Abstract
Stenotrophomonas maltophilia is a multidrug resistant pathogen associated with high mortality and morbidity in patients having compromised immunity. The efflux systems of S. maltophilia include SmeABC and SmeDEF proteins, which assist in acquisition of multiple-drug-resistance. In this study, proteome based mapping was utilized to find out the potential drug targets for S. maltophilia strain k279a. Various tools of computational biology were applied to remove the human-specific homologous and pathogen-specific paralogous sequences from the bacterial proteome. The CD-HIT analysis selected 4315 proteins from total proteome count of 4365 proteins. Geptop identified 407 essential proteins, while the BlastP revealed approximately 85 non-homologous proteins in the human genome. Moreover, metabolic pathway and subcellular location analysis were performed for essential bacterial genes, to describe their role in various cellular processes. Only two essential proteins (Acyl-[acyl-carrier-protein]—UDP-N acetyl glucosamine O-acyltransferase and D-alanine-D-alanine ligase) as candidate for potent targets were found in proteome of the pathogen, in order to design new drugs. An online tool, Swiss model was employed to model the 3D structures of both target proteins. A library of 5000 phytochemicals was docked against those proteins through the molecular operating environment (MOE). That resulted in to eight inhibitors for both proteins i.e. enterodiol, aloin, ononin and rhinacanthinF for the Acyl-[acyl-carrier-protein]—UDP-N acetyl glucosamine O-acyltransferase, and rhazin, alkannin beta, aloesin and ancistrocladine for the D-alanine-D-alanine ligase. Finally the ADMET was done through ADMETsar. This study supported the development of natural as well as cost-effective drugs against S. maltophilia. These inhibitors displayed the effective binding interactions and safe drug profiles. However, further in vivo and in vitro validation experiment might be performed to check their drug effectiveness, biocompatibility and their role as effective inhibitors.
Introduction
Stenotrophomonas maltophilia is an intensive emergent gram-negative bacterium of the human-ecological origin worldwide. That characteristically impart resistance to different classes of antibiotics and hefty metals [1, 2]. It is responsible for wide scope infections in clinics and local area settings including infection of respiratory tract and septicemia which are pervasive in nature. Whereas infections of bone, joints, urinary tract as well as meningitis are less successive [3].
Owing to adaptive behavior of intrinsic resistance of S. maltophilia through horizontal gene transfer and mutation against anti-microbial utilized beforehand, the medicinal approach is getting popular nowadays [4]. The resistance emerges fundamentally by modifying the medication targets, bypassing molecules, efflux pumps, substance alteration, self-prescription, mutations or by phenotypic variation arising internally or externally by the host [5]. The efflux systems of S. maltophilia include SmeABC and SmeDEF proteins, which assist in acquisition of multiple-drug-resistance [4]. Furthermore, that offer an extreme tendency of fighting against drugs by extraordinary liability outcomes such as trimethoprim/sulfamethoxazole (TMP/SMX), fluoroquinolones and ceftazidime [6, 7]. Consequently it is of imperative significance to identify novel and potent therapeutic targets in S. maltophilia to cope with this multidrug-resistant pathogen successfully.
The enormous progress in computational biology and diversified applications of bioinformatics have gained importance in drug designing thereby reducing the cost and time needed for in vivo screening and testing [8, 9]. The bioinformatics has substantially shortened traditional lab trials through employment of approaches including identification of drug candidates, structure-based designing of drug molecule, screening of antiviral drugs, comparative investigations utilizing genome to recognize host specific targets etc. [10, 11]. Currently the subtractive genomic approach is being focused in order to examine the entire host and proteome of bacterium. This is to recognize the proteins with various therapeutic perspectives solely present in the pathogenic genome, by excluding the homologous proteins of the host [12]. Numerous investigations have already utilized this approach on the multiple pathogenic strains and detailed fruitful identification and acknowledgment of novel species-specific therapeutic targets [13, 14].
The current study involves applying subtractive proteomics approach on the whole proteome of S. maltophilia. Briefly, the proteins which are fundamental to pathogenic survival were prioritized via computational tools and databases. It was followed by eliminating host homology proteins. Merely pathogenic proteins were retained to minimize the accidental therapeutic blockage by the host and involved in the metabolism of host. These proteins were further subjected to prediction of their subcellular localization for recognizing membrane protein followed by the drug-ability analysis. That led to the identification of two virtually hit compounds including Acyl-[acyl-carrier-protein]—UDP-N acetyl glucosamine O-acyltransferase and D-alanine-D-alanine ligase as therapeutic targets in S. maltophilia. These proteins were then docked with phytochemicals, enterodiol, aloin, ononin and rhinacanthinF. That revealed sound molecular interaction and high docking score as well as binding affinity. The potent compounds were also evaluated for drug-likeness and toxicity assessment that may serve as the target for the further optimization of the compounds through experimental study.
Methods
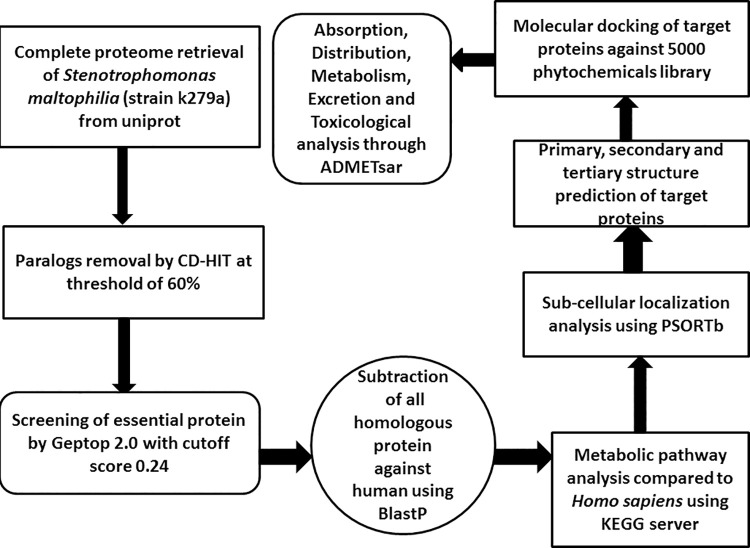
The subtractive genomic approach was employed for analysis of the whole proteome of S. maltophilia (strain k279a) screen immunogenic proteins that may serve as novel drug targets. The overall flowchart of the study is shown (Fig 1).
Fig 1. Overall flow chart of subtractive genomic against S. maltophilia.
This shows analysis of whole proteome of S. maltophilia (strain k279a).
The whole proteome retrieval
First of all the whole proteome of S. maltophilia (strain k279a) was retrieved from Uniprot in the FASTA format.
Identification of paralogous sequences
The whole proteome of S. maltophilia (strain k279a) was subjected to CD-HIT suite. The parameters were set to default except for threshold value kept to 60%. CD-HIT suite is widely employed for comparing and clustering protein and genomic sequences. That is to remove paralogs or redundant proteins [15].
