Abstract
Background
β-Hemolytic streptococci are frequently implicated in necrotizing soft-tissue infections (NSTIs). Clindamycin administration may improve outcomes in patients with serious streptococcal infections. However, clindamycin resistance is growing worldwide, and resistance patterns in NSTIs and their impact on outcomes are unknown.
Methods
Between 2015 and 2018, patients with NSTI at a quaternary referral center were followed up for the outcomes of death, limb loss, and streptococcal toxic shock syndrome. Surgical wound cultures and resistance data were obtained within 48 hours of admission as part of routine care. Risk ratios for the association between these outcomes and the presence of β-hemolytic streptococci or clindamycin-resistant β-hemolytic streptococci were calculated using log-binomial regression, controlling for age, transfer status, and injection drug use–related etiology.
Results
Of 445 NSTIs identified, 85% had surgical wound cultures within 48 hours of admission. β-Hemolytic streptococci grew in 31%, and clindamycin resistance was observed in 31% of cultures. The presence of β-hemolytic streptococci was associated with greater risk of amputation (risk ratio, 1.80; 95% confidence interval, 1.07–3.01), as was the presence of clindamycin resistance among β-hemolytic streptococci infections (1.86; 1.10–3.16).
Conclusions
β-Hemolytic streptococci are highly prevalent in NSTIs, and in our population clindamycin resistance was more common than previously described. Greater risk of limb loss among patients with β-hemolytic streptococci—particularly clindamycin-resistant strains—may portend a more locally aggressive disease process or may represent preexisting patient characteristics that predispose to both infection and limb loss. Regardless, these findings may inform antibiotic selection and surgical management to maximize the potential for limb salvage.
Keywords: NSTI, necrotizing soft-tissue infection, clindamycin, resistance, β-hemolytic streptococci
β-Hemolytic streptococci are commonly identified in necrotizing skin and soft-tissue infections and are associated with risk of amputation. Clindamycin resistance is frequently found in invasive, virulent β-hemolytic streptococci and increases the risk of amputation.
Necrotizing soft-tissue infections (NSTIs) represent a heterogeneous group of rapidly progressive skin and soft-tissue infection. In the United States, there are approximately 3800–5800 cases annually, with reported case-fatality rates for modern series between 8% and 17% as well as substantial morbidity rates and functional limitations [1–4]. NSTIs are caused by a variety of pathogens and can be either monomicrobial or polymicrobial. Although Clostridium perfringens is the most feared organism implicated in NSTIs owing its associated high case-fatality rate [5], streptococcal species are considerably more common [6]. β-Hemolytic streptococcal species, including Streptococcus pyogenes (group A Streptococcus [GAS]), Streptococcus agalactiae (group B Streptococcus [GBS]) and Streptococcus dysgalactiae subspecies, have all been implicated to varying degrees and in different populations [7–13].
Penicillin is the primary treatment for β-hemolytic streptococcal infection. The use of clindamycin is recommended for documented GAS by the Infectious Diseases Society of America [14]. The addition of clindamycin has been associated with improved outcomes in patients with serious streptococcal infections, including those with streptococcal toxic shock syndrome (STSS), and penicillin monotherapy is associated with higher morbidity and mortality rates compared to penicillin plus clindamycin [15–18]. Preclinical studies demonstrate that clindamycin reduces the production of bacterial toxins, which mediate the intense inflammatory response [19–22]. In vivo studies using murine NSTI models have demonstrated that clindamycin leads to a reduction in virulence factor activity among GAS strains [23], but this finding has not been well evaluated in humans.
Unfortunately, clindamycin resistance is emerging in streptococcal species worldwide [24–26]. In the United States, it was encountered in <1% of GAS isolates in 2003 [27]; this increased to 15% in 2015 [28]. Even higher levels of resistance are reported in GBS isolates [29, 30]. Between 2006 and 2010 in France, clindamycin resistance was higher for S. dysgalactiae subsp. than for GAS during the same period [31]. A study in Ontario, Canada, in 2011–2012 identified 14 cases of invasive S. dysgalactiae subsp., of which all were penicillin sensitive but 14% were clindamycin resistant [13].
Antibiotic resistance profiles in NSTI have not been clearly characterized, although clindamycin is strongly recommended as an adjunct antibiotic regimen despite its associated risk of Clostridioides difficile pseudomembranous colitis. Furthermore, it is unknown whether patient outcomes differ based on clindamycin resistance. Using a large, prospective NSTI cohort, we sought to describe clindamycin resistance patterns and determine whether patient outcomes differed based on the presence of β-hemolytic streptococci and as well as clindamycin-resistant isolates. We hypothesized that the presence of β-hemolytic streptococcal species—particularly clindamycin-resistant strains—would be associated with worse outcomes.
