Abstract
People with chronic stress have higher levels of pro-inflammatory cytokines, which enhance their susceptibility to cardiovascular diseases. Diacerein has ability to modulate pro-inflammatory cytokines such as IL-1β and IL-6; however, its efficacy in chronic stress associated cardiovascular diseases is not yet assessed. In this study, we standardized a rat model of chronic unpredictable stress (CUS) demonstrating cardiovascular dysfunctions and further assessed the effect of IL-6 modulator, diacerein, on cardiovascular functions in CUS exposed rats. The CUS procedure consisted of exposing male albino Wistar rats to random stressors, everyday for 8 weeks. The binding affinity of diacerein with IL-6 was ascertained using Docking tools viz AutoDock and SwissDock. Moreover, diacerein was administered (50 mg/kg/day x 20 days P.O) post CUS exposure to rats and the serum IL-6 levels and heart functions of CUS rats were determined by ELISA and ECG-HRV analysis, respectively. 8 weeks of CUS exposure resulted in two-fold increase in serum corticosterone and IL-6 levels in rats. The ECG and HRV analysis of CUS rats showed altered sinus rhythm, elevated heart rate, systolic blood pressure and sympathetic tone. Molecular docking studies revealed diacerein high binding affinity towards IL-6 receptor. The post-treatment of diacerein in CUS rats prevented these cardiovascular dysfunctions. Our findings thus suggests that IL-6 may have a prominent role in chronic stress induced cardiovascular dysfunctions and diacerein, could be used as a preventive measure for such conditions.
Keywords: Chronic unpredictable stress, Interleukin-6, Pro-inflammatory cytokines, Cardiac dysfunction, Diacerein
Chronic unpredictable stress, Interleukin-6, Pro-inflammatory cytokines, Cardiac dysfunction, Diacerein.
1. Introduction
Chronic stress, resulting from prolonged exposure to physical and psychological stressors, is associated with enhanced risk for cardiovascular, metabolic, gastrointestinal and mental disorders (Rozanski et al., 1999; Mayer, 2000; Tamashiro et al., 2011; Golbidi et al., 2015; Tafet and Nemeroff, 2016; Cui et al., 2019). The precise neurobiological mechanisms underlying these associations are not yet clearly understood. However, it has been reported that chronic stress triggers long-term dysregulation of stress hormones (catecholamines and cortisol), which alters the pattern of cytokine release from immune cells and facilitates the production of pro-inflammatory cytokines over anti-inflammatory cytokines, thereby inducing a state of chronic inflammation (Liu et al., 2017).
C-reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and nuclear factor kappa B (NF-κB) are some of the pro-inflammatory proteins or cytokines that are elevated following chronic stress (Miller et al., 2009; Maydych, 2019). Among these cytokines, meta-analysis studies revealed IL-6 to be the consistently elevated cytokine in the blood of patients with post-traumatic stress disorder (PTSD) and depression (Passos et al., 2015). IL-6 and its soluble receptor α (sIL-6Rα) complex play a key role in the transition from acute to chronic inflammation by switching the nature of the leucocyte infiltrate from polymorphonuclear neutrophils to monocyte/macrophages (Hurst et al., 2001; Kaplanski et al., 2003). Moreover, IL-6 also exerts stimulatory effects on T-and B-cells, thereby facilitating chronic inflammatory responses (Gabay, 2006).
Several studies have found significant correlation between long term elevation of IL-6 and cardiovascular diseases resulting in morbidity and mortality (Gabriel et al., 2004; Baune et al., 2011; Zhang et al., 2018; Gager et al., 2020). IL-6 level was found elevated in the heart tissue of patients with dilated cardiomyopathy and end-stage heart failure. Prolonged exposure to IL-6 causes cardiomyocyte injury, cardiac hypertrophy, and loss of cardiac function (Fontes et al., 2015). Further, increased IL-6 level has been associated with atherosclerosis (Wainstein et al., 2017) and production of CRP, an important risk factor for coronary heart diseases (Buckley et al., 2009; Del Giudice and Gangestad, 2018). The pathophysiology of atrial fibrillation has also been linked to IL-6 signaling (Markousis-Mavrogenis et al., 2019). In experimental studies, administration of IL-6 to rats dose-dependently decreased myocardial contractile activity and caused alterations in cardiovascular functional parameters (Janssen et al., 2005). Moreover, double-transgenic mice overexpressing both IL-6 & IL-6 receptors showed cardiac hypertrophy and decline in left ventricular volume (Hirota et al., 1995).
These studies suggest inhibition of IL-6 and its downstream signaling pathways following chronic stress would be an effective strategy to prevent chronic stress-induced cardiovascular disorders. Diacerein, an anthraquinone derivative, belonging to the class of non-steroidal anti-inflammatory drug possess unique pharmacological actions on several cytokines such as IL-1β and IL-6; and is clinically used to treat osteoarthritis (Martel Pelletier and Pelletier, 2010; Pavelka et al., 2016). While the effect of diacerein is well known on IL-1β, recent docking studies revealed that diacerein also disrupts IL-6 signalling by interfering with the binding of IL-6 to its receptor. The study also provided experimental evidence for the inhibitory effects of diacerein on the expression of IL-6 as well as IL-6 receptors (Bharti et al., 2016). Based on these findings, we undertook the study to assess the effect of diacerein on chronic unpredictable stress (CUS) induced rise in IL-6 and its subsequent pathological consequences on cardiovascular functions in a rat model.
2. Material & methods
2.1. Animals
Five weeks old male albino Wistar rats weighing 100 ± 20g were used in experiments. The animals were housed in group of 2–3 rats per cage under controlled ambient environments (12 h light/dark cycle 25±2 °C) in institutional animal house premises and fed a standard pellet diet with free access to water and food. The care of the animals was taken as per guidelines of the committee for the purpose and control and supervision of experiments on animals, Govt of India vide approval number: BBDNIT/IAEC/2019/Oct/03, prior to experimentation.
2.2. Experimental design
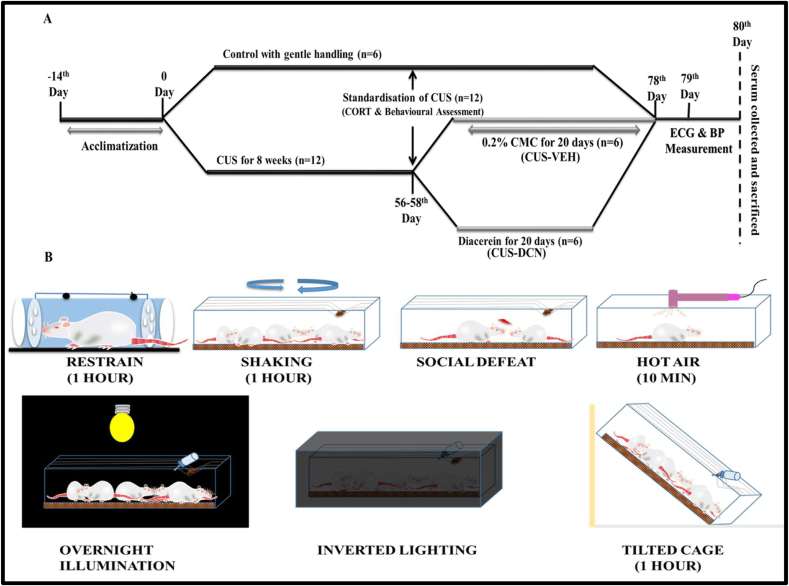
After 2 weeks of acclimatization period, all animals were randomly divided into two groups viz Control and CUS consisting of 6 and 12 animals, respectively in each group. The control group consisted of rats with gentle handling for 78 days along with free access to food and water. The CUS rats group was subjected to a random stressor daily for 8 weeks (Figure 1B). The stressors for CUS paradigm were: restraint– rats were held in restrainers for 1 hour; tilted cage–rat cages were tilted at 45° for 1 hour; shaking–rats were shaken on a mechanical shaker for 1 hour; hot air stream–rats were subjected to hot air from a hair dryer for 10 min; social defeat–rats were exposed to a hostile rat and after being overwhelmed, they were returned back to their original home cages with a cutoff time of 5 min; overnight illumination–rats were introduced to regular room light during the night period; inverted light cycle–regular room light in rats holding area was inverted (off during day time and on during night) (Monteiro et al., 2015). Post 8 weeks of random exposure to stress, CUS rats were subjected to behavioural tests and corticosterone measurement for assessment of stress induction. Further, CUS rats were randomly divided into two groups; CUS-DCN and CUS-VEH group. The CUS-DCN group was treated with diacerein suspended in 0.2% carboxymethylcellulose (CMC) (50 mg/kg, orally) for 20 days post CUS exposure period and continued for 20 days (Abd Allah, 2017; Almezgagi et al., 2020; Upadhyay et al., 2021). The CUS-VEH group was administered 0.2% CMC orally for 20 days. Post-CUS induction and designated treatment, ECG recording, blood pressure measurement was performed on both groups and finally blood samples were collected for biochemical estimations.
