Abstract
The development of interdisciplinary biomedical engineering brings significant breakthroughs to the field of cartilage regeneration. However, cartilage defects are considerably more complicated in clinical conditions, especially when injuries occur at specific sites (e.g., osteochondral tissue, growth plate, and weight-bearing area) or under inflammatory microenvironments (e.g., osteoarthritis and rheumatoid arthritis). Therapeutic implantations, including advanced scaffolds, developed growth factors, and various cells alone or in combination currently used to treat cartilage lesions, address cartilage regeneration under abnormal conditions. This review summarizes the strategies for cartilage regeneration at particular sites and pathological microenvironment regulation and discusses the challenges and opportunities for clinical transformation.
Keywords: Cartilage lesion, Pathological microenvironment, Synergistic effect, Tissue engineering, Cartilage regeneration
Graphical abstract
Highlights
-
•
Tissue engineering for cartilage regeneration in particular sites and pathological microenvironments are discrepant.
-
•
The scaffolds, cells, and cytokines are selected referencing to the specific characteristics of injured cartilage.
-
•
Immunomodulating agents rehabilitate proper microenvironments for regeneration of cartilage under inflammatory conditions.
-
•
Advanced technologies with bionic principles exhibit promising prospects for instructive cartilage regeneration.
1. Introduction
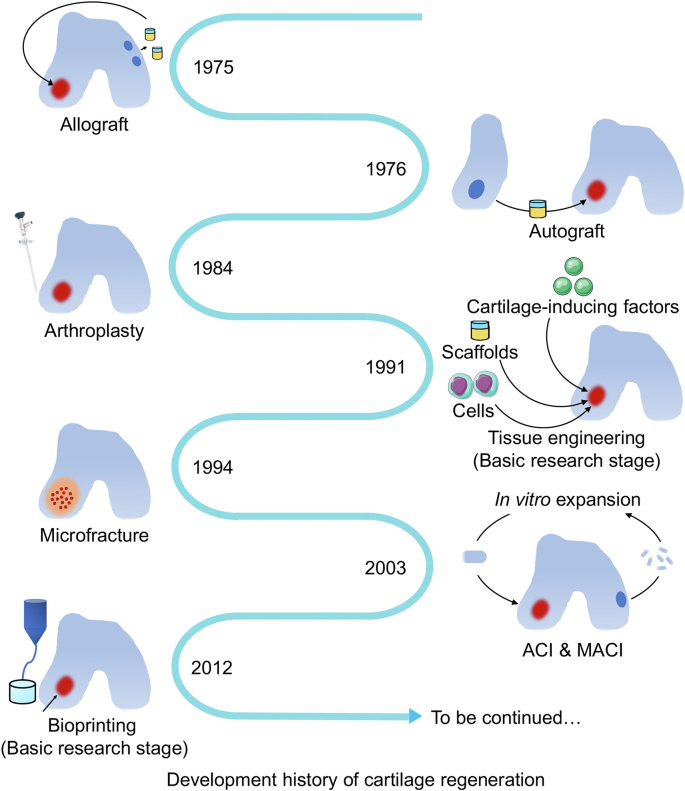
Cartilage damages caused by acute trauma or chronic inflammation represent most frequently joint lesions, resulting in pain, limited mobility, or even limb deformity and disability [1]. The regeneration of articular cartilage remains a significant challenge to date due to its avascular characteristics, making it difficult for nutrients and regenerative stimuli to penetrate the injured area. Moreover, the metabolic activity of chondrocytes is relatively low, rendering it challenging to produce sufficient cartilage matrices to repair the damaged cartilage [2]. Considering the complexity of cartilage regeneration, a series of techniques have been developed (Fig. 1) [3]. Initially, autologous and allogeneic transplantation of cartilage introduced by arthroscopy and joint surface debridement were used for cartilage repair to restore joint morphology while failing to eliminate etiology [[4], [5], [6]]. Subsequently, cell therapy was demonstrated to be beneficial for cartilage regeneration. Microfracture releases stem cells from the marrow blood into the defect area to repair cartilage [7,8]. Furthermore, autologous chondrocyte implantation (ACI) and matrix-induced ACI have become two mainstream clinical cell-based methods, currently regarded as the gold standards in treating sizable articular cartilage defects (≤12 cm2) [[9], [10], [11], [12]]. However, the shortcomings of chondrocyte implantation are also apparent, including the invasiveness of the operation and the relatively long period of chondrocyte expansion before implantation. Therefore, cartilage tissue engineering was employed to construct artificial tissue to fill defects by combining scaffolds, seeding cells, and producing cartilage-inducing factors [13].
Fig. 1.
Development history of articular cartilage repair. Currently used modalities for cartilage repair in clinic include osteochondral allograft transplantation, microfracture, and autologous chondrocyte implantation. The strategy choice for cartilage repair depended on the size and type of the cartilage defect, as well as the age and activity level of patients. Nowadays, the development of tissue engineering has performed promising results in cartilage tissue regeneration.
To this end, numerous unique materials, manufacturing crafts, various sources of cells, and bioactive factors are involved in tissue engineering. A large majority of related studies are still at the stage of primary or preclinical study. Among the techniques established, three-dimensional (3D) printing as an emerging method for scaffold preparation satisfies the need for molding precise shapes for cartilage defects by adjusting the spatial arrangement of printed bioink [14]. Although significant progress has been achieved in treating cartilage defects, meeting the diverse demands of cartilage regeneration remains challenging. The natural morphologies and functions of cartilage are challenging to restore perfectly within current clinical therapeutic methods. Further, various molecular biological factors are involved in cartilage regeneration, whereas most current studies merely focus on gross appearances, ignoring investigating and solving deep-rooted reasons for the diseases.
Different pathological mechanisms induce various cartilage damages. Excessive violence and mechanical loading may cause full-layered articular or osteochondral defects [15,16]. Generally, the weight-bearing area is more commonly affected due to long-term mechanical loading [17]. Moreover, if the cartilage injury occurs at the end of the long bone in the early developmental stage of children, the growth plate may be damaged, which impedes the extension of long bones [18]. Cartilage defects are always accompanied by inflammatory conditions, such as osteoarthritis (OA) and rheumatoid arthritis (RA). The pathological mechanisms of arthritis strain joint instability cause over-expressed inflammatory factors and subsequently lead to cartilage degradation and deterioration [[19], [20], [21], [22]]. These pathological states always happen concurrently, such as co-occurrence of osteochondral defects and OA. However, the pathogenesis of these conditions is independent. Hence, it is essential to realize their etiopathogenesis and pathological features to screen optimal individual therapeutic selection.
Different therapeutic strategies are proposed to deal with various articular cartilage defect situations. For example, defects of full-layered cartilage and in the weight-bearing area require scaffolds with specific structural and mechanical properties to recover articular morphology and withstand loading [23,24]. A biomimetic microenvironment favorable for long bone growth must be provided in growth plate repair to avoid bony bridge formation and prevent angulation deformation [25].
The treatment of cartilage defects under inflammatory conditions differs markedly from that of mechanical injuries. Unregulated cytokine expression and immune disorder during inflammatory processes significantly change the microenvironments of joints, leading to undesirable consequences. For OA, the treatment strategy focuses on alleviating cartilage degradation and cartilage matrix decomposition by restraining relevant cartilage-digesting enzymes [26,27]. However, the treatment of RA mainly relies on the immune regulation of the microenvironment to prevent the invasion of inflammatory factors into cartilage tissue [28,29].
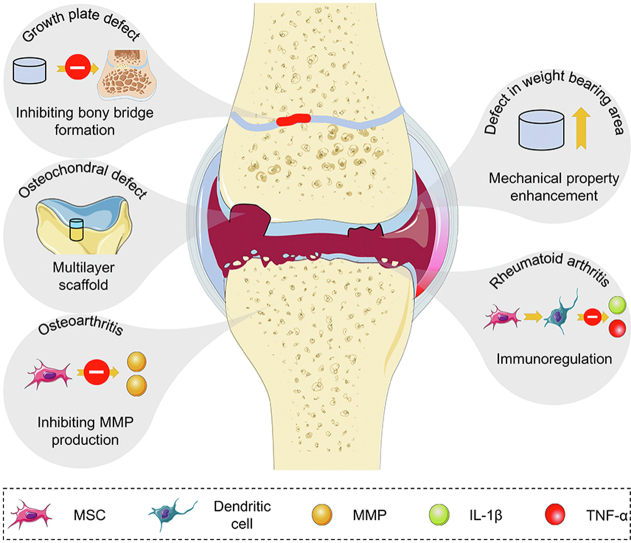
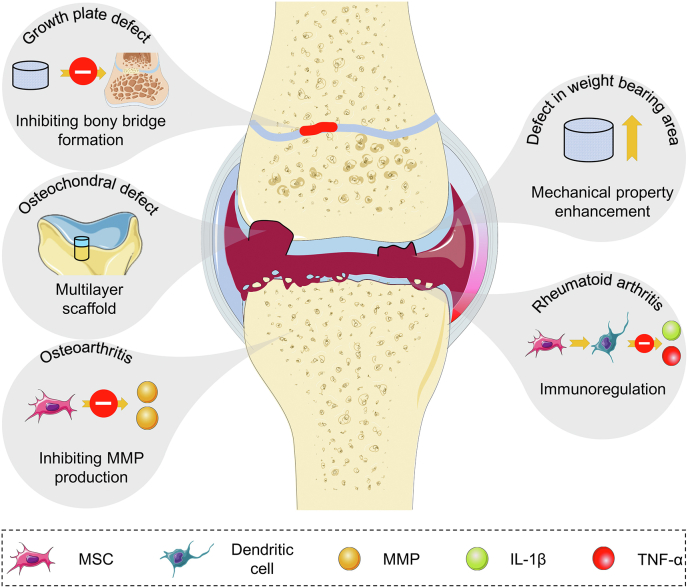
In this review, we discuss different cartilage repair strategies in various pathological conditions. Moreover, we emphasize the importance of microenvironments in the repair process and present the challenges and future directions of cartilage repair approaches (Scheme 1).
Scheme 1.
Therapeutic strategies for cartilage damage under abnormal conditions, such as cartilage regeneration in osteochondral defect, growth plate defect, weight-bearing area defect, and OA and RA conditions. During this process, scaffolds play roles in regenerating multi-layered defects, bearing compression, and hindering heterotopic ossification during growth plate regeneration. Meanwhile, MSCs differentiate into chondrocytes as well as osteoblasts to repair tissue defects and regulate abnormal inflammatory conditions of OA and RA.
2. Structures, components, and functions of cartilage
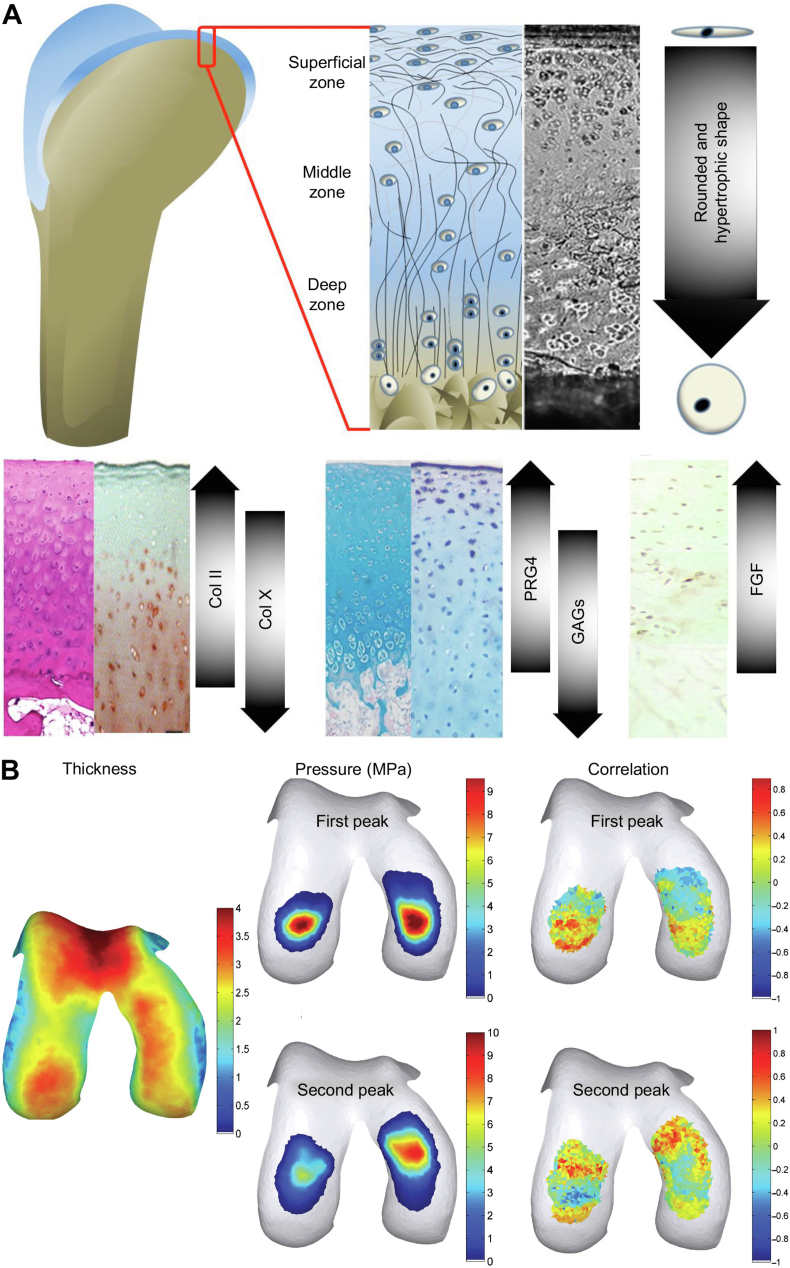
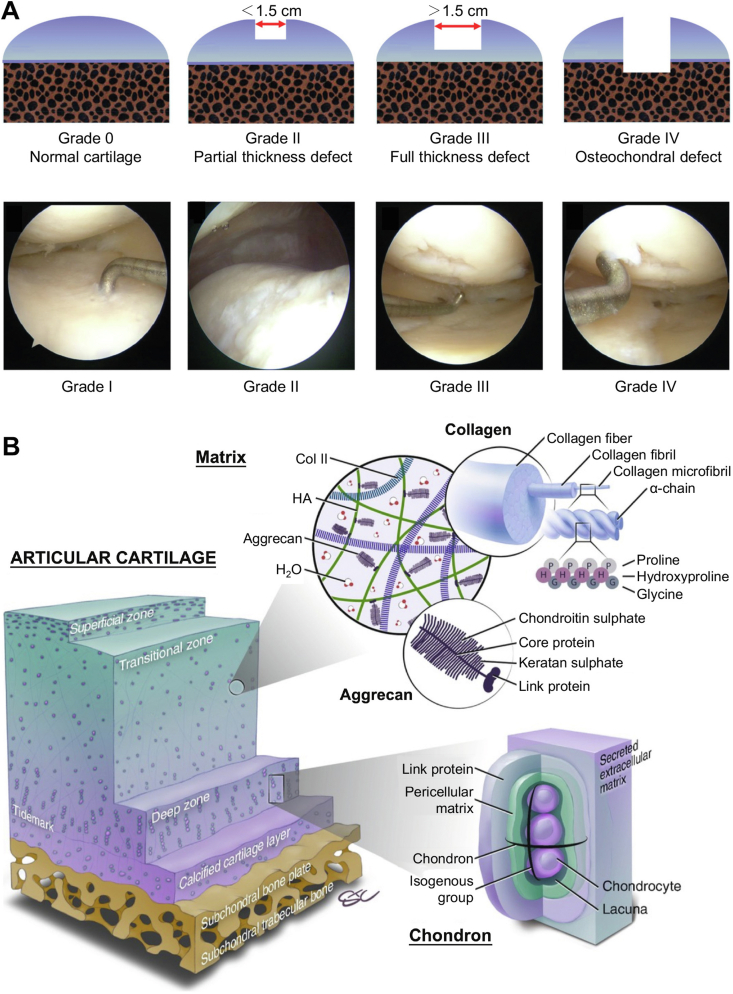
Articular cartilage is a smooth elastic tissue covered with hyaline cartilage on the joint surface, acting as a “bearing" in conjunction with adjacent bones to reduce the friction and impact during physical movement [30]. Articular cartilage tissue has a top-to-bottom four-layered structure, including superficial, transitional, radial, and calcified cartilage zones (Fig. 2A) [[31], [32], [33]]. The morphology of chondrocytes and components in the cartilage matrix differs across various zones. Chondrocytes in the superficial zone are immature, exhibiting an oblate shape, and are individually distributed at the surface of the cartilage. In the deeper zones, chondrocytes become relatively round, hypertrophic, and gather in clusters. In contrast, the calcified cartilage zone contains only a small number of calcified cells [34].
Fig. 2.
Anatomical characteristics of articular cartilage in normal knee [33,39]. (A) Gradient structure and material components in articular cartilage. (B) Average thickness of cartilage, pressure distribution, and correlation of two factors in loading condition of human knee. The map showed the thickness of the local cartilage was correlated with its local pressure. Reproduced with permission [33,39]. Copyright 2015, Mary Ann Liebert, Inc; Copyright 2017, Public Library of Science.
The physiological characteristics of cartilage tissue are caused by the highly hydrated extracellular matrix (ECM) surrounding the chondrocytes. The major components of cartilage ECM include collagen (Col), hyaluronan (HA), proteoglycans (PRGs), other minor proteins, and water. Water constitutes 75% of the weight in cartilage tissue, and Col, mainly type II, IX, and XI Col, comprises approximately 40% of dry cartilage weight, forming a complex network for resisting shear forces. The superficial layer of cartilage contains large amounts of Col II, PRG 4, and fibroblast growth factor (FGF) [35]. Fibers in cartilage are relatively thin and parallel to the articular surface, forming a smooth interface to assist in the transformation of nutrients from the perichondrium.
The composition in cartilage is also varied according to the cartilage position. The contents of Col II, PRG4, and FGF declined with the deepening sites of cartilage, and the reverse happened to the content of Col X and glycosaminoglycan (GAG) [36,37]. A thin layer (2–5 μm thickness) termed “tidemark”, composed of Col fiber and apatite, is observed at the bottom of the radial zone, signifying the radial boundary calcified cartilage zones. The tidemark buffers the impact forces and protects deeper tissue from injuries. The transitional and radial zones are abundant in Col X and GAG. The Col fibers in the tangent plane of articular surface provide support and balance the tensile strength from outside mechanics [38].
Articular cartilage plays a crucial role in lubrication and resistance to compression during joint movement. Under physical loading conditions, the cartilage in the knee joint can bear 2–6 folds the body weight with forces mainly concentrated on the femoral condyle and suffers >10,000 times of friction per day [39]. In the superficial zone, compact Col fibers are parallel to the articular surface and possess a highly elastic modulus (>10 MPa) to maintain the shape without deformation in cartilage tissue. The randomly arranged Col fibers in the middle zone are composed of a complex network responsible for the vertical stress bearing [40]. Furthermore, Col fibers in the deep zone are oriented perpendicular to the surface of the subchondral bone, acting as a transition region between the cartilage and bone for resisting outside mechanics. This construction generates a strong juncture between interfaces, protecting cartilage from injuries after suffering high-energy impact [41]. The interstitial fluid can moreover disperse the force acting on the cartilage in the cartilage matrix. When the cartilage is under pressure, the liquid flow in the cartilage matrix disperses the compression within [42].
The anatomical structure of joints is likewise essential for the transition of forces during the loading process. The shape of a couple of articular surfaces in a joint is morphologically well fitted. Together with the affiliated structures (e.g., meniscus and synovial folds), this specific shape makes the joint integrate the two surfaces. When forces are transiting through the joint, the articular surface ultimately transmits the force and decreases the risk of articular cartilage damage caused by stress concentration [43].
Lubrication is another significant function in articular cartilage that guarantees the movement of joints. The kinetic coefficient of friction in joints is less than 0.005. Sufficient lubrication of synovial fluid and glycoprotein substances can efficiently decrease the degree of wear in cartilage. The synovial fluid filling the joint cavity contains abundant hyaluronic acid, such that the cavity is in a viscoelastic semi-fluid state that significantly reduces friction on the cartilage surface. Furthermore, in the metabolic process of cartilage, a thin layer of glycoprotein biomolecules secreted by chondrocytes prevents cartilage from wear by covering the articular surface with a protective “boundary” [44].
3. Articular cartilage regeneration under abnormal conditions
3.1. Full-layered osteochondral regeneration
Various pathological cartilage conditions, such as cartilage degradation, trauma, OA, and osteochondritis dissecans, give rise to severe cartilage damage. The extent of the full-layered cartilage lesion may even compromise the subchondral bone. The osteochondral defects are classified by the International Cartilage Repair Society (ICRS) on a scale of grades 0–4 according to the defect depth and subchondral bone lesion. Surgical management is required for grade 3–4 defects to reconstruct the full-layered osteochondral structure for the sake of relieving symptoms, such as pain, swelling, and stiffness, and protection from secondary arthritis (Fig. 3A) [[45], [46], [47]]. In contrast to a mere cartilage injury, osteochondral regeneration strategies must recover both zonal articular cartilage and the subchondral bone, restoring structural, biomechanical, and biochemical features consistent with native osteochondral tissue (Fig. 3B) [48]. Accordingly, tissue engineering strategies are employed in osteochondral regeneration, with the three factors, i.e., scaffolds, cells, and bioactive factors, corresponding to the demands of structural reconstruction and tissue activity remodeling.
Fig. 3.
