Supplemental Digital Content is available in the text.
Abstract
Kidney transplant recipients (KTRs) are at increased risk of developing renal cell carcinoma (RCC). The cancer can be encountered at different steps in the transplant process. RCC found during work-up of a transplant candidate needs treatment and to limit the risk of recurrence usually a mandatory observation period before transplantation is recommended. An observation period may be omitted for candidates with incidentally discovered and excised small RCCs (<3 cm). Likewise, RCC in the donor organ may not always preclude usage if tumor is small (<2 to 4 cm) and removed with clear margins before transplantation. After transplantation, 90% of RCCs are detected in the native kidneys, particularly if acquired cystic kidney disease has developed during prolonged dialysis. Screening for RCC after transplantation has not been found cost-effective. Treatment of RCC in KTRs poses challenges with adjustments of immunosuppression and oncologic treatments. For localized RCC, excision or nephrectomy is often curative. For metastatic RCC, recent landmark trials in the nontransplanted population demonstrate that immunotherapy combinations improve survival. Dedicated trials in KTRs are lacking. Case series on immune checkpoint inhibitors in solid organ recipients with a range of cancer types indicate partial or complete tumor response in approximately one-third of the patients at the cost of rejection developing in ~40%.
INTRODUCTION
Historical Perspective
Localized RCC is potentially curable by surgery alone (ie, stage I–III), though recurrence is seen in 3%–30% dependent on stage.1-3 The prognosis of metastasized or locally advanced disease (ie, stage IV) has traditionally been poor due to this tumor’s inherent resistance to standard chemotherapy and radiotherapy.4 Based on advances in the understanding of immuno-oncology and molecular tumor biology, the last decades have brought new major treatment principles to the clinic:
First, involvement of the immune system was long anticipated. Case reports since 1928 described spontaneous regression of metastatic RCC after nephrectomy of the primary tumor.5,6 However, initial experiences from the mid-1980s with immunotherapy were disappointing. Interleukin-2 or interferon alpha were used until mid-2000s to stimulate antitumor immune responses, but enthusiasm was hampered by low response rates (7%–27%) and high rate of toxicity.7-9
The second advance in treatment of RCC was based on improved understanding of the molecular pathways underlying tumor progression. Briefly, both hereditary and sporadic clear-cell RCC display mutations stimulating the vascular endothelial growth factor (VEGF) receptor pathway.1 Tyrosin kinase inhibitors (TKI) and other VEGF receptor inhibitors and mechanistic (mammalian) target of rapamycin (mTOR) inhibitors affect this pathway and improved progression-free10,11 and overall survival,12 with Food and Drug Administration (FDA) approval from 2005 onward.13
Last, immunotherapy has since 2018 been the frontline therapy. Compared with the previous standard of care for metastatic RCC with monotherapy TKI, either a combination of 2 immune checkpoint inhibitors, nivolumab plus ipilimumab,14 or a combination of a checkpoint inhibitor and a TKI, for example, pembrolizumab plus axitinib,15 was found to be superior as the first-line medical treatment in terms of progression-free survival, objective response rate, and overall survival. Both regimens are FDA-approved, though the former is only for intermediate- and high-risk patients. No head-to-head trial between these combination regimens has been undertaken, and recently updated European Association of Urology (EAU) guidelines recommend either as the first-line therapy.3 No systematic trial utilizing immune checkpoint inhibitors in solid organ transplant recipients on immunosuppression has been conducted.
This aim of this review is to summarize the state of evidence for RCC in kidney transplantation, including the epidemiology, pathology, diagnosis, and treatment of RCC.
MATERIALS AND METHODS
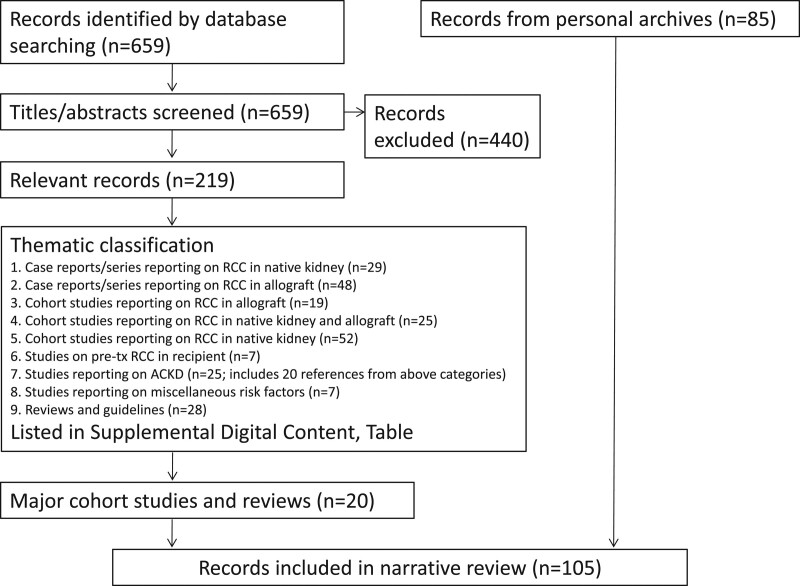
This review is largely based on the current guidelines from EAU and the European Society of Medical Oncology (ESMO), including current electronic updates,3,16,17 supplemented by a literature search in PubMed with the search words including “kidney transplant recipient” or “renal transplant recipient” and “renal cell carcinoma.” This search identified 659 articles on human subjects (search date July 28, 2020). Titles and abstracts were screened by the first author, identifying 219 articles of relevance to the subject (Figure 1 and Table S1, SDC, http://links.lww.com/TP/C193) and prioritizing large cohorts and systematic reviews for inclusion herein (ie, excluding most case reports and case series). Additional articles were selected from reference lists and personal archives. Staging was defined according to The Union for International Cancer Control tumor, node, and metastasis 8th Edition.18
FIGURE 1.
Flowchart of literature search. For Supplemental Digital Content please refer to http://links.lww.com/TP/C193. ACKD, acquired cystic kidney disease; RCC, renal cell carcinoma.
REVEIW
Epidemiology, Classification, and Risk Factors
Kidney transplantation is widely regarded as the best treatment option for patients with kidney failure. Compared with remaining waitlisted in dialysis, kidney transplantation is associated with improved survival19,20 and quality of life21,22 and entails a lower cost for the society.23 Modern immunosuppression has reduced acute rejections to <10%, and >90%–95% of grafts function beyond the first year.24 In parallel, mortality has decreased so that 60%–80% of patients survive >10 y after a first deceased or living donor kidney transplant, respectively.24 Cardiovascular disease has traditionally dominated outcomes in these patients but has now decreased to the extent that, beyond the first year after transplantation, malignancy and infectious diseases have become relatively more frequent.25,26 A recent report from the European Renal Association-European Dialysis and Transplant Association highlights a worrisome increase in cancer mortality among elderly kidney transplant recipients (KTRs) during the last decades,27 though possibly linked to acceptance of more elderly and comorbid patients for kidney transplantation.28 This indicates that transplant physicians need to have increased focus on early detection and possible prevention of death from cancer.
