Abstract
It has been suggested that gene duplication and polyploidization create opportunities for the evolution of novel characters. However, the connections between the effects of polyploidization and morphological novelties have rarely been examined. In this study, we investigated whether petal pigmentation patterning in an allotetraploid Clarkia gracilis has evolved as a result of polyploidization. Clarkia gracilis is thought to be derived through a recent polyploidization event with two diploid species, C. amoena huntiana and an extinct species that is closely related to C. lassenensis. We reconstructed phylogenetic relationships of the R2R3-MYBs (the regulators of petal pigmentation) from two subspecies of C. gracilis and the two purported progenitors, C. a. huntiana and C. lassenensis. The gene tree reveals that these R2R3-MYB genes have arisen through duplications that occurred before the divergence of the two progenitor species, that is, before polyploidization. After polyploidization and subsequent gene loss, only one of the two orthologous copies inherited from the progenitors was retained in the polyploid, turning it to diploid inheritance. We examined evolutionary changes in these R2R3-MYBs and in their expression, which reveals that the changes affecting patterning (including expression domain contraction, loss-of-function mutation, cis-regulatory mutation) occurred after polyploidization within the C. gracilis lineages. Our results thus suggest that polyploidization itself is not necessary in producing novel petal color patterns. By contrast, duplications of R2R3-MYB genes in the common ancestor of the two progenitors have apparently facilitated diversification of petal pigmentation patterns.
Keywords: anthocyanin pigmentation, Clarkia, evolutionary novelty, petal pigmentation patterning, polyploidization, R2R3-MYB transcription factor
Introduction
Since the seminal work of Ohno (1970), duplicated genes have generally been thought to provide important material for the origin of evolutionary novelties. A duplicate gene copy can contribute to genetic and morphological diversification by evolving new gene functions (neofunctionalization), whereas the other copy can maintain the ancestral function (Zhang 2003; Rensing 2014). In addition, whole-genome duplication (WGD) often leads to extensive changes in gene expression, which can potentially produce novel traits (Wang et al. 2012; Shi et al. 2015).
WGD events (involving either allo- or auto-polyploidization) have been common in the evolution of angiosperms (De Bodt et al. 2005; Flagel and Wendel 2009; Rensing 2014). Because angiosperms are the most species-rich group of plants and exhibit a great diversity of morphological and physiological traits, it seems likely that polyploidization has facilitated diversification and speciation in this group. However, the direct connections between the effects of polyploidization and morphological characters are in general unclear, largely because there are few studies that have attempted to ascertain how divergent copies of duplicated genes affect plant development. One impediment to such studies is that gene duplicates often become lost or silenced over short evolutionary timescales (Lynch and Conery 2000, 2003). It is thus difficult to establish whether diverged paralogs represent duplicate copies created by WGD or copies produced by tandem or segmental duplication after WGD and gene loss. This distinction is important because in the former situation, WGD actually provides the raw material for evolutionary novelty, whereas the latter situation is not a direct result of WGD.
This difficulty can be overcome by examining a recent polyploidization event in which the parental species are identifiable, and by following the inheritance, modification, loss, or duplication of individual parental-species gene copies in the polyploid. Furthermore, if the effects of these gene copies on the phenotype can be determined, it should be possible to determine whether and how WGD contributes to the evolution of novel morphological traits. Here, we adopt this approach to examine the effects of polyploidization on the evolution of novel petal pigment pattern elements in the genus Clarkia (Onagraceae).
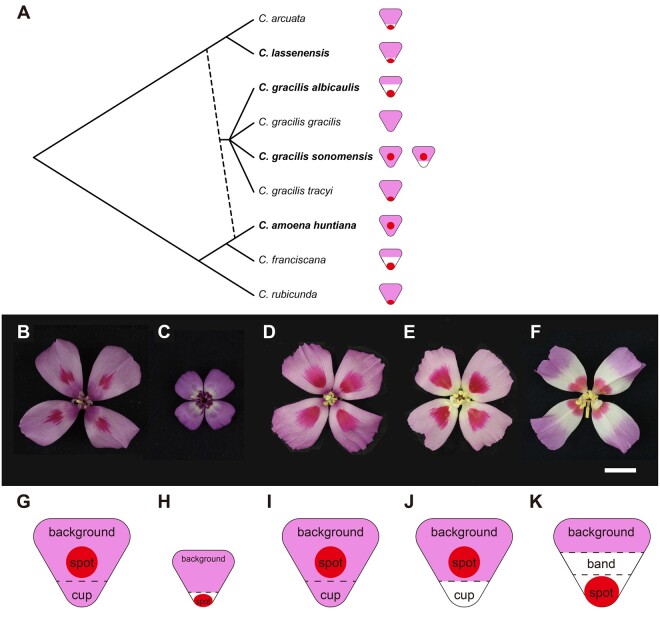
In this study, we examine the allotetraploid Clarkia gracilis (Piper) A. Nelson and J.F. Macbride and its two purported, diploid, progenitor species. Clarkia gracilis is thought to be derived from C. amoena huntiana (Jeps.) H. Lewis and M. Lewis and an extinct species related to C. lassenensis (Eastw.) H. Lewis and M. Lewis and C. arcuata (Kellogg) A. Nelson and J.F. Macbride (Abdel-Hameed and Snow 1968, 1972), which have similar floral color patterns (fig. 1A). We chose C. lassenensis to represent the extinct parental species, because a relatively better chromosome pairing was observed in C. lassenensis × C. gracilis triploids than that in C. arcuata × C. gracilis triploids (Hakansson 1946, cited by Abdel-Hameed and Snow [1972]).
Fig. 1.
Petal pigmentation patterns in Clarkia species. (A) Phylogeny of Clarkia species in section Rhodanthos, adapted from Martins et al. (2017), copyright John Wiley and Sons. Dashed line indicates hybridization to produce the allotetraploid Clarkia gracilis, which has four subspecies. Species examined in this study are indicated in bold. Petal pigmentation patterns are shown by diagrams next to the species names. Flowers of the examined Clarkia (sub)species: (B) C. amoena huntiana; (C) C. lassenensis; (D) pink-cupped C. gracilis sonomensis; (E) white-cupped C. g. sonomensis; and (F) C. g. albicaulis. The scale bar indicates 15 mm. (G–K) The elements of petal pigmentation patterns of these flowers.
Although both of the two progenitor species have a pink petal background, they differ in floral color pattern. Clarkia amoena huntiana petals have red central spots (fig. 1B and G). By contrast, petals of C. lassenensis have red basal spots and narrow white bands above the spots (fig. 1C and H).
