Abstract
Dopamine system deficiencies and associated behavioral phenotypes may be a critical barrier to success in treating stimulant use disorders. Similarities in dopamine dysfunction between cocaine and methamphetamine use disorder but also key differences may impact treatment efficacy and outcome. This review will first compare the epidemiology of cocaine and methamphetamine use disorder. A detailed account of the pharmacokinetic and pharmacodynamic properties associated with each drug will then be discussed, with an emphasis on effects on the dopamine system and associated signaling pathways. Lastly, treatment results from pharmacological clinical trials will be summarized along with a more comprehensive review of the involvement of the trace-amine associated receptor on dopamine signaling dysfunction among stimulants and its potential as a therapeutic target.
Introduction
Stimulants continue to rank as the most commonly abused agents worldwide, second only to cannabis 1. The most frequently abused psychostimulants include cocaine, amphetamine and methamphetamine. Here, amphetamine and methamphetamine will be referred to by the class name, amphetamines, unless the discussion is specific to either amphetamine or methamphetamine. Decades of addiction research using both animal models of addiction and advanced neuroimaging techniques, provide evidence of long-lasting changes in neurochemical networks, particularly the dopaminergic system. These neurochemical abnormalities are a potential substrate for the affective and behavioral sequelae of cycles of addiction, including craving, uncontrolled drug intake and withdrawal 2. Cocaine shares dopamine-enhancing properties with S-methamphetamine and S-amphetamine, but the drug classes differ in their effects on components of the dopamine system. For example, dopamine transporter (DAT) numbers are decreased in chronic methamphetamine users but are either increased or unchanged in chronic cocaine users 3, 4. Pharmacokinetic differences between cocaine and amphetamines may contribute to divergent actions on the dopamine system, including differential agonism of amphetamine-type stimulants and cocaine at the trace amine associated receptor 1 (TAAR1) to modulate dopamine signaling 5.
This paper will review the current understanding of the effects of cocaine and amphetamines on dopamine receptors and transporters and offer hypotheses to explain the similarities and differences between the drug classes, as well as examine the literature on pharmacological approaches to treatment. Lastly, a review of clinical investigations in combination with preclinical evidence may present new and promising therapeutic avenues.
Epidemiology and morbidity
Methamphetamine is the preferred amphetamine in the US and, as the price per gram of methamphetamine continues to decrease and potency and availability increase, so does the number of methamphetamine-related deaths. In Oregon, for example, methamphetamine-related deaths increased over 400% from 2009 to 2018 and outnumbered heroin-related deaths by the end of the decade 6. Over the same time period, methamphetamine accounted for 89% of drug-related violent crimes and 69% of drug-related property crimes. Like methamphetamine, cocaine-related overdose deaths have doubled between 2007-2017 1 with a 34.4% increase between 2016-2017 in the US 7.
The risks of medical morbidity differ between amphetamines and cocaine. A five-fold risk for hemorrhagic stroke is associated with amphetamine use, twice that of cocaine use 8, while a 5.7-fold increased risk for ischemic stroke was associated with cocaine use but not with amphetamine use 8, 9. Furthermore, methamphetamine users were 19% more likely than cocaine users to develop an acute myocardial infarction 10. Although cocaine and amphetamines are both sympathomimetic drugs, differences in rates of cardiovascular and cerebrovascular complications may be attributed to the longer half-life of amphetamines during which cardiac demand is elevated.
Stimulant use is often associated with irritability, interpersonal sensitivity, physical aggression, psychomotor agitation and uncontrolled anger 11, 12. Early abstinence is accompanied with mood disturbances 13, 14, along with elevated levels of depression, anxiety, restlessness and intense drug craving 11, 15-17. Individuals who abuse amphetamines or cocaine can also develop paranoia, hallucinations, and psychotic behavior. Stimulant-induced psychosis, however, is more common with the use of amphetamines than with cocaine 12, possibly due to the longer persistence of amphetamines in the brain causing prolonged activation of mesolimbic dopamine pathways, which may also lead to more violent behaviors 12, 18.
Although both cocaine and amphetamine use have been associated with perpetration of crime and violence, chronic use of methamphetamine has been more closely related to violent behavior than that of cocaine 12, 19. For example, higher psychopathy ratings and number of total criminal convictions were related to lower levels of corticostriatal resting-state functional connectivity in methamphetamine users 20. Thus, reduced cortical regulation of reward networks in methamphetamine users may predispose them to antisocial behavior and criminality. It is unknown, however, whether resting-state functional connectivity is associated with psychopathy or criminal behavior in cocaine users.
Pharmacokinetics
Although amphetamine and methamphetamine have similar subjective effects, methamphetamine is thought to be more addictive 21. The N-methyl group on the molecule renders methamphetamine more lipid-soluble than amphetamine, facilitates the transport of the substance across the blood–brain barrier and inhibits enzymatic degradation by monoamine oxidase (MAO) 22. Methamphetamine also inhibits DAT-mediated dopamine reuptake more efficiently than amphetamine 23.
Both human and animal studies demonstrate differences between cocaine and methamphetamine on dopamine kinetics. Higher levels of intrasynaptic dopamine and slower clearance of methamphetamine compared to cocaine contributes to longer behavioral effects, oxidative stress and damage to the dopaminergic system 24, 25. The duration of action for cocaine is approximately 1-3 hours while that for amphetamines are approximately 8-13 hours 26, 27. In non-human primates, [11C]cocaine and [11C]S-methamphetamine showed differences in brain distribution, kinetics and clearance rates. Although [11C]S-methamphetamine peaked more slowly than [11C]cocaine, it also cleared more slowly and its distribution extended beyond the striatum to cortical brain regions 28. Similarly, in humans, cocaine was concentrated in only the striatum and its uptake and clearance was faster than that of methamphetamine 29.
Pharmacodynamics of the monoamine system
Amphetamines and cocaine inhibit the reuptake of monoamine neurotransmitters, such as norepinephrine, dopamine, and serotonin and thereby enhance the intrasynaptic levels of these molecules. Multiple effects in the central nervous system result from increased cytoplasmic and extracellular concentrations of these neurotransmitters 22. Although both classes have broadly similar neurochemical and behavioral effects, there are important differences, such as the chronic effects of amphetamines on the dopaminergic system due to the intracellular effects at the vesicular monoamine transporter 2 (VMAT2) and TAAR1 receptor, neither of which is affected by cocaine.
Amphetamines are substrates of synaptic monoamine transporters, and can thus affect cytoplasmic concentrations of monoamines by interactions with intracellular targets such as VMAT2 30, 31, MAO 32, tyrosine hydroxylase 32 and the intracellular TAAR1 receptor 5. Amphetamines substantially elevate intracellular dopamine levels by altering dopamine synthesis, packaging and release in the dopaminergic terminal 22. Amphetamines compete with other substrates of VMAT2, a transporter that translocates monoamines from cytosol into synaptic vesicles 30, 31. Amphetamines inhibit the ability of MAO to degrade excess intracellular dopamine 32 and increase the expression of tyrosine hydroxylase 32, the rate limiting step for dopamine biosynthesis 22, resulting in further increase in intracellular dopamine. Amphetamines reduce cytoplasmic dopamine by facilitating dopamine release by reversing the direction of the DAT 22.
