Abstract
Background
Hidradenitis suppurativa (HS) is a chronic, debilitating disease with a profound impact on the quality of life of patients.
Objectives
To describe a rare case of HS with postmenopausal onset, to review the literature data regarding late onset HS and to discuss the current knowledge on the role of endocrine abnormalities in the development of HS.
Case report
We report the case of a 68-year-old patient in whom HS occurred 10 years after menopause. She was referred to our clinic for the presence of an open fistula on the left groin, fibrotic scars and visible alteration of the vulvar anatomy due to numerous surgical interventions. The patient shared features of the metabolic syndrome (obesity, arterial hypertension, dyslipidemia, aortic atherosclerosis), but showed no signs of virilism and no hormonal abnormality. HS was controlled using antiseptics, topical retinoids and antibiotics.
Conclusions
This case is of particular interest given the late onset of HS, long time after menopause. The development of HS requires a complex interaction between genetic predisposing factors, endocrine dysregulation, metabolic alterations, bacterial overgrowth and an aberrant inflammatory response. Evidence points to an important role of sex-hormones in the emergence and progression of the disease, but the underlying mechanisms are still unclear. A better understanding of HS pathogenesis is needed to elucidate the precise way in which endocrine factors influence the disease onset and course. This would guide the way to novel therapies and a better control of this challenging disease.
Keywords: hidradenitis suppurativa, menopause, late onset, androgens, obesity
INTRODUCTION
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease with a complex, multifactorial pathogenesis, manifested by recurrent painful nodules, draining abscesses, fistulae and sinus tracts that evolve into inflamed subcutaneous networks and heal with hypertrophic, disfiguring scars, fibrotic bands, and disabling contractures,affecting areas rich in apocrine glands, particularly the axillary, inguinal and anogenital regions. HS is not an uncommon disease, with a prevalence of 1-4% (1,2). It has a strong predilection for the female gender (F/M=2-3/1)(1-3). The onset of the disease takes place after puberty and peaks in the third decade (1-3).
Initially perceived as an inflammatory disease of the apocrine glands given the anatomical distribution of the lesions, HS is currently considered a follicular occlusion disorder. Genetic susceptibility, immune dysregulation, hormonal factors, obesity, metabolic disorders, diet, smoking, mechanical stress, and alterations of the skin microbiome are decisive contributors to HS development (4).
The diagnosis is based on characteristic clinical findings. As microbial factors play a secondary role, microbial cultures frequently fail to detect pathogenic bacteria. Nevertheless, HS severity significantly increases in case of superinfection. Therefore, bacteriological cultures are mainly useful for the differential diagnosis with cutaneous infections and to guide the treatment in refractory cases (5). Given the lack of specific lesions, diagnosis delay is common, HS patients often undergoing repeated abscess drainage and antibiotic treatment before referral to a dermatologist.
Despite the advances in the understanding of HS pathogenesis, the optimal therapeutic approach remains unclear. Patients need guidance on lifestyle changes, medical, and surgical treatment. They are advised to keep proper hygiene, avoid trauma, lose weight, and stop smoking. Depending on disease severity, the treatment consists of topical/systemic antibiotics, dapsone, antiandrogens, retinoids, immunosuppressive agents. These therapies often yield disappointing results and surgical treatment is required, ranging from electrocautery/laser unroofing of sinus tracts to wide excision of the entire affected area. The latter is the only curative treatment for HS.
The aims of this study are to describe a rare case of HS occurring ten years after menopause, to review the literature data on late onset HS, and to discuss the current knowledge on the contribution of endocrine factors in HS development.
CASE REPORT
We report the case of a 68-year-old female patient who presented for an open fistula on the left groin, fibrotic scars and vulvar anatomy alteration due to numerous surgical interventions (Fig. 1). The patient denied a family history of HS. She was a smoker (14 pack-years) and shared features of metabolic syndrome: body mass index (BMI) of 33.2 kg/m2, grade I essential arterial hypertension, dyslipidemia, aortic atherosclerosis. HS onset had taken place 8 years previously, 10 years after menopause. The anogenital region was the only affected area. The patient had undergone two wide surgical excisions three years previously. Afterwards, the disease remained minimally active. The rest of the physical examination revealed no pathologic findings and no signs of virilism.