Clarkia gracilis is the only tetraploid in section Rhodanthos (fig. 1A). It has four named subspecies, two of which were included in this study. Both have color patterns that differ from those of the two parental species, as well as from each other, and thus represent the evolution of novelty. One is C. g. sonomensis that typically has petals with a pink background and red central spots (fig. 1D and I), whereas one of its variants has a basal petal region lacking pigmentation (i.e., “white cup”; fig. 1E and J). The other subspecies, C. g. albicaulis, has a similar pink petal background, but has a basal spot and a large unpigmented (white) band in the middle of each petal (fig. 1F and K).
Previous studies have demonstrated that in C. g. sonomensis, each of the distinctive pattern elements (background, spot, cup) is controlled by different sets of R2R3-MYB transcriptional regulators (hereafter “MYB”; Martins et al. 2017; Lin and Rausher 2021). MYB1 regulates spot formation, whereas MYB6, MYB11, and MYB12 control background pigmentation (hereafter “background MYBs”), including presence/absence of the white cup (fig. 2). The protein products of these MYBs form complexes with bHLH and WDR proteins to activate the enzyme-coding genes in the anthocyanin biosynthetic pathway (Ramsay and Glover 2005; Xu et al. 2015). Because a single bHLH or WDR gene has broader expression domains and influences more characters than an individual MYB gene, the latter is largely responsible for tissue-specific/pattern-specific expression of anthocyanin pigments (Ramsay and Glover 2005; Albert et al. 2011; Streisfeld and Rausher 2011).
Fig. 2.
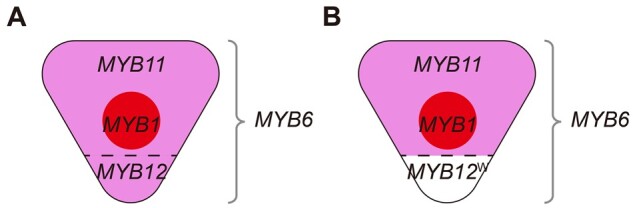
Schematic portrayal of expression domains of R2R3-MYB genes controlling color pattern elements in Clarkia gracilis sonomensis. MYB1 controls spot formation. MYB6 is expressed throughout the petal. MYB11 is expressed in the distal petal region above the cup, whereas MYB12 is expressed in the cup. (A) In conjunction with MYB6, MYB11, and MYB12 produce pigmentation in the distal and cup regions, respectively. (B) In individuals with a white cup, a nonfunctional MYB12W is expressed in the cup (Lin and Rausher 2021).
Because of their central role in regulating color pattern elements, we undertook an examination on the evolution of these MYB genes in C. gracilis and its progenitors to determine whether polyploidization had direct effects on how these genes influenced pattern evolution. Specifically, we ask how changes in copy number, functionality, or expression patterns of these genes contributed to the evolution of color pattern elements.
The effects of polyploidization on these MYB genes could directly affect pattern evolution by three distinct processes.
Process 1
Polyploidization combines MYB genes controlling disparate pattern elements from the two parental species, creating a new pattern that is a combination of elements from the progenitors. For example, in C. g. albicaulis, the basal spot might be produced by the copy of MYB1 controlling the basal spot in C. lassenensis, whereas the petal background pigmentation (including the white band) might be controlled by MYB6, MYB11, and MYB12 inherited from C. a. huntiana. Similarly, the white cup in C. g. sonomensis may reflect inheritance of MYB genes controlling the white cup in C. lassenensis, whereas the central spot may reflect inheritance of MYB1 from C. a. huntiana.
Process 2
Polyploidization results in two copies of orthologous MYB genes from the progenitors, which allows for subsequent neofunctionalization or subfunctionalization to produce new pattern elements. For example, the large white band in the middle of the C. g. albicaulis petal, which is lacking in both progenitors, may reflect neofunctionalization or subfunctionalization of duplicate copies of petal background MYBs inherited from the progenitors.
Process 3
Through interactions between MYB genes from the two parental species, novel patterns that were not present in either parent can be generated. One possibility is epigenetic gene silencing in which an introduced copy of a gene (in this case through polyploidization) results in silencing of a paralogous copy (van der Krol et al. 1990; Rajeevkumar et al. 2015). This type of interaction could also explain the central white band in C. g. albicaulis.
The alternative to a direct effect of WGD on the evolution of color patterns in C. gracilis is that pattern changes are caused by mutations affecting the MYB genes that could have produced the same change in one of the progenitor species. One example would be if the basal spot in C. g. albicaulis resulted from a mutation in the copy of MYB1 inherited from C. a. huntiana, which produces a central spot in C. a. huntiana, rather than from inheritance of the copy of MYB1 from C. lassenensis, which makes C. lassenensis basal-spotted. Another would be if loss of pigmentation in the white cup of C. g. sonomensis represents an independent mutation in the background MYB gene responsible for pigmentation in the cup region rather than from inheritance of the background MYB(s) controlling the white cup in C. lassenensis. Yet another would be if the white band in C. g. albicaulis resulted from a mutation in a background MYB rather than from an interaction between genes inherited from the two progenitors. In each of these cases, polyploidization would not have been necessary in order for the changes to have evolved.
Results
Identification of R2R3-MYBs
Martins et al. (2017) demonstrated that in C. gracilis, CgMYB1 is responsible for initiating spot formation early in the flower bud development. Two different alleles (CgMYB1C and CgMYB1B) at this locus determine whether spotting is central (as in C. g. sonomensis) or basal (as in C. g. albicaulis). Lin and Rausher (2021) showed that three R2R3-MYB genes, CgsMYB6, CgsMYB11, and CgsMYB12, are involved in petal background coloration in C. g. sonomensis (fig. 2). CgsMYB6 is expressed throughout flower bud development and everywhere in the petal, including the basal cup region. It activates all anthocyanin enzyme-coding genes except CgsAns (anthocyanidin synthase). CgsMYB11 is expressed late in the development and activates CgsAns, which completes the expression of all enzyme-coding genes and allows pigments to form. This gene is not expressed, however, in the basal region of the petal (“cup”). Instead, pigmentation in the cup region is controlled by CgsMYB12. Like CgsMYB11, CgsMYB12 is expressed late in development and activates CgsAns in this region. In the C. g. sonomensis individuals having the white cup, the copy of CgsMYB12 (CgsMYB12W) is inactivated due to a premature stop codon.
