Abstract
The onset of random X chromosome inactivation in mouse requires the switch from a symmetric to an asymmetric state, where the identities of the future inactive and active X chromosomes are assigned. This process is known as X chromosome choice. Here, we show that RIF1 and KAP1 are two fundamental factors for the definition of this transcriptional asymmetry. We found that at the onset of differentiation of mouse embryonic stem cells (mESCs), biallelic up‐regulation of the long non‐coding RNA Tsix weakens the symmetric association of RIF1 with the Xist promoter. The Xist allele maintaining the association with RIF1 goes on to up‐regulate Xist RNA expression in a RIF1‐dependent manner. Conversely, the promoter that loses RIF1 gains binding of KAP1, and KAP1 is required for the increase in Tsix levels preceding the choice. We propose that the mutual exclusion of Tsix and RIF1, and of RIF1 and KAP1, at the Xist promoters establish a self‐sustaining loop that transforms an initially stochastic event into a stably inherited asymmetric X‐chromosome state.
Keywords: KAP1, RIF1, Tsix, X chromosome inactivation, Xist
Subject Categories: Chromatin, Transcription & Genomics; Development
Differentiation‐induced Tsix RNA triggers asymmetric distribution of RIF1 and KAP1 on the future inactive and active mouse X chromosomes, respectively, establishing a self‐sustaining Xist expression loop from an initially stochastic event.
Introduction
X chromosome inactivation (XCI) is the process leading to the stable transcriptional silencing of one of the two X chromosomes in female placental mammals, with the aim of equalising the expression of X‐linked genes between males and females (Lyon, 1961). This process represents one of the best‐studied examples of how different nuclear processes, such as epigenetic control, 3D organisation of chromatin contacts, sub‐nuclear positioning and, potentially, replication‐timing regulation, are integrated to achieve transcriptional control. Random XCI (rXCI) is initiated when Xist, an X‐encoded long non‐coding RNA (lncRNA) is up‐regulated from one of the two X chromosomes, the future inactive X chromosome (Xi) (Brockdorff et al, 1991; Brown et al, 1991; Penny et al, 1996; Marahrens et al, 1997). In vivo, this happens around the time of embryo implantation (Monk & Harper, 1978; Rastan, 1982), while in cultured female mouse embryonic stem cells (mESCs), XCI takes place during a narrow time‐window at the onset of differentiation (Wutz & Jaenisch, 2000). Monoallelic up‐regulation of Xist is coupled to loss of pluripotency and several activating and repressing factors of this process have been described (Lee & Lu, 1999; Navarro et al, 2008; Jonkers et al, 2009; Tian et al, 2010; Chureau et al, 2011; Gontan et al, 2012; Makhlouf et al, 2014; Furlan et al, 2018). Guided by the three‐dimensional (3D) conformation of the X chromosome (Engreitz et al, 2013; Simon et al, 2013), Xist spreads in cis and recruits SPEN to enhancers and promoters of the X‐linked genes to trigger their silencing (Chu et al, 2015; McHugh et al, 2015; Moindrot et al, 2015; Monfort et al, 2015; Dossin et al, 2020), and the exclusion of RNA polymerase II (Chaumeil et al, 2006; Kucera et al, 2011). This in turn promotes the recruitment of the Polycomb Repressive Complexes (PRC1/2) and the accumulation of tri‐methylated H3K27 (H3K27me3) (Sun et al, 2006; Zhao et al, 2008) and monoubiquitinated H2AK119 (H2AK119ub) (de Napoles et al, 2004) on the future inactive X chromosome (Xi). Contextually, Lamin B receptor (LBR) tethers the future Xi to the nuclear periphery to facilitate Xist spreading into active gene regions and the maintenance of the silent state (Chen et al, 2016). Finally, the entire Xi switches the replication timing to mid‐late S‐phase (Takagi et al, 1982). The combination of all these events facilitates the attainment of an exceptionally stable transcriptionally silent status, so robustly controlled that it is maintained throughout the entire life of the organism. One of the least understood of all these steps is the mechanism that, in the initiating phase of XCI, directs the random choice of which one of the two Xist alleles to up‐regulate, marking the future Xi, and which to silence (marking the future active X chromosome, Xa). We will refer to this process as the “choice” (Avner & Heard, 2001). This is a key stage, as failure to establish monoallelic Xist expression can result in either both X chromosomes being silenced or both remaining active, consequently leading to embryonic lethality (Takagi & Abe, 1990; Marahrens et al, 1997; Borensztein et al, 2017). Tsix is a lncRNA encoded by a gene that overlaps, in the antisense orientation, with Xist, and plays a well‐established role as an in cis repressor of Xist (Lee & Lu, 1999). In female mESCs, Tsix is bi‐allelically expressed, to become down‐regulated on one of the two X chromosomes, the future Xi, at the onset of differentiation, hence allowing for in cis Xist up‐regulation (Lee et al, 1999; Stavropoulos et al, 2001). The switch to mono‐allelic expression of Tsix is important in determining the destinies of the future Xi (Tsix silenced) and Xa (Tsix transiently maintained). In fact, interfering with the expression of one of the two Tsix alleles in female mESCs results in a non‐random choice, with the Tsix‐defective chromosome pre‐determined as the future Xi (reviewed in (Starmer & Magnuson, 2009)). Although Tsix down‐regulation is essential for in cis up‐regulation of Xist, the molecular mechanism of Tsix‐driven silencing is still unclear. The Tsix terminator region overlaps with the Xist promoter, and Tsix transcription through this region and/or Tsix RNA have been proposed to be essential for Xist repression (Shibata & Lee, 2004) by promoting a transient silenced chromatin state (Navarro et al, 2005, 2006; Sado et al, 2005; Ohhata et al, 2008). The establishment of the opposite Xist/Tsix expression patterns on the two genetically identical X chromosomes must rely on the coordinated asymmetric distribution of activators and/or repressors of transcription.
Mathematical modelling can recapitulate the experimental features of XCI by postulating the existence of an in cis‐negative regulator of Xist (cXR) and an in trans, X‐linked, Xist activator (tXA) (Mutzel et al, 2019). While a cXR is sufficient to explain the maintenance of mono‐allelic Xist expression, a tXA is needed to explain: 1. the establishment of the Xist mono‐allelic expression; 2. the female specificity of XCI; 3. the resolution of bi‐allelic Xist expression detected, to various extents, in different organisms (Mutzel et al, 2019). In mouse, Tsix is the most likely cXR, while RNF12, an X‐linked ubiquitin ligase that functions as a dose‐dependent initiator of XCI (Jonkers et al, 2009; Gontan et al, 2012), has been proposed as one of the potential tXA. However, while overexpression of Rlim (Rnf12) in male cells can induce XCI (Jonkers et al, 2009), its deletion in females is not sufficient to prevent Xist up‐regulation (Shin et al, 2014; Wang et al, 2017). Thus, RNF12 could account for the X‐linked aspects of the tXA function, such as female specificity and resolution of bi‐allelic expression, but one or multiple other transactivators must be contributing to the asymmetric control of Xist expression. Moreover, conceptually, the expression level of a single, X‐linked gene, does not constitute a switch robust or sensitive enough to be the only element to control a clear‐cut bi‐stable state for Xist (active on one and silent on the other allele) (Mutzel & Schulz, 2020). The establishment of in cis, self‐reinforcing and mutually exclusive circuits on the two Xist alleles could create the ultrasensitivity required to generate a binary switch‐type of control (Mutzel & Schulz, 2020). Key to this model, is the idea that the initial stochastic events will trigger a chain of local, mutually exclusive and self‐sustaining events to bookmark both Xi and Xa.
RIF1 is a multifaceted protein, required for the regulation of several of the nuclear processes that take place during XCI. RIF1 is the only known genome‐wide regulator of replication timing (Cornacchia et al, 2012; Hayano et al, 2012; Yamazaki et al, 2012; Hiraga et al, 2014; Peace et al, 2014; Foti et al, 2016; Seller & O'Farrell, 2018). It confines long‐range chromatin contacts within the respective boundaries of the nuclear A/B compartments (Gnan et al, 2021) and plays an as yet unclear function in the control of gene expression (Daxinger et al, 2013; Foti et al, 2016; Tanaka et al, 2016; Zofall et al, 2016; Li et al, 2017; Toteva et al, 2017). RIF1 is an adaptor for Protein Phosphatase 1 (PP1), one of the main Ser/Thr phosphatases in eukaryotic cells (Trinkle‐Mulcahy et al, 2006; Dave et al, 2014; Hiraga et al, 2014, 2017; Mattarocci et al, 2014; Sreesankar et al, 2015; Alver et al, 2017). In Drosophila melanogaster, the RIF1‐PP1 interaction was shown to be essential during embryonic development (Seller & O'Farrell, 2018). In addition, the RIF1‐PP1 interaction is essential for RIF1‐dependent organisation of chromatin contacts (Gnan et al, 2021). Others (Chapman et al, 2013; Daxinger et al, 2013) and we (this work) have observed that mouse RIF1 deficiency is associated with a sex‐linked differential lethality, with the female embryos dying around the time of implantation. These data have suggested that RIF1 could be important during XCI. Here we find that RIF1, present biallelically on the Xist P2‐promoter in female mESCs, becomes asymmetrically enriched at P2 on the future Xi, concomitant with the choice, at the time when Tsix expression switches from bi‐ to mono‐allelic. RIF1 then plays an essential role in Xist up‐regulation, thus determining the future Xi. The removal of RIF1 from the future Xa arises from the KAP1‐dependent increase of Tsix bi‐allelic expression that leads to the choice. Our data identify the KAP1‐dependent regulation of Tsix levels and the consequent transition of RIF1 association with Xist promoter from symmetric to asymmetric, as key steps in the molecular cascade that leads to the identification of the future Xi and Xa.
Results
RIF1 is required for X inactivation during embryonic development and for Xist up‐regulation
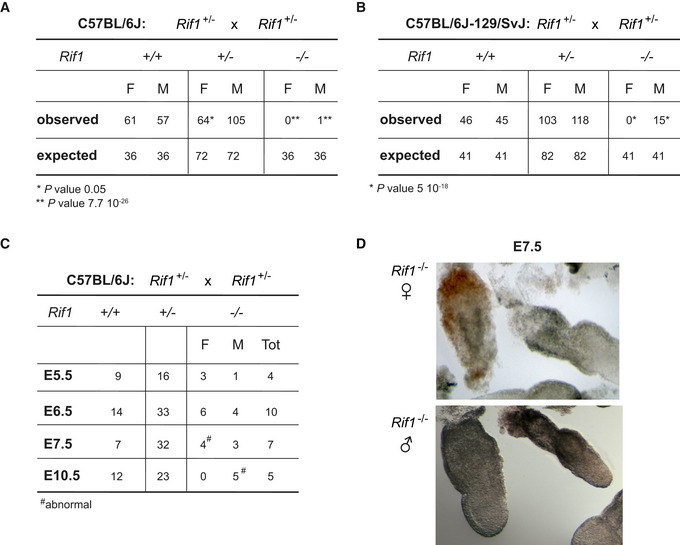
The analysis of the progeny derived from inter‐crosses of mice heterozygous for a Rif1 null allele (Rif1 +/−, Appendix Fig S1A and B) in a pure C57BL/6J genetic background has revealed that Rif1 is essential for embryonic development (Fig 1A). In contrast, in a mixed genetic C57BL/6J‐129/SvJ background, Rif1 deletion results in a differential lethality between the sexes (Fig 1B). Indeed, in this case, only a small proportion of the expected Rif1 −/− mice, exclusively males, are recovered at weaning. In order to pinpoint more precisely the time of the onset of lethality, we have analysed the frequency of recovery of Rif1 −/− embryos at different stages of development in a C57BL/6J pure background. We found that, up to the blastocyst stage (E3.5), there are no obvious differences in the number of male and female Rif1 null and wild‐type embryos recovered (our unpublished observation). However, by E7.5, although still recoverable, Rif1 null female embryos are already dead/abnormal (Fig 1C and D). In contrast, males appear to die only around mid‐gestation (Fig 1C). This early‐onset lethality observed specifically in females suggests that the lack of RIF1 could interfere with the process of XCI, as the timing coincides with the onset of random XCI.
