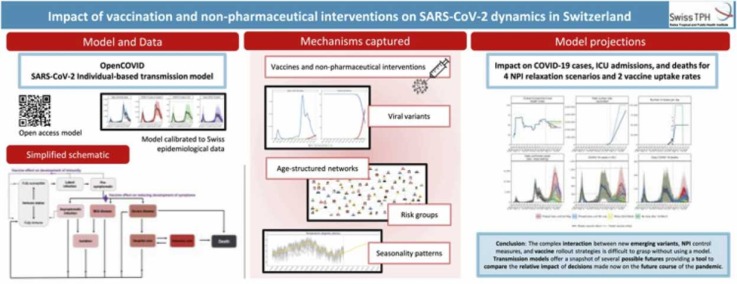
Abstract
Background
As vaccination coverage against SARS-CoV-2 increases amidst the emergence and spread of more infectious and potentially more deadly viral variants, decisions on timing and extent of relaxing effective, but unsustainable, non-pharmaceutical interventions (NPIs) need to be made.
Methods
An individual-based transmission model of SARS-CoV-2 dynamics, OpenCOVID, was developed to compare the impact of various vaccination and NPI strategies on the COVID-19 epidemic in Switzerland. OpenCOVID uses the Oxford Containment Health Index (OCHI) to quantify the stringency of NPIs.
Results
Even if NPIs in place in March 2021 were to be maintained and the vaccine campaigns rollout rapidly scaled-up, a ‘third wave’ was predicted. However, we find a cautious phased relaxation can substantially reduce population-level morbidity and mortality. We find that a faster vaccination campaign can offset the size of such a wave, allowing more flexibility for NPIs to be relaxed sooner. Model outcomes were most sensitive to the level of infectiousness of variants of concern observed in Switzerland.
Conclusion
A rapid vaccination rollout can allow the sooner relaxation of NPIs, however ongoing surveillance of - and swift responses to - emerging viral variants is of utmost importance for epidemic control.
Abbreviation: COVID-19, Coronavirus disease 2019; FOPH, Swiss Federal Office of Public Health; ICU, intensive care unit; NPI, non-pharmaceutical intervention; OCHI, Oxford Containment Health Index; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; VOC, variants of concern
Keywords: COVID-19, Modelling, Vaccination, Non-pharmaceutical intervention, Variants of concern
Graphical Abstract
1. Introduction
The COVID-19 pandemic has caused a public health and economic crisis worldwide. In response to the pandemic, countries implemented non-pharmaceutical interventions (NPIs), but social and economic consequences of certain NPIs made them unsustainable in the long-term. The rollout of COVID-19 vaccines following approval in December 2020 and in early 2021 contributed to a substantial reduction in pandemic burden, thus raising the possibility of relaxing NPIs. While emergence of more transmissible variants presented new challenges, many countries, such as Switzerland, were faced with having to make decisions on when and how to relax NPIs while continuing to protect their population.
Mathematical models have proven to be useful in providing insights to support this type of country-wide decision-making around different control strategies and public health objectives (Flaxman et al., 2020, Scott et al., 2021, Moore et al., 2021). Here, we present an individual-based model, ‘OpenCOVID’, which captures SARS-CoV-2 transmission dynamics using an age-structured population network that includes risk-groups (e.g., healthcare workers or those with comorbidities) and seasonal patterns ( Fig. 1). The model allows for exploration to identify when and by how much NPIs could be relaxed alongside various speeds of vaccine rollout to prevent or limit a potential ‘third wave’ surge of cases, hospitalisations, ICU admissions, and deaths at the national level. Since factors other than control measures can strongly influence the course of the epidemic, using a sensitivity analysis we could examine the potential impact of 1) varying properties of variants of concern (VOC), 2) varying vaccine efficacies, and 3) varying levels of vaccine uptake. We do not consider potential future changes and developments in clinical care and population health; nor potential changes in mass testing or in rates of effective testing, tracing, isolating, and quarantining, or the introduction of new, as of now, interventions.
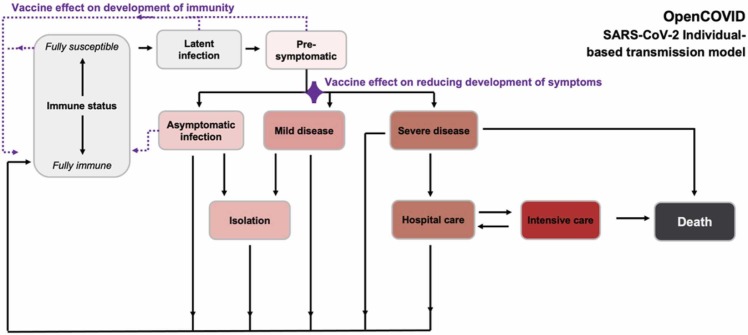
Fig. 1.
Simplified schematic of OpenCOVID model structure. The model captures potential states of individuals. The ‘immune status’ ranges from fully susceptible to fully immune, where any level of immunity is a consequence of previously acquired natural immunity and/or vaccination. Development of immunity is one of the two vaccination effects modelled (indicated by dotted purple lines). Other states include latent infection, pre-symptomatic, and the asymptomatic state from which vaccination may also lead to development of immunity. After infection, some remain asymptomatic, while for others, mild or severe disease progression may occur. The second vaccine effect reduces symptom development (indicated by the purple diamond) as well as potential downstream events (isolation, hospital care, intensive care, and death). Isolation or care (hospital care, intensive care) may be required for those with symptomatic infection, resting in recovery or death. Increasingly darker shading (grey, pink, red, dark grey) indicates increasing severity. Full model details are provided in the Appendix.
As an application of our model, OpenCOVID was calibrated to the Swiss COVID-19 epidemic trajectory, where SARS-CoV-2 began emerging in early 2020, reporting approximately 8725 deaths by the end of January 2021 (FOPH, 2021). In response to the first steep increase in cases (the ‘first wave’) in the spring of 2020, Switzerland introduced a variety of NPIs, such as physical distancing, contact tracing, isolation of contacts, quarantining of confirmed cases, and closure or limited openings of shops and schools, with facemask mandates later introduced. As a result, case numbers, intensive care unit (ICU) admissions, and deaths decreased prior to the summer of 2020, which led to the relaxation of some NPIs. In October 2020, Switzerland experienced a major second wave, as did other European countries, and NPIs were strengthened (FOPH, 2020, ECDC, 2020). The rollout of COVID-19 vaccines in early 2021 raised questions of when and by how much NPIs could be relaxed while still protecting the health of the Swiss population, which we addressed using OpenCOVID and present in this study.
2. Materials and methods
2.1. Model
A new stochastic, discrete-time, individual-based transmission model of SARS-CoV-2 infection and COVID-19 disease, OpenCOVID, was developed. OpenCOVID tracks characteristics of individuals such as age (in one-year age bins), risk-group (namely those with comorbidity), SARS-CoV-2 infection status, COVID-19 disease state, level of immunity, and vaccination details. If infected, an individual's viral load is tracked as a function of time since infection (Appendix Fig. S.5), along with the viral variant with which an individual is infected (inherited from the infector). The model captures viral transmission as infectious and susceptible people come into contact with each other. The probability of transmission is dependent on the viral load of the infectious individual, the variant of the virus being transmitted, and any partial immunity acquired by the susceptible individual (either through previous infection (Hall et al., 2021) or vaccination (Polack et al., 2020; Baden et al., 2021)). Waning immunity was not considered in this study. Further, seasonality affects the probability of transmission (Appendix Fig. S.6) - with lower probabilities in warmer periods - reflecting a larger proportion of people coming into contact outdoors once temperatures warm. Any contact between an infectious and susceptible individual is assumed to carry the same probability of transmission, all else being equal. Human contacts are represented through an age-structured network (Appendix Fig. S.4 (Mossong et al., 2008)). Additional model details are provided in the Appendix. Model code is open source and available from (SwissTPH, 2021).