From petal RNA of the progenitors C. a. huntiana and C. lassenensis, we cloned the full-length or nearly full-length copies of the four R2R3-MYB genes (supplementary fig. S1, Supplementary Material online). Based on the primers used (supplementary table S1, Supplementary Material online), these represent copies putatively orthologous to CgMYB1, CgsMYB6, CgsMYB11, and CgsMYB12. Although cloning these genes from C. g. albicaulis, despite several attempts, we were unable to amplify the putative ortholog of CgsMYB12 from this subspecies. Consistently, our transcriptome data also show that petal background transcriptome assemblies from C. g. albicaulis only reveal MYB6 and MYB11, each of which has one copy (supplementary table S2 and methods S1, Supplementary Material online).
Because each of the two diploid progenitors expresses four R2R3-MYB genes, we would expect the two subspecies of C. gracilis to express eight different copies if there had been no gene loss or gene silencing following polyploidization. However, our recovery of at most four expressed copies suggests that after polyploidization, four of these copies—one copy of each of the four paralogs in the progenitors—have either been lost/downregulated or have evolved sufficiently in sequence that they are no longer amplified by the primers used. The latter is less likely because petal background transcriptome assemblies reveal only one copy of each of the paralogs (three paralogs in C. g. albicaulis as the absence of CgaMYB12 and four paralogs in C. g. sonomensis). In addition, attempts to amplify MYB12 from genomic DNA using multiple primer pairs yielded only one copy from C. g. sonomensis (identical to the transcriptome copy; Lin and Rausher 2021) and only ∼350 bp of Exon 3 from C. g. albicaulis. This suggests that the second copy of MYB12 inherited from the progenitors is fully or partially deleted from C. gracilis. Because we did not attempt to clone the other MYB genes from the C. gracilis genomic DNA, it remains unclear whether the undetected MYB1, MYB6, and MYB11 are lost or simply downregulated.
Phylogenetic Relationships of R2R3-MYBs
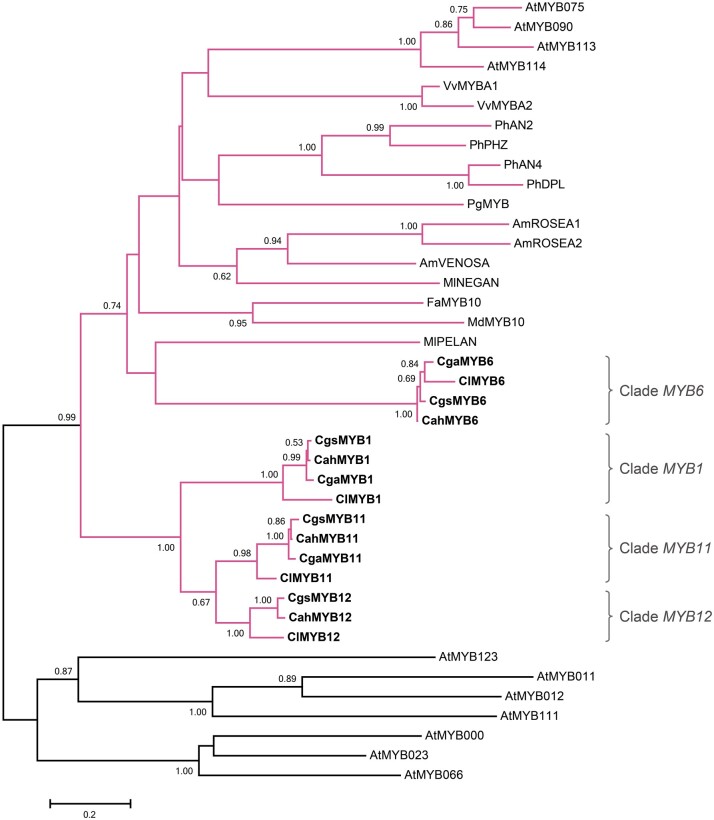
The reconstructed maximum-likelihood gene tree confirms the MYB copies in C. gracilis are orthologs of the genes identified in the progenitors (fig. 3). In particular, these genes form four highly supported clades, each containing one copy from each of the four examined (sub)species, except the MYB12 clade, which lacks the copy from C. g. albicaulis. Moreover, the MYB1, MYB11, and MYB12 genes form a clade separate from the MYB6 clade, which suggests that the former genes are derived from each other through two rounds of duplication prior to the divergence of the two diploid progenitors. Duplication of the ancestral copy first gave rise to MYB1 and the common ancestor of MYB11 and MYB12, and then the second duplication gave rise to MYB11 and MYB12.
Fig. 3.
The maximum-likelihood gene tree of R2R3-MYBs. The clades containing the subgroup 6 R2R3-MYB genes, the regulators of the anthocyanin enzyme-coding genes, are shown in pink. The genes from the Clarkia (sub)species are shown in bold. Branch supports are estimated with 1,000 bootstrap replicates. Only bootstrap values greater than 0.5 are shown. The Arabidopsis thaliana sequences were retrieved from TAIR (https://www.arabidopsis.org/): subgroup 5: AtMYB123 (AT5G35550); subgroup 6: AtMYB75 (AT1G56650), AtMYB90 (AT1G66390), AtMYB113 (AT1G66370), and AtMYB114 (AT1G66380); subgroup 7: AtMYB11 (AT3G62610), AtMYB12 (AT2G47460), and AtMYB111 (AT5G49330); subgroup 15: AtMYB0 (AT3G27920), AtMYB23 (AT5G40330), and AtMYB66 (AT5G14750). Other sequences were retrieved from GenBank: Antirrhinum majus AmROSEA1 (DQ275529), AmROSEA2 (DQ275530), AmVENOSA (DQ275531); Clarkia gracilis albicaulis CgaMYB1 (CgMYB1B, KX592431); C. g. sonomensis CgsMYB1 (CgMYB1C, KX592432); C. lassenensis ClMYB1 (KX592428); Fragaria × ananassa FaMYB10 (EU155162); Malus domestica MdMYB10 (EU518249); Mimulus lewisii MlPELAN (KJ011144), MlNEGAN (KJ011145); Petunia × hybrida PhAN2 (AF146702), PhAN4 (HQ428105), PhDPL (HQ116169), PhPHZ (HQ116170); Punica granatum PgMYB (KF841621); Vitis vinifera VvMYBA1 (AB097923), VvMYBA2 (AB097924).
