Abstract
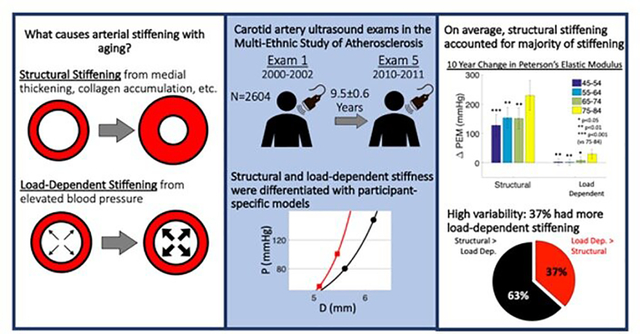
Elastic arteries stiffen via two main mechanisms: 1) load-dependent stiffening from higher blood pressure (BP), and 2) structural stiffening due to changes in the vessel wall. Differentiating these closely coupled mechanisms is important to understanding vascular aging. MESA participants with B-mode carotid ultrasound and brachial BP at Exam 1 and Exam 5 (year 10) were included in this study (n=2604). Peterson’s and Young’s elastic moduli (PEM and YEM) were calculated to represent total stiffness. Structural stiffness was calculated by adjusting PEM and YEM to a standard BP of 120/80 mmHg with participant-specific models. Load-dependent stiffness was the difference between total and structural stiffness. Changes in carotid artery stiffness mechanisms over 10 years were compared by age groups with ANCOVA models adjusted for baseline CVD risk factors. The 75–84 age group had the greatest change in total, structural, and load-dependent stiffening compared to younger groups (p<0.05). Only age and cessation of antihypertensive medication were predictive of structural stiffening, whereas age, race/ethnicity, education, BP, cholesterol, and antihypertensive medication were predictive of increased load-dependent stiffening. On average, structural stiffening accounted for the vast majority of total stiffening, but 37% of participants had more load-dependent than structural stiffening. Rates of structural and load-dependent carotid artery stiffening increased with age. Structural stiffening was consistently observed, and load-dependent stiffening was highly variable. Heterogeneity in arterial stiffening mechanisms with aging may influence CVD development.
Keywords: Aging, Arterial Stiffness, Blood Pressure, Remodeling, Arterial Compliance
Graphical Abstract
Introduction:
Arterial stiffness is associated with increased risk for incident hypertension, cardiovascular disease (CVD), stroke, and damage to end organs with low resistance capillary beds such as the brain and kidneys1–6. Large elastic arteries stiffen via two main mechanisms7: 1) Load-dependent stiffening due to elevated blood pressure increasing collagen fiber loading without an intrinsic change to the artery wall composition, and 2) Structural stiffening due to growth (eg. intima-media thickening), remodeling (eg. elastin fragmentation, collagen accumulation) or both. Prior studies have shown that age is a major determinant of arterial stiffness both in cross-sectional and longitudinal analyses.7–10. It is unclear whether increased arterial stiffness with aging is due to structural changes in the artery wall with aging, a load-dependent response to age-associated increased systolic blood pressure, or a combination of both mechanisms. As highlighted by the 2015 American Heart Association scientific statement on arterial stiffness11, understanding whether the age-associated increase in arterial stiffness is driven by structural or load-dependent mechanisms is an important unanswered question that could yield valuable insights into the physiology of arterial aging. The aim of this study was to evaluate the longitudinal changes in structural and load-dependent carotid artery stiffness in a diverse cohort without baseline CVD.
Methods:
The data are available to other researchers through the National Institutes of Health, National Heart, Lung, and Blood Institute, Biologic Specimen and Data Repository Information Coordinating Center12. Analytic methods may be requested from the author. Researchers with interest in the ultrasound images or other study materials are invited to contact MESA (Multi-Ethnic Study of Atherosclerosis) via the study authors about access to images which are held internally at MESA because of the size of the archive and to protect participant privacy in accordance with participant consent.
Study Participants and Design
The Multi-Ethnic Study of Atherosclerosis (MESA) is a large prospective cohort study investigating the prevalence, causes, and progression of subclinical CVD. MESA has a population-based sample of 6814 men and women aged 45 to 84 years, free of known CVD at baseline, recruited from 6 United States communities. The study objectives and design have been published previously13. All participants gave informed consent for the study protocol, which was approved by the Institutional Review Boards of the ultrasound reading center and all MESA field centers.
The present analyses include a subset of MESA participants with valid carotid distensibility measurements at the first (baseline) and fifth examination who were not missing key covariates (n=2604; online-only Data figure S1: Flow diagram). Demographic, medical history, and laboratory data for the present study were obtained from the first (July 2000 to August 2002) and fifth (April 2010 to February 2012) examinations of the cohort. Hypertension was defined as systolic blood pressure (SBP) ≥140 mm Hg, diastolic blood pressure (DBP) ≥90 mm Hg, or use of antihypertensive medications. Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dL or use of antiglycemic medications. Impaired fasting glucose was defined as blood glucose 100 to 125 mg/dL. Total and high-density lipoprotein cholesterol levels were measured after a 12-hour fast. Low-density lipoprotein cholesterol was calculated.
