Abstract
The inhibitory regulators, known as immune checkpoints, prevent overreaction of the immune system, avoid normal tissue damage, and maintain immune homeostasis during the antimicrobial or antiviral immune response. Unfortunately, cancer cells can mimic the ligands of immune checkpoints to evade immune surveillance. Application of immune checkpoint blockade can help dampen the ligands expressed on cancer cells, reverse the exhaustion status of effector T cells, and reinvigorate the antitumor function. Here, we briefly introduce the structure, expression, signaling pathway, and targeted drugs of several inhibitory immune checkpoints (PD-1/PD-L1, CTLA-4, TIM-3, LAG-3, VISTA, and IDO1). And we summarize the application of immune checkpoint inhibitors in tumors, such as single agent and combination therapy and adverse reactions. At the same time, we further discussed the correlation between immune checkpoints and microorganisms and the role of immune checkpoints in microbial-infection diseases. This review focused on the current knowledge about the role of the immune checkpoints will help in applying immune checkpoints for clinical therapy of cancer and other diseases.
Keywords: immune checkpoint, immunotherapy, cancer, microbiome, PD-1/PD-L1
Introduction
Activation of T cells plays an important role in the process of immunity (Lenschow and Bluestone, 1993). During normal immune response, the process that T cells accept antigen peptides presented by major histocompatibility complex (MHC) on antigen-presenting cells (APCs) via T-cell receptor (TCR) in order to exert its function is called the first signal for T-cell activation. The second signal for T-cell activation is a costimulatory signal which comes from a combination between CD28 on T cells and CD80(B7-1)/CD86(B7-2) on APCs (Lenschow et al., 1996; Nandi et al., 2020). This activation process also requires cytokines such as IL-2 to help. The rightly activated T cells or in tandem with B cells will eliminate threats, while uncontrolled activation of T cells would bring serious consequences such as autoimmune diseases (Takeuchi et al., 2020). Therefore, scientists devoted their lives to shed light on how the immune system regulates itself.
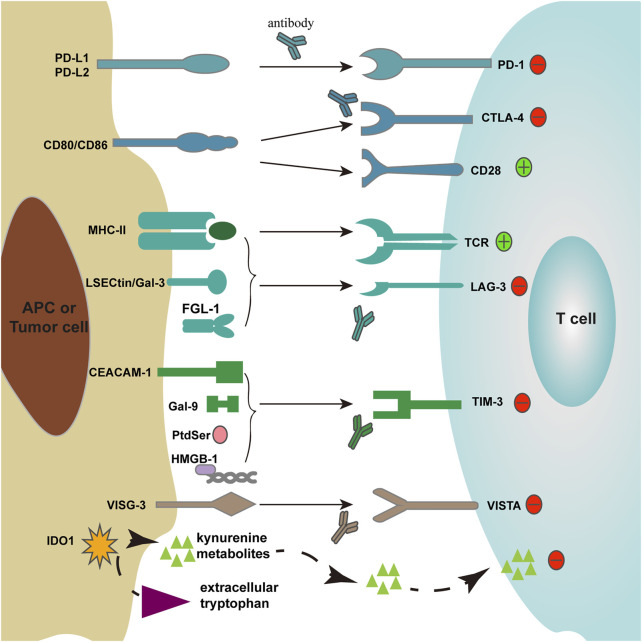
In the last two decades, the understanding of regulatory pathways in immune responses to cancer immunotherapies remains unclear. The enormous progress was made in 1996; Leach and his colleagues (Linsley et al., 1991; Leach et al., 1996) have been validated that blockade of cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) could downregulate T-cell responses and enhance antitumor responses in immunocompetent mouse models. In 2000, Gordon J. Freeman identified that CTLA-4 structurally similar protein-programmed death 1 (PD-1) could bind to its ligand PD-L1 and lead to the inhibition of lymphocyte proliferation (Freeman et al., 2000). The binding of B- and T-cell lymphocyte attenuator (BTLA) to its ligand HVEM may lead to decreased T-cell proliferation and cytokine production (Murphy et al., 2006). The binding of T-cell immunoglobulin and mucin domain-containing 3 (TIM-3) to its ligand galectin-9 could result in T helper 1 (Th1) cell death (Zhu et al., 2005). V-domain Ig suppressor of T-cell activation (VISTA) is a potent T-cell suppressor and inhibits T-cell immune response in animal models (Wang et al., 2011). During these processes, the set of costimulatory or coinhibitory molecules, which regulate the activation, effector functions, and interactions among APCs and T lymphocytes, provides a critical checkpoint in the regulation of T-cell immunity and maintenance of immune homeostasis. As their function in the balance of the immune system, these costimulatory or coinhibitory proteins are defined as immune checkpoint proteins (Figure 1, Table 1). A direct consequence of these findings was to reveal the regulatory pathways involved in immune responses in cancer and infectious diseases.
FIGURE 1.
Immune checkpoint receptors and their ligands. Two signals participate in T-cell activation: 1) T cells recognize antigen presented by MHC-II molecules on APCs through TCR; 2) T cells accept costimulatory signals CD80/CD86 through CD28.
TABLE 1.
The expression and mechanism of the immune checkpoints.
| Immune checkpoints | Expression | Ligand | Mechanisms | PMID |
|---|---|---|---|---|
| PD-1 | Activated T cells, Tregs, B cells, NK cells, DCs, macrophages, and monocytes | PD-L1 and PD-L2 | ITSM recruits SHP-2, which acts as a bridge between two PD-1 molecules and induces inhibitory function of PD-1 | 30851633, 32184441, and 28443090 |
| CTLA-4 | Activated T cells and Tregs | CD80 and CD 86 | Conserved YVKM motif in the cytoplasmic tail of CTLA-4 mediates recruitment of SH2-domain-containing proteins to regulate immune response | 10411922, 18845758, and 29794465 |
| LAG-3 | Activated T cells, Tregs, NK cells, DCs, and B cells | MHC-II, LESCtin, Galectin-3, FGL-1, and α-synuclein | The KIEELE motif is considered to be essential for LAG-3 mediated inhibition | 33488626 and 34067904 |
| TIM-3 | Activated T cells, TH17 cells, Tregs, DCs, NK cells, and monocytes | Galectin-9, CEACAM-1, HMGB-1, and PtdSer | TIM-3 exerts its function through several tyrosine residues | 29069302 and 31676858 |
| VISTA | Myeloid cell, T cells, and Tregs | VSIG-3 and PSGL-1 | VISTA has the potential function of both a receptor and a ligand. The precise mechanism of VISTA needs to be explored | 29375120 and 31690319 |
| IDO1 | A heme-containing enzyme participates in tryptophan metabolism | L-Tryptophan | Accumulation of kynurenine metabolites leads to suppression of T cells and induction of Tregs | 20720200 and 33883013 |
Immune checkpoint proteins have been playing a significant role in inflammatory reactions and cancer immunotherapy. A number of immune checkpoint proteins were shown to be dysregulated in cancers and infectious diseases, including PD-1/PD-L1, CTLA-4, lymphocyte activation 3 (LAG-3), TIM-3, VISTA, and Indoleamine-2,3 dioxygenase 1 (IDO1). These immune checkpoints and other regulatory cells, such as regulatory T cells (Tregs), myeloid-derived suppressors cells (MDSCs), M2 macrophages, and cytokines, are often enhanced during infections and cancers (Pauken and Wherry, 2015). Pathogens can develop immune checkpoints to limit host-protective antigen-specific immune response (Dyck and Mills, 2017). The cancer cells can disrupt the immune response and cleverly escape from immunity by dysregulating immune checkpoint signaling. Many similarities exist between cancer and infectious disease (Hotchkiss and Moldawer, 2014). They can utilize similar receptors to detect damage-associated molecular patterns (DAMP) and pathogen-associated molecular patterns (PAMPs), respectively (Vance et al., 2017). In the meantime, persistent stimulation of the immune system and induction of T-cell-mediated inflammation can be aroused. In pathogen-infected diseases, with elevated expression of the immune checkpoint molecules on T cells as it is in cancer, the immune checkpoint blockade therapy may bring favorable consequences (Wykes and Lewin, 2018). So, agonists of costimulatory signals or antagonists of inhibitory signals function as good ways for cancer therapy and also could help to reverse the state of immune suppression in chronic infection. Some antibodies that targeted immune checkpoint molecules to reverse the suppression of the immune system have been applied in the clinical treatment of cancer (Remon and Besse, 2017; Chen et al., 2019). However, the unexpected events of an immune checkpoint inhibitor (ICI) have emerged as frequent complications at the same time.
Here, we review the mechanisms, functions, and adverse events of common immune checkpoints in cancer and infectious diseases. We also discuss the impact of the bacterial microbiome on the relationship between cancer therapy and the immune system.
Biology of Immune Checkpoint Proteins
PD-1/PD-L1
PD-1 is a 288 amino acid protein that is encoded by the PDCD1 gene and belongs to the immunoglobulin superfamily (Tavares et al., 2018). PD-1 can be expressed on T cells, B cells, natural killer cells (NKs), dendritic cells (DCs), macrophages, and monocytes (Ahmadzadeh et al., 2009). T cells inducibly express PD-1 after activation (Han et al., 2020), while different from other members of the CD28 superfamily, which has Src homology (SH2) binding motifs and/or SH3 binding motifs in their cytoplasmic tail, the cytoplasmic tail of PD-1 possesses a sequence that can form an immunoreceptor tyrosine-based inhibition motif (ITIM) and an immunoreceptor tyrosine-based switch motif (ITSM) that can recruit Src homology 2 domain-containing protein tyrosine phosphatases (SHP-2), resulting in the inhibitory function (Neel et al., 2003; Patsoukis et al., 2020).
The two ligands of PD-1, PD-L1 (also known as B7-H1) and PD-L2 (B7-H2), differ in expression patterns (Panjwani et al., 2018). PD-L1 is expressed on many cells, including B cells, T cells, macrophages, tumor cells, and other tissue cells such as vascular endothelial cells (Ritprajak and Azuma, 2015; Dermani et al., 2019). Ligation of PD-1 and PD-L1 can lead to T-cell dysfunction and anergy, helping PD-L1 expressing tumor cells escape from cytotoxic T-cell-mediated cell death (Ritprajak and Azuma, 2015; Dermani et al., 2019).
PD-1/PD-L1 blockade not only facilitates T-cell function but also restores NKs antitumor response (Hsu et al., 2018). PD-L1 expression on cancer cells resulted in the generation of more aggressive tumors in vivo. Depleting NKs before PD-L1 expressed or not tumor cell implantation resulted in similar growth of tumors and mortality. However, no such effect occurring with depletion of CD4+ and CD8+ T cells indicates that NKs take a vital position in immune checkpoint blockade (Hsu et al., 2018).
It is reported that several signaling pathways would participate in the PD-1/PD-L1 axis. For example, PD-1−PD-L1+ regulatory B cells must exert their immunosuppressive function through activation of the PI3K/AKT/NF-κB signaling pathway in breast cancer (Liu et al., 2021). PTEN is a critical inhibitor of the PI3K/AKT signaling pathway. In microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) gastrointestinal tumors, mutation of PTEN, especially in the phosphatase domain, could be negative predictors of PD-1 blockade treatment (Chida et al., 2021). Blockade of MAPK pathway through MEK1 and two inhibitors prevented the expression of PD-L1 in lung adenocarcinoma cells (Stutvoet et al., 2019), whereas inhibition of ERK could improve the anti-PD-L1 checkpoint blockade effect in preclinical pancreatic ductal adenocarcinoma (Henry et al., 2021). What we have listed above indicates that MAPK pathway activity could also severely influence the PD-L1 axis despite the PI3K pathway. Similarly, using inhibitors of the JAK/STAT pathway, which was reported to suppress PD-L1 upregulation, showed that it can also take part in regulating the PD-L1 axis (Doi et al., 2017).
CTLA-4
CTLA-4 is a 223 amino acid protein, which belongs to the immunoglobulin superfamily and consists of an IgV domain, a transmembrane region, and a cytoplasmic tail containing a conserved YVKM motif (Rowshanravan et al., 2018). Stored in endocytic vesicles, CTLA-4 is transported to the cell membrane to be colocalized with TCR on the cell surface. Dependent on dynamin and clathrin adaptor protein complex (AP2), which targets the YVKM motif, internalization of CTLA-4 from cell surface for degradation and recycling is rapid (usually within minutes) (Shiratori et al., 1997). Then CTLA-4 can be transported to cell membrane again or compartment of lysosome for degradation. Such regulation of AP2 can be disrupted by the phosphorylation of the YVKM motif after T-cell activation (Qureshi et al., 2012). Lipopolysaccharide responsive and beige-like protein (LRBA) may inhibit degradation of CTLA-4 by disrupting transportation of CTLA-4 to the lysosome via binding to YVKM sequence and promote recycling of CTLA-4. Patients with LRBA deficiency raised autoimmunity syndrome designating that accurate CTLA-4 trafficking is important for autoimmune diseases (Lo et al., 2015; Rowshanravan et al., 2018).
The phenomenon that CTLA-4, often expressed on antigen-specific T cells, has a higher affinity (10–100-fold) for CD80 dimer and CD86 monomer than CD28 is considered to be a conventional concept about how CTLA-4 downregulates the immune response (Linsley et al., 1994; van der Merwe et al., 1997). Different from antigen-specific T cells that upregulated CTLA-4 after activation, Tregs constitutively express a high range of CTLA-4 ensuring immune homeostasis and immunosuppressive capacity. Intriguingly, there have been studies proved that CD80 and CD86 on APC can be captured and deleted by CTLA-4 expressed on CD4+CD25+Foxp3+ Tregs (Qureshi et al., 2011; Tekguc et al., 2021), while patients or carriers with CTLA-4 mutation showed diminished Tregs inhibitory function and impaired trans-endocytosis of CD80 (Schubert et al., 2014). These discoveries provide a proper explanation for the rapid endocytic behavior of CTLA-4 that CTLA-4 may exhibit its inhibitory function by trans-endocytosis. Also, there have been studies about other mechanisms undergoing CTLA-4 inhibition. Kong et al. found that protein kinase C-η (PKC-η) was recruited to and physically associated with the CTLA-4 expressed on Tregs in the immunological synapse. PKC-η-deficient Tregs lacked their suppressive function, leading to lymphoproliferation and autoimmune syndromes (Kong et al., 2014). In addition, competitively binding with CD28, CTLA-4 limited the positive costimulation of CD28 by blocking the downstream PI3K/AKT and NF-κB signaling pathway (Pages et al., 1994; Olsson et al., 1999). The anti-CTLA-4 antibody (ipilimumab) eliminated Tregs in an Fc-dependent manner to achieve clinical relief, which may be due to relieved NKs cytotoxicity suppressed by Tregs (Romano et al., 2015; Khan et al., 2020). For anti-CTLA-4 antibodies therapy, CD8+ T cells were required for the therapeutic effect. Fas-FasL and perforin interactions also were important for CTLA-4 blockade (van Elsas et al., 2001).
LAG-3
Firstly identified in 1990 by Triebel and colleagues, lymphocyte activation 3 (LAG-3, CD223), an immune inhibitory receptor, is a 503 amino acid protein encoded by lymphocyte activation gene that is located on chromosome 12, containing eight exons (Triebel et al., 1990; Sierro et al., 2011). Belonging to the Ig superfamily, LAG-3 contains four extracellular Ig-like domains D1, D2, D3, and D4, which share approximately 20% amino acid homology with that of CD4. Comprising unlike intracellular region with CD4, LAG-3 is closely related but exhibits divergent functions with CD4 (Maruhashi et al., 2020). The cytoplasmic tail of LAG-3 has three conserved motifs. The first motif, which has not been considered functional, contains a hypothesized serine phosphorylation site containing two serine residues in humans. It is reported that the second motif, which has conserved six amino acid sequences (KIEELE), plays an important role in dampening T-cell proliferation, cytokine production, and cytolytic function. The third motif is a glutamic acid and proline dipeptide repeat which can colocalize LAG-3 with CD3, CD4, and CD8 molecules (Goldberg and Drake, 2011; Ruffo et al., 2019).
