Abstract
Fucoidan derivatives 10–13, whose basic sugar chains are composed of repeating α(1,4)-linked l-fucopyranosyl residues with different sulfation patterns, were designed and systematically synthesized. A structure–activity relationship (SAR) study examined competitive inhibition by thirteen fucoidan derivatives against heparin binding to the SARS-CoV-2 spike (S) protein. The results showed for the first time that 10 exhibited the highest inhibitory activity of the fucoidan derivatives used. The inhibitory activity of 10 was much higher than that of fondaparinux, the reported ligand of SARS-CoV-2 S protein. Furthermore, 10 exhibited inhibitory activities against the binding of heparin with several mutant SARS-CoV-2 S proteins, but was found to not inhibit factor Xa (FXa) activity that could otherwise lead to undesirable anticoagulant activity.
Synthesized fucoidan derivative 10 exhibited inhibitory activities against the binding of heparin with several SARS-Cov-2 spike proteins, but did not inhibit factor Xa activity that could otherwise lead to undesirable anticoagulant activity.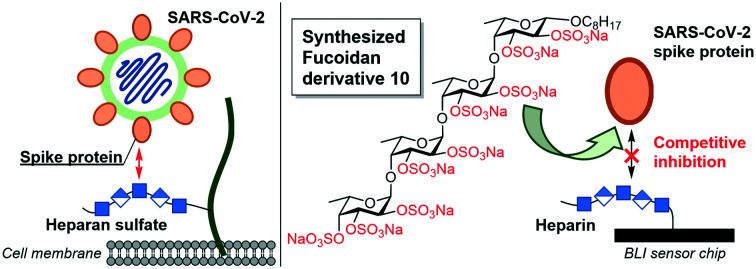
Introduction
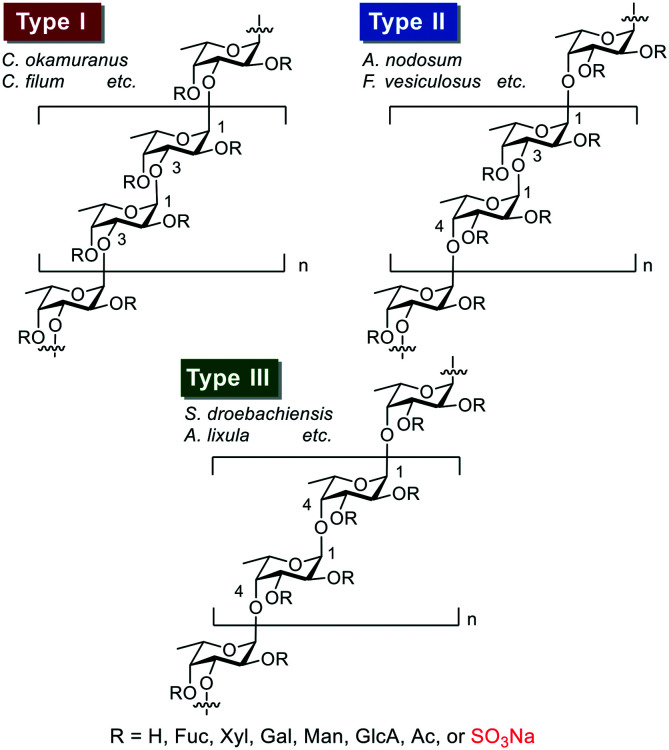
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2), is evolving rapidly worldwide and changing economic and public health conditions. To date, it has infected over 110 million people and caused more than 2.5 million deaths globally,1 prompting rapid vaccine development and distribution and drug discovery research. Various drug candidates in development include agents to interfere with virus attachment to host cells.2 Previous studies suggest that when SARS-CoV-2 enters host cells, SARS-CoV-2 spike (S) protein, consisting of S1 and S2 subunits, interacts with heparan sulfate (HS) on the cell surface through the S1/S2 proteolytic cleavage site. The receptor-binding domain (RBD) in the S1 subunit and/or S2 subunit then facilitates binding to the high affinity receptor angiotensin-converting enzyme 2 (ACE-2) to infect host cells effectively.2,3 Some fucoidans (sulfated polysaccharides derived from seaweed and sea urchin) can reportedly bind to the SARS-CoV-2 S protein