Figure 1. Rif1 deficiency leads to female embryonic lethality at peri‐implantation.
-
A, BTables summarising the number and the sex of the pups recovered at weaning from Rif1 +/− x Rif1 +/− mice inter‐crosses, either in a C57BL/6J (A) or in a mixed C57BL/6J‐129/SvJ genetic background (B). The observed number of mice is compared to the expected number, based on the Mendelian ratio. P calculated by χ2.
-
CThe table summarises the number and the sex of the embryos of the indicated genotypes, recovered from timed matings of Rif1 +/− x Rif1 +/− mice, in a C57BL/6J genetic background. The day of gestation (E) is indicated. (D). Representative images of Rif1 −/− E7.5 embryos, female top and male bottom.
Given the diversity of its roles, RIF1 could act at one or several of the multiple steps during XCI. To dissect at what stage(s) of the process RIF1 is required, we generated female mESCs carrying homozygous conditional Rif1 allele (Rif1Flox/Flox ) and a tamoxifen‐inducible CRE recombinase (Rosa26Cre‐ERT/+ , Buonomo et al, 2009). To trigger XCI in the absence of Rif1, we set up a protocol in which we combined differentiation by embryoid body (EB) formation (Doetschman et al, 1985) and tamoxifen treatment (Fig 2A and Materials and Methods). By RT‐qPCR as well as by RNA sequencing, we found that Rif1 deletion (Fig 2B) severely impairs Xist up‐regulation (Figs 2C, EV1A and B, and EV2A) and, consequently, the enrichment of H3K27me3 on the future Xi (Fig 2D). Failure of Xist up‐regulation in the absence of Rif1 is not due to a general defect in exit from pluripotency (Figs EV1C and EV2B, D and E) or to failed commitment to differentiation (Figs EV1C and EV2C–E). Moreover, during the early stages of differentiation the levels of the main negative regulator of Xist, Tsix, appear to be reduced faster in Rif1 knockout cells compared to the control (Appendix Fig S2A). Finally, the overall dynamics of RNF12 appear comparable between control and Rif1 knockout cells (Fig EV1B and Appendix Fig S2B). Overall, these results indicate that failure of Xist up‐regulation is the likely cause of defective XCI in Rif1 null female embryos and that RIF1 could directly and positively regulate Xist expression.
Figure 2. Rif1 null female mESCs fail to up‐regulate Xist upon differentiation.
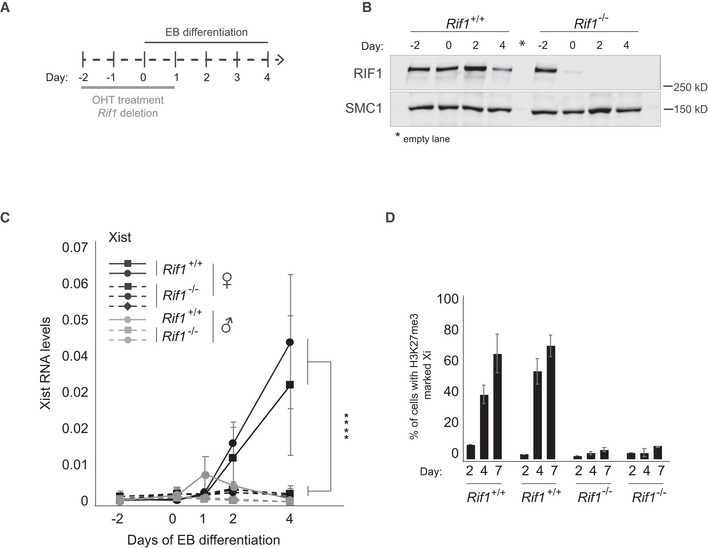
- Overview of the experimental design. Rif1+/+ and Rif1F/F mESCs were grown for 2 days in medium supplemented with 4‐Hydroxytamoxifen (OHT) to induce the translocation of the Cre recombinase into the nucleus, leading to Rif1 deletion in the Rif1F/F cells (Rif1 −/− ). The embryoid body (EB) differentiation protocol was then started to trigger XCI. OHT was kept in the medium during the first 24 h of EB differentiation. Cells were differentiated up to 4 (RNA analysis) or 7 days (H3K27me3 IF).
- Representative western blot to monitor RIF1 levels after Cre‐mediated Rif1 deletion and EB differentiation. SMC1: loading control.
- Time course analysis of Xist RNA expression by RT–qPCR during EB differentiation of Rif1+/+ (Rif1+/+ +OHT) and Rif1 −/− (Rif1F/F +OHT) cells at the indicated timepoints. Rif1+/+ (solid line) and Rif1 −/− (dashed line), female (black) and male (grey). Data are presented as mean ± standard deviation from three (female lines) or two (male lines) independent experiments. Statistical significance was determined using two‐way ANOVA comparing female Rif1+/+ to female Rif1 −/− cell lines (****P ≤ 0.0001). Xist RT‐primers Xist ex7 F and R were used. Values are normalised to a geometric mean consisting of the expression of Gapdh, Ubiquitin and β‐Actin.
- Bar plot summarising the number of cells showing H3K27me3‐marked Xi as a percentage of total cells counted, in Rif1+/+ (Rif1+/+ +OHT) and Rif1 −/− (Rif1F/F +OHT) female mESCs at the indicated days of EB differentiation. Averages ± standard deviation from three (day 4 and 7) and two (day 2) independent experiments (n > 200).
Figure EV1. Transcriptome analysis in Rif1 null ESCs and early‐stage EBs.
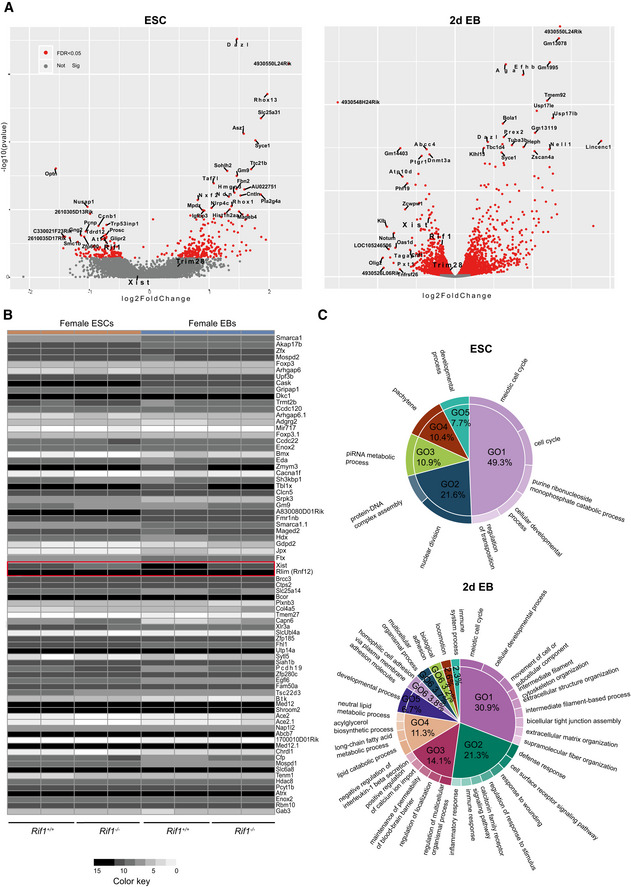
- Volcano plot summarising the top differentially expressed genes in Rif1 −/− (Rif1F/F +OHT) ESCs (left) and EBs (right), compared to Rif1+/+ (Rif1 +/++OHT). Two independent cell lines were analysed per genotype. In red, genes whose differential expression is statistically significant (FDR < 0.05). Trim28 (Kap1) expression is not up‐regulated in Rif1 null cells, while Xist expression levels are significantly down‐regulated in EBs.
- Heat maps summarising the logarithmically transformed, normalised expression levels of randomly chosen X‐linked genes in Rif1+/+ and Rif1 −/− ESCs (brown) and 2 days EBs (blue). Failure to up‐regulate Xist in Rif1 −/− EBs is specific, as other X‐linked genes do not show the same behaviour. Rlim (Rnf12) expression, for example, is not affected by Rif1 deletion (highlighted, along with Xist, in the red box).
- Pie charts summarising the biological processes (GO enrichment analysis, GOrilla) most represented among the genes whose expression is significantly deregulated upon Rif1 deletion in ESCs (top) and EBs (bottom). Differentially expressed genes were obtained from the DESeq2 (adjusted P‐value, 0.05 and log2FCl > 0.5). Genes linked to developmental processes (GO5) represent only a small percentage of differentially expressed genes (about 50% up‐regulated and 50% down‐regulated).
Figure EV2. Rif1 −/− mESCs can exit pluripotency and commit to differentiation.
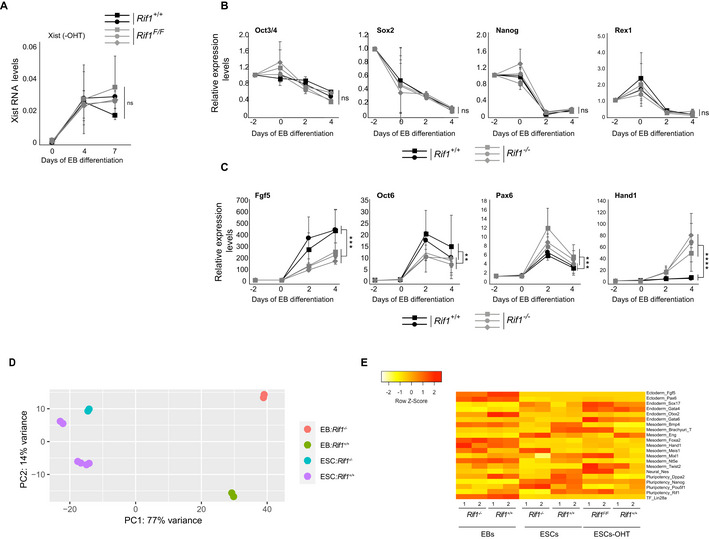
-
AXist RNA levels monitored by RT‐qPCR in two independent Rif1+/+ (black) and three independent Rif1F/F (grey) female mESC lines, during EB differentiation, in the absence of OHT. Xist RT‐primers Xist ex7 F and R were used. Values are normalised to a geometric mean consisting of the expression of Gapdh, Ubiquitin and β‐Actin. Expression levels are plotted as mean ± standard deviation of a minimum of three individual experiments with statistical significance determined using two‐way ANOVA. ns = not significant.
-
B, CTime course analysis of expression levels for the indicated genes, during EB differentiation of two individual Rif1+/+ (Rif1+/+ +OHT, black) and three individual Rif1 −/− (Rif1F/F +OHT, grey) female mESC lines. Expression levels are first normalised to a geometric mean consisting of the expression of Gapdh, Ubiquitin and β‐Actin and then plotted relative to pre‐samples (day −2), as mean ± standard deviation of three or four individual experiments with statistical significance determined using two‐way ANOVA (**P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001 and ns = not significant) comparing Rif1+/+ to Rif1 −/− cells. (B). Pluripotency‐associated genes and (C) genes expressed during early stages of differentiation.
-
DThe analysis of gene expression in Rif1 wild‐type and null female ESCs and EBs shows that the data cluster first according to the cell type (ESCs versus EBs, PC1) and then according to the genotype (Rif1+/+ versus Rif1 −/−, PC2). The wider distance between the gene expression data of wild‐type and Rif1 null EBs as compared to ESCs is in agreement with the mixture of cell types that is characteristic of EBs.
-
EThe analysis of the expression of key lineage markers in Rif1 wild‐type and null female cells, both ESCs and EBs, shows that Rif1 null cells differentiate in all three lineages, with some individual genes displaying some degree of variable level of expression.