Within the clades representing MYB1, MYB11, and MYB12, the orthologs from C. gracilis are more closely related to the ortholog from C. a. huntiana than to the ortholog from C. lassenensis. This pattern, which has high statistical support (bootstrap support ≥0.98, fig. 3), indicates that after polyploidization, it was the orthologs of each of these genes inherited from C. lassenensis that were lost or downregulated. By contrast, the copies of MYB6 in C. gracilis are more similar to the copy from C. lassenensis than to the copy from C. a. huntiana (bootstrap support = 1.00, fig. 3), indicating that in the tetraploid, it was the copy of this gene from C. a. huntiana that was lost or downregulated.
Expression Domains of R2R3-MYBs
The expression patterns of MYB6 and MYB11 in C. g. albicaulis, C. a. huntiana, and C. lassenensis (fig. 4) are consistent with what has been previously reported for C. g. sonomensis (Lin and Rausher 2021). In these three (sub)species, MYB6 is expressed early during flower bud development and remains expressed in all petal sections throughout bud maturation. MYB11 is expressed late in development and is only expressed in the pigmented petal background. Notably, in C. g. albicaulis, the expression of MYB11 does not extend into the region of white band in the middle of the petal.
Fig. 4.
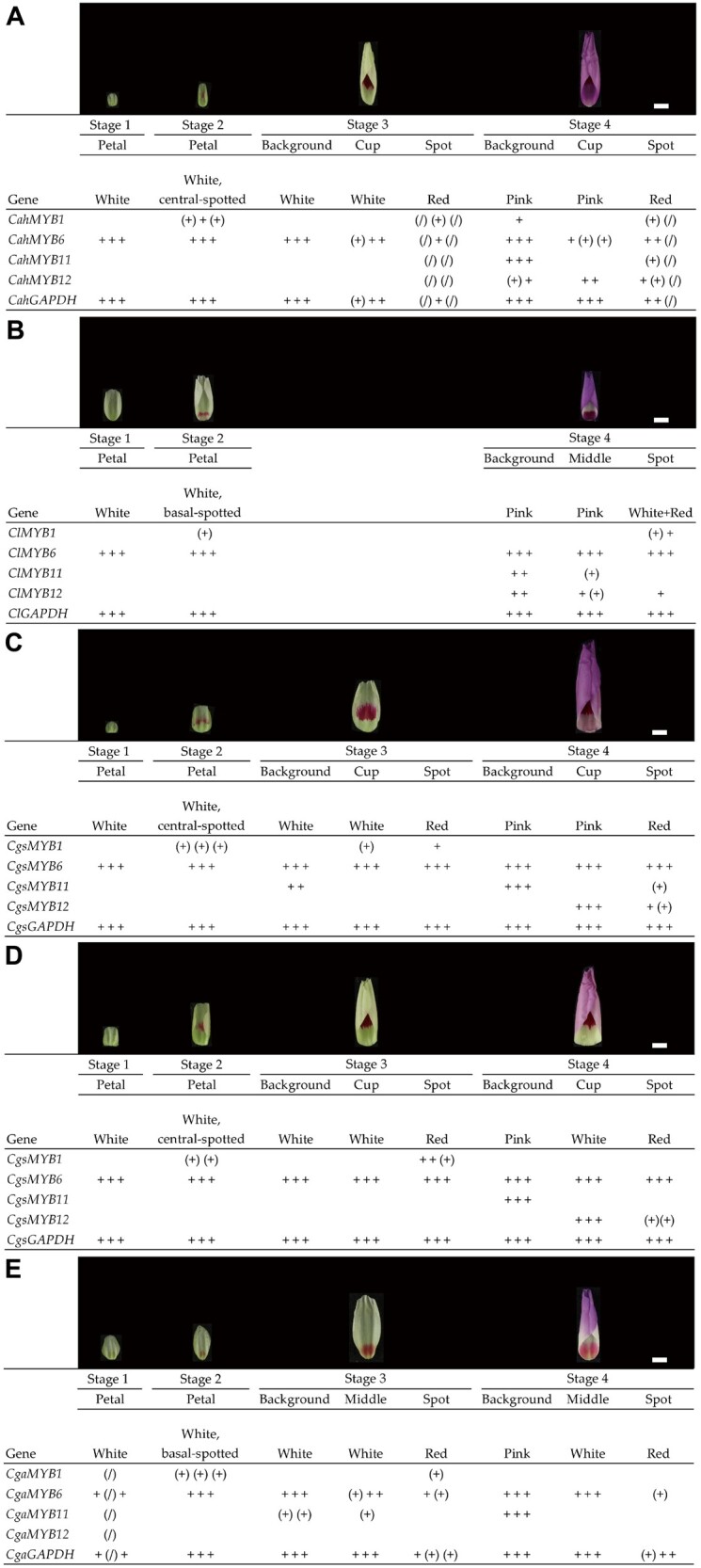
Expression patterns of MYB1, MYB6, MYB11, and MYB12 across the flower bud development in (A) Clarkia amoena huntiana, (B) C. lassenensis, (C) pink-cupped C. g. sonomensis, (D) white-cupped C. g. sonomensis, and (E) C. g. albicaulis. Based on the PCR-band brightness on the gels (see supplementary fig. S2, Supplementary Material online for gel photos), the expression levels were scored as “+,” “(+),” and blank, respectively, representing expressed, weakly expressed, and not expressed. A “+,” “(+),” or blank represents a single plant. A “(/)” indicates a missing data point. A constitutively expressed gene GAPDH was included for cDNA quality control. Pictures above the columns designate the bud phenotypes. The scale bar indicates 5 mm.
By contrast, MYB12 shows a more complex expression pattern (fig. 4). The timing of this gene’s expression is similar in C. g. sonomensis and the two diploid progenitors, being expressed late in the flower bud development (Lin and Rausher 2021). However, its spatial expression domain differs from that of MYB11. In the two progenitors, MYB12 is expressed throughout the petal, including the basal (cup) region. Despite its expression in the cup region, this region is unpigmented in C. lassenensis, which may suggest that this gene is nonfunctional in C. lassenensis, although no evidence of frame shifts or premature stop codons was found in this gene, except a 27-bp insertion in Exon 3 (supplementary fig. S1D, Supplementary Material online). By contrast, in C. g. sonomensis, MYB12 is only expressed in the basal (cup) region, allowing pigmentation of that region (Lin and Rausher 2021). Expression of this gene was not detected in C. g. albicaulis, which, along with absence of MYB11 expression in the middle portion of the petal, is consistent with absence of background pigmentation in the central and basal portions of the C. g. albicaulis petal.
