Abstract
Background and Aims:
To estimate during pregnancy correlations between frequency of self-reported use of marijuana, and quantified marijuana metabolite in biospecimens including urine, sera, and umbilical cord homogenate.
Design:
Prospective cohort
Setting:
Two urban hospitals in Colorado with legal recreational and medicinal marijuana
Participants:
Pregnant women (<16 weeks’ gestation) self-reporting marijuana use
Measurements:
Participants completed a written self-report survey and provided biospecimens at <16 weeks’ gestation (n=46), 18-22 weeks’ gestation (n=43), 32-36 weeks’ gestation (n=39) and delivery (n=37). Self-reported marijuana use frequency was calculated based on past-month days of use multiplied by number of daily uses. Maternal urine and sera were tested for presence (>5ng/mL) of 11-nor-9-carboxy-delta-9-tetrahydrocannabinol (THC-COOH). Liquid chromatography tandem mass spectrometry quantified THC-COOH in umbilical cord homogenate (ng/g). Last marijuana use by any measure was recorded to evaluate the time frame over which THC-COOH remains detectable (>0.10 ng/g) in cord.
Findings:
From December 2017 through May 2019, 51 pregnant women enrolled, and 46 were included in analyses (2 withdrew and 3 had a spontaneous abortion). The majority were normal weight, White or Black race, and insured by Medicaid. At the time of enrollment between seven to 15 weeks’ gestation, 87% had ongoing use by self-report, or positive urine or serum. The majority (33 [66%]) stopped using prior to delivery. Sera and urine results were strongly correlated with self-reported use frequency (Spearman correlation coefficient (r) range 0.70-0.87 across visits, p<0.001), and with each other. There was only one positive cord result when use stopped before 22 weeks. Frequency of self-reported marijuana use at delivery had strong correlation with quantified cord THC-COOH (r=0.80, 95% CI 0.62-0.89).
Conclusions:
Quantified umbilical cord 11-nor-9-carboxy-delta-9-tetrahydrocannabinol (THC-COOH) appears to strongly correlate with frequency of maternal marijuana use in the last month of pregnancy. Earlier use can be measured by either quantitative urine or serum assay.
Introduction
Marijuana is the most commonly used “illicit” drug during pregnancy with a prevalence of use of 3-30% in various populations.1,2 As legalization expands, so has use and perception of safety among pregnant women.3,4 Contradictory scientific evidence and uncertainty regarding the effects of prenatal marijuana use on perinatal outcomes is exacerbated by poor quantification of marijuana exposure with a reliance on self-reported use.5
Prior studies demonstrate poor agreement between self-reported marijuana use and biological sampling in pregnancy. In a multicenter study by Shiono et al, only 31% of the women with a positive serum screen for the psychoactive component of marijuana, delta-9-tetrahydrocannabinol (THC), also reported marijuana use.6 Some of the differences in self-report and biologic sampling may be related to women not wanting to disclose use given the stigma associated with drug use in pregnancy.
Part of the poor agreement between self-report and biologic sampling may also result from limitations in available testing. For instance, urine and serum toxicology tests are positive for marijuana metabolites for 2-3 days following the last use in occasional users and 2-3 weeks in heavier users.7 Other biospecimens such as umbilical cord homogenate (Wharton’s jelly) allow for detection of marijuana metabolite which is thought to reflect use from the second trimester onward, since marijuana metabolites cross the placenta.8,9 There is also likely variation in the limit of detection and quantification based on the laboratory and specimen type. The best methodology for detection and quantification of marijuana exposure throughout pregnancy remains unknown.
Our aim was to collect information about marijuana use prospectively throughout pregnancy in order to evaluate correlations between serial self-report, and quantified marijuana metabolite, 11-nor-9-carboxy-delta-9-THC (THC-COOH) in biologic samples including urine, sera, and umbilical cord homogenate. This aim was accomplished through longitudinal sampling across pregnancy and at the time of delivery. Determining the best methodology for quantification of perinatal marijuana use will improve the rigor of future assessments of the association between marijuana use and pregnancy outcomes.
Methods
Design
This was a prospective longitudinal cohort study of women who self-reported marijuana use at one of their prenatal visits and planned to deliver at one of two large Colorado medical centers.
Sample
One hospital is a tertiary academic center (University of Colorado Hospital [UCH]) located in Aurora, Colorado and one is a safety net hospital, which provides care to all regardless of ability to pay, (Denver Health Hospital [DH]) located in Denver, Colorado, USA. The study was conducted in Colorado following legalization of marijuana for recreational use. Enrollment occurred between December 2017 and September 2018 with follow-up ending at delivery, with the last delivery in May 2019.
