Abstract
Jak3-deficient mice display vastly reduced numbers of lymphoid cells. Thymocytes and peripheral T cells from Jak3-deficient mice have a high apoptotic index, suggesting that Jak3 provides survival signals. Here we report that Jak3 regulates T lymphopoiesis at least in part through its selective regulation of Bax and Bcl-2. Jak3-deficient thymocytes express elevated levels of Bax and reduced levels of Bcl-2 relative to those in wild-type littermates. Notably, up-regulation of Bax in Jak3-deficient T cells is physiologically relevant, as Jak3 Bax double-null mice have marked increases in thymocyte and peripheral T-cell numbers. Rescue of T lymphopoiesis by Bax loss was selective, as mice deficient in Jak3 plus p53 or in Jak3 plus Fas remained lymphopenic. However, Bax loss failed to restore proper ratios of peripheral CD4/CD8 T cells, which are abnormally high in Jak3-null mice. Transplantation into Jak3-deficient mice of Jak3-null bone marrow transduced with a Bcl-2-expressing retrovirus also improved peripheral T-cell numbers and restored the ratio of peripheral CD4/CD8 T cells to wild-type levels. The data support the concepts that Jak kinases regulate cell survival through their selective and cell context-dependent regulation of pro- and antiapoptotic Bcl-2 family proteins and that Bax and Bcl-2 play distinct roles in T-cell development.
The cytokine receptor family controls physiologic responses as diverse as growth, fertility, lactation, hematopoiesis, lymphopoiesis, and the response to pathogens. In part, the selective nature of these responses is regulated through the specific high-affinity interaction of the receptors with their respective ligands. Ligand binding and receptor aggregation activate one or two members of the Janus family of tyrosine kinases (Jak1 to -3 and Tyk2), which then transphosphorylate themselves, their associated receptors, and numerous substrates recruited to the activated receptor complex (10, 22). Shared substrates of Jak kinases include members of the signal transducers and activators of transcription (Stats), which dimerize following Jak-mediated tyrosine phosphorylation, relocalize to the nucleus, and activate a subset of cytokine-inducible genes. The specificity of the cytokine response is therefore at least in part due to the selective activation of dedicated Jaks and Stats (9, 23).
The creation of mice deficient in components of the Jak-Stat pathway by gene targeting approaches has demonstrated diverse but nonredundant roles for these signaling effectors. Deletion of the Jak kinases led to largely predictable phenotypes. For example, deletion of Jak2, which among other signals mediates those emanating from the erythropoietin receptor (65), leads to profound defects in definitive erythropoiesis (43, 46). Furthermore, deletion of Jak3, which is required for interleukin-7 (IL-7), IL-2, IL-4, IL-9, and IL-15 signaling (24), results in a scid-like phenotype with severe defects in lymphopoiesis (44, 47, 59). One prediction was that the deletion of specific Stats that are selectively activated by hemopoietins would recapitulate the phenotypes of Jak-deficient mice. However, Stat-deficient mice display surprisingly restricted phenotypes that represent only a subset of those present in the Jak knockouts (14, 25, 26, 32, 35, 52, 56–58). Thus, other effectors also contribute nonredundant roles to Jak-induced pathways regulating cell proliferation, differentiation, and/or survival.
Cytokine signaling is continuously required to suppress the apoptotic program (64), and the suppression of apoptosis is, in some scenarios, sufficient to permit hematopoietic cell differentiation (15). Attractive targets for Jak kinase-mediated signals regulating survival include the Bcl-2 family of apoptotic regulators, which serve to either suppress (e.g., Bcl-2 or Bcl-XL) or activate (e.g., Bax, Bad, or Bak) the cell death program (66). Indeed, in myeloid cells a Jak2-dependent pathway is necessary and sufficient for cell survival and for the selective regulation of Bcl-XL (45). However, in vivo tests of this paradigm are difficult, as deletion of Jak2 results in midgestation embryonic lethality (43, 46). A more genetically tractable system is the Jak3-deficient mouse, which is viable. Like IL-7 receptor α (IL-7Rα) or common γ chain (the two chains of the IL-7R) deficiency (6, 12, 50), Jak3 deficiency in mice leads to severe defects in T- and B-cell development and function (44, 47, 59). All defects present in Jak3-deficient mice are intrinsic to a common lymphoid progenitor, as lymphopoiesis can be fully restored by transplantation with Jak3-deficient bone marrow transduced with a Jak3-expressing retrovirus (5). In vitro splenocyte analysis has suggested that the defects of Jak3-deficient mice may be related to defects in cell survival (60). Here we report that Jak3 regulates T lymphopoiesis at least in part through its ability to selectively repress the expression of Bax and to induce the expression of Bcl-2. Furthermore, genetic studies demonstrate that the selective regulation of Bax and Bcl-2 by Jak3 is physiologically relevant and that Bcl-2 and Bax play distinct roles in T lymphopoiesis. The data support a model whereby Jak3 selectively regulates the expression of Bax and Bcl-2, and this is sufficient to promote T-cell development.
MATERIALS AND METHODS
Mice.
Jak3−/− C57BL/6 × 129 and bax+/−, p53−/−, mlr-lpr, IL-7Rα−/−, Stat5a/5b+/−, and Rag2−/− C57BL/6 mice were bred and maintained in the animal research facility of St. Jude Children's Research Hospital. IL-7Rα−/− C57BL/6 and p53−/− C57BL/6 mice were obtained from Jackson Laboratories. Peter Doherty (St. Jude Children's Research Hospital) and Rakesh Goorha generously provided mlr-lpr and Rag2−/− C57BL/6 mice, respectively. Jak3−/− bax−/− mice were generated by crossing Jak3−/− mice with the bax+/− mice (bax−/− mice have severe fertility problems [29, 48]). F1 offspring were intercrossed to obtain double-null mice. In a similar fashion, Jak3−/− p53−/− and Jak3−/− lpr mice were generated by crossing Jak3−/− mice with p53−/− or lpr mice, respectively, and double-null mice were obtained by intercrossing F1 mice. All mice were genotyped at weaning by PCR and analyzed at 4 to 6 weeks of age.
In situ cell death (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end-labeling [TUNEL]) analysis
Thymuses from wild-type and Jak3-deficient mice were removed, rinsed in phosphate-buffered saline (PBS), and immediately frozen on dry ice in Tissue Tek O.C.T. compound (Fisher). Embedded tissues were cut into 8-μm sections, fixed for 20 min in 10% buffered formalin, and washed twice with PBS. Tissue sections were permeabilized by incubation for 5 min at 37°C with 10-μg/ml proteinase K solution, washed twice in 1× PBS, and then incubated for 2 min in 0.1% Triton X-100–0.1% sodium citrate at 4°C. The slides were then dried and incubated for 1 h at 37°C with reaction mix from an In Situ Cell Death Assay Kit (Boehringer Mannheim). Slides were washed three times in PBS for 5 min, mounted under antifade Fluoromount, and photographed.
Fluorescence-activated cell sorter (FACS) analysis of apoptosis.
Single-cell suspensions of thymuses and spleens were prepared by passing tissue through a fine-mesh cell strainer with the plunger of a 3-ml syringe. For splenocytes, single-cell suspensions were treated with Gey's solution to lyse red blood cells. Cells were then incubated on ice for 30 min with fluorescein isothiocyanate (FITC)-conjugated anti-CD4, phycoerythrin (PE)-conjugated anti-CD8, and allophycocyanin-conjugated annexin V (Caltag Inc.). The cells were then washed and incubated with 1-μg/ml 7-aminoactinomycin solution (Sigma) for 30 min and analyzed by FACScan (Becton Dickinson).
FACS analyses of Bcl-2, Bax, and Bcl-XL expression.
Single-cell suspensions of thymocytes were prepared, and cells were surface stained with FITC-conjugated anti-CD4 antibody GK1.5 and Cychrome-conjugated anti-CD8 antibody 53-6.7 (Pharmingen) at 4°C for 30 min. The cells were then washed and fixed on ice for 15 min in 1% paraformaldehyde (Ted Pella, Inc.). The cells were then washed once with PBS and incubated with a mouse anti-Bax antibody (2280-MC-100; Genzyme), a hamster anti-Bcl-2 antibody (15021A; Pharmingen,) or a rabbit anti-Bcl-XL antibody (B22630; Transduction Labs) in PBS–5% bovine serum albumin–0.05% saponin (Sigma). The cells were washed twice; PE-conjugated anti-mouse antibody (Caltag) or PE-conjugated anti-hamster or PE-conjugated anti-rabbit antibody (both from Southern Biotechnology Inc.) was added, respectively; and the cells were incubated on ice for 30 min. The cells were then washed and analyzed by FACScan. Parallel analyses of Bax- and Bcl-2-null thymuses and spleens and of Bcl-X-deficient fetal liver-derived hematopoietic cells demonstrated the specificity of each of these antibodies. Isotype-matched control antibodies, purified mouse immunoglobulin G1 (IgG1), hamster IgG (Pharmingen), and normal rabbit Ig (R&D Systems) served as negative controls.
