Abstract
The transcription factor TFIID is a multiprotein complex that includes the TATA box binding protein (TBP) and a number of associated factors, TAFII. Prodos (PDS) is a conserved protein that exhibits a histone fold domain (HFD). In yeast two-hybrid tests using PDS as bait, we cloned the Drosophila TAFII, dTAFII16, as a specific PDS target. dTAFII16 is closely related to human TAFII30 and to another recently discovered Drosophila TAF, dTAFII24. PDS and dTAFII24 do not interact, however, thus establishing a functional difference between these dTAFs. The PDS-dTAFII16 interaction is mediated by the HFD motif in PDS and the N terminus in dTAFII16, as indicated by yeast two-hybrid assays with protein fragments. Luciferase-reported transcription tests in transfected cells show that PDS or an HFD-containing fragment activates transcription only with the help of dTAFII16 and TBP. Consistent with this, the eye phenotype of flies expressing a sev-Ras1 construct is modulated by PDS and dTAFII16 in a gene dosage-dependent manner. Finally, we show that PDS function is required for cell viability in somatic mosaics. These findings indicate that PDS is a novel transcriptional coactivator that associates with a member of the general transcription factor TFIID.
One of the first events in transcription of protein-encoding genes is the assembly of a preinitiation complex that contains RNA polymerase II and general transcription factors such as TFIIA, TFIIB, TFIID, TFIIE, TFIIF, TFIIH, and TFIIJ (24, 52, 56). Assembly is thought to be a sequential process initiated by the nucleation of transcription factors to core promoters or, alternatively, the attachment of preformed complexes, holoenzymes, to target DNA sequences (36).
TFIID is a multiprotein complex formed by the TATA-binding protein (TBP) and several TBP-associated factors, TAFIIs (23, 31, 38, 66, 70). TAFIIs are named according to their apparent or estimated molecular weight (e.g., TAFII30, TAFII105). They are present not only in TFIID but also in other complexes that play additional roles in chromatin remodeling and gene transcription, such as SAGA, PCAF, and TFTC (22, 43, 51, 63, 75). Their presence in multiple protein complexes reflects a variety of functional roles and protein interactions. Some TAFIIs are thought to mediate promoter recognition and selectivity (8, 61, 69). Human TAFII250 exhibits histone acetylase and protein kinase activities that modulate chromatin structure as well as the activity of general transcription factors (11, 46, 49). Other TAFIIs are thought to act as cofactors, since they associate with specific transcription activators. For example, hTAFII130 and its Drosophila homologue, dTAFII110, interact with Sp1 (29, 57, 65) and with the cyclic AMP response element-binding protein (13, 14, 58). In addition, hTAFII105 interacts with NF-κB (77). In vitro experiments using reconstituted TFIID complexes demonstrated a synergistic interaction between two activators, Bicoid and Hunchback, and specific TAFIIs, TAFII110 and TAFII60 (59). In vivo studies in Drosophila support the hypothesis that TAFIIs may serve as targets for enhancer-binding proteins (54, 78). On the other hand, TAFIIs may also serve to mediate signaling pathways; this is the case for hTAFII55, hTAFII30 and hTAFII28, which bind to nuclear receptors for thyroid hormone, estrogen, and retinoic acid, respectively (37, 44). Likewise, hTAFII31 interacts with p53 (40) whereas dTAFII40 interacts with VP16 (21, 35) and p53 (67). TAFIIs thus contribute to gene regulation, acting as cofactors in a wide repertoire of interactions with transcription factors.
Nonetheless, under certain conditions, TAFIIs may not be obligatory components of activated transcription. Yeast and HeLa cells depleted of major TAFIIs can form preinitiation complexes in a single-round transcription assay (47, 50, 71). Although it is possible that TAFIIs are not always required for transcription initiation, it is clear that they are essential for the functional expression of specific genes. In yeast and mammals, TAFII mutations cause cell cycle arrest at phases specific to each TAFII (1, 42, 45, 72, 73). In Drosophila, mutations in TAFII110, TAFII250, and TAFII60 yield lethal phenotypes (FlyBase, on http://flybase.bio.indiana.edu). For any particular type of TAFII, sequences are significantly conserved among yeast, flies, and humans (23, 66). Thirteen are known so far, and this number is not likely to increase much further since the identification has been carried out mostly through direct biochemical purification of whole complexes. Although several of them are usually present in any given complex, it seems that the exquisite control of gene transcription would require a larger repertoire of regulators. It is in this context that the PDS-TAFII16 interaction described here becomes relevant.
prodos (pds) is a recently discovered vital gene from the 16F cluster in the X chromosome of Drosophila (55). The corresponding protein, Prodos (PDS) (submitted to the EMBL database under accession number Y15513), is rich in proline residues and contains a histone fold domain (HFD). This motif is thought to mediate DNA-protein and protein-protein interactions (2) and is found in the four core histones (4) as well as in several TAFIIs (6, 18, 30, 76). The pds gene is expressed throughout development in all tissues analyzed, and the protein localizes to nuclear extracts (our unpublished data). The conserved sequence and the general expression of the gene suggested a basic role in cell biology; we thus set out to identify proteins that might interact with PDS. The yeast two-hybrid test led to the cloning of a new Drosophila TAFII, dTAFII16, with significant homology to human TAFII30. We report the characterization of this member of the TAFII family in Drosophila and its physical interaction with PDS in vivo and in vitro through the N terminus and the HFDs, respectively. We also demonstrate that this association activates gene transcription in transfected cells and that both proteins modulate the expression of a gene construct in the organism.
MATERIALS AND METHODS
Fly strains and genetic procedures.
The mutation pds2270 is an ethyl methanesulfonate (EMS)-induced allele of pds, and pds88 is a P element insertion located 17 bp upstream of the ATG initiation site in the transcription unit (our unpublished data). Deletions Df(1)88-6 and Df(1)88-2 were generated by remobilization of the P insert and characterized by genetic and molecular procedures. Df(1)88-6 deletes approximately 4 kb from the insertion site toward the telomere and removes the adjacent vital gene, HL-XIV, but not scully (55, 68). Df(1)88-2 deletes from the insertion site toward the centromere without affecting the adjacent gene, HL-VII. The pds+ transgene was supplied by the genomic fragment E6L, which contains the complete open reading frame (ORF) and is able to rescue the pds phenotype (55). Two independent viable insertions of genomic transformants, T(2)E6L and T(3)E6L, in the autosomes were tested, yielding the same results. The Df(2)N6 and Df(2)N19 chromosomes were from the Bloomington Stock Center (Indiana University, Bloomington, Ind.). These deficiencies delete the dTAFII16 gene, as we confirmed by PCR. The sev-Ras1V12 construct used was from the CyO-CR2 chromosome, provided by the Umea stock center (Umea University, Umea, Sweden). Enhancers in sev-Ras1V12 consist of three copies of the genomic fragment from 7134 to 7833, whereas those in sE-raftorY9 and sev-hsp-ro correspond to two copies of the fragment from 7319 to 8536 (3, 7). The sev promoter is the fragment from the sevenless gene from −966 to 88, whereas the hsp promoter is the fragment from the hsp70 gene from −250 to 90. The sE-raftorY9 and sev-hsp-ro constructs were provided by E. Hafen (Zürich University, Zürich, Switzerland). Scanning electron micrographs were obtained using Philips XL-30 equipment. Mosaics were generated by X-ray-induced recombination in two genotypes, y w pds2270/f36a and y w pds2270/M(1)n. In the first case, most crossovers are expected to occur between the centromere and the pds locus because of the large amount of intervening DNA. These crossovers generate twin spots that should grow to the same size. In the second case, the mutant clones have a growth advantage over their adjacent nonmutant cells, the M+ condition (15).
Plasmid constructs.
