Abstract
T cells, the critical immune cells of the adaptive immune system, are often dysfunctional in Alzheimer's disease (AD) and are involved in AD pathology. Reports highlight neuroinflammation as a crucial modulator of AD pathogenesis, and aberrant T cells indirectly contribute to neuroinflammation by secreting proinflammatory mediators via direct crosstalk with glial cells infiltrating the brain. However, the mechanisms underlying T‐cell abnormalities in AD appear multifactorial. Risk factors for AD and pathological hallmarks of AD have been tightly linked with immune responses, implying the potential regulatory effects of these factors on T cells. In this review, we discuss how the risk factors for AD, particularly Apolipoprotein E (ApoE), Aβ, α‐secretase, β‐secretase, γ‐secretase, Tau, and neuroinflammation, modulate T‐cell activation and the association between T cells and pathological AD hallmarks. Understanding these associations is critical to provide a comprehensive view of appropriate therapeutic strategies for AD.
Keywords: Alzheimer's disease, hallmarks, neuroinflammation, risk factors, T cells
T‐cell dysfunction is associated with Alzheimer's disease pathology and takes several forms and involves many different factors. In this review, we highlight that T‐cell dysfunction leads to neuroinflammation, a critical modulator of AD pathology. We review the literature pertaining to AD risk factors which contribute to T‐cell dysfunction, including Apolipoprotein E (ApoE), Aβ, α‐secretase, β‐secretase, γ‐secretase, Tau, and neuroinflammation, with a focus on understanding the role of T‐cell dysfunction in AD progression and the possibility of T cells being a target for successful AD treatments
1. INTRODUCTION
Alzheimer's disease (AD), which usually progresses to dementia, is the most prevalent neurodegenerative disease in older adults (Hardy & Higgins, 1992). Symptoms of the disease manifest mainly as deficits in cognitive function, including memory loss, impairment of language, misidentifications, and behavioral disturbances (Burns et al., 2002). Neuropathologically, AD is remarkably characterized by two proteinaceous aggregate hallmarks, including tau‐hyperphosphorylation‐induced intracellular neurofibrillary tangles (NFTs) and extracellular depositions of amyloid plaques induced by beta‐amyloid peptide (Aβ) (Ittner & Gotz, 2011), which both contribute to synaptic damage and neuronal loss.
In the past few decades, various groups have devoted enormous efforts to explore AD pathogenesis and find key risk factors for the prevention and treatment of AD. However, it is disappointing that almost all AD‐related clinical trials to date have failed to reverse cognitive decline and/or brain pathology. Undoubtedly, it is gratifying that there are different reasonable evidence‐based hypotheses relating to underlying causes, such as the amyloid cascade hypothesis, the tau hypothesis, the Apolipoprotein E (ApoE) hypothesis, and the neuroinflammation hypothesis (Jiang et al., 2014; C. C. Liu et al., 2013; Lue et al., 2001; Morales et al., 2014; L. B. Yang et al., 2000).
Although it is widely accepted that neuroinflammation in AD is mediated by microglia and astrocytes, mounting evidence shows that T cells are involved in regulating the inflammatory response in AD through, but not limited to, the following two aspects (Figure 1). First, during AD progression, the permeability of the blood–brain barrier (BBB) gradually increases due to decreased expression of the tight junction molecules ZO1 and occludin in vascular endothelial cells (Carrano et al., 2011; Cheng et al., 2014; Marco & Skaper, 2006). In addition, there is elevated peripheral T‐cell expression of chemokine receptors, such as C‐C motif chemokine receptor type 2 (CCR2), C‐C motif chemokine receptor type 5 (CCR5), and C‐X‐C motif chemokine receptor 2 (CXCR2) (Goldeck et al., 2013; Liu et al., 2010; Town et al., 2005a). Both of these abnormal changes promote T‐cell penetration into brain parenchyma. More important, activated CD8+ T and CD4+ T cells, the two major T‐cell subsets, are neurotoxic and can induce substantial neuronal death through mechanisms dependent on cell–cell contact involving Fas ligand (FasL), lymphocyte function‐associated antigen‐1 (LFA‐1), and CD40 (Giuliani et al., 2003). Increased T‐cell infiltration also promotes crosstalk between T cells and microglia in a process dependent on major histocompatibility complex (MHC) class II, leading to further acceleration of neuroinflammation (Schetters et al., 2017). T‐cell‐derived cytokines can also impact local astrocyte‐expressed chemokine function in inflammatory and neurodegenerative diseases (Williams et al., 2020). Second, peripheral T cells can modulate glial cell neuroinflammation mediated by the release of proinflammatory factors into the central nervous system (CNS), such as interferon gamma (IFN‐γ) (Town et al., 2005b). Activation of peripheral T cells in AD patients is elevated compared to healthy controls, and these T cells further promote peripheral blood mononuclear cells (PBMCs) to release proinflammatory factors including IL‐6, tumor necrosis factor α (TNFα), and IL‐1, all of which are essential immune modulators in AD pathology (Mietelska‐Porowska & Wojda, 2017; Tan et al., 2002).
FIGURE 1.
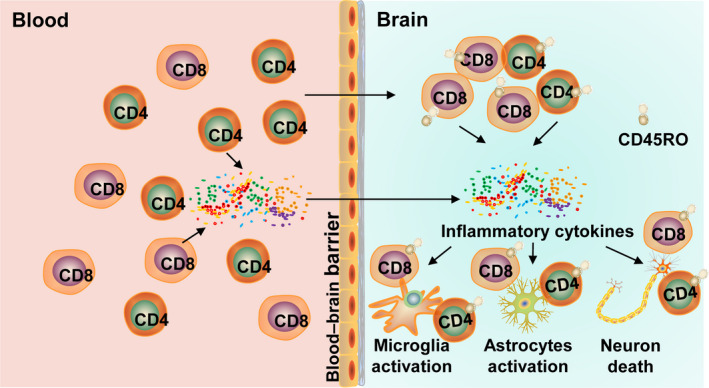
Summary of T‐cell roles in AD. With the development of AD, T‐cell infiltration into the brain increases, and a large number of inflammatory cytokines derived from T cells in the peripheral blood also enter the brain, which eventually exacerbate neuroinflammation and accelerate neuronal death
Despite accumulating evidence suggesting that T cells participate in immunological and pathological stages of AD concomitant with changes in T‐cell phenotype, the mechanism of T‐cell abnormality in AD remains unknown. In this review, we summarize T‐cell dysfunction in AD and demonstrate the association between T cells and the critical pathological features of AD. Furthermore, we propose that aberrant activation of T cells contributes to AD pathogenesis, and that the critical elements of AD can also mediate biological processes involving T cells.
2. T‐CELL ABNORMALITIES IN AD
Although the mechanisms by which T cells contribute to AD pathophysiology are unclear, considerable work has shown that normal T‐cell function and T‐cell markers in both AD mouse models (Table 1) and AD patients are different when compared to measures from respective control groups (Table 2). As early as 1981, for example, Miller and colleagues first demonstrated that concanavalin A (ConA)‐induced T‐cell suppression in AD patients was significantly elevated and lymphocyte proliferation was lower when compared with an elderly control group (Miller et al., 1981). However, another group found no difference in phytohaemagglutinin (PHA)‐induced T‐cell proliferation between T cells derived from AD patients and those from age‐matched controls (Leffell et al., 1985). This discrepancy may be accounted for by different types or intensities of mitogen stimulation used. Although interpretation of mitogen‐induced T‐cell activation is debated due to such discrepancies, variation in T‐cell populations in peripheral blood from both AD mouse models and AD patients appears convincing, as described below. A higher ratio of CD4+/CD8+ T cells was found in the in peripheral blood of AD patients and AD mouse models compared to relevant controls, concomitant with lower CD8+ T‐cell counts and lower total CD3 expression (Hu et al., 1995; Pirttila et al., 1992; Schindowski et al., 2007; Shalit et al., 1995; St‐Amour et al., 2014, 2019). Furthermore, analyses of peripheral blood from AD patients have shown that the number of IL‐2R+, HLA‐DR+, CD25+, and CD28+T cells was significantly higher than controls (Ikeda et al., 1991; Lombardi et al., 1999; Speciale et al., 2007), indicating an immune response in the peripheral system. Similarly, a consistent finding is that the numbers of CD4+ and CD8+ T cells in the brain parenchyma and cerebrospinal fluid (CSF) of AD patients are significantly higher than normal, with CD8+ T cells having the advantage over CD4 + T cells in absolute numbers, and that both subtypes exhibit the CD45RA‐CD45RO+ phenotype (Ferretti et al., 2016; Laurent et al., 2017; Merlini et al., 2018; Rogers et al., 1988; Togo et al., 2002), indicating that infiltrating T cells were activated and may be cytotoxic.
TABLE 1.
Summary of T‐cell abnormalities in AD animal models (studies in chronological order)
| Study | Mouse model | Findings (AD vs WT) |
|---|---|---|
| (Schindowski et al., 2007) | Thy1‐APP751SL · HMG‐PS1M146L mouse | Increased CD4/CD8‐ratio in PBMC |
| Decreased CD3 and CD8 surface expression in PBMC | ||
| Decreased mitogen‐induced activation and proliferation in CD8+ T cells | ||
| (Browne et al., 2013) | APPxPS1 mouse | Increased Th1 and Th17 subsets infiltrate the brain |
| (Robinson et al., 2013) | APPxPS1 mouse | Increased oxidative stress in T cells with age |
| (Zhang et al., 2013) | Injected Aβ1‐42 rat | Increased Th17 subsets infiltrate the brain |
| Increased expression of IL−17, IL−22, and FasL by Th17 cells | ||
| (St‐Amour et al., 2014) | 3xTg‐AD mouse | Decreased CD4+ and CD8+ T cells in the blood |
| Increased CD4/CD8‐ratio in blood | ||
| (McManus et al., 2014) | APPxPS1 mouse | Increased IFNg+and IL−17+T cells infiltration into brains after a respiratory infection |
| (Baruch et al., 2015) | 5xFAD mouse | Increased Treg cells along disease progression in in spleen |
| (Baek et al., 2016) | 3xTg‐AD mouse | Increased CD4/CD8‐ratio in in spleen |
| (Ferretti et al., 2016) | Tg2576 mouse | Increased CD3+T cells infiltrate the brain |
| APPxPS1 mouse | Increased CD3+T cells infiltrate the brain | |
| ArcAb mouse | Increased CD3+T cells infiltrate the brain (CD8+ T being the predominant) | |
| Decreased percentage of IFN+cells in the CD4+ and CD8+ T cells in brain | ||
| (Laurent et al., 2017) | THY‐Tau22 mouse | Increased CD8+ T cells infiltration into the parenchyma |
| (Saksida et al., 2018) | 5xFAD mouse | Increased CD4+ T cells in Peyer's patches (PP) and mesenteric lymph node (MLN) |
| Decreased capacity of T cells to produce IL−17 | ||
| (St‐Amour et al., 2019) | 3xTg‐AD mouse | Decreased T cells numbers in blood |
| Increased activation along with Th17 polarization of T cells | ||
| (Wang et al., 2019) | 5xFAD mouse | Increased T cells, particularly Th1 cells infiltrate the brain |
| Increased the differentiation and proliferation of Th1 cells | ||
| (Yang et al., 2020) | APP/PS1 mouse | Increased proportions of CD4+CD25+Foxp3+ Tregs in the spleen |
| (Unger et al., 2020) | APP/PS1 mouse | Increased numbers of CD8+ T cells in brain parenchyma |
| (Sanchez et al., 2020) | PS19/5xFAD mouse | Increased numbers of memory CD8+ T cells in brains |
TABLE 2.
Summary of T‐cell abnormalities in AD patients (studies in chronological order)
| Study | Population | Findings (AD vs other controls) |
|---|---|---|
| (Miller et al., 1981) | 24 AD | Decreased mitogen‐induced T cells proliferation |
| 32 age‐matched controls | Increased Con A‐induced T cells suppression responses | |
| (MacDonald et al., 1982) | 41 AD | Decreased UCHT3 +T‐cell number in the blood |
| 41 age‐matched controls | ||
| 41 younger controls | ||
| (Skias et al., 1985) | 16 AD | Decreased CD8+ T cell‐mediated suppressor function using a PWM‐induced IgG secretion assay |
| 14 age‐matched controls | ||
| (Leffell et al., 1985) | 30 AD | No changes in PHA‐induced T cells proliferation compared to age‐matched controls |
| 30 age‐matched controls | No changes in T‐cell subsets compared to age‐matched controls | |
| 20 younger controls | Decreased responsiveness of T cells from AD and 30 age‐matched controls compared to younger controls | |
| (Torack 1986) | 95 AD‐DTH treated | Increased suppressor cells activity |
| 61 control‐DTH treated | Decreased Con A‐induced T‐cell responses | |
| 19 AD‐Con A treated | ||
| 11 control‐ Con A treated | ||
| (Gibson et al., 1987) | 9 AD | Decreased mitogen‐induced calcium uptake by T cells |
| 9 age‐matched controls | ||
| (Bartha et al., 1987) | 15 AD | Decreased the AChE activity of T cells in the blood |
| 40 multi‐infarct dementia | ||
| 8 alcoholic dementia | ||
| 30 age‐matched controls | ||
| (McGeer et al., 1988) | 10 AD | Increased the presence of CD4+ and CD8+ T cells in capillaries of the brain (CD8+ T much more prevalent then CD4+ T) |
| 5 age‐matched controls | ||
| (Rogers et al., 1988) | 10 AD | Increased the presence of CD4+ and CD8+ T cells in the brain parenchyma and blood vessels |
| 6 age‐matched controls | ||
| (Leonardi et al., 1989) | 26 AD | Increased T‐cell proliferative response in AMLR |
| 10 age‐matched controls | ||
| (Adunsky et al., 1991) | 22 AD | Increased cytosolic calcium in resting and activated T cells |
| 6 multi‐infarct dementia | ||
| 19 age‐matched controls | ||
| (Rocca et al., 1991) | 20 early‐onset AD | Decreased T cells 3H‐NMS binding sites |
| 15 late‐onset AD | ||
| 86 other neurological disorders | ||
| 60 age‐matched controls | ||
| (Ikeda et al., 1991a) |
13 AD 13 age‐matched controls |
Increased the ratio of T‐cell subsets of CD4+IL−2R+, CD4+HLA‐DR+, CD8+HLA‐DR+, CD8+HLA‐DR+in peripheral blood |
| Decreased the ratio of T‐cell subsets of CD4+CD45R+ in peripheral blood | ||
| (Ikeda et al., 1991b) |
13 AD 13 age‐matched controls |
Increased the ratio of T‐cell subsets of CD4+CD45R‐, CD4+HLA‐DR+in peripheral blood |
| (Pirttila et al., 1992) | 31 AD | Decreased CD8+ T cells number in peripheral blood |
| 35 age‐matched controls | Increased the ratio of CD4+/CD8+ T cells in peripheral blood | |
| 136 other neurological disorders | ||
| (Grossmann et al., 1993) | 6 familial AD | Decreased intracellular calcium response in CD4+ T cells |
| 39 AD | ||
| 11 DS | ||
| (Hartmann et al., 1994) | 14 AD | Increased baseline cytosolic calcium in T cells |
| 14 age‐matched controls | ||
| 14 younger controls | ||
| (Nijhuis et al., 1994) | 30 AD | Decreased sensitivity to DEX in T cells |
| 30 age‐matched controls | ||
| (Adunsky et al., 1995) | 30 AD | Increased cytosolic calcium in resting and activated PBMC |
| 27 age‐matched controls | ||
| (Eckert et al., 1995) | 24 AD | Decreased the amplifying effect of Aβ25‐35 on calcium signaling in PBMC |
| 20 age‐matched controls | ||
| 16 younger controls | ||
| (Hu et al., 1995) | 20 AD | Decreased CD8+ T cells of PBMC in AD and in other forms of dementia |
| 17 other dementia | Increased the ratio of CD4+/CD8+ T cells in peripheral blood | |
| 23 age‐matched controls | ||
| 17 younger controls | ||
| (Shalit et al., 1995) | 12 Mild AD | Increased the ratio of CD4+ T cell of PBMC in moderately AD compared with age‐matched controls |
| 13 Moderately AD | Increased the ratio of HLA‐DR+cell of PBMC in moderately AD compared with age‐matched controls | |
| 13 age‐matched controls | Decreased lymphocytes proliferation but increased IL−2 synthesis induced by PHA in moderately AD compared with age‐matched controls | |
| (Kell et al., 1996) | 24 AD | The percent of IgM+T cells was negatively correlated with MMSE scores |
| 9 other dementia | ||
| 20 age‐matched controls | ||
| (Singh 1996) | 11 AD | Increase sICAM−1 and sCD8 in AD plasma |
| 10 age‐matched controls | ||
| (Bongioanni et al., 1997)a | 35 AD | Decreased number of T‐cell IFN‐γ receptors in PBMC |
| (Bongioanni et al., 1997)b | 35 age‐matched controls | |
| 35 AD | Increased number of T‐cell TNFα receptors(both TNFR1 and TNFR2) in PBMC | |
| 35 age‐matched controls |
Increased number of T‐cell TNFα receptors(both TNFR1 and TNFR2) in PBMC Increased T‐cell IL‐ 6 receptor binding |
|
| (Bongioanni et al., 1998)c | 35 AD | |
| 35 age‐matched controls | ||
| (Lombardi et al., 1999) | 45 AD | Decreased CD8+ T cell in PBMC |
| 45 age‐matched controls | Increased CD4, CD25, and CD28 antigen expression in PBMC | |
| Increased Fas antigen (CD95) expression on CD4+ T cell by anti‐CD3 and hyperthermia mediated‐apoptosis | ||
|
Decreased Fas antigen (CD95) expression on CD8+ T cell by anti‐CD3 and hyperthermia mediated‐apoptosis |
||
| (Sulger et al., 1999) | 27 AD | Increased calcium responses of T cells induced by PHA |
| 27 age‐matched controls | ||
| 27 younger controls | ||
| (Eckert et al., 2001) | 18 AD | Increased apoptotic nucleosomes in native lymphocytes |
| 14 age‐matched controls | Increased apoptotic nucleosomes in activated lymphocytes | |
| (Stieler et al., 2001) | 18 AD | Decreased T‐cell proliferative response induced by PHA |
| 45 age‐matched controls | ||
| (Tan, et al., 2002) | 46 AD | Decreased CD45RA expression and increased CD45RO/RA ratio in CD4+ T cells |
| 37 cognitively abnormal | ||
| 90 age‐matched controls | ||
| (Togo et al., 2002) | 21 AD | Increased number of T cells(CD45RA‐CD45RO+,activeted)entering the brain |
| 36 other dementia | ||
| 3 age‐matched controls | ||
| (Giubilei et al., 2003) | 30 AD | Decreased T cells responses induced by microbial peptides and human mitochondrial |
| 30 age‐matched controls |
Decreased T cells responses induced by microbial peptides and human mitochondrial Increased T cells responses to Aβ with age |
|
| (Monsonego et al., 2003) | 29 AD |
Decreased T cells responses induced by microbial peptides and human mitochondrial Increased T cells responses to Aβ with age Increased frequencies of Aβ‐specific CD4+ T cells in PBMC |
| 22 age‐matched controls | ||
| 13 younger controls | ||
| (Panossian et al., 2003) | 15 AD | Decreased telomere length of T cells |
| 15 age‐matched controls | Increased lymphocytes proliferation induced by PHA | |
| (Lombardi et al., 2004) | 88 AD | Increased DNA fragmentation of T‐cell exposure to IgM anti‐Fas |
|
(Lombardi et al., 2004) (Dorszewska et al., 2005) |
24 age‐matched controls | Increased expression of Fas mRNA and surface Fas receptor on CD45RO+ T lymphocytes |
|
(Lombardi et al., 2004) (Dorszewska et al., 2005) |
34 AD | Increased expression of p53, Bax, PARP in PBMC |
| 44 age‐matched controls | Decreased expression of Bcl−2 in PBMC | |