LAG-3 can be detected from CD4+ and CD8+ T cells, Tregs, NKs, and plasmacytoid DCs and do not express on naive T cells similar to PD-1 and CTLA-4 (Goldberg and Drake, 2011). Activation of LAG-3 can elevate intratumoral Tregs activity, and blocking of it will upregulate T-cell function and reinvigorate CD8+ tumor-infiltrating lymphocytes (TILs) to eliminate tumor cells (Lecocq et al., 2020). CD4+CD25+ Tregs from LAG-3 (−/−) mice exhibited reduced regulatory activity. Treated with anti-LAG-3 antibody, suppression induced by Tregs was inhibited in vitro and in vivo. It is obvious that LAG-3 marks Tregs populations and intermediates their regulatory function (Huang et al., 2004). As a transmembrane protein receptor which is similar to CD4 with greater affinity for MHC-II molecules on APCs (Triebel et al., 1990), there are also other proposed ligands for LAG-3 like galectin-3, fibrinogen-like protein 1 (FGL-1), α-synuclein, and LSECtin (Xu et al., 2014; Kouo et al., 2015; Mao et al., 2016). Recent research showed that FGL-1 worked as an important ligand of LAG-3 in its inhibitory effect on T cells. The expression of LAG-3 can be elevated on exhausted T cells in cancer. FGL-1 is upregulated in several human cancers, and genetic ablation or blockade of the FGL-1/LAG-3 interaction by monoclonal antibodies (mAbs) would enhance T-cell responses and antitumor immunity. Wang et al. expected a poor prognosis in non-small-cell lung cancer (NSCLC) patients with high plasma FGL-1 treated with anti-PD therapy (Wang et al., 2019a). The precise function of ligands of LAG-3 still needs to be clarified.
TIM-3
TIM-3 is a transmembrane protein encoded by HAVCR2 and identified on IFN-γ-producing CD4+ Th1 cells and CD8+ type 1 cytotoxic T cells firstly. Then it is also discovered on monocytes, Tregs, DCs, and NKs (Wolf et al., 2020). The fact that administration of antibody to TIM-3 could enhance Th1-dependent autoimmune disease strongly implying that TIM-3 works as an inhibitory molecule on T-cell function (Monney et al., 2002). Indeed, TIM-3 is found to be coregulated and coexpressed with other immune checkpoint receptors, such as PD-1 and LAG-3 (Chihara et al., 2018). High expression of TIM-3 on effector T cells also indicates severe T-cell exhaustion or dysfunction (Avery et al., 2018).
Without known inhibitory signaling motifs in its cytoplasmic tail, TIM-3 contains five conserved tyrosines to play its role. TIM-3 can be found in lipid rafts and is recruited to the immunological synapse upon T-cell activation (Clayton et al., 2014). TIM-3 interacts with HLA-B associated transcript (BAT3) in ligand unbound form and maintains T-cell activation by recruiting an active form of tyrosine kinase LCK, while in ligand-bound form, tyrosine phosphorylation in its cytoplasmic tail will release BAT from TIM-3 and recruit tyrosine kinase FYN resulting in immune synapse disruption, phosphatase recruitment, and cell apoptosis (van de Weyer et al., 2006; Rangachari et al., 2012).
It has been demonstrated that IL-27/NFIL3 axis promotes permissive chromatin remodeling of the TIM-3 locus, induces TIM-3 expression, and is crucial for the induction of TIM-3 in vivo. IL-27-conditioned Th1 cells exhibit inhibitory function through NFIL3 in intestinal inflammation (Zhu et al., 2015). In human acute myeloid leukemia (AML), activation of TIM-3 works through NF-κB and β-catenin signaling pathways to promote self-renewal of leukemic stem cells (Kikushige et al., 2015). In hepatocellular carcinoma (HCC), TIM-3 was significantly upregulated in NKs and suppressed their cytokine production and cytotoxic activity through inhibiting PI3k/Akt/mTORC1 signaling pathway (Tan et al., 2020).
Different ligands of TIM-3 show various effects. The well-studied ligands of TIM-3 are galectin-9 (Gal-9), carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM-1), high mobility group box-1 protein (HMGB1), and phosphatidylserine (PtdSer). In T cells, ligation between Gal-9 and carbohydrate motifs on the IgV domain of TIM-3 functions in an immunosuppressive way which will induce T-cell apoptosis (Du et al., 2017; Dixon et al., 2018). CEACAM-1 coexpressed with TIM-3 is considered to be required for the regulatory function of TIM-3 (Huang et al., 2015). HMGB1 can bind to DNA released from dying cells and facilitate the uptake of DNA by Toll-like receptors. The interaction between HMGB1 and TIM-3 interferes with the innate immune response induced by nucleic acid (Nogueira-Machado et al., 2011; Chiba et al., 2012; Urban-Wojciuk et al., 2019). PtdSer-TIM-3 interaction shows clues for participating in apoptotic clearance cells, and more consequences between their interaction are waiting to be found (Nakayama et al., 2009).
Gal-9 binding with TIM-3 can cause an influx of calcium and mediate aggregation and apoptosis of effector Th1 cells in vitro. Administration of Gal-9 can result in selective loss of IFN-γ-producing cells and suppression of Th1 autoimmunity (Zhu et al., 2005). PtdSer engagement will induce TIM-3 phosphorylation leading to dysfunction of NKs in HCC (Tan et al., 2020). In head and neck squamous cell cancer (HNSCC), blockade of TIM-3 by mAbs induced the reduction of Tregs and increased IFN-γ production of CD8+ T cells, while the population of CD206+ M2 macrophages was not significantly reduced (Liu et al., 2018). Intriguingly, TIM-3 can also play an immunostimulatory role in NKs, DCs, and macrophages (Gleason et al., 2012; Zhang et al., 2012; Yang et al., 2013; Clayton et al., 2014).
VISTA
V-domain Ig suppressor of T cell activation (VISTA), also termed as PD-1H, B7-H5, V-set immunoregulatory receptor (VSIR), stress-induced secreted protein 1 (SISPQ), and differentiation of embryonic stem cells 1 (Dies1), is a conventional transmembrane protein whose IgV domain homology with PD-L1 and encoded by the gene located on chromosome 10 (Huang et al., 2020). Although containing a similar molecular sequence with the B7 superfamily, VISTA does not possess ITIM/ITAM (immunoreceptor tyrosine-based activation motif). VISTA is expressed on myeloid cells (e.g., monocytes, conventional DCs, macrophages, and circulating granulocytes), T cells, Tregs, and TILs (Hosseinkhani et al., 2021). There are increasing pieces of evidence showing VISTA as a regulatory immune checkpoint. In mice lacking VISTA, they would develop spontaneous T-cell activation, cutaneous lupus erythematosus, and production of inflammatory cytokines and chemokines (Wang et al., 2014; Liu et al., 2015; Han et al., 2019). With the presence of VISTA on erythroid cells, the transformation from naive CD4+ T cells to Tregs would be accelerated through the production of TGF-β (Shahbaz et al., 2018).
Though the binding pattern of VISTA is not clear, several studies showed that VISTA could act as both ligand on APCs and receptor on T cells (Flies et al., 2014; Lines et al., 2014). Researches have reported V-Set and Immunoglobulin domain containing 3 (VSIG-3) as the ligand for VISTA in impeding cytokine and chemokine production (Wang et al., 2019b). In consideration of elevated expression of VISTA or VSIG-3 in many cancers, such as colorectal cancer (CRC), HCC, and intestinal-type gastric cancers, the blockade of the VISTA/VSIG-3 pathway can work as a new target for immune checkpoint therapy. Besides, Alan et al. presented that VISTA can bind to P-selectin glycoprotein ligand-1 (PSGL-1) in a pH-dependent model (Johnston et al., 2019). Meanwhile, a study of VISTA in malignant pleural mesothelioma shows that VISTA expression was associated with better overall survival (OS), suggesting VISTA’s prognostic value (Muller et al., 2020).
IDO1
Indoleamine-2,3 dioxygenase 1 (IDO1) is one of the three enzymes which catalyze the first rate-limiting step in the oxidative metabolism of tryptophan, an essential amino acid for T-cell proliferation and differentiation. It is mainly distributed in DCs, macrophages, and monocytes (Munn and Mellor, 2013).
Tumor cells can recruit IDO-expressed DCs into the tumor microenvironment (TME). Due to the aggregation of IDO, lack of tryptophan will lead to stagnation of T-cell proliferation and differentiation in many ways. First, decreased tryptophan means elevated uncharged Trp-tRNA, which leads to activation of a stress response kinase, general control nonderepressible 2 (GCN2) (Munn et al., 2005). Then eukaryotic initiation factor-2 (eIF-2) is phosphorylated by GCN2, and translation of protein required for generation and proliferation of effector T cells will be limited. Second, degradation of tryptophan results in suppression of mammalian target of rapamycin complex 1 (mTORC1) and PKC-θ associated with induction of autophagy. Apoptosis of effector T cells will be reinforced (Metz et al., 2012). Third, IDO1 can induce Tregs through increased activity of aryl hydrocarbon receptor (AHR) binding with kynurenine, a metabolite of tryptophan (Mezrich et al., 2010). Thus, the unbalanced metabolism of tryptophan can promote tumor development and evade immune detection indicating that the application of IDO1 inhibitor is also a promising means to enhance antitumor immunity in theory. In status quo, clinical application of IDO1 inhibitor displayed a controversial outcome with rare effect on monotherapy and combination therapy. Although the agents might not be suitable for such types of cancer involved in research, they may be helpful in other diseases.
Single Agent and Combined Therapy in Cancer
Balckburn et al. have demonstrated that T-cell function decreases with increased expression of immune checkpoints, so targeting these immune checkpoint proteins to modulate immune responses holds great promise for cancer immunotherapy (Blackburn et al., 2009). The purpose of immune checkpoint blockade is mainly to suppress CD8+ T cells and improve tumor-specific immune response. The mAbs by targeting checkpoints CTLA-4 and PD-1/PD-L1 have achieved the US Food and Drug Administration (FDA) approval for the treatment of different cancers (Peggs et al., 2006; Hodi et al., 2010).
Ipilimumab was the first FDA-approved recombinant humanized anti-CTLA-4 immunoglobulin G1 monoclonal antibody in 2011 for the treatment of advanced melanoma in patients who cannot be surgically cured or have metastasis (Vaddepally et al., 2020). It can also work well with intermediate or poor-risk advanced renal cell carcinoma (RCC), MSI-H/dMMR CRC, metastatic NSCLC, unresectable malignant pleural mesothelioma, and HCC, which have been previously treated with sorafenib, in combination with nivolumab (Pinto et al., 2019; McKay et al., 2020; Baas et al., 2021; Casak et al., 2021; Saung et al., 2021). In 2014, nivolumab and pembrolizumab (PD-1 blockade) were approved by the FDA as a humanized IgG antibody for the treatment of unresectable or metastatic melanoma (Prasad and Kaestner, 2017; Finkelmeier et al., 2018). In 2016, the PD-L1 blockade, atezolizumab, a humanized IgG antibody, officially worked as a second-line treatment for locally advanced or metastatic urothelial carcinoma (Patel et al., 2017). With the maturity of theory and technology, the usage range of PD-1/PD-L1 blockade has gradually expanded, including metastatic nonsquamous NSCLC, advanced RCC, unresectable or metastatic, recurrent HNSCC, MSI-H/dMMR CRC, relapsed or refractory classical Hodgkin lymphoma (cHL), locally advanced or metastatic urothelial carcinoma, cervical cancer, gastric cancer, and esophageal cancer (Ansell et al., 2015; Beckermann et al., 2017; Chae et al., 2018; Lin et al., 2018; Saito et al., 2018; Oliveira et al., 2019; Wang and Li, 2019; Nassar et al., 2020; Wu et al., 2020). Pembrolizumab and nivolumab targeting PD-1 showed promising results in melanoma and NSCLC with an objective response rate (ORR) of 40–45% (Darvin et al., 2018). LAG-3 is coexpressed with many inhibitory immune checkpoints, especially PD-1, and this signifies a more exhausting state than expressing PD-1 alone. Utilization of coblockade for PD-1 and LAG-3 shows better curative effects. Relatlimab (in combination with nivolumab) is the first LAG-3 blocking antibody to demonstrate a benefit for patients in a Phase 3 study (Lipson et al., 2021). IMP321, a recombinant soluble LAG-3 Ig fusion protein of which multiple phases I and phase II trials have been completed, may enhance T-cell response, expand the percentage of long-lived effector-memory CD8+ T cells, and rarely induce immune-related adverse events (irAEs) (Brignone et al., 2009; Wang-Gillam et al., 2013). TIM-3, as an immunoinhibitory molecule, indicates the most terminal state of T cells, whose antibodies are being studied and evaluated for clinical trials, including Sym023 (NCT03489343), TSR-022 (NCT03680508), LY3321367 (NCT03099109), and MBG453 (NCT02608268). Many studies focus on the combination between anti-TIM-3 antibody and anti-PD-1 antibody in patients with advanced relapsed or a refractory solid tumor. There are also some ongoing clinical trials that evaluate the safety and feasibility of different ICIs in various tumors. Therapeutically targeting BTLA, VISTA, TIM-3, and TIGIT remain in preclinical stages to treat advanced solid malignancies (Derre et al., 2010) (NCT02671955, NCT02817633, NCT02608268, and NCT03119428).
The combination of immune checkpoints may improve clinical response rates. CTLA-4 and PD-1 blockade combination could increase effector T-cell infiltration into B16 melanoma in mice (Curran et al., 2010). Nivolumab plus ipilimumab in patients with metastatic melanoma yielded a response rate from 40% with treatment alone to 72% among patients who were PD-L1-positive (Larkin et al., 2015). In an open-label, randomized, phase 3 study (CheckMate 743), the results showed that nivolumab plus ipilimumab prolonged the median of the OS by nearly one-third versus chemotherapy (18.1 versus 14.1 months) and 2-years OS rates by nearly a half (41 versus 27%) (Baas et al., 2021). Early data using relatlimab plus nivolumab showed promising antitumor activity with an 11.5% ORR (NCT01968109). Now more and more researches focus on combination medication on relatlimab in HCC (NCT04658147), melanoma (NCT03743766), refractory MSI-H solid tumor (NCT03607890), HNSCC (NCT04326257), and so on. Although the clinical effectiveness of these ICIs gained great success in cancer immunotherapy, a subset of patients still does not respond to these inhibitors.
There are also some studies that showed that immune checkpoint blockade combined with radiotherapy, chemotherapy, and targeted drugs could improve the antitumor efficacy (Twyman-Saint Victor et al., 2015; Ebert et al., 2016; Shi et al., 2016). In the murine HCC model, combination with anti-TIM-3 and radiotherapy significantly shrink the tumor growth and elongate the OS compared with monotherapy (Kim et al., 2021). In an open-label, randomized, phase III trial (CheckMate 649), nivolumab plus chemotherapy reveals promising prospects than chemotherapy alone with superior OS and progression-free survival (PFS) benefit (Janjigian et al., 2021). Guidelines recommended using atezolizumab plus nab-paclitaxel for first-line treatment of unresectable, locally advanced, or metastatic triple-negative breast cancer (TNBC) with PD-L1 expressed on tumor-infiltrating immune cells. A survival analysis found that the OS, safety outcomes, and occurrence of immune-mediated adverse events of atezolizumab plus nab-paclitaxel were all ameliorated than placebo plus nab-paclitaxel (Emens et al., 2021). A TLR9 binding CpG-ODN adjuvant with a systemic anti-CTLA-4 antibody could increase the survival of mice bearing poorly immunogenic B16 melanoma (Davila et al., 2003).
Immune-Related Adverse Events Induced by ICIs
As we know, immune checkpoint blockade has demonstrated a significant promise in the clinic across a range of cancer indications (Chen and Mellman, 2017). However, the immune checkpoint blockade can reinforce host immunity at an expanse of uncontrolled effects that results in a unique spectrum of toxicities defined as immune-related adverse effects (irAEs) (Xu et al., 2018). The degree of irAEs is divided into five grades, comprising mild, moderate, severe, life-threatening, and death, elucidated on Common Terminology Criteria for adverse events from US National Cancer Institute (Cancer Therapy Evaluation Program, 2017). Some key oncology societies recently published comprehensive guidelines for irAEs, including the American Society of Clinical Oncology (ASCO), the European Society for Medical Oncology (ESMO), the Society for Immunotherapy of Cancer Toxicity Management Working Group, and the National Comprehensive Cancer Network (Connolly et al., 2019; Ramos-Casals et al., 2020). The referred organs/system of irAEs include, but are not limited to, cardiac, dermatological, endocrine, gastrointestinal, neurological, muscular, pulmonary, ocular, renal, skeletal, and systemic toxicities.