Immunofluoresence analyses.
Single-cell suspensions of thymocytes were prepared and washed twice in PBS, and cells were counted after the second wash. Cells were resuspended in a final volume of 200 μl of PBS, and 10-μl aliquots were applied to glass slides. After cells had completely dried, the slides were stored at −20°C until further use. For immunofluorescence the slides were fixed for 20 min in 10% buffered formalin (Fisher), washed twice with PBS for 5 min, and blocked for 1 h at room temperature with 10% bovine serum albumin–PBS. Following blocking, the slides were incubated for 2 h at room temperature with 1:500 dilutions of anti-mouse Bax (N-20 [Santa Cruz Inc.] or 2280-MC-100). Slides were washed three times in PBS and incubated for a further 2 h at room temperature with a 1:50 dilution of Alexa488–goat anti-rabbit antibody (for Bax antibody N-20) and FITC–rabbit anti-mouse antibody (for Bax 2280-MC-100 antibody). Slides were then washed three times in PBS, mounted under antifade Fluoromount (Fisher), and photographed. The procedure was the same for Bcl-2 (15021A; Pharmingen) and Bcl-XL (B22630; Transduction Labs) analysis, and secondary antibodies were FITC–goat anti-hamster antibody for Bcl-2 and Alexa499–goat anti-rabbit antibody for Bcl-XL. For Thy1.2 detection we used PE-conjugated anti-mouse Thy1.2 antibody (Pharmingen).
Semiquantitative RT-PCR analysis.
Single-cell suspensions from wild-type thymocytes (1 mouse) and Jak3−/− thymocytes (10 mice) were prepared. Thymocytes were stained with PE-conjugated anti-CD4 and Cychrome-conjugated anti-CD8 antibodies and sorted into individual DN, DP, CD4+, and CD8+ populations with a Moflo cell sorter (Cytomation, Inc.). The sorted populations were more than 98% pure. Total cellular RNA was extracted from each population with RNAzol B (TEL-TEST, Inc.). Semiquantitative reverse transcription (RT)-PCR was performed to analyze bcl-2, bax, and actin gene expression using the following primers: Bcl-2 (465 bp), 5′-CTGGATCCAGGATAACGGAGGCT-3′ and 5′-TGGCAATTCCTGGTTCGGTTTTCAA-3′; and Bax (473 bp), 5′-GATTGCTGACGTGGACACGGACT-3′ and 5′-TCAGCCCATCTTCTTCCAGATGGT-3′. These primers encompassed one intron to exclude DNA contamination. The primers for actin were 5′-ACTCCTATGTGGGTGACGAG-3′ and 5′-AGGTCCAGACGCAGGATGGC-3′, which amplified a fragment of 380 bp.
Lymphocyte phenotyping by flow cytometry.
Freshly isolated thymocytes or splenocytes and peripheral blood lymphocytes were stained with antibodies against CD4 (GK1.5), CD8 (53-6.7), B220 (RA3-6B2), NK1.1 (PK136), γδ T-cell receptor (GL3), and Fas (Jo-2) (Pharmingen).
Analysis of peripheral blood T- and B-lymphocyte numbers.
The lymphocyte count was manually scored from Wright-stained blood smears, and the total blood cell count was determined with a Coulter Counter. Red blood cells were lysed, and cells were stained with FITC-conjugated anti-CD4, PE-conjugated anti-CD8, Cychrome-conjugated anti-B220, or PE-conjugated anti-NK marker DX5 (Pharmingen) and analyzed by FACScan. T and B cells were then quantitated according to the lymphocyte number and the percentages of T and B cells.
Retroviral transduction and bone marrow transplantation.
The human Bcl-2 gene was cloned into the Moloney stem cell virus-internal ribosomal entry site-green fluorescent protein (MSCV-I-GFP) vector (20) (kindly provided by Robert Hawley). Ten micrograms of this construct was cotransfected with 10 μg of pEQPAM3 into 293T cells, seeded at 3 × 106 per 10-cm-diameter dish 1 day before, by the calcium precipitation method (49). The cells were cultured in 10 ml of Dulbecco modified Eagle medium (Gibco BRL)–10% fetal bovine serum (HyClone). Virus culture supernatant was collected 48 h after transfection, another 10 ml of medium was added, and supernatant was collected again after another 24 h. The supernatants were pooled, filtered, and used to infect the ecotropic virus producer cell line GP+E86 with 6 μg of Polybrene (Sigma) per ml in the culture medium. The infected GFP-positive cells were then sorted by FACS to establish MSCV-Bcl-2-I-GFP virus producer cell lines.
Eight-week-old Jak3−/− or wild-type donor mice were injected peritoneally with 150 mg of 5-fluorouracil per kg of body weight 48 h before bone marrow harvest. Bone marrow cells harvested from both hind legs were prestimulated for 48 h in the presence of 20 ng of IL-3 per ml, 50 ng of IL-6 per ml, and 50 ng of stem cell factor (R&D Systems, Inc.) per ml, followed by coculture with irradiated MSCV-I-GFP or MSCV-Bcl-2-I-GFP virus-producing GP+E86 cells and 6 μg of Polybrene per ml. After 48 h, 1 × 106 to 2 × 106 transduced bone marrow cells were injected into 8-week-old irradiated (900 rads) recipient Jak3-deficient mice.
RESULTS
Loss of Jak3 augments T-cell apoptosis.
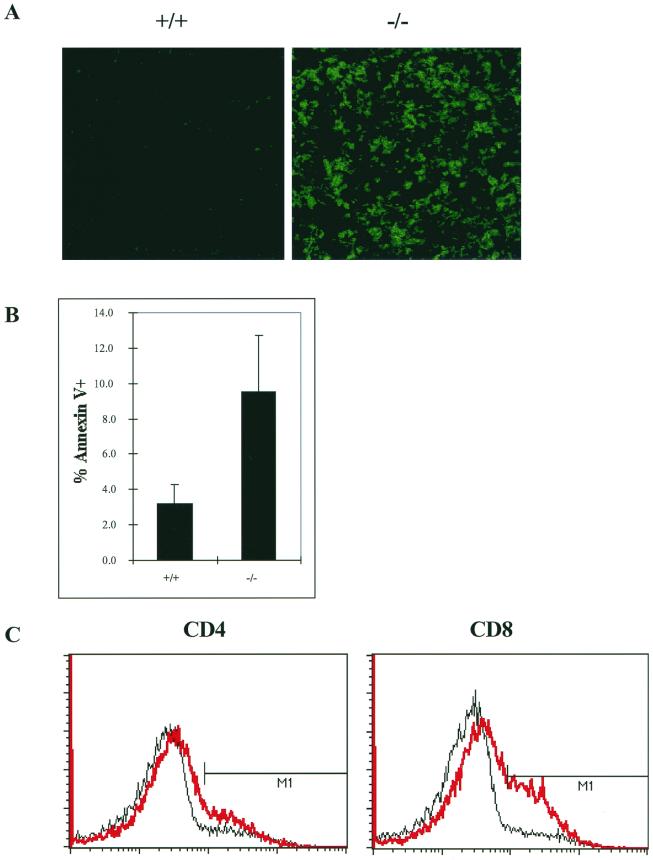
Jak3-deficient mice develop a rudimentary thymus and have severely reduced numbers of T, B, γδ T, and NK cells (44, 47, 59). In addition, Jak3-deficient mice generally display normal ratios of CD4− CD8− double-negative (DN), CD4+ CD8+ double-positive (DP), and CD4+ and CD8+ single-positive cells in the rudimentary thymus, but there is a marked deficit in peripheral CD8+ cell numbers (2, 44). These defects in T lymphopoiesis could reflect a failure of lymphoid progenitors to proliferate and/or survive. To directly assess Jak3-deficient T cells for possible defects in survival, we initially examined the spontaneous rates of apoptosis in thymuses and freshly isolated thymocytes and splenocytes from Jak3-deficient versus wild-type mice. TUNEL analysis demonstrated remarkably high levels of apoptosis in the rudimentary thymuses of 4-week-old Jak3-deficient mice (Fig. 1A). Annexin V staining of total thymocytes confirmed that cells lacking Jak3 had an elevated (threefold) apoptotic index (Fig. 1B), and this phenotype was also evident in each of the Jak3-deficient thymocyte subsets (Fig. 1B legend). Increased levels of apoptosis were also observed in freshly isolated splenic CD4+ and CD8+ cells of Jak3-deficient mice and were particularly elevated in CD8+ cells (Fig. 1C). Thus, both thymic and mature T cells, especially CD8+ cells, of Jak3-deficient mice display high rates of spontaneous apoptosis.
FIG. 1.
