Abstract
Fundamental understandings and precise control of nanoparticle growth in the complex biological environment are crucial to broadening their potential applications in tissue imaging. Herein, we report that glutathione (GSH), a widely used capping ligand for precise control of the size of gold nanoparticle (AuNP) down to single-atom level in test tubes, can also be used to direct the selective growth of the AuNPs in the mitochondria of renal tubule cells as well as hippocampus cells in the tissues. Precise control of this growth process can lead to the formation of both ultrasmall AuNPs with near-infrared luminescence and large plasmonic AuNPs. The observed selective growth of the AuNPs is likely due to unique GSH storage function of the mitochondria. Using a different ligand, β-glucose thiol, we also found that the brush border of the intestine for glucose absorption became the major site for the growth of luminescent AuNPs. These findings suggest that selective growth of AuNPs in the biological tissues can indeed be directed with specific ligands, opening up a new avenue to tissue labeling and future development of artificial bionano hybrid systems.
Keywords: Gold nanoparticles, growth, in situ, imaging, staining, tissue engineering
Graphical Abstract
Fundamental understandings and precise control of engineered nanoparticle growth have led to many breakthroughs in both medical and energy technologies.1–7 For instance, Xia et al. reported the synthesis of mono-dispersed gold nanocages through galvanic replacement1 and further used these nanocages for cancer cell targeting and optical imaging when conjugated with antibodies.8 More recently, ultrasmall gold clusters can be incorporated into the bacteria and enable photosynthesis of acetic acid from CO2 in the nonphotosynthetic bacteria.7 Although many factors are involved in the nanoparticle growth, surface ligand is known to play a key role in confining nanoparticle size, stability, and functionality.2,9–12 With assistance of a variety of surface ligands, many engineered NPs such as gold NPs, iron NPs, and silica NPs can be synthesized with precise control of dimensions and functions.13–15 In particular, the gold nanoparticles (AuNPs) can be prepared with the sizes precisely controlled at a single-atom level.16–18 For example, using p-mercaptobenzoic acid (p-MBA) as surface ligands, a variety of gold nanoclusters (AuNCs) with well-defined structures, such as Au44(p-MBA)26,18 Au102(p-MBA)44,19 Au144(p-MBA)60,20 and so forth, have been prepared. In the aqueous phase, the glutathione (GSH) is the most widely used capping agent in the synthesis of atomically precise AuNCs.21–23 Although significant progression has been made in the control of AuNP growth in the test tubes, continuously advancing understanding and control of AuNP growth in even more complex biological environment is expected to further broaden their potentials.
AuNPs have demonstrated broad applications in the biological imaging as multimodality probes because of their versatile properties such as surface plasmons, fluorescence, and high electron density.8,24–26 More recently, Syed et al. combined tissue clearing technologies with plasmonic AuNPs to further advance their applications in three-dimensional (3D) tissue imaging.6 In particular, signal outputs can be further amplified by in situ growth of AuNPs within cleared tissues, which allowed visualization of only few nanoparticles in the tumor sites. These latest advancements clearly open up a new avenue to biomedical applications of gold nanoparticles. Moreover, it also highlights the importance of deeper understanding and precise control of AuNP growth in the tissue environment. In the present work, we investigated the AuNP growth mainly in kidney tissues in the presence of glutathione, the most abundant intracellular antioxidant that is mainly stored in the mitochondria. Using electron microscopy imaging, we observed the selective distribution of plasmonic gold nanoparticles in the mitochondrial structure of renal tubule cells rather than other cellular organelles in kidney tissues. In the early formation phase, the AuNPs with ultrasmall size (<5 nm) exhibited near-infrared (NIR) luminescence, enabling the multiscale characterization with not only electron microscopy but also fluorescence imaging. Not limited to kidney tissues, GSH-protected AuNPs (GS-AuNPs) can selectively grow in the mitochondria of brain hippocampus with the highest density mediated by glutathione-mitochondrion interaction as well. This selective growth of the AuNPs in the mitochondria is likely because the GSH is known to be actively imported into live cells and selectively stored in mitochondria at high concentration for regulating the molecular machinery of cell death.27 Using β-glucose thiol, we also found the selective growth of AuNPs in the brush border of intestine, which is known for glucose absorption. These findings suggest that the ligand-directed AuNP growth in the biological tissues could open up a new pathway to tissue labeling and imaging.