A newly infected individual will, following a latent period, be assigned through stochastic distributions an age-dependent disease prognosis of either asymptomatic, mild, severe, critical, or eventual death (Fig. 1). These prognosis probabilities are derived from publicly available age-disaggregated morbidity and mortality data (FOPH, 2021, Vaughan et al., 2021). The viral variant an individual is infected with can alter prognosis probabilities, capturing the ability of certain variants to cause increased morbidity and/or mortality (e.g., B.1.1.7 (Davies et al., 2021; Challen et al., 2021)). Cases with prognosis of severe, critical, or eventual death may be admitted to hospital following some delay from symptom onset or may alternatively receive care outside of hospital (e.g., in a care home). Critical cases who are in hospital will be admitted to an ICU, with sufficient capacity assumed in the model. The duration an individual remains in any given disease and/or care state is sampled from a distribution (Appendix Fig. S.8).
2.2. Data
Application of OpenCOVID to the national-level epidemic in Switzerland was informed by publicly available demographic and epidemiological data from the Swiss Federal Office of Public Health, including data on daily age-structured vaccine rates (FOPH, 2021). Climate data originated from MeteoSwiss (FSO, 2021). NPI measures data from various public sources were used to compute national- and cantonal-level interpretations of the OCHI (FOPH, 2020, FOPH, 2021, SwissTPH, 2021). A subset of model parameters (Appendix Table S.1) was calibrated to align model output to six types of epidemiological data, including confirmed cases, patients in hospital, deaths, and prevalence of viral variants (Chen et al., 2021) from 18 February 2020–5 March 2021 (see Fig. 2 and Appendix Fig. S.1-S.3). A log-likelihood objective function was used to measure the overall quality of the model fit weighted for each data type. The effect of NPIs on contact rates was also subject to model calibration, where the OCHI index was proportionally scaled to represent a reduction in contacts. See the Appendix for calibration details and outcomes.
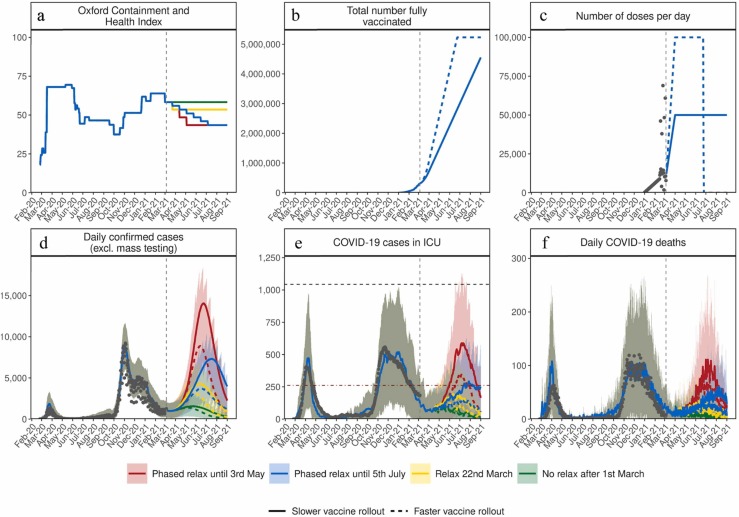
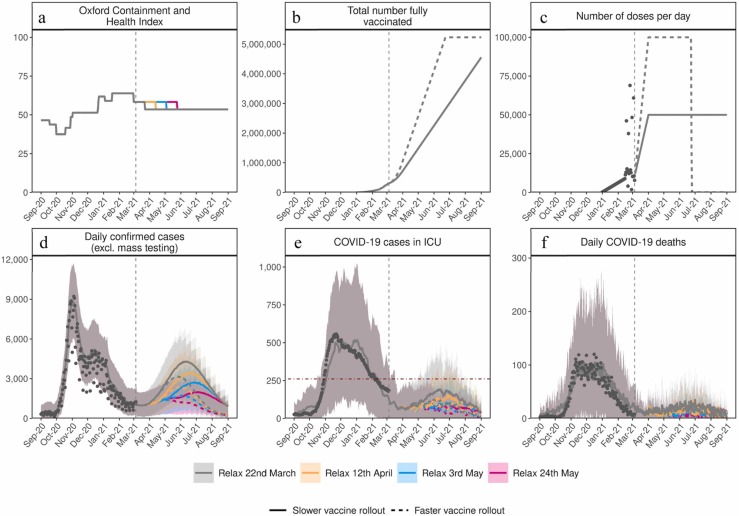
Fig. 2.
Estimated impact of vaccination and NPI relaxation scenarios on the SARS-CoV-2 epidemic in Switzerland. (a) Oxford Containment and Health Index: A measure for the stringency of NPIs from 24 February 2020–21 March 2021 and projections for four relaxation scenarios thereafter. (b) Total number fully vaccinated: Cumulative amount of fully vaccinated persons (assuming two doses). (c) Number of doses per day: Number of vaccine doses administered per day. (d) Daily confirmed COVID-19 cases: Model estimates of the number of confirmed COVID-19 cases per day (not accounting for future testing changes including mass testing). (e) COVID-19 cases in ICU: Model estimates of COVID-19 patients in ICU. (f) Daily COVID-19 deaths: Model estimates of daily COVID-19-related deaths. In all panels, dark grey dots show data to date. Coloured curves show simulation results of different relaxation scenarios and two vaccine rollout scenarios. Vaccination scenarios: Solid curves correspond to a vaccination scenario assuming 50,000 vaccines are administered per day, while the dashed curves correspond to a faster vaccination scenario of 100,000 vaccines per day. NPI scenarios (see details in Table 1): Red represents an NPI relaxation scenario with relaxation steps on 22 March, 12 of April, and 3 of May. Blue represents a slower NPI relaxation scenario compared with the red scenario, with smaller relaxations in three-weekly steps from 22 March to 5 July. Yellow represents an NPI relaxation scenario with relaxation only on 22 March and no further NPI relaxations. Green represents a strategy with no further NPI relaxation after 1 March (an unrealistic scenario of no relaxations through to September 2021, provided as a reference only). The vertical dashed lines represent the date at time of analysis. The horizontal grey dashed line in panel (e) for COVID-19 cases in ICU depicts the estimated maximum national capacity of ICU beds and the horizontal red dashed line 25% ICU capacity. Predictions of confirmed cases, ICU capacity, and mortality are reported as mean estimates with 95% prediction intervals.
2.3. Diagnosis
Upon infection, an individual is assigned a date at which they may potentially be diagnosed as a consequence of test seeking behaviour. The delay between symptom onset and a potential diagnosis for each individual is sampled from a Gaussian distribution. We assume all non-severe cases isolate for a 10-day period immediately following diagnosis. For individuals presenting with severe disease that seek hospital care prior to diagnosis, a test (and consequent diagnosis) is assumed to be carried out once they are admitted to hospital.
In this application for Switzerland, we derive numbers of diagnoses over time directly from data of confirmed COVID-19 cases. All COVID-19 cases that seek hospital care receive a diagnosis. After taking hospitalised diagnoses into account, other individuals with severe disease outside of the hospital setting and individuals with mild disease are randomly selected and assigned a diagnosis in the model. To represent future test-seeking behaviour, the model-calculated proportion of cases diagnosed per infected case over the past 14-days is maintained into the future (Appendix Fig. S.9). This assumption is not robust to major changes in testing policies or behaviours, including, but not limited to, mass testing.
2.4. Immunity
Following a period of infection, surviving individuals recover to a state in which viral shedding no longer occurs. These recovered individuals are assumed to be susceptible to reinfection but are assumed to retain some level of partial immunity, which reduces susceptibility to subsequent exposure. OpenCOVID can capture immunity decay, but for this study we optimistically assume no immunity decay following naturally acquired or vaccine-induced immunity due to the short-term nature of the simulation period. For naturally acquired immunity due to infection, we assume an 83% transmission blocking effect when/if re-exposed (Hall et al., 2021). If a previously infected but recovered individual is later vaccinated, the level of transmission blocking immunity is taken to be the highest of the two independent effects. No synergistic effect is considered. Although the transmission blocking effect of naturally acquired immunity and vaccine-induced-acquired immunity can be of different strengths, they are both included in the overarching ‘immune state’ in the model (Fig. 1).