All plasmids used in the yeast two-hybrid assays were generated by PCR using suitable oligonucleotides and compatible restriction sites. The resulting products were cloned into pACT2 and pAS2-1, which encode the Ga14 activation domain and DNA-binding domain, respectively (Clontech). To generate expression vectors for the fusion protein between glutathione-S-transferase (GST) and PDS, GST-PDS, full-length pds cDNA was SmaI-EcoRI digested from pAS2-1–PDS and cloned into pGEX. His-tagged full-length dTAFII16 cDNA was likewise amplified by PCR and cloned into pRSET. TBP and PDS were fused to a triple HA epitope tag, and dTAFII16 was fused to six myc epitope tags at the start codon by PCR and cloned into RactHAdh (64). The fusion product of PDS and the Ga14 DNA-binding domain was amplified from pAS2-1–PDS and subcloned into RActHAdh. Constructs (G4)5-HSV-TK-Luc and pPac-βgal were a gift of R. Tjian (University of California, Berkeley, Calif.). We generated the construct sev-Luc by replacing the (G4)5-HSV-TK region of the previous vector with the same regulatory (enhancer and promoter) sequences of the sev-Ras1V12 construct (see above). The sev promoter was obtained from vector pSP/HSS and the enhancers were from pSE8/DM30, supplied by D. A. Wassarman (National Institutes of Health, Bethesda, Md.). All plasmids were verified by DNA sequencing. Further details of construct procedures are available on request.
Yeast two-hybrid assay.
The full-length PDS cDNA was used as bait. The cDNA was cloned in plasmid pAS2-1 and introduced into the yeast strain Y190. Colonies expressing the Ga14 DNA-binding domain fused to PDS were identified by immunoblotting with anti-PDS antibody. The second component of the assay, the Ga14 activation domain, was supplied as an embryo cDNA library in pGAD10 (Clontech). The subsequent transformation followed standard procedures (27). A total of 1.4 × 107 library transformants were screened in this manner.
Validation of yeast two-hybrid transformants.
Of 312 transformants able to grow in synthetic complete (SC) medium lacking histidine, only 76 contained plasmids that activated lacZ transcription. To further eliminate false positives, we provoked the loss of “bait” plasmids by streaking the transformants on SC medium containing 2.5 mg of cycloheximide per ml (27). Plasmid loss was verified by replica plating on SC medium lacking tryptophan and leucine. Transformants were mated subsequently to Y187 yeast strains containing pAS2-1–PDS, pAS2-1 alone, or pLAM5′-1 (which expresses a fusion of Ga14 DNA-binding domain and human lamin C). Eleven false positives were discarded since they could activate lacZ transcription in the absence of PDS. Analysis showed that they all encoded typical false positives (28). The remaining clones contained plasmids that activated lacZ transcription only in the presence of the Ga14 DNA-binding domain fusion and PDS but not in the presence of pAS2-1 alone or pLAM5′. In addition, the interaction persisted after plasmid swapping between PDS and the isolated positive clones. Since they fulfilled all possible controls of the yeast two-hybrid assay, we therefore considered the resulting 69 clones to be true positives.
Northern blot analysis and DNA cloning.
Poly(A)+ mRNA was isolated using a QuickPrep Micro mRNA purification kit (Pharmacia Biotech). mRNA (5 μg) from each sample was loaded on 1.5% agarose–formaldehyde gels and transferred to nylon membranes (Bio-Rad). The probe was [γ-32P]dATP radiolabeled by the random-priming method (12). Transcript size was estimated using RNA markers (Gibco-BRL). The partial cDNA found in the yeast two-hybrid assay was used as a probe to screen an adult head λgt11 cDNA library (from E. Meyerowitz, Caltech) at high stringency.
Immunological procedures.
To immunize rabbits, the His-dTAFII16 fusion protein was affinity purified using Ni-nitrilotriacetic acid agarose column (Qiagen). The anti-dTAFII16 monospecific antibody was affinity purified from crude sera using a GST-dTAFII16 column (Pharmacia Biotech) (26) and used for immunoblotting and immunostaining. Whole embryos and third-instar larvae were dissected and fixed for 30 min in freshly prepared 4% paraformaldehyde in phosphate-buffered saline (PBS). Specimens were incubated in crude rabbit serum (1:1,000) or monospecific anti-dTAFII16 (1:200) overnight at 4°C in PBT (0.1% Triton X-100 in PBS), thoroughly washed in PBT, and incubated for 1 h at room temperature in secondary anti-rabbit immunoglobulin G antibody conjugated to Cy3 (Amersham). Preparations were mounted in PBS-glycerol (1:1) and viewed under a confocal microscope (Leica). The anti-myc antibody (1-9E10.2) was a gift of S. Pons (Instituto Cajal, Madrid, Spain), and the anti-HA antibodies (rabbit Y-11 and mouse F-7) were from Santa Cruz Biotechnology.
GST pull-down experiments.
The procedure used for the GST pull-down assay was essentially as described elsewhere (53). Briefly, GST or GST-PDS proteins (5 μg) immobilized on agarose resin (Pharmacia Biotech) were washed extensively with LBST-100 buffer (25 mM HEPES-KOH [pH 7.9], 100 mM NaCl, 6% glycerol, 5 mM MgCl, 1 mM dithiothreitol, 0.05% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 5 mM EDTA). His-dTAFII16 fusion protein (5 μg) was added to the resins, and the interaction assay was carried out in a final volume of 300 μl (for 2 h at 4°C) with gentle shaking. The beads were washed four times with increasing NaCl concentrations (LBST-100, LBST-300, and LBST-500), and the bound proteins were analyzed by immunoblotting using anti-dTAFII16 antibody.
Cell transfection and immunoprecipitation.
Schneider line 2 cells were transfected by the calcium phosphate method (9) at a density of 6 × 106 cells per plate, using 5 μg of plasmid DNA for transfection. After 48 h, the cells were harvested by three freeze-thaw cycles in buffer A (10 mM HEPES-KOH [pH 7.6], 100 mM NaCl, 10% glycerol, 8 mM MgCl, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 0.2 mM EDTA) if they were used for immunoprecipitation or in reporter assay buffer (Promega) if they were used for determination of luciferase activity. A 20-μl volume of cleared lysate was used for each luciferase (Promega) and β-galactosidase assay, and luciferase activity was normalized to that of β-galactosidase. For immunoprecipitation, 100-μl volumes of cell extracts were incubated (for 2 h at room temperature) with 2 μg of the indicated antibody in buffer A to a final volume of 400 μl. Following this, 20 μl of protein A/G agarose (Santa Cruz Biotechnology) was added, and the incubation was continued for 1 h at 4°C. The resin was extensively washed with buffer A, and the bound material was analyzed by immunoblotting. Analysis of the potential interaction is complicated by the fact that the protein constructs, HA-PDS and myc-dTAFII16, have molecular weights very similar to those of the heavy and light immunoglobulin G chains respectively. To avoid this problem, we immunoprecipitated them with a rabbit anti-HA polyclonal (Y-11) and analyzed the result with mouse monoclonal anti-HA (F-7) and anti-myc (1-9E10.2) antibodies.
Nucleotide sequences accession numbers.
dTAFII16 and dTAFII24 sequences have been submitted to the EMBL database under accession numbers AJ237968 and AJ276419, respectively.
RESULTS
PDS interacts with a new Drosophila TAFII.