Cocaine, on the other hand, binds to monoamine transporters and blocks clearance of the transmitters from the synapse, but, because it is not a substrate of the DAT, does not interact with intracellular targets. Although significant attention has been focused on the role of dopamine neurotransmission in psychostimulant addictions 28, it is noteworthy that, among monoamine transporters, cocaine has the highest affinity for the serotonin transporter, whereas amphetamines have the strongest affinity for the norepinephrine transporter 33. The reinforcing properties of cocaine and amphetamines, however, are correlated with DAT affinity and not with affinity for the serotonin or norepinephrine transporters 34, consistent with the central role of dopamine as a mediator of reward. Moreover, dopamine depletion in the nucleus accumbens, but not forebrain norepinephrine depletion 35, markedly attenuates self-administration of cocaine or amphetamines 36-41.
Stimulants and synaptic levels of dopamine
Cocaine and amphetamines both increase intrasynaptic dopamine levels, which significantly contribute to the reinforcing effects of psychostimulants. Cocaine- and methamphetamine-induced increases in intrasynaptic dopamine positively correlates with self-reports of euphoria in humans 42. In rodent models, acute self-administration of cocaine and amphetamines increase extracellular dopamine levels in the nucleus accumbens 43-45. Chronic exposure to stimulants in rodents, however, leads to striatal dopamine depletion. For example, rats receiving a neurotoxic regimen of methamphetamine exhibited a reduction in dopamine concentration of 56% in the caudate nucleus and 30% in the nucleus accumbens 46. Similarly, a recent meta-analysis 3 of seven clinical studies of dopamine release in chronic stimulant users (2 studies of amphetamine, 5 of cocaine) found a robust decrease in dopamine release in patients relative to controls. Postmortem studies in humans also show lower striatal dopamine levels in both cocaine and methamphetamine users compared to controls 47-49.
Stimulant effects on dopamine transporter availability
Neurochemical and biochemical studies of addiction show decreases in brain markers of the degeneration of pre-synaptic dopamine nerve terminals with methamphetamine exposure. Non-human primates exposed to escalating doses of methamphetamine show a reduction in DAT availability 50, 51, and in mice, autoradiographic experiments show DAT depletion as soon as 24 hours after methamphetamine exposure and a 60% depletion 2 days following methamphetamine exposure 52. In addition, in vivo amphetamine and methamphetamine exposure in rats caused a reduction in DAT availability 53, 54. Interestingly, animal studies have found increases, decreases or no change in DAT levels following cocaine administration 55. These discrepant results are partly explained by the length of cocaine administration and number of days of withdrawal before DAT assessment. In non-human primates, decreases in DAT density occur with short cocaine self-administration periods (< 3 months), while longer administration periods (1.5 years) lead to increases in striatal DAT densities 56.
Postmortem studies also found decreased striatal levels of DAT in methamphetamine users 49, 57, while DAT binding was markedly increased in cocaine users 58.. In line with these studies, two recent meta-analyses 3, 4 of human neuroimaging investigations systematically examined the effects of stimulant use on D2/3 and DAT availability. Random effects analyses showed that methamphetamine users had a robust decrease in striatal DAT availability, whereas cocaine users had increased numbers of DAT in the striatum 4. The significant differences between these drugs in the pharmacokinetic clearance rates and pharmacodynamics on DAT levels most likely contribute to differences in DAT availability.
Stimulant effects on dopamine receptor availability
Dopamine D2-type receptor availability has been used as a biomarker for the investigation of postsynaptic dopamine function in both human stimulant users and animals. Similar to human studies showing reduced striatal D2/3 availability in users of both classes of stimulants 3, 4, non-human primates consistently show decreases in striatal D2-type binding following administration of cocaine or methamphetamine 51, 59.
Dopamine D3 receptors also have a role in mediating behavioral responses to psychostimulant drugs. A number of studies using D3-knockout mice suggest that one function of the D3 receptor is to modulate behaviors by inhibiting activation of dopamine D1 and D2 receptors 60, 61. D3-knockout mice exhibit heightened locomotor responses to cocaine, amphetamine and morphine 62 and produce greater conditioned place preference than wild types following low doses of amphetamine 63. However, one study shows that increased D3 receptor binding is associated with an escalation of cocaine intake 64. Pharmacological studies using D3 receptor agonists and antagonists also suggest that D3 receptors play a critical role in the motivation for drugs 65. Selective D3 receptor antagonists show reduced responding for cocaine and methamphetamine in rats during high demand reinforcement schedules 65. Although the exact involvement of D3 receptors in addiction is unclear, the above studies in combination with results showing greater D3 than D2 receptor saturation following stimulant exposure 60, 66 indicate a role for D3 receptors in the biobehavioral response to stimulants.
In vivo neuroimaging studies of human methamphetamine and cocaine dependence have examined D3 receptor density using the radioligand, [11C]-(+)-propyl-hexahydro-naphtho-oxazin ([11C]-(+)-PHNO). Although [11C]-(+)-PHNO lacks absolute specificity for D3 receptors, it does have higher differential binding at this receptor subtype. Methamphetamine users compared to healthy controls exhibit greater relative binding (ratio of D3 to D2 receptors) in the substantia nigra, a region with high expression of D3 receptors 67, and show a decrease in amphetamine-induced D3 binding, suggesting greater amphetamine-induced dopamine release in the substantia nigra 68. Methamphetamine users, however, exhibit less binding in D2-rich striatal regions compared to controls, which may be attributed to low striatal D2-type receptor availability associated with methamphetamine use 67. Cocaine users also exhibit greater D3-type binding in the substantia nigra 69. Furthermore, a postmortem study of cocaine users found an increase in [3H]-(+)-7-OH-DPAT binding in the lateral and medial divisions of the substantia nigra as well as higher D3 receptor binding in the caudate, putamen and nucleus accumbens 70. Although previous human neuroimaging studies with [11C]-(+)-PHNO did not show a reduction in D2/3 receptor availability in cocaine users 69, 71, a more recent study extends these results to show that individuals with cocaine use disorder demonstrate lower binding potential (BPND) in the striatum and greater BPND in the midbrain as well as lower regional striatopallidal D2 binding and greater regional pallidonigral D3 binding in relation to years of cocaine use 72.
The evidence suggests that dopamine D3 compounds are promising candidates for addiction therapies. However, both D3 agonists and antagonists have shown to attenuate some components of the addiction cycle, such as drug seeking, self-administration, and cue- and stress-induced reinstatement 65. While D3 receptor agonists such as 7-OH-DPAT reduce cocaine-seeking behavior, D3 receptor antagonists attenuate cue-reinstatement of drug responding 65. The efficacy of treatments targeting D3 receptors could therefore vary with length of abstinence or functional selectivity.