| (Iarlori et al., 2005) | 40 AD | Decreased expression and production of MCP−1 in PBMC |
| 20 age‐matched controls | Increased expression and production of RANTES in PBMC | |
| (Schindowski et al., 2006) | 34 AD | Increased apoptosis in CD4+ T cells |
| 34 age‐matched controls | Increased expression of Bcl2 in T cells in mild AD | |
| (Zana et al., 2006) | 22 AD | Decreased apoptosis in CD4+ T cells induced by UVB |
| 12 age‐matched controls | ||
| (Man et al., 2007) | 83 AD | Increased expression of MCP−1 in peripheral T cells |
| 70 age‐matched controls | Increased T cells transmigrating the HBMEC monolayer | |
| (Schindowski et al., 2007) | 34 AD | Increased CD4/CD8‐ratio in PBMC |
| 34 age‐matched controls | Decreased CD8 and CD3 expression in PBMC | |
| Increased T‐cell tyrosine phosphorylation induced by mitogen | ||
| Increased number of CD45RO+ CD8 + T cells | ||
| Increased T cells reactivity in PBMC | ||
| (Speciale et al., 2007) | 29 mild AD | Decreased CD8+CD28‐ cells in PBMC |
| 22 moderately AD | Increased CD8+CD28+ cells and CD8+CD71+ cells in PBMC | |
| 51 age‐matched controls | Decreased IL−10 production by PBMC after Aβ stimulate | |
| (Ciccocioppo et al., 2008) | 40 AD | Increased phosphorylation of protein kinase C in Aβ activated T cells |
| 20 age‐matched controls | ||
| 20 younger controls | ||
| (Larbi et al., 2009) | 12 AD | Decreased naïve T cell in PBMC |
| 6 age‐matched controls | Increased EM and TEMRA T cells | |
| 20 younger controls | ||
| (Miscia et al., 2009) | 20 early AD | Increased T‐cell reactivity to Aβ1‐42 |
| 20 severe AD | Increased phosphorylation of PKC‐δ and PKC‐ζ in Aβ activated T cells | |
| 20 age‐matched controls | ||
| 20 younger controls | ||
| (Liu et al., 2010) | 58 AD | Increased CXCR2 expression on peripheral T cells |
| 47 age‐matched controls | ||
| (Pellicano et al., 2010) | 40 AD | Increased CD69 expression on Aβ activated T cells |
| 25 age‐matched controls | Increased CCR2 and CCR5 expression on Aβ activated T cells | |
| (Saresella et al., 2010) | 25 AD | Increased total Treg and PD1+Treg cells |
| 25 MCI | ||
| 55 age‐matched controls | ||
| (Saresella et al., 2011) | 38 AD | Increased activity of Th17 and Th9 cells after Aβ stimulate |
| 34 MCI | ||
| 40 age‐matched controls | ||
| (Goldeck et al., 2013) | 23 AD | Increased CCR4, CCR5, and CCR6 expression on peripheral T cells |
| 20 age‐matched controls | Increased the shift of early‐ to late‐differentiated CD4+ T cells | |
| (Salani et al., 2013) | 13 AD | Increased IL−18Rβ expression on peripheral T cells |
| 24 MCI | ||
| 25 age‐matched controls | ||
| (Westman et al., 2013) | 50 AD | Decreased proportion of CMV Specific CD8+ T cells |
| 50 age‐matched controls | ||
| (Westman et al., 2014) | 30 AD | Increased PBMC inflammatory response in CMV seropositive patients |
| 35 age‐matched controls | ||
| (Busse et al., 2015) | 24 AD | Decreased VGF expression on peripheral T cells |
| 14 age‐matched controls | ||
| (Le Page et al., 2017) | 15 AD | Decreased proportion of Treg cell compare with MCI |
| 13 MCI | ||
| 13 age‐matched controls | ||
| (Terzioğlu et al., 2017) | 30 early‐onset AD | Increased mitochondrial depletion in peripheral CD4+ T cells |
| 30 late‐onset AD | ||
| 30 age‐matched controls | ||
| (Liu et al., 2018) | 17 AD | Increased Let−7b expression on CSF T cells |
| 36 MCI | Increased proportion of T cells in CSF | |
| 41 age‐matched controls | ||
| (Merlini et al., 2018) | 9 AD | Increased extravascular T cells (mostly of the CD8+) in the brain |
| 10 age‐matched controls | ||
| (Oberstein et al., 2018) | 14 AD | Increased proportion of Th17 cells in PBMC |
| 27 MCI | ||
| 13 age‐matched controls | ||
| (Rakic et al., 2018) | 40 AD+systemic infection | Decreased T cells recruitment to the brain after encounter systemic infection |
| 28 AD‐ systemic infection | ||
| 16 controls+systemic infection | ||
| 24 controls‐ systemic infection | ||
| (Tramutola et al., 2018) | 19 AD | Increased protein nitration profile of T cells |
| 19 age‐matched controls | ||
| (Ciccocioppo et al., 2019) | 10 AD | Decreased total and resting Tregs in PBMC |
| 8 AD age‐matched controls | ||
| 10 MS | ||
| 8 MS age‐matched controls | ||
| (Wang et al., 2019) | 31 MCI due to AD | Increased Th1 cell frequency in the blood |
| 40 age‐matched controls | ||
| (Gate et al., 2020) | 28 AD | Increased numbers of CD8+ TEMRA cells in PBMC |
| 8 PD | Increased clonally expanded CD8+ TEMRA cells in the CSF | |
| 31 MCI | ||
| 97 age‐matched controls | ||
| (D’Angelo et al., 2020) | 11 AD | Increased CCR6+ and CCR4+ CD4+ T cells in the blood |
| 6 VaD | ||
| 6 mix dementia | ||
| 17 age‐matched controls | ||
| (Faridar et al., 2020) | 46 AD | Decreased suppressive function of regulatory T cells from peripheral blood |
| 42 MCI | Decreased CD25 mean fluorescence intensity in regulatory T‐cell population | |
| 41 age‐matched controls | ||
| (Amin et al., 2020) | 31 AD | Increased numbers of Th cells in PBMC |
| 31 DLB | ||
| 31 age‐matched controls | ||
| (Dhanwani et al., 2020) | 51 AD | No difference of T‐cell responses to neural autoantigens |
| 54 age‐matched controls |
AD: Alzheimer's Disease; Con A: Concanavalin A; PWM: Pokeweed mitogen; IgG: Immunoglobulin G; PHA: Polyhydroxyalkanoates; DTH: Delayed type hypersensitivity; AChE: Acetylcholinesterase; AMLR: Autologous mixed lymphocyte reaction; 3H‐NMS: 3H ‐N‐methyl‐scopolamine; DS: Down's syndrome; DEX: dexamethasone; PBMC: Peripheral blood mononuclear cells; sICAM‐1: soluble intercellular adhesion molecule‐1;sCD8: soluble CD8; TNFα: Tumor necrosis factor; TNFR1: Tumor necrosis factor receptor1; TNFR2: Tumor necrosis factor receptor2; MMSE: Mini Mental State Examination; MCP‐1: Monocyte chemoattractant protein‐1; RANTES: Chemokine (C‐C motif) ligand 5 (CCL5); UVB: ultraviolet B; HBMEC: Human brain microvascular endothelial cells; EM: Effector memory; TEMRA: Terminally differentiated effector memory; PKC:Protein kinase C; MCI: mild cognitive impairment; CMV: Cytomegalovirus; CSF: Cerebrospinal fluid; MS: Multiple Sclerosis. VaD: Vascular dementia. DLB: Dementia with Lewy bodies.
In addition to apparent changes in the number and proportion of AD‐derived T cells, alterations have also been observed in the intracellular signaling pathway in T cells from AD patients, such as changes in calcium response. For example, there are in vitro data which show that both baseline cytosolic calcium and PHA‐induced calcium responses in T cells from AD patients were higher than measures from control groups (Adunsky et al., 1995; Sulger et al., 1999). However, beta‐amyloid fragment Aβ25‐35 led to a substantial reduction in mitogen‐induced calcium signaling rise in the PBMC of AD patients compared with that of age‐matched controls (Eckert et al., 1995). Therefore, the precise mechanism of calcium homeostasis in T cells may depend on the specific type of stimulus and the local microenvironment but may not rely only on AD status.
T cells from AD patients and AD mouse models are hyperactive to Aβ stimulation, which increases expression of activation marker CD69 and enhances cytokine production (Ciccocioppo et al., 2008; Miscia et al., 2009; Pellicano et al., 2010). Cytokines secreted by activated T cells are significant modulators of microglia and astrocyte function in AD. For example, IL‐10, TNF‐α, IL‐6, IL‐1β, and monocyte chemoattractant protein‐1 (MCP‐1) released from T cells are markedly elevated in AD patients (Lombardi et al., 1999; Man et al., 2007), and microglia and astrocytes are the main targets of these inflammatory factors (Hanisch, 2002; Ramesh et al., 2013). Thus, dysregulation of T cells which overexpress these cytokines is potentially harmful and likely contributes to chronic neuropathology in AD.
Furthermore, proinflammatory cytokines may be responsible for the prevalence of elevated Th1 and Th17 cells in AD (Browne et al., 2013; Oberstein et al., 2018; Saresella et al., 2011; Zhang et al., 2013). Th1 and Th17 cells are two major proinflammatory T‐cell subtypes which are typically elevated in neurodegenerative diseases, including AD. In addition, Th1 cells are significant sources of IFN‐γ secretion. At the same time, microglia and astrocytes can be activated by IFN‐γ and disturb cell homeostasis, thereby contributing to Aβ deposition, impaired synaptic plasticity, and acceleration of cognitive deficits in APP/PS1 mice (Browne et al., 2013; R. J. Kelly et al., 2013). However, injection of Aβ‐specific Th1 cells into 5xFAD mice enhances Aβ uptake due to T‐cell‐activation‐induced expansion of brain‐endogenous MHCII+microglia, which exhibit stronger phagocytic activity (Mittal et al., 2019). These findings suggest that the function of Th1 cells in AD may be linked to disease stage. Therefore, manipulation of Th1 cells at an appropriate period may be a helpful approach for AD therapy.
Alternatively, elevated Th17 cells in AD may also be detrimental and induce neuronal apoptosis (Zhang et al., 2013), through the release of proinflammatory factors such as FasL, IL‐17, and IL‐22. In contrast to the destructive function of Th17 cells, the adoptive transplantation of Th2 cells (one of the immunosuppressive T‐cell subtypes) into APP/PS1 AD mice benefits cognitive function and reduces pathological features (Cao et al., 2009). The function of regulatory T cells (Tregs, another immunosuppressive T‐cell subtype) has also been described in AD. Transient depletion of Tregs in 5xFAD mice is beneficial for Aβ clearance and cognitive function by affecting the choroid plexus, which regulates the recruitment of immunoregulatory cells such as monocyte‐derived macrophages and Tregs into cerebral pathological sites (Baruch et al., 2015). A consistent finding is that IL‐10 (the main Tregs effector cytokine) signaling transduction is accelerated in the brains of AD patients, and IL‐10‐deficient APP/PS1 mice show restricted cerebral amyloidosis and less cognitive decline than controls via a rebalancing of abnormal innate immunity (Guillot‐Sestier et al., 2015). This result suggests that aberrant elevated IL‐10 signaling in AD patients may be a therapeutic target for AD.
In summary, T cells display abnormal phenotypes and dysfunction in AD, and transplantation or deletion of different T‐cell subtypes into AD mice has the potential to alter the progression of AD pathology. Accordingly, AD treatment strategies targeting T cells have been proposed (Table 3). However, it is still unclear whether these changes in T cells are related to disease progression. Thus, the critical question is whether abnormal T‐cell parameters are driving AD progression or do the abnormalities, including the lack of different phenotypes, occur only after onset of AD. Future work is needed to clarify the link between T‐cell abnormalities and disease severity. In particular, analyses of these abnormalities at different AD stages would be beneficial. These results should provide immunology‐based guidance for treating AD.
TABLE 3.
Summary of AD therapy targeting T cells in animal models (studies in chronological order)
| Study | Mouse model | Findings |
|---|---|---|
| (St‐Amour et al., 2014) | 3xTg‐AD mouse | Intravenous immunoglobulin (IVIg) ameliorates cognitive performance and effector T cells are potential pharmacological targets of IVIg in AD model. |
| (Baruch et al., 2015) | 5xFAD mouse | Transient depletion of Foxp3 (+) regulatory T cells (Tregs), or pharmacological inhibition of their activity, mitigates Alzheimer's disease pathology |
| (Guillot‐Sestier et al., 2015) | APP/PS1 mouse | Loss of IL−10, a Treg key cytokine, mitigates synaptic and cognitive deficits |
| (Marsh, Samuel E., et al. 2016) | Rag−5xFAD mouse | Loss of T, B, and natural killer (NK) cells appears to accelerate AD progression |
| (Baek et al., 2016) | 3xTg‐AD mouse | Systemic transplantation of purified Tregs into 3xTg‐AD mice improved cognitive function and reduced deposition of Aβ plaques. |
| (Alves, Sandro et al. 2017) | APP/PS1 mouse | IL−2 administration induces systemic and brain regulatory T cells expansion and improves amyloid pathology, synaptic failure and memory. |
| (Laurent et al., 2017) | THY‐Tau22 mouse | Anti‐CD3 treatment prevented hippocampal T‐cell infiltration and reverted spatial memory deficit |
| (Yang, et al. 2020) | APP/PS1 mouse | Inactivated influenza vaccine (IIV) ameliorates cognitive deficits in APP/PS1 mice by breaking Treg‐mediated systemic immune tolerance. |
| (Brigas, Helena C et al. 2021) | 3xTg‐AD mouse | γδ T cells are the major source of IL−17 in the CNS of 3xTg‐AD mice and neutralization of IL−17 prevents cognitive impairments and synaptic dysfunction |
3. ASSOCIATION BETWEEN T CELLS AND THE CRITICAL FACTORS INVOLVED IN AD
3.1. ApoE modulates T‐cell activation
ApoE is a polymorphic protein involved in lipoprotein conversion and metabolism, produced by organs such as the brain, liver, kidneys, and spleen (Huang & Mahley, 2014). ApoE4, one of the protein isoforms of ApoE, interacts with Aβ more efficiently than ApoE3, which results in increased Aβ deposition and amyloid plaques in AD (Sanan et al., 1994; Schmechel et al., 1993; Strittmatter et al., 1993). ApoE4 can also elicit tau aggregation via the inhibition of noradrenaline transport (Kang et al., 2021). Therefore, AD risk is highly correlated with ApoE alleles (ε4>ε3>ε2), and ApoE4 is the biggest genetic risk factor for sporadic AD (Mahley & Rall, 2000; Theendakara et al., 2018). Besides, ApoE4 not only affects secreted ectodomain APPα (sAPPα) secretion (Cedazo‐Minguez et al., 2001; Vincent & Smith, 2001) but also directly regulates Sirtuin1 protein expression and enzyme activity (Theendakara et al., 2016), which is related to programmed cell death, insulin resistance, and synaptic function, all of which are involved in AD pathogenesis (Theendakara et al., 2018). Recent evidence revealed that ApoE4 can lead to BBB dysfunction in cognitively unimpaired individuals and yet more severe dysfunction in cognitively impaired individuals, independently of CSF Aβ and tau status (Montagne et al., 2020).
It has been shown that plasma lipoproteins containing ApoE have a role in inhibiting T‐cell activation and proliferation induced by PHA in vitro in an ApoE‐concentration‐dependent manner by downregulating the secretion of bioactive IL‐2 (Kelly et al., 1994; Macy et al., 1983). Consistent findings suggest that lack of ApoE exacerbates the production of proinflammatory factors including TNF‐α, IFN‐γ, IL‐12, and IL‐6 during LPS‐induced responses, whereas treatment with exogenous ApoE can normalize these cytokines levels (Ali et al., 2005). Similarly, in another neuroinflammation mouse model induced by ApoE deletion, an ApoE mimetic peptide reversed upregulated expression of proinflammatory factors (IL‐17, IL‐12, TNF‐α, IFN‐γ, IL‐6, and IL‐1β) (Wei et al., 2013). Moreover, two major proinflammatory T‐cell subtypes, Th1 and Th17 cells, were elevated in this model, and IL‐17 levels secreted by Th17 cells were elevated, promoting mononuclear cell infiltration and activation (Gao et al., 2010). Consistent with these results, treatment with IL‐17 antibody led to a significant amelioration of atherosclerotic symptoms (Smith et al., 2010). ApoE also disturbs the balance of Th17 and Treg in the spleen during arteriosclerosis; however, the mechanism remains unknown (Xie et al., 2010). The balance between Th17 and Treg is vital for regulating systemic inflammation and autoimmune response. Th17 cells promote immune system activation and pathogen clearance, while Treg cells protect organisms from damage by attenuating excessive inflammatory response. Therefore, ApoE is extremely important in the maintenance of immune homeostasis. These findings suggest that ApoE exerts an essential effect on the mediation of T‐cell function even though T cells may not be necessary in the formation of early‐early lesions. T cells most likely promote atherosclerosis by modulating monocyte recruitment, proliferation, and cytokine secretion in the later stages of AD.
Notably, humans expressing the ApoE4 isoform show a higher number of activated T cells than those expressing ApoE3 or ApoE2 (Bonacina et al., 2018). Consistently, AD patients with the ApoE allele 4 express higher Fas in T cells than those with the ApoE allele 3 (Lombardi et al., 2004), suggesting that different ApoE alleles are selective for T‐cell activation. Since cholesterol homeostasis in monocyte‐derived macrophages is dependent on ApoE isoform (Cullen et al., 1998), and cholesterol homeostasis has been identified as critical for regulating immune cells, including T cells (Ito et al., 2016; W. Yang et al., 2016; York et al., 2015), we hypothesize that ApoE may affect T‐cell function by promoting cholesterol and lipid metabolism in T cells. Given that ApoE is a crucial risk factor for AD and a significant modulator of T cells, we propose that ApoE may be an immunotherapy target for regulating abnormal T‐cell activation in AD.
3.2. Aβ regulates T‐cell activation
Amyloid precursor protein (APP) is sequentially cleaved by β‐secretase and γ‐secretase in an amyloidogenic pathway to produce Aβ, which aggregates into amyloid plaques (Cole & Vassar, 2007; Selkoe, 2001; Vassar, 2004). It is clear that the level of Aβ in the brain is significantly elevated during the progression of AD, which ultimately results in neuronal death and inflammation. Autoantibodies to Aβ have been detected and were found to be elevated in both AD patients and elderly AD mice, which suggests that Aβ can act as a self‐antigen to initiate humoral immune response (Nath et al., 2003). Moreover, Aβ‐reactive T cells were also detected in the peripheral blood of AD patients (Monsonego et al., 2013). The presentation of peripheral Aβ to T cells is typically detected in lymph glands; however, it has been reported that antigen‐presenting cells (APCs) can present Aβ to T cells infiltrating the parenchyma, although it is unknown why T cells recognize self‐antigen Aβ (Archambault et al., 2005).