RIF1 is a positive regulator of Xist and its binding specifically bookmarks the future Xi
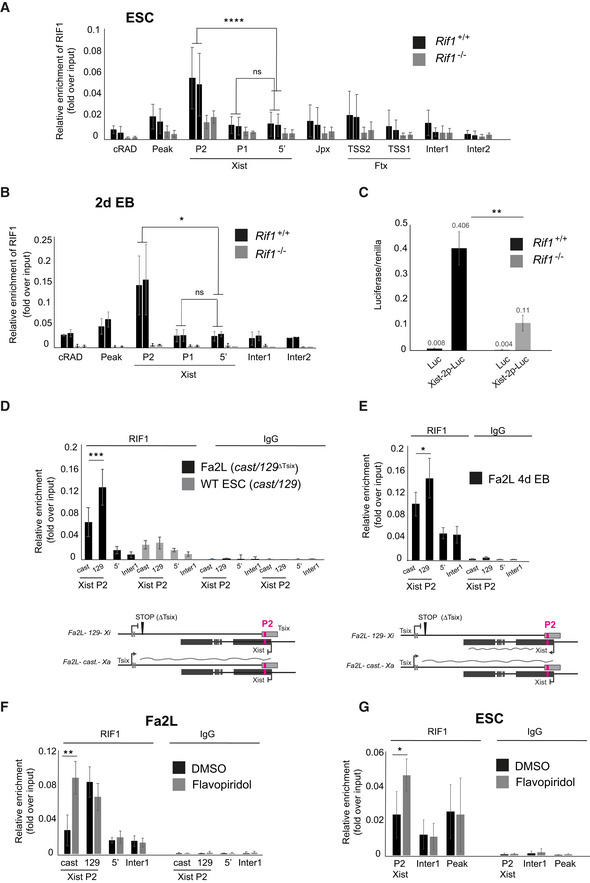
Xist is controlled from two promoters, P1 and P2 (Johnston et al, 1998), separated by a repetitive region essential for the silencing properties of Xist (Wutz et al, 2002). While the epigenetic control of the upstream P1 promoter was shown to be important for Xist regulation (Navarro et al, 2005), P2 appears to serve as an internal regulatory unit, possibly modulating the expression from P1 (Makhlouf et al, 2014). We found that RIF1 is enriched specifically at Xist P2 promoter, both in mESCs (Fig 3A, Appendix Fig S2C and D) and in early EBs (Fig 3B), supporting the hypothesis that RIF1 could be a direct regulator of Xist expression. In agreement with this, we found that P2 harbours two potential RIF1‐binding sites, defined by the presence of a consensus sequence derived from the analysis of RIF1 genome‐wide distribution by ChIP‐seq in female mESCs (Foti et al, 2016) (Appendix Fig S3A). To confirm that RIF1 association with Xist promoter has a positive effect on Xist expression, we used a reporter assay system, where Xist promoter has been cloned upstream of a firefly Luciferase gene (Gontan et al, 2012). We found that, upon differentiation, in the absence of RIF1, the induction of Luciferase from the Xist promoter is significantly reduced (Fig 3C), supporting the hypothesis that RIF1 association with P2 exerts a positive, direct effect on Xist transcription.
Figure 3. RIF1 associates with Xist promoter on the future Xi.
-
A, BRIF1 association with the Xist promoter assessed by ChIP‐qPCR in two independent Rif1+/+ (Rif1+/+ +OHT, black) and two Rif1 −/− (Rif1F/F +OHT, grey) female cell lines, in ESCs (A) and at 2 days of EB differentiation (B). P1 and P2 indicate the two Xist promoters, 5′ indicates a region 2 kb upstream of Xist TSS. Inter1 and 2 are two intergenic regions that serve as negative controls. Peak and cRAD represent two previously identified regions of RIF1 association (positive control). See Appendix Fig S2C for primer positions within Xist. Mean ± standard deviation from three independent experiments (A) and two independent experiments (B). P calculated by Student’s two‐tailed, paired t test comparing RIF1 association in Rif1+/+ cells on Xist P2 and P1 versus 5′. *P ≤ 0.05, ****P ≤ 0.0001 and ns = not significant.
-
CRif1 deletion decreases the efficiency of up‐regulation of a Luciferase reporter under the control of Xist promoter (Xist‐2p‐luc), at 2 days of EB differentiation. As a control (Luc), empty luciferase reporter vector was transfected in parallel. The average of three independent experiments is shown. Error bars indicate the standard deviation. P calculated by Student’s two‐tailed, unpaired t test, for comparison of fold activation of Xist‐2p‐Luc normalised to empty vector (Luc) in Rif1+/+ versus Rif1 −/− cells. **P ≤ 0.01. See Appendix Material and Methods for details about the normalisation.
-
DAssociation of RIF1 with Xist P2 in the Fa2L cells (black) and a wild‐type female mESC line (grey), also harbouring one castaneus and one 129 X chromosome. Allele‐specific ChIP‐qPCR primers were used, cast indicates association with the castaneus Xist P2 and 129 indicates association with the 129 Xist P2. Enrichments are presented relative to input DNA. Mean ± standard deviation from three independent experiments. P calculated by Student’s two‐tailed, paired t test comparing RIF1 association with the castaneus and with the 129 X chromosome Xist P2, ***P ≤ 0.001. Below is the schematic of the Xist/Tsix alleles in the Fa2L undifferentiated cells.
-
EAssociation of RIF1 with Xist P2 in the Fa2L cells (black) upon differentiation. The analysis was performed as in (D). Mean ± standard deviation from three independent experiments. P calculated by Student’s two‐tailed, paired t test comparing RIF1 association with the castaneus and with the 129 X chromosome Xist P2. *P ≤ 0.05. Below is the schematic of the Xist/Tsix alleles in the Fa2L differentiated cells.
-
FQuantification by ChIP‐qPCR of RIF1 association with the indicated regions in the Fa2L cell line, following treatment with DMSO only (black) or flavopiridol (grey). Primers as in (E).
-
GSame as in (F) but for a wild‐type female mESC line. All enrichments are presented relative to input DNA. Mean ± standard deviation from three (F) and two (G) independent experiments are presented. Statistical significance was determined using Student’s two‐tailed, paired t test (*P ≤ 0.05, **P ≤ 0.01 and ns = not significant).
Upon differentiation, Xist is mono‐allelically transcribed, up‐regulated only from the future Xi. If RIF1 acts as a positive regulator of Xist, we would expect it to be associated mono‐allelically, specifically with P2 on the future Xi. In order to test this hypothesis, we have taken advantage of the Fa2L cell line, in which: 1. the two X chromosomes can be discriminated, as one originates from Mus castaneus (cast) and the other from Mus musculus 129/SvJ (129) mouse strains; 2. Xa (cast) and Xi (129) are pre‐determined, as the 129 Tsix allele carries a transcriptional stop signal, approximately 4 kb downstream from the Tsix major promoter (Fig 3D, scheme and (Luikenhuis et al, 2001)). Xist is, therefore, preferentially up‐regulated from the 129‐derived X chromosome. We have analysed the association of RIF1 with Xist P2 promoter of the future Xa and Xi by allele‐specific ChIP‐qPCR (Appendix Fig S3B) and found that RIF1 is preferentially associated with the Xist P2 promoter of the 129 Xist allele (future Xi) in both mESCs (Fig 3D) and upon differentiation (Fig 3E). Importantly, in control wild‐type mESCs (bi‐allelically expressed Tsix), also carrying one cast and one 129 X chromosome, RIF1 is equally distributed on both P2 promoters (Fig 3D). This suggests that the asymmetric association of RIF1 with the future Xi is concomitant with/follows the switch from bi‐ to mono‐allelic Tsix expression that accompanies the choice and allows Xist monoallelic up‐regulation. As in the case of RIF1 conditional cells, depletion of RIF1 in Fa2L cells (Appendix Fig S3C) also compromises Xist up‐regulation (Appendix Fig S3D). These data show that RIF1’s asymmetric association with the future Xi parallels the choice and that it is essential for Xist up‐regulation.
RIF1 asymmetric localisation on the future Xi is driven by Tsix expression
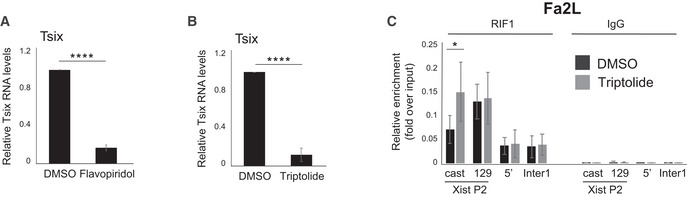
How is the transition from bi‐ to mono‐allelic RIF1 association with Xist promoter regulated? While this would generally be triggered by differentiation, in undifferentiated Fa2L cells it is pre‐determined and RIF1 is preferentially associated with the X chromosome that does not express full‐length Tsix transcript (Fig 3D and E). This suggests that Tsix RNA and/or transcription could destabilise RIF1 association with the Xist promoter. In agreement with this hypothesis, we found that blocking Tsix expression by treating mESCs with the CDK9‐inhibitor flavopiridol, which inhibits transcriptional elongation (Chao & Price, 2001) (Fig EV3A) or, briefly, with triptolide, an inhibitor of transcription initiation (Fig EV3B), is sufficient to revert RIF1 preferential association with the future Xi in the Fa2L cells to a symmetric mode of binding (Figs 3F and EV3C). In addition, flavopiridol treatment of wild‐type mESCs also leads to an increased P2 association of RIF1 (Fig 3G), indicating that this is not an effect specific to the Fa2L cells. Finally, while this work was under review, RIF1 has been found associated with Tsix RNA in mESCs (Aeby et al, 2020), supporting the hypothesis that Tsix RNA can compete for RIF1 association with Xist P2 in the genome.
Figure EV3. Inhibiting Tsix transcription in Fa2L cells reverts RIF1 association with P2 from asymmetric to symmetric.
-
A, BTsix RNA levels following flavopiridol (A) and triptolide (B) treatment, relative to DMSO‐treated Fa2L cells. RNA levels were first normalised to 18S ribosomal RNA and plotted as mean from three individual experiments ± standard deviations. Statistical significance was determined using Student’s two‐tailed unpaired t test (****P ≤ 0.0001).
-
CAllele‐specific RIF1 association with Xist P2 in Fa2L cells following treatment with DMSO only (black) or triptolide (grey). cast indicates association with the castaneus Xist P2 promoter and 129 indicates association with the 129 Xist P2 promoter. Enrichments are presented relative to input DNA. Mean ± standard deviation of three independent experiments. P calculated by Student’s two‐tailed paired t test. (*P ≤ 0.05).
KAP1 is important for the Xa/Xi choice
With the aim of understanding the molecular mechanism by which RIF1 regulates Xist expression, we have investigated whether some of the known transcriptional regulators associated with RIF1 (Sukackaite et al, 2017) are also required for XCI. We focused in particular on KAP1, as KAP1 and RIF1 have already been shown to regulate overlapping targets, such as Dux and MERVLs (Maksakova et al, 2013; Li et al, 2017; Percharde et al, 2018). We found that knock down of Kap1 (Appendix Fig S4A and B) impairs Xist up‐regulation (Figs 4A and EV4A), similarly to the knockout of Rif1. This is not due to compromised exit from pluripotency (Fig EV4B), impaired activation of the differentiation transcriptional program (Fig EV4C) or reduced RIF1 levels (Fig EV4D), suggesting that diminished Xist activation is not a consequence of an overall impaired cell differentiation. In addition, the dynamics of expression of RNF12 appear comparable between control and Kap1 knock down cells (Fig EV4E). However, in contrast to the depletion of Rif1, depletion of Kap1 in Fa2L cells (Appendix Fig S4C), where the choice is pre‐determined, has no consequences for Xist up‐regulation (Fig 4B). These data suggest that KAP1 is required prior to or at the time of the choice, while it is dispensable once Tsix mono‐allelic expression has been established. In agreement with a role during the choice, we found that Kap1 knock down affects Tsix dynamic regulation at the onset of differentiation. In wild‐type cells, during the early stages of differentiation, Tsix levels rise transiently (at 1, or 1 and 2 days of EB differentiation respectively, depending on the culture conditions, Fig 4C and Appendix Fig S2A). The boost corresponds to an increased detection of Tsix RNA from both alleles (Fig 4D), suggesting that this step precedes the switch to Tsix mono‐allelic expression and the consequent choice of Xa/Xi. Upon Kap1 down‐regulation, we found not only a failure in the temporary boost of Tsix levels (Fig 4C) but also a failure to evolve towards Tsix mono‐allelic expression, as Tsix becomes undetectable (Fig 4D). In a situation of pre‐determined choice (Fa2L cells), Tsix levels remain low, even upon differentiation, and Kap1 knock down has no further effect (Appendix Fig S4D).
