Abstract
The COVID-19 pandemic has endangered world health and the economy. As the number of cases is increasing, different companies have started developing potential vaccines using both traditional and nano-based platforms to overcome the pandemic. Several countries have approved a few vaccine candidates for emergency use authorization (EUA), showing significant effectiveness and inducing a robust immune response. Oxford-AstraZeneca, Pfizer-BioNTech’s BNT162, Moderna’s mRNA-1273, Sinovac’s CoronaVac, Johnson & Johnson, Sputnik-V, and Sinopharm’s vaccine candidates are leading the race. However, the SARS-CoV-2 is constantly mutating, making the vaccines less effective, possibly by escaping immune response for some variants. Besides, some EUA vaccines have been reported to induce rare side effects such as blood clots, cardiac injury, anaphylaxis, and some neurological effects. Although the COVID-19 vaccine candidates promise to overcome the pandemic, a more significant and clear understanding is needed. In this review, we brief about the clinical trial of some leading candidates, their effectiveness, and their neutralizing effect on SARS-CoV-2 variants. Further, we have discussed the rare side effects, different traditional and nano-based platforms to understand the scope of future development.
Keywords: Covid-19, Neutralizing antibody, Rare side effects, Vaccine platforms, Variants
Introduction
In August 2019, an unknown virus emerged in the city of China, Wuhan. Later, the virus was identified as a coronavirus (belongs to Coronaviridae family within order Nidovirales, subfamily, Orthocoronaviridae,) named SARS-CoV-2 (Weiskopf et al. 2020; Yu et al. 2020). SARS-CoV-2 is a betacoronavirus. COVID-19 infected patients generally show the symptoms of fever, cough, dyspnea and can transmit the disease (Wu et al. 2020). The severe form of viral infection leads to pneumonia, renal failure, severe acute respiratory distress (ARDS), and occasional death (Andersen et al. 2020). According to a cross-sectional study mainly conducted in China, fever is more prevalent in adults than in children. There was a report of laboratory findings in which elevated lactate dehydrogenase (LDH), elevated C reactive protein (CRP), reduced albumin, and lymphocytes were observed (Benvenuto et al. 2020). Moreover, the Prothrombin and D-dimer increased level in ICU patients were also observed. Patients exhibiting symptoms such as fever, tiredness, rhinitis begin to manifest in the following few days. However, identifying asymptomatic cases is challenging (Wu et al. 2020). Before the outbreak of SARS-CoV-2, two more outbreaks of coronaviruses occurred that caused severe respiratory illness, SARS-CoV in 2002 and MERS-CoV in 2012. However, SARS-CoV-2 was declared much earlier as a global health emergency by WHO because of its rapid infection rate (Lai et al. 2020; Weiskopf et al. 2020). The reproduction number of SARS-CoV-2 is 2–2.5, implying that 2–3 persons can get the illness from an infected patient (Dashraath et al. 2020). In around 75% of SARS-CoV-2 infected individuals, computed tomography (CT) was utilized to detect symptoms (Wu et al. 2020).
As of writing this review, 24,00,70,992 confirmed cases, 48,89,737 deaths, a total of 6,637,457,407 vaccine doses have been administered globally, according to WHO. The genome and structural analysis of SARS-CoV-2 were made available in record time, leading to rapid vaccine production (Andersen et al. 2020; Benvenuto et al. 2020; Wrapp et al. 2020; Yan et al. 2020; Yuan et al. 2020). These findings, together with the efficient delivery of bioinformatic assessments and epitope mapping, have provided critical information for vaccine production beyond live-attenuated and inactivated vaccines (Ahmed et al. 2020; Baruah and Bose 2020; Grifoni et al. 2020; Hoffmann et al. 2020a, b; Lei et al. 2020; Lucchese 2020; Uddin et al. 2020; Walls et al. 2020a, b; Wang et al. 2020a, b, c). Currently, seven candidates are in phase 4 of the clinical trial, AZD122, mRNA-1273, CoronaVac, Ad5-nCoV, BNT162, BBIBP-CorV (Vero Cell) and Ad26.COV2.S (Refer to Table 1).
Table 1.
Vaccine candidates currently in phase 4 of clinical development: As per WHO source, 15th October 2021
| Phase 4 vaccines for COVID-19 | |||||
|---|---|---|---|---|---|
| Candidates | Platform | Developer | Dose | Schedule | Phase |
| mRNA -1273 | RNA based Vaccine | Moderna, National Institute of Allergy and Infectious Diseases (NIAID) | 2 | Day 0 + 28 |
Phase 4 EUCTR2021-003388-38-NL EUCTR2021-002327-90-NL EUCTR2021-003618-37-NO |
| BNT162 (3 LNP-mRNAs)/Comirnaty | RNA based Vaccine | Pfizer/BioNTech, Fosun Pharma | 2 | Day 0 + 21 |
Phase 4 ACTRN12621000661875 EUCTR2021-000412-28-BE EUCTR2021-002327-38-NL EUCTR2021-000893-27-BE EUCTR2021-000930-32-BE EUCTR2021-003388-90-NL EUCTR2021-003618-37-NO |
| ChAdOx1-S (AZD1222) (Covishield) | Viral vector (Non-replicating) | AstraZeneca, University of Oxford | 1–2 | Day 0 + 28 |
Phase 4 EUCTR2021-002327-38-NL ACTRN12621000661875 |
| Ad5-nCoV | Viral vector (Non-replicating) | CanSino Biological Inc./Beijing Institute of Biotechnology | 1 | Day 0 |
Phase 4 |
| BBIBP-CorV (Vero Cell) | Inactivated virus | Sinopharm, China National Biotec Group Co., Beijing Institute of Biological Products | 2 | Day 0 + 21 |
Phase 4 |
| Ad26.COV2.S | Viral vector (non-replicating) | Janssen Pharmaceutical | 1–2 | Day 0 or Day 0 + 56 |
Phase 4 EUCTR2021-002327-38-NL |
| CoronaVac | Inactivated Virus (IV) | Sinovac Research and Development Co., Ltd | 2 | Day 0 + 14 |
Phase 4 NCT04993965 |
Vaccines candidates and different platforms used for the development of COVID-19 vaccine
Every year, 2–3 million fatalities are prevented due to immunization. Numerous illnesses are prevented, and millions of lives are saved due to vaccines every year (https://www.who.int/news-room/fact-sheets/detail/immunization-coverage). Smallpox virus has been completely wiped out and the cases of childhood disease, including polio, measles have been remarkably reduced around the world (Younger et al. 2016). It takes several years of research and studies to successfully develop a safe vaccine before deploying it in clinical use. Typically, after development, various stages are involved in the trial of the vaccine (Shahcheraghi et al. 2021). Phase I is the preclinical phase that facilitates testing on cells, animals, and a few people to confirm the immune system stimulation (Merante 2020). Phase II includes the elderly and children involving hundreds of individuals to further confirm the response of different groups of people (Locht 2020). Finally, Phase III involves thousands of volunteers to check the competence of the vaccine, then the researchers vaccinate the volunteer and wait to see how many vaccinated volunteers get infected (Poland et al. 2020). Vaccine efficacy is defined as the percentage by which the rate of disease’s extent is reduced in the vaccinated group compared to placebo (Singh and Mehta 2016).
According to WHO vaccine landscape data, a total of 320 vaccine candidates are in clinical and pre-clinical development globally. One hundred twenty-six candidates are in the clinical phase and 194 candidates are in the pre-clinical phase. Different developers use different platforms to manufacture a potential and safe vaccine against COVID-19. Among the 126 clinical development candidates, 34% (43 candidates) used protein subunit, 14% (18 candidates) used a non-replication viral vector, 11% (14 candidates) used DNA, 14% (17 candidates) used Inactivated virus, 17% (21 candidates) used RNA, Replicating viral vector used by 2% (2 candidates), 4% (5 candidates) used virus-like particles, 2% (2 candidates) used replicating viral vector with an antigen-presenting cell (APC), 2% (2 candidates) used a live attenuated virus, and 1% (1 candidate) used a non-replicating viral vector with antigen-presenting cell to develop a safe and potential vaccine candidate against SARS-CoV-2. Among the clinical phase vaccines, only 8 candidates are in phase 4, which means, these vaccines are already in the market and available to the general public. 26 vaccine candidates are in phase 3 of clinical development (https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines). Prior DNA and RNA vaccines were not licensed for human use, but these two platforms can be effective in controlling the pandemic. As these two platforms do not need any bioreactor culture techniques as required in inactivated vaccines, DNA and RNA vaccine can be produced rapidly in the laboratory based on genetic sequence of the virus and can fast-track the process in the pandemic (Conforti et al. 2020).
SARS-CoV-2 structural components
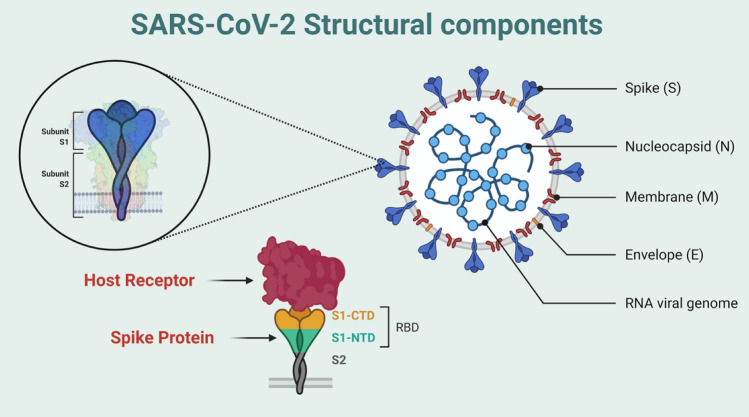
The genome structure of SARS-CoV-2 is about 30 kb (26–32 kb) which is relatively large. The virus codes for several structural, non-structural, and accessory proteins (Li et al. 2020). Four different structural proteins are encoded by the SARS-CoV-2 virus, the spikes (S) (outer spike glycoprotein), envelope (E), membrane (M), and the nucleocapsid (N) (Su et al. 2020) (Fig. 1). The major transmembrane glycoprotein that mediates the binding of receptor and promotes the entrance of the virus is S protein (Su et al. 2020). The non-structural proteins that are vital for their lifecycle and pathogenesis are NSP12, NSP13, NSP3, and NSP5 and the accessory proteins are ORF3a, ORF6, ORF8, ORF7, and ORF9 (Li et al. 2020).
Fig. 1.
Schematic diagram of SARS-CoV-2 with components, enlarged view of Spike and binding of spike protein with host receptor with the receptor binding domain (RBD). (Image created in biorender.com)
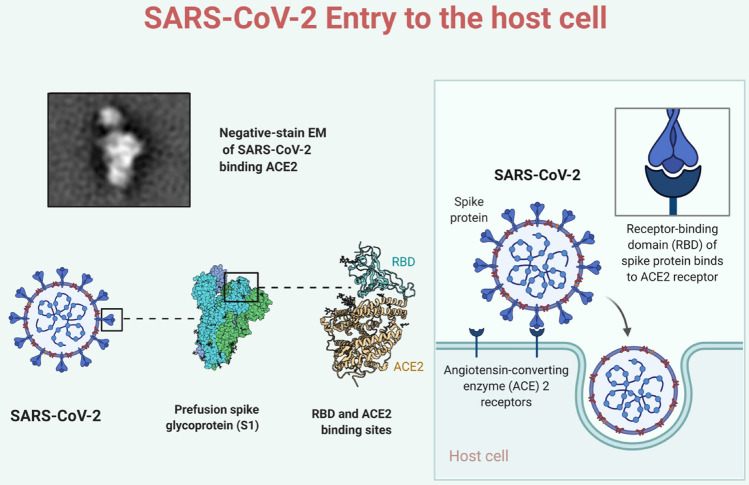
ACE2 receptor and SARS-CoV-2 host entry
SARS-CoV-2 uses angiotensin-converting enzyme-2 (ACE2 receptor) to enter the host, analogue to SARS-CoV and other coronaviruses (Hoffmann et al. 2020a, b; Lu et al. 2020). ACE2 is a membrane-bound peptidase that comprises the N-terminal domain in majority of the proteins receptors, and an extracellularly catalytic site (Chappell 2016; Chappell et al. 2014). SARS-CoV-2 can infect multiple organs, including lungs, stomach, colon, ileum, liver, and kidney, due to the availability of ACE2 receptors on these human tissues (Qi et al. 2020; Zou et al. 2020). In the lungs, alveolar type II cells were proposed as the main target of this virus; Zou et al. reported that these cells express rather a low ACE2 receptor expression (Zou et al. 2020). The S protein contains a binding site that allows it to attach to the ACE2 receptor and helps the virus to enter into the cell. S proteins are the major interest in creating a possible vaccination candidate. S protein has two domains S1 and S2 (Tse et al. 2020). S1 domain facilitates the attachment as it comprises the RBD (receptor binding domain), and the S2 domain aids the virus to host cell fusion (Fig. 2). The primary receptor is ACE2 to initiate the entry of the virus after binding to the RBD, along with another receptor, CD209L with low affinity (Ibrahim et al. 2020). The viral membrane and host cell fuse together by pulling, following the fusion, the S protein conformational changes from pre-fusion to post-fusion (Graham 2020). Only 44% of the whole genomic sequence of S structural protein is shared by MERS-CoV and SARS-CoV, clearly illustrating that S proteins of coronaviruses are quite diverse (Tse et al. 2020). The primary difference between the S protein is mainly the N terminal domain and the receptor-binding domain. Due to the difference, SARS-CoV and MERS-CoV are correlated with different receptors for host entry, however, SARS-CoV and SARS-CoV-2 contributes similar receptors angiotensin-converting enzyme 2 (ACE2) while MERS-CoV enter through dipeptidyl peptidase 4 (DPP4) (Wrapp et al. 2020). Since SARS-CoV-2 and SARS-CoV share the same receptor, there should be cross-reactivity to the SARS-CoV-2 receptor binding domain with the monoclonal antibody that is specific to the SARS-CoV RBD. Results showed no binding or cross-reactivity between the monoclonal antibody and RBD of SARS-CoV-2 despite the similarity in the RBD sequence (Wrapp et al. 2020). In terms of severity and clinical effects, SARS-CoV was more deadly but SARS-CoV-2 spreads more easily and is eminently infectious (https://theconversation.com/the-mysterious-disappearance-of-the-first-sars-virus-and-why-we-need-a-vaccine-for-the-current-one-but-didnt-for-the-other-137583).
Fig. 2.
Enlarge view of S1 domain of spike protein and binding of spike protein with ACE2 receptor to enter the host cell. (Image created in biorender.com)
COVID-19 variants and vaccine effectiveness
The year 2020 was challenging, but 2021 is even more difficult due to the rise of new SARS-CoV-2 strains. The emergence of the novel SARS-CoV-2 strains 501Y.V1 (B.1.1.7) in the UK and 501Y.V2 (B.1.351) in South Africa was attributed to an unanticipated surge in confirmed COVID-19 cases in December 2020 (Fontanet et al. 2021). Several mutations in S protein have recently been identified in Denmark, United States, and Brazil (Kumar et al. 2021). These variants increased the transmission between 40% and 70% due to the mutation (N501Y) at the receptor-binding domain. Two more mutations (E484K and K417N) in the spike protein confer a possible immunological escape to antibodies in the 501Y.V2 variant. Another set of mutations (N501Y, E484K, and K417T) in a new P.1 (501Y.V3) lineage has been reported in Manaus, Brazil, which is alarming (Fontanet et al. 2021). 501Y.V2 shows higher cross-reactivity as it elicits robust neutralizing antibody response against both the original and P.1 variants (Refer to Table 2). A vaccine formulated on the spike protein of 501Y.V2 variant will be promising to elicit cross-reactive neutralizing antibody against COVID-19 virus (Moyo-Gwete et al. 2021).
Table 2.
COVID-19 variants: Showing different names and their documentation
| SARS-CoV-2 variants | Designated as | Earliest documented sample | Designation date | ||
|---|---|---|---|---|---|
| Pango lineage | GISAID clade | WHO label | |||
| B.1.1.7 | GRY | Alpha | VOC | United Kingdom September 2020 | 18 December 2020 |
| B.1.351 | GH/501Y.V2 | Beta | VOC |
South Africa May 2020 |
18 December 2020 |
| P.1 | GR/501Y.V3 | Gamma | VOC |
Brazil November 2020 |
11 January 2021 |
| B.1.617.2§ | G/478 K.V1 | Delta | VOC | India October 2020 |
VOI- 4 April 2021 VOC- 11 May 2021 |
| C.37 | GR/452Q.V1 | Lambda | VOI | Peru December 2020 | 14 June 2021 |
| B.1.621 | GH | Mu | VOI | Colombia January 2021 | 30 August 2021 |
|
B.1.427 B.1.429 |
GH/452R.V1 | Epsilon | VUM |
United states of America March-2020 |
6 July 2021 |
| R.1 | GR | VUM |
Multiple countries January 2021 |
7 April 2021 | |
| B.1.466.2 | GH | VUM | Indonesia November 20,202 | 28 April 2021 | |
| B.1.1.318 | GR | VUM |
Multiple countries January 2021 |
2 June 2021 | |
| B.1.1.519 | GR | VUM |
Multiple countries November 2020 |
2 June 2021 | |
| C.36.3 | GR | VUM |
Multiple countries January 2021 |
16 June 2021 | |
| B.1.214.2 | G | VUM |
Multiple countries November 2020 |
30 June 2021 | |
| B.1.1.523 | GR | VUM |
Multiple countries May 2020 |
14 July 2021 | |
| B.1.619 | G | VUM |
Multiple countries May 2020 |
14 July 2021 | |
| B.1.620 | G | VUM |
Multiple countries November 2020 |
14 July 2021 | |
Several events, such as recombination, single point mutation, insertion, and deletion, alter the pathogenesis of SARS-CoV-2, resulting in diverse variants (Challen et al. 2021). The mutation is frequent in the spike protein and receptor binding domain; RBD is the most divergent, resulting in multiple SARS-CoV-2 variants (Kumar et al. 2021). In a natural infection, the neutralizing antibody majorly targets the spike glycoprotein, and most of the vaccines express the same spike glycoprotein of SARS-CoV-2. Current vaccines are based on the original variant of SARS-CoV-2 that emerged; however, the critical concern remains whether the old version spike glycoprotein may elicit protective effects against the new SARS-CoV-2 variants. Lineages B.1.351, B.1.1.7, and P1 have been identified as having numerous changes in the spike glycoprotein and other proteins from the original SARS-CoV-2 variant from Wuhan. These mutations influence the interaction with the hACE2 receptor, resulting in higher morbidity and mortality (Challen et al. 2021). Spike protein mutated variants have the potential to escape immune responses. A Netherland-based study on health care workers demonstrated that some variants of SARS-CoV-2 may partially escape humoral immune response induced by BTN162b2 or SARS-CoV-2 infection but cannot escape specific CD4+ T cell response including B.1.1.7 and B.1.351. However, two doses of the BTN162b2 vaccine were required to reach the high level of expression of neutralizing antibody and cell-mediated immune response (Geers et al. 2021).
