Abstract
Background
Identifying predictors of preterm birth (PTB) in high-burden regions is important as PTB is the leading cause of global child mortality.
Methods
This analysis was nested in a longitudinal study of human immunodeficiency virus (HIV) incidence in Kenya. HIV-seronegative women enrolled in pregnancy had nucleic acid amplification tests (chlamydia and gonorrhea), rapid plasma reagin (syphilis), wet mount microscopy (Trichomonas and yeast), and Gram stain (bacterial vaginosis); sexually transmitted infection (STI) treatment was provided. PTB predictors were determined using log-binomial regression.
Results
Among 1244 mothers of liveborn infants, median gestational age at enrollment was 26 weeks (IQR, 22–31), and at delivery was 39.1 weeks (IQR, 37.1–40.9). PTB occurred in 302 women (24.3%). Chlamydia was associated with a 1.59-fold (P = .006), gonorrhea a 1.62-fold (P = .04), and incident HIV a 2.08-fold (P = .02) increased PTB prevalence. Vaginal discharge and cervical inflammation were associated with PTB, as were age ≤21 (prevalence ratio [PR] = 1.39, P = .001) and any STI (PR = 1.47, P = .001). Associations with chlamydia and incident HIV remained in multivariable models.
Conclusions
STIs and incident HIV in pregnancy predicted PTB despite treatment, suggesting the need for earlier treatment and interventions to decrease genital inflammation.
Keywords: preterm birth, sexually transmitted infections, chlamydia, gonorrhea, HIV, STI
Pregnant women with chlamydia, gonorrhea, and incident HIV infection had increased risk of preterm birth. Delays in diagnosis and treatment likely attenuated treatment benefit. Point-of-care testing for sexually transmitted infections with same-day treatment may be useful to prevent preterm birth.
Preterm birth (PTB) is the leading cause of neonatal mortality and mortality in children younger than 5 years worldwide [1]. Almost 15 million PTBs occur annually, and sub-Saharan Africa and South Asia together account for an estimated 78.9% of the global burden of PTBs [2].
Determinants of PTB are complex and multifactorial. Maternal infection and inflammation, maternal/fetal hypothalamic pituitary axis activation, decidual hemorrhage, and uterine overdistension are commonly recognized pathways leading to spontaneous PTB [3]. In some studies, maternal sexually transmitted infections (STIs), including chlamydia, gonorrhea, trichomonas, syphilis, and human immunodeficiency virus (HIV), and genital infections, such as bacterial vaginosis and yeast, have been associated with increased risk of PTB [4–10]. However, there is mixed evidence regarding the impact of treatment of STIs or genital infections in decreasing PTB [6, 11–15]. Sociodemographic, behavioral, genetic, and obstetric risk factors also contribute to risk of PTB [16–18].
There are limited data on PTB incidence and risk factors from Africa, despite the region’s disproportionately large contribution to the global burden of PTB. Rates of preterm births in sub-Saharan Africa range from 15% to 21% [2, 19, 20]. Prior studies using cross-sectional or cohort designs in this region have observed associations of HIV, STIs, genital infections, malaria, gestational weight gain, and anemia with PTB [21–23]. There are few longitudinal studies from Africa that have evaluated PTB risk factors with standardized ascertainment of STIs, genital infections, and socioeconomic factors in pregnancy. We conducted a longitudinal study to determine incidence and predictors of PTB in a Kenyan cohort.
METHODS
Study Population
This analysis was nested within a study designed to determine incidence and risk factors for HIV acquisition among pregnant HIV-uninfected pregnant women [24, 25]. Study protocols were approved by the Institutional Review Board of the University of Washington and the Ethics and Research Committee of Kenyatta National Hospital. Pregnant women seeking antenatal care services at 2 public hospitals (Bondo District Hospital and Ahero sub-District Hospital) in Western Kenya were recruited and followed up from 2009 to 2015. Only women testing HIV negative were enrolled. Women who acquired HIV during follow-up were retained.
Study Procedures and Follow-Up
Sociodemographic characteristics and medical and obstetric histories were obtained at enrollment. At the initial study visit (enrollment), clinicians performed a pelvic examination and collected blood and genital specimens for STI screening (median gestational age 26 weeks). Pregnant women and their infants were followed from the initial antenatal visit until 9 months post partum, with 3 visits during pregnancy. At follow-up visits, blood and genital specimens were collected for diagnosis of HIV and STIs. STIs and genital infections were treated at the time of detection (test results). Chlamydia was treated with augmentin 1 g single dose, gonorrhea with cefixime 400 mg single dose, trichomonas with metronidazole 400 mg twice daily for 7 days, and syphilis with benzathine penicillin G 2.4 million units intramuscularly per Kenyan guidelines. Participants with STIs were counseled on disclosing their diagnosis to their sex partner(s) and provided with a partner referral letter or partner treatment [26]. Women who acquired HIV during pregnancy were started on antiretroviral therapy.
Data from delivery facility records were collected regarding gestational age at delivery, delivery type, infant outcome, infant birthweight, duration of ruptured membranes, duration of labor, duration of maternity stay, and complications. For deliveries outside the study sites (at home or at a private facility), delivery and birth weight data was obtained at the first postpartum Maternal Child Health (MCH) clinic visit and abstracted from facility records or government-issued mother-child health booklets.
