Abstract
Nerve guidance conduits (NGCs) have attracted much attention due to their great necessity and applicability in clinical use for the peripheral nerve repair. Great efforts in recent years have been devoted to the development of high-performance NGCs using various materials and strategies. The present review provides a comprehensive overview of progress in the material innovation, structural design, advanced engineering technologies and multi functionalization of state-of-the-art nerve guidance conduits NGCs. Abundant advanced engineering technologies including extrusion-based system, laser-based system, and novel textile forming techniques in terms of weaving, knitting, braiding, and electrospinning techniques were also analyzed in detail. Findings arising from this review indicate that the structural mimetic NGCs combined with natural and synthetic materials using advanced manufacturing technologies can make full use of their complementary advantages, acquiring better biomechanical properties, chemical stability and biocompatibility. Finally, the existing challenges and future opportunities of NGCs were put forward aiming for further research and applications of NGCs.
Keywords: Nerve guidance conduits, Micro-nanofibers, Multi-functional, Surface modification, Microenvironment
Graphical abstract
Highlights
-
•
Advances on materials, structure, multi functionalization, advanced technologies of state-of-the-art NGCs were reviewed.
-
•
Abundant advanced technologies such as extrusion-based, laser-based, and textile techniques were discussed.
-
•
Biomimetic NGCs combined with natural and synthetic materials can make full use of their complementary advantages.
-
•
Challenges and future opportunities of NGCs were discussed, aiming for further research and applications of NGCs.
1. Introduction
Peripheral nerve injuries are commonly occurred as clinical symptoms and constitute 2–5% of all trauma cases [[1], [2], [3]]. Many reasons like traffic accidents, fire, and natural disasters can cause varying degrees of damage to human's peripheral nerve which leads to traumatic or nontraumatic events [4], poor sensation, and paralysis [[5], [6], [7], [8]]. To treat these symptoms, autografts and allografts are frequently used in clinical [[9], [10], [11], [12]]. However, there are two primary limitations regarding the stated treatments respectively, i.e. autografts may cause secondary damage to the human body [13,14], while allografts have the risk of leading to immune rejection symptoms, unmatching nerve length and thickness, as well as the paucity of implantable nerve backup [10,[15], [16], [17], [18], [19]]. Alternatively, much research work in recent years has attempted to develop nerve guidance conduits (NGCs), aiming at making up for the abovementioned shortcomings of autografts and allografts as well as trying to reach the “gold standard” set up by those two major clinical therapies [20,21].
Several requirements are essential to NGCs such as appropriate biocompatibility and degradation rate [[22], [23], [24], [25]], as they allow the NGCs to provide the nerve axons with enough time to grow along the NGCs. On the other hand, they need to have adequate mechanical properties such as tensile strength, ability to resist compression, and ideal elasticity to guarantee the physical space over the two ends of the broken nerve [26]. More importantly, during the regeneration process, the distal tip will expand to form growth cones to explore and detect growth and guidance cues within the surrounding microenvironment. The adhesion and attachment of growth cone to a solid surface is necessary for axon growth. Therefore, developing NGCs with suitable guidance cues is quite strategic in nerve regeneration [23,[27], [28], [29], [30]]. To develop NGCs qualified with the requirements stated above, many efforts have been made [31,32]. However, it is still challenging to develop a NGC matching the “gold standard” satisfactorily [20,33,34].
Herein, this paper reviews the recent advances of NGCs regarding their structures and formation approaches based on different sorts of materials correspondingly. Rapid prototyping (RP) fabrication technologies and textile technologies as two major techniques used to fabricate NGCs were introduced. RP technology includes extrusion-based printing system, inkjet printing system, and laser-based printing system, most of which belong to the so-called “scaffold-based” bioprinting system. The “scaffold-free” bioprinting system which aims at making use of substances directly derived from cells was illustrated. Textile technologies, including woven, knitting, braiding, and electrospinning techniques, were also reviewed due to their advantages such as high efficiency and flexibility. We additionally analyze the functionalization strategies of NGCs including the establishment of an electroactive platform, the coating of graphene conductive substrates, and the embedment of photocatalytic particles. Based on these, the existing challenges and further prospective for NGCs are put forward aiming at implementing NGCs in real applications.
2. Materials choice and structural optimization for NGCs
2.1. Natural materials and related structural design
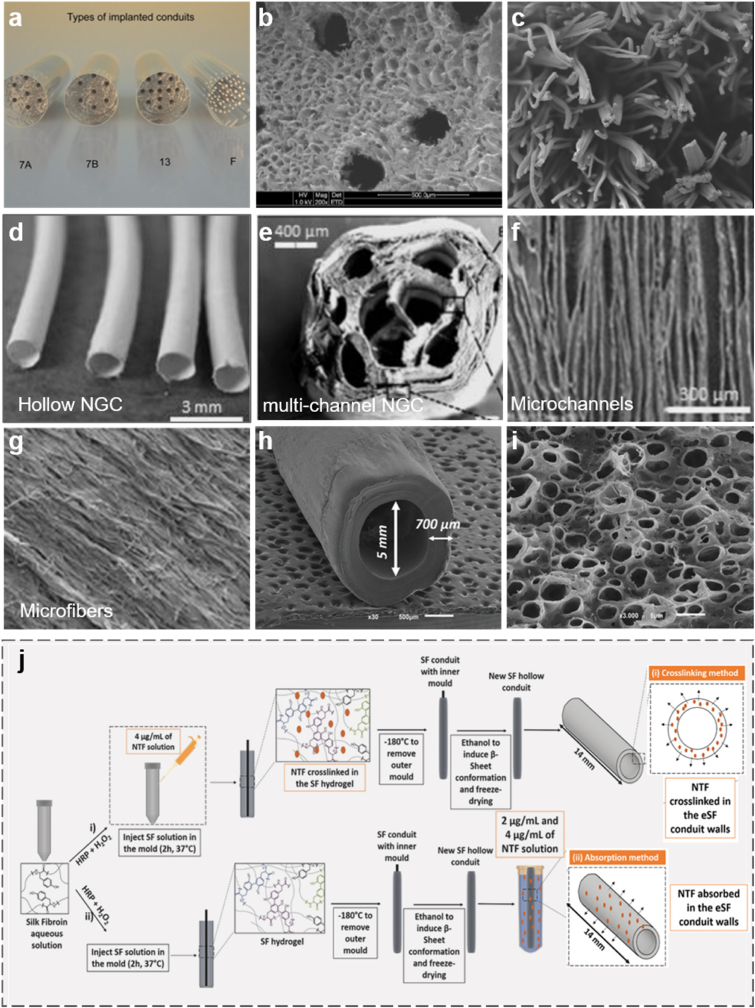
The materials for NGCs mainly involve natural materials, nondegradable synthetic materials, and biodegradable synthetic materials [17,35,36]. Natural materials include biological tissues, such as vascular and musculoskeletal tissues [37], and biomacromolecules, such as chitosan, silk fibroin, collagen, alginate, gelatin, and hyaluronic [[38], [39], [40]]. Composed of repeating glucosamine and N-acetyl glucosamine groups linked with well-sourced β-1, 4 glycoside bond in nature [41], chitosan is popular in biological engineering for its extraordinary properties including sufficient biodegradability, biocompatibility, neglectable toxicity, and suitable biological absorptivity. It attracts much attention in developing bioabsorbable NGCs [42,43]. Chitosan and its composites such as chitosan/chitin and chitosan/polyglycolic acid were utilized to produce artificial nerve grafts in various forms and specifications, and these samples have been demonstrated to have effective repairability by transplanting them into the bodies of rats, rabbits, dogs and monkeys [44,45]. The results also showed that chitosan is beneficial in guiding the orientation of (Schwann cells) SCs and preserving the survival and differentiation of neuronal cells [46]. Chitosan can be blended with silk fibroin (SF) to produce film conduits pre-seeded with adipose-derived stem cells, and there is a significant enhancement of nerve conductivity and functional recovery of nerve axon [47]. Adam et al. demonstrated that conduits consisting of chitosan multifilament yarn or 13-channel microcrystalline chitosan sponge (Fig. 1a) allow the nerve to outgrow through the gap with minor autotomy as well as minor inflammatory reactions [48]. The scanning electron microscopy images of the two types of conduits in Fig. 1b and c present that there are channels and gaps between the fibers caused by microcrystalline chitosan sponge, which is beneficial to facilitate the adhesion and proliferation of neural cells inside this conduit.
Fig. 1.
(a) Four groups of multi-channel conduits with different internal structures: Group 7A and 7B refer to PLGA-based conduits containing microcrystalline chitosan sponge core with 7 channels, the differences of the two groups lie in the molecular weight and pH value of the chitosan solution; Group 13 represents PLGA conduit containing microcrystalline chitosan sponge core with 13 channels; Group F refers to PLGA-based conduit containing chitosan multifilaments. (b) Scanning electron micrograph of the sponge core of 13-channel PLGA conduit. (c) Scanning electron micrograph of PLGA conduit containing chitosan multifilaments (scale bar: 500 μm). (a–c) Reproduced with permission [48]. Copyright 2021, Elsevier. (d) Images of hollow NGC. Reproduced with permission [51]. Copyright 2021, Elsevier. (e) Cross-section diagram of multi-channel NGC. (f) Micrograph of nano fibers on the wall. (g) Transverse-section of nerve guide with aligned nanofibers (scale bar 10 μm). (e, f) Reproduced with permission [54]. Copyright 2021, Elsevier. (h) Scanning electron microscope (SEM) image of NGC produced by the crosslinking method. (i) SEM image of NGC produced by adsorption method. (j) Schematic represents two types of methodologies (crosslinking and adsorption) to produce NGF-loaded SF NGCs. (h–j) Reproduced with permission [53]. Copyright 2021, John Wiley and Sons.
Naturally spun silk from B. mori silkworm has impressive properties such as luster strength, wear-resistance, and anti-wrinkle properties, but photo-induced denaturation and poor biocompatibility of sericin on the surface limits its further applications [49]. One common solution is to produce regenerated silk through physical, chemical, and even genetic treatments. (Silk fibroin) SF, as the main component of the regenerated silk fiber, has a wide range of functional advantages, including sufficient mechanical properties, feasible processability in mild condition, controllable structural design, appropriate degradability, suitable chemical and thermal stability, as well as effectivity in promoting neuronal outgrowth. It has been increasingly recognized as a prospective material for surgical sutures, arterial grafts and heart valves, etc [[50], [51], [52]]. SF is also very attractive for the construction of NGCs, which was intensively reported in previous studies. For example, a SF conduit successfully fabricated with lumens filled with oriented SF filaments was adopted to bridge a 10 mm rat sciatic nerve gap [51]. Observations showed that apart from the negligible systemic inflammation, there were few differences between the SF-based NGC group and the nerve autograft group in terms of the studies of sensory and ventral motor neurons; nevertheless, the SF-based NGC group was witnessed less reversal of muscle atrophy caused by denervation. Another study reported that the SF-based NGCs were fabricated into multi-channeled NGCs (Fig. 1d and e) based on the principles that some channels in the construct's luminal space can help reducing axonal dispersion. But one disadvantage of this structure is that the cell-material interactions are not good enough to enhance axonal pathfinding and SCs migration, but this problem can be improved by building microscale and nanoscale modifications of the luminal architecture in NGCs (Fig. 1f and g) [54].
What's more, one recently published work reported that enzymatically crosslinked SF (eSF) conduits can be developed into a bioactive protein delivery system in aqueous media [52], which means that functional neurotrophic factors (NTF), such as nerve growth factors (NGF) and glial-cell line derived neurotrophic factor (GDNF), can be released from the conduit with a certain profile. Fig. 1j illustrates the step-by-step processes of crosslinking and adsorption methods to produce two types of conduits. The primary difference lies in the way NTF adhere to the conduits. For the first method, NTF are crosslinked in the SF hydrogel, while in the second one, NTF are absorbed in the eSF wall. The morphologies of the two conduits in crosslinked (Fig. 1h) and absorbed (Fig. 1i) NTF show that the conduit wall produced in the first method was smoother than that in another method. In Fig. l j, the porous conduit wall and high surface to volume ratio indicate that the NTF can be retained sufficiently via the second approach. Both NTF and GDNF were examined as functional biological factors and the results reveal that conduits absorbed GDNF with a better release profile and possess the ability to acquire more robust proximal nerve protection. This is because GDNF cannot only promote neurite elongation, as the NGF does, but they can also increase neurite density and improve sensory reinnervation [53]. Therefore, as a versatile biomaterial, SF works as an important substrate no matter it is applied alone or combined with other types of substrates or grow factors.
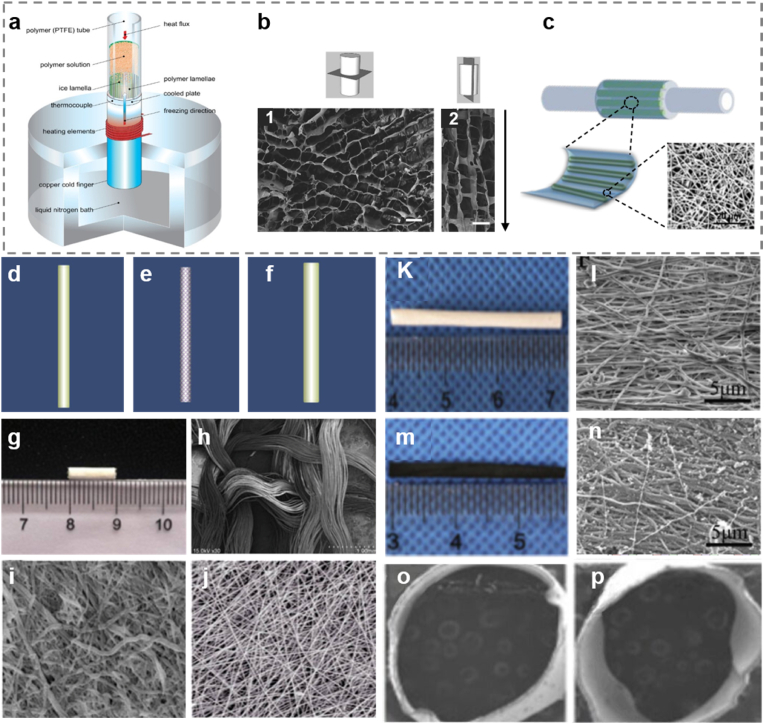
Other natural materials like alginate, collagen, and gelatin have also been investigated widely. Alginate NGCs have the same structure as the connective tissue which can provide a suitable microenvironment for stimulating the regeneration of defected distal and proximal segments [22]. The key benefit of this material is that it has the potential in supporting nerve repair. Besides, the appropriate degradation rate of alginate can provide enough space for axonal sprouting. One NGC made with chitosan and alginate reported previously was fabricated by freeze casting approach (Fig. 2a and b) [54,55]. Fig. 2b1 shows the cross section and Fig. 2b2 illustrates the longitudinal section of the chitosan/alginate NGC. It shows that the sizes of the holes on the spongy conduit are quite even, the black arrow indicates to the oriented growth path of neural cells. Such NGCs show promising characteristics because they are able to support not only neuronal attachment but also neurite axial growth. NGCs made with highly permeable collagen is capable of interacting with surrounding tissue, which is beneficial for enhancing the regeneration rate of the defected nerve fiber [55]. For example, Jin et al. developed a NGC with oriented hydrogel patterns acting as preferable guiding paths through 3D printing on PLCL electrospun nanofiber membrane (Fig. 2c). The work concluded that the NGC containing aligned collagen hydrogel is favorable for peripheral nerve regeneration [56]. Even though gelatin has a series of bio functional properties such as good biocompatibility and biodegradability, cost effectivity and low immunogenicity, this type of NGCs may lead to the collapse of conduits after implantation [22,57], and this issue needs to be improved in the future.