and effectively inhibit virus infection.4 Fucoidan is thus a potential anti-SARS-CoV-2 agent, and elucidation of the detailed structure–activity relationship (SAR) for its binding to the SARS-CoV-2 S protein has attracted much attention.5 In general, fucoidans can be classified into the three groups shown in Fig. 1.6 One group (type I) includes the fucoidans isolated from, for example, Cladosiphon okamuranus and Chorda filum, whose basic sugar chains are composed of repeating α(1,3)-linked l-fucopyranosyl residues. The second group (type II) includes fucoidans isolated from, for example, Ascophyllum nodosum and Fucus vesiculosus, whose basic sugar chains are composed of repeating α(1,3)- and α(1,4)-linked l-fucopyranosyl residues. The third group (type III) includes the fucoidans isolated from, for example, Strongylocentrotus droebachiensis and Arbacia lixula, whose basic sugar chains are composed of repeating α(1,4)-linked l-fucopyranosyl residues. However, the structural heterogeneity and ununiformity of the sulfation patterns of naturally occurring fucoidans makes it difficult to conduct detailed SAR studies. We recently systematically synthesized type I and II fucoidan derivatives 1–9 with different sulfation patterns (Fig. 2) and evaluated their apoptosis-inducing activities against human cancer cells and their binding ability to influenza virus hemagglutinin (HA). The results showed that fucoidan derivative 7 possesses apoptosis-inducing activity against breast cancer MCF-7 and cervical epithelioid carcinoma HeLa cells.7 In addition, fucoidan derivative 5 binds to influenza virus HA at the same binding site as the native sialoside ligand, inhibiting influenza virus infection.8 In this context, here we designed and synthesized type III fucoidan derivatives with different sulfation patterns. In addition, we conducted a SAR study of competitive inhibition using thirteen fucoidan derivatives against heparin binding to the SARS-CoV-2 S protein.
Fig. 1. Three types (I–III) of homofucose chains in fucoidans.
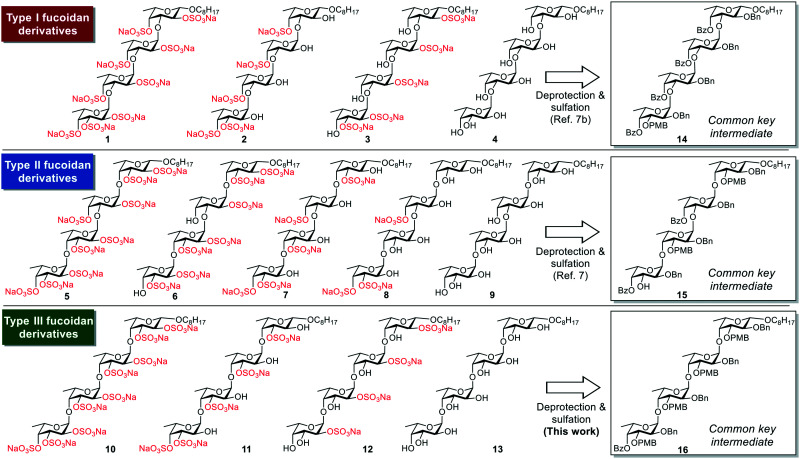
Fig. 2. Chemical structures of the fucoidan derivatives 1–13 and the common key intermediates 14–16.
