Abstract
The gastrointestinal tract (GIT) affects not only local diseases in the GIT but also various systemic diseases. Factors that can affect the health and disease of both GIT and the human body include 1) the mucosal immune system composed of the gut-associated lymphoid tissues and the lamina propria, 2) the intestinal barrier composed of mucus and intestinal epithelium, and 3) the gut microbiota. Selective delivery of drugs, including antigens, immune-modulators, intestinal barrier enhancers, and gut-microbiome manipulators, has shown promising results for oral vaccines, immune tolerance, treatment of inflammatory bowel diseases, and other systemic diseases, including cancer. However, physicochemical and biological barriers of the GIT present significant challenges for successful translation. With the advances of novel nanomaterials, oral nanomedicine has emerged as an attractive option to not only overcome these barriers but also to selectively deliver drugs to the target sites in GIT. In this review, we discuss the GIT factors and physicochemical and biological barriers in the GIT. Furthermore, we present the recent progress of oral nanomedicine for oral vaccines, immune tolerance, and anti-inflammation therapies. We also discuss recent advances in oral nanomedicine designed to fortify the intestinal barrier functions and modulate the gut microbiota and microbial metabolites. Finally, we opine about the future directions of oral nano-immunotherapy.
Keywords: Oral immunotherapy, Nanomedicine, Mucosal Immunity, Peyer’s patches, Lamina propria, Intestinal barrier, Gut microbiota
Graphical Abstract
1. Introduction
The gastrointestinal tract (GIT) is the largest interface in the body that is in direct contact with the external environment [1, 2]. GIT affects not only local diseases in GIT (e.g., inflammatory bowel disease, IBD) but also various systemic diseases (e.g., diabetes and cancer) [3-8]. Thus, therapeutic approaches targeted to GIT have broad applications. When developing therapeutics for GIT, the following factors should be taken into account. 1) The mucosal immune system in GIT is composed of the GALT (the gut-associated lymphoid tissues including Peyer’s patches and isolated lymphoid follicles), lamina propria, and intestinal epithelium [9-11]. 2) The intestinal barrier is composed of mucus and intestinal epithelium [2, 8], and 3) there is a large number (> 1014) and variety (~10,000 species) of gut commensal microbes colonized in GIT [12, 13]. GALT composed of Peyer's patches and lymphoid follicles is a major site for inducing immune responses against antigens trafficked from the gut lumen. After adaptive immune system is activated via the inductive processes in GALT, activated B-cells and T-cells reach the gut lamina propria and other systemic mucosal regions to establish protective immunity [14-16]. On the other hand, when an antigen is processed in the lamina propria, the general immunological consequence is an immune tolerance to that specific antigen [11, 17]. Under the status of certain diseases (e.g., IBD, diabetes, and colorectal cancer), intestinal barrier functions are disrupted, resulting in over-reactive and dysregulated mucosal immune responses against gut-associated antigens [8, 18, 19]. Notably, the gut microbiota closely interacts with the intestinal barrier and mucosal immune system and provides microbial antigens and metabolites [4, 6, 8, 19, 20]. In addition, the composition and dysbiosis of gut microbiota, along with dysfunction of the intestinal barrier and the immune system, have great influences on the development, severity, and treatment of various GIT and systemic diseases [4, 6, 8, 19, 20]. Therefore, there is strong rationale for developing GIT-targeted therapeutics that can modulate the GIT factors.
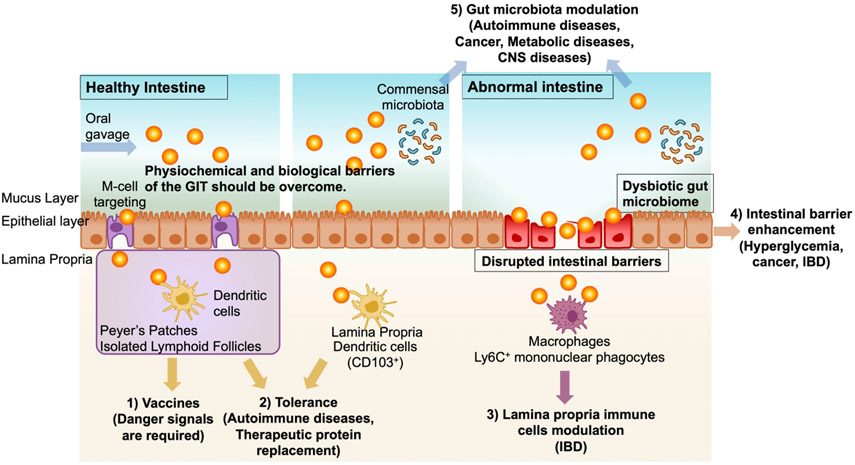
Compared with other routes of administration, oral immunotherapy has clear advantages for modulating the GIT factors, including the ease of targeting GIT, limited systemic drug exposure, simple self-administration, and patient compliance [10]. The major goals and challenges of oral immunotherapies include the following (Fig. 1). 1) Antigens employed in oral vaccine applications should be targeted to microfold (M) cells, which are epithelial cells specialized in transporting antigens from the lumen to lymphoid tissues, such as Peyer’s patches [10]. Immune cells in Peyer’s patches would then elicit antigen-specific immune responses and establish protective immunity in the GI mucosal surfaces as well as in the systemic compartments. 2) On the other hand, antigens taken up in the lamina propria are generally known to induce systemic and mucosal immune tolerance, and this forms the basis of experimental oral immunotherapies against autoimmune diseases and allergies [21]. 3) Furthermore, dysregulated lamina propria is associated with IBD and other GIT disorders; thus, the lamina propria as a potential target for oral immunotherapies [22]. 4) Moreover, disrupted intestinal barrier functions result in numerous pathologies, including IBD. Therefore, the recovery and reinforcement of the intestinal barrier functions are viable therapeutic approaches against GIT disorders [8]. 5) Lastly, emerging evidence indicates that dysbiosis of gut microbiota is linked with numerous diseases, including colitis, diabetes, and colorectal cancer [20, 23-25]. Thus, strategies that can restore the healthy gut microbiome have gained much attention as a new form of oral immunotherapy.
Figure 1. Biomedical applications of oral nano-immunotherapy.
Oral nano-immunotherapy serves as the therapeutic platform for 1) oral vaccines, 2) immune tolerance, 3) treatment of inflammatory bowel diseases, 4) enhancing the intestinal barrier, and 5) modulating the gut microbiota.
To achieve these goals, various therapeutics are under development. In the case of small molecule drugs, only a fraction of orally administered drugs typically reach the target sites in the GIT, while a large portion of the dose is excreted or systemically absorbed, often resulting in toxic systemic exposure and serious side effects [26, 27]. For instance, methylprednisolone can cause thymic involution, insomnia, depression, bone density loss, and moon face, while the side effects of mesalamine include itching, muscle or join pain, swelling of any part of the body, chest pain, heartburn, and dizziness [28, 29]. In contrast, macromolecules administered orally have limited systemic absorption due to their large sizes [30]. However, they are prone to structural changes, degradation, and loss of bioactivity by the harsh conditions of GIT, including the pH variation and proteolytic enzymes [31, 32]. Therefore, there is a great need for new oral drug delivery systems that can effectively protect their cargo from the harsh conditions of the GIT and selectively deliver drugs to desired target sites.
Nanomedicine has attracted much attention due to the abilities of nanoparticles (NPs) to protect cargo molecules from external stresses, deliver drugs to target tissues, and sustain drug release [33]. To achieve these goals, oral nanomedicine should overcome multiple physicochemical and biological barriers in the GIT, including the highly acidic environment in the stomach, pH variation and proteolytic enzymes along the GIT [31, 32]. In the case of NPs with weak acid or base groups, it should be taken into consideration that the pH variation will affect the ionizable groups and morphologies of NPs along the GIT [10]. For NPs targeted to Peyer’s patches or lamina propria, the intestinal epithelium and its mucus-secreting layers are critical barriers to overcome [34, 35]. Thus, it may be suitable to design NPs that adhere to the mucus layer and penetrate the intestinal epithelium. On the other hand, for NPs targeted to the small intestines where the majority of absorption processes occur, the short residence time (3–4 h) in the small intestine present additional challenges for the design and development of small intestine-targeting NPs [36]. Lastly, the design criteria for oral nanomedicine should take into account the properties of target tissues in healthy versus pathological states. For example, since the key features of IBD are leaky inflamed epithelium and loss of mucus layers [8, 19, 22], oral nanomedicine for treating IBD may passively reach the disrupted intestinal barriers and the lamina propria. In contrast, oral nanomedicine intended to modulate the gut microbiome should target the mucus layer since most commensal microbes reside in the mucosal layer in the gut lumen.
In this review paper, we first present the key GIT factors, including the immune system, intestinal barrier, and gut microbiota, and we review physicochemical and biological barriers of the GIT that oral nanomedicine needs to overcome. We also discuss recent progress in oral nanomedicine designed for oral vaccines, oral tolerance, and anti-inflammation applications. Lastly, we highlight the recent developments in oral nanomedicine for modulating the intestinal barrier functions and gut microbiota as a new therapeutic approach against various diseases.
2. Interactions between the immune system, intestinal barrier, and gut microbiome.
2.1. The GIT immune system
It is estimated that GIT harbors up to 70% of lymphocytes in the body, and thus, GIT is the largest immunological organ [1, 2]. The intestinal immune system can be divided into inductive and effector sites. Inductive sites include GALT (e.g., Peyer’s patches and isolated lymphoid follicles) and gut-draining mesenteric lymph nodes (mLNs). The main effector sites are epithelium and lamina propria that contains large populations of activated T-cells and antibody-secreting plasma cells as well as innate immune cells, such as macrophages and dendritic cells (DCs) [9-11]. The gastrointestinal immune system is constantly challenged with antigens from the lumen and therefore must be able to distinguish which antigens should be tolerated (e.g., self-antigens, food, symbiotic microbes) or not (e.g., pathogens, toxins) [11]. Typically, the intestinal immune environment (intestinal epithelium and lamina propria) is immunosuppressive due to the high levels of anti-inflammatory factors, such as IL-10, transforming growth factor (TGF)-β, and retinoic acid [11, 37].
In the intestinal innate immune system, the outermost sentinel is the intestinal epithelium that lines the intestine and serves as a physical barrier between the luminal contents and the host immune system [38]. The intestinal epithelium actively contributes to the innate immune responses and senses microbial organisms via pattern recognition receptors (PRRs), such as Toll like receptors (TLRs) [38]. Furthermore, the epithelium expresses the major histocompatibility complexes and serves as antigen-presenting cells [38]. Macrophages also has important housekeeping roles, including clearance of apoptotic or senescent cells, tissue remodeling, and maintenance of the immunoregulatory gut environment [39]. Under steady state conditions, Ly6Chigh monocytes) constitutively enter the intestinal mucosa (especially in the lamina propria) and differentiate locally into anti-inflammatory mature CX3C chemokine receptor 1(CX3CR1)high F4/80+ macrophages that express scavenger receptors and major histocompatibility complex-II. They are hyporesponsive to pro-inflammatory stimuli but highly phagocytic against invading commensals or pathogens [39, 40]. Furthermore, the resident macrophages help CD103+ DCs to induce oral tolerance by directly sampling the luminal contents via dendrites extended between the cells of the intestinal epithelial barrier [41]. CX3CR1high macrophages also produce a large amounts of IL-10 which enhances the secondary expansion of regulatory T cells (Tregs) in the mucosa and may also condition newly arrived monocytes [40]. In turn, TGF-β generated by Tregs may condition newly extravasated monocytes. Resident macrophages can also help maintain the epithelial integrity by secreting prostaglandin E2, which contributes to physiological tissue remodeling [42].
The gut homeostasis can be perturbed by inflammation or infection. The epithelium plays an important role in the initial colonization and sensing of an infection by PRRs such that it transduces the signal to other innate cells that, in turn, amplify the response [38]. When homeostasis is perturbed by inflammation or infection, the normal pattern of monocyte differentiation is disrupted, leading to the accumulation of potent pro-inflammatory effector Ly6Chigh monocytes and CX3CR1int macrophages [40]. Macrophages sense the perturbation through TLRs and activate intracellular pro-inflammatory signals. Resident monocytes contribute to the recruitment of neutrophils through production of macrophage-derived chemokines [43]. Neutrophils in the blood circulation can sense the chemoattractant gradient and extravasate the vascular endothelium to reach the intestinal lamina propria [43]. Neutrophils are equipped with elegant defense mechanisms, such as nicotinamide adenine dinucleotide phosphate, oxidase-mediated reactive oxygen species burst, antimicrobial peptides, myeloperoxidases, and neutrophil extracellular traps [43]. Neutrophils also contribute to the recruitment of other immune cells and facilitate mucosal healing by releasing mediators necessary for the resolution of inflammation [44]. Importantly, during inflammation, the resident CX3CR1high macrophages retain their anti-inflammatory characteristics, e.g. IL-10 production [39, 40]. Classical monocytes and resident CX3CR1high macrophages play regulatory roles by controlling and removing the activated harmful neutrophils [39, 40, 43, 44].
Amplification of the innate response can have adverse effects on the host. Although the above responses are clearly beneficial, excessive recruitment and accumulation of pro-inflammatory monocytes, activated neutrophils, and other innate immune cells in the intestine under pathological conditions is associated with mucosal injury and debilitating disease symptoms [39, 43, 45, 46]. Furthermore, the production of tumor necrosis factor (TNF)-α from activated innate immune cells has an important role in protecting the host, but when produced in high quantities, or over extended periods of time, it contributes to tissue damage, including DNA damage [47]. As most cells expresses receptors for TNF-α, those can lead to an enhanced accumulation of reactive oxygen species and increased levels of chemokines and other pro-inflammatory responses. TNF-α has promiscuous effects, rendering it an effective therapeutic target in IBD patients [48].
As the primary inductive sites of adaptive immune responses, GALT and mLNs harbor memory B- and T-cells that subsequently migrate to the mucosal effector sites (both gut and systemic mucosal effector sites) via the lymphatic system and exert mucosal immune responses [14, 15] (Fig. 2). GALT contains follicular B cell zones, inter-follicular T cell zones, and antigen-presenting cells (APCs), such as DCs and macrophages [49]. In organized tissues of GALT, such as Peyer’s patches and isolated lymphoid follicles, specialized M cells in the epithelium overlying Peyer’s patches and the lymphoid follicles play a pivotal role in transcellular transport of antigens [11, 50]. M cells pass antigens to DCs that lie either below the epithelium or in a “pocket” created at the basolateral surface of M cells [11, 50]. Antigens can also be directly taken up by DCs in the GALT from the lumen by their extended dendrites [51]. Afterwards, DCs process antigens and present antigenic fragments on their surfaces to activate naïve CD4+ T-cells. Subsequently, CD4+ helper T-cells interact with antigen-specific B-cells, leading to class switching of B-cells, which become immunoglobulin-secreting cells [10, 52]. Activated B-cells leave Peyer’s patches and the lymphoid follicles and reach distant effector sites (i.e., systemic mucosal and gut effector sites), leading to their differentiation and maturation into plasma cells [10, 52]. Alternatively, antigen-carrying DCs themselves could also directly migrate to LNs, interact with germinal centers, and activate humoral and cellular responses, resulting in the migration of activated immune cells (B-cells and T-cells) to distant effector sites [10, 52]. Notably, it remains unclear whether M cell-mediated antigen uptake into GALT has an important role in the induction of oral tolerance against soluble antigens [11, 21, 53-55]. Also, while it seems that antigen uptake by Peyer’s patches and isolated lymphoid follicles play a minor role in oral tolerance [11, 21, 53-55], they may be more important in modulation of immune responses to the gut microbiome [11]. In certain circumstances, low doses of antigens with multiple administrations or high doses of antigens without pro-inflammatory danger signals may cause oral tolerance in the GALT [21]. This should be considered in the development of oral vaccines and immunotherapies for oral tolerance.
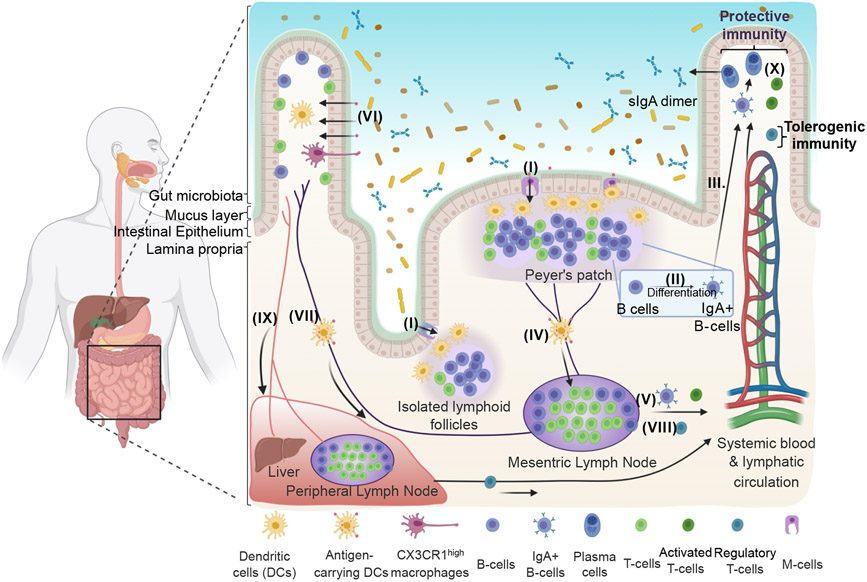
Figure 2. Immune system in the gastrointestinal tract.
(I) Specialized M cells in the epithelium overlying GALT mediates transcellular transport of antigens to DCs. (II) B-cells are activated to immunoglobulin-secreting cells that (III) migrate to the lamina propria and systemic mucosal effector sites and differentiate into IgA-secreting plasma cells. (IV) Antigen-carrying DCs migrate to mLNs to activate B-cells and T-cells, which (V) migrate to distant effector sites through the lymphatic system. (VI) Antigens can be recognized by DCs through diffusion through epithelial tight junctions, transfer across epithelial cells by transcellular routes, exosome-mediated delivery, or capturing from CX3CR1 high macrophages. (VII) Oral tolerance is initiated by antigen-carrying CD103+ DCs that induce Tregs, leading to (VIII) their dissemination to distant effector sites. (IX) Antigens also reach to the liver and peripheral lymph nodes. (X) Oral immunotherapy could trigger protective immunity or tolerogenic immunity, depending on the target cells and local signals. Created by BioRender.com.
The lamina propria (the gut effector site) harbors antigen-specific mucosal effector cells, such as immunoglobulin (Ig) A-producing plasma cells and memory B- and T-cells, and performs mucosal protective activities for maintaining homeostasis [14, 16]. For example, IgA from plasma cells is transported across the epithelium by polymeric Ig receptors [56]. Secretory IgA antibodies prevent the attachment and colonization of pathogens at mucosal surfaces [56]. Other important effector mechanisms that contribute to the host defense against pathogens at lamina propria include locally produced IgM and IgG and mucosal cytotoxic T lymphocytes [57]. Under inflammation as in IBD, the lamina propria is altered, as characterized by increased frequency of activated immune cells [4, 6, 20, 58], leakage of the intestinal barrier [8, 19], penetration of pathogens or gut microbiota [4, 6, 20], and dysregulated immune responses [4, 6, 20, 58]. This renders the lamina propria to be an inflamed environment prone for tissue damage, as observed in IBD [37, 58].
