Abstract
Benefits of phototherapy were characterized in multiple diseases including depression, circadian rhythm disruptions, and neurodegeneration. Studies on migraine and fibromyalgia patients revealed that green light-emitting diodes (GLED) exposure provides a pragmatic and safe therapy to manage chronic pain. In rodents, GLED reversed hypersensitivity related to neuropathic pain. However, little is known about the underlying mechanisms of GLED efficacy. Here, we sought to understand how green light modulates the endogenous opioid system. We first characterized how exposure to GLED stimulates release of β-endorphin and proenkephalin in the central nervous system of male rats. Moreover, by individually editing each of the receptors, we found that μ- and δ-opioid receptors are required for green light’s antinociceptive effect in naïve rats and a model of HIV-induced peripheral neuropathy. We investigated how GLED could increase pain thresholds, and explored its potential in reversing hypersensitivity in a model of HIV-related neuropathy. Through behavioral and gene editing approaches, we identified that green light provides antinociception via modulation of the endogenous opioid system in the spinal cord. This work identifies a previously unknown mechanism by which GLED can improve pain management. Clinical translation of these results will advance the development of an innovative therapy devoid of adverse effects.
Keywords: Phototherapy, Green light, GP120, Neuropathic pain, Endogenous opioids
Introduction
Current approaches to pain management include pharmacotherapies; though effective in some clinical settings, most pharmacologic options come with a host of undesirable adverse effects. For instance, the currently available treatments for peripheral neuropathy induced by human immunodeficiency virus (HIV) have significant side effects, such as headaches, nausea, and fatigue12, 13, 44. Importantly, prolonged opioid intake may be problematic for HIV patients and is not recommended as a long-term solution for HIV-associated pain2, 28. In addition to having safer side effect profiles, nonpharmacological approaches for pain management would be desirable to both patients and clinicians. Therefore, nonpharmacological approaches as a standalone or as a complement to pharmacotherapies may decrease the number of pharmaceuticals used, thereby decreasing the accompanying side effects.
Recently, phototherapy has been explored as an efficacious treatment for multiple pain conditions21, 26, 33, 52. We have previously shown that exposure to green light-emitting diodes (GLEDs) reversed thermal and mechanical hypersensitivities in a nerve injury model of neuropathic pain22. In clinical trials, the Burstein group showed that green light exposure reduced the headache and photophobia pain intensity in ~20% of patients having active migraine attacks40. The analgesic effect of GLED exposure is also supported by two clinical trials investigating its effect on migraine and fibromyalgia31, 32. Exposing patients to GLED for 1–2 hours/day for 10 weeks significantly reduced the number of headache days per month as well as the intensity of migraine headaches and fibromyalgia pain. Patients also reported improved quality of life. Importantly, light of various colors and wavelengths has been used to treat a wide variety of conditions including depression, seasonal affective disorder, circadian rhythm disruptions, and, more recently, neurodegenerative diseases21, 26, 33, 52. Management of circadian rhythm disruptions through light exposure mainly relies on melatonin suppression via stimulation of photoreceptors, including photopigments expressed in intrinsically photosensitive retinal ganglion cells (ipRGCs)5, 33. Interestingly, flickering light can affect neuroinflammation by decreasing microglial activation1, 21, and modulation of neuroinflammation is of particular interest in chronic pain management. In fact, accumulating evidence suggests that central sensitization – a key generator of pain hypersensitivity – is, at least in part, driven by neuroinflammation in the central nervous system (CNS)25. Altogether, phototherapy studies over the past decades have begun to unravel the underlying mechanisms by which light can affect biological processes.
Although the clinical benefit of GLED exposure is clear, the exact mechanisms through which GLED elicits its antinociceptive and analgesic effects have not yet been fully elucidated. Our current understanding of GLED-induced antinociception is that the visual pathway is required, as opaque lenses abolish GLED effect in rats22. Furthermore, GLED was shown to increase PENK mRNA levels in rats, while intrathecal administration of naloxone, an opioid receptor antagonist, suppressed GLED-induced antinociception22. The endogenous opioid system is one of the most studied antinociceptive systems. It is found in central and peripheral neurons that secrete four opioids: β-endorphin, leu- and met-enkephalins, and dynorphins20. Notably, dysregulations of this system are observed in fibromyalgia patients46, and GLED exposure improves fibromyalgia patients’ condition31. Altogether, these observations strongly suggest the involvement of central opioid receptor pathways in GLED-induced antinociception.
Here, we sought to further investigate this central opioid receptor pathway involved in GLED-induced antinociception. We used a CRISPR/Cas9 genome editing approach to reduce the expression of μ-/δ-/κ-opioid receptors in naïve animals to assess the contribution of the different opioid receptors. We also used this approach in the viral envelope glycoprotein 120 (GP120) model of HIV-induced peripheral neuropathic pain to evaluate which actors of the endogenous opioid system were involved in a neuropathic pain model.
Methods
Animals
Pathogen-free male Sprague–Dawley rats (weight at testing 230–280 g; Envigo, Indianapolis, IN) were housed in climate-controlled rooms on a 12-h light/dark cycle and were allowed to have food and water ad libitum. All procedures were approved by the University of Arizona Animal Care and Use Committee and conform to the guidelines for the use of laboratory animals of the National Institutes of Health (publication no. 80-23, 1966). Key experiments were replicated using a randomized, double-blinded protocol. Randomization, exposure of rats to GLED, behavioral testing, unblinding, and data analysis were performed by different individuals.
Intrathecal (IT) Catheter Surgery
Animals were sedated on a thermal pad using 1–5% isoflurane in medical grade oxygen. An approximate 1.5 cm longitudinal incision is made from the back ridge of skull to C1. The atlanto-occipital membrane was exposed by using a muscle retractor (Fine Science Tools, 17003-03). A shallow 1–2 mm T-shaped incision was created to puncture membrane. A sterile polyethylene catheter (BD Intramedic, 427401) was then inserted flat to the skull and slowly advanced toward lumbar enlargement of spinal cord. The catheter knot loop was then secured into muscle mass above membrane using 3-0 silk suture and the skin incision was closed with 5-0 monofilament suture material.
GP120 Induced Neuropathy
On days 7, 10 and 13 post-surgery, GP120 HIV envelope protein (NIH AIDS Reagent Program-Fisher BioServices, 4961, Germantown, MD) was injected into the intrathecal space. Catheter injections were performed using a gastight syringe (Hamilton, 1702) equipped with a 30-gauge beveled needle. Total injection volume was 15 μL, comprised of 5 μL of GP120 at 60 ng/μL, diluted in sterile saline, followed by 10 μL of sterile saline. Control animals received injection of 15 μL sterile saline.
Light Emitting Diodes (LED)
All visible spectrum LED flex strips were purchased from ledsupply.com (VT, USA). The specifications of the LEDs were: (i) #LS-AC50-GR-006, 525 nanometer wavelength (i.e., green), 8 watts, 120 Volts, 120 degree beam angle; and (ii) #LS-AC50-WW-006, white, 9.6 watts, 120 Volts, 120 degree beam angle. LED strips were affixed on the top of racks where rats were housed, allowing global diffusion of light (100 Lux). Rats were exposed to the various LED in these cages with full access to food and water in a dark room devoid of any other source of light. Following behavioral assessment, the rats were returned to their cages for additional LED exposure. At the end of daily testing, the rats were returned to their regular animal room where they were exposed to room light illuminated with Sylvania Octron 3500K F032/835 model which is 48” in length and power output of 32-Watt fluorescent bulbs producing an intensity of 250 lux. A lux meter (Tondaj LX1010B, Amazon.com) was used to determine the illuminance and luminous emittance of the LED strips. Our own observations concluded that maximum effects of GLED exposure occurred after 6 days, hence in our experiments rats were exposed to 6 days of GLED or WLED.
Thermal sensory thresholds
Paw withdrawal latencies were determined as described by Hargreaves et al.17. Rats were acclimated within Plexiglas enclosures on a clear glass plate maintained at room temperature. A radiant heat source (high-intensity projector lamp) was focused onto the plantar surface of the hind paw. When the paw was withdrawn, a motion detector halted the stimulus and a timer. A maximal cutoff of 33.5 sec was used to prevent tissue damage.
