Abstract
Objective
Evidence of the effects of long-term fine particulate matter (PM2.5) exposure on hypertension and blood pressure is limited for populations exposed to high levels of PM2.5. We aim to assess associations of long-term exposure to PM2.5 with hypertension prevalence and blood pressure, and further explore the subpopulation differences and effect modification by participant characteristics in these associations in China.
Methods
We analysed cross-sectional data from 883 827 participants aged 35–75 years in the China Patient-Centred Evaluative Assessment of Cardiac Events Million Persons Project. Data from the monitoring station were used to estimate the 1-year average concentration of PM2.5. The associations of PM2.5 exposure with hypertension prevalence and blood pressure were investigated by generalised linear models, with PM2.5 included as either linear or spline functions.
Results
The 1-year PM2.5 exposure of the study population ranged from 8.8 to 93.8 µg/m3 (mean 49.2 µg/m3). The adjusted OR of hypertension prevalence related to a 10 μg/m3 increase in 1-year PM2.5 exposure was 1.04 (95% CI, 1.02 to 1.05). Each 10 μg/m3 increment in PM2.5 exposure was associated with increases of 0.19 mm Hg (95% CI, 0.10 to 0.28) and 0.13 mm Hg (95% CI, 0.08 to 0.18) in systolic blood pressure and diastolic blood pressure, respectively. The concentration–response curves for hypertension prevalence and systolic blood pressure showed steeper slopes at higher PM2.5 levels; while the curve for diastolic blood pressure was U-shaped. The elderly, men, non-current smokers and obese participants were more susceptible to the exposure of PM2.5.
Conclusions
Long-term exposure to PM2.5 is associated with higher blood pressure and increased risk of hypertension prevalence. The effects of PM2.5 on hypertension prevalence become more pronounced at higher PM2.5 levels. These findings emphasise the need to reduce air pollution, especially in areas with severe air pollution.
Keywords: epidemiology, public health, hypertension
Strengths and limitations of this study.
The large size of our study allowed us to comprehensively assess these associations among a diverse spectrum of population across a wider range of PM2.5 concentrations in China.
The large number of participants with hypertension and high level of PM2.5 exposure (eg, >35 µg/m3), enabled us to examine these associations with greater precision and sufficient statistical power.
Given the nature of the cross-sectional study design, the causal relationship could not be established.
A selection bias is possible because our analysis was restricted to participants with available PM2.5 data.
Introduction
Hypertension is the leading risk factor for death globally.1 Although hypertension is a worldwide public health concern, three-quarters of the world’s population with the condition are living in low-income and middle-income countries (LMICs).2 The causes of hypertension are complex. Apart from genetic predisposition, social determinants and lifestyle factors, air pollution, especially fine particulate matter with an aerodynamic diameter of 2.5 µm or less (PM2.5), may also contribute to increased risk for hypertension.3 4
Over the past few years, a growing body of epidemiological evidence indicated the association of long-term exposure to PM2.5 with blood pressure and hypertension.5–10 However, most of the studies were undertaken in high-income countries.8 10 Compared with high-income countries, exposure to PM2.5 is substantially higher in LMICs. Furthermore, prior studies have largely focused on specific populations or regions.5–7 Therefore, further studies are needed to assess the associations of PM2.5 with blood pressure and prevalence of hypertension among a wider spectrum of populations with high PM2.5 concentrations, especially in LMICs.
China is experiencing a growing epidemic of hypertension and is estimated to have 300 million individuals with hypertension by 2025.11 Meanwhile, outdoor PM2.5 has become one of China’s most serious environmental problems with population-weighted annual means of PM2.5 ranging from 19.1 to 79.3 µg/m³ in 2015.12 A deeper understanding of the chronic health effects of PM2.5 on hypertension prevalence and blood pressure in moderate to high PM2.5 concentrations will help to develop policies to improve air quality and combat the hypertension epidemic in China.
Accordingly, incorporating PM2.5 data with a large-scale population-based screening project in China, the China Patient-Centred Evaluative Assessment of Cardiac Events (PEACE) Million Persons Project, we aimed to: (1) explore the association of long-term PM2.5 exposure with blood pressure level and hypertension prevalence, and evaluate subpopulation differences and effect modification by characteristics of participants in these associations; (2) assess the concentration–response relationships of long-term PM2.5 exposure with hypertension prevalence and blood pressure.