It is known that chloroauric acid (HAuCl4) and thiol (R-SH, such as GSH) in solution readily form the thiolated gold precursor ([Au-SR]), which then dissociates into thiol-protected AuNPs (Figure 1a).28,29 Herein, the AuNP formation in solution was first investigated systematically under different conditions (see Methods and Figure S1) where the HAuCl4 and GSH can be reduced by formaldehyde30 to GS-AuNPs under controlled kinetics (Figure S1). Transmission electron microscopy (TEM) imaging and Fourier transform-infrared (FT-IR) analysis were used to confirm the size evolution of AuNPs and surface coating of GSH ligand (Figures S2 and S3). To conduct the in situ AuNP growth, biological tissues (prefixed kidney slides) were immersed in 4% formaldehyde with selected thiol (R-SH, ~0.5 mM GSH) and incubated for 30 min at room temperature (Figure 1b). The HAuCl4 was then added to a predetermined gold-to-thiol molar ratio of 1:1 (Figures 1c and S1), and the solution was mixed well by gently swirling and then incubated for overnight. On Day 1, the solution changed the color gradually from transparent to light brown-red (consistent with AuNP formation in solution in Figure S1), indicating the formation of ultrasmall GS-AuNPs (<5 nm, Figure S2). In the meantime, there were some AuNPs formed locally in the kidney cortex (Day 1, Figure 1d,e). With time proceeded, these localized AuNPs in tissues evolved into larger AuNPs (plasmonic, >5 nm) and predominantly formed in renal tubule structures (Day 2, Figure 1d–f). Interestingly, some renal tubules (T1) were heavily stained with plasmonic AuNPs (red color, Figure 1f) as shown in the zoom-in image, whereas the neighboring renal tubules (T2, contrasted with DAPI staining) as well as other nephron segments showed no such AuNP growth at high density even at longer time, suggesting a selective formation of AuNPs in the kidney tissues. However, this AuNP distribution with high selectivity cannot be obtained by incubating the tissues with presynthesized GS-AuNPs, indicating the in situ growth of AuNPs is right within the renal tubules rather than the binding of AuNPs (formed in solution) to these specific structures. On the other hand, we changed the ligand to a nonspecific poly(ethylene glycol) thiol (mPEG-SH) ligand for in situ AuNP growth under the same conditions (gold-to-thiol ratio, 1:1, Figure S4) and found that there was minimal growth of mPEG-AuNPs in the kidney tissues (Figure S5). To be noted, using other nonspecific thiols (such as cysteine, thioguanosine, mercaptobenzoic acid, and so forth) could not lead to such selective AuNP growth as well, and large AuNPs would rapidly form in solution if no thiol ligand was added for protection. These results suggested that this in situ AuNP growth in tissues was primarily directed by the glutathione ligand.
Figure 1.
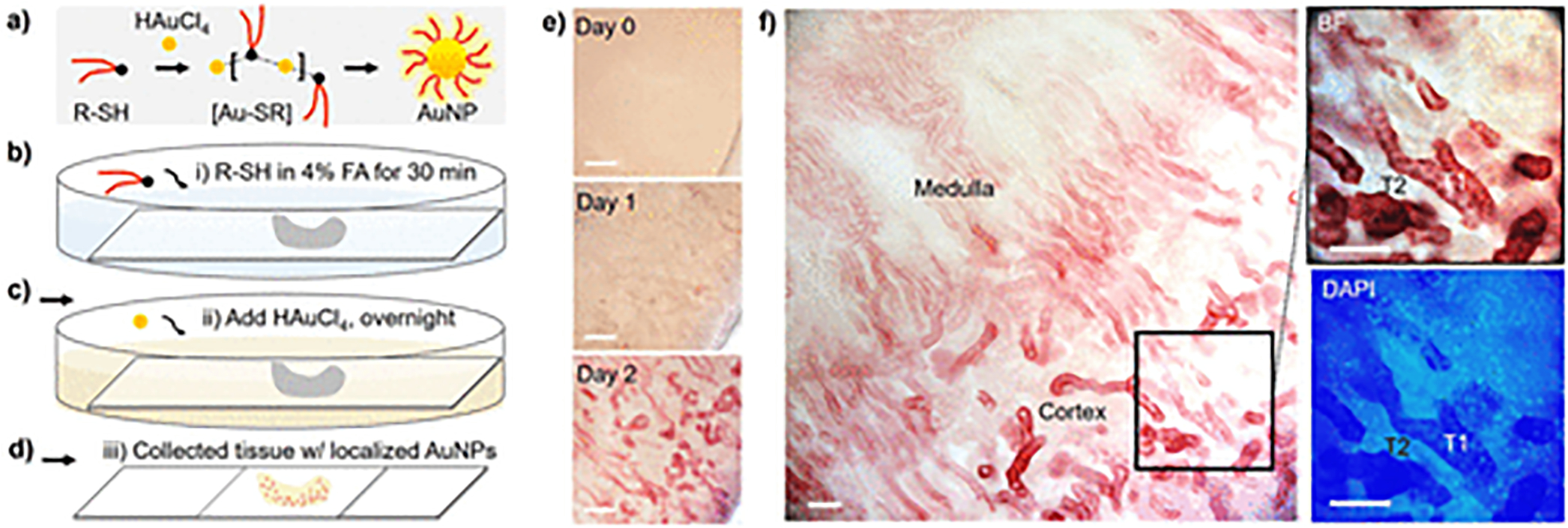
In situ growth of AuNPs in biological (kidney) tissues. (a) Formation process of thiol-protected AuNPs in aqueous solution. The gold ions (HAuCl4) and thiol ligand (R-SH) in solution can readily form thiolated gold precursor ([Au-SR]) and then dissociate into thiol-protected AuNPs. (b–d) Scheme of in situ AuNP formation process in biological tissues (see Methods in SI for experimental details). FA, formaldehyde. (e,f) Time evolution of the AuNP growth in kidney tissues. The AuNPs (plasmonic, red color) showed preferential distribution in the selected tubules (high in T1, but low in T2, f) instead of other nephron segments in kidney cortex. Scale bar, 0.2 mm (e); 0.1 mm (f).
