Abstract
Pyrethroid insecticide use has increased over recent years because of their low to moderate acute toxicity in mammals. However, there is increasing concern over the potential detrimental effects of pyrethroids on developing animals. Most recently, we have shown that developmental exposure to deltamethrin results in long-term neurobehavioral effects. Pyrethroids exert their toxicity by acting on the voltage-gated sodium channel (Nav), delaying channel inactivation and causing hyperexcitability in the nervous system. Previous in vitro studies found that exposure to agents that increase Na+ in flux, including deltamethrin decreased Nav mRNA expression. However, it is unknown whether this occurs in vivo. To determine whether developmental pyrethroid exposure decreases Nav mRNA expression, pregnant mice were exposed to the pyrethroid deltamethrin (0 or 3 mg/kg) every three days throughout gestation and lactation. Nav mRNA expression was measured in the striatum and cortex of the offspring at 10–11 months of age, a time at which behavioral abnormalities were still observed. Developmental exposure to deltamethrin decreased expression of Nav mRNA in a region- and isoform-specific fashion by 24–50%. Deltamethrin exposure also resulted in the persistent down-regulation of brain-derived neurotrophic factor (Bdnf) in the striatum by 66% but not in the cortex, suggesting a plausible mechanism for some of the associated behavioral effects observed previously. Taken together these data suggest that developmental deltamethrin exposure results in persistent deficits in Nav and BDNF mRNA expression that may contribute to long-term behavioral deficits.
Keywords: Pyrethroid, Deltamethrin, Sodium channel, BDNF, Neurodevelopmental
1. Introduction
Pyrethroid insecticides are potent neurotoxic insecticides that account for over 25% of the total annual pesticide use in the world (Casida and Quistad, 1998; Horton et al., 2011; Morgan, 2012). The primary mechanism by which pyrethroids exert their neurotoxic effects in insects involves delaying the inactivation of voltage-gated sodium channels (Nav) (Narahashi, 1971). This delayed inactivation increases sodium in flux, action potential generation, neuronal excitability and ultimately leads to conduction block, particularly with Type II pyrethroids, convulsions and death, if the dose is high enough (Narahashi, 1996).
Nav are integral membrane proteins principally composed of a large (~260 kDa) α subunit that forms a central ion-conducting pore and one or more smaller auxiliary β subunits that are important modifiers of channel gating kinetics, cell-surface expression, and cell-cell interactions (Isom, 2001). The mammalian genome contains 10 sodium channel α subunit isoforms and four β subunits isoforms, yielding multiple subunit combinations, some of which are differentially sensitive to the effects of pyrethroids (Meacham et al., 2008; Soderlund, 2012). In an oocyte expression system, deltamethrin has the most pronounced effect on sodium currents through Nav1.3/β3 (Meacham et al., 2008) and Nav1.6/β1 + β2 channels (Tan and Soderlund, 2010). This may be particularly important to the potential developmental neurotoxicity of deltamethrin, as Nav1.3/β3 channel complexes are highly expressed in the developing rodent brain (Albrieux et al., 2004) and the Nav1.6 isoform is abundantly expressed in the nodes of Ranvier, dendrites, and synapses (Caldwell et al., 2000).
Although there is a general lack of information in the literature describing the developmental neurotoxicity of pyrethroids (Shafer et al., 2005), developing animals are more susceptible to the acute toxic effect of type II pyrethroids (Sheets et al., 1994). This greater susceptibility has been ascribed to lower metabolic detoxication enzymes compared to adults (Cantalamessa, 1993; Sheets et al., 1994). However, the greater abundance of the highly sensitive Nav1.3/β3 channels during neuronal development may also contribute to the increased susceptibility of the developing central nervous system (Meacham et al., 2008). Determining mechanism (s) of the potential developmental neurotoxicity of pyrethroids is particularly important because there is documented exposure of pregnant women and children to pyrethroids (Whyatt et al., 2002; Berkowitz et al., 2003; Heudorf et al., 2004; Bouwman et al., 2006; Barr et al., 2010), and the number of pyrethroid poisonings in children reported to poison control centers has increased recently (Power and Sudakin, 2007). More recently, we reported higher levels of pyrethroid metabolites in the urine of children increased likelihood of ADHD diagnosis (Wagner-Schuman et al., 2015) and that low-level deltamethrin exposure of mice during gestation resulted in hyperactivity and impulsive-like behavior in their male offspring (Richardson et al., 2015).
Previous in vitro studies found that exposure to compounds that delay Nav inactivation, such as veratridine and scorpion toxin, decreased Nav protein levels (Dargent and Couraud, 1990) and mRNA expression (Lara et al., 1996; Magby and Richardson, 2015), possibly as a compensatory mechanism to reduce hyperexcitability in the cells. The mechanism of this down-regulation is not well established, but may include calpain activation. Additionally, in vitro studies reported that the down-regulation of sodium channels occurs only in brain slices from immature animals and not in slices obtained from adult animals (Dargent et al., 1994). These findings suggest a unique susceptibility of the developing nervous system to agents that increase Nav influx that is likely independent of differences in detoxication capacity. However, it is not known whether in vivo exposure to agents that delay Nav inactivation, such as pyrethroids, causes similar effects.
In this study, we report that developmental exposure to the pyrethroid deltamethrin results in long-term down-regulation of Nav mRNA expression in vivo. This down-regulation is correlated with decreased brain-derived neurotrophic factor (BDNF) an important growth factor that is regulated by neuronal activity (Lu, 2003; Imamura et al., 2006; Sharma et al., 2008; Hara et al., 2009; Ihara et al., 2012). Thus, developmental exposure to deltamethrin appears to result in long-term changes in Nav expression, which could contribute to the persistent alterations of neuronal function and long-term behavioral deficits observed in our previous study.
2. Materials and methods
2.1. Chemicals
Deltamethrin was purchased from ChemService (99.5% purity and lot 418–66B; West Chester, PA). All other reagents were purchased from Sigma-Aldrich unless otherwise noted.
2.2. In vivo exposure
Male and female C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) at 6–8 weeks of age. Mice were maintained on a 12:12 light/dark cycle with food and water available ad libitum. All procedures were conducted in accordance with the NIH Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at Rutgers-Robert Wood Johnson Medical School.