These mutations are creating a significant impact on vaccine development and their efficacy. The leading vaccine candidates Novavax and AstraZeneca, show the efficacy of 85.6% and 74.6% against B.1.1.7, respectively. The B.1.351 variant is of more concern because the leading candidates Novavax, AstraZeneca, and Johnson’s & Johnson's vaccines reported very low efficacy of 49.4%, < 25%, and 57%, respectively (Kumar et al. 2021). mRNA-1273 is highly effective against B.1.1.7 (Alpha) and B.1.351 (Beta), whether symptomatic or asymptomatic. mRNA-1273 is 88.1 percent and 100 percent effective against B.1.1.7 infection after a single and second dosage, and 61.3 percent and 94.6 percent effective against B.1.351 infection after a single and second dosage, respectively. Against the wild type, it shows 94.1 effectiveness against symptomatic SARS-CoV-2 infection (Chemaitelly et al. 2021). A two-dose regimen of NVX-CoV2373 conferred 89.7% protection against a blend of prototype and variant Covid-19, demonstrated high efficacy against the B.1.1.7 variant, and had a reassuring safety profile (Heath et al. 2021).
The delta variant is another variant of concern, first detected in India, characterized by the spike protein mutations T19R, Δ157-158, L452R, T478K, D614G, P681R, and D950N (Lopez Bernal et al. 2021). Recent reports said that the delta variant reduces sensitivity to some monoclonal antibodies, including bamlinivimab and polyclonal antibodies. Similarly, the B.1.351 was also found to escape some monoclonal antibodies (Moyo-Gwete et al. 2021). The transmissibility is estimated to have 60% more than the alpha variant (Planas et al. 2021). BNT162b2 effectiveness after 2 doses, for alpha variant, 93.7%; and for delta variant, 88.0% was reported. ChAdOX ncov-19 effectiveness after two doses, for alpha variant, 67.0%; for delta variant, 74.5% was observed. There was a modest difference reported after two vaccination doses between the alpha and the delta variant. However, the absolute difference was observed after single-dose; the vaccines were 30.7% and 48.7% effective against delta and alpha variants, respectively (Lopez Bernal et al. 2021). Development and updates on the leading candidates against B.1.351 are urgent to generate protection. To generate actionable data, create a framework for broader genomic surveillance and timely analysis of novel variations (Kumar et al. 2021). Repeated formulation of COVID-19 vaccines may be needed to control the SARS-CoV-2 transmission. Surveillance of SARS-CoV-2 variants and the sharing of variant-specific PCR primers might assist in tracking their spread, particularly in resource-limited countries. For seroneutralisation and cellular immunity functional testing against newly found variations, a central library of sera and cells from individuals with previous infection or prior vaccination with existing COVID-19 vaccines should be constructed. This repository might provide standard recommendations outlining a minimal set of epitopes that should be introduced in future COVID-19 vaccines. Adaptive and reactive production of COVID-19 vaccines is crucial, and vaccines must be affordable and accessible on a global scale (Fontanet et al. 2021).
Some of the leading vaccine candidates
ChAdOx1-S/AZD1222 (Covishield)
The University of Oxford collaborated with AstraZeneca, a British pharmaceutical firm, to develop AZD1222, a Chimpanzee non-replicating viral vector vaccine (VVnr) formerly known as ChAdOx1. Currently, two companies are manufacturing AZD1222. The vaccine is manufactured by SK Bioscience Co. Ltd, named ChAdOx1-S, and the Serum Institute of India produces COVISHIELD, ChAdOx1 nCoV-19 Coronavirus Vaccine. High antibody response was recorded when demonstrated in pig models (Graham et al. 2020). The journal Lancet reported the result after the completion of phase 1/2. They recruited 1077 participants in the UK between the ages18-55 and conducted a single-blinded randomized trial in five trial sites. The participants were randomly assigned, in a 1:1 fashion (n = 543 each group), to receive a dose of 5 × 1010 viral particles of ChAdOx1 or a single intramuscular injection of meningococcal conjugate vaccine (MenACWY) as a control (Folegatti et al. 2020). A group of 10 participants was cut out for the second dose after 28 days following the first dose. To further monitor of the adverse effect, the participants were divided based on prophylactic paracetamol. From the AZD1222 group, fifty-six out of 543 participants and fifty-seven out of 534 placeboes licensed meningococcal group was administered paracetamol. The result showed a low degree of adverse events, like pain, tenderness, fatigue, and headache, in the prophylactic paracetamol group comparing no prophylaxis groups. The same result was observed in the placebo groups. Neutropenia observed in the AZD1222 group was ~ 46% (25 out of 54) and ~ 7% (3 out of 44) in the control MenACWY group participants (Folegatti et al. 2020). In South Africa, an ongoing trial with 2000 volunteers aged between 18 and 65 with or without HIV is in process to check for an immune response (COVID-19 Vaccine (ChAdOx1 NCoV-19) Trial in South African Adults With and Without HIV-Infection—Full Text View—ClinicalTrials.Gov 2021). A phase IIb/III trial included 12,330 healthy volunteers in the UK, including 5 year olds. The participants, who are at greater risk, are divided into groups based on their age, 5–12 years old and above 70 years old, including the cohorts of extreme demographics among the volunteers (Investigating a Vaccine Against COVID-19—Full Text View—ClinicalTrials.Gov 2021).
AstraZeneca released an update on the efficacy of AZD1222 on 25 March 2021. The analysis included 32,449 trial participants, in which a total of 190 symptomatic cases was included for primary efficacy analysis. Participants were randomly divided into 2:1 fashion between AZD1222 group and placebo MenACWY groups. The US phase III primary analysis showed that AZD1222 has 76% efficacy against the symptomatic COVID-19 cases, 100% efficacy for critically ill and hospitalized patients, and 85% efficacy against symptomatic in participants aged between 65 years and up (AZD1222 US Phase III Primary Analysis Confirms Safety and Efficacy 2021).
mRNA-1273
Moderna is an American-based company in Cambridge, Massachusetts, that developed an mRNA-based vaccine, mRNA-1273, collaborating with the National Institute of Allergy and Infectious Diseases (NIAID). mRNA-1273 is a lipid-nanoparticle (LNP) encapsulated mRNA that expresses the prefusion stabilized spike glycoprotein (Corbett et al. 2020a, b). In the early stage of human testing, the mRNA-1273 vaccine encouraged safety and immunogenicity and demonstrated protection in early animal-challenge experiments (Corbett et al. 2020a, b; Jackson et al. 2020). Employing the mRNA that codes for the spike protein of the virus, they mark the spike protein for destruction in the immune cells once the vaccine is administered into the host body (Triggle et al. 2020). To accelerate the vaccine production Moderna's mRNA-1273 vaccine is included in the Operation Warp initiative. Currently, it is in Phase 4. BNT162b2 is another mRNA vaccine that recently demonstrated vaccine efficacy and safety (Sharma et al. 2020). The results of the mRNA-1273 vaccination against SARS-CoV-2 in non-human primates were published in the New England Journal of Medicine. Using mRNA-1273 produced influential SARS-CoV-2 neutralizing activity. The lungs were healthy and protected in the lower and upper airways. The non-human primates were administered with 10 μg or 100 μg of mRNA-1273 or no vaccine. mRNA-1273 vaccination activates the type 1 helper T-cells responses biasing the CD4+T cell responses, and there was an inadequate response of Th2 or CD8+ T-cell (Corbett et al. 2020a, b) . Nature published the preclinical trial data. They immunize the mice model by administering intramuscularly with either 0.01 μg, 0.1, or 1 μg dose of vaccine. It was shown in the result that, with the 1 μg dose, there was a high pseudo-virus-neutralizing antibody response (NAb) (Sharma et al. 2020). Phase 1 trial was performed on 45 healthy adults aged 18–55. These participants received two doses of mRNA-1273 after 28 days following the first dose. All the participants formed three groups (n = 15) according to the administration doses, 25 μg, 100 μg, or 250 μg (Jackson et al. 2020). After administering the first dose of vaccination, higher antibody responses were reported. Five participants (33%) in the 25 μg group, 10 participants (67%) in the 100 μg group, and 8 participants (53%) in the 250 μg group were reported with adverse events with mild or moderate in severity. After the second dose, solicited systemic adverse events were reported more common, 7 out of 13 (54%) participants in the 25 μg group, 15 of 15 (100%) participants in the 100 μg group, and 14 in the 250 μg group. Three participants from the 250 μg groups were reported with one or more severe adverse events. In the case of fever, no participants had fever after the first dose. In the 2nd dose of vaccination, no participants in the 25 μg group, 6 participants (60%) in the 100 μg, and 8 participants (57%) in the 250 μg group were reported fever (Jackson et al. 2020).
A phase 1 trial was conducted on 40 older adults aged between 56 and 70 years or 71 years or more, stratified in this way. All the participants received two doses of either 25 μg or 100 μg of mRNA-1273 which was administered after 28 days following the first dose. The result showed that mild or moderate adverse events were associated with mRNA-1273. The study also supported the use of 100 μg doses in the phase III vaccine trial as the 100 μg dose group showed higher binding and neutralizing antibody titers than the 25 μg group (Anderson et al. 2020). A random phase 3 trial was conducted, during which thirty-thousand volunteers received two intramuscular injections of mRNA-1273 or placebo at ninety-nine U.S. locations. The injections of 100 μg of mRNA-1273 or placebo were administered 28 days apart. 2.2% had serological, virological, or both evidence of SARS-CoV-2, so more than 96% of total participants were administered with both injections. The result showed 94.1% vaccine efficacy at preventing COVID-19, including the severe condition (Baden et al. 2021).
CoronaVac
Sinovac Biotech Ltd is a China-based pharmaceutical company developing an inactivated vaccine adjuvanted with aluminum. Mice were administered with 1.5 μg or 3 μg or 6 μg doses of the CoronaVac along with an Aluminium adjuvant or a saline placebo. The animal models confirmed sufficient neutralizing antibody titer levels and specific IgG response in the pre-clinical data. In the macaque monkey, no ADE (Antibody-dependent enhancement) was noted. Unlike the controlled group, when macaques were challenged with the SARS-CoV-2 virus, they noted that macaques are protected from SARS-CoV-2 with decreased viral load. In the 6 μg dose, there was also no side effect, no appetite change, or mental status changes noted in the macaque monkey, and ensuring the safety in the case of inactivated vaccine is important (Gao et al. 2020).
A press release for their phase-1 study showed that 143 healthy participants were recruited. All the participants were between the age group of 18–59 for a randomized trial. No results regarding the phase-1 study were made available (Sinovac Says Its Covid-19 Vaccine Generated Immune Responses—STAT 2021). The phase-2 trial recruited 600 participants who were between the ages group of 18–59. It was also a randomized, double-blinded trial. Participants were split into two dual-dose programs. One of the programs had a 0 and 14 day schedule, and the other had a 0 and 28 day schedule. In each schedule, 120 participants were given the 3 µg, 120 participants were given the 6 µg, and 60 participants were given a placebo. Pain and swelling were the local adverse effects in mild and somewhat moderate amounts in both the programs. The pain was mostly reported effect in both schedules (Zhang et al. 2020a, b). Out of 300 participants, 61 participants (20.3%) (0 to 14-day schedule) and 31 participants (10.3%) (0 to 28-day schedule) complained of pain at the injection site. However, these effects were resolved within 3 days, and severe grade-3 adverse effects were not reported. For both 3 µg and 6 µg, neutralizing antibody (NAb) responses were high in both schedules. Twenty-eight days after the second dose, participants in 0 and 14 day schedules had stable NAb levels, and in 0 and 28 days, participants had increased NAb levels. For specific antibodies, a similar pattern was also observed. In older adults, it was observed that NAb levels diminished with an increase in age, requiring high doses (Zhang et al. 2020a, b). In this report, global race for Covid-19 vaccines T-cell immunity was not analyzed. An in-depth study of the immune response generated by the CoronaVac vaccine is required. To rule out the risk of Antibody-dependent enhancement, information about the T-cell response of the vaccine is necessary because it is related to the use of inactivated vaccines. The pre-clinical trials showed no immunopathological findings, and in human trials, it is yet to be seen if a similar pattern is observed or not. For trials in Indonesia and Brazil, Sinovac is planning to get the 3 µg dose in the 0, 14 day and 0, 28 day schedules on a large scale (Indonesia Testing Location for China’s Sinovac Phase 3 Clinical Trial 2021; Zhang et al. 2020a, b). In Brazil, Sinovac plans to recruit 8874 health care workers over age 18 years and assess the vaccine over 0 and 14 day schedules. It will be interesting to see the results of these large-scale efficacy trials in older people over 60 years (Clinical Trial of Efficacy and Safety of Sinovac’s Adsorbed COVID-19 (Inactivated) Vaccine in Healthcare Professionals—Full Text View—ClinicalTrials.Gov 2021).
BNT162 (3 LNP-mRNAs), also known as Comirnaty
Another mRNA-based vaccine was developed by a German-based company named BioNTech, an American company named Pfizer, and a pharmaceutical company Fosun. The mRNA-based vaccine encodes the SARS-CoV-2 RBD domain. This vaccine is named BNT162, and it works by incorporating modified mRNA and including the T4 fibrin-derived trimerization domain, enhancing the immune response. In the USA, for their 1/2 phase trials, they recruited 45 healthy volunteers between 18 and 55 years. They were split into groups of 12 for doses 10, 30, and 100 µg, and another group of 9 volunteers received a placebo. The two doses of 10 μg and 30 μg were given 20 days apart intramuscularly. The second dose was never given to the 100 μg group.
Based on the data collected, elevated levels of IgG were seen in the volunteers. The IgG level heightened in the participants after 7 days of second dose administration (28 day mark), and it stayed elevated until 14 days after the second dose administration (35 day mark). Participants who were administered the 100 μg dose showed an increase in IgG level after 21 days of taking the first dose, but it did not increase after that (Mulligan et al. 2020a). Elevation in IgG level was observed in NAb titers 21 days after the first dose and 7 days after administering the second dose (28 day mark). No observable data was there for the 100 μg administered group because they did not receive the second dose. However, some results showed no significant changes in 30 μg and 100 μg administered groups after the first dose. The data collected showed that 10 μg and 30 μg doses were suitable candidates, and they proceeded for future trials (Mulligan et al. 2020b). For the BNT162 vaccine, dose-dependent Grade 1 to Grade 2 local or systemic reactions were observed. Mild or moderate pain at the injection site were frequent events, but for 100 μg doses, one severe event was noted. The common after-effects observed were headaches, chills, fatigue, muscle, and joint aches. These effects increased depending on the dose. Some severe effects after the second dose were resolved within a day. Fever was reported in a few patients post administration of first and second doses but was also resolved in a day. Some patients saw grade 3 effects like sleep disturbance and pyrexia, but no Grade 4 adverse effects were observed. Few patients had decreased lymphocyte and neutrophil count, which became normal after 6–8 days of vaccination, and other laboratory values did not change much (Mulligan et al. 2020b). A phase 2/3 trial was conducted on healthy adults between 18 and 55 years and 65–85 years. The trial was a randomized, observer-blinded dose-escalation, placebo-controlled study to administer two doses at 21 days intervals. Participants can receive a placebo or two lipids nanoparticle formulated; nucleoside modified RNA vaccine candidates, BNT162b1 or BNT162b2. BNT162b1 candidate encodes the receptor-binding domain of the SARS-CoV-2, and BNT162b2 encodes a full-length spike of SARS-CoV-2 that is a perfused stabilized membrane-anchored to the virus. All the participants were split into 13 groups of 15 participants. In each group, 12 participants received vaccine doses, and 3 received placebo. BNT162b1 and BNT162b2 brought out similar doses that neutralized SARS-CoV-2 geometric mean titers (GMTs) in both younger and older adults. In older adults, BNT162b2 was related to lower systemic reactogenicity. The conclusion of the safety and efficacy evaluation of BNT162 is currently underway (Walsh et al. 2020).
