Abstract
Purpose:
Radium-223 is approved for metastatic castration-refractory prostate cancer (mCRPC) based on improved overall survival, and delay in skeletal related events. However, it is not associated with PSA or radiographic response, which poses a challenge in real-time assessment of its efficacy. Surrogate markers of treatment outcomes may facilitate tailoring treatment duration with radium-223, by limiting the duration of therapy with radium-223 in these patients. Here, we sought to investigate the utility of bone metabolic markers (BMM) as surrogate markers of response to radium-223 in mCRPC.
Experimental Design:
A prospective phase II trial of radium-223 plus enzalutamide (RE) versus enzalutamide (Enza) alone was designed to assess surrogacy of BMMs with respect to response to radium-223. Enza was used as a comparator in lieu of placebo due to the progressive disease. Co-primary endpoints were relative change in serum BMM N-telopeptide (NTP) levels from baseline to 6 months between the two arms and safety and feasibility of the combination.
Results:
Thirty-nine men were randomized to RE (n=27) or Enza (n=12). Combination was safe and feasible. Primary endpoint was met. A statistically significant relative change to NTP ratios between arms (0.64, 95% CI 0.51—0.81; P=0.00048) favored RE versus Enza. Overall, BMMs decreased with the RE therapy compared to Enza. Improved PSA response rate in RE versus Enza (P=0.024), correlated with decline in BMM.
Conclusions:
BMMs declined significantly with combination therapy, and were associated with improved outcomes. Upon external validation, BMMs may emerge as surrogate markers to monitor treatment with radum-223 in real-time.
Keywords: Radium-223, enzalutamide, metastatic castration resistant prostate cancer, bone-specific marker
Translational Relevance
Bony metastases occur in up to 90% of patients with mCRPC and are associated with significant pain, limited mobility, and increased risk for fractures. To date, radium-223, a calcium-mimetic radiopharmaceutical that accumulates in bones and emits alpha radiation, is the only approved bone-targeting agent associated with improved survival outcomes in mCRPC. Currently, response to radium-223 cannot be accurately assessed or monitored by imaging, clinical symptoms, laboratory values, or biomarkers. Surrogate markers of treatment outcomes may facilitate tailoring treatment duration with radium-223. In this study we investigated the utility of five bone metabolic markers as surrogate markers of response to radium-223. In our study, treatment with radium-223 was associated with decrease in serum BMM and this correlated with improved outcomes. Furthermore, these data may allow BMM to be utilized for assessing response to therapy in future trials of novel radio-isotopes such as next generation alpha and beta emitters.
Introduction
Over the past decade, the treatment landscape for metastatic castration-resistant prostate cancer (mCRPC) has evolved to include cytotoxic chemotherapy (cabazitaxel, docetaxel), androgen axis inhibitors (enzalutamide (Enza), abiraterone, and apalutamide), bone-targeted therapy (radium-223), and a prostate cancer therapeutic vaccine (Sipuleucel-T). Bony metastases occur in up to 90% of patients with mCRPC and are associated with significant pain, limited mobility, and increased risk for fractures [1, 2]. To date, radium-223, a calcium-mimetic radiopharmaceutical of alpha radiation, is the only bone-targeted agent associated with improved survival outcomes approved for mCRPC [3]. Radium-223 complexes with bone mineral hydroxyapatite at areas of increased bone turnover thereby concentrating at areas of bone metastases.[4] Radium-223 was approved on the basis of the ALSYMPCA trial which reported a reduction in the risk of death and symptomatic skeletal events with treatment [5, 6]. The recommended treatment regimen is 6 doses of radium-223 at a 4-week interval. Currently, response to radium-223 cannot be accurately assessed or monitored by imaging, clinical symptoms, laboratory values, or biomarkers. Surrogate markers of treatment outcomes with radium-223 are needed to improve tailoring of treatment duration according to response. Identification of real-time markers of response would aid in determining which patients should stop treatment early due to a lack of response.
Bone-metabolic markers (BMM) have shown promise as predictive biomarkers of response for bone-targeted therapies in mCRPC. The SWOG 0421 phase 3 trial of docetaxel with or without atrasentan (a bone-targeting agent) for mCRPC, reported that at week 9 of treatment, increasing levels of BMMs N-telopeptide (NTP), pyridinoline (PYR), and alkaline phosphatase were associated with inferior overall survival (OS). In addition, patients with high baseline levels of BMMs had poor prognosis, but also derived survival benefit from treatment with atrasentan [7]. Finally, continuous decline in BMMs during treatment was associated with improved survival in a pooled retrospective analysis of three clinical trials in patients with metastatic prostate cancer [8].
In the present report, results from a prospective, randomized trial of radium-223 and enzalutamide (RE) versus Enza monotherapy are presented. The underlying hypothesis was that treatment with radium-223 will lead to a decline in BMMs, and associate with improved treatment outcomes. Thus, BMMs may emerge as surrogate biological markers to guide the duration of radium-223 treatment.