Figure 1.
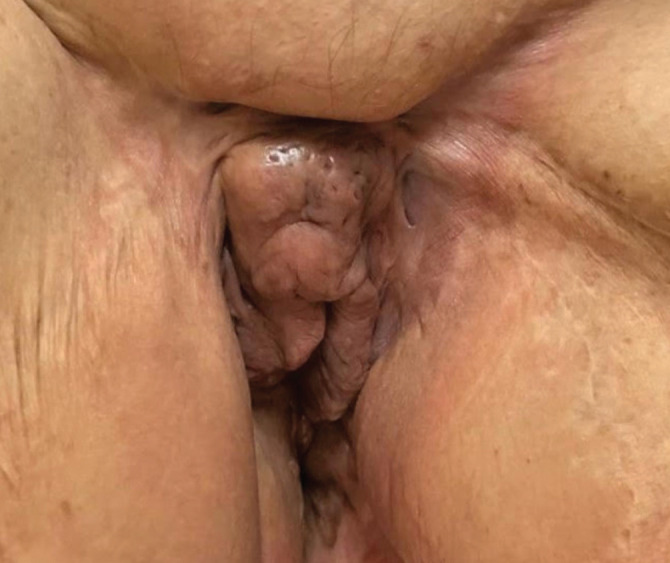
HS, Hurley stage II.
Laboratory test results were normal, including plasma estradiol 103pmol/L, testosterone 1.12 nmol/L and dehydroepiandrosterone-sulphate (DHEA-S)148 mg/dL. Normal reference ranges according to the patient’s age and gender: estradiol 18.4-505 pmol/L, testosterone 0.101-1.42 nmol/L and DHEA-S 9.4-246 mg/dL.
Pelvic magnetic resonance imaging showed an 8 cm sinus tract in the deep subcutaneous tissue of the left groin that extended posteriorly to the fat tissue of the thigh root and vulva, and ended imprecisely, in a mass of inflammatory tissue (Fig. 2). The patient was recommended local antiseptics, topical retinoids and clindamycin gel, which successfully controlled the disease.
Figure 2.
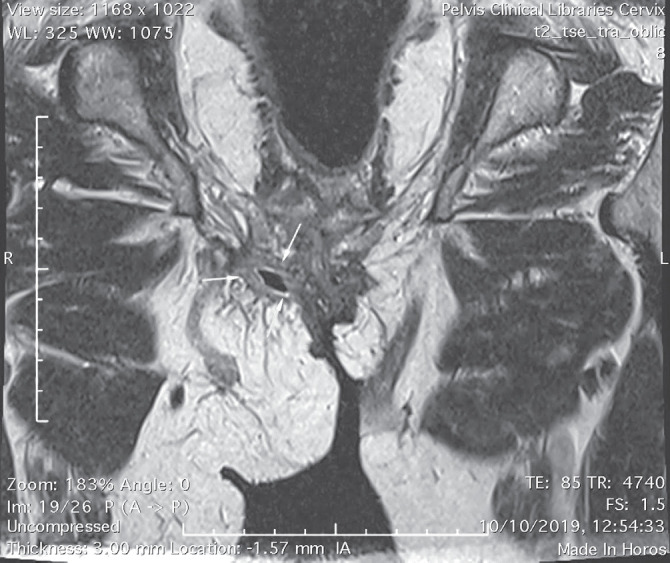
Elongated sinus tract located in the subcutaneous tissue of the left groin.
DISCUSSION
HS onset after menopause is extremely rare, very few cases having been reported. The first report of late onset HS dates from 1988 (6). In 1996, Barth et al. discussed the primary development of HS in postmenopausal women, but only described one such case (7).
The pathogenesis of HS is incompletely elucidated. Follicular epithelium hyperplasia and infundibular hyperkeratosis, leading to an expanding keratinous plug, represent the primary events. A site of weak structural support exists at the junction of sebaceous glands and follicular ducts. Because of increasing pressure in this area, the follicle wall ruptures and the follicular content is released, activating the innate immune system and ensuing an intense, self-perpetuating foreign body type inflammatory response (8). Other factors that contribute to the marked perifolliculitis are follicular immune system deficiencies allowing bacterial overgrowth and/or immune dysregulations associated with an exaggerated inflammatory response to commensal flora (9). Inflammation extends to adjacent apocrine glands in approximately 20% of cases (10). Eventually, the growth of residual unphagocytosed keratinocytes of the ruptured follicular epithelium or follicular stem cells epithelialize the communicating sinuses generated by tissue necrosis (8).
