Abstract
Cac3p/Msi1p, the Saccharomyces cerevisiae homolog of retinoblastoma-associated protein 48 (RbAp48), is a component of chromatin assembly factor I (CAF-I), a complex that assembles histones H3 and H4 onto replicated DNA. CAC3 overexpression also suppresses the RAS/cyclic AMP (cAMP) signal transduction pathway by an unknown mechanism. We investigated this mechanism and found that CAC3 suppression of RAS/cAMP signal transduction was independent of either CAC1 or CAC2, subunits required for CAF-I function. CAC3 suppression was also independent of other chromatin-modifying activities, indicating that Cac3p has at least two distinct, separable functions, one in chromatin assembly and one in regulating RAS function. Unlike Cac1p, which localizes primarily to the nucleus, Cac3p localizes to both the nucleus and the cytoplasm. In addition, Cac3p associates with Npr1p, a cytoplasmic kinase that stablizes several nutrient transporters by antagonizing a ubiquitin-mediated protein degradation pathway. Deletion of NPR1, like overexpression of Cac3p, suppressed the RAS/cAMP pathway. Furthermore, NPR1 overexpression interfered with the ability of CAC3 to suppress the RAS/cAMP pathway, indicating that extra Cac3p suppresses the RAS/cAMP pathway by sequestering Npr1p. Deletion of NPR1 did not affect the quantity, phosphorylation state, or localization of Ras2p. Consistent with the idea that Npr1p exerts its effect on the RAS/cAMP pathway by antagonizing a ubiquitin-mediated process, excess ubiquitin suppressed both the heat shock sensitivity and the sporulation defects caused by constitutive activation of the RAS/cAMP pathway. Thus, CAC3/MSI1 regulates the RAS/cAMP pathway via a chromatin-independent mechanism that involves the sequestration of Npr1p and may be due to the increased ubiquitination of an Npr1p substrate.
Chromatin assembly factor I (CAF-I) is a complex of three proteins that has been purified from both mammalian and yeast cells that assembles histones H3 and H4 onto newly replicated DNA (24, 38). The chromatin assembly complex (CAC) is the combined complex of CAF-I with histones H3 and H4 (48). The three CAF-I proteins from Saccharomyces cerevisiae are designated Cac1p, Cac2p, and Cac3p and correspond to the human CAF-I proteins p150, p60, and p48, respectively. Deletion of any one of the three CAC genes in S. cerevisiae results in multiple phenotypes, including the derepression of telomere-adjacent genes, mislocalization of the telomere-binding protein Rap1p, and an increase in sensitivity to UV radiation (11, 24). However, deletion of any one or all three of the CAC genes is not lethal, indicating that there must be other activities in S. cerevisiae capable of chromatin assembly. Loss of one of the CAC genes coupled with loss of one of the HIR genes (which control histone H2A and H2B function) leads to synergistic defects in chromatin structure and decreased growth rates (22, 33).
CAC1 and CAC2 mRNAs are coordinately regulated, with expression peaking in the G1 phase of the cell cycle (39). In contrast, CAC3 mRNA levels do not change through the cell cycle (39). All three Cac proteins copurify (23, 24, 28), but the majority of p48 in human cells is found in a large complex that does not include p150 or p60 (28). Vertebrate p48 also copurifies with the histone deacteylase HDAC1 (43) and with pRb, the product of the retinoblastoma susceptibility gene (32), which acts as a tumor suppressor. The closest Cac3p homolog in S. cerevisiae is Hat2p, the subunit of histone acetyltransferase I that is necessary for association with histones H3 and H4 (31). Viewed together, these data suggest that Cac3p/p48 plays multiple roles in the deposition and modification of histones (34).
Cac3p appears to have at least one role unrelated to its function in chromatin assembly and/or histone modification. CAC3 was originally isolated as MSI1, a multicopy suppressor of IRA1 (35), which is a negative regulator of the RAS/cyclic AMP (cAMP) pathway. High-copy CAC3 reduces cAMP levels in ira1 and RAS2G19V strains, mitigating the heat shock sensitivity and sporulation deficiency of these strains. High-copy CAC3 also suppresses snf1 and snf4 mutations by decreasing cAMP levels (18). Overexpression of human p48, like CAC3/MSI1, can suppress the RAS/cAMP pathway in S. cerevisiae (32, 35).
The RAS/cAMP pathway has been well characterized in the yeast Saccharomyces. Two genes in S. cerevisiae, RAS1 and RAS2, are structural and functional homologs of human ras genes (8, 20), oncogenic mutations of which are found in 90% of pancreatic, 50% of colon, and 30% of lung adenocarcinomas as well as in 50% of thyroid tumors and 30% of myeloid leukemias (3). In yeast cells, Ras controls the metabolic state and alters the stress response of the cell through modulation of cAMP levels (5). Cdc25p stimulates Ras activity by promoting the exchange of GDP for GTP through the stabilization of Ras in a nucleotide-free state (16). Ira1p and Ira2p negatively regulate Ras proteins by stimulating their intrinsic GTPase activity (29). The RAS2G19V allele, which is analogous to the most common oncogenic mutation in human cancers (3), encodes a protein that binds GTP normally but fails to hydrolyze it (30), resulting in constitutively active Ras protein. Activated Ras protein stimulates adenylyl cyclase (Cyr1p/Cdc35p), thereby promoting production of cAMP. cAMP binds to Bcy1p, the negative regulatory subunit of protein kinase A (PKA), and disassociates it from the catalytic subunit (encoded by any one of the three genes TPK1, -2, or -3) to yield enhanced kinase activity. PKA activation leads to glycogen utilization, increased glycolysis, the induction of many growth-related genes (5), and reduced expression of genes encoding heat shock proteins Hsp72, Hsp41 (37), and Hsp12 (47). Cells containing an activated RAS/cAMP pathway (those containing RAS2G19V, bcy1Δ, or ira1Δ mutations) mate poorly, contain low levels of storage carbohydrates, and are sensitive to transient heat shock.
The mechanism by which Cac3p suppresses the heat shock sensitivity and the sporulation defect of cells with an activated RAS/cAMP pathway is not known. High-copy CAC3 suppresses phenotypes caused by the constitutively active RAS2G19V allele or by deletion of the negative regulator IRA1 but does not suppress these phenotypes when the pathway is activated by deletion of BCY1 (35). We investigated the mechanism by which Cac3p overproduction suppresses the Ras2pG19V oncoprotein. We found that the ability of CAC3 overexpression to suppress the RAS/cAMP pathway is independent of its role in CAF-I-mediated chromatin assembly and its putative role in histone modification activities. This result is similar to the recently published results of Zhu et al. (51). Consistent with this, a significant proportion of Cac3p resides in the cytoplasm, in contrast to Cac1p, which is primarily nuclear. We identified Npr1p as a protein that physically interacts with Cac3p and found that loss of Npr1p function suppresses the RAS/cAMP pathway in a manner indistinguishable from CAC3 overexpression. The Npr1p kinase is known to antagonize the ubiquitin-mediated inactivation of several transporter proteins; similarly, we have found that overexpression of polyubiquitin is also capable of suppressing RAS2G19V-induced heat shock sensitivity. Our results suggest that CAC3 sequesters and thereby inactivates the Npr1p kinase, which effectively reduces the activity of the RAS/cAMP pathway.
MATERIALS AND METHODS
Strains and plasmids.
Escherichia coli strains XL1-Blue and DH5α were used for all standard plasmid preparations and manipulations (1). pML9 for the disruption of NPR1 was provided by J. Heitman (26). pJW192, encoding a Ras2p-green fluorescent protein (GFP) fusion, was provided by J. Rine (4). pCUP1-myc-UBI4 was provided by M. Hochstrasser (9). pbcy1::URA3 has been described (45). Triple-hemagglutinin (HA) epitope-tagged Npr1p carrying a mutation that presumably blocks its kinase activity was a generous gift from Yu Jiang. This mutation, D579E, was created on the basis of homology to mutations known to inactivate other kinases, although the effect of the mutation on Npr1 kinase activity has not been demonstrated.
pGBT9-CAC3 was amplified from yeast strain Y294 by PCR amplification using the oligonucleotides 5′-GGCCGGGGATCCATGAATCAGTGCGCGAAGG-3′ and 5′-GGGCCCGTCGACTCACGAATGTCCAACAAGGTTTCC-3′. The PCR product was cloned into pGBT9C which had been digested with BamHI and SalI. pGBT9C is the pGBT9 vector which has been altered in the reading frame of the multiple cloning site by the addition of two additional guanine residues before the EcoRI site and was a generous gift from Clint McDonald. YEp55-CAC3 was constructed by amplifying the CAC3 gene from pGBT9-CAC3 by PCR using the oligonucleotides 5′-GGCCGGGTCGACATGAATCAGTGCGCGAAGG-3′ and 5′-GGGCCCGGATCCTCACGAATGTCCAACAAGGTTTCC-3′ and was cloned into YEp55S that had also been digested with SalI and BamHI. The multiple cloning site of this vector had been modified through the introduction of a SalI restriction site by Corey Davis and was a gift from him. pRS406-Ras2Val19 was constructed by isolating the 2.1-kb genomic EcoRI-HindIII fragment containing the RAS2G19V allele and cloned into pRS406 that had been digested with EcoRI and HindIII. pGAD1-CAC1 was cloned from a yeast genomic library constructed by Stan Fields and was a generous gift from Mark Rose.