The role of Aβ‐reactive T cells is complicated and controversial. On the one hand, it has been shown that Aβ‐reactive T cells in certain AD mouse models are beneficial for Aβ clearance via enhancement of microglial activation in an IFN‐γ‐dependent manner (Fisher et al., 2010; Monsonego et al., 2006). On the other hand, Aβ‐reactive T cells may be detrimental because they promote pathogenic immune responses in AD. These abnormal T cells lead to strong secretion of proinflammatory factors, including TNF‐α, IL‐1β, and IL‐6, contributing to chronic neuroinflammation and neurotoxicity (Mietelska‐Porowska & Wojda, 2017). Although the beneficial and detrimental effects of Aβ‐reactive T cells in Aβ pathology are still not fully elucidated, it has well accepted that Aβ as a specific antigen can be captured by APCs and then be recognized by T cells to induce T‐cell activation and proliferation. These processes are tightly related to AD pathology.
In addition to being presented as a specific antigen to T cells, Aβ may have a more direct effect on the regulation of T‐cell function; evidence had revealed that T cells can synthesize and secret APP upon activation to initiate the immune response (Bullido et al., 1996; Monning et al., 1990, 1992). Consistently, the lymphoblastoid cell line established from familial Alzheimer's disease (FAD) expresses higher APP than control patients (Matsumoto & Fujiwara, 1991). Therefore, T cells from FAD may express a higher level of APP. Furthermore, with the characteristics of a cell adhesion molecule, APP can bind to extracellular matrix components including collagen and laminin (Sondag & Combs, 2006), implying a role for APP in cell adhesion, cell–extracellular matrix contact, or cell–cell contact during T‐cell recruitment and infiltration.
Strikingly, data obtained in vitro have shown that synthetic APP peptides stimulate the proliferation of resting lymphocytes from young and old healthy donors, which correlates with IL‐2 expression (Trieb et al., 1996). It is a consistent finding that Aβ stimulation in vitro also significantly enhances T‐cell proliferation derived from AD patients and healthy elderly individuals (Jozwik et al., 2012). Moreover, exposure of Aβ to human activated T cells in vitro leads to increased secretion of proinflammatory factors, including IL‐6 and TNF‐α. However, administration of Aβ in vivo attenuates inflammation and reverses paralysis in a Th1/Th17‐induced experimental autoimmune encephalomyelitis (EAE) mouse model (Grant et al., 2012). Notably, both Aβ40 and Aβ42 directly inhibit CD4+ T‐cell activation and proliferation under aCD3/aCD28 antibody stimulation, and Aβ42 exhibits higher inhibitory properties. Moreover, APP expression in other leukocytes, such as monocytes, induces CCR5 expression and proinflammatory cytokine release (Giri et al., 2005; Sondag & Combs, 2006; Vehmas et al., 2004), which could, in part, modulate T‐cell activation.
Although the mechanisms whereby Aβ and APP regulate cellular T‐cell processes have not been fully elucidated, previous studies have suggested that Aβ can regulate T‐cell function in at least three distinct ways: (1) Aβ can be presented by APCs to T cells as an antigen and promote T‐cell activation, (2) Aβ precursor protein endogenously expressed in T cells or exogenous Aβ directly modulate T‐cell function, and (3) Aβ precursor protein expressed in monocytes induces proinflammatory cytokines which mediate T‐cell function indirectly. However, the exact mechanism that Aβ regulates the function of T cells may differ between different disease models and may be associated with the severity of the disease. It is reasonable to suggest that strategies which target Aβ for AD treatment may disturb the functional regulation of T cells by Aβ.
3.3. Alpha secretases regulate T‐cell function
Alpha secretases (α‐secretases) are members of the ADAM (a disintegrin and metalloproteinase) family, which cleave within the Aβ peptide to produce sAPPα and C83 in the non‐amyloidogenic pathway (Zhang et al., 2011). sAPPα, but not secreted ectodomain APPβ (sAPPβ), protects neurons against Aβ‐induced cytotoxicity and is thought to be a neurotrophic and neuroprotective factor (Tackenberg & Nitsch, 2019). Therefore, α‐secretases can facilitate AD prevention, not only by competitive cleavage of APP to preclude the formation of Aβ peptide but also by providing neuroprotective agents. Additionally, decreased activity of α‐secretase was observed in AD patients (Colciaghi et al., 2002; Kim et al., 2009). Therefore, pharmacological intervention targeting α‐secretase may provide a potential therapy for AD.
ADAM9, ADAM10, and ADAM17 (tumor necrosis factor‐α‐converting enzyme, TACE) have been identified as having α‐secretase activity (Buxbaum et al., 1998; Lammich et al., 1999). It has recently been shown that ADAM9 drives Th17‐cell development by producing bioactive transforming growth factor β1 (TGFβ1), and that T cells lacking ADAM fail to induce Th17‐dependent experimental autoimmune encephalomyelitis (Umeda et al., 2021). Besides, ADAM10 and ADAM17 were shown to regulate T‐cell function via the cleavage of lymphocyte activation gene 3 (LAG3), which must be cleaved to allow normal T‐cell activation (Li et al., 2007). ADAM10 and ADAM17 were also identified as major sheddases of T‐cell immunoglobulin and mucin domain 3 (Tim‐3), whereas Tim‐3 dampened the T‐cell response by inducing cell death (Moller‐Hackbarth et al., 2013). Thus, impaired activity of α‐secretases in AD may contribute to T‐cell abnormalities.
Levels of ADAM9 are significantly elevated in T cells from patients with systemic lupus erythematosus, and thus, T‐cell activity may regulate ADAM9 expression (Umeda et al., 2021). Indeed, further analysis revealed that the transcription factor inducible cAMP early repressor (ICER) can directly bind to ADAM9 promoter and to promote ADAM9 transcription during T‐cell activation (Umeda et al., 2021). Moreover, TCR signaling transduction can also induce enzymatic activity of ADAM17 in a PKCθ‐dependent manner (Li et al., 2007). Given that ADAM exhibits neuroprotective properties in AD, T‐cell‐induced modulation of ADAM activity could be an alternative target for AD treatment.
3.4. T‐cell function is associated with β‐secretase
Beta‐secretase, known as β‐site amyloid precursor protein cleaving enzyme 1 (BACE1), cleaves the extracellular domain of APP to produce Aβ. The concentration and enzymatic activity of BACE1 in the CSF and blood of AD patients are significantly higher than that of control participants, indicating that BACE1 is a promising candidate biological marker of AD (Ewers et al., 2011; Hampel & Shen, 2009; Shen et al., 2018). Based on the amyloid hypothesis, Aβ is considered one of the leading potential causes of AD, so inhibition of BACE1 to reduce the production of Aβ is considered to be an effective strategy for AD treatment (Yan & Vassar, 2014). Besides APP, BACE1 has other substrates, including low‐density lipoprotein receptor‐related protein (LRP) (von Arnim et al., 2005), the voltage‐gated sodium channel (Nav1) β2 subunit (Navβ2) (von Arnim et al., 2005), and neuregulin‐1 (NRG1) (X. Hu et al., 2006; Willem et al., 2006), most of which are related to neuron and nervous system function. Furthermore, a recent study showed a BACE1‐dependent insulin receptor (IR) reduction in the liver of AD patients, which concluded that IRs are an additional novel substrate for BACE1, highlighting the role of BACE1 in type II diabetes and other metabolic disorders which are associated with underlying chronic insulin resistance (Meakin et al., 2018).
In addition to the nervous system and hepatic metabolism, there are also immune‐system‐related substrates of BACE1. For example, it has been shown that P‐selectin glycoprotein ligand‐1 (PSLG‐1) is a substrate for BACE1 and the cleavage site has been identified using mass spectrometry (Lichtenthaler et al., 2003). It is expressed in endothelial cells and leukocytes, including T cells, and binds to L‐selectin, E‐selectin, and P‐selectin to mediate monocyte adhesion during inflammation (da Costa Martins et al., 2007; Moore, 1998). In addition, there are another two inflammatory proteins, interleukin‐1 receptor 2 (IL‐1R2) and β‐galactoside α2, 6‐sialyltransferase (ST6Gal1), that are cleaved by BACE1 (Kuhn et al., 2007; Sugimoto et al., 2007). Correspondingly, BACE1 expression is tightly regulated by immune regulators, including nuclear factor kappa B (NF‐κB) (Cai et al., 2011), specificity protein 1 (Sp1) (Christensen et al., 2004), nuclear factor of activated T cells (NFAT) (Cho et al., 2008), GATA Binding Protein 1 (GATA‐1) (Lange‐Dohna et al., 2003), signal transducer and activator of transcription3 (STAT3) (L. Liu et al.,2013) and STAT1(Cho et al., 2007), all of which are key T‐cell transcription factors. More importantly, IFN‐γ, one of the proinflammatory factors secreted predominantly by T cells, has been reported to stimulate BACE1 expression and sAPPβ production (Hong et al., 2003). Surprisingly, recent studies identified that CD4+ T cells highly expressed BACE1, and BACE1 mediated T‐cell activation in the EAE and AD mouse model (Dai et al., 2021; Hernandez‐Mir et al., 2019).
In conclusion, these findings implicate an unexpected relationship between BACE1 and T cells. This relevance is bilateral because, on the one hand, changes of T‐cell‐related activity regulate BACE1 expression and activity. On the other hand, BACE1 may modulate T‐cell function via cleavage of various substrates expressed on leukocytes, including T cells.
3.5. The Role of γ‐secretase in T‐cell function
Gamma‐secretase, another crucial enzyme for the cleavage of APP into Aβ, is a membrane protein complex composed of four individual proteins: presenilin enhancer 2 (PEN2), presenilins (PS), nicastrin, and anterior pharynx defective 1 (APH‐1) (De Strooper, 2003; Kimberly et al., 2003). PSEN1 and PSEN2 are two homologous genes, respectively, encoding PS1 and PS2, representing the major catalytic subunits of γ‐secretase (Edbauer et al., 2003; Wolfe et al., 1999). PS1 and PS2 mutations are reportedly responsible for early‐onset familial Alzheimer's disease (EOFAD) (Haass & De Strooper, 1999; Sherrington et al., 1995; Sorbi et al., 1995). More than 70 known type I integral membrane proteins can be cleaved by γ‐secretase within their transmembrane domains (Wolfe, 2019). Among them, the best‐studied substrate of γ‐secretase is APP due to its relevance in AD (De Strooper et al., 1998). APP is consecutively cleaved by β‐secretase and γ‐secretase, which finally yield Aβ40 or Aβ42, the secreted ectodomain APPβ (sAPPβ) and APP intracellular domain (AICD) (Y. W. Zhang et al., 2011). Moreover, AICD displays transcriptional regulatory function by binding to target genes, including the tumor suppressor TP53, the Aβ‐degrading enzyme neprilysin (NEP), LRP1, and the epidermal growth factor receptor (EGFR) (Multhaup et al., 2015).
In addition to APP, the most studied substrate of γ‐secretase is the Notch receptor family (Shih Ie & Wang, 2007), which release the Notch intracellular domain (NICD) during the proteolysis for translocation to the nucleus and activation of transcription factors involved in cell development and cell fate determination (Henrique & Schweisguth, 2019; Kopan & Ilagan, 2009; Shih Ie & Wang, 2007). Over past decades, it was elucidated that the essential function of Notch signaling is mediated by γ‐secretase in the biological processes of T cells. Treatment with γ‐secretase inhibitors or deletion of Notch in hematopoietic progenitors and common lymphoid precursors impair the development of T cells (Hadland et al., 2001; Radtke et al., 1999; Wilson et al., 2001); conversely, retroviruses can induce continuous expression of Notch1 in hematolymphoid progenitors leading to thymic‐independent T‐cell development (Pui et al., 1999). Unsurprisingly, clinical trials of AD treatment with γ‐secretase inhibitors have failed due to their inhibition of Notch cleavage, resulting in abnormalities in the skin, gastrointestinal tract, thymus, and spleen (Imbimbo & Giardina, 2011). Notch‐signaling‐dependent regulation is necessary for the crucial proteins of T‐cell activation and differentiation, including NF‐κB, T‐bet, IFN‐γ, and TGF‐β, and this regulation can be abolished by γ‐secretase inhibitors (Amsen et al., 2015; Minter et al., 2005; Palaga et al., 2003; Samon et al., 2008; Shin et al., 2006). The Helbig and Maekawa research groups have shown that Notch signaling can prolong activated CD4+ T‐cell longevity and promote memory CD4+ T‐cell maintenance (Helbig et al., 2012; Maekawa et al., 2015). Furthermore, CD44, a critical regulator that controls T‐cell development and function (Baaten et al., 2012; Graham et al., 2007), has been identified as a novel substrate of γ‐secretase in vitro (Lammich et al., 2002), implying a novel mechanism of γ‐secretase in the regulation of T‐cell function.
In summary, the role of γ‐secretase in most biological T‐cell processes suggests that γ‐secretase inhibitors should be used with caution to avoid affecting normal physiological T‐cell function. Conversely, γ‐secretase inhibitor treatment may be an effective strategy for the rescue of abnormal T‐cell function under certain conditions.
3.6. T cells contribute to Tau pathology
Tau is a microtubule‐binding protein abundant in neurons and is mainly localized on axons and dendrites (Hirokawa et al., 1996; Ittner et al., 2010; Konzack et al., 2007; Utton et al., 2002). The primary role of tau in neurons is to modulate the stability of axonal microtubules by interacting with tubulin. While phosphorylation is a common post‐translational modification of tau (Cleveland et al., 1977; Ksiezak‐Reding et al., 2003; Sengupta et al., 1998), abnormal hyperphosphorylation of tau leads to NFT formation and is neurotoxic in neurodegenerative diseases, including AD (Kenessey et al., 1995; Kopke et al., 1993; Ksiezak‐Reding et al., 1992). Under pathological conditions, excessive or abnormal phosphorylation of tau which aggregates into NFTs is responsible for synaptic dysfunction and neuronal death (Guo et al., 2017).
Tau‐specific CD4+ T cells are widely distributed in the peripheral blood from the general population (Lindestam Arlehamn et al., 2019). These cells collected from either young or healthy elderly donors exhibited reactivity to tau‐ and p‐tau‐derived peptides associated with IL‐5 and IFN‐γ. The presence of the tau‐autoreactive T cells indicates that the thymic selection of CD4+ T cells is not sufficient to eliminate these cells. Furthermore, extravascular T cells are observed in the brains, specifically in the hippocampus, of AD patients, most of which are CD8+ T cells. These extravascular T cells are correlated with tau pathology but not with Aβ pathology (Merlini et al., 2018), suggesting that T cells could be critical for driving the tau‐dependent phase of the AD pathology.
Interestingly, T‐cell infiltration has been discovered positively correlated with p‐tau load in the inferior parietal lobule, middle temporal gyrus, and medial frontal gyrus of AD patients (Zotova et al., 2013). In addition, T‐cell infiltration was also observed in the cortex of frontotemporal dementia patients with P301L tau mutation and the hippocampus of THY‐Tau22 mouse model (Laurent et al., 2017). These data support an instrumental role of tau‐driven pathophysiology in brain T‐cell infiltration. However, the underlying molecular mechanisms are not clear.
All these findings together suggest that T cells can respond to, as well as contribute to, tau‐driven pathology. Given the high levels of tau aggregation in the elderly, T‐cell responses to tau may contribute to the progression of neurological diseases, including AD and PD. As tau‐driven pathology in AD induces synaptic loss, cytoskeletal dysfunction, and impair axonal transport, tau‐targeted therapeutic strategies have been proposed. Immunization against tau has shown great potential to treat tau pathology by inhibiting tau transmission and aggregation (Kfoury et al., 2012; Yoshiyama et al., 2013). Furthermore, it has been demonstrated that passive immunization may be more effective and safer than active immunization (Spillantini & Goedert, 2013). It is worth pointing out that it is not known whether tau‐targeted treatment is involved in the regulation of T‐cell response and whether this occurs without breaking down the functional immune homeostasis.
3.7. T‐cell activity is associated with neuronal loss
Brain atrophy caused by neuronal and synaptic loss is one of the definitive pathological lesions observed in AD. Several neuronal death mechanisms have been determined in AD (Cotman & Su, 1996; Niikura et al., 2006). Substantial evidence suggests that Aβ plays a significant role in initiating neurotoxicity and neuronal dysfunction and results in neuronal death. Accumulated Aβ‐initiated toxicities are characterized by mitochondrial dysfunction, oxidative stress, and calcium dyshomeostasis in neurons (Canevari et al., 2004; Caughey & Lansbury, 2003; LaFerla, 2002). Aβ also alters the acetylcholinesterase (AChE) activity, promotes the modification of c‐Fos with O‐linked β‐N‐acetyl glucosamine (O‐GlcNAc), and increases p‐tau expression in neurons, all of which contribute to neuronal apoptosis (Choi et al., 2019; Song et al., 2018). Moreover, Aβ oligomers trigger cyclin‐dependent kinase‐5 (Cdk5) activation, which thereafter mediates neuronal apoptosis via induction of p53 phosphorylation (Lapresa et al., 2019). In addition to the toxic effects of Aβ, inhibition of the proteasome is sufficient to induce neuronal apoptosis by an increase in poly‐ADP‐ribosylation, elevated activation of caspase‐3‐like proteases, and accelerated amylospheroid (Keller & Markesbery, 2000; Komura et al., 2019; Qiu et al., 2000). Widespread proinflammatory factors in the brain such as TNF‐α, IFN‐γ, and IL‐1β also have significant detrimental effects on neurons (Barker et al., 2001; Brown & Neher, 2010; Combs et al., 2001; Rothwell, 2003).
The migration of T cells into the CNS parenchyma during the pathological process of AD has attracted attention due to the crosstalk between neurons and T cells. Nitsch and colleagues were first to present the process of direct physical contact between neurons and T cells in living brain tissue (Nitsch et al., 2004). Notably, they showed that T‐cell–neuronal interactions induced calcium oscillations in neurons, resulting in neuronal death; however, a combination of glutamate receptor antagonists and perforin blockade were sufficient to inhibit this detrimental effect on neuronal calcium levels. Later, researchers pointed out that, although CD8+ T cells exerted more toxicity than CD4+ T cells in a neuronal co‐cultured system, CD4+ T cells can promote cytotoxicity of CD8+ T‐cell neurons via induction of violent inflammation (Zhao et al., 2018). This phenomenon may be due to the lack of MHC II molecule expression on neurons; thus, CD4+ T cells cannot directly interact with neurons in an antigen‐specific way, while neurons can constitutively express the MHC I molecule recognized by CD8+ T cells. Significantly, T cells can also contribute to neuronal loss by releasing proinflammatory cytokines. For instance, IL‐17 secreted by Th17 cells induces neuronal cell death by IL‐17—IL‐17R signaling and NF‐κB activation (Sommer et al., 2018). A consistent finding has been shown that Th17 cells infiltrating the brain can directly induce the death of dopaminergic neurons and promote the activation of glial cells via LFA‐1/ICAM‐1‐mediated intercellular communication involving c‐Jun NH(2)‐terminal kinase (JNK)/ activator protein 1 (AP‐1) signal activation in neurons (Liu et al., 2017).
It has been argued that T cells exhibit neuroprotective properties aside from neurotoxic effects during pathological situations (Baek et al., 2016; Chiu et al., 2008; Endo et al., 2015; Moalem et al., 1999). Most importantly, T cells are indispensable for spatial learning and the maintenance of neurogenesis under physiological situations in adulthood (Ziv et al., 2006). More work is required to discriminate the contributions from different T‐cell subtypes to neurons in neurodegenerative disorders and to determine their pathogenic and neuroprotective properties. Understanding the particular effects of T cells in different diseases may provide important information to be carefully considered when developing therapeutic strategies.
