Abstract
Management of a transgender (TG) woman's gender dysphoria is individualized to address the sources of her distress. This typically involves some combination of psychological therapy, hormone modulation, and surgical intervention. Breast enhancement is the most commonly pursued physical modification in this population. Because hormone manipulation provides disappointing results for most TG women, surgical treatment is frequently required to achieve the goal of a feminine chest. Creating a female breast from natal male chest anatomy poses significant challenges; the sexual dimorphism requires a different approach than that used in cisgender breast augmentation. The options and techniques used continue to evolve as experience in this field grows.
Keywords: chest feminization, gender affirmation, transwomen
Introduction
Gender dysphoria is recognized in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) as an incongruence between gender assigned at birth and one's self-identified gender. The absence of the secondary sexual traits associated with gender identity may serve as a significant source of gender dysphoria among some transgender (TG) individuals. As such, incongruent physical appearance can be as great a contributor to dysphoria as being the target of misgendering pronouns. To address this source of anxiety, management of gender dysphoria sometimes includes efforts to realign physical traits with an individual's gender identity.1
Mammary development begins during the early stages of fetal growth with breast-specific progenitor cells detectable as early as 5 weeks of gestation in both male and female embryos, although puberty is the point at which sexual dimorphism arises. Influenced by the hormonal milieu that is characteristic of adolescence, these morphological differences are highlighted, and the development of secondary sexual characteristics becomes apparent.
To produce these changes in TG individuals, hormone replacement therapy (HRT) and sometimes medication to suppress endogenous steroid hormone effects are used, although consistent adoption of consensus guidelines on ideal regimens, dosages, and monitoring is lacking.2–5
When gender dysphoria persists after a prescribed HRT regimen has been used for a minimum time interval (typically 1–2 years), surgical intervention may be appropriate. The gender-affirming procedures performed depend largely on patients' priorities (i.e., the greatest sources of gender dysphoria), although chest operations are the most commonly performed. For the individual transitioning from female to male, this would involve mastectomy. For male to female (MTF) persons, HRT occasionally results in satisfactory breast development. Otherwise, breast reconstruction/augmentation may be pursued. It is the last situation that presents the greatest challenge in chest surgery. In this review, we discuss the full range of options available to MTF patients seeking chest feminization.
Hormone Therapy to Enhance Breast Development
Role of hormone replacement
HRT in TG patients, sometimes referred to as cross-sex hormone therapy, utilizes formulations of human sex hormones, analogs, or inhibitors to induce hormone-responsive tissue changes, namely secondary sexual characteristics, that align with the individual's gender identity. In 2017, the Endocrine Society published guidelines for gender-affirming HRT prescribing, but these amount to more of a generalized framework drawn from current prescribing practices rather than evidence-based recommendations.6 Ultimately, choice of HRT and starting dosages are at the discretion of the prescribing clinician. Physicians should weigh individual risks and benefits, as well as patient goals to select the appropriate regimen. In the past, TG patients have felt compelled to obtain HRT through unconventional and unsanctioned means, such as black-market suppliers, or travel to foreign dispensaries. The primary reason appears to be a lack of acceptance and understanding of gender-affirming HRT and TG patients in general by mainstream medical professionals. This has sowed a simmering level of distrust of the medical profession within the TG community.7 Use of unregulated formulations has further added to the heterogeneity of HRT regimens to include experimental dosages, frequencies, and routes of administration outside the monitoring of a qualified practitioner, raising serious safety concerns.
Estrogen
17β-Estradiol (the primary circulating form of estrogen in humans, often abbreviated as E2) is a critical component of the estrogen signaling pathway that leads to phenotypic changes associated with the adult female. In natal females, ovarian granulosa cells and corpora lutea produce the bulk of E2, although some is also synthesized in adrenal glands and adipose tissue. In natal males, the lower levels of E2 come primarily from Leydig cells in the testis. As one of the several steps within the steroid hormone production pathway beginning with cholesterol, aromatase catalyzes the conversion of testosterone to E2.
E2-induced gene transcription manifests phenotypically in various ways. Breast tissue, for example, responds to E2 with increased breast duct development, fat deposition, and stromal connective tissue growth.8 Although embryonic breast buds undergo rudimentary differentiation from the ectoderm during gestation, a more significant phase of development begins at puberty under the influence of various hormones, most notably estrogen. In MTF individuals, E2 therapy has a dual effect on secondary sexual characteristics: it partially suppresses unwanted male sexual characteristics while promoting female ones. Furthermore, E2 therapy is associated with gynoid fat redistribution resulting in increased fat deposition in the hips, breasts, and thighs.2–4
Exogenous estrogen as HRT can be administered in a number of ways. Oral formulations are used most commonly and, through first pass circulation, lead to increased sex hormone-binding globulin (SHBG) levels that lower the bioavailability of testosterone. This is a potential benefit for MTF patients whose endogenous testosterone antagonizes some of the effects of E2. Skin preparations include patches, sprays, gels, and creams that have to be used continuously to achieve desired levels. Periodic (every 1–2 weeks) injection of a lipid emulsion containing E2 (e.g., estradiol valerate) provides a third route of administration, although higher peak concentrations may lead to undesirable side effects.5 Sublingual formulations and implanted subcutaneous drug-eluding pellets have not been thoroughly studied and so data on safety and efficacy are lacking.9
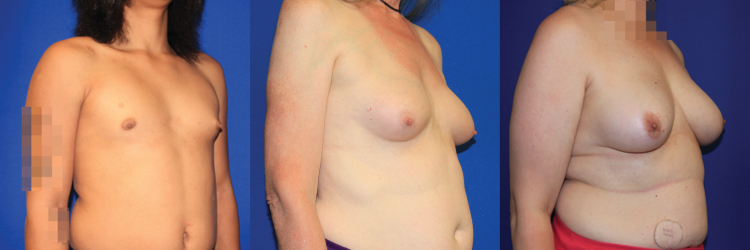
The effect of E2 on breast growth is highly variable among TG patients (Fig. 1). Although only a small percentage experience the equivalent of complete adult breast development after an average of 2 years of hormone therapy, the rest have subjectively disappointing results and turn to alternative methods of breast augmentation. In general, breast buds become palpable under the areola after 3–6 months of HRT, and growth plateaus within 2–3 years of initiation.10 Although aggressive regimens that utilize higher doses of E2 early in the therapeutic course may induce rapid growth initially, they carry the risk of premature termination in ductal branching leading to an arrest in growth at Tanner stage III or IV.11 Even with physician supervision and modern hormone therapy regimens that implement gradual dose escalation, MTF patients are unlikely to reach Tanner stage V breast development.12,13 Reviewing conflicting literature on the matter, the conclusion by de Blok et al. seems to most accurately describe the effect of E2 dosage on breast size: the two are independent. This is supported by the fact that reported data vary widely and fail to demonstrate a correlation between expected breast growth and HRT alone.3,14 One theory to explain this observation is that both genetic and epigenetic factors influence hormone responsiveness in breast tissue,15,16 but evidence also suggests that the variable effects of E2 on endogenous testosterone suppression and SHBG concentration is a confounding component of HRT.17
FIG. 1.