Laboratory Testing
Laboratory assays have previously been described [24]. Briefly, Neisseria gonorrhea and Chlamydia trachomatis were diagnosed using the Gen-Probe/Hologic APTIMA Combo-2 assay at the University of Nairobi/Washington Research Laboratory at Coast Hospital in Mombasa, Kenya. Treponema pallidum was diagnosed by the rapid plasma reagin test (Becton and Dickinson). Bacterial vaginosis (BV) was diagnosed using Amsel and Nugent criteria from gram-stained vaginal smears, and those with BV by either test were defined as having BV. Endocervical Gram stains were examined for polymorphonuclear neutrophils (PMNs) with cervical inflammation defined as >10 PMNs/high power field (hpf). Trichomonas vaginalis and Candida were identified by microscopy visualization of vaginal wet mount. During monthly follow-up in pregnancy, blood was tested for HIV using pooled nucleic acid amplification tests.
Gestational Age Estimation
Infants were considered PTB if born alive before 37 weeks gestation using the World Health Organization definition for PTB [2]. Gestational age at birth was calculated using data from an algorithm of prenatal ultrasounds (US), last menstrual period (LMP), and fundal height (FH). US dating was used if available. If no US data were available and LMP date was accurate and available, LMP was used. If LMP was missing and FH was available, FH was used. Gestational age estimates were based on LMP for 87.1% (1084), US for 9.4% (117), and FH for 3.5% (43). In the subset of women with all 3 measures (n = 94) there was good consistency between measures. The estimated percentage of preterm births was 26.2% by LMP, 24.0% by FH, and 16.2% by US. Women with estimated gestational age above 45 weeks and below 26 weeks were excluded from the analysis.
Statistical Methods
Stata SE version 15 (StataCorp) was used for all analyses. Log binomial regression was used to identify correlates of PTB. Covariates included a priori defined variables based on a review of the literature and hypothesized relationships between maternal STI and birth outcomes. Age and education were dichotomized at their medians to simplify interpretation of prevalence ratios (PRs). Several well-defined risk factors for PTB (eg, smoking, preeclampsia, diabetes) were not evaluated. Prior preterm delivery and placental insufficiency were not assessed. Multivariable regression models were built using covariates that were statistically significant (P ≤ .05) in univariate analyses (P < .05). Because a multivariable model with 3 individual STIs (chlamydia, HIV, and gonorrhea) would not converge, a model including only the 2 most statistically significant (chlamydia and HIV) is reported.
RESULTS
Participant Characteristics
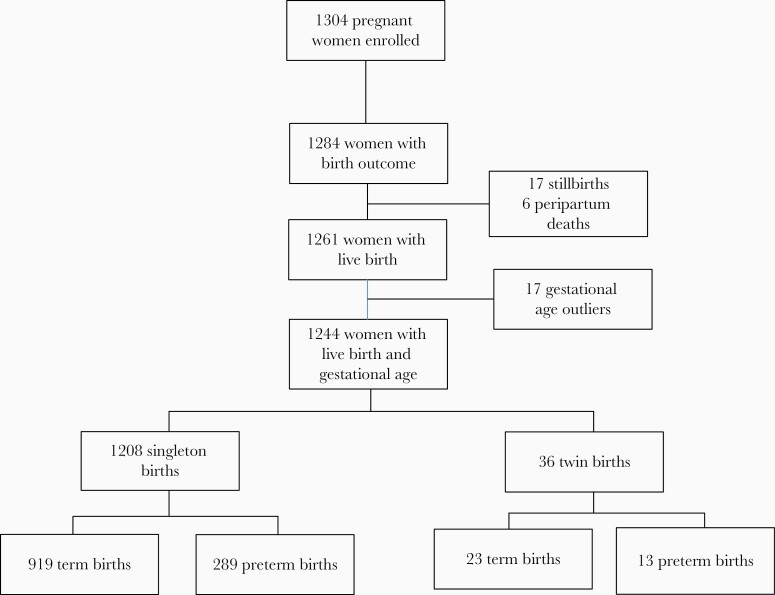
Among 1304 pregnant women enrolled, 1284 women had a birth outcome recorded (Figure 1); 17 deliveries without a valid gestational age were excluded as were 17 stillbirths, and 6 peripartum deaths leaving 1244 women in the PTB analysis. Among these 1244 women, median maternal age at enrollment was 22 years (interquartile range [IQR], 19–27 years); 46.4%% of women were ≤21 years old (Table 1). Median education was 8 years (IQR, 7–10 years). Most women were married (78.5%) and in monogamous relationships (85.3%). Tobacco and alcohol use were rare (<1% for both). Some women (10.4%) reported a history of sex work (defined as providing sexual services in exchange for money or goods). Most (87.6%) women had ≤3 lifetime sexual partners and 92.4% had a current partner. Approximately one-third of women were primigravid. Women with symptoms consistent with malaria received malaria testing; malaria was detected clinically in 5.0% of participants. Median gestational age at enrollment was 26 weeks (IQR, 22–31 weeks). Other complications of pregnancy were rare, with few women reporting gestational hypertension (1.4%), vaginal bleeding (0.4%), or preterm premature rupture of membranes (0.1%).
Figure 1.
Study flow chart.
Table 1.
