Abstract
Amplification of the Neu (ErbB-2 or HER-2) receptor tyrosine kinase occurs in 20 to 30% of human mammary carcinomas, correlating with a poor clinical prognosis. We have previously demonstrated that four (Y1144 Y1201, Y1227 and Y1253) of the five known Neu autophosphorylation sites can independently mediate transforming signals. The transforming potential of two of these mutants correlates with their capacity to recruit Grb2 directly to Y1144 (YB) or indirectly through Shc to Y1227 (YD). Here, we demonstrate that these transformation-competent neu mutants activate extracellular signal-regulated kinases and stimulate Ets-2-dependent transcription. Although the transforming potential of three of these mutants (YB, YD, and YE) was susceptible to inhibition by Rap1A, a genetic antagonist of Ras, the transforming potential of YC was resistant to inhibition by Rap1A. To further address the significance of these ErbB-2-coupled signaling molecules in induction of mammary cancers, transgenic mice expressing mutant Neu receptors lacking the known autophosphorylation sites (NYPD) or those coupled directly to either Grb2 (YB) or Shc (YD) adapter molecules were derived. In contrast to the NYPD strains, which developed focal mammary tumors after a long latency period with low penetrance, all female mice derived from YB and YD strains rapidly developed mammary tumors. Although female mice from several independent YB or YD lines developed mammary tumors, the YB strains developed lung metastases at substantially higher rates than the YD strains. These observations argue that Grb2 and Shc play important and distinct roles in ErbB-2/Neu-induced mammary tumorigenesis and metastasis.
Neu (ErbB-2), the epidermal growth factor receptor, ErbB-3, and ErbB-4 are transmembrane receptor tyrosine kinases (RTKs) which comprise the class I or ErbB RTK family (reviewed in references 21, and 35). Neu or ErbB-2 amplification and elevated expression has been implicated in the etiology of human ovarian and breast cancers and correlates with a poor clinical prognosis in breast cancer patients (3, 44, 45). Moreover, anti-ErbB-2 antibodies demonstrate efficacy for treatment of breast cancer patients with elevated ErbB-2 levels (4). Direct evidence supporting a role for neu in mammary tumorigenesis derives from observations made with transgenic mice expressing oncogenic forms of the neu oncogene under the transcriptional control of mouse mammary tumor virus (MMTV) enhancer. Mammary epithelial cell-specific expression of wild-type Neu or constitutively active Neu or ErbB-2 alleles in the mammary epithelia of transgenic mice results in the induction of metastatic mammary tumors which histologically resemble human comedocarcinomas (2, 6, 18, 19, 30). Taken together, these data suggest that activation of ErbB-2 or Neu plays a causal role in mammary tumorigenesis.
Despite the importance of Neu in human malignancies, the molecular mechanism by which this RTK transforms cells and confers metastatic potential is not known. Following Neu receptor dimerization, class I RTKs become phosphorylated predominantly at discrete tyrosine residues within a 200 to 300-amino-acid carboxyl-terminal region. These phosphorylated tyrosines provide binding sites for a variety of cytoplasmic Src homology 2 (SH2) and/or protein tyrosine binding (PTB) domain-containing proteins involved in transducing proliferative/transforming or differentiating signals to the nucleus. SH2 and PTB domains directly interact with phosphotyrosyl proteins in a sequence-specific manner (reviewed in reference 36), thereby conferring specificity to RTK signaling.
Many reports have identified the signaling proteins which interact with Neu, yet the role that each molecule plays in signal transduction is less clear. A number of SH2 and PTB domain-containing proteins have been implicated in Neu signaling; these intracellular signaling molecules include phospholipase Cγ1 (PLCγ1), c-Src, Crk, Grb7, and proteins which modulate Ras activity either by promoting active Ras-GTP complex formation through Sos GDP/GTP exchange proteins (Grb2, Shc, and Nck) or by accelerating the hydrolysis of Ras-GTP to its inactive Ras-GDP state (Ras–GTPase-activating protein) (12, 13, 24, 27, 31–34, 37, 41, 46). While the binding sites on Neu for many of these SH2 and PTB domain-containing proteins are unclear, deletion or mutation of Neu tyrosine phosphorylation sites can dramatically affect the transforming activity of Neu (1, 5, 9, 10, 39, 40). There is little consensus as to the relative importance of these tyrosine autophosphorylation sites, although it appears that no single site is required to mediated transformation.
To systematically address the role of tyrosine phosphorylation sites in Neu-mediated transformation, we used a strategy initially described for the platelet-derived growth factor receptor (48). In this study, individual tyrosine residues were restored to a mitogenically inactive mutant platelet-derived growth factor receptor containing tyrosine-to-phenylalanine changes at the known tyrosine phosphorylation sites to create a series of phosphorylation add-back mutants. To address the functional significance of specific signaling molecules in Neu-mediated transformation, we have generated a similar series of mutant activated neu receptors that either lack all known tyrosine autophosphorylation sites (Neu tyrosine phosphorylation deficient [NYPD]) or possess only one of these sites in isolation (add-back mutants NT-YA to -YE) (9). For purposes of simplicity, we refer to each of these add-back mutants as YA (tyrosine residue 1028), YB (tyrosine residue 1144), YC (tyrosine residue 1201), YD (tyrosine residue 1227), and YE (tyrosine residue 1253). In established fibroblasts, reconstitution of single phosphorylation sites to NYPD, creating a series of add-back mutants, reveals that four of five phosphorylation sites (sites B through E) can independently mediate transforming signals, whereas tyrosine 1028 (site A) functions to repress transforming signals from the receptor (9). While Grb2 associates directly with Y1144 (site B) and indirectly through tyrosine-phosphorylated Shc proteins at Y1226/7 (site D), the molecular basis for transformation activity from sites C (Y1201) and E (Y1253) has yet to be elucidated.
Here we have assessed the importance of Ras signaling pathway in mediating transforming activity of Neu add-back mutants YB, YC, YD, and YE. The results revealed that coexpression of genetic antagonist of Ras signaling, Rap1A, could effectively interfere with three of the four add-back mutants (YB, YD, and YE) but was ineffective in inhibiting a transforming signal from the YC mutant. Despite differences in the response to Rap1A expression, all four transforming add-back mutants were capable of activating the mitogen-activated protein (MAP) kinase cascade and of inducing the transcription of an ets-2 reporter gene. Although Grb2 binds directly to the YB (Y1144) phosphorylation site and indirectly to YD (Y1226/7) through the Shc adapter protein, microinjection experiments reveal that unlike site B, the Shc binding site does not require Grb2 activity to mediate DNA synthesis. These observations suggest that although these Neu autophosphorylation sites are functionally redundant in cellular transformation, they use distinct signaling effector mechanisms to activate Ets-2 and MAP kinase pathways.
To further explore the biological importance of Neu-coupled Shc and Grb2 signaling in mammary tumorigenesis, we have generated transgenic mice by activated neu alleles of NYPD, YB, and YD under the transcriptional control of the MMTV long terminal repeat (LTR). Mammary epithelial cell-specific expression of the YB and YD neu alleles resulted in the rapid induction of multifocal mammary tumors. In contrast, expression of the NYPD mutant resulted the generation of focal mammary tumors only after a long latency period. Although YB and YD mutant alleles were capable of efficiently inducing mammary cancers, the YB-induced tumors metastasized with much higher frequency than YD-induced mammary tumors. Taken together, these observations suggest that Grb2 and Shc have distinct biological effects on Neu-induced mammary metastasis.
MATERIALS AND METHODS
Cell lines and transformation assays.
Rat1 fibroblasts and 293T epithelial cells were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), amphotericin B (Fungizone), and penicillin-streptomycin or gentamicin. Contransfections in Fig. 1 were carried out using LipofectAmine reagent (GibcoBRL). Rat1 fibroblasts were seeded at 3 × 105 cells in 35-mm-diameter tissue culture dishes following the manufacturer's instructions with 100 ng of transforming plasmid or vector and 1 μg of inhibitory plasmid (pKRev-1/Rap1A) or empty vector (SV2Neo). At confluency, contents of the plates were split into two 60-mm-diameter plates, which were maintained in 4% FBS-supplemented DMEM for 14 days, with a change of medium every third day. Plates were maintained in supplemented DMEM for 14 days, with a change of medium every third day. Plates were stained with Giemsa stain, and the foci were counted from six plates per construct. Relative transformation potential was normalized to that obtained with activated Neu (NT) in the presence of SV2Neo in each experiment multiplied by 100. EKOI fibroblasts, established from Ets-2-deficient teratocarcinoma cells (55), were maintained in DMEM supplemented with 10% fetal calf serum.
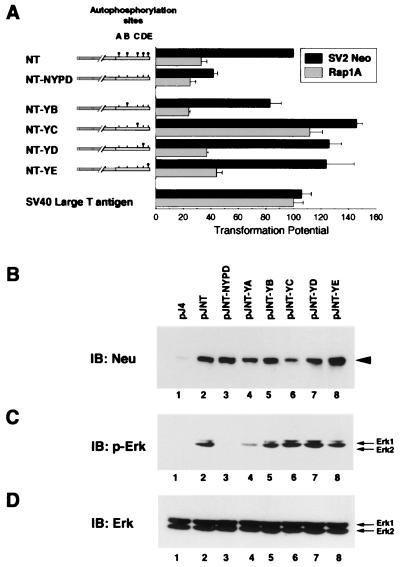
FIG. 1.
