Summary
Background
Obesity, cancer and diabetes frequently coexist. The association of glycaemic variability (GV) and obesity with cancer events had not been explored in diabetes.
Methods
In the prospective Hong Kong Diabetes Register cohort (1995-2019), we used cox proportional hazards models to examine the risk associations of GV with all-site cancer (primary outcome) and cause-specific death (secondary outcome). We also explored the joint association of obesity and GV with these outcomes and site-specific cancer. We expressed GV using HbA1c variability score (HVS) defined as percentage of HbA1c values varying by 0.5% compared with values in preceding visit.
Findings
We included 15,286 patients (type 2 diabetes: n=15,054, type 1 diabetes: n=232) with ≥10 years of diabetes and ≥3 years of observation (51.7% men, age (mean±SD): 61.04±10.73 years, HbA1c: 7.54±1.63%, body mass index [BMI]: 25.65±3.92 kg/m2, all-site cancer events: n=928, cancer death events: n=404). There were non-linear relationships between HVS and outcomes but there was linearity within the high and low HVS groups stratified by the median (IQR) value of HVS (42.31 [27.27, 56.28]). In the high HVS group, the adjusted hazard ratios (aHR) of each SD of HVS was 1.15 (95% CI: 1.04, 1.26) for all-site cancer (n=874). The respective aHRs for breast (n=77), liver (n=117) and colorectal (n=184) cancer were 1.44 (1.07, 1.94), 1.37 (1.08, 1.74), and 1.09 (0.90, 1.32). In the high GV group, the respective aHRs were 1.21 (1.06, 1.39), 1.27 (1.15, 1.40), and 1.15 (1.09, 1.22) for cancer, vascular, and noncancer nonvascular death. When stratified by obesity (BMI ≥25 kg/m2), the high HVS & obese group had the highest aHRs of 1.42 (1.16, 1.73), 2.44 (1.24, 4.82), and 2.63 (1.45, 4.74) respectively for all-site, breast, and liver cancer versus the low GV & non-obese group. The respective aHRs were 1.45 (1.07, 1.96), 1.47 (1.12, 1.93), and 1.35 (1.16, 1.57) for cancer, vascular, and noncancer nonvascular death.
Interpretation
Obesity and high GV were associated with increased risk of all-site, breast, liver cancer, and cancer-specific death in T2D.
Funding
The Chinese University of Hong Kong Diabetes Research Fund
Keywords: diabetes, glycaemic variability, obesity, cancer and all cause death
Abbreviation: aHR, adjusted hazard ratio; ALT, alanine aminotransferase; BMI, body mass index; BP, blood pressure; CI, confidence interval; CVD, cardiovascular disease; EMR, electronic medical record; GV, glycaemic variability; HA, Hospital Authority; HDLC, high-density lipoprotein cholesterol; HKDR, Hong Kong Diabetes Register; HR, hazard ratio; HVS, HbA1c variability score; IQR, inter‐quartile range; LDLC, low-density lipoprotein cholesterol; LLD, lipid lowering drug; MD, median; Mn, mean; OGLDs, oral glucose lowering drugs; RAS, renin angiotensin system; SD, standard deviation; SDIM, SD independent of mean; T2D, type 2 diabetes; TC, total cholesterol; TG, triglyceride
Research in context.
Evidence before this study
We searched MEDLINE, PubMed, and relevant references using the terms “HbA1c variability”, "diabetes", and “cancer”. Articles published in English up to September 31, 2020, were included. We found only one published observational study on the risk association of HbA1c variability with cancer in patients with type 2 diabetes. This study included 2,640 patients observed for a mean period of 4.1 years and used standard deviation (SD) of all HbA1c measures to define GV for association analysis.
Added value of this study
In this long-term prospective study of patients with diabetes for at least 10 years, GV estimated by glycated haemoglobin (A1c) variability score (HVS), SD of A1c (SD_A1c), and SD independent of mean (SDIM) was associated with increased risk of all-site cancer, liver and breast cancer, and cancer-specific death. Stratified by the median of HVS, the high GV & obese group had the highest risk for all-site, site-specific cancer and cancer death compared with the low GV & low BMI group. One in four patients belonged to this high-risk group, in whom optimizing glycaemic control and body weight might reduce the risk of cancer and cancer death.
Implications of all available evidence
Cancer is a leading cause of death in diabetes. Our results suggested optimizing control of body weight and glycaemia in obese patients with fluctuating glycaemic control might reduce the growing burden of cancer in diabetes.
Alt-text: Unlabelled box
Introduction
Cancer is emerging as a leading cause of death in diabetes1 which was associated with 1.5-2 folds increased risk of all-site cancer2 except for prostate.3 In a review of 18 meta-analyses, 74% of the meta-analyses supported the risk association of diabetes with cancer although only 26% of the compiled evidence had more than 1,000 cases. Besides, the nature of the diabetes-cancer association remained unclear.4 Wide glycaemic excursion induced oxidative stress, inflammation, and endothelial dysfunction.5,6 Suboptimal quality of care and self-management could lead to wide glycaemic variability (GV) and poor outcomes.7 Herein, one-time blood glucose measure could not capture the time-varying nature of glycaemic levels.8 Long term GV based on multiple measurements of clinic-based glycated haemoglobin (HbA1c) values was associated with hospitalizations, macro/microvascular complications9 and premature mortality10 in both type 1 and type 2 diabetes (T2D).11,12 To date, only one study including 2,640 patients with T2D observed for 4.1 years reported association of cancer risk with GV expressed as standard deviation (SD) of mean HbA1c.13
Diabetes, obesity and cancer frequently coexist. In a population-based study from Sweden, obesity was associated with an increased risk of 15 types of cancer with the highest standardized incidence ratio of 3.3 for Hodgkin lymphoma in men.14 In a meta-analysis including 221 datasets, every 5 kg/m2 increase in body mass index (BMI) was associated with increased risks of common and rare malignancies.15 Besides, obesity is a strong risk factor for vascular complications and premature mortality in diabetes.16 Both hyperglycaemia and obesity shared common pathways such as oxidative stress, inflammation and endothelial dysfunction to cause organ damage5,6 although their joint associations with all-site cancer and cancer-related death have not been explored.
