Abstract
Endocrine disrupting chemicals (EDCs) are ubiquitous in the environment and involve diverse chemical-receptor interactions that can perturb hormone signaling. The Organization for Economic Co-operation and Development has validated several EDC-receptor bioassays to detect endocrine active chemicals and has established guidelines for regulatory testing of EDCs. Focus on testing over the past decade has been initially directed to EATS modalities (estrogen, androgen, thyroid, and steroidogenesis) and validated tests for chemicals that exert effects through non-EATS modalities are less established. Due to recognition that EDCs are vast in their mechanisms of action, novel bioassays are needed to capture the full scope of activity. Here, we highlight the need for validated assays that detect non-EATS modalities and discuss major international efforts underway to develop such tools for regulatory purposes, focusing on non-EATS modalities of high concern (i.e., retinoic acid, aryl hydrocarbon receptor, peroxisome proliferator-activated receptor, and glucocorticoid signaling). Two case studies are presented with strong evidence amongst animals and human studies for non-EATS disruption and associations with wildlife and human disease. This includes metabolic syndrome and insulin signaling (case study 1) and chemicals that impact the cardiovascular system (case study 2). This is relevant as obesity and cardiovascular disease represent two of the most significant health-related crises of our time. Lastly, emerging topics related to EDCs are discussed, including recognition of crosstalk between the EATS and non-EATS axis, complex mixtures containing a variety of EDCs, adverse outcome pathways for chemicals acting through non-EATS mechanisms, and novel models for testing chemicals. Recommendations and considerations for evaluating non-EATS modalities are proposed. Moving forward, improved understanding of the non-EATS modalities will lead to integrated testing strategies that can be used in regulatory bodies to protect environmental, animal, and human health from harmful environmental chemicals.
Keywords: Metabolic syndrome, Retinoic acid, Hypertension, Lipids, Aryl-hydrocarbon, Endocrine disruption
Highlights
-
•
Validated tests for EDCs that exert effects through non-EATS modalities are lacking.
-
•
EDC exposure are linked to metabolic syndrome and effects on the cardiovascular system.
-
•
Interaction between EATS and non-EATS axis must be integrated in adverse outcome pathways.
-
•
Chemical testing is needed to reveal the multiplicity of chemical interactions to non-EATS pathways.
-
•
Reporter cell lines should be expanded in testing of EDCs to capture non-EATS interactions.
Abbreviation list
- ACE1
angiotensin converting enzyme-1
- ACTH
adrenocorticotropic hormone
- AHR
aryl hydrocarbon receptor
- ANGII
angiotensin II
- AOs
adverse outcomes
- AOP
adverse outcome pathway
- AR
androgen receptor
- ARNT
AHR nuclear translocator
- BAIAP2
BAR/IMD domain containing adaptor protein 2a
- BaP
benzo[a]pyrene
- BP-3
benzophenone 3
- BPA
bisphenol A
- BPF
bisphenol F
- BPS
bisphenol S
- CARs
constitutive androstane receptor
- CCL2
chemokine C–C motif ligand-2
- C/EBP-α:
CAAT enhancer 507binding protein alpha
- CDKN1c
cyclin-dependent kinase inhibitor 1C
- CVD
cardiovascular disease
- CYP1A
cytochrome P4501A
- CYP3A65
cytochrome P450, family 3, subfamily A, polypeptide 65
- DDT
1,1,1-trichloro-2,2-bis (p-chlorophenyl)ethan
- DEHP
2-ethylhexyl) phthalate
- EATS
estrogen, androgen, thyroid, and steroidogenesis' modalities
- EDC
endocrine disrupting chemical
- EFSA
European Food Safety Authority
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- ER
estrogen receptor
- FKBP5
FKBP prolyl isomerase 5
- GCs
glucocorticoids
- GILZ
TSC22 domain family, member 3
- GR
glucocorticoid receptor
- HPA
hypothalamic-pituitary-adrenal
- LPL:
lipoprotein lipase
- KE
key event
- KER
key event relationship
- MI (surgery)
myocardial infarction
- MIE
molecular initiating event
- MoA
mode of action
- MBP
monobutyl phthalate
- MMCPS
mouse mast cell proteases
- MR
mineralocorticoid receptor
- NRF2
nuclear factor erythroid 2-related factor 2
- OECD
Organization for Economic Co-operation and Development
- p53
tumor protein P53
- PCBs
polychlorinated biphenyls
- PEPCK
phosphoenolpyruvate carboxykinase
- POPs
persistent organic pollutants
- PFASs
per- and polyfluoroalkyl substances
- PFDA
perfluorodecanoic acid
- PFHxS
perfluorohexanesulfonic acid
- PFNA
perfluorononanoic acid
- PFOA
perfluorooctanoic acid
- PFOS
perfluorooctanesulfonic acid
- PPAR
Peroxisome proliferator-activated receptor
- PR
progesterone receptor
- PXR
pregnane X receptor (encoding gene; a.k.a. nuclear receptor subfamily 1, group I, member 2)
- qAOPs
quantitative adverse outcome pathways
- RAR
retinoic acid receptor
- RORs
RAR-related orphan receptors
- RXR
retinoid X receptor
- SOX9b
SRY-box transcription factor 9 b
- SREBP-1c
sterol regulatory element binding protein-1c
- TBBA
tetrabrominated bisphenol A
- TBT
tributyltin
- TCDD
2,3,7,8-309tetrachlorodibenzo-p-dioxin
- TCS
triclosan
- TDCIPP
tris 517(1,3-dichloroisopropyl) phosphate
- VDR
vitamin D receptor
- WBCs
white blood cells
- XRE
xenobiotic response element
1. Evaluation and regulation of endocrine disruptors: the EATS and non-EATS pathways
Worldwide investigations and decades of study into endocrine disrupting chemicals (EDCs) have yielded iterative and tiered screening strategies, innovative applications in technologies, and has culminated into mandates to prioritize, screen, and regulate endocrine disruptors (Robitaille et al., this issue). At a time when society, governments, and industry are increasingly aware of wildlife and human health concerns related to perturbations in endocrine systems, regulations for EDC continue to be fiercely debated (e.g., what to regulate, how to regulate, and where to regulate) and concepts related to dose-response-thresholds, cumulative effects, and population-level consequences are actively discussed. In the European Union (EU), EDCs are regulated under Registration, Evaluation, Authorization and Restriction of Chemicals (REACH), and continue to be high on the agenda for risk assessors, policy advisors, and lawmakers. EDCs are no longer recognized as a “country-specific issue”; in fact, scientists have recently called for a multifaceted international program to collate surveillance data on EDCs worldwide to identify hazardous chemicals more expeditiously for subsequent regulation (Kassotis et al., 2020).
Currently, there are several guidelines and specific programs designed to manage EDCs. The Organization for Economic Co-operation and Development (OECD) published a Revised Guidance Document 150 on Standardized Test Guidelines for Evaluating Chemicals for Endocrine Disruption (updated in 2018) to harmonize approaches for chemical testing for EDC modalities. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) (No 528/2012 and No 1107/2009) formulates the criteria for labelling a substance an “EDC”, and proposes tiered testing strategies. Other programs addressing EDCs are the United States Environmental Protection Agency (USEPA) Endocrine Disruptor Screening Program (EDSP) Tier as well as programs under The Chemicals Management Plan (CMP) in Canada, tasked in prioritization and management of chemical substances. Consortia projects in Europe are addressing endocrinology, chemical exposures, and metabolic diseases (subsequently discussed below). There is increased urgency worldwide to expand screening capabilities to improve regulatory policies that protect wildlife and human health.
Endocrine disruptors are detected in an array of environmental matrices (e.g., water, dust, soil, air particulates) (Metcalfe et al., this issue) and involve diverse chemical-receptor interactions which can perturb hormone signaling. As per the OECD “An endocrine disruptor is an exogenous substance or mixture that alters function(s) of the endocrine system and consequently causes adverse health effects in an intact organism, or its progeny, or (sub)populations”. Endocrine disruptors can act through different mechanisms that do not necessarily act independently from one another. EDCs (1) mimic/inhibit the binding of a hormone to its receptor; (2) disrupt the synthesis or metabolism of the hormone or signaling system; or (3) alter the transport of the hormone to and within the target tissue. These concepts have been formulated with input from academic, government, and industry-partner working groups within the OECD, aimed to provide guidance to environmental scientists and regulators on EDC testing methodology and background, standardization, and execution of bioassays to detect endocrine-disrupting activity and interpretation of the data. The OECD has validated several in vitro EDC-receptor based bioassays as well as in vivo bioassays to detect endocrine disruption (ED) activity of test chemicals; this has resulted in defined guidelines for EDCs such as xenoestrogens (e.g., the estrogen receptor transactivation assay (OECD TG 455 and TG 457) (Robitaille et al., this issue). Perhaps not unexpected, the primary focus of these scientific and regulatory efforts has been on the so called “EATS pathway”, which include several bioassays to detect activation/inhibition of estrogen, androgen, and thyroid receptor signaling as well as steroidogenesis (“E-A-T-S”). Based upon efforts of the OECD and the Endocrine Disrupter Testing and Assessment (EDTA) program as well as the European Chemicals Agency, the “EATS modalities” were primarily conceptualized by the following OECD tests: E-modality, Output data from the ToxCastER Bioactivity Model or ‘Uterotrophic bioassay in rodents’ (OECD test guideline 440); A-modality, Hershberger bioassay (OECD test guideline 441); S-modality: H295R steroidogenesis assay (OECD TG 456) and the aromatase assay (OPPTS 890.1200). Thyroid hormone assays were also included. The EATS modalities have been discussed recently for testing plant-based chemicals (Day et al., 2018) and has been effectively described recently by Kucheryavenko et al. (2020). While the EATS modalities for EDCs have been investigated intensively via tiered methodologies (Robitaille et al., this issue), this has not yet been the case for many other hormone systems (i.e., non-EATS).
In recent years, chemicals that disrupt non-EATS modalities have garnered significant interest from regulators and industry, and there is a need to develop new test methods to screen chemicals that can potentially alter these hormone signaling pathways. Non-EATS pathways include retinoic acid, peroxisome proliferator-activated receptors (PPARs), insulin receptor signaling, gastrointestinal hormones, and cardiovascular-related hormones among others, while the EATS modalities are primarily associated with reproduction (sex steroid hormones and steroidogenesis) and development/metabolism (thyroid hormone). A focus on EATS is not surprising, given that decades of investigations into endocrine disruption in wildlife and mammals revealed reproductive impacts by chemicals such as organochlorine pesticides (e.g., DDT, dieldrin), polychlorinated biphenyls, and polybrominated biphenyls (Gellert and Wilson, 1979; Jefferies and French, 1972; Jugan et al., 2010; Rolland, 2000; Tyler et al., 1998). Compared to sex steroids (hypothalamic-pituitary-gonadal axis) (Marlatt et al. this issue, Delbès et al. this issue, Lacouture et al. this issue) and thyroid hormones (hypothalamus-pituitary-thyroid axis) (Thambirajah et al. this issue), less attention has been given to other endocrine signals required for homeostasis in hormone regulated organs (e.g., brain, heart, gastrointestinal system, liver, pancreas, and intestine) (Fig. 1). Validated in vitro and in vivo assays are thus needed to detect disruptions in endocrine systems associated with these tissues following chemical exposures or mixtures thereof. In addition, such assays are needed to monitor other environmental matrices, such as waste-water effluent for hormonally active agents that may act through non-EATS modalities, such as pharmaceuticals, plant protection products, and industrial chemicals (Boberg et al., 2020).
Fig. 1.
The Non-EATS pathways include hormones understudied in environmental toxicology, such as leptin, angiotensin, ghrelin, insulin, and other hormones. Cell based assays to test for novel pathways of endocrine disruption are urgently needed moving forward.
In this critical review, we discuss the need for validated assays that detect non-EATS modalities and discuss major international efforts underway to develop such tools for regulatory purposes. We also present examples of environmental contaminants that alter specific non-EATS pathways, with the understanding that a comprehensive discussion of all non-EATS modalities is beyond the purview of this review. As such, we focus on some of the modalities of highest concern (i.e., retinoic acid, PPARs, aryl hydrocarbon receptor, and glucocorticoid signaling). Following this, we present two case studies with significant evidence amongst animals and human epidemiological studies for non-EATS disruption and disease. These include metabolic syndrome and insulin signaling (case study 1) and chemicals that affect the cardiovascular system (case study 2). Lastly, we consider emerging topics to improve knowledge regarding chemicals that act through non-EATS modalities, including recognition of crosstalk between the EATS and non-EATS axes, complex mixtures containing diverse EDCs, adverse outcome pathways for chemicals acting through non-EATS mechanisms, and novel approaches for testing chemicals. Lastly, we point out recommendations and considerations for non-EATS modalities.
2. Why the need for validated assays to test non-EATS modalities? A disease perspective
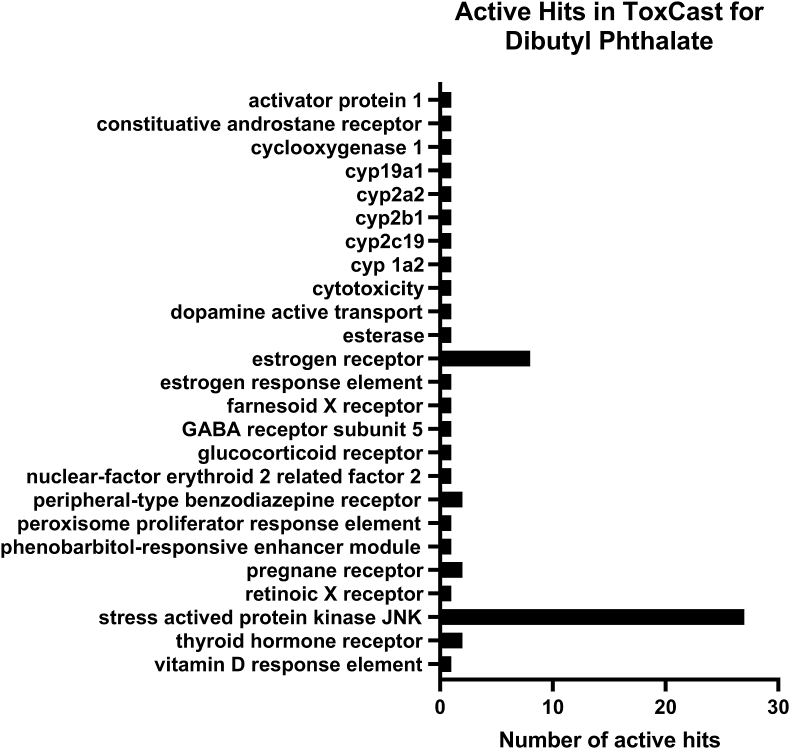
Global and national efforts, such as the high-throughput chemical screening initiatives of the United States Environmental Protection Agency (EPA) ToxCast/Tox21 programs, have revealed widespread promiscuity of chemical interaction with protein receptors and enzymes. Additional bioassays designed to test specific pathways should subsequently validate such data. For example, active hits in the database for dibutyl phthalate (Fig. 2) reveal that it may affecting many receptors, such as estrogen receptor, pregnane X receptor, and a number of cytochrome p450 enzymes. As a result, our current understanding for most chemicals is that their biological activity can occur via different molecular initiating events (MIEs) dependent upon the specific target organ (e.g., liver versus the ovary) and physiologic status of the individual. Currently, there are several receptor-based assays that detect EATS-mediated effects via receptor transactivation assays (e.g., estrogen and androgen receptor agonism/antagonism) or protein binding assays (e.g., transthyretin binding, thyroid peroxidase inhibition) (Robitaille et al., this issue). However, there is growing recognition that validated bioassays are needed to interrogate pathways related to the non-EATS modalities (Andersson et al., 2018). Acetochlor for example is a herbicide that, based upon ToxCast cell assay data for nuclear receptors, appears to activate pregnane X receptor, peroxisome proliferator-activated receptor gamma, retinoid X receptor alpha, and vitamin D response element (non-EATS modalities), but not estrogen, androgen, nor thyroid receptors (EATS modalities) (Fig. 3). Such information is increasingly important as environmental exposures to chemicals are associated with a myriad of animal and human diseases with complex etiology (Vaudin et al. this issue, Plante et al. this issue).
Fig. 2.
Number of active hits in the ToxCast database for dibutyl phthalate.
Fig. 3.
