Abstract
Study Objectives
Recent work on US Whites from clinical samples used obstructive sleep apnea (OSA) symptoms to generate phenotypes for individuals with moderate-severe OSA which suggested 3 to 5 symptom classes. However, it is unknown whether similar classes generalize to diverse Hispanics/Latino adults. Therefore, we sought to fill this gap by empirically deriving sleep phenotypes among a large sample of diverse Hispanics/Latinos.
Methods
We used data from The Hispanic Community Health Study/Study of Latinos (HCHS/SOL; 2008–2011), a prospective cohort study designed using a multisite multistage probability sample of adults 18–74 years old. The subpopulation of interest included participants with moderate-severe OSA symptoms (≥15 respiratory event index (REI) events per hour; n = 1,605). We performed latent class analysis for complex survey data using 15 common OSA symptoms (e.g. Epworth Sleepiness Scale) and 4 comorbidities to identify phenotype classes.
Results
Average age was 52.4 ± 13.9 years and 34.0% were female. Mean REI was 33.8 ± 22.5 events per hour. Fit statistics and clinical significance suggested that a three-class solution provided the best fit to the data. The three phenotypes were: (1) Minimally Symptomatic (47.7%), (2) Excessive sleepiness (37.1%), and (3) Disturbed Sleep (15.2%). Sensitivity models were consistent with the main proposed solution.
Conclusions
Derived sleep phenotypes among diverse Hispanic/Latinos were consistent with recent findings from the Sleep Apnea Global Interdisciplinary Consortium, but we found notable differences in class prevalence relative to Whites. Further research is needed to link derived sleep phenotypes to health comorbidities in diverse populations.
Keywords: obstructive sleep apnea, latent class analysis, sleep phenotypes, Hispanics/Latinos
Statement of Significance.
Sleep apnea subtypes of diverse Latinos are not well understood in sleep medicine. We used data from a large, diverse, and representative cohort study of US Latinos to identify sleep apnea phenotypes and link them to sociocultural characteristics and comorbid health conditions. Our study provides data-driven validated sleep apnea phenotypes like the Sleep Apnea Global Interdisciplinary Consortium, but with notable differences compared to non-Hispanic whites. We propose that the empirically derived sleep apnea phenotypes reported herein will help our understanding of sleep apnea among diverse Latinos and culturally tailor precise therapeutic approaches.
Introduction
The United States (US) population is becoming increasingly diverse. Hispanics/Latinos represent 18.0% of the US population and are projected to increase rapidly in coming decades [1]. Health disparities among Hispanics/Latinos are evident; including higher rates of diabetes and cognitive impairment compared to Whites [2, 3]. Sleep disorders may precede these health disorders and have been associated with a higher risk for heart failure, glucose intolerance, and decline in cognitive function [4–6]. In addition to its health sequelae, sleep disturbances also place a high economic burden. Recent estimates indicate that close to 450 billion dollars of economic output in the US are lost to sleep insufficiency alone [7]. Therefore, more research is needed to characterize sleep within the Hispanic/Latino population.
Demographic factors may influence sleep within the Hispanic/Latino population. For example, data from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) indicate that 25% of Puerto Ricans and 20% of Dominicans and South Americans sleep less than 7 h a day [8]. Another HCHS/SOL study found that Central Americans and Cubans had the most sleepiness symptoms, while Dominicans and Puerto Ricans had the least [9]. Gender/sex differences have also been observed: Mexican men had longer sleep duration compared to Mexican women [10], and are more likely to have obstructive sleep apnea (OSA) compared to women [9].
The prevalence of OSA in predominantly non-Hispanic whites ranges from 6% to 17% [11] and was up to 49% in older adults. Of importance, the home sleep apnea test, allow to measure the respiratory event index (REI), as the number of apnea and hypopnea events in an hour, and the % measures the oxygen desaturation during these events. The prevalence of OSA (REI3% ≥ 15) was 9.8% in middle-aged and older Latinos. The same study attributed increased hypertension and diabetes in Latinos to untreated OSA [9]. Indeed, OSA has also been linked to increased mortality and several cardiovascular risks such as heart failure and stroke [12, 13].
The presentation of OSA symptoms varies across individuals. Recent work has uncovered important heterogeneities in symptoms across patients with OSA and suggested that subgroups can be described using symptom profiles. For example, Ye et al. used latent class analysis (LCA) on 19 sleep symptoms and 4 comorbid conditions to derive 3 distinct derived sleep phenotypes using the Icelandic Sleep Apnea Cohort (ISAC) [14]. Their findings suggest the existence of three groups with select symptoms consistent with (1) minimally symptomatic, (2) disturbed sleep, and (3) excessive sleepiness. Follow-up studies from the ISAC cohort have shown that the effectiveness of treatment differs across OSA subtypes [15]. Kim et al. confirmed these three classes in a community-dwelling Korean population-based study [16]. More recently, data from the Sleep Apnea Global Interdisciplinary Consortium (SAGIC) uncovered five classes, including the three reported in Ye et al., in a primarily non-Hispanic white (58.8%) and Asian (21.1%) sample [17]. The two additional classes include individuals with (1) upper airway symptoms dominant and (2) sleepiness dominant. The Sleep Heart Health Study (SHHS) [18], in addition to the three main clusters, found a moderately sleepy group. The Canadian Study [19] found four clusters: the three main clusters and an “excessive sleepiness with disturbed sleep group.” To date, there is a paucity of data on OSA symptoms in diverse Hispanic/Latino populations.
To address research gaps in characterizing sleep among diverse Hispanics/Latinos, we used data-driven approaches to extract OSA subtypes from a large community-dwelling sample of Hispanics/Latinos in the US. We hypothesized that the three groups found by Ye et al. [14] and other researchers will provide a good fit to the data. However, we expected that the prevalence of these phenotypes would vary relative to non-admixed non-Hispanic white populations, and that women and Hispanics/Latinos of Puerto Rican and Dominican heritage would have symptom profiles closer to the disturbed sleep group proposed by Ye and colleagues (e.g. difficulties falling asleep, waking up at night, and issues falling back asleep after waking up).
Methods
Data
We used baseline data from the HCHS/SOL, an ongoing population-based prospective cohort study of diverse Hispanics/Latinos. Data were collected from four major US metropolitan areas with substantial Hispanic/Latino concentrations (Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA) and include targeted area representation of the following Hispanic/Latino heritage: Central American, Cuban, Dominican, Mexican, Puerto Rican, and South American. Each site enrolled around 4,000 community-dwelling adults, for a total of 16,415 participants (18–74 years). Detailed methodological discussions of the HCHS/SOL sampling design and methods have been published elsewhere [20]. Institutional review boards at each site approved the study protocol and participants provided written informed consent.
Sleep questionnaires
The original aims of the HCHS/SOL sleep study were to evaluate the associations between sleep apnea and cardiovascular risks among Hispanics/Latinos. Questionnaires were administered in English or Spanish, depending on the participant’s preferred language. A modification of the SHHS Sleep habits questionnaire, the International Restless Leg Syndrome questionnaire, [21] as well as the Epworth Sleepiness Scale questionnaire were administered to assess different health risk factors, including OSA. As described elsewhere, participants used an ARES Unicorder 5.2; B-Alert (Carlsbad, CA) to quantify the REI of 3% oxygen desaturation (REI3%) and 4% oxygen desaturation (REI4%) [9].