Breeding and deltamethrin treatments were performed as described previously (Richardson et al., 2015). Briefly, pregnant female mice were individually housed and fed 0 or 3 mg/kg deltamethrin dissolved in corn oil and administered in a small amount of peanut butter every 3 days throughout gestation and lactation, starting at gestation day 6 and ending at weaning on PND25. This dosing paradigm was used to reduce handling stress on the pregnant female (Caudle et al., 2005) and the dose administered every 3 days to allow for significant clearance of the compound (Ruzo et al., 1979). This dose of deltamethrin is lower than the developmental NOAEL (12 mg/kg;(Kavlock et al., 1979)) and was determined based on our previous work that demonstrated no overt toxicity to the dam or offspring (Armstrong et al., 2013). Male mice were sacrificed by decapitation at approximately 10–11 months of age (n = 5–7 with each animal representing an individual litter). These mice were littermates of those previously reported to display hyperactivity and impulsive-like behavior at this time point (Richardson et al., 2015). Males were chosen for these experiments to eliminate the possibility of estrogen-related cyclicity, as estrogen levels affect Nav expression (Hu et al., 2012; Wang et al., 2013), and because of the male predominant behavioral deficits observed following developmental deltamethrin exposure (Richardson et al., 2015). Brains were removed, and the frontal cortex and striatum dissected on ice, and frozen in liquid nitrogen. Samples were stored at −80 °C until RNA isolation.
2.3. Quantitative real-time polymerase chain reaction (qPCR)
qPCR was performed as described previously (Richardson et al., 2008; Fortin et al., 2013). Briefly, total RNA was isolated using Qiagen RNeasy mini kits and RNA (0.5 μg) was reverse-transcribed using Superscript II (Invitrogen, Carlsbad, CA). QPCR reactions were performed in duplicate using an ABI 7900HT and SYBR Green (Applied Biosystems, Carlsbad, CA) detection. β-actin was used as the normalizing gene and data were calculated using the 2ΔΔCt method as described previously (Richardson et al., 2008). Primers were designed using the Primer Blast program (NCBI) and are listed in Supplemental Table 1.
2.4. Statistical analysis
All statistical analyses were performed on raw data. Data were analyzed using Student’s t-test to determine effects on individual isoforms within a single brain region.
3. Results
Developmental deltamethrin exposure did not result in alteration of maternal weight gain during pregnancy nor body weight of the offspring at sacrifice (Supplemental Fig. 1). Likewise, no overt signs of pyrethroid toxicity were observed in the dam or offspring.
3.1. Regional differences in Nav gene expression in the cortex and striatum
As an initial step in characterizing the effects of developmental deltamethrin exposure on Nav expression in 10–11 month old offspring, we first assessed whether there were regional differences in Nav isoform expression. The relative abundance of Nav isoform expression was Nav1.2 > Nav1.6 > Nav1.1 > Nav1.3, regardless of brain region examined. Comparing the two regions, in the cortex, Nav1.2 (11%) and Nav1.6 (21%) were more highly expressed, while Nav1.1 (40%) and Nav1.3 (54%) were more highly expressed in the striatum (Fig. 1) when normalized to cortex. The relative abundance of β subunits in the cortex was Navβ2 > Navβ1 > Navβ 3> Navβ4 whereas the striatum Nav β2 > Nav β1 > Nav β4 > Navβ3. Comparing the two regions the Navβ2 (50%) and Navβ4 (12x fold) were more highly expressed in the striatum than in the cortex, when normalized to cortex (Fig. 1).
Fig. 1.
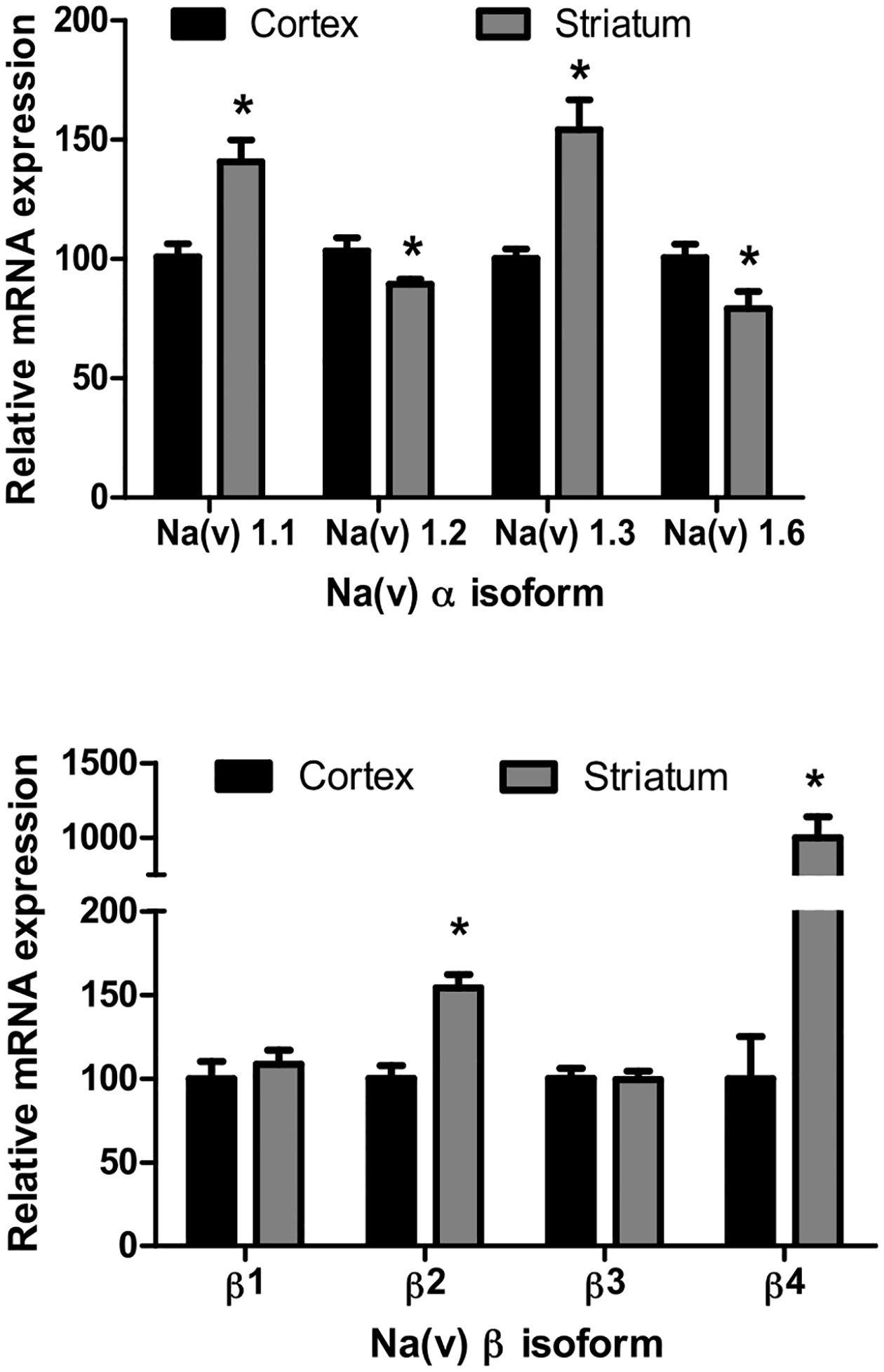
Regional Differences in Basal Expression of α subunit Sodium Channel mRNA in Frontal Cortex and Striatum. Data represent expression of Nav mRNA in the (Top) α subunits (Bottom) β subunits. Regional differences in basal isoform expression comparing cortex to striatum using the cortex as the normalized value. Data represent 2ΔΔCt analysis of qPCR data expressed as percentage of cortex values. * = p< 0.05, n = 5–7. Error bars represent SEM.