Activation of nuclear receptor reporter assays for the herbicide acetochlor. Several non-EATS modalities are active below cytotoxicity for the chemical. Orange circles indicate specific assays, and the Scaled Top values are the relative response activity for all tests. AC50 (activity concentration at 50% of maximal activity) is calculated based upon the Hill and Gain-Loss models. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The WHO in 2018 reported that more than half of the human deaths worldwide were primarily due to a short list of 10 conditions or diseases, and on this list were diabetes mellitus, heart disease and stroke, Alzheimer's disease, and dementia. Environmental pollution can exacerbate or may even initiate these human health issues (Dendup et al., 2018; Hudda et al., 2021; Li et al., 2021). Global concerns about non-communicable disease prevalence have reached epidemic levels for cardiovascular disease, hypertension, and obesity; arguably, these diseases, in addition to cancer, represent some of the most significant health-related crisis of our time. As such, a deeper understanding into risk factors associated with disease etiology is desperately needed to mitigate adverse health outcomes (Ho et al., this issue). Multiple risk factors exist for disease (e.g., age, diet, exercise, and lifestyle), and intertwined with these risk factors is dysfunction in endocrine systems. For example, a lack of exercise and poor diet can lead to obesity; underlying this condition is diabetes type II and the loss in the ability to adequately regulate glucose. The obese phenotype involves several hormone systems including insulin, leptin, and gut-brain peptides (e.g., ghrelin, neuropeptide Y). As another example, hypertension and cardiovascular disease often involve overactive signaling in the angiotensin-renin system (RAS) and dysfunction in the adrenal system (epinephrine and norepinephrine). Environmental chemical exposures can perturb the delicate balance in these endocrine systems over time, acting to exacerbate, accelerate, and worsen disease outcomes. Environmental pollution is one of the main causes for human disease and premature deaths. Chemical exposures have been estimated to result in 9 million premature deaths per year (estimated in 2015) (Landrigan et al., 2018). As such, there is a pressing global need for a battery of in vitro and in vivo assays that can detect diverse chemical MoAs.
Here we note that disruption in non-EATS modalities also occurs in wildlife. Less is understood about diseases and the role of environmental pollution within the context of animal and wildlife health. Invertebrates, fish, amphibians, reptiles, birds, and mammals are different in hormone peptide structure and endocrine system organization. Investigations utilizing a comparative endocrinology approach will undoubtedly reveal differences in sensitivity and resilience to chemicals with non-EATS modalities across species, similar to that reported for the EATS modalities (McArdle et al., 2020). It is also important to note that there are evolutionary conserved hormone-signaling pathways that are targets of hormonally active environmental toxicants. Based on this understanding, efforts are needed to validate non-EATS bioassays in multiple species, to capture the full scope and potential of chemicals for endocrine disruption.
3. Current international undertakings for the Non-EATS
Regulation of EDCs is high on the agenda for many countries in North/South America, Asia, and European Union (Barton-Maclaren et al. this issue). In the EU for example, EDCs are regulated under REACH, which oversees regulations related to biocides and pesticides (Kassotis et al., 2020). Current activities by the European Commission (EC) include rigorous Fitness Checks of EDCs and the organization of annual forums on EDCs. European efforts in the past have focused on EATS related endpoints and the EC rightly recognizes this as a gap in current EDC testing strategies. Therefore in 2017, the EC launched the call SC1–BHC-27-2018 ‘New testing and screening methods to identify endocrine disrupting chemicals (EDCs)’, to fill in gaps and to address the lack of validated test methods also for screening non-EATS endpoints, which resulted in eight funded projects. Three projects focus on metabolism disrupting chemicals (MDCs), which are EDCs that interfere with metabolism leading to altered energy balance. Such disruption in turn is associated with metabolic diseases such as obesity, type II diabetes and non-alcoholic fatty liver disease (Heindel et al., 2017; Nadal et al., 2017). For these chemicals, the GOLIATH, OBERON and EDCMET consortia aim to develop assays targeting the main nuclear receptors and tissues involved in metabolic diseases (Audouze et al., 2020; Küblbeck et al., 2020; Legler et al., 2020). Although few test guidelines exist for thyroid disruption (EATS modality), no validated in vitro alternatives exist and the ATHENA, ERGO and SCREENED projects are establishing methods on different modes of thyroid disruption using advanced cell cultures and alternative animal models (Holbech et al., 2020; Kortenkamp et al., 2020; Moroni et al., 2020). The ENDpoiNTs project will aid in the understanding of the mode of action (MoA) of EDCs on the neuroendocrine axis by establishing new methodologies (Lupu et al., 2020), whereas the FREIA project addresses the lack of test methods for female reproduction (Duursen et al., 2020). All projects focus on the development of novel methodologies, ranging from in silico (QSARs, molecular docking, PBTK), to in vitro and in vivo bioassays. Data from these models will be supplemented with multi-omics approaches to aid in elucidating MOA and Adverse Outcome Pathway (AOP) development. The most promising assays will be brought forward to OECD for validation. The overarching goal is to develop frameworks of integrated testing strategies for non-EATS, including AOPs and ultimately the development of Integrated Approaches to Testing and Assessment (IATA). To create synergies between the projects and to avoid overlap, the 8 projects formed the European Cluster on Identification of Endocrine Disruptors, EURION (https://eurion-cluster.eu).
Over the past decade, several in vitro cell-based and in vivo animal assays (e.g., transgenic fish, amphibians, and mammals) have been established and validated for the EATS pathways, and investigations have compared the efficacy, detection limits, and robustness and reliability of these receptor assays (Robataille et al., this issue). Nuclear hormone receptor-based transactivation assays, commonly used for EATS assessments, are also available for retinoid receptors (retinoid X receptor, retinoic acid receptor), the aryl hydrocarbon receptor and the PPARs (PPAR-α and PPAR-γ), are included in high throughput screens conducted by the US EPA and ToxCast (Villeneuve et al., 2019) (Fig. 3); these are recognized as Level 2 in vitro mechanistic screening assays by the OECD Conceptual Framework (Andersson et al., 2018). In the following section, we highlight some non-EATS modalities of concern, describing examples of in vitro and in vivo assays when available, discussing their strengths and limitations.
4. Non-EATS modalities of concern for hormonally active agents
4.1. Retinoic acid
Retinoic acid (RA) is a natural derivative of vitamin A and is an essential dietary requirement in vertebrates. Retinoic acid acts as a morphogen in vertebrate development, regulating the development of the retina, brain, and the heart. Retinoic acid is also involved in metabolism, reproduction, immune response, and cell proliferation (Cunningham and Duester, 2015; Hall et al., 2011; Maden, 2001). Therefore, RA acts via multiple signaling pathways, requiring high coordination along both temporal and spatial scales. Production of RA is mediated in two consecutive steps: (1) Retinol, directly derived from Vitamin A, is converted to retinal by alcohol dehydrogenases (ADHs) and retinol dehydrogenases (RDHs); (2) Following an oxidation step, retinal is converted to RA by retinaldehyde dehydrogenases (RALDH). RA availability is also regulated by a family of CYP26 enzymes which metabolize RA into more polar metabolites. RA biological activity is mediated via binding to retinoid receptors: the retinoic acid receptors (RARs) and the retinoid X receptors (RXR). When activated, RXR and RAR form heterodimers (RAR/RXR) and are translocated to the nucleus to bind to retinoic acid response elements (RAREs) to regulate transcription of target genes. On the other hand, in the absence of RA, RARs can act as active repressors. The fine tuning of heterodimer activity is regulated by the interplay between RAR- and RXR-ligands, where each subunit retains its intrinsic properties in terms of ligand and co-regulators binding (Le Maire et al., 2019). RXR can also form heterodimeric complexes with many other nuclear receptors. Interestingly, heterodimers can be divided in two categories: permissive (activated by ligands of either RXR or its partner) and non-permissive (only activated by the partner's ligand while RXR is silent). While transcriptional regulation of non-permissive heterodimers is under tight control and directly mediated by hormones (similar to that of TRs and RARs), permissive heterodimers result in a cooperative and synergistic responses whereby a relatively small change in the abundance of ligand can trigger robust transcriptional activation. This activation results in robust biological responses, as is the case for PPARs and constitutive androstane receptor (CARs) among others (Evans and Mangelsdorf, 2014).
Although binding assays are useful to assess interactions between nuclear receptors and EDCs, as demonstrated for organotins such as TBT that have strong binding affinities to human RXR (Nishikawa et al., 2004), they do not inform about the functionality and consequences of binding. In contrast, RAR and RXR transactivation reporter assays and cis-activation of the retinoic acid response element (RARE) by RAR/RXR heterodimers include the use of reporter genes, which can be used to assess agonistic and antagonistic activities. For example, a yeast two hybrid-assay using recombined human RXR gene and reporter gene yeast was used to test RXR agonistic and antagonistic potency of different families of chemicals including phenols, bisphenol A derivatives, and pesticides. Results showed that 10 out of the 16 chemicals tested showed agonistic activities (Li et al., 2008). RAR assays were identified as one of the most positive predictors when developing a rat predictive model that associated in vitro high-throughput screening data from ToxCastDB and in vivo adverse outcomes from developmental toxicity studies from ToxRefDB (Sipes et al., 2011). Moreover, analysis of the ToxCastDB and ToxRefDB data have identified the retinoid pathway as a major component in models for male reproductive developmental defects (Leung et al., 2016). Therefore, the study of the RA pathway has been proposed as a target to identify molecular initiation events (MIEs) of altered RA homeostasis. As such, RA in vitro bioassays can be a useful tool for the study of non-EATs MoA of EDCs.
4.2. Aryl-hydrocarbon receptor
The aryl hydrocarbon receptor (AHR) is a member of the basic helix-loop-helix (bHLH), Per-Arnt-Sim (PAS) family known for its high binding affinity to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Sato et al., 2008). Inactive AHR exists in the cytoplasm bound to chaperones such as HSP90 and ARA9. Upon binding to dioxin, AHR forms a heterodimer with the AHR nuclear translocator (ARNT) in the nucleus (Reyes et al., 1992). This complex then transactivates specific target genes such as xenobiotic-metabolizing enzymes like cytochrome P4501A (Cyp1A) by binding to xenobiotic response element (XRE) sequences in their promoter region. In addition to direct transcriptional regulation, AHR controls genes involved in immune and reproduction through interactions with other transcription factors such as nuclear factor-kappa B or estrogen receptor (Wada et al., 2016). It is now well accepted that AHR participates in dozens of signaling pathways involved in essential life processes including development and metabolism (Nebert, 2017). A recent study in human also found an association between AHR activation and obesity (Shahin et al., 2020). Multispecies studies suggest that, among metabolic pathways, lipid metabolism appears markedly affected by AHR activation. Some of the first evidence of AHR involvement in lipid metabolism came from a rodent ARH knock-down model which appeared to be protected against diet-induced obesity and diet-related metabolic complications such as liver steatosis (Xu et al., 2015; Zhu et al., 2019; Gourronc et al., 2020). However, tissue specific models of AHR loss provide different results compared to whole body knockouts. For example, tissue-specific inhibition of AHR through expression of Cre from an adiponectin promoter (i.e., in mature adipocytes) caused an increase in obesity in mice on a high fat diet (Baker et al., 2015). In addition, other studies demonstrate that inhibition of AHR by the AHR antagonist α-naphthoflavone prevents obesity and fatty liver in male and female mice (Moyer et al., 2017; Rojas et al., 2020). On the contrary, AHR activation in rodents by environmental contaminants is associated with spontaneous hepatic steatosis characterized by the accumulation of triglycerides (Dong et al., 2019; Lee et al., 2010; Nebert, 2017), cholesterol biosynthesis impairments (Dornbos et al., 2019; Sato et al., 2008; Zhu et al., 2019) and systemic metabolic dysfunction (Zhang et al., 2015). The effects of AHR ligand like dioxin on lipogenesis appear to be concentration dependent and non-monotonous like other effects of endocrine disruptors (Baker et al., 2015). Indeed, low concentrations (<0.1 nM) of dioxin, as well as coplanar PCBs (3.4 μM), promote differentiation of adipocytes in mice, whereas higher concentrations of these AHR ligands (10 nM dioxin and 34 μM coplanar PCBs) inhibited adipocyte differentiation. Taken together, these results suggest that AHR-mediated regulation of body weight may result from the combined effects of activation of AHR in various cell types (Baker et al., 2015) and that AHR expression and AHR ligand doses has to be considered. Environmental data linking AHR activation and lipid metabolism impairments are scarce, despite strong foundational understanding of AHR pathway in sentinel species like fish or amphibians (Reynaud and Deschaux, 2006; Reynaud et al., 2012). However, pioneering studies using Xenopus tropicalis demonstrated the capacity of benzo[a]pyrene (50 ng/L) at inducing liver steatosis and metabolism impairments in frogs (Regnault et al., 2016), suggesting that AHR-regulation of metabolic pathways are conserved among species.
AHR involvement in metabolic disruption has been largely neglected despite a considerable amount of literature linking its activation by environmental contaminants to (eco)-toxicological aspects. AHR is now considered as an endocrine disruptor target like other EATS receptors (Tq et al., 2020); despite this knowledge, much remains unknown about the link between AHR-elicited gene expression and metabolic impairments, and cell-based bioassays are needed to highlight potential metabolic disruptors acting through AHR binding. In this way, luciferase reporter gene assays for AHR have been developed for chemical screening and have proven their efficacy to detect potential AHR activators in environmental mixture (Boonen et al., 2020).
4.3. Peroxisome proliferator-activated receptors (PPAR): focus on PPARγ
Peroxisome proliferator-activated receptors are nuclear receptors activated by fatty acids, pharmacological ligands, and other xenobiotics involved in energy homeostasis (glucose and fatty acid metabolism), inflammation, cell proliferation, adipose tissue differentiation and essentially, all aspects of development. There are three distinct PPARs in mammals, chicken, Xenopus and up to four have been found in some fish species, each one expressed in different tissues which carry unique and diverse functions. For example, PPARα plays a key role in regulating signals in the liver and brown adipose tissue. However, for brevity we focus primarily on PPARγ. Obesogens are EDCs that are capable of binding to and activating PPARγ/RXRα heterodimer complexes (Grun et al., 2006). The PPARγ/RXRα complex is known to be a positive regulator of adipocyte differentiation and lipid biosynthesis (Rosen et al., 1999). Organotins have been found to cause obesogenic phenotypes in rodents by binding to and activating both PPARγ and more potently RXRα (Kanayama et al., 2005) and can alter lipid homeostasis, adipogenesis, and lipid accumulation (Grun et al., 2006). Reproduction in mammals is also regulated by the coordination of PPARs and RXR, for example during oocyte and spermatocyte maturation by regulating steroidogenesis or the levels of inflammatory lipids contained in milk produced in the mammary glands (Wan et al., 2007; Yang et al., 2006). Several chemicals are activators of PPARs in both mammalian and non-mammalian species (reviewed in (Abbott, 2009)). Various in vitro assays have been developed to test disruption of the PPAR signaling and this is a focus of the EU program GOLIATH; however, currently there are no validated methods and PPAR assays are proposed candidates outlined in OECD DRP No.178. As for other nuclear receptors, transactivation reporter assays demonstrate functional activation of PPARs when screening EDCs, allowing for the identification of molecular initiation events leading to adverse outcomes (Seimandi et al., 2005).
When considering PPAR pathways related to obesity and metabolism, the OECD proposes to assess in vitro transactivation of different PPARs in reporter gene assays and adipocyte differentiation in models like 3T3-L1 cells and in vivo peroxisome proliferation, and lipid accumulation (LeBlanc et al., 2011). Similarly, to the case of glucocorticoids (see below), the OECD proposed the addition of these new endpoints to existing guidelines in birds, amphibians, fish, and mammals (TG 206, 229, 230, 415, 416, 440, 441, and 443). In addition to the study of weight gain, which could certainly assess the obesogenic properties of the studied compounds, both the PPARγ-RXRα system and its downstream cascades provide excellent targets to assess considering the obesity epidemic. The binding of PPARγ-RXRα heterodimer to peroxisome proliferators response elements (PPREs) triggers the expression of several genes, including several apolipoproteins, phosphoenolpyruvate carboxykinase (pepck), and/or lipoprotein lipase (lpl), among others (Berger and Moller, 2002; IJpenberg et al., 1997), which could serve as biomarkers of exposure. Several obesogens, like TBT, exert multiple effects in mammalian, fish, and amphibian models through PPARγ-RXRα (Capitão et al., 2017; Heindel et al., 2017). Some of these effects may also be trans-generationally inherited, most likely via epigenetic mechanisms.