Enrollment Characteristics During Pregnancy Among Women With Live Births
| Characteristics | n | Value |
|---|---|---|
| Sociodemographic | ||
| Age, y, median (IQR) | 1244 | 22 (19–27) |
| Age ≤ 21 y | 1244 | 46.4 (577) |
| Education No. of y, median (IQR) | 1244 | 8 (7–10) |
| Primary school education, 1–8 y | 1244 | 64.0 (796) |
| Secondary school education, 9–12 y | 1244 | 30.6 (380) |
| Any college education | 1244 | 5.5 (68) |
| Employed | 1244 | 44.1 (548) |
| Relationship status | ||
| Married | 1244 | 78.5 (976) |
| Never married | 1244 | 19.2 (239) |
| Widowed or divorced | 1244 | 2.3 (29) |
| Polygamous | 974 | 14.7 (148) |
| Length of relationship, y, median (IQR) | 1002 | 4 (1–8) |
| Partner’s age, y, median (IQR) | 1012 | 29 (25–35) |
| Sexual history | ||
| Total number of lifetime sex partners | 1244 | |
| 1 | 27.1 (337) | |
| 2–3 | 60.5 (752) | |
| > 3 | 12.4 (155) | |
| Age of sexual debut, y, median (IQR) | 1181 | 16 (15–18) |
| Obstetric history | ||
| Primigravida | 1244 | 30.7 (382) |
| Primiparous | 1207 | 27.6 (333) |
| History of prior miscarriage | 860 | 11.6 (100) |
| History of prior stillbirth | 859 | 3.4 (28) |
| Maternal health in pregnancy | ||
| Gestational age at enrollment, wk, median (IQR) | 1244 | 26 (22–31) |
| Hemoglobin, g/dL, median (IQR) | 1094 | 10.7 (9.3–11.8) |
| Anemiaa | 1094 | 55.9 (612) |
| Malaria infectionb | 1244 | 5.0 (62) |
| Gestational hypertensionc | 1244 | 1.4 (17) |
| Vaginal bleeding | 1244 | 0.4 (5) |
| Delivery | ||
| Vaginal delivery | 1244 | 96.1 (1196) |
| Health facility delivery | 1244 | 62.6 (779) |
| Prolonged rupture of membranesd | 149 | 7.4 (11) |
| Twin birth | 1244 | 2.9 (36) |
| Female infante | 1244 | 47.8 (594) |
| Birth weight, kg,e median (IQR) | 1146 | 3.5 (3.0–3.8) |
| Gestational age, wk, median (IQR) | 1244 | 39.1 (37.1–40.9) |
| HIV status | ||
| Incident HIV between enrollment and delivery | 1244 | 0.8 (10) |
| Partner(s) reported HIV positive | 1149 | 1.4 (16) |
| Partner(s) reported HIV negative | 1149 | 70.4 (809) |
| Partner(s) HIV status unknown | 1149 | 28.2 (324) |
| Sexually transmitted infections | ||
| Previous history of STIs | 1244 | 6.7 (83) |
| STI or HIV during current pregnancy | 1244 | 17.5 (218) |
| Trichomoniasis | 1244 | 10.4 (129) |
| Chlamydia | 1241 | 5.4 (67) |
| Gonorrhea | 1204 | 2.4 (29) |
| Syphilis | 985 | 1.2 (12) |
| Genital infections | ||
| Bacterial vaginosis | 1225 | 24.7 (302) |
| Yeast infection | 1244 | 25.2 (313) |
| Abnormal vaginal dischargef | 1243 | 20.0 (248) |
| Cervical inflammationg | 1234 | 14.9 (184) |
Data are % (No.) except where indicated.
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range.
aAnemia defined as hemoglobin < 11 g/dL.
bMalaria testing not routinely performed at study visits but results reported if malaria detected.
cGestational hypertension defined as systolic blood pressure ≥ 140 mmHg or diastolic ≥ 90 mmHg without proteinuria.
dProlonged rupture of membranes defined as >18 hours.
eFor first born infant if a twin birth.
fAbnormal vaginal discharge reported on pelvic examination or self-reported.
gCervical inflammation defined as >10 polymorphonuclear leukocytes/high power field.
Birth Outcomes
The median gestational age at delivery was 39.1 weeks (IQR, 37.1–40.9 weeks). Among the 1244 live birth deliveries, 302 (24.3%) were preterm births and 36 (2.9%) were twin births (Figure 1). Of all births, 195 (15.7%) were late preterm (gestational age 34–36 weeks), 94 (7.6%) were very preterm (gestational age 28–33 weeks), and 13 (1.1%) were extremely preterm (gestational age <28 weeks). Most women delivered vaginally (96.1%); 62.6% delivered at a health facility with the remainder delivering at home. The median birth weight was 3.5 kg (IQR, 3.0–3.8 kg).
STIs and Genital Infections
At enrollment during pregnancy, trichomonas was detected in 129 women (10.4%), chlamydia in 67 (5.4%), gonorrhea in 29 (2.4%), and syphilis in 12 women (1.2%) (Table 1). Ten women (0.8%) who were HIV seronegative at enrollment acquired HIV by the time of delivery. Of the 10 women with incident HIV infection during pregnancy, 3 had chlamydia, 1 had gonorrhea, 1 had trichomonas, and 4 had BV. Overall, 218 women (17.5%) had an STI (trichomonas, chlamydia, syphilis, gonorrhea, or incident HIV) detected in pregnancy. BV and yeast infections were detected in 184 (23.3%) and 313 (23.3%) women, respectively. Twenty percent of women reported abnormal vaginal discharge and 14.9% had evidence of nonspecific inflammation on endocervical Gram stain (>10 PMNs/hpf).
Correlates of Preterm Birth
Women <21 years of age were significantly more likely to experience PTB (PR, 1.39; 95% confidence interval [CI], 1.14–1.70; P = .001; Table 2). Having less than a primary school education (≤8 years) was associated with a higher prevalence of PTB (PR, 1.33; 95% CI, 1.07–1.65; P = .01). Employment status, marital status, having a current sex partner, or a woman’s total number of lifetime sex partners were not associated with PTB. Most pregnancy-related variables including parity, anemia, infant sex, and type or location of delivery were not associated with PTB, while twin gestation (n = 36), a known risk factor for PTB, was associated with a slightly higher prevalence of PTB (PR, 1.51; 95% CI, .97–2.36; P = .07).
