Abstract
There is an established literature on the symptoms and complications of COVID‐19 but the after‐effects of COVID‐19 are not well understood with few studies reporting persistent symptoms and quality of life. We aim to evaluate the pooled prevalence of poor quality of life in post‐acute COVID‐19 syndrome (PCS) and conducted meta‐regression to evaluate the effects of persistent symptoms and intensive care unit (ICU) admission on the poor quality of life. We extracted data from observational studies describing persistent symptoms and quality of life in post‐COVID‐19 patients from March 10, 2020, to March 10, 2021, following PRISMA guidelines with a consensus of two independent reviewers. We calculated the pooled prevalence with 95% confidence interval (CI) and created forest plots using random‐effects models. A total of 12 studies with 4828 PCS patients were included. We found that amongst PCS patients, the pooled prevalence of poor quality of life (EQ‐VAS) was (59%; 95% CI: 42%–75%). Based on individual factors in the EQ‐5D‐5L questionnaire, the prevalence of mobility was (36, 10–67), personal care (8, 1–21), usual quality (28, 2–65), pain/discomfort (42, 28–55), and anxiety/depression (38, 19–58). The prevalence of persistent symptoms was fatigue (64, 54–73), dyspnea (39.5, 20–60), anosmia (20, 15–24), arthralgia (24.3, 14–36), headache (21, 3–47), sleep disturbances (47, 7–89), and mental health (14.5, 4–29). Meta‐regression analysis showed the poor quality of life was significantly higher among post‐COVID‐19 patients with ICU admission (p = 0.004) and fatigue (p = 0.0015). Our study concludes that PCS is associated with poor quality of life, persistent symptoms including fatigue, dyspnea, anosmia, sleep disturbances, and worse mental health. This suggests that we need more research on PCS patients to understand the risk factors causing it and eventually leading to poor quality of life.
Keywords: health‐related quality of life, long COVID‐19, persistent symptoms, post‐acute COVID‐19 syndrome, post‐COVID syndrome
1. INTRODUCTION
The COVID‐19 virus was first reported in Wuhan China in December 2019. Since its inception, the virus has spread globally and resulted in a pandemic. As of March 2021, the virus has infected 125 million people and has resulted in 2.7 million deaths worldwide. 1 Over the last year, there has been remarkable scientific progress in uncovering the disease mechanism and creating vaccines against the virus. Although there now seems to be light at the end of a long tunnel, the virus still continues to infect people. Although the symptoms of COVID‐19 in the majority of cases are limited to fever, fatigue, cough, diarrhea, anosmia, and headache, in some cases it can cause more severe complications including end‐organ damage. 2 , 3 Manifestations of end‐organ damage can include but are not limited to acute respiratory distress syndrome (ARDS), cardiac injuries (ventricular arrhythmias and hemodynamic instability), thrombotic manifestations, renal, hepatic, and gastrointestinal damage. 4 , 5 , 6 There is well‐known literature on the acute manifestations of the COVID‐19 as well as the complications, but long‐term COVID‐19 effects after recovery or discharge from the hospital have not been established well.
According to the CDC/IDSA, post‐acute COVID‐19 syndrome (PCS) is defined as an ongoing symptomatic illness in patients who have recovered from their initial COVID‐19 infection. 7 , 8 The type of persistent symptoms, their prevalence, duration, and severity following recovery of COVID‐19, as well as risk factors causing them, are still under investigation. A few studies have reported a wide array of persistent symptoms after COVID‐19 hospitalizations as well as outpatient recovery. These persistent symptoms include fatigue, dyspnea, anosmia, sleeping difficulties, chest pain, headache, cough, and mental health problems. 6 , 9 , 10 , 11 , 12 , 13 , 14 , 15 The mechanisms behind these symptoms are not very well understood. One study suggested that they may be associated with active long‐term biochemical and inflammatory response pathways. 16 Another explanation is that these manifestations may arise because of hypoxia and hypoxemia secondary to the destruction of capillaries. 17 However, more studies are required to determine the exact cause of these persistent symptoms. These long‐term symptoms may have a significant effect on the quality of life and cause posttraumatic stress disorder (PTSD). 9 , 11
Persistent symptoms after post‐viral infection is not a novel concept as there is evidence of similar effects seen in SARS 18 , 19 , 20 and MERS. 21 Studies have shown that patients infected with SARS often experienced long‐term fatigue, myalgia encephalomyelitis, anorexia, and hypocortisolism. 18 , 19 Furthermore, MERS survivors who required intensive care unit (ICU) admission have been shown to have a significantly lower quality of life compared with patients in general ward admission. 21 SARS and MERS survivors both may suffer long‐term psychological consequences including depression and PTSD. 20 , 21 Although we are aware that increased severity regarding SARS and MERS may lead to long‐term symptoms, the COVID‐19 severity, as a risk factor for developing PCS and poor quality of life, is still a debated topic. A few published studies have presented different findings. For example, one group of researchers found no difference in persistent symptoms and HRQoL between ICU and ward patients, 15 another study has reported a significant drop in EQ. 5D in ICU (68%) versus ward (45%) patients. 14 More evidence is required on this before we can make any definitive conclusions.
