Supplemental Digital Content is available in the text.
Keywords: bladder cancer, CXCL5, epithelial–mesenchymal transition, metastasis, stemness
Abstract
In our previous studies, we found that T24 lung metastatic cancer cells showed high invasion and metastasis abilities and cancer stem cell characteristics compared with T24 primary cancer cells. By screening for the expression of CXC chemokines in both cell lines, we found that CXCL5 is highly expressed in T24-L cells. The aim of this study is to shed light on the relationship of CXCL5 with epithelial–mesenchymal transition (EMT) and cancer stem cells (CSCs). RNAi technology was used to decrease CXCL5 expression in the T24-L cell line, and the EMT and CSCs of the shCXCL5 group and the control group were compared. The CXCR2 inhibitor SB225002 was used to inhibit the receptor of CXCL5 to determine the effect of the CXCL5/CXCR2 axis. The knockdown of CXCL5 expression in T24-L cells reduced their EMT and CSC characteristics. RT-PCR and Western blot analyses revealed the downregulation of N-cadherin, Vimentin and CD44. In addition, when CD44 expression was knocked down, the EMT ability of the cells was also inhibited. This phenomenon was most pronounced when both CXCL5 and CD44 were knocked down. CXCL5 and CD44 can affect the EMT and stem cell capacity of T24-L cells through some interaction.
Introduction
Bladder cancer is the 10th most common form of cancer worldwide, and it is the sixth most common form of cancer and ninth leading cause of cancer-related death among men [1]. CXCL5, which is a kind of CXC chemokine [2], has been found to play an important role in tumor growth, invasion and metastasis after binding to its receptor, CXCR2 [3]. Many studies have indicated that CXCL5 is highly expressed in many cancers, such as stomach cancer, prostatic cancer and pancreatic cancer [4–7]. In our previous studies, we found that CXCL5 was overexpressed in bladder cancer tissues compared with tumor-free bladder tissues, and the expression of CXCL5 was positively correlated with the pathological grade, clinical stage and lymph node metastasis of bladder cancer; our other study showed that CXCL5 contributed to BCa migration and invasion by binding to its receptor, CXCR2, which led to the upregulation of MMP2/MMP9 through the activation of PI3K/AKT signaling [8].
Epithelial–mesenchymal transition (EMT) plays a crucial role during cancer invasion and metastasis and is associated with tumor progression. Cancer stem cells (CSCs), also known as tumor-initiating cells or tumorigenic cells, are equipped with stem properties and possess the abilities of self-renewal, high proliferation and multipotential differentiation [9]. Scientific studies have shown that both EMT and CSCs play crucial roles in tumor metastasis, therapeutic resistance and recurrence. The EMT status of CSCs has an important effect on tumor resistance, recurrence and metastasis. EMT can also affect the proliferation and metastasis of cancer cells and improve the self-renewal ability of cancer cells, thus improving the stemness of cancer cells [10,11]. CD44, as a marker of CSCs, has been implicated in the regulation of hepatocellular carcinoma invasion and metastasis by regulating EMT in recent research [12].
The T24-t-primary (T24-P) and T24-t-lung-metastatic (T24-L) bladder cancer cell lines were established from T24 primary tumor and lung metastasis cells in a mouse model [13], and our previous studies found that T24-L cells have stronger invasion, metastasis and chemical resistance abilities than T24-P cells in vitro and in vivo [14,15]. In another study of T24-L cells, when compared with T24-P cells, T24-L cells showed stronger stemness and higher CD44 expression [16]. Therefore, in this study, we hypothesize that the high expression of CXCL5 leads to increased migration and invasion capacities and stemness of T24-L cells.
Materials and methods
Cell culture
Lung metastatic T24-L and primary T24-P subline cells expressing luciferase were generated as previously described and maintained in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, San Diego, California, USA) supplemented with an antibiotic solution (penicillin 100 U/ml and streptomycin 100 g/ml; HyClone Laboratories, Logan, Utah, USA), 10% fetal bovine serum (FBS) and 400 mg/L G418 at 37°C with 5% CO2 in a humidified incubator [13]. For SB225002 treatment, the 5 mM stock solution in DMSO was added to the culture medium to achieve the appropriate concentration of 5 μM and was then incubated with the cells for 24 h; DMSO without SB225002 was used as a control.
Chemicals and reagents
DMEM, FBS and penicillin-streptomycin were purchased from Thermo Fisher Scientific (Waltham, Massachusetts, USA). Transwell chambers with an 8-μm pore size were obtained from Millipore (Darmstadt, Germany). G418 and SB225002 were purchased from TargetMol (Boston, Massachusetts, USA).
RNA extraction and quantitative RT-PCR
RNA was isolated using the RNeasy kit (Qiagen, Valencia, California, USA) and reverse-transcribed with the Revert Aid kit (MBI Fermentas, St. Leon-Rot, Germany) according to the manufacturer’s instructions. cDNA was subjected to real-time PCR in a volume of 25 μl in an iCycler thermal cycler (Bio-Rad, Hercules, California, USA) using iQ SYBR Green Supermix (Bio-Rad) with the gene-specific primers. qRT-PCR was performed as previously described, and the relative CXC, E-cad, N-cad, Vimentin and CD44 mRNA expression levels were calculated after normalizing to GAPDH. All the measurements were performed in triplicate. The sequences of the primer pairs are shown in Table 1.
Table 1.