Jak3-deficient thymocytes and splenocytes have high rates of spontaneous apoptosis. (A) In situ analysis of apoptosis by TUNEL assays. Thymuses were isolated from 4-week-old wild-type (+/+) and Jak3-deficient (−/−) mice, and TUNEL assays were performed as described in Materials and Methods. Representative thymuses are shown. Magnification, ×21 (original magnification, ×25). (B) Annexin V staining of freshly isolated thymocytes from 4-week-old wild-type and Jak3-deficient mice. Six mice from each group were analyzed. The percentages of annexin V-positive cells in thymocyte subsets were as follows: DN, +/+ = 5.2 ± 2.4 and Jak3−/− = 10.4 ± 6.3; DP, +/+ = 3.4 ± 0.5 and Jak3−/− = 9.9 ± 3.3; CD4+, +/+ = 4.2 ± 1.4 and Jak3−/− = 7.3 ± 1.5; CD8+, +/+ = 4.5 ± 0.9 and Jak3−/− = 9.5 ± 3.3. (C) Annexin V staining of apoptotic cells from freshly isolated splenocytes from wild-type (black line) versus Jak3-deficient (red line) mice. The percentages of apoptotic cells (annexin V-positive cells; M1) were as follows: wild type, CD4 = 10 and CD8 = 8; Jak3 deficient, CD4 = 18 and CD8 = 29. Data represent two independent experiments.
Jak3 is required for appropriate regulation of Bax and Bcl-2 during T-cell development.
A Jak2 kinase-dependent signal is required for the survival of myeloid progenitors and for the selective regulation of Bcl-XL by cytokines (45). We therefore reasoned that the apoptotic defects manifest in Jak3-deficient T cells could be due to inappropriate regulation of Bcl-2 family proteins. Gene targeting approaches in mice suggested Bcl-2, Bax, and/or Bcl-XL as potential Jak3-dependent mediators. Bcl-2-deficient mice exhibit normal thymic development yet display marked lympho-aplasia of mature T cells, with particularly profound deficits in peripheral CD8+ cells (42, 63). By contrast, deletion of Bax results in modest lymphoid hyperplasia (29). Furthermore, chimeric mice generated using Bcl-X-deficient embryonic stem cells in Rag2−/− hosts demonstrated that Bcl-X loss leads to a modest reduction in the numbers of all thymocyte subsets (38). We therefore assessed the expression of Bcl-2, Bcl-XL, and Bax expression in T-cell subsets of Jak3-deficient and wild-type mice using FACS and immunofluorescence analyses with antibodies specific for each of these proteins.
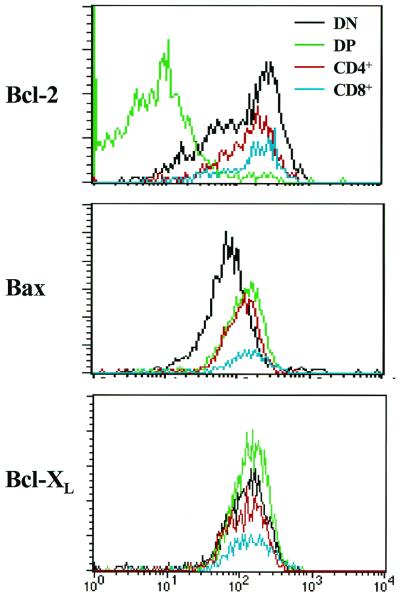
IL-7 signaling is functional in DN and CD4 and CD8 single-positive thymocytes yet is impaired in DP thymocytes (55). We therefore initially assessed whether the levels of Bcl-2, Bcl-XL, and Bax were regulated during T-cell development by FACS analysis of 4-week-old wild-type thymocyte subsets. The specificity of each antibody used for FACS was confirmed by isotype controls and by the analysis of cells derived from Bcl-2-, Bcl-X-, and Bax-deficient mice (data not shown). As expected (62), Bcl-2 levels were dramatically lower in DP versus DN and CD4+ and CD8+ cells (Fig. 2). However, in contrast to previous studies (16), Bcl-XL levels were unchanged in thymocyte subsets (Fig. 2). Interestingly, Bax levels were elevated in DP versus DN thymocytes, and higher levels of Bax were also present in CD4+ and CD8+ cells.
FIG. 2.
Expression of Bcl-2, Bax, and Bcl-XL during T-cell development. Thymocytes were isolated from 4-week-old wild-type mice and stained with Bcl-2, Bax, and Bcl-XL antibodies and analyzed by FACS. Scans shown are representative of four independent analyses.
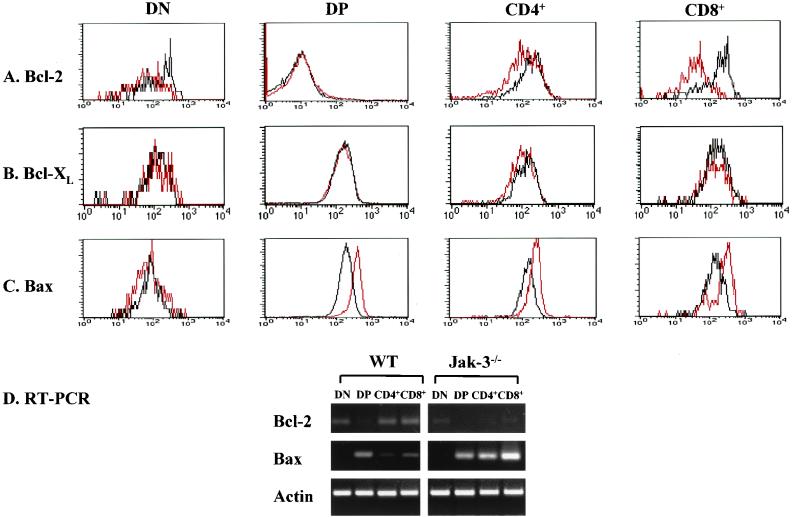
Jak3-deficient mice have on average only 5% of the thymocyte numbers present in wild-type mice (44, 59), and these are highly apoptotic (Fig. 1). DN, CD4+, and CD8+ thymocytes comprise approximately 1, 10, and 5% of total thymocytes, precluding the analysis of Bcl-2, Bcl-XL, and Bax expression in individual T-cell subpopulations of Jak3-deficient mice by Western blot assay. We therefore compared levels of Bcl-2, Bcl-XL, and Bax in wild-type and Jak3-deficient thymocytes by FACS (Fig. 3) and immunofluorescence staining (Fig. 4). Analyses of Bcl-2 levels in IL-7Rα-deficient thymocytes established that Bcl-2 was reduced in DN and single-positive thymocytes from these mice (1). Similarly, Bcl-2 levels were diminished in Jak3-deficient DN, CD4+, and especially CD8+ thymocytes relative to levels of Bcl-2 present in these thymic subsets of wild-type mice (Fig. 3A). Therefore, the reductions in Bcl-2 seen in IL-7Rα-deficient thymocytes are due to defects in Jak3 signaling, but the defects in Bcl-2 levels in Jak3-deficient CD8+ thymocytes appear more profound. As expected, the very low levels of Bcl-2 present in wild-type DP thymocytes were equivalent to those present in Jak3-deficient DP cells (Fig. 3A). Immunofluorescence analyses of total thymocytes and of FACS-sorted thymocytes confirmed that Bcl-2 levels were reduced in DN, CD4+, and CD8+ Jak3-deficient thymocytes, and this was again especially manifest in CD8+ cells (Fig. 4A and data not shown). Therefore, Jak3 is required to sustain Bcl-2 protein expression in T-cell subsets that rely on IL-7 signaling for survival.
FIG. 3.
Jak3-deficient T cells have defects in the expression of Bcl-2 and Bax. (A to C) Thymocytes from 4-week-old wild-type (black lines) and Jak3-deficient (red lines) mice were isolated and analyzed for levels of Bcl-2, Bcl-XL, and Bax by FACS. Signals were Bcl-2, Bax, and Bcl-XL specific based on the analyses of isotype controls and from analyses of thymocytes from Bax- and Bcl-2-deficient mice and of fetal liver-derived hematopoietic progenitors from Bcl-X-deficient mice (data not shown). Representative data are shown from FACS analyses of five age-matched pairs of mice. (D) Semiquantitative RT-PCR was performed to examine bcl-2, bax, and actin mRNA levels in DN, DP, CD4+, and CD8+ thymocytes isolated from 1 wild-type mouse and 10 pooled Jak3−/− mice. Actin was used as a control to show that equal amounts of cDNA templates were used for each cell population.