Methods
Study population
Our study population is derived from the China PEACE Million Persons Project, which has been described previously.13 In brief, we selected county-level regions using a convenience sampling strategy in all 31 provinces in mainland China from September 2014 to March 2019. These regions are designated as rural counties or urban districts according to urban–rural division codes of the National Bureau of Statistics of China.14 Local residents aged 35–75 years, who were currently registered in the selected region’s Hukou (a record officially identifying a person as a resident of an area) or had lived in the region for at least 6 of the previous 12 months, were enrolled in this project. After excluding participants with missing data on education level (n=13 714), body mass index (n=321) or blood pressure measurement (n=2), we further excluded participants with systolic blood pressure (SBP) ≥250 mm Hg or diastolic blood pressure (DBP) ≥150 mm Hg to minimise the potential bias due to measurement errors in blood pressure values (n=73). Finally, we included 883 827 participants in the study sample.
Data collection and variable definitions
Data collection for each participant were performed by trained personnel with a standardised in-person interview and a medical examination. Information on sociodemographic status (age, gender and education level), lifestyle (smoking and alcohol use), medical history and medication use were collected. Medication use was determined by asking participants whether they had taken prescribed medications for controlling blood pressure or glucose in the past 2 weeks. Those who answered ‘yes’ and knew the drug names were asked to report the name, dose and frequency of each drug. Those who did not remember the exact dose stated the number of pills or tablets taken (online supplemental file 1).
bmjopen-2021-050159supp001.pdf (135.5KB, pdf)
The blood pressure of each participant was measured twice on the right upper arm after 5 min of rest in a seated position with a standardised electronic blood pressure monitor (Omron HEM-7430). If the difference between the two SBP readings was greater than 10 mm Hg, a third measurement was obtained and the average of the last two readings was used. Hypertension was defined as SBP of 140 mm Hg or higher, DBP of 90 mm Hg or higher or use of antihypertensive medications, which is consistent with the US Joint National Committee and Chinese definitions.15 16 Body mass index (BMI) was defined as weight in kilograms divided by height in square metres. Obesity was defined as 28.0 kg/m2 or higher, based on recommendations from the Working Group on Obesity in China.17
Exposure assessment
We geocoded each participant’s current address (either rural counties or urban districts) into latitude and longitude data and identified air monitors located within 10 km (online supplemental file 2). The average distance between the address of participants and assigned monitors was 2.7 (IQR 1.2–3.5) kilometres. The measurements from these monitors strictly followed the methodological standards set by the State Environmental Protection Administration of China. For each participant, daily average PM2.5 concentrations measured at the nearest monitors to the residence were used to estimate PM2.5 exposure. The 1-year average concentration before the medical examination was calculated and treated as an indicator of long-term exposure to PM2.5. In the present study, we included participants with more than 330 valid PM2.5 values for assessing long-term exposure, to ensure that, for each participant, the missing rate of PM2.5 data in the preceding 1 year of medical examination is less than 10%.18
Statistical analysis
Continuous variables were reported as means with SD; categorical variables were presented as percentages. We developed mixed models with a logit link function to assess the effect of long-term exposure to PM2.5 on hypertension prevalence. To assess the association of PM2.5 and blood pressure, SBP and DBP were modelled using linear regressions with township-specific random intercepts. For each of these analyses, we started with a model (model 1) which only included age and sex. We then incrementally adjusted for additional covariates. The second model (model 2) included model 1 and socioeconomic factors (education level and urbanity). The third model (model 3) included model 2 and cardiovascular disease risk factors (BMI, smoking status, alcohol consumption and diabetes). Models of blood pressure were controlled for hypertensive medication use; while models of hypertension were not, as hypertension medication use was a component of the outcome definition. To account for potential time-variant factors, we additionally adjusted for the day of week (one indicator variable per day) and season of measurement (summer: June–August; fall: September–November; winter: December–February; spring: March–May). We also used restricted cubic splines to characterise the concentration–response (C–R) relationships of PM2.5 with hypertension prevalence and blood pressure.19 In addition, to examine effect modification by age, gender, smoking status, alcohol consumption, diabetes and obesity, each potential modifier was tested by adding an interaction term in the regression model separately and testing its statistical significance as well as the association per categories of the tested variable through subgroup analyses.