Ras is required for transformation from activated Neu autophosphorylation mutants that stimulate Erk. (A) Ras requirements for Neu add-back-mediated transformation. Activated Neu (NT) is depicted with its tyrosine phosphorylation sites ( ) at residues 1028 (site A), 1144 (site B), 1201 (site C), 1226/7 (site D), and 1253 (site E). NT-NYPD and the derived add-back mutants NT-YB through NT-YE contain tyrosine to phenylalanine (█) mutations at the indicated tyrosine phosphorylation sites. Focus formation assays were carried out in Rat1 fibroblasts transfected with plasmids encoding the indicated Neu mutants or SV40 large T antigen along with either a control vector (SV2Neo) or one encoding Rap1A. Transforming activity (± standard error) was determined from triplicates in three experiments and is expressed relative to NT with SV2Neo. (B to D) 293T cells were transfected with pJ4Ω-derived plasmids encoding Neu phosphorylation mutants. Immunoblots (IB) containing 50 μg of total protein were analyzed with anti-Neu (B) and phospho-Erk1/2 (p-Erk) (C) antibodies, and the membrane in panel C was reprobed with Erk1/2-specific antisera (D).
Erk activation and Ets-2-dependent transcription in transient transfections.
293T cells were seeded at 75% confluency in 60-mm-diameter tissue culture dishes in 10% FBS-supplemented DMEM and transfected the following day with DNA-liposome complexes containing 12 μg of plasmid DNA and 48 μl of LipofectAmine reagent for 5 h. Following 48 h, the medium was replaced with serum-free DMEM to reduce background extracellular signal-regulated kinase (Erk) phosphorylation, and cells were further incubated for 3 to 5 h prior to lysis in hypotonic lysis buffer (20 Tris-HCl [pH 7.5], 2mM EDTA, 2 mM EGTA, 10 mM NaF, 10 mM sodium pyrophosphate) supplemented with 1 mM Na3VO4 and the protease inhibitors aprotinin and leupeptin at 10 μg/ml. Following 20 min, NaCl was added to 400 mM (final concentration), and the mixture was further incubated for 20 min. Immunoblots containing 50 μg of total protein were analyzed using antiphosphotyrosine (PY20), anti-Erk (C14; Santa Cruz Biotechnology), or anti-phospho-Erk (New England Biolabs) antibodies. E18 luciferase and FNEts2 and FNEts2 A72 expression plasmids were provided by C. Hauser (14). EKO1 cells (8.0 × 104) were plated in 12-well dishes 24 hs prior to transfection and then transfected by the calcium phosphate coprecipitation method. The day after transfection, the medium was changed to DMEM containing 0.5% fetal calf serum and incubated for an additional 24 h before lysates were processed for luciferase and β-galactosidase activity.
Affinity purification, immunoprecipitation, and immunoblotting.
Cells were washed twice in 1 × phosphate-buffered saline (PBS) at 4°C, and lysates were made in PLC lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM EGTA, 1.5 mM MgCl2, 10 mM NaF, 10 mM sodium pyrophosphate, 1 mM Na3VO4, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml). Tumors were ground to a powder under liquid nitrogen, and lysates were made in PLC lysis buffer. Neu was immunoprecipitated with protein G-conjugated monoclonal antibody 7.16.4. The immunoprecipitates were washed five to seven times in PLC lysis buffer prior to immunoblot analyses with either anti-Neu (Ab3; 1:1,000; Oncogene Science) or anti-phosphotyrosine (PY20; Transduction Laboratories) antibodies as described elsewhere (9). Grb2 (C23; 1:400; Santa Cruz) and Shc (S14630; 1:1,000; Transduction Laboratories) were detected using rabbit polyclonal antibodies. The peptides pYE (biotin-FEGTPTAENPE[pY]LGLDVPV) and pYC (biotin-FAFGGAVENPE[pY]LVPREGT), synthesized by Research Genetics, were resuspended in 20 mM buffered HEPES and brought to pH 7.0. Peptides were incubated with or without calf intestinal alkaline phosphatase (60 U/μg) at 37°C for 2 h. Peptides were immobilized to streptavidin-agarose, washed extensively in PLC lysis buffer, and aliquoted such that each affinity purification mixture contained 1 μg of immobilized peptide. Protein lysates (500 μg) from Rat1 cells were incubated at 4°C with immobilized peptides on a rotating platform for 1 h and washed four to six times in PLC lysis buffer, and specific complexes were subjected to anti-Crk immunoblot analysis.
GST fusion production and direct affinity assay.
Escherichia coli BL21 (Stratagene) was transformed with pGSTag-derived glutathione S-transferase (GST), GST-PLCγ, and GST-Crk plasmids, and glutathione fusion proteins were purified as described elsewhere (9). Direct binding assays were performed as follows: Serial dilutions (0.01 to 1 μg) of GST fusion proteins were spotted onto nitrocellulose membranes and air dried. Membranes were blocked in 0.3% gelatin in 0.1% Tris-buffered saline–Tween (TBST-G) for 1 h. Membranes were then rinsed twice with TBST and incubated with peptides (1 μg/ml) in TBST-G for 1 h at room temperature. Blots were washed thrice for 10 min with TBST and incubated with a 1:2,500 dilution of 125I-streptavidin (IM236; Amersham) in TBST-G for 1 h. Blots were washed again, dried, and autoradiographed.
Grb2 inhibition of DNA synthesis.
DNA synthesis was assayed after metabolic incorporation of 5-bromo-2′-deoxyuridine (BrdU) (cell proliferation kit; Amersham) as described previously (9, 52). Cells growing on glass cover slips were deprived of serum overnight (∼18 h) and then noninjected or microinjected with 0.5 × PBS containing biotinylated Grb2 SH2 domain (2 mg/ml). After incubation at 37°C for 2 h, fresh medium containing 10% FBS and BrdU was added, and the coverslips were incubated at 37°C for 18 h. Following fixation, BrdU incorporated into DNA was visualized with a BrdU antibody and a rhodamine-labeled secondary antibody to mouse immunoglobulin. Microinjected cells were identified by staining with fluorescein isothiocyanate-labeled avidin (Jackson Immunology Laboratory). Not less than 100 cells were injected on each coverslip, and DNA synthesis was determined as the percentage of microinjected cells staining positively for BrdU incorporation. Equivalent results were obtained from two independent experiments, and results from one such experiment are shown. In noninjected cells, the percentage of BrdU-labeled cells was determined for not less than 200 cells counted from randomly selected fields of view. Cells were visualized for simultaneous red and green fluorescence by using the appropriate filter sets (Carl Zeiss Ltd.).
Generation of transgenic mice, tumor kinetics, and histological evaluation.
cDNAs corresponding to the autophosphorylation mutants (NYPD, YB, and YD) containing an activating extracellular deletion (8142) (42) were excised from pJ4Ω HindIII-EcoRI fragments and inserted into the corresponding sites of p206 under the transcriptional control of the MMTV LTR followed by simian virus 40 (SV40) polyadenylation sequence. Transgenic mice were generated by pronuclear injection of zygotes obtained from FVB × N intercrosses as described elsewhere (43). All mice were obtained from Taconic Farms (Germantown, Pa). Founder animals were identified by Southern blot analyses using an SV40 poly(A)-specific 32P-labeled probe, and progeny were routinely detected by PCR with transgene-specific oligonucleotides. Nulliparous and multiparous female mice were monitored for mammary tumor formation by physical palpation of the mammary glands. Tumor kinetics were obtained from nulliparous animals from MMTV/neundl-NYPD (line 10 [NYPD10]), -YB (line 2 [YD2]), and -YD (line 5 [YD5]) strains. Whole-mount preparations of the number 4 mammary gland was completed as described elsewhere (50). Tissue was fixed in phosphate-buffered 4% paraformaldehyde or formalin at 4°C, transferred to 70% ethanol, and blocked in paraffin. Sections (4 μm) were stained with hematoxylin and eosin (Anatomical Pathology, McMaster University). Lung metastases were evaluated by microscopic analyses of lung sections.
RESULTS
Neu transforming activity functions through distinct Rap1A-sensitive and -resistant pathways.
Our previous observations have implicated the Ras signaling pathway in Neu-induced DNA synthesis (9). To further assess the role of the Ras signaling pathway in Neu-mediated transformation, we have coexpressed a competitive inhibitor of Ras, known as Rap1A, with the various Neu autophosphorylation mutants. Rap1A functions as a dominant Ras inhibitor by titrating critical Ras substrates to an inactive Rap1A -substrate (20, 38, 47) and has the ability to revert v-Ki-ras-transformed cells when overexpressed (26). To accomplish this, we cotransfected Rap1A with various Neu mutants into Rat1 fibroblasts and assessed transformation in focus formation assays. Expression of Rap1A induced a threefold reduction in NT transformation relative to empty vector, demonstrating a role for Ras in this process, but had little effect on NYPD transformation (Fig. 1A). Interestingly, Rap1A similarly inhibited transformation from YB, YD, and YE, whereas YC appeared to be largely Ras independent in this assay. The YA mutant was not included in these analyses, since we have previously demonstrated that this mutant is completely transformation defective (9). The ability of Rap1A to interfere with the transforming activity of these add-back mutants appeared to be specific to Ras since Rap1A had little effect on the transforming activity of SV40 large T antigen, (lt) which is known to transform cells in a Ras-independent manner (25). These results demonstrate that three of four transforming add-back mutants are susceptible to this genetic inhibitor of Ras function.