These locally formed AuNPs in tissues can be readily visualized under electron microscope where the renal proximal tubule (PT, with microvilli) and distal tubule (DT) cells in the kidney cortex are shown in Figure 2a. The AuNPs were found predominately inside the mitochondria of both PT and DT cells with high density and were almost evenly distributed inside the mitochondria as a single nanoparticle (Figure 2b,c). By counting the AuNPs in different cellular compartments (cytoplasm, nucleus, and mitochondrial areas in both PT and DT cells), we found that the most abundant AuNPs were formed in the DT mitochondria (DT-M, 34.1 ± 5.9 NPs per area of 200 nm × 200 nm, Figure 2d, which was 1.8 times higher than that of PT mitochondria (PT-M, 19.5 ± 3.5 NPs per area of 200 nm × 200 nm) and about 15 times higher than those of the other regions (~2 NPs per area of 200 nm × 200 nm). In addition, these localized AuNPs showed the narrow and similar size distribution in both DT and PT mitochondria (14.8 ± 2.2 and 13.7 ± 2.0 nm, respectively, Figure 2e,f). This in situ AuNP growth in both DT and PT mitochondria may attribute to unique storage function of cellular mitochondria to GSH molecules.27 The AuNP growth of the most abundance in DT cells is mainly because the mitochondrial density in DT cells is the highest among all the cells along the nephron,31–33 which is also evident from the TEM imaging (Figure 2a). Therefore, these T1 structures heavily stained with AuNPs in Figure 1f can be identified as the renal DTs. With such highly selective in situ AuNP growth by using GSH, these results indicate that the ligand-directed in situ AuNP growth may occur in some specific cellular compartments in the complex tissues due to the ligand-environment interaction.
Figure 2.
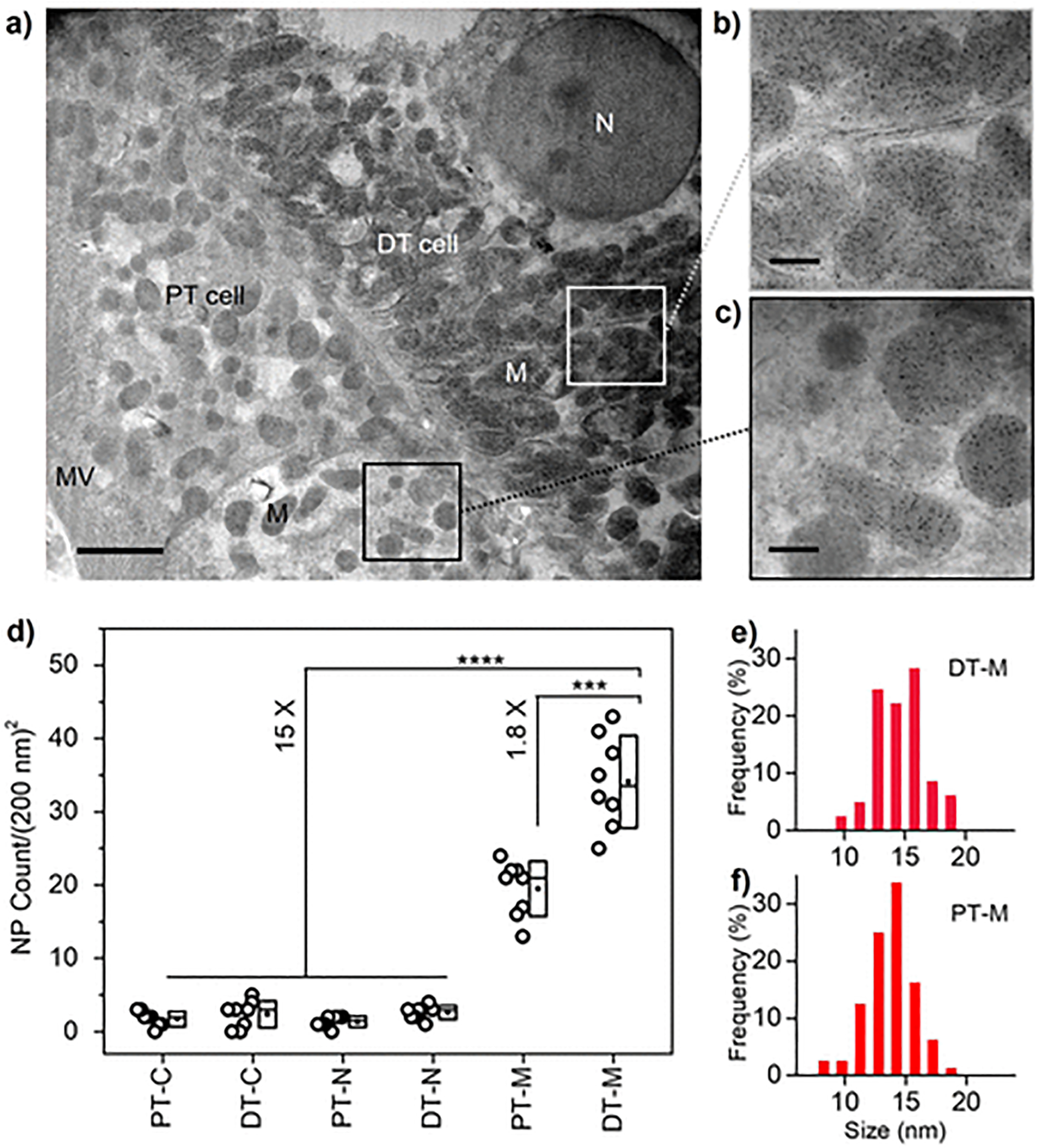
Subcellular distribution of in situ formed AuNPs in renal tubule cells. (a–c) TEM imaging of the renal tubule cells with in situ growth of plasmonic GS-AuNPs. DT, distal tubule; PT, proximal tubule; N, nucleus; M, mitochondrion; MV, microvilli. Scale bar, 2 μm (a); 400 nm (b,c). (d) NP counts in different regions of interest, ROIs (per area of 200 nm × 200 nm) in PT and DT cells. PT cytoplasm, PT-C; DT cytoplasm, DT-C; PT nucleus, PT-N; DT nucleus, DT-N; PT mitochondrion, PT-M; DT mitochondrion, DT M. ***P < 0.0005, ****P < 0.0001 (n = 8, One-Way ANOVA). TEM imaging of the blank kidney was shown in Figure S6. (e,f) Size distribution of in situ formed AuNPs in DT and PT mitochondria.
