Abstract
Purpose
The Coronavirus disease 2019 (COVID-19) pandemic is one of the most devastating global problems. Regarding the lack of disease-specific treatments, repurposing drug therapy is currently considered a promising therapeutic approach in pandemic situations. Recently, the combination therapy of Janus kinase (JAK) inhibitor baricitinib has been authorized for emergency COVID-19 hospitalized patients; however, this strategy's safety, drug-drug interactions, and cellular signaling pathways remain a tremendous challenge.
Methods
In this study, we aimed to provide a deep insight into the baricitinib combination therapies in severe COVID-19 patients through reviewing the published literature on PubMed, Scopus, and Google scholar databases. We also focused on cellular and subcellular pathways related to the synergistic effects of baricitinib plus antiviral agents, virus entry, and cytokine storm (CS) induction. The safety and effectiveness of this strategy have also been discussed in moderate to severe forms of COVID-19 infection.
Results
The severity of COVID-19 is commonly associated with a dysregulated immune response and excessive release of pro-inflammatory agents, resulting in CS. It has been shown that baricitinib combined with antiviral agents could modulate the inflammatory response and provide a series of positive therapeutic outcomes in hospitalized adults and pediatric patients (age ≥ two years old).
Conclusion
Baricitinib plus the standard of care treatment might be a potential strategy in hospitalized patients with severe COVID-19.
Keywords: Baricitinib, Cytokine storm, Severe COVID-19, Janus kinase pathway, Repurposing drug therapy
Introduction
The most recent COVID-19 (Coronavirus disease 2019) pneumonia caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has seriously threatened public health worldwide. The COVID-19 characterizes by multi-systemic manifestations, which can be varied between different age groups. The main clinical symptoms in COVID-19 patients include fever, cough, and dyspnea. While fatigue, headache, anosmia, ageusia, cutaneous manifestations, and gastrointestinal involvement have been reported as minor symptoms of the illnesses [1–4]. Based on a cohort study from China, COVID-19 severity can be classified into (i) mild to moderate (∽81% patients with mild symptoms up to mild pneumonia), (ii) severe (∽14% of patients with dyspnea, hypoxia, or more than 50% lung involvement), and (iii) critical (∽5% of patients with respiratory failure, shock, or multiorgan system dysfunction) [5, 6]. The most important risk factors for severe illness are age and comorbidities, including cardiovascular disease, diabetes, chronic respiratory disease, malignancy, obesity, and male gender [2, 7, 8].
Many studies showed that antiretroviral treatment with remdesivir and steroids reduced mortality. However, not all people with SARS-CoV-2 infection are eligible to start treatment with remdesivir, and in people with severe disease, mortality is still high, and new treatments are needed [9]. Regarding the lack of disease-specific therapies and urgent need to develop therapeutic approaches to manage COVID-19, repurposing drugs used for similar conditions can be considered a potentially useful option [10, 11]. Based on genomic homology between SARS-CoV-2 with SARS-CoV (∽79.5%), and MERS-CoV (∽50%) [12], it has been suggested that drugs in the treatment of SARS or MERS may be beneficial in COVID-19 patients [13]. However, SARS-CoV-2 displayed higher transmissibility and lower mortality rate than SARS outbreaks in 2003 [14].
It has been postulated that COVID-19 severity can be associated with dysregulated inflammatory responses, including excessive interleukins (ILs) and cytokine production; hence, inhibition of the pro-inflammatory cascades using Janus kinase (JAK) inhibitors may modulate the inflammatory response and improve clinical outcomes in severe cases of infection [15]. On 19 November 2020, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for baricitinib combined with antiviral remdesivir to treat hospitalized COVID-19 adults (2 years of age or older) and pediatric patients requiring supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO) [16]. However, this EUA was revised on 28 July 2021 to meet the safety issues and protect public health and no longer requires baricitinib to be used in combination with remdesivir. Based on the scope of the latest version of this authorization, baricitinib can be used to treat COVID-19 in pediatric patients and hospitalized adults, 2 years of age or older, receiving supplemental oxygen, non-invasive or invasive mechanical ventilation, or ECMO [17]. It should be noted that the Infectious Diseases Society of America (IDSA) recommends baricitinib administration for severe COVID-19 patients who require high-flow oxygen/non-invasive ventilation [18].
Baricitinib (an IL-6 receptor antibody) is a selective inhibitor of JAK 1 and 2, approved for the treatment of active rheumatoid arthritis (RA) when patients have shown an inadequate response to other medicines such as tumor necrosis factor (TNF) inhibitors [10]. Based on a double-blind, randomized placebo-controlled trial, combination therapy baricitinib with remdesivir reduced recovery time and accelerated clinical status improvement in hospitalized COVID-19 patients, compared to subjects who received placebo plus remdesivir alone, notably among those receiving high-flow oxygen or non-invasive ventilation [15].
Regarding the complex immunopathogenesis nature of COVID-19, several immunotherapeutic strategies have been developed to target the hyper-inflammatory state and treat the severe phase of illnesses, which have shown promising clinical outcomes [19, 20]. Although immunomodulation by JAK inhibitor baricitinib is now used to manage the severe COVID-19, there are concerns about its further suppression on type I and III interferons (IFNs), which are essential in antiviral defense. In addition, the starting time for such medication is critical because early or delayed administration may be associated with potential risks, including compromise antiviral immunity or irreversible organ damages [21]. Consequently, further studies are needed to evaluate this multimodal treatment’s efficacy, adverse effects, safety, further advantages, and unresolved challenges. In the current study, we aimed to provide a deep insight into the combination therapies with baricitinib in severe COVID-19 patients through reviewing literature published on PubMed, Scopus, and Google scholar databases. We also focus on cellular and subcellular pathways related to the synergistic effects of JAK inhibitors and antiviral agents. Besides, the clinical outcomes of baricitinib combination therapy, its safety, and effectiveness have been discussed in moderate to severe forms of COVID-19 infection.
Pharmacotherapies against COVID-19
Different repurposing drug therapies, including antiviral, anti-inflammatory, antiprotozoal, and fibrinolytic agents, have been investigated during the COVID-19 pandemic [9, 22, 23]. In this respect, the National Institutes of Health (NIH) and FDA recommended using remdesivir and convalescent blood products as the most promising agents for COVID-19 treatment [10]. There are two potential ways to develop drugs against the SARS-CoV-2 virus: (i) targeting the host cell biology, which interferes in the viral entrance, transformation, and replication, and (ii) targeting the virus biology and components [24]. It should be noted that the host cell targeting with novel agents may lead to a series of unwanted side effects in the same target tissue or other tissues. Besides, a high rate of the mutations in the virus genome may induce drug resistance into the novel agents used for targeting the SARS-CoV-2 virus biology [25]. Since most viruses can affect the cells similarly, finding a broad-spectrum antiviral agent may be a promising way to control the virus disease outbreak [26]. According to the clinical data, combination therapies are mostly recommended options in hospitalized patients of COVID-19 [27]. From different points of view, combination therapies usually show several advantages compared to monotherapy, including decreased drug resistance and increased tolerability to adverse effects due to the reduction in the dosage of each drug in combination form [6]. It has been shown that anti-inflammatory agents were not beneficial for patients during the previous pandemic diseases such as SARS and MERS. However, in SARS-CoV-2, anti-inflammatory and immune-modulating agents have their place in the therapeutic regimen due to the extensive hyper-inflammation reactions in the severe forms of the disease [27]. Tocilizumab (anti-IL-6 receptor monoclonal antibody) is an adjunctive therapy administrated to control the cytokine release syndrome caused by hyper-inflammatory responses in severe COVID-19 patients. The clinical efficacy of human monoclonal antibodies such as sarilumab, anakinra, and ruxolitinib has been evaluated to manage cytokine storm (CS) in severe respiratory failure by COVID-19 [28]. Substantial information on the efficacy and safety of these trials can be found on ClinicalTrials.gov database (http://clinicaltrials.gov).