Osteochondral structure and ICRS classification system of osteochondral defects [45,48]. (A) Schematic and arthroscopic view of ICRS classification system addressing osteochondral defects. (B) Extracellular layered structure of osteochondral units and main components in cartilage matrix. Reproduced with permission [45,48]. Copyright 2020, John Wiley & Sons Inc.; Copyright 2021, KeAi Communications Co. Ltd.
3.1.1. Multilayered scaffolds for reconstruction of layered structure of osteochondral defects
Initially, scaffold-free strategies were developed by injecting stem cells, N-acetyl-d-glucosamine, and growth factors into the cartilage defect area. Although they hold advantages, such as low cost and short operation time, scaffold-free strategies rarely yield satisfying results due to the failure to restore cartilage smoothness and integrity, such that the rough articular surface would consistently injure cartilage tissue [[49], [50], [51]]. The defect depth was considered as the leading cause of unfavorable outcomes, as injected substances could hardly penetrate the injured area to yield any therapeutic effect. Therefore, researchers developed scaffold-based methods to provide a substrate to guide therapeutic factors into deep sites.
Monophasic substances, referring to materials of homogeneous composition and structure, were selected as scaffolds for osteochondral defect implantation. The thermo-sensitive poly(lactic-co-glycolic acid)−poly(ethylene glycol)−poly(lactic-co-glycolic acid) (PLGA−PEG−PLGA) hydrogel and poly(lactic acid)/poly(glycolic acid) (PLA/PGA) scaffolds were successively developed. Various stem cells were incorporated into the scaffolds to promote cartilage regeneration [52,53]. These scaffolds constructed an appropriate niche to promote chondrogenesis differentiation, and the metabolic labeling results showed an increasing cartilage-specific matrix deposited around seeding cells [54]. Liu et al. found that a full-layered cartilage structure could be recovered by adjusting the components in a cell-loaded hydrogel [55]. The implantation of bone marrow-mesenchymal stem cell (BM-MSC)-seeded poly(l-alanine-co-l-phenylalanine)-block-poly(ethylene glycol)-block-poly(l-alanine-co-l-phenylalanine) (PAF−PEG−PAF) thermo-sensitive hydrogel led to the regeneration of hyaline-like cartilage and simultaneously reduced fibrous tissue formation in rabbit osteochondral defects. Hence, it was concluded that applying biomimetic synthetic materials that accurately imitated the ECM of natural cartilage would be helpful for the regeneration of high-quality cartilage tissue.
Based on this theory, an anisotropy scaffold mimicking the native cartilage zonal structure was developed. Using a temperature gradient-guided thermal-induced phase separation technique, the cartilage ECM powder was gradually deposited onto a sliced bovine cartilage scaffold [56]. The compressive modulus of this oriented scaffold was superior to those of random scaffolds. The BM-MSC-incorporated scaffold was implanted into the goat articular cartilage defect for 24 weeks. The consequently repaired cartilage surface was flat and consisted of hyaloid cartilage tissue [57].
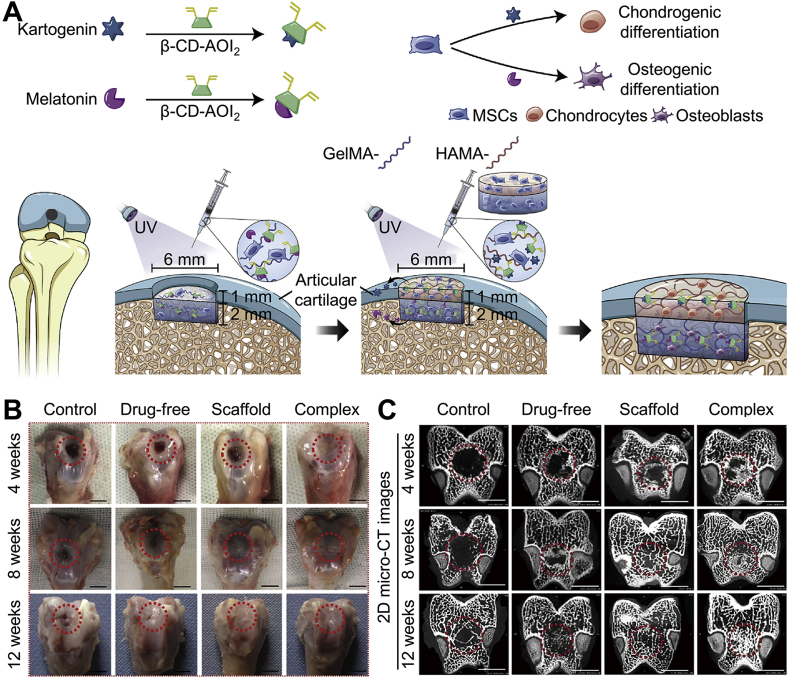
The repair of osteochondral defects is a complex process requiring the reconstruction of the subchondral bone and cartilage layers. Monophasic scaffolds are incompetent to reconstruct cartilage-bone units. Accordingly, a bi-layered scaffold strategy was employed to solve this problem, mimicking natural cartilage and subchondral bone structures. Col II, gelatin, and various hydrogels are primarily used to construct the upper layer of bi-layered scaffold. The porous structures of these materials are similar to the native cartilage ECM. Hydroxyapatite (HA) and Col I are two materials generally used for constructing the lower layer of bi-layered scaffold, which can induce subchondral bone regeneration [[58], [59], [60], [61]]. Furthermore, osteogenic and chondrogenic differentiation inducers, small molecules, and tissue-specific peptides were added into the bone and cartilage phase of a bi-layered hydrogel scaffold to further induce biphasic differentiation in osteochondral defect repairing (Fig. 4) [62,63].
Fig. 4.
Osteochondral defects repaired by bi-layered scaffold loading dual-phase inducers [62]. (A) Scheme of a biphasic hydrogel with controllable release of stem cell differentiation inducer molecules, kartogenin for cartilage repair, and melatonin for bone regeneration for simultaneous repair of osteochondral defect. (B) Gross images of articular specimens were obtained in week 4, 8, and 12. The scale bar is 5 mm. (C) 2D micro-CT images of specimens in week 4, 8, and 12. Scale bar is 5 mm. Reproduced with permission [62]. Copyright 2020, Elsevier B.V.
To improve the integrity of scaffolds with the surrounding tissue, Wu et al. created a photocurable methacrylate silk fibroin to seal the gap between the bi-layered silk scaffold and adjacent cartilage. Under the high bioactivity of this sealing hydrogel, native BM-MSCs migrated to scaffold-native tissue interface to promote cartilage regeneration [64]. Recently, several bi-layered scaffolds, including Agili-C™, BioMatrix CRD®, TruFit® Plug, OsseoFit® Plug, and so forth, for the osteochondral defect treatment have been on the stage for clinical trials or approved for clinical application [46]. However, bi-layered scaffolds also have limitations that may result in abnormal differentiation of stem cells, leading to uncontrollable heterotopic ossification in cartilage tissue and impairment of the function of regenerated cartilage tissue. Remarkably, studies found a calcified layer between the cartilage and subchondral bone, forming a natural barrier to separate these two issues. Accordingly, a sandwich-like tri-layered scaffold was developed. In comparison with the bi-layered structure, the tri-layered scaffold contains a biomimetic calcified layer. They can separate the cartilage and bone layer in regenerated osteochondral tissue to prevent undesired ossification in the cartilage layer [57,65].
Based on the multiphasic scaffold theory, Sun et al. created a gradient-structured scaffold, mimicking a four-layered osteochondral construct for anisotropic osteochondral regeneration. Porous poly(ε-caprolactone) (PCL) scaffolds with a gradient pore size (750, 550, 350, and 150 μm) from bottom to top were fabricated via 3D bioprinting. The gradient structure enabled BM-MSCs to present a series of gradient forms from hypertrophy to shrinkage, similar to the arrangement of chondrocytes in native cartilage tissue. BM-MSCs residing in four different layers showed directional cartilage matrix secretion, verifying that the mechanical stimulation given by pore size change would achieve anisotropic osteochondral regeneration [66]. Similarly, Qiao et al. designed a bioprinting three-layered scaffold by altering the intersection angle and spacing of stent in each layer to imitate the mechanical gradient of natural osteochondral units [67].
3.1.2. Adipose-derived mesenchymal cells are preferred for full-layered osteochondral regeneration
It is noteworthy that seed cells are a decisive factor in cartilage regeneration, and chondrocytes as in situ cells are the preliminary option. A study demonstrated that more functional cartilaginous tissues were regenerated following the implantation of chondrocytes [68]. However, the clinical application of chondrocytes is hindered by several limitations: The harvesting process of chondrocytes is complex, and their extraction rate is relatively low. Moreover, a propensity for chondrocyte dedifferentiation, as well as a dramatic reduction in cartilage-related matrix secretion, was also observed when chondrocytes were cultured in vitro [69,70]. Subsequently, stem cells were used as an alternative to chondrocytes because of their clonogenicity, self-renewal, and controllable differentiation capability [71].
Specifically, mesenchymal stem cells (MSCs) are the most commonly used stem cell type in tissue engineering. BM-MSCs and adipose-derived mesenchymal cells (AD-MSCs) are the two main cell lineages used in cartilage repair. Numerous studies verified the therapeutic potential of BM-MSCs and AD-MSCs in the regeneration of full-layered cartilage defects. However, there is still no consensus on which cell type is more effective for cartilage repair. Zhou et al. demonstrated that AD-MSCs showed lower transcriptome homogeneity and higher immunosuppressive capacity than BM-MSCs. After analyzing the clinical trials of OA treatment with MSCs, the result showed that the therapeutic effect of AD-MSCs was more stable than that of BM-MSCs. Furthermore, a comparison of the metabolic profiles of both cell types revealed that AD-MSCs had a superior capability to maintain stemness [72]. Despite all this, further studies are still warranted to guide a more precise clinical application of MSCs in full-layered cartilage regeneration.
The recruitment of MSCs to the injury sites to repair cartilage defects is another feasible strategy. Nevertheless, only a small percentage of cells can reach the target tissue following systemic administration. Therefore, modifying BM-MSC-specific affinity peptides on the demineralized bone matrix to recruit MSCs derived from subchondral bone may also serve as a strategy to repair cartilage defects in vivo [73,74].
3.1.3. Bioactive factors are required to facilitate both cartilage and bone layer
Bioactive factors are crucial in treating osteochondral defects. Growth factors are among the most frequently used chondrogenic differentiation factors in cartilage regeneration, and their effectiveness in treating osteochondral defects has been extensively verified [75]. Furthermore, unique chondrogenic−inducing factors, such as small molecule drugs and peptides, have been shown to have a higher chondrogenic capability [[76], [77], [78]]. Moreover, trophic factors derived from MSCs paracrine also facilitate cartilage tissue regeneration [79].
Bioactive factors promoting osteochondral regeneration are required to facilitate cartilage and bone layer repair simultaneously. Relative researchers identified that transforming growth factors (TGFs), bone morphogenetic proteins (BMPs), and insulin-like growth factors (IGFs) all play roles in osteochondral regeneration by improving cell arrangement and PRG deposition in the cartilage layer, as well as inducing new bone formation in subchondral bone layer [80].
The discovery of exosome, a small vesicle (diameter: 40–100 μm) secreted from cells, is potentially the critical mediator for cartilage repair in MSC therapies [81]. Zhang et al. injected MSC exosome into the joint cavity to treat osteochondral defect, achieving satisfactory regenerative efficacy [82]. In their subsequent study, exosome secreted by MSCs exhibited multiple effects, i.e., promotion of cell proliferation, cartilage matrix deposition, and immunoregulation, in osteochondral defect repair, and the conclusion was drawn that exosome was superior to the implantation of stem cells, e.g., cell-free, ready-to-use, and more amenable for administration [83]. In another study, the combination of exosome therapy and 3D printing was applied to repair the osteochondral defect. Columnar cartilage ECM/GelMA/exosome scaffold was cast via stereolithographic 3D printing. The released exosome was demonstrated to facilitate osteochondral defect regeneration by inducing chondrocyte migration and enhancing chondrocyte mitochondrial content [84].
Various physiological states influence cartilage formation and enhance the repair outcomes of osteochondral defects. Hypoxia was reported as being indispensable for promoting the chondrogenesis of MSCs. The hypoxic microenvironment of joint cavity and hypoxia-inducible factors, such as hypoxia-inducible factor-1 α (HIF-1α), play a crucial role in inducing chondrogenesis of MSCs [85]. HIF facilitated chondrogenesis by upregulating Sox9 expression, the mechanism of which mainly involves its inhibition of peroxisome proliferator-activated receptor γ2 promoter through the activation of PI3K/Akt/FoxO pathway to suppress adipogenesis [86]. O'Reilly et al. created a computational model to assess crucial parameters in the repair process of osteochondral defects, such as oxygen diffusion coefficient, oxygen tension, and oxygen limit [87]. This research allowed the prediction and regulation of the osteochondral repair process by manipulating oxygen-related parameters in peripheral tissue [[88], [89], [90]].
3.2. Growth plate defect regeneration
The growth plate is a cartilaginous tissue responsible for bone growth located at the end of the long bone in children. The growth plate maintains its activated state from birth to adolescence and starts ossifying from age 14 to 25, implying that the bone gradually loses its growth ability [91,92]. Unlike the four-layered structure of articular cartilage, the growth plate has three distinct zones: The resting zone, proliferation zone, and hypertrophic zone. The resting zone establishes a proper cell niche for chondrocytes, making reserves for further cell activities. Bone growth is an intermittent event modulated by activation of the growth plate [93]. Parathyroid hormone-related protein (PTHrP) secreted by cells in the resting zone and Indian hedgehog (IHH) secreted from hypertrophic chondrocytes are two main modulators regulating growth plate homeostasis.
Once PTHrP becomes dominant, quiescent cells stored in the resting zone are activated and migrate to the proliferation zone for proliferation and mineralization, depositing bone matrix for bone growth. Conversely, IHH is an inhibitor of PTHrP, which is responsible for chondrocyte hypertrophy [94,95]. Hypertrophic chondrocytes in the growth plate are primary functional cells associated with endochondral bone formation, and they can instruct adjacent perichondrial cells to differentiate into osteoblasts and deposit bone matrix on periosteal to form a bone collar [96]. Furthermore, hypertrophic chondrocytes induce blood vessels to penetrate the growth plate center in the presence of vascular endothelial growth factor (VEGF) to form the ossification center, where osteogenesis is actively mobilized to replace former cartilage tissue and elongate the long bone [97].
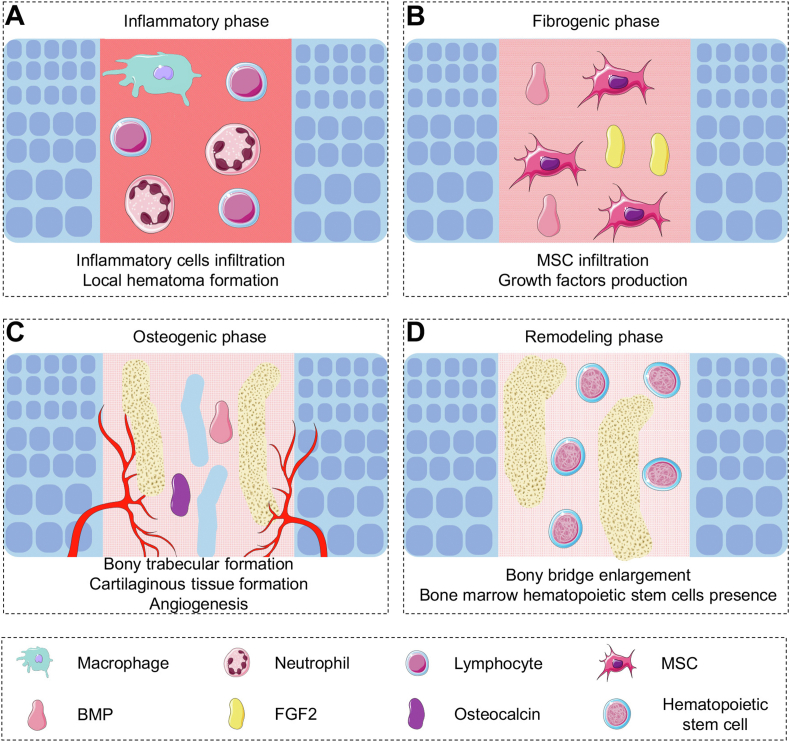
More than 15% of long bone fractures in children injure the growth plate [98,99]. Notably, the injuries to the growth plate always lead to severe consequences, such as unequal length of lower limbs or even disability [100]. The Salter−Harris classification system is commonly used to distinguish the types of growth plate injuries and assess the prognosis and therapeutic outcomes. Type I and II fractures do not injure the growth plate or blood supply of the long bone, and the prognosis is always satisfactory. However, after a Type III or IV fracture, the growth plate regeneration process proceeds through four stages, the inflammatory phase, fibrogenic phase, osteogenic phase, and remodeling phase, and the prognosis is always less than satisfactory through natural restoration (Fig. 5) [101].
Fig. 5.
Four phases of growth plate regeneration. (A) Inflammatory phase: Infiltration and expression of multiple inflammatory factors. (B) Fibrogenic phase: Production of growth factors and tissue organization. (C) Osteogenic phase: Formation of vessels, bony trabecular, and cartilaginous tissue. (D) Remodeling phase: Bony bridge enlargement and dechondrogenesis.
3.2.1. Controllable growth factors releasing imitates developmental biology in growth plate
A series of bio-factors and signaling pathways participate in growth plate regeneration. Tumor necrosis factor-α (TNF-α) was expressed in the process of growth plate regeneration [102]. In the initial stage, upregulated TNF-α is relevant to the local inflammatory condition and activates the proliferation and migration of MSCs into the osteogenic phase [103]. Similarly, platelet-derived growth factor (PDGF) also participates in the migration of MSCs [104]. In the later stage of growth plate regeneration, VEGF and BMP are responsible for tissue remodeling by reconstructing the blood supply and inducing osteogenic differentiation of infiltrated MSCs [[105], [106], [107], [108]]. To restore the natural function of growth plates, it is necessary to construct a biomimetic biochemical microenvironment during growth plate regeneration by incorporating growth factors into scaffolds and controlling their releasing order. Initially, the growth factors were directly added to the scaffolds to regulate bone growth [109,110]. However, the added growth factors cannot continuously work until the bone is mature, a process that takes years. Therefore, a gene therapy approach was introduced to solve this problem.
Target genes of growth factors were transferred into stem cells, inducing constant expression of the growth factors to promote osteogenesis differentiation [111]. Pedro et al. identified growth plate ECM-specific proteins that support stable chondrogenesis of MSCs, which provide a unique regeneration strategy to repair growth plates by autologous growth plate matrix supporting growth factors [112]. Notably, adequate attention must be paid to the metabolism characteristics of the growth plate, as they serve as a critical target in modulating growth plate regeneration.
The anabolic metabolism of cells in the growth plate is highly activated, such that it continuously induces endochondral ossification. Moreover, HIFs have been identified as an essential regulator of the endochondral ossification pathway [113]. The low blood supply in the growth plate creates a hypoxic condition within it, hence upregulating the genes related to lactate synthesis, glycolytic enzymes, and glucose utilization. Furthermore, glycolysis was impaired in the HIFs−deficient growth plate, which led to the apoptosis of chondrocytes and limb shortening in long-term observations. In the case of growth plate injury, local hypoxia conditions may be disturbed due to hemorrhage. The balance of anabolism is disrupted, leading to decreased chondrogenic differentiation and bone matrix deposition [114]. Hence, it is crucial to retain hypoxic conditions or induce expression of HIFs in promoting growth plate regeneration to restore the metabolism function of chondrocytes in the growth plate.
3.2.2. Bone marrow-mesenchymal stem cells have been verified to promote growth plate regeneration
Stem cells, particularly MSCs, are an ideal cell source for growth plate regeneration owing to their controllable differentiation ability. McCarty et al. implanted a BM-MSCs-loaded gel scaffold into a growth plate defect in the rabbit. Denser fibrous tissue was formed in the defect sites five weeks after implantation [115]. In another study, Chung et al. embedded MSCs into the growth plate defect. The injury area was filled with regenerated cartilage tissue similar to that of the native growth plate [116]. The allogeneic and autogenous transplantations of MSCs did not show significant differences in treating growth plate injury in the rabbit, indicating no apparent difference in the effectiveness of different transplantation procedures [117]. Furthermore, a long-term study showed that BM-MSCs could recover the function of growth plate by improving angular deformity, and a higher concentration of BM-MSCs (>1.5 × 106/mL) was beneficial for regeneration of the growth plate [118]. Chondrocytes extracted from the proliferating zone in the growth plate showed higher potential for cartilage regeneration than chondrocytes obtained from articular cartilage. Moreover, chondrocytes in growth plate exhibit significant advantages over cells extracted from articular cartilage in cell viability, proliferation rate, and chondrogenic capability [119].
To date, there is a gap in the research regarding the use of chondrocytes extracted from the growth plate for growth plate regeneration in situ, which has significant research value, as chondrocytes in the growth plate can be harvested and implanted in situ. Nevertheless, the quantum of chondrocytes harvested from the growth plate is limited due to insufficient donor area [120].