2.5. Non-pharmaceutical interventions (NPIs)
In OpenCOVID, NPIs can curb the spread of SARS-CoV-2 in an otherwise unprotected population by reducing the number of potentially transmissible pairwise contacts. In Switzerland, NPIs have targeted several aspects of public life, including closure of shops, restaurants, and other entertainment venues, restrictions in sizes of spontaneous gatherings, the cancellation of events, and facemask mandates in publicly accessible spaces. The Oxford Containment and Health Index (OCHI) is a quantity that is proportional to the amount (or stringency) that such measures are in place at a given moment in time (Hale et al., 2021). The level of cantonal and federal measures are based on publicly available information (SwissTPH, 2021). This publicly available information is translated into 16 OCHI levels for Switzerland, together with a calibrated multiplicative scaling parameter is used in our model to capture the effect of NPIs in reducing the effective daily number of contacts. Switzerland collects 16 OCHI-related variables and attributes them to 13 variables that make up the OCHI. This analysis was conducted in early March 2021 prior to a Federal decision for potential relaxation of NPIs planned to begin 22 March.
2.6. Variants
OpenCOVID tracks the transmission of multiple viral variants. Variants are imported into the modelled population 7-days prior to being identified in national genomic surveillance data (Appendix Fig. S.3). We assume the variant with which an individual is infected is inherited from the infector. At the time of analysis, B.1.1.7 was the dominant variant in Switzerland, replacing D614G (FOPH, 2021, Chen et al., 2021). We modelled three variants in this study: D614G, B.1.1.7, and B.1.351. A 60% increase in B.1.1.7 transmissibility relative to D614G best matched variant prevalence data between January 2021 and February 2021 (Appendix Fig. S.3). For B.1.351, a 10% increase in transmissibility relative to D614G was assumed (Reichmuth et al., 2021). For the primary results reported in this study, we assumed no increased probability of morbidity or mortality due to viral variants. However, we assess the impact of this scenario in a sensitivity analysis.
2.7. Vaccines
Vaccine rollout was modelled according to the strategy defined by the Swiss Federal Office of Public Health (FOPH) of 50,000–100,000 doses per day (FOPH, 2021). A timeline of vaccination rollout released prior to the modelled scenarios is presented in Table 1. Fully susceptible, partially susceptible, and actively infected individuals not in hospital can potentially receive a vaccine. Vaccination is modelled using two properties: first, to trigger an immune response that blocks transmission for a proportion of exposure events, and second, to reduce the probability of developing symptoms if infection does occur. Upon vaccination, there is a time delay until the full efficacy of the vaccine is realised; a sigmoidal curve is used to represent this growth in vaccine effect. Vaccine efficacy, delay to full efficacy, transmission blocking effect, and number of doses required is vaccine specific (Appendix Table S.5). In this study, we consider only mRNA vaccines and assume all vaccinated individuals receive two doses spaced by 3-weeks with vaccination reaching maximum efficacy 28-days after the first dose (Polack et al., 2020, Baden et al., 2021). We assume the vaccine is 80% transmission blocking and has a further 75% probability of preventing symptoms leading to the observed 95% vaccine efficacy reported in clinical trials (Polack et al., 2020, Baden et al., 2021). In this study, we do not model decay of vaccine efficacy over time or reduction in vaccine efficacy due to variants of concern, but the model is able to capture changes to these assumptions.
Table 1.
Summary of model scenarios from Figs. 2 and 3 Oxford Containment and Health Index (OCHI) levels are detailed in the Materials and methods.
| Scenario | NPI relaxation speed | NPI OCHI level on 22 March |
NPI OCHI level on 12 April |
NPI OCHI level on 3 May |
NPI OCHI level on 5 July |
Vaccination speed | VOC transmission | Vaccine transmission blocking |
|---|---|---|---|---|---|---|---|---|
| 1A) RED | NPI relaxation steps on 22 March, 12 April, and 3 May 2021 | 53.5 similar to levels in early June 2020 |
48.5 similar to levels in early July 2020 |
43.5 similar to levels at the end of September 2020 |
43.5 similar to levels at the end of September 2020 |
50,000 per day (solid curve) or 100,000 per day (dashed curve) |
Baseline – 60% higher transmission | Baseline – 80% transmission blocking |
| 1B) BLUE | Small NPI relaxations every 3-weeks from 22 March to 5 July 2021 | 55.9 similar to levels in early June 2020 |
53.5 similar to levels in early June 2020 |
51.0 similar to levels in November and December 2020 |
43.5 similar to levels at the end September 2020 |
50,000 per day (solid curve) or 100,000 per day (dashed curve) |
Baseline – 60% higher transmission | Baseline – 80% transmission blocking |
| 1C) YELLOW | NPI relaxation step on 22 March 2021 with no further relaxation (in accordance with FOPH communication 17 February 2021) | 55.9 similar to levels in early June 2020 |
55.9 similar to levels in early June 2020 |
55.9 similar to levels in early June 2020 |
55.9 similar to levels in early June 2020 |
50,000 per day (solid curve) or 100,000 per day (dashed curve) |
Baseline – 60% higher transmission | Baseline – 80% transmission blocking |
| 1D) GREEN | No further relaxation after 1 March 2021 | 58.3 since 1 March 2021 |
58.3 since 1 March 2021 |
58.3 since 1 March 2021 |
58.3 since 1 March 2021 |
50,000 per day (solid curve) or 100,000 per day (dashed curve) |
Baseline – 60% higher transmission | Baseline – 80% transmission blocking |
2.8. Model simulations
This analysis and COVID-19 model outcomes are conducted at the national level, and therefore do not capture the substantial heterogeneity within or between Swiss cantons but this model can also be applied at the subnational level. All outcomes are reported as mean estimates alongside prediction intervals representing parameter and stochastic uncertainty. Model simulations were initiated on 18 February 2020, 7-days before the first cases were confirmed for three consecutive days (25–28 February 2020) in Switzerland. All model processes were computed at 1-day time intervals. A number of initial cases were imported into the population, which were then able to cause onwards infection. A number of infections are also imported into the population at each time step. Furthermore, new virus variants are initiated by importing a number of new cases of each variant at a given time in alignment with the point the particular variant was first identified in Switzerland. All three importation rates are found through model calibration (see the following section). The number of individuals simulated in the model is capped at a predefined number (one million individuals for all simulations), with a population scaling factor applied to all relevant model outputs to represent a one-to-one scale for the Swiss population of 8.5 million.
Numerous model outputs are captured and reported temporally, including number of infections, diagnosed infections, morbidity, and mortality estimates. Where appropriate, metrics are disaggregated by age and variant of concern (Appendix Figs. S.2 and S.3).
2.9. Scenarios
The OpenCOVID model was used to predict national-level epidemic trajectories from early March to early September 2021. We did not simulate beyond this date due to substantial uncertainty around duration of naturally acquired and vaccine-induced immunity, the impact of additional new variants, and changes in NPIs, adherence, and testing.
Two vaccination rollout speeds (slower, 50,000 doses per day, and faster, 100,000 doses per day) and a range of NPI relaxations were modelled. Vaccine rollout up to 5 March 2021 was modelled as per publicly available data (FOPH, 2021). Vaccine eligible individuals were assigned to one of five priority groups according to age, comorbidity, residential location, and profession, and vaccines were not given to hesitant individuals. Priority groups were modelled with subsequent vaccination with two doses of an mRNA vaccine following the Swiss FOPH strategy (FOPH, 2021)(see Table 2 for further details). As per Swiss vaccination guidelines at the time of analysis, individuals under 18 years of age were not considered eligible for vaccination. We assumed that 75% of each priority group was willing to be vaccinated. NPI relaxation scenarios were modelled as described in Table 1. An NPI ‘relaxation step’ translates to approximately 5 points on the OCHI, reflecting the set of NPI relaxations (further described in the Appendix) proposed by the Swiss Federal Council to occur initially on 1 April 2021 (subsequently re-scheduled to 22 March 2021 (FOPH, 2021). For all scenarios, we assumed consistent adherence to measures over time. Similar levels of testing to those from early February 2021 to early March 2021 was assumed for these simulations, the potential impact of mass testing or widespread testing outreach was not modelled.
