Abstract
Background
The number of breast cancer survivors increases, but information about long-term adverse health effects in breast cancer survivors is sparse. We aimed to get an overview of the health effects for which survivors visit their general practitioner up to 14 years after diagnosis.
Methods
We retrieved data on 11,671 women diagnosed with breast cancer in 2000–2016 and 23,242 age and sex matched controls from the PSCCR-Breast Cancer, a database containing data about cancer diagnosis, treatment and primary healthcare. We built Cox regression models for 685 health effects, with time until the health effect as the outcome and survivor/control and cancer treatment as predictors. Models were built separately for four age groups (aged 18/44, 45/59, 60/74 and 75/89) and two follow-up periods (1/4 and 5/14 years after diagnosis).
Results
229 health effects occurred statistically significantly more often in survivors than in controls (p < 0.05). Health effects varied by age, time since diagnosis and treatment, but coughing, respiratory and urinary infections, fatigue, sleep problems, osteoporosis and lymphedema were statistically significantly increased in breast cancer survivors. Osteoporosis and chest symptoms were associated with hormone therapy; respiratory and skin infections with chemotherapy and lymphedema and skin infections with axillary dissection.
Conclusions
Breast cancer survivors may experience numerous adverse health effects up to 14 years after diagnosis. Insight in individual risks may assist healthcare professionals in managing patient expectations and improve monitoring, detection and treatment of adverse health effects.
Highlights
-
•
Breast cancer survivors experience adverse effects up to 14 years after diagnosis.
-
•
Most common were respiratory/urinary infections, fatigue and sleep problems.
-
•
Adverse effects vary by age, time since diagnosis and treatment.
-
•
Our results may aid monitoring, detection and treatment of adverse effects.
1. Background
Breast cancer is the most prevalent cancer among women worldwide [1]. Due to advances in early detection and better treatment, survival of breast cancer has increased considerably during recent decades [[2], [3], [4]]. Currently, over 85% of breast cancer patients survive more than 5 years [[5], [6], [7]] and about 80% survive more than 10 years after the diagnosis [8,9].
Many breast cancer survivors experience a wide range of physical, emotional, psychological and cognitive health effects related to their cancer or the treatment they receive [[10], [11], [12], [13], [14], [15], [16], [17], [18]]. Commonly reported adverse health effects are lymphedema [10,11,13,14,[16], [17], [18]], bone loss [[10], [11], [12], [13],16], heart failure [10,11,13,14,16,18,19], fatigue [12,[14], [15], [16], [17], [18]], neuropathy [[12], [13], [14],16,17] and arthralgia [11,14,[16], [17], [18]].
Insight into adverse health effects is important for both survivors and healthcare professionals. Knowledge and awareness about possible adverse health effects may aid monitoring, early detection and treatment of these effects and thereby possibly limit their burden [20]. Besides, it may help healthcare professionals to improve the guidance they give to survivors regarding possible adverse effects. If survivors are assisted to interpret their symptoms, this might help them cope better with symptoms and improve their wellbeing [21].
Despite numerous studies addressing breast cancer survivorship, there are still some important knowledge gaps [22]. First, there is a paucity of research on long-term cancer survivors [22]. Most studies are cross-sectional and focus on the first two years after diagnosis. Longitudinal studies also focussing on survivors more than five years after diagnosis would be relevant as some health effects, such as heart failure, may not develop until more than ten years after diagnosis [11,13,16]. Other adverse health effects, such as bone loss, usually develop shortly after diagnosis, but may deteriorate over time [11,13,16].
Second, although almost a third of breast cancer patients are aged over 65 at diagnosis [23], research on this older age group is scarce [22]. Age can have a large effect on the occurrence of symptoms. For instance, premature menopause and fertility problems are especially relevant in younger women, whereas diseases such as heart failure and osteoporosis are more relevant in older women, who already have a high background risk for these diseases [10,11,14].
Finally, existing studies mostly focused on one or more specific adverse health effects or were based on patient-reported health effects. Health effects that were not addressed in these studies, or that patients or healthcare providers did not relate to breast cancer, may therefore have been underreported.
We aimed to close these knowledge gaps by studying the occurrence of adverse health effects breast cancer survivors of all ages, up to 14 years after diagnosis, compared to age-matched control women without breast cancer. We studied health effects presented in primary care, as this is where patients present their symptoms once active treatment has finished. Assuming that we would identify adverse health effects that are more prevalent in breast cancer patients than in control women, we aimed to disseminate our results in a clear-cut and patient-tailored manner that is applicable in daily practice.