Participants
Women who self-reported any marijuana use during pregnancy to a healthcare professional were eligible for inclusion if they had a live, singleton gestation and received prenatal care prior to 16 weeks’ gestation by best available dating. Standard American College of Obstetricians and Gynecologists (ACOG) dating criteria based on last menstrual period and ultrasound were employed.10 Women were excluded if they did not speak English or Spanish, had altered mental status that would result in inability to provide informed consent, planned to deliver at a hospital other than the recruitment sites, or were unwilling to participate in all study procedures including survey assessments for self-reported marijuana use and biospecimen collection.
Procedures
There were four study visits at the following times: (1) enrollment at the time of a prenatal care visit at less than 16 weeks’ gestation, (2) between 18 to 22 weeks’ gestation, (3) between 32 to 36 weeks’ gestation, (4) during the delivery admission. These gestational age windows were selected as routine prenatal visits occur during these time windows. At all study visits, participants were asked to complete a survey with detailed questions about self-reported use. In addition, at each study visit women were asked to provide both a urine and serum sample.
Data
Data regarding tobacco and other drug use was collected by self-report survey and medical record abstraction including both self-report and urine toxicology testing performed for clinical care.
At each visit, a 20-item survey about marijuana use and reasons for use was administered to all participants. Questions delineated frequency (number of days per month and number of uses per day), mode of use (eg inhaled, vaped, edibles, topical, other), and reasons for use (eg recreation, anxiety, depression, for sleep, for appetite, other) over the past 30 days (Figure S1). Marijuana use frequency was estimated based on past-month days of use multiplied by the self-reported number of uses per day. The survey included questions about whether frequency of use changed during pregnancy and why. In addition, participants were queried about second-hand marijuana exposure prior to delivery.
Urine was collected in a sterile urine cup using a clean catch technique. Urine samples were refrigerated and then frozen as soon as possible after collection. Serum samples were sent to the central clinical laboratory at each site for processing and then were frozen at −80°C until they were shipped for analysis. All antenatal urine and serum samples were collected and processed within 1 hour. All delivery samples were collected and processed within 2 hours.
At the time of delivery, a segment of the umbilical cord for THC-COOH assay by liquid chromatography mass-spectrometry (LC-MS/MS) was obtained. An umbilical cord segment is routinely collected as part of standard clinical care at both sites. After collection of fetal blood for clinical purposes, the umbilical cord segment was drained of remaining blood, washed with saline, dried, placed in a sterile specimen cup and refrigerated. A 6-inch segment of umbilical cord was required for drug assay. Specimens were refrigerated until they could be frozen to −80°C. All cord samples were processed and frozen within two hours of delivery.
Measures
Urine, serum and umbilical cord assays were performed at ARUP Laboratories (Salt Lake City, Utah), an accredited national reference laboratory that is an enterprise of the University of Utah, Department of Pathology. Urine and serum samples were shipped frozen and thawed just prior to analysis. Quantitative urine testing was performed for THC-COOH via LC-MS/MS on 0.5mL of urine. Results were reported quantitatively using a clinically available assay. Quantitative serum testing was performed for THC-COOH via LC-MS/MS on 0.5 mL of serum. Results were reported quantitatively using a clinically available assay. The reporting threshold was 5 ng/mL for both urine and serum.
Umbilical cord segments were transported frozen and thawed just prior to analysis for THC-COOH by LC-MS/MS. All methods for cord homogenate assays were developed and validated by ARUP Laboratories.11 Briefly, cord tissue was sliced, weighed and homogenized. Supernatant was subjected to hydrolysis, solid phase extraction, concentration and reconstitution. Extracts were analyzed by LC-MS/MS. THC-COOH was reported quantitatively with a detection threshold of 0.10 ng/g.
Baseline characteristics were collected based on both electronic medical record abstraction and participant self-report at the enrollment study visit including maternal age, weight, gestational age at enrollment, self-reported race and ethnicity, insurance status, employment, education level, and current relationship status. Weight was categorized into underweight, normal weight, overweight, and obese (Class I [30.0-34.9 kg/m2], II [35.0-39.9 kg/m2], or III [≥ 40.0 kg/m2]).
Sample Size
A sample size calculation was performed based on the research objective to assess the correlation between umbilical cord homogenate THC-COOH at the time of delivery and frequency of self-reported use in the past 30 days. This particular correlation was selected for sample size calculation as the umbilical cord specimen was the final collected specimen in the study protocol following delivery. We calculated that with 30 participants, we could achieve a two-sided 95% confidence interval that does not cross zero when the estimate of Spearman's rank correlation is 0.40 or more, representing moderate correlation.
All participants in the study received usual medical care with respect to substance use and were encouraged to stop using marijuana in pregnancy consistent with ACOG recommendations.12 Enrollment in the study did not preclude practitioners from testing or screening for substance use for clinical purposes. No study assay results were provided for clinical purposes. Study visits were conducted in research offices separate from routine prenatal care to preserve confidentiality. All biologic specimens were labeled with only a participant identification number using barcode technology. The results were reported to the investigators by participant identification number and entered into Research Electronic Data Capture (REDCap), a web-based secure application housed at the University of Colorado.13 A Federal Certificate of Confidentiality was obtained from the National Institutes of Health prior to study initiation. The study was approved by the Colorado Multiple Institutional Review Board (COMIRB). All participants provided written informed consent for participation in the study.