Analyses were conducted with SAS V.9.4, 64-bit Windows (SAS Institute, Cary, North Carolina). All tests of significance were two-tailed, with a level of significance set at an alpha of 0.05.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Result
Study population and baseline characteristics
We included a total of 883 827 participants distributed in 83 county-level regions of mainland China (online supplemental file 3). The mean age was 55.5 years, 60.4% were women, 43.1% had hypertension, mean SBP was 143.5 mm Hg, mean DBP was 83.1 mm Hg and 19.4% were taking antihypertensive medications. The 1-year PM2.5 exposure of the total study population ranged from 8.8 to 93.8 µg/m3 (mean 49.2 μg/m3). There were 864 119 (97.8%), 842 356 (95.3%) and 706 415 (79.9%) participants with 1-year PM2.5 exposure higher than 15 μg/m3 (WHO Interim Target 3 (IT-1)), 25 μg/m3 (WHO Interim Target 2 (IT-2)) and 35 μg/m3 (WHO Interim Target 3 (IT-3)), respectively (online supplemental file 4)
Associations of long-term PM2.5 exposure with hypertension prevalence
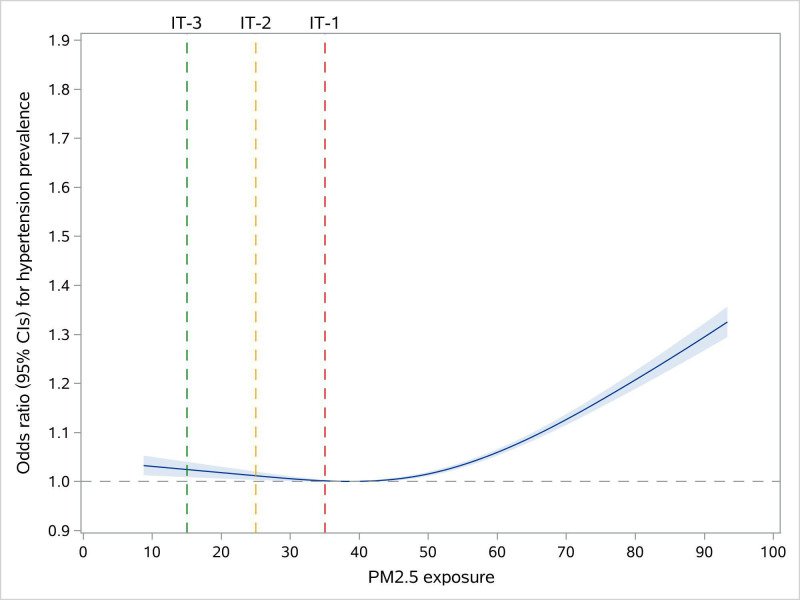
After adjusting for sociodemographic characteristics and cardiovascular risk factors, the OR of hypertension was 1.09 (95% CI: 1.08 to 1.10) for each 10 µg/m3 increase in PM2.5. The adjustment for day of week and season of blood pressure measurements resulted in a slight decrease in the effect estimate of the association of PM2.5 exposure with hypertension prevalence (OR: 1.04, 95% CI: 1.02 to 1.05) (online supplemental file 5). For the C–R relationship, the curve showed steeper slopes at high PM2.5 exposure levels (ie, higher than ~50 µg/m3). Compared with individuals with PM2.5 exposure of 38.5 μg/m3, the adjusted ORs for hypertension of individuals with 15, 25 and 35 μg/m3 of PM2.5 exposure were 1.02 (95% CI: 1.01 to 1.04), 1.01 (95% CI: 1.00 to 1.02) and 1.00 (95% CI: 1.00 to 1.00), respectively (figure 1).
Figure 1.
Concentration–response functions of the long-term exposure to PM2.5 with hypertension prevalence.
Associations of long-term PM2.5 exposure with systolic and diastolic blood pressure
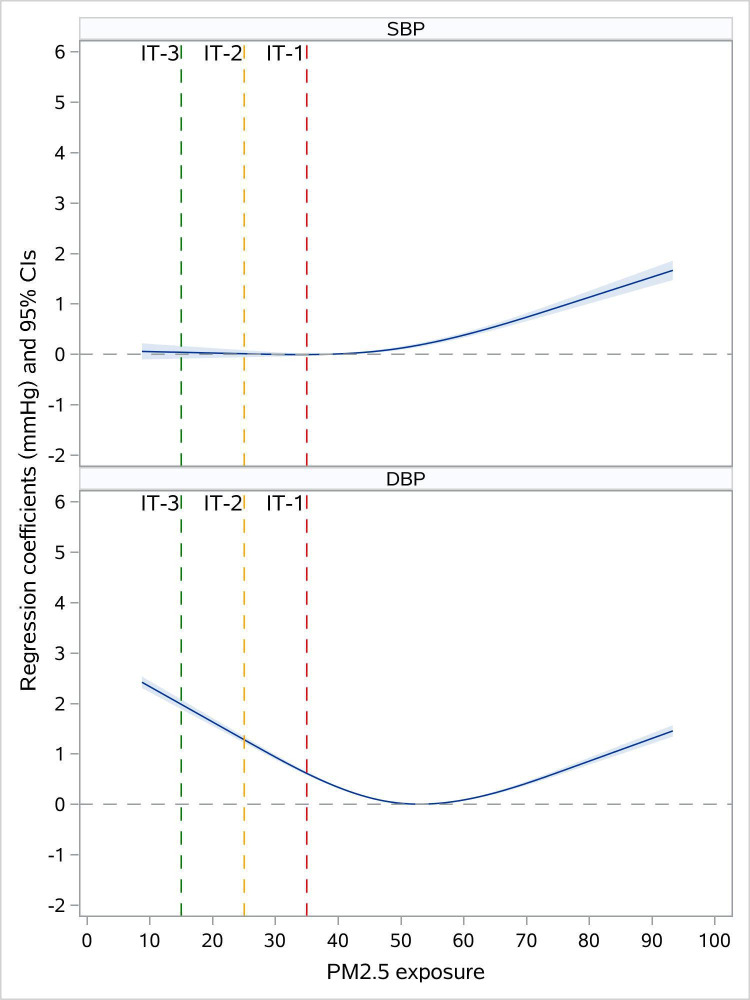
In the adjusted model 4, each 10 μg/m3 increment was associated with increases of 0.50 mm Hg (95% CI: 0.41 to 0.59) in SBP and 0.23 mm Hg (95% CI: 0.18 to 0.28) in DBP. After adjustment for the day of week and season, there was some reduction in the effect estimates of the associations between PM2.5 exposure and blood pressure (0.19 mm Hg (95% CI: 0.10 to 0.28) in SBP; 0.13 mm Hg (95% CI: 0.08 to 0.18) in DBP) (online supplemental file 6). In addition, we found that the shapes of the C–R curves for SBP and DBP were different. The fitted C–R functions showed upward trends with greater effect estimates of PM2.5 at higher concentrations for SBP but were generally U-shaped for DBP. (figure 2).