SARS-CoV-2 cell penetration pathway
The SARS-CoV-2 virus, closely related to the genus Betacoronavirus, belongs to the Orthocoronavirinae subfamily and Coronaviridae family [29]. It is a positive-sense single-stranded RNA virion with a 29,881 bp genome length. The essential structural proteins in SARS-CoV-2 are spike (S), matrix (M), small envelope (E), and nucleocapsid (N) proteins. Homotrimeric S protein, as a critical protein for the fusion and recognition of the virus, is composed of an extracellular N-terminus, a transmembrane domain, and a short intracellular C-terminus. Each of the trimers of S protein is coated with 66 N-linked host-derived glycans to escape from the host immune cells [15]. The glycosylated S protein consists of S1 and S2 subunits [30]. The S1 subunit is responsible for interaction with angiotensin-converting enzyme 2 (ACE-2) receptor and determination of the cellular tropism with receptor-binding domain (RDB) [29]. The S2 subunit is involved in viral fusion [31] by heptad repeat 1 (HR1) and heptad repeat 2 (HR2) domains [30]. The S1 interaction with ACE-2 promotes the dissociation of S1 and transformation of meta-stable pre-fusion S2 to the more-stable post-fusion form [27]. In post-fusion, the HR2 conformation changes to a rigid helix with a flexible loop that interacts with HR1 and can lead to a six-helical bundle S2 [30].
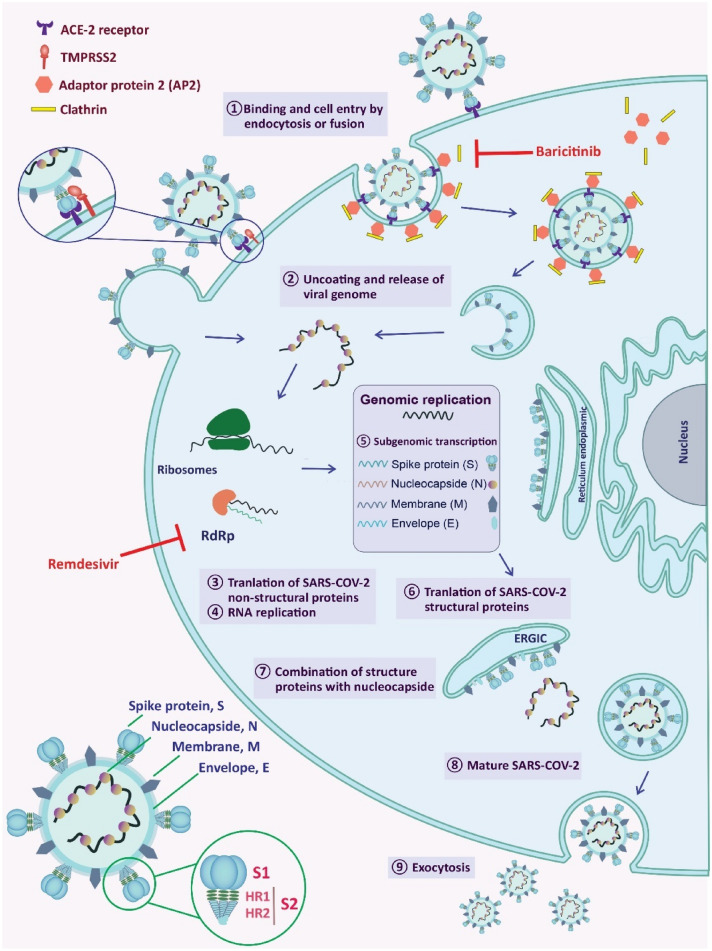
The SARS-CoV-2 entry into the host cells can be mediated by fusion and endocytosis pathways (Fig. 1). Fusion mechanism is usually triggered through (i) the recognizing of ACE-2 receptor by RBD, (ii) subsequent interaction between the S protein and ACE-2, and (iii) cleavage and activation of the S protein into the S1 and S2 at the S1/S2 cleavage site by transmembrane serine protease 2 (TMPRSS2) [30, 32, 33]. The SARS-CoV-2, like the other viruses, takes advantage of receptor-mediated endocytosis [31, 32], which is regulated by the numb-associated kinase (NAK) family, including adaptor-associated protein kinase (AAK-1) and cyclin G-associated kinase (GAK) [34]. The activated AAK-1 and GAK lead to the phosphorylation of the adaptor protein 2 (AP2) complex and consequently promote the clathrin-dependent endocytosis of the virus [35]. So far, two possible mechanisms have been suggested for the SARS-CoV-2 endocytosis. In the first theory, the virus binds to the ACE-2 receptor outside of the membrane. The endocytosis process initiates by assembling clathrin, coat protein, in the inner side of the plasma membrane to form a vesicle (Fig. 1). In the second theory, SARS-CoV-2 utilizes the clathrin-dependent endocytosis directly to get the entrance permission. Following the virus entry, the viral RNA is released into the cytoplasmic space [30], and the production of the structural and non-structural proteins is initiated in the infected cells [36]. Finally, the virions of coronaviruses are formed by budding into the endoplasmic reticulum-Golgi intermediate compartments (ERGIC) [15] and released as new viral particles (Fig. 1) [30]. Based on recently published data, there is an abundant expression of endocytosis markers in nasal epithelial cells such as dynamin, GTPase, clathrin, and caveolae-mediated ones, where the fusion and macropinocytosis of the viruses can be significantly raised, unlike the pneumocytes cells [24]. One of the promising therapeutic approaches is blocking the virus entry by targeting AAK-1, GAK, and ACE-2 receptors and inhibiting its intracellular assembly [31]. However, it has been postulated that anti-hypertensive drugs, which act against ACE-2 receptor, could increase the risk of SARS-CoV-2 infection [37] or mortality [38].
Fig. 1.