The influence of endocrine factors in HS development is controversial. HS predilection for the female gender, its typical onset after puberty, frequent perimenstrual relapses, exacerbations induced by progestins, the amelioration of the disease during pregnancy and after menopause, and the response to antiandrogenic therapies suggest a hormone dysfunction (11). These changes parallel the fluctuation of estradiol and progesterone levels, which rise in pregnancy and decline before menses and after menopause. Moreover, the characteristic onset at puberty corresponds to the rise in androgen production. HS worsening during the luteal phase of the menstrual cycle follows an increased release of ovarian androgens (7). A downturn in androgen level is physiologic with aging and could explain the rarity of HS onset after menopause (6).
Although earlier studies reported increased serum testosterone level, higher free androgen index (FAI) and an association between HS and irregular menses and virilisation (12), further research did not confirm these findings. Increased serum androgens and FAI in HS patients may be explained by low sex hormone binding globulin (SHBG) levels associated with obesity, which is frequent in this population.
Since serum androgen levels are generally normal in HS patients, an increased peripheral conversion of androgens and higher end-organ sensitivity to androgens have been suspected (13). The skin has important endocrine functions. Among them, the synthesis of cholesterol, sex-hormones and glucocorticoids by sebocytes, which possess a rich arsenal of enzymes and control local endocrine homeostasis. Sebaceous glands and terminal hair follicles are the most important conversion site of DHEA-S to DHEA by steroid sulfatase (13). DHEA is produced in the skin both from DHEA-S of adrenal origin and cutaneous cholesterol. Sebocytes possess 3β-hydroxysteroid dehydrogenase (HSD), predominantly type-1, which converts DHEA to androstenedione, the latter being further converted to testosterone by 17β-HSD type-5. 5α-reductase (5αR), especially type-1, turns testosterone into dihydrotestosterone (DHT). 5αR also acts directly on androstenedione, generating 5α-androstanedione, transformed into DHT by 17β-HSD (13).
Sebaceous glands density, morphology and function were investigated in HS patients. Kamp et al. demonstrated that the volume of sebaceous glands in these patients is seven times smaller than that of healthy controls, associated with decreased production of sebum and antimicrobial peptides (14). While less sebum weakens the follicle wall, antimicrobial peptides deficiency favours the proliferation of commensal bacteria and promotes perifollicular inflammation (14). Sebaceous glands shrinkage is also observed in apparently healthy skin. Therefore, the reduction of sebaceous glands volume and the infundibulofolliculitis precede follicular occlusion.
Apocrine glands also possess enzymes involved in the metabolism of androgens. 5αR activity in apocrine glands from HS areas does not differ from that of normal controls, whereas 3β-HSD and 17β-HSD display a significantly lower activity in HS (15). As all other cutaneous adnexa, apocrine glands are androgen-dependent. HS severity also parallels their activity. Their secretion begins at puberty and reaches a peak in adult life. These glands enlarge and enhance their secretion during the premenstrual phase and menstruation and decrease in size after menopause. Their activity declines during pregnancy. Moreover, ongoing dilatation of the apocrine gland tubules could explain the low probability of duct occlusion and disease development after menopause (6). However, no difference in the number, volume or morphology of apocrine glands in HS patients compared with healthy controls has been demonstrated (6).
Considering all these, dysfunction of the androgen receptors (AR) in sebaceous and apocrine glands was hypothesized. The expression of AR and estrogen receptors in apocrine glands from HS and healthy skin was compared, but no significant difference was noticed (16), suggesting that other factors that influence access to AR, such as diet and obesity are key elements in HS pathogenesis.
