Abstract
Objective:
Despite rotator cuff disease being one of the most common causes of shoulder pain, its pathogenesis and biology are poorly understood. In this study, we synthesized evidence from studies reporting associations for aging and smoking status in relation to rotator cuff disease.
Design:
A systematic review was performed using multiple databases (PubMed, Embase, Cochrane, CINAHL, and Science Direct). Articles that met our eligibility criteria and presented data on the association between aging and/or smoking status and rotator cuff disease were included. We performed meta-analyses and report cumulative effects using odds ratios (OR) and corresponding 95% confidence intervals (CI).
Results:
Of the 212 articles eligible for full-text review, seven studies reported on the relationship between aging and rotator cuff disease and ten studies reported on the relationship between smoking and rotator cuff disease. Aging was consistently associated with increased odds of having rotator cuff disease when assessed continuously (per 10-year increase: OR= 1.20, 95% CI: 1.18, 1.21) or categorically [<40 years old versus: (a) 40–44 years old (OR= 2.71, 95% CI: 1.78, 4.13), (b) 45–49 years old (OR= 4.33, 95% CI: 2.88, 6.55), and (c) 50 years of age or older (OR= 6.97, 95% CI: 4.85, 10.01)]. Assessing studies that reported smoking status as current smokers versus non-smokers, current smokers were more likely to have rotator cuff disease (OR= 1.94, 95% CI: 1.52, 2.48). However, a statistically significant association was not found when never smokers were compared to former smokers (OR= 1.08, 95% CI: 0.97, 1.20) and to current smokers (OR= 0.97, 95% CI: 0.87, 1.07).
Conclusion:
In this systematic review and meta-analysis, increasing age was a strong risk factor for rotator cuff disease. The finding that current smokers are more likely to have rotator cuff disease as compared with non-smokers implies that cessation of smoking can potentially lead to mitigation of this risk factor.
Keywords: Rotator cuff disease, risk factor, aging, smoking
INTRODUCTION
Rotator cuff disease includes a broad spectrum of pathology encompassing rotator cuff tears, tendinosis, calcific tendinitis, bursitis, and more. Rotator cuff disease is one of the most common causes of shoulder pain1, accounting for up to 70% of shoulder complaints in the adult population2, and is a leading cause of shoulder-related disability3. It accounts for over 4.5 million physician visits annually4 and incurs an economic burden of $3 to $5 billion per year in the United States5. Despite this, the pathophysiology of rotator cuff disease is poorly understood. Etiologies of rotator cuff disease are multifactorial and include a combination of major extrinsic trauma, microtrauma resulting from daily wear and tear, and natural degeneration over time6. Tendon hypovascularity of the rotator cuff may also contribute to decreased healing ability and predispose to further injury7. Aging and smoking result in metabolic changes and hypovascularity8 and thus are both potential risk factors for rotator cuff degeneration.
In the literature, aging and smoking have been associated with an increased risk of rotator cuff disease to varying degrees. There is robust and mounting evidence that suggests aging is a risk factor for rotator cuff disease9. However, the data on the impact of smoking as a risk factor has been inconsistent. While a previous systematic review in by Bishop et al. in 2015 concluded an association between smoking and rotator cuff disease10, several other studies have found no association11–13. To our knowledge, no studies systematically quantify and summarize these associations. Hence, we synthesized evidence from existing literature to provide qualitative and quantitative summaries on the association between two important risk factors, aging and smoking, and the risk of rotator cuff disease.
MATERIALS AND METHODS
Search Strategy