Materials and Methods
Study Design and Participants
Patients with progressive mCRPC were recruited to a phase II clinical trial of RE versus Enza alone. Enza was used as a comparator in lieu of placebo due to the progressive nature of patient’s disease. Standard dose of Enza (160mg orally daily) and standard dose of radium-223, i.e. 55 kBq/kg IV every 4 weeks for 6 doses) were used. The study had an initial non-randomized single arm, feasibility cohort (RE, n = 8 patients) followed by a randomized (2:1) group comparing RE versus Enza alone (n = 39 evaluable patients; supplementary figure 1). The design included a feasibility and safety cohort to ensure there was no significant immediate toxicities observed with the combination, which was novel at the time of conceptualization of the study. This study was conducted in accordance with the Declaration of Helsinki, approved by the Institutional Review Board of the University of Utah (IRB 68770), and is registered with clinicaltrials.gov under accession NCT02199197. Written informed consent was obtained from all patients.
Patients with progressive mCRPC who had prior docetaxel treatment or were ineligible or refused docetaxel, and who were candidates for treatment with RE or Enza were eligible for inclusion. Patients had to be ≥18 years of age with a life expectancy ≥6 months, and documented mCRPC as defined by disease progression on continuous androgen deprivation therapy (ADT) with a castrate level of serum testosterone (≤50ng/dL). Metastatic disease had to be evidenced by baseline imaging studies (bone scan and/or computed tomography (CT) scan, or magnetic resonance imaging (MRI) of the abdomen and pelvis) within 28 days of registration. Key eligibility criteria also included histologic confirmation of adenocarcinoma, ECOG performance status ≤ 2, disease progression on prior therapy by PCWG2 criteria, no evidence of visceral metastasis, and adequate liver, kidney and bone marrow function at baseline. Patients were excluded if previously treated with Enza or radium-223.
A comprehensive panel of BMMs, including N-terminal propeptide of type 1 collagen (P1NP), NTP, bone alkaline phosphatase (BAP), C-telopeptide (CTP) and PYR were measured at baseline and monthly for 6 months. Baseline imaging included CT chest, abdomen and pelvis and a nuclear medicine bone scan. Imaging was repeated after 3 months, and 6 months on treatment, and/or at the time of PSA or clinical disease progression if occurred earlier. Subjects remained on study treatment for up to 6 months. Patients could continue Enza after completion of the 6-month study duration. A total of 2 years long-term follow-up was planned to assess for OS and potential long-term toxicity.
Conduct of this clinical trial was approved by the local institutional review board.
Study Endpoints
The study had two co-primary endpoints. First was comparison of the change in serum NTP levels, from baseline to after 6 months on treatment (or at disease progression whichever occurred first), between the two treatment arms. The second co-primary endpoint of safety compared the incidence of grade ≥3 hematologic adverse events (AEs) in all patients treated on the combination arm to the incidence reported in the ALSYMPCA trial [5]. Pre-specified secondary endpoints included PSA progression-free survival (PFS); PSA response rate reported as a 50% (PSA50) and 90% (PSA90) reduction from baseline; radiographic PFS (rPFS); radiographic disease control rate (rDCR, defined as complete response, partial response or stable disease); OS (defined as time from treatment initiation to death from any cause); BAP PFS; changes to BMMs (P1NP, NTP, BAP, CTP, and PYR); and AEs of special interest. Only patients enrolled in the randomized portion of the trial were evaluated for the primary endpoint comparing change in NTP levels. All patients in the combination arm were evaluated for the primary safety endpoint, and all patients from both arms were evaluated for the secondary endpoints. Radiographic assessments were made by independent radiologist review, un-blinded to study arm allocation. A description of arm allocation to the different study endpoints is shown in supplementary figure 1.
Statistics
The change in log2 NTP was compared between treatment arms using an ANCOVA model with the final value as response, treatment group as predictor, and initial log2 NTP as an adjustment variable. Based on the data from the SWOG 0421 study [7], the estimated baseline mean and standard deviation of log2 NTP were 3.95 nM and 1.14 nM respectively. We further assumed the Pearson correlation of baseline and follow-up NTP was 0.50. With these assumptions and with 27 evaluable patients in the RE arm, and 12 evaluable patients in the ENZA arm, there was 85% power to detect a 2.2-fold difference between treatment arms at the two-sided 0.05 significance level.
For the co-primary endpoint of safety, an overall proportion of subjects with grade 3 or higher hematologic AEs, significantly higher than those reported for the radium-223 arm in the ALSYMPCA trial [5], would be evidence of unacceptable toxicity. For the comparison of 4/35 events in the current study versus 128/600 events in the ALSYMPCA trial [5], a maximum likelihood test from a Poisson model was used. With a safety population of 35 evaluable patients, ≥13 events of grade ≥3 hematologic AEs would indicate an unacceptable rate of hematologic AEs. An exact binomial test was performed comparing the proportion of neutropenia, anemia, or thrombocytopenia subjects with grade ≥3 events in the study to the proportions of neutropenia, anemia, or thrombocytopenia subjects in the ALSYMPCA trial [5]. Exact 90% confidence intervals were calculated for AEs and serious AEs. With 35 evaluable patients an exact 90% confidence interval for an AE will extend no more than 17.5% from the observed proportion, and there is a 90% probability that an AE with a probability of 6.4% would be observed at least once. For the secondary endpoint of safety, Fisher’s exact test two-sided was used to compare AE between the combination and monotherapy arms.