Figure 4. KAP1 regulates the Xa/Xi choice through Tsix.
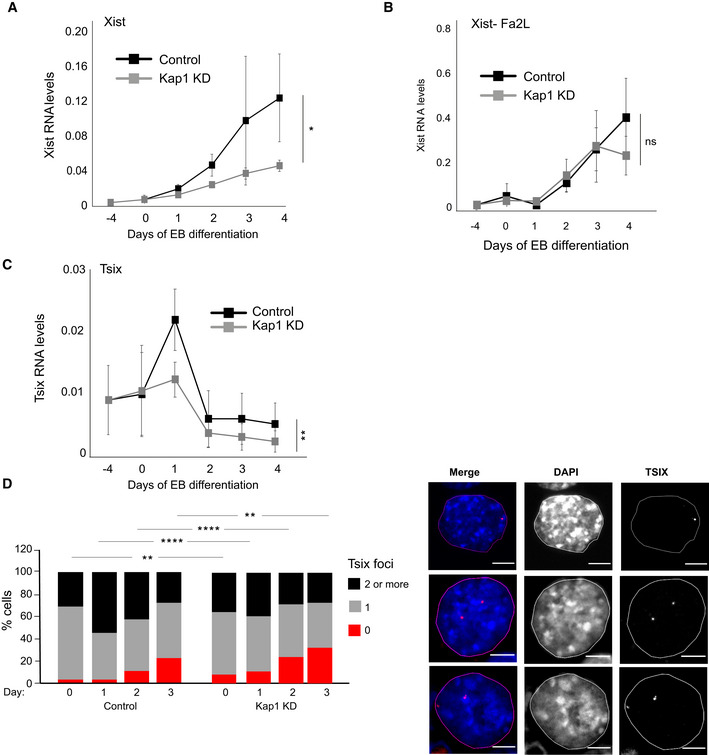
- Time course analysis of Xist expression by RT–qPCR during EB differentiation of female mESCs following knock down of Luciferase (Control, black) and Kap1 (Kap1 KD, grey), at the indicated timepoints. Data are presented as mean ± standard deviation from three independent experiments. Statistical significance was determined using two‐way ANOVA. Xist primers Xist ex3 F and Xist ex4 R were used. Normalisation was performed using a geometric mean consisting of the expression of Rplp0, Ubiquitin and Sdha (*P ≤ 0.05).
- RT–qPCR analysis of Xist expression levels during differentiation of the Fa2L cells, following expression of shRNA against Luciferase (Control, black) and Kap1 (Kap1 KD, grey), at the indicated timepoints. Mean ± standard deviation from a minimum of three independent experiments is presented. Two‐way ANOVA was used to determine statistical significance. ns = not significant.
- Tsix RNA levels in female mESCs infected with shRNA directed against Luciferase (Control, black) and KAP1 (Kap1 KD, grey), during differentiation. Mean ± standard deviation values from four independent experiments are shown. Statistical significance was determined using two‐way ANOVA. (**P ≤ 0.01). Values have first been normalised to a geometric mean consisting of the expression of Rplp0, Ubiquitin and Sdha.
- RNA FISH analysis of Tsix expression during differentiation of female mESCs expressing an shRNA directed against Luciferase (control) or against Kap1 (Kap1 KD). Left: Cells with no (0, red), one (1, grey) and two or more (2, black) Tsix foci were counted in two independent experiments, shown averaged. Statistical significance was determined by χ2. A minimum of 110 cells were counted per time point for each line (**P ≤ 0.01, ****P ≤ 0.0001). Right: examples of cells with one (top) or two (central and bottom) Tsix FISH signals. Scale bars: 5 μm.
Figure EV4. Defective Xist up‐regulation in KAP1 knock down cells is not due to lack of differentiation.
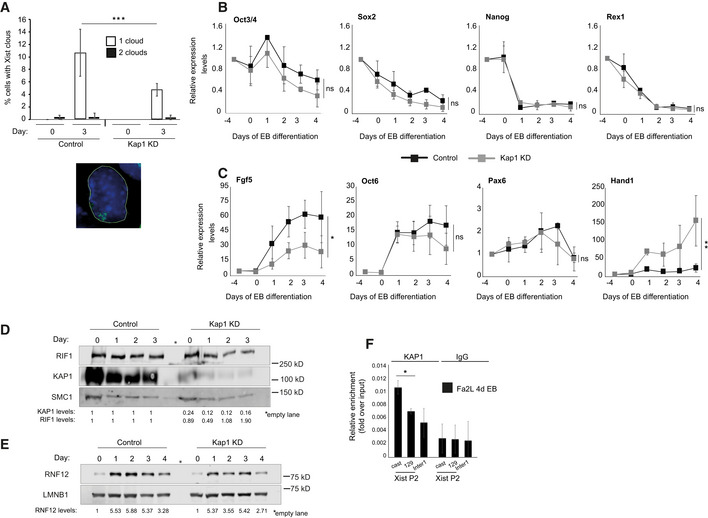
-
AQuantification of Xist cloud formation (one or two clouds, open or filled bars respectively) by RNA FISH in Control and Kap1 KD female mESCs, differentiated into EBs for 3 days. The average of two experiments is shown. N > 400 cells. The error bars represent ± standard deviation. P was calculated by χ2 (***P ≤ 0.001). Below is an example of Xist cloud RNA FISH signal. Green = Xist, blue = DAPI.
-
B, CTime course analysis of gene expression levels quantified by RT‐qPCR during EB differentiation of female mESCs infected with shRNA directed against Luciferase (control, black) and Kap1 (grey), at the indicated timepoints. Values have first been normalised to a geometric mean consisting of the expression of Rplp0, Ubiquitin and Sdha and then presented relative to pre‐samples (day: −4) Mean ± standard deviation from three independent experiments are shown. Statistical significance was determined using two‐way ANOVA (*P ≤ 0.05, **P ≤ 0.01 and ns = not significant). (B). pluripotency‐associated genes and (C) genes expressed during early stages of differentiation.
-
DRepresentative western blot analysis of RIF1 and KAP1 levels in Kap1 KD female mESCs. SMC1: loading control. Quantification of RIF1 and KAP1 protein levels normalised to SMC1 and relative to control cells are shown below.
-
ERepresentative western blot analysis of RNF12 levels in proteins extracted from female mESC infected with shRNA directed against Luciferase (control) and Kap1, at the indicated timepoints during EB differentiation. LAMIN B1 (LMNB1): loading control. Below is the quantification of RNF12 protein levels relative to day 0 of control and Kap1 KD cells respectively. Values normalised to LMNB1.
-
FUsing allele‐specific primers, ChIP‐qPCR was used to analyse the association of KAP1 with Xist P2 in differentiating Fa2L cells. cast indicates association with the castaneus Xist P2 and 129 indicates association with the 129 Xist P2. Enrichments are presented relative to input DNA. Mean ± standard deviation from a minimum of three independent experiments. P was calculated by Student’s two‐tailed paired t test (*P ≤ 0.05).
In summary, the failure to up‐regulate Xist caused by Rif1 deletion and by Kap1 knock down have very different causes. While RIF1 is directly required to promote Xist up‐regulation, KAP1’s function is to drive the transient increase of Tsix levels that precedes the choice. The consequent failure to up‐regulate Xist when Kap1 is knocked down could be caused, in this case, by a failure to execute the choice. The low, bi‐allelic Tsix levels typical of mESCs instead evolve directly towards an absence of Tsix.
RIF1 negatively regulates KAP1 association with the Xist promoter/Tsix terminator in mESCs
KAP1 is a multifunctional protein, and a key global regulator of transcription, involved in several aspects of gene expression modulation. Through its interaction with the H3K9 histone methyltransferase SetDB1, KAP1 can promote transcriptional silencing. Alternatively, it can modulate transcriptional or transcript levels, either regulating the release of RNA polymerase II proximal pausing from the promoter (especially at genes encoding for lncRNAs (Bunch et al, 2016)), or as part of the 7SK complex (McNamara et al, 2016). This is a ribonucleoprotein complex with roles both at the promoter and in the transcriptional termination of several genes, including several lncRNAs (Castelo‐Branco et al, 2013).
To gain an insight into the mechanism by which KAP1 regulates Tsix levels, we have analysed KAP1 distribution along Tsix regulatory regions. Consistent with a function during the choice, we could not detect KAP1 on any of the regions examined in mESCs. Instead, we found that KAP1 was specifically recruited to Xist P2 promoter at the onset of differentiation, around the time when Tsix levels are boosted (Fig 5A). Taking advantage of the Fa2L cells, we could also determine that KAP1 associates preferentially with Xist P2 of the future Xa (castaneus allele, Figs 5B and EV4F). KAP1 and RIF1 occupy, therefore, the same region, but with complementary spatial (Xa versus Xi) and temporal dynamics (KAP1 appears on Xist P2 on the Xa when RIF1 leaves it). In order to understand if these events are coordinated, we have investigated whether RIF1 regulates KAP1 association with Xist P2. We found that Rif1 deletion leads to KAP1 binding to Xist promoter, even in undifferentiated cells (Fig 5C). This is not due to a general increase of Kap1 expression (Fig EV1A), KAP1 protein levels (Fig EV5A) or its overall binding to chromatin (Fig EV5B). Moreover, KAP1 enrichment is specific for Xist promoter, as other regions known to be associated with KAP1 that we have tested, like Zfp629 (Fig 5C and our unpublished observation) (Ding et al, 2018), did not show an increased KAP1 association upon Rif1 deletion. Importantly, the effect of RIF1 deficiency is unlikely to be due to an indirect, general remodelling of the Xist promoter chromatin, as the association of another P2‐specific transcription factor and Xist activator, Yin‐Yang‐1 (YY1) (Makhlouf et al, 2014), is unchanged in Rif1 knockout cells (Fig EV5C). We also found that knocking down Rif1 in undifferentiated Fa2L cells (Fig EV5D) facilitates KAP1 association with Xist P2 (Fig 5D) comparably to what happens in Rif1 conditional cells upon induction of Rif1 deletion (Fig 5C). Specifically, KAP1 gains access to the future Xi (129 allele, carrying the truncated Tsix allele), where normally RIF1 is preferentially localised (Fig 3D and E). Overall, these data indicate that, in mESCs, RIF1 is symmetrically associated with Xist P2 on both X chromosomes, protecting P2 from the binding of KAP1. Upon triggering differentiation, the bi‐allelic increase of Tsix levels weakens RIF1 association with DNA, facilitating the transition of RIF1 to an asymmetric association with one of the two Xist promoters, the future Xi, and the consequent association of KAP1 with the other Xist promoter, on the future Xa. This event, in turn, sustains the KAP1‐dependent increase of Tsix levels that precedes the switch to Tsix mono‐allelic expression and the choice, further reinforcing RIF1 exclusion from P2 on the future Xa.
Figure 5. RIF1 negatively regulates KAP1 association with Xist P2.