3.2.3. Scaffolds play roles in defect occupying in case of endochondral ossification
Excessive osteogenic differentiation may occur in the growth plate regeneration process, and the redundant bony formation in the growth plate may cause growth arrest and angular deformity of long bones [121]. Numerous attempts have been made to avert the bony formation in the cartilage tissue, such as filling the defects with adipose tissue or muscle to hinder bone regeneration [122]. However, these techniques cannot fully restore the morphology and function of the growth plate. Accordingly, scaffolds for growth plate repair must meet a unique requirement distinct from those for articular cartilage repair, and scaffolds must be biodegradable and occupy the defect area in case of bony bridge formation before complete regeneration of growth plate. Both natural materials and synthetic polymers scaffolds verified promising outcomes in the regeneration of growth plate [25,[123], [124], [125]].
Natural hydrogels including alginate, agarose, and chitosan are deemed as appropriate materials for growth plate regeneration. These hydrogels can provide a proper 3D microenvironment to support cell proliferation. In addition, the interconnected pores in hydrogels also facilitate the distribution of nutrition, benefiting cell survival and metabolism [126]. Moreover, ECM derived from growth plates is also used as a scaffold for in situ repair. The matrix components, growth factor contents, and tissue structures in these ECM scaffolds are highly consistent with the natural growth plate, enabling chondrogenic differentiation of seeded MSCs. Further, the safety of ECM scaffolds is promising as well, as immunogenicity in these ECM scaffolds is eliminated by decellularization [127].
The main advantage of synthetic polymers is their tunable degradation rate. Optimally, the degradation period of the scaffold can match the cartilage regeneration process. In that case, scaffold occupation of the defect area avoids redundant bony formation until complete cartilage regeneration. PLGA, PLA, and PCL are commonly selected for growth plate regeneration. By adjusting the molecular weight, the degradation time can be regulated from months to years to satisfy various demands for tissue engineering [128,129]. It is generally accepted that the ideal degradation time of scaffolds for the regeneration of growth plate is within 2–3 months. Composite materials are created to integrate the advantages of various materials. For example, to overcome the low cell affinity limitation of synthetic polymers, Wang et al. established a composite scaffold by synthetizing PLGA, Col, and silk fibroin together, achieving the fusion of biocompatibility and excellent physicochemical properties in an individual scaffold [130].
3.3. Articular cartilage regeneration in weight-bearing area
The articular surface is divided into weight-bearing and non-weight-bearing areas according to the force distribution of the joint surface. In knee joints, for example, the medial and lateral condyles belong to the weight-bearing area, while the intercondylar and supracondylar fossa belongs to the non-weight-bearing area. In the stance posture, lower limb alignment crosses straight through the weight-bearing area (Fig. 2B) [39]. The articular cartilage in the weight-bearing area receives more compression and transmits and absorbs pressure [131]. Different loading conditions also result in anatomical characteristic discrepancies between cartilage tissue in weight-bearing and non-weight-bearing areas. Compared with the non-weight-bearing area, the articular cartilage is thicker in the weight-bearing area. The cartilage in the weight-bearing area has a higher PRG content than that in the non-weight-bearing area. Furthermore, the Col fiber arrangement in the weight-bearing area is more uniform than that in the non-weight-bearing area, resulting in an elastic modulus difference at the micro-level. In addition, the chondrocytes in the weight-bearing area are larger and present a more irregular shape. The morphological changes of chondrocytes may be attributed to the necrosis of chondrocytes and higher cell metabolism, which are also major contributors to OA development [132,133].
3.3.1. Large animals are more appropriate for establishment of cartilage defects in weight-bearing area
More than 75% of cartilage defects in the weight-bearing areas are accompanied by OA [134]. Thus, it is essential to establish an optimal model of large animals for whom the weight-bearing and non-weight-bearing regions can be distinguished. For example, Gong et al. used pigs as experimental animals. Cartilage defect 1 cm in diameter was created on the femoral trochlea, mimicking the cartilage lesion that occurs in the weight-bearing area. After the implantation of AD-MSCs-embedded PGA mesh for six months, the morphology of repaired articular surface was similar to the native tissue, which demonstrated an effective therapeutic strategy for cartilage regeneration in the weight-bearing area [135]. Several studies also showed that cartilage could be better repaired in weight-bearing conditions than non-weight-bearing ones, which can be explained by the loading force transporting biological signals to MSCs and activating the proliferation and differentiation abilities [136].
The technique of autologous living hyaline-like cartilaginous graft (hCG) was preclinically verified on pigs. Because of the wide cartilage surface of knees in the pig, autologous hCG can be harvested on the non-weight-bearing area of articular cartilage and was replanted into the cartilage defects in the weight-bearing area. Six months after implantation, hCG engraftment restored cartilage thickness promoted its integration with surrounding cartilage tissue, and produced abundant cartilage-specific matrix molecules [137].
3.3.2. Scaffolds for weight-bearing area requires a satisfying mechanical property
Tissue engineering scaffolds must have sufficient mechanical strength to maintain stability under physiological loading conditions. Generally, natural materials, e.g., chitosan- or HA-based materials, are not appropriate to repair defects in weight-bearing areas due to their low mechanical properties [138,139]. Compared to natural materials, synthetic materials have more muscular mechanical strength and have been widely used in repairing cartilage defects in weight-bearing areas [140,141].
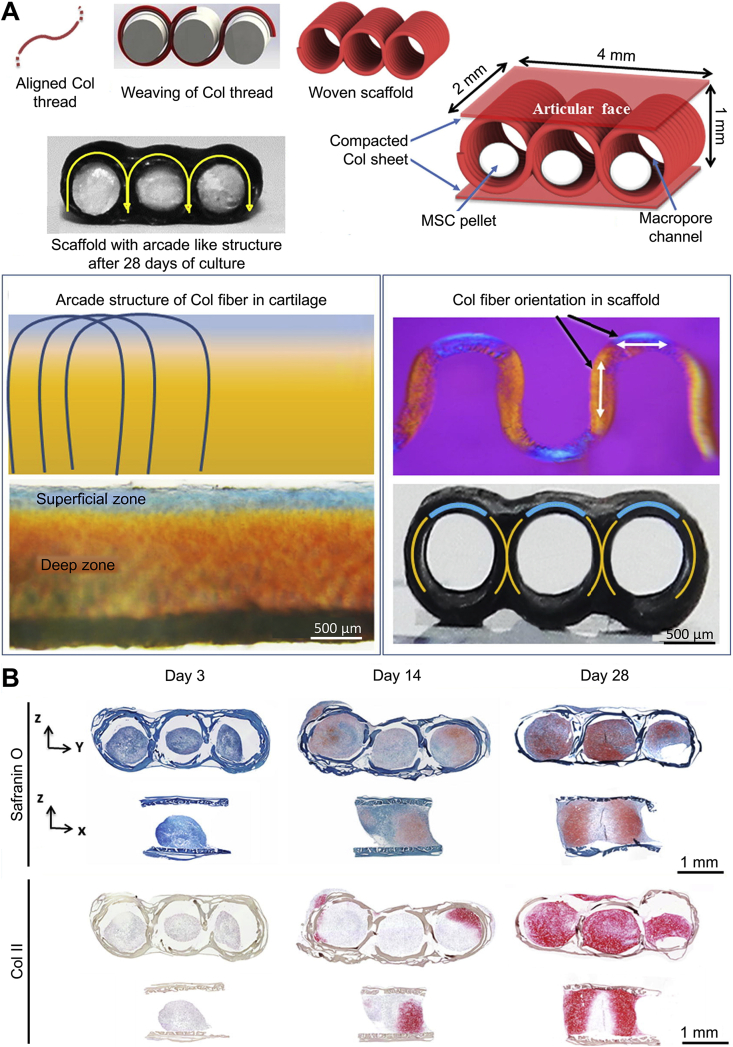
Notably, the mechanical properties of scaffolds can be enhanced by altering their inner structure. Younesi et al. developed a scaffold composed of arcade-like Col fibers with a modulus of 0.83 ± 0.39 MPa, which initially met the mechanical requirement for repairing cartilage tissue in the weight-bearing area. After 28 days of culture, the scaffold modulus increased by 60%, owing to the deposition of the cartilage matrix, and the fatigue property of scaffolds likewise improved (Fig. 6) [142].
Fig. 6.
Col-based woven cartilage scaffold for cartilage repair in the weight-bearing area [142]. (A) Fabrication and morphological characterization of arcade-like Col fiber scaffold. Polarized optical microscopy shows the similarities in the orientations of Col fibers in native articular cartilage and Col scaffold. (B) Histology and immunohistochemistry of the scaffold on day 3, 14, and 28. Formation of the cartilage matrix in the scaffold after long-term in vitro culture. Reproduced with permission [142]. Copyright 2016, Elsevier B.V.
3.3.3. Adipose-derived mesenchymal cells present a better loading tolerance in weight-bearing area implantation
Excessive mechanical loading also results in a high-pressured, hypoxic, and nutrient-deficient microenvironment, inducing decreased cell viability or apoptosis. According to Zhou et al., the energy production of AD-MSCs is independent of aerobic respiration via mitochondria in contrast to BM-MSCs. Furthermore, AD-MSCs have a lower apoptosis rate than BM-MSCs cultured in hypoxia and serum deprivation medium, indicating that AD-MSCs have a better capacity to tolerate hypoxia and non-nutritive conditions [72]. Notably, a proper hypoxic microenvironment promotes the proliferation of AD-MSCs, and postpones cell aging to a certain extent. Enhanced levels of chondrogenic markers, including Sox9 and Col ll were detected in hypoxia-induced AD-MSCs, verifying an excellent potential for cartilage regeneration in the weight-bearing area [143].
3.3.4. Proper mechanical stimulation promotes cartilage regeneration in weight-bearing area
Whether the weight-bearing area must bear loads immediately after cell implantation surgery is still controversial. Proper mechanical stimulation that can be transferred into biochemical signals to modulate cell behaviors was illustrated to promote cartilage regeneration [144]. For example, a periodic pressure acting on chondrocytes in vitro increases the expression of integrin α, which facilitates cell adhesion with ECM. The mitogen-activated protein kinase (MAPK) pathway was extensively studied in transferring mechanical loading into differentiation-inducing signals. MSCs located in the weight-bearing area receives mechanical signals from integrins that are transferred from ECM. The cytoskeleton deformation activates downstream signals, including p38, ERK, JNK, and so forth, causing the upregulation of Sox9 and induction of chondrogenic differentiation [145,146]. However, overloading conditions in joints, such as excessive sport, obesity, and trauma, may increase pro-inflammatory cytokines, reduce Col II and aggrecan (AGG), and even chondrocyte apoptosis. Therefore, it is essential to establish a proper loading condition to facilitate cartilage regeneration [147].
Joint distraction can avoid bearing loading and cartilage surface collapse to maintain regional stability at the early stage after scaffold implantation [148]. Baboolal et al. further investigated the therapeutic mechanism of joint distraction in cartilage lesions [149]. After applying the distraction device in the injured cartilage area, a large amount of HA expression was found in the joint distraction group compared to the non-distraction group, indicating that joint distraction assisted adhesion of MSCs and provided an optimal microenvironment for the chondrogenic differentiation of MSCs. Furthermore, a movable hinge can be equipped on the joint distraction device to offer controllable stress on the articular cartilage surface to stimulate cartilage regeneration [150]. In some cases with severe cartilage damage and long-term joint space stenosis, achieving an appropriate soft tissue balance remains challenging. Thus, joint distraction can ameliorate soft tissue contraction and restore the tissue tension balance [151]. However, the complications of joint distraction must be considered, including pin tract infection and joint stiffness. Although the pin track infection rarely develops to osteomyelitis, it would increase the risk of prosthesis infection if a joint replacement were required in the subsequent treatment.
3.4. Articular cartilage regeneration under osteoarthritis
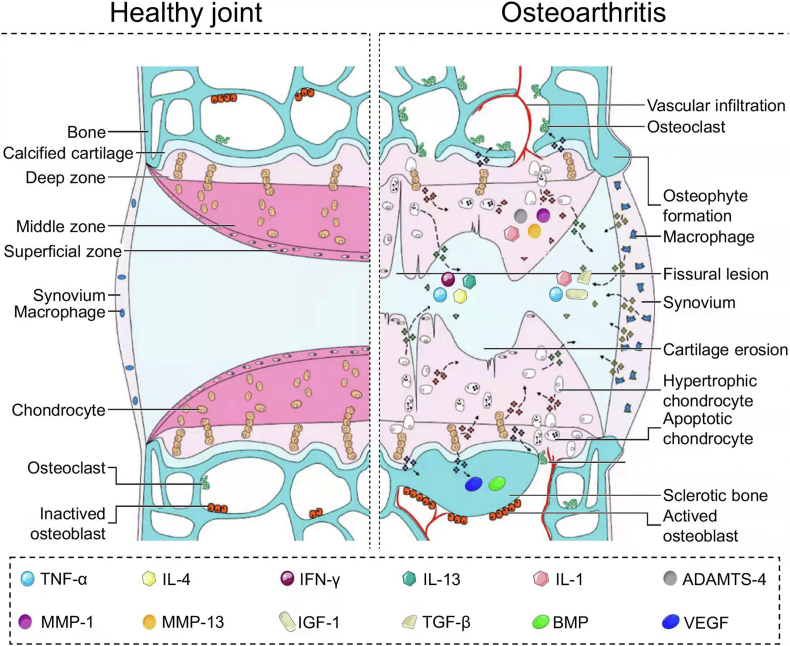
OA is one of the most prevalent joint diseases, resulting in pain, deformity, and even disability of joints. According to the report of the World Health Organization, approximately 27% of individuals aged >60 years suffer from OA, and knee joints are the most commonly affected [152]. With the development of OA, continuous abrasion in joints engenders progressive deterioration of articular cartilage accompanied by subchondral bone defect, joint space narrowing, and osteoporosis formation (Fig. 7) [[153], [154], [155]].
Fig. 7.
Signaling pathways in progression of OA [155]. Inflammatory factors, e.g., TNF-α, IFN-γ, IL-4, and IL-13, expressed from hypertrophic chondrocytes and infiltrated into joint cavity. Inflammatory factors, e.g., IL-1, ADAMTS-4, MMP-1, and MMP-13, expressed from hypertrophic chondrocytes. Growth factors, e.g., VEGF and BMP, expressed from chondrocytes were secreted into the subchondral bone to remodel the morphology of bone. Inflammatory factors, e.g., TNF-α, IL-1, IGF-1, and TGF-β, expressed from macrophages in the synovium were secreted into the joint cavity. Reproduced with permission [155]. Copyright 2015, Elsevier B.V.
3.4.1. Matrix deterioration is intuitionistic view of osteoarthritis
During OA development, multiple tissues are involved in joints, including articular cartilage, subchondral bone, and other peri/intra-articular tissues. Matrix deterioration and remodeling are the predominant appearances [156]. At the early stage of OA, the water content in cartilage is increased, causing tissue swelling. Further, the contents of PRG and AGG, as crucial components in the cartilage matrix, are also reduced. Microcracks are formed on the surface of superficial zones, fragments, and ruptures, occurring in the cartilage matrix [157]. With OA progression, the calcified cartilage area expanded, accompanied by angiogenesis and innervation, and the area of cartilage damage can reach the calcified zone in depth. Due to an uneven mechanical microenvironment, the subchondral bone is remodeled to adapt to intense loading. The osteoblasts are activated by mechanotransduction of signal factors, causing subchondral bone sclerosis and the formation of osteophyte and bone cysts [158]. Tissue lesions, including meniscal tears, synovial membrane hypertrophy, inflammatory infiltrate, muscle atrophy, and ligament stiffness, also arise, jointly contributing to OA symptoms [159].
The leading cause of such problems is the increased expression of protease in articular cartilage and subchondral bone. Matrix metalloproteinases (MMPs), one of the collagenase families activated by metal ions, are essential biological indicators for OA diagnosis owing to their extremely high-level expression in the synovium and synovial fluid of OA patients [160]. The pathogenic mechanism of MMPs in OA involves degradation of the cartilage matrix by splitting Col. MMP-13 is closely related to OA, owing to its high efficiency in degrading the cartilage matrix. MMP-14 can activate gelatinase, which leads to a chain reaction in cartilage destruction. Therefore, another therapeutic strategy for OA focuses on reducing the secretion of MMPs [161,162]. Targeting MMP inhibitors were thus proposed, aiming to restrain the redundant expression of MMPs in cartilage. However, this approach was generally criticized due to its relatively low selectivity. Aside from pathogenesis in OA, MMPs also play pivotal physiological roles in angiogenesis, neural plasticity, wound healing, and glucose metabolism. However, existing MMP inhibitors may cause severe side effects, such as cardiovascular and nerve injury, diabetes mellitus, and delayed healing of wounds [163].
Exosome secreted by MSCs was verified to significantly reduce the expression of MMP-2 and MMP-13 [164]. MSC exosome has been shown to promote the proliferation of MSCs with enhanced cartilage matrix synthesis by downregulating the expression of MMPs in OA joints with the effect of delaying cartilage degeneration. Lai et al. revealed that MSC exosome contained >850 gene products, and large amounts of nucleic acids constructed a complex interaction network in regulating OA states [165]. Although the gene product contents in exosomes varied slightly according to different sources of MSCs, the therapeutic efficiency of heterologous exosome in OA was almost similar.
In molecular biology, IHH signaling pathways are mainstream regulators contributing to cartilage matrix destruction and OA development. The IHH pathway activates by binding IHH and PTCH-1, the membrane receptor, enabling a series of cascade reactions, resulting in the amplification of GLI1. GLI1, as a core gene in the activation of the IHH pathway, promotes the expression of prostaglandin E2 (PGE2), nitric oxide (NO), and MMPs in articular cartilage, thus causing pain and cartilage matrix degradation in OA [166]. Targeting the IHH signaling pathway, several small molecules, including cyclopamine, statins, lithium chloride, icariin, and ipriflavone, are identified to down-regulate MMPs in cartilage by inhibiting the IHH pathway [167]. With the aid of a small interfering RNA technique, IHH can also be entirely knocked down to inhibit hypertrophic chondrocyte markers, ameliorate cartilage quality, and up-regulate cartilage matrix deposition [168].
3.4.2. Biomechanical disorder and inflammatory affection commonly cause osteoarthritis
It is commonly accepted that biomechanical disorder and inflammatory action participate in OA development. Obesity or overload of exercises is considered as an inducing factor of OA, with an approximately 4 and 6.5 folds increase in the risk of OA in obese populations and athletes, respectively [169]. The combination of longitudinal load and transversal wear results in cartilage damage, forming deep cracks and exfoliative injury on the cartilage surface [170,171]. Moreover, muscle and ligament weakness may increase instability and malalignment in joints, exacerbating cartilage destruction [172]. Beyond mechanical factors, high-expressed inflammatory factors including interleukin-1 (IL-1), IL-6, TNF-α, and VEGF are also detected in the synovial fluid and synovium of OA joints, demonstrating that inflammation is involved in OA pathogenesis. These inflammatory factors can induce chondrocyte apoptosis, activate MMPs, influence Col fiber arrangement, and reduce cartilage matrix deposition to destroy OA cartilage further (Fig. 8) [173,174]. Accordingly, surgical and chemical inductions are the two main OA animal modeling methods to mimic disease pathogeny.
Fig. 8.
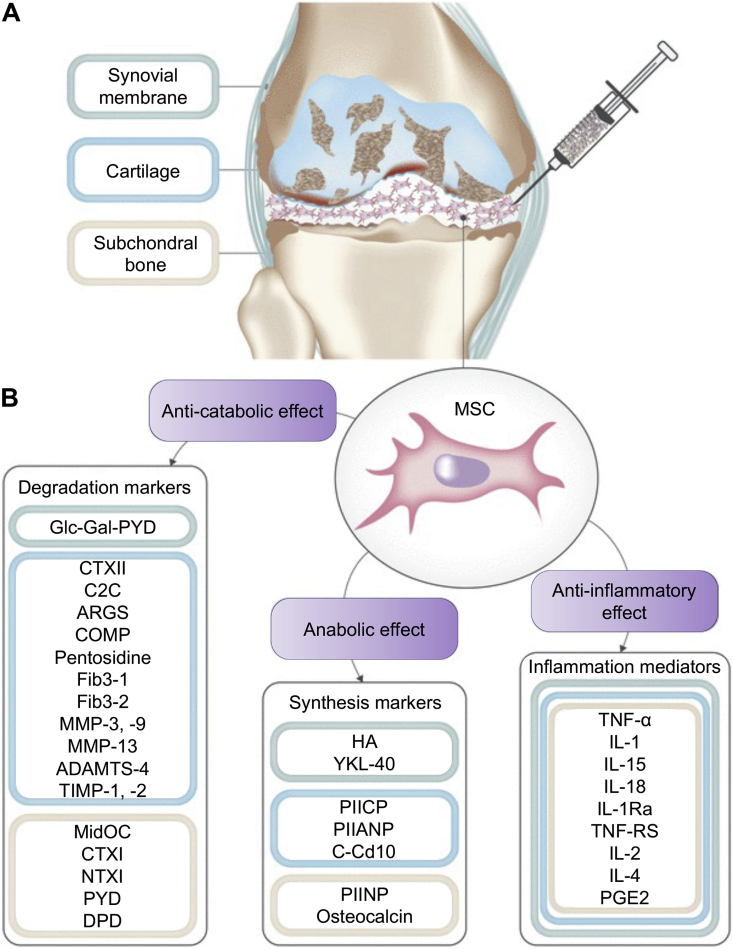
Mechanism of MSC therapy in OA [171]. (A) Intra-articular injection of MSCs into an OA joint. (B) Cartilage degradation markers and inflammation mediators were downregulated, and cartilage synthesis markers were upregulated after injection. The anti-catabolic, anti-anabolic, and anti-inflammatory effects of MSCs are the main therapeutic effect against OA. Monitor for the variation of these markers had been proposed as a method to evaluate the efficacy of MSC therapies in OA. Reproduced with permission [171]. Copyright 2019, Springer.
