Abstract
Two distinct signaling pathways regulate the survival of interleukin-3 (IL-3)-dependent hematopoietic progenitors. One originates from the membrane-proximal portion of the cytoplasmic domain of the IL-3 receptor (βc chain), which is shared by IL-3 and granulocyte-macrophage colony-stimulating factor and is involved in the regulation of Bcl-xL through activation of STAT5. The other pathway emanates from the distal region of the βc chain and overlaps with downstream signals from constitutively active Ras proteins. Although the latter pathway is indispensable for cell survival, its downstream targets remain largely undefined. Here we show that the expression of Bim, a member of the BH3-only subfamily of cell death activators, is downregulated by IL-3 signaling through either of two major Ras pathways: Raf/mitogen-activated protein kinase and the phosphatidylinositol 3-kinase/mammalian target of rapamycin. Akt/phosphokinase B does not appear to play a significant role in this regulatory cascade. Bim downregulation has important implications for cell survival, since enforced expression of this death activator at levels equivalent to those induced by cytokine withdrawal led to apoptosis even in the presence of IL-3. We conclude that Bim is a pivotal molecule in cytokine regulation of hematopoietic cell survival.
Homeostasis, responses to emergency situations, and the transition from emergency to normal status in the hematopoietic system are all regulated by cytokines, through control of cell division, differentiation, and survival. Cytokine regulation of apoptosis not only plays a critical role in normal hematopoiesis but also can contribute to leukemogenesis, when one or more control elements fail to function properly. In recent attempts to clarify the transduction pathways through which cytokines control cell survival, we found that such signaling can involve multiple independent pathways (20, 31).
By contrast, cell death decisions are implemented through an evolutionarily conserved mechanism (or general apoptosis program) in which members of the Bcl-2 superfamily play the central roles (reviewed in references 1 and 6). The anti- or proapoptotic family members regulate the translocation of cytochrome c from mitochondria to the cytosol, an event that ultimately activates the caspase cascade, while the BH3-only subfamily of cell death activators inhibit the function of the antiapoptotic Bcl-2 family members by binding to them. In the nematode Caenorhabditis elegans, Egl-1, the sole BH3-only death activator in this organism, functions at the most upstream point of this system to inhibit the function of Ced-9, again the sole antiapoptotic member of the Bcl-2 family in C. elegans (8). In mammals, six proteins have been isolated as members of the BH3-only subfamily and five have been identified as antiapoptotic and three as proapoptotic members of the Bcl-2 family. Redundancy in each category of the Bcl-2 superfamily could be explained, at least partially, by the tissue- and/or stimulus-specific response of each family member. For instance, the antiapoptotic Bcl-w protein is required for the support of Sertoli cells in the testes but not of other cells, as demonstrated in studies with Bcl-w-deficient mice (39), while Bid, a member of BH3-only subfamily, is involved in Fas-mediated apoptosis in hepatocytes (48). Thus, several members of the Bcl-2 superfamily might play key roles in the response of hematopoietic progenitors to cytokine withdrawal.
Previous studies have demonstrated the importance of Ras pathways, not only in oncogenesis when constitutively activated by oncogenic Ras mutants (reviewed in reference 26) but also in the regulation of apoptosis in hematopoietic progenitors (reviewed in reference 33). The membrane-distal region of the cytoplasmic domain of the common β (βc) chain is required for both the survival and activation of Ras in interleukin-3 (IL-3)-dependent hematopoietic cells (28). Recently, both Raf/mitogen-activated protein kinase (MAPK) and rapamycin/wortmannin-sensitive (most probably phosphatidylinositol 3-kinase [PI3-K]-dependent) pathways downstream of active Ras were found to prevent apoptosis in cytokine-deprived hematopoietic cells (30). These findings suggest that certain Bcl-2 family members under the control of Ras pathways may be pivotal factors in the cytokine-mediated regulation of apoptosis in hematopoietic progenitors.
We and others reported previously that the expression levels of Bcl-xL are downregulated in cytokine-deprived murine IL-3-dependent cells and that enforced expression of Bcl-xL in these cells markedly delays apoptosis due to IL-3 deprivation (31, 32). We also demonstrated that signals originating from the proximal portion of the βc chain, but not from Ras pathways, are required for the induction of Bcl-xL expression (31), in agreement with recent published results indicating that STAT5 is involved in the transcriptional regulation of Bcl-xL (15, 42, 43). Thus, although Bcl-xL is considered to be an important target of cytokine-dependent survival pathways, it is probably not the Bcl-2 family member predicted to interact with Ras pathways.
Another candidate is Bad, a BH3-only subfamily member that is inactivated by the phosphorylation of two serine residues through Akt and other kinases downstream of Ras pathways (12, 13, 50). However, the contribution of Akt/Bad pathways to the apoptosis regulation system in hematopoietic cells has been controversial. Some investigators contend that Akt reverses apoptosis caused by cytokine withdrawal but does so much less efficiently than activated Ras does (2, 44), while others rule out the involvement of Akt/Bad pathways in the survival of hematopoietic progenitors (3, 16, 41).
A final candidate is Bim/Bod. This BH3-only death activator was isolated independently by two groups that exploited its ability to bind Bcl-2 or Mcl1 (18, 34). Alternative splicing gives rise to three variants, BimEL, BimL, and BimS, each of which contains the BH3 domain and functions as a death inducer. In certain cell types, BimEL and BimL, but not BimS, bind to an Mr = 8,000 dynein light chain, LC8 (also known as PIN or Dlc-1) (11, 25, 27), which mediates the function of the former two isoforms (38). The biological relevance of Bim to the control of hematopoiesis was indicated in a recent study of Bim-deficient mice, in which the homeostasis of the lymphoid system was disrupted without resulting in apparent adverse effects on other organs (4). Here we identify Bim downregulation through either of two major Ras pathways (Raf/MAPK or PI3-K/mTOR) as a pivotal step in the maintenance of hematopoietic cell survival under the control of cytokines.
MATERIALS AND METHODS
Cell culture and cell growth assay.
Murine IL-3-dependent (Baf-3, FL5.12, and 32D) cells were cultured in RPMI 1640 medium containing 10% fetal calf serum, 20 mM HEPES, 50 μM 2-mercaptoethanol, and 0.5% 10T1/2 conditioned medium as a source of murine IL-3. In some experiments, recombinant mouse IL-3 (Wako Pure Chemical, Osaka, Japan) was used at the concentrations indicated in the figure legends. To deplete IL-3, we washed the cells twice with IL-3-free growth medium. Viable-cell counts were determined by trypan blue dye exclusion in triplicate assays. BOSC23 cells, an ecotropic retrovirus packaging cell line, were purchased from the American Type Culture Collection and cultured in Dulbecco's modified Eagle's medium containing 10% fetal calf serum.
Enforced expression of genes of interest in Baf-3 cells.
Stable transfectants of truncated forms of the human granulocyta-macrophage colony-stimulating factor (GM-CSF) receptor and dexamethasone (Dex)-inducible Ras mutants were established in Baf-3 cells, as described previously (30, 40). Zn-inducible cells were generated by previously published methods (24). Transfected gene products were induced by the addition of ZnSO4 in culture medium at 100 μM unless otherwise specified in the figure legends. The BimELns mutant, which expresses BimEL alone, was made by replacing a nucleotide in the splicing donor site for BimL mRNA without amino acid replacement. Transfectants were maintained in medium containing either 1 mg of G418 per ml or 200 μg of hygromycin per ml. For retrovirus-mediated gene expression, we constructed a control CD8-expressing vector plasmid (pMX/IRES-CD8) from the pMX retroviral vector (a gift of T. Kitamura) (36) by inserting an IRES-CD8 cassette in which the mouse CD8 cDNA was fused in frame to the internal ribosomal entry site (IRES) sequence. Particular genes were expressed by inserting their cDNAs immediately after the 5′ long terminal repeat sequence. A dominant negative mutant of Akt (MAA-phosphokinase B [PKB]) was made by including the K179M, T308A, and S473A substitutions as described by Wang et al. (46). Retrovirus was made by the method of Onishi et al. (36) using BOSC23 cells. Retroviral infection of Baf-3 cells and the selection of CD8-positive cells with a CD8 monoclonal antibody and MACS separation columns (Miltenyi Biotec) were performed by a method described previously (31). The selection procedure was repeated until more than 95% cells were positive for CD8 by flow cytometry.