On the other hand, the lamina propria could also serve as an inductive site for oral tolerance. Antigen uptake by CD103+ DCs in the lamina propria induces oral tolerance to soluble antigens [11, 17]. Antigens can be recognized by DCs [11] through 1) diffusion through the epithelial tight junctions [59], 2) transfer across epithelial cells by transcellular routes [59], 3) exosome-mediated delivery [60], and 4) capturing from CX3CR1 high macrophages [41]. Oral tolerance is initiated by antigen-carrying CD103+ DCs migrating from lamina propria into mLNs [17, 61]. In mLNs, retinoic acid (RA) produced from vitamin A by retinal dehydrogenase of DCs and local stromal cells induces the expression of gut-homing receptors α4β7 integrin and C-C motif chemokine receptor 9 on antigen-specific T-cells, and TGF-β dependent differentiation of Foxp3+ Tregs [62, 63]. Tregs re-enter the intestinal lamina propria and undergo secondary expansion under the influence of IL-10 produced by CX3CR1high macrophages [64, 65]. Subsequently, Tregs enter other immunologic regions via the lymphatic circulation and/or systemic blood circulation, establishing systemic oral tolerance [11, 66]. Antigens taken up into Peyer’s patches or the lamina propria may also reach the liver where sinusoidal endothelial cells, tolerogenic conventional DCs, or plasmacytoid DCs induce systemic tolerance [67-69]. When antigens reach peripheral lymph nodes and are presented by resident DCs in the absence of co-stimulation, systemic tolerance may also occur [11].
2.2. The intestinal barrier
The intestinal barrier is composed of a single layer of intestinal epithelial cells that participate in the induction and maintenance of innate immunity [2, 8]. Additional intestinal cells include goblet cells, Paneth cells, M cells, intestinal epithelial stem cells, and enteroendocrine cells [34, 70]. Among these, enterocytes, goblet cells, and M cells play crucial roles in gut protection, transport, and immunity [10, 34, 70, 71]. The mucus layer on the GIT epithelium protects the body from the invasion of pathogenic threats [34, 35] (Fig. 3). Mucus, primarily composed of mucins secreted by goblet cells, is essentially a hydrogel (N95% water) consisting of a mixture of proteins, carbohydrates, lipids, salts, and antibodies [35, 72, 73]. The shielding and lubricating functions of mucus have a crucial role in maintaining an intestinal homeostasis [74]. The mucus layer directly interfaces with the gut microbiota [75, 76] and provides attachment sites to glycan-binding components of microorganisms, thus affecting the colonization of microorganisms [77, 78]. Mucin glycans also serves as nutrition for mucus-associated bacteria, so-called ‘mucolytic bacteria’ [79], favoring their replication [80, 81]. These leads to the selection of various commensal microbes that make up our gut microbiota [75, 82]. Moreover, some commensal microbial species, such as Lactobacillus spp [83, 84], Bifidobacterium longum [85], Lactobacillus reuteri [86], and Akkermansia muciniphila [87-92], promote the mucin production and increase the mucus layer thickness. Commensal bacteria provide protection against pathogenic microbes by increasing the mucus production and offering an additional intestinal barrier that prevents the adhesion of pathogenic microbes [93, 94] (Fig. 3). Viscosity of the mucus layer also limits the motility of microbes [93], thus protecting the underlying epithelium against microbes. Collectively, the integrity of the mucus barrier is crucial for the protective functions of the GIT [75, 80, 82, 93, 94].
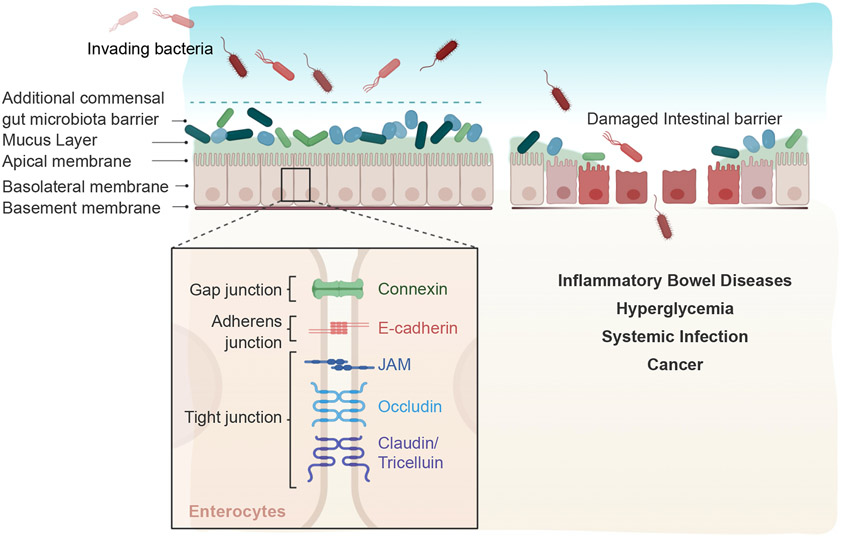
Figure 3. Maintenance of the intestinal barrier functions.
The intestinal barrier is composed of intestinal epithelium and mucosal layer. Epithelial cells are connected by a series of intercellular tight junctions, which are responsible for the intestinal barrier functions. The mucosal and gut microbiota layers protect the body from pathogens. Disrupted of intestinal barrier is associated with various diseases, including IBD, hyperglycemia, infection, and cancer. Created by BioRender.com.
The intestinal barrier function is crucial to maintaining tissue homeostasis [8]. The connection between individual epithelial cells is held by a series of intercellular tight junctions, composed of junctional adhesion molecules, occludin, claudin, and tricelluin [95, 96]. Tight junction is the apical junction along the lateral surface and is directly responsible for intestinal barrier functions [95, 96]. When the integrity of the cell-cell junctions is disrupted, unrestricted passage of pathogens and molecules across the epithelial layers could occur [8]. Furthermore, the mucus layers and gut microbiota are also important for the intestinal barrier functions [75]. The intestinal epithelial barrier is constantly being challenged by the gut microbiota, food, and food-associated microbes, and dysregulated intestinal barrier functions, epithelial integrity, and cell-cell junctions are associated with diverse pathological states, IBD [7, 97-99], autoimmune diseases [100], and systemic infection [101] (Fig. 3). Inactivation of the IBD susceptibility gene, C1orf106 (chromosome 1 open reading frame 106), decreased the intestinal barrier function, thereby triggering intestinal inflammation and IBD [99, 102, 103]. C1orf106 protein has an important role in maintaining an appropriate level of cytohesin 1 protein in mature epithelia. Cytohesins are activators of the Ras guanosine triphosphatase ARF (ADP ribosylation factor 6), which directs endocytic internalization of cadherins. Downregulation of the ARF6 activity is important in maintaining the stability of tight junctions. Depletion of C1orf106 led to an abnormally high amount of cytohesin and excessive ARF6 activation. This in turn increases cadherin endocytosis and tight junction permeability. The passage of bacterial components, debris, and other antigenic molecules through the leaky tight junction structures cause immune response, inflammation, and tissue damage. Also, a pathogenic bacterium, Enterococcus gallinarum, can induce intestinal barrier defects and translocate to LNs and liver, triggering autoimmune diseases, such as systemic lupus erythematosus [100]. In addition, hyperglycemia, associated with diabetes and other metabolic syndromes, can disrupt the intestinal barrier, leading to intestinal inflammation and infection [101]. Chronic hyperglycemia affects the barrier functions through metabolic and transcriptional reprogramming of the glucose transporter GLUT2 in intestinal epithelial cells, leading to the dissemination of bacterial byproducts and systemic inflammation [101]. Overall, these studies have shown the crucial roles of intestinal barrier functions in protection against IBD and other systemic diseases. However, the mechanism by which the intestinal barrier becomes leaky is unclear and requires further investigation.
2.3. The gut microbiota
The human body is inhabited by trillions of microorganisms comprised of bacteria, fungi, and virus [12]. It is estimated that more than 1014 microorganisms of ~ 10,000 bacterial species colonize the GIT [12, 13]. The gut microbiota supplements the intestinal barrier by forming additional barriers in the mucus layer that separate pathogens, particles, and pollutants from the internal milieu [75, 93, 104]. Notably, the gut microbiome has established a symbiotic relationship with our immune system and intestinal barrier [2, 4, 6, 8, 19, 75]. As an example of the host-microbiome symbiosis, some microbiomes such as Akkermansia muciniphila feeds on the mucin layer, while commensal microbiotas are required for fucosylation of the mucus layer and its full intestinal barrier functions [107-109]. The gut microbiotas also participate in the maturation and functions of the innate immune system, including secretion of antimicrobial peptides (α-defensin, interactions with TLRs on Paneth cells; β-defensin, interactions with TLRs on epithelial cells) and production of IL-22, IL-17, and IL-10 [110]. Thus, the gut microbiome-host interactions play crucial roles in the development, maturation, and maintenance of the immune system [105, 106] (Fig. 4).
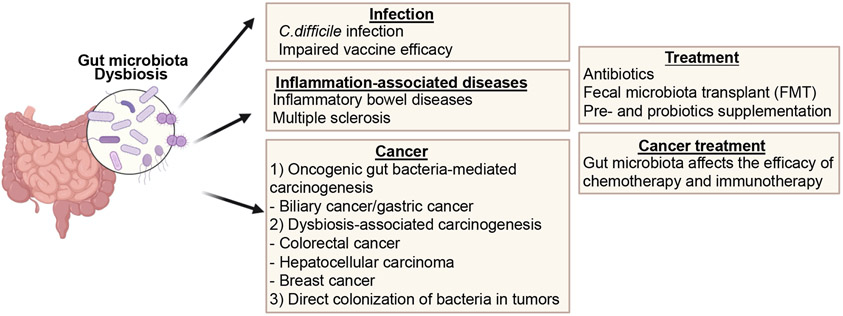
Figure 4. The role of the gut microbiota in various diseases.
Dysbiotic gut microbiota is associated with various local and systemic diseases, including infection, inflammation-associated diseases, and cancer. Created by BioRender.com.
Gut microbiota diversity refers to the number of different commensal microbial species present in an individual. Gut dysbiosis is typically characterized by reduced microbial diversity and substantial shifts in the resident microbial species [5, 106]. Dysbiotic gut microbiota increases the susceptibility to infection and leads to impaired vaccine response, as observed in the setting of malnutrition [5, 112, 113]. A prototypic example of dysbiosis is Clostridioides difficile infection, which causes rapid disruption of gut microbial communities [114, 115]. This leads to an enrichment of primary bile acids and simple carbon compounds, leading to the germination and growth of C. difficile [116]. In addition, IBD patients have dysbiotic gut microbiome [4, 6, 20], disrupted intestinal barriers [8, 19], and dysregulated mucosal immune responses [4, 6, 20]. Other pathologic conditions, including multiple sclerosis (MS), are also associated with dysbiotic gut microbiota [23, 117]. MS is characterized by immune-mediated destruction of myelin in the central nervous system, and the gut microbiota is known to influence this process in genetically susceptible individuals. Depletion of gut commensal bacteria by antibiotics-treatment ameliorated the development of experimental autoimmune encephalomyelitis (EAE) in mice [117]. In contrast, intraperitoneal antibiotic-treatment had minimal effects on the gut microbiota and induction of EAE, suggesting that induction of EAE was affected by the gut microbiome [117]. Furthermore, epidemiological studies of patients with MS have indicated shifts in specific bacterial taxa among MS patients [23]. Taken together, these studies have shown the link between dysregulated gut microbiota and various GIT diseases; thus, modulation of dysregulated microbiota via the use of antibiotics, fecal microbiota transplantation (FMT), or supplementation of probiotics (i.e., a mixture of beneficial commensal microbes) may serve as preventive measures or potential treatments against Clostridioides difficile infection [118, 119], IBD [120-124], and MS [125-127].
The gut microbiome is also increasingly recognized for its role in carcinogenesis. Known oncogenic gut bacteria include Salmonella enterica subspecies enterica serovar Typhi [128] and Helicobacter spp. [129] in biliary cancer and Helicobacter pylori in gastric cancer [130, 131]. Oncogenic gut bacteria contribute to carcinogenesis by trigger local chronic inflammation, and some bacteria, such as H. pylori, have direct genotoxic effects on mucosal cells [130]. Furthermore, preclinical and clinical studies have indicated dysbiosis as an oncogenic driver in colorectal cancer (CRC) [132, 133]. Various microbial species, such as Bacteroides fragilis [134-136], Fusobacterium nucleatum [132, 137, 138], Escherichia coli [139], and Campylobacter jejuni [140], are associated with CRC carcinogenesis and metastasis. Dysregulated gut microbiota also has been implicated in hepatocellular carcinoma (HCC) [141] and breast cancer [142]. Intestinal bacterial components, metabolites, and byproducts could be transported to the liver through the portal venous system, causing inflammatory changes and hepatotoxicity. For example, N-nitroso compounds generated in the gut are hydroxylated to their toxic intermediates by the isozyme system in the liver [143]. Similarly, microbial derivatives of bile acids are implicated in carcinogenesis. As unabsorbed bile acids are antimicrobial, bacteria transform bile acids (cholic acid and chenodeoxycholic acid) to secondary bile acids (deoxycholic acid and lithocholic acid) with carcinogenic properties [24, 144]. The dysregulated gut microbiota may also promote breast cancer formation by gut microbiota-mediated effects on estrogen metabolism, energy metabolism, and obesity [145, 146].
Colonization of bacteria in the tumor microenvironment can also directly impact the growth and immune response of cancer cells, either by direct interactions with the cancer cells or by inducing inflammation or immunosuppression [147, 148]. Nejman, et al. recently profiled the microbiota associated with tumors from cancer patients and reported distinct organ-specific compositions of microbiome in breast, lung, ovary, pancreas, melanoma, and brain tumors [149]. Notably, there was a strong correlation between the intratumor bacteria and the gut bacterial population. Compromised integrity of the gastric epithelial layer may contribute to bacterial translocation to distal tumors, as recently demonstrated in pancreatic adenocarcinoma. Interestingly, tumor-colonized bacteria may also contribute to resistance to chemotherapy [150]. For example, Mycoplasma in pancreatic tumors mediates resistance to gemcitabine by metabolizing it into an inactive form [151]. These studies have shown pro-tumoral properties of the gut microbiome.
In contrast, other studies have reported anti-tumoral effects of “beneficial” gut microbiota [152]. Disruption of the gut microbiota by broad-spectrum antibiotics negatively impacted the patient outcomes in immune checkpoint blockade therapies [153, 154], thus highlighting the importance of the commensal microbiota in regulating immune response during cancer immunotherapy. Furthermore, preclinical studies have shown that administration and subsequent gut colonization of commensal bacteria, such as Akkermansia muciniphila [155], Bacteroides fragilis [156], and Bifidobacterium spp. [157], enhanced the anti-tumor efficacy of immune checkpoint blockade therapies. In addition, anti-tumor effects of cyclophosphamide have been partially attributed to the changes in the gut microbiota [150]. Thus, modulation of the microbiome by removing “harmful” carcinogenic bacteria or transplanting “beneficial” microbes with anti-tumor properties may lead to new therapeutic strategies against cancer.
3. Oral nanomedicine for immune system modulation in the lamina propria
Since effector immune cells are located in the lamina propria, approaches that can modulate lamina propria immune cells are being pursued for the treatment of IBD and other GIT-associated diseases. For example, infiltration of macrophages and neutrophils in the lamina propria is the hallmark of IBD [158, 159]. Thus, macrophages and neutrophils in the lamina propria are potential therapeutic targets in IBD [22]. If drugs can be specifically delivered to the target immune cells by NPs, this would maximize the efficacy of drugs while minimizing toxicity [22]. To achieve this goal, NPs should overcome the biological and physicochemical barriers in the GIT. They include the pH variation and proteolytic enzymes along the GIT [31, 32]. Drug penetration through the mucosal layer and intestinal epithelium are additional barriers to overcome [10]. Differential residence times in small intestine (3-4 h) and colon (1-2 days) are additional factors to consider [36]. Notably, due to the denuded mucus layer and disrupted intestinal epithelium associated with intestinal inflammation in IBD [8, 19], it may be possible to deliver drug-loaded NPs passively to the inflamed site through the leaky epithelium [22]. (Table 1)
Table 1.
Key design criteria to consider for GIT-targeted oral NPs.
| Oral vaccine | Oral tolerance | Modulation of lamina propria immune cells |
Intestinal barrier enhancement |
Modulation of gut microbiota |
|
|---|---|---|---|---|---|
| Harsh GIT conditions (pH variation, proteolytic enzymes) | Should consider (antigens should be protected by NPs encapsulation) | Could consider when loaded drugs or targeting ligands are vulnerable (drugs should be protected by NPs encapsulation, and targeting ligands should be protected, for example by embedding NPs in hydrogels) | |||
| Short residence time in small intestine | Should consider (antigens should be delivered to APCs in the inductive sites of small intestines) | Should consider if targeting the small intestine | |||
| Penetration through the intestinal barrier (mucus layer, epithelium) | Should consider (Both M-cells and epithelium are located underneath the epithelium) | Should consider (The lamina propria is located underneath the epithelium) when the intestinal barrier structure is intact | Should consider penetrating through the mucus layer for epithelium targeting when the intestinal barrier structure is intact | ||
| Not applicable for certain diseases such as IBD with denuded mucus layer and disrupted intestinal epithelium | |||||
| Targeting strategy | - M-cell targeting - Epithelium targeting |
- M-cell targeting - CD103+ DC targeting |
- Targeting innate immune cells (e.g., macrophages, neutrophils, Ly6C+ mononuclear phagocytes) | - Mucus targeting - Epithelium targeting |
- Mucus targeting - Large intestine lumen targeting |
| Other considerations | - Should consider using immunostimulatory adjuvants | - Should consider using immunomodulatory agents | |||
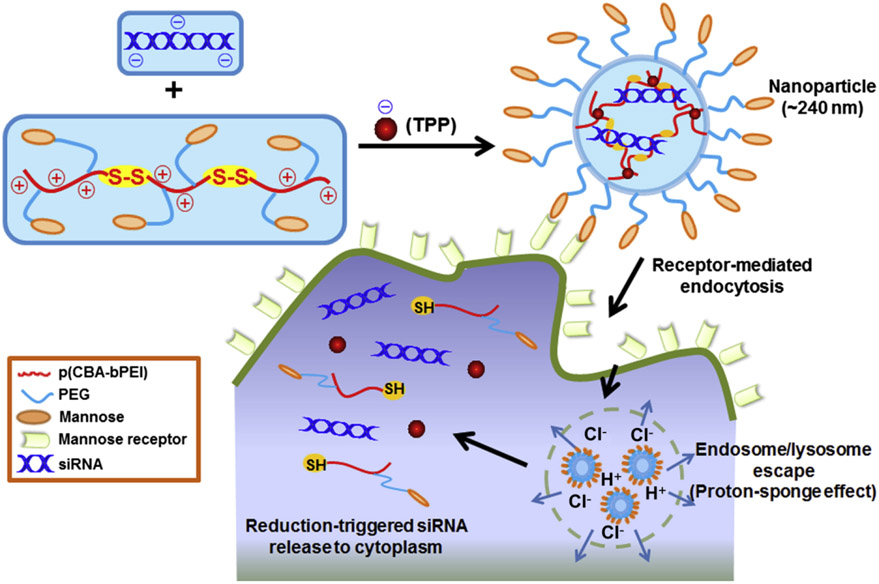
For the treatment of IBD, NP-based targeting of overexpressed surface receptors on activated macrophages has been explored. For instance, mannose receptors [160] and macrophage galactose-type lectin [161] are highly expressed on activated macrophages in inflammatory conditions. Macrophage-targeted NPs have been developed (Fig. 5). These NPs are composed of mannosylated bioreducible cationic polymer, sodium triphosphate, and TNF-α siRNA [162]. These NPs showed efficient macrophage targeting ability without significant uptake by epithelial cells. This led to strong anti-inflammation activity in a murine model of dextran sodium sulfate (DSS)-induced colitis. Furthermore, galactosylated trimethyl chitosan-cysteine NPs were reported to target macrophages via macrophage galactose-type lectin while carrying siRNA against mitogen-activated protein kinase kinase kinase kinase 4, a key upstream mediator of TNF-α production [163]. Oral administration of these NPs ameliorated DSS-induced colitis. Additionally, TNFα-siRNA loaded polylactic acid-(polyethylene glycol) (PEG) NPs grafted with the Fab’ portion of F4/80 antibody exhibited macrophage-targeting ability in vivo with promising therapeutic efficacy in the DSS-induced colitis model [164]. However, there is a possibility of degradation of the grafted ligand anti-F4/80 antibody during GIT transit; thus, loading of polylactic acid-PEG NPs into a colon-specific biodegradable hydrogel (chitosan/alginate) protected the Fab’ fragments on NPs, leading to improved colon-specific delivery of NPs and therapeutic efficacy [164].