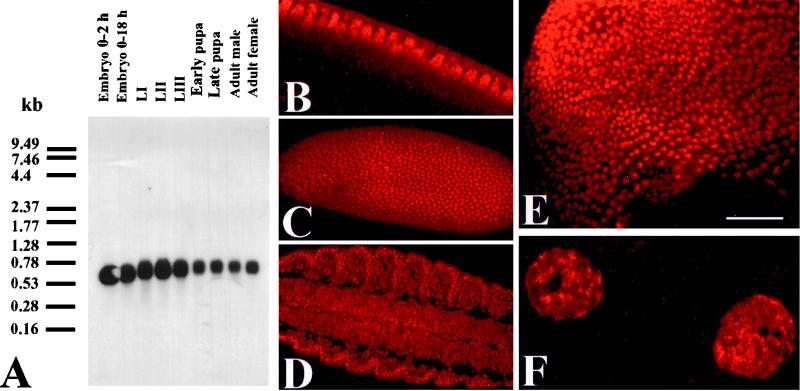
To identify proteins that might interact with PDS, we carried out a yeast two-hybrid screen using a previously isolated (our unpublished data) full-length pds cDNA as bait (16). Of 1.4 × 107 clones screened, 69 true positives were isolated from the initial 76 transformants (see Materials and Methods). On sequencing, all 69 clones were found to contain an identical insert of 485 bp. This large number of clones probably results from the high efficiency of the transformation process. Using this insert as a probe, we screened an adult head cDNA library and isolated a transcript 17 bp longer, which includes a putative translation start site. A polyadenylation consensus signal is located at position 482 (74), but no splice signals are found (48), and comparison with the corresponding genomic sequence does not reveal alternative splicing. Furthermore, expressed sequence tag (EST) database searches revealed one EST (accession number AI388776) with a sequence identical to that of our cDNA, which was reported to the EMBL database under accession number AJ237968. A more recent report indicates the presence of a longer (770-bp) cDNA (accession number AJ243837) (19). The ORF in both sequences, however, are coincident, and the discrepancy between the two mRNA sizes [0.7 and 0.95 kb respectively, including the poly(A) tracts] pertains to the 5′ untranslated region only. The conceptual translation of the single ORF produces a 146-amino-acid protein with significant identity to human TAFII30 (Fig. 1). The theoretical molecular mass of the Drosophila protein is 16 kg mol−1. Based on this value and the sequence identity to hTAFII30, we named the gene dTAFII16. Northern blot analyses indicate that the gene is expressed as a single mRNA band detected throughout all developmental stages (Fig. 2). The size of our cDNA matches that of the corresponding mRNA relatively well, after allowing for poly(A) tracts. The broad band indicates a size of 530 to 700 bp and suggests different levels of polyadenylation. To resolve the cellular localization of the protein, we stained larvae with a monospecific anti-dTAFII16 antibody using the preimmune serum as a negative control. The specific signal was detected exclusively in the nuclei of all cell types at all developmental stages including the blastoderm prior to cellularization (Fig. 2). At variance with a recent report (19), we failed to detect any cytoplasmic signal. To address this discrepancy, we transfected cells with an expression vector containing myc-tagged dTAFII16 and stained them with an anti-myc antibody (see below). This procedure confirmed the nuclear localization (data not shown). Within the nucleus, dTAFII16 is localized in multiple hot spots, leaving a single (occasionally double) major signal-deprived site that seems to correspond to the nucleolus (Fig. 2F).
FIG. 1.
Protein sequence alignment among dTAFII16, dTAFII24, and hTAFII30. Identical residues are shaded. Note the high sequence conservation toward the C terminus. Accession numbers for dTAFII16 and dTAFII24 are AJ237968 and AJ276419, respectively.
FIG. 2.
dTAFII16 expression. (A) Developmental Northern blot hybridized with the 502-nucleotide adult head cDNA probe. (B to F) Confocal images of immunostaining with a crude anti-dTAFII16 rabbit serum (B to E) or a monospecific anti-dTAFII16 antibody (F) visualized by fluorochrome Cy3. (B) Preblastoderm stage during cellularization. (C) Blastoderm stage. (D) Embryo stage 15 showing the central nervous system, muscles, and epidermis. (E) Third-instar imaginal wing disk. Note the nuclear localization of the signal in all tissues and developmental stages. (F) Detail of two Malpighian tubule cells. Note the spotty signal within the nucleus, except for the nucleoli, and its absence from the cytoplasm. Bar, 12 μm (B), 130 μm (C), 190 μm (D), 30 μm (E), and 2 μm (F).
The PDS-dTAFII16 interaction is reproduced in vitro and in vivo.
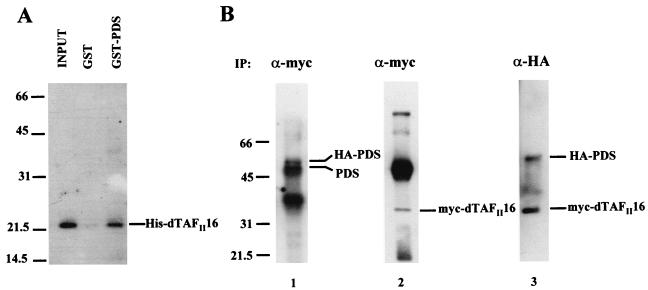
The isolation of a large number of identical interactors in the yeast two hybrid assay suggested that their binding is relevant. To further analyze the association of PDS and dTAFII16, we carried out GST pull-down and coimmunoprecipitation (co-IP) assays. For the in vitro GST pull-down experiment, dTAFII16 was tagged with histidine (His-dTAFII16) and PDS was fused to GST. His-dTAFII16 was eluted through two types of agarose columns, with bound GST-PDS or GST alone. The experiment was performed three times, yielding reproducible results. His-dTAFII16 binds to GST-PDS but fails to bind to GST alone (Fig. 3A), indicating that the interaction is specific, at least in this in vitro assay. In vivo evidence for this association was obtained in a co-IP assay (Fig. 3B). Two types of plasmids expressing myc-tagged dTAFII16 (myc-dTAFII16) and hemagglutinin-tagged PDS (HA-PDS) were used to transiently transfect Schneider 2 cells. The expression of both proteins was verified in blots stained with antibodies against myc and HA tags. Mouse monoclonal anti-myc and rabbit polyclonal anti-HA antibodies were used for IP. Anti-myc precipitates were probed with a rabbit polyclonal anti-PDS (Fig. 3B, lane 1), stripped, and reprobed with anti-myc antibody (lane 2). Conversely, precipitates elicited by rabbit polyclonal anti-HA antibody were probed with a mouse monoclonal anti-HA and the anti-myc antibodies (lane 3). The data show that anti-HA antibody precipitates myc-dTAFII16 when coexpressed with HA-PDS and anti-myc antibody does so with HA-PDS when coexpressed with myc-dTAFII16. Irrespective of the antibody used to elicit IP, therefore, there is co-IP of the other protein partner. As negative controls (data not shown), in cell extracts transfected with single constructs, HA-PDS precipitates with anti-HA but not with anti-myc whereas myc-dTAFII16 precipitates only with anti-myc. These results demonstrate that the PDS-TAFII16 association is specific and takes place in living cells, most probably in the nucleus, where both localize.
FIG. 3.
Physical association of PDS with dTAFII16. Abbreviations: (A) GST pull-down experiment between a GST-PDS fusion protein and a histidine-tagged dTAFII16. (B) Coimmunoprecipitation of HA-tagged PDS with myc-tagged dTAFII16. The antibodies used for the immunoprecipitation (IP) are indicated above each lane. The blot in lane 1 was probed with anti-PDS antibody, stripped, and reprobed with anti-myc antibody (α-myc) (lane 2). The anti-PDS antibody thus reveals the HA-tagged as well as the constitutive PDS products. In lane 3, the precipitate was induced with rabbit anti-HA antibody (α-HA) and then probed with mouse anti-HA antibody (see Materials and Methods) in addition to the anti-myc antibody.
The interaction is mediated by the PDS HFD and the dTAFII16 amino terminus.
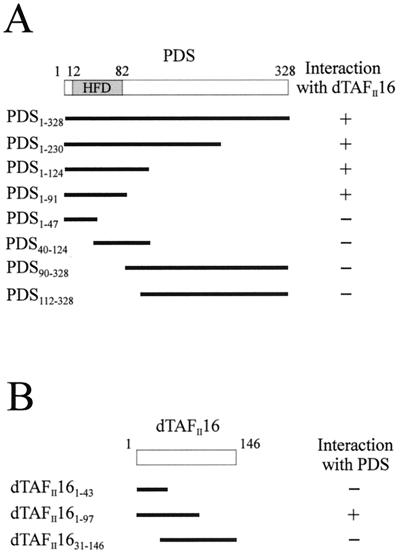
PDS contains a putative HFD between amino acids 12 and 82. In addition, abundant proline residues are found C-terminal to this domain, albeit not clustered in a recognizable motif. The yeast two-hybrid system using eight different PDS fragments served to dissect the PDS domain responsible for the interaction with dTAFII16 (Fig. 4). The same control procedures as in the yeast two-hybrid screen were used. All N-terminal fragments that included the first 90 amino acids of PDS were able to bind dTAFII16, whereas those comprising only the C-terminal regions did not. In addition, a small fragment including the HFD motif (PDS1–91) sustained the interaction whereas fragments that split HFD (PDS1–47 and PDS40–124) did not. HFD thus appears necessary and sufficient to mediate the binding between PDS and dTAFII16. Although dTAFII16 is a small protein without recognizable structural motifs, we attempted to identify a putative critical region for the interaction with PDS. The three fragments used (Fig. 4B) allowed identification of the N terminus as a requirement for this association. Attempts to further subdivide this region failed to sustain the interaction.
FIG. 4.
Dissection of the interaction of PDS and dTAFII16 in yeast. (A) Interaction of PDS fragments with dTAFII16. The HFD motif in PDS extends from amino acids 12 to 82. (B) Interaction of dTAFII16 fragments with PDS.