Immunoprecipitation and immunoblot analysis.
For immunoblot analysis, cells were solubilized in Nonidet P-40 (NP-40) lysis buffer (150 mM NaCl, 1.0% NP-40, 50 mM Tris [pH 8.0]) containing protease inhibitor mixture (Complete; Roche Molecular Biochemicals); total cellular proteins were separated by sodium dodecyl sulfate-polyacrylanide gel electrophoresis (SDS-PAGE). For detecting the phosphorylation of MAPK, a phosphatase inhibitor mixture (50 mM sodium fluoride, 10 mM sodium pyrophosphate, 2 mM sodium orthovanadate) was added to the lysis buffer. Cell lysates extracted from 106 living cells were applied per lane unless otherwise specified in the figure legends. After their wet electrotransfer onto polyvinylidene difluoride membranes, the proteins were detected with appropriate antibodies following standard procedures. The blots were then stained with primary antibodies followed by horseradish peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin secondary antibodies and subjected to chemiluminescence detection as specified by the manufacturer (Amersham). Metabolic labeling and immunoprecipitation were performed by previously described standard methods (49).
Phosphatase treatment of Bim.
Bim was immunoprecipitated from 107 Baf-3 cells overexpressing BimEL and BimL proteins. The protein A-Sepharose-coated beads–Bim complexes were resuspended in 50 μl of the reaction buffer (100 mM NaCl, 50 mM Tris, 10 mM MgCl2, 1 mM dithiothreitol [pH 7.9] at 25°C) containing protease inhibitor mixture. After 5 U of calf intestinal alkaline phosphatase (New England Biolabs, Beverly, Mass.) were added, the samples were incubated at 37°C for 30 min. Some samples contained the phosphatase inhibitor mixture described above. The protein A-Sepharose-coated beads were pelleted by centrifugation, washed three times with NP-40 lysis buffer, resuspended in gel-loading buffer, and examined by immunoblot analysis.
Immunocomplex kinase assay.
Akt was immunoprecipitated with Akt antibody (New England Biolabs) from 107 parental Baf-3 cells or cells expressing a dominant negative form of Akt. The protein A-Sepharose-coated beads–Akt complexes were resuspended in 50 μl of reaction buffer (200 mM HEPES-NaOH, 100 mM MgCl2, 100 mM MnCl2 [pH 7.4]) containing 100 μg of histone H2B (Roche Molecular Biochemicals) per ml, 5 μM ribosomal ATP, and 10 μCi of [γ-32P]ATP. After incubation of the mixture for 30 min at room temperature, gel-loading buffer was added to the beads, which were then boiled for 5 min. Samples were separated on SDS–15% polyacrylamide gels and viewed by autoradiography.
Analysis of mRNA expression.
Total cellular RNA was isolated with the RNeasy kit as specified by the manufacturer (Qiagen, Hildes, Germany). The RNase protection assay was performed with a commercial kit (Ambion, Austin, Tex.) as specified by the, manufacturer. Briefly, RNA samples (10 μg each) were hybridized with an 32S-UTPαS-labeled RNA probe, which protects a 265-bp fragment in mouse BimEL and BimL mRNA and a 210-bp fragment in BimS mRNA. After single-stranded RNA was digested by RNase A, samples were separated on a 5% acrylamide–8 M urea gel and viewed by autoradiography.
Reagents.
Bim polyclonal antibodies were raised against glutathione S-transferase fusion proteins containing amino acids 9 to 53 of mouse BimL as previously described (22). mTOR and AU-1 monoclonal antibodies were described previously (17). Bcl-x and Bcl-2 polyclonal antibodies were purchased from Transduction Laboratories (Lexington, Ky.), while Akt and phosphorylated (Ser473) Akt-specific antibodies were purchased from New England Biolabs. Etoposide, wortmannin, and rapamycin were purchased from Sigma (St. Louis, Mo).
RESULTS
Bim is induced in cytokine-deprived murine IL-3-dependent cells.
We first analyzed the expression of Bcl-2 superfamily members in Baf-3 murine IL-3-dependent pro-B-lymphoid cells in the presence or absence of IL-3. As previously reported by us and others (29, 31, 32), the expression levels of Bcl-xL were downregulated in IL-3-starved Baf-3 cells (Fig. 1A, top panel). In addition, when cells were cultured in IL-3-containing medium, Bim mRNA was barely detectable by the RNase protection assay but its expression was rapidly induced by IL-3 starvation (Fig. 1B, top panel). By immunoblot analysis, we detected a small amount of BimEL and BimL but no BimS in cells cultured in the presence of IL-3, while increased amounts of all three Bim proteins were observed within 24 h in cells cultured in the absence of IL-3 (Fig. 1C, top panel). BimEL proteins expressed in cells cultured in IL-3-containing medium seemed to migrate with several additional bands, indicating that BimEL could be phosphorylated by IL-3-signaling pathways. Bim was similarly induced by IL-3 withdrawal in FL5.12 pro-B-lymphoid and 32D myeloid cells (data not shown).
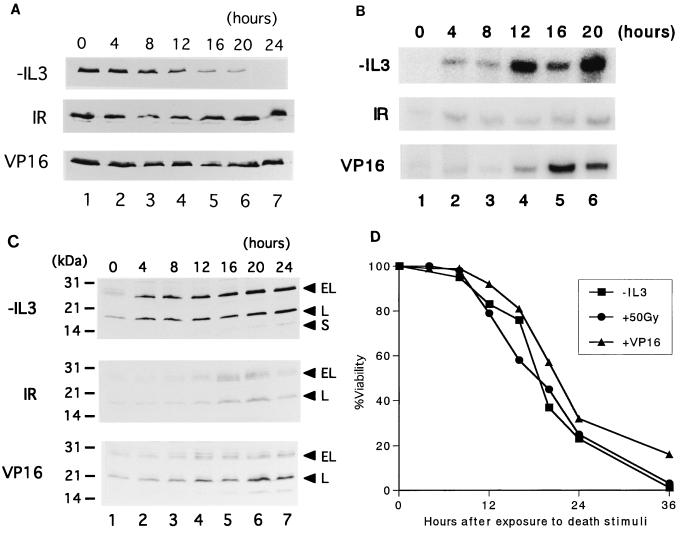
FIG. 1.
Expression of Bcl-xL and Bim in Baf-3 cells undergoing apoptosis induced by various death stimuli. (A to C) Baf-3 cells were cultured in IL-3-free medium (top panel), treated with 50 Gy of ionizing radiation (IR) and cultured with IL-3 (middle panel), or cultured in medium containing IL-3 and etoposide (VP16) at 3 μg/ml (bottom panel) for the indicated times. The expression of Bcl-xL protein (A), Bim mRNA (B), or Bim protein (C) was detected by specific antibodies for each protein (A and C) or by an RNase protection assay with a cDNA probe designed to detect mouse BimEL and BimL mRNA (B). (D) Percent viability, determined by trypan blue dye exclusion of Baf-3 cells treated with the various cell death inducers at the time of sample collection.
To determine whether the simultaneous upregulation of Bim and downregulation of Bcl-xL is specific for cells undergoing apoptosis due to IL-3 withdrawal or is a common response of Baf-3 cells to various apoptotic stimuli, we treated Baf-3 cells in the presence of IL-3 with 50 Gy of ionizing radiation or etoposide at 3 μg/ml, each of which induces apoptosis in roughly the same time as IL-3 withdrawal does (5) (Fig. 1D). This experiment showed that there was little change in protein expression levels of Bcl-xL (Fig. 1A, middle and bottom panels). Although Bim expression was somewhat induced by these death stimuli, the induction levels were not so prominent as that induced by IL-3 starvation (Fig. 1B and C, middle and bottom panels), indicating that upregulation of Bim accompanied by downregulation of Bcl-xL appears specific for cells undergoing apoptosis induced by cytokine withdrawal.