More interestingly, in the early formation phase the strong NIR luminescence29 was detected from these in situ formed AuNPs of ultrasmall size in the kidney tissues (Day 1, Figure 3a). Similarly, the biogenic ultrasmall AuNPs in solution exhibited the NIR emission peaks at 730 and 800 nm under the excitation at 350 nm (Figure 3b), consistent with previously reported NIR luminescent AuNPs or AuNCs.7,34–36 Notably, the NIR signal was decreased at high HAuCl4 concentration due to the accelerated AuNP evolution to larger size, whereas the NIR signal on tissues was also lowered with reduced contrast at low HAuCl4 concentration. Confocal fluorescence microscopy imaging studies show that these locally formed luminescent GS-AuNPs distributed predominately in selected renal tubules rather than other nephron structures such as glomerulus (Figure 3c), confirming the above-mentioned results. More specifically, the AuNPs were heavily localized at the basolateral side of cytoplasm area in the renal DTs (Figure 3d), where the mitochondria of highest density are known to form bundles of filaments and anchor to the basement membrane of distal tubules.31 In contrast, we found that neither mPEG-SH nor presynthesized NIR luminescent GS-AuNPs showed any preferential distribution in the same tissues (Figure S7), which was also consistent with the results for plasmonic AuNPs. These results indicate that the NIR luminescence of the AuNPs can be used to reflect the interaction of GSH with local mitochondria and resulting nanoparticle localization.
Figure 3.
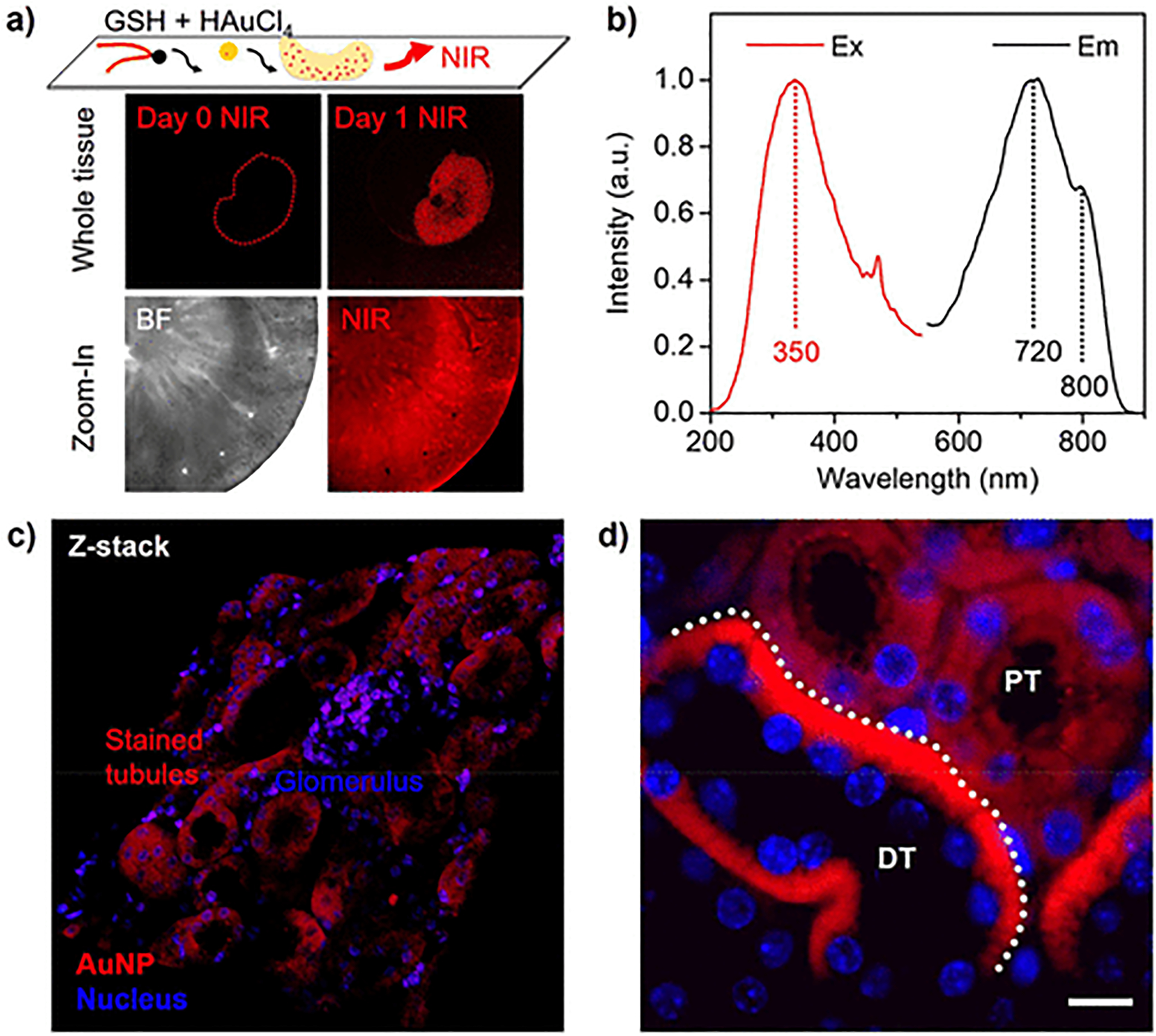
GSH-directed growth of NIR luminescent AuNPs in kidney tissues. (a) Whole-tissue NIR fluorescence imaging of the kidney before (Day 0) and after (Day 1) the formation of GS-AuNPs and zoom-in images of bright field (BF) and near-infrared (NIR) imaging of the kidney cortex after the in situ AuNP growth. (b) NIR luminescence spectra of the GS-AuNPs. (c) Confocal fluorescence microscopy (z-stack) image of kidney cortex with localized GS-AuNPs (full-scale width, 320 μm). (d) Microscopy imaging for the kidney slides (4 μm) with in situ formed ultrasmall GS-AuNPs. Scale bar, 10 μm. DT, distal tubule; PT, proximal tubule.
