SUMMARY
About half of the world’s population and 80% of the world’s biodiversity can be found in the tropics. Many diseases are specific to the tropics, with at least 41 diseases caused by endemic bacteria, viruses, parasites, and fungi. Such diseases are of increasing concern, as the geographic range of tropical diseases is expanding due to climate change, urbanization, change in agricultural practices, deforestation, and loss of biodiversity. While traditional medicines have been used for centuries in the treatment of tropical diseases, the active natural compounds within these medicines remain largely unknown. In this review, we describe infectious diseases specific to the tropics, including their causative pathogens, modes of transmission, recent major outbreaks, and geographic locations. We further review current treatments for these tropical diseases, carefully consider the biodiscovery potential of the tropical biome, and discuss a range of technologies being used for drug development from natural resources. We provide a list of natural products with antimicrobial activity, detailing the source organisms and their effectiveness as treatment. We discuss how technological advancements, such as next-generation sequencing, are driving high-throughput natural product screening pipelines to identify compounds with therapeutic properties. This review demonstrates the impact natural products from the vast tropical biome have in the treatment of tropical infectious diseases and how high-throughput technical capacity will accelerate this discovery process.
KEYWORDS: drug development, infectious disease, microbiology, natural products, tropics
INTRODUCTION
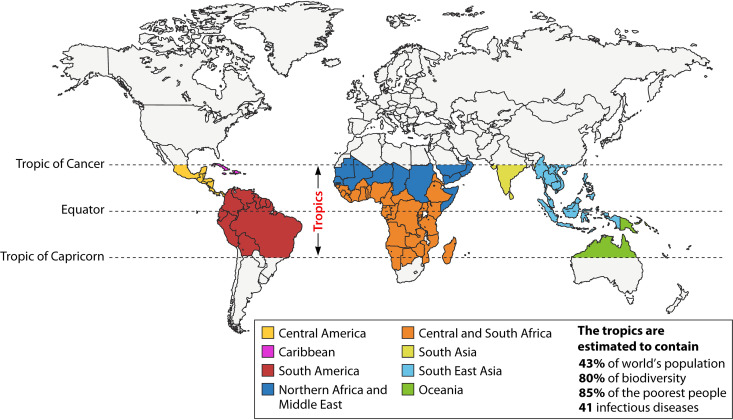
The tropics occupy a large area of the Earth’s landmass from the Tropic of Cancer to the Tropic of Capricorn (Fig. 1). Tropical diseases are caused by a wide variety of pathogens, including bacteria, viruses, parasites, and fungi, that spread through various modes of transmission. The WHO defines 41 different tropical diseases, of which 21 are classified as neglected tropical diseases (NTDs) (Table 1). Traditional medicines have been used for centuries for the treatment of tropical diseases (1). Products of plants such as Cinchona and Artemisia are effectively used even today for the treatment of malaria (2). Plants are a promising source of traditional medicines, as many plants are safe with few side effects even when taken orally for prolonged periods. The long history of screening plant species by humans over millennia has led to deep-rooted knowledge of many plants that are beneficial when used correctly. Plants are also affordable and generally do not require cold-chain storage (3). The WHO has established its Traditional Medicine Strategy, which has guidelines for the assessment of herbal medicines (4). However, the active compounds of many such medicines have not been identified. Despite the encouraging identification of the neuropathic pain drug ω-conotoxin from the marine snail Conus magus in 1999 (5), the majority of plant and animal products have not yet been systematically investigated.
FIG 1.
World map showing tropical regions. The geographical area between the Tropic of Cancer and the Tropic of Capricorn defines the tropics and occupies a large area of the Earth’s landmass and oceans. The tropics span 5 continents and are home to 43% of the world’s population, 80% of the biodiversity, 85% of the poorest people, and 41 infectious diseases. (Adapted from reference 243 with permission of the publisher.)
TABLE 1.
Tropical infectious diseases
| Organism type | Disease | NTDa | Pathogen(s) | Transmission | Location(s) of ongoing/recent major outbreaksb | Reference |
|---|---|---|---|---|---|---|
| Bacterium | Buruli ulcer (Bairnsdale ulcer, Daintree ulcer) | * | Mycobacterium ulcerans | Unclear (evidence for cutaneous contamination from infected aquatic insects, Naucoris spp. and Dyplonychus spp., and bite of infected mosquitoes) | NA | 27 |
| Cholera | Vibrio cholerae | Ingestion of contaminated food/water | 2018: Algeria, Niger, Zimbabwe 2017: DRC, Kenya, Mozambique, Somalia, Zambia 2016: Yemen 2015: DRC, Iraq, Tanzania |
15 | ||
| Leprosy (Hansen's disease) | * | Mycobacterium leprae | Unclear (evidence for contamination through skin-to-skin contact with an infected individual and inhalation of contaminated droplets) | NA | 26 | |
| Melioidosis | Burkholderia pseudomallei | Ingestion, inhalation of contaminated dust/water Contact with contaminated soil |
NA | 244 | ||
| Mycetoma (actinomycetoma) | * | Streptomyces somaliensis, Nocardia brasiliensis, Nocardia otitidiscaviarum, Actinomadura madurae, Actinomadura pelletieri, Pleurostomophora ochracea | Contact of epithelia with contaminated soil or water | NA | 245 | |
| Trachoma | * | Chlamydia trachomatis | Direct or indirect (shared towels and clothes, flies) contact with eye or nose discharge of an infected individual | NA | 246 | |
| Tuberculosis | Mycobacterium tuberculosis | Inhalation of contaminated droplets | NA | 12 | ||
| Yaws | * | Treponema pallidum pertenue | Skin-to-skin contact with an infected individual | 2019: Ghana 2017: Cameroon |
NA | |
| African trypanosomiasis (African sleeping sickness) | * | Trypanosoma brucei gambiense, Trypanosoma brucei rhodesiense | Bite of an infected tsetse fly (Glossina spp.) | NA | 22 | |
| American trypanosomiasis (Chagas disease) | * | Trypanosoma cruzi | Bite of infected triatomine bug (Triatoma spp., Rhodnius spp.) Ingestion of contaminated food In utero transmission |
NA | 19 | |
| Dracunculiasis (Guinea worm disease) | * | Dracunculus medinensis | Ingestion of water contaminated with infected copepods | 2020: Ethiopia 2019: Angola, Cameroon, Chad 2018: Angola, Chad, RSS 2017: Chad, Ethiopia 2016: Chad, Ethiopia, RSS 2015: Chad, Ethiopia, Mali, RSS |
NA | |
| Echinococcosis | * | Echinococcus granulosus, E. multilocularis, E. vogeli, E. oligarthrus | Ingestion of contaminated food/water/soil | NA | 16 | |
| Parasite | Foodborne trematodiases | * | Liver flukes (Clonorchis sinensis, Fasciola gigantica, F. hepatica, Opisthorchis felineus, O. viverrini) Lung flukes (Paragonimus spp.) Intestinal flukes (Echinostoma spp., Fasciolopsis buski) |
Ingestion of contaminated food | NA | 247 |
| Leishmaniasis | * | Leishmania spp. | Bite of an infected sand fly (Phlebotomus spp., Lutzomyia spp.) | 2019: Kenya 2018: Libya 2017: Kenya |
248 | |
| Lymphatic filariasis | * | Wuchereria bancrofti, Brugia malayi, B. timori | Bite of an infected female mosquito (Anopheles spp.) | NA | NA | |
| Malaria | Plasmodium falciparum, P. vivax, P. ovale, P. knowlesi, P. malariae | Bite of an infected mosquito (Anopheles spp., Culex spp., Aedes spp., Mansonia spp.) | 2020: Vanuatu, Zimbabwe 2019: Burundi (249), Sudan 2018: Brazil 2017: Cape Verde, Costa Rica |
20 | ||
| Onchocerciasis | * | Onchocerca volvulus | Bite of an infected black fly (Simulium spp.) | NA | NA | |
| Scabies | * | Sarcoptes scabiei var. hominis | Skin-to-skin contact with an infected individual | NA | 13 | |
| Schistosomiasis | * | Schistosoma mansoni, S. japonicum, S. mekongi, S. guineensis, S. haematobium | Contact with contaminated water | NA | 250 | |
| Soil-transmitted helminth (STH) infections | * | Ancylostoma duodenale, Necator americanus, Ascaris lumbricoides, Trichuris trichiura | Contact with contaminated soil | NA | NA | |
| Strongyloidiasis | Strongyloides stercoralis | Contact with contaminated soil | NA | 251 | ||
| Taeniasis, cysticercosis | * | Taenia solium, T. saginita, T. asiatica | Ingestion of raw or undercooked infected beef or pork meat | NA | 252 | |
| Virus | Chikungunya | * | Chikungunya virus (CHIKV) | Bite of an infected female mosquito (Aedes spp.) | 2019: DRC 2018: Sudan 2017: Italy, Kenya, France 2016: Argentina, Kenya 2015: Senegal |
21 |
| Crimean-Congo hemorrhagic fever | Crimean-Congo hemorrhagic fever virus (CCHFV) | Bite of an infected tick (Hyalomma spp.) Contact with body fluids/tissues of infected livestock Contact with body fluids of an infected individual |
2020: Mali | 17 | ||
| Dengue | * | Dengue virus (DENV) | Bite of an infected female mosquito (Aedes aegypti) | 2020: Chile, Costa Rica, Indonesia, Peru, Singapore 2019: Afghanistan, Bangladesh (253), French Polynesia (254), Jamaica, Mayotte, Pakistan, Sudan 2018: Réunion Island 2017: Burkina Faso, Côte d’Ivoire, Sri Lanka, Sudan 2016: Uruguay 2015: Egypt |
18 | |
| Ebola hemorrhagic fever | Ebola virus (EBOV) | Contact with an infected animal (e.g., fruit bat or nonhuman primate) Contact with body fluids of an EBOV-infected individual or dead body Contact with contaminated objects (e.g., clothes, bedding, needles, and medical equipment) Sexual transmission from semen of men who have recovered from EBOV infection |
2020: DRC 2019: Uganda 2018: DRC 2017: DRC 2013–2016: West Africac |
14 | ||
| Japanese encephalitis | Japanese encephalitis virus (JEV) | Bites of an infected mosquito (Aedes spp., Anopheles spp., Culex spp., Mansonia spp.) | NA | 255 | ||
| Lassa fever | Lassa virus | Contact with urine or feces of Mastomys natalensis rats (handling rats, eating contaminated food, touching contaminated household items, transepithelial contamination) Contact with body fluids of an infected individual |
2020: Nigeria 2019: Nigeria 2018: Nigeria 2017: Nigeria 2016: Benin, Nigeria |
NA | ||
| Marburg hemorrhagic fever | Marburg virus (MARV) | Contact with an infected fruit bat (Rousettus aegyptiacus) Contact with body fluids of an infected individual |
2017: Uganda | 256 | ||
| Rabies | * | Rabies virus | Transcutaneous contamination with saliva of infected animals (e.g., bats, dogs) | 2016: Bhutan (257) | 258 | |
| Rift Valley fever | Rift Valley fever virus (RVFV) | Bite of an infected mosquito (Anopheles spp., Culex spp., Aedes spp., Mansonia spp.) Contact with body fluids/tissues of infected livestock |
2018: Kenya, Mayotte 2016: Niger 2015: Mauritania (259) |
260 | ||
| Tick-borne encephalitis | Tick-borne encephalitis virus (TBEV) | Bite of an infected tick (Ixodidae spp.) Ingestion of raw milk from infected goats/sheep/cows |
NA | 261 | ||
| West Nile fever | West Nile virus (WNV) | Bites of an infected mosquito (Culex spp.) Contact with body fluids/tissues of infected animals In utero transmission |
NA | 262 | ||
| Yellow fever | Yellow fever virus (YFV) | Bite of an infected female mosquito (Aedes spp., Haemogogus spp.) | 2020: Ethiopia, Uganda 2019: Mali 2018: Ethiopia, RSS 2017: Brazil, Nigeria 2016: Angola, Brazil, DRC, Uganda |
263 | ||
| Zika | Zika virus (ZIKV) | Bite of an infected female mosquito (Aedes spp.) In utero transmission Contact with genital fluids of an infected individual |
2015: Region of the Americas and othersd | 23 | ||
| Fungus | Chromoblastomycosis | * | Phialophora verrucosa, Fonsecaea pedrosoi, F. compacta, Cladophialophora carrionii, Rhinocladiella aquaspersa | Unclear (evidence for transcutaneous contamination) | NA | 264 |
| Lobomycosis (lacaziosis) | Lacazia loboi | Unclear (evidence for transcutaneous contamination) | NA | 25 | ||
| Paracoccidioidomycosis | Paracoccidioides brasiliensis | Inhalation of spores | 2015: Brazil (35) | 265 | ||
| Mycetoma (eumycetoma) | * | Madurella mycetomatis, Curvularia lunata, Falciformispora senegalensis, Falciformispora thompkinsii, Trematosphaeria grisea, Exophiala jeanselmei, Medicopsis romeroi, Acremonium spp., Fusarium spp., Neotestudina rosatii, Aspergillus nidulans, A. flavus, Microsporum ferrugineum, M. audouinii, M. langeronii, Scedosporium apiospermum, S. boydii | Contact of epithelia with contaminated soil or water | NA | 245 | |
| Rhinosporidiosis | Rhinosporidium seeberi | Unclear (evidence for transepithelial contamination following contact with stagnant water) | NA | 24 | ||
| Sporotrichosis | Sporothrix schenckii | Transcutaneous inoculation from contaminated plant matter and infected cats Inhalation or ingestion of spores |
NA | 266 | ||
| Talaromycosis (penicilliosis marneffei) | Talaromyces marneffei | Transcutaneous inoculation from contaminated plant matter Inhalation or ingestion of spores |
NA | 267 |
NTD, neglected tropical disease (indicated by asterisk).
Recent and ongoing major outbreaks reported at https://www.cdc.gov, https://www.afro.who.int (WHO Regional Office for Africa), https://www.emro.who.int (WHO Regional Office for the Eastern Mediterranean), https://www.paho.org (WHO Regional Office for the Americas), and https://www.who.int, unless otherwise stated, over the last 5 years. The date indicates the start of the outbreak. DRC, Democratic Republic of the Congo; RSS, Republic of South Sudan; NA, not applicable.
West Africa includes Guinea, Liberia, Mali, Nigeria, Senegal, and Sierra Leone.
Region of the Americas and others includes Bolivia, Brazil, Cape Verde, Caribbean Islands (Aruba, Barbados, Bonaire, Cuba, Curaçao, Dominica, Dominican Republic, Grenadines, Guadeloupe, Haiti, Jamaica, Martinique Island, Puerto Rico, Saint Lucia, Saint Martin, Saint Vincent, Trinidad and Tobago, U.S. Virgin Islands), Chile, Colombia, Costa Rica, Ecuador, El Salvador, French Guiana, Guatemala, Guyana, Honduras, Maldives, Mexico, Nicaragua, Panama, Paraguay, Peru, Suriname, Venezuela, and Vietnam.
Natural products often possess a high degree of bioavailability in comparison to their synthetic counterparts (6). Therefore, it is surprising that not more natural product-based drug candidates have been identified. It is important to reflect upon this, given the recent technical advances used for the screening of natural products. Typically, it takes about 10 years and US$300 million to US$500 million in research and development (R&D) expenditure for a new product to be released into the market (7). Therefore, many pharmaceutical companies are unenthusiastic about developing drugs for tropical diseases that are primarily targeted to emerging economies of low- and middle-income countries. However, recent technological advances have made the process time efficient and cost-effective, providing an unprecedented opportunity for researchers and pharmaceutical companies to identify novel bioactive leads for commercialization (6, 8). About 50% of known plant species are thought to originate in the tropics, and one-third of those used in R&D are found in rainforests; therefore, an important opportunity also awaits developing economies of the tropics. With increasing pressure from climate change and deforestation on biodiversity, it is important for developing nations to consider both the protection of intellectual property rights of traditional knowledge holders and the overall conservation and sustainable use of medicinal plants (6). Pragmatic ways that provide access to modern health care while incorporating these considerations are urgently needed.
In this review, we describe tropical infectious diseases, the pathogens causing them, their modes of transmission, recent major outbreaks, and their geographic locations. We further detail current preventative and therapeutic treatments for tropical diseases, including any commercially licensed vaccines and promising vaccine candidates under investigation. We discuss a range of new technologies that are used for natural product discovery and drug development from natural resources focusing on high-throughput screens (HTS) and omics technologies. Finally, we discuss both approved natural products and molecules used to treat tropical diseases and additional natural products possessing antimicrobial activity with treatment potential. We hope our review will revitalize interest in natural products and drug discovery and encourage more researchers and companies to utilize recent technological advancements made.
INFECTIOUS DISEASES OF THE TROPICS
The WHO defines tropical diseases as all diseases that occur solely or principally in the tropics (Fig. 1) (9). However, this umbrella term often also includes any infectious disease that occurs in hot and humid climate. Tropical diseases are an enormous public health burden, with an estimated 1 billion people affected by at least one tropical disease, representing a significant impact on the health of people living in the tropical and subtropical regions of the world (10) (Fig. 1). Tropical diseases, including neglected tropical diseases (NTDs), are caused by a wide variety of pathogens, including bacteria, viruses, fungi, and parasites (Table 1). NTDs receive less attention from the scientific community and stakeholders of the developed countries than other tropical diseases (11). To address this shortcoming, multiple local and global nonprofit organizations such as Mission to Save the Helpless (MITOSATH, Nigeria) and the Drugs for Neglected Diseases initiative (DNDi, Switzerland) have been established to improve the health and enhance the quality of life of people affected by NTDs. The tropical diseases encompassed by these definitions are changing, with the WHO recently updating their NTD portfolio to include mycetoma, chromoblastomycosis, and scabies (69th World Health Assembly, 2016; 10th Meeting of the Strategic and Technical Advisory Group for Neglected Tropical Diseases, 2017).
Tropical diseases spread through various modes of transmission (Table 1). They can be transmitted via direct or indirect contact with infected individuals through bodily fluids or surfaces (e.g., yaws, scabies, Ebola), as well as by the inhalation of contaminated airborne droplets (e.g., tuberculosis [TB]) (12–14). Transmission may also occur by ingesting contaminated food and/or water (e.g., cholera and echinococcosis) in unsanitary environments, which persist in many tropical and subtropical countries today (15, 16). Many viral and parasitic tropical diseases are vector-borne, with transmission occurring through the bite of infected vectors, including hemipterans (Chagas disease), flies (e.g., African trypanosomiasis, leishmaniasis, onchocerciasis), mosquitoes (e.g., lymphatic filariasis, malaria, chikungunya, and dengue) and ticks (Crimean-Congo hemorrhagic fever) (17–22), among others. In utero transmission has also been reported for tropical diseases such as Zika virus and Chagas disease (19, 23). While the transmission mode is known for most tropical diseases, it remains unknown for Buruli ulcer and leprosy (both listed in the WHO NTD portfolio) as well as for some fungal infections, including chromoblastomycosis, lobomycosis, and rhinosporidiosis (24–27).
Many tropical diseases have recently been or are currently responsible for major outbreaks (e.g., dracunculiasis, leishmaniasis, malaria, chikungunya, dengue, Ebola, yellow fever, and paracoccidioidomycosis) (Table 1). Although most of these outbreaks occur within the tropics, some have occurred in countries with more temperate climates. For example, France and Italy have reported outbreaks of autochthonous chikungunya in 2015 and 2016, respectively (28, 29). Similarly, locally acquired cases of Crimean-Congo hemorrhagic fever were reported in Spain in 2016 and 2018 (30). More recently, in 2019, France reported its first locally acquired case of Zika virus, which is also believed to be the first case recorded in Europe (31). While the number of total cases in each instance was relatively small (8 chikungunya cases in France, 436 chikungunya cases in Italy, 2 Crimean-Congo hemorrhagic fever cases in Spain, and 3 Zika cases in France), it illustrated the potential for such diseases in temperate climates. Such outbreaks outside the tropics highlight the potential risk for tropical diseases to spread globally; of particular concern are some of the vector-borne tropical diseases for which the competent vectors, including mosquitos, ticks, tsetse flies, and triatome bugs, are widely distributed around the world (28–32). Further, climate change, urbanization, change in agricultural practices, deforestation, and loss of biodiversity have all been implicated in increasing the potential spread of tropical diseases (33–36).
Immunization and treatment options differ widely across tropical diseases (Table 2). Currently, commercially licensed vaccines are available for only 8 of the 41 tropical diseases (cholera, TB, dengue, Ebola, Japanese encephalitis, rabies, tick-borne encephalitis, and yellow fever) (37–43), with licensing differing between countries. Many vaccine candidates are under investigation (Table 2) both for tropical diseases without any available licensed vaccine and for diseases with a current vaccine, such as TB. Indeed, the only available TB vaccine, bacillus Calmette-Guérin (Mycobacterium bovis BCG), provides partial protection in children but diminishes over time and is insufficient against pulmonary TB in adults (44). Although curative and/or symptomatic treatments are available for most tropical diseases, their practical efficacy remains challenged by a variety of technical, economic, and biological limitations (Table 2). With the exception of the WHO/UNICEF oral rehydration solution developed to treat cholera, the treatment of tropical diseases often relies on drugs that require strict storage conditions (45, 46). A cold chain is often unreliable or nonexistent for the tropical and subtropical regions, compromising the stability and treatment efficacy of the drugs. Additionally, the treatment of many tropical diseases may be negatively impacted by a lack of qualified health workers in the local community. For example, early intravenous injection is crucial in the treatment of many diseases (Table 2). Furthermore, access to treatment can also be impeded by the relatively high costs associated with effective drugs. For onchocerciasis and lymphatic filariasis, this economic hurdle has been overcome by the creation of the Mectizan (ivermectin) donation program (47). Finally, the global rise in antibiotic, antiparasitic, and antifungal resistance also represents a major threat to the successful treatment and management of tropical diseases (48). Unfortunately, for some tropical diseases such as dracunculiasis, lobomycosis, and rhinosporidiosis, there is currently no treatment or vaccine available, and physical extraction of the pathogens or surgical excision remains the only available option (Table 2). New treatment options are urgently needed, with discoveries from natural product platforms showing potential for the treatment and management of many tropical diseases.
TABLE 2.