Enforced expression of Bim induces apoptosis in Baf-3 cells.
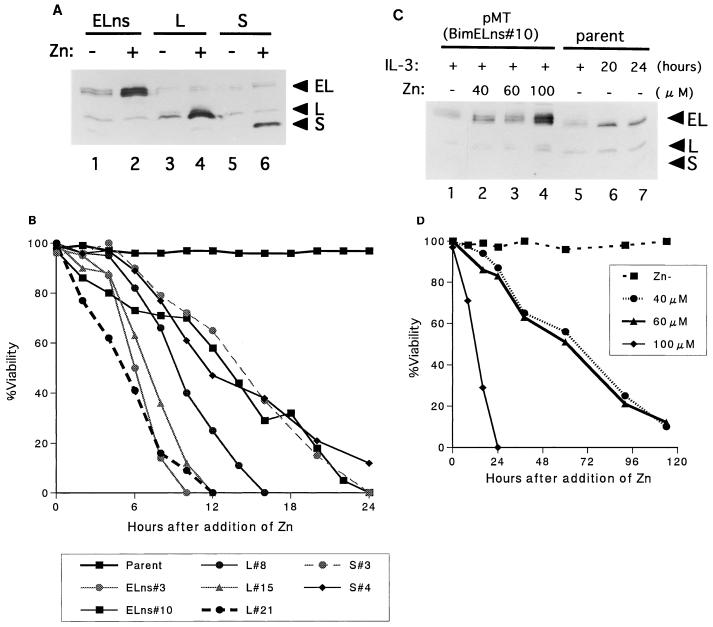
To test whether Bim protein contributes to apoptosis induced by IL-3 starvation, we established Baf-3 cells that expressed each Bim protein after Zn induction. Because the coding region of BimEL cDNA contains both the alternative splicing donor and acceptor sites for BimL mRNA, cells transduced with native BimEL cDNA expressed roughly equal amounts of BimEL and BimL proteins (see Fig. 3A, lane 3). Thus, before determining the biological activity of each Bim protein, we mutated the splice donor site in BimEL cDNA to generate BimEL alone (see Materials and Methods). As shown in Fig. 2A, we obtained clones that expressed BimEL, BimL, and BimS protein upon induction with Zn at 100 μM. Cells strongly expressing any form of Bim protein rapidly underwent apoptosis, even when the culture medium contained an excess dose of IL-3 (Fig. 2B). To determine the antiapoptotic effects of Bim proteins expressed at levels equivalent to those in IL-3-starved Baf-3 cells, we induced the BimEL protein with lower concentrations of Zn, 40 or 60 μM (Fig. 2C). Cells cultured under these conditions still underwent apoptosis in the presence of IL-3 (Fig. 2D); similar results were obtained with clones expressing BimL (data not shown). Taken together, these data indicate that expression levels of Bim associated with IL-3 withdrawal are high enough to reverse the antiapoptotic effects of IL-3.
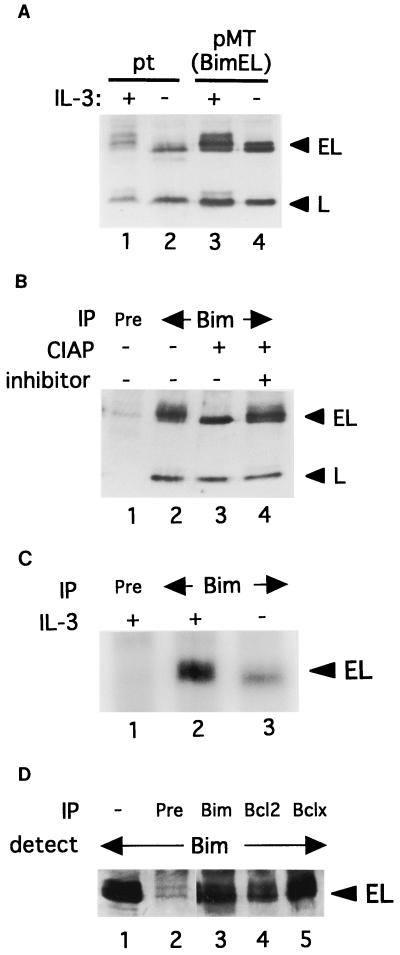
FIG. 3.
Bim phosphorylation. (A) Immunoblot analysis using the Bim antibody with lysates from parental Baf-3 cells (pt) (lanes 1 and 2) or cells overexpressing BimEL and BimL (lanes 3 and 4) cultured in the presence (lanes 1 and 3) or absence (lanes 2 and 4) of IL-3. To demonstrate an extra band comigrating with BimL, we added five times more cell extract to lane 1 than to the other lanes. (B) Lysates from Baf-3 cells overexpressing BimEL and BimL proteins were immunoprecipitated (IP) with preimmune serum (lane 1) or Bim antibody (lanes 2 to 4). After treatment with CIAP in the absence (lane 3) or presence (lane 4) of phosphatase inhibitor, precipitants were separated with SDS-PAGE and Bim proteins were then detected with Bim antibody. (C) Baf-3 cells overexpressing BimEL protein were cultured in medium containing [32P]orthophosphate in the presence (lanes 1 and 2) or absence (lane 3) of IL-3 for 4 h. Immunoprecipitates obtained by preimmune serum (lane 1) or Bim antibody (lanes 2 and 3) were separated by SDS-PAGE. (D) Lysates from Baf-3 cells overexpressing BimEL protein were immunoprecipitated with preimmune serum (lane 2) or antibody against Bim (lane 3), Bcl-2 (lane 4), or Bcl-x (lane 5). After immunoprecipitation and SDS-PAGE followed by blotting, Bim proteins were detected with Bim antibody. Lane 1 is a positive control to detect BimEL protein in whole-cell lysates.
FIG. 2.
Induction of apoptosis by each Bim isoform. (A) Immunoblot analysis of Baf-3 cells engeneered to express BimEL (lanes 1 and 2), BimL (lanes 3 and 4), or BimS (lanes 5 and 6) in the absence (lanes 1, 3, and 5) or presence (lanes 2, 4, and 6) of zinc (100 μM). Samples were collected 4 h after the addition of Zn. (B) Percent viability of cells expressing BimEL, BimL, or BimS after the addition of Zn (100 μM) in the presence of IL-3 (5 ng/ml). (C) Baf-3 cells inducibly expressing BimEL, pMT(BimELns clone 10), were cultured in IL-3-containing medium (5 ng/ml) with various Zn concentrations for 4 h (lanes 1 to 4). For comparison of expression levels, cell extracts from parental Baf-3 cells cultured in the absence of IL-3 for the indicated times (lanes 5 to 7) were blotted on the same membrane. Bim proteins were detected by immunoblot analysis. (D) Percent viability of pMT(BimELns clone 10) cells cultured in IL-3-containing medium (5 ng/ml) with various concentration of Zn.
BimEL and BimL are phosphorylated by IL-3 signaling.
The additional bands of BimEL observed in the immunoblot analysis of lysates from Baf-3 cells cultured in IL-3-containing medium (Fig. 1C, lane 1) raised the possibility that Bim is phosphorylated through IL-3 signaling. Overexposure of immunoblots revealed that not only BimEL, but also BimL, corresponded to a slowly migrating band in lysates from cells growing in IL-3-containing medium but not those cultured in IL-3-free medium (Fig. 3A, lanes 1 and 2). Extracts from cells overexpressing BimEL and BimL proteins from the pMT-BimEL vector showed a similar pattern of Bim protein expression (lanes 3 and 4). To test whether these additional bands are phosphorylated Bim proteins, we treated immunoprecipitated Bim proteins with calf intestine alkaline phosphatase (CIAP). All Bim proteins were detected by Bim antibody but not by preimmune serum in immunoprecipitates from cells overexpressing BimEL and BimL (Fig. 3B, lanes 1 and 2). The more slowly migrating bands of BimEL and BimL were eliminated, apparently by a shift to the faster-migrating bands due to treatment with CIAP lacking phosphatase inhibitor (lane 3), but were retained by treatment with CIAP containing phosphatase inhibitor (lane 4), indicating that BimEL and BimL are phosphorylated through IL-3 signaling. To confirm these results, we labeled Baf-3 cells overexpressing BimEL with [32P]orthophosphate for 2 h, with or without IL-3, and immunoprecipitated Bim protein. The results in Fig. 3C clearly show that BimEL protein was hyperphosphorylated in the presence of IL-3.