NPR1 (with its start and stop codons) was amplified from W303 genomic DNA by PCR and cloned into pCR-II (Invitrogen). The XhoI-HindIII fragment containing the NPR1 gene was subcloned into pRSET-B (Invitrogen) to yield pRSET-NPR1. pRSET-NPR1 was digested with XbaI and the fragment was cotransformed into S. cerevisiae with pGalSET984 (10) linearized with XhoI. In vivo recombination between these two DNA fragments yielded pGalSET-NPR1 (10). Similarly, CAC3 was amplified from W303 genomic DNA by PCR and cloned into pCR-II. The BamHI-HindIII fragment of this plasmid containing the CAC3 gene was subcloned into pRSET-B. The XbaI fragment containing CAC3 and XhoI-linearized pGalSET985 were cotransformed into yeast cells, selecting for in vivo recombination. Immunoblotting confirmed that galactose induction of strains carrying either pGalSET-NPR1 or pGalSET-CAC3 generated an epitope-tagged protein of the expected size (data not shown). The protein encoded by YEp55-CAC3 was tagged at the carboxy terminus with GFP, using the PCR-mediated technique described by Longtine et al. (25). URA3 was integrated adjacent to the left telomere of chromosome VII as described (12).
The yeast strains used in this study are listed in isogenic groups in Table 1. Strains were grown in standard laboratory SD complete (SDC) medium with the appropriate amino acid dropouts (15). Genetic crosses, sporulation, dissection, and transformation were performed as described (15). Auxotrophic markers were swapped as described (7). The [rho−] strain was made by growth of the parental strain in the presence of ethidium bromide (25 μg/ml).
TABLE 1.
Yeast strains used
| Isogenic group and strain | Relevant characteristic(s) | Source or reference | |
|---|---|---|---|
| W303 | |||
| YJB195 | MATaura3-1 ade2-1 his3-11 leu2-3,112 can1-100 trp1-1 | Berman lab | |
| YJB209 | MATα ura3-1 ade2-1 his3-11 leu2-3,112 can1-100 trp1-1 | Berman lab | |
| YJB334 | MATa/α ura3-1/ura3-1 ade2-1/ ade2-1 his3-11/his3-11 leu2-3,112/leu2-3,112 can1-100/can1-100 trp1-1/trp1-1 | Berman lab | |
| YJB1358 | YJB209 cac1-1 | 11 | |
| YJB1599 | YJB209 cac2::TRP1 | 24 | |
| YJB1786 | YJB209 cac3::hisG VIIL::URA3-TEL | This study | |
| YJB2235 | YJB195 URA3::RAS2G19V | This study | |
| YJB2237 | YJB209 cac1-1 URA3::RAS2G19V | This study | |
| YJB2320 | YJB195 URA3::RAS2G19V YEp55-CAC3 | This study | |
| YJB2322 | YJB209 cac1-1 URA3::RAS2G19V YEp55-CAC3 | This study | |
| YJB2543 | YJB195 sir3::TRP1 | Berman lab | |
| YJB2546 | YJB195 gcn5::URA3 | Berman lab | |
| YJB3135 | YJB195 gcn5::ura3::TRP1 URA3::RAS2G19V | This study | |
| YJB3141 | YJB195 gcn5::ura3::TRP1 URA3::RAS2G19V YEp55-CAC3 | This study | |
| YJB3554 | YJB195 npr1::LEU2 bcy1::URA3 | This study | |
| YJB3563 | YJB209 cac2::TRP1 URA3::RAS2G19V | This study | |
| YJB3610 | YJB209 VIIL::URA3-TEL pGalSET | This study | |
| YJB3670 | YJB195 sch9::TRP1 YCP50-RAS2G18A G19V | 50a | |
| YJB3674 | YJB195 sch9::TRP1 YCP50-RAS2G18A G19V YEP55-CAC3 | This study | |
| YJB3712 | YJB195 npr1::LEU2 | This study | |
| YJB3721 | YJB334 ura3-1/URA3::RAS2G19V YEp55-CAC3 | This study | |
| YJB3723 | YJB195 npr1::LEU2 URA3::RAS2G19V | This study | |
| YJB3724 | YJB2235 pCUP1-myc-UB14 | This study | |
| YJB3737 | YJB334 ura3-1/URA3::RAS2G19V | This study | |
| YJB3739 | YJB334 YEp55-CAC3 | This study | |
| YJB3763 | YJB195 sir3::TRP1 URA3::RAS2G19V | This study | |
| YJB3773 | YJB195 sir3::TRP1 URA3::RAS2G19V YEp55-CAC3 | This study | |
| YJB3785 | MATahis3 Leu2-3, 112 trp1 ura3-1 | 4 | |
| YJB3791 | YJB334 ura3-1/URA3::RAS2G19V npr1::LEU2/npr1::LEU2 | This study | |
| YJB3792 | YJB334 npr1::LEU2/npr1::LEU2 | This study | |
| YJB3863 | YJB195 URA3::RAS2G19V pGalSET-NPR1 | This study | |
| YJB3875 | YJB195 URA3::RAS2G19V pGalSET-NPR1 pGalSET-CAC3 | This study | |
| YJB3876 | YJB209 cac2::TRP1 URA3::RAS2G19V YEp55-CAC3 | This study | |
| YJB3896 | YJB3785 pGFP-RAS2 | This study | |
| YJB3897 | YJB3785 npr1::LEU2 pGEP-RAS2 | This study | |
| YJB3898 | YJB3785 YEp55-CAC3 pGFP-RAS2 | This study | |
| YJB3954 | YJB195 pGalSET-NPR1 | This study | |
| YJB4034 | YJB3785 YEp55-CAC3 | This study | |
| YJB4317 | YJB4034 YEp55-CAC3-GEP | This study | |
| YJB4506 | YJB4317 YEp55-CAC3-GFP [rho−] | This study | |
| YJB4632 | YJB209 gpa2::TRP1 | 50a | |
| YJB4635 | YJB209 gpr1::HIS3 | 50a | |
| YJB5235 | YJB195 URA3::RAS2G19V gpa2::TRP1 YEp55-CAC3 | This study | |
| YJB5243 | YJB195 URA3::RAS2G19V gpa2::TRP1 | 50a | |
| YJB5237 | YJB195 URA3::RAS2G19V gpr1::HIS3 YEp55-CAC3 | This study | |
| YJB5245 | YJB195 URA3::RAS2G19V gpr1::HIS3 | 50a | |
| YJB5522 | YJB3723 pCUP1-myc-UB14 | This study | |
| YJB5523 | YJB3737 pCUP1-myc-UB14 | This study | |
| YSJ328 | YJB3954 VIIL::URA3-TEL | This study | |
| Y2799 | MATα ura3-1 ade2-1 his3-11 leu2-3,112 can1-100 trp1-1 npr1::LEU2 pRS424 pGalSET-CAC3 | This study | |
| Y2800 | MATα ura3-1 ade2-1 his3-11 leu2-3,112 can1-100 trp1-1 npr1::LEU2 pRS424- 3HA-NPR1 pGalSET-CAC3 | This study | |
| Y2801 | MATα ura3-1 ade2-1 his3-11 leu2-3,112 can1-100 trp1-1 npr1::LEU2 pRS424-3HA- NPR1-KD pGalSET-CAC3 | This study | |
| YPH49/50 | |||
| YJB1583 | MATaura3-52 lys2-801 ade2-101 trp1-ars1 his3-200 leu2Δ1 | 31 | |
| YJB1584 | YJB1583 hat1::HIS3 | 31 | |
| YJB1585 | YJB1583 hat2::TRP1 | 31 | |
| YJB1586 | YJB1583 hat1::HIS3 hat2::TRP1 | 31 | |
| YJB2349 | YJB1583 URA3::RAS2G19V | This study | |
| YJB2350 | YJB1583 hat1::HIS3 URA3::RAS2G19V | This study | |
| YJB2366 | YJB1583 URA3::RAS2G19V YEp55-CAC3 | This study | |
| YJB2367 | YJB1583 hat1::HIS3 URA3::RAS2G19V YEp55-CAC3 | This study | |
| YJB2648 | YJB1583 hat2::TRP1 URA3::RAS2G19V | This study | |
| YJB2658 | YJB1583 hat2::TRP1 URA3::RAS2G19V YEp55-CAC3 | This study | |
| YJB2672 | YJB1583 hat1::HIS3 hat2::TRP1 URA3::RAS2G19V | This study | |
| YJB2673 | YJB1583 hat1::HIS3 hat2::TRP1 URA3::RAS2G19V YEp55-CAC3 | This study | |
| YJB2697 | YJB1583 bcy1::URA3 | This study | |
| YJB2781 | YJB1583 bcy1::URA3 YEp55-CAC3 | This study | |
| YDS3 | |||
| YJB2431 | MATα ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 | 35a | |
| YJB2432 | YJB2431 hda1::TRP1 | 35a | |
| YJB2433 | YJB2431 rpd3::LEU2 | 35a | |
| YJB2434 | YJB2431 hda1::TRP1 rpd3::LEU2 | 35a | |
| YJB2474 | YJB2431 URA3::RAS2G19V | This study | |
| YJB2475 | YJB2431 hda1::TRP1 URA3::RAS2G19V | This study | |
| YJB2476 | YJB2431 rpd3::leu2::HIS3 URA3::RAS2G19V | This study | |
| YJB2474 | YJB2431 hda1::TRP1 rpd3::leu2::HIS3 URA3::RAS2G19V | This study | |
| YJB2529 | YJB2431 URA3::RAS2G19V YEp55-CAC3 | This study | |
| YJB2530 | YJB2431 rpd3::leu2::HIS3 URA3::RAS2G19V YEp55-CAC3 | This study | |
| YJB2531 | YJB2431 hda1::TRP1 URA3::RAS2G19V YEp55-CAC3 | This study | |
| YJB2532 | YJB2431 hda1::TRP1 rpd3::leu2::HIS3 URA3::RAS2G19V YEp55-CAC3 | This study | |
| HF7c | |||
| YJB1325 | MATaura3-52 his3-200 ade2-101 lys2-801 trp1-901 leu2-3,112 gal4-542 gal180-538 LYS2::GAL1-HIS3 | ||
| URA3::(GAL4 17-mers)3-CYC1-lacZ | Clontech Inc. | ||
| YJB3197 | YJB1325 pGBT9-Cac3p pGAD1-CAC1 | This study | |
| YJB3438 | YJB1325 pGBT9-Cac3p pGAD1-NPR1561–605 | This study | |
| L1356 | |||
| YJB2136 | MATaura3-52 his3-200 leu2-1 met8-1 ilv1-1 trp1-901 cac3-878 lys2::his3Δ4 | 33 | |
| YJB2137 | YJB2136 hir3::LEU2 | 33 | |
| YJB2998 | YJB2136 hir3::leu2::TRP1 URA3::RAS2G19V | This study | |
| YJB2999 | YJB2136 hir3::leu2::TRP1 URA3::RAS2G19V YEp55-CAC3 | This study | |
| YJB3016 | YJB2136 URA3::RAS2G19V | This study | |
| YJB3017 | YJB2136 URA3::RAS2G19V YEp55-CAC3 | This study | |
| Σ1278b | |||
| YJB2444 | MATa/α ura3-52/ura3-52 | 24a | |
| YJB3514 | YJB2444 pGalSET | This study | |
| YJB3515 | YJB2444 pGalSET-CAC3 | This study | |
| YJB5723 | YJB5724 npr1::LEU2/npr1::LEU2 | 26 | |
| YJB5724 | MATa/α ura3-52/ura3-52 | 26 |
Heat shock and sporulation assays.