Hong Kong has a universal healthcare system with all publicly-funded hospitals and clinics operated by the Hospital Authority which shared the same electronic medical record (EMR) system. Due to the non-compulsory nature of private insurance, the majority of patients with chronic diseases requiring long term medications and hospitalizations due to acute illness are managed in the public sector. The Hong Kong Diabetes Register (HKDR), established since 1995, is a research-driven quality improvement program consisting of periodic structured assessments where patients consented to having their data collected for research and publication purpose. The HKDR is linked to the territory-wide EMR system with hospitalization, laboratory, and death records using a unique identifier.17 We leveraged the long history of HKDR with repeat HbA1c measurements and curated a subgroup of patients with at least 10 years of diabetes duration before cancer occurred or censor date to evaluate the risk association of GV with all-site (primary outcome) and cancer-specific death (secondary outcome). We also explored the joint risk association of obesity and GV with these outcomes and site-specific cancer.
Methods
Patients
Since 1995, every week, 30-50 patients were referred from hospital- and community-based clinics to the Diabetes Centre at the Prince of Wales Hospital (PWH) to undergo comprehensive assessment. Given the 20-year history of HKDR2 and in light of the long latent period before cancer occurrence, we selected patients with at least 10 years of disease duration as a reasonable time frame for risk analysis of cancer events. Other inclusion criteria were Chinese ethnicity and no prior history of cancer. Based on published methodology11 , we included patients with at least 5 HbA1c measurements, observation for at least 3 years and no missing data for multivariate analysis (Supplementary Figure 1A). The study was approved by the Joint Chinese University of Hong Kong—New Territories East Cluster Clinical Research Ethics Committee with adherence to the Declaration of Helsinki.
Baseline measurement
The HKDR protocol for comprehensive assessment (eye, feet, blood and urine) after an overnight fast was adapted from the European DiabCare protocol.18 Types of diabetes was based on physician diagnosis with T1D defined as presentation with ketoacidosis and/or continuous requirement of insulin within one year of diagnosis. All other patients were considered to have T2D. Since increased risk of cancer and association of GV with diabetes complications had been reported in T1D and T2D,2 we included both types of diabetes in our analysis. Structured record forms were used to document demographics, clinical measurements (e.g. blood pressure [BP]), body weight, height and waist circumference), medical history, drug usage and laboratory results (HbA1c, plasma glucose, lipid profile (total cholesterol [TC], high-density lipoprotein cholesterol [HDLC], low-density lipoprotein cholesterol [LDLC], and triglyceride [TG]), renal and liver function) for definition of micro/macroalbuminuria, hypertension, retinopathy, and neuropathy (supplementary methods). We used BMI ≥25 kg/m2 to define obesity in Asians according to the World Health Organization.19
HbA1c measurement during follow‐up
We retrieved all HbA1c values measured during outpatient and inpatient settings from the day of enrolment to the HKDR until the first hospitalization with cancer, death, or censor date of 31st December 2019, whichever came earlier.
Outcome definition
We used ICD-9 codes (140-209) to identify first hospitalization due to all-site cancer (primary outcome). Prostate cancer (code 185) was excluded from analysis of cancer incidence due to its negative risk association with diabetes.3 We explored risk analysis of 3 cancer types including breast, liver, and colorectal cancer (174-175, 155, 153-154) in the HKDR, but not for other cancer types due to small sample size. For the secondary outcome, we used ICD-10 codes to classify death due to cancer (C00-97), vascular (I00-99), and noncancer, nonvascular causes (J, N, A, B, K, L, S, T, V, W, X, and Y).20 Prostate cancer was included in the analysis of cancer death due to association of diabetes with increased risk of prostate cancer-specific death.21
Statistical analysis
Assuming a 4% prevalence for all-site cancer in patients with long duration of T2D22 and a hazard ratio (HR) of 1.27 associated with GV index for all-site cancer,13 a sample size of 15,098 would give a power of 0.8 with an alpha (p) value less than 0.05 to test the primary hypothesis of association of GV with all-site cancer. In this analysis, 15,286 patients fulfilled the criteria and were included in our multivariate analysis. All other analyses were exploratory and hypothesis-generating due to small sample size.
We used mean±standard deviation (SD) or median (inter‐quartile range [IQR]) to describe continuous variables, and proportions to describe categorical variables. The follow‐up time lasted from date of enrolment to date of first cancer diagnosis, death, or censor date of 31 December 2019, whichever came first. We calculated and expressed GV using HbA1c variability score (HVS),11 SD of HbA1c (SD_A1c), and SD independent of mean (SDIM).10 The HVS11 measured the number of HbA1c values in a patient where the HbA1c had changed by 0.5% compared with the previous value. SDIM was calculated as 100 × SD/meanb, where b is the regression coefficient based on natural logarithm of SD on natural logarithm of time-weighted average HbA1c (mA1c) (Supplementary figure 2). The mA1c was calculated using trapezoidal integration of the area under the curve (AUC) of HbA1c from baseline to the first event divided by the observation period.11 We included patients with at least 5 HbA1c measurements for more precise calculation of AUC and HVS.11 We excluded 3 years of HbA1c measurements prior to first cancer event to avoid reverse causality.
We performed cubic spline analysis with 3-knot (25th, 50th, and 75th percentiles) of HVS to assess its linearity with all-site, breast, liver, colorectal cancer, and cause-specific death. There was non-linearity between HVS and main outcomes in the whole group but linearity within the high and low HVS group stratified by the median value of HVS. Thus, within each group, we analysed HVS as a continuous variable and estimated the HR of each SD of HVS with the outcomes. We used multivariate Cox regression models adjusted for confounders to estimate HR with 95% confidence interval (CI), after confirming no violation of the proportional hazard assumption using Schoenfeld residuals. Covariates were selected based on prior knowledge including age, sex, disease duration, and mA1c in model 1. Model 2 was additionally adjusted for BMI, HDLC, TG (quartiles), LDLC, alanine aminotransferase (ALT), alcohol and tobacco use, estimated glomerular filtration rate (eGFR), microalbuminuria, macroalbuminuria, use of oral glucose lowering drugs (OGLDs), insulin, lipid lowering drugs (LLDs), and renin angiotensin system inhibitors (RASi), history of cardiovascular disease (CVD) and heart failure at baseline.
We explored the joint risk association of obesity and HVS with the outcomes. We used the low GV and non-obesity group as the reference group and reported the HRs in different categories. We used Fine-Gray competing risk model to adjust for all-cause death in the analysis of all-site cancer and, cause-specific death in the analysis of cancer-specific death. We explored the risk association of SD_A1c and SDIM with all-site cancer and cancer-specific death. We stratified patients by baseline HbA1c at 7% to evaluate the association of SD of HVS with outcomes in the high and low HVS group. We repeated the risk analyses of HVS in all patients irrespective of disease duration and in patients with T2D only. Patients with missing covariates for multivariate analysis were excluded to produce unbiased estimates and conservative results.23 We compared the baseline clinical characteristics between patients with and without missing data. We used R statistical software (version 4.0.3, U.S) to perform the analysis. A p value (2-sided) less than 0.05 was considered significant.