Table 2.
Univariate Correlates of Preterm Birth, <37 Weeks Gestational Age
| Variable | Preterm | Term | PR (95% CI) | P Value | ||
|---|---|---|---|---|---|---|
| n | Value | n | Value | |||
| Demographics | ||||||
| Age ≤ 21 y | 302 | 54.6 | 942 | 43.7 | 1.39 (1.14–1.70) | .001 |
| Education ≤ 8 y, primary school | 302 | 70.2 | 942 | 62.0 | 1.33 (1.07–1.65) | .01 |
| Employed | 302 | 40.4 | 942 | 45.2 | 0.86 (.70–1.05) | .1 |
| Married | 302 | 75.5 | 942 | 79.4 | 0.85 (.68–1.06) | .1 |
| Total number of lifetime sex partners, median (IQR) | 302 | 2 (1–3) | 942 | 2 (1–3) | 0.93 (.85–1.01) | .1 |
| Obstetric | ||||||
| History of prior live birth | 302 | 99.0 | 942 | 97.9 | 1.88 (.65–5.42) | .2 |
| History of prior stillbirth or miscarriage | 300 | 8.7 | 942 | 10.4 | 0.86 (.60–1.22) | .4 |
| Anemia | 252 | 57.9 | 802 | 57.9 | 1.00 (.81–1.25) | 1.0 |
| Vaginal delivery | 302 | 96.0 | 942 | 96.2 | 0.95 (.69–1.31) | .8 |
| Health facility delivery | 302 | 59.9 | 942 | 63.5 | 0.89 (.73–1.09) | .3 |
| Twin birth | 302 | 4.3 | 941 | 2.4 | 1.51 (.97–2.36) | .07 |
| Female infanta | 302 | 44.7 | 941 | 48.8 | 0.88 (.72–1.07) | .2 |
| Symptomatic malariab | 302 | 5.3 | 942 | 4.9 | 1.07 (.69–1.65) | .8 |
| Sexually transmitted infections | ||||||
| Any STIc | 302 | 23.8 | 942 | 15.5 | 1.47 (1.18–1.84) | .001 |
| Trichomoniasis | 302 | 12.6 | 942 | 9.7 | 1.24 (.93–1.66) | .1 |
| Chlamydia | 301 | 8.3 | 940 | 4.5 | 1.59 (1.14–2.20) | .006 |
| Gonorrhea | 301 | 4.0 | 940 | 2.0 | 1.62 (1.03–2.55) | .04 |
| Syphilis | 230 | 2.2 | 755 | 0.9 | 1.80 (.91–3.55) | .09 |
| HIV | 301 | 1.7 | 941 | 0.5 | 2.08 (1.11–3.90) | .02 |
| Genital infections | ||||||
| Bacterial vaginosis | 294 | 27.2 | 931 | 23.9 | 1.14 (.92–1.43) | .2 |
| Yeast | 302 | 28.2 | 942 | 16.3 | 1.07 (.86–1.33) | .5 |
| Abnormal vaginal discharge | 301 | 24.3 | 942 | 18.6 | 1.28 (1.03–1.61) | .03 |
| Cervical inflammationc | 298 | 18.5 | 936 | 13.8 | 1.29 (1.01–1.65) | .04 |
| Genital infections stratified by STI status | ||||||
| Bacterial vaginosis, STI negative | 227 | 27.8 | 787 | 21.9 | 1.27 (.99–1.64) | .06 |
| Bacterial vaginosis, STI positive | 67 | 25.4 | 144 | 34.7 | 0.73 (.46–1.17) | .2 |
| Yeast, STI negative | 230 | 27.4 | 796 | 25.5 | 1.23 (.85–1.76) | .3 |
| Yeast, STI positive | 72 | 30.6 | 146 | 30.8 | 0.99 (.66–1.50) | 1.0 |
| Abnormal vaginal discharge, STI negative | 229 | 22.3 | 796 | 15.7 | 1.38 (1.06–1.80) | .02 |
| Abnormal vaginal discharge, STI positive | 72 | 30.6 | 146 | 34.3 | 0.89 (.59–1.35) | .6 |
| Cervical inflammation,d STI negative | 227 | 13.2 | 792 | 11.7 | 1.11 (.79–1.55) | .5 |
| Cervical inflammation,d STI positive | 71 | 35.2 | 144 | 25.0 | 1.37 (.93–2.02) | .1 |
Data are % except where indicated.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; IQR, interquartile range; PR, prevalence ratio; STI, sexually transmitted infection.
aFirst birth for twin births.
bMalaria testing was conducted only for women with symptoms consistent with malaria as part of clinical care.
cAny STI includes trichomonas, chlamydia, syphilis, gonorrhea, and HIV.
dCervical inflammation defined as >10 polymorphonuclear leukocytes/high power field.