Paolo et al. declared that irAEs occurring in patients treated with ipilimumab were dose-dependent (Ascierto et al., 2017). Generically, the earliest and the most frequent symptom that showed up during ICI therapy (both anti-CTLA-4 and anti-PD-1) was dermatological changes (Sandigursky and Mor, 2018). A meta-analysis of irAEs in phase III randomized controlled trials of lung cancer proposed that the most frequent irAEs were diarrhea, skin rash, and hypothyroidism (Berti et al., 2021). Another network meta-analysis specifically presented that the main irAEs of ipilimumab were related to the gastrointestinal system (diarrhea, 29%) and skin (rash, 31%), while nivolumab and pembrolizumab were referred to as less frequency in irAEs with maculopapular rash (13%), erythema (4%), hepatitis (3%), arthralgia (12%), hypothyroidism (8%), and hyperglycemia (6%), respectively (Almutairi et al., 2020). A retrospective analysis about North American Intergroup trial E1609 with 1,673 patients proclaimed that grade 1-2 irAEs were associated with longer relapse-free survival (RFS) and OS versus no irAEs, while grade 3-4 showed lesser benefit from RFS and no benefit from OS (Tarhini et al., 2021). Combined immunotherapy could induce more severe and sustained irAEs than monotherapy (Choi and Lee, 2020).
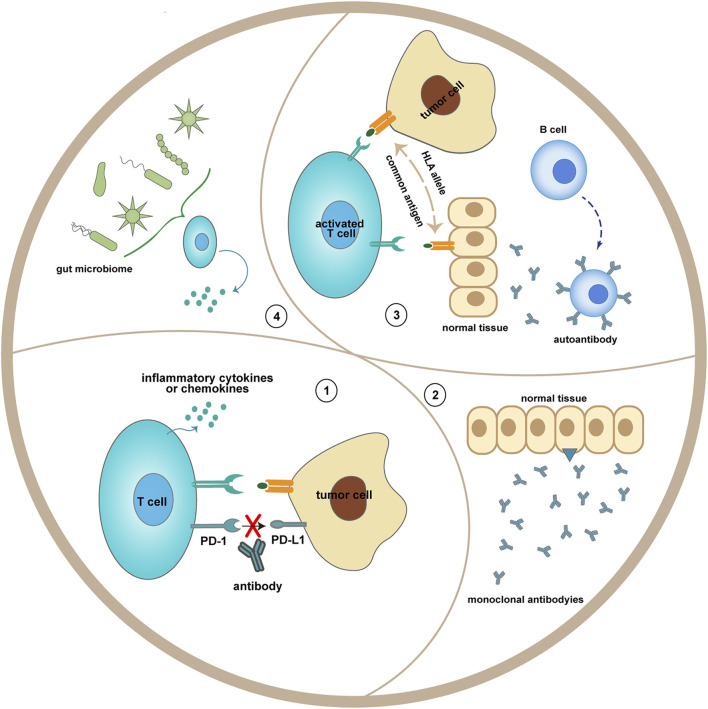
T cells can undergo spontaneous differentiation into Tfh cells in CTLA-4-deficient mice, while not in CD28-deficient mice, they might be applied to explain lethal multiorgan autoimmune symptoms in CTLA4−/− mice (Walker, 2017). As precise mechanisms of irAEs have not been elucidated, some potential ones have been proposed: 1) Increased production of proinflammatory cytokines or chemokines can lead to immune-related damage in tissue which is anatomically prone. 2) Enhanced differentiation of lymphocytes containing T cells and B cells contributes to overpriming of T-cell-mediated immunity and overproduction of autoantibodies (Risbjerg et al., 2020; Ho et al., 2021). 3) Related to off-target effects of ICIs, hypophysitis induced by ipilimumab might be ascribed to targeting CTLA-4 expressed on pituitary tissues (Iwama et al., 2014). 4) The composition and percentage of the commensal microbiome may influence the curative effect for patients treated with ICI (Figure 2). The conclusion discovered from several kinds of research said that various irAEs were associated with the different superior microbiome, application of antibiotics was linked to poor prognosis, and fecal microbiota transplantation (FMT) could reduce immune colitis (Pierrard and Seront, 2019; Hommes et al., 2020; Andrews et al., 2021; Seton-Rogers, 2021). 5) Genetic susceptibility includes HLA haplotypes (Stamatouli et al., 2018).
FIGURE 2.
Potential mechanisms of immune-related adverse events. 1) Blocking the interaction between PD-1 on T cells and PD-L1 on tumor cells may enhance the release of inflammatory cytokines from T cells. 2) Monoclonal antibodies, like anti-CTLA-4, may recognize antigen presented by the normal tissue (hypothalamic and pituitary tissues). 3) Overresponse of naive lymphocytes could proliferate autoreactive T cells and B cells. 4) The gut microbiome, which may be altered after ICI treatment, may influence T-cell function.
For the treatment of irAEs, there have been some guidelines providing algorithms for most of the frequently occurring irAEs. 1) Before ICI initiation, patients’ condition should be evaluated, including family history, general physical condition, and baseline laboratory tests (Ramos-Casals et al., 2020). 2) For those suffering grade I or II irAEs in hardly lethal organs, they could continue/hold immunotherapy. Otherwise, they would better take immunosuppressive or immune-modulating drugs, including corticosteroids, as first-line medicine to control irAEs and relieve clinical symptoms (Esfahani et al., 2020). 3) For those who may bring irreversible or fatal consequences, it is necessary to withhold ICIs and apply steroids or other immunosuppressants immediately (Brahmer et al., 2021). 4) Individual basis should be taken into account when resuming discontinued ICIs owing to irAEs. There are also artificial solutions such as developing engineering antibodies that can induce responsive immune defense and limit systemic exposure of CTLA-4 blockade at the same time (Pai et al., 2019; Lacouture et al., 2021).
Microbiome Related to ICI
With an estimated average of 3.8*1013 commensal bacterial resident in a 70 kg “reference man,” it is fluent in believing that gastrointestinal microbes play an important role in immunity (Sender et al., 2016). To date, there have been some oncogenic gut bacteria such as Salmonella typhi, Helicobacter spp., and Helicobacter pylori (Schwabe and Jobin, 2013; Gagnaire et al., 2017). On the contrary, some bacteria are thought to be beneficial for the proliferation of effector T cells and enhance antitumor efficacy (Pickard et al., 2017; Roy and Trinchieri, 2017). It is harder for mice supported in antibiotic exposed or germ-free conditions to benefit from CTLA-4 blockade versus those in specific pathogen-free environments (Vetizou et al., 2015). Thus, the linkage between microbiome and ICI needs to be elucidated (Table 2).
TABLE 2.
The role of immune checkpoints in bacteria-related diseases.
| Associated immune checkpoints | Associated bacteria/diseases | Related immune or other cells | Influence on efficacy | Mechanism | PMID |
|---|---|---|---|---|---|
| PD-L1 | Oral administration of Bifidobacterium in melanoma | Antigen-specific CD8+ T cells | Bifidobacterium-treated mice showed a better antitumor effect compared to non-Bifidobacterium-treated mice | Oral administration of Bifidobacterium alone improved tumor control to the same degree as anti-PD-L1 therapy, and combination treatment nearly abolished tumor outgrowth. Augmented dendritic cell function leading to enhanced CD8+ T-cell priming and accumulation in the tumor microenvironment mediated the effect. Commensal Bifidobacterium-derived signals modulate the activation of DCs in the steady state, which in turn supports improved effector function of tumor-specific CD8+ T cells | 26541606 |
| PD-1 | Akkermansia muciniphila in epithelial tumors | CCR9+CXCR3+CD4+ T lymphocytes | Enhancing the antitumor effect of PD-1 blockade | Oral gavages with A. muciniphila and E. hirae increased the efficacy of PD-1 blockade with respect to tumor growth and A. muciniphila and E. hirae induced dendritic cells to secrete IL-12, a Th1 cytokine involved in the immunogenicity of PD-1 blockade in eubiotic conditions. Oral supplementation with A. muciniphila after FMT with nonresponder feces restored the efficacy of PD-1 blockade in an interleukin-12-dependent manner by increasing the recruitment of CCR9+CXCR3+CD4+ T lymphocytes into mouse tumor beds | 29097494 |
| PD-1 | Faecalibacterium | CD8+ T cell | Patients with high Faecalibacterium abundance had a significantly prolonged PFS versus those with a low abundance | Patients with a high abundance of Clostridiales, Ruminococcaceae, or Faecalibacterium in the gut had higher levels of effector CD4+ and CD8+ T cells in the systemic circulation with a preserved cytokine response to anti-PD-1 therapy, whereas patients with a higher abundance of Bacteroidales in the gut microbiome had higher levels of regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSC) in the systemic circulation, with a blunted cytokine response | 29097493 |
| PD-L1 | Staphylococcus aureus bacterial pneumonia in mouse model | CD4+ T cell; CD8+ T cell | Anti-PD-L1 therapy did not alter survival in this pneumonia model | Low dose- (LD-) or high dose- (HD-) SA: LD-SA and HD-SA produced lethality of 15 and 70% respectively by 168 h. At 24 h, LD-infected animals exhibited increased lung monocyte PD-L1 expression (p = 0.0002) but lower bacterial counts (p = 0.0002) compared to HD-animals. By 48 h, infection induced lung neutrophil or macrophage PD-L1 expression (p < 0.0001) | 34009385 |
| PD-1 | Mycobacterium tuberculosis infection of rhesus macaques | Mtb-specific CD4 T cells | Animals treated with anti-PD-1 monoclonal antibody developed worse disease and higher granuloma bacterial loads compared | IFN-γ and TNF have both been previously implicated in increased growth of Mtb after PD-1 blockade. Inflammatory pathways (TNF; IFN-γ), normally important for host defense, are required for the exacerbation of Mtb infection after PD-1 blockade | 33452107 |
| PD-1 | Helicobacter pylori in NSCLC | CD8+ T cell | H. pylori seropositivity associated with a decreased NSCLC patient OS and PFS on anti-PD-1 therapy | H. pylori seropositivity is associated with reduced effectiveness of anti-PD1 immunotherapy in patients with NSCLC. H. pylori infection affects not only DC function but also that of monocytes and/or macrophages. Indeed, in humans, it observed a decreased number of cells from the monocyte lineage and a substantially decreased expression of genes induced by type I interferon, IFN-γ, and IL-6 in the tumors of infected patients with NSCLC undergoing anti-PD1 treatment | 34253574 |
| PD-1 | Streptococcus pneumoniae infection in mice | Pneumococcal capsule-specific B cells | PD-1 expression on B cells suppresses protective humoral immune responses to Streptococcus pneumoniae | B-cell-intrinsic PD-1 expression suppresses the protective humoral immune response to the capsule of S. pneumoniae. The selective suppression of TI-2 (T-cell-independent type 2) Ab responses by PD-1 interactions with B7-H1 and B7-DC points to a novel role for PD-1 in regulating Ag-specific B-cell responses to carbohydrate Ags | 25624454 |
| CTLA-4 | Faecalibacterium genus and other Firmicutes in melanoma patients | Peripheral blood Tregs | Longer progression-free survival and overall survival and more frequent occurrence of ipilimumab-induced colitis | The Inducible T-cell COStimulator (ICOS) molecule is significantly upregulated on CD4+ T cells after ipilimumab (an immune checkpoint inhibitor (ICI) targeting CTLA-4) treatment in patients who belong to Faecalibacterium- driven cluster A | 28368458 |
| CTLA-4 | Oral administration of Bacteroides fragilis, Bacteroides thetaiotaomicron, Burkholderia cepacia, or a combination of B. fragilis and Burkholderia cepacia | B. fragilis-specific T cells | Eliciting the antitumor immune response | The geodistribution of Bf (B. fragilis) in the mucosal layer of the intestine and its association with Burkholderiales recognized through the pyrin/caspase-1 inflammasome, synergizing with TLR2/TLR4 signaling pathways, may account for the immunomodulatory effects of anti-CTLA-4 Ab | 26541610 |
| CTLA-4 | Staphylococcus aureus infection in mice | Low level of IL-6 production; high level of monocyte chemoattractant protein-1 | It attenuates disease severity but may prolong the healing time required for S. aureus skin infections, having no impact on bacterial clearance in skin tissues | The pathogenic role of T-cell activation in certain S. aureus infections and the potential use of CTLA4 Ig to diminish tissue damage in those conditions | 28264025 |
| CTLA-4 | Mycobacterium tuberculosis BCG infection | Enhances mycobacterial-infection-induced lymphocyte expansion and effector cell cytokine production in the draining lymph node but does not alter the number or function of lymphocytes at the primary site of infection | Enhancing immune response in the mediastinal lymph node with no improvement in clearance of mycobacteria in the lungs, liver, or spleen | CTLA- 4 blockade increased the antigen-specific expansion and differentiation of lymphocytes in the draining lymph node that is typically induced in response to a BCG lung infection | 10417139 |
| CTLA-4 | Helicobacter pylori infection in mice | Regulation of balance between Th1 and Th2 response | Inhibition of the development of gastric inflammation, accompanied by an increasing ratio of H. pylori-specific IgG1/IgG2a in serum | The predominance of Th2 response by CTLA-4 blockade leads to an inhibition of the development of gastric inflammation. CTLA-4 signaling could contribute to the regulation of Th subsets and the development of gastric inflammation in H. pylori infection | 14678261 |
| CTLA-4 | Listeria monocytogenes infection | Listeria monocytogenes-specific CD4+ and CD8+ T cells | Increasing numbers of CD4+ and CD8+ T cells and conferring stronger and rapid bacterial clearance | Blockade of CTLA-4 results in increased numbers of L. monocytogenes-specific CD4+ and CD8+ T cells after primary infection with attenuated L. monocytogenes and confers more rapid bacterial clearance after secondary challenge with virulent L. monocytogenes | 19191906 |
| CTLA-4 | Mice infected with Nippostrongylus brasiliensis | T cells | Profound reduction in adult worm numbers and early termination of parasite egg production | The ability of CTLA-4 blockade to accelerate primary immune responses to a protective level during an acute infection indicates its potential as an immunotherapeutic tool for dealing with infectious agents | 9221747 |
| PD-1 and CTLA-4 | Fungal sepsis in mice | Reverse sepsis-induced suppression of IFN-gamma and increased expression of MHC-II on APCs | Improving survival in bacterial sepsis | Blockade of cytotoxic T-lymphocyte antigen-4 (CTLA-4), a second negative costimulatory molecule that is upregulated in sepsis and acts like PD-1 to suppress T-cell function, also improved survival in fungal sepsis | 23663657 |
| LAG-3 | Mycobacterium tuberculosis | CD4+ T cells and NK cells | Enhancing high bacterial burdens | Our data show that LAG-3 expressed primarily on CD4þ T cells, presumably by regulatory T cells but also by natural killer cells. The expression of LAG-3 coincides with high bacterial burdens and changes in the host type 1 helper T-cell response. LAG-3 marks a subpopulation of Tregs that are highly active and produce high levels of the cytokine IL-10, which are recruited to the lungs of primates with uncontrolled Mtb replication | 25549835 |
| LAG-3 | Mycobacterium tuberculosis | CD4+ T cells | Modulating adaptive immunity | LAG-3 may modulate adaptive immunity to Mtb infection by interfering with the mitochondrial apoptosis pathway | 28880895 |
| LAG-3 | Staphylococcus aureus and Streptococcus pyogenes | MAIT | Main coinhibitory molecule expressed by SEB-exposed MAIT cells | SEB-induced upregulation of LAG-3 on MAIT cells appears to rely on IL-12 and IL-18. SEB-induced MAIT cell anergy can be reversed by blocking LAG-3 | 28632753 |
| LAG-3 | Plasmodium parasites | T cell | A novel therapeutic strategy for this devastating infectious disease | Expression of the inhibitory receptors PD-1 and LAG-3 on CD4þ T cells and their reduced IL-2 production are common characteristic features of Plasmodium infection | 26696540 |
| LAG-3 | Sepsis | CD4+ T cells; CD8+ T cells | Improving the survival and bacterial clearance in septic mice | LAG-3 was upregulated on CD4+ and CD8+ T cells, CD19+ B cells, natural killer cells, CD4+CD25+ regulatory T cells, and dendritic cells. Both LAG-3 knockout and anti-LAG-3 antibody had a positive effect on survival and on blood or peritoneal bacterial clearance in mice undergoing CLP. Cytokine levels and T-cell apoptosis decreased in anti-LAG-3 antibody-treated mice. Induced T-cell apoptosis decreased, whereas interferon γ secretion and proliferation were improved by anti-LAG-3 antibody in vitro. Interleukin 2 receptor was upregulated on T cells in both wild-type and LAG-3 knockout mice undergoing CLP | 32347939 |
| IDO1 | Mycobacterium tuberculosis | Macrophage, CD141+ tolerogenic DCs, and myeloid-lineage cells | M. tuberculosis bacterial burden promotes dysregulated homing of CD4+ T cells in the T-cell zone of iBALT and poor restoration of CD4+ T cells in the lung interstitium | The macaque model of M. tuberculosis infection showed IDO-expressing cells in the macrophage-rich layer of granulomas, which likely serves to prevent optimal interactions between CD4+ T cells and M. tuberculosis-infected antigen-presenting cells (APCs). Moreover, increased expression of IDO1 correlated with M. tuberculosis bacterial burden, and IDO1 expression was also associated with poorly formed iBALT | 32544085 |
| IDO1 | Autoimmune epididymitis | Plasmacytoid dendritic cells and regulatory T cells | Ido1 responds differently to autoimmune-mediated inflammation in the testis compared with the epididymis | IDO1 is known for its tolerogenic and immunosuppressive properties, exerted by modulating plasmacytoid dendritic cells and regulatory T cells. Ido1 responds differently to autoimmune-mediated inflammation in the testis compared with the epididymis | 32383098 |