In this meta‐analysis, we aim to evaluate the pooled prevalence of poor quality of life in patients post‐COVID‐19. We also aim to perform meta‐regression to evaluate the effects of persistent symptoms and ICU admission on the poor quality of life.
2. METHODS
2.1. Endpoints
2.1.1. Primary aim
We aim to evaluate the pooled prevalence of poor quality of life in patients post‐COVID‐19 using both the EQ‐VAS scale and ED‐5Q‐5L questionnaire.
Poor quality of life is assessed using:
-
1)
VAS scale (0–100): The EQ‐VAS is a patient's subjective assessment of generic health ranging from 0 to 100, with higher scores representing better subjective health experience.
-
2)
The EQ‐5D‐5L is a validated questionnaire to evaluate a patient's quality of life by assessing the following five factors: mobility, self‐care, usual activities, pain or discomfort, and anxiety or depression. Categorization within each factor is divided into five levels that range from no problems to extreme problems 22 and https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/.
2.1.2. Secondary aim
We also aim to evaluate the pooled prevalence of persistent symptoms in PCS and perform a meta‐regression to evaluate the effects of persistent symptoms and ICU admission on the poor quality of life.
2.2. Search strategy and selection criteria
A systematic review was performed using the PRISMA protocol. 23 We searched PubMed for observational studies that described post‐COVID‐19 recovery symptoms and quality of life from March 10, 2020, to March 10, 2021, following keyword/MESH terms: Persistent COVID‐19 [Title/Abstract] OR long‐term COVID‐19[Title/Abstract] OR post‐COVID syndrome [Title/Abstract] OR post‐acute COVID‐19 syndrome [Title/Abstract] OR Health‐related Quality of Life [Title/Abstract]. All studies describing persistent COVID‐19 symptoms and quality of life were included. Literature other than observational studies, non‐English literature, non‐full text, and animal studies were excluded. The flow diagram of the literature search and study selection process is described in Figure 1.
Figure 1.
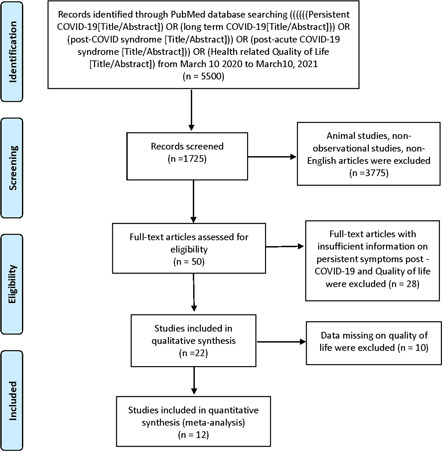
Flow diagram of literature search and study selection process of quality‐of‐life post‐COVID‐19
2.3. Study selection
Abstracts and full‐length articles were reviewed for the availability of data on the poor quality of life in COVID‐19 patients after recovery for quantitative analysis. PM and UP independently screened all of the identified studies and assessed full texts to determine eligibility. Any disagreement was resolved through consensus.
2.4. Data extraction
Data were extracted by two authors (P. M. and U. P.). The descriptive variables extracted were the author's name, country, study type, follow‐up time, the severity of COVID‐19, sample size, mean age and percentage of males, persistent symptoms, and instrument used to measure QoL are presented in Table 1.
Table 1.