Transgender women experience variable degrees of breast development when taking hormone replacement therapy.
de Blok et al. published their observations after following 224 patients with sequential breast measurements during 1 year of E2 HRT.14 In this cohort they found that the majority of breast development took place within the first 6 months of HRT and all but ceased in the final 6 months of estrogen therapy. The authors calculated each patient's breast cup size and found that less than half achieved the smallest cup size (AAA) and merely 3.6% achieved greater than an A cup size. Similar studies have found varying degrees of breast development on E2 HRT, but these data underscores the variability in patient responses to hormone therapy and breast growth.
The duration of E2 therapy necessary to induce maximal breast development has not been well established. A 1986 study of the longitudinal effects of E2 administration in conjunction with anti-androgens showed significant breast growth by 2 years of continuous therapy.18 Wierckx et al. further corroborated this with another MTF cohort, concluding that maximal growth occurs by the end of 2 years of E2 HRT.19 A few other studies have shown that growth may continue for up to 3 years of continuous therapy, which aligns more with the timeframe that breast growth is observed in peripubertal cisgender females.3,18,20,21
Progestins
With the goal of improved breast growth, progestins are also sometimes supplemented in MTF individuals. Although almost exclusively studied in conjunction with estrogen in postmenopausal cisgender women, the combination of E2 and progesterone has fallen out of favor in that group because of the increased risk of developing breast cancer, although there are demonstrable benefits for coronary health.22 Subsequent studies have shown that progestin use alone (without estrogen) does not increase breast cancer risk.23
The use of progestins in MTF patients for breast development is controversial. Because of the role they play in mammary development at a cellular level, some practitioners believe that progestins play a necessary role in achieving full breast development, although some studies have shown this not to be the case.24 Further deterring from progestin use are the several described adverse effects, including depression, lipid metabolism changes, and weight gain, to name a few.25
Androgen suppression
In contrast to E2, androgens have an inhibitory effect on breast development.26 E2 HRT leads to variable amounts of endogenous testosterone suppression and so chemical means of further lowering androgen production in MTF patients have been investigated. Drugs such as spironolactone are often prescribed as testosterone suppressants in an attempt to block the virilizing effects of circulating androgens, although some studies suggest that commonly used agents do not have significant effects on E2 levels.17 The proposed mechanisms of action involve blockage of androgen receptor binding and direct suppression of testosterone secretion. Potential side effects include low blood pressure and hyperkalemia. 5-Alpha reductase inhibitors (finasteride and dutasteride) are antiandrogens used as treatments for baldness that work by blocking the conversion of testosterone to its more active form, 5-alpha-dihydrotestosterone.
Suppression of gonadal hormone production can be achieved with gonadotropin-releasing hormone (GnRH) agonists such as leuprolide and goserelin. These agents block GnRH and thus the release of luteinizing hormone and follicle-stimulating hormone. Although highly effective at blocking the endogenous production of testosterone, cost limits the widespread use of GnRH analogs.
Orchiectomy provides the most reliable method of reducing endogenous testosterone production to the level of cisgender females.2,27 The overpowering effects of androgens are exemplified by the suppression of secondary sexual development in cisgender females with adrenal tumors.28 Removing the influence of testosterone, particularly in younger natal male patients, arrests the development of other secondary sexual changes such as masculinization of the voice, facial and body hair growth, android fat distribution, and muscle development.29
Surgical Breast Enhancement
The most recent edition (Vol. 7) of the World Professional Association for Transgender Health (WPATH) Standards of Care outlines criteria for performing gender-affirming breast enhancement.30 For this undertaking to be considered medically necessary, patients must demonstrate persistent, well-documented gender dysphoria, including assessment by an appropriately trained mental health professional. Presurgical hormone therapy is not an explicit prerequisite, although it is recommended that HRT be used for at least 12 months to help overall results of chest feminization. Although studies show improved body satisfaction after initiating hormone therapy, up to 70% of patients will seek surgical breast augmentation after using HRT.31–33 There is strong evidence that combined medical and surgical therapy for breast enhancement improves the management of gender dysphoria.34 Despite WPATH guidelines and evidence of benefit, public and private medical insurance in the United States does not consistently cover feminizing chest procedures.30,35,36
Implant-based augmentation
Given the modest breast development experienced by most MTF patients using HRT, it is not surprising that many seek out surgical augmentation to achieve their goals. Although patients are clearly happier to have some breast growth after using HRT, their breasts are often still diminutive and unnatural appearing. In fact, surveys of MTF patients have shown that while HRT alone contributes to improved body image, those who underwent surgical enhancement of their breasts reported significantly better body satisfaction scores31 and experienced an increased quality of life.37,38
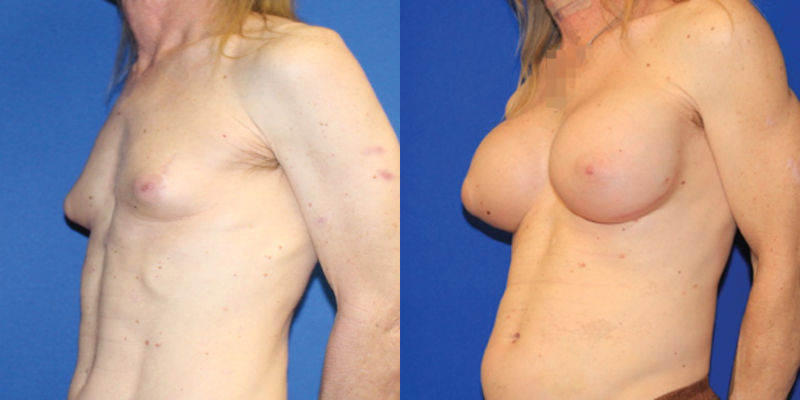
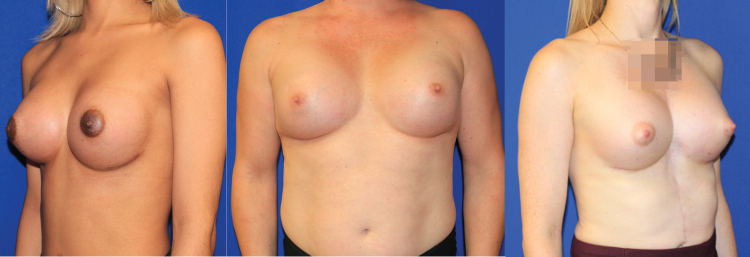
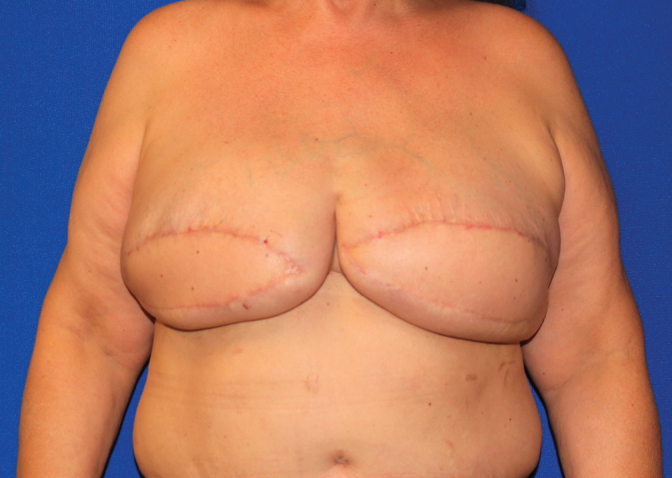
There are no definitive published recommendations on how best to approach breast augmentation in MTF patients and much of what is available in the literature are single-surgeon reports with small cohorts. From the literature it is evident that a significant majority of MTF individuals desire or elect to pursue some form of surgical intervention, with breast implant augmentation rates up to 67% in some studies.37 One of the earliest reviews is by Kanhai et al., who reported 201 MTF patients treated between 1979 and 1997 with implant-based breast augmentation following a minimum of 1.5 years of HRT.39 Of note, average implant size used in gender-affirming breast augmentation increased over the span of this study and has trended upward in subsequent studies.34 On occasion, when the breast skin envelope is especially deficient, use of a two-stage approach starting with placement of subpectoral or prepectoral tissue expanders may be helpful to prepare a more voluminous pocket while establishing a new inframammary fold (IMF) (Fig. 2).