4.4. Glucocorticoids/mineralocorticoids
As pointed out above, obesity is arguably one of the most concerning scientific issues to be addressed for the non-EATS MoA of environmental chemicals. The condition can be induced at different levels of biological organization, for example via altered adipocyte differentiation and deposition, disruption in glucose metabolism, lipid and energy homeostasis, or dysregulation of appetite and satiety, among other mechanisms. The OECD proposes three main routes of chemical disruption that could lead to chemical-induced weight gain. The first involves estrogenic receptor (ER-) activation. This is discussed in Robataille et al. (this issue) and will not be discussed here, although this connection is a strong example of how EATS and non-EATS pathways can intersect to determine health outcomes. The second involves the formation of PPARγ-RXRα heterodimer, and the third mechanism involves glucocorticoid receptor -GR- signaling (LeBlanc et al., 2011). While the activation of PPARγ-RXRα complex by obesogens (e.g., bisphenols, organotin, PCBs or some perfluorinated compounds) are well documented and primarily lead to increased adipogenesis via increased expression of apolipoproteins, activation/inhibition of GR involves perhaps a more intricate network of downstream signaling pathways. These pathways are not yet fully elucidated when it comes to EDCs and represent an understudied receptor-mediated mechanism. This is a significant knowledge gap as glucocorticoids (GCs) are involved, not only in gluconeogenesis, lipolysis, and food intake regulation, but also with the immune system, stress, and development. Noteworthy is that the related nuclear receptor mineralocorticoid receptor (MR) is also implicated with adipogenesis and obesity in mice (LeBlanc et al., 2011), along with salt and water retention which are significant risk factors for hypertension. Since both gluco- and mineralocorticoids are synthesized by the HPA (hypothalamic-pituitary-adrenal) axis, the HPA axis is currently underrepresented in non-EATS toxicity tests. This is concerning because the occurrence of both natural and synthetic glucocorticoids in the environment has been reported. This pathway, along with the PPARγ-RXRα pathway, has been recommended by the EFSA (Committee, 2013) as pathways of concern and those that should be further developed in EDC screening.
Regarding the HPA axis, OECD recommends performing in vivo bioassays to assess: (1) stress response, (2) adrenal corticosteroid synthesis, and (3) ACTH release (LeBlanc et al., 2011). Nevertheless, the proposed addition of these new endpoints to the guidelines in birds, amphibians, fish, and mammals (TG 206, 229, 230, 231, 408, 415, 440, 441, and GD 140) which are focused on classical EATS modalities could be insufficient, considering the complexity and the wide spectrum of effects that EDCs could mediate via non-EATS disruption. For example, GCs exposure can alter behavior, plasma glucose concentration and glycogen storage, energy expenditure, and triglycerides accumulation in fish including gilthead seabream, fathead minnows and zebrafish (Jerez-Cepa et al., 2019; Kugathas et al., 2013; McNeil et al., 2016). It can also increase plasma corticosterone, decrease GR activity (in brain) and increase food intake in amphibians (Xenopus laevis) (Hu et al., 2008). In several species of mammals, GCs exposure has been related to long-lasting and deleterious effects on body, brain, behavior, and HPA axis (Edwards and Burnham, 2001). Similarly, hypercorticosteronemia and 11β-Hydroxysteroid dehydrogenase disruptions have been reported in obese rats (Livingstone et al., 2000). Moreover, glucocorticoid exposure together with stress seems to play a major role in the development and maintenance of obesity in humans (van der Valk et al., 2018), which also seems to be a side effect in GCs treatments (Wung et al., 2008). Although several transcriptomic and metabolic biomarkers require further validation, some in vivo bioassays have been proposed. For example, 11 glucocorticoid-responsive genes (pepck, baiap2, pxr, several mmcps, cdkn1c, fkbp5, cyp3a65, sox9b or gilz) have been reported in zebrafish, both in adults and larvae after exposure to GCs (Chen et al., 2016).
4.5. Other non-EATS modalities
Lastly, we note that modalities discussed above are by no means complete and there exist several hormone systems that have yet to be addressed in the context of environmental risk assessment of EDCs. The neuroendocrine system for example has been reviewed by others, highlighting the interaction of specific environmental chemicals with neuropeptides and neurohormones (i.e., neuroendocrine disruption) (León-Olea et al., 2014, Vaudin et al. this issue). Another non-EATS axis that is understudied is the gastrointestinal hormonal system. Secretin, glucagon, vasoactive intestinal peptide, gastrin, cholecystokinin, and somatostatin are major signaling molecules regulating the gastrointestinal track and chemical exposures can also disrupt these hormones (Lee et al., 2000). In addition, research into the gut-brain axis has revealed relationships between gut dysbiosis, the neuroendocrine system, and obesity (Wu et al., 2019), suggesting that neuroendocrine disruption may affect gut physiology and vice versa. Another component to be addressed is that of the gastrointestinal microbiota. Environmental chemical exposures involve oral routes of exposure, and the microbiota is often the first line of defense against mitigating toxicity through biotransformation and metabolism of chemicals. Undoubtedly, the microbiota has a key role for EDC action and constitutes a novel mechanism yet to be explored (Gálvez-Ontiveros et al., 2020; Roman et al., 2019). Incorporating such knowledge into chemical screening strategies are anticipated to become more prevalent over time.
5. Case study 1: metabolic syndrome and non-EATS modalities
There is good evidence from both epidemiological and experimental studies that EDCs can affect cellular metabolism, and data suggest these effects can manifest early in utero. Because adverse effects by EDC may also lead to metabolic diseases such as metabolic syndrome and type 2 diabetes, this subclass of EDC has been referred to as metabolic disruptors (Casals-Casas and Desvergne, 2011) and later metabolic disrupting chemicals (Heindel et al., 2017). Similar to the effects of chemicals that act through the EATS modalities, the capacity of EDCs to interact with nuclear receptors or hormone signaling explains the wide range of metabolic perturbations reported in multiple studies, reinforcing the concept of associating endocrine and metabolic disruption (Casals-Casas and Desvergne, 2011). Most surprising perhaps is that epidemiology data suggest critical periods of EDC exposure during development that influence the later-life onset of type 2 diabetes, including preconception and gestation, early infancy, the adiposity rebound period between 5 and 7 years of age, and puberty (Heindel et al., 2017; Mimoto et al., 2017). EDCs implicated in diabetes pathogenesis include various inorganic and organic molecules of synthetic origin, including arsenic, bisphenol A, phthalates, polychlorinated biphenyls, and organochlorine pesticides (see (Sargis and Simmons, 2019; Simhadri et al., 2020). Thus, a broad range of chemicals found in the environment can exert metabolic effects in organisms.
Early evidence that developmental EDC exposure alters metabolic health came from studies exposing pregnant rats to bisphenol A (BPA). BPA exposure (0.1 and 1.2 mg BPA/kg/day) resulted in increases in offspring body weights that persisted into adulthood (Rubin et al., 2001) and caused metabolic syndrome in offspring (50 μg/kg/day), including obesity, dyslipidemia, hyperleptinemia, hyperglycemia, hyperinsulinemia, glucose intolerance and insulin resistance (Dunder et al., 2018; Wei et al., 2011). Several mechanisms have been proposed to explain these effects including epigenetic modifications (Alavian‐Ghavanini and Rüegg, 2018) and placental transfer during the prenatal life (Akash et al., 2020). Different mechanisms have been also proposed to explain the direct effects of BPA including the estrogen-receptor dependent alteration of adipogenic gene expression (Ariemma et al., 2016; Ohlstein et al., 2014), mitochondrial-dependent apoptosis resulting in pancreatic β-cell dysfunction (Lin et al., 2013) and its capacity to interact with insulin signaling (Le Magueresse-Battistoni et al., 2018).
BPA is not the only EDC with such effects on metabolism in mammals. Other EDCs have been experimentally validated to have a role in metabolic disorders. For example, in vivo studies also demonstrate that prenatal exposure to tributyltin (0.1 μg/kg/day) results in lipid accumulation in adipose tissues and hepatic steatosis in newborn mice via the RXR-PPARγ pathway (Bertuloso et al., 2015). Other studies also reveal metabolic effects of EDCs. Prenatal exposure of rats to nonylphenol (200 mg/kg/day) induces glucose metabolism disorder in male F1 rats through abnormal pancreatic expression of glucokinase and uncoupling protein-2 (Heindel and Blumberg, 2019; Yang et al., 2017). Exposure of pregnant rats to 4-tert-octylphenol (100 or 500 mg/kg/day) negatively regulates the expression of lipogenic enzymes and associated transcription factors such as C/EBP-α (CAAT enhancer binding protein alpha) and SREBP-1c (sterol regulatory element binding protein-1c) in both liver and adipose tissue, resulting in altered fat metabolism (Kim et al., 2015). Gestational exposure of rats to di(2-ethylhexyl) phthalate (1, 10 and 100 mg/kg/day) induces pancreatic beta-cell dysfunction and gluco-metabolic abnormalities in the F1 offspring (Rajesh and Balasubramanian, 2015). Perinatal exposure to 1,1,1-trichloro-2,2-bis (p-chlorophenyl)ethane (DDT, 1.7 mg/kg/day) induced impaired glucose tolerance later in life in mice (La Merrill et al., 2014). Thus, there are several chemicals that can exert metabolic effects in mammals.
Metabolic disruption by EDCs has also been reported in non-mammalian models. In zebrafish (Danio rerio), recent studies indicate that TBT (10 and 50 ng/L), bisphenol A (100 μg/L), triclosan (TCS, 200 μg/L), tetrabrominated bisphenol A (TBBPA, 0.5 μmol/L), tris (1,3-dichloroisopropyl) phosphate (TDCIPP, 0.5 μmol/L), benzophenone 3 (BP-3, 0.5 μmol/L), (2-ethylhexyl) phthalate (DEHP, 50 μg/L) and monobutyl phthalate (MBP, 10 mg/L) markedly affect lipid metabolism and energetic metabolic processes in larvae and adult liver (Lyssimachou et al., 2015; Mu et al., 2018; Sun et al., 2020; Tao et al., 2020). A comparative analysis using zebrafish eleutheroembryos exposed to BPA, TBT, PFOS and E2 showed that the yolk sac area of exposed embryos increased in size with increasing concentrations of all tested compounds (Martínez et al., 2019a). Considering the yolk sac is the main reservoir of lipids in the embryo, this observation suggests that multiple chemicals can affect lipid stores. In the case of BPA (0.1–4 mg/L), results showed its effects were beyond their well-known estrogenicity, showing complex patterns of toxic effects that included visual disruption and obesogenic effects as evident from the appearance of yolk sac malabsorption syndrome and lipid and metabolism disruption at the transcriptomic and metabolomic level (Martínez et al., 2019a; Ortiz-Villanueva et al., 2017). Several mechanisms have been proposed to explain these observed effects on lipid metabolism, which could differ along the different lipid classes (Martínez et al., 2020a), but also disruption in some energetic metabolic processes seemed to be maintained during part of the life cycle (Martínez et al., 2020b). On the other hand, TBT alteration of the yolk sac area was partially attributed to developmental disruption, since TBT exposures (1–32 mg/L) caused a general developmental delay (diapause-arrest effect) which affected steroids and cell cycle metabolic pathways (Martínez et al., 2020c). Despite the observed distinct effects at the morphological and molecular level, similar underlying metabolic responses to BPA, TBT and PFOS were observed that affected the metabolism of glycerophospholipids, which was associated with altered absorption of the yolk sac (Ortiz-Villanueva et al., 2018). TBT significantly affects the transcription of key factors and enzymes involved in adipogenesis and lipogenesis including pparγ and srebp1 (Lyssimachou et al., 2015) accompanied with increased adiposity at 15 days post fertilization (den Broeder et al., 2017). The exact mode of action of TBT remains elusive, but recent studies point in the direction of RXRα mediated alterations in the epigenetic modifier enhancer of zeste 2 (Ezh2) (den Broeder et al., 2020). TCS and BPA impair lipid beta-oxidation, increasing the expression of liver fatty acid synthetase and promoting hepatic inflammation (Sun et al., 2020). DEHP as another example exerts its obesogenic action by up-regulating hepatic pparα and srebp proteins and by stimulating de novo fatty acid synthesis (Migliarini et al., 2011), and its metabolite MEHP has been shown to alter epigenetic pathways in eleutheroembryos linked to metabolic endpoints (Legler et al., 2017). These mechanisms present new opportunities for potential assays in chemical screening programs.
There are also some data from studies in amphibians that point to metabolic disruption via non-EATS modalities. These include studies investigating TBT exposure (10–100 nM) in Xenopus, which demonstrated its ability to activate RXR/PPARγ pathways and suggested evolutionary conservation of these signals among vertebrates (Grun et al., 2006; Maradonna and Carnevali, 2018). Moreover, both acute and chronic exposure to BaP and TCS induce marked metabolic disorders in Xenopus tropicalis associated with impaired lipid and carbohydrate metabolism (Regnault et al., 2016, 2018; Usal et al., 2019). Molecular mechanisms clearly demonstrate that insulin-regulated processes are affected by EDC exposure. Indeed, female Xenopus tropicalis exposed from the tadpole stage to benzo(a)pyrene or TCS at concentrations of 50 ng/L display glucose intolerance syndrome, liver steatosis, liver mitochondrial dysfunction, liver transcriptomic signature, and pancreatic insulin hypersecretion, each of which indicate a prediabetes state. The exposed animals produce progeny that metamorphose later, are smaller and lighter at metamorphosis, and have reduced reproductive success (Regnault et al., 2018; Usal et al., 2019) in addition to displaying metabolic impairments at the adult stage (Usal et al., 2021). In addition, disruption of lipid, carbohydrate and protein metabolism following insecticide or pesticide mixture exposure has also been reported in amphibians (Glinski et al., 2018; Gurushankara et al., 2007; Wolmarans et al., 2018).
Taken together, metabolic disruption appears to be a frequent phenomenon observed in animal taxa as an outcome to chemical exposure. Mechanisms involved in metabolic dysfunction are varied, and include PPAR signaling, effects on insulin, crosstalk with estrogen receptors and EATS pathways, among others. Clinical signs observed amongst vertebrates indicate obesogenic phenotypes (i.e., dyslipidemia, hyperglycemia, etc.) following exposure to ubiquitous chemicals like BPA and phthalates. We point out that these examples are from vertebrate studies, however it is important to state that metabolic disruption has also been reported extensively in invertebrates (Fuertes et al., 2019; Jordão et al., 2015). As mentioned, EDCs exert effects on multiple systems, through non-EATS modalities. In the second case study, we discuss BPA and phthalates in more detail as EDCs that affect the cardiovascular system.
6. Case study 2: cardiovascular disease (CVD) and non-EATS modalities
6.1. Bisphenols and cardiovascular disease
In the case of BPA, most early studies detected a significant increase in risk for angina, myocardial infarction, and coronary heart disease in people with the highest levels in urine (Lang et al., 2008; Melzer et al., 2010, 2012). Later studies confirmed these findings (Cai et al., 2020; Moon et al., 2020) in several countries (Bae et al., 2012; Jiang et al., 2020; Salamanca-Fernández et al., 2020). Additional concerns have also been reported for analogs of BPA, bisphenol S (BPS) and bisphenol F (BPF) (Jiang et al., 2020) and revealed country-specific differences in human exposure to EDCs (Wang et al., 2020). These data support the point that EDC exposure is not uniform worldwide and that differences in epidemiological findings may reflect the amount and types of chemical exposure in the test populations.
Experimental evidence supports epidemiological studies that demonstrate a negative association between EDC exposure and CVD. CVD is associated with chronic increased inflammation. In rodent experiments designed to replicate long term exposure from low dose 0.5 μg/kg/day to the multiple doses used in the CLARITY-BPA study BPA found increased oxidative stress (Gear et al., 2017; Kasneci et al., 2017; Patel et al., 2014) and increased infiltration of pro-inflammatory innate immune cells in mice exposed to 50 mg/kg/day BPA in the absence of external stress (Reventun et al., 2020). Whereas treatment with an ERβ antagonist obliterated the pro-inflammatory impact of BPA on macrophage polarization in vitro, ERβ-deficient mice chronically exposed to BPS exposure displayed increased expression of inflammatory markers in the infarct (Kasneci et al., 2017). Exposure of cardiomyocytes derived from embryonic stem cells of human or mouse origin can test for the direct effects of EDCs and analogs. Exposure of BPA at levels detected in human blood, ~8 ng/ml, human cardiomyocytes derived from male (H1, XY karyotype) and female (H9, XX karyotype) stem cells found increased expression of genes known to be involved in cardiac development, increased cardiac cell size indicating hypertrophy, reduced ATP content and increased calcineurin signaling, a key regulator of energy metabolism which is modulated by calcium (Cheng et al., 2020; Lee et al., 2019). This pattern is distinct from that of estrogen where E2 reduced calcineurin signaling. Inclusion of an inhibitor of mitochondrial fission, Mdivi-1, effectively ablated the BPA effects suggesting BPA-stimulated modification of the calcineurin - DRP1 pathway.
Likewise, in mouse stem cell-derived cardiomyocytes, BPA, and BPAF at doses from 8 to 1000 ng/ml, increased production of reactive oxygen species via increases in eNOS (Yang et al., 2020). When antagonism testing was expanded to include non-ER inhibiting chemicals, in contrast to the results in other systems, PR antagonists reduced BPA-mediated reductions in calcium homeostasis and arrhythmogenesis downstream of CamKII activation (Lee et al., 2019; Ma et al., 2017). In other studies, inflammation is apparent in the cardiovascular system with BPA exposure. For example, vascular smooth muscle cells treated with BPA at 10 nM had increased expression of proinflammatory markers TNFα and IL-6. These increases were attributed to increased AngII were reduced by Losartan, an angiotensin II type 1 receptor antagonist treatment (Gao et al., 2019). Supporting these findings, BPA-exposure of female rats at levels from 5 to 500 μg/kg/day revealed increased in eNOS and angiotensin converting enzyme 1 (ACE1) and exposure of human cardiomyocytes to 10 nM BPA increased eNOS, ACE1 as well as proinflammatory IL-8 and NFkB (Klint et al., 2017). Similarly, BPA-mediated increases in serum ANGII and increased angiotensin 1 receptor found after liver ischemia/reperfusion injury in rats was reduced by Losartan (Zhang et al., 2020). Thus, experimental evidence points to BPA-mediated inflammation and oxidative damage via ANGII as a potential mechanism for adverse effects on the heart.