B-Mode Ultrasound and Brachial Blood Pressure Measurements
At examination 1, B-mode ultrasound video-loop recordings of a longitudinal section of the distal right common carotid artery were recorded on S-VHS videotapes using a Logiq 700 ultrasound system (General Electric Medical Systems; transducer frequency 13 MHz). Videotaped images were digitized at high resolution and frame rates using a medical digital recording device (PACSGEAR, Pleasanton, CA), which were converted into DICOM-compatible digital records. At examination 5, a similar protocol was performed using the same ultrasound and digitizing equipment; however, the video output was directly digitized using the same medical digital recording settings without use of videotape. Certified and trained sonographers from all 6 MESA sites used selected reference images from examination 1 to match the scanning conditions of the initial study, including common carotid artery display depth, angle of approach, surrounding tissues and internal landmarks, degree of jugular venous distension, and ultrasound system settings. After 10 minutes of rest in the supine position and immediately before ultrasound image acquisition, repeated measures of brachial blood pressures were obtained using a standardized protocol with an automated upper arm sphygmomanometer (DINAMAP; GE Medical Systems, Milwaukee, WI). Ultrasound images were reviewed and interpreted by the MESA Carotid Ultrasound Reading Center (the University of Wisconsin Atherosclerosis Imaging Research Program, Madison, WI). Systolic and diastolic diameters were determined as the largest and smallest diameters during the cardiac cycle. All measurements were made manually tracing a 1 cm long segment and performed in triplicate from 2 to 3 consecutive cardiac cycles. Internal and external artery diameters were measured using Access Point Web version 3.0 (Freeland Systems LLC, Carmel, IN).
Carotid Artery Stiffness
Peterson’s Elastic Modulus (PEM) was calculated14:
| #(1) |
where Ds represents the internal arterial diameter at peak systole, Dd represents the internal diameter at end-diastole, and Δp represents the brachial blood pressure difference between the systolic and diastolic measurements (pulse pressure). Young’s elastic modulus (YEM) was calculated14:
| #(2) |
where h is the carotid artery wall thickness at end diastole.
To differentiate the structural and load-dependent components of carotid artery stiffness, a participant-specific exponential model was used to describe arterial mechanics15 using a non-linear stiffness parameter at the common reference pressure of 120/80 mmHg.
PEM and YEM were calculated at this reference pressure to represent the structural arterial stiffness. All participants were compared at the same reference pressure. The mathematical equations are included as a supplement. The load-dependent arterial stiffness was calculated as the difference between total PEM and YEM calculated at the individuals’ measured BP and the structural arterial stiffness (Figure 1). An individual’s load-dependent stiffness will be positive if their BP is greater than the 120/80 reference pressure and negative if their BP is less than the 120/80 reference pressure.
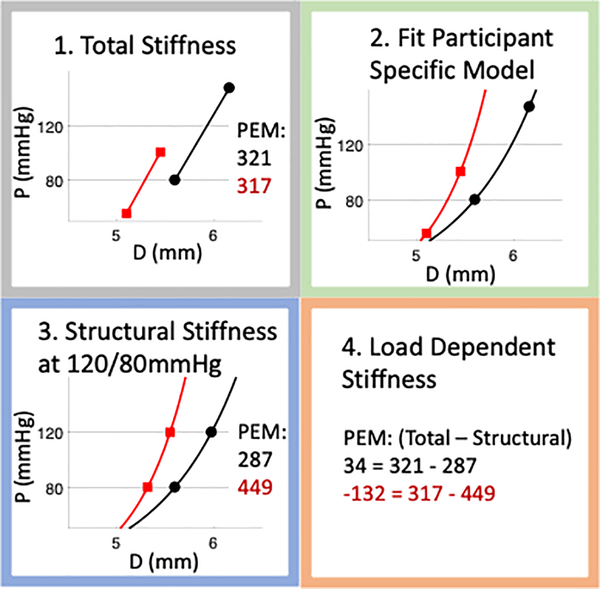
Figure 1:
Graphical representation of methods used to differentiate structural and load-dependent stiffness. Representative results are shown for two participants (one in red, one in black) who had similar total Peterson’s elastic modulus (PEM) (321 vs 317 mmHg), but via different mechanisms. One participant (red lines and text) had higher structural PEM (449 vs 287 mmHg) while the other participant (black lines and font) had higher load-dependent PEM (34 vs −132 mmHg). For graphs: the y axis label “P” is Pressure (mmHg), the x axis label “D” is Diameter (mm).
Statistical Analysis
Results are reported as mean and standard deviation (SD) for continuous variables. Categorical variables are reported as percentages.
Baseline age was classified into 4 categories by decade: 45–54, 55–65, 65–74, and 75–84. Differences in carotid artery stiffness parameters from baseline to examination 5 between age categories were assessed using ANCOVA models. ANCOVA models were adjusted for baseline co-variates: sex, race/ethnicity, study site, education level, income, traditional CVD risk factors (body mass index, diabetes mellitus status, SBP, smoking status, lipids), baseline use of lipid lowering medications, and use of antihypertensive medications (1. never treated with antihypertensive medication, 2. continuous treatment with antihypertensive medication, 3. started treatment with antihypertensive medication, and 4. stopped treatment with antihypertensive medication). ANCOVA model results used a Bonferroni correction to account for multiple comparisons and results are presented as estimated means and 95% confidence intervals. To identify the impact that the reference pressure of 120/80 mmHg had on our calculations, a sensitivity analysis was performed by repeating ANCOVA analyses with reference pressures of 105/70 mmHg, 135/90 mmHg, and 160/90 mmHg. A second set of ANCOVA models was also created that included all co-variates in the primary models and added the change in intima-medial thickness, diastolic diameter, systolic BP, and pulse-pressure as co-variates from Exam 1 to Exam 5.