Sleep variables
In line with published work [14, 16–19], we accounted for 15 OSA symptoms and 4 comorbid health factors. A detailed list of these measures and their scales is included in Supplementary Table S1. We included covariates relating to sleep patterns (e.g. waking up during sleep, napping, having trouble while falling asleep), covariates relating to sleepiness (e.g. dozing off while watching television, dozing off while driving), insomnia (e.g. having trouble falling asleep), and four major comorbidities (i.e. hypertension, obstructive lung disease, cardiovascular disease, and diabetes).
All variables were dichotomized so that individuals reporting a symptom as present once a week. The Epworth Sleep Scale (ESS) was treated as a continuous measure. We classified individuals as meeting criteria for restless legs syndrome if they reported (1) experiencing a desire to move due to discomfort, (2) they felt they needed to move to relieve their discomfort, and (3) if symptoms were worse later in the day or at night. Participants who responded “don’t know” were classified as not having the symptom and those who refused to answer as missing. Individuals with three or more missing covariates were excluded from the analysis. We did not perform imputation on missing covariates since LCA calculates estimates based on all available information.
Sociodemographic variables
Sociodemographic variables include age, sex, self-reported Hispanic/Latino heritage (Central American, Cuban, Dominican, Mexican, Puerto Rican, South American, other/mixed), language preference (English, Spanish), and acculturation (language and social acculturation). Previous research characterizes acculturation as “psychological, behavioral and attitudinal changes that occur when individuals and groups from different cultures come into prolonged contact with each other and whereby individuals adopt attitudes, values, customs, beliefs, and behaviors of another culture” [22, 23]. Acculturation, specifically negative acculturation, has been linked to accelerated decline in health and increase in morbidities [24, 25].
Cardiovascular risk variables.
Cardiovascular risk variables include high-density lipoprotein (mg/dL), triglycerides (mg/dL), total cholesterol (mg/dL), body mass index (BMI; kg/m2), and presence or absence (binary) of the following: current smoker, current alcohol use, hypertension (National Health and Nutrition Examination Survey definition [26]), type 2 diabetes (American Diabetes Association definition [27]; fasting glucose ≥ 126 mg/dL, post-OGTT ≥ 200 ng/DL, or A1C ≥ 6.5%), obstructive lung disease (Spirometry was performed using American Thoracic Society and European Respiratory Society guidelines [28]. HCHS/SOL reference equations for spirometry have also been published [29]; FEV1/FVC < 0.70 or lower limit.), self-reported cardiovascular disease (CVD) including coronary heart disease (based on angina, heart attack, and coronary heart disease), self-reported heart failure, Framingham CVD composite criterion [30], self-reported stroke or transient ischemic attack (TIA), and self-reported physical and mental health composite scores (12-item Short-Form Health Survey; SF-12 [31]).
Analytic sample
From the 16,415 participants in the cohort, n = 1,138 did not participate in the sleep study and an additional n = 808 had insufficient sleep data for analysis. We included n = 1,623 with moderate to severe OSA (REI3% ≥ 15 events per hour). We further excluded n = 18 participants who did not complete the sleep questionnaire. The final analytic sample was n = 1,605. Included participants were more likely to be male, less likely to have graduated high school, had higher BMI, and were on average 12-years older compared to the overall population, consistent with known risk factors for OSA.
Analysis
Mplus Automation software version 8 was used to fit a series of latent class models iteratively up to 10 potential class solutions. LCAs are probabilistic models and have been used to create classification in other clinical settings, which were used to generate the phenotypes in this study [32, 33]. The first model included the sleep variables as well as four comorbidities: hypertension, diabetes, presence of self-reported cardiovascular disease, and obstructive lung disease. All LCA models accounted for the HCHS/SOL survey weights to adjust for nonresponse bias and allow generalization to the target population. We used full information maximum likelihood to fit the LCAs and all models also accounted for data clustering and stratification to reflect the complex sampling design of the HCHS/SOL data.
In line with published recommendations on assessment on the number of classes derived from LCA, we used several statistical fit indices to adjudicate model fit across the iterated solutions [34]. We used the Bayesian information criterion, Akaike information criterion, entropy, and Vuong-Lo-Mendell Rubin to determine the optimal solution. Results from the LCA can be found in Table 1. For the chosen solution, we generated summary tables to characterize the sleep symptoms and sleep health sociodemographic and general health conditions, and cardiovascular risk and disease profiles of the generated phenotypes. We tested overall differences between groups using survey adjusted chi-squared tests for categorical measures and t-tests for continuous measures. Additionally, we calculated and tested pairwise odds ratios to test for potential differences in characteristics between classes. To facilitate understanding of the distribution of the sleep symptoms across the chosen solution, we generated heatmaps to visualize symptoms prevalence.
Table 1.
Latent class analysis model fit statistics of sleep phenotypes in individuals with moderate to severe sleep apnea (REI ≥ 15). Unweighted n = 1,605
| Solution | LL | Scaling correction factor | Free parameters | AIC | BIC | SSABIC | Entropy | VLMR P |
LMR P |
AICC |
|---|---|---|---|---|---|---|---|---|---|---|
| C2 | −20,949.12 | 2.2891 | 44 | 41,986.24 | 42,223 | 42,083.22 | 0.766 | 0.000 | 0.000 | 41,988.78 |
| C3 | −20,453.12 | 2.2333 | 66 | 41,038.23 | 41,393.37 | 41,183.7 | 0.825 | 0.041 | 0.043 | 41,043.98 |
| C4 | −20,232.00 | 2.2434 | 88 | 40,639.99 | 41,113.51 | 40,833.95 | 0.789 | 0.500 | 0.502 | 40,650.32 |
| C5 | −20,092.13 | 2.0964 | 110 | 40,404.26 | 40,996.16 | 40,646.71 | 0.804 | 0.311 | 0.312 | 40,420.61 |
| C6 | −19,960.73 | 2.3792 | 132 | 40,185.47 | 40,895.74 | 40,476.4 | 0.797 | 0.773 | 0.773 | 40,209.32 |
| C7 | −19,872.23 | 2.6249 | 154 | 40,052.46 | 40,881.12 | 40,391.89 | 0.785 | 0.718 | 0.718 | 40,085.39 |
| C8 | −19,779.57 | 2.225 | 176 | 39,911.13 | 40,858.17 | 40,299.05 | 0.823 | 0.243 | 0.243 | 39,954.76 |
| C9 | −19,705.95 | 2.119 | 198 | 39,807.9 | 40,873.31 | 40,244.31 | 0.84 | 0.409 | 0.410 | 39,863.95 |
| C10 | −19,637.82 | 2.1967 | 220 | 39,715.63 | 40,899.43 | 40,200.53 | 0.814 | 0.730 | 0.730 | 39,785.89 |
*P values from non-survey adjusted LCA models.
C# indicates the number of classes estimated in the model.