Figure 1.
A schematic representation of the experimental design and the timeline of experiments. (A) Initially rats were randomly assigned to two groups Control and CUS wherein control rats were gently handled during the course of study and CUS group rats were exposed to 8 weeks of chronic unpredictable stress (CUS) followed by standardisation. CUS group was further randomly divided in two groups: Vehicle (CUS-VEH) and CUS-DCN, wherein CUS-DCN group received diacerein (50 mg/kg in 0.2% CMC) for 20 days and Vehicle group received vehicle alone for 20 days. Post treatment both groups were assessed for physiological and biochemical changes. (B) The CUS paradigm consisted of exposing rats randomly to following stressors daily for 8 weeks: restraint – rats were restrained in a restrainer for 1 hour; shaking - rats were shaked in a shaker for 1 hour; social defeat – the rats were placed in a cage along with an aggressive rat, and after being defeated, they were returned to their former cages; hot air stream – rats were subjected to hot air stream from a hair dryer for 10 min; overnight illumination – rats were constantly exposed to light during day as well as night time; inverted light cycle – the regular lighting environment in the animal room was reversed (off during the day and on at night); tilted cage – the rat cage was inclined up to 45° angle for 1 hour.
2.3. Body weight assessment
Rats were weighed at the beginning of the study and thereafter every week of the CUS exposure duration. At the end of CUS exposure, change in body weight was represented as % weight variation determined by the following formula (1) (Mishra et al., 2016)
| Weight Variation (%) = [(Final weight-Initial weight)/Final Weight] X 100 | (1) |
2.4. Behavioural assessment
2.4.1. Forced swimming test (FST)
Rats were placed individually in a clear glass cylinder (40 cm height and 18 cm diameter) filled with water (21–23 °C) to a depth of 30 cm to keep the rats' hind paws from touching the bottom surface. A 15-minute warm-up was followed by a 5-minute test 24 hours later. A video camera was used to record the duration of immobility in rats which was later evaluated manually. Each rat was dried gently using a soft towel, kept in a warm environment, and then housed in a dry cage with proper bedding, food, and water after completing the FST protocol (Arndt et al., 2015).
2.4.2. Elevated plus-maze test (EPMT)
The elevated plus-maze test was conducted on a plus maze, raised 72.4 cm from the floor, with two opposing open arms (50.8 cm × 10.2 cm) and two opposing closed arms (50.8 cm × 10.2 cm × 40.6 cm). The rats were left in the middle of the maze facing the closed arm and allowed to freely explore all four arms of the maze for 5 min. Movement of stressed and control rats in open and close arms was recorded (Rau et al., 2015). The maze was cleaned with 70% alcohol prior to each testing to eliminate any odor related clues.
2.4.3. Novelty suppressed feeding test (NSFT)
In NSFT, rats were first subjected to fasting for 24 hours with free access to water during the period. The test was performed in an open field Plexiglas chamber (100 cm × 100 cm × 60 cm) illuminated from above. Food pellets were kept in the middle of the chamber in a shallow plastic container. Each rat was released from one of the corners and allowed to explore the chamber with a cut-off time of 5 min. Latency to the first bite of food was measured and compared between groups. The open field box was cleaned with 70% alcohol prior to each testing and fresh food pellets were used every time to eliminate any odor related clues (Hattiangady et al., 2014).
2.5. Corticosterone measurement
Corticosterone levels in serum were measured using a commercial ELISA test kit (Cat. No. ER0859) provided by Wuhan Fine Biological Technology Co., Ltd., China, as per instructions provided by the manufacturer. The wells of the plate were initially loaded with 50 μL of standard or sample. 50 μL of prepared biotin antibody was immediately added to each well with gentle taping, followed by incubation at 37°C for 45 min. The solution in the wells was discarded after incubation, and the wells were gently washed with prepared wash buffer thrice. Thereafter, 100 μL of HRP-Streptavidin solution was loaded into each well and incubated for 30 min at 37°C. The well solution was again discarded and washed five times, followed by the addition of 90 μL of tetramethylbenzidine (TMB) in each well. The plate was incubated for 10–20 min at 37°C and subsequently, stop solution (50 μL) was added to each well. The color change was noted and the resulting reaction was immediately read at 450nm using an ELISA plate reader (Abd El-Fattah et al., 2018).
2.6. Molecular docking studies
Docking studies were performed to assess the interaction between IL-6 receptor and diacerein, using AutoDock 4.0 (Morris et al., 1998) and SwissDock (Grosdidier et al., 2011). Briefly, the crystal structure of the extracellular domains of the human Interleukin-6 receptor (IL-6R) alpha chain was retrieved from Protein Data Bank (PDB) with PDBID: 1N26 (http://www.rcsb.org/pdb). The active site domain of this structure was determined by BIOVIA Discovery Studio visualizer. Further, PDBQT files were generated for ligand-free IL-6R alpha-chain and diacerein, followed by the generation of grid files. The docking analysis was then performed on AutoDock 4.0 and binding affinities (kcal/mol) along with number of probable hydrogen bonds were determined. The best binding pose was selected and portrayed. Moreover, we further validated the docking results using an online web server named SwissDock (http://www.swissdock.ch.). The analysis consisted of submitting the IL-6R protein (PDBID:1N26) structure in PDB format and the ligand (diacerein) structure in mol2 format on the server. The SwissDock server generated results as downloadable zip files which were extracted to view the different docking poses of diacerein with IL-6R protein.
2.7. Electrocardiography - heart rate variability (ECG-HRV) analysis
Rats were anaesthetized with a combination of diazepam (5 mg/kg, i.m.) and ketamine hydrochloride (100 mg/kg, i.m.) and placed on a board in a supine position. The negative and positive platinum hook electrodes were positioned on the ventral and dorsal thorax of rats on the epidermis. In contrast, the neutral electrode was positioned on the epidermis of the peritoneal region. The other ends of electrodes were connected to a Bio-amplifier (ML-136) and a power lab (ML-826) module attached to a computer for data display and storage. Electrocardiography (ECG) recording was performed for 10 min which was then analysed using the in-built modules of ECG and Heart Rate Variability (HRV) of Lab Chart Pro-8 software (AD Instruments, Australia). The data thus obtained was further analysed using an online web server, MetaboAnalyst 4.0 for principal component analysis and partial least squares – discriminant analysis (Chong et al., 2019; Rawat et al., 2019).
Chemometric methods are typically used for multivariate statistical data analysis. It allows the data embedded in a multivariate data table to be visualized in terms of images and/or graphs. Principal Component Analysis (PCA) is a linear transformation algorithm that converts a higher dimensional dataset into two lower dimensional data matrices known as score (T) matrix and loading (P) matrix. The rows of the score matrix are regarded as the score vectors and represent the main components (PCs), while the loading matrix columns are termed the corresponding loading vectors. The most typical statistical analysis model used for statistical inference investigation is Partial Least Squares Discriminant Analysis (PLS-DA). The regression extension of the PCA takes advantage of the class information to try to maximize the distinction between observation classes. Furthermore, the PLS-DA analysis studies also assist in ascertaining the ECG-HRV indices that can differentiate between the respective groups. Specifically, the index values of Variable Importance in Projection (VIP) score in PLS-DA are employed to discover the ECG-HRV indices of discriminatory relevance.
2.8. Blood pressure measurement
The systolic blood pressure measurement of rats was carried out using non-invasive blood pressure (NIBP) module of the Power Lab system (AD instrument, Australia). NIBP utilizes a pulse transducer and tail-cuff to compute blood pressure based on the periodical obstruction of tail blood flow. Briefly, the rats were gently held in a perspex restrainer of appropriate size. A towel was placed over the restrainer, which helped the animal to remain calm. A tail-cuff was placed over the tail of the animal and positioned so that the pulse transducer lies in close proximity to the tail vein. The animal is then allowed to acclimatize for at least 30 min. Meanwhile, in LabChart, channels were setup to record pressure and pulse signals. The range of pulse channel and pressure channel was set at 50 mV and 1 V, respectively. Unit conversion was adjusted as 0 V = 0 mmHg and 1 V = 300 mmHg. The blood pressure was then recorded using the start/stop button on the NIBP controller (Alamgeer et al., 2013).