Vero cell
Sinopharm is working on two inactivated vaccines in cooperation with the Wuhan Institute of Biological Products and the Beijing Institute of Biological Products. Both of them are in phase-3 trials. JAMA Wuhan Institute of Biological Products released its test results for phase-1 and phase-2 randomized, double-blinded clinical trials in the journal. Ninety-six participants were recruited for the Phase-1 trial. They were between 18 and 59 and were assigned equally in one of three dose groups: 2.5 μg, 5 μg, 10 μg for Global Race for COVID-19 vaccine, or an aluminum adjuvant placebo group. Three intramuscular shots at days 0, 28 and 56 were given to the participants (Xia et al. 2020). Adverse effects were reported by 20.8% of participants (5 out of 24) in the low dose for 7 days. For the medium-dose group, 16.7% of participants (4 out of 24) and the high dose group, 25% of participants (6 out of 24) reported adverse effects. Mild and self-resolving issues like pain at injection sites and fever were commonly reported. High NAb response, after 14 days of third vaccination (day 70), was recorded with seroconversion being observed in the low and high-dose participant groups. In the medium-dose group, 95.8% of participants (23 out of 24) showed high NAb responses (Xia et al. 2020). Specific antibody response at high level was generated in the phase-1 trial, and all the participants' seroconversion were observed. For the phase-2 trial, 224 participants were recruited. They were between 18 and 59 years and were equally assigned into two double dose programs 0 days and 14 days or 0 days and 21 days. In both the schedules, 84 participants were administered the medium dose of 5 μg, and 28 participants were administered the aluminum adjuvant placebo group. For the immunogenicity component of phase-2 trials, only half of the participants were analyzed in both groups. For example, 42 participants in the 5 μg group and 14 participants in the placebo group were considered for the 0 and 14 day schedule. However, in safety analysis, 84 participants in the 5 μg group and 28 participants in the placebo group were analyzed. Adverse effects were observed for 0 and 14 days scheduled in both the groups. In the 5 μg group, 6% of participants (5 out of 84) and 14.3% of participants (4 out of 28) experienced adverse effects for placebo. In the 0 and 28 days schedules, for the 5 μg group, 19% of participants (16 out of 84) and the placebo group, 17.9% of participants (5 out of 28) showed adverse effects (Xia et al. 2020). Mild, self-resolving effects like fever and pain at the injection site were commonly reported, just like trial 1. In both the schedules, the high-level reutilizing antibody response was observed with a 97.6% (41 out of 42) seroconversion for both. The specific antibody response was substantially higher for the 0 and 21day schedules than for the 0 and 14-day schedules. Seroconversion was low in the case of 0-and 14-day schedules which were 85.7% (36 out of 42), but for 0 and 21 day schedules, it was 100%. This result showed a correlation between the gap of the vaccine taken and showed a higher immune response for the vaccine when taken in a large gap. The response from T cells was not tested in any trial, so it was not concluded if the vaccine can cause vaccine-associated enhanced respiratory disease (VAERD) (Xia et al. 2020). The problem needed to be resolved in large-scale efficacy trials to check both humoral and cellular immune responses. The phase-2 trial report did not analyze all the participants for the immunogenicity component, which could have created a false sense of security while interpreting the result of an elevated humoral immune response. Results were released for the pre-clinical trials by the Beijing Institute of Biological Products. In different animal species like rats, mice, macaques, and cynomolgus monkeys, the NAb response was high at all three doses (2 μg or 4 μg or 8 μg) along with the aluminum adjuvant (Wang et al. 2020a, b, c). Two doses of 2 μg were sufficient to elicit a high immune response. The ADE in macaques was also reported negative (Wang et al. 2020a, b, c). The company recruited 1120 healthy participants in the age range of 18–59 years and conducted a randomized, double-blinded parallel phase 1/2 trial. There were groups according to dose and age dependency, which included all the participants, and they had to receive the inactivated vaccine or a placebo. Humoral and cellular responses and some adverse effects were observed (Chinese Clinical Trial Register (ChiCTR)—The World Health Organization International Clinical Trials Registered Organization Registered Platform 2021). High antibody response was also noticed without any significant adverse effect, according to the press release. After that, no further data was published (Covid-19 Latest News: Sinopharm Virus Vaccine Safe in Testing—Bloomberg 2021). Because of few active cases in China, the phase-3 trials are supposed to be conducted in the United Arab Emirates by Sinopharm on about 15,000 participants (Chinese COVID-19 Vaccine Candidate the First to Start Phase 3 Clinical Trials Worldwide—Global Times 2021).
Ad5-nCoV [recombinant novel coronavirus vaccine (adenovirus type 5 vector)]
The People’s Republic of China military has been granted permission to use an Adenovirus vaccine developed by CanSino Biologics Inc. after clinical trials established that it is safe and effective (NCT04341389). Ad5-nCoV is a virus that produces the S protein via a replication-defective human Ad5 vector. This vector was used to create the Ebola vaccine (Ad5-EBO). CanSino Biologics Inc. developed a vaccine (Ad5-nCoV) and conducted clinical trials to demonstrate its safety and efficacy (NCT04341389). Neutralizing antibodies against RBD and S proteins and specific T cell responses increased fourfold in phase I clinical studies (Alturki et al. 2020; Belete 2021; Zhu et al. 2020).
Johnson & Johnson
Janssen Pharmaceuticals Johnson & Johnson developed a non-replicating viral vector vaccine, Ad26.CoV2. S. This is in phase-4 of clinical trials. (WHO Vaccine landscape) The phase I-IIa clinical trial results showed the efficiency of the vaccine against the B.1.351 variant. Induction of humoral and cellular responses against SARS-CoV-2 original strain WA1/2020, B.1.1.7, CAL.20C, P.1, and B.1.351 was seen in twenty vaccinated participants. After 71 days of vaccination, median pseudo virus neutralizing antibody titres against P.1 and B.1.351 strains were five fold and 3.3-fold lower than the WA1/2020 strain. The median binding antibody titers were lower against B.1.351 and P.1 variants than WA1/2020 (Alter et al. 2021). For emergency use, the Food and Drug Administration (FDA) and WHO have approved the use of this vaccine. The recommended duration between the 1st and 2nd doses is at least 47 days for people over 18 years old. The efficacy was seen to be 66.0% in phase-3 trials (Sheikh et al. 2021). The phase-3 trial was randomized, double-blind, placebo-controlled, and conducted on adult participants in a 1:1 ratio. Participants were administered a single dose (5 × 1010 viral particles) of Ad26.CoV2.S or placebo. The efficacy and safety were assessed in patients having moderate to severe-critical symptoms and negative for SARS-CoV-2 14 days and 28 days after Ad26.CoV2. S administration in the per-protocol population. 19,630 negatively tested participants and 19,691 placebo administered participants were in the per-protocol population who received Ad26.CoV2.S. 464 participants were in moderate to a severe-critical condition in 468 confirmed symptomatic cases where symptoms began at least 14 days after administration. The vaccine efficacy was observed as 66.9%. The onset of symptoms in 66 cases who had moderate to severe critical infections and 193 placebo-administered cases after at least 28 days of administration were observed. The efficacy was seen to be 66.1%. In severe-critical cases, efficacy was seen to be 76.7% and 85.4% in cases where onset occurred at least after 14 days of administration and 28 days of the administration, respectively—reactogenicity with Ad26.CoV2 S administered cases were higher than placebo administered cases. Ad26.CoV2. S administered cases showed mild to moderate and transient reactogenicity. Severe adverse effects were balanced in these two groups. The number of deaths was 3 in the vaccine group, which were not related to COVID-19, and 16 in the placebo group, among which 5 cases were COVID-19 related. The result showed that Ad26.CoV2. S is effective and safe against SARS-CoV-2 (NCT04505722) (Sadoff et al. 2021; Stephenson et al. 2021).
Neutralization antibody and SARS-CoV-2 variants
The neutralizing antibody targets the spike (S trimer) protein. The S trimer consists of 3 copies of the S1 and S2 subunit each. The RBD of SARS-CoV-2 and other coronaviruses at the S1 domains bind to the ACE2 receptor only when it is in ‘up’ confirmation. When the RBD is in ‘down’ confirmation, it is closed. A subset of neutralizing antibodies that blocks the viral entry by blocking the ACE2 receptor and RBD binding is isolated from the convalescent donors. These blocking NAbs are composed of different heavy chains encoded by VH3-55, VH3-66, VH3-30, and other variety of heavy chains (Barnes et al. 2020; Wang et al. 2020a, b, c; Wang et al. 2021). The neutralizing antibodies are essential keys in immune responses for protection and treatment against any viral disease. Besides, it also plays a significant role in virus clearance. The NAbs specific to the pathogen can block viral infection, can be induced through viral infection or vaccination (Wu et al. 2021). Many proteins, including Structural and non-structural proteins of SARS-CoV-2, can be targeted by neutralizing antibodies. The two main structural proteins for targeting are nucleoprotein and spike protein. The nucleoprotein is surrounded by a viral cellular membrane, making it less preferable in serosurvey than spike protein. The robustness of antibodies with better functionality and longevity remains unanswered (Wajnberg et al. 2020).
In a longitudinal assessment of SARS-CoV-2 infection, the multifaceted immune response was found for 3 months—individuals with mild covid-19 induced SARS-CoV-2 specific IgG, IgG + RBD specific neutralizing antibodies. The memory CD4 + immune response was maintained throughout the study. Increased in NAbs, IgG + classical memory B cells with B cell receptors that formed NAbs, Th1 cytokine-producing CXCR5 + circulating T follicular helper cells and CXCR5- non-T follicular helper cells, proliferating CXCR3 + CD4 + memory cells, and IFN-gamma-producing CD8 + T cells were found in the recovered individuals (Rodda et al. 2021).
A preliminary study (lentiviral pseudotype assay): A study on the dynamics of neutralizing antibodies was conducted on 30 SARS-CoV-2 infected patients coincident with the IgG antibody and proinflammatory cytokines during the acute and convalescent phases to provide critical information to help with the COVID-19 vaccine development. The experiment found out that there was a parallel correlation between the NAbs and IgG antibody levels; the NAbs titer increased as the IgG level increased. The NAbs specific to SARS-CoV-2 were low after the symptom onset for 7–10 days. After 3–7 weeks passed, NAb titer was shown to increase and reached its maiden peak on day 33. However, there was a decline in the NAb titer after 3 months, from 93.3% of patients. Furthermore, a strong positive correlation between NAb titer and levels of plasma proinflammatory cytokines, including stem cell factor, TNF-related apoptosis-inducing ligand, and macrophage colony-stimulating factor, was found (Wang et al. 2021).
Humoral immune responses are generally characterized by primary IgM antibody responses, followed by secondary IgG, IgA, and IgE antibody responses, linked with immunological memory. IgG, IgA, and IgE specific to SARS-CoV-2 elicited, but IgA dominates SARS-CoV-2 neutralization compared to primary immune response, IgG antibody (Sterlin et al. 2021).
A New York-based study suggests that more than 90% of participants seroconvert and showed robust IgG antibodies in mild to moderate SARS-CoV-2 infection. The study also found the stability of antibody titer for 3 months and a modest decline in 5 months (Wajnberg et al. 2020).
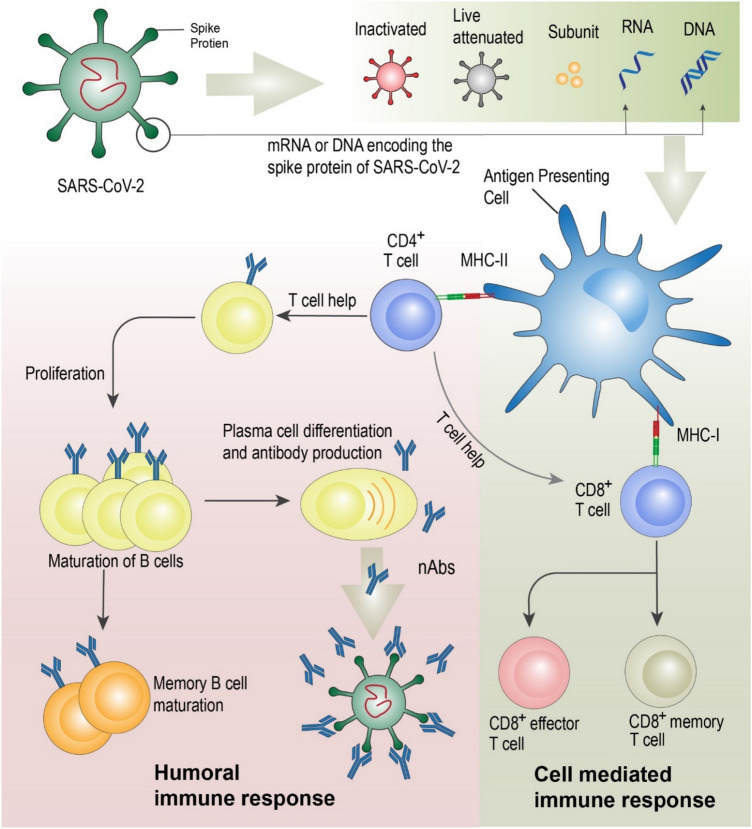
Another preliminary study (lentiviral pseudotype assay): NAb specific to SARS-CoV-2 are detected on days 10–15 after the commencement of the infection and remain subsequently. Spike binding antibody and plasma NAbs titer was higher in the elderly and middle-aged patients than in younger patients. There was a varied titer level of NAbs in patients, with some having a high titer level and some having an undetectable titer level. The study also found that the NAbs were positively correlated with plasma CRP levels. An association between the humoral and cell-mediated immunity was observed as the NAbs were negatively correlated with the lymphocytes counts in the infected patients at the time of admission during the study (Wu et al. 2021). Refer to Fig. 3 for the activation and correlation between humoral, cell mediated and neutralizing antibody.
Fig. 3.
Both conventional and nano based vaccine moves to the antigen presenting cell where major histone compatibility factors (MHC I and MHC II) presents the antigen to induce humoral and cell mediated immune response
It has been seen that patients with severe coronavirus disease have more functional SARS-CoV-2 specific neutralizing antibodies than non-severe patients. The mean neutralizing antibody titer was about five-fold and seven folds higher in severe patients against SARS-CoV-2 pseudovirus and live virus, respectively. These studies have significant implications for individuals involved in plasma therapy, the isolation of neutralizing monoclonal antibodies, and immune determinants (Wang et al. 2020a, b, c). Two specific monoclonal antibodies, CB6 and CA1, were isolated from SASRS-CoV-2 convalescent patients. These antibodies demonstrated robust neutralizing activity in vitro against the pseudo-SARS-CoV-2 virus. However, CB6 has better neutralizing activity than CA1. Both MAbs inhibit pseudovirus transduction into Huh7, Calu-3, and HEK293T cells (Shi et al. 2020). Currently, for COVID-19 treatment, three monoclonal antibody products have been approved by FDA. These products are Bamlinivimab plus etesevimab, Casirivimab plus imdevimab, and Sortovimab. Bamlinivimab plus etesevimab distribution was first paused due to susceptibility concern for P.1 and B.1.1.7 variants but later resumed the distribution (Anti-SARS-CoV-2 Monoclonal Antibodies|COVID-19 Treatment Guidelines 2021). (https://www.covid19treatmentguidelines.nih.gov/therapies/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/).
Traditional vaccine platform uses in COVID-19 vaccine development
Live attenuated vaccines
Live attenuated vaccines (LAV) are the most immunogenic vaccines without any adjuvant due to their effectiveness to provoke the immune system or immunity to mimic the natural infection (Minor 2015). Live attenuated viruses contain viable viruses with low virulence properties, live attenuated viruses are weak enough not to cause any diseases in a typical immune system. The virulence property of the virus is reduced or eliminated typically by site-directed mutagenesis or using chemicals to suppress the disease-causing gene without losing the genome and antigenicity of the virus (Graham et al. 2013). They reproduce slowly in the body, hence providing a long-term immunity against the pathogen as it remains a continuous antigen source. Live attenuated vaccines reduce the need for booster doses with single immunization (Minor 2015). Protection through live attenuated vaccine can be lifelong because LAV elicits both innate and adaptive immune systems; additionally, the production is economically feasible (Vignuzzi et al. 2008). Live attenuated vaccines are not suitable for the elderly, children due to the reversion of the weakened virus to virulent strain again; however, this is not general for every virus (Bull 2015). Live attenuated vaccine is mature and emerged as the front runner in the vaccine development for the SARS-CoV-2 (Shin et al. 2020). New technologies like genetic code expansion are being used to create a high reproductive with a genetically stable live attenuated vaccine (Si et al. 2016). Synthetic genomics is the most recent approach to synthesizing recombinant SARS-CoV-2 viruses from viral DNA fragments (Thao et al. 2020; Xie et al. 2020). Reverse genetic techniques are being used in live, weakened vaccines to effectively inactivate a non-structural peptide protein (nsp 14) and remove the envelope E protein in the pathogenic virus (Graham et al. 2013). BCG, Bacilli Calmette-Guerin vaccine is a live vaccine extensively being used since 1921 to prevent tuberculosis and leprosy (Fatima et al. 2020). In a phase 3 trial of the BCG vaccine (NCT04327206) in Western Australian hospitals, the scientist concluded that the BCG vaccine effectively minimized the incidence of SARS-CoV-2 in different hospitals of children as the vaccine improved the immune system and lowered the infection rate of SARS-CoV-2. Another study from Radboud University of Netherlands (NCT04328441) is going on to see the effectiveness of BCG on the health workers who took part in COVID-19 patient care. By 2022 these two studies will publish their results (Bhagavathula et al. 2020). Several vaccine development companies are working to find a suitable and effective vaccine worldwide. Serum Institute of India is now developing the Covi-Vac vaccine, which is currently in phase 1 of the clinical trial. Table 3 gives a brief overview of the live attenuated vaccine candidates.
Table 3.
