 linked CLA4 was assayed by growth on medium containing 5′-FOA. The cla4K549R allele was unable to replace wild-type CLA4 (Fig. 3B). Taken together, the inability of cla4(ΔPAK) and cla4K549R to substitute for wild-type CLA4 implies that both Cla4p activities are required for viability in a strain lacking NCS1.
FIG. 3.
CLA4 functions are required for viability in ncs1Δ cells. (A) Cla4p lacking the PAK domain cannot support viability in the absence of Ncs1p. Strains SY3364, SY3365, SY3380 (cla4Δ::TRP1 ura3::cla4ΔPAK-MYC::URA3 <YCpHIS3cla4-75>), and SY3379 (ncs1Δ::LEU2 cla4Δ::TRP1 ura3::cla4ΔPAK-MYC::URA3 <YCpHIS3cla4-75>) (sectors 1 to 4, respectively) were grown at 25°C on YEPD medium; single colonies were assayed for growth by streaking onto prewarmed YEPD medium and incubating for 3 days at 37°C. (B) Cells lacking Ncs1p require an active Cla4p kinase. Yeast strain SY3378 (ncs1Δ::HIS3 cla4Δ::TRP1 <pRS316ADE8CLA4>) was transformed with pRS315 (sector a), pRS315ADE8cla4K549R (which encodes a kinase-dead Cla4p), (sector b), or pRS315ADE8CLA4 (sector c), and the ability of pRS315ADE8cla4K549R to substitute for pRS316ADE8CLA4 was assayed by growth on synthetic complete medium lacking leucine (left) or supplemented with 5′-FOA (right) for 3 days at 30°C.
ncs1Δ::LEU2 cla4Δ cells arrest prior to the G2/M transition.
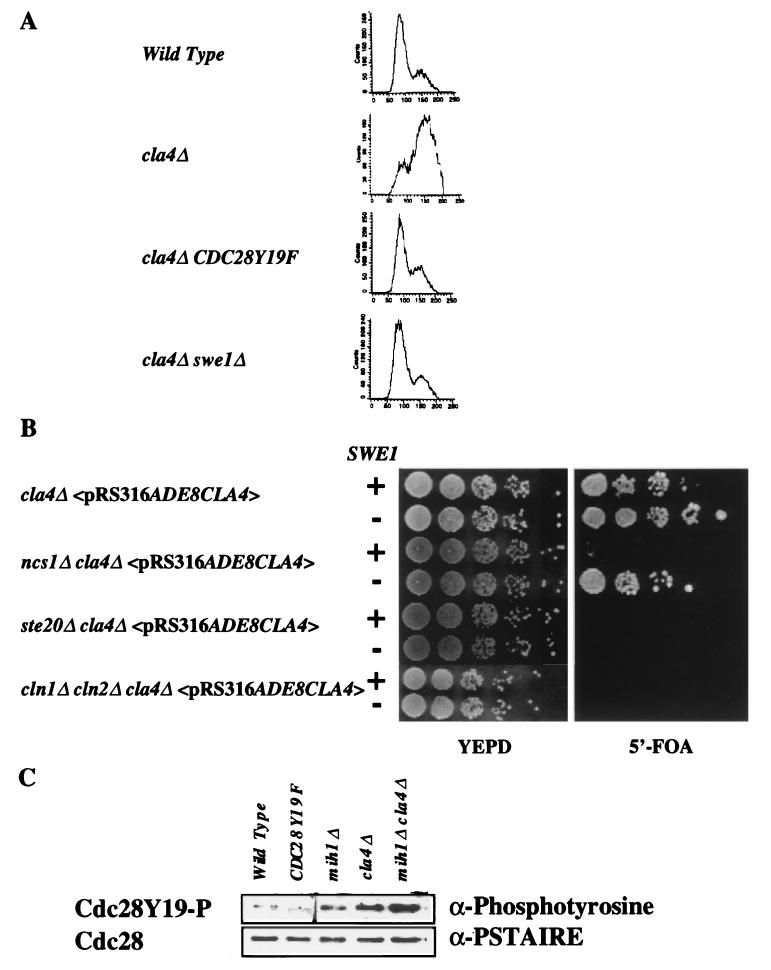
The hyperpolarized bud phenotype of cla4Δ mutants can be interpreted as a delay in the switch from apical bud growth to isotropic growth that occurs late in G2 (17, 24, 25, 41) and is reminiscent of defects conferred by alterations in the expression of SWE1 and CLB2, known regulators of Cdc28p kinase activity. This G2 delay is thought to be the result of activation of a cell cycle checkpoint that monitors the presence and growth of the bud. Phosphorylation of Tyr19 in Cdc28p by Swe1p inhibits Clbp-Cdc28p kinase activity and prevents progression to anaphase. We therefore examined other cell cycle events in cla4Δ, ncs1Δ::LEU2, and cla4Δ ncs1Δ::LEU2 mutants. Using FACS analysis, we analyzed the DNA content of a cla4Δ mutant to determine whether there was indeed a delay in the G2 phase of the cell cycle. Asynchronous cultures of four strains, wild type, cla4Δ, cla4Δ CDC28Y19F, and cla4Δ swe1Δ—were grown to mid-log phase, and their DNA was stained with propidium iodide (Fig. 4A). The wild-type culture contained a high percentage of cells with a 1C DNA content whereas the cla4Δ culture contained a high percentage of cells with a 2C DNA content, implying an accumulation of cells that have replicated their DNA and have yet to undergo mitosis. Mutation of CDC28 to CDC28Y19F or deletion of SWE1 restored the 1C/2C ratio of cla4Δ mutants to that seen for the wild-type strain. We examined the bud morphology of these strains and found that CDC28Y19F and swe1Δ suppressed the hyperpolarized bud phenotype (data not shown).
FIG. 4.