PCR primers
| GeneID | Gene | Primers |
|---|---|---|
| 284340 | CXCL1 | TGCTGCCACTAATGCTGATGT |
| CTCAGGAACCAATCTTTGCACT | ||
| 9832 | CXCL2 | TCCAAGAAAGGGCGAAATAAGG |
| TGCAGCTCTATCTGAATGTCTGT | ||
| 2921 | CXCL3 | CGCCCAAACCGAAGTCATAG |
| GCTCCCCTTGTTCAGTATCTTTT | ||
| 5196 | CXCL4 | CTGAAGAAGATGGGGACCTG |
| AGAGCCACTAACACGTAGCCT | ||
| 6374 | CXCL5 | AGCTGCGTTGCGTTTGTTTAC |
| TGGCGAACACTTGCAGATTAC | ||
| 6372 | CXCL6 | AGAGCTGCGTTGCACTTGTT |
| GCAGTTTACCAATCGTTTTGGGG | ||
| 5473 | CXCL7 | GTAACAGTGCGAGACCACTTC |
| CTTTGCCTTTCGCCAAGTTTC | ||
| 3576 | CXCL8 | ACTGAGAGTGATTGAGAGTGGAC |
| AACCCTCTGCACCCAGTTTTC | ||
| 4283 | CXCL9 | CCAGTAGTGAGAAAGGGTCGC |
| AGGGCTTGGGGCAAATTGTT | ||
| 3627 | CXCL10 | GTGGCATTCAAGGAGTACCTC |
| TGATGGCCTTCGATTCTGGATT | ||
| 6373 | CXCL11 | GACGCTGTCTTTGCATAGGC |
| GGATTTAGGCATCGTTGTCCTTT | ||
| 6387 | CXCL12 | ATTCTCAACACTCCAAACTGTGC |
| ACTTTAGCTTCGGGTCAATGC | ||
| 10563 | CXCL13 | GCTTGAGGTGTAGATGTGTCC |
| CCCACGGGGCAAGATTTGAA | ||
| 9547 | CXCL14 | CGCTACAGCGACGTGAAGAA |
| GTTCCAGGCGTTGTACCAC | ||
| 2649 | E-cad | CGAGAGCTACACGTTCACGG |
| GGGTGTCGAGGGAAAAATAGG | ||
| 2385 | N-cad | TTTGATGGAGGTCTCCTAACACC |
| ACGTTTAACACGTTGGAAATGTG | ||
| 7431 | Vimentin | AGTCCACTGAGTACCGGAGAC |
| CATTTCACGCATCTGGCGTTC | ||
| 4319 | MMP9 | AGACCTGGGCAGATTCCAAAC |
| CGGCAAGTCTTCCGAGTAGT | ||
| 960 | CD44 | CTGCCGCTTTGCAGGTGTA |
| CATTGTGGGCAAGGTGCTATT | ||
| 8626 | p63 | CCACCTGGACGTATTCCACTG |
| TCGAATCAAATGACTAGGAGGGG | ||
| 2597 | GAPDH | AACAGCGACACCCATCCTC |
| CATACCAGGAAATGAGCTTGACAA |
Western blot analysis
Equal amounts of whole-cell extracts were resolved by 10% SDS-PAGE and transferred to PVDF membranes. The membranes were blocked in 5% nonfat dry milk in TBST and incubated (2 h) with the indicated primary antibodies (CXCL5, E-cad, N-cad, Vimentin, CD44, P-AKT, T-AKT, P-NFκB and T- NFκB; 1:1000 dilution), followed by incubation (1 h) with goat anti-rabbit or anti-mouse secondary antibodies (1:5000 dilution; Sigma-Aldrich). Immunoreactivity was visualized by an enhanced chemiluminescence reagent (Perkin Elmer Cetus, Foster City, California, USA). To demonstrate equal loading, the blots were stripped and reprobed with a specific antibody against GAPDH (1:5000 dilution; Sigma-Aldrich). The antibodies against CXCL5, E-cad, N-cad, Vimentin, CD44, P-AKT, T-AKT, P-NFκB and T- NFκB were purchased from Abcam Biotechnology (Abcam, Cambridge, UK). All the measurements were performed in triplicate.
Knockdown of CXCL5 and CD44
Lentivirus plasmids containing short hairpin RNA (shRNA) targeting CXCL5 (shCXCL5) and CD44 (shCD44) and a negative control shRNA (shCon) were designed and produced by GeneChem (Shanghai, China). For transfection, T24-L cells were seeded in 6-well plates and allowed to attach overnight; then, the culture medium was replaced with transfection-enhancing solution with lentivirus at an MOI of 30 and 50 μg/ml polybrene. After 16 h of transfection, the transfection medium was replaced with normal medium. The cells were harvested for passage or testing when they reached 80% confluence.
Wound-healing assay
A wound-healing assay was carried out to determine the invasion and migration abilities of the tumor cells. shCon and shCXCL5 cell lines were seeded into six-well plates and grown to 80–90% confluence. A sterilized 200-microliter pipette tip was used to generate wounds across the cell monolayer, and the debris was washed with PBS. The migration of the cells into the wound was then observed at different time points. The cells that migrated into the wounded area or protruded from the border of the wound were visualized and photographed with an inverted microscope at different time points. A total of nine areas were randomly selected in each well at 100× magnification, and the cells in three wells of each group were quantified in each experiment.