PTB was significantly associated with the presence of any maternal STI during pregnancy (trichomonas, chlamydia, syphilis, gonorrhea, or incident HIV) with a PR of 1.47 (95% CI, 1.18–1.84; P = .001). Assessing each STI separately, PTB was significantly associated with maternal chlamydia infection (PR, 1.59; 95% CI, 1.14–2.20; P = .006). There was also an increased risk of PTB with gonorrhea (PR, 1.62; 95% CI, 1.03–2.55; P = .04) and incident HIV (PR, 2.08; 95% CI; 1.11–3.90; P = .02). While prevalence of PTB was higher for women with trichomoniasis and syphilis, these associations were not statistically significant. Inflammation on endocervical Gram stain (>10 PMNs/hpf) was associated with PTB (PR, 1.29; 95% CI, 1.01–1.65; P = .04) as was abnormal vaginal discharge (PR, 1.28; 95% CI, 1.03–1.61; P = .03). The association between vaginal discharge and PTB was stronger in the subset of women without an STI (PR, 1.38; 95% CI, 1.06–1.80; P = .02). There was no significant association between PTB and yeast infection or bacterial vaginosis. In analyses stratified for gestational age at STI screening <20 weeks or ≥20 weeks, we found no association between STI and PTB for those screened at <20 weeks (PR, 0.97; 95% CI, .48–1.96; P = .939), while there was a significant relationship between STI and PTB among those screened ≥20 weeks (PR, 1.56; 95% CI, 1.23–1.98; P < .001).
Three multivariable models were constructed, each including variables associated with PTB in univariate analyses (Table 3). Young maternal age (PR, 1.33; 95% CI, 1.09–1.62; P = .005) and any STI remained independently associated with increased PTB in a model including those variables and education, abnormal vaginal discharge, and cervical inflammation. In a second model in which “any STI” excluded trichomonas, all relationships were retained. In the third model, young maternal age (PR, 1.35; 95% CI, 1.11–1.65; P = .003), low education (PR, 1.29; 95% CI, 1.04–1.60; P = .02), chlamydia (PR, 1.42; 95% CI, 1.04–1.95; P = .03), and incident HIV (PR, 2.32; 1.65–3.26; P < .001) were associated with increased PTB in a model including those variables and including abnormal vaginal discharge and cervical inflammation.
Table 3.
Multivariable Correlates of Preterm Birth
| Variables in the Model | Adjusted PR (95% CI) | P Value |
|---|---|---|
| Model 1 (n = 1234) | ||
| Age ≤ 21 y | 1.33 (1.09–1.62) | .005 |
| Education ≤ 8 y, primary school | 1.22 (.98–1.52) | .07 |
| Any STIa | 1.31 (1.04–1.66) | .02 |
| Abnormal vaginal discharge | 1.10 (.87–1.39) | .5 |
| Cervical inflammation | 1.17 (.91–1.50) | .2 |
| Model 2 (n = 1234) | ||
| Age ≤ 21 y | 1.31 (1.07–1.60) | .008 |
| Education ≤ 8 y, primary school | 1.24 (1.00–1.54) | .047 |
| Any STIb | 1.49 (1.13–1.97) | .005 |
| Abnormal vaginal discharge | 1.12 (.89–1.42) | .320 |
| Cervical inflammation | 1.20 (.93–1.54) | .149 |
| Model 3 (n = 1230)c | ||
| Age ≤ 21 y | 1.35 (1.11–1.65) | .003 |
| Education ≤ 8 y, primary school | 1.29 (1.04–1.60) | .02 |
| Chlamydia | 1.42 (1.04–1.95) | .03 |
| HIV | 2.32 (1.65–3.26) | <.001 |
| Abnormal vaginal discharge | 1.06 (.85–1.34) | .6 |
| Cervical inflammation | 1.23 (.96–1.57) | .1 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; PR, prevalence ratio.
aAny STI includes trichomonas, chlamydia, syphilis, gonorrhea, and HIV.
bAny STI includes chlamydia, syphilis, gonorrhea, and HIV.
cModel with chlamydia, gonorrhea, and HIV would not converge so only chlamydia and HIV are included.
DISCUSSION
We found substantial risk of PTB in this cohort of young women in Western Kenya. Younger mothers and those with lower education had significantly increased risk of PTB and those diagnosed with any STI during pregnancy had a 1.47-fold increased likelihood of PTB despite microbiologic diagnosis and treatment of STIs. Chlamydia, gonorrhea, and incident HIV infection during pregnancy were each associated with a 1.6- to 2.1-fold increased risk of PTB, despite diagnosis and treatment. Our observed rate of PTB is higher than some prior studies in sub-Saharan Africa, with rates of 15% to 21% [2, 19, 20]. This may be in part due to the young age of our cohort, which enrolled women starting at age 14; almost half were adolescents ≤21 years of age. Given increased PTB risk among young women, the high proportion of young women in the cohort likely contributed to high PTB risk. Prevalence of STIs and genital infections was high, both of which would be expected to increase the PTB rate. Recent studies have observed rising STI prevalence among young women in sub-Saharan Africa, which is likely to adversely influence regional PTB rates and infant morbidity and mortality [27, 28].