Yeast cells were grown to saturation on appropriate dropout medium with either 2% glucose or 2% galactose present as the carbon source. Cells were collected by centrifugation, washed once, and resuspended in water. Aliquots were incubated at 55°C for the time periods indicated. After cooling to room temperature, cells were serially diluted 10-fold, plated, and incubated at 30°C for 2 days. Resulting colonies were counted and normalized to the number of viable cells in an aliquot that was not exposed to 55°C. To determine sporulation efficiency, diploid strains were grown overnight in appropriate dropout medium supplemented with 2% galactose to induce the expression of genes under control of the GAL1 promoter, as appropriate. Cells were collected by centrifugation, washed twice, and allowed to sporulate for 3 days in 1% potassium acetate at room temperature, with shaking. Diploids and tetrads (at least 300 cells of each strain) were counted by microscopic observation.
Two-hybrid screen.
An S. cerevisiae genomic DNA two-hybrid library in pGAD1, -2, or -3 (6) was kindly provided by P. Siliciano (University of Minnesota) and transformed into HF7c containing pGBT9-CAC3 as bait. Transformed cells which contained interacting fusion proteins were selected by plating on SDC lacking Leu, Trp, and His and supplemented with 5 mM 3-amino-1,2,4-triazole (3AT). Transformants that contained GAL4 were identified by PCR and discarded. Surviving yeast strains were cured of either plasmid to ensure that growth on the selective medium was dependent on the presence of both plasmids. Inserts in pGAD were amplified directly from the yeast strain by PCR using primers flanking the multiple cloning site. Amplified DNA was sequenced using a nested primer.
Coimmunoprecipitation.
Yeast strains YSJ401, YSJ402, and YSJ403 were grown in SC lacking Leu and Trp containing 2% galactose for 2 days. Cells were collected by centrifugation and washed once with water and once with 10mM sodium azide. Cell pellets were then frozen at −70°C. Cells were then resuspended in 200 μl of buffer C (20 mM HEPES-KOH [pH 7.4], 150 mM NaCl, 1 mM EDTA, 5 mM MgCl2, 2 mM phenylmethylsulfonyl fluoride plus 1 μg of pepstatin A, 0.5 μg of leupeptin, and 2 μg of aprotinin per ml) and lysed by vortexing with glass beads. Buffer C (200 μl) was then added, and unlysed cells, cellular debris, and glass beads were removed by centrifugation. Lysate (70 μg in 450 μl) was added to 20 μl of anti-HA affinity matrix (Roche) that had been blocked with bovine serum albumin (BSA, 2 mg/ml) in buffer C. Loading buffer (5 × sodium dodecyl sulfate [SDS]) was added to an aliquot of the input as a control. The lysate was allowed to bind to the affinity matrix for 3 h at 4°C. The affinity beads and bound proteins were pelleted by centrifugation, and the supernatant was retained. The 5 × SDS loading buffer was added to an aliquot of the supernatant as a control. Beads were then washed four times with buffer C. After the final wash, beads were boiled for 3 min in 50 μl of 1 × SDS sample buffer, and an aliquot (15 μl) of each sample was loaded onto an 8% polyacrylamide–SDS gel and subjected to polyacrylamide gel electrophoresis (PAGE). Samples were then transferred onto a polyvinylidene difluoride membrane and probed with monoclonal antibodies against the T7 epitope (Novagen) or the HA epitope (12CA5). Immunoreactivity was detected using ECL (Amersham).
Microscopy and pseudohyphal assays.
To determine the subcellular localization of Cac3p-GFP, YJB4506 was grown in SDC lacking Trp and supplemented with 2% raffinose, 0.5% galactose, and 10 ng of DAPI (4′,6′-diamidino-2-phenylindole) for 4 h at 30°C. Cells were viewed using a Nikon Eclipse E800 photomicroscope equipped with differential interference contrast and fluorescence optics using a 100 × 1.3-numerical-aperture plan apo objective. Digital images were collected using a CoolCam liquid-cooled, three-chip color charge-coupled device camera (Cool Camera Company, Decatur, Ga.) and captured to a Pentium II 300-MHz personal computer using Image Pro Plus version 4.0 software (Media Cybernetics, Silver Spring, Md.). Pseudohyphal growth was assayed as described by Lorenz and Heitman (26). Σ1278b strains carrying either a CAC3 overexpression plasmid or the parental plasmid were grown overnight in either glucose or galactose. Cells were then plated on limiting nitrogen medium with either glucose (SLAD) or raffinose and galactose (SLADG) as a carbon source (26) and grown for 3 days at 30°C before being photographed.
Metabolic labeling and immunoprecipitation.
32P metabolic labeling and Ras2p immunoprecipitation were performed essentially as described by Whistler and Rine (50). In brief, wild-type or npr1Δ cells were grown overnight in YPAD medium, washed in SDC low-phosphate medium, and grown in 10 ml of this medium at 30°C for 2 h. Then 2 mCi of H332PO4 (ICN) was added, and the culture was incubated at 30°C for an additional 3 h. Cells were collected by centrifugation, washed in NLB buffer (50 mM Tris [pH 7.5], 20 mM MgCl2, 100 mM NaCl, 0.1% Nonidet P-40, 1 mM dithiothreitol, 1% aprotinin, 1 mM phenylmethylsulfonyl fluoride) and stored at −70°C overnight. Cells were resuspended in 0.5 ml of NLB and lysed by bead beating. Unlysed cells and cell debris were removed by centrifugation. BSA-treated charcoal was added to the lysates, which were vortexed and centrifuged several times to remove all traces of the charcoal. Then 20 μl of either protein A-agarose or anti-Ras2-agarose (clone Y13-259; Calbiochem) was added to the lysate and mixed at 4°C for 2 h. Beads were washed three times with NLB and three times with NLB without detergent. Proteins were eluted from the beads by incubating at 70°C for 10 min in 20 μl of SDS loading buffer. Proteins were separated by SDS-PAGE through a 9% gel, which was fixed and dried, and and bands were quantitated by PhosphoImager analysis (Molecular Dynamics).
RESULTS
CAC3 suppression of the RAS/cAMP pathway is independent of CAF-I.
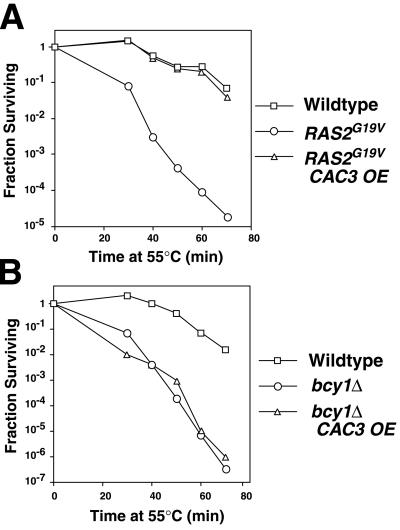
To understand the molecular mechanism by which CAC3 overexpression affects the RAS/cAMP pathway, we first established a quantitative assay for heat shock sensitivity, a phenotype caused by activation of the RAS/cAMP pathway. Cells were grown to saturation, aliquoted and incubated at 55°C for different lengths of time, cooled, serially diluted, and plated on nonselective medium. Resulting colonies were counted after 2 days at 30°C. Yeast cells carrying the dominant RAS2G19V allele have a constitutively activated RAS/cAMP pathway (30) and are sensitive to heat shock (Fig. 1A). Overexpression of CAC3 suppressed the heat shock sensitivity of a strain carrying the RAS2G19V allele (35) (Fig. 1A), restoring wild-type heat shock resistance. In contrast, heat shock sensitivity caused by deletion of BCY1, which encodes the negative regulator of PKA, was not suppressed by CAC3 overexpression (35) (Fig. 1B). Thus, our quantitative heat shock sensitivity assay confirmed the reported ability of CAC3 overexpression to suppress the RAS/cAMP pathway when it is activated by a RAS2G19V allele but not when it is activated by BCY1 deletion.
FIG. 1.
CAC3 overexpression suppresses the RAS/cAMP signal transduction pathway between RAS and PKA. The heat shock resistance of (A) isogenic wild-type (YJB195), RAS2G19V (YJB2235), and RAS2G19V CAC3-over expressing (OE) (YJB2320) strains and (B) isogenic wild-type (YJB1583), bcy1Δ (YJB2697), and bcy1Δ CAC3-overexpressing (YJB2781) strains was determined by incubation at 55°C for the indicated time periods as described in Materials and Methods.