FIG. 2.
This thin patient had inadequate envelope to achieve her desired breast volume with an implant alone. She opted for a staged approach, here shown before and after a tissue expander was placed under the breast skin and fully inflated with saline. At a second stage, the permanent implant will replace the expander.
Anatomic considerations
As in cisgender female breast augmentation, there is some variability in surgical technique among plastic surgeons approaching chest feminization. Scar position (periareolar, IMF, axillary, or periumbilical) and plane of implant placement (submuscular, subglandular, or dual plane) are a few examples. Sexual dimorphism has some bearing on the different approaches to the natal male and female chest. Miller et al. advocated the use of an IMF incision with a submuscular pocket, citing the need for upper-pole shaping by the pectoralis major muscle given the paucity of overlying glandular tissue and to avoid overfilling of the superior pole.34 The authors add that patients infrequently possessed enough subcutaneous tissue in the superior and inferior breast hemispheres (often referred to in plastic surgery literature as the upper and lower “poles”) to adequately support an implant. This is contrasted by our experience and that of others who feel that a subglandular approach better fills the breast without the tightness of the pectoralis major muscle to displace the implant too far laterally or inferiorly.37
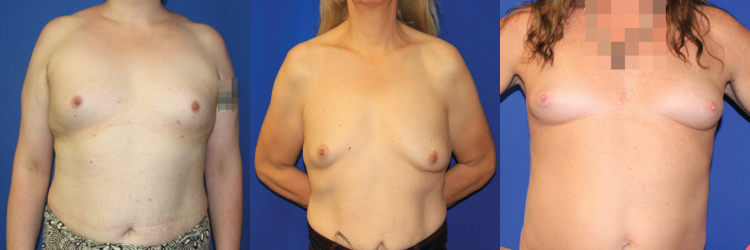
Nipple–areola complex (NAC) position in relation to the breast mound is another consideration. In a natal male, the NAC is naturally more ovoid and lateral, whereas a feminine breast has its rounder, larger NAC centrally placed over the point of maximal projection.40,41 Centering the implant under the NAC in an MTF patient can be problematic, however. If the NACs are too far apart, the patient will have a space between the breasts greater than desired (Fig. 3). Many patients express the desire to have “cleavage” and to have their breasts touch one another. Although this is not always achievable, a compromise is sometimes necessary to address the wish for more medially positioned breasts while avoiding excessively lateral NACs on the breast mound, as demonstrated in Figure 4. In younger patients who have initiated hormonal modulation or in those who naturally have gender-neutral chest anatomy, a more ideal result can be achieved with implants. Repositioning the NACs is difficult, even with crescentic mastopexies, and often results in significant scarring without much NAC movement.42
FIG. 3.
The typical adult natal male chest has nipple–areola complexes that are far apart, with a paucity of upper pole skin and high, flat inframammary folds.
FIG. 4.
There is sometimes a need to compromise between centering the nipple–areola complexes over the implants and avoiding too much space between the breasts.
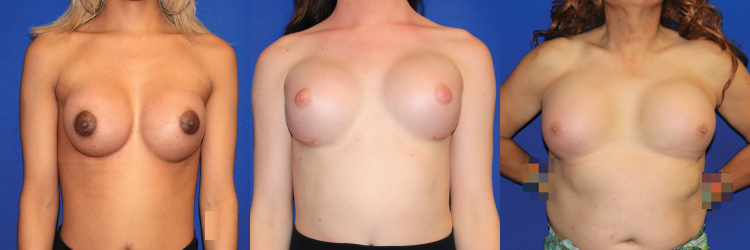
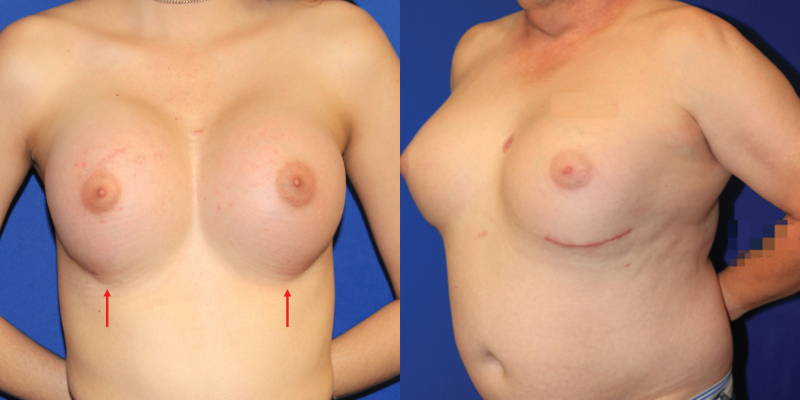
Another anatomic consideration in gender-affirming breast reconstruction is the position and contour of the IMF. The point of attachment between the chest wall and a natal male's breast skin is less well defined than in a female breast, although this IMF is often apparent in overweight natal male patients. When augmenting the cisgender female breast, the IMF is respected and kept intact to preserve breast shape, allowing the lower pole skin to stretch without disrupting this important landmark. In TG women, the IMF is typically either ill-defined or too high and flat to allow for a satisfactory result.34 Releasing the natural attachments sometimes results in an effaced, poorly defined IMF (Fig. 5). Some authors advocate for immediate IMF reconstruction by suturing the dermis to the chest wall or using matrices (acellular biologics or prosthetic mesh) to reinforce the fold, thus maintaining implant position and providing an overall more ideal breast shape (Fig. 6).43–45
FIG. 5.
