Abstract
Life-threatening hypoglycemia is a limiting factor in the management of type 1 diabetes. People with diabetes are prone to develop hypoglycemia because they lose physiological mechanisms that prevent plasma glucose levels from falling. Among these so-called counterregulatory responses, secretion of glucagon from pancreatic α-cells is preeminent. Glucagon, a hormone secreted in response to a lowering in glucose concentration, counteracts a further drop in glycemia by promoting gluconeogenesis and glycogenolysis in target tissues. In diabetes, however, α-cells do not respond appropriately to changes in glycemia and, thus, cannot mount a counterregulatory response. If the α-cell could be targeted therapeutically to restore its ability to prevent hypoglycemia, type 1 diabetes could be managed more efficiently and safely. Unfortunately, the mechanisms that allow the α-cell to respond to hypoglycemia have not been fully elucidated. We know even less about the pathophysiological mechanisms that cause α-cell dysfunction in diabetes. Based on published findings and unpublished observations, and taking into account its electrophysiological properties, we propose here a model of α-cell function that could explain its impairment in diabetes. Within this frame, we emphasize those elements that could be targeted pharmacologically with repurposed U.S. Food and Drug Administration–approved drugs to rescue α-cell function and restore glucose counterregulation in people with diabetes.
Introduction
Glucose counterregulation is the sum of physiological processes that help prevent life-threatening hypoglycemia. The key counterregulatory factors are glucagon, epinephrine, growth hormone, and cortisol (1–3). This order reflects not only their relative importance but also the sequence of activation as hypoglycemia develops. The multiplicity of factors makes hypoglycemia a rare event in healthy people, but people with diabetes are prone to developing hypoglycemia. In particular, patients with type 1 diabetes and, to a lesser extent, type 2 diabetes lose the glucagon response to a drop in glycemia (4). This triggers a series of events that can lead to hard-to-control, brittle diabetes. Because of life-threatening hypoglycemia, the management of diabetes is complex. Philip Cryer, a leading scientist in diabetes research, once stated that if it were not for the devastating effects of hypoglycemia, diabetes would be rather easy to treat (5).
Glucagon plays a preeminent role in defending against hypoglycemia. Glucagon is a peptide hormone secreted from pancreatic α-cells that reside in the pancreatic islet. Under nondiabetic conditions, α-cells secrete glucagon when plasma glucose concentrations drop. Glucagon’s major effect is to prevent glucose concentrations from further falling by promoting gluconeogenesis and glycogenolysis in target organs. In diabetes, α-cell secretion of glucagon is deranged: it is elevated and does not respond appropriately to changes in glycemia (6). This impaired α-cell function increases the risk of the dangerous iatrogenic hypoglycemia that results from therapeutic insulin excess. Increased blood glucagon levels (hyperglucagonemia), on the other hand, are now considered a hyperglycemic factor in diabetes (7,8). That glucagon is not only needed to prevent hypoglycemia but also is diabetogenic poses a therapeutic conundrum: how can we target the α-cell to restore glucose counterregulation while avoiding secondary hyperglycemic effects?
The purpose of this perspective is to discuss strategies aimed at solving this challenge. A major barrier in this field is that the pathophysiological mechanisms that lead to α-cell dysfunction in diabetes are not known. Even worse, we still do not have a unified canonical mechanistic model for the regulation of glucagon secretion (for a recent comprehensive review of α-cell physiology, see Gilon [6]). Thus, we will first touch on basic conceptual issues related to α-cell biology that could provide hints about where to start in our quest to improve α-cell function. We will then discuss therapeutic approaches based on autocrine and paracrine signaling and the methods that can be used to test these ideas. This perspective focuses on the very specific task of preventing hypoglycemia and does not deal with the other roles the α-cell plays in regulating blood levels of free fatty acids and amino acids (9).
The α-Cell Is a Social Animal That Thrives in a Cell Collective
Have you noticed that, no matter what animal species you examine, α-cells always share the pancreatic islet with other endocrine cells? Islets do not start this way. Endocrine cells of the different types are initially segregated but later in development coalesce to form the endocrine cell collectives we call islets (10). Although we do not know the mechanisms, this is clearly a directed morphogenic process. Not only are endocrine cells instructed to be close to each other but they also establish numerous channels of communication with their neighbors. The list of islet paracrine factors keeps increasing (6), and juxtacrine signaling with adjacent cells has been reported (11). That these anatomical and functional features evolved and are conserved across species indicates that the signaling complexity within the pancreatic islet provides a selective advantage. In other words, it seems to be a good design. The narrative thread in this perspective is that α-cells become dysfunctional in diabetes because these structural and functional arrangements are destroyed.
Pancreatic islets seem unnecessarily complicated given that blood glucose concentration is a simple physiological variable that should be easy to regulate. In principle, based on how different their functional roles appear to be preventing hypoglycemia versus hyperglycemia, α-cells and β-cells could live separate lives in their own dedicated mini organs (e.g., in Alphaville and Betatown). Instead, pancreatic islets have a mixed composition that makes them look like small brains. To biologists this complexity may appear redundant and wasteful, but for engineers to complicate and specialize to optimize is a design principle (12).
Scientists are recognizing the advantages a complex islet composition provides. Early on, researchers determined that factors released from β-cells inhibit α-cells (13). The value of this type of interaction could be grasped immediately because it prevents glucagon from countering the hypoglycemic effects of insulin. Until recently, β-cell–to–α-cell communication was thought to be a unidirectional affair. It had been shown in vitro that glucagon stimulates β-insulin secretion (14), but researchers in our field could not envisage an in vivo scenario in which factors derived from α-cells could affect β-cells. Glucagon and insulin secretion from isolated human islets, however, overlap over most of the normoglycemic range (15). It took several recent papers to demonstrate that, although apparently paradoxical, α-cells contribute to amplifying insulin secretion in vivo (15–19). By releasing acetylcholine, glucagon, and GLP-1, α-cells have now been shown to have a major impact on β-cell function. In what could be seen as anticipatory regulation, α-cell input primes the β-cell for upcoming increases in glucose concentration. This allows the β-cell to secrete more efficiently because it is continuously on call and does not need to be awakened from a completely rested, inactivated state. We propose here that β-cells also prime α-cells. To appreciate this, we need to consider the electrical behavior of the α-cell.
The Electrical Behavior of the α-Cell Is Central to Glucagon Secretion
α-Cells are excitable cells. This means that they are capable of producing regenerative action potentials by activating voltage-gated ion channels at the plasma membrane. Generating these action potentials is an expensive endeavor. It has been calculated that these regenerative (pulsatile, digital) potentials cost cells more than 100 times the energy graded (continuous, analog) potentials would consume (20,21). Action potentials are extremely fast and last a few milliseconds. Not surprisingly, they are commonly used by neurons, which signal at a very fast pace. Not all endocrine cells are excitable, but those in the pancreas are. Indeed, pancreatic endocrine cells are conspicuously similar in their electrical behavior to neurons (and, thus, were mistakenly thought to be derived from ectodermal progenitors). That α- and β-cells are excitable is rather surprising, because these cells regulate physiological variables such as plasma glucose concentration in a time dimension that is orders of magnitude away from the speed and rate of action potentials (minutes versus milliseconds). What advantage does this metabolically costly electrical behavior provide the α-cell?