The cla4Δ ncs1Δ::LEU2 defect results in a G2 delay that is suppressed by deletion of SWE1. (A) Strains SY3357, SY3360, SY3392, and SY3391 were grown to mid-log phase in YEPD medium at 30°C. The cells were fixed, stained with propidium iodide, and subjected to FACS analysis. (B) Strains expressing wild-type SWE1 (SY3362, SY3378, SY3368, and SY3504) or lacking SWE1 (SY3392 carrying pRS316ADE8CLA4, SY3403, SY3404, and SY3505) were constructed and grown on YEPD medium at 25°C. After 3 days, dilutions of cells were spotted onto rich medium (YEPD) or onto synthetic complete medium supplemented with 5′-FOA and incubated for 3 days at 25°C. (C) Deletion of CLA4 increases the phosphotyrosine content of Cdc28p. Whole-cell extracts were prepared from exponentially growing strains SY3357, SY3390, SY3393, SY3360, and SY3398. Cdc28p was enriched from the extracts using p13SUC1-conjugated agarose beads. The beads were washed, and the eluted proteins were analyzed by Western analysis using antibodies that recognize phosphotyrosine (4G18) and the PSTAIRE amino acid motif of Cdc28p. The band that appears in the Cdc28Y19F sample of the upper panel (antiphosphotyrosine blot) differs in mobility from Cdc28p. We therefore conclude that it is not Cdc28p but another protein that is phosphorylated on a tyrosine residue(s), but only in Cdc28Y19F cells.
Does the ability to bypass the elongated bud phenotype of cla4Δ cells restore viability to ste20Δ cla4Δ, cln1Δ cln2Δ cla4Δ, or ncs1Δ cla4Δ cells? To test this possibility, cla4Δ swe1Δ cells were crossed to either ste20Δ cla4Δ <pRS316ADE8CLA4>, cln1Δ cln2Δ cla4Δ <pRS316ADE8CLA4>, or ncs1Δ cla4Δ <pRS316ADE8CLA4>. The diploids were then sporulated, and the ability of spores to survive without wild-type CLA4 assayed by growth on medium supplemented with 5′-FOA. As expected, cla4Δ cells were able to grow on medium supplemented with 5′-FOA whether or not SWE1 was deleted (Fig. 4B). Strikingly, deletion of SWE1 suppressed the lethality of the ncs1Δ cla4Δ double mutation. This result contrasts with that obtained for ste20Δ cla4Δ and cln1Δ cln2Δ cla4Δ mutants: swe1Δ did not restore viability to these mutants. Analogous results were obtained using the CDC28Y19F allele instead of swe1Δ. Although CDC28Y19F did not suppress the lethality of ste20Δ cla4Δ, we examined whether it suppressed the hyperpolarized bud morphology by constructing a ste20Δ cla4Δ CDC28Y19F strain carrying plasmid borne cla4-75. At the restrictive temperature, the buds formed by this strain had nearly wild-type morphology and the septin ring was located at the mother/daughter junction of the bud neck (data not shown), indicating that CDC28Y19F suppresses both the morphology defect and the defect in septin ring mislocalization.
To confirm that the G2 delay seen in cla4Δ strains was at the level of Cdc28p activation, we determined the extent of Cdc28p phosphorylation at position 19 by Western analysis using an antibody directed against phosphotyrosine (see Materials and Methods). As anticipated, a modest amount of phosphotyrosine that comigrated with Cdc28p was detected in the wild-type strain (Fig. 4C, lane 1), while no phosphotyrosine-associated Cdc28p was detected in the strain expressing CDC28Y19F (lane 2).
Cells lacking MIH1, which encodes the phosphatase that dephosphorylates residue Tyr19 (Y19) (44), had relatively high (approximately 11-fold higher than wild-type) levels of phosphotyrosine-associated Cdc28p (lane 3). cla4Δ cells also had relatively high (approximately 34-fold higher than wild-type) levels of phosphotyrosine-associated Cdc28p (lane 4). Interestingly, the increase in the amount of phosphotyrosine Cdc28p was higher in cla4Δ mih1Δ mutants than in either single mutant (approximately 50-fold above the wild-type level) (lane 5). On the other hand, the ratio of phosphorylated Cdc28p to total Cdc28p was no greater in extracts from ncs1Δ::LEU2 mih1Δ cells than in extracts from mih1Δ cells (data not shown). These data suggest that the regulation of inhibitory phosphorylation of Cdc28p in a cla4Δ strain can occur independently of Mih1p. Moreover, the lack of difference in Cdc28p phosphorylation between ncs1Δ::LEU2 mih1Δ and mih1Δ cells implies that the effect of Ncs1p on Cdc28p is dependent on Mih1p and that NCS1 may regulate MIH1.
As another measure of G2 progression, we examined mitotic spindle length. The formation of short spindles occurs during S phase and is dependent on the activity of Clb3p and Clb4p forms of Cdc28p kinase but not on DNA replication (12, 40). The mitotic cyclin forms of Cdc28p kinase, Clb1p-Cdc28p and Clb2p-Cdc28p, promote the elongation of the spindles toward the distal poles of both mother and daughter cells (12). Elongation of the spindles and the switch from apical to isotropic bud growth appear to be concurrent and dependent on mitotic cyclin/Cdc28p kinase activity. The relative position and length of the mitotic spindle, therefore, may act as an effective cytological marker for the switch from apical to isotropic growth. Because cla4Δ cells exhibit a G2 delay, we asked whether they also have a defect in spindle elongation or whether such a defect might be apparent in ncs1Δ::LEU2 cla4Δ double mutants.
A set of strains was synchronized in G1 by the presence of α-factor for 1.5 h and then shifted to 37°C for 4 h to deactivate the temperature-sensitive cla4-75 allele. After the initial incubation at 37°C, the cells were washed and incubated at 37°C for 1.25 h in medium lacking α-factor. At that time, more than 73% of the cells of each culture were budded. The cells were fixed and stained with α-tubulin. In the wild-type strain (SY3357), 74% (181 of 244) of the cells had elongated spindles projecting into the daughter bud. Strains lacking either NCS1 (SY3364) or CLA4 (SY3360 containing cla4-75) yielded elongated spindles in 67% (140 of 208) or 81% (171 of 210) of the cells, respectively. The ncs1Δ::LEU2 cla4Δ cla4-75 strain (SY3365), however, produced elongated spindles in only 20% (70 of 346) of the cells.
In the foregoing experiments, we observed numerous cells with elongated buds as well as elongated spindles in the cla4Δ strain. This observation seems to be at odds with the reported coupling of spindle elongation and the switch from apical to isotropic bud growth (24, 31). One possible explanation is that the overlap in the functions of Ste20p and Cla4p is enough to allow Ste20p to partially act on behalf of Cla4p. This redundancy may provide enough kinase activity to allow spindle elongation but not enough to promote the switch from apical to isotropic bud growth. Alternatively, CLA4 may function to couple spindle elongation to the switch from apical to isotropic bud growth. The possibility that the cla4-75 allele is leaky and provides sufficient activity for one function and not the other is unlikely because a strain lacking CLA4 produced similar results (data not shown).
Cla4p physically interacts with Cdc28p.