PDS activates transcription in cells.
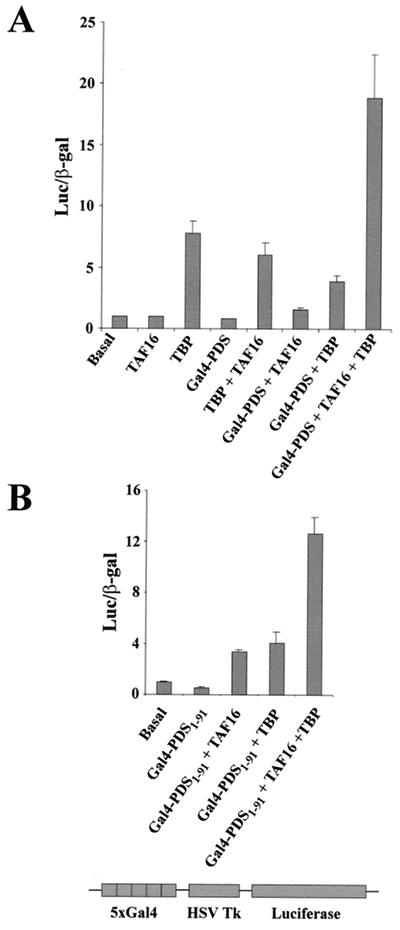
To test the biological significance of the interaction described above, we tested its role in gene transcription by using transfected Schneider 2 cells. As a reporter, we monitored the enzymatic activity of luciferase. The luciferase cDNA is under the control of the herpes simplex virus thymidine kinase promoter fused to an enhancer element containing five upstream activation sequence sites (Gal4 targets). lacZ under the control of the actin promoter, which contains neither enhancers nor Gal4 sites, served as an internal reference. The reporter and the internal control were transfected in the absence or presence of plasmids driving the expression of Gal4-PDS, myc-dTAFII16, or HA-TBP. Transfection with Gal4-PDS or myc-dTAFII16 had no effect on luciferase activity with respect to basal levels. Transfection with HA-TBP, alone or in addition to myc-dTAFII16 or Gal4-PDS, led to relatively weak activation. Following cotransfection with the three proteins (Gal4-PDS, myc-dTAFII16, and HA-TBP), however, a strong increase in luciferase activity (18-fold with respect to the basal level) was detected (Fig. 5A). The experiment was reproduced four times, yielding consistent results. The activation levels observed with the three proteins are equivalent to those reported in a similar experiment using the transcriptional activator SplA (see Fig. 3C in reference 25). Transfection with Gal4-PDS, alone or in addition to mycTAFII16 or HA-TBP, failed to elicit significant levels of activation. This observation would argue against a role for PDS as a transcriptional activator, since the endogenous TFIID components, including TAFII16 which is expressed in Schneider 2 cells (data not shown), may have contributed to the putative role of PDS. It should be noticed, however, that the transcriptional activity of the luciferase reporter in this type of cell depends on the high expression levels of the transfected plasmids. It is quite likely that if any one of the required components is present at low concentration, it will become a limiting factor. In fact, this is the key feature on which this test is based. Comparing the activation levels elicited by each protein combination, we can conclude that PDS is able to activate transcription when fused to Gal4 using dTAFII16 as a cofactor. This experiment provides additional evidence on two important issues, (i) that the PDS-TAFII16 interaction occurs in vivo and (ii) that dTAFII16 is a bona fide TAFII because it requires TBP to promote gene transcription.
FIG. 5.
PDS and dTAFII16 mediate transcription activation. Schneider 2 cells were cotransfected with PDS, TAFII16, and TBP in various combinations of tagged products (see the text). A 5-μg sample of each plasmid DNA was used. Histogram values indicate the recorded luciferase activity normalized with respect to the internal control of β-galactosidase (β-gal) (see Materials and Methods). Basal activity refers to the data obtained after transfection with luciferase- and lacZ-containing plasmids. (A) Assays with full-length PDS fused to Gal4BD. (B) Assays with a PDS fragment that includes the HFD motif. HSV Tk, herpes simplex virus thymidine kinase.
In line with the data from the yeast two-hybrid test, we assayed whether a PDS fragment containing the HFD motif will be able to activate transcription of the luciferase reporter; Fig. 5B shows that this is the case. The same small PDS fragment, PDS1–91, used in the yeast two-hybrid tests yielded significant activation only if co-transfected with TAFII16 and TBP. This result demonstrates that the activation effect observed with the whole PDS protein can be reproduced (70% activation with respect to the whole PDS protein) by a fragment including the HFD motif, further supporting the role of this structural motif in the interaction with dTAFII16. The data obtained so far indicate that the function of PDS in Drosophila might be to activate gene transcription by recruiting dTFIID components.
PDS and dTAFII16 modulate phenotype expression in Drosophila.
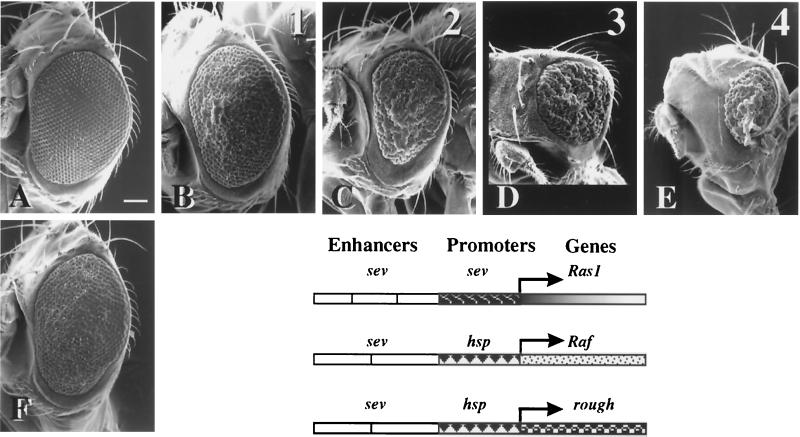
The expression of a constitutively active Ras1 allele under the control of eye-specific sevenless regulatory sequences (sev-Ras1V12) resulted in the transformation of cone cells into R7 photoreceptors, which roughen the eye (compare Fig. 6A and C) (17). This effect has been instrumental in isolating components of the Ras1 signaling pathway (33). Mutations that modify this phenotype are candidate genes for components of the Ras1 signaling cascade or transcription factors that control the expression of the sev-Ras1V12 construct or its downstream genes. In this way, several TAFIIs have been identified as mutant suppressors of the rough eye phenotype (60). We used the same approach to test the potential effects of PDS and dTAFII16. The data show that the sev-Ras1V12 phenotype is modulated by PDS in a gene dosage-dependent manner. Heterozygotes for mutant alleles (pds2270 and pds88) or deletions [Df(1)88-6 and Df(1)88-2] of the gene partially suppressed the eye roughening (Fig. 6B), whereas increased expression of PDS (Fig. 6D and E) using one or two copies of a pds+ transgene (see Materials and Methods) progressively aggravated it. The suppression effect was also observed in heterozygotes of dTAFII16 deletions [Df(2)N6 and Df(2)N19] (Fig. 6F). In this case, however, increased dosage could be assayed since dTAFII16+ transgenes are not yet available. The combined reduction of gene dosage for PDS and dTAFII16 did not show further suppression of the sev-Ras1V12-induced eye phenotype (data not shown). Taken together, these observations indicate that both proteins act through the same mechanism or pathway.
FIG. 6.
PDS and dTAFII16 modulate the phenotypic expression of a sev-Ras1V12 construct. Representative scanning electron micrographs of eyes of adult females expressing one copy of the sev-Ras1V12 construct with variable number of pds+ gene copies. (A) Wild type; (B) pds2270/+; CyO-CR2, sev-Ras1V12/+; (C) CyO-CR2, sev-Ras1V12/+; (D) CyO-CR2, sev-Ras1V12/+;T(3)E6L/+; (E) CyO-CR2, sev-Ras1V12/+;T(3)E6L/ T(3)E6L; (F) CyO-CR2, sev-Ras1V12/Df(2) N6. Numbers 1 to 4 above each panel indicate the number of functional copies of pds+ in the corresponding genotypes. Note the pds gene dosage dependence of the eye-roughening phenotype. Also note in panel F the partial suppression elicited by the heterozygous deletion for dTAFII16. Consistent results were obtained with other pds− [Df(1)88-6 and Df(1)88-2] or dTAFII16− [Df(2)N19] chromosomes (data not shown). Anterior is to the left, dorsal is up. Bar, 90 μm. The diagram shows the structures of the three eye-roughening producing constructs assayed.