Exploratory analyses were also performed after grouping participants into those with greater load-dependent stiffening vs those with greater structural stiffening. Since this was not pre-specified, differences in continuous variables between the groups were assessed by calculating effect size (Hedge’s g).
Results
Participant Characteristics
Participant characteristics are presented in Table 1. Participants were 59.9 ± 9.4 years old at baseline (45–54: 35% [n=907], 55–64: 31% [n=798], 65–74: 27% [n=712], 75–84: 7% [n=184]) and 54% were female. Participants identified as: 39% white, 25% black, 14% Chinese, and 21% Hispanic. The average time from baseline to follow up at examination 5 was 9.5 ± 0.6 years. SBP, pulse pressure, carotid artery wall thickness, and carotid artery diameter all increased over the duration from baseline to examination 5.
Table 1:
Participant Characteristics at Baseline and Exam 5
| N=2604 | Baseline | Examination 5 |
|---|---|---|
| Age (years) | 59.9 ± 9.4 | 69.3 ± 9.3 |
| Female (n, %) | 1392 (53.5%) | |
| Race/Ethnicity (n, %) | ||
| White | 1020 (39.2%) | |
| Black | 656 (25.2%) | |
| Chinese | 373 (14.3%) | |
| Hispanic | 555 (21.3%) | |
| Blood Pressure Parameters (mmHg) | ||
| SBP | 123.2 ± 19.9 | 129.4 ± 18.7 |
| DBP | 71.7 ± 10.1 | 69.4 ± 9.7 |
| Pulse pressure | 51.6 ± 15.5 | 60.1 ± 15.1 |
| Hypertension (n, %) | 1074 (41.2%) | 1597 (61.3) |
| Hypertension Meds (n, %) | 740 (28.4%) | 1363 (52.3%) |
| Diabetes Mellitus Status (n, %) | ||
| Impaired fasting glucose | 313 (12.0%) | 549 (21.1%) |
| Untreated | 39 (1.5%) | 38 (1.5%) |
| Treated | 177 (6.8%) | 411 (15.8%) |
| Lipids (mg/dL) | ||
| Total Cholesterol | 193.6 ± 33.8 | 183.3 ± 36.6 |
| Low-density lipoprotein Cholesterol | 117.4 ± 30.5 | 105.6 ± 32.0 |
| High-density lipoprotein Cholesterol | 51.8 ± 15.0 | 56.7 ± 17.0 |
| Triglycerides | 122.3 ± 62.7 | 105.2 ± 51.9 |
| Lipid-lowering Meds (n, %) | 383 (14.7%) | 978 (37.6%) |
| Body mass index (kg/m2) | 27.7 ± 5.0 | 27.9 ± 5.3 |
| Smoking (n, %) | - | - |
| Current | 288 (11.1%) | 188 (7.2%) |
| Former | 924 (35.5%) | 1185 (45.5%) |
| Carotid Artery Dimensions | - | - |
| Wall Thickness (mm) | 0.74 ± 0.15 | 0.82 ± 0.16 |
| PSI Diameter (mm) | 6.26 ± 0.74 | 6.43 ± 0.80 |
| EDI Diameter (mm) | 5.80 ± 0.70 | 5.99 ± 0.76 |
| Stiffness Parameters (mmHg) | - | - |
| Total PEM | 353 ± 200 | 460 ± 312 |
| Structural PEM | 374 ± 189 | 482 ± 295 |
| Load-Dependent PEM | −21 ± 68 | −23 ± 88 |
| Total YEM | 2865 ± 1700 | 3481 ± 2555 |
| Structural YEM | 3140 ± 1660 | 3760 ± 2448 |
| Load-Dependent YEM | −275 ± 669 | −279 ± 761 |
All values mean and standard deviation. SBP – Systolic blood pressure, DBP – Diastolic blood pressure, PSI Diameter – Peak systolic internal diameter, EDI Diameter– End diastolic internal diameter, PEM – Peterson’s Elastic Modulus, YEM – Young’s Elastic Modulus
Change in Peterson’s Elastic Modulus
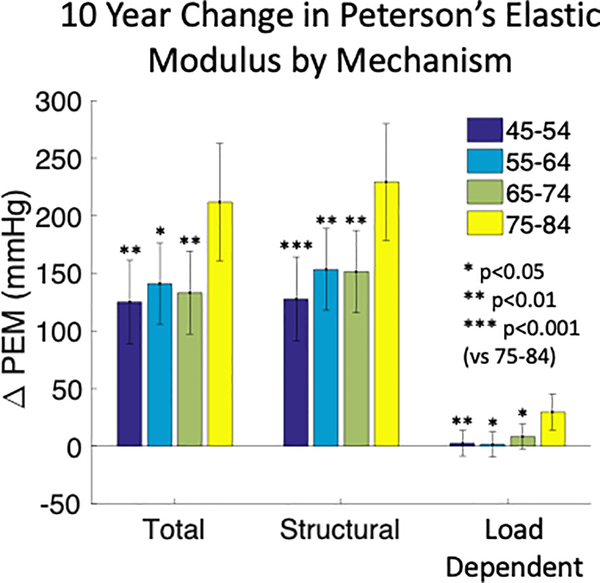
The mean total PEM increased during the study period (Table 1). Total PEM increased the most in 75–84 year old (at baseline) participants compared to younger participants (Δ212 ± 26 vs Δ122 ± 18 mmHg; p<0.05, Figure 2). In addition to age category, self-identification with black race and stopping antihypertensive medication prior to exam 5 assessment were associated with a greater increase in total PEM. Conversely, having more than high school education and greater baseline SBP were associated with less increase in total PEM (Table 2). Structural PEM similarly increased over the study period (Table 1) and the change was also greatest in 75–84 year old participants compared to younger age groups (Δ 229 ± 26 vs Δ 144 ± 18 mmHg; p<0.01, Figure 2). Stopping antihypertensive medication was associated with greater increase in structural PEM (Table 2). For the 78 participants who stopped using antihypertensive medications, the estimated increase in structural PEM (Δ 210 ± 33 mmHg) was greater than for participants who were continuously treated (Δ 141 ± 17 mmHg) or were never treated (Δ 139 ± 18 mmHg). On average, load-dependent PEM did not increase during the study period (Table 1) although 75–84 year old participants had an increase that was not observed in younger participants (Δ 30 ± 8 vs Δ 4 ± 6 mmHg; p<0.05, Figure 2). Self identification with black race was associated with greater increase in load-dependent PEM (Table 2). Self-identification with Chinese ethnicity, more than high school education, starting antihypertensive medication, greater baseline SBP, and greater baseline HDL cholesterol were associated with less increase in load-dependent PEM (Table 2).