2. Methods
2.1. Data
Data included in the Primary and Secondary Cancer Care Registry-Breast Cancer (PSCCR-Breast Cancer) were derived from two databases. Data on breast cancer diagnosis and treatment were derived from the Netherlands Cancer Registry (NCR) [24], which contains data on all cancer patients diagnosed in all Dutch hospitals since 1989. Data on health problems routinely recorded by GPs were derived from Nivel Primary Care Database (Nivel-PCD) [25]. Currently, data are collected from about 500 general practices spread throughout the Netherlands, which is a representative sample of about 10% of all Dutch general practices. In the Netherlands, all inhabitants are required to register with a general practitioner, who acts as the gatekeeper to secondary care [26]. Health problems are coded according to the International Classification of Primary Care (ICPC) [27].
In the absence of a unique identifier, patient records in both databases were linked using a deterministic linkage method, based on a combination of date of birth, sex and postal code. Linkage was performed by a trusted third party that removed all directly identifiable data before making the datasets available to the researchers [28].
2.2. Survivor and control selection
All women diagnosed with stage I-III breast cancer between 2000 and 2016, aged 18–89 at diagnosis, for whom general practice data were available for 1–14 years after diagnosis (n = 11,671) were selected from the PSCCR-Breast Cancer. As a comparison group, we selected two controls for each survivor (i.e. women without breast cancer of a similar age (+- 5 years) and from the same GP practice (n = 23,342)) from Nivel-PCD.
2.3. Statistical analysis
To identify for which health effects survivors visit their GP more often than control patients, we built Cox regression models. These models were built in two steps. For step 1, we modelled the frequency of each of the 685 health effects (i.e. ICPC codes [27]) between survivors and controls. These models were built separately for four age groups (15–44 (premenopausal), 45 to 59 (perimenopausal), 60 to 74 (postmenopausal) and 75 to 89 (elderly)) and two follow-up periods (1–4 years after diagnosis (hospital follow-up) and 5–14 years after diagnosis). For the age group 75 to 89, only the period of 1–4 years after diagnosis was included, as the number of survivors in this age group who had follow-up in the period 5–14 years after diagnosis was too limited. This gave a total of 4795 Cox regression models with time to the first occurrence of the health effect as the outcome and survivor/control as the predictor.
For step 2, we selected all models in which the health effect was statistically significantly more frequent in survivors than controls and refined each model by adding treatment variables as predictors (axillary dissection (yes/no), adjuvant radiotherapy (yes/no and right/left), (neo)adjuvant chemotherapy (yes/no) and adjuvant hormone therapy (yes/no)). Type of surgery (amputation/breast conserving) was not included as this was closely correlated with receiving radiation therapy. Age, as a centred continuous variable, was included to account for age effects within the age groups. A p-value <0.05 was considered statistically significant. No correction for multiple testing was performed, as we deemed it more important to find possible adverse effects than to exclude possible false-positive effects. Bootstrapping with 50 replications was used to improve accuracy of models. Predicted probabilities at 5 and 15 years were calculated for cases and controls using the predict command. Discrimination of models was assessed using Gönen-Heller's C. All analyses were performed using Stata/SE 15.1.
2.4. Ethical statement
At cancer diagnosis, the hospital informs the patient through a brochure that their data are included in the NCR. All general practices participating in the Nivel-PCD inform their patients, via a poster in the waiting room or via their website, that their data are included in Nivel-PCD. Both databases have an opt-out procedure. According to Dutch legislation, it is not necessary to obtain informed consent from patients or approval from a medical ethics committee for this type of observational study (Dutch Civil Law, Article 7: 458). Use of data for this study was approved by the Privacy review boards of NCR and Nivel-PCD.
3. Results
3.1. Study population
A total of 11,671 survivors and 23,342 age-matched controls were included in the study (Table 1). Among them were a considerable number of long-term survivors and older women; for 7422 survivors (64%), follow-up exceeded the period of five years after diagnosis and 1110 (10%) were aged 75 or older at diagnosis.
Table 1.