3.2. Developmental deltamethrin exposure reduces expression of Nav
In the frontal cortex, offspring developmentally exposed to deltamethrin exhibited a significant 30% reduction in Nav1.3 mRNA expression compared to controls at 10–11 months of age (Fig. 2A). There was also a significant reduction (26%) in expression of the β3 subunit in the frontal cortex of exposed mice (Fig. 2B).
Fig. 2.
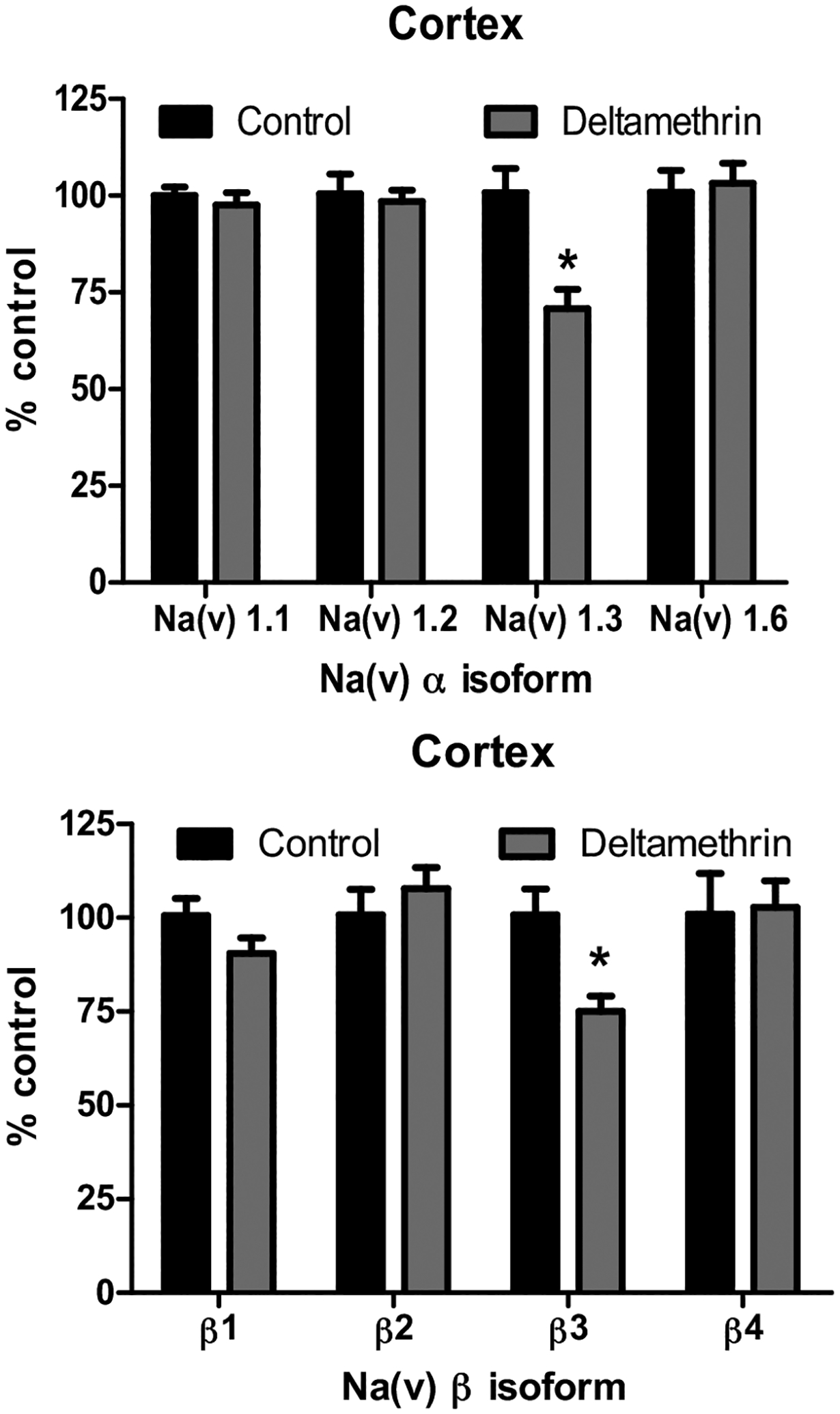
Expression of Nav mRNA in the Frontal Cortex Following Developmental Deltamethrin Exposure. (Top) Cortex, α isoforms. (Bottom) Cortex, β isoforms. All isoforms are compared to control value for that region. Data represent 2ΔΔCt analysis of qPCR data, expressed as percentage of control. Treatment-related differences for each isoform was determined via Student’s t-test * = p< 0.05, n = 5–7. Error bars represent SEM.
In contrast to the isoform-specific down-regulation of Nav in the cortex, developmental deltamethrin exposure broadly decreased mRNA expression of all the Nav isoforms assayed in the striatum, as Nav1.1, Nav1.2, Nav1.3, and Nav1.6 were significantly decreased by 24–26% (Fig. 3A). Similar to the α subunits, the β subunits were more broadly affected by deltamethrin in the striatum, with significant reductions of β1, β3, and β4 by 18–27% (Fig. 3B).
Fig. 3.