METHODS
Data Collection
All adult patients presenting with necrotizing skin and soft-tissue infections at Harborview Medical Center, Seattle, Washington, were included in a prospective, internal registry beginning in January of 2015. Harborview is a 413-bed acute care hospital that serves as a public safety-net hospital for King County, as well as the level I trauma and burn center for Washington, Alaska, Montana, and Idaho. Cases were confirmed by review of operative and admission records. American Association for the Surgery of Trauma grade IV and V soft-tissue infections were considered NSTIs for the purposes of this study [32]. Patients with surgical wound cultures obtained within 48 hours of admission were eligible for inclusion in this study.
Wound cultures—either culture swabs of typically sterile spaces or tissue cultures—were obtained as part of routine patient care. The clindamycin resistance of β-hemolytic streptococcal species (GAS, GBS, or S. dysgalactiae subsp.) was determined using standard procedures involving clindamycin- and erythromycin-impregnated disk diffusion to evaluate for resistance; inducible versus constitutive mechanism of resistance was determined by the shape of the zone of inhibition [33].
The primary outcomes were in-hospital death, amputation among patients with limb involvement, and early STSS among patients with β-hemolytic streptococcal species. The presence of STSS within 24 hours of admission was determined based on the Centers for Disease Control and Prevention criteria: hypotension (systolic blood pressure <90 mm Hg) and organ failure (characterized by creatinine level >2 m/dL; platelet count <100 × 103/µL; aspartate aminotransferase, alanine aminotransferase, or total bilirubin level >2 times the upper limit of normal; or acute respiratory distress syndrome) [34]. Key covariates—age, whether the patient was transferred from another facility, and whether the infection was injection drug use related—were chosen a priori. This study was performed with the approval of the University of Washington Institutional Review Board, and informed consent was waived.
Statistical Analysis
Data were analyzed using R software (version 3.6.1). Categorical variables were reported as absolute values and percentages, and continuous variables as medians and interquartile ranges. Log-binomial regression was used to estimate risk ratios (RRs), controlling for potential confounders (injection drug use, transfer status, and age). Unadjusted and adjusted RRs are presented with associated 95% confidence intervals (CIs).
RESULTS
Patient and Infection Characteristics
Between 2015 and 2018, 445 adult patients with NSTI were identified. Of these, 435 (98%) underwent ≥1 surgical debridement at our institution; 377 (87%) had intraoperative wound cultures obtained as surgical specimens within 48 hours of admission and were included in this study (Table 1). Patients were predominantly middle-aged (median age, 55 years; interquartile range, 44–62 years), male (66%), and white (78%). The majority of patients were transferred from another institution (88%). Diabetes was the most common preexisting condition. The perineum was the most frequently involved site (37%), followed by the leg (33%). β-Hemolytic streptococcal species were common among infections involving the leg and arm. Nearly a third of infections had no clear cause (eg, trauma, postoperative).
Table 1.
Demographic and Clinical Characteristics of Patients With Necrotizing Soft-tissue Infections and Culture Data, Overall (n = 377) and by Presence (n = 118) or Absence (n = 259) of β-Hemolytic Streptococci
| Characteristic | Patients, No. (%)a | ||
|---|---|---|---|
| Total (n = 377) | β-Hemolytic Streptococci | ||
| Absent (n = 259) | Present (n = 118) | ||
| Male sex | 248 (65.8) | 164 (63.3) | 84 (71.2) |
| Age, median (IQR), y | 55.0 (44.0–62.0) | 56.0 (44.5–63.0) | 53.0 (43.3–60.8) |
| Race | |||
| White | 294 (78.0) | 202 (78.0) | 92 (78.0) |
| Black | 19 (5.0) | 14 (5.4) | 5 (4.2) |
| Native American | 11 (2.9) | 8 (3.1) | 3 (2.5) |
| Asian | 6 (1.6) | 4 (1.5) | 2 (1.7) |
| Other/unknown | 47 (12.5) | 31 (12.0) | 16 (13.6) |
| Transfer patient | 332 (88.1) | 238 (91.9) | 94 (79.7) |
| Preexisting conditions | |||
| Chronic renal failure | 26 (6.9) | 23 (8.9) | 3 (2.5) |
| Diabetes | 166 (44.0) | 117 (45.2) | 49 (41.5) |
| Cigarette smoking | 72 (19.1) | 53 (20.5) | 19 (16.1) |
| Drug abuse | 68 (18.0) | 44 (17.0) | 24 (20.3) |
| Body site(s) involved | |||
| Head | 8 (2.1) | 6 (2.3) | 2 (1.7) |
| Chest | 20 (5.3) | 13 (5.0) | 7 (5.9) |
| Arm | 64 (17.0) | 38 (14.7) | 26 (22.0) |
| Abdomen | 42 (11.1) | 40 (15.4) | 2 (1.7) |
| Perineum | 139 (36.9) | 111 (42.9) | 28 (23.7) |
| Leg | 124 (32.9) | 67 (25.9) | 57 (48.3) |
| Multiple sites | 19 (5.0) | 15 (5.8) | 4 (3.4) |
| Infection etiology | |||
| Boil/furuncle | 65 (17.2) | 50 (19.3) | 15 (12.7) |
| Trauma | 45 (11.9) | 20 (7.7) | 25 (21.2) |
| Chronic wound | 42 (11.1) | 23 (8.9) | 19 (16.1) |
| Injection drug use related | 48 (12.7) | 36 (13.9) | 12 (10.2) |
| Perirectal abscess | 17 (4.5) | 15 (5.8) | 2 (1.7) |
| Postoperative | 21 (5.6) | 18 (6.9) | 3 (2.5) |
| Idiopathic | 118 (31.3) | 83 (32.0) | 35 (29.7) |
| Other | 21 (5.7) | 13 (5.5) | 7 (5.8) |
| Polymicrobial infection | 275 (72.9) | 193 (74.5) | 83 (69.5) |
Abbreviation: IQR, interquartile range.