Figure 2:
Estimated means and 95% confidence intervals for total, structural, and load-dependent changes in carotid artery Peterson’s elastic modulus (PEM) over 10 years of aging by age groups. Older individuals (75–84, yellow bars) had greater rates of total, structural, and load-dependent stiffening. ANCOVA model co-variates were: sex, race/ethnicity, education level, income, smoking status, diabetes mellitus status, systolic blood pressure, total cholesterol, HDL cholesterol, BMI, antihypertensive medication, and lipid lowering medication.
Table 2:
Multivariate ANCOVA Model for Change in Peterson’s Elastic Modulus*
| Change in Total PEM | ||
| Age Category (vs 75–84) | β | P-value |
| 45–54 | −129 | <0.001 |
| 55–64 | −104 | <0.001 |
| 65–74 | −99 | <0.001 |
| Black (vs mean) | 26 | 0.02 |
| More than HS Education (vs did not graduate HS) | −41 | 0.046 |
| HTN Medication cessation (vs Untreated) | 105 | 0.002 |
| Systolic BP (per 10 mmHg) | −19 | <0.001 |
| Change in Structural PEM | ||
| Age Category (vs 75–84) | ||
| 45–54 | −102 | <0.001 |
| 55–64 | −76 | 0.001 |
| 65–74 | −78 | 0.001 |
| HTN Medication cessation (vs Untreated) | 89 | 0.007 |
| Change in Load-Dependent PEM | ||
| Age Category (vs 75–84) | ||
| 45–54 | −27 | <0.001 |
| 55–64 | −28 | <0.001 |
| 65–74 | −21 | 0.002 |
| Black (vs mean) | 9 | <0.001 |
| Chinese (vs mean) | −20 | 0.002 |
| More than HS Education (vs did not graduate HS) | −12 | 0.05 |
| Starting HTN Medication (vs Untreated) | −11 | 0.02 |
| Systolic BP (per 10 mmHg) | −17 | <0.001 |
| HDL Cholesterol (per 10 mg/dL) | −6 | <0.001 |
Only significant predictors are included in the table
PEM – Peterson’s elastic modulus, HS – high school, HTN med – antihypertensive medication, BP – blood pressure
In a second set of ANCOVA models that incorporated the change in IMT, diastolic diameter, systolic BP, and pulse-pressure as co-variates (Online supplement); race/ethnicity and educational attainment were no longer significantly associated with changes in total and load-dependent PEM. Cessation of antihypertensive medication was still associated with increased total PEM (β=74mmHg, p=0.02) and structural PEM (β=71mmHg, p=0.02). Starting antihypertensive medication was no longer associated with the change in load-dependent PEM. The change in IMT, diastolic diameter, systolic BP, and pulse-pressure were all significantly associated with changes in total, structural, and load-dependent PEM (Online Supplement ). Changes in systolic BP and pulse-pressure had opposite associations with the different stiffness mechanisms. Increased change in systolic BP increased total PEM (β=27mmHg per 10mmHg, p<0.001), decreased structural PEM (β=−39mmHg per 10mmHg, p<0.001), and increased load-dependent PEM (β=69mmHg per 10mmHg, p<0.001). Increased change in pulse-pressure increased total PEM (β=40mmHg per 10mmHg, p<0.001), increased structural PEM (β=81mmHg per 10mmHg, p<0.001), and increased load-dependent PEM (β=−41mmHg per 10mmHg, p<0.001).