Identification of essential proteins
The Geptop 2.0 server was used to retrieve essential proteins of S. maltophilia. That server is used for the detection of essential genes taking into account comparison of the phylogeny and orthology of provided query protein with datasets of essential genes.
Identification of essential non-homologous proteins
The essential proteins were submitted to Blastpagainst host proteome with a threshold of e-value 10−4, with the query coverage and identity of more than 70% and 30%, respectively. The purpose was to identify those proteins which are non-homologous to the host.
Analysis of metabolic pathways
The essential proteins of S. maltophilia were analyzed through KEGG automatic annotation server. The pathways unique to S. maltophilia (strain k279a) and absent in humans were selected [19] at KEGG [20].
Subcellular localization analysis
The target proteins were subjected to the identification of subcellular localization of metabolic proteins of S. maltophilia by the PSORTb tool to enable identification of these predicted therapeutic targets.
Selection of membrane proteins by drug-ability
In order to screen for the uniqueness of putative targets, Drug-Bank 5.1.0 database set to default limitations was used. Consequently, the proteins with significant hit higher to threshold with pre-treated drug targets displayed common functions. These were proceeded further for drug-able testing.
Primary and secondary structure analysis of target proteins
The evaluations of primary structure of selected proteins were done through EXPASY. It was followed by prediction of secondary structure through PSIPRED. That generated outcome based on feed-forward neural networks [16].
Furthermore, SignalP-5.0 server was used for the prediction of location for signal peptide and their protein cleavage sites. Subsequently these targets were tested for transmembrane topology via TMHMM tool. Which relies on Hidden Markov model for the prediction and so on predicts transmembrane helices and precisely distinguishes soluble from membrane proteins [17].
Structure prediction and validation
An online tool, Swiss-Model was employed to predict the 3D structure of putative proteins. That tool identifies the template, aligns it with the target sequence, constructs and evaluates quality of the 3D model [18]. The Chimera Structure Visualization software [19] was used to visualize and Galaxy WEB server was used to refine the models. The quality of model was evaluated using SAVES server which analyzes them on the basis of ERRAT [20], WHATCHECK [21] and PROCHECK [22].
Compounds library preparation and molecular docking
For docking, the 2D conformation of compounds were downloaded from the PubChem [23] followed by protonation and energy minimization in MOE software and further added to the database. These compounds were then docked with putative proteins via the MOE software [24].
Physiochemical property profiling and toxicity predictions
Molinspiration server was used to analyze the molecular descriptors and drug likeliness properties of compounds. In fact that gives a prediction based ‘rule of five’ (Ro5) [25]. AdmetSAR database was used to indicate the pharmacokinetic properties such as ADMET toxicity of the compounds [26]. ProTox-II webserver used for selected molecules were subjected to various toxicity screening endpoints models. That is a web server designed to predict the toxicity of various toxicological endpoints for different chemical compounds [27].
Results
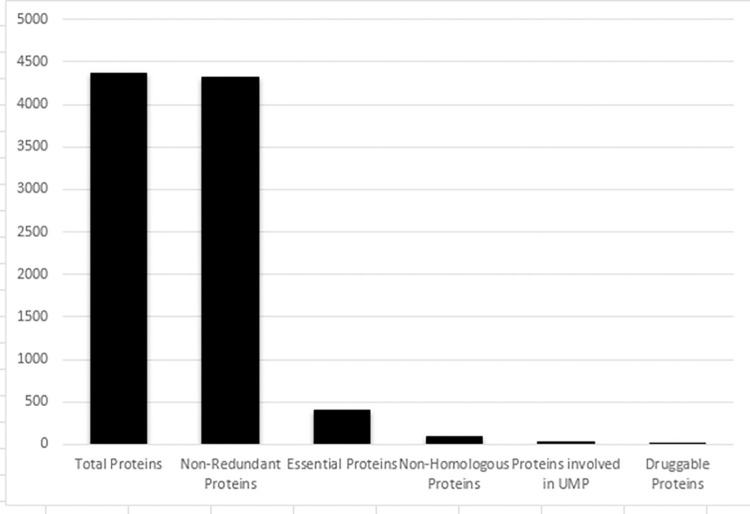
This study was done to recognize the novel drug targets in S. maltophilia. In this study, the subtractive genomic approach is employed for seeking therapeutic target proteins which are indispensable for the bacterial survival but absent in the host. The insight of that approach is shown (Fig 2).
Fig 2. Summary for the detection of novel drug targets in S. maltophilia.
This shows protein counts of selected paralogous sequences, essential proteins, non-homologous proteins and drug target proteins.
Selection of paralogous sequences
The proteome of S. maltophilia strain was based on 4365 proteins which were subsequently subjected to the CD-HIT server that facilitated selection of paralogous sequences. It was followed by excluding paralogous sequences showing similarity more than 60% thereby retaining back to 4315 non paralogous proteins.
Selection of essential proteins
The screening of 4315 non-paralogous proteins using Geptop 2.0 server resulted in 407 essential proteins. Actually designing of antibacterial compounds relies on docking and hinders with essential proteins [28].
Selection of non-homologous proteins
The cellular proteins of humans evolved to be homologous with bacteria [29] that necessitate the therapeutics to be non-homologous to humans to avoid cross-reactivity. Out of 407 proteins subjected to BlastP, 85 revealed as non-homologous.
Metabolic pathway analysis
The analysis of those 85 non homologous proteins appeared to be involved in 33 pathways explored with KEGG. Among these 33 pathways, 13 were predicted to be particular for the S. maltophilia and remaining to be common for the S. maltophilia as well as host. Briefly, 27 essential proteins are revealed to participate in 13 pathways (Table 1). Among these 27 proteins which were analyzed in KEGG database, 24 proteins were found to participate in common metabolic pathways and rest of three namely chromosomal replication initiator protein DnaA, D-alanine-D-alanine ligase and Acyl-[acyl-carrier-protein]—UDP-N acetyl glucosamine O-acyltransferase were observed to participate in the pathogen specific pathways. Hence, continued further for analysis.