In an attempt to discriminate between PDS and dTAFII16 affecting sev-Ras1V12 transcription or the subsequent signaling cascade, we assayed two other available constructs, sE-RaftorY9 and sev-hsp-ro. The three constructs differ in the enhancer/promoter sequences or the gene driven (Fig. 6). The previous gene dosage genotypes involving PDS or dTAFII16 show no modification of the eye phenotype elicited by either construct (data not shown). Since Ras1 and Raf activate the same or very similar pathways, it is unlikely that the differential effect observed with these two constructs could be attributed to a selective effect of PDS and dTAFII16 on genes in the cascade. Similarly, the lack of effect the sev-hsp-ro construct points to the sev promoter as a likely target for PDS-dTAFII16. Since the genomic segments containing the sev enhancers are not identical between the first two constructs, however, sev enhancers cannot be formally excluded as a target for PDS-dTAFII16. To further explore the role of PDS on transcription activation, and in particular whether PDS could target the sevenless promoter or enhancer sequences, we assayed a novel luciferase-expressing construct, sev-luc, containing the same regulatory sequences as the sev-Ras1V12 construct (see Materials and Methods). No significant effect on luciferase expression was found in cells transfected with PDS, dTAFII16, TBP, or their combinations (data not shown). This result indicates that PDS is unlikely to be a transcriptional activator per se or, alternatively, that its native target is not included in the sev-promoter/sev-enhancer sequences. Nonetheless, since both the Gal4-driven luciferase transcription in cells and the sev-driven Ras1 phenotypes in flies indicate that PDS plays a role in activation, it can be concluded that PDS is a coactivator that recruits components of the TFIID and requires an additional mechanism to bind DNA.
Exploring interactions with other TFIID components.
hTAFII30 is reported to interact with TBP, hTAFII250, hTAFII20, and itself, forming homomultimeres (32). Using the yeast two-hybrid system, we tested whether dTAFII16 could interact with dTBP, dTAFII30α (the Drosophila homologue of hTAFII20), or itself. All these assays yielded negative results (data not shown), suggesting that hTAFII30 and dTAFII16 do not sustain the same interactions within TFIID. EST database searches revealed another Drosophila EST (accession number AI457031), however, encoding a protein very similar to dTAFII16 and hTAFII30. The gene maps to the 23A6-7 chromosome band, adjacent and in opposite orientation to dTAFII16. We sequenced this genomic region and the EST in full to confirm the ORF. The revised sequence has been submitted to the EMBL database (accession number AJ276419). It indicates a second hTAFII30 homologue, with a theoretical molecular mass of 18.5 kg mol−1. Both Drosophila homologues of hTAFII30 have been reported recently, and the name dTAFII24 was assigned to the second element (19). Given the similarity between dTAFII16 and dTAFII24, we used the yeast two-hybrid assay to find whether PDS would interact with the new homologue; the result was negative. In addition, since the N terminus of dTAFII16 mediates the interaction with PDS (Fig. 4B) and since both dTAFIIs deviate in their N-terminal sequences (Fig. 1), it is likely that this part of the protein sustains differential interactions of these TAFIIs. These results demonstrate that dTAFII16 and dTAFII24 are functionally distinct. Nevertheless, we further assayed whether dTAFII16 and dTAFII24 could interact in Drosophila, mimicking the reported homodimerization of hTAFII30. The result from the yeast two-hybrid assay was, however, negative (data not shown). The same experimental approach also yielded a negative result to the question whether PDS could interact directly with TBP. This last observation is coherent with the requirement for the triple cotransfection (PDS, dTAFII16, and TBP) in the transcription activation assays (Fig. 5).
pds function is required for cell survival.
The cellular effects of PDS depletion can be evaluated in mosaics. To that end, transheterozygous y w pds2270/f36a larvae were irradiated to obtain marked twin spots screened in the dorsal notum. In addition, y w pds2270/M(1)n larvae were similarly treated to generate w pds2270 M+clones marked in the eye. No mutant clones were detected in either system, and control cases appeared at the expected ratios (Table 1). It should be noted that mutant eye cells, which are M+, would have had a growth advantage over the surrounding cells (15). These data demonstrate that PDS is a cell vital function, at least for integument cells.
TABLE 1.
pds somatic mosaicsa
| Region | Locus | No. of clones |
|---|---|---|
| Notum | f36a | 19 |
| y − f36a | 2 | |
| y | 3 | |
| Eye | w pds | 0 |
| Control | 17 |
Notum clones marked with f36a result from crossovers between this marker and the centromere. An equivalent number of y-marked twins was expected if pds was a viable mutation. The only two cases of y − f36a twins detected must correspond to crossovers between pds and f36a loci. Clones marked with y result from crossovers distal to f36a. The sizes of all clones ranged from one to four bristles. Eye clones marked with w are also M(1)n+ and must include pds. Control clones in the eye are scored on Dp(1;3)JC153 bearing siblings. This duplication carries a normal copy of pds gene.
DISCUSSION
The data show that Drosophila PDS and TAFII16 interact specifically, leading to transcription activation through the TFIID complex. The structural motifs in each protein that mediate the interaction have been dissected, and the results indicate that PDS should be considered a coactivator of gene expression. Since PDS is a vital cell function and since both proteins are expressed in all cell types, it can be deduced that the interaction is functional in many genes. In addition, since both proteins appear to have conserved homologues and requirements, their interaction is expected to be functional in mammals as well.
Functional interaction of the HFD.
PDS fragments that contain the HFD motif sustain the PDS-dTAFII16 association, demonstrating that this is a functional domain. For the other partner, dTAFII16, the N terminus is necessary and sufficient for the interaction to take place. HFD is a motif conserved in all core histones and several TAFIIs. It is formed by a long central α-helix flanked by two smaller ones. The central helix acts as a dimerization interface in a head-to-tail fashion described as a “handshake” structure (2, 76). To date, most HFD-mediated interactions have been documented between proteins containing the same motif. A precedent for interaction between HFD- and non-HFD-containing proteins is, nonetheless, known for the trimeric CBF/NF-Y transcription factor (41). The case of PDS-dTAFII16 reported here would add another such example. Current databases include over 1,000 HFD-containing proteins (5), suggesting that the number and diversity of processes mediated by this motif will increase substantially.
The association has been reproduced both in vitro and in vivo, showing that it is specific and functionally relevant for gene transcription. Further, the luciferase transcription assay demonstrates that the functional expression of this interaction also requires TBP. This result argues in favor of TFIID as the likely transcription factor through which PDS elicits transcription activation. dTAFII16 has recently been shown to participate in TFIID but not in other complexes (19). Moreover, the absence of dTAFII16 from nucleoli is consistent with the fact that ribosomal gene transcription does not involve TFIID.
Two conserved proteins, PDS and dTAFII16.
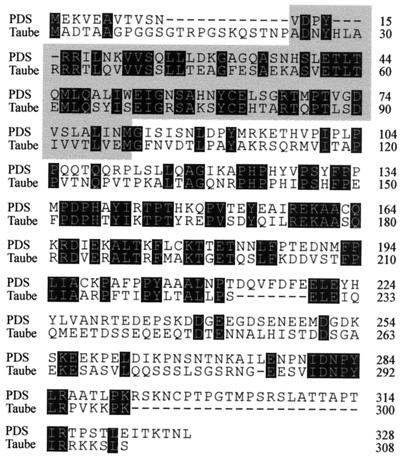
pds is a recently discovered Drosophila gene (our unpublished data) (accession number Y15513). Databases also include the murine protein Taube nuss (accession number AAG01682), which is 35% identical to PDS and has an HFD motif toward its N terminus (Fig. 7). In addition, we found that PDS is a vital cell function in Drosophila and Taube is reported to be essential for pluripotent cells in the mouse. Based on these structural and functional features, it is likely that the two proteins are homologues. Among human database sequences, a clot (Hs. 139179) of eight ESTs (accession numbers AI151131, AI027324, AI027325, AI655948, AI738942, AA983184, AA641254, and N71758) also shows close homology to the Drosophila PDS.
FIG. 7.