Surgical techniques, such as meniscectomy and cruciate ligament resection, mimic the natural progression of OA by creating an unstable condition in the joints [175,176]. Recent studies have shown that large animal models generated more similar clinical OA pathological changes than small animals [177]. The latter approach was to inject chemical agents (e.g., collagenase, protease, and iodoacetate) into the joint to destroy the structure of cartilage ECM and accelerate cartilage degeneration [178,179]. Chemical induction usually has a shorter modeling duration within 4–6 weeks, forming a typical OA manifestation [180]. Currently, in vitro OA mimicking models provide a more convenient method for analyzing OA treatment effects, conveniently avoiding ethical issues. Yeung et al. co-cultured MSCs with excised osteochondral tissues from OA patients [181]. After exposure to the OA mimicking microenvironment, the levels of inflammatory cytokines in MSCs were significantly upregulated, similarly to the natural OA condition.
Weight loss is an effective method to relieve the excessive load on cartilage. A report indicates that losing 1 kg can reduce about 40 N of compressive load [182]. Moreover, a series of adipose-derived cytokines, such as leptin and adiponectin, were also related to OA [183]. The weight loss strategy can decrease the secretion of these cytokines, suppressing inflammation in OA [184]. Joint realignment and muscle exercise also help to alleviate biomechanical disorders [185,186]. With these rehabilitation means, the articular surface matching can be ameliorated in unstable joints to recover force transmission. Symptoms, such as pain and joint deformity, are also improved by restoring mechanical stability in joints.
The mechanism of MSC therapies in treating OA is to delay cartilage degradation and recover the balance of chondrocyte metabolism. Furthermore, eliminating inflammatory factors in deteriorated cartilage is another therapeutic objective [154,187]. Multiple cytokines manipulate chondrogenic differentiation and immune regulatory functions of MSCs to delay the degradation process of cartilage in OA [188]. MSC therapy consistently combines with chondrogenic factors. Among them, plate-rich plasma (PRP) is one of the most commonly used chondrogenic−inducing agents. The cartilage matrix deposition was significantly increased when MSCs were cultured in PRP, as PRP contains abundant growth factors, such as basic FGF and TGF-β [189]. Numerous studies demonstrated the chondral-promotive effect of MSCs in PRP by facilitating cartilage matrix production. However, the application of PRP remains limited because its composition is complex and obscure, which must be further refined. Moreover, gene therapy techniques can selectively transport inflammatory antagonists-related genes into the deceased joint to inhibit OA progression and slow cartilage deterioration. IL-1 is a crucial inflammatory cytokine mediating the anabolic disorder of cartilage in RA joints. Frisbie et al. transported the IL-1 receptor antagonists gene carried by adenovirus vector into the OA joints of equine and found the transported gene can be expressed steadily for over 28 days. The OA symptoms were significantly relieved [190].
OA-modifying drugs have been developed to relieve pain and suppress inflammation. Furthermore, micro RNA (miRNA), as well as long noncoding RNA (lncRNA), can also be employed as cargo to transport into OA joints for modulating the inflammation state [[191], [192], [193]]. However, systemic administration or local joint injection may give rise to problems, such as low drug efficacy and overly rapid clearance rates [194,195]. Thus, nanoparticle-based technology is introduced as a unique drug delivery system to enhance drug efficiency. Nanoparticles, commonly sized between 10 and 1000 nm, possess unique characteristics in enhancing drug stability, avoiding drug dispersion, and improving drug-targeting efficacy. Liposomes, dendrimers, metal nanoparticles, and various polymer nanoparticles are suitable as drug nanocarriers in OA management [196,197]. By selecting materials and adjusting the particle size, drug loading properties can also be controlled for optimal releasing and function enhancement [198]. In addition, targeting ligands can be modified on the surface of nanoparticles to facilitate nanoparticle−cartilage interaction. By conjugating the Col II-targeting peptide (WYRGRL) on the surface of the metal−organic framework (MOF)-decorated mesoporous polydopamine, this nanoparticle actively targeted the Col II chain in cartilage ECM and exhibited anti-oxidation and anti-apoptosis capability with the ability to impede cartilage degradation in OA [199]. Moreover, some nanoparticles display specific physicochemical properties that respond to chemical, light, thermal, and magnetic stimuli, playing roles in aiding drug infiltration and symptom relief [200,201].
Nuclear factor-κB (NF-κB), IHH, and HIF-1 are other pivotal inflammatory pathways in mediating OA occurrence and progression, which can be deemed target spots to suppress the expression of inflammatory cytokines. Moreover, miRNA can modify post-transcriptional regulation by mediating transcription. miRNA-140, miRNA-145, miRNA-146, miRNA-194a, and miRNA-199a regulate the hypertrophy and proteolytic enzyme synthesis of chondrocytes to inhibit cartilage damage and OA progression. By systematic administration, intra-articular injection, carrier-mediated transporting, and ultrasound transmission, miRNA can be delivered into OA joints and play various roles [202].
3.4.3. Age and gender are pivotal risk factors of osteoarthritis
Age is closely related to OA occurrence. According to a prevalence study in the USA, more than 19% of the population aged over 45 present radiographic OA [203]. Apart from cumulated impact and friction injuries, abnormal cell functions in aged chondrocytes also contribute to cartilage failure in OA. First, the chondrocyte number is reduced in the aging process due to cell death and apoptosis. A study reported that only 2/3 cell density remains in articular cartilage at the age of 70 compared to 30 [204]. The aged chondrocytes also exhibit a senescent phenotype, indicating decreased synthetic activity and imbalance of homeostasis [205]. Furthermore, the responsiveness of growth factors in aged chondrocytes is weakened due to the reduced number of signaling receptors on the cell surface [206]. Thus, aged chondrocytes forfeit cell−cell and cell−matrix crosstalk, losing chondrogenic and matrix deposition capability.
Aging is an irreversible process, and anti-senescence chondrocytes methods are still challenging to develop in practical terms. To date, several plant-derived small molecules were reported to play roles in restoring the function of aged chondrocytes. Flavonoids enable the inhibition of chondrocyte apoptosis and modulate the expression of inflammatory factors. Glycosides can likewise improve the functions of aged chondrocytes. After being treated with clematis saponins extract from Clematis Florida Thunb, apoptosis, depolarization of the mitochondrial membrane, and caspase-3 activity was suppressed, indicating the apoptosis protecting function for aged cells [207]. Remarkably, the cartilage repair effect of MSC therapy is also closely related to the donor's age. The stemness, proliferation, and differentiation capability of BM-MSCs were diminished in aged donors. Due to the autologous MSCs, transplantation is still the general trend, considering the clinical histocompatibility and ethics issues.
The aging of OA patients may impair the repair function of MSCs. An immediate study performed by Matthew et al. recaptures the public attention in microfracture, an ordinary technique that has already existed for over 60 years. They demonstrated that the progressive function loss of skeletal stem cells (SSCs) was associated with aging. Through microfracture, SSCs were released to the defect area. The low function state of SSCs can be reversed, triggered by the microfracture process, and resided on the cartilage surface to restore cartilage lesion [208].
Females were shown to have a higher OA incidence, and estrogen is deemed as the main modulating factor [209]. Cartilage is the target tissue of estrogen, as estrogen receptors massively exist on the chondrocyte surface. In estrogen receptor gene knockout mice, mature cartilage is disturbed [210]. Estrogen plays significant physiological role in cartilage homeostasis. OA incidence is significantly increased in the menopause population. Estrogen deficiency weakens the protection effect of articular cartilage, causing the high expression of inflammatory factors, including IL-1, TNF-α, and MMPs [[211], [212], [213]]. Through hormone replacement therapy, supplemental estrogen can alleviate local inflammatory reactions and protect cartilage degradation in OA. In a clinical trial, cartilage volume rose by 7.7% after five years of estrogen replacement therapy [214].
3.5. Articular cartilage regeneration under rheumatoid arthritis
Compared to OA, RA is a systemic inflammatory disease caused by autoimmune disorders. The joints are the most likely injured, frequently resulting in joint stiffness, swelling, and even terminal joint deformity, which markedly reduces life expectancy. The comparison of OA and RA is presented in Table 1 [215].
Table 1.
Comparison of risk factors, symptoms, mechanisms and pathological features between OA and RA [215].
| OA | RA | |
|---|---|---|
| Risk factor | Aging, excess loading, obesity | Autoimmune abnormality |
| Symptom | Joint swelling, pain, temporary joint stiffness, joint movement limitation, joint locking, joint deformity | Multiple joint swelling, continuous joint stiffness |
| Mechanism | Biomechanical disorder, inflammation, and chondrocyte aging commonly contribute to OA progression, causing cartilage matrix deterioration | Autoimmune disorder leads to synovial inflammation, and pannus erodes cartilage and subchondral bone |
| Pathological feature | 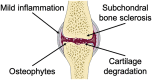 |
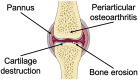 |
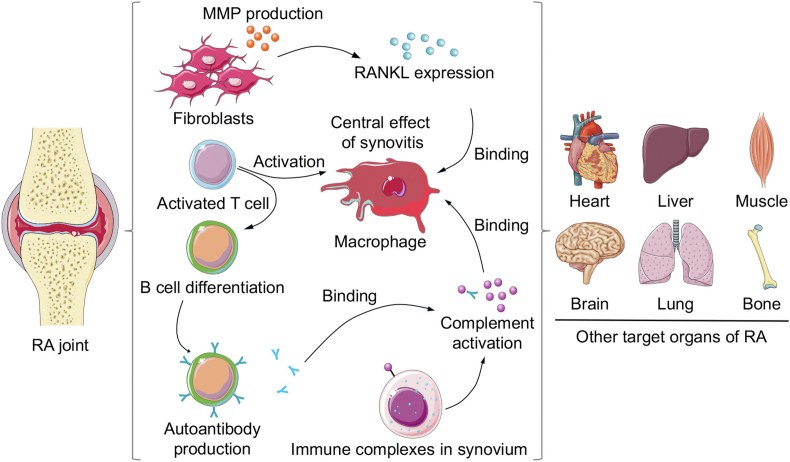
The pathological progression and exact cause of RA are still ambiguous. As far as is known, cellular and humoral immunity are involved in RA occurrence and progression, where macrophages act as core factors. Initially, inflammatory cells (e.g., lymphocytes, monocytes, and fibroblast-like synoviocytes) infiltrate the synovial tissue, causing edema and hyperplasia. External or autoantigen are presented from antigen-presenting cells to naive T cells and trigger T cells activation in the synovium by IL-2 and IL-21. Subsequently, IL-7 and interferon-γ (IFN-γ) expressed by activated T cells contribute to the macrophage activation contained in the joint cavity. Antibodies and rheumatoid factors produced by plasma cells bind with and activate macrophages. In addition, IL-1, IL-6, and TNF-α derived from activated macrophages are the main pathogenic molecules leading to cartilage damage by stimulating MMPs release from chondrocytes and fibroblasts and bone resorption by activating osteoclasts (Fig. 9) [22,216,217].
Fig. 9.
Schematic illustration of immune disorders and other target organs of RA. Macrophage as the key modulator is involved in RA progression. Cell immunity, humoral immunity and complement immunity all participate in immune disorder and cartilage destruction. Other organs, including heart, liver, muscle, brain, lung, and bone, are also affected by RA.
Multiple small joints are first involved in RA, accompanied by joint swelling and morning stiffness. Afterward, large bilateral joints, including the shoulder, elbow, knee, and ankle, can be affected, and joint symptoms, including swelling, pain, and dysfunction, are gradually presented. A comprehensive evaluation of the symptoms mentioned above and serology testing can be used as accurate bases for diagnosing RA patients [218].
3.5.1. Medical treatment as first-line choice for early-stage rheumatoid arthritis
Although RA is incurable, medical treatment is still helpful in the early stage. Nonsteroidal anti-inflammatory drugs (NSAIDs) are effective in relieving RA-related symptoms. Furthermore, disease-modifying anti-rheumatic drugs (DMARDs) are developed to inhibit RA progression and become a routine RA management medication. Conventional synthetic DMARDs, including methotrexate (MTX), sulfasalazine (SSZ), and leflunomide (LEF), are first-generation drugs with no therapeutic target and severe side effects. Target and biological DMARDs were subsequently developed to target block the RA-associated molecules, such as TNF (e.g., etanercept, infliximab, and adalimumab), IL-6 (e.g., tocilizumab and sarilumab), and JAK (e.g., tofacitinib and baricitinib), which were more secure for patients with fewer tolerability profiles. DMARDs have been verified to postpone RA symptoms and radiological progression, which significantly improve the life quality for RA patients [219,220]. However, these drugs only delay the progression of cartilage degradation rather than repair the damaged cartilage [221].
Although increased permeability results from angiogenesis and widening endothelial cell gaps within RA joints, local drug concentrations remain limited due to their avascular physiological characteristics. The intra-articular injection can directly deliver drugs to the target tissue, but repeated injections may increase the risk of joint infection. Hence, an efficient drug delivery system assisting routine administration is required to promote curative effect for RA.
The nanoscale drug delivery systems emerged as an advanced technology with various advantages, including a sound encapsulating effect, controllable releasing behavior, and precise targeting administration [222]. Thus far, liposomes and polymer nanoparticles are commonly adopted as nanoscale delivery nanocarriers for RA treatment. Liposomes are spherical nanoparticles constructed by lipid bilayer membranes with promising tissue permeability. A previous study reported that a 40 times higher MTX concentration remaining in the joint was detected in the liposome delivery group compared with the non-liposome group [223]. Moreover, small-size liposomes are more beneficial for uptaking than large-size ones [224].
Nevertheless, the limitations of liposomes acting as drug nanocarriers are worth noting, and the stability of liposomes is relatively poor, which may cause the leakage of encapsulated drugs when the lipid bilayer membrane is destructed [225]. Polymers, including PLA, PLGA, PEG, and chitosan, are reconstructed into the nanoscale particles and used to deliver RA treatment drugs [[226], [227], [228]]. The structures of these polymer nanoparticles are more stable compared to liposomes. The drug-carried nanoparticles can be guided to the target organ and exert effects due to their charged characteristics. However, activated immune cells in patients with RA may recognize and phagocytose the exotic nanoparticles, consequently impairing delivery efficiency by decreasing effective doses [229]. Hence, an active targeting drug delivery system is designed according to the inflammatory situation in RA.
Mediating from the interactions between ligands and receptors, drug-loaded nanoparticles are linked to inflammatory cells or cytokines and are transported to the RA joint by chemotaxis [230]. HA can specifically bind with CD44, an adhesion receptor widely presented on epithelial cells and activated lymphocytes [231]. Heo et al. fabricated polymer micelles by covalently combining HA and cholic acid. They found that the HA micelle intake by macrophages overtook that of the HA-free micelle [232]. Another highly expressed molecule found on macrophages is the folic acid receptor, which draws extensive attention and is modified on the surface of FITC-MTX-contained PLGA nanoparticle. Subsequently, a concentration of FITC fluorescence was observed residing in the joint, demonstrating a significant targeting effect [233]. Similarly, polypeptides, antibodies, and metal nanoclusters are also identified as potential targets for drug delivery into the RA joint. Moreover, the microenvironments in inflammatory RA joints are altered with a higher local temperature (>37.5 °C) and acidic conditions (pH 5–6), which can be used as a responding target for drug release. Nanoparticles bound with hydrazone are sensitive to temperature and pH variation. When these substances flow through diseased joints, the hydrazone bond breaks due to microenvironment alteration, leading to the de-crosslinking of nanoparticles that ultimately targets drug delivery into RA joints [234].
3.5.2. Mesenchymal stem cells therapy and immunotherapy play crucial roles in ameliorating autoimmune disorder in rheumatoid arthritis
Reports indicate that MSCs exhibit promising immunosuppressive functions in RA treatment. MSCs inhibit the proliferation of T cells and the maturation of monocytes, suppressing the cytotoxic lymphocytes production via antigen presentation by dendritic cells [29]. Moreover, MSCs down-regulate the TNF-α and IL-10 production in dendritic cells, reducing the IFN-γ from natural killer cells and T-helper-1 cells and increasing the expression of IL-4 in T-helper-2 cells at the same time [[235], [236], [237]].
The application of MSC therapies to suppress systemic symptoms of RA has been widely studied. The intra-peritoneal injection of MSCs into mice was reported to prevent RA progression [238]. A more refined study showed that the expression of pro-inflammatory cytokines, such as IL-10 and TNF-α, was inhibited after the administration of MSCs [239]. Considering that MSCs possess both immunomodulation and tissue-regeneration capacities, they are deemed an optimal choice for the treatment of RA, as they simultaneously exert anti-inflammatory effects and repair injured cartilage tissue [240].
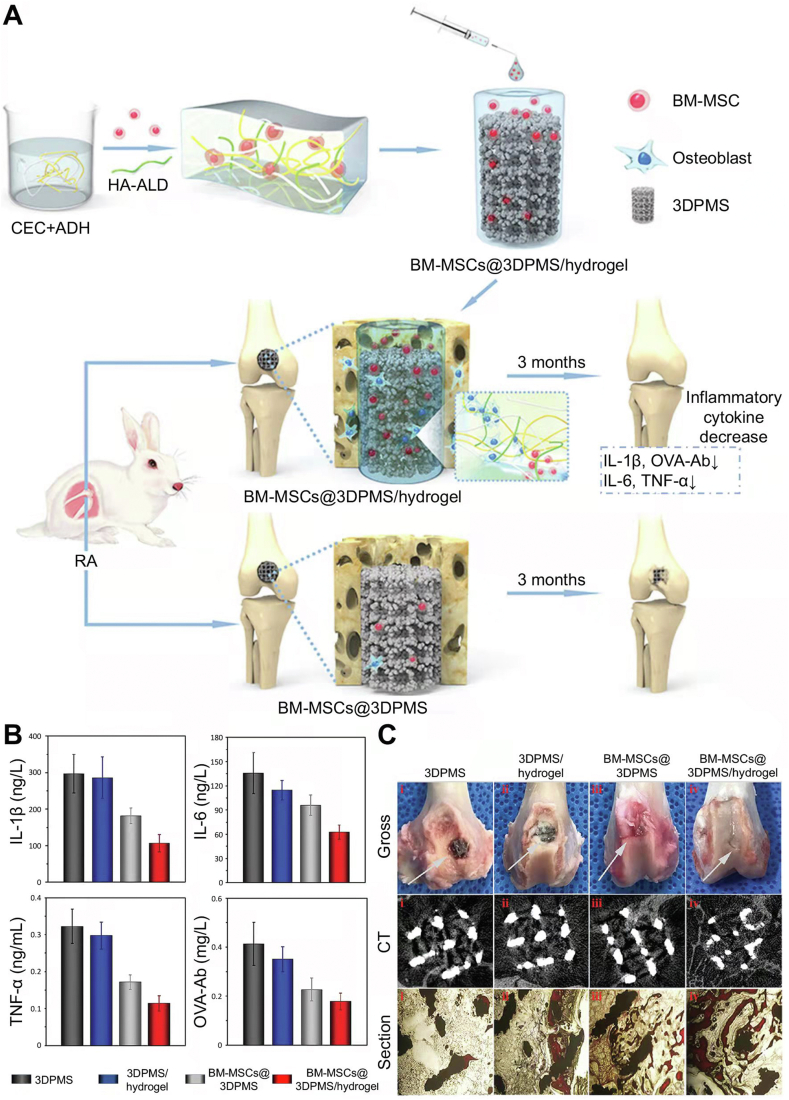
Our research group has conducted several relevant studies using a tissue engineering approach to repair damaged cartilage and regulate immune disorders in RA. First, we attempted to combine BM-MSC therapy with microfracture in treating the rabbit RA model. During the cartilage repair process, the numbers of CD4+ and CD8+ T lymphocytes and inflammatory cytokines in peripheral blood were reduced [241]. The subsequent study targeted high-quality cartilage repair by developing a fibrin gel combined with PLGA−PEG−PLGA hydrogel as a scaffold to treat cartilage defects in RA rabbit models [242,243]. Recently, we focused on improving bone quality in the RA joint to extend the life of the prosthesis. A polysaccharide hydrogel/BM-MSCs/porous titanium scaffold complex was constructed and implanted in the knee joints of RA rabbits to mimic total knee arthroplasty surgery. BM-MSCs were sustained released with the hydrogel degradation and played roles in RA treatment. Local symptoms, such as joint swelling and redness and systemic inflammatory indications, were relieved. Moreover, bone ingrowth into porous prosthesis was achieved after eliminating the RA inflammatory conditions (Fig. 10) [244].
Fig. 10.
Polysaccharide hydrogel/BM-MSCs/porous titanium scaffold complex for RA treatment and bone reconstruction around RA joint [244]. (A) Schematic illustration of hydrogel preparation, hydrogel/BM-MSCs/porous titanium scaffold complex synthesis, and in vivo treating effect evaluation. (B) RA-related inflammatory factor (IL-1β, IL-6, TNF-α, and OVA-Ab) levels in peripheral blood after implantation in RA rabbit model. (C) Bone ingrowth effect assessed by the gross view, CT scanning, and histological section. Reproduced with permission [244]. Copyright 2019, John Wiley & Sons, Inc.