Schematic representation of SARS-CoV-2 structure, virus entry, and replication in host cells. Virus cell entry by endocytosis is a clathrin-dependent route. Baricitinib effect is usually related to the irregularity in AP2-associated protein kinase 1 and inhibiting SARS-CoV-2 cellular entry through the clathrin-dependent pathway. Antiviral function of remdesivir is related to inhibiting the RNA-dependent RNA polymerase (RdRp)
Cytokine storm signaling pathway
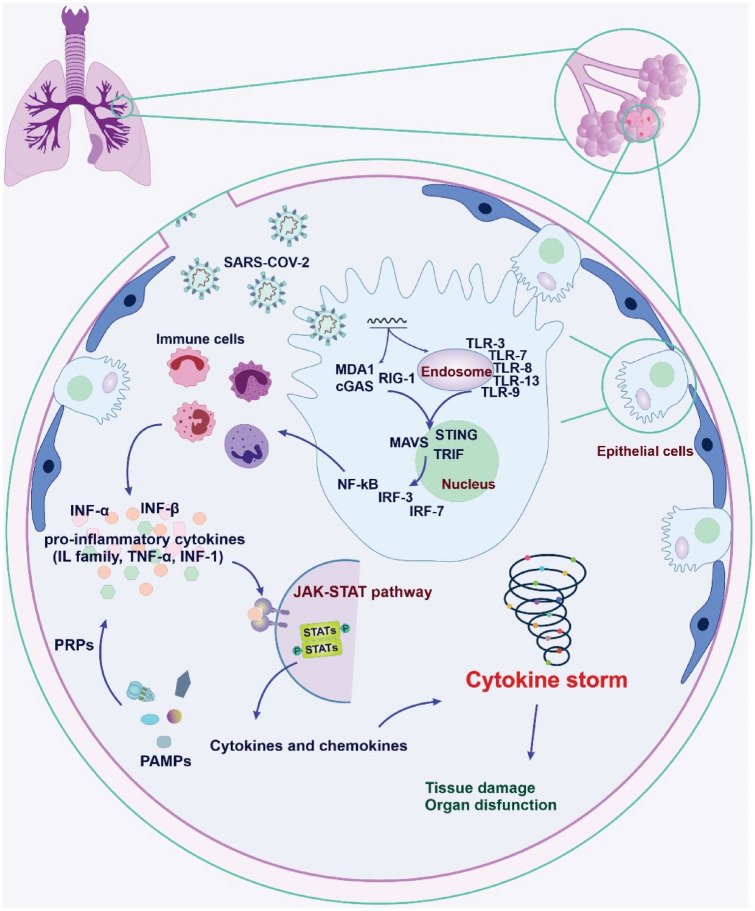
The innate immune system is the first line of the immune defense against pathogens; however, it can lead to some illnesses when it works out of control. The uncontrolled and excessive release of pro-inflammatory agents and cytokines causes CS. Based on the clinical data from severe COVID-19 patients, there is a significant release of the inflammatory factors, including IL-6, IL-10, and TNF-α. Further, patients in an intensive care unit (ICU) show a more considerable increment in IL-2, IL-7, IL-10, TNF-α, and granulocyte-colony-stimulating factor (GCSF) [30, 39]. Thus, it has been suggested that immunomodulation may be a beneficial therapeutic option to overcome such situations in the severe form of the disease. As shown in Fig. 2, the pattern recognition receptors (PRRs) of the host immune cells detect and respond to the pathogen-associated molecular patterns (PAMPs) [40]. The PAMPs are the molecular structures such as peptidoglycans and lipopolysaccharides essential for the pathogens’ life cycle, and many of them cannot be found in the host. However, one of the exceptions is the pathogen-derived nucleic acids.
Fig. 2.
Schematic depicting the cytokine storm (CS) mechanisms in severe COVID-19 patients. The pattern recognition receptors (PRRs) detect and respond to the pathogen-associated molecular patterns (PAMPs). Toll-like receptors (TLRs) are expressed by immune and non-immune cells. Retinoid acid-inducible gene I (RIG-I), cyclic GMP-AMP synthase (cGAS), and melanoma differentiation-associated gene 5 (MDA-5) are receptors for detecting the viral genome in the cytoplasm. The activation of these sensors leads to an increase of TIR-domain-containing adaptor inducing interferon β (TRIF), stimulation of interferon genes (STINGs), and mitochondrial antiviral-signaling protein (MAVS). The activation of interferon regulatory factor-3, 7 (IRF-3, IRF-7), and nuclear factor-kappa (NF-κB) initiate a subcellular signaling cascade, induce interferons (INFs) and pro-inflammatory cytokines, subsequently leading to CS phenomena
There are two groups of viral RNA and DNA sensors based on their intracellular localization [41]. Toll-like receptors (TLRs) are a group of innate immune systems localized on the endosomal membrane of the immune cells (e.g., TLR-3, TLR-7, TLR-8, TLR-9, and TLR-13) [29, 41]. The second group of receptors that are responsible for detecting the viral genome in the cytoplasm includes retinoid acid-inducible gene I (RIG-I) [41], cyclic GMP-AMP synthase (cGAS), and melanoma differentiation-associated gene 5 (MDA-5) [42]. These complex sensors lead to the increment of TIR-domain-containing adaptor inducing IFN-β (TRIF), stimulation of IFN genes (STINGs), and mitochondrial antiviral-signaling protein (MAVS), which can initiate a series of subcellular signaling cascade [29, 43]. In the following, the activation of IFN regulatory factor-3, 7 (IRF-3, IRF-7), and nuclear factor-kappa-light-chain-enhancer of activated B cells (NF-κB) lead to the induction of numerous INF-α and INF-β, and some pro-inflammatory cytokines (e.g., IL-1β, TNF-α) [41, 44]. Following, the INF-1 prompts a large group of antivirals by activating the STAT and JAK pathways [41].
Inhibition of Janus kinase pathway
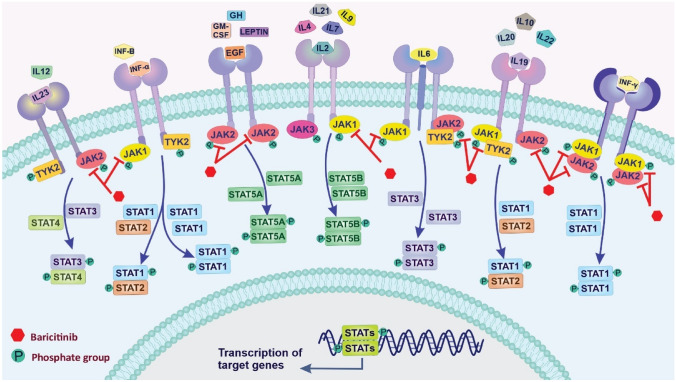
The JAK/STAT (Janus kinase/signal transducers and activators of transcription) signaling pathway is related to various biological processes in the body, including cell proliferation, differentiation, migration, survival, apoptosis, immune regulation, and hematopoiesis [45]. It has been recently indicated that this pathway is involved in the SARS-CoV-2-induced CS syndrome [46]. The JAK/STAT signaling pathway consists of three main components: (i) tyrosine kinase-associated receptor, (ii) JAK, and (iii) STAT [47]. Four JAK family members JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2), are receptor-associated tyrosine kinases, which JAK1, JAK2, and TYK2 are expressed in a wide verity of mammalian tissues, whereas JAK3 is in the hematopoietic cells. They can transmit extracellular signals from many pro-inflammatory cytokines to STATs molecules [48]. The STAT family (STAT1–STAT6), as a downstream target of JAKs, is one of the most crucial cytokine-activated transcription factors in the immune response process and plays a vital role in the activation of signal and transcription in the neuronal and cytokine-mediated signaling pathways [49]. There are two canonical and non-canonical ways of stimulating the JAK-STAT pathway. The canonical JAK-STAT activates via high-affinity interaction between extracellular signaling of cytokines and their cognate receptors [50]. Various cytokines and growth factors can participate in signal transmission through this pathway, including ILs, granulocyte–macrophage colony-stimulating factor (GM-CSF), epidermal growth factor (EGF), growth hormone (GH), platelet-derived growth factor (PDGF), and IFNs [51]. JAK phosphorylates the tyrosine residues of many target proteins; thus, it affects signal translocation from the extracellular to the intracellular [52]. JAK activation mediates phosphorylation of the STATs, leading to form dimers of phosphorylated STATs (STAT-STAT). Dimer of STATs can translocate into the nucleus, bind to specific DNA sequences, and transmit extracellular cytokine signals into transcriptional responses [53]. This pathway can be inactivated by negative regulators such as suppressors of cytokine signaling (SOCS), protein inhibitors of activated STATs (PIAS), and protein tyrosine phosphatases (PTPs) [54]. Other signaling molecules are involved in the JAK pathway, such as PI3 kinase (PI3K), mitogen-activated kinase (MAPK), extracellular receptor kinase (ERK) [50].