Interim analysis of Grade ≥3 hematologic toxicity in the first 10 patients recruited to the combination arm (radium-223 + enzalutamide) was separately evaluated as required by the sponsor (Bayer pharmaceutical). The null hypotheses were based on proportions reported in the ALSYMPCA trial [5]. The purpose of the interim analysis was to formally review safety data and assess whether continued enrollment of subjects was acceptable. The safety data in this interim analysis was reviewed by the institutional data safety review committee. A proportion of subjects with grade 3 or higher hematologic AEs, significantly more than those reported for the radium-223 arm of the phase 3 ALSYMPCA [5] trial would be evidence of unacceptable toxicity. In the radium 223 arm of the Phase 3 ALSYMPCA trial [5] 12.7% (76/600) subjects experience grade ≥3 anemia, 39/600 (6.5%) experienced grade ≥3 thrombocytopenia, and 13/600 (2.2%) experienced grade ≥3 neutropenia.
The Wilcoxon rank sum test with continuity correction, two sided was used to compare response rates between the combination and monotherapy arms.
Univariate associations between changes in bone markers (log2 baseline minus log2 final value) and outcomes in all patients (N = 47) was performed. Ordinal outcomes, radiographic response (progressive disease, stable disease, partial response and complete response) and PSA response (<30%, 30–49%, 50–89%, ≥90%), were examined in the context of proportional odds logistic regression models, binary outcomes were assessed in the context of logistic regression models, and time to event outcomes were assessed in the context of Cox proportional hazards models. For the ordinal outcomes (radiographic and PSA response), the odds ratios represent the increase in odds that a patient’s response is better per unit best response in marker. For example, a patient with a 1-unit greater best response in N-Telopeptide, Cross-Linked than another patient (corresponding to a twice greater decline because of the log2 scale), we would estimate to have 6.11 fold increased odds of having a better response.
All statistical analysis were performed using the R-statistical package v.3.5.2 (https://www.r-project.org/).
Results
Patient Characteristics
Between July 2014 and November 2017, a total of 49 patients were enrolled in this study. Baseline demographics and clinical characteristics for 8 patients treated with the combination RE therapy in the initial non-randomized single arm feasibility cohort, followed by 41 patients in the randomized (2:1) to either the combination or monotherapy arms respectively are described in Table 1. At time of data cutoff, the median duration of follow-up was 26.0 months and 21.0 months in the RE and Enza arms respectively. Only 2 of 49 patients (one in each arm) refused and did not receive a concomitant bone strengthening therapy (zoledronic acid or denosumab) at enrollment and throughout the study. Twenty-one out of thirty-five (60%) patients in the combination arm and nine out of fourteen (64%) patients treated with Enza had prior progression on abiraterone. Twelve of thirty-five (34%) of RE patients and 3 of 12 (25%) Enza patients had prior progression on docetaxel. Of the patients randomized to Enza, four were subsequently were treated with radium-223, three are still on Enza, three received chemotherapy and two patients chose to pursue hospice because of rapidly progressing disease.
Table 1.
Baseline characteristics of patients in initial and randomized cohorts.
| Variable | Randomized (2:1) |
||
|---|---|---|---|
| Radium + Enza (n=8) | Radium + Enza (n=27) | Enza (n=14) | |
| Age (years), Median (Range) | 77 (58 – 88) | 70 (56 – 84) | 71 (55 – 82) |
| PSA (ng/mL) , Median (Range) | 72.4 (15.9 – 501.5) | 18.9 (3.4 – 144.7) | 26.6 (4 – 261.3) |
| Alkaline Phosphatase (U/L) , Median (Range) | 98 (53 – 394) | 81 (29 – 254) | 96 (44 – 202) |
| Hemoglobin (g/dL) , Median (Range) | 12.7 (10.3 – 15.8) | 14.2 (11.5 – 19) | 13.4 (10.6 – 15.3) |
| Albumin (g/dL) , Median (Range) | 3.9 (3.6 – 4.3) | 4.1 (3.5 – 4.5) | 4.1 (3.7 – 4.4) |
| ECOG 0, n (%) | 2 (25%) | 15 (56%) | 7 (50%) |
| ECOG 1, n (%) | 6 (75%) | 12 (44%) | 7 (50%) |
Serum bone metabolic markers
A statistically significant relative change to NTP ratios between arms (0.64, 95% CI 0.51—0.81; P=0.00048) was observed and favored the combination RE versus Enza arm (Table 2A). The estimated NTP relative changes in each arm are 0.84 (95% CI 0.75–0.95) and 1.34 (95% CI 0.99– 1.82) in the RE and Enza arms respectively (Table 2B). This reflects a relative 39% decrease in RE versus Enza arm. Overall, BMMs decreased with the RE therapy compared to Enza alone, with the exception of PYR which had a similar change in both treatment arms (Table 2A). A decreased ratio in all BMMs between treatment arms was statistically significant with exception of CTP and PYR (Table 2B).