Results and discussion
Synthesis of type III fucoidan derivatives 10–13
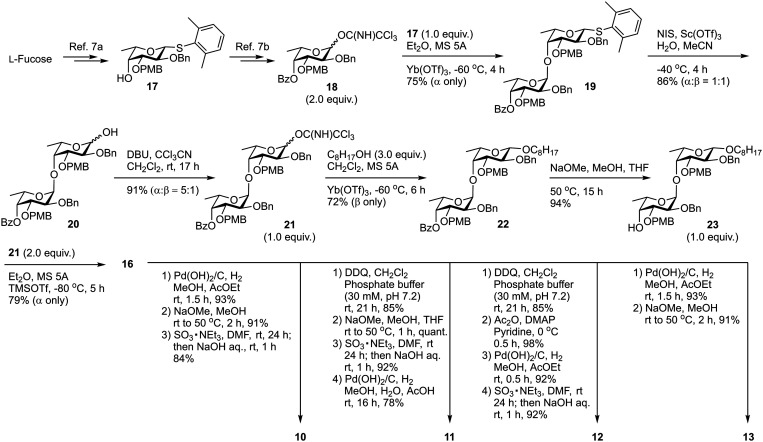
The designed type III fucoidan derivatives 10–13 with different sulfation patterns (2,3-O-sulfated type 10, 3-O-sulfated type 11, 2-O-sulfated type 12 and non-sulfated type 13) are shown in Fig. 2. As shown in Scheme 1, we planned to synthesize 10–13 utilizing common key intermediate 16 with three types of protecting groups (benzyl (Bn), benzoyl (Bz) and p-methoxybenzyl (PMB)) at appropriate positions. Inititially we synthesized 16. Thioglycoside 17 and trichloacetimidate 18 were prepared from l-fucose in 8 and 11 steps, respectively. Chemoselective glycosylation of 17 and 18 using Yb(OTf)3 as an activator at −60 °C gave 19 with complete α-stereoselectivity. Hydrolysis of 19 using NIS/Sc(OTf)3, followed by trichloroacetimidate formation, provided disaccharide donor 21. Coupling reaction of 21 and 1-octanol using Yb(OTf)3 at −60 °C proceeded to give 22 with complete β-stereoselectivity. Deprotection of the Bz group on 22 affored disaccharide acceptor 23 in high yield. Next, we examined the glycosylation of 21 and 23 using TMSOTf in Et2O at −80 °C. The reaction proceeded smoothly to provide the desired key intermediate 16 in 79% yield as a single isomer. We next synthesized the designed type III fucoidan derivatives 10–13 from 16. Deprotection of the Bn and PMB groups on 16 under hydrogenolysis conditions, followed by methanolysis, provided the non-sulfated fucoidan derivative 13. Sulfation of 13 using SO3·NEt3 complex in DMF gave all-sulfated 10. Alternatively, selective deprotection of the PMB groups on 16 using DDQ, followed by acetylation, hydrogenolysis, sulfation using the SO3·NEt3 complex in DMF and saponification, provided 2-O-sulfated tetrasaccharide 12. Deprotection of the PMB groups on 16, followed by methanolysis, sulfation, and hydrogenolysis, afforded the desired 3-O-sulfated tetrafucoside 11.
Scheme 1. Synthetic scheme for 16 and type III fucoidan derivatives 10–13. PMB = p-methoxybenzyl; Et = ethyl; Me = methyl; Bn = benzyl; Bz = benzoyl; Tf = trifluoromethanesulfonyl; NIS = N-iodosuccinimide; MS = molecular sieves; DBU = 1,8-diazabicyclo[5,4,0]undec-7-ene; THF = tetrahydrofuran; TMS = trimethylsilyl; DDQ = 2,3-dichloro-5,6-dicyano-p-benzoquinone; Ac = acetyl; DMF = N,N-dimethylformamide; DMAP = 4-dimethylaminopyridine.