We found associations of STIs with PTB consistent with other studies [4, 5, 7, 8, 10, 29]. Chlamydia, gonorrhea, and incident HIV were significantly associated with PTB, and there were trends for associations with syphilis and trichomonas and for bacterial vaginosis (among women without an STI). In previous studies, chlamydia has been associated with 1.35- to 3.9-fold increased odds of PTB [7, 8, 10, 30, 31]. Some recent studies have not observed an association between chlamydia diagnosis in pregnancy and PTB. However, these studies had differences in setting, timing of detection of infection or coverage of treatment, and study design (serologic retrospective assessment, propensity-matched, or registry-based analysis) [5, 32, 33]. Maternal gonorrhea in pregnancy predicted PTB in our study, consistent with other studies [4, 5, 7]. Trichomonas has been associated with PTB, with a pooled relative risk of 1.34 (95% CI, 1.19–1.51) in a meta-analysis [34, 35], similar to the trend for elevated risk in our study. BV and syphilis have been associated with PTB, similar to trends in our study [5, 29, 36]. The absence of an association between yeast infection and PTB in our study is also consistent with prior studies [37, 38]. Women in our study received STI treatment; nevertheless, PTB risk was elevated even among those treated for STIs. There is evidence that STIs and genital infections have lingering effects on PTB risk, despite treatment [6, 7]. In a prior study, chlamydia treatment before 20 weeks gestation decreased PTB risk compared to treatment after 20 weeks [6]. We similarly found that among women screened for STIs at <20 weeks, STIs were not associated with PTB while among those screened at >20 weeks, STIs were significantly associated with PTB. Given the lag in treatment in our study due to turnaround time for results and clinic return, STI diagnosis/treatment <24 weeks may be sufficient for prevention of PTB. In another study, risk of PTB was elevated in women who had an STI more than a year prior to pregnancy [7]. Median gestational age at enrollment in our cohort was 26 weeks, suggesting that women were treated after the inflammatory cascade associated with STIs had already influenced likelihood of PTB. Our findings are also consistent with conflicting results of clinical trials of antimicrobials to prevent PTB [11, 15]. Not only was testing conducted relatively late in pregnancy in our study, but laboratory testing for chlamydia and gonorrhea was conducted off-site delaying return of results and treatment and partner treatment was not uniformly utilized by women. Newer point-of-care STI testing options are promising for implementation during pregnancy earlier in antenatal care [39].
Our finding of an association with cervical inflammation and PTB suggests local inflammation is in the mediating pathway of STIs and PTB. Early activation of these inflammatory pathways, triggered by genital tract infection, can lead to preterm labor and PTB. Addressing cervical inflammation may provide a more proximate intervention to prevent PTB. The initiation of normal term labor involves secretion of cytokines at the maternal/fetal interface, which triggers a proinflammatory cascade. Inflammatory cells have been consistently associated with cervical remodeling in preterm labor [40]. The recent ASPIRIN trial found that low-dose aspirin decreased PTB, though mechanisms of effect are undefined and could include antiplatelet or anti-inflammatory pathways [41]. Combining early STI diagnosis and treatment with low-dose aspirin could be useful in young women at risk for PTB in sub-Saharan Africa.
Prevalent HIV infection has been associated with PTB in numerous studies [21]. However, to our knowledge, this is the first study to demonstrate an association with incident HIV infection. This suggests that either recruitment of inflammatory cells to the cervix or systemic inflammation during HIV acquisition in pregnancy contributes to PTB risk. In addition to prompt diagnosis and treatment of incident HIV during pregnancy, these women may benefit from monitoring or anti-inflammatory treatment to prevent PTB.
We found that younger women had increased risk of PTB, consistent with prior studies [42]. Increased risk in this population may include both biologic and psychosocial mechanisms. Younger women in this cohort more frequently had STIs than older women. However, young women had increased risk of PTB even in analyses adjusted for presence of STIs. Short cervix length and irregular menstrual cycles may increase the risk of PTB in young women who conceive within 2 years of menarche [43]. Younger women are prone to early vaginal bleeding and have a more alkaline genital tract, which increases susceptibility to infections associated with risk of PTB [44]. Antenatal and obstetric care services that are friendly, as well as more intensive community-based services, for younger women may improve pregnancy outcomes for this population. Similar to prior studies, lower maternal education contributed to higher risk of PTB [45]. Low education may be a proxy for poorer nutritional status, sociodemographic status, or access to antenatal care, all which also contribute to risk for PTB and STIs.
Our study had limitations. Our estimation of gestational age for infants was based primarily on LMP, given limited availability of US dating. First trimester US dating is the most accurate method to establish gestational age; however, dating based on LMP is a simple, low-cost method and remains the most commonly used method in resource-limited settings [46, 47]. LMP dating can be biased by irregularity or individual variations in the length of the menstrual cycle and recall biases by the mother [47]. LMP dating tends to overestimate gestational age at the extremes of gestation, thereby underestimating PTB; however, we observed high rates of PTB and plausible predictors of PTB using this approach [46]. Among women with other measures of gestational age (FH and US), we found good consistency between LMP, FH, and US. However, unreliable gestational age estimation likely led to some misclassification bias for the main study outcome. We were unable to assess some risk factors (preeclampsia, placental insufficiency, preterm premature rupture of membranes (PPROM), and substance use) because they were not ascertained. We did not assess history of prior PTB, a consistent predictor of PTB in other studies. Our study did not examine subsets of spontaneous versus medically indicated PTB; given low reports of maternal complications such as preeclampsia, medically indicated PTB was rare in this cohort. Syphilis results were obtained from medical records and did not include a confirmatory test or rapid plasma reagin titer. Lastly, there was limited retesting of participants with STIs to confirm microbiologic cure. Although referral and treatment for sex partners was provided, it is possible that women who were treated for STIs were reinfected by their partners during their pregnancy.
Despite the growing contribution of PTB to neonatal and child mortality, few prevention strategies are available. Further research is needed to elucidate the pathophysiology and mechanisms of PTB. This study underscores the high burden of PTB, and the need to prioritize interventions for younger women and optimize both detection and treatment of STIs and genital infections prior to conception or early in pregnancy.
Notes
Acknowledgments. We thank the study participants, the Mama Salama Study team for their participation, and the participating Maternal Child Health (MCH) clinics.
Financial support. This work was supported by the National Institutes of Health (NIH) grant (P01 HSD 064915); and the Center for AIDS Research (CFAR) (grant number P30 AI27757). The Mama Salama Study Team was supported by the University of Washington Global Center for Integrated Health of Women, Adolescents, and Children.