Finally, MYB1 expression in all four (sub) species (fig. 4) appears to be consistent with the pattern previously reported for C. g. sonomensis (Martins et al. 2017): its expression is limited to spots. We have found that in C. a. huntiana, MYB1 expression was detected in a background sample at Stage 4 (fig. 4A). Given that this happened in only one out of three samples, we suspect that this result may reflect contamination of that sample. In C. g. sonomensis, however, we also found that it is expressed at appreciable levels in the cup region (fig. 4C). We suspect that this reflects expression in the small basal spot at the most proximal part of the cup region. This spot appears phenotypically similar to the central spot, being red rather than pink, suggesting they are activated by the same MYB copy.
In C. g. albicaulis and C. lassenensis, MYB1 expression is restricted to the region of the basal spots. Although this pattern suggests that spots in these two (sub)species are homologous, the gene tree indicates that this is not the case. Specifically, the basal spot in C. g. albicaulis is produced by a copy of MYB1 that is more similar to, and thus inherited from, the central-spotted C. a. huntiana (fig. 3). This pattern implies that the basal position of the spot in C. g. albicaulis evolved independently after polyploidization. In particular, Martins et al. (2017) demonstrated that this shift in spot position was caused by a mutation in the cis-regulatory region of CgMYB1. Because this mutated copy in C. g. albicaulis was derived from the copy of MYB1 inherited from the central-spotted C. a. huntiana, the basal position of the spot represents convergence on the basal-spotted phenotype exhibited by C. lassenensis rather than homology.
Effect of Polyploidization on Mutation Rates
Evidence in other systems indicates that polyploidization increases transposable element (TE) activity (Parisod and Senerchia 2012; Ramachandran et al. 2020), which is expected to cause an increase in TE-induced mutations (Wicker et al. 2016). Consequently, polyploidization could have indirectly facilitated the observed color-pattern changes in C. gracilis by increasing mutation rates. However, we see little increased mutation rate in C. gracilis. For MYB1, MYB11, and MYB12, dS (synonymous substitution rate, a proxy for mutation rate) is larger along the branches leading to the polyploid C. gracilis than the branch leading to C. amoena; by contrast, in MYB6 it is lower compared with the C. lassenensis branch (supplementary table S3, Supplementary Material online). There is thus no consistent pattern. Unfortunately, the sample of genes is too small to be analyzed statistically.
Discussion
A Model for the Evolution of Petal Color Patterns in C. gracilis
The results reported here suggest a model for the evolution of petal color patterns in the tetraploid C. gracilis (fig. 5). This model makes the following assumptions: 1) pigmentation in the petal background requires expression of a functional MYB6, which activates all anthocyanin enzyme-coding genes except Ans, and either MYB11 or MYB12, which activate Ans (Lin and Rausher 2021) and 2) MYB1 activates at least Ans in the regions where spots form.
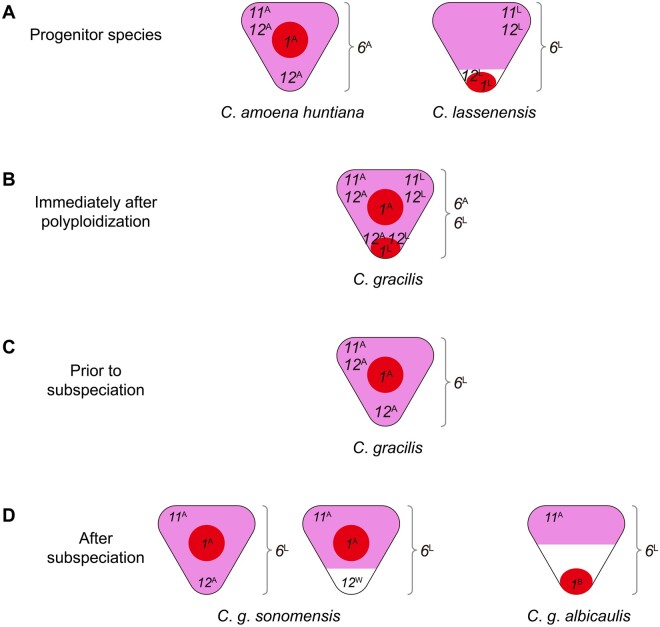
Fig. 5.
A model for the evolution of petal pigmentation patterning in Clarkia gracilis. (A) R2R3-MYB genes in the progenitor species of C. gracilis, C. amoena huntiana, and C. lassenensis. R2R3-MYB genes are designated by number and letter: 1, MYB1; 6, MYB6; 11, MYB11; 12, MYB12; and A, the copy from C. a. huntiana; L, the copy from C. lassenensis. The positions of the numbers indicate the expression domains of the R2R3-MYB genes. 6 (MYB6) is shown beside the petal because it is expressed throughout the whole petal. Colored areas indicate regions in which pigmentation is visible: red for spot formation, pink for background pigmentation, white for unpigmentation. (B) Immediately after polyploidization, genes from the two progenitors were all combined in the ancestor of C. gracilis. (C) Four changes occurred prior to subspeciation: gene loss/downregulation of MYB1L, MYB6A, MYB11L, and MYB12L. (D) Five changes occurred after subspeciation: expression domain contraction of MYB12A in C. g. sonomensis; generating MYB12W by a loss-of-function mutation in C. g. sonomensis; gene loss/downregulation of MYB12A in C. g. albicaulis; expression domain contraction of MYB11A in C. g. albicaulis; generating MYB1B by a cis-regulatory mutation in C. g. albicaulis.
In the progenitors (fig. 5A), MYB1 is expressed in regions that become spots, primarily centrally in C. a. huntiana (MYB1A) and basally in C. lassenensis (MYB1L). MYB6A (in C. a. huntiana) and MYB6L (in C. lassenensis) are expressed throughout the petal. The MYB11 copies in C. a. huntiana and C. lassenensis (MYB11A andMYB11L, respectively) are expressed in the distal regions of the petal, promoting, in conjunction with MYB6, background pigmentation in those regions. Pigmentation in the basal (cup) region in C. a. huntiana is controlled by MYB12A that is expressed in both distal and basal regions. Similarly, in C. lassenensis, MYB12L is expressed throughout the petal, but there is a basal petal area that lacks any pigmentation. Because MYB11L is not expressed in this region, a functional copy of MYB12L would be required for pigmentation. Thus, one explanation for the white cup in C. lassenensis is that MYB12L is nonfunctional. However, we cannot rule out the possibility that some other factor (such as an inhibitor) is preventing pigmentation in this area.
Immediately after polyploidization (fig. 5B), C. gracilis presumably expressed all eight copies of these genes. The entire petal background would have been pigmented because there would have been functional MYB6A and MYB6L, and either MYB11s or MYB12s expressed in all petal regions. In particular, the cup region would be pigmented because MYB12A was functional. Expression of MYB1A would presumably have produced a central spot and MYB1L a basal spot. The petal would thus have had a pink background with two spots (fig. 5B).