Characteristics of survivors and controls.
| Survivors (N = 11,671) | Controls (N=23,342) | |||
|---|---|---|---|---|
| Age at diagnosisa | n | % | n | % |
| 18 to 44 | 1583 | 14% | 3166 | 14% |
| 45 to 59 | 4920 | 42% | 9840 | 42% |
| 60 to 74 | 4058 | 35% | 8116 | 35% |
| 75 to 89 | 1110 | 10% | 2220 | 10% |
| Year of diagnosisa | ||||
| 2000–2005 | 2518 | 22% | 5036 | 22% |
| 2006–2009 | 3915 | 34% | 7830 | 34% |
| 2010–2015 | 5238 | 45% | 10,476 | 45% |
| Follow-up in period | ||||
| 1–4 years after diagnosisa | 8042 | 69% | 16,084 | 69% |
| 5–14 years after diagnosisa | 7442 | 64% | 14,884 | 64% |
| Tumour stage | ||||
| I | 5579 | 48% | ||
| II | 4841 | 42% | ||
| III | 1251 | 11% | ||
| Lateralisation | ||||
| Left | 5904 | 51% | ||
| Right | 5764 | 49% | ||
| Unknown | 3 | 1% | ||
| Type of surgery | ||||
| No surgery | 190 | 2% | ||
| Breast conserving | 6772 | 58% | ||
| mputation | 4686 | 40% | ||
| Unknown/Other | 23 | 0% | ||
| Axillary dissection | 4519 | 39% | ||
| Radiotherapy | 8116 | 70% | ||
| Chemotherapy | 5059 | 43% | ||
| Hormone therapy | 6149 | 53% | ||
For controls: after diagnosis of breast cancer of (matched) survivor.
In the survivor group, treatment characteristics differed by age group (eTables 1 and 2a-2d). In survivors aged 18 to 44, the majority (82%) received a combination of surgery and chemotherapy, often combined with radio or hormone therapy. In survivors aged 45 to 59, the majority (73%) received surgery and radiotherapy, often combined with chemotherapy or hormonal therapy. Most survivors aged 60 to 74 (74%) received surgery and radiotherapy, often without additional treatment or combined with hormone therapy. In the group aged 75 to 89, the majority of survivors received surgery (85%), often combined with hormone therapy.
3.2. Adverse health effects
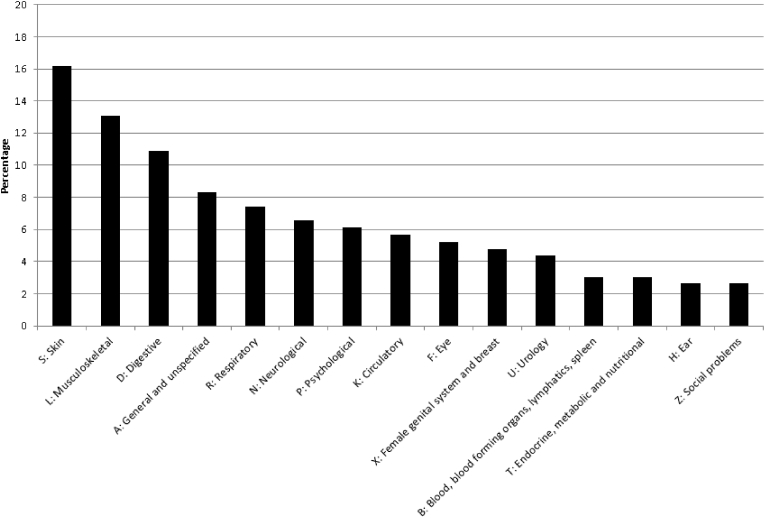
In 612 of the 4795 models that we tested (13%), the health effect was statistically significantly more prevalent in survivors than controls. These 612 models were for 229 separate health effects. Adverse health effects were most frequently related to the skin (16%) and the musculoskeletal system (13%) (see Fig. 1). Adverse health effects differed by age at diagnosis and time since diagnosis. We will discuss the most important findings below.
Fig. 1.
Type of health problem (ICPC chapter) of the identified adverse health effects that were statistically significantly more prevalent in survivors than controls.
3.3. One to four years after diagnosis
Most adverse health effects shortly after completion of treatment were related to the musculoskeletal system, the skin and the category ‘general and unspecified’ in all age groups. The percentage of adverse health effects related to the digestive system was somewhat higher in the older age groups, while the percentage of adverse health effects related to the female genital system was somewhat higher in the younger age groups (Table 2).
Table 2.
