Key Points
Question
Is the implementation of the Medicare national coverage determination (NCD) associated with use of next-generation sequencing by insurance and racial and ethnic categories?
Findings
In this cohort study of 92 687 patients with lung, breast, colon, and skin cancer, NCD implementation was associated with a slower rate of increase in next-generation sequencing use for patients with patient assistance programs compared with Medicare beneficiaries. Implementation of the NCD was not associated with narrowing of racial and ethnic disparities among Medicare beneficiaries alone or the overall insured population.
Meaning
These findings suggest that implementation of the Medicare NCD for next-generation sequencing did not result in equal increased use across insurance types or racial and ethnic groups.
Abstract
Importance
In March 2018, Medicare issued a national coverage determination (NCD) for next-generation sequencing (NGS) to facilitate access to NGS testing among Medicare beneficiaries. It is unknown whether the NCD affected health equity issues for Medicare beneficiaries and the overall population.
Objective
To examine the association between the Medicare NCD and NGS use by insurance types and race and ethnicity.
Design, Setting, and Participants
A retrospective cohort analysis was conducted using electronic health record data derived from a real-world database. Data originated from approximately 280 cancer clinics (approximately 800 sites of care) in the US. Patients with advanced non–small cell lung cancer (aNSCLC), metastatic colorectal cancer (mCRC), metastatic breast cancer (mBC), or advanced melanoma diagnosed from January 1, 2011, through March 31, 2020, were included.
Exposure
Pre- vs post-NCD period.
Main Outcomes and Measures
Patients were classified by insurance type and race and ethnicity to examine patterns in NGS testing less than or equal to 60 days after diagnosis. Difference-in-differences models examined changes in average NGS testing in the pre- and post-NCD periods by race and ethnicity, and interrupted time-series analysis examined whether trends over time varied by insurance type and race and ethnicity.
Results
Among 92 687 patients with aNSCLC, mCRC, mBC, or advanced melanoma, mean (SD) age was 66.6 (11.2) years, 51 582 (55.7%) were women, and 63 864 (68.9%) were Medicare beneficiaries. The largest racial and ethnic categories according to the database used and further classification were Black or African American (8605 [9.3%]) and non-Hispanic White (59 806 [64.5%]). Compared with Medicare beneficiaries, changes in pre- to post-NCD NGS testing trends were similar in commercially insured patients (odds ratio [OR], 1.03; 95% CI, 0.98-1.08; P = .25). Pre- to post-NCD NGS testing trends increased at a slower rate among patients in assistance programs (OR, 0.93; 95% CI, 0.87-0.99; P = .03) compared with Medicare beneficiaries. The rate of increase for patients receiving Medicaid was not statistically significantly different compared with those receiving Medicare (OR, 0.92; 95% CI, 0.84-1.01; P = .07). The NCD was not associated with statistically significant changes in NGS use trends by racial and ethnic groups within Medicare beneficiaries alone or across all insurance types. Compared with non-Hispanic White individuals, increases in average NGS use from the pre-NCD to post-NCD period were 14% lower (OR, 0.86; 95% CI, 0.74-0.99; P = .04) among African American and 23% lower (OR, 0.77; 95% CI, 0.62-0.96; P = .02) among Hispanic/Latino individuals; increases among Asian individuals and those with other races and ethnicities were similar.
Conclusions and Relevance
The findings of this study suggest that expansion of Medicare-covered benefits may not occur equally across insurance types, thereby further widening or maintaining disparities in NGS testing. Additional efforts beyond coverage policies are needed to ensure equitable access to the benefits of precision medicine.
This cohort study examines changes in next-generation sequencing among individuals of different races and ethnicities and different levels of insurance coverage following implementation of the Medicare national coverage determination.
Introduction
Precision medicine has changed the nature of treatment practices for patients with cancer. During the past decade, an increasing number of biomarkers have been identified across various cancers.1,2,3 The clinical benefits of biomarker testing for patients with advanced or metastatic cancer are substantial, as the increased use of appropriate targeted therapies is associated with improved survival rates.4,5
Guidelines of the National Comprehensive Cancer Network recommend biomarker testing across multiple cancer types.1,2,3,6 Next-generation sequencing (NGS) testing has become increasingly important because it enables identification of multiple biomarkers simultaneously and efficiently while minimizing the number of biopsies required. Next-generation sequencing testing adoption rates have increased in recent years, with rates for advanced non–small cell lung cancer (aNSCLC), metastatic colorectal cancer (mCRC), metastatic breast cancer (mBC), and advanced melanoma increasing from less than 1% in 2011 to approximately 40% in 2019.7 In 2017, 3 of 4 oncologists reported using NGS testing to direct treatment to genomically matched therapies.8,9 Next-generation sequencing testing in oncology has also facilitated clinical research furthering advancement of precision medicine.10
Despite recognized clinical benefits of biomarker testing, variability in health care coverage policies has posed a significant barrier to obtaining NGS testing for patients with cancer.11 Specifically, coverage policy differences have limited the accessibility of this type of testing for select patients based on insurance plan.12,13,14 In a survey of more than 3000 patients with cancer and survivors, 33% of respondents with private insurance, 13% of respondents with employer-provided insurance, and 3% of respondents with Medicare coverage reported that biomarker testing was not covered by their insurer.15
In 2018, the Centers for Medicare & Medicaid Services issued a national coverage determination (NCD) for initial and further NGS testing for all solid tumors with a companion diagnostic claim.16 Although previous studies suggest that coverage changes may increase NGS testing, less is known about the potential association with health equity in both Medicare beneficiaries and the broader insured population.7,14 In a recent study, the NCD led to greater NGS testing for Medicare beneficiaries as well as commercially insured patients7; other research has also suggested that the NCD may influence commercial coverage.14 However, it is unclear whether the NCD has influenced NGS testing coverage by other insurance types, such as Medicaid, which has a larger population of minority racial and ethnic groups that may experience poorer care and outcomes.17 Previous research has shown that African American individuals and Medicaid beneficiaries with aNSCLC may be less likely to receive NGS testing.11 To inform future equitable health coverage policies, it is important to evaluate whether different coverage policies contribute to health inequities, especially given the variations in patient demographics across insurance types.