Let us first take a look at how the α-cell’s electrical behavior impacts glucagon secretion. α-Cells sense glucose like β-cells: glucose entering the cell is metabolized to generate ATP, and the resulting increase in the ATP/ADP ratio closes ATP-dependent K+ channels, thus depolarizing the membrane. Although both cells are equipped with similar ATP-dependent K+ channels, glucose regulates glucagon secretion under conditions when insulin secretion does not occur. ATP-dependent K+ channels in α-cells already have some activity in the absence of glucose and become strongly inhibited at low glucose concentrations (22). This closure of ATP-dependent K+ channels depolarizes the membrane and elicits action potentials that overshoot, that is, they peak at positive membrane potentials (23,24). This large depolarization activates the Ca2+ influx through high-voltage–gated P/Q-type Ca2+ channels that is required for glucagon secretion (23). When glucose levels increase, closure of residual ATP-dependent K+ channel activity further depolarizes the membrane but paradoxically inhibits glucagon. This is because during increased depolarization, voltage-gated Na+ and N-type Ca2+ channels inactivate, which reduces the size of action potentials and decreases glucagon secretion (22,24). It is therefore clear that small changes in the size of the action potential can initiate or stop glucagon secretion (22,25,26). Thus, information about the probability of glucagon release is encoded in the height and width of action potentials. This is important for our discussion below but still does not answer why expensive excitability was favored evolutionarily over cheaper graded signals in regulating glucagon secretion.
It is unlikely that the kinetics of glucagon responses benefit from the speed of individual action potentials. Being small, α-cells also do not need regenerative action potentials to transmit information over large distances, as neurons do. Action potentials, however, have other features: 1) they have a threshold, which means that a lot of subthreshold analog computing can be done before they are triggered; 2) they are regenerative, all-or-none signals, which means that they cancel the noise of analog signals; and 3) they are very robust and likely the only signals that depolarize the cell membrane to levels that activate the Ca2+ influx needed for exocytosis. The α-cell processes and integrates impinging inputs (e.g., changes in glucose concentration, paracrine signals derived from neighboring cells, and circulating adrenaline) and produces an executive summary in the shape of changes in membrane potential that determine the frequency and size of action potentials. The inbuilt threshold mechanism of action potentials filters out irrelevant influences and attenuates noise. By being sparse, action potentials may help α-cells save hormone. This is only possible because action potentials reliably couple to exocytosis, thereby guaranteeing a glucagon response.
Another important feature of electrical behavior is adaptation. Electrical signals decay over time and distance due to passive properties of the membrane as well as to inactivation of ion channels or desensitization of ionotropic receptors. We already saw how Na+ and Ca2+ channel inactivation can shape α-cell secretory responses. If this state persists, α-cells may still generate action potentials but not at the size needed for glucagon secretion. Importantly, under these conditions, α-cells may become refractory to changes in glucose concentration. Not being able to recover from adaptation may explain why α-cells fail to respond to hypoglycemia in diabetes.
Revisiting the “Switch-Off” Hypothesis of Glucagon Secretion
All secretory products from β- and δ-cells, be it γ-aminobutyric acid (GABA), insulin, Zn2+, serotonin, or somatostatin, have been reported to inhibit α-cells and decrease glucagon secretion. This paracrine signaling is seen as an important mechanism to prevent glucagon secretion during hyperglycemia. This, however, may be only part of what these factors accomplish. We propose that paracrine inhibition is a critical mechanism to reset α-cells and enable responses to a drop in glycemia. To explain this, let us revisit the “switch-off” hypothesis of glucagon secretion.
The intraislet insulin hypothesis (27,28) and its newer iteration, the β-cell switch-off hypothesis (29,30), propose that a sudden cessation of insulin secretion from β-cells in the islet during hypoglycemia is a necessary signal for the glucagon response from neighboring α-cells. Importantly, Robertson and colleagues (30) demonstrated that reestablishing the switch-off signal restores glucagon responses to hypoglycemia in an animal model of type 1 diabetes. The physiological mechanisms in the α-cell, however, were not explored. Do α-cells just secrete glucagon once freed from the inhibitory influence from β- and δ-cells, or are active processes involved? It turns out that α-cells secrete several molecules that provide positive feedback, thus instructing the α-cell to release more glucagon: glutamate, acetylcholine (our unpublished observations), and glucagon itself (31). These signaling molecules trigger different signaling cascades: membrane depolarization, Ca2+ release from intracellular stores, and cAMP increases, respectively. Antagonizing membrane receptors for these signaling molecules strongly inhibits glucagon secretion in response to decreases in glucose concentration (31). Thus, the α-cell response to hypoglycemia relies on redundant positive feedback.
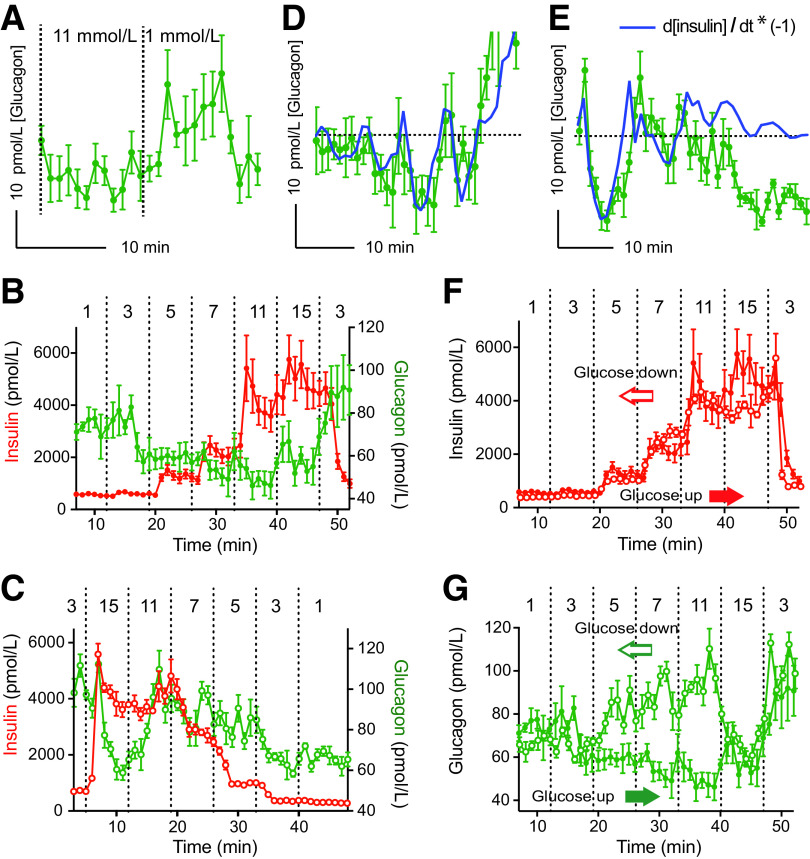
Although rare in nature because of its destabilizing potential, positive feedback allows the α-cells to mount a robust and rapid response once glucose levels drop. However, as discussed above, α-cells show adaptation in their electrical behavior. If so, glucagon secretion should be transient, not sustained, if conditions persist. This phenomenon has not been studied systematically, but in vitro studies show that glucagon responses are relatively brief (Fig. 1) (29,32). Inspecting the literature reveals that α-cells respond similarly in vivo in several mammalian species (Fig. 4 in Li et al. [33]; Fig. 1 in Munoz et al. [34]; Fig. 2 in Biggers et al. [35]; Fig. 2 in Canniff et al. [36]; Fig. 7 in Stern et al. [37]; Fig. 2 and 3 in Yue et al. [38]). In hypoglycemic clamps or prolonged hypoglycemia, glucagon levels wane while other counterregulatory responses are maintained (35,36,39) (Fig. 3 in Bolli et al. [40]; Fig. 2 in Rickels et al. [41]; Fig. 1 in Kendall et al. [42]; see also Garber et al. [43] and Santiago et al. [44]). By exposing human islets to a stepping up and down in glucose concentration, we further found that glucagon secretion does not seem to follow absolute glucose levels (Fig. 1). Instead, it is stimulated by changes in glucose concentration. Even a drop from 15 to 11 mmol/L, both hyperglycemic concentrations, elicited glucagon secretion (see also Santiago et al. [44]). Thus, α-cells seem to be differential detectors of decreases in glucose levels, not proportional detectors of absolute glucose concentration as β-cells are. Importantly, the glucagon response depended on the preceding glucose concentration: the concentration-response relationship when glucose was stepped up dramatically differed from that when glucose was stepped down (Fig. 1). These findings suggest that α-cell behavior shows hysteresis, that is, it is shaped by its history.