REI, respiratory event index; LL, log likelihood; AIC, Akaike information criterion; BIC, Bayesian information criterion; SSABIC, sample size adjusted BIC; VLMR, Vuong-Lo-Mendell Rubin; LMR, Lo-Mendell-Rubin; AICC, sample corrected Akaike information criterion.
Sensitivity analysis
We conducted two sets of sensitivity models. First, we refit the LCAs to include only the sleep-related variables. We did so to examine the extent of change in the generated phenotypes as a result of excluding comorbid conditions from the classification process. As with the primary analyses, we characterize the sleep symptoms and sleep health, sociodemographic and general health conditions, and cardiovascular risk and disease profiles of the generated phenotypes.
Second, we validated our results using hierarchical clustering on principal components (HCPC) techniques. To start, multiple correspondence analysis (MCA) was used to generate the principal components for categorical variables. For missing data under MCA (around 1%), we replaced missing ESS scores with the median score and performed hot-deck imputation [35] using the VIM (version 6.1.0) package in R on missing categorical indicators. Details of hot-deck imputation are provided elsewhere [36, 37]. Briefly, this imputation technique sorts individuals by responses and replaces missing values based on the value of the closest matching pair. We used the FactoMineR package to perform the MCA, and scree tests to determine the optimal number of extracted dimensions. Subsequently, we used HCPC with the ward method to determine the ideal number of classes. Results were generated using the NbClust package, which calculates up to 23 indices to determine the optimal solution. As with the primary analyses, we generated descriptive tables and a heatmap to characterize the sleep symptoms of the adopted solution through this method. Lastly, we tabulated classification from the primary solution and MCA/HCPC model.
Results
Target population characteristics
The average age was 52.4 ± 13.9 years and 34.0% were female. Overall, 54.9% were hypertensive, and 8.4% had obstructive lung disease. The average respiratory event index (REI3%) was 33.8 ± 22.5. The most-reported sleep symptoms were snoring (76.8%), dozing off while watching television (72.4%), and waking up several times at night (62.3%).
Model selection
The statistical fit indices generated across the solutions of the full LCA models and the models not accounting for comorbidities are presented in Table 1 and Supplementary Table S3, respectively. Across models, the sample size corrected Akaike and Bayesian information criterion decreased consistently as the number of classes increased suggesting a better fit. The descent of both criteria narrowed and stabilized after the three-class solution. Additionally, the Vuong-Lo-Mendell Rubin p-value became insignificant with four classes thus rejecting a better fit relative to the three-class solution. The entropy value for the three-class model was 0.82 suggesting good fit and decremented when larger classes were fit. The fit statistics were consistent in identifying the three-class solution as providing optimal fit for the LCA model that excluded comorbid conditions (Supplementary Table S3). The MCA/HCPC models also pointed to a three-class solution as providing the best fit.
Phenotypes characteristics
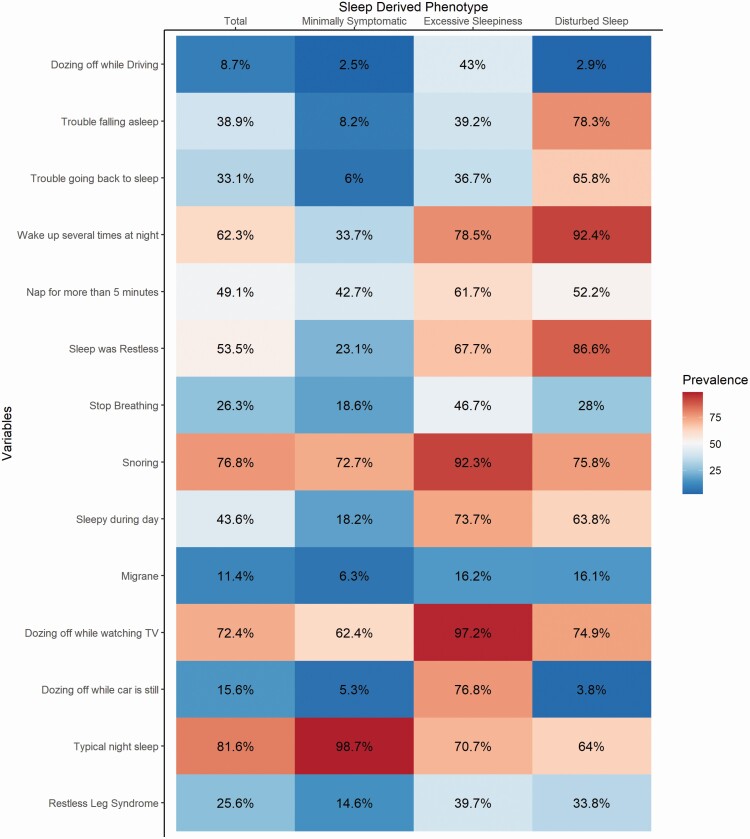
Based on the fit criteria, we selected the three-class solution as providing optimal fit to the data. Based on our examination of OSA symptoms prevalence within each class, we adopted a naming convention similar to Ye et al. [14] since the OSA profiles were consistent with their findings: The most common derived sleep phenotype was (1) minimally symptomatic (47.7%), (2) disturbed sleep (37.1%), and (3) excessive sleepiness (15.2%). Representation of OSA symptoms for these classes can be seen in Figure 1. We found significant and consistent differences in OSA symptoms across the three derived sleep phenotypes (Table 2). For example, the excessive sleepiness group was more likely to report snoring, dozing while watching TV, or driving compared to the other derived sleep phenotypes. The disturbed sleep phenotype was associated with trouble falling asleep, awaking at night, and trouble going back to sleep. The minimally symptomatic phenotype had the lowest prevalence on every OSA symptom.
Figure 1.
Symptom profile of primary latent class solution with HCHS/SOL individuals REI3% ≥ 15.All variables are presented using the prevalence in % term.
TV, television.
Table 2.
Sleep symptoms (prevalence or mean) by derived sleep phenotypes from LCA solution, HCHS/SOL (2008–2011)
| Total (n = 1,605) |
Minimally symptomatic (n = 780) |
Excessive sleepiness (n = 248) |
Disturbed sleep (n = 577) |
P | |
|---|---|---|---|---|---|
| Sleep symptoms included in LCA† | |||||
| Dozing off while driving | 8.7 | 2.5 | 43.0 | 2.9 | 0.001 |
| Trouble falling asleep | 38.9 | 8.2 | 39.2 | 78.3 | 0.001 |
| Trouble going back to sleep | 33.1 | 6.0 | 36.7 | 65.8 | P < 0.001 |
| Wake up several times at night | 62.3 | 33.7 | 78.5 | 92.4 | P < 0.001 |
| Nap for more than 5 min | 49.1 | 42.7 | 61.7 | 52.2 | P < 0.001 |
| Restless sleep | 53.5 | 23.1 | 67.7 | 86.6 | P < 0.001 |
| Stop breathing | 26.3 | 18.6 | 46.7 | 28.0 | P < 0.001 |
| Snoring | 76.8 | 72.7 | 92.3 | 75.8 | P < 0.001 |
| Sleepy during day | 43.6 | 18.2 | 73.7 | 63.8 | P < 0.001 |
| Migraine | 11.4 | 6.3 | 16.2 | 16.1 | P < 0.001 |
| Dozing off while watching TV | 72.4 | 62.4 | 97.2 | 74.9 | P < 0.001 |
| Dozing off while car is still | 15.6 | 5.3 | 76.8 | 3.8 | P < 0.001 |
| Sound or restful sleep | 81.6 | 98.7 | 70.7 | 64.0 | P < 0.001 |
| Restless legs | 25.6 | 14.6 | 39.7 | 33.8 | P < 0.001 |
| ESS score* | 6.73 (5.65) | 4.39 (3.67) | 15.71 (4.53) | 6.03 (3.56) | P < 0.001 |
| Sleep variables not included in LCA* | |||||
| REI3% | 33.76 (22.53) | 31.40 (18.19) | 42.50 (30.28) | 33.40 (23.02) | P < 0.001 |
| REI4% | 26.42 (21.95) | 24.16 (17.62) | 36.05 (30.23) | 25.51 (21.93) | P < 0.001 |
| Minimum oxygen saturation (%) | 77.64 (8.43) | 77.84 (8.09) | 75.42 (9.94) | 78.24 (8.07) | 0.006 |
*Means and standard deviations are presented.