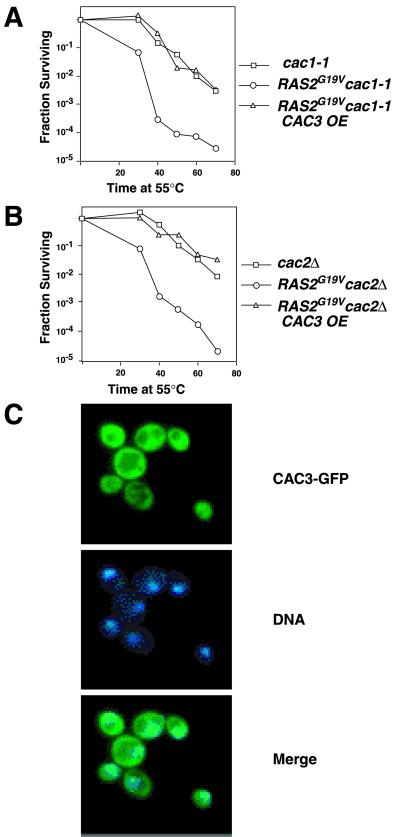
To determine if the CAF-I complex mediates CAC3 suppression of the RAS/cAMP pathway, perhaps by affecting the expression of one or more genes that alter RAS/cAMP signal transduction, we asked if CAC3 overexpression could suppress the RAS/cAMP pathway in the absence of functional CAF-I. We found that loss of CAC1 function did not affect the heat shock sensitivity of RAS2G19V cells and CAC3 overexpression suppressed the heat shock sensitivity of cac1-1 RAS2G19V cells (Fig. 2A). Similarly, deletion of CAC2 had no effect on the heat shock sensitivity of RAS2G19V cells or on the ability of CAC3 overexpression to suppress the heat shock sensitivity (Fig. 2B). Thus, the ability of CAC3 to affect the RAS/cAMP pathway did not require the presence of active CAF-I and must be independent of CAF-I-mediated chromatin assembly function.
FIG. 2.
CAC1 and CAC2 are not required for CAC3 to suppress the RAS/cAMP pathway. The heat shock sensitivity of (A) cac1-1 (YJB1358), RAS2G19V cac1-1 (YJB2237), RAS2G19V cac1-1 CAC3-overexpressing (OE) (YJB2322) or (B) cac2Δ (YJB1599), RAS2G19V cac2Δ (YJB3563), and RAS2G19V cac2Δ CAC3-overexpressing (YJB3876) was determined as described for Fig. 1. (C) Cells carrying Cac3p-GFP (YJB4034) were stained with DAPI and analyzed by fluorescence microscopy to observe both GFP (top panel) and DNA (middle panel). The merged image (bottom panel) shows that Cac3p-GFP localizes throughout the cytoplasm and nucleus. Darker regions correspond to the vacuoles.
The ability of Cac3p to function independently of Cac1p and Cac2p is supported by the previous observations that the cell cycle regulation of CAC3 transcripts is different from that of CAC1 and CAC2 transcripts (39) and that Cac3p/p48 does not always copurify with Cac1p and Cac2p (28). Consistent with this, we found that Cac3p-GFP had a localization pattern different from that of Cac1p: Cac3p-GFP appeared to be diffuse and localized throughout the nucleus and cytoplasm (but not in the vacuoles) (Fig. 2C) during all stages of the cell cycle (S. Enomoto and J. Berman, unpublished data). In contrast, epitope-tagged Cac1p localized primarily to large foci within the nucleus, even when expressed from a high-copy-number vector (11).
CAC3 suppression of the RAS/cAMP pathway is independent of several histone-modifying activities.
Cac3p and other p48-related proteins are involved in several chromatin-related functions. For example, CAC3 has been shown to antagonize the Sin3p-Rpd3p histone deacetylation complex (42). Additionally, Cac3p has high sequence similarity to RbAp48p and RbAp46, which are subunits of histone modification enzymes such as histone deacetylase I (HDAC1) and histone acetylases (43, 49). Therefore, we asked if Cac3p affects the RAS/cAMP pathway through its association with histone deacetylases such as Rpd3p and Hda1p. The heat shock sensitivity of RAS2G19V strains lacking RPD3 and/or HDA1 did not differ from that of otherwise wild-type RAS2G19V strains (Table 2). Furthermore, there was no difference in the degree to which CAC3 overexpression suppressed the heat shock sensitivity of these strains (Table 2).
TABLE 2.
Effect of CAC3 overexpression on RAS/cAMP pathway activation in different mutant strainsa
| Notation type | Strain | Relevant genotype | Heat shock response |
|---|---|---|---|
| RAS/cAMP pathway | YJB195 | Wild type | Resistant |
| YJB2235 | RAS2G19V | Sensitive | |
| YJB2320 | RAS2G19V YEp55-CAC3 | Resistant | |
| YJB2697 | bcy1Δ | Sensitive | |
| YJB2781 | bcy1Δ YEp55-CAC3 | Sensitive | |
| Chromatin assembly | YJB2237 | RAS2G19V cac1-1 | Sensitive |
| YJB2322 | RAS2G19V cac1-1 YEp55-CAC3 | Resistant | |
| YJB3563 | RAS2G19V cac2Δ | Sensitive | |
| YJB3876 | RAS2G19V cac2Δ YEp55-CAC3 | Resistant | |
| Histone acetyltransferases | YJB2350 | RAS2G19V hat1Δ | Sensitive |
| YJB2367 | RAS2G19V hat1Δ YEp55-CAC3 | Resistant | |
| YJB2648 | RAS2G19V hat2Δ | Sensitive | |
| YJB2658 | RAS2G19V hat2Δ YEp55-CAC3 | Resistant | |
| YJB2672 | RAS2G19V hat1Δ hat2Δ | Sensitive | |
| YJB2673 | RAS2G19V hat1Δ hat2Δ YEp55-CAC3 | Resistant | |
| YJB3135 | RAS2G19V gcn5Δ | Sensitive | |
| YJB3141 | RAS2G19V gcn5Δ YEp55-CAC3 | Resistant | |
| Histone deacetylases | YJB2475 | RAS2G19V hda1Δ | Sensitive |
| YJB2531 | RAS2G19V hda1Δ YEp55-CAC3 | Resistant | |
| YJB2476 | RAS2G19V rpd3Δ | Sensitive | |
| YJB2530 | RAS2G19V rpd3Δ YEp55-CAC3 | Resistant | |
| YJB2474 | RAS2G19V had1Δrpd3Δ | Sensitive | |
| YJB2532 | RAS2G19V had1Δrpd3Δ YEp55-CAC3 | Resistant | |
| Histone transcription | YJB2998 | RASG19V hir3Δ | Sensitive |
| YJB2999 | RAS2G19V hir3Δ YEp55-CAC3 | Resistant | |
| Resistant | |||
| Silencing maintenance | YJB3763 | RAS2G19V sir3Δ | Sensitive |
| YJB3773 | RAS2G19V sir3Δ YEp55-CAC3 | Resistant | |
| Cell signaling | YJB3670 | RAS2G18A G19V sch9Δ | Sensitive |
| YJB3674 | RAS2G18A G19V sch9Δ YEp55-CAC3 | Resistant | |
| YJB5245 | RAS2G19V gpr1Δ | Sensitive | |
| YJB5237 | RAS2G19V gpr1Δ YEp55-CAC3 | Resistant | |
| YJB5243 | RAS2G19V gpa2Δ | Sensitive | |
| YJB5235 | RAS2G19V gpa2Δ YEp55-CAC3 | Resistant |
Sensitive indicates that the heat shock response was indistinguishable from that of an isogenic RAS2G19V strain (YJB2235, -2349, -2474, and -3016). Resistant indicates that the heat shock response was indistinguishable from that of an isogenic RAS2 strain (YJB195, -1583, -2431, and -2136).
Based on the similarity between Cac3p and Hat2p, a factor that facilitates the association of Hat1p with histones H3 and H4 (31), we investigated the ability of CAC3 to suppress RAS2G19V-induced heat shock sensitivity in strains lacking genes encoding histone acetyltransferase components. The heat shock sensitivity of RAS2G19V strains carrying deletions in HAT1, HAT2, HAT1 and HAT2, or GCN5 was measured in the presence and absence of a plasmid overexpressing CAC3. In all of these strains, heat shock sensitivity and CAC3 suppression of this sensitivity were not significantly different from what was found in the isogenic wild-type RAS2G19V strain (Table 2). In addition, we asked if deletion of HIR3, which encodes a histone transcriptional regulator that has synergistic effects with CAC mutants, or of SIR3, which encodes a component of silent chromatin, would affect RAS2G19V-induced heat shock sensitivity in the presence and absence of CAC3 overexpression. Again, the heat shock sensitivity and the ability of CAC3 overexpression to suppress this heat shock sensitivity were not significantly different from the isogenic wild-type strain (Table 2). Thus, CAC3 overexpression does not appear to affect the RAS/cAMP pathway by functioning as a component of a histone modification complex.
CAC3 suppression of the RAS/cAMP pathway is independent of the GPA2/GPR1 signaling pathway.
A parallel pathway for the activation of PKA utilizes the membrane proteins Gpa2p and Gpr1p. Gpa2p is a Gα-like protein which activates adenylyl cyclase in response to extracellular glucose (44). Sch9p is a protein kinase that contributes to the heat shock response independently of the RAS/cAMP and GPA2/GPR1 pathways (27). Thevelein and de Winde (44) hypothesized the existence of a Saccharomyces protein containing WD40 repeat motifs which could function as a Gβ-like protein, suppressing the function of Gpa2p. As CAC3 contains WD40 repeat motifs, we hypothesized that CAC3 overexpression might decrease the levels of intracellular cAMP by suppressing the activity of Gpa2p. To test this possibility, we asked if components of the GPA2 pathway were required for CAC3-mediated suppression of RAS2G19V-induced heat shock sensitivity. We found that RAS2G19V strains lacking GPA2 or GPR1 remained sensitive to heat shock and that CAC3 overexpression effectively suppressed the heat shock sensitivity of these strains (Table 2). Similarly, cells lacking functional SCH9 were also sensitive to heat shock, and this sensitivity was still suppressed by CAC3. Thus, signal transduction through the GPA2/GPR1 or SCH9 pathway is not required for suppression of RAS2G19V by CAC3 overexpression and Cac3p is not acting as a Gβ-like protein to directly suppress Gpa2p.