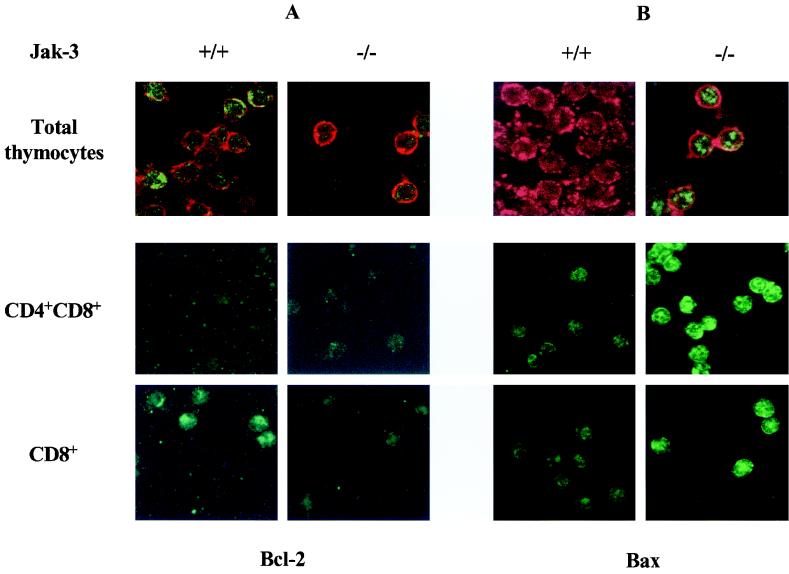
FIG. 4.
Bax expression is elevated and Bcl-2 expression is suppressed in thymocytes of Jak3-deficient mice. Bcl-2 and Bax expression in total, DP, and CD8+ thymocytes is shown. Thymocytes were isolated from 4-week-old wild-type and Jak3−/− mice. Bcl-2- and Bax-specific antibody staining is shown in green. To identify T cells in bulk thymocyte preparations (top panels), the slides were also costained with the T-cell marker Thy1.2 (red staining). Note that only a proportion of total thymocytes from wild-type mice stain strongly with antibody to Bcl-2 (A, upper left panel), as Bcl-2 is not expressed in DP thymocytes (Fig. 2), but that Bcl-2 levels are much lower in total thymocytes from Jak3−/− mice. Representative fields are shown. Magnification of all cells is ×52 (original magnification, ×63).
In addition to obvious reductions in Bcl-2 levels in Jak3-deficient thymocytes, FACS and immunofluorescence analyses surprisingly demonstrated that levels of Bax were elevated at least threefold in DP, CD4+, and CD8+ Jak3-deficient thymocytes relative to the levels expressed in wild-type T cells (Fig. 3C and 4B). Therefore, one unanticipated function of Jak3 signaling in T cells is to suppress the expression of Bax. Despite these changes in Bcl-2 and Bax, the levels of Bcl-XL were essentially equivalent in all T-cell subsets of Jak3-deficient and wild-type mice (Fig. 3B and data not shown). Thus, the loss of Jak3 is associated with selective changes in both Bax and Bcl-2 protein levels.
To address whether changes in the levels of Bcl-2 and Bax proteins in Jak3-deficient thymocytes reflected changes in gene expression, we pooled thymocytes from 10 Jak3-deficient mice, sorted them by FACS, prepared RNA, and analyzed bcl-2 and bax expression by semiquantitative RT-PCR. For comparison we prepared RNA from age-matched wild-type thymocyte subsets. These data clearly demonstrated that bax RNA levels were augmented in Jak3-deficient CD4+ and CD8+ thymocytes (at least threefold) relative to those in wild-type thymocytes (Fig. 3D). Furthermore, bcl-2 RNA levels were obviously diminished in Jak3-deficient DN, CD4+, and CD8+ thymocytes (Fig. 3D). Therefore, inappropriate levels of Bax and Bcl-2 in Jak3-deficient thymocytes are largely accounted for by changes in gene expression.
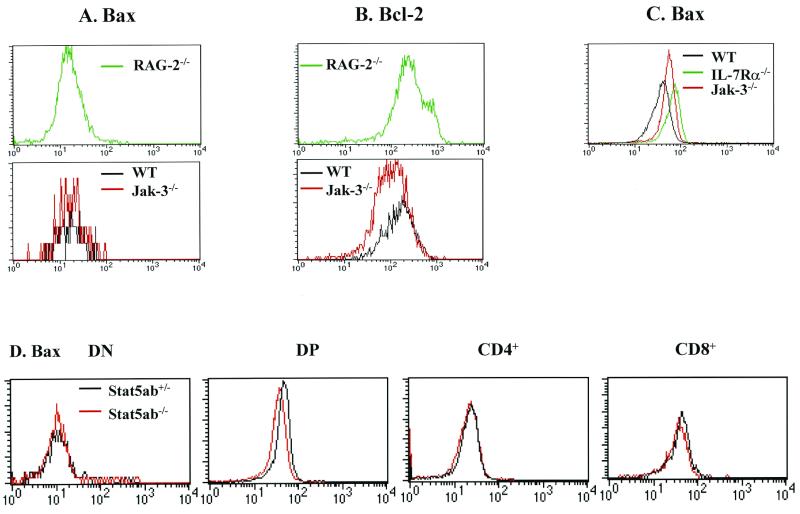
The alterations in Bax and Bcl-2 regulation in Jak3-deficient thymocytes could simply reflect defects in development rather than being associated with their apoptotic phenotype. If so, then other knockout mice defective in T-cell development might also display inappropriate expression of Bax and Bcl-2. To address this issue, we analyzed Bax (Fig. 5A) and Bcl-2 (Fig. 5B) expression in Rag2-deficient mice, which fail to develop beyond the DN stage due to a defect in T-cell receptor rearrangement (53). Parallel FACS analyses demonstrated that Bax levels were essentially equivalent in Rag2-deficient versus wild-type DN thymocytes (Fig. 5A). Furthermore, Bcl-2 levels in Rag2-deficient thymocytes were essentially equivalent to those present in wild-type DN thymocytes (Fig. 5B). Therefore, the failure of a Jak3-dependent signal to suppress Bax levels and to sustain Bcl-2 expression is specific and not simply a consequence of any defect that perturbs T-cell development.
FIG. 5.
Bax and Bcl-2 expression in Rag2-, IL-7Rα-, and Stat5a/Stat5b-deficient mice. (A) FACS analysis of Bax levels in Rag2-deficient mice versus those present in wild-type and Jak3-deficient DN thymocytes. Note that the numbers of DN thymocytes are elevated in Rag2−/− mice relative to those present in Jak3-deficient and wild-type mice. (B) FACS analysis of Bcl-2 levels in Rag2-deficient mice versus those present in wild-type and Jak3-deficient DN thymocytes. (C) Bax levels are also elevated in IL-7Rα−/− thymocytes. CD3-positive total thymocytes were harvested from 4-week old wild-type, Jak3-deficient, and IL-7Rα−/− mice and directly compared for their levels of Bax by FACS analyses. (D) Bax levels in FACS-analyzed Stat5a/Stat5b-deficient thymocytes were compared to those in heterozygous littermates. Data shown are representative of two separate analyses of IL-7Rα- and Rag2-deficient mice and three analyses of Stat5a/Stat5b-deficient mice.
Inactivation of IL-7Rα in mice results in defective lymphopoiesis akin to that seen in Jak3-deficient mice (50). Thus, a logical prediction was that IL-7Rα-deficient thymocytes should also have alterations in Bax levels. Indeed, a direct comparison by FACS analyses demonstrated that Bax expression was elevated in IL-7Rα-deficient total thymocytes relative to that in wild-type T cells (Fig. 5C). Activation of Jak3 by IL-7R elicits the tyrosine phosphorylation and activation of Stat5a and Stat5b (23). Although Stat5a/Stat5b-deficient mice do not display a defect in T-cell development (57), it was formally possible that the changes in Bax levels observed in Jak3-deficient T cells were Stat5a or Stat5b dependent. However, Bax levels were equivalent in Stat5a/Stat5b-deficient and Stat5a/Stat5b heterozygous thymocytes (Fig. 5D). Therefore, the alterations in Bax expression in Jak3-deficient thymocytes appear to be mediated through an IL-7-to-Jak3 pathway but are independent of activation of Stat5a or Stat5b.
Bax loss promotes thymic development in Jak3-deficient mice.