Because phosphorylated Bad cannot interact with members of the antiapoptotic Bcl-2 family (50), we tested whether phosphorylated Bim proteins can bind to Bcl-xL and Bcl-2. Extracts of Baf-3 cells inducibly expressing BimEL were immunoprecipitated with preimmune serum (Fig. 3D, lane 2), anti-Bim (lane 3), anti-Bcl-2 (lane 4), and anti-Bcl-x (lane 5) antibodies, and the proteins bound to protein A-Sepharose-coated beads were separated and blotted. Immunodetection by Bim antiserum revealed that both the hyperphosphorylated and hypophosphorylated forms of BimEL are coimmunoprecipitated with Bcl-2 and Bcl-x (lanes 4 and 5). Similar results were obtained with extracts from BimL-expressing cells (data not shown). Thus, unlike the case with Bad, phosphorylation of Bim does not affect the ability of the protein to bind to members of the antiapoptotic Bcl-2 family.
Either the Ras/Raf/MAPK or the Ras/PI3-K pathway is sufficient to downregulate Bim expression.
To identify the signaling protein(s) through which IL-3 regulates Bim expression, we first used Baf-3 cells expressing the human βc chain truncated at amino acid 544 (β544 cells). Stimulation of the receptor with human GM-CSF activates signaling molecules near the membrane-proximal region of the βc chain, such as JAK2/STAT5, but does not affect components of the Ras signaling pathways (40).
As expected, the β544 cells grew well in IL-3-containing medium but underwent rapid apoptosis in the absence of the cytokine. When cultured with human GM-CSF, the cells proliferated for approximately 72 h essentially the same rate as did cells in IL-3-containing medium with no apparent loss of viability; thereafter, they underwent cell cycle arrest followed by rapid apoptosis, as in earlier studies (28, 31) (Fig. 4A). Immunoblot analysis showed that the expression levels of Bim remained unaltered for about 12 h, subsequently reaching levels equivalent to those in IL-3-starved Baf-3 cells (Fig. 4B, upper panel). By contrast, as we previously reported (31), Bcl-xL protein levels were maintained for more than 5 days before the cells underwent apoptosis (Fig. 4B, bottom panel). These results indicate that signals originating from the membrane-distal region of the βc chain are required for the regulation of Bim expression and hematopoietic cell survival.
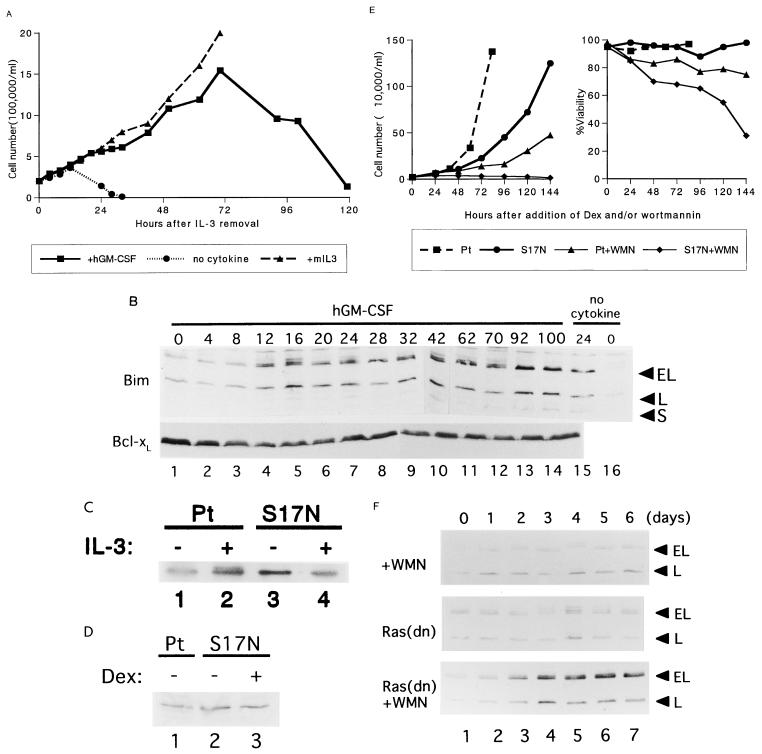
FIG. 4.
Identification of upstream pathways regulating Bim expression. (A) IL-3 was washed from the culture medium of β544 cells, and the cells were cultured in the presence of human GM-CSF (hGM-CSF) or murine IL-3 (mIL3) or in the absence of cytokines. The number of viable cells was determined by trypan blue dye exclusion. (B) Immunoblot analysis using lysates from β544 cells cultured in human GM-CSF (lanes 1 to 14) or in the absence of cytokines (lanes 15 and 16) for the indicated times. Bim (top panel) and Bcl-xL (bottom panel) proteins were detected by specific antibodies to each protein. (C) Phosphorylation of p42/MAPK. Parental Baf-3 cells (lanes 1 and 2) or cells expressing Ras(S17N) induced by the addition of 10−7 M Dex (lanes 3 and 4) were cultured in IL-3-free medium for 2 h (lanes 1 and 3), after which IL-3 was added at 20 ng/ml for 5 min (lanes 2 and 4). p42/MAPK was detected by immunoblot analysis using a specific monoclonal antibody. Cell extracts from 105 cells were loaded per lane. (D) Parental Baf-3 cells (lane 1) or cells containing Dex-inducible Ras(S17N) in the presence or absence of Dex (lanes 2 and 3) were cultured in IL-3-containing medium. The phosphorylated form of Akt was detected with antibody specifically recognizing phosphorylated Akt at Ser473. (E) Parental Baf-3 cells and cells expressing Ras(S17N) were cultured in the presence or absence of wortmannin (WMN). The number of viable cells (left panel) and the percent viability determined by trypan blue dye exclusion (right panel) are shown. The results are from a representative experiment; similar results were obtained in three other independent experiments. (F) Immunoblot analysis with Bim antibodies. Parental Baf-3 cells treated with wortmannin at 0.5 μM (top panel) or cells expressing Ras(S17N) induced by addition of 10−7 M Dex in the absence (middle panel) or presence (bottom panel) of wortmannin were cultured in IL-3-containing medium (5 ng/ml). Cell extracts were prepared after the indicated times.
To determine the downstream pathways of the distal βc chain that regulate Bim expression, we used Baf-3 cells conditionally expressing a dominant negative Ras protein, Ras(S17N), and the PI3-K inhibitor wortmannin. Induction of Ras(S17N) in Baf-3 cells by Dex abrogated the activation of MAPK by IL-3 stimulation (Fig. 4C) but did not affect the activation of PI3-K, as judged by the phosphorylation of Akt (Fig. 4D). In agreement with earlier reports (35, 45), expression of Ras(S17N) in Baf-3 cells impeded cell growth but did not induce apoptosis (Fig. 4E). Baf-3 cells cultured in wortmannin at 0.5 μM also proliferated more slowly, but only a limited number of cells underwent apoptosis. However, when Baf-3 cells expressing Ras(S17N) were cultured in medium containing wortmannin, they virtually all underwent apoptosis, confirming the notion that at least one of the Ras-activated pathways is required for cell survival (28).
Bim expression was downregulated in both Baf-3 cells expressing Ras(S17N) and those cultured with wortmannin (Fig. 4F, top and middle panels). When cells expressing Ras(S17N) were cultured in wortmannin, Bim proteins were induced after 48 to 72 h (bottom panel), a time comparable to that when Bim protein was induced in β544 cells cultured in human GM-CSF (Fig. 4B). These results indicate that activation of either the Ras/MAPK or Ras/PI3-K pathway is sufficient to suppress Bim expression.
Involvement of mTOR in cytokine-regulated cell survival.