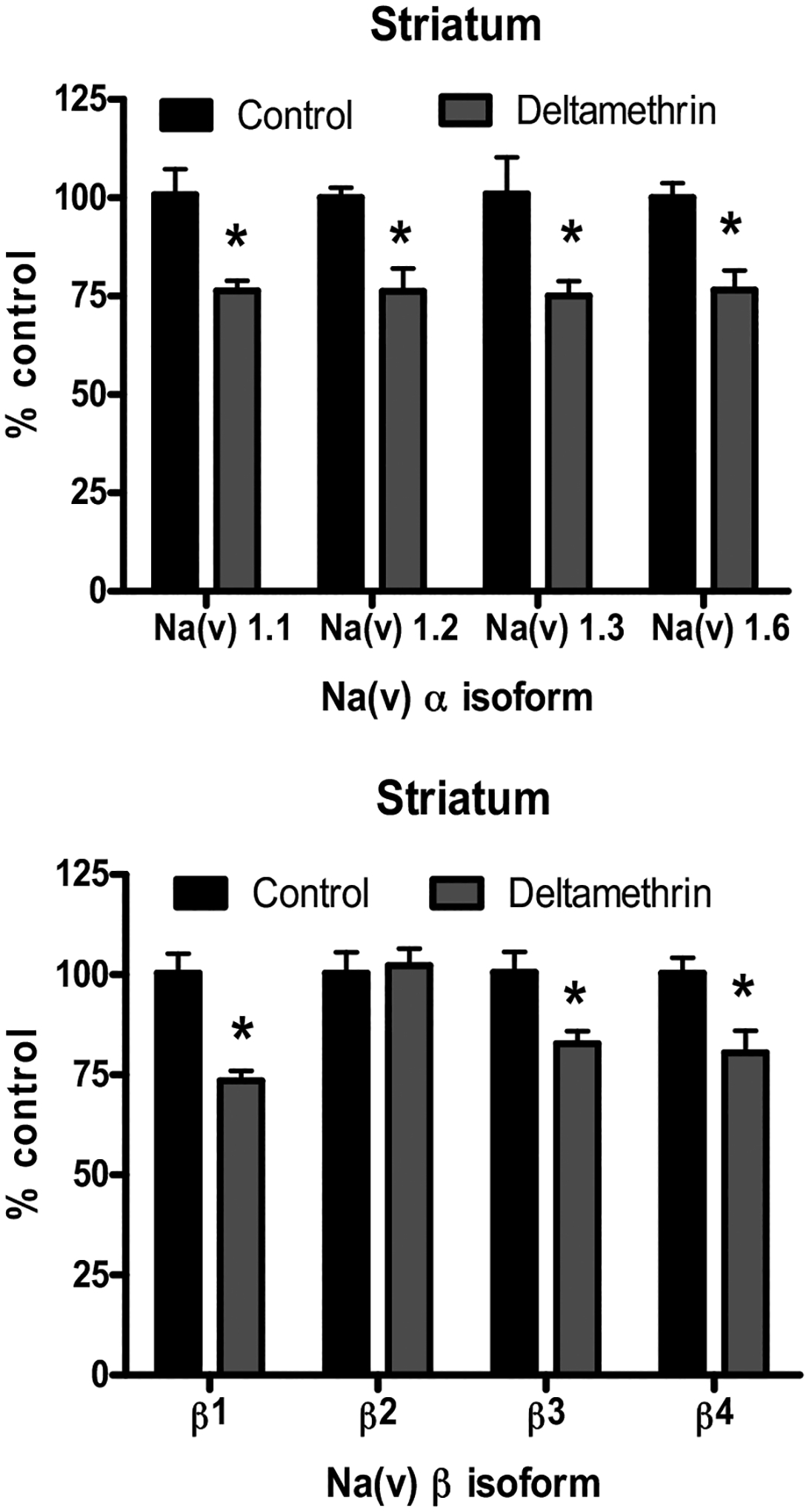
Expression of Nav mRNA in the Striatum Following Developmental Deltamethrin Exposure. (Top) Striatum, α isoforms. (Bottom) Striatum β isoforms. All isoforms are compared to control value for that region. Data represent 2ΔΔCt analysis of qPCR data, expressed as percentage of control. Treatment-related differences for each isoform was determined via Student’s t-test * = p< 0.05, n = 5–7. Error bars represent SEM.
3.3. Developmental deltamethrin exposure reduces BDNF mRNA in the striatum but not the cortex of male mice
Brain-derived neurotrophic factor (BDNF) expression is correlated with neuronal activity (Heese et al., 2000; Lu, 2003; Imamura et al., 2006; Abuhatzira et al., 2007; Sharma et al., 2008; Ihara et al., 2012). To determine whether decreased Nav expression results in reduced neuronal activity, we measured BDNF mRNA as a proxy for neuronal activity. In mice developmentally exposed to deltamethrin BDNF mRNA in the cortex was not changed. However, in the striatum of mice exposed to deltamethrin, BDNF mRNA was reduced by 66% compared to control animals (Fig. 4).
Fig. 4.