3.8. T cells contribute to neuroinflammation
Pathogenesis of AD is not limited to neuronal loss but also extends to extensive glial cell activation (Lee & Landreth, 2010; Medeiros & LaFerla, 2013; Molofsky et al., 2012; Morales et al., 2013; Paresce et al., 1996). In AD, neuroinflammation is a common phenomenon activated by amyloid plaques and NFTs. It contributes to pathogenesis just as much as plaques and tangles, perhaps even more so, and there is evidence to suggest that neuroinflammation is a critical modulator of AD development (Heneka et al., 2015; Jiang et al., 2014; R. Li et al., 2004; Van Eldik et al., 2016; L. Yang et al., 2002).
Microglia, the most abundant resident innate immune cells in the brain, are vital cellular mediators for initiating neuroinflammatory responses. Various immune‐related receptors are expressed on microglia cell membranes, including scavenger receptors, chemokine receptors, cytokine receptors, and pattern‐recognition receptors (PRRs), which can bind with proinflammatory mediators to trigger microglial activation (Kierdorf & Prinz, 2013). Furthermore, Genome‐Wide Association Studies (GWAS) have also determined many gene mutations related to an elevated risk of late‐onset AD (LOAD) and most are expressed abundantly in microglia, such as triggering receptor expressed on myeloid cells 2 protein (TREM2), complement receptor type 1 (CR1) and CD33 (Karch & Goate, 2015). Recently, high‐throughput sequencing methods at the single‐cell level (scRNA‐seq) have been applied to study microglial function and heterogeneity throughout microglial lifespan and AD (Hammond et al., 2019; Keren‐Shaul et al., 2017; Q. Li et al., 2019; Mathys et al., 2017; Zeisel et al., 2015). Mathys and colleagues first used the scRNA approach to track activation of microglia during the neurodegenerative processes in CK‐p25 mice (Mathys et al., 2017), which mimic the major pathological hallmarks of AD, such as impaired synaptic plasticity, upregulated Aβ, and neuronal death. They found that proinflammatory factors TNF‐α and macrophage migration inhibitory factor (MIF) were upregulated in the early microglial response state (1‐week post p25 induction), suggesting that inflammation occurs as early as Aβ production and may initiate cascading effects that ultimately lead to neuronal loss and cognitive dysfunction. Astrocytes are another key regulator of neuroinflammation. During neuroinflammation, destructive signaling pathways in astrocyte are triggered by IL‐17, sphingolipids, and LacCer, followed by activation of the NF‐κB‐dependent and STAT3‐dependent transcription of proinflammatory factors, which finally contributes to neuroinflammation and promotes neurodegenerative disorders.
It is clear that T cells infiltrate the CNS and promote neuroinflammation during the pathogenesis of AD (Hoppmann et al., 2015; Laurent et al., 2017; Mietelska‐Porowska & Wojda, 2017; Raveney et al., 2015; Wimmer et al., 2019; Yu et al., 2015). Th1 and Th17 cells significantly accumulate in the brains of APP/PS1 mice; however, only Aβ‐specific Th1 cells adoptively transplanted to APP/PS1 mice lead to a deficit in cognitive function (Browne et al., 2013). These Aβ‐specific Th1 cells promote microglia activation and neuroinflammation via IFN‐γ production, and administration of a neutralizing IFN‐γ antibody reverses the outcomes of Th1 cells on microglia activation and Aβ deposition. In addition, microglial cells co‐cultured with Aβ‐specific Th1 cells or Aβ‐specific Th17 cells induce proinflammatory cytokine production in microglia, which can be attenuated by Th2 cells (McQuillan et al., 2010), indicating that the regulation of microglia activation by T cells occurs in a cell‐type‐dependent manner. Surprisingly, T cells, especially brain‐resident CD4+ T cells, can regulate microglia activation and are required for microglia maturation (Pasciuto et al., 2020). The absence of CD4+ T cells traps microglia in a fetal‐like transcriptional state and results in defective synaptic pruning and depression‐like mouse behavior. Besides, T cells can positively contribute to astrocyte activation and then exacerbate neuroinflammation. T‐cell‐derived IFN‐γ induces astrocyte proliferative response in vitro and promotes brain reactive astrogliosis (Yong et al., 1991). Moreover, IL‐17, produced by Th17 cells, has been repeatedly identified as an effective astrocyte stimulator. IL‐17 stimulates inducible nitric oxide synthase activation (Trajkovic et al., 2001), regulates macrophage inflammatory proteins‐1α (MIP‐1α) expression via PI3K/Akt and NF‐κB pathways (Yi et al., 2014), and enhances the IL‐6 signaling pathway (Ma et al., 2010) in astrocytes. In addition to activating microglia and astrocytes, T cells may also promote brain inflammation by inducing myeloid cells, including dendritic cells (DCs) and macrophages associated with secretion of TNF‐α, IL1β, and IL‐6 (Town et al., 2005b). More critically, T cells not only contribute to neuroinflammation but also initiate neuroinflammation in an MHCII‐dependent manner. In this way, conventional DCs process and present myelin antigen to parenchymal T cells and then trigger T cells to infiltrate the CNS to initiate neuroinflammation (Mundt et al., 2019).
Of particular note, T cells also exhibit neuroprotective properties by regulating the trophic/cytotoxic glial cell balance and restoring glial cell activation (Beers et al., 2008). CD4+ T‐cell‐derived IL‐10 and IL‐4 are two immunoregulatory factors with important neuroprotective properties, involving the inhibition of microglia with subsequent reduction of nitric oxide and TNF‐α levels (Chao et al., 1993; Frenkel et al., 2005).
Summarizing, these results highlight T cells as an essential modulator in mediating neuroinflammation, which is achieved by activating microglia and astrocytes and releasing proinflammatory factors, implicating T cells as a potential immunotherapy target for neuroinflammation in neurodegenerative disease.
4. CONCLUDING REMARKS
In this article, we first reviewed the abnormal behavior of T cells in the progression of AD. Although the significance of T cells in AD pathogenesis is still hotly debated, there is convincing evidence from pre‐clinical, epidemiological, and genetic studies which indicate that the immune system is closely involved in AD and that T cells contribute to the pathological responses of AD (Mietelska‐Porowska & Wojda, 2017; Schellenberg, 2012; Wyss‐Coray & Rogers, 2012).
We then discussed in detail factors which affect T‐cell function in AD. We summarized that the ApoE might affect T‐cell activation via regulation of lipid metabolism. In contrast, the mechanisms by which Aβ regulates T‐cell function are multiplex, including antigen presentation, direct modulation of endogenous or exogenous Aβ, and indirect activation via monocytes. Besides, tau‐driven pathology also contributes to T‐cell activation, although the exact mechanism remains unknown. Moreover, α‐, β‐, and γ‐secretase, three crucial APP enzymes, modulate T‐cell function, in a manner which is dependent for the most part on the cleavage of specific substrates. As for neuronal loss, we should notice the pathogenic contribution of T cells and the neuroprotective properties of different T‐cell subsets. Lastly, neuroinflammation, one of the crucial hallmarks and contributors of AD, can facilitate the activation and recruitment of T cells into the brain. At the same time, activated T cells exacerbate AD pathology via the activation of microglia and astrocytes and through the release of proinflammatory cytokines.
In conclusion, we summarized that AD risk factors and hallmarks can modulate T‐cell function, and abnormal activation of T cells in AD can also act on these critical factors, ultimately exacerbating AD pathology (Figure 2). Thus, understanding these causal associations may provide important insights into developing effective therapeutics. We also proposed that targeted treatment based on these risk factors and hallmarks may cause changes in the normal T‐cell phenotypes and peripheral immune responses under the physiological state. Therefore, the critical question is how to identify and limit the potential side effects of AD‐related factor‐based therapies on the normal T‐cell function.
FIGURE 2.
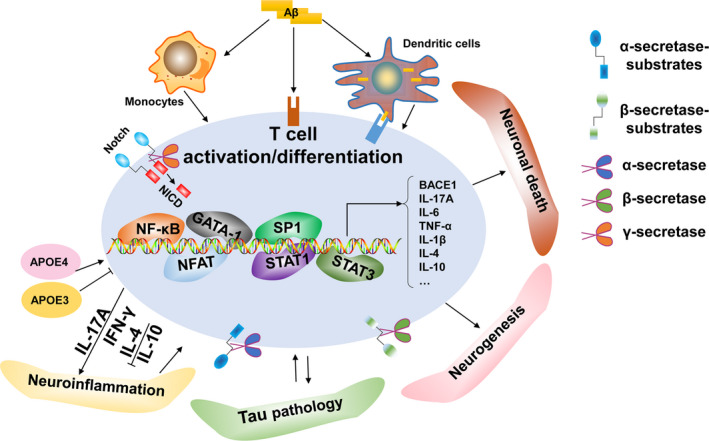
Schematic representation of the association between T cells and AD hallmarks. (1) ApoE is a key risk factor for AD and is also a significant T‐cell modulator, a function which may be ApoE‐allele‐dependent. (2) (a) Aβ can be presented by APCs to T cells as an antigen and promote T‐cell activation; (b) Aβ precursor protein endogenously expressed in T cells or exogenous Aβ directly modulates T‐cell function; (c) Aβ precursor protein expressed in monocytes induce proinflammatory cytokines to indirectly mediate T‐cell function. (3) α‐secretase mediates T‐cell function via the cleavage of diverse substrates, whereas T‐cell activation promotes α‐secretase activity. (4) T‐cell‐related biological changes regulate BACE1 expression and activity. Conversely, BACE1 may modulate T‐cell function via the cleavage of various substrates expressed on T cells. (5) The Notch receptor family are substrates of γ‐secretase, which releases the Notch intracellular domain (NICD) during proteolysis for translocation to the nucleus and activation of transcription factors involved in T‐cell development and T‐cell fate determination. (6) T cells are correlated with tau pathology and tau‐driven pathology may also induce excessive activation of T cells. (7) T cells migrate into the CNS parenchyma during the pathological progression of AD and contribute to neuronal death while T cells are also neuroprotective for spatial learning and the maintenance of neurogenesis under physiological situations. (8) T cells infiltrate the CNS and promote neuroinflammation during the pathogenesis of AD. Notably, T cells may also exhibit neuroprotective properties by regulating trophic/cytotoxic glia balance and restored glial activation
COMPETING INTERESTS
All authors declare that they have no competing financial interests.
AUTHOR CONTRIBUTIONS
Linbin Dai and Yong Shen made substantial contributions to the review including writing the manuscript, preparing the figures and tables, and discussing the content.
ACKNOWLEDGEMENTS
This work was supported by the National Key Plan for Scientific Research and Development of China (2020YFA0509304), the Chinese Academy of Sciences (QYZDY‐SSW‐SMC012 and XDB39000000), the National Natural Sciences Foundation of China (31530089), and the Fundamental Research Funds for the Central Universities (YD2070002003).
Dai, L. , & Shen, Y. (2021). Insights into T‐cell dysfunction in Alzheimer's disease. Aging Cell, 20, e13511. 10.1111/acel.13511
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
REFERENCES
- Adunsky, A. , Diver‐Haber, A. , Becker, D. , & Hershkowitz, M. (1995). Basal and activated intracellular calcium ion concentrations in mononuclear cells of Alzheimer's disease and unipolar depression. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 50(4), B201–204. 10.1093/gerona/50a.4.b201 [DOI] [PubMed] [Google Scholar]
- Ali, K. , Middleton, M. , Pure, E. , & Rader, D. J. (2005). Apolipoprotein E suppresses the type I inflammatory response in vivo. Circulation Research, 97(9), 922–927. 10.1161/01.RES.0000187467.67684.43 [DOI] [PubMed] [Google Scholar]
- Alves, S. , Churlaud, G. , Audrain, M. , Michaelsen‐Preusse, K. , Fol, R. , Souchet, B. , Braudeau, J. , Korte, M. , Klatzmann, D. , & Cartier, N. (2017). Interleukin‐2 improves amyloid pathology, synaptic failure and memory in Alzheimer's disease mice. Brain : a journal of neurology, 140(3), 826–842. [DOI] [PubMed] [Google Scholar]
- Amsen, D. , Helbig, C. , & Backer, R. A. (2015). Notch in T Cell Differentiation: All Things Considered. Trends in Immunology, 36(12), 802–814. 10.1016/j.it.2015.10.007 [DOI] [PubMed] [Google Scholar]
- Archambault, A. S. , Sim, J. , Gimenez, M. A. , & Russell, J. H. (2005). Defining antigen‐dependent stages of T cell migration from the blood to the central nervous system parenchyma. European Journal of Immunology, 35(4), 1076–1085. 10.1002/eji.200425864 [DOI] [PubMed] [Google Scholar]
- Baaten, B. J. , Tinoco, R. , Chen, A. T. , & Bradley, L. M. (2012). Regulation of Antigen‐Experienced T Cells: Lessons from the Quintessential Memory Marker CD44. Frontiers in Immunology, 3, 23. 10.3389/fimmu.2012.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek, H. , Ye, M. , Kang, G. H. , Lee, C. , Lee, G. , Choi, D. B. , Jung, J. , Kim, H. , Lee, S. , Kim, J. S. , Lee, H.J. , Shim, I. , Lee, J. H. , & Bae, H. (2016). Neuroprotective effects of CD4+CD25+Foxp3+ regulatory T cells in a 3xTg‐AD Alzheimer's disease model. Oncotarget, 7(43), 69347–69357. 10.18632/oncotarget.12469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker, V. , Middleton, G. , Davey, F. , & Davies, A. M. (2001). TNFalpha contributes to the death of NGF‐dependent neurons during development. Nature Neuroscience, 4(12), 1194–1198. 10.1038/nn755 [DOI] [PubMed] [Google Scholar]
- Baruch, K. , Rosenzweig, N. , Kertser, A. , Deczkowska, A. , Sharif, A. M. , Spinrad, A. , Tsitsou‐Kampeli, A. , Sarel, A. , Cahalon, L. , & Schwartz, M. (2015). Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer's disease pathology. Nature Communications, 6, 7967. 10.1038/ncomms8967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers, D. R. , Henkel, J. S. , Zhao, W. , Wang, J. , & Appel, S. H. (2008). CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proceedings of the National Academy of Sciences, 105(40), 15558–15563. 10.1073/pnas.0807419105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonacina, F. , Coe, D. , Wang, G. , Longhi, M. P. , Baragetti, A. , Moregola, A. , Garlaschelli, K. , Uboldi, P. , Pellegatta, F. , Grigore, L. , Da Dalt, L. , Annoni, A. , Gregori, S. , Xiao, Q. , Caruso, D. , Mitro, N. , Catapano, A. L. , Marelli‐Berg, F. M. , & Norata, G. D. (2018). Myeloid apolipoprotein E controls dendritic cell antigen presentation and T cell activation. Nature Communications, 9(1), 10.1038/s41467-018-05322-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigas, H. C. , Ribeiro, M. , Coelho, J. E. , Gomes, R. , Gomez‐Murcia, V. , Carvalho, K. , Faivre, E. , Costa‐Pereira, S. , Darrigues, J. , de Almeida, A. A. , Buée, L. , Dunot, J. , Marie, H. , Pousinha, P. A. , Blum, D. , Silva‐Santos, B. , Lopes, L. V. , & Ribot, J. C. (2021). IL‐17 triggers the onset of cognitive and synaptic deficits in early stages of Alzheimer's disease. Cell reports, 36(9), 109574. 10.1016/j.celrep.2021.109574 [DOI] [PubMed] [Google Scholar]
- Brown, G. C. , & Neher, J. J. (2010). Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Molecular Neurobiology, 41(2–3), 242–247. 10.1007/s12035-010-8105-9 [DOI] [PubMed] [Google Scholar]
- Browne, T. C. , McQuillan, K. , McManus, R. M. , O'Reilly, J. A. , Mills, K. H. , & Lynch, M. A. (2013). IFN‐gamma Production by amyloid beta‐specific Th1 cells promotes microglial activation and increases plaque burden in a mouse model of Alzheimer's disease. The Journal of Immunology, 190(5), 2241–2251. 10.4049/jimmunol.1200947 [DOI] [PubMed] [Google Scholar]
- Bullido, M. J. , Munoz‐Fernandez, M. A. , Recuero, M. , Fresno, M. , & Valdivieso, F. (1996). Alzheimer's amyloid precursor protein is expressed on the surface of hematopoietic cells upon activation. Biochimica Et Biophysica Acta, 1313(1), 54–62. 10.1016/0167-4889(96)00015-8 [DOI] [PubMed] [Google Scholar]
- Burns, A. , Byrne, E. J. , & Maurer, K. (2002). Alzheimer's disease. Lancet, 360(9327), 163–165. 10.1016/S0140-6736(02)09420-5 [DOI] [PubMed] [Google Scholar]