Fig. 2.
(a) Schematic diagram of freeze casting setup of the chitosan/alginate scaffold. (b) Transverse section and longitudinal section of the scaffold made by freeze casting technique. Reproduced with permission [55]. Copyright 2021, The Royal Society (U.K.). (c) Schematic diagram of manufacturing collagen hydrogel NGC by 3D printing on electrospun nanofiber membrane. Reproduced with permission [56]. Copyright 2021, Royal Society of Chemistry. (d) The first layer of the SF/PLGA conduit made with electrospun SF membrane. (e) The second layer of the SF/PLGA conduit, in which the PLGA net around the electrospun nanofibers. (f) The third layer of the SF/PLGA conduit made of another electrospun SF membrane. (g) A macroscopic view of the conduit. (h) SEM picture of the PLGA layer. (i) The inner wall of the ESP-NGC. (j) Electrospun SF fibers (Scale bars: 1.0 mm in h, 10.0 μm in i and j) [69]. Copyright 2015, Wolters Kluwer Medknow Publications. (k) Picture of PLCL/SF NGC. (l) Scanning electron micrograph of the nanofibrous surface of PLCL/SF NGC. (m) Picture of Ppy-coated NGC. (n) Scanning electron micrograph of the nanofibrous surface of Ppy-coated NGC. (o) Cross-sectional view of PLCL/SF NGC. (p) Cross-sectional view of Ppy-coated NGC. (scale bars: 5 μm). (k–p) Reproduced with permission [71]. Copyright 2021, Elsevier.
2.2. Synthetic materials and related structural optimization
Silicone gel, as the first-generation synthetic material, was early applied in NGCs with low degradability and expansion capacity, however, it can lead to nerve compression and thus the applications were limited in the long run [58]. Its alternatives, other biodegradable synthetic polymers including polylactide (PLA), polyglycolide (PGA), polycaprolactone (PCL) as well as their copolymers, such as poly (lactic co-glycolic acid) (PLGA) are much more attractive for the fabrication of NGCs. The bioabsorbable corrugated nerve conduit based on PGA is the first porous, absorbable nerve conduits produced in laboratory [59]. Even though PGA has high tensile modulus and ideal mechanical properties, its mechanical strength can decrease dramatically within a short time on account of degradation. Its degradation products are acidic, which is not favorable within the human body [44,59,60]. Ring-opening co-polymerization method can be adopted to synthesize PLGA and the obtained PLGA was characterized by infrared spectroscopy and gel-permeation chromatography [[61], [62], [63], [64]]. Zhou et al. carried out biocompatibility test of PLGA conduits in vitro and in vivo, the results demonstrated that the extracts from PLGA are non-cytotoxic. Their work has also indicated that PLGA has good biocompatibility and the ability to promote the regeneration of injured peripheral nerves [61].
2.3. Synergistic effects of natural and synthetic materials
Comparing natural polymers with synthetic polymers, the former ones are less toxic and more biocompatible, in which some compositions can animate cellular proliferation and migration [65]. However, they usually have poor mechanical properties [66]. To solve this issue, researchers have tried to combine natural and synthetic polymers [67,68]. Sheikh et al. mixed SF and PCL to form colloidal solution to prepare nano fibers by electrospinning technique. The nano fibers produced by the mixed solution have better hygroscopicity and mechanical properties compared with the nano fibers made from pure SF [59]. In order to optimize the advantages of both natural and synthetic materials, Wang et al. developed a novel NGC made from SF and PLGA through electrospinning and weaving (ESP-NGC) [69,70]. It can be seen that the electrospun SF fibers constitute the outer and inner layers, and the PLGA fibers form the middle layer (Fig. 2d–f). The conduit has a length of ∼1 cm (Fig. 2g), the corresponding morphology of the woven layer, inner wall, and electrospun fibers are shown in Fig. 2 h-j, respectively. The microscope images of the electrospun nanofibers demonstrated that this technique can fabricate scaffold with nanoscale dimensions and high porosity, which is beneficial to provide a good biological environment for cell adhesion, growth, proliferation and transport of nutrients. The PLGA braiding net in the middle of this conduit can provide sufficient mechanical strength and luminal space for axons to grow across. Further experimental results in vivo demonstrated that the ESP-NGCs had a mild inflammatory reaction and high biocompatibility as well as ideal tensile stiffness.
Another practice using three types of materials is the poly (l-lactic acid-co-ε-caprolactone)/silk fibroin (PLCL/SF) NGCs coated by polypyrrole (Ppy). Ppy has been intensively studied due to its biocompatibility and conductivity, however, the conductive polymer of Ppy is insoluble and brittle, causing great difficulty while developing tissue scaffold by traditional technology. Electrospinning is a common method to prepare nanofibrous scaffold for tissue engineering, it is able to make Ppy coated PLCL/SF scaffold [71]. In the article, PLCL/SF NGCs without Ppy coating are the control group. Fig. 2k illustrates the image of the conduit with 3.2 cm in length, of which the round cross section and outer wall are shown in Fig. 2o and l, respectively. The outer wall with thousands of microfibers intimating the extracellular matrix is beneficial for high-density cell and tissue cultures. Fig. 2m and n presents the conduit coated with Ppy layer from two aspects: macroscopic image and surface morphology. Fig. 2p illustrates the microscopic transverse section of Ppy coated conduit. It can be seen in Fig. 2p that the cross-sectional area is not round enough and there are nanoparticles distributed on the Ppy coating in Fig. 2n which impair its surface smoothness compared with that in Fig. 2l. The thickness of the PLCL/SF layer achieved by the electrospinning technique can be well controlled by altering the time of electrospinning. Under immunofluorescence and observation with SEM and TEM, it showed that the nerve regeneration effect is similar to the autograft group, which is better than the controlling group. This result indicated that Ppy can work as an effective functionalized coating material in optimizing the guiding properties of NGCs.
3. Manufacturing and functionalization techniques for NGCs
3.1. Rapid prototyping fabrication techniques
Rapid prototyping (RP) technology is a fast fabrication method to form 3D constructs. It mainly contains extrusion-based system, inkjet printing system, and laser-based system, which have been widely used to build NGCs in tissue engineering [72,73].
3.1.1. Extrusion-based printing system
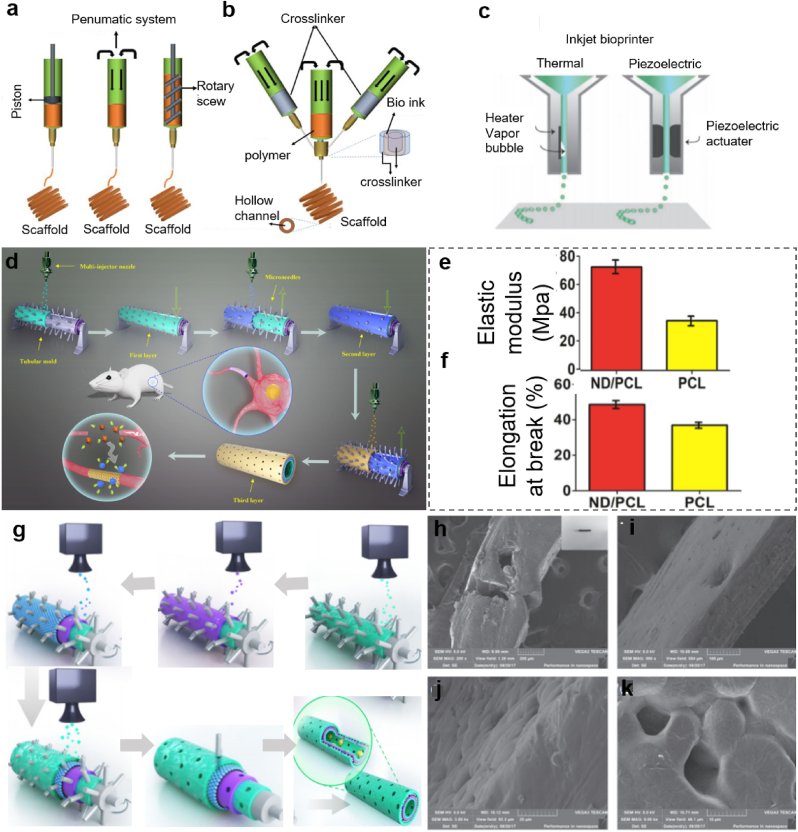
The extrusion-based bioprinting technique combines two processes: extrusion and bioprinting. They are controlled by a fluid-dispensing system and 3D spatial automatic movement controlling system, respectively [74]. Using either pneumatic or mechanical (including piston or screw) dispensers (Fig. 3a), the extrusion printing system can dispense ink to form the required object. Particularly, 3D bioprinting technique is one of the most widely researched techniques to fabricate NGCs by taking advantage of the ability to deposit high cell densities, and to precisely control the constructs in three dimensions. Moreover, the nozzle size and shape design are flexible, which facilitate the improvement of the performance of NGCs during construction [74]. The coaxial nozzle system is a more advanced 3D bioprinter based on the extrusion-based bioprinting technique, making the printing process more favorable for NGC fabrication. Yu et al. introduced a coaxial nozzle method where two different fluids of polymer flow through the tubes, and then they combined to produce uniform filaments [74]. Gao et al. improved the printing process by motorizing x, y stages with the coaxial nozzle to form hollow filament at a specific location [75].
Fig. 3.
Schematic of two different 3D bio-printing methods and major functions. (a) Extrusion-based 3D printing set-up. (b) Coaxial nozzle set-up for 3D printing. (a, b) Reproduced with permission [74]. Copyright 2021, John Wiley and Sons. (c) Inkjet-printing set-up. (c) Reproduced with permission [82]. Copyright 2021, John Wiley and Sons. (d) Schematic introduction on the manufacturing process of the nano diamond/polycaprolactone (ND/PCL) NGC and its in vivo performance. (e) Elasticity evaluation of ND/PCL and PCL NGCs. (f) The average elongation at break of ND/PCL and PCL NGCs. (d–f) Reproduced with permission [84]. Copyright 2019, Springer Nature. (g) Illustration of the layer-by-layer casting method of the graphene NGC. (h–k) SEM images of the nano porous and the multi-layered 3D structure of the graphene NGC. (g–k) Reproduced with permission [85]. Copyright 2018, Springer Nature.
Fig. 3b shows an extrusion-based printer composed of a small nozzle and an automatic robotic mechanism responsible for dispensing the biomaterial solution and controlling the motion in x, y and z directions [76,77]. With high precision and flexibility, the printer is superior for the fabrication of performance controllable NGCs. By increasing the amounts of nozzles, the bioprinters with multiple nozzles can be used to dispense different materials, both of which work as separate print heads or with coaxial nozzles [74]. During the printing process, the flowability of the biomaterial solution, especially the viscosity, plays an important role in deciding the accurate position of the scaffold [[78], [79], [80]]. Low viscosity materials are proven to be more qualified for maintaining cell viability during and after printing, but materials with higher viscosity can ensure better support and fidelity required during the printing process. Therefore, for extrusion-based printing system, it is crucial to figure out a critical point of viscosity of the biomaterial solutions [71].
3.1.2. Inkjet printing system
Inkjet printing system is able to deposit tiny droplets of polymer solution along the x, y and z axes in a highly controlled manner to repeatedly produce patterns on substrates (Fig. 3c) [[81], [82]]. It contains two types of working principles: thermal and piezoelectric inkjet bioprinters (Fig. 3c). The bio-ink should maintain in liquid form so that it can immediately solidify to form droplets upon being deposited into the 3D structure. The deposition volume of this printing method is rather small compared with the other two types of the printing system, and therefore, it enables to achieve a better printing resolution. However, the limited upper viscosity of bio-ink is one concern regarding this technique because it requires the materials squeezed from the nozzles to be in a form of droplets. This problem leads to other drawbacks such as unreliable cell encapsulation and frequent clogging of nozzles. Currently, different approaches such as using a neutrally buoyant suspension have been reported to result in a much more uniform distribution of cells and the elimination of clogging in the cell-laden bio-ink, which can make this technique more versatile in practice [83].
Inkjet printing machines can be properly adjusted to fit the needs of NGCs construction so as to improve the cell-biomaterial interactions. For example, Qian et al. innovatively constructed a new conduit structure to fabricate microporous nano diamonds/polycaprolactone (ND/PCL) nerve conduit through the methods of multilayered spraying and gradient dotting [84]. The schematic of the manufacturing process in Fig. 3c shows that the printer contains a multi-injector nozzle, which is different from the original inkjet printing machine. Besides, the receiver is a needle-contained rotating tubular in order to make the tubular structure with multi pores conveniently. This tubular mold is to receive ND/PCL suspension sprayed from the vertical multi-injector nozzle, and thus, the first layer is formed on the tubular mold. After that, the second layer can be sprayed on the first layer with staggered microspores. Unlike conventional overlapped pores, staggered microspores are able to prevent fibroblasts to grow inside and do not impede the process of nutrient exchange. The outermost layer is constructed similarly by spraying suspension onto the two layers below (Fig. 3d). Appropriate flexibility and receptivity can be achieved by this concentric three-layer structure. The average elastic modulus of the ND/PCL NGCs can reach approximately 70 MPa, which is almost two times as strong as that of the PCL control group. The elongation at break of the ND/PCL NGCs can reach about 49%, and this value is also higher than that of the control group. Because of the great biocompatibility and excellent neural maintenance, nerve cells seeded on ND-coated sheets performed similarly to those of cells cultured on protein-coated biomaterials in terms of cell attachment, neurite outgrowth, excitability as well as functional electrical activity. Besides, the images of regenerated sciatic nerve morphology on ND/PCL NGCs, PCL NGCs, and autograft confirmed that ND/PCL NGCs have the potential to promote nerve repair. The axon regrowth and remyelination results within ND/PCL also supported the facts depicted above.
Qian et al. reported a method to improve cell adhesion of NGCs by using polydopamine (PDA), arginylglycylaspartic acid (RGD), and graphene via dot spraying 3D bio-printing technology [85]. In this printing system, the spray nozzle contains several injectors to accelerate the printing speed which is different from the inkjet printing, because the latter method requires a more accurate printing result and therefore, it has to strictly control the size, viscosity and position of the materials squeezed from the nozzle. The fabrication process can be seen in Fig. 3g. The outer and inner green layers are made of the mixture of PDA and RGD; the graphene/PCL mixed layer is represented by the single purple layer; the blue layer is another graphene/PCL mixed layer. The morphology of this nano porous and multilayered 3D tubular structure in Fig. 3h–k shows that there are tiny bulges on the surface of the conduit, providing a suitable cultivation platform for SCs. The diameters of micro porous on conduit wall are rather even because they are formed by microneedles in Fig. 3g with the same diameter sizes and the 3D bioprinter. These porous pores are important for exchanges of nutrients and oxygen as well as cell proliferation. The successful axonal regrowth and remyelination implicate that this structure has potentials in clinical use to promote peripheral nerve regeneration. The key innovation of this work lies in the nano porous structure fabricated by the novel dot spraying printing method, as well as the graphene-based material to improve electric conductivity.