aData represent no. (%) of patients unless otherwise specified.
Microbiology, Antibiotic, and Sensitivity Data
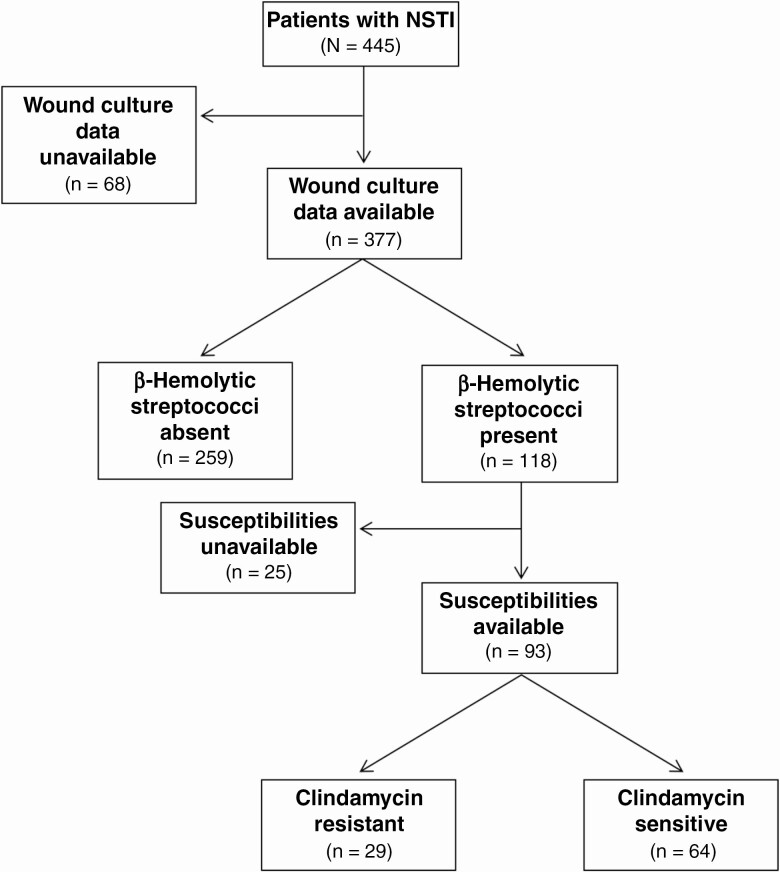
Figure 1 provides a flowchart of patient culture data. There were 120 β-hemolytic streptococcal isolates identified among 118 patients. Twenty-five patients (21%) with β-hemolytic streptococci lacked susceptibility data. Among those with susceptibility data, 31% (29 of 93) were found to have a clindamycin-resistant β-hemolytic streptococcal species. Clindamycin resistance was observed in 26% of GAS isolates (19 of 73), 50% of GBS isolates (9 of 18), and 67% of S. dysgalactiae subsp. isolates (2 of 3). Resistance was constitutive in 33% of resistant isolates (10 of 30; 2 of 19 GAS, 8 of 9 GBS, and 0 of 2 S. dysgalactiae subsp. isolates).
Figure 1.
Flowchart outlining patient culture characteristics. Abbreviation: NSTI, necrotizing soft-tissue infection.
Clindamycin resistance was more common among patients who presented primarily to our institution (57%) than in those who were transferred (23%). Transfer patients were 59% less likely to have a clindamycin-resistant strain, controlling for injection drug use (RR, 0.41; 95% CI, .24–.72).
Table 2 summarizes the organisms most frequently observed in wound cultures. Overall, the majority of infections were polymicrobial (73%). Of the β-hemolytic streptococci, GAS was more likely to be found alone, whereas GBS and S. dysgalactiae subsp. were more commonly part of a polymicrobial culture. β-Hemolytic streptococci were most frequently cocultured with Staphylococcus species: 47% were cocultured with a Staphylococcus species, of which 13% were cultured with methicillin-resistant and 15% with methicillin-sensitive Staphylococcus aureus. Among patients with wound cultures, 5% also had a positive blood culture during the same time period.
Table 2.