Live attenuate Vaccine candidates in development for SARS-CoV-2 virus
| Live Attenuated Vaccine candidates (Source: WHO as per 15th October 2021) | |||||
|---|---|---|---|---|---|
| Candidate | Dose | Schedule | Route of Administration | Phase | Developer |
| COVI-VAC | 1–2 |
Day 0 or Day 0 + 28 |
Intranasal |
Phase 1 |
Codagenix/Serum Institute of India |
| MV-014–212, expresses the spike (S) protein | 1 | Day 0 | Intranasal |
Phase 1 |
Meissa Vaccines, Inc |
Viral vector vaccines
Vaccines based on viral vectors use a vector which is a modified version of another harmless virus, not SARS-CoV-2, to deliver information and instruction to the host cells. This viral vector uses our cell's machinery to manufacture a non-harmful piece of SARS-CoV-2; generally, a spike protein, to trigger the immune system (Understanding Viral Vector COVID-19 Vaccines|CDC 2021). Viruses from mammals have been repurposed and engineered for a variety of vaccine applications. For COVID-19, several non-replicating adenoviral vector vaccine options are being developed. The leading adenovirus type 5 vector (Ad5-nCoV) and chimpanzee adenovirus vaccine vector (ChAdOx1) are the leading adenoviral vectors in SARS-CoV-2 clinical trials as of 16 March 2020 and 31 March 2020, respectively. Adenoviral vectors' broad tissue tropism, natural adjuvant properties, and scalability are all advantages. Pre-existing immunity of the pre-adenoviral vectored vaccine in humans is a problem and disadvantage for adenoviral vector platforms, as it may reduce the adenoviral vector efficacy (Xiang et al. 2002). Although pre-existing immunity to Ad5 is widespread, its clinical use continues, and alternatives like ChAdOx1 with low human seroprevalence are being established (Dicks et al. 2012; Fausther-Bovendo and Kobinger 2014). Table 4 gives a brief overview of the viral vector vaccine candidates.
Table 4.
Viral vector vaccine candidates for COVID-19
| Viral Vector-Non -replicating (Phase 3 and above) Vaccine candidates (Source: WHO as per 15th October 2021) | |||||
|---|---|---|---|---|---|
| Candidate | Dose | Schedule | Route of Administration | Phase | Developer |
| ChAdOx1-S—(AZD1222) (Covishield) | 1–2 | Day 0 + 28 | Intramuscular |
Phase 4 EUCTR2021-002327-38-NL ACTRN12621000661875 |
AstraZeneca, University of Oxford |
| Recombinant novel coronavirus vaccine (Adenovirus type 5 vector) | 1 | Day 0 | Intramuscular |
Phase 4 |
CanSino Biological Inc./Beijing Institute of Biotechnology |
| Gam-COVID-Vac Adeno-based (rAd26-S + rAd5-S) | 2 | Day 0 + 21 | Intramuscular |
Phase 3 |
Gamaleya Research Institute; Health Ministry of the Russian Federation |
| DelNS1-2019-nCoV-RBD-OPT1 | 2 | Day 0 + 28 | Intranasal |
Phase-3 ChiCTR2100051391 |
University of Hong Kong, Xiamen university and Beijing Wantai Biological pharmacy |
| Ad26.COV2.S | 1–2 | Day 0 or Day 0 + 56 | Intramuscular |
Phase 4 EUCTR2021-002327-38-NL |
Janssen Pharmaceutical |
Gam-COVID-Vac, commonly called Sputnik-V, is a non-replicating adenovirus-based vaccine developed by the Gamaleya National Centre of Epidemiology and Microbiology, Russia. Sputnik-V was developed using two different viral vectors, rAd type 26 (rAd26) and rAd type 5 (rAd5). Phase-I and II results showed no severe adverse effects. A stable humoral and cellular immune response was seen in almost every participant. The vaccine is in Phase-3 of a clinical trial. The interim clinical efficacy result showed the effectiveness of this vaccine in 21,862 participants in Moscow, Russia. The randomized, double-blind placebo-controlled phase-3 trial showed an efficacy of 91.6% against SARS-CoV-2. The Ministry of Health of the Russian Federation has approved the use of the Sputnik-V vaccine. Over 50 Countries like India, Serbia, and Belarus, ordered Sputnik-V from Russia (Logunov et al. 2021). The duration recommended between 1st dose and the second dose is 21 days (Sheikh et al. 2021).
Inactivated vaccines
Vaccines that induce immunity utilizing inactivated pathogens have a long history in pandemic response. Although this type of vaccination has traditionally been the most successful, its long processing time has disadvantaged it in the current COVID-19 pandemic. The phase 3 clinical trial for the most promising SARS-CoV-2 vaccine candidates is in progress(Izda et al. 2021). SARS-CoV-2 viral subtypes are propagated using Vero (African Green Monkey) cell lines in this vaccine. Beta-propiolactone is used to inactivate the virus after it is extracted, and the viral particle is then adsorbed onto an adjuvant (aluminum hydroxide). Antiviral immunity development is being investigated at 14 and 28 days after vaccination, with differences in booster vaccine timing and dosage and an assessment of two booster doses. These inactivated viral vaccines tend to have fewer side effects as compared to other vaccine groups. The majority of systemic side effects were mild, with no serious ones reported, although redness and pain at the injection site were normal. All adverse reactions were gone 72 h after the vaccine was given (Xia et al. 2020; Zhang et al. 2020a, b). Table 5 gives a brief overview of the inactivated vaccine candidates.
Table 5.
Phase 3 and above Inactivated virus vaccine candidates for COVID-19
| Inactivated Virus Vaccine Candidates Phase 3 and above (Source: WHO as per 15th October 2021) | |||||
|---|---|---|---|---|---|
| Candidate | Dose | Schedule | Route of Administration | Phase | Developer |
| BBIBP-CorV (Vero Cell) | 2 | Day 0 + 21 | Intramuscular |
Phase 4 |
Sinopharm, China National Biotec Group Co, Beijing Institute of Biological Products |
| CoronaVac | 2 | Day 0 + 14 | Intramuscular |
Phase 4 |
Sinovac Research and Development Co., Ltd |
| Vero cell | 2 | Day 0 + 21 | Intramuscular |
Phase 3 ChiCTR2000034780 ChiCTR2000039000 |
Sinopharm and China National Biotec Group Co and Wuhan Institute of Biological Products |
| SARS-CoV-2 vaccine (vero cells) | 2 | Day 0 + 28 | Intramuscular |
Phase 3 |
Institute of Medical Biology and Chinese Academy of Medical Sciences |
| QazCovid-in® | 2 | Day 0 + 21 | Intramuscular |
Phase 3 |
Research Institute for Biological Safety Problems, Rep of Kazakhstan |
| BBV152 | 2 | Day 0 + 14 | Intramuscular |
Phase 3 CTRI/2020/11/028976 |
Bharat Biotech International Limited |
| VLA2001 | 2 | Day 0 + 21 | Intramuscular |
Phase-3 |
Valneva, National Institute for Health research, United Kingdom |
| ERUCOV-VAC | 2 | Day 0 + 21 | Intramuscular |
Phase-3 |
Erciyes University, Turkey |
| Inactivated vaccine (Vero cell) | 2 | Day 0 + 28 | Intramuscular |
Phase-3 |
Shenzhen Kangtai Biological products Co., Ltd |
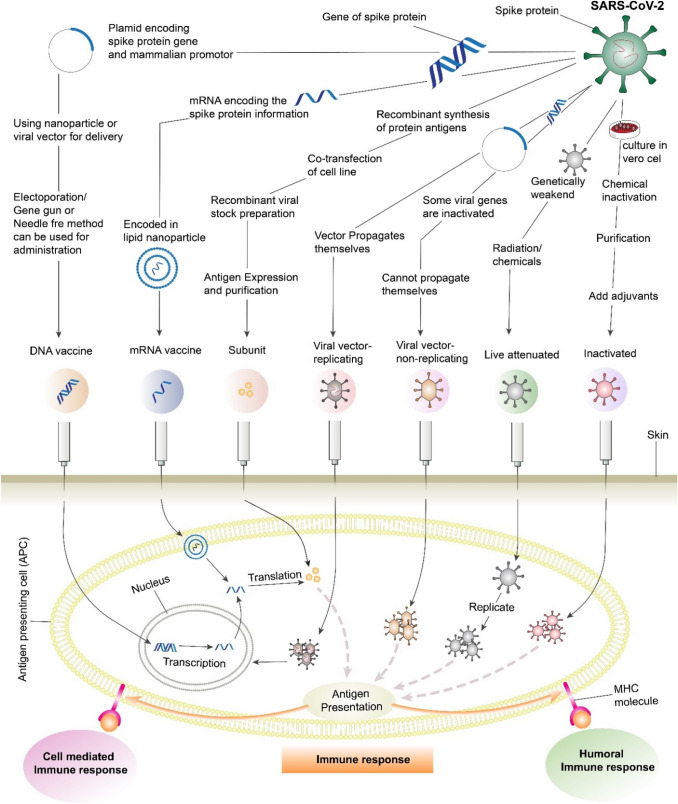
Nano-based vaccines in the development of a potential COVID-19 vaccine
Viruses are nanoscale structures and, therefore, can be considered naturally produced nanomaterials; LAVs, IVs, and viral vectors can be examples of nanotechnologies. Nanoparticles and viruses have similar length scales, which is why nanotechnology applications to vaccine production and immune engineering are so efficient (Shin et al. 2020).The use of nanomaterials as a carrier of antigenic components is a new approach in vaccine design technology (Fig. 4). The fundamental three interactions that are associated with antigen and nanoparticles are adsorption, entrapment, and conjugation. Nanomaterials such as nano polymers, liposomes, inorganic nanoparticles, carbon-based nanomaterials, and quantum dots are conventional vehicles for nucleic acid and subunit vaccines. These nanomaterials are already being utilized to develop vaccines for illnesses such as HIV, malaria, Ebola, toxoplasmosis, and influenza. The cellular toxicity of nanomaterials and the requirement for an adjuvant are the two significant limitations of nano-based vaccines (Kato et al. 2019; Raghuwanshi et al. 2012).
Fig. 4.
Diagrammatic representation of preparation and administration of different vaccine to induce immune response
Subunit vaccine
Subunit vaccines contain the antigen, typically a pathogen-driven protein with immunogenicity that can activate the host immune system. Adjuvants are required for subunit vaccinations to boost the immune response that is safe and can be easily manufactured by using the technique of DNA recombination. Many manufactures started developing subunit vaccines using spike S spike glycoprotein and its fragments, including S1, S2 domain, and receptor-binding domain (RBD) (Uddin et al. 2020). In an experiment on monkeys utilizing the recombinant receptor-binding domain (RBD) as an antigen, viral loads were shown to be lower in the lungs and oropharynx, and it was beneficial in preventing pneumonia in MERS-CoV (Zhang et al. 2020a, b). S protein N terminal domains (NTD), E, N, M, and NSPs similar to receptor binding domain of the S protein showed receptor-binding activity to carbohydrates. For instance, binding properties to the carbohydrate of IBV M41 strain is related to the N-terminal domain of the S protein; hence this domain can be the vaccine candidate as antigen for the development of a vaccine (Lan et al. 2015). Clover Biopharmaceuticals has constructed an S-Trimer, SARS-CoV-2 S protein trimer vaccine utilizing its proprietary Trimer-tag (Uddin et al. 2020).
Moreover, Novavax Inc has developed a recombinant nanoparticle constructed on full-length spike glycoprotein of wild-type SARS-CoV-2. A robust CD4 + and CD8 + T cell immune response with the dominant CD4 + biased Th1 response phenotype was reported (Keech et al. 2020). Nanoparticles can be employed to target multiepitope formulation of a subunit vaccine. A study demonstrates that a nanoparticle immunogen displaying 60 RBD of SARS-CoV-2 with the ability to self-assemble increases the neutralizing antibody titer ~ 10 folds higher than the prefusion-stabilized spike. Further, the study stated that this process could be highly scalable due to the self-assembly property of the immunogens, and the multiple distinct epitopes employed in the nanoparticle may defy the immunogenic scape of the SARS-CoV-2 variants (Walls et al. 2020a, b). Adjuvating the nanoparticle-based subunit vaccines can stimulate durable neutralizing antibodies against SARS-CoV-2 infection (Arunachalam et al. 2021). In animal experiments, it has been seen that several nanocarriers such as ferritin, polymersomes, Heptad repeats, and mesoporous silica nanoparticles can elicit potent humoral and cell-mediated immune responses against SARS-CoV-2 (Lam et al. 2021; Ma et al. 2020; Powell et al. 2021; Qiao et al. 2021). Table 6 gives a brief overview of the protein subunit vaccine candidates.
Table 6.
Protein subunit vaccine candidates that are currently in phase 3 of clinical development
| Candidate | Dose | Schedule | Route of Administration | Phase | Developer |
|---|---|---|---|---|---|
| SARS-CoV-2 rS/Matrix M1-Adjuvant | 2 | Day 0 + 21 | Intramuscular |
Phase 3 EUCTR2020-004123-16-GB |
Novavax |
| CHO Cell | 2–3 | Day 0 + 28 or Day 0 + 28 + 56 | Intramuscular |
Phase 3 |
Anhui Zhifei Longcom Biopharmaceutical and Institute of Microbiology, Chinese Academy of Sciences |
| VAT00002 | 2 | Day 0 + 21 | Intramuscular |
Phase 3 PACTR202011523101903 |
Sanofi Pasteur + GSK |
| FINLAY-FR-2 anti-SARS-CoV-2 Vaccine | 2 | Day 0 + 28 | Intramuscular |
Phase 3 RPCEC00000354 |
Instituto Finlay de Vacunas |
| EpiVacCorona | 2 | Day 0 + 21 | Intramuscular |
Phase 3 |
Federal Budgetary Research Institution State Research Center of Virology and Biotechnology “Vector” |
| CIGB-66 | 3 | Day 0 + 14 + 28 or Day 0 + 28 + 56 | Intramuscular |
Phase 3 RPCEC00000359 |
Center for Genetic Engineering and Biotechnology (CIGB) |
| SCB-2019 + AS03 | 2 | Day 0 + 21 | Intramuscular |
Phase-3 |
Clover Biopharmaceuticals Inc./GSK/Dynavax |
| COVAX-19 | 2 | Day 0 + 21 | Intramuscular |
Phase-3 IRCT20150303021315N24 |
Vaxine Pty Ltd./CinnaGen CO |
| MVC-COV1901 | 2 | Day 0 + 28 | Intramuscular |
Phase-3 |
Medigen Vaccine Biologics + Dynavax + National Institute of Allergy and Infectious Diseases (NIAID) |
| Recombinant SARS-Cov-2 vaccine | 2 | Day 0 + 28 | Intramuscular |
Phase-3 NCT04887207 |
West China hospital + Sichuan University WestVac Biopharma Co., Ltd |
| BECOV2 | 2 | Day 0 + 28 | Intramuscular |
Phase-3 CTRI/2021/08/036074 |
Biological E. Ltd |
| Nanocovax | 2 | Day 0 + 21 | Intramuscular |
Phase-3 |
Nanogen Pharmaceutical Biotechnology |
| GBP510 | 2 | Day 0 + 28 | Intramuscular |
Phase-3 |
SK Bioscience Co., Ltd and CEPI |
| Razi Cov Pars | 3 | Day 0 + 21 + 51 | IM and IN |
Phase-3 IRCT20210206050259N3 |
Razi vaccine and Serum research institute |
DNA vaccines
DNA vaccines (DVs) contain a plasmid incorporating a particular gene that encodes the antigens classified from the pathogenic microorganism. Pathogenic microorganism activates typically the immune system stimulated by the antigenic protein. However, the desired gene is now carried by the bacterial plasmid that delivers the antigenic encoding desired gene into the host cell and translates to activate the immune system. The DNA vaccine elicits both humoral and cell-mediated immunity and induces long-term immunity that protects from the disease effectively in the future (Hobernik and Bros 2018).
Nanotechnology promises to deliver next-generation vaccines and fight against COVID-19 (Mufamadi 2020). Nanoparticle provides an alternative yet safe and potential approach in vaccine delivery systems (Theobald 2020). Nanocarriers protect and shield the DNA vaccine from degradation by DNases and other enzymes (Cai et al. 2018). Liposomes or lipid nanoparticles, polymers, chitosan-based, functionalized silica nanoparticles can be employed in DNA vaccine delivery systems, as revealed in various animal experiments (Mucker et al. 2020; Tatlow et al. 2020; Theobald 2020; Zhao et al. 2013; Zhao et al. 2021). The alternative ways to increase the efficiency of DNA vaccine delivery could be the addition of adjuvants and applying a transcutaneous microneedle delivery system. In melanoma studies, microneedle applied transcutaneously showed a higher fold of IgG antibody and excess CD8 + T cell response to inhibit the melanoma. A preclinical study on H1N1 examined that DNA vaccine incorporated in liposomes can be delivered orally, resulting in higher IgG titer and activity of T cell response (Park et al. 2021). A new concept arises in COVID-19 development through inhalation of DNA vaccine directly by using nebulizer or metered-dose inhaler. If a naked DNA vaccine is delivered, it demonstrates lower immunogenicity. As DNA persists in a negative charge, it faces repulsion from the negatively charged lipid layer of the cell. So, a cationic nanoparticle, chitosan, can be a preferred delivery system to pass the genetic material through the mucosal surface. Chitosan has the advantage of mucoadhesion, non-immunogenicity, and high solubility (Tatlow et al. 2020). DNA vaccines can be administered to the host body through different routes such as Intramuscularly, intradermally, and subcutaneously. These routes primarily address the monocytes and keratinocytes and antigen-presenting cells near the injection site (Hengge et al. 1995; Marino et al. 2011; Porgador et al. 1998). DNA vaccines are more economically feasible, easy to manufacture, and safe handling (Prazeres and Monteiro 2014). More than ten vaccine candidates for COVID-19 are in clinical trials. There were two parts to the Phase 1/2 clinical studies, Part A and B. Inovio recruited 40 healthy adults for part A, aged 19–50, to test the safety and immunological response of the vaccination in South Korea (Phase II/III Study of COVID-19 DNA Vaccine (AG0302-COVID19)—Full Text View—ClinicalTrials.Gov 2021). There were two parts to the Phase 1/2 clinical studies, Part A and B. Inovio recruited 40 healthy adults for part A, aged 19–50, to test the safety and immunological response of the vaccination in South Korea (Phase I/II Study of Intracutaneous Inoculation of COVID-19 DNA Vaccine (AG0302-COVID19) 2021). In the various field of therapeutics, including therapy of cancer (Fioretti et al. 2014), infectious disease (Maslow 2017), allergies (Scheiblhofer et al. 2018), and autoimmune diseases (Zhang and Nandakumar 2018), DNA vaccines can be applied. FDA and USDA have authorized vaccinations against canine melanoma and West Nile Virus in horses for veterinary use only; there are currently no fully approved DNA vaccines for human use (Atherton et al. 2016; Dauphin and Zientara 2007). However, India has recently approved a three-dose DNA-based vaccine (ZyCoV-D) developed by Zydus Cadila under emergency use authorization against COVID-19, the first DNA vaccine to administer in humans. ZyCoV-D reported 67% protection against symptomatic SARS-CoV-2 infection (Mallapaty 2021). Previously DNA vaccine clinical trial was performed against HIV to evaluate the prophylactic and therapeutic effect, which was an early human clinical trial with DNA vaccine, there was no significant immune response, but potential immunogenicity was observed. Another clinical trial targeting the Hepatitis B virus showed that the humoral response was induced in patients who typically did not respond to the conventional vaccine (Rottinghaus et al. 2003). Table 7 gives a brief overview of the DNA vaccine candidates.