On July 10, 2019, we performed a comprehensive literature search of multiple databases (PubMed, Embase, Cochrane, CINAHL, and Science Direct) on the relationship between various putative risk factors and rotator cuff disease. The search terms applied for each database may be found in the Supplementary section. An initial total of 7,332 articles (including duplicates) were identified after the database search and manual bibliography review. Studies were excluded for the following reasons: was published in a language other than English, was not an original research article (editorials, opinions, systematic reviews, and meta-analyses), only an abstract could be found, was unrelated to rotator cuff disease, evaluated surgical outcomes or procedures, assessed genetics as a risk factor, or was a description of a case report or series. This screening yielded a total of 212 studies. Full-text review of these articles was subsequently performed. Observational studies (cohort, cross-sectional and case-control) that provided ratio effect estimates (odds ratio, risk ratio or hazard ratio) or provided sufficient data to compute these estimates for aging or smoking were included (Figure 1). In order to ensure relatively stable effect estimates, we only included studies that had at least 30 rotator cuff disease cases and controls each. When two or more articles reported effect estimates from overlapping populations, only the article containing the larger sample size was used. This study conforms to PRISMA guidelines and reports the required information accordingly (see Supplementary Checklist)
Figure 1:
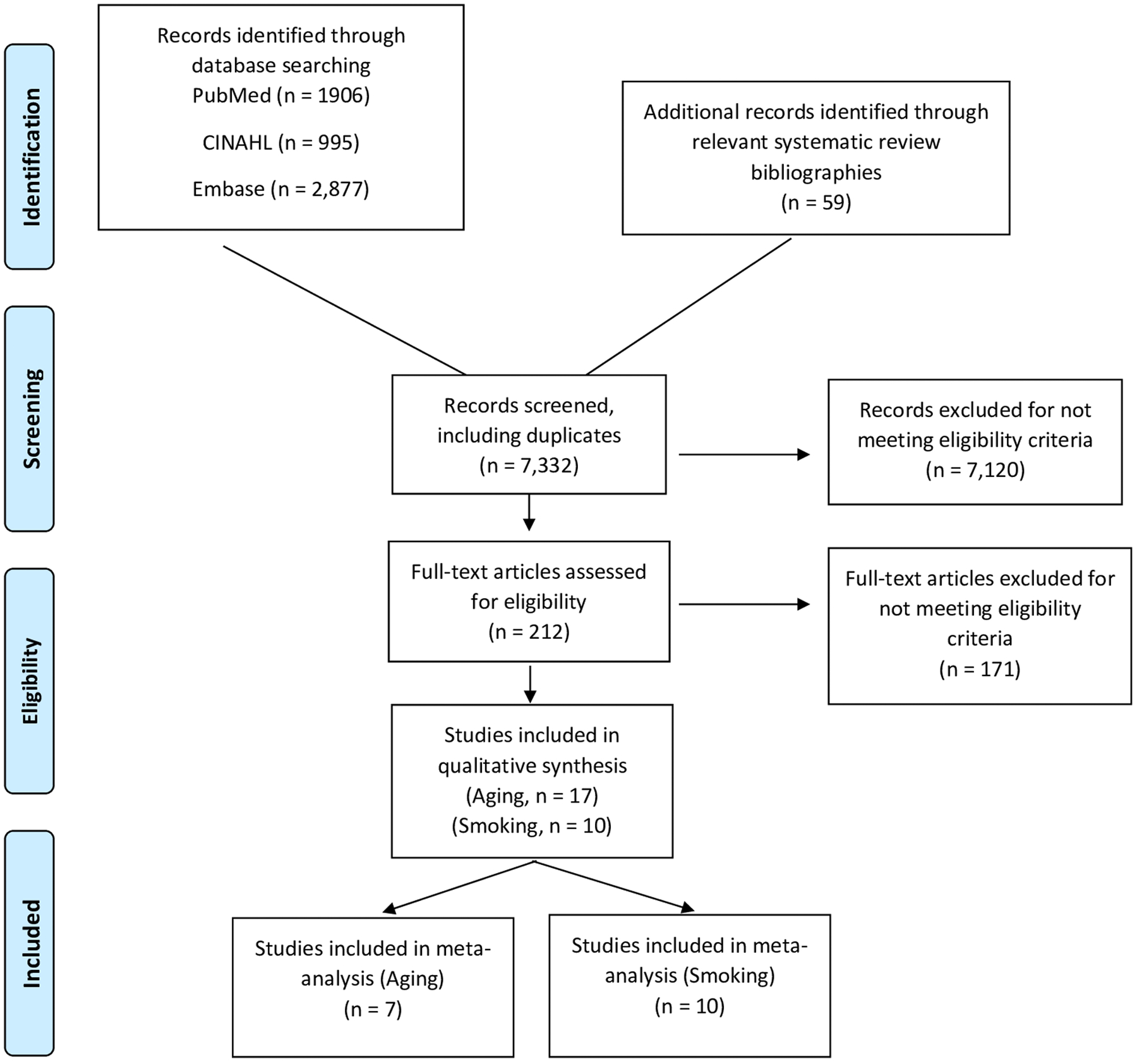
Flow chart of study selection
Assessment of Rotator Cuff Disease
The primary outcome for this meta-analysis was rotator cuff disease interpreted as a broad composite outcome. This approach was used because articles were inconsistent in distinguishing between rotator cuff tear and rotator cuff disease. Thus, the definition of rotator cuff disease in our study encompasses the following terms: rotator cuff disease, rotator cuff tear, rotator cuff injury, rotator cuff syndrome, rotator cuff tendinopathy, rotator cuff tendonitis, and rotator cuff tendinosis. We accepted the diagnosis of rotator cuff disease as presented in each of the included studies. Articles that studied post-traumatic rotator cuff disease were excluded.
Assessment of Risk Factors
In this systematic review, we focused on aging and smoking as the exposures of interest in relation to rotator cuff disease. We included studies that reported on the association between aging and rotator cuff disease if age was assessed as a continuous variable or categorical variable. Studies only reporting summary measures, such as mean or median age by case-control status, were not considered to be eligible for meta-analysis. We included studies that reported on the association between smoking and rotator cuff disease if smoking was assessed as a binary variable (yes or no) or smoking status was classified as never smokers, former smokers, or current smokers. All included studies classified smoking as traditional tobacco smoking (as opposed to the use of alternative smokeless tobacco, “vaping”, electronic cigarettes, etc.).
Data Abstraction
A standardized approach was used to abstract data to be recorded as the following fields from each article: study title, publication date, journal, first author, study design, rotator cuff disease specifics (tear, syndrome, disease, or tendonitis), definition for diagnosis of rotator cuff disease (imaging with magnetic resonance imaging, computerized tomography, ultrasound, surgical repair codes, medical notes), smoking status, age categories, number of cases and controls, risk factor and outcome specific sample size (exposed-cases, unexposed cases, exposed controls, unexposed controls) to compute effect estimate when available, unadjusted effect estimate, and multivariable adjusted effect estimate if provided. When multiple effect estimates were presented or could be computed for different definitions of rotator cuff disease, estimates were abstracted and flagged to avoid double counting of overlapping samples in a given meta-analysis set. When estimates were presented for two or more independent populations in a given study (for example men and women, and as a composite), then all three estimates were abstracted for potential sensitivity analyses.