Figure 2.
Concentration–response functions of the long-term exposure to PM2.5 with systolic blood pressure (SBP) and diastolic blood pressure (DBP).
Subpopulation difference and effect modification
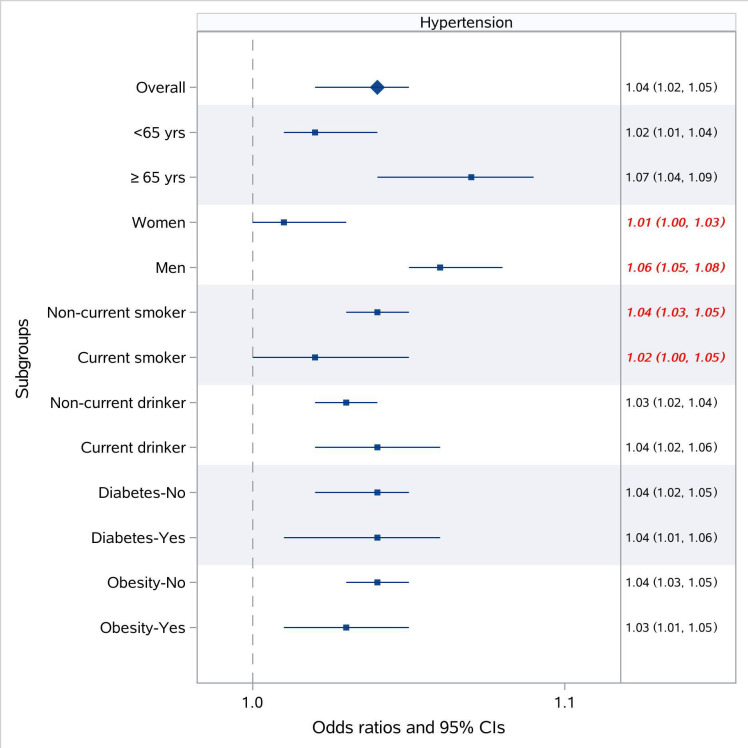
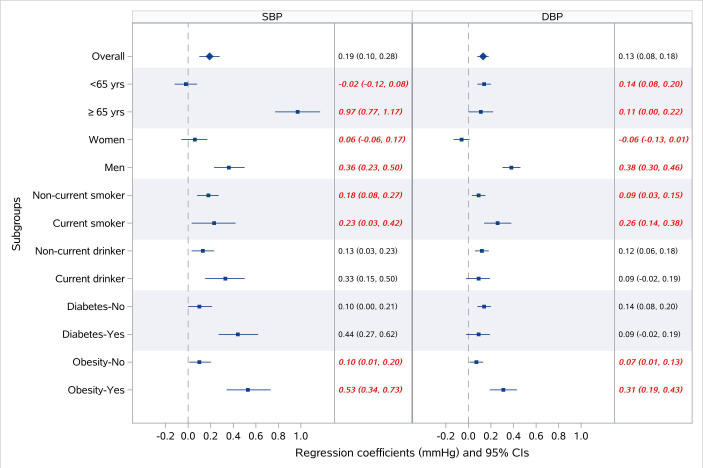
The associations of PM2.5 exposure with hypertension were stronger among men and non-current smokers, compared with their counterparts (figure 3). Gender significantly modified the effects of PM2.5 exposure on all three outcomes (all p for interaction <0.05) with stronger associations among men (eg, OR for hypertension per 10 μg/m3 increase in PM2.5 of 1.01 (95% CI: 1.00 to 1.03) for women, and 1.06 (95% CI: 1.05 to 1.08) for men); while these associations were not modified by alcohol consumption and prevalence of diabetes (all p for interaction >0.05). Although smoking status was the effect modifiers in the associations of PM2.5 exposure with SBP, DBP and hypertension, the impacts of smoking on these associations differed depending on the outcome. We observed greater effect estimates of PM2.5 exposure for SBP (elevation in SBP per 10 μg/m3 increase in PM2.5 of 0.97 mm Hg (95% CI: 0.77 to 1.17) for the elderly, and −0.02 mm Hg (95% CI: −0.12 to 0.08) for their younger counterparts), while smaller estimates for DBP among the elderly (elevation in DBP per 10 μg/m3 increase in PM2.5 of 0.11 mm Hg (95% CI: 0.00 to 0.22) for the elderly, and 0.14 mm Hg (95% CI: 0.08 to 0.20) for their younger counterparts). Besides, obesity was also found to be an effect modifier in the associations of PM2.5 exposure with SBP and DBP, with greater effect estimates in obese participants (figure 4).
Figure 3.
Stratified analysis of the association of long-term exposure to PM2.5 with hypertension prevalence.
Figure 4.
Stratified analysis of the association of long-term exposure to PM2.5 with systolic blood pressure (SBP) and diastolic blood pressure (DBP).
Discussion
In this study, we investigated the associations between long-term exposure to PM2.5 and hypertension prevalence, SBP and DBP. We found that PM2.5 was associated with increased risk of hypertension prevalence and elevation of blood pressure. Non-linearity in these associations was also observed. The C–R curves for hypertension and SBP showed steeper slopes for PM2.5 concentration above 50 μg/m3; while the C–R curve for PM2.5–DBP was U-shaped, with the turning point around 50 μg/m3. The elderly, men, non-current smokers and obese participants appeared to be more susceptible to the exposure of PM2.5.
