ABSTRACT
Children are seldom affected by severe forms of severe acute respiratory syndrome coronavirus type 2 (SARS-CoV2) infection; however, the impact of comorbidities in the clinical presentation and outcome of SARS-CoV2 in children is poorly characterized including that of chronic liver disease (CLD) and those taking immunosuppressive medications for autoimmune liver disease or following liver transplantation (LT). Although not the main target organ, a spectrum of liver involvement has been described in children infected with SARS-CoV2 and those presenting with Multisystem Inflammatory Syndrome in Children (MIS-C). The Hepatology Committee of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the Society of Pediatric Liver Transplantation (SPLIT) present an evidence-based position paper on liver involvement in children with SARS-CoV2 infection and its impact on those with CLD as well as LT recipients. All children may exhibit acute liver injury from SARS-CoV2 infection, and those with CLD and may experience hepatic decompensation. Preventative and therapeutic measures are discussed.
Keywords: autoimmune hepatitis, autoimmune liver disease, coronavirus disease 2019, COVID-19, immunosuppression, liver transplantation, multisystem inflammatory syndrome in children, MIS-C, PIMS-TS, severe acute respiratory syndrome coronavirus type 2, SARS-CoV2
What Is Known
Coronavirus disease 2019 (COVID-19) has challenged the healthcare systems and made difficult the access to care of children with chronic liver conditions.
There is concern that children with chronic liver disease, or immunosuppressed for autoimmune liver disease or liver transplant, can be at higher risk from COVID-19.
What Is New
Children with chronic liver disease may be at higher risk of developing severe COVID-19 and can experience decompensation of end stage liver disease during severe acute respiratory syndrome coronavirus type 2 infection.
Routine discontinuation of immunosuppressive medications is not advised for liver transplanted children or those with autoimmune liver disease, owing to the absence of evidence of a greater risk from COVID-19.
Severe acute respiratory syndrome coronavirus type 2 (SARS-CoV2)-associated liver injury is defined as the spectrum of liver damage that occurs during infection and disease, or correlated with coronavirus disease 2019 (COVID-19) treatment, with or without pre-existing liver disease (1,2). SARS-CoV2 may cause liver injury with multiple mechanisms. Viral entry into hepatocytes and cholangiocytes allows direct cytopathic effects from active replication (3). Immune-mediated liver injury following the inflammatory response, hypoxic and vascular insults, and anti-SARS-CoV2 treatments are thought to be other means of damage (4,5). In adults, the role of a preexisting liver disease in magnifying SARS-CoV2-associated liver injury is well documented (6). Uncertainties still exist regarding whether children with chronic liver disease (CLD) or those on immunomodulatory and immunosuppressive treatments for liver conditions have an additional clinical risk of severe SARS-CoV2 infection. This document focuses on the impact of SARS-CoV2 infection on children with CLD, including those with end-stage liver disease, liver transplant (LT) recipients and pre-transplant candidates.
OBJECTIVES
To formulate evidence-based position statements using current knowledge of clinical and therapeutic management of acute liver injury during SARS-CoV2 infection, including COVID-19 and Multisystem Inflammatory Syndrome in Children (MIS-C);
To formulate evidence-based position statements using current knowledge for the clinical and therapeutic management of SARS-CoV2 infection in children with CLD (including children with autoimmune liver disease maintained on immunosuppressive, immunomodulator, or biologic therapies) and in children after LT.
METHODS
The Hepatology Committee of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the Society of Pediatric Liver Transplantation (SPLIT) sponsored this manuscript. A working group consisting of selected ESPGHAN members (G.I., E.N., and P.C.) and SPLIT members (M.Ma., N.E., M.K., S.L., M.Mi., V.N.) drafted this position paper. Representatives of the respective societies reviewed and approved the manuscript.