In vitro migration and invasion assay
Chambers (Millipore, Billerica, Massachusetts, USA) with or without Matrigel (BD Biosciences, San Jose, California, USA) bedding were placed in 24-well plates. Cells (1.5 × 104 for migration and 4 × 104 for invasion) that had been starved overnight were added to the upper chamber in 200 μl of serum-free medium, and 500 μl of complete medium was added to the bottom chamber. After 24 h of incubation, the cells in the upper chamber were fixed with 4% paraformaldehyde and stained for 15 min with 4 g/L crystal violet. The cells on the underside of the chambers were counted under 100× magnification with a microscope after the topside of the filter was wiped. All the measurements were performed in triplicate.
Clonogenic assay
A clonogenic assay was conducted to evaluate clonogenicity. Briefly, shCon cells and shCXCL5 cells were seeded in 6-well plates (Corning) at a clonal density (i.e. 2000 cells per well) and cultured for 14 days prior to fixing in 4% paraformaldehyde and staining with crystal violet. The number of clones containing more than 20 cells was counted. Representative fields were photographed. All the measurements were performed in triplicate.
Tumorsphere formation assay
To evaluate tumorsphere growth and formation, shCon cells and shCXCL5 cells were plated at a density of 4000 cells per well in 24-well ultralow attachment plates (Corning Inc., Corning, New York, USA) in serum-free DMEM/F12 supplemented with 20 ng/ml human EGF, 10 ng/ml human bFGF, 2% B27 and 1% N2 supplement (Invitrogen). The cells were incubated in a humidified atmosphere at 37°C with 5% CO2. Two weeks later, the plates were analyzed for tumorsphere formation and quantified using an inverted microscope. All the measurements were performed in triplicate.
Statistical analysis
All the assays were repeated in triplicate in three independent experiments, and the quantitative data are presented as the mean ± SEM. The differences between two groups were compared by two-tailed Student’s t test. The survival curves were plotted using Kaplan–Meier analysis. In all cases, P < 0.05 was considered statistically significant. All the statistical analyses were performed using SPSS 15.0 (SPSS Inc., Chicago, Illinois, USA).
Results
CXCL5 expression and knockdown efficiency in the T24-L cell line
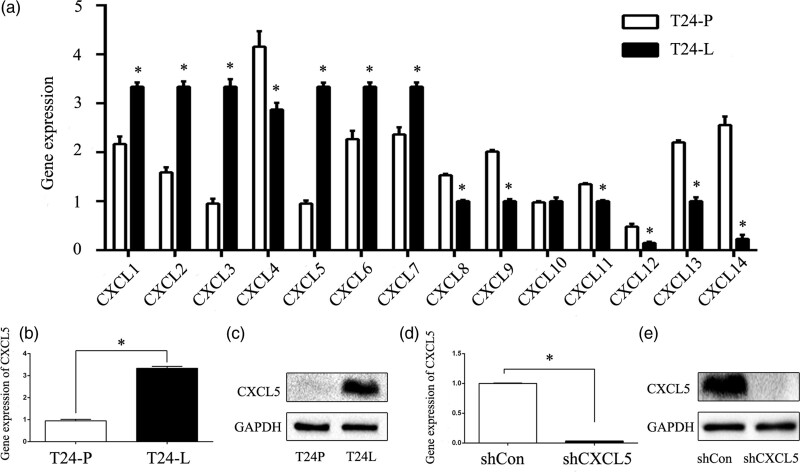
Our previous study demonstrated that T24-L cells exhibit higher invasiveness, metastasis and stemness than T24-P cells. In this study, we further used in vitro models of primary bladder carcinoma and pulmonary metastasis cancer to screen the CXC chemokine family by qRT-PCR (Fig. 1a). We found that the expression of CXCL5 in the T24-L cell line was higher than that in the T24-P cell line (Fig. 1b), and this finding was verified by Western blot (Fig. 1c). Lentivirus plasmids containing short hairpin RNA (shRNA) targeting CXCL5 (shCXCL5) and a negative control shRNA (shCon) were transfected into the T24-L cells, and the total RNA and protein were extracted after 72 h. The mRNA (Fig. 1d) and protein (Fig. 1e) expression of CXCL5 in the T24-L cell line was effectively inhibited after transfection, and the difference was statistically significant (P < 0.05), indicating that this model could be used for further studies.
Fig. 1.
High CXCL5 expression in T24-L cells and the knockdown efficiency of shCXCL5. (a) The gene expression of the CXC chemokine family members was screened in the T24-P and T24-L cell lines by qRT-PCR. *P < 0.05 versus T24-P cells; (b) quantitative RT-PCR analysis of the CXCL5 mRNA levels in T24-P and T24-L cells. *P < 0.05 versus T24-P cells; (c) western blot analysis of CXCL5 expression in T24-P and T24-L cells; GAPDH was used as the loading control; (d) quantitative RT-PCR analysis of the CXCL5 mRNA levels after knocking down CXCL5 expression in T24-L cells. *P < 0.05 versus shCon cells; (e) western blot analysis of CXCL5 expression in the shCon and shCXCL5 groups; GAPDH was used as the loading control.