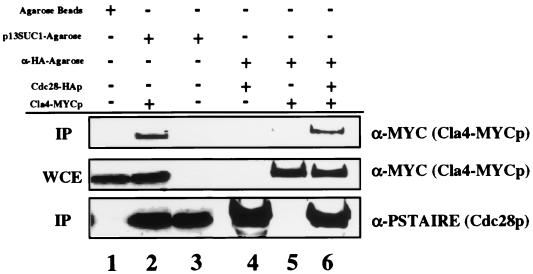
Given the genetic interactions between CLA4 and CLN1/CLN2 and between CLA4 and CDC28, we examined whether Cla4p and Cdc28p exist in a complex. To test this idea, we performed a Cdc28p pull-down experiment. Extracts were prepared from a strain expressing an epitope-tagged version of Cla4p, Cla4-MYCp (2), and Cdc28p was precipitated by using agarose-conjugated p13SUC1, a protein known to associate with cyclin-dependent kinases. The MYC antibody recognized a single protein of approximately 93 kDa (Fig. 5, lane 2). Cla4-MYCp was detected in the p13SUC1 precipitates (lane 2), suggesting a direct physical association between Cla4p and Cdc28p or protein that interacts with Cdc28p. Cla4-MYCp was not precipitated when p13SUC1 was not present on the agarose beads (lane 1). To ensure that the interaction between Cla4-MYCp and Cdc28p is specific for Cdc28p and not another protein that could be recognized by the p13SUC1-conjugated agarose beads, we performed a similar experiment using cells expressing Cdc28-HAp (53). Precipitation with the antibody that recognizes the HA epitope led to coprecipitation of Cla4p (lane 6). We estimate the amount of Cla4-MYCp that associates with Cdc28-HAp to be approximately 1 to 5% of the starting material, whereas the amount of Cla4-MYCp that was pulled down with the p13SUC1-agarose to be 20 to 30% of the starting material. The difference between the experiments may in part be due to the different methods used to isolate Cdc28p: p13SUC1 can recognize all Cdc28p molecules in the extracts, whereas the HA antibody recognizes Cdc28-HAp but not wild-type Cdc28p.
FIG. 5.
Cla4p binds to Cdc28p. Cdc28p was enriched from whole-cell extracts of strains SY3409 and SY3357 (lanes 2 and 3, respectively) using p13SUC1-agarose beads or from extracts of SY3357 carrying pSF19 (CDC28-HA) (lane 4), SY3409 (lane 5), and SY3409 carrying pSF19 (lane 6), using HA antibody-conjugated agarose. Neither Cdc28p nor Cla4-MYC could be enriched from extracts using undecorated agarose beads (lane 1; SY3409). Top, Western (immunoprecipitation [IP]) analysis of Cla4-MYCp associated with Cdc28p-coated beads; middle, amount of Cla4-MYCp present in the whole-cell extract (WCE); bottom, amount of Cdc28p associated with the agarose beads.
NCS1 encodes a protein with sequence similarity to the PTPA family of enzymes.
The LEU2-marked transposon based insertion was mapped to ORF YIL153w on chromosome IX by rescuing the marked locus and sequencing the flanking regions of DNA (2). The marker insertion was at codon 86 of the ORF (Fig. 6A). The presumed protein sequence of YIL153w shows high sequence identity to the PTPA family of protein phosphatase regulators (5). The family includes members from Homo sapiens (38% identity to Ncs1p), Drosophila melanogaster (29% identity to Ncs1p), and Schizosaccharomyces pombe (54% identity to Ncs1p). The PTPA family of regulators, including the yeast homologs, have been shown to increase the phosphotyrosyl phosphatase activity of PP2As in vitro (56); however, the in vivo function of these proteins is not known.
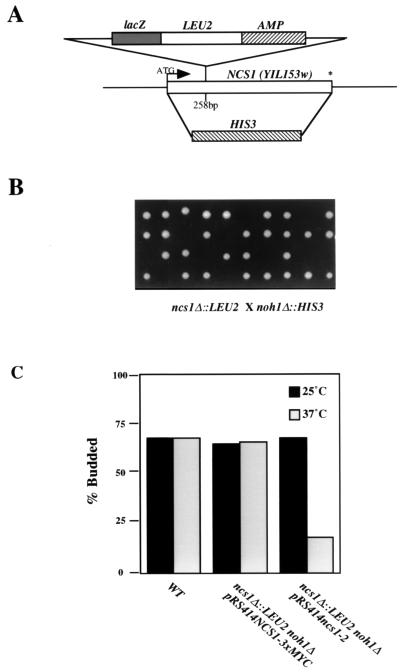
FIG. 6.
Schematic representation of NCS1 null alleles and phenotypic analysis of ncs1Δ::LEU2 noh1Δ::HIS3 double-mutant strains. (A) Schematic representation of the NCS1 alleles, ncs1Δ::LEU2 and ncs1Δ::HIS3, used throughout this study. The putative translation start site is shown with an arrow. The termination codon is denoted by an asterisk. (B) Analysis of ncs1Δ noh1Δ double mutants. Tetrads dissected from the meiotic progeny of diploid MATa/MATα ncs1Δ::LEU2/NCS1 NOH1/noh1Δ::HIS3 were placed on YEPD medium and incubated at 30°C for 3 days. (C) Phenotypic analysis of cells lacking PTPA function. The budding index (percent budded cells/total number of cells) of asynchronously growing cultures was measured for strains SY3357, SY3383, and SY3382 after growth of cultures in YEPD medium at 25°C followed by a shift to 37°C for 5 h. WT, wild type.
A second gene, YPL152w, also encodes a protein with sequence similarity to the PTPA family. NCS1 and YPL152w (which we designate NOH1; also called RRD2 [39]) show a great deal of sequence similarity (179 of 393 amino acid residues) but are only 25% identical. As an initial examination of PTPA function, we sought to determine the effect of loss of PTPA function on cell growth. Although loss of either NCS1 or NOH1 had no effect on growth or morphology, deletion of both genes led to inviability. In particular, the spores of the double mutants germinated but could undergo fewer than five rounds of division (Fig. 6B). Examination of the double-mutant microcolonies revealed cells that were rounded and unbudded. To investigate this phenotype further, a temperature-sensitive NCS1 allele (ncs1-2) was created by the error-prone PCR technique (Materials and Methods). The plasmid-borne recessive ncs1-2 allele (pRS414ncs1-2) was transformed into the MATa ncs1Δ::LEU2 strain, and the resulting strain was mated to a MATα noh1Δ::HIS3 strain. The resulting diploid was sporulated, and ncs1Δ::LEU2 noh1Δ::HIS3 segregants containing plasmid borne ncs1-2 were identified. These segregants were unable to grow at 37°C, and approximately 82% of the cells arrested with a round and unbudded morphology (Fig. 6C).