Statistical Analysis
We used inverse-variance weighted fixed effects meta-analysis for each of the risk factors and rotator cuff disease. We did not perform random effects analyses due to the small number of studies in each meta-analysis set. We chose odds ratio as the effect estimate of choice as most studies reported this estimate or provided numbers to compute this estimate. If a study report estimated men and women separately (considered as independent, mutually exclusive populations), both estimates were considered from the same study in a given meta-analysis set. Multivariable adjusted estimates were given preference over reported unadjusted estimates. In the absence of both estimates, crude odds ratios were manually calculated when possible. Since the association between aging and rotator cuff disease was reported in different ways (age-categories) across studies, four meta-analysis sets were constructed based on available data. For studies reporting the association between age as a continuous variable in relation to rotator cuff disease, the resulting odds ratios were first computed to provide an interpretation of per 10-year-increase in age and then meta-analyzed together. Categorical reports of aging were summarized into the following groups: 40–44 years, 45–49 years, and >50 years, all compared to <40 years of age (referent category) in relation to rotator cuff disease. Information regarding smoking was summarized into three meta-analysis sets: studies reporting smokers versus non-smokers; former smokers versus never smokers, and current smokers versus never smokers. For each meta-analysis set we report odds ratios and corresponding 95% confidence intervals along with I2 statistic for assessing heterogeneity. All analyses were conducted in STATA/MP 16.0 using the metan package.
RESULTS
Of the 212 articles eligible for full-text review, seven studies reported on the relationship between age and rotator cuff disease14–20 and ten studies reported on the relationship between smoking and rotator cuff disease12,14,19–26 (Table 1; Appendix 1).
Table I:
Characteristics of studies included in the systematic review
| Study | Age in Years (range) and Country | Eligibility Criteria | Number of Cases and Controls | Risk Factor Values in Cases; Controls (Presented as Number of participants (%) or Mean Values (SD) unless otherwise noted) | Results |
|---|---|---|---|---|---|
| Applegate et al (2017) | Cases: 45.6 Control: 41.6 USA |
Workers from 17 diverse production facilities in Wisconsin, Utah, and Illinois participating in a hand study, exclusions based on severe hand deformities, arthritis or pending retirement. Rotator cuff tendinopathy was defined as both glenohumeral joint pain in the prior month and positive supraspinatus test. Controls are study participants with no rotator cuff tendinopathy |
156 Cases 1070 Controls |
Age: 45.6; 41.6 Smoker: 44 (28.2%); 295 (27.6%) Former Smoker: 34 (21.8%); 265 (24.8%) |
Age OR: 1.03 (1.02–1.05) Smoker OR: 0.98 (0.66–1.45) Former Smoker OR: 0.84 (0.55–1.29) (ref=never smoker) |
| Baumgarten et al (2009) | Cases: 62.6 Control: 49.2 (18–94) USA |
18 years or older with diagnostic shoulder ultrasound for unilateral, atraumatic shoulder pain and full or partial tear. Exclusion criteria including previous shoulder surgery history, history of major shoulder trauma, bilateral symptoms Controls were patients evaluated for pain without rotator cuff tears |
375 Cases 209 Controls |
Age: 62.5; 49.0 History of smoking: 61.9%; 48.3% Smoking within past 10 years: 35.2%; 29.9% Mean Packs per day: 1.2; 1.1 Mean years of smoking: 23.4, 20.2 Mean pack-years: 30.1, 22.0 |
Age: p<0.001 Smoking OR: 1.74 (1.23–2.44) Within 10 years OR: 4.24 (1.75–10.25) < 1 pack/day OR: 1.08 p=0.79 1–2 packs/day OR: 1.66 p=0.009 >2 packs/day OR: 3.35 p=0.0007 |
| Bodin et al. 2012(1) | 38.7 France |
96 cases were diagnosed with RCS based on intermittent pain for more than 4 of the past 7 days worsened by elevation and positive shoulder tests. 1,360 workers without RCS as controls |
96 Cases 1360 Controls |
Please see paper for incidence of RCS broken down by work and personal characteristic as too detailed to present | Men: Age 45–49 OR: 4.7 (2.2–10.0); (ref<40 yrs) Women: Age: 50–59 OR: 5.4 (2.3–13.2); (ref. <40 yrs) |
| Bodin et al (2012)(2) | 38.7 France |
Cases were French workers completing questionnaire with shoulder pain and diagnosed with RCS Controls were French workers with or without shoulder pain and no RCS based on intermittent pain for more than 4 of the past 7 days worsened by elevation and positive shoulder tests |
274 Cases 3435 Controls |
Please see paper for incidence of RCS broken down by work and personal characteristic as too detailed to present | Men RCS OR: Age≥55: 7.8 (3.7–16.5) (ref<35) Women RCS OR: Age≥55: 8.0 (3.7–17.6) (ref<35) |
| Chung et al (2016) | 60.1, 46–76 Korea |
Patients with chronic, symptomatic full thickness RCT surgically treated at institution; Controls are age and sex matched patients who visited hospital for regular health examination with no shoulder symptoms or disease |
48 Cases 48 Controls |
Smoking: 6 (12.5%); 7 (14.6%) | Smoking: P=0.765 |
| Djerbi et al (2015) | Cases: 57.8 Controls: 59.4 France |
Patients undergoing arthroscopic RC repair Controls are patients with asymptomatic shoulders in same orthopedic unit |