Pharmacological studies also indicate a role for D1-type dopamine receptors in psychostimulant reinforcement 73, 74. Self-administration studies show that rats and non-human primates self-administer full D1-type agonists and that systemically administered D1-type antagonists decrease the reinforcing efficacy of cocaine 75-79. Cocaine self-administration paradigms in rats and in non-human primates show a down-regulation of striatal D1 receptors after a withdrawal period of 18-24 hours 80, 81. The effects of methamphetamine on D1 receptors after a withdrawal period of less than 24 hours are inconclusive 82-84; however, persistent D1 receptor upregulation (21 days) is reported in the substantia nigra 83, but not in the striatum 82, 83.
While cocaine exposure causes reduced dopamine D1 receptor levels in animals, human in vivo and ex vivo studies show no differences in D1 receptor binding in cocaine users. A PET study using the ligand [11C]NNC112 (8-chloro-7-hydroxy-3-methyl-5-(7-benzofuranyl)-2,3,4,5-tetrahydro-IH-3-benzazepine) showed no differences between cocaine-dependent and control subjects in D1 receptor availability 85. However, despite no differences in D1 receptor binding, D1 receptor availability was negatively associated with the choice to self-administer cocaine. Similar results were obtained in methamphetamine use disorder, with no significant differences in D1 binding compared to controls 86. In a postmortem study, cocaine users show no difference in D1 receptor protein immunoreactivity while methamphetamine users show an increase in the nucleus accumbens compared to controls 87. Together, the results suggest that D1 receptors are somewhat protected from the stimulant-induced increases in synaptic dopamine, perhaps because of relatively low D1 receptor affinity for dopamine compared to D2 receptors.
Summary of MA and Cocaine effects on dopamine targets
Overall, chronic stimulant use is associated with decreased dopamine release and D2 receptor availability compared to controls but amphetamines cause substantial decreases in DAT binding and potentially a bigger effect on D1 receptor availability than for cocaine (Figure 1). The magnitude of deficiencies in dopamine release and D2 receptor availability between cocaine and amphetamines has not been well studied nor have the interactions between these markers of dopamine signaling been evaluated within or across cocaine- and methamphetamine-use disorder. Studies that directly compare abnormalities in dopamine function between stimulant classes and the interaction with dopaminergic markers has the potential to critically inform medication development and may improve the efficacy of current treatment avenues as summarized below.
Figure 1. Mechanistic comparisons of methamphetamine and cocaine and the implications for treatment.
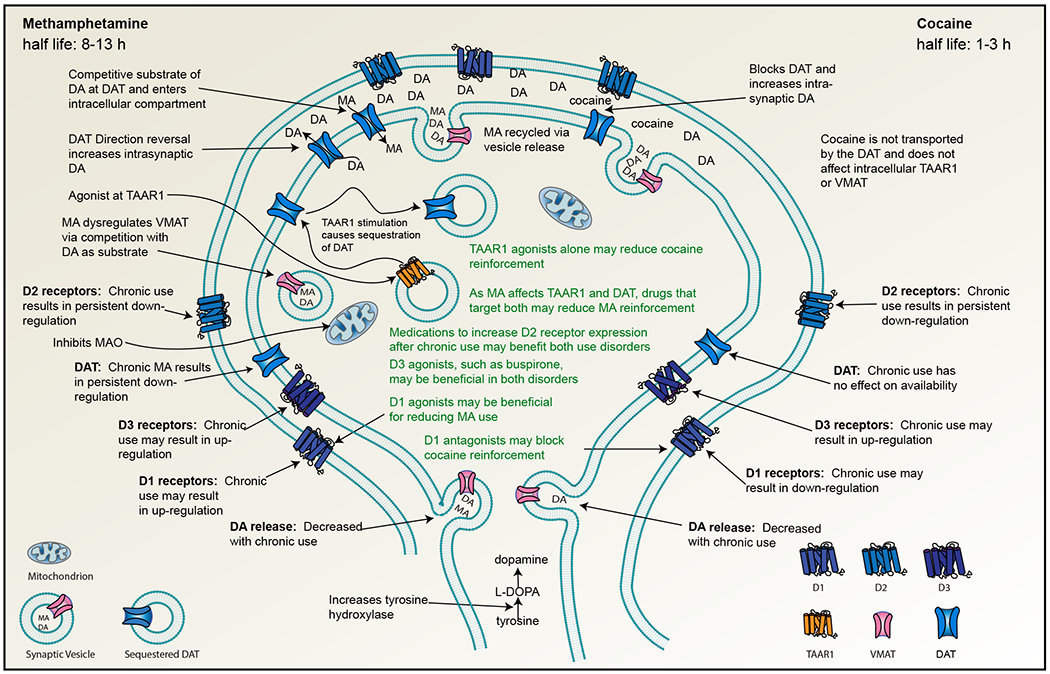
Similarities and differences between methamphetamine and cocaine on dopamine signaling may confer differences in treatment approaches.
Treatment
Despite high rates of attrition and relapse 88, 89, cognitive behavioral therapy, contingency management 90, 91, and motivational interviewing 91, 92 are the current options for treating stimulant dependence. Although there are no approved pharmacological treatments for stimulant dependence, many clinical trials are testing potential pharmacotherapies, many of which target the dopamine system.
Several recent systematic reviews and meta-analyses have investigated pharmacological treatments for amphetamine and cocaine use disorders. Meta-analyses specifically investigating the use of prescription psychostimulants for amphetamine 93, 94 and cocaine 95 use disorders, showed no effect on sustained abstinence from amphetamines 93, 94; however, there was an effect for sustained abstinence from cocaine with higher doses of psychostimulants, particularly prescription amphetamines 95. Meta-analyses investigating multiple drug classes showed that bupropion, psychostimulants, and topiramate may improve abstinence in cocaine use disorder 96. For methamphetamine use disorder, anticonvulsants, antipsychotics, opioid receptor antagonists, varenicline, and atomoxetine were ineffective; however, methylphenidate did show some promise in reducing methamphetamine use 97. Taken together, these somewhat unremarkable pharmacotherapy results for both cocaine and amphetamine treatment indicate that other options should also be evaluated as potential targets for treatment.
Trace amine associated receptor 1 (TAAR1) and new treatment approaches.