Identification of Npr1p as a Cac3p-interacting protein.
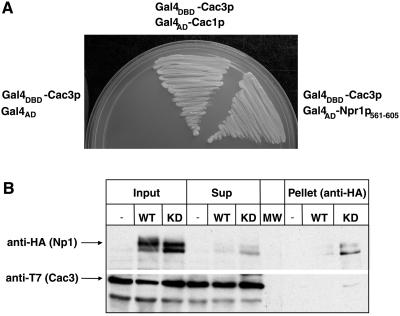
To identify factors that interact with Cac3p and that may be required for CAC3 suppression of the RAS/cAMP pathway, we isolated genes encoding proteins that interact with Cac3p using the yeast two-hybrid system (6). Cac3p was fused to the Gal4p DNA-binding domain and used to screen a library of S. cerevisiae genes fused to the Gal4p activation domain. One gene identified in this screen, CAC1, was subsequently used as a positive control in the screen. Further screening identified a clone containing the codons for amino acids 561 to 605 of NPR1. NPR1 encodes a nitrogen permease reactivator, a putative serine/threonine protein kinase required to regulate the posttranslational stability of several permeases, including Mep2p (26), Gap1p (41), and Tat2p (36). Although the Cac3p-Npr1p interaction is weaker than the Cac3p-Cac1p interaction, it was consistently and reproducibly detected. To biochemically confirm this protein-protein interaction, we constructed strains expressing T7 epitope-tagged Cac3p and either HA-tagged Npr1p or an HA-tagged, kinase-dead version of Npr1p. We immunoprecipitated HA-tagged Npr1 using anti-HA antibodies under nondenaturing conditions, fractionated the immunoprecipitates, and then probed for Npr1p and Cac3p by immunoblotting. As shown in Fig. 3B, Cac3p was coimmunoprecipitated with the putative kinase-dead version of Npr1p, although not with the wild-type version of the protein. This confirms the interaction between Npr1p and Cac3p detected by two-hybrid analysis but suggests that the interaction with the wild-type protein may be relatively transient. Loss of Npr1p kinase activity evidently stabilizes this transient interaction. To the best of our knowledge, neither interaction between Npr1p and the CAF-I complex (or other aspects of chromatin metabolism) nor any connection between Npr1p and the RAS/cAMP pathway has been reported previously.
FIG. 3.
Cac3p interacts with Npr1p in a two-hybrid assay. (A) Gal4DBD-Cac3p/Gal4AD (YJB3523), Gal4DBD-Cac3p/Gal4AD-Cac1p (YJB3197), and Gal4DBD-Cac3p/Gal4AD-Npr1p561–605 (YJB3438) strains carrying the HIS3 gene under control of the GAL1 promoter were plated on SDC lacking Leu, Trp, and His and with 5 mM 3AT. Growth indicates a positive interaction between the Gal4 DNA-binding domain (DBD) and Gal4 activation domain (AD) fusion proteins. (B) Cac3p coimmunoprecipitates with Npr1p. Yeast strains overexpressing T7 epitope-tagged Cac3p either without (lane —) or with the cooverexpression of HA epitope-tagged wild-type Npr1p (WT) or kinase-dead Npr1p (KD) were immunoprecipitated under native conditions with anti-HA antiserum. Levels of Npr1p and Cac3p were determined from the input, supernatants (sup), and pellets by immunoblotting with either anti-HA or anti-T7 antiserum. Lane MW, size markers.
Deletion of NPR1 yields the same phenotype as does CAC3 overexpression.
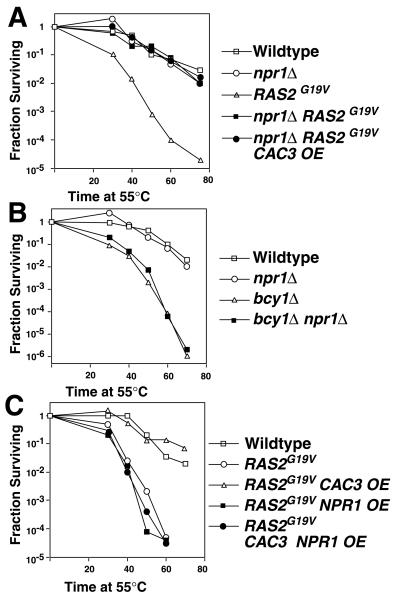
To determine if the interaction of Cac3p with Npr1p was responsible for suppression of the RAS/cAMP pathway, NPR1 was deleted in a wild-type or RAS2G19V background, and the resulting strains were assayed for heat shock sensitivity. Deletion of NPR1 had no affect on the heat shock sensitivity of a wild-type strain but fully suppressed the heat shock sensitivity induced by the RAS2G19V allele (Fig. 4A). In contrast, deletion of NPR1, similar to overexpression of CAC3, had no effect on the heat shock sensitivity of a bcy1Δ strain (Fig. 4B).
FIG. 4.
Deletion of NPR1 suppresses the RAS/cAMP pathway between RAS and PKA. The heat shock resistance of (A) wild-type (YJB195), npr1Δ (YJB3712), RAS2G19V (YJB2235), RAS2G19V npr1Δ (YJB2723), and RAS2G19V npr1Δ CAC3-overexpressing (OE) (YJB4017), and (B) bcy1Δ (YJB2697) and bcy1Δ npr1Δ (YJB3554) strains was determined. (C) The heat shock resistance of isogenic wild-type (YJB195), RAS2G19V (YJB2235), and RAS2G19V strains carrying high-copy CAC3 (YJB2320), NPR1 (YJB3863) and both CAC3 and NPR1 (YJB3875) was determined.
Activation of the RAS/cAMP pathway causes a sporulation defect (21), which can be suppressed by overexpression of CAC3 (35) (Table 3). Similar to CAC3 overexpression, deletion of both copies of NPR1 suppressed the sporulation defect caused by the RAS2G19V allele (Table 3). The fact that CAC3 overexpression or NPR1 deletion can suppress two distinctly different phenotypes of an activated RAS/cAMP pathway indicates that this effect is specific to this pathway and does not reflect a generalized increase in heat tolerance. Thus, the phenotypes of strains lacking NPR1 are indistinguishable from the phenotypes of strains overexpressing CAC3: both mutations suppress the RAS/cAMP signal transduction pathway when it is activated by a RAS2G19V allele but not by BCY1 deletion.
TABLE 3.
Deletion of NPR1, like overexpression of CAC3, suppresses the sporulation defect of RAS2G19V strains
| Strain | Relevant genotype | Sporulation efficiency (%) |
|---|---|---|
| YJB334 | Wild type | 14.9 |
| YJB3737 | RAS2G19V | 4.8 |
| YJB3792 | npr1Δ/npr1Δ | 23.0 |
| YJB3791 | RAS2G19V npr1Δ/npr1Δ | 18.1 |
| YJB3739 | YEp55-CAC3 | 47.2 |
| YJB3721 | RAS2G19V YEp55-CAC3 | 34.0 |
| YJB5523 | RAS2G19V pCUP1-myc-UBI4 | 18.1 |
These data suggest a model in which excess Cac3p interacts with Npr1p, sequestering the kinase so that it is not available for its role in the RAS/cAMP pathway. The sequestration model predicts that (i) CAC3 overexpression in an npr1Δ strain would confer no additional resistance to heat shock, (ii) the ability of an npr1Δ mutation to suppress RAS2G19V phenotypes would not depend on the presence of a functional CAC3 gene, and (iii) elevated levels of NPR1 would provide excess copies of Npr1p and negate the effect of CAC3 overexpression. Consistent with the first prediction, no additional heat shock resistance was observed when CAC3 was overexpressed in an npr1Δ strain (Fig. 4A), which is consistent with the idea that CAC3 overexpression and NPR1 deletion are suppressing the RAS/cAMP pathway by a common mechanism. Consistent with the second prediction, RAS2G19V cells carrying deletions of both NPR1 and CAC3 remained heat shock resistant like the RAS2G19V npr1Δ cells (data not shown). Consistent with the third prediction, RAS2G19V cells remained sensitive to heat shock when both CAC3 and NPR1 were overexpressed from the GAL1 promoter (Fig. 4C).
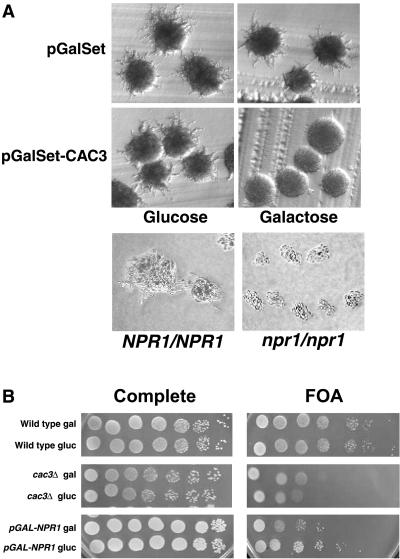
In addition, the sequestration model predicts that if excess Cac3p sequesters Npr1p, then the phenotypes of cells overexpressing CAC3 should resemble the known phenotypes of cells lacking NPR1. Npr1p is required for the stable expression of the Mep2p ammonium transporter, which is necessary for pseudohyphal growth in diploid strains in the Σ1278b strain background. Homozygous deletion of NPR1 in the diploid Σ1278b strain background results in cells that produce fewer pseudohyphae (Fig. 5A, bottom panels), apparently due to the loss of Mep2p function (26). As predicted by the sequestration model, overexpression of CAC3 in a diploid Σ1278b strain, like deletion of NPR1 in these strains, greatly reduced the number and length of pseudohyphae formed (Fig. 5A, upper panels). Thus, CAC3 overexpression and NPR1 deletion both result in identical phenotypes for suppression of the RAS/cAMP pathway and for pseudohyphal growth.