206 Cases 100 Controls |
Age: 57.8; 59.4 Smoker: 54%; 10% |
Multivariate analysis OR(CI): Smoker: 8.715 (4.192–18.118) P<0.0001 Univariate analysis RCT Occurrence OR(CI): Age: 1.546 (0.96–2.5) P=0.3441 |
| Lee et al. (2015) | Cases: 61.3 (46–86) Controls: 57.1 (46–76) Korea |
Patients with symptoms for more than 3 months, >45 years old, had ultrasound evaluation, no bilateral shoulder pain Cases had full or partial tear upon ultrasound examination Controls had no rotator cuff tear upon examination |
140 Cases 176 Controls |
Age: 61.3; 57.1 Smoking: 39 (27.9%); 27 (15.3%) |
Smoking OR: 2.08 (1.15–3.76) P=0.017 Age: 1.06 (1.03–1.09) |
| Lin et al. (2015) | 48.8 Taiwan |
Subset of National Health Insurance Research Database diagnosed with Diabetes or hyperlipidemia. Cases were diagnosed with RCD Exclusion criteria includes less than 30 years old, died before 2001, rheumatoid arthritis, previous RCD diagnosis, shoulder fractures Controls were in database without RCD |
26664 Cases 472014 Controls |
||
| Pasaretti et al. (2013) | Cases: 64 (54–78) Controls: 66 (58–72) Italy |
Patients treated arthroscopically for full-thickness rotator cuff tear and controls with no history of shoulder pathologies. Exclusion criteria included previous shoulder operation, inflammatory or rheumatologic joint disease, primary osteoarthritis, BMI>25,hypertension, diabetes, hypercholesteremia Controls included consecutive patients with no history of shoulder pathology seen at outpatient hospital clinic |
249 full thickness RCT 356 Controls |
Age: 64; 66 Smoking: 102 (41.0%); 176 (41.1%) |
Age OR: 0.96(0.94–0.98) P<0.001 Smoking OR: 0.71 (0.51–0.98) P=0.004 |
| Rechardt et al. (2010) | 6,237 Men and women residing in Finland over the age of 30 taking Health 2000 survey. Exclusion criteria included missing information on shoulder disorders, rheumatoid arthritis, positive rheumatoid factor Cases had chronic rotator tendinopathy, defined as history of rotator cuff pain |
152 Cases 5165 Controls |
Former Smoker: M 3.5% F: 3.1% Current Smokers > 20 pack years: M 3.4% F 1.2% |
Former Smoker OR: M: 1.3 (0.7–2.3) F: 1.0 (0.5–1.8) Current Smoker > 20 pack years OR: M: 1.3 (0.5–3.3) F: 0.4 (0.1–1.7) |
|
| Roquelaure et al. (2011) | 38.7 France |
3710 French workers, distribution similar to regional workforce, completing questionnaire regarding personal factors and work exposures | 261 Cases 3321 Controls |
Univariate analysis Age OR (I year increment) M: 1.07 (1.05–1.09) F: 1.08 (1.06–1.10) |
|
| Seidler et al. (2011) | 25–65 Germany |
German or Turkish speaking residents of regions in Hesse, Germany with partial or total supraspinatus tendon tears diagnosed by MRI after presenting with shoulder pain. Controls are randomly selected residents of same geographical regions |
443 Cases 300 Controls |
Age: 53.5; 45.0 | |
| Shinagawa et al. (2018) | >50 Japan |
Patients older than 50 years undergoing shoulder x-ray, ultrasound or MRI examination for shoulder pain without avascular necrosis, severe deformity of glenoid or humerus Cases had partial or full-thickness rotator cuff tears Controls did not have rotator cuff tears or osteoarthritis |
112 cases 183 controls |
Age: 70 (8.7); 63 (9.3) Smoker (%): 24.1;18.7 |
Multivariable analysis Age, per 10 years 1.95 (1.37–2.78) P<.001 Nonsmoker to smoker 1.07 (0.56–2.01) P=.858 |
| Silverstein et al. (2008) | Cases:41.8 Controls:39.3 USA |
Workers at manufacturing worksites in Washington State answering health and work questionnaire. Exclusion criteria included part-time workers, mobile jobs, more than 4 tasks, or temporary workers Cases were diagnosed with rotator cuff syndrome including shoulder symptoms and positive physical exam with no history of acute trauma or rheumatoid arthritis Controls were study participants without RCS |
55 Cases 678 Controls |
Age: 41.8; 39.3 Current smokers: 29.1%; 29.8% Past Smokers: 18.2%; 20.9% |
Full model OR Age: 1.02 (0.99–1.05) |
| Titchener et al. (2014) | 55 UK |
Cases were randomly selected patients with recorded diagnosis of RCD within THIN database of UK patient records. Controls were matched by age, sex, and general practice without RCD |
5000 Cases 5000 controls |
Current smoker: 925 (18.5%); 826 Ex-smoker: 981 (18.5%); 763 (16.7%) |
Multivariate analysis Current smoker: 0.94 (.83–1.06) Ex-smoker: 1.09 (0.96–1.23) |
Age and Rotator Cuff Disease
Of the seven studies that reported on the relationship between age and rotator cuff disease, five were cross-sectional studies, one was a cohort study, and one was a case-control study. Three studies reported on rotator cuff syndrome, two studies reported on rotator cuff tear, one study reported on rotator cuff tendinopathy, and one study reported on rotator cuff disease. On aggregate, these studies comprised of 27,787 cases compared to 478,926 controls.
Five studies assessed age as a continuous variable in its relationship with rotator cuff disease14,16,17,19,20 (Figure 2). Roquelaure et al.17 provided separate estimates for men and women, allowing for six independent effect estimates for meta-analysis. In our meta-analysis in which age was assessed as a continuous variable, a ten-year increase in age was associated with a 1.20-fold increase in odds of having rotator cuff disease (OR= 1.20, 95% CI: 1.18, 1.21) (Table 2).