Figure 5. Oral nanomedicine for targeting the lamina propria.
Schematic illustration of TPP(sodium triphosphate)-PPM(a mannosylated bioreducible cationic polymer)/siRNA NP (NP) formation and macrophage-targeting delivery, and the release of siRNAs to the cytoplasm. Reproduced with permission from [162].
Ly6C+ inflammatory leukocytes in inflamed intestines in IBD also may serve as a target for nanomedicine [165]. Lipid-based NPs carrying IL-10 mRNA achieved a selective expression of IL-10 among Ly6C+ inflammatory leukocytes via targeting with anti-Ly6C antibody, resulting in a favorable therapeutic efficacy in a DSS colitis model [166]. Other siRNA-loaded NPs have been reported to target inflammatory leukocytes. Epithelial cells and immune cells increase the expression of cyclin D1 (CyD1) in IBD [167]. NPs carrying siRNA CyD1 and targeted to inflamed leukocytes inhibited CyD1 mRNA and inflammatory responses in the DSS colitis model [168]. Similarly, anti-Ly6C antibody-grafted lipid-based NPs carrying siRNA against Interferon Regulatory Factor-8, an immunomodulatory protein, targeted to inflammatory Ly6C+ leukocytes blocked Interferon Regulatory Factor-8 mRNA and significantly decreased the differentiation, polarization, and activation of mononuclear phagocytic cells [169].
Another emerging area in the cell-specific active targeting approaches is to explore naturally occurring extracellular vesicles, such as exosomes derived from edible plants [170, 171]. For example, phosphatidylethanolamine and phosphatidylcholine-enriched grapefruit-derived edible nanovesicles were taken up by intestinal macrophages via the clathrin-dependent pathway and micropinocytosis due to the enrichment of phosphatidylethanolamine and phosphatidylcholine on the outer layer [172]. Thus, nanovesicles loaded with methotrexate exhibited a macrophage-targeting ability and ameliorated DSS-induced murine colitis [172]. Furthermore, TGF-β1 gene-modified DC-derived exosomes induced CD4+Foxp3+ Tregs while decreasing helper T-cells (Th17) at inflammatory sites in GIT, exerting efficacy against DSS-induced colitis in mice [173, 174].
In addition to IBD, GIT-targeted NPs, antigen-carrying NPs with immunomodulatory drugs, such as vitamin D and rapamycin [175, 176], have been shown to induce tolerogenic immune responses. These tolerance-inducing NPs may prevent anti-protein drug antibody responses [177] and could be used to treat various diseases, including arthritis [178], allergy [179, 180] and diabetes [181]. For more detailed information, readers are referred to section 6.2. Oral nanomedicines for immune tolerance.
4. Oral nanomedicine for modulation of the intestinal barrier
Disrupted intestinal barrier is associated with inflammation and systemic infection, and this may lead to the leakage of bacteria or their byproducts into the underlying tissues and systemic circulation [8]. Thus, restoring the integrity of the disrupted intestinal barrier may have beneficial effects against GIT-associated diseases [8]. For example, in a mouse model of T cell-mediated acute diarrhea, pharmacological agents for controlling actomyosin contractility or endocytosis reduced the symptoms of acute diarrhea [182]. Furthermore, stabilization of C1orf106, which modulates the tight junction proteins [99, 102, 103], may offer a therapeutic strategy for improving intestinal barrier functions in IBD. In addition, repairing the epithelial layer can strengthen the intestinal barrier functions and may serve as a new approach for the prevention and treatment of IBD [8]. Also, differentiation of intestinal cells fortifies the intestinal crypts, thus providing another potential target for improving the intestinal barrier functions [8].
Oral nanomedicine designed to overcome the biological and physicochemical barriers of the GIT may offer new ways to deliver drugs to the intestinal epithelium and lamina propria (Table 1). Various NP-based strategies have been explored for regulating the intestinal barrier functions as the potential treatments against IBD. Muco-adhesive NPs can deliver drugs to the mucus layer of small or large intestines [183]. Since mucins are composed of hydrophilic components (single-chain amino acid backbone with branched oligosaccharide side chains), NPs should contain hydrophilic functional groups, such as carboxyl or hydroxyl, to facilitate the formation of hydrogen bonds between mucins and NPs [184, 185]. Furthermore, mucus can be targeted by exploiting the charge interactions between the anionic charge of mucus and cationic polymers, such as chitosan [186]. Synthetic polymers (e.g., acrylic acid derivative/polyacrylate) and natural polymers (e.g., hyaluronic acid, cellulose derivative, chitosan, alginates, and pectin) that have been shown to non-specifically adhere to mucins could be explored for mucus-targeted therapies [22]. In addition, tomato lectins and bacterial adhesins have been examined for constructing mucin-binding NPs [187].
Notably, as the mucus layer is thin in the inflamed regions in IBD patients [18, 188, 189], this should be taken into account when designing mucus-targeting NPs for the treatment of IBD. Size and charge of the NP platforms influence their targeting to inflamed epithelium [22, 31, 190]. Several studies have demonstrated that NPs with less than 200 nm in diameter and a negative surface charge showed better tissue-penetrating activity for IBD treatment as these characteristics allowed for interactions with positively charged proteins expressed on the damaged epithelium of IBD [22, 31, 190]. To specifically target the inflamed epithelium, active targeting strategies against receptors/molecules upregulated on the inflamed epithelium have been explored. For example, Peptide transporter 1, an oligopeptide transporter, is overexpressed in the colonic epithelium of IBD patients [191-193]. KPV (Lys-Pro-Val) peptide has a high affinity to Peptide transporter 1 and exerts anti-inflammatory effects by restoring inflamed epithelium functions [194]. KPV-based NPs alleviated inflammation by accelerating mucosal healing [195]. Also, Intercellular Adhesion Molecule 1 (ICAM-1) is significantly upregulated in inflamed intestinal mucosal tissues and microvasculature of colon in IBD, colon adenomas, and colon adenocarcinoma [196-198]. Thus, ICAM-1 may serve as a target for oral nanomedicine. For example, anti-ICAM-1 antibody-coated polystyrene NPs have been reported [199]. However, after oral administration, these NPs were mainly deposited in the stomach and duodenum, and approximately 60% of anti-ICAM-1 antibody on NPs was rapidly degraded by GIT enzymes, thus presenting a challenge for successful oral delivery. Transferrin receptor (TfR) is another target that is overexpressed in both the basolateral and apical membranes of enterocytes in inflamed colon [200]. Orally administered anti-TfR-antibody-conjugated NPs targeted the inflamed colon [200]. Thus, ICAM-1 and TfR targeting strategies have the potential to target drugs to inflamed tissues and restore intestinal barrier functions although more research is required to protect targeting ligands from degradation in the GIT.
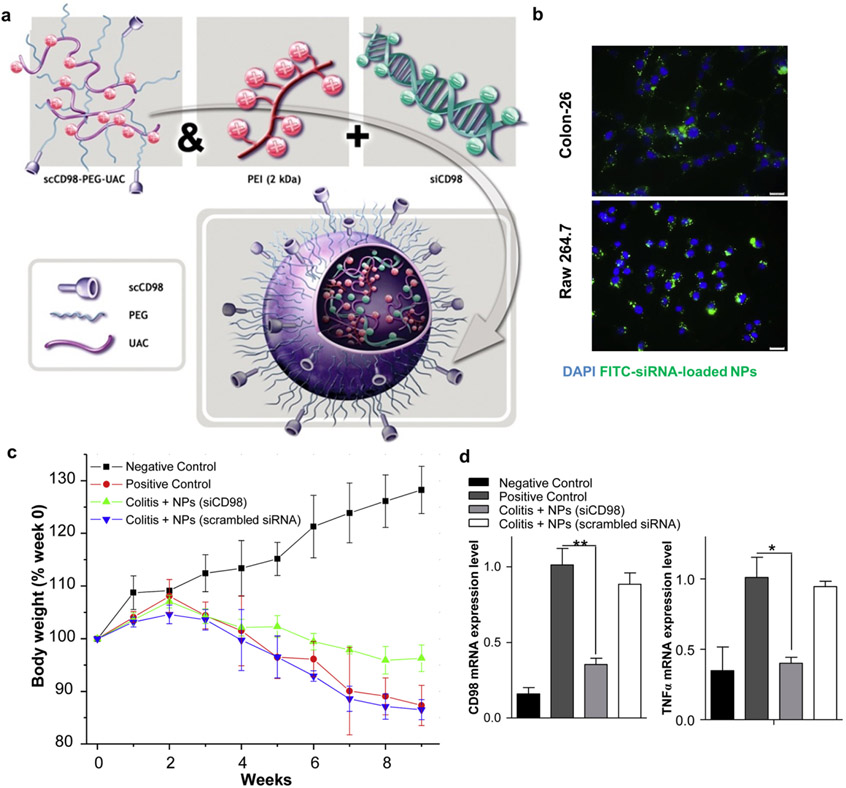
Since restoration of the intestinal barrier functions and modulation of activated immune cells are both important in the management of IBD, targeting molecules overexpressed on both colonic epithelium and activated immune cells may achieve synergistic therapeutic activity. For instance, both CD98 and CD44 are overexpressed on the colonic epithelium and activated immune cells, thus serving as potential targets [201-203]. An oral hydrogel has been developed for delivering CD98 siRNA-loaded NPs decorated with single-chain CD98 antibody on their surface [204] (Fig. 6). In murine colitis models, orally administered hydrogels targeted CD98-overexpressing macrophages and inflamed epithelium, beneficially modulating their functions. Furthermore, hyaluronic acid has also been used as an active targeting ligand on NPs. Hyaluronic acid is a natural polysaccharide composed of N-acetylglucosamine and D-glucuronic acid units and is generally considered nontoxic and biodegradable. Hyaluronic acid binds to its receptor CD44, which is overexpressed on inflamed epithelium and activated inflammatory cells (e.g., macrophages) [205-207]. Hyaluronic acid-functionalized polymeric NPs carrying CD98 siRNA and anti-inflammatory curcumin protected the mucosal layer and modulated the inflammatory functions of activated macrophages, alleviating mucosal inflammation [208]. Also, polymeric NPs functionalized with hyaluronic acid and loaded with KPV accelerated mucosal healing and alleviated inflammation in a murine model of DSS-induced colitis [190].
Figure 6. Oral nanomedicine for improving the intestinal barrier functions.
a, Self-assembly procedure of single-chain CD98 antibody-functionalized siRNA-loaded NPs. b, Specificity of scCD98-functionalized FITC-siRNA–loaded NPs (green) against in vitro colonic epithelial cells (Colon-26 cells; top), macrophages (RAW 267.4; bottom). c-d, In vivo therapeutic efficacy of siCD98 NPs as measured by body weight changes (c), CD98 mRNA levels (d; left), and TNF-alpha mRNA levels (d; right) in the colon of DSS-induced colitis mice. Reproduced with permission from [204].
The intestinal mucosa of IBD patients is characterized by overproduction of reactive oxygen species and imbalance of antioxidants, which lead to oxidative mucosal injury [209-211]. To address these issues, MeO-PEG-b-PMOT amphiphilic block copolymer-based micelles with stable nitroxide radicals in a hydrophobic segment have been developed [212]. These micelles significantly accumulated in the colonic mucosa area, especially in inflammatory sites, protected epithelial regions from oxidative damages, and exhibited potent therapeutic efficiency in a murine model of DSS colitis.
Overall, these studies have shown that oral nanomedicine may modulate the induction, development, and severity of local GIT diseases and provide a new pathway for treating other systemic diseases.
5. Oral nanomedicine for gut microbiome manipulation
Gut microbiome is intricately linked to immune activation and tolerance as well as various pathologies. Thus, strategies that can modulate the gut microbiome, especially via gut-targeted NPs and microparticles, are being explored [12, 213]. Gut microbiome-targeted NPs should also overcome the biological and physicochemical barriers of GIT [31, 32]. Notably, unlike other systems mentioned above, these NPs would target commensal microbes residing in the gut lumen and thus do not need to penetrate through the gut epithelium (Table 1).
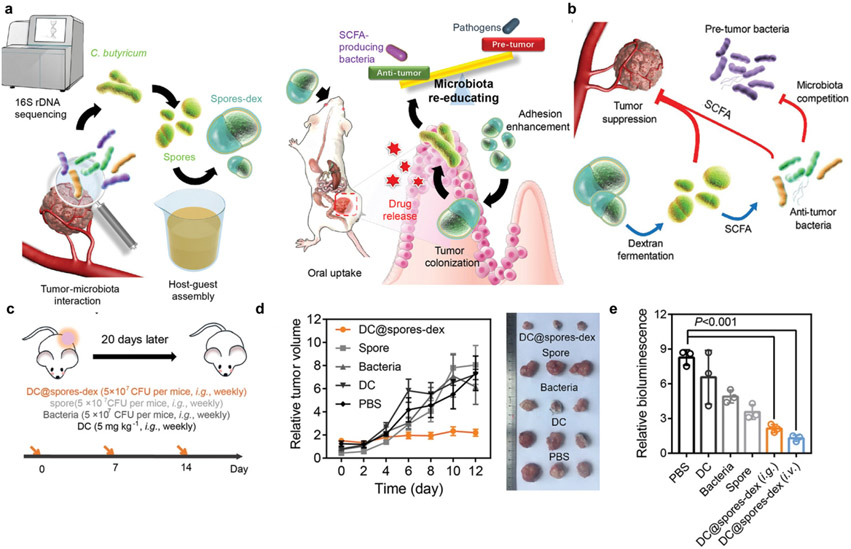
Various nanomedicine systems have been reported for their ability to modulate the gut microbiota as anti-cancer platforms. For example, Fe@Fe3O4 NPs conjugated with ginsenoside Rg3 (NpRg3) have been developed for the treatment of HCC. Ginsenoside Rg3 was used as an autophagy inhibitor and sensitizer of doxorubicin-mediated anti-cancer activity against HCC [214]. Notably, this nanocomposite altered the gut microbiome composition, elevating the relative abundances of Bacteroidetes and Verrucomicrobia while decreasing Firmicutes. Also, NpRg3 significantly decreased 3-indolepropionic acid and urea, which are important metabolites during HCC development, while increasing free fatty acids. Thus, NpRg3 inhibited HCC growth and lung metastasis by remodeling the unbalanced gut microbiota and metabolism. In addition, gut microbe-targeting strategies have been explored for the treatment of CRC. A phage-guided irinotecan-dextran hybrid nanosystem was designed to target a carcinogenic bacterium F. nucleatum and a probiotic bacterium C. butyricum as a potential therapy against CRC [215]. In this system, a phage was utilized to specifically lyse F. nucleatum, which is known to induce chemo-resistance and immunosuppression. In addition, dextran was introduced in the nanosystem to promote the proliferation of C. butyricum, which suppresses the growth of colorectal carcinoma and induces anti-tumor immune responses via production of short-chain fatty acids [216]. This combination strategy showed synergistic anti-cancer activity against CRC. Furthermore, dextran capsules co-loaded with C. butyricium (used as a probiotic) and diclofenac (used as a chemotherapeutic agent) have been shown to exert synergistic anti-cancer activity against CRC [217] (Fig. 7). As an alternative strategy, NPs with intrinsic antimicrobial properties have been employed to deplete harmful bacteria as a new form anti-cancer therapy. Silver NPs with antimicrobial properties have been shown to reduce intratumoral bacteria associated with resistance to cancer therapy and exert anti-tumor effects against pancreatic cancer in mice [218]. Interestingly, there is a case report of a patient with refractory metastatic head and neck squamous cell cancer who had sustained radiographic resolution of cancer after consuming home-made silver NPs daily for 3 months [219]. Based on this anecdotal example and prior preclinical evidence, the authors suggested that silver NPs should be explored further for their safety and efficacy against head and neck cancer.
Figure 7. Oral nanomedicine for modulating the gut microbiome for anti-cancer therapy.
a, Dextran-encapsulated probiotics (C. butyricum) (Spores-dex) regulate gut microbiota and suppress colon cancer. b, Short-chain fatty acids, one of the microbial metabolites, regulate gut microbiota and suppress tumor growth. c-e, In vivo therapeutic efficacy of diclofenac-loaded spores-dex (DC@spores-dex) in mice bearing subcutaneous (c, d) or orthotopic CT26 tumors (c, e). Reproduced with permission from [217].
Platform technologies other than nanomedicine are also reported to modulate the gut microbiota for a potential therapy against cancer. We have recently screened the FDA’s list of ingredients generally recognized as safe and found that oral administration of inulin, a polysaccharide dietary fiber found in chicory root and Jerusalem artichoke, improved the anti-tumor efficacy of immune checkpoint blocker therapy [220]. Based on this, we engineered “colon-retentive” inulin gel to target “beneficial” commensal microbes prevalent in colon. Oral inulin gel treatments in tumor-bearing mice increased the relative abundances of key commensal microbes known for their “beneficial” roles in T cell immunity (e.g., Akkermansia, Lactobacillus, Roseburia) and their short-chain fatty acids as metabolites. This led to enhanced memory recall response for IFN-γ+CD8+ T-cells and establishment of stem-like Tcf1+PD-1+CD8+ T-cells within the tumor microenvironment. Orally administered inulin gel achieved synergy with systemic immune checkpoint blocker therapy in multiple murine tumor models, thus highlighting the potential benefits of targeting the gut microbiome for improving cancer immunotherapy.