Figure 1.
The dynamics of glucagon secretion do not reflect absolute glucose levels. Glucagon and insulin secretion were measured in perifusion experiments of isolated human islets stimulated with changes in glucose concentration. A: Glucagon secretion, to a drop in glucose concentration from 11 to 1 mmol/L, is transient. B: Glucagon and insulin secretion in response to stepwise increases in glucose concentration, the conventional way to determine glucose dependency in the field of islet biology. C: Glucagon and insulin secretion in response to stepwise decreases in glucose concentration. Notice that glucagon secretion does not continue to increase as glucose levels decrease. D and E: Glucagon secretion (green symbols) tightly follows the inverted first derivative of insulin secretion (blue line, calculated from data shown in B and C and adjusted for a 3-min lag). At low levels of insulin secretion (last 20 min in E, also see C), glucagon secretion no longer follows the change in insulin secretion. F: The concentration–response relationship of insulin secretion was very similar when glucose levels were increased (glucose up) or decreased (glucose down). Data are those shown in panels B and C, with data in C reversed and aligned in time to that for the increase in glucose. G: The concentration–response relationship of glucagon secretion depended very much on whether glucose levels were increased or decreased stepwise. Data are from experiments performed by Cabrera et al. (31,80) (n = 4 technical replicates).
Figure 4.
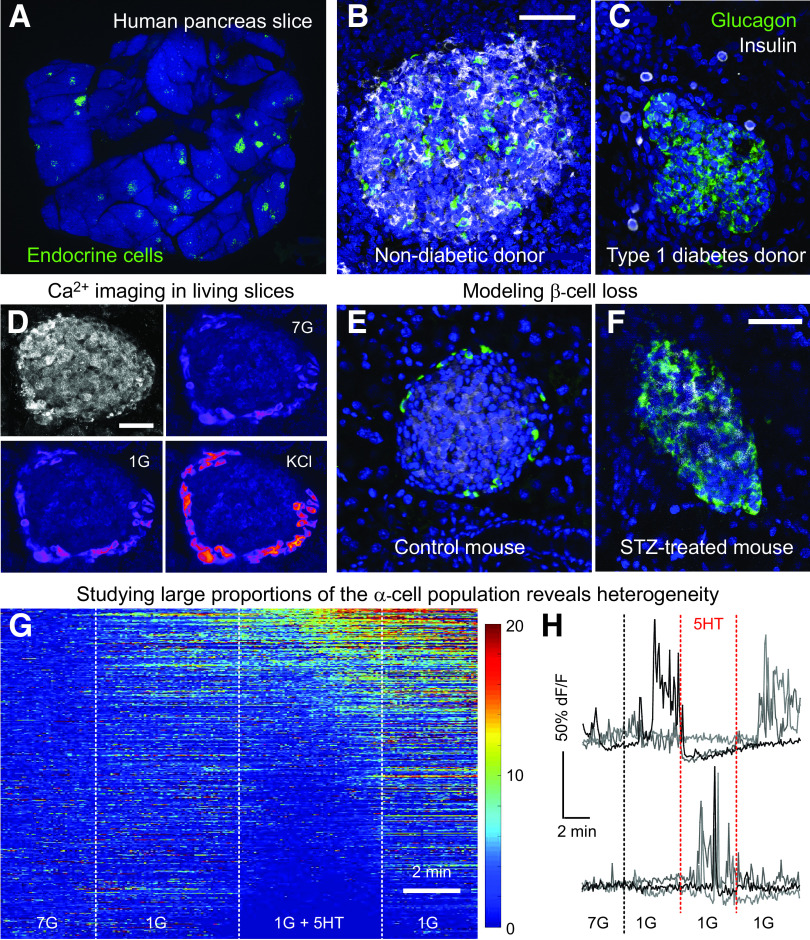
Using living pancreas slices to study α-cell function. A: A human pancreas slice immunostained for endocrine markers (green) after a physiologic experiment shows widespread distribution of islets embedded in the exocrine pancreas. B and C: Confocal images showing islets immunostained for insulin and glucagon in slices from a donor without diabetes (B) and a donor with type 1 diabetes (C). D: Ca2+ imaging performed in a living pancreas slice from a mouse expressing the genetically encoded Ca2+ indicator GCamP6 in α-cells. The islet stands out by its backscatter (top left). The fluorescence intensity of GCamP6 (in pseudocolor scale) increases in response to a drop in glucose from 7 to 1 mmol/L (1G) or to KCl depolarization. E and F: The loss of β-cells seen in type 1 diabetes can be mimicked in the mouse by using streptozotocin. Notice the similarity of the islet in a slice from a streptozotocin-treated mouse to the islet from the type 1 diabetes donor in C (same settings as those in B and C). G and H: Using Ca2+ imaging to interrogate large numbers of α-cells reveals differential responses to lowering the glucose concentration and to serotonin (10 μmol/L). Panel G shows a heatmap of GCamP6 fluorescence intensity over time, with each row representing a single α-cell (total of 555 α-cells, pooled from 8 islets, 8 slices, and 4 mice). Scale shows dF/F of GCamP6 fluorescence intensity. F: Representative traces of data shown in G illustrating the heterogeneity of responses to serotonin in the α-cell population. Scale bars: 50 μmol/L.
Figure 2.
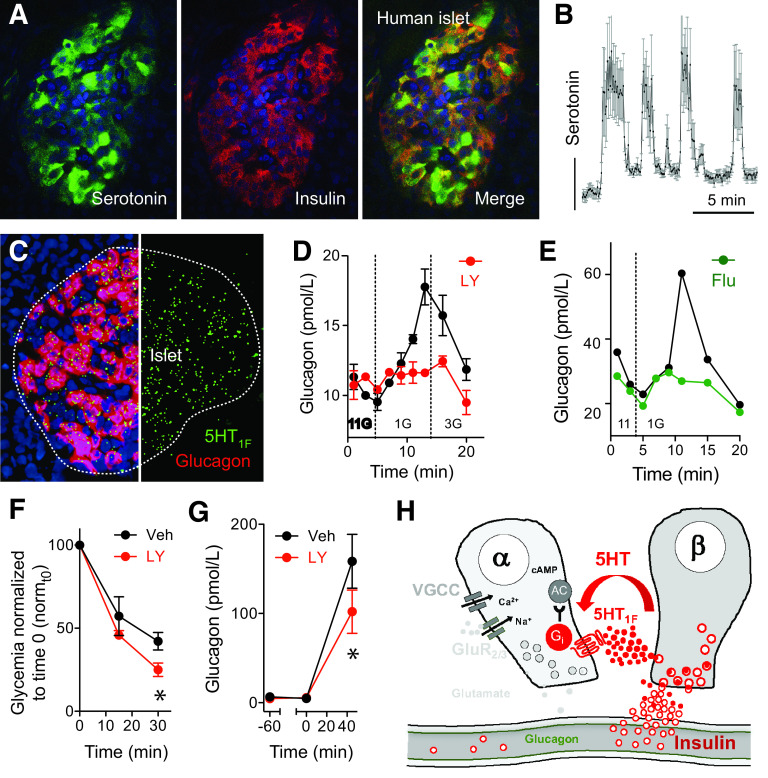
β-Cell–derived serotonin inhibits glucagon secretion. A: Maximal projection of confocal images showing serotonin labeling in an islet in a human pancreatic section. Serotonin colocalizes with insulin. B: Serotonin is secreted in pulses form human islets maintained at 11 mmol/L glucose. C: In situ hybridization for the serotonin receptor 5HT1F shows colocalization with transcripts for glucagon. D: Glucagon secretion in response to a drop in glucose concentration from 11 to 1 mmol/L is inhibited by the 5HT1F receptor antagonist LY344864 (100 nmol/L) in human islets. E: Glucagon secretion in response to a drop in glucose concentration from 11 to 1 mmol/L is inhibited by the SSRI fluvoxamine (Flu; 500 nmol/L). F: Insulin-induced hypoglycemia was exacerbated in the presence of LY344864 (LY) (n = 12 mice per group). Veh, vehicle. G: Increases in glucagon plasma levels stimulated by decreasing glycemia with insulin (1 unit/kg) were inhibited in the presence of the 5HT1F receptor agonist LY344864 (1 mg/kg, i.v.; n = 5 mice per group). H: Model of how β-cells release serotonin to regulate glucagon secretion in human islets. AC, adenylate cyclase; Gi, Galphai; GluR2/3, iGluRs of the AMPA type composed by GluR2 and GluR3 subunits; VGCC, voltage-gated Ca2+ channels. Data were adapted from Almaça et al. (32).