MSC therapy for RA entered clinical trials in 2010. Over the past decade, ten trials have been completed, demonstrating preliminary results of MSC therapy for RA in clinical application. The first pilot clinical trial was conducted by the Stem Cell Research Center of Korea. Four RA patients were involved in this trial who received therapy of autologous AD-MSCs multiple times and were monitored for 13 months. This study was deemed the first credible clinical evidence to support the effect of MSCs in RA management and to identify a safe dose of AD-MSCs infusion (8 × 108/patients) with no adverse effects [245]. Subsequently, 172 RA patients were treated with allogenic UC-MSCs (4 × 107/patient) with a single dose for three years in a trial by the Hospital of People's Liberation Army Air Force in China. Serum markers, including rheumatoid factor (RF), C-reactive protein (CRP), anti-cyclic citrullinated peptide (CCP, IL-6, and TNF-α, were significantly down-regulated compared to the group treated with DMARDs, indicating a conspicuous remission of the disease [246].
In another Chinese study, intravenous injection of 106 allogeneic UC-MSCs per kg body weight was used to treat 53 RA patients with the resistance of DMARDs. Consequently, the efficacy and biosafety of therapy were confirmed [247]. In other completed or functional studies, the promising effect and biosafety of MSC therapy for RA were repeatedly demonstrated. However, a conclusion on the types, sources, and doses of MSC therapy for RA management was lacking. Notably, the patients enrolled in these trials were mainly at the terminal stage of RA. Thus, the effect of MSC therapy in delaying or even reversing RA progression in the early stage still needs further investigation [248].
One promising treatment for RA is targeted therapy with genetic methods through the inhibition of pro-inflammatory cytokines. IL-1β and TNF-α are two key factors mediating the development of RA. Researchers aim to restrain the effect of cytokines by blocking receptor binding via competitive inhibition. Recombinant IL-1 receptor antagonist (anakinra) and monoclonal antibodies directed against TNF-α (e.g., infliximab and adalimumab) have been reported to improve RA-related joint symptoms. Furthermore, the recombinant IL-1 receptor antagonist (anakinra) was approved by the US Food and Drug Administration (FDA) for clinical treatment of RA [249,250]. Currently, Au cluster treatment exhibited potential in treating RA. The Au cluster inhibited osteoclast activation by downregulating the receptor activator of NF-κB ligand (RANKL) and reversed bone and cartilage damage in RA. However, Au cluster efficacy has not been proved by clinical application. Its treatment results and toxic side effects must be studied in detail [251].
4. Current challenges and future directions
Because of the complexity of cartilage defects, various tissue engineering strategies have been developed to deal with different cartilage injuries. This study classified five commonly clinical cartilage defect types (full-layered, osteochondral, growth plate, weight-bearing area, and OA and RA) and summarized the current treatment techniques for cartilage defects under different conditions. Overall, bionic techniques are essential for cartilage repair in these cases. Here, we present our expectations of bionic technology to treat unconventional cartilage defects to propose structure simulation and functional enhancement of the repaired tissue.
4.1. Structure simulation
Structure simulation is considered as a critical factor in selecting biomaterials for cartilage tissue repair to obtain properties similar to native cartilage. The multi-layered scaffolds for osteochondral defects and high-strength scaffolds for weight-bearing cartilage defects are simple patterns of bionic techniques. However, currently available materials fail to mimic the structural properties of native cartilage completely. The development of bioprinting techniques allows the fabrication of bioactive scaffolds with micrometer-scaled accuracy [252,253]. By adjusting the component of seeded cells and carriers, specific bioactive scaffolds with different structures can be conveniently designed and produced.
Articular cartilage consists of superficial, transitional, radial, and calcified cartilage zones. The cell phenotype and cartilage matrix distribution also change in different layers [32]. To fabricate a biomimetic cartilage scaffold, other cells can be isolated and reseeded into appropriate layers of scaffolds. The introduction of cell-loaded bioinks into 3D bioprinters offers the possibility to construct a full-layered cartilage scaffold that mimics the native structure of cartilage tissue.
The Col fiber density arranged in cartilage is uniform, leading to variable mechanical strength. Stent alignment could be designed to stimulate micro-Col fiber distribution by altering interval spacing or the stacking mode. Bioprinting provides a more precise approach to control the stent position and embodies repetitive basic geometric modules to fill scaffolds [254]. According to the natural fiber distribution in natural cartilage, the geometric modules and unit compactness in scaffolds can be predesigned accurately to perfectly imitate cartilage micromorphology and obtain biomimetic mechanical distribution in the scaffold.
4.2. Function enhancement
As mentioned above, cytokines play a crucial role in regulating long bone extension during growth plate repair. They are also predominant in repairing cartilage under OA and RA, where the repair process involves a complex network and interactions of cytokines at both local and systemic levels [19,92,215]. Indeed, stem cell implantation is an effective strategy in regulating cytokines, such that MSCs can promote growth factor expression and inhibit inflammatory factor secretion. Therefore, combining MSCs with traditional anti-arthritis drugs may contribute to a synthetic effect in treating OA and RA. However, the doses of addictive drugs are limited by their cytotoxicity, and they cannot provide a long-term effective concentration in the whole process of cartilage regeneration. Hence, we hypothesized that MSC functions might be precisely and continuously enhanced through gene remodeling according to different conditions.
Bone development is controlled by various signaling pathways regulating proteins in the growth plate. Sox9 and Runx2 are essential for converting MSCs into chondrocytes and subsequently driving chondrocytes into the hypertrophic phenotype [255,256]. In addition, FGFs and BMPs are responsible for successive bone formation [257]. It would be ideal for achieving sustainable target protein expression and ensuring the sustained release of cytokines in regulating bone regeneration in a biomimetic manner. Transfecting genes into MSCs to establish a group of gene-modeling cells provides long-term self-synthesized growth factors instead of short-term explosive release by delivering growth factors in the lesion area. However, mimicking the expression pattern of cytokines in the developmental biology approach is still a considerable challenge. For instance, Sox9 and Runx2 are expected to be expressed in the initial period of chondrogenesis, followed by FGFs and BMPs to induce the subsequent bone formation process.
From the aspect of molecular biology, the occurrence of OA and RA is closely associated with their specific gene products, such as abnormally high expression of MMPs, ADAMTs, and RhoA in OA [216,[258], [259], [260]]. The effective inhibition of the expression of inflammatory-related genes postpones the progression of OA and RA. Therefore, introducing the silenced gene is deemed an effective method to restrain the expression of inflammatory-related genes.
5. Conclusions and perspectives
The current cartilage regeneration target is not merely confined to a simple tissue formation but focuses on enabling cartilage regeneration under abnormal conditions to imitate clinical conditions. Likewise, treatment requirements also vary according to different injured sites or primary disease status.
As discussed above, the full-layered osteochondral defects, growth plate defects, and cartilage lesions in the weight-bearing areas represent three commonly injured sites, posing numerous challenges to restore cartilage shape and functions. Priorities in repairing cartilage in specific locations aim to recover tissue morphology similar to host tissue, and shaping the scaffold to mimic native tissue structure in different injured sites is common.
Cartilage defects always accompany inflammatory conditions, especially OA and RA. However, optimal cartilage regeneration can be achieved only in inflammatory diseases that are well controlled. OA is characterized by irreversible cartilage degradation, and MMPs were verified as critical pathogenic factors by accelerating cartilage matrix enzymolysis. By introducing biological agents, such as PRP and exosomes, MMPs are effectively down-regulated. For RA treatment, the immunomodulation capability of MSCs is available to suppress RA-related inflammatory factors, such as IL-6 and TNF-α. In addition, multiple targeted agents, such as recombinant IL-1 receptor antagonist (e.g., anakinra) and monoclonal antibodies directed against TNF-α (e.g., infliximab and adalimumab), have entered clinical trials with the promise of restoring a favorable microenvironment for cartilage regeneration while treating RA.
The rapid advancement of engineering and biological techniques brings novel possibilities in cartilage regeneration. 3D bioprinting allows the customized scaffold to exhibit controllable structure, anisotropy, and cell distribution, highly similar to native cartilage tissue. Genetic engineering genes of cytokines can be transferred into seed cells to obtain a sustained and steady expression of required cytokines after implantation. Cartilage formation-related genes and arthritis inhibition genes are both suitable for transfected into seed cells from two reversal aspects.
Overall, several cases and therapeutic expectations of cartilage injured patients continue to rise annually, putting forward higher requirements for the cartilage regeneration effect. This review offers a preliminary summary of cartilage regeneration modalities with advanced scaffolds, growth factors, and cells under abnormal conditions and provides direction for subsequent studies in this field.
CRediT authorship contribution statement
Zhonghan Wang: Methodology, Validation, Formal analysis, Resources, Writing – original draft, Visualization. Hanxiang Le: Methodology, Validation, Formal analysis, Resources, Writing – review & editing. Yanbing Wang: Validation, Resources, Writing – review & editing. He Liu: Writing – review & editing, Supervision, Funding acquisition. Zuhao Li: Validation, Resources, Writing – review & editing. Xiaoyu Yang: Writing – review & editing, Supervision. Chenyu Wang: Writing – review & editing, Supervision. Jianxun Ding: Conceptualization, Writing – review & editing, Supervision, Funding acquisition. Xuesi Chen: Conceptualization, Writing – review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 82102358, 82001971, 81701811, and 81772456), the Science and Technology Development Program of Jilin Province (Grant Nos. 20200802008GH, 20200404202YY, 20200404190YY, 20200404140YY, 20200403088SF, 20190304123YY, 20190201068JC, 20180623050TC, and 20180201041SF), the Health Department Program of Jilin Province (Grant Nos. 2020Q018, 2019SCZT001, 2019SCZT014, 2019SRCJ001, and 2017F007), the Youth Talents Promotion Project of Jilin Province (Grant No. 192004), and the Interdisciplinary Research Funding Program for Doctoral Candidates of Jilin University (Grant No. 41900200861).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Moran C.J., Pascual-Garrido C., Chubinskaya S., Potter H.G., Warren R.F., Cole B.J., Rodeo S.A. Restoration of articular cartilage. J. Bone Joint Surg. Am. 2014;96(4):336–344. doi: 10.2106/JBJS.L.01329. [DOI] [PubMed] [Google Scholar]
- 2.Hunziker E.B. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10(6):432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 3.Kwon H., Brown W.E., Lee C.A., Wang D., Paschos N., Hu J.C., Athanasiou K.A. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 2019;15(9):550–570. doi: 10.1038/s41584-019-0255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gross A.E., Silverstein E.A., Falk J., Falk R., Langer F. The allotransplantation of partial joints in the treatment of osteoarthritis of the knee. Clin. Orthop. Relat. Res. 1975;108:7–14. doi: 10.1097/00003086-197505000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Hesse W., Hesse I. Long-term results after cartilage transplantation. Langenbecks Arch. Chir. Suppl. 1976:292–295. [PubMed] [Google Scholar]
- 6.Friedman M.J., Berasi C.C., Fox J.M., Del Pizzo W., Snyder S.J., Ferkel R.D. Preliminary results with abrasion arthroplasty in the osteoarthritic knee. Clin. Orthop. Relat. Res. 1984;182:200–205. [PubMed] [Google Scholar]
- 7.Gordon K.R. Microfracture patterns of abrasive wear striations on teeth indicate directionality. Am. J. Phys. Anthropol. 1984;63(3):315–322. doi: 10.1002/ajpa.1330630308. [DOI] [PubMed] [Google Scholar]
- 8.Chen M., Li Y., Liu S., Feng Z., Wang H., Yang D., Guo W., Yuan Z., Gao S., Zhang Y., Zha K., Huang B., Wei F., Sang X., Tian Q., Yang X., Sui X., Zhou Y., Zheng Y., Guo Q. Hierarchical macro-microporous WPU-ECM scaffolds combined with microfracture promote in situ articular cartilage regeneration in rabbits. Bioact. Mater. 2021;6(7):1932–1944. doi: 10.1016/j.bioactmat.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994;331(14):889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 10.Bartlett W., Skinner J.A., Gooding C.R., Carrington R.W., Flanagan A.M., Briggs T.W., Bentley G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: A prospective, randomised study. J. Bone Joint Surg. Br. 2005;87(5):640–645. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 11.Becher C., Cantiller E.B. Focal articular prosthetic resurfacing for the treatment of full-thickness articular cartilage defects in the knee: 12-year follow-up of two cases and review of the literature. Arch. Orthop. Trauma. Surg. 2017;137(9):1307–1317. doi: 10.1007/s00402-017-2717-8. [DOI] [PubMed] [Google Scholar]
- 12.Schneider T.E., Karaikudi S. Matrix-induced autologous chondrocyte implantation (MACI) grafting for osteochondral lesions of the talus. Foot Ankle Int. 2009;30(9):810–814. doi: 10.3113/FAI.2009.0810. [DOI] [PubMed] [Google Scholar]
- 13.Shahgaldi B., Amis A., Heatley F., McDowell J., Bentley G. Repair of cartilage lesions using biological implants. A comparative histological and biomechanical study in goats. J. Bone Joint Surg. Br. 1991;73-B(1):57–64. doi: 10.1302/0301-620X.73B1.1991776. [DOI] [PubMed] [Google Scholar]
- 14.Cui X., Breitenkamp K., Finn M.G., Lotz M., D'Lima D.D. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng. Part A. 2012;18(11–12):1304–1312. doi: 10.1089/ten.tea.2011.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haklar U., Tuzuner T., Uygur I., Kocaoglu B., Guven O. The effect of overlapping on the primary stability of osteochondral grafts in mosaicplasty. Knee Surg. Sports Traumatol. Arthrosc. 2008;16(7):651–654. doi: 10.1007/s00167-008-0526-x. [DOI] [PubMed] [Google Scholar]
- 16.Li X., Wei J., Aifantis K.E., Fan Y., Feng Q., Cui F.Z., Watari F. Current investigations into magnetic nanoparticles for biomedical applications. J. Biomed. Mater. Res. A. 2016;104(5):1285–1296. doi: 10.1002/jbm.a.35654. [DOI] [PubMed] [Google Scholar]
- 17.Jerosch J., Filler T., Peuker E. Is there an option for harvesting autologous osteochondral grafts without damaging weight-bearing areas in the knee joint? Knee Surg. Sports Traumatol. Arthrosc. 2000;8(4):237–240. doi: 10.1007/s001670000122. [DOI] [PubMed] [Google Scholar]
- 18.Samsa W.E., Zhou X., Zhou G. Signaling pathways regulating cartilage growth plate formation and activity. Semin. Cell Dev. Biol. 2017;62:3–15. doi: 10.1016/j.semcdb.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldring S.R., Goldring M.B. Changes in the osteochondral unit during osteoarthritis: Structure, function and cartilage-bone crosstalk. Nat. Rev. Rheumatol. 2016;12(11):632–644. doi: 10.1038/nrrheum.2016.148. [DOI] [PubMed] [Google Scholar]
- 20.Liu H., Ding J.X., Wang J.C., Wang Y.A., Yang M.D., Zhang Y.B., Chang F., Chen X.S. Remission of collagen-induced arthritis through combination therapy of microfracture and transplantation of thermogel-encapsulated bone marrow mesenchymal stem cells. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0120596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aho O.M., Finnila M., Thevenot J., Saarakkala S., Lehenkari P. Subchondral bone histology and grading in osteoarthritis. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0173726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo Q., Wang Y., Xu D., Nossent J., Pavlos N.J., Xu J. Rheumatoid arthritis: Pathologicall mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:15. doi: 10.1038/s41413-018-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou C.L., Rivera A.L., Williams V., Welter J.F., Mansour J.M., Drazba J.A., Sakai T., Baskaran H. Micrometer scale guidance of mesenchymal stem cells to form structurally oriented large-scale tissue engineered cartilage. Acta Biomater. 2017;60:210–219. doi: 10.1016/j.actbio.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Woude J.T.A.D., Wiegant K., van Roermund P.M., Intema F., Custers R.J.H., Eckstein F., van Laar J.M., Mastbergen S.C., Lafeber F.P.J.G. Five-year follow-up of knee distraction: Clinical benefit and cartilaginous tissue repair in an open uncontrolled prospective study. Cartilage. 2017;8(3):263–271. doi: 10.1177/1947603516665442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shukrimi A.B., Afizah M.H., Schmitt J.F., Hui J.H. Mesenchymal stem cell therapy for injured growth plate. Front. Biosci. 2013;5:774–785. doi: 10.2741/s407. [DOI] [PubMed] [Google Scholar]
- 26.Bravo B., Arguello J.M., Gortazar A.R., Forriol F., Vaquero J. Modulation of gene expression in infrapatellar fat pad-derived mesenchymal stem cells in osteoarthritis. Cartilage. 2018;9(1):55–62. doi: 10.1177/1947603516686144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J.Q., Wu C.S., Wang D., Wang L.C., Sun S. Acetylsalicylic acid combined with diclofenac inhibits cartilage degradation in rabbit models of osteoarthritis. Exp. Ther. Med. 2016;12(4):2177–2182. doi: 10.3892/etm.2016.3560. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Cao W., Cao K., Cao J.C., Wang Y., Shi Y.F. Mesenchymal stem cells and adaptive immune responses. Immunol. Lett. 2015;168(2):147–153. doi: 10.1016/j.imlet.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Tyndall A. Mesenchymal stromal cells and rheumatic disorders. Immunol. Lett. 2015;168(2):201–207. doi: 10.1016/j.imlet.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Armiento A.R., Stoddart M.J., Alini M., Eglin D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018;65:1–20. doi: 10.1016/j.actbio.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 31.McDaniel D., Tilton E., Dominick K., Flory K., Ernest T., Johnson J.C., Main D.C., Kondrashov P. Histological characteristics of knee menisci in patients with osteoarthritis. Clin. Anat. 2017;30(6):805–810. doi: 10.1002/ca.22920. [DOI] [PubMed] [Google Scholar]
- 32.Tatman P.D., Gerull W., Sweeney-Easter S., Davis J.I., Gee A.O., Kim D.H. Multiscale biofabrication of articular cartilage: Bioinspired and biomimetic approaches. Tissue Eng. B Rev. 2015;21(6):543–559. doi: 10.1089/ten.TEB.2015.0142. [DOI] [PubMed] [Google Scholar]