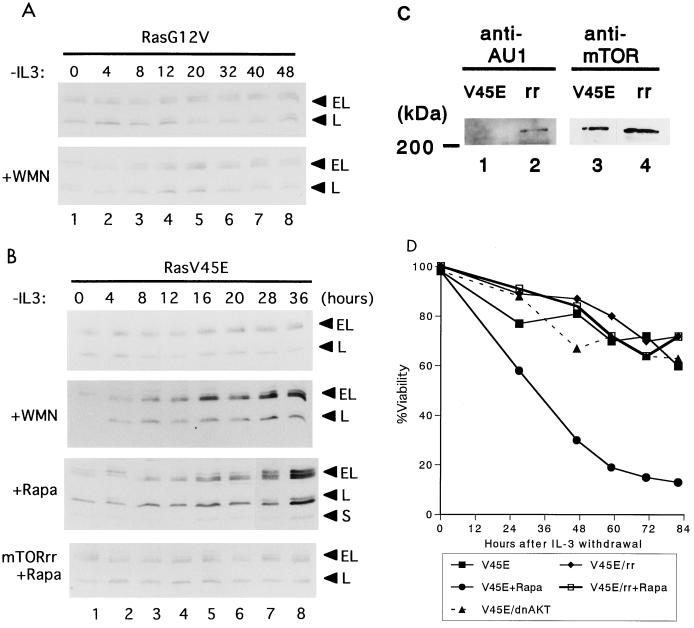
To further dissect the Ras/PI3-K signaling pathways, we used Baf-3 cells conditionally expressing constitutively active Ras mutants induced by Dex. We and others previously reported (28, 31) that the Ras(G12V) mutant, when expressed in Baf-3 cells, activates both the Raf/MAPK and PI3-K pathways, while the Ras(G12V/V45E) mutant activates the PI3-K but not the Raf/MAPK pathway, as judged by the phosphorylation of p42/MAPK, Akt, or p70/S6 kinase (Table 1). Baf-3 cells expressing either Ras mutant survive for prolonged times in IL-3-free medium, while the antiapoptotic effect of Ras(G12V/V45E) but not that of Ras(G12V) is reversed by either the PI3-K inhibitor wortmannin or the mTOR inhibitor rapamycin (Table 1; Fig. 5D)
TABLE 1.
Summary of activation of Ras mutants
| Mutant | Phosphorylation of:
|
% Viability 48 h after IL-3 starvation | ||
|---|---|---|---|---|
| p42/MAPK | p70/S6K | Akt | ||
| Ras(G12V) | + | + | + | >90 |
| Ras(G12V) + wortmannin | + | − | − | 80 |
| Ras(G12V) + rapamycin | + | − | + | 80 |
| Ras(G12V/V45E) | − | + | + | 75 |
| Ras(G12V/V45E) + wortmannin | − | − | − | <10 |
| Ras(G12V/V45E) + rapamycin | − | − | + | <20 |
FIG. 5.
mTOR participates in the regulation of Bim by PI3-K pathways. (A and B) Immunoblot analysis with Bim antibodies. Cells were cultured in IL-3-free medium for the indicated times. Baf-3 cells containing Dex-inducible Ras(G12V) were cultured with 10−7 M Dex in the absence (upper panel) or presence (lower panel) of wortmannin (WMN) (0.5 μM) (A). Baf-3 cells containing Dex-inducible Ras(G12V/V45E) were cultured with 10−7 M Dex (top panel), in the presence of wortmannin (upper middle panel) or rapamycin (Rapa) (10 ng/ml; lower middle panel); Baf-3 cells containing Dex-inducible Ras(G12V/V45E) were engeneered to express an AU1-tagged rapamycin-resistant form of mTOR (AU1-mTOR-rr) (see Materials and Methods) and were cultured with rapamycin (bottom panel) (B). (C) Immunoblot analysis of AU1-tagged mTOR-rr and an endogenous mTOR protein in cell extracts from Baf-3 cells containing Dex-inducible Ras(G12V/V45E) (V45E; lanes 1 and 3) or expressing AU1-mTOR-rr (rr, lanes 2 and 4), using monoclonal antibodies specific for AU1 (lanes 1 and 2) and mTOR (lanes 3 and 4). (D) Percent viability of cells expressing Ras(G12V/V45E), with or without mTOR-rr (rr), in the presence or absence of rapamycin in IL-3-free medium. Also shown is that of cells expressing Ras(G12V/V45E) and MAA-PKB in IL-3-free medium.
To test whether Bim expression is involved in the wortmannin- or rapamycin-sensitive pathways, we performed immunoblot analysis using cell lysates from Baf-3 cells that expressed either Ras(G12V) or Ras(G12V/V45E), with or without wortmannin or rapamycin, in IL-3-free medium. As expected, Bim was downregulated in cells expressing Ras(G12V) whether or not wortmannin was present (Fig. 5A). Downregulation of Bim was also observed in Baf-3 cells expressing Ras(G12V/V45E) (Fig. 5B, top panel); however, in contrast to Ras(G12V), this effect was reversed by wortmannin or rapamycin (top and lower middle panels), suggesting that rapamycin-sensitive pathways (mTOR/S6-K) play a role in the antiapoptotic pathways downstream of PI3-K.
To confirm that mTOR is involved in the regulation of cytokine-mediated cell survival, we used a mutant form of mTOR that is resistant to rapamycin (Ser2035 →Ile; designated mTOR-rr) (7). Retrovirus containing the cDNA of mTOR-rr, tagged with the AU1 sequence at its 5′ end and murine CD8 (lyt-2) as a marker, was transfected to Baf-3 cells inducibly expressing Ras(G12V/V45E) (see Materials and Methods). CD8-expressing cells, selected by magnetic beads, were demonstrated to express mTOR-rr by immunoblot analysis with a monoclonal antibody specific for the AU1 peptide (Fig. 5C, lane 2), which comigrated with endogenously expressed mTOR protein (lane 3). These cells were then cultured in IL-3-free, Dex-containing medium, with or without rapamycin. As shown in Fig. 5D, cells expressing both Ras(G12V/V45E) and mTOR-rr survived in the presence as well as the absence of rapamycin. Moreover, Bim was downregulated in these cells (Fig. 5B, bottom panel), indicating that mTOR contributes to cell survival by downregulating Bim expression.
Lack of Akt involvement in cytokine-mediated regulation of apoptosis.
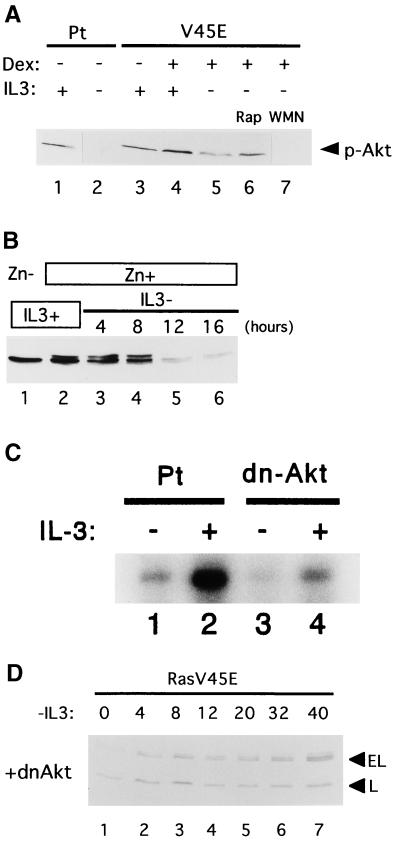
The apparent reversal of the antiapoptotic effects of Ras(G12V/V45E) by rapamycin raised questions about the contribution of Akt to downstream regulation of apoptosis through Ras pathways. Indeed, the phosphorylation levels of Akt in dying cells [Ras(G12V/V45E)-expressing cells cultured in IL-3-free medium with rapamycin] (Fig. 6A, lane 6) were equivalent to those in healthy cells (i.e., those without rapamycin or parental Baf-3 cells cultured in IL-3-containing medium) (lanes 1 and 5). To directly assess the regulatory role of Akt in Baf-3 cells, we established cells that expressed myristylated Akt (myr-Akt) after Zn induction. Although myr-Akt was readily induced with Zn, migrating slightly slower than endogenous Akt (Fig. 6B, lane 2), its expression was quickly downregulated when IL-3 was removed. Substitution of a retroviral long terminal repeat for the metallothionein promoter failed to stimulate constant expression of myr-Akt in IL-3-starved Baf-3 cells (data not shown), suggesting that this myristylated protein is downregulated by posttranscriptional mechanisms. Because rapid downregulation of myr-Akt was also observed in 32D and FL5.12 cells (data not shown), we could not determine the antiapoptotic effect of myr-Akt.