Knockdown of CXCL5 induces MET and inhibits migration and invasion of T24-L cells
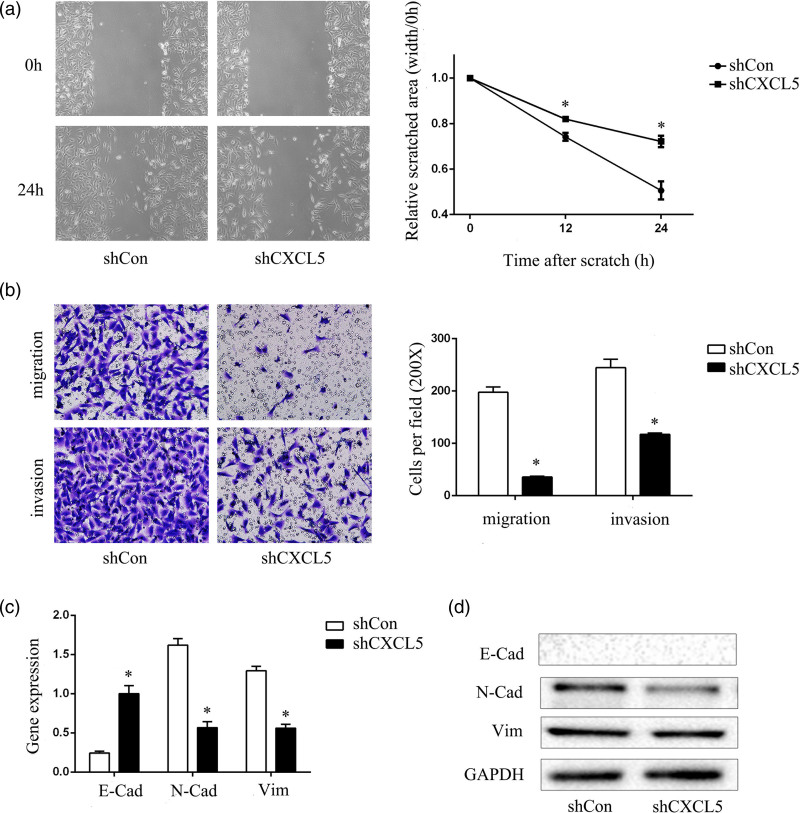
As previously shown, T24-L cells have stronger invasion and metastasis abilities than T24-P cells [14,15]. In this study, after the successful construction of the CXCL5 knockdown model in T24-L cells, we compared the migration and invasive abilities of the shCon group with those of the shCXCL5 group. The wound-healing assay suggested that the efficiency with which cells migrated into the wound area or protruded from the border of the wound significantly decreased after knockdown of CXCL5 (Fig. 2a), and the invasion and migration abilities also significantly decreased, as shown by the transwell migration and invasion assays (Fig. 2b). Moreover, because of CXCL5 knockdown, the reduced expression of mesenchymal markers (N-cadherin and Vimentin) in the T24-L cells was observed by Western blot (Fig. 2c,d). These results demonstrated that the knockdown of CXCL5 induced a transition to the epithelial phenotype and inhibited the migration and invasion abilities.
Fig. 2.
Knockdown of CXCL5 inhibits migration and invasion and induces MET in T24-L cells. (a) Wound-healing assay showed the healing ability of the shCon group and shCXCL5 group under 100× magnification after 24 h, and the quantitative results are shown on the right side, *P < 0.05 versus shCon cells; (b) migration and invasion of the shCon group and shCXCL5 group were evaluated under 200× magnification after 24 h, and the quantitative results are shown on the right side, *P < 0.05 versus shCon cells; (c) the E-cad, N-cad, and Vimentin mRNA levels were detected by quantitative RT-PCR, *P < 0.05 versus shCon cells; (d) the E-cad, N-cad and vimentin proteins were detected by Western blot, and GAPDH was used as the loading control, *P < 0.05 versus shCon cells. MET, mes-enchymal-epithelial transition.
Knockdown of CXCL5 suppresses cancer stem cell characteristics and downregulates CD44 expression in T24-L cells
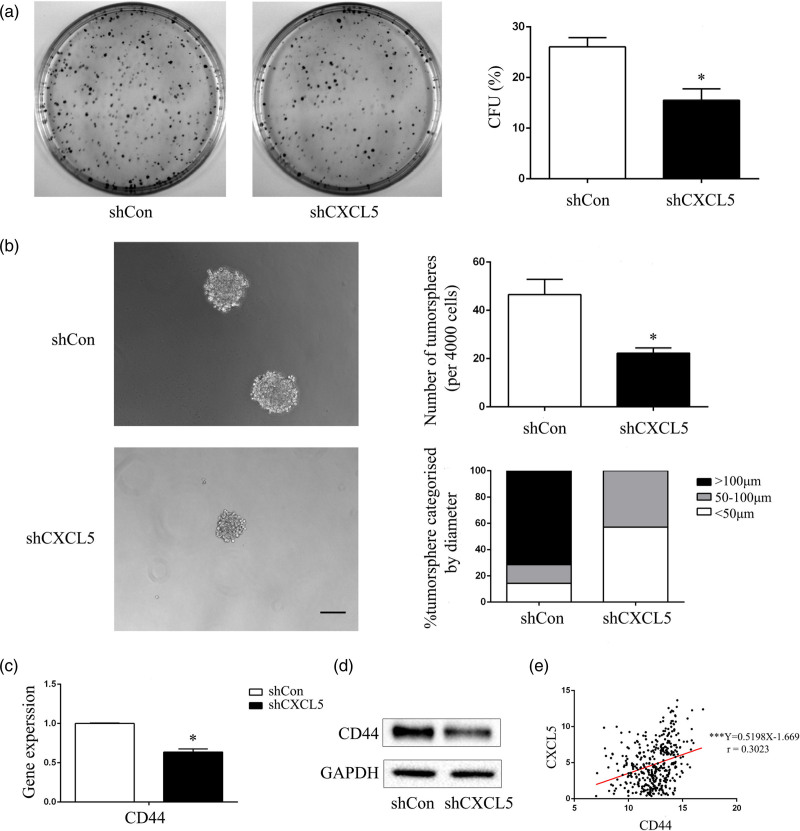
Compared with T24-P cells, T24-L cells exhibited stronger stemness and higher expression of the tumor stem cell factor CD44 in a previous study [16]. In this study, the shCon and shCXCL5 cells were cultured in serum-free stem cell medium. After 14 days, tumorsphere cells of 50–100 μm in diameter were observed. The self-renewal capacity of the cells was assessed by dispersing them into single cells and growing them at a density of 2000 cells/ml to form tumorspheres. After 14 days, the shCXCL5 group generated fewer tumorspheres with sizes larger than 100 μm in diameter. The tumorsphere formation rate of the shCXCL5 cells was significantly lower than that of the shCon cells. We also analyzed the size of the tumorspheres and found that the shCXCL5 cells generated tumorspheres of smaller sizes. The percentage of tumorspheres with a diameter larger than 100 μm was significantly lower in the shCXCL5 cells than in the shCon cells (Fig. 3b). qRT-PCR (Fig. 3c) and Western blot (Fig. 3d) assays showed that the CSC marker CD44 was downregulated after the knockdown of CXCL5. Interestingly, when analyzing