Table 2.
| Scenario | NPI relaxation speed (blue scenario from Fig. 2) OCHI levels on 22 March of 55.9, 12 April of 53.5, 3 May of 51.0, 5 July of 43.5 |
Vaccination speed | Variants of concern (VOC) |
Vaccine transmission blocking | Vaccination coverage (accounting for hesitancy or increased acceptance among groups P2-P5 for certain scenarios) |
|---|---|---|---|---|---|
| 3A) DARK ORANGE | Small NPI relaxations every 3-weeks between 22 March and 5 July | 100,000 doses per day | Baseline – 60% higher transmission | Lower transmission blocking - 60% transmission blocking | Baseline – 75% coverage for P1-P5 |
| 3B) LIGHT ORANGE | Small NPI relaxations every 3-weeks between 22 March and 5 July | 100,000 doses per day | Baseline – 60% higher transmission | Higher transmission blocking – 95% transmission blocking | Baseline – 75% coverage for P1-P5 |
| 4A) DARK GREEN | Small NPI relaxations every 3-weeks between 22 March and 5 July | 100,000 doses per day | Baseline – 60% higher transmission | Baseline – 80% transmission blocking | 75% coverage for P1 60% coverage for P2-P5 reflecting higher vaccine hesitancy |
| 4B) LIGHT GREEN | Small NPI relaxations every 3-weeks between 22 March and 5 July | 100,000 doses per day | Baseline – 60% higher transmission | Baseline – 80% transmission blocking | 75% coverage for P1 90% coverage for P2-P5 reflecting lower vaccine hesitancy |
| 5A) DARK PURPLE | Small NPI relaxations every 3-weeks between 22 March and 5 July | 100,000 doses per day | Higher transmission – 70% higher transmission of B.1.1.7 relative to D614G | Baseline – 80% transmission blocking | Baseline – 75% coverage for P1-P5 |
| 5B) LIGHT PURPLE | Small NPI relaxations every 3-weeks between 22 March and 5 July | 100,000 doses per day | Lower transmission – 50% higher transmission of B.1.1.7 relative to D614G | Baseline – 80% transmission blocking | Baseline – 75% coverage for P1-P5 |
| 6A) DARK PINK | Small NPI relaxations every 3-weeks between 22 March and 5 July | 100,000 doses per day | Baseline – 60% higher transmission and with 50% higher mortality of B.1.1.7 relative to D614G | Lower transmission blocking – 60% transmission blocking | Baseline – 75% coverage for P1-P5 |
P1 (highest priority, adults older than 75 years of age); P2 (65–75 years, 18–64 years with comorbidity, and healthcare workers); P3 (household members of those at high-risk from groups P1 and P2); P4 (18–64 years in communal facilities and their caregivers); P5 (18–65 years of age with no comorbidities and not in communal facilities). See the Appendix for additional details.
2.10. Sensitivity analysis
To assess the sensitivity of our findings, we simulated scenarios that independently varied several key model parameters related to vaccine characteristics and currently circulating variants of concern (see Table 2 for details).
3. Results and discussion
3.1. Scenario projections
Through fitting to national-level data, the model estimated that 18–26% of the Swiss population had been exposed to SARS-CoV-2 by the end of February 2021, approximately aligned with seroprevalence surveys (Stringhini et al., 2021, West et al., 2020). It was assumed that variant B.1.1.7 is 60% more transmissible than the previously dominant variant D614G (Binois et al., 2018). For control measures in place from January to February 2021, this increased transmissibility translates to a relative transmission advantage of 1.3–1.4 over this period.
Findings from our scenario analyses suggest that even if NPIs in place in early March 2021 would have been maintained through to September 2021 (an unlikely scenario), daily cases, hospitalisations, and deaths would have increased ( Fig. 2, Fig. 3) and would have resulted in a third wave. Any relaxation of NPIs during the month of April was also predicted to lead to increases in all three indicators ( Fig. 4, Fig. 5). This is due to 1) increased rates of human contact following relaxation of NPIs, 2) the majority of the population still being immunologically naïve (i.e., no prior infection or vaccination)(Appendix Fig. S.10), and 3) increasing incidence of VOC with higher transmissibility. Since these findings may be sensitive to uncertainties in VOC properties, these factors were further explored via sensitivity analysis. Scenario details and assumptions are provided in Table 1, Table 2.
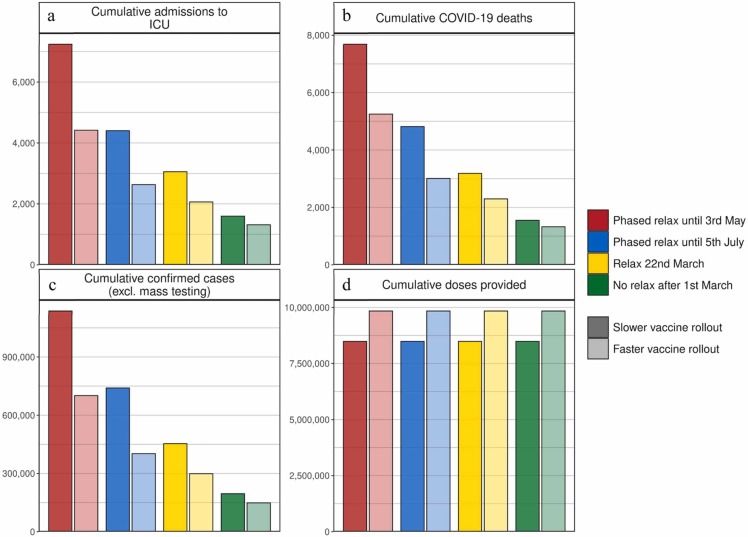
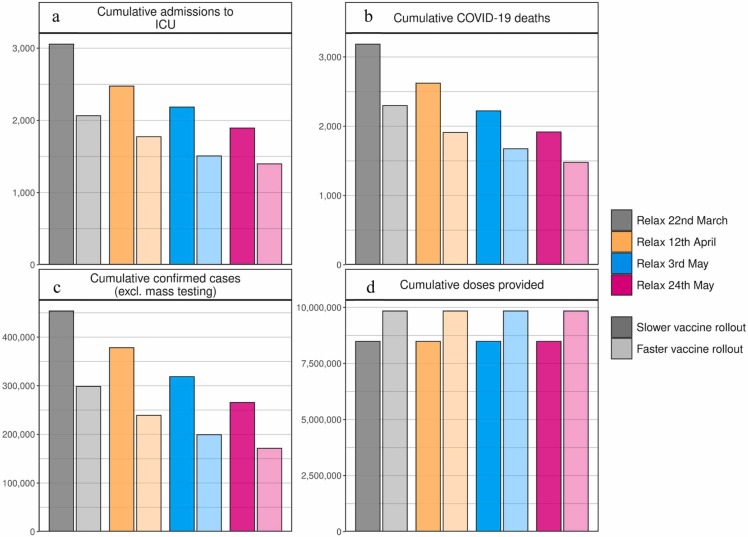
Fig. 3.
Cumulative estimates of vaccine doses provided, confirmed cases, ICU admissions, and COVID-19 deaths from 6 March to 1 September 2021. (a) Cumulative admissions to ICU, (b) cumulative COVID-19 deaths, (c) cumulative confirmed COVID-19 cases (excluding mass testing), and (d) cumulative vaccine doses provided. Bar colours indicate NPI relaxation and vaccination scenarios as per Fig. 2, darker shaded bars correspond to vaccination scenarios with 50,000 vaccines administered per day, lighter shaded bars to faster vaccination scenarios with 100,000 vaccines administered per day.
Fig. 4.
Comparison of the impact of delaying single-step NPI relaxation and vaccination scenarios on SARS-CoV-2 dynamics in Switzerland over time. (a) Oxford Containment and Health Index: A measure of the stringency of NPI measures from 24 February 2020 until 21 March 2021 and for four exemplar relaxation scenarios with one step relaxation. NPI scenarios: Grey curves represent an NPI relaxation scenario with relaxation only on 22 March with no further NPI relaxation (as detailed in the yellow scenario in Table 1); orange curves have the same level of relaxation but implemented three-weeks later on 12 April; blue curves have the same relaxation on 3 May, and pink curves the same relaxation on 24 May. (b) Total number fully vaccinated: Cumulative number of fully vaccinated persons (assuming two doses) shown daily. (c) Number of doses per day: Number of vaccine doses administered per day. (d) Daily confirmed cases (excl. mass testing): Model estimates of the number of confirmed COVID-19 cases per day excluding mass testing and not accounting for testing changes including mass testing. (e) COVID-19 cases in ICU: Model estimates of COVID-19 patients in ICU. (f) Daily COVID-19 deaths: Model estimates of daily COVID-19-related deaths. Grey dots show data available at the time of analysis. Coloured curves show simulation results for the different relaxation scenarios with the two vaccine rollout scenarios. Vaccination scenarios: Solid curves correspond to vaccination scenarios assuming 50,000 vaccine doses are administered per day, while dashed curves correspond to a faster vaccination scenario with 100,000 vaccines per day. Vertical grey dashed lines represent the date at the time of simulation (March 2021). Horizontal red dashed line in panels (e) for COVID-19 cases in ICU depicts a level of 25% of ICU capacity. Predictions of confirmed cases, cases in ICU, and deaths (panels d-f) are mean estimates with 95% prediction intervals.