Tactile thresholds
The assessment of tactile sensory thresholds was determined by measuring the withdrawal response to probing the hindpaw with a series of calibrated fine (von Frey) filaments. Each filament was applied perpendicularly to the plantar surface of the paw of rats held in suspended wire mesh cages. Withdrawal threshold was determined by sequentially increasing and decreasing the stimulus strength (the “up and down” method), and data were analyzed with the nonparametric method of Dixon, as described by Chaplan and colleagues8 and expressed as the mean withdrawal threshold.
Tissue collection
Generation of spinal cord lysates: spinal cords were harvested by hydraulic extrusion from Sprague-Dawley rats, and tissue lysates were generated by homogenization and trituration in lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton-X-100, 0.5% sodium deoxycholate,1 mM EDTA, 0.1% SDS, pH 7.4). To isolate dorsal horns, the lumbar part of the spinal cord was hemisected prior to homogenization. Lysis buffer was supplemented with protease inhibitor cocktail (Bimake, B14002), phosphatase inhibitors (Bimake, B15002), and Pierce universal nuclease (Fisher Scientific, PI88701). Protein concentrations were determined using BCA protein assay (ThermoFisher Scientific, PI23225).
Blood collection: animals prepared for terminal collection were first sedated via an anesthetic cocktail comprised of Ketamine (80 mg/mL)/Xylazine (12 mg/mL) delivered by intraperitoneal injection. The rat was positioned on its back and the abdominal/chest cavity was opened to clearly expose the heart. The left ventricle was punctured using a 25-gauge beveled needle with gentle suction applied via 3–5 mL syringe to withdraw the required blood volume.
Cerebrospinal fluid (CSF) collection: animals prepared for terminal collection were first sedated via an anesthetic cocktail comprised of Ketamine (80 mg/mL)/Xylazine (12 mg/mL) delivered by intraperitoneal injection. An approximate 1.5 cm longitudinal incision was made from the back ridge of skull to C1. The atlanto-occipital membrane was exposed by using a muscle retractor (Fine Science Tools, 17003-03). A shallow 1–2 mm T-shaped incision was made to puncture membrane. A pool of CSF was immediately visible upon membrane puncture. Fluid was recovered using 22 to 23-gauge blunted needle with gentle suction generated with 1 mL syringe to collect required volume.
Enzyme-linked immunosorbent assay (ELISA) for endogenous opioids
Rat ELISA assays were purchased from MyBioSource (San Diego, CA) to measure serum and CSF levels of endogenous opioids (β-endorphin, #MBS452166; Proenkephalin, #MBS726498 and Dynorphin, #MBS720677). Procedures were conducted according to the manufacturers’ instructions. Colorimetric detection was based on H2O2/TMB reaction. To determine the optical density of each well, a Biotek Epoch 2.0 microplate reader was set to 450 nm. Determination of endogenous opioid involved triplicate determinations for each sample.
gRNA strategy for opioid receptor gene targeting
Our strategy to truncate μ- (ENSRNOG00000018191), δ- (ENSRNOG00000010531), or κ- (ENSRNOG00000007647) opioid receptors focused on targeting the rat genes using a guide RNA (gRNA) as described previously37–39. The target exons were selected to ensure total removal of one of the receptors while ensuring minimal to none off-target activity of the Cas9 enzyme as we and others verified before29, 39. The gRNA sequences for oprm1 (GGCAACCAGTCCGATCCATG, on 73.9, off 90), oprd1 (gACGCATTGGCGCTCGCACTG, on 67.4, off 47.6) and oprk1 (gTTGGGGAGTAGGCAAGCACT, on 65.4, off 38.0) were inserted into the Esp3I restriction site of the pL-CRISPR.EFS.tRFP lentiplasmid (Cat#57819, Addgene, Cambridge, MA)18 a plasmid that allows for simultaneous expression of the Cas9 enzyme and the gRNA. The control gRNA was the empty plasmid which contained the scaffold, but no homing sequence. All plasmids were verified by Sanger sequencing (Eurofins, Louisville, KY).
In vivo transfection of CRISPR plasmids
For in vivo transfection, the indicated plasmids were diluted to 0.4 μg/μl in 5% sterile glucose solution. Then, Turbofect in vivo transfection reagent (Cat#R0541, Thermo Fisher Scientific) was added following manufacturer’s instructions. The plasmid complexes were administered intrathecally, approximately between L5 and L6 (15 μL per animal).
Western blotting
For detection of μ-/δ-/κ-opioid receptors (MOR/DOR/KOR), 10 μg of proteins isolated from lumbar dorsal horns were loaded into wells of 3–8% gradient SDS-PAGE gels (Criterion XT, Biorad). Proteins were transferred to ø0.2 μm PVDF membranes (Millipore #ISEQ00005) after activation in 100% methanol. Membranes were immunoblotted with MOR (1/500, Abcam #Ab10275), DOR (1/1000, Millipore #Ab1560) or KOR antibodies (1/500, Invitrogen #44302G), after a blocking step for 45 minutes in Tris-buffered saline, 0.1% Tween, 5% non-fat dry milk, pH 7.6. Membranes were then incubated overnight with antibodies diluted in Tris-buffered saline, 0.1% Tween, pH 7.6, with 2% non-fat dry milk. Goat anti-rabbit fused to horseradish peroxidase - HRP (1/10000, Jackson Immunoresearch; room temperature, 2h) were used as secondary antibodies. Protein bands were detected with Azure Sapphire Biomolecular Imager (Azure Biosystems) after applying chemiluminescent reagent for 2 minutes (ThermoFisher Scientific #34557). Bands were quantified for optical densitometry using Image J software (National Institute of Health). Actin protein levels were quantified in total lysates for loading control (Sigma Aldrich #1978).
Statistical analysis
All data were expressed as mean ± standard error of the mean (SEM), unless stated otherwise. Statistical analysis was run using GraphPad Prism software 8.0 (San Diego, CA). All data were first tested for Gaussian distribution and heteroscedasticity, using Shapiro-Wilk and Barlett’s tests, respectively. The statistical significance of differences between means was determined by parametric and non-parametric analyses followed by post-hoc comparisons. Differences were considered to be significant if the probability value p≤0.05. No outlier data were removed. All data were plotted using Prism software.
Data and materials availability
All data is available in the main text and figures. Raw data will be provided upon request to corresponding author.
Results
Green LED (GLED) stimulates secretion of β-endorphin, proenkephalin, but not dynorphin in CSF of naïve male rats
Our previous studies demonstrated the benefits of GLED for managing pain in both fibromyalgia and migraine patients31, 32. Moreover, we also reported that GLED provided both thermal and mechanical antinociception in rat models of acute and chronic pain22. Importantly, our reports indicated that GLED stimulates PENK mRNA expression, and GLED-induced antinociception was abolished when rats were injected with naloxone, a μ-opioid receptor antagonist22. These observations suggested the possibility that GLED effects are contingent on modulation of the endogenous opioid system. Therefore, we evaluated endogenous opioid levels in naïve rats, from both serum and cerebrospinal fluid (CSF), six days following exposure to green or white (control) light. Using ELISAs, our results showed no differences in serum levels of β-endorphin, proenkephalin or dynorphin following exposure to GLED (Figs. 1A, B & C). However, CSF levels of β-endorphin were increased from 13.86±2.54 pg/mL to 40.63±7.76 pg/mL, and levels of proenkephalin from 2.43±0.01 ng/mL to 3.24±0.14 ng/mL (Figs. 1D & E). Levels of dynorphin remained unchanged (Fig. 1F). These results identify a previously unknown mechanism, with GLED increasing levels of endogenous opioid specifically in the CSF. This observation suggests that antinociceptive properties of GLED are mediated through central rather than peripheral mechanisms.
Figure 1: Green LED light (GLED) stimulates secretion of β-endorphin, proenkephalin, but not dynorphin in CSF of naïve rats.