Given that activation of Ras results in the initiation of a kinase cascade terminating in the phosphorylation and activation of Erks (29), we investigated whether Neu add-back mutants were capable of stimulating Erk phosphorylation. To accomplish this, we transfected 293T cells with empty vector or those encoding NT or the derived mutants and assessed Erk phosphorylation with anti-Erk phosphospecific antisera (Fig. 1C). These analyses revealed that NT and each transforming add-back mutant activates Erks whereas NYPD fails to due so, despite expressing comparable amounts of Neu (Fig. 1B). Thus, activated Neu and the YB, YD, and YE add-back mutants both activate Ras signaling and display Ras-dependent transforming activities, whereas YC expression induces Erk phosphorylation but is not inhibited by Rap1A overexpression.
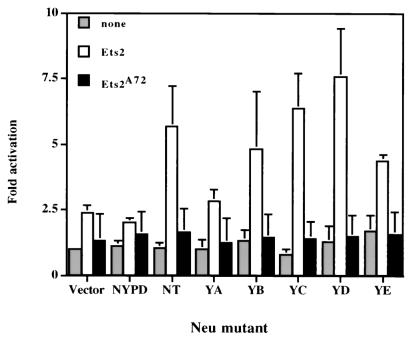
Another important indicator of Ras activity is its ability to activate certain classes of transcription factors. Indeed, activated Ras is known to stimulate the transcriptional activity of the Ets-2 transcription factor through the MAP kinase phosphorylation of threonine 72 in the Ets-2 pointed domain (56). To explore whether Ets-2-dependent transcription was differentially effected by the different Neu add-back mutants, the various Neu add-back mutants were cotransfected with an Ets-2-dependent luciferase reporter (E18) (12) and expression vectors harboring either wild-type Ets-2 or mutant Ets-2 bearing an alanine substitution for threonine 72 (Ets-2 A72) (Fig. 2). Cotransfection of control vector NYPD or YA mutants with the Ets-2 expression vector resulted in a modest twofold activation of the reporter construct. In contrast, cotransfection of NT, YB, YC, YD, and YE mutants resulted in a 5- to 10-fold stimulation of Ets-2-dependent transcription (Fig. 2). Stimulation of the Ets-2 reporter construct by these neu mutants was dependent on the conservation of the Ets-2 MAP kinase phosphorylation site since cotransfection of the Ets-2 A72 mutant failed to activate the Ets-2 reporter construct. Taken together, these observations suggest that transformation-competent neu mutants funnel through the MAP kinase signaling pathway and stimulate Ets-2-dependent transcription through the Ets-2 MAP kinase phosphorylation site (threonine 72).
FIG. 2.
Neu mutants induce Ets-2 transcriptional activation through threonine 72. The E18 luciferase reporter construct was cotransfected into EKO1 Ets-2-deficient cells with the various Neu add-back mutants, wild-type ets-2 expression vector (FNEts2) (open box), or mutant ets-2 with alanine substitution at threonine 72 (FNEts2 A72) (closed box). Also included was a β-actin-driven β-galactosidase expression vector, which served as an internal control for transfection efficiency. The ratio of luciferase activity relative to that observed for the empty pJ4 expression vector was used to calculate fold activation.
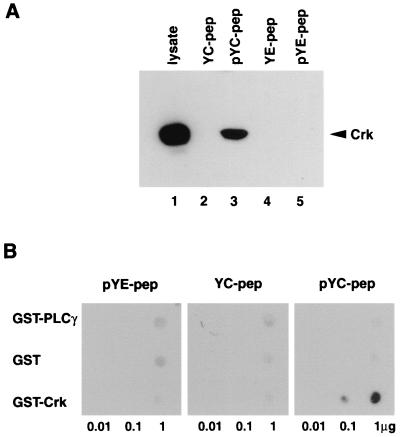
While all of the transforming neu mutants were capable of stimulating MAP kinase activity and activating Ets-2-dependent transcription, YC-mediated transformation remained refractory to Rap1A inhibition (Fig. 1A). One potential explanation for these observations is that the transforming signal from the YC mutant is not dependent on Ras activity. Indeed, it has recently been demonstrated that the Crk1/2 adapter protein recruits the C3G exchange factor, which in turn can activate Erk kinases in a Ras-independent fashion (22, 23). To explore the possibility that site C interacts with Crk, we generated chemically synthesized phosphorylated and nonphosphorylated peptides spanning site C from positions −11 to +7 relative to the phosphotyrosine residue. To control for nonspecific binding, we also included peptides directed to the Rap1A-sensitive E site. The amino terminus of each peptide was covalently linked to biotin, thus providing a means of immobilizing the peptides via streptavidin-conjugated agarose beads. To determine whether Crk interacted with site C, affinity purification assays were carried out with the immobilized peptides to site E or C followed by immunoblot analyses with Crk-specific antisera. The results revealed that the site C specifically associated with Crk protein whereas the phosphorylated site E peptide failed to associate with Crk (compare lanes 3 and 5 in Fig. 3A). In parallel assays, we have shown that the site E peptide is capable of associating with a number of unidentified cellular proteins. Furthermore, Crk-specific binding is dependent on phosphorylation of tyrosine residues since dephosphorylated site C peptide showed no evidence of stable association.
FIG. 3.
Crk interacts with phosphopeptides containing tyrosine 1201 (site C) in vitro. (A) Immobilized phosphorylated and unphosphorylated peptides (1 μg) were incubated with 500 μg of Rat1 fibroblast lysates. Associated proteins were electrophoresed on a sodium dodecyl sulfate–12% polyacrylamide gel and subjected to anti-Crk immunoblot analysis. The migration of Crk is indicated. (B) Membrane-immobilized GST fusion proteins were incubated with phosphorylated and unphosphorylated peptides (1 μg/ml). Membranes were subsequently probed with 125I-streptavidin and autoradiographed. Serial dilutions of fusion (0.01 to 1 μg) are indicated.
To assess whether the phosphorylated YC peptide could directly associate with the SH2 domain of Crk, a direct binding assay was performed. Membrane-immobilized GST fusion proteins containing the SH2 domain of Crk or PLCγ were probed with either phosphorylated peptides made to site C or E or a control dephosphorylated C peptide. The results revealed that only the GST-Crk fusion protein could directly bind the phosphorylated YC peptide (Fig. 3B). Taken together, these results suggest that theRap1A refractory site C binds Crk in a phosphospecific manner in vitro.
YB and YD mediate Grb2-dependent and -independent signals, respectively.
Previous studies have indicated that YB and YD mutants independently mediate transforming signals through their ability to directly recruit Grb2 (YB) or indirectly sequester Grb2 via its interaction with the Shc adapter protein (YD). The specificity of binding partners for YB and YD was further confirmed by the creation of secondary mutations in the consensus binding sites for Grb2 and Shc adapter proteins, respectively (D. Dankort and W. J. Muller, unpublished observations). To determine the requirement for Grb2 in mediating signals from Neu, we microinjected a dominant negative inhibitor of Grb2 into established cell lines expressing the various add-back Neu mutants and assessed the ability of this Grb2 inhibitor to interfere with Neu-induced DNA synthesis. Because the dominant negative inhibitor of Grb2 comprises only the Grb2-SH2 domain fused to GST and lacks SH3 domains, it can compete with the endogenous Grb2 for receptor binding but cannot couple to downstream Sos exchange factors to activate the Ras signaling pathway (50).
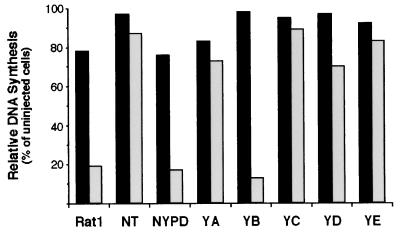
Microinjection of the Grb2 SH2 domain into Rat1 fibroblasts expressing NT (Fig. 4) had a minimal effect on the capacity of these cells to undergo DNA synthesis as assessed by BrdU incorporation (90% of uninjected controls), suggesting that there exists at least one Grb2-independent signal emanating from Neu. Inhibition of Grb2 function dramatically reduced DNA synthesis in NYPD cells and control Rat1 fibroblasts. The potent inhibition of these cell lines likely reflects a dependence on serum factors for initiating DNA synthesis. In contrast, cell lines expressing YA, YC, and YE were largely Grb2 independent in these assays (80 to 90% of noninjected controls). Although Grb2 binds to both sites B and D, microinjection of the Grb2 inhibitor strongly suppressed YB-induced DNA synthesis (13% of uninjected controls) and had only marginal effect on BrdU incorporation in YD-expressing cells (72% of noninjected controls) (Fig. 4). These data are consistent with the view that the primary Grb2-dependent signal from Neu is mediated by the YB phosphorylation site.