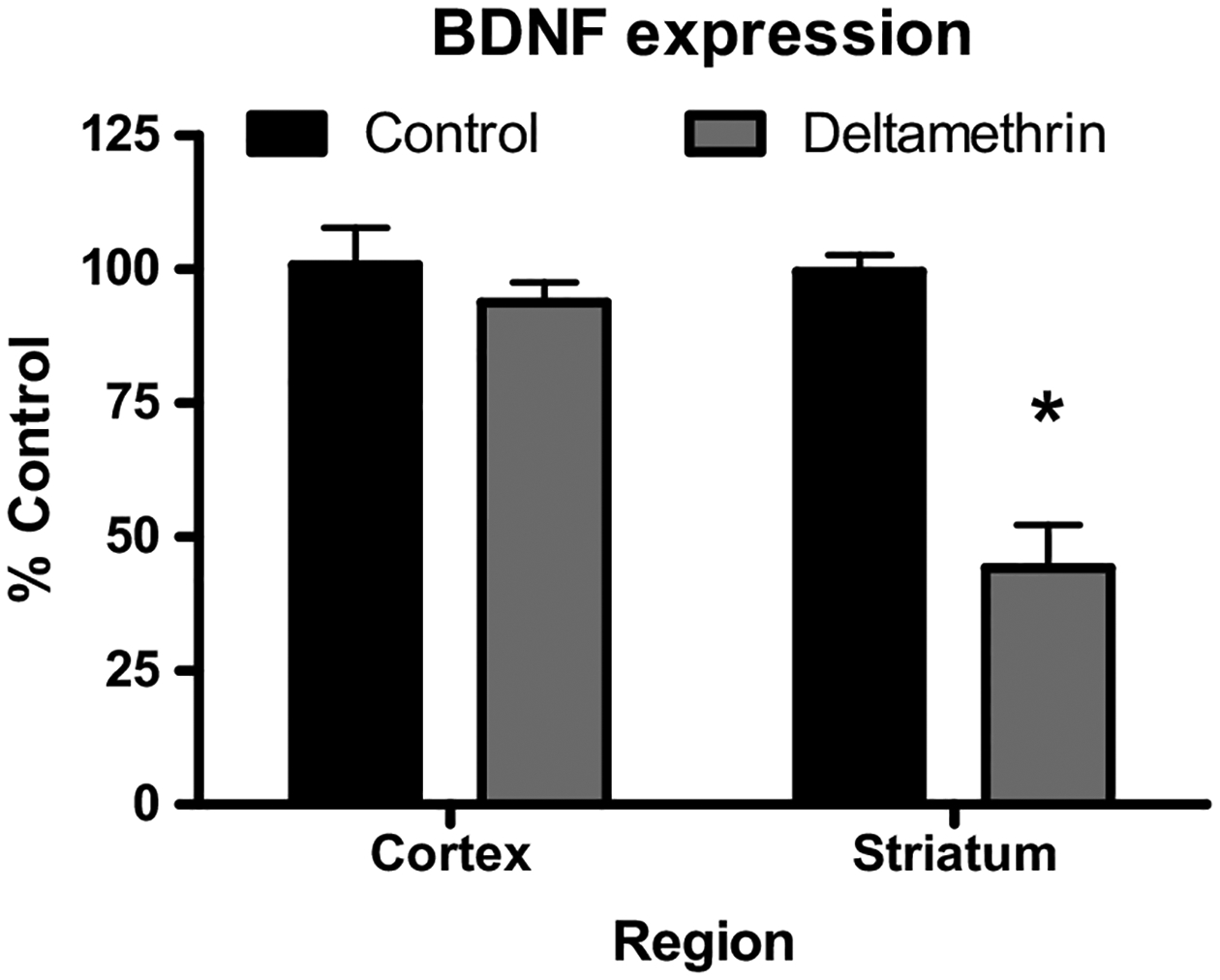
Developmental Deltamethrin Reduces BDNF mRNA in the Striatum following Developmental Deltamethrin Exposure. Cortical and Striatal BDNF mRNA expression. BDNF mRNA was compared to control values for that region. Data represent 2ΔΔCt analysis of qPCR data, expressed as percentage of control. Treatment-related differences for each region was determined via Student’s t-test * = p< 0.05, n = 5–7. Error bars represent SEM.
4. Discussion
Previous in vitro studies found that exposure to compounds that delay Nav inactivation reduces Nav mRNA and protein (Dargent and Couraud, 1990; Dargent et al., 1994, 1995; Lara et al., 1996; Paillart et al., 1996; Magby and Richardson, 2015), but it is unknown whether this occurs in vivo. Here, we report that developmental deltamethrin exposure caused a significant reduction of both Nav α and β subunit mRNAs in the brain. We also show that developmental deltamethrin results in persistent down-regulation of BDNF mRNA. These data demonstrate that developmental deltamethrin exposure causes long-term alterations in the expression of Nav and BDNF, both of which are essential to proper brain development and function.
Measuring Nav mRNA in the striatum and cortex yielded brain region-specific differences in basal Nav mRNA expression as well as differences in deltamethrin-induced down-regulation. A recent paper reported that Nav1.1,1.2, and 1.6 protein levels were higher in the striatum compared to the frontal cortex in nerve terminal preparations from rats (Westphalen et al., 2010). Consistent with previous results (Brysch et al., 1991), the frontal cortex expressed more Nav1.2 mRNA than the striatum, which may partially explain why the overall expression of Nav isoforms in the frontal cortex was not as affected by developmental deltamethrin exposure compared to the striatum. In contrast, the striatum has higher expression of Nav1.1 and Nav1.3 isoforms, which is also consistent with previous studies (Beckh et al., 1989; Shah et al., 2001). Nav1.3 is especially sensitive to pyrethroid modification, whereas the relative sensitivity of Nav1.1 is currently unknown, and Nav 1.3 is more highly expressed during development in the rodent brain (Albrieux et al., 2004; Meacham et al., 2008). Taken together, these data suggest that the higher expression of pyrethroid-sensitive Nav isoforms in the striatum, such as Nav1.3 and perhaps Nav1.1, may be important contributing factors to the regional differences observed in the down-regulation of Nav isoforms following developmental deltamethrin exposure. Further, decreased expression of the corresponding β subunit isoforms likely contributes to the differential effects observed, as levels and isoform specificity of the β subunit have profound effects on Nav gating kinetics, activity, and mRNA expression (O’Malley and Isom, 2015).
From a mechanistic standpoint, we recently reported that deltamethrin decreased Nav mRNA through increases in intracellular calcium and activation of calpain (Magby and Richardson, 2015). Importantly, these effects were blocked by inclusion of tetrodotoxin in the culture media, demonstrating the requirement of interaction with Nav to produce down-regulation. Although this is the first report of which we are aware to report decreased Nav expression in vivo following pyrethroid exposure, deltamethrin and similar compounds that delay Nav inactivation are not alone in their ability to alter Nav mRNA expression. There are a number of other models in which Nav expression is altered in vivo. For example, kainate-induced seizures transiently increased Nav mRNAs encoding for Nav1.2 and Nav1.3 in adult rats (Bartolomei et al., 1997). In animal models, hypoxia causes Na+-dependent depolarization following the insult (Fung and Haddad, 1997). In turn, hypoxia can either increase (Xia et al., 2000; Zhao et al., 2005) or decrease (Xia and Haddad, 1999; Gu and Haddad, 2001; Zhao et al., 2005) Nav expression, depending on age, duration of exposure and brain region. Chronic hypoxia in adult animals causes decreased Nav protein and mRNA expression, which coincides with a net decrease in neuronal excitability (Gu and Haddad, 2001). Thus, there appears to be the potential for developmental deltamethrin to cause long-term alterations in neuronal excitability based on its ability to down-regulate Nav.