Tropical infectious diseases: current treatments and vaccinesa
| Organism type | Disease | Current treatment(s) | Commercially licensed vaccine(s)b | Vaccine candidates under investigation [reference(s)] |
|---|---|---|---|---|
| Bacterium | Buruli ulcer (Bairnsdale ulcer, Daintree ulcer) | Antibiotics: rifampin, clarithromycin, streptomycin Symptomatic treatment: wound care, lymphedema management, skin grafting, physiotherapy (27) Disadvantages: Older patients may suffer from hearing loss, dizziness, and imbalance. |
NA | e.g., MUL_3720 and Hsp18-based vaccines (268–271) |
| Cholera | Moderate dehydration: oral administration of WHO/UNICEF oral rehydration solution Severe dehydration: intravenous administration of rehydration fluids plus antibiotic treatment Symptomatic treatment: zinc therapy for children <5 years (45, 46) Disadvantages: Antibiotics can cause nausea and vomiting and should not be given to patients with only some or no diarrhea. |
Two types of licensed Vibrio cholera vaccines are commercially available: inactivated (Shanchol [Shantha Biotec]; Euvichol-Plus [Eubiologics]; Dukoral [SBL Vaccines]) and live attenuated (VaxChora [Emergent Biosolutions]) (37). | e.g., Dukoal, Shanechol, MORC-Vax (272) | |
| Leprosy (Hansen's disease) | Paucibacillary leprosy—antibiotics: rifampin, dapsone Multibacillary leprosy—antibiotics: rifampin, clofazimine, dapsone (273) Disadvantages: Antibiotics have to be taken for longer duration with follow up every 6 months for 10 years. Multidrug therapy does not provide cure in all cases of leprosy. |
NA | e.g., Th1-biasing adjuvant formulation; glucopyranosyl lipid adjuvant in stable emulsion (GLA-SE, LepVax) (274–276) | |
| Melioidosis | Acute phase (10–14 days)—antibiotics: intravenous administration of ceftazidime or meropenem Elimination phase (3–6 months)—antibiotics: oral administration of SMX-THT or amoxicillin-clavulanic acid (277, 278) Disadvantages: Single-drug antibiotic therapy is only partially effective. Combined antibiotic therapy must be used for extended periods. SMX-THT resistance reported in Thai isolates. |
NA | e.g., purN mutant (ΔpurN) (279, 280) | |
| Mycetoma (actinomycetoma) | Antibiotics: amikacin, rifampin, SMX-THT, amoxicillin-clavulanate, imipenem, gentamicin, doxycycline (245) Disadvantages: Less effective and with many side effects, and the patients should be followed closely to assess them clinically and biochemically. |
NA | e.g., epitope-based vaccine FFKEHGVPL (281, 282) | |
| Trachoma | Antibiotics: azithromycin, doxycycline, erythromycin, levoflaxin, ofloxacin Symptomatic treatment: surgery (246, 283) Disadvantages: It can take decades to evaluate the desired primary end point of trachoma treatment after the start of the intervention. Trials suggest merely a lowering of the risk, not a cure. |
NA | e.g., subunit Chlamydia vaccine (C. muridarum recombinant MOMP [rMOMP], native trimeric conformation [nMOMP]) (284, 285) | |
| Tuberculosis | Antibiotic treatment of M. tuberculosis infection varies depending on infection form (i.e., active or latent infection), antibiotic resistance (i.e., drug-resistant or multidrug-resistant infection), infected individuals (e.g., pregnant women, children), and coinfection status (e.g., HIV infection). Isoniazid, rifampin, rifapentine, pyrazinamide, and ethambutol are some of the main antibiotics currently used (12) Disadvantages: Multidrug-resistant tuberculosis (MDR-TB) is resistant to both isoniazid (INH) and rifampin (RFP). These antibiotics have many side effects, including gastrointestinal disturbance, psychiatric disorder, arthralgia, dermatological effects, ototoxicity, nephrotoxicity, peripheral neuropathy, hypothyroidism, and epileptic seizures. |
The BCG vaccine (live attenuated Mycobacterium bovis strain) is the only commercially licensed TB vaccine. | e.g., protein-subunit vaccine M72/AS01E, live attenuated VPM1002, MTBVAC (12, 44, 286, 287) | |
| Yaws | Antibiotic: azithromycin Alternative antibiotics: benzathine penicillin, doxycycline (288) Disadvantages: Painful during deep i.m. injection of antibiotics, allergy to penicillin, structural and logistic problems related to treatment. |
NA | Single-dose azithromycin for the treatment of yaws. (NIH, U.S. National Library of Medicine, ClinicalTrials.gov.) | |
| Parasite | African trypanosomiasis (African sleeping sickness) |
T. brucei gambiense—antiparasitics: pentamidine, eflornithine, NECT, melarsoprol, fexinidazole T. brucei rhodesiense—antiparasitics: suramin, melarsoprol (289) Disadvantages: Very toxic, prevalence in impoverished regions of Africa places economic constraints, small number of expensive drugs with limited efficacy and serious side effects and which are difficult to administer. |
NA | e.g., invariant surface glycoproteins (ISGs), conserved variant surface glycoprotein (VSG) (290, 291) |
| American trypanosomiasis (Chagas disease) | Antiparasitics: benznidazole, nifurtimox (292) Disadvantages: Significant side effects, efficacy decreases with length of the infection; treatment success difficult to measure; can take years before patients become seronegative (average, 16 years). |
NA | e.g., recombinant proteins (Tc24, TSA-1 with Th1 adjuvant) (293, 294) | |
| Dracunculiasis (Guinea worm disease) | No commercially licensed antiparasitic drug to treat Dracunculus medinensis infection Physical extraction |
NA | NA | |
| Echinococcosis | Antiparasitics: mebendazole, albendazole, praziquantel Symptomatic treatment: surgery or PAIR (percutaneous aspiration, injection of chemicals, and reaspiration) (295) Disadvantages: Gold standard methods to determine efficacy of medical treatment, biological status, effective dose not available. No standardized diagnostic and monitoring methods for long-term follow-up. Treatment outcomes improve when surgery is combined with drugs; timing of chemotherapy pre/postsurgery unclear. |
NA | e.g., epitope-based vaccine (A5YTY7, A0A068WVL6) (296) | |
| Foodborne trematodiases | Antiparasitics: pranziquantel, triclabendazole, nitazoxanide Disadvantages: Few drugs are available, and therefore potential for emerging drug resistance is high. Reliable tests to detect parasites are not available; potential for misdiagnosis and incorrect treatment is high. |
NA | e.g., recombinant Ov-TSP-2 and −3, C. sinensis CsTP 22.3 kDa (297, 298) | |
| Leishmaniasis | Antiparasitics: sodium stibogluconate, pentavalent antimonials, amphotericin B, paromomycin, miltefosine Disadvantages: 60% of patients unresponsive, drug resistance common, combination therapy required, intramuscular or intravenous injections per day for 20–28 days lead to toxicity, drug efficacy compromised due to parenteral route of administration. |
NA | e.g., ChAd63-KH (299, 300) | |
| Lymphatic filariasis | Antiparasitics: diethylcarbamazine, ivermectin, albendazole, doxycycline Disadvantages: Temporarily clear microfilariae but not adult worms; where filariasis coexists with Loa loa, neurologic decline and encephalopathy are causes for concern. |
NA | e.g., thioredoxin peroxidase (TPX), collagen 4 (Col4) (301–303) | |
| Malaria | Antiparasitic treatment of Plasmodium species infection varies depending on two main factors: severity status (i.e., uncomplicated, severe, cerebral) and parasite species. Atovaquone and proguanil, artemether and lumefantrine, quinine sulfate and doxycycline, mefloquine, chloroquine phosphate, primaquine phosphate, and hydroxychloroquine are some of the main antiparasitics currently used (304). Disadvantages: Rampant drug resistance, questionable safety of antimalarials, side effects such as headache, dyspepsia, diarrhea, etc. Limited data are available on their efficacy in treatment of drug-resistant and non-falciparum strains. Difficult to achieve required drug concentration in infants. |
N/A | e.g., PfSPZ vaccine, chimpanzee adenovirus serotype 63 (ChAd63) (305) | |
| Onchocerciasis (river blindness) | Antiparasitics: ivermectin, moxidectin (306) Disadvantages: Questions remain if drugs can eliminate disease in areas of very high endemicity and loiasis coendemicity, due to severe reactions in people with Loa loa microfilaremia. Drug-resistant parasites are emerging following many years of treatment. Safe dose in children not determined. |
NA | e.g., recombinant proteins—Ov-103 and Ov-RAL-2 (307) | |
| Scabies | Antiparasitics: ivermectin, permethrin, crotamiton (13) Disadvantages: Neurotoxicity has been reported in children with widespread skin damage. Potential for emergence of drug resistance. Harmful effects on health and environment. Reinfection and recrudescence are common. |
NA | e.g., recombinant Sarcoptes scabiei chitinase-like protein 5 (rSsCLP5-based) vaccine (308, 309) | |
| Schistosomiasis | Antiparasitic: praziquantel (250) Disadvantages: Treatment does not prevent transmission or reinfection in areas of endemicity, as it is ineffective against juvenile parasites; prevalence will decrease only if more than 70% of the community participates; growing concerns regarding resistance, chemical residues, and cost. |
NA | e.g., recombinant Sh28GST/Alhydrogel (310–313) | |
| STH infections | Antiparasitic: albendazole or mebendazole Disadvantages: Increasing drug resistance, treatment often followed by rapid reinfection. |
NA | e.g., rAc-MTP-1, rAc-16 (314, 315) | |
| Strongyloidiasis | Antiparasitic: ivermectin Disadvantages: Development of drug resistance as parasite remains in the body for a long time, lack of standardization of antihelmintic treatment, toxicity, no test to detect cure currently available. |
NA | e.g., DNA immunization (Sseat-6 gene), Ss-IR (S. stercoralis immune-reactive antigen), srHSP60 (316) | |
| Taeniasis, cysticercosis | Taeniasis antiparasitics: praziquantel, niclosamide Cysticercosis antiparasitics: praziquantel, albendazole Symptomatic treatment: corticosteroids, antiepileptic drugs (neurocysticercosis), surgical extraction (depending on localization of the cysts) (317) Disadvantages: Death of the parasite between the 2nd and 5th day of treatment triggers neurological symptoms and, rarely, can be fatal. Side effects of praziquantel include malaise, headache, dizziness, nausea, fever, bloody diarrhea, etc. Side effects of albendazole include hepatotoxicity, alopecia, headache, nausea, urticaria. |
NA | e.g., recombinant vaccines (TSOL18 and TSOL45) (318, 319) | |
| Virus | Chikungunya | No commercially licensed antiviral drug to treat CHIKV infection Symptomatic treatment: rest, prevention of dehydration, administration of pain relief drugs (acetaminophen or paracetamol) to reduce fever and relieve some symptoms. Aspirin and other nonsteroidal anti-inflammatory drugs can be administered once DENV infection is ruled out (21). Disadvantages: Long-term pain management required for some with recurring joint pain in 20% of patients after 1 year. |
NA | e.g., live attenuated vaccine (TSI-GSD-218), live recombinant vaccine (MV-CHIKV), virus-like-particle vaccine (VRC-CHKVLP059-00-VP) (320, 321) |
| Crimean-Congo hemorrhagic fever | No commercially licensed antiviral drug to treat CCHFV infection Symptomatic treatment: intravenous fluids and electrolyte supplementation, oxygen therapy, coinfection treatment (17) Disadvantages: Requires high-level isolation facilities with proper biocontainment procedures. |
NA | e.g., CCHFV Bulgarian vaccine, CCHFV DNA vaccine (322, 323) | |
| Dengue | No commercially licensed antiviral drug to treat DENV infection Mild infection: treatment of symptoms with pain relief drugs (acetaminophen or paracetamol) to reduce fever and relieve some symptoms Severe infection: supportive hospital therapy (18) Disadvantages: For cases progressing to dengue hemorrhagic fever, patient requires hospitalization and extensive monitoring (recommended 4-h checks) during onset of critical phase. Vaccine requires strict cold-chain storage. |
A licensed live attenuated recombinant DENV vaccine is commercially available: CYD-TDV, Dengvaxia, Sanofi Pasteur (38). | e.g., tetravalent dengue vaccine (CYD-TDV), Sanofi Pasteur’s Dengvaxia (38, 324) | |
| Ebola hemorrhagic fever | No commercially licensed antiviral drug to treat EBOV infection Symptomatic treatment: intravenous fluids and electrolyte supplementation, oxygen therapy, antiemetic drug treatment, antidiarrheal drug treatment, coinfection treatment (14) Disadvantages: Requires high-level isolation facilities with proper biocontainment procedures. Vaccine requires strict cold-chain storage. |
A live attenuated recombinant licensed EBOV vaccine is commercially available: rVSV-ZEBOV, Ervebo, Merck. | e.g., inactivated EBOVΔVP3, Ad5.EBOV GP + Ad5.EBOV NP, Ad5.EBOVGPΔTM + Ad5.EBOV (325, 326) | |
| Japanese encephalitis | No commercially licensed antiviral drug to treat JEV infection Symptomatic infection: supportive hospital therapy, including neurological observation, intravenous fluids and electrolyte supplementation, administration of pain relief drugs to reduce fever and relieve some symptoms, rest (255) Disadvantages: Confirmation of suspected severe cases requires CT/MRI scans, spinal fluid extraction. Inactivated vaccines require 2 doses, and others require cold-chain storage. |
Three types of JEV vaccines are commercially licensed: inactivated (Ixiaro, Valneva Austria GmbH; JE-VAX, Sanofi Pasteur), live attenuated (CD.JEVAX, CDIBP), and recombinant (IMOJEV, Sanofi Pasteur) (39). | NA | |
| Lassa fever | No commercially licensed antiviral drug to treat Lassa virus infection Symptomatic treatment: intravenous fluids and electrolyte supplementation, oxygen therapy, coinfection treatment Disadvantages: Hospitalization required in severe cases. Ribavirin used for treatment in early stages, but it is not available in many regions and is suspected to be toxic and teratogenic. |
NA | e.g., ChAdOx1-Lassa-GP (327) | |
| Marburg hemorrhagic fever | No commercially licensed antiviral drug to treat MARV infection Symptomatic treatment: intravenous fluids and electrolyte supplementation, oxygen therapy, coinfection treatment (256) Disadvantages: Severity of disease require hospitalization in intensive care for all affected. |
NA | e.g., inactivated MARV, VRO-MARV GP, VRO-MARV NP (325, 328, 329) | |
| Rabies | Postexposure prophylaxis (before symptom onset): extensive wound washing, immediate vaccination, and administration of rabies immunoglobulin (if classified as severe exposure) (258) Disadvantages: Vaccine is effective after exposure but not after development of symptoms. Virtually always fatal after symptoms develop. |
A licensed inactivated rabies virus vaccine is commercially available: Rabipur/Rabipor/Rabavert, GSK; Imovax Rabies, Sanofi Pasteur (40, 41). | NA | |
| Rift Valley fever | No commercially licensed antiviral drug to treat RVFV infection Mild and short-duration infection: no specific treatment required, pain relief drugs can be used to reduce fever and relieve some symptoms Severe infections: supportive hospital therapy Disadvantages: Hospitalization required in severe cases, but treatment is generally limited to supportive care. |
NA | e.g., TSI-GSD-200, TSI-GSD-223 (330) | |
| Tick-borne encephalitis | No commercially licensed antiviral drug to treat TBEV infection Symptomatic treatment to treat neurologic symptoms (261) Disadvantages: Severe cases require hospitalization, including tracheal intubation and respiratory support. Vaccines not widely available. |
A licensed inactivated TBEV vaccine is commercially available: Encepur, GSK; TICOVAC/FSME-IMMUN, Pfizer; EnceVir, NPO Microgen (42). | NA | |
| West Nile fever | No commercially licensed antiviral drug to treat WNV infection Mild infection: no specific treatment required, pain relief drugs can be used to reduce fever and relieve some symptoms Severe infection: supportive hospital therapy (262) Disadvantages: Hospitalization required in severe cases and can require CT/MRI scans, spinal fluid extraction. |
NA | e.g., Hydrovax-001, ChimaeriVax-WN02, rWN/DEN4Δ30 (331–334) | |
| Yellow fever | No commercially licensed antiviral drug to treat YFV infection Mild infection: rest, dehydration prevention by drinking, administration of pain relief drugs to reduce fever and relieve some symptoms Severe infection: supportive hospital therapy Disadvantages: Hospitalization required in severe cases; however, treatment is generally limited to supportive care. Vaccine requires cold-chain storage. |
A licensed live attenuated YFV vaccine is commercially available: YF17D, YF-VAX/Stamaril, Sanofi Pasteur (43) | NA | |
| Zika | No commercially licensed antiviral drug to treat ZIKV infection Mild infection: rest, dehydration prevention by drinking, treatment of symptoms with pain relief drugs (acetaminophen or paracetamol) to reduce fever and relieve some symptoms Severe infection: supportive hospital therapy Disadvantages: Hospitalization required in severe cases. Pregnant women require monthly monitoring for fetal growth. |
NA | e.g., DNA vaccines (VRC5283, VRC5288, GLS5700), mRNA vaccines (mRNA-1325, mRNA-1893) (335, 336) | |
| Fungus | Chromoblastomycosis | Antifungals: itraconazole, thiabendazole, posaconazole, voriconazole, terbinafine, flucytosine, fluconazole, ketoconazole, amphotericin B Symptomatic treatment: heat treatment, cryotherapy, surgery (264) Disadvantages: Amphotericin B targets cholesterol-containing membranes, leading to cellular toxicity in humans. Side effects are significant, and therefore amphotericin B is used only for critically ill patients with serious fungal infections. Side effects of common antifungals include headaches, diarrhea, rash, nausea, and muscle or joint pains. Surgery is not usually recommended, as it is thought to facilitate spread of disease. |
NA | DNA-hsp65 vaccine (337) |
| Lobomycosis (lacaziosis) | No commercially licensed antifungal drug to treat Lacazia loboi infection No standard treatment is available to date; surgical excision and successful treatment protocols have been reported (25, 338, 339) Disadvantages: Recurrence is common after surgery due to contaminated tools or incomplete removal due to difficulty in demarcating the lesion site. Treatment with common antifungals including amphotericin B has been found to be inadequate. |
NA | NA | |
| Mycetoma (eumycetoma) | Antifungals: ketoconazole, itraconazole, voriconazole, posaconazole, terbinafine Symptomatic treatment: surgery, amputation (245) Disadvantages: Long treatment course. Side effects of common antifungals include headaches, diarrhea, rash, nausea, and muscle or joint pains. Amputation causes significant loss of life quality. |
NA | e.g., peptides KYLQ, FEYARKHAF, FFKEHGVPL (282) | |
| Paracoccidioidomycosis | Antifungals: itraconazole, amphotericin B, SMX-THT Supportive hospital therapy for severe infection (265) Disadvantages: Hospitalization required in severe cases. Side effects of common antifungals include headaches, diarrhea, rash, nausea, and muscle or joint pains. |
NA | e.g., antigen gp43, peptide P10 (340–342) | |
| Rhinosporidiosis | Combination of surgical excision and supportive medical therapy (dapsone, amphotericin B) (24) Disadvantages: Recurrence is common after excision. Treatment with amphotericin B results in significant cellular toxicity (as described above). Disease can occasionally be resistant to dapsone and may require combination therapy with other drugs. |
NA | NA | |
| Sporotrichosis | Oral administration of saturated solution of potassium iodide Antifungals: amphotericin B, itraconazole, terbinafine (266) Disadvantages: Potassium iodide treatment typically effective for skin infection only. Side effects of common antifungals include headaches, diarrhea, rash, nausea, and muscle or joint pains. Treatment with amphotericin B results in significant cellular toxicity (as described above). |
NA | e.g., humanized antibody (MAbP6E7) (343) | |
| Talaromycosis (penicilliosis marneffei) | Antifungals: amphotericin B, itraconazole, voriconazole (267) Disadvantages: Side effects of antifungals include headaches, diarrhea, rash, nausea, and muscle or joint pains. Treatment with amphotericin B results in significant cellular toxicity (as described above). |
NA | NA |
BCG, bacillus Calmette-Guérin; CCHFV: Crimean-Congo hemorrhagic fever virus; CHIKV, chikungunya virus; DENV, dengue virus; EBOV, Ebola virus; JEV, Japanese encephalitis virus; MARV, Marburg virus; NECT, nifurtimox-eflornithine combination therapy; RVFV, Rift valley fever virus; SMX-THT, sulfamethoxazole-trimethoprim (cotrimoxazole); STH, soil-transmitted helminth; TBEV, tick-borne encephalitis virus; WNV, West Nile virus; YFV, yellow fever virus; ZIKV, Zika virus; i.m., intramuscular; CT/MRI, computed tomography/magnetic resonance imaging; Th1, T helper 1; NA, not applicable.
Licensing may vary between countries.
NATURAL PRODUCTS AND BIODISCOVERY POTENTIAL OF THE TROPICAL BIOME
Broadly, natural products can be defined as any metabolites produced by living organisms that are largely obtained from plants, animals, and marine and microscopic organisms. Metabolites include primary and secondary metabolites. While primary metabolites such as proteins, carbohydrates, and fats are vital for the growth and development of a living organism, secondary metabolites such as alkaloids, terpenoids, and flavonoids are responsible for its survival and defense against competitors and intruders (49). These natural products are being exploited and manipulated by humans for developing novel drugs (see Drug Development from Natural Resources and Therapeutic Solutions for Infectious Diseases of the Tropics, below). With an estimated 300,000 to 500,000 plant species and ca. 2 million lower classes of organisms, these resources are considered the chemotherapeutic pool, which can be exploited for developing drugs (50).
It is estimated that 50% of known plant species originate in the tropics (Fig. 1), with 14,000 species identified from the Amazon region alone (51). Similarly, the tropical Far North Queensland region of Australia is rich in rainforest (covering 3.6 million ha) and reef biomes, and its Wet Tropics World Heritage Area alone is home to over 2,800 plant species, including 700 endemic species that occur nowhere else on Earth (52). Approximately one-third of the medicinal plants used in the research and development of pharmaceutical drugs are found in rainforests (47). However, only a limited number of tropical plants and animals have been considered for medical uses and therefore provide an unprecedented opportunity for researchers and pharmaceutical companies to identify novel bioactive leads for potential commercialization.
Within the plant kingdom, the focus of pharmaceutical research has been on flowering plants, whereas mangroves and nonflowering plants, such as mosses, ferns, hornworts, cycads, liverworts, and lycopods, remain barely studied for drug development to date and represent an untapped source of novel compounds. Similarly, tropical lichens, fungi, insects, snails, reptiles, spiders, scorpions, and amphibians are not well characterized and are worthy of pharmaceutical exploration.
DRUG DEVELOPMENT FROM NATURAL RESOURCES
Developing drugs from natural sources is a lengthy and tedious endeavor. The biodiscovery pathways include specimen identification and collection, extraction and isolation, identification, and bioactivity testing (53). The most common challenge faced by researchers in translating laboratory discoveries to commercial drugs is access to sufficiently large quantities of biological samples and lead compounds, which is considered a “valley of death.” This bottleneck could be overcome through strategic collaboration between chemists (with expertise in natural products and organic synthetic chemistry), biologists (with expertise in biological processes and sample collection), immunologists (with expertise in cell- and animal-based assays), and bioinformaticians (to develop discovery platforms using large-scale genome sequence mining and shotgun metagenomics) (54).
Strategies for Drug Development from Natural Resources
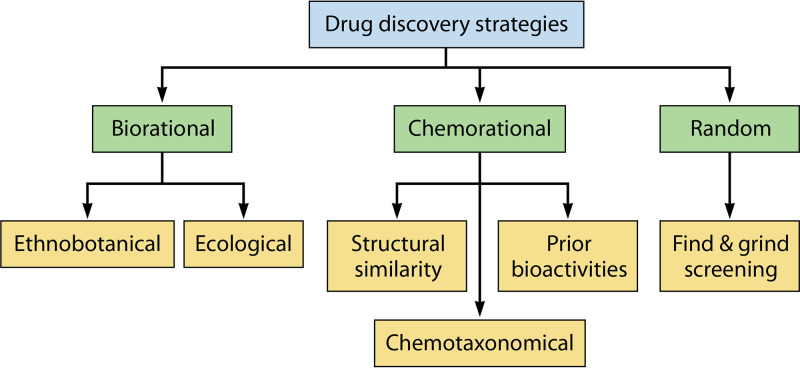
It is important to understand the existing techniques, technologies, expertise, and financial resources within the pharmaceutical field in order to devise an efficient drug discovery strategy (55). Several natural products containing compounds with activity against tropical disease-causing pathogens have been discovered. However, due to the high failure rate and the significant investment required to take a promising raw natural product forward, very few compounds have overcome the bottleneck toward becoming a new standard-of-care treatment for a tropical pathogen. Currently, the most common strategies used for discovering novel drugs from natural resources are (i) the random approach based on a “find and isolate” method, (ii) the biorational approach based on ecological and ethnobotanical methods, and (iii) the chemorational approach based on chemotaxonomical considerations (56) (Fig. 2). The last strategy uses information on plant-specific chemotypes, structural similarity, and reported bioactivities (57) to guide drug screening processes. Of these three strategies, the biorational approach, especially ethnobotany-guided screening, is the most efficient one. For example, 80% of 122 plant-derived drugs were discovered based on an ethnodirected biorational approach (58). This high hit rate of novel drugs or drug leads is mainly attributable to their extended clinical uses in traditional medicines.
FIG 2.
Strategies for searching for novel drugs from natural products. Common strategies for discovering novel drugs from natural resources include random, chemorational, and biorational approaches. The biorational approach relies on ethnobotanically focused screening and ecologically directed screening. The chemorational approach is directed by chemotaxonomical considerations. The random approach relies on high-throughput screening with no prior ethnopharmacological uses or chemotypical rationality.
Techniques for Drug Development from Natural Resources
A range of technologies spanning low to medium throughput has been available for decades, allowing the screening of viable pathogens responsible for tropical diseases. The bioassay screening protocols include in vitro, ex vivo, and in vivo models. For intracellular pathogens (e.g., Trypanosoma cruzi and Plasmodium spp.), cell-based screening methods adapted from conventional mammalian cell monitoring have been developed, such as the WST-1 assay (water-soluble tetrazolium) (59–61) or cell death monitoring with an array of fluorescent probes (62–65). While useful, these assays require careful consideration of the cell types used, as this choice can heavily influence the screening outcomes (66). For larger extracellular pathogens, particularly helminths, techniques are more challenging to develop. Nonetheless, a range of screening techniques have emerged over the past decade with various levels of scalability. These techniques include manual or automated video assessment (67), impedance motility monitoring (68, 69), enzymatic alamarBlue reduction (70), colorimetric (71), fluorescence (72, 73), and lactate or luminescent assays (74, 75).
Screening natural product libraries or raw products for their potential bioactive effect on pathogens can be a daunting task unless a high-throughput screen (HTS) can be developed for the target disease organism. Workflows that incorporate multiwell plates (e.g., 96 or 384 wells) can ideally be handled by robotics to allow for optimal HTS. Challenges arise when developing HTS for larger organisms, such as helminths. Often, the parasite life cycle stage that is key for treating clinical manifestation in humans is challenging to produce in sufficient quantities in the lab for adequate testing. Additionally, the physical size of the worm (millimeters to tens of centimeters long) can make large-scale handling (manual) of the parasite extremely difficult. Therefore, in many studies, the only feasible option for drug screening is to use analogues akin to the target macroscopic organism, such as easily available microscopic larval stages, or related microscopic model organisms, such as Caenorhabditis elegans (76, 77). While this allows simple HTS, the applicability to the desired target needs to be assessed appropriately. The limited applicability of these methods was recently highlighted in a study screening 1,280 compounds, in which neither the hookworm larva or C. elegans models demonstrated high fidelity as analogue models for detecting toxicity against the adult hookworm, the desired target that infects humans (78).
While the gold standard for evaluating antihelminth activity when screening drugs is visual phenotypic assessment of the parasite, the past decade has seen rapid advancement in adapting a range of HTS technologies (79). Some impedance-based methods based on either commercial cell monitoring products such as the xCELLigence system (ACEA Biosciences, Inc.) or custom “in-house” systems designed from the ground up for targeted purposes have been adapted and improved to allow antihelminth activity to be evaluated based on helminth mobility measurement (68, 80). While ultimately applicable to HTS, these methods so far have not been used for natural product screening beyond small research laboratory-based proof-of-principle studies, exploring 10 to 50 products at a time (81–84). Additionally, HTS of drugs against macroscopic disease-causing agents has been taking advantage of the development of advanced automated microscopy (85, 86). The automated imaging of a 12- to 384-well plate(s) allows, for a reasonably low cost, assessment of pathogen viability, and therefore the drug screening can be performed by a simple visualization of the pathogen mobility.
While many of these HTS techniques are commonly used in research laboratories worldwide, pathogens that require biocontainment higher than biosafety level 2 (BSL-2) (e.g., Mycobacterium tuberculosis, rabies, Rift Valley fever virus [RVFV], and West Nile virus [WNV] require BSL-3; Ebola virus [EBOV], Lassa virus, and Marburg virus [MARV] require BSL-4) can be uniquely challenging, especially in the tropics, where the proportion of low-income countries remains relatively high. Advances in modern robotics have made possible the incorporation of such technologies up to BSL-4 biocontainment capacities, allowing HTS of drugs for a range of deadly pathogens, including EBOV (87). However, capability will always be limited, and extensive safety restrictions limit full incorporation of HTS methods. One alternative to phenotypic screening that bypasses the parasite supply or safety limitations is virtual drug design based on protein sequences (88–91); while still a technically challenging and expensive method, it is slowly becoming more readily available with increasing computing power coupled with a decrease in the cost of sequencing technologies.
Omics Technologies for Drug Development from Natural Resources
As critical as the search strategies are, the success of drug discovery and development also relies heavily on the successful adaptation of advanced technologies to the discovery platforms. In many countries, increasingly affordable technological innovation in the areas of genomics, metagenomics, proteomics, and metabolomics have revolutionized the drug discovery programs (53). While genomics-, transcriptomics- and proteomics-based approaches have been extensively used to better understand the biology of parasitic helminths and facilitate development of diagnostics and therapeutics, metabolomics-based approaches have been largely overlooked. High-throughput technologies and software need to be integrated to enable big data generation, mining, and interpretation of the results.
Genomics and metagenomics.
Increasingly affordable sequencing technologies are changing how potential pharmaceutical drugs are being identified from natural products. While industrial investment in research programs aimed at discovering natural products suitable for pharmaceuticals has decreased in recent years (92), the use of next-generation sequencing technologies offers new screening pathways for targeted natural product discovery. Two sequencing applications in particular have the potential to revolutionize natural product discovery: large-scale genome sequence mining (93) and shotgun metagenomics (94). Large-scale genomic mining is a targeted approach, where the entire genome sequence from organisms of interest is interrogated in order to identify previously uncharacterized natural products. In contrast, shotgun metagenomics is an untargeted approach, where all sequences present in a community/environment are interrogated for novel natural products; however, with this approach, the organism of origin may not be known.
Many natural products have been discovered using genomics technologies. For example, genome mining of individual species led to the discovery of the novel polyphenolic polyketide antibiotic clostrubin from Clostridium beijerinckii, a strictly anaerobic bacterium (95), in addition to novel aminocoumarins from the uncommon actinomycete Catenulispora acidiphila DSM 44928 (96). To date, most sequence-based natural product discoveries have relied on individual genome sequences; however, the growth of high-quality, publicly available sequence data is enabling the simultaneous genome mining of thousands of species. For example, a recent study mined 10,000 actinomycetes in a search for novel phosphonic acids, an important class of natural products with known antimicrobial, antiviral, antimalarial, and herbicidal activities. This study identified a new archetypical pathway for phosphonate biosynthesis in addition to 11 previously undescribed phosphonic acid natural products (97). The authors propose their methodology as a generalizable framework suitable for the rapid discovery of other natural product classes in order to discover lead compounds suitable for the pharmaceutical industry (97).
Functional metagenomics are also being used as screening tools for natural product discovery at both the species level (e.g., Strepomyces [98]) and in complex environment samples (e.g., marine [99]). These methods are becoming increasingly popular for accessing bacterially encoded secondary metabolites, as it gives access to products from the majority of bacteria that are not readily culturable. Shotgun metagenomic sequencing has several advantages in that it is unbiased and requires no species-specific lab-based preparation, but most critically, it allows access to all the organisms’ collective genomes and thus provides a snapshot of the bioactive potential of entire bacterial populations in a single experiment. Additionally, the genetic information encoding the relevant biological activities are typically clustered on bacterial genomes, meaning that with limited starting material, it is possible to capture sequence describing the biological pathway of interest.
The last decade has seen an acceleration in the sequencing of microbial, fungal, and plant genomes, with tens of thousands of genomes now available in public archives, including GenBank and Ensembl. Despite the generation of this large volume of data, there exists a bottleneck in our ability to process and analyze these data in a meaningful way. In natural drug discovery, genome mining techniques have emerged as an approach to identify potential products of interest (100, 101), where secondary metabolites from biosynthetic gene clusters that encode novel bioactive metabolites are identified. In recent years, software to support genome mining have significantly matured for microbes and fungi. For example, AntiSMASH (antibiotics and secondary metabolite analysis shell) (102) uses computational methods to rapidly identify, annotate, and analyze secondary metabolite biosynthesis gene clusters identified in bacterial and fungal genomes. Many other software tools that exist are typically specific to either an organism group(s) or pathways. These include SMURF for fungal metabolites (103), BAGEL3 for prokaryotes (104), PRISM (Prediction Informatics for Secondary Metabolome) for microbial organisms (105), IMG/ABC for storing experimentally validated BCGs (106), and ASMPKS for predicting modular polyketide synthases (107). While progress has been made in regard to microbes and fungal genomes, tools available for plant-based drug discoveries are significantly lagging.
Proteomics.
Over the last few decades, numerous proteomic approaches have been developed and applied to facilitate the process of identifying protein and small molecule drug candidates. Typically, bioactivity or phenotype-based drug discovery involves the development and execution of bioassay screens to guide the isolation of the active fraction leading to the eventual identification of the active compounds (108, 109). Mass spectrometry, with its ability to identify small molecules and proteins through their fragment peptides, is an integral step in both proteomics and metabolomics (see “Metabolomics,” below) and has been used to characterize small molecules and natural products since the 1960s (110).
Recent improvements have been made in both the utility and sensitivity of mass spectrometers (111). An example of this progress is the recently released high-performance mass spectrometer Orbitrap Fusion Lumos Tribrid. With a resolution of up to 1,000,000 full width at half maximum (FWHM) values at m/z 200, this mass spectrometer combines Orbitrap, quadrupole, and linear ion trap technologies in one acquisition path, which allows it to acquire a more complex spectra at a higher rate (112, 113).
Other forms of chemistry or affinity-based fraction selection techniques range from simple solvent extractions (114) to molecular affinity, as mentioned above, as well as more complex techniques such as photoaffinity labeling, which allows potential drug compounds to be labeled with a photo cross-linker and a purification tag (109). Modern techniques taking advantage of newly discovered biochemical interactions between proteins and their ligands, such as the cellular thermal shift assay, have shown promise as drug discovery techniques. The cellular thermal shift assay is based on the rationale that protein stability can be altered by ligand binding (115), and it was recently demonstrated that studying the shift in the heat denaturation curves of the cellular proteomes after exposure to lead compounds can identify effective binding partners (116).
Novel extraction technologies have also been developed to address chemical and biological constraints and to improve overall extraction and downstream detection efficiency. These include high-intensity pulsed electric fields combined with semibionic extraction (117), highly sensitive supercritical fluid extraction (118), high-speed countercurrent chromatography (119), and sequential extractions combining multiple techniques to extract compounds with different properties from a single source (120).
Metabolomics.
Metabolomics uses multiple technologies, including high-performance liquid chromatography (HPLC), infrared spectroscopy (IR), gas chromatography-mass spectrometry (GC-MS), liquid chromatography-mass spectrometry (LC-MS), and nuclear magnetic resonance (NMR) (53). Metabolomics platforms are increasingly being used for a variety of applications, including diagnosis of diseases, infections, host-parasite interaction, biomarker and drug lead discoveries, drug target identification, drug interaction, and personalized treatments (121). Metabolomics techniques are also emerging for the identification of the secreted metabolites by tropical canine parasites, such as hookworm, tapeworm, and roundworm (122, 123). There is a need to apply metabolomics to identify biomarker compounds for many other tropical parasites in order to understand the mechanisms responsible for their parasitism and host immune evasion.