Non-mammalian studies support observations of BPA-induced cardiotoxicity via increased in oxidative stress. Danio rerio (zebrafish) have been used to study in vivo effects of EDCs. BPA, its metabolites and BPAF exposure reduced heart size, increased expression of genes predicted to activate innate immune cells, reduced expression of antioxidant enzyme expression, altered heart valve architecture and impaired heart function (Brown et al., 2019; Gu et al., 2020; Moreman et al., 2018). RNA-Seq data from zebrafish embryos exposed to BPS indicated increases in iNOS, and identified genes predicted to lead to activation of innate immune cells (Qiu et al., 2020). Importantly, the level of BPS in these last experiments was below the level US FDA guideline for BPA amounts permitted in drinking water. These data support the idea that the impact of bisphenols may be in part conserved and replicated in zebrafish and rodents. The data support the notion that multiple models are necessary to fully describe the range of EDC effects.
Despite many years of dedicated study knowledge gaps remain, and with increased use of bisphenol analogs, are expanding. In silico docking experiments have confirmed expected BPA binding to ER and ERR receptors but also identified the potential for binding with other receptors such as RAR, RXR, CAR, PPAR-γ, enzymes such as MMP9, clock regulating proteins, such as CLK1, signaling pathway proteins such as PKCa, ECM regulating proteins such as TGF-β and VCAM1, immune cell ligands such as CCL2 and CXCL10 (Cavaliere et al., 2020; Delfosse et al., 2012; Montes-Grajales and Olivero-Verbel, 2013). All these proteins are detected in heart tissue and cells and are linked to pathology. Predicted binding of bisphenols to PPARs and RXRs is analog specific. In a study of 18 analogs, predicted PPAR-α and PPAR-γ binding of BPPH, BPG and BPC2 was similar to the known ligand fenofibrate and greater than that predicted for BPA or BPS (Sharma et al., 2018). In predicted binding to RXR-α, BPPH, BPAF and BPZ binding was scored as similar to that of prostacyclin and predicted binding to RXR-γ, BPAF and BPC was similar to that of the score for rosiglitazone. These differences have been independently verified in zebrafish (Pinto et al., 2019), where BPA analogs such as BPC did not replicate the parent compound. Importantly, some species differences in receptor response to BPA complicate determination of mechanism. For example, BPA does not interact with mouse PXR, but is an agonist with murine PXR. Thus, BPA-mediate increased of atherosclerosis were only demonstrable in mice which expressed the human PXR protein (Sui et al., 2018). Thus, BPA and its analogs may have a species dependent, individual receptor binding and different toxicity profiles across multiple receptors.
6.2. Phthalates and cardiovascular disease
Phthalate exposure is also a potential contributor to cardiovascular dysfunction (Just et al., 2012; Mariana et al., 2018). Maternal exposure to DEHP results in congenital heart defects and altered expression of important cardiac transcription factors in children (Snijder et al., 2012; Tang et al., 2019; Wang et al., 2015). Similarly, early life phthalate exposure can lead to vascular adaptations that increase the risk of cardiovascular disease later in life (Sol et al., 2020). For example, an increased urinary phthalate concentration was associated with elevated systolic blood pressure (Gao and Wang, 2014). In elderly populations, the risk of coronary heart disease, atherosclerosis, and downstream complications, including MI, increases with the concentration of circulating phthalates (Lind and Lind, 2011; Olsén et al., 2012).
Experimental data links phthalate exposure and cardiac dysfunction. The impact of phthalate exposure on cardiac electrophysiology and contractile performance is well documented in ex vivo preparations and isolated mammalian cells. Cardiomyocytes are connected to each other via a highly organized framework consisting of mechanical and electrical connections (Vermij et al., 2017). DEHP from 1 to 50 μg/ml slowed electrical conduction in rat cardiomyocytes in a dose-dependent manner, with increased exposure exacerbating an arrhythmogenic phenotype (Gillum et al., 2009). Phthalate exposure at doses expected in the pediatric patient population, of ~0.17 μg/kg/day, increased NOS3 expression, interfered with cardiac electrophysiology, induced heart rate variability and reduce cardiovascular reactivity in male mice (Jaimes et al., 2017). Similarly, in isolated and intact rat hearts, phthalate exposure decreased heart rate in a concentration-dependent manner. Furthermore, phthalate exposed hearts show edema of myocardial tissue, and increased inflammatory cell infiltration and myocardial cell necrosis (Wang et al., 2018). Other experiments reveal direct adverse effects on cardiomyocytes. In human stem-cell derived cardiomyocytes, exposure to phthalates at clinically relevant doses affected calcium handling and intercellular cardiomyocyte connectivity (Jaimes et al., 2019; Posnack et al., 2015).
Similar to BPA, alternative animal models support phthalate-induced cardiotoxicity. For example, acute exposure to DEHP inhibited contractile function of chick embryonic cardiomyocytes and induced cell death after 24 h (Rubin and Jaeger, 1973). DEHP exposure in male quail results in increased cardiomyocyte swelling and muscle fiber dilation (Wang et al., 2019). Phthalate treatment of chicken cardiomyocytes induces cardiomyocyte hypertrophy via mitochondrial dysfunction (Cai et al., 2019). As in testing to assess BPA toxicity, developmental exposure of zebrafish to phthalates induces cardiac morphological abnormalities, pericardial edema, and reduced cardiac function (Pu et al., 2020; Sun and Li, 2019; Sun and Liu, 2017).
Taken together, there is strong evidence from epidemiological, molecular to phenotypic viewpoints that the cardiovascular system is significantly impacted by chemicals detected in the environment. EDCs appear to affect the cardiovascular system via an array of signaling pathways and bioassays in utilizing cardiomyocytes will be increasingly important as additional contaminants are revealed and associate with increased risk to CVD.
7. Emerging topics and considerations for Non-EATS modalities and risk assessment of EDCs
7.1. Endocrine crosstalk with other systems
Hormones do not operate in isolation, and there can be significant crosstalk amongst systems. Interaction between the non-EATS and EATS axes following exposures to EDCs are evidenced through metabolic disruption or AHR activation and reproductive function. Indeed, life-history theory states that animals must partition limited resources between growth metabolic homeostasis, and reproduction (Lardner and Loman, 2003). For many EDCs, a direct link between metabolism and reproductive deficits are difficult to establish as some chemicals can exert effects on both processes. For example, triphenyltin has been shown in mammals and aquatic species to impair endocrine, metabolism, neurological and reproductive dysfunction (He et al., 2020). More recently, transgenerational metabolic impairments induced by the EDCs benzo[a]pyrene and triclosan have been associated with reproduction defects in frogs (Regnault et al., 2018; Usal et al., 2020). In this way, the metabolic effects via non-EATS modalities should be considered within the context of the EATS axis to appreciate the full scope of organismal impact. Here we provide three brief examples of how chemicals acting through EATS and non-EATS modalities can impact reproduction, metabolism, and the immune system.
7.1.1. Crosstalk between estrogen and aryl hydrocarbon receptors: focus on reproduction
AHR and estrogen receptor signaling has been widely described in literature and it is now acknowledged that AHR ligands affect reproductive function considerably (Tarnow et al., 2019). AhR appears to modulate estrogen signaling both positively and negatively depending on the cellular context (Ohtake et al., 2011). For example, dioxin has been shown to inhibit the estrogen-induced vitellogenin response through AHR, leading to reproduction deficits in zebrafish (Bugel et al., 2013). Human exposure to dioxin has been shown to induce adverse endocrine effects, such as alterations in sex ratio in children of exposed parents (Karmaus et al., 2002). Dioxins have also been shown to have estrogenic effects including the stimulation of uterine enlargement, and the induction of estrogen-responsive genes (Brauze et al., 1997; Boveroff et al., 2006). Different mechanisms have been proposed to explain Ah-R and estrogen receptor (ER) cross-talk. Ah-R and ER may compete for common cofactors needed for ER and AHR signaling like the AHR nuclear translocator (ARNT). AHR may regulate the levels of circulating E2 by controlling the gene expression of cytochromes P450 involved in estrogen production from cholesterol. AHR may regulate the levels of circulating E2 by controlling the gene expression of cytochromes P450 involved in estrogen production from cholesterol. AHR has been also shown to mediate the assembly of a CUL4B-based ubiquitin ligase complex and promotes the degradation of ER (Ohtake et al., 2011) Finally, AHR may compete with ER for promoter binding leading to inhibition of transcription (Swedenborg and Pongratz, 2010). In conclusion, the physiological role of the AHR and ER as well as their complex mutual crosstalk remain to be determined as do resulting impacts on human health. With more and more endogenous AHR ligands being discovered, the potential impact of such substances on estrogen signaling must be studied in more detail (Tarnow et al., 2019).
7.1.2. Phthalate-mediated cross talk among the EATS and non-EATS pathways
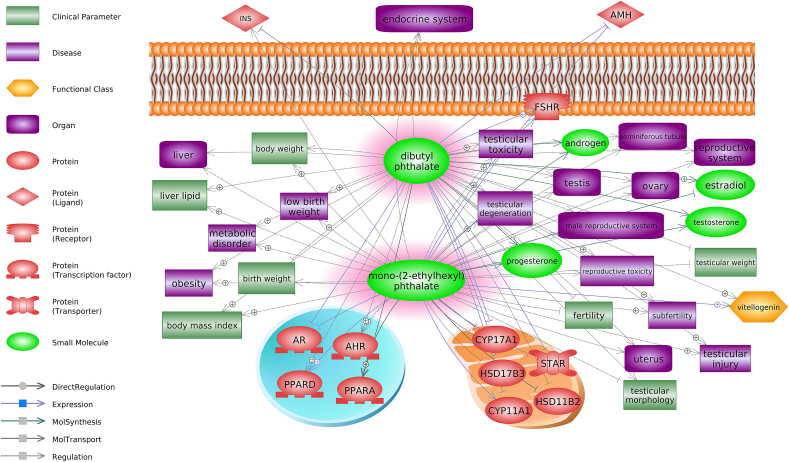
Phthalates are chemicals that are used as plastic additives to protect material from degradation, UV damage, and to improve structural flexibility and resilience. In terms of toxicity assessments, perhaps the two most well studied processes impacted by exposure to phthalates include reproduction and metabolism (Fig. 4). Another example is that of phthalates and the cardiovascular system. Phthalates are reported to act as anti-androgens (EATS pathway) but are also EDCs implicated in interfering with PPAR activation and expression (Casals-Casas and Desvergne, 2011). DEHP, a high-production phthalate representative has been implicated as a PPAR-γ activator (Ito et al., 2019). DEHP exposure upregulates PPAR-γ, which results in a metabolic remodeling of cardiomyocytes (Posnack Nikki et al., 2012). Disruption in cardiomyocyte metabolism by DEHP exposure has led to elevated inflammatory and oxidative stress markers that can sensitize the heart to ischemic injury and ventricular dysfunction (Amara et al., 2019). While phthalates show a high binding efficiency with PPARs, they demonstrate a greater preference for RXRs, as well as their downstream target genes in vitro (Cocci et al., 2015; Sarath Josh et al., 2014). In other settings, such as in the nervous system, the toxicity of phthalates is mediated via the aryl hydrocarbon receptor (AhR) (Wójtowicz et al., 2017, 2019). Thus, phthalates likely interfere with different receptors depending on the context. However, more work is needed to elucidate between the different pathways of interference depending on the mode of phthalate toxicity.
Fig. 4.
Multiple non-EATS pathways are altered by exposure to phthalates. AHR, aryl hydrocarbon receptor; AMH, anti-Mullerian hormone; AR, androgen receptor; CYP11A1, cytochrome P450 family 11 subfamily A member 1; CYP17A1, cytochrome P450 family 17 subfamily A member 1; FSHR, follicle stimulating hormone receptor; HSD11B2, hydroxysteroid 11-beta dehydrogenase 2; HSD17B3, hydroxysteroid 17-beta dehydrogenase 3; INS, insulin; PPARA, peroxisome proliferator activated receptor alpha; PPARD, peroxisome proliferator activated receptor delta, STAR, steroidogenic acute regulatory protein.
7.1.3. Crosstalk between sex steroids, glucocorticoids, and the immune system
EDCs that act as estrogens and androgens can modulate the immune system following classical EATS modalities (LeBlanc et al., 2011). However, the immune system can also be modulated through additional mechanisms, e.g., non-EATS pathways. For example, there is significant crosstalk between the immune system, sex steroid signaling, and the HPA axis (e.g., glucocorticoids repress the expression of pro-inflammatory cytokines and increase the transcription of anti-inflammatory proteins) or vitamin D pathway (e.g. the VDR regulation and function in T cells) (Feldman et al., 2013; Newton et al., 2017). Indeed, not only do GR and VDR play an important role in regulation of immunity, but they can also modulate other nuclear receptors, including AhR, PPARs, RARs, RXRs or RORs (Arakawa, 2014). Nevertheless, the current in vivo bioassays that assess the immunotoxicity of chemicals (for example, OECD TG 407 and 443, and others (Dusinska et al., 2017) mainly focus on “downstream effects” (e.g., immunoglobulin levels, lymphocyte proliferation, cytokine expression, resistance against infection) rather than “upstream effects” which could more clearly reveal the MoA of the chemical being tested.
Vitamin D and PPAR signaling pathways are two of the seven main routes proposed to be assessed by OECD in endocrine disruption and their roles are well documented in the regulation of the immune system (LeBlanc et al., 2011). For non-EATS immunotoxic EDCs, we highlight per- and polyfluoroalkyl substances (PFAS) which exert their action mostly via PPARα (Corsini et al., 2014). Exposure to PFOS, PFOA, PFNA and PFDA have been associated with immune suppression (reduced hemocyte cell viability) in invertebrates (Perna viridis) (Liu and Gin, 2018). Immunoglobulin levels were also observed to decrease in birds (Gallus gallus) after exposure to PFOS, while their plasma lysozyme activity was increased (Peden-Adams et al., 2009). In another study, PFHxS increased trematode infections in amphibian larvae (Lithobates pipiens) (Brown et al., 2020). In mammals (rodents), several PFASs exerted a wide spectrum of effects, e.g. suppressed antibody production and altered T-cell populations in mice exposed to PFOA and/or PFOS (Corsini et al., 2014).
In addition, epidemiological studies link PFASs exposure in humans to alterations in cytokines and interleukins, autoimmune responses as asthma, allergies and dermatitis, and viral infections; and several correlations between their concentrations with WBCs and immunoglobulins levels were reported (Liew et al., 2018). We point out here isoforms of PPAR are involved in modulating the immune specifically (PPARγ) (Martin, 2010), thus EDCs that exert immune system dysregulation via PPAR-α/γ isoforms are also expected to disrupt lipid and adipogenesis pathways.
Along with PFASs, other chemicals have also been reported to alter the immune system via non-EATS modality, as GCs via HPA axis and TBT via vitamin D pathway (as above mentioned), phthalates (probably via PPARα/γ (Hurst and Waxman, 2003), bisphenol A (although in this case is more difficult to discern if the stronger effect is via PPAR -non-EATS- o via ER -EATS-), several phenols (not only via ER -EATS- but also via direct impact on signaling pathways (Nowak et al., 2019), and dioxins and PCBs (via AhR (Larigot et al., 2018) to name a few. As such, PPAR (specially α isoform), AhR, vitamin D and HPA axis pathways along with classical immunotoxicology endpoints should be considered in the assessment of non-EATS immunotoxicity by EDCs.
7.2. Screening complex mixtures in the natural environment
The impact of the chemical environment is often more complex than the sum or synergistic effects of the individual chemicals present. Given that organisms are unlikely ever exposed to a single chemical, determining the overall impact of mixture exposures becomes compelling. Exposure studies in zebrafish exposed to chemicals identified in river water revealed that the effects of the mixture were not mediated by a single receptor and could not be predicted based on the readout of the individual chemicals (Kinch et al., 2016). In contrast, exposure of zebrafish embryos to a mixture of POPs revealed PFOS as the sole chemical responsible for behavioral effects (Khezri et al., 2017), with to a disruption in calcium signaling (Christou et al., 2020). The concentrations of known chemicals detected in a source of drinking water in Belgium were tested for agonistic and antagonistic effects using a panel of rat and human cells expressing AhR, ER, AR, PR and GR promoters linked to a luciferase reporter gene (Tq et al., 2020). Similarly, mixtures of BPA, BPS and BPF had greater agonistic on ER activity and antagonist activity on AR activity with no activity on AhR in reporter cells lines than individual chemicals (Park et al., 2020).