Fig. 5.
Cumulative estimates of confirmed cases, ICU admissions, and COVID-19 deaths, as well as vaccine doses provided between 6 March and 1 September 2021 for various delays in single-step NPI relaxation scenarios. (a) Cumulative admissions to ICU, (b) cumulative COVID-19 deaths, (c) cumulative confirmed COVID-19 cases (excluding mass testing), and (d) cumulative vaccine doses provided. Bar colours indicate NPI relaxation and vaccination scenarios as per Fig. 4 with darker shaded bars corresponding to the slower vaccination scenario with 50,000 vaccinations per day and lighter shaded bars to the faster vaccination with 100,000 vaccinations per day.
The size of the resulting third wave was strongly dependent on the timing and amount of NPI relaxation. Faster and stronger relaxations led to larger third waves, while slower relaxations resulted in a smaller, but also a delayed third wave peak (Fig. 2). If relaxation was particularly strong, either in large steps or via quick successive relaxations (e.g., the ‘red’ scenario), we estimated that ICU occupancy would increase above 25% capacity, a key indicator for decision-makers (FOPH, 2021). The size of the projected potential third wave could have been further substantially reduced with a higher vaccination rate. We found that increasing vaccination rates from 50,000 to 100,000 doses per day (0·6% and 1·2% of the Swiss population, respectively) resulted in a halved and slightly earlier third wave peak. Furthermore, for the more gradual phased relaxation scenario, this increased vaccination rate resulted in a substantial reduction in ICU occupancy and deaths over the period from March to September 2021.
The impact of delaying NPI relaxation was explored by comparing a relaxation on 22 March with the same relaxation delayed by 3-, 6-, or 9-weeks (Fig. 4). Simulations indicated that delaying relaxation could lead to fewer cases, less morbidity, and less mortality. However, similar gains could also be achieved through faster vaccination, thus facilitating a more flexible epidemic exit strategy. Scenarios involving quicker NPI relaxation led to more person-to-person contact and increased transmission (Fig. 2). In turn, such scenarios had earlier levels of population-level natural immunity, which along with vaccination-induced immunity, built-up incrementally until the summer of 2021 (Appendix Fig. S.10). In general, such scenarios result in a predicted large wave of infections, high ICU occupancy, and increased deaths in spring and into summer 2021 (Fig. 2, Fig. 3). The peak in cases occurred when there were a sufficient number of people with natural immunity due to infection or vaccine-induced immunity, so while NPI measures were in place, transmission largely decreased. Subsequent decay in new daily cases occurs because fewer individuals were susceptible, and along with NPI measures having remained in place, resulted in an effective reproduction number below one. When projections increased towards Switzerland’s maximum ICU capacity or to death rates as high as observed in previous waves, this type of trajectory over a prolonged period would become increasingly unlikely as additional measures would likely be implemented.
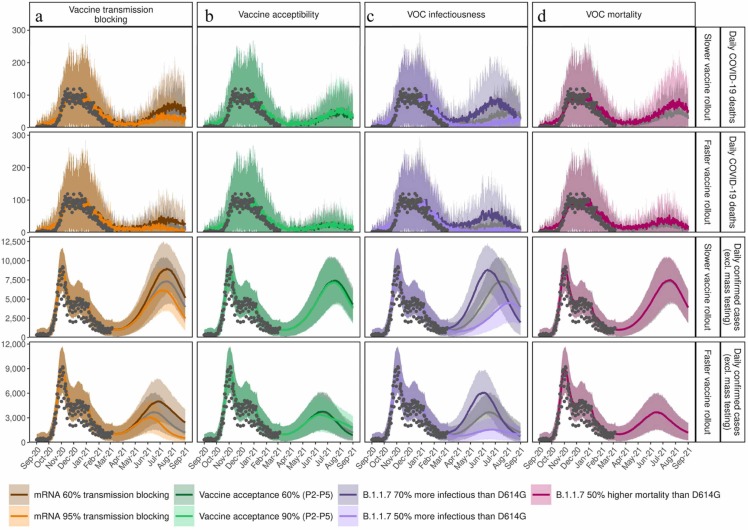
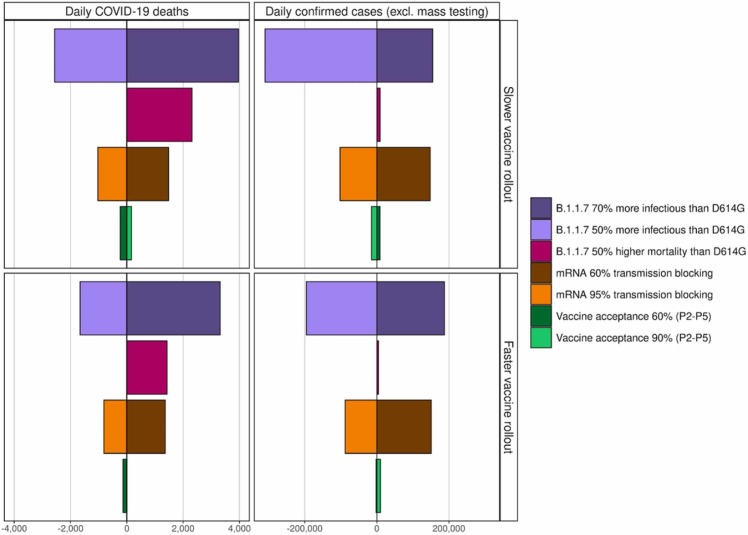
Assumptions around increased transmission of and mortality from new VOC, vaccine properties, and vaccine hesitancy were further explored. The biggest driver of impact from vaccination and NPI relaxation on mortality was the level of increased transmission from VOC ( Fig. 6, Fig. 7). If new variants are more transmissible than assumptions explored here (i.e., greater than 70%), then there is considerable risk of overwhelming the health system. Furthermore, depending on the NPI relaxation strategy and speed of vaccination, if new variants have 50% higher mortality than D614G (Davies et al., 2021, Challen et al., 2021), then 41–44% more deaths could have occurred from March 2021 to September 2021. Even with faster vaccination, if individuals who were vaccinated but then became infected caused partial onward transmission (i.e., assuming less than 65% transmission blocking), an even slower NPI relaxation would have been needed to avoid subsequent re-strengthening of measures. Regardless of vaccination speed, for the scenario with higher vaccine acceptability (90%) among groups P2-P5, fewer cases were predicted during the third peak of the epidemic, compared with lower acceptability of 60% (Fig. 6b).
Fig. 6.
Sensitivity of predictions for daily confirmed cases and COVID-19 deaths given assumptions for vaccine transmission blocking, vaccine acceptability, infectiousness of VOC, and potential increased mortality from VOC.
Fig. 7.
Cumulative mean impact of slower relaxation until 5 July represented by the blue reference scenario from Fig. 2 and Table 1 on daily COVID-19 deaths and daily confirmed cases given the sensitivity analysis from Fig. 6 and Table 2.