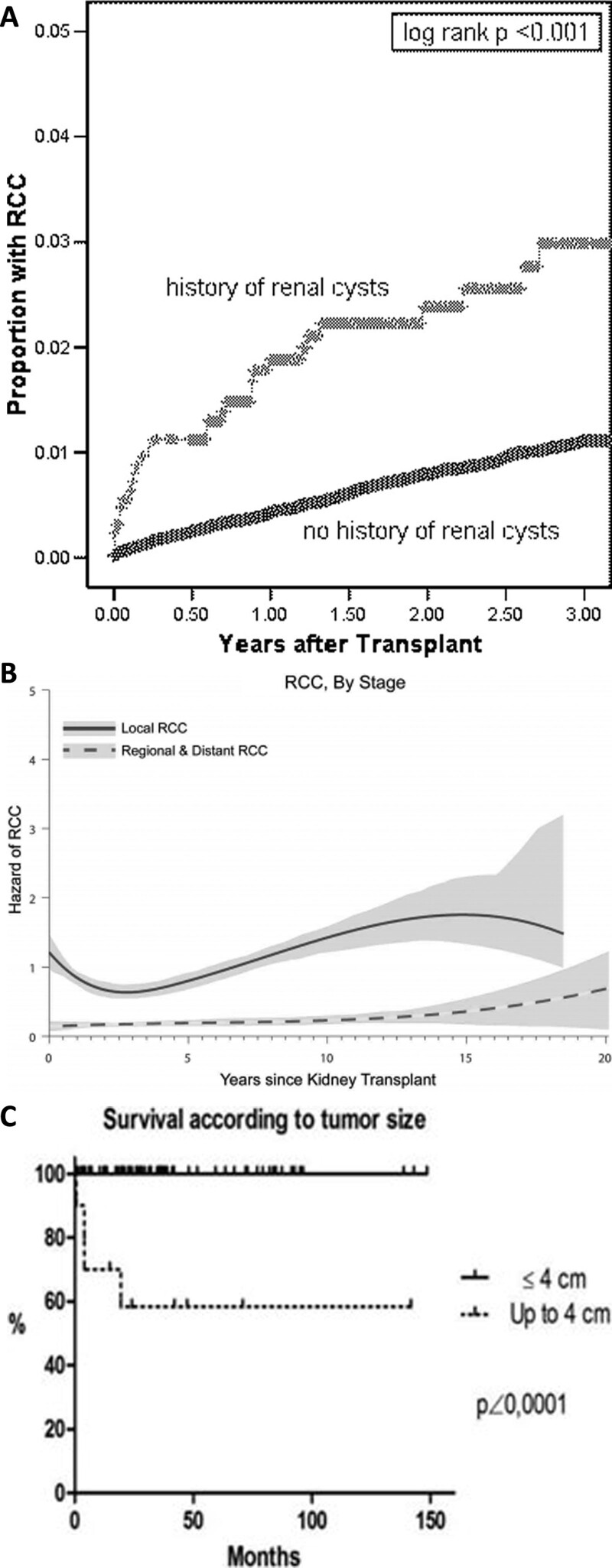
Overall, KTRs have a 2- to 4-fold increased risk for cancer compared with the general population, most pronounced for c ancers associated with UV radiation (ie, skin cancer) and infection-related cancer (eg, Ebstein–Barr virus–induced lymphoma) but also extending to several cancer types unrelated to infection.29 The risk of RCC in KTRs is about 5–10 times higher compared with the general population,29 and predominantly (~90%) encountered in the native kidneys, rarely in the kidney allograft.30,31 The absolute risk of RCC in KTRs, however, is relatively low. An early single-center study where routinely ipsilateral native nephrectomy was performed at transplantation detected RCC in 4.2 % of KTRs,32 but publications addressing large epidemiological cohorts identify RCC in <1% of KTRs (Table 1). Acquired cystic kidney disease (ACKD) is a risk factor for RCC development (Figure 2A) as detailed below. Most tumors are localized (Figure 2B), and small tumors herald a good prognosis (Figure 2C). Interestingly, a 1.5 to 3 times increased risk of kidney cancer has also been found in patients with lung, heart, or liver transplants.43 This could indicate an increased risk for RCC related to certain immunosuppressive drugs, although the increased detection of RCC due to frequent radiological imaging in transplant recipients (versus the general population) is also plausible.
TABLE 1.
RCC occurrence in KTRs, including the largest cohorts (N > 5000 KTRs)a
| References | No. of transplants | Study details | Calendar period; (follow-up time) | No. of tumors (%) in native kidney | No. of tumors (%) in allograft | No. histology (%) | ||
|---|---|---|---|---|---|---|---|---|
| ccRCC | Papillary RCC | Other | ||||||
| Hoover and Fraumeni33 | 6297 | Multinational Tx registry study | 1951-1971 | Category “other” ie ≤12 (≤0.2%) | ||||
| Wunderlich et al34 | 10 997 | Survey of 38 German Tx centers, 27 (71%) responded | 1990-1998 | 16 (0.15%) | ||||
| Einollahi et al35 | 5532 | 5 Tx centers in Iran | 1984-2008 | 4 (0.07%) | 1 (0.02%) | |||
| Hurst et al36 | 40 821 | USRDS billing claims, location in native kidney vs allograft not discernible | 2000-2005 (within 3 y after tx) | 368 (0.9%) | ||||
| Tillou et al37 | 41 806 | Survey of 32 French Tx centers. Yearly us screening since 2007 | 1988-2009 | 79 (0.2%) | 32 (40%) | 44 (56%) | 3 (4%) | |
| Desai et al38 | 21 029 | UK study on donor origin cancer | 2001-2010 | 6 (0.03%) | ||||
| Smith et al39 | 10 474 | NAPRTCS pediatric registryb | 1987-2009 (until 21st birthday) | 3 (0.03%) | 2 (0.02%) | |||
| Zhang et al40 | 30 632 | Several Chinese databases | 1974-2014 | 42 (0.1%) | ||||
| Karami et al31 | 116 208 | US Transplant Cancer Match Study | 1987-2010 | 683 (0.6%)c | 219 (32%) | 191 (28%) | 273 (40%) | |
| Ranasinghe et al41 | 8850 | ANZDATA | 2000-2012 | 47 (0.5%) | 8 (0.09%) | |||
| Eggers et al42 | 5250 | Hannover, Germany. | 1970-end unspecified | 61 (1.2%) | 20 (0.4%) | |||
aSmaller cohorts and case studies are shown in the SDC, http://links.lww.com/tp/c193.
bNorth American Pediatric Renal Transplant and Collaborative Studies (NAPRTCS)-registry including 120 centers in United States, Canada, Mexico, Costa Rica; data on pediatric KTRs until 21st birthday.
cSubset analysis indicated 11% were in the allograft.
ANZDATA, Data from the Australia and New Zealand Dialysis and Transplant Registry; ccRCC, clear-cell RCC; KTR, kidney transplant recipient; NAPRTCS, North American Pediatric Renal Trials and Collaborative Studies; RCC, renal cell carcinoma; Tx, transplantation; us, ultrasound; USRDS, United States Renal Data System.
FIGURE 2.
Epidemiology of RCC in kidney transplant recipients. A, Time to RCC, by history of renal cyst. Adapted from Hurst et al36 with permission. B, Risk of local or regional/distant RCC after kidney transplant. Vertical axis shows hazard in units of “per 1000 person-y.” Adapted from Karami et al31 with permission. C, Survival after diagnosis of RCC in allograft, by size of tumor. Adapted from Tillou et al37 with permission. RCC, renal cell carcinoma.
RCC accounts for 80%–90% of all kidney cancers,3,16 the majority of the remaining being urothelial (transitional cell) cancers of the kidney pelvis, which are reviewed elsewhere.44 The classification further subdivides RCCs based on morphological and cytogenetic characteristics,45 with clear-cell RCC being the most common type (~75%–85%) followed by papillary (type I and II; ~10%–15%), chromophobe, and several less common subtypes.46
In the general population, kidney cancer is among the 10 most common cancer types, accounting for 5% of all cancer in men and 3% in women, with a peak incidence between 60 and 70 y of age.3,16 Its incidence rose during the latter 2–3 decades and then levelled off, correlating with the establishment of radiological imaging and increased detection of lower-stage tumors. The known risk factors for RCC include older age, male gender, tobacco smoking, obesity, and ACKD, whereas evidence is inconclusive on diabetes, physical inactivity, dietary factors, and occupational carcinogens.3,16 On a population level, smoking cessation and decreased obesity may be the most effective prophylactic measures.47 Although most RCC are sporadic, 5%–8% are part of hereditary syndromes, with 10 germline syndromes currently known.3 Genetic testing has been recommended for patients with multiple or bilateral RCC or in the presence of associated disorders/phenotypes.16
Chronic kidney disease of any cause uniquely predisposes for RCC through accumulation of cystic degenerative changes in the native kidneys, with RCC developing from the cyst walls. Such ACKD is reported in 5% to 20% of patients initiating dialysis, and in close to all patients after ~10 y of dialysis, independent of cause of CKD and dialysis modality (ie, hemodialysis or peritoneal dialysis).48,49 One study found RCC in nearly 20% of patients with ACKD, but in only 0.5% of patients without ACKD.50 Thus, ACKD may (along with tuberous sclerosis complex and von Hippel–Lindau disease) be viewed as a premalignant disease.49
The RCCs developing in CKD are often multicentric and bilateral but herald a better prognosis compared with sporadic RCC.3 Whether this relates to earlier detection or specific CKD-related factors is unknown. The histologic appearance mirrors sporadic cases, though papillary RCC is more common, followed by clear-cell RCC. A variant specific for CKD is termed acquired cystic disease–associated RCC.