Figure 3.
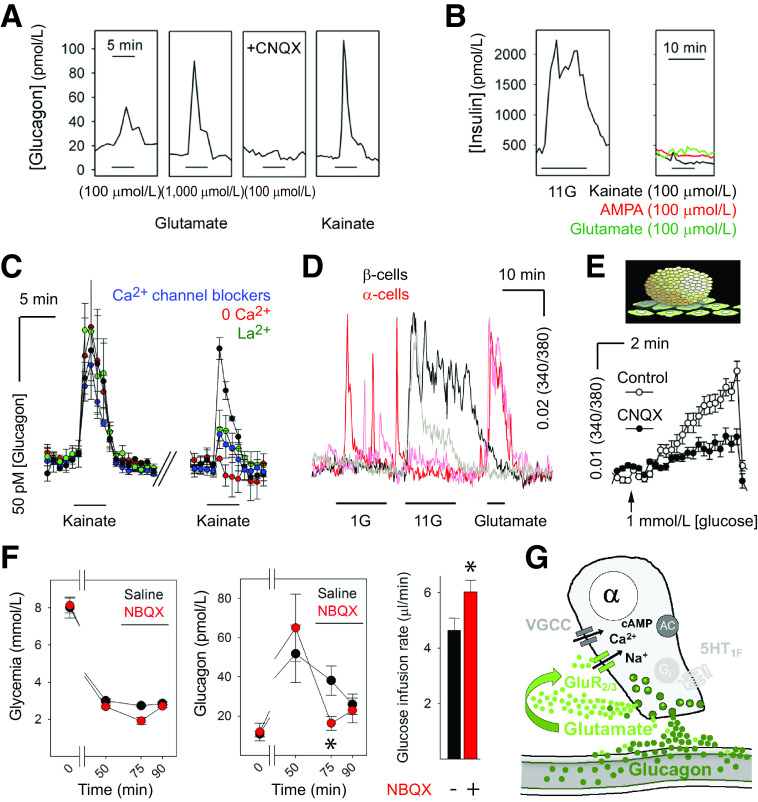
Glutamate receptor signaling forms a positive autocrine loop that amplifies glucagon secretion. A: Glutamate induced glucagon responses in human islets that could be blocked by the AMPA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (10 μmol/L). The iGluR agonists kainate and AMPA (both 100 μmol/L) also elicited strong glucagon secretion. B: Insulin release was induced by high glucose (11 mmol/L; 11G) but not by kainate (representative of six islet preparations). C: Glucagon secretion from human islets in response to kainate was blocked by Ca2+ channel blockers and was strongly reduced in the absence of nominal Ca2+ in the solution. D: Ca2+ imaging of dispersed human β-cells (black traces) and α-cells (red traces) showed that only α-cells responded to glutamate. E: Glucagon secretion in response to a drop from 11 to 1 mmol/L glucose concentration, as measured by biosensor cells, was inhibited in the presence of CNQX (10 μmol/L). F, left: Hyperinsulinemic-hypoglycemic clamp to provide a constant hypoglycemic stimulus at ∼3 mmol/L blood glucose concentration was induced with insulin infusion in mice. Glucagon secretion in response to hypoglycemia was significantly diminished in mice after infusion of the iGluR antagonist NBQX (10 mg/kg; red symbols; n = 7) compared with saline-infused mice (black symbols; n = 3; repeated-measures ANOVA, P < 0.05). Bar indicates drug infusion. Notice that the glucose infusion rate needed to maintain glycemia after drug infusion was significantly larger in NBQX-treated mice than in saline-treated mice (not shown; n = 3; Student’s t test, P < 0.05). G: Model of how α-cells release glutamate to amplify glucagon secretion in human islets. GluR2/3, iGluRs of the AMPA type composed by GluR2 and GluR3 subunits; VGCC, voltage-gated Ca2+ channels. Data werae adapted from Cabrera et al. (31).
The basis for hysteresis is likely to be found in the adaptation (and recovery from it) that determines the α-cell’s electrical behavior. In this context, inhibition may play a role that is equivalent to stimulation. Although counterintuitive, inhibition can be an instructive signal shaping cellular responses in other tissues. In a fascinating study about signal transmission in the retina, it was shown that, at the first synapse in the visual pathway between cones and “off” bipolar cells, the amplitudes of postsynaptic membrane depolarizations depended on the preceding synaptic inhibition (45).
We propose this analogy: when the influence of β- and δ-cells decreases once glucose levels drop, positive feedback stimulates α-cells, but glucagon secretion eventually subsides because Na+ and Ca2+ channels inactivate and glutamate receptors desensitize. Once glucose levels rise (in part by the glucagon burst), β- and δ-cells start releasing a panoply of inhibitory factors that allow α-cells to recover from inactivation and desensitization. Among these factors, insulin acts by translocating GABAA receptors to the α-cell membrane (46). Interstitial GABA then activates these receptors and hyperpolarizes the α-cell (46,47). The hyperpolarization relieves inactivation of Na+ and Ca2+ channels. Because glucagon and glutamate secretion also subsides, glutamate receptors recover from desensitization (48). A subsequent drop in glucose concentration reduces inhibitory input from β- and δ-cells and KATP channel activity in α-cells, leading to glucagon secretion (49). The inhibition of α-cells not only serves to avoid cosecretion of glucagon with insulin but also is crucial for resetting the α-cell, enabling it to respond to the next decrease in glucose. Thus, inhibition by its neighbors is a priming signal for α-cells.
The emerging scheme is that intraislet signaling is orchestrated in ways that anticipate need. It is essential for β-cells to be prepared for upcoming increases in glycemia. The islet arrangement also prepares α-cells to respond to and prevent hypoglycemia. This mutual anticipatory regulation makes the islet ensemble much stronger than its individual parts. The anticipatory regulation prepares the islet to cope with needs (e.g., the constant changes in glycemia) before they lead to errors (hyper- or hypoglycemia). This is different from and more efficient than feedback control after the error occurs. Alone, α-cells would not be able to secrete glucagon appropriately, as seen in type 1 diabetes.
Targeting Intrinsic Signaling Mechanisms to Reverse the α-Cell’s Glucose Blindness
What do α-cells go through when diabetes ensues? Reports on the number of α- and δ-cells left in islets of individuals with type 1 diabetes indicate that the autoimmune attack works with surgical precision to exclusively destroy β-cells (50,51). The presence of killer T cells and high levels of cytokines may enrich transcripts of genes related to immune activation and allograft rejection (52), but otherwise α-cells survive and respond to stimulation (e.g., with arginine [6]). Loss of glucose counterregulation develops years after diagnosis, when α-cells have been exposed to a new world in which β-cells are absent, sympathetic innervation recedes, and uncontrolled glycemia prevails (5,53,54). Two recent studies showed that Ca2+ channel activity and gene expression decrease in the α-cell in type 1 diabetes, suggesting that the cell loses part of its potential for excitability (52,55). We reason that loss of electrical excitability could also be caused by chronic adaptation. Importantly, by then, the priming effects from β-cells are gone. In the absence of the inhibitory influence of β-cells, α-cells cannot repolarize their membrane potential (needed to reverse ion channel inactivation), cannot recover from receptor desensitization (needed to reestablish positive feedback), and, thus, cannot fire full-blown action potentials (needed for the glucagon response to a drop in glycemia). At the same time, without inhibition of cAMP production by β-cell–derived serotonin (32), cAMP levels now promote increased basal levels of glucagon secretion. As a result, the α-cell secretes unsolicited glucagon and is blind to changes in glucose levels.