To our knowledge, no studies have yet examined the association between the NGS NCD and disparities in NGS testing in either the Medicare population, for whom the NCD was implemented, or the broader insured population because other insurance types may adopt the guidance of the NCD. Therefore, this study aimed to examine whether the Medicare NCD was associated with changes in NGS testing trends among patients with commercial insurance, with Medicaid, or in patient assistance programs (PAPs), and assess whether access afforded through the NCD was associated with racial and ethnic disparities in NGS testing trends among Medicare beneficiaries and the overall population.
Methods
Data Source
We conducted a retrospective analysis using the Flatiron Health electronic health record–derived deidentified nationwide longitudinal database. This database has increasingly become a research resource in the past 5 years and includes patients diagnosed with cancer on or after January 1, 2011.18,19,20,21,22,23 During the study period, deidentified data originated from approximately 280 US cancer clinics (approximately 800 sites of care).24 Most patients in the database were in community oncology settings; relative community and academic proportions may vary depending on study cohort. The deidentified patient-level data include structured and unstructured data curated via technology-enabled abstraction. Unstructured data, such as confirmation of cancer diagnosis and stage, are manually abstracted by clinical oncology nurses or tumor registrars.25 The protocol was approved by an independent institutional review board service (WCG IRB) before the onset of the study and included a waiver of informed consent because the data are deidentified and subject to obligations to prevent reidentification and protect patient confidentiality. This study followed the applicable portions of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.26
Patient Population
Selected patients (age ≥18 years) had a diagnosis of aNSCLC, mCRC, mBC, or advanced melanoma from January 1, 2011 (January 1, 2013, for mCRC) through March 31, 2020. We chose these tumors given the frequency of NGS testing and the number of targeted therapies available for each cancer type.1,2,3,6,27 Inclusion criteria required a clinic visit within 90 days of diagnosis in the community practice setting. Academic practice settings were excluded because of potential incomplete capture of biomarker testing from scanned documents (eg, pathologic and biomarker test reports) from external laboratories. Patients with multiple tumors were classified according to the tumor type diagnosed for the earliest episode of advanced or metastatic cancer. Exclusion criteria included an advanced or metastatic diagnosis in another cancer type before an advanced or metastatic diagnosis in NSCLC, mCRC, mBC, or advanced melanoma, or an NGS test before the date of the advanced or metastatic cancer diagnosis. Patients were required to have 60 days or more of follow-up between the advanced or metastatic diagnosis and study cutoff to identify NGS testing.
Construction of Variables
We used biomarker testing variables derived by Flatiron Health, based on abstracted data from electronic health records, to identify NGS testing. Evidence of NGS testing was identified by searching terms (eg, next-generation sequencing), NGS technology platforms (eg, Illumina HiSeq), and specific NGS tests (eg, FoundationOne CDx). Any use of NGS testing was defined as an NGS test, regardless of the number of genes in the panel. We excluded RNA-sequencing NGS tests and germline or hereditary NGS tests.
We categorized patients into year-quarters based on their advanced or metastatic cancer diagnosis date, which could occur at their initial diagnosis (de novo) or after early-stage disease diagnosis that later progressed (recurrent). The following stages were included for each cancer cohort: aNSCLC cohort (stage IIIB or IV disease), mCRC (stage IV disease), mBC (stage IV disease), and advanced melanoma (stage III or IV disease).
We developed an insurance classification hierarchy based on date of diagnosis: (1) Medicare (ie, patients aged ≥65 years), (2) Medicaid, (3) PAP (ie, evidence of PAP, not Medicaid group), (4) commercial (ie, evidence of commercial, but not Medicare, Medicaid, or PAP), and (5) other or unknown (ie, patients with missing or other types of insurance) groups. Racial and ethnic group status of participants was obtained via patient self-report based on electronic health record–abstracted patient-reported data. The Flatiron database has the following race categories: Asian, Black or African American, Hispanic or Latino White, and Other. Separately, the database has an Ethnicity variable that captures Hispanic or Latino (yes/no). Using that information, patients were categorized as Asian, Black or African American (hereafter, African American), Hispanic/Latino, non-Hispanic White (hereafter, White), Other, and missing.
NGS Testing Rate
We defined the NGS testing rate as the number of patients with an NGS test less than or equal to 60 days following an advanced or metastatic cancer diagnosis divided by the total number of patients diagnosed in the quarter. The 60-day window was based on clinical opinion regarding the time in which NGS testing is assumed to provide clinical utility. We designated the quarters after the policy effect date as the post-NCD period and the quarters before that as the pre-NCD period. We assessed changes in NGS testing use from the pre-NCD to post-NCD period.
Statistical Analysis
Descriptive statistics, including means and percentages, were calculated to compare differences in baseline patient characteristics by race and ethnicity. We conducted an interrupted time-series analysis to examine changes in NGS testing trends from the pre-NCD to the post-NCD period by race and ethnicity, insurance, and factors contributing to disparities between race and ethnicity and insurance, while adjusting for other covariates. Other covariates included age at advanced or metastatic cancer diagnosis, sex, race and ethnicity, region where the patient lives, tumor type, and insurance. The interrupted time-series model was fitted as a logistic regression model using separate intercepts and slopes in the pre- and post-NCD periods for each covariate (main effects only, no interactions between covariates). We assumed no level change between the pre- and post-NCD period within each covariate. Missing insurance type and race and ethnicity categories were included in the interrupted time-series model but excluded in the difference-in-differences analysis of race and ethnicity. We estimated the main effects on the proportion of patients who received NGS tests using weighted marginal means, with weights chosen based on the relative frequency in the data of patients with various covariate combinations. Estimates of NGS testing before the NCD were based on predicted values from the model at quarter 3, 2016, while estimates of NGS testing after the NCD were based on model predicted values at quarter 2, 2019.