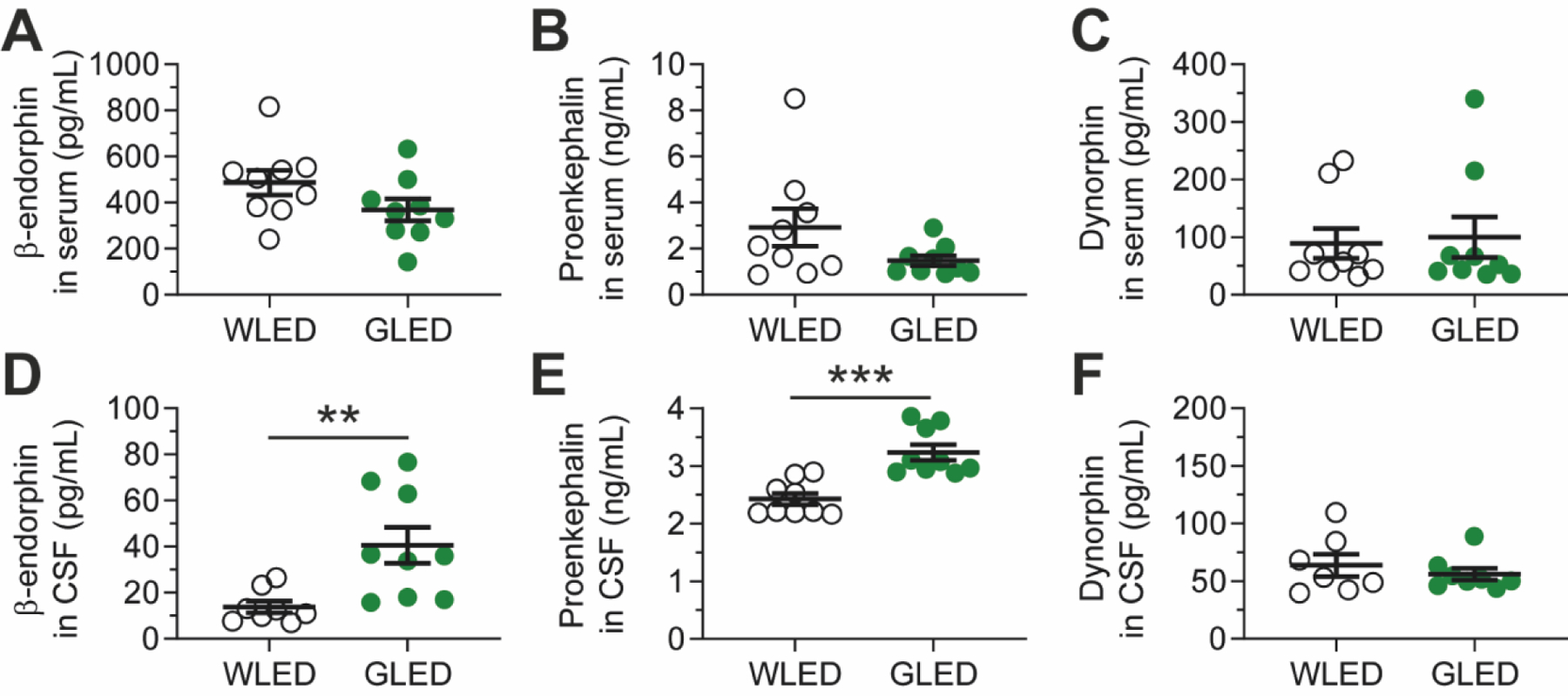
Endogenous opioid levels in rats after exposure to white (WLED) or GLED. Rats were exposed to GLED or WLED for 6 days; 8h/day, 100 Lux. Blood and CSF were collected at the end of exposure. Levels of β-endorphin (A), Proenkephalin (B) and Dynorphin (C) were analyzed through ELISA in serum, as well as in CSF (D, E, F, respectively) (n=7–9, Mann-Whitney nonparametric test, **p<0.01, ***p<0.001). GLED exposure resulted in increased levels of β-endorphin and Proenkephalin in CSF, but not in serum. No changes were observed in Dynorphin levels. Results are expressed in mean±SEM.
Validation of genomic editing of opioid receptors in the spinal cord of male rats
Our previous study showed increased levels of spinal cord proenkephalin-A mRNA in the rat neuropathic pain model of L5 and L6 spinal nerve ligations following GLED exposure22. Therefore, we focused our attention on the role played by the spinal cord in mediating the effects of GLED. To confirm the involvement of endogenous opioids in GLED-induced antinociception, we edited the receptors using a Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/Cas9 approach. Plasmids allowing for simultaneous expression of Cas9 and a guide RNA (gRNA) unique for each opioid receptor (OR) were injected intrathecally (Fig. 2A) prior to exposure to GLED.
Figure 2: Validation of genomic editing of opioid receptors in the spinal cord.
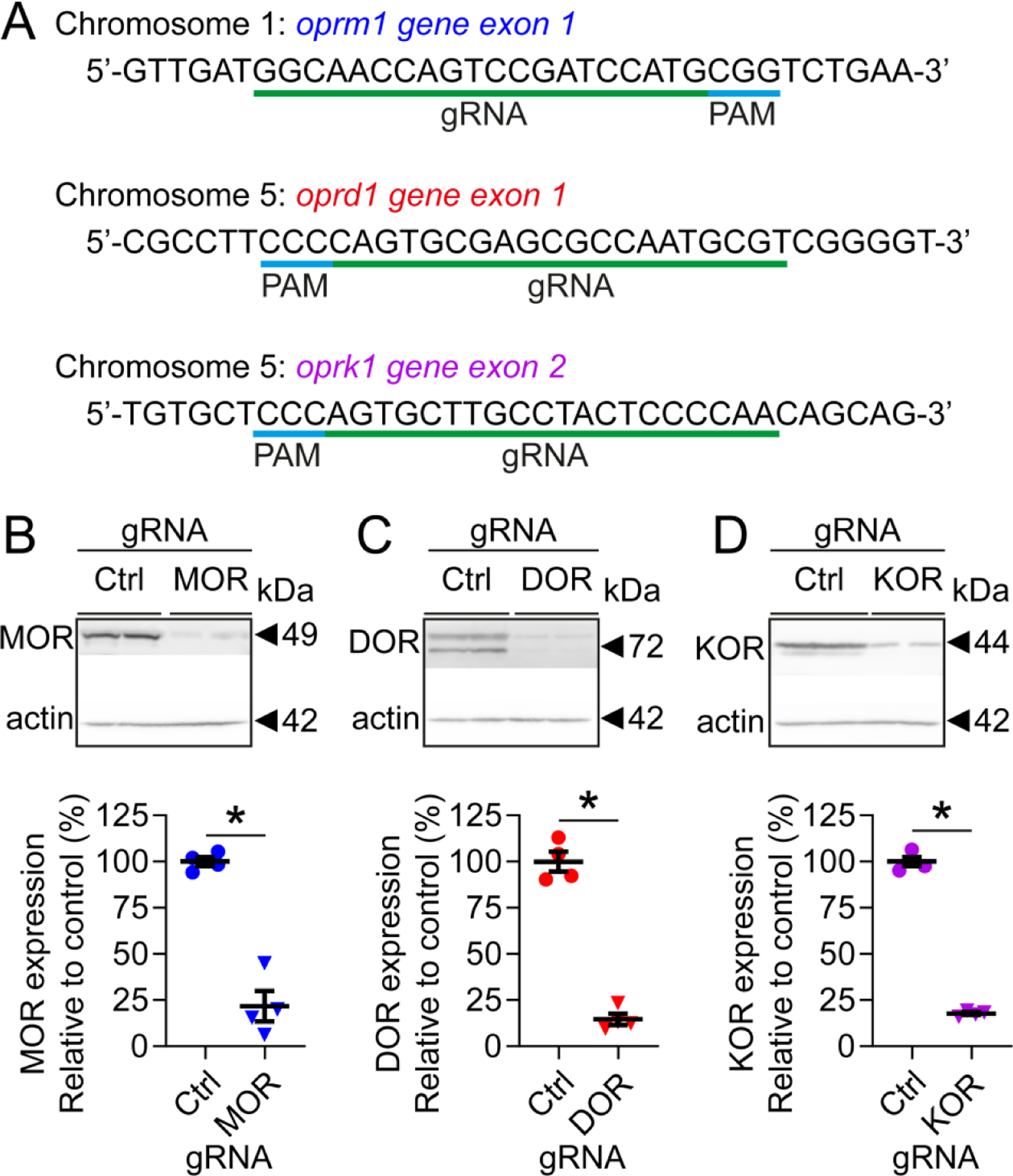
Rats were intrathecally injected with plasmids encoding Cas9 and gRNAs targeting μ- (MOR), δ- (DOR), or κ-opioid receptors (KOR). Two weeks following injection, dorsal horns of the spinal cord were collected, and levels or each opioid receptor were assessed by western blot. (A) Sequences of gRNA specifically targeting MOR, DOR, or KOR. Representative western blots illustrating levels of MOR (B), DOR (C), or KOR (D) in rats injected with CRISPR/Cas9 constructs, and quantification of their respective levels of expression. Targeting opioid receptors resulted in a robust decrease of receptor expression in the dorsal horn of the spinal cord. Results are expressed in mean±SEM, n=4, Mann-Whitney nonparametric test, *p<0.05.