Table 7.
DNA based COVID-19 vaccine candidates in phase 2 and above
| DNA based Vaccine candidates currently in Phase 2/3 and above (Source: WHO as per 15th October 2021) | |||||
|---|---|---|---|---|---|
| Candidate | Dose | Schedule | Route of Administration | Phase | Developer |
| nCov vaccine | 3 | Day 0 + 28 + 56 | Intradermal |
Phase 3 CTRI/2020/07/026352 |
Zydus Cadila |
| AG0301-COVID19 | 2 | Day 0 + 14 | Intramuscular |
Phase 2/3 |
AnGes and Takara Bio and Osaka University |
| INO-4800 + electroporation | 2 | Day 0 + 28 | Intradermal |
Phase 2/3 |
Inovio Pharmaceuticals and International Vaccine Institute and Advaccine (Suzhou) Biopharmaceutical Co., Ltd |
| GX-19 N | 2 | Day 0 + 28 | Intramuscular |
Phase-2/3 |
Genexine consortium |
RNA vaccine
mRNA vaccines resemble the natural infection caused by the virus by retaining a short synthetic viral mRNA that encodes only the specific required antigen (Pardi et al. 2018). mRNA carries the information from the DNA that encodes the protein to the ribosome that translates the protein. Two platforms are mainly present for mRNA vaccine development: non-replicating mRNA and self-amplifying mRNA (saRNA). The self-amplifying mRNA encodes the specific required antigen and the whole machinery of the viral replication. In 1989, as therapeutics, mRNA was initially promoted as an invitro broadly applicable transfection technique (Malone et al. 1989). In recent years several mRNA vaccine platforms have become more advanced, validating the studies of immunogenicity safety and efficacy like modified mRNA vaccine in Zika virus (Pardi et al. 2017), against Influenza A virus (Petsch et al. 2012), and non-viral delivery of self-amplifying RNA vaccine (Geall et al. 2012). Plasmids from Trinidad donkey Venezuelan equine encephalitis virus strains (VEEV) are used to make saRNA vaccines. The VEEV structural coding regions are then substituted with SARS-CoV-2 pre-fusion Spike protein, while the VEEV alphavirus self-amplifying coding region is preserved. saRNA vaccines show opportunities to make a possible vaccination as they can induce a more robust immune response than non-replicating mRNA vaccines. The spike protein and replicon contain a lengthy RNA sequence which is one significant drawback for saRNA vaccines. Arcturus/DUKE-NS, Imperial College in London, and the University of Washington are studying the saRNA vaccine to develop a potential candidate against SARS-CoV-2. saRNa constructs with adjuvants and is embedded in different forms of nanoparticles (de Alwis et al. 2020; Erasmus et al. 2020; McKay et al. 2020). Table 8 gives a brief overview of the RNA-based vaccine candidates.
Table 8.
RNA based vaccine currently in phase 3 or above in clinical development for COVID-19
| RNA based Vaccine candidates currently in Phase 3 and above (Source: WHO as per 15th October 2021) | |||||
|---|---|---|---|---|---|
| Candidate | Dose | Schedule | Route of Administration | Phase | Developer |
| mRNA -1273 | 2 | Day 0 + 28 | Intramuscular |
Phase 4 EUCTR2021-002327-38-NL EUCTR2021-003388-90-NL EUCTR2021-003618-37-NO |
Moderna, National Institute of Allergy and Infectious Diseases (NIAID) |
| BNT162 (3 LNP-mRNAs) /Comirnaty | 2 | Day 0 + 21 | Intramuscular |
Phase 4 ACTRN12621000661875 EUCTR2021-000412-28-BE EUCTR2021-002327-38-NL EUCTR2021-000893-27-BE EUCTR2021-000930-32-BE EUCTR2021-003388-90-NL EUCTR2021-003618-37-NO |
Pfizer/BioNTech, Fosun Pharma |
| CVnCoV Vaccine | 2 | Day 0 + 28 | Intramuscular |
Phase 3 |
CureVac AG |
| ARCoV | 2 |
Day 0 + 14 or Day 0 + 28 |
Intramuscular |
Phase-3 |
AMS, Walvax Biotechnology and Suzhou Abogen Biosciences |
BNT16b2 and mRNA-1273 mRNA vaccines are among the most outstanding achievements in next-generation vaccine development that showed a significant role in filling the need for global vaccine demand. Both the vaccines are composed of lipid nanoparticles that show an efficacy of ~ 95% (Khurana et al. 2021). Lipid nanoparticles have various advantages that make them suitable as a vehicle to deliver mRNAs, such as the biocompatibility to human use, stability during the passage, efficient encapsulation of the informant, and increased cellular uptake in vivo (Khurana et al. 2021; Park et al. 2021). The 5′ and 3′ untranslated regions of mRNA sequences can be engineered systematically to the nanoparticle to ensure efficient delivery (Zeng et al. 2020).
Efficacy of the vaccines
With vaccine production and clinical trials ongoing, the issue about how much efficacy is required for a vaccination to be immunogenic arises. While further research is needed, initial research studies indicate that while an efficacy of greater than or equal to 70% is required to eradicate the illness, a prophylactic shot with a 70% efficacy would still have a significant considerable influence and could lead to the virus’s elimination if sufficient means of social distancing are implemented (Makhoul et al. 2020). A D614G mutation in the spike S protein, G to A base shift from the original Wuhan strain, has been identified predominantly in Europe and has been linked to increased transmissibility and viral load, but further research is required to assess its effect on clinical outcomes (Korber et al. 2020). The D614G mutation is identified on the spike S protein but not in the RBD; instead, it is present between the individual spike protomers that can provide stability by hydrogen bonding, implies that, while it may influence the virus's infectivity, it should not have a significant impact on the efficacy of vaccines and, as a result, NAbs released against the RBD (Grubaugh et al. 2020). Several vaccine candidates gave opportunistic results toward the efficacy of hospitalized patients. More than a hundred and fifty vaccine candidates are in pre-clinical trials, which might have better efficacy than the current vaccine.
Rare adverse effects of COVID-19 vaccines (Refer to Table 9)
Table 9.
List of common and rare side effects post COVID-19 vaccination
| Vaccine candidates | Common side effects | Rare side effects |
|---|---|---|
| AstraZeneca |
Pain in arms Influenza like symptoms Nausea |
Thrombosis with thrombocytopenia syndrome (TTS) |
| Pfizer (BNT162) |
Allergy Pain, redness, swelling at injection site Fever Fatigue Headache Nausea Vomiting Itching Chills Joint pain |
Anaphylactic shock (rare) Myocarditis (Rare) |
| Moderna (mRNA-1273) |
Allergy Pain, redness, swelling at injection site Fever Fatigue Headache Nausea Vomiting Itching Chills Joint pain |
Anaphylactic shock Myocarditis |
| Johnson & Johnson |
Pain, redness, swelling at injection site Fever Fatigue Headache Muscle pain Chills Joint pain Nausea Vomiting |
Thrombosis with thrombocytopenia syndrome (TTS) Guillain–Barre syndrome(GBS) |
|
CoronaVac (Sinovac Biotech) |
Injection site pain Fatigue Muscle pain Diarrhea |
Bell’s palsy |
| Vero cell (Sinopharm) |
Injection site pain Fatigue Headache Lethargy Tenderness Backpain Nausea Abdominal pain |
Abdominal discomfort Loss of smell Facial swelling Tinnitus |
| Ad5-nCoV (CanSino) |
Injection site pain Fever Fatigue Headache Muscle pain |
No serious adverse effect has been reported |
| Sputnik V |
Flu-like illness Injection site pain Headache Asthenia |
No serious adverse effect found post vaccination |
Myocarditis/perimyocarditis
The mRNA vaccine (BNT162b2 and mRNA-1273) has shown excellent reliability to reach the global vaccine demand against COVID-19. However, few myocarditis/perimayocarditis have been reported in young children adults from different countries such as the United States, Israel, and the European Economic area. Smallpox, Influenza, Hepatitis B vaccine, Varicella vaccine are also associated with myocarditis. Israel was the first country to mass vaccinate all age groups, allowing a temporal correlation between the vaccine and potential issues to be described. Israel reported at least 60 first cases in April, then the US Ministry of Defence tracked 14 at the same time, followed by European Medical Agency in May with 107 cases of myocarditis within young males. From 19 December 2020 to 11 June 2021, 1226 cases of myocarditis were reported to Vaccine Adverse Event Reporting System. The cases are mild and temporarily exist but can cause severe effects on adolescents. Pleuritic chest pain, dyspnoea, or palpitations are the symptoms that can be noticed especially in younger children with myocarditis besides, epigastric pain, profuse sweating, tachycardia, hypotension can also be observed in some cases (Albert et al. 2021; Hasnie et al. 2021; Maron et al. 2021; McLean and Johnson 2021; Patrignani et al. 2021; Snapiri et al. 2021; Vogel 2021). According to multiple case reports, most of the symptoms for myocarditis arise within 1–4 days after the first or second dose vaccination of BNT162b2 or mRNA-1273. Younger males, 12–29 years, are predominantly affected; however, a 56-year-old case was also reported with previously SARS-CoV-2 infection but was healthy at the time of vaccination (Maron et al. 2021; McLean and Johnson 2021; Patrignani et al. 2021; Snapiri et al. 2021). Cardiac magnetic resonance imaging was an effective tool for the diagnosis of myocarditis. Elevated troponin level was reported in almost every case; in some cases, creative protein and creatine kinase, CK-MB, and B-type natriuretic peptide levels were also reported higher (Albert et al. 2021; Hasnie et al. 2021; Maron et al. 2021; McLean and Johnson 2021; Patrignani et al. 2021; Snapiri et al. 2021). Different drugs were introduced to treat and elevate the symptoms of myocarditis. Aspirin, colchicine, ibuprofen, beta-blockers, steroids or corticosteroids, intravenous anti-inflammatory drugs were used for the recovery of patients (Albert et al. 2021; Hasnie et al. 2021; Maron et al. 2021; McLean and Johnson 2021; Patrignani et al. 2021; Vogel 2021).
Thrombosis with thrombocytopenia syndrome (TTS)
Thrombosis with thrombocytopenia syndrome (TTS) involving acute arterial and venous thrombosis is a rare side effect reported in few vaccine candidates. On 13th April 2021, the FDA and CDC paused the vaccine use because of associated risk factors, mainly in women aged < 50. AstraZeneca and Janssen are adenoviral vector-based vaccines that showed similar cases of TTS in Europe and the U.S., respectively. Out of 15 TTS patients, 12 had cerebral venous sinus thrombosis (CVST) after Janssen vaccine administration. Among 15 patients, 13 TTS-affected women were 18–49 years old, and two were of age > 50. Some had a medical history of obesity, hypertension, hypothyroidism, and oral contraceptive use (MacNeil et al. 2021). The European Medicines Agency (EMA) claims that blood clotting because of low platelet count is a “very rare” (D’agostino et al. 2021; Mahase 2021) and possible side effects of COVID-19 vaccination. EMA reported 8 cases in the U.S due to the Janssen vaccine, 287 cases in Europe because of AstraZeneca vaccine, 25 cases for Pfizer, and 5 cases for Moderna vaccine since 4th April (Mahase 2021; Mohseni Afshar et al. 2021).
The blood clots were observed in unusual body parts like the brain and abdomen. EMA reported 86 people with blood clots in the abdomen and brain after 2 weeks of AstraZeneca vaccine administration. Some of them were not even administered heparin but showed heparin-induced thrombocytopenia (HIT) (Ledford 2021). The treatment method for Vaccine-induced thrombotic thrombocytopenia (VITT) is almost similar to the HIT. Any administration of Heparin or platelet transfusion should be stopped. Possible treatment methods are high doses of intravenous immunoglobulins (IVIG), non-heparin-based anticoagulants, corticosteroid administration, and plasma exchange. After clinical stabilization, oral anticoagulants such as apixaban, rivaroxaban can be given (Iba et al. 2021; Mohseni Afshar et al. 2021; Siegler et al. 2021).
Neurological effects
Post COVID-19 vaccination, rare neurological effects have been reported in a few patients. A 61-year-old man and an 82-year-old woman developed the Guillain–Barre Syndrome (GBS) post-vaccination with Pfizer (Waheed et al. 2021) and Moderna (Matarneh et al. 2021) vaccines, respectively. GBS is an inflammatory polyradiculoneuropathy disease affecting peripheral nerves and nerve roots, associated with viral infections. In both cases, symptoms like difficulty in walking, weakness in extremities and muscles were common. In the 82-year-old lady, symptoms like body ache started after the first week of administration and worsened during the second week. Lumbar puncture and cerebrospinal fluid analysis confirmed the development of GBS. Intravenous immunoglobulin (IVIG) was given for treatment for up to 5 days, and after 3 days, improvement was observed in her (Waheed et al. 2021). In the 61-year-old male, symptoms like weakness in proximal and upper extremities were reported 4 days after receiving the second dose of Moderna vaccine. His cerebrospinal fluid analysis and electrodiagnostic tests showed an acute demyelinating polyneuropathy. After 5 days of IVIG administration, his condition also improved (Matarneh et al. 2021).
Severe allergic reactions/anaphylaxis
The mRNA vaccines Moderna and Pfizer/BioNTech have been reported to cause life-threatening allergic reaction anaphylaxis in different countries, including the United States, the United Kingdom, and Singapore (Garvey and Nasser 2021; Lim et al. 2021; Shimabukuro 2021a). In the United States, VAERS reported 108 cases and 175 cases of severe allergic reaction post-vaccination (in some cases first dose or second dose) of Moderna and Pfizer, respectively. 10 (from 108 cases) and 21 (from 175 cases) patients were determined as anaphylaxis from these cases. In the United States, it is determined that the rate of anaphylaxis for Pfizer and Moderna is 2.7 and 11.1 cases per million doses, respectively (Shimabukuro 2021a, b). The mRNA vaccines are carried by lipid nanoparticles that contain an excipient called PEG-2000 and polysorbate. There are some structural similarities between Polysorbate and PEG molecules. The cause of these rare adverse effects is unclear, but it has been postulated that the polyethylene glycol (PEG) in BNT62b2 and polysorbate-80 in mRNA-1273 could be the culprit (Garvey and Nasser 2021; Pegs and Interestingly 2021; Sellaturay et al. 2021). Several reports indicate that people with a history of an allergic reaction, PEG allergic, asthma, rhinitis, and urticaria are more prone to develop severe allergic reactions (Do et al. 2021; Lim et al. 2021; Sellaturay et al. 2021; Shimabukuro 2021a, b). From the 108 and 175 cases, 10 cases (9 with previous allergic history) and 21 (17 with previous allergic history) cases of anaphylaxis were found, respectively (Shimabukuro 2021a, b). Symptoms such as edema, globus sensation, rash, wheezing, flushing, breathlessness, throat closure, and swelling, and generalized urticaria were reported through various case studies (Do et al. 2021; Garvey and Nasser 2021; Lim et al. 2021; Sellaturay et al. 2021; Shimabukuro 2021a). The onset of symptoms varies from minutes to hours; however, the median onset time post-vaccination was recorded about 7.5 min (actual range is 1–45 min) in Moderna and 13 min (actual range 2–150 min) in the Pfizer vaccine (Shimabukuro 2021a, b). The reported patients were administered with intramuscular injection of epinephrine as a treatment due to the life-threatening nature of anaphylaxis (Shimabukuro 2021b).
Orofacial effects
The two main mRNA vaccines for COVID-19, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) have shown orofacial side effects in a few people. Data collected from the United States, Canada, the European Union, and the United Kingdom showed common side effects such as swelling of lips, face, throat, and tongue. A rare side effect like temporary one-sided facial drooping, otherwise called Bell’s palsy, in people who previously used cosmetic injections on their faces was reported by healthcare providers (Cirillo 2021; Sofi-Mahmudi 2021).
Rare side effects in cancer patients
In a few cancer patients and non-cancer patients with or without any family history of cancer, side effects like axillary lymphadenopathy at the ipsilateral injection site were reported after BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) administration. A clinical test report of five patients with unilateral axillary lymphadenopathy showed that they were administered the COVID-19 vaccine (Özütemiz et al. 2021). The PET/CT, MRI, US, Mammogram, and histopathological tests of affected patients aged 32–59 showed benign lymph node reactivation. The thickness and diameter after administration of the vaccine were different from before. According to the Center for Disease Control and Prevention (CDC), axillary adenopathy at the injection site/arm was reported as a typical local reaction after Moderna vaccine administration. In the 18–64 age group, the adenopathy was seen after the first dose and second dose administration in 11.6% and 16% of people, respectively. People administered with Pfizer vaccine reported adenopathy after 2–4 days of vaccination which made clear relation between lymphadenopathy and vaccines (Hiller et al. 2021; Mehta et al. 2021; Özütemiz et al. 2021).