FIG. 5.
CAC3 and NPR1 are mutally antagonistic. (A) CAC3 overexpression suppresses pseudohyphal growth. Σ1278B strains carrying either pGalSet (YJB3514) or pGalSet-CAC3 (YJB3515) were grown in either galactose (to induce CAC3 expression) or glucose (to repress CAC3 expression). The cells were then plated on either SLAD or SLADG medium to induce pseudohyphal growth either with or without CAC3 expression. Representative colonies were photographed after 3 days of growth at 30°C. For comparison, wild-type (YJB5724) and npr1/npr1 (YJB5723) Σ1278B strains are also shown. (B) NPR1 overexpression decreases telomeric silencing. Serial dilutions (1:10) of wild-type (YJB3610), cac3 (YJB1786), and pGalSET-NPR1 (YSJ328) strains were plated on either complete medium or medium with 5-fluoroorotic acid (FOA). Yeast cells were grown in medium containing either glucose (gluc) or galactose (gal), as indicated. Plates were photographed after 2 days of growth at 30°C.
Furthermore, the sequestration model predicts that overexpression of NPR1 should produce the same phenotypes as deletion of CAC3. Kaufman et al. (24) showed that deletion of CAC3 leads to a decrease in transcriptional silencing of a URA3 reporter gene at the telomere on chromosome VII by about 25-fold. The NPR1 gene was placed on a plasmid under control of the GAL1, 10 promoter to be induced in the presence of galactose and repressed in the presence of glucose. NPR1-overexpressing cells, like cac3Δ cells, have a modest decrease in telomeric silencing of approximately 16-fold (Fig. 5B). Taken together, these data are consistent with our proposed model that excess Cac3p binds to Npr1p and sequesters the kinase, resulting in a set of phenotypes that are indistinguishable from those caused by npr1Δ.
Npr1p does not affect the phosphorylation state or localization of Ras2p.
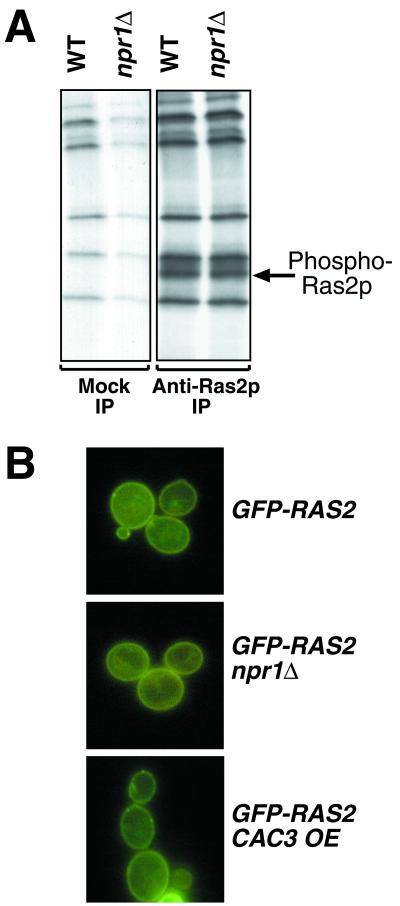
Ras2p is a membrane-associated phosphoprotein whose activity is increased by phosphorylation of Ser-214 (50) by an unknown kinase. Npr1p is a putative serine/threonine kinase that presumably phosphorylates several membrane proteins (26, 36, 41). To test the hypothesis that Npr1p may be the kinase that phosphorylates Ras2p, we metabolically labeled wild-type and npr1Δ cells with 32P, immunoprecipitated Ras2p, performed SDS-PAGE, and detected phospho-Ras2p by autoradiography. The amount and electrophoretic mobility of phospho-Ras2p were not affected by deletion of NPR1 (Fig. 6A), indicating that Npr1p does not affect the degree of Ras2p phosphorylation either directly or indirectly. Immunoblot experiments did not detect any change in the quantity of total Ras2p in RAS2G19V cells overexpressing Cac3p or missing Npr1p (data not shown). Furthermore, recombinant Npr1p did not phosphorylate recombinant human Ha-Ras (data not shown). Thus, it appears unlikely that Npr1p modulates the RAS/cAMP pathway by affecting the phosphorylation of Ras. We cannot rule out the formal possibility that Npr1p is one of several kinases that can phosphorylate Ras in vivo, although this scenario cannot explain how loss of Npr1p function alone could suppress the RAS/cAMP pathway while not causing any detectable change in the degree of Ras phosphorylation.
FIG. 6.
Npr1p does not affect the phosphorylation or localization of Ras2p. (A) Wild-type (WT) (YJB195) and npr1Δ (YJB3712) cells were grown in the presence of [32P]orthophosphate. Lysates were immunoprecipitated (IP) with protein A-agarose (mock) or anti-Ras2p conjugated to agarose. Bound proteins were eluted by denaturation, separated by SDS-PAGE, and detected by autoradiography. The arrow indicates phospho-Ras2p. (B) Localization of GFP-Ras2p was determined by fluorescence microscopy in wild-type (YJB3896) and npr1Δ (YJB3897) strains and cells expressing extra copies of CAC3 (YJB3898).
In order to function correctly, Ras2p must localize to the plasma membrane. Mislocalization of Ras2p decreases its signaling activity and effectively suppresses the constitutively active RAS2G19V allele (2, 4). Thus, we asked if deletion of NPR1 or overexpression of CAC3 altered the membrane localization of Ras2p. When the RAS2 product is fused to GFP, the plasma membranes of yeast cells fluoresce, indicating that Ras2p is located at the membrane (4) (Fig. 6B). Deletion of NPR1 or overexpression of CAC3 did not alter the peripheral localization pattern of Ras2p-GFP (Fig. 6B), suggesting that suppression of the RAS2G19V allele by excess Cac3p is not due to an alteration in the subcellular distribution of Ras2p.
CAC3 overexpression and npr1Δ lead to indistinguishable phenotypes, leading us to propose a model in which Cac3p binds and sequesters Npr1p. Another possibility is that overexpression of CAC3 decreases the quantity of Npr1p, perhaps by targeting Npr1p for destruction. To test this possibility, we used a series of yeast strains containing an epitope-tagged NPR1 gene (36). The overexpression of CAC3 in either the presence or absence of the RAS2G19V allele had no effect on the amount of Npr1p present in the cell (data not shown). Thus, consistent with the sequestration model, excess copies of Cac3p did not destabilize Npr1p, yet still caused the same phenotypes as in an npr1Δ strain.
Ubiquitin overexpression can suppress the RAS/cAMP pathway.
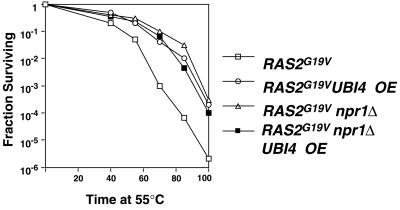
Npr1p is thought to stabilize Mep2p and other permeases by phosphorylating the protein, which then reduces the protein's ubiquitination and subsequent inactivation (26, 41). We hypothesized that, in a similar manner, Npr1p may stabilize another substrate protein that is important for the full function of the RAS/cAMP pathway by inhibiting its ubiquitination. Thus, deletion of NPR1 would be expected to increase the ubiquitination of putative substrate proteins. Furthermore, if this hypothesis is correct, overexpression of UBI4, the gene encoding polyubiquitin, would be expected to increase the ubiquitination of this substrate protein. Consistent with this expectation, overexpression of Myc epitope-tagged UBI4 from the copper-inducible CUP1 promoter on a high-copy plasmid suppressed RAS2G19V-induced heat shock sensitivity (Fig. 7). Additionally, overexpression of UBI4 in a RAS2G19V npr1Δ strain conferred no additional heat shock resistance, suggesting that these two genes suppress the RAS/cAMP signal transduction pathway by a common mechanism. Furthermore, overexpression of UBI4 suppressed the sporulation defect of RAS2G19V cells (Table 3). Thus, overexpression of polyubiquitin suppressed activated RAS2 phenotypes in a manner indistinguishable from either CAC3 overexpression or NPR1 deletion. This result is consistent with the hypothesis that NPR1 and ubiquitin have antagonistic roles in the suppression of the RAS/cAMP pathway.
FIG. 7.
Overexpression of polyubiquitin (UBI4) suppresses the RAS2G19V-induced heat shock sensitivity. The heat shock resistance of RAS2G19V (YJB2235), RAS2G19V UBI4-overexpressing (OE) (YJB27334), RAS2G19V npr1Δ (YJB3723), and RAS2G19V npr1 UBI4-overexpressing (YJB5522) strains in the presence and absence of NPR1 and UB14 overexpression were determined as for Fig. 1.
DISCUSSION
Cac3p has at least two separable functions.
Cac3p is the smallest subunit of CAF-I, which assemble histones H3 and H4 tetramers onto newly replicated DNA (24, 38). In addition, excess Cac3p suppresses the activated RAS/cAMP pathway (35) (Fig. 1) in a CAF-I independent manner (Fig. 2), a result consistent with a recent report (51). Because CAC3 acts antagonistically with the Sin3p-Rpd3p complex (42) and the mammalian Cac3p homolog (RbAp48) is associated with chromatin-modifying enzymes such as histone deacetylases and histone acetylases (43, 49), we asked if the CAC3 suppression of RAS2G19V required histone-modifying activities such as the histone deacetylases Rpd3p and Hda1p or the histone acetyltransferases Hat1p, Hat2p, and Gcn5p. Interestingly, we found that CAC3 suppression of RAS2G19V was not dependent upon any of the histone deacetylases or histone acetylases tested, nor was it dependent upon the histone regulator Hir3p or the silent chromatin component Sir3p (Table 2). Furthermore, several histone deacetylase complexes, including those containing Rpd3p and Hda1p, have been characterized in biochemical fractionation studies, and Cac3p has not been isolated as a component of these complexes (M. Grunstein, personal communication). Therefore, we conclude that the role of Cac3p in the RAS/cAMP signal transduction pathway is distinct and separable from the role of Cac3p in CAF-I-dependent chromatin assembly and histone modification. Consistent with this conclusion, CAC3 mRNA does not share the cell cycle-dependent expression pattern identified for CAC1 and CAC2 (39), not all Cac3p/p48 copurifies with the CAF-I complex (28, 32, 43), and the subcellular localization of Cac3p is distinct from that of Cac1p (Fig. 2C).