Type of health problem (ICPC chapter) of the identified adverse health effects by and age and time after diagnosis (n = 569).
| Period |
Age at diagnosis |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1–4 years after diagnosis |
18 to 44 |
45 to 59 |
60 to 74 |
75 to 89 |
||||
| N = 51 |
N = 104 |
N = 102 |
N = 77 |
|||||
| n | % | n | % | n | % | n | % | |
| A: General and unspecified | 7 | 14% | 11 | 11% | 13 | 13% | 11 | 14% |
| B: Blood, blood forming organs, lymphatics, spleen | 0 | 0% | 2 | 2% | 2 | 2% | 2 | 3% |
| D: Digestive | 4 | 8% | 8 | 8% | 10 | 10% | 9 | 12% |
| F: Eye | 3 | 6% | 2 | 2% | 2 | 2% | 3 | 4% |
| H: Ear | 0 | 0% | 2 | 2% | 1 | 1% | 2 | 3% |
| K: Circulatory | 2 | 4% | 8 | 8% | 5 | 5% | 5 | 6% |
| L: Musculoskeletal | 12 | 24% | 21 | 20% | 14 | 14% | 15 | 19% |
| N: Neurological | 0 | 0% | 5 | 5% | 6 | 6% | 2 | 3% |
| P: Psychological | 3 | 6% | 3 | 3% | 9 | 9% | 5 | 6% |
| R: Respiratory | 3 | 6% | 9 | 9% | 8 | 8% | 5 | 6% |
| S: Skin | 8 | 16% | 21 | 20% | 19 | 19% | 11 | 14% |
| T: Endocrine, metabolic and nutritional | 2 | 4% | 0 | 0% | 3 | 3% | 0 | 0% |
| U: Urology | 3 | 6% | 3 | 3% | 4 | 4% | 4 | 5% |
| X: Female genital system and breast | 4 | 8% | 7 | 7% | 4 | 4% | 3 | 4% |
| Z: Social problems | 0 | 0% | 2 | 2% | 2 | 2% | 0 | 0% |
| Age at diagnosis |
||||||
|---|---|---|---|---|---|---|
| 5–14 years after diagnosis |
18–44 yrs |
45–59 yrs |
60–74 yrs |
|||
| N = 38 |
N = 99 |
N = 98 |
||||
| n | % | n | % | n | % | |
| A: General and unspecified | 6 | 16% | 11 | 11% | 9 | 9% |
| B: Blood, blood forming organs, lymphatics, spleen | 1 | 3% | 4 | 4% | 3 | 3% |
| D: Digestive | 4 | 11% | 13 | 13% | 9 | 9% |
| F: Eye | 2 | 5% | 2 | 2% | 6 | 6% |
| H: Ear | 0 | 0% | 0 | 0% | 2 | 2% |
| K: Circulatory | 0 | 0% | 9 | 9% | 6 | 6% |
| L: Musculoskeletal | 6 | 16% | 13 | 13% | 16 | 16% |
| N: Neurological | 0 | 0% | 3 | 3% | 6 | 6% |
| P: Psychological | 2 | 5% | 6 | 6% | 5 | 5% |
| R: Respiratory | 4 | 11% | 9 | 9% | 9 | 9% |
| S: Skin | 6 | 16% | 19 | 19% | 17 | 16% |
| T: Endocrine, metabolic and nutritional | 1 | 3% | 1 | 1% | 4 | 4% |
| U: Urology | 4 | 11% | 5 | 5% | 6 | 6% |
| X: Female genital system and breast | 2 | 5% | 1 | 1% | 3 | 3% |
| Z: Social problems | 2 | 5% | 1 | 1% | 0 | 0% |
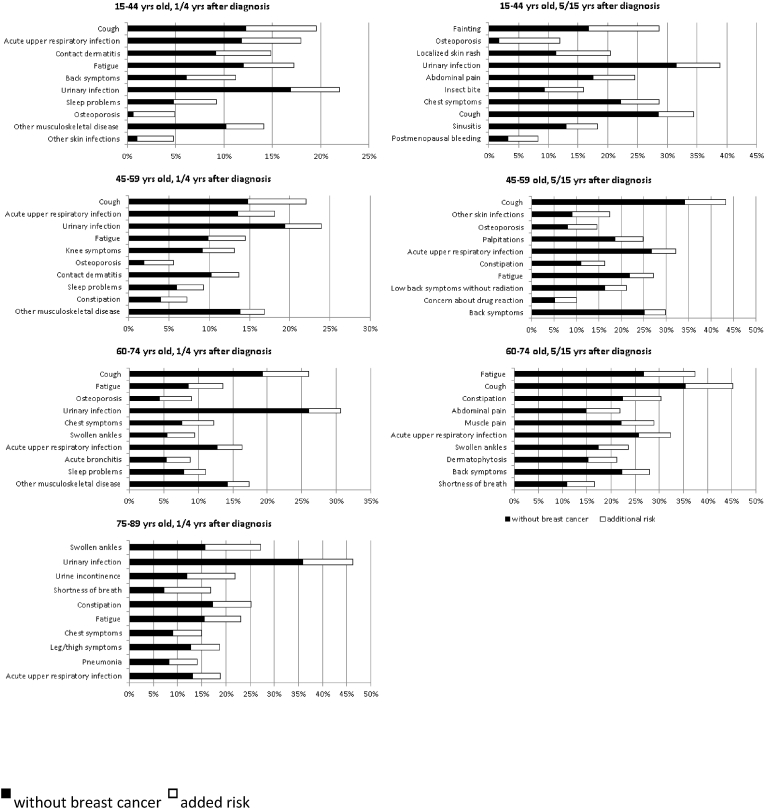
The adverse health effects with the highest additional risks were very similar in all but the oldest age group (age>=75). In all age groups, coughing was the adverse health effect for which survivors had the largest additional risk. Besides, large additional risks were found for acute upper respiratory infection, fatigue, cystitis, sleep problems, osteoporosis and ‘other musculoskeletal disease’. In the oldest age group (75–89), swollen ankles, cystitis and urinary incontinence showed the largest additional risks (Fig. 2).