†% are presented.
P value: Pearson’s chi square test for continuous variables; Regression-based F test for categorical variables.
ESS, Epworth Sleepiness Scale; LCA, latent class analysis; REI, respiratory event index.
The minimally symptomatic class was more likely to be male (76%) and had the lowest BMI, REI3%, and REI4% averages relative to the other phenotypes. Individuals in this group performed better (higher) on the mental and physical health measures and had an ESS score of 4.4 ± 3.7. This group was the least acculturated compared to the other classes; it also had the highest Spanish language preference of the three groups. The minimally symptomatic group had the lowest prevalence of OSA symptoms and cardiovascular comorbidities relative to the other phenotypes but had a prevalence of obstructive lung disease that was like other groups (Tables 2–7).
Table 3.
Baseline sociodemographic and health characteristics by primary solution of three derived sleep phenotypes, HCHS/SOL (2008–2011)
| Total | Minimally symptomatic | Excessive sleepiness | Disturbed sleep | P | |
|---|---|---|---|---|---|
| Unweighted n | 1,605 | 780 | 248 | 577 | |
| % of total | 100.0 | 47.7 | 15.2 | 37.1 | |
| Males† | 934 (66.0) | 520 (75.5) | 150 (67.3) | 264 (53.3) | P < 0.001 |
| Background† | |||||
| Central American | 154 (6.2) | 77 (6.2) | 31 (9.3) | 46 (5.1) | P < 0.001 |
| Cuban | 281 (26.9) | 145 (31.1) | 42 (26.3) | 94 (21.8) | |
| Dominican | 117 (8.5) | 49 (6.8) | 15 (5.2) | 53 (12.1) | |
| Mexican | 632 (34.9) | 326 (36.0) | 88 (34.8) | 218 (33.4) | |
| Puerto Rican | 287 (16.7) | 104 (11.4) | 55 (19.6) | 128 (22.4) | |
| South American | 90 (3.8) | 52 (4.5) | 12 (2.9) | 26 (3.4) | |
| Other | 42 (2.9) | 26 (4.1) | 5 (1.9) | 11 (1.8) | |
| Language preference† | |||||
| Spanish | 1,361 (81.8) | 682 (86.2) | 202 (80.0) | 477 (76.7) | P = 0.017 |
| English | 244 (18.2) | 98 (13.8) | 46 (20.0) | 100 (23.3) | |
| Age years* | 52.41 (13.93) | 52.13 (13.98) | 52.48 (13.19) | 52.95 (14.10) | P = 0.812 |
| SF-12 physical component score* | 46.75 (11.13) | 49.33 (9.37) | 44.33 (12.79) | 44.57 (11.56) | P < 0.001 |
| SF-12 mental component score* | 49.83 (12.11) | 53.40 (10.87) | 47.93 (12.19) | 46.00 (12.07) | P < 0.001 |
| SASH acculturation language* | 1.92 (1.14) | 1.78 (1.04) | 2.03 (1.20) | 2.03 (1.21) | P = 0.007 |
| SASH acculturation social* |
2.19 (0.62) | 2.16 (0.59) | 2.19 (0.60) | 2.22 (0.65) | P = 0.460 |
| Insured† | 791 (57.5) | 349 (53.3) | 111 (53.0) | 331 (64.8) | P = 0.006 |
*Means and standard deviations are presented.
†Counts and % are presented.
P value: Pearson’s chi square test for continuous variables; Regression-based F test for categorical variables.
SF-12, 12-Item short-form survey.
Table 4.
Baseline cardiovascular characteristics by primary solution derived sleep phenotype
| Total | Minimally symptomatic | Excessive sleepiness | Disturbed sleep | P | |
|---|---|---|---|---|---|
| Major comorbidities included in LCA† | |||||
| Hypertension | 54.9 | 52.6 | 57.4 | 56.8 | P = 0.450 |
| Diabetes | 34.1 | 30.4 | 37.7 | 37.5 | P = 0.119 |
| Framingham CVD | 37.6 | 27.7 | 41.6 | 48.8 | P < 0.001 |
| Obstructive lung disease | 8.4 | 9.2 | 12.7 | 5.6 | P = 0.036 |
| Major comorbidities not included in LCA | |||||
| HDL* (mg/dL) | 44.38 (11.56) | 44.76 (12.18) | 43.93 (11.34) | 44.17 (10.83) | P = 0.648 |
| Total cholesterol* (mg/dL) | 206.21 (46.36) | 206.14 (44.82) | 203.87 (45.39) | 207.46 (48.98) | P = 0.758 |
| Triglycerides* (mg/dL) | 173.00 (146.37) | 166.51 (115.59) | 165.96 (94.23) | 184.23 (191.10) | P = 0.272 |
| BMI* (kg/m2) | 33.81 (6.83) | 32.97 (6.43) | 35.04 (7.70) | 34.21 (6.66) | P = 0.003 |
| Current smoker† | 18.9 | 19.1 | 18.2 | 18.8 | P = 0.974 |
| Alcohol use† | 47.4 | 49.8 | 46.8 | 44.5 | P = 0.387 |
| CHD† | 7.7 | 6.3 | 11.5 | 7.9 | P = 0.167 |
| Heart failure† | 3.1 | 3.1 | 3.5 | 2.8 | P = 0.899 |
| Stroke/TIA† | 4.4 | 3.0 | 3.1 | 6.6 | P = 0.040 |
*Means and standard deviations are presented.
†% are presented.
P value: Pearson’s chi square test for continuous variables; Regression-based F test for categorical variables.
HDL, high-density lipoproteins; BMI, body mass index; CHD, coronary heart disease; TIA, transient ischemic attack; LCA, latent class analysis; CVD, cardiovascular disease. This is a 0/1 indicator variable that defines a composite CVD definition based on the Framingham Study criterion (http://www.framinghamheartstudy.org/risk/gencardio.html).
Table 5.