Based on the synthetic lethality exhibited by three sets of mutants, ste20Δ cla4Δ, ncs1Δ::LEU2 cla4Δ, and ncs1Δ::LEU2 noh1Δ, we hypothesized that STE20 and NOH1 might show genetic interactions. However, we were unable to detect new phenotypes for ste20Δ noh1Δ double mutants, nor did we detect new phenotypes when ste20Δ ncs1Δ::LEU2 and noh1Δ cla4Δ double mutants were constructed (Table 4), providing further evidence that the overlap in NCS1 and CLA4 function is physiologically different from the overlap in STE20 and CLA4 function.
TABLE 4.
Effects of gene deletions on viability
| Mutation(s) | Viability
|
||
|---|---|---|---|
| 25°C | 30°C | 37°C | |
| cla4Δ | + | + | + |
| ncs1Δ::LEU2 | + | + | + |
| ncs1Δ::HIS3 | + | + | + |
| ste20Δ | + | + | + |
| noh1Δ | + | + | + |
| pph21Δ | + | + | + |
| pph22Δ | + | + | + |
| ppg1Δ | + | + | + |
| sit4Δ | + | + | +/− |
| pph3Δ | + | + | + |
| ssd1Δ | + | + | + |
| cln3Δ | + | + | + |
| pph21Δ pph22Δ | − | − | − |
| pph21Δ cla4Δ | + | + | + |
| pph22Δ cla4Δ | + | + | + |
| ppg1Δ cla4Δ | + | + | + |
| sit4Δ cla4Δ | − | − | − |
| pph3Δ cla4Δ | + | + | + |
| ncs1Δ::LEU2 cla4Δ | − | − | − |
| noh1Δ cla4Δ | + | + | + |
| ste20Δ ncs1Δ::LEU2 | + | + | + |
| noh1Δ ncs1Δ::LEU2 | − | − | − |
| ssd1Δ ncs1Δ::LEU2 | + | + | + |
| cln3Δ ncs1Δ::LEU2 | + | + | − |
| ste20Δ noh1Δ | + | + | + |
| sit4Δ noh1Δ | + | + | +/− |
| sit4Δ ncs1Δ::LEU2 | + | + | +/− |
Strains lacking the gene for the PP2A-like phosphatase, SIT4, require CLA4.
The homology between Ncs1p and the PTPA family of proteins suggests that Ncs1p may regulate a PP2A. Indeed, van Hoof et al. (56) have shown that the proteins encoded by both of the S. cerevisiae PTPA homologs can increase the specific activity of a PP2A, PP2AD, for a phosphotyrosyl-containing substrate. However, PP2AD is a mammalian enzyme, and we sought to identify a yeast target of Ncs1p. We speculated that the relevant target would show genetic interactions with CLA4. In S. cerevisiae there are four PP2As and one PP2A-like phosphatase, Sit4p. We first tested SIT4 by mating SY3361 (MATα cla4Δ) to strain SY3389 (MATa sit4::HIS3). After sporulation and tetrad (n = 20) dissection, no sit4Δ cla4Δ double mutants were recovered (Table 4). In contrast, strains lacking both SIT4 and NCS1 appeared phenotypically similar in terms of growth and morphology to a strain lacking only SIT4 (Table 4). SIT4 exhibits synthetic lethality with two other genes, SSD1 and CLN3; we therefore asked whether NCS1 would show interactions with these genes. We were able to construct ncs1Δ::LEU2 ssd1Δ and ncs1Δ::LEU2 cln3Δ double-mutant strains. The ncs1Δ::LEU2 ssd1Δ double mutant was wild type for growth at 25 and 37°C (Table 4) and had no morphological abnormalities (data not shown). The ncs1Δ::LEU2 cln3Δ double mutant, on the other hand, grew at wild-type rates at 25°C but was temperature sensitive for growth at 37°C (Table 4), accumulating unbudded cells at the restrictive temperature.
Of the PP2A family, Pph21p and Pph22p contribute the majority of the PP2A activity. As for sit4Δ strains, strains lacking PPH21 and PPH22 are severely impaired for growth or dead, depending on the genetic background (54). In our genetic background (S288C), pph21Δ pph22Δ double mutants are inviable (Table 4), dividing only two to three times after germination. As done for SIT4, we constructed diploids heterozygous at the CLA4 locus and the PPH21 and PPH22 loci. After sporulation, analysis of the tetrads revealed no observable growth phenotype in the pph21Δ cla4Δ or the pph22Δ cla4Δ double mutant compared to the respective congenic pph21Δ, pph22Δ, and cla4Δ strains (Table 4). Likewise, no genetic interactions between CLA4 and the other two PP2A genes, PPH3 and PPG1, were observed (Table 4). Although these data do not rule out an interaction between Pph21p, Pph22p, Pph3p, or Ppg1p and Ncs1p, they do suggest that the essential function shared by CLA4 and NCS1 does not involve PPH21, PPH22, PPH3, or PPG1.
Ncs1p and Noh1p physically interact with Sit4p.
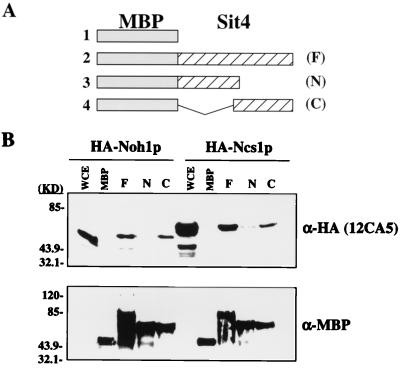
Given the genetic interactions between CLA4 and both NCS1 and SIT4, we asked whether the PTPA proteins and Sit4p interact biochemically. A binding assay was conducted using fragments of Sit4p that had been expressed in bacteria as malE-encoded MBP fusion proteins and then attached to amylose resin. These fragments included the full-length Sit4 protein, a C-terminal truncation encoding only the first 164 amino acids, and an N-terminal deletion of the first 161 amino acids leaving only the Sit4p catalytic domain. As a source of Ncs1p and Noh1p, we prepared whole-cell extracts from a yeast strain in which Ncs1p and Noh1p were overexpressed from the GAL1 promoter as HA fusion proteins. The expression of either pGAL-3xHA-NCS1 or pGAL-3xHA-NOH1 was able to restore viability to ncs1Δ::LEU2 noh1Δ double mutants (data not shown). The HA-Ncs1p or HA-Noh1p-containing extracts were incubated with amylose resin decorated with Sit4p, and the bound PTPA protein was detected by Western analysis. As seen in Fig. 7B, both HA-Ncs1p and HA-Noh1p associate with full-length Sit4p as well as the C-terminal catalytic domain. HA-Ncs1p, but not HA-Noh1p, exhibited weak interaction with the N-terminal regulatory domain of Sit4p. These data show that the S. cerevisiae PTPA proteins are capable of interacting with PP2As, possibly regulating the phosphatase activity through interaction with the phosphatase catalytic domain.