Role of the funding source
The funders had no role in study design, data collection, data analysis, interpretation, and writing of the manuscript.
Results
After excluding patients with a history of cancer (n=2,047) and before curating a subgroup for risk analysis, 2,237 patients (264,096 patient-years) developed cancer with an incidence rate of 8.47 per 1,000 patient-years. In the selected cohort of 15,286 patients with diabetes (T2D: n=15,054, T1D: n=232), 928 patients developed cancer after a median (IQR) follow-up period of 11.91 (8.26, 15.26) years (181,990 patient-years) with an incidence rate of 5.10 per 1,000 patient-years. The risk of liver, pancreas, endometrium, colon/rectum, breast, and bladder cancer is known to be increased in patients with diabetes. Other cancers including lung and genitourinary other than bladder cancer did not appear to be associated with an increased risk in diabetes.2 In the top 6 cancer types, we selected colorectal (n=184), liver (n=117), and breast cancer (n=77) for sub-analysis. For other cancer types including pancreatic cancer (n=35), the small sample size did not allow subgroup analysis. (supplementary Figure 1B). Amongst 928 patients with cancer events, 349 patients died from cancer. The median diabetes duration was 19.17 (14.59, 25.04) years and the median number of HbA1c measurements was 17.10,27 The median value of number of HbA1c measurements per year was 2.38 (1.45, 3.29).
Clinical characteristics
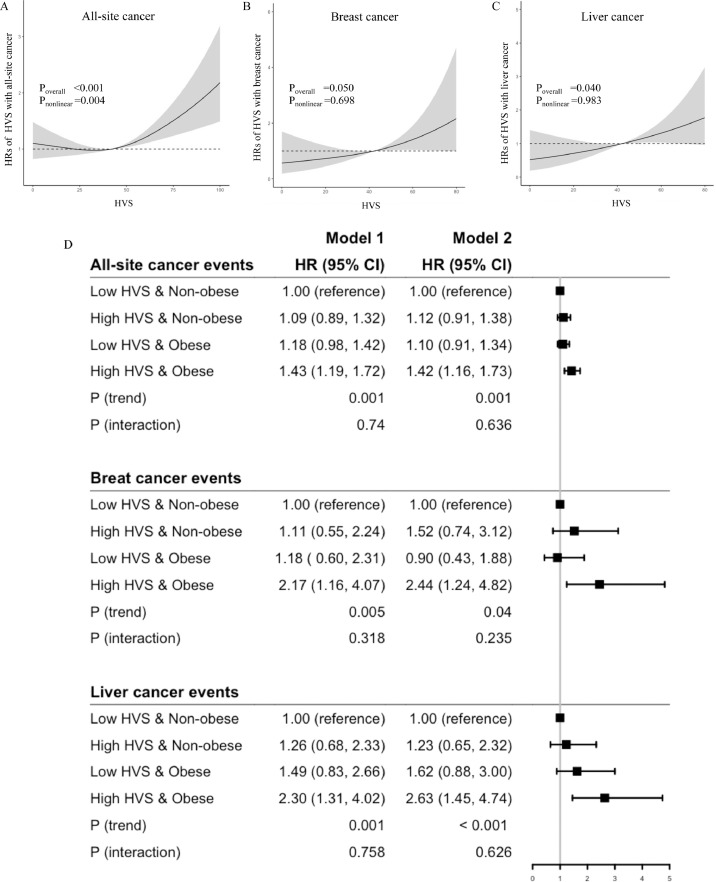
Spline analysis indicated non-linearity between HVS and risk of all-site cancer (Poverall <0.001, Pnonlinear =0.004) (Figure 1A). When stratified by the median value of HVS (42.31 [27.27, 56.28]), there was linearity within the high HVS and low HVS groups. Table 1 shows the clinical profiles and outcomes between the low and high GV groups. The latter group was younger, had longer diabetes duration, and was less likely to have a family history of diabetes. Overall, they had worse risk factors with higher usage of OGLDs and insulin but were less likely to use BP lowering drugs and LLDs and more likely to die (Table 1). The excluded patients were more likely to be men. Overall, they had worse risk profiles and more comorbidities but less likely to be treated with OGLDs, insulin, and RASi and had higher death rates (supplementary table 1).
Figure 1.
Cubic spline analysis of hazard ratios (HR) with HbA1c variability score (HVS) for all-site (A), breast (B) and liver cancer events (C). There was non-linearity of HVS with all-site cancer but linearity within the high HVS and low HVS group stratified by median of HVS. The joint associations of obesity (body mass index ≥25 kg/m2) and HVS stratified by median value of HVS were expressed as forest plots for all-site, breast, and liver cancer events, where low HVS & Non-obese group was used as the reference group (D).
Model 1: adjusted for time weighted mean A1c (mA1c), age, sex, and disease duration. Model 2: Model 1 plus BMI, use of tobacco and alcohol, HDL-cholesterol (HDLC), triglyceride (TG) (quantiles), LDL-cholesterol (LDLC), Alanine transferase (ALT), estimated glomerular filtration rate (eGFR), microalbuminuria, and macroalbuminuria, use of oral glucose lowering drugs (OGLDs), insulin, lipid lowering drugs (LLDs), and renin angiotensin system inhibitors (RASi), and history of cardiovascular disease (CVD) and heart failure. HRs are expressed with 95% CIs in parentheses.
Table 1.