Table 1. Essential non-homologous proteins involved in 27 unique metabolic pathways.
| SrNo. | Protein Name (ID) | Unique Pathway | Common pathway |
|---|---|---|---|
| 1 | Bifunctional protein (B2FHY5) | Sml00541-O-Antigen nucleotide sugar biosynthesis | Sml00520-Amino sugar and nucleotide sugar metabolism |
| Sml01100-Metabolic pathways | |||
| 2 | Ubiquinone/menaquinone biosynthesis C-methyl transferase (B2FUU6) | sml01110-Biosynthesis of secondary metabolites | Sml01240 -Biosynthesis of cofactors |
| Sml00130-Ubiquinone and other terpenoid-quinone biosynthesis | |||
| sml01100 -Metabolic pathways | |||
| 3 | UDP-N-acetyl glucosamine 1-carboxyvinyltransferase (B2FRX1) | Sml00550-Peptidoglycan biosynthesis | sml01100-Metabolic pathways |
| Sml00520-Amino sugar and nucleotide sugar metabolism | |||
| 4 | D-alanine—D-alanine ligase (B2FNN9) | Sml01502-Vancomycin resistance | |
| Sml00550-Peptidoglycan biosynthesis | |||
| Sml00473-D-Alanine metabolism | |||
| 5 | Phosphatidate cytidylyltransferase (B2FIA3) | sml01110-Biosynthesis of secondary metabolites | sml01100-Metabolic pathways |
| sml00564-Glycerophospholipid metabolism | |||
| 6 | B2FNL3 | sml01110-Biosynthesis of secondary metabolites | sml01100-Metabolic pathways |
| sml01240- Biosynthesis of cofactors | |||
| sml00740-Riboflavin metabolism | |||
| 7 | UDP-N-acetylmuramate—L-alanine ligase (B2FNN8) | Sml00550-Peptidoglycan biosynthesis | Sml00471-D-Glutamine and D-glutamate metabolism |
| sml01100-Metabolic pathways | |||
| 8 | UDP-2,3-diacylglucosamine hydrolase (B2FQP4) | Sml00540- Lipopolysaccharide biosynthesis | sml01100-Metabolic pathways |
| 9 | Acyl-[acyl-carrier-protein]—UDP-N acetyl glucosamine O-acyltransferase (B2FHN6) | Sml01503- Cationic antimicrobial peptide (CAMP) resistance. | |
| Sml00540- Lipopolysaccharide biosynthesis | |||
| 10 | 2-Dehydro-3-deoxyphosphooctonate aldolase (B2FK85) | Sml00540- Lipopolysaccharide biosynthesis | sml01100-Metabolic pathways |
| 11 | Histidine biosynthesis bifunctional protein (B2FPM1) | sml01110-Biosynthesis of secondary metabolites | sml01100-Metabolic pathways |
| sml01230-Biosynthesis of amino acids | |||
| sml00340-Histidine metabolism | |||
| 12 | Shikimate kinase (B2FQI7) | sml01110-Biosynthesis of secondary metabolites | sml01100-Metabolic pathways |
| sml01230-Biosynthesis of amino acids | |||
| sml00400-Phenylalanine, tyrosine and tryptophan biosynthesis | |||
| 13 | Acetyl-coenzyme A carboxylase (B2FHN1) | sml01110-Biosynthesis of secondary metabolites | Sml00061-Fatty acid biosynthesis |
| sml01120-Microbial metabolism in diverse environments | sml01100-Metabolic pathways | ||
| sml01200- Carbon metabolism | |||
| sml01212-Fatty acid metabolism | |||
| sml00640- Propanoate metabolism | |||
| sml00620- Pyruvate metabolism | |||
| 14 | Glutamyl-tRNA reductase (B2FQ15) | sml01110-Biosynthesis of secondary metabolites | sml01100-Metabolic pathways |
| sml01120-Microbial metabolism in diverse environments | Sml01240 -Biosynthesis of cofactors | ||
| Sml00860- Porphyrin and chlorophyll metabolism | |||
| 15 | 3-Methyl-2-oxobutanoate hydroxymethyltransferase (B2FL67) | sml01110-Biosynthesis of secondary metabolites | Sml00770- Pantothenate and CoA biosynthesis |
| sml01100-Metabolic pathways | |||
| Sml01240 -Biosynthesis of cofactors | |||
| 16 | 4-Hydroxy-3-methylbut-2-enyl diphosphate reductase (B2FU83) | sml01110-Biosynthesis of secondary metabolites | Sml00900- Terpenoid backbone biosynthesis |
| sml01100-Metabolic pathways | |||
| 17 | Chorismate synthase (B2FP01) | sml01110-Biosynthesis of secondary metabolites | sml01100-Metabolic pathways |
| sml01230-Biosynthesis of amino acids | |||
| sml00400-Phenylalanine, tyrosine and tryptophan biosynthesis | |||
| 18 | Chromosomal replication initiator protein DnaA (B2FUW1) | Sml02020- Two-component system | |
| 19 | 3-deoxy-manno-octulosonate cytidylyltransferase (B2FK23) | Sml00540- Lipopolysaccharide biosynthesis | sml01100-Metabolic pathways |
| 20 | Tetraacyldisaccharide 4’-kinase (B2FK22) | Sml00540- Lipopolysaccharide biosynthesis | sml01100-Metabolic pathways |
| 21 | Oxygen-dependent coproporphyrinogen-III oxidase (B2FND0) | sml01110-Biosynthesis of secondary metabolites | sml01100-Metabolic pathways |
| sml00860-Porphyrin and chlorophyll metabolism | |||
| Sml01240 -Biosynthesis of cofactors | |||
| 22 | Pantothenate synthetase (B2FL68) | sml01110-Biosynthesis of secondary metabolites | sml01100-Metabolic pathways |
| sml00770-Pantothenate and CoA biosynthesis | |||
| Sml01240 -Biosynthesis of cofactors | |||
| Sml00410- beta-Alanine metabolism | |||
| 23 | Protein translocase subunit SecA (B2FPB2) | Sml02024- Quorum sensing | Sml03060- Protein export |
| Sml03070- Bacterial secretion system | |||
| 24 | Acetyl-coenzyme A carboxylase carboxyl transferase subunit beta (B2FNY8) | sml01110-Biosynthesis of secondary metabolites | sml01100-Metabolic pathways |
| sml01120-Microbial metabolism in diverse environments | sml01212-Fatty acid metabolism | ||
| sml00640- Propanoate metabolism | |||
| sml00620- Pyruvate metabolism | |||
| Sml00061-Fatty acid biosynthesis | |||
| sml01200- Carbon metabolism | |||
| 25 | Succinyl-diaminopimelate desuccinylase (B2FIC0) | Sml00300- Lysine biosynthesis | sml01100-Metabolic pathways |
| sml01120-Microbial metabolism in diverse environments | sml01230-Biosynthesis of amino acids | ||
| 26 | 4-hydroxy-tetrahydrodipicolinate reductase (B2FQ70) | Sml00300- Lysine biosynthesis | sml01100-Metabolic pathways |
| sml01120-Microbial metabolism in diverse environments | sml01230-Biosynthesis of amino acids | ||
| sml01110-Biosynthesis of secondary metabolites | |||
| sml00261- Monobactam biosynthesis | |||
| 27 | Glycerol-3-phosphate dehydrogenase [NAD(P)+] (B2FHD8) | sml01110-Biosynthesis of secondary metabolites | Sml00564-Glycerophospholipid metabolism |
Subcellular localization prediction
The prediction of subcellular location is a quick way to obtain protein as it facilitates the steps required to purify in the experimental setup. That is done by determining its location i.e. whether cytoplasmic or membranous. The prediction retrieved via PSORTb revealed those proteins to be cytoplasmic in nature (Table 2).