A recently recognized critical difference between amphetamines and cocaine is the agonism of the former 5, but not the latter, at the intracellular TAAR1 receptor 98. TAAR1, a G-protein receptor, is expressed in dopaminergic brain regions, including ventral tegmental area (VTA), substantia nigra pars compacta and ventral (including nucleus accumbens) and dorsal striatum. A mutation in mouse Taar1 that produces a non-functional receptor accounts for 60% of the variance in methamphetamine intake in lines of mice selected to either drink or avoid a methamphetamine solution 99, 100. The methamphetamine high drinking line was homozygous for the non-functional Taar1 allele, while methamphetamine low drinking mice had at least one allele that produced a functional receptor. As amphetamine-induced dopamine release is increased in Taar1 knockout mice 101, the blunted effect of methamphetamine in mice with functional TAAR1 receptors is likely due to the reduction in TAAR1 mediated dopamine signaling. This observation is consistent with human studies showing that striatal dopamine signaling is related to addiction severity and treatment response 102, 103 and that dopamine release is positively related to the reinforcing effects of drugs 42. Humans, who almost all have functional TAAR1 receptors, readily self-administer methamphetamine, as do rats and non-human primates. Therefore, TAAR1 influences in humans may be more complicated than in mice. Polymorphisms in the TAAR1 gene produce functional, sub-functional and non-functional variants 104, although the sub-functional or non-functional variants are rare. Recently, Loftis et al. 105 found that a synonymous single nucleotide polymorphism (SNP) in the human TAAR1 gene was associated with increased craving in methamphetamine users. Roles for non-functional TAAR1 genotypes have been proposed in obesity 106, schizophrenia 107 and ‘mental illness’ 108.
TAAR1 influence is implicated in cell bodies (VTA) and terminal field levels (nucleus accumbens and striatum) of dopamine neurons 108-110. The potential sites of TAAR1 action are determined by the requirement that amphetamines be transported into neurons via the DAT in order to interact with the intracellular TAAR1 receptor 111. In midbrain dopamine neurons (likely a major site for TAAR1 effects), amphetamine stimulation of TAAR1 initiates a complex cascade of effects that includes internalization of the DAT and the excitatory amino acid transporter 3 (EAAT3); the latter clears glutamate from the peri-neuronal space. As single dose stimulation of TAAR1 by amphetamine results in initial internalization of the DAT but subsequent termination of internalization, it is possible that chronic methamphetamine use results in persistent DAT trafficking. As cocaine is not transported by the DAT and is not a TAAR1 ligand, this effect would not be expected with cocaine. This is consistent with the robust reductions in DAT numbers in methamphetamine users but increases in cocaine users 3, 4.
In addition to dopaminergic effects, a role for glutamate in TAAR1-mediated effects of methamphetamine is also strongly supported. For example, there are TAAR1-mediated effects of methamphetamine on glutamate regulation of VTA dopamine neurons 112, 113. Furthermore, incubation of methamphetamine craving depends on methamphetamine-induced plasticity of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) transmission in the nucleus accumbens 114, 115. Finally, activation of downstream targets of striatal projections is dependent in large part upon striatal and VTA excitation by glutamatergic inputs. Changes in the strength of these inputs (synaptic plasticity) will no doubt modulate the response of downstream regions to dopamine.
As noted above, chronic use of amphetamines and cocaine have differential effects on dopamine targets. Decreased DAT in (meth)amphetamine users is possibly due to amphetamine agonism at the TAAR1 receptor as well as kinetic mechanisms. As cocaine is not transported, the increase in DAT number in chronic cocaine users likely results from compensatory upregulation of the DAT in response to continued blockade.
TAAR1 and Implications for treatment
Several full and partial agonists at the TAAR1 receptor have been developed recently 116-118. As TAAR1 stimulation decreases dopamine release in the striatum, animal studies have examined the effect of TAAR1 agonists on addictive behaviors [See 119 for review]. Interestingly, despite the disjunct actions of methamphetamine and cocaine at TAAR1 at the synaptic level, the partial TAAR1 agonist, RO5263397, reduces development of sensitization and cue-induced reinstatement for both substances 110 and the compound presents as a possible model agent for methamphetamine replacement therapy 120. TAAR1 receptor activation decreases dopamine signaling in mesocorticolimbic and corticostriatal networks 109, 110, while treatment with a TAAR1 antagonist 116 or knocking out the Taar1 rodent gene 101 is associated with increased striatal dopamine signaling. Therefore, the effects of TAAR1 agonists on addictive behavior associated with both stimulant classes is hypothesized to derive from reduced striatal dopamine release. Emphasizing the importance of TAAR1 in methamphetamine addiction, a recent paper found increased craving for methamphetamine in a subset of humans with a common TAAR1 genotype 105.
There are no differentiating effects of TAAR1 on amphetamine or cocaine self-administration in animal models and cocaine is not an agonist for TAAR1. The use of TAAR1 agonists and the associated effect on dopamine signaling, however, may still be beneficial for cocaine-use disorder, a population where DAT expression is intact. The influence of time course may be critical in testing and evaluating TAAR1 agents. The acute effects of methamphetamine agonism at TAAR1 is DAT sequestration, followed by inhibition of sequestration via two different G protein signaling pathways. TAAR1 agonists, secondary to blunting dopamine release, may have similar effects on initial drug use or maintenance in animal models. As methamphetamine agonism of TAAR1 likely contributes, in part, to the consistent reports of decreased DAT, and methylphenidate has shown some promise in methamphetamine addiction, perhaps the use of TAAR1-targeted pharmacotherapies in conjunction with methylphenidate may increase therapeutic effectiveness of these medications. There are, however, currently no clinical trials to test TAAR1 agonists in human addictive disorders, although two TAAR1 agonists are in phase II trials for the treatment of schizophrenia. Differences in the efficacies of TAAR1 agents in amphetamine vs. cocaine use disorders may become apparent when testing the effect of TAAR1 agents in chronically using populations.
Conclusion
Together, the results support targeting the neurobiological pathology of the dopamine system as a promising avenue for the treatment of stimulant use disorders. This is in line with studies showing that, in both cocaine and methamphetamine use disorders, higher levels of D2-type receptor availability and dopamine release is associated with better response to contingency management 102, 121. In addition to protecting against relapse and contributing to the success of treatment, improvements in dopamine signaling may also ameliorate the behavioral and cognitive phenotypes that accompany addiction 122. Cocaine and methamphetamine show similarities in dopamine dysfunctions; however, the magnitude of severity of dopamine deficiencies between stimulants play key roles in medication effectiveness along with some key differences, such as DAT or TAAR1 expression. In addition to the differences between cocaine and amphetamines on dopamine function as factors in the efficacy of treatment options, the lack of compliance and differences in the frequency and duration of treatments may also have impact on the lack of definitive results in clinical trials. In addition, psychiatric comorbidity, baseline drug use, levels of education and neurocognitive impairments also complicate treatment outcomes 123. While these variables need to be considered when examining the success or failure of pharmacotherapies, they are insufficient if individual variability in neurobiological factors that determine the response to medications exists between treatment-seeking participants. Future studies investigating new pharmacotherapies while considering the interactions between psychosocial variables, psychiatric comorbidities, demographic variables and neurobiological variability would greatly advance the development of tailored treatment approaches for stimulant use disorders.