Other examples underscoring exposures to mixtures include food packaging products and medical devices. In one study, extractions from a set of 20 different food packaging materials identified between 16 and 47 compounds per material; these were analyzed using AR, ER, AhR, PPAR-γ, Nrf2, p53 and Ames mutagen assays using a suite of cell lines (Rosenmai et al., 2017). These analyses revealed that the response of mixtures could not be predicted from analyses of the single chemicals. Another example includes plasticizers. Bisphenols are used in medical devices and can leach into patients. Studies in newborns and those undergoing surgery to repair heart malformations revealed significant exposure to BPA and DEHP that was attributed to medical devices (Gaynor et al., 2019; Stroustrup et al., 2020). Increased BPA and DEHP were detected in adults within 12 h of cardiac surgery that required a cardiopulmonary pump (Huygh et al., 2015; Shang et al., 2019). Exposure of mice to this mixture while recovering from MI surgery found a reduced ability to recover fully when compared with control unexposed mice (Shang et al., 2019).
7.3. Adverse outcome pathways and the Non-EATS
One approach for integrating EATS and non-EATS modalities into risk assessment include quantitative adverse outcome pathways (qAOPs) which incorporate quantitative descriptors for key event relationships (KERs) (Perkins et al., 2019). Indeed, dose-response patterns (and sometimes also temporal patterns) are assessed between key events which not only reinforced the linkages between KEs (causality vs coincidence) but also ease the decision making in regulatory contexts (Conolly et al., 2017). The modelling approaches for descriptors can vary from probabilistic to deterministic and are accompanied by mathematical expressions that can present different equations (Spinu et al., 2020).
Although a relatively new concept, several qAOPs have already been proposed for both EATS and non-EATS. For EATS modalities, qAOPs have been developed for reproductive impairment in fish via aromatase inhibition, altered swimming performance in fish via inhibition of thyroid hormone synthesis/degradation, and diverse adverse outcomes (AOs) involved in reproduction and development in vertebrates via activation of ERα (Knapen et al., 2020). For non-EATS modalities, qAOPs concerning immunosuppression and decreased egg production in fish because of GR signaling have been developed (Margiotta-Casaluci et al., 2016), as well as early life stage mortality in birds and fishes via AhR (Doering et al., 2018), and liver steatosis in humans via PPARα (Perkins et al., 2019), among others. It is important to note that both AOPs and qAOPs are extended and cover more than one MIE, several interconnected KEs and several AOs (Conolly et al., 2017). In this way and taken into consideration all the receptors that a certain EDC can interact with at the same time, both EATS and non-EATS modalities can become integrated into the same qAOP. For example, Margiotta-Casaluci et al. (2016) proposed the qAOP of the active metabolite of a synthetic glucocorticoid (beclomethasone dipropionate) that lead to immunosuppression not only via AR, but also via PR and GR. Although EATs and non-EATS present different MIEs and early-KEs, they could exert the same late-KEs and AOs. For example, in Fig. 5 we present a simplified AOP for liver steatosis both via EATS and non-EATS (adapted from [(Mellor et al., 2016), with information from AOPwiki 36, 57, 60, 318]). AOP Wiki (https://aopwiki.org/wiki/index.php/Aop:34) for example describes the key events include mitochondrial impairment, triglyceride accumulation, and cytoplasm distortion. Underlying mechanisms involve inhibition of beta-oxidation and the de novo increases in fatty acids (Fig. 5).
Fig. 5.
Generalized AOP for liver steatosis both via EATS and non-EATS (adapted from [(Mellor et al., 2016), with information from AOPwiki 36, 57, 60, 318]). ER, estrogen receptor; LXR, liver X receptor; PXR, pregnane X receptor; PPARγ/β/α, peroxisome proliferator activated receptor gamma/beta/alpha, respectively; AhR, aryl hydrocarbon receptor; GR, glucocorticoid receptor; RAR, retinoic acid receptor.
7.4. Research models and computational approaches for the Non-EATS
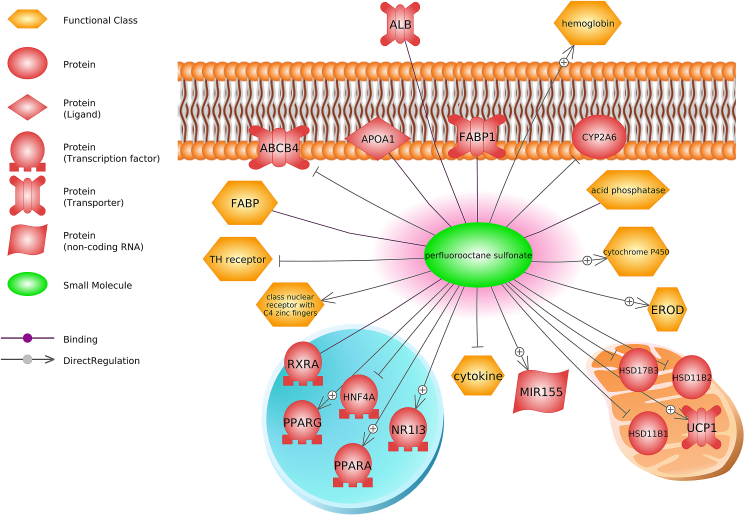
There are new opportunities using genetic engineering, computational toxicology, and multi-omics data sets to elucidate non-EATS modalities for EDCs. CRISPR-based gene editing approaches show great promise for screening EDCs for mechanisms of action, as in the case of TCS and liver cells (Xia et al., 2016) and CRISPR/Cas9 knock-in models of zebrafish have shown promise for detecting EDCs in wastewater (Abdelmoneim et al., 2020). High throughput molecular and biochemical assays are also playing a key role in elucidating EDC effects across cell and animal models. These methods include transcriptomics, proteomics, and metabolomics, each of which aims to capture the full repertoire of transcripts, proteins, or metabolites respectively within the cell. The use of omics technologies has led to new research on non-EATS endocrine pathways, drawing attention to those pathways not previously suspected as targets for chemicals traditionally categorized into EATS modalities. The use of omics technologies has led to new research and adds evidence on existing data on non-EATS endocrine pathways, drawing attention to those pathways not previously suspected as targets for chemicals traditionally categorized into EATS modalities (Franco et al., 2020; Jordão et al., 2015). Two examples include immune system effects (predicted to act via vitamin D signaling) and MoA of PFOS (predicted to act via PPARα) (Martínez et al., 2019b, 2020c) (Fig. 6).
Fig. 6.
Perfluorooctane sulfonate is predicted to affect proteins and functional classes related to immune, steroidogenesis, and metabolism. Abbreviations: HSD17B3, hydroxysteroid 17-beta dehydrogenase 3; ABCB4, ATP binding cassette subfamily B member 4; ALB, albumin; APOA1, apolipoprotein A1; CYP2A6, cytochrome P450 family 2 subfamily A member 6; FABP1, fatty acid binding protein 1; HNF4A, hepatocyte nuclear factor 4 alpha; HSD11B1, hydroxysteroid 11-beta dehydrogenase 1; HSD11B2, hydroxysteroid 11-beta dehydrogenase 2; MIR155, microRNA 155; NR1I3, nuclear receptor subfamily 1 group I member 3; PPARA, peroxisome proliferator activated receptor alpha; PPAR-Γ, peroxisome proliferator activated receptor gamma; RXRA, retinoid X receptor alpha; UCP1, uncoupling protein 1.
The strength of leveraging computational and omics methodology into high throughput chemical screening is that novel hormone signaling pathways are potentially revealed, generating new hypotheses for investigations (i.e., non-EATS pathways). Moreover, this approach can be useful for complex environmental mixtures. In this regard, concentration-dependent transcriptomic studies in vitro bioassays, have been used following a tiered approach to identify and select bioassays based on nuclear receptors (AhR, ER, AR and Nrf2) for water quality assessment (Fang et al., 2020).
8. Final recommendation
Test guidelines based on the examination of specific phenotypic endpoints, e.g., effects on reproduction, growth or development, and the study of single molecular pathways mostly related to estrogenic, androgenic, thyroidal and steroidogenic (EATS) related pathways are currently those most prevalent in literature and chemical screening programs (Kucheryavenko et al., 2020). To date however, testing methodologies are insufficient at addressing specific aspects of EDCs. Computational insight from Tox21 and ToxCast highlights the extent of receptor promiscuity and hormone crosstalk for several chemicals and novel MoAs through the non-EATS modality. Table 1 summarizes several non-EATS interactions between chemicals and receptors. Although these high-throughput approaches show great promise in identifying chemicals that act for example, as obesogens, care must be taken by extrapolating results to higher biological levels as data do not always correlate between HTS receptor activation to phenotypic assays (Auerbach et al., 2016; Janesick et al., 2016). Several software tools are also available to predict endocrine activity related to both EATS and non-EATS modalities. Andersson and colleagues (Andersson et al., 2018) highlight useful software tools available for predictive toxicology regarding the non-EATS, which include MolCode Toolbox (AhR), Virtual Toxab (GRs, AhRs, PPARs), Endocrine Disruptome (GRs, PPAR, RXRs), and Danish (Q)SATR DB (PXR) to name but a few. Recommendations and consideration for evaluating non-EATS modalities include the following:
-
1)
Imperative to detect potential disruptions and non-EATS pathways to develop new regulations for developing safer chemicals and protect environmental, animal, and human health.
-
2)
A non-targeted approach to toxicity testing is needed to reveal the multiplicity of chemical interactions to non-EATS pathways.
-
3)
Multiple tissues in multiple species and the inclusion of males and females in testing is essential to fully describe non-EATS chemical toxicities.
-
4)
Consideration that analogs may have different affinities or even no affinity for their non-EATS interactions. Class effects should not be assumed without testing.
-
5)
Relevant dosing to reflect levels experienced by the general population, those occupationally exposed should be a focal point for non-EATS interactions.
-
6)
The use of reporter cell lines should be expanded in testing of endocrine disruptors to reflect the possibility for non-EATS interactions. However, there can be limitations as cell lines do not completely recapitulate in vivo exposures, nor do they necessarily yield accurate data for all species. Nevertheless, such assays coupled to other approaches like omics, have provided viable targets of endocrine disruptors for obesogens and cardiovascular dysregulation.
Table 1.
Examples of Non-EATS modalities perturbed by different chemical classes and specific chemicals.
| Chemical | Receptor and/or enzyme |
|---|---|
| General Examples | |
| Coplanar PCBs | AhR |
| Dioxins | AhR |
| Organotins | PPARγ, RXRα |
| Bisphenols | PPARγ, RXRα |
| Polychlorinated biphenyls (PCBs) | PPARγ, RXRα |
| Phthalates | PPARs, RXRs, AHR |
| Polyfluoroalkyl substances (PFAS) | PPARα |
| Specific Examples | |
| Dibutyl phthalate | PXR, cytochrome p450 enzymes |
| TBT (tributyltin) | PPARγ, RXRα |
| BPA (bisphenol A) | PPAR, RXR, RAR, CAR |
| TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) | AhR |
| Di(2- ethylhexyl) phthalate | PPARγ |
| α-naphthoflavone | AHR (inhibition) |
| Benzo[a]pyrene | AhR |
| Bisphenol AF | RXR-γ |
| Bisphenol Z | RXR-γ |
| Prostacyclin | RXR-γ |
| Beclomethasone dipropionate | PR, GR |
| Perfluorooctane sulfonic acid | PPARα |
Taken together, while much data has been generated regarding chemicals with novel targets related to endocrine disruption, the remains a significant number of hormones (non-EATS modalities) for which we know little in terms of chemical interactions and perturbation.
Author contributions
Christopher J. Martyniuk: conceptualization, writing (original draft, review & editing), visualization; Rubén Francisco Martínez López: writing (original draft, review & editing), Laia Navarro-Martin: writing (original draft, review & editing); Jorke H. Kamstra: writing (original draft, review & editing); Adam Schwendt: writing (original draft, review & editing), Stephane Reynaud: writing (review & editing); Lorraine Chalifour writing (original draft, review & editing).
Funding
LNM was supported by a H2020-Marie Skłodowska-Curie Action MSCA-IF-RI- 2017 awarded by the European Commission (ref. 797725-EpiSTOX). JK was funded by the European Union's Horizon 2020 research and innovation program under grant agreement GOLIATH No. 825489. AS and LEC were supported by a Grant-in-Aid from the Heart and Stroke Foundation of Canada.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
.
Acknowledgements
We thank the Intersectorial Centre for Endocrine Disruptor Analysis (ICEDA)'s researcher network that facilitated this Special Issue.