Relevant clinical questions were formulated (Table 1) by the leads of the working group (G.I., M.Ma.) and agreed upon by the other members. Relative position statements intended to answer the questions were based on available evidence from selected key publications cited in PubMed (www.ncbi.nlm.nih.gov/pubmed) and Embase (www.embase.com/#search) from inception to May 1, 2021. The following search words were used “severe acute respiratory syndrome coronavirus type 2”, “SARS-CoV-2”, “SARS-CoV2”, “coronavirus disease 2019”, “COVID-19”, “multisystem inflammatory syndrome in children”, “MIS-C”, “PIMS”, “acute liver injury”, “immunosuppressive agents”, “liver transplantation”, “immunosuppression”, “chronic liver disease”, “infant”, “child” and “adolescent”, “vaccine”. Fundamental characteristics of the abstracts judged pertinent to the review were noted and full-length articles or reviews were selected from the abstracts. Citations were chosen based on their relevance to the context. Due to the paucity of well-characterized pediatric data, relevant adult studies and guidelines were evaluated, and extrapolations from adult literature were included where cited in the manuscript.
TABLE 1.
Research questions addressed by the working group
| Does the diagnostic accuracy of molecular analysis by nucleic acid amplification and serologic tests for SARS-CoV2 differ between children with CLD or immunosuppressed for LT and the pediatric general population? |
| Does SARS-CoV2 infection cause acute liver injury in children with or without CLD? |
| Does MIS-C cause acute liver injury in children? |
| Is CLD a risk factor for acquiring SARS-CoV2 or for a more severe infection in children? |
| Does SARS-CoV2 infection cause acute liver failure in children? |
| Balancing the risk of a hepatic disease flare and the estimated risk of SARS-CoV2 infection, should uninfected children with CLD on treatment with immunomodulators and biologic therapies continue their medical treatment? |
| Balancing the risk of a hepatic disease flare and the estimated risk of SARS-CoV2 infection, should infected children with liver diseases on treatment with immunomodulators and biologic therapies continue their medical treatment? |
| Balancing the risk of graft rejection and the estimated risk of SARS-CoV2 infection, should uninfected LT children continue their immunosuppressive regime? |
| Balancing the risk of graft rejection and the estimated risk of SARS-CoV2 infection, should infected LT children continue their immunosuppressive regime? |
| Balancing the risk of the condition underlying indication to liver transplantation and the estimated risk of SARS-CoV2 infection, should deceased donor liver transplant be avoided in case of ongoing SARS-CoV2 donor/recipient infection? |
| Are the same behavioral measures used by the general population during the pandemic (e.g., hand hygiene, and social distancing) recommended for decreasing the risk of contracting SARS-CoV2 in children with CLD, immunosuppressed for autoimmune liver diseases, and recipients of LT? |
CLD = chronic liver disease; LT = liver transplantation; SARS-CoV2 = severe acute respiratory syndrome coronavirus type 2.
CONSENSUS AND VOTING
A consensus was formally achieved utilizing a structured quantitative method. The working group members voted on each recommendation using a 4-point scale (1: completely disagree; 2: disagree; 3: agree; 4: completely agree), and votes are reported for each recommendation. It was decided in advance that consensus was reached if >75% of the working group members voted 3 or 4. Consensus agreement was achieved for all questions.
Epidemiology and Clinical Features of Severe Acute Respiratory Syndrome Coronavirus Type 2 Infection: Relevance to Pediatric Hepatology
Clinical Burden of Severe Acute Respiratory Syndrome Coronavirus Type 2 in Children
As of July 2021, the World Health Organization (WHO) has confirmed 182,000,000 cases of COVID-19 worldwide, including 3,950,000 deaths, now tempered by the administration of 2,900,000,000 vaccine doses (7). The exact burden in children is poorly known. Once infected, up to 50% of children remain asymptomatic. Cough and/or fever are the most common presenting symptoms in children (9). Fatigue, headache, myalgia, nasal congestion, rhinorrhea, loss of taste or smell, sore throat, dyspnea, abdominal pain, diarrhea, nausea/vomiting, and poor appetite are also observed. In children with chronic disease of the native liver, 50% presented with respiratory symptoms, 47% with fever and 18% were asymptomatic. In children with LT, 36% presented with respiratory symptoms, followed by 34% with fever and 27% were asymptomatic (8). The clinical presentation of MIS-C can be severe, including hypotension and shock, while other symptoms include fever, abdominal pain, vomiting, diarrhea, skin rash and mucocutaneous lesions (9). Compared to adults, children are at lower risk of hospitalization and death from SARS-CoV2 infection, and are less likely to develop severe disease requiring intensive care unit (ICU) management, mechanical ventilation, and vasopressor support (10). Compared to LT recipients, children with CLD (including children with end-stage liver disease) are more likely to be hospitalized and require intensive care (8).