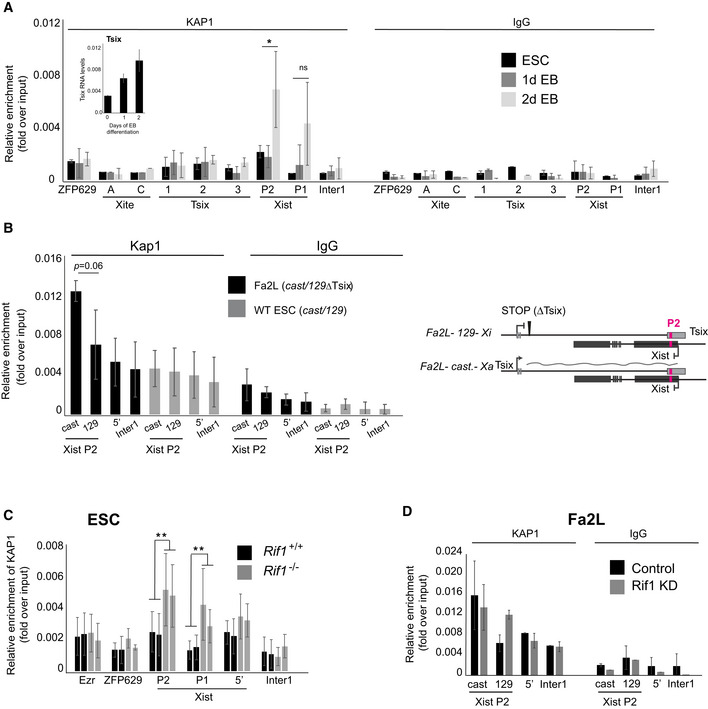
- ChIP‐qPCR analysis of KAP1 association with the indicated sites in wild‐type female mESCs (Rif1+/+ , same as used in Fig 3B but without OHT) and during early differentiation. ZFP629 is a well‐characterised KAP1 associated region (positive control). Xite A and C indicate two regions within the Tsix enhancer Xite, Tsix region 1 indicates Tsix major promoter, Tsix region 2 indicates the Dxpas34 region, Tsix region 3 indicates a region slightly downstream of the Dxpas34 region. P1 and P2 indicate the two Xist promoters, 5′ indicates a region 2 kb upstream of Xist TSS. Inter1 is an intergenic region. See Appendix Fig S2C for the positions of the primers within Xist and Tsix. The data are presented as mean ± standard deviation from three (2d EB and 1d EB) and two (ESCs) independent experiments. Statistical significance was calculated by Student’s two‐tailed unpaired t test comparing RIF1 association with Xist P2 and P1 in 2d EB versus 1d EB (*P ≤ 0.05 and ns = not significant). In the inset, Tsix RNA levels were quantified by RT‐qPCR during the differentiation of wild‐type female ESCs shown in Fig 4F. The average of two experiments is shown. Tsix values are normalised to a geometric mean consisting of the expression of Rplp0, Ubiquitin and Sdha. Error bars indicate standard deviations.
- Using allele‐specific primers, ChIP‐qPCR was used to measure the association of KAP1 with Xist P2 in the Fa2L cells (black) and a wild‐type female mESC line also harbouring one castaneus and one 129 X chromosome (grey). cast indicates association with the castaneus Xist P2 and 129 indicates association with the 129 Xist P2. Enrichments are presented relative to input DNA. Mean ± standard deviation from a minimum of three independent experiments. Statistical significance was determined by Student’s two‐tailed, paired t test. Below is the schematic of the Xist/Tsix alleles in the Fa2L cells.
- KAP1 association with Xist promoter in two independent Rif1+/+ (Rif1+/+ +OHT, black) and two Rif1 −/− (Rif1F/F +OHT, grey) female mESC cell lines. Ezr is an additional region known to be associated with KAP1 in mESCs. Enrichments are presented relative to input DNA. Mean ± standard deviation from a minimum of three independent experiments per cell line are displayed. Statistical significance was determined using Student’s two‐tailed, unpaired t test comparing the KAP1 association with Xist P2 and P1 in Rif1+/+ versus Rif1 −/− cells (**P ≤ 0.01).
- Allele‐specific KAP1 association with Xist P2 in Fa2L cells following knock down of Luciferase (Control, black) and Rif1 (Rif1 KD, grey). cast indicates association with the castaneus Xist P2 promoter and 129 indicates association with the 129 Xist P2 promoter. Enrichments are presented relative to input DNA. Average ± standard deviation of two independent experiments.
Figure EV5. The overall binding of KAP1 to chromatin is unaffected in Rif1 −/− mESCs.

- RIF1 and KAP1 levels analysed by western blot in protein extracts from two Rif1+/+ (Rif1+/+ +OHT) and two Rif1 −/− (Rif1F/F +OHT) independent female mESC lines, following 2 days of OHT treatment and at 2 days EB. SMC1: loading control. Below are the quantifications of RIF1 and KAP1 protein levels shown as relative levels compared to one of the Rif1+/+ cells. Values normalised to SMC1.
- Western blot analysis of KAP1 levels in protein extracts from the indicated cell fractions, from two Rif1+/+ (Rif1+/+ +OHT) and two Rif1 −/− (Rif1F/F +OHT) independent female mESC lines, following 2 days of OHT treatment. β‐TUBULIN: marker for the cytosolic fraction. LMNB1 and histone H2A: markers for the chromatin/insoluble fraction. Below are the quantifications of KAP1 protein levels detected in the chromatin fractions shown as relative levels compared to one of the control cells. Values normalised to H2A.
- YY1 association with the Xist promoter in two independent Rif1+/+ (Rif1+/+ +OHT, black) and two Rif1 −/− (Rif1F/F +OHT, grey) female mESC lines, analysed by ChIP‐qPCR. Ex1 indicates a region within Xist exon 1, 2.5 kb downstream of the Xist transcriptional start site (TSS), P2 indicates Xist promoter P2 spanning the YY1 consensus motif. Peg3 indicates a known YY1‐associated site on the Peg3 gene. Data from two independent experiments are presented.
- Western blot analysis of RIF1 levels in protein extracts from Fa2L cells after Rif1 knock down. SMC1: loading control. Below is the quantification of RIF1 protein levels compared to control cells.
KAP1 recruits the 7SK complex to Tsix terminator
The timing of recruitment, the RIF1‐dependent regulation and the preferential enrichment on the future Xa support the idea that KAP1 functions by promoting the choice, possibly in cis. The association of KAP1 with Xist P2 promoter on the future Xa suggests that KAP1 could repress Xist. However, Kap1 knock down does not induce precocious up‐regulation of Xist (Figs 4A and EV4A), nor does KAP1 early association with Xist P2 promoter in Rif1 null mESCs and EBs lead to increased tri‐methylation of histone H3K9 (Fig 6A and B). These observations do not support the hypothesis of KAP1 regulating the choice through Xist repression. An alternative hypothesis is that KAP1 could instead regulate Tsix either by controlling its transcriptional termination and, consequently, RNA stability (reviewed in Peck et al, 2019), or by promoting the formation of a terminator–promoter‐positive feedback loop (Tan‐Wong et al, 2008), to boost Tsix transcription. Xist P2 promoter, in fact, overlaps with Tsix transcriptional terminator. In support of either of these hypotheses, we have found that, as in the case of KAP1, the 7SK complex component HEXIM1 is also enriched on Xist promoter/Tsix terminator in Rif1 knockout mESCs (Fig 6C), and it is associated with the future Xa in Fa2L cells, in a KAP1‐dependent manner (Fig 6D and E). Overall, these data suggest that KAP1 could promote the choice of the future Xa by sustaining in cis the increase of Tsix levels that would stabilise the asymmetric RIF1 distribution.
Figure 6. KAP1 recruits the 7SK complex to the Xist P2 promoter/Tsix terminator of the future Xa.

-
A, BH3K9me3 association with the Xist promoter (P1 and P2) in two independent Rif1+/+ and Rif1 −/− cell lines, analysed by ChIP‐pPCR in mESCs (A) and 2d EBs (B). Myf5 serves as a positive control region, β actin negative. Average ± standard deviation of two independent experiments.
-
CHEXIM1 association with the Xist promoters (P1 and P2) in two independent Rif1+/+ and two Rif1 −/− mESC lines, analysed by ChIP‐qPCR. Tkbp1 and Myf5 are two control regions. As in the case of KAP1 association, deletion of Rif1 induces accumulation of HEXIM1 on Xist P1 and P2. Average ± standard deviation of two independent experiments. P values were calculated by two‐tailed, unpaired, equal variance t test. (**P ≤ 0.01, ***P ≤ 0.001).
-
DWestern blot analysis of KAP1 levels in protein extracts from Fa2L cells after Kap1 knock down. SMC1: loading control. Quantification of KAP1 levels normalised to SMC1 and relative to Luciferase control cells are shown below.
-
EUpon infection of Fa2L cells with shRNA against Kap1 or control, against Luciferase, HEXIM1 association with P2 was analysed, on both alleles, by ChIP‐qPCR. As in the case of KAP1, HEXIM1 shows preferential association with Xist P2 on the Cast allele (future Xa, Control). The association is lost upon knock down of Kap1 (Kap1 KD). Myf5 serves as a negative and Neat1 as a positive control region. (*P ≤ 0.05, **P ≤ 0.01).
Discussion
While marsupials have adopted an imprinted X inactivation strategy, eutherians have evolved a mechanism based on the random choice of the X chromosome to be inactivated. The latter can contribute to a higher degree of resistance of females to pathogenic X‐linked mutations and increase phenotypic diversity. Despite its importance, the mechanisms guiding the random choice are still unclear, partially because of the randomness and consequent heterogeneity in the cell population, partially because of the inaccessibility of the early embryos, where the process takes place naturally and, finally, because of the inherent difficulty of identifying asymmetry involving two identical chromosomes.
Several lines of evidence suggest that Tsix is involved in the choice‐making process. For example, introduction of a stop codon that blocks Tsix transcript before its overlap with Xist (Luikenhuis et al, 2001), or deletions of its major promoter (Vigneau et al, 2006), or of the GC‐rich repeat region that immediately follows it (Dxpas34) (Lee & Lu, 1999), or insertion of a gene trap in the same region, that abolishes the production of Tsix RNA (Sado et al, 2001), result in a non‐random choice, with the Tsix‐defective chromosome as the future Xi. Moreover, monoallelic down‐regulation of Tsix levels by deleting Xite, a cis‐acting element that positively regulates Tsix, also skews the choice (Ogawa & Lee, 2003). Interestingly, Xist itself can influence the choice, in a yet‐to‐be‐understood feedback control loop. Xist ectopic up‐regulation can in fact skew the choice in favour of the Xist‐overexpressing chromosome (Newall et al, 2001; Nesterova et al, 2003).
Our experiments show that RIF1 association with the Xist P2 promoter is negatively regulated by Tsix expression or RNA levels. Tsix could, therefore, be the determinant of the asymmetric association of RIF1 with the future Xi at the choice. We would like to propose a model (Fig 7) whereby, at the onset of differentiation, the transient, bi‐allelic increase of Tsix levels will promote a weaker or more dynamic association of RIF1 with Xist P2, thus creating a window of opportunity for KAP1 stochastic association with either allele. The KAP1‐bound allele will go on to sustain higher Tsix steady‐state levels in cis, thus skewing RIF1 association with the opposite allele, and initiating a self‐reinforcing loop on the future Xa. On the future Xi, RIF1 will promote Xist up‐regulation, thus establishing the inactivation. The negative effect of RIF1 on KAP1 association with Xist promoter in ESCs is at the heart of the mutual exclusion, reinforced by KAP1’s positive effect on the levels of Tsix, that is, in turn, a negative regulator of RIF1 association with Xist promoter. How RIF1 excludes KAP1 is currently unclear, but we can envisage at least two potential mechanisms, based either on RIF1/KAP1 competition for binding to a shared site, RNA or protein partner, or through KAP1 de‐phosphorylation by RIF1‐associated PP1. Phosphorylation of KAP1 has indeed been shown to regulate KAP1 association with heterochromatin protein 1 (HP1) (Chang et al, 2008).
Figure 7. Model for RIF1 and KAP1‐dependent bookmarking of Xi and Xa respectively.