References
- Abbott B.D. Review of the expression of peroxisome proliferator-activated receptors alpha (PPARα), beta (PPARβ), and gamma (PPARγ) in rodent and human development. Reprod. Toxicol. 2009;27:246–257. doi: 10.1016/j.reprotox.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Abdelmoneim A., et al. Fluorescent reporter zebrafish line for estrogenic compound screening generated using a CRISPR/Cas9-mediated knock-in system. Toxicol. Sci. 2020;173:336–346. doi: 10.1093/toxsci/kfz224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akash M.S.H., et al. Bisphenol A-induced metabolic disorders: from exposure to mechanism of action. Environ. Toxicol. Pharmacol. 2020;77:103373. doi: 10.1016/j.etap.2020.103373. [DOI] [PubMed] [Google Scholar]
- Alavian‐Ghavanini A., Rüegg J. Understanding epigenetic effects of endocrine disrupting chemicals: from mechanisms to novel test methods. Basic Clin. Pharmacol. Toxicol. 2018;122:38–45. doi: 10.1111/bcpt.12878. [DOI] [PubMed] [Google Scholar]
- Amara I., et al. Di (2-ethylhexyl) phthalate induces cardiac disorders in BALB/c mice. Environ. Sci. Pollut. Res. Int. 2019;26:7540–7549. doi: 10.1007/s11356-019-04219-w. [DOI] [PubMed] [Google Scholar]
- Andersson N., et al. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA J. 2018;16(6) doi: 10.2903/j.efsa.2018.5311. ECHA-18-G-01-EN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa Y. Immunotoxicity risk assessment for chemicals and regulatory science. Ind. Health. 2014;52:87–89. doi: 10.2486/indhealth.51_101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariemma F., et al. Low-dose bisphenol-A impairs adipogenesis and generates dysfunctional 3T3-L1 adipocytes. PloS One. 2016;11 doi: 10.1371/journal.pone.0150762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audouze K., et al. Integrative strategy of testing systems for identification of endocrine disruptors inducing metabolic disorders—an introduction to the OBERON project. Int. J. Mol. Sci. 2020;21:2988. doi: 10.3390/ijms21082988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach S., et al. Prioritizing environmental chemicals for obesity and diabetes outcomes research: a screening approach using ToxCast™ high-throughput data. Environ. Health Perspect. 2016;124:1141–1154. doi: 10.1289/ehp.1510456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae S., et al. Associations of bisphenol A exposure with heart rate variability and blood pressure/novelty and significance. Hypertension. 2012;60:786–793. doi: 10.1161/HYPERTENSIONAHA.112.197715. [DOI] [PubMed] [Google Scholar]
- Baker N.A., Shoemaker R., English V., Larian N., Sunkara M., Morris A.J., Walker M., Yiannikouris F., Cassis L.A. Effects of adipocyte aryl hydrocarbon receptor deficiency on PCB-induced disruption of glucose homeostasis in lean and obese mice. Environ. Health Perspect. 2015;123(10):944–950. doi: 10.1289/ehp.1408594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton-Maclaren, et al. Innovation in regulatory approaches for Endocrine-Disrupting Chemicals (EDCs): the journey to risk assessment modernization in Canada. Environ. Res.. [DOI] [PubMed]
- Berger J., Moller D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- Bertuloso B.D., et al. Tributyltin chloride leads to adiposity and impairs metabolic functions in the rat liver and pancreas. Toxicol. Lett. 2015;235:45–59. doi: 10.1016/j.toxlet.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Boberg J., et al. Using assessment criteria for pesticides to evaluate the endocrine disrupting potential of non-pesticide chemicals: case butylparaben. Environ. Int. 2020;144:105996. doi: 10.1016/j.envint.2020.105996. [DOI] [PubMed] [Google Scholar]
- Boonen I., et al. Assessing the receptor-mediated activity of PAHs using AhR-, ERα- and PPARγ- CALUX bioassays. Food Chem. Toxicol. 2020;145:111602. doi: 10.1016/j.fct.2020.111602. [DOI] [PubMed] [Google Scholar]
- Brown A.R., et al. Cardiovascular effects and molecular mechanisms of bisphenol A and its metabolite MBP in zebrafish. Environ. Sci. Technol. 2019;53:463–474. doi: 10.1021/acs.est.8b04281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S.R., et al. Perfluoroalkyl substances increase susceptibility of northern leopard frog tadpoles to trematode infection. Environ. Toxicol. Chem. 2020 doi: 10.1002/etc.4678. [DOI] [PubMed] [Google Scholar]
- Bugel S.M., et al. Inhibition of vitellogenin gene induction by 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin is mediated by aryl hydrocarbon receptor 2 (AHR2) in zebrafish (Danio rerio) Aquat. Toxicol. 2013;126:1–8. doi: 10.1016/j.aquatox.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Cai J., et al. Taxifolin ameliorates DEHP-induced cardiomyocyte hypertrophy via attenuating mitochondrial dysfunction and glycometabolism disorder in chicken. Environ. Pollut. 2019;255:113155. doi: 10.1016/j.envpol.2019.113155. [DOI] [PubMed] [Google Scholar]
- Cai S., et al. Relationship between urinary bisphenol a levels and cardiovascular diseases in the U.S. adult population, 2003–2014. Ecotoxicol. Environ. Saf. 2020;192:110300. doi: 10.1016/j.ecoenv.2020.110300. [DOI] [PubMed] [Google Scholar]
- Capitão A., et al. Obesogens in the aquatic environment: an evolutionary and toxicological perspective. Environ. Int. 2017;106:153–169. doi: 10.1016/j.envint.2017.06.003. [DOI] [PubMed] [Google Scholar]
- Casals-Casas C., Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu. Rev. Physiol. 2011;73:135–162. doi: 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- Cavaliere F., et al. Molecular modelling methods in food safety: bisphenols as case study. Food Chem. Toxicol. 2020;137:111116. doi: 10.1016/j.fct.2020.111116. [DOI] [PubMed] [Google Scholar]
- Chen Q., et al. Glucocorticoid activity detected by in vivo zebrafish assay and in vitro glucocorticoid receptor bioassay at environmental relevant concentrations. Chemosphere. 2016;144:1162–1169. doi: 10.1016/j.chemosphere.2015.09.089. [DOI] [PubMed] [Google Scholar]
- Cheng W., et al. Low doses of BPA induced abnormal mitochondrial fission and hypertrophy in human embryonic stem cell-derived cardiomyocytes via the calcineurin-DRP1 signaling pathway: a comparison between XX and XY cardiomyocytes. Toxicol. Appl. Pharmacol. 2020;388:114850. doi: 10.1016/j.taap.2019.114850. [DOI] [PubMed] [Google Scholar]
- Cocci P., et al. Effects of diisodecyl phthalate on PPAR:RXR-Dependent gene expression pathways in sea bream hepatocytes. Chem. Res. Toxicol. 2015;28:935–947. doi: 10.1021/tx500529x. [DOI] [PubMed] [Google Scholar]
- Committee E.S. Scientific Opinion on the hazard assessment of endocrine disruptors: scientific criteria for identification of endocrine disruptors and appropriateness of existing test methods for assessing effects mediated by these substances on human health and the environment. EFSA Journal. 2013;11:3132. [Google Scholar]
- Conolly R.B., et al. Quantitative adverse outcome pathways and their application to predictive toxicology. Environ. Sci. Technol. 2017;51:4661–4672. doi: 10.1021/acs.est.6b06230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsini E., et al. Perfluorinated compounds: emerging POPs with potential immunotoxicity. Toxicol. Lett. 2014;230:263–270. doi: 10.1016/j.toxlet.2014.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou M., et al. Calcium signaling as a possible mechanism behind increased locomotor response in zebrafish larvae exposed to a human relevant persistent organic pollutant mixture or PFOS. Environ. Res. 2020;187:109702. doi: 10.1016/j.envres.2020.109702. [DOI] [PubMed] [Google Scholar]
- Cunningham T.J., Duester G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat. Rev. Mol. Cell Biol. 2015;16:110–123. doi: 10.1038/nrm3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day P., Green R.M., Gross M., Weltje L., Wheeler J.R. Endocrine Disruption: current approaches for regulatory testing and assessment of plant protection products are fit for purpose. Toxicol. Lett. 2018;296:10–22. doi: 10.1016/j.toxlet.2018.07.011. [DOI] [PubMed] [Google Scholar]
- Delfosse V., et al. Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109:14930–14935. doi: 10.1073/pnas.1203574109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbès, G., et al. Effects of EDCs on gonad development: mechanistic insights from fish and mammals. Environ. Res.. [DOI] [PubMed]
- den Broeder M.J., et al. Inhibition of methyltransferase activity of enhancer of zeste 2 leads to enhanced lipid accumulation and altered chromatin status in zebrafish. Epigenet. Chromatin. 2020;13:5. doi: 10.1186/s13072-020-0329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Broeder M.J., et al. Altered adipogenesis in zebrafish larvae following high fat diet and chemical exposure is visualised by stimulated Raman scattering microscopy. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18040894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendup T., et al. Environmental risk factors for developing type 2 diabetes mellitus: a systematic review. Int. J. Environ. Res. Publ. Health. 2018;15 doi: 10.3390/ijerph15010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering J.A., et al. A cross-species quantitative adverse outcome pathway for activation of the aryl hydrocarbon receptor leading to early life stage mortality in birds and fishes. Environ. Sci. Technol. 2018;52:7524–7533. doi: 10.1021/acs.est.8b01438. [DOI] [PubMed] [Google Scholar]
- Dong M., et al. Dose-dependent effects of triclocarban exposure on lipid homeostasis in rats. Chem. Res. Toxicol. 2019;32:2320–2328. doi: 10.1021/acs.chemrestox.9b00316. [DOI] [PubMed] [Google Scholar]
- Dornbos P., et al. Characterizing the role of HMG-CoA reductase in aryl hydrocarbon receptor-mediated liver injury in C57BL/6 mice. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-019-52001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunder L., et al. Low-dose developmental bisphenol A exposure alters fatty acid metabolism in Fischer 344 rat offspring. Environ. Res. 2018;166:117–129. doi: 10.1016/j.envres.2018.05.023. [DOI] [PubMed] [Google Scholar]
- Dusinska M., et al. Immunotoxicity, genotoxicity and epigenetic toxicity of nanomaterials: new strategies for toxicity testing? Food Chem. Toxicol. 2017;109:797–811. doi: 10.1016/j.fct.2017.08.030. [DOI] [PubMed] [Google Scholar]
- Duursen M., et al. Safeguarding female reproductive health against endocrine disrupting chemicals—the FREIA project. Int. J. Mol. Sci. 2020;21:3215. doi: 10.3390/ijms21093215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards H.E., Burnham W.M. The impact of corticosteroids on the developing animal. Pediatr. Res. 2001;50:433–440. doi: 10.1203/00006450-200110000-00003. [DOI] [PubMed] [Google Scholar]
- Evans R.M., Mangelsdorf D.J. Nuclear receptors, RXR, and the big bang. Cell. 2014;157:255–266. doi: 10.1016/j.cell.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W., et al. A tiered approach for screening and assessment of environmental mixtures by omics and in vitro assays. Environ. Sci. Technol. 2020;54:7430–7439. doi: 10.1021/acs.est.0c00662. [DOI] [PubMed] [Google Scholar]
- Feldman D., et al. The vitamin D receptor and T cell function. Front. Immunol. 2013;18 doi: 10.3389/fimmu.2013.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco M.E., et al. Metabolomic-based assessment reveals dysregulation of lipid profiles in human liver cells exposed to environmental obesogens. Toxicol. Appl. Pharmacol. 2020;398:115009. doi: 10.1016/j.taap.2020.115009. [DOI] [PubMed] [Google Scholar]
- Fuertes I., et al. Time-dependent transcriptomic responses of Daphnia magna exposed to metabolic disruptors that enhanced storage lipid accumulation. Environ. Pollut. 2019;249:99–108. doi: 10.1016/j.envpol.2019.02.102. [DOI] [PubMed] [Google Scholar]
- Gálvez-Ontiveros Y., et al. Endocrine disruptors in food: impact on gut microbiota and metabolic diseases. Nutrients. 2020;12 doi: 10.3390/nu12041158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F., et al. Involvement of estrogen receptor and GPER in bisphenol A induced proliferation of vascular smooth muscle cells. Toxicol. Vitro. 2019;56:156–162. doi: 10.1016/j.tiv.2019.01.012. [DOI] [PubMed] [Google Scholar]
- Gao X., Wang H.S. Impact of bisphenol a on the cardiovascular system - epidemiological and experimental evidence and molecular mechanisms. Int. J. Environ. Res. Publ. Health. 2014;11:8399–8413. doi: 10.3390/ijerph110808399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor J.W., et al. Perioperative exposure to suspect neurotoxicants from medical devices in newborns with congenital heart defects. Ann. Thorac. Surg. 2019;107:567–572. doi: 10.1016/j.athoracsur.2018.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gear R., et al. Effects of bisphenol A on incidence and severity of cardiac lesions in the NCTR-Sprague-Dawley rat: a CLARITY-BPA study. Toxicol. Lett. 2017;275:123–135. doi: 10.1016/j.toxlet.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert R.J., Wilson C. Reproductive function in rats exposed prenatally to pesticides and polychlorinated biphenyls (PCB) Environ. Res. 1979;18:437–443. doi: 10.1016/0013-9351(79)90119-1. [DOI] [PubMed] [Google Scholar]
- Gillum N., et al. Clinically relevant concentrations of di (2-ethylhexyl) phthalate (DEHP) uncouple cardiac syncytium. Toxicol. Appl. Pharmacol. 2009;236:25–38. doi: 10.1016/j.taap.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinski D.A., et al. Endogenous and exogenous biomarker analysis in terrestrial phase amphibians (Lithobates sphenocephala) following dermal exposure to pesticide mixtures. Environ. Chem. 2018;16:55–67. doi: 10.1071/EN18163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourronc F.A., Markan K.R., Kulhankova K., Zhu Z., Sheehy R., Quelle D.E., Zingman L.V., Kurago Z.B., Ankrum J.A., Klingelhutz A.J. Pdgfrα-Cre mediated knockout of the aryl hydrocarbon receptor protects mice from high-fat diet induced obesity and hepatic steatosis. PloS One. 2020;15(7) doi: 10.1371/journal.pone.0236741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun F., et al. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol. Endocrinol. 2006;20:2141–2155. doi: 10.1210/me.2005-0367. [DOI] [PubMed] [Google Scholar]
- Gu J., et al. Oxidative stress in bisphenol AF-induced cardiotoxicity in zebrafish and the protective role of N-acetyl N-cysteine. Sci. Total Environ. 2020;731:139190. doi: 10.1016/j.scitotenv.2020.139190. [DOI] [PubMed] [Google Scholar]
- Gurushankara H., et al. Impact of malathion stress on lipid metabolism in Limnonectus limnocharis. Pestic. Biochem. Physiol. 2007;88:50–56. [Google Scholar]
- Hall J.A., et al. The role of retinoic acid in tolerance and immunity. Immunity. 2011;35:13–22. doi: 10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., et al. Review on endocrine disrupting toxicity of triphenyltin from the perspective of species evolution: aquatic, amphibious and mammalian. Chemosphere. 2020:128711. doi: 10.1016/j.chemosphere.2020.128711. [DOI] [PubMed] [Google Scholar]
- Heindel J.J., Blumberg B. Environmental obesogens: mechanisms and controversies. Annu. Rev. Pharmacol. Toxicol. 2019;59:89–106. doi: 10.1146/annurev-pharmtox-010818-021304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel J.J., et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017;68:3–33. doi: 10.1016/j.reprotox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, V., et al. Endocrine disruptors: challenges and future directions in epidemiologic research. Environ. Res.. [DOI] [PubMed]
- Holbech H., et al. ERGO: breaking down the wall between human health and environmental testing of endocrine disrupters. Int. J. Mol. Sci. 2020;21:2954. doi: 10.3390/ijms21082954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F., et al. Programming neuroendocrine stress Axis Activity by exposure to glucocorticoids during postembryonic development of the frog, Xenopus laevis. Endocrinology. 2008;149:5470–5481. doi: 10.1210/en.2008-0767. [DOI] [PubMed] [Google Scholar]
- Hudda N., et al. Hypertension; 2021. Effect of Reducing Ambient Traffic-Related Air Pollution on Blood Pressure: A Randomized Crossover Trial. Hypertensionaha12015580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst C.H., Waxman D.J. Activation of PPARα and PPARγ by environmental phthalate monoesters. Toxicol. Sci. 2003;74:297–308. doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- Huygh J., et al. Considerable exposure to the endocrine disrupting chemicals phthalates and bisphenol-A in intensive care unit (ICU) patients. Environ. Int. 2015;81:64–72. doi: 10.1016/j.envint.2015.04.008. [DOI] [PubMed] [Google Scholar]
- IJpenberg A., et al. Polarity and specific sequence requirements of peroxisome proliferator-activated receptor (PPAR)/retinoid X receptor heterodimer binding to DNA A functional analysis of the malic enzyme gene PPAR response element. J. Biol. Chem. 1997;272:20108–20117. doi: 10.1074/jbc.272.32.20108. [DOI] [PubMed] [Google Scholar]
- Ito Y., et al. Di(2-ethylhexyl) phthalate-induced toxicity and peroxisome proliferator-activated receptor alpha: a review. Environ. Health Prev. Med. 2019;24:47. doi: 10.1186/s12199-019-0802-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaimes R., et al. Plastics and cardiovascular health: phthalates may disrupt heart rate variability and cardiovascular reactivity. Am. J. Physiol. Heart Circ. Physiol. 2017;313:H1044–H1053. doi: 10.1152/ajpheart.00364.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaimes R., 3rd, et al. Plasticizer interaction with the heart: chemicals used in plastic medical devices can interfere with cardiac electrophysiology. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.119.007294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janesick A.S., et al. On the utility of ToxCast™ and ToxPi as methods for identifying new obesogens. Environ. Health Perspect. 2016;124:1214–1226. doi: 10.1289/ehp.1510352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies D., French M. Changes induced in the pigeon thyroid by p, p′-DDE and Dieldrin. J. Wildl. Manag. 1972:24–30. [Google Scholar]