THERAPEUTIC SOLUTIONS FOR INFECTIOUS DISEASES OF THE TROPICS
Approved Therapeutic Molecules Derived from Natural Products
Historically, natural products have been employed in the treatment of many diseases affecting humans. The Dictionary of Natural Products (https://www.routledge.com/go/the_dictionary_of_natural_products), an authoritative and comprehensive database on natural products, lists 270,000 chemical entities, some of which are considered vital components of many modern drugs (124). Newman and Cragg recently reported that 1,881 naturally derived drugs were discovered and approved as drug entities between 1981 and 2019 (125). It is also estimated that approximately half of all medications validated between 1981 and 2010 have been sourced from natural products (126). As an example, out of 15 antiparasitic compounds used between 1981 and 2014, 60% of these have their origins in natural products (127). However, the majority of them are either semisynthetic or mimics of the natural bioactive compounds, resulting in a significantly smaller percentage (5%) of natural products sourced directly from nature and used as therapeutic molecules (126). This section highlights the most commonly used approved pharmaceutical drugs sourced from natural products.
Tetracyclines.
Tetracyclines are a family of broad-spectrum antimicrobial agents that were discovered in the 1940s. They are still used for their antimicrobial activity against a range of microbes implicated in many diseases, including some tropical diseases like malaria, where it is used as primary treatment for mefloquine-resistant Plasmodium falciparum (128), as well as trachoma and yaws (129, 130). Chlortetracycline (initially called aureomycin due to its yellow color) and oxytetracycline (initially called terramycin, in reference to terra, Latin for earth) were the first of the tetracyclines isolated from Streptomyces aureofaciens and Streptomyces rimosus, respectively (131–133). Similar molecules of the same class were subsequently extracted from S. aureofaciens, S. rimosus, and Streptomyces viridofaciens (tetracycline and demeclocycline) or synthesized through modification of natural products (e.g., doxycycline, lymecycline, methacycline, minocycline, rolitetracycline) (131, 134). Tetracyclines are known to bind to the bacterial 30S ribosomal subunit to reversibly inhibit bacterial protein synthesis and blocking them from growing or replicating further, a mode of action called “bacteriostatic” (129). However, since the isolation of the first tetracycline-resistant bacterium, Shigella dysenteriae (135), multiple microbial species have been reported to have acquired resistance to the natural (first-generation and some second-generation) tetracyclines, leading to the introduction of newer (third-generation) synthetic tetracyclines (136–138).
Quinine.
Quinine is a basic alkaloid prepared from the bark of the Cinchona plant. The WHO recommends its use in combination with clindamycin in the management of uncomplicated P. falciparum malaria in pregnant women who are within their first trimester. In the first trimester of pregnancy, quinine is also recommended for treatment of chloroquine-resistant Plasmodium vivax malaria (139). Additionally, quinine administration has been recommended for severe malaria in adults and children if artesunate and artemether (see “Artemisin” below) are not available (139). The mechanism behind quinine’s antimalaria action is not fully understood. It was demonstrated that its antimalaria activity could be from the ability of its quinoline group to cap hemozoin, which is crystallized from heme, as the parasite digests hemoglobin in red blood cells (140). Heme is chemically destructive and causes cellular damage through various means, such as oxidative stress and cytoskeletal protein disruption (141). Quinoline capping of hemozoin crystals prevents the parasite from detoxifying heme into insoluble and inert heme, thereby allowing free heme to build up, poisoning the parasite (140). Lastly, it must be noted that quinine has considerable adverse effects, which can range from impairment of hearing, tinnitus, headaches, and nausea to vertigo, vomiting, and loss of vision (142). Despite this, quinine remains a viable alternative to many approved pharmaceutical drugs due to its low cost and the emergence of resistance to other common antimalarials.
Artemisinin.
The use of the Artemisia annua plant, also known as sweet wormwood, to treat intermittent fevers among other indications has been documented in the Chinese materia medica in the late 1960s (143). In the early 1970s, artemisinin, a sesquiterpene lactone, was identified as responsible for the antimalarial activity of A. annua (144). Although the use of nonpharmaceutical forms of A. annua is not recommended by the WHO, artemether, artesunate, and dihydroartemisinin, its more stable semisynthetic derivatives, are included in the artemisinin-based combination therapy (ACT) recommended for treating malaria (139, 144). Both artemether and artesunate are metabolized by the body into dihydroartemisinin, which has various toxic effects against the parasite, including alkylation and misfolding of proteins initiated by free radicals created from the cleavage of the endoperoxide bridge found within the dihydroartemisinin molecule (144–146). ACTs constitute first-line therapies for most indications of malaria, including severe malaria. Depending on the indication, these compounds are frequently used in combination with other long-acting synthetic antimalarials, such as lumefantrine or amodiaquine (139). This is because artemisinin-based compounds have a short half-life and the longer-lasting synthetic compounds can continue to provide antimalarial activity to prevent the rise of drug resistance after artemisinin reaches subtherapeutic concentrations in the body (147).
NATURAL PRODUCT DISCOVERIES FOR THE TREATMENT OF TROPICAL DISEASES
Increasingly, natural products are being examined for their suitability in the treatment of tropical diseases caused by bacteria, virus, parasites, and fungi. There are many studies highlighting the effectiveness of natural products in treating tropical diseases.
Bacteria
In a very comprehensive review of recipes used by traditional healers in Burundi, Ngezahayo and colleagues recently identified a list of 155 different plant species belonging to 51 families and 139 genera used to prepare treatments for microbial tropical diseases of bacterial origin (148). Similarly, based on local folklore, Gupta et al. have collected 35 different plant species from India with anecdotal evidence of antituberculosis activity (149). Upon further examination, the ethanol extracts of 11 of those plants showed clear antimycobacterial activity (Table 3). There is also evidence that many plants from the Ivory Coast, Ghana, and Benin used to treat Buruli ulcer contain active ingredients with in vitro and in vivo activity against Mycobacterium ulcerans (150–156) (Table 3). Additionally, a plant-based treatment using Capparis zeylanica has been associated with a reduction of the diarrhea in patients suffering from cholera (157) (Table 3). Several other studies have described plant-based treatments of cholera (148), leprosy (148, 158, 159), and yaws (148); however, these studies do not provide clear evidence of the implication of the natural products in the improvement of the symptoms. Traditionally, most of these plant-based treatments are applied either as a maceration, powder, or decoction, indicating that the active ingredients within some of these plants may have topical and/or oral antibacterial activity.
TABLE 3.
Active compounds from natural products with activity against tropical disease-causing bacteriaa
| Disease | Description and pathogenesis | Geographical distribution | Product family/class | Extracts/natural product | Product source or origin | Extraction method | Efficacy/assessment model | Biological activity | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|
| Buruli ulcer (BU) | BU is caused by Mycobacterium ulcerans. Pathogenesis of BU relies on mycolactone, a polyketide-derived macrolide. Its mode of transmission remains poorly understood, but the current hypothesis is that the disease is transmitted from stagnant bodies of water or mosquitoes. | BU was first described in Australia but has been reported from 33 countries worldwide, including West Africa, Central and South America, and the Western Pacific. About 73% of the total global cases have been reported from Côte d’Ivoire, Ghana, and Benin. | Naphthofurans | Rifampin | Amycolatopsis rifamycinica | Extracted from fermentation culture of the bacterium | Clinically used for treating BU | Oral administration of rifampin (10 mg/kg orally once daily) | 27, 344 |
| Benzene and substituted derivatives | Streptomycin | Streptomyces griseus | Extracted from fermentation culture of the bacterium | Clinically used for treating BU | Intramuscular injection, 15 mg/kg of body weight for 8 weeks | 345 | |||
| Alkaloids | Holadysamine | Holarrhena floribunda | 50 g of powder was macerated and extracted using 70% ethanol. | In vitro: well diffusion assays | Compound inhibited the growth of M. ulcerans at MIC of 50 μg/ml | 155 | |||
| Holophyllinol | Compound inhibited the growth of M. ulcerans at MIC of 125 μg/ml | ||||||||
| Holamine | |||||||||
| Holaphyllamine | |||||||||
| Crude extract | Moringa oleifera | Extracted with water | Children with skin lesions clinically suggestive of BU (2–15 years old) were given normal diet spiked with 330 ml of M. oleifera/child at each meal. | Children’s ulcers decreased from 72 mm to 48 mm on day 56 after administration of water extract of (330 ml) M. oleifera. | 154 | ||||
| Aglaonema commutatum | Leaves boiled in water for 5 min | 200 μg/ml of extract was prepared and diluted with medium (1st to 8th dilution); MIC was determined at final concentrations (25% [vol/vol] to 0.20% [vol/vol]) corresponding to 50 μg/ml to 0.4 μg/ml. |
In vitro activity with MIC of 40 μg/ml |
346 | |||||
| Aloe vera | Leaves macerated in water | ||||||||
| Alstonia boonei | |||||||||
| Capsicum annum | Fruit macerated in water | ||||||||
| Gratiola officinalis | Bark boiled in water for 20 min | In vitro activity with MIC of 1.56–25 μg/ml | |||||||
| Jatropha curcas | Leaves macerated in 70% ethanol | In vitro activity with MIC of 250 μg/ml | |||||||
| Spigelia anthelmia | Leaves and grains boiled in water for 5 min | In vitro activity with MIC of 6.25–25 μg/ml | |||||||
| Syzygium aromaticum | Seeds boiled in water for 20 min | In vitro activity with MIC of 25 μg/ml | |||||||
| Zea mays and Spigelia anthelmia | Grains and leaves boiled in water for 5 min | In vitro activity with MIC of 6.25–25 μg/ml | |||||||
| Zanthoxylum zanthoxyloides | Roots boiled in water for 20 min | In vitro activity with MIC of 12.5–25 μg/ml | |||||||
| Trachoma | Trachoma is a bacterial infection of the eyes and genitals, which is spread through flies or direct contact. The eye infection can lead to blindness. | Trachoma is widespread across Africa, Asia, and Central and South America, with the highest prevalence in Ethiopia and South Sudan. | Flavonoids | Baicalin | Scutellariae baicalensis | Purchased commercially, dissolved in DMSO | Female mice were infected with Chlamydia trachomatis, followed by 1 mM intravaginal rinse treatment. | Reduced bacterial counts by 78% after 5 days and 99.9% after 11 days | 178 |
| Flavonoids | Luteolin | Wide range of plants such as trees, herbs, and vegetables. Sourced from Extrasynthese, Genay, France | Purchased commercially, dissolved in DMSO | Chlamydia trachomatis bacterial challenge, followed by 2 mg/kg intraperitoneal injection treatment | Showed 25% and 37% fewer pathogen-positive mice at days 6 and 13, respectively | ||||
| Flavonoids | Catechin | Various vascular plants, sourced from tea leaves | Boiling in water | In vitro HL cells (human airway epithelium line) cultured with bacteria and treatments applied in culture medium | A 0.4-mg/ml concentration applied topically was completely inhibitory | ||||
| Polyphenols | Flavones, flavonols, coumarins, gallates | Various vascular plants | Purchased commercially, dissolved in DMSO | In vitro HL cells (human airway epithelium line) cultured with bacteria and treatments applied in culture medium | A 50 μM concentration was highly active (85%–100% inhibition) | ||||
| Tuberculosis | TB is a pulmonary disease that is initiated by the deposition of Mycobacterium tuberculosis, contained in aerosol droplets, onto lung alveolar surfaces. The progression of the disease can have several outcomes, determined largely by the response of the host immune system. | Most new cases of TB are in Asia and Africa. | Quinonoids | Plumbagin and crotonate plumbagin | Root of Plumbago indica Linn collected from Orissa, India | Multiple extraction methods | Broth microdilution assay Resazurin microplate assay (REMA) |
Inhibition of thymidylate synthase MIC of 0.25–16 μg/ml | 160 – 162 |
| Flavonoid | Kaempferol and its benzyl derivative | Leaf extract of Rhoeo spathacea, Pluchea indica from Indonesia | In silico modeling of molecular structures | AutoDock Vina, followed by 50-ns molecular dynamics simulation using YASARA | In silico inhibitor of the CYP121 M. tuberculosis enzyme | 163 | |||
| Naphthoquinones | Maritinone | Stem bark extract of Diospyros anisandra | Maceration and liquid-liquid fractionation | Cytotoxicity assay using Vero cells and peripheral blood mononuclear cells | Bacteriostatic activity MIC of 1.56–3.33 μg/ml | 164 | |||
| 3,3′-Biplumbagin | Bacteriostatic activity MIC of 1.56–3.33 μg/ml | ||||||||
| Iridoids-plumeride | Plumericin/isoplumericin | Stem bark of Plumeria bicolor | Extracted by methanol | Tetrazolium bromide assay | Bacteriostatic activity MIC of 1.5–2.1 μg/ml | 165 | |||
| Piperidines | Dipiperidine derivatives | Piper nigrum | Not applicable | Luciferase growth inhibition assay, in vivo M. tuberculosis induced weight loss in mouse and human trials | Disease reduction and bacterial growth inhibition | 170 | |||
| Bacteriostatic activity MIC in the range of 4.0–32.0 μg/ml | 171 – 174 | ||||||||
| Gallic acid-derivatives | 3-O-methyl-alkylgallates | Loranthus micranthus | Maceration | Bactericidal assay | Bacteriostatic activity MIC of 6.25 μM | 166 | |||
| Coumarin-type compound | Collinin | Zanthoxylum schinifolium found in Korea, China, and Japan | Extracted by methanol and isolated using HPLC | Microbial cell viability assay | Bacteriostatic activity MIC of 3.13–6.25 μg/ml in culture broth and 6.25–12.5 μg/ml inside cells | 167 | |||
| Acridone alkaloid | (i) Hydroxy-1, 3-dimethoxy-10-methyl-9-acridone, (ii) 1-hydroxy-3-methoxy-10-methyl-9-acridone, (iii) 3-hydroxy-1, 5, 6-trimethoxy-9-acridone |
Stem bark of Zanthoxylum leprieurii from Mpigi District, Uganda | Crude extract extracted with methanol column chromatography | Microplate alamarBlue assay | Bacteriostatic activity MIC of 5.1 μg/ml | 168 | |||
| Cucurbitacins | Ursolic acid | Ripe deseeded fruit of Citrullus colocynthis collected from Rajasthan, India | Extracted with petroleum ether, chloroform, methanol, and water | Maceration chromatography, bacterial viability assay | Bacteriostatic activity MIC of 50 μg/ml | 169 | |||
| Cucurbitacins | Cucurbitacin | Ripe deseeded fruit of Citrullus colocynthis collected from Rajasthan, India | Extracted with petroleum ether, chloroform, methanol, and water | Maceration chromatography, bacterial viability assay | Bacteriostatic activity MIC of 25 μg/ml | 169 | |||
| Sponge-derived bengamide | Bengamide B | Tedania sp. collected from East Diamond Islet, Queensland, Australia | Marine sponge extract, isolated using HPLC | Intracellular mycobacterial activity assay | Interference with methionine aminopeptidase activity MIC of 0.39–1.56 μg/ml | 175 | |||
| Alkaloid | Halicyclamine A | Haliclona sp. | Marine sponge extract, extracted with methanol and isolated using HPLC | MTT assay | Bacteriostatic activity MIC of 1–5 μg/ml under aerobic and hypoxic conditions | 176 | |||
| Steroidal alkaloid | Plakinamide P | Plakina sp. collected from Crooked Island, Bahamas | Marine sponge extract, extracted with heptane, ethyl acetate-ethanol mixture, ethanol, and methanol HPLC | Autoluminescence bacterial viability assay | Bactericidal activity MIC of 1.8 μg/ml | 347 | |||
| Not specifically identified in this paper, but some active products were discussed | Bark of Alstonia scholaris | Plant material collected from four districts in Madhya Pradesh were dried, ground, and then extracted with 95% ethanol to obtain crude extract. | In vitro measurement of plant extract bacteriostatic activity against 7 strains of M. tuberculosis via resazurin microtiter plate assay (REMA) compared to cytotoxicity against THP-1 macrophage cells measured via flow cytometry to derive a selectivity index (IC50 cells/MIC) | Selectivity index of 4 to >8 | 149 | ||||
| Roots of Glycyrrhiza glabra | Selectivity index of 8 to >32 | ||||||||
| Seeds of Holarrhena antidysenterica | Selectivity index of 8 to >32 | ||||||||
| Fruits of Mallotus philippensis | Selectivity index of 8 to >32 | ||||||||
| Tubers of Eulophia nuda | Selectivity index of 8 to 32 | ||||||||
| Leaves of Cocculus hirsutus | Selectivity index of 4 to >8 | ||||||||
| Tubers of Pueraria tuberosa | Selectivity index of 1 to 8 | ||||||||
| Roots of Cyperus rotundus | Selectivity index of 16 to >64 | ||||||||
| Rhizome of Curcuma caesia | Selectivity index of 4 to 16 | ||||||||
| Floral head of Sphaeranthus indicus | Selectivity index of 8 to 16 | ||||||||
| Roots of Plumbago zeylanica | Selectivity index of 32 to 64 | ||||||||
DMSO, dimethyl sulfoxide; HPLC, high-performance liquid chromatography; MTT, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide; IC50, 50% inhibitory concentration.
In contrast to the vast knowledge about the source organisms (mainly plants), relatively little is known about the identity of the active compounds within these organisms exerting antimicrobial activity (Table 3). Over the last few years, some groups around the world have isolated a number of natural compounds with proven in vitro activity against M. tuberculosis (Table 3). Plumbagin and other quinonoid compounds have shown a strong bacteriostatic effect in a number of independent studies (160–162). In addition, various other plant-derived compounds, such as kaempferol (163), maritinone (164), 3,3′-biplumbagin (164), plumericin and isoplumericin (165), 3-O-methyl-alkylgallates (166), collinin (167), acridone alkaloids (168), ursolic acid (169), and cucurbitacin (169), have been reported to have bacteriostatic effects on M. tuberculosis in vitro (Table 3). Importantly, piperidine-based active compounds isolated from Piper nigrum have already progressed to preclinical and clinical trials in animals and humans (170–174) (Table 3). Another promising source for antituberculosis drugs appears to be marine sponges (175–177) (Table 3). Quan and colleagues recently reported on the antituberculosis activity of bengamide B derived from the marine sponge Tedania sp. (175). Other studies have demonstrated strong bacteriostatic effects of halicyclamine A (176) and plakinamide P (177), derived from the sponges Haliclona sp. and Plakina sp., respectively. In a study from Benin, four steroidal alkaloids (holadysamine, holophyllinol, holamine, and holaphyllamine) from the plant species Holarrhena floribunda showed bacteriostatic activity against Mycobacterium ulcerans (155). Several active natural products, including baicalin, luteolin, catechins, flavonoids, and polyphenols, from a variety of tropical plants have also been shown to improve the eyesight of patients infected with Chlamydia trachomatis (178). Preclinical studies in mouse models of cholera have also shown a reduction in diarrhea when mice were treated with farnesol isolated from the Acacia farnesiana tree (179) or with sulfated polysaccharides isolated from the red seaweed Gracilaria cervicornis (180).
Parasites
Multiple natural products have been shown to have antiparasitic activity against pathogens responsible for lymphatic filariasis (181–186), leishmaniasis (187), and Chagas disease (187) (Table 4). Similarly, a large range of natural products from the tropics have been commonly used for treating malaria (188–190) (Table 4). More recently, by combining nanoparticle technologies and natural extracts from the leaves of the plant Indigofera oblongifolia, Dkhil et al. have demonstrated the promising antiplasmodial and hepatoprotective activity of silver nanoparticles in a Plasmodium chabaudi-infected mouse model (191). Nanocarrier-based drug delivery systems have received enormous attention in the past few years (192). Antimicrobial drugs become more efficacious when adsorbed, entrapped, or linked to polysaccharides such as dextran, besides increasing the surface area for the drug and achieving targeted drug delivery (193). A snapshot of intravenously administered tetramethylrhodamine-isothiocyanate (TRITC)-conjugated dextran in the blood vessels of mice with malaria is shown (Fig. 3). Dextran-based carriers are being tested for many diseases, including for codelivery of buparvaquone (BPQ) and polymyxin B (PB) against leishmaniasis and to enhance cross presentation of subunit vaccines against melioidosis (194, 195). Two active compounds against Plasmodium spp., cryptolepine and dichroine, extracted from Cryptolepis sanguinolenta and Dichroa febrifuga (Ch’ang Shan) roots, respectively, have been identified (196–198) (Table 4). Recently, bisaprasin, a bromotyrosine alkaloid isolated from Aplysinella rhax, a Fijian marine sponge, has exhibited moderate antiparasitic activity against P. falciparum (199) (Table 4).
TABLE 4.
Active compounds from natural products with activity against tropical disease-causing parasites
| Disease | Description and pathogenesis | Geographical distribution | Product family/class | Extracts/natural product | Product source or origin | Extraction method | Efficacy/assessment model | Biological activity | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|
| American trypanosomiasis (Chagas disease) | A vector-borne parasitic disease caused by blood-sucking bugs infected with Trypanosoma cruzi and spread through bites from insect vectors | Americas | Solenopsins alkaloids | Solenopsin | Solenosis invicta, S. saevissima | Fire ants’ mounds isolated; solenopsin alkaloids extracted with hexane and further purified with hexane–acetone silica columns; alkaloids compared with known mass spectra. | In vitro: Tested against proliferation of T. cruzi epimastigote forms of Dm-28c and CL-Brener. | Solenopsin extracts used at 0.25–0.5 × IC50 values for up to 8 days; growth capacity recovered after solenopsins were removed (inhibition reversible). | 208 |
| Bromotyrosine alkaloids | Bisaprasin | Aplysinella rhax | Marine sponge extract partitioned using modified Kupchan partitioning technique; reversed‐phase solid‐phase extraction used for further fractionation | In vitro: T. cruzi Tulahuen C2C4 strain, expressing LacZ; L6 rat skeletal muscle cells with 5 μl of compound | Moderate parasiticidal activity; IC50, 19 μM | 348 | |||
| Unspecified | Handroanthus impetiginosa | Crude extracts prepared from powdered aerial parts; macerated in hydroalcoholic solution followed by evaporation at 35 degrees for 2 h | In vitro: Cytotoxicity measured in MTT assay (concentrations from 1.0 to 0.03125 kg body/ml) against Trypanosoma cruzi trypomastigotes (CL strain; Brener) and murine J774.G8 macrophages. | Lethal to T. cruzi trypomastigotes at 0.5, 0.25, and 0.125 kg body/ml of H. impetiginosus extract | 187 | ||||
| Ageratum conyzoides | A. conyzoides extract toxic at most of the concentrations (exception, 0.0625 kg body/ml) | ||||||||
| Ruta graveolens | R. graveolens extract showed increased mortality of trypomastigotes compared to H. impetiginosa extract. | ||||||||
| Cell invasion inhibition: Pretreatment with A. conyzoides, H. impetiginosus, and R. graveolens reduced no. of Trypanosoma cruzi trypomastigote-infected cells | |||||||||
| Sesquiterpene lactones | Psilostachyin (Psi) | Ambrosia tenuifolia | Unspecified | In vitro: Growth inhibition of T. cruzi epimastigotes, (percent inhibition and IC50) estimated in the presence of hemin concentrations of 0–20 kg body/liter. | Maximum inhibitory capacity without hemin (IC50, 4.74 μM). Reduction of IC50 value in the presence of 20 kg body/liter of hemin. Psilostachyin A: EC50 at 24 h of 33 ± 1 μg/ml against bloodstream trypomastigotes. In vivo: Reductions in parasitemia from 40 to 70% at 0.5–50 mg/kg; Psilostachyin A did not protect against animal mortality | 209, 210 | |||
| In vivo: Swiss-Webster mice (one female and one male) treated intraperitoneally or orally at 25–400 mg/kg; MTD values determined on animal survival rates and behavior alteration after 48 h | |||||||||
| Psilostachyin C (PsiC) |
Ambrosia scabra | Extraction using the aerial parts of A. scabra by maceration with dichloromethane and methanol at room temp. | In vitro: Bloodstream trypomastigotes cultured with 0.1–100 μg/ml PsiC; peritoneal macrophages infected with transfected trypomastigotes expressing β-galactosidase cultured with 0.01–10 μg/ml to 10 μg/ml. | Increased no. of epimastigotes observed after treatment with GSH and PsiC compared with PsiC alone. In vivo: PsiC-treated animals showed a twofold reduction in parasites, but succumbed to infection from day 20 (30-day survival rate of 20% vs 0% in untreated mice). | 349 | ||||
| In vivo: CF1 and C3H/HeN mice infected i.p. with bloodstream T. cruzi trypomastigotes; treated daily with 1 mg/kg body/day of PsiC. Parasitemia and mice mortality assessed. | |||||||||
| Human African trypanosomiasis | A vector-borne parasitic disease caused by insect vectors tsetse flies infected with Trypanosoma brucei and spread through bites from the insect. | Sub-Saharan Africa | Phenolic compounds | Bisdemethoxycurcumin, demethoxycurcumin, oregonin, broussochalcone A, 3-deoxysappanchalcone, xanthoangelol, 7-(4”-hydroxy-3”-methoxyphenyl)-1-phenylhept-4-en-3-one, 4-hydroxy-3-methoxycinnamaldehyde, obovatal, honokiol, 1’S-1’-acetoxychavicol acetate, saucerneol D, manassantin A, manassantin B, kushenol F, apigenin, eupatilin, morusin, 3-deoxysappanone B, 6,8-diprenylorobol, genistin, sophoricoside | Curcuma longa, Alnus japonica, Broussonetia papyrifera, Caesalpinia sappan, Angelica keiskei, Cinnamomum cassia, Machilus thunbergii, Alpinia galanga, Saururus chinensis, Sophora flavescens, Agrimonia pilosa, Artemisia vulgaris, Morus alba | Identified from the Chungnam National University (Korea) proprietary library of 440 natural products from medicinal plants. | In vitro: growth inhibition assay of bloodstream form T. brucei brucei strain 427 treated with test compounds compared to cytotoxicity assays of HEK239T and HepG2 cell lines treated with test compounds to derive a selectivity index (CC50 cells/EC50 T. brucei brucei) | Selectivity index ranging from 2.29 to >46.34 | 211 |
| Guajanolide sesquiterpene lactone | Cynaropicrin | Centaurea salmantica L. (Asteraceae) | Dried aerial parts of C. salmantica were defatted with n-hexane (Scharlau), dried and then extracted exhaustively with ethyl acetate (Scharlau) yielding crude extract. |
In vitro: Cardiac cells were infected with Y and Colombiana strains of T. cruzi with increasing nontoxic concentrations of the compounds. Death rates and EC50 were calculated In vivo: Swiss-Webster mice (one female and one male) treated intraperitoneally or orally at 25–400 mg/kg; MTD values determined on animal survival rates and behavior alteration after 48 hours |
Cynaropicrin moderately effective on intracellular proliferative forms (EC50 at 48 h of >0.75 μg/ml). In vivo: Cynaropicrin (25 and 50 mg/kg/day i.p.) showed no reduction in parasitemia; 100% mortality in all groups. | 210, 212 | |||
| Sesquiterpene lactone | Deoxyelephantopin | Elephantopus scaber Linn. | Dried powder of E. scaber soaked with methanol for 3 days. Extracts concentrated with rotary vacuum evaporator. | In vitro: T. brucei rhodesience (strain STIB 900) parasite and mouse skeletal (L-6) cell were incubated with compound at 90–0.123 μg/ml over 72 h | Ethyl acetate fraction showed highest antitrypanosomal activity (0.21 ± 0.04 μg/ml) with a lower cytotoxicity (6.25 ± 0.77 μg/ml). | 350 | |||
| Leishmaniasis | A parasitic disease caused by Leishmania parasites and spread through bites from sand flies | Tropics and subtropics of Africa and Asia, and southern Europe | Lignans | Cubebin | Piper cueba | Materials extracted with n-hexane, ethyl acetate, and methanol and concentrated under vacuum on rotary evaporator | In vitro: L. donovani promastigote MTT cell cytotoxicity assay. In vivo: L. donovani amastigotes (intracardiac injection) in golden hamsters were administered ± compound for 10 days. | P. cubeba showed > 90% inhibition of promastigotes of L. donovani at 100 μg/ml; extracts from P. retrofractum >75% inhibition at 20 μg/ml. In vivo: Treatment with piplartine (30 mg/kg/10 ml i.p.) reduced parasitic burden and spleen weight. | 200 |
| Amides | Piplartine | ||||||||
| Multiple families | Quinic acid, catequin/epicatequin, ellagic acid, gallocatechol as well as myricetin, quercetin, cafeoil, and their associated derivatives, were identified from analyzed extracts | Psidium brownianum | Crushed leaves were extracted with 100% ethanol for 96 h, filtered, then dried in a rotary evaporator. Flavonoid fractions were extracted from the ethanol extract with hexane, chloroform, then ethyl acetate. Tannic fractions were extracted from the ethanol extract with 7:3 acetone/water, filtered and concentrated, then further extracted with petroleum ether. | In vitro Leishmanicide assay against epimastigotes compared to cytotoxicity against NCTC 929 fibroblasts exposed to 1,000 and 500 μg/ml of the respective extract | 84.02–94.25% of epimastigote forms killed compared to 38.86–40.97% fibroblasts at 1,000 μg/ml | 201 | |||
| Psidium guajava | 46.6–95.55% of epimastigote forms killed compared to 36.66–37.9% of fibroblasts at 1,000 μg/ml | ||||||||