3.1.3. Laser-based printing system
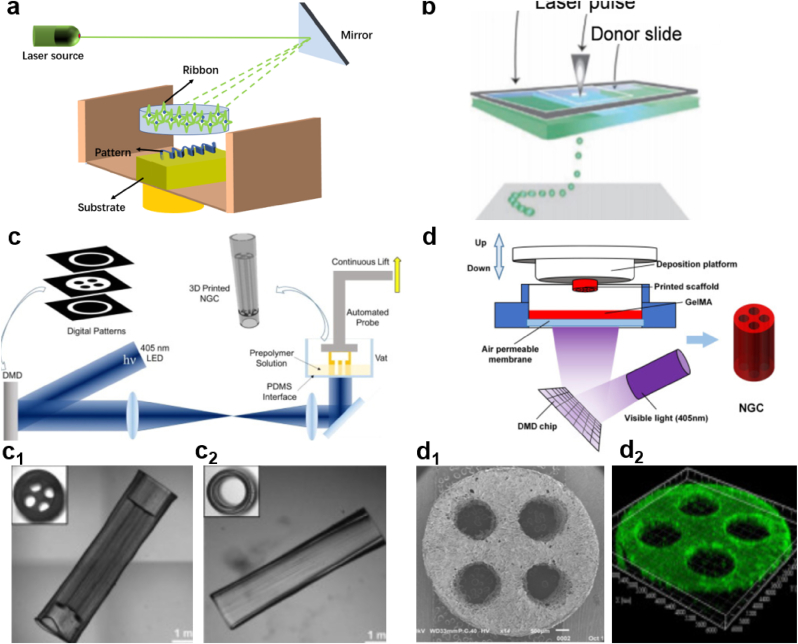
Laser-based printing technique using laser energy to transfer prepared liquid from biomaterials to substrates is widely applied for the fabrication of tissue constructs or high precision patterning of biologics (Fig. 4a) [86,87]. Some studies also explored this printing technique to develop single and multi-lumen NGCs using stereolithography (SLA) to achieve complex NGC structure. This is realized by photopolymerizing individual points with each layer [88,89]. Table 1 summarizes the properties and characteristics of the three types of 3D printing techniques introduced above. Another 3D bioprinting system based on laser is called laser-induced forward transfer (LIFT). This is a 2D/3D bioprinting technique that has been successfully implemented for biological materials, like peptides, DNA and cells, and has been utilized in the fabrication of NGCs for nerve tissue regeneration [90,91]. This technology makes use of a donor slide and two layers: a laser energy absorbing layer and a cell-containing bio-ink layer. On the absorbing layer, the laser pulse can cause local evaporation and thus propel the bio-ink toward the slide of collector (Fig. 4b) [92]. Since LIFT is a nozzle-free technique, nozzle clogging and shear-induced cell damage can be well avoided. However, this method is challenged by its long material preparation time, contaminations of a small amount of metallic parts, damages to cells caused by exposure to laser and expensive cost [93].
Fig. 4.
(a) Diagram of laser-based printing system. Reproduced with permission [86]. Copyright 2021, John Wiley and Sons. (b) Schematic of a laser-induced transfer printing system. Reproduced with permission [92]. Copyright 2021, John Wiley and Sons. (c) Schematic diagram illustrating the fabrication process of the customized NGC by using a rapid continuous 3D printing system. (c1-c2) The real figures of the 3D printed NGCs with one and four channels, respectively. (c, c1 and c2) Reproduced with permission [94]. Copyright 2021, Elsevier. (d) Schematic presenting the DLP-based manufacturing process of NGC made of GelMA. (d1) Confocal image of live/dead cell staining on the GelMA NGC. (d2) SEM image of the transverse section of the four-channel NGC. (d, d1 and d2) Reproduced with permission [95]. Copyright 1969, Elsevier.
Table 1.
Comparison of the extrusion-based and laser-based 3D bioprinting techniques.
| Parameters | Extrusion-based | Inkjet printing | Laser-based | Refs |
|---|---|---|---|---|
| Part 1 Parameters of 3D bioprinting techniques | ||||
| Scaffold/non-scaffold base | Scaffold-based and scaffold-free | Scaffold-based | Scaffold-based | |
| Printing resolution (μm) | 200 | 200 | Cell size | [80,83] |
| Printing homogeneity | uniform | Non-uniform | Non-uniform | [73,76] |
| Printing cost | low | Medium | High | [74,76] |
| Printing speed | Slow (10–50 mm/s) | Fast (1–10000 droplets per second) | Medium (200–1600 mm/s) | [76,80] |
| Part 2 Parameter of bio-ink | ||||
| Cell density | High,cell-only bio-ink | Low | High | [76,80] |
| Material choices for bio-ink | Cell-free, cell-laden, cell-only bio-ink | Cell-free, cell-laden bio-ink | Cell-laden bio-ink | [76,80] |
| Part 3 Advantages and disadvantages of bioprinting methods | ||||
| Advantages | High cell density, spatial controllability, capable to support scaffold-free printing | High speed, medium resolution and cost | No clogging issue | [76,80] |
| Disadvantages | Moderate speed, clogging issue | Low upper viscosity of bio-ink, clogging issue, low cell concentration, ununiform droplet size | Difficult to target cell position, high cost, low cell viability, un-uniform size of ink droplet | |
Because of limitations of laser-based printing systems, another laser-based printing system, digital light processing (DLP), has attracted great attention. This system is similar but superior to SLA. The machine contains a digital micro-mirror device (DMD) which promotes rapid crosslinking in a complete layer instead of a single dot in SLA [94]. One study reported the fabrication of four-lumen NGCs by this rapid continuous 3D printing method (Fig. 4c): the 405 nm-length LED light resource irradiates on the DMD containing specific patterns, can photopolymerize on the polymer solution. The DMD device can be controlled by user-defined computer-aided design (CAD) to produce customized NGCs models. Fig. 4c1 and 4c2 show two types of physical conduits with four channels and one channel fabricated by this method. The manufacturing process is able to produce a precise and complex nerve guidance scaffold in a short period of time, which was significantly more advantageous than the previous SLA printing technology. What's more, the printed NGCs are capable of guiding peripheral nerve regeneration in a specific direction with good recovery of motor and sensory function.
Inspired by this 3D bioprinting technique, another group of researchers introduced a gelatin methacrylate-based (GelMA) NGCs with multiple channels supported by the DLP-based 3D printing system (Fig. 4d) [95]. The printing system is similar to the one shown in Fig. 4c. The difference is that the materials used in the latter one are pure GelMA with optimized printing parameters instead of the combination of GelMA and polyethylene glycol diacrylate (PEGDA). Fig. 4d1 shows the SEM image of the flat and smooth transverse section of multi-channel NGC printed by the DLP system. The image of the NGC presents a dense skin of the conduit wall with uniformly interconnected micropores. Such skin is beneficial to support cell adhesion and facilitate nutrients exchange, indicating the great potential of the NGCs in generating peripheral nerve recovery. In addition to that, the image in Fig. 4d2 also demonstrated that the fabricated conduits can not only support the proliferation as well as migration of PC12 cells but also stimulate the neural crest stem cells to grow into neurons.
3.2. Scaffold-free bio-printing system
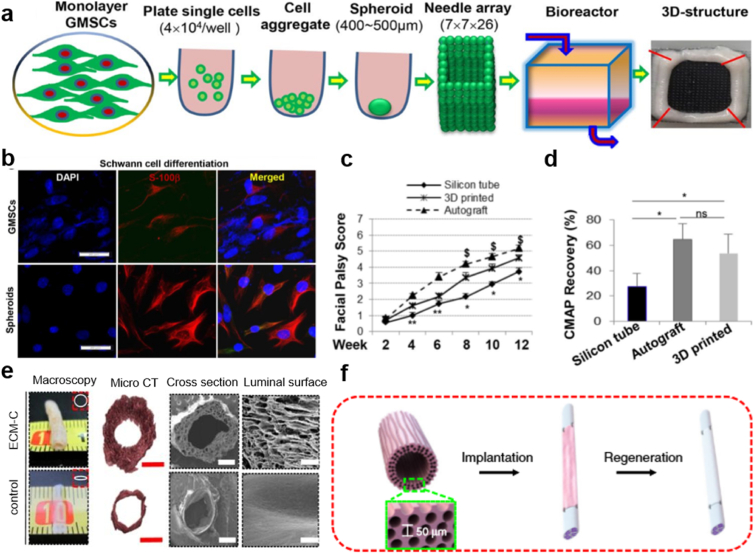
The above-mentioned bioprinting techniques belong to the “scaffold-based” bioprinting system because they make use of biomaterials (such as hydrogel) with or without living cells (in this situation, the cells are distributed on top of the material as biological guiding cues). Therefore, it is easy to figure out that the cell density is rather small because of the limitation of other biomaterials [[96], [97], [98]]. While compared to the scaffold-based system, the scaffold-free bioprinting system can achieve higher cell density because it is realized by biological self-assembly, an additive manufacturing strategy, aiming at using materials directly derived from tissue such as cell spheroids, extracellular matrix or tissue fusion and mini tissues as building blocks [[99], [100], [101], [102]]. This method is considered to be promising due to its extraordinary biocompatibility and favorable immune responses as a result of improved cell-to-cell interactions and better biomimicry [[103], [104], [105], [106]]. Zhang et al. explored a scaffold-free 3D bio-printed technique with human gingiva-derived mesenchymal stem cells (GMSCs) [107]. The primary principle of this approach is self-assembly/self-organization of multiple cell spheroids derived from GMSCs, which are used as building blocks in the fabrication of NGCs. The formation of this 3D structure was achieved by assembling GMSCs spheroids into a 9 × 9 mm square needle-array under the instruction of software-designed configuration (Fig. 5a). Fig. 5b illustrates the differentiation of cells dissociated from both MSC spheroids and their adherent counterpart. It demonstrated that GMSC spheroids provide cells with a better condition to differentiate into Schwann-like cells. The clinical outcome shown in Fig. 5c and d represents that the facial palsy score and the compound muscle action potential of the 3D printed tubular are higher than the silicon tube control group, indicating an enhanced biological function compared with the controlling group in the in vivo test. In addition, the scaffold-free bioprinting system has little damage to the cells caused by large sheer forces in scaffold-based bioprinting system and the biological properties, while generative capabilities are significantly improved. However, the most urgent challenge is to find an alternative and easily accessible source of stem cells to fabricate bioengineered nerve tissues. To solve this problem, studies have attempted to utilize numerous adult tissues including the oral and craniofacial tissues to confer neural crest stem-like cells properties [108,109]. It was demonstrated that human normal dermal fibroblasts can be successfully generated into spheroids and developed into bio 3D conduits to regenerate peripheral nerve in a rat sciatic nerve model [110].
Fig. 5.
(a) Schematic of the fabrication procedures for 3D bioprinting scaffold-free NGCs from GMSC spheroids. (b) Seeded onto poly-d-lysine precoated 4-well chamber slides, the GMSC spheroid cells (spheroids) and the adherent counterparts (GMSCs) were cultured under Schwann cell differentiation conditions for 14 days. (c) Postoperative facial palsy scores at different time points. (d) The recordings of the vibrissa muscles through compound muscle action potential (CMAP). (a–d) Reproduced with permission [107]. Copyright 2018, Springer Nature. (e) Schematic steps of implanting ECM-C scaffolds. (f) Macroscopic appearances and microstructures of ECM NGCs, indicating the shape of the scaffolds. (e, f) Reproduced with permission [112]. Copyright 2019, Springer Nature.
Apart from stem cells used as building blocks, extracellular matrix (ECM) also plays a special but nonnegligible role in scaffold-free tissue engineering. Therefore, abundant efforts have shifted to develop ECM scaffolds by decellularizing the cultured cells or native tissues [111]. Derived from the cultured cells or native tissues, ECM scaffolds are compatible with human tissues and can trigger ideal immune responses with superior biocompatibility. Based on this concept, Zhu et al. chose extracellular matrix as the main material for building NGCs [112]. The lack of hierarchical porous structure generates the idea of making parallel microchannels on ECM scaffold to guide the directional migration and spatial organization of cells. Fig. 5e shows the appearances of the scaffold and its counterpart made of solid silicon. It is quite apparent that the luminal wall of the scaffold is far thicker and rougher than that in the control group, indicating a better guiding and supporting effect of the experimental group. Fig. 5f illustrates the formation process of the scaffold: firstly, design and fabricate sacrificial templates with aligned polymeric microfibers; then, implant the templates into rat subcutaneous pockets for cellularization and tissue formation; finally, remove the template and cellular component. The guiding property of ECM on cells was also studied in vitro test comparing to that of the control. The results showed that the longitudinally aligned microchannels (∼40 μm) offer noticeable guidance for Schwann cells. The functional recovery of nerves and alleviation of the gastrocnemius muscle atrophy have also been observed. This accounts for the synergy effect of parallel microchannels and ECM nanofibers.
3.3. Textile bioengineering technologies for NGCs
3.3.1. Woven, knitting, and braiding technologies
In addition to the newly grown-up fabrication technologies, it is not negligible to keep an eye on the advanced engineering fabrication method: biomedical textile technology, which has been used in the manufacturing process of a wide range of physiological conduits such as stent/scaffold/graft/prosthesis of intestine [113], tracheobronchial [114], esophageal [115], nerve [31], ligament [116], and blood vessel [117,118]. It is a promising technology due to its bionic surface morphology, flexible forming method, appropriate mechanical properties and lower production cost [119,120]. The superiorities of biomedical textile technologies for the fabrication of NGCs have attracted much attention during the past decades. NGCs based on biomedical textile technologies have many outstanding advantages: they are faster and easier to carry out than the 3D printing techniques; it is more practical to fabricate the conduits with the similar size as well as the shape to physiological conduits; the materials are easier to prepare, etc [121].