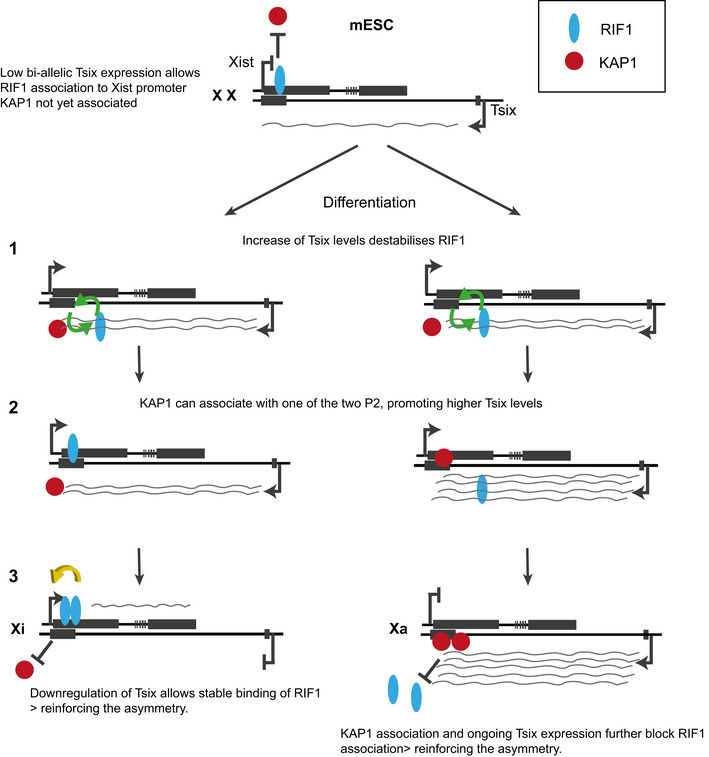
The low bi‐allelic expression of Tsix in mESCs allows the association of RIF1 with P2 on both Xist alleles. However, the presence of pluripotency‐dependent inhibitors will not allow Xist up‐regulation, despite the presence of RIF1. (1) Upon differentiation, the increase in Tsix levels weakens the association of RIF1 with P2. This opens the opportunity for a stochastic KAP1 binding to P2 of one of the two alleles (2). KAP1 is required for sustained high levels of Tsix, further reinforcing RIF1 exclusion from the KAP1‐bound/Tsix high allele and establishing the asymmetry. It is not known whether KAP1 gains access to P2 to promote the increase of Tsix levels first, or whether the increase of Tsix levels is initially triggered by a differentiation‐dependent factor. (3). The pluripotency Xist inhibitors having been silenced, RIF1 promotes Xist expression on the future Xi. A self‐sustainable binary switch is thus created and it consolidates the choice of the future Xi and Xa.
In support of our model, we have shown that, the association of KAP1 with the P2 region upon differentiation coincides with the detection of higher levels of Tsix RNA (Fig 5A), and this increase is dependent upon KAP1 (Fig 4C and D). The molecular mechanism by which KAP1 modulates Tsix levels is currently unknown. The data presented here suggest that KAP1 could modulate in cis Tsix transcriptional up‐regulation, termination and/or RNA stability through the 7SK complex. Finally, we cannot exclude a model where KAP1 promotes Tsix increase in trans, through a yet unknown differentiation‐induced factor. In this case, the association of KAP1 with Xist P2 could contribute in cis to the identification of Xa, by establishing a stable repression of Xist promoter, with RIF1 shielding the future Xi by excluding KAP1. Although our data do not support the hypothesis of KAP1‐dependent silencing of Xist (Figs 4A and EV4A) through H3K9me3 (Fig 6A and B), KAP1 could promote repression through a different mechanism, for example, DNA methylation (Coluccio et al, 2018).
Our data show that the increase of Tsix that precedes and, possibly, leads to a proficient choice, requires KAP1. It has been previously shown that failure to set up the choice as a consequence of homozygous deletion of Tsix, leads to a mixture of cells showing either no Xist up‐regulation or bi‐allelic up‐regulation during differentiation (Lee, 2002, 2005). This is different from what we observe in Kap1 knock down cells, where we detect defective Xist up‐regulation, but not bi‐allelic expression. Nonetheless, a situation where, from the start of the process in ESCs, Tsix is always absent, as in the case of Tsix −/−, is clearly different from the system where Tsix levels remain physiological until differentiation is triggered, as in the case of Kap1 knock down (Fig 4D).
The early embryonic lethality of Rif1 −/− females described here contrasts with the milder effect of Xist conditional inactivation in the epiblast described previously (Yang et al, 2016). However, beside the technical differences between a conditional system, where the efficiency of the deletion can be lower than 100%, and a knockout, RIF1 has at least two other key roles, in the regulation of the replication timing program (Cornacchia et al, 2012; Hayano et al, 2012; Yamazaki et al, 2012; Foti et al, 2016) and replication fork protection (Buonomo et al, 2009; Garzon et al, 2019). In fact, depending on the genetic background, most or some of the male embryos also die, although later during development (this work). We cannot, therefore, exclude that the early female lethality could derive from a synthetic effect of multiple problems, added on top of the failure of X inactivation.
In summary, we propose that, during the stochastic phase of the choice of the future Xi, Tsix‐dependent destabilisation of the symmetric association of RIF1 with Xist P2 promoter sets in motion the establishment of two, mutually exclusive circuits that will identify Xi and Xa. RIF1’s presence on P2, inhibiting KAP1 and promoting Xist expression will identify the future Xi. On the other allele, KAP1’s presence on P2, sustaining Tsix levels and, thus, helping to exclude RIF1, will identify the Xa. The initial stochastic binding of KAP1 will thus become a binary switch, where a bi‐stable, self‐sustaining circuitry on the two X chromosomes is propagated.
Materials and Methods
mESC differentiation
Wild‐type ESCs were plated onto non‐coated Petri dishes at a concentration of 1 × 106 cells/ 10 cm2, in a volume of 10 ml medium lacking 2i and LIF. At day 4 of differentiation the aggregated EBs were gently transferred to gelatinised tissue culture dishes. Medium was gently changed every 48 h with minimal disruption of the EBs. EBs were grown for up to 4 or 7 days in total. In experiments where cell differentiation was combined with Rif1 deletion, the differentiation was preceded by 48 h of 4‐hydroxytamoxifen (OHT, #H7904, Sigma‐Aldrich) treatment, at a concentration of 200 nM in ES medium containing LIF and 2i. Differentiation was then started with 2 × 106 cells/ 10 cm2 dish for Rif1+/+ and 2.5 × 106 cells/ 10 cm2 for Rif1F/F cells in a medium lacking 2i and LIF but containing 200 nM OHT. On day 1 of differentiation, the medium was replaced with a medium without OHT. On day 4 of differentiation, the EBs were transferred to gelatinised tissue culture dishes as above.
KAP1, RIF1 and HEXIM1 ChIP
Chromatin immunoprecipitation was performed according to Bulut‐Karslioglu et al, 2012). Briefly, for RIF1, KAP1 and HEXIM1 ChIP, collected cells were first cross‐linked using 2 mM disuccinimidyl glutarate (DSG, # BC366 Synchem UG & Co. KG) in PBS for 45 min at RT while rotating, washed twice in PBS, followed by 10 min of additional cross‐linking in 1% formaldehyde (#252549, Sigma‐Aldrich) in cross‐linking buffer (50 mM HEPES pH 7.8, 150 mM NaCl, 1 mM EDTA and 500uM EGTA) at RT. Cross‐linking was followed by 5 min quenching in 0.125 M glycine at RT, washed twice in cold PBS and resuspended in lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris‐HCl pH 8.1, supplemented with protease inhibitor cocktail, #11873580 001, Roche). Chromatin fragmentation was performed using Soniprep 150 to produce a distribution of fragments enriched between 300 and 400 bp. The lysate was pre‐cleared by centrifugation at low speed 400 g for 20 min at 4°C. Chromatin was quantified using Qubit dsDNA High Sensitivity assay kit (#Q32854, Life Technologies). Immunoprecipitation was performed by incubating 100 μg of chromatin diluted in 10 volumes of Dilution buffer (1% Triton X‐100, 2 mM EDTA, 167 mM NaCl, 20 mM Tris‐HCl pH 8.1, including Protease Inhibitor) overnight rotating at 4°C together with either α‐KAP1, α‐RIF1 or α‐HEXIM1 antibodies (see Appendix Table S2) or IgG only control (#sc‐2026, Santa Cruz), 10% of chromatin was isolated as input control. The following day, 50 μl of Dynabeads protein G slurry (#10004D, Thermo Fisher) per ChIP sample was added and incubated rotating for another 2 h at 4°C. The beads were magnet‐separated and washed twice with low salt buffer (0.1% SDS, 1% Triton X‐100, 2 mM EDTA, 150 mM NaCl, 20 mM Tris‐HCl pH8.1), one time each with high salt buffer (0.1% SDS, 1% Triton X‐100, 2 mM EDTA, 500 mM NaCl, 20 mM Tris‐HCl pH8.1), LiCl buffer (0.25 M LiCl, 0.5% NP‐40, 0.5% sodium deoxycholate, 1 mM EDTA,10 mM Tris‐HCl pH 8.1) and finally TE. Each wash was performed for 5 min. on a rotating wheel at 4°C and all buffers were supplemented with protease inhibitor cocktail (#11873580 001, Roche). Prior to elution, samples were rinsed once in TE without protease inhibitor. ChIP‐DNA was eluted from the beads by rotating at RT for 1 h in elution buffer (1% SDS, 100 mM NaHCO3). Beads were separated and the supernatants as well as input samples were subjected to RNAse A (#R5250, Sigma‐Aldrich) treatment (37.5 µg/sample) for 1 h at 37°C followed by de‐cross‐linking using Proteinase K (#P6556, Sigma‐Aldrich) treatment (45 µg/sample) overnight at 60°C. The following day, ChIP‐DNA and input samples were purified using ChIP DNA Clean and Concentrator kit (#D5205, Zymo Research) and the retrieved DNA as well as input DNA was quantified using Qubit dsDNA High Sensitivity assay kit (#Q32854, Life Technologies). The concentration of ChIP‐DNA and input samples was adjusted to maintain a similar ratio of ChIP‐DNA:INPUT between different ChIP experiments. qPCRs were performed using the SYBR Green reaction mix (#04887352001, Roche) on a LightCycler 96 Instrument (Roche), following standard protocols. Enrichments over input control were calculated for each respective primer set. Primer sequences are presented in Appendix Table S3.
RNA extraction, reverse transcription and RT–qPCR
Frozen cell pellets were lysed and homogenised using QIAshredder column (#79656, QIAGEN) followed by RNA extraction using the RNeasy kit (#74106, QIAGEN) according to the manufacturer’s instructions. On‐column DNAse treatment was performed at 25–30°C for 20 min. using RQ1 RNase‐Free DNase (#M6101, Promega). After elution, a second round of DNAse treatment was performed using 8 U of DNase/sample, incubated at 37°C for 20 min. The reaction was terminated by adding 1 μl of RQ1 DNase Stop Solution and incubated at 65°C for 10 min. RNA was quantified using Nanodrop, and cDNA synthesis was performed using RevertAid H Minus First Strand cDNA kit (#K1632, Thermo Scientific) using random hexamer priming. qPCRs were performed using the SYBR Green reaction mix (#04887352001, Roche) on a LightCycler 96 Instrument, following standard protocols. Gene expression data were normalised against a geometric mean generated by RT‐qPCR of either: Gapdh, Ubiquitin and β‐Actin or Rplp0, Ubiquitin and Sdha. For flavopiridol‐ or triptolide‐treated cells, gene expression levels were normalised against 18S ribosomal RNA. Primer sequences are presented in Appendix Table S4.
Additional material and method descriptions can be found in the Appendix Material and Methods.