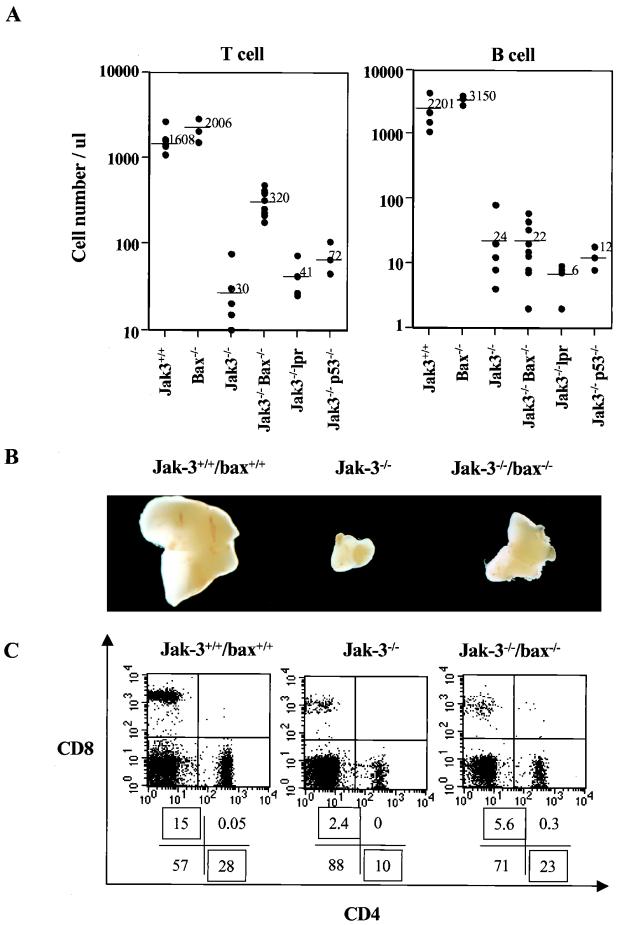
Consistent with a physiologic role in T-cell development, Bax-deficient mice have increased numbers of all T-cell subsets, but the normal ratios of these cells are maintained (29). To test whether up-regulation of Bax could account for a proportion of the defects in Jak3-deficient mice, we generated mice deficient in both Jak3 and Bax. Strikingly, the numbers of peripheral T cells from all Jak3 bax double-null mice were at least 10-fold higher than those present in their Jak3−/− bax+/− or Jak3−/− bax+/+ littermates (Fig. 6A and data not shown). There was also a significant increase in thymus size in double-null mice relative to that in Jak3-deficient bax+/− or bax+/+ littermates (Fig. 6B). Thus, the elevated levels of Bax in Jak3-deficient mice contribute to defects in T lymphopoiesis in these mice. However, Bax loss failed to rescue defects in the numbers of peripheral B cells, γδ T cells, or NK cells (Fig. 6A and data not shown). Furthermore, when double-null peripheral splenic T cells were examined for their CD4+/CD8+ ratios, it was evident that Bax loss also failed to compensate for the exacerbated defects inherent to peripheral Jak3-deficient CD8+ cells (Fig. 6C). Thus, signals other than Bax loss are required to rescue apoptotic defects of Jak3-deficient peripheral CD8+ cells and for the development of Jak3-deficient B, γδ T, or NK cells.
FIG. 6.
Bax loss promotes T lymphopoiesis in Jak3-deficient mice. (A) Absolute numbers of peripheral T and B cells were determined in 6- to 8-week-old wild-type (+/+), Jak3−/−, Jak3 bax double-null, Jak3 p53 double-null, and Jak3−/− lpr mice. Lymphocyte counts were manually scored from Wright-stained blood smears, and the percentages of T versus B cells were determined by FACS analysis with antibodies to CD4, CD8, and B220. Each symbol indicates an individual mouse, and the bars and adjacent numbers denote the mean values. (B) Thymuses from 4-week-old wild-type, Jak3-deficient, and Jak3 bax double-null mice were surgically removed and photographed. (C) Bax loss fails to compensate for peripheral CD8 defects inherent to Jak3-deficient mice. Spleens were isolated from 4-week-old wild-type, Jak3-deficient, and Jak3 bax double-null mice, and the proportions of peripheral splenic CD4 to CD8 cells were determined by FACS analyses. The mean average of CD4 to CD8 splenocytes was 2:1 in wild-type mice, 8:1 in Jak3−/− mice, and 6:1 in Jak3 bax double-null mice (n = 5 mice for each group).
Bax is sometimes required, in a cell context-specific fashion, as a mediator of p53-induced apoptosis (8, 29, 34, 67). Although loss of p53 does not alter lymphopoiesis, p53-deficient mice are highly prone to the development of T-cell lymphomas by 4 to 6 months of age (13). Loss of p53 can, however, compensate for some of the T-cell developmental defects observed in CD3α-chain-deficient, Rag1- or Rag2-deficient, and scid mice (18, 31, 39, 40). We therefore addressed the relevance of p53 to the defects in lymphopoiesis of Jak3-deficient mice. p53 protein levels were not elevated in T cells of Jak3-deficient versus wild-type mice (data not shown). Furthermore, the generation and analysis of Jak3 p53 double-null mice demonstrated that these animals remained lymphopenic and failed to show any rescue in the size of the thymus (Fig. 6A and data not shown). Therefore, the high levels of apoptosis of Jak3-deficient T cells are p53 independent.
The ability of Bax loss to promote T lymphopoiesis of Jak3-deficient mice could also be considered nonspecific, and defects could therefore possibly be corrected by the loss of other apoptotic regulators. Particularly relevant was the possible role of the Fas pathway in the defects in Jak3-deficient mice. Fas is an important regulator of peripheral T-cell apoptosis, and in the absence of this pathway, for example in lpr (mutant Fas receptor) or gld (mutant Fas ligand) mice, there are marked lymphoproliferative syndromes (41). One unexplained feature of peripheral Jak3-deficient CD4+ and CD8+ cells is a marked up-regulation in their levels of Fas (2, 54; data not shown). We therefore also generated Jak3−/− lpr offspring. These mice were equivalent to Jak3-deficient mice in their defects in T-, B-, and NK-cell lymphopoiesis (Fig. 6A and data not shown), and loss of Fas function did not rescue the apoptotic defects inherent to peripheral Jak3-deficient CD4+ and CD8+ cells (data not shown). Therefore, the ability of Bax loss to promote T lymphopoiesis of Jak3-deficient mice is not simply due to the removal of any proapoptotic regulator, and the apoptotic defects in Jak3-deficient T cells are independent of p53 or Fas.
Bcl-2 is necessary and sufficient to restore CD8+ T-cell development in Jak3-deficient mice.
Genetic analysis of the interplay between Bcl-2 and Bax in T lymphopoiesis has demonstrated additive effects of Bax deficiency and Bcl-2 overexpression, suggesting that Bax and Bcl-2 do not function in a linear pathway (28). Consistent with this notion, Jak3-deficient CD8+ cells express markedly reduced levels of Bcl-2 (Fig. 3 and 4) and peripheral CD8+ numbers remain underrepresented in Jak3 bax double-null mice (Fig. 6C). To address whether Bcl-2 overexpression was sufficient to restore lymphopoiesis in Jak3-deficient mice, we used retrovirus-mediated gene transfer of bone marrow from Jak3-deficient mice. Transduction of Jak3-deficient bone marrow with a Jak3-expressing retrovirus and transplantation into irradiated Jak3-deficient mice rescues all hematopoietic defects in these mice (5). We therefore generated a recombinant human bcl-2 retrovirus using a vector backbone (MSCV-I-GFP [20]) successfully used in the Jak3 virus transduction experiments. Virus-transduced cells can be identified by virtue of the GFP gene, which is expressed in cis from an internal ribosome entry site.
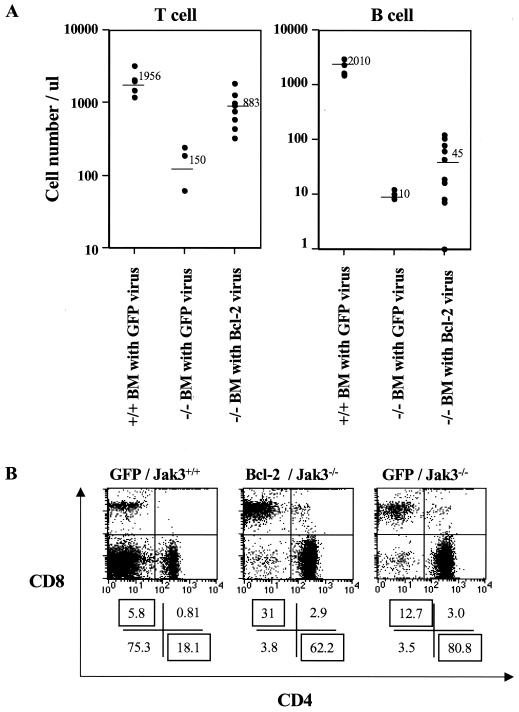
Bone marrow was harvested from wild-type or Jak3-deficient mice, pooled, and then transduced with MSCV-Bcl-2-I-GFP or with the vector control virus MSCV-I-GFP. Vector-only- or Bcl-2 virus-transduced wild-type or Jak3-null bone marrow was then transplanted into irradiated Jak3-deficient mice. Two months following transplant, the animals were analyzed for reconstitution of the lymphoid system. As expected, transduced bone marrow derived from wild-type mice efficiently reconstituted both T and B lymphopoiesis in Jak3-deficient mice, whereas Jak3-deficient bone marrow transduced with control MSCV-I-GFP failed to reconstitute lymphopoiesis (Fig. 7A). By contrast, transplantation of MSCV-Bcl-2-I-GFP-transduced Jak3-deficient bone marrow reconstituted peripheral T-cell numbers in each Jak3-null recipient (mean of 800 T cells per μl of peripheral blood) to levels approaching those present in Jak3-deficient mice transplanted with wild-type bone marrow transduced with the MSCV-I-GFP control virus. FACS analyses of GFP fluorescence demonstrated that >95% of MSCV-Bcl2-I-GFP-reconstituted Jak3-deficient T cells expressed GFP, whereas the percentages of GFP-positive T cells were much lower when vector-only transduced bone marrow from wild-type mice was used (10 to 20% [data not shown]). Furthermore, FACS analyses demonstrated that Bcl-2 virus-reconstituted Jak3-deficient T cells expressed high levels of human Bcl-2 (data not shown). Thus, there is a profound selection for T cells expressing Bcl-2 when they are deficient in Jak3. Furthermore, Bcl-2 overexpression restored the ratio of peripheral CD4+ to CD8+ cells to those present in wild-type mice (Fig. 7B). However, MSCV-Bcl-2-I-GFP-reconstituted Jak3-deficient mice remained defective in their numbers of B, γδ T, and NK cells (Fig. 7A and data not shown). Finally, the apoptotic index in MSCV-Bcl-2-I-GFP-reconstituted Jak3-deficient peripheral CD8+ cells was significantly lower than that of Jak3-deficient mice (data not shown), confirming that the selective advantage imparted by Bcl-2 was due to its ability to suppress apoptosis. Thus, the severely reduced levels of Bcl-2 in Jak3-deficient CD8+ cells are relevant to their exacerbated apoptotic defects. Furthermore, the ability of Bcl-2 overexpression, but not Bax loss, to compensate for this CD8+ defect supports the model that Bax and Bcl-2 function in an additive fashion to regulate T lymphopoiesis (28).