| IDO1 | Coxiella burnetii | Host cells | IDO1 production as a key cell-autonomous defense mechanism that limits infection by C. burnetii | IDO1 contributes to IFN-γ-mediated restriction of C. burnetii. IDO1 is an enzyme that catabolizes cellular tryptophan to kynurenine metabolites, thereby reducing tryptophan availability in cells. Cells deficient in IDO1 function were more permissive for C. burnetii replication when treated with IFN-γ, and supplementing IFN-γ-treated cells with tryptophan enhanced intracellular replication. Additionally, ectopic expression of IDO1 in host cells was sufficient to restrict replication of C. burnetii in the absence of IFN-γ signaling. Using differentiated THP1 macrophage-like cells, it was determined that IFN-γ activation resulted in IDO1 production and that supplementation of IFN-γ-activated THP1 cells with tryptophan enhanced C. burnetii replication | 31461509 |
| IDO1 | Mycobacterium tuberculosis | Macrophages | IDO is associated with Mtb immune escape | Rv1737c is predominantly expressed by the Mtb in latent infection. In this study, we have characterized the Rv1737c functions in the recruitment and activation of macrophages, which play a cardinal role in innate and adaptive immunity. Rv1737c induced the tolerogenic phenotype of macrophages by upregulating the expression of indoleamine-2,3-dioxygenase 1 (IDO1) | 31326120 |
| IDO1 | Staphylococcus aureus and Toxoplasma gondii | Human retinal pigment epithelial (hRPE) cells | Inhibiting the growth of T. gondii and S. aureus | We found that an IFN-γ stimulation of hPRE cells induced the expression of IDO1, which inhibited the growth of T. gondii and S. aureus. Costimulation with IFN-γ, interleukin-1 beta, and tumor necrosis factor alpha induced a strong expression of iNOS. The iNOS-derived nitric oxide production was dependent on cell-culture conditions; however, it could not cause antimicrobial effects. iNOS did not act synergistically with IDO1. Instead, iNOS activity inhibited IDO1-mediated tryptophan degradation and bacteriostasis | 31267172 |
| IDO1 | Chlamydia trachomatis | Human endometrial carcinoma cell line; peripheral blood mononuclear cells | IDO1 catalyzes the degradation of tryptophan, which can eliminate C. trachomatis infection in vitro | In PBMCs infected with C. trachomatis there was a significant upregulation in IDO1 levels, which was independent of IFN-γ. In fact, C. trachomatis infection in PBMCs failed to induce IFN-γ levels in comparison to the uninfected culture | 30832593 |
| IDO1 | Uropathogenic Escherichia coli | epithelial cell | IDO1 activity regulates PMN chemotaxis in response to epithelial bacterial infection | The idea of an expanded role for IDO in innate cellular responses through the AHR-mediated effects of kynurenine metabolites on neutrophil function, in addition to the previously identified roles in adaptive immune regulation | 26857571 |
| IDO1 | Candida albicans | Treg | Contributing to establish immune tolerance and allow fungi colonization | Subsequent inflammatory Th1-type immunity was modulated by induced Treg cells, which required the TRIF pathway as well, and acted through activation of IDO in dendritic cells and Th17 cell antagonism (17947673) | 17947673; 15728500 |
| IDO1 | Aspergillus species | Treg | Controlling fungal burdens of Aspergillus species by activating distinct populations of Treg cells | S. cerevisiae has only one IDO gene (BNA2) and, to date, it has only been associated with one function, NAD + synthesis | 21170645 |
| IDO1 | A primary fungal in pulmonary paracoccidioidomycosis | T cell | Controlling fungal loads and immunity, the impairment of IDO1 activity could play a major role in the pathogenesis of severe forms of human pulmonary | IDO inhibition was shown to induce increased fungal loads in resistant and susceptible mice concomitantly with increased induction of NO synthesis | 25411790 |
| VISTA | Experimental autoimmune encephalomyelitis | CD4+ effector T cells; CD8+ effector T cells | Treatment with VISTA-blocking mAb led to more severe disease in the EAE mode | VISTA overexpression on tumor cells interferes with protective antitumor immunity in vivo in mice. These findings show that VISTA, a novel immunoregulatory molecule, has functional activities that are nonredundant with other Ig superfamily members and may play a role in the development of autoimmunity and immune surveillance in cancer | 21383057 |
| TIM-3 | Mycobacterium tuberculosis | Macrophage | TIM-3-immunoglobulin fusion protein reduced the M. tuberculosis burden in M. tuberculosis-infected mice. TIM-3/Gal-9 pathway in triggering antibacterial activity in M. tuberculosis-infected human macrophages | The TH1 cell surface molecule TIM-3 has evolved to inhibit the growth of intracellular pathogens via its ligand Gal9, which in turn inhibits expansion of effector TH1 cells to prevent further tissue inflammation | 20937702; 23180810 |
Clinical studies have reported that bacterial species can be differentially abundant in responders versus nonresponders (Katayama et al., 2019). Through feeding with B. fragilis, immunization with B. fragilis polysaccharides, or adoptive B. fragilis-specific T cells transfer, mice that failed in CTLA-4 blockade could regain their immunity. Transplantation of microbiota from melanoma patients to mice proved that B. fragilis favored the CTLA-4 blockade (Vetizou et al., 2015). In metastatic melanoma, Chaput et al. reported that patients with enriched Faecalibacterium and other Firmicutes as baseline microbiota presented a better prognosis than those with Bacteroides. However, the Bacteroidetes bring little colitis than Faecalibacterium (Chaput et al., 2017). In linkage with this, Gopalakrishnan et al. discovered that Faecalibacterium was enriched in responders, while Bacteroides thetaiotaomicron was enriched in nonresponders in melanoma patients (Gopalakrishnan et al., 2018). Using 16S ribosomal RNA gene sequencing, Matson et al. found out that Bifidobacterium longum, Collinsella aerofaciens, and Enterococcus faecium were more abundant in anti-PD-1 responders with metastatic melanoma (Matson et al., 2018).
The potential mechanisms through which the immune response is regulated by the microbiome may be as follows (Mazmanian et al., 2005; Helmink et al., 2019; Hayase and Jenq, 2021): 1) Through linkage between PAMPs and pattern recognized receptors (PRRs, such as Toll-like receptors), the adaptive immune response can be activated by APCs. 2) Cancer cells can bear cross-reactive neoantigens with microbiota, thus inducing an immune response. 3) Cytokines secreted by APCs or lymphocytes can be altered with specific metabolites or bacterial byproducts. 4) Metabolites entering the bloodstream could elicit a systemic response.
It also has been reported that irAEs induced by CTLA-4 occur most commonly and frequently at sites of the GI tract rich in bacteria. Disrupting the gut microbiota via antibiotics could potentially impair antitumor immune responses as well as response to immune checkpoint blockade (Helmink et al., 2019). Reconstruction of GI microbiome using FMT from healthy or responding donors shows a promising therapeutic effect with ICI-associated colitis relief and proportion of Tregs increase (Wang et al., 2018).
Still, the limitations of FMT should be taken into consideration. The connection between favorable microbiota and certain immune checkpoint blockade needs to be cleared. There could be adverse events induced by FMT, as we talked about above in IrAEs, either.
Immune Checkpoint Molecules in Virus-Infected Diseases
In chronic viral infection and cancer, due to long-term and low magnitude exposure to antigen, that T cell progressively loses its effector function with elevated coinhibitory receptor constitutive expression in order to diminish tissue damage is called “T-cell exhaustion.” Many pathogens and cancers promote inhibitory interactions to escape immune surveillance (Table 3). Thus, reversing the T-cell state is regarded as an effective solution in infectious diseases.
TABLE 3.
The role of immune checkpoints in virus infection diseases.
| Associated immune checkpoints | Associated virus | Associated diseases | Related immune cells or other cells | Influence on efficacy | Mechanism | PMID |
|---|---|---|---|---|---|---|
| PD-1 | HBV | Acute or chronic HBV infection disease | HBsAg-specific B cell unable to mature into Ab-secreting cells and displayed increased expression of CD21lo and PD-1 | Anti-PD-1 antibodies could partially restore HBsAg-specific B-cell maturation | HBV infection has a marked impact on global and HBV-specific humoral immunity, yet HBsAg-specific B cells are amenable to a partial rescue by B-cell maturing cytokines and PD-1 blockade | 30084841 |
| PD-1 | HCV | Chronic HCV-infected chimpanzees | Restoring intrahepatic CD4+ and CD8+ T-cell immunity | Significant reduction in HCV viremia in responder animal | Successful PD-1 blockade likely requires a critical threshold of preexisting virus-specific T cells in liver and warrants consideration of therapeutic vaccination strategies in combination with PD-1 blockade to broaden narrow responses | 23980172 |
| PD-1 | HIV | HIV-infected patients | Increasing CD8+ T cells in patients with chronic HIV infection | Cof blocking CD39/adenosine and PD-1 signaling showed a synergic effect in restoring CD8+ T-cell function (secrete functional cytokines and kill autologous reservoir cells) in vitro | Combined blockade of CD39/adenosine and PD-1 signaling in vitro may exert a synergistic effect in restoring CD8+ T-cell function in HIV-1-infected patients | 34177939 |
| PD-1 | CMV | Chronic CMV infection after renal transplantation | Higher positive rate of PD-1 in CMV-specific CD4+ T cell from viremic transplant recipients, loss of IL-2 production | Blockade of PD-1/PD-L1 could reverse functional anergy of CMV-specific CD4+ T cell and increase 10-fold proliferation in CMV-specific CD4+ T cell | Expression of PD-1 defines a reversible defect of CMV-specific CD4 T cells that are associated with viremia, and blocking PD-1 signaling may provide a potential target for enhancing the function of exhausted T cells in chronic CMV infection | 18510628 |
| PD-1 | HPV | HPV associated squamous cell carcinoma of the head and neck | Cancer cells | Pembrolizumab was tolerated with 17% grade 3-4 irAEs; the overall response was 25% in HPV-positive patients | Greater antitumor activity was recorded in patients with squamous cell carcinoma tumors of the head and neck that expressed higher levels of PD-L1 and interferon-γ-related genes. Thus, pembrolizumab might represent a new treatment approach for patients with squamous cell carcinoma of the head and neck | 27247226 |
| CTLA-4 | LCMV | Mice chronically infected with LCMV | Virus-specific CD8+ T cell | Blockade of the CTLA-4 had no effect on either T-cell function or viral control | Inhibition mediated by PD-1 requires close proximity of PD-1 to the site of TCR engagement and does not signal in the absence of a TCR signal. Following crosslinking by PD-1 ligand, the immunoreceptor tyrosine-based switch motif (ITSM) in the cytoplasmic domain of PD-1 is phosphorylated and recruits the phosphatases SHP-1 and SHP-2. These phosphatases act on proximal signaling kinases of the TCR pathway, reducing the TCR signal and leading to diminished T-cell activation and cytokine production. Therefore, under conditions of persistent antigen, T cells may modulate their responsiveness by upregulating inhibitory receptors such as PD-1 that attenuate TCR signaling | 16382236 |
| CTLA-4 | HBV | Chronic HBV infection | CTLA-4 is upregulated on HBV-specific CD8+ T cells with the highest level of Bim protein | Blocking CTLA-4 can increase the expansion of IFN-gamma producing HBV-specific CD8+ T cells | CTLA-4 is expressed by HBV-specific CD8+ T cells with high levels of Bim and helps to drive this proapoptotic phenotype | 21360567 |
| CTLA-4 | HCV | Patients with hepatocellular carcinoma and chronic HCV infection | Cancer cells | Anti-CTLA-4 showed a good safety profile; no patients needed steroids due to severe irAEs; disease control rate was 76.4% | HCV-specific CD8+ T cells that are exhausted express various inhibitory receptors, including CTLA-4 that acts synergistically with the programmed cell death-1 receptor (PD-1) to enforce their exhaustion state. Moreover, CTLA-4 is preferentially upregulated in PD-1+ T cells from the liver of chronically HCV-infected patients. It seems possible that the revival of antiviral T-cell immunity in patients with long-lasting chronic HCV infection following tremelimumab therapy may result from increased CD4+ T cell help and recovery of CD8+ T-cell exhaustion | 23466307 |
| CTLA-4 | HIV | HIV-infected patients | No pattern was noted regarding the change from baseline in CD4 or CD8 T cells | No serious adverse events or dose-limiting toxicities and ipilimumab were associated with variations in HIV RNA | Ipilimumab treatment of an HIV-infected patient on antiretroviral therapy increased CD4+ T cells, predominantly total memory and effector-memory cells, postinfusion along with transient increases in CD8+ T cells without change in cell activation. Furthermore, ipilimumab increased cell-associated unspliced HIV RNA and a subsequent decline in plasma HIV RNA | 29879143 |
| CTLA-4 | HIV | HIV-infected patients | CTLA-4 was upregulated in HIV-specific CD4+ T cells but not CD8+ T cells | CTLA-4 expression correlated positively with disease progression and negatively with the capacity of CD4+ T cells to produce interleukin 2 in response to viral antigen. In vitro blockade of CTLA-4 augmented HIV-specific CD4+ T-cell function | CTLA-4 ligation can suppress effector T-cell functions both directly through CTLA-4 expressed on effector cells and indirectly through CTLA-4 expressed on CD4+CD25+ Treg cells. A CTLA-4-mediated effect of Treg cells can probably occur in vivo both by direct T-cell-T-cell contact and indirectly by induction of indoleamine-2,3-dioxygenase in dendritic cells | 17906628 |
| PD-1/CTLA-4 | HAV | HAV-associated hepatitis | Isolated PBMC, PD-1, and CTLA-4 on T cells were measured by flow cytometry | Significantly higher expression of PD-1 and CTLA-4 on T cells consistent with a viral-protective effect of PD-1 and CTLA-4, thereby preventing the destruction of virus-infected hepatocytes in AHA | The changing expression of PD-1 and CTLA-4 during the symptomatic and recovery phases of AHA points to the protective effects of these inhibitory molecules, perhaps by suppressing the activity of cytotoxic T cells, thereby preventing the induced fulminant destruction of HAV-infected hepatocytes | 26347518 |
| PD-1/CTLA-4 | EBV | Intraperitoneally inject EBV-infected human cord blood into NSG mice | Increasing EBV-specific T-cell response and enhancing tumor infiltration by CD4+ and CD8+ T cells | Combination of PD-1/CTLA-4 blockade reduced the size of lymphoma, decreased the number of both latently and lytically EBV-infected B cells | PD-1/CTLA-4 blockade markedly increases EBV-specific T-cell responses and is associated with enhanced tumor infiltration by CD4+ and CD8+ T cells | 27186886 |
| PD-1/CTLA-4 | SIV | SIV-infected long-term antiretroviral therapy-treated rhesus macaques | Decreasing total and intact SIV-DNA in CD4+ T cells and B-cell follicles | Inducing robust latency reversal and reducing total levels of integrated virus. No enhanced SIV-specific CD8+ T-cell responses or viral control | Dual CTLA-4/PD-1 blockade produced a significant reduction in cell-associated SIV-DNA within LN CD4+ TEM, the CD4+ T-cell subpopulation most activated from combined treatment. Importantly, in situ hybridization assays demonstrated a significant reduction in the number of vRNA+ and vDNA + cells following dual CTLA-4/PD-1 blockade in the LN, including in the BCF | 32284611 |
| LAG-3 | HIV | AIDS | T cell | High viral load, faster disease progression, and rapid return of viremia following treatment interruption | Although mechanisms and functions of LAG-3 remain controversial, LAG-3 clearly inhibits immune responses. If LAG-3 blockade improves immune function during HIV infection, it could help deplete the HIV reservoir by reversing latency and restoring immunity of exhausted cells | 30653605 |