FIG. 7.
In vitro association of PTPA proteins with Sit4p. (A) Schematic representation of bacterially expressed MBP-Sit4p fusion proteins used in the assay. 1, MBP; 2, MBP–full-length Sit4p (F); 3, MBP–N-terminal regulatory domain of Sit4p (amino acids 1 to 164) (N); 4, MBP–C-terminal catalytic domain of Sit4p (amino acids 161 to 302) (C). (B) Yeast PTPA proteins associate with the MBP-Sit4p fusion proteins, as shown by Western analysis using the HA antibody 12CA5 as a probe (top) and the α-MBP antibody (New England Biolabs, Inc.) as a loading control) (bottom). WCE, whole-cell extract.
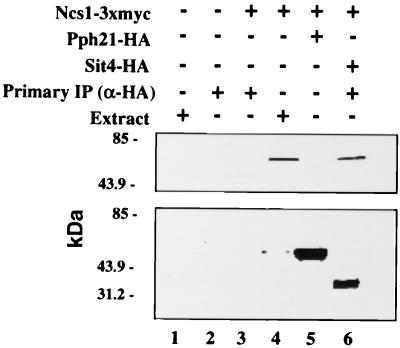
We extended our analysis of the interactions between Ncs1p and PP2As to include an examination of potential physical interactions in vivo. Specifically, we compared the association of Ncs1p with Pph21p and Sit4p. Strains carrying plasmids YEpURA3ADH-SIT4-HA and YEp13NCS1-3xMYC or plasmids YEp24PPH21-HA and YEp13NCS1-3xMYC were grown to mid-log phase, and extracts were prepared. The protein phosphatases were precipitated with antibodies to the HA epitope, and the potential association with Ncs1p was assessed by Western analysis. Ncs1p was present in the Sit4p precipitates but not the Pph21p precipitates (Fig. 8, lanes 5 and 6). Of course, this result does not exclude a physical interaction between Ncs1p and Pph21p; the interaction may simply be below our limits of detection. These results suggest that Ncs1p and Sit4p are part of a complex in vivo and are consistent with the notion that Sit4p is a target of Ncs1p.
FIG. 8.
Ncs1p coimmunoprecipitates with Sit4p from whole-cell extracts (WCE). Lane 1, SY3357 (wild type; WCE); lane 2, SY3357 (wild type; immunoprecipitate [IP]); lane 3, SY3394 (ncs1Δ) carrying YEp13NCS1-3xMYC (IP); lane 4, SY3394 (ncs1Δ) carrying YEp13NCS1-3xMYC (WCE); lane 5, SY3394 (ncs1Δ) carrying YEp13NCS1-3xMYC plus YEp24PPH21-HA (IP); lane 6, SY3394 (ncs1Δ) carrying YEp13NCS1-3xMYC plus YEpURA3ADH-SIT4-HA (IP).
DISCUSSION
Yeast contains two related PAKs, Ste20p and Cla4p, that have individual functions but also appear to share an additional essential function. This latter conclusion is based on the synthetic lethality of ste20Δ and cla4Δ mutations (7, 15). We sought new mutations that are lethal in a cla4Δ background in an effort to identify putative targets or regulators of STE20 function and more generally to learn about the essential function(s) shared by CLA4 and STE20. This effort identified 10 complementation groups, one of which, NCS1, is the focus of this report. Ncs1p is a member of the conserved PTPA family of proteins, which are capable of increasing the phosphotyrosyl phosphatase activity of PP2As in vitro (5). We show that Ncs1p binds to, and thereby presumably regulates, the PP2A-like phosphatase, Sit4p. Because loss of SIT4 is lethal in a cla4Δ background, we infer that loss of the Ncs1p-Sit4p interaction is responsible for the lethality of ncs1Δ cla4Δ strains. NCS1 function has been explored in previous studies, which showed that cells lacking NCS1 have a higher mutagenesis rate, are more resistant to rapamycin and caffeine, and are more sensitive to vanadate than are wild-type cells (39). We show that together with CLA4, NCS1 has a role in the cell cycle that is manifest at the G2/M transition, perhaps by regulating Cdc28p kinase activity. In addition, together with NOH1, a gene that encodes a closely related PTPA that also binds to Sit4p, NCS1 has a role in the G1 phase of the cell cycle.
Interaction of Cla4p with Cdc28p.
CLA4 has been reported to have important roles in maintaining actin cytoskeletal polarity during both G1 and G2 phases of the cell cycle (15); its enzymatic activity is maximal in G2 (2). A second role during G1 is to promote normal septin organization (26). We have shown that cla4Δ mutants exhibit a G2-delay that appears to reflect activation of a morphogenesis checkpoint because the delay can be overcome by mutations in CDC28 (CDC28Y19F) or SWE1. Moreover, these mutations largely restore normal morphology to cla4Δ strains. In principle, activation of the checkpoint could be the consequence of perturbation of actin cytoskeleton or septin organization. In either event, we suggest that CLA4 is intimately tied to the checkpoint mechanism, because we can detect a Cdc28p-Cla4p complex. Thus, these reports that a protein known to interact with septins also interacts with Cdc28p parallel the finding that Swe1p, known to interact with and regulate Cdc28p, also interacts with the septin ring (30).
The morphogenesis checkpoint can be activated by increasing the activity of Swe1p, which phosphorylates Cdc28p residue Y19 and thereby inactivates it, or by decreasing the activity of Mih1p, the phosphatase that dephosphorylates Y19 (3, 44, 50). We have observed an increase in Cdc28Y19p phosphorylation in cla4Δ mutants, consistent with the view presented above that a checkpoint is activated in these mutants. Does Cla4p influence Mih1p or Swe1p activity? Cells lacking both CLA4 and MIH1 have higher levels of phosphorylated Cdc28Y19p than do cells lacking either protein singly. Moreover, cla4Δ mih1Δ cells have a growth defect compared to cla4Δ or mih1Δ cells. Together, these results imply that Cla4p and Mih1p regulate Cdc28p by different mechanisms. Because Swe1p is the only enzyme, other than Mih1p, shown to catalyze Cdc28Y19p phosphorylation/dephosphorylation events, by default we suggest that Cla4p regulates Swe1p. This is an attractive possibility given that both proteins interact with Cdc28p and with the septin ring (7, 26, 30, 41, 49).