Transgender female patients shown with effaced inframammary folds (arrows); a nonideal result that may require use of sutures or mesh to restore definition.
FIG. 6.
Three transgender women with implant-based breast augmentation are shown. The ideal feminine inframammary fold is curved and low enough to allow for adequate lower pole shape and volume.
Fat grafting and fillers
Autologous fat transfer has been used for over 100 years in both reconstructive and cosmetic surgical practice, although currently used techniques and instruments were largely developed during the late twentieth century.46 In all kinds of breast surgery, fat grafts are often used to affect breast contour. Fat grafts have also been used in both cisgender and TG women to enhance breast volume, although the amount that can be transferred in one operation is limited by the nature of grafted tissue: all grafts need to be in contact with vascularized tissues to establish a blood supply and survive (at best, only ∼50–60% of transferred fat survives).47 As a result, this procedure often requires multiple attempts to achieve noticeable volume enhancement.48 Surgeons may utilize this option to supplement the medial and superior portions of the breast but the tremendous time and effort to achieve goal size and shape make this approach to primary augmentation less practical for most MTF patients.34 Fat grafting also carries some risk. If inadvertently injected intravascularly, fat can embolize and cause cardiovascular collapse, multiorgan failure, or even death. Fat that does not survive (i.e., “fat necrosis”) may lead to hard lumps and/or oil cysts in the breasts. Calcified deposits of fat necrosis may also be confused for suspicious calcifications associated with breast malignancy on imaging studies, although some authors dispute this concern.49
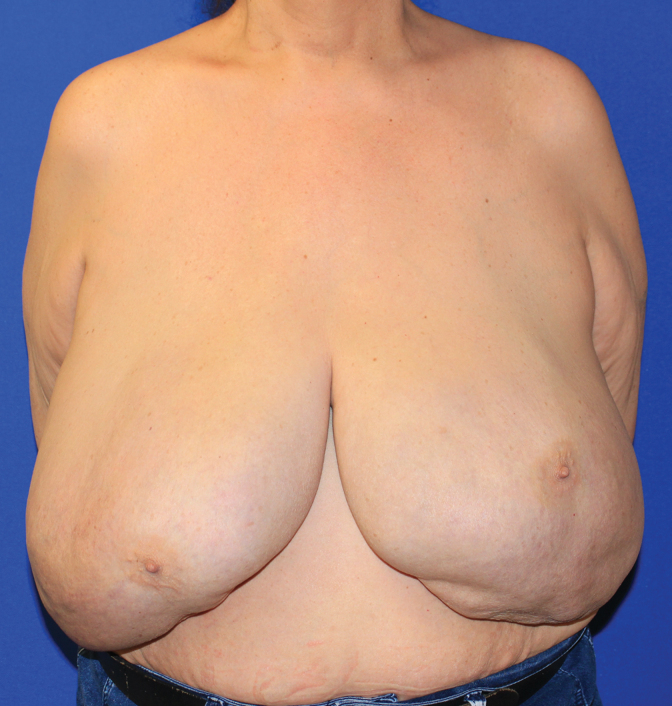
Inorganic fillers, such as injected silicone, are not approved for primary breast augmentation in the United States, although they have served as an inexpensive alternative approach to breast augmentation employed by unscrupulous practitioners. A range of nonmedical grade fillers used include mixtures of caulking, oil, and commercial silicone. For marginalized patients without economic means, desperation has led too many TG women to seek such injections.50,51 Complications such as infection, nonhealing wounds, pain, deformity, and even embolism are possible, and can be life threatening.52–55 Chronic breast granulomas are frequently seen after silicone injection and may require mastectomy if infection or extrusion occurs, posing significant reconstructive challenges35 (Fig. 7).
FIG. 7.
Granulomas of the breast may result from injection of materials such as silicone. The patient shown had injections performed decades before and now is seeking breast reduction.
Autologous tissue augmentation
Although breast implants improve quality of life for a significant number of MTF patients, there are limitations and sometimes disappointing results for a variety of reasons. Larger, more broad-chested patients require a greater volume to achieve breast sizes that are proportional to their body frames. Commercially available implants often do not have the volume or dimensions needed to help patients achieve their goals. An alternative to implant-based reconstruction frequently offered to cisgender women utilizes autologous tissue “flaps.” Flap-based breast reconstruction requires greater surgical technical expertise, prolonged time under anesthesia, a sufficient donor site, and lengthier recovery, but for selected TG women, this may be the most ideal solution.
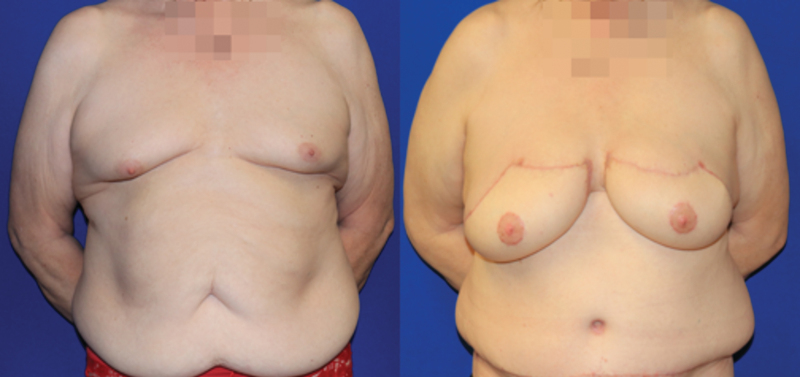
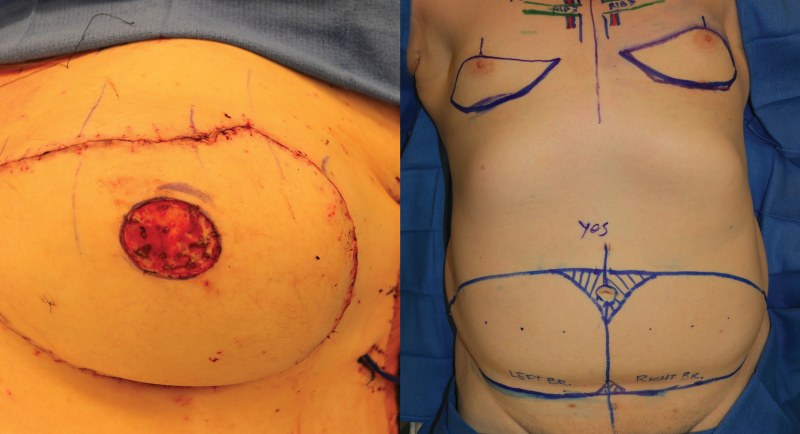
Virtually all the countless publications on flap tissue donor sites, technique, and patient-reported outcomes focus on cisgender women. At the time of writing this review, there is only one case report of autologous breast reconstruction in a TG woman, performed as a salvage procedure after treatment of silicone-injection granulomatosis of the breast.35 In this report, abdominal flaps were used, a muscle-sparing transverse rectus abdominis myocutaneous (TRAM) flap on one side and a deep inferior epigastric perforator (DIEP) flap on the other. As of this publication, our group performed the first reported bilateral DIEP flap primary breast augmentation procedure in a TG woman (Fig. 8). The pedicle of the tissue flap, the deep inferior epigastric vessels, can be anastomosed to either the internal mammary or thoracodorsal vessels, as in cisgender breast reconstruction.