Baseline clinical characteristics and glycaemic indexes in patients with diabetes and disease duration ≥ 10 years stratified by HbA1c variability score (HVS) median value followed up between 1995 and 2019
| Low HVS group (< 42.31) | High HVS group (≥ 42.31) | |
|---|---|---|
| n | 7662 | 7624 |
| Clinical profiles at baseline | ||
| Age (years) | 59.04 (11.59) | 58.11 (12.95) |
| Men (n, %) | 3846 (50.2) | 3910 (51.3) |
| Diabetes duration at baseline (years) | 7.56 (6.79) | 9.48 (7.56) |
| Family history of diabetes (n, %) | 3984 (52.0) | 3795 (49.8) |
| Use of tobacco (n, %) | ||
| Current | 700 (9.1) | 1030 (13.5) |
| Ex | 1351 (17.6) | 1490 (19.5) |
| Non-smoker | 5611 (73.2) | 5104 (66.9) |
| Use of alcohol (n, %) | ||
| Ex | 799 (10.4) | 957 (12.6) |
| Never | 5098 (66.5) | 5198 (68.2) |
| Occasional | 1355 (17.7) | 977 (12.8) |
| Regular | 410 (5.4) | 492 (6.5) |
| Body mass index (kg/m2) | 25.42 (4.00) | 25.75 (4.21) |
| Waist hip ratio | 0.91 (0.07) | 0.92 (0.08) |
| Systolic blood pressure (mmHg) | 133.70 (18.56) | 135.35 (19.53) |
| Diastolic blood pressure (mmHg) | 76.14 (10.54) | 76.38 (10.77) |
| Sensory neuropathy (n, %) | 695 (9.1) | 1149 (15.1) |
| Retinopathy (n, %) | 1608 (21.0) | 2441 (32.0) |
| History of cardiovascular disease (n, %) | 1914 (25.0) | 1803 (23.6) |
| History of heart failure (n, %) | 271 (3.5) | 310 (4.1) |
| Glycaemic variability indexes | ||
| Fasting plasma glucose (mmol/L) | 7.10 [6.10, 8.40] | 8.30 [6.80, 10.40] |
| Baseline HbA1c (%) | 7.03 (1.27) | 8.13 (1.75) |
| HbA1c measurements per year | 2.62 (1.39) | 2.92 (1.57) |
| Time weighted mean A1c (mA1c) (%) | 6.99 (0.74) | 8.08 (1.05) |
| HbA1c variability score (HVS) | 25.34 (11.65) | 58.18 (12.01) |
| Standard deviation of HbA1c (SD_A1c) | 0.55 (0.16) | 1.32 (0.49) |
| Standard deviation independent of mean (SDIM) | 0.38 (0.09) | 0.76 (0.25) |
| Laboratory results at baseline | ||
| Triglyceride (mmol/L) | 1.30 [0.90, 1.80] | 1.40 [1.00, 2.01] |
| Triglyceride quantile (n, %) | ||
| Quantile 1 (0-0.97 mmol/L) | 2110 (27.5) | 1769 (23.2) |
| Quantile 2 (0.97-1.35) | 2018 (26.3) | 1900 (24.9) |
| Quantile 3 (1.35-2.0) | 2072 (27.0) | 2046 (26.8) |
| Quantile 4 (≥ 2.0) | 1462 (19.1) | 1909 (25.0) |
| Total cholesterol (mmol/L) | 4.78 (0.96) | 4.93 (1.07) |
| HDL-cholesterol (mmol/L) | 1.30 [1.10, 1.51] | 1.21 [1.03, 1.50] |
| LDL-cholesterol (mmol/L) | 2.73 (0.91) | 2.86 (1.02) |
| Estimated GFR (ml/min/1.73m2) | 81.96 (21.14) | 80.83 (24.40) |
| Albuminuria (n, %) | 2537 (33.1) | 3556 (46.6) |
| Microalbuminuria (n, %) | 668 (8.7) | 1193 (15.6) |
| Macroalbuminuria (n, %) | 1869 (24.4) | 2363 (31.0) |
| Alanine transferase (mmol/L) | 28.23 (27.92) | 30.72 (35.28) |
| Bilirubin (mmol/L) | 11.38 (5.60) | 10.81 (5.71) |
| Use of medications at baseline | ||
| Oral glucose lowering drugs (n, %) | 6098 (79.6) | 6177 (81.0) |
| Insulin (n, %) | 1010 (13.2) | 2150 (28.2) |
| Lipid lowering drugs (n, %) | 3146 (41.1) | 2703 (35.5) |
| Blood pressure lowering drugs (n, %) | 4498 (58.7) | 4313 (56.6) |
| Renin angiotensin system inhibitors (n, %) | 2798 (36.5) | 2782 (36.5) |
| Follow-up duration | 11.70 (5.02) | 12.12 (5.56) |
| Death (n, %) | 905 (11.8) | 1892 (24.8) |
Mean (SD) or median (IQR) or number (%). GFR: glomerular filtration rate
Risk association of HVS with all-site, breast, liver, and colorectal cancer
In the high GV group, per SD increase of HVS was associated with an adjusted HR (aHR) of 1.15 (1.04, 1.26) for all-site cancer, but not in the low GV group (0.97 [0.87, 1.09]) (table 2). For the three cancer events, there was linearity between HVS and cancer risk (Pnonlinear =0.698 for breast, Pnonlinear =0.983 for liver, Pnonlinear =0.437 for colorectal cancers) (Figure 1B-C, and supplementary figure 3). The linear relationship was significant in liver cancer (Poverall =0.040), but not in breast cancer (Poverall =0.050) and colorectal cancer (Poverall =0.497). For breast, liver, and colorectal cancer, each SD of HVS was associated with aHRs of 1.44 (1.07, 1.94), 1.37 (1.08, 1.74) and 1.09 (0.90, 1.32) respectively (table 2).
Table 2.
Cox regression model on HbA1c variability score (HVS) analysed as a continuous variable either in the whole group or stratified into high and low HVS group by the median value of HVS for all-site, breast, liver and colorectal cancer and cause-specific death in patients with diabetes ≥10 years expressed as hazard ratio for each standard deviation increment of HVS.