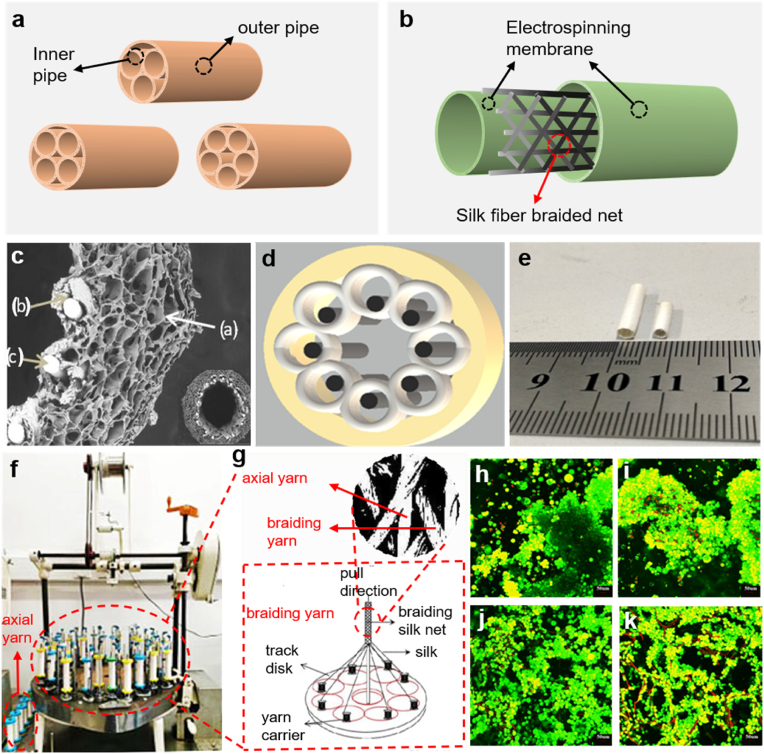
Woven, knitting and braiding technologies are three main methods in textile field used to fabricate NGCs [122]. The woven construct is achieved by interconnecting the warp and weft yarns according to different fabric structures such as plain, satin and twill, etc. Therefore, the mechanical strength and the size of pores can be tailored by changing the characteristics of yarns, the fabrication structure and the arrangement of yarns per inch [123]. In practice, the weaving technology is usually used to produce two or three-dimensional, lightweight, and flexible structures in tissue engineering such as vascular, chest wall and cartilage tissue, etc [124,125]. This is because the woven fabrics have more outstanding mechanical properties in the in-plane direction than in longitudinal and transverse direction when the fabric rolling up to form a tubular structure. But in order to improve the mechanical properties in woven conduits, the weaving looms and parameters can be designed and adjusted [126]. Knitting fabric is formed by interlacing yarns in different types of knitting loops in two-dimensional (2D) and three-dimensional (3D) to fabricate complex knitted patterns and this technology is able to provide mechanical strength in in-plane and across-plane direction [127]. Even though bio-textiles with knitted structures are widely applied in biomedical field and this technique is relatively mature [128,129], it is rather hard for the knitting machine to fabricate the conduits with a diameter between 0.8 mm and 5 mm, which limits the application of this technique. What's more, despite the stretchability of knitted construct and exquisitely controlled microstructure achieved by computer aided system, it is still difficult to create a small diameter tubular construct with adjustable properties in different directions [130]. The braiding technique allows winding a group of yarn with a certain bias along the length of the conduits so as to produce the fabric [131]. According to the thickness of braided fabrics, the braiding technology can be sorted into two kinds of techniques: 2D and 3D techniques. As for conduits fabricated by 2D braiding technique, the thickness of the conduit wall is no larger than three times of the fiber diameter, while for conduits fabricated by 3D braiding technique, the thickness of that is at least three times of the fiber diameter. Because the thickness of the conduit wall is very thin (around 0.1–0.5 mm), it is hard to achieve such a small scope through 3D braiding technique. Therefore, the 2D braiding technique is more applicable not only because of the thickness of the conduit wall it can achieve, but also the appropriate elasticity and smooth surface of conduits it can realize. Besides, the 2D technique features for its high production efficiency as well as an uncomplex mechanism [132]. Based on the braiding technique, a group of researchers developed a pipe-in-pipe conduit which is formed by 3–5 inner pipes and 1 outer pipe wrap around the inner pipes (Fig. 6a) [133]. The biomimetic structure inspired by the anatomy of the nerve fiber bundle aims to lead the direction of nerve regeneration by providing sufficient cell adhesion space. This NGC has been proved to have good mechanical properties and a gradient of degradation.
Fig. 6.
(a) Schematic diagram of NGCs with pipe-in-pipe structure [133]. (b) Schematic of NGCs with sandwich structure [13]. (c) The SEM image of the cross-section of the Mg/Silk-CS/SF NGCs. (d) Schematic illustration of Mg/Silk-CS/SF NGCs in cross-sectional direction. (e) Photographs of the Mg/Silk-CS/SF NGCs. (c–e) Reproduced with permission [134]. Copyright 2021, Elsevier. (f) Photographs of the vertical spindle braiding machine. (g) Schematic of local structural details of the braiding machine. (h–k) Live staining images of SCs after the 3-week cultivation in four nerve conduits (SF/CS-2mm, SF/CS-3mm, Mg-SF/CS-2mm, Mg-SF/CS-3mm). Reproduced with permission.
Apart from the unique pipe-in-pipe conduit, a previous study reported a NGC with sandwich structure composed of three layers: inner electrospinning membrane, middle braiding layer, and outer electrospinning membrane (Fig. 6b) [13]. The braided fiber net in the middle layer acts as a strong scaffold to improve the mechanical properties of the conduit. The two electrospinning membranes with 3D porous structures resembling extracellular matrix, are beneficial for cells to attach, migrate and proliferate [132]. It is worth noting that the fabrication density as well as the fabrication angel are two key factors in determining the mechanical properties of the braided net [13]. The fabrication parameters of such braiding NGCs have been optimized to improve the radial compression capacity as well as axial tensile strength. Further in vivo tests of the NGCs with sandwich structure showed that it possesses sufficient mechanical properties even though they are not as strong as the pipe-in-pipe structural NGCs.
Zhang et al. reported a Magnesium/Silk-Chitosan/Silk Fibroin (Mg/Silk-CS/SF) NGC which was composed of two layers: one outer sponge layer and one inner braiding layer (Fig. 6c) [134]. The innovation of this work lies in its novel braiding structure: 8.89 tex degummed silk yarn served as braiding yarn, Mg wire (0.1 mm in diameter) served as the axial yarn. The existence of Mg wire not only helps to improve the mechanical properties (such as axial tensile strength, the resistant to compression and the inner maximum pressure, etc.), but also provides a series of physiological benefits (such as participating the synthesis of proteins in central nervous system, activating various enzymes in the body and regulating neuromuscular activity). Fig. 6d represents this design in a more concise way. It presents the transverse section of the sample: the grey cylinders illustrate the Mg wire working as the axial yarn while the small white circles around the grey cylinders illustrate the braiding yarns. Fig. 6e shows the conduit samples sized of 3 mm and 2 mm in diameter respectively. Fig. 6f and g illustrate the images of a vertical spindle braiding machine. The optimized setting parameters of this machine vary according to sample diameters. The braiding silk yarn is 8.89 tex in linear density and the Mg wire is 0.1 mm in diameter. Fig. 6h–k present the proliferation behavior of SCs on four conduits with different diameters and materials. The results indicate that the four NGCs have good biocompatibility, and the cells can adhere and proliferate well on the conduit wall. However, compared to the conduits without Mg filaments, the distribution of cells on conduits containing Mg filaments is more uniform along the conduit, which is dyed in red, indicating that the Mg filaments have the ability to guide the adhesion of cells during the dissolution process.
3.3.2. Electrospinning
Electrospinning is a kind of micro/nano-techniques with the ability to endow samples with a large surface area in a small volume [135]. Fig. 7a and b display the image and schematic diagram of a typical electrospinning machine. It can produce nanofibers to mimic ECM environment and promote nerve tissue regeneration by allowing cells to proliferate in a 3D microenvironment composed of ECM components such as nanostructured collagen, laminin, fibronectin, elastin within NGCs [136,137]. The polymer solution in the injector can be squeezed out by the syringe pump at a low speed, which can be set according to polymer characteristics. The liquid on the tip of the needle can form nanofibers under an external electrostatic field between the spinneret and the collector (those two devices are placed apart for 10–20 cm in distance). The electrostatic field stretches the polymer solution into a conical droplet, and then nanofibers are developed continuously [138]. Many parameters such as electrospinning solution, the humidity of the environment, voltage, polymer feed rate, and needle-target distance are vital to the process [139,140]. Electrospinning technology is quite inclusive in terms of the choices of polymer materials, synthetic and natural polymers or copolymers are all feasible in this fabrication technique [141]. It has been demonstrated that NGCs need to maintain a balance between physical stiffness as well as cellular compatibility [142]. Compared to natural materials, most of synthetic materials possess poor hydrophilic properties and lack cell-binding sites [142]. Thus, most scaffolds contain electrospinning membrane are made of natural polymers or natural-based composites, such as collagen, cellulose, chitosan/gelatin, SF, natural polymer/PCL, natural polymer/PLA, natural polymer/PLGA, natural polymer/Poly(ethylene oxide) (PEO) and natural polymer/conductive polymers (CPs) [143].
Fig. 7.
(a) Image of the electrospinning apparatus. (b) Schematic illustration of a simplified electrospinning apparatus and simulated fabrication process of nanofibers. (a, b) Reproduced with permission [136]. Copyright 2020, Taylor & Francis. (c) Schematic illustration of preparing electrospinning membrane and NGC as well as the conduit implantation. (d) Image of the multilayered NGC. (e) Local microscopic image of the transverse section of NGC. (f) SEM image of the PCL nanofiber membrane magnified by 10.0k. (g) SEM image of the composite nanofiber membrane (with Fe3O4-MNPs) magnified by 5.0k. (h) The release profile of MLT from both single-layered and multi-layered NGCs during the 21-day assessment. (c–h) Reproduced with permission [144]. Copyright 2021, John Wiley and Sons. (i) Picture of the profiled multi-pin electrospinning setup: (1) high voltage power source (2) spinneret drive arrangement (3) profiled multi-pin spinneret (4) stationary fat collector. (5) polymer reservoir (6) Direct current (DC) high voltage cable (7) grounding cable (8) pneumatic circuit (9) profiled multi-pin magnified view. (j) Microscopic image of multi-pin electrospun nanofibers with nanoparticle distribution. (k) X Elemental mapping of zinc oxide nanoparticle encapsulated with PVA electrospun nanofiber. (i–k) Reproduced with permission [151]. Copyright 2020, Springer Nature.
Various NGCs have been successfully developed through electrospinning combined with multi functions and features. One study reported a novel NGC based on electrospinning techniques with different solutions consists of three layers. The outer layer is made from PCL solution, the middle layer contains Fe3O4 magnetic nanoparticles (Fe3O4-MNPs) in the previous PCL solution, while the inner layer solution is made up of Melatonin (MLT) instead of Fe3O4-MNPs [144]. The reason why Fe3O4-MNPs were chosen is that the magnetic nanoparticles have been reported to be able to induce the axonal extension under an external magnetic field without side effects [145,146]. Fig. 7c is a schematic diagram representing the manufacturing process of this conduit. It should be noted that the first bottle paralleled to the three bottles contains all the three contents; this is because the solution is spun in only one layer to act as a control group. Fig. 7d shows the picture of NGCs, the pores on the wall have the function of improving the permeability of the conduits. Fig. 7e is the microscope image of the transverse section of the multilayered NGCs indicating a flat and smooth cross-sectional part. Fig. 7f and g illustrate the microscope images of PCL nanofiber membrane and composite nanofiber membrane (contains Fe3O4-MNPs), respectively. The microbeads on the composite nanofibers were proven to facilitate the encapsulation and release of drug and bioactive agents. Fig. 7h shows the release profile of melatonin of multilayered NGCs compared with the single-layered NGCs. The result suggested that MLT is more effectively released, which will timely suppress the oxidative stress and inflammatory response. Combined with other assessments, the article reached the conclusion that the multilayered NGCs show promising evidence for peripheral nerve repair due to the anti-oxidative, anti-inflammatory and axonal growth-promoting properties of MLT and Fe3O4-MNPs.
In order to improve the biological functions of the electrospinning membrane used in NGCs, this technology has been modified in several ways. For example, researchers identified that Mg can support nerve regeneration. The presence of Mg slowed the rate of muscle atrophy by initially providing an electrically conductive path for transmission of nerve impulses across the gap, and thus stimulating the muscle, which then, in turn, might slow atrophy [147,148]. To apply this finding into the electrospinning technique, researchers have tried to add MgO powder at nano-scale into SF solution in a proper proportion, so as to enhance the conductivity of nanofibers. Meanwhile, the electrospinning instrument has experienced improvements based on the existing limitations. In practice, SF embedded with nanoparticles usually affects solution viscosity, surface tension and solvent volatility, which makes it difficult to spin nanofibers with the existing needle electrospinning systems. What's more, the bottlenecks such as low production rate, the demand of high voltage to form Taylor cone and needle blocking, restrict the application of the original needle electrospinning machine [149,150]. Therefore, to solve these problems, previous work reported a multi-pin high production electrospinning machine (Fig. 7i). The machine possesses 21 numbers of half-sphere shaped profiled spins on a circular disk with a spacing of approximately 15 mm each (Fig. 7i3). In order to test the function of this machine, they prepared polyvinyl alcohol (PVA) polymer solution with ZnO nanoparticles as electrospinning solution. The elemental mapping images in Fig. 7j and k demonstrate that the encapsulated nanoparticles can equally disperse into the PVA nanofiber mat throughout the fiber production process by using this novel apparatus [151]. This improvement provided a method to spin nanofibers containing nanoparticles as uniform as possible. For NGCs, previous research has also proved that nanoparticles can help to increase the mechanical properties of NGCs besides the advantages such as exceptional size, surface functionalization and chemical stability [152]. Therefore, this multi-pin high production electrospinning machine has a profound influence on the fabrication of nanofiber membrane and the application in NGCs, which deserves further research for the fabrication of high-performance NGCs [153,154].
3.4. Electroactive techniques for functionalization of NGCs
3.4.1. Wireless bioresorbable electronic system
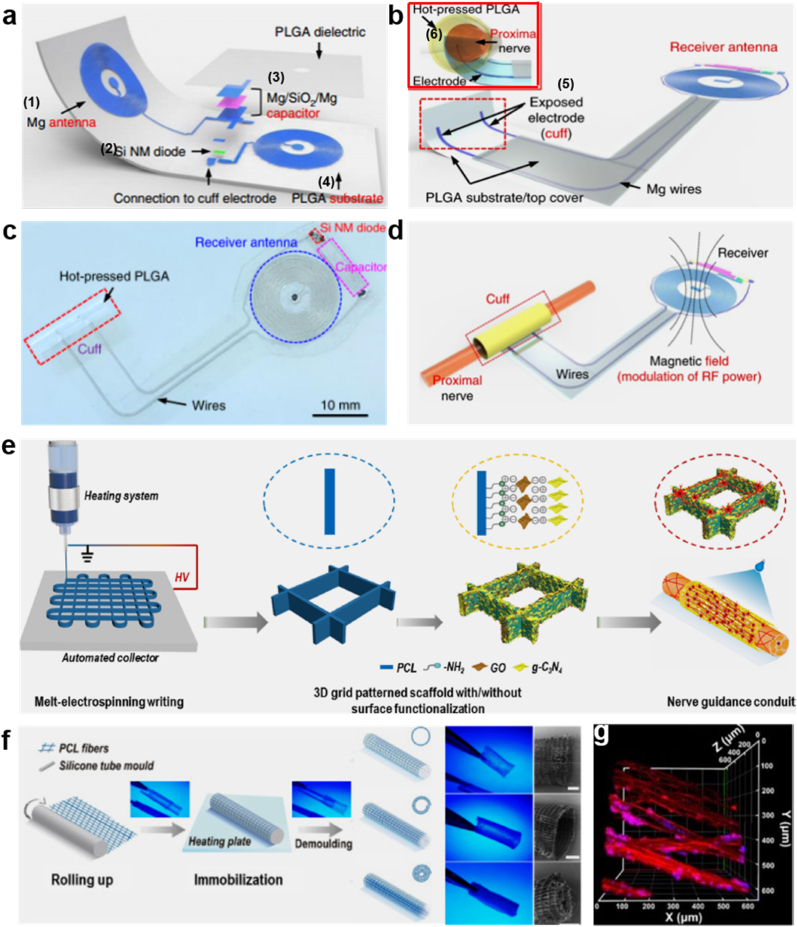
Recent studies demonstrated that electrical simulation plays an important role in fabricating the regeneration of nerve tissues. It is revealed that the electrical simulation can improve the generation and growth of nerve tissue [155]. In addition to the pharmacological approaches (like growth factors, immunosuppressants) to accelerate and enhance nerve regeneration in rodent models, direct intraoperative electrical stimulation of injured nerve tissue was also demonstrated to enhance and accelerate functional recovery [156]. It suggested a novel nonpharmacological, bioelectric form of therapy that could complement the existing surgical approaches. However, there are some limitations, i.e. the existing protocols are constrained to intraoperative use and have limited therapeutic benefits [157]. Being inspired by this direct intraoperative electrical stimulation technique, one study reported a platform for wireless and programmable electrical peripheral nerve stimulation to break the limitation. It enabled electrical stimulation of injured nerves to facilitate nerve regeneration [158]. Fig. 8a highlights the design features and key materials of this technology. The device contains a wireless receiver acting as a radio frequency power harvester includes an inductor (1), a radio frequency diode (2), a Mg/SiO2/Mg capacitor (3), and a PLGA substrate (4). If this system is folded in half, a device containing a double coil inductor can be formed accordingly. Fig. 8b presents the electrode and cuff interface for nerve stimulation. It contains metal electrodes (5) inserted in PLGA substrate. The cuff was created by rolling this system into a cylinder as shown in Fig. 8b (6). Fig. 8c is a vertical view of the complete system and Fig. 8d shows the operation process of this wireless platform.