the mRNA expression of CXCL5 and CD44 in 400 BLCA patients recorded in the TCGA database, we found that the expression of CXCL5 was positively associated with the expression of CD44 (r = 0.3023), and the difference was statistically significant (P < 0.001) (Fig. 3e). These results demonstrated that the expression of the chemokine CXCL5 was positively related to the expression of CD44 and further verified the conclusion that CXCL5 could increase the CSC properties of T24-L cells.
Fig. 3.
Knockdown of CXCL5 suppresses CSC characteristics and downregulates the expression of the CSC markers CD44 and p63 in T24-L cells. (a) Photographs of the colonies from shCon and shCXCL5 cells, and the colony-forming units rate (CFU%) of the shCon group and shCXCL5 group were calculated and are shown on the right side, *P < 0.05 versus shCon cells; (b) the tumorsphere formation ability of the shCon and shCXCL5 cells was evaluated under 200× magnification, Bar = 50 μm. The tumorsphere number and the percentage of tumorspheres with diameters <5, 50–100 or >100 μm were calculated and are shown on the right side. qRT-PCR (c) and Western blot (d) analysis were used to detect the mRNA and protein expression of CD44, *P < 0.05 versus shCon cells. (e) Correlation analysis of CXCL5 and CD44 mRNA expression in 400 BCa patients in the TCGA database; the correlation coefficient was 0.3023, ***P < 0.001. CSC, cancer stem cells.
The interaction of CXCL5 and its receptor, CXCR2, promotes invasion, migration and cancer stem cell characteristics of T24-L cells
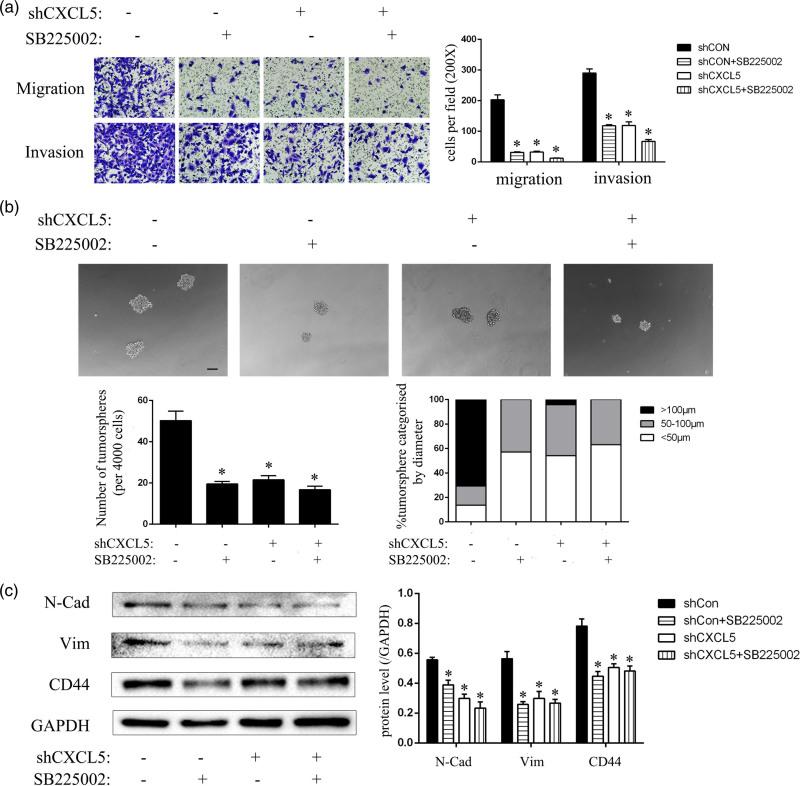
As CXCR2 is the receptor of the chemokine CXCL5, we hypothesized that CXCL5 may interact with CXCR2 to promote the invasive and migratory capabilities and cancer stem cell properties of T24-L cells; thus, we used the CXCR2 receptor antagonist SB225002 to inhibit the binding of CXCL5 and CXCR2. The results of the transwell assay showed that in both the migration and invasion assays, the number of cells on the underside of the inserts in the shCon group was significantly reduced after SB225002 treatment; however, the number of cells on the underside of the inserts in the shCXCL5 group did not obviously decrease after SB225002 treatment (Fig. 4a). Additionally, in the tumorsphere assay, the shCon with SB225005 group generated fewer tumorspheres with sizes larger than 100 μm in diameter. The tumorsphere formation rate of the cells was also significantly lower. The percentage of tumorspheres with a diameter larger than 100 μm was significantly lower after SB225002 treatment (Fig. 4b). In addition, we extracted the protein of the SB225002 treatment group and the solvent control group and detected the expression of N-Cadherin, Vimentin and CD44. In the shCon group, N-Cadherin, Vimentin and CD44 protein expression was significantly reduced after SB225002 treatment but showed no significant change in the shCXCL5 group after SB225002 treatment (Fig. 4c). These results demonstrated that CXCL5 combined with CXCR2 to promote the invasion and migration abilities and cancer stem cell properties of T24-L cells, but the inhibition of CXCR2 reversed this process.