FIG. 8.
The first reported primary bilateral gender-affirming breast reconstruction using deep inferior epigastric perforator flaps (before and after).
This approach has the advantage of augmenting not just breast volume but also breast skin. The envelope of both upper and lower poles is typically tight in the natal male. Unlike most cisgender women having breast reconstruction after mastectomy, the TG woman often lacks sufficient skin and volume. Fortunately, the free tissue transferred to the chest brings both. As a result, the shape of both poles can be controlled much more precisely than with implants. The IMF position and shape can also be customized to create the desired breast shape (Fig. 9).
FIG. 9.
Autologous flaps allow for more control over breast shape, including the inframammary fold position and upper pole skin envelope.
Android and gynoid fat distribution differ in ways that affect flap tissue donor sites as well.56 Truncal fat in the MTF patient is less central and more widely distributed across the lower abdomen with a straighter waistline and narrower relative circumference at the hips. Removal of central abdominal tissue may leave the abdominal flanks looking fuller with the unintended consequence of accentuating a lack of feminine hip contour. As a result, the traditional DIEP or TRAM flap suffers from a less ideal donor site in the natal male. Other tissue donor sites include the upper inner thighs (the transverse upper gracilis flap), the buttocks (the superior gluteal artery perforator flap), and the flanks (the lumbar artery perforator flap). These flaps were all designed to exploit typical gynoid fat distribution and are similarly less ideal for the natal male body habitus, although not absolutely contraindicated. Patients considering autologous tissue transfer should be assessed at each of these sites to identify the ideal donor tissue.
Another option to be considered and which has been used in MTF breast reconstruction is the latissimus dorsi myocutaneous (LDMC) flap. As in cisgender breast and chest wall reconstruction, a deficit of skin on the anterior chest can be enhanced with unilateral or bilateral LDMC flaps. In breast reconstruction and augmentation, this typically requires use of an implant to provide volume while the flap primarily enhances the breast envelope (Fig. 10). This approach is most ideal for thinner patients with inadequate abdominal donor tissue or a history of abdominal surgery that makes the abdominal tissue unusable.
FIG. 10.
Latissimus dorsi myocutaneous flaps, typically with implants, provide another autologous alternative when skin envelope is inadequate.
The advantages of autologous reconstruction are several fold. Avoiding the need for implant surveillance and periodic implant replacements as well as the risks associated with implants such as rupture, migration, infection, capsular contracture, and implant-associated syndromes, are just a few of the upsides.57 Some authors advocate prophylactically exchanging implants every 10 years, although the vast majority of implants are intact a decade after implantation.58 The flexibility that the autologous approach affords in creating a natural-appearing breast is significant. In the senior author's approach, however, autologous reconstruction requires grafting of the NAC, invariably resulting in loss of NAC sensation. The length of surgery and potential for donor site complications are also disadvantages of the flap approach to breast reconstruction.
As indicated by the lack of literature, primary use of autologous tissue in gender-affirming chest feminization is largely untested. It is by no means clear if public or private medical insurers in the United States will consistently pay for the autologous option. However, patient demand for these procedures may very well rise owing to recent concerns surrounding implant-related illnesses and breast implant-associated anaplastic large cell lymphoma, coupled with rising societal acceptance of gender-affirming procedures that has driven a steady increase in the number of breast augmentations performed for gender dysphoria annually.59
Breast cancer surveillance
There is currently no widely accepted approach to breast cancer screening in the TG population, although breast cancer certainly occurs in both TG men and women, with or without a history of exogenous hormone therapy.60 Some reviews of the literature addressing this topic focus on risk calculations, considering factors such as age, family history, and duration of hormone therapy to determine the initiation and frequency of breast imaging.36,60–62 de Blok et al. reported on the elevated incidence of breast cancer among 2260 Dutch TG women on HRT compared with cisgender men (although not as high as age-matched cisgender women).63 Our group recommends counseling TG women taking HRT on the need for lifelong cancer surveillance and follows the same risk-adjusted guidelines given to cisgender women.
Future Directions
Owing to the risks involved with autologous tissue flap or implant-based procedures, including but not limited to flap failure, donor site morbidity, infection, or capsular contracture, new approaches to breast reconstruction will inevitably arise.64,65 In this regard, tissue engineering and regenerative medicine technology hold promise for mitigating these concerns.
Current tissue engineering techniques focus on using biological or synthetic scaffolds to support the growth of new adipose tissue. Although autologous fat grafting has its advantages, its efficacy is limited by resorption and potential for volume loss over extended periods of time, underscoring the importance of developing durable scaffolds.66 Biologic and synthetic scaffolds, composed of collagen or decellularized tissue matrix and thermoplastic polymers, respectively, provide structural support until the transferred soft tissue can regenerate and support itself.67–70 With currently available materials and tissue engineering techniques, synthetic scaffolds are favored over biologic ones because of more reliable structural support.71 These synthetic scaffolds typically employ either a hydrogel filler or a solid structural component that degrades as the tissue matures.69,71 Although these scaffold composites have shown promise, they are limited in their ability to permit revascularization of larger tissue volumes.72 The advancement of interdisciplinary research in nanobiotechnology, materials science, biochemistry, and bioengineering will help facilitate the use of graft-compatible scaffolds in clinical applications.