Fig. 8.
(a) Schematic illustration of the electroactive wireless platform design. (b) Illustration of the rolled-up system acting as a cuff with electrodes interfacing with the nerve. (c) Image of the ultimately formed device. (d) Schematic illustration of the operation procedure, containing the nerve interface. (a–d) Reproduced with permission [158]. Copyright 2021, Springer Nature. (e) Schematic of 3D polymeric grid-patterned scaffolds fabricated by visible-light photocatalyst to decorate NGCs for promoting peripheral neural regeneration. (f) Scheme illustration of the construction process. Digital photos of various NGCs were taken under UV lamp irradiation and the SEM images on the right show one head of the conduits. (g) 3D reconstruction on the luminal surface of the conduit. Cells were stained with class III β-tubulin (TUJ1) (red) and Hoechst (blue). (e–g) Reproduced with permission [159]. Copyright 2021, Elsevier. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To evaluate the nerve repair effects in animal models, the reported bioresorbable nerve stimulators were implanted in a dorsolateral gluteal-muscle-splitting incision exposing the sciatic nerve. During the recovery stage, the improvement in muscle activation showed that electrical stimulation may increase the rate of axonal regeneration and muscle reinnervation. It also showed that repeated 1-h daily applications of electrical stimulation in the early stages of recovery bring advantages to improve the rate and degree of nerve regeneration as well as the recovery of muscle function compared to the existing standard of care. These findings have laid an engineering foundation for the development of bioresorbable electronic implants, which serve as a novel platform with the function of bioelectronic interventions. Moreover, this system has been widely used for various tissues and organ systems.
3.4.2. 3D tubular conductive substrate coatings
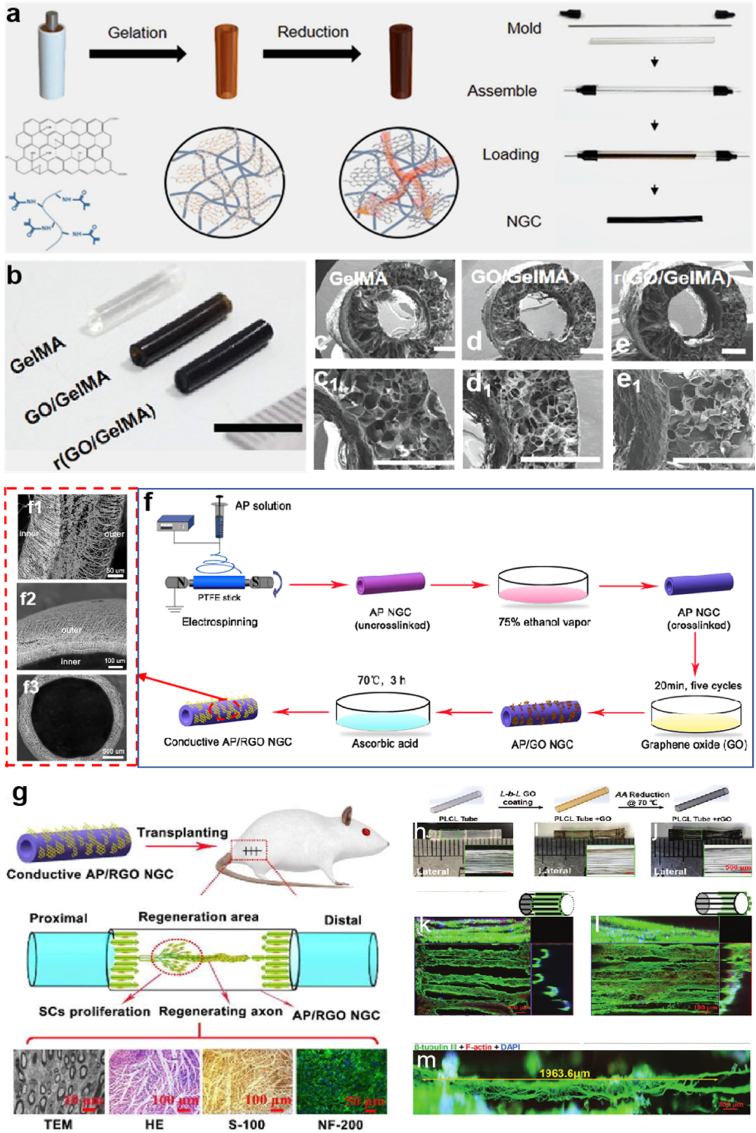
Recently, conductive carbon nanomaterials such as carbon nanotubes, graphene, and nano diamonds have been explored for biomedical applications due to their remarkable advantages such as mechanical properties and electrical conductivity [[159], [160], [161], [162], [163], [164]]. Most studies put an emphasis on graphene oxide (GO) and reduced graphene oxide (rGO) because of their intriguing not only conductivity but also biocompatibility [165,166]. The studies also demonstrated that graphene enables various cells (including neural stem cells, pluripotent stem cells and mesenchymal stem cells) to better adhere, grow, proliferate and differentiate on the surface of conductive carbon nanomaterials [[167], [168], [169]]. For example, Zhang et al. proposed a new 3D anisotropic photocatalytic nerve guidance conduits achieved by using methods of melt electro writing (MEW) (Fig. 8e) and surface functionalization [159]. This study aimed to build PCL grid patterned scaffold (Fig. 8f) decorated with graphtic carbon nitride (g-C3N4) and graphemes oxide (GO) nanosheets. Fig. 8e illustrates three primary steps of fabricating 3D polymeric grid-patterned scaffolds. In the first step, the PCL microarchitectures with anisotropic, mechanically stable grid patterns were fabricated via MEW method; then, by sequentially decorating the architectures with graphene oxide nanosheets and g-C3N4 nanoparticles, a photocatalytic scaffold was achieved. Thirdly, the photocatalytic NGC was assembled based on the microarchitectures. Fig. 8f presents each step clearly from rolling up and immobilization to demolding. The digital photos and SEM images are shown in the two right columns. MEW enables nerves to grow better along the axis at an optimized parameter, which can be adjusted freely. The visible-light photocatalysis can improve the neurite growth of nerve cells by the function of the GO and g-C3N4. PC12 cells grown on the nerve conduit were found to distribute their neurites along the polymeric backbone (Fig. 8g). Actually, this method is an alternative to the direct electrical stimulation strategy introduced in the previous example because the conduits here are functionalized by optoelectronic conversion triggered by photocatalytic systems, such as reduced graphene oxide (rGO)/Titanium dioxide. What's more, researchers fabricated conductive NGCs by polymerizing GO and gelatin-methacrylate (GelMA), and then chemically reduced them to form final r(GO/GelMA) (Fig. 9a) [170]. This type of conduits displayed low Young's modulus, good electrical conductivity, flexibility, durability, permeability, and low impedances at a wide frequency range. In vitro and in vivo studies revealed that r(GO/GelMA) can support PC12 neural cell growth and differentiation, as well as facilitate neural regrowth, myelination, and functional regeneration of nerve tissues and muscle tissues. Fig. 9b represents the macroscopic image of the NGCs made of GelMA, GO/GelMA, r(GO/GelMA). Fig. 9c-e1 illustrate the SEM images of the corresponding NGCs, respectively. Another study also introduced a strategic design of NGCs made of electrospun Antheraea pernyi SF (ApF)/(Poly(l-lactic acid-co-caprolactone)) (PLCL) and the rGO coating layer [171]. The step-by-step manufacturing process is shown in Fig. 9f, and the local microscopic images of the novel NGC are illustrated in Fig. 9f1-f3 respectively. After being implanted in vivo, the TEM image in Fig. 9g illustrates that there were no significant differences in the number of myelinated nerve fibers regenerated in the AP/RGO NGC group and the autograft group. The HE staining image shows negligible inflammation and AP/RGO NGC regenerate a great deal of nerve fibers. The S-100 and NF-200 result confirmed that the regenerated nerve recovery absolutely exist in AP/RGO NGC.
Fig. 9.
(a) Schematic diagram briefly described the fabrication of the conductive r(GO/GelMA) hydrogel conduit with an annulus mold. (b) An image showed side views of GelMA, GO/GelMA, and r(GO/GelMA) conduits with an inner diameter of 1.2 mm and wall thickness of 0.5 mm (scale bar: 10 mm). SEM images showed cross sections of the produced (c, c1) GelMA, (d, d1) GO/GelMA (e, e1) r(GO/GelMA) conduits. (a-e1) Reproduced with permission [170]. Copyright 2021, John Wiley and Sons. (f) Schematic diagram explaining the way to fabricate the conductive AP/RGONGC. (f1-f3) SEM images of the conductive AP/RGO NGC from different dimensions. (g) In vivo implantation and microscopic assessment image abstract of NGCs. (f–g) Reproduced with permission [171]. Copyright 2021, Elsevier. (h–j) Schematic illustration of 3D tube with axially oriented PLCL microfibers under GO and r-GO coating; macroscopic view of the tubular from lateral and transverse aspects. (k–m) PC12 cell neurite outgrowths on rGO-encapsulated 3D tubular under confocal microscopy. (h–m) Reproduced with permission [176]. Copyright 2021, John Wiley and Sons.
Because the dimension and shape of the micropattern have a great impact on neurogenesis, it is quite applicable to combine the physical cues and electric-conductive materials to achieve a win-win effect [[172], [173], [174], [175]]. Researchers speculated that 3D micropatterns containing graphene can favorably modulate the mobility and differentiation of neural cells under electrical stimulation (ES) and subsequently facilitating the development of a 3D neuronal-like network. Fig. 9h–j show one of the 3D tubular structures consisting of axially oriented PLCL microfibers [176]. The 3D tubes of PLCL fibers were processed to be conductive substrates by the layer-by-layer GO coating and ascorbic acid enabled rGO reduction. PC12 cells and mouse hippocampal neurons were cultured on the obtained conductive substrates for 14 days under electrical stimulation, the outgrowth of neurites was observed to follow the rGO-encapsulated tubes (Fig. 9k-m) despite some branching and random growth of neurites. It was concluded that coating GO/rGO onto the surface of 3D PLCL microfiber templates was an effective strategy to obtain conductive scaffolds with both structural complexity and high electrical conductivity, which can enable the axon to form a hierarchical neuronal-like network with the guidance from conductive substrates. What makes this functionalization idea special is that this innovation has strategically combined two types of physical cues (namely micropatterns and electrical stimulation) to achieve a more effective regeneration, which deserves further exploration. Table 2 summarizes six design strategies discussed above and the in vivo experiment results for NGCs. It showed that both biological (e. g. using stem cells and human fibroblasts) and physical (e. g. using nano diamond and electrical stimulation) modification can achieve reasonable regeneration effects (e. g. excellent mechanical characteristics, improved sensory and CAMP recovery, larger number of axons, etc.).
Table 2.
Research progress of conduit development for nerve regeneration.
| Conduit/device materials (main) | Structures | Fabrication method | In vitro/vivo | Study period | Findings | Refs |
|---|---|---|---|---|---|---|
| Nano diamond/polycaprolactone (ND/PCL) | Multilayered, microporous nerve bridges | Multilayered spraying manufacturing process | In vitro & in vivo | 4 m | Excellent mechanical characteristics; Improved locomotor and sensory recovery; Improved bioelectrical activity; Enhanced skeletal muscle regeneration (compared with auto graft) |
[84] |
| Human gingiva-derived mesenchymal stem cells (GMSCs) | Scaffold-free neural constructs | 3D bio-printer system and allow to mature in a bioreactor | In vivo | 12 w | Similar compound muscle action potential (CMAP) recovery to the autograft transplantation group; Similarly organized nerve fascicles to those transplanted autografts | [107] |
| Human fibroblasts | Scaffold-free neural constructs | 3D bio-printer system and allow to mature in a bioreactor | In vivo | 8 w | The expression of SCs was promoted by bio 3D conduits; Lower muscle atrophy in bio 3D conduits; Higher mean CMAP in bio 3D conduits (compared with silicon conduit) | [110] |
| Metal strip (Mg or Mo) PLGA Silicon nanomembrane Silicon dioxide | A wireless platform made by a collection of circuit elements and substrates | – | In vivo | 10 w | Therapeutic electrical stimulation increased the rate of recovery, reduced the time of nerve reinnervation; Higher EMG amplitude in the presence of electrical stimulation |
[158] |
| Extracellular matrix (ECM) | ECM with parallel micro channels (ECM-C) | Design and fabricate sacrificial templates; Implant templates in vivo for cellularization and tissue formation; Remove template and cellular component | In vitro & in vivo | 16 w | CAMP ratios of ECM-C-regenerated neo-nerves were less than autograft but higher than silicone rods; Muscle fiber size of ECM-C-regenerated neo-nerves was smaller than autograft but both were higher than the silicone rods |
[112] |
4. Challenge and prospects
It is still challengeable to apply the NGCs in real applications for large nerve injury defects, even though the research of them has experienced a long period of time. Autografts and allografts are still the most widely applied in clinical treatment as different-design NGCs need to get through strict medical experiments before being successfully manufactured into applicable products. Because of the scarcity of autografts and allografts, NGCs are vitally important to repair massive nerve injuries. Optimal NGCs must be equipped with sufficient softness as well as flexibility, which should be regarded as a specific assessment standard during the evaluation process of the experiment. The in vivo tests of most reported NGCs are far from optimal to completely reach the “gold standard” set by autograft therapy. Therefore, much more efforts are still needed to do further research and set a series of standard to regulate the relevant parameters of NGCs. Because of the anatomical structure of the nerve transverse section, it is of great importance to make sure that the conduits can shape the movement of SCs and neuronal cells, so as to reduce the escape of axons. In this consideration, several strategies regarding structure, material, and functionalization for the conduits need to be investigated to improve the efficiency of nerve repair.
The fabrication techniques such as extrusion-based, inkjet-based, and laser-based 3D bioprinting technologies are also of vital significance for the development of NGCs. One challengeable issue for the extrusion-based and inkjet-based techniques is the lack of suitable bio-inks for regenerating larger 3D constructs. It is quite demanding to develop unique bio-inks, enabling to strike a balance between biological competence (such as cellular survival) and mechanical properties. The digital light processing technology for laser-based printing of NGCs has opened up an opportunity for printing complex 3D conduits in a more rapid and accurate manner. It has superior speed and scalability than the other laser-based bio-printers such as stereolithography. Besides, textile forming technologies have gained increasing attention because of their versatility such as controlled microarchitecture, high permeability to nutrients, outstanding mechanical properties, and directionality to cells. Inserting living cells into fibers can improve the biological properties of this type of conduits. However, it is challengeable to produce cell-laden fibers for enhanced nerve repair. Even though the fibers are mechanically strong and can be fabricated into complex structure, encapsulating cells inside the fibers is rather difficult because of the harsh manufacturing process, which should be further explored.