Study characteristics and persistent symptoms and quality of life instrument used in individual studies
| Author, country | Sample size (N) | Follow‐up after discharge (days), mean (±SD/range) | Mean age | Male (N) | ICU | Persistent symptoms | Quality of life (QoL) instruments |
|---|---|---|---|---|---|---|---|
| Chopra et al., USA 24 | 1250 | 60 | 62 | 648 | 165 | Cough, dyspnea, anosmia, chest pain, mental health | EQ‐VAS |
| Carfi et al., Italy 25 | 143 | 36.1 (12.9) | 56 | 90 | 18 | Fatigue, cough, dyspnea, anosmia, headache, arthralgia, chest pain | EQ‐VAS |
| Haplin et al., UK 14 | 100 | 48 (10.3) | 58.5 | 54 | 32 | Fatigue, dyspnea, mental health |
EQ‐VAS EQ‐5D‐5L |
| Taboada et al., Spain 10 | 91 | 180 | 65 | 59 | 91 | Fatigue, cough, anosmia, arthralgia, chest pain, sleeping disturbances |
EQ‐VAS EQ‐5D‐5L |
| Jacobs et al., USA 6 | 183 | 35 | 57 | 112 | Fatigue, cough, dyspnea, anosmia, headache, arthralgia | EQ‐VAS | |
| Tabacof et al., USA 26 | 84 | 151 | 44 | 26 | Fatigue, headache | EQ‐VAS | |
| Valent et al., France 27 | 54 | 90 | 62 | 39 | 54 | NA |
EQ‐VAS EQ‐5D‐5L |
| Arab‐Zozani et al., Iran 11 | 409 | 30 | 58.4 | 247 | 74 | NA | EQ‐5D‐5L |
| Mandal et al., UK 28 | 384 | 54 (47–59) | 59.9 | 238 | Fatigue, cough, dyspnea, anosmia, headache, arthralgia, sleeping disturbances, mental health | EQ‐VAS | |
| Huang et al., China 29 | 1733 | 153 (146–160) | 57 | 897 | 76 | Fatigue, cough, dyspnea, anosmia, headache, arthralgia, chest pain, sleeping disturbances |
EQ‐VAS EQ‐5D‐5L |
| Garrigues et al., France 15 | 120 | 110.9 (±11.1) | 63.2 | 75 | 24 | Fatigue, cough, dyspnea, anosmia, chest pain, sleeping disturbances | EQ‐VAS |
| Moreno‐Pérez et al., Spain 30 | 277 | 77 (72–85) | 62 | 146 | 24 | Fatigue, cough, dyspnea, anosmia, headache, arthralgia | EQ‐VAS |
| Total | 4828 | ||||||
2.5. Data‐analysis
We used the MetaXL software to estimate the pooled prevalence, 95% confidence interval (95% CI) of poor quality of life (EQ‐VAS and ED‐EQ‐5L questionnaires), and persistent symptoms amongst COVID‐19 patients post‐recovery. Meta‐regression was performed to evaluate the effects of persistent symptoms, ICU admission, and age on the poor quality of life of post‐COVID‐19 patients. Comprehensive Meta‐Analysis software (Biostat Inc.) was used to estimate correlation coefficient (r), 95% CI, odds ratios [e^ coefficient], p‐value, and I 2 using a random‐effects model due to expected heterogeneity. I 2 values of 25%, 50%, and 75% represented low, medium, and high heterogeneity. p < 0.05 was considered significant.
3. RESULTS
Initially, 1725 publications were screened. Out of which 50 full‐text articles were assessed for eligibility using inclusion and exclusion criteria. 28 studies were excluded because they had no information on the poor quality of life and persistent symptoms. After a detailed assessment, as of March 10, 2021, a total of 12 studies were selected to calculate the pooled prevalence of poor quality of life and persistent symptoms in post‐recovery COVID‐19 patients (Table 1 and Figure 1). There were seven studies from the UK/Europe, three from the USA, one each from Iran and China. The follow‐up time from discharge of the COVID‐19 patients ranged from 30 to 180 days. The mean age of adults in our meta‐analysis was 58.75 (44–65) years old.
3.1. Pooled prevalence of poor quality of life and persistent symptoms in post‐recovery COVID‐19 patients
The pooled prevalence of poor quality of life (EQ‐VAS) was (59%, 95% CI: 42%–75%, p < 0.0001) amongst post‐recovery COVID‐19 patients (Table 2 and Figure 2).
Table 2.
Pooled prevalence of quality of life and persistent symptoms
| Variable | Studies | Total cases | Cases affected | Pooled prevalence | 95% confidence interval (CI) (%) |
|---|---|---|---|---|---|
| EQ‐VAS | |||||
| Poor quality of life | 7 | 1108 | 539 | 59% | (42–74) |
| 5Q‐5D‐5L | |||||
| Mobility | 5 | 2241 | 401 | 36% | (9–66) |
| Self‐care | 5 | 2241 | 91 | 8% | (1–21) |
| Usual activity | 5 | 2230 | 279 | 28% | (2–65) |
| Pain/discomfort | 5 | 2235 | 683 | 41.5% | (28–55 |
| Anxiety/depression | 5 | 2236 | 680 | 37.5% | (19–58) |
| Persistent symptoms | |||||
| Fatigue | 9 | 2929 | 1787 | 63.9% | (54–73) |
| Dyspnea | 9 | 3278 | 693 | 39.5% | (20–60) |
| Anosmia | 8 | 3194 | 573 | 20% | (15–24) |
| Cough | 7 | 1505 | 333 | 22.5% | (16–30) |
| Sleep disturbances | 4 | 2142 | 759 | 47% | (7–89) |
| Chest pain | 5 | 2497 | 171 | 10% | (5–16) |
| Headache | 5 | 2218 | 175 | 21% | (3–47) |
| Arthralgia | 6 | 2496 | 356 | 24.3% | (14–36) |
| Mental health/PTSD | 3 | 972 | 115 | 14.5% | (4–29) |
Figure 2.