FIG. 4.
Effect of Grb2 SH2 domain microinjection on DNA synthesis from add-back cell lines. Cells rendered quiescent through serum deprivation were either uninjected (black bars) or microinjected with 1 μg of biotinylated Grb2 SH2 domain (grey bars) and released from quiescence via addition of serum, at which time BrdU added. The percentages of BrdU-positive injected or noninjected cells are graphically depicted. DNA synthesis was determined as the percentage of microinjected cells (not less than 100 cells for each line) staining positively for BrdU incorporation. Equivalent results were obtained from two independent experiments, and results from one such experiment are shown.
Direct Grb2 recruitment by Neu is associated with a metastatic tumor phenotype.
Although these observations suggest that recruitment of Grb2 or Shc to Neu may result in the activation of distinct effector pathways, the in vivo relevance of these Neu-coupled pathways is unclear. Thus, we tested the effects of activation of these coupled signaling pathways in mammary tumorigenesis and metastasis by targeting expression of NYPD, YB, and YD to the mammary epithelium of transgenic mice. These different neu phosphorylation mutants were placed in the context of an activating extracellular deletion (NDL1) which efficiently induces metastatic mammary tumors in transgenic mice (43). While this deletion mutant transforms at approximately 50% of the level of NT, the relative transforming potential of the derived add-back mutants in culture remains the same, suggesting that these mutants function similarly in a different activating background (not shown).
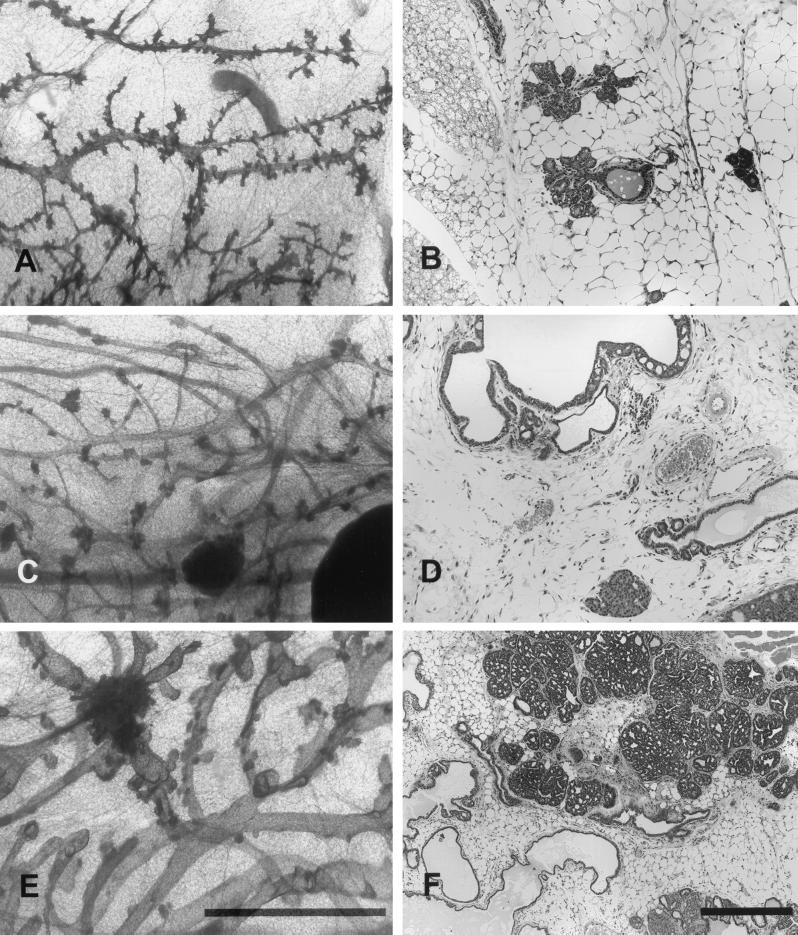
Multiple independent MMTV/NYPD, MMTV/YB, and MMTV/YD transgene lines were generated by pronuclear injection of fertilized mouse zygotes. Expression of the transgene in derived mammary RNA samples was verified by reverse transcription-PCR (data not shown). A number of expressing founder animals did not pass the transgene to their progeny; thus, we restricted our subsequent analyses to two strains for each phosphorylation allele (NYPD10, NYPD11, YB2, YB6, YD5, and YD6). We performed whole-mount analyses on virgin female mammary glands to ascertain the phenotypic consequences of expression of these various neu mutants. These analyses revealed that mammary expression of NYPD led to significant alveolar development, histologically resembling normal mammary epithelium (Fig. 5A and B). In contrast to this relatively normal developmental profile, the MMTV/YD-derived mammary glands possessed dense hyperplastic foci that were histologicaly composed of solid glandular arrays of epithelial cells (Fig. 5C and D). MMTV/YB whole mounts display histological abnormalities which include ductal ectasia with scattered foci of papillary epithelial hyperplasia (Fig. 5E and F).
FIG. 5.
Mammary gland architecture of MMTV/activated neu mutants. Photoimages show whole-mount preparations (A, C, and E) and comparable microscopic fields (B, D, and F) of mammary glands of mice harboring NYPD10 (A and B), YD5 (C and D), and YB2 (E and F) neu autophosphorylation mutants. All mammary glands are from 12 to 14-week-old virgin female mice. Note that all mammary whole mounts have some degree of alveolar development and foci of intraluminal hyperplasia. The YB mutant mice had the most severe ductal ectasia with scattered foci of papillary hyperplasia (E and F). The YD mutant strain had less ductal ectasia, but the hyperplastic lesions were composed of solid glandular arrays (C and D). The NYPD mutant had significant lobular development that tended to follow normal histological patterns (A and B). (Normal mammary gland morphologies for the FVB strain can be viewed online [http://ccm.ucdavis.edu/tgmouse/wmtable.htm].) The size bars in panels E and F indicate 1 and 0.1 mm, respectively.
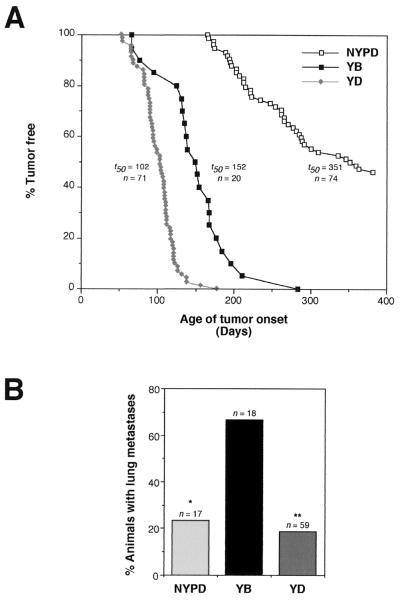
Given the observed abnormal development with the various mutant neu strains, we monitored cohorts of virgin female mice from representative NYPD (NYPD10) YB (YB2), and YD (YD5) strains for the development of mammary tumors. Mammary epithelial expression of activated YB and YD alleles efficiently induced mammary tumors at rates comparable to those for other MMTV-driven activated alleles (30, 43) (Fig. 6A). While female YB or YD neu transgenic mice both developed mammary tumors with complete penetrance at average age of onset of 152 and 102 days, respectively (Fig. 6A; Table 1), the mammary tumors that arose in the YD strains tended to be multifocal in origin (>10 tumors per mouse) whereas those of the YB strains generally were focal in nature. In contrast, focal mammary tumors arose only after a long latency period in the NYPD females (Fig. 6A, average age of onset= 252 days), and tumors arose with a penetrance of either 13 or 50%, depending on the line, after a 1-year observation period (Table 1). Limited analyses of independent NYPD, YB, and YD transgenic strains (NYPD11, YB6, and YD6) revealed similar times of tumor onset and phenotypes (Table 1), suggesting that observed characteristics of these strains were independent of integration site.
FIG. 6.
Mammary tumorigenesis and metastasis in transgenic mice expressing neu autophosphorylation mutants. (A) Mammary tumor kinetics in YB 2, YD 5, and NYPD 10 mice. Age indicated is that (days) at which a mammary tumor was first palpable in each transgenic strain. The number of female animals analyzed for each strain (n) and median age at which tumors are palpable (t50) are also shown. The mean tumor latencies for YB and YD are statistically different (P = 0.002, Students t test). (B) Percentage of tumor-bearing animals with metastatic lesions in the lung. The percentage of all tumor-bearing mice harboring lung metastases is indicated for each genotype; the number of animals analyzed is indicated (n). While there are no statistically significant differences between occurrence of lung metastastes in NYPD and YD animals, Fisher exact tests demonstrate significant differences between occurrence of metastases in YB versus NYPD (∗, P = 0.012) and YD (∗∗, P = 0.0002) mice.
TABLE 1.