We created a difference-in-differences logistic regression model28 to assess changes in average pre-and post-NDS NGS testing by race and ethnicity. We assessed the suitability of the difference-in-differences model via testing for parallel trends using a χ2 test on the interaction term in a logistic regression analysis of deviance by quarter and by race and ethnicity using pre-NCD period data.
To assess whether factors contributed to disparities in NGS testing, we used the interrupted time-series model with race and ethnicity as the independent variable and sequentially added covariates to assess the change in the differences in pre- and post-NCD NGS testing for White patients vs the other groups. Analyses were conducted in R, version 3.6.3.29 Estimated marginal means and 95% CIs were generated using the R package emmeans.30 We set the statistical significance threshold with 2-sided testing a priori at P < .05.
Results
Patient Demographic Characteristics
Among 92 687 patients with aNSCLC, mCRC, mBC, or advanced melanoma, the mean (SD) age was 66.6 (11.2) years; most patients were women (51 582 [55.7%]), located in the South region (41 679 [45.0%]), and had aNSCLC (50 326 [54.3%]). The race and ethnicity groups included African American (8605 [9.3%], Asian (2246 [2.4%], Hispanic/Latino (4473 [4.8%]), White (59 806 [64.5%]), and Other (American Indian or Alaska Native, Hawaiian or Pacific Islander, and race descriptions that fall into multiple race categories) (8240 [10.1%]) individuals. Data on 9317 patients were missing (Table 1). Medicare was the most common insurance type (63 864 [68.9%]), followed by commercial insurance (14 409 [15.5%]), PAP (4912 [5.3%]), and Medicaid (3083 [3.3%]). African American individuals composed a larger proportion of the population with Medicaid coverage (664 of 3083 [21.5%]) compared with commercial insurance (1349 of 14 409 [9.4%]) or Medicare (5500 of 63 864 [8.6%]). White individuals composed a larger portion of the population with commercial insurance (9023 of 14 409 [62.6%]) or Medicare (42 350 of 63 864 [66.3%]) compared with Medicaid (1590 of 3083 [51.6%]).
Table 1. Demographic Characteristics of Patients Overall and by Race and Ethnicity.
| Characteristic | No. (%) | ||||||
|---|---|---|---|---|---|---|---|
| Non-Hispanic White (n = 59 806) | African American (n = 8605) | Asian (n = 2246) | Hispanic/Latino (n = 4473) | Other (n = 8240)a | Missing (n = 9317) | Overall (n = 92 687) | |
| Age at diagnosis, y | |||||||
| Mean (SD) | 67.2 (10.9) | 64.0 (11.5) | 65.3 (12.0) | 63.5 (12.7) | 66.4 (11.2) | 67.5 (11.0) | 66.6 (11.2) |
| Median (range) | 69.0 (18.0-85.0) | 65.0 (21.0-85.0) | 67.0 (23.0-85.0) | 65.0 (18.0-85.0) | 68.0 (18.0-85.0) | 69.0 (20.0-85.0) | 68.0 (18.0-85.0) |
| Sex | |||||||
| Female | 33 037 (55.2) | 5111 (59.4) | 1204 (53.6) | 2649 (59.2) | 4562 (55.4) | 5019 (53.9) | 51 582 (55.7) |
| Male | 26 769 (44.8) | 3494 (40.6) | 1042 (46.4) | 1824 (40.8) | 3678 (44.6) | 4298 (46.1) | 41 105 (44.3) |
| Tumor | |||||||
| Breast | 10 814 (18.1) | 2020 (23.5) | 390 (17.4) | 1251 (28.0) | 1586 (19.2) | 1576 (16.9) | 17 637 (19.0) |
| CRC | 12 278 (20.5) | 2223 (25.8) | 601 (26.8) | 1594 (35.6) | 1876 (22.8) | 1755 (18.8) | 20 327 (21.9) |
| Melanoma | 3504 (5.9) | 30 (0.3) | 12 (0.5) | 99 (2.2) | 316 (3.8) | 436 (4.7) | 4397 (4.7) |
| NSCLC | 33 210 (55.5) | 4332 (50.3) | 1243 (55.3) | 1529 (34.2) | 4462 (54.2) | 5550 (59.6) | 50 326 (54.3) |
| Region | |||||||
| Midwest | 11 032 (18.4) | 920 (10.7) | 154 (6.9) | 194 (4.3) | 1029 (12.5) | 940 (10.1) | 14 269 (15.4) |
| West | 8698 (14.5) | 387 (4.5) | 1103 (49.1) | 1300 (29.1) | 2741 (33.3) | 2030 (21.8) | 16 259 (17.5) |
| South | 25 658 (42.9) | 6047 (70.3) | 507 (22.6) | 2303 (51.5) | 3551 (43.1) | 3613 (38.8) | 41 679 (45.0) |
| Northeast | 12 670 (21.2) | 1050 (12.2) | 463 (20.6) | 612 (13.7) | 894 (10.8) | 2211 (23.7) | 17 900 (19.3) |
| Unknown | 1748 (2.9) | 201 (2.3) | 19 (0.8) | 64 (1.4) | 25 (0.3) | 523 (5.6) | 2580 (2.8) |
| Insurance | |||||||
| Medicare | 42 350 (70.8) | 5500 (63.9) | 1389 (61.8) | 2616 (58.5) | 5558 (67.5) | 6451 (69.2) | 63 864 (68.9) |
| Medicaid | 1590 (2.7) | 664 (7.7) | 92 (4.1) | 224 (5.0) | 295 (3.6) | 218 (2.3) | 3083 (3.3) |
| Commercial | 9023 (15.1) | 1349 (15.7) | 430 (19.1) | 850 (19.0) | 1283 (15.6) | 1474 (15.8) | 14 409 (15.5) |
| PAP | 3245 (5.4) | 436 (5.1) | 146 (6.5) | 218 (4.9) | 509 (6.2) | 358 (3.8) | 4912 (5.3) |
| Other/unknown | 3598 (6.0) | 656 (7.6) | 189 (8.4) | 565 (12.6) | 595 (7.2) | 816 (8.8) | 6419 (6.9) |
Abbreviations: CRC, colorectal cancer; NSCLC, non–small cell lung cancer; PAP, patient assistance program.