| Unspecified | Handroanthus impetiginosa | Crude extracts prepared from powdered aerial parts of the plant; macerated in hydroalcoholic solution, followed by evaporation | In vitro: Cytotoxicity measured using MTT assay with extract concentrations of 1.0 to 0.03125 mg/ml | A. conyzoides extract caused increased mortality in L. amazonensis promastigote; treatment with H. impetiginosus showed lower parasite mortality; treatment with R. graveolens showed significant increase in mortality rate. Invasion assay: Leishmania amazonensis pretreated with A. conyzoides and R. graveolens exhibited decreased entry into macrophages; H. impetiginosus had no impact on invasion and infection. | 187 | ||||
| Ageratum conyzoides | |||||||||
| Ruta graveolens | |||||||||
| Sesquiterpene lactone | Parthenolide | Tanacetum parthenium | Maceration in ethanol-water and extraction at room temp in the dark. | In vitro: Antiamastigote activity was assessed using L. amazonensis-infected (MHOM/BR/75/Josefa strain) J774G8 macrophages treated with compound. | Extract inhibited promastigote growth (IC50, 29 μg/ml after 48 h). Parthenolide (IC50, 0.37 μg/ml) induced partial lysis of the promastigotes at 5 μg/ml. At 1 μg/ml, it resulted in 90% growth inhibition. Cytotoxic assay: Macrophages treated with parthenolide showed CC50 of 14 μg/ml. | 203 | |||
| Purchased from Sigma-Aldrich (Germany) | In vitro: Parasite growth assay on L. amazonesis treated with 70% parthenolide | IC50 of 1.3 μM and IC90 of 3.3 μM | 204 | ||||||
| Sesquiterpene lactone-rich fraction | Extraction of aerial parts of plant with ethanol and distilled water followed by sequential chromatography with hexane, ethyl acetate, dichloromethane, and methanol. | In vivo: Mice infected with L. amazonensis metacyclic promastigotes by injection into the footpad. Mice were treated intramuscularly with test fraction after lesion development. Mice were assessed for lesion growth and parasite load | Sesquiterpine lactone-rich-fragment-treated mice had reduced lesion development. Parasite load was significantly reduced | 202 | |||||
| Lymphatic filariasis | A parasitic disease caused by micropic, thread-like worms transmitted via mosquito bites | Africa, the Americas, Pacific and Asia | Galactolipids | 1, 2-di-O-linolenoyl-3-O-α-galactopyranosyl-(1→6)-O-β-galactopyranosyl glycerol | Bauhinia racemosa | Successive column chromatographic separations on normal and reverse phase silica gel was used to extract subfraction F4 | In vivo: Active compounds identified in n-butanol fraction F4 were evaluated intraperitoneally (i.p.) for five consecutive days using 50 mg/kg | Galactolipid 1, 2, and 3 had 58.3%, 45.8%, and 54.15% adult worm mortality, respectively, with long chain fatty acid alcohol 8 showing 49.95% adulticidal activity over control. | 205 |
| Unknown | Hexane fraction | Botryocladia leptopoda | 95% ethanol was used to extract powdered, dried material with the combined extract concentrated at below 50°C in a rotavapour and dried under high vacuum. Ethanol extract fractionation was done into four parts: hexane, chloroform, n-butanol soluble, and n-butanol insoluble. | In vitro: Crude extracts/fractions tested against adult female parasites of L. sigmodontis, A. viteae, and B. malayi. The crude extract was tested at 250 μg/ml while the fractions were tested at various serial twofold dilutions from 500 down to 15.6 μg/ml. Worms were incubated in triplicate at 37°C overnight in the RPMI 1640 medium containing antibiotics. | Slight decrease in microfilarial levels was observed on day 8 post-treatment in both L. sigmodontis- and A. viteae-infected animals. Microfilarial levels continuously decreased until the end of the observation period, finishing with decreases in microfilarial density of 82% and 97%, respectively. | 181 | |||
| Triterpenoids | Oleanonic acid/oleanolic acid | Lantana camara | Combined extract concentrated under reduced pressure at below 40°C , then dried under high vacuum. Fractionation done with different solvents to obtain fractions including n-hexane, chloroform, n-butanol, and aqueous fractions. | In vivo: Mastomys rat showing rises in microfilaremia that had 2–3-mo-old A. viteae infections or 5–6-mo-old B. malayi infections were selected. Extracts suspended in 0.1% Tween 80 and administered orally at varying doses for 5 days. Two pure compounds were administered both orally and intraperitoneally. | 95.04% reduction in microfilaremia on day 8 when treated with 1 g/kg of crude extract for 5 consecutive days. The crude extract caused high mortality in the adult B. malayi transplanted gerbil model on day 42 (72%) or day 60 (80%) of subcutaneous or oral treatment. | 182 | |||
| Unknown | Hexane fraction | Butea monosperma | Powdered Butea monosperma L. plant leaves were extracted with petroleum ether, hexane ethanol, and methanol. Extract was dissolved in dimethylsulfoxide to obtain different concentrations for further study. | In vitro: Crude methanol, hexane-ethanol, and antibiotic ciprofloxacin extracts were used for antifilarial screening on adult Setaria cervi. | Methanol concn (0.25 to 5.0 mg/ml), hexane-ethanol concentrations (0.50 to 10.0 mg/ml) and ciprofloxacin concn (1.0 to 20.0 mg/ml) caused complete immobilization of the worms at 5 to 24 h exposure. | 351 | |||
| Flavonoids | 4,5-diethyl-3′-ethoxy-pyro-flavone | Vitex negundo | Powdered Vitex negundo L. leaves were extracted with petroleum ether (60°C–80°C), chloroform, ethyl acetate, and methanol using the percolation method. | In vitro: 0.01% streptopenicillin and 10% heat-inactivated fetal calf serum were mixed in DMEM and the worms added. 100 μl diluted extract of Vitex negundo was added and worms incubated at 37°C for 24 h in 5% CO2 incubator and motility observed. | Vitex negundo fraction 3 concentrations from 0.005 to 0.02 mg/ml caused complete immobilization of the worms at incubation at 2 h. | 352 | |||
| Phenylpropanoids | Coumarins | Aegle marmelos Corr. | Leaves from Aegle marmelos Corr. were extracted by 70% ethanolic extraction process. | In vitro: 100 microfilariae mixed in 100 μl of RPMI of were added to each well. Plates were incubated at 37°C for 48 hours in 5% CO2 incubator. Microfilaria motility assessed after incubation by microscopy. | Dose-dependent loss of microfilaria motility observed for herbal extracts from Aegle marmelos Corr. leaves. | 353 | |||
| Unknown | Hexane fraction | Caesalpinia bonducella | Kernel seeds air dried and extracted with 95% ethanol with the extract concentrated under reduced pressure <45°C using a Rotavapor | In vitro: Microfilaricidal activity was assessed in animals killed on day 42 or 91 post-treatment. Cottom rat pleural cavities and various tissues from Mastomys coucha were examined in saline for motility and cell adhesion on the worm surface. | With L. sigmodontis on cotton rats, the extract of seed kernel showed 60.7, 72.4, and 98.4% microfilaricidal action respectively on days 8, 21, and 42 post-treatment with 96.0% overall adulticidal activity. | 186 | |||
| Limonoid | Gedunin | Xylocarpus granatum | 1 kg of air-dried, powdered fruits were extracted with 50% ethanol and combined extracts filtered, concentrated under reduced pressure below 50°C. The powder was fractionated into chloroform-soluble and chloroform-insoluble fractions by maceration. | In vitro: Actively motile female worms exposed to various concentrations in 48-well culture plate containing 1,000 μl media. RPMI 1640 medium containing antibiotics and 10% fetal bovine serum was used. Following drug exposure, worm motility was recorded microscopically. | The crude aqueous ethanolic extract of fruit of Xylocarpus killed adult B. malayi and microfilariae at 125 and 62.5 μg/ml. This compares to the standard drug ivermectin, which killed adult worms at 7.8 μg/ml and microfilariae at 125 μg/ml concn. | 185 | |||
| Malaria | A mosquito-borne parasitic disease transmitted via mosquito bites | Sub-Saharan Africa and South Asia | Indoloquinolone alkaloid | Cryptolepine | Cryptolepis sanguinolenta | 650 g of powdered roots boiled for 30 min in 5 liters of distilled water which was decanted and filtered. Filtrate freeze-dried to obtain crude extract referred to as cryptolepis (CPS). | In vitro: Cryptolepine mixed with the aqueous root extract of C. sanguinolenta were used to assess gametocyte survival after drug exposure using a resazurin-based assay. | Cryptolepis sanguinolenta and its major alkaloid, cryptolepine exhibited high inhibitory activity against late-stage P. falciparum gametocytes (NF54). | 354 |
| Quinazolinone alkaloid | Febrifugine | Dichroa febrifuga | Dried roots were ground and macerated in 14 liters of methanol at room temp for a wk. After filtration, solvent was evaporated to obtain crude methanol extract, which was then suspended with the alkaloidal portion separated by chromatography on a silica gel 60 column | In vitro: Mice were treated orally or subcutaneously daily from day 3-10 to day 10 with either candidate antimalarial drugs or vehicle alone (negative control). Five doses for each group were tested. Films were Giemsa stained and examined microscopically to determine parasitemia level. | R237645 (halofuginone) was found to be the most active febrifugine analog against the parasites. | 355 | |||
| Bromopyrrole alkaloids | Bisaprasin | Aplysinella rhax | The sponge sample was extracted with methanol followed by DCM and dried and partitioned following the modified Kupchan liquid‐liquid partitioning technique. | In vitro: Compounds 1–6 and 9–10 were tested in duplicate with a 16-point dose-response curve (starting concentrations of 63–115 μM). Following incubation, LDH activity was measured. | Bisaprasin, a biphenylic dimer derived from psammaplin A, showed moderate activity at 19 and 29 μM against T. cruzi Tulahuen C4 while psammaplin A showed activity at 30 and 60 μM against P. falciparum 3D7 strain. | 348 | |||
| Not determined | Cochlospermum planchonii | N’Dribala extract was prepared daily by decoction of 50 g of dried Cochlospermum planchonii tuberous root powder mixed with 1,500 ml of boiling water. | In vivo: Parasite density was estimated by two readers. Full clinical examination and blood sampling of patients was conducted on days 0, 2, and 5. | At D2, 17 N’Dribala-treated patients had Pd > 500 parasites/mm3 compared to all CQ-treated patients, with Pd < 500 parasites/mm3. At D5, seven N’Dribala-treated patients had Pd > 500 parasites/mm3, with 57% of CQ-treated and 52% N’Dribala-treated patients having Pd = 0 | 356 | ||||
| Schistosomiasis | A parasitic disease caused by worms transmitted through contact with contaminated freshwater | Africa, Asia and the Americas | Lactones | Vernodalin | Vernonia amygdalina | 1 kg of air-dried plant leaves was ground coarsely and added to a stoppered container with water. Mixture was strained, the damp solid material pressed, and the combined liquid clarified by filtration. | In vitro: 1.0 ml from each of the homogenates added to test tubes and left undisturbed for 10 min at 25°C. Mixture incubated for 5 min at 55°C, then removed and cooled with running water. Absorbance read at 650 nm against the blank. | Treatment of the intermediary host Bulinus truncatus with various concentrations of Vernonia amygdalina leaf extracts caused reductions in the activity of acetylcholinesterase in snail haemolymph, muscle, hepatopancreas, and intestine. | 357 |
| Phenolics | Aspidin | Dryopteris spp. | Dihydroartemisinin was ground in a ball miller with dimethyl sulfoxide, Tween 80, and 1% carboxymethylcellulose sodium, yielding suspension solutions containing 8, 12, 16, or 24 g dihydroarteminisin. | In vitro: Adult worm pairs were incubated for 24 h with phloroglucinol derivatives (range 10-100 μM). Viability assays were performed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. | All worm pairs died after 24 h of incubation with aspidin, flavaspidic acid, methylene-bis-aspidinol, and desaspidin. Worms showed decreased motor activity with tegumental alterations that were incubated with aspidin and flavaspidic acid, while worms showed decreased motor activity without tegumental alterations when incubated with methylene-bis-aspidinol and desaspidin. | 207 | |||
| Phloroglucinols | Flavaspidic acid | Dryopteris spp. | |||||||
| Phloroglucinol | Methylene-bis-aspidinol | Dryopteris spp. | |||||||
| Alkyl-phenylketones | Desaspidin | Dryopteris spp. | |||||||
MTD, maximum tolerated dose; GSH, glutathione; i.p., intraperitoneally; CC50, 50% cytotoxic concentration; MTT, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide; DMEM, Dulbecco modified Eagle medium; DCM, dichloromethane; LDH, lactate dehydrogenase; Pd, parasite density; CQ, chloroquine.
FIG 3.
Visualizing drug delivery in the microvasculature. MacGreen mice were infected with Plasmodium berghei ANKA for subsequent intravital imaging of the brain microvasculature when the mice were showing clinical signs of malaria. Blood vessels were infused with tetramethylrhodamine-isothiocyanate (TRITC)-conjugated dextran (red). Moderate levels of leukocyte accumulation are seen both within and outside the blood vessels (green).
Regarding leishmaniasis, cubebin isolated from Piper cubeba has been described as a potent parasiticidal (200) (Table 4). A recent study conducted by de Souza and colleagues has found that the Psidium brownianum-derived natural compounds quercetin, myricetin, and gallic acid as well as the Psidium guajava-derived ethanolic extract and the flavonoid and tannic fractions show antiparasitic activity against both Leishmania brasiliensis and Leishmania infantum parasites (201) (Table 4). Similarly, a sesquiterpene lactone, parthenolide, derived from Tanacetum parthenium, shows antileishmania activity against Leishmania amazonensis in both in vitro and in vivo studies (202–204). In the context of lymphatic filariasis, galactolipids isolated from the leaves of the tropical tree Bauhinia racemosa have been characterized as promising antifilarial agents in both in vitro and in vivo models (205) (Table 4). Several in vitro studies have identified promising natural compounds for treating schistosomiasis; among them are vernodalin, aspidin, flavaspidic acid, methylene-bis-aspidinol, and desaspidin (206, 207) (Table 4). The current antihelminthic drug, praziquantel, is not effective against the Schistosoma mansoni schistosomulum stage. Recently, four lead natural chemotherapeutic agents, including isomyristicin, bergapten, luteolin, and linalool oxide acetate, which were discovered from medicinal plants, showed efficacy against the schistosomulum stage and against multiple phylogenetically distinct parasites (82). Finally, some studies focusing on Chagas diseases have identified solenopsin alkaloids extracted from fire ants (Solenosis invicta and Solenosis saevissima), bisaprasin, and psilostachyin and psilostachyin C (sesquiterpene lactones isolated from Ambrosia tenuifolia and Ambrosia scabra) as promising antitrypanosomal agents (199, 208–210) (Table 4). Of note, the antiparasitic activity of the natural compounds described by de Souza and colleagues against L. brasiliensis and L. infantum was also observed against Trypanosoma cruzi (201). In the context of human African trypanosomiasis (HAT), phenolic compounds extracted from medicinal plants in vitro (211) and a sesquiterpene lactone, cynaropicrin (from Asteraceae plants in vivo), have exhibited potent activity against T. brucei (210, 212). Further reviews of active natural products against tropical parasites can be found in studies by Cockram and Smith (213) and Herrera Acevedo et al. (190).
Viruses
Natural products as potential novel therapeutics have been explored, especially in the context of dengue fever (214, 215). Oliveira and colleagues provide a detailed review of natural products that showed antiviral activities against dengue virus (DENV) and chikungunya virus (216). A Malaysian open-label randomized controlled trial (RCT) that included patients suffering from dengue fever and dengue hemorrhagic fever has demonstrated that papaya extract was effective in improving symptoms by increasing the platelet count, as well as the expression of some genes implicated in the de novo regeneration of platelets, less than 2 days after receiving the first dose of fresh Carica papaya leaf juice (217) (Table 5). Similar results have been obtained in an Indonesian RCT in which C. papaya leaf extract was encapsulated and administered orally (218) (Table 5). Additionally, antiviral activity against the four serotypes of DENV (DENV-1, -2, -3, and -4) has also been reported for Lippia species plants (219) (Table 5). Inhibitory activities of natural products against Ebola have also been explored, and aqueous extract of Prunella vulgaris has been shown to be a potent inhibitor of EBOV entry in vitro (220) (Table 5).
TABLE 5.
Active compounds from natural products with activity against tropical disease-causing virusesa
| Disease | Description and pathogenesis | Geographical distribution | Product family/class | Extracts/natural product | Product source or origin | Extraction method | Efficacy/assessment model | Biological activity | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Chikungunya | Chikungunya virus is transmitted through the bite of an infected mosquito | Mostly Africa, Asia, and the Americas | Diterpenoid esters and potentially other unidentified families | Several identified and unidentified compounds | 11 Euphorbia species plants collected from Corsica island, France | Solid-liquid or liquid-liquid extraction with ethyl acetate, methanol, or water from powdered plant material, such as whole plant, leaves, stems, roots, or aerial parts. Forty-five extracts from parts of 11 Euphorbiaceae plants were tested. | In vitro cell quantification of Vero cell cultures treated with plant extracts only or chikungunya virus inoculum and plant extract to derive a selectivity index (CC50 Vero cells/EC50 virus). | Selectivity index ranging from 1 to >47 (e.g., 4.1 μg/ml EC50 virus vs >100 μg/ml CC50 Vero cells). | 229 |
| Flavonoids | Luteolin and apigenin (major constituents of the fraction with antiviral activity) | Cynodon dactylon plants collected in the Western Ghats region around the Madurai District of Tamil Nadu, India | Powdered plant material defatted with petroleum ether, extracted with ethanol, and fractionated through silica gel. Five fractions were screened in a pilot, with 1 displaying sufficient antiviral activity for further testing. | In vitro cell quantification of Vero cells treated with titrated active fraction compared to Vero cells incubated with chikungunya virus inoculum, followed by treatment with titrated active fraction. Validated by reverse transcription-PCR of chikungunya virus E2 gene. | Expressed as a percentage of the untreated control, 25 μg/μl of active fraction resulted in 88% antiviral activity while displaying minimal cytotoxicity. Band displaying E2 gene product was significantly reduced. | 228 | |||
| Macrocyclic lactone | Abamectin | Streptomyces avermitilis | Identified from 2,933 compounds from three compound libraries: Spectrum (MicroSource Discovery Systems), NIH Clinical Collection I, and an FDA-approved drug library (ENZO Life Sciences). | In vitro cell quantification of BHK-21 and Huh-7.5 cells treated with test compounds and quantification of viral replication in chikungunya virus-infected BHK-21 and Huh-7.5 cells treated with test compounds to derive a selectivity index (CC50 cells/EC50 virus). Validated by reverse transcription-PCR and Western blotting of chikungunya virus RNA and proteins. | Selectivity index of 19.2 in BHK-21 cells and 10.9 in Huh-7.5 cells. Bands for chikungunya virus RNA and proteins are significantly reduced. | 230 | |||
| Ivermectin | Selectivity index of 62.4 in BHK-21 cells and 4.1 in Huh-7.5 cells. Bands for chikungunya virus RNA and proteins are significantly reduced. | ||||||||
| Benzylisoquinoline alkaloids | Berberine | Berberis vulgaris | Selectivity indexes of >55.64 in BHK-21 cells and >52.6 in Huh-7.5 cells. Bands for chikungunya virus RNA and proteins are significantly reduced. | ||||||
| Dengue | Dengue viruses (DENV-1, -2, -3, and -4) are spread through the bite of an infected Aedes species mosquito | Mostly Africa, Asia, and South America | Polysaccharides | Galactan | Cryptonemia crenulata collected from Cupe's beach, Pernambuco State, Brazil | Powdered whole-plant material extracted with water, followed by KCl fractionation and DEAE-Sephacel chromatography. | In vitro plaque reduction and virus yield inhibition assay on cell lines exposed to different dengue virus strains. | IC50 of 5.2–13.9 μg/ml against DENV-3 plaque formation or virus yield in three cell lines. IC50 of 3.6–14.7 μg/ml against DENV-2 plaque formation or virus yield in three cell lines. | 221 |
| Kappa carrageenan | Gymnogongrus griffithsiae collected in Caioba, Parana State, Brazil | Powdered whole-plant material extracted with water, followed by KCl fractionation. | IC50 of 5.2–13.9 μg/ml against DENV-3 plaque formation or virus yield in three cell lines. IC50 of 0.31–1.8 μg/ml against DENV-2 plaque formation or virus yield in three cell lines. | ||||||
| Flavonoid | Baicalein | Scutellaria baicalensis | Purchased from the Indofine Chemical Company (USA). | In vitro cytotoxicity compared to focus formation unit reduction and DENV-2 RNA quantitative reverse transcription-PCR of Vero cells infected with DENV-2 after various conditions of baicalein treatment to derive a selectivity index (CC50 cells/IC50 virus). | Selectivity index of 16.1 for antiviral adsorption activity. Selectivity index of 17.8 for postviral adsorption activity. Selectivity index of 21.3 for antiviral activity during continuous treatment. | 222 | |||
| Protoberberine alkaloid | Palmatine | Coptis chinensis | Purchased from Chengdu Mansite Pharmaceutical Company (China). | In vitro cytotoxicity compared to virus titer measurements from Vero cells infected with DENV-2 to derive a selectivity index (CC50 Vero cells/EC50 virus). | Selectivity index of 39. | 223 | |||
| Triterpenoid | Glycyrrhizin | Glycyrrhiza uralensis, G. glabra | Purchased from Sigma-Aldrich Chimie SARL (France). | In vitro cytotoxicity in confluent or proliferating Vero cells compared to virus-induced pathogenicity measurements from Vero cells infected with DENV-1, -2, and -4 to derive a selectivity index (CC50 Vero cells/EC50 virus). | Selectivity index of 6 for DENV-1 and DENV-2. Selectivity index of 4 for DENV-4. | 224 | |||
| Not specifically identified | Carica papaya | Fresh juice extracted using a juice extractor from Sekaki variant C. papaya leaves sourced from a private plantation in Selangor, Malaysia. | Open-label randomized controlled human trial involving recruited patients from the dengue ward in a hospital at Selangor, Malaysia. Patients received 50 g of fresh juice, 15 min after breakfast, for 3 days. | Increased platelet count and increased ALOX12 and PTAFR expression in patients from the intervention group. | 217 | ||||
| Capsules containing material extracted from C. papaya leaves with ethanol registered for sale in Indonesia. | Human trial involving dengue patients in Indonesia who received 24 capsules of C. papaya leaf extract taken twice daily. | Increased platelet counts, shorter hospitalization, and increased stability of hematocrit in patients from the intervention group. | 218 | ||||||
| Multiple chemical classes | Carvone/limonene/bicyclosesquiphellandrene/piperitenone/piperitone/β-bourboneno | Lippia alba | Experimental plant material was grown from propagation cuttings collected from the Cundinamarca and Antioquia regions in Colombia. Essential oil mixtures were extracted from plant material via microwave-assisted hydrodistillation in a Clevenger-type apparatus and identified through chromatography and spectroscopy. | In vitro cytotoxicity in Vero cells compared to virus titer measurements from Vero cells infected with treated DENV-1, -2, -3, and -4 to derive a selectivity index (CC50 Vero cells/EC50 virus). | Selectivity index of 4–349. | 219 | |||
| Geranial/neral/limonene/1,8-cineole/spathulenol/geraniol/trans-β-cariofilene/nerol/geranyl acetate | Lippia citriodora | Selectivity index of 2–30. | |||||||
| Ebola | A rare and deadly viral disease caused by genus Ebolavirus. The disease is transmitted through direct contact with an infected animal or sick or dead Ebola virus-infected human | Sub-Saharan Africa | Bisbenzylisoquinoline alkaloid | Tetrandrineb | Stephaniae tetrandra and other Menispermacae plant species | Tetrandrine was purchased from an unspecified commercial vendor. | In vivo mouse model of EBOV infection via the i.p. route. Mice were treated i.p. with tetrandrine periodically. Viral load in blood was measured via plaque-forming assays. | Significant protection against EBOV-induced weight loss, mortality, and clinical score. 1,000-fold decrease in viral load at day 3 of infection. | 232 |
| Not specifically identified | Prunella vulgaris | Dried fruit spikes were purchased from Tong Ren Tang Health Pharmaceutical Co., Ltd. (China), and were soaked and then boiled in deionized water. | In vitro viral infection inhibition assay on pretreated Vero E6 cells infected with recombinant eGFP-Zaire EBOV virus. | >50% reduction in infection in the presence of 6.25 μg/ml of aqueous Prunella vulgaris extract. | 220 | ||||
| Japanese encephalitis | Japanese encephalitis is a flavivirus transmitted via bite from an infected Culex species mosquito | Asia and the western Pacific | Triterpenoid | Glycyrrhizin | Glycyrrhiza uralensis, G. glabra | Purchased from Sigma-Aldrich Chimie SARL (France). | In vitro cytotoxicity in confluent or proliferating Vero cells compared to virus-induced pathogenicity measurements of Vero cells infected with JEV to derive a selectivity index (CC50 Vero cells/EC50 virus). | Selectivity index of 7. | 224 |
| Dihydroxyanthraquinone | Aloe-emodin | Rheum palmatum | Purchased from Sigma Chemical Co. (USA). | In vitro cytotoxicity in HL-CZ and TE-671 cells compared to a plaque reduction assay on HL-CZ and TE-671 cells pretreated with aloe-emodin and then infected with T1P1 strain of JEV to derive a therapeutic index (CC50 cells/IC50 virus). | Therapeutic index of >1,500. | 225 | |||
| West Nile fever | A mosquito-borne disease caused by vectors infected with West Nile virus spread through bite of an infected mosquito | Africa, parts of Europe, the Americas, Middle East, West Asia, and Australia | Triterpenoid | Glycyrrhizin | Glycyrrhiza uralensis, G. glabra | Purchased from Sigma-Aldrich Chimie SARL (France). | In vitro cytotoxicity in confluent or proliferating Vero cells compared to virus-induced pathogenicity measurements from Vero cells infected with WNV to derive a selectivity index (CC50 Vero cells/EC50 virus). | Selectivity index of 11 for proliferating healthy cells and >13 for confluent healthy cells. | 224 |
| Protoberberine alkaloid | Palmatine | Coptis chinensis | Purchased from Chengdu Mansite Pharmaceutical Company (China). | In vitro cytotoxicity compared to virus titer measurements from Vero cells infected with WNV to derive a selectivity index (CC50 Vero cells/EC50 virus). | Selectivity index of 286. | 223 | |||
| Yellow fever | A rare mosquito-borne viral infection spread through bite of an infected Aedes or Haemagogus species mosquito | Tropical and subtropical areas of Africa and South America | Terpenes | Carvone/limonene/bicyclosesquiphellandrene | Lippia alba collected from Jordan Sube, Colombia | Essential oil mixtures were extracted by microwave-assisted hydrodistillation in a Clevenger-type apparatus, followed by chromatographic and spectroscopic identification. | In vitro cytotoxicity in Vero cells compared to a plaque reduction assay of Vero cells infected with YFV treated with extracted essential oils to derive a CC50/MIC ratio. | CC50/MIC ratio of 22.9. | 226 |
| Carvacrol/thymol/γ-terpinene | Lippia origanoides collected from Jordan Sube, Colombia | CC50/MIC ratio of 26.4. | |||||||
| trans-Sabinene hydrate/thymol/carvacryl methyl ether/γ-terpineno/p-cimene | Oreganum vulgare collected from Marinilla, Colombia | CC50/MIC ratio of 26.5. | |||||||
| α-Thujone/β-thujone/1,8-cineole/trans-carveol/trans-carveol/sabinene | Artemisia vulgaris collected from Armenia, Colombia | CC50/MIC ratio of 8.8. | |||||||
| Protoberberine alkaloid | Palmatine | Coptis chinensis | Purchased from Chengdu Mansite Pharmaceutical Company (China). | In vitro cytotoxicity compared to virus titer measurements from Vero cells infected with YFV to derive a selectivity index (CC50 Vero cells/EC50 virus). | Selectivity index of 141. | 223 | |||
| Triterpenoid | Glycyrrhizin | Glycyrrhiza uralensis, G. glabra | Purchased from Sigma-Aldrich Chimie SARL (France). | In vitro cytotoxicity in confluent or proliferating Vero cells compared to virus-induced pathogenicity measurements from Vero cells infected with two strains of YFV to derive a selectivity index (CC50 Vero cells/EC50 virus). | Selectivity index of 5 to >6 against both strains of YFV. | 224 | |||
| Zika | A mosquito-borne viral infection spread through bite of an infected Aedes species mosquito, by sex, and by pregnant mother to fetus | Africa, Asia, and the Americas | Anthraquinone | Emodin | Rheum palmatum, Cassia obtusifolia, Polygonum multiflorum, Aloe barbadensis | Not mentioned. | In vitro cytotoxicity in Vero E6 cells compared to plaque-forming measurements from Vero E6 cells incubated with ZIKV to derive a selectivity index (CC50 Vero cells/IC50 virus). | Selectivity index of 21.44. | 231 |
| Benzylisoquinoline alkaloids | Berberine | Berberis vulgaris | Selectivity index of 5.65. | ||||||
| Triterpenoid | Glycyrrhizin | Glycyrrhiza uralensis, G. glabra | Purchased from Sigma-Aldrich Chimie SARL (France). | In vitro cytotoxicity in confluent or proliferating Vero cells compared to virus-induced pathogenicity measurements from Vero cells infected with ZKV to derive a selectivity index (CC50 Vero/EC50 virus). | Selectivity index of >7. | 224 | |||
i.p., intraperitoneal; eGFP, enhanced green fluorescent protein; ALOX12, arachidonate 12-lipoxygenase; PTAFR, platelet-activating factor receptor.
Tetrandrine, originally isolated from Chinese and Japanese herbs, is now produced synthetically.