Odds ratio of derived sleep phenotypes by sleep symptoms
| Disturbed sleep (ref) vs excessive sleepiness | Disturbed sleep (ref) vs minimally symptomatic | Minimally symptomatic (ref) vs excessive sleepiness | ||||
|---|---|---|---|---|---|---|
| Odds ratio | P | Odds ratio | P | Odds ratio | P | |
| Sleep symptoms included in LCA† | ||||||
| Dozing off while driving | 25.074 | 0.001 | 0.845 | P = 0.691 | 29.690 | P < 0.001 |
| Trouble falling asleep | 0.179 | P < 0.001 | 0.025 | P < 0.001 | 7.256 | P < 0.001 |
| Trouble going back to sleep | 0.302 | P < 0.001 | 0.033 | P<0.001 | 9.148 | P < 0.001 |
| Wake up several times at night | 0.300 | P < 0.001 | 0.042 | P < 0.001 | 7.187 | P < 0.001 |
| Nap for more than 5 minutes | 1.476 | P = 0.083 | 0.685 | P = 0.018 | 2.156 | P < 0.001 |
| Sleep was restless | 0.322 | P < 0.001 | 0.046 | P < 0.001 | 6.960 | P < 0.001 |
| Stop breathing | 2.255 | P < 0.001 | 0.587 | P = 0.004 | 3.840 | P < 0.001 |
| Snoring | 3.830 | P < 0.001 | 0.849 | P = 0.377 | 4.154 | P < 0.001 |
| sleepy during day | 1.592 | P = 0.046 | 0.126 | P < 0.001 | 12.585 | P < 0.001 |
| Migraine | 1.011 | P = 0.969 | 0.352 | P < 0.001 | 2.872 | P < 0.001 |
| Dozing off while watching television | 11.666 | P < 0.001 | 0.556 | P = 0.001 | 20.963 | P < 0.001 |
| Dozing off while car is still | 84.280 | P < 0.001 | 1.424 | P = 0.349 | 59.188 | P < 0.001 |
| Sound or restful sleep | 1.361 | P = 0.153 | 42.367 | P < 0.001 | 0.032 | P < 0.001 |
| Restless legs | 1.288 | P = 0.238 | 0.333 | P < 0.001 | 3.863 | P < 0.001 |
†% are presented.
Odds ratios were calculated using survey weighted logic regression models.
LCA, latent class analysis.
Table 6.
Mean comparisons across sleep health and cardiovascular risk indicators for primary LCA solution
| Minimally symptomatic | Excessive sleepiness | Disturbed sleep | |
|---|---|---|---|
| β [95% CI] | β [95% CI] | β [95% CI] | |
| Age years | 52.13 [50.77;53.49] | 52.48 [50.16;54.80] | 52.95 [50.89;55.01] |
| REI3% | 31.40 [29.89;32.92]B | 42.50 [37.88;47.11]C | 33.40 [30.73;36.07] |
| REI4 % | 24.16 [22.67;25.64]B | 36.05 [31.45;40.64]C | 25.51 [23.12;27.90] |
| Minimum oxygen saturation | 77.84 [77.20;78.48]B | 75.42 [73.93;76.92]C | 78.24 [77.32;79.16] |
| SF-12 physical component score | 49.33 [48.50;50.16]B,C | 44.33 [42.17;46.49] | 44.57 [43.28;45.87] |
| SF-12 mental component score | 53.40 [52.46;54.34]B,C | 47.93 [46.08;49.78] | 46.00 [44.37;47.63] |
| HDL (mg/dL) | 44.76 [43.67;45.84] | 43.93 [42.24;45.62] | 44.17 [42.97;45.37] |
| Total cholesterol (mg/dL) | 206.14 [202.25;210.02] | 203.87 [196.62;211.12] | 207.46 [201.99;212.93] |
| Triglycerides (mg/dL) | 166.51 [157.11;175.91] | 165.96 [152.67;179.25] | 184.23 [164.07;204.38] |
| BMI (kg/m2) | 32.97 [32.43;33.51]B,C | 35.04 [33.80;36.27] | 34.21 [33.31;35.10] |
| Language acculturation | 1.78 [1.68;1.88]B,C | 2.03 [1.84;2.22] | 2.03 [1.87;2.19] |
| Social acculturation | 2.16 [2.10;2.21] | 2.19 [2.10;2.28] | 2.22 [2.13;2.31] |
Group differences testing for continuous variables calculated through survey adjusted linear regression of the clustering variable on latent class membership.
B: Group differences significant at P < 0.05 relative to the daytime sleepiness.
C: Group differences significant at P < 0.05 relative to the disturbed sleep.
LCA, latent class analysis; REI, respiratory event index; HDL, high-density lipoproteins; BMI, body mass index; SF-12, 12-Item short-form survey.
Table 7.
Odds ratio comparisons across cardiovascular risk indicators for primary LCA solution
| Disturbed sleep (ref) vs excessive sleepiness | Disturbed sleep (ref) vs minimally symptomatic | Minimally symptomatic (ref) vs excessive sleepiness | ||||
|---|---|---|---|---|---|---|
| OR | P | OR | P | OR | P | |
| Included in the LCA model | ||||||
| Hypertension | 1.027 | P = 0.900 | 0.844 | P = 0.297 | 1.217 | P = 0.318 |
| Diabetes | 1.008 | P = 0.972 | 0.729 | P = 0.073 | 1.383 | P = 0.119 |
| Framingham CVD | 0.748 | P = 0.175 | 0.401 | P < 0.001 | 1.862 | P = 0.002 |
| Obstructive lung disease | 2.436 | P = 0.015 | 1.696 | P = 0.058 | 1.436 | P = 0.259 |
| Not included in the LCA model | ||||||
| Male | 1.804 | P = 0.008 | 2.706 | P < 0.001 | 0.667 | P = 0.050 |
| Cigarettes | 0.963 | P = 0.879 | 1.018 | P = 0.923 | 0.945 | P = 0.816 |
| Alcohol | 1.100 | P = 0.658 | 1.238 | P = 0.192 | 0.889 | P = 0.547 |
| CHD | 1.515 | P = 0.193 | 0.788 | P = 0.406 | 1.923 | P = 0.065 |
| Heart failure | 1.276 | P = 0.635 | 1.125 | P = 0.778 | 1.135 | P = 0.817 |
| Stroke/TIA | 0.462 | P = 0.105 | 0.444 | P = 0.032 | 1.042 | P = 0.929 |
Odds ratios were calculated using survey weighted logic regression models.
OR, odds ratios; LCA, latent class analysis; CHD, coronary heart disease; TIA, transient ischemic attack; CVD, cardiovascular disease.
The disturbed sleep group had the highest female composition (53% male). Average BMI, REI3%, and REI4% were slightly above the minimally symptomatic group, but below the excessive sleepiness group. Individuals in this group had the highest prevalence of insomnia-related symptoms. For example, individuals in this group had high rates of trouble falling asleep (78%), waking up several times at night (92%), and trouble going back to sleep (65.8%). The percentage of Dominicans and Puerto Ricans was higher compared to other classes. The average ESS score of individuals in this group was 6.0 ± 3.6. In addition to the higher levels of acculturation compared to the other groups, they were also the most likely to have insurance and had the lowest Spanish language preference of the three groups. The disturbed sleep group had higher odds of stroke/TIA history compared to the minimally symptomatic group (Tables 2–7).