Intestinal inflammation is generally associated with significant decreases in the population of Verrucomicrobia, Bacteroidetes, and Firmicutes, especially in bacterial species of Akkermansia muciniphila, Clostridium XIVα, Lactobacillus, Clostridium coccoides, and Clostridium leptum [221-226]. Intestinal inflammation is also associated with substantial increases in the communities of Actinobacteria and Proteobacteria, especially Enterobacteriaceae [221, 222]. Thus, various probiotic strategies have been explored as a potential therapy against IBD. For example, oral administration of a probiotic strain Lactococcus lactis engineered to express anti-inflammatory interleukin-10 (IL-10) restored intestinal homeostasis functions and prevented mice from DSS-induced colitis and the onset of colitis in IL-10−/− mice [227]. Furthermore, other probiotic strategies using engineered Lactobacillus casei [228, 229], Lactococcus plantarum [230, 231], and Streptococcus gordonii [232], have been reported for the treatment of IBD. These engineered probiotics are thought to colonize the gut and produce recombinant proteins with beneficial roles, thus affecting the microbial ecosystem and reshaping the microbiota structure.
Plant-derived NPs based on edible prebiotics could be used to target microbes. Orally administered ginger-derived lipid NPs (GDLPs) were found to target the Lactobacillus rhamnosus GG (LGG) in a lipid-dependent manner [233]. GDLPs carrying mdo-miR7267-3p microRNA mediated targeting of LGG monooxygenase, increased indole-3-carboxaldehyde, and induced IL-22 production, leading to improved intestinal barrier functions in a murine model of DSS colitis. These findings showed that edible plant-based NPs could be used to target microbes and alleviate inflammation in IBD.
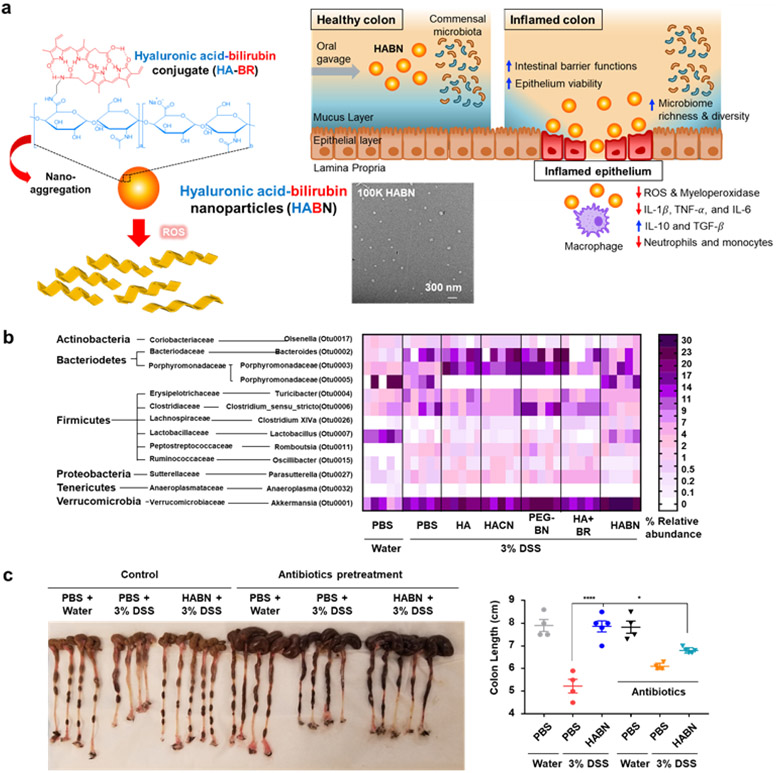
Pathogenesis of IBD is associated with disrupted intestinal barrier functions [8, 19], imbalance of the gut microbiome [4, 6, 20], and subsequent dysregulated mucosal immune responses to the gut commensal bacteria [4, 6, 20]. Thus, targeting all these factors simultaneously may lead to improved results in IBD management. Recently, we reported the development of hyaluronic acid-bilirubin NPs (HABN) that can modulate the gut microbiome, restore the intestinal barrier functions, and exert anti-inflammation effects in a murine model of DSS colitis [234] (Fig. 8). HABN administered orally altered the gut microbiome, increased the diversity and relative abundance of Akkermansia muciniphila (known to induce protective intestinal barrier), Clostridium XIVα (known to induce regulatory CD4 T-cells), and Lactobacillus (known to have anti-inflammation effects) (Fig. 8b). Notably, the anti-inflammatory effects of HABN were abrogated by antibiotic-mediated depletion of the gut microbes, indicating the crucial role of the gut microbiome in HABN-based therapy (Fig. 8c). HABN restored the protective intestinal barrier functions and anti-inflammatory immune responses in the gut epithelium, leading to amelioration of DSS-induced colitis (Fig. 8c).
Figure 8. Oral nanomedicine for altering the gut microbiome for IBD therapy.
a, Schematic of hyaluronic acid-bilirubin NPs (HABN) self-assembled from hyaluronic acid-bilirubin conjugate (HA–BR) and their TEM images. HABN accumulates in inflamed colon and exerts therapeutic effects against acute colitis by targeted modulation of immune systems, intestinal barrier, and gut microbiota. b, Orally administered HABN modulates the gut microbiome in DSS-colitis mice. Heatmap of the relative abundance of family-level taxa (rows) in each mouse (columns). c, In vivo therapeutic efficacy of HABN in DSS-colitis mice. Antibiotics partially reduced the efficacy of HABN, showing the importance of HABN-mediated modulation of the gut microbiota. Reproduced with permission from [234].
These studies have shown the therapeutic potential of gut microbiome-targeted approaches against cancer and IBD. Furthermore, in the future, gut microbiome-targeted strategies should be examined for treating other metabolic diseases and mental health illnesses, such as obesity and Alzheimer's disease, that have been shown to be associated with dysbiotic gut microbiome.
6. Oral nanomedicine for vaccines and immune tolerance
Oral vaccination strategies can generate both humoral and cellular immunity with innate and adaptive components, leading to successful mucosal and systemic protective immunity [14, 235, 236]. Similarly, successful oral tolerance strategies would generate antigen-specific tolerance in the local intestinal mucosal regions and the systemic compartments [11, 14, 21]. Induction of either protective or tolerogenic immune responses requires: (1) successful delivery of the intact and active antigen to the intestines, (2) transport across the mucosal barrier, and (3) subsequent immune modulation (protective immunity for vaccine, but tolerogenic immunity for tolerance) with APCs [30, 32, 237]. In this regard, oral nanomedicine offers potential solutions to these challenges. Encapsulation or entrapment of antigens within NPs protects the cargo molecules against pH- and enzyme-mediated degradation, while preventing their dilution over the large surface area of the GIT [10, 22]. Furthermore, NPs may efficiently deliver an antigenic payload to phagocytic APCs through passive or active targeting [10, 21], thereby stimulating antigen-specific cellular and humoral responses (Table 1).
When developing oral vaccine and tolerance strategies, a delicate balance between immune tolerance, anergy/deletion, and protective immune response should be considered. First, the dose of antigens delivered should be considered for successful oral vaccination and tolerance. Generally, a higher dose of antigen is needed to induce protective immune responses in GALT when compared to traditional parenteral immunizations [238]. However, since too high doses are known to induce anergy/deletion instead of protective immune responses, it is necessary to deliver adequate doses of antigens with oral nanomedicine for protective immunity [11, 239]. On the other hand, it should be noted that repeated oral administrations of antigens in low doses may trigger a Treg-based tolerogenic response [11, 239]. Second, for successful oral vaccination, co-delivery of antigens with immunostimulatory adjuvants is needed to avoid immune tolerance [236]. For oral tolerance, co-delivery antigens with immunomodulatory drugs, such as vitamin D [175, 176] or rapamycin [240-242], should be considered for inducing oral tolerance. Another important factor is the target tissue site. Whereas antigen delivery targeted to M cells in GALT is needed for successful oral vaccination [243], antigen delivery to CD103+ DCs in the lamina propria is known to induce immune tolerance [11]. On the other hand, delivery of lower doses of antigens to M cells without danger signals could also induce tolerogenic immune responses[243].
6.1. Oral nanomedicine for vaccine applications
Licensed oral vaccines are currently based on live-attenuated organisms and inactivated vaccines [9, 235] that elicit broad and robust immune responses that are characterized by serum (IgG) and mucosal (IgA) antibodies and effector and memory T-cells. In contrast, there is no licensed subunit-based oral vaccine in part due to the delivery challenges presented by the GIT system [10]. To efficiently deliver antigens via the oral route, it is necessary to 1) protect the payload during the transit against the harsh conditions of the GIT tract [31, 32]; 2) deliver a sufficient amount of antigens to APCs in the inductive sites through the mucosal and epithelial layers within the short residence time (3-4 hr) in the small intestine [36]; and 3) boost immune responses by incorporating adjuvants or using vaccine delivery vehicles with inherent adjuvant properties [236] (Table 1).
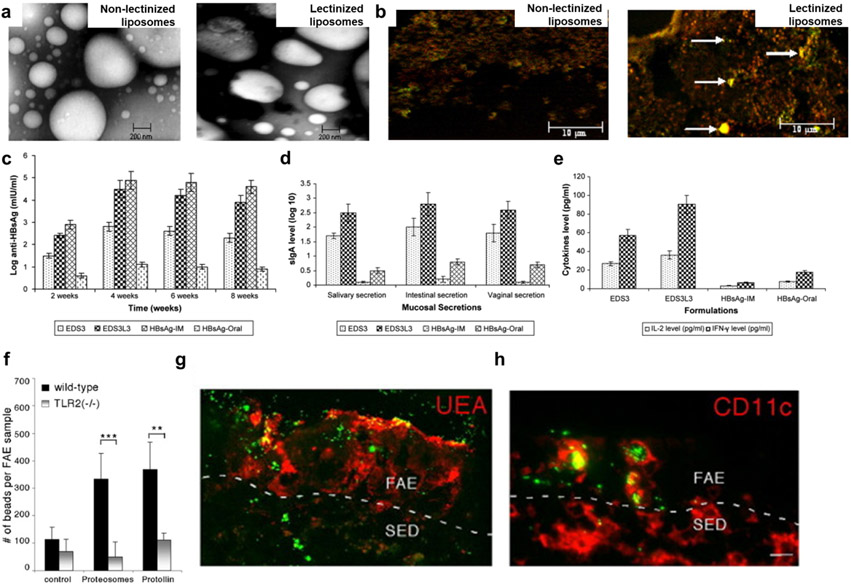
Oral nanomedicine carrying antigens for vaccines should be transported from the intestinal lumen into the GALT via M cells and other enterocytes. M cells efficiently internalize and transport particulate matter (e.g., bacteria, viruses) to the underlying Peyer's Patches and are therefore desirable targets for oral vaccine design [52, 244]. M cells express multiple receptors suitable for targeting. They include α-L-fucose resides (interacting with lectins [245]), β1 integrin (interacting with cRGD [246]), claudin 4 (interacting with claudin 4-targeting peptides [247]), and glycoprotein 2 (interacting with FimH [248] or other GP2 ligands [249]). For example, in oral immunization experiments, lectinized liposomes were able to effectively target M cells in Peyer’s patches, resulting in mucosal responses with high antibody titers [250] (Fig. 9a-e).
Figure 9. Oral nanomedicine for vaccine applications.
a, TEM images of non-lectinized liposomes and lectinized liposomes. b, In vivo M cell-targeting ability of lectinized liposomes (shown by arrows) c-e, In vivo protective immunity induced by orally administered liposomes carrying hepatitis B surface antigen as measured by serum antibody levels (c), sIgA levels in mucosal secretion (d), and cytokine (IL-2 and IFN-γ) levels in mouse spleen homogenates (e). f-g, In vivo enhancement of TLR2-mediated transepithelial transport of orally administered proteosomes, as measured by counting the number of microspheres in FAE (f) and immunofluorescence images with microspheres (green) associated with M cells (red; g) and intraepithelial CD11c+ DCs (red; h) in the FAE of Peyer's patch. Panels a-e and f-g are reproduced with permission from [250] and [254], respectively.
While M cells are an attractive target for oral vaccination, M cells comprise < 5% of the follicle-associated epithelium [251]. Therefore, targeting normal gut epithelial cells is an alternative strategy for oral vaccination. A variety of lectins (N-acetyl-D-glucosamine and sialic acid residues) and PRRs expressed on epithelial cells have been examined for transepithelial transport [10, 243, 252]. Wheat germ agglutinin, which can bind to N-acetyl-D-glucosamine and sialic acid residues [243, 253], as well as Toll-like receptor (TLR) agonists [252] have been used as targeting ligands for epithelial cells. TLR agonists, particularly those for TLR-2 and TLR-4, have been shown to enhance the transport of microparticles across the intestinal lumen into follicle-associated epithelium [254, 255] (Fig. 9f-h). On the other hand, natural polymers, such as CD44-targeting hyaluronic acid [190, 234] and muco-adhesive chitosan [186], can bind to intestinal epithelium; thus, NPs based on natural polymers with intrinsic targeting ability should be explored further.
For prophylactic oral vaccines, it is necessary to boost immune responses with adjuvants. Cholera toxin from Vibrio Cholerae and heat-labile enterotoxin from enterotoxigenic E.coli are potent mucosal adjuvants, but these native toxins pose safety issues [256]. Molecular adjuvants that are widely examined include TLR agonists, such as lipopolysaccharide or monophosphoryl lipid A for TLR4 activation [257], flagellin for TLR5 [258], and CpG deoxyoligonucleotides for TLR9 [259]. Oral NPs incorporated with both antigens and TLR agonists have been shown to generate immune response [260]. For NPs with intrinsic adjuvanticity, such as polyanhydride materials [261], they could modulate immune response without the requirement of adjuvants.
The novel coronavirus, SARS-CoV-2 causing pneumonia-associated respiratory disorder (COVID-19), has led to an unprecedented international health crisis [262]. Oral immunization is a viable strategy that could prevent respiratory illnesses. For example, an oral influenza vaccine has shown promising results in a phase II clinical trial [263], highlighting the potential of oral vaccination against respiratory pathogens. In addition, orally administered adenovirus-vector based vaccine expressing a SARS-CoV-2 antigen and dsRNA adjuvant, termed VXA-CoV-2-1, yielded positive preliminary data from a phase 1 clinical trial (NCT04563702). Enteric coating was used to protect the active ingredient from the stomach’s acidic environment. VXA-CoV-2-1 was generally well-tolerated while triggering immune responses against SARS-CoV-2, as shown by increased CD8+ cytotoxic T-cell response against the viral Spike protein and increased plasma cells and pro-inflammatory Th1 cytokines. VXA-CoV-2-1 also induced serum and nasal IgA responses. More oral nanomedicine vaccines for COVID-19 should be designed, developed, and clinically tested.
6.2. Oral nanomedicine for immune tolerance
While our immune system can detect and eliminate foreign pathogens by generating systemic immune responses, the immune system can inadvertently focus its attack on the host [264, 265]. These inappropriate, auto-reactive humoral or cellular immune responses are underlying causes of autoimmune diseases, such as rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosus, and type 1 diabetes [264, 265]. Autoimmune reactions inflict serious damage to cells and organs, sometimes with fatal consequences. In addition, anti-drug antibody responses, caused immune responses directed against therapeutic drugs, are major challenges. Therapeutic protein replacement is a routine treatment for genetic deficiencies, such as hemophilia and lysosomal storage diseases, but many patients develop neutralizing antibodies against the recombinant biologics [266, 267]. For example, coagulation factor VIII (F.VIII) and acid α-glucosidase (GAA) have been used as therapeutic protein replacement for hemophilia A and B [266] and Pompe disease (autosomal recessive lysosomal storage disorder) [267], but a subset of patients develops antibodies against the recombinant proteins, presenting a major hurdle for successful treatments [266, 267]. Thus, oral tolerance strategies have been explored to induce immunosuppressive immune responses against recombinant protein drugs (for the prevention of anti-drug antibodies) [177, 268] as well as autoantigens (for the treatment of autoimmune diseases) [178, 269, 270].
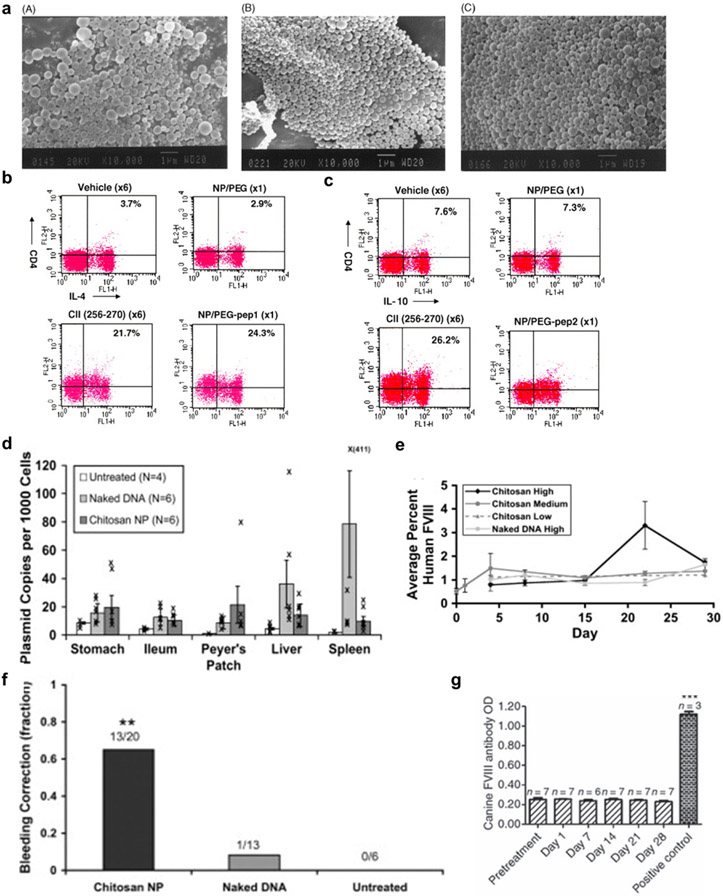
Even though oral tolerance strategies have shown promising results in preclinical studies, several disadvantages, including the pH variation, protein degradation, requirement for frequent dosing, and high cost, limit their wide applications [21]. Oral NPs may overcome these limitations owing to the stability of autoantigens incorporated into NPs, controlled and prolonged release of autoantigens, and cell- and tissue-specific targeting ability of NPs [21] (Table 1). For example, polylactic-co-glycolic acid NPs delivering collagen type II generated tolerogenic activity against collagen by expanding TGFβ-secreting Th3 regulatory T-cells in a murine model of arthritis [178]. In another study, collagen peptide conjugated to PEG suppressed collagen-induced arthritis by inducing tolerance against collagen [269] (Fig. 10a-c). Furthermore, cDNA complexed with chitosan has been employed for oral immune tolerance. Nanoencapsulation through the electrostatic interactions between cDNA and chitosan protected cDNA from digestion. As chitosan binds non-specifically to the intestinal mucus layers, oral administration of cDNA-chitosan complexes led to their accumulation in the intestinal epithelium, and NPs carrying F.VIII cDNA were effective in hemophilia A knockout mice (Fig. 10d-g) [268]. Functional F.VIII protein was detected in plasma at a peak level of 2–4% F.VIII activity, and 13 out of 30 mice showed a phenotypic correction in the bleeding challenge [268] (Fig. 10e and 10f). Another study also determined that neutralizing F.VIII antibody was not detected in the blood of mice treated with canine F.VIII cDNA-loaded chitosan, indicating proper induction of oral tolerance [177] (Fig. 10g).