| LAG-3 | HBV | Hepatocellular carcinoma | CD8 (+) T cells | Acting as a suppressor of HBV-specific | Since LAG-3 is an inhibitory molecule that plays a downregulatory role on T-cell responses, we found the correlation between LAG-3 expression and HBV-specific CD8+ T cells dysfunction | 23261718 |
| LAG-3 | HPV | OPSCC | CD8 (+) T cells | HPV-related OPSCC might be more susceptible to single or combined anti-LAG-3 antibody therapy than HPV-negative OPSCC patients | Possible reasons for this may be the interrelationship of multiple components in the tumor immune microenvironment, as it has been reported that the coexpression of LAG-3 with other inhibitory molecules such as TIM-3 or PD-1 induces the exhaustion of immune cells, resulting in downregulated cytokine expression | 33396515 |
| LAG-3 | HCV | Follicular lymphoma | CD8 T cells | Inhibiting cell proliferation, cytotoxicity function, and cytokine production | LAG-3 expression could be substantially upregulated on CD4+ or CD8+ T cells by IL-12, a cytokine that has been shown to induce T-cell exhaustion and be increased in the serum of lymphoma patients. Furthermore, we found that blockade of both PD-1 and LAG-3 signaling enhanced the function of intratumoral CD8+ T cells resulting in increased IFN-γ and IL-2 production | 28977875 |
| LAG-3 | LCMV | Chronic viral infections | CD8 T cells | LAG-3 is continuously upregulated on LCMV-specific exhausted CD8 T cells; it alone does not significantly contribute to T-cell exhaustion | LAG-3 is upregulated on LCMV-specific exhausted CD8 T cells; it does not significantly contribute to T-cell exhaustion alone. To effectively interfere with T-cell exhaustion, it is very likely that several inhibitory receptors will have to be targeted simultaneously | 19880580 |
| IDO1 | HIV | HIV-1 infection | CD4+ T cells | IDO may represent a critical initiating event that results in inversion of the T(H)17/T (reg) balance and in the consequent maintenance of a chronic inflammatory state in progressive HIV disease | IDO1-dependent tryptophan catabolism may be an important link between immune activation and the gradual decline of immune function seen in progressive HIV infection | 20484731 |
| IDO1 | HPV16 | Head and neck squamous cell carcinomas | HPV16-specific CD8+ T cells | The HPV16 CTL epitopes identified in this study, in combination with blockade of HPV + HNSCC-specific PD-1/IDO-1 checkpoints, may be useful for targeted immunotherapy | Our findings implicate mechanisms of T-cell escape in HPV + HNSCC, wherein high tumoral HPV-antigen load results in high expression of immune dysfunction genes on tumor cells (e.g., IDO-1) and dysfunction of HPV-specific CTLs (e.g., E7; E2-CTLs). HPV + HNSCCs expressing IDO-1 might similarly be driven by HPV-specific-CTL infiltration in response to high tumoral HPV-antigen load | 30154146 |
| IDO1 | HPV | Chronic infection | Invariant natural killer T; T cell | Induction of IDO1 in HPV-infected skin contributes to evasion of host immunity | Inhibiting IDO activity using 1-methyl-DL-tryptophan (1-D/L-MT) promotes K14E7 skin graft rejection. Increased IDO1 expression and activity in K14E7 skin require IFN-g and invariant natural killer T (iNKT) cells, both of which have been shown to negatively regulate T-cell effector function and suppress K14E7 graft rejection. Furthermore, DCs from K14E7 skin express higher levels of IFN-g receptor (IFN-gR) than DCs from control skin | 23652797 |
| VISTA | HIV | AIDS | CD4+ and CD8+ T cells | Gal-9 and VISTA expression was associated with impaired T-cell effector functions | A dramatic reduction in the production of cytokines by T cells expressing PD-1, CD160, CD39, TIM-3, and VISTA. In contrast to other coinhibitory molecules, the pattern of cytokine production was not different between 2B4+ and 2B42 CD4+ T cells, and interestingly 2B4+ CD8+ T cells exhibited higher cytokine production capabilities compared with 2B42 CD8+ T cells | 32205423 |
| TIM-3 | HIV | AIDS | T cells | Blocking the TIM-3 signaling pathway restored proliferation and enhanced cytokine production in HIV-1-specific T cells | In progressive HIV-1 infection, TIM-3 expression was upregulated on HIV-1-specific CD8 + T cells. TIM-3-expressing T cells failed to produce cytokine or proliferate in response to antigen and exhibited impaired Stat5, Erk1/2, and p38 signaling. Blocking the TIM-3 signaling pathway restored proliferation and enhanced cytokine production in HIV-1-specific T cells | 19001139 |
| TIM-3 | HCV | HCV infection | HCV-specific CTLs | Blockade of either PD-1 or TIM-3 enhanced in vitro proliferation of HCV-specific CTLs to a similar extent, whereas cytotoxicity against a hepatocyte cell line that expressed cognate HCV epitopes was increased exclusively by TIM-3 blockade | Early accumulation of PD-1+TIM-3+ T cells is associated with functional impairment and consequently with the development of persistent HCV. The present study provides a basis for improving current therapies by simultaneous blockade of multiple inhibitory pathways that could result in additive efficacy without excessive toxicity | 21084749 |
| TIM-3 | LCMV | Chronic LCMV infection | CD8 T cell | Targeting both PD-1 and TIM-3 is an effective immune strategy for treating chronic viral infections | Whereas TIM-3 was only transiently expressed by CD8 T cells after acute infection, virus-specific CD8 T cells retained high TIM-3 expression throughout chronic infection. The majority (approximately 65–80%) of lymphocytic choriomeningitis virus-specific CD8 T cells in lymphoid and nonlymphoid organs coexpressed TIM-3 and PD-1. This coexpression of TIM-3 and PD-1 was associated with more severe CD8 T-cell exhaustion in terms of proliferation and secretion of effector cytokines such as IFN-γ, TNF-α, and IL-2. Interestingly, CD8 T cells expressing both inhibitory receptors also produced the suppressive cytokine IL-10. Most importantly, combined blockade of TIM-3 and PD-1 pathways in vivo synergistically improved CD8 T-cell responses and viral control in chronically infected mice | 20679213 |
| TIM-3 | Friend virus | Acute Friend virus-induced disease | CD8 T cell | Combined blockade of PD-1 and TIM-3 during the priming/differentiation phase rescued FV-specific CD8 (+) T cells from becoming terminally exhausted, resulting in improved CD8 (+) T-cell functionality and virus control | TIM-3 and CTLA-4 were recently found to be overexpressed on HIV- and hepatitis C virus-specific CD4+ and CD8+ T cells and to act to suppress effector functions of activated T cells. Upregulation of LAG-3 was also shown to correlate with the impaired effector functions and exhaustion of CD8+ T cells | 20351188 |
| TIM-3 | HBV | Chronic HBV infection | CD4+ and CD8+ T cells | Overexpression of TIM-3 is involved in disease progression of CHB and that TIM-3 may participate in skewing of Th1/Tc1 response, which contributes to the persistency of HBV infection | The expression of TIM-3 is upregulated on circulating CD4+ and CD8+ T cells in CHB patients. TIM-3 was highly expressed on T cells from AHB patients as well; however, its expression decreased dynamically in the convalescence phase. TIM-3 expression positively correlated with disease severity and negatively correlated with Th1/Tc1 response in CHB patients | 21392402 |
In mice with chronic LCMV infection, blockade of PD-1 restored CD8+ T cell function, suggesting that T-cell exhaustion is reversible. In patients with chronic hepatitis B, CTLA-4 blockade can reinvigorate hepatitis B virus- (HBV-) specific CD8+ T cells in both intrahepatic and peripheral compartments (Cao et al., 2018). With the coinhibition of PD-1 and CTLA-4, the effector function of HCV-specific CD8+ T cells can be restored in chronic hepatitis C patients (Cho et al., 2017). Meanwhile, inhibition of PD-1 can induce the production of cytokines (e.g., IFN-γ) in HIV/HBV-specific CD8+ T cells to enhance immune response (Jubel et al., 2020). Coexpressing with PD-1, LAG-3, TIM-3, and TIGIT blockade can also reverse dysfunctional T-cell responses and reduce cytokines production. It is widely known that TIM-3 is highly upregulated on virus and tumor Ag-specific CD8+ T cells, and antagonizing TIM-3 helps restore the function of CD8+ T cells (Clayton et al., 2014). Expression of LAG-3 has been reported to be associated with a reduction in invariant NKTs IFN-γ production during chronic HIV infection (Juno et al., 2015).
Discussion and Future Perspectives
Immune checkpoints are some vital regulators of the immune system. Now in most referred contexts, immune checkpoints are equivalent to inhibitor regulators of the immune system. Despite the immune checkpoint molecules that we have discussed above, there are still other immune checkpoint molecules, such as BTLA, KIR, A2AR, B7-H4, NOX2, HO-1, and SIGLEC7. Besides, the stimulatory immune checkpoints are also promising targets for immune therapy, such as CD40, CD122, CD137, OX40, and GITR. Relying on neoantigen expressed on tumor cells, T cells can target and exclude potential threats. So as to escape from host immunity, tumor cells requisition inhibitory molecules to bind and silence immune cells. The availability of immune checkpoint blockade as one of the effective supplemental methods for tumor treatment has been verified. However, some tumors show low immunogenicity and cannot respond effectively to immune checkpoint blockade. For initially responding tumors, selection of low immunogenic clones and inducement of tolerance due to tumor heterogeneity will develop frequent relapses and even hyperprogression in nonresponders, of which the range was between 4 and 29% (Denis et al., 2020). Such phenomenon is known as resistance (Sharma et al., 2017). The mechanisms of resistance can be divided into intrinsic and extrinsic (Figure 3). The intrinsic mechanisms are composed of lack of tumor antigen presentation, alteration of several inhibitory signaling pathways, and upregulation of other immune checkpoints. The extrinsic mechanisms are predominantly referred to as various elements in the TME (Baxter et al., 2021). To reverse the resistance and ameliorate patients’ symptoms, researchers came up with the idea to turn the “cold” immune response to “hot.” The strategies applied under such fundamental idea consist of turning down the volume of inhibitory immune signals, triggering T-cell priming, increasing the costimulatory signals, and modulation of the TME (Attili et al., 2021; Weiss and Sznol, 2021).
FIGURE 3.
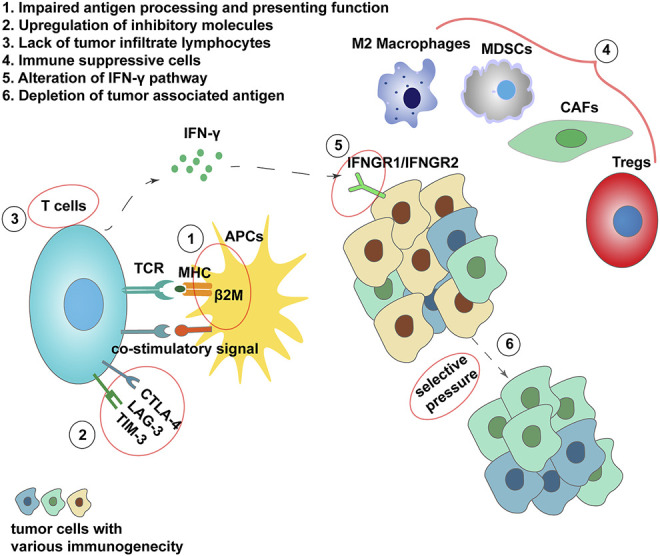
Mechanisms of resistance from ICI treatment. 1) β2M mutations lead to loss of HLA and antigen-presenting function. 2) Additional inhibitory signals expression. 3) Little tumor-infiltrating lymphocytes present in the tumor microenvironment resulting in nonresponse. 4) Immune suppressive cells in TME. 5) Loss of IFN-γ sensitivity. 6) Formation of low immunogenicity clone under selective pressure.
Meanwhile, the sailing of drug development is never smooth. Hundreds of clinical trials to develop new agents targeted at immune checkpoints have been terminated due to low responsiveness and fatal irAEs. IrAEs induced by ICI are an impassable mountain lying in front of us, with death as the most severe consequence. The clinical trial testing sym022 (anti-LAG-3 mAb) in humans with metastatic cancer, solid tumors, or lymphoma exhibits an unwanted outcome with high progression and irAEs rate (NCT03489369). In addition, the mechanisms under ICI still need to be shed light on.
In conclusion, despite the shortcomings of immune checkpoint blockade in clinical application, it is a promising strategy for cancer therapy, with a considerable proportion of applicants achieving an objective response. Further studies are needed to be explored to elucidate precise mechanisms, achieve potential will, and ameliorate adverse events to benefit more patients with tumors and other diseases.
Author Contributions
Literature search: HZ and YY, tables and figures: JY and YZ, writing the original manuscript: XC and YZ; editing manuscript: JY; review and editing manuscript: JL, MZ, and YZ.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
References
- Ahmadzadeh M., Johnson L. A., Heemskerk B., Wunderlich J. R., Dudley M. E., White D. E., et al. (2009). Tumor Antigen-specific CD8 T Cells Infiltrating the Tumor Express High Levels of PD-1 and Are Functionally Impaired. Blood 114 (8), 1537–1544. 10.1182/blood-2008-12-195792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almutairi A. R., McBride A., Slack M., Erstad B. L., Abraham I. (2020). Potential Immune-Related Adverse Events Associated with Monotherapy and Combination Therapy of Ipilimumab, Nivolumab, and Pembrolizumab for Advanced Melanoma: A Systematic Review and Meta-Analysis. Front. Oncol. 10, 91. 10.3389/fonc.2020.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews M. C., Duong C. P. M., Gopalakrishnan V., Iebba V., Chen W.-S., Derosa L., et al. (2021). Gut Microbiota Signatures Are Associated with Toxicity to Combined CTLA-4 and PD-1 Blockade. Nat. Med. 27 (8), 1432–1441. 10.1038/s41591-021-01406-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell S. M., Lesokhin A. M., Borrello I., Halwani A., Scott E. C., Gutierrez M., et al. (2015). PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin's Lymphoma. N. Engl. J. Med. 372 (4), 311–319. 10.1056/NEJMoa1411087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascierto P. A., Del Vecchio M., Robert C., Mackiewicz A., Chiarion-Sileni V., Arance A., et al. (2017). Ipilimumab 10 Mg/kg versus Ipilimumab 3 Mg/kg in Patients with Unresectable or Metastatic Melanoma: a Randomised, Double-Blind, Multicentre, Phase 3 Trial. Lancet Oncol. 18 (5), 611–622. 10.1016/S1470-2045(17)30231-0 [DOI] [PubMed] [Google Scholar]
- Attili I., Tarantino P., Passaro A., Stati V., Curigliano G., de Marinis F. (2021). Strategies to Overcome Resistance to Immune Checkpoint Blockade in Lung Cancer. Lung Cancer 154, 151–160. 10.1016/j.lungcan.2021.02.035 [DOI] [PubMed] [Google Scholar]
- Avery L., Filderman J., Szymczak-Workman A. L., Kane L. P. (2018). Tim-3 Co-stimulation Promotes Short-Lived Effector T Cells, Restricts Memory Precursors, and Is Dispensable for T Cell Exhaustion. Proc. Natl. Acad. Sci. USA 115 (10), 2455–2460. 10.1073/pnas.1712107115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P., Scherpereel A., Nowak A. K., Fujimoto N., Peters S., Tsao A. S., et al. (2021). First-line Nivolumab Plus Ipilimumab in Unresectable Malignant Pleural Mesothelioma (CheckMate 743): a Multicentre, Randomised, Open-Label, Phase 3 Trial. The Lancet 397 (10272), 375–386. 10.1016/S0140-6736(20)32714-8 [DOI] [PubMed] [Google Scholar]
- Baxter M. A., Middleton F., Cagney H. P., Petty R. D. (2021). Resistance to Immune Checkpoint Inhibitors in Advanced Gastro-Oesophageal Cancers. Br. J. Cancer 125 (8), 1068–1079. 10.1038/s41416-021-01425-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckermann K. E., Johnson D. B., Sosman J. A. (2017). PD-1/PD-L1 Blockade in Renal Cell Cancer. Expert Rev. Clin. Immunol. 13 (1), 77–84. 10.1080/1744666X.2016.1214575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti A., Bortolotti R., Dipasquale M., Kinspergher S., Prokop L., Grandi G., et al. (2021). Meta-analysis of Immune-Related Adverse Events in Phase 3 Clinical Trials Assessing Immune Checkpoint Inhibitors for Lung Cancer. Crit. Rev. Oncology/Hematology 162, 103351. 10.1016/j.critrevonc.2021.103351 [DOI] [PubMed] [Google Scholar]
- Blackburn S. D., Shin H., Haining W. N., Zou T., Workman C. J., Polley A., et al. (2009). Coregulation of CD8+ T Cell Exhaustion by Multiple Inhibitory Receptors during Chronic Viral Infection. Nat. Immunol. 10 (1), 29–37. 10.1038/ni.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J. R., Abu-Sbeih H., Ascierto P. A., Brufsky J., Cappelli L. C., Cortazar F. B., et al. (2021). Society for Immunotherapy of Cancer (SITC) Clinical Practice Guideline on Immune Checkpoint Inhibitor-Related Adverse Events. J. Immunother. Cancer 9 (6), e002435. 10.1136/jitc-2021-002435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignone C., Escudier B., Grygar C., Marcu M., Triebel F. (2009). A Phase I Pharmacokinetic and Biological Correlative Study of IMP321, a Novel MHC Class II Agonist, in Patients with Advanced Renal Cell Carcinoma. Clin. Cancer Res. 15 (19), 6225–6231. 10.1158/1078-0432.CCR-09-0068 [DOI] [PubMed] [Google Scholar]
- Cancer Therapy Evaluation Program (2017). CTCAE. Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf .
- Cao H., Zhang R., Zhang W. (2018). CTLA-4 Interferes with the HBV-specific T cell Immune Response (Review). Int. J. Mol. Med. 42 (2), 703–712. 10.3892/ijmm.2018.3688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casak S. J., Marcus L., Fashoyin-Aje L., Mushti S. L., Cheng J., Shen Y.-L., et al. (2021). FDA Approval Summary: Pembrolizumab for the First-Line Treatment of Patients with MSI-H/dMMR Advanced Unresectable or Metastatic Colorectal Carcinoma. Clin. Cancer Res. 27 (17), 4680–4684. 10.1158/1078-0432.CCR-21-0557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae Y. K., Arya A., Iams W., Cruz M. R., Chandra S., Choi J., et al. (2018). Current Landscape and Future of Dual Anti-CTLA4 and PD-1/pd-L1 Blockade Immunotherapy in Cancer; Lessons Learned from Clinical Trials with Melanoma and Non-small Cell Lung Cancer (NSCLC). J. Immunotherapy Cancer 6 (1), 39. 10.1186/s40425-018-0349-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput N., Lepage P., Coutzac C., Soularue E., Le Roux K., Monot C., et al. (2017). Baseline Gut Microbiota Predicts Clinical Response and Colitis in Metastatic Melanoma Patients Treated with Ipilimumab. Ann. Oncol. 28 (6), 1368–1379. 10.1093/annonc/mdx108 [DOI] [PubMed] [Google Scholar]
- Chen D. S., Mellman I. (2017). Elements of Cancer Immunity and the Cancer-Immune Set point. Nature 541 (7637), 321–330. 10.1038/nature21349 [DOI] [PubMed] [Google Scholar]
- Chen Y., Zhou Y., Tang L., Peng X., Jiang H., Wang G., et al. (2019). Immune-Checkpoint Inhibitors as the First Line Treatment of Advanced Non-small Cell Lung Cancer: A Meta-Analysis of Randomized Controlled Trials. J. Cancer 10 (25), 6261–6268. 10.7150/jca.34677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Baghdadi M., Akiba H., Yoshiyama H., Kinoshita I., Dosaka-Akita H., et al. (2012). Tumor-infiltrating DCs Suppress Nucleic Acid-Mediated Innate Immune Responses through Interactions between the Receptor TIM-3 and the Alarmin HMGB1. Nat. Immunol. 13 (9), 832–842. 10.1038/ni.2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida K., Kawazoe A., Kawazu M., Suzuki T., Nakamura Y., Nakatsura T., et al. (2021). A Low Tumor Mutational Burden and PTEN Mutations Are Predictors of a Negative Response to PD-1 Blockade in MSI-H/dMMR Gastrointestinal Tumors. Clin. Cancer Res. 27 (13), 3714–3724. 10.1158/1078-0432.CCR-21-0401 [DOI] [PubMed] [Google Scholar]
- Chihara N., Madi A., Kondo T., Zhang H., Acharya N., Singer M., et al. (2018). Induction and Transcriptional Regulation of the Co-inhibitory Gene Module in T Cells. Nature 558 (7710), 454–459. 10.1038/s41586-018-0206-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H., Kang H., Lee H., Kim C. (2017). Programmed Cell Death 1 (PD-1) and Cytotoxic T Lymphocyte-Associated Antigen 4 (CTLA-4) in Viral Hepatitis. Ijms 18 (7), 1517. 10.3390/ijms18071517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Lee S. Y. (2020). Clinical Characteristics and Treatment of Immune-Related Adverse Events of Immune Checkpoint Inhibitors. Immune Netw. 20 (1), e9. 10.4110/in.2020.20.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton K. L., Haaland M. S., Douglas-Vail M. B., Mujib S., Chew G. M., Ndhlovu L. C., et al. (2014). T Cell Ig and Mucin Domain-Containing Protein 3 Is Recruited to the Immune Synapse, Disrupts Stable Synapse Formation, and Associates with Receptor Phosphatases. J.I. 192 (2), 782–791. 10.4049/jimmunol.1302663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly C., Bambhania K., Naidoo J. (2019). Immune-Related Adverse Events: A Case-Based Approach. Front. Oncol. 9, 530. 10.3389/fonc.2019.00530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran M. A., Montalvo W., Yagita H., Allison J. P. (2010). PD-1 and CTLA-4 Combination Blockade Expands Infiltrating T Cells and Reduces Regulatory T and Myeloid Cells within B16 Melanoma Tumors. Proc. Natl. Acad. Sci. 107 (9), 4275–4280. 10.1073/pnas.0915174107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvin P., Toor S. M., Sasidharan Nair V., Elkord E. (2018). Immune Checkpoint Inhibitors: Recent Progress and Potential Biomarkers. Exp. Mol. Med. 50 (12), 1–11. 10.1038/s12276-018-0191-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila E., Kennedy R., Celis E. (2003). Generation of Antitumor Immunity by Cytotoxic T Lymphocyte Epitope Peptide Vaccination, CpG-Oligodeoxynucleotide Adjuvant, and CTLA-4 Blockade. Cancer Res. 63 (12), 3281–3288. [PubMed] [Google Scholar]
- Denis M., Duruisseaux M., Brevet M., Dumontet C. (2020). How Can Immune Checkpoint Inhibitors Cause Hyperprogression in Solid Tumors. Front. Immunol. 11, 492. 10.3389/fimmu.2020.00492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermani F. K., Samadi P., Rahmani G., Kohlan A. K., Najafi R. (2019). PD‐1/PD‐L1 Immune Checkpoint: Potential Target for Cancer Therapy. J. Cel Physiol 234 (2), 1313–1325. 10.1002/jcp.27172 [DOI] [PubMed] [Google Scholar]
- Derré L., Rivals J.-P., Jandus C., Pastor S., Rimoldi D., Romero P., et al. (2010). BTLA Mediates Inhibition of Human Tumor-specific CD8+ T Cells that Can Be Partially Reversed by Vaccination. J. Clin. Invest. 120 (1), 157–167. 10.1172/JCI40070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon K. O., Das M., Kuchroo V. K. (2018). Human Disease Mutations Highlight the Inhibitory Function of TIM-3. Nat. Genet. 50 (12), 1640–1641. 10.1038/s41588-018-0289-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T., Ishikawa T., Okayama T., Oka K., Mizushima K., Yasuda T., et al. (2017). The JAK/STAT Pathway Is Involved in the Upregulation of PD-L1 Expression in Pancreatic Cancer Cell Lines. Oncol. Rep. 37 (3), 1545–1554. 10.3892/or.2017.5399 [DOI] [PubMed] [Google Scholar]
- Du W., Yang M., Turner A., Xu C., Ferris R., Huang J., et al. (2017). TIM-3 as a Target for Cancer Immunotherapy and Mechanisms of Action. Ijms 18 (3), 645. 10.3390/ijms18030645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck L., Mills K. H. G. (2017). Immune Checkpoints and Their Inhibition in Cancer and Infectious Diseases. Eur. J. Immunol. 47 (5), 765–779. 10.1002/eji.201646875 [DOI] [PubMed] [Google Scholar]
- Ebert P. J. R., Cheung J., Yang Y., McNamara E., Hong R., Moskalenko M., et al. (2016). MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity 44 (3), 609–621. 10.1016/j.immuni.2016.01.024 [DOI] [PubMed] [Google Scholar]
- Emens L. A., Adams S., Barrios C. H., Diéras V., Iwata H., Loi S., et al. (2021). First-line Atezolizumab Plus Nab-Paclitaxel for Unresectable, Locally Advanced, or Metastatic Triple-Negative Breast Cancer: IMpassion130 Final Overall Survival Analysis. Ann. Oncol. 32 (8), 983–993. 10.1016/j.annonc.2021.05.355 [DOI] [PubMed] [Google Scholar]
- Esfahani K., Elkrief A., Calabrese C., Lapointe R., Hudson M., Routy B., et al. (2020). Moving towards Personalized Treatments of Immune-Related Adverse Events. Nat. Rev. Clin. Oncol. 17 (8), 504–515. 10.1038/s41571-020-0352-8 [DOI] [PubMed] [Google Scholar]
- Finkelmeier F., Waidmann O., Trojan J. (2018). Nivolumab for the Treatment of Hepatocellular Carcinoma. Expert Rev. Anticancer Ther. 18 (12), 1169–1175. 10.1080/14737140.2018.1535315 [DOI] [PubMed] [Google Scholar]
- Flies D. B., Han X., Higuchi T., Zheng L., Sun J., Ye J. J., et al. (2014). Coinhibitory Receptor PD-1H Preferentially Suppresses CD4+ T Cell-Mediated Immunity. J. Clin. Invest. 124 (5), 1966–1975. 10.1172/JCI74589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G. J., Long A. J., Iwai Y., Bourque K., Chernova T., Nishimura H., et al. (2000). Engagement of the PD-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation. J. Exp. Med. 192 (7), 1027–1034. 10.1084/jem.192.7.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnaire A., Nadel B., Raoult D., Neefjes J., Gorvel J.-P. (2017). Collateral Damage: Insights into Bacterial Mechanisms that Predispose Host Cells to Cancer. Nat. Rev. Microbiol. 15 (2), 109–128. 10.1038/nrmicro.2016.171 [DOI] [PubMed] [Google Scholar]
- Gleason M. K., Lenvik T. R., McCullar V., Felices M., O'Brien M. S., Cooley S. A., et al. (2012). Tim-3 Is an Inducible Human Natural Killer Cell Receptor that Enhances Interferon Gamma Production in Response to Galectin-9. Blood 119 (13), 3064–3072. 10.1182/blood-2011-06-360321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. V., Drake C. G. (2010). LAG-3 in Cancer Immunotherapy. Curr. Top. Microbiol. Immunol. 344, 269–278. 10.1007/82_2010_114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan V., Spencer C. N., Nezi L., Reuben A., Andrews M. C., Karpinets T. V., et al. (2018). Gut Microbiome Modulates Response to Anti-PD-1 Immunotherapy in Melanoma Patients. Science 359 (6371), 97–103. 10.1126/science.aan4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Vesely M. D., Yang W., Sanmamed M. F., Badri T., Alawa J., et al. (2019). PD-1H (VISTA)-mediated Suppression of Autoimmunity in Systemic and Cutaneous Lupus Erythematosus. Sci. Transl. Med. 11 (522), eaax1159. 10.1126/scitranslmed.aax1159 [DOI] [PubMed] [Google Scholar]
- Han Y., Liu D., Li L. (2020). PD-1/PD-L1 Pathway: Current Researches in Cancer. Am. J. Cancer Res. 10 (3), 727–742. [PMC free article] [PubMed] [Google Scholar]
- Hayase E., Jenq R. R. (2021). Role of the Intestinal Microbiome and Microbial-Derived Metabolites in Immune Checkpoint Blockade Immunotherapy of Cancer. Genome Med. 13 (1), 107. 10.1186/s13073-021-00923-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmink B. A., Khan M. A. W., Hermann A., Gopalakrishnan V., Wargo J. A. (2019). The Microbiome, Cancer, and Cancer Therapy. Nat. Med. 25 (3), 377–388. 10.1038/s41591-019-0377-7 [DOI] [PubMed] [Google Scholar]
- Henry K. E., Mack K. N., Nagle V. L., Cornejo M., Michel A. O., Fox I. L., et al. (2021). ERK Inhibition Improves Anti-PD-L1 Immune Checkpoint Blockade in Preclinical Pancreatic Ductal Adenocarcinoma. Mol. Cancer Ther. 20, 2026–2034. 10.1158/1535-7163.MCT-20-1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A. K., Ho A. M.-H., Cooksley T., Nguyen G., Erb J., Mizubuti G. B. (2021). Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitor Therapy. Anesth. Analg 132 (2), 374–383. 10.1213/ANE.0000000000005029 [DOI] [PubMed] [Google Scholar]
- Hodi F. S., O'Day S. J., McDermott D. F., Weber R. W., Sosman J. A., Haanen J. B., et al. (2010). Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 363 (8), 711–723. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommes J. W., Verheijden R. J., Suijkerbuijk K. P. M., Hamann D. (2020). Biomarkers of Checkpoint Inhibitor Induced Immune-Related Adverse Events-A Comprehensive Review. Front. Oncol. 10, 585311. 10.3389/fonc.2020.585311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinkhani N., Derakhshani A., Shadbad M. A., Argentiero A., Racanelli V., Kazemi T., et al. (2021). The Role of V-Domain Ig Suppressor of T Cell Activation (VISTA) in Cancer Therapy: Lessons Learned and the Road Ahead. Front. Immunol. 12, 676181. 10.3389/fimmu.2021.676181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss R. S., Moldawer L. L. (2014). Parallels between Cancer and Infectious Disease. N. Engl. J. Med. 371 (4), 380–383. 10.1056/NEJMcibr1404664 [DOI] [PubMed] [Google Scholar]
- Hsu J., Hodgins J. J., Marathe M., Nicolai C. J., Bourgeois-Daigneault M.-C., Trevino T. N., et al. (2018). Contribution of NK Cells to Immunotherapy Mediated by PD-1/pd-L1 Blockade. J. Clin. Invest. 128 (10), 4654–4668. 10.1172/JCI99317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.-T., Workman C. J., Flies D., Pan X., Marson A. L., Zhou G., et al. (2004). Role of LAG-3 in Regulatory T Cells. Immunity 21 (4), 503–513. 10.1016/j.immuni.2004.08.010 [DOI] [PubMed] [Google Scholar]
- Huang X., Zhang X., Li E., Zhang G., Wang X., Tang T., et al. (2020). VISTA: an Immune Regulatory Protein Checking Tumor and Immune Cells in Cancer Immunotherapy. J. Hematol. Oncol. 13 (1), 83. 10.1186/s13045-020-00917-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.-H., Zhu C., Kondo Y., Anderson A. C., Gandhi A., Russell A., et al. (2015). CEACAM1 Regulates TIM-3-Mediated Tolerance and Exhaustion. Nature 517 (7534), 386–390. 10.1038/nature13848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwama S., De Remigis A., Callahan M. K., Slovin S. F., Wolchok J. D., Caturegli P. (2014). Pituitary Expression of CTLA-4 Mediates Hypophysitis Secondary to Administration of CTLA-4 Blocking Antibody. Sci. Transl. Med. 6 (230), 230ra245. 10.1126/scitranslmed.3008002 [DOI] [PubMed] [Google Scholar]
- Janjigian Y. Y., Shitara K., Moehler M., Garrido M., Salman P., Shen L., et al. (2021). First-line Nivolumab Plus Chemotherapy versus Chemotherapy Alone for Advanced Gastric, Gastro-Oesophageal junction, and Oesophageal Adenocarcinoma (CheckMate 649): a Randomised, Open-Label, Phase 3 Trial. Lancet 398 (10294), 27–40. 10.1016/S0140-6736(21)00797-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. J., Su L. J., Pinckney J., Critton D., Boyer E., Krishnakumar A., et al. (2019). VISTA Is an Acidic pH-Selective Ligand for PSGL-1. Nature 574 (7779), 565–570. 10.1038/s41586-019-1674-5 [DOI] [PubMed] [Google Scholar]
- Jubel J. M., Barbati Z. R., Burger C., Wirtz D. C., Schildberg F. A. (2020). The Role of PD-1 in Acute and Chronic Infection. Front. Immunol. 11, 487. 10.3389/fimmu.2020.00487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juno J. A., Stalker A. T., Waruk J. L., Oyugi J., Kimani M., Plummer F. A., et al. (2015). Elevated Expression of LAG-3, but Not PD-1, Is Associated with Impaired iNKT Cytokine Production during Chronic HIV-1 Infection and Treatment. Retrovirology 12, 17. 10.1186/s12977-015-0142-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y., Yamada T., Shimamoto T., Iwasaku M., Kaneko Y., Uchino J., et al. (2019). The Role of the Gut Microbiome on the Efficacy of Immune Checkpoint Inhibitors in Japanese Responder Patients with Advanced Non-small Cell Lung Cancer. Transl Lung Cancer Res. 8 (6), 847–853. 10.21037/tlcr.2019.10.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M., Arooj S., Wang H. (2020). NK Cell-Based Immune Checkpoint Inhibition. Front. Immunol. 11, 167. 10.3389/fimmu.2020.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikushige Y., Miyamoto T., Yuda J., Jabbarzadeh-Tabrizi S., Shima T., Takayanagi S.-i., et al. (2015). A TIM-3/Gal-9 Autocrine Stimulatory Loop Drives Self-Renewal of Human Myeloid Leukemia Stem Cells and Leukemic Progression. Cell Stem Cell 17 (3), 341–352. 10.1016/j.stem.2015.07.011 [DOI] [PubMed] [Google Scholar]