In the canonical pathway, unphosphorylated STATs are usually located in the cytoplasm. In the non-canonical, some unphosphorylated STATs localized on heterochromatin in the nucleus associated with specific proteins contribute to maintaining the heterochromatin state [50]. Increasing the phosphorylation of the STAT by JAK or other tyrosine kinases reduces the amount of unphosphorylated STATs on heterochromatin. Dispersed phosphorylated STATs can affect total transcriptional events through binding to cognitive sites in euchromatin, chromatin modification, and inducing gene expression [55]. As CS is relatively common in severe cases of COVID-19, anti-inflammation therapy may help prevent further injury. A variety of JAK inhibitors, including baricitinib (JAK1/2 inhibitor), ruxolitinib (oral JAK1/2 inhibitor), upadacitinib (JAK1 inhibitor), tofacitinib (JAK1, JAK 2, JAK3, and TYK2 inhibitor), and fedratinib (JAK2 inhibitor) [56], are currently approved for the treatment of many inflammation-driven pathologies [57]. For the management of COVID-19-associated CS, FDA authorized combination therapy with baricitinib (JAK1/2 inhibitor), which can be applicable for hospitalized COVID-19 patients. The JAK/STAT signaling pathway and inhibition effects of baricitinib have been shown in Fig. 3.
Fig. 3.
Schematic representation of the JAK/STAT signaling pathway and inhibition effects of baricitinib. Various cytokines and growth factors can participate in signal transmission through the JAK pathway, including different interleukins (ILs), interferons (INFs α, β, and γ), granulocyte–macrophage colony-stimulating factor (GM-CSF), growth hormone (GH), epidermal growth factor (EGF), and leptin hormone. Interaction between the receptors and ligands leads to phosphorylation of the tyrosine residues of many target proteins by JAK enzymes and affects signal translocation from the extracellular to the intracellular. Then, phosphorylated STATs form dimers (STAT-STAT) can translocate into the nucleus, bind to specific DNA sequences, and transmit extracellular cytokine signals into transcriptional responses
Baricitinib
Baricitinib (Olumiant) has been approved for the treatment of moderate to severe RA. The most common drugs against RA are corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), TNF inhibitors, and JAK inhibitors [58]. Chronic inflammatory autoimmune disease RA results from an imbalance between anti-inflammatory and pro-inflammatory cytokines and loss of immunologic tolerance of autoreactive immune cells. As a fundamental insight, cytokines are now known as the main actors of autoimmunity disorders, and biologic agents targeting cytokines considered to treat immune-mediated diseases [57].
Baricitinib, as a reversible JAK inhibitor, has a high affinity to AAK-1 and a lower affinity to GAK [59]. It acts through selective inhibiting of JAK1/JAK2 enzymes, targeting cytokine, and stimulation of growth factor receptor result in a reduction of downstream immune cell function [60]. Baricitinib interrupts the cytokines’ signaling pathway, including IL-6, IL-2, IL-10, INF-γ, and GM-CSF, which are elevated in the hyper-inflammatory situations due to COVID-19 infection [61, 62].
Compared to the other JAK inhibitors, baricitinib exhibits antiviral effects in clinically relevant serum concentrations and tolerable therapeutic dosage range [31]. The function of the baricitinib against COVID-19 is usually related to the irregularity in AP2-associated protein kinase 1 and inhibiting SARS-CoV-2 cellular entry. It is proposed that baricitinib shows antiviral effects by inhibiting upregulated INF-1 caused by the ACE-2 receptor and subsequently blocking the cell entry of viruses [63]. Hence, baricitinib can prevent cell entry and SARS-CoV-2-initiated inflammation responses [62] through clathrin-mediated endocytosis inhibition [59, 62]. As mentioned in the previous section, ACE-2 receptors represent cellular ports to enter the SARS-CoV-2 into the lung cells. Several clinical trials have been conducted regarding the role of baricitinib in the management of COVID-19 (Table 1). In this respect, decreased inflammatory markers and improved oxygenation have been reported in COVID-19 patients treated with baricitinib [64, 65]. In a most updated meta-analysis of randomized controlled trials, it has been demonstrated that JAK inhibitors (e.g., baricitinib) in hospitalized COVID-19 patients can significantly reduce the risk of death and mechanical ventilation or extracorporeal membrane oxygenation by 43% and 36%, respectively, compared to standard of care alone [66]. In another meta-analysis of randomized controlled trials, the safety and effectiveness of JAK inhibitors for hospitalized COVID-19 have also been demonstrated [67]. In an observational and longitudinal trial, COVID-19 patients treated with baricitinib showed rapid recovery of circulating T and B cell frequencies, a marked reduction in serum levels of cytokines (e.g., IL-6, IL-1β, and TNF-α), and an increased level of antibody against the SARS-CoV-2 spike protein. In addition, the need for oxygen therapy and a progressive increase in the P/F (PaO2, oxygen partial pressure/FiO2, fraction of inspired oxygen) ratio were markedly decreased [68]. The effectiveness and safety of baricitinib have been investigated in an observational retrospective study on hospitalized severe COVID-19 patients. From the results, clinical improvements have been detected in patients without relevant adverse events (AEs) and 100% overall survival [69]. However, side effects such as upper respiratory tract infections, nausea, cold sores, and shingles may limit the broad application of baricitinib in COVID-19 patients.
Table 1.
Clinical trials related to the baricitinib for COVID-19 treatment
| Study title | Status* | Number enrolled | Phase | Ages eligible for study | Reference |
|---|---|---|---|---|---|
| Baricitinib therapy in COVID-19 | Completed | 12 | Phase 2/3 | 18–85 years (adult, older adult) | [79], NCT04358614 |
| A study of baricitinib (ly3009104) in participants with COVID-19 | Completed | 1585 | Phase 3 | 18 years and older (adult, older adult) | [15], NCT04421027 |
| Treatment of moderate to severe coronavirus disease (COVID-19) in hospitalized patients | Recruiting | 800 | Phase 2 | 18 years and older (adult, older adult) | NCT04321993 |
| Baricitinib in Hospitalized Covid-19 Patients with Diabetes Mellitus | Recruiting | 382 | Phase 3 | 18 years and older (adult, older adult) | NCT04970719 |
| Baricitinib Compared to Standard Therapy in Patients With COVID-19 (BARICIVID-19) | Not yet recruiting | 126 | Phase 2 | 18 years and older (adult, older adult) | NCT04393051 |
| Baricitinib for corona virus pneumonia (COVID-19): a therapeutic trial (BREATH) | Not yet recruiting | 13 | Phase 2 | 18–74 years (adult, older adult) | NCT04399798 |
| A Study of Baricitinib (LY3009104) in Children With COVID-19 (COV-BARRIER-PEDS) (COV-BARRIER) | Not yet recruiting | 24 | Phase 3 | 2–18 years (child, adult) | NCT05074420 |
| Efficacy and Safety of Baricitinib in Patients with Moderate and Severe COVID-19 | Not yet recruiting | 480 | Phase 3 | 18–80 years (adult, older adult) | NCT05056558 |
| Clinical-epidemiological Characterization of COVID-19 Disease in Hospitalized Older Adults (COVID-AGE) | Completed | 576 | – | 70 years and older (older adult) | NCT04362943 |
*Please refer to https://clinicaltrials.gov/ for more details
Baricitinib combination therapies against COVID-19
Some clinical studies have evaluated the safety and efficacy of combination therapies plus baricitinib in COVID-19 patients. In a cohort non-controlled retrospective combinational study, using a short course of baricitinib plus hydroxychloroquine in 15 patients was efficient (73% of the patients); however, the relation of this regimen with clinical improvements remains unclear and should be further investigated in randomized, controlled clinical trials [70]. In a clinical study for predicting the mortality of 150 patients in Wuhan, the evidence showed that most mortalities are due to CS and myocarditis. There is also a relation between cytokine elevation and the severity of COVID-19 disease [29]. In a case report study, a 50-year-old man with follicular non-Hodgkin lymphoma successfully recovered from COVID-19 after receiving remdesivir, baricitinib, tocilizumab hydroxychloroquine, and broad-spectrum antibiotics [71]. In another study by Izumo and colleagues on 44 patients with severe COVID-19, it has been shown that combination therapy using baricitinib, remdesivir, and dexamethasone (BRD) was effective, and the incidence rate of AEs was low. Based on the results, the BRD therapy in most patients (90%, 17/19 patients) leads to avoiding the need for mechanical ventilation, reducing the duration of the hospitalization, supplemental oxygen therapy, and recovery time [72]. An observational cohort study has shown that a combination of baricitinib with corticosteroids in patients with moderate to severe SARS-CoV-2 pneumonia can be associated with a more remarkable improvement in pulmonary function when compared with corticosteroids alone [73].