Two weeks following a single CRISPR/Cas9 injection, protein levels of μ-/δ-/κ-opioid receptors were decreased by ~78% in the spinal dorsal horn of rats (Figs. 2B–D).
μ- and δ-opioid receptors are required for GLED-mediated antinociception in male rats
To assess if editing of opioid receptors affects GLED-mediated antinociception, rats were exposed to WLED (white light-emitting diodes) or GLED for 6 days, based on the results we obtained in our previous study22. Thermal sensitivity was evaluated over the whole experiment, to evaluate potential effects of genome editing (Fig. 3A). Depletion of the ORs did not alter basal thermal sensitivity (Figs. 3 B–D). In contrast, deletion of either δ- or μ-opioid receptor prevented GLED-induced antinociception (Figs. 3C–D). Rats that were injected with a gRNA specific for κ-opioid receptor presented similar behavior compared to control gRNA-injected rats (Fig. 3B). These observations implicate the endogenous opioid system in GLED antinociception and demonstrate that both δ- or μ-opioid receptors at the lumbar level of the spinal cord are required to attenuate thermally evoked responses.
Figure 3: μ- and δ-opioid receptors are required for GLED-mediated antinociception.
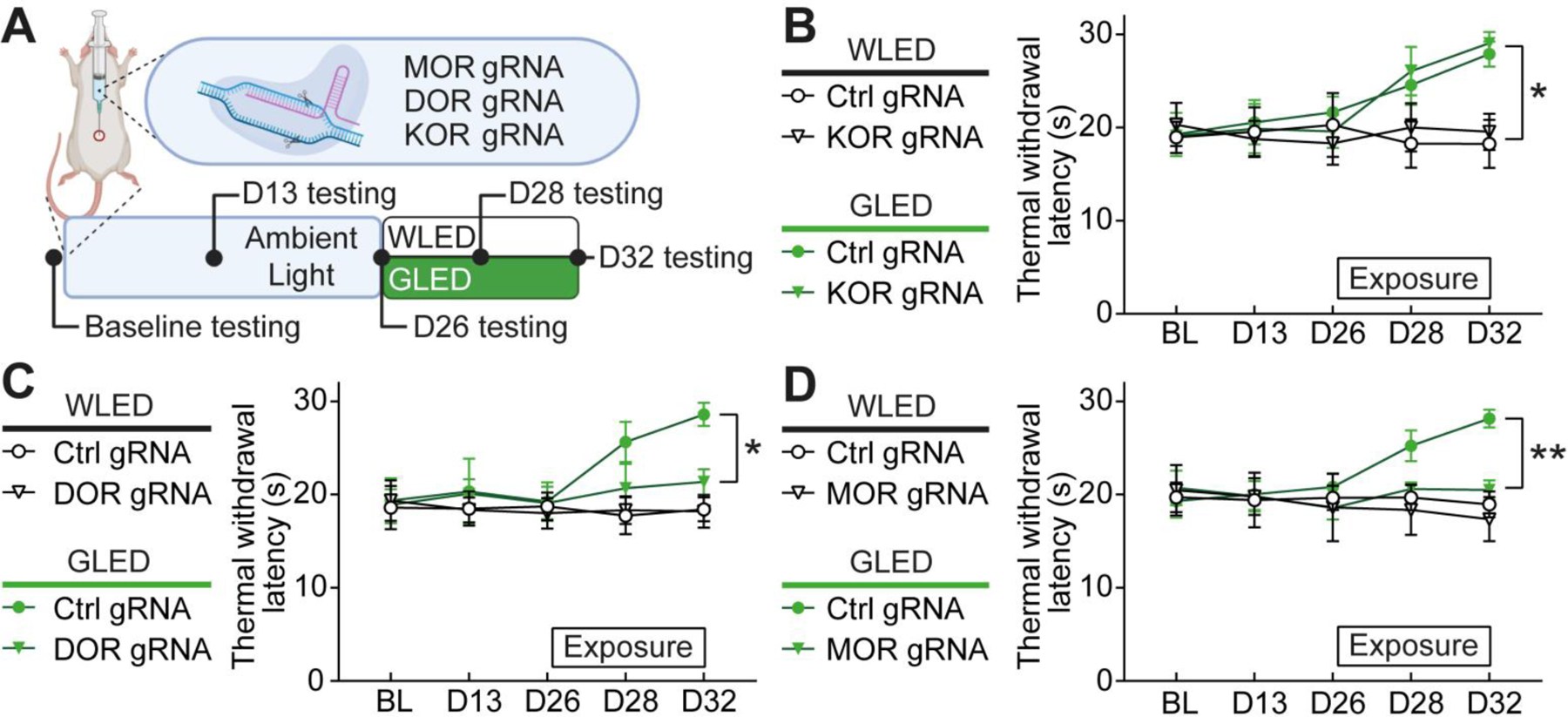
(A) Experimental design. After baseline testing (BL) for thermal sensitivity (Hargreaves test), rats were injected intrathecally with plasmids carrying control, μ- (MOR), δ- (DOR) or κ-opioid receptor (KOR) gRNA, and the CRISPR-associated endonuclease 9 (Cas9). Thermal sensitivity was measured before light exposure at days 13 and 26, and after exposure to white LED (WLED) or GLED at days 28 and 32. Rats were exposed to 100 Lux light over 6 days after D26 for 8h/day. (B) Thermal withdrawal latencies in rats injected with control or KOR gRNA, before and after exposure to WLED or GLED. KOR editing did not alter GLED antinociceptive effect (n=8, parametric two-way ANOVA followed by Tukey’s posthoc test, *p<0.05). (C) Thermal withdrawal latencies in rats injected with control or DOR gRNA, before and after exposure to WLED or GLED. DOR editing reduced GLED antinociceptive effect (n=8, parametric two-way ANOVA followed by Tukey’s posthoc test, *p<0.05). (D) Thermal withdrawal latencies in rats injected with control or MOR gRNA, before and after exposure to WLED or GLED. MOR editing blocked GLED antinociceptive effect (n=6–8, parametric two-way ANOVA followed by Tukey’s posthoc test, **p<0.01).
GLED induces long-lasting antinociceptive effect in the GP120 model of chronic neuropathic pain of male rats
As increasing evidence showed that GLED is effective in multiple pain models22, we next explored the potential benefits of GLED phototherapy in a model of HIV-related chronic neuropathic pain. Distal symmetric polyneuropathy is one of the neurological complications related to human immunodeficiency virus (HIV); neurotoxicity resulting from the virus and its products has been proposed to play a crucial role in this polyneuropathy47. Studies conducted in rodents demonstrated that injections of envelope glycoprotein GP120 of the HIV induce thermal and mechanical hypersensitivity between 4 and 7 days after injections, lasting over 40 days34, 49, 53. Therefore, we exposed rats injected three times with GP120 to either 100 Lux of white (control) or green LED, 8 hours a day, 7 days after GP120 injections. Rats injected with GP120 protein and exposed to control white light (WLED) displayed thermal and mechanical hypersensitivities over at least 15 days (Fig. 4). When exposed to green light, hypersensitivity to mechanical stimulus was significantly decreased after 2 days of exposure. Antinociception lasted until 14 days, demonstrating long-lasting effect of GLED even after exposure termination (Fig. 4A). Mechanical sensitivity measurements suggested that the antinociceptive effect is characterized by a biphasic response, as the maximum effect lasted 3 days, followed by a less pronounced desensitization that was stable over 5 days. Thermal sensitivity assessment presented similar results, although a biphasic response was not observed. In GLED-exposed rats, thermal withdrawal latencies were increased after 5 days of exposure compared to rats exposed to WLED. Interestingly, thermal antinociception followed a slightly different timeline when compared to mechanical sensitivity; thermal sensitivity measures revealed that antinociception only lasted until day 12, i.e. 6 days following exposure termination, whereas mechanical sensitivity measures revealed antinociception lasting until day 14, 8 days following exposure termination (Fig. 4B). In the GP120 model of neuropathic pain, a 6-day exposure of GLED provided long-lasting antinociception.