- 33.Pearle A.D., Warren R.F., Rodeo S.A. Basic science of articular cartilage and osteoarthritis. Clin. Sports Med. 2005;24(1):1–12. doi: 10.1016/j.csm.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Lakstins K., Arnold L., Gunsch G., Khan S., Moore S., Purmessur D. Characterization of bovine and canine animal model cartilage endplates and comparison to human cartilage endplate structure, matrix composition, and cell phenotype. JOR Spine. 2020;3(4):e1116. doi: 10.1002/jsp2.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li A., Wei Y., Hung C., Vunjak-Novakovic G. Chondrogenic properties of collagen type XI, a component of cartilage extracellular matrix. Biomaterials. 2018;173:47–57. doi: 10.1016/j.biomaterials.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Bornes T.D., Adesida A.B., Jomha N.M. Mesenchymal stem cells in the treatment of traumatic articular cartilage defects: A comprehensive review. Arthritis Res. Ther. 2014;16(5):432. doi: 10.1186/s13075-014-0432-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silverberg J.L., Barrett A.R., Das M., Petersen P.B., Bonassar L.J., Cohen I. Structure-function relations and rigidity percolation in the shear properties of articular cartilage. Biophys. J. 2014;107(7):1721–1730. doi: 10.1016/j.bpj.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teshima R., Nawata K., Hagino H., Morio Y., Inoue M., Irizawa Y. Effects of weight bearing on the tidemark and osteochondral junction of articular cartilage: Histomorphometric analyses of 7 normal femoral head. Acta Orthop. Scand. 1999;70(4):381–386. doi: 10.3109/17453679908997828. [DOI] [PubMed] [Google Scholar]
- 39.Van Rossom S., Smith C.R., Zevenbergen L., Thelen D.G., Vanwanseele B., Van Assche D., Jonkers I. Knee cartilage thickness, T1rho and T2 relaxation time are related to articular cartilage loading in healthy adults. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0170002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akizuki S., Mow V.C., Muller F., Pita J.C., Howell D.S., Manicourt D.H. Tensile properties of human knee joint cartilage: I. Influence of ionic conditions, weight bearing, and fibrillation on the tensile modulus. J. Orthop. Res. 1986;4(4):379–392. doi: 10.1002/jor.1100040401. [DOI] [PubMed] [Google Scholar]
- 41.Schinagl R.M., Gurskis D., Chen A.C., Sah R.L. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J. Orthop. Res. 1997;15(4):499–506. doi: 10.1002/jor.1100150404. [DOI] [PubMed] [Google Scholar]
- 42.Moutos F.T., Freed L.E., Guilak F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat. Mater. 2007;6(2):162–167. doi: 10.1038/nmat1822. [DOI] [PubMed] [Google Scholar]
- 43.Armiento A.R., Stoddart M.J., Alini M., Eglin D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018;65:1–20. doi: 10.1016/j.actbio.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 44.Jahn S., Seror J., Klein J. Lubrication of articular cartilage. Annu. Rev. Biomed. Eng. 2016;18:235–258. doi: 10.1146/annurev-bioeng-081514-123305. [DOI] [PubMed] [Google Scholar]
- 45.Zhou F., Chu L., Liu X., He Z., Han X., Yan M., Qu X., Li X., Yu Z. Subchondral trabecular microstructure and articular cartilage damage variations between osteoarthritis and osteoporotic osteoarthritis: A cross-sectional cohort study. Front. Med. 2021;8:617200. doi: 10.3389/fmed.2021.617200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou L., Gjvm V.O., Malda J., Stoddart M.J., Lai Y., Richards R.G., Ki-Wai Ho K., Qin L. Innovative tissue-engineered strategies for osteochondral defect repair and regeneration: Current progress and challenges. Adv. Healthc. Mater. 2020 doi: 10.1002/adhm.202001008. [DOI] [PubMed] [Google Scholar]
- 47.Slattery C., Kweon C.Y. Classification in brief: Outerbridge classification of chondral lesions. Clin. Orthop. Relat. Res. 2018;476(10):2101–2104. doi: 10.1007/s11999.0000000000000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei W., Dai H. Articular cartilage and osteochondral tissue engineering techniques: Recent advances and challenges. Bioact. Mater. 2021;6(12):4830–4855. doi: 10.1016/j.bioactmat.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang N.J., Lin Y.T., Lin C.C., Wang H.C., Hsu H.C., Yeh M.L. The repair of full-thickness articular cartilage defect using intra-articular administration of N-acetyl-D-glucosamine in the rabbit knee: Randomized controlled trial. Biomed. Eng. Online. 2015;14:105. doi: 10.1186/s12938-015-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahmoud E.E., Adachi N., Mawas A.S., Gaarour O.S., Ochi M. Coculturing of mesenchymal stem cells of different sources improved regenerative capability of osteochondral defect in the mature rabbit: An in vivo study. J. Orthop. Surg. 2019;27(2) doi: 10.1177/2309499019839850. 2309499019839850. [DOI] [PubMed] [Google Scholar]
- 51.Chang F., Ishii T., Yanai T., Mishima H., Akaogi H., Ogawa T., Ochiai N. Repair of large full-thickness articular cartilage defects by transplantation of autologous uncultured bone-marrow-derived mononuclear cells. J. Orthop. Res. 2008;26(1):18–26. doi: 10.1002/jor.20470. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y., Zhang J., Chang F., Xu W., Ding J. Repair of full-thickness articular cartilage defect using stem cell-encapsulated thermogel. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;88:79–87. doi: 10.1016/j.msec.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 53.He A., Liu L., Luo X., Liu Y., Liu Y., Liu F., Wang X., Zhang Z., Zhang W., Liu W., Cao Y., Zhou G. Repair of osteochondral defects with in vitro engineered cartilage based on autologous bone marrow stromal cells in a swine model. Sci. Rep. 2017;7:40489. doi: 10.1038/srep40489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loebel C., Kwon M.Y., Wang C., Han L., Mauck R.L., Burdick J.A. Metabolic labeling to probe the spatiotemporal accumulation of matrix at the chondrocyte-hydrogel interface. Adv. Funct. Mater. 2020:1909802. doi: 10.1002/adfm.201909802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu H., Cheng Y., Chen J., Chang F., Wang J., Ding J., Chen X. Component effect of stem cell-loaded thermosensitive polypeptide hydrogels on cartilage repair. Acta Biomater. 2018;73:103–111. doi: 10.1016/j.actbio.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 56.Antich C., Jimenez G., de Vicente J., Lopez-Ruiz E., Chocarro-Wrona C., Grinan-Lison C., Carrillo E., Montanez E., Marchal J.A. Development of a biomimetic hydrogel based on predifferentiated mesenchymal stem-cell-derived ECM for cartilage tissue engineering. Adv. Healthc. Mater. 2021 doi: 10.1002/adhm.202001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang T., Zhang H., Zhang L., Jia S., Liu J., Xiong Z., Sun W. Biomimetic design and fabrication of multilayered osteochondral scaffolds by low-temperature deposition manufacturing and thermal-induced phase-separation techniques. Biofabrication. 2017;9(2) doi: 10.1088/1758-5090/aa7078. [DOI] [PubMed] [Google Scholar]
- 58.Kim H.W., Kim H.E., Salih V. Stimulation of osteoblast responses to biomimetic nanocomposites of gelatin-hydroxyapatite for tissue engineering scaffolds. Biomaterials. 2005;26(25):5221–5230. doi: 10.1016/j.biomaterials.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 59.Li X., Ding J., Wang J., Zhuang X., Chen X. Biomimetic biphasic scaffolds for osteochondral defect repair. Regen. Biomater. 2015;2(3):221–228. doi: 10.1093/rb/rbv015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Getgood A., Henson F., Skelton C., Brooks R., Guehring H., Fortier L.A., Rushton N. Osteochondral tissue engineering using a biphasic collagen/GAG scaffold containing rhFGF18 or BMP-7 in an ovine model. J. Exp. Orthop. 2014;1(1):13. doi: 10.1186/s40634-014-0013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suo H., Chen Y., Liu J., Wang L., Xu M. 3D printing of biphasic osteochondral scaffold with sintered hydroxyapatite and polycaprolactone. J. Mater. Sci. 2021;56(29):16623–16633. [Google Scholar]
- 62.Liu X., Chen Y., Mao A.S., Xuan C., Wang Z., Gao H., An G., Zhu Y., Shi X., Mao C. Molecular recognition-directed site-specific release of stem cell differentiation inducers for enhanced joint repair. Biomaterials. 2020;232:119644. doi: 10.1016/j.biomaterials.2019.119644. [DOI] [PubMed] [Google Scholar]
- 63.Guo J.L., Kim Y.S., Koons G.L., Lam J., Navara A.M., Barrios S., Xie V.Y., Watson E., Smith B.T., Pearce H.A., Orchard E.A., van den Beucken J., Jansen J.A., Wong M.E., Mikos A.G. Bilayered, peptide-biofunctionalized hydrogels for in vivo osteochondral tissue repair. Acta Biomater. 2021;128:120–129. doi: 10.1016/j.actbio.2021.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu X., Zhou M., Jiang F., Yin S., Lin S., Yang G., Lu Y., Zhang W., Jiang X. Marginal sealing around integral bilayer scaffolds for repairing osteochondral defects based on photocurable silk hydrogels. Bioact. Mater. 2021;6(11):3976–3986. doi: 10.1016/j.bioactmat.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Levingstone T.J., Matsiko A., Dickson G.R., O'Brien F.J., Gleeson J.P. A biomimetic multi-layered collagen-based scaffold for osteochondral repair. Acta Biomater. 2014;10(5):1996–2004. doi: 10.1016/j.actbio.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 66.Sun Y., You Y., Jiang W., Wang B., Wu Q., Dai K. 3D bioprinting dual-factor releasing and gradient-structured constructs ready to implant for anisotropic cartilage regeneration. Sci. Adv. 2020;6(37) doi: 10.1126/sciadv.aay1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qiao Z., Lian M., Han Y., Sun B., Zhang X., Jiang W., Li H., Hao Y., Dai K. Bioinspired stratified electrowritten fiber-reinforced hydrogel constructs with layer-specific induction capacity for functional osteochondral regeneration. Biomaterials. 2021;266:120385. doi: 10.1016/j.biomaterials.2020.120385. [DOI] [PubMed] [Google Scholar]
- 68.Wang C., Feng N., Chang F., Wang J., Yuan B., Cheng Y., Liu H., Yu J., Zou J., Ding J., Chen X. Injectable cholesterol-enhanced stereocomplex polylactide thermogel loading chondrocytes for optimized cartilage regeneration. Adv. Healthc. Mater. 2019;8(14) doi: 10.1002/adhm.201900312. [DOI] [PubMed] [Google Scholar]
- 69.Ferruzzi A., Calderoni P., Grigolo B., Gualtieri G. Autologous chondrocytes implantation: Indications and results in the treatment of articular cartilage lesions of the knee. Chir. Organi Mov. 2004;89(2):125–134. [PubMed] [Google Scholar]
- 70.Richardson J.B., Caterson B., Evans E.H., Ashton B.A., Roberts S. Repair of human articular cartilage after implantation of autologous chondrocytes. J. Bone Joint Surg. Br. 1999;81(6):1064–1068. doi: 10.1302/0301-620x.81b6.9343. [DOI] [PubMed] [Google Scholar]
- 71.Schmal H., Kowal J.M., Kassem M., Seidenstuecker M., Bernstein A., Bottiger K., Xiong T., Sudkamp N.P., Kubosch E.J. Comparison of regenerative tissue quality following matrix-associated cell implantation using amplified chondrocytes compared to synovium-derived stem cells in a rabbit model for cartilage lesions. Stem Cell. Int. 2018;2018:4142031. doi: 10.1155/2018/4142031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou W., Lin J., Zhao K., Jin K., He Q., Hu Y., Feng G., Cai Y., Xia C., Liu H., Shen W., Hu X., Ouyang H. Single-cell profiles and clinically useful properties of human mesenchymal stem cells of adipose and bone marrow origin. Am. J. Sports Med. 2019;47(7):1722–1733. doi: 10.1177/0363546519848678. [DOI] [PubMed] [Google Scholar]
- 73.Huang H., Zhang X., Hu X., Shao Z., Zhu J., Dai L., Man Z., Yuan L., Chen H., Zhou C., Ao Y. A functional biphasic biomaterial homing mesenchymal stem cells for in vivo cartilage regeneration. Biomaterials. 2014;35(36):9608–9619. doi: 10.1016/j.biomaterials.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 74.Ullah M., Liu D.D., Thakor A.S. Mesenchymal stromal cell homing: Mechanisms and strategies for improvement. iScience. 2019;15:421–438. doi: 10.1016/j.isci.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lopez-Ruiz E., Jimenez G., Garcia M.A., Antich C., Boulaiz H., Marchal J.A., Peran M. Polymers, scaffolds and bioactive molecules with therapeutic properties in osteochondral pathologies: What's new? Expert Opin. Ther. Pat. 2016;26(8):877–890. doi: 10.1080/13543776.2016.1203903. [DOI] [PubMed] [Google Scholar]
- 76.Li X., Ding J., Zhang Z., Yang M., Yu J., Wang J., Chang F., Chen X. Kartogenin-incorporated thermogel supports stem cells for significant cartilage regeneration. ACS Appl. Mater. Interfaces. 2016;8(8):5148–5159. doi: 10.1021/acsami.5b12212. [DOI] [PubMed] [Google Scholar]
- 77.Fan L., Chen J., Tao Y., Heng B.C., Yu J., Yang Z., Ge Z. Enhancement of the chondrogenic differentiation of mesenchymal stem cells and cartilage repair by ghrelin. J. Orthop. Res. 2019;37(6):1387–1397. doi: 10.1002/jor.24224. [DOI] [PubMed] [Google Scholar]
- 78.Muttigi M.S., Kim B.J., Choi B., Yoshie A., Kumar H., Han I., Park H., Lee S.H. Matrilin-3 codelivery with adipose-derived mesenchymal stem cells promotes articular cartilage regeneration in a rat osteochondral defect model. J. Tissue Eng. Regen. Med. 2018;12(3):667–675. doi: 10.1002/term.2485. [DOI] [PubMed] [Google Scholar]
- 79.Meirelles Lda S., Fontes A.M., Covas D.T., Caplan A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20(5–6):419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 80.Kim K., Lam J., Lu S., Spicer P.P., Lueckgen A., Tabata Y., Wong M.E., Jansen J.A., Mikos A.G., Kasper F.K. Osteochondral tissue regeneration using a bilayered composite hydrogel with modulating dual growth factor release kinetics in a rabbit model. J. Control.. Release. 2013;168(2):166–178. doi: 10.1016/j.jconrel.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reyes R., Pec M.K., Sanchez E., del Rosario C., Delgado A., Evora C. Comparative, osteochondral defect repair: Stem cells versus chondrocytes versus bone morphogenetic protein-2, solely or in combination. Eur. Cell. Mater. 2013;25:351–365. doi: 10.22203/ecm.v025a25. [DOI] [PubMed] [Google Scholar]
- 82.Zhang S., Chu W.C., Lai R.C., Lim S.K., Hui J.H., Toh W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24(12):2135–2140. doi: 10.1016/j.joca.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 83.Zhang S., Chuah S.J., Lai R.C., Hui J.H.P., Lim S.K., Toh W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. doi: 10.1016/j.biomaterials.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 84.Chen P., Zheng L., Wang Y., Tao M., Xie Z., Xia C., Gu C., Chen J., Qiu P., Mei S., Ning L., Shi Y., Fang C., Fan S., Lin X. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 2019;9(9):2439–2459. doi: 10.7150/thno.31017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ranera B., Remacha A.R., Alvarez-Arguedas S., Castiella T., Vazquez F.J., Romero A., Zaragoza P., Martin-Burriel I., Rodellar C. Expansion under hypoxic conditions enhances the chondrogenic potential of equine bone marrow-derived mesenchymal stem cells. Vet. J. 2013;195(2):248–251. doi: 10.1016/j.tvjl.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 86.Kim K.H., Song M.J., Chung J., Park H., Kim J.B. Hypoxia inhibits adipocyte differentiation in a HDAC-independent manner. Biochem. Biophys. Res. Commun. 2005;333(4):1178–1184. doi: 10.1016/j.bbrc.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 87.O'Reilly A., Kelly D.J. Role of oxygen as a regulator of stem cell fate during the spontaneous repair of osteochondral defects. J. Orthop. Res. 2016;34(6):1026–1036. doi: 10.1002/jor.23110. [DOI] [PubMed] [Google Scholar]
- 88.Campbell J.J., Lee D.A., Bader D.L. Dynamic compressive strain influences chondrogenic gene expression in human mesenchymal stem cells. Biorheology. 2006;43(3,4):455–470. [PubMed] [Google Scholar]
- 89.Ning T., Guo J., Zhang K., Li K., Zhang J., Yang Z., Ge Z. Nanosecond pulsed electric fields enhanced chondrogenic potential of mesenchymal stem cells via JNK/CREB-STAT3 signaling pathway. Stem Cell Res. Ther. 2019;10(1):45. doi: 10.1186/s13287-019-1133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang K., Fan Y., Dunne N., Li X. Effect of microporosity on scaffolds for bone tissue engineering. Regen. Biomater. 2018;5(2):115–124. doi: 10.1093/rb/rby001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iannotti J.P. Growth plate physiology and pathology. Orthop. Clin. North Am. 1990;21(1):1–17. [PubMed] [Google Scholar]
- 92.Burdan F., Szumilo J., Korobowicz A., Farooquee R., Patel S., Patel A., Dave A., Szumilo M., Solecki M., Klepacz R., Dudka J. Morphology and physiology of the epiphyseal growth plate. Folia Histochem. Cytobiol. 2009;47(1):5–16. doi: 10.2478/v10042-009-0007-1. [DOI] [PubMed] [Google Scholar]
- 93.Wang X., Li Z., Wang C., Bai H., Wang Z., Liu Y., Bao Y., Ren M., Liu H., Wang J. Enlightenment of growth plate regeneration based on cartilage repair theory: A review. Front. Bioeng. Biotechnol. 2021;9:654087. doi: 10.3389/fbioe.2021.654087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hallett S.A., Ono W., Ono N. Growth plate chondrocytes: Skeletal development, growth and beyond. Int. J. Mol. Sci. 2019;20(23):6009. doi: 10.3390/ijms20236009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haseeb A., Lefebvre V. Isolation of mouse growth plate and articular chondrocytes for primary cultures. Methods Mol. Biol. 2021;2245:39–51. doi: 10.1007/978-1-0716-1119-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Street J., Bao M., deGuzman L., Bunting S., Peale F.V., Ferrara N., Steinmetz H., Hoeffel J., Cleland J.L., Daugherty A., van Bruggen N., Redmond H.P., Carano R.A.D., Filvaroff E.H. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. P. Natl. Acad. Sci. USA. 2002;99(15):9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cooper K.L., Oh S., Sung Y., Dasari R.R., Kirschner M.W., Tabin C.J. Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature. 2013;495(7441):375–378. doi: 10.1038/nature11940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eid A.M., Hafez M.A. Traumatic injuries of the distal femoral physis. Retrospective study on 151 cases. Injury. 2002;33(3):251–255. doi: 10.1016/s0020-1383(01)00109-7. [DOI] [PubMed] [Google Scholar]
- 99.Mizuta T., Benson W.M., Foster B.K., Paterson D.C., Morris L.L. Statistical analysis of the incidence of physeal injuries. J. Pediatr. Orthop. 1987;7(5):518–523. doi: 10.1097/01241398-198709000-00003. [DOI] [PubMed] [Google Scholar]
- 100.Drenkard L.M.M., Kupratis M.E., Li K., Gerstenfeld L.C., Morgan E.F. Local changes to the distal femoral growth plate following injury in mice. J. Biomech. Eng. 2017;139(7) doi: 10.1115/1.4036686. [DOI] [PubMed] [Google Scholar]
- 101.Brown J.H., DeLuca S.A. Growth plate injuries: Salter-Harris classification. Am. Fam. Physician. 1992;46(4):1180–1184. [PubMed] [Google Scholar]
- 102.Xian C.J., Zhou F.H., McCarty R.C., Foster B.K. Intramembranous ossification mechanism for bone bridge formation at the growth plate cartilage injury site. J. Orthop. Res. 2004;22(2):417–426. doi: 10.1016/j.orthres.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 103.Zhou F.H., Foster B.K., Sander G., Xian C.J. Expression of proinflammatory cytokines and growth factors at the injured growth plate cartilage in young rats. Bone. 2004;35(6):1307–1315. doi: 10.1016/j.bone.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 104.Chung R., Foster B.K., Zannettino A.C.W., Xian C.J. Potential roles of growth factor PDGF-BB in the bony repair of injured growth plate. Bone. 2009;44(5):878–885. doi: 10.1016/j.bone.2009.01.377. [DOI] [PubMed] [Google Scholar]
- 105.Brandi M.L., Collin-Osdoby P. Vascular biology and the skeleton. J. Bone Miner. Res. 2006;21(2):183–192. doi: 10.1359/JBMR.050917. [DOI] [PubMed] [Google Scholar]
- 106.Macsai C.E., Georgiou K.R., Foster B.K., Zannettino A.C.W., Xian C.J. Microarray expression analysis of genes and pathways involved in growth plate cartilage injury responses and bony repair. Bone. 2012;50(5):1081–1091. doi: 10.1016/j.bone.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 107.Long F., Shi H., Li P., Guo S., Ma Y., Wei S., Li Y., Gao F., Gao S., Wang M., Duan R., Wang X., Yang K., Sun W., Li X., Li J., Liu Q. A SMOC2 variant inhibits BMP signaling by competitively binding to BMPR1B and causes growth plate defects. Bone. 2021;142:115686. doi: 10.1016/j.bone.2020.115686. [DOI] [PubMed] [Google Scholar]
- 108.Su Y.W., Wong D.S.K., Fan J., Chung R., Wang L., Chen Y., Xian C.H., Yao L., Wang L., Foster B.K., Xu J., Xian C.J. Enhanced BMP signalling causes growth plate cartilage dysrepair in rats. Bone. 2021;145:115874. doi: 10.1016/j.bone.2021.115874. [DOI] [PubMed] [Google Scholar]
- 109.Clark A., Hilt J.Z., Milbrandt T.A., Puleo D.A. Treating proximal tibial growth plate injuries using poly(lactic-co-glycolic acid) scaffolds. Biores. Open Access. 2015;4(1):65–74. doi: 10.1089/biores.2014.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sundararaj S.K., Cieply R.D., Gupta G., Milbrandt T.A., Puleo D.A. Treatment of growth plate injury using IGF-I-loaded PLGA scaffolds. J. Tissue Eng. Regen. Med. 2015;9(12):202–209. doi: 10.1002/term.1670. [DOI] [PubMed] [Google Scholar]