Statistical Analysis
Descriptive statistics including frequency, mean and standard deviation, were applied to report marijuana use patterns and reasons for use. The Spearman correlation and 95% confidence intervals (CI) between the various measures of marijuana use are reported for each study visit. Women with missing data for either component of the correlation at each study visit were not included in that particular correlation analysis. We also conducted a sensitivity analysis in which we repeated all correlation analyses after excluding women who self-reported no use.
In addition, we estimated a general linear mixed effects regression model that accounted for repeated measures within participants, and to examine correlation between different sample types. In this model, we assumed a linear relationship between self-reported frequency and log-transformed urine and serum measured THC-COOH, and that self-reported use was measured without error as an independent variable. The outcome was biologically detected THC-COOH. We specified a random effect for the type of sample, repeated measures for sample type, and nested visit number within participants. No adjustments were made for within-hospital clustering given that all biospecimen assays were performed at a central laboratory, and all study procedures were completed by a single research team. Correlation between urine and serum THC-COOH was reported. The statistical analysis plan was not pre-registered on a publicly available platform. Therefore, results should be considered exploratory.
Data analysis was performed using SAS software, Version 9.4 of the SAS System for Windows. Copyright © 2006 SAS Institute Inc., Cary, NC, USA. Graphics were created using GraphPad Prism version 9.0 for Windows, GraphPad Software, La Jolla California USA. Sample size estimate completed with PASS Power Analysis and Sample Size Software, NCSS, LLC. Kaysville, Utah, USA.
Results
In total, 73 women were approached for the study, and 51 enrolled. Five women, two who withdrew and three with pregnancies that ended in spontaneous abortion prior to 20 weeks’ gestation, were excluded from all analyses. Of the 46 included women, 37 women delivered at one of the enrollment sites. Participation in the collection of biospecimens and questionnaire data was variable by study participant (Figure 1). Baseline marijuana use of participants who did not provide survey results or biospecimens at delivery were compared to those who did; there was no statistical difference in baseline use. Women were enrolled between seven to 15 weeks’ gestation. Descriptive characteristics of the study participants are included in Table 1.
Figure 1.
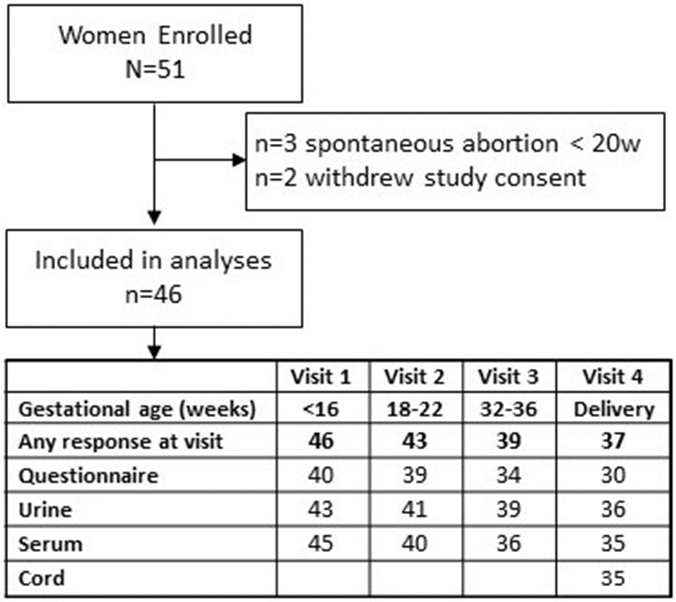
Study Participants
Table 1.