Table 2A.
Relative ratio of changes to bone metabolic markers.
| Bone Marker | Ratio of Changes Between Arms (95% CI) | P-value |
|---|---|---|
| BAP | 0.38 (0.27−0.53) | <0.00001 |
| CTP | 0.70 (0.48−1.00) | 0.07 |
| NTP | 0.64 (0.51−0.81) | 0.00048 |
| P1NP | 0.55 (0.37−0.81) | 0.00291 |
| PYR | 1.00 (0.79−1.27) | 0.99 |
Ratio between arms = Radium-223 + Enzalutamide/Enzalutamide. Bone alkaline phosphatase (BAP), C-telopeptide (CTP), intact N-terminal propeptide (NTP), N-terminal propeptide of type 1 collagen (P1NP), and pyridinoline (PYR).
Table 2B.
Estimated fold change for bone metabolic markers in each arm.
| Bone Marker | Radium + Enza Arm (N=35) | Enza Arm (N=12) |
|---|---|---|
| Fold Change (95% CI) | Fold Change (95% CI) | |
| BAP | 0.48 (0.39 – 0.60) | 1.20 (0.85 – 1.69) |
| CTP | 0.97 (0.79 – 1.19) | 1.32 (0.87 – 2.01) |
| NTP | 0.86 (0.78 – 0.96) | 1.34 (0.99 – 1.81) |
| P1NP | 0.52 (0.41 – 0.65) | 0.91 (0.62 – 1.33) |
| PYR | 1.19 (1.05 – 1.34) | 1.21 (0.94 – 1.56) |
Fold changes (post/pre) and 95% confidence intervals are reported. The mean and standard deviation of the differences were calculated on the log scale. The exponential function was used to convert to a fold change. Bone alkaline phosphatase (BAP), C-telopeptide (CTP), intact N-terminal propeptide (NTP), N-terminal propeptide of type 1 collagen (P1NP), and pyridinoline (PYR).
There was evidence that declines in bone markers is associated with better outcomes, and conversely increases in bone markers may be associated with worse outcomes (Table 3). Notably, declines in NTP showed statistically significant relationships with improved radiographic response, PSA response, radiographic DCR, and radiographic PFS. BAP, CTP, P1NP, and PYR showed similar trends, but with less consistent statistical significance. See supplemental table 2 for timing to nadir of BMM.
Table 3.
Associations between changes in bone markers and outcomes in all patients (N = 47).
| Best response in bone markera | Radiographic Objective Responseb | PSA50 Responsec | PSA90 Responsec | Disease Control Rated | PSA PFSe | Radiographic PFSe |
|---|---|---|---|---|---|---|
| BAP | 0.74, 95% CI (0.23, 2.40), P=0.62 |
1.13,
95% CI (1.39, 6.84), P=0.01 |
1.39, 95% CI (0.75, 2.56), P=0.29 |
2.11, 95% CI (0.90, 4.96), P=0.09 |
0.72, 95% CI (0.48, 1.06), P=0.09 |
0.62,
95% CI (0.39, 0.96), P=0.03 |
| CTP | 0.82, 95% CI (0.21, 3.30), P=0.78 |
1.32, 95% CI (0.66, 2.66), P=0.44 |
0.88, 95% CI (0.44, 1.76), P=0.72 |
5.06,
95% CI (1.44, 17.75), P=0.01 |
0.79, 95% CI (0.51, 1.22), P=0.29 |
0.68, 95% CI (0.41, 1.14), P=0.15 |
| P1NP | 1.17, 95% CI (0.35, 3.91), P=0.79 |
3.20,
95% CI (1.33, 7.69), P=0.01 |
1.51, 95% CI (0.78, 2.90), P=0.22 |
1.72, 95% CI (0.71, 4.15), P=0.23 |
0.75, 95% CI (0.52, 1.08), P=0.12 |
0.64,
95% CI (0.42, 0.97), P=0.04 |
| NTP | 0.72, 95% CI (0.11, 4.64), P=0.73 |
4.55,
95% CI (1.21, 17.13), P=0.03 |
1.11, 95% CI (0.39, 3.16), 0.84 |
17.90,
95% CI (2.14, 150.03), P=0.01 |
0.59, 95% CI (0.28, 1.25), P=0.17 |
0.43,
95% CI (0.20, 0.91), P=0.03 |
| PYR | 0.30, 95% CI (0.04, 2.39), P=0.26 |
1.10, 95% CI (0.34, 3.53), P=0.87 |
1.36, 95% CI (0.40, 4.62), P=0.63 |
3.86, 95% CI (0.85, 17.52), P=0.08 |
0.62, 95% CI (0.29, 1.32), P=0.22 |
0.78, 95% CI (0.36, 1.69), P=0.54 |
log2 baseline minus log2 final value;
Binary response (PR+CR and PD+SD), reference level of PD+SD;
Binary response of PSA50 (PSA<50, PSA≥50), and binary response of PSA90 (PSA<90, PSA≥90);
Binary radiographic disease control rate (SD+PR+CR);
Hazard ratio. Bone alkaline phosphatase (BAP), C-telopeptide (CTP), intact N-terminal propeptide (NTP), N-terminal propeptide of type 1 collagen (P1NP), and pyridinoline (PYR).