Summary of Organisms Observed in Surgical Wound Cultures
| Organisms | Patients, No. (%) | ||||
|---|---|---|---|---|---|
| Total (n = 377) | Polymicrobial | β-Hemolytic Streptococci | |||
| No (n = 102) | Yes (n = 275) | Absent (n = 259) | Present (n = 118)a | ||
| Streptococcus spp. | 209 (55.4) | 43 (42.2) | 118 (100) | 91 (35.1) | NA |
| GAS | 75 (19.9) | 32 (31.4) | 75 (63.6) | NA | NA |
| GBS | 37 (9.8) | 4 (3.9) | 37 (31.4) | NA | NA |
| S. dysgalactiae subsp. | 8 (2.1) | 0 (0) | 8 (6.8) | NA | NA |
| Staphylococcus spp. | 155 (41.1) | 21 (20.6) | 134 (48.7) | 100 (38.6) | 55 (46.6) |
| MRSA | 41 (10.9) | 11 (10.8) | 30 (10.9) | 26 (10.0) | 15 (12.7) |
| MSSA | 37 (9.8) | 7 (6.9) | 30 (10.9) | 19 (7.3) | 18 (15.3) |
| Gram-negative rods | 135 (35.8) | 4 (3.9) | 131 (47.6) | 106 (40.9) | 29 (24.6) |
| Mixed anaerobic flora | 192 (50.9) | 0 (0) | 192 (69.8) | 157 (60.6) | 35 (29.7) |
| Candida spp. | 38 (10.1) | 4 (3.9) | 34 (12.4) | 28 (10.8) | 10 (8.5) |
Abbreviations: GAS, group A Streptococcus; GBS, group B Streptococcus; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; NA, not applicable.
aSome patients had >1 β-hemolytic streptococcal species.
Within 12 hours of admission, 94% of patients in whose cultures β-hemolytic streptococci ultimately grew had received clindamycin, and 69% had received penicillin. At our institution, patients with suspected NSTI typically receive a high dose of clindamycin: 1200 mg intravenously every 6 hours [35]. Two of the 7 who did not receive clindamycin, and 19 of the 25 who did not receive penicillin reported an allergy to that particular medication. One patient received linezolid in place of clindamycin, and 12 of the 25 patients who did not receive penicillin received an alternate β-lactam. C. difficile colitis occurred in 3% of the overall NSTI cohort, 4% of those with β-hemolytic streptococci, and 3.4% of those with clindamycin-resistant isolates.
Treatment and Clinical Outcomes
Overall, the median hospital length of stay was 19 days (interquartile range, 11–30 days), though intensive care unit stays were relatively short, with few ventilator days (Table 3). Amputation was required in 23% of patients with limb involvement overall and was more common among patients with β-hemolytic streptococci (occurring in 32% vs 15% of those without β-hemolytic streptococci). Unadjusted and adjusted RR estimates for the associations between the presence and absence of β-hemolytic streptococci and the outcomes of interest are provided in Table 4. The presence of β-hemolytic streptococci was associated with greater risk of amputation (adjusted RR, 1.79; 95% CI, 1.07–3.01). This association did not differ depending on whether the β-hemolytic streptococci were part of a monomicrobial or polymicrobial infection. STSS early in a patient’s hospital courses was rare, occurring in only 5% of patients with β-hemolytic streptococci; however, 33% patients (2 of 6) with early STSS died, compared with 9% (10 of 112) without STSS. The overall mortality rate was 15% in our study cohort. No differences in mortality rate were observed based on the presence or absence of β hemolytic streptococci.
Table 3.
Clinical Outcomes in Patients With Necrotizing Soft-tissue Infections and Culture Data, Overall (n = 377) and by Presence (n = 118) or Absence (n = 259) of β-Hemolytic Streptococci
| Outcome | Total (n = 377) | β-Hemolytic Streptococci | |
|---|---|---|---|
| Absent (n = 259) | Present (n = 118) | ||
| Ventilator-free days, median (IQR)a | 26 (24–28) | 26 (24–28) | 26 (24–28) |
| ICU-free days, median (IQR)a | 24 (20–25) | 24 (20–25) | 24 (20–25) |
| Hospital LOS, median (IQR), d | 19 (11–30) | 18 (10–31) | 21 (11–39) |
| Debridements, median (IQR), no. | 3 (2–4) | 3 (2–4) | 3 (2–4) |
| Clostridioides difficile colitis, no. (%) | 12 (3.2) | 7 (2.7) | 5 (4.2) |
| STSS, no. (%) | NA | NA | 6 (5.1) |
| Amputation, no. (%)b | 42/187 (22.5) | 16/105 (15.2) | 26/82 (31.7) |
| Death, no. (%) | 55 (14.6) | 43 (16.6) | 12 (10.2) |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; NA, not applicable; STSS, streptococcal toxic shock syndrome.
aOf 28 days.
bAmong patients with extremity involvement.
Table 4.