Fig. 2.
Heading: Health effects with highest additional risk by age and time since diagnosis.
3.4. Five to fourteen years after diagnosis
In this follow-up period, most adverse health effects were associated with the musculoskeletal system, the skin and the category ‘general and unspecified’ in all three age groups (the oldest age group was not examined, as mentioned previously). Adverse health effects were also quite often related to the digestive and respiratory system in all age groups. In the youngest age group (18–45) adverse health effects were also related to the urological system. (Table 2).
The adverse health effects with the largest additional risks differed widely between the three age groups. Only coughing had a large additional risk in all age groups. In the youngest group of survivors, aged 18 to 44 at diagnosis, adverse health effects with the largest additional risks were fainting, osteoporosis and localised skin rash. In those aged 45 to 49, coughing, skin infections and osteoporosis had the largest additional risks. In those aged 60 to 74, fatigue, coughing and constipation had the largest additional risks (Fig. 2).
3.5. Treatment effects
We used the refined regression models from step 2 to assess whether adverse health effects were associated with specific treatments. Overall performance of the models was fair. The mean C-statistic was 0.61 (range 0.52–0.79). The treatment effects that were found in more than one age group or follow-up period were: an increased risk for osteoporosis, chest symptoms, blepharitis and general deterioration associated with hormone therapy; increased risk for acute upper respiratory infections, osteoporosis and skin infections/rashes associated with chemotherapy; and increased risk for lymphedema and skin infections associated with axillary dissection.
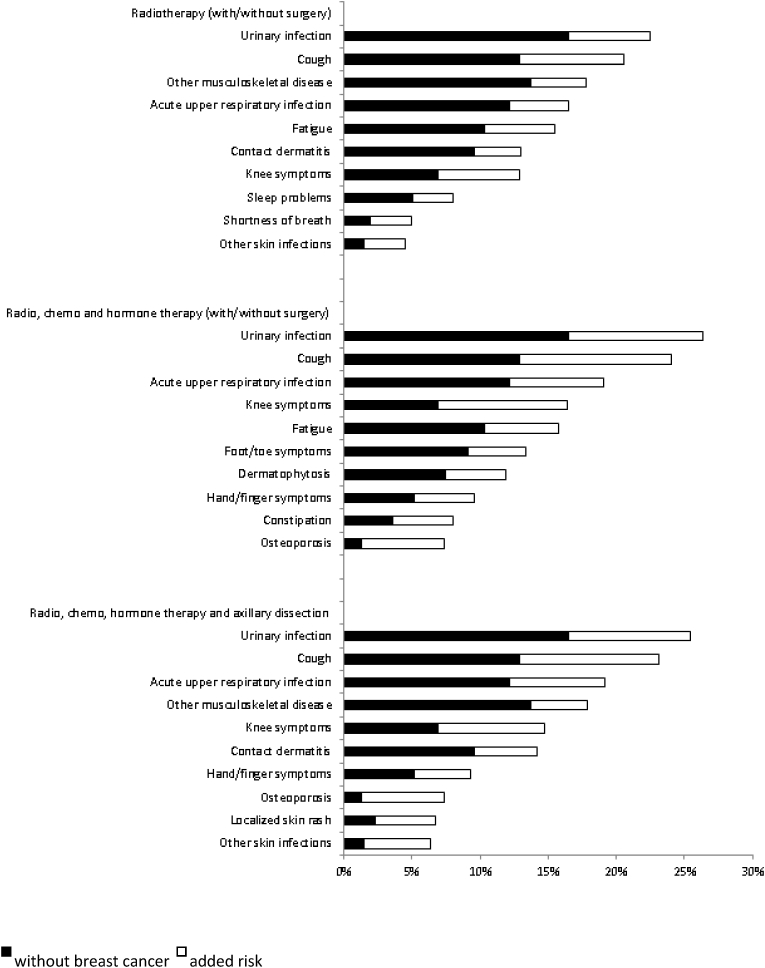
As it is not possible to present results of all 569 models in this paper, we show results for three common treatment regimens in Fig. 3. Additional examples are presented in eFigures 1a to 1e. An overview of all results is available at the EVIDENCIO medical prediction platform https://www.evidencio.com/models/share/1901?signature=c33c2d2278fd6ac6c31550cbac7f7f48e9a7d309ec02b1f4436b7e2fba5617a0).