Treatment Outcomes
At the time of data cutoff, median PSA PFS was 9.86 months (95% CI, 4.67 – 22.5) in the RE arm, and 3.34 months (95% CI, 2.66 to not estimable [NE]) in the Enza arm (HR 0.79 [95% CI 0.36 – 1.75]; P = 0.60; Figure 1A). Median rPFS was 11.31 months (95% CI, 9.07 – NE) in the RE arm and 5.62 months (95% CI, 2.76 – NE) in the Enza arm (HR 0.54 [95% CI 0.22 – 1.32]; P = 0.20; Figure 1B). Median OS was 26.0 months (95% CI, 17.6 – NE) in the RE arm and 21.0 months (95% CI, 16.6 – NE) in the Enza arm (HR 0.54 [95% CI 0.22 – 1.32]; P = 0.40; Figure 1C). A statistically significant difference in median BAP PFS was noted with the median not reached in the RE arm and 4.34 months (95% CI, 2.76 – NE) in the Enza arm (HR 0.12 [95% CI 0.03 – 0.50]; P = 0.0007; Figure 1D).
Figure 1.
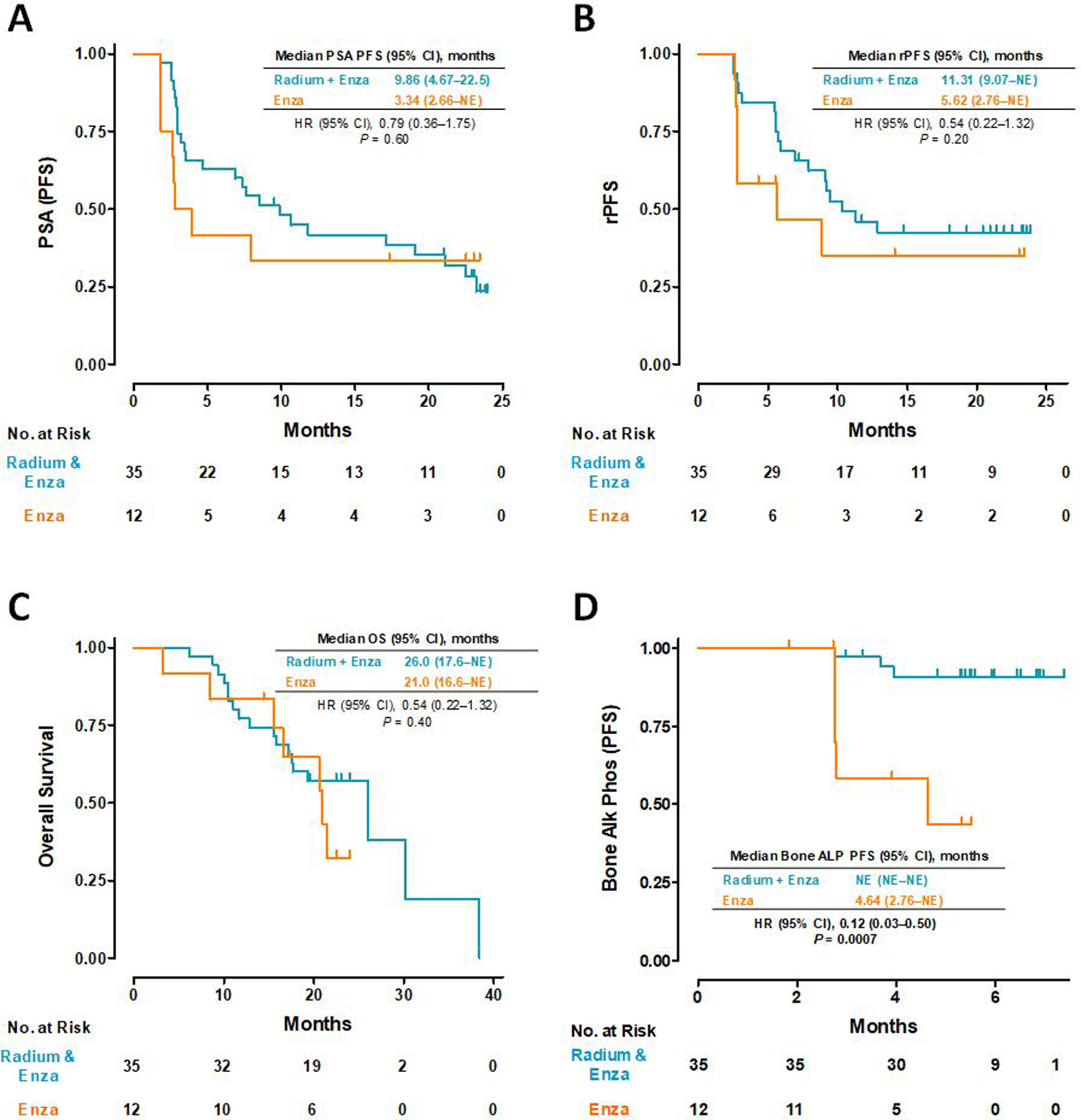
Kaplan-Meier plots for (A) PSA progression-free survival, (B) radiographic progression-free survival, (C) overall survival, and (D) bone alkaline phosphatase progression-free survival.