| Event/Total | HR (95% CI) | P value | HR (95% CI) | P value | |
|---|---|---|---|---|---|
| All-site cancer events | Model 1 | Model 2 | |||
| Low HVS group | 429/7767 | 1.03 (0.87, 1.09) | 0.609 | 0.97 (0.87, 1.09) | 0.602 |
| High HVS group | 499/7519 | 1.13 (1.03, 1.24) | 0.010 | 1.15 (1.04, 1.26) | 0.006 |
| Site-specific cancer events | |||||
| Breast cancer | 77/15286 | 1.30 (0.97, 1.75) | 0.081 | 1.44 (1.07, 1.94) | 0.017 |
| Liver cancer | 117/15286 | 1.37 (1.09, 1.74) | 0.008 | 1.37 (1.08, 1.74) | 0.010 |
| Colorectal cancer | 184/15286 | 1.08 (0.90, 1.30) | 0.422 | 1.09 (0.90, 1.32) | 0.371 |
| Cause-specific Death | |||||
| Cancer Death | |||||
| Low HVS group | 167/7767 | 0.90 (0.76, 1.08) | 0.270 | 0.88 (0.73, 1.05) | 0.161 |
| High HVS group | 237/7519 | 1.19 (1.05, 1.36) | 0.008 | 1.21 (1.06, 1.39) | 0.005 |
| Vascular Death | |||||
| Low HVS group | 171/7767 | 1.00 (0.83, 1.20) | 0.975 | 0.92 (0.76, 1.10) | 0.361 |
| High HVS group | 410/7519 | 1.27 (1.15, 1.39) | < 0.001 | 1.27 (1.15, 1.40) | < 0.001 |
| Noncancer and Nonvascular Death | |||||
| Low HVS group | 589/7767 | 1.27 (1.15, 1.40) | < 0.001 | 1.16 (1.05, 1.29) | < 0.001 |
| High HVS group | 1243/7519 | 1.18 (1.11, 1.24) | < 0.001 | 1.15 (1.09, 1.22) | 0.004 |
Model 1: adjusted for time weighted mean A1c (mA1c), age, sex, and disease duration. Model 2: Model 1 plus BMI, use of tobacco and alcohol, HDL-cholesterol (HDLC), triglyceride (TG) (quantiles), LDL-cholesterol (LDLC), Alanine transferase (ALT), estimated glomerular filtration rate (eGFR), microalbuminuria, and macroalbuminuria, use of oral glucose lowering drugs (OGLDs), insulin, lipid lowering drugs (LLDs), and renin angiotensin system inhibitors (RASi), and history of cardiovascular disease (CVD) and heart failure. HRs are expressed with 95% CIs in parentheses.
Risk association of HVS with all-site, breast, and liver cancer events in obese patients
The obese group had aHRs of 1.19 (1.04, 1.36) for all-site cancer, 1.97 (1.32, 2.94) for liver cancer, but not significant for breast and colorectal cancer (supplementary table 5) compared with the non-obese group. We stratified patients by obesity and median value of HVS into 4 groups: 1) low GV & non-obese; 2) high GV & non-obese; 3) low GV& obese; 4) high GV & obese. Using the low GV & non-obese group as reference, there was a linear trend in the high GV & obese group having the highest aHR of 1.42 (1.16, 1.73), 2.44 (1.24, 4.82), and 2.63 (1.45, 4.74) for all-site, breast, and liver cancer albeit without interaction (Figure 1D).
Risk association of HVS with cause-specific death
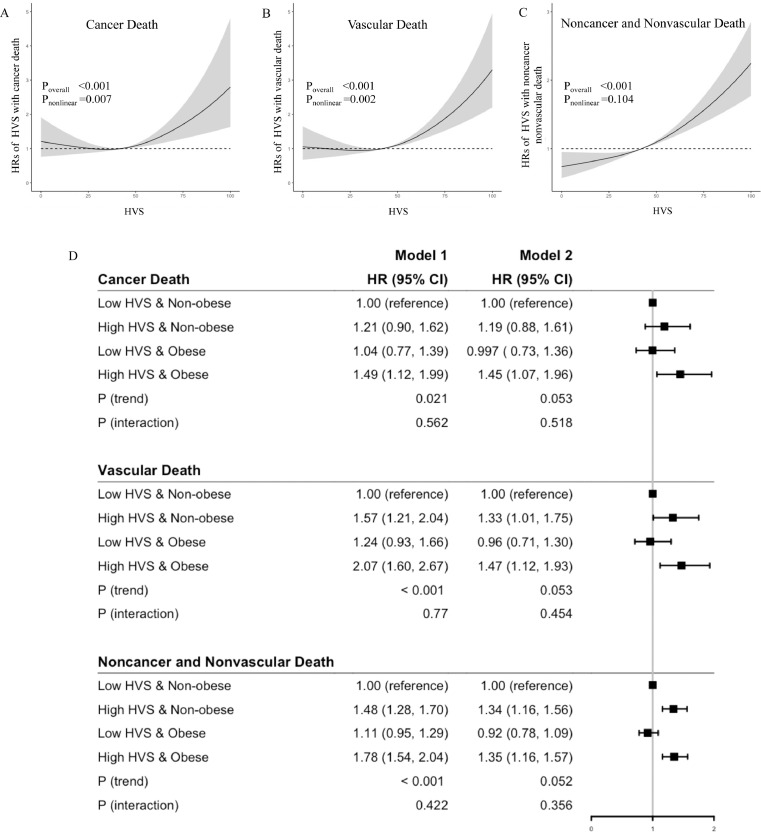
Spline analysis indicated non-linearity between HVS and death due to cancer (Poverall <0.001, Pnonlinear =0.007) and vascular (Poverall <0.001, Pnonlinear =0.002), but not for noncancer nonvascular causes (Poverall <0.001, Pnonlinear =0.104). There was a linearity within the high and low GV groups (Figure 3A-C). In the high HVS group, each SD of HVS was associated with aHR of 1.21 (1.06, 1.39) for cancer death but not in the low HVS group (0.88 [0.73, 1.05]) (table 2). In the high GV group, each SD of HVS was associated with respective aHRs of 1.27 (1.15, 1.40) and 1.15 (1.09, 1.22) for vascular and noncancer nonvascular death (table 2). Using the low GV & non-obese group as a reference, the high GV & obese group had the highest respective HRs of 1.45 (1.07, 1.96), 1.41 (1.12, 1.93), and 1.35 (1.16, 1.57) for cancer, vascular, and noncancer nonvascular death without interaction (Figure 1D).
Figure 2.
Cubic spline analysis of hazard ratios (HR) with HbA1c variability score (HVS) for death due to cancer (A), vascular (B) and noncancer nonvascular causes (C). There was non-linearity of HVS with cancer and vascular death but linearity within the high HVS and low HVS group stratified by median of HVS. The joint associations of obesity (body mass index ≥25 kg/m2) and HVS stratified by median value of HVS were expressed as forest plots of HRs for deaths due to cancer, vascular and noncancer nonvascular causes where low HVS & Non-obese group was used as the reference group (D).
Model 1: adjusted for time weighted mean A1c (mA1c), age, sex, and disease duration. Model 2: Model 1 plus BMI, use of tobacco and alcohol, HDL-cholesterol (HDLC), triglyceride (TG) (quantiles), LDL-cholesterol (LDLC), Alanine transferase (ALT), estimated glomerular filtration rate (eGFR), microalbuminuria, and macroalbuminuria, use of oral glucose lowering drugs (OGLDs), insulin, lipid lowering drugs (LLDs), and renin angiotensin system inhibitors (RASi), and history of cardiovascular disease (CVD) and heart failure. HRs are expressed with 95% CIs in parentheses.