Time of tumor onset and metastases in MMTV transgenic mice expressing Neu autophosphorylation mutants
| Strain | Avg age of breast tumor
onseta (n)
|
Penetrance of breast tumorsb | Metastases (30–60 days with tumor)c | |
|---|---|---|---|---|
| Nulliparous animals | Multiparous animals | |||
| NYPD10 | 252 ± 59 (40) | 163 ± 14 (3) | 59 (40/68) | 38 (3/8) |
| NYPD11 | 255 ± 74 (13) | 182 ± 65 (2) | 13 (13/51) | 0 (0/1) |
| YB2 | 152 ± 47 (20) | 170 ± 33 (2) | 100 (20) | 50 (2/4) |
| YB6 | 136 ± 40 (4) | 268 ± 54 (2) | ND | 100 (1/1) |
| YD5 | 102 ± 22 (71) | 109 ± 20 (9) | 100 (71) | 10 (3/26) |
| YD6 | 128 ± 22 (2) | 274d (1) | 100 (2) | ND |
Average age (days) of palpable breast tumor ± standard deviation and (in parentheses) the number of animals with tumors. Nulliparous animals were virgins at the time of breast tumor detection, and multiparous animals had at least one pregnancy.
Penetrance in percent breast tumor phenotype, determined in nulliparous animals. Numbers in parentheses are the number of animals that developed breast tumors within 365 days over the number of observed animals. ND, not determined.
Percentage of animals which possessed lung metastases at time of necropsy and had a palpable breast tumor for more than 30 but less than 60 days prior to necropsy. Numbers in parentheses indicate the number of animals with metastases over the number examined.
This animal was a founder.
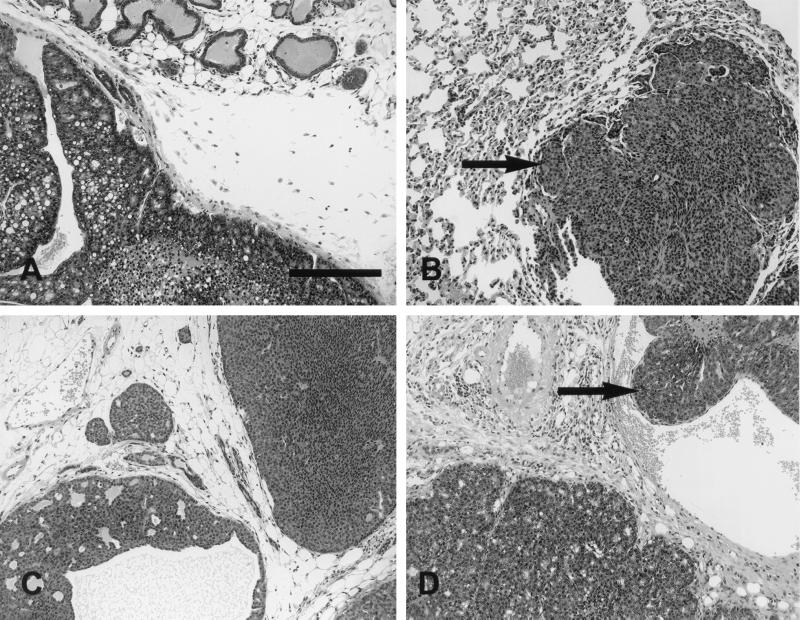
Although expression of the YB and YD transgenes was capable of efficiently inducing mammary tumors, there were marked differences of the histopathology of these tumors. YD transgene expression was primarily associated with the induction of solid comedo-type tumors reminiscent of tumors induced by activated neu (Fig. 7C), while the YB-induced mammary tumors were comprised of papillary fronds (Fig. 7D). The tumors induced by mammary epithelial expression of NYPD also resembled the parental activated neu-induced tumors (Fig. 7A and B). In addition to the striking morphological differences between the various neu mutants, these strains differed markedly in the relative rates of metastatic progression observed. Overall, while tumor-bearing NYPD (4 of 17) and YD (11 of 59) animals developed lung metastases at similar frequencies, YB mice developed lung metastases at a significantly higher rate (12 of 18 animals) (Fig. 6B). To ensure that metastases did not reflect differences in tumor onset, metastasis was assessed in a cohort of animals bearing mammary tumors for 30 to 60 days (Table 1). During this observation period, 50% of the YB tumor-bearing mice develop metastatic lesions in the lung, whereas only 10% of the YD animals exhibited detectable metastatic lesions (Fig. 6B; Table 1). It should be noted that due to the greater number of mammary tumors detected on the YD females, the tumor burden in the YD animals was considerably greater than that in the YB strains. Therefore, the differences in metastatic behavior between these tumor cell types is even greater than these pathological analyses would suggest. Female YD mice carrying tumors for extended periods (3 months after initial palpation) eventually developed metastatic mammary tumors due to the extensive tumor load, but the percentage of animals with such lesions was significantly lower than in YB mice. Interestingly, mammary tumors induced by the NYPD neu mutant displayed similar levels of metastatic infiltration. Taken together, these observations suggest that Neu receptors coupled specifically to the Grb2 adapter protein have enhanced metastatic potential compared to Neu receptor coupled to the Shc adapter protein.
FIG. 7.
Histopathology of mammary tumors induced by the various neu autophosphorylation mutants. Photoimages show mammary tumors from mice expressing the neu autophosphorylation mutants NYPD (line 10) (A and B), YD (line 6) (C), and YB (line 2) (D). All three animals exhibited well-differentiated glandular patterns. The tumors were composed of cells with relatively small, oval to round nuclei without significant pleomorphism. The cells were cytologically identical to the cells seen in all Neu-induced tumors. YB consistently had a better-differentiated glandular pattern (D). The size bar indicates 0.1 mm.
Biochemical analyses of mammary tumors induced by the mutant Neu receptors.
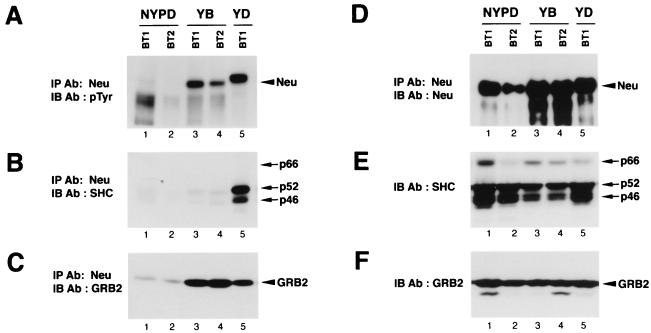
To ensure that Neu was activated to the same extent in these tumors, Neu immunoprecipitates were subjected to immunoblot analyses with antiphosphotyrosine antibodies. As expected, tyrosine-phosphorylated Neu was detected in tumors derived from the YB and YD mutants (Fig. 8A,lanes 3 to 5), whereas Neu from NYPD-induced tumors was poorly tyrosine phosphorylated, suggesting that the major in vivo tyrosine autophosphorylation sites had been eliminated (lanes 1 and 2). The differences in the levels of tyrosine-phosphorylated Neu were not reflective of the amount of Neu expressed, since comparable levels of Neu were detected in these samples (Fig. 8D). These Neu immunoprecipitates were subjected to immunoblot analyses with Grb2 and Shc-specific antibodies. While these tumor samples expressed comparable Grb2 and Shc levels (Fig. 8E and F), Shc solely interacted with Neu from YD-induced mammary tumors (Fig. 8B, lane 5), whereas Grb2 was coupled to both YB and YD but not NYPD Neu receptors (Fig. 8C, lanes 3 to 5). Taken together, these observations indicate that mutant Neu receptors that are expressed in these tumors are associated with their expected substrates or partners.
FIG. 8.
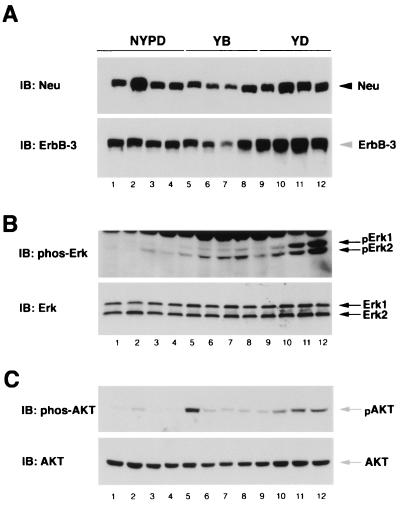
Neu is tyrosine phosphorylated in YB and YD-derived but not NYPD-derived mammary tumors. Neu was immunoprecipitated (IP) from the indicated breast tumor lysates. Two-thirds of each immunoprecipitate was electrophoresed and subjected to phosphotyrosine (pTyr) (A), Shc (B), and Grb2 (C) immunoblot (IB) analyses. Ab, antibody. (D) Neu immunoblot of the remaining portion of each Neu immunoprecipitate. Equivalent amounts (20 μg) of the same protein lysates were subjected to immunoblot analyses with Shc (E)- or Grb2 (F)-specific antisera. Tumor lysates were derived from NYPD11, YB6, and YD6 animals. The migration of YD in lane 5 of panel A is a gel artifact, as Neu from the same immunoprecipitate comigrates with the other mutant molecules (D).