Shingles
After administration of COVID-19 Vaccine, a 68-year-old male, having a past medical history of hypertension, anxiety, and dysrhythmia, reported varicella-zoster virus (VZV) reactivation. His symptoms were stinging sensation, and pain emerged from right chest to back. Few pinheaded vesicular lesions were observed in his right mammary area and back, which started 5 days after his second dose vaccination. Treatment started with the administration of valaciclovir, paracetamol for pain, and a cream for the lesions (Aksu and Öztürk 2021). A study of ninety-one patients suffering from shingles or varicella-zoster virus (VZV) reactivation post-COVID-19 vaccination was done. The results showed that sixteen had hypertension, twelve had autoimmune disorders, and nine were on immunosuppressants among all the participants. All of them developed symptoms of VZV reactivation after 5.8 days of vaccination. Valacyclovir oral administration was used for treatment (Triantafyllidis et al. 2021).
ANCA-Associated Vasculitis and Glomerulonephritis:
After administration of the COVID-19 vaccine, the onset of Antineutrophil cytoplasmic antibody ANCA-Associated Vasculitis (AAV) has been observed in a few people. AAV is a small vessel vasculitis that occurs by the presence of antibodies in cytoplasmic granules of neutrophils. After the first dose of Pfizer-BioNTech vaccine administration in a 78-year-old woman, renal-limited anti-myeloperoxidase (MPO) was onset. The woman had a past medical history of type-2 diabetes, hypertension, and paroxysmal atrial fibrillation—symptoms like nausea, vomiting, and diarrhea post-vaccination in early February 2021. Sixteen days post-vaccination and a few weeks post-vaccination showed an improvement in her urinalysis and serum creatinine level. However, after the second dose administration, she observed nausea, vomiting, diarrhea, and a new symptom-lethargy. Her abdomen and pelvis tomography result was normal, but the manual urine microscopy confirmed that her anti-MPO antibody titer was elevated. For 3 days, she was administered intravenous methylprednisolone and prednisone 1 mg/kg after that, daily. The kidney biopsy and light microscopy confirmed that she had crescentic necrotizing glomerulonephritis and moderate interstitial inflammation. The immunofluorescence showed that she had pauci-immune glomerulonephritis and was diagnosed with renal-limited MPO-AAV. After administration of rituximab, her serum creatinine level improved to 2.25 mg/dL during discharge, and after a month of follow-up, it fell to 1.75 mg/dL (Shakoor et al. 2021). A similar case of ANCA-glomerulonephritis was observed in a 52-year man 2 weeks post vaccination with Moderna mRNA vaccine. On April 15, 2021, the person reported a headache that started the day after his vaccination. He had hypertension in the past. Serological tests, Kidney biopsy, and immunofluorescence confirmed the person had pauci-immune necrotizing and crescentic glomerulonephritis. Rituximab and continuous dialysis were used for treatment (Sekar et al. 2021). In another study conducted on thirteen people (age between 19 and 83), eight people showed onset of Glomerulonephritis, and five people had relapsed after being vaccinated with mRNA COVID-19 vaccine candidates like Moderna and Pfizer. mRNA vaccines are effective in inducing immune response immediately. However, the onset of such rare side effects in a few people has been creating hesitancy, and in the future, more experimentation is needed to solve these side effects (Klomjit et al. 2021).
Conclusion
A vaccine is required to halt the worldwide spread of the SARS-CoV-2 virus. Any consistent, reliable vaccine, safe, long-lasting, and widely available is a suitable candidate. However, viral particles can evolve and mutate, making vaccines ineffective; consequently, creating an effective and safe vaccine in advance of potential SARS-CoV-2 variant outbreaks is necessary. A vaccination with higher safety and fewer side effects is required, and a single vaccine that is effective to all age groups, including hospitalized patients, is essential. Despite substantial progress and favorable outcomes from vaccine candidate trials, many problems remain, along with logistical challenges of mass manufacturing and distribution of millions or billions of doses to the global population, which will undoubtedly be the biggest bottleneck in the pipeline. However, both conventional and next-generation vaccines gave an opportunistic approach to whether the nanotechnology-based vaccine needs more research and collaborations for future vaccine development against infectious disease.
Funding
This research receives no external funding.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Faizan Zarreen Simnani, Email: fzsimnani@gmail.com.
Dibyangshee Singh, Email: dibyasingh123.ds@gmail.com.
Ramneet Kaur, Email: 1989ramneet@gmail.com.
References
- Ahmed SF, Quadeer AA, McKay MR. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020 doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksu SB, Öztürk GZ. A rare case of shingles after covid-19 vaccine: is it a possible adverse effect? Clin Exp Vaccine Res. 2021;10(2):198–201. doi: 10.7774/CEVR.2021.10.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert E, Aurigemma G, Saucedo J, Gerson DS. Myocarditis following COVID-19 vaccination. Radiol Case Rep. 2021;16(8):2142–2145. doi: 10.1016/j.radcr.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter G, Yu J, Liu J, Chandrashekar A, Borducchi EN, Tostanoski LH, McMahan K, Jacob-Dolan C, Martinez DR, Chang A, Anioke T. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature. 2021;596(7871):268–272. doi: 10.1038/S41586-021-03681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alturki SO, Alturki SO, Connors J, Cusimano G, Kutzler MA, Izmirly AM, Haddad EK. The 2020 pandemic: current SARS-CoV-2 vaccine development. Front Immunol. 2020 doi: 10.3389/fimmu.2020.01880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Res. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott AB, Flach B, Lin BC, Doria-Rose NA, O’Dell S, Schmidt SD, Corbett KS, Swanson PA, Padilla M, Beigel JH. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anti-SARS-CoV-2 Monoclonal Antibodies | COVID-19 Treatment Guidelines. (2021). https://www.covid19treatmentguidelines.nih.gov/therapies/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/. Accessed 11 Oct 2021
- Arunachalam PS, Walls AC, Golden N, Atyeo C, Fischinger S, Li C, Aye P, Navarro MJ, Lai L, Edara VV, Röltgen K, Rogers K, Shirreff L, Ferrell DE, Wrenn S, Pettie D, Kraft JC, Miranda MC, Kepl E, Pulendran B (2021) Adjuvanting a subunit SARS-CoV-2 nanoparticle vaccine to induce protective immunity in non-human primates. BioRxiv, 2021.02.10.430696. Doi: 10.1101/2021.02.10.430696
- Atherton MJ, Morris JS, McDermott MR, Lichty BD. Cancer immunology and canine malignant melanoma: a comparative review. Vet Immunol Immunopathol. 2016;169:15–26. doi: 10.1016/j.vetimm.2015.11.003. [DOI] [PubMed] [Google Scholar]
- AZD1222 US Phase III primary analysis confirms safety and efficacy (2021) https://www.astrazeneca.com/media-centre/press-releases/2021/azd1222-us-phase-iii-primary-analysis-confirms-safety-and-efficacy.html. Accessed 30 Mar 2021
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Zaks T. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CO, Jette CA, Abernathy ME, Dam KMA, Esswein SR, Gristick HB, Malyutin AG, Sharaf NG, Huey-Tubman KE, Lee YE, Robbiani DF, Nussenzweig MC, West AP, Bjorkman PJ. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588(7839):682–687. doi: 10.1038/s41586-020-2852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruah V, Bose S. Immunoinformatics-aided identification of T cell and B cell epitopes in the surface glycoprotein of 2019-nCoV. J Med Virol. 2020;92(5):495–500. doi: 10.1002/jmv.25698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belete TM. Review on up-to-date status of candidate vaccines for COVID-19 disease. Infect Drug Resist. 2021;14:151–161. doi: 10.2147/IDR.S288877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenuto D, Giovanetti M, Ciccozzi A, Spoto S, Angeletti S, Ciccozzi M. The 2019-new coronavirus epidemic: evidence for virus evolution. J Med Virol. 2020;92(4):455–459. doi: 10.1002/jmv.25688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagavathula AS, Aldhaleei W, Rovetta A, Rahmani J. Vaccines and drug therapeutics to lock down novel coronavirus disease 2019 (COVID-19): a systematic review of clinical trials. Cureus. 2020 doi: 10.7759/cureus.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull JJ. Evolutionary reversion of live viral vaccines: can genetic engineering subdue it? Virus Evol. 2015 doi: 10.1093/ve/vev005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai P, Zhang X, Wang M, Wu YL, Chen X. Combinatorial nano-bio interfaces. ACS Nano. 2018;12(6):5078–5084. doi: 10.1021/acsnano.8b03285. [DOI] [PubMed] [Google Scholar]
- Challen R, Brooks-Pollock E, Read JM, Dyson L, Tsaneva-Atanasova K, Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021;372:2–3. doi: 10.1136/bmj.n579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell MC. Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am J Physiol Heart Circ Physiol. 2016;310(2):H137–H152. doi: 10.1152/ajpheart.00618.2015.(AmericanPhysiologicalSociety). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell MC, Marshall AC, Alzayadneh EM, Shaltout HA, Diz DI. Update on the angiotensin converting enzyme 2-angiotensin (1–7)-Mas receptor axis: fetal programing, sex differences, and intracellular pathways. Front Endocrinol. 2014 doi: 10.3389/fendo.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, Tang P, Hasan MR, Malek JA, Coyle P, Ayoub HH, Al Kanaani Z, Al Kuwari E, Jeremijenko A, Kaleeckal AH, Latif AN, Shaik RM, Abdul Rahim HF, Nasrallah GK, Al Kuwari MG, Al Romaihi HE, Abu-Raddad LJ. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.3.5.1 variants and severe COVID-19 disease in Qatar. Nat Med. 2021;27(9):1614–1621. doi: 10.1038/s41591-021-01446-y. [DOI] [PubMed] [Google Scholar]
- Chinese Clinical Trial Register (ChiCTR)—The world health organization international clinical trials registered organization registered platform (2021) http://www.chictr.org.cn/showprojen.aspx?proj=52227. Accessed 30 Mar 2021
- Chinese COVID-19 vaccine candidate the first to start phase 3 clinical trials worldwide—Global Times (2021) https://www.globaltimes.cn/content/1192598.shtml. Accessed 30 Mar 2021
- Cirillo N. Reported orofacial adverse effects of COVID-19 vaccines: the knowns and the unknowns. J Oral Pathol Med. 2021;50(4):424–427. doi: 10.1111/jop.13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical Trial of Efficacy and Safety of Sinovac’s Adsorbed COVID-19 (Inactivated) Vaccine in Healthcare Professionals—Full Text View—ClinicalTrials.gov. (2021) https://clinicaltrials.gov/ct2/show/NCT04456595. Accessed 30 Mar 2021
- Conforti A, Marra E, Roscilli G, Palombo F, Ciliberto G, Aurisicchio L. Are genetic vaccines the right weapon against COVID-19? Mol Ther. 2020;28(7):1555–1556. doi: 10.1016/j.ymthe.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett K, Edwards D, Leist S, Abiona O, Boyoglu-Barnum S, Gillespie R, Himansu S, Schäfer A, Ziwawo C, DiPiazza A, Dinnon K, Elbashir S, Shaw C, Woods A, Fritch E, Martinez D, Bock K, Minai M, Nagata B, Graham B (2020a) SARS-CoV-2 mRNA vaccine development enabled by prototype pathogen preparedness. BioRxiv : The Preprint Server for Biology, 2020.06.11.145920. Doi: 10.1101/2020.06.11.145920
- Corbett KS, Flynn B, Foulds KE, Francica JR, Boyoglu-Barnum S, Werner AP, Flach B, O’Connell S, Bock KW, Minai M, Nagata BM, Andersen H, Martinez DR, Noe AT, Douek N, Donaldson MM, Nji NN, Alvarado GS, Edwards DK, Graham BS. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med. 2020;383(16):1544–1555. doi: 10.1056/nejmoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covid-19 Latest News: Sinopharm Virus Vaccine Safe in Testing—Bloomberg (2021) https://www.bloomberg.com/news/articles/2020-06-28/sinopharm-says-2nd-covid-vaccine-found-to-be-safe-in-testing. Accessed 30 Mar 2021
- COVID-19 Vaccine (ChAdOx1 nCoV-19) Trial in South African Adults With and Without HIV-infection—Full Text View—ClinicalTrials.gov. (2021) https://clinicaltrials.gov/ct2/show/NCT04444674. Accessed 30 Mar 2021
- D’agostino V, Caranci F, Negro A, Piscitelli V, Tuccillo B, Fasano F, Sirabella G, Marano I, Granata V, Grassi R, Pupo D, Grassi R, Buonaguro FM (2021) Personalized medicine case report a rare case of cerebral venous thrombosis and disseminated intravascular coagulation temporally associated to the COVID-19 vaccine administration. J Pers Med [DOI] [PMC free article] [PubMed]
- Dashraath P, Wong JLJ, Lim MXK, Lim LM, Li S, Biswas A, Choolani M, Mattar C, Su LL. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol. 2020;222(6):521–531. doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauphin G, Zientara S. West Nile virus: recent trends in diagnosis and vaccine development. Vaccine. 2007;25(30):5563–5576. doi: 10.1016/j.vaccine.2006.12.005. [DOI] [PubMed] [Google Scholar]
- de Alwis R, Gan ES, Chen S, Shan Leong Y, Cheng Tan H, Zhang SL, Yau C, Matsuda D, Allen E, Hartman P, Alayyoubi M, Bhaskaran H, Dukanovic A, Bao B, Vega J, Roberts S, Gonzalez JA, Sablad M, Taylor W, Eong Ooi E (2020) A single dose of self-transcribing and replicating RNA based SARS-CoV-2 1 vaccine produces protective adaptive immunity. Mice. 2 3. BioRxiv, 2020.09.03.280446. Doi: 10.1101/2020.09.03.280446
- Dicks MDJ, Spencer AJ, Edwards NJ, Wadell G, Bojang K, Gilbert SC, Hill AVS, Cottingham MG. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLoS ONE. 2012 doi: 10.1371/journal.pone.0040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do TC, Konig J, Hochfelder J, Siegel S, Gans M. Polyethylene glycol and polysorbate testing in twelve patients prior to or after COVID-19 vaccine administration. Ann Allergy Asthma Immunol. 2021 doi: 10.1016/j.anai.2021.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erasmus JH, Khandhar AP, Walls AC, Hemann EA, O’Connor MA, Murapa P, Archer J, Leventhal S, Fuller J, Lewis T, Draves KE, Randall S, Guerriero KA, Duthie MS, Carter D, Reed SG, Hawman DW, Feldmann H, Gale M, Fuller DH (2020) Single-dose replicating RNA vaccine induces neutralizing antibodies against SARS-CoV-2 in nonhuman primates. In bioRxiv (p. 2020.05.28.121640). bioRxiv. 10.1101/2020.05.28.121640
- Fatima S, Kumari A, Das G, Dwivedi VP. Tuberculosis vaccine: a journey from BCG to present. Life Sci. 2020;252:117594. doi: 10.1016/j.lfs.2020.117594. [DOI] [PubMed] [Google Scholar]
- Fausther-Bovendo H, Kobinger GP. Pre-existing immunity against Ad vectors: humoral, cellular, and innate response, what’s important? Hum Vaccines Immunother. 2014;10(10):2875–2884. doi: 10.4161/hv.29594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioretti D, Iurescia S, Rinaldi M. Recent advances in design of immunogenic and effective naked DNA vaccines against cancer. Recent Pat Anti-Cancer Drug Discov. 2014;9(1):66–82. doi: 10.2174/1574891X113089990037. [DOI] [PubMed] [Google Scholar]
- Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, Dold C, Faust SN, Finn A, Flaxman AL, Hallis B, Heath P, Jenkin D, Lazarus R, Makinson R, Yau Y. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanet A, Autran B, Lina B, Kieny MP, Karim SSA, Sridhar D. SARS-CoV-2 variants and ending the COVID-19 pandemic. Lancet. 2021;397(10278):952–954. doi: 10.1016/S0140-6736(21)00370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, Li Y, Zhu L, Wang N, Lv Z, Gao H, Ge X, Kan B, Hu Y, Liu J, Cai F, Jiang D, Yin Y, Qin C, Qin C. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey LH, Nasser S. Anaphylaxis to the first COVID-19 vaccine: is polyethylene glycol (PEG) the culprit? Br J Anaesth. 2021;126(3):e106–e108. doi: 10.1016/j.bja.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geall AJ, Verma A, Otten GR, Shaw CA, Hekele A, Banerjee K, Cu Y, Beard CW, Brito LA, Krucker T, O’Hagan DT, Singh M, Mason PW, Valiante NM, Dormitzer PR, Barnett SW, Rappuoli R, Ulmer JB, Mandl CW. Nonviral delivery of self-amplifying RNA vaccines. Proc Natl Acad Sci USA. 2012;109(36):14604–14609. doi: 10.1073/pnas.1209367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers D, Shamier MC, Bogers S, den Hartog G, Gommers L, Nieuwkoop NN, Schmitz KS, Rijsbergen LC, van Osch JAT, Dijkhuizen E, Smits G, Comvalius A, van Mourik D, Caniels TG, van Gils MJ, Sanders RW, Oude Munnink BB, Molenkamp R, de Jager HJ, GeurtsvanKessel CH. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol. 2021;6(59):1–15. doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BS. Rapid COVID-19 vaccine development. Science. 2020;368(6494):945–946. doi: 10.1126/science.abb8923. [DOI] [PubMed] [Google Scholar]