The phylogenetic evidence indicates that prior to subspeciation (fig. 5C), MYB1L, MYB6A, MYB11L, and MYB12L were either lost or downregulated. We infer that MYB1L, the MYB1 copy from C. lassenensis, was lost or downregulated because the MYB1 copies in the two C. gracilis subspecies are more similar to MYB1A, the C. a. huntiana copy. This inference is also consistent with the central location of the spot in C. g. sonomensis. Since functional copies of all four genes remained and were presumably expressed, the petals of the common ancestor of the two C. gracilis subspecies had a pink background throughout the petal and a single central spot (fig. 5C).
After subspeciation (fig. 5D), C. g. sonomensis underwent two changes: 1) the expression domain of MYB12A contracted, such that it is expressed only in the proximal (cup) region and (2) a new, loss-of-function mutation occurred in that gene, producing MYB12W (Lin and Rausher 2021), creating the pink/white cup polymorphism. In C. g. albicaulis, MYB12A became downregulated or was nonfunctionalized or deleted. Additionally, the expression domain of MYB11A in C. g. albicaulis contracted to just the most distal region of the petal. This contraction produced a white band in the middle of the petal, where neither MYB11A nor MYB12A is expressed. We do not know, however, whether this contraction is due to a change in MYB11A itself, or in upstream regulators or inhibitors.
Finally, based on the orthology of MYB1 in the two C. gracilis subspecies, a cis-regulatory mutation in MYB1A produced a new allele, MYB1B, which shifted its expression domain to the basal region (Martins et al. 2017). This allele became fixed in C. g. albicaulis, shifting the spot position from central to basal.
This model highlights the role of ancestral gene duplication prior to tetraploidization in facilitating the evolution of novel characters, specifically the white band in C. g. albicaulis and the white cup in C. g. sonomensis. Both of these novel pattern elements evolved because an ancestral duplication produced the paralogs MYB11 and MYB12. After this duplication, their expression domains diverged, such that in the progenitor species, MYB12 was expressed throughout the petal, whereas MYB11 was not expressed in the cup region. This expression domain divergence was further enhanced in C. g. sonomensis, with MYB12 expressed only in the cup region. This spatial differentiation allowed a functionally inactivating mutation in MYB12 (MYB12W) to produce an unpigmented area in only part of the petal (the white cup) in C. g. sonomensis. Additionally, contraction of the MYB11 expression domain in C. g. albicaulis created a region in the center of the petal in which neither MYB11 nor MYB12 was expressed, producing the white band.
Effects of Polyploidization on Petal Color Patterns
In the model described above, there are nine evolutionary changes to R2R3-MYB genes in the polyploid C. gracilis. Four of them occurred prior to subspeciation: 1) gene loss or gene downregulation of MYB1L; 2) gene loss/downregulation of MYB6A; 3) gene loss/downregulation of MYB11L; and 4) gene loss/downregulation of MYB12L. The others occurred after subspeciation: 5) MYB12A expression domain contraction in C. g. sonomensis; 6) a loss-of-function mutation in MYB12W in C. g. sonomensis; 7) gene loss/downregulation of MYB12A in C. g. albicaulis; 8) MYB11A expression domain contraction in C. g. albicaulis; and 9) a cis-regulatory mutation in MYB1B in C. g. albicaulis. Some of these changes (e.g., 6, 8, and 9) affect petal pigmentation patterning. However, none of the changes affecting patterning appear to be the direct result of polyploidization. Rather, they appear to be evolutionary changes within lineages of C. gracilis that occurred after one copy of each of the four MYBs had been lost or silenced—changes that could have occurred in a diploid species.
In particular, for the two novel phenotypic changes that occurred in C. gracilis, we found no evidence for any of the three processes involving direct effects of polyploidization described in the introduction. One phenotypic change is a candidate for Process 1, the combining of MYB genes for different elements of the two progenitors to create a new pattern: the combination of a central spot (present in C. a. huntiana) with a white cup (present in C. lassenensis) in C. g. sonomensis. However, we have shown that the white cup in this species is derived from a mutation in the copy of MYB12 inherited from C. a. huntiana as well. We note, however, that MYB1, MYB11, and MYB12 from C. a. huntiana have been combined with MYB6 from C. lassenensis, as envisioned in Process 1. Although neither of the novel patterns we identified appear to be caused by Process 1, we cannot rule out subtle changes that we have not quantified.
The second phenotypic change—the presence of a white band in the center of the C. g. albicaulis petals—is a candidate for either Process 2 or 3. Process 2, which involves differentiation of the expression domain of two MYB orthologs, could not have occurred because only one copy of each paralog remained prior to subspeciation, which in turn occurred before the evolution of the white band. Process 3, which involves interactions between MYB genes from different parents, could not have occurred because if it had, it presumably would have occurred at the time of polyploidization or soon thereafter, and we would thus expect both C. gracilis subspecies to exhibit the central white band. Since it does not appear in C. g. sonomensis, any gene interaction was likely not occurring at the time of subspeciation. Instead, the white band is found only in C. g. albicaulis and clearly results in a loss of expression (or gene loss) of MYB12A and contraction of the domain of MYB11A in that lineage.
One possible limitation of this study is that we have not characterized upstream regulators of the MYB genes, partly because the regulation of MYB-bHLH-WDR genes themselves is less understood (Xu et al. 2015). It is possible that an upstream regulator may have diverged in a way that would be a direct effect of WGD. For example, consider a regulator of MYB1. Although only one copy of this gene (MYB1A) is present in C. gracilis, it is possible that two copies of its regulator may be present, one inherited from each progenitor. Initially, the expression domain of both copies would produce a central spot. However, if the expression domain of one of the regulator copies shifted to the base of the petal, that would also cause a shift in the spot position, and could account for the basal spot in C. g. albicaulis. This would reflect Process 2 in the introduction. We know, however, that the basal spot position in C. g. albicaulis is cause by a cis-regulatory mutation in MYB1A itself, ruling out this possibility.