Unadjusted and Adjusted Risk Ratio Estimates for the Occurrence of Amputation or Death, Comparing Patients With or Without β-Hemolytic Streptococci
| Outcome | RR (95% CI) | |
|---|---|---|
| Unadjusted | Adjusteds | |
| Amputationb | ||
| β-Hemolytic streptococci absent | Reference | Reference |
| β-Hemolytic streptococci present | 2.08 (1.20–3.61) | 1.79 (1.07–3.01) |
| Death | ||
| β-Hemolytic streptococci absent | Reference | Reference |
| β-Hemolytic streptococci present | 0.61 (.34–1.12) | 0.72 (.40–1.31) |
Abbreviations: CI, confidence interval; RR, risk ratio.
aAdjusted for injection drug use, transfer status, and age by quintile.
bAmong patients with extremity involvement (n = 187).
Unadjusted and adjusted RR estimates for the associations between clindamycin resistance among patients with β-hemolytic streptococci and the outcomes of interest are provided in Table 5. In univariate analysis, there were no observed differences in amputation, STSS, or mortality rate based on clindamycin susceptibility. In prespecified multivariate analysis, clindamycin resistance was associated with 86% greater risk of amputation (adjusted RR, 1.86; 95% CI, 1.10–3.16) than in patients with clindamycin-sensitive β-hemolytic streptococci, but no appreciably altered risk of early STSS (1.23; .25–6.08) or death (1.38; .41–4.63) was observed.
Table 5.
Unadjusted and Adjusted Risk Ratio Estimates Comparing Patients With or Without Clindamycin-resistant Strains Among Those With β-Hemolytic Streptococci
| RR (95% CI) | ||
|---|---|---|
| Unadjusted | Adjusteda | |
| Amputationb | ||
| Clindamycin sensitive | Reference | Reference |
| Clindamycin resistant | 1.07 (.49–2.32) | 1.86 (1.10–3.16) |
| STSS | ||
| Clindamycin sensitive | Reference | Reference |
| Clindamycin resistant | 1.10 (.21–5.69) | 1.23 (.25–6.08) |
| Death | ||
| Clindamycin sensitive | Reference | Reference |
| Clindamycin resistant | 0.83 (.21–2.90) | 1.38 (.41–4.63) |
Abbreviations: CI, confidence interval; RR, risk ratio; STSS, streptococcal toxic shock syndrome.
aAdjusted for injection drug use, transfer status, and age by quintile.
bAmong patients with extremity involvement (n = 73).
DISCUSSION
Using one of the largest prospectively collected NSTI databases, we observed that β-hemolytic streptococci were frequently identified in NSTI surgical wound cultures, and—for infections involving extremities—were associated with a greater risk of amputation compared with infections not involving β-hemolytic streptococci. Clindamycin-resistant streptococcal infections were also associated with a relatively greater risk of amputation than those involving clindamycin-sensitive streptococci.
The bacterial etiology of necrotizing infections involving the limb as a risk factor for amputation has not been fully elucidated. Although infection with Clostridium has previously been associated with amputation in NSTI, an association between streptococcal infection and limb loss is less clear [5, 36]. In our study, it was unclear whether β-hemolytic streptococcal species—through rapidly progressive and extensive necrosis preventing limb salvage—are the primary determinant for amputation or instead signify other patient conditions that both predispose to amputation (eg, peripheral vascular disease or diabetes) and provide an ideal environment for β-hemolytic streptococcal infection. Streptococcus spp. in particular are not infrequently found in chronic wounds associated with diabetes or peripheral vascular disease [37].
Diabetes was particularly common in our population, and though no indicator of underlying vascular disease was available, a chronic wound was noted as the cause of infection in 16% of patients with β-hemolytic streptococcal species. A post hoc analysis controlling for chronic wound as infection etiology—a potential surrogate for peripheral vascular disease—notably increased the strength of the association between β-hemolytic streptococci and amputation (adjusted RR, 3.37; 95% 1.93–5.87). Despite this, the possibility of residual confounding by other predisposing conditions may limit interpretation.
Clindamycin resistance among β-hemolytic streptococci was more prevalent than previously reported. One recently published study of 5 Scandinavian centers focusing on GAS and S. dysgalactiae subsp. NSTI found that only 1 of 113 streptococcal isolates were resistant to clindamycin [7]. In our cohort, resistance was particularly common among patients presenting directly to our hospital. This may be related to the fact that our institution is a safety net for the underserved and those living homeless in our large, urban community. As such, the prevalence of clindamycin resistance reported in this study may not be generalizable to other institutions, which often have their own resistance patterns and antibiograms. However, differential frequencies of resistance observed in patients originating from rural or urban centers may be useful in guiding antibiotic selection and highlight the need for intensive surveillance of resistance patterns.