Figure 4: GLED induces long-lasting antinociceptive effect in the GP120 model of chronic neuropathic pain.
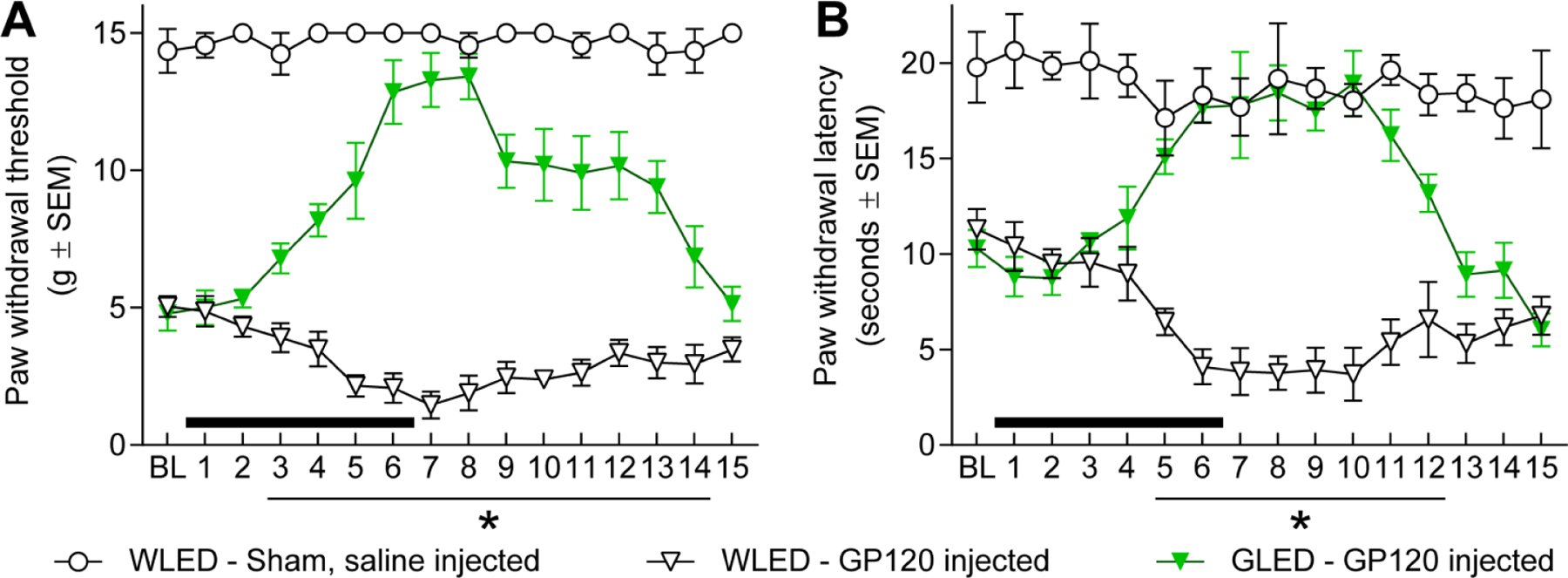
Both mechanical (Von Frey test) and thermal (Hargreaves test) hypersensitivity were assessed in rats to examine GLED’s antinociceptive effect. Neuropathic pain was induced with three intrathecal injections of GP120. Seven days after the last injection of GP120 or saline solution, rat sensitivities to thermal and mechanical stimuli were measured. Following measurement of baseline (BL), rats were exposed to white LED (WLED) or GLED, 100 Lux 8h/day, for 6 days (black bars). (A) Mechanical hypersensitivity was assessed every day for 15 days. Light exposure started after BL measurements. 6 days of GLED exposure produced significant antinociception over 11 days compared to WLED exposed animals (nSHAM=4, nWLED=6, nGLED=7, mean±SEM, parametric two-way ANOVA, followed by Tukey’s posthoc, *p<0.05). (B) Thermal hypersensitivity was measured over 15 days. 6 days of GLED exposure produced significant antinociception over 8 days compared to WLED exposed animals (nSHAM=4, nWLED=6, nGLED=7, mean±SEM, parametric two-way ANOVA, followed by Tukey’s posthoc, *p<0.05).
GLED antinociception in GP120 neuropathic pain model requires μ- and δ-opioid receptors in male rats
Finally, we tested if GLED-induced antinociception relies on OR-dependent mechanisms in the GP120 model of chronic neuropathic pain. Rats were injected with CRISPR/Cas9 constructs carrying gRNAs specific for one of the ORs or a control gRNA, and then subsequently injected with GP120. Following development of the neuropathy (7 days following the last injection of GP120), rats were exposed to WLED or GLED for 8h/day for 6 days. Two days following the beginning of the exposure, mechanical hypersensitivity in control rats exposed to GLED started to decrease compared to WLED exposed rats (Fig. 5). Rats did not present any differences in mechanical sensitivity whether κ-opioid receptors were depleted or not, confirming our previous results on naïve rats (Fig. 5A). In contrast, gene editing of δ- (DOR) or μ-opioid receptors (MOR) strongly altered GLED effects. Mechanical hypersensitivity in GLED conditions was increased in the DOR-depleted rats, even though rats remained significantly less sensitive than control rats exposed to WLED (Fig. 5B). On the other hand, MOR deletion blocked GLED-induced antinociception compared to WLED conditions (Fig. 5C).
Figure 5: GLED antinociception in GP120 neuropathic pain model requires μ- and δ-opioid receptors.