2.9. IL-6 measurement
IL-6 levels in serum of the respective groups (i.e., control, CUS-VEH, and CUS-DCN) were determined using the enzyme-linked immunosorbent assay (ELISA) Kit as per manufacturer's instructions (Abcam, United States). In brief, 100 μL of sample or standard was added to the wells of the kit plate and incubated at room temperature for 2.5 hours. Post incubation, the solution from the wells was discarded and wells were gently washed with 1X wash buffer. 100 μL of prepared biotin antibody was then added to each well, followed by incubation at room temperature for 1 hour with gentle shaking. The solution from the wells was again discarded and washed. Thereafter, 100 μL of 1X HRP-Streptavidin solution was added into each well and incubated for 45 min at room temperature with gentle shaking. The well solution was again discarded and washed, followed by the addition of TMB one-step development solution (100 μL) in each well. The plate is again incubated for 30 min at room temperature and subsequently, stop solution (50 μL) is added to each well. The resulting reaction was immediately read at 450nm using an ELISA plate reader (Chen et al., 2012).
2.10. Statistical analysis
All data were presented as mean ± SD and statistical analysis was carried out using GraphPad Prism software (8.01). For comparison involving only two groups, unpaired student t-test was used and for comparison between more than two groups, one-way ANOVA followed by Tukey's multiple comparisons test was employed. Results with (P < 0.05∗), (P < 0.01∗∗), (P < 0.001∗∗∗), (P < 0.0001∗∗∗∗) were considered as statistically significant.
3. Results
3.1. Effect of chronic unpredictable stress on body weight
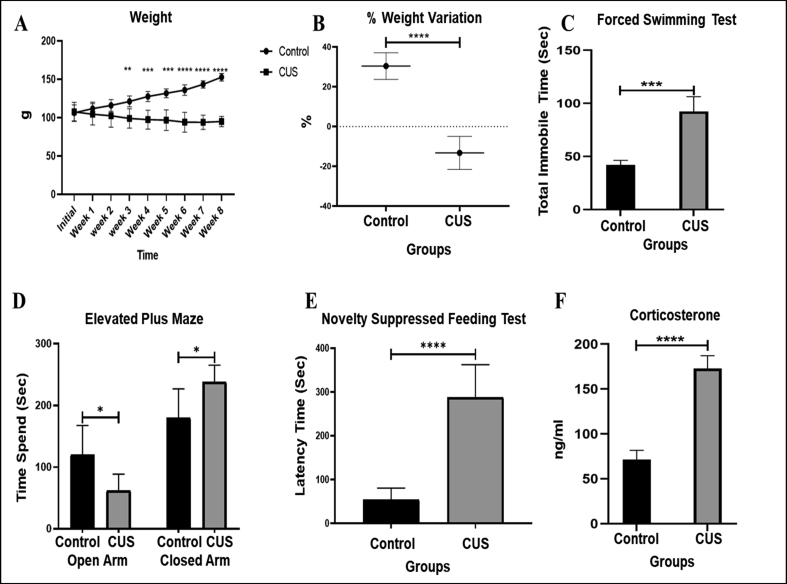
Reduced body weight is an important feature of stressed animals (Monteiro et al., 2015). The CUS paradigm used in our study also resulted in reduced weight gain in CUS rats compared to the control group that gained weight over the time. A significant (P = 0.004) difference in body weight of CUS vs. control rats appeared in the 3rd week which was observed till the end of the stress exposure period. The negative values of % weight variation (P < 0.0001) observed in CUS rats indicated that some rats at the end of CUS exposure weighed even lesser then their initial weight at the start of the experiment (Figure 2A).
Figure 2.
Standardisation of CUS paradigm in rats. (A) The body weight of control and CUS group were measured every week, before the start of stress protocol till the end. The CUS group animals gained less weight compared to control group. (B) Body weight assessment revealed significantly less weight variation (%) in CUS rats depicting weight loss as well as less weight gain in rats exposed to 8 weeks of CUS. (C) In force swimming test the immobility time was found significantly increased in CUS rats compared to control. (D) Elevated plus maze test revealed increased time spent in closed arms by CUS rats compared to control group. (E) Novelty suppressed feeding test revealed increased latency to the first bite by CUS rats, revealing depressive behaviour. (F) Serum corticosterone level was significantly elevated in CUS rats after 8-week of stress paradigm. All parameters were compared between the CUS and control groups using unpaired t-test (∗P<0.05, ∗∗ P<0.01, ∗∗∗ P<0.001, ∗∗∗∗ P<0.0001).
3.2. Effect of chronic unpredictable stress on behavioural responses
Anxiety/depressive-like behavioural response in rats is a strong predictor of stress-related conditions (Sequeira-Cordero et al., 2019) and these behavioural changes are frequently assessed using FST, EPMT and NSFT. FST is one of the most accepted animal model to study depressive-like behaviour in rodents, where immobility time is used as a measure of depression. The 8 week stress paradigm resulted in a significantly (P = 0.0005) higher immobility time in CUS rats compared to control rats in FST (92.25 vs. 42 s, Figure 2C). The EPMT revealed that CUS rats spent significantly (P < 0.05) more time in closed arm compared to control rats (238.33 ± 26.89 vs. 179.66 ± 47.27 s, respectively), suggesting induced unconditioned anxiety-like behaviour in rats (Figure 2D). The NSFT, on the other hand, assesses the latency of food-deprived rats for their first bite when presented with food. The CUS exposed rats showed increased latency to first bite (288 ± 74.30 vs. 54 ± 26.51 s, respectively; P < 0.0001) indicating decreased motivation levels and depression-like behaviour (Figure 2E).
3.3. Effect of chronic unpredictable stress on corticosterone level in serum samples
Elevated serum corticosterone is a well-known marker for presence of stress in animals. A two-fold increase in corticosterone level was observed in serum of CUS rats, compared to the control rats (172.77 ± 14.24 vs. 71.36 ± 10.42 ng/ml, respectively; P < 0.0001) (Figure 2F).
3.4. Effect of chronic unpredictable stress on cardiac functions
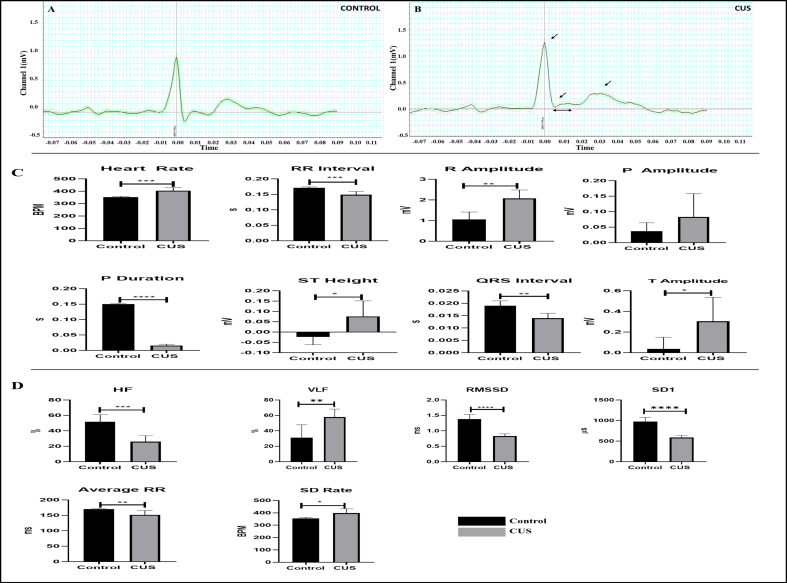
ECG recordings revealed significantly elevated heart rate (404.725 ± 26.35 vs. 351.725 ± 6.224 bpm; P = 0.0007) in CUS exposed rats (Figure 3). The sinus rhythm of CUS rats showed elevated R Amplitude (2.071 ± 0.41 vs. 1.058 ± 0.355 mV; P = 0.0010), ST height (0.076 ± 0.075 vs. -0.024 ± 0.038 mV; P = 0.0155) and T amplitude (0.305 ± 0.232 vs. 0.037 ± 0.113 mV; P = 0.0292) together with decreased P duration (0.016 ± 0.004 vs. 0.15 ± 0.002 s; P < 0.0001), RR interval (0.149 ± 0.01 vs. 0.171 ± 0.003 s; P = 0.0004) and QRS interval (0.014 ± 0.002 vs. 0.019 ± 0.002 s; P = 0.0015). The time-domain analysis of HRV revealed lowered average RR (151.625 ± 13.446 vs. 170.05 ± 1.879 s; P = 0.0077), RMSSD (0.83 ± 0.074 vs. 1.378 ± 0.149 %; P < 0.0001) and SD1 (P < 0.0001). The frequency-domain analysis revealed significantly decreased HF (25.98 ± 7.852 vs. 51.628 ± 9.43 %; P = 0.0005). Furthermore, persistent exposure to stress caused a significant (P = 0.0001) rise in systolic blood pressure in CUS animals (133.56 ± 7.82 mmHg) compared to control animals (109.94 ± 5.09 mmHg) (Figure 3).