Binding affinity of heparin to SARS-CoV-2 S protein
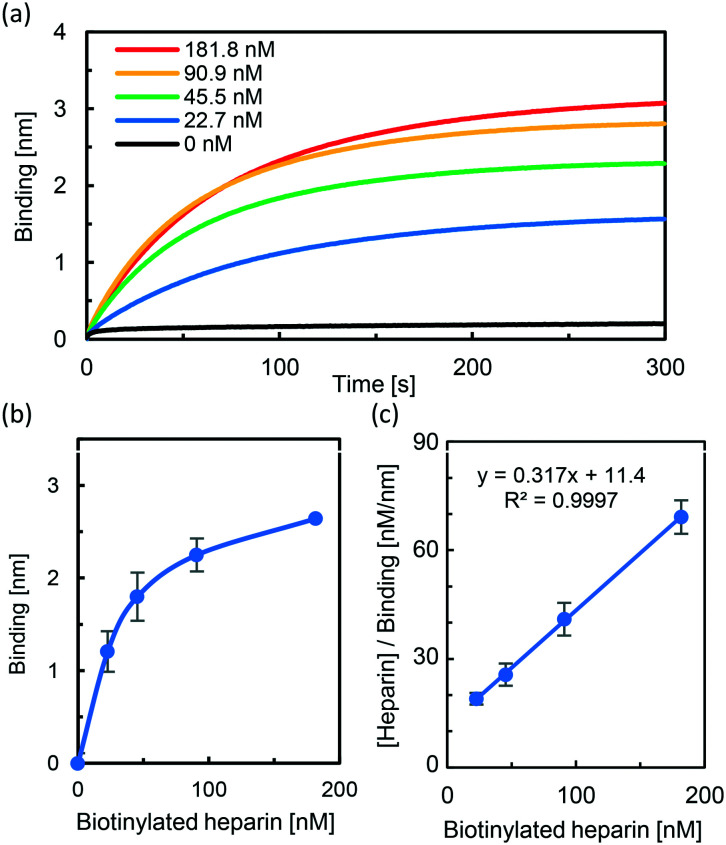
With the thirteen designed fucoidan derivatives in hand, we used bio-layer interferometry (BLI) to study the binding of the fucoidan derivatives to wild type SARS-CoV-2 S protein (S1 and S2). First, the BLI sensor tips functionalized with streptavidin were modified with different concentrations (0, 22.7, 45.5, 90.9, and 181.8 nM) of heparin–biotin. Next, each sensor chip was treated with 1 μM SARS-CoV-2 S protein (S1 and S2) in TBS (20 mM Tris, pH 7.4, containing 0.02% BSA) for 5 min. The corresponding BLI spectra for real-time monitoring of binding between heparin and SARS-CoV-2 S protein are shown in Fig. 3a. The BLI response signal increased in a concentration-dependent manner, and the dissociation constant (Kd) was calculated using the 1 : 1 Langmuir model to be 36.0 nM (Fig. 3b and c), in good agreement with reported data.2b
Fig. 3. Partial BLI sensor-grams for measuring the binding affinities of SARS-CoV-2 S protein to heparin. (a) Biotinylated heparin (0–181.8 nM) was immobilized to streptavidin biosensors which were then exposed to SARS-CoV-2 S protein (1 μM). (b) Plot of the BLI signal of the heparin-dose response of the binding affinity of SARS-CoV-2 S protein. (c) Binding kinetics was analyzed by fitting the 1 : 1 Langmuir model to evaluate the dissociation constant (Kd).