American Indian or Alaska Native, Hawaiian or Pacific Islander, and race descriptions that fall into multiple race categories.
Proportion Using Pre- and Post-NCD NGS Testing
Demographic differences in NGS testing for race and ethnicity, region, insurance, age, sex, and tumor type before and after NCD are presented in Figure 1. Next-generation sequencing testing increased across all covariate-defined subpopulations in the post-NCD period compared with the pre-NCD period. Estimates of the proportion of patients tested across all covariates widened from a range of 3.5% to 16.6% in the pre-NCD period to 10.3% to 44.6% in the post-NCD period.
Figure 1. Demographic Differences in Next-Generation Sequencing (NGS) Testing Stratified by Pre– and Post–National Coverage Determination (NCD) Periods.
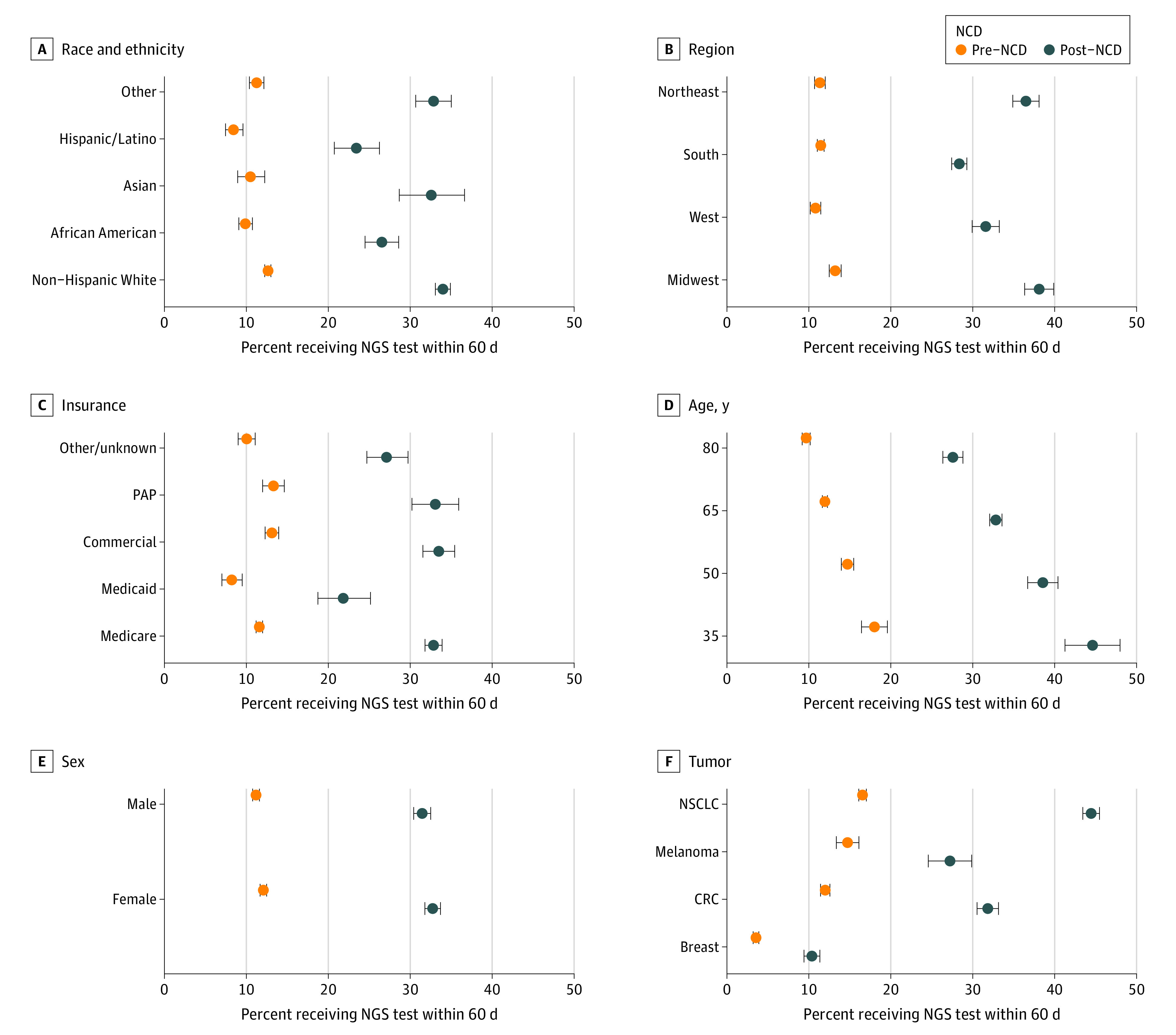
CRC indicates colorectal cancer; NSCLC, non–small cell lung cancer; and PAP, patient assistance program.
The percentage of African American and Hispanic/Latino patients who received NGS testing was lower than the percentage of White individuals who received NGS testing in both the pre- and post-NCD periods. Asian American patients and patients of other races and ethnicities had similar use of NGS testing compared with White individuals in both periods. In both the pre- and post-NCD periods, the percentage of NGS-tested patients in the West and South regions was lower than that of the Midwest region, and the percentage of NGS-tested Medicaid beneficiaries was lower than that of the Medicare and commercially insured populations. Furthermore, the gap in NGS testing between patients in the South and those in the Midwest increased by 8 percentage points, from a 1.70-percentage-point gap before NCD to a 9.7-percentage point gap after NCD. The NGS testing gap between the West and Midwest regions increased by 4.1 percentage points, from a 2.4-percentage-point gap before NCD to a 6.5-percentage-point gap after NCD. The gap for Medicaid beneficiaries increased in the after NCD period by 5.6-percentage points (3.4-percentage-point gap before NCD to 9.0-percentage-point gap after NCD) compared with Medicare beneficiaries and 6.8 percentage points (4.9-percentage-point gap before NCD to 11.7-percentage-point gap after NCD) compared with commercially insured beneficiaries.