Figure 3.
Effect of chronic unpredictable stress on cardiac function in CUS rats. (A–B) represents the average ECG pattern of a sample control and CUS rat, respectively. CUS rat has significantly altered R amplitude, T amplitude with elevated ST segment compared to control. (C) represents the effect of CUS on various ECG parameters. ECG analysis revealed significantly elevated heart rate (HR), R Amplitude T Amplitude along with decreased RR interval, P duration and QRS interval in CUS rats. (D) represents the effect of chronic stress on various HRV indices. HRV analysis by LabChart revealed that chronic stress resulted in reduced HF, RMSSD, SD1, indicating dominance of SNS and diminished vagal tone. All parameters were compared between the CUS and control groups using unpaired t-test (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001).
3.5. Docking studies of diacerein for IL-6 interaction
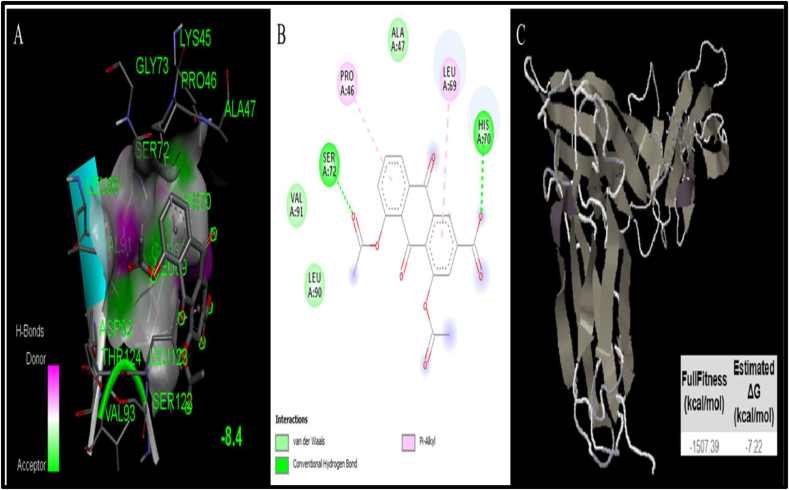
Docking studies predict binding interaction between receptor and its ligand. Two docking software's viz. AutoDock 4.0 and SwissDock, were used to identify the binding properties of diacerein with IL-6. The binding affinity of diacerein to IL-6 receptor were -8.4 kcal/mol and -7.22 kcal/mol as measured by AutoDock 4.0 and SwissDock, respectively. These binding affinities were attributed to 2 hydrogen bonds and 2 pi-alkyl bonds. The amino acids involved in the interaction include LYS 45, GLY 73, PRO 46, ALA 47, SER 72, LEU 90, HIS 70, VAL 91, GLN 68, LEU 69, ASP 92, THR 124, LEU 123, VAL 93, SER 122 (Table 1). Diacerein and IL-6 receptor complex, along with the amino acids involved in binding is shown in Figure 4.
Table 1.
Binding affinities of Diacerein with IL-6 (PDB:1N26). Comparative studies were performed using AutoDock 4.0.
| Lead molecule | Target with PDB | Amino acids involved in Interactions | Affinity (kcal/mol) | H-bonds |
|---|---|---|---|---|
| Diacerein | IL-6 (PDB: 1N26) | LYS 45, GLY 73,PRO 46, ALA 47, SER 72, LEU 90, HIS 70, VAL 91, GLN 68, LEU 69, ASP 92, THR 124, LEU 123, VAL 93, SER 122 | -8.4 | 2 |
Figure 4.
Docking studies of diacerein with IL-6 (PDB:1N26). (A) The output of AutoDock 3D diagram shows the binding site residues of IL-6 protein with the diacerein, with binding affinity of -8.44 kcal/mol. The residues in the binding site are shown in green color. Diacerein is shown in gray color. (B) The 2D structure exhibits different types of interactions formed between IL-6R and diacerein. The dark green and purple dotted lines indicate H-bond and Pi-Alkyl interactions between IL-6R and diacerein, respectively. (C) The interaction result of IL-6R with the diacerein and the obtained ΔG value was -7.22 kcal/mol.
3.6. Effect of diacerein treatment on chronic stress-induced change in peripheral IL-6
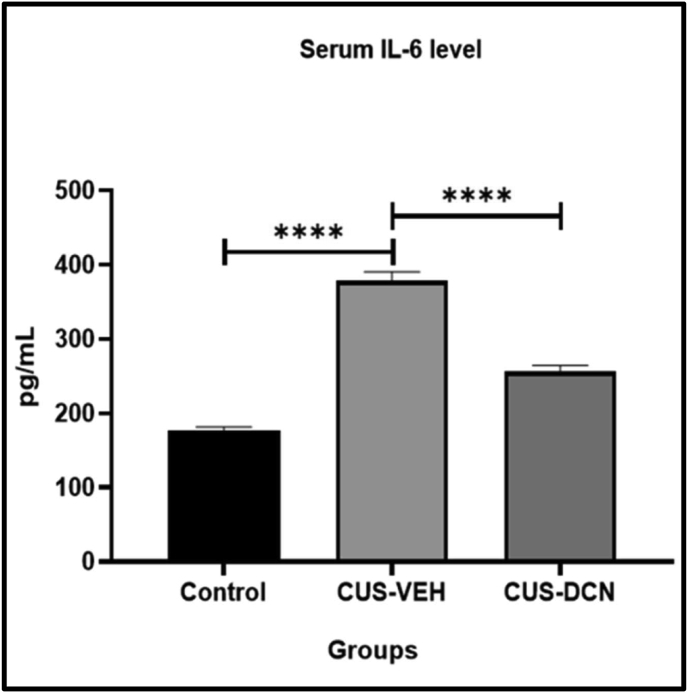
The effect of diacerein treatment on IL-6 levels in CUS rats was quantified in serum after 3 weeks of CUS exposure. A two-fold increase in IL-6 level was observed in serum samples of CUS-VEH rats, compared to the control group (378.29 ± 12.12 vs. 176.29 ± 5.29 pg/mL, respectively; P < 0.0001). Twenty days of diacerein treatment significantly prevented the CUS induced rise in serum IL-6 levels (256.34 ± 8.2 vs. 378.29 ± 12.12 pg/mL; P < 0.0001) of rats (Figure 5).
Figure 5.
Effect of diacerein on IL-6 expression in CUS rats. Serum analysis revealed significant rise in IL-6 levels in CUS rats, which was significantly reduced in rats treated with diacerein. Values are presented as Mean ± SD; each group contains 6 animals. All parameters were compared to CUS group using one-way ANOVA test (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001).