SAR study using 1–13 for competitive inhibition against heparin binding to SARS-CoV-2 S protein
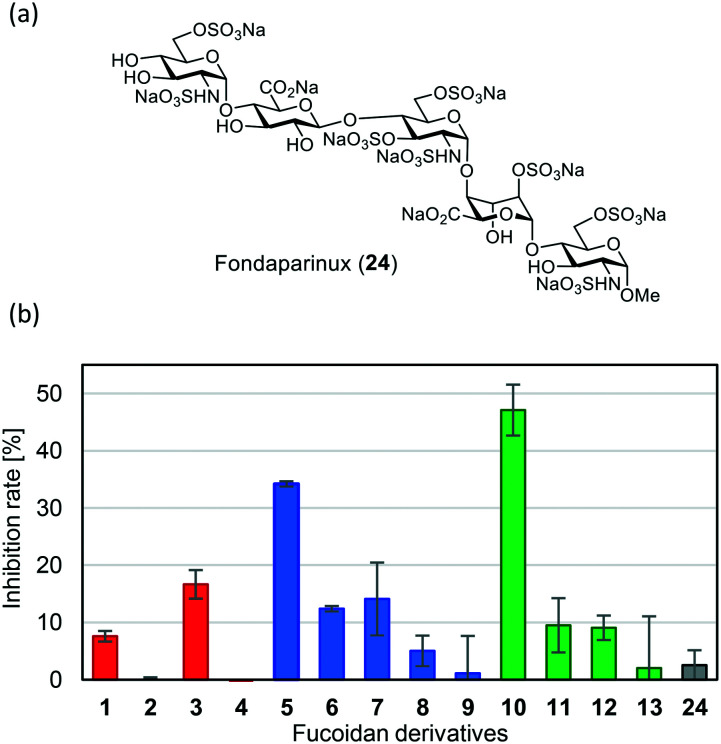
Next, to measure the inhibitory activities of fucoidan derivatives 1–13 for the binding between SARS-CoV-2 S protein and heparin, BLI biosensors with immobilized heparin were dipped into a solution of SARS-CoV-2 S protein (1 μM) and each fucoidan derivative 1–13 (50 μM) in TBS. The FDA-approved heparin drug fondaparinux (24)9 (Fig. 4a), a known binder3c to SARS-CoV-2 S protein, was used as a control. The results are shown in Fig. 4. All-sulfated type 10, with a type III sugar backbone chain, exhibited the highest inhibitory activity, followed by all-sulfated type 5 with a type II sugar backbone chain. Non-sulfated type 4, 9, and 13 showed almost no inhibitory activity. Interestingly, all-sulfated type 1 with a type I sugar backbone chain exhibited low inhibitory activity (less than 10%). These results clearly indicate that slight differences in the sugar backbone chain and in the pattern of sulfate groups in 1 to 13 significantly affect inhibitory activity. Furthermore, the inhibitory activity of 10 was much higher than that of 24.
Fig. 4. (a) Chemical structure of fondaparinux (24). (b) Inhibitory activities of fucoidan derivatives 1–13 and 24 for binding between SARS-CoV-2 S protein and heparin. BLI biosensors with immobilized heparin were dipped into a solution of SARS-CoV-2 S protein (1 μM) and each fucoidan derivative 1–13 and 24 (50 μM) in 0.02% BSA/TBS. Inhibition rate (%) was determined by the decrease in the BLI signal.