Fig. 4.
The interaction of CXCL5 and its receptor, CXCR2, promotes invasion, migration and CSC characteristics of T24-L cells. (a) The migration and invasion abilities of the shCon and shCXCL5 groups treated with SB225002 or DMSO (as a negative control) were evaluated under 200× magnification. The quantitative results are shown on the right side, *P < 0.05 versus the control group. (b) The tumorsphere formation ability of the shCon and shCXCL5 groups treated with or without SB225002 and processed in advance were evaluated under 200× magnification. Bar = 50 μm. The tumorsphere number and the percentage of tumorspheres with diameters <50 μm, 50–100 μm, or >100 μm were calculated and are shown on the lower side, *P < 0.05 versus control group; (c) The N-cad, vimentin, CD44 proteins were detected by Western blot, and GAPDH was used as the loading control. The quantitative results are shown on the right side, *P < 0.05 versus control group. CSC, cancer stem cells.
CXCL5 and CD44 interact to regulate epithelial–mesenchymal transition and cancer stem cells characteristics in T24-L cells
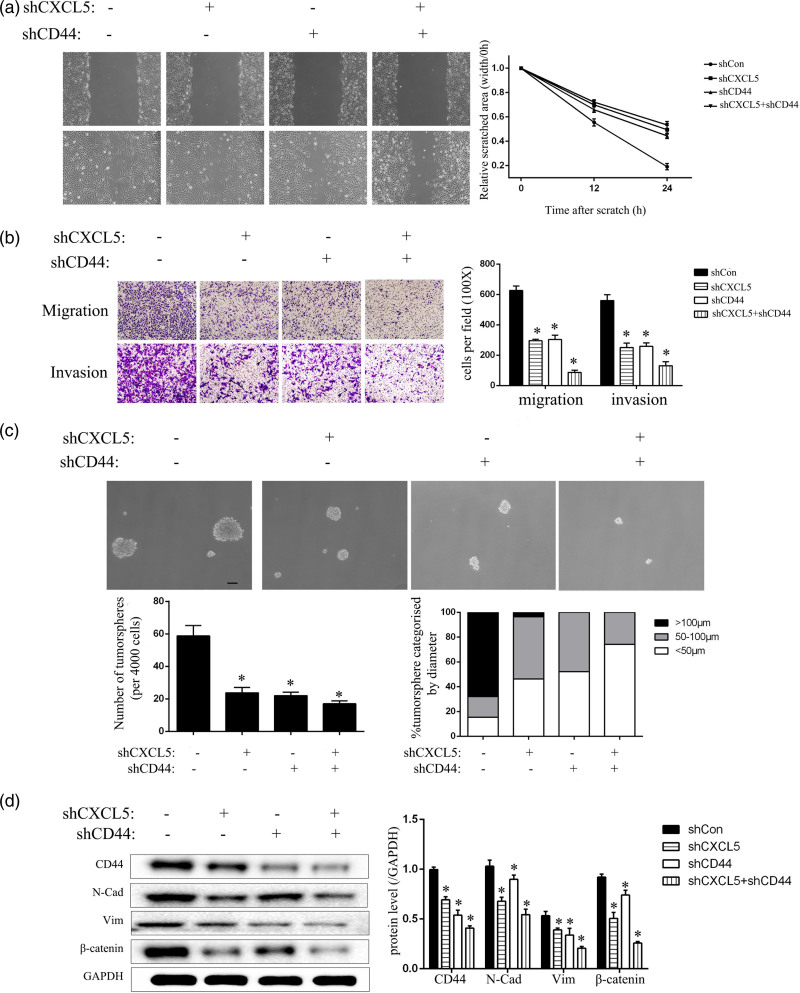
To investigate the interaction between CXCL5 and CD44, we further reduced the expression of CD44 by lentiviral transfection and then established shCD44 and shCXCL5-shCD44 models. Both the scratch test and the transwell assay showed that the invasion and migration abilities of the T24-L cells were decreased after CD44 expression was decreased (Fig. 5a,b). Similarly, the CSC characteristics of the cells were also decreased when the CXCL5 and CD44 levels were both reduced, and these cells formed the fewest number of tumorspheres with the smallest diameters (Fig. 5c). The detection of protein levels also verified this phenomenon (Fig. 5d).