FIG. 7.
Overexpression of Bcl-2 promotes T lymphopoiesis and increases peripheral CD8+ cells in Jak3-deficient mice. (A) Bone marrow transplantation of irradiated Jak3-deficient mice with progenitors infected with a Bcl-2-expressing retrovirus. Sublethally irradiated (900 rads) Jak3-deficient mice were transplanted with MSCV-I-GFP-infected wild-type (+/+) or Jak3−/− bone marrow (BM) or with MSCV-Bcl-2-I-GFP-infected Jak3-deficient bone marrow. Two months following transplant the peripheral blood was analyzed for lymphocyte numbers. Lymphocyte counts and FACS analysis of B220, CD4, or CD8 expression were used to determine the absolute number of T and B cells. Each symbol indicates the analysis of an individual mouse, and the bars and adjacent numbers denote the mean value. (B) CD4 and CD8 FACS analysis profile of splenocytes from Jak3-deficient mice reconstituted with Bcl-2-overexpressing Jak3-null bone marrow (Bcl-2/Jak3−/−), MSCV-I-GFP-expressing Jak3-null bone marrow (GFP/Jak3−/−), or MSCV-I-GFP-expressing wild-type bone marrow (GFP/Jak3+/+). FACS analyses shown are representative of three individual mice analyzed. Ratios of CD4 to CD8 cells were 2:1 for GFP/Jak3+/+ mice or Bcl-2/Jak3−/− mice, whereas they were 8:1 in GFP/Jak3−/− mice.
DISCUSSION
Jak3 associates with the γc chain of IL-7R, and activation of Jak3 by IL-7 is critical for T-cell development, as deletion of Jak3 recapitulates the defects observed in IL-7Rα- or γc-chain-deficient mice (6, 12, 44, 47, 50, 59). Defects in Jak3-null mice are intrinsic to a lymphoid progenitor (5) and are not due to defects in lymphocyte development per se, as there are proper ratios of thymocyte subsets. Rather, the defects of these knockouts are largely quantitative, resulting from the failure of these cells to grow and/or survive. Gain-of-function studies using lymphoid cell-specific Bcl-2 transgenic mice have suggested that it is the survival, rather than the proliferative, signal emanating from IL-7R that is necessary and sufficient to promote T lymphopoiesis (1, 30, 33). Our findings support this concept and suggest that Jak3 functions as a critical mediator of the IL-7 survival pathway by selectively regulating the expression of Bax and Bcl-2.
Jak kinases provide required survival signals that regulate the expression of Bcl-2 family proteins
Several lines of evidence support the concept that one nonredundant function of Jak kinases is to provide a survival signal. Firstly, Jak3 knockout mice display high levels of spontaneous apoptosis in the thymus and periphery (Fig. 1) (60). Secondly, cells expressing mutant cytokine receptors that fail to activate Jak kinases cannot support cell survival (27, 36). By contrast, cytokine receptors selectively defective in activating phosphatidylinositol-3′ kinase, phospholipase C-γ, Ras–Raf-1, and STATs retain their capacity to suppress cell death (45, 51). Finally, Jak kinase functions are required for the proper regulation of the Bcl-2 family proteins. Two striking examples underscore this concept. First, as shown here, Jak3 loss leads to the up-regulation of Bax expression in DP, CD4+, and CD8+ thymocytes and to the reduction of Bcl-2 levels in DN, CD4+, and especially CD8+ thymocytes (Fig. 3 and 4). These changes are in part accounted for by alterations in RNA levels (Fig. 3D) and are physiologically relevant, as either Bax loss or Bcl-2 overexpression promotes T-cell development in Jak3-deficient mice. By crossing the common γ-chain knockout to a transgenic mouse expressing a truncated version of γc, Tsujino et al. (61) have suggested that Bcl-2 regulation in peripheral CD4+ T cells and DN thymocytes is independent of Jak3. By contrast, our results suggest that regulation of Bcl-2 in DN, CD4+, and CD8+ thymocytes is strictly dependent upon Jak3, and this result is consistent with the phenotypes of the Jak3 and common γ-chain knockouts, which are essentially the same. Thus, Jak3 is the critical nonredundant effector of common γ chain-mediated signaling, and its targets include Bcl-2 and Bax. Although the signaling pathway by which Jak3 regulates Bax expression is not resolved, in thymocytes this clearly does not involve Stat5a or Stat5b (Fig. 5D). Second, in myeloid progenitors, Jak2 is specifically required to promote survival and for the selective regulation of Bcl-XL (45).
An interesting feature of Jak-mediated survival pathways is their cell context-specific nature. Firstly, there is selective regulation of different Bcl-2 family members. For example, Jak3 is essential for proper regulation of Bax and Bcl-2 RNA and protein in thymocyte subsets (Fig. 3 and 4), whereas Jak2 regulates the expression of Bcl-XL RNA and protein in myeloid progenitors (45). Secondly, all lymphoid defects of Jak3-deficient mice can be rescued by reconstitution of bone marrow with a Jak3-expressing retrovirus (5), but a retrovirus expressing Bcl-2 fails to rescue B-, γδ T-, or NK-cell numbers in Jak3-deficient recipients. Similarly, bcl-2 transgenes fail to rescue B- or NK-cell development in IL-7Rα-deficient mice (1, 33), and loss of bax also has no consequence on Jak3-deficient B-, γδ T-, or NK-cell development. Thus, an unknown but Jak3-dependent survival signal may contribute to this arm of the immune system.
In addition to their requirement for regulating Bcl-2 family proteins, Jak kinases may regulate additional survival pathways. In particular, the rescue of Jak3- and IL-7Rα-deficient T-cell defects by Bcl-2 gain-of-function strategies (1, 33) or by Bax loss in Jak3-deficient mice is certainly not complete. One possibility is that other Bcl-2 family members are required for proper T lymphopoiesis. In support of this concept, chimeric analyses have suggested that Bcl-XL plays a quantitative role in thymocyte development and mice deficient in the proapoptotic family member Bim have defects in T-cell development (3). However, we have not observed changes in Bcl-XL or Bim levels in Jak3-null versus wild-type T cells (Fig. 3 and data not shown). A second mechanism could involve posttranslational modifications of Bcl-2 family members. For example, overexpression studies have shown that cytokines activate protein kinase B (PKB; also called Akt)- and PKA-dependent phosphorylation of the cell death agonist Bad (11, 19), which displaces Bad from Bcl-XL to promote cell survival (68). Thus, in lymphoid cells a Jak3-dependent signal may regulate the activity of PKB or PKA to phosphorylate Bcl-2 family members or other apoptotic targets such as caspase 9 or the forkhead transcription factor (4, 7). Finally, a Jak-dependent signal may also regulate localization of Bcl-2 family proteins. In healthy cells Bax is present in the cytosol yet relocalizes to mitochondria after cells receive an apoptotic signal (17, 21). The signaling events regulating Bax relocalization are unresolved, but once they are identified, genetic tests of this pathway in Jak3-deficient mice may also be warranted.
The requirement for Jak3-mediated regulation of Bcl-2 and Bax for T-cell development is additive.