Change in Young’s Elastic Modulus
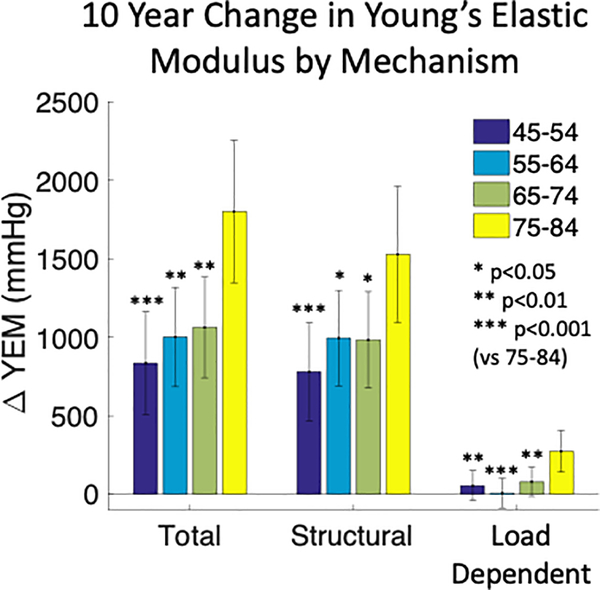
The mean total YEM increased during the study period (Table 1). Total YEM increased the most in 75–84 year old participants compared to younger participants (Δ 1801 ± 231 vs Δ 966 ± 164 mmHg; p<0.01, Figure 3). In addition to age category, stopping antihypertensive medication was associated with greater increase in total YEM while high school education and greater baseline systolic BP were associated with less increase in total YEM (Table 3). Structural YEM similarly increased over the study period (Table 1) and the change was also greatest 75–84 year old participants compared to younger participants (Δ 1529 ± 221 vs Δ 920 ± 157 mmHg; p<0.01, Figure 3). In additional to age category, stopping antihypertensive medication was associated with greater increase in structural YEM (Table 3). The average load-dependent YEM did not increase during the study period (Table 1) although 75–84 year old participants had an increase that was not observed in younger participants (Δ 273 ± 67 vs Δ 47 ± 48 mmHg; p<0.01, Figure 3). In additional to age category, self-identification with black race and stopping antihypertensive medication were associated with greater increase in load-dependent YEM (Table 3). Self-identification with Chinese ethnicity, starting antihypertensive medication, greater baseline systolic BP, and greater baseline HDL cholesterol were associated with less increase in load-dependent YEM (Table 3).
Figure 3:
Estimated means and 95% confidence intervals for total, structural, and load-dependent changes in carotid artery Young’s elastic modulus (YEM) over 10 years of aging. Older individuals (75–84) had greater rates of total, structural, and load-dependent stiffening. ANCOVA model co-variates were: sex, race/ethnicity, education level, income, smoking status, diabetes mellitus status, systolic blood pressure, total cholesterol, HDL cholesterol, BMI, antihypertensive medication, and lipid lowering medication.
Table 3:
Multivariate ANCOVA Model for Change in Young’s Elastic Modulus*
| Change in Total YEM | ||
| Age Category (vs 75–84) | β | P-value |
| 45–54 | −966 | <0.001 |
| 55–64 | −800 | <0.001 |
| 65–74 | −738 | <0.001 |
| HS Education (vs did not graduate HS) | −380 | 0.04 |
| HTN Medication cessation (vs Untreated) | 1010 | 0.001 |
| Systolic BP (per 10 mmHg) | −140 | <0.001 |
| Change in Structural YEM | ||
| Age Category (vs 75–84) | ||
| 45–54 | −747 | <0.001 |
| 55–64 | −533 | 0.008 |
| 65–74 | −544 | 0.006 |
| HTN Medication cessation (vs Untreated) | 840 | 0.003 |
| Change in Load-Dependent YEM | ||
| Age Category (vs 75–84) | ||
| 45–54 | −219 | <0.001 |
| 55–64 | −267 | <0.001 |
| 65–74 | −194 | 0.001 |
| Black (vs mean) | 58 | <0.001 |
| Chinese (vs mean) | −193 | <0.001 |
| HTN Medication cessation (vs Untreated) | 170 | 0.05 |
| Starting HTN Medication (vs Untreated) | −115 | 0.003 |
| Systolic BP (per 10 mmHg) | −167 | <0.001 |
| HDL Cholesterol (per 10 mg/dL) | −44 | <0.001 |
Only significant predictors are included in the table
YEM – Young’s elastic modulus, HS – high school, HTN med – antihypertensive medication, BP – blood pressure
In a second set of ANCOVA models that incorporated the change from Exam 1 to Exam 5 in IMT, diastolic diameter, systolic BP, and pulse-pressure as co-variates (Online Supplement ); race/ethnicity and educational attainment were no longer significantly associated with changes in YEM. Cessation of antihypertensive medication was still associated with increased total YEM (β=657mmHg, p=0.01) and structural YEM (β=596mmHg, p=0.02). Antihypertensive medication usage was no longer associated with the change in load-dependent YEM. Changes in systolic BP and pulse-pressure had opposite associations with the different stiffness mechanisms. Increased change in systolic BP increased total YEM (β=193mmHg per 10mmHg, p<0.001), decreased structural YEM (β=−419mmHg per 10mmHg, p<0.001), and increased load-dependent YEM (β=612mmHg per 10mmHg, p<0.001). Increased change in pulse-pressure increased total YEM (β=299mmHg per 10mmHg, p<0.001), increased structural YEM (β=737mmHg per 10mmHg, p<0.001), and increased load-dependent YEM (β=−438mmHg per 10mmHg, p<0.001).
Differences in Stiffness Mechanisms Based on Reference Pressure
Complete results from ANCOVA models using reference pressures of 105/70, 135/90, and 160/90 mmHg are presented in the supplementary data. Increasing the reference pressure from 120/80 to 135/90 or 160/90 increased the change in structural carotid artery stiffness and decreased the change in load-dependent carotid artery stiffness over the study period. Decreasing the reference pressure to 105/70 had the opposite effect. For structural stiffness, statistical comparisons between age groups were unaffected by changing the reference pressure. For load-dependent stiffness, decreasing the reference pressure did not affect statistical results by age category, but increasing the reference pressure changed several comparisons between the 75–84 age group and younger age groups to be non-significant. Besides age, the other strong predictors of change in load-dependent stiffness (race/ethnicity, starting antihypertensive medication, systolic BP, HDL cholesterol) were largely still significant predictors after increasing the reference pressure.