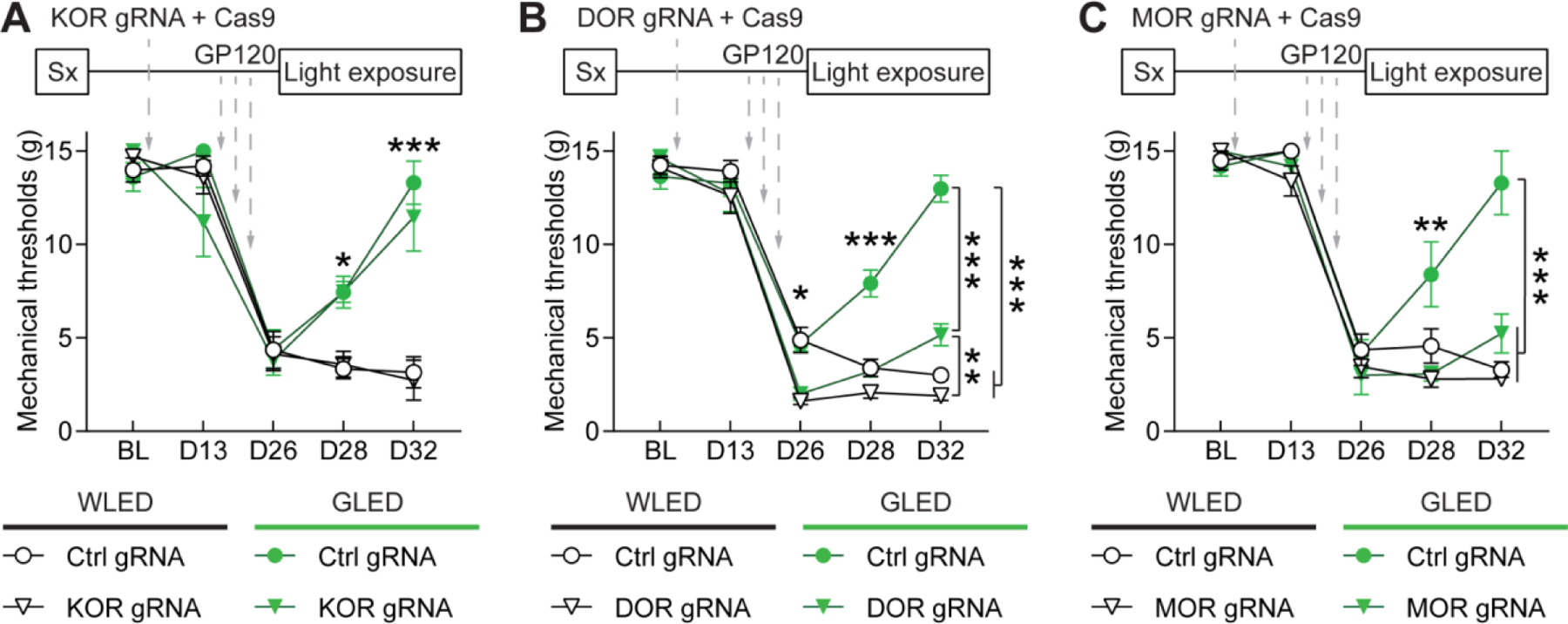
Rats underwent intrathecal (IT) catheter surgery (Sx), and baseline mechanical sensitivity thresholds (BL) were measured two weeks after surgery using Von Frey Filaments. After baseline testing, rats were injected intrathecally with plasmids encoding control, μ- (MOR), δ- (DOR) or κ-opioid receptor (KOR) gRNAs, and the CRISPR-associated endonuclease 9 (Cas9). Mechanical sensitivity was measured two weeks after gene editing, and rats received three IT injections of GP120 (3×300 ng) over a week. One week after the last injection (at D26), hypersensitivity was evaluated to confirm establishment of neuropathic pain. Then rats were then exposed to WLED or GLED for 6 days (8h/day, 100 Lux), and mechanical thresholds were measured at days 28 and 32. (A) Mechanical withdrawal thresholds in rats injected with control or KOR gRNA, before and after exposure to WLED or GLED. KOR editing did not alter GLED antinociceptive effect (n=6, parametric two-way ANOVA followed by Tukey’s posthoc test, *p<0.05, ***p<0.001). (B) Mechanical withdrawal thresholds in rats injected with control or DOR gRNA, before and after exposure to WLED or GLED. DOR editing significantly reduced GLED antinociceptive effect in GP120 rats but did not completely block antinociception compared to WLED rats (n=7–8, parametric two-way ANOVA followed by Tukey’s posthoc test, *p<0.05, **p<0.01, ***p<0.001). (C) Mechanical withdrawal thresholds in rats injected with control or MOR gRNA, before and after exposure to WLED or GLED. MOR editing blocked GLED antinociceptive effect (n=6, parametric two-way ANOVA followed by Tukey’s posthoc test, **p<0.01, ***p<0.001).
Altogether, our results demonstrated that antinociception induced by GLED exposure increases levels of endogenous opioids specifically in the spinal cord, and its effect is mediated through both δ- and μ-opioid receptors. Moreover, we demonstrated the efficacy of GLED in another model of chronic neuropathic pain.
Discussion
The endogenous opioid system is composed of neurons producing three endogenous opioid neurotransmitters that act both peripherally and centrally20; β-endorphin and enkephalins are anti-nociceptive agents, whereas dynorphins can elicit both pro- and anti-nociceptive effects51. Upon noxious stimulation, pain causes a release of endogenous opioids that act on their cognate receptors to inhibit nociceptive transmission20. In naive rats, GLED exposure significantly increased the CSF levels of β-endorphin and proenkephalin, but not dynorphin. This is consistent with our previous study showing increased levels of spinal cord proenkephalin-A mRNA in the rat neuropathic pain model of L5 and L6 spinal nerve ligations following GLED exposure22. As both β-endorphin and enkephalins induce antinociception51, the increased levels of these endogenous opioids could contribute to the reversal of thermal and mechanical hypersensitivity in rats treated with GP120 glycoprotein. In the spinal cord, μ- and κ-opioid receptors are mostly expressed in the superficial laminae of the dorsal horn, whereas δ-opioid receptors are more widely expressed throughout the laminae and into the ventral horn20. β-endorphin binds primarily to μ-opioid receptors. Leu- and met-enkephalin are agonists of the δ-opioid receptor, and to a lesser extent the μ-opioid receptor. Lastly, dynorphin exerts its effect primarily through stimulation of κ-opioid receptors51.
To assess the contribution of the spinal opioid receptors in mediating the effects of GLED exposure, we utilized the CRISPR/Cas9 genome editing approach to block the expression of the μ-, δ-, or κ-opioid receptors. μ- or δ-opioid receptors appeared to be key actors in GLED-induced antinociception. On the other hand, gene editing of κ-opioid receptors did not impede GLED exposure from reversing the thermal and mechanical hypersensitivity. We obtained similar effects in the GP120 model of neuropathic pain. The differential involvement of the ORs in antinociception may be due to difference in binding affinity of the endogenous opioids for their respective receptors. β-endorphin and enkephalins primarily act on the μ- and δ-opioid receptors, respectively. GLED exposure did not affect dynorphin levels, which binds to the κ-opioid receptor. As a result, deleting κ-opioid receptors did not result in any effect on GLED-induced pain relief. Interestingly, knocking down the DORs in the GP120 model of neuropathic pain, not naïve rats, further decreased the threshold for mechanical hypersensitivity suggesting an intrinsic role for the DORs in mediating mechanical hypersensitivity in pathological environments. This finding is supported by other groups4, 15. While the CRISPR plasmids were administered intrathecally at approximately L5 and L6 levels, it is possible that their effects took place at different levels of locations. However, this is unlikely given the small volume infused (15 μL).
While our findings implicate the endogenous opioid system in mediating the hypersensitivity reversal of GLED exposure, the exact mechanisms of action by which GLED can increase endogenous opioid levels remain unknown. Bruguerolle & Labrecque described a circadian rhythm of expression of endogenous opioids, where β-endorphin levels are highest in the morning7, 48. The suprachiasmatic nucleus (SCN), the principal monitor of circadian rhythms, receives direct retinal inputs36. A recent study analyzing gene expression in the SCN revealed that proenkephalin-A is expressed in this nucleus54. Therefore, the SCN could be one of the first gateways in GLED antinociception. On the other hand, the optic tract connects to the olivary pretectal nucleus (OPN), which in turn connects to the rostral ventromedial medulla (RVM)10. The RVM contains endogenous opioids-expressing neurons3, and our previous study demonstrated that RVM inhibition blocked GLED-induced antinociception22. Therefore, the increased levels of endogenous opioids induced by GLED could originate from the RVM or from spinal interneurons through descending pathways involving the RVM.
This work also identifies the efficacy of GLED in the GP120-HIV model of chronic neuropathic pain. A study led by Martin Adler demonstrated how GP120 could affect opioid-induced analgesia; a GP120 injection in the periaqueductal grey (PAG) reduced morphine antinociception in the hotplate test9. Furthermore, GP120 is important in the apoptotic death of T cells, and a knock-out of μ-opioid receptor results in decreased apoptosis in mice35. These studies illustrate the importance of the opioid system in the GP120 model.
The inflammatory system also plays a crucial role in the establishment of GP120-induced hypersensitivity34, 50, 55. Milligan et al. have shown that spinal microglia are essential in mediating thermal and mechanical hypersensitivity, and intrathecal injection of tumor necrosis factor-alpha (TNFα) siRNA or soluble TNF receptor reduced GP120-induced hypersensitivity55. Interestingly, the SCN regulates inflammatory responses, and this effect is mediated through non-image forming cells, known as Intrinsically Photosensitive Retinal Ganglion Cells (ipRGCs)14, 48. Disruption of SCN-driven circadian rhythms increases secretion of pro-inflammatory cytokines and consequently enhances proinflammatory immune responses48. Numerous studies have shown that different wavelengths of light can strongly affect inflammatory processes10, thus raising the necessity to analyze GLED influence on neuroinflammation.