A double-blinded randomized control clinical trial showed that baricitinib plus remdesivir is more efficient in reducing recovery time (1–8 days) of hospitalized patients (1034 patients) than remdesivir plus placebo, especially in patients requiring high-flow oxygenation or non-invasive ventilation. Besides, a decrease in the severe adverse effects from 21 to 16% with lower ventilation time has been detected. In addition, these patients showed 30% higher chances of improvement in clinical status at day 15 than the control group [27]. Based on a systematic literature review, in the available randomized controlled trials, baricitinib plus remdesivir was effective in patients with non-invasive ventilation, which confirmed the European League Against Rheumatism (EULAR) points on COVID-19 pathophysiology and immunomodulatory treatment from the rheumatology perspective [74]. Besides, it has been shown that baricitinib combination therapy can reduce recovery time in hospitalized cancer patients infected with COVID-19, required oxygen therapy, and non-invasive ventilation [75]. In a case report study, combination therapy with methylprednisolone, baricitinib, and remdesivir was effective against critical COVID-19 patients with lung cancer [76]. Further clinical trials related to the different combinational strategies of baricitinib and remdesivir with other repurposing drugs have been shown in Table 2.
Table 2.
Clinical trials related to the combination therapies of baricitinib and remdesivir for COVID-19 patients
| Study title | Status* | Number enrolled | Phase | Ages eligible for study | Reference | |
|---|---|---|---|---|---|---|
| Baricitinib combinations | Baricitinib in symptomatic patients infected by COVID-19 | Not yet recruiting | 200 |
Phase 2 Phase 3 |
18–85 years (adult, older adult) | NCT04320277 |
| Clinical trial to evaluate efficacy of 3 types of treatment in patients with pneumonia by COVID-19 | Active, not recruiting | 168 | Phase 2 | 18 years and older (adult, older adult) | NCT04346147 | |
| Multi-arm therapeutic study in pre-ICU patients admitted with COVID-19—repurposed drugs (TACTIC-R) | Recruiting | 1167 | Phase 4 | 18 years and older (adult, older adult) | [64], NCT04390464 | |
| Randomized evaluation of COVID-19 Therapy | Recruiting | 45,000 |
Phase 2 Phase 3 |
Child, adult, older adult | [89], NCT04381936 | |
| Baricitinib, placebo and antiviral therapy for the treatment of patients with moderate and severe COVID-19 | Terminated | 6 | Phase 2 | 18 years and older (adult, older adult) | NCT04373044 | |
| Remdesivir combinations | Comparison of remdesivir versus lopinavir/ ritonavir and remdesivir combination in COVID-19 patients | Recruiting | 90 | Phase 4 | 18–80 years (adult, older adult) | NCT04738045 |
| ACTIV-5/big effect trial (BET-B) for the treatment of COVID-19 | Recruiting | 400 | Phase 2 | 18–99 years (adult, older adult) | NCT04583969 | |
| ACTIV-5/big effect trial (BET-A) for the treatment of COVID-19 | Active, not recruiting | 167 | Phase 2 | 18–99 years (adult, older adult) | NCT04583956 | |
| Safety, tolerability and pharmacokinetics of inhaled nanoparticle formulation of remdesivir (GS-5734) and NA-831 (NEUROSIVIR) | Recruiting | 45 | Phase 1 | 21 years to 50 years (adult) | NCT04480333 | |
| Trial to determine the efficacy/safety of plitidepsin vs control in patients with moderate COVID-19 infection (Neptuno) | Recruiting | 609 | Phase 3 | 18 years and older (adult, older adult) | NCT04784559 | |
| Trial of treatments for COVID-19 in hospitalized adults (DisCoVeRy) | Recruiting | 2416 | Phase 3 | 18 years and older (adult, older adult) | [18], NCT04315948 | |
| Adaptive COVID-19 Treatment Trial 3 (ACTT-3) | Completed | 969 | Phase 3 | 18 years and older (adult, older adult) | NCT04492475 | |
| Study to evaluate the efficacy and safety of remdesivir in participants with severely reduced kidney function who Are hospitalized for coronavirus disease 2019 (COVID-19) (REDPINE) | Recruiting | 1116 | Phase 3 | 12 years and older (child, adult, older adult) | NCT04745351 | |
| INF-β 1B and remdesivir for COVID-19 | Recruiting | 100 | Phase 2 | 18 years and older (adult, older adult) | NCT04647695 | |
| Study of merimepodib in combination with remdesivir in adult patients with advanced COVID-19 | Terminated | 44 | Phase 2 | 18 years and older (adult, older adult) | NCT04410354 | |
| Antiviral activity and safety of remdesivir in Bangladeshi patients with severe corona virus disease (COVID-19) | Completed | 60 | Phase 2 | 18 years and older (adult, older adult) | [90], NCT04596839 | |
| Investigational treatments for COVID-19 in tertiary care hospital of Pakistan | Completed | 600 | Not applicable | 18 years to 90 years (adult, older adult) | NCT04492501 | |
| A study to evaluate the efficacy and safety of remdesivir plus tocilizumab compared with remdesivir plus placebo in hospitalized participants with severe COVID-19 pneumonia (REMDACTA) | Completed | 649 | Phase 3 | 12 years and older (child, adult, older adult) | NCT04409262 | |
| Baricitinib plus Remdesivir | Efficacy of remdesivir and baricitinib for the treatment of severe COVID-19 patients | Recruiting | 150 | Phase 3 | Child, adult and older adult | NCT04693026 |
| Adaptive COVID-19 treatment trial 2 (ACTT-2) | Completed | 1033 | Phase 3 | 18 years to 99 years (adult, older adult) | [27], NCT04401579 | |
| Adaptive COVID-19 treatment trial 4 (ACTT-4) | Active, not recruiting | 1010 | Phase 3 | 18 years to 99 years (adult, older adult) | NCT04640168 | |
| Factorial randomized trial of remdesivir and baricitinib plus dexamethasone for COVID-19 (the AMMURAVID Trial) (AMMURAVID) | Not yet recruiting | 4000 | Phase 3 | 18 years and older (adult, older adult) | NCT04832880 | |
*Please refer to https://clinicaltrials.gov/ for more details
Baricitinib safety and possible adverse effects
The FDA has authorized baricitinib for emergency use in hospitalized adults and pediatric patients of COVID-19 under an EUA guideline until the declaration is terminated or the authorization revoked. In this regard, there are crucial safety issues related to the unapproved use of baricitinib under emergency conditions that should be considered; however, there is limited information on the potential risks of this treatment in COVID-19 patients. Despite the beneficial outcomes of baricitinib in controlling the inflammatory conditions, its administration may associate with some AEs such as serious infection, thrombosis, and hypersensitivity reactions [77]. Liver toxicity, gastrointestinal complication, and cardiovascular side effects may also occur during baricitinib therapy. It has been reported that the baricitinib therapy may be associated with the occurrence of infectious disease and reactivation of latent infections such as tuberculosis (TB), hepatitis B, Epstein–Barr virus, varicella-zoster, and herpes simplex [78]. There is a negligible TB risk in RA patients treated with JAK inhibitor baricitinib [79]. Thus, baricitinib administration should be avoided in COVID-19 patients with known active TB unless the potential benefits outweigh the potential risks of baricitinib treatment in patients with serious active infections other than COVID-19 or chronic/recurrent infections [18].