Pathophysiology of the Severe Acute Respiratory Syndrome Coronavirus Type 2-Related Systemic and Hepatic Injury
SARS-CoV2 infects cells via the angiotensin-converting enzyme 2 (ACE2) and CD147 receptors, and exploits the cleavage of the viral S glycoprotein by the host transmembrane protease serum 2 (TMPRSS2). The virus primarily targets lung cells, undergoing intracellular replication. An innate immune response is triggered, releasing cytokines that promote a SARS-CoV2 specific T and B cell response. In some cases, the overproduction of cytokines can lead to severe lung inflammation, multi-organ failure, and death (11,12). A reduced expression of the SARS-CoV2 receptors in respiratory epithelia, a lower threshold of interferon (IFN) antiviral response, a better monocyte/macrophage functioning, and other specific means of viral tolerance could explain the good outcomes generally displayed in childhood infection compared to adults (13–15).
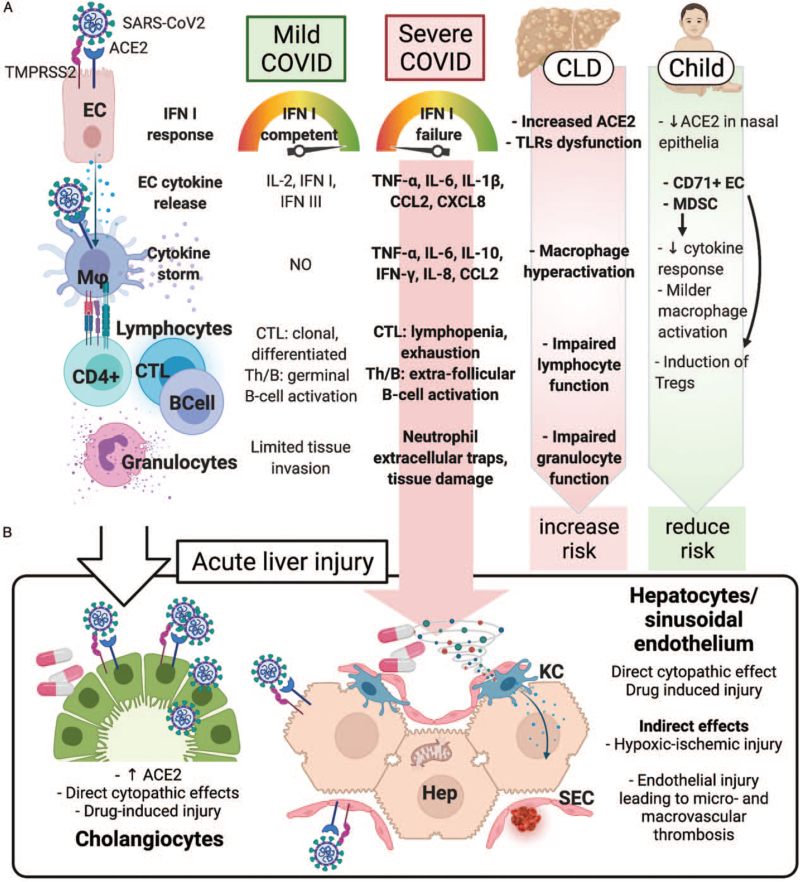
ACE2, TMPRSS2, and CD147 are also present on hepatocytes and cholangiocytes, and ACE2 expression is increased in fibrotic and cirrhotic livers (16,17). Along with the over-expression of pro-inflammatory cytokines, infected hepatocytes downregulate key metabolic processes, accounting, at least in part, for the histological and biochemical features of the SARS-CoV2 liver involvement (18). Cytopathic effects are also mediated by SARS-CoV2-induced hepatocyte mitochondrial dysfunction and impaired autophagy (19,20). Indirect means of SARS-CoV2-related liver injury are thought to be the systemic inflammatory interleukin (IL)-6 and IL-10 cytokine milieu (21), hypoxic-ischemic damage, vascular endothelial changes (22), and drug-induced liver injury from SARS-CoV2 directed therapies (23,24). Figure 1 summarizes the influence of CLD on the pathophysiology of COVID-19, and the mechanisms of acute liver injury (ALI).