Our study contributes to the existing scientific literature in several ways. First, by including participants in a national cross-sectional survey with long-term exposure to PM2.5 ranged from 8.8 to 93.8 µg/m3, we are able to comprehensively assess the association of PM2.5 exposure with hypertension prevalence and blood pressure among a more diverse spectrum of population with a wider range of PM2.5 concentrations. We found exposure to PM2.5 was positively associated with hypertension prevalence and blood pressure. This echoed the data showing that there was an absolute increase of 139 million individuals with hypertension in China during a decade from 2002 to 2013/2014,20 with the national annual mean PM2.5 increasing from 39.5 to 47 μg/m3 between 2000 and 2013.21 Specifically, in some high-polluted areas such as Beijing-Tianjin-Hebei region of 2013, the annual average concentrations of PM2.5 had reached 98.9 μg/m3, and daily average concentrations had exceeded 300 μg/m3.22 23 Furthermore, the magnitude of the effects for each 10 μg/m3 increment in PM2.5 was also similar compared with other studies.5 7 24–26 For example, one study found that each 10 μg/m3 in PM2.5 was associated with increases of 0.45 and 0.07 mm Hg in SBP and DBP, respectively.24 And ORs for hypertension prevalence related to a 10 μg/m3 increase in PM2.5 were ranged from 1.01 to 1.14 in prior studies.5 25 26 It is also noteworthy that others have reported no or inconsistent associations.27 28 Adar et al found no associations between exposures to PM2.5 and blood pressure based on a longitudinal cohort.27 However, this study only included a small fraction of Chinese populations (10%), and was conducted in the USA with a mean annual average PM2.5 of 17 μg/m3, which was lower than the present study (49.2 μg/m3).
Second, we provided new information on the C–R relationship between long-term PM2.5 exposure and hypertension prevalence. Previous research reported a U-shaped relationship with a threshold PM2.5 of 47.9 μg/m3.5 However, in our study, the risk of hypertension associated with PM2.5 became even more pronounced when the exposure was extended to higher levels. This finding was in line with a prior study based on prospective cohorts showed that there was a stepwise increase in the risk of developing hypertension with increasing quartiles of long-term PM2.5 exposure.29 This result suggests that, for a given decrease in the concentration of PM2.5, a greater reduction in excess hypertension prevalence would be obtained in highly polluted regions compared with regions with low to moderate levels of PM2.5 exposure. In this respect, the implication of air quality improvements in highly polluted regions of China in recent years would be more profound. It has shown that from 2013 to 2018, the annual average concentration of PM2.5 in Beijing-Tianjin-Hebei region has declined by 49%.30 Considering the population size and baseline PM2.5 levels in these areas, the public health impact related to PM2.5 reduction would be huge.