Another possibility involves the expression domain contractions of MYB11 and MYB12 that occurred in C. gracilis. These contractions can be explained by changes in their regulators consistent with Process 2. For example, if C. gracilis retained both copies of a regulator of MYB11A, initially both would cause background pigmentation in the entire petal except for the cup region. If there was subsequent expression domain subfunctionalization, however, the potential for the formation of a white band would be present. Specifically, if the expression domain of one copy of the regulator contracted to just the distal portion of the petal, whereas the expression domain of the second copy contracted to the central portion of the petal, and this was followed by downregulation or loss of function of the second regulator copy, a white band would be produced. This scenario would be an example of Process 2 and would thus be a direct effect of polyploidization. However, other processes that are not direct effects can also produce a white band. For example, if one of the regulators is lost, the remaining regulator may undergo a contraction in the expression domain to the distal portion of the petal. In this situation, there would be no MYB expressed in the central portion to activate Ans, complete the pathway, and cause pigments to be expressed. Although at present, we cannot distinguish between these two types of processes, the appearance of the novel white band in C. g. albicaulis is certainly consistent with processes that could operate regardless of whether WGD had occurred.
Although we obtained no evidence for a direct effect of polyploidization on genetic changes contributing to pigment pattern evolution in the (sub)species of Clarkia examined, it is possible that there are more subtle indirect effects. One possibility is that polyploidization caused increased mutation rates that contributed to the observed changes. Previous studies have suggested that polyploidization can increase transposition rates of TEs (Parisod and Senerchia 2012; Ramachandran et al. 2020) and thus increase mutation rates. Although the primary effects of increased transposition may be the inactivation of genes, they may also cause single base pair changes in flanking regions when they excise (Wicker et al. 2016). However, our analysis of mutation rates provides little evidence for increased mutation rates in C. gracilis, although admittedly the sample size is very limited. Increased rates of gene inactivation remain a possibility.
Conclusions
Although it has been suggested that polyploidization creates opportunities for the evolution of novel characters, this suggestion is based largely on the observation that morphological novelties (e.g., floral forms; Zahn et al. 2005) have often arisen after polyploidization. However, there have been very few prior investigations that have attempted to determine whether the genome-combining effects of polyploidization itself are responsible for those changes. In this study, we provide evidence indicating that the evolution of novel phenotypes after tetraploidization in C. gracilis was likely not caused by the effects of polyploidization itself, but represent evolutionary changes that could have occurred if polyploidization had not taken place—the equivalent changes could have occurred in a diploid species. Although it is dangerous to make generalizations based on one study, our results suggest that polyploidization itself may not contribute to trait diversification as much as is currently believed. Rather, ancient duplications of R2R3-MYB genes before polyploidization have apparently facilitated diversification of petal pigmentation patterns in C. gracilis. Our study also supports the common observation that evolutionary changes in floral pigmentation are accomplished primarily through the modification of R2R3-MYB genes or their expression.
Materials and Methods
Plant Growth
Methods for germination of the seeds of C. a. huntiana, C. lassenensis, and C. g. albicaulis (see supplementary table S4, Supplementary Material online for voucher information) and growth of these plants were described in Lin and Rausher (2021).
Cloning of the R2R3-MYB Genes
We amplified the coding regions of MYB6, MYB11, and MYB12 from C. a. huntiana, C. lassenensis, and C. g. albicaulis with the primers listed in supplementary table S1, Supplementary Material online. We also amplified MYB1 from C. a. huntiana because the available sequence in GenBank is only 271-bp long (GenBank accession no. KX592430).
Total RNA of the collected/dissected petals (see supplementary fig. S2, Supplementary Material online) was extracted using Spectrum Plant Total RNA Kit (Sigma-Aldrich, St. Louis, MO, USA). cDNA was synthesized following the methods described in Supporting Information Methods S4 in Lin and Rausher (2021). Amplification and sequencing of these four R2R3-MYB genes were conducted following Supporting Information Methods S5 in Lin and Rausher (2021). The sequences generated in this study were deposited at NCBI under GenBank accession numbers MT796894–MT796902.
Phylogenetic Analysis
The nucleotide sequences of MYB1, MYB6, MYB11, and MYB12 from C. a. huntiana, C. lassenensis, C. g. albicaulis, and C. g. sonomensis and the subgroup 6 R2R3-MYBs from Arabidopsis thaliana, Antirrhinum majus, Fragaria × ananassa, Malus domestica, Mimulus lewisii, Petunia × hybrida, Punica granatum, and Vitis vinifera were aligned using MUSCLE (Edgar 2004). A maximum-likelihood phylogenetic tree was constructed using PhyML version 20120412 (http://www.atgc-montpellier.fr/phyml, last accessed August 20, 2021; Guindon et al. 2010). The GTR+I + G (I = 0.080, G = 1.806) substitution model was used as determined based on the Akaike Information Criterion by Smart Model Selection version 1.8.1 (Lefort et al. 2017), which was integrated into PhyML. Clade support was estimated with 1,000 bootstrap replicates.
Semiquantitative Assessment of Gene Expression across Flower Bud Developmental Stages
To examine the expression patterns of MYB1, MYB6, MYB11, and MYB12, we collected flower buds from three plants each of C. a. huntiana, C. lassenensis, and C. g. albicaulis. The flower buds were collected at four different stages that color appears in different pattern elements: 1) white petal; 2) central or basal spot appearing, depending on species; 3) central or basal spot well defined; and 4) background and cup colors appearing. The larger petals (Stages 3 and 4) were dissected into sections as illustrated in supplementary figure S2, Supplementary Material online. For C. lassenensis, Stages 2 and 3 were combined into Stage 2 due to a small petal size. Samples of pink-cupped C. g. sonomensis (fig. 1D) and white-cupped C. g. sonomensis (fig. 1E) from Lin and Rausher (2021; Types I and III, respectively) were also included for the comparison purpose.
Each of the cDNA samples prepared as described above was diluted to 2.5 ng/μl for semiquantification of gene expression. PCR reactions were conducted using Taq DNA Polymerase (New England BioLabs, Ipswich, MA, USA) with the primers listed in supplementary table S1, Supplementary Material online. The thermoprofile included: denaturation at 95 °C for 2 min, followed by 35 cycles of 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s, and final extension at 72 °C for 2 min. PCR products were visualized on 2% agarose gels. Gel photographs are shown in supplementary figure S2, Supplementary Material online. The brightness of PCR bands reflects the expression levels of the tested genes, which was scored as expressed (“+”), weakly expressed (“(+)”), or not expressed (blank). We also labeled the missing data as “(/).”