Clindamycin resistance was also associated with greater risk of amputation. Clindamycin may play an important role in minimizing the locally destructive nature of β-hemolytic streptococcal infections, and resistance may mitigate this. Alternatively, patients with clindamycin-resistant strains may have had more interaction with the healthcare system and this may simply be a marker of underlying medical conditions that predispose a patient to amputation. However, in additional analyses we found no association between clindamycin resistance and the presence of a number of preexisting conditions, including diabetes or a chronic extremity wound. Given the risk associated with direct admission compared with being transferred in, there may also be an association with higher-risk exposures to individuals or at-risk groups colonized or infected with clindamycin-resistant β-hemolytic streptococci. The RR estimate relating clindamycin resistance to amputation changed considerably after controlling for potential confounders, which were selected a priori. Transfer status in particular is likely contributing to confounding, as it was noted to be associated with lower risk of clindamycin resistance in this study and has been shown to be associated with greater likelihood of amputation in NSTI [38].
Clindamycin is thought to have a bacteriostatic, and possibly bactericidal, effect in Streptococcus species by binding ribosomal subunits and inhibiting protein synthesis, and is believed to improve outcomes by inhibiting the production of key virulence proteins. Resistance is possible through a number of mechanisms, including modification of ribosomal targets through mutation or methylation, or increased efflux [39]. One would expect clindamycin resistance to mitigate the benefit of clindamycin on clinical outcomes. However, because nearly every patient in this cohort received clindamycin on admission, it was impossible to assess whether the previously described benefit of adjunct clindamycin administration is negated by clindamycin resistance. This leads us to postulate whether linezolid, an oxazolidinone that inhibits bacterial protein synthesis, may reduce virulence factor and the production of toxins in clindamycin-resistant isolates, and potentially improve outcomes. Although linezolid has been shown to be effective in uncomplicated skin and soft-tissue infections, the clinical utility of linezolid as an alternative to clindamycin will need to be further evaluated in NSTI.
Beyond limb NSTI, the predictive value of the presence of β-hemolytic streptococci—and clindamycin resistance in β-hemolytic streptococci in NSTI—may be limited, because no differences in risk of death or development of STSS early in hospitalization were observed. Our study was underpowered to detect small differences in the occurrence of these outcomes, because mortality rates and the prevalence of STSS were both low. Alternatively, this finding may reflect that β-hemolytic streptococci play a relatively minor role in the systemic manifestations of NSTI.
We opted to use wound culture data rather than blood cultures for this study. Blood cultures are generally inefficient at identifying pathogen(s) responsible for infection [40]. In our cohort, few patients had positive blood culture results, and even fewer were positive for streptococci species. Culture data were included in this analysis only if the culture had been obtained within 48 hours of admission; this was intended to minimize potential for detecting colonizing—rather than disease causing—organisms. We believe that wound culture data provide a useful insight into the pathophysiology of NSTIs, because the majority of NSTIs are polymicrobial, and wound cultures reflect their complex, diverse bacterial milieu, without necessarily definitively pinpointing the causative organism(s). The diversity of bacterial profiles that have been described in NSTIs makes it difficult to draw conclusions regarding necessary and sufficient conditions for their development; however, a secondary analysis of our data suggested that the association with amputation did not differ based on whether or not the β-hemolytic streptococci were part of a monomicrobial or polymicrobial infection.
This study has a few limitations. Wound culture data were missing in 15% of patients, and of those with streptococcal isolates, 21% lacked resistance data. This missingness is likely related to transfer status and debridement history, because outside cultured data were unavailable, and a small fraction of patients were transferred after having been adequately debrided or died before operative intervention was possible. The data set lacked information on some preexisting conditions that may affect limb loss. However, in an analysis that used the presence of a chronic wound as a surrogate for peripheral vascular disease, the observed association between β-hemolytic streptococci and amputation was strengthened. In addition, neither a chronic wound nor diabetes was associated with clindamycin resistance. The timing of amputation relative to admission was not available; however, early amputation may represent more advanced disease on presentation, whereas later amputation may be more amenable to the potential benefits of clindamycin. Finally, the majority of patients were transferred from another institution. This may select for extreme cases, which require higher levels of care that are more appropriate for a quaternary referral center and may limit generalizability.
In summary, the data presented in this study suggest that among patients with extremity NSTI, knowledge of the organism involved, as well as its resistance to clindamycin, can help predict the need for limb amputation. We also demonstrated a high degree of clindamycin resistance within our community, particularly among highly virulent, invasive Streptococcus species. The empiric use of clindamycin and the potential use of alternative antimicrobial agents (eg, linezolid) in clinical settings with high prevalence of clindamycin resistance require further evaluation. These results also highlight the need for additional inquiry into the complex microenvironment and host-organism interactions that facilitate the development of necrotizing infections, in order to identify modifiable patient or disease characteristics and additional interventions to facilitate limb salvage.
Notes
Financial support. This work was supported by the National Institutes of Health (grant T32 5T32GM121290 to D. L. H).