Table 2. Sub cellular localization prediction of proteins involved in unique metabolic pathways.
Selection of drug-able proteins
Those 3 putative proteins were subjected to the Drug Bank. Two of them were found to be significantly similar to drug entries of the database, either to FDA approved or experimental drugs. These might act as potential novel drug targets (Table 3).
Table 3. Drug-able target proteins analysed via DrugBank.
| Sr. No. | Accession No. | Protein Name | DrugBank ID | Drug Name | Category | Organism |
|---|---|---|---|---|---|---|
| 1 | B2FNN9 | D-alanine—D-alanine ligase | DB07805 | 3-CHLORO-2,2-DIMETHYL-N-[4 (TRIFLUOROMETHYL)PHENYL]PROPANAMIDE | Experimental Approved | Staphylococcus aureus(strainCOL) |
| DB00260 | ||||||
| Escherichia coli (strain K12) | ||||||
| Cycloserine | ||||||
| 2 | B2FHN6 | Acyl-[acyl-carrier-protein]—UDP-N acetyl glucosamine O-acyltransferase | DB01694 | D-tartaric acid | Experimental | Helicobacter-pylori (strain ATCC 700392 /26695) |
| DB08558 | 2-HYDROXYMETHYL-6-OCTYLSULFANYL-TETRAHYDRO-PYRAN-3,4,5-TRIOL | Experimental | ||||
| Helicobacter pylori (strain ATCC 700392 / 26695) |
Structural analysis of target protein
The analysis of primary structure revealed that the D-alanine—D-alanine ligase protein and Acyl-[acyl-carrier-protein]—UDP-N acetyl glucosamine O-acyltransferase harbor the molecular mass of 21.07 kDaand 28.1 kDa, respectively. Moreover, their isoelectric points were 4.87 and 6.47, grand averages of hydropathic (GRAVY) were 0.004 and -0.100, terminating amino acid at the N-terminus of protein was lysine and methionine and the instability indices were 30.98 and 18.93, respectively. As these proteins carried isoelectric points below 7 so that is an indication of positively charged proteins. Moreover, the GRAVY computed values explained them as hydrophilic and unstable.
Further, the secondary structure of Acyl-[acyl-carrier-protein]—UDP-N acetyl glucosamine O-acyltransferase and D-alanine-D-alanine ligase protein showed that they have 27.38% and 32.19% of alpha helices, 24.33% and 18.44% extended, and 48.29% and 49.38% of random coils, respectively. Both proteins displayed no beta turns.
The signal peptide probability values of Acyl-[acyl-carrier-protein]—UDP-N acetyl glucosamine O-acyltransferase and D-alanine-D-alanine ligase were obtained by SignalP. These values were 0.012 and 0.021, respectively. That was indicating the absence of signal peptide in these protein targets. The TMHMM showed absence of transmembrane helices in both putative proteins.
The 3D structure prediction and validation
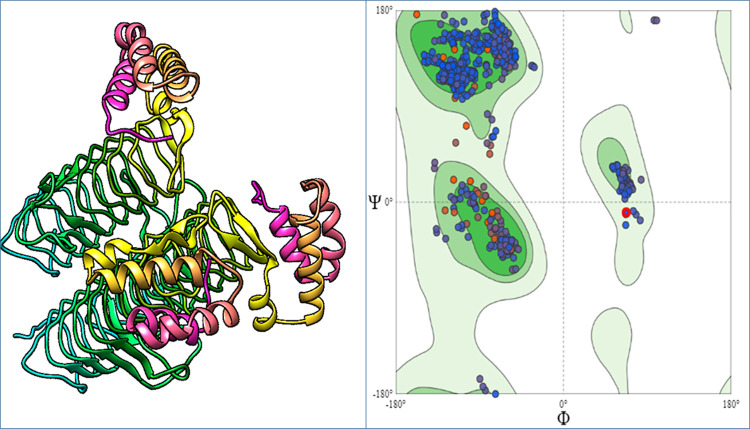
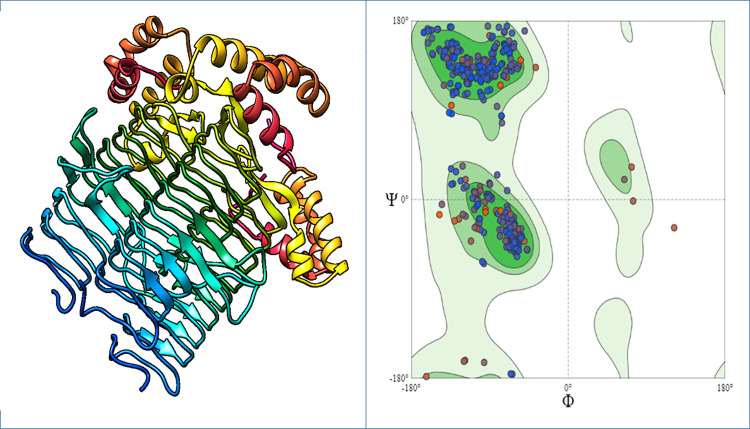
The structures of both proteins Acyl-[acyl-carrier-protein]—UDP-N acetyl glucosamine O-acyltransferase and D-alanine-D-alanine ligase were predicted by the Swiss-Model with confidence level of 100% and coverages were 92% and 98%, respectively. The SAVES analyzed those models by visualizing through Verify 3D, WHATCHECK, Prove, PROCHECK and ERRAT. The RAMPAGE server generated the Ramachandran plot for Acyl-[acyl-carrier-protein]—UDP-N acetyl glucosamine O-acyltransferase. This displayed that 96.9% of residues were found in the most favored regions, 0.3% of residues in additional allowed regions, 2.8% of residues in generously allowed region and none in disallowed region. According to the Ramachandran plot generated for D-alanine-D-alanine ligase, 85.8% of residues were found in the most favored region, while 13.7% and 13.2% of amino acids resided in additional allowed regions, and 0.8% and 1.0% of residues are found in generously allowed region and none in disallowed region (Figs 3 & 4).
Fig 3. Structure of the D-alanine-D-alanine ligase protein.
(A) Three dimensional structure of D-alanine-D-alanine ligase protein. (B) Ramachandran Plot of D-alanine-D-alanine ligase protein.
Fig 4. Structure of the Acyl-[acyl-carrier-protein]—UDP-N acetyl glucosamine O-acyltransferase protein.
(A) Three dimensional structure of Acyl-[acyl-carrier-protein]—UDP-N acetyl glucosamine O-acyltransferase protein. (B) Ramachandran Plot of Acyl-[acyl-carrier-protein]—UDP-N acetyl glucosamine O-acyltransferase protein.