Because this ligand-directed growth of AuNPs could respond differently to the renal subcellular structures owing to the GSH-mediated interaction with mitochondria, we continued to understand and substantiate this GSH-directed AuNP growth by using a different biological tissue. The mitochondria have been reported to have great impact on the brain cognition and function,37,38 thus we next chose brain tissues to see whether there was the selective growth of the GS-AuNPs. Similar to the kidney slides, we found that the brain slides after 1 day NP growth showed the strong NIR signals as well (Figure S8a,b), indicating the in situ growth of large amount of ultrasmall GS-AuNPs. More specifically, in the hippocampus there were several types of cells with locally formed GS-AuNPs at the highest selectivity, including the cornu ammonis (CA) pyramidal cells and dentate gyrus (DG) granular cells (Figure 4a,b).39 From the confocal fluorescence microscopy, the formed luminescent GS-AuNPs were highly confined in the DG regions surrounding the nuclei (Figure S8c,d). The mitochondria in the brain are known to undergo the continuous process of distribution throughout the cell to reach the sites of high energy demand,38,40 especially the hippocampus. Therefore, this in situ AuNP formation with high selectivity in hippocampus cells may also attribute to the strong interaction and related function between GSH and mitochondria. We next used the TEM imaging to characterize the local distribution of these GS-AuNPs in the subcellular structures of the hippocampus cells. Consistent with the observation from the kidneys, GS-AuNPs were selectively grown in the cellular mitochondria with high density, whereas there was low NP distribution in nuclei (Figure 4c–e). Consistent with the observation from the kidneys, these results derived from the brain tissues suggested the GSH-directed AuNP growth in biological tissues originated from the interaction of GSH ligand to the cellular mitochondrial structures. However, it should be noted that we also observed the formation of ultrasmall AuNPs in the other organells such as endoplasmic reticulum in addition to mitochondria in the brain tissues (Figure 4c). This finding implies that some other compartments might also be involved in GSH storage, which demads the further investigation.
Figure 4.
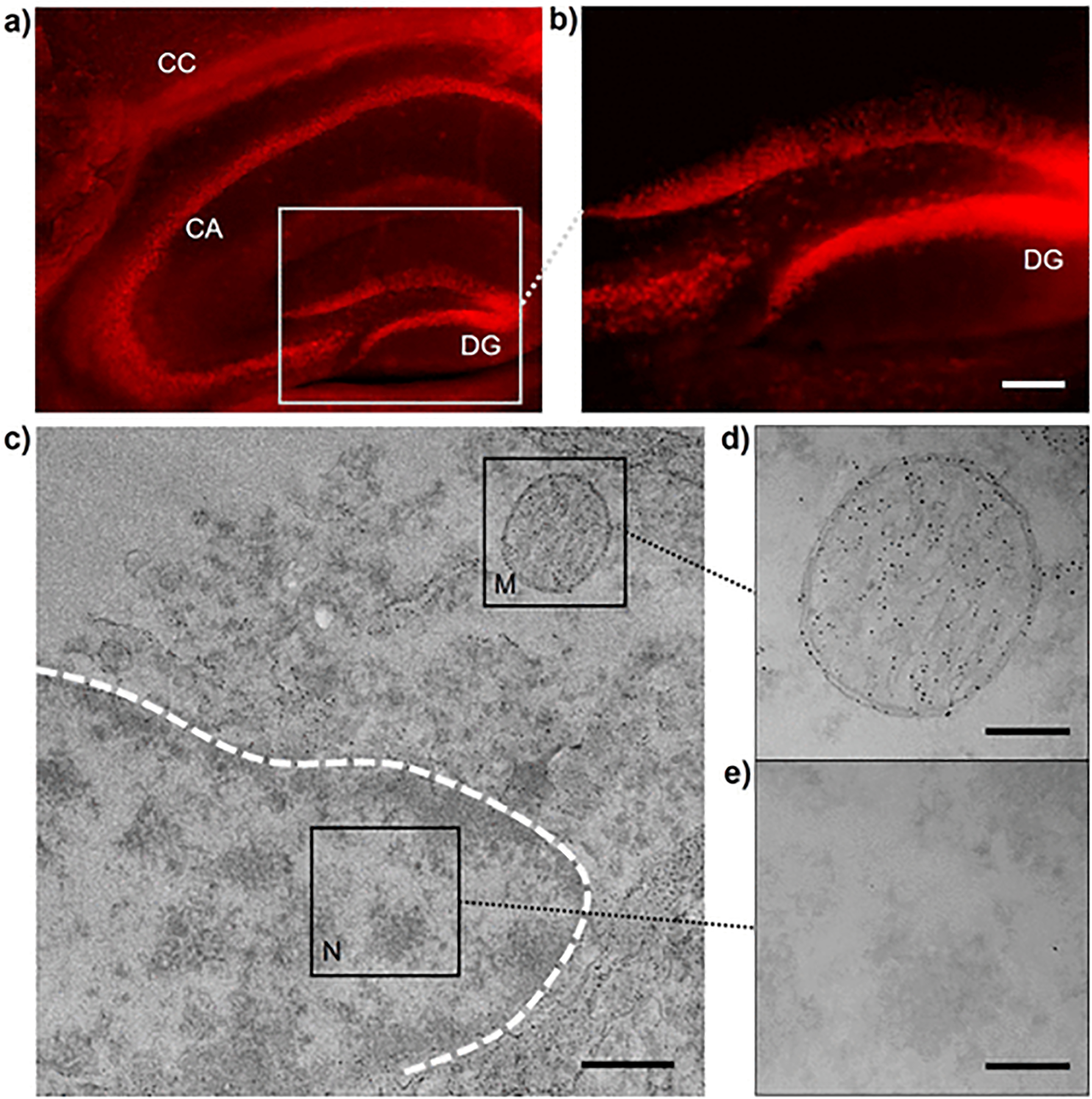
GSH-directed growth of AuNPs in brain hippocampus. (a,b) NIR fluorescent microscopy imaging of GS-AuNPs in brain hippocampus. Scale bar, 100 μm. (c–e) TEM imaging of subcellular area of hippocampus cells with GS-AuNPs growth. M, mitochondrion; N, nucleus. Scale bar, 500 nm (c); 200 nm (d,e).