- Buxbaum, J. D. , Liu, K. N. , Luo, Y. , Slack, J. L. , Stocking, K. L. , Peschon, J. J. , Johnson, R. S. , Castner, B.J. , Cerretti, D.P. ,& Black, R. A. (1998). Evidence that tumor necrosis factor alpha converting enzyme is involved in regulated alpha‐secretase cleavage of the Alzheimer amyloid protein precursor. Journal of Biological Chemistry, 273(43), 27765–27767. 10.1074/jbc.273.43.27765 [DOI] [PubMed] [Google Scholar]
- Cai, Z. , Zhao, Y. , Yao, S. , & Bin Zhao, B. (2011). Increases in beta‐amyloid protein in the hippocampus caused by diabetic metabolic disorder are blocked by minocycline through inhibition of NF‐kappaB pathway activation. Pharmacological Reports, 63(2), 381–391. 10.1016/s1734-1140(11)70504-7 [DOI] [PubMed] [Google Scholar]
- Canevari, L. , Abramov, A. Y. , & Duchen, M. R. (2004). Toxicity of amyloid beta peptide: tales of calcium, mitochondria, and oxidative stress. Neurochemical Research, 29(3), 637–650. [DOI] [PubMed] [Google Scholar]
- Cao, C. , Arendash, G. W. , Dickson, A. , Mamcarz, M. B. , Lin, X. , & Ethell, D. W. (2009). Abeta‐specific Th2 cells provide cognitive and pathological benefits to Alzheimer's mice without infiltrating the CNS. Neurobiology of Diseases, 34(1), 63–70. 10.1016/j.nbd.2008.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrano, A. , Hoozemans, J. J. M. , van der Vies, S. M. , Rozemuller, A. J. M. , van Horssen, J. , & de Vries, H. E. (2011). Amyloid Beta induces oxidative stress‐mediated blood‐brain barrier changes in capillary amyloid angiopathy. Antioxidants & Redox Signaling, 15(5), 1167–1178. 10.1089/ars.2011.3895 [DOI] [PubMed] [Google Scholar]
- Caughey, B. , & Lansbury, P. T. (2003). Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annual Review of Neuroscience, 26, 267–298. 10.1146/annurev.neuro.26.010302.081142 [DOI] [PubMed] [Google Scholar]
- Cedazo‐Minguez, A. , Wiehager, B. , Winblad, B. , Huttinger, M. , & Cowburn, R. F. (2001). Effects of apolipoprotein E (apoE) isoforms, beta‐amyloid (Abeta) and apoE/Abeta complexes on protein kinase C‐alpha (PKC‐alpha) translocation and amyloid precursor protein (APP) processing in human SH‐SY5Y neuroblastoma cells and fibroblasts. Neurochemistry International, 38(7), 615–625. [DOI] [PubMed] [Google Scholar]
- Chao, C. C. , Molitor, T. W. , & Hu, S. (1993). Neuroprotective role of IL‐4 against activated microglia. The Journal of Immunology, 151(3), 1473–1481. [PubMed] [Google Scholar]
- Cheng, X. , He, P. , Yao, H. , Dong, Q. , Li, R. , & Shen, Y. (2014). Occludin deficiency with BACE1 elevation in cerebral amyloid angiopathy. Neurology, 82(19), 1707–1715. 10.1212/WNL.0000000000000403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu, I. M. , Chen, A. , Zheng, Y. , Kosaras, B. , Tsiftsoglou, S. A. , Vartanian, T. K. , Brown, R. H. , & Carroll, M. C. (2008). T lymphocytes potentiate endogenous neuroprotective inflammation in a mouse model of ALS. Proceedings of the National Academy of Sciences, 105(46), 17913–17918. 10.1073/pnas.0804610105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, H. J. , Jin, S. M. , Youn, H. D. , Huh, K. , & Mook‐Jung, I. (2008). Disrupted intracellular calcium regulates BACE1 gene expression via nuclear factor of activated T cells 1 (NFAT 1) signaling. Aging Cell, 7(2), 137–147. 10.1111/j.1474-9726.2007.00360.x [DOI] [PubMed] [Google Scholar]
- Cho, H. J. , Kim, S. K. , Jin, S. M. , Hwang, E. M. , Kim, Y. S. , Huh, K. , & Mook‐Jung, I. (2007). IFN‐gamma‐induced BACE1 expression is mediated by activation of JAK2 and ERK1/2 signaling pathways and direct binding of STAT1 to BACE1 promoter in astrocytes. Glia, 55(3), 253–262. 10.1002/glia.20451 [DOI] [PubMed] [Google Scholar]
- Choi, H. , Kim, C. , Song, H. , Cha, M. Y. , Cho, H. J. , Son, S. M. , Kim, H. J. , & Mook‐Jung, I. (2019). Amyloid beta‐induced elevation of O‐GlcNAcylated c‐Fos promotes neuronal cell death. Aging Cell, 18(1), e12872. 10.1111/acel.12872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, M. A. , Zhou, W. , Qing, H. , Lehman, A. , Philipsen, S. , & Song, W. (2004). Transcriptional regulation of BACE1, the beta‐amyloid precursor protein beta‐secretase, by Sp1. Molecular and Cellular Biology, 24(2), 865–874. 10.1128/mcb.24.2.865-874.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo, F. , Lanuti, P. , Marchisio, M. , Gambi, F. , Santavenere, E. , Pierdomenico, L. , Bascelli, A. , Velluto, L. , Gambi, D. , & Miscia, S. (2008). Expression and phosphorylation of protein kinase C isoforms in Abeta(1–42) activated T lymphocytes from Alzheimers disease. International Journal of Immunopathology Pharmacology, 21(1), 23–33. 10.1177/039463200802100104 [DOI] [PubMed] [Google Scholar]
- Cleveland, D. W. , Hwo, S. Y. , & Kirschner, M. W. (1977). Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. Journal of Molecular Biology, 116(2), 227–247. 10.1016/0022-2836(77)90214-5 [DOI] [PubMed] [Google Scholar]
- Colciaghi, F. , Borroni, B. , Pastorino, L. , Marcello, E. , Zimmermann, M. , Cattabeni, F. , Padovani, A. , & Di Luca, M. (2002). [alpha]‐Secretase ADAM10 as well as [alpha]APPs is reduced in platelets and CSF of Alzheimer disease patients. Molecular Medicine, 8(2), 67–74. [PMC free article] [PubMed] [Google Scholar]
- Cole, S. L. , & Vassar, R. (2007). The Alzheimer's disease beta‐secretase enzyme, BACE1. Molecular Neurodegeneration, 2, 22. 10.1186/1750-1326-2-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs, C. K. , Karlo, J. C. , Kao, S. C. , & Landreth, G. E. (2001). beta‐Amyloid stimulation of microglia and monocytes results in TNFalpha‐dependent expression of inducible nitric oxide synthase and neuronal apoptosis. Journal of Neuroscience, 21(4), 1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman, C. W. , & Su, J. H. (1996). Mechanisms of neuronal death in Alzheimer's disease. Brain Pathology, 6(4), 493–506. 10.1111/j.1750-3639.1996.tb00878.x [DOI] [PubMed] [Google Scholar]
- Cullen, P. , Cignarella, A. , Brennhausen, B. , Mohr, S. , Assmann, G. , & von Eckardstein, A. (1998). Phenotype‐dependent differences in apolipoprotein E metabolism and in cholesterol homeostasis in human monocyte‐derived macrophages. Journal of Clinical Investigation, 101(8), 1670–1677. 10.1172/JCI119887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa Martins, P. , García‐Vallejo, J. J. , van Thienen, J. V. , Fernandez‐Borja, M. , van Gils, J. M. , Beckers, C. , Horrevoets, A. J. , Hordijk, P. L. , & Zwaginga, J. Jan (2007). P‐selectin glycoprotein ligand‐1 is expressed on endothelial cells and mediates monocyte adhesion to activated endothelium. Arteriosclerosis, Thrombosis, and Vascular Biology, 27(5), 1023–1029. 10.1161/ATVBAHA.107.140442 [DOI] [PubMed] [Google Scholar]
- Dai, L. , Wang, Q. , Lv, X. , Gao, F. , Chen, Z. , & Shen, Y. (2021). Elevated β‐secretase 1 expression mediates CD4+ T cell dysfunction via PGE2 signalling in Alzheimer's disease. Brain, Behavior, and Immunity, 98, 337–348. 10.1016/j.bbi.2021.08.234 [DOI] [PubMed] [Google Scholar]
- De Strooper, B. (2003). Aph‐1, Pen‐2, and Nicastrin with Presenilin generate an active gamma‐Secretase complex. Neuron, 38(1), 9–12. [DOI] [PubMed] [Google Scholar]
- De Strooper, B. , Saftig, P. , Craessaerts, K. , Vanderstichele, H. , Guhde, G. , Annaert, W. , Von Figura, K. , & Van Leuven, F. (1998). Deficiency of presenilin‐1 inhibits the normal cleavage of amyloid precursor protein. Nature, 391(6665), 387–390. 10.1038/34910 [DOI] [PubMed] [Google Scholar]
- Eckert, A. , Forstl, H. , Hartmann, H. , Czech, C. , Monning, U. , Beyreuther, K. , & Muller, W. E. (1995). The amplifying effect of beta‐amyloid on cellular calcium signalling is reduced in Alzheimer's disease. NeuroReport, 6(8), 1199–1202. 10.1097/00001756-199505300-00031 [DOI] [PubMed] [Google Scholar]
- Edbauer, D. , Winkler, E. , Regula, J. T. , Pesold, B. , Steiner, H. , & Haass, C. (2003). Reconstitution of gamma‐secretase activity. Nature Cell Biology, 5(5), 486–488. 10.1038/ncb960 [DOI] [PubMed] [Google Scholar]
- Endo, F. , Komine, O. , Fujimori‐Tonou, N. , Katsuno, M. , Jin, S. , Watanabe, S. , Sobue, G. , Dezawa, M., Wyss‐Coray, T. , & Yamanaka, K. (2015). Astrocyte‐derived TGF‐beta1 accelerates disease progression in ALS mice by interfering with the neuroprotective functions of microglia and T cells. Cell Reports, 11(4), 592–604. 10.1016/j.celrep.2015.03.053 [DOI] [PubMed] [Google Scholar]
- Ewers, M. , Cheng, X. , Zhong, Z. , Nural, H. F. , Walsh, C. , Meindl, T. , Teipel, S. J. , Buerger, K. , He, P. , Shen, Y. , & Hampel, H. (2011). CSF‐BACE1 activity associated with decreased hippocampus volume in Alzheimer's disease. Journal of Alzheimer's Disease, 25(2), 373–381. 10.3233/JAD-2011-091153 [DOI] [PubMed] [Google Scholar]
- Ferretti, M.T. , Merlini, M. , Späni, C. , Gericke, C. , Schweizer, N. , Enzmann, G. , Engelhardt, B. , Kulic, L. , Suter, T. , & Nitsch, R. M. (2016).T‐cell brain infiltration and immature antigen‐presenting cells in transgenic models of Alzheimer's disease‐like cerebral amyloidosis. Brain, Behavior, and Immunity, 54, 211–225. 10.1016/j.bbi.2016.02.009 [DOI] [PubMed] [Google Scholar]
- Fisher, Y. , Nemirovsky, A. , Baron, R. , & Monsonego, A. (2010). T cells specifically targeted to amyloid plaques enhance plaque clearance in a mouse model of Alzheimer's disease. PLoS One, 5(5), e10830. 10.1371/journal.pone.0010830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel, D. , Huang, Z. , Maron, R. , Koldzic, D. N. , Moskowitz, M. A. , & Weiner, H. L. (2005). Neuroprotection by IL‐10‐producing MOG CD4+ T cells following ischemic stroke. Journal of the Neurological Sciences, 233(1–2), 125–132. 10.1016/j.jns.2005.03.022 [DOI] [PubMed] [Google Scholar]
- Gao, Q. , Jiang, Y. , Ma, T. , Zhu, F. , Gao, F. , Zhang, P. , Guo, C. , Wang, Q. , Wang, X. , Ma, C. , Zhang, Y. , Chen, W. , & Zhang, L. (2010). A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. The Journal of Immunology, 185(10), 5820–5827. 10.4049/jimmunol.1000116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri, R. K. , Rajagopal, V. , Shahi, S. , Zlokovic, B. V. , & Kalra, V. K. (2005). Mechanism of amyloid peptide induced CCR5 expression in monocytes and its inhibition by siRNA for Egr‐1. American Journal of Physiology. Cell Physiology, 289(2), C264–276. 10.1152/ajpcell.00461.2004 [DOI] [PubMed] [Google Scholar]
- Giuliani, F. , Goodyer, C. G. , Antel, J. P. , & Yong, V. W. (2003). Vulnerability of human neurons to T cell‐mediated cytotoxicity. The Journal of Immunology, 171(1), 368–379. 10.4049/jimmunol.171.1.368 [DOI] [PubMed] [Google Scholar]
- Goldeck, D. , Larbi, A. , Pellicanó, M. , Alam, I. , Zerr, I. , Schmidt, C. , Fulop, T. , & Pawelec, G. (2013). Enhanced Chemokine Receptor Expression on Leukocytes of Patients with Alzheimer's Disease. PLoS One, 8(6), e66664. 10.1371/journal.pone.0066664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, V. A. , Marzo, A. L. , & Tough, D. F. (2007). A role for CD44 in T cell development and function during direct competition between CD44+ and CD44‐ cells. European Journal of Immunology, 37(4), 925–934. 10.1002/eji.200635882 [DOI] [PubMed] [Google Scholar]
- Grant, J. L. , Ghosn, E. E. , Axtell, R. C. , Herges, K. , Kuipers, H. F. , Woodling, N. S. , & Steinman, L. (2012). Reversal of paralysis and reduced inflammation from peripheral administration of beta‐amyloid in TH1 and TH17 versions of experimental autoimmune encephalomyelitis. Science Translational Medicine, 4(145), 145ra105. 10.1126/scitranslmed.3004145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot‐Sestier, M. V. , Doty, K. R. , Gate, D. , Rodriguez, J. Jr , Leung, B. P. , Rezai‐Zadeh, K. , & Town, T. (2015). Il10 deficiency rebalances innate immunity to mitigate Alzheimer‐like pathology. Neuron, 85(3), 534–548. 10.1016/j.neuron.2014.12.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, T. , Noble, W. , & Hanger, D. P. (2017). Roles of tau protein in health and disease. Acta Neuropathologica, 133(5), 665–704. 10.1007/s00401-017-1707-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass, C. , & De Strooper, B. (1999). The presenilins in Alzheimer's disease–proteolysis holds the key. Science, 286(5441), 916–919. 10.1126/science.286.5441.916 [DOI] [PubMed] [Google Scholar]
- Hadland, B. K , Manley, N. R , Su, D. M , Longmore, G. D , Moore, C. l , Wolfe, M. S , Schroeter, E. H , & Kopan, R. (2001). Gamma ‐secretase inhibitors repress thymocyte development. Proceedings of the National Academy of Sciences, 98(13), 7487–7491. 10.1073/pnas.131202798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond, T. R. , Dufort, C. , Dissing‐Olesen, L. , Giera, S. , Young, A. , Wysoker, A. , Walker, A. J. , Gergits, F. , Segel, M. , Nemesh, J. , Marsh, S. E. , Saunders, A. , Macosko, E. , Ginhoux, F. , Chen, J. , Franklin, R. J. M. , Piao, X. , McCarroll, S. A. , & Stevens, B. (2019). Single‐cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell‐state changes. Immunity, 50(1), 253–271 e256. 10.1016/j.immuni.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel, H. , & Shen, Y. (2009). Beta‐site amyloid precursor protein cleaving enzyme 1 (BACE1) as a biological candidate marker of Alzheimer's disease. Scandinavian Journal of Clinical and Laboratory Investigation, 69(1), 8–12. 10.1080/00365510701864610 [DOI] [PubMed] [Google Scholar]
- Hanisch, U. K. (2002). Microglia as a source and target of cytokines. Glia, 40(2), 140–155. 10.1002/glia.10161 [DOI] [PubMed] [Google Scholar]
- Hardy, J. A. , & Higgins, G. A. (1992). Alzheimer's disease: the amyloid cascade hypothesis. Science, 256(5054), 184–185. 10.1126/science.1566067 [DOI] [PubMed] [Google Scholar]
- Helbig, C. , Gentek, R. , Backer, R. a , de Souza, Y. , Derks, I. a m , Eldering, E. , Wagner, K. , Jankovic, D. , Gridley, T. , Moerland, P. D , Flavell, R. A , & Amsen, D. (2012). Notch controls the magnitude of T helper cell responses by promoting cellular longevity. Proceedings of the National Academy of Sciences of the United States of America, 109(23), 9041–9046. 10.1073/pnas.1206044109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka, M. T. , Carson, M. J. , Khoury, J. E. , Landreth, G. E. , Brosseron, F. , Feinstein, D. L. , Jacobs, A. H. , Wyss‐Coray, T. , Vitorica, J. , Ransohoff, R. M. , Herrup, K. , Frautschy, S. A. , Finsen, B. , Brown, G. C. , Verkhratsky, A. , Yamanaka, K. , Koistinaho, J. , Latz, E. , Halle, A. , Petzold, G. C. , Town, T. , Morgan, D. , Shinohara, M. L. , Perry, V. H. , Holmes, C. , Bazan, N. G. , Brooks, D. J. , Hunot, S. , Joseph, B. , Deigendesch, N. , Garaschuk, O. , Boddeke, E. , Dinarello, C. A. , Breitner, John C. , Cole, G. M. , Golenbock, D. T. , & Kummer, M. P. (2015). Neuroinflammation in Alzheimer's disease. The Lancet Neurology, 14(4), 388–405. 10.1016/S1474-4422(15)70016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrique, D. , & Schweisguth, F. (2019). Mechanisms of Notch signaling: A simple logic deployed in time and space. Development, 146(3), 10.1242/dev.172148 [DOI] [PubMed] [Google Scholar]
- Hernandez‐Mir, G. , Raphael, I. , Revu, S. , Poholek, C. H. , Avery, L. , Hawse, W. F. , Kane, L. P. , & McGeachy, M. J. (2019). The Alzheimer's Disease‐Associated Protein BACE1 Modulates T Cell Activation and Th17 Function. The Journal of Immunology, 203(3), 665–675. 10.4049/jimmunol.1800363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa, N. , Funakoshi, T. , Sato‐Harada, R. , & Kanai, Y. (1996). Selective stabilization of tau in axons and microtubule‐associated protein 2C in cell bodies and dendrites contributes to polarized localization of cytoskeletal proteins in mature neurons. Journal of Cell Biology, 132(4), 667–679. 10.1083/jcb.132.4.667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, H. S. , Hwang, E. M. , Sim, H. J. , Cho, H. J. , Boo, J. H. , Oh, S. S. , Kim, S. U. , & Mook‐Jung, I. (2003). Interferon gamma stimulates beta‐secretase expression and sAPPbeta production in astrocytes. Biochemical and Biophysical Research Communications, 307(4), 922–927. 10.1016/s0006-291x(03)01270-1 [DOI] [PubMed] [Google Scholar]