Estimating Effects of Polyploidization on Mutation Rates
Some evidence suggests that an indirect effect of polyploidization is an increase in mutation rates, which could facilitate subsequent evolution. We thus compared synonymous substitution rates (dS, a proxy for mutation rate) of the MYB genes in C. gracilis to those in either C. a. huntiana or C. lassenensis whichever was closest to C. gracilis. Specifically, we used the CODEML program in PAML (Yang 2007) to estimate dS along branches of the MYB gene tree (supplementary fig. S3, Supplementary Material online). In this tree, we constrained the two C. gracilis genes (from the two subspecies) to be most closely related. We ran models without and with selection (Models 7 and 8, respectively) and chose the dS estimates along branches from the better model (Model 8, based on their likelihoods). The models were run with the following parameters: fix_alpha=0, clock=0, CodonFreq=0, and method=0. To estimate dS for C. gracilis, we averaged the values for the two C. gracilis branches (representing the two subspecies) and added that average to the dS value for the branch subtending them. This value was then compared with the neighboring branch (either C. a. huntiana for MYB1, MYB11, and MYB12, or C. lassenensis for MYB6). Note that these values represent equal times because they are descended from a common node.
Supplementary Material
Supplementary data are available at Molecular Biology and Evolution online.
Supplementary Material
Acknowledgments
This work was supported by National Science Foundation Grant DEB1542387 to M.D.R. and by the Duke Biology grant to R.-C.L. We are grateful to Talline Martins and Rancho Santa Ana Botanical Garden for providing seeds. We thank Duke University Greenhouse for plant care. We also thank members of the Rausher lab for constructive feedback.
References
- Abdel-Hameed F, Snow R.. 1968. Cytogenetic studies in Clarkia, section Primigenia. IV. A cytological survey of Clarkia gracilis. Am J Bot. 55(9):1047–1054. [Google Scholar]
- Abdel-Hameed F, Snow R.. 1972. The origin of the allotetraploid Clarkia gracilis. Evolution 26(1):74–83. [DOI] [PubMed] [Google Scholar]
- Albert NW, Lewis DH, Zhang H, Schwinn KE, Jameson PE, Davies KM.. 2011. Members of an R2R3-MYB transcription factor family in Petunia are developmentally and environmentally regulated to control complex floral and vegetative pigmentation patterning. Plant J. 65(5):771–784. [DOI] [PubMed] [Google Scholar]
- De Bodt S, Maere S, Van de Peer Y.. 2005. Genome duplication and the origin of angiosperms. Trends Ecol Evol. 20(11):591–597. [DOI] [PubMed] [Google Scholar]
- Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32(5):1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel LE, Wendel JF.. 2009. Gene duplication and evolutionary novelty in plants. New Phytol. 183(3):557–564. [DOI] [PubMed] [Google Scholar]
- Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O.. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 59(3):307–321. [DOI] [PubMed] [Google Scholar]
- Lefort V, Longueville J-E, Gascuel O.. 2017. SMS: smart model selection in PhyML. Mol Biol Evol. 34(9):2422–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R-C, Rausher MD.. 2021. R2R3-MYB genes control petal pigmentation patterning in Clarkia gracilis ssp. sonomensis (Onagraceae). New Phytol. 229(2):1147–1162. [DOI] [PubMed] [Google Scholar]
- Lynch M, Conery JS.. 2000. The evolutionary fate and consequences of duplicate genes. Science 290(5494):1151–1155. [DOI] [PubMed] [Google Scholar]
- Lynch M, Conery JS.. 2003. The evolutionary demography of duplicate genes. J Struct Funct Genom. 3:35–44. [PubMed] [Google Scholar]
- Martins TR, Jiang P, Rausher MD.. 2017. How petals change their spots: cis-regulatory re-wiring in Clarkia (Onagraceae). New Phytol. 216(2):510–518. [DOI] [PubMed] [Google Scholar]
- Ohno S. 1970. Evolution by gene duplication. New York: Springer-Verlag. [Google Scholar]
- Parisod C, Senerchia N.. 2012. Responses of transposable elements to polyploidy. In: Grandbastien M-A, Casacuberta JM, editors. Plant transposable elements. Berlin (Germany: ): Springer-Verlag. p. 147–168. [Google Scholar]
- Rajeevkumar S, Anunanthini P, Sathishkumar R.. 2015. Epigenetic silencing in transgenic plants. Front Plant Sci. 6:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran D, McKain MR, Kellogg EA, Hawkins JS.. 2020. Evolutionary dynamics of transposable elements following a shared polyploidization event in the tribe Andropogoneae. G3 (Bethesda). 10(12):4387–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay NA, Glover BJ.. 2005. MYB–bHLH–WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci. 10(2):63–70. [DOI] [PubMed] [Google Scholar]
- Rensing SA. 2014. Gene duplication as a driver of plant morphogenetic evolution. Curr Opin Plant Biol. 17:43–48. [DOI] [PubMed] [Google Scholar]
- Shi X, Zhang C, Ko DK, Chen ZJ.. 2015. Genome-wide dosage-dependent and -independent regulation contributes to gene expression and evolutionary novelty in plant polyploids. Mol Biol Evol. 32(9):2351–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisfeld MA, Rausher MD.. 2011. Population genetics, pleiotropy, and the preferential fixation of mutations during adaptive evolution. Evolution 65(3):629–642. [DOI] [PubMed] [Google Scholar]
- van der Krol AR, Mur LA, Beld M, Mol JN, Stuitje AR.. 1990. Flavonoid genes in petunia: addition of a limited number of gene copies may lead to a suppression of gene expression. Plant Cell 2(4):291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang X, Paterson AH.. 2012. Genome and gene duplications and gene expression divergence: a view from plants. Ann N Y Acad Sci. 1256(1):1–14. [DOI] [PubMed] [Google Scholar]
- Wicker T, Yeisoo Y, Haberer G, Mayer KFX, Marri PR, Rounsley S, Chen M, Zuccolo A, Panaud O, Wing RA, et al. 2016. DNA transposon activity is associated with increased mutation rates in genes of rice and other grasses. Nat Commun. 7:12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Dubos C, Lepiniec L.. 2015. Transcriptional control of flavonoid biosynthesis by MYB–bHLH–WDR complexes. Trends Plant Sci. 20(3):176–185. [DOI] [PubMed] [Google Scholar]
- Yang Z. 2007. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 24(8):1586–1591. [DOI] [PubMed] [Google Scholar]
- Zahn LM, Kong H, Leebens-Mack JH, Kim S, Soltis PS, Landherr LL, Soltis DE, dePamphilis CW, Ma H.. 2005. The evolution of the SEPALLATA subfamily of MADS-box genes: a preangiosperm origin with multiple duplications throughout angiosperm history. Genetics 169(4):2209–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. 2003. Evolution by gene duplication: an update. Trends Ecol Evol. 18(6):292–298. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.