NCS1 plays a role in G1 and at the G2/M transition.
One perspective of CLA4 function has come from the analysis of cla4Δ ste20Δ mutants (7, 15). These studies revealed that the septin ring that separates the mother cell from the growing bud did not function properly in the double mutant. In particular, new surface growth occurred on the mother side of the ring rather than on the daughter side. In contrast, we find that the septin ring is maintained at the bud neck in cla4Δ ncs1Δ mutants, implying that at least with respect to this property the septin ring is normal. The different phenotypes of ncs1Δ cla4Δ and ste20Δ cla4Δ mutants suggest two possible relationships between NCS1 and STE20. First, if NCS1 is in some way related to STE20, NCS1 must orchestrate only a subset of STE20 functions. Alternatively, NCS1 and STE20 functions may be unrelated. In this case, the lethality of ncs1Δ cla4Δ and ste20Δ cla4Δ strains must have different explanations; that is, loss of NCS1 reveals a different cla4Δ defect than does the loss of STE20. We favor the latter possibility because CDC28Y19F suppresses the lethality of ncs1Δ cla4Δ mutants but not ste20Δ cla4Δ mutants, even though CDC28Y19F restores bud morphology and the septin ring to its normal bud neck location in ste20Δ cla4Δ cells.
The foregoing discussion suggests that NCS1 has a function that is evident at the G2/M transition in the cell cycle. We believe that NCS1 also has a function in the G1 phase. This conclusion follows from the observation that cells lacking both identified PTPA subunit genes, NCS1 and NOH1, arrest as unbudded cells.
Sit4p as a target of PTPA function.
What are the targets of NCS1 (and NOH1) action? Having isolated the yeast PTPA gene (NCS1) in our genetic screen, we sought to identify the phosphatase target(s) of Ncs1p activity. We reasoned that one kind of target should (i) be a PP2A, (ii) interact physically with Ncs1p (and potentially Noh1p), and (iii) have mutant phenotypes in common with ncs1Δ, for example, synthetic lethality with cla4Δ. Only one of the five known yeast PP2As, Sit4p, satisfies these three criteria. Rempola et al. have argued that Pph22p might be a target of Ncs1p (Rrd1p) because overexpression of PPH22 can partially suppress the lethality associated with loss of PTPA function (39). However, the degree of suppression and the absence of data showing a physical interaction between the PTPA subunits and Pph22p detract from their argument. In addition, we were unable to detect an in vivo interaction between Ncs1p and Pph21p, the nearest homolog of Pph22p, or between PPH22 and CLA4.
The lack of genetic interactions between CLA4 and other phosphatase structural genes, however, does not rule out the possibility that Ncs1p interacts with other phosphatase catalytic subunits. Indeed, we believe Ncs1p has other targets, based on two observations. First, both Ncs1p and Noh1p interact with the catalytic domain of Sit4p. However, the phenotype associated with the loss of both NCS1 and NOH1 (growth arrest) is more severe than the phenotype associated with the loss of SIT4 in our strain background. If Sit4p were the only target of PTPA function, then one would expect that loss of both PTPA genes and the loss of SIT4 would confer the same phenotype(s) or, if the phenotypes were different, that the loss of SIT4 would be more severe given that the Sit4p phosphoserine/threonine as well as phosphotyrosine phosphatase activity is absent. This is not the case. Second, the phenotype of a sit4Δ noh1Δ strain is no more severe than that of either single mutant. If Sit4p were the only target of Ncs1p, then one would expect an noh1Δ sit4Δ strain to be phenotypically similar to an noh1Δ ncs1Δ strain. Again, this is not the case. Therefore, we conclude that Sit4p is one, but not the only, target of PTPA function.
ACKNOWLEDGMENTS
We thank Charlie Boone, Kim Arndt, Danny Lew, Kim Nasmyth, Fred Cross, Michael Stark, Megan Keniry, Kunliang Guan, and Xiaoli Zhan for providing plasmids, Mike Snyder for providing the yeast transposon library, and John Pringle, John Chant, Mike Marusich, and the University of Oregon Monoclonal Antibody Facility for providing antibodies. We are forever grateful to Daciana Margineantu, April Goehring, Diamond Bob Deschenes, Laurie Graham, Liz Conibear, and Tom Stevens for technical assistance, advice, and reagents. We also thank members of the Sprague lab for helpful comments and discussions. FACS analysis was performed by the University of Iowa Flow Cytometry Facility. DNA sequencing was performed by Yanling Wang.
This work was supported by National Research Service Award GM-18002-03 (to D.A.M.) and by grant GM30027 from the National Institutes of Health (to G.F.S.).