While the pathogenic mechanisms linking androgens to HS development continue to puzzle researchers, the benefit of antiandrogen therapies in some HS patients cannot be disputed. Current guidelines recommend the use of cyproterone acetate combined with ethinyl estradiol in female patients with menstrual abnormalities or hyperandrogenism (17). Finasteride and spironolactone are useful alternatives.
The hypothesis of hyperandrogenism was not supported in our patient given the absence of clinical signs of virilism, hyperseborrhea and the normal serum androgen levels.
Another argument in favour of the endocrine influence in HS is the high frequency of obesity, 60–88% of HS patients being overweight/obese (8). HS severity correlates with BMI and weight loss invariably leads to clinical improvement. Metabolic imbalance is clearly related to HS, frequently associated with metabolic syndrome (8).
The adipose tissue is a veritable endocrine organ, with the capacity to produce androgens. Furthermore, SHBG is down regulated in obese patients (7), leading to an elevated FAI at tissue level. Most importantly, obesity is associated with resistin and chemerin production, adipokines that induce insulin resistance, glucose and lipid metabolism abnormalities, and promote cutaneous immune cell infiltration (18). Insulin resistance promotes HS activity, as increased plasma insulin and insulin-like growth factor (IGF)-1 sensitize AR that control cell growth, normally inaccessible to circulating androgens (19). Carbohydrate–rich diets and whey in dairy products increase insulin levels, while casein increases IGF-1 (14, 19). Milk also contains 5α-DHT and other 5α-reduced-hormones (14), raising the exposure of folliculo pilosebaceous units to androgens.
Aromatase expression in the adipose tissue induces peripheral conversion of testosterone to estradiol. Estrogens stimulate Il-12 production and Thelper-1 responses, amplifying inflammation (20). Obesity is associated with a proinflammatory state, adding to the chronic inflammation encountered in HS. Thus, a hypoglycemic, diary free diet and maintaining/achieving a normal BMI are recommended in all HS patients.
In conclusion, the case of our patient is of particular interest given the late onset of HS. We believe that HS is a heterogeneous disease, in which the subjacent endocrine alterations vary from patient to patient. We emphasize the need for further studies on HS pathophysiology, as yet unknown predisposing factors are most probably involved in the occurrence of HS after menopause.
This would guide the way to novel therapies and better control of this challenging disease.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Calao M, Wilson JL, Spelman L, Billot L, Rubel D, Watts A, Jemec GBE. Hidradenitis Suppurativa (HS) prevalence, demographics and management pathways in Australia: A population-based cross-sectional study. PLoS One. 2018;13(7) doi: 10.1371/journal.pone.0200683. e0200683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Revuz JE, Canoui-Poitrine F, Wolkenstein P, Viallette C, Gabison G, Pouget F, Poli F, Faye O, Roujeau JC, Bonnelye G, Grob JJ, Bastuji-Garin S. Prevalence and factors associated with hidradenitis suppurativa: Results from two case-control studies. J Am Acad Dermatol. 2008;59(4):596–601. doi: 10.1016/j.jaad.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 3.Morss PC, Porter ML, Savage KT, Rosales Santillan M, Giannotti N, Kimball AB. Investigating race and gender in age at onset of hidradenitis suppurativa. J Eur Acad Dermatology Venereol. 2020;34(3):e139–e141. doi: 10.1111/jdv.16095. [DOI] [PubMed] [Google Scholar]
- 4.Kurayev A, Ashkar H, Saraiya A, Gottlieb AB. Hidradenitis Suppurativa: Review of the Pathogenesis and Treatment. J Drugs Dermatol. 2016;15(8):1017–1022. [PubMed] [Google Scholar]