3.7. Effect of diacerein treatment on chronic stress-induced change in ECG-HRV parameters and systolic blood pressure
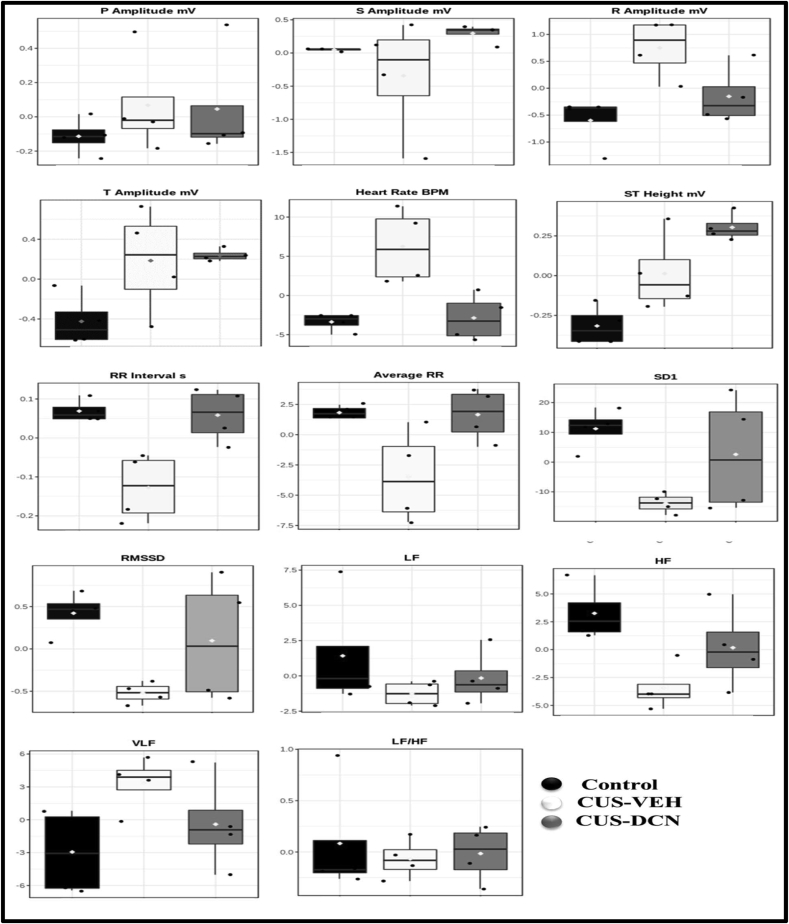
The ECG paradigm is regarded as a reflection of cardiac activity. Diacerein treatment significantly prevented the CUS-induced rise in R Amplitude, P Amplitude, and Heart Rate in the treated group. However, the treatment failed to normalize elevated ST height and T amplitude induced by chronic stress. In HRV, all components, including RMSSD, HF, SD1, except VLF, were decreased in chronic stress conditions as depicted in Box-cum-whisker plots. Administration of diacerein in CUS rats significantly prevented the CUS-induced reduction in HF, SD1 RMSSD, representing restoration of sympatho-vagal balance. Moreover, the CUS induced rise in VLF was also prevented by diacerein treatment (Figure 6).
Figure 6.
Effect of diacerein treatment on ECG and HRV parameters of CUS rats. Box-cum-whisker plots showing quantitative variations in relative signal integrals of various important components of ECG and HRV. In the box plot, the boxes denote interquartile ranges, the horizontal line inside the box denotes the median, and the bottom and top boundaries are 25th and 75th percentiles, respectively. Lower and upper whiskers are 5th and 95th percentiles, respectively.
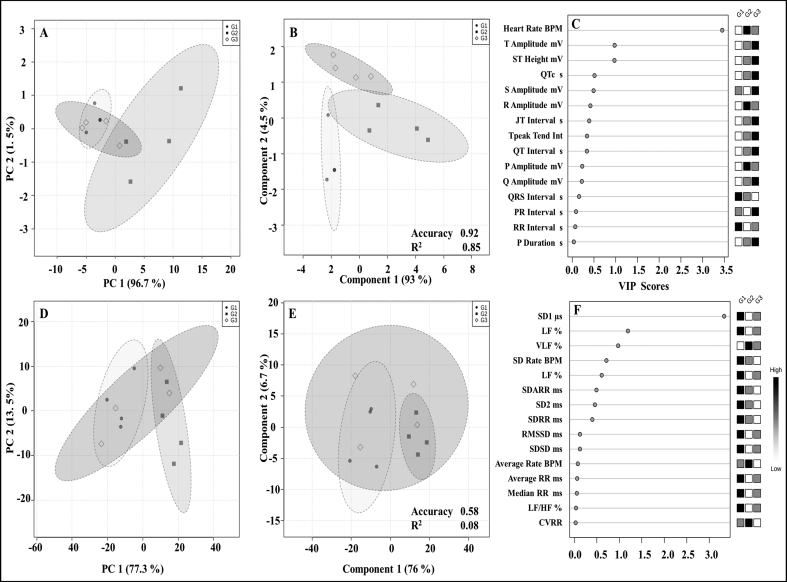
PCA analysis of ECG-HRV indices in the respective groups suggests therapeutic potential of diacerein treatment in CUS rats. The diacerein treatment group showed a compressive shift towards the control group, which was further validated by the PLS-DA model. The prediction accuracy of the PLS-DA model in ECG and HRV was found to be 0.92 and 0.58, respectively. The VIP score of the PLS-DA model with all important variables of ECG-HRV indices is shown in Figure 7. In the present study, there were five ECG parameters (Heart Rate, T amplitude, ST height, QTc and S amplitude) which showed a higher score than the threshold value of 0.5. In addition, six HRV parameters (SD1, HF, VLF, SD Rate, LF and SDARR) were higher than the threshold value of 0.5 VIP score.
Figure 7.
PCA, PLS-DA and VIP score of ECG and HRV. (A–B) represents the PCA and PLS-DA plot of ECG parameters involving all groups, respectively, with statistical values in the form of regression (R2 0.85). (D–E) represents the PCA and PLS-DA plot of HRV parameters involving all groups, respectively, with statistical values in the form of regression (R2 0.58). Circles indicate the 95% confidence interval for each class. The LOOCV method is used in cross-validation, using accuracy as a performance measure in the MetaboAnalyst web server. (C&F) represents the VIP scores of ECG and HRV in decreasing order of their respective VIP score values. G1, G2 and G3 indicate control, CUS-VEH and CUS-DCN groups, respectively.
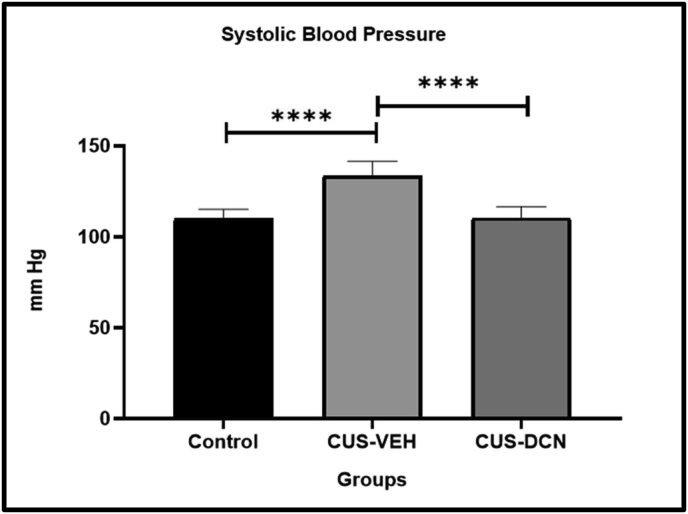
Interestingly, diacerein treatment also significantly (P < 0.0001) prevented the CUS induced rise in systolic BP (110.20 ± 6.24 mmHg) of CUS rats (Figure 8).
Figure 8.
Effect of diacerein treatment on CUS induced rise in systolic blood pressure. The CUS paradigm resulted in an increase in systolic blood pressure. This rise in systolic BP was prevented in diacerein-treated CUS rats. Values are presented as Mean ± SD; each group contains 6 animals. All parameters were compared to CUS group using one-way ANOVA test (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001).
4. Discussion
Stress is part of everyday life however prolonged stress can have serious consequences on human health. Various individuals, such as night-shift workers, time zone travelers, intensive care personnel, military personnel, and others, are particularly vulnerable to the negative effects of long-term stress. Prolonged stress can reduce longevity as well as enhance morbidity rate in susceptible individuals (Salleh, 2008). Most cardiologists believe that increased stress levels, together with inadequate diet and lack of physical activity, may enhance the susceptibility of urbanites to cardiovascular ailments. According to studies, people who suffer from stress-related anxiety and depression have two-fold increased risk of developing heart-related diseases (Batty et al., 2014). There is a well-established correlation between cardiac arrhythmia and psychological stress (Buckley and Shivkumar, 2016). Holistic interventions such as meditation, yoga, and exercise may help to reduce the harmful impact of chronic stress. Nevertheless, blockade of chronic stress associated patho-mechanisms could be an effective strategy to prevent the harmful consequences of chronic stress on cardiovascular functions.