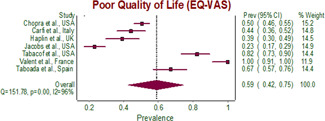
Forest plot of pooled prevalence of poor quality of life (EQ‐VAS) in post‐COVID‐19 patients. CI, confidence interval
Four studies have reported mean EQ‐VAS. The pooled mean EQ‐VAS of poor quality of life was 81.1, 95% CI: 75.6–86.5) (Table 3 and Figure 3).
Table 3.
Pooled mean using a random‐effects model of four studies reporting mean EQ‐VAS
| Study name | Mean | Standard error | Variance | Lower limit | Upper limit |
|---|---|---|---|---|---|
| Mandal et al., UK | 90 | 0.94 | 0.89 | 88.2 | 91.9 |
| Huang et al., China | 80 | 0.35 | 0.13 | 79.3 | 80.7 |
| Garrigues et al., France | 70.3 | 1.96 | 3.85 | 66.5 | 74.2 |
| Moreno‐Pérez et al., Spain | 83 | 0.89 | 0.79 | 81.3 | 84.7 |
| Random effects model | 81.1 | 2.8 | 7.82 | 75.6 | 86.5 |
Figure 3.
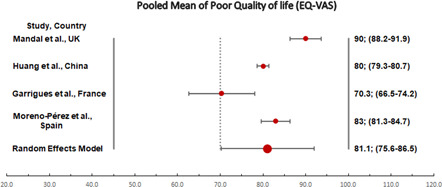
Forest plot of pooled mean of poor quality of life (EQ‐VAS) in post‐COVID‐19 patients
The pooled prevalence of individual factors in EQ‐5D‐5L questionnaire estimating poor quality of life was mobility (36%, 95% CI: 10%–67%), personal care (8%, 95% CI: 1%–21%), usual quality (28%, 95% CI: 2%–65%), pain/discomfort (42%, 95% CI: 28%–55%), anxiety/depression (38%, 95% CI: 19%–58%) (Table 2 and Figure 4A–E).
Figure 4.
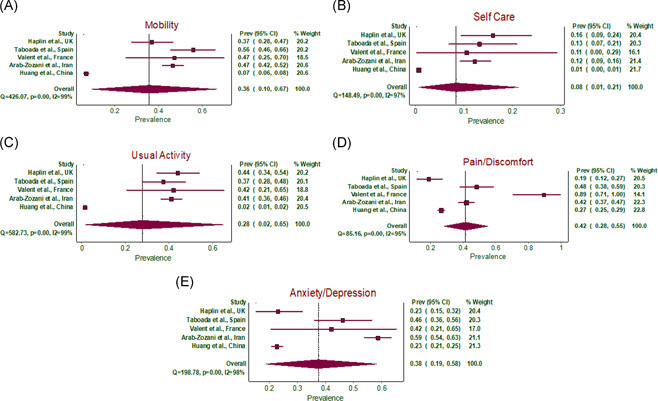
(A) Forest plot of pooled prevalence of mobility in EQ‐5D‐5L questionnaire in post‐COVID‐19 patients. (B) Forest plot of pooled prevalence of self‐care in EQ‐5D‐5L questionnaire in post‐COVID‐19 patients. (C) Forest plot of pooled prevalence of usual activity in EQ‐5D‐5L questionnaire in post‐COVID‐19 patients. (D) Forest plot of pooled prevalence of pain/discomfort in EQ‐5D‐5L questionnaire in post‐COVID‐19 patients. (E) Forest plot of pooled prevalence of anxiety/depression in EQ‐5D‐5L questionnaire in post‐COVID‐19 patients
There was a total of nine most commonly reported persistent symptoms identified in post‐COVID‐19 patients in the literature reviewed. The prevalence of all the persistent symptoms is presented in Table 2. The most common manifestations were fatigue (64%, 95% CI: 54–73), cough (22.5%, 95% CI: 16–30), dyspnea (39.5%, 95% CI: 20–60), anosmia (20%, 95% CI: 15–24), arthralgia (24.3%, 95% CI: 14–36), chest pain (10%, 95% CI: 5–16), headache (21%, 95% CI: 3–47), sleep disturbances (47%, 95% CI: 7–89), and mental Health problems (14.5%, 95% CI: 4–9) (Figures S1–S9).