Natural compounds such as galactan (221), kappa carrageenan (221), baicalein (222), and palmatine (223) have been shown to have antiviral activity against DENV-2, whereas glycyrrhizin has displayed antiviral effects against both DENV-1 and DENV-2 (224) (Table 5). Furthermore, glycyrrhizin has also shown antiviral activity against Japanese encephalitis virus (JEV), West Nile virus (WNV). and yellow fever virus (YFV) (224) (Table 5). Antiviral activity against WNV and YFV has also been described for palmatine (223), whereas anti-JEV activity has been reported for aloe emodin, a natural anthraquinone derived from Rheum palmatum (225) (Table 5). Additionally, a Colombian study revealed that essential oils extracted from Lippia alba, Lippia origanoides, Oreganum vulgare, and Artemisia vulgaris show inhibitory effects against YFV (226) (Table 5). The natural compounds from these plants include, among others, carvone, limonene, bicyclosesquiphellandrene, carvacrol, thymol, gamma-terpinene, trans-sabinene hydrate, and sabinene (226) (Table 5). Antiviral activity against other mosquito-transmitted viruses, including chikungunya virus (CHIKV) and Zika virus (ZIKV), has also been demonstrated for jatrophane esters (227), luteolin (228), apigenin (228), ethanolic extracts from several Euphorbia species (229), abamectin and ivermectin (230), berberine (230, 231), and emodin (231) (Table 5). Finally, tetrandine isolated from radix stephaniae tetrandrine, the dried root of Stephania tetrandra, was described as a potent EBOV entry inhibitor (232) (Table 5).
Fungi
Several studies have described plant-based treatments for various tropical fungal diseases (233–237) (Table 6). Interestingly, propolis (bee glue) from the honey bee Apis mellifera also demonstrates strong fungicidal effects against Paracoccidioides brasiliensis and Sporothrix schenckii (238, 239). Although the active antifungal product within propolis remains elusive, it was shown that p-coumaric acid appears to be the major compound (239).
TABLE 6.
Active compounds from natural products with activity against tropical disease-causing fungia
| Disease | Description and pathogenesis | Geographical distribution | Product family/class | Extracts/natural product | Product source | Extraction method | Efficacy/assessment model | Biological activity | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|
| Paracoccidioidomycosis | Fungal infection caused by the fungus Paracoccidioides | Central and South America | Flavonoids | Chalconoids | Cinnamomum verum | Nanoemulsion | In vitro: Antifungal activities of the compounds 2′ chalc and NLS + 2′ chalc were tested at different concentrations, 0.24–250 μg/ml, in a 96-well plate. MICs were observed via color change. MFCs were assessed for growth. | The MIC is the lowest compound concentration at which colonies were observed. NLS + 2′ chalc showed significant antifungal activity. | 240 |
| Terpenes | Methyl linolenate | Baccharis dracunculifolia | Dried plant part was extracted with ethanol and fractionated through silica gel. 105 fractions were collected; ursolic acid, methyl linolenate, caryophyllene oxide, and trans-nerolidol were isolated. | In vitro: Fungal strains inoculated with the fractions were visually compared with drug-free growth control. The MIC for which the well was optically clear was observed. | The MIC is the lowest compound concentration at which colonies were observed. | 241 | |||
| Caryophyllene oxide | Baccharis dracunculifolia | ||||||||
| Trans-nerolidol | Baccharis dracunculifolia | ||||||||
| Terpenes | Caryophyllene, kaurenoic acid, copalic acid | Copaifera langsdorffii | Copaiba resin oil was extracted from the trunk of a C. langsdorffii tree. A nanoemulsion of copaiba resin oil (CopaPlu) was formed by dissolving the extract and Pluronic F-127 in ethanol, followed by evaporation at 60°C. | In vitro: Copaiba resin oil and CopaPlu were tested at different concentrations against isolates of P. lutzi, P. brasiliensis, P. americana, and P. restrepiensis. The MIC was noted, and the MFC of 20 ml of each well with no visible fungal growth was examined. | Copaíba resin oil and CopaPlu inhibited the growth of all isolates. | 235 | |||
| Terpenes | Ethyl hydrocinnamate, spathulenol | Baccharis dracunculifolia | Aerial parts of B. dracunculifolia and P. regnellii were extracted by maceration with 80% ethanol and concentrated with hydroalcoholic extract, distilled in water, and extracted with hexane dichloromethane and ethyl acetate. | In vitro: Fungal strains were inoculated with the extracts and visually compared with drug-free growth control. The MIC for which the well was optically clear was observed. | The hexane fractions from both B. dracunculifolia and P. regnellii showed the best MIC value (7.8–30 μg/ml) against P. brasiliensis. | 236, 241 | |||
| Phenols | 1-Methoxy-4-(1 -propenyl)benzene, apiol | Piper regnellii | |||||||
| Crude propolis | Apis mellifera | Frozen propolis was ground and extracted with ethanol. The extracts were filtered and diluted in distilled water. | In vitro: The number of CFU of P. brasiliensis was assessed. | Fungicidal activities of macrophages of P. brasiliensis. | 238 | ||||
| Chromoblastomycosis | Chronic fungal disease caused by a variety of genera of the order Chaetothyriales | Africa and South America | Not specifically identified | Pterocaulon alopecuroides | Dried, powdered aerial parts of P. alopecuroides were macerated in methanol for 3 days at a ratio of 1:10 (wt/vol). | In vitro: The absence of organism growth indicates fungicidal activity. | Crude methanolic extract of P. alopecuroides inhibited organism growth of all chromoblastomycosis agents tested. | 233 | |
| Mycetomas | Chronic fungal infection of the skin and subcutaneous tissue caused by eumycetoma found in soil and water. | Sub-Saharan Africa, Central and South America, Asia | Phytosterols | Stigmatriene | Boswellia papyrifera | Dried, powdered samples were macerated in methanol for 7 days at room temperature. Crude methanol extract, hexane fraction, and a defatted methanol fraction were obtained from the vacuum-dried methanolic extract through liquid-liquid separation using hexane. | In vitro: Isolates were cultured for 10 days at 37°C. The MIC was defined as the first well in which no growth was visible. | Although all crude methanolic extracts were able to inhibit M. mycetomatis, lower concentrations of stigmatriene inhibited M. mycetomatis the most. | 234 |
| Crude extract | Acacia nubica | ||||||||
| Crude extract | Nigella sativa | ||||||||
| Sporotrichosis | Fungal infection caused by the fungus Sporothrix. | South America, China, South Africa | O-glucosyl-flavonoids, 16 prenylated benzophenone | Vismia guianensis | A mixture of stem bark or leaves of V. guianensis powder was soaked in ethanol and solvent solution at 10% (wt/vol), filtered, and reextracted with solvents, and extract was evaporated and lyophilized. |
In vitro: V. guianensis extracts were tested in S. schenckii isolates from humans. The MIC is the lowest antifungal agent concentration that inhibits fungal growth. Mice: Oral administration at a dose of 10 mg/kg body weight. |
In vitro: Treatment of S. schenckii with V. guianensis extracts inhibits the growth of the organism significantly. Mice: Clinically used for treating S. schenckii. |
237 | |
| Crude propolis | Apis mellifera | Hydroalcoholic extract of frozen brown propolis was evaporated, and dry matter was dissolved in phosphate buffer, emulsified, filtered, and diluted in sterile distilled water. | In vitro: Hydroalcoholic extract of brown propolis was tested in vitro against Sporothrix brasiliensis isolates. The MIC was noted, and the MFC of 10 ml of each well with no visible fungal growth was examined. | Treatment of Sporothrix brasiliensis with brown Brazilian propolis inhibited growth of 100% of the isolates. | 239 | ||||
Drugs are currently in (pre)clinical trials. 2’ chalc, 2’-hydroxychalcone; NLS, nanoemulsion; MFC, minimum fungicidal concentration.
Some studies have also investigated the antifungal activity of compounds isolated from natural products (Table 6). Medina-Alarcon and colleagues have reported that chalconoids derived from Cinnamomum verum display fungicidal activity against Paracoccidiodes brasiliensis and Paracoccidioides lutzii (240). Fungicidal effects against paracoccidioidomycosis have also been demonstrated with methyl linolenate, caryophyllene oxide, and trans-nerolidol derived from the Brazilian medicinal plant Baccharis dracunculifolia (241).
CONCLUSIONS
Here, we examine how natural products are being harnessed to develop solutions to the diseases of the tropics. Our literature search revealed that while there is vast knowledge of medicinal use from many tropical plants and animals, overall, the underlying active compounds remain largely unknown or else restricted to crude extracts. Further, while many emerging natural products have been tested for their efficacy against various tropical diseases, much of the work currently is done using in vitro assays. Given that the Amazon rainforest alone harbors at least 15,000 plant species (242) and the Great Barrier Reef is home to 1,400 species of coral, 3,000 species of mollusks, and 630 species of echinoderms (Australian Government Great Barrier Reef Marine Park Authority; http://www.gbrmpa.gov.au/, accessed 20 April 2020), the potential reservoir of natural products for treating tropical diseases is enormous. While we describe many natural products containing compounds with activity against tropical disease-causing pathogens, we find that there is currently a discovery bottleneck due to the high failure rate and the significant investment required to take a promising raw natural product forward in order to become a new standard-of-care treatment for a tropical pathogen. This limitation is being overcome with improved high-throughput technical capacity for natural product isolation and identification in many tropical areas of the world, ensuring that more of these natural reservoirs will likely reveal their pharmaceutical secrets in the near future.
ACKNOWLEDGMENTS
This work was supported by the National Health and Medical Research Council (NHMRC) of Australia through a Career Development Fellowship (APP1140709) to A.K. and a CJ Martin Early Career Fellowship (APP5121190) to M.A.F. M.J.S. is funded by NIH-NCI R01 grant 2R01CA164719-06A1, P.W. is funded by an NHMRC Ideas grant (APP1183323), and S.P. is supported by an AITHM Research Support Grant.
We declare that no conflicts of interest exist.
Biographies

Oyelola Adegboye, Ph.D., is a chartered biostatistician and a lecturer at James Cook University, College of Public Health, Medical and Veterinary Sciences. Dr. Adegboye completed his M.Sc. Biostatistics in 2010 at Hasselt University, Belgium, and obtained his Ph.D. in Statistics from the University of the Western Cape, South Africa, in 2014. He was previously a clinical research biostatistician in infectious disease at the Australian Institute of Tropical Health and Medicine, James Cook University. His current research interests focus on understanding the exposure-response relationship in tropical disease, One Health, and environmental studies. He has strong scholarly interests in spatial epidemiology, exposure science, and global health.

Matt A. Field, Ph.D., is a Principal Senior Research Fellow in Bioinformatics at the Australian Institute of Tropical Health and Medicine at James Cook University. He completed two Bachelor of Science degrees in computer science and biology from the University of British Columbia, followed by a Ph.D. in medical science in 2015. He currently holds an NHMRC CJ Martin Early Career Research Fellowship and is a founder and codirector of the Centre for Tropical Bioinformatics and Molecular Biology. He specializes in developing high-throughput bioinformatics analysis pipelines.

Andreas Kupz, Ph.D., is a microbiologist and immunologist and leads the tuberculosis immunology group at the Australian Institute of Tropical Health and Medicine as an NHMRC Career Development Fellow. Dr. Kupz completed his M.Sc./Diplom in biology at Humboldt-University in Berlin and a Ph.D. in microbiology and immunology at the University of Melbourne. He performed his postdoctoral training at the Max-Planck Institute for Infection Biology in Germany as an NHMRC CJ Martin Research Fellow. His interests include microbiology, immunology, and vaccine development. His current research focuses on interactions between the intracellular pathogenic microbes Mycobacterium tuberculosis and Toxoplasma gondii and the host immune system and their implications for the development of new vaccines.

Saparna Pai, Ph.D., is a Research Fellow at the Australian Institute of Tropical Health and Medicine, James Cook University, with 12 years’ experience in the field of cellular immunology. Most of this time is devoted to investigating the role of T cells in controlling two of the world's deadliest infectious diseases, malaria and tuberculosis. Dr. Pai began her research career as a Ph.D. scholar at the Indian Institute of Science, Bangalore, India. She later moved to QIMR Berghofer Medical Research Institute, Brisbane, Australia, to undertake Ph.D. studies on immune evasion of the host cytotoxic T lymphocyte response by Epstein-Barr virus. She spent the first 7 years of her postdoctoral career at the Translational Research Institute, Brisbane, in the area of dendritic cell biology. Her motivation to study infection in an in vivo setting led her to the University of Sydney, where she successfully generated a whole new multiphoton microscopy approach for the study of vascular inflammation.

Dileep Sharma, Ph.D., is a clinician researcher currently working as a Senior Lecturer and discipline lead in Periodontology, College of Medicine and Dentistry, James Cook University, Australia. Dr. Sharma completed his Ph.D. at Griffith University, Australia (2016). Previously, he completed his Bachelor’s degree in Dentistry and a Clinical Master’s in Periodontology from Rajiv Gandhi University of Health Sciences India. He started his teaching tenure in 2006 and has accumulated over a decade of teaching and research experience in various universities in both India and Australia. He has been research active for the last 15 years and leads a research team working on the development of novel approaches in managing periodontal diseases using natural products or phytochemicals.

Michael J. Smout, Ph.D., is a Senior Research Fellow at the Australian Institute of Tropical Health and Medicine at James Cook University. His postgraduate research initially explored skin cancer, followed by respiratory syncytial virus. Eventually, he was inspired by parasitic worms and ways to stop these infections with projects exploring vaccines for hookworms and schistosomes. His Ph.D. study focused on the carcinogenic secretions of the Thai liver fluke at the QIMR Berghofer Medical Research Institute and the University of Queensland. Since graduating in 2010, he has held research positions at James Cook University, based in Cairns, Australia. While his focus is still the carcinogenic host-parasite relationship of parasitic fluke “venom,” he has also explored other toxins from the wide range of fascinating deadly venoms of Australian animals, such as jellyfish, land and sea snakes, stonefish, and scorpions.

Phurpa Wangchuk, Ph.D., is a natural product chemist and leads the nature-based drug discovery group at the Australian Institute of Tropical Health and Medicine. Dr. Wangchuk completed his M.Sc. in medicinal chemistry and Ph.D. in natural product-based drug discovery from the University of Wollongong, Australia. He obtained his postgraduate diploma (health research methodology) from the University of Copenhagen, Denmark, and his B.Sc. (Biology and Chemistry as majors) from Delhi University, India. Prior to moving to Australia in 2014, Dr. Wangchuk led a research and development team at Manjong Sorig Pharmaceuticals, Ministry of Health, in Bhutan. His interests include natural products, metabolomics, and small molecule drug discovery from medicinal plants, fungi, and helminths. His current research focuses on isolation and drug lead identification for inflammatory bowel disease based on Aboriginal medicinal plants and mountaintop plants affected by climate change.

Yide Wong, Ph.D., is currently a Postdoctoral Research Fellow in the Molecular Helminthology group at the Australian Institute of Tropical Health and Medicine. His Ph.D., which was completed in 2017, involved investigating potential immunotherapies against Epstein-Barr virus-associated cancers at the QIMR Berghofer Medical Research Institute and the University of Queensland. Dr. Wong was previously a Postdoctoral Research Fellow studying immune modulation with natural and nonnatural compounds in the Molecular Immunology group at the Australia Institute of Tropical Health and Medicine. His interests include immunotherapy, human research, mass spectrometry, and proteomics. Dr. Wong’s current research focuses on proteomics around the host-parasite interaction, immunoparasitology, and general immunoproteomics.
Claire Loiseau, Ph.D., is an immunologist with interest in infectious diseases. She was a postdoc in the Malaria Immunology group at the Australian Institute of Tropical Health and Medicine at James Cook University. Her research interests focused on identifying the molecular and cellular mechanisms of the human immune responses to Plasmodium parasites by which high and low immune responders can be discriminated.
REFERENCES
- 1.Pan SY, Litscher G, Gao SH, Zhou SF, Yu ZL, Chen HQ, Zhang SF, Tang MK, Sun JN, Ko KM. 2014. Historical perspective of traditional indigenous medical practices: the current renaissance and conservation of herbal resources. Evid Based Complement Alternat Med 2014:525340. 10.1155/2014/525340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mojab F. 2012. Antimalarial natural products: a review. Avicenna J Phytomed 2:52–62. [PMC free article] [PubMed] [Google Scholar]
- 3.Artnzen CJ. 1997. Edible vaccines. Public Health Rep 112:190–197. [PMC free article] [PubMed] [Google Scholar]
- 4.Anonymous. 2013. WHO traditional medicine strategy: 2014–2023. WHO, Geneva, Switzerland. [Google Scholar]
- 5.Burns LH, Jin Z, Bowersox SS. 1999. The neuroprotective effects of intrathecal administration of the selective N-type calcium channel blocker ziconotide in a rat model of spinal ischemia. J Vasc Surg 30:334–343. 10.1016/S0741-5214(99)70145-X. [DOI] [PubMed] [Google Scholar]
- 6.Harvey AL, Edrada-Ebel R, Quinn RJ. 2015. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov 14:111–129. 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- 7.Willcox ML, Cosentino MJ, Pink R, Bodeker G, Wayling S. 2001. Natural products for the treatment of tropical diseases. Trends Parasitol 17:58–60. 10.1016/s1471-4922(00)01870-5. [DOI] [PubMed] [Google Scholar]
- 8.Lautié E, Russo O, Ducrot P, Boutin JA. 2020. Unraveling plant natural chemical diversity for drug discovery purposes. Front Pharmacol 11:397. 10.3389/fphar.2020.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zumla A, Ustianowski A. 2012. Tropical diseases: definition, geographic distribution, transmission, and classification. Infect Dis Clin North Am 26:195–205. 10.1016/j.idc.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitra AK, Mawson AR. 2017. Neglected tropical diseases: epidemiology and global burden. Trop Med Infect Dis 2:36. 10.3390/tropicalmed2030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engels D, Zhou XN. 2020. Neglected tropical diseases: an effective global response to local poverty-related disease priorities. Infect Dis Poverty 9:10. 10.1186/s40249-020-0630-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, Ginsberg A, Swaminathan S, Spigelman M, Getahun H, Menzies D, Raviglione M. 2016. Tuberculosis. Nat Rev Dis Primers 2:16076. 10.1038/nrdp.2016.76. [DOI] [PubMed] [Google Scholar]
- 13.Chandler DJ, Fuller LC. 2019. A review of scabies: an infestation more than skin deep. Dermatology 235:79–90. 10.1159/000495290. [DOI] [PubMed] [Google Scholar]
- 14.Jacob ST, Crozier I, Fischer WA, II, Hewlett A, Kraft CS, Vega MA, Soka MJ, Wahl V, Griffiths A, Bollinger L, Kuhn JH. 2020. Ebola virus disease. Nat Rev Dis Primers 6:13. 10.1038/s41572-020-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weil AA, Ryan ET. 2018. Cholera: recent updates. Curr Opin Infect Dis 31:455–461. 10.1097/QCO.0000000000000474. [DOI] [PubMed] [Google Scholar]
- 16.Wen H, Vuitton L, Tuxun T, Li J, Vuitton DA, Zhang W, McManus DP. 2019. Echinococcosis: advances in the 21st century. Clin Microbiol Rev 32 10.1128/CMR.00075-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shayan S, Bokaean M, Shahrivar MR, Chinikar S. 2015. Crimean-Congo hemorrhagic fever. Lab Med 46:180–189. 10.1309/LMN1P2FRZ7BKZSCO. [DOI] [PubMed] [Google Scholar]
- 18.Guzman MG, Gubler DJ, Izquierdo A, Martinez E, Halstead SB. 2016. Dengue infection. Nat Rev Dis Primers 2:16055. 10.1038/nrdp.2016.55. [DOI] [PubMed] [Google Scholar]
- 19.Álvarez-Hernández DA, Franyuti-Kelly GA, Díaz-López-Silva R, González-Chávez AM, González-Hermosillo-Cornejo D, Vázquez-López R. 2018. Chagas disease: current perspectives on a forgotten disease. Rev Med Hosp Gen (Mex) 81:154–164. 10.1016/j.hgmx.2016.09.010. [DOI] [Google Scholar]
- 20.Ashley EA, Pyae Phyo A, Woodrow CJ. 2018. Malaria. Lancet 391:1608–1621. 10.1016/S0140-6736(18)30324-6. [DOI] [PubMed] [Google Scholar]
- 21.Silva JVJ, Jr, Ludwig-Begall LF, Oliveira-Filho EF, Oliveira RAS, Duraes-Carvalho R, Lopes TRR, Silva DEA, Gil L. 2018. A scoping review of chikungunya virus infection: epidemiology, clinical characteristics, viral co-circulation complications, and control. Acta Trop 188:213–224. 10.1016/j.actatropica.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy PGE. 2019. Update on human African trypanosomiasis (sleeping sickness). J Neurol 266:2334–2337. 10.1007/s00415-019-09425-7. [DOI] [PubMed] [Google Scholar]
- 23.Pierson TC, Diamond MS. 2018. The emergence of Zika virus and its new clinical syndromes. Nature 560:573–581. 10.1038/s41586-018-0446-y. [DOI] [PubMed] [Google Scholar]
- 24.Das S, Kashyap B, Barua M, Gupta N, Saha R, Vaid L, Banka A. 2011. Nasal rhinosporidiosis in humans: new interpretations and a review of the literature of this enigmatic disease. Med Mycol 49:311–315. 10.3109/13693786.2010.526640. [DOI] [PubMed] [Google Scholar]
- 25.Francesconi VA, Klein AP, Santos AP, Ramasawmy R, Francesconi F. 2014. Lobomycosis: epidemiology, clinical presentation, and management options. Ther Clin Risk Manag 10:851–860. 10.2147/TCRM.S46251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White C, Franco-Paredes C. 2015. Leprosy in the 21st century. Clin Microbiol Rev 28:80–94. 10.1128/CMR.00079-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yotsu RR, Suzuki K, Simmonds RE, Bedimo R, Ablordey A, Yeboah-Manu D, Phillips R, Asiedu K. 2018. Buruli ulcer: a review of the current knowledge. Curr Trop Med Rep 5:247–256. 10.1007/s40475-018-0166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calba C, Guerbois-Galla M, Franke F, Jeannin C, Auzet-Caillaud M, Grard G, Pigaglio L, Decoppet A, Weicherding J, Savaill MC, Munoz-Riviero M, Chaud P, Cadiou B, Ramalli L, Fournier P, Noel H, De Lamballerie X, Paty MC, Leparc-Goffart I. 2017. Preliminary report of an autochthonous chikungunya outbreak in France, July to September 2017. Euro Surveill 22:17-00647. 10.2807/1560-7917.ES.2017.22.39.17-00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindh E, Argentini C, Remoli ME, Fortuna C, Faggioni G, Benedetti E, Amendola A, Marsili G, Lista F, Rezza G, Venturi G. 2019. The Italian 2017 outbreak chikungunya virus belongs to an emerging Aedes albopictus-adapted virus cluster introduced from the Indian subcontinent. Open Forum Infect Dis 6:ofy321. 10.1093/ofid/ofy321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Negredo A, de la Calle-Prieto F, Palencia-Herrejon E, Mora-Rillo M, Astray-Mochales J, Sanchez-Seco MP, Bermejo Lopez E, Menarguez J, Fernandez-Cruz A, Sanchez-Artola B, Keough-Delgado E, Ramirez de Arellano E, Lasala F, Milla J, Fraile JL, Ordobas Gavin M, Martinez de la Gandara A, Lopez Perez L, Diaz-Diaz D, Lopez-Garcia MA, Delgado-Jimenez P, Martin-Quiros A, Trigo E, Figueira JC, Manzanares J, Rodriguez-Baena E, Garcia-Comas L, Rodriguez-Fraga O, Garcia-Arenzana N, Fernandez-Diaz MV, Cornejo VM, Emmerich P, Schmidt-Chanasit J, Arribas JR, Crimean Congo Hemorrhagic Fever@Madrid Working Group . 2017. Autochthonous Crimean-Congo hemorrhagic fever in Spain. N Engl J Med 377:154–161. 10.1056/NEJMoa1615162. [DOI] [PubMed] [Google Scholar]
- 31.Giron S, Franke F, Decoppet A, Cadiou B, Travaglini T, Thirion L, Durand G, Jeannin C, L'Ambert G, Grard G, Noel H, Fournet N, Auzet-Caillaud M, Zandotti C, Aboukais S, Chaud P, Guedj S, Hamouda L, Naudot X, Ovize A, Lazarus C, de Valk H, Paty MC, Leparc-Goffart I. 2019. Vector-borne transmission of Zika virus in Europe, southern France, August 2019. Euro Surveill 24:1900655. 10.2807/1560-7917.ES.2019.24.45.1900655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nash D, Mostashari F, Fine A, Miller J, O'Leary D, Murray K, Huang A, Rosenberg A, Greenberg A, Sherman M, Wong S, Layton M, West Nile Outbreak Response Working Group . 2001. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med 344:1807–1814. 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- 33.McGuinness SL, Boyd R, Kidd S, McLeod C, Krause VL, Ralph AP. 2016. Epidemiological investigation of an outbreak of cutaneous sporotrichosis, Northern Territory, Australia. BMC Infect Dis 16:16. 10.1186/s12879-016-1338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Messina JP, Brady OJ, Golding N, Kraemer MUG, Wint GRW, Ray SE, Pigott DM, Shearer FM, Johnson K, Earl L, Marczak LB, Shirude S, Davis Weaver N, Gilbert M, Velayudhan R, Jones P, Jaenisch T, Scott TW, Reiner RC, Jr, Hay SI. 2019. The current and future global distribution and population at risk of Dengue. Nat Microbiol 4:1508–1515. 10.1038/s41564-019-0476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.do Valle ACF, Marques de Macedo P, Almeida-Paes R, Romao AR, Lazera MDS, Wanke B. 2017. Paracoccidioidomycosis after highway construction, Rio de Janeiro, Brazil. Emerg Infect Dis 23:1917–1919. 10.3201/eid2311.170934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellwanger JH, Kulmann-Leal B, Kaminski VL, Valverde-Villegas JM, Veiga A, Spilki FR, Fearnside PM, Caesar L, Giatti LL, Wallau GL, Almeida SEM, Borba MR, Hora VPD, Chies JAB. 2020. Beyond diversity loss and climate change: impacts of Amazon deforestation on infectious diseases and public health. An Acad Bras Cienc 92:e20191375. 10.1590/0001-3765202020191375. [DOI] [PubMed] [Google Scholar]
- 37.Wierzba TF. 2019. Oral cholera vaccines and their impact on the global burden of disease. Hum Vaccin Immunother 15:1294–1301. 10.1080/21645515.2018.1504155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas SJ, Yoon IK. 2019. A review of Dengvaxia(R): development to deployment. Hum Vaccin Immunother 15:2295–2314. 10.1080/21645515.2019.1658503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hegde NR, Gore MM. 2017. Japanese encephalitis vaccines: immunogenicity, protective efficacy, effectiveness, and impact on the burden of disease. Hum Vaccin Immunother 13:1–18. 10.1080/21645515.2017.1285472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kessels J, Tarantola A, Salahuddin N, Blumberg L, Knopf L. 2019. Rabies post-exposure prophylaxis: a systematic review on abridged vaccination schedules and the effect of changing administration routes during a single course. Vaccine 37(Suppl 1):A107–A117. 10.1016/j.vaccine.2019.01.041. [DOI] [PubMed] [Google Scholar]
- 41.Denis M, Knezevic I, Wilde H, Hemachudha T, Briggs D, Knopf L. 2019. An overview of the immunogenicity and effectiveness of current human rabies vaccines administered by intradermal route. Vaccine 37(Suppl 1):A99–A106. 10.1016/j.vaccine.2018.11.072. [DOI] [PubMed] [Google Scholar]
- 42.Ruzek D, Avsic Zupanc T, Borde J, Chrdle A, Eyer L, Karganova G, Kholodilov I, Knap N, Kozlovskaya L, Matveev A, Miller AD, Osolodkin DI, Overby AK, Tikunova N, Tkachev S, Zajkowska J. 2019. Tick-borne encephalitis in Europe and Russia: review of pathogenesis, clinical features, therapy, and vaccines. Antiviral Res 164:23–51. 10.1016/j.antiviral.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Collins ND, Barrett AD. 2017. Live attenuated yellow fever 17D vaccine: a legacy vaccine still controlling outbreaks in modern day. Curr Infect Dis Rep 19:14. 10.1007/s11908-017-0566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersen P, Scriba TJ. 2019. Moving tuberculosis vaccines from theory to practice. Nat Rev Immunol 19:550–562. 10.1038/s41577-019-0174-z. [DOI] [PubMed] [Google Scholar]
- 45.Bhattacharya SK. 2003. An evaluation of current cholera treatment. Expert Opin Pharmacother 4:141–146. 10.1517/14656566.4.2.141. [DOI] [PubMed] [Google Scholar]
- 46.Qadir MI, Arshad A, Ahmad B. 2013. Zinc: role in the management of diarrhea and cholera. World J Clin Cases 1:140–142. 10.12998/wjcc.v1.i4.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gustavsen KM, Colatrella BD, McCoy T. 2018. For as long as necessary: examining 30 years of MSD's focus on achieving elimination of onchocerciasis and lymphatic filariasis. Int Health 10:i3–i6. 10.1093/inthealth/ihx038. [DOI] [PubMed] [Google Scholar]
- 48.Akinsolu FT, Nemieboka PO, Njuguna DW, Ahadji MN, Dezso D, Varga O. 2019. Emerging resistance of neglected tropical diseases: a scoping review of the literature. Int J Environ Res Public Health 16:1925. 10.3390/ijerph16111925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dias DA, Urban S, Roessner U. 2012. A historical overview of natural products in drug discovery. Metabolites 2:303–3336. 10.3390/metabo2020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bohlin L, Göransson U, Alsmark C, Wedén C, Backlund A. 2010. Natural products in modern life science. Phytochem Rev 9:279–301. 10.1007/s11101-009-9160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dotson JD. 2019. Plants and animals found in tropical regions. Types of environmental ecosystems. https://sciencing.com/plants-animals-found-tropical-regions-6562132.html.