Previous studies found that acute exposure to pyrethroids can rapidly alter BDNF mRNA and protein in vitro and in vivo (Imamura et al., 2000, 2002, 2005, 2006; Ihara et al., 2012). Studies identifying pyrethroid induced BDNF induction report the induction is likely due to an acute increase in neuronal depolarization and subsequent increase in Ca2+ in flux (Imamura et al., 2006; Ihara et al., 2012; Matsuya et al., 2012). In cultured cortical neurons, 1 μM deltamethrin or in vivo administration of 25 mg/kg deltamethrin to seven-week old rats caused rapid increases in BDNF mRNA and protein in the cerebral cortex and hippocampus. In vitro studies reported that BDNF induction by veratridine was prevented by pre-treatment with permethrin and to a lesser extent deltamethrin (Imamura et al., 2000, 2002, 2005). Imamura et al. (2000) suggested that the repression of BDNF and c-fos is through effects on calcium signaling and inhibition of the transcription factor AP-1 binding in response to permethrin exposure. However, it is possible that down-regulation of Nav could play a role in reducing activity, BDNF expression and a secondary rise in intracellular Ca2+, because Nav can be removed from the cell membrane within 15 min of exposure to an Nav activator and decreased expression of Nav resulted in reduced excitability and action potential firing in neurons (Dargent et al., 1994; Imamura et al., 2000; Kalume et al., 2007). Because BDNF expression is closely tied to neuronal activity decreased BDNF mRNA in the striatum, but not the cortex of exposed animals, is likely indicative of reduced neuronal activity. Indeed, neuronal activity regulates the transcription of BDNF mRNA, transport of BDNF mRNA and protein, and the secretion of BDNF protein(Lu, 2003). There is likely further connection between the effects on BDNF, as the behavioral profile reported in mice developmentally exposed to deltamethrin (i.e., ADHD-like phenotype including hyperactivity and impulsive-like behavior) is similar to that observed in BDNF heterozygous mice (Monteggia et al., 2007). Further, alterations in BDNF have been linked to ADHD and risk factors for ADHD, including maternal smoking (Yochum et al., 2014). However, a direct link between decreased BDNF mRNA and Nav mRNA expression remains to be established.
In this study, we chose to focus on mRNA expression because antibodies obtained from several commercial sources did not provide single bands for quantification of Nav protein levels in mouse brain by western immunoblotting (data not shown). Nonetheless, previous studies reported that Nav mRNA expression is reflective of changes in sodium channel protein levels, as measured by radiolabeled saxitoxin binding (Xia and Haddad, 1999), neuronal excitability, and electrophysiological recordings of isolated Na+ currents (Gu and Haddad, 2001). Importantly, the reduction in Nav was similar in magnitude to previous in vitro studies (Dargent et al., 1994). Future studies, including electrophysiological and other protein measurement approaches, are required to definitively correlate mRNA, function and protein levels.
5. Conclusions
Based on the data presented here, we conclude that alterations of neuronal activity due to deltamethrin exposure during the perinatal period have long lasting impacts on Nav expression. Whether or not these changes result in alterations of neuronal excitability and functional alterations in neurotransmission is currently under investigation. Because of the importance of BDNF to neuronal development and its role in long-term potentiation it is vital to fully understand how pyrethroid exposure down-regulates BDNF mRNA. In light of previously reported association between pyrethroid exposure and ADHD (Wagner-Schuman et al., 2015) and the observation of ADHD-like behaviors in animals exposed to deltamethrin (Richardson et al., 2015), determining whether or not alterations in Nav expression, manifesting as changes in neuronal excitability or the down-stream effects of excitability on neuro-transmitter or neurotrophin systems, could play a role in this phenotype could provide new insight into the pathophysiology of behavioral dysfunction.
Supplementary Material
Acknowledgements
This work was supported in part by National Institute of Health Grants R21ES013828,R01ES015991 and P30ES005022 and a graduate research fellowship to JPM from Bristol Myers Squibb.
Footnotes
Conflict of interest
The authors declare they have no conflict of interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neuro.2016.04.002.
References
- Abuhatzira L, Makedonski K, Kaufman Y, Razin A, Shemer R, 2007. MeCP2 deficiency in the brain decreases BDNF levels by REST/CoREST-mediated repression and increases TRKB production. Epigenetics 2, 214–222. [DOI] [PubMed] [Google Scholar]
- Albrieux M, Platel JC, Dupuis A, Villaz M, Moody WJ, 2004. Early expression of sodium channel transcripts and sodium current by cajal-retzius cells in the preplate of the embryonic mouse neocortex. J. Neurosci 24, 1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong LE, Driscoll MV, Donepudi AC, Xu J, Baker A, Aleksunes LM, Richardson JR, Slitt AL, 2013. Effects of developmental deltamethrin exposure on white adipose tissue gene expression. J. Biochem. Mol. Toxicol 27, 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Olsson AO, Wong LY, Udunka S, Baker SE, Whitehead RD, Magsumbol MS, Williams BL, Needham LL, 2010. Urinary concentrations of metabolites of pyrethroid insecticides in the general U.S. population: national Health and Nutrition Examination Survey 1999–2002. Environ. Health Perspect 118, 742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomei F, Gastaldi M, Massacrier A, Planells R, Nicolas S, Cau P,1997. Changes in the mRNAs encoding subtypes I, II and III sodium channel alpha subunits following kainate-induced seizures in rat brain. J. Neurocytol 26, 667–678. [DOI] [PubMed] [Google Scholar]