Baseline Characteristics of Study Participants by Site
| Characteristic | Value | Overall N=46 |
Site 1 n=25 |
Site 2 n=21 |
|---|---|---|---|---|
| Maternal weight category at enrollment | Underweight | 2 (4) | 1 (4) | 1 (5) |
| Normal weight | 17 (37) | 8 (32) | 9 (43) | |
| Pre-obese | 11 (24) | 8 (32) | 3 (14) | |
| Obesity Class I | 11 (24) | 4 (16) | 7 (33) | |
| Obesity Class II | 4 (9) | 3 (12) | 1 (5) | |
| Obesity Class III | 1 (2) | 1 (4) | 0 (0) | |
| Maternal age at enrollment (years) | 18-<25 | 10 (22) | 5 (20) | 5 (24) |
| 25-<30 | 17 (37) | 9 (36) | 8 (38) | |
| 30-<35 | 14 (30) | 9 (36) | 5 (24) | |
| 35-<40 | 4 (9) | 1 (4) | 3 (14) | |
| 40+ | 1 (2) | 1 (4) | 0 (0) | |
| Mean maternal age at enrollment (years) | Mean (SD) | 28.5 (4.6) | 28.7 (4.6) | 28.1 (4.8) |
| Gestational age at enrollment (weeks) | 7-8 | 2 (4) | 2 (8) | 0 (0) |
| 9-12 | 23 (50) | 12 (48) | 11 (52) | |
| 13-15 | 21 (46) | 11 (44) | 10 (48) | |
| Race / Ethnicity | Hispanic1 | 12 (26) | 4 (16) | 8 (38) |
| Black | 17 (37) | 8 (32) | 9 (43) | |
| White | 17 (37) | 13 (52) | 4 (19) | |
| Insurance | Private insurance | 6 (13) | 6 (24) | 0 (0) |
| Medicaid | 40 (87) | 19 (76) | 21 (100) | |
| Current employment status | Employed full-time | 16 (35) | 10 (40) | 6 (29) |
| Employed part-time | 8 (17) | 5 (20) | 3 (14) | |
| Student | 4 (9) | 3 (12) | 1 (5) | |
| Homemaker | 8 (17) | 5 (20) | 3 (14) | |
| Out of work/looking for work | 4 (9) | 1 (4) | 3 (14) | |
| Out of work and not currently looking for work | 2 (4) | 0 (0) | 2 (10) | |
| Unable to work | 4 (9) | 1 (4) | 3 (14) | |
| Education level | No high school diploma | 4 (9) | 1 (4) | 3 (14) |
| High school diploma or equivalent | 18 (39) | 8 (32) | 10 (48) | |
| Trade/technical/vocational | 4 (9) | 3 (12) | 1 (5) | |
| Some college, no degree | 12 (26) | 7 (28) | 5 (24) | |
| Associate's degree | 4 (9) | 2 (8) | 2 (10) | |
| Bachelor's degree | 3 (7) | 3 (12) | 0 (0) | |
| Master's degree | 1 (2) | 1 (4) | 0 (0) | |
| Relationship Status | Married/domestic partnership | 16 (35) | 10 (40) | 6 (29) |
| Engaged | 7 (15) | 5 (20) | 2 (10) | |
| In a serious relationship but not married/engaged | 19 (41) | 9 (36) | 10 (48) | |
| Divorced or separated | 1 (2) | 1 (4) | 0 (0) | |
| Not currently in a relationship | 3 (7) | 0 (0) | 3 (14) |
Note: Data are presented as n (%) unless otherwise specified.
All participants classified as Hispanic ethnicity are Hispanic White.
All participants had reported marijuana use at some point during the pregnancy to a healthcare practitioner. Ongoing marijuana use was confirmed at enrollment by self-report on survey, or THC-COOH detected in the urine or serum in 87% of the participants (Table 2). By the time of delivery, 16 (44%) had evidence of ongoing use by either self-report or positive biospecimen. At enrollment, many participants (n=36 [78%]) reported a decrease in marijuana use during pregnancy compared to before pregnancy, and only one (2%) reported an increase in use during pregnancy. The most frequently reported reason for decreasing use was concern for adverse fetal effects (n=14 [30%]). Only four women reported a decrease in use because of maternal risks and three reported a lack of interest in marijuana use while pregnant. Most participants smoked marijuana with a minority reporting the use of edibles, concentrates, tinctures, and topical products (Table 2).
Table 2.
Perinatal marijuana use in a longitudinal cohort by survey self-report, urine and serum (N=46)
| Marijuana Use Measure | Visit 1 <16 wks |
Visit 2 18-22 wks |
Visit 3 32-36 wks |
Visit 4 Delivery |
|---|---|---|---|---|
| n=46 | n=43 | n=39 | n=36 | |
| Any marijuana use (self, urine, or serum) | 39 (85) | 34 (79) | 22 (56) | 15 (42) |
| n=45 * | n=43 | n=39 | n=35 | |
| Self-report | ||||
| ongoing use | 16 (36) | 16 (37) | 10 (26) | 10 (29) |
| plans to stop, has not yet stopped | 11 (24) | 5 (12) | 4 (10) | 0 (0) |