Successful kidney transplantation may reduce the size of cysts and prevalence of ACKD, but it has been debated if transplantation also decreases the long-term risk of RCC development in native kidneys.48,49 Transplantation normalizes kidney function but adds immunosuppression to the risk of cancer development. In an ANZDATA study by Vajdic et al,51 kidney cancer incidence was increased about 10-fold in patients both on dialysis and after transplantation when compared with the general population, whereas another study from the USRDS by Kasiske et al52 reported an increased risk of kidney cancer (39%) early after transplant compared with patients remaining waitlisted. A recent study of >200 000 patients from the Scientific Registry of Transplant Recipients assessed cancer patterns across periods of dialysis versus periods with a functioning graft.53 Due to the size of the study, one could evaluate risk for development of specific cancer types and the relationship toward immunosuppression and poor kidney function. The kidney cancer incidence was clearly increased during periods of dialysis.53
Pathogenesis
Both clear-cell RCC and papillary RCC originate from the proximal tubule.1 For clear-cell RCC, discovery of the von Hippel–Lindau tumor-suppressor gene (VHL) in 1993 improved our understanding of its pathogenesis and heralded development of the VEGF receptor inhibitors and other TKIs. The gene product, VHL protein, inhibits hypoxia-inducible factors, which if unopposed will constitutively stimulate growth factors including VEGF, and thereby epithelial cell proliferation. Additional genetic alterations accrue over time and may worsen the prognosis. Interestingly, several of these genes are located on the short arm of chromosome 3 with the VHL gene. Thus, clear-cell RCC is a heterogeneous disease in terms of molecular biology, presentation, and prognosis.54 Other genetic aberrations underlie the development of papillary RCC type 1 and 2, with frequent alterations in MET- and NRF2-ARE pathways, respectively. As for clear-cell RCC, most papillary RCCs are sporadic but may occur in distinct familial syndromes. Chromophobe RCC should be differentiated from oncocytoma,16 with frequent mutated tumor suppressor protein 53 (TP53) and involvement of the mTOR pathway. Other types of RCCs include the aggressive collecting duct carcinoma and renal medullary carcinoma, the latter associated with sickle cell disease, including sickle cell trait. Noteworthy, about 15% of renal tumors are benign, including angiomyolipoma and renal oncocytoma, with further elaboration in the current EAU guidelines.3
Diagnosis
In the general population, more than half of RCC are incidentally detected early by radiologic imaging for other diseases or unspecific symptoms.3 As symptoms tend to occur late, about 25% of patients present with advanced disease when the tumor extends to neighboring structures or has spread. In about one-third of symptomatic patients, paraneoplastic symptoms can be observed, sometimes related to secretion of various vasoactive peptides or hormones, such as parathyroid hormone–related protein, erythropoietin, gonadotropins, human chorionic somatomammotropin, adrenocorticotropic hormone–like substance, renin, glucagon, or insulin. Symptoms and findings may include weight loss, night sweats, fatigue, edema, liver dysfunction, pain, cough, anemia, erythrocytosis, hypercalcemia, and other metabolic disturbances. This varied presentation historically coined RCC as the internist’s tumor; however, in the present era, it has been renamed as the radiologist’s tumor.55
Radiology (computed tomography [CT] or magnetic resonance imaging) before and after intravenous contrast is used to demonstrate enhancement of renal masses indicating the presence of a solid tumor (versus benign cyst) although differentiating malignancy from the benign oncocytoma or fat-free angiomyolipoma may require biopsy.3 Similarly, contrast-enhanced CT has been the basis for characterization of renal cysts according to the Bosniak classification,56 with enhancement of thickened walls or septa indicating malignancy. Magnetic resonance imaging or contrast-enhanced ultrasound may be superior for cyst classification. If malignancy is highly suspected, biopsy is not required before surgery (radical or partial nephrectomy), but chest, abdominal, and pelvic CT are required for staging. According to the EAU and ESMO guidelines, there is consensus that skeletal metastases are symptomatic, and that imaging of bone or brain is needed only on clinical indication.3,16 Limited evidence for the sensitivity of fluorodeoxyglucose positron emission tomography leaves guidelines arguing against its use. Systemic therapy may be indicated without nephrectomy in poor prognosis patients with metastatic disease57; in such instance, a metastasis may be easier to biopsy than the primary kidney tumor to provide a histopathological diagnosis. Other possible indications for biopsy include small masses to be treated with ablative therapy or followed by active surveillance. For percutaneous biopsy, a coaxial cannula is recommended to avoid tumor seeding the biopsy canal,3 targeting at least 2 biopsy cores and avoiding central necrotic areas. Tumor seeding has been regarded as extremely rare (0.01%)58 though a recent report of 7 cases of tumor seeding in 218 partial nephrectomies indicates heightened awareness may be necessary.59 Lower accuracy is reported for biopsy of cystic lesions and is not recommended unless there are areas of solid tissue (Bosniak IV cysts). Caution is required with biopsies, as the negative predictive value is only 63%60; moreover, oncocytoma (benign) and chromophobe RCC may appear similar on core biopsy.61
Treatment of RCC in the General Population
Treatment options for RCC include surgery, ablation, TKIs and immunotherapy, with active surveillance as an option for slowly growing small lesions in patients at high surgical risk.3 Surgery has become less mutilating with nephron-sparing (ie, partial nephrectomy) and minimal-invasive (ie, laparoscopy or robot-assisted) approaches. Thermal ablative therapies (cryotherapy, microwave, or radiofrequency ablation) show promising results,62 and for metastatic RCC, prognosis has improved with newer oncologic treatments targeting tyrosin kinases and immunotherapy with immune checkpoint inhibitor combinations. mTOR inhibition is less effective but may still be used as the third- or fourth-line drug option. Standard chemotherapy is ineffective. An updated and detailed treatment algorithm is presented in the 2020 revision of the EAU guidelines3 and will be summarized below. Currently, most trial evidence is for treatment of clear-cell RCC.
For localized disease (stages I–III), surgical resection is curative in a majority, though recurrence is seen in 3%–30% dependent on stage.1-3 Partial nephrectomy preserves kidney function and is preferred for stage T1 tumors and may be considered for some T2 tumors. It is uncertain if regional lymph node resection improves prognosis. Currently, there is a weak recommendation for resection of clinically suspected metastatic nodes (on imaging or by intraoperative assessment), and for patients with adverse clinical features, including a large diameter of the primary tumor. For small tumors <4 cm (stage T1a) in patients at increased operative risk, thermal ablation or active surveillance can be considered. The risk of local recurrence and metastasis associated with these approaches is very low in absolute numbers (1%–2%). Several trials demonstrate that adjuvant therapy with TKI did not improve overall survival, and none carries European Medicines Agency approval for this indication, whereas sunitinib carries FDA approval as adjuvant therapy based on improved disease-free survival. However, disease-free survival is poorly correlated with overall survival in RCC. Trials are ongoing with adjuvant everolimus (NCT01120249) and with different immune checkpoint inhibitors (Nivolumab plus ipilimumab, NCT03138512; Nivolumab, NCT03055013; Pembrolizumab, NCT03142334; Atezolizumab, NCT03024996). Recent data indicate that active surveillance may also be a viable option for small tumors. Note that active surveillance is different from watchful waiting, that is, the former includes planned follow-up to detect progression. In particular, tumors <2 cm have little metastatic potential.
For advanced/metastatic RCC with resectable and few (≤3) metastases, radical nephrectomy and metastasectomy should be considered. Cytoreductive nephrectomy is recommended if radical treatment is planned for oligometastatic disease.3 Oligometastases can be treated with surgery, stereotactic radiotherapy, and other ablative techniques. Other nonresectable metastatic RCC are medically treated. In the latter case, performing an up-front cytoreductive nephrectomy (ie, nephrectomy and surgery to remove as much of the tumor as possible) did not improve outcomes compared with TKI alone in poor prognosis patients and is currently not recommended by the EAU guidelines, although there is a weak recommendation to consider cytoreductive nephrectomy after documented response to oncological treatment. Studies are ongoing for cytoreductive nephrectomy in the era of immunotherapy (NORDIC-SUN, NCT03977571, CYTOSHRINK, and NCT04090710).
Until recently, the first-line therapy for metastatic RCC consisted of monotherapy with a TKI. Advances with immune checkpoint inhibitor combinations have improved prognosis and changed recommendations. Recent trials in the nontransplant population are presented in Table 2. Albeit oncologic outcomes and overall survival have improved, complete responses are still rare (~10% with double checkpoint blockade), and for most patients, drug therapy continues to be palliative. Thus, asymptomatic patients with low-volume metastatic disease in nonthreatening locations can initially be followed for progressive disease before commencing active treatment.
TABLE 2.