- Beckh S, Noda M, Lubbert H, Numa S, 1989. Differential regulation of three sodium channel messenger RNAs in the rat central nervous system during development. EMBO J. 8, 3611–3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz GS, Obel J, Deych E, Lapinski R, Godbold J, Liu Z, Landrigan PJ, Wolff MS, 2003. Exposure to indoor pesticides during pregnancy in a multiethnic, urban cohort. Environ. Health Perspect 111, 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwman H, Sereda B, Meinhardt HM, 2006. Simultaneous presence of DDT and pyrethroid residues in human breast milk from a malaria endemic area in South Africa. Environ. Pollut 144, 902–917. [DOI] [PubMed] [Google Scholar]
- Brysch W, Creutzfeldt OD, Luno K, Schlingensiepen R, Schlingensiepen KH, 1991. Regional and temporal expression of sodium channel messenger RNAs in the rat brain during development. Exp. Brain Res 86, 562–567. [DOI] [PubMed] [Google Scholar]
- Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR, 2000. Sodium channel Na(v)1.6 is localized at nodes of ranvier, dendrites, and synapses. Proc. Natl. Acad. Sci. U. S. A 97, 5616–5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalamessa F, 1993. Acute toxicity of two pyrethroids, permethrin, and cypermethrin in neonatal and adult rats. Arch. Toxicol 67, 510–513. [DOI] [PubMed] [Google Scholar]
- Casida JE, Quistad GB, 1998. Golden age of insecticide research: past, present, or future? Annu. Rev. Entomol 43, 1–16. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Wang M, Miller GW, 2005. Perinatal heptachlor exposure increases expression of presynaptic dopaminergic markers in mouse striatum. Neurotoxicology 26, 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargent B, Couraud F, 1990. Down-regulation of voltage-dependent sodium channels initiated by sodium in flux in developing neurons. Proc. Natl. Acad. Sci. U. S. A 87, 5907–5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargent B, Paillart C, Carlier E, Alcaraz G, Martin-Eauclaire MF, Couraud F, 1994. Sodium channel internalization in developing neurons. Neuron 13, 683–690. [DOI] [PubMed] [Google Scholar]
- Dargent B, Jullien F, Couraud F, 1995. Internalization of voltage-dependent sodium channels in fetal rat brain neurons: a study of the regulation of endocytosis. J. Neurochem 65, 407–413. [DOI] [PubMed] [Google Scholar]
- Fortin MC, Aleksunes LM, Richardson JR, 2013. Alteration of the expression of pesticide-metabolizing enzymes in pregnant mice: potential role in the increased vulnerability of the developing brain. Drug Metab. Dispos 41, 326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung ML, Haddad GG, 1997. Anoxia-induced depolarization in CA1 hippocampal neurons: role of Na+-dependent mechanisms. Brain Res. 762, 97–102. [DOI] [PubMed] [Google Scholar]
- Gu XQ, Haddad GG, 2001. Decreased neuronal excitability in hippocampal neurons of mice exposed to cyclic hypoxia. J. Appl. Physiol 91, 1245–1250. [DOI] [PubMed] [Google Scholar]
- Hara D, Fukuchi M, Miyashita T, Tabuchi A, Takasaki I, Naruse Y, Mori N, Kondo T, Tsuda M, 2009. Remote control of activity-dependent BDNF gene promoter-i transcription mediated by REST/NRSF. Biochem. Biophys. Res. Commun 384, 506–511. [DOI] [PubMed] [Google Scholar]
- Heese K, Otten U, Mathivet P, Raiteri M, Marescaux C, Bernasconi R, 2000. GABA(B) receptor antagonists elevate both mRNA and protein levels of the neurotrophins nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) but not neurotrophin-3 (NT-3) in brain and spinal cord of rats. Neuropharmacology 39, 449–462. [DOI] [PubMed] [Google Scholar]
- Heudorf U, Angerer J, Drexler H, 2004. Current internal exposure to pesticides in children and adolescents in Germany: urinary levels of metabolites of pyrethroid and organophosphorus insecticides. Int. Arch. Occup. Environ. Health 77, 67–72. [DOI] [PubMed] [Google Scholar]
- Horton MK, Jacobson JB, McKelvey W, Holmes D, Fincher B, Quantano A, Diaz BP, Shabbazz F, Shepard P, Rundle A, Whyatt RM, 2011. Characterization of residential pest control products used in inner city communities in New York City. J. Expo. Sci. Environ. Epidemiol 21, 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Wang Q, Wang P, Wang W, Qian W, Xiao H, Wang L, 2012. 17beta-estradiol regulates the gene expression of voltage-gated sodium channels: role of estrogen receptor alpha and estrogen receptor beta. Endocrine 41, 274–280. [DOI] [PubMed] [Google Scholar]
- Ihara D, Fukuchi M, Honma D, Takasaki I, Ishikawa M, Tabuchi A, Tsuda M, 2012. Deltamethrin, a type II pyrethroid insecticide, has neurotrophic effects on neurons with continuous activation of the BDNF promoter. Neuropharmacology 62, 1091–1098. [DOI] [PubMed] [Google Scholar]
- Imamura L, Hasegawa H, Kurashina K, Hamanishi A, Tabuchi A, Tsuda M, 2000. Repression of activity-dependent c-fos and brain-derived neurotrophic factor mRNA expression by pyrethroid insecticides accompanying a decrease in Ca(2+) influx into neurons. J. Pharmacol. Exp. Ther 295, 1175–1182. [PubMed] [Google Scholar]
- Imamura L, Hasegawa H, Kurashina K, Matsuno T, Tsuda M, 2002. Neonatal exposure of newborn mice to pyrethroid (permethrin) represses activity-dependent c-fos mRNA expression in cerebellum. Arch. Toxicol 76, 392–397. [DOI] [PubMed] [Google Scholar]
- Imamura L, Kurashina K, Kawahira T, Omoteno M, Tsuda M, 2005. Additional repression of activity-dependent c-fos and BDNF mRNA expression by lipophilic compounds accompanying a decrease in Ca2+ in flux into neurons. Neurotoxicology 26, 17–25. [DOI] [PubMed] [Google Scholar]
- Imamura L, Yasuda M, Kuramitsu K, Hara D, Tabuchi A, Tsuda M, 2006. Deltamethrin, a pyrethroid insecticide, is a potent inducer for the activity-dependent gene expression of brain-derived neurotrophic factor in neurons. J. Pharmacol. Exp. Ther 316, 136–143. [DOI] [PubMed] [Google Scholar]
- Isom LL, 2001. Sodium channel beta subunits: anything but auxiliary. Neuroscientist 7, 42–54. [DOI] [PubMed] [Google Scholar]
- Kalume F, Yu FH, Westenbroek RE, Scheuer T, Catterall WA, 2007. Reduced sodium current in Purkinje neurons from Nav1.1 mutant mice: implications for ataxia in severe myoclonic epilepsy in infancy. J. Neurosci 27, 11065–11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock R, Chernoff N, Baron R, Linder R, Rogers E, Carver B, Dilley J, Simmon V, 1979. Toxicity studies with decamethrin, a synthetic pyrethroid insecticide. J. Environ. Pathol. Toxicol 2, 751–765. [PubMed] [Google Scholar]
- Lara A, Dargent B, Julien F, Alcaraz G, Tricaud N, Couraud F, Jover E, 1996. Channel activators reduce the expression of sodium channel alpha-subunit mRNA in developing neurons. Brain Res. Mol. Brain Res 37, 116–124. [DOI] [PubMed] [Google Scholar]