The excessive sleepiness group was composed of 67% males, had the highest prevalence of obstructive lung disease, highest average BMI, REI3%, REI4%. The average ESS score was 15.7 ± 4.6 with 61% of individuals reporting they nap for more than 5 min, 73.7% feel sleepy during the day, and 97.2% may fall asleep or feel sleepy watching television. Insomnia-related symptoms were also higher for this group compared to the Minimally symptomatic group (Tables 2–7).
Similar trends in summary fit statistics were observed in the LCA solution without comorbidities, thus we also selected three-class solution. The three classes found in this solution with largely similar results to those with comorbidities (Supplementary Tables S4–S6).
Validation with MCA/HCPC
The three-class solution was validated by the MCA/HCPC models with 11 indices (e.g. silhouette) pointing to optimal fit and evidence for the highest relative loss of inertia through this solution. Cross-validation using different variables (e.g. no comorbidities) as well as different clustering techniques (hierarchical clustering, LCA) showed consistency of classes across models. The same three phenotypes with similar prevalence were extracted; however, we found differences in comorbidities prevalence through this extraction method. For a visual representation of these classes, see Supplementary Figure S1. While there were some differences between these two solutions, the OSA symptoms, as well as socio-demographics of each group, were largely equivalent (Tables 2 and Supplementary Tables S7–S9).
We tested the consistency of classification between the LCA and MCA/HCPC models. The minimally symptomatic group was the most consistent; 96% of individuals were classified as minimally symptomatic under both methods. The disturbed sleep phenotype was also stable at 83%. The excessive sleepiness was the least stable, with only a 67% overlap. These results were consistent in the models without comorbidities (Supplementary Table S10). Lastly, we tested whether recoding “don’t know” answers could affect models by recoding them to missing. While the subpopulation was smaller, cross-tabulation with the original LCA model shows that results are consistent with the original model (Supplementary Table S11).
Discussion
Using three clustering algorithms and covariates, we showed evidence for three sleep phenotypes: (1) minimally symptomatic, (2) disturbed sleep, and (3) excessive sleepiness among diverse Hispanics/Latinos. We also found noteworthy differences in comorbidity profiles across the three phenotypes. The excessive sleepiness group had an increased prevalence of obstructive lung disease, while the disturbed sleep group had higher odds of self-reported cerebrovascular disease and/or TIA. Hispanics/Latinos overall had higher cardiovascular risk (diabetes and hypertension) and cardiovascular disease compared to the populations represented in the ISAC and SAGIC cohorts [14, 17], and our results underscore the importance of considering these factors [38–40] in the context of sleep in this population. The prevalence of obstructive lung disease in our cohort was lower compared to the ISAC cohort (8.4% HCHS/SOL vs. 18.7% ISAC).
Our study is partly consistent with previous research of predominantly non-Hispanic White and Korean samples [14, 16, 17]. We provide added evidence on the stability of OSA symptom classes across populations, and our findings help generalize symptom heterogeneity to diverse community-dwelling Hispanics/Latinos. Our study is unique in that it points to differences in the prevalence of symptoms classes across populations. Our representative target populations had a higher prevalence of minimally symptomatic individuals compared to the ISAC and SAGIC samples and a smaller excessive sleepiness group compared to ISAC, SAGIC, and Korean samples. Furthermore, the Central/South Americans in SAGIC (6.6% of cohort) were less prevalent in the disturbed sleep group, but we did not see the same relationship in our cohort. These results have implications for targeted treatment strategies in Hispanics/Latinos. For example, some evidence [15] suggests that OSA patients in the excessive sleepiness group have better positive airway pressure (PAP) adherence compared to the other two groups. Future work should examine the implications of different treatment modalities across sleep-derived phenotypes among Hispanics/Latinos.
We posit several explanations for differences in prevalence by cohorts. First, our findings are based on community-dwelling adults, whereas ISAC and SAGIC focused on clinical samples, suggesting a referral bias in the latter studies. Second, despite consistent selection on moderate and severe apnea (REI3% ≥ 15), our target population is relatively young and had a lower OSA symptom load, most likely a consequence of our study design. Indeed, a recent study using a population-based cohort, found that sleep measures did not significantly differ between OSA and non-OSA individuals [41]. Third, the sociodemographic, cultural, and health profiles of our target population are distinct from those of non-Hispanic whites and Koreans. Our findings point to distinct socio-demographic (e.g. sex and Hispanic/Latino background) and cardiovascular and pulmonary profiles that could guide research and clinical work on Hispanics/Latinos as well as the non-Latino population. Socioeconomic characteristics [42, 43], cultural practices (e.g. acculturation [44, 45]), and health conditions (e.g. BMI [46]) influence sleep health, sleep management, and clinical management and treatment of symptoms. Kim et al.’s [16] community-dwelling Korean population-based study also found a high prevalence of minimally symptomatic patients (55.7%) in a healthier population. However, they reported a much lower prevalence of disturbed sleep relative to our cohort (38.1% in our cohort vs. 14.5%). The Sleep Heart Health study [18], a population-based study, reported around 12% disturbed sleep, but the Canadian Study [19] reported around 30% disturbed sleep and found another cluster with disturbed sleep symptoms. We found a disproportionate representation of Puerto Ricans, Dominicans, and women in the disturbed sleep group compared to the minimally symptomatic and excessive sleepiness groups. We posit the following explanations for these differences: Previous studies on HCHS/SOL cohort have found female, but not male, Puerto Ricans had the highest risk for three or more cardiovascular risk factors [40]. The majority of the Dominican and Puerto Ricans in our cohort came from the field center in the Bronx and thus may experience more environmental noise from living in a dense urban environment which could have affected sleep [47]. Results from HCHS/SOL have shown Dominicans and Puerto Ricans had the highest depressive symptoms of all groups [48], and sleep disturbances are common in individuals with depression [49, 50]. Similar to other studies [14, 16, 17], we found that despite greater numbers of men to women in each group, women were overrepresented in the disturbed sleep group, underscoring the importance of characterizing individuals into OSA subgroups. Women usually report more insomnia-like symptoms compared to men [51]. Women also have shorter apnea/hypopnea events [52] and greater reporting of somatic symptoms among women compared to men [53].
Our findings suggest that, despite evidence for moderate to severe OSA (REI3% ≥ 15), a large portion of individuals meeting clinical OSA criteria could go undiagnosed and untreated due to minimum symptoms/complaints present. Even compared to other population-based studies like the Sleep Heart Health Study [18] and the Canadian Study [19], there was an overrepresentation of minimally symptomatic individuals in our cohort. On the one hand, fewer OSA symptoms are potential markers of a better quality of life. Excessive sleepiness and insomnia have been linked with decrements in health quality of life [54], while many studies have shown no relation between AHI and quality of life [54–56]. On the other hand, fewer symptoms presentation could lead to longer periods of undiagnosed disorders and portend more severe downstream development of adverse health risks (e.g. hypertension [57]) and negative health outcomes. Underdiagnosis of OSA can lead to substantial adverse downstream health outcomes including cognitive impairment and dementia [58]. OSA may impact overall health through amplification of oxidative stress, endothelial dysfunction, and increased inflammation [59, 60].