Trials of immunotherapy combination regimens in treatment-naive metastatic RCC.a
| Trial | CheckMate 214.Motzer et al14 | KEYNOTE-426.Rini et al15 | JAVELIN 101.Motzer et al63 | IMmotion151.Rini et al64 | ||||
|---|---|---|---|---|---|---|---|---|
| Treatment arm | Nivo + Ipi (n = 425)b | Sun (n = 422)b | Pembro + Axi (n = 432) | Sun (n = 429) | Avelu + Axi (n = 442) | Sun (n = 444) | Atezo + Beva (n = 454) | Sun (n = 461) |
| ORR (%) | 42 | 27 | 59.3 | 35.7 | 51.4 | 25.7 | 37 | 33 |
| CRR (%) | 9 | 1 | 5.8 | 1.9 | 3.4 | 1.8 | 5 | 2 |
| Median progression-free survival, mo | 11.6 | 8.4 | 15.1 | 11.1 | 13.8 | 8.4 | 11.2 | 8.4 |
| Hazard ratio for disease progression or death | 0.82 (P = 0.03, not significant per prespecified 0.009 threshold) | 0.69 (95% CI, 0.57-0.84; P < 0.001) | 0.69 (95% CI, 0.56-0.84; P < 0.001).PD-L1+: 0.61 (95% CI, 0.47-0.79; P < 0.001) | 0.83 (95% CI, 0.70-0.97).PD-L1+: 0.74 (95% CI, 0.57-0.96; P = 0.02) | ||||
| Median overall survival, mo | NR | 26.0 | NR | NR | NR | NR | 33.6 | 34.9 |
| Hazard ratio for death | 0.63 (99.8% CI, 0.44-0.89; P < 0.001) | 0.53 (95% CI, 0.38-0.74; P < 0.0001) | 0.78 (95% CI, 0.55-1.08; P = 0.14) | 0.93 (95% CI, 0.76-1.14) | ||||
Atezo, atezolizumab; Avelu, avelumab; Axi, axitinib; Beva, bevacizumab; CI, confidence interval; CRR, complete response rate; Ipi, ipilimumab; Nivo, nivolumab; NR, not reached; ORR, objective response rate; PD-L1+, programmed cell death 1 ligand 1 positive; Pembro, pembrolizumab; RCC, renal cell carcinoma; Sun, sunitinib.
aTransplant recipients excluded.
bIntermediate or poor prognostic risk groups.
Risk classification of metastatic disease is based on models developed in the era of first-line TKI monotherapy but has also been validated in the recent trials involving immune checkpoint inhibitors. The International Metastatic Renal Cell Carcinoma Database Consortium risk model stratify patients into favorable/low risk (no risk factors), intermediate risk (1–2 risk factors), or poor/high risk (3–6 risk factors) using the following 6 risk factors: Karnofsky performance status <80%, time from diagnosis to treatment <12 mo, hemoglobin less than the lower reference limit, corrected serum calcium >2.4 mmol/L (10.0 mg/dL), absolute neutrophil count greater than the upper reference limit, platelets greater than the upper reference limit. In the era of the first-line TKI monotherapy, median survival is 43, 23, and 8 mo for these risk groups.
The currently recommended first-line therapy for metastatic RCC involves either a combination of 2 immune checkpoint inhibitors (nivolumab plus ipilimumab) in intermediate- or poor-risk group patients or a combination of a checkpoint inhibitor and a TKI (axitinib plus pembrolizumab) in all risk groups. Compared with sunitinib, both regimens improved progression-free survival, objective response rate, and overall survival, though for the former (nivolumab plus ipilimumab), this did not extend to International Metastatic Renal Cell Carcinoma Database Consortium low-risk patients. Beneficial treatment effects were seen regardless of PD-L1 status, and PD-L1 status is currently not used for patient selection. The 2 regimens have not been directly compared head to head. For patients who cannot receive or tolerate immune checkpoint inhibitors, a TKI is recommended.
Second lines of therapy have less evidence and may include TKIs not used in the first-line treatment. An mTOR inhibitor is not recommended before third or fourth line. Of note, immunotherapy is also supported by recent epidemiologic evidence even in advanced cancer with brain metastasis.65
Most trials have focused on clear-cell RCC. Nonclear-cell RCC seems to be less responsive to TKI and mTOR inhibitors than clear-cell RCC and patients should be referred to clinical studies if available. The recent immunotherapy combination regimens hold approvals regardless of RCC histology. For patients not included in studies, nonclear-cell RCC can be treated similarly to clear-cell RCC.66
Issues Specific to Kidney Transplantation
RCC in the Donor Kidney
The incidence of donor-transmitted RCC is unknown. Transplantation of kidneys after ex vivo excision of small tumors has been reported since 1982.67 A systematic review of published cases until June 2017 identified 109 such transplantations.68 Most excised tumors were RCC (81%), mean tumor size was 2 cm (range 0.5–6.0 cm; predominantly <4 cm), and nucleolar/Fuhrman grade I–II in 93%, all had clear margins. Mean follow-up was 39.9 mo, there was only 1 local tumor relapse (after 9 y), other outcomes were inconsistently reported. From studies with available data, 5-y patient and graft survival were 92% and 95.6%, respectively. Limitations included a relatively short follow-up and risk of publication bias. The authors suggested preoperative percutaneous biopsy to exclude grade 4 RCC and frozen section to ensure complete resection. Most transplant programs restrict these “restored” kidneys to patients >60 y of age or who have dialysis access problems and require recipient consent.68
The case series of restored kidneys most often describe incidentally discovered tumors in living or deceased donors though some transplant programs also utilize kidneys from patients undergoing nephrectomy for a known RCC. The latter has been pioneered by Australian transplant centers, and a recent report summarize that among 107 such transplantations, only 2 recurrences (1.87%) were seen after a median of 7 y of follow-up, making a strong argument for the low oncological risk.69 However, most patients with a small RCC require only partial nephrectomy, ruling out donation.70 Details of surgical technique and monitoring are reviewed elsewhere.71
There is no consensus on the acceptable size of tumor before restoration of a donor kidney for transplantation though several reviews mention <4 cm,68,70 some <3 cm.29 The Council of Europe stratify risk according to size and grade of the RCC, that is, <1, 1–4, and 4–7 cm as minimal, low, and intermediate risk, respectively, conditional on nucleolar/Fuhrman grade I–II.72 Higher nucleolar/Fuhrman grades (III/IV) are considered high risk for transmission. Similar risk categories are reported from the US-based Disease Transmission Advisory Committee of the Organ Procurement and Transplantation Network–United Network for Organ Sharing,73 though the low-risk category was restricted from 1 to 2.5 cm. Use of the contralateral kidney and other organs is considered minimal risk with RCC ≤ 4 cm and nucleolar/Fuhrman grade I–II.72 A previous RCC in the donor history is regarded similar to the above categories within the first 5 y after treatment.72
The unintentional transplantation of a kidney with an undetected RCC is a rare event and until December 2012 reported in only 20 patients according to a systematic review.74 Although prognosis may be more benign than after transmission of other cancer types, that is, over 70% of recipients survived for at least 24 mo after transplantation, reports of detrimental transmissions also exist.72 Publication bias is likely. The World Health Organization and the Italian national Transplant Center encourage to report cases to a surveillance database, the NOTIFY library (www.notifylibrary.org).
RCC in the Kidney Transplant Candidate
Recipient evaluation before transplantation aims to exclude cancer for 2 main reasons: (1) to avoid aggravating the prognosis of any cancer with immunosuppression, and (2) to avoid transplantation in patients with a short life expectancy as donor organs are a scarce resource.29 To avoid aggravating the risk of early recurrence, an observation period between completion of curative cancer therapy and transplantation (or enlisting) is usually required. Previously, general guidelines varied in their recommendation for an observation period between 2 and 5 y, though for small or incidentally discovered RCC, no observation period was required.75 The 2020 KDIGO Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation provides an ungraded recommendation to “screen candidates at increased risk for RCC (eg, ≥3 y dialysis, family history of renal cancer, acquired cystic disease, or analgesic nephropathy) with ultrasonography”.76 Long-term smoking is also mentioned as a risk factor. Further, the guideline states that renal tumors ≤1 cm are no exclusion for transplantation, curatively treated RCC < 3 cm requires no observation period, and for “early” or “large and invasive” kidney cancer, observation periods of 2 and 5 y are suggested, respectively. It is advised that oncologists, transplant nephrologists, patients, and their caregivers should be involved in the decision to transplant patients in remission from cancer.76
The long observation periods mentioned above has recently been challenged. Our group analyzed cancer recurrence deaths from the national transplant program in Norway, in which only a 1-y cancer-free observation period before transplantation (or enlisting) is required. We found no association between observation periods and all-cause or cancer-specific mortality.77 Among 100 KTRs with a pretransplant RCC, 13 died from a posttransplant recurrent RCC; however, none of these 13 patients had a short (<2 y) observation period, and 7 of 13 had an observation period of >5 y. Similar findings were noted from an analysis of 501 patients with renal-malignancy–associated cause of end-stage kidney from the United States.78 Observation period was not associated with cancer-specific mortality, and overall survival was better with shorter (0–2 y) than longer observation periods. Additionally, a French study on 143 KTRs with a previous RCC found recurrence in 13 at a mean of 3 y after transplantation, again without any association with observation period.79 Collectively, these publications find lack of benefits with prescribing long observation periods, and that the resulting prolonged time in dialysis may worsen the overall prognosis.