| reports quit, used within 24 hrs | 2 (4) | 2 (5) | 0 (0) | 0 (0) |
| reports quit, used < 30 days ago | 5 (11) | 7 (16) | 5 (13) | 4 (11) |
| reports quit, last use > 30 days ago | 11 (24) | 13 (30) | 20 (51) | 21 (60) |
| n=44 * | n=43 | n=36 | n=33 | |
| Days of use in past 30 days by self-report | ||||
| Zero days | 10 (23) | 11 (26) | 18 (50) | 19 (58) |
| 1-6 days | 4 (9) | 8 (19) | 7 (19) | 3 (9) |
| 7-13 days | 5 (11) | 6 (14) | 2 (6) | 1 (3) |
| 14-20 days | 8 (18) | 6 (14) | 6 (17) | 4 (12) |
| 21-30 days | 17 (39) | 12 (28) | 3 (8) | 6 (18) |
| n=44 * | n=43 | n=36 | n=33 | |
| Number of uses per day | ||||
| One time | 15 (33) | 12 (30) | 17 (52) | 13 (39) |
| Two to three times | 18 (39) | 18 (45) | 11 (33) | 18 (55) |
| Four to five times | 13 (28) | 10 (25) | 4 (12) | 1 (3) |
| More than five times | 0 (0) | 0 (0) | 1 (3) | 1 (3) |
| n=46 * | n=42 | n=36 | n=34 | |
| Type of marijuana use | ||||
| Buds (flowers) | 42 (91) | 37 (88) | 33 (92) | 33 (97) |
| Edibles | 13 (28) | 16 (38) | 7 (19) | 12 (35) |
| Concentrate (hash, oil, wax, shatter) | 14 (30) | 10 (24) | 8 (22) | 6 (18) |
| Drinks | 1 (2) | 1 (2) | 0 (0) | 0 (0) |
| Tincture (under the tongue) | 2 (4) | 4 (10) | 2 (6) | 2 (6) |
| Topical (spray, lotion, skin product) | 2 (4) | 5 (12) | 2 (6) | 2 (6) |
| Other | 1 (2) | 0 (0) | 2 (6) | 0 (0) |
| n=43 | n=41 | n=39 | n=36 | |
| Urine THC-COOH positive | 33 (77) | 29 (71) | 19 (49) | 14 (39) |
| n=45 | n=40 | n=36 | n=35 | |
| Serum THC-COOH positive | 32 (71) | 26 (65) | 16 (44) | 13 (37) |
Denominator available for each item is reported. Missing data are present at each visit due to some participants not providing complete data or biosamples. For example, one of the 46 participants did not provide self-report data at the first visit but provided all other data at that visit and for the duration of the study. THC-COOH is 11-nor-9-carboxy-delta-9-tetrahydrocannabinol.
Overall, 33% of participants reported concurrent tobacco use at the time of enrollment. Among those still participating at the time of delivery, only 11% reported ongoing tobacco use. There were 5 (11%) women who reported the use of other drugs at study enrollment. Three (7%) reported use of prescription opioids, one reported both methamphetamine and cocaine use, and one other reported only cocaine use. At the time of delivery, 2 (6%) reported prescription opioid use but no other drug use was reported by remaining participants.
At the time of enrollment, 29 (63%) reported using marijuana to help with nausea. Other reported reasons for use were help with sleep (n=19, 41%), anxiety (n=12, 26%), pain (n=9, 20%), avoidance of weight gain (n=6, 13%), habit (n=6, 13%), and recreation (n=3, 7%). At the time of delivery, the number of women reporting use to help with nausea decreased to 22 (59%). The number of participants using marijuana to help with pain and anxiety increased to 15 (41%) for both indications. Only one woman reported use for recreation at the time of delivery, and five women reported ongoing use due to habit.
Plots of self-reported frequency of use, quantified serum THC-COOH, and quantified urine THC-COOH for individual participants across study visits are available as supplemental material (Figure S2). Correlations between the different measures of marijuana use are reported in Figure 2. After excluding women with no self-reported use, all correlation results were similar. In the regression model analysis, across all visits and within each participant, serum and urine THC-COOH were highly correlated (0.91).
Figure 2.
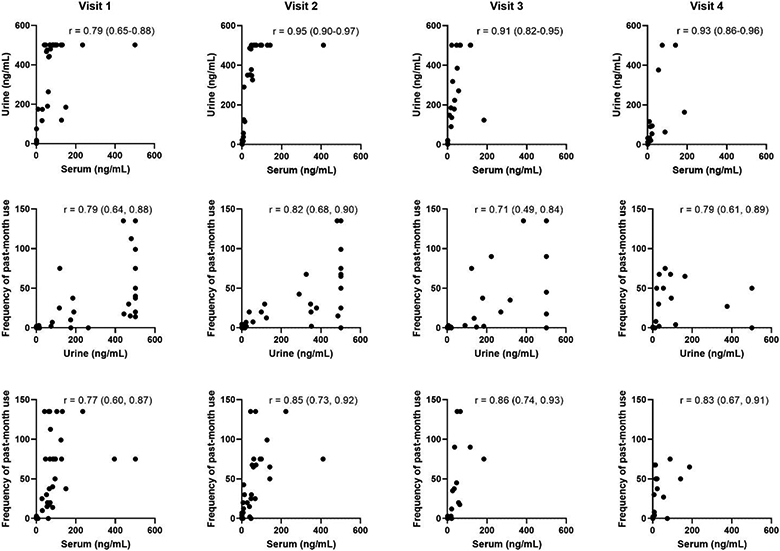
Correlations between self-reported frequency of past-month use, serum THC-COOH, and urine THC-COOH at each of the study visits. All values are in ng/mL. Spearman’s correlation coefficients are reported with 95% confidence intervals. Clustering of values at 500 ng/mL for urine represents the upper limit of detection of the assay.