Fig. 3.
Example of health effects for a woman 52 years old at diagnosis for the period 1–4 years after diagnosis, according to therapy received.
4. Discussion
To our knowledge, this is the first study examining the full range of adverse health effects in breast cancer survivors of all ages (≤89) and up to 14 years after diagnosis as presented in the primary care setting. Our results provide an overview of the adverse health effects for which survivors visit their general practitioner up to 14 years after diagnosis and could provide an indication whether health effects that survivors experience may be breast-cancer related.
Several adverse health effects presented in this paper are well-described adverse health effects in breast cancer survivors, e.g. bone loss [[10], [11], [12], [13],16] and fatigue [12,[14], [15], [16], [17], [18]], which supports the validity of our results. However, we also found lesser-known adverse health effects, notably different types of infections such as urinary, respiratory and skin infections. Although these infections may seem minor, survivors in all age groups appeared to be at increased risk up to 14 years after diagnosis, which may suggest long-term immunological consequences. These infections are currently not mentioned in breast cancer survivorship care guidelines [29,30]. They may have been missed by previous studies, as these studies did not focus on infections, or survivors may not have reported them in questionnaires because they did not relate infections to breast cancer.
Some of the well-known adverse effects, such as lymphedema [10,11,13,14,[16], [17], [18]] and heart failure [10,11,13,14,16,18], did not appear in our analyses as prominently as we had expected. For lymphedema, a possible explanation could be that survivors visit a physiotherapist and not their general practitioner. Another explanation may be that lymphedema has no separate ICPC code, but is included in the ICPC code ‘Other disorders of blood/lymph/spleen’. This non-specific code makes it more likely that GPs register codes like shoulder pain or arm problems in survivors presenting with lymphedema. Heart failure is specifically associated with anthracyclines, trastuzumab [31] or (left-sided) radiotherapy [32]. It is therefore only seen in a subgroup of survivors, which may explain why it did not show up as an important adverse effect in the broad group of all breast cancer survivors.
A strength of our study is the large, nationally representative databases that we used for this study. This allowed us to include a large unselected population-based sample of women with breast cancer, aged up to 89 years at diagnosis (almost 12,000 in total). The combination of detailed diagnostic and treatment data with primary care data allowed us to study health problems for which survivors visited their general practitioner rather than the hospital. Also, long-term follow-up was available, which enabled us to study long-term adverse health effects. The comparison with age-matched women without breast cancer allowed us to adjust for the age-related background risk of health effects.
It should be stressed that our study only identified adverse health effects that were presented to the GP. This is important as breast cancer survivors experiencing adverse health effects often do not present them to a healthcare provider. We cross-validated our findings in a survey we recently conducted among 400 breast cancer survivors [33]. Almost all women diagnosed with breast cancer reported adverse health effects, most commonly fatigue, memory/concentration problems and menopausal complaints. However, they often did not visit a healthcare provider. For example, only 27% of survivors experiencing fatigue and 8% of those experiencing memory/concentration problems visited a healthcare provider for this problem.
Health professionals can use our results to inform survivors and make them aware of possible adverse health effects. This might lead to earlier recognition and treatment of adverse health effects, and may ultimately improve quality of care and survivors’ quality of life. Besides, they can use our results when women visit them with symptoms that are possibly associated with their previous breast cancer diagnosis and treatment. This may help survivors to cope with symptoms and might reduce their anxiety if they see that their symptoms also occur in women without a history of breast cancer.