Similar to the glutathione, we further found that the in situ formed AuNPs, under the direction of another specific ligand, β-glucose thiol, were selectively localized in the brush border of small intestine (lumen membrane, tunica mucosa, Figure 5) rather than other regions (submembrane, tunica submucosa; and muscle, tunica muscularis). This was due to the strong affinity and interaction of glucose moiety to the brush border membrane of the intestine as previously reported.41 Electron microscopy imaging also confirmed the highly localized β-glucose-AuNPs in these brush border regions compared to the cytoplasm and nucleus areas of intestinal cells (Figure S9). This further indicates that the ligand-directed growth of AuNPs may serve as a novel strategy to selectively label specific subcellular compartments or ultrafine structures in biological tissues via nanoparticle labeling and imaging. It is worth noting that the major challenge for highly selective nanoparticle labeling is to use the specific ligand-tissue interaction to direct the AuNP localization and formation under controlled kinetics, and we believe with rational design and extensive investigation this AuNP labeling method may be also achieved with many other biological (normal or pathological) organs and tissues.
Figure 5.
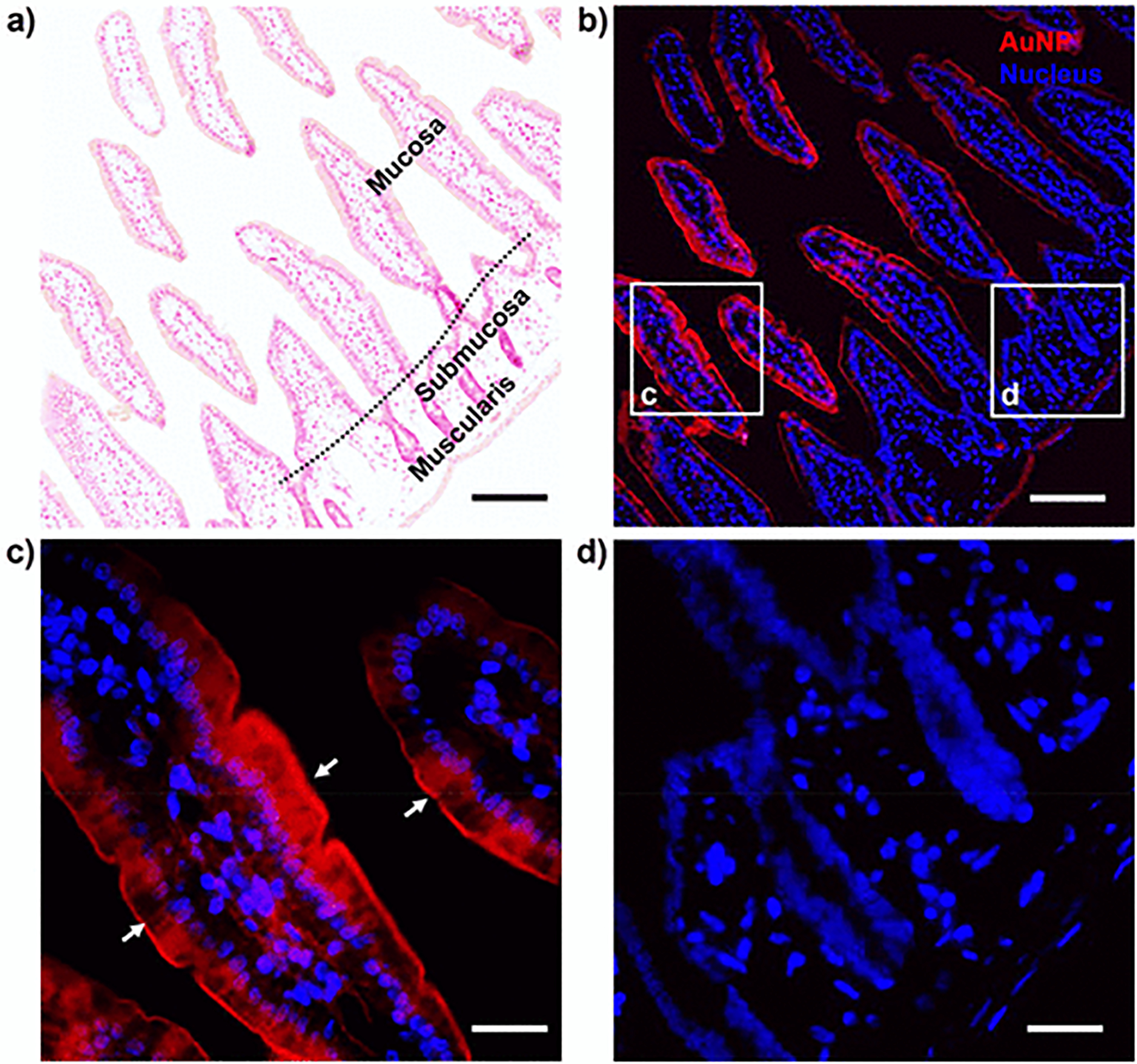
Glucose-directed in situ growth of β-glucose-AuNPs in small intestine. (a) Histological imaging (H&E staining) of the small intestine. (b–d) Fluorescence imaging of small intestine with in situ growth of NIR luminescent β-glucose-AuNPs. Directed by β-glucose thiol, these NIR luminescent AuNPs were predominately localized at the brush border of small intestine. The TEM imaging in Figure S9 also confirmed these results. Scale bar, 100 μm (a,b); 25 μm (c,d).