Binding affinity of 10 to several SARS-CoV-2 S proteins
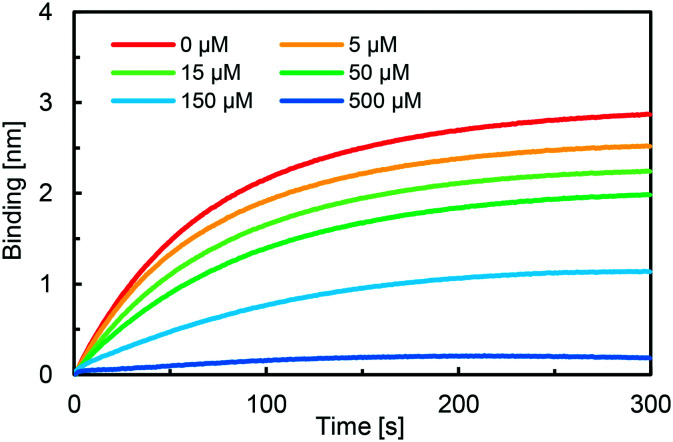
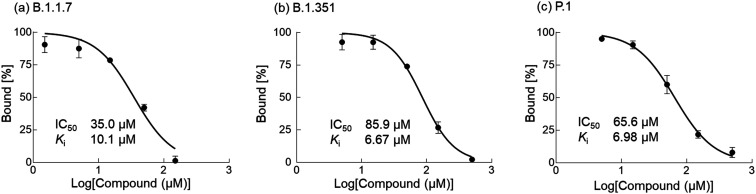
Next, we measured the binding affinity of 10 to SARS-CoV-2 S protein using a competition assay. As shown in Fig. 5, the BLI signal decreased in a dose-dependent manner when the biosensor with immobilized heparin was incubated with a solution of SARS-CoV-2 S protein (1 μM) and different concentrations of 10 (0–500 μM). The IC50 value was calculated by nonlinear regression analysis using GraphPad Prism software to be 86.9 μM. In addition, the Ki value was calculated to be 3.02 μM using the Cheng–Prusoff equation,10 indicating high affinity of compound 10 to SARS-CoV-2 S protein. Next, to show the generality and efficiency of compound 10, the binding affinities of 10 to mutated SARS-CoV-2 S proteins derived from variants of concern (B.1.1.7, B.1.351, and P.1)11 were evaluated by the same competition assay. It was found that 10 effectively bound to the three mutant SARS-CoV-2 S proteins in a dose dependent manner. The IC50 values were 35.0 μM, 85.9 μM and 65.6 μM for B.1.1.7, B.1.351, and P.1, respectively, as shown in Fig. 6, and the Ki values were calculated to be 10.1 μM, 6.67 μM and 6.98 μM, respectively. These results indicate that 10 can effectively inhibit the binding process between mutant SARS-CoV-2 S proteins and surface heparin.
Fig. 5. Partial BLI sensor-grams of the competition assay between 10 and heparin. Biosensors with immobilized heparin were dipped into a solution containing SARS-CoV-2 S protein (1 μM) and 10 (0–500 μM). IC50 was evaluated by the inhibition rate using GraphPad Prism.
Fig. 6. Binding affinities of 10 to the mutant SARS-CoV-2 S proteins (B.1.1.7, B.1.351 and P.1). BLI biosensors with immobilized heparin were dipped into a solution of 10 (1.5–500 μM) and each mutant SARS-CoV-2 S protein (1 μM) in 0.02% BSA/TBS. Bound (%) was determined by the response of the BLI signal. IC50 values were calculated using GraphPad Prism software. Ki values of 10 to the mutant SARS-CoV-2 S proteins were calculated based on the IC50 values according to the Cheng–Prusoff equation.
Anti-factor Xa activities of 10 and 24
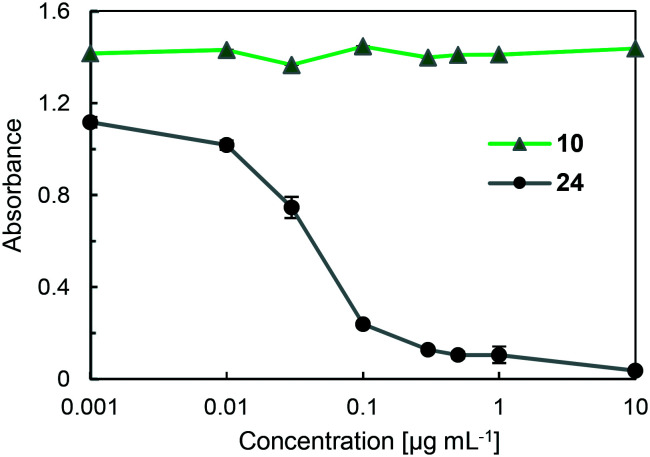
Anti-coagulant activity is a possible and serious side effect of the use of heparin drugs, including 24, because these drugs can bind to antithrombin (AT III) and inactivate factor Xa (FXa), which leads to blood clot formation by converting prothrombin to thrombin through the prothrombinase complex.12 Thus, to show the efficacy of fucoidan derivative 10, the enzyme inhibitiory activity of 10 against FXa was examined using a Fxa-specific chromogenic substrate in Tris-EDTA buffer (50 mM Tris, 175 mM NaCl, 7.5 mM EDTA, 0.1% PEG, 0.9 g L−1 NaN3, pH 8.4). The results are summarized in Fig. 7. When 24 was used as a control compound, significant inhibitory activity was observed in a concentration-dependent manner. In sharp contrast, when compound 10 was used under the same conditions, no inhibitory activity against FXa was observed even at a high concentration (10 μg mL−1), indicating that compound 10 is a promising candidate as an effective SARS-CoV-2 entry inhibitor targeting SARS-CoV-2 S protein.