3.2. Meta‐regression showing correlation of persistent symptoms, ICU admission, and age with poor quality of life
We performed meta‐regression on 7 studies with poor quality of life using EQ‐VAS. Meta‐regression analysis showed poor quality of life was significantly higher among post‐COVID‐19 patients with ICU admission (r: 0.014; odds ratio [OR]: 1.01; 95% CI: 1–1.02; I 2: 82.96%; p = 0.004) and fatigue (r: 0.06; OR: 1.06; 95% CI: 1.02–1.09; I 2: 95.45%; p = 0.0015] (Figure 5A,B). There was no significant correlation between dyspnea, anosmia, and poor quality of life (r: −0.001; OR: 0.99; 95% CI: 0.94–1.04; I 2: 92.39%; p = 0.77) (r: −0.06; OR: 0.94; 95% CI: 0.84–0.94; I 2: 94.54%; p = 0.353), respectively (Supporting Information figure).
Figure 5.
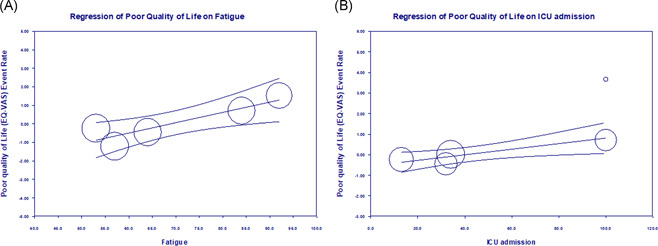
(A) Meta‐regression between the poor quality of life (log‐event) and fatigue in post‐COVID‐19 patients. (B) Meta‐regression between the poor quality of life (log‐event) and ICU admission in post‐COVID‐19 patients. ICU, intensive care unit
4. DISCUSSION
In our meta‐analysis, we found that 58% of the post‐COVID‐19 patients had reported poor quality of life. In post‐COVID‐19 patients, the pooled analysis of individual factors in the EQ‐5Q‐5L questionnaire showed that 41.5% had pain/discomfort, 37.5% had anxiety/depression, followed by 36% problems with mobility, 28% problems with usual activities, and only 8% having self‐care problems. These results signify that the majority of COVID‐19 patients post‐recovery have a poor quality of life posing challenges to patients, healthcare providers, and public health practitioners.
A few studies have reported that PCS results in a poor quality of life and 11 , 14 , 25 , 27 , 30 one potential explanation for this phenomenon is that COVID‐19 may result in PTSD. A hypothesis for the development of PTSD is that it may have a higher incidence in people who have comorbidities that are associated with higher COVID‐19 mortality rates. In addition, no visitor hospital policies during COVID‐19 may have led to more fear and a greater degree of stress than an otherwise healthy individual. 31 As a result, during times of fear and worry, patients did not have access to loved ones which may have compounded these feelings and contributed to the development of PTSD. Different studies have found the prevalence of PTSD to be approximately 15%–20% among hospitalized COVID‐19 survivors. 9 , 32 There is evidence of PTSD in post‐COVID‐19 but the risk factors causing it is not clearly described and need further evidence. 32 A study conducted by Chang et al. found no significant differences in the development of PTSD between age, sex, hospitalization time, and duration after discharge. Another explanation for the lower quality of life can be attributed to the financial costs associated with Covid hospitalization. 32 Chopra et al. found that almost half of their study patients reported having a financial impact as a result of COVID‐19 hospitalization. Nearly 10% reported that they had used all of their savings and had to ration food, heat, housing, and other medications. 24 Moreover, in many patients, the persistent symptoms force them to have reduced hours at work or quit altogether which may increase their financial distress. 24 In addition, many patients who return from the hospital and have ongoing symptoms may have faced prolonged social isolation which in turn negatively impacts their mental health and their perceived quality of life. 33 Even though any one of these factors alone can reduce the quality of life (QOL), the combination of all of these factors most likely has additive effects resulting in drastic effects on QOL.