Figure 10. Oral nanomedicine for inducing immune tolerance.
a, SEM images of (A) NP/pep (type II collagen peptide), (B) NP/PEG-pep1 (mPEG-SPDP-peptide), and (C) NP/PEG-pep2 (mPEG-OD-peptide). b-c, In vivo IL-4 (b) and IL-10 (c)-producing T-cell induction activity of NP/PEG-pep1 or NP/PEG-pep2 in the Peyer's patches of DBA/1 mice, as analyzed by FACS. d, In vivo biodistribution of orally administered FVIII DNA-chitosan NPs as measured by quantitative PCR g-h, In vivo functional FVIII protein production (g) and phenotypic correction (h) activities of orally administered FVIII DNA-chitosan NPs, measured by a tail-clip assay. i, In vivo tolerance induction activity against functional FVIII protein of orally administered FVIII DNA-chitosan NPs in hemophilia A mice, as measured by ELISA-mediated detection of plasma FVIIII antibody levels. Panels a-d, e-h, and i are reproduced with permission from [269], [268], and [177], respectively.
Cholera toxin B subunit, CTB, is a promising adjuvant for oral tolerance. CTB binds to GM1 ganglioside expressed on live intestinal epithelial cells and facilitates uptake into the lamina propria via actin- and ATP-dependent processes [271, 272]. Biodistribution of CTB-antigen conjugate delivered orally was investigated using CTB-GFP fusion protein [273]. After oral administration of chloroplast transgenic leaf material containing CTB–GFP fusion protein in mice, GFP protein was detected in the ileum, liver, and spleen, especially within macrophages and DCs. As DCs have a crucial role in induction of oral tolerance, CTB may serve as a vehicle to deliver autoantigens to DCs of the lamina propria. Indeed, CTB-coupled autoantigens have been shown to suppress DC activation and induce Foxp3+ Treg. NOD mice administered orally with CTB fused to GAD65531–545 peptide exhibited reduced pancreatic inflammation and delayed the onset of hyperglycemia [274]. However, both CTB and antigens may be degraded and hydrolyzed in the harsh conditions of GIT before reaching the target site. Thus, stable co-delivery of CTB and antigens via NPs would be an interesting future direction of the study. In another example, nano-sized recombinant vaccinia virus harboring CTB fused to the proinsulin gene and C-terminal peptide from glutamate decarboxylase has been developed [181]. Oral administration of the system in NOD mice reduced hyperglycemia and insulitis, whereas the control groups developed hyperglycemia. Moreover, plant-based platforms may offer an alternative method for oral tolerance [275]. Oral administration of powdered rice seeds expressing T-cell epitopes induced allergen-specific oral tolerance and improved symptoms against allergies triggered by pollen or mite [179, 180].
7. Perspectives, challenges, and future research directions
As discussed in this review article, various GIT factors, including the mucosal immune system, the intestinal barrier, and gut microbiota, affect not only GIT local diseases but also various systemic diseases. Oral nanomedicine allows for new therapeutic approaches that could overcome the physicochemical and biological barriers of GIT and selectively deliver drug cargo to the GIT target sites. Thus, oral nanomedicine may lead to new strategies for oral vaccines, tolerance against autoimmune diseases (e.g., multiple sclerosis, rheumatoid arthritis), and treatment of IBD, cancer, and metabolic diseases (e.g., obesity, diabetes). Compared with other routes of drug administration, the oral administration route has clear advantages, such as limited drug exposure in the systemic compartment, simple self-administration, patient compliance, and ease of distribution. In addition, the gut microbiota plays a crucial role in maintaining the homeostasis of the GIT, and dysbiotic gut microbiota is associated with pathogenesis of various diseases.
Despite the progress made over the last decade in oral nanomedicines, many challenges remain to be addressed for successful their clinical translation. 1) Variations in immune responses generated by oral vaccines has been shown to be dependent on the nutrition and health of the GIT system. For example, tropical enteropathy can cause child undernutrition, intestinal absorption, and inflammatory disorders that diminish the efficacy of oral immunization [276]. 2) Better understanding of the dose and the antigen release kinetics is required for developing effective approaches for inducing protective immunity or immune tolerance. 3) NPs should release their cargo at the target sites without premature release, which can cause systemic absorption-mediated side effects. To prevent premature release, stable drug loading in nanoformulations is required, but the increased stability of the formulation could also lead to a poor drug-release profile at the target sites. Thus, differences (enzyme, pH-, GSH levels, and reactive oxygen species) between non-target and target sites should be exploited further for tissue-specific release of drugs [33]. 4) Many NP systems reported in the literature have complex structures, and large scale manufacturing of NPs and associated quality control issues have hampered clinical translation [33]. Thus, the design of oral nanomedicine systems should be as simple as possible. 5) When developing oral nanomedicine targeting the gut microbiome, it should be noted that the gut microbiota [277] may vary, depending on the age, environmental exposure, health status, genetics, geography, and diet of the population. Also, exercise [278], antibiotic use [153, 224], and surgical interventions [279] are known to alter the gut microbiome, thus presenting additional factors to consider for the treatment strategies.
Furthermore, we believe oral nanomedicine should be explored further for treating diseases in the central nervous system (CNS). Notably, the gut-brain axis is an emerging area with intensive research interest [280]. The GIT is considered the “second brain” as there are a half billion neurons innervating the gut [25, 281]. Gut microbes and their microbial metabolites affect the development and homeostasis of the CNS [280, 281]. Gut bacteria communicate with the brain through receptors expressed on vagal nerves in the mucosal and muscular layers with their ligands, such as cholecystokinin, exogenous peptides, toxins, ghrelin, serotonin, and glucagon-like peptide-1 [282]. Neurodegenerative diseases, such as Alzheimer’s disease [283, 284] and Parkinson’s disease [285], are associated with dysbiotic gut microbiota, their metabolites, and amyloid biofilm. Thus, these are pharmacological targets, and future research effort should focus on developing oral nanomedicine that can target and normalize the gut-brain axis for potential treatment of CNS diseases.
In summary, oral nanomedicine provides a viable strategy for modulating the gut microbiota and microbial metabolites and offers promising therapeutic platforms for various biomedical applications.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2021R1F1A106212711). Also, this work was supported in part by the US National Institutes of Health (NIH) (R01DE030691, R01DK125087, R01AI127070, R01NS122536, R01CA210273, and U01CA210152). We thank Ms. Marisa Aikins for critical review of the manuscript.
Abbreviations
- APCs
Antigen-presenting cells
- ARF
ADP ribosylation factor 6
- C1orf106
Chromosome 1 open reading frame 106
- CRC
Colorectal cancer
- CTB
Cholera toxin B subunit
- CX3CR1
CX3C chemokine receptor 1
- CyD1
Cyclin D1
- DCs
Dendritic cells
- DSS
Dextran sodium sulfate
- EAE
Experimental autoimmune encephalomyelitis
- F.VIII
Coagulation factor VIII
- GAA
Acid alpha-glucosidase
- GALT
the gut-associated lymphoid tissues
- GIT
Gastrointestinal tract
- HABN
Hyaluronic acid-bilirubin nanoparticles
- HCC
Hepatocellular carcinoma
- IBD
Inflammatory bowel diseases
- ICAM-1
Intercellular adhesion molecule 1
- Ig
Immunoglobulin
- ILC
Innate lymphoid cells
- LNs
Lymph nodes
- M-cells
Microfold cells
- mLN
Mesentric lymph node
- MS
Multiple sclerosis
- NK cells
Natural killer cells
- NPs
Nanoparticles
- PEG
Polyethylene glycol
- PRRs
Pattern recognition receptors
- TfR
Transferrin receptor
- TGF-β
Transforming growth factor-beta
- TLRs
Toll like receptors
- TNF-α
Tumor necrosis factor-alpha
- Tregs
Regulatory T-cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
A patent application (WO/2021/061789) for inulin gel-based in situ modulation of the gut microbiome has been filed with J.J.M. as an inventor.
References
- [1].Pabst R, Russell MW, Brandtzaeg P, Tissue distribution of lymphocytes and plasma cells and the role of the gut, Trends Immunol, 29 (2008) 206–208; author reply 209-210. [DOI] [PubMed] [Google Scholar]
- [2].Takiishi T, Fenero CIM, Camara NOS, Intestinal barrier and gut microbiota: Shaping our immune responses throughout life, Tissue Barriers, 5 (2017) e1373208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Song W, Tiruthani K, Wang Y, Shen L, Hu M, Dorosheva O, Qiu K, Kinghorn KA, Liu R, Huang L, Trapping of Lipopolysaccharide to Promote Immunotherapy against Colorectal Cancer and Attenuate Liver Metastasis, Adv Mater, 30 (2018) e1805007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kostic AD, Xavier RJ, Gevers D, The microbiome in inflammatory bowel disease: current status and the future ahead, Gastroenterology, 146 (2014) 1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E, Dysbiosis and the immune system, Nat Rev Immunol, 17 (2017) 219–232. [DOI] [PubMed] [Google Scholar]
- [6].Round JL, Mazmanian SK, The gut microbiota shapes intestinal immune responses during health and disease, Nat Rev Immunol, 9 (2009) 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Khor B, Gardet A, Xavier RJ, Genetics and pathogenesis of inflammatory bowel disease, Nature, 474 (2011) 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Citi S, Intestinal barriers protect against disease, Science, 359 (2018) 1097–1098. [DOI] [PubMed] [Google Scholar]
- [9].Marasini N, Skwarczynski M, Toth I, Oral delivery of nanoparticle-based vaccines, Expert Rev Vaccines, 13 (2014) 1361–1376. [DOI] [PubMed] [Google Scholar]
- [10].Vela Ramirez JE, Sharpe LA, Peppas NA, Current state and challenges in developing oral vaccines, Adv Drug Deliv Rev, 114 (2017) 116–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pabst O, Mowat AM, Oral tolerance to food protein, Mucosal Immunol, 5 (2012) 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Javed I, Cui X, Wang X, Mortimer M, Andrikopoulos N, Li Y, Davis TP, Zhao Y, Ke PC, Chen C, Implications of the Human Gut-Brain and Gut-Cancer Axes for Future Nanomedicine, ACS Nano, 14 (2020) 14391–14416. [DOI] [PubMed] [Google Scholar]
- [13].Cresci GA, Bawden E, Gut Microbiome: What We Do and Don't Know, Nutr Clin Pract, 30 (2015) 734–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Thakur A, Foged C, Nanoparticles for mucosal vaccine delivery, Nanoengineered Biomaterials for Advanced Drug Delivery 2020, pp. 603–646. [Google Scholar]
- [15].Fujkuyama Y, Tokuhara D, Kataoka K, Gilbert RS, McGhee JR, Yuki Y, Kiyono H, Fujihashi K, Novel vaccine development strategies for inducing mucosal immunity, Expert Rev Vaccines, 11 (2012) 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brandtzaeg P, Mucosal immunity: induction, dissemination, and effector functions, Scand J Immunol, 70 (2009) 505–515. [DOI] [PubMed] [Google Scholar]
- [17].Pabst O, Bernhardt G, Forster R, The impact of cell-bound antigen transport on mucosal tolerance induction, J Leukoc Biol, 82 (2007) 795–800. [DOI] [PubMed] [Google Scholar]
- [18].Swidsinski A, Loening-Baucke V, Theissig F, Engelhardt H, Bengmark S, Koch S, Lochs H, Dorffel Y, Comparative study of the intestinal mucus barrier in normal and inflamed colon, Gut, 56 (2007) 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Turner JR, Intestinal mucosal barrier function in health and disease, Nat Rev Immunol, 9 (2009) 799–809. [DOI] [PubMed] [Google Scholar]
- [20].Halfvarson J, Brislawn CJ, Lamendella R, Vazquez-Baeza Y, Walters WA, Bramer LM, D'Amato M, Bonfiglio F, McDonald D, Gonzalez A, McClure EE, Dunklebarger MF, Knight R, Jansson JK, Dynamics of the human gut microbiome in inflammatory bowel disease, Nat Microbiol, 2 (2017) 17004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang X, Sherman A, Liao G, Leong KW, Daniell H, Terhorst C, Herzog RW, Mechanism of oral tolerance induction to therapeutic proteins, Adv Drug Deliv Rev, 65 (2013) 759–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yang C, Merlin D, Nanoparticle-Mediated Drug Delivery Systems For The Treatment Of IBD: Current Perspectives, Int J Nanomedicine, 14 (2019) 8875–8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, Patel B, Mazzola MA, Liu S, Glanz BL, Cook S, Tankou S, Stuart F, Melo K, Nejad P, Smith K, Topcuolu BD, Holden J, Kivisakk P, Chitnis T, De Jager PL, Quintana FJ, Gerber GK, Bry L, Weiner HL, Alterations of the human gut microbiome in multiple sclerosis, Nat Commun, 7 (2016) 12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, Hara E, Ohtani N, Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome, Nature, 499 (2013) 97–101. [DOI] [PubMed] [Google Scholar]
- [25].Ridaura V, Belkaid Y, Gut microbiota: the link to your second brain, Cell, 161 (2015) 193–194. [DOI] [PubMed] [Google Scholar]
- [26].Lee Y, Kim J, Kim H, Kang S, Yoon JH, Kim DD, Kim YM, Jung Y, N-succinylaspart-1-yl celecoxib is a potential colon-specific prodrug of celecoxib with improved therapeutic properties, J Pharm Sci, 101 (2012) 1831–1842. [DOI] [PubMed] [Google Scholar]
- [27].Lee Y, Kim J, Kim W, Nam J, Jeong S, Lee S, Yoo JW, Kim MS, Jung Y, Celecoxib coupled to dextran via a glutamic acid linker yields a polymeric prodrug suitable for colonic delivery, Drug Des Devel Ther, 9 (2015) 4105–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Buchman AL, Side effects of corticosteroid therapy, Journal of clinical gastroenterology, 33 (2001) 289–294. [DOI] [PubMed] [Google Scholar]
- [29].Sehgal P, Colombel JF, Aboubakr A, Narula N, Systematic review: safety of mesalazine in ulcerative colitis, Alimentary pharmacology & therapeutics, 47 (2018) 1597–1609. [DOI] [PubMed] [Google Scholar]
- [30].Ibraheem D, Elaissari A, Fessi H, Administration strategies for proteins and peptides, Int J Pharm, 477 (2014) 578–589. [DOI] [PubMed] [Google Scholar]
- [31].Zhang S, Langer R, Traverso G, Nanoparticulate Drug Delivery Systems Targeting Inflammation for Treatment of Inflammatory Bowel Disease, Nano Today, 16 (2017) 82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Renukuntla J, Vadlapudi AD, Patel A, Boddu SH, Mitra AK, Approaches for enhancing oral bioavailability of peptides and proteins, Int J Pharm, 447 (2013) 75–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mura S, Nicolas J, Couvreur P, Stimuli-responsive nanocarriers for drug delivery, Nat Mater, 12 (2013) 991–1003. [DOI] [PubMed] [Google Scholar]
- [34].Pelaseyed T, Bergstrom JH, Gustafsson JK, Ermund A, Birchenough GM, Schutte A, van der Post S, Svensson F, Rodriguez-Pineiro AM, Nystrom EE, Wising C, Johansson ME, Hansson GC, The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system, Immunol Rev, 260 (2014) 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ensign LM, Cone R, Hanes J, Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers, Adv Drug Deliv Rev, 64 (2012) 557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mudie DM, Amidon GL, Amidon GE, Physiological parameters for oral delivery and in vitro testing, Mol Pharm, 7 (2010) 1388–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Abraham C, Medzhitov R, Interactions between the host innate immune system and microbes in inflammatory bowel disease, Gastroenterology, 140 (2011) 1729–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hold GL, Mukhopadhya I, Monie TP, Innate immune sensors and gastrointestinal bacterial infections, Clin Dev Immunol, 2011 (2011) 579650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bain CC, Mowat AM, Macrophages in intestinal homeostasis and inflammation, Immunol Rev, 260 (2014) 102–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, Mowat AM, Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors, Mucosal Immunol, 6 (2013) 498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC, CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance, Science, 307 (2005) 254–258. [DOI] [PubMed] [Google Scholar]
- [42].Grainger JR, Wohlfert EA, Fuss IJ, Bouladoux N, Askenase MH, Legrand F, Koo LY, Brenchley JM, Fraser ID, Belkaid Y, Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection, Nat Med, 19 (2013) 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fournier BM, Parkos CA, The role of neutrophils during intestinal inflammation, Mucosal Immunol, 5 (2012) 354–366. [DOI] [PubMed] [Google Scholar]
- [44].Sylvia CJ, The role of neutrophil apoptosis in influencing tissue repair, J Wound Care, 12 (2003) 13–16. [DOI] [PubMed] [Google Scholar]
- [45].Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E, Innate lymphoid cells--a proposal for uniform nomenclature, Nat Rev Immunol, 13 (2013) 145–149. [DOI] [PubMed] [Google Scholar]
- [46].Poggi A, Benelli R, Vene R, Costa D, Ferrari N, Tosetti F, Zocchi MR, Human Gut-Associated Natural Killer Cells in Health and Disease, Front Immunol, 10 (2019) 961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].den Hartog G, Crowe SE, Das S, Ernst PB, Gut Barrier: Innate Immunity, Physiology of the Gastrointestinal Tract 2018, pp. 663–670. [Google Scholar]
- [48].Dulai PS, Sandborn WJ, Next-Generation Therapeutics for Inflammatory Bowel Disease, Curr Gastroenterol Rep, 18 (2016) 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Brandtzaeg P, Pabst R, Let's go mucosal: communication on slippery ground, Trends Immunol, 25 (2004) 570–577. [DOI] [PubMed] [Google Scholar]
- [50].Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P, The immune geography of IgA induction and function, Mucosal Immunol, 1 (2008) 11–22. [DOI] [PubMed] [Google Scholar]
- [51].Lelouard H, Henri S, De Bovis B, Mugnier B, Chollat-Namy A, Malissen B, Meresse S, Gorvel JP, Pathogenic bacteria and dead cells are internalized by a unique subset of Peyer's patch dendritic cells that express lysozyme, Gastroenterology, 138 (2010) 173–184 e171-173. [DOI] [PubMed] [Google Scholar]