It should be noted that there is a wide range of applications in electrical stimulation, which is considered as a crucial scheme of functionalization because the passive electrical stimulators were promised to be an optimal treatment for nerve regeneration. To achieve this, the electric field and several conductive materials can be applied to NGCs. Among the conductive materials, the carbon-based materials seem to be feasible as they are equipped with conductive functionalities and they can tailor the cell-material interactions due to the high surface area to volume ratio. Notably, even though previous studies have attached great attention to the results that the electrical stimulation can have impacts on nerve regeneration, there are limited articles reporting the optimized intensity of electric current under specific situations and the mechanism behind this phenomenon. These limitations open up opportunities for the development of the abovementioned conductive conduits to be more accurate. Nanoparticles, like ZnO, and MgO as well as magnetic Fe3O4 nanoparticles were proven useful in contributing to the repair of damaged nerve tissue because of their exceptional sizes, surface functionalization, along with their electrical, magnetic, and bioactive properties. These substances can be further applied in the constructs but there are still some technical challenges such as the way to evenly distribute nanoparticles in substrates, and the appropriate dosage and concentration of particles required for improved repair effects. In addition to the specific modification, biological cells such as human gingiva-derived mesenchymal stem cells, human fibroblasts, and extracellular matrix acting as “building blocks” indeed outperform the cell-free and neurotrophic factor-free counterparts, providing great opportunities and ideas for the development of NGCs in the future. However, this approach can lead to challenges such as the way to maintain suitable cultivating conditions for stem cell activity to fulfill the requirement of printing technology which allows no damage on the embedded cells during the printing process in view of the fragility of these biomolecules.
5. Conclusions
In summary, the recent progress of NGCs from material choices, structural design, and manufacturing techniques to functionalization approaches was reviewed. It shows that the implantable devices made from combinative materials and technologies possess better properties in terms of mechanical properties and biological functions compared to those made from sole material and technology. An increasing focus on functional materials such as a series of carbon-based materials and nano particles utilized as modification factors was put forward to increase biodegradation stability, biocompatibility, mechanical properties and biological stability of NGCs. RP technologies (e.g. extrusion-based printing, inkjet printing, and laser-based printing) and textile technologies (e.g. woven, knitting, braiding, and electrospinning) have been demonstrated as the prospective fabrication approaches of NGCs. Appropriate functionalization such as topological patterning and electrical stimulation can yield significant improvement of NGCs. Topological patterning usually provides directional guidance for neurite outgrowth, while the mechanism for this function needs to be identified further. NGCs made from rGO to achieve electrical stimulation can regulate cell adhesion as well as mobility under electric field. However, the degradation rate of rGO NGCs still remains unfavorable and the mechanism behind the function of electrical stimulation is still unclear in this field. Furthermore, electric field strength and scaffold conductivity are two crucial factors in regulating neural behavior, which deserves further research to facilitate the development of functional conduits for nerve repair.
Author contributions
This manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. Yixin Yan, Ruotong Yao and Jingyuang Zhao were mainly in charge of collecting and summarizing recent resources related to nerve guidance conduits, Xiaoqin Wang and Yi Li instructed making supplements to the details of the content. Shujun Zhang, Lirong Duan and Tian Wang made the contributions to editing the nine pictures in this work. Kaili Chen, Jinping Guan and Zhaozhu Zheng ultimately proofread the manuscript in grammar and format styles. Zekun Liu and Gang Li instructed the writing, organization and logical structure of this review.
Declaration of competing interest
The authors have nothing to disclose regarding the conflict of interest.
Acknowledgements
This work was financially supported by National Key R&D Program of China (2021YFE0111100 and 2019YFE0117700) and the “Top six talent peaks” program of Jiangsu (GDZB-035) and Science and Technology Project of Nantong (JC2020082). We would like to thank for the support of China National Textile and Apparel Council (J202002) and joint scientific research project of Sino-foreign cooperative education platform of Jiangsu Higher Education Institutions (5011500720) and projects with numbers FZ20190257, XJFZ/2021/7 and 2021fx010104.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Zekun Liu, Email: zekun.liu@manchester.ac.uk.
Yi Li, Email: henry.yili@manchester.ac.uk.
Gang Li, Email: tcligang@suda.edu.cn.
References
- 1.Ahn H.S., Hwang J.Y., Kim M.S., et al. Carbon-nanotube-interfaced glass fiber scaffold for regeneration of transected sciatic nerve. Acta Biomater. 2015;13:324–334. doi: 10.1016/j.actbio.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 2.Osborne N.R., Anastakis D.J., Davis K.D. Peripheral nerve injuries, pain, and neuroplasticity. J. Hand Ther. 2018;31:184–194. doi: 10.1016/j.jht.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Babaei-Ghazani A., Eftekharsadat B., Samadirad B., et al. Traumatic lower extremity and lumbosacral peripheral nerve injuries in adults: electrodiagnostic studies and patients symptoms. J. Forensic Leg. Med. 2017;52:89–92. doi: 10.1016/j.jflm.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Geissler J., Stevanovic M. Management of large peripheral nerve defects with autografting. Injury. 2019;50:S64–S67. doi: 10.1016/j.injury.2019.10.051. [DOI] [PubMed] [Google Scholar]
- 5.Chang W., Shah M.B., Lee P., et al. Tissue-engineered spiral nerve guidance conduit for peripheral nerve regeneration. Acta Biomater. 2018;73:302–311. doi: 10.1016/j.actbio.2018.04.046. [DOI] [PubMed] [Google Scholar]
- 6.Romeo-Guitart D., Casas C. Network-centric medicine for peripheral nerve injury: treating the whole to boost endogenous mechanisms of neuroprotection and regeneration. Neural Regener. Res. 2019;14:1122–1128. doi: 10.4103/1673-5374.251187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith S., Knight R. second ed. Springer London; London: 2011. Surgical Disorders of the Peripheral Nerves. [Google Scholar]
- 8.Yang W., Ting L., Liangsong S., et al. Spontaneous peripheral nerve palsy with hourglass-like fascicular constriction in the upper extremity. J. Neurosurg. JNS. 2019;131:1876–1886. doi: 10.3171/2018.8.JNS18419. [DOI] [PubMed] [Google Scholar]
- 9.Rbia N., Shin A.Y. The role of nerve graft substitutes in motor and mixed motor/sensory peripheral nerve injuries. J. Hand Surg. 2017;42:367–377. doi: 10.1016/j.jhsa.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Gao Y., Wang Y.L., Kong D., et al. Nerve autografts and tissue-engineered materials for the repair of peripheral nerve injuries: a 5-year bibliometric analysis. Neural Regener. Res. 2015;10:1003–1008. doi: 10.4103/1673-5374.158369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeBrun D.G., Sneag D.B., Feinberg J.H. Surgical treatment of iatrogenic nerve injury following arthroscopic capsulolabral repair. J. Hand Ther. 2021 doi: 10.1016/j.jhsa.2021.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Kornfeld T., Vogt P.M., Radtke C. Nerve grafting for peripheral nerve injuries with extended defect sizes. Wien Med. Wochenschr. 2019;169:240–251. doi: 10.1007/s10354-018-0675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y., et al. Jiangnan University; 2016. Preparation of Composite Silk Fibroin Nerve Guidance Conduits for Peripheral Nerve Regeneration. Doctoral Thesis. [Google Scholar]
- 14.Li G., Li Y., Chen G.Q., He J.H., et al. Silk-based biomaterials in biomedical textiles and fiber-based implants. Adv. Healthcare Mater. 2015;4:1134–1151. doi: 10.1002/adhm.201500002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arslantunali D., Dursun T., Yucel D., et al. Peripheral nerve conduits: technology update. Med. Devices (Auckl.) 2014;7:405–424. doi: 10.2147/MDER.S59124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nectow A.R., Marra K.G., Kaplan D.L. Biomaterials for the development of peripheral nerve guidance conduits. Tissue Eng., Part B. 2011;18:40–50. doi: 10.1089/ten.teb.2011.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houshyar S., Bhattacharyya A., Shanks R. Peripheral nerve conduit: materials and structures. ACS Chem. Neurosci. 2019;10:3349–3365. doi: 10.1021/acschemneuro.9b00203. [DOI] [PubMed] [Google Scholar]
- 18.Roballo K.C.S., Bushman J. Evaluation of the host immune response and functional recovery in peripheral nerve autografts and allografts. Transpl. Immunol. 2019;53:61–71. doi: 10.1016/j.trim.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Leckenby J.I., Furrer C., Haug L., et al. A retrospective case series reporting the outcomes of avance nerve allografts in the treatment of peripheral nerve injuries. Plast. Reconstr. Surg. 2020;145:368e. doi: 10.1097/PRS.0000000000006485. [DOI] [PubMed] [Google Scholar]
- 20.Pinho A.C., Fonseca A.C., Serra A.C., et al. Peripheral nerve regeneration: current status and new strategies using polymeric materials. Adv. Healthcare Mater. 2016;5:2732–2744. doi: 10.1002/adhm.201600236. [DOI] [PubMed] [Google Scholar]
- 21.Carriel V., Alaminos M., Garzón I., et al. Tissue engineering of the peripheral nervous system. Expert Rev. Neurother. 2014;14:301–318. doi: 10.1586/14737175.2014.887444. [DOI] [PubMed] [Google Scholar]
- 22.Farokhi M., Mottaghitalab F., Shokrgozar M.A., et al. Prospects of peripheral nerve tissue engineering using nerve guide conduits based on silk fibroin protein and other biopolymers. Int. Mater. Rev. 2016;62:367–391. [Google Scholar]
- 23.Sarker M.D., Naghieh S., McInnes A.D., et al. Regeneration of peripheral nerves by nerve guidance conduits: influence of design, biopolymers, cells, growth factors, and physical stimuli. Prog. Neurobiol. 2018;171:125–150. doi: 10.1016/j.pneurobio.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Yu W., Zhao W., Zhu C., et al. Sciatic nerve regeneration in rats by a promising electrospun collagen/poly(ε-caprolactone) nerve conduit with tailored degradation rate. BMC Neurosci. 2011;12:68. doi: 10.1186/1471-2202-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alessandrino A., Fregnan F., Biagiotti M., et al. SilkBridge: a novel biomimetic and biocompatible silk-based nerve conduit. Biomater. Sci. 2019;7:4112–4130. doi: 10.1039/c9bm00783k. [DOI] [PubMed] [Google Scholar]
- 26.Chiono V., Tonda-Turo C. Trends in the design of nerve guidance channels in peripheral nerve tissue engineering. Prog. Neurobiol. 2015;131:87–104. doi: 10.1016/j.pneurobio.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Kim S.M., Lee M.S., Jeon J., et al. Biodegradable nerve guidance conduit with microporous and micropatterned poly(lactic-co-glycolic acid)-accelerated sciatic nerve regeneration. Macromol.Biosci. 2018;18 doi: 10.1002/mabi.201800290. [DOI] [PubMed] [Google Scholar]
- 28.Önger M.E., Delibaş B., Türkmen A.P., et al. The role of growth factors in nerve regeneration. J. Biomech. Eng. 2016;10:285–291. doi: 10.5582/ddt.2016.01058. [DOI] [PubMed] [Google Scholar]
- 29.de Luca A.C., Lacour S.P., Raffoul W., et al. Extracellular matrix components in peripheral nerve repair: how to affect neural cellular response and nerve regeneration. Neural Regener. Res. 2014;9:1943–1948. doi: 10.4103/1673-5374.145366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson B.N., Jia X. 3D printed nerve guidance channels: computer-aided control of geometry, physical cues, biological supplements and gradients. Neural Regener. Res. 2016;11:1568–1569. doi: 10.4103/1673-5374.193230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y.S., Hsieh C.L., Tsai C.C., et al. Peripheral nerve regeneration using silicone rubber chambers filled with collagen, laminin and fibronectin. Biomaterials. 2000;21:1541–1547. doi: 10.1016/s0142-9612(00)00028-4. [DOI] [PubMed] [Google Scholar]
- 32.Kiyotani T., Teramachi M., Takimoto Y., et al. Nerve regeneration across a 25-mm gap bridged by a polyglycolic acid-collagen tube: a histological and electrophysiological evaluation of regenerated nerves. Brain Res. 1996;740:66–74. doi: 10.1016/s0006-8993(96)00848-7. [DOI] [PubMed] [Google Scholar]
- 33.Mobini S., Spearman B.S., Lacko C.S., et al. Recent advances in strategies for peripheral nerve tissue engineering. Curr. Opin. in Biomed. Eng. 2017;4:134–142. [Google Scholar]
- 34.Gu X., Ding F., Williams D.F. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials. 2014;35:6143–6156. doi: 10.1016/j.biomaterials.2014.04.064. [DOI] [PubMed] [Google Scholar]
- 35.Kehoe S., Zhang X.F., Boyd D. FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury. 2012;43:553–572. doi: 10.1016/j.injury.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 36.Duffy P., McMahon S., Wang X., et al. Synthetic bioresorbable poly-alpha-hydroxyesters as peripheral nerve guidance conduits; a review of material properties, design strategies and their efficacy to date. Biomater. Sci. 2019;7:4912–4943. doi: 10.1039/c9bm00246d. [DOI] [PubMed] [Google Scholar]
- 37.McCullen S.D., Chow A.G.Y., Stevens M.M. In vivo tissue engineering of musculoskeletal tissues. Curr. Opin. Biotechnol. 2011;22:715–720. doi: 10.1016/j.copbio.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Han D., Cheung K.C. Biodegradable cell-seeded nanofiber scaffolds for neural repair. Polymers. 2011;3:1684–1733. [Google Scholar]
- 39.Asadpour S., Kargozar S., Moradi L., et al. Natural biomacromolecule based composite scaffolds from silk fibroin, gelatin and chitosan toward tissue engineering applications. Int. J. Biol. Macromol. 2020;154:1285–1294. doi: 10.1016/j.ijbiomac.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Farokhi M., Mottaghitalab F., Shokrgozar M.A., et al. Prospects of peripheral nerve tissue engineering using nerve guide conduits based on silk fibroin protein and other biopolymers. Int. Mater. Rev. 2017;62:367–391. [Google Scholar]