Sensitivity analyses
After adjusting for competing risk of death for all-site cancer and cause-specific death for cancer death, the aHRs of HVS in the high HVS group for both outcomes remained constant (supplementary table 2). Using other indices of GV, each SD increment of SD_A1c (median [IQR] 0.83 [0.57, 1.19]) and SDIM (0.57 [0.39, 0.68]) were associated with aHRs of 1.5 (1.30, 1.74) and 2.03 (1.65, 2.50) for all-site cancer. The respective aHRs were 1.37 (1.10, 1.71) and 1.64 (1.23, 2.18) for cancer death (supplementary table 3). We included all patients irrespective of disease duration (n=18,494) with a median duration of diabetes 17.08 (11.77, 23.73) and found non-linear relationships between HVS and all-site cancer events and cancer death (Poverall <0.001, Pnonlinear <0.001). In the high HVS group, the aHR of each SD of HVS for all-site cancer and cancer death remained the same (supplementary table 4). In the whole group, obesity was associated with an increased risk for all-site cancer and liver cancer.
Stratified by median value of HbA1c measurements per year (2.38), patients with frequent HbA1c measurements had higher HVS, worse cardiometabolic risk profiles and were more likely to have complications and treated with multiple drugs than those with less frequent HbA1c measurements (supplementary table 6). In both groups, there was linearity between HVS and outcomes. In patients with frequent HbA1c measurements, the aHRs of SD of HVS for all-site cancer and cancer death were 1.21 (1.07, 1.37) and 1.33 (1.10, 1.61). The respective HRs were 1.14 (1.00, 1.30) and 1.15 (0.95, 1.39) in those with less frequent HbA1c measurements (supplementary table 7). In patients with baseline HbA1c ≥7%, there was non-linearity between HVS and outcomes. In the high HVS group, for each SD of HVS, the respective aHRs were 1.19 (1.06, 1.33) and 1.22 (1.04, 1.44) for all-site cancer and cancer death (supplementary table 8). Analysed by sex, the joint association of obesity and high HVS with all-site cancer was significant only in women with interaction (supplementary table 9). Additional analyses showed similar baseline data between patients fulfilling inclusion criteria with and without missing data (supplementary table 10). Repeat analysis after excluding 1.5% of patients with T1D with 2 cancer events and no cancer death showed similar results (data not shown).
Discussion
To our best knowledge, this is the first report on the association of GV and obesity withcancer and cancer-specific death in patients with diabetes. In this register-based cohort analysis, we included 15,286 Chinese patients with diabetes for at least 10 years, 98.5% of them having T2D. After nearly 12 years, 928 developed all-site cancer. We excluded HbA1c values measured within 3 years of cancer to minimize reverse causality and found linearity between cancer incidence and SD_A1c and SDIM. However, there was nonlinearity between HVS and all-site cancer and cancer death, albeit linearity in low and high HVS groups stratified by median value of HVS. In the high HVS group, each SD of HVS was associated with aHR of 1.15 for all-site cancer and 1.21 for cancer death. Compared with the low HVS & non-obese group, the high HVS & obese group had 2.5 aHR for all-site, breast and liver cancer and 1.35-1.45 aHR for cancer, vascular, and noncancer nonvascular death. In sensitivity analyses, these associations remained unchanged irrespective of disease duration and number of HbA1c measurements. Taken together, our results supported the importance of optimizing glycaemic and body weight control to reduce the risk of cancer and cancer death especially in patients with poor and fluctuating glycaemic control.
Comparison with other studies
In a cohort of 2,640 Japanese patients with T2D followed up for 4.1 years, GV expressed as SD of all HbA1c measurements, was associated with all-site cancer including prostate cancer with a HR of 1.2.13 Our cohort had considerably greater power, including 15,286 patients (928 cancer events) with a median diabetes duration of 19.17 years and a median follow-up period of 11.91 years. Leveraging these data, we used HVS, SD_A1c, and SDIM to define GV and explored their causal and latent effects on cancer and cancer death. In the aforementioned study,13 the researchers only used SD to define GV and did not exclude HbA1c measured within a few years prior to cancer. Compared with SD_A1c and SDIM, the use of HVS was more meaningful to practitioners in making clinical decisions.
Risk association of HVS with all-site cancer and cancer-specific death, stratified by frequency of HbA1c measurements and baseline HbA1c
Patients with poor glycaemic control tended to have higher HbA1c, more frequent measurements of HbA1c and worse GV, which might be closely linked. In our analysis, patients with frequent HbA1c measurements had higher HVS and worse clinical profiles than those with less frequent HbA1c measurements. In the former group, the aHR of each SD increment of HVS with all-site cancer and cancer death was also numerically higher than that in the latter group. Amongst patients with baseline HbA1c ≥7%, the association of SD of HVS with all-site cancer and cancer death was only significant in the high HVS group. These findings supported our primary analysis regarding the hazards of fluctuating glycaemia on cancer risk, especially in patients with poor glycaemic control.
GV and site-specific cancer
In our analysis, we examined colorectal, liver, and breast cancer, which were known to be positively associated with diabetes.2 Although pancreatic and endometrial cancer were also known to be associated with diabetes, the number of cases were too few for separate analysis. We found linearity between HVS and liver and breast cancer, but not colorectal cancer. In a Korean national cohort study, high GV was associated with an increased risk of hepatocellular carcinoma (HR: 1.27 [1.17-1.38]),24 which accorded with our results although we also found association of high HVS and obesity with liver cancer. In the Nurses' Health Study, women with T2D had 1.17-fold (1.01, 1.35) increased risk of breast cancer versus those without diabetes, especially in postmenopausal (1.16) versus premenopausal women (0.83).25 In our patients with diabetes, each SD of HVS was associated with aHR of 1.44 for breast cancer. However, we did not have information on menopausal status and the limited breast cancer events (n=77) did not allow meaningful subgroup analysis. We excluded 54 patients with prostate cancer for all-site cancer analysis due to its known negative association with T2D.3 In a meta-analysis of 17 cohort studies, T2D was associated with 29% increased risk for death due to prostate cancer.21 Thus, we included these patients in the all-cause and cancer-specific death analysis.
Risk association of obesity and GV with cancer
Obesity was often used to explain the frequent co-occurrence of diabetes and cancer.26 Inflammation and oxidative stress are common pathways shared by hyperglycaemia and obesity.27 The risk associations of overweight or obesity with cancer at different sites are well recognized.28 However, in a Korean population-based study, fasting plasma glucose was linearly associated with the risk of all-site cancer in non-obese, overweight, and obese subjects.29 In a multi-ethnic population-based study conducted in the USA, people with the highest and lowest BMI had the highest risk of all-cause death with a ‘U’ shaped relationship, irrespective of age, sex, and ethnicities.30 In our study, using Asian definition,9 obesity was associated with all-site and liver cancer, but not for all-cause or cause-specific death, probably due to small sample size. More importantly, our analysis revealed that obese patients with high HVS had the highest risk for cancer and cancer-specific death. The lack of interaction, which might be due to small sample size, suggested that control of body weight and glycaemia might have independent benefits on reducing cancer risk, albeit intervention studies are needed to test the hypothesis.