- [52].Brayden DJ, Jepson MA, Baird AW, Keynote review: intestinal Peyer's patch M cells and oral vaccine targeting, Drug Discov Today, 10 (2005) 1145–1157. [DOI] [PubMed] [Google Scholar]
- [53].Fujihashi K, Dohi T, Rennert PD, Yamamoto M, Koga T, Kiyono H, McGhee JR, Peyer's patches are required for oral tolerance to proteins, Proc Natl Acad Sci U S A, 98 (2001) 3310–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Spahn TW, Fontana A, Faria AM, Slavin AJ, Eugster HP, Zhang X, Koni PA, Ruddle NH, Flavell RA, Rennert PD, Weiner HL, Induction of oral tolerance to cellular immune responses in the absence of Peyer's patches, Eur J Immunol, 31 (2001) 1278–1287. [DOI] [PubMed] [Google Scholar]
- [55].Spahn TW, Weiner HL, Rennert PD, Lugering N, Fontana A, Domschke W, Kucharzik T, Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer's patches, Eur J Immunol, 32 (2002) 1109–1113. [DOI] [PubMed] [Google Scholar]
- [56].Mantis NJ, Rol N, Corthesy B, Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut, Mucosal Immunol, 4 (2011) 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Strbo N, Pahwa S, Kolber MA, Gonzalez L, Fisher E, Podack ER, Cell-secreted Gp96-Ig-peptide complexes induce lamina propria and intraepithelial CD8+ cytotoxic T lymphocytes in the intestinal mucosa, Mucosal Immunol, 3 (2010) 182–192. [DOI] [PubMed] [Google Scholar]
- [58].de Souza HS, Fiocchi C, Immunopathogenesis of IBD: current state of the art, Nat Rev Gastroenterol Hepatol, 13 (2016) 13–27. [DOI] [PubMed] [Google Scholar]
- [59].Menard S, Cerf-Bensussan N, Heyman M, Multiple facets of intestinal permeability and epithelial handling of dietary antigens, Mucosal Immunol, 3 (2010) 247–259. [DOI] [PubMed] [Google Scholar]
- [60].Karlsson M, Lundin S, Dahlgren U, Kahu H, Pettersson I, Telemo E, "Tolerosomes" are produced by intestinal epithelial cells, Eur J Immunol, 31 (2001) 2892–2900. [DOI] [PubMed] [Google Scholar]
- [61].Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Forster R, Pabst O, Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells, J Exp Med, 203 (2006) 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y, Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid, J Exp Med, 204 (2007) 1775–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY, Retinoic acid imprints gut-homing specificity on T cells, Immunity, 21 (2004) 527–538. [DOI] [PubMed] [Google Scholar]
- [64].Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Muller W, Sparwasser T, Forster R, Pabst O, Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria, Immunity, 34 (2011) 237–246. [DOI] [PubMed] [Google Scholar]
- [65].Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M, Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis, Nat Immunol, 10 (2009) 1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Tomura M, Honda T, Tanizaki H, Otsuka A, Egawa G, Tokura Y, Waldmann H, Hori S, Cyster JG, Watanabe T, Miyachi Y, Kanagawa O, Kabashima K, Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice, J Clin Invest, 120 (2010) 883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Dou L, Ono Y, Chen YF, Thomson AW, Chen XP, Hepatic Dendritic Cells, the Tolerogenic Liver Environment, and Liver Disease, Semin Liver Dis, 38 (2018) 170–180. [DOI] [PubMed] [Google Scholar]
- [68].Thomson AW, Knolle PA, Antigen-presenting cell function in the tolerogenic liver environment, Nat Rev Immunol, 10 (2010) 753–766. [DOI] [PubMed] [Google Scholar]
- [69].Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, Trinchieri G, Kaiserlian D, Plasmacytoid dendritic cells mediate oral tolerance, Immunity, 29 (2008) 464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Peterson LW, Artis D, Intestinal epithelial cells: regulators of barrier function and immune homeostasis, Nat Rev Immunol, 14 (2014) 141–153. [DOI] [PubMed] [Google Scholar]
- [71].Kunisawa J, Kurashima Y, Kiyono H, Gut-associated lymphoid tissues for the development of oral vaccines, Adv Drug Deliv Rev, 64 (2012) 523–530. [DOI] [PubMed] [Google Scholar]
- [72].Lai SK, Wang YY, Hanes J, Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues, Adv Drug Deliv Rev, 61 (2009) 158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lai SK, Wang YY, Wirtz D, Hanes J, Micro- and macrorheology of mucus, Adv Drug Deliv Rev, 61 (2009) 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Atuma C, Strugala V, Allen A, Holm L, The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo, Am J Physiol Gastrointest Liver Physiol, 280 (2001) G922–929. [DOI] [PubMed] [Google Scholar]
- [75].Paone P, Cani PD, Mucus barrier, mucins and gut microbiota: the expected slimy partners?, Gut, 69 (2020) 2232–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Arike L, Hansson GC, The Densely O-Glycosylated MUC2 Mucin Protects the Intestine and Provides Food for the Commensal Bacteria, J Mol Biol, 428 (2016) 3221–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ouwerkerk JP, de Vos WM, Belzer C, Glycobiome: bacteria and mucus at the epithelial interface, Best Pract Res Clin Gastroenterol, 27 (2013) 25–38. [DOI] [PubMed] [Google Scholar]
- [78].Bergstrom KS, Xia L, Mucin-type O-glycans and their roles in intestinal homeostasis, Glycobiology, 23 (2013) 1026–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Li H, Limenitakis JP, Fuhrer T, Geuking MB, Lawson MA, Wyss M, Brugiroux S, Keller I, Macpherson JA, Rupp S, Stolp B, Stein JV, Stecher B, Sauer U, McCoy KD, Macpherson AJ, The outer mucus layer hosts a distinct intestinal microbial niche, Nat Commun, 6 (2015) 8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Etienne-Mesmin L, Chassaing B, Desvaux M, De Paepe K, Gresse R, Sauvaitre T, Forano E, de Wiele TV, Schuller S, Juge N, Blanquet-Diot S, Experimental models to study intestinal microbes-mucus interactions in health and disease, FEMS Microbiol Rev, 43 (2019) 457–489. [DOI] [PubMed] [Google Scholar]
- [81].Hansson GC, Mucins and the Microbiome, Annu Rev Biochem, 89 (2020) 769–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wang BX, Wu CM, Ribbeck K, Home, sweet home: how mucus accommodates our microbiota, FEBS J, 288 (2021) 1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Bron PA, Kleerebezem M, Brummer RJ, Cani PD, Mercenier A, MacDonald TT, Garcia-Rodenas CL, Wells JM, Can probiotics modulate human disease by impacting intestinal barrier function?, Br J Nutr, 117 (2017) 93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Sicard JF, Le Bihan G, Vogeleer P, Jacques M, Harel J, Interactions of Intestinal Bacteria with Components of the Intestinal Mucus, Front Cell Infect Microbiol, 7 (2017) 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Schroeder BO, Birchenough GMH, Stahlman M, Arike L, Johansson MEV, Hansson GC, Backhed F, Bifidobacteria or Fiber Protects against Diet-Induced Microbiota-Mediated Colonic Mucus Deterioration, Cell Host Microbe, 23 (2018) 27–40 e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ahl D, Liu H, Schreiber O, Roos S, Phillipson M, Holm L, Lactobacillus reuteri increases mucus thickness and ameliorates dextran sulphate sodium-induced colitis in mice, Acta Physiol (Oxf), 217 (2016) 300–310. [DOI] [PubMed] [Google Scholar]
- [87].Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD, Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity, Proc Natl Acad Sci U S A, 110 (2013) 9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, Bae JW, An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice, Gut, 63 (2014) 727–735. [DOI] [PubMed] [Google Scholar]
- [89].Wu W, Lv L, Shi D, Ye J, Fang D, Guo F, Li Y, He X, Li L, Protective Effect of Akkermansia muciniphila against Immune-Mediated Liver Injury in a Mouse Model, Front Microbiol, 8 (2017) 1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].van der Lugt B, van Beek AA, Aalvink S, Meijer B, Sovran B, Vermeij WP, Brandt RMC, de Vos WM, Savelkoul HFJ, Steegenga WT, Belzer C, Akkermansia muciniphila ameliorates the age-related decline in colonic mucus thickness and attenuates immune activation in accelerated aging Ercc1 (-/Delta7) mice, Immun Ageing, 16 (2019) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Barcena C, Valdes-Mas R, Mayoral P, Garabaya C, Durand S, Rodriguez F, Fernandez-Garcia MT, Salazar N, Nogacka AM, Garatachea N, Bossut N, Aprahamian F, Lucia A, Kroemer G, Freije JMP, Quiros PM, Lopez-Otin C, Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice, Nat Med, 25 (2019) 1234–1242. [DOI] [PubMed] [Google Scholar]
- [92].Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, Myridakis A, Delzenne NM, Klievink J, Bhattacharjee A, van der Ark KC, Aalvink S, Martinez LO, Dumas ME, Maiter D, Loumaye A, Hermans MP, Thissen JP, Belzer C, de Vos WM, Cani PD, A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice, Nat Med, 23 (2017) 107–113. [DOI] [PubMed] [Google Scholar]
- [93].Donaldson GP, Lee SM, Mazmanian SK, Gut biogeography of the bacterial microbiota, Nat Rev Microbiol, 14 (2016) 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kim YS, Ho SB, Intestinal goblet cells and mucins in health and disease: recent insights and progress, Curr Gastroenterol Rep, 12 (2010) 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zihni C, Mills C, Matter K, Balda MS, Tight junctions: from simple barriers to multifunctional molecular gates, Nat Rev Mol Cell Biol, 17 (2016) 564–580. [DOI] [PubMed] [Google Scholar]
- [96].Van Itallie CM, Anderson JM, Claudins and epithelial paracellular transport, Annu Rev Physiol, 68 (2006) 403–429. [DOI] [PubMed] [Google Scholar]
- [97].Buckley A, Turner JR, Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease, Cold Spring Harb Perspect Biol, 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Luissint AC, Parkos CA, Nusrat A, Inflammation and the Intestinal Barrier: Leukocyte-Epithelial Cell Interactions, Cell Junction Remodeling, and Mucosal Repair, Gastroenterology, 151 (2016) 616–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Mohanan V, Nakata T, Desch AN, Levesque C, Boroughs A, Guzman G, Cao Z, Creasey E, Yao J, Boucher G, Charron G, Bhan AK, Schenone M, Carr SA, Reinecker HC, Daly MJ, Rioux JD, Lassen KG, Xavier RJ, C1orf106 is a colitis risk gene that regulates stability of epithelial adherens junctions, Science, 359 (2018) 1161–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Manfredo Vieira S, Hiltensperger M, Kumar V, Zegarra-Ruiz D, Dehner C, Khan N, Costa FRC, Tiniakou E, Greiling T, Ruff W, Barbieri A, Kriegel C, Mehta SS, Knight JR, Jain D, Goodman AL, Kriegel MA, Translocation of a gut pathobiont drives autoimmunity in mice and humans, Science, 359 (2018) 1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Thaiss CA, Levy M, Grosheva I, Zheng D, Soffer E, Blacher E, Braverman S, Tengeler AC, Barak O, Elazar M, Ben-Zeev R, Lehavi-Regev D, Katz MN, Pevsner-Fischer M, Gertler A, Halpern Z, Harmelin A, Aamar S, Serradas P, Grosfeld A, Shapiro H, Geiger B, Elinav E, Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection, Science, 359 (2018) 1376–1383. [DOI] [PubMed] [Google Scholar]
- [102].Manzanillo P, Mouchess M, Ota N, Dai B, Ichikawa R, Wuster A, Haley B, Alvarado G, Kwon Y, Caothien R, Roose-Girma M, Warming S, McKenzie BS, Keir ME, Scherl A, Ouyang W, Yi T, Inflammatory Bowel Disease Susceptibility Gene C1ORF106 Regulates Intestinal Epithelial Permeability, Immunohorizons, 2 (2018) 164–171. [DOI] [PubMed] [Google Scholar]
- [103].Tang J, Zhang CB, Lyu KS, Jin ZM, Guan SX, You N, Huang M, Wang XD, Gao X, Association of polymorphisms in C1orf106, IL1RN, and IL10 with post-induction infliximab trough level in Crohn's disease patients, Gastroenterol Rep (Oxf), 8 (2020) 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Goto Y, Kiyono H, Epithelial barrier: an interface for the cross-communication between gut flora and immune system, Immunol Rev, 245 (2012) 147–163. [DOI] [PubMed] [Google Scholar]
- [105].Belkaid Y, Hand TW, Role of the microbiota in immunity and inflammation, Cell, 157 (2014) 121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zheng D, Liwinski T, Elinav E, Interaction between microbiota and immunity in health and disease, Cell Res, 30 (2020) 492–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Johansson ME, Jakobsson HE, Holmen-Larsson J, Schutte A, Ermund A, Rodriguez-Pineiro AM, Arike L, Wising C, Svensson F, Backhed F, Hansson GC, Normalization of Host Intestinal Mucus Layers Requires Long-Term Microbial Colonization, Cell Host Microbe, 18 (2015) 582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Schroeder BO, Fight them or feed them: how the intestinal mucus layer manages the gut microbiota, Gastroenterol Rep (Oxf), 7 (2019) 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Bry L, Falk PG, Midtvedt T, Gordon JI, A model of host-microbial interactions in an open mammalian ecosystem, Science, 273 (1996) 1380–1383. [DOI] [PubMed] [Google Scholar]
- [110].Cheng HY, Ning MX, Chen DK, Ma WT, Interactions Between the Gut Microbiota and the Host Innate Immune Response Against Pathogens, Front Immunol, 10 (2019) 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wieers G, Belkhir L, Enaud R, Leclercq S, Philippart de Foy JM, Dequenne I, de Timary P, Cani PD, How Probiotics Affect the Microbiota, Front Cell Infect Microbiol, 9 (2019) 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Huda MN, Lewis Z, Kalanetra KM, Rashid M, Ahmad SM, Raqib R, Qadri F, Underwood MA, Mills DA, Stephensen CB, Stool microbiota and vaccine responses of infants, Pediatrics, 134 (2014) e362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Oh JZ, Ravindran R, Chassaing B, Carvalho FA, Maddur MS, Bower M, Hakimpour P, Gill KP, Nakaya HI, Yarovinsky F, Sartor RB, Gewirtz AT, Pulendran B, TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination, Immunity, 41 (2014) 478–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Schubert AM, Rogers MA, Ring C, Mogle J, Petrosino JP, Young VB, Aronoff DM, Schloss PD, Microbiome data distinguish patients with Clostridium difficile infection and non-C. difficile-associated diarrhea from healthy controls, mBio, 5 (2014) e01021–01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Zhang L, Dong D, Jiang C, Li Z, Wang X, Peng Y, Insight into alteration of gut microbiota in Clostridium difficile infection and asymptomatic C. difficile colonization, Anaerobe, 34 (2015) 1–7. [DOI] [PubMed] [Google Scholar]
- [116].Winston JA, Theriot CM, Impact of microbial derived secondary bile acids on colonization resistance against Clostridium difficile in the gastrointestinal tract, Anaerobe, 41 (2016) 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, Kasper LH, Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis, J Immunol, 183 (2009) 6041–6050. [DOI] [PubMed] [Google Scholar]
- [118].van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, Speelman P, Dijkgraaf MG, Keller JJ, Duodenal infusion of donor feces for recurrent Clostridium difficile, N Engl J Med, 368 (2013) 407–415. [DOI] [PubMed] [Google Scholar]
- [119].Juul FE, Garborg K, Bretthauer M, Skudal H, Oines MN, Wiig H, Rose O, Seip B, Lamont JT, Midtvedt T, Valeur J, Kalager M, Holme O, Helsingen L, Loberg M, Adami HO, Fecal Microbiota Transplantation for Primary Clostridium difficile Infection, N Engl J Med, 378 (2018) 2535–2536. [DOI] [PubMed] [Google Scholar]
- [120].Wang SL, Wang ZR, Yang CQ, Meta-analysis of broad-spectrum antibiotic therapy in patients with active inflammatory bowel disease, Exp Ther Med, 4 (2012) 1051–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, Armstrong D, Marshall JK, Kassam Z, Reinisch W, Lee CH, Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial, Gastroenterology, 149 (2015) 102–109 e106. [DOI] [PubMed] [Google Scholar]
- [122].Rossen NG, Fuentes S, van der Spek MJ, Tijssen JG, Hartman JH, Duflou A, Lowenberg M, van den Brink GR, Mathus-Vliegen EM, de Vos WM, Zoetendal EG, D'Haens GR, Ponsioen CY, Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis, Gastroenterology, 149 (2015) 110–118 e114. [DOI] [PubMed] [Google Scholar]
- [123].Costello SP, Soo W, Bryant RV, Jairath V, Hart AL, Andrews JM, Systematic review with meta-analysis: faecal microbiota transplantation for the induction of remission for active ulcerative colitis, Aliment Pharmacol Ther, 46 (2017) 213–224. [DOI] [PubMed] [Google Scholar]
- [124].Bak SH, Choi HH, Lee J, Kim MH, Lee YH, Kim JS, Cho YS, Fecal microbiota transplantation for refractory Crohn's disease, Intest Res, 15 (2017) 244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kouchaki E, Tamtaji OR, Salami M, Bahmani F, Daneshvar Kakhaki R, Akbari E, Tajabadi-Ebrahimi M, Jafari P, Asemi Z, Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled trial, Clin Nutr, 36 (2017) 1245–1249. [DOI] [PubMed] [Google Scholar]
- [126].Tankou SK, Regev K, Healy BC, Tjon E, Laghi L, Cox LM, Kivisakk P, Pierre IV, Hrishikesh L, Gandhi R, Cook S, Glanz B, Stankiewicz J, Weiner HL, A probiotic modulates the microbiome and immunity in multiple sclerosis, Ann Neurol, 83 (2018) 1147–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Makkawi S, Camara-Lemarroy C, Metz L, Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS, Neurol Neuroimmunol Neuroinflamm, 5 (2018) e459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Di Domenico EG, Cavallo I, Pontone M, Toma L, Ensoli F, Biofilm Producing Salmonella Typhi: Chronic Colonization and Development of Gallbladder Cancer, Int J Mol Sci, 18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Huang Y, Fan XG, Wang ZM, Zhou JH, Tian XF, Li N, Identification of helicobacter species in human liver samples from patients with primary hepatocellular carcinoma, J Clin Pathol, 57 (2004) 1273–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Wang F, Meng W, Wang B, Qiao L, Helicobacter pylori-induced gastric inflammation and gastric cancer, Cancer Lett, 345 (2014) 196–202. [DOI] [PubMed] [Google Scholar]
- [131].Ishaq S, Nunn L, Helicobacter pylori and gastric cancer: a state of the art review, Gastroenterol Hepatol Bed Bench, 8 (2015) S6–S14. [PMC free article] [PubMed] [Google Scholar]