Fig. 7. Anti-FXa activities of 10 and fondaparinux (24) (0.001–10 μg mL−1) in the presence of ATIII using an FXa specific chromogenic substrate in Tris-EDTA buffer. The absorbance was measured at 405 nm on a plate reader. The results are expressed as means ± the standard deviation (n = 3).
Conclusions
In conclusion, purpose-designed fucoidan derivatives 10–13 possessing a type III sugar backbone chain with different sulfation patterns were systematically synthesized. A SAR study of competitive inhibition by fucoidan derivatives 1–13 against heparin binding to the SARS-CoV-2 S protein showed for the first time that 10 exhibited the highest inhibitory activity of the compounds synthesized, and the inhibitory activity of 10 was much higher than that of the known binder fondaparinux (24). Furthermore, 10 exhibited significant inhibitory activities for binding not only between heparin and wild type SARS-CoV-2 S protein, but also the three mutant SARS-CoV-2 S proteins of the variants of concern B.1.1.7, B.1.351 and P.1. Moreover, 10 did not inhibit FXa activity that could lead to undesirable anticoagulant activity. These results are expected to contribute to the development of effective new SARS-CoV-2 entry inhibitors targeting SARS-CoV-2 S protein.
Author contributions
DT and KT conceived and directed the project. TK, AS, SK, SK and YK performed the chemical syntheses. TK, AS and TK performed the binding experiments. IA and TW prepared the mutant SARS-CoV-2 S proteins. The first draft of this manuscript was prepared by TK and DT, and the final draft was edited by all the authors.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This research was supported in part by JSPS KAKENHI Grant Number JP19H02724 in Scientific Research (B), JST CREST Grant Number JPMJCR20R3, and Keio Gijuku Academic Development Funds.