FIG. 6.
(A) Phosphorylation of Akt in Baf-3 cells. Parental (Pt) Baf-3 cells were cultured in the presence (lane 1) or absence (lane 2) of IL-3 for 12 h. Baf-3 cells containing Dexinducible Ras(G12V/V45E) were cultured in IL-3-containing medium without (lane 3) or with (lane 4) 10−7 M Dex for 16 h. The Dex-treated cells were then cultured in IL-3-free, Dex-containing medium for 12 h without wortmannin (WMN) or rapamycin (Rap) (lane 5), with 10 ng/ml rapamycin (lane 6), or with 100 nM wortmannin (lane 7). Akt phosphorylated at Ser473 (top panel) was detected using specific antibodies. (B) Expression of total Akt protein in IL-3-starved Baf-3 cells as detected by immunoblot analysis with antibody recognizing both phosphorylated and nonphosphorylated Akt. Baf-3 cells engineered to express myr-Akt upon addition of Zn were cultured in IL-3-containing medium in the absence (lane 1) or presence (lane 2) of 100 μM Zn for 16 h. The cells were then transferred into IL-3-free medium containing Zn for the indicated times. (C) Akt kinase assay. Parental cells (Pt) (lanes 1 and 2) and cells expressing a dominant-negative form of Akt (dn-Δkt) (lanes 3 and 4) were starved of IL-3 for 2 h (lanes 1 and 3) and then cultured in the presence of IL-3 (20 ng/ml) for 5 min (lanes 2 and 4). The kinase activity of Akt was determined by histone H2B phosphorylation in immunoprecipitates using Akt antibody. (D) Baf-3 cells containing Dex-inducible Ras(G12V/V45E) were retrovirally engineered to express a dominant negative form of Akt (MAA-PKB) (dnΔkt) and then were cultured in IL-3-free, Dex-containing medium for the indicated times.
We then used a dominant negative form of Akt (MAA-PKB; see Materials and Methods) (46) to investigate the possible role of Akt kinase in apoptosis regulation by Ras/PI3-K pathways. Retrovirus containing the cDNAs of MAA-PKB and CD8 was used to infect Baf-3 cells inducibly expressing Ras(G12V/V45E). Although selected cells lacked Akt kinase activity (Fig. 6C), there were no discernible differences in survival between cells expressing Ras(G12V/V45E) alone and those expressing MAA-PKB in addition to the Ras mutant in IL-3-free medium (Fig. 5D). Moreover, Bim expression was suppressed in these cells (Fig. 6D), suggesting that Akt is not required for suppression of this BH3 family member. These results, taken together, show that Akt does not appear to contribute to the cytokine-mediated survival of murine IL-3-dependent hematopoietic cell lines.
DISCUSSION
In earlier studies, we identified two distinct signaling pathways that appear to regulate cell survival. One originates from the membrane-proximal portion of the βc chain and is involved in the rapid and stable regulation of Bcl-xL, while the other emanates from the distal region of the βc chain and overlaps with the downstream pathways of constitutively active Ras proteins (31). The first pathway has been described by others (37), and recently STAT5 was shown to play a pivotal role in upregulating Bcl-xL through signals from the proximal domain of the βc chain (15, 42, 43). However, such induction of Bcl-xL is not sufficient to protect cells from apoptosis (28) (see Fig. 4B, where human GM-CSF was used to stimulate β544 cells). Because Ras is activated through signals from the distal βc chain (40) and because an oncogenic Ras mutant [Ras(G12V)] rescued β544 cells from apoptosis (28), it appears likely that in addition to Bcl-xL one or more genes downstream of Ras(G12V) are required for cell survival. The findings presented here implicate Bim, a member of the BH3-only subfamily of cell death activators, as a key target in the regulation of apoptosis by cytokines, acting through Ras pathways (summarized in Fig. 7). Importantly Bim expression was downregulated not only through constitutively active Ras proteins but also through the physiologically relevant Ras/Raf or Ras/PI3-K pathway (Fig. 4E).
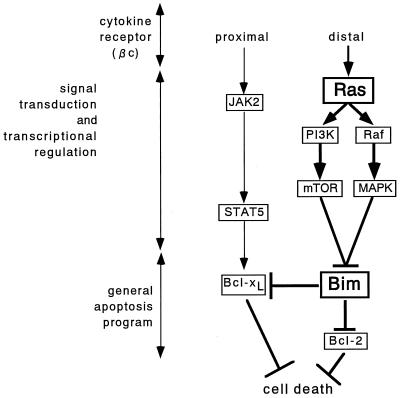
FIG. 7.
Proposed pathways for the cytokine regulation of Baf-3 cell survival. Signals arising from the membrane-proximal portion of the βc chain contribute to the rapid and stable expression of Bcl-xL through activation of STAT5 but downregulate Bim only transiently. By contrast, signals from the membrane-distal domain of the βc chain activate the P13-K/mTOR and Raf/MAPK pathways through Ras stimulation. Either pathway can downregulate Bim expression. When suppressed, Bim cannot inhibit the antiapoptotic functions of Bcl-2 and Bcl-xL and thus contributes to the cytokine-mediated survival of Baf-3 cells.
The biological significance of Bim expression in hematopoietic cells was demonstrated by several experiments. First, in the presence of cytokine, mRNA and protein expression of Bim was very low in the murine IL-3-dependent cells used in this study, which included both lymphoid (Baf-3 and FL5.12) and myeloid (32D) cells. Second, the induction of Bim protein in Baf-3 cells uniformly correlated with cell fate (Fig. 4 to 6). Third, enforced expression of Bim at levels equivalent to those in IL-3-deprived Baf-3 cells induced apoptosis in the presence of IL-3 (Fig. 2C and D), suggesting that Bim might be able to induce cell death by itself. The expression level-dependent apoptosis was also noted in studies with Bim-deficient mice: that is, lymphocytes isolated from Bim−/− mice were much more resistant to apoptosis than were those from wild-type mice, while cells from Bim+/− mice showed intermediate resistance (4).
There are at least two other mechanisms by which Bim could be regulated by cytokines. One is phosphorylation. Like expression level-dependent regulation, signals from the distal portion of the βc chain are required for stable phosphorylation of the Bim protein. Signals from the proximal βc chain can also phosphorylate Bim protein, but only for a limited time (Fig. 4B). The function of Bad, another member of the BH3-only family of death activators, is regulated through the phosphorylation of two serine residues, which causes the loss of its capacity to bind to antiapoptotic Bcl-2 family members, such as Bcl-2 or Bcl-xL (12, 13, 50). Unlike Bad, however, hyperphosphorylated forms of Bim also bind to Bcl-2 or Bcl-xL as avidly as hypophosphorylated forms do (Fig. 3D). We cannot exclude the possibility that the phosphorylation of Bim affects its function through mechanisms other than the regulation of binding to members of the antiapoptotic Bcl-2 family. Even so, it is likely to be a relatively minor mechanism, at least in Baf-3 cells, because enforced expression of BimEL and BimL proteins in Baf-3 cells induced rapid apoptosis in the presence of IL-3, even though both Bim proteins were hyperphosphorylated by IL-3 signaling (Fig. 2 and 3C).