Cac3p suppression of the RAS/cAMP pathway is mediated by Npr1p.
A C-terminal fragment of Npr1p, which includes a portion of the kinase domain, interacted with Cac3p in a yeast two-hybrid screen and biochemically (Fig. 3). Consistent with the hypothesis that Cac3p overexpression reduces the effective activity of Npr1p, the suppression phenotypes of npr1Δ strains (e.g., heat shock resistance, sporulation, and loss of pseudohyphal growth) were indistinguishable from the phenotypes of strains overexpressing CAC3 (Fig. 4 and 5). Both mutations were capable of suppressing two different phenotypes caused by the RAS2G19V allele but not the same phenotypes resulting from deletion of the negative regulator BCY1. This demonstrates that overexpression of CAC3 and deletion of NPR1 suppress the pathway in an indistinguishable manner, suggesting a common suppression mechanism which is dependent on the RAS/cAMP pathway. Furthermore, overexpression of CAC3 in an npr1Δ strain did not enhance the suppression phenotype, supporting the idea that Cac3p affects the RAS/cAMP pathway by reducing the level of active, available Npr1p. Finally, cooverexpression of NPR1 blocked the ability of CAC3 overexpression to reduce the heat shock sensitivity of a RAS2G19V strain (Fig. 4C), supporting the model that excess Cac3p binds and sequesters Npr1p.
NPR1 encodes a nitrogen permease reactivator, a putative serine/threonine kinase that affects the activity of several nutrient transporters (13, 14), including Gap1p, the general amino acid permease (46); Mep2p, an ammonium permease (26); Pcp1p, a spermidine transporter (19); and Tat2p, the tryptophan transporter (36). NPI1/RSP5, encoding a ubiquitin-protein ligase, antagonizes the activity of NPR1 in many cases (14, 17). The currrent model postulates that phosphorylation of a transporter by Npr1p affects ubiquitination and subsequent proteolysis of that transporter, stabilizing nutrient-repressible permeases such as Gap1p (40, 41) but promoting degradation of constitutive permeases such as Tat2p (36). In its interaction with the RAS/cAMP pathway, NPR1 acts antagonistically with ubiquitin. We found that overexpression of polyubiquitin suppressed RAS2G19V-induced heat shock sensitivity in a manner that was indistinguishable from the suppression observed in npr1Δ strains (Fig. 7). The target of rapamycin (TOR) nutrient signaling pathway leads to the phosphorylation and subsequent inhibition of Npr1p (36). For more than 15 years, we have known that NPR1 is a key regulator of nitrogen metabolism (14) and that RAS/cAMP pathway is the principal regulator of carbon metabolism in Saccharomyces (5). This work is the first reported example of cross-talk between these two metabolic regulatory pathways. We have demonstrated that Npr1p affects the activity of the RAS/cAMP signal transduction pathway, providing a heretofore unrecognized connection between the carbon and nitrogen signaling pathways.
The precise mechanism by which Npr1p affects the RAS/cAMP pathway remains unknown. Neither the TOR1-1 or TOR2-1 mutation nor treatment with rapamycin had any effect on the ability of either CAC3 overexpression or NPR1 deletion to suppress the RAS2G19V phenotype (data not shown). This indicates that the TOR-dependent phosphorylation state of Npr1p does not affect the role of Npr1p in RAS/cAMP signaling. Since Npr1p affects the stability of a number of proteins and since overexpression of polyubiquitin yields the same phenotype as loss of Npr1p, we surmise that Npr1p activates or potentiates the RAS/cAMP pathway by stabilizing one or more intermediates in the pathway. Furthermore, the fact that overexpression of CAC3 or deletion of NPR1 suppresses RAS2G19V-induced phenotypes but not the same phenotypes resulting from deletion of BCY1 suggests that Npr1p acts between Ras-induced synthesis of cAMP and cAMP-mediated activation of the A kinase. Deletion of NPR1 does not affect the phosphorylation, ubiquitination, localization, or abundance of Ras2p (Fig. 6 and data not shown), nor does deletion of NPR1 affect the levels of Cdc25p, the guanine nucleotide exchanger for Ras (L. Schneper and J. R. Broach, unpublished observations).
Zhu and colleagues (51) recently reported that CAC3 overexpression suppressed the RAS/cAMP pathway when that pathway was activated by deletion of PDE1 and PDE2 or by an activated TPK2 mutation. However, CAC3 overexpression did not affect the total level of extractable PKA kinase activity. Their data indicated that CAC3 suppressed the RAS/cAMP pathway in a CAC1-independent and BCY1-dependent fashion which is not fully understood (51). The results presented here support and extend their conclusions by indicating that Npr1p is the target of Cac3p that modulates the RAS/cAMP signal transduction pathway.
ACKNOWLEDGMENTS
We thank C. Asleson, J. Beckerman, J. Whistler, and members of the Berman laboratory for helpful discussions. We thank C. Davis, S. Fields, D. Gottschling, M. Grunstein, M. Hall, J. Heitman, M. Hochstrasser, Y. Jiang, P. Kaufman, S. Liebman, C. McDonald, M. Parthun, J. Rine, M. Rose, and P. Siliciano for generously providing plasmids and strains and M. Grunstein for discussing results prior to publication.
S.D.J. was supported by a postdoctoral fellowship from the National Institute of General Medical Sciences, 1 F32 GM19065-01. This work was supported by National Institutes of Health grants CA41086 to J. Broach and GM38626 to J. Berman.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1989. [Google Scholar]
- 2.Bhattacharya S, Chen L, Broach J R, Powers S. Ras membrane targeting is essential for glucose signalling but not for viability in yeast. Proc Natl Acad Sci USA. 1995;92:2984–2988. doi: 10.1073/pnas.92.7.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bos J L. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 4.Boyartchuk V L, Ashby M N, Rine J. Modulation of Ras and a-factor function by carboxyl-terminal proteolysis. Science. 1997;275:1796–1800. doi: 10.1126/science.275.5307.1796. [DOI] [PubMed] [Google Scholar]
- 5.Broach J R, Deschenes R J. The function of RAS genes in Saccharomyces cerevisiae. Adv Cancer Res. 1990;54:79–139. doi: 10.1016/s0065-230x(08)60809-x. [DOI] [PubMed] [Google Scholar]
- 6.Chien C-T, Bartel P L, Sternglanz R, Fields S. The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc Natl Acad Sci USA. 1991;88:9578–9582. doi: 10.1073/pnas.88.21.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cross F R. ‘Marker swap’ plasmids: convenient tools for budding yeast molecular genetics. Yeast. 1997;13:647–653. doi: 10.1002/(SICI)1097-0061(19970615)13:7<647::AID-YEA115>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 8.DeFeo-Jones D, Tatchell K, Robinson L C, Sigal I S, Vass W C, Lowy D R, Scolnick E M. Mammalian and yeast ras gene products: biological function in their heterologous systems. Science. 1985;228:179–184. doi: 10.1126/science.3883495. [DOI] [PubMed] [Google Scholar]
- 9.Ellison M J, Hochstrasser M. Epitope-tagged ubiquitin: a new probe for analyzing ubiquitin function. J Biol Chem. 1991;266:21150–21157. [PubMed] [Google Scholar]
- 10.Enomoto S, Chen G, Berman J. Vectors for expressing T7 epitope-and His6 affinity-tagged fusion proteins in S. cerevisiae. BioTechniques. 1998;24:782–788. doi: 10.2144/98245st01. [DOI] [PubMed] [Google Scholar]
- 11.Enomoto S, McCune-Zierath P D, Gerami-Nejad M, Sanders M A, Berman J. RLF2, a subunit of yeast chromatin assembly factor-I, is required for telomeric chromatin function in vivo. Genes Dev. 1997;11:358–370. doi: 10.1101/gad.11.3.358. [DOI] [PubMed] [Google Scholar]
- 12.Gottschling D E, Aparicio O M, Billington B L, Zakian V A. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell. 1990;63:751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- 13.Grenson M. Inactivation-reactivation process and repression of permease formation regulate several ammonia-sensitive permeases in the yeast Saccharomyces cerevisiae. Eur J Biochem. 1983;133:135–139. doi: 10.1111/j.1432-1033.1983.tb07438.x. [DOI] [PubMed] [Google Scholar]
- 14.Grenson M. Study of the positive control of the general amino-acid permease and other ammonia-sensitive uptake systems by the product of the NPR1 gene in the yeast Saccharomyces cerevisiae. Eur J Biochem. 1983;133:141–144. doi: 10.1111/j.1432-1033.1983.tb07439.x. [DOI] [PubMed] [Google Scholar]
- 15.Guthrie C, Fink G R. Guide to yeast genetics and molecular biology. San Diego, Calif: Academic Press; 1991. [Google Scholar]
- 16.Haney S A, Broach J R. Cdc25p, the guanine nucleotide exchange factor for the Ras proteins of Saccharomyces cerevisiae, promotes exchange by stabilizing Ras in a nucleotide-free state. J Biol Chem. 1994;269:16541–16548. [PubMed] [Google Scholar]
- 17.Hein C, Springael J Y, Volland C, Haguenauer-Tsapis R, Andre B. NPI1, an essential yeast gene involved in induced degradation of Gap1 and Fur4 permeases, encodes the Rsp5 ubiquitin-protein ligase. Mol Microbiol. 1995;18:77–87. doi: 10.1111/j.1365-2958.1995.mmi_18010077.x. [DOI] [PubMed] [Google Scholar]