Given that these Neu mutants remain coupled to their cognate adapters in vivo, we further investigated their signaling capabilities. Tumor lysates were subjected to immunoblot analyses with antibodies specific to the activating phosphorylation sites of Erk1/2 and Akt. While Erk1/2 activation was observed in YB- and YD-derived mammary tumor lysates (Fig. 9B, lanes 5 to 12), Erk was poorly activated in the NYPD-induced mammary tumors (Fig. 9B, lanes 1 to 4) despite expressing similar levels of Neu or Erk in these tumor samples (Fig. 8A, and 9B). These observations argue that recruitment of either Grb2 or Shc to Neu is required for efficient activation of a Ras signaling pathway.
FIG. 9.
Activation of Erk and Akt phosphorylation in YB- and YD-derived but not NYPD-derived mammary tumors. Equivalent amounts (60 μg) of lysates from NYPD, YB, and YD tumors were subjected to Neu and ErbB-3 immunoblot (IB) analyses (A). The same lysates were similarly analyzed for phospho-Erk1/2 and Erk (B) and phospho-Akt and Akt (C) levels as indicated. Each tumor lysate was derived from an independent animal: NYPD10 (lanes 1 to 3), NYPD11 (lane 4), YB2 (lane 5), YB6 (lanes 6 and 7), YB7 (lane 8), YD5 (lanes 9 to 11), and YD6 (lane 12).
Activation of the phosphatidylinositol 3′-kinase (PI-3′ kinase) through Neu's transactivation of the ErbB-3 receptor ultimately results in the stimulation of Akt serine kinase (51). Because the tumor samples possessed elevated levels of ErbB-3 capable of interacting with PI-3′ kinase, we next examined whether a downstream kinase, Akt, was activated in this system. Despite similar ErbB-3 and Akt-1 levels, Akt-1 was phosphorylated in YB and YD but not NYPD tumors (Fig. 9C). Together, these data suggest that mammary tumors expressing YB and YD, but not NYPD, activate two Ras effector pathways, resulting in the activation of both Erk and Akt kinases.
DISCUSSION
The ability of activated growth factor RTKs to induce cellular proliferation and differentiation is dependent on their capacity to associate with and activate a number of substrates. We have demonstrated that Neu-mediated transformation is dependent on at least four independent tyrosine autophosphorylation sites that can functionally substitute for each other (9). Two of these functionally redundant sites (YB and YD) have been shown to specifically couple to either the Grb2 or Shc adapter protein 9). Here we have demonstrated that YB and YD are involved in activation of the Ras/Erk signaling pathway. Although both of these phosphorylation sites are capable of recruiting the Grb2 adapter protein, only the YB phosphorylation site is dependent on a functional Grb2 protein. To further explore the biological properties of these Neu-coupled signaling pathways, we have generated transgenic mice that express activated versions of Neu coupled specifically to either the Grb2 (YB) or Shc (YD) adapter protein or a Neu receptor lacking the major Neu tyrosine autophosphorylation sites (NYPD) in the mammary epithelium. The results of these analyses revealed that both the YB and YD strains were capable of efficiently inducing mammary tumors, whereas the NYPD strains developed focal mammary tumors only after a long latency period. However, only the YB-induced mammary tumors were able to metastasize efficiently to distal sites. Taken together, these observations argue that the YB and YD phosphorylation mutants are involved in activating both distinct and common effector pathways.
The transforming potential of the different neu add-back mutants correlated with their capacity to stimulate the Erk kinase cascade (Fig. 1C). Although all add-back neu mutants were capable of efficiently activating the Erk kinase pathway, the transforming activity of three of these mutants (YB, YD, and YE) was inhibited by Rap1A, which acts as a competitive inhibitor of Ras (20, 26, 38, 47). In contrast, the YC mutant appeared refractory to Rap1A-mediated inhibition. One potential explanation for this observation is that the YC phosphorylation site recruits a signaling pathway that operates independently of the Ras signaling pathway. Indeed, it has been demonstrated that the Crk adapter protein recruits the Rap1A-specific C3G exchange factor (16), and this in turn results in stimulation of MAP kinase pathway (22, 23). Given the observation that Crk specifically interacts with site C in vitro (Fig. 3), it is likely that site C is actually involved in the activation of Rap1A and stimulation of the Erk kinases, thus explaining its resistance to Rap1A inhibition. Further evidence supporting the contention that site C funnels through a signaling pathway distinct from the other transforming add-back mutants stems from recent observations that reovirus infection selectively kills Ras-transformed cells through lytic virus production (8). We and our collaborators have recently demonstrated that reovirus infection of YB-, YD-, and YE-expressing cells results in virus-mediated cell lysis but is incapable of killing cells transformed by the YC mutant (P. Lee, D. Dankort, and W. J. Muller, unpublished observations). Taken together, these data again argue that both Ras-dependent and Ras-independent pathways are involved in Neu-mediated transformation.
We have conducted further detailed mutagenesis analysis of the Grb2 and Shc binding sites to determine that the YB and YD phosphorylation sites are highly specific binding sites for these adapter proteins. The results of these analyses demonstrate that the transforming potential of these mutants is directly correlated with ability of these sites to recruit Grb2 and Shc, respectively (not shown). Although YB and YD mutants are capable of either directly or indirectly recruiting the Grb2 protein, only the YB Neu mutant is dependent on Grb2 function to stimulate DNA synthesis (Fig. 6). These observations argue that Shc has the ability to induce DNA synthesis in a Grb2-independent manner. Consistent with this view, it has recently been demonstrated that tyrosines 239 and 240 within Shc are capable of stimulating mitogenesis and cell survival independent of the Grb2/Ras signaling pathway (16, 17). Although the Shc-associated proteins have yet to be identified, several unidentified proteins specifically associate with Shc through these tyrosine phosphorylation sites (49). Identification of these novel proteins associated with Shc will provide important insight into the role of Shc in Neu-mediated proliferation.
Further evidence supporting the contention that Shc and Grb2 utilize distinct effector pathways stems from studies of transgenic mice expressing the YB and YD Neu mutants in the mammary epithelium. Mammary epithelial expression of either YB or YD efficiently induced mammary tumors in these strains after a latency period ranging from 102 to 152 days (Fig. 6). In contrast, a maximum of 50% (depending on the NYPD line) of NYPD female transgenic mice developed focal mammary tumors after a 351-day observation period. These observations argue that coupling of Neu to either the Grb2 (YB strains) or Shc (YD strains) adapter protein is able to efficiently promote transformation of mammary epithelial cells. In fact, the 100% of the mammary tumor phenotype is higher than the 80% penetrance phenotype observed with the comparable activated neu strains (43). The difference in biological behavior of these strains may reflect the fact that the latter activated neu strains still carry the negative regulatory tyrosine residue (tyrosine 1028) whereas the YB and YD strains do not (9).
The potent transforming activity of YD and YB strains was associated with their capacity to couple to both the Erk and Akt kinase cascades. In addition, mammary tumor progression in the YB- and YD-induced mammary tumors was also associated with a dramatic upregulation of the ErbB-3 RTK and activation of the associated PI-3′ kinase signaling pathway. Thus, stimulation of both Ras and PI-3′ kinase-dependent pathways was sufficient to promote efficient mammary tumor induction. In contrast to these observations, the NYPD-induced mammary tumors failed to exhibit significant activation of either Erk or Akt kinase, suggesting that mammary tumorigenesis in this model system occurs through signaling pathways independent of either Ras or PI-3′ kinase. Indeed, we have recently demonstrated that the NYPD mutant still retains the capacity to couple to the Src family kinases and itself retains wild-type kinase activity in vitro (Dankort and Muller, unpublished). Conceivably, stimulation of the Src family kinases may be responsible for the residual transforming activity exhibited by the NYPD neu mutant. Indeed, it has been demonstrated that mammary epithelial expression of activated Src is capable of inducing focal mammary tumors after a long latency period (53). Another possible mechanism by which the NYPD neu mutant is capable of inducing mammary tumors is through reversion of specific Neu autophosphorylation sites. Indeed, previous studies have shown that 10% of the mammary tumors arising in transgenic mice expressing a mutant polyomavirus middle T oncogene decoupled from the Shc adapter molecule possess reversion mutations that restore the ability of Shc to efficiently couple to polyomavirus middle T antigen (54). Finally, it is also possible that the NYPD mutant induces tumorigenesis through the formation of specific heterodimers with other members of the epidermal growth factor receptor family. In this regard, we have noted that tumors derived from NYPD strains express elevated levels of ErbB-3 (Fig. 9). Future studies with these NYPD strains should provide important insight into the contribution of each of these coupled signaling pathways in Neu-mediated tumorigenesis and metastasis.
Although mammary epithelial expression of either the YB or YD mutant efficiently induced mammary tumors, histological examination of these Neu-induced mammary tumors have revealed that they have distinct morphologies (Fig. 5 and 7). In particular, the YB tumors displayed papillary morphology, whereas the YD-induced tumors developed a nodular phenotype similar to that displayed by the parental activated neu strains. The differences in the morphologies may reflect differences in the repertoire of signaling proteins coupled to Shc and Grb2. Indeed, it has been demonstrated that in addition to Grb2, Shc couples to several unidentified proteins (49). Moreover, in certain cell types Shc can signal independent of Grb2/Ras activation through a pathway involving the upregulation of the c-Myc transcription (16). Taken together, these observations argue that YB and YD neu mutants function through both common and distinct signaling pathways.