- Lu B, 2003. BDNF and activity-dependent synaptic modulation. Learn. Mem 10, 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magby JP, Richardson JR, 2015. Role of calcium and calpain in the downregulation of voltage-gated sodium channel expression by the pyrethroid pesticide deltamethrin. J. Biochem. Mol. Toxicol 29, 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuya Y, Ihara D, Fukuchi M, Honma D, Itoh K, Tabuchi A, Nemoto H, Tsuda M, 2012. Synthesis and biological evaluation of pyrethroid insecticide-derivatives as a chemical inducer for BDNF mRNA expression in neurons. Bioorg. Med. Chem 20, 2564–2571. [DOI] [PubMed] [Google Scholar]
- Meacham CA, Brodfuehrer PD, Watkins JA, Shafer TJ, 2008. Developmentally-regulated sodium channel subunits are differentially sensitive to alpha-cyano containing pyrethroids. Toxicol. Appl. Pharmacol 231, 273–281. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ, 2007. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol. Psychiatry 61, 187–197. [DOI] [PubMed] [Google Scholar]
- Morgan MK, 2012. Children’s exposures to pyrethroid insecticides at home: a review of data collected in published exposure measurement studies conducted in the United States. Int. J. Environ. Res. Public Health 9, 2964–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T, 1971. Mode of action of pyrethroids. Bull. World Health Organ 44, 337–345. [PMC free article] [PubMed] [Google Scholar]
- Narahashi T, 1996. Neuronal ion channels as the target sites of insecticides. Pharmacol. Toxicol 79, 1–14. [DOI] [PubMed] [Google Scholar]
- O’Malley HA, Isom LL, 2015. Sodium channel ‘ subunits: emerging targets in channelopathies. Annu. Rev. Physiol 77, 481–504. doi: 10.1146/annurev-physiol-021014-071846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillart C, Boudier JL, Boudier JA, Rochat H, Couraud F, Dargent B, 1996. Activity-induced internalization and rapid degradation of sodium channels in cultured fetal neurons. J. Cell Biol 134, 499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power LE, Sudakin DL, 2007. Pyrethrin and pyrethroid exposures in the United States: a longitudinal analysis of incidents reported to poison centers. J. Med. Toxicol 3, 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JR, Caudle WM, Wang MZ, Dean ED, Pennell KD, Miller GW, 2008. Developmental heptachlor exposure increases susceptibility of dopamine neurons to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)in a gender-specific manner. Neurotoxicology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JR, Taylor MM, Shalat SL, Guillot TS 3rd, Caudle WM, Hossain MM, Mathews TA, Jones SR, Cory-Slechta DA, Miller GW, 2015. Developmental pesticide exposure reproduces features of attention deficit hyperactivity disorder. FASEB J. 29, 1960–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzo LO, Engel JL, Casida JE, 1979. Decamethrin metabolites from oxidative, hydrolytic, and conjugative reactions in mice. J. Agric. Food Chem 27, 725–731. [DOI] [PubMed] [Google Scholar]
- Shafer TJ, Meyer DA, Crofton KM, 2005. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ. Health Perspect 113, 123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah BS, Stevens EB, Pinnock RD, Dixon AK, Lee K, 2001. Developmental expression of the novel voltage-gated sodium channel auxiliary subunit beta3, in rat CNS. J. Physiol 534, 763–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma RP, Tun N, Grayson DR, 2008. Depolarization induces downregulation of DNMT1 and DNMT3a in primary cortical cultures. Epigenetics 3. [DOI] [PubMed] [Google Scholar]
- Sheets LP, Doherty JD, Law MW, Reiter LW, Crofton KM,1994. Age-dependent differences in the susceptibility of rats to deltamethrin. Toxicol. Appl. Pharmacol 126, 186–190. [DOI] [PubMed] [Google Scholar]
- Soderlund DM, 2012. Molecular mechanisms of pyrethroid insecticide neurotoxicity: recent advances. Arch. Toxicol 86, 165–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Soderlund DM, 2010. Divergent actions of the pyrethroid insecticides S-bioallethrin, tefluthrin, and deltamethrin on rat Na(v)1.6 sodium channels. Toxicol. Appl. Pharmacol 247, 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner-Schuman M, Richardson JR, Auinger P, Braun JM, Lanphear BP, Epstein JN, Yolton K, Froehlich TE, 2015. Association of pyrethroid pesticide exposure with attention-deficit/hyperactivity disorder in a nationally representative sample of U.S. children. Environ. Health 14, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Cao J, Hu F, Lu R, Wang J, Ding H, Gao R, Xiao H, 2013. Effects of estradiol on voltage-gated sodium channels in mouse dorsal root ganglion neurons. Brain Res. 1512, 1–8. [DOI] [PubMed] [Google Scholar]
- Westphalen RI, Yu J, Krivitski M, Jih TY, Hemmings HC Jr., 2010. Regional differences in nerve terminal Na+ channel subtype expression and Na+ channel-dependent glutamate and GABA release in rat CNS. J. Neurochem 113, 1611–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, Camann DE, Kinney PL, Reyes A, Ramirez J, Dietrich J, Diaz D, Holmes D, Perera FP, 2002. Residential pesticide use during pregnancy among a cohort of urban minority women. Environ. Health Perspect 110, 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Haddad GG, 1999. Effect of prolonged O2 deprivation on Na+ channels: differential regulation in adult versus fetal rat brain. Neuroscience 94, 1231–1243. [DOI] [PubMed] [Google Scholar]
- Xia Y, Fung ML, O’Reilly JP, Haddad GG, 2000. Increased neuronal excitability after long-term O(2) deprivation is mediated mainly by sodium channels. Brain Res. Mol. Brain Res 76, 211–219. [DOI] [PubMed] [Google Scholar]
- Yochum C, Doherty-Lyon S, Hoffman C, Hossain MM, Zelikoff JT, Richardson JR, 2014. Prenatal cigarette smoke exposure causes hyperactivity and aggressive behavior: role of altered catecholamines and BDNF. Exp. Neurol 254, 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Xue J, Gu XQ, Haddad GG, Xia Y, 2005. Intermittent hypoxia modulates Na+ channel expression in developing mouse brain. Int. J. Dev. Neurosci 23, 327–333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