Regulation of the subcellular localization of Bim protein by cytokines is another mechanism. Puthalakath et al. reported (38) that Bim protein forms a complex with an Mr 8,000 dynein light chain, LC8 (11, 25, 27), which functions as a component of the dynein motor complex. In the presence of IL-3, the Bim-LC8 complex binds to the intermediate chain of the dynein motor complex on the microtubules, physically separated from antiapoptotic Bcl-2 family members on the surface of mitochondria. IL-3 withdrawal dissociates the Bim-LC8 complex from the dynein intermediate chain by undetermined mechanisms, enabling Bim to bind to antiapoptotic Bcl-2 family members and inhibit their function. Expression levels of LC8 in Baf-3 or 32D cells were very low; indeed, the LC8 mRNA in these cells was not detectable by Northern blot analysis using a unique probe, despite the fact that high-level expression was readily detected in tissues and organs with the same probe (our unpublished data). In addition, as discussed above, enforced (but not excessive) expression of Bim induces apoptosis in Baf-3 cells, even in cultures containing excess concentrations of IL-3 (Fig. 2C and D). All evidence considered, we prefer Bim expression levels to phosphorylation or subcellular localization as the means by which cytokines regulate apoptosis in these particular cell systems we used. Further investigation is needed to substantiate this interpretation with native hematopoietic progenitors.
The biological significance of PI3-K and the lack of significance of Raf/MAPK pathways in cell survival were established by using nerve growth factor to stimulate rat pheochromocytoma PC-12 cells (47). Akt, a downstream kinase of PI3-K, was then shown to be involved in the PI3-K-mediated survival of cerebellum granular cells (14). In these cells, a dominant negative form of Akt blocks the prosurvival effects of insulin-like growth factor I, while a constitutively active form of Akt promotes cell survival without neurotropic factors. Moreover, rapamycin does not affect cell survival, indicating that Akt (not mTOR) plays the major role in the regulation of apoptosis in neuronal cells. By contrast, both Raf/MAPK and PI3-K can protect hematopoietic progenitors from apoptosis (28, 30), while the role of Akt in this system has been controversial. Although several reports indicate a marginal capacity of Akt to reverse apoptosis due to cytokine withdrawal (2, 44), accumulating evidence refutes a contribution of this protein to cell survival (3, 16, 41). Our data not only support these previous negative findings but also suggest that mTOR is a key downstream mediator of hematopoietic progenitor cell survival, a role supported by recent observations on mTOR in human rhabdomyosarcoma cells (17). In light of the above observations, it is not surprising that Bad phosphorylation, which occurs downstream of Akt, does not play an important role in the cytokine-mediated survival of hematopoietic cells (16).
Aberrant control of apoptosis in hematopoietic progenitors may predispose the cells to leukemic conversion. Several chimeric gene products formed by nonrandom chromosomal translocations, such as the BCR-ABL tyrosine kinase implicated in chronic myelogenous leukemia (CML) and the E2A-HLF transcription factor in adolescent acute lymphoblastic leukemia (ALL) (19, 21) can block apoptosis due to cytokine withdrawal when expressed in Baf-3 cells (9, 10, 23). In the present study, Bim expression was not affected by the E2A-HLF chimera in Baf-3 cells (data not shown); however, it was suppressed in Baf-3 cells expressing BCR-ABL kinase and in BCR-ABL-positive human leukemia cell lines established from ALL or CML patients (our unpublished results). Indeed, the accumulation of mature hematopoietic cells, a prominent feature of CML, is also seen in Bim-deficient mice (4), suggesting that Bim downregulation might be involved in the pathogenesis of CML. Whether Bim contributes to leukemogenesis induced by BCR-ABL or oncogenic Ras mutants is currently under investigation in our laboratory.
ACKNOWLEDGMENTS
We are indebted to T. Kitamura for providing the pMX retrovirus vector and to John Gilbert for editorial review.
This research was supported by Grants-in-Aid from the Ministry of Education, Science and Culture of Japan, by the Uehara Memorial Foundation and the Japan Leukemia Research Fund.
REFERENCES
- 1.Adams J M, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed N N, Grimes H L, Bellacosa A, Chan T O, Tsichlis P N. Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:3627–3632. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao H, Jacobs-Helber S M, Lawson A E, Penta K, Wickrema A, Sawyer S T. Protein kinase B (c-Akt), phosphatidylinositol 3-kinase, and STAT5 are activated by erythropoietin (EPO) in HCD57 erythroid cells but are constitutively active in an EPO-independent, apoptosis-resistant subclone (HCD57-SREI cells) Blood. 1999;93:3757–3773. [PubMed] [Google Scholar]
- 4.Bouillet P, Metcalf D, Huang D C S, Tarlinton D M, Kay T W H, Koentgen F, Adams J M, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 5.Britos-Bray M, Ramirez M, Cao W, Wang X, Liu P P, Civin C I, Friedman A D. CBFβ-SMMHC, expressed in M4eo acute myeloid leukemia, reduces p53 induction and slows apoptosis in hematopoietic cells exposed to DNA-damaging agents. Blood. 1998;92:4344–4352. [PubMed] [Google Scholar]
- 6.Chao D T, Korsmeyer S J. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Zheng X F, Brown E J, Schreiber S L. Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289-kDa FKBP12-rapamycin-associated protein and characterization of a critical serine residue. Proc Natl Acad Sci USA. 1995;92:4947–4951. doi: 10.1073/pnas.92.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conradt B, Horvitz H R. The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein CED-9. Cell. 1998;93:519–529. doi: 10.1016/s0092-8674(00)81182-4. [DOI] [PubMed] [Google Scholar]
- 9.Cortez D, Kadlec L, Pendergast A M. Structural and signaling requirements for BCR-ABL-mediated transformation and inhibition of apoptosis. Mol Cell Biol. 1995;15:5531–5541. doi: 10.1128/mcb.15.10.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortez D, Stoica G, Pierce J H, Pendergast A M. The BCR-ABL tyrosine kinase inhibits apoptosis by activating a Ras-dependent signaling pathway. Oncogene. 1996;13:2589–2594. [PubMed] [Google Scholar]
- 11.Crépieux P, Kwon H, Leclerc N, Spencer W, Richard S, Lin R, Hiscott J. IκBα physically interacts with a cytoskeleton-associated protein through its signal response domain. Mol Cell Biol. 1997;17:7375–7385. doi: 10.1128/mcb.17.12.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 13.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 14.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 15.Dumon S, Santos S C, Debierre-Grockiego F, Gouilleux-Gruart V, Cocault L, Boucheron C, Mollat P, Gisselbrecht S, Gouilleux F. IL-3 dependent regulation of Bcl-xL gene expression by STAT5 in a bone marrow derived cell line. Oncogene. 1999;18:4191–4199. doi: 10.1038/sj.onc.1202796. [DOI] [PubMed] [Google Scholar]
- 16.Hinton H J, Welham M J. Cytokine-induced protein kinase B activation and Bad phosphorylation do not correlate with cell survival of hemopoietic cells. J Immunol. 1999;162:7002–7009. [PubMed] [Google Scholar]
- 17.Hosoi H, Dilling M B, Shikata T, Liu L N, Shu L, Ashmun R A, Germain G S, Abraham R T, Houghton P J. Rapamycin causes poorly reversible inhibition of mTOR and induces p53-independent apoptosis in human rhabdomyosarcoma cells. Cancer Res. 1999;59:886–894. [PubMed] [Google Scholar]
- 18.Hsu S Y, Lin P, Hsueh A J. BOD (Bcl-2-related ovarian death gene) is an ovarian BH3 domain-containing proapoptotic Bcl-2 protein capable of dimerization with diverse antiapoptotic Bcl-2 members. Mol Endocrinol. 1998;12:1432–1440. doi: 10.1210/mend.12.9.0166. [DOI] [PubMed] [Google Scholar]