Protein sequence alignment between PDS and the recently reported murine protein Taube nuss. Identical residues are shaded. Note the presence of a HFD domain (shaded) in both proteins. Accession numbers for PDS and Taube are Y15513 and AAG01682, respectively.
Although dTAFII16 and dTAFII24 are considered sequence homologues of hTAFII30, detailed analysis of the three protein sequences indicates that dTAFII16 is a closer relative of hTAFII30 (45% identity) than of dTAFII24 (38% identity). The two protein sequences diverge in their N terminus, and this is the region that mediates the selective interaction dTAFII16-PDS. It thus seems that the two TAFIIs are functionally different in Drosophila. In this context, specific mutant traits would be expected for these two genes, although no mutations have thus far been identified in either of them to confirm this point. We used PCR to analyze all available lethal mutations in the area [l(2)AB2, l(2)AB3, l(2)AB4, l(2)AB5, and l(2)AB6] (10, 39), and none showed sequence alterations in the ORF or the genomic regions of either dTAFII. The chromosome Df(2)N6 is the only genetic variant available, and it deletes both dTAFII genes. Since additional genes are included, however, the lethality of this chromosome cannot be attributed unequivocally to the dTAFII genes.
In addition to the sequence conservation and its functional association with TBP for gene transcription, dTAFII16 localizes to the nucleus at all developmental stages and tissues, as do all other TAFIIs. In this context, we cannot confirm the reported dual nuclear and cytoplasmic localization of dTAFII16 (19). It may be relevant that the antibodies used in the previous study were raised against peptide sequences whereas those used here were obtained after immunization with the whole protein. Data based on monospecific antibodies, crude serum, and labeling of myc-tagged dTAFII16 localize the signal in multiple spots within the nucleus only (Fig. 2). The hot spots at which dTAFII16 appears to accumulate may correspond to transcriptionally active regions.
Molecular mechanism and biological function of PDS.
Transfection experiments in Schneider 2 cells show that PDS activates transcription when fused to the Gal4 DNA binding domain in a dTAFII16- and TBP-dependent manner. The gene dosage effects on the expression of a Ras1 construct also support the role of PDS as an activator rather than a repressor. In principle, this function could be achieved either as a bona fide transcription activator binding directly to DNA and recruiting TFIID to specific promoters or as a cofactor linking a true activator to the transcription complex. Several arguments support PDS as a cofactor for gene transcription. Cells transfected with PDS only fail to activate transcription of the luciferase reporter from the sev-Luc construct. This experiment was designed to test whether PDS could use enhancer or promoter sequences from the sevenless gene as target, in view of the effects observed on the eye phenotype of sev-Ras1V12 and the pds gene dosage. It thus appears that to activate transcription, PDS requires association with a DNA-binding activator. In the experiments with the upstream activation sequence-Luc construct (Fig. 5), this effect was provided by the DNA-binding domain of Gal4. This procedure of tethering proteins to a promoter has served to infer a role of coactivators for many components of several transcriptional complexes (20, 34, 62). The gene dosage modulation of the eye phenotype is probably due to the titration effect on native components of the transcription machinery.
Through which transcription complex does PDS function? Extensive biochemical work on preinitiation complex purification in several organisms has failed to identify PDS so far, suggesting that PDS is not a constitutive member of TFIID, and we have shown here that PDS does not interact with TBP directly. These features prevent consideration of PDS as a TAF, at least formally. Taken together, the available data indicate that PDS is a novel transcriptional coactivator in which HFD is the functional domain that mediates the interaction with dTAFII16; this mechanism underlies a biological function essential for cell survival. This observation, in addition to its generalized expression in all tissues throughout development, as well as its conserved sequence and requirement for cell survival, suggests that many genes will be the target of PDS-mediated activation.
ACKNOWLEDGMENTS
We thank R. Tjian for plasmids (G4)5-HSV-TK-Luc and pPac-βgal, D. A. Wassarman for plasmids pSP/HSS and pSE8/DM30, S. Pons for anti-myc antibody (1-9E10.2), H. Bellen and the Bloomington and Umeå stock centers for mutant strains, and E. Hafen for Raf and rough constructs. G. Marques provided key training for experimental procedures. Laboratory members read the text critically.
This research was funded by grants 08.5/43/98 and PM96-006 from the Comunidad Autónoma de Madrid (CAM) and the Spanish Ministry of Culture (DGICYT), respectively. A. H.-H. is a CAM-funded postdoctoral fellow.
REFERENCES
- 1.Apone L M, Virbasius C M, Reese J C, Green M R. Yeast TAF(II)90 is required for cell-cycle progression through G2/M but not for general transcription activation. Genes Dev. 1996;10:2368–2380. doi: 10.1101/gad.10.18.2368. [DOI] [PubMed] [Google Scholar]
- 2.Arents G, Burlingame R W, Wang B C, Love W E, Moudrianakis E N. The nucleosomal core histone octamer at 3.1 A resolution: a tripartite protein assembly and a left-handed superhelix. Proc Natl Acad Sci USA. 1991;88:10148–10152. doi: 10.1073/pnas.88.22.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basler K, Siegrist P, Hafen E. The spatial and temporal expression pattern of sevenless is exclusively controlled by gene-internal elements. EMBO J. 1989;8:2381–2386. doi: 10.1002/j.1460-2075.1989.tb08367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baxevanis A D, Arents G, Moudrianakis E N, Landsman D. A variety of DNA-binding and multimeric proteins contain the histone fold motif. Nucleic Acids Res. 1995;23:2685–2691. doi: 10.1093/nar/23.14.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baxevanis A D, Landsman D. Histone Sequence Database: new histone fold family members. Nucleic Acids Res. 1998;26:372–375. doi: 10.1093/nar/26.1.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birck C, Poch O, Romier C, Ruff M, Mengus G, Lavigne A C, Davidson I, Moras D. Human TAF(II)28 and TAF(II)18 interact through a histone fold encoded by atypical evolutionary conserved motifs also found in the SPT3 family. Cell. 1998;94:239–249. doi: 10.1016/s0092-8674(00)81423-3. [DOI] [PubMed] [Google Scholar]
- 7.Bowtell D D, Lila T, Michael W M, Hackett D, Rubin G M. Analysis of the enhancer element that controls expression of sevenless in the developing Drosophila eye. Proc Natl Acad Sci USA. 1991;88:6853–6857. doi: 10.1073/pnas.88.15.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalkley G E, Verrijzer C P. DNA binding site selection by RNA polymerase II TAFs: a TAF(II)250-TAF(II)150 complex recognizes the initiator. EMBO J. 1999;18:4835–4845. doi: 10.1093/emboj/18.17.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherbas L, Moss R, Cherbas P. Transformation techniques for Drosophila cell lines. In: Goldstein L S B, Fyrberg E A, editors. Drosophila melanogaster:practical uses in cell and molecular biology. New York, N.Y: Academic Press, Inc.; 1994. pp. 161–179. [DOI] [PubMed] [Google Scholar]
- 10.DiAntonio A, Parfitt K D, Schwarz T L. Synaptic transmission persists in synaptotagmin mutants of Drosophila. Cell. 1993;73:1281–1290. doi: 10.1016/0092-8674(93)90356-u. [DOI] [PubMed] [Google Scholar]