In summary, we reported in situ selective growth of AuNPs in kidney and brain tissues, which can be directed by a specific thiol ligand, glutathione. We found that in situ formed plasmonic AuNPs were selectively localized in the mitochondrial structures in tubule cells of the kidney as well as brain hippocampus cells. Moreover, in the early formation phase the ultrasmall-sized AuNPs with NIR luminescence were locally formed in these tissues under the controlled kinetics, which allowed not only electron microscopy imaging but also fluorescence imaging of mitochondria in the biological tissues. Not limited to glutathione, we also found that β-glucose thiol enabled the selective growth of NIR-emitting AuNPs in the brush border membrane of the intestine. The observation of ligand-directed selective growth of luminescent or plasmonic AuNPs in the specific organelle of biological tissues might open up a new path for tissue labeling and allow multiple imaging modalities (including electron microscopy, near-infrared luminescence, surface plasmon resonance,42 scattering,6 and so forth) to probe nanoparticle growth at an inclusive and tunable spatial resolution ranging from millimeter (10−3 m) down to nanometer scale (10−9 m), which normally cannot be achieved with small molecular dyes6 nor with other metal staining methods.43,44 With more investigation in comparing the AuNP growth in the normal and diseased (kidney) tissues, such in situ AuNP growth may serve as a promising metal-based staining method for ex vivo disease detection and diagnosis (Figure S10). In addition, the fundamental understandings derived from the studies also lay down a foundation for further development of artificial bionano hybrid systems.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the NIH (1R01DK103363 and 1R01DK115986), CPRIT (RP160866), and Welch Research Foundation [AT-1974-20180324 (J.Z.)] and Cecil H. and Ida Green Professorship (J.Z.) from The University of Texas at Dallas.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.nanolett.9b04911.
Methods, related results, and additional figures (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Sun Y; Xia Y Science 2002, 298 (5601), 2176–2179. [DOI] [PubMed] [Google Scholar]
- (2).Wang G; Huang T; Murray RW; Menard L; Nuzzo RG J. Am. Chem. Soc 2005, 127 (3), 812–813. [DOI] [PubMed] [Google Scholar]
- (3).Wei H; Wang Z; Zhang J; House S; Gao Y-G; Yang L; Robinson H; Tan LH; Xing H; Hou C; et al. Nat. Nanotechnol 2011, 6 (2), 93. [DOI] [PubMed] [Google Scholar]
- (4).Huang X; Tang S; Mu X; Dai Y; Chen G; Zhou Z; Ruan F; Yang Z; Zheng N Nat. Nanotechnol 2011, 6 (1), 28–32. [DOI] [PubMed] [Google Scholar]
- (5).Liu Y; Goebl J; Yin Y Chem. Soc. Rev 2013, 42 (7), 2610–2653. [DOI] [PubMed] [Google Scholar]
- (6).Syed AM; Sindhwani S; Wilhelm S; Kingston BR; Lee DS; Gommerman JL; Chan WC J. Am. Chem. Soc 2017, 139 (29), 9961–9971. [DOI] [PubMed] [Google Scholar]
- (7).Zhang H; Liu H; Tian Z; Lu D; Yu Y; Cestellos-Blanco S; Sakimoto KK; Yang P Nat. Nanotechnol 2018, 13 (10), 900. [DOI] [PubMed] [Google Scholar]
- (8).Chen J; Saeki F; Wiley BJ; Cang H; Cobb MJ; Li Z-Y; Au L; Zhang H; Kimmey MB; Li; Xia Y Nano Lett. 2005, 5 (3), 473–477. [DOI] [PubMed] [Google Scholar]
- (9).Tao AR; Habas S; Yang P Small 2008, 4 (3), 310–325. [Google Scholar]
- (10).Wu B; Zheng N Nano Today 2013, 8 (2), 168–197. [Google Scholar]
- (11).Xia Y; Xiong Y; Lim B; Skrabalak SE Angew. Chem., Int. Ed 2009, 48 (1), 60–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Walkey CD; Olsen JB; Guo H; Emili A; Chan WCW J. Am. Chem. Soc 2012, 134 (4), 2139–2147. [DOI] [PubMed] [Google Scholar]
- (13).Jin R; Zeng C; Zhou M; Chen Y Chem. Rev 2016, 116 (18), 10346–10413. [DOI] [PubMed] [Google Scholar]
- (14).Sun Y; Zuo X; Sankaranarayanan SK; Peng S; Narayanan B; Kamath G Science 2017, 356 (6335), 303–307. [DOI] [PubMed] [Google Scholar]