REFERENCES
- 1.Baudin A, Ozier-Kalogeropoulos O, Denouel A, Lacroute F, Cullin C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 1993;21:3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benton B K, Tinkelenberg A, Gonzalez I, Cross F R. Cla4p, a Saccharomyces cerevisiae Cdc42p-activated kinase involved in cytokinesis, is activated at mitosis. Mol Cell Biol. 1997;17:5067–5076. doi: 10.1128/mcb.17.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booher R N, Deshaies R J, Kirschner M W. Properties of Saccharomyces cerevisiae wee1 and its differential regulation of p34CDC28 in response to G1 and G2 cyclins. EMBO J. 1993;12:3417–3426. doi: 10.1002/j.1460-2075.1993.tb06016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns N, Grimwade B, Ross-Macdonald P B, Choi E Y, Finberg K, Roeder G S, Snyder M. Large-scale analysis of gene expression, protein localization, and gene disruption in Saccharomyces cerevisiae. Genes Dev. 1994;8:1087–1105. doi: 10.1101/gad.8.9.1087. [DOI] [PubMed] [Google Scholar]
- 5.Cayla X, Van Hoof C, Bosch M, Waelkens E, Vandekerckhove J, Peeters B, Merlevede W, Goris J. Molecular cloning, expression, and characterization of PTPA, a protein that activates the tyrosyl phosphatase activity of protein phosphatase 2A. J Biol Chem. 1994;269:15668–15675. [PubMed] [Google Scholar]
- 6.Conibear E, Stevens T H. Vps52p, Vps53p, and Vps54p form a novel multisubunit complex required for protein sorting at the yeast late Golgi. Mol Biol Cell. 2000;11:305–323. doi: 10.1091/mbc.11.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cvrckova F, De Virgilio C, Manser E, Pringle J R, Nasmyth K. Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes Dev. 1995;9:1817–1830. doi: 10.1101/gad.9.15.1817. [DOI] [PubMed] [Google Scholar]
- 8.Di Como C J, Arndt K T. Nutrients, via the Tor proteins, stimulate the association of Tap42 with type 2A phosphatases. Genes Dev. 1996;10:1904–1916. doi: 10.1101/gad.10.15.1904. [DOI] [PubMed] [Google Scholar]
- 9.Eby J J, Holly S P, van Drogen F, Grishin A V, Peter M, Drubin D G, Blumer K J. Actin cytoskeleton organization regulated by the PAK family of protein kinases. Curr Biol. 1998;8:967–970. doi: 10.1016/s0960-9822(98)00398-4. [DOI] [PubMed] [Google Scholar]
- 10.Evangelista M, Blundell K, Longtine M S, Chow C J, Adames N, Pringle J R, Peter M, Boone C. Bni1p, a yeast formin linking cdc42p and the actin cytoskeleton during polarized morphogenesis. Science. 1997;276:118–122. doi: 10.1126/science.276.5309.118. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Sarabia M J, Sutton A, Zhong T, Arndt K T. SIT4 protein phosphatase is required for the normal accumulation of SWI4, CLN1, CLN2, and HCS26 RNAs during late G1. Genes Dev. 1992;6:2417–2428. doi: 10.1101/gad.6.12a.2417. [DOI] [PubMed] [Google Scholar]
- 12.Fitch I, Dahmann C, Surana U, Amon A, Nasmyth K, Goetsch L, Byers B, Futcher B. Characterization of four B-type cyclin genes of the budding yeast Saccharomyces cerevisiae. Mol Biol Cell. 1992;3:805–818. doi: 10.1091/mbc.3.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagen D C, McCaffrey G, Sprague G F., Jr Pheromone response elements are necessary and sufficient for basal and pheromone-induced transcription of the FUS1 gene of Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:2952–2961. doi: 10.1128/mcb.11.6.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Healy A M, Zolnierowicz S, Stapleton A E, Goebl M, DePaoli-Roach A A, Pringle J R. CDC55, a Saccharomyces cerevisiae gene involved in cellular morphogenesis: identification, characterization, and homology to the B subunit of mammalian type 2A protein phosphatase. Mol Cell Biol. 1991;11:5767–5780. doi: 10.1128/mcb.11.11.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holly S P, Blumer K J. PAK-family kinases regulate cell and actin polarization throughout the cell cycle of Saccharomyces cerevisiae. J Cell Biol. 1999;147:845–856. doi: 10.1083/jcb.147.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson D I. Cdc42: an essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol Mol Biol Rev. 1999;63:54–105. doi: 10.1128/mmbr.63.1.54-105.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaiser C, Michaelis S, Mitchell A. Methods in yeast genetics: a Cold Spring Harbor Laboratory course manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 19.Kim H B, Haarer B K, Pringle J R. Cellular morphogenesis in the Saccharomyces cerevisiae cell cycle: localization of the CDC3 gene product and the timing of events at the budding site. J Cell Biol. 1991;112:535–544. doi: 10.1083/jcb.112.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kranz J E, Holm C. Cloning by function: an alternative approach for identifying yeast homologs of genes from other organisms. Proc Natl Acad Sci USA. 1990;87:6629–6633. doi: 10.1073/pnas.87.17.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Leberer E, Dignard D, Harcus D, Thomas D Y, Whiteway M. The protein kinase homologue Ste20p is required to link the yeast pheromone response G-protein beta gamma subunits to downstream signalling components. EMBO J. 1992;11:4815–4824. doi: 10.1002/j.1460-2075.1992.tb05587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leberer E, Wu C, Leeuw T, Fourest-Lieuvin A, Segall J E, Thomas D Y. Functional characterization of the Cdc42p binding domain of yeast Ste20p protein kinase. EMBO J. 1997;16:83–97. doi: 10.1093/emboj/16.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lew D J, Reed S I. A cell cycle checkpoint monitors cell morphogenesis in budding yeast. J Cell Biol. 1995;129:739–749. doi: 10.1083/jcb.129.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lew D J, Reed S I. Cell cycle control of morphogenesis in budding yeast. Curr Opin Genet Dev. 1995;5:17–23. doi: 10.1016/s0959-437x(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 26.Longtine M S, Theesfeld C L, McMillan J N, Weaver E, Pringle J R, Lew D J. Septin-dependent assembly of a cell cycle-regulatory module in Saccharomyces cerevisiae. Mol Cell Biol. 2000;20:4049–4061. doi: 10.1128/mcb.20.11.4049-4061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma H, Kunes S, Schatz P J, Botstein D. Plasmid construction by homologous recombination in yeast. Gene. 1987;58:201–216. doi: 10.1016/0378-1119(87)90376-3. [DOI] [PubMed] [Google Scholar]
- 28.Ma X J, Lu Q, Grunstein M. A search for proteins that interact genetically with histone H3 and H4 amino termini uncovers novel regulators of the Swe1 kinase in Saccharomyces cerevisiae. Genes Dev. 1996;10:1327–1340. doi: 10.1101/gad.10.11.1327. [DOI] [PubMed] [Google Scholar]