- Kim K. J., Lee H. W., Seong J. (2021). Combination Therapy with anti‐T‐cell Immunoglobulin and Mucin‐domain Containing Molecule 3 and Radiation Improves Antitumor Efficacy in Murine Hepatocellular Carcinoma. J. Gastroenterol. Hepatol. 36 (5), 1357–1365. 10.1111/jgh.15319 [DOI] [PubMed] [Google Scholar]
- Kong K.-F., Fu G., Zhang Y., Yokosuka T., Casas J., Canonigo-Balancio A. J., et al. (2014). Protein Kinase C-η Controls CTLA-4-Mediated Regulatory T Cell Function. Nat. Immunol. 15 (5), 465–472. 10.1038/ni.2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouo T., Huang L., Pucsek A. B., Cao M., Solt S., Armstrong T., et al. (2015). Galectin-3 Shapes Antitumor Immune Responses by Suppressing CD8+ T Cells via LAG-3 and Inhibiting Expansion of Plasmacytoid Dendritic Cells. Cancer Immunol. Res. 3 (4), 412–423. 10.1158/2326-6066.CIR-14-0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacouture M. E., Sibaud V., Gerber P. A., van den Hurk C., Fernández-Peñas P., Santini D., et al. (2021). Prevention and Management of Dermatological Toxicities Related to Anticancer Agents: ESMO Clinical Practice Guidelines☆. Ann. Oncol. 32 (2), 157–170. 10.1016/j.annonc.2020.11.005 [DOI] [PubMed] [Google Scholar]
- Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J. J., Cowey C. L., Lao C. D., et al. (2015). Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 373 (1), 23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach D. R., Krummel M. F., Allison J. P. (1996). Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 271 (5256), 1734–1736. 10.1126/science.271.5256.1734 [DOI] [PubMed] [Google Scholar]
- Lecocq Q., Keyaerts M., Devoogdt N., Breckpot K. (2020). The Next-Generation Immune Checkpoint LAG-3 and its Therapeutic Potential in Oncology: Third Time's a Charm. Ijms 22 (1), 75. 10.3390/ijms22010075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow D. J., Bluestone J. A. (1993). T Cell Co-stimulation and In Vivo Tolerance. Curr. Opin. Immunol. 5 (5), 747–752. 10.1016/0952-7915(93)90132-c [DOI] [PubMed] [Google Scholar]
- Lenschow D. J., Walunas T. L., Bluestone J. A. (1996). CD28/B7 System of T Cell Costimulation. Annu. Rev. Immunol. 14, 233–258. 10.1146/annurev.immunol.14.1.233 [DOI] [PubMed] [Google Scholar]
- Lin W., Chen M., Hong L., Zhao H., Chen Q. (2018). Crosstalk between PD-1/pd-L1 Blockade and its Combinatorial Therapies in Tumor Immune Microenvironment: A Focus on HNSCC. Front. Oncol. 8, 532. 10.3389/fonc.2018.00532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lines J. L., Pantazi E., Mak J., Sempere L. F., Wang L., O'Connell S., et al. (2014). VISTA Is an Immune Checkpoint Molecule for Human T Cells. Cancer Res. 74 (7), 1924–1932. 10.1158/0008-5472.CAN-13-1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Urnes M., Grosmaire L. S., Damle N. K., Ledbetter J. A. (1991). CTLA-4 Is a Second Receptor for the B Cell Activation Antigen B7. J. Exp. Med. 174 (3), 561–569. 10.1084/jem.174.3.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Greene J. L., Brady W., Bajorath J., Ledbetter J. A., Peach R. (1994). Human B7-1 (CD80) and B7-2 (CD86) Bind with Similar Avidities but Distinct Kinetics to CD28 and CTLA-4 Receptors. Immunity 1 (9), 793–801. 10.1016/s1074-7613(94)80021-9 [DOI] [PubMed] [Google Scholar]
- Lipson E. J., Tawbi H. A.-H., Schadendorf D., Ascierto P. A., Matamala L., Gutiérrez E. C., et al. (2021). Relatlimab (RELA) Plus Nivolumab (NIVO) Versus NIVO in First-Line Advanced Melanoma: Primary Phase III Results from RELATIVITY-047 (CA224-047). J. Clin. Oncol. 39, 9503. 10.1200/JCO.2021.39.15_suppl.9503 [DOI] [Google Scholar]
- Liu J.-F., Wu L., Yang L.-L., Deng W.-W., Mao L., Wu H., et al. (2018). Blockade of TIM3 Relieves Immunosuppression through Reducing Regulatory T Cells in Head and Neck Cancer. J. Exp. Clin. Cancer Res. 37 (1), 44. 10.1186/s13046-018-0713-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Yuan Y., Chen W., Putra J., Suriawinata A. A., Schenk A. D., et al. (2015). Immune-checkpoint Proteins VISTA and PD-1 Nonredundantly Regulate Murine T-Cell Responses. Proc. Natl. Acad. Sci. USA 112 (21), 6682–6687. 10.1073/pnas.1420370112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Wei F., Wang J., Yu W., Shen M., Liu T., et al. (2021). Myeloid-derived Suppressor Cells Regulate the Immunosuppressive Functions of PD-1−pd-L1+ Bregs through PD-L1/PI3K/AKT/NF-κB axis in Breast Cancer. Cell Death Dis 12 (5), 465. 10.1038/s41419-021-03745-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo B., Zhang K., Lu W., Zheng L., Zhang Q., Kanellopoulou C., et al. (2015). Patients with LRBA Deficiency Show CTLA4 Loss and Immune Dysregulation Responsive to Abatacept Therapy. Science 349 (6246), 436–440. 10.1126/science.aaa1663 [DOI] [PubMed] [Google Scholar]
- Mao X., Ou M. T., Karuppagounder S. S., Kam T.-I., Yin X., Xiong Y., et al. (2016). Pathological α-synuclein Transmission Initiated by Binding Lymphocyte-Activation Gene 3. Science 353 (6307), aah3374. 10.1126/science.aah3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruhashi T., Sugiura D., Okazaki I.-m., Okazaki T. (2020). LAG-3: from Molecular Functions to Clinical Applications. J. Immunother. Cancer 8 (2), e001014. 10.1136/jitc-2020-001014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson V., Fessler J., Bao R., Chongsuwat T., Zha Y., Alegre M.-L., et al. (2018). The Commensal Microbiome Is Associated with Anti-PD-1 Efficacy in Metastatic Melanoma Patients. Science 359 (6371), 104–108. 10.1126/science.aao3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian S. K., Liu C. H., Tzianabos A. O., Kasper D. L. (2005). An Immunomodulatory Molecule of Symbiotic Bacteria Directs Maturation of the Host Immune System. Cell 122 (1), 107–118. 10.1016/j.cell.2005.05.007 [DOI] [PubMed] [Google Scholar]
- McKay R. R., McGregor B. A., Xie W., Braun D. A., Wei X., Kyriakopoulos C. E., et al. (2020). Optimized Management of Nivolumab and Ipilimumab in Advanced Renal Cell Carcinoma: A Response-Based Phase II Study (OMNIVORE). Jco 38 (36), 4240–4248. 10.1200/JCO.20.02295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz R., Rust S., Duhadaway J. B., Mautino M. R., Munn D. H., Vahanian N. N., et al. (2012). Ido Inhibits a Tryptophan Sufficiency Signal that Stimulates mTOR: A Novel Ido Effector Pathway Targeted by D-1-Methyl-Tryptophan. Oncoimmunology 1 (9), 1460–1468. 10.4161/onci.21716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezrich J. D., Fechner J. H., Zhang X., Johnson B. P., Burlingham W. J., Bradfield C. A. (2010). An Interaction between Kynurenine and the Aryl Hydrocarbon Receptor Can Generate Regulatory T Cells. J.I. 185 (6), 3190–3198. 10.4049/jimmunol.0903670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monney L., Sabatos C. A., Gaglia J. L., Ryu A., Waldner H., Chernova T., et al. (2002). Th1-specific Cell Surface Protein Tim-3 Regulates Macrophage Activation and Severity of an Autoimmune Disease. Nature 415 (6871), 536–541. 10.1038/415536a [DOI] [PubMed] [Google Scholar]
- Muller S., Victoria Lai W., Adusumilli P. S., Desmeules P., Frosina D., Jungbluth A., et al. (2020). V-domain Ig-Containing Suppressor of T-Cell Activation (VISTA), a Potentially Targetable Immune Checkpoint Molecule, Is Highly Expressed in Epithelioid Malignant Pleural Mesothelioma. Mod. Pathol. 33 (2), 303–311. 10.1038/s41379-019-0364-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn D. H., Mellor A. L. (2013). Indoleamine 2,3 Dioxygenase and Metabolic Control of Immune Responses. Trends Immunol. 34 (3), 137–143. 10.1016/j.it.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn D. H., Sharma M. D., Baban B., Harding H. P., Zhang Y., Ron D., et al. (2005). GCN2 Kinase in T Cells Mediates Proliferative Arrest and Anergy Induction in Response to Indoleamine 2,3-dioxygenase. Immunity 22 (5), 633–642. 10.1016/j.immuni.2005.03.013 [DOI] [PubMed] [Google Scholar]
- Murphy K. M., Nelson C. A., Šedý J. R. (2006). Balancing Co-stimulation and Inhibition with BTLA and HVEM. Nat. Rev. Immunol. 6 (9), 671–681. 10.1038/nri1917 [DOI] [PubMed] [Google Scholar]
- Nakayama M., Akiba H., Takeda K., Kojima Y., Hashiguchi M., Azuma M., et al. (2009). Tim-3 Mediates Phagocytosis of Apoptotic Cells and Cross-Presentation. Blood 113 (16), 3821–3830. 10.1182/blood-2008-10-185884 [DOI] [PubMed] [Google Scholar]
- Nandi D., Pathak S., Verma T., Singh M., Chattopadhyay A., Thakur S., et al. (2020). T Cell Costimulation, Checkpoint Inhibitors and Anti-tumor Therapy. J. Biosci. 45, 50. 10.1007/s12038-020-0020-2 [DOI] [PubMed] [Google Scholar]
- Nassar A. H., Mouw K. W., Jegede O., Shinagare A. B., Kim J., Liu C.-J., et al. (2020). A Model Combining Clinical and Genomic Factors to Predict Response to PD-1/pd-L1 Blockade in Advanced Urothelial Carcinoma. Br. J. Cancer 122 (4), 555–563. 10.1038/s41416-019-0686-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel B. G., Gu H., Pao L. (2003). The 'Shp'ing News: SH2 Domain-Containing Tyrosine Phosphatases in Cell Signaling. Trends Biochem. Sci. 28 (6), 284–293. 10.1016/S0968-0004(03)00091-4 [DOI] [PubMed] [Google Scholar]
- Nogueira-Machado J. A., Volpe C. M. d. O., Veloso C. A., Chaves M. M. (2011). HMGB1, TLR and RAGE: a Functional Tripod that Leads to Diabetic Inflammation. Expert Opin. Ther. Targets 15 (8), 1023–1035. 10.1517/14728222.2011.575360 [DOI] [PubMed] [Google Scholar]
- Oliveira A. F., Bretes L., Furtado I. (2019). Review of PD-1/pd-L1 Inhibitors in Metastatic dMMR/MSI-H Colorectal Cancer. Front. Oncol. 9, 396. 10.3389/fonc.2019.00396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson C., Riebeck K., Dohlsten M., Michaëlsson E. (1999). CTLA-4 Ligation Suppresses CD28-Induced NF-Κb and AP-1 Activity in Mouse T Cell Blasts. J. Biol. Chem. 274 (20), 14400–14405. 10.1074/jbc.274.20.14400 [DOI] [PubMed] [Google Scholar]
- Pagès F., Ragueneau M., Rottapel R., Truneh A., Nunes J., Imbert J., et al. (1994). Binding of Phosphatidyl-Inositol-3-OH Kinase to CD28 Is Required for T-Cell Signalling. Nature 369 (6478), 327–329. 10.1038/369327a0 [DOI] [PubMed] [Google Scholar]
- Pai C.-C. S., Simons D. M., Lu X., Evans M., Wei J., Wang Y.-h., et al. (2018). Tumor-conditional Anti-CTLA4 Uncouples Antitumor Efficacy from Immunotherapy-Related Toxicity. J. Clin. Invest. 129 (1), 349–363. 10.1172/JCI123391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjwani P. K., Charu V., DeLisser M., Molina-Kirsch H., Natkunam Y., Zhao S. (2018). Programmed Death-1 Ligands PD-L1 and PD-L2 Show Distinctive and Restricted Patterns of Expression in Lymphoma Subtypes. Hum. Pathol. 71, 91–99. 10.1016/j.humpath.2017.10.029 [DOI] [PubMed] [Google Scholar]
- Patel R., Bock M., Polotti C. F., Elsamra S. (2017). Pharmacokinetic Drug Evaluation of Atezolizumab for the Treatment of Locally Advanced or Metastatic Urothelial Carcinoma. Expert Opin. Drug Metab. Toxicol. 13 (2), 225–232. 10.1080/17425255.2017.1277204 [DOI] [PubMed] [Google Scholar]
- Patsoukis N., Duke-Cohan J. S., Chaudhri A., Aksoylar H.-I., Wang Q., Council A., et al. (2020). Interaction of SHP-2 SH2 Domains with PD-1 ITSM Induces PD-1 Dimerization and SHP-2 Activation. Commun. Biol. 3 (1), 128. 10.1038/s42003-020-0845-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken K. E., Wherry E. J. (2015). Overcoming T Cell Exhaustion in Infection and Cancer. Trends Immunol. 36 (4), 265–276. 10.1016/j.it.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peggs K. S., Quezada S. A., Korman A. J., Allison J. P. (2006). Principles and Use of Anti-CTLA4 Antibody in Human Cancer Immunotherapy. Curr. Opin. Immunol. 18 (2), 206–213. 10.1016/j.coi.2006.01.011 [DOI] [PubMed] [Google Scholar]
- Pickard J. M., Zeng M. Y., Caruso R., Núñez G. (2017). Gut Microbiota: Role in Pathogen Colonization, Immune Responses, and Inflammatory Disease. Immunol. Rev. 279 (1), 70–89. 10.1111/imr.12567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrard J., Seront E. (2019). Impact of the Gut Microbiome on Immune Checkpoint Inhibitor Efficacy-A Systematic Review. Curr. Oncol. 26 (6), 395–403. 10.3747/co.26.5177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto J. A., Raez L. E., Oliveres H., Rolfo C. C. (2019). Current Knowledge of Ipilimumab and its Use in Treating Non-small Cell Lung Cancer. Expert Opin. Biol. Ther. 19 (6), 509–515. 10.1080/14712598.2019.1610380 [DOI] [PubMed] [Google Scholar]
- Prasad V., Kaestner V. (2017). Nivolumab and Pembrolizumab: Monoclonal Antibodies against Programmed Cell Death-1 (PD-1) that Are Interchangeable. Semin. Oncol. 44 (2), 132–135. 10.1053/j.seminoncol.2017.06.007 [DOI] [PubMed] [Google Scholar]
- Qureshi O. S., Kaur S., Hou T. Z., Jeffery L. E., Poulter N. S., Briggs Z., et al. (2012). Constitutive Clathrin-Mediated Endocytosis of CTLA-4 Persists during T Cell Activation. J. Biol. Chem. 287 (12), 9429–9440. 10.1074/jbc.M111.304329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi O. S., Zheng Y., Nakamura K., Attridge K., Manzotti C., Schmidt E. M., et al. (2011). Trans-endocytosis of CD80 and CD86: a Molecular Basis for the Cell-Extrinsic Function of CTLA-4. Science 332 (6029), 600–603. 10.1126/science.1202947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Casals M., Brahmer J. R., Callahan M. K., Flores-Chávez A., Keegan N., Khamashta M. A., et al. (2020). Immune-related Adverse Events of Checkpoint Inhibitors. Nat. Rev. Dis. Primers 6 (1), 38. 10.1038/s41572-020-0160-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangachari M., Zhu C., Sakuishi K., Xiao S., Karman J., Chen A., et al. (2012). Bat3 Promotes T Cell Responses and Autoimmunity by Repressing Tim-3-Mediated Cell Death and Exhaustion. Nat. Med. 18 (9), 1394–1400. 10.1038/nm.2871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remon J., Besse B. (2017). Immune Checkpoint Inhibitors in First-Line Therapy of Advanced Non-small Cell Lung Cancer. Curr. Opin. Oncol. 29 (2), 97–104. 10.1097/CCO.0000000000000351 [DOI] [PubMed] [Google Scholar]