Figure 2: Forest plots depicting associations between aging and rotator cuff disease.

A: Age as continuous; B: Age as categorical 40–44 vs. < 40 years (reference); C: Age as categorical 45–49 vs. < 40 years (reference); D: Age as categorical >=50 vs. < 40 years (reference)
Table II:
Meta-analysis of studies evaluating aging and smoking in association with Rotator Cuff Disease
| Characteristic | N-estimates | OR | 95% CI | I2 |
|---|---|---|---|---|
| Aging - RCD | ||||
| Continuous | 5 | 1.20 | (1.18, 1.21) | 94% |
| 40–44 vs. <40 | 3 | 2.71 | (1.78, 4.13) | 0% |
| 45–49 vs. <40 | 3 | 4.33 | (2.86, 6.55) | 0% |
| >=50 vs. <40 | 3 | 6.97 | (4.85, 10.01) | 0% |
| Smoking - RCD | ||||
| Former Smoker vs. Never Smoker | 6 | 1.08 | (0.97, 1.21) | 0% |
| Current Smoker vs. Never Smoker | 6 | 0.97 | (0.87, 1.07) | 37% |
| Yes vs. No | 5 | 1.94 | (1.12, 2.48) | 82% |
OR = Odds Ratio; 95% CI = 95% Confidence Interval; I2 = Percent variation due to heterogeneity; RCD = Rotator Cuff Disease; RCT = Rotator Cuff Tear; All meta-analysis estimates were computed using inverse variance-weighted fixed-effects models.
Bodin et al.15 and Seidler et al.18 stratified the relationship between age and rotator cuff disease into five-year increments. Bodin et al.15 provided separate estimates for men and women. In our meta-analysis in which age was assessed as a categorical variable, an increase in age was associated with an increase odds of rotator cuff disease when participants less than 40 years of age were compared to participants between 40–44 years of age (OR= 2.71, 95% CI: 1.78, 4.13), participants between 45–49 years of age (OR= 4.33, 95% CI: 2.88, 6.55), and participants 50 years of age or greater (OR= 6.97, 95% CI: 4.85, 10.01) (Table 2).
Smoking and Rotator Cuff Disease
Of the ten studies that reported on the relationship between smoking status and rotator cuff disease, five were cross-sectional studies, four were case-control studies, and one was a cohort study. Six studies reported on rotator cuff tear, one study reported on rotator cuff syndrome, one study reported on rotator cuff tendinitis, one study reported on rotator cuff tendinopathy, and one study reported on rotator cuff disease. On aggregate, these studies comprised of 6,493 cases compared to 12,985 controls.
Five studies reported the relationship between smoking and rotator cuff disease as current smoking status (yes versus no)19,21–24 (Figure 3). In our meta-analysis based on these studies, current smokers were approximately two times more likely to have rotator cuff disease compared with non-smokers (OR= 1.94, 95% CI: 1.52, 2.48) (Table 2).
Figure 3: Forest plots depicting associations between smoking status and rotator cuff disease.

A: Former smokers vs. never smokers (reference); B: Current smokers vs. never smokers (reference); C; Smoking status yes or no (reference)
Five studies evaluated smoking status by categorizing participants as never smokers, former smokers, and current smokers12,14,20,25,26 (Figure 3). Odds ratios were reported for former smokers and current smokers using never smokers as the reference category. Rechardt et al.26 provided separate estimates for men and women. In our meta-analysis, compared with never smokers, former smokers were 1.08 times as likely of having rotator cuff disease (OR= 1.08, 95% CI: 0.97, 1.20) and current smokers were 0.97 times as likely to have rotator cuff disease (OR= 0.97, 95% CI: 0.87, 1.07) (Table 2).
DISCUSSION
In this systematic review and meta-analysis, we assessed evidence on the association of two risk factors—age and smoking—with the diagnosis of rotator cuff disease. Our meta-analysis shows that there is a strong association between increased age and rotator cuff disease. The relationship between smoking status and rotator cuff disease is not as clear. However, when dichotomized as current smoking status, current smokers were substantially more likely to have rotator cuff disease.
Evidence of association between increased age and rotator cuff disease was robust across the meta-analyses. Aging was consistently associated with increased odds of rotator cuff disease when considered as a continuous or categorical variable. The mechanism by which age is associated with rotator cuff disease is likely due to degenerative changes in the tendon27. Several mechanisms are postulated in the literature to explain this relationship including increased microvascular compromise with age28, thinning and disorientation of collagen fibers at the cuff insertion29,30, and reduction in tensile strength31. These mechanisms can suggest a potential target for future studies on therapeutics for modification of intrinsic age-related tendon changes.
A previous systematic review and pooled analysis in 2014 by Teunis et al.9 found a strong association between increasing age and rotator cuff disease. The study estimated the unadjusted pooled prevalence of rotator cuff abnormalities in several populations including asymptomatic patients, symptomatic patients, the general population (including cadavers), and patients after shoulder dislocation. Our review finds a positive association between aging and rotator cuff disease even after considering estimates adjusted for others factors that are correlated with aging. Our study helps to quantify the risk of rotator cuff disease associated with age across the literature.