FIGURE 1.
Pathophysiology of the liver injury in SARS-CoV2 infection. (A) Immune pathophysiology of mild and severe COVID-19. In severe cases, viral and host factors may result in an inhibited IFN type I response, resulting in a proinflammatory cytokines and chemokines production. This milieu causes lung recruitment of macrophages and T cells, responsible for a cytokine storm, causing further neutrophil recruitment and NETosis, ultimately leading to tissue injury, diverted adaptive immune response, systemic inflammation, and organ dysfunction, including liver. In children, the detrimental role of a preexisting chronic liver disease (due to increased hepatocyte ACE2 expression, innate and adaptive immune dysfunction, macrophage hyperactivation) is counterbalanced by the immunotolerant phenotype associated with the young age. (B) Acute liver injury during SARS-CoV2 infection can occur via direct and indirect mechanisms. The systemic inflammatory cytokine milieu causes sinusoidal endothelial activation, with subsequent thrombosis and hypoxic-ischemic injury. Direct cytopathic effects, and drug-induced injury are also possible especially in cholangiocytes. ACE2 = angiotensin-converting enzyme 2; CCL2 = C–C motif chemokine ligand 2; CD4+ = CD4+ lymphocyte; CD71+ EC = CD71+ erythroid precursors; CLD = chronic liver disease; CTL = cytotoxic T cell; CXCL8 = C–X–C motif chemokine ligand 8; EC = epithelial (respiratory) cell; Hep = hepatocyte; IFN = interferon; IL-2, -6, -8, -10 = interleukin-2, -6, -8, -10; KC = Kupffer cell; Mϕ = macrophage; MDSC = myeloid-derived suppressor cells; SEC = sinusoidal endothelial cell; Th = helper T cell; TLRs = Toll-like receptors; TMPRSS2 = transmembrane protease, serine 2; TNF-α = tumor necrosis factor-α.
Psychosocial Burden of Severe Acute Respiratory Syndrome Coronavirus Type 2 and Health Equity
COVID-19 has further revealed global health inequities with differential access to testing, medical care, therapeutics, and vaccination further driven by structural and systemic racism. In the United Kingdom, Black and South Asian individuals and in the United States, Black, Hispanic, and Native American individuals have disproportionately higher rates of diagnosis, hospitalization, and mortality than white individuals (25). To prevent the ongoing disproportionate risk of mortality, equitable distribution of COVID-19 vaccination is critical and a global ethical imperative. Children, especially those with the least access to resources, remain deeply impacted from the pandemic with reduced access to healthcare, vaccinations and school, with rising food insecurity (26). Social isolation has led to increasing rates of depression, anxiety and suicidal ideation in children and adolescents (27,28).