In our meta‐analysis, the most common persistent symptoms associated with PCS include fatigue, dyspnea, anosmia, cough, sleep disturbances, arthralgia, headache, and mental health/PTSD, which is consistent with the reported literature. The mechanism behind these long‐term symptoms in post‐COVID‐19 patients is not well understood and is currently an area of investigation. A study conducted by Doykov et al. found that inflammatory markers (cytokines), and upregulation of mitochondrial proteins (peroxiredoxin 3 and carbamoyl phosphate synthase) correspond to mitochondrial stress in COVID‐19 patients 40–60 days post‐infection. 16 The increased cytokines including IL‐6, IL‐8, and TNFα, may cause cytokine dysregulation and eventually lead to cytokine release storms. In addition, the coronavirus can spread through nerves, to the CNS as evidenced by autopsies that have found viral proteins in the brainstem and cranial nerves. The combination of the cytokine storm and CNS entry of the virus can cause neuroinflammation which can lead to prolonged generalized symptoms including fatigue, headache, myalgias, and dyspnea. 34 However, there have been other theories that focus more on neurological issues secondary to vascular disruption, instead of direct neuronal penetration. Moreover, to our knowledge, there has been no intrinsic mechanism that has been described for why PCS patients suffer from sleep disturbances. This finding may be explained by the fact that PCS patients have higher stress levels and psychological issues such as PTSD inhibiting them to relax and may manifest as sleep disturbances. 9 , 32 Furthermore, upper respiratory infections (URIs) have been shown to increase cough reflex sensitivity. This cough sensitivity can be enhanced for months after a viral infection. 35 Finally, there have been multiple mechanisms proposed for COVID‐related anosmia. One study showed that a specialized group of cells in the olfactory epithelium express high levels of ACE 2 receptor, which is used by coronavirus to invade the cells and cause infection. As the support network for olfactory cells is affected, olfactory cells may not properly develop resulting in loss of smell. 36 Another mechanism that could potentially explain anosmia has its basis in inflammatory products. As the olfactory bulb is immunogenic, inflammatory products released as a result of COVID‐19 may lead to selective damage of cells resulting in anosmia. 37 Although there is still debate over the exact mechanisms behind these long‐term symptoms, one thing remains clear; the presence of these symptoms has significant psychological impacts including mental health implications, increased stress, and decreased HRQoL 10 , 11 , 14
As the relationship of COVID‐19 severity in developing PCS and decreasing HRQoL has not been widely studied, relatively little is known about their association. A few studies on this have reported different results which may be partially attributed to differences in samples and methodology. A study conducted by Garrigues et al. found no differences between ICU versus Wards' patients both in the frequency of patients that developed PCS and reported decreased HRQoL based on COVID‐19 severity, while another study concluded that COVID‐19 severity is related to a worse HRQoL. 14 , 15 Many studies have established that once a patient develops PCS, they are likely to report having a decreased HRQoL. 11 , 14 , 25 , 30
According to the literature, there seems to be a trend of older people experiencing PCS. 6 , 28 This finding is not surprising as increased age results in increased home stenosis and a weakened immune system. Additionally, many of the studies conducted on PCS use data from hospitalized patients which tend to be older. Many studies that we included in our analysis also involve hospitalized COVID‐19 patients with a mean age of approximately 60 years. 6 , 28 To our knowledge, there is no strong evidence of PCS in younger populations. Although it is very likely that age is a risk factor for PCS, it needs further investigation in younger populations before we can make a definitive conclusion.
Viral infections causing persistent symptoms are not a new concept. SARS and MERS both cause persistent post‐infection symptoms in addition to psychological sequelae. The long‐term effects of SARS include chronic fatigue, myalgia encephalomyelitis, anorexia, and pulmonary damage. 17 , 18 , 38 Although the literature on MERS is not as extensive as it is on SARS, MERS has been linked to long‐term insomnia, impaired pulmonary function, and feelings of suicide. Both SARS and MERS have been shown to result in long‐term PTSD, depression, and anxiety disorder, with MERS resulting in a higher prevalence of these disorders. 39 These symptoms have been reported to persist many years post‐infection. Perhaps, it should not be surprising to see similar manifestations in COVID‐19 survivors, as the virus is closely related to SARS and MERS. What still remains to be determined with COVID‐19 is the severity and duration of PCS symptoms.
4.1. Strength and limitations
A limitation of this meta‐analysis is the heterogeneity of the included studies which can be explained by the different tools used to assess the quality of life in different populations of different countries and different follow‐up periods. Another limitation is the majority of the studies had followed up COVID‐19 patients who were hospitalized and elderly populations. Hence, more studies on PCS need to be conducted in both younger and outpatient settings as well to have more strong evidence. Additionally, studies included in meta‐analysis have different follow‐up times with a mean range from 36 to 153 days but still reported persistent symptoms even after long follow‐up periods. Despite these limitations, our meta‐analysis of 4828 confirmed post‐recovery COVID‐19 patients suggests poor quality of life, persistent symptoms, and worse mental health in these patients. These findings may help in developing a better management plan for the COVID‐19 patients' post‐recovery to avoid developing PCS and also the treatment guidelines of PCS patients. There are few hypotheses supporting these findings but there is no definitive answer to why certain patients develop PCS and its overall mechanism of action. Understanding the risk factors and pathophysiology will allow us to better manage these PCS patients.