The relationship between smoking status and rotator cuff disease is inconsistent in current literature. Studies that compared current smokers versus non-smokers found that current smokers were more likely to have rotator cuff disease. Paradoxically and conversely, studies that compared current smokers, former smokers, and never smokers did not show an association between smoking status and rotator cuff disease. Studies assessing smoking as a dichotomous variable were more likely to report stronger effect estimates and were also more likely to report statistically significant associations than studies that evaluated smoking status as current smokers, former smokers, and never smokers. Although we were not able to formally assess evidence for small-study bias due to the few number of studies in each analysis category, this possibility is likely. Smaller studies generally tend to dichotomize exposure variables due to reduced sample sizes and may be more likely report estimates with “statistical significance” and omit estimates not reaching such a threshold. The largest studies evaluated in this meta-analysis12,14,26 did not find any meaningful associations between former smoking and rotator cuff disease and between current smoking and rotator cuff disease, both compared to never smoking.
A previous systematic review in 2015 by Bishop et al.10 concluded that smoking is associated with rotator cuff tears, shoulder dysfunction, and shoulder symptoms. Our systematic review differs from this previous review in a few ways. Our review does not look at patient-reported shoulder symptoms but instead focuses on studies with diagnosed rotator cuff disease. In addition, our review builds on the previous review by adding a greater number of relevant studies in the analysis. Finally, we perform a meta-analysis in our study.
Several mechanisms have been postulated in the literature to explain the possible association between smoking and rotator cuff disease. Nicotine is a vasoconstrictive agent32. This may exacerbate the relative vascular insufficiency of the rotator cuff, particularly at its insertion8, leading to degeneration via hypoxia and apoptosis33. A similar mechanism may be of importance in tendon-to-bone healing34 after rotator cuff surgery35. Another hypothesis is that there is deficient collagen synthesis in smokers leading to tendon deterioration and healing impairment36. Some studies have suggested that smoking increases the quantity of pro-inflammatory cytokines such as IL-1, which is associated with the development of symptomatic shoulder pathology10.
Smoking is a modifiable risk factor and data on its association with rotator cuff disease is conflicting. It can affect surgical decision-making. Surgeons may be concerned about poor outcomes in patients that smoke after rotator cuff surgery, which may preclude them from having access to surgical treatment. While we did not specifically explore the impact of smoking status on recovery time after surgical rotator cuff repair, our study shows that the risk of rotator cuff disease is significant among current smokers as compared to others. This finding emphasizes that risk mitigation may be possible with cessation of smoking. Given the importance and potential association of smoking with the etiology of rotator cuff disease, larger and better-designed studies are needed to provide more definitive evidence on this issue.
One limitation of our study is that we did not assess the possibility that former smokers may regress towards the risk-profile of the non-smoking population after years of non-smoking (as seen in lung cancer, for example). While this may be able to explain the lack of association between former smokers and rotator cuff disease when compared to never smokers, it would not explain the lack of association between current smokers and rotator cuff disease. Since our analysis was limited to smoking, the effects of vaping cannot be ascertained from our study.
Other limitations of our meta-analysis are inherent to the studies that are included in our synthesis. There is a lack of standardization across studies. This applies to definitions of cases and controls of rotator cuff disease and the heterogeneity of diagnostic criteria used for rotator cuff disease. Likewise, the reporting of smoking status in the literature is variable and limited. As an example, most studies do not report pack-years and instead only report never/former/current smoking status. Pack-years may provide important information that could be used to demonstrate a possible dose-effect of smoking. Consequently, a synthesis of the literature as it currently stands is severely limited in making any inferences regarding specific conditions relating to the rotator cuff (rotator cuff tears, tendonitis) separately. To address these shortcomings, researchers should consider using more precise and better-defined outcomes (e.g. studying rotator cuff tears specifically instead of rotator cuff disease or rotator cuff syndrome) to provide a basis for clear and actionable messaging in future investigations.
Furthermore, although our analysis included more total studies looking at the relationship between smoking than age, there were significantly more cases and controls in the age studies. Additionally, our analysis for age was driven by the Lin et al. study due to its large sample size.
CONCLUSION
In this systematic review and meta-analysis, we present evidence to suggest that aging is a strong risk factor for rotator cuff disease. The mechanisms by which age is related to rotator cuff disease can suggest a potential target for future studies on therapeutics for modification of intrinsic age-related tendon changes. The finding that current smokers are more likely to have rotator cuff disease as compared with non-smokers implies that cessation of smoking can potentially lead to mitigation of this risk factor. Given the importance and potential association of smoking with the etiology of rotator cuff disease, larger and better-designed studies are needed to provide more definitive evidence on this issue.
Supplementary Material
Appendix Table I: Cases and controls of included studies N/A: Not applicable; studies with N/A were not included in the meta-analysis indicated for aging or smoking.
What is Known:
The etiology of rotator cuff disease is poorly understood. Many have hypothesized that aging and smoking are risk factors for rotator cuff disease, but these associations have not been systematically reviewed and summarized in the literature.
What is New:
In our systematic review and meta-analysis, increasing age was found to be a robust risk factor for rotator cuff disease. Although evidence on the relationship of smoking with rotator cuff disease was conflicting, our finding that current smokers were at a higher risk for rotator cuff disease as compared with non-smokers implies that cessation of smoking can potentially lead to mitigation of this risk factor.
Funding statement:
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01 AR074989. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Ayush Giri was a scholar of the Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) program [2K12HD043483 (PI: Katherine Hartmann)] and a recipient of the NIDDK Research Scientist Development Award [1K01DK120631-01A1] when this work was performed.
Footnotes
Author Disclosures:
Alan Z. Grusky: No disclosures.
Ayush Giri: No disclosures.
Deirdre O’Hanlon: No disclosures.
Nitin B. Jain: No disclosures.