Potential conflicts of interest. B. R. reports personal fees from Gilead, University of North Carolina, and PATH, outside the submitted work. R. S. M. reports grants from Hologic Corporation and personal fees from Lupin Pharmaceuticals, outside the submitted work. G. J.-S. reports personal fees from University of Washington, during the conduct of the study; grants from Thrasher and International Maternal Pediatric Adolescent Clinical Trials Network (IMPAACT); personal fees from UpToDate; and other shares from Malaika, outside the submitted work. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015; 385:430–40. [DOI] [PubMed] [Google Scholar]
- 2. Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019; 7:e37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gravett MG, Rubens CE, Nunes TM; GAPPS Review Group . Global report on preterm birth and stillbirth (2 of 7): discovery science. BMC Pregnancy Childbirth 2010; 10 (Suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adachi K, Klausner JD, Xu J, et al. ; NICHD HPTN 040 Study Team . Chlamydia trachomatis and Neisseria gonorrhoeae in HIV-infected pregnant women and adverse infant outcomes. Pediatr Infect Dis J 2016; 35:894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baer RJ, Chambers CD, Ryckman KK, Oltman SP, Rand L, Jelliffe-Pawlowski LL. An evaluation of sexually transmitted infection and odds of preterm or early-term birth using propensity score matching. Sex Transm Dis 2019; 46:389–94. [DOI] [PubMed] [Google Scholar]
- 6. Folger AT. Maternal Chlamydia trachomatis infections and preterm birth: the impact of early detection and eradication during pregnancy. Matern Child Health J 2014; 18:1795–802. [DOI] [PubMed] [Google Scholar]
- 7. Liu B, Roberts CL, Clarke M, Jorm L, Hunt J, Ward J. Chlamydia and gonorrhoea infections and the risk of adverse obstetric outcomes: a retrospective cohort study. Sex Transm Infect 2013; 89:672–8. [DOI] [PubMed] [Google Scholar]
- 8. Rours GI, Duijts L, Moll HA, et al. Chlamydia trachomatis infection during pregnancy associated with preterm delivery: a population-based prospective cohort study. Eur J Epidemiol 2011; 26:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Slyker JA, Patterson J, Ambler G, et al. Correlates and outcomes of preterm birth, low birth weight, and small for gestational age in HIV-exposed uninfected infants. BMC Pregnancy Childbirth 2014; 14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mann JR, McDermott S, Gill T. Sexually transmitted infection is associated with increased risk of preterm birth in South Carolina women insured by Medicaid. J Matern Fetal Neonatal Med 2010; 23:563–8. [DOI] [PubMed] [Google Scholar]
- 11. Owens DK, Davidson KW, Krist AH, et al. Screening for bacterial vaginosis in pregnant persons to prevent preterm delivery: US preventive services task force recommendation statement. JAMA 2020; 323:1286–92. [DOI] [PubMed] [Google Scholar]
- 12. Medley N, Vogel JP, Care A, Alfirevic Z. Interventions during pregnancy to prevent preterm birth: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev 2018; ( 11):CD012505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cluver C, Novikova N, Eriksson DO, Bengtsson K, Lingman GK. Interventions for treating genital Chlamydia trachomatis infection in pregnancy. Cochrane Database Syst Rev 2017; ( 9):CD010485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roberts CL, Algert CS, Rickard KL, Morris JM. Treatment of vaginal candidiasis for the prevention of preterm birth: a systematic review and meta-analysis. Syst Rev 2015; 4:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Subramaniam A, Abramovici A, Andrews WW, Tita AT. Antimicrobials for preterm birth prevention: an overview. Infect Dis Obstet Gynecol 2012; 2012:157159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amjad S, MacDonald I, Chambers T, et al. Social determinants of health and adverse maternal and birth outcomes in adolescent pregnancies: a systematic review and meta-analysis. Paediatr Perinat Epidemiol 2019; 33:88–99. [DOI] [PubMed] [Google Scholar]
- 17. Afulani PA, Altman M, Musana J, Sudhinaraset M. Conceptualizing pathways linking women’s empowerment and prematurity in developing countries. BMC Pregnancy Childbirth 2017; 17:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown HK, Speechley KN, Macnab J, Natale R, Campbell MK. Biological determinants of spontaneous late preterm and early term birth: a retrospective cohort study. BJOG 2015; 122:491–9. [DOI] [PubMed] [Google Scholar]
- 19. Feresu SA. Annual distribution of births and deaths outcomes at Harare Maternity Hospital, Zimbabwe. Cent Afr J Med 2010; 56:30–41. [PubMed] [Google Scholar]
- 20. Olusanya BO, Ofovwe GE. Predictors of preterm births and low birthweight in an inner-city hospital in sub-Saharan Africa. Matern Child Health J 2010; 14:978–86. [DOI] [PubMed] [Google Scholar]
- 21. Abrams ET, Milner DA Jr, Kwiek J, et al. Risk factors and mechanisms of preterm delivery in Malawi. Am J Reprod Immunol 2004; 52:174–83. [DOI] [PubMed] [Google Scholar]
- 22. Watson-Jones D, Changalucha J, Gumodoka B, et al. Syphilis in pregnancy in Tanzania. I. Impact of maternal syphilis on outcome of pregnancy. J Infect Dis 2002; 186:940–7. [DOI] [PubMed] [Google Scholar]