Some evidence also implicates the central nervous system in mediating the effects of GLED exposure. For example, Noseda et al. identified a retino-thalamo-cortical pathway of light exacerbating migraine headache pain. The authors hypothesized that green light exposure reduced the pain intensity in about 20% of patients having active migraine attack may be due to inactivation of the retino-thalamo- cortical nervous system40, 41.
Therefore, GLED exposure may be producing antinociception by several mechanisms. For example, it is possible that GLED exposure mediates its pain modulating effects through inactivation of the retino-thalamo-cortical pathway and increased release of endogenous opioids in the spinal cord. GLED could also exert its effect via inactivation of microglial cells as well as other unidentified mechanism(s) involved in inflammatory pain.
This study confirms our previous hypothesis: GLED induces antinociception, at least in part, through endogenous opioid system stimulation22. It is most likely that GLED exposure induced antinociception in our study by acting through the visual system22. Interestingly, our group found that exposure to red light through the visual system produced thermal and mechanical hypersensitivity in rats27. Additionally, wearing eyeglasses that filter out red light resulted in significant reduction of photophobia in migraine patients19. Therefore, the red color seems to play a role in exacerbating pain in both humans and rodents. On the other hand, GLED exposure in both humans and rats decreased pain sensitivity, indicating shared mechanisms between humans and rats22, 32, 40. Our similar conclusions in rodents and humans raise the question of a common mechanism of pain modulation by light in these two species, characterized by relatively different photoreception systems. The main difference is in cone composition; rodents have only two types of cones and visual range shifted to shorter wavelength (ultraviolet to green light), while humans can perceive longer wavelength (red light)23, 24, 45. Therefore, it is unclear if humans and rodents can perceive the same colors. On the other hand, rods and cones are not the only photosensitive cells in the visual system. IpRGCs are photoreceptors common to humans and rodents that are known to regulate non-visual functions such as circadian rhythms5, 16. The ipRGCs project to several brain areas involved in pain modulation such as the spinal trigeminal nucleus and rostral ventromedial medulla6, 11, 30, 40–43. Therefore, the ipRGCs may be the target for GLED exposure to produce antinociception and reversal of thermal and mechanical hypersensitivity.
A study limitation is the focus on male rats. Female rats may have a different behavioral and biochemical responses to GLED. We have shown that GLED was effective in women with fibromyalgia and migraine. So, it is likely that GLED will have similar effects in female rats. The effects of GLED on female rats will be further investigated in our next studies.
While there are still many unanswered questions about the mechanism(s) of GLED-induced antinociception and the reversal of thermal and mechanical hypersensitivity, it is clear that it has antinociceptive effects on biological systems. Given the lack of side effects seen in clinical trials with humans exposed to GLED31, 32 coupled with its availability, ease of use, and implementation, it is reasonable to investigate the effects of GLED on HIV-induced painful peripheral neuropathy in humans.
Highlights.
Green light reverses hypersensitivity associated with HIV-related neuropathy.
Green light increased the CSF levels of β-endorphin and proenkephalin.
Mu- and δ-opioid receptors are required for green light-mediated antinociception.
Perspective.
Development of new pain management therapies, especially for HIV patients, is crucial as long-term opioid prescription is not recommended due to adverse side effects. Green light addresses this necessity. Characterizing the underlying mechanisms of this potentially groundbreaking and safe antinociceptive therapy will advance its clinical translation.
Disclosure
This research was supported by National Center for Complementary and Integrative Health [R01AT009716, 2017] (M.M.I.), National Institute of Health [K08 NS104272, 2017] (A.P.) the University of Arizona CHiLLi Initiative (M.M.I.), and the Comprehensive Chronic Pain and Addiction Center-University of Arizona (M.M.I and A.P.). Drs. Ibrahim and Khanna have a patent issued through the University of Arizona for using green light therapy for the management of pain. All other authors have no conflict of interest to report. None of the authors of the manuscript received any remuneration or any reimbursement or honorarium in any other manner. The authors are not affiliated with any vendor or pharmaceutical company associated with this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Refrences
- 1.Adaikkan C, Middleton SJ, Marco A, Pao PC, Mathys H, Kim DN, Gao F, Young JZ, Suk HJ, Boyden ES, McHugh TJ, Tsai LH. Gamma Entrainment Binds Higher-Order Brain Regions and Offers Neuroprotection. Neuron. 102:929–943 e928, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Addis DR, DeBerry JJ, Aggarwal S. Chronic Pain in HIV. Mol Pain. 16:1744806920927276, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagley EE, Ingram SL. Endogenous opioid peptides in the descending pain modulatory circuit. Neuropharmacology. 173:108131, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardoni R, Tawfik VL, Wang D, Francois A, Solorzano C, Shuster SA, Choudhury P, Betelli C, Cassidy C, Smith K, de Nooij JC, Mennicken F, O’Donnell D, Kieffer BL, Woodbury CJ, Basbaum AI, MacDermott AB, Scherrer G. Delta opioid receptors presynaptically regulate cutaneous mechanosensory neuron input to the spinal cord dorsal horn. Neuron. 81:1312–1327, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedrosian TA, Nelson RJ. Timing of light exposure affects mood and brain circuits. Transl Psychiatry. 7:e1017, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benarroch EE. The melanopsin system: Phototransduction, projections, functions, and clinical implications. Neurology. 76:1422–1427, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Bruguerolle B, Labrecque G. Rhythmic pattern in pain and their chronotherapy. Adv Drug Deliv Rev. 59:883–895, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 53:55–63, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Kirby LG, Palma J, Benamar K, Geller EB, Eisenstein TK, Adler MW. The effect of gp120 on morphine’s antinociceptive and neurophysiological actions. Brain Behav Immun. 25:1434–1443, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng K, Martin LF, Slepian MJ, Patwardhan A, Ibrahim MM. Mechanisms and Pathways of Pain Photobiomodulation: A Narrative Review. The Journal of Pain. Epub: Feb 23, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolgonos S, Ayyala H, Evinger C. Light-induced trigeminal sensitization without central visual pathways: another mechanism for photophobia. Invest Ophthalmol Vis Sci. 52:7852–7858, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elafros MA, Birbeck GL, Gardiner JC, Siddiqi OK, Sikazwe I, Paneth N, Bositis CM, Okulicz JF. Patient-Reported Adverse Effects Associated with Combination Antiretroviral Therapy and Coadministered Enzyme-Inducing Antiepileptic Drugs. Am J Trop Med Hyg. 96:1505–1511, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson JM. SSRI Antidepressant Medications: Adverse Effects and Tolerability. Prim Care Companion J Clin Psychiatry. 3:22–27, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Y, Liao HW, Do MT, Yau KW. Non-image-forming ocular photoreception in vertebrates. Curr Opin Neurobiol. 15:415–422, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaveriaux-Ruff C, Karchewski LA, Hever X, Matifas A, Kieffer BL. Inflammatory pain is enhanced in delta opioid receptor-knockout mice. Eur J Neurosci. 27:2558–2567, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green A, Cohen-Zion M, Haim A, Dagan Y. Evening light exposure to computer screens disrupts human sleep, biological rhythms, and attention abilities. Chronobiol Int. 34:855–865, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 32:77–88, 1988 [DOI] [PubMed] [Google Scholar]