Third, we found evidence of non-linearity in the relationships of PM2.5 exposure with SBP and DBP. Interestingly, the shapes of curves for SBP and DBP were different. Although the relevant mechanism remains unclear, pathophysiological changes, such as systemic inflammation, atherosclerosis, endothelial dysfunction and increased arterial stiffness,4 may have contributed to the observed patterns. The increased arterial stiffness induced by PM2.5 would initially lead to elevation in SBP and decline in DBP, creating an increased pulse pressure.31 While with further increase of arterial stiffness, the heart rates got higher to maintain the stroke volume, which could result in the rise of DBP afterward. In addition, prior studies have reported the effects of PM2.5 exposure on pulse pressures showing a tendency of rising first and then declining at higher PM2.5 levels, which partially supports this hypothesis.24
Fourth, we assessed the subpopulation differences through stratified analyses and identified the susceptible individuals to the exposure of PM2.5. We observed the large effect estimates of PM2.5 on SBP among the elderly. Elderly subjects may commonly represent a higher prevalence of pre-existing cardiovascular and respiratory diseases, which may confer susceptibility to PM2.5. Also, the results showed that PM2.5 exposure had larger effects on hypertension in men, and such increased susceptibility may be related to sex-related differences in the deposition localisation and rates of air pollutants; specifically, men have larger airways and slightly lower airway reactivity.32 Furthermore, we found stronger associations of PM2.5 and blood pressure in obese participants. The greater response observed in obese individuals may be attributed to the higher deposition rate of PM2.5 exposure. This has been demonstrated in overweight children, where there was an increase in tidal volume and resting minute ventilation.33 Additionally, smoking status was found to be an effect modifier with smaller effects on hypertension among current smokers. This finding is also supported by prior research.8 24 One possible explanation is that smoking and PM2.5 exposure may share the same pathways in mediating cardiovascular effects and smoking may play a dominant role in smokers. Thus, exposure to PM2.5 might not exert additional harmful effects via the same pathway.24 There is also some potential that the greater effect size in non-current smokers could also be connected to some of them being advised to quit smoking because of multiple comorbidities.
Our study should also be interpreted in the context of several limitations. First, given the nature of the cross-sectional study design, the causal relationship could not be established. Additional research is needed to examine these relationships in a prospective manner. Second, a selection bias is possible because our analysis was restricted to participants with available PM2.5 data. Third, while we included a number of potential confounders in the analyses, there might be unmeasured confounders that affected the observed associations. In particular, we were unable to control for other confounders such as diet and physical activity, because these data were only available in a subset of the China PEACE Million Persons Project cohort. Fourth, we used the data from the fixed monitors to estimate the exposure of PM2.5 and did not account for residential proximity to major roads, time-activity patterns and indoor-related characteristics, which would likely result in non-differential measurement errors. However, this approach is commonly used and previous research has indicated that PM2.5 exposure estimated by the nearest monitor was highly correlated with other sophisticated approaches.34 Fifth, other gaseous pollutants, such as NOx and ozone, temperature and noise were not included in this study. As a result, we were not able to determine whether the observed effects were specifically attributable to PM2.5 or the combined effects of these factors.
In conclusion, our study demonstrated that long-term exposure to PM2.5 was positively associated with blood pressure and hypertension prevalence. The effect of PM2.5 on hypertension prevalence was more pronounced at higher PM2.5 concentrations. Our findings reinforce the need to develop comprehensive strategies for addressing air pollution problems, especially for areas with severe air pollution.
Supplementary Material
Acknowledgments
We thank all study individuals for their participation and appreciate the contributions made by project teams at the National Centre for Cardiovascular Diseases in the realms of project design, operations and data collection.
Footnotes
Contributors: JS and XZ conceived of this article. JS wrote the manuscript with further contributions from XZ, YG, XL, EM, MR, GT, DZ, WZ, JL and MAS. JS and SH completed all the statistical analyses. All authors interpreted data, contributed to critical revisions and approved the final version of the article. XZ accepts full responsibility for the finished work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: This project was supported by the National Key Research and Development Program (2016YFE0103800) from the Ministry of Science and Technology of China, the CAMS Innovation Fund for Medical Science (2016-I2M-1–006), the Major Public Health Service Project from the Ministry of Finance and National Health and Family Planning Commission of China and the 111 Project (B16005) from the Ministry of Education of China.