- Jerez-Cepa I., et al. What can we learn from glucocorticoid administration in fish? Effects of cortisol and dexamethasone on intermediary metabolism of gilthead seabream (Sparus aurata L.) Comp. Biochem. Physiol. Mol. Integr. Physiol. 2019;231:1–10. doi: 10.1016/j.cbpa.2019.01.010. [DOI] [PubMed] [Google Scholar]
- Jiang S., et al. Association of bisphenol A and its alternatives bisphenol S and F exposure with hypertension and blood pressure: a cross-sectional study in China. Environ. Pollut. 2020;257:113639. doi: 10.1016/j.envpol.2019.113639. [DOI] [PubMed] [Google Scholar]
- Jordão R., et al. Obesogens beyond vertebrates: lipid perturbation by tributyltin in the Crustacean Daphnia magna. Environ. Health Perspect. 2015;123:813–819. doi: 10.1289/ehp.1409163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jugan M.-L., et al. Endocrine disruptors and thyroid hormone physiology. Biochem. Pharmacol. 2010;79:939–947. doi: 10.1016/j.bcp.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Just A.C., et al. Prenatal exposure to butylbenzyl phthalate and early eczema in an urban cohort. Environ. Health Perspect. 2012;120:1475–1480. doi: 10.1289/ehp.1104544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama T., et al. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor γ/retinoid X receptor pathway. Mol. Pharmacol. 2005;67:766–774. doi: 10.1124/mol.104.008409. [DOI] [PubMed] [Google Scholar]
- Kasneci A., et al. From the cover: lifelong exposure of C57bl/6n male mice to bisphenol A or bisphenol S reduces recovery from a myocardial infarction. Toxicol. Sci. 2017;159:189–202. doi: 10.1093/toxsci/kfx133. [DOI] [PubMed] [Google Scholar]
- Kassotis C.D., et al. Endocrine-disrupting chemicals: economic, regulatory, and policy implications. The Lancet Diabetes & Endocrinology. 2020;8:719–730. doi: 10.1016/S2213-8587(20)30128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., et al. The adverse effect of 4-tert-octylphenol on fat metabolism in pregnant rats via regulation of lipogenic proteins. Environ. Toxicol. Pharmacol. 2015;40:284–291. doi: 10.1016/j.etap.2015.06.020. [DOI] [PubMed] [Google Scholar]
- Kinch C.D., et al. Adverse morphological development in embryonic zebrafish exposed to environmental concentrations of contaminants individually and in mixture. Aquat. Toxicol. 2016;175:286–298. doi: 10.1016/j.aquatox.2016.03.021. [DOI] [PubMed] [Google Scholar]
- Khezri A., et al. A mixture of persistent organic pollutants and perfluorooctanesulfonic acid induces similar behavioural responses, but different gene expression profiles in zebrafish larvae. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klint H., et al. Low-dose exposure to bisphenol A in combination with fructose increases expression of genes regulating angiogenesis and vascular tone in juvenile Fischer 344 rat cardiac tissue. Ups. J. Med. Sci. 2017;122:20–27. doi: 10.1080/03009734.2016.1225870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapen D., et al. Toward an AOP network-based tiered testing strategy for the assessment of thyroid hormone disruption. Environ. Sci. Technol. 2020;54:8491–8499. doi: 10.1021/acs.est.9b07205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortenkamp A., et al. Removing critical gaps in chemical test methods by developing new assays for the identification of thyroid hormone system-disrupting chemicals—the athena project. Int. J. Mol. Sci. 2020;21:3123. doi: 10.3390/ijms21093123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küblbeck J., et al. The EDCMET project: metabolic effects of endocrine disruptors. Int. J. Mol. Sci. 2020;21:3021. doi: 10.3390/ijms21083021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucheryavenko O., Vogl S., Marx-Stoelting P. eISBN; 2020. Endocrine Disruptor Effects on Estrogen, Androgen and Thyroid Pathways: Recent Advances on Screening and Assessment; pp. 1–24. 978-1-83916-073-8. [DOI] [Google Scholar]
- Kugathas S., et al. Metabolic and reproductive effects of relatively low concentrations of beclomethasone dipropionate, a synthetic glucocorticoid, on fathead minnows. Environ. Sci. Technol. 2013;47:9487–9495. doi: 10.1021/es4019332. [DOI] [PubMed] [Google Scholar]
- La Merrill M., et al. Perinatal exposure of mice to the pesticide DDT impairs energy expenditure and metabolism in adult female offspring. PloS One. 2014;9 doi: 10.1371/journal.pone.0103337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacouture, A., et al. Impacts of Endocrine-Disrupting Chemicals on prostate function & cancer. Environ. Res. [DOI] [PubMed]
- Landrigan P.J., et al. The Lancet Commission on pollution and health. Lancet. 2018;391:462–512. doi: 10.1016/S0140-6736(17)32345-0. [DOI] [PubMed] [Google Scholar]
- Lang I.A., et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. J. Am. Med. Assoc.: JAMA, J. Am. Med. Assoc. 2008;300:1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- Lardner B., Loman J. Growth or reproduction? Resource allocation by female frogs Rana temporaria. Oecologia. 2003;137:541–546. doi: 10.1007/s00442-003-1390-5. [DOI] [PubMed] [Google Scholar]
- Larigot L., et al. AhR signaling pathways and regulatory functions. Biochimie open. 2018;7:1–9. doi: 10.1016/j.biopen.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Magueresse-Battistoni B., Multigner L., Beausoleil C., Rousselle C. Effects of bisphenol A on metabolism and evidences of a mode of action mediated through endocrine disruption. Mol. Cell. Endocrinol. 2018;475:74–91. doi: 10.1016/j.mce.2018.02.009. [DOI] [PubMed] [Google Scholar]
- Le Maire A., et al. Regulation of RXR-RAR heterodimers by RXR-and RAR-specific ligands and their combinations. Cells. 2019;8:1392. doi: 10.3390/cells8111392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc G.A., et al. Organisation for Economic Cooperation and Development; Paris, France: 2011. Draft Detailed Review Paper State of the Science on Novel in Vitro and in Vivo Screening and Testing Methods and Endpoints for Evaluating Endocrine Disruptors. [Google Scholar]
- Lee H.-M., et al. Endocrine disruptive effects of polychlorinated aromatic hydrocarbons on intestinal cholecystokinin in rats*. Endocrinology. 2000;141:2938–2944. doi: 10.1210/endo.141.8.7626. [DOI] [PubMed] [Google Scholar]
- Lee J.H., et al. A novel role for the dioxin receptor in fatty acid metabolism and hepatic steatosis. Gastroenterology. 2010;139:653–663. doi: 10.1053/j.gastro.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., et al. Inhibitory effect of octyl-phenol and bisphenol A on calcium signaling in cardiomyocyte differentiation of mouse embryonic stem cells. J. Physiol. Pharmacol. 2019;70 doi: 10.26402/jpp.2019.3.10. [DOI] [PubMed] [Google Scholar]
- Legler J., et al. The GOLIATH project: towards an internationally harmonised approach for testing metabolism disrupting compounds. Int. J. Mol. Sci. 2020;21:3480. doi: 10.3390/ijms21103480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legler J., et al. 2017. Differential DNA Methylation at Conserved Non-genic Elements and Evidence for Transgenerational Inheritance Following Developmental Exposure to Mono (2-ethylhexyl) Phthalate and 5-azacytidine in Zebrafish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León-Olea M., et al. Current concepts in neuroendocrine disruption. Gen. Comp. Endocrinol. 2014;203:158–173. doi: 10.1016/j.ygcen.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung M.C., et al. Systems toxicology of male reproductive development: profiling 774 chemicals for molecular targets and adverse outcomes. Environ. Health Perspect. 2016;124:1050–1061. doi: 10.1289/ehp.1510385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., et al. A two-hybrid yeast assay to quantify the effects of xenobiotics on retinoid X receptor-mediated gene expression. Toxicol. Lett. 2008;176:198–206. doi: 10.1016/j.toxlet.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Li Y., et al. The association between toxic pesticide environmental exposure and Alzheimer's disease: a scientometric and visualization analysis. Chemosphere. 2021;263:128238. doi: 10.1016/j.chemosphere.2020.128238. [DOI] [PubMed] [Google Scholar]
- Liew Z., et al. Developmental exposures to perfluoroalkyl substances (PFASs): an update of associated health outcomes. Current environmental health reports. 2018;5:1–19. doi: 10.1007/s40572-018-0173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., et al. Exposure to bisphenol A induces dysfunction of insulin secretion and apoptosis through the damage of mitochondria in rat insulinoma (INS-1) cells. Cell Death Dis. 2013;4 doi: 10.1038/cddis.2012.206. e460-e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind P.M., Lind L. Circulating levels of bisphenol A and phthalates are related to carotid atherosclerosis in the elderly. Atherosclerosis. 2011;218:207–213. doi: 10.1016/j.atherosclerosis.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Liu C., Gin K.Y.H. Immunotoxicity in green mussels under perfluoroalkyl substance (PFAS) exposure: reversible response and response model development. Environ. Toxicol. Chem. 2018;37:1138–1145. doi: 10.1002/etc.4060. [DOI] [PubMed] [Google Scholar]
- Livingstone D.E.W., et al. Understanding the role of glucocorticoids in obesity: tissue-specific alterations of corticosterone metabolism in obese zucker Rats1. Endocrinology. 2000;141:560–563. doi: 10.1210/endo.141.2.7297. [DOI] [PubMed] [Google Scholar]
- Lupu D., et al. The ENDpoiNTs project: novel testing strategies for endocrine disruptors linked to developmental neurotoxicity. Int. J. Mol. Sci. 2020;21:3978. doi: 10.3390/ijms21113978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssimachou A., et al. The mammalian “obesogen” tributyltin targets hepatic triglyceride accumulation and the transcriptional regulation of lipid metabolism in the liver and brain of zebrafish. PloS One. 2015;10 doi: 10.1371/journal.pone.0143911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., et al. Progesterone protects against bisphenol A-induced arrhythmias in female rat cardiac myocytes via rapid signaling. Endocrinology. 2017;158:778–790. doi: 10.1210/en.2016-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M. Role and distribution of retinoic acid during CNS development. Int. Rev. Cytol. 2001;209:1–77. doi: 10.1016/s0074-7696(01)09010-6. [DOI] [PubMed] [Google Scholar]
- Maradonna F., Carnevali O. Lipid metabolism alteration by endocrine disruptors in animal models: an overview. Front. Endocrinol. 2018;9:654. doi: 10.3389/fendo.2018.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margiotta-Casaluci L., et al. Internal exposure dynamics drive the adverse outcome pathways of synthetic glucocorticoids in fish. Sci. Rep. 2016;6:1–13. doi: 10.1038/srep21978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariana M., et al. Cardiovascular response of rat aorta to di-(2-ethylhexyl) phthalate (DEHP) exposure. Cardiovasc. Toxicol. 2018;18:356–364. doi: 10.1007/s12012-017-9439-6. [DOI] [PubMed] [Google Scholar]
- Martin H. Role of PPAR-gamma in inflammation. Prospects for therapeutic intervention by food components. Mutat. Res. Fund Mol. Mech. Mutagen. 2010;690:57–63. doi: 10.1016/j.mrfmmm.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Martínez R., et al. Changes in lipid profiles induced by bisphenol A (BPA) in zebrafish eleutheroembryos during the yolk sac absorption stage. Chemosphere. 2020;246:125704. doi: 10.1016/j.chemosphere.2019.125704. [DOI] [PubMed] [Google Scholar]
- Martínez R., et al. Acute and long-term metabolic consequences of early developmental Bisphenol A exposure in zebrafish (Danio rerio) Chemosphere. 2020;256:127080. doi: 10.1016/j.chemosphere.2020.127080. [DOI] [PubMed] [Google Scholar]
- Martínez R., et al. Transcriptomic effects of tributyltin (TBT) in zebrafish eleutheroembryos. A functional benchmark dose analysis. J. Hazard Mater. 2020:122881. doi: 10.1016/j.jhazmat.2020.122881. [DOI] [PubMed] [Google Scholar]
- Martínez R., et al. Morphometric signatures of exposure to endocrine disrupting chemicals in zebrafish eleutheroembryos. Aquat. Toxicol. 2019;214:105232. doi: 10.1016/j.aquatox.2019.105232. [DOI] [PubMed] [Google Scholar]
- Martínez R., et al. Unravelling the mechanisms of PFOS toxicity by combining morphological and transcriptomic analyses in zebrafish embryos. Sci. Total Environ. 2019;674:462–471. doi: 10.1016/j.scitotenv.2019.04.200. [DOI] [PubMed] [Google Scholar]
- Marlatt, V., et al. Evidence for a generalized decreased of the reproductive status in wildlife and in human (including the sex dymorphism effect). Environ. Res..
- McArdle M.E., et al. Critical review of read‐across potential in testing for endocrine‐related effects in vertebrate ecological receptors. Environ. Toxicol. Chem. 2020;39:739–753. doi: 10.1002/etc.4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil P.L., et al. Physiological and behavioral effects of exposure to environmentally relevant concentrations of prednisolone during zebrafish (Danio rerio) embryogenesis. Environ. Sci. Technol. 2016;50:5294–5304. doi: 10.1021/acs.est.6b00276. [DOI] [PubMed] [Google Scholar]
- Mellor C.L., et al. The identification of nuclear receptors associated with hepatic steatosis to develop and extend adverse outcome pathways. Crit. Rev. Toxicol. 2016;46:138–152. doi: 10.3109/10408444.2015.1089471. [DOI] [PubMed] [Google Scholar]
- Melzer D., et al. Urinary bisphenol A concentration and angiography-defined coronary artery stenosis. PloS One. 2012;7 doi: 10.1371/journal.pone.0043378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer D., et al. Association of urinary bisphenol A concentration with heart disease: evidence from NHANES 2003/06. PloS One. 2010;5 doi: 10.1371/journal.pone.0008673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe, C., et al. State-of-the-art monitoring, chemical analysis,and environmental levels of EDCs. Environ. Res..
- Migliarini B., et al. Perspectives on endocrine disruptor effects on metabolic sensors. Gen. Comp. Endocrinol. 2011;170:416–423. doi: 10.1016/j.ygcen.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Mimoto M.S., et al. Polluted pathways: mechanisms of metabolic disruption by endocrine disrupting chemicals. Current environmental health reports. 2017;4:208–222. doi: 10.1007/s40572-017-0137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes-Grajales D., Olivero-Verbel J. Computer-aided identification of novel protein targets of bisphenol A. Toxicol. Lett. 2013;222:312–320. doi: 10.1016/j.toxlet.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Moon S., et al. Effects of bisphenol A on cardiovascular disease: an epidemiological study using National Health and Nutrition Examination Survey 2003-2016 and meta-analysis. Sci. Total Environ. 2020:142941. doi: 10.1016/j.scitotenv.2020.142941. [DOI] [PubMed] [Google Scholar]
- Moreman J., et al. Estrogenic mechanisms and cardiac responses following early life exposure to bisphenol A (BPA) and its metabolite 4-Methyl-2,4-bis( p-hydroxyphenyl)pent-1-ene (MBP) in zebrafish. Environ. Sci. Technol. 2018;52:6656–6665. doi: 10.1021/acs.est.8b01095. [DOI] [PubMed] [Google Scholar]
- Moroni L., et al. SCREENED: a multistage model of thyroid gland function for screening endocrine-disrupting chemicals in a biologically sex-specific manner. Int. J. Mol. Sci. 2020;21:3648. doi: 10.3390/ijms21103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer B.J., et al. Obesity and fatty liver are prevented by inhibition of the aryl hydrocarbon receptor in both female and male mice. Nutr. Res. (N.Y.) 2017;44:38–50. doi: 10.1016/j.nutres.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X., Huang Y., Li J., Yang K., Yang W., Shen G., Li X., Lei Y., Pang S., Wang C., Li X. New insights into the mechanism of phthalate-induced developmental effects. Environ. Pollut. 2018;241:674–683. doi: 10.1016/j.envpol.2018.05.095. [DOI] [PubMed] [Google Scholar]
- Nadal A., et al. Endocrine-disrupting chemicals and the regulation of energy balance. Nat. Rev. Endocrinol. 2017;13:536. doi: 10.1038/nrendo.2017.51. [DOI] [PubMed] [Google Scholar]
- Nebert D.W. Aryl hydrocarbon receptor (AHR):“pioneer member” of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of “sensors” of foreign and endogenous signals. Prog. Lipid Res. 2017;67:38–57. doi: 10.1016/j.plipres.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton R., et al. Glucocorticoid and cytokine crosstalk: feedback, feedforward, and co-regulatory interactions determine repression or resistance. J. Biol. Chem. 2017;292:7163–7172. doi: 10.1074/jbc.R117.777318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa J.-i., et al. Involvement of the retinoid X receptor in the development of imposex caused by organotins in gastropods. Environ. Sci. Technol. 2004;38:6271–6276. doi: 10.1021/es049593u. [DOI] [PubMed] [Google Scholar]
- Nowak K., et al. Immunomodulatory effects of synthetic endocrine disrupting chemicals on the development and functions of human immune cells. Environ. Int. 2019;125:350–364. doi: 10.1016/j.envint.2019.01.078. [DOI] [PubMed] [Google Scholar]
- Ohlstein J.F., et al. Bisphenol A enhances adipogenic differentiation of human adipose stromal/stem cells. J. Mol. Endocrinol. 2014;53:345. doi: 10.1530/JME-14-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsén L., et al. Associations between circulating levels of bisphenol A and phthalate metabolites and coronary risk in the elderly. Ecotoxicol. Environ. Saf. 2012;80:179–183. doi: 10.1016/j.ecoenv.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Ortiz-Villanueva E., et al. Assessment of endocrine disruptors effects on zebrafish (Danio rerio) embryos by untargeted LC-HRMS metabolomic analysis. Sci. Total Environ. 2018;635:156–166. doi: 10.1016/j.scitotenv.2018.03.369. [DOI] [PubMed] [Google Scholar]
- Ortiz-Villanueva E., et al. Metabolic disruption of zebrafish (Danio rerio) embryos by bisphenol A. An integrated metabolomic and transcriptomic approach. Environ. Pollut. 2017;231:22–36. doi: 10.1016/j.envpol.2017.07.095. [DOI] [PubMed] [Google Scholar]