Grouping by Mechanism
On average, structural stiffening accounted for the vast majority of total stiffening, but 27% (n=700) and 37% (n=954) of participants had more load-dependent than structural stiffening based on PEM and YEM respectively. At baseline, participants with greater load-dependent stiffening were found to have lower SBP and DBP compared to participants with greater structural stiffening (small-to-moderate effect, Hedges g = −0.27 to −0.49). The participants with greater load-dependent stiffening were also found to have greater total stiffness (small-to-moderate effect, Hedges g = 0.31 to 0.56), greater structural stiffness (moderate-to-large effect Hedges g = 0.50 to 0.77), lower load-dependent stiffness (small-to-moderate effect Hedges g = −0.40 to −0.52), and a thinner carotid artery wall (Hedges g = −0.19 to −0.36). Demographics, diabetes mellitus status, smoking status, and lipid levels were similar among participants with greater load-dependent stiffening compared to participants with greater structural stiffening. Of note, participants with greater load-dependent stiffening were distributed evenly across age groups. Complete results of the exploratory analysis are presented in Online Supplement.
Discussion
This study is the first to quantify the age-associated longitudinal changes in structural and load-dependent carotid artery stiffness in a large, multi-ethnic cohort study with a decade of observation. The major finding of this study was that, on average, the vast majority of the age-associated increases in carotid artery stiffness were due to structural stiffening intrinsic to the arterial wall rather than being a concomitant effect of increased systolic BP with aging. The rates of both structural and load-dependent carotid artery stiffness increased with age. There was also large individual variability, with up to 37% of participants having greater load-dependent than structural stiffening, which was evenly distributed evenly across age groups. The methods of this study will be useful to evaluate if therapies can be targeted to reduce structural arterial stiffness and if the deleterious CVD outcomes associated with greater stiffening can also be reduced.
Increased structural arterial stiffness is largely due to changes in the arterial wall including elastin degradation and collagen accumulation7. New elastin is not generated following the perinatal period and the half-life of elastin is on the order of decades16, therefore the progressive loss of arterial elastin is expected with aging. The combination elastin degradation and collagen accumulation with aging likely contribute to our finding that the rate of structural carotid artery stiffening increased with age. Besides age, the only predictor of increased changes in structural stiffness in primary ANCOVA models was stopping antihypertensive therapy. In secondary models, greater increases in intima-media thickness were also associated with smaller increases in structural stiffness (Online Supplement ). Intima-media thickening occurs at a greater rate in middle-aged individuals compared to older individuals10 and may help prevent increases in structural arterial stiffness. Antihypertensive therapy use, baseline SBP, and baseline HDL cholesterol levels were all predictive of changes in load-dependent stiffness. This suggests that load-dependent stiffness is more modifiable than structural stiffness via traditional CVD risk factors, particularly as it relates to individual hypertension management. This discrepancy may also underly why some but not all studies have found improvements in arterial stiffness with treatment of dyslipidemia17–19. The methods of this study will be useful in the assessment of therapies and interventions targeted at reducing structural arterial stiffness.
Van der Bruggen et al. used similar methods in the CATOD study to quantify changes in structural carotid artery stiffness in hypertensive individuals (58±9 years old) over a 3-year period20. The major discrepancies were that van der Bruggen’s group found a greater rate of change in structural stiffness and decreases in load-dependent stiffness while we found, on average, no change in load-dependent stiffness. These differences are likely due to the shorter follow up period and enrollment criteria in CATOD that only included hypertensive individuals with less race/ethnicity and sex diversity than the MESA cohort.
Increased arterial stiffness is believed to be both a cause of hypertension and a consequence of hypertension, creating a positive and detrimental feedback loop through increased pulse pressure and both changes and structural stiffening over time2. However, clinical studies utilizing simultaneous carotid ultrasound and tonometry have shown that structural stiffness is not increased in hypertensive individuals21,22, indicating that increased arterial stiffness in hypertension is primarily due to load-dependent stiffening. The results of our longitudinal analysis support this finding as baseline systolic blood pressure was a significant predictor of changes in load-dependent stiffness but not changes in structural stiffness, which was predominantly driven by age. Systolic blood pressure and pulse pressure also had different longitudinal associations as greater increases in systolic blood pressure increased load-dependent stiffness while greater increases in pulse pressure increased structural stiffness. The associations between changes in blood pressure and arterial stiffness are likely bi-directional2. With regards to hypertension treatment, previous analysis in MESA found that starting and stopping antihypertensive medications were associated with decreased and increased carotid artery stiffness respectively10. The results of the present analysis suggest that starting antihypertensive medication predominately decreased load-dependent stiffness while stopping antihypertensive medication had negative effects on both structural and load-dependent stiffness over the 10 year follow up period. The mechanisms of how starting or stopping antihypertensive therapy may affect arterial stiffness also are likely dependent on the duration of therapy and the degree of blood pressure control23.
While structural stiffening, on average, accounted for the vast majority of increases in carotid artery stiffness over a decade of aging, there was large variability with over 1/3 of participants having greater increases in load-dependent YEM than structural YEM. These participants had a unique pattern of baseline CVD risk factors with lower blood pressure and thinner carotid artery walls, but higher total and structural carotid artery stiffness measures. The observed heterogeneity in mechanisms of arterial stiffness may impact the development of hypertension, CVD, or end organ damage. In patients with end stage renal failure, PWV not decreasing following a successful reduction in BP, possibly indicating elevated structural stiffness, was an independent predictor of both cardiovascular and all-cause mortality24. The association of arterial stiffness with incident hypertension, CVD events, and end organ outcomes, including kidney disease, should be a focus of future studies.