Another potentially important phenotypic difference between the YB and YD strains is that only the YB strains efficiently develop metastatic lesions. The difference in the metastatic properties of these neu mutants is not due to difference in rate of tumor development since the nonmetastatic YD tumors actually develop a much greater tumor load. One potential explanation for the differential metastatic properties of the YB-induced tumors is that Neu is coupled to signaling pathways that confer enhanced metastatic properties to these cells. Although the molecular basis for this difference in metastatic phenotype is unclear, we have previously shown that the dosage of Grb2 can have a profound effect on tumor induction in transgenic mice (7). In addition to affecting tumor induction, reduction of Grb2 levels also impairs ductal outgrowth of the mammary epithelium (7), suggesting that it may be involved in promoting epithelial cell migration. Direct recruitment of Grb2 to the Met receptor appears to confer metastatic potential to engineered cell lines (15), and there appears to be a direct link with Grb2 to sustain myoblast proliferation and/or survival during migration from the somites in Met receptor knock-in mice (28). Given the importance of migration in metastatic disease, it is conceivable that the YB mutant, through its direct interaction with Grb2, promotes metastasis through stimulation of cell migration and or survival of migrating cells. In this regard, it has been demonstrated that phosphospecific antibodies to Neu tyrosine phosphorylation site 1253 (YE) appear to detect a minority of ErbB-2 human breast cancers that exhibit the invasive phenotype (11). It is conceivable that other ErbB-2 phosphorylation sites such as YB (tyrosine 1144) may also predict particularly aggressive human breast cancer phenotypes. Future studies with these mutant Neu strains and conditional ablation of Grb2 in mice should provide important insights into the molecular basis of metastatic progression.
ACKNOWLEDGMENTS
We thank Luika Timmerman for comments. We are also grateful to Monica Graham, Linda Wei, and Judy Walls for technical support.
This work was supported by a CBCRI grant awarded to W.J.M. This work was also partially supported by grants awarded to R.G.O. (National Cancer Institute grant CA74507 and Cancer Center Support grant P30 CA30199). D.D. was supported by scholarships from Cancer Research Society and NSERC. W.J.M. is supported by an MRC Scientist award.
REFERENCES
- 1.Alroy I, Soussan L, Seger R, Yarden Y. Neu differentiation factor stimulates phosphorylation and activation of the Sp1 transcription factor. Mol Cell Biol. 1999;19:1961–1972. doi: 10.1128/mcb.19.3.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrechek E R, Hardy W R, Siegel P M, Rudnicki M A, Cardiff R D, Muller W J. Amplification of neu/erbB-2 oncogene in a novel model of mammary tumorigenesis. Proc Natl Acad Sci USA. 2000;97:3444–3447. doi: 10.1073/pnas.050408497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrulis I L, Bull S B, Blackstein M E, Sutherland D, Mak C, Sidlofsky S, Pritzker K P, Hartwick R W, Hanna W, Lickley L, Wilkinson R, Qizilbash A, Ambus U, Lipa M, Weizel H, Katz A, Baida M, Mariz S, Stoik G, Dacamara P, Strongitharm D, Geddie W, McCready D. neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer. Toronto Breast Cancer Study Group. J Clin Oncol. 1998;16:1340–1349. doi: 10.1200/JCO.1998.16.4.1340. [DOI] [PubMed] [Google Scholar]
- 4.Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz C C, Dantis L, Sklarin N T, Seidman A D, Hudis C A, Moore J, Rosen P P, Twaddell T, Henderson I C, Norton L. Phase II study of weekly intravenous trastuzumab (Herceptin) in patients with HER2/neu-overexpressing metastatic breast cancer. Semin Oncol. 1999;26:78–83. [PubMed] [Google Scholar]
- 5.Ben-Levy R, Paterson H F, Marshall C J, Yarden Y. A single autophosphorylation site confers oncogenicity to the Neu/ErbB-2 receptor and enables coupling to the MAP kinase pathway. EMBO J. 1994;13:3302–3311. doi: 10.1002/j.1460-2075.1994.tb06632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouchard L, Lamarre L, Tremblay P J, Jolicoeur P. Stochastic appearance of mammary tumors in transgenic mice carrying the MMTV/ c-neu oncogene. Cell. 1989;57:931–936. doi: 10.1016/0092-8674(89)90331-0. [DOI] [PubMed] [Google Scholar]
- 7.Cheng A M, Saxton T M, Sakai R, Kulkarni S, Mbamalu G, Vogel W, Tortorice C G, Cardiff R D, Cross J C, Muller W J, Pawson T. Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell. 1998;95:793–803. doi: 10.1016/s0092-8674(00)81702-x. [DOI] [PubMed] [Google Scholar]
- 8.Coffey M C, Strong J E, Forsyth P A, Lee P W. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 9.Dankort D L, Wang Z, Blackmore V, Moran M F, Muller W J. Distinct tyrosine autophosphorylation sites negatively and positively modulate Neu-mediated transformation. Mol Cell Biol. 1997;17:5410–5425. doi: 10.1128/mcb.17.9.5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Fiore P P, Segatto O, Lonardo F, Fazioli F, Pierce J H, Aaronson S A. The carboxy-terminal domains of ErbB-2 and epidermal growth factor receptor exert different regulatory effects on intrinsic receptor tyrosine kinase function and transforming activity. Mol Cell Biol. 1990;10:2749–2756. doi: 10.1128/mcb.10.6.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiGiovanna M P, Carter D, Flynn S D, Stern D F. Functional assay for HER-2/neu demonstrates active signalling in a minority of HER-2/neu-overexpressing invasive human breast tumours. Br J Cancer. 1996;74:802–806. doi: 10.1038/bjc.1996.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fazioli F, Kim U H, Rhee S G, Molloy C J, Segatto O, Di Fiore P P. The ErbB-2 mitogenic signaling pathway: tyrosine phosphorylation of phospholipase C-gamma and GTPase-activating protein does not correlate with ErbB-2 mitogenic potency. Mol Cell Biol. 1991;11:2040–2048. doi: 10.1128/mcb.11.4.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiddes R J, Campbell D H, Janes P W, Sivertsen S P, Sasaki H, Wallasch C, Daly R J. Analysis of Grb7 recruitment by heregulin-activated erbB receptors reveals a novel target selectivity for erbB3. J Biol Chem. 1998;273:7717–7724. doi: 10.1074/jbc.273.13.7717. [DOI] [PubMed] [Google Scholar]
- 14.Galang C K, Garcia-Ramirez J, Solski P A, Westwick J K, Der C J, Neznanov N N, Oshima R G, Hauser C A. Oncogenic Neu/ErbB-2 increases ets, AP-1, and NF-kappaB-dependent gene expression, and inhibiting ets activation blocks Neu-mediated cellular transformation. J Biol Chem. 1996;271:7992–7998. doi: 10.1074/jbc.271.14.7992. [DOI] [PubMed] [Google Scholar]
- 15.Giordano S, Bardelli A, Zhen Z, Menard S, Ponzetto C, Comoglio P M. A point mutation in the MET oncogene abrogates metastasis without affecting transformation. Proc Natl Acad Sci USA. 1997;94:13868–13872. doi: 10.1073/pnas.94.25.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gotoh N, Tojo A, Shibuya M. A novel pathway from phosphorylation of tyrosine residues 239/240 of Shc, contributing to suppress apoptosis by IL-3. EMBO J. 1996;15:6197–204. [PMC free article] [PubMed] [Google Scholar]
- 17.Gotoh N, Toyoda M, Shibuya M. Tyrosine phosphorylation sites at amino acids 239 and 240 of Shc are involved in epidermal growth factor-induced mitogenic signaling that is distinct from Ras/mitogen-activated protein kinase activation. Mol Cell Biol. 1997;17:1824–1831. doi: 10.1128/mcb.17.4.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guy C T, Cardiff R D, Muller W J. Activated neu induces rapid tumor progression. J Biol Chem. 1996;271:7673–7678. doi: 10.1074/jbc.271.13.7673. [DOI] [PubMed] [Google Scholar]
- 19.Guy C T, Webster M A, Schaller M, Parsons T J, Cardiff R D, Muller W J. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu C-D, Kariya K, Kotani G, Shirouzu M, Yokoyama S, Kataoka T. Coassociation of Rap1A and Ha-Ras with Raf-1 N-terminal region interferes with Ras-dependent activation of Raf-1. J Biol Chem. 1997;272:11702–11705. doi: 10.1074/jbc.272.18.11702. [DOI] [PubMed] [Google Scholar]
- 21.Hynes N E, Stern D F. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta. 1994;1198:165–184. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 22.Ichiba T, Hashimoto Y, Nakaya M, Kuraishi Y, Tanaka S, Kurata T, Mochizuki N, Matsuda M. Activation of C3G guanine nucleotide exchange factor for Rap1 by phosphorylation of tyrosine 504. J Biol Chem. 1999;274:14376–14381. doi: 10.1074/jbc.274.20.14376. [DOI] [PubMed] [Google Scholar]