Electronic supplementary information (ESI) available. See DOI: 10.1039/d1md00264c
Notes and references
- World Health Organization, Coronavirus disease (COVID-19) situation reports, WHO, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (2021) [Google Scholar]
- (a) Dimitrov D. S. Nat. Rev. Microbiol. 2004;2:109. doi: 10.1038/nrmicro817. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu L. Chopra P. Li X. Bouwman K. M. Tompkins S. M. Wolfert M. A. de Vries R. P. Boons G.-J. ACS Cent. Sci. 2021;7:1009. doi: 10.1021/acscentsci.1c00010. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hu B. Guo H. Zhou P. Shi Z. Nat. Rev. Microbiol. 2021;19:141. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Kim S. Y. Jin W. Sood A. Montgomery D. W. Grant O. C. Fuster M. M. Fu L. Dordick J. S. Woods R. J. Zhang F. Linhardt R. J. Antiviral Res. 2020;181:104873. doi: 10.1016/j.antiviral.2020.104873. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Clausen T. M. Sandoval D. R. Spliid C. B. Pihl J. Perrett H. R. Painter C. D. Narayanan A. Majowicz S. A. Kwong E. M. McVicar R. N. Thacker B. E. Glass C. A. Yang Z. Torres J. L. Golden G. J. Bartels P. L. Porell R. N. Garretson A. F. Laubach L. Feldman J. Yin X. Pu Y. Hauser B. M. Caradonna T. M. Kellman B. P. Martino C. Gordts P. L. M. S. Chanda S. K. Schmidt A. G. Godula K. Leibel S. L. Jose J. Corbett K. D. Ward A. B. Carlin A. F. Esko J. D. Cell. 2020;183:1043. doi: 10.1016/j.cell.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hao W. Ma B. Li Z. Wang X. Gao X. Li Y. Qin B. Shang S. Cui S. Tan Z. Sci. Bull. 2021;66:1205. doi: 10.1016/j.scib.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Kwon P. S. Oh H. Kwon S.-J. Jin W. Zhang F. Fraser K. Hong J. J. Linhardt R. J. Dordick J. S. Cell Discovery. 2020;6:50. doi: 10.1038/s41421-020-00192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tandon R. Sharp J. S. Zhang F. Pomin V. H. Ashpole N. M. Mitra D. McCandless M. G. Jin W. Liu H. Sharma P. Linhardt R. J. J. Virol. 2021;95:e01987. doi: 10.1128/JVI.01987-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W. Zhang W. Mitra D. McCandless M. G. Sharma P. Tandon R. Zhang F. Linhardt R. J. Int. J. Biol. Macromol. 2020;163:1649. doi: 10.1016/j.ijbiomac.2020.09.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Cumashi A. Ushakova N. A. Preobrazhenskaya M. E. D'Incecco A. Piccoli A. Totani L. Tinari N. Morozevich G. E. Berman A. E. Bilan M. I. Usov A. I. Ustyuzhanina N. E. Grachev A. A. Sanderson C. J. Kelly M. Rabinovich G. A. Iacobelli S. Nifantiev N. E. Glycobiology. 2007;17:541. doi: 10.1093/glycob/cwm014. [DOI] [PubMed] [Google Scholar]; (b) Pomin V. H. Mouraõ P. A. S. Glycobiology. 2008;18:1016. doi: 10.1093/glycob/cwn085. [DOI] [PubMed] [Google Scholar]
- (a) Arafuka S. Koshiba N. Takahashi D. Toshima K. Chem. Commun. 2014;50:9831. doi: 10.1039/C4CC03544E. [DOI] [PubMed] [Google Scholar]; (b) Kasai A. Arafuka S. Koshiba N. Takahashi D. Toshima K. Org. Biomol. Chem. 2015;13:10556. doi: 10.1039/C5OB01634G. [DOI] [PubMed] [Google Scholar]
- Kosono S. Kasai A. Komaba S. Matsubara T. Sato T. Takahashi D. Toshima K. Chem. Commun. 2018;54:7467. doi: 10.1039/C8CC03865A. [DOI] [PubMed] [Google Scholar]
- Bauer K. A. Hawkins D. W. Peters P. C. Petitou M. Herbert J. M. van Boeckel C. A. A. Meuleman D. G. Cardiovasc. Drug Rev. 2002;20:37. doi: 10.1111/j.1527-3466.2002.tb00081.x. [DOI] [PubMed] [Google Scholar]
- Cheng Y. Prusoff W. H. Biochem. Pharmacol. 1973;22:3099. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Harvey W. T. Carabelli A. M. Jackson B. Gupta R. K. Thomson E. C. Harrison E. M. Ludden C. Reeve R. Rambaut A. COVID-19 Genomiccs UK (COG-UK) Consortium Peacock S. J. Rovertson D. L. Nat. Rev. Microbiol. 2021;19:409. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boeckel C. A. A. Petitou M. Angew. Chem., Int. Ed. Engl. 1993;32:1671. doi: 10.1002/anie.199316713. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.