The fact that the loss of Jak3 differentially affects the regulation of Bcl-2 and Bax in T cells is consistent with the concept that Bcl-2 and Bax play distinct roles in T lymphopoiesis. Our analyses and those of others (37, 62) have shown that Bcl-2 RNA and protein levels are low in DP thymocytes but higher in DN, CD4+, and CD8+ cells (Fig. 2 and 3D). IL-7 and Jak3 signaling is impaired in DP thymocytes (22, 55), and DP cells express high levels of Bax and reduced levels of Bcl-2 (Fig. 2 and 3), underscoring the physiological role of the Jak3-to-Bax/Bcl-2 pathway. It was perhaps not surprising that IL-7Rα- and Jak3-deficient thymocytes had comparable deficits in Bcl-2 (data not shown) (1), since Jak3 is required for IL-7 signaling, but the fact that Bax regulation was also altered in IL-7Rα- and Jak3-deficient thymocytes was unanticipated (Fig. 5C). Alterations of Bcl-2 and Bax expression in Jak3-deficient thymocytes are not simply due to a blockade in T-cell development, as the changes are not observed in Rag2-deficient mice (Fig. 5).
In contrast to the observed changes in Bcl-2, the levels of Bax present in DP, CD4+, and CD8+ thymocytes cannot simply be accounted for by Jak3 activity. For example, levels of Bax RNA and protein in DN Jak3-deficient and wild-type cells are low but essentially equivalent (Fig. 2 and 3), despite the fact that IL-7-to-Jak3 signaling is required for expansion of this subset. Furthermore, wild-type CD4+ and CD8+ thymocytes express higher levels of Bax RNA and protein than do DN cells (Fig. 2 and 3D), despite IL-7-to-Jak3 signaling in all these three subsets. Thus, other signaling pathways must also participate in the regulation of Bax during T lymphopoiesis.
If Bcl-2 and Bax were the sole mediators of T-lymphoid survival in the IL-7-to-Jak3 pathway, one would predict that the T-cell phenotypes of Bcl-2-, Bax-, IL-7Rα-, and Jak3-deficient mice would be concordant. This is not the case. In contrast to the scid-like phenotypes of IL-7Rα- and Jak3-deficient mice, the phenotypes of Bcl-2- and Bax-deficient mice suggest only partial nonredundant functions in T lymphopoiesis (28, 29, 63). Mice lacking Bcl-2 initially display normal T-cell development, yet these cells undergo massive apoptosis at 4 to 8 weeks of age (42, 63). Thus, Bcl-2 is not strictly required for T lymphopoiesis but rather is necessary to sustain cell survival. Furthermore, similar to the Jak3 deficiency, loss of Bcl-2 results in especially marked defects in CD8+ numbers (63), underscoring the physiological role of the Jak3-to-Bcl-2 pathway in the maintenance of this T-cell subset. Bax-deficient mice have on average a 1.6-fold increase in all thymocyte subsets but have normal ratios of T-cell subsets and do not display a lymphoproliferative phenotype (29). However, the loss of T cells in IL-7Rα- or Jak3-deficient mice is consistent with the notion that Bax RNA and protein levels are repressed by this pathway and that Bax proapoptotic functions are manifest only when the Jak3 pathway is disrupted.
Genetic studies using Bcl-2- and Bax-deficient mice, combined with gain-of-function studies using Bcl-2 transgenic mice, have suggested that Bcl-2 and Bax play distinct roles in T-cell development (28). Loss of Bax rescues the apoptotic phenotype of Bcl-2-deficient mice, demonstrating that Bax is downstream of Bcl-2. However, Bcl-2 still displays gain-of-function activity in the absence of Bax, indicating that each regulates apoptosis independently in an additive fashion. This model is supported by our data. In particular, Bcl-2 overexpression rescues the marked defects in Jak3-deficient peripheral CD8+ cells, whereas Bax loss does not. Therefore, the data support the model that Jak3 is required to correctly regulate the expression of Bax and Bcl-2 in specific T-cell contexts, that these pathways are independent, and that both contribute to T-cell development.
ACKNOWLEDGMENTS
We are grateful for the outstanding technical assistance of Chunying Yang, Elsie White, Rob Jeffers, Jinling Wang, Evan Parganas, Linda Snyder, and Kristen Rothammer. We thank Peter Doherty and Rakesh Goorha for providing mlr-lpr and Rag2-deficient mice, respectively. We also thank the staff of our Animal Resources Center. We also thank Peter McKinnon, Dario Vignali, and members of our laboratories for their suggestions and Richard Cross and Richard Ashmun for their help with FACS analysis.
This work was supported in part by grants CA76379 and DK44158 (J.L.C), DK42932 (J.N.I), CA63230 (G.P.Z.), and P01HL 53749 (E.F.V.); Cancer Center CORE grant CA21765; the ASSISI Foundation of Memphis; and the American Lebanese Syrian Associated Charities.
REFERENCES
- 1.Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman I L. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 1997;89:1033–1041. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 2.Baird A M, Thomis D C, Berg L J. T cell development and activation in Jak3-deficient mice. J Leukoc Biol. 1998;63:669–677. doi: 10.1002/jlb.63.6.669. [DOI] [PubMed] [Google Scholar]
- 3.Bouillet P, Metcalf D, Huang D C, Tarlinton D M, Kay T W, Kontgen F, Adams J M, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 4.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 5.Bunting K D, Sangster M Y, Ihle J N, Sorrentino B P. Restoration of lymphocyte function in Janus kinase 3-deficient mice by retroviral-mediated gene transfer. Nat Med. 1998;4:58–64. doi: 10.1038/nm0198-058. [DOI] [PubMed] [Google Scholar]
- 6.Cao X, Shores E W, Hu-Li J, Anver M R, Kelsall B L, Russell S M, Drago J, Noguchi M, Grinberg A, Bloom E T, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 7.Cardone H M, Roy N, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E, Frisch S, Reed J C. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 8.Chong J M, Murray M R, Gosink E C, Russell H R C, Srinivasan A, Kapsetaki M, Korsmeyer S J, McKinnon P J. Atm and Bax cooperate in ionizing radiation-induced apoptosis in the central nervous system. Proc Natl Acad Sci USA. 2000;97:889–894. doi: 10.1073/pnas.97.2.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darnell J E., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 10.Darnell J E, Jr, Kerr I M, Stark G R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 11.del Peso, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 12.DiSanto J P, Muller W, Guy-Grand D, Fischer A, Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain Proc. Natl Acad Sci USA. 1995;92:377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donehower L A, Harvey M, Slagle B L, McArthur M J, Montgomery C A, Jr, Butel J S, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 14.Durbin J E, Hackenmiller R, Simon M C, Levy D E. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 15.Fairbairn L J, Cowling G J, Reipert B M, Dexter T M. Suppression of apoptosis allows differentiation and development of a multipotent hemopoietic cell line in the absence of added growth factors. Cell. 1993;74:823–832. doi: 10.1016/0092-8674(93)90462-y. [DOI] [PubMed] [Google Scholar]
- 16.Grillot D A, Merino R, Nunez G. Bcl-XL displays restricted distribution during T cell development and inhibits multiple forms of apoptosis but not clonal deletion in transgenic mice. J Exp Med. 1995;182:1973–1983. doi: 10.1084/jem.182.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross A, Jockel J, Wei M C, Korsmeyer S J. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haks C M, Krimpenfort P J, van den Brakel H, Kruisbeek A M. Pre-TCR signaling and inactivation of p53 induces crucial cell survival pathways in pre-T cells. Immunity. 1999;11:91–101. doi: 10.1016/s1074-7613(00)80084-9. [DOI] [PubMed] [Google Scholar]
- 19.Harada H, Becknell B, Wilm M, Mann M, Huang L J, Taylor S S, Scott J D, Korsmeyer S J. Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol Cell. 1999;3:413–422. doi: 10.1016/s1097-2765(00)80469-4. [DOI] [PubMed] [Google Scholar]
- 20.Hawley R G, Fong A Z, Burns B F, Hawley T S. Transplantable myeloproliferative disease induced in mice by an interleukin 6 retrovirus. J Exp Med. 1992;176:1149–1163. doi: 10.1084/jem.176.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu Y T, Wolter K G, Youle R J. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci USA. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ihle J N. Cytokine receptor signalling. Nature. 1995;377:591–594. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- 23.Ihle J N. STATs: signal transducers and activators of transcription. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- 24.Ihle J N, Thierfelder W, Teglund S, Stravapodis D, Wang D, Feng J, Parganas E. Signaling by the cytokine receptor superfamily. Ann N Y Acad Sci. 1998;865:1–9. doi: 10.1111/j.1749-6632.1998.tb11157.x. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan H M, Schindler U, Smiley S T, Grusby M J. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan H M, Sun Y L, Hoey T, Grusby M J. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 27.Kinoshita T, Yokota T, Arai K, Miyajima A. Suppression of apoptotic death in hematopoietic cells by signalling through the IL-3/GM-CSF receptors. EMBO J. 1995;14:266–275. doi: 10.1002/j.1460-2075.1995.tb07000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knudson C M, Korsmeyer S J. Bcl-2 and Bax function independently to regulate cell death. Nat Genet. 1997;16:358–363. doi: 10.1038/ng0897-358. [DOI] [PubMed] [Google Scholar]