Venous thromboembolism is another AE that may be occurred during baricitinib administration, so COVID-19 patients with deep-vein thrombosis or pulmonary embolism status should be evaluated promptly and treated appropriately. In addition, baricitinib therapy should be discontinued in patients with severe hypersensitivity reactions [18, 80].
Therefore, evaluating the laboratory values, including estimated glomerular filtration rate (eGFR), liver enzymes, and complete blood count, is one of the critical issues before initiation of baricitinib therapy. Accordingly, dose adjustments should be followed according to the recommended clinical guideline for COVID-19 patients with abnormal renal, hematological, and hepatic laboratory values. Besides, baricitinib therapy is not recommended in patients with severe renal and hepatic impairment unless the potential benefit outweighs the potential risk [18].
The safety and clinical impact of baricitinib therapy (4 mg/day) has been investigated in a pilot study on 12 patients with moderate COVID-19. Based on these preliminary data, baricitinib plus lopinavir/ritonavir have significantly improved the clinical and laboratory parameters and reduced severity progression. Besides, after 2 weeks of treatment, no serious infections, cardiovascular and hematologic AEs have been reported [81]. In another clinical study on 507 adult COVID-19 patients, treatment with baricitinib plus remdesivir resulted in serious infection, venous thromboembolism, and pulmonary embolism in 6%, 4%, and 1% of patients, respectively, which was not statistically significant compared to the group treated with remdesivir alone [82]. Indeed, these results cannot be generalized to all COVID-19 patients, and further controlled, more extensive studies should be conducted to confirm the long-term safety of the treatment [81]. Overall, the risk of AEs in hospitalized severe or critical COVID-19 patients receiving baricitinib was not greater than those not receiving baricitinib. Thus, the guideline panel suggests baricitinib in addition to the standard of care for patients hospitalized with severe COVID-19 [18].
Treatment guideline recommended for baricitinib combination therapies
The severe COVID-19 infection is related to dysregulation of pro-inflammatory and cytokines, so the administration of JAK inhibitor plus antiviral agents might be a promising approach. Based on recently published reports, baricitinib coupled with remdesivir can control COVID-19 in adult patients. It seems baricitinib can be a suitable candidate for combination therapy because of its low plasma protein binding and low interaction with drug transporters and cytochromes P450 (CYPs) enzymes. It should be noted that baricitinib has minimal interaction with the direct-acting antivirals against SARS-CoV-2, including remdesivir, favipiravir, and lopinavir [62]. Besides, the combination of baricitinib and remdesivir reduces the aberrant inflammatory response and viral replication and provides a series of positive therapeutic outcomes [34, 59]. However, the consequent immunosuppression following baricitinib administration leads to a delay in the clearance of virus load. Recently, baricitinib has been authorized by the FDA for hospitalized adults and pediatric patients older than 2 years in emergencies. Based on the NIH COVID-19 treatment guidelines, baricitinib and remdesivir have been recommended to treat COVID-19 in hospitalized, non-intubated patients who require oxygen supplementation. The combination of baricitinib or tocilizumab with dexamethasone alone or dexamethasone plus remdesivir (NCT04421027) has been recommended in hospitalized COVID-19 patients on high-flow oxygen or non-invasive ventilation, whose have evidence of clinical progression or increased markers of inflammation. Baricitinib dose is dependent on the eGFR, and the duration of the therapy is recommended up to 14 days or until hospital discharge. In a low level of eGFR (< 15 mL/min/1.73 m2), baricitinib is not recommended. It has been suggested that if neither baricitinib nor tocilizumab is available or feasible to administrate, tofacitinib and IV sarilumab can be used instead of baricitinib and tocilizumab, respectively. In addition, remdesivir administration is not recommended at eGFR < 30 mL/min/1.73 m2. Clinical trials to evaluate the safety and efficacy of remdesivir plus baricitinib against COVID-19 are listed in Table 2. Based on the scope of the latest version of EUA authorization, it no longer requires baricitinib to be used in combination with remdesivir [18]. In addition, patients who receive baricitinib for the treatment of COVID-19 should not receive tocilizumab or other immunomodulators as no adequate evidence is available for its combined administration [83].
Final remarks and conclusion
Immunomodulation therapy by baricitinib has been recently authorized in hospitalized COVID-19 patients. Baricitinib can interrupt the signaling of multiple cytokines and alleviate the host’s hyper-inflammatory responses caused by increased IL-6 and IFNs α and β [10, 59, 84]. IFNs, as one of the most potent antiviral mediators, prevent virus replication during the early stage of the infection. These molecules, through the activation of the JAK-STAT signaling pathway (JAK1/JAK2), result in the upregulation of several IFN-stimulated genes (ISGs), which can rapidly increase the antiviral effects inside the infected cells [85]. Thus, the administration of JAK inhibitors such as baricitinib in viral infections may seem a limited therapeutic option. However, it has been shown that the high level of IFNs α and β can be beneficial in the early stage of SARS and MERS, whereas it may lead to damages in the later stage of the diseases [86, 87]. Therefore, JAK-STAT inhibitors might be a potential strategy in more severe COVID-19 patients. Besides, baricitinib may show antiviral effects through targeting the host cell entities are involved in virus entry into the cells [59]. Regarding the possible interaction of baricitinib with drug-metabolizing enzymes such as CYPs [15, 84], baricitinib, along with antiviral agents such as remdesivir, may be considered as a suitable candidate for combination strategies. Although physicians were concerned about potential AEs of baricitinib combination therapy (e.g., immunosuppression, secondary infections, and thrombosis), fortunately, this combination therapy showed significantly fewer adverse effects in clinical subjects. However, the exact mechanisms of combination treatment with baricitinib are not fully understood.
In conclusion, there is no fully effective and specific drug for treating COVID-19 infection so far. Therefore, early identification and isolation of patients and timely initiation of supportive care (e.g., respiratory support and micronutrient therapy) to prevent the progression of infection play an essential role in disease management [88]. There is no need to administrate the JAK inhibitors in patients with mild to moderate symptoms (∽81%) without requiring hospital care, and viruses can be cleared from the body through IFN-mediated signaling pathways. In hospitalized adults and pediatric patients (age ≥ 2 years old), baricitinib therapy has been recommended as an emergency. Consequently, for choosing a treatment option in COVID-19, several factors, including the general condition of patients, stage of the disease, the clinical status of patients, drug–drug interaction, and safety issues, must be evaluated to increase the chance of the treatment and improve the clinical outcomes. Finally, it seems that simultaneous applying of combination therapy and drug repurposing can be a promising approach to deal with the pandemic situation.
Acknowledgements
The authors would like to acknowledge the Connective Tissue Diseases Research Center, Infectious and Tropical Diseases Research Center at Tabriz University of Medical Sciences.
Author contributions
MAK, LR, and MT researched and drafted the manuscript and prepared figures and tables. AS and LR conceived and designed research, and revised the context and finalized the manuscript.
Funding
The authors received no financial support for the preparation of this manuscript.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All the authors gave consent for the submission and publication of the manuscript.
Footnotes
Mostafa Akbarzadeh-Khiavi and Mitra Torabi have an equal contribution as the joint first authors.
Contributor Information
Leila Rahbarnia, Email: le.rahbarnia@gmail.com.
Azam Safary, Email: azamsafary@yahoo.com.