- [132].Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, Enk J, Bar-On Y, Stanietsky-Kaynan N, Coppenhagen-Glazer S, Shussman N, Almogy G, Cuapio A, Hofer E, Mevorach D, Tabib A, Ortenberg R, Markel G, Miklic K, Jonjic S, Brennan CA, Garrett WS, Bachrach G, Mandelboim O, Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack, Immunity, 42 (2015) 344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Tsilimigras MC, Fodor A, Jobin C, Carcinogenesis and therapeutics: the microbiota perspective, Nat Microbiol, 2 (2017) 17008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Purcell RV, Pearson J, Aitchison A, Dixon L, Frizelle FA, Keenan JI, Colonization with enterotoxigenic Bacteroides fragilis is associated with early-stage colorectal neoplasia, PLoS One, 12 (2017) e0171602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Boleij A, Hechenbleikner EM, Goodwin AC, Badani R, Stein EM, Lazarev MG, Ellis B, Carroll KC, Albesiano E, Wick EC, Platz EA, Pardoll DM, Sears CL, The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients, Clin Infect Dis, 60 (2015) 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Mangerich A, Knutson CG, Parry NM, Muthupalani S, Ye W, Prestwich E, Cui L, McFaline JL, Mobley M, Ge Z, Taghizadeh K, Wishnok JS, Wogan GN, Fox JG, Tannenbaum SR, Dedon PC, Infection-induced colitis in mice causes dynamic and tissue-specific changes in stress response and DNA damage leading to colon cancer, Proc Natl Acad Sci U S A, 109 (2012) E1820–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, Barnes R, Watson P, Allen-Vercoe E, Moore RA, Holt RA, Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma, Genome Res, 22 (2012) 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, El-Omar EM, Brenner D, Fuchs CS, Meyerson M, Garrett WS, Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment, Cell Host Microbe, 14 (2013) 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C, Intestinal inflammation targets cancer-inducing activity of the microbiota, Science, 338 (2012) 120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].He Z, Gharaibeh RZ, Newsome RC, Pope JL, Dougherty MW, Tomkovich S, Pons B, Mirey G, Vignard J, Hendrixson DR, Jobin C, Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin, Gut, 68 (2019) 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Mima K, Nakagawa S, Sawayama H, Ishimoto T, Imai K, Iwatsuki M, Hashimoto D, Baba Y, Yamashita YI, Yoshida N, Chikamoto A, Baba H, The microbiome and hepatobiliary-pancreatic cancers, Cancer Lett, 402 (2017) 9–15. [DOI] [PubMed] [Google Scholar]
- [142].Garcia-Castillo V, Sanhueza E, McNerney E, Onate SA, Garcia A, Microbiota dysbiosis: a new piece in the understanding of the carcinogenesis puzzle, J Med Microbiol, 65 (2016) 1347–1362. [DOI] [PubMed] [Google Scholar]
- [143].Tiso M, Schechter AN, Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions, PLoS One, 10 (2015) e0119712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Ridlon JM, Harris SC, Bhowmik S, Kang DJ, Hylemon PB, Consequences of bile salt biotransformations by intestinal bacteria, Gut Microbes, 7 (2016) 22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Kwa M, Plottel CS, Blaser MJ, Adams S, The Intestinal Microbiome and Estrogen Receptor-Positive Female Breast Cancer, J Natl Cancer Inst, 108 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Shapira I, Sultan K, Lee A, Taioli E, Evolving concepts: how diet and the intestinal microbiome act as modulators of breast malignancy, ISRN Oncol, 2013 (2013) 693920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Hsu RY, Chan CH, Spicer JD, Rousseau MC, Giannias B, Rousseau S, Ferri LE, LPS-induced TLR4 signaling in human colorectal cancer cells increases beta1 integrin-mediated cell adhesion and liver metastasis, Cancer Res, 71 (2011) 1989–1998. [DOI] [PubMed] [Google Scholar]
- [148].Yu LX, Schwabe RF, The gut microbiome and liver cancer: mechanisms and clinical translation, Nat Rev Gastroenterol Hepatol, 14 (2017) 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, Rotter-Maskowitz A, Weiser R, Mallel G, Gigi E, Meltser A, Douglas GM, Kamer I, Gopalakrishnan V, Dadosh T, Levin-Zaidman S, Avnet S, Atlan T, Cooper ZA, Arora R, Cogdill AP, Khan MAW, Ologun G, Bussi Y, Weinberger A, Lotan-Pompan M, Golani O, Perry G, Rokah M, Bahar-Shany K, Rozeman EA, Blank CU, Ronai A, Shaoul R, Amit A, Dorfman T, Kremer R, Cohen ZR, Harnof S, Siegal T, Yehuda-Shnaidman E, Gal-Yam EN, Shapira H, Baldini N, Langille MGI, Ben-Nun A, Kaufman B, Nissan A, Golan T, Dadiani M, Levanon K, Bar J, Yust-Katz S, Barshack I, Peeper DS, Raz DJ, Segal E, Wargo JA, Sandbank J, Shental N, Straussman R, The human tumor microbiome is composed of tumor type-specific intracellular bacteria, Science, 368 (2020) 973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, Schlitzer A, Ginhoux F, Apetoh L, Chachaty E, Woerther PL, Eberl G, Berard M, Ecobichon C, Clermont D, Bizet C, Gaboriau-Routhiau V, Cerf-Bensussan N, Opolon P, Yessaad N, Vivier E, Ryffel B, Elson CO, Dore J, Kroemer G, Lepage P, Boneca IG, Ghiringhelli F, Zitvogel L, The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide, Science, 342 (2013) 971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, Gavert N, Zwang Y, Cooper ZA, Shee K, Thaiss CA, Reuben A, Livny J, Avraham R, Frederick DT, Ligorio M, Chatman K, Johnston SE, Mosher CM, Brandis A, Fuks G, Gurbatri C, Gopalakrishnan V, Kim M, Hurd MW, Katz M, Fleming J, Maitra A, Smith DA, Skalak M, Bu J, Michaud M, Trauger SA, Barshack I, Golan T, Sandbank J, Flaherty KT, Mandinova A, Garrett WS, Thayer SP, Ferrone CR, Huttenhower C, Bhatia SN, Gevers D, Wargo JA, Golub TR, Straussman R, Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine, Science, 357 (2017) 1156–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA, The microbiome, cancer, and cancer therapy, Nat Med, 25 (2019) 377–388. [DOI] [PubMed] [Google Scholar]
- [153].Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, Long N, Plodkowski AJ, Arbour KC, Chaft JE, Rouche JA, Zitvogel L, Zalcman G, Albiges L, Escudier B, Routy B, Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer, Ann Oncol, 29 (2018) 1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, Dai RM, Kiu H, Cardone M, Naik S, Patri AK, Wang E, Marincola FM, Frank KM, Belkaid Y, Trinchieri G, Goldszmid RS, Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment, Science, 342 (2013) 967–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragon L, Jacquelot N, Qu B, Ferrere G, Clemenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L, Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors, Science, 359 (2018) 91–97. [DOI] [PubMed] [Google Scholar]
- [156].Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Berard M, Nigou J, Opolon P, Eggermont A, Woerther PL, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M, Zitvogel L, Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota, Science, 350 (2015) 1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, Chang EB, Gajewski TF, Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy, Science, 350 (2015) 1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Schoultz I, Keita AV, Cellular and Molecular Therapeutic Targets in Inflammatory Bowel Disease-Focusing on Intestinal Barrier Function, Cells, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Butin-Israeli V, Bui TM, Wiesolek HL, Mascarenhas L, Lee JJ, Mehl LC, Knutson KR, Adam SA, Goldman RD, Beyder A, Wiesmuller L, Hanauer SB, Sumagin R, Neutrophil-induced genomic instability impedes resolution of inflammation and wound healing, J Clin Invest, 129 (2019) 712–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Wileman TE, Lennartz MR, Stahl PD, Identification of the macrophage mannose receptor as a 175-kDa membrane protein, Proc Natl Acad Sci U S A, 83 (1986) 2501–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].van Vliet SJ, Saeland E, van Kooyk Y, Sweet preferences of MGL: carbohydrate specificity and function, Trends Immunol, 29 (2008) 83–90. [DOI] [PubMed] [Google Scholar]
- [162].Xiao B, Laroui H, Ayyadurai S, Viennois E, Charania MA, Zhang Y, Merlin D, Mannosylated bioreducible nanoparticle-mediated macrophage-specific TNF-alpha RNA interference for IBD therapy, Biomaterials, 34 (2013) 7471–7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Zhang J, Tang C, Yin C, Galactosylated trimethyl chitosan-cysteine nanoparticles loaded with Map4k4 siRNA for targeting activated macrophages, Biomaterials, 34 (2013) 3667–3677. [DOI] [PubMed] [Google Scholar]
- [164].Laroui H, Viennois E, Xiao B, Canup BS, Geem D, Denning TL, Merlin D, Fab'-bearing siRNA TNFalpha-loaded nanoparticles targeted to colonic macrophages offer an effective therapy for experimental colitis, J Control Release, 186 (2014) 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Dearling JL, Daka A, Veiga N, Peer D, Packard AB, Colitis ImmunoPET: Defining Target Cell Populations and Optimizing Pharmacokinetics, Inflamm Bowel Dis, 22 (2016) 529–538. [DOI] [PubMed] [Google Scholar]
- [166].Veiga N, Goldsmith M, Granot Y, Rosenblum D, Dammes N, Kedmi R, Ramishetti S, Peer D, Cell specific delivery of modified mRNA expressing therapeutic proteins to leukocytes, Nat Commun, 9 (2018) 4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Maclachlan I, siRNAs with guts, Nat Biotechnol, 26 (2008) 403–405. [DOI] [PubMed] [Google Scholar]
- [168].Peer D, Park EJ, Morishita Y, Carman CV, Shimaoka M, Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target, Science, 319 (2008) 627–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Veiga N, Goldsmith M, Diesendruck Y, Ramishetti S, Rosenblum D, Elinav E, Behlke MA, Benhar I, Peer D, Leukocyte-specific siRNA delivery revealing IRF8 as a potential anti-inflammatory target, J Control Release, 313 (2019) 33–41. [DOI] [PubMed] [Google Scholar]
- [170].Xiao J, Feng S, Wang X, Long K, Luo Y, Wang Y, Ma J, Tang Q, Jin L, Li X, Li M, Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables, PeerJ, 6 (2018) e5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Yang C, Zhang M, Merlin D, Advances in Plant-derived Edible Nanoparticle-based lipid Nano-drug Delivery Systems as Therapeutic Nanomedicines, J Mater Chem B, 6 (2018) 1312–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Wang B, Zhuang X, Deng ZB, Jiang H, Mu J, Wang Q, Xiang X, Guo H, Zhang L, Dryden G, Yan J, Miller D, Zhang HG, Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit, Mol Ther, 22 (2014) 522–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Cai Z, Zhang W, Yang F, Yu L, Yu Z, Pan J, Wang L, Cao X, Wang J, Immunosuppressive exosomes from TGF-beta1 gene-modified dendritic cells attenuate Th17-mediated inflammatory autoimmune disease by inducing regulatory T cells, Cell Res, 22 (2012) 607–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Ma ZJ, Wang YH, Li ZG, Wang Y, Li BY, Kang HY, Wu XY, Immunosuppressive Effect of Exosomes from Mesenchymal Stromal Cells in Defined Medium on Experimental Colitis, Int J Stem Cells, 12 (2019) 440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Suaini NH, Zhang Y, Vuillermin PJ, Allen KJ, Harrison LC, Immune Modulation by Vitamin D and Its Relevance to Food Allergy, Nutrients, 7 (2015) 6088–6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Hufnagl K, Jensen-Jarolim E, Vitamin A and D in allergy: from experimental animal models and cellular studies to human disease, Allergo J Int, 27 (2018) 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Dhadwar SS, Kiernan J, Wen J, Hortelano G, Repeated oral administration of chitosan/DNA nanoparticles delivers functional FVIII with the absence of antibodies in hemophilia A mice, J Thromb Haemost, 8 (2010) 2743–2750. [DOI] [PubMed] [Google Scholar]
- [178].Kim WU, Lee WK, Ryoo JW, Kim SH, Kim J, Youn J, Min SY, Bae EY, Hwang SY, Park SH, Cho CS, Park JS, Kim HY, Suppression of collagen-induced arthritis by single administration of poly(lactic-co-glycolic acid) nanoparticles entrapping type II collagen: a novel treatment strategy for induction of oral tolerance, Arthritis Rheum, 46 (2002) 1109–1120. [DOI] [PubMed] [Google Scholar]
- [179].Takagi H, Hiroi T, Yang L, Tada Y, Yuki Y, Takamura K, Ishimitsu R, Kawauchi H, Kiyono H, Takaiwa F, A rice-based edible vaccine expressing multiple T cell epitopes induces oral tolerance for inhibition of Th2-mediated IgE responses, Proc Natl Acad Sci U S A, 102 (2005) 17525–17530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Suzuki K, Kaminuma O, Yang L, Takai T, Mori A, Umezu-Goto M, Ohtomo T, Ohmachi Y, Noda Y, Hirose S, Okumura K, Ogawa H, Takada K, Hirasawa M, Hiroi T, Takaiwa F, Prevention of allergic asthma by vaccination with transgenic rice seed expressing mite allergen: induction of allergen-specific oral tolerance without bystander suppression, Plant Biotechnol J, 9 (2011) 982–990. [DOI] [PubMed] [Google Scholar]
- [181].Denes B, Krausova V, Fodor N, Timiryasova T, Henderson D, Hough J, Yu J, Fodor I, Langridge WH, Protection of NOD mice from type 1 diabetes after oral inoculation with vaccinia viruses expressing adjuvanted islet autoantigens, J Immunother, 28 (2005) 438–448. [DOI] [PubMed] [Google Scholar]
- [182].Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, Clarke LL, Mrsny RJ, Turner JR, Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo, J Clin Invest, 115 (2005) 2702–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Melero A, Draheim C, Hansen S, Giner E, Carreras JJ, Talens-Visconti R, Garrigues TM, Peris JE, Recio MC, Giner R, Lehr CM, Targeted delivery of Cyclosporine A by polymeric nanocarriers improves the therapy of inflammatory bowel disease in a relevant mouse model, Eur J Pharm Biopharm, 119 (2017) 361–371. [DOI] [PubMed] [Google Scholar]
- [184].Witten J, Samad T, Ribbeck K, Selective permeability of mucus barriers, Curr Opin Biotechnol, 52 (2018) 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Chen EY, Daley D, Wang YC, Garnica M, Chen CS, Chin WC, Functionalized carboxyl nanoparticles enhance mucus dispersion and hydration, Sci Rep, 2 (2012) 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Ho DK, Frisch S, Biehl A, Terriac E, De Rossi C, Schwarzkopf K, Lautenschlager F, Loretz B, Murgia X, Lehr CM, Farnesylated Glycol Chitosan as a Platform for Drug Delivery: Synthesis, Characterization, and Investigation of Mucus-Particle Interactions, Biomacromolecules, 19 (2018) 3489–3501. [DOI] [PubMed] [Google Scholar]
- [187].Irache JM, Durrer C, Duchene D, Ponchel G, Bioadhesion of lectin-latex conjugates to rat intestinal mucosa, Pharm Res, 13 (1996) 1716–1719. [DOI] [PubMed] [Google Scholar]
- [188].Qin X, Damage of the Mucus Layer: The Possible Shared Critical Common Cause for Both Inflammatory Bowel Disease (IBD) and Irritable Bowel Syndrome (IBS), Inflamm Bowel Dis, 23 (2017) E11–E12. [DOI] [PubMed] [Google Scholar]
- [189].van der Post S, Jabbar KS, Birchenough G, Arike L, Akhtar N, Sjovall H, Johansson MEV, Hansson GC, Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis, Gut, 68 (2019) 2142–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Xiao B, Xu Z, Viennois E, Zhang Y, Zhang Z, Zhang M, Han MK, Kang Y, Merlin D, Orally Targeted Delivery of Tripeptide KPV via Hyaluronic Acid-Functionalized Nanoparticles Efficiently Alleviates Ulcerative Colitis, Mol Ther, 25 (2017) 1628–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Zucchelli M, Torkvist L, Bresso F, Halfvarson J, Hellquist A, Anedda F, Assadi G, Lindgren GB, Svanfeldt M, Janson M, Noble CL, Pettersson S, Lappalainen M, Paavola-Sakki P, Halme L, Farkkila M, Turunen U, Satsangi J, Kontula K, Lofberg R, Kere J, D'Amato M, PepT1 oligopeptide transporter (SLC15A1) gene polymorphism in inflammatory bowel disease, Inflamm Bowel Dis, 15 (2009) 1562–1569. [DOI] [PubMed] [Google Scholar]
- [192].Wang Y, Hu Y, Li P, Weng Y, Kamada N, Jiang H, Smith DE, Expression and regulation of proton-coupled oligopeptide transporters in colonic tissue and immune cells of mice, Biochem Pharmacol, 148 (2018) 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Wang CY, Liu S, Xie XN, Tan ZR, Regulation profile of the intestinal peptide transporter 1 (PepT1), Drug Des Devel Ther, 11 (2017) 3511–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Dalmasso G, Charrier-Hisamuddin L, Nguyen HT, Yan Y, Sitaraman S, Merlin D, PepT1-mediated tripeptide KPV uptake reduces intestinal inflammation, Gastroenterology, 134 (2008) 166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Wu Y, Sun M, Wang D, Li G, Huang J, Tan S, Bao L, Li Q, Li G, Si L, A PepT1 mediated medicinal nano-system for targeted delivery of cyclosporine A to alleviate acute severe ulcerative colitis, Biomater Sci, 7 (2019) 4299–4309. [DOI] [PubMed] [Google Scholar]
- [196].Danese S, Semeraro S, Marini M, Roberto I, Armuzzi A, Papa A, Gasbarrini A, Adhesion molecules in inflammatory bowel disease: therapeutic implications for gut inflammation, Dig Liver Dis, 37 (2005) 811–818. [DOI] [PubMed] [Google Scholar]
- [197].Binion DG, West GA, Volk EE, Drazba JA, Ziats NP, Petras RE, Fiocchi C, Acquired increase in leucocyte binding by intestinal microvascular endothelium in inflammatory bowel disease, Lancet, 352 (1998) 1742–1746. [DOI] [PubMed] [Google Scholar]
- [198].Kelly CP, O'Keane JC, Orellana J, Schroy PC 3rd, Yang S, LaMont JT, Brady HR, Human colon cancer cells express ICAM-1 in vivo and support LFA-1-dependent lymphocyte adhesion in vitro, Am J Physiol, 263 (1992) G864–870. [DOI] [PubMed] [Google Scholar]
- [199].Mane V, Muro S, Biodistribution and endocytosis of ICAM-1-targeting antibodies versus nanocarriers in the gastrointestinal tract in mice, Int J Nanomedicine, 7 (2012) 4223–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Harel E, Rubinstein A, Nissan A, Khazanov E, Nadler Milbauer M, Barenholz Y, Tirosh B, Enhanced transferrin receptor expression by proinflammatory cytokines in enterocytes as a means for local delivery of drugs to inflamed gut mucosa, PLoS One, 6 (2011) e24202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Xiao B, Yang Y, Viennois E, Zhang Y, Ayyadurai S, Baker M, Laroui H, Merlin D, Glycoprotein CD98 as a receptor for colitis-targeted delivery of nanoparticle, J Mater Chem B, 2 (2014) 1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Kucharzik T, Lugering A, Yan Y, Driss A, Charrier L, Sitaraman S, Merlin D, Activation of epithelial CD98 glycoprotein perpetuates colonic inflammation, Lab Invest, 85 (2005) 932–941. [DOI] [PubMed] [Google Scholar]