Mechanisms of GV in cancer
In patients with high HVS, each SD of HVS was associated with a HR of 1.20 for all-site cancer and cancer-specific death. Glycaemic variability could activate release of inflammatory cytokines, attract local adhesion of monocytes and macrophages, and increase oxidative stress,31,32 which portended endothelial dysfunction and generalised vasculopathy.5,6 This unfavourable metabolic milieu could inhibit cellular apoptosis and promote proliferation of neoplastic cells.33,34 Experimentally, use of RASi might attenuate inflammation and oxidative stress by reducing C reactive protein and nitric oxide synthase.35 In a smaller HKDR cohort, we had reported a reduced risk of all-site cancer with RASi and statins use, and HbA1c<7% at enrolment.36 In this analysis, we had adjusted for the use of RASi and statin at baseline. Given the long follow-up period, the use of statins and RASi in some patients might have attenuated these risk associations.
Study limitations
Our study had several limitations. Although metformin is well known to be associated with a reduced risk of cancer,37 due to the complex usage of multiple drugs and a relatively small sample size, we did not adjust for the time-varying use of drugs. The selection criteria of long disease duration and inclusion of at least 5 HbA1c measurements might limit the generalizability of our results. The excluded patients had worse risk factors and more comorbidities with less intensive treatment, which might underlie their higher mortality rate. However, our sensitivity analyses including all patients irrespective of disease duration and number of HbA1c measurements per year did not alter our conclusions. We did not have data on lifestyle changes but had adjusted for the use of alcohol and tobacco at baseline. Due to the pragmatic nature of the HKDR, we did not record socioeconomic status. Since blood pressure and body weight were not routinely captured in usual clinic visits, we were unable to include these measures as time-varying covariates. Given that we have already transformed the HbA1c measures into various indexes of GV and that no other time-varying variables were included in the model, we applied Cox model only adjusting for baseline covariates to elucidate the risk associations of GV indexes with cancer outcomes. The diagnosis of cancer was based on the first hospitalization record with possible missed diagnosis due to subclinical cancer or diagnosis in the private sector. By excluding 3 years of HbA1c values after enrolment, we have minimized bias due to reverse causality. Selection bias was possible with patients enrolled in the HKDR having more advanced disease with referral to the Diabetes Centre for structured assessment. Since autoimmune antibodies or C peptide were not routinely measured, we acknowledged possible misclassification of T1D and T2D. Since patients with T1D also had increased risk of cancer,38 we did not exclude patients with T1D, who accounted for 1.5% (232 cases) of all patients with only 2 cancer events in our primary analysis. Exclusion of patients with T1D did not alter our results which were largely reflective of T2D. Lastly, we did not have a validation cohort of a general population, although our results might motivate similar analysis in nationwide cohorts.
Conclusion
In our analysis, high GV was associated with increased risks of all-site, liver, breast cancer, and cancer death, especially in those with obesity and poor glycaemic control. With a declining trend of vascular death, aging and increasing population of young patients who face long disease duration, the dual burden of diabetes and cancer will continue to rise.39 Given the availability of technologies that can reduce obesity and glycaemic variability and that 1 in 4 patients were obese with high GV who had 2-fold increased risk of cancer and related deaths, identification of these high risk patients for intensive management will have important implications on personal and public health.
Declaration of interests
JC reported received research grants, honorarium and speakers’ fees from Applied Therapeutics, Astra Zeneca, Bayer, Boehringer Ingelheim, Celltrion, Hua Medicine, Lee Powder, Lilly, Merck Sharpe Dohme, Merck Serono, Pfizer, Sanofi, Servier and Virtus Pharmaceutical. EC reported receiving grants from Lee Powder and Sanofi. RCW reported receiving grants from AstraZeneca, Bater, MSD, Novo Nordisk, Tricia Inc, and Boehringer Ingelheim. APSK has received research grants and/or speaker honoraria from Abbott, Astra Zeneca, Eli-Lilly, Merck Serono, Nestle, and Novo Nordisk. Other coauthors did not have any conflict of interest relevant to the manuscript.
Acknowledgments
Contributors
All authors have made substantial contributions to conception and design, revised the article, and approved the final version to be published. DM additionally contributed to data analysis and interpretation and written the manuscript. JCNC additionally contributed to supervision of this study, who has full access to all the data in this study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgements
This project was supported by the CUHK Direct Grant and CUHK Diabetes Research and Education Fund of the Department of Medicine and Therapeutics, Faculty of Medicine, CUHK.
Data sharing statement
Due to patient's consent and institutional regulation, we can only share summary data with researchers who have submitted research proposal to ensure that our data are suitable for their intended analysis. Please send request to Prof Juliana Chan at jchan@cuhk.edu.hk.
Editor note: The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100315.