REFERENCES
- 1.Mitchell C, Adebajo A, Hay E, Carr A. Shoulder pain: diagnosis and management in primary care. BMJ. 2005;331(7525):1124–1128. doi: 10.1136/bmj.331.7525.1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker-Bone K, Palmer KT, Reading I, Coggon D, Cooper C. Prevalence and impact of musculoskeletal disorders of the upper limb in the general population. Arthritis Rheum. 2004;51(4):642–651. doi: 10.1002/art.20535 [DOI] [PubMed] [Google Scholar]
- 3.Chakravarty K, Webley M. Shoulder joint movement and its relationship to disability in the elderly. J Rheumatol. 1993;20(8):1359–1361. [PubMed] [Google Scholar]
- 4.Aleem AW, Brophy RH. Outcomes of rotator cuff surgery: what does the evidence tell us? Clin Sports Med. 2012;31(4):665–674. doi: 10.1016/j.csm.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 5.Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: An analysis of utility scores and cost-effectiveness. Journal of Shoulder and Elbow Surgery. 2007;16(2):181–187. doi: 10.1016/j.jse.2006.06.013 [DOI] [PubMed] [Google Scholar]
- 6.Barr KP. Rotator cuff disease. Phys Med Rehabil Clin N Am. 2004;15(2):475–491. doi: 10.1016/j.pmr.2004.03.002 [DOI] [PubMed] [Google Scholar]
- 7.Katzer A, Wening JV, Becker-Männich HU, Lorke DE, Jungbluth KH. [Rotator cuff rupture. Vascular supply and collagen fiber processes as pathogenetic factors]. Unfallchirurgie. 1997;23(2):52–59. doi: 10.1007/bf02628150 [DOI] [PubMed] [Google Scholar]
- 8.Carbone S, Gumina S, Arceri V, Campagna V, Fagnani C, Postacchini F. The impact of preoperative smoking habit on rotator cuff tear: cigarette smoking influences rotator cuff tear sizes. Journal of Shoulder and Elbow Surgery. 2012;21(1):56–60. doi: 10.1016/j.jse.2011.01.039 [DOI] [PubMed] [Google Scholar]
- 9.Teunis T, Lubberts B, Reilly BT, Ring D. A systematic review and pooled analysis of the prevalence of rotator cuff disease with increasing age. J Shoulder Elbow Surg. 2014;23(12):1913–1921. doi: 10.1016/j.jse.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 10.Bishop JY, Santiago-Torres JE, Rimmke N, Flanigan DC. Smoking Predisposes to Rotator Cuff Pathology and Shoulder Dysfunction: A Systematic Review. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2015;31(8):1598–1605. doi: 10.1016/j.arthro.2015.01.026 [DOI] [PubMed] [Google Scholar]
- 11.Grusky AZ, Song A, Kim P, et al. Factors Associated With Symptomatic Rotator Cuff Tears: The Rotator Cuff Outcomes Workgroup Cohort Study. Am J Phys Med Rehabil. 2021;100(4):331–336. doi: 10.1097/PHM.0000000000001684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Titchener AG, White JJE, Hinchliffe SR, Tambe AA, Hubbard RB, Clark DI. Comorbidities in rotator cuff disease: a case-control study. J Shoulder Elbow Surg. 2014;23(9):1282–1288. doi: 10.1016/j.jse.2013.12.019 [DOI] [PubMed] [Google Scholar]
- 13.Fehringer EV, Sun J, VanOeveren LS, Keller BK, Matsen FA. Full-thickness rotator cuff tear prevalence and correlation with function and co-morbidities in patients sixty-five years and older. Journal of Shoulder and Elbow Surgery. 2008;17(6):881–885. doi: 10.1016/j.jse.2008.05.039 [DOI] [PubMed] [Google Scholar]
- 14.Applegate KA, Thiese MS, Merryweather AS, et al. Association Between Cardiovascular Disease Risk Factors and Rotator Cuff Tendinopathy: A Cross-Sectional Study. J Occup Environ Med. 2017;59(2):154–160. doi: 10.1097/JOM.0000000000000929 [DOI] [PubMed] [Google Scholar]
- 15.Bodin J, Ha C, Chastang J-F, et al. Comparison of risk factors for shoulder pain and rotator cuff syndrome in the working population. Am J Ind Med. 2012;55(7):605–615. doi: 10.1002/ajim.22002 [DOI] [PubMed] [Google Scholar]
- 16.Lin TT-L, Lin C-H, Chang C-L, Chi C-H, Chang S-T, Sheu WH-H. The effect of diabetes, hyperlipidemia, and statins on the development of rotator cuff disease: a nationwide, 11-year, longitudinal, population-based follow-up study. Am J Sports Med. 2015;43(9):2126–2132. doi: 10.1177/0363546515588173 [DOI] [PubMed] [Google Scholar]
- 17.Roquelaure Y, Bodin J, Ha C, et al. Personal, biomechanical, and psychosocial risk factors for rotator cuff syndrome in a working population. Scand J Work Environ Health. 2011;37(6):502–511. doi: 10.5271/sjweh.3179 [DOI] [PubMed] [Google Scholar]
- 18.Seidler A, Bolm-Audorff U, Petereit-Haack G, et al. Work-related lesions of the supraspinatus tendon: a case-control study. Int Arch Occup Environ Health. 2011;84(4):425–433. doi: 10.1007/s00420-010-0567-6 [DOI] [PubMed] [Google Scholar]