In this study, we first validated a rat model of chronic stress by exposing rats to 8 weeks of random psychological and physical stressors and subsequently assessing them for presence of stress and cardio-vascular complications. We observed stunted growth, elevated corticosterone levels and depressive-like behaviours in CUS rats post 8 weeks of random stress exposure which are the characteristic features of stressed animals (Monteiro et al., 2015). Corticotrophin-releasing factor (CRF) possess strong anorectic effects and hyperactivation of the CRF parvocellular paraventricular nucleus of the hypothalamus (PVNp) neurons in response to chronic stress (Lenglos et al., 2013), which might have caused a sustained decrease in body weight gain in CUS exposed rats. The overactive Hypothalamus Pituitary Adrenal axis and overactive sympatho-adrenomedullary axis caused elevated corticosterone levels and anxiety/depressive-like behaviour, respectively (Sharpley, 2009).
Chronic stress leads to detrimental effects on heart contractility and conduction properties (Crestani, 2016). The cardiac functions assessment of CUS rats by ECG analysis revealed persistent elevation of heart rate, which is consistent with several reports of resting tachycardia in animal models of chronic stress (Crestani, 2016). An increase in average R amplitude, T Amplitude, P Amplitude and ST Height of the sinus rhythm, was observed in animals exposed to chronic stress, suggesting remodeling of the heart's electrical conduction system. These changes in the sinus rhythm are associated with altered morphology and function of the heart. R Amplitude accounts for ventricular activity, a rise in R amplitude is indicative of increase in ventricular dilation, which is associated with myocardial infarction (MI) (Surawicz and Knilans, 2008). P amplitude controls the enlargement of the atrium, and increased P amplitude is suggestive of right atrial enlargement. ST-segment represents the interval between depolarisation and repolarisation of the ventricles and elevated ST segment is usually associated with MI (Bin-Jaliah, 2017). T Amplitude accounts for ventricular repolarisation. Tikkanen et al. (2015) has demonstrated the association of altered T Amplitude with cardiovascular disorders (Tikkanen et al., 2015). Previous studies also suggests that rats exposed to stress displayed cardiac hypertrophy, altered sinus rhythm, and enhanced susceptibility to cardiac arrhythmias (Carnevali et al., 2013; Sgoifo et al., 2015). Stress has also shown to alters left atrial electrophysiology, indicating that stress promotes adverse transient electrical changes to provide the necessary substrate for atrial fibrillation (O'Neal et al., 2017).
Heart Rate Variability serves as a non-invasive technique to evaluate autonomic regulations of the heart. Root mean square of successive beat-to-beat differences (RMSSD) and SD1 values are time domain HRV indices. Both RMSSD and SD1 values were low in chronically stressed rats, suggesting decreased parasympathetic (vagal) tone (Shaffer and Ginsberg, 2017; De Souza Filho et al., 2019). Low frequency power (LF), high frequency power (HF) and HF/LF are frequency domain in HRV indices. The HF power reflects parasympathetic activity, whereas the LF power reflects a combination of sympathetic and parasympathetic activities. The LF/HF ratio is used to capture the relative balance of parasympathetic and sympathetic activity (Gehrmann et al., 2000; Mishra et al., 2018). The reduced LF/HF ratio in chronically stressed rats suggests sympatho-vagal imbalance induced by chronic stress. Moreover, lower HF value also suggests decreased vagal tone in chronically stressed rats.
In our studies, elevated IL-6 levels in serum of CUS rats was found even 3 weeks after the last day of stress exposure which is consistent with previous reports that showed elevated pro-inflammatory cytokines following chronic stress in the brain and periphery (Yang et al., 2015; Munshi et al., 2020). The release of pro-inflammatory cytokines from macrophages in the periphery and microglia in the brain is caused via the hyperactivated adrenergic system and corticosterone resistance (Leonard, 2010). Vagal (cholinergic) flow has also been shown to regulate the release of pro-inflammatory cytokines in the brain and periphery (Tagliari et al., 2011; Pavlov and Tracey, 2012). HRV analysis revealed reduced vagal tone in CUS rats in our study. Reduced vagal flow leads to decreased activity of the α7 nicotinic acetylcholine receptor (α7nAChR). The α7nAChR inhibits NF-κB nuclear translocation and activates janus kinase 2/signal transducer and activator of transcription 3 (JAK2–STAT3) mediated signaling cascade in macrophages and other immune cells thereby inhibiting the production of pro-inflammatory cytokines, such as IL-6 (Pavlov, 2019).
Based on a previous study by Bharti et al. (2016), we investigated diacerein for its ability to reduce IL-6 expression and IL-6 interaction with its receptors (Bharti et al., 2016). The computational docking studies using Autodock 4.0 showed a binding energy of -8.44 kcal/mol, indicating that diacerein has high affinity for IL-6 receptors. Moreover, we further validated our results using another docking software named SwissDock, which represented a binding affinity of -7.22 kcal/mol for the IL-6 receptor. The binding sites (LYS 45, GLY 73, PRO 46, ALA 47, SER 72, LEU 90, HIS 70, VAL 91, GLN 68, LEU 69, ASP 92, THR 124, LEU 123, VAL 93, SER 122) were similar to the binding sites for IL-6 inhibitors previously reported (Kumar Maurya et al., 2019).
Treatment of CUS rats with diacerein prevented the CUS induced rise in serum IL-6 levels. This effect could be similar to the effects of antipsychotic drugs, where continued inhibition of dopamine D2 receptors by anti-psychotics results in declined synthesis of dopamine in the limbic system. Moreover, the chronic stress induced alterations in ECG parameters were also prevented in diacerein treated CUS rats. The increase in R, T and P amplitude of sinus rhythm was prevented; however, it failed to prevent the elevated ST height and T amplitude. Moreover, the disturbance in sympatho-vagal balance in CUS rats was also prevented with diacerein treatment that was evidenced by normalized HRV indices viz RMSSD, SD1 and HF values. Thus, diacerein demonstrated protective effects against chronic stress-induced abnormalities in heart function as well as autonomic flow to heart. This effect could be attributed to reduced IL-6 levels in serum of diacerein treated CUS rats. Previous studies have reported expression of IL-6 receptors in sympathetic neurons and contribution of increased cerebrospinal fluid IL-6 to sympathetic dysregulation (März et al., 1998; Helwig et al., 2008).
Animal models have consistently failed to replicate epidemiological findings that associate chronic stress with hypertension in humans. However, several studies in which rats were subjected to variable stress for an extended period of time and the tail-cuff method used to assess arterial pressure, reported persistently elevated blood pressure (Crestani, 2016). Inflammation of forebrain and hindbrain nuclei which regulates the sympathetic nervous system outflow from the brain to the periphery is believed to play a key element in the development of neurogenic hypertension (Winklewski et al., 2015). The inflammation mediated enhanced sympathetic nervous system outflow causes enhanced catecholamine release which increases heart rate and blood pressure. Moreover, Esteve et al. (2007) has also reported a negative correlation between IL-6 levels and endothelium-dependent vasodilation (Esteve et al., 2007). This endothelium-dependent vasodilation is found impaired in patients with essential hypertension that underscores the enhanced IL-6 levels association with elevated blood pressure. Nevertheless, the precise relationship between systolic blood pressure and IL-6 is still obscure. We observed high systolic BP in chronically stressed rats which was prevented with diacerein treatment. This protective effect on BP could be attributed to IL-6 inhibitory effect of diacerein. Various studies have also reported that hypertension facilitates remodeling of the structure and functions of the heart, which ultimately causes alterations in the ECG-HRV paradigm (Ogunsua et al., 2015). In our studies, we have found protective effect of diacerein on ECG-HRV and systolic BP which could be interrelated.
Thus, our data supports the notion that diacerein could be used as a preventive measure in high-risk populations exposed to chronic stress associated cardiac dysfunctions. Diacerein cardioprotective effect was also reported previously by Torina et al. (2015) revealing that diacerein treatment post MI, attenuated left ventricular remodeling and improved cardiac functions in rats (Torina et al., 2015). Furthermore, studies by Fouad et al. (2020) and Calisto et al. (2012) demonstrated that administration of diacerein in control rats alone has no side-effect on inflammatory markers (Fouad et al., 2020) (Calisto et al., 2012). As mentioned previously, diacerein also inhibits IL-1β, hence its cardioprotective effect cannot be exclusively assigned to IL-6 inhibition. Therefore, further studies are warranted to understand the effect of diacerein on various pro-inflammatory cytokines in chronic stress associated cardiac dysfunctions.