Acknowledgements
This work was supported in part by the Department of Veterans Affairs Clinical Sciences Research and Development Merit Review Program, I01 CX001558-01A1 (WFH); Department of Justice 2010-DD-BX-0517 (WFH); National Institute on Drug Abuse P50DA018165 (WFH) and R21 DA047602-01A1 (WFH); Oregon Clinical and Translational Research Institute, 1 UL1 RR024140 01 from the National Center for Research Resources, a component of the National Institutes of Health and National Institute of Health Roadmap for Medical Research (WFH) and National Institute on Alcohol Abuse and Alcoholism R21 AA020039 (WFH). Dr. Kohno was supported by Department of Veterans Affairs Clinical Sciences Research and Development Career Development Award CX001790, Oregon Health & Science University Collins Medical Trust Award APSYC0249, Medical Research Foundation of Oregon APSYC0250 and Center for Women’s Health Circle of Giving GPSYC0287A.
Footnotes
Publisher's Disclaimer: Disclaimer
The contents of this paper do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.World Drug Report 2020. (United Nations publication, Sales No. E.20.XI.6). [Google Scholar]
- 2.Koob GF. Circuits, drugs, and drug addiction. Adv Pharmacol 1998; 42: 978–982. [DOI] [PubMed] [Google Scholar]
- 3.Ashok AH, Mizuno Y, Volkow ND, Howes OD. Association of Stimulant Use With Dopaminergic Alterations in Users of Cocaine, Amphetamine, or Methamphetamine: A Systematic Review and Meta-analysis. JAMA Psychiatry 2017; 74(5): 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Proebstl L, Kamp F, Manz K, Krause D, Adorjan K, Pogarell O et al. Effects of stimulant drug use on the dopaminergic system: A systematic review and meta-analysis of in vivo neuroimaging studies. Eur Psychiatry 2019; 59: 15–24. [DOI] [PubMed] [Google Scholar]
- 5.Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol 2001; 60(6): 1181–1188. [DOI] [PubMed] [Google Scholar]
- 6.Oregon-Idaho High Intensity Drug Trafficking Area. Annual Report 2019. [Google Scholar]
- 7.Drug Enforcement Administration. 2019 National drug threat assessment Annual Drug Report. US Department of Justice, Drug Enforcement Administration: Springfield, Virginia, 2019. [Google Scholar]
- 8.Westover AN, McBride S, Haley RW. Stroke in young adults who abuse amphetamines or cocaine: a population-based study of hospitalized patients. Arch Gen Psychiatry 2007; 64(4): 495–502. [DOI] [PubMed] [Google Scholar]
- 9.Cheng YC, Ryan KA, Qadwai SA, Shah J, Sparks MJ, Wozniak MA et al. Cocaine Use and Risk of Ischemic Stroke in Young Adults. Stroke 2016; 47(4): 918–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callaghan RC, Halliday M, Gatley J, Sykes J, Taylor L, Benny C et al. Comparative hazards of acute myocardial infarction among hospitalized patients with methamphetamine-or cocaine-use disorders: A retrospective cohort study. Drug Alcohol Depend 2018; 188: 259–265. [DOI] [PubMed] [Google Scholar]
- 11.Zweben JE, Cohen JB, Christian D, Galloway GP, Salinardi M, Parent D et al. Psychiatric symptoms in methamphetamine users. Am J Addict 2004; 13(2): 181–190. [DOI] [PubMed] [Google Scholar]
- 12.Boles S, Miotto K. Substance abuse and violence A review of the literature. Aggression and violent behavior 2003; 8: 155–174. [Google Scholar]
- 13.Vik PW. Methamphetamine use by incarcerated women: comorbid mood and anxiety problems. Womens Health Issues 2007; 17(4): 256–263. [DOI] [PubMed] [Google Scholar]
- 14.Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse M, Ang A, Rawson R et al. Identifying methamphetamine users at risk for major depressive disorder: findings from the methamphetamine treatment project at three-year follow-up. Am J Addict 2008; 17(2): 99–102. [DOI] [PubMed] [Google Scholar]
- 15.London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Archives of General Psychiatry 2004; 61: 73–84. [DOI] [PubMed] [Google Scholar]
- 16.Newton TF, Kalechstein AD, Duran S, Vansluis N, Ling W. Methamphetamine abstinence syndrome: Preliminary findings. American Journal on Addictions 2004; 13: 248–255. [DOI] [PubMed] [Google Scholar]
- 17.Semple SJ, Zians J, Strathdee SA, Patterson TL. Psychosocial and behavioral correlates of depressed mood among female methamphetamine users. J Psychoactive Drugs 2007; Suppl 4: 353–366. [DOI] [PubMed] [Google Scholar]
- 18.Alexander PD, Gicas KM, Willi TS, Kim CN, Boyeva V, Procyshyn RM et al. A comparison of psychotic symptoms in subjects with methamphetamine versus cocaine dependence. Psychopharmacology (Berl) 2017; 234(9–10): 1535–1547. [DOI] [PubMed] [Google Scholar]
- 19.Gizzi MC, Gerkin P. Methamphetamine use and criminal behavior. Int J Offender Ther Comp Criminol 2010; 54(6): 915–936. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman WF, Jacobs MB, Dennis LE, McCready HD, Hickok AW, Smith SB et al. Psychopathy and Corticostriatal Connectivity: The Link to Criminal Behavior in Methamphetamine Dependence. Front Psychiatry 2020; 11: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor S, Lewis C, Olive M. The neurocircuitry of illicit psychostimulant addiction: acute and chronic effects in humans. Substance Abuse and Rehabilitation 2013; 4: 29–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karila L, Weinstein A, Aubin HJ, Benyamina A, Reynaud M, Batki SL. Pharmacological approaches to methamphetamine dependence: a focused review. Br J Clin Pharmacol 2010; 69(6): 578–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodwin JS, Larson GA, Swant J, Sen N, Javitch JA, Zahniser NR et al. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J Biol Chem 2009; 284(5): 2978–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohno M, Link J, Dennis LE, McCready H, Huckans M, Hoffman WF et al. Neuroinflammation in addiction: A review of neuroimaging studies and potential immunotherapies. Pharmacol Biochem Behav 2019; 179: 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities: classical and emerging mechanisms. Ann N Y Acad Sci 2010; 1187: 101–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jufer RA, Wstadik A, Walsh SL, Levine BS, Cone EJ. Elimination of cocaine and metabolites in plasma, saliva, and urine following repeated oral administration to human volunteers. J Anal Toxicol 2000; 24(7): 467–477. [DOI] [PubMed] [Google Scholar]
- 27.Harris DS, Boxenbaum H, Everhart ET, Sequeira G, Mendelson JE, Jones RT. The bioavailability of intranasal and smoked methamphetamine. Clin Pharmacol Ther 2003; 74(5): 475–486. [DOI] [PubMed] [Google Scholar]