Potential conflicts of interest. E. M. B. received a grant from Atox Bio for an necrotizing soft-tissue infection clinical trial outside the scope of this study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Audureau E, Hua C, de Prost N, et al. ; Henri Mondor Hospital Necrotizing Fasciitis group . Mortality of necrotizing fasciitis: relative influence of individual and hospital-level factors, a nationwide multilevel study, France, 2007-12. Br J Dermatol 2017; 177:1575–82. [DOI] [PubMed] [Google Scholar]
- 2. Psoinos CM, Flahive JM, Shaw JJ, et al. Contemporary trends in necrotizing soft-tissue infections in the United States. Surgery 2013; 153:819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harbrecht BG, Nash NA. Necrotizing soft tissue infections: a review. Surg Infect (Larchmt) 2016; 17:503–9. [DOI] [PubMed] [Google Scholar]
- 4. Hakkarainen TW, Burkette Ikebata N, Bulger E, Evans HL. Moving beyond survival as a measure of success: understanding the patient experience of necrotizing soft-tissue infections. J Surg Res 2014; 192:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anaya DA, McMahon K, Nathens AB, Sullivan SR, Foy H, Bulger E. Predictors of mortality and limb loss in necrotizing soft tissue infections. Arch Surg 2005; 140:151–8. [DOI] [PubMed] [Google Scholar]
- 6. Louis A, Savage S, Utter GH, Li SW, Crandall M. NSTI organisms and regions: a multicenter study from the American Association for the Surgery of Trauma. J Surg Res 2019; 243:108–13. [DOI] [PubMed] [Google Scholar]
- 7. Bruun T, Rath E, Bruun Madsen M, et al. Risk factors and predictors of mortality in streptococcal necrotizing soft-tissue infections: a multicenter prospective study. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gardam MA, Low DE, Saginur R, Miller MA. Group B streptococcal necrotizing fasciitis and streptococcal toxic shock-like syndrome in adults. Arch Intern Med 1998; 158:1704–8. [DOI] [PubMed] [Google Scholar]
- 9. Umemura H, Hiragushi K, Sasaki S, et al. A male with group B streptococcal necrotizing fasciitis at multiple sites secondary to multifocal septic arthritis. Acta Derm Venereol 2015; 95:614–5. [DOI] [PubMed] [Google Scholar]
- 10. Wong CH, Kurup A, Tan KC. Group B Streptococcus necrotizing fasciitis: an emerging disease? Eur J Clin Microbiol Infect Dis 2004; 23:573–5. [DOI] [PubMed] [Google Scholar]
- 11. Gandhi P, Singh S, Farkas A. Group B streptococcal necrotising fasciitis following normal vaginal delivery. J Obstet Gynaecol 2009; 29:554. [DOI] [PubMed] [Google Scholar]
- 12. Bruun T, Kittang BR, de Hoog BJ, et al. Necrotizing soft tissue infections caused by Streptococcus pyogenes and Streptococcus dysgalactiae subsp. equisimilis of groups C and G in western Norway. Clin Microbiol Infect 2013; 19:E545–50. [DOI] [PubMed] [Google Scholar]
- 13. Anantha RV, Kasper KJ, Patterson KG, Zeppa JJ, Delport J, McCormick JK. Fournier’s gangrene of the penis caused by Streptococcus dysgalactiae subspecies equisimilis: case report and incidence study in a tertiary-care hospital. BMC Infect Dis 2013; 13:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stevens DL, Bisno AL, Chambers HF, et al. Executive summary: practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:147–59. [DOI] [PubMed] [Google Scholar]
- 15. Zimbelman J, Palmer A, Todd J. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J 1999; 18:1096–100. [DOI] [PubMed] [Google Scholar]
- 16. Linnér A, Darenberg J, Sjölin J, Henriques-Normark B, Norrby-Teglund A. Clinical efficacy of polyspecific intravenous immunoglobulin therapy in patients with streptococcal toxic shock syndrome: a comparative observational study. Clin Infect Dis 2014; 59:851–7. [DOI] [PubMed] [Google Scholar]
- 17. Mulla ZD, Leaverton PE, Wiersma ST. Invasive group A streptococcal infections in Florida. South Med J 2003; 96:968–73. [DOI] [PubMed] [Google Scholar]
- 18. Carapetis JR, Jacoby P, Carville K, Ang SJ, Curtis N, Andrews R. Effectiveness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group a streptococcal infections. Clin Infect Dis 2014; 59:358–65. [DOI] [PubMed] [Google Scholar]
- 19. Sriskandan S, McKee A, Hall L, Cohen J. Comparative effects of clindamycin and ampicillin on superantigenic activity of Streptococcus pyogenes. J Antimicrob Chemother 1997; 40:275–7. [DOI] [PubMed] [Google Scholar]