Of the 35 women with cord segments available at the time of delivery, 40% (14/35) reported ongoing marijuana use or use within the past 30 days. There was strong correlation between self-reported frequency of use over the past 30 days and umbilical cord THC-COOH (Figure 3), but weaker correlation between self-reported use at each visit and umbilical cord THC-COOH at delivery (Figure 4). Among 8 women who reported cessation before 22 weeks, one cord result was positive (12.5%). Among 17 women with self-reported use at the 32-36 week visit for whom cord was available at delivery, 76.5% (13/17) had detectable cord THC-COOH, and among 14 with ongoing use at delivery, 93% (13/17) had detectable cord THC-COOH. 48% (14/29) reported second-hand exposure to marijuana use at the time of delivery. Among women who denied use by self-report, 21% (4/19) reported second-hand exposure; of these 4 participants, 3 had cord available at delivery and 1 had a positive cord homogenate for THC-COOH.
Figure 3.
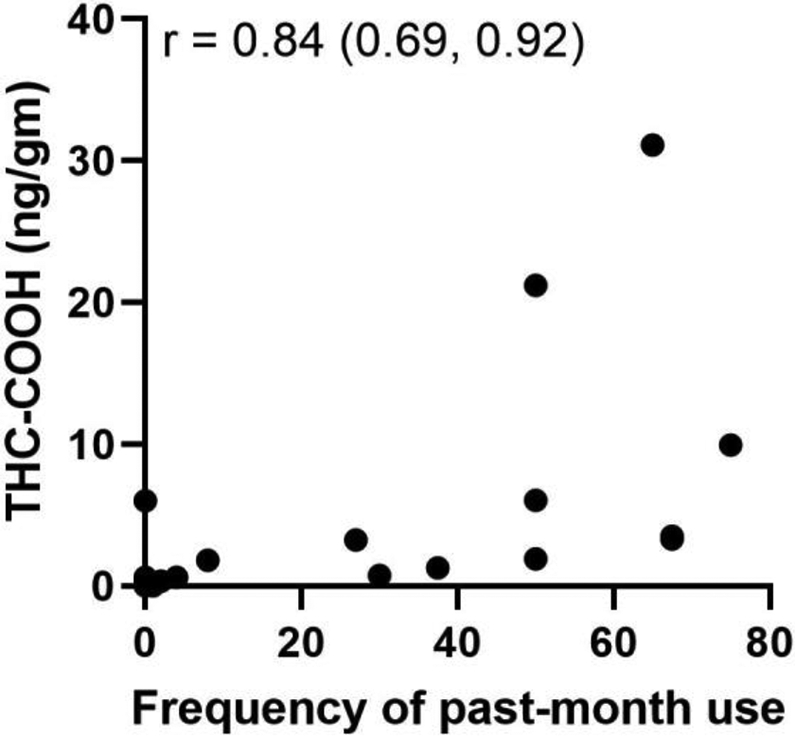
Correlation between self-reported past 30-day frequency of marijuana use and quantified THC-COOH umbilical cord homogenate assay results. Reported values for THC-COOH are in ng/g. Spearman’s correlation coefficient is reported with 95% confidence intervals.
Figure 4.
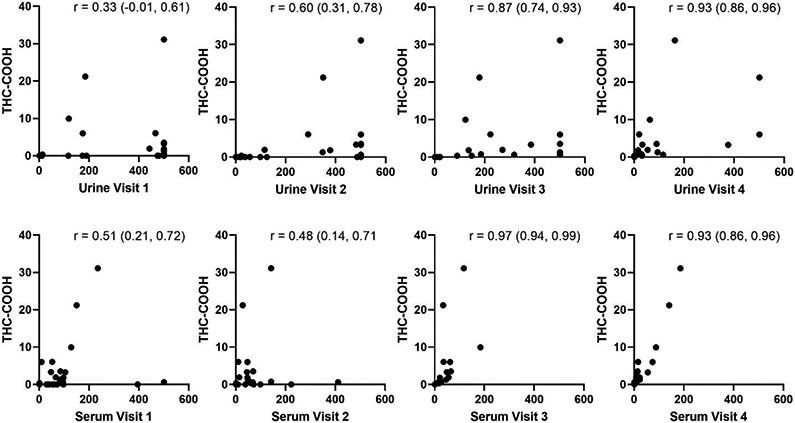
Correlation between THC-COOH in cord at delivery with serum and urine at each visit. Urine and serum values are in ng/mL. Umbilical cord THC-COOH values are reported in ng/g. Spearman’s correlation coefficients are reported on each panel with 95% confidence intervals. Clustering of values at 500 ng/mL for urine represents the upper limit of detection of the assay.
Of the 11 participants who self-reported no use at visit 2, THC-COOH was detected in the urine and serum of two participants (both positive for urine and serum). Of the 18 who self-reported no use at visit 3, THC-COOH was detected in the urine for 3 of them. Among the 3 with detectable THC-COOH in urine, serum was not available for one, was undetectable for one, and detectable for the other. Of 19 who self-reported no use at delivery, THC-COOH was present in both urine and serum for 1 participant.