- 111.Gelse K., Jiang Q.J., Aigner T., Ritter T., Wagner K., Poschl E., von der Mark K., Schneider H. Fibroblast-mediated delivery of growth factor complementary DNA into mouse joints induces chondrogenesis but avoids the disadvantages of direct viral gene transfer. Arthritis Rheum. 2001;44(8):1943–1953. doi: 10.1002/1529-0131(200108)44:8<1943::AID-ART332>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 112.Diaz-Payno P.J., Browe D.C., Cunniffe G.M., Kelly D.J. The identification of articular cartilage and growth plate extracellular matrix-specific proteins supportive of either osteogenesis or stable chondrogenesis of stem cells. Biochem. Bioph. Res. Co. 2020;528(2):285–291. doi: 10.1016/j.bbrc.2020.05.074. [DOI] [PubMed] [Google Scholar]
- 113.Tchetina E.V., Markova G.A. Regulation of energy metabolism in the growth plate and osteoarthritic chondrocytes. Rheumatol. Int. 2018;38(11):1963–1974. doi: 10.1007/s00296-018-4103-4. [DOI] [PubMed] [Google Scholar]
- 114.Araldi E., Schipani E. Hypoxia, HIFs and bone development. Bone. 2010;47(2):190–196. doi: 10.1016/j.bone.2010.04.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McCarty R.C., Xian C.J., Gronthos S., Zannettino A.C., Foster B.K. Application of autologous bone marrow derived mesenchymal stem cells to an ovine model of growth plate cartilage injury. Open Orthop. J. 2010;4:204–210. doi: 10.2174/1874325001004010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chung R., Xian C.J. Recent research on the growth plate: Mechanisms for growth plate injury repair and potential cell-based therapies for regeneration. J. Mol. Endocrinol. 2014;53(1):45–61. doi: 10.1530/JME-14-0062. [DOI] [PubMed] [Google Scholar]
- 117.Planka L., Gal P., Kecova H., Klima J., Hlucilova J., Filova E., Amler E., Krupa P., Kren L., Srnec R., Urbanova L., Lorenzova J., Necas A. Allogeneic and autogenous transplantations of MSCs in treatment of the physeal bone bridge in rabbits. BMC Biotechnol. 2008;8:70. doi: 10.1186/1472-6750-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Azarpira M.R., Shahcheraghi G.H., Ayatollahi M., Geramizadeh B. Tissue engineering strategy using mesenchymal stem cell-based chitosan scaffolds in growth plate surgery: A preliminary study in rabbits. Orthop. Traumatol. Surg. Res. 2015;101(5):601–605. doi: 10.1016/j.otsr.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 119.Liu Z.M., Shen P.C., Lu C.C., Chou S.H., Tien Y.C. Characterization of the proliferating layer chondrocytes of growth plate for cartilage regeneration. Tissue Eng. Part A. 2019;25(5–6):364–378. doi: 10.1089/ten.TEA.2018.0110. [DOI] [PubMed] [Google Scholar]
- 120.Tomaszewski R., Wiktor L., Gap A. Enhancement of cartilage repair through the addition of growth plate chondrocytes in an immature skeleton animal model. J. Orthop. Surg. Res. 2019;14(1):260. doi: 10.1186/s13018-019-1302-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cepela D.J., Tartaglione J.P., Dooley T.P., Patel P.N. Classifications in brief: Salter-Harris classification of pediatric physeal fractures. Clin. Orthop. Relat. Res. 2016;474(11):2531–2537. doi: 10.1007/s11999-016-4891-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yamasaki A., Itabashi M., Sakai Y., Ito H., Ishiwari Y., Nagatsuka H., Nagai N. Expression of type I, type II, and type X collagen genes during altered endochondral ossification in the femoral epiphysis of osteosclerotic (oc/oc) mice. Calcif. Tissue Int. 2001;68(1):53–60. doi: 10.1007/BF02685003. [DOI] [PubMed] [Google Scholar]
- 123.Chung R., Foster B.K., Xian C.J. Preclinical studies on mesenchymal stem cell-based therapy for growth plate cartilage injury repair. Stem Cell. Int. 2011;2011:570125. doi: 10.4061/2011/570125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Y., Liu X., Zeng L., Zhang J., Zuo J., Zou J., Ding J., Chen X. Polymer fiber scaffolds for bone and cartilage tissue engineering. Adv. Funct. Mater. 2019;29(36):1903279. [Google Scholar]
- 125.Ding J., Zhang J., Li J., Li D., Xiao C., Xiao H., Yang H., Zhuang X., Chen X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019;90:1–34. [Google Scholar]
- 126.Bonhome-Espinosa A.B., Campos F., Durand-Herrera D., Sanchez-Lopez J.D., Schaub S., Duran J.D.G., Lopez-Lopez M.T., Carriel V. In vitro characterization of a novel magnetic fibrin-agarose hydrogel for cartilage tissue engineering. J. Mech. Behav. Biomed. Mater. 2020;104:103619. doi: 10.1016/j.jmbbm.2020.103619. [DOI] [PubMed] [Google Scholar]
- 127.Cunniffe G.M., Diaz-Payno P.J., Sheehy E.J., Critchley S.E., Almeida H.V., Pitacco P., Carroll S.F., Mahon O.R., Dunne A., Levingstone T.J., Moran C.J., Brady R.T., O'Brien F.J., Brama P.A.J., Kelly D.J. Tissue-specific extracellular matrix scaffolds for the regeneration of spatially complex musculoskeletal tissues. Biomaterials. 2019;188:63–73. doi: 10.1016/j.biomaterials.2018.09.044. [DOI] [PubMed] [Google Scholar]
- 128.Wan L., Zhang Y. Jointly modified mechanical properties and accelerated hydrolytic degradation of PLA by interface reinforcement of PLA-WF. J. Mech. Behav. Biomed. Mater. 2018;88:223–230. doi: 10.1016/j.jmbbm.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 129.Yan B., Zhang Z., Wang X., Ni Y., Liu Y., Liu T., Wang W., Xing H., Sun Y., Wang J., Li X.F. PLGA-PTMC-cultured bone mesenchymal stem cell scaffold enhances cartilage regeneration in tissue-engineered tracheal transplantation. Artif. Organs. 2017;41(5):461–469. doi: 10.1111/aor.12805. [DOI] [PubMed] [Google Scholar]
- 130.Wang J., Yang Q., Cheng N., Tao X., Zhang Z., Sun X., Zhang Q. Collagen/silk fibroin composite scaffold incorporated with PLGA microsphere for cartilage repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;61:705–711. doi: 10.1016/j.msec.2015.12.097. [DOI] [PubMed] [Google Scholar]
- 131.Pinskerova V., Iwaki H., Freeman M.A. The shapes and relative movements of the femur and tibia at the knee. Orthopä. 2000;29(Suppl 1):S3–S5. doi: 10.1007/pl00003679. [DOI] [PubMed] [Google Scholar]
- 132.Bagi C.M., Zakur D.E., Berryman E., Andresen C.J., Wilkie D. Correlation between muCT imaging, histology and functional capacity of the osteoarthritic knee in the rat model of osteoarthritis. J. Transl. Med. 2015;13:276. doi: 10.1186/s12967-015-0641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Eggli P.S., Hunziker E.B., Schenk R.K. Quantitation of structural features characterizing weight- and less-weight-bearing regions in articular cartilage: A stereological analysis of medial femoral condyles in young adult rabbits. Anat. Rec. 1988;222(3):217–227. doi: 10.1002/ar.1092220302. [DOI] [PubMed] [Google Scholar]
- 134.McDevitt C.A., Muir H. Biochemical changes in the cartilage of the knee in experimental and natural osteoarthritis in the dog. J. Bone Joint Surg. Br. 1976;58(1):94–101. doi: 10.1302/0301-620X.58B1.131804. [DOI] [PubMed] [Google Scholar]
- 135.Gong L., Zhou X., Wu Y., Zhang Y., Wang C., Zhou H., Guo F., Cui L. Proteomic analysis profile of engineered articular cartilage with chondrogenic differentiated adipose tissue-derived stem cells loaded polyglycolic acid mesh for weight-bearing area defect repair. Tissue Eng. Part A. 2014;20(3–4):575–587. doi: 10.1089/ten.tea.2013.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sah R.L. Interface and bulk regions in the repair, regeneration, and replacement of articular cartilage. J. Musculoskelet. Neuronal Interact. 2004;4(4):393–395. [PubMed] [Google Scholar]
- 137.Peck Y., He P., Chilla G.S., Poh C.L., Wang D.A. A preclinical evaluation of an autologous living hyaline-like cartilagious graft for articular cartilage repair: A pilot study. Sci. Rep. 2015;5:16225. doi: 10.1038/srep1622510.1038/srep16225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Choi B., Kim S., Fan J.B., Kowalski T., Petrigliano F., Evseenko D., Lee M. Covalently conjugated transforming growth factor-β1 in modular chitosan hydrogels for the effective treatment of articular cartilage defects. Biomater. Sci. 2015;3(5):742–752. doi: 10.1039/c4bm00431k. [DOI] [PubMed] [Google Scholar]
- 139.Palumbo F.S., Fiorica C., Di Stefano M., Pitarresi G., Gulino A., Agnello S., Giammona G. In situ forming hydrogels of hyaluronic acid and inulin derivatives for cartilage regeneration. Carbohydr. Polym. 2015;122:408–416. doi: 10.1016/j.carbpol.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 140.Vahedi P., Roshangar L., Jarolmasjed S., Shafaei H., Samadi N., Soleimanirad J. Effect of low-intensity pulsed ultrasound on regenerative potential of transplanted ASCs-PCL construct in articular cartilage defects in sheep. Indian J. Anim. Sci. 2016;86(10):1111–1114. [Google Scholar]
- 141.Cha S.R., Cho S.A., Lee S.E., Jang N.K., Cho S.J., Song J.E., Khang G. Evaluation of the therapeutic potential in vitro and in vivo of the SIS/PLGA scaffolds for costal cartilage regeneration. Macromol. Res. 2016;24(5):400–408. [Google Scholar]
- 142.Younesi M., Goldberg V.M., Akkus O. A micro-architecturally biomimetic collagen template for mesenchymal condensation based cartilage regeneration. Acta Biomater. 2016;30:212–221. doi: 10.1016/j.actbio.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cui L., Wu Y., Cen L., Zhou H., Yin S., Liu G., Liu W., Cao Y. Repair of articular cartilage defect in non-weight bearing areas using adipose derived stem cells loaded polyglycolic acid mesh. Biomaterials. 2009;30(14):2683–2693. doi: 10.1016/j.biomaterials.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 144.Engler A.J., Sen S., Sweeney H.L., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 145.Hu B., Xu C., Cao P., Tian Y., Zhang Y., Shi C., Xu J., Yuan W., Chen H. TGF-β stimulates expression of chondroitin polymerizing factor in nucleus pulposus cells through the Smad3, RhoA/ROCK1, and MAPK signaling pathways. J. Cell. Biochem. 2018;119(1):566–579. doi: 10.1002/jcb.26215. [DOI] [PubMed] [Google Scholar]
- 146.Guo J.B., Liang T., Che Y.J., Yang H.L., Luo Z.P. Structure and mechanical properties of high-weight-bearing and low-weight-bearing areas of hip cartilage at the micro- and nano-levels. BMC Muscoskel. Disord. 2020;21(1):1–9. doi: 10.1186/s12891-020-03468-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Davis S., Roldo M., Blunn G., Tozzi G., Roncada T. Influence of the mechanical environment on the regeneration of osteochondral defects. Front. Bioeng. Biotechnol. 2021;9:603408. doi: 10.3389/fbioe.2021.603408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nishino T., Chang F., Ishii T., Yanai T., Mishima H., Ochiai N. Joint distraction and movement for repair of articular cartilage in a rabbit model with subsequent weight-bearing. J. Bone Joint Surg. Br. 2010;92(7):1033–1040. doi: 10.1302/0301-620X.92B7.23200. [DOI] [PubMed] [Google Scholar]
- 149.Baboolal T.G., Mastbergen S.C., Jones E., Calder S.J., Lafeber F.P., McGonagle D. Synovial fluid hyaluronan mediates MSC attachment to cartilage, a potential novel mechanism contributing to cartilage repair in osteoarthritis using knee joint distraction. Ann. Rheum. Dis. 2016;75(5):908–915. doi: 10.1136/annrheumdis-2014-206847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mastbergen S. Joint distraction and cartilage regeneration − what is the basis for structural repair? Ann. Rheum. Dis. 2013;72(Suppl 3):A38. [Google Scholar]
- 151.Jansen M., Mastbergen S., Van Empelen M., Kester E., Lafeber F., Custers R. Knee joint distraction as standard of care treatment for knee osteoarthritis: A comparison with clinical trial patients. Ann. Rheum. Dis. 2019;78:505–506. [Google Scholar]
- 152.Li M.H., Xiao R., Li J.B., Zhu Q. Regenerative approaches for cartilage repair in the treatment of osteoarthritis. Osteoarthritis Cartilage. 2017;25(10):1577–1587. doi: 10.1016/j.joca.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 153.Freitag J., Bates D., Boyd R., Shah K., Barnard A., Huguenin L., Tenen A. Mesenchymal stem cell therapy in the treatment of osteoarthritis: Reparative pathways, safety nad efficacy − a review. BMC Muscoskel. Disord. 2016;17:230. doi: 10.1186/s12891-016-1085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Abramson S.B., Attur M. Developments in the scientific understanding of osteoarthritis. Arthritis Res. Ther. 2009;11(3):227. doi: 10.1186/ar2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Glyn-Jones S., Palmer A.J., Agricola R., Price A.J., Vincent T.L., Weinans H., Carr A.J. Osteoarthritis, Lancet. 2015;386(9991):376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 156.Mamidi M.K., Das A.K., Zakaria Z., Bhonde R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthritis Cartilage. 2016;24(8):1307–1316. doi: 10.1016/j.joca.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 157.Heinegard D., Saxne T. The role of the cartilage matrix in osteoarthritis. Nat. Rev. Rheumatol. 2011;7(1):50–56. doi: 10.1038/nrrheum.2010.198. [DOI] [PubMed] [Google Scholar]
- 158.Maldonado M., Nam J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. BioMed Res. Int. 2013;2013:284873. doi: 10.1155/2013/284873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Goldring M.B., Goldring S.R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. N. Y. Acad. Sci. 2010;1192:230–237. doi: 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]
- 160.Mort J.S., Billington C.J. Articular cartilage and changes in arthritis: Matrix degradation. Arthritis Res. 2001;3(6):337–341. doi: 10.1186/ar325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wilkinson D.J., Falconer A.M.D., Wright H.L., Lin H., Yamamoto K., Cheung K., Charlton S.H., Arques M.D.C., Janciauskiene S., Refaie R., Rankin K.S., Young D.A., Rowan A.D. Matrix metalloproteinase-13 is fully activated by neutrophil elastase and inactivates its serpin inhibitor, α-1 antitrypsin: Implications for osteoarthritis. FEBS J. 2021 doi: 10.1111/febs.16127. [DOI] [PubMed] [Google Scholar]
- 162.Zhao Q.H., Lin L.P., Guo Y.X., Zou R., Wang Z., Shi Z.P., Fu-Qing L. Matrix metalloproteinase-13, NFκB p65 and interleukin-1β are associated with the severity of knee osteoarthritis. Exp. Ther. Med. 2020;19(6):3620–3626. doi: 10.3892/etm.2020.8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Castañeda S., Vicente E.F. Osteoarthritis: More than cartilage degradation, clinical reviews in bone and mineral metabolism. Clin. Rev. Bone Miner. Metabol. 2017;15(2):69–81. [Google Scholar]
- 164.Zhang S., Teo K.Y.W., Chuah S.J., Lai R.C., Lim S.K., Toh W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35–47. doi: 10.1016/j.biomaterials.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 165.Lai R.C., Tan S.S., Teh B.J., Sze S.K., Arslan F., de Kleijn D.P., Choo A., Lim S.K. Proteolytic potential of the MSC exosome proteome: Implications for an exosome-mediated delivery of therapeutic proteasome. Int. J. Proteomics. 2012;2012:971907. doi: 10.1155/2012/971907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ingham P.W., Nakano Y., Seger C. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat. Rev. Genet. 2011;12(6):393–406. doi: 10.1038/nrg2984. [DOI] [PubMed] [Google Scholar]
- 167.Xiao W.F., Li Y.S., Deng A., Yang Y.T., He M. Functional role of hedgehog pathway in osteoarthritis. Cell Biochem. Funct. 2020;38(2):122–129. doi: 10.1002/cbf.3448. [DOI] [PubMed] [Google Scholar]
- 168.Zhou J., Chen Q., Lanske B., Fleming B.C., Terek R., Wei X., Zhang G., Wang S., Li K., Wei L. Disrupting the Indian hedgehog signaling pathway in vivo attenuates surgically induced osteoarthritis progression in Col2a1-CreERT2; Ihhfl/fl mice. Arthritis Res. Ther. 2014;16(1):R11. doi: 10.1186/ar4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sharma L. Osteoarthritis of the knee. N. Engl. J. Med. 2021;384(1):51–59. doi: 10.1056/NEJMcp1903768. [DOI] [PubMed] [Google Scholar]
- 170.Roemhildt M.L., Beynnon B.D., Gauthier A.E., Gardner-Morse M., Ertem F., Badger G.J. Chronic in vivo load alteration induces degenerative changes in the rat tibiofemoral joint. Osteoarthritis Cartilage. 2013;21(2):346–357. doi: 10.1016/j.joca.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Arno S., Bell C.P., Uquillas C., Borukhov I., Walker P.S. Tibiofemoral contact mechanics following a horizontal cleavage lesion in the posterior horn of the medial meniscus. J. Orthop. Res. 2015;33(4):584–590. doi: 10.1002/jor.22809. [DOI] [PubMed] [Google Scholar]
- 172.Felson D.T., Goggins J., Niu J., Zhang Y., Hunter D.J. The effect of body weight on progression of knee osteoarthritis is dependent on alignment. Arthritis Rheum. 2004;50(12):3904–3909. doi: 10.1002/art.20726. [DOI] [PubMed] [Google Scholar]
- 173.Adler N., Schoeniger A., Fuhrmann H. Effects of transforming growth factor-β and interleukin-1β on inflammatory markers of osteoarthritis in cultured canine chondrocytes. Am. J. Vet. Res. 2017;78(11):1264–1272. doi: 10.2460/ajvr.78.11.1264. [DOI] [PubMed] [Google Scholar]
- 174.Maksymowych W.P., Russell A.S., Chiu P., Yan A., Jones N., Clare T., Lambert R.G. Targeting tumour necrosis factor alleviates signs and symptoms of inflammatory osteoarthritis of the knee. Arthritis Res. Ther. 2012;14(5):R206. doi: 10.1186/ar4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chu J.G., Dai M.W., Wang Y., Tian F.M., Song H.P., Xiao Y.P., Shao L.T., Zhang Y.Z., Zhang L. Strontium ranelate causes osteophytes overgrowth in a model of early phase osteoarthritis. BMC Muscoskel. Disord. 2017;18(1) doi: 10.1186/s12891-017-1399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Piombo V. Small animal models to understand pathogenesis of osteoarthritis and use of stem cell in cartilage regeneration. Cell Biochem. Funct. 2017;35(1):3–11. doi: 10.1002/cbf.3246. [DOI] [PubMed] [Google Scholar]
- 177.Murphy J.M., Fink D.J., Hunziker E.B., Barry F.P. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48(12):3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 178.Lee S.Y., Lee S.H., Na H.S., Kwon J.Y., Kim G.Y., Jung K., Cho K.H., Kim S.A., Go E.J., Park M.J., Baek J.A., Choi S.Y., Jhun J., Park S.H., Kim S.J., Cho M.L. The therapeutic effect of STAT3 signaling-suppressed MSC on pain and articular cartilage damage in a rat model of monosodium iodoacetate-induced osteoarthritis. Front. Immunol. 2018;9:2881. doi: 10.3389/fimmu.2018.02881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li C., He Y., Li Y., Wang G., Liu D., Cai G., He C. A novel method to establish the rabbit model of knee osteoarthritis: Intra-articular injection of SDF-1 induces OA. BMC Muscoskel. Disord. 2021;22(1):329. doi: 10.1186/s12891-021-04188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nwosu L.N., Mapp P.I., Chapman V., Walsh D.A. Relationship between structural pathology and pain behaviour in a model of osteoarthritis (OA) Osteoarthritis Cartilage. 2016;24(11):1910–1917. doi: 10.1016/j.joca.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yeung P., Zhang W., Wang X.N., Yan C.H., Chan B.P. A human osteoarthritis osteochondral organ culture model for cartilage tissue engineering. Biomaterials. 2018;162:1–21. doi: 10.1016/j.biomaterials.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 182.Chen L., Zheng J.J.Y., Li G., Yuan J., Ebert J.R., Li H., Papadimitriou J., Wang Q., Wood D., Jones C.W., Zheng M. Pathogenesis and clinical management of obesity-related knee osteoarthritis: Impact of mechanical loading. J. Orthop. Translat. 2020;24:66–75. doi: 10.1016/j.jot.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Richette P., Poitou C., Garnero P., Vicaut E., Bouillot J.L., Lacorte J.M., Basdevant A., Clement K., Bardin T., Chevalier X. Benefits of massive weight loss on symptoms, systemic inflammation and cartilage turnover in obese patients with knee osteoarthritis. Ann. Rheum. Dis. 2011;70(1):139–144. doi: 10.1136/ard.2010.134015. [DOI] [PubMed] [Google Scholar]
- 184.Messier S.P., Gutekunst D.J., Davis C., DeVita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52(7):2026–2032. doi: 10.1002/art.21139. [DOI] [PubMed] [Google Scholar]
- 185.Messier S.P., Mihalko S.L., Legault C., Miller G.D., Nicklas B.J., DeVita P., Beavers D.P., Hunter D.J., Lyles M.F., Eckstein F., Williamson J.D., Carr J.J., Guermazi A., Loeser R.F. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: The IDEA randomized clinical trial. J. Am. Med. Assoc. 2013;310(12):1263–1273. doi: 10.1001/jama.2013.277669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.van Outeren M.V., Waarsing J.H., Brouwer R.W., Verhaar J.A.N., Reijman M., Bierma-Zeinstra S.M.A. Is a high tibial osteotomy (HTO) superior to non-surgical treatment in patients with varus malaligned medial knee osteoarthritis (OA)? A propensity matched study using 2 randomized controlled trial (RCT) datasets. Osteoarthritis Cartilage. 2017;25(12):1988–1993. doi: 10.1016/j.joca.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 187.Wang H., Wang Q.G., Yang M.J., Yang L.L., Wang W.L., Ding H.B., Zhang D., Xu J., Tang X.Z., Ding H.T., Wang Q.F. Histomorphology and innate immunity during the progression of osteoarthritis: Does synovitis affect cartilage degradation? J. Cell. Physiol. 2018;233(2):1342–1358. doi: 10.1002/jcp.26011. [DOI] [PubMed] [Google Scholar]