Previous studies have investigated the utility of mesenchymal stem cells in promoting adipose regeneration and retention. An example is the discovery that adipose-derived stem cells in autologous lipoaspirate implanted into a scaffold significantly increased the volume of viable adipose tissue.73 However, this technique is not without its risks, as stem cells may have a detrimental effect by stimulating breast cancer recurrence in mastectomy patients.74
Experimental tissue models utilizing induced pluripotent stem cells (iPSCs) and three-dimensional (3D) organoid cultures have demonstrated their usefulness in understanding normal tissue and disease development but might also be employed as breast reconstruction tools in the future, allowing for the in vitro growth, expansion, and transplantation of patient-derived tissues. Human iPSCs (hiPSCs) have the ability to differentiate into multiple cell types, including neurons, hepatocytes, and adipocytes.75–77 Mammary-like organoids expressing common breast, luminal, and basal markers, have been generated from hiPSCs, revealing that hiPSCs can be directed toward mammary lineage differentiation in vitro.78 Primary mammary epithelial cells from patient-derived tissue have also been shown to grow into complex breast tissues.79 Once mature, these tissues, grown in vitro in a 3D hydrogel scaffold, exhibited ductal and lobular morphologies similar to that of the human breast and were responsive to hormones.79 One daunting obstacle is to differentiate iPSCs into breast epithelial, fat, and other cell types and direct them to grow into scaffold-supported breast tissue structures.
The advantage of utilizing hiPSC and 3D primary culture models is the ability for personalized modeling. It is worth mentioning that mammary tissue grown from these methods will not display high immunogenicity, a property that has been a disadvantage of biological scaffolds. One group has shown that a combination of gel breast implant, acellular dermal scaffold, and regenerative cells within processed fat can be successfully utilized in breast reconstruction.80 In the future, it is possible that breast augmentation and reconstruction procedures will employ mammary tissue or fat grown from hiPSCs or in primary culture ex vivo, thereby eliminating the risks associated with prosthetic implants.
Conclusions
Gender-affirming breast feminization typically involves a combination of hormonal manipulation and surgical management, although no one approach works for all patients. Peer-reviewed data support breast enhancement as one part of an overall strategy to manage gender dysphoria and improve the quality of life for many TG women. Because much of our experience in breast surgery comes from treatment of cisgender women, more and larger studies are needed to better understand the optimal approach to the TG patient.
Abbreviations Used
- 3D
three-dimensional
- DIEP
deep inferior epigastric perforator
- GnRH
gonadotropin-releasing hormone
- hiPSC
human iPSC
- HRT
hormone replacement therapy
- IMF
inframammary fold
- iPSC
induced pluripotent stem cell
- LDMC
latissimus dorsi myocutaneous
- MTF
male to female
- NAC
nipple–areola complex
- SHBG
sex hormone-binding globulin
- TG
transgender
- TRAM
transverse rectus abdominis myocutaneous
- WPATH
World Professional Association for Transgender Health
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Xiaojiang Cui was supported by the National Institutes of Health (Grant No. 2R01CA151610), Department of Defense (Grant No. W81XWH-18-1-0067), Samuel Oschin Cancer Institute Discovery Fund Award and Community Outreach and Engagement Developmental Fund Award, and the Margie and Robert E. Petersen Foundation. No other funding sources to report.
Cite this article as: Patel H, Samaha Y, Ives G, Lee T-Y, Cui X, Ray E (2021) Chest feminization in male-to-female transgender patients: a review of options, Transgender Health 6:5, 244–255, DOI: 10.1089/trgh.2020.0057.
References
- 1. Beckwith N, Reisner SL, Zaslow S, et al. Factors associated with gender-affirming surgery and age of hormone therapy initiation among transgender adults. Transgend Health. 2017;2:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tangpricha V, Den Heijer M. Oestrogen and anti-androgen therapy for transgender women. Lancet Diabetes Endocrinol. 2017;5:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hembree WC, Cohen-Kettenis P, Delemarre-Van De Waal HA, et al. Endocrine treatment of transsexual persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2009;94:3132–3154. [DOI] [PubMed] [Google Scholar]
- 4. Fabris B, Bernardi S, Trombetta C. Cross-sex hormone therapy for gender dysphoria. J Endocrinol Invest. 2015;38:269–282. [DOI] [PubMed] [Google Scholar]
- 5. Gooren LJ, Giltay EJ, Bunck MC. Long-term treatment of transsexuals with cross-sex hormones: extensive personal experience. J Clin Endocrinol Metab. 2008;93:19–25. [DOI] [PubMed] [Google Scholar]
- 6. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:3869–3903. [DOI] [PubMed] [Google Scholar]
- 7. Poteat T, Wirtz AL, Radix A, et al. HIV risk and preventive interventions in transgender women sex workers. Lancet. 2015;385:274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arendt LM, Kuperwasser C. Form and function: how estrogen and progesterone regulate the mammary epithelial hierarchy. J Mammary Gland Biol Neoplasia. 2015;20:9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iwamoto SJ, Defreyne J, Rothman MS, et al. Health considerations for transgender women and remaining unknowns: a narrative review. Ther Adv Endocrinol Metab. 2019;10;30:10.:2042018819871166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sonnenblick EB, Shah AD, Goldstein Z, Reisman T. Breast imaging of transgender individuals: a review. Curr Radiol Rep. 2018;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seal LJ. A review of the physical and metabolic effects of cross-sex hormonal therapy in the treatment of gender dysphoria. Ann Clin Biochem. 2016;53:10–20. [DOI] [PubMed] [Google Scholar]
- 12. T'Sjoen G, Arcelus J, Gooren L, et al. Endocrinology of transgender medicine. Endocr Rev. 2019;40:97–117. [DOI] [PubMed] [Google Scholar]
- 13. Maycock LB, Kennedy HP. Breast care in the transgender individual. J Midwifery Womens Health. 2014;59:74–81. [DOI] [PubMed] [Google Scholar]
- 14. de Blok CJM, Klaver M, Wiepjes CM, et al. Breast development in transwomen after 1 year of cross-sex hormone therapy: results of a prospective multicenter study. J Clin Endocrinol Metab. 2018;103:532–538. [DOI] [PubMed] [Google Scholar]
- 15. Wade TD, Zhu G, Martin NG. Body mass index and breast size in women: same or different genes? Twin Res Hum Genet. 2010;13:450–454. [DOI] [PubMed] [Google Scholar]