Our data suggest that minimally symptomatic Hispanics/Latinos may face unique barriers to achieving diagnosis and treatment. For example, over half of the individuals in the minimally symptomatic group did not have health insurance (53%), had the lowest language acculturation of the three groups, and highest Spanish language preference. Spanish language preference is associated with lower rates of insurance and access health care [61], and this is in addition to lower access to healthcare among US Hispanics/Latinos, even after adjusting for income and health insurance [62]. Overall, these factors may present as barriers for both diagnosis and treatment of OSA. US nativity could also influence OSA classification. US-born Mexican Americans have higher rates of short sleep (both <7 and <6 h) and report more insomnia and excessive sleepiness symptoms compared to immigrants (also known as the “Hispanic Paradox”) [10]. Future studies should account for acculturation and other sociocultural factors (years in the US, health literacy, nativity, etc.). Prospective studies are needed to determine if derived sleep phenotypes have a differential incidence of health outcomes/chronic illness.
Strengths and limitations
To our knowledge, this is the first study that provides estimates for empirical classifications of OSA symptoms among diverse community-dwelling Hispanics/Latino adults in the US. Our study benefits from the design of the HCHS/SOL cohort, the large sample size relative to existing studies, and the potential generalizability to a wider range of the OSA spectrum compared to clinical samples. Our findings provide additional evidence for the validity and the generalizability of classes currently reported in the literature.
Our study has some limitations. First, our selected symptom items did not exactly match the ones reported in previous studies. However, the criteria in this study include largely similar array of OSA symptom comparable to previously published work [14, 16, 17]. This potentially limits comparisons of specific derived sleep phenotypes and their correlates across studies. Second, community-based population studies may have confounding conditions that lead to systematic errors (e.g. through differential measurement). HCHS/SOL minimized the influence of these confounders by creating strict regulations and guidelines across testing centers with centrally trained bilingual staff and technicians administering the tests in the individual’s preferred language. As with other studies, using subjective data can also introduce classification error which can have implications for interpretation in clinical settings. Even though we provided cardiovascular information for each cluster, given the scope of the paper, we did not perform any multivariable modeling to link our extracted phenotypes to the prevalence and incidence of cardiovascular disease and risk factors. Furthermore, the adherence in classification between LCA and MCA models was mixed for the excessive sleepiness group. Specifically, we found that 33% of individuals were classified out of the excessive sleepiness group by the MCA algorithm and classified as minimally or disturbed sleep. However, this reclassification was primarily driven by the MCA algorithm’s selection relative to the “sleepy while driving” item. Otherwise, our findings show that the symptoms were consistent with what the LCA classified as excessive sleepiness. Further explorations are required to validate which of the two classification methods is more precise. Finally, given the scope of the study, we did not test for invariance in phenotype composition across socio-demographically interesting groups. Future studies should explore whether the LCA classifications generated through this work are invariant to language. This can potentially provide supportive evidence that language differences in questionnaire compositions (e.g. through translation) did not lead to differential classifications. We believe that the differences in phenotype characteristics published in this work provide a starting point for future work focused on testing the stability of phenotypes across demographic and other health characteristics.
Conclusion
We reported three OSA phenotypes among diverse Hispanics/Latinos; a large US population that has been understudied in sleep research. These derived phenotypes were comparable to previous studies of non-Latinos. However, we found notable differences in the prevalence of these classes relative to non-Hispanic white, suggesting that other biopsychosocial and lifestyle factors such as diet, environment, and physical activity may contribute to derived sleep phenotypes among Hispanics/Latinos. Our reported empirically derived OSA phenotypes in Hispanics/Latinos may guide research focused on health outcomes (e.g. dementia) and could inform clinical diagnoses, development of targeted therapeutics, and allocation of public health resources. There is value in relating these symptom-based classes to physiological endotypes (arousal threshold, neuromuscular collapsibility, loop gain, etc.) which would provide specific therapeutic targets as well as risk prediction.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of HCHS/SOL for their important contributions. A complete list of staff and investigators has been provided by Sorlie P., et al. in Ann Epidemiol. 2010 Aug;20:642–649 and is also available on the study website http://www.cscc.unc.edu/hchs/. The authors thank the reviewers of this manuscript for the time and effort they invested in reading and commenting on our study.
Funding
The Hispanic Community Health Study/Study of Latinos was carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following Institutes/Centers/Offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Center on Minority Health and Health Disparities, the National Institute of Deafness and Other Communications Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and the Office of Dietary Supplements. Additionally, Kevin González, Dr. Wassim Tarraf, and Dr. Hector Gonzalez are funded by R56AG48642.
Disclosure Statement
Financial Disclosure: The author of this manuscript does not report any financial arrangements of connections.
Nonfinancial Disclosure: The author of this manuscript does not report any conflicts of interest.
References
- 1. Colby SL, et al. Projections of the Size and Composition of the US Population: 2014 to 2060. Population Estimates and Projections. Washington, DC: US Census Bureau; 2017. [Google Scholar]
- 2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2020. [Google Scholar]
- 3. Noble JM, et al. Type 2 diabetes and ethnic disparities in cognitive impairment. Ethn Dis. 2012;22(1):38–44. [PMC free article] [PubMed] [Google Scholar]
- 4. Punjabi NM, et al. ; Sleep Heart Health Study Investigators. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160(6):521–530. [DOI] [PubMed] [Google Scholar]
- 5. Yenokyan G, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure. Circulation. 2010;122:352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramos AR, et al. Obstructive sleep apnea and neurocognitive function in a Hispanic/Latino population. Neurology. 2015;84(4):391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hafner M, et al. Why sleep matters-the economic costs of insufficient sleep: a cross-country comparative analysis. Rand Health Q. 2017;6(4):11. [PMC free article] [PubMed] [Google Scholar]
- 8. Patel SR, et al. Social and health correlates of sleep duration in a US hispanic population: results from the Hispanic Community Health Study/Study of Latinos. Sleep. 2015;38(10):1515–1522. doi: 10.5665/sleep.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Redline S, et al. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med. 2014;189(3):335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seicean S, et al. An exploration of differences in sleep characteristics between Mexico-born US immigrants and other Americans to address the Hispanic paradox. Sleep. 2011;34(8):1021–1031. doi: 10.5665/SLEEP.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Senaratna CV, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. [DOI] [PubMed] [Google Scholar]
- 12. Yamashiro Y, et al. Why should sleep apnea be diagnosed and treated? Clin Pulmonary Med. 1994;1(4):250–259. [Google Scholar]
- 13. He J, et al. Mortality and apnea index in obstructive sleep apnea. Experience in 385 male patients. Chest. 1988;94(1):9–14. [PubMed] [Google Scholar]