It is important to stress that our results are not meant to give a precise estimation of the exact risk for a specific adverse effect in an individual woman. Nor are they meant to be used in the process of making treatment decisions, as an accurate risk prediction is needed to weigh the pros and cons of a certain treatment. However, they can be used to predict which health effects a woman is more likely to visit her GP for. Before giving this information, healthcare professionals should discuss a patient's preferences, as some women prefer to receive detailed information about all possible adverse health effects and others only want to receive general information about the most important risks. Besides, health care professionals should be aware that many people have difficulties interpreting risks, so it is advisable to explain them in multiple ways, e.g. both percentages and frequencies [34].
In conclusion, we found that breast cancer survivors can experience numerous adverse health effects up to 14 years after diagnosis. Our findings can assist healthcare professionals in managing patient expectations, and can improve monitoring, early detection and treatment of adverse health effects, possibly leading to better quality of care and health-related quality of life.
Funding
This work was supported by the Netherlands Organisation for Health Research and Development (project number 80-84800-98-34003). The funder had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Declaration of competing interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.12.001.
Contributor Information
Marianne J. Heins, Email: m.heins@nivel.nl.
PSCCR group:
Annette Berendsen, Daan Brandenbarg, Anneriet Dassen, Agnes Jager, Jacqueline Hugtenburg, and Gerda van der Weele
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca - Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C.E., Ma J., Goding Sauer A., Newman L.A., Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. Ca - Cancer J Clin. 2017;67(6):439–448. doi: 10.3322/caac.21412. [DOI] [PubMed] [Google Scholar]
- 3.Carioli G., Malvezzi M., Rodriguez T., Bertuccio P., Negri E., La Vecchia C. Trends and predictions to 2020 in breast cancer mortality in Europe. Breast. 2017;36:89–95. doi: 10.1016/j.breast.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Carioli G., Malvezzi M., Rodriguez T., Bertuccio P., Negri E., La Vecchia C. Trends and predictions to 2020 in breast cancer mortality: americas and Australasia. Breast. 2018;37:163–169. doi: 10.1016/j.breast.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. Ca - Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. 2018. [DOI] [PubMed] [Google Scholar]
- 6.Woods L.M., Rachet B., O'Connell D.L., Lawrence G., Coleman M.P. Are international differences in breast cancer survival between Australia and the UK present amongst both screen-detected women and non-screen-detected women? survival estimates for women diagnosed in West Midlands and New South Wales 1997-2006. Int J Cancer. 2016;138(10):2404–2414. doi: 10.1002/ijc.29984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allemani C., Matsuda T., Di Carlo V., et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Netherlands Comprehensive Cancer Organisation (IKNL) Dutch cancer figures: survival invasive breast cancer. https://www.cijfersoverkanker.nl/selecties/Dataset_1/img5d3aa6c49925e Available at:
- 9.American Cancer Society . American Cancer Society; Atlanta: 2019. Breast cancer Facts & figures 2019. [Google Scholar]
- 10.Agrawal S. Late effects of cancer treatment in breast cancer survivors. South Asian J Cancer. 2014;3(2):112–115. doi: 10.4103/2278-330X.130445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodai B.I., Tuso P. Breast cancer survivorship: a comprehensive review of long-term medical issues and lifestyle recommendations. Perm J. 2015;19(2):48–79. doi: 10.7812/TPP/14-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brem S., Kumar N.B. Management of treatment-related symptoms in patients with breast cancer. Clin J Oncol Nurs. 2011;15(1):63–71. doi: 10.1188/11.CJON.63-71. [DOI] [PubMed] [Google Scholar]
- 13.Ewertz M., Jensen A.B. Late effects of breast cancer treatment and potentials for rehabilitation. Acta Oncol. 2011;50(2):187–193. doi: 10.3109/0284186X.2010.533190. [DOI] [PubMed] [Google Scholar]
- 14.Ganz P.A., Goodwin P.J. Breast cancer survivorship: where are we today? Adv Exp Med Biol. 2015;862:1–8. doi: 10.1007/978-3-319-16366-6_1. [DOI] [PubMed] [Google Scholar]