- 41.Cheng M., Deng J., Yang F., et al. Study on physical properties and nerve cell affinity of composite films from chitosan and gelatin solutions. Biomaterials. 2003;24:2871–2880. doi: 10.1016/s0142-9612(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 42.Yuan Y., Zhang P., Yang Y., et al. The interaction of Schwann cells with chitosan membranes and fibers in vitro. Biomaterials. 2004;25:4273–4278. doi: 10.1016/j.biomaterials.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Z., Zhao Z., Zheng Z., et al. Functionalization of polyethylene terephthalate fabrics using nitrogen plasma and silk fibroin/chitosan microspheres. Appl. Surf. Sci. 2019:143481. [Google Scholar]
- 44.Wang X., Hu W., Cao Y., et al. Dog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graft. Brain. 2005;128:1897–1910. doi: 10.1093/brain/awh517. [DOI] [PubMed] [Google Scholar]
- 45.Muxika A., Etxabide A., Uranga J., et al. Chitosan as a bioactive polymer: processing, properties and applications. Int. J. Biol. Macromol. 2017;105:1358–1368. doi: 10.1016/j.ijbiomac.2017.07.087. [DOI] [PubMed] [Google Scholar]
- 46.Jiang M., Zhuge X., Yang Y., et al. The promotion of peripheral nerve regeneration by chitooligosaccharides in the rat nerve crush injury model. Neurosci. Lett. 2009;454:239–243. doi: 10.1016/j.neulet.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 47.Wei Y., Gong K., Zheng Z., et al. Chitosan/silk fibroin-based tissue-engineered graft seeded with adipose-derived stem cells enhances nerve regeneration in a rat model. J. Mater. Sci. Mater. Med. 2011;22:1947–1964. doi: 10.1007/s10856-011-4370-z. [DOI] [PubMed] [Google Scholar]
- 48.Wlaszczuk A., Marcol W., Kucharska M., et al. Poly(D,L-Lactide-Co-Glycolide) tubes with multifilament chitosan yarn or chitosan sponge core in nerve regeneration. J. Oral Maxillofac. Surg. 2016;74 doi: 10.1016/j.joms.2016.07.009. 2327.e2321-2327.e2312. [DOI] [PubMed] [Google Scholar]
- 49.Phillips D.M., Drummy L.F., Naik R.R., et al. Regenerated silk fiber wet spinning from an ionic liquid solution. J. Mater. Chem. 2005;15:4206–4208. [Google Scholar]
- 50.Yang Y., Ding F., Wu J., et al. Development and evaluation of silk fibroin-based nerve grafts used for peripheral nerve regeneration. Biomaterials. 2007;28:5526–5535. doi: 10.1016/j.biomaterials.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Magaz A., Faroni A., Gough J.E., et al. Bioactive silk-based nerve guidance conduits for augmenting peripheral nerve repair. Adv. Healthcare Mater. 2018;7:1800308. doi: 10.1002/adhm.201800308. [DOI] [PubMed] [Google Scholar]
- 52.Uebersax L., Mattotti M., Papaloïzos M., et al. Silk fibroin matrices for the controlled release of nerve growth factor (NGF) Biomaterials. 2007;28:4449–4460. doi: 10.1016/j.biomaterials.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 53.Carvalho C.R., Chang W., Silva-Correia J., et al. Engineering silk fibroin-based nerve conduit with neurotrophic factors for proximal protection after peripheral nerve injury. Adv. Healthcare Mater. 2021;10:2000753. doi: 10.1002/adhm.202000753. [DOI] [PubMed] [Google Scholar]
- 54.Dinis T.M., Elia R., Vidal G., Dermigny Q., et al. 3D multi-channel bi-functionalized silk electrospun conduits for peripheral nerve regeneration, Behav. Biomed. Mater. 2015;41:43–45. doi: 10.1016/j.jmbbm.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 55.Francis N.L., Hunger P.M., Donius A.E., et al. An ice-templated, linearly aligned chitosan-alginate scaffold for neural tissue engineering. J. Biomed. Mater. Res. 2013;101:3493–3503. doi: 10.1002/jbm.a.34668. [DOI] [PubMed] [Google Scholar]
- 56.Yoshii S., Oka M., Shima M., et al. 30 mm regeneration of rat sciatic nerve along collagen filaments. Brain Res. 2002;949:202–208. doi: 10.1016/s0006-8993(02)03149-9. [DOI] [PubMed] [Google Scholar]
- 57.Yoo J., Park J.H., Kwon Y.W., et al. Augmented peripheral nerve regeneration through elastic nerve guidance conduits prepared using a porous PLCL membrane with a 3D printed collagen hydrogel. Biomater. Sci. 2020;8:6261–6271. doi: 10.1039/d0bm00847h. [DOI] [PubMed] [Google Scholar]
- 58.Azami M., Ai J., Ebrahimi-Barough S., et al. In vitro evaluation of biomimetic nanocomposite scaffold using endometrial stem cell derived osteoblast-like cells. Tissue Cell. 2013;45:328–337. doi: 10.1016/j.tice.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 59.Cunha C., Panseri S., Antonini S. Emerging nanotechnology approaches in tissue engineering for peripheral nerve regeneration. Nanomed. Nanotechnol. Biol. Med. 2011;7:50–59. doi: 10.1016/j.nano.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Teuschl A.H., Schuh C., Halbweis R., et al. A new preparation method for anisotropic silk fibroin nerve guidance conduits and its evaluation in vitro and in a rat sciatic nerve defect model. Tissue Eng. C Methods. 2016;21:945–957. doi: 10.1089/ten.tec.2014.0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gerth D.J., Tashiro J., Thaller S.R. Clinical outcomes for Conduits and Scaffolds in peripheral nerve repair. World J. Clin. Cases. 2015;3:141–147. doi: 10.12998/wjcc.v3.i2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Z.H., Liu X.P., Liu L.H. Preparation and biocompatibility of poly(L-lactide-co-glycolide) scaffold materials for nerve conduits, des. Monom. Polym. 2012;11:447–456. [Google Scholar]
- 63.Kapoor D.N., Bhatia A., Kaur R., et al. PLGA: a unique polymer for drug delivery. Ther. Deliv. 2015;6:41–58. doi: 10.4155/tde.14.91. [DOI] [PubMed] [Google Scholar]
- 64.Makadia H.K., Siegel S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011;3:1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gunatillake P.A., Adhikari R. Biodegradable synthetic polymers for tissue engineering. Eur. Cell. Mater. 2003;5:1–16. doi: 10.22203/ecm.v005a01. [DOI] [PubMed] [Google Scholar]
- 66.Asti A., Gioglio L. Natural and synthetic biodegradable polymers: different scaffolds for cell expansion and tissue formation. Int. J. Artif. Organs. 2014;37:187–205. doi: 10.530/ijao.5000307. [DOI] [PubMed] [Google Scholar]
- 67.Gu X., Ding F., Yang Y., et al. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog. Neurobiol. 2011;93:204–230. doi: 10.1016/j.pneurobio.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 68.Janouskova O. Synthetic polymer scaffolds for soft tissue engineering. Physiol. Res. 2018;67:S335–S348. doi: 10.33549/physiolres.933983. [DOI] [PubMed] [Google Scholar]
- 69.Wang Y.l., Gu X.m., Kong Y., et al. Electrospun and woven silk fibroin/poly(lactic-co- glycolic acid) nerve guidance conduits for repairing peripheral nerve injury. Neural Regener. Res. 2015;10:1635–1642. doi: 10.4103/1673-5374.167763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wegst U.G.K., Schecter M., Donius A.E., et al. Biomaterials by freeze casting. Philos. Trans. R. Soc. 2010;368:2099–2121. doi: 10.1098/rsta.2010.0014. [DOI] [PubMed] [Google Scholar]
- 71.Sun B., Zhou Z., Li D., et al. Polypyrrole-coated poly(l-lactic acid-co-ε-caprolactone)/silk fibroin nanofibrous nerve guidance conduit induced nerve regeneration in rat. Mater. Sci. Eng. C. 2019;94:190–199. doi: 10.1016/j.msec.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 72.Vijayavenkataraman S. Nerve guide conduits for peripheral nerve injury repair: a review on design, materials and fabrication methods. Acta Biomater. 2020;106:54–69. doi: 10.1016/j.actbio.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 73.Sarker M., Naghieh S., McInnes A.D., et al. Strategic design and fabrication of nerve guidance conduits for peripheral nerve regeneration. Biotechnol. J. 2018;13 doi: 10.1002/biot.201700635. [DOI] [PubMed] [Google Scholar]
- 74.Ozbolat I.T., Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016;76:321–343. doi: 10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 75.Khalil S., Sun W. Biopolymer deposition for freeform fabrication of hydrogel tissue constructs. Mater. Sci. Eng. C. 2007;27:469–478. [Google Scholar]
- 76.Zhang Z., Wang B., Hui D., et al. 3D bioprinting of soft materials-based regenerative vascular structures and tissues. Composites, Part B. 2017;123:279–291. [Google Scholar]
- 77.Gao Q., He Y., Fu J.Z., et al. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials. 2015;61:203–215. doi: 10.1016/j.biomaterials.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 78.Naghieh S., Badrossamay M., Foroozmehr E., et al. A review on properties of natural and synthetic based electrospun fibrous materials for bone tissue engineering. J. Pharm. Anal. 2018;8 doi: 10.3390/membranes8030062. 277-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Naghieh S., Foroozmehr E., Badrossamay M., et al. Combinational processing of 3D printing and electrospinning of hierarchical poly(lactic acid)/gelatin-forsterite scaffolds as a biocomposite: mechanical and biological assessment. Mater. Des. 2017;133:128–135. [Google Scholar]
- 80.Jones N. The print revolution: three-dimensional printers are opening up new worlds to research. Nature (London) 2012;487 22-22. [Google Scholar]
- 81.Wang X., Rijff B.L., Khang G. A building-block approach to 3D printing a multichannel, organ-regenerative scaffold. J. Tissue Eng. Regener. Med. 2017;11:1403–1411. doi: 10.1002/term.2038. [DOI] [PubMed] [Google Scholar]
- 82.Naghieh S., Sarker M., Izadifar M., et al. Dispensing-based bioprinting of mechanically-functional hybrid scaffolds with vessel-like channels for tissue engineering applications – a brief review. J. Mech. Behav. Biomed. Mater. 2018;78:298–314. doi: 10.1016/j.jmbbm.2017.11.037. [DOI] [PubMed] [Google Scholar]
- 83.Singh M., Haverinen H.M., Dhagat P., et al. Inkjet printing—process and its applications. Adv. Mater. 2010;22:673–685. doi: 10.1002/adma.200901141. [DOI] [PubMed] [Google Scholar]
- 84.Qian Y., Cheng Y., Ouyang Y., et al. Multilayered spraying and gradient dotting of nanodiamond–polycaprolactone guidance channels for restoration of immune homeostasis. NPG Asia Mater. 2019;11 doi: 10.1038/s41427-019-0136-8. [DOI] [Google Scholar]
- 85.Qian Y., Zhao X., Han Q., et al. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat. Commun. 2018;9:323. doi: 10.1038/s41467-017-02598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Malda J., Visser J., Melchels F.P. 25th anniversary article: engineering hydrogels for biofabrication. Adv. Mater. 2013;25:5011–5028. doi: 10.1002/adma.201302042. [DOI] [PubMed] [Google Scholar]
- 87.Hospodiuk M., Dey M., Sosnoski D., et al. The bioink: a comprehensive review on bioprintable materials. Biotechnol. Adv. 2017;35:217–239. doi: 10.1016/j.biotechadv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 88.Arcaute K., Mann B.K., Wicker R.B. Fabrication of off-the-shelf multilumen poly(ethylene glycol) nerve guidance conduits using stereolithography. Tissue Eng. C Methods. 2011;17:27–38. doi: 10.1089/ten.TEC.2010.0011. [DOI] [PubMed] [Google Scholar]
- 89.Petcu E.B., Midha R., McColl E., et al. 3D printing strategies for peripheral nerve regeneration. Biofabrication. 2018;10 doi: 10.1088/1758-5090/aaaf50. [DOI] [PubMed] [Google Scholar]
- 90.Hribar K.C., Soman P., Warner J., et al. Light-assisted direct-write of 3D functional biomaterials. Lab Chip. 2014;14:268–275. doi: 10.1039/c3lc50634g. [DOI] [PubMed] [Google Scholar]
- 91.Mandrycky C., Wang Z., Kim K., et al. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016;34:422–434. doi: 10.1016/j.biotechadv.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Duocastella M., Fernández-Pradas J.M., Morenza J.L., et al. Novel laser printing technique for miniaturized biosensors preparation. Sensor. Actuator. B Chem. 2010;145:596–600. [Google Scholar]
- 93.Ng W.L., Lee J.M., Zhou M., et al. Vat polymerization-based bioprinting—process, materials, applications and regulatory challenges. Biofabrication. 2020;12 doi: 10.1088/1758-5090/ab6034. [DOI] [PubMed] [Google Scholar]
- 94.Zhu W., Tringale K.R., Woller S.A., et al. Rapid continuous 3D printing of customizable peripheral nerve guidance conduits. Mater. Today. 2018;21:951–959. doi: 10.1016/j.mattod.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ye W., Li H., Yu K. 3D printing of gelatin methacrylate-based nerve guidance conduits with multiple channels. Mater. Des. 2020;192:108757. [Google Scholar]
- 96.Murphy S.V., Atala A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 97.Chia H.N., Wu B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015;9 doi: 10.1186/s13036-015-0001-4. 4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guvendiren M., Lu H.D., Burdick J.A. Shear-thinning hydrogels for biomedical applications. Soft Matter. 2012;8:260–272. [Google Scholar]
- 99.Yu Y., Moncal K.K., Li J., et al. Three-dimensional bioprinting using self-assembling scalable scaffold-free “tissue strands” as a new bioink. Sci. Rep. 2016;6:28714. doi: 10.1038/srep28714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ozbolat I.T., Peng W., Ozbolat V. Application areas of 3D bioprinting. Drug Discov. Today. 2016;21:1257–1271. doi: 10.1016/j.drudis.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 101.Miller J.S., Stevens K.R., Yang M.T., et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012;11:768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Karoly J., Adrian N., Vladimir M., et al. Engineering biological structures of prescribed shape using self-assembling multicellular systems. Proc. Natl. Acad. Sci. Unit. States Am. 2004;101:2864–2869. doi: 10.1073/pnas.0400164101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cesarz Z., Tamama K. Spheroid culture of mesenchymal stem cells. Stem Cell. Int. 2016;2016:9176357. doi: 10.1155/2016/9176357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Norotte C., Marga F.S., Niklason L.E., et al. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30:5910–5917. doi: 10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jakab K., Norotte C., Damon B., et al. Tissue engineering by self-assembly of cells printed into topologically defined structures. Tissue Eng. 2008;14:413–421. doi: 10.1089/tea.2007.0173. [DOI] [PubMed] [Google Scholar]
- 106.Laschke M.W., Menger M.D. Life is 3D: boosting spheroid function for tissue engineering. Trends Biotechnol. 2017;35:133–144. doi: 10.1016/j.tibtech.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Q., Nguyen P.D., Shi S., et al. 3D bio-printed scaffold-free nerve constructs with human gingiva-derived mesenchymal stem cells promote rat facial nerve regeneration. Sci. Rep. 2018;8:6634. doi: 10.1038/s41598-018-24888-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kaltschmidt B., Kaltschmidt C., Widera D. Adult craniofacial stem cells: sources and relation to the neural crest. Stem Cell Rev. Rep. 2012;8:658–671. doi: 10.1007/s12015-011-9340-9. [DOI] [PubMed] [Google Scholar]
- 109.La Noce M., Mele L., Tirino V., et al. Neural crest stem cell population in craniomaxillofacial development and tissue repair. Eur. Cell. Mater. 2014;28:348–357. doi: 10.22203/ecm.v028a24. [DOI] [PubMed] [Google Scholar]