- 16. Li J, Foo JN, Schoof N, et al. Large-scale genotyping identifies a new locus at 22q13.2 associated with female breast size. J Med Genet. 2013;50:666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leinung MC, Feustel PJ, Joseph J. Hormonal treatment of transgender women with oral estradiol. Transgend Health. 2018;3:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meyer WJ, 3rd, Webb A, Stuart CA, et al. Physical and hormonal evaluation of transsexual patients: a longitudinal study. Arch Sex Behav. 1986;15:121–138. [DOI] [PubMed] [Google Scholar]
- 19. Wierckx K, Van Caenegem E, Schreiner T, et al. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: results from the European network for the investigation of gender incongruence. J Sex Med. 2014;11:1999–2011. [DOI] [PubMed] [Google Scholar]
- 20. Klaver M DM, Schreiner T, Fishcer AD, T'Sjoen G, den Heijer. Cross-sex hormone therapy and the effects on fat distribution in transgender persons. Program of the 24th WPATH Symposium Amsterdam, The Netherlands, 2016. [Google Scholar]
- 21. Bannink EM, van Sassen C, van Buuren S, et al. Puberty induction in Turner syndrome: results of oestrogen treatment on development of secondary sexual characteristics, uterine dimensions and serum hormone levels. Clin Endocrinol (Oxf). 2009;70:265–273. [DOI] [PubMed] [Google Scholar]
- 22. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. [DOI] [PubMed] [Google Scholar]
- 23. Vaz-Luis I, Partridge AH. Exogenous reproductive hormone use in breast cancer survivors and previvors. Nat Rev Clin Oncol. 2018;15:249–261. [DOI] [PubMed] [Google Scholar]
- 24. Oriel KA. Clincial update: medical care of transsexual patients. J Gay Lesbian Med Assoc. 2000;4:185–194. [Google Scholar]
- 25. Tangpricha V, Ducharme SH, Barber TW, Chipkin SR. Endocrinologic treatment of gender identity disorders. Endocr Pract. 2003;9:12–21. [DOI] [PubMed] [Google Scholar]
- 26. Dimitrakakis C, Bondy C. Androgens and the breast. Breast Cancer Res. 2009;11:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prior JC, Vigna YM, Watson D. Spironolactone with physiological female steroids for presurgical therapy of male-to-female transsexualism. Arch Sex Behav. 1989;18:49–57. [DOI] [PubMed] [Google Scholar]
- 28. Forsbach G, Guitron-Cantu A, Vazquez-Lara J, et al. Virilizing adrenal adenoma and primary amenorrhea in a girl with adrenal hyperplasia. Arch Gynecol Obstet. 2000;263:134–136. [DOI] [PubMed] [Google Scholar]
- 29. Melmed S, Polonsky K, Reed Larsen P, Kronenberg H. Williams Textbook of Endocrinology. Elsevier Health Sciences. [Google Scholar]
- 30. Coleman E BW, Botzer M, et al. Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. Int J Transgend. 2012;7:165–232. [Google Scholar]
- 31. van de Grift TC, Elaut E, Cerwenka SC, et al. Effects of medical interventions on gender dysphoria and body image: a follow-up study. Psychosom Med. 2017;79:815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wierckx K, Gooren L, T'Sjoen G. Clinical review: breast development in trans women receiving cross-sex hormones. J Sex Med. 2014;11:1240–1247. [DOI] [PubMed] [Google Scholar]
- 33. Seal LJ, Franklin S, Richards C, et al. Predictive markers for mammoplasty and a comparison of side effect profiles in transwomen taking various hormonal regimens. J Clin Endocrinol Metab. 2012;97:4422–4428. [DOI] [PubMed] [Google Scholar]
- 34. Miller TWS, Massie J, Morrison S, Satterwhite T. Breast augmentation in male-to-female transgender patients: technical considerations and outcomes. JPRAS Open. 2019;21:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morrison SD, Massie JP, Kneib CJ, et al. The use of autologous tissue for chest feminization in gender-affirming surgery. Plast Reconstr Surg. 2020;145:228e–229e. [DOI] [PubMed] [Google Scholar]
- 36. Van Boerum MS, Salibian AA, Bluebond-Langner R, Agarwal C. Chest and facial surgery for the transgender patient. Transl Androl Urol. 2019;8:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kanhai RC, Hage JJ, Asscheman H, Mulder JW. Augmentation mammaplasty in male-to-female transsexuals. Plast Reconstr Surg. 1999;104:542–549; discussion 550–541. [DOI] [PubMed] [Google Scholar]
- 38. Weigert R, Frison E, Sessiecq Q, et al. Patient satisfaction with breasts and psychosocial, sexual, and physical well-being after breast augmentation in male-to-female transsexuals. Plast Reconstr Surg. 2013;132:1421–1429. [DOI] [PubMed] [Google Scholar]
- 39. Kanhai RC, Hage JJ, Karim RB. Augmentation mammaplasty in male-to-female trans-sexuals: facts and figures from Amsterdam. Scand J Plast Reconstr Surg Hand Surg. 2001;35:203–206. [DOI] [PubMed] [Google Scholar]
- 40. Beckenstein MS, Windle BH, Stroup RT Jr. Anatomical parameters for nipple position and areolar diameter in males. Ann Plast Surg. 1996;36:33–36. [DOI] [PubMed] [Google Scholar]
- 41. Shulman O, Badani E, Wolf Y, Hauben DJ. Appropriate location of the nipple-areola complex in males. Plast Reconstr Surg. 2001;108:348–351. [DOI] [PubMed] [Google Scholar]
- 42. Choi M, Frey JD, Salibian AA, Karp NS. Nipple-areola complex malposition in nipple-sparing mastectomy: a review of risk factors and corrective techniques from greater than 1000 reconstructions. Plast Reconstr Surg. 2017;140:247e–257e. [DOI] [PubMed] [Google Scholar]
- 43. Roh DS, Treiser MD, Lafleur EH, Chun YS. Technique to promote symmetry in 2-staged bilateral breast reconstruction in the setting of unilateral postmastectomy radiation. Ann Plast Surg. 2017;78:386–391. [DOI] [PubMed] [Google Scholar]
- 44. Cook LJ, Kovacs T. Novel devices for implant-based breast reconstruction: is the use of meshes to support the lower pole justified in terms of benefits? A review of the evidence. Ecancermedicalscience. 2018;12:796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Margulies IG, Salzberg CA. The use of acellular dermal matrix in breast reconstruction: evolution of techniques over 2 decades. Gland Surg. 2019;8:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bellini E, Grieco MP, Raposio E. The science behind autologous fat grafting. Ann Med Surg (Lond). 2017;24:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Parrish JN, Metzinger SE. Autogenous fat grafting and breast augmentation: a review of the literature. Aesthet Surg J. 2010;30:549–556. [DOI] [PubMed] [Google Scholar]
- 48. Morrison SD, Wilson SC, Mosser SW. Breast and body contouring for transgender and gender nonconforming individuals. Clin Plast Surg. 2018;45:333–342. [DOI] [PubMed] [Google Scholar]