- 52.Metcalfe DJ, Ford AJ. 2009. A re-evaluation of Queensland’s wet tropics based on primitive plants. Pac Conserv Biol 15:80–86. 10.1071/PC090080. [DOI] [Google Scholar]
- 53.Wangchuk P, Loukas A. 2018. Techniques and technologies for the biodiscovery of novel small molecule drug lead compounds from natural products, p 435–465. In Mandal SC, Mandal V, Konishi T (ed), Natural products and drug discovery. Elsevier, Amsterdam, Netherlands. 10.1016/b978-0-08-102081-4.00016-2. [DOI] [Google Scholar]
- 54.Newman DJ. 2016. Developing natural product drugs: supply problems and how they have been overcome. Pharmacol Ther 162:1–9. 10.1016/j.pharmthera.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 55.Verpoorte R. 1999. Chemodiversity and the biological role of secondary metabolites, some thoughts for selecting plant material for drug development, p 11–23. In Bohlin L, Bruhn JG (ed), Bioassay methods in natural product research and drug development, vol 43. Kluwer Academic Publishers, Dordrecht, The Netherlands. [Google Scholar]
- 56.Yeshi K, Ruscher R, Hunter L, Daly NL, Loukas A, Wangchuk P. 2020. Revisiting inflammatory bowel disease: pathology, treatments, challenges and emerging therapeutics including drug leads from natural products. J Clin Microbiol 9:1273. 10.3390/jcm9051273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hostettmann K, Marston A, Wolfender JL. 1995. Strategy in the search for new biologically active plant constituents, p 17–45. In Hostettmann K, Marston A, Mailard M, Hamburger M (ed), Phytochemistry of plants used in traditional medicine. Oxford University Press, Inc, New York, NY. [Google Scholar]
- 58.Fabricant DS, Farnsworth NR. 2001. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect 109:69–75. 10.2307/3434847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teichmann K, Kuliberda M, Schatzmayr G, Hadacek F, Joachim A. 2012. In vitro determination of anticryptosporidial activity of phytogenic extracts and compounds. Parasitol Res 111:231–240. 10.1007/s00436-012-2824-y. [DOI] [PubMed] [Google Scholar]
- 60.Ledoux A, St-Gelais A, Cieckiewicz E, Jansen O, Bordignon A, Illien B, Di Giovanni N, Marvilliers A, Hoareau F, Pendeville H, Quetin-Leclercq J, Frederich M. 2017. Antimalarial activities of alkyl cyclohexenone derivatives isolated from the leaves of Poupartia borbonica. J Nat Prod 80:1750–1757. 10.1021/acs.jnatprod.6b01019. [DOI] [PubMed] [Google Scholar]
- 61.Jansen O, Tits M, Angenot L, Nicolas JP, De Mol P, Nikiema JB, Frederich M. 2012. Anti-plasmodial activity of Dicoma tomentosa (Asteraceae) and identification of urospermal A-15-O-acetate as the main active compound. Malar J 11:289. 10.1186/1475-2875-11-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kessler RL, Gradia DF, Pontello Rampazzo R.dC, Lourenço ÉE, Fidêncio NJ, Manhaes L, Probst CM, Ávila AR, Fragoso SP. 2013. Stage-regulated GFP expression in Trypanosoma cruzi: applications from host-parasite interactions to drug screening. PLoS One 8:e67441. 10.1371/journal.pone.0067441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Melo ARB, Maciel Higino TM, da Rocha Oliveira ADP, Fontes A, da Silva DCN, de Castro M, Dantas Lopes JA, de Figueiredo R. 2020. Lippia sidoides and Lippia origanoides essential oils affect the viability, motility and ultrastructure of Trypanosoma cruzi. Micron 129:102781. 10.1016/j.micron.2019.102781. [DOI] [PubMed] [Google Scholar]
- 64.Leidenberger M, Voigtlander C, Simon N, Kappes B. 2017. SYBR((R)) green I-based fluorescence assay to assess cell viability of malaria parasites for routine use in compound screening. Methods Mol Biol 1601:97–110. 10.1007/978-1-4939-6960-9_9. [DOI] [PubMed] [Google Scholar]
- 65.Duffy S, Avery VM. 2013. Identification of inhibitors of Plasmodium falciparum gametocyte development. Malar J 12:408. 10.1186/1475-2875-12-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Franco CH, Alcantara LM, Chatelain E, Freitas-Junior L, Moraes CB. 2019. Drug discovery for Chagas disease: impact of different host cell lines on assay performance and hit compound selection. Trop Med Infect Dis 4:82. 10.3390/tropicalmed4020082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paveley RA, Mansour NR, Hallyburton I, Bleicher LS, Benn AE, Mikic I, Guidi A, Gilbert IH, Hopkins AL, Bickle QD. 2012. Whole organism high-content screening by label-free, image-based Bayesian classification for parasitic diseases. PLoS Negl Trop Dis 6:e1762. 10.1371/journal.pntd.0001762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rinaldi G, Loukas A, Brindley PJ, Irelan JT, Smout MJ. 2015. Viability of developmental stages of Schistosoma mansoni quantified with xCELLigence worm real-time motility assay (xWORM). Int J Parasitol Drugs Drug Resist 5:141–148. 10.1016/j.ijpddr.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smout MJ, Kotze AC, McCarthy JS, Loukas A. 2010. A novel high throughput assay for anthelmintic drug screening and resistance diagnosis by real-time monitoring of parasite motility. PLoS Negl Trop Dis 4:e885. 10.1371/journal.pntd.0000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mansour NR, Bickle QD. 2010. Comparison of microscopy and Alamar blue reduction in a larval based assay for schistosome drug screening. PLoS Negl Trop Dis 4:e795. 10.1371/journal.pntd.0000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tritten L, Braissant O, Keiser J. 2012. Comparison of novel and existing tools for studying drug sensitivity against the hookworm Ancylostoma ceylanicum in vitro. Parasitology 139:348–357. 10.1017/S0031182011001934. [DOI] [PubMed] [Google Scholar]
- 72.Peak E, Chalmers IW, Hoffmann KF. 2010. Development and validation of a quantitative, high-throughput, fluorescent-based bioassay to detect schistosoma viability. PLoS Negl Trop Dis 4:e759. 10.1371/journal.pntd.0000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peak E, Hoffmann KF. 2011. Cross-disciplinary approaches for measuring parasitic helminth viability and phenotype. An Acad Bras Cienc 83:649–662. 10.1590/s0001-37652011000200024. [DOI] [PubMed] [Google Scholar]
- 74.Howe S, Zophel D, Subbaraman H, Unger C, Held J, Engleitner T, Hoffmann WH, Kreidenweiss A. 2015. Lactate as a novel quantitative measure of viability in Schistosoma mansoni drug sensitivity assays. Antimicrob Agents Chemother 59:1193–1199. 10.1128/AAC.03809-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lalli C, Guidi A, Gennari N, Altamura S, Bresciani A, Ruberti G. 2015. Development and validation of a luminescence-based, medium-throughput assay for drug screening in Schistosoma mansoni. PLoS Negl Trop Dis 9:e0003484. 10.1371/journal.pntd.0003484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leung CK, Deonarine A, Strange K, Choe KP. 2011. High-throughput screening and biosensing with fluorescent C. elegans strains. J Vis Exp 2011:2745. 10.3791/2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Solis GM, Petrascheck M. 2011. Measuring Caenorhabditis elegans life span in 96 well microtiter plates. J Vis Exp 2011:2496. 10.3791/2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elfawal MA, Savinov SN, Aroian RV. 2019. Drug screening for discovery of broad-spectrum agents for soil-transmitted nematodes. Sci Rep 9:12347. 10.1038/s41598-019-48720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weng HB, Chen HX, Wang MW. 2018. Innovation in neglected tropical disease drug discovery and development. Infect Dis Poverty 7:67. 10.1186/s40249-018-0444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sundaraneedi MK, Tedla BA, Eichenberger RM, Becker L, Pickering D, Smout MJ, Rajan S, Wangchuk P, Keene FR, Loukas A, Collins JG, Pearson MS. 2017. Polypyridylruthenium(II) complexes exert anti-schistosome activity and inhibit parasite acetylcholinesterases. PLoS Negl Trop Dis 11:e0006134. 10.1371/journal.pntd.0006134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wangchuk P, Apte SH, Smout MJ, Groves PL, Loukas A, Doolan DL. 2018. Defined small molecules produced by Himalayan medicinal plants display immunomodulatory properties. Int J Mol Sci 19:3490. 10.3390/ijms19113490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wangchuk P, Pearson MS, Giacomin PR, Becker L, Sotillo J, Pickering D, Smout MJ, Loukas A. 2016. Compounds derived from the Bhutanese daisy, Ajania nubigena, demonstrate dual anthelmintic activity against Schistosoma mansoni and Trichuris muris. PLoS Negl Trop Dis 10:e0004908. 10.1371/journal.pntd.0004908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chawla K, Modena MM, Ravaynia PS, Lombardo FC, Leonhardt M, Panic G, Burgel SC, Keiser J, Hierlemann A. 2018. Impedance-based microfluidic assay for automated antischistosomal drug screening. ACS Sens 3:2613–2620. 10.1021/acssensors.8b01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ravaynia PS, Lombardo FC, Biendl S, Dupuch MA, Keiser J, Hierlemann A, Modena MM. 2020. Parallelized impedance-based platform for continuous dose-response characterization of antischistosomal drugs. Adv Biosyst 4:e1900304. 10.1002/adbi.201900304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Storey B, Marcellino C, Miller M, Maclean M, Mostafa E, Howell S, Sakanari J, Wolstenholme A, Kaplan R. 2014. Utilization of computer processed high definition video imaging for measuring motility of microscopic nematode stages on a quantitative scale: “The Worminator.” Int J Parasitol Drugs Drug Resist 4:233–243. 10.1016/j.ijpddr.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marcellino C, Gut J, Lim KC, Singh R, McKerrow J, Sakanari J. 2012. WormAssay: a novel computer application for whole-plate motion-based screening of macroscopic parasites. PLoS Negl Trop Dis 6:e1494. 10.1371/journal.pntd.0001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rasmussen L, Tigabu B, White EL, Bostwick R, Tower N, Bukreyev A, Rockx B, LeDuc JW, Noah JW. 2015. Adapting high-throughput screening methods and assays for biocontainment laboratories. Assay Drug Dev Technol 13:44–54. 10.1089/adt.2014.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chauhan N, Poddar R. 2019. In silico pharmacophore modeling and simulation studies for searching potent antileishmanials targeted against Leishmania donovani nicotinamidase. Comput Biol Chem 83:107150. 10.1016/j.compbiolchem.2019.107150. [DOI] [PubMed] [Google Scholar]
- 89.Adeyemi OS, Atolani O, Awakan OJ, Olaolu TD, Nwonuma CO, Alejolowo O, Otohinoyi DA, Rotimi D, Owolabi A, Batiha GE. 2019. In vitro screening to identify anti-Toxoplasma compounds and in silico modeling for bioactivities and toxicity. Yale J Biol Med 92:369–383. [PMC free article] [PubMed] [Google Scholar]
- 90.Yunta MJR, Dietrich RC. 2019. Tropical and subtropical parasitic diseases: targets for a new approach to virtual screening. Mol Inform 38:e1900052. 10.1002/minf.201900052. [DOI] [PubMed] [Google Scholar]
- 91.Stevanovic S, Sencanski M, Danel M, Menendez C, Belguedj R, Bouraiou A, Nikolic K, Cojean S, Loiseau PM, Glisic S, Baltas M, Garcia-Sosa AT. 2019. Synthesis, in silico, and in vitro evaluation of anti-leishmanial activity of oxadiazoles and indolizine containing compounds flagged against anti-targets. Molecules 24:1282. 10.3390/molecules24071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Beutler JA. 2009. Natural products as a foundation for drug discovery. Curr Protoc Pharmacol Chaper 9:Unit 911. 10.1002/0471141755.ph0911s46. [DOI] [PubMed] [Google Scholar]
- 93.Zerikly M, Challis GL. 2009. Strategies for the discovery of new natural products by genome mining. Chembiochem 10:625–633. 10.1002/cbic.200800389. [DOI] [PubMed] [Google Scholar]
- 94.Milshteyn A, Schneider JS, Brady SF. 2014. Mining the metabiome: identifying novel natural products from microbial communities. Chem Biol 21:1211–1223. 10.1016/j.chembiol.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pidot S, Ishida K, Cyrulies M, Hertweck C. 2014. Discovery of clostrubin, an exceptional polyphenolic polyketide antibiotic from a strictly anaerobic bacterium. Angew Chem Int Ed Engl 53:7856–7859. 10.1002/anie.201402632. [DOI] [PubMed] [Google Scholar]
- 96.Zettler J, Xia H, Burkard N, Kulik A, Grond S, Heide L, Apel AK. 2014. New aminocoumarins from the rare actinomycete Catenulispora acidiphila DSM 44928: identification, structure elucidation, and heterologous production. Chembiochem 15:612–621. 10.1002/cbic.201300712. [DOI] [PubMed] [Google Scholar]
- 97.Ju KS, Gao J, Doroghazi JR, Wang KK, Thibodeaux CJ, Li S, Metzger E, Fudala J, Su J, Zhang JK, Lee J, Cioni JP, Evans BS, Hirota R, Labeda DP, van der Donk WA, Metcalf WW. 2015. Discovery of phosphonic acid natural products by mining the genomes of 10,000 actinomycetes. Proc Natl Acad Sci USA 112:12175–12180. 10.1073/pnas.1500873112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Iqbal HA, Low-Beinart L, Obiajulu JU, Brady SF. 2016. Natural product discovery through improved functional metagenomics in Streptomyces. J Am Chem Soc 138:9341–9344. 10.1021/jacs.6b02921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Trindade M, van Zyl LJ, Navarro-Fernandez J, Abd Elrazak A. 2015. Targeted metagenomics as a tool to tap into marine natural product diversity for the discovery and production of drug candidates. Front Microbiol 6:890. 10.3389/fmicb.2015.00890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bachmann BO, Van Lanen SG, Baltz RH. 2014. Microbial genome mining for accelerated natural products discovery: is a renaissance in the making? J Ind Microbiol Biotechnol 41:175–184. 10.1007/s10295-013-1389-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Challis GL. 2008. Genome mining for novel natural product discovery. J Med Chem 51:2618–2628. 10.1021/jm700948z. [DOI] [PubMed] [Google Scholar]
- 102.Blin K, Medema MH, Kottmann R, Lee SY, Weber T. 2017. The antiSMASH database, a comprehensive database of microbial secondary metabolite biosynthetic gene clusters. Nucleic Acids Res 45:D555–D559. 10.1093/nar/gkw960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khaldi N, Seifuddin FT, Turner G, Haft D, Nierman WC, Wolfe KH, Fedorova ND. 2010. SMURF: genomic mapping of fungal secondary metabolite clusters. Fungal Genet Biol 47:736–741. 10.1016/j.fgb.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Heel AJ, de Jong A, Montalban-Lopez M, Kok J, Kuipers OP. 2013. BAGEL3: automated identification of genes encoding bacteriocins and (non-)bactericidal posttranslationally modified peptides. Nucleic Acids Res 41:W448–W453. 10.1093/nar/gkt391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Skinnider MA, Dejong CA, Rees PN, Johnston CW, Li H, Webster AL, Wyatt MA, Magarvey NA. 2015. Genomes to natural products PRediction Informatics for Secondary Metabolomes (PRISM). Nucleic Acids Res 43:9645–9662. 10.1093/nar/gkv1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hadjithomas M, Chen IM, Chu K, Ratner A, Palaniappan K, Szeto E, Huang J, Reddy TB, Cimermancic P, Fischbach MA, Ivanova NN, Markowitz VM, Kyrpides NC, Pati A. 2015. IMG-ABC: a knowledge base to fuel discovery of biosynthetic gene clusters and novel secondary metabolites. mBio 6:e00932. 10.1128/mBio.00932-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tae H, Kong EB, Park K. 2007. ASMPKS: an analysis system for modular polyketide synthases. BMC Bioinformatics 8:327. 10.1186/1471-2105-8-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Henke MT, Kelleher NL. 2016. Modern mass spectrometry for synthetic biology and structure-based discovery of natural products. Nat Prod Rep 33:942–950. 10.1039/c6np00024j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kubota K, Funabashi M, Ogura Y. 2019. Target deconvolution from phenotype-based drug discovery by using chemical proteomics approaches. Biochim Biophys Acta Proteins Proteom 1867:22–27. 10.1016/j.bbapap.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 110.Yates JR, III, 2011. A century of mass spectrometry: from atoms to proteomes. Nat Methods 8:633–637. 10.1038/nmeth.1659. [DOI] [Google Scholar]
- 111.Yates JR, III, 2019. Recent technical advances in proteomics. F1000Res 8:F1000 Faculty Rev-351. 10.12688/f1000research.16987.1. [DOI] [Google Scholar]
- 112.Senko MW, Remes PM, Canterbury JD, Mathur R, Song Q, Eliuk SM, Mullen C, Earley L, Hardman M, Blethrow JD, Bui H, Specht A, Lange O, Denisov E, Makarov A, Horning S, Zabrouskov V. 2013. Novel parallelized quadrupole/linear ion trap/Orbitrap tribrid mass spectrometer improving proteome coverage and peptide identification rates. Anal Chem 85:11710–11714. 10.1021/ac403115c. [DOI] [PubMed] [Google Scholar]
- 113.Hoegg ED, Godin S, Szpunar J, Lobinski R, Koppenaal DW, Marcus RK. 2019. Coupling of an atmospheric pressure microplasma ionization source with an Orbitrap Fusion Lumos Tribrid 1M mass analyzer for ultra-high resolution isotopic analysis of uranium. J Anal At Spectrom 34:1387–1395. 10.1039/C9JA00154A. [DOI] [Google Scholar]
- 114.Kupchan SM, Britton RW, Lacadie JA, Ziegler MF, Sigel CW. 1975. Tumor inhibitors. 100. Isolation and structural elucidation of bruceantin and bruceantinol, new potent antileukemic quassinoids from Brucea antidysenterica. J Org Chem 40:648–654. 10.1021/jo00893a023. [DOI] [PubMed] [Google Scholar]
- 115.Molina DM, Jafari R, Ignatushchenko M, Seki T, Larsson EA, Dan C, Sreekumar L, Cao Y, Nordlund P. 2013. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 341:84–87. 10.1126/science.1233606. [DOI] [PubMed] [Google Scholar]
- 116.Savitski MM, Reinhard FBM, Franken H, Werner T, Savitski MF, Eberhard D, Molina DM, Jafari R, Dovega RB, Klaeger S, Kuster B, Nordlund P, Bantscheff M, Drewes G. 2014. Tracking cancer drugs in living cells by thermal profiling of the proteome. Science 346:1255784. 10.1126/science.1255784. [DOI] [PubMed] [Google Scholar]
- 117.He G, Yin Y, Yan X, Wang Y. 2017. Semi‐bionic extraction of effective ingredient from fishbone by high intensity pulsed electric fields. J Food Process Eng 40:e12392. 10.1111/jfpe.12392. [DOI] [Google Scholar]
- 118.Hofstetter R, Fassauer GM, Link A. 2018. Supercritical fluid extraction (SFE) of ketamine metabolites from dried urine and on-line quantification by supercritical fluid chromatography and single mass detection (on-line SFE-SFC-MS). J Chromatogr B Analyt Technol Biomed Life Sci 1076:77–83. 10.1016/j.jchromb.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 119.Gan Z, Liang Z, Chen X, Wen X, Wang Y, Li M, Ni Y. 2016. Separation and preparation of 6-gingerol from molecular distillation residue of Yunnan ginger rhizomes by high-speed counter-current chromatography and the antioxidant activity of ginger oils in vitro. J Chromatogr B Analyt Technol Biomed Life Sci 1011:99–107. 10.1016/j.jchromb.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 120.Morales D, Piris AJ, Ruiz‐Rodriguez A, Prodanov M, Soler‐Rivas C. 2018. Extraction of bioactive compounds against cardiovascular diseases from Lentinula edodes using a sequential extraction method. Biotechnol Prog 34:746–755. 10.1002/btpr.2616. [DOI] [PubMed] [Google Scholar]
- 121.Wishart DS. 2016. Emerging applications of metabolomics in drug discovery and precision medicine. Nat Rev Drug Discov 15:473–484. 10.1038/nrd.2016.32. [DOI] [PubMed] [Google Scholar]
- 122.Wangchuk P, Constantinoiu C, Eichenberger RM, Field M, Loukas A. 2019. Characterization of tapeworm metabolites and their reported biological activities. Molecules 24:1480. 10.3390/molecules24081480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wangchuk P, Lavers O, Wishart DS, Loukas A. 2020. Excretory/secretory metabolome of the zoonotic roundworm parasite Toxocara canis. Biomolecules 10:1157. 10.3390/biom10081157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wangchuk P. 2018. Therapeutic applications of natural products in herbal medicines, biodiscovery programs, and biomedicine. J Biol Active Products Nat 8:1–20. 10.1080/22311866.2018.1426495. [DOI] [Google Scholar]
- 125.Newman DJ, Cragg GM. 2020. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod 83:770–803. 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 126.Mushtaq S, Abbasi BH, Uzair B, Abbasi R. 2018. Natural products as reservoirs of novel therapeutic agents. EXCLI J 17:420–451. 10.17179/excli2018-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Newman DJ, Cragg GM. 2016. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79:629–661. 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 128.Smilack JD. 1999. The tetracyclines. Mayo Clin Proc 74:727–729. 10.4065/74.7.727. [DOI] [PubMed] [Google Scholar]
- 129.Roberts MC. 2003. Tetracycline therapy: update. Clin Infect Dis 36:462–467. 10.1086/367622. [DOI] [PubMed] [Google Scholar]
- 130.Gaillard T, Madamet M, Pradines B. 2015. Tetracyclines in malaria. Malar J 14:445. 10.1186/s12936-015-0980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chopra I, Roberts M. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev 65:232–260. 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Waksman SA, Geiger WB, Reynolds DM. 1946. Strain specificity and production of antibiotic substances. VII. Production of actinomycin by different actinomycetes. Proc Natl Acad Sci USA 32:117–120. 10.1073/pnas.32.5.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nelson ML, Levy SB. 2011. The history of the tetracyclines. Ann N Y Acad Sci 1241:17–32. 10.1111/j.1749-6632.2011.06354.x. [DOI] [PubMed] [Google Scholar]
- 134.Shutter MC, Akhondi H. 2020. Tetracycline. In StatPearls. StatPearls Publishing, Treasure Island, FL. [PubMed] [Google Scholar]
- 135.Falkow S. 1975. Infectious multiple drug resistance. Pion Ltd, London, United Kingdom. [Google Scholar]
- 136.Chopra I. 2001. Glycylcyclines: third-generation tetracycline antibiotics. Curr Opin Pharmacol 1:464–469. 10.1016/S1471-4892(01)00081-9. [DOI] [PubMed] [Google Scholar]
- 137.Chopra I. 2002. New developments in tetracycline antibiotics: glycylcyclines and tetracycline efflux pump inhibitors. Drug Resist Updat 5:119–125. 10.1016/s1368-7646(02)00051-1. [DOI] [PubMed] [Google Scholar]
- 138.Speer BS, Shoemaker NB, Salyers AA. 1992. Bacterial resistance to tetracycline: mechanisms, transfer, and clinical significance. Clin Microbiol Rev 5:387–399. 10.1128/CMR.5.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.WHO. 2015. WHO guidelines for the treatment of malaria, 3rd ed. WHO, Geneva, Switzerland. [Google Scholar]
- 140.Kapishnikov S, Staalsø T, Yang Y, Lee J, Pérez-Berná AJ, Pereiro E, Yang Y, Werner S, Guttmann P, Leiserowitz L, Als-Nielsen J. 2019. Mode of action of quinoline antimalarial drugs in red blood cells infected by Plasmodium falciparum revealed in vivo. Proc Natl Acad Sci USA 116:22946–22952. 10.1073/pnas.1910123116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kumar S, Bandyopadhyay U. 2005. Free heme toxicity and its detoxification systems in human. Toxicol Lett 157:175–188. 10.1016/j.toxlet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 142.Achan J, Talisuna AO, Erhart A, Yeka A, Tibenderana JK, Baliraine FN, Rosenthal PJ, D'Alessandro U. 2011. Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar J 10:144. 10.1186/1475-2875-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hsu E. 2006. The history of qing hao in the Chinese materia medica. Trans R Soc Trop Med Hyg 100:505–508. 10.1016/j.trstmh.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 144.Faurant C. 2011. From bark to weed: the history of artemisinin. Parasite 18:215–218. 10.1051/parasite/2011183215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bridgford JL, Xie SC, Cobbold SA, Pasaje CFA, Herrmann S, Yang T, Gillett DL, Dick LR, Ralph SA, Dogovski C, Spillman NJ, Tilley L. 2018. Artemisinin kills malaria parasites by damaging proteins and inhibiting the proteasome. Nat Commun 9:1–9. 10.1038/s41467-018-06221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Talman AM, Clain J, Duval R, Ménard R, Ariey F. 2019. Artemisinin bioactivity and resistance in malaria parasites. Trends Parasitol 35:953–963. 10.1016/j.pt.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 147.Eastman RT, Fidock DA. 2009. Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat Rev Microbiol 7:864–874. 10.1038/nrmicro2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ngezahayo J, Havyarimana F, Hari L, Stevigny C, Duez P. 2015. Medicinal plants used by Burundian traditional healers for the treatment of microbial diseases. J Ethnopharmacol 173:338–351. 10.1016/j.jep.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 149.Gupta VK, Kaushik A, Chauhan DS, Ahirwar RK, Sharma S, Bisht D. 2018. Anti-mycobacterial activity of some medicinal plants used traditionally by tribes from Madhya Pradesh, India for treating tuberculosis related symptoms. J Ethnopharmacol 227:113–120. 10.1016/j.jep.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 150.Tsouh Fokou PV, Nyarko AK, Appiah-Opong R, Tchokouaha Yamthe LR, Addo P, Asante IK, Boyom FF. 2015. Ethnopharmacological reports on anti-Buruli ulcer medicinal plants in three West African countries. J Ethnopharmacol 172:297–311. 10.1016/j.jep.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 151.Trébissou J, Bla K, Yapo A, Yapi H, Djaman A. 2014. Therapeutic survey on traditional treatment of Buruli ulcer in Côte d'Ivoire. J Microbiol Biotechnol 4:52–56. [Google Scholar]
- 152.Adjet A, Fokou G, Kouame D, Mosi L. 2013. Soins de l’ulcere de Buruli dans le district sanitaire de Yamoussoukro. Conférence Internationale Africa 2013 sur l’Ecosanté.