- 19.Hunger S P, Ohyashiki K, Toyama K, Cleary M L. HLF, a novel hepatic bZIP protein, shows altered DNA-binding properties following fusion to E2A in t(17; 19) acute lymphoblastic leukemia. Genes Dev. 1992;6:1608–1620. doi: 10.1101/gad.6.9.1608. [DOI] [PubMed] [Google Scholar]
- 20.Ikushima S, Inukai T, Inaba T, Nimer S D, Cleveland J L, Look A T. Pivotal role for the NFIL3/E4BP4 transcription factor in IL-3-mediated survival of pro-B lymphocytes. Proc Natl Acad Sci USA. 1997;94:2609–2614. doi: 10.1073/pnas.94.6.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inaba T, Roberts W M, Shapiro L H, Jolly K W, Raimondi S C, Smith S D, Look A T. Fusion of the leucine zipper gene HLF to the E2A gene in human acute B-lineage leukemia. Science. 1992;257:531–534. doi: 10.1126/science.1386162. [DOI] [PubMed] [Google Scholar]
- 22.Inaba T, Shapiro L H, Funabiki T, Sinclair A E, Jones B G, Ashmun R A, Look A T. DNA-binding specificity and trans-activating potential of the leukemia-associated E2A-HLF fusion protein. Mol Cell Biol. 1994;14:3403–3413. doi: 10.1128/mcb.14.5.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inaba T, Inukai T, Yoshihara T, Seyschab H, Ashmun R A, Canman C E, Laken S J, Kastan M B, Look A T. Reversal of apoptosis by the leukemia-associated E2A-HLF chimeric transcription factor. Nature. 1996;382:541–544. doi: 10.1038/382541a0. [DOI] [PubMed] [Google Scholar]
- 24.Inukai T, Inaba T, Ikushima S, Look A T. The AD1 and AD2 transactivation domains of E2A are essential for the antiapoptotic activity of the E2A-HLF chimeric oncoprotein. Mol Cell Biol. 1998;18:6035–6043. doi: 10.1128/mcb.18.10.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaffrey S R, Snyder S H. PIN: an associated protein inhibitor of neuronal nitric oxide synthase. Science. 1996;274:774–777. doi: 10.1126/science.274.5288.774. [DOI] [PubMed] [Google Scholar]
- 26.Joneson T, Bar Sagi D. Ras effectors and their role in mitogenesis and oncogenesis. J Mol Med. 1997;75:587–593. doi: 10.1007/s001090050143. [DOI] [PubMed] [Google Scholar]
- 27.King S M, Barbarese E, Dillman III J F, Patel-King R S, Carson H, Pfister K K. Brain cytoplasmic and flagellar outer arm dyneins share a highly conserved Mr 8,000 light chain. J Biol Chem. 1996;271:19358–19366. doi: 10.1074/jbc.271.32.19358. [DOI] [PubMed] [Google Scholar]
- 28.Kinoshita T, Yokota T, Arai K, Miyajima A. Suppression of apoptotic death in hematopoietic cells by signaling through the IL-3/GM-CSF receptors. EMBO J. 1995;14:266–275. doi: 10.1002/j.1460-2075.1995.tb07000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinoshita T, Yokota T, Arai K, Miyajima A. Regulation of Bcl-2 expression by oncogenic Ras protein in hematopoietic cells. Oncogene. 1995;10:2207–2212. [PubMed] [Google Scholar]
- 30.Kinoshita T, Shirouzu M, Kamiya A, Hashimoto K, Yokoyama S, Miyajima A. Raf/MAPK and rapamycin-sensitive pathways mediate the anti-apoptotic function of p21Ras in IL-3-dependent hematopoietic cells. Oncogene. 1997;15:619–627. doi: 10.1038/sj.onc.1201234. [DOI] [PubMed] [Google Scholar]
- 31.Kuribara R, Kinoshita T, Miyajima A, Shinjyo T, Yoshihara T, Inukai T, Ozawa K, Look A T, Inaba T. Two distinct interleukin-3-mediated signal pathways, Ras-NFIL3(E4BP4) and Bcl-xL, regulate the survival of murine pro-B lymphocytes. Mol Cell Biol. 1999;19:2754–2762. doi: 10.1128/mcb.19.4.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leverrier Y, Thomas J, Perkins G R, Mangeney M, Collins M K L, Marvel J. In bone marrow derived Baf-3 cells, inhibition of apoptosis by IL-3 is mediated by two independent pathways. Oncogene. 1997;14:425–430. doi: 10.1038/sj.onc.1200845. [DOI] [PubMed] [Google Scholar]
- 33.Miyajima A, Ito Y, Kinoshita T. Cytokine signaling for proliferation, survival, and death in hematopoietic cells. Int J Hematol. 1999;69:137–146. [PubMed] [Google Scholar]
- 34.O'Connor L, Strasser A, O'Reilly L A, Hausmann G, Adams J M, Cory S, Huang D C. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okuda K, Ernst T J, Griffin J D. Inhibition of p21ras activation blocks proliferation but not differentiation of interleukin-3-dependent myeloid cells. J Biol Chem. 1994;269:24602–24607. [PubMed] [Google Scholar]
- 36.Onishi M, Kinoshita S, Morikawa Y, Shibuya A, Phillips J, Lanier L L, Gorman D M, Nolan G P, Miyajima A, Kitamura T. Application of retrovirus-mediated expression cloning. Exp Hematol. 1996;24:324–329. [PubMed] [Google Scholar]
- 37.Packham G, White E L, Eischen C M, Yang H, Parganas E, Ihle J N, Grillot D A, Zambetti G P, Nunez G, Cleveland J L. Selective regulation of Bcl-XL by a Jak kinase-dependent pathway is bypassed in murine hematopoietic malignancies. Genes Dev. 1998;12:2475–2487. doi: 10.1101/gad.12.16.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puthalakath H, Huang D C, O'Reilly L A, King S M, Strasser A. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999;3:287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- 39.Ross A J, Waymire K G, Moss J E, Parlow A F, Skinner M K, Russell L D, MacGregor G R. Testicular degeneration in Bclw-deficient mice. Nat Genet. 1998;18:251–256. doi: 10.1038/ng0398-251. [DOI] [PubMed] [Google Scholar]
- 40.Sato N, Sakamaki K, Terada N, Arai K, Miyajima A. Signal transduction by the high-affinity GM-CSF receptor: two distinct cytoplasmic regions of the common β subunit responsible for different signaling. EMBO J. 1993;12:4181–4189. doi: 10.1002/j.1460-2075.1993.tb06102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheid M P, Duronio V. Dissociation of cytokine-induced phosphorylation of Bad and activation of PKB/akt: involvement of MEK upstream of Bad phosphorylation. Proc Natl Acad Sci USA. 1998;95:7439–7444. doi: 10.1073/pnas.95.13.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silva M, Benito A, Sanz C, Prosper F, Ekhterae D, Nunez G, Fernandez-Luna J L. Erythropoietin can induce the expression of bcl-x(L) through Stat5 in erythropoietin-dependent progenitor cell lines. J Biol Chem. 1999;274:22165–22169. doi: 10.1074/jbc.274.32.22165. [DOI] [PubMed] [Google Scholar]
- 43.Socolovsky M, Fallon A E, Wang S, Brugnara C, Lodish H F. Fetal anemia and apoptosis of red cell progenitors in Stat5a-/-5b-/- mice: a direct role for Stat5 in Bcl-X(L) induction. Cell. 1999;98:181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 44.Songyang Z, Baltimore D, Cantley L C, Kaplan D R, Franke T F. Interleukin 3-dependent survival by the Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:11345–11350. doi: 10.1073/pnas.94.21.11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terada K, Kaziro Y, Satoh T. Ras is not required for the interleukin 3-induced proliferation of a mouse pro-B cell line, Baf-3. J Biol Chem. 1995;270:27880–27886. doi: 10.1074/jbc.270.46.27880. [DOI] [PubMed] [Google Scholar]
- 46.Wang Q, Somwar R, Bilan P J, Liu Z, Jin J, Woodgett J R, Klip A. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol. 1999;19:4008–4018. doi: 10.1128/mcb.19.6.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao R, Cooper G M. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- 48.Yin X M, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth K A, Korsmeyer S J. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 49.Yoshihara T, Inaba T, Shapiro L H, Kato J, Look A T. E2A-HLF-mediated cell transformation requires both the trans-activation domains of E2A and the leucine zipper dimerization domain of HLF. Mol Cell Biol. 1995;15:3247–3255. doi: 10.1128/mcb.15.6.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zha J, Harada H, Yang E, Jockel J, Korsmeyer S J. Serine phosphorylation of death agonist Bad in response to survival factor results in binding to 14-3-3 not Bcl-xL. Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]