- 29.Knudson C M, Tung K S, Tourtellotte W G, Brown G A, Korsmeyer S J. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 30.Kondo M, Akashi K, Domen J, Sugamura K, Weissman I L. Bcl-2 rescues T lymphopoiesis, but not B or NK cell development, in common gamma chain-deficient mice. Immunity. 1997;7:155–162. doi: 10.1016/s1074-7613(00)80518-x. [DOI] [PubMed] [Google Scholar]
- 31.Liao J M, Zhang X X, Hill R, Gao J, Qumsiyeh M B, Nichols W, Van Dyke T. No requirement for V(D)J recombination in p53-deficient thymic lymphoma. Mol Cell Biol. 1998;18:3495–3501. doi: 10.1128/mcb.18.6.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X, Robinson G W, Wagner K U, Garrett L, Wynshaw-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- 33.Maraskovsky E, O'Reilly L A, Teepe M, Corcoran L M, Peschon J J, Strasser A. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell. 1997;89:1011–1019. doi: 10.1016/s0092-8674(00)80289-5. [DOI] [PubMed] [Google Scholar]
- 34.McCurrach M E, Connor T M, Knudson C M, Korsmeyer S J, Lowe S W. bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis Proc. Natl Acad Sci USA. 1997;94:2345–2349. doi: 10.1073/pnas.94.6.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meraz M A, White J M, Sheehan K C, Bach E A, Rodig S J, Dighe A S, Kaplan D H, Riley J K, Greenlund A C, Campbell D, Carver-Moore K, DuBois R N, Clark R, Aguet M, Schreiber R D. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 36.Miura O, Cleveland J L, Ihle J N. Inactivation of erythropoietin receptor function by point mutations in a region having homology with other cytokine receptors. Mol Cell Biol. 1993;13:1788–1795. doi: 10.1128/mcb.13.3.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore N C, Anderson G, Williams G T, Owen J J, Jenkinson E J. Developmental regulation of bcl-2 expression in the thymus. Immunology. 1994;81:115–119. [PMC free article] [PubMed] [Google Scholar]
- 38.Motoyama N, Wang F, Roth K A, Sawa H, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S, et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- 39.Nacht M, Jacks T. V(D)J recombination is not required for the development of lymphoma in p53-deficient mice. Cell Growth Differ. 1998;9:131–138. [PubMed] [Google Scholar]
- 40.Nacht M, Strasser A, Chan Y R, Harris A W, Schlissel M, Bronson R T, Jacks T. Mutations in the p53 and SCID genes cooperate in tumorigenesis. Genes Dev. 1996;10:2055–2066. doi: 10.1101/gad.10.16.2055. [DOI] [PubMed] [Google Scholar]
- 41.Nagata S, Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 42.Nakayama K, Negishi I, Kuida K, Sawa H, Loh D Y. Targeted disruption of Bcl-2 alpha beta in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc Natl Acad Sci USA. 1994;91:3700–3704. doi: 10.1073/pnas.91.9.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neubauer H, Cumano A, Muller M, Wu H, Huffstadt U, Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 44.Nosaka T, van Deursen J M, Tripp R A, Thierfelder W E, Witthuhn B A, McMickle A P, Doherty P C, Grosveld G C, Ihle J N. Defective lymphoid development in mice lacking Jak3. Science. 1995;270:800–802. doi: 10.1126/science.270.5237.800. [DOI] [PubMed] [Google Scholar]
- 45.Packham G, White E L, Eischen C M, Yang H, Parganas E, Ihle J N, Grillot D A, Zambetti G P, Nunez G, Cleveland J L. Selective regulation of Bcl-XL by a Jak kinase-dependent pathway is bypassed in murine hematopoietic malignancies. Genes Dev. 1998;12:2475–2487. doi: 10.1101/gad.12.16.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parganas E, Wang D, Stravopodis D, Topham D J, Marine J C, Teglund S, Vanin E F, Bodner S, Colamonici O R, van Deursen J M, Grosveld G, Ihle J N. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 47.Park S Y, Saijo K, Takahashi T, Osawa M, Arase H, Hirayama N, Miyake K, Nakauchi H, Shirasawa T, Saito T. Developmental defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity. 1995;3:771–782. doi: 10.1016/1074-7613(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 48.Perez G I, Robles R, Knudson C M, Flaws J A, Korsmeyer S J, Tilly J L. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet. 1999;21:200–203. doi: 10.1038/5985. [DOI] [PubMed] [Google Scholar]
- 49.Persons D A, Mehaffey M G, Kaleko M, Nienhuis A W, Vanin E F. An improved method for generating retroviral producer clones for vectors lacking a selectable marker gene. Blood Cells Mol Dis. 1998;24:167–182. doi: 10.1006/bcmd.1998.0184. [DOI] [PubMed] [Google Scholar]
- 50.Peschon J J, Morrissey P J, Grabstein K H, Ramsdell F J, Maraskovsky E, Gliniak B C, Park L S, Ziegler S F, Williams D E, Ware C B, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quelle F W, Wang J, Feng J, Wang D, Cleveland J L, Ihle J N, Zambetti G P. Cytokine rescue of p53-dependent apoptosis and cell cycle arrest is mediated by distinct Jak kinase signaling pathways. Genes Dev. 1998;12:1099–1107. doi: 10.1101/gad.12.8.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimoda K, van Deursen J, Sangster M Y, Sarawar S R, Carson R T, Tripp R A, Chu C, Quelle F W, Nosaka T, Vignali D A, Doherty P C, Grosveld G, Paul W E, Ihle J N. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 53.Shinkai Y, Rathbun G, Lam K P, Oltz E M, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall A M, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 54.Sohn S J, Forbush K A, Nguyen N, Witthuhn B, Nosaka T, Ihle J N, Perlmutter R M. Requirement for Jak3 in mature T cells: its role in regulation of T cell homeostasis. J Immunol. 1998;160:2130–2138. [PubMed] [Google Scholar]
- 55.Sudo T, Nishikawa S, Ohno N, Akiyama N, Tamakoshi M, Yoshida H. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc Natl Acad Sci USA. 1993;90:9125–9129. doi: 10.1073/pnas.90.19.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA. 1997;94:3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teglund S, McKay C, Schuetz E, van Deursen J M, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle J N. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 58.Thierfelder W E, van Deursen J M, Yamamoto K, Tripp R A, Sarawar S R, Carson R T, Sangster M Y, Vignali D A, Doherty P C, Grosveld G C, Ihle J N. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 59.Thomis D C, Gurniak C B, Tivol E, Sharpe A H, Berg L J. Defects in B lymphocyte maturation and T lymphocyte activation in mice lacking Jak3. Science. 1995;270:794–797. doi: 10.1126/science.270.5237.794. [DOI] [PubMed] [Google Scholar]
- 60.Thomis D C, Lee W, Berg L J. T cells from Jak3-deficient mice have intact TCR signaling, but increased apoptosis. J Immunol. 1997;159:4708–4719. [PubMed] [Google Scholar]
- 61.Tsujino S, Di Santo J P, Takaoka A, McKernan T L, Noguchi S, Taya C, Yonekawa H, Saito T, Taniguchi, T. T, Fujii H. Differential requirement of the cytoplasmic subregions of gamma c chain in T cell development and function. Proc Natl Acad Sci USA. 2000;97:10514–10519. doi: 10.1073/pnas.180063297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Veis D J, Sentman C L, Bach E A, Korsmeyer S J. Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T lymphocytes. J Immunol. 1993;151:2546–2554. [PubMed] [Google Scholar]
- 63.Veis D J, Sorenson C M, Shutter J R, Korsmeyer S J. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 64.Williams G T, Smith C A, Spooncer E, Dexter T M, Taylor D R. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature. 1990;343:76–79. doi: 10.1038/343076a0. [DOI] [PubMed] [Google Scholar]
- 65.Witthuhn B A, Quelle F W, Silvennoinen O, Yi T, Tang B, Miura O, Ihle J N. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74:227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- 66.Yang E, Korsmeyer S J. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996;88:386–401. [PubMed] [Google Scholar]
- 67.Yin C, Knudson C M, Korsmeyer S J, Van Dyke T. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature. 1997;385:637–640. doi: 10.1038/385637a0. [DOI] [PubMed] [Google Scholar]
- 68.Zha J, Harada H, Yang E, Jockel J, Korsmeyer S J. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14 to 3 to 3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]