Molecular docking
The minimum binding energy and scoring function of each docked ligand are shown (Table 4). The LigX interaction diagrams showed that Acyl-[acyl-carrier-protein]—UDP-N acetyl glucosamine O-acyltransferase (B2FHN6) interacts with the enterodiol, aloin, ononin and rhinacanthinF having binding scores -11.36, -17.44, -15.42 and -12.86, respectively. While D-alanine-D-alanine ligase (B2FNN9) interacted with ligands including rhazin, alkannin beta, aloesin and ancistrocladine having the docking scores -12.58, -12.57, -14.39 and -14.41, respectively. All these compounds exhibited RMSD below 3 which is an indication of the sound interaction. The 2D and 3D interaction diagrams are displayed (Figs 5 & 6).
Table 4. The table displays the docking score and RMSD value for the compounds.
| PubChem ID | Compound Name | S-score | RMSD_Refine |
|---|---|---|---|
| 115089 | Enterodiol | -11.36 | 3.0 |
| 14989 | Aloin | -17.44 | 1.7 |
| 442813 | Ononin | -15.42 | 2.5 |
| 10411189 | RhinacanthinF | -12.86 | 1.4 |
| 21160714 | Rhazin | -12.58 | 2.4 |
| 442720 | Alkannin beta, beta-dimethylacrylate | -12.57 | 1.4 |
| 160190 | Aloesin | -14.39 | 2.6 |
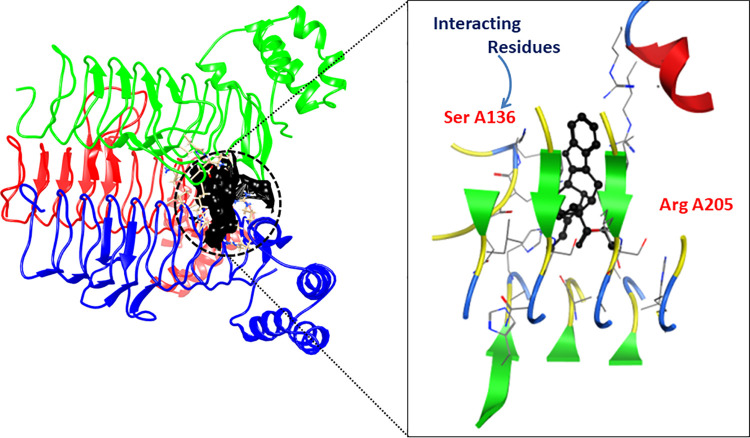
Fig 5. 2D and 3D interaction diagram of Acyl-[acyl-carrier-protein]—UDP-N acetyl glucosamine O-acyltransferase protein.
The structure shows complex with Enterodiol.
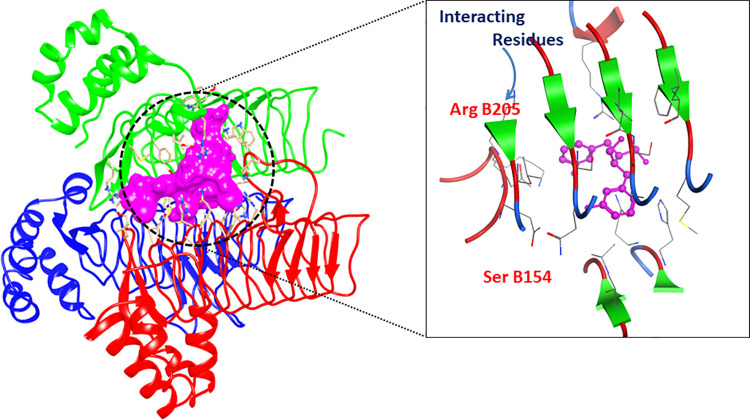
Fig 6. 2D and 3D interaction diagram of D-alanine-D-alanine ligase protein.
The structure shows complex with Rhazin.
ADMET/Drug scans results
The drug likeliness of compounds was predicted through the Molinspiration server, based on the Ro5. The selected candidates indicate zero violations to Lipinski’s Ro5 and showed acceptable drug-like properties (Table 5). All candidate compounds were assessed for the pharmacokinetic properties through the AdmetSAR server for drug likeliness (Table 6).
Table 5. Results of inhibitors examined for Lipinski rule.
| PubChem ID | Compound Name | Molecular Weight | Number of HBA | Number of HBD | MlogP |
|---|---|---|---|---|---|
| 115089 | Enterodiol | 302.37 | 4 | 4 | 2.10 |
| 14989 | Aloin | 418.40 | 7 | 3 | 0.89 |
| 442813 | Ononin | 430 | 9 | 4 | 0.65 |
| 10411189 | RhinacanthinF | 444.44 | 9 | 0 | 2.52 |
| 21160714 | Rhazin | 352.43 | 4 | 2 | 2.57 |
| 442720 | Alkannin beta, beta-dimethylacrylate | 370.40 | 6 | 2 | 3.67 |
| 160190 | Aloesin | 394.38 | 9 | 5 | -0.55 |
| 161741 | Ancistrocladine | 407.51 | 5 | 2 | 5.14 |
Table 6. ADMET properties for compounds as predicted by admetSAR server.
| Compounds | Enterodiol | Aloin | Ononin | RhinacanthinF | Rhazin | Alkannin beta, beta-dimethylacrylate | Aloesin | Ancistrocladine |
|---|---|---|---|---|---|---|---|---|
| Absorption | ||||||||
| Blood Brain Barrier | + | - | - | - | + | - | - | - |
| Human Intestinal Absorption | + | + | - | + | + | + | + | + |
| Metabolism | ||||||||
| P-glycoprotein substrate | - | - | - | - | + | - | - | + |
| CYP1A2 Inhibitor | No | No | No | Yes | Yes | Yes | No | No |
| CYP 450 2C9 Inhibitor | No | No | No | Yes | No | Yes | No | No |
| CYP 450 2D9 Inhibitor | No | No | No | No | No | No | No | Yes |
| CYP 450 2C19 Inhibitor | Yes | No | No | Yes | No | Yes | No | No |
| CYP 450 3A4 Inhibitor | No | No | No | Yes | No | No | Yes | Yes |
| Distribution | ||||||||
| Subcellular Localization | Mitocho-ndria | Mitocho-ndria | Mitochon-dria | Mitochon-dria | Mitochon-dria | Mitochon-dria | Mitochon-dria | Mitochon-dria |
| Toxicity | ||||||||
| AMES Toxicity | No | No | No | No | No | No | No | No |
Toxicity assessment
The rat oral acute toxicity (LD50) as mg/kg, toxicity classes (I–VI) predicted with accuracy in percent, the prediction of hepatotoxicity and cytotoxicity with their probability are indicated (Table 7).