Competing interests: JL discloses that she is a recipient of research grants from the government of China, through Fuwai Hospital, for research to improve the management of hypertension and blood lipids, and to improve care quality and patient outcomes of cardiovascular disease; is a recipient of research agreement with Amgen, through National Centre for Cardiovascular Diseases (NCCD) and Fuwai Hospital, for a multicentre trial to assess the efficacy and safety of Omecamtiv Mecarbil, and for dyslipidemic patient registration; is a recipient of a research agreement with Sanofi, through Fuwai Hospital, for a multicentre trial on the effects of sotagliflozin; is a recipient of a research agreement with University of Oxford, through Fuwai Hospital, for a multicentre trial of empagliflozin and was a recipient of a research agreement, through NCCD, from AstraZeneca for clinical research methods training.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. The China PEACE Million Persons Project is a major national programme, and as the government policy stipulates, it is not permissible for the researchers to make the raw data publicly available at this time. All data generated during this study are included in this manuscript and its supplementary information files.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
The central ethics committee at the China National Centre for Cardiovascular Diseases approved this project (Ethical code 2014-574). All enrolled participants provided written informed consent.
References
- 1.GBD 2017 Risk Factor Collaborators . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018;392:1923–94. 10.1016/S0140-6736(18)32225-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control. Circulation 2016;134:441–50. 10.1161/CIRCULATIONAHA.115.018912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajagopalan S, Al-Kindi SG, Brook RD. Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:2054–70. 10.1016/j.jacc.2018.07.099 [DOI] [PubMed] [Google Scholar]
- 4.Carey RM, Muntner P, Bosworth HB, et al. Prevention and control of hypertension: JACC health promotion series. J Am Coll Cardiol 2018;72:1278–93. 10.1016/j.jacc.2018.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie X, Wang Y, Yang Y, et al. Long-Term Effects of Ambient Particulate Matter (With an Aerodynamic Diameter ≤2.5 μm) on Hypertension and Blood Pressure and Attributable Risk Among Reproductive-Age Adults in China. J Am Heart Assoc 2018;7:e008553. 10.1161/JAHA.118.008553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong G-H, Qian ZM, Xaverius PK, et al. Association between long-term air pollution and increased blood pressure and hypertension in China. Hypertension 2013;61:578–84. 10.1161/HYPERTENSIONAHA.111.00003 [DOI] [PubMed] [Google Scholar]
- 7.Liu C, Chen R, Zhao Y, et al. Associations between ambient fine particulate air pollution and hypertension: a nationwide cross-sectional study in China. Sci Total Environ 2017;584-585:869–74. 10.1016/j.scitotenv.2017.01.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuks KB, Weinmayr G, Foraster M, et al. Arterial blood pressure and long-term exposure to traffic-related air pollution: an analysis in the European study of cohorts for air pollution effects (escape). Environ Health Perspect 2014;122:896–905. 10.1289/ehp.1307725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz J, Alexeeff SE, Mordukhovich I, et al. Association between long-term exposure to traffic particles and blood pressure in the Veterans administration normative aging study. Occup Environ Med 2012;69:422–7. 10.1136/oemed-2011-100268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan SH, Van Hee VC, Bergen S, et al. Long-Term air pollution exposure and blood pressure in the sister study. Environ Health Perspect 2015;123:951–8. 10.1289/ehp.1408125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet 2005;365:217–23. 10.1016/S0140-6736(05)17741-1 [DOI] [PubMed] [Google Scholar]
- 12.Cohen AJ, Brauer M, Burnett R, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the global burden of diseases study 2015. Lancet 2017;389:1907–18. 10.1016/S0140-6736(17)30505-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu J, Xuan S, Downing NS, et al. Protocol for the China peace (patient-centered Evaluative assessment of cardiac events) million persons project pilot. BMJ Open 2016;6:e010200. 10.1136/bmjopen-2015-010200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Compilation rules of zoning codes for statistics and urban-rural division codes. Available: http://www.stats.gov.cn/tjsj/tjbz/200911/t20091125_8667.html [Accessed 01 Jul 2021].