- 23.Ishimaru S, Williams R, Clark E, Hanafusa H, Gaul U. Activation of the Drosophila C3G leads to cell fate changes and overproliferation during development, mediated by the RAS-MAPK pathway and RAP1. EMBO J. 1999;18:145–155. doi: 10.1093/emboj/18.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janes P W, Daly R J, deFazio A, Sutherland R L. Activation of the Ras signalling pathway in human breast cancer cells overexpressing erbB-2. Oncogene. 1994;9:3601–3608. [PubMed] [Google Scholar]
- 25.Jelinek M A, Hassell J A. Reversion of middle T antigen-transformed Rat-2 cells by Krev-1: implications for the role of p21c-ras in polyomavirus-mediated transformation. Oncogene. 1992;7:1687–1698. [PubMed] [Google Scholar]
- 26.Kitayama H, Matsuzaki T, Ikawa Y, Noda M. Genetic analysis of the Kirsten-ras-revertant 1 gene: potentiation of its tumor suppressor activity by specific point mutations. Proc Natl Acad Sci USA. 1990;87:4284–4287. doi: 10.1073/pnas.87.11.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luttrell D K, Lee A, Lansing T J, Crosby R M, Jung K D, Willard D, Luther M, Rodriguez M, Berman J, Gilmer T M. Involvement of pp60c-src with two major signaling pathways in human breast cancer. Proc Natl Acad Sci USA. 1994;91:83–87. doi: 10.1073/pnas.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maina F, Casagranda F, Audero E, Simeone A, Comoglio P M, Klein R, Ponzetto C. Uncoupling of Grb2 from the Met receptor in vivo reveals complex roles in muscle development. Cell. 1996;87:531–542. doi: 10.1016/s0092-8674(00)81372-0. [DOI] [PubMed] [Google Scholar]
- 29.Marshall C J. Ras effectors. Curr Opin Cell Biol. 1996;8:197–204. doi: 10.1016/s0955-0674(96)80066-4. [DOI] [PubMed] [Google Scholar]
- 30.Muller W J, Sinn E, Pattengale P K, Wallace R, Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988;54:105–115. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- 31.Muthuswamy S K, Muller W J. Activation of Src family kinases in Neu-induced mammary tumors correlates with their association with distinct sets of tyrosine phosphorylated proteins in vivo. Oncogene. 1995;11:1801–1810. [PubMed] [Google Scholar]
- 32.Muthuswamy S K, Muller W J. Activation of the Src family of tyrosine kinases in mammary tumorigenesis. Adv Cancer Res. 1994;64:111–123. doi: 10.1016/s0065-230x(08)60836-2. [DOI] [PubMed] [Google Scholar]
- 33.Muthuswamy S K, Muller W J. Direct and specific interaction of c-Src with Neu is involved in signaling by the epidermal growth factor receptor. Oncogene. 1995;11:271–279. [PubMed] [Google Scholar]
- 34.Muthuswamy S K, Siegel P M, Dankort D L, Webster M A, Muller W J. Mammary tumors expressing the neuproto-oncogene possess elevated c-Src tyrosine kinase activity. Mol Cell Biol. 1994;14:735–743. doi: 10.1128/mcb.14.1.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olayioye M A, Neve R M, Lane H A, Hynes N E. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pawson T. Protein modules and signalling networks. Nature. 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 37.Ricci A, Lanfrancone L, Chiari R, Belardo G, Pertica C, Natali P G, Pelicci P G, Segatto O. Analysis of protein-protein interactions involved in the activation of the Shc/Grb-2 pathway by the ErbB-2 kinase. Oncogene. 1995;11:1519–1529. [PubMed] [Google Scholar]
- 38.Sakoda T, Kaibuchi K, Kishi K, Kishida S, Doi K, Hoshino M, Hattori S, Takai Y. smg/rap1/Krev-1 p21s inhibit the signal pathway to the c-fos promoter/enhancer from c-Ki-ras p21 but not from c-raf-1 kinase in NIH3T3 cells. Oncogene. 1992;7:1705–1711. [PubMed] [Google Scholar]
- 39.Segatto O, King C R, Pierce J H, Di Fiore P P, Aaronson S A. Different structural alterations upregulate in vitro tyrosine kinase activity and transforming potency of the erbB-2gene. Mol Cell Biol. 1988;8:5570–5574. doi: 10.1128/mcb.8.12.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Segatto O, Lonardo F, Wexler D, Fazioli F, Pierce J H, Bottaro D P, White M F, Di Fiore P P. The juxtamembrane regions of the epidermal growth factor receptor and gp185erbB-2determine the specificity of signal transduction. Mol Cell Biol. 1991;11:3191–3202. doi: 10.1128/mcb.11.6.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segatto O, Pelicci G, Giuli S, Digiesi G, Di Fiore P P, McGlade J, Pawson T, Pelicci P G. Shc products are substrates of erbB-2 kinase. Oncogene. 1993;8:2105–2112. [PubMed] [Google Scholar]
- 42.Siegel P M, Dankort D L, Hardy W R, Muller W J. Novel activating mutations in the neuproto-oncogene involved in induction of mammary tumors. Mol Cell Biol. 1994;14:7068–7077. doi: 10.1128/mcb.14.11.7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siegel P M, Ryan E D, Cardiff R D, Muller W J. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. EMBO J. 1999;18:2149–2164. doi: 10.1093/emboj/18.8.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slamon D J, Clark G M, Wong S G, Levin W J, Ullrich A, McGuire W L. Human breast cancer: correlation of relapse and survival with the amplification of the HER2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 45.Slamon D J, Godolphin W, Jones L A, Holt J A, Wong S G, Keith D E, Levin W J, Stuart S G, Udove J, Ullrich A, Press M F. Studies of the HER-2/neu proto-oncogene in human breast and ovaian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 46.Stein D, Wu J, Fuqua S A, Roonprapaunt C, Yajnik V, D'Eutachio P, Moskow J J, Buchberg A M, Osborne C K, Margolis B. The SH2 protein GRB7 is coamplified, overexpressed and in a tight complex with HER2 in breast cancer. EMBO J. 1994;13:1331–1340. doi: 10.1002/j.1460-2075.1994.tb06386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urano T, Emkey R, Feig L A. Ral-GTPases mediate a distinct downstream signaling pathway from Ras that facilitates cellular transformation. EMBO J. 1996;15:810–816. [PMC free article] [PubMed] [Google Scholar]
- 48.Valius M, Kazlauskas A. Phospholipase C-gamma 1 and phosphatidylinositol 3 kinase are the downstream mediators of the PDGF receptor's mitogenic signal. Cell. 1993;73:321–334. doi: 10.1016/0092-8674(93)90232-f. [DOI] [PubMed] [Google Scholar]
- 49.van der Geer P, Wiley S, Gish G D, Pawson T. The Shc adaptor protein is highly phosphorylated at conserved, twin tyrosine residues (Y239/240) that mediate protein-protein interactions. Curr Biol. 1996;6:1435–1444. doi: 10.1016/s0960-9822(96)00748-8. [DOI] [PubMed] [Google Scholar]
- 50.Vonderhaar B K, Greco A E. Lobulo-alveolar development of mouse mammary glands is regulated by thyroid hormones. Endocrinology. 1979;104:409–418. doi: 10.1210/endo-104-2-409. [DOI] [PubMed] [Google Scholar]
- 51.Wallasch C, Weiss F U, Niederfellner G, Jallal B, Issing W, Ullrich A. Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. EMBO J. 1995;14:4267–4275. doi: 10.1002/j.1460-2075.1995.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Moran M F. Requirement for the adapter protein GRB2 in EGF receptor endocytosis. Science. 1996;272:1935–1939. doi: 10.1126/science.272.5270.1935. [DOI] [PubMed] [Google Scholar]
- 53.Webster M A, Cardiff R D, Muller W J. Induction of mammary epithelial hyperplasias and mammary tumors in transgenic mice expressing a murine mammary tumor virus/activated c-src fusion gene. Proc Natl Acad Sci USA. 1995;92:7849–7853. doi: 10.1073/pnas.92.17.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Webster M A, Hutchinson J N, Rauh M J, Muthuswamy S K, Anton M, Tortorice C G, Cardiff R D, Graham F L, Hassell J A, Muller W J. Requirement for both Shc and phosphatidylinositol 3′kinase signaling pathways in polyomavirus middle T-mediated mammary tumorigenesis. Mol Cell Biol. 1998;18:2344–2359. doi: 10.1128/mcb.18.4.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamamoto H, Flannery M L, Kupriyanov S, Pearce J, McKercher S R, Henkel G W, Maki R A, Werb Z, Oshima R G. Defective trophoblast function in mice with a targeted mutation of Ets2. Genes Dev. 1998;12:1315–1326. doi: 10.1101/gad.12.9.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang B S, Hauser C A, Henkel G, Colman M S, Van Beveren C, Stacey K J, Hume D A, Maki R A, Ostrowski M C. Ras-mediated phosphorylation of a conserved threonine residue enhances the transactivation activities of c-Ets1 and c-Ets2. Mol Cell Biol. 1996;16:538–547. doi: 10.1128/mcb.16.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]