Screening for RCC After Transplantation
There are no clinical trials of cancer screening in KTRs demonstrating improved survival. Although the risk of cancer is high, so is the competing risk of noncancer death, precluding generalizations from cancer screening trials in the general population.29 A recent review highlighted the discrepancies and other shortcomings of cancer screening guidelines in transplant recipients, including the lack of clinical trial evidence and involvement of key stakeholders.80 The review found inconsistent recommendations to screen for RCC in KTRs, with the 2002 European Best Practice Guidelines in favor and the 2011 Renal Association Clinical Practice Guidelines in disfavor of screening, while 5 other guidelines provided no clear recommendations. The 2009 KDIGO clinical practice guideline for the care of kidney transplant recipients notes that several centers are screening for RCC in KTRs without evidence of a mortality benefit.81 Further, the KDIGO states that screening will likely detect many unimportant lesions though may be beneficial in certain high-risk groups such as KTRs with prior RCC, tuberous sclerosis, ACKD, and analgesic nephropathy, and a randomized controlled study is adviced.81 A Markov model of ultrasound screening for RCC found minimal survival benefit, that is, only 2 deaths avoided from RCC per 1000 KTRs screened annually for 62 y, at an incremental cost-effectiveness ratio of >$300 000 per life-y saved.82 In conclusion, in most KTRs, there is little evidence to support screening for RCC.
Treatment of RCC in KTRs
No specific guidelines exist for the evaluation or treatment of tumors in renal allografts or RCC in KTRs.83 In practice, treatment strategies outlined for the general population are used as long as appropriate with some modifications. Localized RCC in dysfunctional native kidneys is often treated with nephrectomy, whereas for localized RCC in the allograft, several case reports describe successful nephron sparing surgery or ablative therapy.44,84 A systematic review on solid masses in kidney allografts found 175 tumors reported in 163 patients, mostly clear-cell RCC (46%) or papillary RCC (42%).2 The majority were treated with partial nephrectomy (68%), fewer with allograft nephrectomy (19%), radiofrequency ablation (10%), and cryoablation (2%). Cancer recurrence after partial nephrectomy was 3.6% after 3.1 y, which is similar to nontransplanted patients.2
For metastatic RCC in KTRs, it is not known if immune checkpoint inhibitor combination therapy provides survival benefit, as recently shown in nontransplanted patients. Case reports caution that maintenance immunosuppression may render the checkpoint inhibitors less effective and there is a high risk of rejection and graft loss.85 A recent systematic review on immune checkpoint inhibitors in solid organ recipients identified 83 treated patients, of whom 53 were KTRs.86 Most patients had melanoma or other skin cancers, only 2 had RCC. Acute rejections were seen in 39.8%, often (71%) leading to graft failure. Stable or responsive disease was noted in 3 (3.6%) and 23 (27.7%) patients, respectively. Tumor response was not associated with immune-related adverse events, time since transplant, specific immunosuppressant drugs, or rejection. Receipt of at least 1 immunosuppressant drug other than steroids was associated with less rejection but also a trend for lower progression-free survival. The median survival was 36 wk, 48 (57.8%) patients died, mostly attributed to tumor progression. Even though 19.3% were alive and free from rejection and tumor progression at the end of study, this review highlights the difficult tradeoffs facing transplant specialists and oncologists managing solid organ recipients with cancer.87 Clearly, better predictive biomarkers are needed to decipher which patients will benefit from these treatments.
Although immunosuppression is a recognized risk factor for development of malignancies, the optimal adjustments to maintenance immunosuppression in KTRs with de novo cancer are uncertain.88 For some malignancies, that is, posttransplant lymphoproliferative disease and Kaposi sarcoma, a reduction of immunosuppression89 or switch to mTOR inhibitor90 has been shown to facilitate tumor regression, respectively. For other cancer types, reduction of immunosuppression has not proven beneficial. One cohort study found that immunosuppression reduction did not prolong cancer-free survival.91 A recent study from France in kidney and liver transplant recipients with de novo malignancy found that optimal oncologic treatment and introduction of mTOR inhibition was associated with improved survival.92 Notably from this study, optimal oncologic therapy was delivered in 80% and 38% of patients with localized and advanced cancer, respectively, and among the latter, 27% had only supportive care.92 For RCC, no trials of mTOR inhibition included KTRs, and other trials of mTOR inhibition in KTRs warrant caution. A systematic review and meta-analysis of 5876 KTRs randomized to sirolimus immunosuppression found a 40% reduced risk of malignancy, driven by a 56% reduced risk of nonmelanoma skin cancer though at a 43% increased risk of mortality.93 Moreover, in organ transplant recipients, a switch to mTOR inhibition seems to be most effective in early-stage cancer. For instance, in KTRs with skin cancer, the effect of mTOR conversion is significant only in subgroups with less advanced disease at baseline, ie, ≤1 previous skin cancer.94 Similarly, in a randomized study in liver transplant recipients, sirolimus conversion for hepatocellular carcinoma surprisingly only benefited patients with low-risk cancer.95 Thus, the effect of mTOR conversion in KTRs with metastatic RCC would need confirmation in a randomized trial. Nevertheless, reduction in the maintenance immunosuppression regimen is customary in KTRs with active cancer.29 No particular pattern of reduction is discernible from the current literature.96 Reduction or stopping the antimetabolite would be beneficial to avoid leukopenia, others would advocate for switching the calcineurin-inhibitor to mTOR, particularly knowing the benefits of mTOR inhibitors as treatment options for nontransplanted patients with RCC. For patients planning immune checkpoint inhibitor treatment, some would favor an mTOR combined with 10 mg of prednisolone,97 though as stated above, there is no clinical supporting evidence.86 The option of graftectomy and stopping all immunosuppression followed by checkpoint inhibition has, to our knowledge, not been explored, though stopping immunosuppression was recommended in the era of interleukin 2 treatment.98
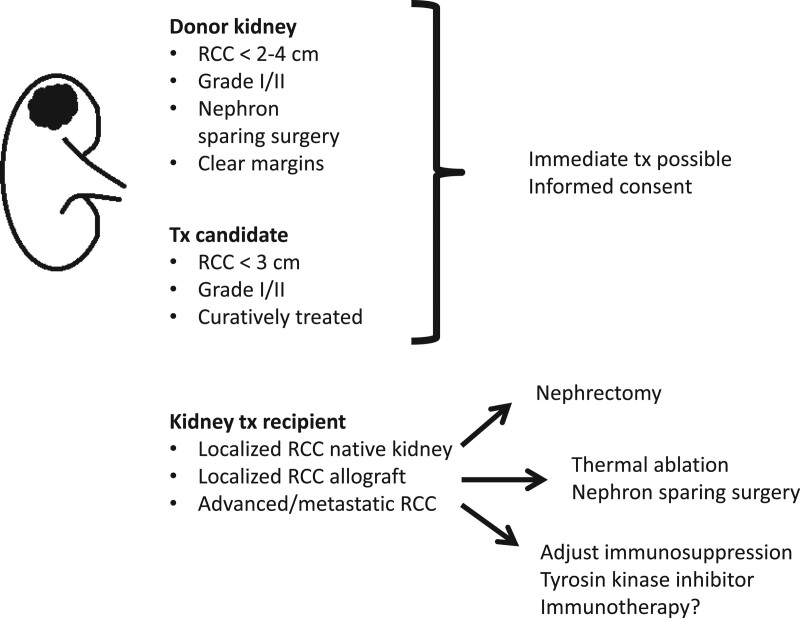
With these highly uncertain outcomes of treatment, shared decision making between a fully informed patient, the oncologist and transplant nephrologist is essential. Many patients will likely decline immunotherapy, in particular if frail or unable/unwilling to return to dialysis (in whom rejections are indeed life threatening). No treatment, an introduction of mTOR inhibitor, and/or therapy with TKI are all valid options. No trial evidence exists for TKI in KTRs, few case reports show effect in localized99,100 and metastatic101-103 disease, though as for immune checkpoint inhibitors, toxicity may limit treatment.104,105 Based on the current available data, we present our suggested recommendations as an overview in Figure 3 and with details in Table 3.