The NCD and Changes in NGS Use Trends
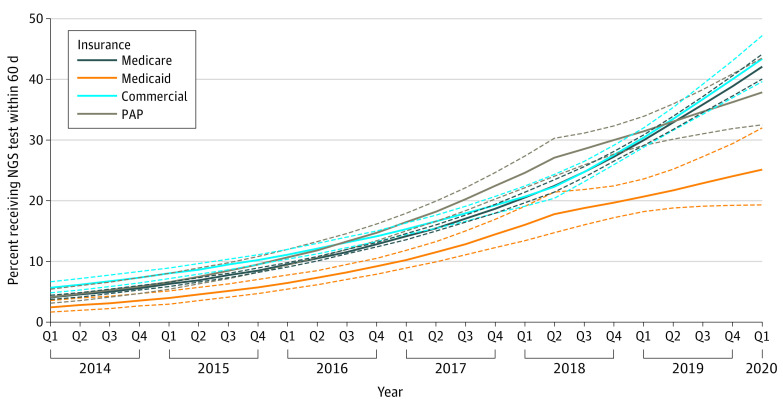
The association between the Medicare NCD and changes in pre- to post-NCD NGS testing trends between insurance types is presented in Figure 2. The odds of testing per quarter for Medicare beneficiaries increased from the pre- to post-NCD period, from 12% to 14%. Pre- to post-NCD NGS testing trends were similar among commercially insured beneficiaries and Medicare beneficiaries (odds ratio [OR], 1.03; 95% CI, 0.98-1.08; P = .25). In contrast, pre- to post-NCD NGS testing trends increased at a slower rate among the PAP beneficiaries (OR, 0.93; 95% CI, 0.87-0.99; P = .03) compared with Medicare beneficiaries. The rate of increase for Medicaid beneficiaries was slower but not statistically significant when compared with Medicare beneficiaries (OR, 0.92; 95% CI, 0.84-1.01; P = .07).
Figure 2. Association Between Medicare National Coverage Determination (NCD) and Changes in Next-Generation Sequencing (NGS) Testing Trends by Insurance Typea.
PAP indicates patient assistance program.
aInterrupted time-series model was adjusted for race and ethnicity, region, tumor type, sex, and age at diagnosis of metastasis. Dashed lines represent 95% CIs.
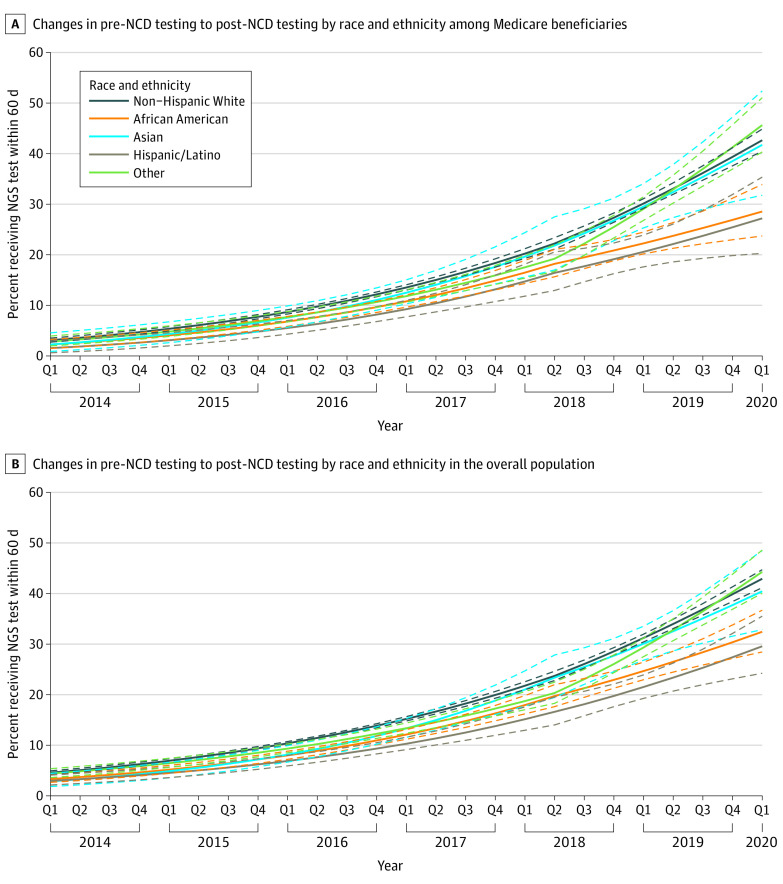
There was no statistically significant difference (P > .05 for all comparisons) in the change in pre- to post-NCD testing trends for any racial and ethnic groups relative to White individuals among Medicare beneficiaries alone (Figure 3A) as well as among the overall patient population (Figure 3B).
Figure 3. Association Between Medicare National Coverage Determination (NCD) and Changes in Next-Generation Sequencing Testing Trends by Race and Ethnicity Among Patients With Medicare and Overall Patients.
A, Interrupted time-series model in patients with Medicare was adjusted for region, tumor type, sex, and age at metastatic diagnosis. B, Interrupted time-series model was adjusted for region, tumor type, sex, age at metastatic diagnosis, and insurance type. Dashed lines represent 95% CI.