Reestablishing these regulatory mechanisms seems a logical and plausible way to reverse the α-cell’s glucose blindness. We propose that activating endogenous paracrine and autocrine signaling pathways in the islet can restore the α-cell’s ability to respond to hypoglycemia in type 1 diabetes. Targeting receptors for these signaling pathways could 1) provide inhibitory inputs that allow recovery from adaptation and, thus, reset α-cell function and 2) potentiate endogenous positive feedback to promote α-cell responses to a lowering in glucose concentration. We focus on signaling pathways for which there is substantial support from the literature and for which drugs are already approved for use in human beings. There is a panoply of pharmacological agents in the clinic or in preclinical studies for other conditions that can be repurposed to target these receptors in α-cells in type 1 diabetes (Table 1).
Table 1.
Pharmacological compounds targeting paracrine and autocrine signaling pathways
| Drug name | Property | Tested effect on α-cells | Stage |
|---|---|---|---|
| Glutamate | Natural agonist | Stimulates (in vivo*, in vitro†) | Experimental‡ |
| AMPA | iGluR agonist | Stimulates (in vivo, in vitro) | Experimental‡ |
| Kainate | iGluR agonist | Stimulates (in vivo, in vitro) | Experimental‡ |
| CNQX | iGluR antagonist | Inhibits (in vitro) | Experimental |
| DNQX | iGluR antagonist | Inhibits (in vitro) | Experimental |
| Perampanel | iGluR antagonist | Not tested yet | FDA approved |
| Cyclothiazide | iGluR PAM | Not tested yet | FDA approved |
| Mibampator | iGluR PAM | Not tested yet | Phase II |
| Aniracetam | iGluR PAM | Not tested yet | Over the counter |
| Serotonin | Natural agonist | Inhibits (in vitro) | Experimental |
| LY344864 | HTR1F agonist | Inhibits (in vivo, in vitro) | Experimental‡ |
| Lasmiditan | HTR1F agonist | Not tested yet | Phase II |
| Methysergide | HTR1F antagonist | Not tested yet | FDA approved |
| Reserpine | Blocker of VMAT | Stimulates (in vitro) | FDA approved |
| Somatostatin | Natural agonist | Inhibits | Experimental |
| Octreotide | SSTR agonist | Inhibits | FDA approved |
| Cyclosomatostatin | SSTR antagonist | Stimulates (in vitro) | Experimental |
| GABA | Natural agonist | Inhibits | Food supplement |
Perampanel is the first and only FDA-approved noncompetitive AMPA receptor antagonist used as an adjunctive therapy in the treatment of focal seizures with or without secondary generalized seizures. Cyclothiazide (Anhydron) is used to treat edema caused by disease (e.g., congestive heart failure or hepatic cirrhosis). Mibampator (LY451395) is used in clinical trials for the treatment of Alzheimer's disease (clinical trials reg. no. NCT00051909, ClinicalTrials.gov). Aniracetam is an over-the-counter drug used for its nootropic (cognition-enhancing) and central stimulant effects; it is approved in some countries. Lasmiditan is in phase II and III clinical trials for the acute treatment of migraine (clinical trial reg nos. NCT00384774 and NCT02605174, ClinicalTrials.gov). Methysergide is used in the treatment of migraine and cluster headaches. Use of this drug is discontinued in the U.S. Reserpine is an antihypertensive drug also used as an antipsychotic. Its clinical utility is limited due to its adverse effects profile. CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; DNQX, 6,7-dinitroquinoxaline-2,3-dione; HTR, serotonin receptor; PAM, positive allosteric modulator; SSTR, somatostatin receptor antagonist; VMAT, vesicular monoamine transporter.
Effects in vivo were tested in mice only.
Effects in vitro were tested on human and mouse islets.
Does not cross blood-brain barrier; has only peripheral effects.
We recently showed that human β-cells produce and secrete serotonin when stimulated (32). Serotonin secretion from β-cells decreases cAMP levels in neighboring α-cells via 5HT1F receptors and inhibits glucagon secretion (32,56–58). Importantly, in the islet, expression of the 5HT1F receptor is restricted to the α-cell (32,59,60). We found that without serotonergic input, α-cells lose their ability to regulate glucagon secretion in response to changes in glucose concentration (Fig. 2). This suggests that diminished serotonergic control of α-cells can cause glucose blindness and the uncontrolled glucagon secretion associated with diabetes. Because of comorbidity of diabetes and depression, patients with diabetes are frequently treated with selective serotonin reuptake inhibitor (SSRI) antidepressants. The hypoglycemic effects of SSRIs have been proposed to be beneficial for patients with diabetes (61). In our hands (Fig. 2) (32), SSRIs increased serotonin levels within the islet and inhibited glucagon secretion from α-cells. Thus, modulation of serotonin signaling in the islet represents a drug intervention opportunity. This may also be true for GABA signaling, which is also reduced in type 1 diabetes (47). Although this requires experimental confirmation at the islet level, GABA could be provided as a food supplement to reset α-cells (Table 1).
SSRIs, however, may also render patients with diabetes susceptible to life-threatening hypoglycemia. Indeed, patients on long-term SSRI treatment have poor counterregulatory responses (62–66). This exemplifies the potential dangers of targeting α-cells to control hyperglucagonemia and emphasizes the need for combining approaches.
Perhaps more important than treating hyperglucagonemia is reestablishing glucose-dependent glucagon secretion to prevent life-threatening hypoglycemia. For almost three decades it has been known that glutamate stimulates glucagon secretion via ionotropic glutamate receptors (iGluRs) of the AMPA type (67). Since then, studies have demonstrated that human α-cells express high levels of iGluRs (31,60,68). Importantly, glutamate and other agonists (e.g., kainate) act on iGluRs of the AMPA/kainate type, resulting in membrane depolarization, opening of voltage-gated Ca2+ channels, increase in cytoplasmic free Ca2+ concentration, and enhanced glucagon release (approximately fivefold increase in secretion) (Fig. 3) (31). Because these effects are very strong and not seen in human β-cells, activation of iGluRs is used in several laboratories as a means to specifically stimulate α-cells in human islets (69–71; for similar results in mouse α-cells, see Cho et al. [72] and Zhang et al. [73]). Studies further determined that a lowering in glucose concentration results in the release of glutamate from the α-cell (31,74). Importantly, in vivo blockade of iGluRs in the mouse reduces glucagon secretion and exacerbates insulin-induced hypoglycemia (31). The glutamate autocrine feedback loop thus endows the α-cell with the ability to effectively potentiate its own secretory activity.
It is very likely that α-cells are capable of secreting glutamate in type 1 diabetes given that glutamate is cosecreted with glucagon and α-cells still efficiently release glucagon granules (e.g., in response to arginine [6]). In a background of persisting glutamate and glucagon secretion, it would make little sense to further stimulate and desensitize the receptors by direct stimulation with glutamate receptor agonists. Instead, we propose to use positive allosteric modulators of AMPA receptors prescribed to improve cognitive impairment in dementia (75). This group of drugs includes many compounds of different chemical classes approved for use in human beings. Positive allosteric modulators potentiate AMPA receptor signaling only in the presence of glutamate and, hence, do not activate the receptor directly (76). To our knowledge (PubMed and Google searches), these drugs have not been tested in the context of activating pancreatic α-cells to prevent hypoglycemia. One positive allosteric modulator, Aniracetam, is sold in Europe as a prescription drug. Despite lack of approval from the U.S. Food and Drug Administration (FDA), the drug is readily available over the counter in the U.S. as a dietary supplement. Aniracetam and other FDA-approved modulators (Table 1) could be tested for their potential to reverse α-cell glucose blindness in preclinical trials.