FIGURE 3.
Overview of recommendations. Please refer to Table 3 for details.
TABLE 3.
Suggested recommendations.
| RCC in donor |
|---|
| • Donor kidney can be used for transplantation after excision of RCC if size <2–4 cm, nucleolar grade ≤II and clear surgical margins. Most data are for the clear-cell type. |
| • Preferentially used to higher-risk recipients (age above 60 y, dialysis access problems), and after informed consent. |
| • For a deceased donor, if RCC ≤ 4 cm and nucleolar grade ≤ II, the contralateral kidney can be used. |
| • For a donor with a previous RCC, precautions apply as above until 5 y after treatment.RCC in transplant candidate |
| • Incidentally detected small renal masses (≤1 cm) are no contraindication to transplantation. |
| • It is reasonable routinely to screen candidates for RCC with ultrasound or other modality if they are at high risk for RCC (eg, ≥3 y dialysis, family history of renal cancer, ACKD, analgesic nephropathy, or long-term smoking). |
| • A curatively treated RCC <3 cm requires no observation period before transplantation. |
| • Larger (>3 cm) curatively treated RCCs require an observation period before transplantation, the length of which is debatable. Our center use 1 y, after which a thorough reexamination for recurrence is required. The KDIGO guidelines suggest longer observation periods of 2–5 y in “early” or “large and invasive” kidney cancer, respectively. |
| Screening for RCC in kidney transplant recipient |
| • Routine screening for RCC not recommended for average risk individuals. |
| • Not cost-effective. |
| • May be considered in high-risk groups (see above). |
| Treatment of RCC in KTRs |
| • For localized RCC in native kidney, nephrectomy is suggested. |
| • For localized RCC in allograft, nephron sparing surgery or ablative therapy is suggested. |
| • For advanced or metastatic RCC, shared decision making with involvement of the patient, caregivers, oncologist and transplant nephrologist is paramount. |
| ∘ There are no randomized controlled studies to guide recommendations. |
| ∘ Consider risks and consequences of rejection (ie, able or willing to return to dialysis?) |
| ∘ Consider adjustment of immunosuppression: minimization and/or switch to mTOR inhibitor (the latter delayed until after potential surgery, as wound healing is impaired by mTOR inhibitors). Graftectomy and complete withdrawal of immunosuppression is an option though rarely reported. |
| ∘ Consider immunotherapy combination or tyrosin kinase inhibitor |
| ∘ For patients who are frail, elderly and/or in poor prognosis groups, maintained standard immunosuppression is a valid option to maximize graft survival and quality of life. |
ACKD, acquired cystic kidney disease; KDIGO, Kidney Disease: Improving Global Outcomes; KTR, kidney transplant recipient; mTOR, mechanistic (mammalian) target of rapamycin; RCC, renal cell carcinoma.
CONCLUSIONS
KTRs are at increased risk of RCC, in particular of the native kidneys. Screening for RCC in all KTRs is not cost effective but may be of value in high-risk subsets, such as patients with a previous RCC and patients with known ACKD. Localized RCC is treated similar to the nontransplanted population. For advanced RCC, there is currently no trial evidence for the optimal immunosuppression regimen or oncological treatment. Prospectively collected data and randomized trials are urgently needed.
Supplementary Material
Footnotes
The authors declare no conflicts of interest.
D.O.D. performed the literature search, drafted the manuscript, and approved the final version. M.S., C.W.L., K.B., N.W., and K.M. contributed literature references, revised the manuscript, and approved the final version.
Supplemental Visual Abstract; http://links.lww.com/TP/C194.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–2490. [DOI] [PubMed] [Google Scholar]
- 2.Griffith JJ, Amin KA, Waingankar N, et al. Solid renal masses in transplanted allograft kidneys: a closer look at the epidemiology and management. Am J Transplant. 2017;17:2775–2781. [DOI] [PubMed] [Google Scholar]
- 3.Ljungberg B, Albiges L, Bensalah K, et al. Renal cell carcinoma, European Association of Urology (EAU) Guidelines. 2020. Available at http://uroweb.org/guidelines/compilations-of-all-guidelines/. Accessed October 11, 2020.
- 4.Amato RJ. Chemotherapy for renal cell carcinoma. Semin Oncol. 2000;27:177–186. [PubMed] [Google Scholar]
- 5.Bumpus HC. The apparent disappearance of pulmonary metastasis in a case of hypernephroma following nephrectomy. J Urol. 1928;20:185–191. [Google Scholar]
- 6.Vogelzang NJ, Priest ER, Borden L. Spontaneous regression of histologically proved pulmonary metastases from renal cell carcinoma: a case with 5-year followup. J Urol. 1992;148:1247–1248. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Lotze MT, Yang JC, et al. Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J Natl Cancer Inst. 1993;85:622–632. [DOI] [PubMed] [Google Scholar]
- 8.Fyfe G, Fisher RI, Rosenberg SA, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–696. [DOI] [PubMed] [Google Scholar]
- 9.McDermott DF, Regan MM, Clark JI, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133–141. [DOI] [PubMed] [Google Scholar]
- 10.Escudier B, Eisen T, Stadler WM, et al. ; TARGET Study Group. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. [DOI] [PubMed] [Google Scholar]
- 11.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. [DOI] [PubMed] [Google Scholar]
- 12.Hudes G, Carducci M, Tomczak P, et al. ; Global ARCC Trial. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. [DOI] [PubMed] [Google Scholar]
- 13.American Society of Clinical Oncology. Kidney Cancer Progress Timeline. Available at https://www.asco.org/research-guidelines/cancer-progress-timeline/kidney-cancer. Accessed October 11, 2020.
- 14.Motzer RJ, Tannir NM, McDermott DF, et al. ; CheckMate 214 Investigators. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378:1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rini BI, Plimack ER, Stus V, et al. ; KEYNOTE-426 Investigators. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–1127. [DOI] [PubMed] [Google Scholar]
- 16.Escudier B, Porta C, Schmidinger M, et al. ; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30:706–720. [DOI] [PubMed] [Google Scholar]
- 17.Renal Cell Carcinoma. ESMO Clinical Practice Guidelines - eUpdates February 2020. Available at https://www.esmo.org/guidelines/genitourinary-cancers/renal-cell-carcinoma. Accessed October 11, 2020.