Table 7. Prediction of class and accuracy, organ toxicity, oral acute toxicity and genetic toxicity endpoints of candidate compounds.
| Sr. No | Compound name | Oral LD50 value (mg/Kg) | Predicted toxicity class | Prediction accuracy (%) | Hepato-toxicity | Proba-bility | Cytotoxicity | Proba-bility |
|---|---|---|---|---|---|---|---|---|
| 1 | Enterodiol | 2950 | V | 69.26% | Inactive | 0.80 | Inactive | 0.93 |
| 2 | Aloin | 221 | III | 68.07% | Inactive | 0.85 | Inactive | 0.83 |
| 3 | Ononin | 3100 | V | 64.71% | Inactive | 0.83 | Inactive | 0.58 |
| 4 | RhinacanthinF | 4000 | V | 68.07% | inactive | 0.83 | Inactive | 0.95 |
| 5 | Rhazin | 300 | III | 68.07% | Inactive | 0.85 | Inactive | 0.69 |
| 6 | Alkannin beta, beta-dimethylacrylate | 1000 | IV | 72.9% | Inactive | 0.54 | Inactive | 0.88 |
| 7 | Aloesin | 832 | IV | 67.38% | Inactive | 0.80 | Inactive | 0.78 |
| 8 | Ancistrocladine | 450 | IV | 69.26% | inactive | 0.64 | Inactive | 0.51 |
Among these compounds, enterodiol, ononin and rhinacanthinF exhibited the highest toxicity. Those belong to the class V i.e. prescribed as harmful when swallowed (2000 < LD50 ≤ 5000) with accuracy of 69.26%, 64.71% and 68.7%, respectively. While ononin predicted to have class III (50 < LD50 ≤ 300) prescribed as toxic if swallowed with accuracy of 68.07%. Moreover, other compounds like alkannin beta, aloesin and ancistrocladine belonging to class IV i.e prescribed as harmful after swallowing (300 < LD50 ≤ 2000) with accuracy of 72.9%, 67.38% and 69.26%, respectively. While the compound Rhazin was predicted to have class III (50 < LD50 ≤ 300) prescribed as toxic if swallowed with accuracy of 68.07%. All these compounds were predicted to show hepatotoxicity and cytotoxicity inactive with probability values are shown (Figs 7 & 8).
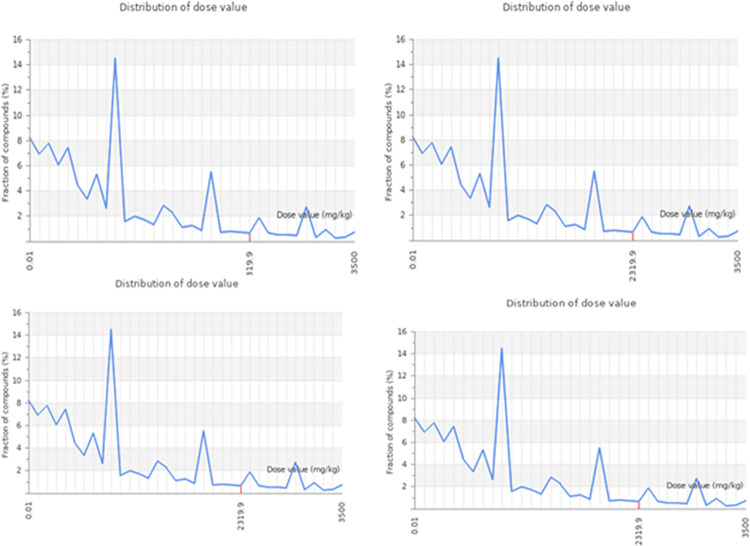
Fig 7. Graphical representation of predicted dose value distribution for Acyl-[acyl-carrier-protein]—UDP-N acetyl glucosamine O-acyltransferase protein.
In this graph, x-axis represents distribution of dose value and y-axis represents fraction of compounds.
Fig 8. Graphical representation of predicted dose value distribution for D-alanine-D-alanine ligase.
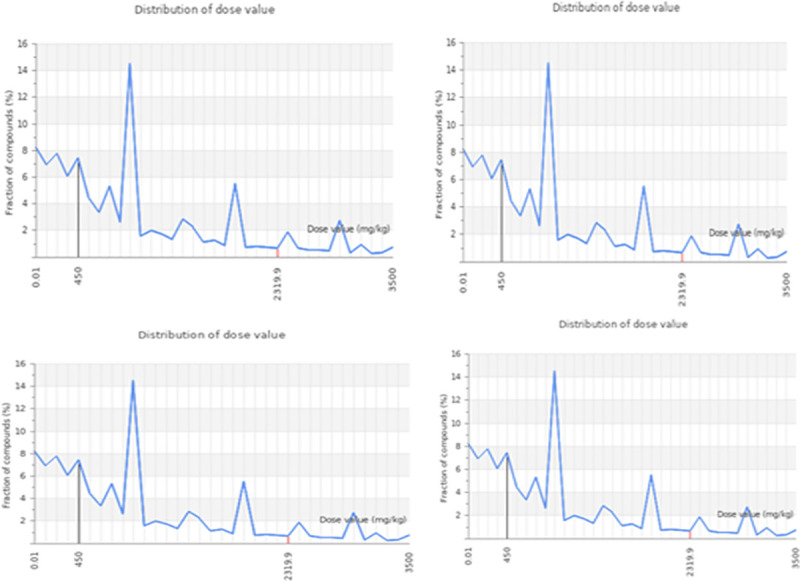
In this graph, x-axis represents distribution of dose value and y-axis represents fraction of compounds.
Discussion
Stenotrophomonas maltophilia (strain k279a) is a multidrug-resistant (MDR) bacterium. There is currently no effective vaccine for that but frequent and thorough hand washing can prevent person-to-person transmission [1]. Recent advances in the disciplines of bioinformatics as well as computational biology have created a variety of approaches to drug designing and in silico analysis, reducing the time and expenses associated with trial and error of ions devoted to drug development [30].
The whole proteome of S. maltophilia (strain k279a) contained 4365 proteins, was analyzed through CD-HIT that eliminated all the redundant proteins and provided a group of 4315 non-redundant proteins. For the survival of bacteria, essential genes are necessary [31]. Essential genes are preferred targets for vaccine development and antibacterial drugs [32]. Thus 407 essential genes were screened from non-redundant proteins. These genes could be homologous to human [33]. Thus, targeting such genes may interfere with human metabolism and might be fatal. The possibility of cross-reactivity as well as adverse events might be reduced by the selection of non-homologous proteins that are not found in Homo sapiens [34]. To avoid such undesirable circumstances and toxicity, we screened 85 non-homologous proteins. It might be the best strategy to target and develop inhibitors against non-homologous sequences for the production of new drugs [35].
Only two proteins Acyl-[acyl-carrier-protein]—UDP-N acetyl glucosamine O-acyltransferase and D-alanine-D-alanine ligase were involved in a unique metabolic pathway. Different tools were applied to determine the sequence and structural features as well as functions and localization of that protein. Both proteins were found to be cytoplasmic as predicted by PSORTb [36]. A proper identification of the potential drug targets and inhibitors is crucial for the treatment of this disease due to their emerging multidrug resistance (MDR) patterns. In this study, a systematic subtractive approach was implemented for the identification of novel therapeutic targets of S. maltophilia through genome-wide metabolic pathway analysis of the essential genes and proteins. ADMET analyses were also made for the identification of potential inhibitors as well. Then, we found unique proteins as novel targets. Therapeutic targets and its inhibitors might give some breakthrough to treat Stenotrophomonas maltophilia efficiently in in vitro [37].