- 188.Glyn-Jones S., Palmer A.J.R., Agricola R., Price A.J., Vincent T.L., Weinans H., Carr A.J. Osteoarthritis, Lancet. 2015;386(9991):376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 189.Ramezanifard R., Kabiri M., Hanaee Ahvaz H. Effects of platelet rich plasma and chondrocyte co-culture on MSC chondrogenesis, hypertrophy and pathological responses. EXCLI J. 2017;16:1031–1045. doi: 10.17179/excli2017-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Frisbie D.D., Nixon A.J. Insulin-like growth factor 1 and corticosteroid modulation of chondrocyte metabolic and mitogenic activities in interleukin 1-conditioned equine cartilage. Am. J. Vet. Res. 1997;58(5):524–530. [PubMed] [Google Scholar]
- 191.He J., Wang L., Ding Y., Liu H., Zou G. lncRNA FER1L4 is dysregulated in osteoarthritis and regulates IL-6 expression in human chondrocyte cells. Sci. Rep. 2021;11(1):13032. doi: 10.1038/s41598-021-92474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Si H.B., Zeng Y., Liu S.Y., Zhou Z.K., Chen Y.N., Cheng J.Q., Lu Y.R., Shen B. Intra-articular injection of microRNA-140 (miRNA-140) alleviates osteoarthritis (OA) progression by modulating extracellular matrix (ECM) homeostasis in rats. Osteoarthritis Cartilage. 2017;25(10):1698–1707. doi: 10.1016/j.joca.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 193.Steinecker-Frohnwieser B., Lohberger B., Eck N., Mann A., Kratschmann C., Leithner A., Kullich W., Weigl L. Nuclear magnetic resonance therapy modulates the miRNA profile in human primary OA chondrocytes and antagonizes inflammation in Tc28/2a cells. Int. J. Mol. Sci. 2021;22(11) doi: 10.3390/ijms22115959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bajpayee A.G., Grodzinsky A.J. Cartilage-targeting drug delivery: Can electrostatic interactions help? Nat. Rev. Rheumatol. 2017;13(3):183–193. doi: 10.1038/nrrheum.2016.210. [DOI] [PubMed] [Google Scholar]
- 195.Han Y., Yang J., Zhao W., Wang H., Sun Y., Chen Y., Luo J., Deng L., Xu X., Cui W., Zhang H. Biomimetic injectable hydrogel microspheres with enhanced lubrication and controllable drug release for the treatment of osteoarthritis. Bioact. Mater. 2021;6(10):3596–3607. doi: 10.1016/j.bioactmat.2021.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jin G.Z. Current nanoparticle-based technologies for osteoarthritis therapy. Nanomaterials. 2020;10(12):2368–2388. doi: 10.3390/nano10122368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chang M.C., Chiang P.F., Kuo Y.J., Peng C.L., Chen K.Y., Chiang Y.C. Hyaluronan-loaded liposomal dexamethasone-diclofenac nanoparticles for local osteoarthritis treatment. Int. J. Mol. Sci. 2021;22(2):665–684. doi: 10.3390/ijms22020665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li X., Dai B., Guo J., Zheng L., Guo Q., Peng J., Xu J., Qin L. Nanoparticle-cartilage interaction: Pathology-based intra-articular drug delivery for osteoarthritis therapy. Nano-Micro Lett. 2021;13(1):1–48. doi: 10.1007/s40820-021-00670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xue S., Zhou X., Sang W., Wang C., Lu H., Xu Y., Zhong Y., Zhu L., He C., Ma J. Cartilage-targeting peptide-modified dual-drug delivery nanoplatform with NIR laser response for osteoarthritis therapy. Bioact. Mater. 2021;6(8):2372–2389. doi: 10.1016/j.bioactmat.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wei Y., Yan L., Luo L., Gui T., Jang B., Amirshaghaghi A., You T., Tsourkas A., Qin L., Cheng Z. Phospholipase A2 inhibitor-loaded micellar nanoparticles attenuate inflammation and mitigate osteoarthritis progression. Sci. Adv. 2021;7(15) doi: 10.1126/sciadv.abe6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kang L.J., Yoon J., Rho J.G., Han H.S., Lee S., Oh Y.S., Kim H., Kim E., Kim S.J., Lim Y.T., Park J.H., Song W.K., Yang S., Kim W. Self-assembled hyaluronic acid nanoparticles for osteoarthritis treatment. Biomaterials. 2021;275:120967. doi: 10.1016/j.biomaterials.2021.120967. [DOI] [PubMed] [Google Scholar]
- 202.Zhao L., Huang J., Fan Y., Li J., You T., He S., Xiao G., Chen D. Exploration of CRISPR/Cas9-based gene editing as therapy for osteoarthritis. Ann. Rheum. Dis. 2019;78(5):676–682. doi: 10.1136/annrheumdis-2018-214724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bijlsma J.W., Berenbaum F., Lafeber F.P. Osteoarthritis: An update with relevance for clinical practice. Lancet. 2011;377(9783):2115–2126. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 204.Vignon E., Arlot M., Patricot L.M., Vignon G. The cell density of human femoral head cartilage. Clin. Orthop. Relat. Res. 1976;121:303–308. [PubMed] [Google Scholar]
- 205.Martin J.A., Buckwalter J.A. Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology. 2002;3(5):257–264. doi: 10.1023/a:1020185404126. [DOI] [PubMed] [Google Scholar]
- 206.Blaney Davidson E.N., Scharstuhl A., Vitters E.L., van der Kraan P.M., van den Berg W.B. Reduced transforming growth factor-β signaling in cartilage of old mice: Role in impaired repair capacity. Arthritis Res. Ther. 2005;7(6):R1338–R1347. doi: 10.1186/ar1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lee H., Zhao X., Son Y.O., Yang S. Therapeutic single compounds for osteoarthritis treatment. Pharmaceuticals. 2021;14(2):131–149. doi: 10.3390/ph14020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Murphy M.P., Koepke L.S., Lopez M.T., Tong X., Ambrosi T.H., Gulati G.S., Marecic O., Wang Y., Ransom R.C., Hoover M.Y., Steininger H., Zhao L., Walkiewicz M.P., Quarto N., Levi B., Wan D.C., Weissman I.L., Goodman S.B., Yang F., Longaker M.T., Chan C.K.F. Articular cartilage regeneration by activated skeletal stem cells. Nat. Med. 2020;26(10) doi: 10.1038/s41591-020-1013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Richette P., Corvol M., Bardin T. Estrogens, cartilage, and osteoarthritis. Joint Bone Spine. 2003;70(4):257–262. doi: 10.1016/s1297-319x(03)00067-8. [DOI] [PubMed] [Google Scholar]
- 210.Korach K.S., Couse J.F., Curtis S.W., Washburn T.F., Lindzey J., Kimbro K.S., Eddy E.M., Migliaccio S., Snedeker S.M., Lubahn D.B., Schomberg D.W., Smith E.P. Estrogen receptor gene disruption: Molecular characterization and experimental and clinical phenotypes. Recent Prog. Horm. Res. 1996;51:159–186. [PubMed] [Google Scholar]
- 211.Ravn P., Warming L., Christgau S., Christiansen C. The effect on cartilage of different forms of application of postmenopausal estrogen therapy: Comparison of oral and transdermal therapy. Bone. 2004;35(5):1216–1221. doi: 10.1016/j.bone.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 212.Xu X., Li X., Liang Y., Ou Y., Huang J., Xiong J., Duan L., Wang D. Estrogen modulates cartilage and subchondral bone remodeling in an ovariectomized rat model of postmenopausal osteoarthritis. Med. Sci. Monit. 2019;25:3146–3153. doi: 10.12659/MSM.916254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hang X., Zhang Z., Niu R., Wang C., Yao J., Xu Y., Tao J., Li L., Chen F. Estrogen protects articular cartilage by downregulating ASIC1a in rheumatoid arthritis. J. Inflamm. Res. 2021;14:843–858. doi: 10.2147/JIR.S295222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wluka A.E., Davis S.R., Bailey M., Stuckey S.L., Cicuttini F.M. Users of oestrogen replacement therapy have more knee cartilage than non-users. Ann. Rheum. Dis. 2001;60(4):332–336. doi: 10.1136/ard.60.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Smolen J.S., Aletaha D., McInnes I.B. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 216.Kastrinaki M.C., Papadaki H.A. Mesenchymal stromal cells in rheumatoid arthritis: Biological properties and clinical applications. Curr. Stem Cell Res. Ther. 2009;4(1):61–69. doi: 10.2174/157488809787169084. [DOI] [PubMed] [Google Scholar]
- 217.Choy E.H., Panayi G.S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 2001;344(12):907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 218.Bhattacharya N. Rheumatoid arthritis. N. Engl. J. Med. 2004;351(13):1360–1361. [PubMed] [Google Scholar]
- 219.Park S.K., Hwang P.H., Yun S.K., Kim H.U., Park J. Tumor necrosis factor α blocker-induced erythrodermic sarcoidosis in with juvenile rheumatoid arthritis: A case report and review of the literature. Ann. Dermatol. 2017;29(1):74–78. doi: 10.5021/ad.2017.29.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gunasekera W.M., Kirwan J.R. Rheumatoid arthritis: Previously untreated early disease. BMJ Clin. Evid. 2016;2016 [PMC free article] [PubMed] [Google Scholar]
- 221.Dulic S., Vasarhelyi Z., Sava F., Berta L., Szalay B., Toldi G., Kovacs L., Balog A. T-cell subsets in rheumatoid arthritis patients on long-term anti-TNF or IL-6 receptor blocker therapy. Mediat. Inflamm. 2017;2017 doi: 10.1155/2017/6894374. 6894374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mitragotri S., Yoo J.W. Designing micro- and nano-particles for treating rheumatoid arthritis. Arch Pharm. Res. 2011;34(11):1887–1897. doi: 10.1007/s12272-011-1109-9. [DOI] [PubMed] [Google Scholar]
- 223.Tarner I.H., Muller-Ladner U. Drug delivery systems for the treatment of rheumatoid arthritis. Expet Opin. Drug Deliv. 2008;5(9):1027–1037. doi: 10.1517/17425247.5.9.1027. [DOI] [PubMed] [Google Scholar]
- 224.van den Hoven J.M., Van Tomme S.R., Metselaar J.M., Nuijen B., Beijnen J.H., Storm G. Liposomal drug formulations in the treatment of rheumatoid arthritis. Mol. Pharm. 2011;8(4):1002–1015. doi: 10.1021/mp2000742. [DOI] [PubMed] [Google Scholar]
- 225.Xiao S., Tang Y., Lv Z., Lin Y., Chen L. Nanomedicine − Advantages for their use in rheumatoid arthritis theranostics. J. Control. Release. 2019;316:302–316. doi: 10.1016/j.jconrel.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 226.Higaki M., Ishihara T., Izumo N., Takatsu M., Mizushima Y. Treatment of experimental arthritis with poly(D,L-lactic/glycolic acid) nanoparticles encapsulating betamethasone sodium phosphate. Ann. Rheum. Dis. 2005;64(8):1132–1136. doi: 10.1136/ard.2004.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Oliveira I.M., Gonçalves C., Reis R.L., Oliveira J.M. Engineering nanoparticles for targeting rheumatoid arthritis: Past, present, and future trends. Nano Res. 2018;11(9):4489–4506. [Google Scholar]
- 228.Jabbari N., Eftekhari Z., Roodbari N.H., Parivar K. Evaluation of encapsulated eugenol by chitosan nanoparticles on the aggressive model of rheumatoid arthritis. Inter. Immunopharmcol. 2020;85:106554. doi: 10.1016/j.intimp.2020.106554. [DOI] [PubMed] [Google Scholar]
- 229.Zielinska A., Carreiro F., Oliveira A.M., Neves A., Pires B., Venkatesh D.N., Durazzo A., Lucarini M., Eder P., Silva A.M., Santini A., Souto E.B. Polymeric nanoparticles: Production, characterization, toxicology and ecotoxicology. Molecules. 2020;25(16):3731–3751. doi: 10.3390/molecules25163731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lyu J., Wang L., Bai X., Du X., Wei J., Wang J., Lin Y., Chen Z., Liu Z., Wu J., Zhong Z. Treatment of rheumatoid arthritis by serum albumin nanoparticles coated with mannose to target neutrophils. ACS Appl. Mater. Interfaces. 2021;13(1):266–276. doi: 10.1021/acsami.0c19468. [DOI] [PubMed] [Google Scholar]
- 231.Runnels H.A., Weber G.L., Min J., Kudlacz E.M., Zobel J.F., Donovan C.B., Thiede M.A., Zhang J., Alpert R.B., Salafia M.A., Milici A.J., Burdette D., Bell R.R., Beebe J.S., Xu X. PF-03475952: A potent and neutralizing fully human anti-CD44 antibody for therapeutic application in inflammatory diseases. Adv. Ther. 2010;27(3):168–180. doi: 10.1007/s12325-010-0010-0. [DOI] [PubMed] [Google Scholar]
- 232.Heo R., Park J.S., Jang H.J., Kim S.H., Shin J.M., Suh Y.D., Jeong J.H., Jo D.G., Park J.H. Hyaluronan nanoparticles bearing γ-secretase inhibitor: In vivo therapeutic effects on rheumatoid arthritis. J. Control. Release. 2014;192:295–300. doi: 10.1016/j.jconrel.2014.07.057. [DOI] [PubMed] [Google Scholar]
- 233.Poh S., Chelvam V., Kelderhouse L.E., Ayala-Lopez W., Vaitilingam B., Putt K.S., Low P.S. Folate-conjugated liposomes target and deliver therapeutics to immune cells in a rat model of rheumatoid arthritis. Nanomedicine. 2017;12(20):2441–2451. doi: 10.2217/nnm-2017-0166. [DOI] [PubMed] [Google Scholar]
- 234.Liu X.M., Quan L.D., Tian J., Laquer F.C., Ciborowski P., Wang D. Syntheses of click PEG-dexamethasone conjugates for the treatment of rheumatoid arthritis. Biomacromolecules. 2010;11(10):2621–2628. doi: 10.1021/bm100578c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jones E., Churchman S.M., English A., Buch M.H., Horner E.A., Burgoyne C.H., Reece R., Kinsey S., Emery P., McGonagle D., Ponchel F. Mesenchymal stem cells in rheumatoid synovium: Enumeration and functional assessment in relation to synovial inflammation level. Ann. Rheum. Dis. 2010;69(2):450–457. doi: 10.1136/ard.2008.106435. [DOI] [PubMed] [Google Scholar]
- 236.Ansboro S., Roelofs A.J., De Bari C. Mesenchymal stem cells for the management of rheumatoid arthritis: Immune modulation, repair or both? Curr. Opin. Rheumatol. 2017;29(2):201–207. doi: 10.1097/BOR.0000000000000370. [DOI] [PubMed] [Google Scholar]
- 237.Han Y., Pang X., Pi G. Biomimetic and bioinspired intervention strategies for the treatment of rheumatoid arthritis. Adv. Funct. Mater. 2021:2104640. [Google Scholar]
- 238.Augello A., Tasso R., Negrini S.M., Cancedda R., Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56(4):1175–1186. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- 239.Choi J.J., Yoo S.A., Park S.J., Kang Y.J., Kim W.U., Oh I.H., Cho C.S. Mesenchymal stem cells overexpressing interleukin-10 attenuate collagen-induced arthritis in mice. Clin. Exp. Immunol. 2008;153(2):269–276. doi: 10.1111/j.1365-2249.2008.03683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lopa S., Colombini A., Moretti M., de Girolamo L. Injective mesenchymal stem cell-based treatments for knee osteoarthritis: From mechanisms of action to current clinical evidences. Knee Surg. Sports Traumatol. Arthrosc. 2019;27(6):2003–2020. doi: 10.1007/s00167-018-5118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Liu H., Ding J., Wang C., Wang J., Wang Y., Yang M., Jia Y., Zhang Y., Chang F., Li R., Chen X. Intra-articular transplantation of allogeneic BMMSCs rehabilitates cartilage injury of antigen-induced arthritis. Tissue Eng. Part A. 2015;21(21–22):2733–2743. doi: 10.1089/ten.TEA.2014.0666. [DOI] [PubMed] [Google Scholar]
- 242.Liu H., Ding J., Wang J., Wang Y., Yang M., Zhang Y., Chang F., Chen X. Remission of collagen-induced arthritis through combination therapy of microfracture and transplantation of thermogel-encapsulated bone marrow mesenchymal stem cells. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0120596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Liu H., Ding J., Li C., Wang C., Wang Y., Wang J., Chang F. Hydrogel is superior to fibrin gel as matrix of stem cells in alleviating antigen-induced arthritis. Polymers. 2016;8(5):182. doi: 10.3390/polym8050182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zhao Y., Wang Z., Jiang Y., Liu H., Song S., Wang C., Li Z., Yang Z., Liu H., Wang J., Yang B., Lin Q. Biomimetic composite scaffolds to manipulate stem cells for aiding rheumatoid arthritis management. Adv. Funct. Mater. 2019;29(30):1807860. [Google Scholar]
- 245.Ra J.C., Kang S.K., Shin I.S., Park H.G., Joo S.A., Kim J.G., Kang B.C., Lee Y.S., Nakama K., Piao M., Sohl B., Kurtz A. Stem cell treatment for patients with autoimmune disease by systemic infusion of culture-expanded autologous adipose tissue derived mesenchymal stem cells. J. Transl. Med. 2011;9:181. doi: 10.1186/1479-5876-9-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wang L., Wang L., Cong X., Liu G., Zhou J., Bai B., Li Y., Bai W., Li M., Ji H., Zhu D., Wu M., Liu Y. Human umbilical cord mesenchymal stem cell therapy for patients with active rheumatoid arthritis: Safety and efficacy. Stem Cell. Dev. 2013;22(24):3192–3202. doi: 10.1089/scd.2013.0023. [DOI] [PubMed] [Google Scholar]
- 247.Yang Y., He X., Zhao R., Guo W., Zhu M., Xing W., Jiang D., Liu C., Xu X. Serum IFN-γ levels predict the therapeutic effect of mesenchymal stem cell transplantation in active rheumatoid arthritis. J. Transl. Med. 2018;16(1):165. doi: 10.1186/s12967-018-1541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lopez-Santalla M., Fernandez-Perez R., Garin M.I. Mesenchymal stem/stromal cells for rheumatoid arthritis treatment: An update on clinical application. Cells. 2020;9(8):1852. doi: 10.3390/cells9081852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Abramson S.B., Amin A. Blocking the effects of IL-1 in rheumatoid arthritis protects bone and cartilage. Rheumatology. 2002;41(9):972–980. doi: 10.1093/rheumatology/41.9.972. [DOI] [PubMed] [Google Scholar]
- 250.Chan J.M., Villarreal G., Jin W.W., Stepan T., Burstein H., Wahl S.M. Intraarticular gene transfer of TNFR: Fc suppresses experimental arthritis with reduced systemic distribution of the gene product. Mol. Ther. 2002;6(6):727–736. doi: 10.1006/mthe.2002.0808. [DOI] [PubMed] [Google Scholar]
- 251.Gao F., Yuan Q., Cai P., Gao L., Zhao L., Liu M., Yao Y., Chai Z., Gao X. Au clusters treat rheumatoid arthritis with uniquely reversing cartilage/bone destruction. Adv. Sci. 2019;6(7):1801671. doi: 10.1002/advs.201801671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Apelgren P., Amoroso M., Lindahl A., Brantsing C., Rotter N., Gatenholm P., Kolby L. Chondrocytes and stem cells in 3D-bioprinted structures create human cartilage in vivo. PLoS One. 2017;12(12) doi: 10.1371/journal.pone.0189428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Cunniffe G.M., Gonzalez-Fernandez T., Daly A., Sathy B.N., Jeon O., Alsberg E., Kelly D.J. Three-dimensional bioprinting of polycaprolactone reinforced gene activated bioinks for bone tissue engineering. Tissue Eng. Part A. 2017;23(17–18):891–900. doi: 10.1089/ten.tea.2016.0498. [DOI] [PubMed] [Google Scholar]
- 254.Wang Z., Wang C., Li C., Qin Y., Zhong L., Chen B., Li Z., Liu H., Chang F., Wang J. Analysis of factors influencing bone ingrowth into three-dimensional printed metal scaffolds: A review. J. Alloys Compd. 2017;717:271–285. [Google Scholar]
- 255.Gibson J.D., O'Sullivan M.B., Alaee F., Paglia D.N., Yoshida R., Guzzo R.M., Drissi H. Regeneration of articular cartilage by human ESC-derived mesenchymal progenitors treated sequentially with BMP-2 and Wnt5a. Stem Cell Transl. Med. 2017;6(1):40–50. doi: 10.5966/sctm.2016-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.McGee-Lawrence M.E., Carpio L.R., Bradley E.W., Dudakovic A., Lian J.B., van Wijnen A.J., Kakar S., Hsu W., Westendorf J.J. Runx2 is required for early stages of endochondral bone formation but delays final stages of bone repair in Axin2-deficient mice. Bone. 2014;66:277–286. doi: 10.1016/j.bone.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lee J., Lee J.Y., Chae B.C., Jang J., Lee E., Son Y. Fully dedifferentiated chondrocytes expanded in specific mesenchymal stem cell growth medium with FGF2 obtains mesenchymal stem cell phenotype in vitro but retains chondrocyte phenotype in vivo. Cell Transplant. 2017;26(10):1673–1687. doi: 10.1177/0963689717724794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Jablonska-Trypuc A., Matejczyk M., Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2016;31:177–183. doi: 10.3109/14756366.2016.1161620. [DOI] [PubMed] [Google Scholar]
- 259.Ichiseki T., Shimazaki M., Ueda Y., Ueda S., Tsuchiya M., Souma D., Kaneuji A., Kawahara N. Intraarticularly-injected mesenchymal stem cells stimulate anti-inflammatory molecules and inhibit pain related protein and chondrolytic enzymes in a monoiodoacetate-induced rat arthritis model. Int. J. Mol. Sci. 2018;19(1):203. doi: 10.3390/ijms19010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lu X.Y., Fukumoto S., Yamada Y., Evans C.A., Diekwisch T.G.H., Luan X.H. Ameloblastin, an extracellular matrix protein, affects long bone growth and mineralization. J. Bone Miner. Res. 2016;31(6):1235–1246. doi: 10.1002/jbmr.2788. [DOI] [PubMed] [Google Scholar]