Time series for daily COVID-19 deaths (rows 1 and 2) and daily confirmed COVID-19 cases (excluding mass testing) (rows 3 and 4) between 1 September 2020 and 1 September 2021 with slower relaxations every three-weeks from 22 March to 5 July corresponding to the blue scenario with either a slower vaccination of 50,000 vaccines per day (rows 1 and 3) or faster vaccination with 100,000 per day (rows 2 and 4). Results of the blue scenario are represented by grey dots, and colours show the impact of (a) vaccine transmission blocking, influenced by the assumption for the transmission blocking property of the vaccine. Dark orange curves represent 60% vaccine transmission blocking, light orange curves 95% transmission blocking, and grey curves the best estimate for 80% transmission blocking used in Fig. 2, Fig. 3. The transmission blocking property of the vaccine is offset with the symptom blocking property, so the vaccine always has an efficacy of 95% in reducing symptoms. (b) Vaccine acceptability, in terms of coverage, represents assumed vaccine hesitancy resulting in a change in vaccination coverage. Dark green curves represent 60% coverage, light green curves 90% coverage, and grey curves the best estimate of 75% coverage used in Fig. 2, Fig. 3. (c) VOC infectiousness represents assumed increased transmission for variant B.1.1.7. Dark purple curves show a 70% increased transmissibility (with transmission advantage of 1.4–1.5), light purple curves 50% increased transmissibility (transmission advantage 1.2–1.3), and grey curves, the reference scenario, 60% increased transmissibility (transmission advantage 1.3–1.4) used in Fig. 2, Fig. 3. (d) VOC mortality with dark pink curves representing an assumed 50% increased mortality for variant B.1.1.7 compared with D614G, while grey curves the same mortality assumptions as for variants that emerged in 2020. Predictions of confirmed cases and mortality are reported as mean estimates with 95% prediction intervals.
Estimated impact on (a) daily COVID-19-related deaths and (b) daily confirmed COVID-19 cases (excluding mass testing) from 1 September 2020–1 September 2021 given the different assumptions for infectiousness of VOC, potential increased mortality from VOC, and vaccine transmission blocking and vaccine acceptability as illustrated in Fig. 6. Colour and shading schemes are identical to those from Fig. 6. Dividing horizontal lines at zero indicate reference cumulative mean estimates from Fig. 3, for the dark blue bars modelled with 50,000 vaccines per day or light blue bars with 100,000 vaccines per day both representing a slow, phased NPI relaxation scenario of three-weekly steps from 22 March to 5 July 2021. The influence of assuming higher transmission of variant B.1.1.7 is represented by dark purple bars with 70% higher transmissibility (corresponding to a transmission advantage of 1.4–1.5) and by light purple bars with 50% higher transmissibility (transmission advantage 1.2–1.3) over the reference of 60% transmissibility for D614G. The influence of assuming 50% higher mortality for B.1.1.7 is represented by dark pink bars. Influence of the assumption for the transmission blocking property of the vaccine is illustrated by dark orange bars representing a vaccine with 60% transmission blocking efficacy or by light orange bars 95% transmission blocking. The transmission blocking property of the vaccine is offset with the symptom blocking property, so the vaccine always has a 95% efficacy for reducing symptoms. Influence of the assumption for vaccine hesitancy, resulting in a change of vaccination coverage, is represented by dark green bars showing 60% coverage (acceptance) or by light green bars with 90% coverage compared with the reference 75% coverage. For all bars, negative values to the left of the horizontal lines indicate fewer cases or deaths were predicted compared with the reference scenario, and positive values to the right, more deaths or cases were predicted.
3.2. Interpretation of findings
The OpenCOVID model has proven to be useful for comparing scenarios of varying NPI relaxation strategies, vaccination rollout strategies, and vaccination efficacies, as well as for examining the impact of new variants on transmission dynamics. Even accounting for uncertainty around the relative transmission advantage of new variants, all scenarios indicated that faster vaccination and more cautious, phased, NPI relaxation would lead to more optimistic COVID-19 incidence and mortality. This in turn would lead to reduced risk of surpassing 25% ICU capacity from COVID-19 patients and hence to a reduced need to have re-strengthened measures in spring or summer 2021 in Switzerland. Higher than 25% ICU occupancy from COVID-19 patients has the potential to fully exhaust capacity when ICU occupancy from other ailments is considered (FOPH, 2021, Zhao et al., 2020).
We found that significant delays exist between changing NPI policies and the measurable effect on case numbers, hospitalisations, and ICU admissions. Specifically, the consequence of a relaxation was not observed in projections of ICU admissions until at least four- to six-weeks later. Given these delays, making decisions to relax NPI measures made more frequently than every four- to six- weeks (ScienceTaskForce, 2020) runs the risk of causing undesirable knock-on effects, including mounting pressure on ICUs and a later need to re-strengthen measures. Furthermore, if NPIs are relaxed too frequently or too strongly before a maximum ICU capacity trigger is reached, a larger peak in ICU occupancy is likely to occur and a stronger reactive strengthening of NPIs may be required. Decisions around trigger points for changing NPI measures should take this delay into account to better control ICU occupancy.
Without sufficient person-to-person contact reduction from NPIs, we found that a vaccination strategy of 50,000 to 100,000 doses per day (as was scheduled at the time of the analysis) would be insufficient to control COVID-19-related mortality. Crucially, given the increased transmissibility of variant B.1.1.7, even without any further NPI relaxation after 1 March 2021, a third wave was expected, and, at the time of writing, cases were already increasing across Europe. Therefore, any relaxation of NPIs would likely have led to another wave of cases; however, faster vaccine uptake, alongside a more gradual NPI relaxation, would have minimised this wave and its associated mortality. Our phased relaxation scenarios indicated that if NPIs would have been relaxed too soon or too aggressively, the third wave that could have resulted would have had the potential to overload the Swiss health system (e.g., the red scenario shown in Fig. 2) and could thus have prompted the need to re-strengthen NPIs.
In retrospect, our scenario design differed somewhat from the vaccine rollout and NPI strategies as they actually occurred in Switzerland from 6 March to 1 September 2021 (see Appendix for details). Whilst the actual vaccine rollout did reach levels of 100,000 doses per day, this occurred somewhat later than assumed in the ambitious vaccine scenarios. OCHI values from the actual implemented NPIs approximately reflected levels assumed in scenario 1C (yellow curves in Fig. 2). In general, the model represented the magnitude of most metrics reasonably well, however, timing was not well captured as peaks were observed quicker than the model had predicted (Appendix Fig. S12). One potential factor for this discrepancy could be the difference between assumed and actual time to peak vaccine rollout. Broadly, the model was too optimistic in terms of ICU occupancy, and too pessimistic in terms of deaths (Appendix Fig. S12). Whilst the total numbers vaccinated in the ambitious vaccine scenarios were somewhat similar to those actually observed, a higher vaccine coverage was achieved among age groups most at risk of COVID-19-related death (70–79 and 80 +) than the assumed 75% (Appendix Fig. S13). This likely contributed to the overestimate of deaths. Further, lower vaccine coverage among age groups 30–39, 40–49, and 50–59 were achieved in reality compared with the assumed 75% coverage modelled. These age groups are more likely to require hospital and ICU care relative to the risk of death, which may be contributing to underestimate of ICU occupancy.
Other key factors also influenced projections including uncertainty surrounding infectiousness of the alpha variant, B.1.1.7, which was the dominant strain in Switzerland at the beginning of the analysis period. At the time of analysis, less was known about variant transmissibility; however, a study published after this analysis was completed reported that B.1.1.7 was found to be up to 90% more transmissible than its predecessors (Davies et al., 2021). It is therefore possible that B.1.1.7 was and is more infectious than the 60% higher transmissibility we assumed relative to D614G (and more infectious still than the 70% higher transmission simulated as part of the sensitivity analysis shown in Fig. 6c).
Our results, which are based on the Swiss COVID-19 epidemic, but can be interpreted on a more generalisable scale, suggest that to safeguard against substantial future waves, rigorous and continued monitoring of vaccination uptake, NPI adherence, and viral variant emergence is required. Whilst vaccination is ongoing, it is critical to continuously assess the impact of each NPI relaxation over a sufficient length of time before committing to additional relaxations. Effective communication with the public is key to ensure the successful uptake of vaccines and to ensure that NPI measures, including hand hygiene, physical distancing, and facemask use, continue to be followed. Even once active recruitment for individuals to get additional vaccine doses ends, facemasks, and some level of physical distancing will likely still be required (Christie et al., 2021), and for this reason we modelled the lowest level of NPIs to be roughly equivalent to measures that were in place in Switzerland in September 2020.