An online tool, Swiss-model was employed to model the 3D structure of Acyl-[acyl-carrier-protein]—UDP-N acetyl glucosamine O-acyltransferase and D-alanine-D-alanine ligase proteins [38]. The prediction of 3D structures provided the great aid in studying protein functions, dynamics, ligand interactions and other protein components [39]. Analysis of the Ramachandran plot showed that most residues were present in the acceptable as well as favored areas and few residues in the disallowed regions [40]. The ERRAT quality factor and z-score proved that structures of the Acyl-[acyl-carrier-protein]—UDP-N acetyl glucosamine O-acyltransferase and D-alanine-D-alanine ligase protein were of good quality.
Molecular docking was performed to find out the compounds exhibiting the best residue interaction with the target protein [26]. Out of 5000 docked molecules, eight (8) top molecules for both proteins: enterodiol, aloin, ononin, rhinacanthinF, rhazin, alkannin beta, aloesin and ancistrocladine were selected based on low score i.e. rmsd < 3 and different interacting residues. Based on "Lipinski’s Rule of Five" molecular profile and drug probability of these eight compounds were assessed. Those compounds were then tested for penetration of the blood-brain barrier (BBB), Human intestinal absorption (HIA) as well as AMES monitoring. Predicting the ADMET properties is a significant indicator of the behavior, toxicity level and fate of the drug candidate in the human body [41]. It provides a likelihood of the candidate’s ability to enter the intestinal absorption, metabolism, blood-brain barrier, subcellular localization and most significantly the level of harm that it can cause to the body [42]. The superfamily cytochrome P450 consists of isoforms such as CYP2A6, CYP1A2, CYP2C9, CYP2D6, CYP2C19, CYP3A4 and CYP2E1 which are involved in drug metabolism as well as hepatic clearance. So, inhibiting the cytochrome P450 isoforms can result in drug-drug interaction that hinders the metabolism of concomitant drugs that cause its accumulation to toxic levels [43]. Admet SAR showed that drugs exhibit localization in mitochondria. The compound localized in mitochondria show no toxicity. The ADMET profile of those compounds indicated that they have no adverse effects on absorption [44].
Various toxicity modules were subjected to the eight compounds obtained after the virtual screening [42]. Toxicity evaluation results revealed that none of the compounds was found to be cytotoxic, hepatotoxic as well as mutagenic [43].
Conclusions
The subtractive genomics approach in our study has indicated two proteins of S. maltophilia as novel drug targets. The probability of cross reactivity seem to be ruled out between drugs and host proteins because there was no similarity between the proteome and ‘anti-targets’. Thus development of the putative target against S. maltophilia might be significantly effective for the eradication of otherwise resulting disease.
Acknowledgments
We are thankful to Department of Bioinformatics and Biotechnology, Government College University, Faisalabad, Pakistan for providing computational facilities for successful completion of this project.
Data Availability
All relevant data are within the manuscript.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Brooke J.S., Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clinical microbiology reviews, 2012. 25(1): p. 2–41. doi: 10.1128/CMR.00019-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Looney W.J., Narita M., and Mühlemann K., Stenotrophomonas maltophilia: an emerging opportunist human pathogen. The Lancet infectious diseases, 2009. 9(5): p. 312–323. doi: 10.1016/S1473-3099(09)70083-0 [DOI] [PubMed] [Google Scholar]
- 3.Nseir S., et al., Intensive care unit-acquired Stenotrophomonas maltophilia: incidence, risk factors, and outcome. Critical Care, 2006. 10(5): p. 1–9. doi: 10.1186/cc5063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sánchez M.B., Antibiotic resistance in the opportunistic pathogen Stenotrophomonas maltophilia. Frontiers in microbiology, 2015. 6: p. 658. doi: 10.3389/fmicb.2015.00658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright G.D., Molecular mechanisms of antibiotic resistance. Chemical communications, 2011. 47(14): p. 4055–4061. doi: 10.1039/c0cc05111j [DOI] [PubMed] [Google Scholar]
- 6.Brooke J.S., New strategies against Stenotrophomonas maltophilia: a serious worldwide intrinsically drug-resistant opportunistic pathogen. Expert review of anti-infective therapy, 2014. 12(1): p. 1–4. doi: 10.1586/14787210.2014.864553 [DOI] [PubMed] [Google Scholar]
- 7.Chang Y.T., et al., Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Frontiers in microbiology, 2015. 6: p. 893. doi: 10.3389/fmicb.2015.00893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barh D., et al., In silico subtractive genomics for target identification in human bacterial pathogens. Drug Development Research, 2011. 72(2): p. 162–177. [Google Scholar]
- 9.Lin X., Li X., and Lin X., A review on applications of computational methods in drug screening and design. Molecules, 2020. 25(6): p. 1375. doi: 10.3390/molecules25061375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu H., Big data and artificial intelligence modeling for drug discovery. Annual review of pharmacology and toxicology, 2020. 60: p. 573–589. doi: 10.1146/annurev-pharmtox-010919-023324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lakshmi P. and Ramyachitra D., Review About Bioinformatics, Databases, Sequence Alignment, Docking, and Drug Discovery, in Statistical Modelling and Machine Learning Principles for Bioinformatics Techniques, Tools, and Applications. 2020, Springer. p. 11–23. [Google Scholar]
- 12.Chakrabarty R.P., et al., Identification and qualitative characterization of new therapeutic targets in Stenotrophomonas maltophilia through in silico proteome exploration. Microbial pathogenesis, 2020. 149: p. 104293. doi: 10.1016/j.micpath.2020.104293 [DOI] [PubMed] [Google Scholar]
- 13.Hossain T., et al., Application of the subtractive genomics and molecular docking analysis for the identification of novel putative drug targets against Salmonella enterica subsp. enterica serovar Poona. BioMed research international, 2017. 2017. doi: 10.1155/2017/3783714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mondal S.I., et al., Identification of potential drug targets by subtractive genome analysis of Escherichia coli O157: H7: an in silico approach. Advances and applications in bioinformatics and chemistry: AABC, 2015. 8: p. 49. doi: 10.2147/AABC.S88522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y., et al., CD-HIT Suite: a web server for clustering and comparing biological sequences. Bioinformatics, 2010. 26(5): p. 680–682. doi: 10.1093/bioinformatics/btq003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birzele F. and Kramer S., A new representation for protein secondary structure prediction based on frequent patterns. Bioinformatics, 2006. 22(21): p. 2628–2634. doi: 10.1093/bioinformatics/btl453 [DOI] [PubMed] [Google Scholar]
- 17.Krogh A., et al., Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. Journal of molecular biology, 2001. 305(3): p. 567–580. doi: 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
- 18.Waterhouse A., et al., SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic acids research, 2018. doi: 10.1093/nar/gky427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pettersen E.F., et al., UCSF Chimera—a visualization system for exploratory research and analysis. Journal of computational chemistry, 2004. 25(13): p. 1605–1612. doi: 10.1002/jcc.20084 [DOI] [PubMed] [Google Scholar]
- 20.Colovos C. and Yeates T.O., Verification of protein structures: patterns of nonbonded atomic interactions. Protein science, 1993. 2(9): p. 1511–1519. doi: 10.1002/pro.5560020916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hooft R.W., et al., Errors in protein structures. Nature, 1996. 381(6580): p. 272. doi: 10.1038/381272a0 [DOI] [PubMed] [Google Scholar]
- 22.Laskowski R.A., et al., AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. Journal of biomolecular NMR, 1996. 8(4): p. 477–486. doi: 10.1007/BF00228148 [DOI] [PubMed] [Google Scholar]
- 23.Bolton E.E., et al., PubChem: integrated platform of small molecules and biological activities, in Annual reports in computational chemistry. 2008, Elsevier. p. 217–241. [Google Scholar]
- 24.Inc., C.C.G., Molecular operating environment (MOE). 2016, Chemical Computing Group Inc 1010 Sherbooke St. West, Suite# 910, Montreal, QC, Canada, H3A 2R7.