- 15.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth joint National Committee (JNC 8). JAMA 2014;311:507–20. 10.1001/jama.2013.284427 [DOI] [PubMed] [Google Scholar]
- 16.Liu L-S, Writing Group of 2010 Chinese Guidelines for the Management of Hypertension . [2010 Chinese guidelines for the management of hypertension]. Zhonghua Xin Xue Guan Bing Za Zhi 2011;39:579–615. [PubMed] [Google Scholar]
- 17.Zhou B-F, Cooperative Meta-Analysis Group of the Working Group on Obesity in China . Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci 2002;15:83–96. [PubMed] [Google Scholar]
- 18.Bennett DA. How can I deal with missing data in my study? Aust N Z J Public Health 2001;25:464–9. 10.1111/j.1467-842X.2001.tb00294.x [DOI] [PubMed] [Google Scholar]
- 19.Desquilbet L, Mariotti F. Dose‐response analyses using restricted cubic spline functions in public health research. Stat Med 2010;29:1037–57. 10.1002/sim.3841 [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Yang L, Wang L, et al. Burden of hypertension in China: a nationally representative survey of 174,621 adults. Int J Cardiol 2017;227:516–23. 10.1016/j.ijcard.2016.10.110 [DOI] [PubMed] [Google Scholar]
- 21.Liang F, Xiao Q, Huang K, et al. The 17-y spatiotemporal trend of PM 2.5 and its mortality burden in China. Proc Natl Acad Sci U S A 2020;117:25601–8. 10.1073/pnas.1919641117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Xiong Q, Wu G, et al. Spatio-Temporal Variation Characteristics of PM2.5 in the Beijing-Tianjin-Hebei Region, China, from 2013 to 2018. Int J Environ Res Public Health 2019;16:4276. 10.3390/ijerph16214276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batterman S, Xu L, Chen F, et al. Characteristics of PM2.5 Concentrations across Beijing during 2013-2015. Atmos Environ 2016;145:104–14. 10.1016/j.atmosenv.2016.08.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Z, Guo C, Lau AKH, et al. Long-Term exposure to fine particulate matter, blood pressure, and incident hypertension in Taiwanese adults. Environ Health Perspect 2018;126:017008. 10.1289/EHP2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai Y, Zhang B, Ke W, et al. Associations of short-term and long-term exposure to ambient air pollutants with hypertension: a systematic review and meta-analysis. Hypertension 2016;68:62–70. 10.1161/HYPERTENSIONAHA.116.07218 [DOI] [PubMed] [Google Scholar]
- 26.Lin H, Guo Y, Zheng Y, et al. Long-Term Effects of Ambient PM2.5 on Hypertension and Blood Pressure and Attributable Risk Among Older Chinese Adults. Hypertension 2017;69:806–12. 10.1161/HYPERTENSIONAHA.116.08839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adar SD, Chen Y-H, D'Souza JC, et al. Longitudinal analysis of long-term air pollution levels and blood pressure: a cautionary tale from the multi-ethnic study of atherosclerosis. Environ Health Perspect 2018;126:107003. 10.1289/EHP2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S-Y, Wu C-F, Lee J-H, et al. Associations between long-term air pollutant exposures and blood pressure in elderly residents of Taipei City: a cross-sectional study. Environ Health Perspect 2015;123:779–84. 10.1289/ehp.1408771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang K, Yang X, Liang F, et al. Long-Term exposure to fine particulate matter and hypertension incidence in China. Hypertension 2019;73:1195–201. 10.1161/HYPERTENSIONAHA.119.12666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhai S, Jacob DJ, Wang X, et al. Fine particulate matter (PM<sub>2.5</sub>) trends in China, 2013–2018: separating contributions from anthropogenic emissions and meteorology. Atmos Chem Phys 2019;19:11031–41. 10.5194/acp-19-11031-2019 [DOI] [Google Scholar]
- 31.Bavishi C, Goel S, Messerli FH. Isolated systolic hypertension: an update after sprint. Am J Med 2016;129:1251–8. 10.1016/j.amjmed.2016.08.032 [DOI] [PubMed] [Google Scholar]
- 32.Yunginger JW, Reed CE, O'Connell EJ, et al. A community-based study of the epidemiology of asthma. Incidence rates, 1964-1983. Am Rev Respir Dis 1992;146:888–94. 10.1164/ajrccm/146.4.888 [DOI] [PubMed] [Google Scholar]
- 33.Bennett WD, Zeman KL. Effect of body size on breathing pattern and fine-particle deposition in children. J Appl Physiol 2004;97:821–6. 10.1152/japplphysiol.01403.2003 [DOI] [PubMed] [Google Scholar]
- 34.Miller KA, Spalt EW, Gassett AJ, et al. Estimating ambient-origin PM2.5 exposure for epidemiology: observations, prediction, and validation using personal sampling in the Multi-Ethnic Study of Atherosclerosis. J Expo Sci Environ Epidemiol 2019;29:227–37. 10.1038/s41370-018-0053-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-050159supp001.pdf (135.5KB, pdf)
Data Availability Statement
No data are available. The China PEACE Million Persons Project is a major national programme, and as the government policy stipulates, it is not permissible for the researchers to make the raw data publicly available at this time. All data generated during this study are included in this manuscript and its supplementary information files.