The NCD and Changes in Average NGS Use From Before to After NCD
Within the overall patient population, although African American and Hispanic/Latino individuals had approximately 2.5 to 3 times greater odds of receiving NGS testing after vs before NCD, the increase in odds of testing was 14% less (OR, 0.86; 95% CI, 0.74-0.99; P = .04) in African American and 23% less (OR, 0.77; 95% CI, 0.62-0.96; P = .02) in Hispanic/Latino individuals than in White individuals. The increase in odds of receiving NGS testing after NCD were similar for the Asian (OR, 1.19; 95% CI, 0.93-1.53; P = .17) and other racial and ethnic groups (OR, 1.02; 95% CI, 0.88-1.17; P = .83) compared with the White group (eFigure, A in the Supplement). Similar results were observed in the Medicare beneficiaries alone (eFigure, B in the Supplement).
Factors Associated With Race and Ethnicity Disparities in NGS Testing
Potential factors associated with the disparities in NGS testing by race and ethnicity are presented in Table 2. The sequential addition of variables resulted in small changes in the OR for NGS testing among African American vs White individuals (ranging from −0.2 to 0.07). Similarly, the sequential addition of variables resulted in minimal changes in the OR (ranging from −0.02 to 0.09) for NGS testing among Hispanic/Latino vs White individuals.
Table 2. Factors Contributing to the Disparity Gaps in Next-Generation Sequencing Testing by Race/Ethnicity.
| Race/ethnicity | Model | Pre-NCD | Post-NCD | ||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| African American | No adjustment | 0.72 (0.65-0.79) | <.01 | 0.63 (0.56-0.70) | <.001 |
| Adjusted for age and sex | 0.70 (0.63-0.77) | <.01 | 0.63 (0.56-0.69) | <.001 | |
| Adjusted for age, sex, and tumor | 0.73 (0.66-0.80) | <.01 | 0.63 (0.56-0.70) | <.001 | |
| Adjusted for age, sex, tumor, and region | 0.74 (0.67-0.81) | <.01 | 0.69 (0.61-0.76) | <.001 | |
| Adjusted for age, sex, tumor, and insurance type | 0.75 (0.68-0.82) | <.01 | 0.64 (0.57-0.72) | <.001 | |
| Adjusted for age, sex, tumor, insurance type, and region | 0.76 (0.68-0.84) | <.01 | 0.70 (0.62-0.78) | <.001 | |
| Asian | No adjustment | 0.79 (0.65-0.93) | .03 | 0.96 (0.79-1.13) | .96 |
| Adjusted for age and sex | 0.77 (0.63-0.90) | .01 | 0.95 (0.78-1.12) | .93 | |
| Adjusted for age, sex, and tumor | 0.77 (0.52-0.70) | .02 | 0.93 (0.76-1.11) | .86 | |
| Adjusted for age, sex, tumor, and region | 0.81 (0.66-0.95) | .08 | 0.92 (0.75-1.10) | .83 | |
| Adjusted for age, sex, tumor, and insurance type | 0.77 (0.63-0.91) | .02 | 0.94 (0.76-1.11) | .89 | |
| Adjusted for age, sex, tumor, insurance type, and region | 0.81 (0.66-0.96) | .09 | 0.94 (0.76-1.11) | .89 | |
| Hispanic/Latino | No adjustment | 0.55 (0.47-0.62) | <.01 | 0.50 (0.42-0.57) | <.001 |
| Adjusted for age and sex | 0.53 (0.45-0.60) | <.01 | 0.49 (0.41-0.57) | <.001 | |
| Adjusted for age, sex, and tumor | 0.61 (0.52-0.70) | <.01 | 0.55 (0.46-0.64) | <.001 | |
| Adjusted for age, sex, tumor, and region | 0.63 (0.54-0.72) | <.01 | 0.58 (0.48-0.68) | <.001 | |
| Adjusted for age, sex, tumor, and insurance type | 0.62 (0.53-0.71) | <.01 | 0.56 (0.47-0.65) | <.001 | |
| Adjusted for age, sex, tumor, insurance type, and region | 0.64 (0.55-0.73) | <.01 | 0.59 (0.50-0.69) | <.001 | |
| Non-Hispanic White | 1 [Reference] | 1 [Reference] | |||
| Othera | No adjustment | 0.84 (0.76-0.91) | <.01 | 0.94 (0.85-1.04) | .62 |
| Adjusted for age and sex | 0.83 (0.76-0.91) | <.01 | 0.94 (0.84-1.03) | .54 | |
| Adjusted for age, sex, and tumor | 0.85 (0.77-0.93) | <.01 | 0.93 (0.84-1.03) | .50 | |
| Adjusted for age, sex, tumor, and region | 0.87 (0.79-0.95) | .02 | 0.93 (0.83-1.03) | .56 | |
| Adjusted for age, sex, tumor, and insurance type | 0.85 (0.77-0.93) | <.01 | 0.94 (0.84-1.04) | .64 | |
| Adjusted for age, sex, tumor, insurance type, and region | 0.88 (0.79-0.96) | .02 | 0.95 (0.85-1.05) | .75 | |
Abbreviations: NCD, national coverage determination; OR, odds ratio.
American Indian or Alaska Native, Hawaiian or Pacific Islander, and race descriptions that fall into multiple race categories.
Discussion
In this real-world analysis of a large, US-based oncology electronic health record database, we found that increased NGS testing in Medicare beneficiaries after NCD did not result in an equal increased use across other insurance types. Specifically, PAP beneficiaries had slower growth rates of NGS testing following the NCD announcement compared with Medicare or commercial insurance beneficiaries. Each insurance type comprised a different racial and ethnic demographic composition, and the NCD was associated with different NGS use trends among these populations. Testing rates in the African American and Hispanic/Latino groups were lower than those of the White group during both pre- and post-NCD periods.