- 25.Lipinski C.A., et al., Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings1. Advanced drug delivery reviews, 2001. 46(1–3): p. 3–26. doi: 10.1016/s0169-409x(00)00129-0 [DOI] [PubMed] [Google Scholar]
- 26.Yang H., et al., admetSAR 2.0: web-service for prediction and optimization of chemical ADMET properties. Bioinformatics, 2019. 35(6): p. 1067–1069. doi: 10.1093/bioinformatics/bty707 [DOI] [PubMed] [Google Scholar]
- 27.Banerjee P., et al., ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic acids research, 2018. 46(W1): p. W257–W263. doi: 10.1093/nar/gky318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang R., Ou H.Y., and Zhang C.T., DEG: a database of essential genes. Nucleic acids research, 2004. doi: 10.1093/nar/gkh024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hediger M.A., Turk E., and Wright E.M., Homology of the human intestinal Na+/glucose and Escherichia coli Na+/proline cotransporters. Proceedings of the National Academy of Sciences, 1989. 86(15): p. 5748–5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asalone K.C., Nelson M.M., and Bracht J.R., Novel Sequence Discovery by Subtractive Genomics. J Vis Exp, 2019(143). doi: 10.3791/58877 [DOI] [PubMed] [Google Scholar]
- 31.Ahmad S., et al., Subtractive Genomics, Molecular Docking and Molecular Dynamics Simulation Revealed LpxC as a Potential Drug Target Against Multi-Drug Resistant Klebsiella pneumoniae. Interdiscip Sci, 2019. 11(3): p. 508–526. doi: 10.1007/s12539-018-0299-y [DOI] [PubMed] [Google Scholar]
- 32.Kaur H., Kalia M., and Taneja N., Identification of novel non-homologous drug targets against Acinetobacter baumannii using subtractive genomics and comparative metabolic pathway analysis. Microb Pathog, 2021. 152: p. 104608. doi: 10.1016/j.micpath.2020.104608 [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez R.L., et al., Iterative subtractive binning of freshwater chronoseries metagenomes identifies over 400 novel species and their ecologic preferences. Environ Microbiol, 2020. 22(8): p. 3394–3412. doi: 10.1111/1462-2920.15112 [DOI] [PubMed] [Google Scholar]
- 34.Chandela R., et al., Identification of Therapeutic drug Target of Stenotrophomonas maltophilia through subtractive genomic approach and in-silico screening based on 2D similarity filtration and Molecular Dynamic Simulation. Comb Chem High Throughput Screen, 2020. doi: 10.2174/1871520620666201123094330 [DOI] [PubMed] [Google Scholar]
- 35.Shahid F., et al., In Silico Subtractive Proteomics Approach for Identification of Potential Drug Targets in Staphylococcus saprophyticus. Int J Environ Res Public Health, 2020. 17(10). doi: 10.3390/ijerph17103644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abuthakir M.H.S., et al., Putative Drug Target Identification in Tinea Causing Pathogen Trichophyton rubrum Using Subtractive Proteomics Approach. Curr Microbiol, 2020. 77(10): p. 2953–2962. doi: 10.1007/s00284-020-02114-z [DOI] [PubMed] [Google Scholar]
- 37.Studer G., et al., QMEANDisCo-distance constraints applied on model quality estimation. Bioinformatics, 2020. 36(6): p. 1765–1771. doi: 10.1093/bioinformatics/btz828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koutsouleris N., et al., Multimodal Machine Learning Workflows for Prediction of Psychosis in Patients With Clinical High-Risk Syndromes and Recent-Onset Depression. JAMA Psychiatry, 2021. 78(2): p. 195–209. doi: 10.1001/jamapsychiatry.2020.3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kryshtafovych A., et al., Critical assessment of methods of protein structure prediction (CASP)-Round XIII. Proteins, 2019. 87(12): p. 1011–1020. doi: 10.1002/prot.25823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinzi L. and Rastelli G., Molecular Docking: Shifting Paradigms in Drug Discovery. Int J Mol Sci, 2019. 20(18). doi: 10.3390/ijms20184331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desale V.J., et al., Synthesis, admetSAR predictions, DPPH radical scavenging activity and potent anti-mycobacterial studies of hydrazones of substituted 4-(anilinomethyl)benzohydrazides (Part 2). Curr Comput Aided Drug Des, 2020. [DOI] [PubMed] [Google Scholar]
- 42.Adki K.M., Murugesan S., and Kulkarni Y.A., In Silico and In Vivo Toxicological Evaluation of Paeonol. Chem Biodivers, 2020. 17(10): p. e2000422. doi: 10.1002/cbdv.202000422 [DOI] [PubMed] [Google Scholar]
- 43.García-Jacas C.R., et al., Enhancing Acute Oral Toxicity Predictions by using Consensus Modeling and Algebraic Form-Based 0D-to-2D Molecular Encodes. Chem Res Toxicol, 2019. 32(6): p. 1178–1192. doi: 10.1021/acs.chemrestox.9b00011 [DOI] [PubMed] [Google Scholar]
- 44.Samonis G, Karageorgopoulos DE, Maraki S, Levis P, Dimopoulou D, Spernovasilis NA, et al. Stenotrophomonas maltophilia infections in a general hospital: patient characteristics, antimicrobial susceptibility, and treatment outcome. PloS one. 2012. May 18; 7(5):e37375. doi: 10.1371/journal.pone.0037375 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.