A key issue for potential therapeutic strategies is how treatments will interfere with the effects of the prevailing glycemia on α-cells. The α-cell neighbors provide inhibitory input under hyperglycemic conditions, while potential therapeutic strategies would be present under hypoglycemic and hyperglycemic conditions. However, glucagon responses do not depend on an absolute plasma glucose concentration but rather on decrements in the glucose concentration (Fig. 1) (44). In principle, this should make interventions glycemia independent. It would also be beneficial if the treatment did not indiscriminately increase basal glucagon secretion. These issues, however, need to be resolved experimentally in preclinical studies.
Technical Approaches to Study α-Cell Function in Type 1 Diabetes
Beyond the conceptual roadblocks that have prevented a better understanding of what goes awry with α-cells, major barriers are likely logistical and technical: it is difficult to study islets from donors with type 1 diabetes. Until recently there was no coordinated effort to procure pancreases from these donors for physiological studies. Even now that there is the Network for Pancreatic Organ Donors with Diabetes (nPOD) (77), the number of cases dedicated to functional studies is still small (5–10 pancreases per year nationwide). A further problem is that it is difficult to isolate islets from these donors because the islet structure breaks down due to β-cell loss. In a heroic and unprecedented effort, Brissova et al. (55) were able to isolate islets from donors with type 1 diabetes, record insulin and glucagon secretion, and confirm loss of glucose counterregulation in vitro.
An alternative to isolating islets is to produce living slices from these pancreases, as we recently reported (78). Using pancreas tissue slices from donors with type 1 diabetes allowed assessing physiological processes critical for disease development, such as insulin secretion, β-cell responses, endocrine cell morphology, and immune infiltration within the same donor organ. We are currently extending these studies to figure out how α-cell function changes in type 1 diabetes and if their dysfunction can be reversed (Fig. 4). An interesting approach is to purify human α-cells from islets of deceased donors and reaggregate them with or without β-cells (79). These studies already demonstrated the importance of cell-to-cell contacts between α- and β-cells for adequate inhibition of glucagon secretion when the glucose concentration increases. Future studies should test the response to a decrease in glucose concentrations, which is relevant for glucose counterregulation.
While we wait for increased availability of tissues from donors with type 1 diabetes, we can still benefit from mouse models. We can take advantage of the fact that mice lose their ability to respond to hypoglycemia after their β-cells are destroyed by the toxin streptozotocin. This model does not recapitulate the natural history of type 1 diabetes, but it mimics the loss of β-cells and counterregulatory responses to hypoglycemia seen in human beings. For certain aspects of paracrine and autocrine regulation, it has been shown that receptor signaling pathways are similar in the mouse. These can be readily manipulated in vivo in the mouse to regulate α-cell function and plasma glucagon levels (Fig. 2 and 3) (31,32). In those instances, the mouse could be an adequate experimental model as a first step toward more relevant preclinical studies in human beings.
Concluding Remarks
Recurrent hypoglycemia due to defective glucose counterregulation represents a morbidity and mortality risk that prevents more aggressive insulin therapy in type 1 diabetes. In this context, and given its dominant role in glucose counterregulation, understanding how α-cell function is disrupted in type 1 diabetes should be a research priority. However, studying α-cells in type 1 diabetes has hit major conceptual, logistic, and technological roadblocks in the past. What we propose is biased by our own work (Fig. 5). Still, it takes into account what is known about the α-cell’s electrical properties and its autocrine and paracrine regulation. This model is not complete. Indeed, it may be too early to propose a model for how α-cells respond to hypoglycemia. A concerted effort is needed to study the α-cell as much as the β-cell has been studied. The methods conventionally used to investigate islet physiology may not be appropriate or are very difficult to apply to α-cells in islets from donors with type 1 diabetess. We need additional approaches, such as living pancreas slices, to help overcome these limitations. Like the morphological studies that changed our view of how immune cells infiltrate the human islet in type 1 diabetes (54), we expect future studies to impact our model of α-cell dysfunction in this devastating condition. Moreover, if we identify the signaling pathways that become defective in type 1 diabetes, we could target them pharmacologically to correct glucagon secretion. Bringing to light the basic mechanisms of dysfunction will guide us in our quest to reverse glucose blindness in α-cells.
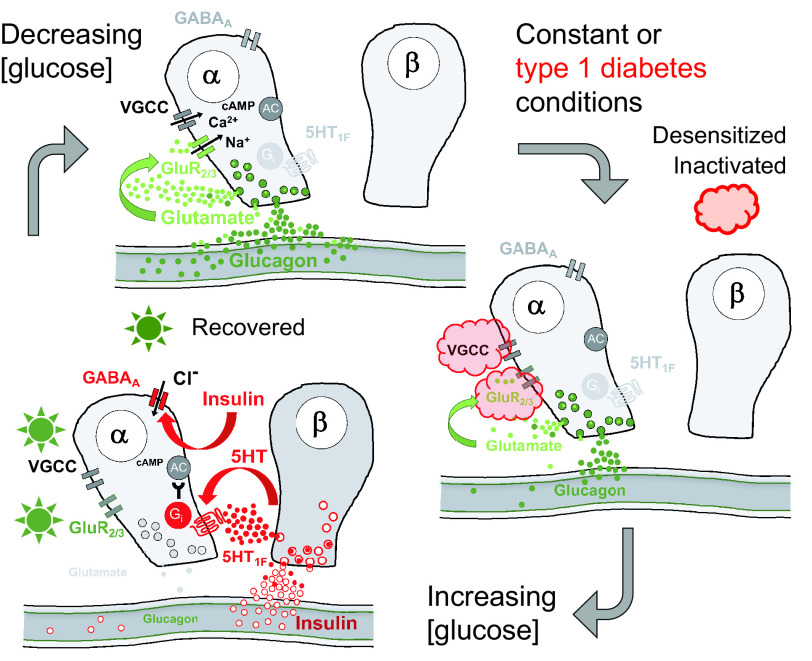
Figure 5.
Proposed model of regulation of glucagon secretion. α-Cells are activated by a lowering of glucose concentration. Under constant conditions, glucagon secretion wanes because voltage-gated channels inactivate and ionotropic glutamate receptors desensitize (red cloud). When glucose levels increase, β-cells secrete insulin and other paracrine factors that inhibit α-cells, among others, by hyperpolarizing the cell via GABAA receptors. This allows voltage-gated ion channel and ionotropic glutamate receptors to recover from inactivation and desensitization (green star). In type 1 diabetes, α-cells are locked in the refractory state shown on the right because they cannot be reset by β-cell input. GluR2/3, iGluRs of the AMPA type composed by GluR2 and GluR3 subunits; VGCC, voltage-gated Ca2+ channels.