5. Conclusion
Chronic stress is one of the major risk factors for cardiovascular diseases. Our findings suggest long-term chronic stress can trigger cardiovascular dysfunctions resulting in cardiac arrhythmia and hypertension. We further found elevated pro-inflammatory cytokine, IL-6, in chronically stressed rats suggesting IL-6 involvement in cardiac dysfunctions induced by prolonged stress. Moreover, diacerein due to its inhibitory effects on pro-inflammatory cytokines prevented chronic stress induced cardiac arrhythmia and rise in blood pressure which could be used as a preventive medicine for such conditions.
Declarations
Author contribution statement
Vipul Agarwal; Arjun Singh Kaushik: Performed the experiments and contributed equally.
Mujeeba Rehman; Rishabh Chaudhary: Analyzed and interpreted the data.
Talha Jawaid; Mehnaz Kamal: Contributed reagents, materials, analysis tools or data.
Vikas Mishra: Conceived and designed the experiments; wrote the paper.
Funding statement
Dr. Vikas Mishra was supported by University Grants Commission (F. 30–460/2019 (BSR), and Vipul Agarwal was supported by Indian Council of Medical Research (3/1/2(12)/CVD/NCD-II).
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abd Allah O.M. Beneficial effects of diacerein on adipokines and pro-inflammatory cytokines involved in diet-induced nonalcoholic steatohepatitis in rats. Int. J. Basic Clin. Pharmacol. 2017;6(4):811. [Google Scholar]
- Abd El-Fattah A.A., et al. Resveratrol and dimethyl fumarate ameliorate depression-like behaviour in a rat model of chronic unpredictable mild stress. Brain Res. 2018;1701:227–236. doi: 10.1016/j.brainres.2018.09.027. [DOI] [PubMed] [Google Scholar]
- Alamgeer, et al. Antihypertensive activity of aqueous-methanol extract of Berberis orthobotrys Bien Ex Aitch in rats. Trop. J. Pharmaceut. Res. 2013;12(3):393–399. [Google Scholar]
- Almezgagi M., et al. Diacerein: recent insight into pharmacological activities and molecular pathways. Biomed. Pharmacother. 2020;131:110594. doi: 10.1016/j.biopha.2020.110594. [DOI] [PubMed] [Google Scholar]
- Arndt D.L., Peterson C.J., Cain M.E. Differential rearing alters forced swim test behavior, fluoxetine efficacy, and post-test weight gain in male rats. PLoS One. 2015;10(7):e0131709. doi: 10.1371/journal.pone.0131709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty G.D., et al. Psychological distress and risk of peripheral vascular disease, abdominal aortic aneurysm, and heart failure: pooling of sixteen cohort studies. Atherosclerosis. 2014;236(2):385–388. doi: 10.1016/j.atherosclerosis.2014.06.025. [DOI] [PubMed] [Google Scholar]
- Baune B.T., et al. Systemic inflammation (interleukin 6) predicts all-cause mortality in men: results from a 9-year follow-up of the MEMO study. Age. 2011;33(2):209–217. doi: 10.1007/s11357-010-9165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti R., et al. Diacerein-mediated inhibition of IL-6/IL-6R signaling induces apoptotic effects on breast cancer. Oncogene. 2016;35(30):3965–3975. doi: 10.1038/onc.2015.466. [DOI] [PubMed] [Google Scholar]
- Bin-Jaliah I. La quercetina inhibe el infarto al miocardio inducido por estrés crónico en ratas. Int. J. Morphol. 2017;35(4):1363–1369. [Google Scholar]
- Buckley U., Shivkumar K. Stress-induced cardiac arrhythmias: the heart-brain interaction. Trends Cardiovasc. Med. 2016:78–80. doi: 10.1016/j.tcm.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley D.I., et al. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. preventive services task force. Ann. Intern. Med. 2009:483–495. doi: 10.7326/0003-4819-151-7-200910060-00009. [DOI] [PubMed] [Google Scholar]
- Calisto K.L., et al. Diacerhein attenuates the inflammatory response and improves survival in a model of severe sepsis. Crit. Care. 2012;16(4) doi: 10.1186/cc11478. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Carnevali L., et al. Vagal withdrawal and susceptibility to cardiac arrhythmias in rats with high trait aggressiveness. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0068316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.C., et al. Wound repair and anti-inflammatory potential of Lonicera japonica in excision wound-induced rats. BMC Compl. Alternative Med. 2012;12 doi: 10.1186/1472-6882-12-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J., Wishart D.S., Xia J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Prot. Bioinform. 2019;68(1) doi: 10.1002/cpbi.86. [DOI] [PubMed] [Google Scholar]
- Crestani C.C. Emotional stress and cardiovascular complications in animal models: a review of the influence of stress type. Front. Physiol. 2016:251. doi: 10.3389/fphys.2016.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui B., et al. Stress-induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem-like cells. J. Clin. Invest. 2019;129(3):1030–1046. doi: 10.1172/JCI121685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza Filho L.F.M., et al. Evaluation of the autonomic nervous system by analysis of heart rate variability in the preterm infants. BMC Cardiovasc. Disord. 2019;19(1) doi: 10.1186/s12872-019-1166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M., Gangestad S.W. Rethinking IL-6 and CRP: why they are more than inflammatory biomarkers, and why it matters. Brain Behav. Immun. 2018;70:61–75. doi: 10.1016/j.bbi.2018.02.013. [DOI] [PubMed] [Google Scholar]
- Esteve E., et al. Serum interleukin-6 correlates with endothelial dysfunction in healthy men independently of insulin sensitivity. Diabetes Care. 2007;30(4):939–945. doi: 10.2337/dc06-1793. [DOI] [PubMed] [Google Scholar]
- Fontes J.A., Rose N.R., Čiháková D. The varying faces of IL-6: from cardiac protection to cardiac failure. Cytokine. 2015:62–68. doi: 10.1016/j.cyto.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad A.A., Abdel-Aziz A.M., Hamouda A.A.H. Diacerein downregulates NLRP3/caspase-1/IL-1β and IL-6/STAT3 pathways of inflammation and apoptosis in a rat model of cadmium testicular toxicity. Biol. Trace Elem. Res. 2020;195(2):499–505. doi: 10.1007/s12011-019-01865-6. [DOI] [PubMed] [Google Scholar]
- Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006;8(SUPPL. 2) doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel A.S., et al. IL-6 levels in acute and post myocardial infarction: their relation to CRP levels, infarction size, left ventricular systolic function, and heart failure. Eur. J. Intern. Med. 2004;15(8):523–528. doi: 10.1016/j.ejim.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Gager G.M., et al. Interleukin-6 level is a powerful predictor of long-term cardiovascular mortality in patients with acute coronary syndrome. Vasc. Pharmacol. 2020;135 doi: 10.1016/j.vph.2020.106806. [DOI] [PubMed] [Google Scholar]
- Gehrmann J., et al. Phenotypic screening for heart rate variability in the mouse. Am. J. Physiol. Heart Circ. Physiol. 2000;279(2 48-2) doi: 10.1152/ajpheart.2000.279.2.H733. [DOI] [PubMed] [Google Scholar]
- Golbidi S., Frisbee J.C., Laher I.X. Chronic stress impacts the cardiovascular system: animal models and clinical outcomes. Am. J. Physiol. Heart Circ. Physiol. 2015:H1476–H1498. doi: 10.1152/ajpheart.00859.2014. [DOI] [PubMed] [Google Scholar]
- Grosdidier A., Zoete V., Michielin O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011;39(SUPPL. 2) doi: 10.1093/nar/gkr366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B., et al. Object location and object recognition memory impairments, motivation deficits and depression in a model of Gulf War illness. Front. Behav. Neurosci. 2014;8(MAR) doi: 10.3389/fnbeh.2014.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helwig B.G., et al. Central nervous system administration of interleukin-6 produces splenic sympathoexcitation. Auton. Neurosci.: Basic and Clinical. 2008;141(1–2):104–111. doi: 10.1016/j.autneu.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota H., et al. Continuous activation of gp130, a signal-transducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice. Proc. Natl. Acad. Sci. U. S. A. 1995;92(11):4862–4866. doi: 10.1073/pnas.92.11.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst S.M., et al. IL-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14(6):705–714. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- Janssen S.P.M., et al. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation. 2005;111(8):996–1005. doi: 10.1161/01.CIR.0000156469.96135.0D. [DOI] [PubMed] [Google Scholar]