Previous analysis of arterial stiffness in MESA has identified associations between carotid artery stiffness, race/ethnicity, and socioeconomic status8,10. Novel findings of this study were that race/ethnicity and markers of socioeconomic status (indicated by higher education levels) were significantly associated with changes in load-dependent stiffening but not with structural stiffening over the 10-year period. This finding suggests that disparities in carotid artery stiffness are primarily due to blood pressure control, which is clearly linked to socioeconomic status25, not due to intrinsic differences in the artery wall material. Limited access to healthcare and substandard insurance coverage have been identified as key factors driving racial/ethnic disparities in blood pressure control25. Measuring differences in the mechanisms of arterial stiffness and targeting at risk racial and ethnic groups could be a novel way to attempt to improve these inequities in addition to improving healthcare access and insurance coverage.
Limitations:
The associations reported in this study cannot confirm causation due to the non-randomized design. The participants in this analysis were only a subset of the MESA study. There may be a survivorship bias where participants who participated in exam 5 were healthier than the original MESA cohort but we expect that this bias would increase the chances of a null finding. Like most epidemiological studies, brachial artery blood pressures were used in place of carotid artery blood pressures when calculating carotid artery stiffness. The difference between peripheral and central blood pressure decreases with age, which would also result in methodological bias towards a null finding. Sensitivity analysis showed that comparisons of load-dependent stiffness between age groups were dependent on the choice of reference pressure. Differentiating structural from load-dependent stiffness inherently requires comparing stiffness at a common reference blood pressure for all participants. We chose a reference pressure of 120/80, in part based prior studies suggesting that arterial stiffness measures should be reported at a standardized loading condition that lies in normal physiological ranges26 and that a pressure of 120/80 mmHg more or less represents the common perception of “normal” resting BP27. The other reason for choosing 120/80 mmHg was to facilitate the comparison of quantitative results between studies20. Older studies have utilized simultaneous carotid tonometry and carotid ultrasound to calculate structural stiffness at 10021 or 110 mmHg22. Lastly, this study did not measure carotid-femoral pulse wave velocity (cf-PWV), which is considered the gold standard measure of arterial stiffness11.
Perspectives
The major finding of this study was that, on average, the vast majority of the age-associated increases in carotid artery stiffness were due to structural stiffening intrinsic to the arterial wall rather than being a concomitant effect of increased systolic BP. The longitudinal rates of structural and load-dependent carotid artery stiffening increased with age. There was high individual variability with a large portion of participants having greater load-dependent than structural stiffening. The heterogeneity in arterial stiffening mechanisms with aging may influence CVD development and warrants future investigation.
Supplementary Material
Novelty and Significance.
What Is New?
This study is the first to quantify the age-associated longitudinal changes in structural and load-dependent carotid artery stiffness in a large, multi-ethnic cohort study with a decade of observation.
On average, the vast majority of the age-associated increases in carotid artery stiffness were due to structural stiffening intrinsic to the arterial wall rather than being a concomitant effect of increased systolic BP with aging.
There was high individual variability; 37% of participants had more load-dependent than structural stiffening.
What Is Relevant?
The longitudinal rates of structural and load-dependent carotid artery stiffening increased with age.
Results suggest that load-dependent stiffness could be a more modifiable component of arterial stiffness compared to the structural stiffness component.
Load dependent stiffness could be novel target for hypertension management and antihypertensive therapy.
Summary
Over a decade of aging, structural carotid artery stiffening was consistently observed, and load dependent carotid artery stiffening was highly variable.
Acknowledgements
We thank the staff and study participants of the Multi-Ethnic Study of Atherosclerosis. We also acknowledge Drs. Robyn McClelland, Emmanuel Sampene, and Zhanhai Li for helpful discussions regarding our statistical approach.
Sources of Funding
MESA and the MESA SHARe project are conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with MESA investigators. Support for MESA is provided by contracts N01-HC95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC95166, N01-HC-95167, N01-HC-95168, N01-HC-95169 and CTSA UL1-RR-024156. Dr. Pewowaruk was supported by a T32 HL 07936 Ruth L. Kirschstein National Research Service Award from the National Heart Lung and Blood Institute to the University of Wisconsin-Madison Cardiovascular Research Center. This manuscript does not necessarily represent the views or opinions of MESA or the NHLBI. This material is the result of work supported with resources and the use of facilities at the William S. Middleton Memorial VA Hospital, Madison, WI.
Footnotes
Disclosures
The authors have no conflicts of interest, financial or otherwise, to disclose.