- 18.Brierley JD, Gospodarowicz MK, Wittekind C, eds. IUCC TNM Classification of Malignant Tumours. 8th ed. John Wiley & Sons Inc.; 2016. [Google Scholar]
- 19.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. [DOI] [PubMed] [Google Scholar]
- 20.Heldal K, Hartmann A, Grootendorst DC, et al. Benefit of kidney transplantation beyond 70 years of age. Nephrol Dial Transplant. 2010;25:1680–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jofré R, López-Gómez JM, Moreno F, et al. Changes in quality of life after renal transplantation. Am J Kidney Dis. 1998;32:93–100. [DOI] [PubMed] [Google Scholar]
- 22.Liem YS, Bosch JL, Arends LR, et al. Quality of life assessed with the Medical Outcomes Study Short Form 36-Item Health Survey of patients on renal replacement therapy: a systematic review and meta-analysis. Value Health. 2007;10:390–397. [DOI] [PubMed] [Google Scholar]
- 23.Kontodimopoulos N, Niakas D. An estimate of lifelong costs and QALYs in renal replacement therapy based on patients’ life expectancy. Health Policy. 2008;86:85–96. [DOI] [PubMed] [Google Scholar]
- 24.United States Renal Data System. 2019 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2019. [Google Scholar]
- 25.Ying T, Shi B, Kelly PJ, et al. Death after kidney transplantation: an analysis by era and time post-transplant. J Am Soc Nephrol. 2020;31:2887–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ku E, McCulloch CE, Ahearn P, et al. Trends in cardiovascular mortality among a cohort of children and young adults starting dialysis in 1995 to 2015. JAMA Netw Open. 2020;3:e2016197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boenink R, Stel VS, Waldum-Grevbo BE, et al. Data from the ERA-EDTA Registry were examined for trends in excess mortality in European adults on kidney replacement therapy. Kidney Int. 2020;98:999–1008. [DOI] [PubMed] [Google Scholar]
- 28.Foster BJ. Survival improvements for Europeans with ESKD. Kidney Int. 2020;98:834–836. [DOI] [PubMed] [Google Scholar]
- 29.Au E, Wong G, Chapman JR. Cancer in kidney transplant recipients. Nat Rev Nephrol. 2018;14:508–520. [DOI] [PubMed] [Google Scholar]
- 30.Leveridge M, Musquera M, Evans A, et al. Renal cell carcinoma in the native and allograft kidneys of renal transplant recipients. J Urol. 2011;186:219–223. [DOI] [PubMed] [Google Scholar]
- 31.Karami S, Yanik EL, Moore LE, et al. Risk of renal cell carcinoma among kidney transplant recipients in the United States. Am J Transplant. 2016;16:3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denton MD, Magee CC, Ovuworie C, et al. Prevalence of renal cell carcinoma in patients with ESRD pre-transplantation: a pathologic analysis. Kidney Int. 2002;61:2201–2209. [DOI] [PubMed] [Google Scholar]
- 33.Hoover R, Fraumeni JF, Jr. Risk of cancer in renal-transplant recipients. Lancet. 1973;2:55–57. [DOI] [PubMed] [Google Scholar]
- 34.Wunderlich H, Wilhelm S, Reichelt O, et al. Renal cell carcinoma in renal graft recipients and donors: incidence and consequence. Urol Int. 2001;67:24–27. [DOI] [PubMed] [Google Scholar]
- 35.Einollahi B, Simforoosh N, Lessan-Pezeshki M, et al. Genitourinary tumor following kidney transplantation: a multicenter study. Transplant Proc. 2009;41:2848–2849. [DOI] [PubMed] [Google Scholar]
- 36.Hurst FP, Jindal RM, Graham LJ, et al. Incidence, predictors, costs, and outcome of renal cell carcinoma after kidney transplantation: USRDS experience. Transplantation. 2010;90:898–904. [DOI] [PubMed] [Google Scholar]
- 37.Tillou X, Doerfler A, Collon S, et al. ; “Comité de Transplantation de l’Association Française d’Urologie (CTAFU)”. De novo kidney graft tumors: results from a multicentric retrospective national study. Am J Transplant. 2012;12:3308–3315. [DOI] [PubMed] [Google Scholar]
- 38.Desai R, Collett D, Watson CJ, et al. Cancer transmission from organ donors-unavoidable but low risk. Transplantation. 2012;94:1200–1207. [DOI] [PubMed] [Google Scholar]
- 39.Smith JM, Martz K, McDonald RA, et al. Solid tumors following kidney transplantation in children. Pediatr Transplant. 2013;17:726–730. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Ma L, Xie Z, et al. Epidemiology of post-transplant malignancy in Chinese renal transplant recipients: a single-center experience and literature review. Med Oncol. 2014;31:32. [DOI] [PubMed] [Google Scholar]
- 41.Ranasinghe WK, Suh N, Hughes PD. Survival outcomes in renal transplant recipients with renal cell carcinoma or transitional cell carcinoma from the ANZDATA database. Exp Clin Transplant. 2016;14:166–171. [DOI] [PubMed] [Google Scholar]
- 42.Eggers H, Güler F, Ehlers U, et al. Renal cell carcinoma in kidney transplant recipients: descriptive analysis and overview of a major German transplant center. Future Oncol. 2019;15:3739–3750. [DOI] [PubMed] [Google Scholar]
- 43.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hickman LA, Sawinski D, Guzzo T, et al. Urologic malignancies in kidney transplantation. Am J Transplant. 2018;18:13–22. [DOI] [PubMed] [Google Scholar]
- 45.Moch H, Cubilla AL, Humphrey PA, et al. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur Urol. 2016;70:93–105. [DOI] [PubMed] [Google Scholar]
- 46.Patard JJ, Leray E, Rioux-Leclercq N, et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol. 2005;23:2763–2771. [DOI] [PubMed] [Google Scholar]
- 47.Tahbaz R, Schmid M, Merseburger AS. Prevention of kidney cancer incidence and recurrence: lifestyle, medication and nutrition. Curr Opin Urol. 2018;28:62–79. [DOI] [PubMed] [Google Scholar]
- 48.Bonsib SM. Renal cystic diseases and renal neoplasms: a mini-review. Clin J Am Soc Nephrol. 2009;4:1998–2007. [DOI] [PubMed] [Google Scholar]
- 49.Mühlfeld A, Boor P. Aquired cystic kidney disease and malignant neoplasms. Feehally J, Floege J, Tonelli M, et al., eds. In: Comprehensive Clinical Nephrology. 6th ed. Elsevier; 2019:1022–1027. [Google Scholar]
- 50.Schwarz A, Vatandaslar S, Merkel S, et al. Renal cell carcinoma in transplant recipients with acquired cystic kidney disease. Clin J Am Soc Nephrol. 2007;2:750–756. [DOI] [PubMed] [Google Scholar]
- 51.Vajdic CM, McDonald SP, McCredie MR, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823–2831. [DOI] [PubMed] [Google Scholar]
- 52.Kasiske BL, Snyder JJ, Gilbertson DT, et al. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4:905–913. [DOI] [PubMed] [Google Scholar]
- 53.Yanik EL, Clarke CA, Snyder JJ, et al. Variation in cancer incidence among patients with ESRD during kidney function and nonfunction intervals. J Am Soc Nephrol. 2016;27:1495–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Babaian KN, Delacroix SE, Wood CG, et al. Kidney Cancer. Skorecki K, Chertow GM, Marsden PA, et al., eds. In: Brenner and Rector’s The Kidney. 10th ed. Elsevier; 2016:1368–1388. [Google Scholar]
- 56.Silverman SG, Pedrosa I, Ellis JH, et al. Bosniak classification of cystic renal masses, version 2019: an update proposal and needs assessment. Radiology. 2019;292:475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Méjean A, Ravaud A, Thezenas S, et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N Engl J Med. 2018;379:417–427. [DOI] [PubMed] [Google Scholar]
- 58.Caoili EM, Davenport MS. Role of percutaneous needle biopsy for renal masses. Semin Intervent Radiol. 2014;31:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Macklin PS, Sullivan ME, Tapping CR, et al. Tumour seeding in the tract of percutaneous renal tumour biopsy: a report on seven cases from a UK tertiary referral centre. Eur Urol. 2019;75:861–867. [DOI] [PubMed] [Google Scholar]
- 60.Campbell S, Uzzo RG, Allaf ME, et al. Renal mass and localized renal cancer: AUA guideline. J Urol. 2017;198:520–529. [DOI] [PubMed] [Google Scholar]
- 61.Patel HD, Druskin SC, Rowe SP, et al. Surgical histopathology for suspected oncocytoma on renal mass biopsy: a systematic review and meta-analysis. BJU Int. 2017;119:661–666. [DOI] [PubMed] [Google Scholar]
- 62.Rivero JR, De La Cerda J, III, Wang H, et al. Partial nephrectomy versus thermal ablation for clinical stage T1 renal masses: systematic review and meta-analysis of more than 3,900 patients. J Vasc Interv Radiol. 2018;29:18–29. [DOI] [PubMed] [Google Scholar]
- 63.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rini BI, Powles T, Atkins MB, et al. ; IMmotion151 Study Group. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393:2404–2415. [DOI] [PubMed] [Google Scholar]
- 65.Amin S, Baine MJ, Meza JL, et al. Association of immunotherapy with survival among patients with brain metastases whose cancer was managed with definitive surgery of the primary tumor. JAMA Netw Open. 2020;3:e2015444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahrens M, Hartmann A, Bergmann L. What is new in the diagnosis and therapy of renal cell carcinoma?. Dtsch Med Wochenschr. 2020;145:734–739. [DOI] [PubMed] [Google Scholar]
- 67.Stubenbord WT, Cheigh JS, Riggio RR. Kidney transplantation immediately following excision of a malignant tumor from the donor kidney: a case report with long-term follow-up. Transplant Proc. 1982;14:775–776. [PubMed] [Google Scholar]
- 68.Hevia V, Hassan Zakri R, Fraser Taylor C, et al. Effectiveness and harms of using kidneys with small renal tumors from deceased or living donors as a source of renal transplantation: a systematic review. Eur Urol Focus. 2019;5:508–517. [DOI] [PubMed] [Google Scholar]
- 69.He B, Ng ZQ, Mou L, et al. Long-term outcome of kidney transplant by using restored kidney grafts after tumour ex vivo excision - a prospective study. Transpl Int. 2020;33:1253–1261. [DOI] [PubMed] [Google Scholar]
- 70.Flechner SM, Campbell SC. The use of kidneys with small renal tumors for transplantation: who is taking the risk? Am J Transplant. 2012;12:48–54. [DOI] [PubMed] [Google Scholar]
- 71.Lugo-Baruqui A, Guerra G, Arocha A, et al. Use of kidneys with small renal tumors for transplantation. Curr Urol Rep. 2016;17:3. [DOI] [PubMed] [Google Scholar]
- 72.European Directorate for the Quality of Medicines & HealthCare of the Council of Europe. Guide to the quality and safety of organs for transplantation (Council of Europe, Strasbourg, France, 2018). Available at https://www.edqm.eu/en/news/new-release-7th-edition-guide-quality-and-safety-organs-transplantation. Accessed October 11, 2020.