- Hoppmann, N. , Graetz, C. , Paterka, M. , Poisa‐Beiro, L. , Larochelle, C. , Hasan, M. , Lill, C. M. , Zipp, F. , & Siffrin, V. (2015). New candidates for CD4 T cell pathogenicity in experimental neuroinflammation and multiple sclerosis. Brain, 138(4), 902–917. 10.1093/brain/awu408 [DOI] [PubMed] [Google Scholar]
- Hu, G. R. , Walls, R. S. , Creasey, H. , McCusker, E. , & Broe, G. A. (1995). Peripheral blood lymphocyte subset distribution and function in patients with Alzheimer's disease and other dementias. Australian and New Zealand Journal of Medicine, 25(3), 212–217. 10.1111/j.1445-5994.1995.tb01525.x [DOI] [PubMed] [Google Scholar]
- Hu, X. , Hicks, C. W. , He, W. , Wong, P. , Macklin, W. B. , Trapp, B. D. , & Yan, R. (2006). Bace1 modulates myelination in the central and peripheral nervous system. Nature Neuroscience, 9(12), 1520–1525. 10.1038/nn1797 [DOI] [PubMed] [Google Scholar]
- Huang, Y. , & Mahley, R. W. (2014). Apolipoprotein E: Structure and function in lipid metabolism, neurobiology, and Alzheimer's diseases. Neurobiology of Disease, 72, 3–12. 10.1016/j.nbd.2014.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, T. , Yamamoto, K. , Takahashi, K. , Kaneyuki, H. , & Yamada, M. (1991). Interleukin‐2 receptor in peripheral blood lymphocytes of Alzheimer's disease patients. Acta Psychiatrica Scandinavica, 84(3), 262–265. 10.1111/j.1600-0447.1991.tb03141.x [DOI] [PubMed] [Google Scholar]
- Imbimbo, B. P. , & Giardina, G. A. (2011). Gamma‐secretase inhibitors and modulators for the treatment of Alzheimer's disease: disappointments and hopes. Current Topics in Medicinal Chemistry, 11(12), 1555–1570. 10.2174/156802611795860942 [DOI] [PubMed] [Google Scholar]
- Ito, A. , Hong, C. , Oka, K. , Salazar, J. V. , Diehl, C. , Witztum, J. L. , Diaz, M. , Castrillo, A. , Bensinger, S. J. , Chan, L. , & Tontonoz, P. (2016).Cholesterol accumulation in CD11c(+) immune cells is a causal and targetable factor in autoimmune disease. Immunity, 45(6), 1311–1326. 10.1016/j.immuni.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittner, L. M. , & Gotz, J. (2011). Amyloid‐beta and tau–a toxic pas de deux in Alzheimer's disease. Nature Reviews Neuroscience, 12(2), 65–72. 10.1038/nrn2967 [DOI] [PubMed] [Google Scholar]
- Ittner, L. M. , Ke, Y. D. , Delerue, F. , Bi, M. , Gladbach, A. , van Eersel, J ., Wölfing, H. , Chieng, B. C. , Christie, M. J. , Napier, I. A. , Eckert, A. , Staufenbiel, M. , Hardeman, E. , & Gotz, J. (2010). Dendritic function of tau mediates amyloid‐beta toxicity in Alzheimer's disease mouse models. Cell, 142(3), 387–397. 10.1016/j.cell.2010.06.036 [DOI] [PubMed] [Google Scholar]
- Jiang, H. , He, P. , Xie, J. , Staufenbiel, M. , Li, R. , & Shen, Y. (2014). Genetic deletion of TNFRII gene enhances the Alzheimer‐like pathology in an APP transgenic mouse model via reduction of phosphorylated IkappaBalpha. Human Molecular Genetics, 23(18), 4906–4918. 10.1093/hmg/ddu206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozwik, A. , Landowski, J. , Bidzan, L. , Fulop, T. , Bryl, E. , & Witkowski, J. M. (2012). Beta‐amyloid peptides enhance the proliferative response of activated CD4CD28 lymphocytes from Alzheimer disease patients and from healthy elderly. PLoS One, 7(3), e33276. 10.1371/journal.pone.0033276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, S. S. , Ahn, E. H. , Liu, X. , Bryson, M. , Miller, G. W. , Weinshenker, D. , & Ye, K. (2021). ApoE4 inhibition of VMAT2 in the locus coeruleus exacerbates Tau pathology in Alzheimer's disease. Acta Neuropathologica, 142(1), 139–158. 10.1007/s00401-021-02315-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch, C. M. , & Goate, A. M. (2015). Alzheimer's disease risk genes and mechanisms of disease pathogenesis. Biological Psychiatry, 77(1), 43–51. 10.1016/j.biopsych.2014.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, J. N. , & Markesbery, W. R. (2000). Proteasome inhibition results in increased poly‐ADP‐ribosylation: Implications for neuron death. Journal of Neuroscience Research, 61(4), 436–442. 10.1002/1097-4547(20000815)61:4<436:AID‐JNR10>3.0.CO;2‐Z. [DOI] [PubMed] [Google Scholar]
- Kelly, M. E. , Clay, M. A. , Mistry, M. J. , Hsieh‐Li, H. M. , & Harmony, J. A. (1994). Apolipoprotein E inhibition of proliferation of mitogen‐activated T lymphocytes: production of interleukin 2 with reduced biological activity. Cellular Immunology, 159(2), 124–139. 10.1006/cimm.1994.1302 [DOI] [PubMed] [Google Scholar]
- Kelly, R. J. , Minogue, A. M. , Lyons, A. , Jones, R. S. , Browne, T. C. , Costello, D. A. , Denieffe, S. , O'Sullivan, C. , Connor, T. J. , & Lynch, M. A. (2013). Glial Activation in AbetaPP/PS1 Mice is Associated with Infiltration of IFNgamma‐Producing Cells. Journal of Alzheimer's Disease, 37(1), 63–75. 10.3233/JAD-130539 [DOI] [PubMed] [Google Scholar]
- Kenessey, A. , Yen, S. H. , Liu, W. K. , Yang, X. R. , & Dunlop, D. S. (1995). Detection of D‐aspartate in tau proteins associated with Alzheimer paired helical filaments. Brain Research, 675(1–2), 183–189. 10.1016/0006-8993(95)00061-t [DOI] [PubMed] [Google Scholar]
- Keren‐Shaul, H. , Spinrad, A. , Weiner, A. , Matcovitch‐Natan, O.t , Dvir‐Szternfeld, R. , Ulland, T. K. , David, E. , Baruch, K. , Lara‐Astaiso, D. , Toth, B. , Itzkovitz, S. , Colonna, M. , Schwartz, M. , & Amit, I. (2017). A Unique microglia type associated with restricting development of Alzheimer's disease. Cell, 169(7), 1276–1290 e1217. 10.1016/j.cell.2017.05.018 [DOI] [PubMed] [Google Scholar]
- Kfoury, N. , Holmes, B. B. , Jiang, H. , Holtzman, D. M. , & Diamond, M. I. (2012). Trans‐cellular propagation of Tau aggregation by fibrillar species. Journal of Biological Chemistry, 287(23), 19440–19451. 10.1074/jbc.M112.346072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierdorf, K. , & Prinz, M. (2013). Factors regulating microglia activation. Frontiers in Cellular Neuroscience, 7, 44. 10.3389/fncel.2013.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M. , Suh, J. , Romano, D. , Truong, M. H. , Mullin, K. , Hooli, B. , Norton, D. , Tesco, G. , Elliott, K. , Wagner, S. L. , Moir, R. D. , Becker, K. D. , & Tanzi, R. E. (2009). Potential late‐onset Alzheimer's disease‐associated mutations in the ADAM10 gene attenuate {alpha}‐secretase activity. Human Molecular Genetics, 18(20), 3987–3996. 10.1093/hmg/ddp323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberly, W. T. , LaVoie, M. J. , Ostaszewski, B. L. , Ye, W. , Wolfe, M. S. , & Selkoe, D. J. (2003). Gamma‐secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph‐1, and Pen‐2. Proceedings of the National Academy of Sciences, 100(11), 6382–6387. 10.1073/pnas.1037392100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komura, H. , Kakio, S. , Sasahara, T. , Arai, Y. , Takino, N. , Sato, M. , Satomura, K. , Ohnishi, T. , Nabeshima, Y. I. , Muramatsu, S. I. , Kii, I. , Hoshi, M. , (2019). Alzheimer Abeta Assemblies Accumulate in Excitatory Neurons upon Proteasome Inhibition and Kill Nearby NAKalpha3 Neurons by Secretion. iScience, 13, 452‐477. doi: 10.1016/j.isci.2019.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konzack, S. , Thies, E. , Marx, A. , Mandelkow, E. M. , & Mandelkow, E. (2007). Swimming against the tide: mobility of the microtubule‐associated protein tau in neurons. Journal of Neuroscience, 27(37), 9916–9927. 10.1523/JNEUROSCI.0927-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan, R. , & Ilagan, M. X. (2009). The canonical Notch signaling pathway: unfolding the activation mechanism. Cell, 137(2), 216–233. 10.1016/j.cell.2009.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopke, E. , Tung, Y. C. , Shaikh, S. , Alonso, A. C. , Iqbal, K. , & Grundke‐Iqbal, I. (1993). Microtubule‐associated protein tau. Abnormal phosphorylation of a non‐paired helical filament pool in Alzheimer disease. Journal of Biological Chemistry, 268(32), 24374–24384. [PubMed] [Google Scholar]
- Ksiezak‐Reding, H. , Liu, W. K. , & Yen, S. H. (1992). Phosphate analysis and dephosphorylation of modified tau associated with paired helical filaments. Brain Research, 597(2), 209–219. 10.1016/0006-8993(92)91476-u [DOI] [PubMed] [Google Scholar]
- Ksiezak‐Reding, H. , Pyo, H. K. , Feinstein, B. , & Pasinetti, G. M. (2003). Akt/PKB kinase phosphorylates separately Thr212 and Ser214 of tau protein in vitro. Biochimica et Biophysica Acta (BBA) ‐ Molecular Basis of Disease, 1639(3), 159–168. 10.1016/j.bbadis.2003.09.001 [DOI] [PubMed] [Google Scholar]
- Kuhn, P. H. , Marjaux, E. , Imhof, A. , De Strooper, B. , Haass, C. , & Lichtenthaler, S. F. (2007). Regulated intramembrane proteolysis of the interleukin‐1 receptor II by alpha‐, beta‐, and gamma‐secretase. Journal of Biological Chemistry, 282(16), 11982–11995. 10.1074/jbc.M700356200 [DOI] [PubMed] [Google Scholar]
- LaFerla, F. M. (2002). Calcium dyshomeostasis and intracellular signalling in Alzheimer's disease. Nature Reviews Neuroscience, 3(11), 862–872. 10.1038/nrn960 [DOI] [PubMed] [Google Scholar]
- Lammich, S. , Kojro, E. , Postina, R. , Gilbert, S. , Pfeiffer, R. , Jasionowski, M. , Haass, C. ,& Fahrenholz, F. (1999). Constitutive and regulated alpha‐secretase cleavage of Alzheimer's amyloid precursor protein by a disintegrin metalloprotease. Proceedings of the National Academy of Sciences United States of America, 96(7), 3922–3927. 10.1073/pnas.96.7.3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammich, S. , Okochi, M. , Takeda, M. , Kaether, C. , Capell, A. , Zimmer, A. K. , Edbauer, D. , Walte, J. , Steiner, H. , & Haass, C. (2002). Presenilin‐dependent intramembrane proteolysis of CD44 leads to the liberation of its intracellular domain and the secretion of an Abeta‐like peptide. Journal of Biological Chemistry, 277(47), 44754–44759. 10.1074/jbc.M206872200 [DOI] [PubMed] [Google Scholar]
- Lange‐Dohna, C. , Zeitschel, U. , Gaunitz, F. , Perez‐Polo, J. R. , Bigl, V. , & Roßner, S. (2003). Cloning and expression of the rat BACE1 promoter. Journal of Neuroscience Research, 73(1), 73–80. 10.1002/jnr.10639 [DOI] [PubMed] [Google Scholar]
- Lapresa, R. , Agulla, J. , Sanchez‐Moran, I. , Zamarreno, R. , Prieto, E. , Bolanos, J. P. , & Almeida, A. (2019). Amyloid‐ss promotes neurotoxicity by Cdk5‐induced p53 stabilization. Neuropharmacology, 146, 19–27. 10.1016/j.neuropharm.2018.11.019 [DOI] [PubMed] [Google Scholar]
- Laurent, C. , Dorothée, G. , Hunot, S. , Martin, E. , Monnet, Y. , Duchamp, M. , Dong, Y. , Légeron, F. P. , Leboucher, A. , Burnouf, S. , Faivre, E. , Carvalho, K. , Caillierez, R. , Zommer, N. , Demeyer, D. , Jouy, N. , Sazdovitch, V. , Schraen‐Maschke, S. , Delarasse, C. , Buée, L. , & Blum, D. (2017). Hippocampal T cell infiltration promotes neuroinflammation and cognitive decline in a mouse model of tauopathy. Brain, 140(1), 184–200. 10.1093/brain/aww270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C. Y. , & Landreth, G. E. (2010). The role of microglia in amyloid clearance from the AD brain. Journal of Neural Transmission (Vienna), 117(8), 949–960. 10.1007/s00702-010-0433-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffell, M. S. , Lumsden, L. , & Steiger, W. A. (1985). An analysis of T lymphocyte subpopulations in patients with Alzheimer's disease. Journal of the American Geriatrics Society, 33(1), 4–8. 10.1111/j.1532-5415.1985.tb02851.x [DOI] [PubMed] [Google Scholar]
- Li, N. , Wang, Y. , Forbes, K. , Vignali, K. M. , Heale, B. S. , Saftig, P. , Hartmann, D. , Black, R. A. , Rossi, J. J. , Blobel, C. P. , Dempsey, P. J. , Workman, C. J. , & Vignali, D. A. A. (2007). Metalloproteases regulate T‐cell proliferation and effector function via LAG‐3. EMBO Journal, 26(2), 494–504. 10.1038/sj.emboj.7601520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Cheng, Z. , Zhou, L. , Darmanis, S. , Neff, N. F. , Okamoto, J. , Gulati, G. , Bennett, M. L. , Sun, L. O. , Clarke, L. E. , Marschallinger, J. , Yu, G. , Quake, S. R. , Wyss‐Coray, T. , & Barres, B. A. (2019). Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single‐cell RNA sequencing. Neuron, 101(2), 207–223 e210. 10.1016/j.neuron.2018.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R. , Yang, L. , Lindholm, K. , Konishi, Y. , Yue, X. , Hampel, H. , Zhang, D. & Shen, Y. (2004). Tumor necrosis factor death receptor signaling cascade is required for amyloid‐beta protein‐induced neuron death. Journal of Neuroscience, 24(7), 1760–1771. 10.1523/JNEUROSCI.4580-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler, S. F. , Dominguez, D.I. , Westmeyer, G. G. , Reiss, K. , Haass, C. , Saftig, P. , De Strooper, B. , & Seed, B. (2003). The cell adhesion protein P‐selectin glycoprotein ligand‐1 is a substrate for the aspartyl protease BACE1. Journal of Biological Chemistry, 278(49), 48713–48719. 10.1074/jbc.M303861200 [DOI] [PubMed] [Google Scholar]
- Lindestam Arlehamn, Cecilia. S. , Pham, J. , Alcalay, R. N. , Frazier, A. , Shorr, E. , Carpenter, C. , Sidney, J. , Dhanwani, R. , Agin‐Liebes, J. , Garretti, F. , Amara, A. W. , Standaert, D. G. , Phillips, E. J. , Mallal, S. A. , Peters, B. , Sulzer, D. , & Sette, A. (2019). Widespread Tau‐Specific CD4 T cell reactivity in the general population. Journal of Immunology 203(1), 84–92. 10.4049/jimmunol.1801506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C. C. , Kanekiyo, T. , Xu, H. , & Bu, G. (2013). Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nature Reviews Neurology, 9(2), 106–118. 10.1038/nrneurol.2012.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. , Martin, R. , Kohler, G. , & Chan, C. (2013). Palmitate induces transcriptional regulation of BACE1 and presenilin by STAT3 in neurons mediated by astrocytes. Experimental Neurology, 248, 482–490. 10.1016/j.expneurol.2013.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. J. , Guo, D. W. , Tian, L. , Shang, D. S. , Zhao, W. D. , Li, B. , Fang, W. G. , Zhu, L. , & Chen, Y. H. (2010). Peripheral T cells derived from Alzheimer's disease patients overexpress CXCR2 contributing to its transendothelial migration, which is microglial TNF‐alpha‐dependent. Neurobiology of Aging, 31(2), 175–188. 10.1016/j.neurobiolaging.2008.03.024 [DOI] [PubMed] [Google Scholar]
- Liu, Z. , Huang, Y. , Cao, B. B. , Qiu, Y. H. , & Peng, Y. P. (2017). Th17 Cells Induce Dopaminergic Neuronal Death via LFA‐1/ICAM‐1 Interaction in a Mouse Model of Parkinson's Disease. Molecular Neurobiology, 54(10), 7762–7776. 10.1007/s12035-016-0249-9 [DOI] [PubMed] [Google Scholar]
- Lombardi, V. R. , Fernandez‐Novoa, L. , Etcheverria, I. , Seoane, S. , & Cacabelos, R. (2004). Association between APOE epsilon4 allele and increased expression of CD95 on T cells from patients with Alzheimer's disease. Methods and Findings in Experimental and Clinical Pharmacology, 26(7), 523–529. 10.1358/mf.2004.26.7.863735 [DOI] [PubMed] [Google Scholar]
- Lombardi, V. R.M. , Garcia, M. , Rey, L. , & Cacabelos, R. (1999). Characterization of cytokine production, screening of lymphocyte subset patterns and in vitro apoptosis in healthy and Alzheimer's Disease (AD) individuals. Journal of Neuroimmunology, 97(1–2), 163–171. 10.1016/S0165-5728(99)00046-6 [DOI] [PubMed] [Google Scholar]
- Lue, L. F. , Rydel, R. , Brigham, E. F. , Yang, L. B. , Hampel, H. , Murphy, G. M. Jr , Brachova, L. , Yan, S. D. , Walker, D. G. , Shen, Y. , & Rogers, J. (2001). Inflammatory repertoire of Alzheimer's disease and nondemented elderly microglia in vitro. Glia, 35(1), 72–79. 10.1002/glia.1072 [DOI] [PubMed] [Google Scholar]
- Ma, X. , Reynolds, S. L. , Baker, B. J. , Li, X. , Benveniste, E. N. , & Qin, H. (2010). IL‐17 enhancement of the IL‐6 signaling cascade in astrocytes. The Journal of Immunology, 184(9), 4898–4906. 10.4049/jimmunol.1000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macy, M. , Okano, Y. , Cardin, A. D. , Avila, E. M. , & Harmony, J. A. (1983). Suppression of lymphocyte activation by plasma lipoproteins. Cancer Research, 43(5), 2496s–2502s. [PubMed] [Google Scholar]