Author contributions
EE created the cellular system, performed the majority of the experiments and co‐wrote the manuscript. RF initiated the project and performed some of the early experiments, like the staining of E3.5 embryos. LMP performed some of the ChIP experiments, the triptolide treatment, and the Luciferase assay. LB, AC and NBR performed KAP1 KD, RNA FISH and its analysis. GK analysed the RNA seq data, supervised by MV. NBR was supervised by AC, who also critically read the manuscript. FC and AP isolated and stained the E5.5 embryos. SBCB conceived the project, performed some of the experiments and wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Appendix
Expanded View Figures PDF
EVFigures
Uncropped_Fig2
Uncropped_Fig6
Acknowledgements
We acknowledge David Kelly from the COIL facility, WTCCB, University of Edinburgh; Emerald Perlas from the Histology Facility of the Epigenetics & Neurobiology Unit, EMBL Rome; Violetta Parimbeni for mouse husbandry, (Epigenetics & Neurobiology Unit, EMBL Rome). We thank Phil Avner (Epigenetics & Neurobiology Unit, EMBL Rome) for advice, reagents, support, discussions and critically reading the manuscript. Rafael Galupa (EMBL Heidelberg) and Jacqueline Mermoud (University of Marburg) are thanked for critically reading the manuscript. Titia de Lange (The Rockefeller University) is thanked for initially supporting the generation of the Rif1 knockout mice. Joost Gribnau and Cristina Gontan (Erasmus MC, University Medical Center, Rotterdam) are thanked for the Xist‐luciferase reporter plasmid. Andrew Jarman and Petra zur Lage (Centre for Discovery Brain Sciences, Edinburgh) and Sally Lowell (MRC Centre for Regenerative Medicine, Edinburgh) are all thanked for providing reagents. EE received funding from the European Union’s Horizon 2020 research and the Marie Skłodowska‐Curie Individual Fellowship grant agreement No. 660985 and from the ERC consolidator award 726130 to SCBB. LP and LB were funded by the ERC consolidator award 726130 to SBCB. RF was funded by the EMBL Interdisciplinary Postdoc (EIPOD) fellowship under Marie Curie Actions (COFUND). GK acknowledges funding from the IMPRS‐BAC. AC is funded by a Rett Syndrome Research Trust (RSRT), BARTSCHARITY grants, and intramural QMUL support.
The EMBO Journal (2021) 40: e105862.
Data availability
The RNA‐seq data have been deposited in the GEO database (GSE165704) and are available at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE165704.
References
- Aeby E, Lee HG, Lee YW, Kriz A, Del Rosario BC, Oh HJ, Boukhali M, Haas W, Lee JT (2020) Decapping enzyme 1A breaks X‐chromosome symmetry by controlling Tsix elongation and RNA turnover. Nat Cell Biol 22: 1116–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alver RC, Chadha GS, Gillespie PJ, Blow JJ (2017) Reversal of DDK‐mediated MCM phosphorylation by Rif1‐PP1 regulates replication initiation and replisome stability independently of ATR/Chk1. Cell Rep 18: 2508–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avner P, Heard E (2001) X‐chromosome inactivation: counting, choice and initiation. Nat Rev Genet 2: 59–67 [DOI] [PubMed] [Google Scholar]
- Borensztein M, Syx L, Ancelin K, Diabangouaya P, Picard C, Liu T, Liang JB, Vassilev I, Galupa R, Servant N et al (2017) Xist‐dependent imprinted X inactivation and the early developmental consequences of its failure. Nat Struct Mol Biol 24: 226–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockdorff N, Ashworth A, Kay GF, Cooper P, Smith S, McCabe VM, Norris DP, Penny GD, Patel D, Rastan S (1991) Conservation of position and exclusive expression of mouse Xist from the inactive X chromosome. Nature 351: 329–331 [DOI] [PubMed] [Google Scholar]
- Brown CJ, Ballabio A, Rupert JL, Lafreniere RG, Grompe M, Tonlorenzi R, Willard HF (1991) A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature 349: 38–44 [DOI] [PubMed] [Google Scholar]
- Bulut‐Karslioglu A, Perrera V, Scaranaro M, de la Rosa‐Velazquez IA, van de Nobelen S, Shukeir N, Popow J, Gerle B, Opravil S, Pagani M et al (2012) A transcription factor‐based mechanism for mouse heterochromatin formation. Nat Struct Mol Biol 19: 1023–1030 [DOI] [PubMed] [Google Scholar]
- Bunch H, Lawney BP, Burkholder A, Ma D, Zheng X, Motola S, Fargo DC, Levine SS, Wang YE, Hu G (2016) RNA polymerase II promoter‐proximal pausing in mammalian long non‐coding genes. Genomics 108: 64–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomo SB, Wu Y, Ferguson D, de Lange T (2009) Mammalian Rif1 contributes to replication stress survival and homology‐directed repair. J Cell Biol 187: 385–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo‐Branco G, Amaral PP, Engstrom PG, Robson SC, Marques SC, Bertone P, Kouzarides T (2013) The non‐coding snRNA 7SK controls transcriptional termination, poising, and bidirectionality in embryonic stem cells. Genome Biol 14: R98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CW, Chou HY, Lin YS, Huang KH, Chang CJ, Hsu TC, Lee SC (2008) Phosphorylation at Ser473 regulates heterochromatin protein 1 binding and corepressor function of TIF1beta/KAP1. BMC Mol Biol 9: 61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao SH, Price DH (2001) Flavopiridol inactivates P‐TEFb and blocks most RNA polymerase II transcription in vivo. J Biol Chem 276: 31793–31799 [DOI] [PubMed] [Google Scholar]
- Chapman JR, Barral P, Vannier JB, Borel V, Steger M, Tomas‐Loba A, Sartori AA, Adams IR, Batista FD, Boulton SJ (2013) RIF1 is essential for 53BP1‐dependent nonhomologous end joining and suppression of DNA double‐strand break resection. Mol Cell 49: 858–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumeil J, Le Baccon P, Wutz A, Heard E (2006) A novel role for Xist RNA in the formation of a repressive nuclear compartment into which genes are recruited when silenced. Genes Dev 20: 2223–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK, Blanco M, Jackson C, Aznauryan E, Ollikainen N, Surka C, Chow A, Cerase A, McDonel P, Guttman M (2016) Xist recruits the X chromosome to the nuclear lamina to enable chromosome‐wide silencing. Science 354: 468–472 [DOI] [PubMed] [Google Scholar]
- Chu C, Zhang QC, da Rocha ST, Flynn RA, Bharadwaj M, Calabrese JM, Magnuson T, Heard E, Chang HY (2015) Systematic discovery of Xist RNA binding proteins. Cell 161: 404–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chureau C, Chantalat S, Romito A, Galvani A, Duret L, Avner P, Rougeulle C (2011) Ftx is a non‐coding RNA which affects Xist expression and chromatin structure within the X‐inactivation center region. Hum Mol Genet 20: 705–718 [DOI] [PubMed] [Google Scholar]
- Coluccio A, Ecco G, Duc J, Offner S, Turelli P, Trono D (2018) Individual retrotransposon integrants are differentially controlled by KZFP/KAP1‐dependent histone methylation, DNA methylation and TET‐mediated hydroxymethylation in naive embryonic stem cells. Epigenetics Chromatin 11: 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornacchia D, Dileep V, Quivy JP, Foti R, Tili F, Santarella‐Mellwig R, Antony C, Almouzni G, Gilbert DM, Buonomo SB (2012) Mouse Rif1 is a key regulator of the replication‐timing programme in mammalian cells. EMBO J 31: 3678–3690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave A, Cooley C, Garg M, Bianchi A (2014) Protein phosphatase 1 recruitment by Rif1 regulates DNA replication origin firing by counteracting DDK activity. Cell Rep 7: 53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daxinger L, Harten SK, Oey H, Epp T, Isbel L, Huang E, Whitelaw N, Apedaile A, Sorolla A, Yong J et al (2013) An ENU mutagenesis screen identifies novel and known genes involved in epigenetic processes in the mouse. Genome Biol 14: R96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Bergmaier P, Sachs P, Klangwart M, Ruckert T, Bartels N, Demmers J, Dekker M, Poot RA, Mermoud JE (2018) The CUE1 domain of the SNF2‐like chromatin remodeler SMARCAD1 mediates its association with KRAB‐associated protein 1 (KAP1) and KAP1 target genes. J Biol Chem 293: 2711–2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R (1985) The in vitro development of blastocyst‐derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol 87: 27–45 [PubMed] [Google Scholar]
- Dossin F, Pinheiro I, Zylicz JJ, Roensch J, Collombet S, Le Saux A, Chelmicki T, Attia M, Kapoor V, Zhan Y et al (2020) SPEN integrates transcriptional and epigenetic control of X‐inactivation. Nature 578: 455–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engreitz JM, Pandya‐Jones A, McDonel P, Shishkin A, Sirokman K, Surka C, Kadri S, Xing J, Goren A, Lander ES et al (2013) The Xist lncRNA exploits three‐dimensional genome architecture to spread across the X chromosome. Science 341: 1237973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti R, Gnan S, Cornacchia D, Dileep V, Bulut‐Karslioglu A, Diehl S, Buness A, Klein FA, Huber W, Johnstone E et al (2016) Nuclear architecture organized by Rif1 underpins the replication‐timing program. Mol Cell 61: 260–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan G, Gutierrez Hernandez N, Huret C, Galupa R, van Bemmel JG, Romito A, Heard E, Morey C, Rougeulle C (2018) The Ftx noncoding locus controls X chromosome inactivation independently of Its RNA products. Mol Cell 70: 462–472.e8 [DOI] [PubMed] [Google Scholar]
- Garzon J, Ursich S, Lopes M, Hiraga SI, Donaldson AD (2019) Human RIF1‐protein phosphatase 1 prevents degradation and breakage of nascent DNA on replication stalling. Cell Rep 27: 2558–2566.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnan S, Flyamer IM, Klein KN, Castelli E, Rapp A, Maiser A, Chen N, Weber P, Enervald E, Cardoso MC et al (2021) Nuclear organisation and replication timing are coupled through RIF1‐PP1 interaction. Nat Commun 12: 2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gontan C, Achame EM, Demmers J, Barakat TS, Rentmeester E, van IJcken W, Grootegoed JA, Gribnau J (2012) RNF12 initiates X‐chromosome inactivation by targeting REX1 for degradation. Nature 485: 386–390 [DOI] [PubMed] [Google Scholar]
- Hayano M, Kanoh Y, Matsumoto S, Renard‐Guillet C, Shirahige K, Masai H (2012) Rif1 is a global regulator of timing of replication origin firing in fission yeast. Genes Dev 26: 137–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S, Alvino GM, Chang F, Lian HY, Sridhar A, Kubota T, Brewer BJ, Weinreich M, Raghuraman MK, Donaldson AD (2014) Rif1 controls DNA replication by directing Protein Phosphatase 1 to reverse Cdc7‐mediated phosphorylation of the MCM complex. Genes Dev 28: 372–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga SI, Ly T, Garzon J, Horejsi Z, Ohkubo YN, Endo A, Obuse C, Boulton SJ, Lamond AI, Donaldson AD (2017) Human RIF1 and protein phosphatase 1 stimulate DNA replication origin licensing but suppress origin activation. EMBO Rep 18: 403–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CM, Nesterova TB, Formstone EJ, Newall AE, Duthie SM, Sheardown SA, Brockdorff N (1998) Developmentally regulated Xist promoter switch mediates initiation of X inactivation. Cell 94: 809–817 [DOI] [PubMed] [Google Scholar]
- Jonkers I, Barakat TS, Achame EM, Monkhorst K, Kenter A, Rentmeester E, Grosveld F, Grootegoed JA, Gribnau J (2009) RNF12 is an X‐Encoded dose‐dependent activator of X chromosome inactivation. Cell 139: 999–1011 [DOI] [PubMed] [Google Scholar]
- Kucera KS, Reddy TE, Pauli F, Gertz J, Logan JE, Myers RM, Willard HF (2011) Allele‐specific distribution of RNA polymerase II on female X chromosomes. Hum Mol Genet 20: 3964–3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JT (2002) Homozygous Tsix mutant mice reveal a sex‐ratio distortion and revert to random X‐inactivation. Nat Genet 32: 195–200 [DOI] [PubMed] [Google Scholar]
- Lee JT (2005) Regulation of X‐chromosome counting by Tsix and Xite sequences. Science 309: 768–771 [DOI] [PubMed] [Google Scholar]