Discussion
In a cohort of women in a state with legalized marijuana, biologic sampling of urine, serum and umbilical cord homogenate correlated well with self-reported marijuana use patterns on a detailed questionnaire. Quantification of THC-COOH in umbilical cord homogenate was strongly correlated with frequency of maternal marijuana use in the last month of pregnancy. However, umbilical cord testing only detected use prior to 22 weeks’ gestation in one participant, and infrequently detected use reported at 32 to 36 weeks’ gestation. As a result, future studies focusing on marijuana use in the last month of pregnancy should consider umbilical cord testing. In contrast, earlier use could be measured by quantitative urine or serum assay, which were highly correlated with self-report and each other.
Prior studies of the association between prenatal marijuana use and pregnancy outcomes are limited by reliance on self-reported use. Self-report underestimated prenatal marijuana use by at least two to three fold compared to urine toxicology testing in prior studies. A retrospective cohort study of pregnant women seeking care in the Kaiser Permanente Northern California system demonstrated that 7.1% used marijuana but only 54% of these women were detected by self-report; the remainder were ascertained by universal urine toxicology testing.14 A prospective cohort study performed at the same two sites as this study demonstrated that self-reported use on an anonymous survey occurred in 6.0% of participants, while umbilical cord homogenate testing for THC-COOH was positive in 22.4%.15 A reliance on self-reported use in the perinatal population may bias cohort studies of the association between marijuana use and pregnancy outcomes towards the null.16 In contrast, we identified a strong correlation between self-report and biological sampling. This strong correlation likely reflects the recruitment of a population of women who openly acknowledged marijuana use and were willing to enroll in a study specifically examining the relationship between self-report and biological sampling over time. It also supports the validity of the self-report survey in the setting of legalized medicinal and recreational marijuana.
We also demonstrated that quantified THC-COOH in the umbilical cord does not seem to be valuable for ascertainment of more remote use, even use in the third trimester at 32 to 36 weeks’ gestation. Other studies had similar findings suggesting that meconium may be a more sensitive means of ascertaining use that does not continue until the time of delivery.17 This difference in detection could be related to the fact that THC-COOH in meconium is more concentrated than in cord tissue, which makes it easier to detect analytically in meconium. However, meconium is challenging to collect and many institutions now use cord homogenate for neonatal testing because of the ease of collection and use of an otherwise discarded specimen. We were precluded from collecting meconium for the purposes of this study by the institutional review board, as a positive test would require mandatory reporting in Colorado, even in a research setting.
Importantly, just over 50% of the women enrolled in the study who self-reported marijuana use in pregnancy stopped using marijuana prior to the time of delivery. All women received standard counseling regarding marijuana cessation which, at the time of the study, included healthcare practitioner counseling and a handout from the Colorado Department of Public Health and Environment about possible harms of marijuana use in pregnancy. The high proportion of women with ongoing use who reported reasons for use such as help with sleep, pain, anxiety and nausea demonstrates the importance of helping women find safe alternatives to marijuana for the treatment of common symptoms in pregnancy. In addition, effective interventions are needed to help motivated women stop using marijuana in pregnancy as many participants cited concerns for fetal risks but still were not able to stop using marijuana.
The strengths of this study include conducting study procedures in the setting of legalized medical and recreational marijuana, which may have resulted in participants being more forthcoming about use. We prospectively enrolled women and collected biospecimens at multiple time points across pregnancy to evaluate correlation between biological samples and self-reported use across gestation. Participants provided detailed data about frequency of use and mode of use improving the rigor of our correlation analyses. However, diverse modes of consumption, product types, and product THC-COOH concentrations could have contributed to scatter in correlation analyses at higher use frequencies.
Our study has several limitations. Despite using a detailed survey to gather data regarding marijuana use, we were not able to directly quantify exposure as we did not have the ability to analyze the products that participants were using to accurately determine the THC concentration. In addition, variable length of THC-COOH assay positivity based on chronicity of use could have led to persistently positive results for those with more chronic use across study visits even after cessation.18 While we enrolled 51 women and attempted to follow them longitudinally with an experienced study team, participation was variable and only 22 women completed all study procedures. However, we had 33 women with umbilical cord homogenate available for analysis and 30 of them also had self-reported data regarding frequency of use, which allowed us to meet our a priori sample size minimum of 30 participants. Finally, urine THC-COOH concentrations could have varied based on unmeasured factors such as urine dilution.
Utilization of biological samples for ascertainment and quantification of marijuana use will be critical in advancing work in the field to evaluate associations between perinatal marijuana use and maternal and neonatal outcomes. The prevalence of marijuana use and perception of safety of use in pregnancy are both increasing.3,4,19 As legalization of marijuana continues to expand across the United States, rigorous evaluations of anticipated outcomes of perinatal marijuana use are needed to better educate women about anticipated risks thereby allowing them to make informed decisions about use.