- 29.Martin H, Mendoza A, Rodriguez-Pachon J M, Molina M, Nombela C. Characterization of SKM1, a Saccharomyces cerevisiae gene encoding a novel Ste20/PAK-like protein kinase. Mol Microbiol. 1997;23:431–444. doi: 10.1046/j.1365-2958.1997.d01-1870.x. [DOI] [PubMed] [Google Scholar]
- 30.McMillan J N, Longtine M S, Sia R A, Theesfeld C L, Bardes E S, Pringle J R, Lew D J. The morphogenesis checkpoint in Saccharomyces cerevisiae: cell cycle control of Swe1p degradation by Hsl1p and Hsl7p. Mol Cell Biol. 1999;19:6929–6939. doi: 10.1128/mcb.19.10.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendenhall M D, Hodge A E. Regulation of Cdc28 cyclin-dependent protein kinase activity during the cell cycle of the yeast Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1998;62:1191–1243. doi: 10.1128/mmbr.62.4.1191-1243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Millward T A, Zolnierowicz S, Hemmings B A. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci. 1999;24:186–191. doi: 10.1016/s0968-0004(99)01375-4. [DOI] [PubMed] [Google Scholar]
- 33.Muhlrad D, Hunter R, Parker R. A rapid method for localized mutagenesis of yeast genes. Yeast. 1992;8:79–82. doi: 10.1002/yea.320080202. [DOI] [PubMed] [Google Scholar]
- 34.Nickels J T, Broach J R. A ceramide-activated protein phosphatase mediates ceramide-induced G1 arrest of Saccharomyces cerevisiae. Genes Dev. 1996;10:382–394. doi: 10.1101/gad.10.4.382. [DOI] [PubMed] [Google Scholar]
- 35.O'Rourke S M, Herskowitz I. The Hog1 MAPK prevents cross talk between the HOG and pheromone response MAPK pathways in Saccharomyces cerevisiae. Genes Dev. 1998;12:2874–2886. doi: 10.1101/gad.12.18.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pringle J R, Preston R A, Adams A E, Stearns T, Drubin D G, Haarer B K, Jones E W. Fluorescence microscopy methods for yeast. Methods Cell Biol. 1989;31:357–435. doi: 10.1016/s0091-679x(08)61620-9. [DOI] [PubMed] [Google Scholar]
- 37.Ramer S W, Davis R W. A dominant truncation allele identifies a gene, STE20, that encodes a putative protein kinase necessary for mating in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1993;90:452–456. doi: 10.1073/pnas.90.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramotar D, Belanger E, Brodeur I, Masson J Y, Drobetsky E A. A yeast homologue of the human phosphotyrosyl phosphatase activator PTPA is implicated in protection against oxidative DNA damage induced by the model carcinogen 4-nitroquinoline 1-oxide. J Biol Chem. 1998;273:21489–21496. doi: 10.1074/jbc.273.34.21489. [DOI] [PubMed] [Google Scholar]
- 39.Rempola B, Kaniak A, Migdalski A, Rytka J, Slonimski P P, di Rago J P. Functional analysis of RRD1 (YIL153w) and RRD2 (YPL152w), which encode two putative activators of the phosphotyrosyl phosphatase activity of PP2A in Saccharomyces cerevisiae. Mol Gen Genet. 2000;262:1081–1092. doi: 10.1007/pl00008651. [DOI] [PubMed] [Google Scholar]
- 40.Richardson H, Lew D J, Henze M, Sugimoto K, Reed S I. Cyclin-B homologs in Saccharomyces cerevisiae function in S phase and in G2. Genes Dev. 1992;6:2021–2034. doi: 10.1101/gad.6.11.2021. [DOI] [PubMed] [Google Scholar]
- 41.Richman T J, Sawyer M M, Johnson D I. The Cdc42p GTPase is involved in a G2/M morphogenetic checkpoint regulating the apical-isotropic switch and nuclear division in yeast. J Biol Chem. 1999;274:16861–16870. doi: 10.1074/jbc.274.24.16861. [DOI] [PubMed] [Google Scholar]
- 42.Roberts R L, Fink G R. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev. 1994;8:2974–2985. doi: 10.1101/gad.8.24.2974. [DOI] [PubMed] [Google Scholar]
- 43.Rothstein R J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- 44.Russell P, Moreno S, Reed S I. Conservation of mitotic controls in fission and budding yeasts. Cell. 1989;57:295–303. doi: 10.1016/0092-8674(89)90967-7. [DOI] [PubMed] [Google Scholar]
- 45.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 46.Schneider B L, Seufert W, Steiner B, Yang Q H, Futcher A B. Use of polymerase chain reaction epitope tagging for protein tagging in Saccharomyces cerevisiae. Yeast. 1995;11:1265–1274. doi: 10.1002/yea.320111306. [DOI] [PubMed] [Google Scholar]
- 47.Sherman F, Fink G R, Hicks J B. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 48.Shu Y, Yang H, Hallberg E, Hallberg R. Molecular genetic analysis of Rts1p, a B′ regulatory subunit of Saccharomyces cerevisiae protein phosphatase 2A. Mol Cell Biol. 1997;17:3242–3253. doi: 10.1128/mcb.17.6.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shulewitz M J, Inouye C J, Thorner J. Hs17 localizes to a septin ring and serves as an adapter in a regulatory pathway that relieves tyrosine phosphorylation of Cdc28 protein kinase in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:7123–7137. doi: 10.1128/mcb.19.10.7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sia R A, Bardes E S, Lew D J. Control of Swe1p degradation by the morphogenesis checkpoint. EMBO J. 1998;17:6678–6688. doi: 10.1093/emboj/17.22.6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sneddon A A, Cohen P T, Stark M J. Saccharomyces cerevisiae protein phosphatase 2A performs an essential cellular function and is encoded by two genes. EMBO J. 1990;9:4339–4346. doi: 10.1002/j.1460-2075.1990.tb07883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sorger P K, Murray A W. S-phase feedback control in budding yeast independent of tyrosine phosphorylation of p34cdc28. Nature. 1992;355:365–368. doi: 10.1038/355365a0. [DOI] [PubMed] [Google Scholar]
- 54.Stark M J. Yeast protein serine/threonine phosphatases: multiple roles and diverse regulation. Yeast. 1996;12:1647–1675. doi: 10.1002/(SICI)1097-0061(199612)12:16%3C1647::AID-YEA71%3E3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 55.Sutton A, Immanuel D, Arndt K T. The SIT4 protein phosphatase functions in late G1 for progression into S phase. Mol Cell Biol. 1991;11:2133–2148. doi: 10.1128/mcb.11.4.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Hoof C, Janssens V, Dinishiotu A, Merlevede W, Goris J. Functional analysis of conserved domains in the phosphotyrosyl phosphatase activator. Molecular cloning of the homologues from Drosophila melanogaster and Saccharomyces cerevisiae. Biochemistry. 1998;37:12899–12908. doi: 10.1021/bi980496l. [DOI] [PubMed] [Google Scholar]
- 57.Zhao Y, Boguslawski G, Zitomer R S, DePaoli-Roach A A. Saccharomyces cerevisiae homologs of mammalian B and B′ subunits of protein phosphatase 2A direct the enzyme to distinct cellular functions. J Biol Chem. 1997;272:8256–8262. doi: 10.1074/jbc.272.13.8256. [DOI] [PubMed] [Google Scholar]