- 15.Harrington C.B., Hansen J.A., Moskowitz M., Todd B.L., Feuerstein M. It's not over when it's over: long-term symptoms in cancer survivors--a systematic review. Int J Psychiatr Med. 2010;40(2):163–181. doi: 10.2190/PM.40.2.c. [DOI] [PubMed] [Google Scholar]
- 16.Kenyon M., Mayer D.K., Owens A.K. Late and long-term effects of breast cancer treatment and surveillance management for the general practitioner. J Obstet Gynecol Neonatal Nurs : J Obstet Gynecol Neonatal Nurs. 2014;43(3):382–398. doi: 10.1111/1552-6909.12300. [DOI] [PubMed] [Google Scholar]
- 17.Lovelace D.L., McDaniel L.R., Golden D. Long-term effects of breast cancer surgery, treatment, and survivor care. J Midwifery Wom Health. 2019;64(6):713–724. doi: 10.1111/jmwh.13012. [DOI] [PubMed] [Google Scholar]
- 18.Sisler J., Chaput G., Sussman J., Ozokwelu E. Follow-up after treatment for breast cancer: practical guide to survivorship care for family physicians. Can Fam Physician. 2016;62(10):805–811. [PMC free article] [PubMed] [Google Scholar]
- 19.Boerman L.M., Maass S., van der Meer P., et al. Long-term outcome of cardiac function in a population-based cohort of breast cancer survivors: a cross-sectional study. Eur J Cancer. 2017;81:56–65. doi: 10.1016/j.ejca.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Chopra I., Chopra A. Follow-up care for breast cancer survivors: improving patient outcomes. Patient Relat Outcome Meas. 2014;5:71–85. doi: 10.2147/PROM.S49586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badana A.N.S., Marino V.R., Templeman M.E., et al. Understanding the roles of patient symptoms and subjective appraisals in well-being among breast cancer patients. Support Care Cancer. 2019;27(11):4245–4252. doi: 10.1007/s00520-019-04707-2. [DOI] [PubMed] [Google Scholar]
- 22.Jacobsen P.B., Rowland J.H., Paskett E.D., et al. Identification of key gaps in cancer survivorship research: findings from the American society of clinical oncology survey. Journal of oncology practice. 2016;12(3):190–193. doi: 10.1200/JOP.2015.009258. [DOI] [PubMed] [Google Scholar]
- 23.American Cancer Society . American Cancer Society; Atlanta: 2019. Cancer Facts & figures 2019. [Google Scholar]
- 24.Netherlands Comprehensive Cancer Organisation (IKNL) About the NCR. https://www.iknl.nl/en/ncr Available at:
- 25.Nivel. Nivel Primary Care Database. Available at: https://nivel.nl/en/nivel-primary-care-database.
- 26.Kroneman M., Boerma W., van den Berg M., Groenewegen P., de Jong J., van Ginneken E. Netherlands: health system review. Health Syst Transit. 2016;18(2):1–240. [PubMed] [Google Scholar]
- 27.Lamberts H., Wood M. Oxford University Press; Oxford: 1987. ICPC, International Classification of primary care. [Google Scholar]
- 28.Heins M, De Ligt KM, Verloop J, Siesling S, Korevaar J. Opportunities and obstacles in linking large health care registries: the primary secondary cancer care Registry - breast cancer. Submitted. [DOI] [PMC free article] [PubMed]
- 29.Spronk I., Korevaar J.C., Schellevis F.G., Albreht T., Burgers J.S. Evidence-based recommendations on care for breast cancer survivors for primary care providers: a review of evidence-based breast cancer guidelines. BMJ open. 2017;7(12) doi: 10.1136/bmjopen-2016-015118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Runowicz C.D., Leach C.R., Henry N.L., et al. American cancer society/American society of clinical oncology breast cancer survivorship care guideline. Ca - Cancer J Clin. 2016;66(1):43–73. doi: 10.3322/caac.21319. [DOI] [PubMed] [Google Scholar]
- 31.Bowles E.J., Wellman R., Feigelson H.S., et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012;104(17):1293–1305. doi: 10.1093/jnci/djs317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rehammar J.C., Jensen M.B., McGale P., et al. Risk of heart disease in relation to radiotherapy and chemotherapy with anthracyclines among 19,464 breast cancer patients in Denmark, 1977-2005. Radiother Oncol. 2017;123(2):299–305. doi: 10.1016/j.radonc.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Ligt K.M., Heins M., Verloop J., Smorenburg C.H., Korevaar J.C., Siesling S. Patient-reported health problems and healthcare use after treatment for early-stage breast cancer. Breast. 2019;46:4–11. doi: 10.1016/j.breast.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong K.A., Metlay J.P. Annals clinical decision making: communicating risk and engaging patients in shared decision making. Ann Intern Med. 2020;172(10):688–692. doi: 10.7326/M19-3495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.