Fig. 5.
CXCL5 and CD44 interact to regulate EMT and CSC characteristics of T24-L cells. (a) Wound-healing assay showed the healing ability after the knockdown of CXCL5 or CD44 or both under 100× magnification after 24 h, and the quantitative results are shown on the right side, *P < 0.05 versus shCon cells; (b) the migration and invasion abilities of each group were evaluated under 100× magnification. The quantitative results are shown on the right side, *P < 0.05 versus the shCon group; (c) the tumorsphere formation ability was evaluated in each group under 200× magnification. Bar = 50 μm. The tumorsphere number and the percentage of tumorspheres with diameters <50 μm, 50–100 μm or >100 μm were calculated and are shown on the lower side, *P < 0.05 versus the shCon group; (d) the CD44, N-cad, vimentin and β-catenin proteins were detected by Western blot, and GAPDH was used as the loading control. The quantitative results are shown on the right side, *P < 0.05 versus the shCon group. CSC, cancer stem cells; EMT, epithelial–mesenchymal transition.
Discussion
CXCL5, as a kind of CXC chemokine, has been shown to play an important role in tumor growth, invasion and metastasis after binding to its receptor, CXCR2. Classic research indicates that CXCL5 is highly expressed in a variety of cancers, contributes to EMT by binding to its receptor, and plays a crucial role in tumor invasion, metastasis and progression.
In recent years, the discovery of CSCs has provided new insights into the study of tumors. CSCs are a small fraction of tumor cells that have morphological markers and functional properties that are associated with normal stem cells and play a key role in tumorigenesis and progression [17]. However, unlike stem cells in nontumor tissues, non-CSCs in cancer tissues can spontaneously differentiate into CSCs and regenerate cancer potential [18]. Current research indicates that CSCs are closely related to EMT [19]. CD44, as a marker of CSCs, has been found to regulate the invasion and metastasis of hepatocellular carcinoma by affecting EMT [20]; activation of EMT programs in cancer cells can also lead to the transformation of non-CSCs into CSCs [11,21–22]. However, the relationship between chemokines and CSCs is rarely reported.
In a previous study, we established two bladder cancer cell lines, namely, T24-t-primary (T24-P) and T24-t-lung metastasis (T24-L). It was proven that T24-L cells showed higher invasive and migratory capabilities than T24-P cells in vitro and in vivo, and T24-L cells had stronger stemness and higher expression of the tumor stem cell factor CD44. In this study, to explore the relationship of chemokines with EMT and CSCs, we used qRT-PCR to screen the expression of CXC chemokines 1–14 in both cell lines and found that CXCL5 is highly expressed in T24-L cells. To elucidate the function of CXCL5, we inhibited its expression in T24-L cells by lentiviral transfection and found that the invasion and migration abilities were inhibited, the expression of N-Cadherin and Vimentin was decreased, and the CSC characteristics and CD44 expression were decreased. In addition, we also searched and analyzed the patients in the TCGA database and found that CXCL5 and CD44 showed a strong positive correlation in 400 cases of patient mRNA expression. This finding suggests that the chemokine CXCL5 has a strong relationship with CSCs and may be able to alter the ability of cancer cells to migrate and invade by regulating their gene expression. Moreover, the same phenomenon occurred after the inhibition of CXCR2, indicating that CXCL5 works after binding to its receptor, CXCR2. For further validation, we continued to establish T24-L cell lines with low expression of CD44 and dual inhibition of CXCL5 and CD44 by lentiviral transfection. As a result, when CD44 expression was knocked down, invasion and migration were inhibited. When both CXCL5 and CD44 were knocked down, the invasion and migration abilities and CSC characteristics were decreased. Thus, chemokines and CSCs affect the progression of cancer cells through interactions.
Zhou et al. [23] and Zhao et al.’s [24] research confirmed that the CXCL5/CXCR2 axis can enhance the level of p-AKT. According to the theory of Smith et al. [25] and Haria et al. [26], NF-κB can function upstream to regulate CD44 expression. Therefore, we hypothesized and confirmed that CXCL5/CXCR2 can regulate CD44 expression via the AKT/NF-κB pathway (Supplemental Fig. 1, Supplemental digital content 1, http://links.lww.com/ACD/A402). However, due to the complexity of the tumor microenvironment, we believe that this pathway may be only one of the pathways activated by the interaction between CXCL5 and CD44, and the deeper mechanism of action requires further confirmation. However, there is no doubt that CXCL5 has a great effect on the progression of cancer; thus, CXCL5 can be used as a new therapeutic target to eliminate CSCs in future research on targeted cancer therapy.
Acknowledgements
We acknowledge and appreciate our colleagues for their valuable efforts and comments on this paper.
The manuscript has been approved by all authors for publication.
This work supported by the National Natural Science Foundation of China (No: 81201134 and 81572520).