Improvements in PSA30, 50, and 90 response rates were observed in patients in the RE arm compared to the Enza arm. The categorical PSA response significantly favored the RE arm compared to the Enza arm (P = 0.024; Table 4). In the RE arm, radiographic disease control rate rDCR was 89% (31/35 patients), with 28 patients had stable disease (80%), 4 had progressive disease (11%), 3 had a partial response (9%), and no complete responses (P = 0.058; Table 4). In contrast, in the Enza arm, rDCR was 67% (8/12 patients), with 8 stable disease (67%), 4 progressive disease (33%), and no partial or complete responses. As of data cutoff, 2 patients in the Enza arm had not yet experienced radiographic or symptomatic disease progression compared to 9 patients receiving RE.
Table 4.
PSA and radiographic response rates in the radium + enzalutamide arm versus enzalutamide monotherapy arm.
| Marker/Response | Radium + Enza Arm N=35 (%) | Enza Arm N=12 (%) |
P-value* |
|---|---|---|---|
| PSA | |||
| <30 | 6 (17%) | 6 (50%) | 0.024 |
| ≥30 | 5 (14%) | 2 (17%) | |
| ≥50 | 9 (26%) | 2 (17%) | |
| ≥90 | 15 (43%) | 2 (17%) | |
|
| |||
| Radiographic | |||
| CR | 0 (0%) | 0 (0%) | 0.058 |
| PR | 3 (9%) | 0 (0%) | |
| SD | 28 (80%) | 8 (67%) | |
The Wilcoxon rank sum test with continuity correction, two sided was used to compare response rates between the combination and monotherapy arms. Complete Response (CR); Partial Response (PR); Stable Disease (SD).
Interim Safety and Overall Safety Analysis
A pre-specified interim analysis of safety (mandated by the study sponsor, Bayer Pharma) was conducted in the first 10 patients that completed treatment on the combination arm or discontinued study treatment early (Supplementary Table 1). Enrollment was continued after no safety signals were detected.
There was no significant difference in observed grade ≥3 hematologic toxicities in patients treated with RE in our study compared to the ALSYMPCA trial (P = 0.91, Table 5A). [5] . When comparing toxicity profiles between treatment arms, the overall incidence of AEs was higher in the RE arm compared to Enza; however, grade≥3 AEs were similar in both arms (Table 5B). No pathological fractures were observed in any patient enrolled in this trial at the time of data cutoff, i.e. a median follow-up of 26.0 months in the RE combination arm and 21.0 months in the Enza arm. The cytopenias in the RE patients were of expected frequency and duration compared to radium-223 alone in the ALSYMPCA trial. No patients required growth factor support or a bone marrow biopsy due to severe or prolonged cytopenias.
Table 5A.
Comparison of frequency of grade ≥3 cytopenias between radium-223 + enzalutamide arm with ALSYMPCA trial.
| AEs | Agarwal et al N=35 |
95% CI | Parker et al [4] (ALSYMPCA) N=600 |
P-value (one sided) |
|---|---|---|---|---|
| Hematologic, N (%) a | 4 (13.8%) | 4.2% - 30.5% | 128 (21.3%) | 0.91 |
| Neutropenia, N (%) b | 3 (8.6%) | 1.8% - 23.1% | 13 (2.2%) | 0.041 |
| Anemia, N (%) b | 0 (0%) | 0% - 10% | 76 (12.7%) | 1.00 |
| Thrombocytopenia, N (%) b | 1 (2.8%) | 0% - 14.9% | 39 (6.5%) | 0.90 |
Hematologic AEs includes all neutropenia, anemia, and thrombocytopenia events and is used for comparison to the ALSYMPCA trial [5, 6]. For the comparison of 4/35 events in the Agarwal et al study versus 128/600 events in the ALSYMPCA trial [5, 6], a maximum likelihood test from a Poisson model was used. With a safety population of 35 evaluable patients, ≥13 events of grade ≥3 hematologic AEs would indicate an unacceptable rate of hematologic AEs.
Table 5B.