Appendix. Supplementary materials
Reference
- 1.Pearson-Stuttard J, Bennett J, Cheng YJ, Vamos EP, Cross AJ, Ezzati M, et al. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 2021;9(3):165–173. doi: 10.1016/S2213-8587(20)30431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60(4):207–221. doi: 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- 3.Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearson-Stuttard J, Papadimitriou N, Markozannes G, Cividini S, Kakourou A, Gill D, et al. Type 2 Diabetes and Cancer: An Umbrella Review of Observational and Mendelian Randomization Studies. Cancer Epidemiol Biomarkers Prev. 2021;30(6):1218–1228. doi: 10.1158/1055-9965.EPI-20-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106(16):2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 6.Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349–1354. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 7.Luk AO, Ma RC, Lau ES, Yang X, Lau WW, Yu LW, et al. Risk association of HbA1c variability with chronic kidney disease and cardiovascular disease in type 2 diabetes: prospective analysis of the Hong Kong Diabetes Registry. Diabetes Metab Res Rev. 2013;29(5):384–390. doi: 10.1002/dmrr.2404. [DOI] [PubMed] [Google Scholar]
- 8.Habib SL, Rojna M. Diabetes and Risk of Cancer. ISRN Oncology. 2013;2013 doi: 10.1155/2013/583786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergenstal RM, Kerr MSD, Roberts GJ, Souto D, Nabutovsky Y, Hirsch IB. Flash CGM Is Associated With Reduced Diabetes Events and Hospitalizations in Insulin-Treated Type 2 Diabetes. Journal of the Endocrine Society. 2021;5(4) doi: 10.1210/jendso/bvab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Echouffo-Tcheugui JB, Zhao S, Brock G, Matsouaka RA, Kline D, Joseph JJ. Visit-to-Visit Glycemic Variability and Risks of Cardiovascular Events and All-Cause Mortality: The ALLHAT Study. Diabetes Care. 2019;42(3):486–493. doi: 10.2337/dc18-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S, Nemeth I, Donnelly L, Hapca S, Zhou K, Pearson ER. Visit-to-Visit HbA(1c) Variability Is Associated With Cardiovascular Disease and Microvascular Complications in Patients With Newly Diagnosed Type 2 Diabetes. Diabetes care. 2020;43(2):426–432. doi: 10.2337/dc19-0823. [DOI] [PubMed] [Google Scholar]
- 12.Sheng CS, Tian J, Miao Y, Cheng Y, Yang Y, Reaven PD, et al. Prognostic Significance of Long-term HbA(1c) Variability for All-Cause Mortality in the ACCORD Trial. Diabetes Care. 2020;43(6):1185–1190. doi: 10.2337/dc19-2589. [DOI] [PubMed] [Google Scholar]
- 13.Saito Y, Noto H, Takahashi O, Kobayashi D. Visit-to-Visit Hemoglobin A1c Variability Is Associated With Later Cancer Development in Patients With Diabetes Mellitus. The Cancer Journal. 2019;25(4):1–13. doi: 10.1097/PPO.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 14.Wolk A, Gridley G, Svensson M, Nyrén O, McLaughlin JK, Fraumeni JF, et al. A prospective study of obesity and cancer risk (Sweden) Cancer Causes & Control. 2001;12(1):13–21. doi: 10.1023/a:1008995217664. [DOI] [PubMed] [Google Scholar]
- 15.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 16.Chobot A, Górowska-Kowolik K, Sokołowska M. Jarosz-Chobot P. Obesity and diabetes-Not only a simple link between two epidemics. Diabetes Metab Res Rev. 2018;34(7):e3042. doi: 10.1002/dmrr.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan JCN, Lim LL, Luk AOY, Ozaki R, Kong APS, Ma RCW, et al. From Hong Kong Diabetes Register to JADE Program to RAMP-DM for Data-Driven Actions. Diabetes Care. 2019;42(11):2022–2031. doi: 10.2337/dci19-0003. [DOI] [PubMed] [Google Scholar]
- 18.Piwernetz K, Home PD, Snorgaard O, Antsiferov M, Staehr-Johansen K, Krans M. Monitoring the targets of the St Vincent Declaration and the implementation of quality management in diabetes care: the DIABCARE initiative. The DIABCARE Monitoring Group of the St Vincent Declaration Steering Committee. Diabet Med. 1993;10(4):371–377. doi: 10.1111/j.1464-5491.1993.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 19.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 20.Wu H, Lau ES, Ma RC, Kong AP, Wild SH, Goggins W, et al. Secular trends in all-cause and cause-specific mortality rates in people with diabetes in Hong Kong, 2001–2016: a retrospective cohort study. Diabetologia. 2020;63(4):757–766. doi: 10.1007/s00125-019-05074-7. [DOI] [PubMed] [Google Scholar]
- 21.Lee J, Giovannucci E, Jeon JY. Diabetes and mortality in patients with prostate cancer: a meta-analysis. Springerplus. 2016;5(1):1548. doi: 10.1186/s40064-016-3233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong AP, Yang X, So WY, Luk A, Ma RC, Ozaki R, et al. Additive effects of blood glucose lowering drugs, statins and renin-angiotensin system blockers on all-site cancer risk in patients with type 2 diabetes. BMC Med. 2014;12:76–87. doi: 10.1186/1741-7015-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang H. The prevention and handling of the missing data. Korean J Anesthesiol. 2013;64(5):402–406. doi: 10.4097/kjae.2013.64.5.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoo JJ, Cho EJ, Han K, Heo SS, Kim BY, Shin DW, et al. Glucose Variability and Risk of Hepatocellular Carcinoma in Patients with Diabetes: A Nationwide Population-Based Study. Cancer Epidemiol Biomarkers Prev. 2021;30(5):974–981. doi: 10.1158/1055-9965.EPI-20-1654. [DOI] [PubMed] [Google Scholar]
- 25.Michels KB, Solomon CG, Hu FB, Rosner BA, Hankinson SE, Colditz GA, et al. Type 2 Diabetes and Subsequent Incidence of Breast Cancer in the Nurses’ Health Study. Diabetes Care. 2003;26(6):1752–1758. doi: 10.2337/diacare.26.6.1752. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher EJ, LeRoith D. Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol Rev. 2015;95(3):727–748. doi: 10.1152/physrev.00030.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. 2019;11(3):45–63. [PMC free article] [PubMed] [Google Scholar]
- 28.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 29.Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol. 2005;23(21):4742–4754. doi: 10.1200/JCO.2005.11.726. [DOI] [PubMed] [Google Scholar]
- 30.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355(8):763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 31.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. Jama. 2006;295(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 32.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 33.Park MH, Hong JT. Roles of NF-κB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells. 2016;5(2):15–20. doi: 10.3390/cells5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-Biological Interactions. 2006;160(1):1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4(12):977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 36.Kong AP, Yang X, So WY, Luk A, Ma RC, Ozaki R, et al. Additive effects of blood glucose lowering drugs, statins and renin-angiotensin system blockers on all-site cancer risk in patients with type 2 diabetes. BMC Med. 2014;12:76. doi: 10.1186/1741-7015-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, DeCensi A, et al. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer prevention research. 2014;7(9):867–885. doi: 10.1158/1940-6207.CAPR-13-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carstensen B, Read SH, Friis S, Sund R, Keskimäki I, Svensson A-M, et al. Cancer incidence in persons with type 1 diabetes: a five-country study of 9,000 cancers in type 1 diabetic individuals. Diabetologia. 2016;59(5):980–988. doi: 10.1007/s00125-016-3884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan JCN, Lim LL, Wareham NJ, Shaw JE, Orchard TJ, Zhang P, et al. The Lancet Commission on diabetes: using data to transform diabetes care and patient lives. Lancet. 2021;396(10267):2019–2082. doi: 10.1016/S0140-6736(20)32374-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.