Diagnosis, Management Strategies and Monitoring in Children with Severe Acute Respiratory Syndrome Coronavirus Type 2 Infection with Chronic Liver Diseases or Following Liver Transplantation
General Management of Children With Chronic Liver Disease or Liver Transplantation Recipients with Acute Severe Acute Respiratory Syndrome Coronavirus Type 2 Infection
The general management of SARS-CoV2 infection in children with CLD or LT recipients does not differ from that of healthy children. Initial efforts should be focused on infection prevention through infection control measures. Infected children should be isolated to contain virus transmission in the hospital, community facility, or home. The decision on whether to admit a child with SARS-CoV2 infection depends on the clinical presentation, stratification of other potential risk factors (including time from LT, degree of immunosuppression and/or other co-morbid conditions) and on the feasibility of caring for the child at home. Four illness severity categories (mild, moderate, severe, and critical) have been described (29–31). Mild or moderate SARS-CoV2 infection (without or with clinical signs of non-severe pneumonia, respectively) should be managed with supportive care only. Severe pneumonia (tachypnea, and hypoxia) and critical (acute respiratory distress syndrome, sepsis, and septic shock) SARS-CoV2 infection requires treatment with antivirals and glucocorticoid steroids together with respiratory and hemodynamic support. Antimicrobials should not be administered unless there is clinical suspicion of superimposed opportunistic infection. In hospitalized children, the clinical condition will guide the intensity of monitoring by routine blood sampling, including liver function tests, blood gas analysis, serum inflammatory markers, blood/urine/sputum cultures, urinalysis, and serial chest radiologic imaging.
Diagnostic Testing
Recommendations for SARS-CoV2 testing have been developed by major national and international health agencies and apply to both children with and without underlying liver disease (32–34). The diagnosis of acute SARS-CoV2 infection relies on viral testing by molecular analysis of nucleic acid amplification, polymerase chain reaction (PCR), or antigen detection from nasal, nasopharyngeal, or oropharyngeal samples (34,35). Molecular assays (nucleic acid amplification and PCR) have emerged as the “gold standard” for testing a child for acute SARS-CoV2 infection (36). Sensitivity of antigen tests is generally lower than that of nucleic acid amplification assays, and more time-dependent; however, antigen tests have low cost and some are approved for point-of-care use delivering rapid results. The advantages of antigen tests need to be balanced against lower sensitivity, especially among asymptomatic individuals and in children. In children with mild respiratory symptoms, the sensitivity of the nasopharyngeal antigen test was 45–62% compared with 83% in adults (37–39). Confirmatory testing with a nucleic acid amplification test should be considered after negative antigen test results in symptomatic individuals and after positive antigen test results in asymptomatic individuals (40).
Serologic assays for SARS-CoV2 infection demonstrating antibodies against SARS-CoV2 in serum are not recommended for the diagnosis of acute infection. Serologic assays may be used together with viral detection tests in the clinical assessment of individuals presenting late in their course and specifically, for children suspected to have MIS-C. Antibody testing is essential for surveillance, epidemiological studies, and determining whether the tested individual was previously infected (41).
Statement:
1. The diagnostic approach and the general management strategies of children with SARS-CoV2 infection do not differ between those with and without CLD or in those who have undergone LT.
(Agreement: 4/4/4/4/4/4/4/4/4)
Acute Liver Injury and Implications for Chronic Liver Conditions
Severe Acute Respiratory Syndrome Coronavirus Type 2 Infection
SARS-CoV2 infection has been associated with ALI with varying degrees of severity. In children, as in adults, most patients have mild-to-moderate ALI (transaminases <2 to 2–5 times the upper limit of normal), and the severity of liver involvement parallels the seriousness of SARS-CoV2 infection in general (42–44). More severe liver injury, characterized as transaminases >5 times the upper limit of normal, is seen in the setting of shock, hypoxia/respiratory compromise, longer overall hospital length of stay, and higher serum inflammatory markers. Liver injury may be due to direct viral cytotoxic effects or indirect injury secondary to cytokine-mediated global inflammatory responses and ischemia (45,46). The presence of ACE2 receptors in biliary and liver epithelial cells predispose the liver to potential targeting by SARS-CoV2 infection. Cholestatic jaundice has been described in adolescents as a possible presentation of SARS-CoV2 infection (47). Additional liver injury may result from drug-induced liver injury from SARS-CoV2 directed therapies (48). Severe SARS-CoV2 infection with ALI may be challenging to differentiate from MIS-C, a multisystem inflammatory condition that may include significant liver injury described in detail below (49).