- 153.Addo P, Quartey M, Abbas M, Adu-Addai B, Owusu E, Okang I, Dodoo A, De Souza D, Ofori-Adjei D. 2007. In-vitro susceptibility of Mycobacterium ulcerans to herbal preparations. Internet J Trop Med 4. [Google Scholar]
- 154.Kodia M, Trébissou J, Crezoit Y, Eyangoh S, Asse H. 2014. Effects of Moringa oleifera, a plant extract coded obayokou on ulcers caused by Mycobacterium ulcerans in children under 15 years in Côte d'Ivoire. Int J Pharm Sci Drug 6:137–139. [Google Scholar]
- 155.Yemoa A, Gbenou J, Affolabi D, Moudachirou M, Bigot A, Anagonou S, Portaels F, Martin A, Quetin-Leclercq J. 2015. Beninese medicinal plants as a source of antimycobacterial agents: bioguided fractionation and in vitro activity of alkaloids isolated from Holarrhena floribunda used in traditional treatment of Buruli ulcer. BioMed Res Int 2015:835767. 10.1155/2015/835767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kone M, Vangah-Manda O, Kouakou H, Yapo AP, Bléyéré N, Datté Y. 2007. Influence de Sacoglottis gabonensis (Baille) Urban et de Okoubaka aubrevillei Normand et Pellegrin sur la croissance in vitro de Mycobacterium ulcerans. Le Pharmacien D’Afrique 206:17–22. [Google Scholar]
- 157.Arulmozhi P, Vijayakumar S, Praseetha PK, Jayanthi S. 2019. Extraction methods and computational approaches for evaluation of antimicrobial compounds from Capparis zeylanica L. Anal Biochem 572:33–44. 10.1016/j.ab.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 158.Aswal S, Kumar A, Semwal RB, Chauhan A, Kumar A, Lehmann J, Semwal DK. 2019. Drimia indica: a plant used in traditional medicine and its potential for clinical uses. Medicina (Kaunas) 55:255. 10.3390/medicina55060255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rahman AHMM. 2013. Ethno-medico-botanical studies on cucurbits of Rajshahi division, Bangladesh. J Medicinal Plants Studies 1:118–125. [Google Scholar]
- 160.Dey D, Ray R, Hazra B. 2014. Antitubercular and antibacterial activity of quinonoid natural products against multi-drug resistant clinical isolates. Phytother Res 28:1014–1021. 10.1002/ptr.5090. [DOI] [PubMed] [Google Scholar]
- 161.Mathew R, Kruthiventi AK, Prasad JV, Kumar SP, Srinu G, Chatterji D. 2010. Inhibition of mycobacterial growth by plumbagin derivatives. Chem Biol Drug Des 76:34–42. 10.1111/j.1747-0285.2010.00987.x. [DOI] [PubMed] [Google Scholar]
- 162.Sarkar A, Ghosh S, Shaw R, Patra MM, Calcuttawala F, Mukherjee N, Das Gupta SK. 2020. Mycobacterium tuberculosis thymidylate synthase (ThyX) is a target for plumbagin, a natural product with antimycobacterial activity. PLoS One 15:e0228657. 10.1371/journal.pone.0228657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Prasasty VD, Cindana S, Ivan FX, Zahroh H, Sinaga E. 2020. Structure-based discovery of novel inhibitors of Mycobacterium tuberculosis CYP121 from Indonesian natural products. Comput Biol Chem 85:107205. 10.1016/j.compbiolchem.2020.107205. [DOI] [PubMed] [Google Scholar]
- 164.Uc-Cachon AH, Borges-Argaez R, Said-Fernandez S, Vargas-Villarreal J, Gonzalez-Salazar F, Mendez-Gonzalez M, Caceres-Farfan M, Molina-Salinas GM. 2014. Naphthoquinones isolated from Diospyros anisandra exhibit potent activity against pan-resistant first-line drugs Mycobacterium tuberculosis strains. Pulm Pharmacol Ther 27:114–120. 10.1016/j.pupt.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 165.Kumar P, Singh A, Sharma U, Singh D, Dobhal MP, Singh S. 2013. Anti-mycobacterial activity of plumericin and isoplumericin against MDR Mycobacterium tuberculosis. Pulm Pharmacol Ther 26:332–335. 10.1016/j.pupt.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 166.Rehberg N, Omeje E, Ebada SS, van Geelen L, Liu Z, Sureechatchayan P, Kassack MU, Ioerger TR, Proksch P, Kalscheuer R. 2019. 3-O-methyl-alkylgallates inhibit fatty acid desaturation in Mycobacterium tuberculosis. Antimicrob Agents Chemother 63 10.1128/AAC.00136-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kim S, Seo H, Mahmud HA, Islam MI, Lee BE, Cho ML, Song HY. 2018. In vitro activity of collinin isolated from the leaves of Zanthoxylum schinifolium against multidrug- and extensively drug-resistant Mycobacterium tuberculosis. Phytomedicine 46:104–110. 10.1016/j.phymed.2018.04.029. [DOI] [PubMed] [Google Scholar]
- 168.Bunalema L, Fotso GW, Waako P, Tabuti J, Yeboah SO. 2017. Potential of Zanthoxylum leprieurii as a source of active compounds against drug resistant Mycobacterium tuberculosis. BMC Complement Altern Med 17:89. 10.1186/s12906-017-1602-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mehta A, Srivastva G, Kachhwaha S, Sharma M, Kothari SL. 2013. Antimycobacterial activity of Citrullus colocynthis (L.) Schrad. against drug sensitive and drug resistant Mycobacterium tuberculosis and MOTT clinical isolates. J Ethnopharmacol 149:195–200. 10.1016/j.jep.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 170.Bogatcheva E, Hanrahan C, Nikonenko B, de los Santos G, Reddy V, Chen P, Barbosa F, Einck L, Nacy C, Protopopova M. 2011. Identification of SQ609 as a lead compound from a library of dipiperidines. Bioorg Med Chem Lett 21:5353–5357. 10.1016/j.bmcl.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pasca MR, Degiacomi G, Ribeiro AL, Zara F, De Mori P, Heym B, Mirrione M, Brerra R, Pagani L, Pucillo L, Troupioti P, Makarov V, Cole ST, Riccardi G. 2010. Clinical isolates of Mycobacterium tuberculosis in four European hospitals are uniformly susceptible to benzothiazinones. Antimicrob Agents Chemother 54:1616–1618. 10.1128/AAC.01676-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Trefzer C, Skovierova H, Buroni S, Bobovska A, Nenci S, Molteni E, Pojer F, Pasca MR, Makarov V, Cole ST, Riccardi G, Mikusova K, Johnsson K. 2012. Benzothiazinones are suicide inhibitors of mycobacterial decaprenylphosphoryl-beta-D-ribofuranose 2'-oxidase DprE1. J Am Chem Soc 134:912–915. 10.1021/ja211042r. [DOI] [PubMed] [Google Scholar]
- 173.Makarov V, Lechartier B, Zhang M, Neres J, van der Sar AM, Raadsen SA, Hartkoorn RC, Ryabova OB, Vocat A, Decosterd LA, Widmer N, Buclin T, Bitter W, Andries K, Pojer F, Dyson PJ, Cole ST. 2014. Towards a new combination therapy for tuberculosis with next generation benzothiazinones. EMBO Mol Med 6:372–383. 10.1002/emmm.201303575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lechartier B, Cole ST. 2015. Mode of action of clofazimine and combination therapy with benzothiazinones against Mycobacterium tuberculosis. Antimicrob Agents Chemother 59:4457–4463. 10.1128/AAC.00395-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Quan DH, Nagalingam G, Luck I, Proschogo N, Pillalamarri V, Addlagatta A, Martinez E, Sintchenko V, Rutledge PJ, Triccas JA. 2019. Bengamides display potent activity against drug-resistant Mycobacterium tuberculosis. Sci Rep 9:14396. 10.1038/s41598-019-50748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Arai M, Sobou M, Vilcheze C, Baughn A, Hashizume H, Pruksakorn P, Ishida S, Matsumoto M, Jacobs WR, Jr, Kobayashi M. 2008. Halicyclamine A, a marine spongean alkaloid as a lead for anti-tuberculosis agent. Bioorg Med Chem 16:6732–6736. 10.1016/j.bmc.2008.05.061. [DOI] [PubMed] [Google Scholar]
- 177.Rodrigues Felix C, Roberts JC, Winder PL, Gupta R, Diaz MC, Pomponi SA, Wright AE, Rohde KH. 2019. Plakinamine P, a steroidal alkaloid with bactericidal activity against Mycobacterium tuberculosis. Mar Drugs 17:707. 10.3390/md17120707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Potroz MG, Cho NJ. 2015. Natural products for the treatment of trachoma and Chlamydia trachomatis. Molecules 20:4180–4203. 10.3390/molecules20034180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.da Costa DS, Negreiros PDS, da Silva VG, Nunes DB, Acha BT, Quintans-Junior LJ, Antunes de S, Araujo A, Araujo TSL, Sousa FBM, Medeiros JVR, Lima F, da Silva FI, Magalhaes Costa RK, Santos RFD, Oliveira RCM. 2020. Antidiarrheal activity of farnesol in rodents: pharmacological actions and molecular docking. Eur J Pharmacol 874:172986. 10.1016/j.ejphar.2020.172986. [DOI] [PubMed] [Google Scholar]
- 180.Bezerra FF, Lima GC, Sousa NA, Sousa WM, Costa LEC, Costa DSD, Barros FCN, Medeiros JVR, Freitas ALP. 2018. Antidiarrheal activity of a novel sulfated polysaccharide from the red seaweed Gracilaria cervicornis. J Ethnopharmacol 224:27–35. 10.1016/j.jep.2018.05.033. [DOI] [PubMed] [Google Scholar]
- 181.Lakshmi V, Kumar R, Gupta P, Varshney V, Srivastava M, Dikshit M, Murthy P, Misra-Bhattacharya S. 2004. The antifilarial activity of a marine red alga, Botryocladia leptopoda, against experimental infections with animal and human filariae. Parasitol Res 93:468–474. 10.1007/s00436-004-1159-8. [DOI] [PubMed] [Google Scholar]
- 182.Misra N, Sharma M, Raj K, Dangi A, Srivastava S, Misra-Bhattacharya S. 2007. Chemical constituents and antifilarial activity of Lantana camara against human lymphatic filariid Brugia malayi and rodent filariid Acanthocheilonema viteae maintained in rodent hosts. Parasitol Res 100:439–448. 10.1007/s00436-006-0312-y. [DOI] [PubMed] [Google Scholar]
- 183.Sahare K, Anandhraman V, Meshram V, Meshram S, Reddy M, Tumane P, Goswami K. 2008. Anti-microfilarial activity of methanolic extract of Vitex negundo and Aegle marmelos and their phytochemical analysis. Indian J Exp Biol 46:128–131. [PubMed] [Google Scholar]
- 184.Sahare K, Anandharaman V, Meshram V, Meshram S, Gajalakshmi D, Goswami K, Reddy M. 2008. In vitro effect of four herbal plants on the motility of Brugia malayi microfilariae. Indian J Med Res 127:467–472. [PubMed] [Google Scholar]
- 185.Misra S, Verma M, Mishra SK, Srivastava S, Lakshmi V, Misra-Bhattacharya S. 2011. Gedunin and photogedunin of Xylocarpus granatum possess antifilarial activity against human lymphatic filarial parasite Brugia malayi in experimental rodent host. Parasitol Res 109:1351–1360. 10.1007/s00436-011-2380-x. [DOI] [PubMed] [Google Scholar]
- 186.Gaur R, Sahoo M, Dixit S, Fatma N, Rastogi S, Kulshreshtha D, Chatterjee R, Murthy P. 2008. Antifilarial activity of Caesalpinia bonducella against experimental filarial infections. Indian J Med Res 128:65–70. [PubMed] [Google Scholar]
- 187.Teixeira TL, Teixeira SC, da Silva CV, de Souza MA. . 2014. Potential therapeutic use of herbal extracts in trypanosomiasis. Pathog Glob Health 108:30–36. 10.1179/2047773213Y.0000000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Willcox ML, Bodeker G. 2004. Traditional herbal medicines for malaria. BMJ 329:1156–1159. 10.1136/bmj.329.7475.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yerbanga RS, Lucantoni L, Lupidi G, Dori GU, Tepongning NR, Nikiema JB, Esposito F, Habluetzel A. 2012. Antimalarial plant remedies from Burkina Faso: their potential for prophylactic use. J Ethnopharmacol 140:255–260. 10.1016/j.jep.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 190.Herrera Acevedo C, Scotti L, Feitosa Alves M, Formiga Melo Diniz MDF, Scotti MT. 2017. Computer-aided drug design using sesquiterpene lactones as sources of new structures with potential activity against infectious neglected diseases. Molecules 22:79. 10.3390/molecules22010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dkhil MA, Abdel-Gaber R, Alojayri G, Al-Shaebi EM, Qasem MAA, Murshed M, Mares MM, El-Matbouli M, Al-Quraishy S. 2020. Biosynthesized silver nanoparticles protect against hepatic injury induced by murine blood-stage malaria infection. Environ Sci Pollut Res Int 27:17762–17769. 10.1007/s11356-020-08280-8. [DOI] [PubMed] [Google Scholar]
- 192.Ishak RA, Osman R, Awad GA. 2016. Dextran-based nanocarriers for delivery of bioactives. Curr Pharm Des 22:3411–3428. 10.2174/1381612822666160209152329. [DOI] [PubMed] [Google Scholar]
- 193.Arora D, Sharma N, Sharma V, Abrol V, Shankar R, Jaglan S. 2016. An update on polysaccharide-based nanomaterials for antimicrobial applications. Appl Microbiol Biotechnol 100:2603–2615. 10.1007/s00253-016-7315-0. [DOI] [PubMed] [Google Scholar]
- 194.Schully KL, Bell MG, Prouty AM, Gallovic MD, Gautam S, Peine KJ, Sharma S, Bachelder EM, Pesce JT, Elberson MA, Ainslie KM, Keane-Myers A. 2015. Evaluation of a biodegradable microparticulate polymer as a carrier for Burkholderia pseudomallei subunit vaccines in a mouse model of melioidosis. Int J Pharm 495:849–861. 10.1016/j.ijpharm.2015.09.059. [DOI] [PubMed] [Google Scholar]
- 195.Monteiro LM, Löbenberg R, Fotaki N, de Araújo GLB, Cotrim PC, Bou-Chacra N. 2019. Co-delivery of buparvaquone and polymyxin B in a nanostructured lipid carrier for leishmaniasis treatment. J Glob Antimicrob Resist 18:279–283. 10.1016/j.jgar.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 196.Grellier P, Ramiaramanana L, Millerioux V, Deharo E, Schrével J, Frappier F, Trigalo F, Bodo B, Pousset J-L. 1996. Antimalarial activity of cryptolepine and isocryptolepine, alkaloids isolated from Cryptolepis sanguinolenta. Phytother Res 10:317–321. . [DOI] [Google Scholar]
- 197.Cheng Fang TSU. 1947. Chang shan in the treatment of malaria. J Trop Med Hyg 50:75–77. [PubMed] [Google Scholar]
- 198.Jang CS, Fu FY. 1948. Pharmacology of ch'ang shan (Dichroa febrifuga) a Chinese antimalarial herb. Nature 161:400. 10.1038/161400b0. [DOI] [PubMed] [Google Scholar]
- 199.Oluwabusola ET, Tabudravu JN, Al Maqbali KS, Annang F, Perez-Moreno G, Reyes F, Jaspars M. 2020. Antiparasitic activity of bromotyrosine alkaloids and new analogues isolated from the Fijian marine sponge Aplysinella rhax. Chem Biodivers 17:32000335. 10.1002/cbdv.202000335. [DOI] [PubMed] [Google Scholar]
- 200.Bodiwala HS, Singh G, Singh R, Dey CS, Sharma SS, Bhutani KK, Singh IP. 2007. Antileishmanial amides and lignans from Piper cubeba and Piper retrofractum. J Nat Med 61:418–421. 10.1007/s11418-007-0159-2. [DOI] [Google Scholar]
- 201.de Souza CES, da Silva ARP, Gomez MCV, Rolóm M, Coronel C, da Costa JGM, Sousa AK, Rolim LA, de Souza FHS, Coutinho HDM. 2017. Anti-Trypanosoma, anti-Leishmania, and cytotoxic activities of natural products from Psidium brownianum Mart. ex DC. and Psidium guajava var. Pomifera analysed by LC-MS. Acta Trop 176:380–384. 10.1016/j.actatropica.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 202.Rabito MF, Britta EA, Pelegrini BL, Scariot DB, Almeida MB, Nixdorf SL, Nakamura CV, Ferreira ICP. 2014. In vitro and in vivo antileishmania activity of sesquiterpene lactone-rich dichloromethane fraction obtained from Tanacetum parthenium (L.) Schultz-Bip. Exp Parasitol 143:18–23. 10.1016/j.exppara.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 203.Tiuman TS, Ueda-Nakamura T, Cortez DAG, Dias Filho BP, Morgado-Díaz JA, de Souza W, Nakamura CV. 2005. Antileishmanial activity of parthenolide, a sesquiterpene lactone isolated from Tanacetum parthenium. Antimicrob Agents Chemother 49:176–182. 10.1128/AAC.49.11.176-182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tiuman TS, Ueda-Nakamura T, Alonso A, Nakamura CV. 2014. Cell death in amastigote forms of Leishmania amazonensis induced by parthenolide. BMC Microbiol 14:152. 10.1186/1471-2180-14-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sashidhara KV, Singh SP, Misra S, Gupta J, Misra-Bhattacharya S. 2012. Galactolipids from Bauhinia racemosa as a new class of antifilarial agents against human lymphatic filarial parasite, Brugia malayi. Eur J Med Chem 50:230–235. 10.1016/j.ejmech.2012.01.057. [DOI] [PubMed] [Google Scholar]
- 206.Jisaka M, Kawanaka M, Sugiyama H, Takegawa K, Huffman MA, Ohigashi H, Koshimizu K. 1992. Antischistosomal activities of sesquiterpene lactones and steroid glucosides from Vernonia amygdalina, possibly used by wild chimpanzees against parasite-related diseases. Biosci Biotechnol Biochem 56:845–846. 10.1271/bbb.56.845. [DOI] [PubMed] [Google Scholar]
- 207.Magalhães LG, Kapadia GJ, da Silva Tonuci LR, Caixeta SC, Parreira NA, Rodrigues V, Da Silva Filho AA. 2010. In vitro schistosomicidal effects of some phloroglucinol derivatives from Dryopteris species against Schistosoma mansoni adult worms. Parasitol Res 106:395–401. 10.1007/s00436-009-1674-8. [DOI] [PubMed] [Google Scholar]
- 208.Silva R, Fox EGP, Gomes FM, Feijo DF, Ramos I, Koeller CM, Costa TFR, Rodrigues NS, Lima AP, Atella GC, Miranda K, Schoijet AC, Alonso GD, de Alcantara Machado E, Heise N. 2020. Venom alkaloids against Chagas disease parasite: search for effective therapies. Sci Rep 10:10642. 10.1038/s41598-020-67324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sülsen VP, Puente V, Papademetrio D, Batlle A, Martino VS, Frank FM, Lombardo ME. 2016. Mode of action of the sesquiterpene lactones psilostachyin and psilostachyin C on Trypanosoma cruzi. PLoS One 11:e0150526. 10.1371/journal.pone.0150526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.da Silva CF, da Gama Jaen Batista D, Siciliano De Araújo J, Batista MM, Lionel J, Mello de Souza E, Hammer ER, Bernardino da Silva P, De Mieri M, Adams M, Zimmermann S, Hamburger M, Brun R, Schühly W, de Nazare Correia Soeiro M. 2013. Activities of psilostachyin A and cynaropicrin against Trypanosoma cruzi in vitro and in vivo. Antimicrob Agents Chemother 57:5307–5314. 10.1128/AAC.00595-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sun YN, No JH, Lee GY, Li W, Yang SY, Yang G, Schmidt TJ, Kang JS, Kim YH. 2016. Phenolic constituents of medicinal plants with activity against Trypanosoma brucei. Molecules 21:480. 10.3390/molecules21040480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zimmermann S, Kaiser M, Brun R, Hamburger M, Adams M. 2012. Cynaropicrin: the first plant natural product with in vivo activity against Trypanosoma brucei. Planta Med 78:553–556. 10.1055/s-0031-1298241. [DOI] [PubMed] [Google Scholar]
- 213.Cockram PE, Smith TK. 2018. Active natural product scaffolds against trypanosomatid parasites: a review. J Nat Prod 81:2138–2154. 10.1021/acs.jnatprod.8b00159. [DOI] [PubMed] [Google Scholar]
- 214.Kadir SLA, Yaakob H, Zulkifli RM. 2013. Potential anti-dengue medicinal plants: a review. J Nat Med 67:677–689. 10.1007/s11418-013-0767-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sarala N, Paknikar S. 2014. Papaya extract to treat dengue: a novel therapeutic option? Ann Med Health Sci Res 4:320–324. 10.4103/2141-9248.133452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Oliveira AF, Teixeira RR, Oliveira AS, Souza AP, Silva ML, Paula SO. 2017. Potential antivirals: natural products targeting replication enzymes of dengue and chikungunya viruses. Molecules 22:505. 10.3390/molecules22030505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Subenthiran S, Choon TC, Cheong KC, Thayan R, Teck MB, Muniandy PK, Afzan A, Abdullah NR, Ismail Z. 2013. Carica papaya leaves juice significantly accelerates the rate of increase in platelet count among patients with dengue fever and dengue haemorrhagic fever. Evid Based Complement Alternat Med 2013:1–7. 10.1155/2013/616737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yunita F, Hanani E, Kristianto J. 2012. The effect of Carica papaya L. leaves extract capsules on platelets count and hematocrit level in dengue fever patient. Int J Med Aromat Plants 2:573–578. [Google Scholar]
- 219.Ocazionez RE, Meneses R, Torres FA, Stashenko E. 2010. Virucidal activity of Colombian Lippia essential oils on dengue virus replication in vitro. Mem Inst Oswaldo Cruz 105:304–309. 10.1590/s0074-02762010000300010. [DOI] [PubMed] [Google Scholar]
- 220.Zhang X, Ao Z, Bello A, Ran X, Liu S, Wigle J, Kobinger G, Yao X. 2016. Characterization of the inhibitory effect of an extract of Prunella vulgaris on Ebola virus glycoprotein (GP)-mediated virus entry and infection. Antiviral Res 127:20–31. 10.1016/j.antiviral.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Talarico LB, Pujol CA, Zibetti RG, Faria PC, Noseda MD, Duarte ME, Damonte EB. 2005. The antiviral activity of sulfated polysaccharides against dengue virus is dependent on virus serotype and host cell. Antiviral Res 66:103–110. 10.1016/j.antiviral.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 222.Zandi K, Teoh B-T, Sam S-S, Wong P-F, Mustafa MR, AbuBakar S. 2012. Novel antiviral activity of baicalein against dengue virus. BMC Complement Altern Med 12:214. 10.1186/1472-6882-12-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Jia F, Zou G, Fan J, Yuan Z. 2010. Identification of palmatine as an inhibitor of West Nile virus. Arch Virol 155:1325–1329. 10.1007/s00705-010-0702-4. [DOI] [PubMed] [Google Scholar]
- 224.Crance JM, Scaramozzino N, Jouan A, Garin D. 2003. Interferon, ribavirin, 6-azauridine and glycyrrhizin: antiviral compounds active against pathogenic flaviviruses. Antiviral Res 58:73–79. 10.1016/S0166-3542(02)00185-7. [DOI] [PubMed] [Google Scholar]
- 225.Lin C-W, Wu C-F, Hsiao N-W, Chang C-Y, Li S-W, Wan L, Lin Y-J, Lin W-Y. 2008. Aloe-emodin is an interferon-inducing agent with antiviral activity against Japanese encephalitis virus and enterovirus 71. Int J Antimicrob Agents 32:355–359. 10.1016/j.ijantimicag.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Meneses R, Ocazionez RE, Martínez JR, Stashenko EE. 2009. Inhibitory effect of essential oils obtained from plants grown in Colombia on yellow fever virus replication in vitro. Ann Clin Microbiol Antimicrob 8:8. 10.1186/1476-0711-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Nothias-Scaglia L-F, Retailleau P, Paolini J, Pannecouque C, Neyts J, Dumontet V, Roussi F, Leyssen P, Costa J, Litaudon M. 2014. Jatrophane diterpenes as inhibitors of chikungunya virus replication: structure-activity relationship and discovery of a potent lead. J Nat Prod 77:1505–1512. 10.1021/np500271u. [DOI] [PubMed] [Google Scholar]
- 228.Murali KS, Sivasubramanian S, Vincent S, Murugan SB, Giridaran B, Dinesh S, Gunasekaran P, Krishnasamy K, Sathishkumar R. 2015. Anti-chikungunya activity of luteolin and apigenin rich fraction from Cynodon dactylon. Asian Pac J Trop Med 8:352–358. 10.1016/S1995-7645(14)60343-6. [DOI] [PubMed] [Google Scholar]
- 229.Nothias-Scaglia LF, Dumontet V, Neyts J, Roussi F, Costa J, Leyssen P, Litaudon M, Paolini J. 2015. LC-MS(2)-Based dereplication of Euphorbia extracts with anti-chikungunya virus activity. Fitoterapia 105:202–209. 10.1016/j.fitote.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 230.Varghese FS, Kaukinen P, Gläsker S, Bespalov M, Hanski L, Wennerberg K, Kümmerer BM, Ahola T. 2016. Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses. Antiviral Res 126:117–124. 10.1016/j.antiviral.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 231.Batista M, Braga A, Campos G, Souza M, Matos R, Lopes T, Candido N, Lima M, Machado F, Andrade S, Bittar C, Nogueira M, Carneiro B, Mariutti R, Arni R, Calmon M, Rahal P. 2019. Natural products isolated from oriental medicinal herbs inactivate Zika virus. Viruses 11:49. 10.3390/v11010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sakurai Y, Kolokoltsov AA, Chen C-C, Tidwell MW, Bauta WE, Klugbauer N, Grimm C, Wahl-Schott C, Biel M, Davey RA. 2015. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science 347:995–998. 10.1126/science.1258758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Daboit TC, Stopiglia CD, von Poser GL, Scroferneker ML. 2010. Antifungal activity of Pterocaulon alopecuroides (Asteraceae) against chromoblastomycosis agents. Mycoses 53:246–250. 10.1111/j.1439-0507.2009.01704.x. [DOI] [PubMed] [Google Scholar]
- 234.Elfadil H, Fahal A, Kloezen W, Ahmed EM, van de Sande W. 2015. The in vitro antifungal activity of Sudanese medicinal plants against Madurella mycetomatis, the eumycetoma major causative agent. PLoS Negl Trop Dis 9:e0003488. 10.1371/journal.pntd.0003488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.do Carmo Silva L, Miranda M, de Freitas JV, Ferreira SFA, de Oliveira Lima EC, de Oliveira CMA, Kato L, Terezan AP, Rodriguez AFR, Faria F, de Almeida Soares CM, Pereira M. 2020. Antifungal activity of copaiba resin oil in solution and nanoemulsion against Paracoccidioides spp. Braz J Microbiol 51:125–134. 10.1007/s42770-019-00201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Johann S, Cisalpino PS, Watanabe GA, Cota BB, de Siqueira EP, Pizzolatti MG, Zani CL, de Resende MA. 2010. Antifungal activity of extracts of some plants used in Brazilian traditional medicine against the pathogenic fungus Paracoccidioides brasiliensis. Pharm Biol 48:388–396. 10.3109/13880200903150385. [DOI] [PubMed] [Google Scholar]
- 237.Oliveira AH, de Oliveira GG, Carnevale Neto F, Portuondo DF, Batista-Duharte A, Carlos IZ. 2017. Anti-inflammatory activity of Vismia guianensis (Aubl.) Pers. extracts and antifungal activity against Sporothrix schenckii. J Ethnopharmacol 195:266–274. 10.1016/j.jep.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 238.Murad JM, Calvi SA, Soares AM, Bankova V, Sforcin JM. 2002. Effects of propolis from Brazil and Bulgaria on fungicidal activity of macrophages against Paracoccidioides brasiliensis. J Ethnopharmacol 79:331–334. 10.1016/s0378-8741(01)00404-4. [DOI] [PubMed] [Google Scholar]
- 239.Waller SB, Peter CM, Hoffmann JF, Picoli T, Osorio LD, Chaves F, Zani JL, de Faria RO, de Mello JR, Meireles MC. 2017. Chemical and cytotoxic analyses of brown Brazilian propolis (Apis mellifera) and its in vitro activity against itraconazole-resistant Sporothrix brasiliensis. Microb Pathog 105:117–121. 10.1016/j.micpath.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 240.Medina-Alarcon KP, L Singulani J, Dutra LA, S Pitangui N, Pereira-da-Silva MA, Dos Santos MB, Ayusso GM, Regasini LO, Soares CP, Chorilli M, Mendes-Giannini MJ, Fusco-Almeida AM. 2020. Antifungal activity of 2'-hydroxychalcone loaded in nanoemulsion against Paracoccidioides spp. Future Microbiol 15:21–33. 10.2217/fmb-2019-0095. [DOI] [PubMed] [Google Scholar]
- 241.Johann S, Oliveira FB, Siqueira EP, Cisalpino PS, Rosa CA, Alves TM, Zani CL, Cota BB. 2012. Activity of compounds isolated from Baccharis dracunculifolia D.C. (Asteraceae) against Paracoccidioides brasiliensis. Med Mycol 50:843–851. 10.3109/13693786.2012.678903. [DOI] [PubMed] [Google Scholar]
- 242.Cardoso D, Särkinen T, Alexander S, Amorim AM, Bittrich V, Celis M, Daly DC, Fiaschi P, Funk VA, Giacomin LL, Goldenberg R, Heiden G, Iganci J, Kelloff CL, Knapp S, Cavalcante de Lima H, Machado AFP, Dos Santos RM, Mello-Silva R, Michelangeli FA, Mitchell J, Moonlight P, de Moraes PLR, Mori SA, Nunes TS, Pennington TD, Pirani JR, Prance GT, de Queiroz LP, Rapini A, Riina R, Rincon CAV, Roque N, Shimizu G, Sobral M, Stehmann JR, Stevens WD, Taylor CM, Trovó M, van den Berg C, van der Werff H, Viana PL, Zartman CE, Forzza RC. 2017. Amazon plant diversity revealed by a taxonomically verified species list. Proc Natl Acad Sci USA 114:10695–10700. 10.1073/pnas.1706756114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.State of the Tropics. 2020. State of the tropics 2020 report. James Cook University, Townsville, Australia. https://www.jcu.edu.au/state-of-the-tropics/publications/state-of-the-tropics-2020-report. [Google Scholar]
- 244.Wiersinga WJ, Virk HS, Torres AG, Currie BJ, Peacock SJ, Dance DAB, Limmathurotsakul D. 2018. Melioidosis. Nat Rev Dis Primers 4:17107. 10.1038/nrdp.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zijlstra EE, van de Sande WWJ, Welsh O, Mahgoub ES, Goodfellow M, Fahal AH. 2016. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis 16:100–112. 10.1016/S1473-3099(15)00359-X. [DOI] [PubMed] [Google Scholar]
- 246.Mohammadpour M, Abrishami M, Masoumi A, Hashemi H. 2016. Trachoma: past, present and future. J Curr Ophthalmol 28:165–169. 10.1016/j.joco.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Nyindo M, Lukambagire A-H. 2015. Fascioliasis: an ongoing zoonotic trematode infection. BioMed Res Int 2015:786195. 10.1155/2015/786195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Torres-Guerrero E, Quintanilla-Cedillo MR, Ruiz-Esmenjaud J, Arenas R. 2017. Leishmaniasis: a review. F1000Res 6:750. 10.12688/f1000research.11120.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lok P, Dijk S. 2019. Malaria outbreak in Burundi reaches epidemic levels with 5.7 million infected this year. BMJ 366:l5104. 10.1136/bmj.l5104. [DOI] [PubMed] [Google Scholar]