Article Information
Funding. This work was supported by the Diabetes Research Institute Foundation and National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, grants R56DK084321 (A.C.), R01DK084321 (A.C.), R01DK111538 (A.C.), R01DK113093 (A.C.), U01DK120456 (A.C.), R33ES025673 (A.C.), and R21ES025673 (A.C.) and the Leona M. and Harry B. Helmsley Charitable Trust grants G-2018PG-T1D034 and G-1912-03552.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
References
- 1. Rizza RA, Cryer PE, Gerich JE. Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. Effects of somatostatin and combined alpha- and beta-adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin-induced hypoglycemia. J Clin Invest 1979;64:62–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gerich JE. Lilly lecture 1988. Glucose counterregulation and its impact on diabetes mellitus. Diabetes 1988;37:1608–1617 [DOI] [PubMed] [Google Scholar]
- 3. Mitrakou A, Ryan C, Veneman T, et al. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol 1991;260:E67–E74 [DOI] [PubMed] [Google Scholar]
- 4. Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science 1973;182:171–173 [DOI] [PubMed] [Google Scholar]
- 5. Cryer PE. Hypoglycemia is the limiting factor in the management of diabetes. Diabetes Metab Res Rev 1999;15:42–46 [DOI] [PubMed] [Google Scholar]
- 6. Gilon P. The role of α-cells in islet function and glucose homeostasis in health and type 2 diabetes. J Mol Biol 2020;432:1367–1394 [DOI] [PubMed] [Google Scholar]
- 7. Holst JJ, Holland W, Gromada J, et al. Insulin and glucagon: partners for life. Endocrinology 2017;158:696–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Unger RH, Raskin P, Srikant CB, Orci L. Glucagon and the A cells. Recent Prog Horm Res 1976;33:477–517 [DOI] [PubMed] [Google Scholar]
- 9. Campbell JE, Newgard CB. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat Rev Mol Cell Biol 2021;22:142–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jeon J, Correa-Medina M, Ricordi C, Edlund H, Diez JA. Endocrine cell clustering during human pancreas development. J Histochem Cytochem 2009;57:811–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hutchens T, Piston DW. EphA4 receptor forward signaling inhibits glucagon secretion from α-cells. Diabetes 2015;64:3839–3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Glegg GL. The science of design. Cambridge, University Press, 1973 [Google Scholar]
- 13. Asplin CM, Paquette TL, Palmer JP. In vivo inhibition of glucagon secretion by paracrine beta cell activity in man. J Clin Invest 1981;68:314–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huypens P, Ling Z, Pipeleers D, Schuit F. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia 2000;43:1012–1019 [DOI] [PubMed] [Google Scholar]
- 15. Rodriguez-Diaz R, Molano RD, Weitz JR, et al. Paracrine interactions within the pancreatic islet determine the glycemic set point. Cell Metab 2018;27:549–558.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Traub S, Meier DT, Schulze F, et al. Pancreatic α-cell-derived glucagon-related peptides are required for β cell adaptation and glucose homeostasis. Cell Rep 2017;18:3192–3203 [DOI] [PubMed] [Google Scholar]
- 17. Capozzi ME, Svendsen B, Encisco SE, et al. β Cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight 2019;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Svendsen B, Larsen O, Gabe MBN, et al. Insulin secretion depends on intra-islet glucagon signaling. Cell Rep 2018;25:1127–1134 [DOI] [PubMed] [Google Scholar]
- 19. Zhu L, Dattaroy D, Pham J, et al. Intra-islet glucagon signaling is critical for maintaining glucose homeostasis. JCI Insight 2019;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 2001;21:1133–1145 [DOI] [PubMed] [Google Scholar]
- 21. Sengupta B, Laughlin SB, Niven JE. Consequences of converting graded to action potentials upon neural information coding and energy efficiency. PLOS Comput Biol 2014;10:e1003439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. MacDonald PE, De Marinis YZ, Ramracheya R, et al. A K ATP channel-dependent pathway within alpha cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biol 2007;5:e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramracheya R, Ward C, Shigeto M, et al. Membrane potential-dependent inactivation of voltage-gated ion channels in alpha-cells inhibits glucagon secretion from human islets. Diabetes 2010;59:2198–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Q, Ramracheya R, Lahmann C, et al. Role of KATP channels in glucose-regulated glucagon secretion and impaired counterregulation in type 2 diabetes. Cell Metab 2013;18:871–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Q, Dou H, Rorsman P. “Resistance is futile?”—paradoxical inhibitory effects of KATP channel closure in glucagon-secreting α-cells. J Physiol 2020;598:4765–4780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Watts M, Ha J, Kimchi O, Sherman A. Paracrine regulation of glucagon secretion: the β/α/δ model. Am J Physiol Endocrinol Metab 2016;310:E597–E611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Samols E, Harrison J. Intraislet negative insulin-glucagon feedback. Metabolism 1976;25(Suppl. 1):1443–1447 [DOI] [PubMed] [Google Scholar]
- 28. Banarer S, McGregor VP, Cryer PE. Intraislet hyperinsulinemia prevents the glucagon response to hypoglycemia despite an intact autonomic response. Diabetes 2002;51:958–965 [DOI] [PubMed] [Google Scholar]
- 29. Hope KM, Tran PO, Zhou H, Oseid E, Leroy E, Robertson RP. Regulation of alpha-cell function by the beta-cell in isolated human and rat islets deprived of glucose: the “switch-off” hypothesis. Diabetes 2004;53:1488–1495 [DOI] [PubMed] [Google Scholar]
- 30. Zhou H, Tran PO, Yang S, et al. Regulation of alpha-cell function by the beta-cell during hypoglycemia in Wistar rats: the “switch-off” hypothesis. Diabetes 2004;53:1482–1487 [DOI] [PubMed] [Google Scholar]
- 31. Cabrera O, Jacques-Silva MC, Speier S, et al. Glutamate is a positive autocrine signal for glucagon release. Cell Metab 2008;7:545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Almaça J, Molina J, Menegaz D, et al. Human beta cells produce and release serotonin to inhibit glucagon secretion from alpha cells. Cell Rep 2016;17:3281–3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li NX, Brown S, Kowalski T, et al. GPR119 agonism increases glucagon secretion during insulin-induced hypoglycemia. Diabetes 2018;67:1401–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Muñoz A, Hu M, Hussain K, Bryan J, Aguilar-Bryan L, Rajan AS. Regulation of glucagon secretion at low glucose concentrations: evidence for adenosine triphosphate-sensitive potassium channel involvement. Endocrinology 2005;146:5514–5521 [DOI] [PubMed] [Google Scholar]
- 35. Biggers DW, Myers SR, Neal D, et al. Role of brain in counterregulation of insulin-induced hypoglycemia in dogs. Diabetes 1989;38:7–16 [DOI] [PubMed] [Google Scholar]
- 36. Canniff KM, Smith MS, Lacy DB, Williams PE, Moore MC. Glucagon secretion and autonomic signaling during hypoglycemia in late pregnancy. Am J Physiol Regul Integr Comp Physiol 2006;291:R788–R795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stern JH, Smith GI, Chen S, Unger RH, Klein S, Scherer PE. Obesity dysregulates fasting-induced changes in glucagon secretion. J Endocrinol 2019;243:149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yue JT, Burdett E, Coy DH, Giacca A, Efendic S, Vranic M. Somatostatin receptor type 2 antagonism improves glucagon and corticosterone counterregulatory responses to hypoglycemia in streptozotocin-induced diabetic rats. Diabetes 2012;61:197–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. De Feo P, Perriello G, De Cosmo S, et al. Comparison of glucose counterregulation during short-term and prolonged hypoglycemia in normal humans. Diabetes 1986;35:563–569 [PubMed] [Google Scholar]
- 40. Bolli GB, Gottesman IS, Cryer PE, Gerich JE. Glucose counterregulation during prolonged hypoglycemia in normal humans. Am J Physiol 1984;247:E206–E214 [DOI] [PubMed] [Google Scholar]