- 28.Feltenstein MW, See RE. Systems level neuroplasticity in drug addiction. Cold Spring Harb Perspect Med 2013; 3(5): a011916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fowler JS, Volkow ND, Logan J, Alexoff D, Telang F, Wang GJ et al. Fast uptake and long-lasting binding of methamphetamine in the human brain: comparison with cocaine. NeuroImage 2008; 43(4): 756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vergo S, Johansen JL, Leist M, Lotharius J. Vesicular monoamine transporter 2 regulates the sensitivity of rat dopaminergic neurons to disturbed cytosolic dopamine levels. Brain Res 2007; 1185: 18–32. [DOI] [PubMed] [Google Scholar]
- 31.Volz TJ, Hanson GR, Fleckenstein AE. The role of the plasmalemmal dopamine and vesicular monoamine transporters in methamphetamine-induced dopaminergic deficits. J Neurochem 2007; 101(4): 883–888. [DOI] [PubMed] [Google Scholar]
- 32.Pereira FC, Lourenco ES, Borges F, Morgadinho T, Ribeiro CF, Macedo TR et al. Single or multiple injections of methamphetamine increased dopamine turnover but did not decrease tyrosine hydroxylase levels or cleave caspase-3 in caudate-putamen. Synapse 2006; 60(3): 185–193. [DOI] [PubMed] [Google Scholar]
- 33.Ritz MC, Kuhar MJ. Relationship between self-administration of amphetamine and monoamine receptors in brain: comparison with cocaine. J Pharmacol Exp Ther 1989; 248(3): 1010–1017. [PubMed] [Google Scholar]
- 34.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 1987; 237(4819): 1219–1223. [DOI] [PubMed] [Google Scholar]
- 35.Roberts DC, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav 1977; 6(6): 615–620. [DOI] [PubMed] [Google Scholar]
- 36.Lyness WH, Friedle NM, Moore KE. Destruction of dopaminergic nerve terminals in nucleus accumbens: effect on d-amphetamine self-administration. Pharmacol Biochem Behav 1979; 11(5): 553–556. [DOI] [PubMed] [Google Scholar]
- 37.Roberts DC, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav 1980; 12(5): 781–787. [DOI] [PubMed] [Google Scholar]
- 38.Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berl) 1984; 84(2): 167–173. [DOI] [PubMed] [Google Scholar]
- 39.Caine SB, Koob GF. Effects of dopamine D-1 and D-2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J Pharmacol Exp Ther 1994; 270(1): 209–218. [PubMed] [Google Scholar]
- 40.Caine SB, Koob GF. Effects of mesolimbic dopamine depletion on responding maintained by cocaine and food. J Exp Anal Behav 1994; 61(2): 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koob GF, Caine B, Markou A, Pulvirenti L, Weiss F. Role for the mesocortical dopamine system in the motivating effects of cocaine. NIDA Res Monogr 1994; 145: 1–18. [PubMed] [Google Scholar]
- 42.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Wong C et al. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J Pharmacol Exp Ther 1999; 291(1): 409–415. [PubMed] [Google Scholar]
- 43.Weiss F, Markou A, Lorang MT, Koob GF. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Res 1992; 593(2): 314–318. [DOI] [PubMed] [Google Scholar]
- 44.O’Dell SJ, Weihmuller FB, Marshall JF. Multiple methamphetamine injections induce marked increases in extracellular striatal dopamine which correlate with subsequent neurotoxicity. Brain Res 1991; 564(2): 256–260. [DOI] [PubMed] [Google Scholar]
- 45.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 1988; 85(14): 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallace TL, Gudelsky GA, Vorhees CV. Methamphetamine-induced neurotoxicity alters locomotor activity, stereotypic behavior, and stimulated dopamine release in the rat. J Neurosci 1999; 19(20): 9141–9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson JM, Levey AI, Bergeron C, Kalasinsky K, Ang L, Peretti F et al. Striatal dopamine, dopamine transporter, and vesicular monoamine transporter in chronic cocaine users. Ann Neurol 1996; 40(3): 428–439. [DOI] [PubMed] [Google Scholar]
- 48.Little KY, Krolewski DM, Zhang L, Cassin BJ. Loss of striatal vesicular monoamine transporter protein (VMAT2) in human cocaine users. Am J Psychiatry 2003; 160(1): 47–55. [DOI] [PubMed] [Google Scholar]
- 49.Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 1996; 2(6): 699–703. [DOI] [PubMed] [Google Scholar]
- 50.Melega WP, Jorgensen MJ, Lacan G, Way BM, Pham J, Morton G et al. Long-term methamphetamine administration in the vervet monkey models aspects of a human exposure: brain neurotoxicity and behavioral profiles. Neuropsychopharmacology 2008; 33(6): 1441–1452. [DOI] [PubMed] [Google Scholar]
- 51.Groman SM, Lee B, Seu E, James AS, Feiler K, Mandelkern MA et al. Dysregulation of D(2)-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. J Neurosci 2012; 32(17): 5843–5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu JP, Xu W, Angulo JA. Disparity in the temporal appearance of methamphetamine-induced apoptosis and depletion of dopamine terminal markers in the striatum of mice. Brain Res 2005; 1049(2): 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.German CL, Hanson GR, Fleckenstein AE. Amphetamine and methamphetamine reduce striatal dopamine transporter function without concurrent dopamine transporter relocalization. J Neurochem 2012; 123(2): 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thanos PK, Kim R, Delis F, Rocco MJ, Cho J, Volkow ND. Effects of chronic methamphetamine on psychomotor and cognitive functions and dopamine signaling in the brain. Behav Brain Res 2017; 320: 282–290. [DOI] [PubMed] [Google Scholar]
- 55.Izenwasser S The role of the dopamine transporter in cocaine abuse. Neurotox Res 2004; 6(5): 379–383. [DOI] [PubMed] [Google Scholar]
- 56.Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci 2001; 21(8): 2799–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kitamura O Detection of methamphetamine neurotoxicity in forensic autopsy cases. Leg Med (Tokyo) 2009; 11 Suppl 1: S63–65. [DOI] [PubMed] [Google Scholar]
- 58.Little KY, McLaughlin DP, Zhang L, McFinton PR, Dalack GW, Cook EH Jr. et al. Brain dopamine transporter messenger RNA and binding sites in cocaine users: a postmortem study. Arch Gen Psychiatry 1998; 55(9): 793–799. [DOI] [PubMed] [Google Scholar]
- 59.Narendran R, Martinez D. Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse 2008; 62(11): 851–869. [DOI] [PubMed] [Google Scholar]
- 60.Richtand NM, Woods SC, Berger SP, Strakowski SM. D3 dopamine receptor, behavioral sensitization, and psychosis. Neurosci Biobehav Rev 2001; 25(5): 427–443. [DOI] [PubMed] [Google Scholar]
- 61.Xu M, Koeltzow TE, Santiago GT, Moratalla R, Cooper DC, Hu XT et al. Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron 1997; 19(4): 837–848. [DOI] [PubMed] [Google Scholar]
- 62.Pritchard LM, Newman AH, McNamara RK, Logue AD, Taylor B, Welge JA et al. The dopamine D3 receptor antagonist NGB 2904 increases spontaneous and amphetamine-stimulated locomotion. Pharmacol Biochem Behav 2007; 86(4): 718–726. [DOI] [PubMed] [Google Scholar]
- 63.Khroyan TV, Baker DA, Fuchs RA, Manders N, Neisewander JL. Differential effects of 7-OH-DPAT on amphetamine-induced stereotypy and conditioned place preference. Psychopharmacology (Berl) 1998; 139(4): 332–341. [DOI] [PubMed] [Google Scholar]