Statement:
2. SARS-CoV2 infection causes ALI of varying degrees to patients with and without underlying liver disease by multiple potential mechanisms.
(Agreement: 4/4/4/4/4/4/4/4/3)
Multisystem Inflammatory Syndrome in Children
MIS-C (or PIMS-TS, Pediatric Multisystem Inflammatory Syndrome temporally associated with SARS-CoV2) is a rare but severe late complication described in children associated with previous or ongoing SARS-CoV2 infection (50–53). Unlike severe pediatric COVID-19, the syndrome mainly affects children without comorbidities (54). It is defined by the presence of prolonged fever and elevated inflammatory markers, coagulopathy, acute gastrointestinal involvement, evidence of previous or ongoing SARS-CoV2 infection, associated with two of the following: mucocutaneous inflammatory involvement; hypotension or shock; clinical, laboratory or echocardiographic features of myocardial dysfunction (55). Liver involvement in COVID-19 and MIS-C are distinct entities (56). In MIS-C, the injury is usually limited to modest increases in serum transaminases; however, ALF has been described in critically ill children with MIS-C with multi-organ failure (43). Elevated transaminases occur in 24–83% of patients with MIS-C (43,49,57–63), usually in the early phase of the syndrome. Notably, half of patients with MIS-C reported persistently elevated liver enzymes one month after discharge (43). Cantor et al (43) retrospectively assessed liver involvement in children with MIS-C and found that 43% had elevated transaminases. Patients with elevated transaminases had lower albumin levels but similar prothrombin time compared to patients without liver injury, and exhibited more severe cardiovascular involvement, higher rates of shock at presentation, greater respiratory support requirement and longer hospitalization times. A trend towards higher body mass index (BMI) was observed in children with MIS-C and elevated transaminases (43).
Statement:
3. Children diagnosed with MIS-C can have acute liver injury with elevated liver enzymes that is most commonly self-limited, though ALF has also been reported.
(Agreement: 4/4/4/4/4/4/4/4/3)
Children with Underlying Chronic Liver Disease
Patients with CLD represent a vulnerable population with a theoretically increased risk of infection and/or severe COVID-19 (64). Initial reports suggested that CLD was associated with increased disease severity (65–67), although subsequent analyses have challenged this conclusion (67–70). Severe COVID-19, however, is uncommon in children (71,72). In a multicenter cohort (2780 patients from the United States, including children >10 years), patients with a preexisting liver disease had a higher risk of mortality (relative risk [RR]: 3.0; 95% confidence interval [CI]: 1.5 – 6.0; P = 0.001), even after propensity matching for metabolic comorbid indicators (BMI, diabetes, hypertension); the risk was even higher for patients with cirrhosis (RR: 4.6; 95% CI: 2.6–8.3; P < 0.001) (73); however, there are insufficient data to draw definite conclusions in children. Metanalyses of the published literature in adults and children with SARS-CoV2 infection have identified several medical conditions correlated with higher disease severity and need for intensive care (74). Some of the factors identified include diabetes, hypertension, coronary artery and cardiovascular disease, chronic pulmonary disease, malignancy, chronic kidney disease, older age, and male gender; though the heterogeneity between studies varied substantially.
In a retrospective study, adult patients with NAFLD had a higher risk of COVID-19 disease progression (6.6% vs 44.7%; P < 0.001) and persistently abnormal liver function (70% vs 11.1%; P < 0.001) compared to patients without NAFLD (75). In another multicenter observational study on 363 adults, patients with NAFLD had significant higher rates of ICU admission (50.9% vs 35.2%; P = 0.0095) and need for mechanical ventilation (49.1% vs 30.4%; P = 0.006) (76).