Incidence of AEs of special interest, comparison between treatment arms.
| RE (N = 35) | Enza (N = 14) | a P-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AEs Grades | All | 1–2 | 3–4 | All | 1–2 | 3–4 | All | 3–4 | |
| Leukopenia, N (%) | 20 (57.1%) | 17 (48.6%) | 3 (8.6%) | 0 | 0 | 0 | 0.0002 | 0.55 | |
| Diarrhea, N (%) | 19 (54.3%) | 18 (51.4%) | 1 (2.9%) | 1 (7.1%) | 1 (7.1%) | 0 | 0.004 | 1.0 | |
| Fatigue, N (%) | 16 (45.7%) | 15 (42.9%) | 1 (2.9%) | 3 (21.4%) | 2 (14.3%) | 1 (7.1%) | 0.20 | 0.49 | |
| Arthralgia, N (%) | 7 (20%) | 7 (20%) | 0 | 4 (28.6%) | 3 (21.4%) | 1 (7.1%) | 0.52 | 0.49 | |
| Neutropenia, N (%) | 14 (40%) | 11 (31.4%) | 3 (8.6%) | 0 | 0 | 0 | 0.0097 | 0.55 | |
| Bone Pain, N (%) | 5 (14.3%) | 5 (14.3%) | 0 | 2 (14.3%) | 2 (14.3%) | 0 | 0.47 | 1.0 | |
| Anemia, N (%) | 9 (25.7%) | 9 (25.7%) | 0 | 1 (7.1%) | 1 (7.1%) | 0 | 0.24 | 1.0 | |
| Myalgia, N (%) | 8 (22.9%) | 8 (22.9%) | 0 | 3 (21.4%) | 3 (21.4%) | 0 | 1.0 | 1.0 | |
| Thrombocytopenia, N (%) | 7 (20.0%) | 6 (17.1%) | 1 (2.9%) | 0 | 0 | 0 | 0.17 | 1.0 | |
Statistical analysis was performed using Fisher’s exact test two-sided.
Discussion
Currently there are no laboratory or radiographic markers available to differentiate between responders and non-responders to radium-223. BMMs have shown some promise for real-time monitoring of response to radium-223. The results reported here support this contention, since a significant decrease in BMM levels was associated with a corresponding response to treatment. In an exploratory analysis from the ALSYMPCA trial, Sartor and colleagues reported that decrease in total alkaline phosphatase from baseline to 12 weeks of radium-223 was associated with longer OS compared to patients with no decline (17.8 months vs. 10.4 months, P <0.0001) [9]. In SWOG 0421, elevated baseline levels and increasing levels of BMMs (NTP, PYR, BAP, and P1NP) after 9 weeks of atrasentan therapy were associated with worse survival (P<0.001) [7]. In a phase 2 trial of 53 men with mCRPC randomized to either radium-223 and docetaxel or docetaxel alone, alkaline phosphatase and P1NP levels declined more rapidly in the combination arm compared to docetaxel alone [10]. In a phase 2 trial of radium-223 in combination with either sorafenib or pazopanib for patients with mRCC, NTP, CTP, BAP, and P1NP declined significantly between baseline and weeks 8 and 16 of treatment [11].
This study is the first to report on the correlation of BMM profiles with response outcomes and efficacy from a prospective, randomized trial in mCRPC. Furthermore, our findings are consistent with the preliminary safety results from the phase 3 clinical trial (PEACE-3, NCT02194842), which showed RE is safe when used with bone-strengthening agents. The only data currently available from PEACE-3 is the frequency of skeletal fractures, which indicated an absolute increased risk of 33% with RE vs. 13% with Enza alone [12]. This difference in risk was abolished when patients received a bone-strengthening agent at least 6 weeks prior to first treatment with RE [12]. These safety results are in contrast to the phase 3 clinical trial (ERA223) of radium-223 and abiraterone acetate (AA), which was unblinded early following an increased rate of skeletal fractures (29%) and deaths in the combination arm over placebo [13].
In this study the phase 2 clinical trial of RE versus Enza alone met its primary efficacy endpoint of a significant decline in serum NTP levels with the combination therapy versus Enza monotherapy. Additionally, BAP and P1NP, declined significantly more in the combination arm compared to Enza monotherapy. Furthermore, the changes in the bone metabolic markers directly correlated with treatment outcomes (Table 3). Patients in the combination arm demonstrated significantly improved outcomes in PSA response rates and rDCR compared to Enza alone. Our findings are in contrast to the phase 3 ERA223 trial which found no improvement in OS (HR 1.195, P = 0.1280) or radiographic PFS (HR 1.152, 95% CI 0.96 – 1.383) with the combination of radium-223 plus abiraterone over abiraterone alone. This may be due to differences between bone-strengthening therapy use between the trials or differences between abiraterone and enzalutamide when used in combination with radium-223. The ongoing PEACE-3 trial will provide definitive evidence of clinical efficacy and safety of the combination of radium-223 plus enzalutamide in this population. RE was safe in patients with mCRPC, as the incidence of grade ≥3 hematologic AEs were similar to the ALSYMPCA trial. Furthermore, non-hematologic AEs were similar in both arms.
This study is limited by its small sample size and was not powered to compare survival outcomes and efficacy of RE versus Enza alone. Also, this trial did not stipulate the therapy used after disease progression. Differences in subsequent therapy may have influenced the results on overall survival in this study. Addition of a third arm using radium-223 monotherapy would have better defined the impact of radium-223 alone on BMM. However, this arm would not have allowed for correlation of BMM with PSA PFS, radiographic PFS, and objective responses, as monotherapy with radium-223 has never been shown to impact any of these efficacy endpoints. Nevertheless, findings of improved PSA and radiographic response are encouraging.