5. CONCLUSION
In our meta‐analysis, we found that PCS has been associated with poor quality of life, long‐term persistent symptoms including fatigue, dyspnea, anosmia, cough, sleep disturbances, chest pain, arthralgia, and worse overall mental health. Although there is established literature on persistent symptoms associated with PCS, risk factors for developing it still remain unclear. These gaps in our understanding can partially be attributed to the fact that the primary focus to combat the pandemic, was developing vaccines. However, due to the large COVID‐19 infected population which has recovered and some of whom have developed PCS, the healthcare focus should shift in understanding the risk factors causing PCS eventually leading to poor quality of life, and developing follow‐up and treatment strategies accordingly.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
Though this article does not contain any studies with direct involvement of human participants or animals performed by any of the authors, all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
AUTHOR CONTRIBUTIONS
Conceptualization: Preeti Malik; Data curation: Preeti Malik and Urvish Patel. Formal analysis: Preeti Malik. Investigation: Urvish Patel. Methodology: Urvish Patel and Preeti Malik. Software: Preeti Malik. Project administration and Supervision: Preeti Malik and Urvish Patel. Writing—original draft: Karan Patel, Candida Pinto, Richa Jaiswal, Raghavendra Tirupathi and Preeti Malik. Writing—review & editing: Shreejith Pillai and Urvish Patel.
Supporting information
Supporting information.
Malik P, Patel K, Pinto C, et al. Post‐acute COVID‐19 syndrome (PCS) and health‐related quality of life (HRQoL)—A systematic review and meta‐analysis. J Med Virol. 2021;94:253‐262. 10.1002/jmv.27309
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. COVID‐19 Coronavirus Pandemic . Worldometer. 2020. Accessed March 20, 2021. https://www.worldometers.info/coronavirus/#countries
- 2. Patel U, Malik P, Usman MS, et al. Age‐adjusted risk factors associated with mortality and mechanical ventilation utilization amongst COVID‐19 hospitalizations—a systematic review and meta‐analysis. SN Compr Clin Med. 2020:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaur N, Gupta I, Singh H, et al. Epidemiological and clinical characteristics of 6635 COVID‐19 patients: a pooled analysis. SN Compr Clin Med. 2020;2(8):1048‐1052. [DOI] [PMC free article] [PubMed]
- 4. Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID‐19 and multiorgan response. Curr Probl Cardiol. 2020;45(8):100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mokhtari T, Hassani F, Ghaffari N, Ebrahimi B, Yarahmadi A, Hassanzadeh G. COVID‐19 and multiorgan failure: a narrative review on potential mechanisms. J Mol Histol. 2020;51(6):613‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jacobs LG, Gourna Paleoudis E, Lesky‐Di Bari, et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID‐19 infection. PLoS One. 2020;15(12):e0243882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. (IDSA) IDSoA . Post COVID/long COVID. 2021. Accessed March 1, 2021. https://www.idsociety.org/covid-19-real-time-learning-network/disease-manifestations--complications/post-covid-syndrome/#:%7E:text=Preliminary%20reports%20indicate%20some%20patients,to%2Dsevere%20disease
- 8. Mahase E. Covid‐19: What do we know about “long covid”? BMJ. 2020;370:m2815. [DOI] [PubMed] [Google Scholar]
- 9. Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID‐19 four months after hospital discharge. JAMA Netw Open. 2021;4(1):e2036142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taboada M, Moreno E, Cariñena A, et al. Quality of life, functional status, and persistent symptoms after intensive care of COVID‐19 patients. Br J Anaesth. 2021;126(3):e110‐e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arab‐Zozani M, Hashemi F, Safari H, Yousefi M, Ameri H. Health‐related quality of life and its associated factors in COVID‐19 patients. Osong Public Health Res Perspect. 2020;11(5):296‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Galván‐Tejada CE, Herrera‐García CF, Godina‐González S, et al. Persistence of COVID‐19 symptoms after recovery in Mexican population. Int J Environ Res Public Health. 2020;17(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al‐Jahdhami I, Al‐Naamani K, Al‐Mawali A. The post‐acute COVID‐19 syndrome (long COVID). Oman Med J. 2021;36(1):e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID‐19 infection: a cross‐sectional evaluation. J Med Virol. 2021;93(2):1013‐1022. [DOI] [PubMed] [Google Scholar]