- 18.Hubbard E J A, Yang X, Carlson M. Relationship of the cAMP-dependent protein kinase pathway to the SNF1 protein kinase and invertase expression in Saccharomyces cerevisiae. Genetics. 1992;130:71–80. doi: 10.1093/genetics/130.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaouass M, Gamache I, Ramotar D, Audette M, Poulin R. The spermidine transport system is regulated by ligand inactivation, endocytosis, and by the Nrp1p Ser/Thr protein kinase in Saccharomyces cerevisiae. J Biol Chem. 1998;273:2109–2117. doi: 10.1074/jbc.273.4.2109. [DOI] [PubMed] [Google Scholar]
- 20.Kataoka T, Powers S, Camerson S, Fasano O, Goldfarb M, Broach J, Wigler M. Functional homology of mammalian and yeast RAS genes. Cell. 1985;40:19–26. doi: 10.1016/0092-8674(85)90304-6. [DOI] [PubMed] [Google Scholar]
- 21.Kataoka T, Powers S, McGill C, Fasano O, Strathern J, Broach J, Wigler M. Genetic analysis of yeast RAS1 and RAS2 genes. Cell. 1984;37:437–445. doi: 10.1016/0092-8674(84)90374-x. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman P D, Cohen J L, Osley M A. Hir proteins are required for position-dependent gene silencing in Saccharomyces cerevisiae in the absence of chromatin assembly factor I. Mol Cell Biol. 1998;18:4793–4806. doi: 10.1128/mcb.18.8.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufman P D, Kobayashi R, Kessler N, Stillman B. The p150 and p60 subunits of chromatin assembly factor I: a molecular link between newly synthesized histones and DNA replication. Cell. 1995;81:1105–1114. doi: 10.1016/s0092-8674(05)80015-7. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman P D, Kobayashi R, Stillman B. Ultraviolet radiation sensitivity and reduction of telomeric silencing in Saccharomyces cerevisiae cells lacking chromatin assembly factor-I. Genes Dev. 1997;11:345–357. doi: 10.1101/gad.11.3.345. [DOI] [PubMed] [Google Scholar]
- 24a.Liu H, Styles C A, Fink G R. Saccharomyces cerevisiae S288C has a mutation in FL08, a gene required for filamentous growth. Genetics. 1996;144:967–978. doi: 10.1093/genetics/144.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longtine M S, McKenzie I A, Demarini D J, Shah N G, Wach A, Brachat A, Philippsen P, Pringle J R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 26.Lorenz M C, Heitman J. The MEP2 ammonium permease regulates pseudohyphal differentiation in Saccharomyces cerevisiae. EMBO J. 1998;17:1236–1247. doi: 10.1093/emboj/17.5.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorenz M C, Pan X, Harashima T, Cardenas M E, Xue Y, Hirsch J P, Heitman J. The G protein-coupled receptor Gpr1 is a nutrient sensor that regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Genetics. 2000;154:609–622. doi: 10.1093/genetics/154.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marheineke K, Krude T. Nucleosome assembly activity and intracellular localization of human CAF-1 changes during the cell division cycle. J Biol Chem. 1998;273:15279–15286. doi: 10.1074/jbc.273.24.15279. [DOI] [PubMed] [Google Scholar]
- 29.McCormick F. ras GTPase activating protein: signal transmitter and signal terminator. Cell. 1989;56:5–8. doi: 10.1016/0092-8674(89)90976-8. [DOI] [PubMed] [Google Scholar]
- 30.McGrath J P, Capon D J, Goeddel D V, Levinson A D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984;310:644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- 31.Parthun M R, Widom J, Gottschling D E. The major cytoplasmic histone acetyltransferase in yeast: links to chromatin replication and histone metabolism. Cell. 1996;87:85–94. doi: 10.1016/s0092-8674(00)81325-2. [DOI] [PubMed] [Google Scholar]
- 32.Qian Y-W, Wang Y-C J, Hollingsworth R E, Jones D, Ling N, Lee E Y-H P. A retinoblastoma-binding protein related to a negative regulator of Ras in yeast. Nature. 1993;364:648–652. doi: 10.1038/364648a0. [DOI] [PubMed] [Google Scholar]
- 33.Qian Z, Huang H, Hong J Y, Burck C L, Johnston S D, Berman J, Carol A, Liebman S. Yeast Ty1 retrotransposition is stimulated by a synergistic interaction between mutations in chromatin assembly factor I and histone regulatory proteins. Mol Cell Biol. 1998;18:4783–4792. doi: 10.1128/mcb.18.8.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roth S Y, Allis C D. Histone acetylation and chromatin assembly: a single escort, multiple dances? Cell. 1996;87:5–8. doi: 10.1016/s0092-8674(00)81316-1. [DOI] [PubMed] [Google Scholar]
- 35.Ruggieri R, Tanaka K, Nakafuku M, Kaziro Y, Tho-e A, Matsumoto K. MSI1, a negative regulator of the RAS-cAMP pathway in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1989;86:8778–8782. doi: 10.1073/pnas.86.22.8778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35a.Rundlett S E, Carmen A A, Kobayashi R, Bavykin S, Turner B M, Grunstein M. HDA1 and RPD3 are members of distinct yeast histone deacetylase complexes that regulate silencing and transcription. Proc Natl Acad Sci USA. 1996;93:14503–14508. doi: 10.1073/pnas.93.25.14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt A, Beck T, Koller A, Kunz J, Hall M N. The TOR nutrient signalling pathway phosphorylates NPR1 and inhibits turnover of the tryptophan permease. EMBO J. 1998;17:6924–6931. doi: 10.1093/emboj/17.23.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin D-Y, Matsumoto D, Iida H, Uno I, Ishikawa T. Heat shock response in Saccharomyces cerevisiae mutants altered in cyclic AMP-dependent protein phosphorylation. Mol Cell Biol. 1987;7:244–250. doi: 10.1128/mcb.7.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith S, Stillman B. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during replication. Cell. 1989;58:15–25. doi: 10.1016/0092-8674(89)90398-x. [DOI] [PubMed] [Google Scholar]
- 39.Spellman P T, Sherlock G, Zhang M Q, Iyer V R, Anders K, Eisen M B, Brown P O, Botstein D, Futcher B. Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol Biol Cell. 1998;9:3273–3297. doi: 10.1091/mbc.9.12.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Springael J Y, Andre B. Nitrogen-regulated ubiquitination of the Gap1 permease of Saccharomyces cerevisiae. Mol Biol Cell. 1998;9:1253–1263. doi: 10.1091/mbc.9.6.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanbrough M, Magasanik B. Transcriptional and posttranslational regulation of the general amino acid permease of Saccharomyces cerevisiae. J Bacteriol. 1995;177:94–102. doi: 10.1128/jb.177.1.94-102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Z W, Hampsey M. A general requirement for the Sin3-Rpd3 histone deacetylase complex in regulating silencing in Saccharomyces cerevisiae. Genetics. 1999;152:921–932. doi: 10.1093/genetics/152.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taunton J, Hassig C A, Schreiber S L. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science. 1996;272:408–411. doi: 10.1126/science.272.5260.408. [DOI] [PubMed] [Google Scholar]
- 44.Thevelein J M, de Winde J H. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol Microbiol. 1999;33:904–918. doi: 10.1046/j.1365-2958.1999.01538.x. [DOI] [PubMed] [Google Scholar]
- 45.Toda T, Cameron S, Sass P, Zoller M, Scott J D, McMullen B, Hurwitz M, Krebs E G, Wigler M. Cloning and characterization of BCY1, a locus encoding a regulatory subunit of the cyclic AMP-dependent protein kinase in Saccharomyces cerevisiae. Mol Cell Biol. 1987;7:1371–1377. doi: 10.1128/mcb.7.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vandenbol M, Jauniqux J-C, Grenson M. The Saccharomyces cerevisiae NPR1 gene required for the activity of ammonia-sensitive amino acid permeases encodes a protein kinase homolog. Mol Gen Genet. 1990;222:393–399. doi: 10.1007/BF00633845. [DOI] [PubMed] [Google Scholar]
- 47.Varela J C S, Praekelt U M, Meacock P A, Planta R J, Mager W H. The Saccharomyces cerevisiae HSP12 gene is activated by the high-osmolarity glycerol pathway and negatively regulated by protein kinase A. Mol Cell Biol. 1995;15:6232–6245. doi: 10.1128/mcb.15.11.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verreault A, Kaufman P D, Kobayashi R, Stillman B. Nucleosome assembly by a complex of CAF-1 and acetylated histones H3/H4. Cell. 1996;87:95–104. doi: 10.1016/s0092-8674(00)81326-4. [DOI] [PubMed] [Google Scholar]
- 49.Wade P A, Jones P L, Vermaak D, Wolffe A P. A multiple subunit Mi-2 histone deacetylase from Xenopus laevis cofractionates with an associated Snf2 superfamily ATPase. Curr Biol. 1998;8:843–846. doi: 10.1016/s0960-9822(98)70328-8. [DOI] [PubMed] [Google Scholar]
- 50.Whistler J L, Rine J. Ras2 and Ras1 protein phosphorylation in Saccharomyces cerevisiae. J Biol Chem. 1997;272:18790–18800. doi: 10.1074/jbc.272.30.18790. [DOI] [PubMed] [Google Scholar]
- 50a.Xue Y, Battle M, Hirsch J P. GPR1 encodes a putative G protein-coupled receptor that associates with the Gpa2p Gα subunit and functions in a Ras-dependent pathway. EMBO J. 1998;17:1996–2007. doi: 10.1093/emboj/17.7.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu X, Démolis N, Jacquet M, Michaeli T. MSI1 suppresses hyperactive RAS via the cAMP-dependent protein kinase and independently of chromatin assembly factor-1. Curr Genet. 2000;38:60–70. doi: 10.1007/s002940000133. [DOI] [PubMed] [Google Scholar]