References
- 1.Vaira LA, et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: single-center experience on 72 cases. Head Neck. 2020;42:1252–1258. doi: 10.1002/hed.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Vito A, et al. Predictors of infection, symptoms development, and mortality in people with SARS-CoV-2 living in retirement nursing homes. PLoS ONE. 2021;16:e0248009. doi: 10.1371/journal.pone.0248009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geremia N, et al. A case of vasculitis-like skin eruption associated with COVID-19. Infect Dis Clin Pract. 2020;28:e30–e31. doi: 10.1097/IPC.0000000000000952. [DOI] [Google Scholar]
- 4.Alimohamadi Y, et al. Determine the most common clinical symptoms in COVID-19 patients: a systematic review and meta-analysis. J Prev Med Hyg. 2020;61:e304–e312. doi: 10.15167/2421-4248/jpmh2020.61.3.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Ginex T, et al. Host-directed FDA-approved drugs with antiviral activity against SARS-CoV-2 identified by hierarchical in silico/in vitro screening methods. Pharmaceuticals. 2021;14:332. doi: 10.3390/ph14040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow NF-DK, Gierke R, et al. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States. MMWR Morb Mortal Wkly Rep 2020;69:382–6 [DOI] [PMC free article] [PubMed]
- 8.De Vito A, et al. Clinical features, laboratory findings and predictors of death in hospitalized patients with COVID-19 in Sardinia Italy. Eur Rev Med Pharmacol Sci. 2020;24:7861–7868. doi: 10.26355/eurrev_202007_22291. [DOI] [PubMed] [Google Scholar]
- 9.Dallocchio RN, et al. Early combination treatment with existing HIV antivirals: an effective treatment for COVID-19. Eur Rev Med Pharmacol Sci. 2021;25:2435–2448. doi: 10.26355/eurrev_202103_25285. [DOI] [PubMed] [Google Scholar]
- 10.Hossen MS, et al. A review on current repurposing drugs for the treatment of COVID-19: reality and challenges. SN Compr Clin Med 2020; 1–13 [DOI] [PMC free article] [PubMed]
- 11.Saber-Ayad M, Saleh MA, Abu-Gharbieh E. The rationale for potential pharmacotherapy of COVID-19. Pharmaceuticals (Basel) 2020;13 [DOI] [PMC free article] [PubMed]
- 12.Zhu Z, et al. From SARS and MERS to COVID-19: a brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir Res. 2020;21:224. doi: 10.1186/s12931-020-01479-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos J, et al. Repurposing therapeutics for potential treatment of SARS-CoV-2: a review. Viruses 2020;12 [DOI] [PMC free article] [PubMed]
- 14.Martinez MA. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob Agents Chemother. 2020;20:64. doi: 10.1128/AAC.00399-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalil AC, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coronavirus (COVID-19) Update: FDA authorizes drug combination for treatment of COVID-19. 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-drug-combination-treatment-covid-19. (Accessed date 19 Nov 2020)
- 17.Baricitinib EUA Letter of authorization. 2021. https://www.fda.gov/media/143822/download. (Accessed 28 Jul 2021)
- 18.Lilly E. Fact sheet for healthcare providers: emergency use authorization (EUA) of baricitinib. 2021. https://www.fda.gov/media/143823/download. (Accessed date Aug 2021)
- 19.Calabrese C, et al. Practical aspects of targeting IL-6 in COVID-19 disease. Cleve Clin J Med 2020 [DOI] [PubMed]
- 20.Calabrese LH, Calabrese C. Baricitinib and dexamethasone for hospitalized patients with COVID-19. Cleve Clin J Med 2021 [DOI] [PubMed]
- 21.Calabrese LH. Cytokine storm and the prospects for immunotherapy with COVID-19. Cleve Clin J Med. 2020;87:389–393. doi: 10.3949/ccjm.87a.ccc008. [DOI] [PubMed] [Google Scholar]
- 22.Beigel JH, et al. Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med 2020 [DOI] [PubMed]
- 23.Di Castelnuovo A, et al. Heparin in COVID-19 patients is associated with reduced in-hospital mortality: the multicenter Italian CORIST study. Thromb Haemost. 2021;121:1054–1065. doi: 10.1055/a-1347-6070. [DOI] [PubMed] [Google Scholar]
- 24.Glebov OO. Understanding SARS-CoV-2 endocytosis for COVID-19 drug repurposing. FEBS J. 2020;287:3664–3671. doi: 10.1111/febs.15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pachetti M, et al. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant. J Transl Med. 2020;18:179. doi: 10.1186/s12967-020-02344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazzon M, Marsh M. Targeting viral entry as a strategy for broad-spectrum antivirals. F1000Res 2019; 8 [DOI] [PMC free article] [PubMed]
- 27.Beigel JH, et al. Remdesivir for the treatment of Covid-19. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barlow A, et al. Review of emerging pharmacotherapy for the treatment of coronavirus disease 2019. Pharmacotherapy. 2020;40:416–437. doi: 10.1002/phar.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao B, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020 [DOI] [PMC free article] [PubMed]
- 30.Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frediansyah A, et al. Antivirals for COVID-19: a critical review. Clin Epidemiol Glob Health. 2021;9:90–98. doi: 10.1016/j.cegh.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magro G. SARS-CoV-2 and COVID-19: what are our options? Where should we focus our attention on to find new drugs and strategies? Travel Med Infect Dis 2020;37: 101685 [DOI] [PMC free article] [PubMed]
- 33.Kumar P, et al. Role of ACE2 receptor and the landscape of treatment options from convalescent plasma therapy to the drug repurposing in COVID-19. Mol Cell Biochem. 2021;476:553–574. doi: 10.1007/s11010-020-03924-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cingolani A, et al. Baricitinib as rescue therapy in a patient with COVID-19 with no complete response to sarilumab. Infection. 2020;48:767–771. doi: 10.1007/s15010-020-01476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nitulescu GM, et al. Comprehensive analysis of drugs to treat SARS-CoV-2 infection: mechanistic insights into current COVID-19 therapies (Review) Int J Mol Med. 2020;46:467–488. doi: 10.3892/ijmm.2020.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Wilde AH, et al. Host factors in coronavirus replication. Curr Top Microbiol Immunol. 2018;419:1–42. doi: 10.1007/82_2017_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Vito A, et al. Does angiotensin II receptor blockers increase the risk of SARS-CoV-2 infection? A real-life experience. Eur Rev Med Pharmacol Sci. 2021;25:523–526. doi: 10.26355/eurrev_202101_24424. [DOI] [PubMed] [Google Scholar]
- 38.Zhang P, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lotfi F, et al. Micronutrient therapy and effective immune response: a promising approach for management of COVID-19. Infection 2021; 1–15 [DOI] [PMC free article] [PubMed]
- 40.Alexopoulou L, et al. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 41.Wu J, Chen ZJ. Innate immune sensing and signaling of cytosolic nucleic acids. Annu Rev Immunol. 2014;32:461–488. doi: 10.1146/annurev-immunol-032713-120156. [DOI] [PubMed] [Google Scholar]
- 42.Wu J, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seth RB, et al. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Suresh R, Mosser DM. Pattern recognition receptors in innate immunity, host defense, and immunopathology. Adv Physiol Educ. 2013;37(4):284–291. doi: 10.1152/advan.00058.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harrison DA. The Jak/STAT pathway. Cold Spring Harb Perspect Biol 2012;4. [DOI] [PMC free article] [PubMed]
- 46.Satarker S, et al. JAK-STAT pathway inhibition and their implications in COVID-19 Therapy. Postgrad Med 2020; 1–19 [DOI] [PMC free article] [PubMed]
- 47.Li HX, et al. Retinoic acid amide inhibits JAK/STAT pathway in lung cancer which leads to apoptosis. Tumour Biol. 2015;36:8671–8678. doi: 10.1007/s13277-015-3534-8. [DOI] [PubMed] [Google Scholar]