- Park C., et al. The mixture effects of bisphenol derivatives on estrogen receptor and androgen receptor. Environ. Pollut. 2020;260:114036. doi: 10.1016/j.envpol.2020.114036. [DOI] [PubMed] [Google Scholar]
- Patel B.B., et al. Metabolic response to chronic bisphenol A exposure in C57bl/6n mice. Toxicology Reports. 2014;1:522–532. doi: 10.1016/j.toxrep.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden-Adams M.M., et al. Developmental toxicity in white leghorn chickens following in ovo exposure to perfluorooctane sulfonate (PFOS) Reprod. Toxicol. 2009;27:307–318. doi: 10.1016/j.reprotox.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Perkins E.J., et al. Building and applying quantitative adverse outcome pathway models for chemical hazard and risk assessment. Environ. Toxicol. Chem. 2019;38:1850–1865. doi: 10.1002/etc.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto C., et al. Differential activity of BPA, BPAF and BPC on zebrafish estrogen receptors in vitro and in vivo. Toxicol. Appl. Pharmacol. 2019;380:114709. doi: 10.1016/j.taap.2019.114709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante, I., et al. Killing two birds with one stone: pregnancy is a sensitive window for endocrine effects on both the mother and the fetus. Environ. Res.. [DOI] [PubMed]
- Posnack N.G., et al. Exposure to phthalates affects calcium handling and intercellular connectivity of human stem cell-derived cardiomyocytes. PloS One. 2015;10 doi: 10.1371/journal.pone.0121927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posnack Nikki G., et al. Phthalate exposure changes the metabolic profile of cardiac muscle cells. Environ. Health Perspect. 2012;120:1243–1251. doi: 10.1289/ehp.1205056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu S.Y., et al. Effects of phthalate acid esters on zebrafish larvae: development and skeletal morphogenesis. Chemosphere. 2020;246:125808. doi: 10.1016/j.chemosphere.2019.125808. [DOI] [PubMed] [Google Scholar]
- Qiu W., et al. Transcriptomic responses of bisphenol S predict involvement of immune function in the cardiotoxicity of early life-stage zebrafish (Danio rerio) Environ. Sci. Technol. 2020;54:2869–2877. doi: 10.1021/acs.est.9b06213. [DOI] [PubMed] [Google Scholar]
- Rajesh P., Balasubramanian K. Gestational exposure to di (2-ethylhexyl) phthalate (DEHP) impairs pancreatic β-cell function in F1 rat offspring. Toxicol. Lett. 2015;232:46–57. doi: 10.1016/j.toxlet.2014.09.025. [DOI] [PubMed] [Google Scholar]
- Regnault C., et al. Unexpected metabolic disorders induced by endocrine disruptors in Xenopus tropicalis provide new lead for understanding amphibian decline. Proc. Natl. Acad. Sci. Unit. States Am. 2018;115:E4416–E4425. doi: 10.1073/pnas.1721267115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnault C., et al. Metabolic and immune impairments induced by the endocrine disruptors benzo [a] pyrene and triclosan in Xenopus tropicalis. Chemosphere. 2016;155:519–527. doi: 10.1016/j.chemosphere.2016.04.047. [DOI] [PubMed] [Google Scholar]
- Reventun P., et al. Bisphenol A induces coronary endothelial cell necroptosis by activating RIP3/CamKII dependent pathway. Sci. Rep. 2020;10:4190. doi: 10.1038/s41598-020-61014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes H., et al. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992;256:1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- Reynaud S., Deschaux P. The effects of polycyclic aromatic hydrocarbons on the immune system of fish: a review. Aquat. Toxicol. 2006;77:229–238. doi: 10.1016/j.aquatox.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Reynaud S., et al. Toxicokinetic of benzo [a] pyrene and fipronil in female green frogs (Pelophylax kl. esculentus) Environ. Pollut. 2012;161:206–214. doi: 10.1016/j.envpol.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Robitaille, J., et al. Towards regulation of Endocrine-Disrupting Chemicals (EDCs) in water with the use of bioassays – a guide to developing a testing strategy. Environ. Res.. [DOI] [PubMed]
- Rojas I.Y., et al. Reversal of obesity and liver steatosis in mice via inhibition of aryl hydrocarbon receptor and altered gene expression of CYP1B1, PPARα, SCD1, and osteopontin. Int. J. Obes. 2020;44:948–963. doi: 10.1038/s41366-019-0512-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland R.M. A review of chemically-induced alterations in thyroid and vitamin A status from field studies of wildlife and fish. J. Wildl. Dis. 2000;36:615–635. doi: 10.7589/0090-3558-36.4.615. [DOI] [PubMed] [Google Scholar]
- Roman P., et al. Microbiota and organophosphates. Neurotoxicology. 2019;75:200–208. doi: 10.1016/j.neuro.2019.09.013. [DOI] [PubMed] [Google Scholar]
- Rosen E.D., et al. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- Rosenmai A.K., et al. An effect-directed strategy for characterizing emerging chemicals in food contact materials made from paper and board. Food Chem. Toxicol. 2017;106:250–259. doi: 10.1016/j.fct.2017.05.061. [DOI] [PubMed] [Google Scholar]
- Rubin B.S., et al. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ. Health Perspect. 2001;109:675–680. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R.J., Jaeger R.J. Some pharmacologic and toxicologic effects of di-2-ethylhexyl phthalate (DEHP) and other plasticizers. Environ. Health Perspect. 1973;3:53–59. doi: 10.1289/ehp.730353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamanca-Fernández E., et al. Bisphenol A exposure and risk of ischemic heart disease in the Spanish European Prospective Investigation into cancer and nutrition study. Chemosphere. 2020;261:127697. doi: 10.1016/j.chemosphere.2020.127697. [DOI] [PubMed] [Google Scholar]
- Sarath Josh M.K., et al. Phthalates efficiently bind to human peroxisome proliferator activated receptor and retinoid X receptor α, β, γ subtypes: an in silico approach. J. Appl. Toxicol. 2014;34:754–765. doi: 10.1002/jat.2902. [DOI] [PubMed] [Google Scholar]
- Sargis R.M., Simmons R.A. Environmental neglect: endocrine disruptors as underappreciated but potentially modifiable diabetes risk factors. Diabetologia. 2019;62:1811–1822. doi: 10.1007/s00125-019-4940-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., et al. Low-dose dioxins alter gene expression related to cholesterol biosynthesis, lipogenesis, and glucose metabolism through the aryl hydrocarbon receptor-mediated pathway in mouse liver. Toxicol. Appl. Pharmacol. 2008;229:10–19. doi: 10.1016/j.taap.2007.12.029. [DOI] [PubMed] [Google Scholar]
- Seimandi M., et al. Differential responses of PPARalpha, PPARdelta, and PPARgamma reporter cell lines to selective PPAR synthetic ligands. Anal. Biochem. 2005;344:8–15. doi: 10.1016/j.ab.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Shahin N.N., et al. Potential role of aryl hydrocarbon receptor signaling in childhood obesity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020:158714. doi: 10.1016/j.bbalip.2020.158714. [DOI] [PubMed] [Google Scholar]
- Shang J., et al. Recovery from a myocardial infarction is impaired in male C57bl/6 N mice acutely exposed to the bisphenols and phthalates that escape from medical devices used in cardiac surgery. Toxicol. Sci. 2019;168:78–94. doi: 10.1093/toxsci/kfy276. [DOI] [PubMed] [Google Scholar]
- Sharma S., et al. In silico molecular interaction of bisphenol analogues with human nuclear receptors reveals their stronger affinity vs. classical bisphenol A. Toxicol. Mech. Methods. 2018;28:660–669. doi: 10.1080/15376516.2018.1491663. [DOI] [PubMed] [Google Scholar]
- Simhadri J.J., et al. Biomarkers of metabolic disorders and neurobehavioral diseases in a PCB-exposed population: what we learned and the implications for future research. Environ. Res. 2020:110211. doi: 10.1016/j.envres.2020.110211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipes N.S., et al. Predictive models of prenatal developmental toxicity from ToxCast high-throughput screening data. Toxicol. Sci. 2011;124:109–127. doi: 10.1093/toxsci/kfr220. [DOI] [PubMed] [Google Scholar]
- Snijder C.A., et al. Congenital heart defects and parental occupational exposure to chemicals. Hum. Reprod. 2012;27:1510–1517. doi: 10.1093/humrep/des043. [DOI] [PubMed] [Google Scholar]
- Sol C.M., et al. Associations of maternal phthalate and bisphenol urine concentrations during pregnancy with childhood blood pressure in a population-based prospective cohort study. Environ. Int. 2020;138:105677. doi: 10.1016/j.envint.2020.105677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinu N., et al. Quantitative adverse outcome pathway (qAOP) models for toxicity prediction. Arch. Toxicol. 2020;94:1497. doi: 10.1007/s00204-020-02774-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroustrup A., et al. Sources of clinically significant neonatal intensive care unit phthalate exposure. J. Expo. Sci. Environ. Epidemiol. 2020;30:137–148. doi: 10.1038/s41370-018-0069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Y., Park S.H., Wang F., Zhou C. Perinatal bisphenol A exposure increases atherosclerosis in adult male PXR-humanized mice. Endocrinology. 2018;159(4):1595–1608. doi: 10.1210/en.2017-03250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G., Li Y. Exposure to DBP induces the toxicity in early development and adverse effects on cardiac development in zebrafish (Danio rerio) Chemosphere. 2019;218:76–82. doi: 10.1016/j.chemosphere.2018.11.095. [DOI] [PubMed] [Google Scholar]
- Sun G., Liu K. Developmental toxicity and cardiac effects of butyl benzyl phthalate in zebrafish embryos. Aquat. Toxicol. 2017;192:165–170. doi: 10.1016/j.aquatox.2017.09.020. [DOI] [PubMed] [Google Scholar]
- Sun L., et al. Differential mechanisms regarding triclosan vs. bisphenol A and fluorene-9-bisphenol induced zebrafish lipid-metabolism disorders by RNA-Seq. Chemosphere. 2020:126318. doi: 10.1016/j.chemosphere.2020.126318. [DOI] [PubMed] [Google Scholar]
- Tang C., et al. Placental P-glycoprotein inhibition enhances susceptibility to Di-(2-ethylhexyl)-phthalate induced cardiac malformations in mice: a possibly promising target for congenital heart defects prevention. PloS One. 2019;14 doi: 10.1371/journal.pone.0214873. e0214873-e0214873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y., et al. When Pharmaceutical Drugs Become Environmental Pollutants: Potential Neural Effects and Underlying Mechanisms Environ Res the Gills of Adult Zebrafish. vol. 266. Environmental Pollution; (Danio rerio): 2020. Monobutyl phthalate (MBP) induces energy metabolism disturbances in Vaudin, P., et al; p. 115288. [DOI] [PubMed] [Google Scholar]
- Tarnow P., et al. Chemical activation of estrogen and aryl hydrocarbon receptor signaling pathways and their interaction in toxicology and metabolism. Expet Opin. Drug Metabol. Toxicol. 2019;15:219–229. doi: 10.1080/17425255.2019.1569627. [DOI] [PubMed] [Google Scholar]
- Thambirajah, A.A., et al. Disruption by stealth - interference of Endocrine-Disrupting Chemicals on hormonal crosstalk with thyroid axis function in humans and other animals. Environ. Res. [DOI] [PubMed]
- Tq D., et al. In vitro profiling of the potential endocrine disrupting activities affecting steroid and aryl hydrocarbon receptors of compounds and mixtures prevalent in human drinking water resources. Chemosphere. 2020:127332. doi: 10.1016/j.chemosphere.2020.127332. [DOI] [PubMed] [Google Scholar]
- Tyler C., et al. Endocrine disruption in wildlife: a critical review of the evidence. Crit. Rev. Toxicol. 1998;28:319–361. doi: 10.1080/10408449891344236. [DOI] [PubMed] [Google Scholar]
- Usal M., et al. Concomitant exposure to benzo [a] pyrene and triclosan at environmentally relevant concentrations induces metabolic syndrome with multigenerational consequences in Silurana (Xenopus) tropicalis. Sci. Total Environ. 2019;689:149–159. doi: 10.1016/j.scitotenv.2019.06.386. [DOI] [PubMed] [Google Scholar]
- Usal M., et al. Transgenerational metabolic disorders and reproduction defects induced by benzo [a] pyrene in Xenopus tropicalis. Environ. Pollut. 2020:116109. doi: 10.1016/j.envpol.2020.116109. [DOI] [PubMed] [Google Scholar]
- Usal M., et al. Transgenerational metabolic disorders and reproduction defects induced by benzo [a] pyrene in Xenopus tropicalis. Environ. Pollut. 2021;269:116109. doi: 10.1016/j.envpol.2020.116109. [DOI] [PubMed] [Google Scholar]
- van der Valk E.S., et al. Stress and obesity: are there more susceptible individuals? Current Obesity Reports. 2018;7:193–203. doi: 10.1007/s13679-018-0306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermij S.H., et al. Refining the molecular organization of the cardiac intercalated disc. Cardiovasc. Res. 2017;113:259–275. doi: 10.1093/cvr/cvw259. [DOI] [PubMed] [Google Scholar]
- Villeneuve D.L., et al. High‐throughput screening and environmental risk assessment: state of the science and emerging applications. Environ. Toxicol. Chem. 2019;38:12–26. doi: 10.1002/etc.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T., et al. Aryl hydrocarbon receptor plays protective roles against high fat diet (HFD)-induced hepatic steatosis and the subsequent lipotoxicity via direct transcriptional regulation of Socs3 gene expression. J. Biol. Chem. 2016;291:7004–7016. doi: 10.1074/jbc.M115.693655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., et al. Maternal PPARγ protects nursing neonates by suppressing the production of inflammatory milk. Gene Dev. 2007;21:1895–1908. doi: 10.1101/gad.1567207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., et al. Parental occupational exposures to endocrine disruptors and the risk of simple isolated congenital heart defects. Pediatr. Cardiol. 2015;36:1024–1037. doi: 10.1007/s00246-015-1116-6. [DOI] [PubMed] [Google Scholar]
- Wang H., et al. Modulation of heat-shock response is associated with Di (2-ethylhexyl) phthalate (DEHP)-induced cardiotoxicity in quail (Coturnix japonica) Chemosphere. 2019;214:812–820. doi: 10.1016/j.chemosphere.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Wang H., et al. Human exposure of bisphenol A and its analogues: understandings from human urinary excretion data and wastewater-based epidemiology. Environ. Sci. Pollut. Control Ser. 2020;27:3247–3256. doi: 10.1007/s11356-019-07111-9. [DOI] [PubMed] [Google Scholar]
- Wang Z.Z., et al. [Study on the effect of oxidative stress on the cardiac injury induced by MEHP in rats] Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2018;36:485–491. doi: 10.3760/cma.j.issn.1001-9391.2018.07.002. [DOI] [PubMed] [Google Scholar]
- Wei J., et al. Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology. 2011;152:3049–3061. doi: 10.1210/en.2011-0045. [DOI] [PubMed] [Google Scholar]
- Wójtowicz A.K., et al. The action of di-(2-ethylhexyl) phthalate (DEHP) in mouse cerebral cells involves an impairment in aryl hydrocarbon receptor (AhR) signaling. Neurotox. Res. 2019;35:183–195. doi: 10.1007/s12640-018-9946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wójtowicz A.K., et al. Dibutyl phthalate (DBP)-Induced apoptosis and neurotoxicity are mediated via the aryl hydrocarbon receptor (AhR) but not by estrogen receptor alpha (ERα), estrogen receptor beta (ERβ), or peroxisome proliferator-activated receptor gamma (PPARγ) in mouse cortical neurons. Neurotox. Res. 2017;31:77–89. doi: 10.1007/s12640-016-9665-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolmarans N.J., et al. Linking organochlorine exposure to biomarker response patterns in Anurans: a case study of Müller’s clawed frog (Xenopus muelleri) from a tropical malaria vector control region. Ecotoxicology. 2018;27:1203–1216. doi: 10.1007/s10646-018-1972-y. [DOI] [PubMed] [Google Scholar]
- Wu Y., et al. The role of neuropeptide Y and peptide YY in the development of obesity via gut-brain Axis. Curr. Protein Pept. Sci. 2019;20:750–758. doi: 10.2174/1389203720666190125105401. [DOI] [PubMed] [Google Scholar]
- Wung P.K., et al. Effects of glucocorticoids on weight change during the treatment of Wegener's granulomatosis. Arthritis Care Res. 2008;59:746–753. doi: 10.1002/art.23561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P., et al. Functional toxicogenomic assessment of triclosan in human HepG2 cells using genome-wide CRISPR-Cas9 screening. Environ. Sci. Technol. 2016;50:10682–10692. doi: 10.1021/acs.est.6b02328. [DOI] [PubMed] [Google Scholar]
- Xu C.-X., et al. Aryl hydrocarbon receptor deficiency protects mice from diet-induced adiposity and metabolic disorders through increased energy expenditure. Int. J. Obes. 2015;39:1300–1309. doi: 10.1038/ijo.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., et al. The adverse effects of perinatal exposure to nonylphenol on carbohydrate metabolism in male offspring rats. Int. J. Environ. Health Res. 2017;27:368–376. doi: 10.1080/09603123.2017.1373275. [DOI] [PubMed] [Google Scholar]
- Yang Q., et al. Peroxisome proliferator-activated receptor α activation during pregnancy severely impairs mammary lobuloalveolar development in mice. Endocrinology. 2006;147:4772–4780. doi: 10.1210/en.2006-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., et al. Use of embryonic stem cell-derived cardiomyocytes to study cardiotoxicity of bisphenol AF via the GPER/CAM/eNOS pathway. Toxicology. 2020;432:152380. doi: 10.1016/j.tox.2020.152380. [DOI] [PubMed] [Google Scholar]
- Zhang L., et al. Metabolomics reveals that aryl hydrocarbon receptor activation by environmental chemicals induces systemic metabolic dysfunction in mice. Environ. Sci. Technol. 2015;49:8067–8077. doi: 10.1021/acs.est.5b01389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., et al. BPA disrupts 17-estradiol-mediated hepatic protection against ischemia/reperfusion injury in rat liver by upregulating the Ang II/AT1R signaling pathway. Mol. Med. Rep. 2020;22:416–422. doi: 10.3892/mmr.2020.11072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K., et al. Aryl hydrocarbon receptor pathway: role, regulation and intervention in atherosclerosis therapy. Mol. Med. Rep. 2019;20:4763–4773. doi: 10.3892/mmr.2019.10748. [DOI] [PMC free article] [PubMed] [Google Scholar]