- Lee JT, Davidow LS, Warshawsky D (1999) Tsix, a gene antisense to Xist at the X‐inactivation centre. Nat Genet 21: 400–404 [DOI] [PubMed] [Google Scholar]
- Lee JT, Lu N (1999) Targeted mutagenesis of Tsix leads to nonrandom X inactivation. Cell 99: 47–57 [DOI] [PubMed] [Google Scholar]
- Li P, Wang L, Bennett BD, Wang J, Li J, Qin Y, Takaku M, Wade PA, Wong J, Hu G (2017) Rif1 promotes a repressive chromatin state to safeguard against endogenous retrovirus activation. Nucleic Acids Res 45: 12723–12738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luikenhuis S, Wutz A, Jaenisch R (2001) Antisense transcription through the Xist locus mediates Tsix function in embryonic stem cells. Mol Cell Biol 21: 8512–8520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon MF (1961) Gene action in the X‐chromosome of the mouse (Mus musculus L.). Nature 190: 372–373 [DOI] [PubMed] [Google Scholar]
- Makhlouf M, Ouimette JF, Oldfield A, Navarro P, Neuillet D, Rougeulle C (2014) A prominent and conserved role for YY1 in Xist transcriptional activation. Nat Commun 5: 4878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksakova IA, Thompson PJ, Goyal P, Jones SJ, Singh PB, Karimi MM, Lorincz MC (2013) Distinct roles of KAP1, HP1 and G9a/GLP in silencing of the two‐cell‐specific retrotransposon MERVL in mouse ES cells. Epigenetics Chromatin 6: 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marahrens Y, Panning B, Dausman J, Strauss W, Jaenisch R (1997) Xist‐deficient mice are defective in dosage compensation but not spermatogenesis. Genes Dev 11: 156–166 [DOI] [PubMed] [Google Scholar]
- Mattarocci S, Shyian M, Lemmens L, Damay P, Altintas DM, Shi T, Bartholomew CR, Thoma NH, Hardy CF, Shore D (2014) Rif1 controls DNA replication timing in yeast through the PP1 phosphatase Glc7. Cell Rep 7: 62–69 [DOI] [PubMed] [Google Scholar]
- McHugh CA, Chen CK, Chow A, Surka CF, Tran C, McDonel P, Pandya‐Jones A, Blanco M, Burghard C, Moradian A et al (2015) The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature 521: 232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RP, Reeder JE, McMillan EA, Bacon CW, McCann JL, D'Orso I (2016) KAP1 recruitment of the 7SK snRNP complex to promoters enables transcription elongation by RNA polymerase II. Mol Cell 61: 39–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moindrot B, Cerase A, Coker H, Masui O, Grijzenhout A, Pintacuda G, Schermelleh L, Nesterova TB, Brockdorff N (2015) A pooled shRNA screen identifies Rbm15, Spen, and Wtap as factors required for Xist RNA‐Mediated Silencing. Cell Rep 12: 562–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfort A, Di Minin G, Postlmayr A, Freimann R, Arieti F, Thore S, Wutz A (2015) Identification of Spen as a crucial factor for Xist function through forward genetic screening in haploid embryonic stem cells. Cell Rep 12: 554–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk M, Harper M (1978) X‐chromosome activity in preimplantation mouse embryos from XX and XO mothers. J Embryol Exp Morphol 46: 53–64 [PubMed] [Google Scholar]
- Mutzel V, Okamoto I, Dunkel I, Saitou M, Giorgetti L, Heard E, Schulz EG (2019) A symmetric toggle switch explains the onset of random X inactivation in different mammals. Nat Struct Mol Biol 26: 350–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutzel V, Schulz EG (2020) Dosage sensing, threshold responses, and epigenetic memory: a systems biology perspective on random X‐chromosome inactivation. BioEssays 42: e1900163 [DOI] [PubMed] [Google Scholar]
- de Napoles M, Mermoud JE, Wakao R, Tang YA, Endoh M, Appanah R, Nesterova TB, Silva J, Otte AP, Vidal M et al (2004) Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev Cell 7: 663–676 [DOI] [PubMed] [Google Scholar]
- Navarro P, Chambers I, Karwacki‐Neisius V, Chureau C, Morey C, Rougeulle C, Avner P (2008) Molecular coupling of Xist regulation and pluripotency. Science 321: 1693–1695 [DOI] [PubMed] [Google Scholar]
- Navarro P, Page DR, Avner P, Rougeulle C (2006) Tsix‐mediated epigenetic switch of a CTCF‐flanked region of the Xist promoter determines the Xist transcription program. Genes Dev 20: 2787–2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro P, Pichard S, Ciaudo C, Avner P, Rougeulle C (2005) Tsix transcription across the Xist gene alters chromatin conformation without affecting Xist transcription: implications for X‐chromosome inactivation. Genes Dev 19: 1474–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesterova TB, Johnston CM, Appanah R, Newall AE, Godwin J, Alexiou M, Brockdorff N (2003) Skewing X chromosome choice by modulating sense transcription across the Xist locus. Genes Dev 17: 2177–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newall AE, Duthie S, Formstone E, Nesterova T, Alexiou M, Johnston C, Caparros ML, Brockdorff N (2001) Primary non‐random X inactivation associated with disruption of Xist promoter regulation. Hum Mol Genet 10: 581–589 [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Lee JT (2003) Xite, X‐inactivation intergenic transcription elements that regulate the probability of choice. Mol Cell 11: 731–743 [DOI] [PubMed] [Google Scholar]
- Ohhata T, Hoki Y, Sasaki H, Sado T (2008) Crucial role of antisense transcription across the Xist promoter in Tsix‐mediated Xist chromatin modification. Development 135: 227–235 [DOI] [PubMed] [Google Scholar]
- Peace JM, Ter‐Zakarian A, Aparicio OM (2014) Rif1 regulates initiation timing of late replication origins throughout the S. cerevisiae genome. PLoS One 9: e98501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck SA, Hughes KD, Victorino JF, Mosley AL (2019) Writing a wrong: coupled RNA polymerase II transcription and RNA quality control. Wiley Interdiscip Rev RNA 10: e1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny GD, Kay GF, Sheardown SA, Rastan S, Brockdorff N (1996) Requirement for Xist in X chromosome inactivation. Nature 379: 131–137 [DOI] [PubMed] [Google Scholar]
- Percharde M, Lin CJ, Yin Y, Guan J, Peixoto GA, Bulut‐Karslioglu A, Biechele S, Huang B, Shen X, Ramalho‐Santos M (2018) A LINE1‐nucleolin partnership regulates early development and ESC identity. Cell 174: 391–405.e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastan S (1982) Timing of X‐chromosome inactivation in postimplantation mouse embryos. J Embryol Exp Morphol 71: 11–24 [PubMed] [Google Scholar]
- Sado T, Hoki Y, Sasaki H (2005) Tsix silences Xist through modification of chromatin structure. Dev Cell 9: 159–165 [DOI] [PubMed] [Google Scholar]
- Sado T, Wang Z, Sasaki H, Li E (2001) Regulation of imprinted X‐chromosome inactivation in mice by Tsix. Development 128: 1275–1286 [DOI] [PubMed] [Google Scholar]
- Seller CA, O'Farrell PH (2018) Rif1 prolongs the embryonic S phase at the Drosophila mid‐blastula transition. PLoS Biol 16: e2005687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Lee JT (2004) Tsix transcription‐ versus RNA‐based mechanisms in Xist repression and epigenetic choice. Curr Biol 14: 1747–1754 [DOI] [PubMed] [Google Scholar]
- Shin J, Wallingford MC, Gallant J, Marcho C, Jiao B, Byron M, Bossenz M, Lawrence JB, Jones SN, Mager J et al (2014) RLIM is dispensable for X‐chromosome inactivation in the mouse embryonic epiblast. Nature 511: 86–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MD, Pinter SF, Fang R, Sarma K, Rutenberg‐Schoenberg M, Bowman SK, Kesner BA, Maier VK, Kingston RE, Lee JT (2013) High‐resolution Xist binding maps reveal two‐step spreading during X‐chromosome inactivation. Nature 504: 465–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreesankar E, Bharathi V, Mishra RK, Mishra K (2015) Drosophila Rif1 is an essential gene and controls late developmental events by direct interaction with PP1‐87B. Sci Rep 5: 10679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starmer J, Magnuson T (2009) A new model for random X chromosome inactivation. Development 136: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavropoulos N, Lu N, Lee JT (2001) A functional role for Tsix transcription in blocking Xist RNA accumulation but not in X‐chromosome choice. Proc Natl Acad Sci USA 98: 10232–10237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukackaite R, Cornacchia D, Jensen MR, Mas PJ, Blackledge M, Enervald E, Duan G, Auchynnikava T, Kohn M, Hart DJ et al (2017) Mouse Rif1 is a regulatory subunit of protein phosphatase 1 (PP1). Sci Rep 7: 2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun BK, Deaton AM, Lee JT (2006) A transient heterochromatic state in Xist preempts X inactivation choice without RNA stabilization. Mol Cell 21: 617–628 [DOI] [PubMed] [Google Scholar]
- Takagi N, Abe K (1990) Detrimental effects of two active X chromosomes on early mouse development. Development 109: 189–201 [DOI] [PubMed] [Google Scholar]
- Takagi N, Sugawara O, Sasaki M (1982) Regional and temporal changes in the pattern of X‐chromosome replication during the early post‐implantation development of the female mouse. Chromosoma 85: 275–286 [DOI] [PubMed] [Google Scholar]
- Tanaka H, Muto A, Shima H, Katoh Y, Sax N, Tajima S, Brydun A, Ikura T, Yoshizawa N, Masai H et al (2016) Epigenetic regulation of the Blimp‐1 Gene (Prdm1) in B cells involves Bach2 and histone deacetylase 3. J Biol Chem 291: 6316–6330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan‐Wong SM, French JD, Proudfoot NJ, Brown MA (2008) Dynamic interactions between the promoter and terminator regions of the mammalian BRCA1 gene. Proc Natl Acad Sci USA 105: 5160–5165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D, Sun S, Lee JT (2010) The long noncoding RNA, Jpx, is a molecular switch for X chromosome inactivation. Cell 143: 390–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toteva T, Mason B, Kanoh Y, Brogger P, Green D, Verhein‐Hansen J, Masai H, Thon G (2017) Establishment of expression‐state boundaries by Rif1 and Taz1 in fission yeast. Proc Natl Acad Sci USA 114: 1093–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkle‐Mulcahy L, Andersen J, Lam YW, Moorhead G, Mann M, Lamond AI (2006) Repo‐Man recruits PP1 gamma to chromatin and is essential for cell viability. J Cell Biol 172: 679–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau S, Augui S, Navarro P, Avner P, Clerc P (2006) An essential role for the DXPas34 tandem repeat and Tsix transcription in the counting process of X chromosome inactivation. Proc Natl Acad Sci USA 103: 7390–7395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, McCannell KN, Boskovic A, Zhu X, Shin J, Yu J, Gallant J, Byron M, Lawrence JB, Zhu LJ et al (2017) Rlim‐dependent and ‐independent pathways for X chromosome inactivation in female ESCs. Cell Rep 21: 3691–3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wutz A, Jaenisch R (2000) A shift from reversible to irreversible X inactivation is triggered during ES cell differentiation. Mol Cell 5: 695–705 [DOI] [PubMed] [Google Scholar]
- Wutz A, Rasmussen TP, Jaenisch R (2002) Chromosomal silencing and localization are mediated by different domains of Xist RNA. Nat Genet 30: 167–174 [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Ishii A, Kanoh Y, Oda M, Nishito Y, Masai H (2012) Rif1 regulates the replication timing domains on the human genome. EMBO J 31: 3667–3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Kirby JE, Sunwoo H, Lee JT (2016) Female mice lacking Xist RNA show partial dosage compensation and survive to term. Genes Dev 30: 1747–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT (2008) Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science 322: 750–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zofall M, Smith DR, Mizuguchi T, Dhakshnamoorthy J, Grewal SIS (2016) Taz1‐shelterin promotes facultative heterochromatin assembly at chromosome‐internal sites containing late replication origins. Mol Cell 62: 862–874 [DOI] [PMC free article] [PubMed] [Google Scholar]