- 48.Burchill MA, et al. IL-2 receptor β-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 49.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li WX. Canonical and non-canonical JAK-STAT signaling. Trends Cell Biol. 2008;18:545–551. doi: 10.1016/j.tcb.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Shea JJ, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang Y, et al. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41:1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwartz DM, et al. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;16:843. doi: 10.1038/nrd.2017.201. [DOI] [PubMed] [Google Scholar]
- 54.Böhmer FD, Friedrich K. Protein tyrosine phosphatases as wardens of STAT signaling. Jak-stat. 2014;3:e28087. doi: 10.4161/jkst.28087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silver-Morse L, Li WX. JAK-STAT in heterochromatin and genome stability. Jak-stat. 2013;2:e26090. doi: 10.4161/jkst.26090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luo W, et al. Targeting JAK-STAT signaling to control cytokine release syndrome in COVID-19. Trends Pharmacol Sci. 2020;41:531–543. doi: 10.1016/j.tips.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwartz DM, et al. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol. 2016;12:25. doi: 10.1038/nrrheum.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh JA, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1–26. doi: 10.1002/art.39480. [DOI] [PubMed] [Google Scholar]
- 59.Jorgensen SCJ, et al. Baricitinib: a review of pharmacology, safety, and emerging clinical experience in COVID-19. Pharmacotherapy. 2020;40:843–856. doi: 10.1002/phar.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mogul A, Corsi K, McAuliffe L. Baricitinib: the second FDA-approved JAK inhibitor for the treatment of rheumatoid arthritis. Ann Pharmacother. 2019;53:947–953. doi: 10.1177/1060028019839650. [DOI] [PubMed] [Google Scholar]
- 61.Wruck W, Adjaye J. SARS-CoV-2 receptor ACE2 is co-expressed with genes related to transmembrane serine proteases, viral entry, immunity and cellular stress. Sci Rep. 2020;10:21415. doi: 10.1038/s41598-020-78402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Richardson P, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ziegler CGK, et al. SARS-CoV-2 receptor ACE2 Is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–1035.e19. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kulkarni S, et al. Repurposed immunomodulatory drugs for Covid-19 in pre-ICu patients - mulTi-Arm therapeutic study in pre-ICu patients admitted with Covid-19 - Repurposed Drugs (TACTIC-R): A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:626. doi: 10.1186/s13063-020-04535-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moreno-González G, et al. A Phase I/II Clinical Trial to evaluate the efficacy of baricitinib to prevent respiratory insufficiency progression in onco-hematological patients affected with COVID19: a structured summary of a study protocol for a randomised controlled trial. Trials. 2021;22:1–4. doi: 10.1186/s13063-021-05072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patoulias D, et al. Janus kinase inhibitors and major COVID-19 outcomes: time to forget the two faces of Janus! A meta-analysis of randomized controlled trials. Clin Rheumatol, 2021; 1–4 [DOI] [PMC free article] [PubMed]
- 67.Chen CY, et al. Clinical efficacy and safety of Janus kinase inhibitors for COVID-19: A systematic review and meta-analysis of randomized controlled trials. Int Immunopharmacol. 2021;99:108027. doi: 10.1016/j.intimp.2021.108027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bronte V, et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J Clin Invest. 2020;130:6409–6416. doi: 10.1172/JCI141772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iglesias GR, et al. Baricitinib against severe COVID-19: effectiveness and safety in hospitalised pretreated patients. Eur J Hosp Pharm 2021. [DOI] [PMC free article] [PubMed]
- 70.Titanji BK, et al. Use of baricitinib in patients with moderate to severe coronavirus disease 2019. Clin infect Dis Official Pub Infect Diseases Soc Am. 2021;72:1247–1250. doi: 10.1093/cid/ciaa879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sodani P, et al. Successful recovery from COVID-19 pneumonia after receiving baricitinib, tocilizumab, and remdesivir. A case report: Review of treatments and clinical role of computed tomography analysis. Respir Med Case Rep. 2020;31:101115. doi: 10.1016/j.rmcr.2020.101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Izumo T, et al. Clinical impact of combination therapy with baricitinib, remdesivir, and dexamethasone in patients with severe COVID-19. Respir Investig 2021 [DOI] [PMC free article] [PubMed]
- 73.Rodriguez-Garcia JL, et al. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study. Rheumatology. 2021;60:399–407. doi: 10.1093/rheumatology/keaa587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alunno A, et al. Immunomodulatory therapies for SARS-CoV-2 infection: a systematic literature review to inform EULAR points to consider. Ann Rheum Dis. 2021;80:803–815. doi: 10.1136/annrheumdis-2020-219725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giesen N, et al. 2021 update of the AGIHO guideline on evidence-based management of COVID-19 in patients with cancer regarding diagnostics, viral shedding, vaccination and therapy. Eur J Cancer. 2021;147:154–160. doi: 10.1016/j.ejca.2021.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oda N, et al. Successful treatment of critical coronavirus disease 2019 in a patient with lung cancer concomitant with pembrolizumab-induced arthritis by methylprednisolone, baricitinib, and remdesivir. Clin Case Rep. 2021;9:e04459. doi: 10.1002/ccr3.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peng L, et al. A real-world disproportionality analysis of FDA adverse event reporting system (FAERS) events for baricitinib. Expert Opin Drug Saf. 2020;19:1505–1511. doi: 10.1080/14740338.2020.1799975. [DOI] [PubMed] [Google Scholar]
- 78.Assadiasl S, et al. Baricitinib: from rheumatoid arthritis to COVID‐195. J Clin Pharmacol 2021. [DOI] [PMC free article] [PubMed]
- 79.Cantini F, et al. Systematic review on tuberculosis risk in patients with rheumatoid arthritis receiving inhibitors of Janus Kinases. Expert Opin Drug Saf. 2020;19:861–872. doi: 10.1080/14740338.2020.1774550. [DOI] [PubMed] [Google Scholar]
- 80.Hsu JY, et al. Pharmacology and adverse events of emergency-use authorized medication in moderate to severe COVID-19. Pharmaceuticals (Basel) 2021;14. [DOI] [PMC free article] [PubMed]
- 81.Cantini F, et al. Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kalil AC, et al. Baricitinib plus Remdesivir for hospitalized adults with Covid-19. N Engl J Med 2020 [DOI] [PMC free article] [PubMed]
- 83.Bhimraj A, Shumaker AH, Lavergne V, Baden L, Cheng VC, Edwards KM, Gandhi R, Gallagher J, Muller WJ, O'Horo JC, Shoham S, Murad MH, Mustafa RA, Sultan S, Falck-Ytter Y. Infectious diseases society of america guidelines on the treatment and management of patients with COVID-19. 2021; 5.5.1. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. (Accessed date 21 Apr 2020) [DOI] [PMC free article] [PubMed]
- 84.Favalli EG, et al. COVID-19 infection and rheumatoid arthritis: Faraway, so close! Autoimmun Rev 2020;19(5): 102523 [DOI] [PMC free article] [PubMed]
- 85.Fleming SB. Viral Inhibition of the IFN-Induced JAK/STAT signalling pathway: development of live attenuated vaccines by mutation of viral-encoded IFN-antagonists. Vaccines, 2016;4 [DOI] [PMC free article] [PubMed]
- 86.Channappanavar R, et al. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J Clin Invest. 2019;129:3625–3639. doi: 10.1172/JCI126363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mehta P, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Majumder J, Minko T. Recent developments on therapeutic and diagnostic approaches for COVID-19. AAPS J. 2021;23:14. doi: 10.1208/s12248-020-00532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021; 397: 1637–45 [DOI] [PMC free article] [PubMed]
- 90.Ansems K, et al. Remdesivir for the treatment of COVID-19. Cochrane Database Syst Rev. 2021;8:Cd14962. doi: 10.1002/14651858.CD014962. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.