- 64.Groman SM, Hillmer AT, Liu H, Fowles K, Holden D, Morris ED et al. Midbrain D3 Receptor Availability Predicts Escalation in Cocaine Self-administration. Biol Psychiatry 2020; 88(10): 767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neisewander JL, Cheung TH, Pentkowski NS. Dopamine D3 and 5-HT1B receptor dysregulation as a result of psychostimulant intake and forced abstinence: Implications for medications development. Neuropharmacology 2013; 76 Pt B: 301–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Richtand NM. Behavioral sensitization, alternative splicing, and d3 dopamine receptor-mediated inhibitory function. Neuropsychopharmacology 2006; 31(11): 2368–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J et al. Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. J Neurosci 2012; 32(4): 1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boileau I, Payer D, Rusjan PM, Houle S, Tong J, McCluskey T et al. Heightened Dopaminergic Response to Amphetamine at the D3 Dopamine Receptor in Methamphetamine Users. Neuropsychopharmacology 2016; 41(13): 2994–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matuskey D, Gallezot JD, Pittman B, Williams W, Wanyiri J, Gaiser E et al. Dopamine D(3) receptor alterations in cocaine-dependent humans imaged with [(1)(1)C](+)PHNO. Drug Alcohol Depend 2014; 139: 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci 1996; 16(19): 6100–6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matuskey D, Gaiser EC, Gallezot JD, Angarita GA, Pittman B, Nabulsi N et al. A preliminary study of dopamine D2/3 receptor availability and social status in healthy and cocaine dependent humans imaged with [(11)C](+)PHNO. Drug Alcohol Depend 2015; 154: 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Worhunsky PD, Matuskey D, Gallezot JD, Gaiser EC, Nabulsi N, Angarita GA et al. Regional and source-based patterns of [(11)C]-(+)-PHNO binding potential reveal concurrent alterations in dopamine D2 and D3 receptor availability in cocaine-use disorder. Neuroimage 2017; 148: 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology 1996; 14(6): 375–424. [DOI] [PubMed] [Google Scholar]
- 74.Platt DM, Rowlett JK, Spealman RD. Behavioral effects of cocaine and dopaminergic strategies for preclinical medication development. Psychopharmacology (Berl) 2002; 163(3-4): 265–282. [DOI] [PubMed] [Google Scholar]
- 75.Self DW, Stein L. The D1 agonists SKF 82958 and SKF 77434 are self-administered by rats. Brain Res 1992; 582(2): 349–352. [DOI] [PubMed] [Google Scholar]
- 76.Weed MR, Woolverton WL. The reinforcing effects of dopamine D1 receptor agonists in rhesus monkeys. J Pharmacol Exp Ther 1995; 275(3): 1367–1374. [PubMed] [Google Scholar]
- 77.Grech DM, Spealman RD, Bergman J. Self-administration of D1 receptor agonists by squirrel monkeys. Psychopharmacology (Berl) 1996; 125(2): 97–104. [DOI] [PubMed] [Google Scholar]
- 78.Weed MR, Paul IA, Dwoskin LP, Moore SE, Woolverton WL. The relationship between reinforcing effects and in vitro effects of D1 agonists in monkeys. J Pharmacol Exp Ther 1997; 283(1): 29–38. [PubMed] [Google Scholar]
- 79.Self DW, Belluzzi JD, Kossuth S, Stein L. Self-administration of the D1 agonist SKF 82958 is mediated by D1, not D2, receptors. Psychopharmacology (Berl) 1996; 123(4): 303–306. [DOI] [PubMed] [Google Scholar]
- 80.Graziella De Montis M, Co C, Dworkin SI, Smith JE. Modifications of dopamine D1 receptor complex in rats self-administering cocaine. Eur J Pharmacol 1998; 362(1): 9–15. [DOI] [PubMed] [Google Scholar]
- 81.Moore RJ, Vinsant SL, Nader MA, Porrino LJ, Friedman DP. Effect of cocaine self-administration on striatal dopamine D1 receptors in rhesus monkeys. Synapse 1998; 28(1): 1–9. [DOI] [PubMed] [Google Scholar]
- 82.Tomic M, Vukosavic S, Joksimovic J. Acute amphetamine and/or phencyclidine effects on the dopamine receptor specific binding in the rat brain. Eur Neuropsychopharmacol 1997; 7(4): 295–301. [DOI] [PubMed] [Google Scholar]
- 83.Ujike H, Akiyama K, Nishikawa H, Onoue T, Otsuki S. Lasting increase in D1 dopamine receptors in the lateral part of the substantia nigra pars reticulata after subchronic methamphetamine administration. Brain Res 1991; 540(1–2): 159–163. [DOI] [PubMed] [Google Scholar]
- 84.Nonaka R, Moroji T. Effects of chronic methamphetamine treatment on the binding parameters of [3H]SCH 23390, a selective D1-dopamine receptor ligand, in the rat brain. Neurosci Lett 1990; 120(1): 109–112. [DOI] [PubMed] [Google Scholar]
- 85.Martinez D, Slifstein M, Narendran R, Foltin RW, Broft A, Hwang DR et al. Dopamine D1 receptors in cocaine dependence measured with PET and the choice to self-administer cocaine. Neuropsychopharmacology 2009; 34(7): 1774–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Okita K, Morales AM, Dean AC, Johnson MC, Lu V, Farahi J et al. Striatal dopamine D1-type receptor availability: no difference from control but association with cortical thickness in methamphetamine users. Mol Psychiatry 2018; 23(5): 1320–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Worsley JN, Moszczynska A, Falardeau P, Kalasinsky KS, Schmunk G, Guttman M et al. Dopamine D1 receptor protein is elevated in nucleus accumbens of human, chronic methamphetamine users. Mol Psychiatry 2000; 5(6): 664–672. [DOI] [PubMed] [Google Scholar]
- 88.Smout MF, Longo M, Harrison S, Minniti R, Wickes W, White JM. Psychosocial treatment for methamphetamine use disorders: a preliminary randomized controlled trial of cognitive behavior therapy and Acceptance and Commitment Therapy. Subst Abus 2010; 31(2): 98–107. [DOI] [PubMed] [Google Scholar]
- 89.Rawson RA, Marinelli-Casey P, Anglin MD, Dickow A, Frazier Y, Gallagher C et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction 2004; 99(6): 708–717. [DOI] [PubMed] [Google Scholar]
- 90.AshaRani PV, Hombali A, Seow E, Ong WJ, Tan JH, Subramaniam M. Non-pharmacological interventions for methamphetamine use disorder: a systematic review. Drug Alcohol Depend 2020; 212: 108060. [DOI] [PubMed] [Google Scholar]
- 91.Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiber C et al. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction 2006; 101(2): 267–274. [DOI] [PubMed] [Google Scholar]
- 92.Carroll KM, Onken LS. Behavioral therapies for drug abuse. Am J Psychiatry 2005; 162(8): 1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bhatt M, Zielinski L, Baker-Beal L, Bhatnagar N, Mouravska N, Laplante P et al. Efficacy and safety of psychostimulants for amphetamine and methamphetamine use disorders: a systematic review and meta-analysis. Syst Rev 2016; 5(1): 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tardelli VS, Bisaga A, Arcadepani FB, Gerra G, Levin FR, Fidalgo TM. Prescription psychostimulants for the treatment of stimulant use disorder: a systematic review and meta-analysis. Psychopharmacology (Berl) 2020; 237(8): 2233–2255. [DOI] [PubMed] [Google Scholar]