- Maekawa, Y. , Ishifune, C. , Tsukumo, S. , Hozumi, K. , Yagita, H. , & Yasutomo, K. (2015). Notch controls the survival of memory CD4+ T cells by regulating glucose uptake. Nature Medicine, 21(1), 55–61. 10.1038/nm.3758 [DOI] [PubMed] [Google Scholar]
- Mahley, R. W. , & Rall, S. C. Jr (2000). Apolipoprotein E: far more than a lipid transport protein. Annual Review of Genomics and Human Genetics, 1, 507–537. 10.1146/annurev.genom.1.1.507 [DOI] [PubMed] [Google Scholar]
- Man, S. M. , Ma, Y. R. , Shang, D. S. , Zhao, W. D. , Li, B. , Guo, D. W. , Fang, W.G. , Zhu, L. , & Chen, Y. H. (2007). Peripheral T cells overexpress MIP‐1alpha to enhance its transendothelial migration in Alzheimer's disease. Neurobiology of Aging, 28(4), 485–496. 10.1016/j.neurobiolaging.2006.02.013 [DOI] [PubMed] [Google Scholar]
- Marco, S. , & Skaper, S. D. (2006). Amyloid beta‐peptide1‐42 alters tight junction protein distribution and expression in brain microvessel endothelial cells. Neuroscience Letters, 401(3), 219–224. 10.1016/j.neulet.2006.03.047 [DOI] [PubMed] [Google Scholar]
- Mathys, H. , Adaikkan, C. , Gao, F. , Young, J. Z. , Manet, E. , Hemberg, M. , De Jager, P. L. , Ransohoff, R. M. , Regev, A. , & Tsai, L. H. (2017). Temporal tracking of microglia activation in neurodegeneration at single‐cell resolution. Cell Reports, 21(2), 366–380. 10.1016/j.celrep.2017.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto, A. , & Fujiwara, Y. (1991). Abnormal and deficient processing of beta‐amyloid precursor protein in familial Alzheimer's disease lymphoblastoid cells. Biochemical and Biophysical Research Communications, 175(2), 361–365. 10.1016/0006-291x(91)91572-t [DOI] [PubMed] [Google Scholar]
- McManus, R. M. , Higgins, S. C. , Mills, K. H. , & Lynch, M. A. (2014). Respiratory infection promotes T cell infiltration and amyloid‐β deposition in APP/PS1 mice. Neurobiology of aging, 35(1), 109–121. 10.1016/j.neurobiolaging.2013.07.025 [DOI] [PubMed] [Google Scholar]
- McQuillan, K. , Lynch, M. A. , & Mills, K. H. (2010). Activation of mixed glia by Abeta‐specific Th1 and Th17 cells and its regulation by Th2 cells. Brain, Behavior, and Immunity, 24(4), 598–607. 10.1016/j.bbi.2010.01.003 [DOI] [PubMed] [Google Scholar]
- Meakin, P. J. , Mezzapesa, A. , Benabou, Eva , Haas, M. E. , Bonardo, B. , Grino, M. , Brunel, J. M. , Desbois‐Mouthon, C. , Biddinger, S. B. , Govers, R. , Ashford, M. L. J. , & Peiretti, F. (2018). The beta secretase BACE1 regulates the expression of insulin receptor in the liver. Nature Communications, 9(1), 1306. 10.1038/s41467-018-03755-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros, R. , & LaFerla, F. M. (2013). Astrocytes: conductors of the Alzheimer disease neuroinflammatory symphony. Experimental Neurology, 239, 133–138. 10.1016/j.expneurol.2012.10.007 [DOI] [PubMed] [Google Scholar]
- Merlini, M. , Kirabali, T. , Kulic, L. , Nitsch, R. M. , & Ferretti, M. T. (2018). Extravascular CD3+ T Cells in Brains of Alzheimer Disease Patients Correlate with Tau but Not with Amyloid Pathology: An Immunohistochemical Study. Neuro‐Degenerative Diseases, 18(1), 49–56. 10.1159/000486200 [DOI] [PubMed] [Google Scholar]
- Mietelska‐Porowska, A. , & Wojda, U. (2017). T Lymphocytes and Inflammatory Mediators in the Interplay between Brain and Blood in Alzheimer's Disease: Potential Pools of New Biomarkers. Journal of Immunology Research, 2017, 1–17. 10.1155/2017/4626540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, A. E. , Neighbour, P. A. , Katzman, R. , Aronson, M. , & Lipkowitz, R. (1981). Immunological studies in senile dementia of the Alzheimer type: evidence for enhanced suppressor cell activity. Annals of Neurology, 10(6), 506–510. 10.1002/ana.410100603 [DOI] [PubMed] [Google Scholar]
- Minter, L. M. , Turley, D. M. , Das, P. , Shin, H. M. , Joshi, I. , Lawlor, R. G. , & Osborne, B. A. (2005). Inhibitors of gamma‐secretase block in vivo and in vitro T helper type 1 polarization by preventing Notch upregulation of Tbx21. Nature Immunology, 6(7), 680–688. [PubMed] [Google Scholar]
- Miscia, S. , Ciccocioppo, F. , Lanuti, P. , Velluto, L. , Bascelli, A. , Pierdomenico, L. , Genovesi, D. , Di Siena, A. , Santavenere, E. , Gambi, F. , Ausili‐Cèfaro, G. , Grimley, P.M. , Marchisio, M. , & Gambi, D. (2009). Abeta(1–42) stimulated T cells express P‐PKC‐delta and P‐PKC‐zeta in Alzheimer disease. Neurobiology of Aging, 30(3), 394–406. 10.1016/j.neurobiolaging.2007.07.011 [DOI] [PubMed] [Google Scholar]
- Mittal, K. , Eremenko, E. , Berner, O. , Elyahu, Y. , Strominger, I. , Apelblat, D. , Nemirovsky, A. , Spiegel, I. , Monsonego, A. (2019). CD4 T Cells Induce A Subset of MHCII‐Expressing Microglia that Attenuates Alzheimer Pathology. iScience, 16, 298‐311. doi: 10.1016/j.isci.2019.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moalem, G. , Leibowitz‐Amit, R. , Yoles, E. , Mor, F. , Cohen, I. R. , & Schwartz, M. (1999). Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nature Medicine, 5(1), 49–55. 10.1038/4734 [DOI] [PubMed] [Google Scholar]
- Moller‐Hackbarth, K. , Dewitz, C. , Schweigert, O. , Trad, A. , Garbers, C. , Rose‐John, S. , & Scheller, J. (2013). A disintegrin and metalloprotease (ADAM) 10 and ADAM17 are major sheddases of T cell immunoglobulin and mucin domain 3 (Tim‐3). Journal of Biological Chemistry, 288(48), 34529–34544. 10.1074/jbc.M113.488478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky, A. V , Krenick, R. , Ullian, E. , Tsai, H. H , Deneen, B. , Richardson, W. D , Barres, B. A , & Rowitch, D. H (2012). Astrocytes and disease: A neurodevelopmental perspective. Genes & Development, 26(9), 891–907. 10.1101/gad.188326.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mönning, U. , König, G. , Banati, R. B , Mechler, H. , Czech, C. , Gehrmann, J. , Schreiter‐Gasser, U. , Masters, C. l. , & Beyreuther, K. (1992). Alzheimer beta A4‐amyloid protein precursor in immunocompetent cells. Journal of Biological Chemistry, 267(33), 23950–23956. 10.1016/S0021-9258(18)35929-5 [DOI] [PubMed] [Google Scholar]
- Monning, U. , Konig, G. , Prior, R. , Mechler, H. , Schreiter‐Gasser, U. , Masters, C. L. , & Beyreuther, K. (1990). Synthesis and secretion of Alzheimer amyloid beta A4 precursor protein by stimulated human peripheral blood leucocytes. FEBS Letters, 277(1–2), 261–266. 10.1016/0014-5793(90)80861-c [DOI] [PubMed] [Google Scholar]
- Monsonego, A. , Imitola, J. , Petrovic, S. , Zota, V. , Nemirovsky, A. , Baron, R. , Fisher, Y. , Owens, T. , & Weiner, H. L. (2006). Abeta‐induced meningoencephalitis is IFN‐gamma‐dependent and is associated with T cell‐dependent clearance of Abeta in a mouse model of Alzheimer's disease. Proceedings of the National Academy Sciences, 103(13), 5048–5053. 10.1073/pnas.0506209103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsonego, A. , Nemirovsky, A. , & Harpaz, I. (2013). CD4 T cells in immunity and immunotherapy of Alzheimer's disease. Immunology, 139(4), 438–446. 10.1111/imm.12103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne, A. , Nation, D. A. , Sagare, A. P. , Barisano, G. , Sweeney, M. D. , Chakhoyan, A. , Pachicano, M. , Joe, E. , Nelson, A. R. , D’Orazio, L. M. , Buennagel, D. P. , Harrington, M. G. , Benzinger, T. L. S. , Fagan, A. M. , Ringman, J. M. , Schneider, L. S. , Morris, J. C. , Reiman, E. M. , Caselli, R. J. , Chui, H. C. , Tcw, J. , Chen, Y. , Pa, J. , Conti, P. S. , Law, M. , Toga, A. W. , & Zlokovic, B. V. (2020). APOE4 leads to blood‐brain barrier dysfunction predicting cognitive decline. Nature, 581(7806), 71–76. 10.1038/s41586-020-2247-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, K. L. (1998). Structure and function of P‐selectin glycoprotein ligand‐1. Leukaemia & Lymphoma, 29(1–2), 1–15. 10.3109/10428199809058377 [DOI] [PubMed] [Google Scholar]
- Morales, I. , Guzman‐Martinez, L. , Cerda‐Troncoso, C. , Farias, G. A. , & Maccioni, R. B. (2014). Neuroinflammation in the pathogenesis of Alzheimer's disease. A rational framework for the search of novel therapeutic approaches. Frontiers Cell Neuroscience, 8, 112. 10.3389/fncel.2014.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales, I. , Jimenez, J. M. , Mancilla, M. , & Maccioni, R. B. (2013). Tau oligomers and fibrils induce activation of microglial cells. Journal of Alzheimer's Disease, 37(4), 849–856. 10.3233/JAD-131843 [DOI] [PubMed] [Google Scholar]
- Multhaup, G. , Huber, O. , Buee, L. , & Galas, M. C. (2015). Amyloid precursor protein (APP) metabolites APP intracellular fragment (AICD), Abeta42, and Tau in nuclear roles. Journal of Biological Chemistry, 290(39), 23515–23522. 10.1074/jbc.R115.677211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundt, S. , Mrdjen, D. , Utz, S. G. , Greter, M. , Schreiner, B. , & Becher, B. (2019). Conventional DCs sample and present myelin antigens in the healthy CNS and allow parenchymal T cell entry to initiate neuroinflammation. Science Immunology, 4(31), 10.1126/sciimmunol.aau8380 [DOI] [PubMed] [Google Scholar]
- Nath, A. , Hall, E. , Tuzova, M. , Dobbs, M. , Jons, M. , Anderson, C. , Woodward, J. , Guo, Z. , Fu, W. , Kryscio, R. , Wekstein, D. , Smith, C. , Markesbery, W.R. , & Mattson, M. P. (2003). Autoantibodies to amyloid beta‐peptide (Abeta) are increased in Alzheimer's disease patients and Abeta antibodies can enhance Abeta neurotoxicity: implications for disease pathogenesis and vaccine development. Neuromolecular Medicine, 3(1), 29–39. [DOI] [PubMed] [Google Scholar]
- Niikura, T. , Tajima, H. , & Kita, Y. (2006). Neuronal cell death in Alzheimer's disease and a neuroprotective factor, humanin. Current Neuropharmacology, 4(2), 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsch, R. , Pohl, E. E. , Smorodchenko, A. , Infante‐Duarte, C. , Aktas, O. , & Zipp, F. (2004). Direct impact of T cells on neurons revealed by two‐photon microscopy in living brain tissue. Journal of Neuroscience, 24(10), 2458–2464. 10.1523/JNEUROSCI.4703-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberstein, T. J. , Taha, L. , Spitzer, P. , Hellstern, J. , Herrmann, M. , Kornhuber, J. , & Maler, J. M. (2018). Imbalance of circulating Th17 and regulatory T cells in Alzheimer's Disease: A case control study. Frontiers in Immunology, 9, 1213. 10.3389/fimmu.2018.01213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaga, T. , Miele, L. , Golde, T. E. , & Osborne, B. A. (2003). TCR‐mediated Notch signaling regulates proliferation and IFN‐gamma production in peripheral T cells. Journal of Immunology, 171(6), 3019–3024. 10.4049/jimmunol.171.6.3019 [DOI] [PubMed] [Google Scholar]
- Paresce, D. M. , Ghosh, R. N. , & Maxfield, F. R. (1996). Microglial cells internalize aggregates of the Alzheimer's disease amyloid beta‐protein via a scavenger receptor. Neuron, 17(3), 553–565. [DOI] [PubMed] [Google Scholar]
- Pasciuto, E. , Burton, O. T. , Roca, C. P. , Lagou, V. , Rajan, W. D. , Theys, T ., Mancuso, R. , Tito, R. Y. , Kouser, L. , Callaerts‐Vegh, Z. , de la Fuente, A. G. , Prezzemolo, T., Mascali, L. G. , Brajic, A. , Whyte, C. E. , Yshii, L. , Martinez‐Muriana, A. , Naughton, M. , Young, A. , Moudra, A. , Lemaitre, P. , Poovathingal, S. , Raes, J. , De Strooper, B. , Fitzgerald, D. C. , Dooley, J. & Liston, A. (2020). Microglia require CD4 T cells to complete the fetal‐to‐adult transition. Cell, 182(3), 625–640 e624. 10.1016/j.cell.2020.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicanò, M. , Bulati, M. , Buffa, S. , Barbagallo, M. , Di Prima, A. , Misiano, G. , Picone, P. , Di Carlo, M. , Nuzzo, D. , Candore, G. , Vasto, S. , Lio, D. , Caruso, Calogero , & Colonna‐Romano, G. (2010). Systemic immune responses in Alzheimer's disease: in vitro mononuclear cell activation and cytokine production. Journal of Alzheimer's Disease, 21(1), 181–192. 10.3233/JAD-2010-091714 [DOI] [PubMed] [Google Scholar]
- Pirttila, T. , Mattinen, S. , & Frey, H. (1992). The decrease of CD8‐positive lymphocytes in Alzheimer's disease. Journal of the Neurological Sciences, 107(2), 160–165. 10.1016/0022-510X(92)90284-R [DOI] [PubMed] [Google Scholar]
- Pui, J. C. , Allman, D. , Xu, L. , DeRocco, S. , Karnell, F. G. , Bakkour, S. , Lee, J. Y. , Kadesch, T. , Hardy, R. R. , Aster, J. C. , & Pear, W. S. (1999). Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity, 11(3), 299–308. 10.1016/S1074-7613(00)80105-3 [DOI] [PubMed] [Google Scholar]
- Qiu, J. H. , Asai, A. , Chi, S. , Saito, N. , Hamada, H. , & Kirino, T. (2000). Proteasome inhibitors induce cytochrome c‐caspase‐3‐like protease‐mediated apoptosis in cultured cortical neurons. Journal of Neuroscience, 20(1), 259–265. 10.1523/JNEUROSCI.20-01-00259.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke, F. , Wilson, A. , Stark, G. , Bauer, M. , van Meerwijk, J. , MacDonald, H. R. , & Aguet, M. (1999). Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity, 10(5), 547–558. 10.1016/S1074-7613(00)80054-0 [DOI] [PubMed] [Google Scholar]
- Ramesh, G. , MacLean, A. G. , & Philipp, M. T. (2013). Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators of Inflammation, 2013, 1–20. 10.1155/2013/480739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveney, B. J. , Oki, S. , Hohjoh, H. , Nakamura, M. , Sato, W. , Murata, M. , & Yamamura, T. (2015). Eomesodermin‐expressing T‐helper cells are essential for chronic neuroinflammation. Nature Communications, 6, 8437. 10.1038/ncomms9437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, J. , Luber‐Narod, J. , Styren, S. D. , & Civin, W. H. (1988). Expression of immune system‐associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer's disease. Neurobiology of Aging, 9(4), 339–349. 10.1016/S0197-4580(88)80079-4 [DOI] [PubMed] [Google Scholar]
- Rothwell, N. (2003). Interleukin‐1 and neuronal injury: mechanisms, modification, and therapeutic potential. Brain, Behavior, and Immunity, 17(3), 152–157. 10.1016/S0889-1591(02)00098-3 [DOI] [PubMed] [Google Scholar]
- Samon, J. B. , Champhekar, A. , Minter, L. M. , Telfer, J. C. , Miele, L. , Fauq, A. , Das, P. , Golde T. E., & Osborne, B. A. (2008). Notch1 and TGFbeta1 cooperatively regulate Foxp3 expression and the maintenance of peripheral regulatory T cells. Blood, 112(5), 1813–1821. 10.1182/blood-2008-03-144980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanan, D. A , Weisgraber, K. H , Russell, S. J , Mahley, R. W , Huang, D. , Saunders, A. , Schmechel, D. , Wisniewski, T. , Frangione, B. , & Roses, A. D (1994). Apolipoprotein E associates with beta amyloid peptide of Alzheimer's disease to form novel monofibrils. Isoform apoE4 associates more efficiently than apoE3. Journal of Clinical Investigation, 94(2), 860–869. 10.1172/JCI117407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksida, T. , Koprivica, I. , Vujičić, M. , Stošić‐Grujičić, S. , Perović, M. , Kanazir, S. , & Stojanović, I. (2018). Impaired IL‐17 Production in Gut‐Residing Immune Cells of 5xFAD Mice with Alzheimer's Disease Pathology. Journal of Alzheimer's disease : JAD, 61(2), 619–630. 10.3233/JAD-170538 [DOI] [PubMed] [Google Scholar]
- Saresella, M. , Calabrese, E. , Marventano, I. , Piancone, F. , Gatti, A. , Alberoni, M. , Nemni, R. , & Clerici, M. (2011). Increased activity of Th‐17 and Th‐9 lymphocytes and a skewing of the post‐thymic differentiation pathway are seen in Alzheimer's disease. Brain, Behavior, and Immunity, 25(3), 539–547. 10.1016/j.bbi.2010.12.004 [DOI] [PubMed] [Google Scholar]
- Schellenberg, G. D. (2012). International Genomics of Alzheimer's Disease Project (IGAP) genome‐wide association study. Alzheimer's & Dementia: the Journal of the Alzheimer's Association, 8(4), P101.