- 15. Garrigues E, Janvier P, Kherabi Y, et al. Post‐discharge persistent symptoms and health‐related quality of life after hospitalization for COVID‐19. J Infect. 2020;81(6):e4‐e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doykov I, Hällqvist J, Gilmour K, Grandjean L, Mills K, Heywood W. The long tail of Covid‐19?—the detection of a prolonged inflammatory response after a SARS‐CoV‐2 infection in asymptomatic and mildly affected patients [version 2; peer review: 2 approved]. F1000Res. 2021;9:1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Østergaard L. SARS CoV‐2 related microvascular damage and symptoms during and after COVID‐19: Consequences of capillary transit‐time changes, tissue hypoxia and inflammation. Physiol Rep. 2021;9(3):e14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leow MK‐S, Kwek DS‐K, Ng AW‐K, Ong K‐C, Kaw GJ‐L, Lee LS‐U. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS). Clin Endocrinol. 2005;63(2):197‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moldofsky H, Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post‐SARS syndrome; a case‐controlled study. BMC Neurol. 2011;11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee AM, Wong JG, McAlonan GM, et al. Stress and psychological distress among SARS survivors 1 year after the outbreak. Can J Psychiatry. 2007;52(4):233‐240. [DOI] [PubMed] [Google Scholar]
- 21. Batawi S, Tarazan N, Al‐Raddadi R, et al. Quality of life reported by survivors after hospitalization for Middle East respiratory syndrome (MERS). Health Qual Life Outcomes. 2019;17(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Qual Life Res. 2011;20(10):1727‐1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1‐e34. [DOI] [PubMed] [Google Scholar]
- 24. Chopra V, Flanders SA, O'Malley M, Malani AN, Prescott HC. Sixty‐day outcomes among patients hospitalized with COVID‐19. Ann Intern Med. 2020;174:576‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carfì A, Bernabei R, Landi F. Group ftGAC‐P‐ACS. Persistent symptoms in patients after acute COVID‐19. JAMA. 2020;324(6):603‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tabacof L, Tosto‐Mancuso J, Wood J, et al. Post‐acute COVID‐19 syndrome negatively impacts health and wellbeing despite less severe acute infection. medRxiv. 2020:2020.2011.2004.20226126. [Google Scholar]
- 27. Valent A, Dudoignon E, Ressaire Q, Dépret F, Plaud B. Three‐month quality of life in survivors of ARDS due to COVID‐19: a preliminary report from a French academic centre. Anaesth Crit Care Pain Med. 2020;39(6):740‐741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mandal S, Barnett J, Brill SE, et al. ‘Long‐COVID’: a cross‐sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID‐19. Thorax. 2021;76(4):396‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang C, Huang L, Wang Y, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moreno‐Pérez O, Merino E, Leon‐Ramirez J‐M, et al. Post‐acute COVID‐19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021;82(3):378‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Centers for Disease Control and Prevention (CDC) . Management of visitors to healthcare facilities in the context of COVID‐19: non‐US healthcare settings. 2020. Accessed March 1, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/non-us-settings/hcf-visitors.html
- 32. Chang MC, Park D. Incidence of post‐traumatic stress disorder after coronavirus disease. Healthcare. 2020;8(4):373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hwang T‐J, Rabheru K, Peisah C, Reichman W, Ikeda M. Loneliness and social isolation during the COVID‐19 pandemic. Int Psychogeriatr. 2020;32(10):1217‐1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Amruta N, Chastain WH, Paz M, et al. SARS‐CoV‐2 mediated neuroinflammation and the impact of COVID‐19 in neurological disorders. Cytokine Growth Factor Rev. 2021;58:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dicpinigaitis PV, Canning BJ. Is there (will there be) a post‐COVID‐19 chronic cough? Lung. 2020;198(6):863‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jiang K. How COVID‐19 causes loss of smell. 2020. Accessed March 10, 2021.https://hms.harvard.edu/news/how-covid-19-causes-loss-smell
- 37. Gori A, Leone F, Loffredo L, et al. COVID‐19‐related anosmia: the olfactory pathway hypothesis and early intervention. Front Neurol. 2020;11:956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang P, Li J, Liu H, et al. Long‐term bone and lung consequences associated with hospital‐acquired severe acute respiratory syndrome: a 15‐year follow‐up from a prospective cohort study. Bone Res. 2020;8(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta‐analysis with comparison to the COVID‐19 pandemic. Lancet Psychiatry. 2020;7(7):611‐627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