- Risbjerg R. S., Hansen M. V., Sørensen A. S., Kragstrup T. W. (2020). The Effects of B Cell Depletion on Immune Related Adverse Events Associated with Immune Checkpoint Inhibition. Exp. Hematol. Oncol. 9, 9. 10.1186/s40164-020-00167-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritprajak P., Azuma M. (2015). Intrinsic and Extrinsic Control of Expression of the Immunoregulatory Molecule PD-L1 in Epithelial Cells and Squamous Cell Carcinoma. Oral Oncol. 51 (3), 221–228. 10.1016/j.oraloncology.2014.11.014 [DOI] [PubMed] [Google Scholar]
- Romano E., Kusio-Kobialka M., Foukas P. G., Baumgaertner P., Meyer C., Ballabeni P., et al. (2015). Ipilimumab-dependent Cell-Mediated Cytotoxicity of Regulatory T Cells Ex Vivo by Nonclassical Monocytes in Melanoma Patients. Proc. Natl. Acad. Sci. USA 112 (19), 6140–6145. 10.1073/pnas.1417320112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowshanravan B., Halliday N., Sansom D. M. (2018). CTLA-4: a Moving Target in Immunotherapy. Blood 131 (1), 58–67. 10.1182/blood-2017-06-741033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Trinchieri G. (2017). Microbiota: a Key Orchestrator of Cancer Therapy. Nat. Rev. Cancer 17 (5), 271–285. 10.1038/nrc.2017.13 [DOI] [PubMed] [Google Scholar]
- Ruffo E., Wu R. C., Bruno T. C., Workman C. J., Vignali D. A. A. (2019). Lymphocyte-activation Gene 3 (LAG3): The Next Immune Checkpoint Receptor. Semin. Immunol. 42, 101305. 10.1016/j.smim.2019.101305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Kono Y., Murakami Y., Shishido Y., Kuroda H., Matsunaga T., et al. (2018). Highly Activated PD-1/pd-L1 Pathway in Gastric Cancer with PD-L1 Expression. Ar 38 (1), 107–112. 10.21873/anticanres.12197 [DOI] [PubMed] [Google Scholar]
- Sandigursky S., Mor A. (2018). Immune-Related Adverse Events in Cancer Patients Treated with Immune Checkpoint Inhibitors. Curr. Rheumatol. Rep. 20 (10), 65. 10.1007/s11926-018-0770-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saung M. T., Pelosof L., Casak S., Donoghue M., Lemery S., Yuan M., et al. (2021). FDA Approval Summary: Nivolumab Plus Ipilimumab for the Treatment of Patients with Hepatocellular Carcinoma Previously Treated with Sorafenib. Oncol. 26 (9), 797–806. 10.1002/onco.13819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Bode C., Kenefeck R., Hou T. Z., Wing J. B., Kennedy A., et al. (2014). Autosomal Dominant Immune Dysregulation Syndrome in Humans with CTLA4 Mutations. Nat. Med. 20 (12), 1410–1416. 10.1038/nm.3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe R. F., Jobin C. (2013). The Microbiome and Cancer. Nat. Rev. Cancer 13 (11), 800–812. 10.1038/nrc3610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender R., Fuchs S., Milo R. (2016). Revised Estimates for the Number of Human and Bacteria Cells in the Body. Plos Biol. 14 (8), e1002533. 10.1371/journal.pbio.1002533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seton-Rogers S. (2021). Microbiota Links to Immunotherapy Toxicity. Nat. Rev. Cancer 21 (9), 540. 10.1038/s41568-021-00390-w [DOI] [PubMed] [Google Scholar]
- Shahbaz S., Bozorgmehr N., Koleva P., Namdar A., Jovel J., Fava R. A., et al. (2018). CD71+VISTA+ Erythroid Cells Promote the Development and Function of Regulatory T Cells through TGF-β. Plos Biol. 16 (12), e2006649. 10.1371/journal.pbio.2006649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., Hu-Lieskovan S., Wargo J. A., Ribas A. (2017). Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 168 (4), 707–723. 10.1016/j.cell.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Chen L., Wu C., Zhu Y., Xu B., Zheng X., et al. (2016). PD-1 Blockade Boosts Radiofrequency Ablation-Elicited Adaptive Immune Responses against Tumor. Clin. Cancer Res. 22 (5), 1173–1184. 10.1158/1078-0432.CCR-15-1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratori T., Miyatake S., Ohno H., Nakaseko C., Isono K., Bonifacino J. S., et al. (1997). Tyrosine Phosphorylation Controls Internalization of CTLA-4 by Regulating its Interaction with Clathrin-Associated Adaptor Complex AP-2. Immunity 6 (5), 583–589. 10.1016/s1074-7613(00)80346-5 [DOI] [PubMed] [Google Scholar]
- Sierro S., Romero P., Speiser D. E. (2011). The CD4-like Molecule LAG-3, Biology and Therapeutic Applications. Expert Opin. Ther. Targets 15 (1), 91–101. 10.1517/14712598.2011.540563 [DOI] [PubMed] [Google Scholar]
- Stamatouli A. M., Quandt Z., Perdigoto A. L., Clark P. L., Kluger H., Weiss S. A., et al. (2018). Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors. Diabetes 67 (8), 1471–1480. 10.2337/dbi18-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutvoet T. S., Kol A., Vries E. G., Bruyn M., Fehrmann R. S., Terwisscha van Scheltinga A. G., et al. (2019). MAPK Pathway Activity Plays a Key Role in PD‐L1 Expression of Lung Adenocarcinoma Cells. J. Pathol. 249 (1), 52–64. 10.1002/path.5280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y., Hirota K., Sakaguchi S. (2020). Impaired T Cell Receptor Signaling and Development of T Cell-Mediated Autoimmune Arthritis. Immunol. Rev. 294 (1), 164–176. 10.1111/imr.12841 [DOI] [PubMed] [Google Scholar]
- Tan S., Xu Y., Wang Z., Wang T., Du X., Song X., et al. (2020). Tim-3 Hampers Tumor Surveillance of Liver Resident and Conventional NK Cells by Disrupting PI3K Signaling. Cancer Res. 80 (5), canres.2332.2019–1142. 10.1158/0008-5472.CAN-19-2332 [DOI] [PubMed] [Google Scholar]
- Tarhini A. A., Kang N., Lee S. J., Hodi F. S., Cohen G. I., Hamid O., et al. (2021). Immune Adverse Events (irAEs) with Adjuvant Ipilimumab in Melanoma, Use of Immunosuppressants and Association with Outcome: ECOG-ACRIN E1609 Study Analysis. J. Immunother. Cancer 9 (5), e002535. 10.1136/jitc-2021-002535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares A. B. M. L. A., Lima Neto J. X., Fulco U. L., Albuquerque E. L. (2018). Inhibition of the Checkpoint Protein PD-1 by the Therapeutic Antibody Pembrolizumab Outlined by Quantum Chemistry. Sci. Rep. 8 (1), 1840. 10.1038/s41598-018-20325-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekguc M., Wing J. B., Osaki M., Long J., Sakaguchi S. (2021). Treg-expressed CTLA-4 Depletes CD80/CD86 by Trogocytosis, Releasing Free PD-L1 on Antigen-Presenting Cells. Proc. Natl. Acad. Sci. USA 118 (30), e2023739118. 10.1073/pnas.2023739118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triebel F., Jitsukawa S., Baixeras E., Roman-Roman S., Genevee C., Viegas-Pequignot E., et al. (1990). LAG-3, a Novel Lymphocyte Activation Gene Closely Related to CD4. J. Exp. Med. 171 (5), 1393–1405. 10.1084/jem.171.5.1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman-Saint Victor C., Rech A. J., Maity A., Rengan R., Pauken K. E., Stelekati E., et al. (2015). Radiation and Dual Checkpoint Blockade Activate Non-redundant Immune Mechanisms in Cancer. Nature 520 (7547), 373–377. 10.1038/nature14292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban-Wojciuk Z., Khan M. M., Oyler B. L., Fåhraeus R., Marek-Trzonkowska N., Nita-Lazar A., et al. (2019). The Role of TLRs in Anti-cancer Immunity and Tumor Rejection. Front. Immunol. 10, 2388. 10.3389/fimmu.2019.02388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaddepally R. K., Kharel P., Pandey R., Garje R., Chandra A. B. (2020). Review of Indications of FDA-Approved Immune Checkpoint Inhibitors Per NCCN Guidelines with the Level of Evidence. Cancers 12 (3), 738. 10.3390/cancers12030738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Weyer P. S., Muehlfeit M., Klose C., Bonventre J. V., Walz G., Kuehn E. W. (2006). A Highly Conserved Tyrosine of Tim-3 Is Phosphorylated upon Stimulation by its Ligand Galectin-9. Biochem. Biophysical Res. Commun. 351 (2), 571–576. 10.1016/j.bbrc.2006.10.079 [DOI] [PubMed] [Google Scholar]
- van der Merwe P. A., Bodian D. L., Daenke S., Linsley P., Davis S. J. (1997). CD80 (B7-1) Binds Both CD28 and CTLA-4 with a Low Affinity and Very Fast Kinetics. J. Exp. Med. 185 (3), 393–404. 10.1084/jem.185.3.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elsas A., Sutmuller R. P. M., Hurwitz A. A., Ziskin J., Villasenor J., Medema J.-P., et al. (2001). Elucidating the Autoimmune and Antitumor Effector Mechanisms of a Treatment Based on Cytotoxic T Lymphocyte Antigen-4 Blockade in Combination with a B16 Melanoma Vaccine. J. Exp. Med. 194 (4), 481–490. 10.1084/jem.194.4.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance R. E., Eichberg M. J., Portnoy D. A., Raulet D. H. (2017). Listening to Each Other: Infectious Disease and Cancer Immunology. Sci. Immunol. 2 (7), eaai9339. 10.1126/sciimmunol.aai9339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vétizou M., Pitt J. M., Daillère R., Lepage P., Waldschmitt N., Flament C., et al. (2015). Anticancer Immunotherapy by CTLA-4 Blockade Relies on the Gut Microbiota. Science 350 (6264), 1079–1084. 10.1126/science.aad1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L. S. K. (2017). EFIS Lecture: Understanding the CTLA-4 Checkpoint in the Maintenance of Immune Homeostasis. Immunol. Lett. 184, 43–50. 10.1016/j.imlet.2017.02.007 [DOI] [PubMed] [Google Scholar]
- Wang J., Sanmamed M. F., Datar I., Su T. T., Ji L., Sun J., et al. (2019a). Fibrinogen-like Protein 1 Is a Major Immune Inhibitory Ligand of LAG-3. Cell 176 (1-2), 334–347. 10.1016/j.cell.2018.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wu G., Manick B., Hernandez V., Renelt M., Erickson C., et al. (2019b). VSIG-3 as a Ligand of VISTA Inhibits Human T-Cell Function. Immunology 156 (1), 74–85. 10.1111/imm.13001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Le Mercier I., Putra J., Chen W., Liu J., Schenk A. D., et al. (2014). Disruption of the Immune-Checkpoint VISTA Gene Imparts a Proinflammatory Phenotype with Predisposition to the Development of Autoimmunity. Proc. Natl. Acad. Sci. 111 (41), 14846–14851. 10.1073/pnas.1407447111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Rubinstein R., Lines J. L., Wasiuk A., Ahonen C., Guo Y., et al. (2011). VISTA, a Novel Mouse Ig Superfamily Ligand that Negatively Regulates T Cell Responses. J. Exp. Med. 208 (3), 577–592. 10.1084/jem.20100619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li G. (2019). PD-1/PD-L1 Blockade in Cervical Cancer: Current Studies and Perspectives. Front. Med. 13 (4), 438–450. 10.1007/s11684-018-0674-4 [DOI] [PubMed] [Google Scholar]
- Wang Y., Wiesnoski D. H., Helmink B. A., Gopalakrishnan V., Choi K., DuPont H. L., et al. (2018). Fecal Microbiota Transplantation for Refractory Immune Checkpoint Inhibitor-Associated Colitis. Nat. Med. 24 (12), 1804–1808. 10.1038/s41591-018-0238-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang-Gillam A., Plambeck-Suess S., Goedegebuure P., Simon P. O., Mitchem J. B., Hornick J. R., et al. (2013). A Phase I Study of IMP321 and Gemcitabine as the Front-Line Therapy in Patients with Advanced Pancreatic Adenocarcinoma. Invest. New Drugs 31 (3), 707–713. 10.1007/s10637-012-9866-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. A., Sznol M. (2021). Resistance Mechanisms to Checkpoint Inhibitors. Curr. Opin. Immunol. 69, 47–55. 10.1016/j.coi.2021.02.001 [DOI] [PubMed] [Google Scholar]
- Wolf Y., Anderson A. C., Kuchroo V. K. (2020). TIM3 Comes of Age as an Inhibitory Receptor. Nat. Rev. Immunol. 20 (3), 173–185. 10.1038/s41577-019-0224-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Sang M., Liu F., Zhang J., Li W., Li Z., et al. (2020). Epigenetic Modulation Combined with PD-1/pd-L1 Blockade Enhances Immunotherapy Based on MAGE-A11 Antigen-specific CD8+T Cells against Esophageal Carcinoma. Carcinogenesis 41 (7), 894–903. 10.1093/carcin/bgaa057 [DOI] [PubMed] [Google Scholar]
- Wykes M. N., Lewin S. R. (2018). Immune Checkpoint Blockade in Infectious Diseases. Nat. Rev. Immunol. 18 (2), 91–104. 10.1038/nri.2017.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Chen Y.-P., Du X.-J., Liu J.-Q., Huang C.-L., Chen L., et al. (2018). Comparative Safety of Immune Checkpoint Inhibitors in Cancer: Systematic Review and Network Meta-Analysis. BMJ 363, k4226. 10.1136/bmj.k4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F., Liu J., Liu D., Liu B., Wang M., Hu Z., et al. (2014). LSECtin Expressed on Melanoma Cells Promotes Tumor Progression by Inhibiting Antitumor T-Cell Responses. Cancer Res. 74 (13), 3418–3428. 10.1158/0008-5472.CAN-13-2690 [DOI] [PubMed] [Google Scholar]
- Yang X., Jiang X., Chen G., Xiao Y., Geng S., Kang C., et al. (2013). T Cell Ig Mucin-3 Promotes Homeostasis of Sepsis by Negatively Regulating the TLR Response. J.I. 190 (5), 2068–2079. 10.4049/jimmunol.1202661 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Ma C. J., Wang J. M., Ji X. J., Wu X. Y., Moorman J. P., et al. (2012). Tim-3 Regulates Pro- and Anti-inflammatory Cytokine Expression in Human CD14+ Monocytes. J. Leukoc. Biol. 91 (2), 189–196. 10.1189/jlb.1010591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C., Anderson A. C., Schubart A., Xiong H., Imitola J., Khoury S. J., et al. (2005). The Tim-3 Ligand Galectin-9 Negatively Regulates T Helper Type 1 Immunity. Nat. Immunol. 6 (12), 1245–1252. 10.1038/ni1271 [DOI] [PubMed] [Google Scholar]
- Zhu C., Sakuishi K., Xiao S., Sun Z., Zaghouani S., Gu G., et al. (2015). Erratum: Corrigendum: An IL-27/NFIL3 Signalling axis Drives Tim-3 and IL-10 Expression and T-Cell Dysfunction. Nat. Commun. 6, 7657. 10.1038/ncomms8657 [DOI] [PubMed] [Google Scholar]