- 20. Norrby-Teglund A, Kaul R, Low DE, et al. Evidence for the presence of streptococcal-superantigen-neutralizing antibodies in normal polyspecific immunoglobulin G. Infect Immun 1996; 64:5395–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mascini EM, Jansze M, Schouls LM, Verhoef J, Van Dijk H. Penicillin and clindamycin differentially inhibit the production of pyrogenic exotoxins A and B by group A streptococci. Int J Antimicrob Agents 2001; 18:395–8. [DOI] [PubMed] [Google Scholar]
- 22. Stevens DL, Bryant AE, Hackett SP. Antibiotic effects on bacterial viability, toxin production, and host response. Clin Infect Dis 1995; 20:S154–7. [DOI] [PubMed] [Google Scholar]
- 23. Andreoni F, Zürcher C, Tarnutzer A, et al. Clindamycin affects group A Streptococcus virulence factors and improves clinical outcome. J Infect Dis 2017; 215:269–77. [DOI] [PubMed] [Google Scholar]
- 24. Pesola AK, Sihvonen R, Lindholm L, Pätäri-Sampo A. Clindamycin resistant emm33 Streptococcus pyogenes emerged among invasive infections in Helsinki metropolitan area, Finland, 2012 to 2013. Euro Surveill 2015; 20:21117. [DOI] [PubMed] [Google Scholar]
- 25. Lu B, Fang Y, Fan Y, et al. High prevalence of macrolide-resistance and molecular characterization of Streptococcus pyogenes isolates circulating in China from 2009 to 2016. Front Microbiol. 2017; 8:1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Plainvert C, Martin C, Loubinoux J, et al. Highly virulent M1 Streptococcus pyogenes isolates resistant to clindamycin. Med Mal Infect 2015; 45:470–4. [DOI] [PubMed] [Google Scholar]
- 27. Richter SS, Heilmann KP, Beekmann SE, et al. Macrolide-resistant Streptococcus pyogenes in the United States, 2002–2003. Clin Infect Dis 2005; 41:599–608. [DOI] [PubMed] [Google Scholar]
- 28. De Muri GP, Sterkel AK, Kubica PA, Duster MN, Reed KD, Wald ER. Macrolide and clindamycin resistance in group a streptococci isolated from children with pharyngitis. Pediatr Infect Dis J 2017; 36:342–4. [DOI] [PubMed] [Google Scholar]
- 29. Heelan JS, Hasenbein ME, McAdam AJ. Resistance of group B Streptococcus to selected antibiotics, including erythromycin and clindamycin. J Clin Microbiol 2004; 42:1263–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Castor ML, Whitney CG, Como-Sabetti K, et al. Antibiotic resistance patterns in invasive group B streptococcal isolates. Infect Dis Obstet Gynecol 2008; 2008:727505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Loubinoux J, Plainvert C, Collobert G, Touak G, Bouvet A, Poyart C. Adult invasive and noninvasive infections due to Streptococcus dysgalactiae subsp. equisimilis in France from 2006 to 2010. J Clin Microbiol 2013; 51:2724–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ray-Zack MD, Hernandez MC, Younis M, et al. Validation of the American Association for the Surgery of Trauma emergency general surgery grade for skin and soft tissue infection. J Trauma Acute Care Surg 2018; 84:939–45. [DOI] [PubMed] [Google Scholar]
- 33. CLIS. Performance standards for antimicrobial susceptibility testing. 26th ed. CLSI supplement M100S. Wayne, PA: Clinical and Laboratory Standards Institute, 2016. [Google Scholar]
- 34. National Notifiable Diseases Surveillance System, Centers for Disease Control and Prevention. Streptococcal toxic shock syndrome (STSS) (Streptococcus pyogenes): 2010 case definition. Available at: https://wwwn.cdc.gov/nndss/conditions/streptococcal-toxic-shock-syndrome/case-definition/2010/. Accessed 18 March 2020.
- 35. May AK, Stafford RE, Bulger EM, et al. ; Surgical Infection Society . Treatment of complicated skin and soft tissue infections. Surg Infect (Larchmt) 2009; 10:467–99. [DOI] [PubMed] [Google Scholar]
- 36. Khamnuan P, Chongruksut W, Jearwattanakanok K, Patumanond J, Tantraworasin A. Necrotizing fasciitis: epidemiology and clinical predictors for amputation. Int J Gen Med 2015; 8:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wolcott RD, Hanson JD, Rees EJ, et al. Analysis of the chronic wound microbiota of 2963 patients by 16S rDNA pyrosequencing. Wound Repair Regen 2016; 24:163–74. [DOI] [PubMed] [Google Scholar]
- 38. Horn DL, Shen J, Roberts E, et al. Predictors of mortality, limb loss, and discharge disposition at admission amongst patients with necrotizing skin and soft tissue infections. J Trauma Acute Care Surg 2020; 89:186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haenni M, Lupo A, Madec JY. Antimicrobial resistance in Streptococcus spp. Microbiol Spectr 2018; 6:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nannan Panday RS, Wang S, van de Ven PM, Hekker TAM, Alam N, Nanayakkara PWB. Evaluation of blood culture epidemiology and efficiency in a large European teaching hospital. PLoS One 2019; 14:e0214052. [DOI] [PMC free article] [PubMed] [Google Scholar]