- 18.Heckl D, Kowalczyk MS, Yudovich D, Belizaire R, Puram RV, McConkey ME, Thielke A, Aster JC, Regev A, Ebert BL. Generation of mouse models of myeloid malignancy with combinatorial genetic lesions using CRISPR-Cas9 genome editing. Nat Biotechnol. 32:941–946, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoggan RN, Subhash A, Blair S, Digre KB, Baggaley SK, Gordon J, Brennan KC, Warner JE, Crum AV, Katz BJ. Thin-film optical notch filter spectacle coatings for the treatment of migraine and photophobia. J Clin Neurosci. 28:71–76, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holden JE, Jeong Y, Forrest JM. The endogenous opioid system and clinical pain management. AACN Clin Issues. 16:291–301, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, Mathys H, Seo J, Kritskiy O, Abdurrob F, Adaikkan C, Canter RG, Rueda R, Brown EN, Boyden ES, Tsai LH. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature. 540:230–235, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibrahim MM, Patwardhan A, Gilbraith KB, Moutal A, Yang X, Chew LA, Largent-Milnes T, Malan TP, Vanderah TW, Porreca F, Khanna R. Long-lasting antinociceptive effects of green light in acute and chronic pain in rats. Pain. 158:347–360, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs GH. Evolution of colour vision in mammals. Philos Trans R Soc Lond B Biol Sci. 364:2957–2967, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs GH, Fenwick JA, Williams GA. Cone-based vision of rats for ultraviolet and visible lights. J Exp Biol. 204:2439–2446, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology. 129:343–366, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kemper KJ. “Let there be light.” Research on phototherapy, light therapy, and photobiomodulation for healing - Alternative therapy becomes mainstream. Complement Ther Med. 41:A1–A6, 2018 [DOI] [PubMed] [Google Scholar]
- 27.Khanna R, Patwardhan A, Yang X, Li W, Cai S, Ji Y, Chew LA, Dorame A, Bellampalli SS, Schmoll RW, Gordon J, Moutal A, Vanderah TW, Porreca F, Ibrahim MM. Erratum to Development and Characterization of An Injury-free Model of Functional Pain in Rats by Exposure to Red Light’: The Journal of Pain 20 (2019) 1293–1306. J Pain. 20:1509, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krashin DL, Merrill JO, Trescot AM. Opioids in the management of HIV-related pain. Pain Physician. 15:ES157–168, 2012 [PubMed] [Google Scholar]
- 29.Ma H, Marti-Gutierrez N, Park SW, Wu J, Lee Y, Suzuki K, Koski A, Ji D, Hayama T, Ahmed R, Darby H, Van Dyken C, Li Y, Kang E, Park AR, Kim D, Kim ST, Gong J, Gu Y, Xu X, Battaglia D, Krieg SA, Lee DM, Wu DH, Wolf DP, Heitner SB, Belmonte JCI, Amato P, Kim JS, Kaul S, Mitalipov S. Correction of a pathogenic gene mutation in human embryos. Nature. 548:413–419, 2017 [DOI] [PubMed] [Google Scholar]
- 30.Martenson ME, Halawa OI, Tonsfeldt KJ, Maxwell CA, Hammack N, Mist SD, Pennesi ME, Bennett RM, Mauer KM, Jones KD, Heinricher MM. A possible neural mechanism for photosensitivity in chronic pain. Pain. 157:868–878, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin L, Porreca F, Mata EI, Salloum M, Goel V, Gunnala P, Killgore WDS, Jain S, Jones-MacFarland FN, Khanna R, Patwardhan A, Ibrahim MM. Green Light Exposure Improves Pain and Quality of Life in Fibromyalgia Patients: A Preliminary One-Way Crossover Clinical Trial. Pain Med. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin LF, Patwardhan AM, Jain SV, Salloum MM, Freeman J, Khanna R, Gannala P, Goel V, Jones-MacFarland FN, Killgore WD, Porreca F, Ibrahim MM. Evaluation of green light exposure on headache frequency and quality of life in migraine patients: A preliminary one-way cross-over clinical trial. Cephalalgia.333102420956711, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melrose S Seasonal Affective Disorder: An Overview of Assessment and Treatment Approaches. Depress Res Treat. 2015:178564, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, Maier SF, Watkins LR. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 861:105–116, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Moorman J, Zhang Y, Liu B, LeSage G, Chen Y, Stuart C, Prayther D, Yin D. HIV-1 gp120 primes lymphocytes for opioid-induced, beta-arrestin 2-dependent apoptosis. Biochim Biophys Acta. 1793:1366–1371, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morin LP. Neuroanatomy of the extended circadian rhythm system. Exp Neurol. 243:4–20, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moutal A, Cai S, Luo S, Voisin R, Khanna R. CRMP2 is necessary for Neurofibromatosis type 1 related pain. Channels (Austin). 12:47–50, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moutal A, Sun L, Yang X, Li W, Cai S, Luo S, Khanna R. CRMP2-Neurofibromin Interface Drives NF1-related Pain. Neuroscience. 381:79–90, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moutal A, Yang X, Li W, Gilbraith KB, Luo S, Cai S, Francois-Moutal L, Chew LA, Yeon SK, Bellampalli SS, Qu C, Xie JY, Ibrahim MM, Khanna M, Park KD, Porreca F, Khanna R. CRISPR/Cas9 editing of Nf1 gene identifies CRMP2 as a therapeutic target in neurofibromatosis type 1-related pain that is reversed by (S)-Lacosamide. Pain. 158:2301–2319, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noseda R, Bernstein CA, Nir RR, Lee AJ, Fulton AB, Bertisch SM, Hovaguimian A, Cestari DM, Saavedra-Walker R, Borsook D, Doran BL, Buettner C, Burstein R. Migraine photophobia originating in cone-driven retinal pathways. Brain. 139:1971–1986, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, Burstein R. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 13:239–245, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okamoto K, Tashiro A, Chang Z, Bereiter DA. Bright light activates a trigeminal nociceptive pathway. Pain. 149:235–242, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okamoto K, Thompson R, Tashiro A, Chang Z, Bereiter DA. Bright light produces Fos-positive neurons in caudal trigeminal brainstem. Neuroscience. 160:858–864, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Quintero GC. Review about gabapentin misuse, interactions, contraindications and side effects. J Exp Pharmacol. 9:13–21, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rocha FA, Gomes BD, Silveira LC, Martins SL, Aguiar RG, de Souza JM, Ventura DF. Spectral Sensitivity Measured with Electroretinogram Using a Constant Response Method. PLoS One. 11:e0147318, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schrepf A, Harper DE, Harte SE, Wang H, Ichesco E, Hampson JP, Zubieta JK, Clauw DJ, Harris RE. Endogenous opioidergic dysregulation of pain in fibromyalgia: a PET and fMRI study. Pain. 157:221720132225, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schutz SG, Robinson-Papp J. HIV-related neuropathy: current perspectives. HIV AIDS (Auckl). 5:243–251, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Segal JP, Tresidder KA, Bhatt C, Gilron I, Ghasemlou N. Circadian control of pain and neuroinflammation. J Neurosci Res. 96:1002–1020, 2018 [DOI] [PubMed] [Google Scholar]
- 49.Shi L, Wu B, Yi Z, Zhao S, Zou L, Li L, Yuan H, Jia T, Liu S, Liu H, Gao Y, Li G, Xu H, Zhang C, Liang S. P2Y12 shRNA treatment relieved HIV gp120-induced neuropathic pain in rats. Neurochem Int. 112:259–266, 2018 [DOI] [PubMed] [Google Scholar]
- 50.Shi Y, Yuan S, Tang SJ. Morphine and HIV-1 gp120 cooperatively promote pathogenesis in the spinal pain neural circuit. Mol Pain. 15:1744806919868380, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stein C, Gaveriaux-Ruff C, Wood J: The Oxford Handbook of the Neurobiology of Pain, Opioids and Pain. The Oxford Handbook of the Neurobiology of Pain, Oxford Handbooks Online, 2020, pp. 52. [Google Scholar]
- 52.van Maanen A, Meijer AM, van der Heijden KB, Oort FJ. The effects of light therapy on sleep problems: A systematic review and meta-analysis. Sleep Med Rev. 29:52–62, 2016 [DOI] [PubMed] [Google Scholar]
- 53.Wallace VC, Blackbeard J, Segerdahl AR, Hasnie F, Pheby T, McMahon SB, Rice AS. Characterization of rodent models of HIV-gp120 and anti-retroviralassociated neuropathic pain. Brain. 130:2688–2702, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wen S, Ma D, Zhao M, Xie L, Wu Q, Gou L, Zhu C, Fan Y, Wang H, Yan J. Spatiotemporal single-cell analysis of gene expression in the mouse suprachiasmatic nucleus. Nat Neurosci. 23:456–467, 2020 [DOI] [PubMed] [Google Scholar]
- 55.Zheng W, Ouyang H, Zheng X, Liu S, Mata M, Fink DJ, Hao S. Glial TNFalpha in the spinal cord regulates neuropathic pain induced by HIV gp120 application in rats. Mol Pain. 7:40, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]