- [203].Nguyen HT, Merlin D, Homeostatic and innate immune responses: role of the transmembrane glycoprotein CD98, Cell Mol Life Sci, 69 (2012) 3015–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Xiao B, Laroui H, Viennois E, Ayyadurai S, Charania MA, Zhang Y, Zhang Z, Baker MT, Zhang B, Gewirtz AT, Merlin D, Nanoparticles with surface antibody against CD98 and carrying CD98 small interfering RNA reduce colitis in mice, Gastroenterology, 146 (2014) 1289–1300 e1281-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Macdonald DC, Leir SH, Brooks C, Sanders E, Lackie P, Rosenberg W, CD44 isoform expression on colonic epithelium mediates lamina propria lymphocyte adhesion and is controlled by Th1 and Th2 cytokines, Eur J Gastroenterol Hepatol, 15 (2003) 1101–1110. [DOI] [PubMed] [Google Scholar]
- [206].Fromont Hankard G, Cezard JP, Aigrain Y, Navarro J, Peuchmaur M, CD44 variant expression in inflammatory colonic mucosa is not disease specific but associated with increased crypt cell proliferation, Histopathology, 32 (1998) 317–321. [DOI] [PubMed] [Google Scholar]
- [207].Wittig BM, Stallmach A, Zeitz M, Gunthert U, Functional involvement of CD44 variant 7 in gut immune response, Pathobiology, 70 (2002) 184–189. [DOI] [PubMed] [Google Scholar]
- [208].Xiao B, Zhang Z, Viennois E, Kang Y, Zhang M, Han MK, Chen J, Merlin D, Combination Therapy for Ulcerative Colitis: Orally Targeted Nanoparticles Prevent Mucosal Damage and Relieve Inflammation, Theranostics, 6 (2016) 2250–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Simmonds NJ, Rampton DS, Inflammatory bowel disease--a radical view, Gut, 34 (1993) 865–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Xavier RJ, Podolsky DK, Unravelling the pathogenesis of inflammatory bowel disease, Nature, 448 (2007) 427–434. [DOI] [PubMed] [Google Scholar]
- [211].Babbs CF, Oxygen radicals in ulcerative colitis, Free Radic Biol Med, 13 (1992) 169–181. [DOI] [PubMed] [Google Scholar]
- [212].Vong LB, Tomita T, Yoshitomi T, Matsui H, Nagasaki Y, An orally administered redox nanoparticle that accumulates in the colonic mucosa and reduces colitis in mice, Gastroenterology, 143 (2012) 1027–1036 e1023. [DOI] [PubMed] [Google Scholar]
- [213].Song W, Anselmo AC, Huang L, Nanotechnology intervention of the microbiome for cancer therapy, Nat Nanotechnol, 14 (2019) 1093–1103. [DOI] [PubMed] [Google Scholar]
- [214].Ren Z, Chen X, Hong L, Zhao X, Cui G, Li A, Liu Y, Zhou L, Sun R, Shen S, Li J, Lou J, Zhou H, Wang J, Xu G, Yu Z, Song Y, Chen X, Nanoparticle Conjugation of Ginsenoside Rg3 Inhibits Hepatocellular Carcinoma Development and Metastasis, Small, 16 (2020) e1905233. [DOI] [PubMed] [Google Scholar]
- [215].Zheng DW, Dong X, Pan P, Chen KW, Fan JX, Cheng SX, Zhang XZ, Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy, Nat Biomed Eng, 3 (2019) 717–728. [DOI] [PubMed] [Google Scholar]
- [216].Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V, Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis, Immunity, 40 (2014) 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Zheng DW, Li RQ, An JX, Xie TQ, Han ZY, Xu R, Fang Y, Zhang XZ, Prebiotics-Encapsulated Probiotic Spores Regulate Gut Microbiota and Suppress Colon Cancer, Adv Mater, 32 (2020) e2004529. [DOI] [PubMed] [Google Scholar]
- [218].Foulkes R, Ali Asgari M, Curtis A, Hoskins C, Silver-Nanoparticle-Mediated Therapies in the Treatment of Pancreatic Cancer, ACS Applied Nano Materials, 2 (2019) 1758–1772. [Google Scholar]
- [219].Singh J, Moore W, Fattah F, Jiang X, Zheng J, Kurian P, Beg MS, Khan SA, Activity and pharmacology of homemade silver nanoparticles in refractory metastatic head and neck squamous cell cancer, Head Neck, 41 (2019) E11–E16. [DOI] [PubMed] [Google Scholar]
- [220].Han K, Nam J, Xu J, Sun X, Huang X, Animasahun O, Achreja A, Jeon JH, Pursley B, Kamada N, Chen GY, Nagrath D, Moon JJ, Generation of systemic antitumour immunity via the in situ modulation of the gut microbiome by an orally administered inulin gel, Nat Biomed Eng, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Khan I, Ullah N, Zha L, Bai Y, Khan A, Zhao T, Che T, Zhang C, Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome, Pathogens, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Sun Y, Li L, Xia Y, Li W, Wang K, Wang L, Miao Y, Ma S, The gut microbiota heterogeneity and assembly changes associated with the IBD, Sci Rep, 9 (2019) 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H, Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells, Nature, 504 (2013) 446–450. [DOI] [PubMed] [Google Scholar]
- [224].Sartor RB, Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics, Gastroenterology, 126 (2004) 1620–1633. [DOI] [PubMed] [Google Scholar]
- [225].Png CW, Linden SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, Florin TH, Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria, Am J Gastroenterol, 105 (2010) 2420–2428. [DOI] [PubMed] [Google Scholar]
- [226].Fabia R, Ar'Rajab A, Johansson ML, Andersson R, Willen R, Jeppsson B, Molin G, Bengmark S, Impairment of bacterial flora in human ulcerative colitis and experimental colitis in the rat, Digestion, 54 (1993) 248–255. [DOI] [PubMed] [Google Scholar]
- [227].Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, Remaut E, Treatment of murine colitis by Lactococcus lactis secreting interleukin-10, Science, 289 (2000) 1352–1355. [DOI] [PubMed] [Google Scholar]
- [228].Bellavia M, Rappa F, Lo Bello M, Brecchia G, Tomasello G, Leone A, Spatola G, Uzzo ML, Bonaventura G, David S, Damiani P, Hajj Hussein I, Zeenny MN, Jurjus A, Schembri-Wismayer P, Cocchi M, Zummo G, Farina F, Gerbino A, Cappello F, Traina G, Lactobacillus casei and bifidobacterium lactis supplementation reduces tissue damage of intestinal mucosa and liver after 2,4,6-trinitrobenzenesulfonic acid treatment in mice, J Biol Regul Homeost Agents, 28 (2014) 251–261. [PubMed] [Google Scholar]
- [229].Llopis M, Antolin M, Carol M, Borruel N, Casellas F, Martinez C, Espin-Basany E, Guarner F, Malagelada JR, Lactobacillus casei downregulates commensals' inflammatory signals in Crohn's disease mucosa, Inflamm Bowel Dis, 15 (2009) 275–283. [DOI] [PubMed] [Google Scholar]
- [230].Kim MS, Byun JS, Yoon YS, Yum DY, Chung MJ, Lee JC, A probiotic combination attenuates experimental colitis through inhibition of innate cytokine production, Benef Microbes, 8 (2017) 231–241. [DOI] [PubMed] [Google Scholar]
- [231].Kawahara M, Nemoto M, Nakata T, Kondo S, Takahashi H, Kimura B, Kuda T, Anti-inflammatory properties of fermented soy milk with Lactococcus lactis subsp. lactis S-SU2 in murine macrophage RAW264.7 cells and DSS-induced IBD model mice, Int Immunopharmacol, 26 (2015) 295–303. [DOI] [PubMed] [Google Scholar]
- [232].Ricci S, Macchia G, Ruggiero P, Maggi T, Bossu P, Xu L, Medaglini D, Tagliabue A, Hammarstrom L, Pozzi G, Boraschi D, In vivo mucosal delivery of bioactive human interleukin 1 receptor antagonist produced by Streptococcus gordonii, BMC Biotechnol, 3 (2003) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Teng Y, Ren Y, Sayed M, Hu X, Lei C, Kumar A, Hutchins E, Mu J, Deng Z, Luo C, Sundaram K, Sriwastva MK, Zhang L, Hsieh M, Reiman R, Haribabu B, Yan J, Jala VR, Miller DM, Van Keuren-Jensen K, Merchant ML, McClain CJ, Park JW, Egilmez NK, Zhang HG, Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota, Cell Host Microbe, 24 (2018) 637–652 e638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Lee Y, Sugihara K, Gillilland MG 3rd, Jon S, Kamada N, Moon JJ, Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis, Nat Mater, 19 (2020) 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Pasetti MF, Simon JK, Sztein MB, Levine MM, Immunology of gut mucosal vaccines, Immunol Rev, 239 (2011) 125–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Newsted D, Fallahi F, Golshani A, Azizi A, Advances and challenges in mucosal adjuvant technology, Vaccine, 33 (2015) 2399–2405. [DOI] [PubMed] [Google Scholar]
- [237].Mitragotri S, Burke PA, Langer R, Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies, Nat Rev Drug Discov, 13 (2014) 655–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Pavot V, Rochereau N, Genin C, Verrier B, Paul S, New insights in mucosal vaccine development, Vaccine, 30 (2012) 142–154. [DOI] [PubMed] [Google Scholar]
- [239].Mestecky J, Russell MW, Elson CO, Perspectives on mucosal vaccines: is mucosal tolerance a barrier?, J Immunol, 179 (2007) 5633–5638. [DOI] [PubMed] [Google Scholar]
- [240].Zhang P, Tey SK, Koyama M, Kuns RD, Olver SD, Lineburg KE, Lor M, Teal BE, Raffelt NC, Raju J, Leveque L, Markey KA, Varelias A, Clouston AD, Lane SW, MacDonald KP, Hill GR, Induced regulatory T cells promote tolerance when stabilized by rapamycin and IL-2 in vivo, J Immunol, 191 (2013) 5291–5303. [DOI] [PubMed] [Google Scholar]
- [241].Shan J, Feng L, Li Y, Sun G, Chen X, Chen P, The effects of rapamycin on regulatory T cells: its potential time-dependent role in inducing transplant tolerance, Immunol Lett, 162 (2014) 74–86. [DOI] [PubMed] [Google Scholar]
- [242].Kishimoto TK, Maldonado RA, Nanoparticles for the Induction of Antigen-Specific Immunological Tolerance, Front Immunol, 9 (2018) 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Devriendt B, De Geest BG, Goddeeris BM, Cox E, Crossing the barrier: Targeting epithelial receptors for enhanced oral vaccine delivery, J Control Release, 160 (2012) 431–439. [DOI] [PubMed] [Google Scholar]
- [244].Corr SC, Gahan CC, Hill C, M-cells: origin, morphology and role in mucosal immunity and microbial pathogenesis, FEMS Immunol Med Microbiol, 52 (2008) 2–12. [DOI] [PubMed] [Google Scholar]
- [245].Roth-Walter F, Bohle B, Scholl I, Untersmayr E, Scheiner O, Boltz-Nitulescu G, Gabor F, Brayden DJ, Jensen-Jarolim E, Targeting antigens to murine and human M-cells with Aleuria aurantia lectin-functionalized microparticles, Immunol Lett, 100 (2005) 182–188. [DOI] [PubMed] [Google Scholar]
- [246].Garinot M, Fievez V, Pourcelle V, Stoffelbach F, des Rieux A, Plapied L, Theate I, Freichels H, Jerome C, Marchand-Brynaert J, Schneider YJ, Preat V, PEGylated PLGA-based nanoparticles targeting M cells for oral vaccination, J Control Release, 120 (2007) 195–204. [DOI] [PubMed] [Google Scholar]
- [247].Lo DD, Ling J, Eckelhoefer AH, M cell targeting by a Claudin 4 targeting peptide can enhance mucosal IgA responses, BMC Biotechnol, 12 (2012) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Hase K, Kawano K, Nochi T, Pontes GS, Fukuda S, Ebisawa M, Kadokura K, Tobe T, Fujimura Y, Kawano S, Yabashi A, Waguri S, Nakato G, Kimura S, Murakami T, Iimura M, Hamura K, Fukuoka S, Lowe AW, Itoh K, Kiyono H, Ohno H, Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response, Nature, 462 (2009) 226–230. [DOI] [PubMed] [Google Scholar]
- [249].Shima H, Watanabe T, Fukuda S, Fukuoka S, Ohara O, Ohno H, A novel mucosal vaccine targeting Peyer's patch M cells induces protective antigen-specific IgA responses, Int Immunol, 26 (2014) 619–625. [DOI] [PubMed] [Google Scholar]
- [250].Gupta PN, Vyas SP, Investigation of lectinized liposomes as M-cell targeted carrier-adjuvant for mucosal immunization, Colloids Surf B Biointerfaces, 82 (2011) 118–125. [DOI] [PubMed] [Google Scholar]
- [251].Giannasca PJ, Giannasca KT, Leichtner AM, Neutra MR, Human intestinal M cells display the sialyl Lewis A antigen, Infect Immun, 67 (1999) 946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Demento SL, Siefert AL, Bandyopadhyay A, Sharp FA, Fahmy TM, Pathogen-associated molecular patterns on biomaterials: a paradigm for engineering new vaccines, Trends Biotechnol, 29 (2011) 294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Chen H, Torchilin V, Langer R, Lectin-bearing polymerized liposomes as potential oral vaccine carriers, Pharm Res, 13 (1996) 1378–1383. [DOI] [PubMed] [Google Scholar]
- [254].Chabot SM, Chernin TS, Shawi M, Wagner J, Farrant S, Burt DS, Cyr S, Neutra MR, TLR2 activation by proteosomes promotes uptake of particulate vaccines at mucosal surfaces, Vaccine, 25 (2007) 5348–5358. [DOI] [PubMed] [Google Scholar]
- [255].Chabot S, Wagner JS, Farrant S, Neutra MR, TLRs regulate the gatekeeping functions of the intestinal follicle-associated epithelium, J Immunol, 176 (2006) 4275–4283. [DOI] [PubMed] [Google Scholar]
- [256].Holmgren J, Czerkinsky C, Mucosal immunity and vaccines, Nat Med, 11 (2005) S45–53. [DOI] [PubMed] [Google Scholar]
- [257].Casella CR, Mitchell TC, Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant, Cell Mol Life Sci, 65 (2008) 3231–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Honko AN, Mizel SB, Effects of flagellin on innate and adaptive immunity, Immunol Res, 33 (2005) 83–101. [DOI] [PubMed] [Google Scholar]
- [259].Harandi AM, Holmgren J, CpG DNA as a potent inducer of mucosal immunity: implications for immunoprophylaxis and immunotherapy of mucosal infections, Curr Opin Investig Drugs, 5 (2004) 141–145. [PubMed] [Google Scholar]
- [260].Arigita C, Luijkx T, Jiskoot W, Poelen M, Hennink WE, Crommelin DJ, Ley P, Els C, Kersten GF, Well-defined and potent liposomal meningococcal B vaccines adjuvated with LPS derivatives, Vaccine, 23 (2005) 5091–5098. [DOI] [PubMed] [Google Scholar]
- [261].Irache JM, Salman HH, Gomez S, Espuelas S, Gamazo C, Poly(anhydride) nanoparticles as adjuvants for mucosal vaccination, Front Biosci (Schol Ed), 2 (2010) 876–890. [DOI] [PubMed] [Google Scholar]
- [262].Park KS, Sun X, Aikins ME, Moon JJ, Non-viral COVID-19 vaccine delivery systems, Adv Drug Deliv Rev, 169 (2021) 137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Liebowitz D, Gottlieb K, Kolhatkar NS, Garg SJ, Asher JM, Nazareno J, Kim K, McIlwain DR, Tucker SN, Efficacy, immunogenicity, and safety of an oral influenza vaccine: a placebo-controlled and active-controlled phase 2 human challenge study, Lancet Infect Dis, 20 (2020) 435–444. [DOI] [PubMed] [Google Scholar]
- [264].Goodnow CC, Pathways for self-tolerance and the treatment of autoimmune diseases, Lancet, 357 (2001) 2115–2121. [DOI] [PubMed] [Google Scholar]
- [265].Sinha AA, Lopez MT, McDevitt HO, Autoimmune diseases: the failure of self tolerance, Science, 248 (1990) 1380–1388. [DOI] [PubMed] [Google Scholar]
- [266].Dimichele D, Rivard G, Hay C, Antunes S, Inhibitors in haemophilia: clinical aspects, Haemophilia, 10 Suppl 4 (2004) 140–145. [DOI] [PubMed] [Google Scholar]
- [267].Byrne BJ, Falk DJ, Pacak CA, Nayak S, Herzog RW, Elder ME, Collins SW, Conlon TJ, Clement N, Cleaver BD, Cloutier DA, Porvasnik SL, Islam S, Elmallah MK, Martin A, Smith BK, Fuller DD, Lawson LA, Mah CS, Pompe disease gene therapy, Hum Mol Genet, 20 (2011) R61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Bowman K, Sarkar R, Raut S, Leong KW, Gene transfer to hemophilia A mice via oral delivery of FVIII-chitosan nanoparticles, J Control Release, 132 (2008) 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Lee WK, Park JY, Jung S, Yang CW, Kim WU, Kim HY, Park JH, Park JS, Preparation and characterization of biodegradable nanoparticles entrapping immunodominant peptide conjugated with PEG for oral tolerance induction, J Control Release, 105 (2005) 77–88. [DOI] [PubMed] [Google Scholar]
- [270].Weiner HL, da Cunha AP, Quintana F, Wu H, Oral tolerance, Immunol Rev, 241 (2011) 241–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Ruhlman T, Ahangari R, Devine A, Samsam M, Daniell H, Expression of cholera toxin B-proinsulin fusion protein in lettuce and tobacco chloroplasts--oral administration protects against development of insulitis in non-obese diabetic mice, Plant Biotechnol J, 5 (2007) 495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Odumosu O, Nicholas D, Payne K, Langridge W, Cholera toxin B subunit linked to glutamic acid decarboxylase suppresses dendritic cell maturation and function, Vaccine, 29 (2011) 8451–8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Limaye A, Koya V, Samsam M, Daniell H, Receptor-mediated oral delivery of a bioencapsulated green fluorescent protein expressed in transgenic chloroplasts into the mouse circulatory system, FASEB J, 20 (2006) 959–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Gong Z, Pan L, Le Y, Liu Q, Zhou M, Xing W, Zhuo R, Wang S, Guo J, Glutamic acid decarboxylase epitope protects against autoimmune diabetes through activation of Th2 immune response and induction of possible regulatory mechanism, Vaccine, 28 (2010) 4052–4058. [DOI] [PubMed] [Google Scholar]
- [275].Kwon KC, Verma D, Singh ND, Herzog R, Daniell H, Oral delivery of human biopharmaceuticals, autoantigens and vaccine antigens bioencapsulated in plant cells, Adv Drug Deliv Rev, 65 (2013) 782–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Gilmartin AA, Petri WA Jr., Exploring the role of environmental enteropathy in malnutrition, infant development and oral vaccine response, Philos Trans R Soc Lond B Biol Sci, 370 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Environmental Chemicals, the Human Microbiome, and Health Risk: A Research Strategy, Washington (DC), 2017. [PubMed] [Google Scholar]
- [278].Monda V, Villano I, Messina A, Valenzano A, Esposito T, Moscatelli F, Viggiano A, Cibelli G, Chieffi S, Monda M, Messina G, Exercise Modifies the Gut Microbiota with Positive Health Effects, Oxid Med Cell Longev, 2017 (2017) 3831972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Guyton K, Alverdy JC, The gut microbiota and gastrointestinal surgery, Nat Rev Gastroenterol Hepatol, 14 (2017) 43–54. [DOI] [PubMed] [Google Scholar]
- [280].Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K, Gut microbes and the brain: paradigm shift in neuroscience, J Neurosci, 34 (2014) 15490–15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Forsythe P, Kunze WA, Voices from within: gut microbes and the CNS, Cell Mol Life Sci, 70 (2013) 55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Blackshaw LA, Brookes SJ, Grundy D, Schemann M, Sensory transmission in the gastrointestinal tract, Neurogastroenterol Motil, 19 (2007) 1–19. [DOI] [PubMed] [Google Scholar]
- [283].Giovannini MG, Lana D, Traini C, Vannucchi MG, The Microbiota-Gut-Brain Axis and Alzheimer Disease. From Dysbiosis to Neurodegeneration: Focus on the Central Nervous System Glial Cells, J Clin Med, 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Pistollato F, Sumalla Cano S, Elio I, Masias Vergara M, Giampieri F, Battino M, Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease, Nutr Rev, 74 (2016) 624–634. [DOI] [PubMed] [Google Scholar]
- [285].Parashar A, Udayabanu M, Gut microbiota: Implications in Parkinson's disease, Parkinsonism Relat Disord, 38 (2017) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]