This study suggests that increased NGS testing access for Medicare beneficiaries following the NCD differentially affected NGS testing rates in other insurance types. Previous research suggests that the NCD affected private payer decisions to cover NGS testing in patients with cancer.14 Our study supports this finding in that the post-NCD trend of increasing NGS testing seen in Medicare beneficiaries was similarly observed in those with commercial insurance. However, testing rate differences widened or maintained after vs before the NCD in PAP and Medicaid beneficiaries relative to Medicare beneficiaries, suggesting that access to NGS testing did not improve equally among insurance types. Although Medicare is a federal program, Medicaid coverage is determined at the state level; thus, further research examining individual state coverage policies is warranted. This research may further elucidate the underlying factors behind the slower uptake among Medicaid beneficiaries.
Given differences in the racial and ethnic composition of each insurance population, with African American individuals accounting for about 20% of Medicaid beneficiaries and only approximately 10% of Medicare or commercial beneficiaries, we examined the association between the Medicare NCD and accessibility of NGS testing among different racial and ethnic groups. Following the NCD, there was an increase in NGS testing among all racial and ethnic groups across all insurance types. However, the odds of African American and Hispanic/Latino patients receiving NGS testing were less than those of White patients, and there was no statistically significant difference in the rates between Asian and other groups compared with White patients. This finding was also observed for Medicare beneficiaries, suggesting that, despite the efforts of the NCD to provide greater access to NGS testing among beneficiaries, it did not narrow existing disparities in NGS testing among different racial and ethnic groups and instead may have maintained or widened disparities. Previous studies have similarly found widening disparities owing to NCD policy implementations, although potential explanations have not been elucidated.31,32 In these studies, the purpose of the NCD was to restrict the use of certain procedures and treatments to avoid negative outcomes, which disproportionately affected certain populations.31,32 In contrast, the NGS NCD was intended to enable access to a technology deemed reasonable and necessary. However, our study results suggest that the NCD NGS policy coverage change differentially affected members of racial and ethnic categories, even after adjusting for insurance type. These factors should be considered when developing NCD policies to ensure that populations in all demographic groups benefit equally from expanded coverage, with specific attention to groups that may have already been at a disadvantage at the time of NCD. Although policies that encourage state Medicaid programs to follow the guidance of the NCD may help facilitate access to NGS testing for individuals with lower socioeconomic status, further research is needed to understand how to implement NCDs to equitably benefit all beneficiaries.
The inability of the NCD to narrow disparities in NGS testing in patients with cancer indicates that barriers outside of coverage may also be factors in disparities in NGS testing. Although the present study sought to identify factors (eg, insurance type) that may be associated with these differences, no single factor could explain the disparities. Instead, the data suggest that these differences may be multifactorial, and it is important that future studies examine other factors that cannot be measured using electronic health record data to address narrowing this disparity gap.
Limitations
This study has limitations. First, the study was based on research-grade real-world electronic health record data, which may contain incomplete or misclassified data. Approximately 10% of sampled patients were missing racial and ethnic data and 7% were missing insurance type. Although this small sample of patients with partially incomplete data is not likely to significantly influence the final reported results, it cannot be determined whether the unknown data were a source of potential bias. Second, insurance information used to classify patients was based on data from the time of advanced or metastatic cancer diagnosis and as a result may not fully capture cases in which beneficiaries changed insurance coverage. However, because the outcome of interest was NGS testing occurring within 60 days or less of an advanced or metastatic cancer diagnosis, the risk of patients switching insurance within that short time frame is low. Third, this analysis excluded academic sites; therefore, not all US regions may be equally represented. Fourth, in the South, comprehensive biomarker testing is less likely to be covered in Medicaid programs,33 and 45.0% of patients in this study were in the South. It remains unclear how inclusion of academic sites and additional regions in the US would influence these results. Fifth, medically appropriate testing, which did not include NGS, was not captured in the study.
Conclusions
The slower or similar increase in NGS testing in patients with PAP and Medicaid compared with Medicare and commercial insurance suggests that expansion of Medicare-covered benefits may not carry over equally to other insurance types and may widen or maintain disparities in NGS testing. Greater access afforded by the NCD was not associated with narrowing of racial and ethnic disparities in NGS testing, suggesting that additional efforts and measures to improve access to testing, in tandem with reimbursement policies, may be needed to ensure equitable access and realization of the benefits of precision medicine.
eFigure. Association Between the Medicare NCD and Changes in Average NGS Testing Use by Race and Ethnicity Among the Overall Population (A) and Medicare Beneficiaries Only (B)
References
- 1.National Comprehensive Cancer Network . Rectal cancer. NCCN guidelines, version 1.2021. Accessed October 23, 2021. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1461
- 2.National Comprehensive Cancer Network . Breast cancer guidelines. NCCN guidelines, version 8.2021. Accessed October 23, 2021. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1419
- 3.National Comprehensive Cancer Network . Non–small cell lung cancer, version 4.2021. Accessed October 23, 2021. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450
- 4.Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640-649. doi: 10.1056/NEJMoa1916623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.John A, Shah RA, Wong WB, Schneider CE, Alexander M. Value of precision medicine in advanced non–small cell lung cancer: real-world outcomes associated with the use of companion diagnostics. Oncologist. 2020;25(11):e1743-e1752. doi: 10.1634/theoncologist.2019-0864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network . NCCN guidelines. Melanoma: cutaneous, version 2.2021. Accessed October 23, 2021. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1492