- 19.Shinagawa K, Hatta T, Yamamoto N, et al. Critical shoulder angle in an East Asian population: correlation to the incidence of rotator cuff tear and glenohumeral osteoarthritis. J Shoulder Elbow Surg. 2018;27(9):1602–1606. doi: 10.1016/j.jse.2018.03.013 [DOI] [PubMed] [Google Scholar]
- 20.Silverstein BA, Bao SS, Fan ZJ, et al. Rotator cuff syndrome: personal, work-related psychosocial and physical load factors. J Occup Environ Med. 2008;50(9):1062–1076. doi: 10.1097/JOM.0b013e31817e7bdd [DOI] [PubMed] [Google Scholar]
- 21.Baumgarten KM, Gerlach D, Galatz LM, et al. Cigarette smoking increases the risk for rotator cuff tears. Clin Orthop Relat Res. 2010;468(6):1534–1541. doi: 10.1007/s11999-009-0781-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung SW, Yoon JP, Oh K-S, et al. Rotator cuff tear and sarcopenia: are these related? J Shoulder Elbow Surg. 2016;25(9):e249–255. doi: 10.1016/j.jse.2016.02.008 [DOI] [PubMed] [Google Scholar]
- 23.Djerbi I, Chammas M, Mirous M-P, Lazerges C, Coulet B, French Society For Shoulder and Elbow (SOFEC). Impact of cardiovascular risk factor on the prevalence and severity of symptomatic full-thickness rotator cuff tears. Orthop Traumatol Surg Res. 2015;101(6 Suppl):S269–273. doi: 10.1016/j.otsr.2015.06.011 [DOI] [PubMed] [Google Scholar]
- 24.Lee D-H, Lee H-D, Yoon S-H. Relationship of ABO Blood Type on Rotator Cuff Tears. PM R. 2015;7(11):1137–1141. doi: 10.1016/j.pmrj.2015.04.015 [DOI] [PubMed] [Google Scholar]
- 25.Passaretti D, Candela V, Venditto T, Giannicola G, Gumina S. Association between alcohol consumption and rotator cuff tear. Acta Orthop. 2016;87(2):165–168. doi: 10.3109/17453674.2015.1119599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rechardt M, Shiri R, Karppinen J, Jula A, Heliövaara M, Viikari-Juntura E. Lifestyle and metabolic factors in relation to shoulder pain and rotator cuff tendinitis: a population-based study. BMC Musculoskelet Disord. 2010;11:165. doi: 10.1186/1471-2474-11-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milgrom C, Schaffler M, Gilbert S, van Holsbeeck M. Rotator-cuff changes in asymptomatic adults. The effect of age, hand dominance and gender. The Journal of Bone and Joint Surgery British volume. 1995;77-B(2):296–298. doi: 10.1302/0301-620X.77B2.7706351 [DOI] [PubMed] [Google Scholar]
- 28.Mehta S, Gimbel JA, Soslowsky LJ. Etiologic and pathogenetic factors for rotator cuff tendinopathy. Clin Sports Med. 2003;22(4):791–812. doi: 10.1016/s0278-5919(03)00012-7 [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto T, Nobuhara K, Hamada T. Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clin Orthop Relat Res. 2003;(415):111–120. doi: 10.1097/01.blo.0000092974.12414.22 [DOI] [PubMed] [Google Scholar]
- 30.Nho SJ, Yadav H, Shindle MK, Macgillivray JD. Rotator cuff degeneration: etiology and pathogenesis. Am J Sports Med. 2008;36(5):987–993. doi: 10.1177/0363546508317344 [DOI] [PubMed] [Google Scholar]
- 31.Sano H, Ishii H, Yeadon A, Backman DS, Brunet JA, Uhthoff HK. Degeneration at the insertion weakens the tensile strength of the supraspinatus tendon: a comparative mechanical and histologic study of the bone-tendon complex. J Orthop Res. 1997;15(5):719–726. doi: 10.1002/jor.1100150514 [DOI] [PubMed] [Google Scholar]
- 32.Mosely LH, Finseth F. Cigarette smoking: impairment of digital blood flow and wound healing in the hand. Hand. 1977;9(2):97–101. doi: 10.1016/s0072-968x(77)80001-6 [DOI] [PubMed] [Google Scholar]
- 33.Benson RT, McDonnell SM, Knowles HJ, Rees JL, Carr AJ, Hulley PA. Tendinopathy and tears of the rotator cuff are associated with hypoxia and apoptosis. The Journal of Bone and Joint Surgery British volume. 2010;92-B(3):448–453. doi: 10.1302/0301-620X.92B3.23074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galatz LM, Silva MJ, Rothermich SY, Zaegel MA, Havlioglu N, Thomopoulos S. Nicotine Delays Tendon-to-Bone Healing in a Rat Shoulder Model. JBJS. 2006;88(9):2027–2034. doi: 10.2106/JBJS.E.00899 [DOI] [PubMed] [Google Scholar]
- 35.Mallon WJ, Misamore G, Snead DS, Denton P. The impact of preoperative smoking habits on the results of rotator cuff repair. Journal of Shoulder and Elbow Surgery. 2004;13(2):129–132. doi: 10.1016/j.jse.2003.11.002 [DOI] [PubMed] [Google Scholar]
- 36.Jorgensen LN, Kallehave F, Christensen E, Siana JE, Gottrup F. Less collagen production in smokers. Surgery. 1998;123(4):450–455. doi: 10.1016/S0039-6060(98)70167-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Table I: Cases and controls of included studies N/A: Not applicable; studies with N/A were not included in the meta-analysis indicated for aging or smoking.