- 73.Nalesnik MA, Woodle ES, Dimaio JM, et al. Donor-transmitted malignancies in organ transplantation: assessment of clinical risk. Am J Transplant. 2011;11:1140–1147. [DOI] [PubMed] [Google Scholar]
- 74.Xiao D, Craig JC, Chapman JR, et al. Donor cancer transmission in kidney transplantation: a systematic review. Am J Transplant. 2013;13:2645–2652. [DOI] [PubMed] [Google Scholar]
- 75.Batabyal P, Chapman JR, Wong G, et al. Clinical practice guidelines on wait-listing for kidney transplantation: consistent and equitable? Transplantation. 2012;94:703–713. [DOI] [PubMed] [Google Scholar]
- 76.Chadban SJ, Ahn C, Axelrod DA, et al. KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Transplantation. 2020;104(4S1 Suppl 1):S11–S103. [DOI] [PubMed] [Google Scholar]
- 77.Dahle DO, Grotmol T, Leivestad T, et al. Association between pretransplant cancer and survival in kidney transplant recipients. Transplantation. 2017;101:2599–2605. [DOI] [PubMed] [Google Scholar]
- 78.Nguyen KA, Syed JS, Luciano R, et al. Optimizing waiting duration for renal transplants in the setting of renal malignancy: is 2 years too long to wait? Nephrol Dial Transplant. 2017;32:1767–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cognard N, Anglicheau D, Gatault P, et al. Recurrence of renal cell cancer after renal transplantation in a multicenter French cohort. Transplantation. 2018;102:860–867. [DOI] [PubMed] [Google Scholar]
- 80.Acuna SA, Huang JW, Scott AL, et al. Cancer screening recommendations for solid organ transplant recipients: a systematic review of clinical practice guidelines. Am J Transplant. 2017;17:103–114. [DOI] [PubMed] [Google Scholar]
- 81.Kidney Disease: Improving Global Outcomes Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–S155. [DOI] [PubMed] [Google Scholar]
- 82.Wong G, Howard K, Webster AC, et al. Screening for renal cancer in recipients of kidney transplants. Nephrol Dial Transplant. 2011;26:1729–1739. [DOI] [PubMed] [Google Scholar]
- 83.Motta G, Ferraresso M, Lamperti L, et al. Treatment options for localised renal cell carcinoma of the transplanted kidney. World J Transplant. 2020;10:147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Favi E, Raison N, Ambrogi F, et al. Systematic review of ablative therapy for the treatment of renal allograft neoplasms. World J Clin Cases. 2019;7:2487–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Venkatachalam K, Malone AF, Heady B, et al. Poor outcomes with the use of checkpoint inhibitors in kidney transplant recipients. Transplantation. 2020;104:1041–1047. [DOI] [PubMed] [Google Scholar]
- 86.d’Izarny-Gargas T, Durrbach A, Zaidan M. Efficacy and tolerance of immune checkpoint inhibitors in transplant patients with cancer: a systematic review. Am J Transplant. 2020;20:2457–2465. [DOI] [PubMed] [Google Scholar]
- 87.Zwald FO. Transplant-associated cancer in the era of immune checkpoint inhibitors: primum non nocere. Am J Transplant. 2020;20:2299–2300. [DOI] [PubMed] [Google Scholar]
- 88.Krisl JC, Doan VP. Chemotherapy and transplantation: the role of immunosuppression in malignancy and a review of antineoplastic agents in solid organ transplant recipients. Am J Transplant. 2017;17:1974–1991. [DOI] [PubMed] [Google Scholar]
- 89.Reshef R, Vardhanabhuti S, Luskin MR, et al. Reduction of immunosuppression as initial therapy for posttransplantation lymphoproliferative disorder. Am J Transplant. 2011;11:336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nichols LA, Adang LA, Kedes DH. Rapamycin blocks production of KSHV/HHV8: insights into the anti-tumor activity of an immunosuppressant drug. PLoS One. 2011;6:e14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hope CM, Krige AJ, Barratt A, et al. Reductions in immunosuppression after haematological or solid organ cancer diagnosis in kidney transplant recipients. Transpl Int. 2015;28:1332–1335. [DOI] [PubMed] [Google Scholar]
- 92.Rousseau B, Guillemin A, Duvoux C, et al. Optimal oncologic management and mTOR inhibitor introduction are safe and improve survival in kidney and liver allograft recipients with de novo carcinoma. Int J Cancer. 2019;144:886–896. [DOI] [PubMed] [Google Scholar]
- 93.Knoll GA, Kokolo MB, Mallick R, et al. Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta-analysis of individual patient data. BMJ. 2014;349:g6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dantal J, Morelon E, Rostaing L, et al. ; TUMORAPA Study Group. Sirolimus for secondary prevention of skin cancer in kidney transplant recipients: 5-year results. J Clin Oncol. 2018;36:2612–2620. [DOI] [PubMed] [Google Scholar]
- 95.Geissler EK, Schnitzbauer AA, Zülke C, et al. Sirolimus use in liver transplant recipients with hepatocellular carcinoma: a randomized, multicenter, open-label phase 3 trial. Transplantation. 2016;100:116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Bruyn P, Van Gestel D, Ost P, et al. Immune checkpoint blockade for organ transplant patients with advanced cancer: how far can we go? Curr Opin Oncol. 2019;31:54–64. [DOI] [PubMed] [Google Scholar]
- 97.Alhamad T, Venkatachalam K, Linette GP, et al. Checkpoint inhibitors in kidney transplant recipients and the potential risk of rejection. Am J Transplant. 2016;16:1332–1333. [DOI] [PubMed] [Google Scholar]
- 98.Muruve NA, Shoskes DA. Genitourinary malignancies in solid organ transplant recipients. Transplantation. 2005;80:709–716. [DOI] [PubMed] [Google Scholar]
- 99.Hongo F, Oishi M, Ueda T, et al. Complete response of sunitinib therapy for renal cell cancer recurrence in the native kidney after renal transplantation: a case report. BMC Res Notes. 2014;7:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mar N, Houshyar R, Jordan M. Use of neoadjuvant sunitinib in renal cell carcinoma of a transplanted kidney. J Oncol Pract. 2018;14:632–634. [DOI] [PubMed] [Google Scholar]
- 101.Hasegawa Y, Mita K, Matsubara A, et al. Multidisciplinary treatment including sorafenib stabilized the bone metastases of renal cell carcinoma in an immunosuppressed renal transplant recipient. Int J Clin Oncol. 2009;14:465–467. [DOI] [PubMed] [Google Scholar]
- 102.Ruangkanchanasetr P, Kanjanapayak B, Jungmeechoke K. Prolonged survival in renal transplant recipient with advanced renal cell carcinoma by everolimus and sorafenib. Nephrology (Carlton). 2011;16:118–119. [DOI] [PubMed] [Google Scholar]
- 103.Chueh SJ, Sankari BR, Gonzales-Chambers R, et al. Temsirolimus as base immunosuppressant for a recipient with metastatic renal cancer: adequate immunosuppression and oncological control–case report. Transplant Proc. 2014;46:271–273. [DOI] [PubMed] [Google Scholar]
- 104.Zheng I, Alameddine M, Tan Y, et al. Collecting duct carcinoma of the native kidney in a renal transplant recipient. Case Rep Transplant. 2017;2017:4527104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Porta C, Cosmai L, Gallieni M, et al. Renal effects of targeted anticancer therapies. Nat Rev Nephrol. 2015;11:354–370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.