- 23. Watson-Jones D, Weiss HA, Changalucha JM, et al. Adverse birth outcomes in United Republic of Tanzania—impact and prevention of maternal risk factors. Bull World Health Organ 2007; 85:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kinuthia J, Drake AL, Matemo D, et al. HIV acquisition during pregnancy and postpartum is associated with genital infections and partnership characteristics. AIDS 2015; 29:2025–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kinuthia J, Richardson BA, Drake AL, et al. Sexual behavior and vaginal practices during pregnancy and postpartum: implications for HIV prevention strategies. J Acquir Immune Defic Syndr 2017; 74:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Unger JA, Matemo D, Pintye J, et al. Patient-delivered partner treatment for chlamydia, gonorrhea, and trichomonas infection among pregnant and postpartum women in Kenya. Sex Transm Dis 2015; 42:637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Joseph Davey DL, Nyemba DC, Gomba Y, et al. Prevalence and correlates of sexually transmitted infections in pregnancy in HIV-infected and -uninfected women in Cape Town, South Africa. PLoS One 2019; 14:e0218349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kiweewa FM, Brown E, Mishra A, et al. ; MTN-020/ASPIRE Study Team . Acquisition of sexually transmitted infections among women using a variety of contraceptive options: a prospective study among high-risk African Women. J Int AIDS Soc 2019; 22:e25257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Santis M, De Luca C, Mappa I, et al. Syphilis infection during pregnancy: fetal risks and clinical management. Infect Dis Obstet Gynecol 2012; 2012:430585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Silva MJ, Florêncio GL, Gabiatti JR, Amaral RL, Eleutério Júnior J, Gonçalves AK. Perinatal morbidity and mortality associated with chlamydial infection: a meta-analysis study. Braz J Infect Dis 2011; 15:533–9. [DOI] [PubMed] [Google Scholar]
- 31. Martius J, Krohn MA, Hillier SL, Stamm WE, Holmes KK, Eschenbach DA. Relationships of vaginal Lactobacillus species, cervical Chlamydia trachomatis, and bacterial vaginosis to preterm birth. Obstet Gynecol 1988; 71:89–95. [PubMed] [Google Scholar]
- 32. Rantsi T, Joki-Korpela P, Wikström E, et al. Population-based study of prediagnostic antibodies to chlamydia trachomatis in relation to adverse pregnancy outcome. Sex Transm Dis 2016; 43:382–7. [DOI] [PubMed] [Google Scholar]
- 33. Reekie J, Roberts C, Preen D, et al. ; Chlamydia and Reproductive Health Outcome Investigators . Chlamydia trachomatis and the risk of spontaneous preterm birth, babies who are born small for gestational age, and stillbirth: a population-based cohort study. Lancet Infect Dis 2018; 18:452–60. [DOI] [PubMed] [Google Scholar]
- 34. Silver BJ, Guy RJ, Kaldor JM, Jamil MS, Rumbold AR. Trichomonas vaginalis as a cause of perinatal morbidity: a systematic review and meta-analysis. Sex Transm Dis 2014; 41:369–76. [DOI] [PubMed] [Google Scholar]
- 35. Cotch MF, Pastorek JG 2nd, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis 1997; 24:353–60. [DOI] [PubMed] [Google Scholar]
- 36. Korenromp EL, Rowley J, Alonso M, et al. Global burden of maternal and congenital syphilis and associated adverse birth outcomes—estimates for 2016 and progress since 2012. PLoS One 2019; 14:e0211720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Farr A, Kiss H, Holzer I, Husslein P, Hagmann M, Petricevic L. Effect of asymptomatic vaginal colonization with Candida albicans on pregnancy outcome. Acta Obstet Gynecol Scand 2015; 94:989–96. [DOI] [PubMed] [Google Scholar]
- 38. Cotch MF, Hillier SL, Gibbs RS, Eschenbach DA. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol 1998; 178:374–80. [DOI] [PubMed] [Google Scholar]
- 39. Badman SG, Vallely LM, Toliman P, et al. A novel point-of-care testing strategy for sexually transmitted infections among pregnant women in high-burden settings: results of a feasibility study in Papua New Guinea. BMC Infect Dis 2016; 16:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gonzalez JM, Romero R, Girardi G. Comparison of the mechanisms responsible for cervical remodeling in preterm and term labor. J Reprod Immunol 2013; 97:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hoffman MK, Goudar SS, Kodkany BS, et al. ; ASPIRIN Study Group . Low-dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (ASPIRIN): a randomised, double-blind, placebo-controlled trial. Lancet 2020; 395:285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Black AY, Fleming NA, Rome ES. Pregnancy in adolescents. Adolesc Med State Art Rev 2012; 23:123–38, xi. [PubMed] [Google Scholar]
- 43. Stevens-Simon C, Beach RK, McGregor JA. Does incomplete growth and development predispose teenagers to preterm delivery? A template for research. J Perinatol 2002; 22:315–23. [DOI] [PubMed] [Google Scholar]
- 44. Stevens-Simon C, Roghmann KJ, McAnarney ER. Early vaginal bleeding, late prenatal care, and misdating in adolescent pregnancies. Pediatrics 1991; 87:838–40. [PubMed] [Google Scholar]
- 45. Ruiz M, Goldblatt P, Morrison J, et al. Mother’s education and the risk of preterm and small for gestational age birth: a DRIVERS meta-analysis of 12 European cohorts. J Epidemiol Community Health 2015; 69:826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pereira AP, Dias MA, Bastos MH, da Gama SG, Leal Mdo C. Determining gestational age for public health care users in Brazil: comparison of methods and algorithm creation. BMC Res Notes 2013; 6:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. American College Obstetricians and Gynecologists. Method for estimating due date. Committee opinion No. 611. Obstet Gynecol 2014; 124:863–6. [DOI] [PubMed] [Google Scholar]