- Graham RL, Donaldson EF, Baric RS. A decade after SARS: strategies for controlling emerging coronaviruses. Nat Rev Microbiol. 2013;11(12):836–848. doi: 10.1038/nrmicro3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham SP, McLean RK, Spencer AJ, Belij-Rammerstorfer S, Wright D, Ulaszewska M, Edwards JC, Hayes JWP, Martini V, Thakur N, Conceicao C, Dietrich I, Shelton H, Waters R, Ludi A, Wilsden G, Browning C, Bialy D, Bhat S, Lambe T (2020) Evaluation of the immunogenicity of prime-boost vaccination with the replication-deficient viral vectored COVID-19 vaccine candidate ChAdOx1 nCoV-19. In: bioRxiv (p. 2020.06.20.159715). bioRxiv. 10.1101/2020.06.20.159715 [DOI] [PMC free article] [PubMed]
- Grifoni A, Sidney J, Zhang Y, Scheuermann RH, Peters B, Sette A. A sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host Microbe. 2020;27(4):671–680.e2. doi: 10.1016/j.chom.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubaugh ND, Hanage WP, Rasmussen AL. Making sense of mutation: what D614G means for the COVID-19 pandemic remains unclear. Cell. 2020;182(4):794–795. doi: 10.1016/j.cell.2020.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasnie AA, Hasnie UA, Patel N, Aziz MU, Xie M, Lloyd SG, Prabhu SD. Perimyocarditis following first dose of the mRNA-1273 SARS-CoV-2 (Moderna) vaccine in a healthy young male: a case report. BMC Cardiovasc Disord. 2021;21(1):1–6. doi: 10.1186/s12872-021-02183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath PT, Eva Galiza FP, David Neil Baxter M, Boffito M, Browne D, Burns F, Chadwick DR, Clark R, Catherine Cosgrove MBC, Galloway J, Goodman AL, Heer A, Andrew Higham MBC, Iyengar S, Arham Jamal M, Christopher Jeanes M, Philip Kalra MA, Kyriakidou C, McAuley DF, Minton J (2021) Efficacy of the NVX-CoV2373 Covid-19 vaccine against the B117 variant. MedRxiv, 2021.05.13.21256639. 10.1101/2021.05.13.21256639
- Hengge UR, Chan EF, Foster RA, Walker PS, Vogel JC. Cytokine gene expression in epidermis with biological effects following injection of naked DNA. Nat Genet. 1995;10(2):161–166. doi: 10.1038/ng0695-161. [DOI] [PubMed] [Google Scholar]
- Hiller N, Goldberg SN, Cohen-Cymberknoh M, Vainstein V, Simanovsky N. Lymphadenopathy associated with the COVID-19 vaccine. Cureus. 2021 doi: 10.7759/cureus.13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobernik D, Bros M. DNA vaccines—how far from clinical use? Int J Mol Sci. 2018;19(11):3605. doi: 10.3390/ijms19113605MDPIAG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Krüger N, Müller M, Drosten C, Pöhlmann S (2020a) The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv (p. 2020.01.31.929042). bioRxiv. 10.1101/2020.01.31.929042
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba T, Levy JH, Warkentin TE. Recognizing vaccine-induced immune thrombotic thrombocytopenia. Crit Care Med. 2021 doi: 10.1097/ccm.0000000000005211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim IM, Abdelmalek DH, Elshahat ME, Elfiky AA. COVID-19 spike-host cell receptor GRP78 binding site prediction. J Infect. 2020;80(5):554–562. doi: 10.1016/j.jinf.2020.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indonesia Testing Location for China’s Sinovac Phase 3 Clinical Trial (2021) https://trialsitenews.com/indonesia-testing-location-for-chinas-sinovac-phase-3-clinical-trial/. Accessed 30 Mar 2021
- Investigating a Vaccine Against COVID-19—Full Text View—ClinicalTrials.gov. (2021) https://clinicaltrials.gov/ct2/show/study/NCT04400838. Accessed 30 Mar 2021
- Izda V, Jeffries MA, Sawalha AH. COVID-19: a review of therapeutic strategies and vaccine candidates. Clin Immunol. 2021 doi: 10.1016/j.clim.2020.108634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott A, Flach B, Doria-Rose NA, Corbett KS, Morabito KM, O’Dell S, Schmidt SD, Swanson PA, Beigel JH. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Takami Y, Kumar Deo V, Park EY. Preparation of virus-like particle mimetic nanovesicles displaying the S protein of Middle East respiratory syndrome coronavirus using insect cells. J Biotechnol. 2019;306:177–184. doi: 10.1016/j.jbiotec.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, Plested JS, Zhu M, Cloney-Clark S, Zhou H, Smith G, Patel N, Frieman MB, Haupt RE, Logue J, McGrath M, Weston S, Piedra PA, Desai C, Glenn GM. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle. Vaccine. 2020;383(24):2320–2332. doi: 10.1056/NEJMOA2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana A, Allawadhi P, Khurana I, Allwadhi S, Weiskirchen R, Banothu AK, Chhabra D, Joshi K, Bharani KK. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today. 2021;38:101142. doi: 10.1016/J.NANTOD.2021.101142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klomjit N, Alexander MP, Fervenza FC, Zoghby Z, Garg A, Hogan MC, Nasr SH, Minshar MA, Zand L. COVID-19 vaccination and glomerulonephritis. Kidney Int Rep. 2021 doi: 10.1016/J.EKIR.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, Foley B, Giorgi EE, Bhattacharya T, Parker MD, Partridge DG, Evans CM, Freeman TM, de Silva TI, LaBranche CC, Montefiori DC (2020) Spike mutation pipeline reveals the emergence of a more transmissible form of SARS-CoV-2. In bioRxiv. Vol. 4, p. 2020.04.29.069054. bioRxiv. Doi: 10.1101/2020.04.29.069054
- Kumar A, Dowling WE, Román RG, Chaudhari A, Gurry C, Le TT, Tollefson S, Clark CE, Bernasconi V, Kristiansen PA. Status report on COVID-19 vaccines development. Curr Infect Dis Rep. 2021 doi: 10.1007/s11908-021-00752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam JH, Khan AK, Cornell TA, Dress RJ, Chia TW, Yeow WWW, Mohd-Ismail NK, Venkatraman S, Ng KT, Tan Y-J, Anderson DE, Ginhoux F, Nallani M (2021) Next generation vaccine platform: polymersomes as stable nanocarriers for a highly immunogenic and durable SARS-CoV-2 spike protein subunit vaccine. BioRxiv, 2021.01.24.427729. Doi: 10.1101/2021.01.24.427729 [DOI] [PubMed]
- Lan J, Yao Y, Deng Y, Chen H, Lu G, Wang W, Bao L, Deng W, Wei Q, Gao GF, Qin C, Tan W. Recombinant receptor binding domain protein induces partial protective immunity in rhesus macaques against middle east respiratory syndrome coronavirus challenge. EBioMedicine. 2015;2(10):1438–1446. doi: 10.1016/j.ebiom.2015.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledford H. How could a COVID vaccine cause blood clots? Scientists race to investigate. Nature. 2021;592(7854):334–335. doi: 10.1038/d41586-021-00940-0. [DOI] [PubMed] [Google Scholar]
- Lei C, Qian K, Li T, Zhang S, Fu W, Ding M, Hu S. Neutralization of SARS-CoV-2 spike pseudotyped virus by recombinant ACE2-Ig. Nat Commun. 2020;11(1):1–5. doi: 10.1038/s41467-020-16048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, Su X, Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395(10235):1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim XR, Leung BP, Ng CYL, Tan JWL, Chan GYL, Loh CM, Tan GLX, Goh VHH, Wong LT, Chua CR, Tan SC, Lee SSM, Howe HS, Thong BYH, Leong KP. Pseudo-anaphylactic reactions to pfizer bnt162b2 vaccine: report of 3 cases of anaphylaxis post pfizer bnt162b2 vaccination. Vaccines. 2021;9(9):3–9. doi: 10.3390/vaccines9090974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht C. Vaccines against COVID-19. Anaesth Crit Care Pain Med. 2020;39(6):703–705. doi: 10.1016/j.accpm.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, Kovyrshina AV, Lubenets NL, Grousova DM, Erokhova AS, Botikov AG, Izhaeva FM, Popova O, Ozharovskaya TA, Esmagambetov IB, Favorskaya IA, Zrelkin DI, Voronina DV, Shcherbinin DN, Gintsburg AL. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, Myers R, Campbell CNJ, Amirthalingam G, Edmunds M, Zambon M, Brown KE, Hopkins S, Chand M, Ramsay M. Effectiveness of covid-19 vaccines against the B16172 (Delta) variant. N Engl J Med. 2021;385(7):585–594. doi: 10.1056/nejmoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchese G. Epitopes for a 2019-nCoV vaccine. Cell Mol Immunol. 2020;17(5):539–540. doi: 10.1038/s41423-020-0377-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Zou F, Yu F, Li R, Yuan Y, Zhang Y, Zhang X, Deng J, Chen T, Song Z, Qiao Y, Zhan Y, Liu J, Zhang J, Zhang X, Peng Z, Li Y, Lin Y, Liang L, Zhang H. Nanoparticle vaccines based on the receptor binding domain (RBD) and heptad repeat (HR) of SARS-CoV-2 elicit robust protective immune responses. Immunity. 2020;53(6):1315–1330.e9. doi: 10.1016/J.IMMUNI.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil JR, Su JR, Broder KR, Guh AY, Gargano JW, Wallace M, Hadler SC, Scobie HM, Blain AE, Moulia D, Daley MF, McNally VV, Romero JR, Talbot HK, Lee GM, Bell BP, Oliver SE. Updated recommendations from the advisory committee on immunization practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(17):651–656. doi: 10.15585/mmwr.mm7017e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E. AstraZeneca vaccine: blood clots are “extremely rare” and benefits outweigh risks, regulators conclude. BMJ (clin Res Ed) 2021;373:n931. doi: 10.1136/bmj.n931. [DOI] [PubMed] [Google Scholar]
- Makhoul M, Ayoub HH, Chemaitelly H, Seedat S, Mumtaz GR, Al-Omari S, Abu-Raddad LJ (2020) Epidemiological impact of SARS-CoV-2 vaccination: mathematical modeling analyses. medRxiv (p. 2020.04.19.20070805). Doi: 10.1101/2020.04.19.20070805 [DOI] [PMC free article] [PubMed]
- Mallapaty S. India’s DNA COVID vaccine is a world first—more are coming. Nature. 2021;597(7875):161–162. doi: 10.1038/D41586-021-02385-X. [DOI] [PubMed] [Google Scholar]
- Malone RW, Felgner PL, Verma IM. Cationic liposome-mediated RNA transfection. Proc Natl Acad Sci USA. 1989;86(16):6077–6081. doi: 10.1073/pnas.86.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino M, Scuderi F, Provenzano C, Bartoccioni E. Skeletal muscle cells: from local inflammatory response to active immunity. Gene Ther. 2011;18(2):109–116. doi: 10.1038/gt.2010.124. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Udelson JE, Bonow RO, Nishimura RA, Ackerman MJ, Estes NAM, Cooper LT, Link MS, Maron MS. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the advisory committee on immunization practices—United States, June 2021. Morb Mortal Wkly Rep Use. 2021;132(22):e273–e280. doi: 10.1161/CIR.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslow JN. Vaccines for emerging infectious diseases: lessons from MERS coronavirus and Zika virus. Hum Vaccin Immunother. 2017;13(12):2918–2930. doi: 10.1080/21645515.2017.1358325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarneh AS, Al-battah AH, Farooqui K, Ghamoodi M, Alhatou M. COVID-19 vaccine causing Guillain-Barre syndrome, a rare potential side effect. Clin Case Rep. 2021;9(9):1–5. doi: 10.1002/ccr3.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay PF, Hu K, Blakney AK, Samnuan K, Brown JC, Penn R, Zhou J, Bouton CR, Rogers P, Polra K, Lin PJC, Barbosa C, Tam YK, Barclay WS, Shattock RJ. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat Commun. 2020;11(1):1–7. doi: 10.1038/s41467-020-17409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean K, Johnson TJ. Myopericarditis in a previously healthy adolescent male following COVID-19 vaccination: a case report. Acad Emerg Med. 2021;28(8):918–921. doi: 10.1111/acem.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta N, Sales RM, Babagbemi K, Levy AD, McGrath AL, Drotman M, Dodelzon K. Unilateral axillary adenopathy in the setting of COVID-19 vaccine. Clin Imaging. 2021;75:12–15. doi: 10.1016/j.clinimag.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merante D. SARS-CoV-2, from its current highly contagious spreading toward the global development of an effective and safe vaccine: challenges and uncertainties. Expert Opin Drug Saf. 2020;19(7):771–774. doi: 10.1080/14740338.2020.1773789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor PD. Live attenuated vaccines: historical successes and current challenges. Virology. 2015;479–480:379–392. doi: 10.1016/j.virol.2015.03.032. [DOI] [PubMed] [Google Scholar]
- Mohseni Afshar Z, Babazadeh A, Janbakhsh A, Afsharian M, Saleki K, Barary M, Ebrahimpour S. Vaccine-induced immune thrombotic thrombocytopenia after vaccination against Covid-19: a clinical dilemma for clinicians and patients. Rev Med Virol. 2021 doi: 10.1002/rmv.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyo-Gwete T, Madzivhandila M, Makhado Z, Ayres F, Mhlanga D, Oosthuysen B, Lambson BE, Kgagudi P. Cross-reactive neutralizing antibody responses elicited by SARS-CoV-2 501Y.V2 (B.1.351) N Engl J Med. 2021;384(22):2159–2161. doi: 10.1056/nejmc2100365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucker EM, Karmali PP, Vega J, Kwilas SA, Wu H, Joselyn M, Ballantyne J, Sampey D, Mukthavaram R, Sullivan E, Chivukula P, Hooper JW. Lipid nanoparticle formulation increases efficiency of DNA-vectored vaccines/immunoprophylaxis in animals including transchromosomic bovines. Sci Rep. 2020;10(1):1–13. doi: 10.1038/s41598-020-65059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufamadi MS. Nanotechnology shows promise for next-generation vaccines in the fight against COVID-19. MRS Bull. 2020;45(12):981–982. doi: 10.1557/MRS.2020.307. [DOI] [Google Scholar]
- Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Raabe V, Bailey R, Swanson KA, Li P, Koury K, Kalina W, Cooper D, Fontes-Garfias C, Shi PY, Türeci Ö, Tompkins KR, Walsh EE, Jansen KU. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586(7830):589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart SP, Neuzil K, Raabe V, Bailey R, Swanson KA, Li P, Koury K, Kalina W, Cooper D, Fontes-Garfias C, Shi PY, Türeci Ö, Tompkins KR, Walsh EE, Jansen KU (2020b) Phase 1/2 study to describe the safety and immunogenicity of a COVID-19 RNA vaccine candidate (BNT162b1) in adults 18 to 55 years of age: Interim report. medRxiv (p. 2020.06.30.20142570). Doi: 10.1101/2020.06.30.20142570
- Özütemiz C, Krystosek LA, Church AL, Chauhan A, Ellermann JM, Domingo-Musibay E, Steinberger D. Lymphadenopathy in COVID-19 vaccine recipients: diagnostic dilemma in oncologic patients. Radiology. 2021;300(1):E290–E294. doi: 10.1148/radiol.2021210275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, Dowd KA, Sutherland LL, Scearce RM, Parks R, Wagner W, Granados A, Greenhouse J, Walker M, Willis E, Yu JS, McGee CE, Sempowski GD, Mui BL, Weissman D. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543(7644):248–251. doi: 10.1038/nature21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines-a new era in vaccinology. Nat Rev Drug Dis. 2018;17(4):261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KS, Sun X, Aikins ME, Moon JJ. Non-viral COVID-19 vaccine delivery systems. Adv Drug Deliv Rev. 2021;169:137–151. doi: 10.1016/J.ADDR.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrignani A, Schicchi N, Calcagnoli F, Falchetti E, Ciampani N, Argalia G, Mariani A. Acute myocarditis following Comirnaty vaccination in a healthy man with previous SARS-CoV-2 infection. Radiol Case Rep. 2021;16(11):3321–3325. doi: 10.1016/j.radcr.2021.07.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegs S, Interestingly G. COVID-19 vaccine anaphylaxis : IgE, complement or what else ? A reply to: “COVID-19 vaccine anaphylaxis: PEG or not ? Allergy. 2021 doi: 10.1111/all.14725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petsch B, Schnee M, Vogel AB, Lange E, Hoffmann B, Voss D, Schlake T, Thess A, Kallen KJ, Stitz L, Kramps T. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat Biotechnol. 2012;30(12):1210–1216. doi: 10.1038/nbt.2436. [DOI] [PubMed] [Google Scholar]
- Phase I/II study of intracutaneous inoculation of COVID-19 DNA vaccine (AG0302-COVID19) (2021) https://jrct.niph.go.jp/en-latest-detail/jRCT2051200085. Accessed 30 Mar 2021
- Phase II/III Study of COVID-19 DNA Vaccine (AG0302-COVID19)—Full Text View—ClinicalTrials.gov. (2021) https://www.clinicaltrials.gov/ct2/show/NCT04655625. Access 30 Mar 2021
- Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, Planchais C, Porrot F, Robillard N, Puech J, Prot M, Gallais F, Gantner P, Velay A, Le Guen J, Kassis-Chikhani N, Edriss D, Belec L, Seve A, Schwartz O. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596(7871):276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396(10262):1595–1606. doi: 10.1016/S0140-6736(20)32137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porgador A, Irvine KR, Iwasaki A, Barber BH, Restifo NP, Germain RN. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J Exp Med. 1998;188(6):1075–1082. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell AE, Zhang K, Sanyal M, Tang S, Weidenbacher PA, Li S, Pham TD, Pak JE, Chiu W, Kim PS. A Single immunization with spike-functionalized ferritin vaccines elicits neutralizing antibody responses against SARS-CoV-2 in mice. ACS Cent Sci. 2021;7(1):183–199. doi: 10.1021/ACSCENTSCI.0C01405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prazeres DMF, Monteiro GA. Plasmid biopharmaceuticals. Microbiol Spectr. 2014 doi: 10.1128/microbiolspec.plas-0022-2014. [DOI] [PubMed] [Google Scholar]
- Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526(1):135–140. doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L, Chen M, Li S, Hu J, Gong C, Zhang Z, Cao X. A peptide-based subunit candidate vaccine against SARS-CoV-2 delivered by biodegradable mesoporous silica nanoparticles induced high humoral and cellular immunity in mice. Biomater Sci. 2021 doi: 10.1039/D1BM01060C. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Zhao YB, Wang Q, Li JY, Zhou ZJ, Liao CH, Ge XY. Predicting the angiotensin converting enzyme 2 (ACE2) utilizing capability as the receptor of SARS-CoV-2. Microbes Infect. 2020;22(4–5):221–225. doi: 10.1016/j.micinf.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuwanshi D, Mishra V, Das D, Kaur K, Suresh MR. Dendritic cell targeted chitosan nanoparticles for nasal DNA immunization against SARS CoV nucleocapsid protein. Mol Pharm. 2012;9(4):946–956. doi: 10.1021/mp200553x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda LB, Netland J, Shehata L, Pruner KB, Morawski PA, Thouvenel CD, Takehara KK, Eggenberger J, Hemann EA, Waterman HR, Fahning ML, Chen Y, Hale M, Rathe J, Stokes C, Wrenn S, Fiala B, Carter L, Hamerman JA, Pepper M. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell. 2021;184(1):169–183.e17. doi: 10.1016/j.cell.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottinghaus ST, Poland GA, Jacobson RM, Barr LJ, Roy MJ. Hepatitis B DNA vaccine induces protective antibody responses in human non-responders to conventional vaccination. Vaccine. 2003;21(31):4604–4608. doi: 10.1016/S0264-410X(03)00447-X. [DOI] [PubMed] [Google Scholar]
- Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B, Offergeld K. Safety and efficacy of single-dose Ad26COV2S vaccine against covid-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMOA2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiblhofer S, Thalhamer J, Weiss R. DNA and mRNA vaccination against allergies. Pediatr Allergy Immunol. 2018;29(7):679–688. doi: 10.1111/pai.12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar A, Campbell R, Tabbara J, Rastogi P. ANCA glomerulonephritis after the Moderna COVID-19 vaccination. Kidney Int. 2021;100(2):473–474. doi: 10.1016/J.KINT.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellaturay P, Nasser S, Islam S, Gurugama P, Ewan PW. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin Exp Allergy. 2021;51(6):861–863. doi: 10.1111/cea.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahcheraghi SH, Ayatollahi J, Aljabali AA, Shastri MD, Shukla SD, Chellappan DK, Jha NK, Anand K, Katari NK, Mehta M, Satija S, Dureja H, Mishra V, Almutary AG, Alnuqaydan AM, Charbe N, Prasher P, Gupta G, Dua K, Tambuwala MM. An overview of vaccine development for COVID-19. Ther Deliv. 2021;12(3):235–244. doi: 10.4155/tde-2020-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoor MT, Birkenbach MP, Lynch M. ANCA-associated vasculitis following pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021;78(4):611–613. doi: 10.1053/j.ajkd.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma O, Sultan AA, Ding H, Triggle CR. A review of the progress and challenges of developing a vaccine for COVID-19. Front Immunol. 2020;11:585354. doi: 10.3389/fimmu.2020.585354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh AB, Pal S, Javed N, Shekhar R. COVID-19 vaccination in developing nations: challenges and opportunities for innovation. Infect Dis Rep. 2021;13(2):429–436. doi: 10.3390/IDR13020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R, Shan C, Duan X, Chen Z, Liu P, Song J, Song T, Bi X, Han C, Wu L, Gao G, Hu X, Zhang Y, Tong Z, Huang W, Liu WJ, Wu G, Zhang B, Wang L, Yan J. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature. 2020;584(7819):120–124. doi: 10.1038/s41586-020-2381-y. [DOI] [PubMed] [Google Scholar]
- Shimabukuro T. Allergic reactions including anaphylaxis after receipt of the first dose of moderna COVID-19 vaccine—United States, December 21, 2020–January 10, 2021. Am J Transplant. 2021;21(3):1332–1337. doi: 10.1111/ajt.16516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro T. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14–23, 2020. Am J Transplant. 2021;21(3):1332–1337. doi: 10.1111/ajt.16516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MD, Shukla S, Chung YH, Beiss V, Chan SK, Ortega-Rivera OA, Wirth DM, Chen A, Sack M, Pokorski JK, Steinmetz NF. COVID-19 vaccine development and a potential nanomaterial path forward. Nat Nanotechnol. 2020;15(8):646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- Si L, Xu H, Zhou X, Zhang Z, Tian Z, Wang Y, Wu Y, Zhang B, Niu Z, Zhang C, Fu G, Xiao S, Xia Q, Zhang L, Zhou D. Generation of influenza A viruses as live but replication-incompetent virus vaccines. Science. 2016;354(6316):1170–1173. doi: 10.1126/science.aah5869. [DOI] [PubMed] [Google Scholar]
- Siegler JE, Klein P, Yaghi S, Vigilante N, Abdalkader M, Coutinho JM, Abdul Khalek F, Nguyen TN. Cerebral vein thrombosis with vaccine-induced immune thrombotic thrombocytopenia. Stroke. 2021 doi: 10.1161/STROKEAHA.121.035613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Mehta S. The clinical development process for a novel preventive vaccine: an overview. J Postgrad Med. 2016;62(1):4. doi: 10.4103/0022-3859.173187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinovac says its Covid-19 vaccine generated immune responses—STAT (2021) https://www.statnews.com/2020/06/14/sinovac-early-data-covid19-vaccine-generated-immune-responses/. Accessed 30 Mar 2021
- Snapiri O, Rosenberg Danziger C, Shirman N, Weissbach A, Lowenthal A, Ayalon I, Adam D, Yarden-Bilavsky H, Bilavsky E. Transient cardiac injury in adolescents receiving the BNT162b2 mRNA COVID-19 vaccine. Pediatr Infect Dis J. 2021;40(10):E360–E363. doi: 10.1097/INF.0000000000003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofi-Mahmudi A. Orofacial adverse effects of COVID-19 vaccines exist but are rare. Evid Based Dent. 2021;22(2):70–71. doi: 10.1038/s41432-021-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson KE, Le Gars M, Sadoff J, De Groot AM, Heerwegh D, Truyers C, Atyeo C, Loos C, Chandrashekar A, McMahan K, Tostanoski LH, Yu J, Gebre MS, Jacob-Dolan C, Li Z, Patel S, Peter L, Liu J, Borducchi EN, Barouch DH. Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. JAMA J Am Med Assoc. 2021;325(15):1535–1544. doi: 10.1001/JAMA.2021.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claër L, Quentric P, Fadlallah J, Devilliers H, Ghillani P, Gunn C, Hockett R, Mudumba S, Guihot A, Luyt CE, Mayaux J, Beurton A, Fourati S, Bruel T, Gorochov G. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021;13(577):1–11. doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su QD, Yi Y, Zou YN, Jia ZY, Qiu F, Wang F, Yin WJ, Zhou WT, Zhang S, Yu PC, Bi SL, Shen LP, Wu GZ. The biological characteristics of SARS-CoV-2 spike protein Pro330-Leu650. Vaccine. 2020;38(32):5071–5075. doi: 10.1016/j.vaccine.2020.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatlow D, Tatlow C, Tatlow S, Tatlow S. A novel concept for treatment and vaccination against Covid-19 with an inhaled chitosan-coated DNA vaccine encoding a secreted spike protein portion. Clin Exp Pharmacol Physiol. 2020;47(11):1874–1878. doi: 10.1111/1440-1681.13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thao TT, Labroussaa F, Ebert N, V’kovski P, Stalder H, Portmann J, Kelly J, Steiner S, Holwerda M, Kratzel A, Gultom M, Schmied K, Laloli L, Hüsser L, Wider M, Pfaender S, Hirt D, Cippà V, Crespo-Pomar S, Thiel V. Rapid reconstruction of SARS-CoV-2 using a synthetic genomics platform. Nature. 2020;582(7813):561–565. doi: 10.1038/s41586-020-2294-9. [DOI] [PubMed] [Google Scholar]
- Theobald N. Emerging vaccine delivery systems for COVID-19: functionalised silica nanoparticles offer a potentially safe and effective alternative delivery system for DNA/RNA vaccines and may be useful in the hunt for a COVID-19 vaccine. Drug Discov Today. 2020;25(9):1556. doi: 10.1016/J.DRUDIS.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafyllidis KK, Giannos P, Mian IT, Kyrtsonis G, Kechagias KS. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines. 2021;9(9):1013. doi: 10.3390/VACCINES9091013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triggle CR, Bansal D, Farag EABA, Ding H, Sultan AA. COVID-19: learning from lessons to guide treatment and prevention interventions. Msphere. 2020 doi: 10.1128/msphere.00317-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse LV, Meganck RM, Graham RL, Baric RS. The current and future state of vaccines, antivirals and gene therapies against emerging coronaviruses. Front Microbiol. 2020;11:658. doi: 10.3389/fmicb.2020.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Mustafa F, Rizvi TA, Loney T, Al Suwaidi H, Al-Marzouqi AHH, Kamal Eldin A, Alsabeeha N, Adrian TE, Stefanini C, Nowotny N, Alsheikh-Ali A, Senok AC. SARS-CoV-2/COVID-19: viral genomics, epidemiology, vaccines, and therapeutic interventions. Viruses. 2020;12(5):526. doi: 10.3390/v12050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Understanding Viral Vector COVID-19 Vaccines|CDC (2021) https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/viralvector.html. Accessed 30 Mar 2021
- Vignuzzi M, Wendt E, Andino R. Engineering attenuated virus vaccines by controlling replication fidelity. Nat Med. 2008;14(2):154–161. doi: 10.1038/nm1726. [DOI] [PubMed] [Google Scholar]
- Vogel G. Israel reports link between rare cases of heart inflammation and COVID-19 vaccination in young men. Science. 2021 doi: 10.1126/science.abj7796. [DOI] [Google Scholar]
- Waheed S, Bayas A, Hindi F, Rizvi Z, Espinosa PS. Neurological complications of COVID-19: guillain-barre syndrome following pfizer COVID-19 vaccine. Cureus. 2021;13(Cdc):2–5. doi: 10.7759/cureus.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajnberg A, Amanat F, Firpo A, Altman DR, Bailey MJ, Mansour M, McMahon M, Meade P, Mendu DR, Muellers K, Stadlbauer D, Stone K, Strohmeier S, Simon V, Aberg J, Reich DL, Krammer F, Cordon-Cardo C. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls AC, Fiala B, Schäfer A, Wrenn S, Pham MN, Murphy M, Tse LV, Shehata L, O’Connor MA, Chen C, Navarro MJ, Miranda MC, Pettie D, Ravichandran R, Kraft JC, Ogohara C, Palser A, Chalk S, Lee EC, King NP. Elicitation of potent neutralizing antibody responses by designed protein nanoparticle vaccines for SARS-CoV-2. Cell. 2020;183(5):1367–1382.e17. doi: 10.1016/J.CELL.2020.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh EE, Frenck R, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, Swanson KA, Li P, Koury K, Kalina W, Cooper D, Fontes-Garfias C, Shi PY, Türeci Z, Tompkins KR, Gruber WC (2020) RNA-based COVID-19 vaccine BNT162b2 selected for a pivotal efficacy study. medRxiv (p. 2020.08.17.20176651). Doi: 10.1101/2020.08.17.20176651
- Wang C, Li W, Drabek D, Okba NMA, van Haperen R, Osterhaus ADME, van Kuppeveld FJM, Haagmans BL, Grosveld F, Bosch BJ. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun. 2020;11(1):1–6. doi: 10.1038/s41467-020-16256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang Y, Huang B, Deng W, Quan Y, Wang W, Xu W, Zhao Y, Li N, Zhang J, Liang H, Bao L, Xu Y, Ding L, Zhou W, Gao H, Liu J, Niu P, Zhao L, Yang X. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020;182(3):713–721.e9. doi: 10.1016/j.cell.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Liu L, Nair MS, Yin MT, Luo Y, Wang Q, Yuan T, Mori K, Solis AG, Yamashita M, Garg A, Purpura LJ, Laracy JC, Yu J, Joshua-Tor L, Sodroski J, Huang Y, Ho DD. SARS-CoV-2 neutralizing antibody responses are more robust in patients with severe disease. Emerg Microbes Infect. 2020;9(1):2091–2093. doi: 10.1080/22221751.2020.1823890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Long QX, Deng HJ, Hu J, Gao QZ, Zhang GJ, He CL, Huang LY, Hu JL, Chen J, Tang N, Huang AL. Longitudinal dynamics of the neutralizing antibody response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Clin Infect Dis off Publ Infect Dis Soc Am. 2021;73(3):e531–e539. doi: 10.1093/cid/ciaa1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf D, Schmitz KS, Raadsen MP, Grifoni A, Okba NMA, Endeman H, van den Akker JPC, Molenkamp R, Koopmans MPG, van Gorp ECM, Haagmans BL, de Swart RL, Sette A, de Vries RD. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol. 2020 doi: 10.1126/SCIIMMUNOL.ABD2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Biorxiv. 2020 doi: 10.1101/2020.02.11.944462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Liu Y, Yang Y, Zhang P, Zhong W, Wang Y, Wang Q, Xu Y, Li M, Li X, Zheng M, Chen L, Li H. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10(5):766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Haung J, Wang A, Wang Q, Chen J, Xia S, Ling Y, Zhang Y, Xun J, Lu L, Jiang S, Lu H, Wen Y (2021) Neutrilizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patients cohort and their implications. Lancet
- Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, Li X, Peng C, Zhang Y, Zhang W, Yang Y, Chen W, Gao X, You W, Wang X, Wang Z, Shi Z, Wang Y, Yang X, Yang X. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA J Am Med Assoc. 2020;324(10):951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Gao G, Reyes-Sandoval A, Cohen CJ, Li Y, Bergelson JM, Wilson JM, Ertl HCJ. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J Virol. 2002;76(6):2667–2675. doi: 10.1128/jvi.76.6.2667-2675.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Muruato A, Lokugamage KG, Narayanan K, Zhang X, Zou J, Liu J, Schindewolf C, Bopp NE, Aguilar PV, Plante KS, Weaver SC, Makino S, LeDuc JW, Menachery VD, Shi PY. An infectious cDNA clone of SARS-CoV-2. Cell Host Microbe. 2020;27(5):841–848.e3. doi: 10.1016/j.chom.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger DS, Younger APJ, Guttmacher S. Childhood vaccination: implications for global and domestic public health. Neurol Clin. 2016;34(4):1035–1047. doi: 10.1016/j.ncl.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Yu F, Du L, Ojcius DM, Pan C, Jiang S. Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan, China. Microbes Infect. 2020;22(2):74–79. doi: 10.1016/j.micinf.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Wu NC, Zhu X, Lee CCD, So RTY, Lv H, Mok CKP, Wilson IA. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020;368(6491):630–633. doi: 10.1126/science.abb7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Hou X, Yan J, Zhang C, Li W, Zhao W, Du S, Dong Y. Leveraging mRNA sequences and nanoparticles to deliver SARS-CoV-2 antigens in vivo. Adv Mater. 2020;32(40):2004452. doi: 10.1002/ADMA.202004452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Nandakumar KS. Recent advances in the development of vaccines for chronic inflammatory autoimmune diseases. Vaccine. 2018;36(23):3208–3220. doi: 10.1016/j.vaccine.2018.04.062. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zeng H, Gu J, Li H, Zheng L, Zou Q. Progress and prospects on vaccine development against sars-cov-2. Vaccines. 2020 doi: 10.3390/vaccines8020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, Zeng G, Pan HX, Li CG, Kan B, Hu YL, Mao HY, Xin QQ, Chu K, Han WX, Chen Z, Tang R, Yin WD, Chen X, Gong XJ, Qin C, Hu YS, Liu XY, Cui GL, Zhu FC (2020b) Immunogenicity and safety of a SARS-CoV-2 inactivated vaccine in healthy adults aged 18–59 years: report of the randomized, double-blind, and placebo-controlled phase 2 clinical trial. medRxiv (p. 2020.07.31.20161216). Doi: 10.1101/2020.07.31.20161216
- Zhao FJ, Zhang XH, Liu SQ, Zeng TB, Yu J, Gu WM, Zhang YJ, Chen X, Wu YM. Assessment of the immune responses to Treponema pallidum Gpd DNA vaccine adjuvanted with IL-2 and chitosan nanoparticles before and after Treponema pallidum challenge in rabbits. Sci China Life Sci. 2013;56(2):174–180. doi: 10.1007/S11427-012-4434-4. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Ma X, Zhang R, Hu F, Zhang T, Liu Y, Han MH, You F, Yang Y, Zheng W. A novel liposome-polymer hybrid nanoparticles delivering a multi-epitope self-replication DNA vaccine and its preliminary immune evaluation in experimental animals. Nanomed Nanotechnol Biol Med. 2021;35:102338. doi: 10.1016/J.NANO.2020.102338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu FC, Guan XH, Li YH, Huang JY, Jiang T, Hou LH, Li JX, Yang BF, Wang L, Wang WJ, Wu SP, Wang Z, Wu XH, Xu JJ, Zhang Z, Jia SY, Wang BS, Hu Y, Liu JJ, Chen W. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]