References
- 1.Laurent S, Boutouyrie P. Arterial stiffness: A new surrogate end point for cardiovascular disease? Journal of Nephrology. 2007;20(SUPPL. 12):S45–50. [PubMed] [Google Scholar]
- 2.Humphrey JD, Harrison DG, Figueroa CA, Lacolley P, Laurent S. Central Artery stiffness in hypertension and aging a problem with cause and consequence. Circulation Research. 2016;118(3):379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of Cardiovascular Events and All-Cause Mortality With Arterial Stiffness. A Systematic Review and Meta-Analysis. Journal of the American College of Cardiology. 2010;55(13):1318–1327. [DOI] [PubMed] [Google Scholar]
- 4.Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial Stiffness and Cardiovascular Risk in Hypertension. Circulation Research. 2021:864–886. [DOI] [PubMed] [Google Scholar]
- 5.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA - Journal of the American Medical Association. 2012;308(9):875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell GF. Aortic stiffness, pressure and flow pulsatility, and target organ damage. Journal of Applied Physiology. 2018;125(6):1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Rourke MF, Hashimoto J. Mechanical Factors in Arterial Aging. A Clinical Perspective. Journal of the American College of Cardiology. 2007;50(1):1–13. [DOI] [PubMed] [Google Scholar]
- 8.Vaidya D, Heckbert SR, Wasserman BA, Ouyang P. Sex-specific association of age with carotid artery distensibility: Multi-ethnic study of atherosclerosis. Journal of Women’s Health. 2012;21(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Safar ME. Systolic hypertension in the elderly: Arterial wall mechanical properties and the renin-angiotensin-aldosterone system. Journal of Hypertension. 2005;23(4):673–681. [DOI] [PubMed] [Google Scholar]
- 10.Gepner AD, Korcarz CE, Colangelo LA, Hom EK, Tattersall MC, Astor BC, Kaufman JD, Liu K, Stein JH. Longitudinal effects of a decade of aging on carotid artery stiffness : The multiethnic study of atherosclerosis. Stroke. 2014;45(1):48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement from the American Heart Association. Hypertension. 2015;66(3):698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institutes of Health, National Heart, Lung, and Blood Institute. Multi‐Ethnic Study of Atherosclerosis (MESA). Biologic Specimen and Data Repository Information Coordinating Center. https://biolincc.nhlbi.nih.gov/studies/mesa/?q=multi ethnic study of atherosclerosis [Google Scholar]
- 13.Bild DE. Multi-Ethnic Study of Atherosclerosis: Objectives and Design. American Journal of Epidemiology. 2002;156(9):871–881. [DOI] [PubMed] [Google Scholar]
- 14.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. European Heart Journal. 2006;27(21):2588–2605. [DOI] [PubMed] [Google Scholar]
- 15.Spronck B, Heusinkveld MHG, Vanmolkot FH, Roodt JO ‘t, Hermeling E, Delhaas T, Kroon AA, Reesink KD. Pressure-dependence of arterial stiffness. Journal of Hypertension. 2015;33(2):330–338. [DOI] [PubMed] [Google Scholar]
- 16.Humphrey J, Epstein M. Cardiovascular Solid Mechanics: Cells, Tissues, and Organs. Applied Mechanics Reviews. 2002;55(5):B103–B104. [Google Scholar]
- 17.Upala S, Wirunsawanya K, Jaruvongvanich V, Sanguankeo A. Effects of statin therapy on arterial stiffness: A systematic review and meta-analysis of randomized controlled trial. International Journal of Cardiology. 2017;227:338–341. [DOI] [PubMed] [Google Scholar]
- 18.Gepner AD, Lazar K, Van Hulle C, Korcarz CE, Asthana S, Carlsson CM. Effects of Simvastatin on Augmentation Index Are Transient: Outcomes From a Randomized Controlled Trial. Journal of the American Heart Association. 2019;8(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh PC, Han SH, Koh KK, Lee K, Seo JG, Suh SY, Ahn T, Choi IS, Shin EK. Rosuvastatin treatment improves arterial stiffness with lowering blood pressure in healthy hypercholesterolemic patients. International Journal of Cardiology. 2014;176(3):1284–1287. [DOI] [PubMed] [Google Scholar]
- 20.van der Bruggen M, Spronck B, Bos S, Heusinkveld MHG, Taddei S, Ghiadoni L, Delhaas T, Bruno RM, Reesink KD. Pressure-Corrected Carotid Stiffness and Young’s Modulus: Evaluation in an Outpatient Clinic Setting. American Journal of Hypertension. 2021. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laurent S, Caviezel B, Beck L, Girerd X, Billaud E, Boutouyrie P, Hoeks A, Safar M. Carotid artery distensibility and distending pressure in hypertensive humans. Hypertension. 1994;23(6):878–883. [DOI] [PubMed] [Google Scholar]
- 22.Bussy C, Boutouyrie P, Lacolley P, Challande P, Laurent S. Intrinsic stiffness of the carotid arterial wall material in essential hypertensives. Hypertension. 2000;35(5):1049–1054. [DOI] [PubMed] [Google Scholar]
- 23.Gepner AD, Tedla Y, Colangelo LA, Tattersall MC, Korcarz CE, Kaufman JD, Liu K, Burke GL, Shea S, Greenland P, Stein JH. Progression of Carotid Arterial Stiffness with Treatment of Hypertension over 10 Years. Hypertension. 2017;69(1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001;103(7):987–992. [DOI] [PubMed] [Google Scholar]
- 25.Gu A, Yue Y, Desai RP, Argulian E. Racial and Ethnic Differences in Antihypertensive Medication Use and Blood Pressure Control among US Adults with Hypertension: The National Health and Nutrition Examination Survey, 2003 to 2012. Circulation: Cardiovascular Quality and Outcomes. 2017;10(1). [DOI] [PubMed] [Google Scholar]
- 26.Fung YC. Elasticity of soft tissues in simple elongation. The American journal of physiology. 1967;213(6):1532–1544. [DOI] [PubMed] [Google Scholar]
- 27.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Pr. Journal of the American College of Cardiology. 2018;71(19):e127–e248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.