- 110.Yurie H., Ikeguchi R., Aoyama T., et al. The efficacy of a scaffold-free Bio 3D conduit developed from human fibroblasts on peripheral nerve regeneration in a rat sciatic nerve model. PLoS One. 2017;12 doi: 10.1371/journal.pone.0171448. e0171448-e0171448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hussey G.S., Dziki J.L., Badylak S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018;3:159–173. [Google Scholar]
- 112.Zhu M., Li W., Dong X., et al. In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nat. Commun. 2019;10:4620. doi: 10.1038/s41467-019-12545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li G., Chen Y., Hu J., et al. A 5-fluorouracil-loaded polydioxanone weft-knitted stent for the treatment of colorectal cancer. Biomaterials. 2013;34:9451–9461. doi: 10.1016/j.biomaterials.2013.08.055. [DOI] [PubMed] [Google Scholar]
- 114.Schultz P., Vautier D., Chluba J., et al. Survival analysis of rats implanted with porous titanium tracheal prosthesis. Ann. Thorac. Surg. 2002;73:1747–1751. doi: 10.1016/s0003-4975(02)03569-5. [DOI] [PubMed] [Google Scholar]
- 115.Hayashi K., Ando N., Ozawa S., et al. A neo-esophagus reconstructed by cultured human esophageal epithelial cells, smooth muscle cells, fibroblasts, and collagen. Am. Soc. Artif. Intern. Organs J. 2004;50:261–266. doi: 10.1097/01.mat.0000123688.45717.a4. [DOI] [PubMed] [Google Scholar]
- 116.Iannace S., Sabatini G., Ambrosio L., et al. Mechanical behaviour of composite artificial tendons and ligaments. Biomaterials. 1995;16:675–680. doi: 10.1016/0142-9612(95)99693-g. [DOI] [PubMed] [Google Scholar]
- 117.Blanchemain N., Haulon S., Boschin F., et al. Vascular prostheses with controlled release of antibiotics: Part 1: surface modification with cyclodextrins of PET prostheses. Biomol. Eng. 2007;24:149–153. doi: 10.1016/j.bioeng.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 118.Xie X., Zheng X., Han Z., et al. A biodegradable stent with surface functionalization of combined-therapy drugs for colorectal cancer. Adv. Healthcare Mater. 2018;7:1801213. doi: 10.1002/adhm.201801213. [DOI] [PubMed] [Google Scholar]
- 119.Akbari M., Tamayol A., Bagherifard S., et al. Textile technologies and tissue engineering: a path toward organ weaving. Adv. Healthcare Mater. 2016;5:751–766. doi: 10.1002/adhm.201500517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Onoe H., Okitsu T., Itou A., et al. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater. 2013;12:584–590. doi: 10.1038/nmat3606. [DOI] [PubMed] [Google Scholar]
- 121.Liu G.H., Hu H., Zhang P.H., et al. Radial compressive properties of the biodegradable braided regeneration tubes for peripheral nerve repair. J. Ind. Textil. 2006;36:35–46. [Google Scholar]
- 122.Akbari M., Tamayol A., Laforte V., et al. Composite living fibers for creating tissue constructs using textile techniques. Adv. Funct. Mater. 2014;24:4060–4067. doi: 10.1002/adfm.201303655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sun X., Tong L., Wood M.D.K., et al. Effect of stitch distribution on mode I delamination toughness of laminated DCB specimens. Compos. Sci. Technol. 2004;64:967–981. [Google Scholar]
- 124.Moutos F.T., Guilak F. Functional properties of cell-seeded three-dimensionally woven poly(epsilon-caprolactone) scaffolds for cartilage tissue engineering. Tissue Eng. 2010;16:1291–1301. doi: 10.1089/ten.tea.2009.0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guilak F., Moutos F.T., Freed L.E. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat. Mater. 2007;6:162–167. doi: 10.1038/nmat1822. [DOI] [PubMed] [Google Scholar]
- 126.Gloy Y.S., Loehrer M., Lang B., et al. Tubular woven narrow fabrics for replacement of cruciate ligaments. Ann. Biomed. Eng. 2013;41:1950–1956. doi: 10.1007/s10439-013-0806-6. [DOI] [PubMed] [Google Scholar]
- 127.Tamayol A., Akbari M., Annabi N., et al. Fiber-based tissue engineering: progress, challenges, and opportunities. Biotechnol. Adv. 2013;31:669–687. doi: 10.1016/j.biotechadv.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen J.L., Yin Z., Shen W.L., et al. Efficacy of hESC-MSCs in knitted silk-collagen scaffold for tendon tissue engineering and their roles. Biomaterials. 2010;31:9438–9451. doi: 10.1016/j.biomaterials.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 129.Almeida L.R., Martins A.R., Fernandes E.M., et al. New biotextiles for tissue engineering: development, characterization and in vitro cellular viability. Acta Biomater. 2013;9:8167–8181. doi: 10.1016/j.actbio.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 130.Akbari M., Tamayol A., Bagherifard S., et al. Textile technologies and tissue engineering: a path toward organ weaving. Adv. Healthcare Mater. 2016;5:751–766. doi: 10.1002/adhm.201500517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stewart J.D. Peripheral nerve fascicles: anatomy and clinical relevance. Muscle Nerve. 2003;28:525–541. doi: 10.1002/mus.10454. [DOI] [PubMed] [Google Scholar]
- 132.Xue J., Xie J., Liu W., et al. Electrospun nanofibers: new concepts, materials, and applications. Acc. Chem. Res. 2017;50:1976–1987. doi: 10.1021/acs.accounts.7b00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang S. Donghua University; 2013. Preparation and Properties of the Nerve Regeneration Conduit with a Tube in Tube Braided Structure. Master Thesis. [Google Scholar]
- 134.Zhang S.J., Wang J., Zheng Z.Z., et al. Porous nerve guidance conduits reinforced with braided composite structures of silk/magnesium filaments for peripheral nerve repair. Acta Biomater. 2021 doi: 10.1016/j.actbio.2021.07.028. [DOI] [PubMed] [Google Scholar]
- 135.Mishra R.K., Mishra P., Verma K., et al. Electrospinning production of nanofibrous membranes. Environ. Chem. Lett. 2019;17:767–800. [Google Scholar]
- 136.Frost H.K., Andersson T., Johansson S., et al. Electrospun nerve guide conduits have the potential to bridge peripheral nerve injuries in vivo. Sci. Rep. 2018;8:16716. doi: 10.1038/s41598-018-34699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang C.Y., Huang W.Y., Chen L.H., et al. Neural tissue engineering the influence of scaffold surface. J. Mater. Chem. B. 2021;9 doi: 10.1039/d0tb01605e. 567-548. [DOI] [PubMed] [Google Scholar]
- 138.Zha F., Chen W., Zhang L., et al. Electrospun natural polymer and its composite nanofibrous scaffolds for nerve tissue engineering. J. Biomater. Sci. Polym. Ed. 2019:1–30. doi: 10.1080/09205063.2019.1697170. [DOI] [PubMed] [Google Scholar]
- 139.Cheng H., Yang X., Che X., et al. Biomedical application and controlled drug release of electrospun fibrous materials. Mater. Sci. Eng. C. 2018;90:750–763. doi: 10.1016/j.msec.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 140.Ingavle G.C., Leach J.K. Advancements in electrospinning of polymeric nanofibrous scaffolds for tissue engineering. Tissue Eng., Part B. 2013;20:277–293. doi: 10.1089/ten.TEB.2013.0276. [DOI] [PubMed] [Google Scholar]
- 141.Al-Enizi A.M., Zagho M.M., Elzatahry A.A. Polymer-based electrospun nanofibers for biomedical applications. Nanomaterials. 2018;8:259–280. doi: 10.3390/nano8040259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zha F., Chen W., Zhang L., et al. Electrospun natural polymer and its composite nanofibrous scaffolds for nerve tissue engineering. J. Biomater. Sci. Polym. Ed. 2020;31:519–548. doi: 10.1080/09205063.2019.1697170. [DOI] [PubMed] [Google Scholar]
- 143.Teodorescu M., Bercea M., Morariu S. Biomaterials of poly(vinyl alcohol) and natural polymers. Plolym. Rev. 2018;58:247–287. [Google Scholar]
- 144.Chen X., Ge X., Qian Y., et al. Electrospinning multilayered scaffolds loaded with melatonin and Fe3O4 magnetic nanoparticles for peripheral nerve regeneration. Adv. Funct. Mater. 2020;30:2004537. [Google Scholar]
- 145.Zuidema J.M., Provenza C., Caliendo T., et al. Magnetic NGF-releasing PLLA/iron oxide nanoparticles direct extending neurites and preferentially guide neurites along aligned electrospun microfibers. ACS Chem. Neurosci. 2015;6:1781–1788. doi: 10.1021/acschemneuro.5b00189. [DOI] [PubMed] [Google Scholar]
- 146.Giannaccini M., Calatayud M.P., Poggetti A., et al. Magnetic nanoparticles for efficient delivery of growth factors: stimulation of peripheral nerve regeneration. Adv. Healthcare Mater. 2017;6:1601429. doi: 10.1002/adhm.201601429. [DOI] [PubMed] [Google Scholar]
- 147.Vennemeyer J.J., Hopkins T., Hershcovitch M., et al. Initial observations on using magnesium metal in peripheral nerve repair. J. Biomater. Appl. 2015;29:1145–1154. doi: 10.1177/0885328214553135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Monfared A., Ghaee A., Ebrahimi-Barough S. Fabrication of tannic acid/poly(N-vinylpyrrolidone) layer-by-layer coating on Mg-based metallic glass for nerve tissue regeneration application. Colloids Surf. B Biointerfaces. 2018;170:617–626. doi: 10.1016/j.colsurfb.2018.06.060. [DOI] [PubMed] [Google Scholar]
- 149.Kenry, Lim C.T. Nanofiber technology: current status and emerging developments. Prog. Polym. Sci. 2017;70:1–17. [Google Scholar]
- 150.Niu H., Lin T. Fiber generators in needleless electrospinning. J. Nanomater. 2012;2012:725950. [Google Scholar]
- 151.Prabu G.T.V., Dhurai B. A novel profiled multi-pin electrospinning system for nanofiber production and encapsulation of nanoparticles into nanofibers. Sci. Rep. 2020;10:4302. doi: 10.1038/s41598-020-60752-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dong M., Wang X., Chen X.-Z., et al. 3D-Printed soft magnetoelectric microswimmers for delivery and differentiation of neuron-like cells. Adv. Funct. Mater. 2020;30:1910323. [Google Scholar]
- 153.Carvalho C.R., Silva-Correia J., Oliveira J.M., et al. Nanotechnology in peripheral nerve repair and reconstruction. Adv. Drug Deliv. Rev. 2019;148:308–343. doi: 10.1016/j.addr.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 154.Riggio C., Calatayud M.P., Giannaccini M., et al. The orientation of the neuronal growth process can be directed via magnetic nanoparticles under an applied magnetic field. Nanomedicine. 2014;10:1549–1558. doi: 10.1016/j.nano.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 155.Gordon T. Electrical stimulation to enhance axon regeneration after peripheral nerve injuries in animal models and humans. Neurotherapeutics. 2016;13:295–310. doi: 10.1007/s13311-015-0415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nix W.A., Hopf H.C. Electrical stimulation of regenerating nerve and its effect on motor recovery. Brain Res. 1983;272:21–25. doi: 10.1016/0006-8993(83)90360-8. [DOI] [PubMed] [Google Scholar]
- 157.Ray W.Z., Mahan M.A., Guo D., et al. An update on addressing important peripheral nerve problems: challenges and potential solutions. Acta Neurochir. 2017;159:1765–1773. doi: 10.1007/s00701-017-3203-3. [DOI] [PubMed] [Google Scholar]
- 158.Koo J., MacEwan M.R., Kang S.K., et al. Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy. Nat. Med. 2018;24:1830–1836. doi: 10.1038/s41591-018-0196-2. [DOI] [PubMed] [Google Scholar]
- 159.Zhang Z., Jorgensen M.L., Wang Z. 3D anisotropic photocatalytic architectures as bioactive nerve guidance conduits for peripheral neural regeneration. Biomaterials. 2020;253:120108. doi: 10.1016/j.biomaterials.2020.120108. [DOI] [PubMed] [Google Scholar]
- 160.Cha C., Shin S.R., Annabi N., et al. Carbon-based nanomaterials: multifunctional materials for biomedical engineering. ACS Nano. 2013;7:2891–2897. doi: 10.1021/nn401196a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bolotin K.I., Sikes K.J., Jiang Z. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008;146:351–355. [Google Scholar]
- 162.Ryan A.J., Kearney C.J., Shen N., et al. Electroconductive biohybrid collagen/pristine graphene composite biomaterials with enhanced biological activity. Adv. Mater. 2018;30:1706442. doi: 10.1002/adma.201706442. [DOI] [PubMed] [Google Scholar]
- 163.Aydin T., Gurcan C., Taheri H., et al. Springer International Publishing AG; Cham: 2018. Cell Biology and Translational Medicine, Volume 3: Stem Cells, Bio-Materials and Tissue Engineering, Springer International Publishing. [Google Scholar]
- 164.Zhang K., Zheng H., Liang S., et al. Aligned PLLA nanofibrous scaffolds coated with graphene oxide for promoting neural cell growth. Acta Biomater. 2016;37:131–142. doi: 10.1016/j.actbio.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 165.Ding X., Liu H., Fan Y. Graphene-based materials in regenerative medicine. Adv. Healthcare Mater. 2015;4:1451–1468. doi: 10.1002/adhm.201500203. [DOI] [PubMed] [Google Scholar]
- 166.Jaafar E., Kashif M., Sahari S.K., et al. Study on morphological, optical and electrical properties of graphene oxide (GO) and reduced graphene oxide (rGO) Mater. Sci. Forum. 2018;917:112–116. [Google Scholar]
- 167.Wang Y., Chen Y., Lacey S.D., et al. Reduced graphene oxide film with record-high conductivity and mobility. Mater. Today. 2018;21:186–192. [Google Scholar]
- 168.Chen G.Y., Pang D.W.P., Hwang S.M., et al. A graphene-based platform for induced pluripotent stem cells culture and differentiation. Biomaterials. 2012;33:418–427. doi: 10.1016/j.biomaterials.2011.09.071. [DOI] [PubMed] [Google Scholar]
- 169.Akhavan O., Ghaderi E. Flash photo stimulation of human neural stem cells on graphene/TiO2 heterojunction for differentiation into neurons. Nanoscale. 2013;5:10316–10326. doi: 10.1039/c3nr02161k. [DOI] [PubMed] [Google Scholar]
- 170.Park J., Jeon J., Kim B., et al. Electrically conductive hydrogel nerve guidance conduits for peripheral nerve regeneration. Adv. Funct. Mater. 2020;30:2003759. [Google Scholar]
- 171.Wang J., Cheng Y., Chen L., et al. In vitro and in vivo studies of electroactive reduced graphene oxide-modified nanofiber scaffolds for peripheral nerve regeneration. Acta Biomater. 2019;84:98–113. doi: 10.1016/j.actbio.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 172.Chua J.S., Chng C.-P., Moe A.A.K., et al. Extending neurites sense the depth of the underlying topography during neuronal differentiation and contact guidance. Biomaterials. 2014;35:7750–7761. doi: 10.1016/j.biomaterials.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 173.Czöndör K., Garcia M., Argento A., et al. Micropatterned substrates coated with neuronal adhesion molecules for high-content study of synapse formation. Nat. Commun. 2013;4:2252. doi: 10.1038/ncomms3252. [DOI] [PubMed] [Google Scholar]
- 174.Jeong G.S., Chang J.Y., Park J.S., et al. Networked neural spheroid by neuro-bundle mimicking nervous system created by topology effect. Mol. Brain. 2015;8:17. doi: 10.1186/s13041-015-0109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Menaa F., Abdelghani A., Menaa B. Graphene nanomaterials as biocompatible and conductive scaffolds for stem cells: impact for tissue engineering and regenerative medicine. J. Tissue Eng. Regener. Med. 2015;9:1321–1338. doi: 10.1002/term.1910. [DOI] [PubMed] [Google Scholar]
- 176.Wang J., Wang H., Mo X., et al. Reduced graphene oxide-encapsulated microfiber patterns enable controllable formation of neuronal-like networks. Adv. Mater. 2020;32 doi: 10.1002/adma.202004555. [DOI] [PMC free article] [PubMed] [Google Scholar]