- 11.Dikstein R, Ruppert S, Tjian R. TAFII250 is a bipartite protein kinase that phosphorylates the base transcription factor RAP74. Cell. 1996;84:781–790. doi: 10.1016/s0092-8674(00)81055-7. [DOI] [PubMed] [Google Scholar]
- 12.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1984;137:266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- 13.Felinski E A, Quinn P G. The CREB constitutive activation domain interacts with TATA-binding protein-associated factor 110 (TAF110) through specific hydrophobic residues in one of the three subdomains required for both activation and TAF110 binding. J Biol Chem. 1999;274:11672–11678. doi: 10.1074/jbc.274.17.11672. [DOI] [PubMed] [Google Scholar]
- 14.Ferreri K, Gill G, Montminy M. The cAMP-regulated transcription factor CREB interacts with a component of the TFIID complex. Proc Natl Acad Sci USA. 1994;91:1210–1213. doi: 10.1073/pnas.91.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrús A. Parameters of mitotic recombination in minute mutants of Drosophila melanogaster. Genetics. 1975;79:589–599. doi: 10.1093/genetics/79.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 17.Fortini M E, Simon M A, Rubin G M. Signalling by the sevenless protein tyrosine kinase is mimicked by Ras1 activation. Nature. 1992;355:559–561. doi: 10.1038/355559a0. [DOI] [PubMed] [Google Scholar]
- 18.Gangloff Y G, Werten S, Romier C, Carre L, Poch O, Moras D, Davidson I. The human TFIID components TAF(II)135 and TAF(II)20 and the yeast SAGA components ADA1 and TAF(II)68 heterodimerize to form histone-like pairs. Mol Cell Biol. 2000;20:340–351. doi: 10.1128/mcb.20.1.340-351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georgieva S, Kirschner D B, Jagla T, Nabirochkina E, Hanke S, Schenkel H, de Lorenzo C, Sinha P, Jagla K, Mechler B, Tora L. Two novel Drosophila TAF(II)s have homology with human TAF(II)30 and are differentially regulated during development. Mol Cell Biol. 2000;20:1639–1648. doi: 10.1128/mcb.20.5.1639-1648.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez-Couto E, Klages N, Strubin M. Synergistic and promoter-selective activation of transcription by recruitment of transcription factors TFIID and TFIIB. Proc Natl Acad Sci USA. 1997;94:8036–8041. doi: 10.1073/pnas.94.15.8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodrich J A, Hoey T, Thut C J, Admon A, Tjian R. Drosophila TAFII40 interacts with both a VP16 activation domain and the basal transcription factor TFIIB. Cell. 1993;75:519–530. doi: 10.1016/0092-8674(93)90386-5. [DOI] [PubMed] [Google Scholar]
- 22.Grant P A, Schieltz D, Pray-Grant M G, Steger D J, Reese J C, Yates J R, Workman J L. A subset of TAF(II)s are integral components of the SAGA complex required for nucleosome acetylation and transcriptional stimulation. Cell. 1998;94:45–53. doi: 10.1016/s0092-8674(00)81220-9. [DOI] [PubMed] [Google Scholar]
- 23.Hahn S. The role of TAFs in RNA polymerase II transcription. Cell. 1998;95:579–582. doi: 10.1016/s0092-8674(00)81625-6. [DOI] [PubMed] [Google Scholar]
- 24.Hampsey M. Molecular genetics of the RNA polymerase II general transcriptional machinery. Microbiol Mol Biol Rev. 1998;62:465–503. doi: 10.1128/mmbr.62.2.465-503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen S K, Takada S, Jacobson R H, Lis J T, Tjian R. Transcription properties of a cell type-specific TATA-binding protein, TRF. Cell. 1997;91:71–83. doi: 10.1016/s0092-8674(01)80010-6. [DOI] [PubMed] [Google Scholar]
- 26.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 27.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 28.Hengen P N. False positives from the yeast two-hybrid system. Trends Biochem Sci. 1997;22:33–34. doi: 10.1016/s0968-0004(96)30047-9. [DOI] [PubMed] [Google Scholar]
- 29.Hoey T, Weinzierl R O, Gill G, Chen J L, Dynlacht B D, Tjian R. Molecular cloning and functional analysis of Drosophila TAF110 reveal properties expected of coactivators. Cell. 1993;72:247–260. doi: 10.1016/0092-8674(93)90664-c. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann A, Chiang C M, Oelgeschlager T, Xie X, Burley S K, Nakatani Y, Roeder R G. A histone octamer-like structure within TFID. Nature. 1996;380:356–359. doi: 10.1038/380356a0. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann A, Oelgeschlager T, Roeder R G. Considerations of transcriptional control mechanisms: do TFIID-core promoter complexes recapitulate nucleosome-like functions? Proc Natl Acad Sci USA. 1997;94:8928–8935. doi: 10.1073/pnas.94.17.8928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacq X, Brou C, Lutz Y, Davidson I, Chambon P, Tora L. Human TAFII30 is present in a distinct TFIID complex and is required for transcriptional activation by the estrogen receptor. Cell. 1994;79:107–117. doi: 10.1016/0092-8674(94)90404-9. [DOI] [PubMed] [Google Scholar]
- 33.Karim F D, Chang H C, Therrien M, Wassarman D A, Laverty T, Rubin G M. A screen for genes that function downstream of Ras1 during Drosophila eye development. Genetics. 1996;143:315–329. doi: 10.1093/genetics/143.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keaveney M, Struhl K. Activator-mediated recruitment of the RNA polymerase II machinery is the predominant mechanism for transcriptional activation in yeast. Mol Cell. 1998;1:917–924. doi: 10.1016/s1097-2765(00)80091-x. [DOI] [PubMed] [Google Scholar]
- 35.Klemm R D, Goodrich J A, Zhou S, Tjian R. Molecular cloning and expression of the 32-kDa subunit of human TFIID reveals interactions with VP16 and TFIIB that mediate transcriptional activation. Proc Natl Acad Sci USA. 1995;92:5788–5792. doi: 10.1073/pnas.92.13.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koleske A J, Young R A. The RNA polymerase II holoenzyme and its implications for gene regulation. Trends Biochem Sci. 1995;20:113–116. doi: 10.1016/s0968-0004(00)88977-x. [DOI] [PubMed] [Google Scholar]
- 37.Lavigne A C, Mengus G, Gangloff Y G, Wurtz J M, Davidson I. Human TAF(II)55 interacts with the vitamin D(3) and thyroid hormone receptors and with derivatives of the retinoid X receptor that have altered transactivation properties. Mol Cell Biol. 1999;19:5486–5494. doi: 10.1128/mcb.19.8.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee T I, Young R A. Regulation of gene expression by TBP-associated proteins. Genes Dev. 1998;12:1398–1408. doi: 10.1101/gad.12.10.1398. [DOI] [PubMed] [Google Scholar]
- 39.Littleton J T, Bellen H J. Genetic and phenotypic analysis of thirteen essential genes in cytological interval 22F1–2; 23B1–2 reveals novel genes required for neural development in Drosophila. Genetics. 1994;138:111–123. doi: 10.1093/genetics/138.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu H, Levine A J. Human TAFII31 protein is a transcriptional coactivator of the p53 protein. Proc Natl Acad Sci USA. 1995;92:5154–5158. doi: 10.1073/pnas.92.11.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maity S N, de Crombrugghe B. Role of the CCAAT-binding protein CBF/NF-Y in transcription. Trends Biochem Sci. 1998;23:174–178. doi: 10.1016/s0968-0004(98)01201-8. [DOI] [PubMed] [Google Scholar]
- 42.Martin J, Halenbeck R, Kaufmann J. Human transcription factor hTAF(II)150 (CIF150) is involved in transcriptional regulation of cell cycle progression. Mol Cell Biol. 1999;19:5548–5556. doi: 10.1128/mcb.19.8.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez E, Kundu T K, Fu J, Roeder R G. A human SPT3-TAFII31-GCN5-L acetylase complex distinct from transcription factor IID. J Biol Chem. 1998;273:23781–23785. doi: 10.1074/jbc.273.37.23781. [DOI] [PubMed] [Google Scholar]
- 44.May M, Mengus G, Lavigne A C, Chambon P, Davidson I. Human TAF(II28) promotes transcriptional stimulation by activation function 2 of the retinoid X receptors. EMBO J. 1996;15:3093–3104. [PMC free article] [PubMed] [Google Scholar]