- 14. Ye L, et al. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J. 2014;44(6):1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pien GW, et al. Changing faces of obstructive sleep apnea: treatment effects by cluster designation in the icelandic sleep apnea cohort. Sleep. 2018;41(3). doi: 10.1093/sleep/zsx201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim J, et al. Symptom-based subgroups of Koreans with obstructive sleep apnea. J Clin Sleep Med. 2018;14(3):437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Keenan BT, et al. Recognizable clinical subtypes of obstructive sleep apnea across international sleep centers: a cluster analysis. Sleep. 2018;41(3). doi: 10.1093/sleep/zsx214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mazzotti DR, et al. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med. 2019;200(4):493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Allen AH, et al. Symptom subtypes and cognitive function in a clinic-based OSA cohort: a multi-centre Canadian study. Sleep Med. 2020;74:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lavange LM, et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20(8):642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Allen RP, et al. ; Restless Legs Syndrome Diagnosis and Epidemiology workshop at the National Institutes of Health; International Restless Legs Syndrome Study Group. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101–119. [DOI] [PubMed] [Google Scholar]
- 22. Alidu L, et al. A systematic review of acculturation, obesity and health behaviours among migrants to high-income countries. Psychol Health. 2018;33(6):724–745. [DOI] [PubMed] [Google Scholar]
- 23. Crespo CJ, et al. Acculturation and leisure-time physical inactivity in Mexican American adults: results from NHANES III, 1988–1994. Am J Public Health. 2001;91(8):1254–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gorman BK, et al. Gender, acculturation, and health among Mexican Americans. J Health Soc Behav. 2010;51(4):440–457. [DOI] [PubMed] [Google Scholar]
- 25. Finch BK, et al. Could “acculturation” effects be explained by latent health disadvantages among Mexican immigrants? Int Migr Rev. 2009;43(3):471–495. [Google Scholar]
- 26. Cutler JA, et al. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52(5):818–827. [DOI] [PubMed] [Google Scholar]
- 27. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S13–S28. [DOI] [PubMed] [Google Scholar]
- 28. Miller MR, et al. ; ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. [DOI] [PubMed] [Google Scholar]
- 29. LaVange L, et al. Spirometry reference equations from the HCHS/SOL (Hispanic Community Health Study/Study of Latinos). Am J Respir Crit Care Med. 2017;196(8):993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsuji H, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94(11):2850–2855. [DOI] [PubMed] [Google Scholar]
- 31. Ware J Jr, et al. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
- 32. Ganesalingam J, et al. Latent cluster analysis of ALS phenotypes identifies prognostically differing groups. PLoS One. 2009;4(9):e7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dunn KM, et al. Characterizing the course of low back pain: a latent class analysis. Am J Epidemiol. 2006;163(8):754–761. [DOI] [PubMed] [Google Scholar]
- 34. Nylund KL, et al. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Modeling Multidiscip J. 2007;14(4):535–569. [Google Scholar]
- 35. Kowarik A, et al. Imputation with the R Package VIM. J Stat Softw. 2016;74(7):1–16. [Google Scholar]
- 36. Myers TA. Goodbye, listwise deletion: presenting hot deck imputation as an easy and effective tool for handling missing data. Commun Methods Meas. 2011;5(4):297–310. [Google Scholar]
- 37. Hawthorne G, et al. Imputing cross-sectional missing data: comparison of common techniques. Aust N Z J Psychiatry. 2005;39(7):583–590. [DOI] [PubMed] [Google Scholar]
- 38. Castañeda SF, et al. Cardiovascular disease risk factors and psychological distress among Hispanics/Latinos: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Prev Med. 2016;87:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Daviglus ML, et al. Cardiovascular disease risk factors in the Hispanic/Latino population: lessons from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Prog Cardiovasc Dis. 2014;57(3):230–236. [DOI] [PubMed] [Google Scholar]
- 40. Daviglus ML, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308(17):1775–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arnardottir ES, et al. Obstructive sleep apnoea in the general population: highly prevalent but minimal symptoms. Eur Respir J. 2016;47(1):194–202. [DOI] [PubMed] [Google Scholar]
- 42. Guglielmi O, et al. Association between socioeconomic status, belonging to an ethnic minority and obstructive sleep apnea: a systematic review of the literature. Sleep Med. 2019;57:100–106. [DOI] [PubMed] [Google Scholar]
- 43. Moore PJ, et al. Socioeconomic status and health: the role of sleep. Psychosom Med. 2002;64(2):337–344. [DOI] [PubMed] [Google Scholar]
- 44. Hale L, et al. Acculturation and sleep among a multiethnic sample of women: the Study of Women’s Health Across the Nation (SWAN). Sleep. 2014;37(2):309–317. doi: 10.5665/sleep.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Martinez-Miller EE, et al. US acculturation and poor sleep among an intergenerational cohort of adult Latinos in Sacramento, California. Sleep. 2019;42(3). doi: 10.1093/sleep/zsy246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Taheri S, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1(3):e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Muzet A. Environmental noise, sleep and health. Sleep Med Rev. 2007;11(2):135–142. [DOI] [PubMed] [Google Scholar]
- 48. González P, et al. Measurement properties of the Center for Epidemiologic Studies Depression Scale (CES-D 10): findings from HCHS/SOL. Psychol Assess. 2017;29(4):372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Germain A, et al. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23(7):571–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tsuno N, et al. Sleep and depression. J Clin Psychiatry. 2005;66(10):1254–1269. [DOI] [PubMed] [Google Scholar]
- 51. Zhang B, et al. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29(1):85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 52. Borker PV, et al. Non-REM apnea and hypopnea duration varies across population groups and physiologic traits. Am J Respir Crit Care Med. 2021;203:1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Barsky AJ, et al. Somatic symptom reporting in women and men. J Gen Intern Med. 2001;16(4):266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baldwin CM, et al. Sleep disturbances, quality of life, and ethnicity: the Sleep Heart Health Study. J Clin Sleep Med. 2010;6(2):176–183. [PMC free article] [PubMed] [Google Scholar]
- 55. Asghari A, et al. Evaluation of quality of life in patients with obstructive sleep apnea. Eur Arch Otorhinolaryngol. 2013;270(3):1131–1136. [DOI] [PubMed] [Google Scholar]
- 56. Weaver EM, et al. Polysomnography indexes are discordant with quality of life, symptoms, and reaction times in sleep apnea patients. Otolaryngol Head Neck Surg. 2005;132(2):255–262. [DOI] [PubMed] [Google Scholar]
- 57. Marin JM, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307(20):2169–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Daulatzai MA. Evidence of neurodegeneration in obstructive sleep apnea: relationship between obstructive sleep apnea and cognitive dysfunction in the elderly. J Neurosci Res. 2015;93(12):1778–1794. [DOI] [PubMed] [Google Scholar]
- 59. Yamauchi M, et al. Oxidative stress in obstructive sleep apnea. Chest. 2005;127(5):1674–1679. [DOI] [PubMed] [Google Scholar]
- 60. Arnaud C, et al. Obstructive sleep apnea, immuno-inflammation, and atherosclerosis. Paper presented at: Seminars in immunopathology; 2009. [DOI] [PMC free article] [PubMed]
- 61. Pearson WS, et al. Language preference as a predictor of access to and use of healthcare services among Hispanics in the United States. Ethn Dis. 2008;18(1):93–97. [PubMed] [Google Scholar]
- 62. Weinick RM, et al. Racial and ethnic differences in access to and use of health care services, 1977 to 1996. Med Care Res Rev. 2000;57(Suppl 1):36–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.