- 41. Rickels MR, Schutta MH, Mueller R, et al. Islet cell hormonal responses to hypoglycemia after human islet transplantation for type 1 diabetes. Diabetes 2005;54:3205–3211 [DOI] [PubMed] [Google Scholar]
- 42. Kendall DM, Teuscher AU, Robertson RP. Defective glucagon secretion during sustained hypoglycemia following successful islet allo- and autotransplantation in humans. Diabetes 1997;46:23–27 [DOI] [PubMed] [Google Scholar]
- 43. Garber AJ, Cryer PE, Santiago JV, Haymond MW, Pagliara AS, Kipnis DM. The role of adrenergic mechanisms in the substrate and hormonal response to insulin-induced hypoglycemia in man. J Clin Invest 1976;58:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Santiago JV, Clarke WL, Shah SD, Cryer PE. Epinephrine, norepinephrine, glucagon, and growth hormone release in association with physiological decrements in the plasma glucose concentration in normal and diabetic man. J Clin Endocrinol Metab 1980;51:877–883 [DOI] [PubMed] [Google Scholar]
- 45. DeVries SH, Schwartz EA. Kainate receptors mediate synaptic transmission between cones and ‘Off’ bipolar cells in a mammalian retina. Nature 1999;397:157–160 [DOI] [PubMed] [Google Scholar]
- 46. Xu E, Kumar M, Zhang Y, et al. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab 2006;3:47–58 [DOI] [PubMed] [Google Scholar]
- 47. Menegaz D, Hagan DW, Almaça J, et al. Mechanism and effects of pulsatile GABA secretion from cytosolic pools in the human beta cell. Nat Metab 2019;1:1110–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mayer ML, Armstrong N. Structure and function of glutamate receptor ion channels. Annu Rev Physiol 2004;66:161–181 [DOI] [PubMed] [Google Scholar]
- 49. Rorsman P, Salehi SA, Abdulkader F, Braun M, MacDonald PE. K(ATP)-channels and glucose-regulated glucagon secretion. Trends Endocrinol Metab 2008;19:277–284 [DOI] [PubMed] [Google Scholar]
- 50. Stefan Y, Orci L, Malaisse-Lagae F, Perrelet A, Patel Y, Unger RH. Quantitation of endocrine cell content in the pancreas of nondiabetic and diabetic humans. Diabetes 1982;31:694–700 [DOI] [PubMed] [Google Scholar]
- 51. Rahier J, Goebbels RM, Henquin JC. Cellular composition of the human diabetic pancreas. Diabetologia 1983;24:366–371 [DOI] [PubMed] [Google Scholar]
- 52. Camunas-Soler J, Dai XQ, Hang Y, et al. Patch-Seq links single-cell transcriptomes to human islet dysfunction in diabetes. Cell Metab 2020;31:1017–1031.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mundinger TO, Mei Q, Foulis AK, Fligner CL, Hull RL, Taborsky GJ Jr. Human type 1 diabetes is characterized by an early, marked, sustained, and islet-selective loss of sympathetic nerves. Diabetes 2016;65:2322–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Campbell-Thompson M, Fu A, Kaddis JS, et al. Insulitis and β-cell mass in the natural history of type 1 diabetes. Diabetes 2016;65:719–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Brissova M, Haliyur R, Saunders D, et al. α Cell function and gene expression are compromised in type 1 diabetes. Cell Rep 2018;22:2667–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Marco J, Hedo JA, Villanueva ML. Inhibition of glucagon release by serotonin in mouse pancreatic islets. Diabetologia 1977;13:585–588 [DOI] [PubMed] [Google Scholar]
- 57. Pontiroli AE, Micossi P, Foá PP. Effects of serotonin, of its biosynthetic precursors and of the anti-serotonin agent metergoline on the release of glucagon and insulin from rat pancreas. Horm Metab Res 1978;10:200–203 [DOI] [PubMed] [Google Scholar]
- 58. Bennet H, Balhuizen A, Medina A, et al. Altered serotonin (5-HT) 1D and 2A receptor expression may contribute to defective insulin and glucagon secretion in human type 2 diabetes. Peptides 2015;71:113–120 [DOI] [PubMed] [Google Scholar]
- 59. Blodgett DM, Nowosielska A, Afik S, et al. Novel observations from next-generation RNA sequencing of highly purified human adult and fetal islet cell subsets. Diabetes 2015;64:3172–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Segerstolpe Å, Palasantza A, Eliasson P, et al. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab 2016;24:593–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Daubresse JC, Kolanowski J, Krzentowski G, Kutnowski M, Scheen A, Van Gaal L. Usefulness of fluoxetine in obese non-insulin-dependent diabetics: a multicenter study. Obes Res 1996;4:391–396 [DOI] [PubMed] [Google Scholar]
- 62. Deeg MA, Lipkin EW. Hypoglycemia associated with the use of fluoxetine. West J Med 1996;164:262–263 [PMC free article] [PubMed] [Google Scholar]
- 63. Takhar J, Williamson P. Hypoglycemia associated with high doses of sertraline and sulphonylurea compound in a noninsulin-dependent diabetes mellitus patient. Can J Clin Pharmacol 1999;6:12–14 [PubMed] [Google Scholar]
- 64. Sawka AM, Burgart V, Zimmerman D. Loss of hypoglycemia awareness in an adolescent with type 1 diabetes mellitus during treatment with fluoxetine hydrochloride. J Pediatr 2000;136:394–396 [DOI] [PubMed] [Google Scholar]
- 65. Derijks HJ, Heerdink ER, De Koning FH, Janknegt R, Klungel OH, Egberts AC. The association between antidepressant use and hypoglycaemia in diabetic patients: a nested case-control study. Pharmacoepidemiol Drug Saf 2008;17:336–344 [DOI] [PubMed] [Google Scholar]
- 66. Biagetti B, Corcoy R. Hypoglycemia associated with fluoxetine treatment in a patient with type 1 diabetes. World J Clin Cases 2013;1:169–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bertrand G, Gross R, Puech R, Loubatières-Mariani MM, Bockaert J. Glutamate stimulates glucagon secretion via an excitatory amino acid receptor of the AMPA subtype in rat pancreas. Eur J Pharmacol 1993;237:45–50 [DOI] [PubMed] [Google Scholar]
- 68. Li J, Yu Q, Ahooghalandari P, et al. Submembrane ATP and Ca2+ kinetics in α-cells: unexpected signaling for glucagon secretion. FASEB J 2015;29:3379–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Köhler M, Daré E, Ali MY, et al. One-step purification of functional human and rat pancreatic alpha cells. Integr Biol 2012;4:209–219 [DOI] [PubMed] [Google Scholar]
- 70. Shuai H, Xu Y, Yu Q, Gylfe E, Tengholm A. Fluorescent protein vectors for pancreatic islet cell identification in live-cell imaging. Pflugers Arch 2016;468:1765–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Molina J, Rodriguez-Diaz R, Fachado A, Jacques-Silva MC, Berggren PO, Caicedo A. Control of insulin secretion by cholinergic signaling in the human pancreatic islet. Diabetes 2014;63:2714–2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cho JH, Chen L, Kim MH, Chow RH, Hille B, Koh DS. Characteristics and functions of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors expressed in mouse pancreatic alpha-cells. Endocrinology 2010;151:1541–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang H, Liu R, Deng T, et al. The microRNA-124-iGluR2/3 pathway regulates glucagon release from alpha cells. Oncotarget 2016;7:24734–24743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hayashi M, Otsuka M, Morimoto R, et al. Differentiation-associated Na+-dependent inorganic phosphate cotransporter (DNPI) is a vesicular glutamate transporter in endocrine glutamatergic systems. J Biol Chem 2001;276:43400–43406 [DOI] [PubMed] [Google Scholar]
- 75. Partin KM. AMPA receptor potentiators: from drug design to cognitive enhancement. Curr Opin Pharmacol 2015;20:46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Black MD. Therapeutic potential of positive AMPA modulators and their relationship to AMPA receptor subunits. A review of preclinical data. Psychopharmacology (Berl) 2005;179:154–163 [DOI] [PubMed] [Google Scholar]
- 77. Campbell-Thompson M. Organ donor specimens: what can they tell us about type 1 diabetes? Pediatr Diabetes 2015;16:320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Panzer JK, Hiller H, Cohrs CM, et al. Pancreas tissue slices from organ donors enable in situ analysis of type 1 diabetes pathogenesis. JCI Insight 2020;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Liu W, Kin T, Ho S, et al. Abnormal regulation of glucagon secretion by human islet alpha cells in the absence of beta cells. EBioMedicine 2019;50:306–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA 2006;103:2334–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]