- 45.Metzger D, Scheer E, Soldatov A, Tora L. Mammalian TAF(II)30 is required for cell cycle progression and specific cellular differentiation programmes. EMBO J. 1999;18:4823–4834. doi: 10.1093/emboj/18.17.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mizzen C A, Yang X J, Kokubo T, Brownell J E, Bannister A J, Owen-Hughes T, Workman J, Wang L, Berger S L, Kouzarides T, Nakatani Y, Allis C D. The TAF(II)250 subunit of TFIID has histone acetyltransferase activity. Cell. 1996;87:1261–1270. doi: 10.1016/s0092-8674(00)81821-8. [DOI] [PubMed] [Google Scholar]
- 47.Moqtaderi Z, Bai Y, Poon D, Weil P A, Struhl K. TBP-associated factors are not generally required for transcriptional activation in yeast. Nature. 1996;383:188–191. doi: 10.1038/383188a0. [DOI] [PubMed] [Google Scholar]
- 48.Mount S M, Burks C, Hertz G, Stormo G D, White O, Fields C. Splicing signals in Drosophila: intron size, information content, and consensus sequences. Nucleic Acids Res. 1992;20:4255–4262. doi: 10.1093/nar/20.16.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Brien T, Tjian R. Functional analysis of the human TAFII250 N-terminal kinase domain. Mol Cell. 1998;1:905–911. doi: 10.1016/s1097-2765(00)80089-1. [DOI] [PubMed] [Google Scholar]
- 50.Oelgeschlager T, Tao Y, Kang Y K, Roeder R G. Transcription activation via enhanced preinitiation complex assembly in a human cell-free system lacking TAFIIs. Mol Cell. 1998;1:925–931. doi: 10.1016/s1097-2765(00)80092-1. [DOI] [PubMed] [Google Scholar]
- 51.Ogryzko V V, Kotani T, Zhang X, Schlitz R L, Howard T, Yang X J, Howard B H, Qin J, Nakatani Y. Histone-like TAFs within the PCAF histone acetylase complex. Cell. 1998;94:35–44. doi: 10.1016/s0092-8674(00)81219-2. [DOI] [PubMed] [Google Scholar]
- 52.Orphanides G, Lagrange T, Reinberg D. The general transcription factors of RNA polymerase II. Genes Dev. 1996;10:2657–2683. doi: 10.1101/gad.10.21.2657. [DOI] [PubMed] [Google Scholar]
- 53.Ortiz L, Aza-Blanc P, Zannini M, Cato A C, Santisteban P. The interaction between the forkhead thyroid transcription factor TTF-2 and the constitutive factor CTF/NF-1 is required for efficient hormonal regulation of the thyroperoxidase gene transcription. J Biol Chem. 1999;274:15213–15221. doi: 10.1074/jbc.274.21.15213. [DOI] [PubMed] [Google Scholar]
- 54.Pham A D, Muller S, Sauer F. Mesoderm-determining transcription in Drosophila is alleviated by mutations in TAF(II)60 and TAF(II)110. Mech Dev. 1999;84:3–16. doi: 10.1016/s0925-4773(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 55.Prado A, Canal I, Ferrus A. The haplolethal region at the 16F gene cluster of Drosophila melanogaster: structure and function. Genetics. 1999;151:163–175. doi: 10.1093/genetics/151.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roeder R G. The role of general initiation factors in transcription by RNA polymerase II. Trends Biochem Sci. 1996;21:327–335. [PubMed] [Google Scholar]
- 57.Rojo-Niersbach E, Furukawa T, Tanese N. Genetic dissection of hTAF(II)130 defines a hydrophobic surface required for interaction with glutamine-rich activators. J Biol Chem. 1999;274:33778–33784. doi: 10.1074/jbc.274.47.33778. [DOI] [PubMed] [Google Scholar]
- 58.Saluja D, Vassallo M F, Tanese N. Distinct subdomains of human TAFII130 are required for interactions with glutamine-rich transcriptional activators. Mol Cell Biol. 1998;18:5734–5743. doi: 10.1128/mcb.18.10.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sauer F, Hansen S K, Tjian R. Multiple TAFIIs directing synergistic activation of transcription. Science. 1995;270:1783–1788. doi: 10.1126/science.270.5243.1783. [DOI] [PubMed] [Google Scholar]
- 60.Sauer F, Wassarman D A, Rubin G M, Tjian R. TAF(II)s mediate activation of transcription in the Drosophila embryo. Cell. 1996;87:1271–1284. doi: 10.1016/s0092-8674(00)81822-x. [DOI] [PubMed] [Google Scholar]
- 61.Shen W C, Green M R. Yeast TAF(II)145 functions as a core promoter selectivity factor, not a general coactivator. Cell. 1997;90:615–624. doi: 10.1016/s0092-8674(00)80523-1. [DOI] [PubMed] [Google Scholar]
- 62.Stargell L A, Moqtaderi Z, Dorris D R, Ogg R C, Struhl K. TFIIA has activator-dependent and core promoter functions in vivo. J Biol Chem. 2000;275:12374–12380. doi: 10.1074/jbc.275.17.12374. [DOI] [PubMed] [Google Scholar]
- 63.Struhl K, Moqtaderi Z. The TAFs in the HAT. Cell. 1998;94:1–4. doi: 10.1016/s0092-8674(00)81213-1. [DOI] [PubMed] [Google Scholar]
- 64.Swevers L, Cherbas L, Cherbas P, Iatrou K. Bombyx EcR (BmEcR) and Bombyx USP (BmCF1) combine to form a functional ecdysone receptor. Insect Biochem Mol Biol. 1996;26:217–221. doi: 10.1016/0965-1748(95)00097-6. [DOI] [PubMed] [Google Scholar]
- 65.Tanese N, Saluja D, Vassallo M F, Chen J L, Admon A. Molecular cloning and analysis of two subunits of the human TFIID complex: hTAFII130 and hTAFII100. Proc Natl Acad Sci USA. 1996;93:13611–13616. doi: 10.1073/pnas.93.24.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tansey W P, Herr W. TAFs: guilt by association? Cell. 1997;88:729–732. doi: 10.1016/s0092-8674(00)81916-9. [DOI] [PubMed] [Google Scholar]
- 67.Thut C J, Chen J L, Klemm R, Tjian R. p53 transcriptional activation mediated by coactivators TAFII40 and TAFII60. Science. 1995;267:100–104. doi: 10.1126/science.7809597. [DOI] [PubMed] [Google Scholar]
- 68.Torroja L, Ortuno-Sahagun D, Ferrus A, Hammerle B, Barbas J A. scully, an essential gene of Drosophila, is homologous to mammalian mitochondrial type II l-3-hydroxyacyl-CoA dehydrogenase/amyloid-beta peptide-binding protein. J Cell Biol. 1998;141:1009–1017. doi: 10.1083/jcb.141.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Verrijzer C P, Chen J L, Yokomori K, Tjian R. Binding of TAFs to core elements directs promoter selectivity by RNA polymerase II. Cell. 1995;81:1115–1125. doi: 10.1016/s0092-8674(05)80016-9. [DOI] [PubMed] [Google Scholar]
- 70.Verrijzer C P, Tjian R. TAFs mediate transcriptional activation and promoter selectivity. Trends Biochem Sci. 1996;21:338–342. [PubMed] [Google Scholar]
- 71.Walker S S, Reese J C, Apone L M, Green M R. Transcription activation in cells lacking TAFIIS. Nature. 1996;383:185–188. doi: 10.1038/383185a0. [DOI] [PubMed] [Google Scholar]
- 72.Walker S S, Shen W C, Reese J C, Apone L M, Green M R. Yeast TAF(II)145 required for transcription of G1/S cyclin genes and regulated by the cellular growth state. Cell. 1997;90:607–614. doi: 10.1016/s0092-8674(00)80522-x. [DOI] [PubMed] [Google Scholar]
- 73.Wang E H, Tjian R. Promoter-selective transcriptional defect in cell cycle mutant ts13 rescued by hTAFII250. Science. 1994;263:811–814. doi: 10.1126/science.8303298. [DOI] [PubMed] [Google Scholar]
- 74.Wickens M, Stephenson P. Role of the conserved AAUAAA sequence: four AAUAAA point mutants prevent messenger RNA 3′ end formation. Science. 1984;226:1045–1051. doi: 10.1126/science.6208611. [DOI] [PubMed] [Google Scholar]
- 75.Wieczorek E, Brand M, Jacq X, Tora L. Function of TAF(II)-containing complex without TBP in transcription by RNA polymerase II. Nature. 1998;393:187–191. doi: 10.1038/30283. [DOI] [PubMed] [Google Scholar]
- 76.Xie X, Kokubo T, Cohen S L, Mirza U A, Hoffmann A, Chait B T, Roeder R G, Nakatani Y, Burley S K. Structural similarity between TAFs and the heterotetrameric core of the histone octamer. Nature. 1996;380:316–322. doi: 10.1038/380316a0. [DOI] [PubMed] [Google Scholar]
- 77.Yamit-Hezi A, Dikstein R. TAFII105 mediates activation of anti-apoptotic genes by NF-kappaB. EMBO J. 1998;17:5161–5169. doi: 10.1093/emboj/17.17.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou J, Zwicker J, Szymanski P, Levine M, Tjian R. TAFII mutations disrupt Dorsal activation in the Drosophila embryo. Proc Natl Acad Sci USA. 1998;95:13483–13488. doi: 10.1073/pnas.95.23.13483. [DOI] [PMC free article] [PubMed] [Google Scholar]