- 7.Sheinson DM, Wong WB, Flores C, Ogale S, Gross CP. Association between Medicare’s national coverage determination and utilization of next-generation sequencing. JCO Oncol Pract. 2021;17(11):e1774-e1784. doi: 10.1200/OP.20.01023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freedman A, Klabunde C, Wiant K, et al. Use of next-generation sequencing tests to guide cancer treatment: results from a nationally representative survey of oncologists in the United States. JCO Precis Oncol. 2018;2:1-13. doi: 10.1200/PO.18.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barroso-Sousa R, Guo H, Srivastava P, et al. Utilization of tumor genomics in clinical practice: an international survey among ASCO members. Future Oncol. 2019;15(21):2463-2470. doi: 10.2217/fon-2019-0010 [DOI] [PubMed] [Google Scholar]
- 10.Reitsma M, Fox J, Borre PV, et al. Effect of a collaboration between a health plan, oncology practice, and comprehensive genomic profiling company from the payer perspective. J Manag Care Spec Pharm. 2019;25(5):601-611. doi: 10.18553/jmcp.2019.18309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Presley C, Soulos P, Chiang A, et al. Disparities in next generation sequencing in a population-based community cohort of patients with advanced non–small cell lung cancer [abstract]. J Clin Oncol. 2017;35(15 suppl):6563. doi: 10.1200/JCO.2017.35.15_suppl.6563 [DOI] [Google Scholar]
- 12.Messner DA, Al Naber J, Koay P, et al. Barriers to clinical adoption of next generation sequencing: perspectives of a policy Delphi panel. Appl Transl Genom. 2016;10:19-24. doi: 10.1016/j.atg.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu CY, Loomer S, Ceccarelli R, et al. Insurance coverage policies for pharmacogenomic and multi-gene testing for cancer. J Pers Med. 2018;8(2):1-15. doi: 10.3390/jpm8020019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trosman JR, Douglas MP, Liang SY, et al. Insights from a temporal assessment of increases in US private payer coverage of tumor sequencing from 2015-2019. Value Health. 2020;23(5):551-558. doi: 10.1016/j.jval.2020.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cancer Action Network . Cancer biomarker testing: the key to unlocking precision cancer therapy. Accessed April 6, 2021. https://www.fightcancer.org/sites/default/files/Biomarker_One_Pager_7.22.20_0.pdf
- 16.Centers for Medicare & Medicaid Services. National coverage determination (NCD). Next generation sequencing (NGS): 2020. Accessed May 6, 2021. https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=372 [PubMed]
- 17.Walker GV, Grant SR, Guadagnolo BA, et al. Disparities in stage at diagnosis, treatment, and survival in nonelderly adult patients with cancer according to insurance status. J Clin Oncol. 2014;32(28):3118-3125. doi: 10.1200/JCO.2014.55.6258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong W, Wu N, Gupta R, Mansfield AS. Utilization trends and factors associated with ROS1 testing among patients with advanced non–small-cell lung cancer in US community practices. Clin Lung Cancer. 2021;22(3):e470-e480. doi: 10.1016/j.cllc.2020.06.019 [DOI] [PubMed] [Google Scholar]
- 19.Illei PB, Wong W, Wu N, et al. ALK testing trends and patterns among community practices in the United States. JCO Precision Oncology. 2018;2:1-11. doi: 10.1200/PO.18.00159 [DOI] [PubMed] [Google Scholar]
- 20.Whitman ED, Liu FX, Cao X, Diede SJ, Haiderali A, Abernethy AP. Treatment patterns and outcomes for patients with advanced melanoma in US oncology clinical practices. Future Oncol. 2019;15(5):459-471. doi: 10.2217/fon-2018-0620 [DOI] [PubMed] [Google Scholar]
- 21.Hess LM, Cui ZL, Mytelka DS, Han Y, Goodloe R, Schelman W. Treatment patterns and survival outcomes for patients receiving second-line treatment for metastatic colorectal cancer in the USA. Int J Colorectal Dis. 2019;34(4):581-588. doi: 10.1007/s00384-018-03227-5 [DOI] [PubMed] [Google Scholar]
- 22.Quek RGW, Mardekian J. Clinical outcomes, treatment patterns, and health resource utilization among metastatic breast cancer patients with germline BRCA1/2 mutation: a real-world retrospective study. Adv Ther. 2019;36(3):708-720. doi: 10.1007/s12325-018-0867-x [DOI] [PubMed] [Google Scholar]
- 23.Zhang Q, Gossai A, Monroe S, Nussbaum NC, Parrinello CM. Validation analysis of a composite real-world mortality endpoint for patients with cancer in the United States. Health Serv Res. Published online May 17, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma X, Long L, Moon S, Adamson B, Baxi S. Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron Health, SEER, and NPCR. medRxiv. Preprint posted online May 30, 2020. https://www.medrxiv.org/content/10.1101/2020.03.16.20037143v2.full
- 25.Birnbaum B, Nussbaum N, Seidl-Rathkopf K, et al. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv. Preprint posted online January 13, 2020. https://arxiv.org/abs/2001.09765.
- 26.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology. 2007;18(6):800-804. doi: 10.1097/EDE.0b013e3181577654 [DOI] [PubMed] [Google Scholar]
- 27.National Cancer Institute. Targeted therapy to treat cancer. Accessed March 14, 2021. https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies
- 28.Gertler P, Martinez S, Premand P, Rawlings L, Vermeersch C.. Impact Evaluation in Practice, 2nd ed. World Bank; 2016. [Google Scholar]
- 29.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2020. [Google Scholar]
- 30.Lenth RV, Buerkner P, Herve M, Love J, Riebl H, Singmann H. emmeans: estimated marginal means, aka least-squares means. Accessed October 23, 2021. https://github.com/rvlenth/emmeans
- 31.Li M, Schulz R, Chisholm-Burns M, Wang J, Lu ZK. Racial/ethnic and gender disparities in the use of erythropoiesis-stimulating agents and blood transfusions: cancer management under Medicare’s reimbursement policy. J Manag Care Spec Pharm. 2020;26(11):1477-1486. doi: 10.18553/jmcp.2020.26.11.1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicholas LH, Dimick JB. Bariatric surgery in minority patients before and after implementation of a centers of excellence program. JAMA. 2013;310(13):1399-1400. doi: 10.1001/jama.2013.277915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LUNGevity. State Scorecard: State Medicaid coverage policy and impact on lung cancer outcomes. March 2020. Accessed June 1, 2021. https://www.lungevity.org/sites/default/files/state-scorecards/LUNGevity-scorecard-030920.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Association Between the Medicare NCD and Changes in Average NGS Testing Use by Race and Ethnicity Among the Overall Population (A) and Medicare Beneficiaries Only (B)