To conclude, combination therapy with RE is associated with a significant decline in BMM levels compared to Enza alone, and correlated with improved outcomes. Moreover, the combination is safe and feasible when used with concurrent bone-strengthening agents. Following independent validation, BMMs may emerge as surrogate markers to inform and optimize treatment duration with radum-223. Furthermore, these data may position BMM as biomarkers of response to therapy in upcoming trials of novel radio-isotopes such as next generation alpha and beta emitters in the setting of metastatic castration refractory prostate cancer.
Supplementary Material
Acknowledgements
We thank the patients who participated in this clinical trial and their families for ongoing support. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30CA042014 and to N Agarwal 3P30CA042014–25S2. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest Statement
Neeraj Agarwal has consulted for Pfizer, Exelixis, Merck, Argos, EMD Serono, Eisai, Bayer, Novartis, Genentech, BMS, Astra Zeneca, Medivation, Clovis, Foundation One, Astellas, Eli Lilly, Nektar, Active Biotech, Bavarian Nordic, Calithera, Celldex, GlaxoSmithKline, Immunomedics, Janssen, Merck, New Link Genetics, Prometheus, Rexahn, Sanofi, Takeda, Tracon. Benjamin Maughan has consulted for Janssen Oncology, Exelixis, Tempus, Peloton Therapeutics and Astellas. John Hoffman has consulted for Blue Earth Diagnostics. Jared Thorley has consulted for Exelixis and Bristol Myers Squibb. Sumati Gupta reports her spouse has stock ownership in Salarius Pharmaceutical. Roberto Nussenzveig has consulted for Tempus. All authors declare this study was funded with support from Bayer Oncology. Additional support was provided by the Center for Quantitative Cancer Imaging at the Huntsman Cancer Institute, University of Utah.
Ethics Committee Approval
This study was approved by the Institutional Review Board of the University of Utah (IRB 68770), and is registered with clinicaltrials.gov under accession NCT02199197.
References
- 1.Freedland SJ, Richhariya A, Wang H, Chung K, and Shore ND, Treatment patterns in patients with prostate cancer and bone metastasis among US community-based urology group practices. Urology, 2012. 80(2): p. 293–8. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RE, Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res, 2006. 12(20 Pt 2): p. 6243s–6249s. [DOI] [PubMed] [Google Scholar]
- 3.Bellmunt J, Tackling the bone with alpha emitters in metastatic castration-resistant prostate cancer patients. Eur Urol, 2013. 63(2): p. 198–200. [DOI] [PubMed] [Google Scholar]
- 4.Henriksen G, Breistøl K, Bruland ØS, Fodstad Ø, and Larsen RH, Significant antitumor effect from bone-seeking, alpha-particle-emitting (223)Ra demonstrated in an experimental skeletal metastases model. Cancer Research, 2002. 62(11): p. 3120–5. [PubMed] [Google Scholar]
- 5.Parker C, Nilsson S, Heinrich D, et al. , Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med, 2013. 369(3): p. 213–23. [DOI] [PubMed] [Google Scholar]
- 6.Sartor O, Coleman R, Nilsson S, et al. , Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol, 2014. 15(7): p. 738–46. [DOI] [PubMed] [Google Scholar]
- 7.Lara PN Jr., Ely B, Quinn DI, et al. , Serum biomarkers of bone metabolism in castration-resistant prostate cancer patients with skeletal metastases: results from SWOG 0421. J Natl Cancer Inst, 2014. 106(4): p. dju013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Som A, Tu SM, Liu J, et al. , Response in bone turnover markers during therapy predicts overall survival in patients with metastatic prostate cancer: analysis of three clinical trials. Br J Cancer, 2012. 107(9): p. 1547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sartor O, Coleman RE, Nilsson S, et al. , An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann Oncol, 2017. 28(5): p. 1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris MJ, Loriot Y, Fizazi K, et al. , Effects of radium-223 (Ra-223) with docetaxel versus docetaxel alone on bone biomarkers in patients with bone-metastatic castration-resistant prostate cancer (CRPC): A phase I/IIa clinical trial. Journal of Clinical Oncology, 2017. 35(6_suppl): p. 154–154. [Google Scholar]
- 11.McKay RR, Bosse D, Gray KP, et al. , Radium-223 Dichloride in Combination with Vascular Endothelial Growth Factor-Targeting Therapy in Advanced Renal Cell Carcinoma with Bone Metastases. Clin Cancer Res, 2018. 24(17): p. 4081–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tombal BF, Loriot Y, Saad F, et al. , Decreased fracture rate by mandating bone-protecting agents in the EORTC 1333/PEACE III trial comparing enzalutamide and Ra223 versus enzalutamide alone: An interim safety analysis. Journal of Clinical Oncology, 2019. 37(15_suppl): p. 5007–5007. [Google Scholar]
- 13.Smith M, Parker C, Saad F, et al. , Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol, 2019. 20(3): p. 408–419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