- 49. Chopan M, White JA, Sayadi LR, et al. Autogenous fat grafting to the breast and gluteal regions: safety profile including risks and complications. Plast Reconstr Surg. 2019;143:1625–1632. [DOI] [PubMed] [Google Scholar]
- 50. Dangol GMS, Negrete H. Silicone-induced granulomatous reaction causing severe hypercalcemia: case report and literature review. Case Rep Nephrol. 2019;2019:9126172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wallace PM. Finding self: a qualitative study of transgender, transitioning, and adulterated silicone. Health Educ J. 2010;69:439–446. [Google Scholar]
- 52. Bigata X, Ribera M, Bielsa I, Ferrandiz C. Adverse granulomatous reaction after cosmetic dermal silicone injection. Dermatol Surg. 2001;27:198–200. [DOI] [PubMed] [Google Scholar]
- 53. Fox LP, Geyer AS, Husain S, et al. Mycobacterium abscessus cellulitis and multifocal abscesses of the breasts in a transsexual from illicit intramammary injections of silicone. J Am Acad Dermatol. 2004;50:450–454. [DOI] [PubMed] [Google Scholar]
- 54. Gaber Y. Secondary lymphoedema of the lower leg as an unusual side-effect of a liquid silicone injection in the hips and buttocks. Dermatology. 2004;208:342–344. [DOI] [PubMed] [Google Scholar]
- 55. Hage JJ, Kanhai RC, Oen AL, et al. The devastating outcome of massive subcutaneous injection of highly viscous fluids in male-to-female transsexuals. Plast Reconstr Surg. 2001;107:734–741. [DOI] [PubMed] [Google Scholar]
- 56. Nauta AC, Baltrusch KM, Heston AL, et al. Differences in chest measurements between the cis-female and trans-female chest exposed to estrogen and its implications for breast augmentation. Plast Reconstr Surg Global Open. 2019;7:e2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fischer JP, Nelson JA, Au A, et al. Complications and morbidity following breast reconstruction—a review of 16,063 cases from the 2005–2010 NSQIP datasets. J Plast Surg Hand Surg. 2014;48:104–114. [DOI] [PubMed] [Google Scholar]
- 58. Hillard C, Fowler JD, Barta R, Cunningham B. Silicone breast implant rupture: a review. Gland Surg. 2017;6:163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lane M, Ives GC, Sluiter EC, et al. Trends in gender-affirming surgery in insured patients in the United States. Plast Reconstr Surg Global Open. 2018;6:e1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hartley RL, Stone JP, Temple-Oberle C. Breast cancer in transgender patients: a systematic review. Part 1: male to female. Eur J Surg Oncol. 2018;44:1455–1462. [DOI] [PubMed] [Google Scholar]
- 61. Deutsch MB, Radix A, Wesp L. Breast cancer screening, management, and a review of case study literature in transgender populations. Semin Reprod Med. 2017;35:434–441. [DOI] [PubMed] [Google Scholar]
- 62. Narayan A, Lebron-Zapata L, Morris E. Breast cancer screening in transgender patients: findings from the 2014 BRFSS survey. Breast Cancer Res Treat. 2017;166:875–879. [DOI] [PubMed] [Google Scholar]
- 63. de Blok CJM, Wiepjes CM, Nota NM, et al. Breast cancer risk in transgender people receiving hormone treatment: nationwide cohort study in the Netherlands. BMJ. 2019;365:l1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gart MS, Smetona JT, Hanwright PJ, et al. Autologous options for postmastectomy breast reconstruction: a comparison of outcomes based on the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2013;216:229–238. [DOI] [PubMed] [Google Scholar]
- 65. Fischer JP, Nelson JA, Cleveland E, et al. Breast reconstruction modality outcome study: a comparison of expander/implants and free flaps in select patients. Plast Reconstr Surg. 2013;131:928–934. [DOI] [PubMed] [Google Scholar]
- 66. Simonacci F, Bertozzi N, Grieco MP, et al. Procedure, applications, and outcomes of autologous fat grafting. Ann Med Surg (Lond). 2017;20:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Glowacki J, Mizuno S. Collagen scaffolds for tissue engineering. Biopolymers. 2008;89:338–344. [DOI] [PubMed] [Google Scholar]
- 68. Debels H. Advances in tissue engineering; a novel technology making use of an in vivo vascularized chamber. Acta Chir Belg. 2015;115:104–110. [DOI] [PubMed] [Google Scholar]
- 69. Cho SW, Song KW, Rhie JW, et al. Engineered adipose tissue formation enhanced by basic fibroblast growth factor and a mechanically stable environment. Cell Transplant. 2007;16:421–434. [DOI] [PubMed] [Google Scholar]
- 70. Donnely E, Griffin M, Butler PE. Breast reconstruction with a tissue engineering and regenerative medicine approach (systematic review). Ann Biomed Eng. 2020;48:9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gerges I, Tamplenizza M, Martello F, et al. Exploring the potential of polyurethane-based soft foam as cell-free scaffold for soft tissue regeneration. Acta Biomater. 2018;73:141–153. [DOI] [PubMed] [Google Scholar]
- 72. Lovett M, Lee K, Edwards A, Kaplan DL. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev. 2009;15:353–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Debels H, Han, XL, Palmer J, et al. Sustainable fat grafting-optimizing fat grafting in an in vivo tissue engineering chamber model. 23rd Annual Meeting of the European Tissue Repair Society. 2013. Reims, France. [Google Scholar]
- 74. O'Halloran N GK, Dolan E, et al. Evaluating a novel adipose tissue engineering strategy for breast reconstruction postmastectomy. 43rd Sir Peter Freyer Memorial Lecture and Surgical Symposium Ireland. Galway, Ireland, 2018. [Google Scholar]
- 75. Kawamura T, Miyagawa S, Fukushima S, et al. Cardiomyocytes derived from MHC-homozygous induced pluripotent stem cells exhibit reduced allogeneic immunogenicity in MHC-matched non-human primates. Stem Cell Rep. 2016;6:312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tomizawa M, Shinozaki F, Motoyoshi Y, et al. Transcription factors and medium suitable for initiating the differentiation of human-induced pluripotent stem cells to the hepatocyte lineage. J Cell Biochem. 2016;117:2001–2009. [DOI] [PubMed] [Google Scholar]
- 77. Mohsen-Kanson T, Hafner AL, Wdziekonski B, et al. Differentiation of human induced pluripotent stem cells into brown and white adipocytes: role of Pax3. Stem Cells. 2014;32:1459–1467. [DOI] [PubMed] [Google Scholar]
- 78. Qu Y, Han B, Gao B, et al. Differentiation of human induced pluripotent stem cells to mammary-like organoids. Stem Cell Rep. 2017;8:205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sokol ES, Miller DH, Breggia A, et al. Growth of human breast tissues from patient cells in 3D hydrogel scaffolds. Breast Cancer Res. 2016;18:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Maxwell GP, Gabriel A. Bioengineered breast: concept, technique, and preliminary results. Plast Reconstr Surg. 2016;137:415–421. [DOI] [PubMed] [Google Scholar]