- Kaplanski G., et al. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003;24(1):25–29. doi: 10.1016/s1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Kumar Maurya A., et al. Chemical composition and skin inflammation protective profile of pulegone rich essential oil of Mentha arvensis L. Indian J. Exp. Biol. 2019 http://www.pubchem.ncbi.nlm.nih.gov Available at: [Google Scholar]
- Lenglos C., et al. Sex differences in the effects of chronic stress and food restriction on body weight gain and brain expression of CRF and relaxin-3 in rats. Gene Brain Behav. 2013;12(4):370–387. doi: 10.1111/gbb.12028. [DOI] [PubMed] [Google Scholar]
- Leonard E. The concept of depression as a dysfunction of the immune system. Curr. Immunol. Rev. 2010;6(3):205–212. doi: 10.2174/157339510791823835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.Z., Wang Y.X., Jiang C.L. Inflammation: the common pathway of stress-related diseases. Front. Hum. Neurosci. 2017:316. doi: 10.3389/fnhum.2017.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markousis-Mavrogenis G., et al. The clinical significance of interleukin-6 in heart failure: results from the BIOSTAT-CHF study. Eur. J. Heart Fail. 2019;21(8):965–973. doi: 10.1002/ejhf.1482. [DOI] [PubMed] [Google Scholar]
- Martel Pelletier J., Pelletier J.P. Effects of diacerein at the molecular level in the osteoarthritis disease process. Therap. Adv. Musculoskelet. Dis. 2010:95–104. doi: 10.1177/1759720X09359104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- März P., et al. Sympathetic neurons can produce and respond to interleukin 6. Proc. Natl. Acad. Sci. U. S. A. 1998;95(6):3251–3256. doi: 10.1073/pnas.95.6.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maydych V. The interplay between stress, inflammation, and emotional attention: relevance for depression. Front. Neurosci. 2019 doi: 10.3389/fnins.2019.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer E.A. The neurobiology of stress and gastrointestinal disease. Gut. 2000:861–869. doi: 10.1136/gut.47.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Maletic V., Raison C.L. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatr. 2009:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R.K., et al. Palonosetron attenuates 1,2-dimethyl hydrazine induced preneoplastic colon damage through downregulating acetylcholinesterase expression and up-regulating synaptic acetylcholine concentration. RSC Adv. 2016;6(46):40527–40538. [Google Scholar]
- Mishra V., Gautier N.M., Glasscock E. Simultaneous video-EEG-ECG monitoring to identify neurocardiac dysfunction in mouse models of epilepsy. JoVE. 2018;(131):2018. doi: 10.3791/57300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro S., et al. An efficient chronic unpredictable stress protocol to induce stress-related responses in C57BL/6 mice. Front. Psychiatr. 2015;6(FEB) doi: 10.3389/fpsyt.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G.M., et al. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998;19(14):1639–1662. [Google Scholar]
- Munshi S., et al. Repeated stress induces a pro-inflammatory state, increases amygdala neuronal and microglial activation, and causes anxiety in adult male rats. Brain Behav. Immun. 2020;84:180–199. doi: 10.1016/j.bbi.2019.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunsua A.A., et al. Atrial fibrillation and hypertension: mechanistic, epidemiologic, and treatment parallels. Method. DeBakey Cardiovas. J. 2015:228–234. doi: 10.14797/mdcj-11-4-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neal W.T., et al. The association between acute mental stress and abnormal left atrial electrophysiology. J. Cardiovasc. Electrophysiol. 2017;28(10):1151–1157. doi: 10.1111/jce.13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos I.C., et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psy. 2015;2(11):1002–1012. doi: 10.1016/S2215-0366(15)00309-0. [DOI] [PubMed] [Google Scholar]
- Pavelka K., et al. Diacerein: benefits, risks and place in the management of osteoarthritis. An opinion-based report from the ESCEO. Drugs Aging. 2016;33(2):75–85. doi: 10.1007/s40266-016-0347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov V.A. Collateral benefits of studying the vagus nerve in bioelectronic medicine. Bioelectronic Medicine. 2019;5(1) doi: 10.1186/s42234-019-0021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov V.A., Tracey K.J. The vagus nerve and the inflammatory reflex - linking immunity and metabolism. Nat. Rev. Endocrinol. 2012:743–754. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau A.R., et al. Increased basolateral amygdala pyramidal cell excitability may contribute to the anxiogenic phenotype induced by chronic early-life stress. J. Neurosci. 2015;35(26):9730–9740. doi: 10.1523/JNEUROSCI.0384-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat J.K., et al. Transcutaneous vagus nerve stimulation regulates the cholinergic anti-inflammatory pathway to counteract 1, 2-dimethylhydrazine induced colon carcinogenesis in albino wistar rats. Front. Pharmacol. 2019;10(MAY) doi: 10.3389/fphar.2019.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozanski A., Blumenthal J.A., Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99(16):2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- Salleh M.R. Life event, stress and illness. Malays. J. Med. Sci. : MJMS. 2008;15(4):9–18. https://pubmed.ncbi.nlm.nih.gov/22589633 Available at: [PMC free article] [PubMed] [Google Scholar]
- Sequeira-Cordero A., et al. Behavioural characterisation of chronic unpredictable stress based on ethologically relevant paradigms in rats. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-53624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgoifo A., et al. Autonomic dysfunction and heart rate variability in depression. Stress. 2015:343–352. doi: 10.3109/10253890.2015.1045868. [DOI] [PubMed] [Google Scholar]
- Shaffer F., Ginsberg J.P. An overview of heart rate variability metrics and norms. Front. Pub. Healt. 2017;5:258. doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpley C.F. Neurobiological pathways between chronic stress and depression: dysregulated adaptive mechanisms? Clin. Med. Insights Psychiatry. 2009;2 CMPsy.S3658. [Google Scholar]
- Surawicz B., Knilans T.K. 2008. Chou’s Electrocardiography in Clinical Practice: Adult and Pediatric, Chou’s Electrocardiography in Clinical Practice: Adult and Pediatric. [Google Scholar]
- Tafet G.E., Nemeroff C.B. The links between stress and depression: psychoneuroendocrinological, genetic, and environmental interactions. J. Neuropsychiatry Clin. Neurosci. 2016;28(2):77–88. doi: 10.1176/appi.neuropsych.15030053. [DOI] [PubMed] [Google Scholar]
- Tagliari B., et al. Chronic variable stress alters inflammatory and cholinergic parameters in hippocampus of rats. Neurochem. Res. 2011;36(3):487–493. doi: 10.1007/s11064-010-0367-0. [DOI] [PubMed] [Google Scholar]
- Tamashiro K.L., et al. Chronic stress, metabolism, and metabolic syndrome. Stress. 2011:468–474. doi: 10.3109/10253890.2011.606341. [DOI] [PubMed] [Google Scholar]
- Tikkanen J.T., et al. Electrocardiographic T wave abnormalities and the risk of sudden cardiac death: the Finnish perspective. Ann. Noninvasive Electrocardiol. 2015:526–533. doi: 10.1111/anec.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torina A.G., et al. Diacerein improves left ventricular remodeling and cardiac function by reducing the inflammatory response after myocardial infarction. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0121842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay R., et al. Evaluation of prophylactic diacerein treatment on anti-arthritic activity in freund’s complete adjuvant-induced arthritis in rat model. J. Clin. Diagn. Res. 2021;15(1) [Google Scholar]
- Wainstein M.V., et al. Elevated serum interleukin-6 is predictive of coronary artery disease in intermediate risk overweight patients referred for coronary angiography. Diabetol. Metab. Syndrome. 2017;9(1) doi: 10.1186/s13098-017-0266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklewski P.J., et al. Brain inflammation and hypertension: the chicken or the egg? J. Neuroinflammation. 2015 doi: 10.1186/s12974-015-0306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., et al. Changes in proinflammatory cytokines and white matter in chronically stressed rats. Neuropsychiatric Dis. Treat. 2015;11:597–607. doi: 10.2147/NDT.S78131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., et al. Interleukin-6 as a predictor of the risk of cardiovascular disease: a meta-analysis of prospective epidemiological studies. Immunol. Invest. 2018;47(7):689–699. doi: 10.1080/08820139.2018.1480034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.