- 5.Patrascu V, Picleanu AM. Verneuil’s disease clinical evolution and therapeutic aspects of four clinical cases. Dermato Venerol. 2013;58(3):165–181. [Google Scholar]
- 6.Harrison BJ, Read GF, Hughes LE. Endocrine basis for the clinical presentation of hidradenitis suppurativa. Br J Surg. 1988;75(10):972–975. doi: 10.1002/bjs.1800751011. [DOI] [PubMed] [Google Scholar]
- 7.Barth JH, Layton AM, Cunliffe WJ. Endocrine factors in pre- and postmenopausal women with hidradenitis suppurativa. Br J Dermatol. 1996;134(6):1057–1059. [PubMed] [Google Scholar]
- 8.Vekic DA, Frew J, Cains GD. Hidradenitis suppurativa, a review of pathogenesis, associations and management. Part 1. Australas J Dermatol. 2018;59(4):267–277. doi: 10.1111/ajd.12770. [DOI] [PubMed] [Google Scholar]
- 9.Prens E, Deckers I. Pathophysiology of hidradenitis suppurativa: An update. J Am Acad Dermatol. 2015;73(5 Suppl 1):S8–S11. doi: 10.1016/j.jaad.2015.07.045. [DOI] [PubMed] [Google Scholar]
- 10.Jemec GB, Hansen U. The histology of hidradenitis suppurativa. J Am Acad Dermatol. 1996;34(6):994–999. doi: 10.1016/s0190-9622(96)90277-7. [DOI] [PubMed] [Google Scholar]
- 11.Vossen ARJV, van Straalen KR, Prens EP, van der Zee HH. Menses and pregnancy affect symptoms in hidradenitis suppurativa: A cross-sectional study. J Am Acad Dermatol. 2017;76(1):155–156. doi: 10.1016/j.jaad.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Mortimer PS, Dawber RP, Gales MA, Moore RA. Mediation of hidradenitis suppurativa by androgens. Br Med J (Clin Res Ed) 1986;292(6515):245–248. doi: 10.1136/bmj.292.6515.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karagiannidis I, Nikolakis G, Sabat R, Zouboulis CC. Hidradenitis suppurativa/Acne inversa: an endocrine skin disorder? Rev Endocr Metab Disord. 2016;17(3):335–341. doi: 10.1007/s11154-016-9366-z. [DOI] [PubMed] [Google Scholar]
- 14.Kamp S, Fiehn AM, Stenderup K, Rosada C, Pakkenberg B, Kemp K, Dam TN, Jemec GBE. Hidradenitis suppurativa: a disease of the absent sebaceous gland? Sebaceous gland number and volume are significantly reduced in uninvolved hair follicles from patients with hidradenitis suppurativa. Br J Dermatol. 2011;164(5):1017–1022. doi: 10.1111/j.1365-2133.2011.10224.x. [DOI] [PubMed] [Google Scholar]
- 15.Barth JH, Kealey T. Androgen metabolism by isolated human axillary apocrine glands in hidradenitis suppurativa. Br J Dermatol. 1991;125(4):304–308. doi: 10.1111/j.1365-2133.1991.tb14162.x. [DOI] [PubMed] [Google Scholar]
- 16.Buimer MG, Wobbes T, Klinkenbijl JHG, Reijnen MMPJ, Blokx WAM. Immunohistochemical analysis of steroid hormone receptors in hidradenitis suppurativa. Am J Dermatopathol. 2015;37(2):129–132. doi: 10.1097/DAD.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 17.Zouboulis CC, Desai N, Emtestam L, Hunger RE, Ioannides D, Juhasz I, Lapins J, Matusiak L, Prens EP, Revuz J, SchneiderBurrus S, Szepietowski JC, van der Zee HH, Jemec GBE. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29(4):619–644. doi: 10.1111/jdv.12966. [DOI] [PubMed] [Google Scholar]
- 18.Parolini S, Santoro A, Marcenaro E, Luini W, Massardi L, Facchetti F, Communi D, Parmentier M, Majorana A, Sironi M, Tabellini G, Moretta A, Sozzani S. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood. 2007;109(9):3625–3632. doi: 10.1182/blood-2006-08-038844. [DOI] [PubMed] [Google Scholar]
- 19.Melnik BC, Zouboulis CC. Potential role of FoxO1 and mTORC1 in the pathogenesis of Western diet-induced acne. Exp Dermatol. 2013;22(5):311–315. doi: 10.1111/exd.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolk K, Warszawska K, Hoeflich C, Witte E, Schneider-Burrus S, Witte K, Kunz S, Buss A, Roewert HJ, Krause M, Lukowsky A, Volk HD, Sterry W, Sabat R. Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol. 2011;186(2):1228–1239. doi: 10.4049/jimmunol.0903907. [DOI] [PubMed] [Google Scholar]