Several simplifying assumptions were made in the modelled scenarios. At the time of analysis, the extent to which immunity wanes over time was not well understood, thus we assumed no loss of naturally acquired or vaccine-induced immunity for the short period of simulation. It will be crucial to monitor waning immunity over the coming 6- to 12-months, as well as vaccine efficacy on novel variants. Vaccinating people under 18 years of age (since at the time of writing vaccine safety in children had yet to be tested) and delaying of second vaccine doses to increase coverage of first doses (or because of dosing shortages) were not explored, nor were third dose booster vaccines for those over 65 years of age (as they were not approved at the time of writing). We did not consider potential changes in behaviour among people once they were vaccinated. We only considered variants of concern identified through Swiss genomic surveillance data (Chen et al., 2021); B.1.1.7, the most prevalent VOC in Switzerland as of March 2021, and B.1.351. We did not consider P.1 nor the delta variant, B.1.617.2, for which vaccines may be less efficacious (Fontanet et al., 2021, Madhi et al., 2021). As more information about VOC becomes available, additional model simulations will be required to further examine the effects of new variants on the epidemic.
We used the Oxford Containment and Health Index (OCHI) to measure the strength of NPIs. This single-value index integrates 13 different SARS-CoV-2 protection measures. Equal levels of OCHI can be reached by different combinations of NPIs. This means that the scenarios modelled are not specific to a certain defined relaxation scenario, but rather treat the effect of a certain total amount of relaxations as measured by the OCHI. The set of relaxation scenarios modelled in this study was chosen to allow exploration of interactions between different vaccination speeds and delays of relaxation, and do not represent explicit measures that were planned for Switzerland.
While we quantified short-term consequences of COVID-19 such as hospitalisations and mortality, we did not quantify long-term sequelae associated with non-severe and severe cases. While on average COVID-19 symptoms last for two weeks on average, an estimated one in ten people suffer with symptoms for more than 12 weeks defined as ‘long COVID’. Long COVID and disability associated with severe symptoms was not considered; however, the impact of these factors could be substantial, as symptoms, including fatigue, anxiety, joint or muscle pain, and more, could have a considerable impact on physical and mental health, and on workforce participation. In this context, our results were more optimistic since outcomes of long COVID and disability from severe symptoms were not considered. We also did not include economic consequences and secondary health impacts of relaxing and potentially re-strengthening measures. These must be considered as part of any policy decision, alongside additional economic and health analysis, and should be a priority for future modelling analyses.
As for any epidemic model, considerable uncertainty exists when projecting further into the future. While the current pandemic will not end in September 2021, we ended our simulations at that time point due to increasing uncertainty of predicting beyond six-months. Moving forward, it will be important to monitor the effect of waning immunity and any benefit from summertime climatic effects. We optimistically assumed that the seasonal effect of reduced transmission seen in summer 2020 will apply equally to the new variants of concern. It is important to highlight that the numbers of predicted cases are thus influenced by this phenomenon and that the opposite effect will occur in autumn and winter, also supporting advocacy for both high vaccination rates before autumn periods and continued effective monitoring. Precise transmission levels of new variants of concern are not fully known. Transmissibility directly affects the measure of the effective reproductive number. Likewise, the effectiveness of various COVID-19 vaccines on existing and possible new emerging variants is not yet fully known. Changes in testing (including mass testing), changes in NPI measures, and adherence to those measures may result in different future trends. Furthermore, communication of expected epidemic trends may lead to behavioural changes that may make these trends less likely (such as the population becoming more careful and reducing contacts if cases are expected to increase, and vice versa). Such potential behavioural changes were not modelled. Although we took great effort to inform our model with the best available data and assumptions, the purpose of analysis was not to inform future predictions. Instead, our findings provide outcomes for various potential scenarios comparing the relative impact of different SARS-CoV-2 control strategies.
4. Conclusion
Disease models allow us to explore counterfactual scenarios and to counterbalance the human tendency to underestimate exponential growth. The complex interaction between new emerging variants, NPI control measures, and vaccine rollout strategies is difficult to grasp without using a model. Models offer a snapshot of several possible futures. Models provide a tool to compare the relative impact of decisions made now on the future course of the pandemic. With this study, we have addressed policy-related considerations for the Swiss population from a public health point of view as an exemplar application of the model. However, these insights can be applied more broadly to other countries and regions to forecast the impact that response measures will have on SARS-CoV-2 transmission, which we hope will aid in global control of COVID-19.
Funding
This work was supported by the Botnar Research Center for Child Health (DZX2165 to MAP); Swiss National Science Foundation NFP 78 Covid-19 2020 (4079P0_198428) and the Swiss National Science Foundation Professorship of MAP (PP00P3_170702).
CRediT authorship contribution statement
MAP, NC, and AJS conceived the study. AJS and RPD developed the model with input from ELR, MAP, NC, SS, and SLK. Analyses were performed by AJS, ELR, and RPD. AJS and MAP validated the model and analyses. Figure preparation was performed by AJS and ELR. All authors contributed to interpretation of the results, writing the draft and final version of the manuscript, and gave final approval for publication.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgements
The authors want to acknowledge members of the Disease Modelling Unit, Swiss Tropical and Public Health Institute, and also Dylan Muir for their insightful feedback on the modelling and manuscript. We would also like to acknowledge Martin Ackermann, Tanja Stadler, Olivia Keiser, Thomas Van Boeckel, Jacques Fellay, Richard Neher, Emma Hodcraft, Jan-Egbert Sturm, Marius Brülhart, Suzanne Suggs, and Nicola Low for their feedback on this work.
Calculations were performed at sciCORE (http://scicore.unibas.ch/) scientific computing core facility at the University of Basel. This work has been possible thanks to many members of Swiss National COVID-19 Science Taskforce and Swiss Federal Office of Public Health, who contributed to discussions and supported model scenarios and interpretations.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.epidem.2021.100535.
Appendix A. Supplementary material
Supplementary material
.
References
- Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T., COVE Study Group Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binois M., Gramacy R.B., Ludkovski M. Practical Heteroscedastic Gaussian Process Modeling for Large Simulation Experiments. J. Comput. Graph. Statist. 2018;27(4):808–821. doi: 10.1080/10618600.2018.1458625. [DOI] [Google Scholar]
- Challen R., Brooks-Pollock E., Read J.M., Dyson L., Tsaneva-Atanasova K., Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021;372:n579. doi: 10.1136/bmj.n579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Nadeau S.A., Topolsky I., Manceau M., Huisman J.S., Jablonski K.P., Fuhrmann L., Dreifuss D., Jahn K., Beckmann C., Redondo M., Noppen C., Risch L., Risch M., Wohlwend N., Kas S., Bodmer T., Roloff T., Stange M., Egli A., Eckerle I., Kaiser L., Denes R., Feldkamp M., Nissen I., Santacroce N., Burcklen E., Aquino C., de Gouvea A.C., Moccia M.D., Grüter S., Sykes T., Opitz L., White G., Neff L., Popovic D., Patrignani A., Tracy J., Schlapbach R., Dermitzakis E.T., Harshman K., Xenarios I., Pegeot H., Cerutti L., Penet D., Blin A., Elies M., Althaus C.L., Beisel C., Beerenwinkel N., Ackermann M., Stadler T. Quantification of the spread of SARS-CoV-2 variant B.1.1.7 in Switzerland. Epidemics. 2021;37 doi: 10.1016/j.epidem.2021.100480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie A., Mbaeyi S.A., Walensky R.P. CDC interim recommendations for fully vaccinated people: an important first step. JAMA. 2021;325(15):1501–1502. doi: 10.1001/jama.2021.4367. [DOI] [PubMed] [Google Scholar]
- Davies N.G., Jarvis C.I., CMMID COVID- Working G., Edmunds W.J., Jewell N.P., Diaz-Ordaz K., Keogh R.H. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021;593(7858):270–274. doi: 10.1038/s41586-021-03426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., Pearson C., Russell T.W., Tully D.C., Washburne A.D., Wenseleers T., Gimma A., Waites W., Wong K., van Zandvoort K., Silverman J.D., Diaz-Ordaz K., Keogh R., Eggo R.M., Funk S., Jit M., Atkins K.E., Edmunds W.J., CMMID COVID-19 WORKING GROUP, COVID-19 GENOMICS UK (COG-UK) CONSORTIUM Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. JAMA. 2021;372(6538) doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC. Projected baselines of COVID-19 in the EU/EEA and the UK for assessing the impact of de-escalation of measures – 26 May 2020. Stockholm: European Centre for Disease Prevention and Control; 2020 (Available from) https://www.ecdc.europa.eu/sites/default/files/documents/Projected-baselines-COVID-19-for-assessing-impact-measures.pdf.