Supplementary Material
Funding:
Dr. Metz was supported by the National Institute on Child Health and Human Development under award number K12HD001271 and R01DA049832 during the completion of this work. A portion of the work on this project was also funded by the Colorado Clinical and Translational Sciences Institute (CCTSI) under award number UL1RR025780. The cost of some of the assays was internally funded by ARUP Laboratories. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
COI Declaration: The authors do not have any conflicts of interest to report related to this work. Each author has indicated that he/she has met the journal’s requirements for authorship.
Previous Presentation: This work was presented in abstract format at the Society for Maternal-Fetal Medicine Annual Meeting on February 8, 2020 in Grapevine, TX.
References
- 1.Saurel-Cubizolles MJ, Prunet C, Blondel B. Cannabis use during pregnancy in France in 2010. BJOG. 2014;121(8):971–977. [DOI] [PubMed] [Google Scholar]
- 2.Tennes K. Effects of marijuana on pregnancy and fetal development in the human. NIDA Res Monogr. 1984;44:115–123. [PubMed] [Google Scholar]
- 3.Jarlenski M, Zank J, Bodnar LM, Koma JW, Chang JC, Bogen DL. Trends in perception of risk of regular marijuana use among US pregnant and nonpregnant reproductive-aged women. Am J Obstet Gynecol. 2017; 217(6):705–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown QL, Sarvet AL, Shmulewitz D, Martins SS, Wall MM, Hasin DS. Trends in Marijuana Use Among Pregnant and Nonpregnant Reproductive-Aged Women, 2002-2014. JAMA. 2017; 317(2):207–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda; Board on Population Health and Public Health Practice; Health and Medicine Division; National Academies of Sciences. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. http:www.nap.edu/246252017. [PubMed]
- 6.Shiono PH, Klebanoff MA, Nugent RP, et al. The impact of cocaine and marijuana use on low birth weight and preterm birth: a multicenter study. Am J Obstet Gynecol. 1995;172(1 Pt 1):19–27. [DOI] [PubMed] [Google Scholar]
- 7.Niedbala RS, Kardos KW, Fritch DF, et al. Detection of marijuana use by oral fluid and urine analysis following single-dose administration of smoked and oral marijuana. J Anal Toxicol. 2001;25(5):289–303. [DOI] [PubMed] [Google Scholar]
- 8.Montgomery D, Plate C, Alder SC, Jones M, Jones J, Christensen RD. Testing for fetal exposure to illicit drugs using umbilical cord tissue vs meconium. J Perinatol. 2006;26(1):11–14. [DOI] [PubMed] [Google Scholar]
- 9.Chittamma A, Marin SJ, Williams JA, Clark C, McMillin GA. Detection of in utero marijuana exposure by GC-MS, ultra-sensitive ELISA and LC-TOF-MS using umbilical cord tissue. J Anal Toxicol. 2013;37(7):391–394. [DOI] [PubMed] [Google Scholar]
- 10.American College of Obstetricians and Gynecologists. Committee Opinion No. 700. Methods for Estimating the Due Date. Obstet Gynecol 2017. [DOI] [PubMed] [Google Scholar]
- 11.Wu F, Scroggin TL, Metz TD, McMillin GA. Development of a Liquid Chromatography-Tandem Mass Spectrometry Method for the Simultaneous Determination of Four Cannabinoids in Umbilical Cord Tissue. J Anal Toxicol. 2018;42(1):42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American College of Obstetricians and Gynecologist. Committee on Obstetric Practice. Committee Opinion No. 722: Marijuana Use During Pregnancy and Lactation. Obstet Gynecol. 2017;130(4):e205–e209. [DOI] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young-Wolff KC, Tucker LY, Alexeeff S, et al. Trends in Self-reported and Biochemically Tested Marijuana Use Among Pregnant Females in California From 2009-2016. JAMA. 2017;318(24):2490–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metz TD, Silver RM, McMillin GA, et al. Prenatal Marijuana Use by Self-Report and Umbilical Cord Sampling in a State With Marijuana Legalization. Obstet Gynecol. 2019;133(1):98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez CE SJ, Allshouse AA, Hermesch A, Metz TD. Marijuana use in young mothers and adverse pregnancy outcomes: a retrospective cohort study. BJOG. 2019;126(12):1491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colby JM. Comparison of umbilical cord tissue and meconium for the confirmation of in utero drug exposure. Clin Biochem. 2017;50(13-14):784–790. [DOI] [PubMed] [Google Scholar]
- 18.Schep LJ, Slaughter RJ, Glue P, Gee P. The clinical toxicology of cannabis. N Z Med J 2020;133(1523):96–103. [PubMed] [Google Scholar]
- 19.Metz TD, Borgelt LM. Marijuana Use in Pregnancy and While Breastfeeding. Obstet Gynecol. 2018; 132(5):1198–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