- 95.Castells X, Cunill R, Perez-Mana C, Vidal X, Capella D. Psychostimulant drugs for cocaine dependence. Cochrane Database Syst Rev 2016; 9: CD007380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chan B, Kondo K, Freeman M, Ayers C, Montgomery J, Kansagara D. Pharmacotherapy for Cocaine Use Disorder-a Systematic Review and Meta-analysis. J Gen Intern Med 2019; 34(12): 2858–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chan B, Freeman M, Kondo K, Ayers C, Montgomery J, Paynter R et al. Pharmacotherapy for methamphetamine/amphetamine use disorder-a systematic review and meta-analysis. Addiction 2019; 114(12): 2122–2136. [DOI] [PubMed] [Google Scholar]
- 98.Simmler LD, Buchy D, Chaboz S, Hoener MC, Liechti ME. In Vitro Characterization of Psychoactive Substances at Rat, Mouse, and Human Trace Amine-Associated Receptor 1. J Pharmacol Exp Ther 2016; 357(1): 134–144. [DOI] [PubMed] [Google Scholar]
- 99.Harkness JH, Shi X, Janowsky A, Phillips TJ. Trace Amine-Associated Receptor 1 Regulation of Methamphetamine Intake and Related Traits. Neuropsychopharmacology 2015; 40(9): 2175–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Phillips TJ, Shabani S. An animal model of differential genetic risk for methamphetamine intake. Front Neurosci 2015; 9: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lindemann L, Meyer CA, Jeanneau K, Bradaia A, Ozmen L, Bluethmann H et al. Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther 2008; 324(3): 948–956. [DOI] [PubMed] [Google Scholar]
- 102.Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC et al. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry 2011; 168(6): 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry 2012; 17(9): 918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shi X, Walter NA, Harkness JH, Neve KA, Williams RW, Lu L et al. Genetic Polymorphisms Affect Mouse and Human Trace Amine-Associated Receptor 1 Function. PLoS ONE 2016; 11(3): e0152581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Loftis JM, Lasarev M, Shi X, Lapidus J, Janowsky A, Hoffman WF et al. Trace amine-associated receptor gene polymorphism increases drug craving in individuals with methamphetamine dependence. PLoS One 2019; 14(10): e0220270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Muhlhaus J, Dinter J, Jyrch S, Teumer A, Jacobi SF, Homuth G et al. Investigation of Naturally Occurring Single-Nucleotide Variants in Human TAAR1. Front Pharmacol 2017; 8: 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.John J, Kukshal P, Bhatia T, Chowdari KV, Nimgaonkar VL, Deshpande SN et al. Possible role of rare variants in Trace amine associated receptor 1 in schizophrenia. Schizophr Res 2017; 189: 190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rutigliano G, Bräunig J, Del Grande C, Carnicelli V, Masci I, Merlino S et al. Non-Functional Trace Amine-Associated Receptor 1 Variants in Patients With Mental Disorders. Frontiers in Pharmacology 2019; 10(1027). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Berry MD, Gainetdinov RR, Hoener MC, Shahid M. Pharmacology of human trace amine-associated receptors: Therapeutic opportunities and challenges. Pharmacol Ther 2017; 180: 161–180. [DOI] [PubMed] [Google Scholar]
- 110.Pei Y, Asif-Malik A, Canales JJ. Trace Amines and the Trace Amine-Associated Receptor 1: Pharmacology, Neurochemistry, and Clinical Implications. Front Neurosci 2016; 10: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Underhill SM, Hullihen PD, Chen J, Fenollar-Ferrer C, Rizzo MA, Ingram SL et al. Amphetamines signal through intracellular TAAR1 receptors coupled to Galpha13 and GalphaS in discrete subcellular domains. Mol Psychiatry 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Underhill SM, Wheeler DS, Li M, Watts SD, Ingram SL, Amara SG. Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons. Neuron 2014; 83(2): 404–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Underhill SM, Ingram SL, Ahmari SE, Veenstra-VanderWeele J, Amara SG. Neuronal excitatory amino acid transporter EAAT3: Emerging functions in health and disease. Neurochem Int 2019; 123: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Scheyer AF, Loweth JA, Christian DT, Uejima J, Rabei R, Le T et al. AMPA Receptor Plasticity in Accumbens Core Contributes to Incubation of Methamphetamine Craving. Biol Psychiatry 2016; 80(9): 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Murray CH, Loweth JA, Milovanovic M, Stefanik MT, Caccamise AJ, Dolubizno H et al. AMPA receptor and metabotropic glutamate receptor 1 adaptations in the nucleus accumbens core during incubation of methamphetamine craving. Neuropsychopharmacology 2019; 44(9): 1534–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R et al. TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc Natl Acad Sci U S A 2011; 108(20): 8485–8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Revel FG, Meyer CA, Bradaia A, Jeanneau K, Calcagno E, Andre CB et al. Brain-specific overexpression of trace amine-associated receptor 1 alters monoaminergic neurotransmission and decreases sensitivity to amphetamine. Neuropsychopharmacology 2012; 37(12): 2580–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Harmeier A, Obermueller S, Meyer CA, Revel FG, Buchy D, Chaboz S et al. Trace amine-associated receptor 1 activation silences GSK3beta signaling of TAAR1 and D2R heteromers. Eur Neuropsychopharmacol 2015. [DOI] [PubMed] [Google Scholar]
- 119.Liu J, Wu R, Li JX. TAAR1 and Psychostimulant Addiction. Cell Mol Neurobiol 2020; 40(2): 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pei Y, Asif-Malik A, Hoener M, Canales JJ. A partial trace amine-associated receptor 1 agonist exhibits properties consistent with a methamphetamine substitution treatment. Addict Biol 2016. [DOI] [PubMed] [Google Scholar]
- 121.Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry 2011; 17(9): 918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Robertson CL, Ishibashi K, Chudzynski J, Mooney LJ, Rawson RA, Dolezal BA et al. Effect of Exercise Training on Striatal Dopamine D2/D3 Receptors in Methamphetamine Users during Behavioral Treatment. Neuropsychopharmacology 2016; 41(6): 1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dean AC, London ED, Sugar CA, Kitchen CM, Swanson AN, Heinzerling KG et al. Predicting adherence to treatment for methamphetamine dependence from neuropsychological and drug use variables. Drug Alcohol Depend 2009; 105(1-2): 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]