- (15).Ma K; Gong Y; Aubert T; Turker MZ; Kao T; Doerschuk PC; Wiesner U Nature 2018, 558 (7711), 577. [DOI] [PubMed] [Google Scholar]
- (16).Li ZY; Young NP; Di Vece M; Palomba S; Palmer RE; Bleloch AL; Curley BC; Johnston RL; Jiang J; Yuan J Nature 2008, 451 (7174), 46–48. [DOI] [PubMed] [Google Scholar]
- (17).Qian H; Eckenhoff WT; Zhu Y; Pintauer T; Jin R J. Am. Chem. Soc 2010, 132 (24), 8280–8281. [DOI] [PubMed] [Google Scholar]
- (18).Yao Q; Yuan X; Fung V; Yu Y; Leong DT; Jiang D.-e.; Xie J Nat. Commun 2017, 8 (1), 927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Jadzinsky PD; Calero G; Ackerson CJ; Bushnell DA; Kornberg RD Science 2007, 318 (5849), 430–433. [DOI] [PubMed] [Google Scholar]
- (20).Jensen KMØ; Juhas P; Tofanelli MA; Heinecke CL; Vaughan G; Ackerson CJ; Billinge SJL Nat. Commun 2016, 7 (1), 11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Negishi Y; Nobusada K; Tsukuda T J. Am. Chem. Soc 2005, 127 (14), 5261–5270. [DOI] [PubMed] [Google Scholar]
- (22).Yu Y; Chen X; Yao Q; Yu Y; Yan N; Xie J Chem. Mater 2013, 25 (6), 946–952. [Google Scholar]
- (23).Wu Z; Gayathri C; Gil RR; Jin R J. Am. Chem. Soc 2009, 131 (18), 6535–6542. [DOI] [PubMed] [Google Scholar]
- (24).Kim BY; Rutka JT; Chan WC N. Engl. J. Med 2010, 363 (25), 2434–2443. [DOI] [PubMed] [Google Scholar]
- (25).Chan WCW Acc. Chem. Res 2017, 50 (3), 627–632. [DOI] [PubMed] [Google Scholar]
- (26).Sun T; Zhang YS; Pang B; Hyun DC; Yang M; Xia Y Angew. Chem., Int. Ed 2014, 53 (46), 12320–12364. [DOI] [PubMed] [Google Scholar]
- (27).Ribas V; García-Ruiz C; Fernández-Checa JC Front. Pharmacol 2014, 5, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Zhou C; Sun C; Yu M; Qin Y; Wang J; Kim M; Zheng J J. Phys. Chem. C 2010, 114 (17), 7727–7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Xu J; Peng C; Yu M; Zheng J Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 2017, 9 (5), e1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Gomes JF; Garcia AC; Ferreira EB; Pires C; Oliveira VL; Tremiliosi-Filho G; Gasparotto LH Phys. Chem. Chem. Phys 2015, 17 (33), 21683–21693. [DOI] [PubMed] [Google Scholar]
- (31).Reilly RF; Ellison DH Physiol. Rev 2000, 80 (1), 277–313. [DOI] [PubMed] [Google Scholar]
- (32).Pfaller W; Rittinger M Int. J. Biochem 1980, 12 (1), 17–22. [DOI] [PubMed] [Google Scholar]
- (33).Hall AM; Unwin RJ; Parker N; Duchen MR J. Am. Soc. Nephrol 2009, 20 (6), 1293–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Wu Z; Jin R Nano Lett. 2010, 10 (7), 2568–2573. [DOI] [PubMed] [Google Scholar]
- (35).Pyo K; Thanthirige VD; Kwak K; Pandurangan P; Ramakrishna G; Lee D J. Am. Chem. Soc 2015, 137 (25), 8244–8250. [DOI] [PubMed] [Google Scholar]
- (36).Muhammed MAH; Verma PK; Pal SK; Kumar RA; Paul S; Omkumar RV; Pradeep T Chem. - Eur. J 2009, 15 (39), 10110–10120. [DOI] [PubMed] [Google Scholar]
- (37).Picard M; McEwen BS Proc. Natl. Acad. Sci. U. S. A 2014, 111 (1), 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Hagberg H; Mallard C; Rousset CI; Thornton C Lancet Neurol. 2014, 13 (2), 217–232. [DOI] [PubMed] [Google Scholar]
- (39).Bienkowski MS; Bowman I; Song MY; Gou L; Ard T; Cotter K; Zhu M; Benavidez NL; Yamashita S; Abu-Jaber J; Azam S; Lo D; Foster NN; Hintiryan H; Dong H-W Nat. Neurosci 2018, 21 (11), 1628–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Chan YL; Saad S; Machaalani R; Oliver BG; Vissel B; Pollock C; Jones NM; Chen H Front. Mol. Neurosci 2017, 10, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Hopfer U; Nelson K; Perrotto J; Isselbacher KJ J. Biol. Chem 1973, 248 (1), 25–32. [PubMed] [Google Scholar]
- (42).Im H; Shao H; Park YI; Peterson VM; Castro CM; Weissleder R; Lee H Nat. Biotechnol 2014, 32 (5), 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Gibb R; Kolb B J. Neurosci. Methods 1998, 79 (1), 1–4. [DOI] [PubMed] [Google Scholar]
- (44).Chou LY; Fischer HC; Perrault SD; Chan WC Anal. Chem 2009, 81 (11), 4560–4565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