- Schetters, S. T. T. , Gomez‐Nicola, D. , Garcia‐Vallejo, J. J. , & Van Kooyk, Y. (2017). Neuroinflammation: Microglia and T Cells Get Ready to Tango. Frontiers in Immunology, 8, 1905. 10.3389/fimmu.2017.01905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindowski, K. , Eckert, A. , Peters, J. , Gorriz, C. , Schramm, U. , Weinandi, T. , Maurer, K. , Frölich, L. , & Müller, W. E. (2007). Increased T‐cell reactivity and elevated levels of CD8+ memory T‐cells in Alzheimer's disease‐patients and T‐cell hyporeactivity in an Alzheimer's disease‐mouse model: implications for immunotherapy. Neuromolecular Med, 9(4), 340–354. 10.1007/s12017-007-8015-9 [DOI] [PubMed] [Google Scholar]
- Schmechel, D. E , Saunders, A. M , Strittmatter, W. J , Crain, B. J , Hulette, C. M , Joo, S. H , Pericak‐Vance, M. A , Goldgaber, D. , & Roses, A. D (1993). Increased amyloid beta‐peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late‐onset Alzheimer disease. Proceedings of the National Academy of Sciences, 90(20), 9649–9653. 10.1073/pnas.90.20.9649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe, D. J. (2001). Alzheimer's disease: genes, proteins, and therapy. Physiological Reviews, 81(2), 741–766. 10.1152/physrev.2001.81.2.741 [DOI] [PubMed] [Google Scholar]
- Sengupta, A. , Kabat, J. , Novak, M. , Wu, Q. , Grundke‐Iqbal, I. , & Iqbal, K. (1998). Phosphorylation of tau at both Thr 231 and Ser 262 is required for maximal inhibition of its binding to microtubules. Archives of Biochemistry and Biophysics, 357(2), 299–309. 10.1006/abbi.1998.0813 [DOI] [PubMed] [Google Scholar]
- Shalit, F. , Sredni, B. , Brodie, C. , Kott, E. , & Huberman, M. (1995). T lymphocyte subpopulations and activation markers correlate with severity of Alzheimer's disease. Clinical Immunology and Immunopathology, 75(3), 246–250. 10.1006/clin.1995.1078 [DOI] [PubMed] [Google Scholar]
- Shen, Y. , Wang, H. , Sun, Q. , Yao, H. , Keegan, . P. , Mullan, M. , Wilson, J. , Lista, S. , Leyhe, T. , Laske, C.h , Rujescu, D. , Levey, A. , Wallin, A. , Blennow, K. , Li, R. , & Hampel, H. (2018). Increased plasma Beta‐Secretase 1 May predict conversion to Alzheimer's disease dementia in individuals with mild cognitive impairment. Biological Psychiatry, 83(5), 447–455. 10.1016/j.biopsych.2017.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington, R. , Rogaev, E. I , Liang, Y. , Rogaeva, E. A , Levesque, G. , Ikeda, M. , Chi, H. , Lin, C. , Li, G. , Holman, K. , Tsuda, T. , Mar, L. , Foncin, J. F , Bruni, A. C , Montesi, M. P , Sorbi, S. , Rainero, I. , Pinessi, L. , Nee, L. , Chumakov, I. , Pollen, D. , Brookes, A. , Sanseau, P. , Polinsky, R. J , Wasco, W. , Da Silva, H. A R , Haines, J. l , Pericak‐Vance, M. A , Tanzi, R. E , Roses, A. D , Fraser, P. E , Rommens, J. M , & St George‐Hyslop, P. h (1995). Cloning of a gene bearing missense mutations in early‐onset familial Alzheimer's disease. Nature, 375(6534), 754–760. 10.1038/375754a0 [DOI] [PubMed] [Google Scholar]
- Shih Ie, M. , & Wang, T. L. (2007). Notch signaling, gamma‐secretase inhibitors, and cancer therapy. Cancer Research, 67(5), 1879–1882. 10.1158/0008-5472.CAN-06-3958 [DOI] [PubMed] [Google Scholar]
- Shin, H. M. , Minter, L. M. , Cho, O. H. , Gottipati, S. , Fauq, A. H. , Golde, T. E. , Sonenshein, G. E. , & Osborne, B. A. (2006). Notch1 augments NF‐kappaB activity by facilitating its nuclear retention. EMBO Journal, 25(1), 129–138. 10.1038/sj.emboj.7600902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, E. , Prasad, K. M. , Butcher, M. , Dobrian, A. , Kolls, J. K. , Ley, K. , & Galkina, E. (2010). Blockade of interleukin‐17A results in reduced atherosclerosis in apolipoprotein E‐deficient mice. Circulation, 121(15), 1746–1755. 10.1161/CIRCULATIONAHA.109.924886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer, A. , Marxreiter, F. , Krach, F. , Fadler, T. , Grosch, J. , Maroni, M. , Graef, D. , Eberhardt, E. , Riemenschneider, M. J. , Yeo, G. W. , Kohl, Z. , Xiang, W. , Gage, F. H. , Winkler, J. , Prots, I. , & Winner, B. (2018). Th17 lymphocytes induce neuronal cell death in a human iPSC‐based model of Parkinson's disease. Cell Stem Cell, 23(1), 123–131.e126. 10.1016/j.stem.2018.06.015 [DOI] [PubMed] [Google Scholar]
- Sondag, C. M. , & Combs, C. K. (2006). Amyloid precursor protein cross‐linking stimulates beta amyloid production and pro‐inflammatory cytokine release in monocytic lineage cells. Journal of Neurochemistry, 97(2), 449–461. 10.1111/j.1471-4159.2006.03759.x [DOI] [PubMed] [Google Scholar]
- Song, H. , Huang, L. P. , Li, Y. , Liu, C. , Wang, S. , Meng, W. , Wei, S. , Gong, Y. , Liu, X. P. , & Yao, L. H. (2018). Neuroprotective effects of cordycepin inhibit Abeta‐induced apoptosis in hippocampal neurons. Neurotoxicology, 68, 73–80. 10.1016/j.neuro.2018.07.008 [DOI] [PubMed] [Google Scholar]
- Sorbi, S. , Nacmias, B. , Forleo, P. , Piacentini, S. , Sherrington, R. , Rogaev, E. , & Amaducci, L. (1995). Missense mutation of S182 gene in Italian families with early‐onset Alzheimer's disease. Lancet, 346(8972), 439–440. 10.1016/s0140-6736(95)92809-x [DOI] [PubMed] [Google Scholar]
- Speciale, L. , Calabrese, E. , Saresella, M. , Tinelli, C. , Mariani, C. , Sanvito, L. , Longhi, R. , & Ferrante, P. (2007). Lymphocyte subset patterns and cytokine production in Alzheimer's disease patients. Neurobiology of Aging, 28(8), 1163–1169. 10.1016/j.neurobiolaging.2006.05.020 [DOI] [PubMed] [Google Scholar]
- Spillantini, M. G. , & Goedert, M. (2013). Tau pathology and neurodegeneration. The Lancet Neurology, 12(6), 609–622. 10.1016/S1474-4422(13)70090-5 [DOI] [PubMed] [Google Scholar]
- St‐Amour, I. , Bosoi, C. R. , Paré, Isabelle , Ignatius Arokia Doss, P. M. , Rangachari, M. , Hébert, S. S. , Bazin, R. , & Calon, F. (2019). Peripheral adaptive immunity of the triple transgenic mouse model of Alzheimer's disease. Journal of Neuroinflammation, 16(1), 3. 10.1186/s12974-018-1380-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St‐Amour, I. , Pare, I. , Tremblay, C. , Coulombe, K. , Bazin, R. , & Calon, F. (2014). IVIg protects the 3xTg‐AD mouse model of Alzheimer's disease from memory deficit and Abeta pathology. Journal of Neuroinflammation, 11, 54. 10.1186/1742-2094-11-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter, W. J , Weisgraber, K. H , Huang, D. Y , Dong, L. M , Salvesen, G. S , Pericak‐Vance, M. , Schmechel, D. , Saunders, A. M , Goldgaber, D. , & Roses, A. D (1993). Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform‐specific effects and implications for late‐onset Alzheimer disease. Proceedings of the National Academy of Sciences U S A, 90(17), 8098–8102. 10.1073/pnas.90.17.8098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto, I. , Futakawa, S. , Oka, R. , Ogawa, K. , Marth, J. D. , Miyoshi, E. , Taniguchi, N. , Hashimoto, Y. , & Kitazume, S. (2007). Beta‐galactoside alpha2,6‐sialyltransferase I cleavage by BACE1 enhances the sialylation of soluble glycoproteins. A novel regulatory mechanism for alpha2,6‐sialylation. Journal of Biological Chemistry, 282(48), 34896–34903. 10.1074/jbc.M704766200 [DOI] [PubMed] [Google Scholar]
- Sulger, J. , Dumais‐Huber, C. , Zerfass, R. , Henn, F. A. , & Aldenhoff, J. B. (1999). The calcium response of human T lymphocytes is decreased in aging but increased in Alzheimer's dementia. Biological Psychiatry, 45(6), 737–742. 10.1016/S0006-3223(98)00218-2 [DOI] [PubMed] [Google Scholar]
- Tackenberg, C. , & Nitsch, R. M. (2019). The secreted APP ectodomain sAPPalpha, but not sAPPbeta, protects neurons against Abeta oligomer‐induced dendritic spine loss and increased tau phosphorylation. Molecular Brain, 12(1), 27. 10.1186/s13041-019-0447-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, J. , Town, T. , Abdullah, L. , Wu, Y. , Placzek, A. , Small, B. , Kroeger, J. , Crawford, F. , Richards, D. , & Mullan, M. (2002). CD45 isoform alteration in CD4+ T cells as a potential diagnostic marker of Alzheimer's disease. Journal of Neuroimmunology, 132(1–2), 164–172. 10.1016/S0165-5728(02)00309-0 [DOI] [PubMed] [Google Scholar]
- Theendakara, V. , Peters‐Libeu, C. A. , Bredesen, D. E. , & Rao, R. V. (2018). Transcriptional Effects of ApoE4: Relevance to Alzheimer's Disease. Molecular Neurobiology, 55(6), 5243–5254. 10.1007/s12035-017-0757-2 [DOI] [PubMed] [Google Scholar]
- Theendakara, V. , Peters‐Libeu, C. A. , Spilman, P. , Poksay, K. S. , Bredesen, D. E. , & Rao, R. V. (2016). Direct Transcriptional Effects of Apolipoprotein E. Journal of Neuroscience, 36(3), 685–700. 10.1523/JNEUROSCI.3562-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togo, T. , Akiyama, H. , Iseki, E. , Kondo, H. , Ikeda, K. , Kato, M. , Oda, T. , Tsuchiya, K. , & Kosaka, K. (2002). Occurrence of T cells in the brain of Alzheimer's disease and other neurological diseases. Journal of Neuroimmunology, 124(1–2), 83–92. 10.1016/S0165-5728(01)00496-9 [DOI] [PubMed] [Google Scholar]
- Town, T. , Nikolic, V. , & Tan, J. (2005a). The microglial "activation" continuum: from innate to adaptive responses. Journal of Neuroinflammation, 2, 24. 10.1186/1742-2094-2-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town, T. , Tan, J. , Flavell, R. A. , & Mullan, M. (2005b). T‐cells in Alzheimer's disease. NeuroMolecular Medicine, 7(3), 255–264. 10.1385/NMM:7:3:255 [DOI] [PubMed] [Google Scholar]
- Trajkovic, V. , Stosic‐Grujicic, S. , Samardzic, T. , Markovic, M. , Miljkovic, D. , Ramic, Z. , & Stojkovic, M. M. (2001). Interleukin‐17 stimulates inducible nitric oxide synthase activation in rodent astrocytes. Journal of Neuroimmunology, 119(2), 183–191. 10.1016/S0165-5728(01)00391-5 [DOI] [PubMed] [Google Scholar]
- Trieb, K. , Ransmayr, G. , Sgonc, R. , Lassmann, H. , & Grubeck‐Loebenstein, B. (1996). APP peptides stimulate lymphocyte proliferation in normals, but not in patients with Alzheimer's disease. Neurobiology of Aging, 17(4), 541–547. 10.1016/0197-4580(96)00068-1 [DOI] [PubMed] [Google Scholar]
- Umeda, M. , Yoshida, N. , Hisada, R. , Burbano, C. , Orite, S. Y. K. , Kono, M. , Kyttaris, V.C. , Krishfield, S. , Owen, C.A. , & Tsokos, G. C. (2021). ADAM9 enhances Th17 cell differentiation and autoimmunity by activating TGF‐beta1. Proceedings of the National Academy of Science U S A, 118(18), 10.1073/pnas.2023230118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utton, M. A. , Connell, J. , Asuni, A. A. , van Slegtenhorst, M. , Hutton, M. , de Silva, R. , Lees, A. J. , Miller, C. C. J. , & Anderton, B. H. (2002). The slow axonal transport of the microtubule‐associated protein tau and the transport rates of different isoforms and mutants in cultured neurons. Journal of Neuroscience, 22(15), 6394–6400. 10.1523/JNEUROSCI.22-15-06394.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eldik, L. J. , Carrillo, M. C. , Cole, P. E. , Feuerbach, D. , Greenberg, B. D. , Hendrix, J. A. , Kennedy, M. , Kozauer, N. , Margolin, R. A. , Molinuevo, J. L. , Mueller, R. , Ransohoff, R.M. , Wilcock, D.M. , Bain L., & Bales, K. (2016). The roles of inflammation and immune mechanisms in Alzheimer's disease. Alzheimers Dement (N Y), 2(2), 99–109. 10.1016/j.trci.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar, R. (2004). BACE1: the beta‐secretase enzyme in Alzheimer's disease. Journal of Molecular Neuroscience, 23(1–2), 105–114. 10.1385/JMN:23:1-2:105 [DOI] [PubMed] [Google Scholar]
- Vehmas, A. , Lieu, J. , Pardo, C. A. , McArthur, J. C. , & Gartner, S. (2004). Amyloid precursor protein expression in circulating monocytes and brain macrophages from patients with HIV‐associated cognitive impairment. Journal of Neuroimmunology, 157(1–2), 99–110. 10.1016/j.jneuroim.2004.08.035 [DOI] [PubMed] [Google Scholar]
- Vincent, B. , & Smith, J. D. (2001). Astrocytes down‐regulate neuronal beta‐amyloid precursor protein expression and modify its processing in an apolipoprotein E isoform‐specific manner. European Journal of Neuroscience, 14(2), 256–266. [DOI] [PubMed] [Google Scholar]
- von Arnim, C. A. , Kinoshita, A. , Peltan, I. D. , Tangredi, M. M. , Herl, L. , Lee, B. M. , Spoelgen, R. , Hshieh,T. T. , Ranganathan, S. , Battey, F. D. , Liu,C. X. , Bacskai, B. J. , Sever, S. , Irizarry, M. C. , Strickland, D.K. , & Hyman, B. T. (2005). The low density lipoprotein receptor‐related protein (LRP) is a novel beta‐secretase (BACE1) substrate. Journal of Biological Chemistry, 280(18), 17777–17785. 10.1074/jbc.M414248200 [DOI] [PubMed] [Google Scholar]
- Wang, X. , Sun, G. , Feng, T. , Zhang, J. , Huang, X. , Wang, T. , Xie, Z. , Chu, X. , Yang, J. , Wang, H. , Chang, S. , Gong, Y. , Ruan, L. , Zhang, G. , Yan, S. , Lian, W. , Du, C. , Yang, D. , Zhang, Q. , Lin, F. , … Geng, M. (2019). Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids‐shaped neuroinflammation to inhibit Alzheimer's disease progression(Cell research, 29, (10), 787. –803. 10.1038/s41422-019-0216-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, J. , Zheng, M. , Liang, P. , Wei, Y. , Yin, X. , Tang, Y. , & Xue, Y. (2013). Apolipoprotein E and its mimetic peptide suppress Th1 and Th17 responses in experimental autoimmune encephalomyelitis. Neurobiology of Diseases, 56, 59–65. 10.1016/j.yjmcc.2014.02.005, DOI: [DOI] [PubMed] [Google Scholar]
- Willem, M. , Garratt, A. N. , Novak, B. , Citron, M. , Kaufmann, S. , Rittger, A. , DeStrooper, B. , Saftig, P. Birchmeier, C. & Haass, C. (2006). Control of peripheral nerve myelination by the beta‐secretase BACE1. Science, 314(5799), 664–666. 10.1126/science.1132341 [DOI] [PubMed] [Google Scholar]
- Williams, J. L. , Manivasagam, S. , Smith, B. C. , Sim, . Vollmer, L. L. , Daniels, B. P. , Russell, J. H. , & Klein, R. S. (2020). Astrocyte‐T cell crosstalk regulates region‐specific neuroinflammation. Glia, 68(7), 1361–1374. 10.1002/glia.23783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, A. , MacDonald, H. R. , & Radtke, F. (2001). Notch 1‐deficient common lymphoid precursors adopt a B cell fate in the thymus. Journal of Experimental Medicine, 194(7), 1003–1012. 10.1084/jem.194.7.1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer, I. , Tietz, S. , Nishihara, H. , Deutsch, U. , Sallusto, F. , Gosselet, F. , Lyck, R. , Muller, W. A. , Lassmann, H. , & Engelhardt, B. (2019). PECAM‐1 stabilizes blood‐brain barrier integrity and favors paracellular T‐Cell diapedesis across the blood‐brain barrier during neuroinflammation. Frontiers in Immunology, 10, 711. 10.3389/fimmu.2019.00711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe, M. S. (2019). Substrate recognition and processing by gamma‐secretase. Biochim Biophys Acta Biomembr, 10.1016/j.bbamem.2019.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe, M. S. , Xia, W. , Ostaszewski, B. L. , Diehl, T. S. , Kimberly, W. T. , & Selkoe, D. J. (1999). Two transmembrane aspartates in presenilin‐1 required for presenilin endoproteolysis and gamma‐secretase activity. Nature, 398(6727), 513–517. 10.1038/19077 [DOI] [PubMed] [Google Scholar]
- Wyss‐Coray, T. , & Rogers, J. (2012). Inflammation in Alzheimer disease‐a brief review of the basic science and clinical literature. Cold Spring Harbor Perspectives in Medicine, 2(1), 10.1101/cshperspect.a006346 a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, J. J. , Wang, J. , Tang, T. T. , Chen, J. , Gao, X. L , Yuan, J. , Zhou, Z. H. , Liao, M. Y , Yao, Rui , Yu, X. , Wang, D. , Cheng, Y. , Liao, Y. H. , & Cheng, X. (2010). The Th17/Treg functional imbalance during atherogenesis in ApoE(‐/‐) mice. Cytokine, 49(2), 185–193. 10.1016/j.cyto.2009.09.007 [DOI] [PubMed] [Google Scholar]
- Yan, R. , & Vassar, R. (2014). Targeting the beta secretase BACE1 for Alzheimer's disease therapy. The Lancet Neurology, 13(3), 319–329. 10.1016/S1474-4422(13)70276-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, L. B. , Li, R. , Meri, S. , Rogers, J. , & Shen, Y. (2000). Deficiency of complement defense protein CD59 may contribute to neurodegeneration in Alzheimer's disease. Journal of Neuroscience, 20(20), 7505–7509. 10.1523/JNEUROSCI.20-20-07505.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, L. , Lindholm, K. , Konishi, Y. , Li, R. , & Shen, Y. (2002). Target depletion of distinct tumor necrosis factor receptor subtypes reveals hippocampal neuron death and survival through different signal transduction pathways. Journal of Neuroscience, 22(8), 3025–3032. 10.1523/JNEUROSCI.22-08-03025.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, W. , Bai, Y. , Xiong, Y. , Zhang, J. , Chen, S. , Zheng, X. , Meng, X. , Li, L. , Wang, J. , Xu, C. , Yan, C. , Wang, L. , Chang, C. C. Y. , Chang, T. Y. , Zhang, T. , Zhou, P. , Song, B. L. , Liu, W. , Sun, S. C. , Liu, X. , Li, B. L. , & Xu, C. (2016). Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature, 531(7596), 651–655. 10.1038/nature17412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , He, Z. , Xing, Z. , Zuo, Z. , Yuan, L. , Wu, Y. , Jiang, M. , Qi, F. , & Yao, Z. (2020). Influenza vaccination in early Alzheimer's disease rescues amyloidosis and ameliorates cognitive deficits in APP/PS1 mice by inhibiting regulatory T cells. Journal of neuroinflammation, 17(1), 65. 10.1186/s12974-020-01741-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, H. , Bai, Y. , Zhu, X. , Lin, L. , Zhao, L. , Wu, X. , Buch, S. , Wang, L. , Chao, J. , & Yao, H. (2014). IL‐17A induces MIP‐1alpha expression in primary astrocytes via Src/MAPK/PI3K/NF‐kB pathways: implications for multiple sclerosis. Journal of Neuroimmune Pharmacology: the Official Journal of the Society on NeuroImmune Pharmacology, 9(5), 629–641. 10.1007/s11481-014-9553-1 [DOI] [PubMed] [Google Scholar]
- Yong, V. W. , Moumdjian, R. , Yong, F. P. , Ruijs, T. C. , Freedman, M. S. , Cashman, N. , & Antel, J. P. (1991). Gamma‐interferon promotes proliferation of adult human astrocytes in vitro and reactive gliosis in the adult mouse brain in vivo. Proceedings of the National Academy of Science, 88(16), 7016–7020. 10.1073/pnas.88.16.7016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- York, A. G. , Williams, K. J. , Argus, J. P. , Zhou, Q. D. , Brar, G. , Vergnes, L. , Gray, E. E. , Zhen, A. , Wu, N. C. , Yamada, D. H. , Cunningham, C. R. , Tarling, E. J. , Wilks, M. Q. , Casero, D. , Gray, D. H. , Yu, A. K. , Wang, E. S. , Brooks, D. G. , Sun, R. , Kitchen, S. G , Wu, T. T. , Reue, K. , Stetson, D. B , & Bensinger, S. J. (2015).Limiting Cholesterol Biosynthetic Flux Spontaneously Engages Type I IFN Signaling. Cell, 163(7), 1716–1729. 10.1016/j.cell.2015.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama, Y. , Lee, V. M. , & Trojanowski, J. Q. (2013). Therapeutic strategies for tau mediated neurodegeneration. Journal of Neurology, Neurosurgery and Psychiatry, 84(7), 784–795. 10.1136/jnnp-2012-303144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J. , Zhou, X. , Chang, M. , Nakaya, M. , Chang, J. H. , Xiao, Y. , William Lindsey, J. , Dorta‐Estremera, S. , Cao, W. , Zal, A. , Zal, T. , & Sun, S. C. (2015). Regulation of T‐cell activation and migration by the kinase TBK1 during neuroinflammation. Nature Communications, 6, 6074. 10.1038/ncomms7074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel, A. , Muñoz‐Manchado, A. B. , Codeluppi, S. , Lönnerberg, P. , La Manno, G. , Juréus, A. , Marques, S. , Munguba, H. , He, L. , Betsholtz, C. , Rolny, C. , Castelo‐Branco, G. , Hjerling‐Leffler, J. , & Linnarsson, S. (2015). Brain structure. Cell types in the mouse cortex and hippocampus revealed by single‐cell RNA‐seq. Science, 347(6226), 1138–1142. 10.1126/science.aaa1934 [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Ke, K. F. , Liu, Z. , Qiu, Y. H. , & Peng, Y. P. (2013). Th17 cell‐mediated neuroinflammation is involved in neurodegeneration of abeta1‐42‐induced Alzheimer's disease model rats. PLoS One, 8(10), e75786. 10.1371/journal.pone.0075786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. W. , Thompson, R. , Zhang, H. , & Xu, H. (2011). APP processing in Alzheimer's disease. Molecular Brain, 4, 3. 10.1186/1756-6606-4-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, D. , Feng, F. , Zhao, C. , Wu, F. , Ma, C. , Bai, Y. , Guo, J. , & Li, H. (2018). Role of perforin secretion from CD8+ T‐cells in neuronal cytotoxicity in multiple sclerosis. Neurological Research, 40(1), 62–67. 10.1080/01616412.2017.1398371 [DOI] [PubMed] [Google Scholar]
- Ziv, Y. , Ron, N. , Butovsky, O. , Landa, G. , Sudai, E. , Greenberg, N. , Cohen, H. , Kipnis, J. , & Schwartz, M. (2006). Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nature Neuroscience, 9(2), 268–275. 10.1038/nn1629 [DOI] [PubMed] [Google Scholar]
- Zotova, E. , Bharambe, V. , Cheaveau, M. , Morgan, W. , Holmes, C. , Harris, S. , Neal, J. Love, S. Nicoll, J. & Boche, D. (2013). Inflammatory components in human Alzheimer's disease and after active amyloid‐beta42 immunization. Brain, 136(Pt 9), 2677–2696. 10.1093/brain/awt210 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.