M.Z. Z., W.Y. W. and H. Z. X performed the experiments. L. W. analyzed and interpreted the data. W. Y. W. and Y. Y. Y wrote the manuscript. S. Y. L. and J. H. F. provided critical suggestions. J. H. F. revised the manuscript critically for important intellectual content and designed and supervised the project. All authors discussed the results and commented on the manuscript.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Weiyi Wang and Mengzhao Zhang contributed equally to the writing of this article.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.anti-cancerdrugs.com.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Rollins BJ. Inflammatory chemokines in cancer growth and progression. Eur J Cancer 2006; 42:760–767. [DOI] [PubMed] [Google Scholar]
- 3.Kakinuma T, Hwang ST. Chemokines, chemokine receptors, and cancer metastasis. J Leukoc Biol 2006; 79:639–651. [DOI] [PubMed] [Google Scholar]
- 4.Kuo PL, Chen YH, Chen TC, Shen KH, Hsu YL. CXCL5/ENA78 increased cell migration and epithelial-to-mesenchymal transition of hormone-independent prostate cancer by early growth response-1/snail signaling pathway. J Cell Physiol 2011; 226:1224–1231. [DOI] [PubMed] [Google Scholar]
- 5.Miyazaki H, Patel V, Wang H, Edmunds RK, Gutkind JS, Yeudall WA. Down-regulation of CXCL5 inhibits squamous carcinogenesis. Cancer Res 2006; 66:4279–4284. [DOI] [PubMed] [Google Scholar]
- 6.Kawamura M, Toiyama Y, Tanaka K, Saigusa S, Okugawa Y, Hiro J, et al. CXCL5, a promoter of cell proliferation, migration and invasion, is a novel serum prognostic marker in patients with colorectal cancer. Eur J Cancer 2012; 48:2244–2251. [DOI] [PubMed] [Google Scholar]
- 7.Zhou SL, Dai Z, Zhou ZJ, Wang XY, Yang GH, Wang Z, et al. Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology 2012; 56:2242–2254. [DOI] [PubMed] [Google Scholar]
- 8.Gao Y, Guan Z, Chen J, Xie H, Yang Z, Fan J, et al. CXCL5/CXCR2 axis promotes bladder cancer cell migration and invasion by activating PI3K/AKT-induced upregulation of MMP2/MMP9. Int J Oncol 2015; 47:690–700. [DOI] [PubMed] [Google Scholar]
- 9.Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 2011; 481:85-89. [DOI] [PubMed] [Google Scholar]
- 10.Tomaskovic-Crook E, Thompson EW, Thiery JP. Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res 2009; 11:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133:704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao Y, Ruan B, Liu W, Wang J, Yang X, Zhang Z, et al. Knockdown of CD44 inhibits the invasion and metastasis of hepatocellular carcinoma both in vitro and in vivo by reversing epithelial-mesenchymal transition. Oncotarget 2015; 6:7828–7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karam JA, Huang S, Fan J, Stanfield J, Schultz RA, Pong RC, et al. Upregulation of TRAG3 gene in urothelial carcinoma of the bladder. Int J Cancer 2011; 128:2823–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang T, Fan J, Wu K, Zeng J, Sun K, Guan Z, et al. Roles of HIF-1α in a novel optical orthotopic spontaneous metastatic bladder cancer animal model. Urol Oncol 2012; 30:928–935. [DOI] [PubMed] [Google Scholar]
- 15.Wu K, Zeng J, Zhou J, Fan J, Chen Y, Wang Z, et al. Slug contributes to cadherin switch and malignant progression in muscle-invasive bladder cancer development. Urol Oncol 2013; 31:1751–1760. [DOI] [PubMed] [Google Scholar]
- 16.Wu K, Ning Z, Zeng J, Fan J, Zhou J, Zhang T, et al. Silibinin inhibits β-catenin/ZEB1 signaling and suppresses bladder cancer metastasis via dual-blocking epithelial-mesenchymal transition and stemness. Cell Signal 2013; 25:2625–2633. [DOI] [PubMed] [Google Scholar]
- 17.Hemmings C. The elaboration of a critical framework for understanding cancer: the cancer stem cell hypothesis. Pathology 2010; 42:105–112. [DOI] [PubMed] [Google Scholar]
- 18.Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol 2017; 14:611–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat Med 2009; 15:1010–1012. [DOI] [PubMed] [Google Scholar]
- 20.Jayachandran A, Dhungel B, Steel JC. Epithelial-to-mesenchymal plasticity of cancer stem cells: therapeutic targets in hepatocellular carcinoma. J Hematol Oncol 2016; 9:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells - what challenges do they pose? Nat Rev Drug Discov 2014; 13:497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morel AP, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One 2008; 3:e2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou SL, Zhou ZJ, Hu ZQ, Li X, Huang XW, Wang Z, et al. CXCR2/CXCL5 axis contributes to epithelial-mesenchymal transition of HCC cells through activating PI3K/Akt/GSK-3β/Snail signaling. Cancer Lett 2015; 358:124–135. [DOI] [PubMed] [Google Scholar]
- 24.Zhao JK, Ou BC, Han DP, Wang PX, Zong YP, Zhu CC, et al. Tumor-derived CXCL5 promotes human colorectal cancer metastasis through activation of the ERK/Elk-1/Snail and AKT/GSK3 beta/beta-catenin pathways. Mol Cancer 2017; 16:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haria D, Trinh BQ, Ko SY, Barengo N, Liu JS, Naora H. The homeoprotein DLX4 stimulates NF-kappa B activation and CD44-mediated tumor-mesothelial cell interactions in ovarian cancer. Am J Pathol 2015; 185:2298–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith SM, Lyu YL, Cai L. NF-κB affects proliferation and invasiveness of breast cancer cells by regulating CD44 expression. PLoS One 2014; 9:e106966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.