- Flaxman S., Mishra S., Gandy A., Unwin H.J.T., Mellan T.A., Coupland H., Whittaker C., Zhu H., Berah T., Eaton J.W., Monod M., Imperial College COVID-19 Response Team, Ghani A.C., Donnelly C.A., Riley S., Vollmer M.A.C., Ferguson N.M., Okell L.C., Bhatt S. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020;584(7820):257–261. doi: 10.1038/s41586-020-2405-7. [DOI] [PubMed] [Google Scholar]
- Fontanet A., Autran B., Lina B., Kieny M.P., Karim S.S.A., Sridhar D. SARS-CoV-2 variants and ending the COVID-19 pandemic. Lancet. 2021;397(10278):952–954. doi: 10.1016/s0140-6736(21)00370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOPH . Federal Office of Public Health,; Switzerland: 2020. COVID-19 Switzerland: Easing and tightening of nationwide measures: Tables represent situation from 27 April to 2 November 2020 Liebefeld.https://www.bag.admin.ch/dam/bag/en/dokumente/mt/k-und-i/aktuelle-ausbrueche-pandemien/2019-nCoV/covid-19-tabelle-lockerung.pdf.download.pdf/Easing_of_measures_and_possible_next_steps.pdf (Available from) [Google Scholar]
- FOPH . Federal Office of Public Health,; Switzerland: 2021. Coronavirus: Situation in Switzerland, as of 22 March 2021 Liebefeld.https://www.bag.admin.ch/bag/de/home/krankheiten/ausbrueche-epidemien-pandemien/aktuelle-ausbrueche-epidemien/novel-cov/situation-schweiz-und-international.html] (Available from) [Google Scholar]
- FOPH . Switzerland: Federal Office of Public Health; 2021. COVID-19 Switzerland: Further procedures with regard to the national measures, dated 17 February 2021 Liebefeld.https://www.bag.admin.ch/dam/bag/de/dokumente/mt/k-und-i/aktuelle-ausbrueche-pandemien/2019-nCoV/Unterlagen-Konsultationen-Kantone/begleitdokument-bes-lage-lockerung-1.pdf.download.pdf/Begleitdokument%20f%C3%BCr%20die%20Kantone.pdf (Available from) [Google Scholar]
- FSO MeteoSwiss. Neuchâtel: Federal Statistical Office. 2021 https://www.meteoswiss.admin.ch/home.html?tab=overview (Available from) [Google Scholar]
- Hale T., Angrist N., Goldszmidt R., Kira B., Petherick A., Phillips T., Webster S., Cameron-Blake E., Hallas L., Majumdar S., Tatlow H. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker) Nat. Human Behav. 2021;5(4):529–538. doi: 10.1038/s41562-021-01079-8. [DOI] [PubMed] [Google Scholar]
- Hall V.J., Foulkes S., Charlett A., Atti A., Monk E.J.M., Simmons R., Wellington E., Cole M.J., Saei A., Oguti B., Munro K., Wallace S., Kirwan P.D., Shrotri M., Vusirikala A., Rokadiya S., Kall M., Zambon M., Ramsay M., Brooks T., Brown C.S., Chand M.A., Hopkins S., SIREN Study Group SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN) Lancet. 2021;397(10283):1459–1469. doi: 10.1016/s0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhi S.A., Baillie V., Cutland C.L., Voysey M., Koen A.L., Fairlie L., Padayachee S.D., Dheda K., Barnabas S.L., Bhorat Q.E., Briner C., Kwatra G., Ahmed K., Aley P., Bhikha S., Bhiman J.N., Bhorat A.E., du Plessis J., Esmail A., Groenewald M., Horne E., Hwa S.H., Jose A., Lambe T., Laubscher M., Malahleha M., Masenya M., Masilela M., McKenzie S., Molapo K., Moultrie A., Oelofse S., Patel F., Pillay S., Rhead S., Rodel H., Rossouw L., Taoushanis C., Tegally H., Thombrayil A., van Eck S., Wibmer C.K., Durham N.M., Kelly E.J., Villafana T.L., Gilbert S., Pollard A.J., de Oliveira T., Moore P.L., Sigal A., Izu A., NGS-SA Group, Wits-VIDA COVID Group Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med. 2021;384(20):1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S., Hill E.M., Tildesley M.J., Dyson L., Keeling M.J. Vaccination and non-pharmaceutical interventions for COVID-19: a mathematical modelling study. Lancet Infect. Dis. 2021;21(6):793–802. doi: 10.1016/s1473-3099(21)00143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossong J., Hens N., Jit M., Beutels P., Auranen K., Mikolajczyk R., Massari M., Salmaso S., Tomba G.S., Wallinga J., Heijne J., Sadkowska-Todys M., Rosinska M., Edmunds W.J. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5(3) doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck RW Jr, Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C., C4591001 Clinical Trial Group Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmuth M., Schibler M., Suter F., Huber M., Trkola A., Hasse B., Nilsson J., Buonomano R., Wepf A., Karrer U., Neher R. Transmission of SARS-CoV-2 variants in Switzerland. 2021 https://ispmbern.github.io/covid-19/variants/ (Available from) [Google Scholar]
- ScienceTaskForce, 2020. Effect of measures – Response to questions from FOPH 17th April 2020. Bern/Basel: National COVID-19 Science Task Force. (Available from) https://sciencetaskforce.ch/policy-brief/effect-of-measures/.
- Scott N., Palmer A., Delport D., Abeysuriya R., Stuart R.M., Kerr C.C., Mistry D., Klein D.J., Sacks-Davis R., Heath K., Hainsworth S.W., Pedrana A., Stoove M., Wilson D., Hellard M.E. Modelling the impact of relaxing COVID-19 control measures during a period of low viral transmission. Med. J. Aust. 2021;214(2):79–83. doi: 10.5694/mja2.50845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringhini S., Zaballa M.E., Perez-Saez J., Pullen N., de Mestral C., Picazio A., Pennacchio F., Wisniak A., Richard A., Baysson H., Loizeau A., Balavoine J.F., Trono D., Pittet D., Posfay-Barbe K., Flahault A., Chappuis F., Kherad O., Vuilleumier N., Kaiser L., Azman A.S., Guessous I., Specchio-COVID19 Study Group Seroprevalence of anti-SARS-CoV-2 antibodies after the second pandemic peak. Lancet Infect Dis. 2021;21(5):600–601. doi: 10.1016/s1473-3099(21)00054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SwissTPH . Basel: Swiss Tropical and Public Health Institute; 2021. COVID measures by canton Basel.https://github.com/SwissTPH/COVID_measures_by_canton (Available from) [Google Scholar]
- SwissTPH . Basel: Swiss Tropical and Public Health Institute; 2021. OpenCOVID source code Basel.https://github.com/SwissTPH/OpenCOVID (Available from) [Google Scholar]
- Vaughan T., Chen C., Ashcroft P., Lethinen S., Angst D., B S., et al. Swiss Federal Office of Public Health and OpenZH; Switzerland: 2021. CH Covid-19 Dashboard Zurich.https://ibz-shiny.ethz.ch/covidDashboard/ (Available from) [Google Scholar]
- West E.A., Anker D., Amati R., Richard A., Wisniak A., Butty A., Albanese E., Bochud M., Chiolero A., Crivelli L., Cullati S., d’Acremont V., Epure A.M., Fehr J., Flahault A., Fornerod L., Frank I., Frei A., Michel G., Gonseth S., Guessous I., Imboden M., Kahlert C.R., Kaufmann L., Kohler P., Mösli N., Paris D., Probst-Hensch N., Rodondi N., Stringhini S., Vermes T., Vollrath F., Puhan M.A., Corona Immunitas Research Group Corona Immunitas: study protocol of a nationwide program of SARS-CoV-2 seroprevalence and seroepidemiologic studies in Switzerland. Int. J. Public Health. 2020;65(9):1529–1548. doi: 10.1007/s00038-020-01494-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Tepekule B., Criscuolo N.G., Wendel Garcia P.D., Hilty M.P., Risc-Icu Consortium Investigators In S., Fumeaux T., Van Boeckel T. icumonitoring.ch: a platform for short-term forecasting of intensive care unit occupancy during the COVID-19 epidemic in Switzerland. Swiss Med. Wkly. 2020;150 doi: 10.4414/smw.2020.20277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material