- 250.McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou XN. 2018. Schistosomiasis. Nat Rev Dis Primers 4:13. 10.1038/s41572-018-0013-8. [DOI] [PubMed] [Google Scholar]
- 251.Greaves D, Coggle S, Pollard C, Aliyu SH, Moore EM. 2013. Strongyloides stercoralis infection. BMJ 347:f4610. 10.1136/bmj.f4610. [DOI] [PubMed] [Google Scholar]
- 252.Okello AL, Thomas LF. 2017. Human taeniasis: current insights into prevention and management strategies in endemic countries. Risk Manag Healthc Policy 10:107–116. 10.2147/RMHP.S116545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Hsan K, Hossain MM, Sarwar MS, Wilder-Smith A, Gozal D. 2019. Unprecedented rise in dengue outbreaks in Bangladesh. Lancet Infect Dis 19:1287. 10.1016/S1473-3099(19)30616-4. [DOI] [PubMed] [Google Scholar]
- 254.Aubry M, Mapotoeke M, Teissier A, Paoaafaite T, Dumas-Chastang E, Giard M, Cao-Lormeau VM. 2019. Dengue virus serotype 2 (DENV-2) outbreak, French Polynesia, 2019. Euro Surveill 24:1900407. 10.2807/1560-7917.ES.2019.24.29.1900407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Turtle L, Solomon T. 2018. Japanese encephalitis—the prospects for new treatments. Nat Rev Neurol 14:298–313. 10.1038/nrneurol.2018.30. [DOI] [PubMed] [Google Scholar]
- 256.Brauburger K, Hume AJ, Muhlberger E, Olejnik J. 2012. Forty-five years of Marburg virus research. Viruses 4:1878–1927. 10.3390/v4101878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Tenzin T, Namgyal J, Letho S. 2017. Community-based survey during rabies outbreaks in Rangjung town, Trashigang, eastern Bhutan, 2016. BMC Infect Dis 17:281. 10.1186/s12879-017-2393-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Fooks AR, Cliquet F, Finke S, Freuling C, Hemachudha T, Mani RS, Muller T, Nadin-Davis S, Picard-Meyer E, Wilde H, Banyard AC. 2017. Rabies. Nat Rev Dis Primers 3:17091. 10.1038/nrdp.2017.91. [DOI] [PubMed] [Google Scholar]
- 259.Bob NS, Ba H, Fall G, Ishagh E, Diallo MY, Sow A, Sembene PM, Faye O, El Kouri B, Sidi ML, Sall AA. 2017. Detection of the northeastern African Rift Valley fever virus lineage during the 2015 outbreak in Mauritania. Open Forum Infect Dis 4:ofx087. 10.1093/ofid/ofx087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Boshra H, Lorenzo G, Busquets N, Brun A. 2011. Rift Valley fever: recent insights into pathogenesis and prevention. J Virol 85:6098–6105. 10.1128/JVI.02641-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Bogovic P, Strle F. 2015. Tick-borne encephalitis: a review of epidemiology, clinical characteristics, and management. World J Clin Cases 3:430–441. 10.12998/wjcc.v3.i5.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Colpitts TM, Conway MJ, Montgomery RR, Fikrig E. 2012. West Nile virus: biology, transmission, and human infection. Clin Microbiol Rev 25:635–648. 10.1128/CMR.00045-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Douam F, Ploss A. 2018. Yellow fever virus: knowledge gaps impeding the fight against an old foe. Trends Microbiol 26:913–928. 10.1016/j.tim.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Queiroz-Telles F, de Hoog S, Santos DW, Salgado CG, Vicente VA, Bonifaz A, Roilides E, Xi L, Azevedo CM, da Silva MB, Pana ZD, Colombo AL, Walsh TJ. 2017. Chromoblastomycosis. Clin Microbiol Rev 30:233–276. 10.1128/CMR.00032-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Marques SA. 2013. Paracoccidioidomycosis: epidemiological, clinical, diagnostic and treatment up-dating. An Bras Dermatol 88:700–711. 10.1590/abd1806-4841.20132463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Barros MB, de Almeida Paes R, Schubach AO. 2011. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev 24:633–654. 10.1128/CMR.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Cao C, Xi L, Chaturvedi V. 2019. Talaromycosis (Penicilliosis) due to Talaromyces (Penicillium) marneffei: insights into the clinical trends of a major fungal disease 60 years after the discovery of the pathogen. Mycopathologia 184:709–720. 10.1007/s11046-019-00410-2. [DOI] [PubMed] [Google Scholar]
- 268.Huygen K, Adjei O, Affolabi D, Bretzel G, Demangel C, Fleischer B, Johnson RC, Pedrosa J, Phanzu DM, Phillips RO, Pluschke G, Siegmund V, Singh M, van der Werf TS, Wansbrough-Jones M, Portaels F. 2009. Buruli ulcer disease: prospects for a vaccine. Med Microbiol Immunol 198:69–77. 10.1007/s00430-009-0109-6. [DOI] [PubMed] [Google Scholar]
- 269.Hart BE, Lee S. 2016. Overexpression of a Mycobacterium ulcerans Ag85B-EsxH fusion protein in recombinant BCG improves experimental Buruli ulcer vaccine efficacy. PLoS Negl Trop Dis 10:e0005229. 10.1371/journal.pntd.0005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Mangas KM, Tobias N, Marion E, Babonneau J, Marsollier L, Porter JL, Pidot SJ, Wong CY, Jackson DC, Chua BY, Stinear TP. 2020. High antibody titres induced by protein subunit vaccines against Buruli ulcer using Mycobacterium ulcerans antigens Hsp18 and MUL_3720. bioRxiv 10.1101/2020.02.16.951533:2020.02.16.951533. [DOI] [PMC free article] [PubMed]
- 271.Phillips RO, Robert J, Abass KM, Thompson W, Sarfo FS, Wilson T, Sarpong G, Gateau T, Chauty A, Omollo R. 2020. Rifampicin and clarithromycin (extended release) versus rifampicin and streptomycin for limited Buruli ulcer lesions: a randomised, open-label, non-inferiority phase 3 trial. Lancet 395:1259–1267. 10.1016/S0140-6736(20)30047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Shaikh H, Lynch J, Kim J, Excler JL. 2020. Current and future cholera vaccines. Vaccine 38(Suppl 1):A118–A126. 10.1016/j.vaccine.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 273.Worobec SM. 2012. Current approaches and future directions in the treatment of leprosy. Res Rep Trop Med 3:79–91. 10.2147/RRTM.S27395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Duthie MS, Pena MT, Ebenezer GJ, Gillis TP, Sharma R, Cunningham K, Polydefkis M, Maeda Y, Makino M, Truman RW, Reed SG. 2018. LepVax, a defined subunit vaccine that provides effective pre-exposure and post-exposure prophylaxis of M. leprae infection. NPJ Vaccines 3:12. 10.1038/s41541-018-0050-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Coppola M, van den Eeden SJF, Robbins N, Wilson L, Franken K, Adams LB, Gillis TP, Ottenhoff THM, Geluk A. 2018. Vaccines for leprosy and tuberculosis: opportunities for shared research, development, and application. Front Immunol 9:308. 10.3389/fimmu.2018.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Malathi M, Thappa DM. 2013. Fixed-duration therapy in leprosy: limitations and opportunities. Indian J Dermatol 58:93–100. 10.4103/0019-5154.108029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Dance D. 2014. Treatment and prophylaxis of melioidosis. Int J Antimicrob Agents 43:310–318. 10.1016/j.ijantimicag.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Sullivan RP, Ward L, Currie BJ. 2019. Oral eradication therapy for melioidosis: Important but not without risks. Int J Infect Dis 80:111–114. 10.1016/j.ijid.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 279.Estes DM, Dow SW, Schweizer HP, Torres AG. 2010. Present and future therapeutic strategies for melioidosis and glanders. Expert Rev Anti Infect Ther 8:325–338. 10.1586/eri.10.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Johnson MM, Ainslie KM. 2017. Vaccines for the prevention of melioidosis and glanders. Curr Trop Med Rep 4:136–145. 10.1007/s40475-017-0121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Fahal AH. 2011. Mycetoma, p 565–568. In Guerrant RL, Walker DH, Weller PF (ed), Tropical infectious diseases, 3rd ed. Elsevier Health Sciences, Amsterdam, The Netherlands. [Google Scholar]
- 282.Mohammed AA, ALnaby AM, Sabeel SM, AbdElmarouf FM, Dirar AI, Ali MM, Khandgawi MA, Yousif AM, Abdulgadir EM, Sabahalkhair MA, Abbas AE, Hassan MA. 2018. Epitope-based peptide vaccine against fructose-bisphosphate aldolase of Madurella mycetomatis using immunoinformatics approaches. Bioinform Biol Insights 12:1177932218809703. 10.1177/1177932218809703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.WHO. 2016. WHO guidelines for the treatment of Chlamydia trachomatis. WHO, Geneva, Switzerland. [PubMed] [Google Scholar]
- 284.de la Maza LM, Zhong G, Brunham RC. 2017. Update on Chlamydia trachomatis vaccinology. Clin Vaccine Immunol 24:e00543-16. 10.1128/CVI.00543-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Mabey D, Solomon A. 2003. The effect of antibiotic treatment on active trachoma and ocular Chlamydia trachomatis infection. Expert Rev Anti Infect Ther 1:209–216. 10.1586/14787210.1.2.209. [DOI] [PubMed] [Google Scholar]
- 286.Hatherill M, White RG, Hawn TR. 2019. Clinical development of new TB vaccines: recent advances and next steps. Front Microbiol 10:3154. 10.3389/fmicb.2019.03154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Yang TW, Park HO, Jang HN, Yang JH, Kim SH, Moon SH, Byun JH, Lee CE, Kim JW, Kang DH. 2017. Side effects associated with the treatment of multidrug-resistant tuberculosis at a tuberculosis referral hospital in South Korea: a retrospective study. Medicine 96:e7482. 10.1097/MD.0000000000007482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Marks M. 2018. Advances in the treatment of yaws. Trop Med Infect Dis 3:92. 10.3390/tropicalmed3030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Bouteille B, Buguet A. 2012. The detection and treatment of human African trypanosomiasis. Res Rep Trop Med 3:35–45. 10.2147/RRTM.S24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Black SJ, Mansfield JM. 2016. Prospects for vaccination against pathogenic African trypanosomes. Parasite Immunol 38:735–743. 10.1111/pim.12387. [DOI] [PubMed] [Google Scholar]
- 291.Vigneron A, O'Neill MB, Weiss BL, Savage AF, Campbell OC, Kamhawi S, Valenzuela JG, Aksoy S. 2020. Single-cell RNA sequencing of Trypanosoma brucei from tsetse salivary glands unveils metacyclogenesis and identifies potential transmission blocking antigens. Proc Natl Acad Sci USA 117:2613–2621. 10.1073/pnas.1914423117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Meymandi S, Hernandez S, Park S, Sanchez DR, Forsyth C. 2018. Treatment of Chagas disease in the United States. Curr Treat Options Infect Dis 10:373–388. 10.1007/s40506-018-0170-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Dumonteil E, Herrera C, Buekens P. 2019. A therapeutic preconceptional vaccine against Chagas disease: a novel indication that could reduce congenital transmission and accelerate vaccine development. PLoS Negl Trop Dis 13:e0006985. 10.1371/journal.pntd.0006985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Dumonteil E. 2009. Vaccine development against Trypanosoma cruzi and Leishmania species in the post-genomic era. Infect Genet Evol 9:1075–1082. 10.1016/j.meegid.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 295.Velasco-Tirado V, Alonso-Sardon M, Lopez-Bernus A, Romero-Alegria A, Burguillo FJ, Muro A, Carpio-Perez A, Munoz Bellido JL, Pardo-Lledias J, Cordero M, Belhassen-Garcia M. 2018. Medical treatment of cystic echinococcosis: systematic review and meta-analysis. BMC Infect Dis 18:306. 10.1186/s12879-018-3201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Miles S, Portela M, Cyrklaff M, Ancarola ME, Frischknecht F, Duran R, Dematteis S, Mourglia-Ettlin G. 2019. Combining proteomics and bioinformatics to explore novel tegumental antigens as vaccine candidates against Echinococcus granulosus infection. J Cell Biochem 120:15320–15336. 10.1002/jcb.28799. [DOI] [PubMed] [Google Scholar]
- 297.Chaiyadet S, Sotillo J, Krueajampa W, Thongsen S, Brindley PJ, Sripa B, Loukas A, Laha T. 2019. Vaccination of hamsters with Opisthorchis viverrini extracellular vesicles and vesicle-derived recombinant tetraspanins induces antibodies that block vesicle uptake by cholangiocytes and reduce parasite burden after challenge infection. PLoS Negl Trop Dis 13:e0007450. 10.1371/journal.pntd.0007450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Lee DH, Kim AR, Lee SH, Quan FS. 2017. Virus-like particles vaccine containing Clonorchis sinensis tegumental protein induces partial protection against Clonorchis sinensis infection. Parasit Vectors 10:626. 10.1186/s13071-017-2526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Srivastava S, Shankar P, Mishra J, Singh S. 2016. Possibilities and challenges for developing a successful vaccine for leishmaniasis. Parasit Vectors 9:277. 10.1186/s13071-016-1553-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Osman M, Mistry A, Keding A, Gabe R, Cook E, Forrester S, Wiggins R, Di Marco S, Colloca S, Siani L, Cortese R, Smith DF, Aebischer T, Kaye PM, Lacey CJ. 2017. A third generation vaccine for human visceral leishmaniasis and post kala azar dermal leishmaniasis: first-in-human trial of ChAd63-KH. PLoS Negl Trop Dis 11:e0005527. 10.1371/journal.pntd.0005527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kalyanasundaram R, Khatri V, Chauhan N. 2020. Advances in vaccine development for human lymphatic filariasis. Trends Parasitol 36:195–205. 10.1016/j.pt.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Anand SB, Murugan V, Prabhu PR, Anandharaman V, Reddy MVR, Kaliraj P. 2008. Comparison of immunogenicity, protective efficacy of single and cocktail DNA vaccine of Brugia malayi abundant larval transcript (ALT-2) and thioredoxin peroxidase (TPX) in mice. Acta Trop 107:106–112. 10.1016/j.actatropica.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 303.Gnanasekar M, Rao KV, He Y-X, Mishra PK, Nutman TB, Kaliraj P, Ramaswamy K. 2004. Novel phage display-based subtractive screening to identify vaccine candidates of Brugia malayi. Infect Immun 72:4707–4715. 10.1128/IAI.72.8.4707-4715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.CDC. 2020. Malaria in the United States: treatment tables. https://www.cdc.gov/malaria/resources/pdf/Malaria_Treatment_Table.pdf. Accessed 15 April 2020.
- 305.Draper SJ, Sack BK, King CR, Nielsen CM, Rayner JC, Higgins MK, Long CA, Seder RA. 2018. Malaria vaccines: Recent advances and new horizons. Cell Host Microbe 24:43–56. 10.1016/j.chom.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Opoku NO, Bakajika DK, Kanza EM, Howard H, Mambandu GL, Nyathirombo A, Nigo MM, Kasonia K, Masembe SL, Mumbere M, Kataliko K, Larbelee JP, Kpawor M, Bolay KM, Bolay F, Asare S, Attah SK, Olipoh G, Vaillant M, Halleux CM, Kuesel AC. 2018. Single dose moxidectin versus ivermectin for Onchocerca volvulus infection in Ghana, Liberia, and the Democratic Republic of the Congo: a randomised, controlled, double-blind phase 3 trial. Lancet 392:1207–1216. 10.1016/S0140-6736(17)32844-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Lustigman S, Makepeace BL, Klei TR, Babayan SA, Hotez P, Abraham D, Bottazzi ME. 2018. Onchocerca volvulus: the road from basic biology to a vaccine. Trends Parasitol 34:64–79. 10.1016/j.pt.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Liu X, Walton S, Mounsey K. 2014. Vaccine against scabies: necessity and possibility. Parasitology 141:725–732. 10.1017/S0031182013002047. [DOI] [PubMed] [Google Scholar]
- 309.Shen N, Zhang H, Ren Y, He R, Xu J, Li C, Lai W, Gu X, Xie Y, Peng X, Yang G. 2018. A chitinase-like protein from Sarcoptes scabiei as a candidate anti-mite vaccine that contributes to immune protection in rabbits. Parasit Vectors 11:599. 10.1186/s13071-018-3184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Hillyer GV. 2005. Fasciola antigens as vaccines against fascioliasis and schistosomiasis. J Helminthol 79:241–247. 10.1079/joh2005304. [DOI] [PubMed] [Google Scholar]
- 311.Egesa M, Hoffmann KF, Hokke CH, Yazdanbakhsh M, Cose S. 2017. Rethinking schistosomiasis vaccine development: synthetic vesicles. Trends Parasitol 33:918–921. 10.1016/j.pt.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 312.Stutzer C, Richards SA, Ferreira M, Baron S, Maritz-Olivier C. 2018. Metazoan parasite vaccines: present status and future prospects. Front Cell Infect Microbiol 8:67. 10.3389/fcimb.2018.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Molehin AJ. 2020. Schistosomiasis vaccine development: update on human clinical trials. J Biomed Sci 27:28. 10.1186/s12929-020-0621-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Noon JB, Aroian RV. 2017. Recombinant subunit vaccines for soil-transmitted helminths. Parasitology 144:1845–1870. 10.1017/S003118201700138X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Fujiwara RT, Zhan B, Mendez S, Loukas A, Bueno LL, Wang Y, Plieskatt J, Oksov Y, Lustigman S, Bottazzi ME, Hotez P, Bethony JM. 2007. Reduction of worm fecundity and canine host blood loss mediates protection against hookworm infection elicited by vaccination with recombinant Ac-16. Clin Vaccine Immunol 14:281–287. 10.1128/CVI.00404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Levenhagen MA, Conte H, Costa-Cruz JM. 2016. Current progress toward vaccine and passive immunization approaches for Strongyloides spp. Immunol Lett 180:17–23. 10.1016/j.imlet.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 317.Zammarchi L, Bonati M, Strohmeyer M, Albonico M, Requena-Mendez A, Bisoffi Z, Nicoletti A, Garcia HH, Bartoloni A, Group CPS, The COHEMI Project Study Group . 2017. Screening, diagnosis and management of human cysticercosis and Taenia solium taeniasis: technical recommendations by the COHEMI project study group. Trop Med Int Health 22:881–894. 10.1111/tmi.12887. [DOI] [PubMed] [Google Scholar]
- 318.Gonzalez AE, Gauci CG, Barber D, Gilman RH, Tsang VC, Garcia HH, Verastegui M, Lightowlers MW. 2005. Vaccination of pigs to control human neurocysticercosis. Am J Trop Med Hyg 72:837–839. 10.4269/ajtmh.2005.72.837. [DOI] [PubMed] [Google Scholar]
- 319.Flisser A, Gauci CG, Zoli A, Martinez-Ocaña J, Garza-Rodriguez A, Dominguez-Alpizar JL, Maravilla P, Rodriguez-Canul R, Avila G, Aguilar-Vega L, Kyngdon C, Geerts S, Lightowlers MW. 2004. Induction of protection against porcine cysticercosis by vaccination with recombinant oncosphere antigens. Infect Immun 72:5292–5297. 10.1128/IAI.72.9.5292-5297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Gao S, Song S, Zhang L. 2019. Recent progress in vaccine development against chikungunya virus. Front Microbiol 10:2881. 10.3389/fmicb.2019.02881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Wressnigg N, Hochreiter R, Zoihsl O, Fritzer A, Bezay N, Klingler A, Lingnau K, Schneider M, Lundberg U, Meinke A, Larcher-Senn J, Corbic-Ramljak I, Eder-Lingelbach S, Dubischar K, Bender W. 2020. Single-shot live-attenuated chikungunya vaccine in healthy adults: a phase 1, randomised controlled trial. Lancet Infect Dis 20:1193–1203. 10.1016/S1473-3099(20)30238-3. [DOI] [PubMed] [Google Scholar]
- 322.Dowall SD, Carroll MW, Hewson R. 2017. Development of vaccines against Crimean-Congo haemorrhagic fever virus. Vaccine 35:6015–6023. 10.1016/j.vaccine.2017.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Spik K, Shurtleff A, McElroy AK, Guttieri MC, Hooper JW, Schmaljohn C. 2006. Immunogenicity of combination DNA vaccines for Rift Valley fever virus, tick-borne encephalitis virus, Hantaan virus, and Crimean Congo hemorrhagic fever virus. Vaccine 24:4657–4666. 10.1016/j.vaccine.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 324.Deng SQ, Yang X, Wei Y, Chen JT, Wang XJ, Peng HJ. 2020. A review on dengue vaccine development. Vaccines 8:63. 10.3390/vaccines8010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Suschak JJ, Schmaljohn CS. 2019. Vaccines against Ebola virus and Marburg virus: recent advances and promising candidates. Hum Vaccin Immunother 15:2359–2377. 10.1080/21645515.2019.1651140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Sullivan NJ, Geisbert TW, Geisbert JB, Shedlock DJ, Xu L, Lamoreaux L, Custers JHHV, Popernack PM, Yang Z-Y, Pau MG, Roederer M, Koup RA, Goudsmit J, Jahrling PB, Nabel GJ. 2006. Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs. PLoS Med 3:e177. 10.1371/journal.pmed.0030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Purushotham J, Lambe T, Gilbert SC. 2019. Vaccine platforms for the prevention of Lassa fever. Immunol Lett 215:1–11. 10.1016/j.imlet.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Hevey M, Negley D, Pushko P, Smith J, Schmaljohn A. 1998. Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates. Virology 251:28–37. 10.1006/viro.1998.9367. [DOI] [PubMed] [Google Scholar]
- 329.Ignatyev GM, Agafonov AP, Streltsova MA, Kashentseva EA. 1996. Inactivated Marburg virus elicits a nonprotective immune response in rhesus monkeys. J Biotechnol 44:111–118. 10.1016/0168-1656(95)00104-2. [DOI] [PubMed] [Google Scholar]
- 330.Ikegami T. 2019. Candidate vaccines for human Rift Valley fever. Expert Opin Biol Ther 19:1333–1342. 10.1080/14712598.2019.1662784. [DOI] [PubMed] [Google Scholar]
- 331.Ulbert S. 2019. West Nile virus vaccines—current situation and future directions. Hum Vaccin Immunother 15:2337–2342. 10.1080/21645515.2019.1621149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Woods CW, Sanchez AM, Swamy GK, McClain MT, Harrington L, Freeman D, Poore EA, Slifka DK, Poer DeRaad DE, Amanna IJ, Slifka MK, Cai S, Shahamatdar V, Wierzbicki MR, Amegashie C, Walter EB. 2019. An observer blinded, randomized, placebo-controlled, phase I dose escalation trial to evaluate the safety and immunogenicity of an inactivated West Nile virus vaccine, HydroVax-001, in healthy adults. Vaccine 37:4222–4230. 10.1016/j.vaccine.2018.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Barrett PN, Terpening SJ, Snow D, Cobb RR, Kistner O. 2017. Vero cell technology for rapid development of inactivated whole virus vaccines for emerging viral diseases. Expert Rev Vaccines 16:883–894. 10.1080/14760584.2017.1357471. [DOI] [PubMed] [Google Scholar]
- 334.Dayan GH, Pugachev K, Bevilacqua J, Lang J, Monath TP. 2013. Preclinical and clinical development of a YFV 17 D-based chimeric vaccine against West Nile virus. Viruses 5:3048–3070. 10.3390/v5123048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Poland GA, Ovsyannikova IG, Kennedy RB. 2019. Zika vaccine development: current status. Mayo Clin Proc 94:2572–2586. 10.1016/j.mayocp.2019.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Pattnaik A, Sahoo BR, Pattnaik AK. 2020. Current status of Zika virus vaccines: successes and challenges. Vaccines (Basel) 8:266. 10.3390/vaccines8020266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Siqueira IM, Ribeiro AM, Nobrega YK, Simon KS, Souza AC, Jeronimo MS, Cavalcante Neto FF, Silva CL, Felipe MS, Bocca AL. 2013. DNA-hsp65 vaccine as therapeutic strategy to treat experimental chromoblastomycosis caused by Fonsecaea pedrosoi. Mycopathologia 175:463–475. 10.1007/s11046-012-9599-7. [DOI] [PubMed] [Google Scholar]
- 338.Bustamante B, Seas C, Salomon M, Bravo F. 2013. Lobomycosis successfully treated with posaconazole. Am J Trop Med Hyg 88:1207–1208. 10.4269/ajtmh.12-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Araujo MG, Cirilo NS, Santos S, Aguilar CR, Guedes ACM. 2018. Lobomycosis: a therapeutic challenge. An Bras Dermatol 93:279–281. 10.1590/abd1806-4841.20187044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Taborda CP, Uran ME, Nosanchuk JD, Travassos LR. 2015. Paracoccidioidomycosis: challenges in the development of a vaccine against an endemic mycosis in the Americas. Rev Inst Med Trop Sao Paulo 57(Suppl 19):21–24. 10.1590/S0036-46652015000700005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Travassos LR, Taborda CP. 2012. New advances in the development of a vaccine against paracoccidioidomycosis. Front Microbiol 3:212. 10.3389/fmicb.2012.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Silva LBR, Dias LS, Rittner GMG, Munoz JE, Souza ACO, Nosanchuk JD, Travassos LR, Taborda CP. 2017. Dendritic cells primed with Paracoccidioides brasiliensis peptide P10 are therapeutic in immunosuppressed mice with paracoccidioidomycosis. Front Microbiol 8:1057. 10.3389/fmicb.2017.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.de Almeida SR. 2019. Advances in vaccine development against sporotrichosis. Curr Trop Med Rep 6:126–131. 10.1007/s40475-019-00183-0. [DOI] [Google Scholar]
- 344.Tanywe A, Fernandez RS. 2017. Effectiveness of rifampicin-streptomycin for treatment of Buruli ulcer: a systematic review. JBI Database System Rev Implement Rep 15:119–139. 10.11124/JBISRIR-2016-003235. [DOI] [PubMed] [Google Scholar]
- 345.Woodruff HB. 2014. Selman A. Waksman, winner of the 1952 Nobel Prize for physiology or medicine. Appl Environ Microbiol 80:2–8. 10.1128/AEM.01143-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Addo P, Quartey M, Abbas M, Adu-Addai B, Owusu E, Okang I, Dodoo A, de Souza D, Ankrah N, Ofori-Adjei D. 2008. In-vitro susceptibility of Mycobacterium ulcerans to herbal preparations. Internet J Trop Med 4. [Google Scholar]
- 347.Rodrigues Felix C, Roberts JC, Winder PL, Gupta R, Diaz MC, Pomponi SA, Wright AE, Rohde KH. 2019. Plakinamine P, a steroidal alkaloid with bactericidal activity against Mycobacterium tuberculosis. Marine Drugs 17:707. 10.3390/md17120707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Oluwabusola ET, Tabudravu JN, Al Maqbali KS, Annang F, Pérez-Moreno G, Reyes F, Jaspars M. 2020. Antiparasitic activity of bromotyrosine alkaloids and new analogues isolated from the Fijian marine sponge Aplysinella rhax. Chem Biodivers 17:e2000335. 10.1002/cbdv.202000335. [DOI] [PubMed] [Google Scholar]
- 349.Sülsen VP, Frank FM, Cazorla SI, Barrera P, Freixa B, Vila R, Sosa MA, Malchiodi EL, Muschietti LV, Martino VS. 2011. Psilostachyin C: a natural compound with trypanocidal activity. Int J Antimicrob Agents 37:536–543. 10.1016/j.ijantimicag.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 350.Geetha BS, Nair MS, Latha PG, Remani P. 2012. Sesquiterpene lactones isolated from Elephantopus scaber L. inhibits human lymphocyte proliferation and the growth of tumour cell lines and induces apoptosis in vitro. J Biomed Biotechnol 2012:721285. 10.1155/2012/721285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Deshmukh M, Sahare KN, Patidar RK, Mahajan B, Singh V. 2014. Antifilarial activity of Butea monosperma L. leaves extracts against Setaria cervi. Trends Vector Res Parasitol 1:1–5. 10.7243/2054-9881-1-1. [DOI] [Google Scholar]
- 352.Rana G. 2018. Inhibition efficiency of a newly isolated flavonoid compound from Vitex negundo L. leaves against cattle-endosymbiont Setaria cervi: phytomedicine for lymphatic filariasis. Parasite Epidemiol Control 3:88–95. 10.1016/j.parepi.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Sharma RD, Veerpathran AR, Dakshinamoorthy G, Sahare KN, Goswami K, Reddy MV. 2010. Possible implication of oxidative stress in anti filarial effect of certain traditionally used medicinal plants in vitro against Brugia malayi microfilariae. Pharmacognosy Res 2:350–354. http://www.phcogres.com/article/2010/2/6/1041030974-849075453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Forkuo AD, Ansah C, Mensah KB, Annan K, Gyan B, Theron A, Mancama D, Wright CW. 2017. In vitro anti-malarial interaction and gametocytocidal activity of cryptolepine. Malar J 16:496. 10.1186/s12936-017-2142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Jiang S, Zeng Q, Gettayacamin M, Tungtaeng A, Wannaying S, Lim A, Hansukjariya P, Okunji CO, Zhu S, Fang D. 2005. Antimalarial activities and therapeutic properties of febrifugine analogs. Antimicrob Agents Chemother 49:1169–1176. 10.1128/AAC.49.3.1169-1176.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Benoit-Vical F, Valentin A, Da B, Dakuyo Z, Descamps L, Mallié M. 2003. N'Dribala (Cochlospermum planchonii) versus chloroquine for treatment of uncomplicated Plasmodium falciparum malaria. J Ethnopharmacol 89:111–114. 10.1016/S0378-8741(03)00277-0. [DOI] [PubMed] [Google Scholar]
- 357.Eze JC, Okafor F, Nwankwo NE, Okeke ES, Onwudiwe NN. 2020. Schistosomiasis prevention option: toxicological evaluation of Vernonia amygdalina on the tissues of Bulinus truncatus at different pH conditions. Heliyon 6:e04796. 10.1016/j.heliyon.2020.e04796. [DOI] [PMC free article] [PubMed] [Google Scholar]