Focusing on pediatrics, in a metanalysis on 285,004 children with confirmed SARS-CoV2 infection, 9353 (3.3%) had at least one underlying comorbidity, of which 5.4% were obese. Among 507 obese children, 64 had severe COVID-19 or required ICU admission, with a calculated risk of severity of 2.87 (95% CI 1.16–7.07) (77). Obesity and NAFLD are associated with increased production of pro-inflammatory cytokines like IL-6 or tumor necrosis factor (TNF)-α by adipose cells and Kupffer cells (78). A dysregulated hepatic innate immunity may contribute to worse outcomes with SARS-CoV2 infection. Obesity is the most common comorbidity reported in children with severe SARS-CoV2 infection (8,79–81). Over half of children who require hospitalization for severe illness have an underlying medical condition, most commonly obesity (8,65,81–83). Most children have mild clinical symptoms, though hepatic manifestations can be significant (82,84–88).
Since the proportion of NAFLD patients in published obese cohorts remains unknown, the question as to whether NAFLD is an independent risk factor of COVID-19 severity warrants further studies in children. Nevertheless, regarded adult data, children with NAFLD especially those with obesity, should be considered a risk group for severe COVID-19.
For children with end stage liver disease listed for LT, two patients developed new onset ascites in the setting of SARS-CoV2 infection and were reported to the International NASPGHAN/SPLIT registry. One child has subsequently been transplanted and the other child remains listed for transplantation at the time of writing (10).
Statements:
4. Children with end stage liver disease may experience hepatic decompensation during SARS-CoV2 infection.
(Agreement: 4/4/4/4/4/4/4/4/3)
5. Children with CLD, including obese patients with suspected or documented NAFLD, may be at higher risk of developing severe COVID-19.
(Agreement: 4/4/4/4/4/4/4/3/3)
Acute Liver Failure
While ALI with preserved synthetic function is common in patients with SARS-CoV2 infection, ALF is rare with few cases reported in the literature (89–94). Children surviving ALF without the requirement for LT have been reported (10). Transcriptome analysis of liver tissue collected during autopsy of subjects who succumbed to severe COVID-19 revealed transcriptional shifts resulting in tissue remodeling, mitochondrial dysfunction and lower hepatic detoxification resulting in the clinically observed liver injury and dysfunction (95). The death of an 11-year-old patient from complications of ALF secondary to COVID-19 has been reported. Additionally, successful LT has been performed in a child with ALF secondary to COVID-19 (96,97). ALF is seen more often in patients with severe illness and multiorgan dysfunction. The etiology of ALF in this setting may be the result of direct viral cytotoxicity, immune mediated liver injury (cytokine storm) and/or drug-induced liver injury (90). A comprehensive analysis to better understand which patients are at risk of developing ALF in the setting of SARS-CoV2 infection is needed.
Statement:
6. ALF in SARS-CoV2 infection is rare. The role of SARS-CoV2 infection in causing pediatric ALF, leading to death or LT, is uncertain.
(Agreement: 4/4/4/4/4/4/3/3/3)
Liver Transplant Recipients and Immunocompromised Children
Impact of Immunosuppressive Drugs in Severe Acute Respiratory Syndrome Coronavirus Type 2 Infection
As previously observed in SARS-CoV1 and MERS-CoV epidemics, morbidity related to SARS-CoV2 infection is mediated by exaggerated immune responses rather than the direct cytopathic effect of the virus (98). Although there was initial concern that immunocompromised patients could be at increased risk to develop severe SARS-CoV2 infection, studies involving pediatric and adult solid organ transplant recipients have not demonstrated worse outcomes or increased mortality. Immunosuppression maintenance in solid organ transplant adult recipients has not been associated with a more severe COVID-19 course (8,99–101). Moreover, corticosteroids have been reported to reduce 28-day mortality in an open-label randomized controlled trial in adults, with the greatest benefit in those requiring invasive mechanical ventilation (102). In other studies, corticosteroid use was associated with a shorter stay in the intensive care unit and decreased need for invasive ventilation (103,104). Calcineurin inhibitors have a potential role in inhibiting Coronavirus replication (105), and data from the European Liver Transplant Registry demonstrated an association of tacrolimus treatment with less severe SARS-CoV2 adult infections (106). In addition, antimetabolites such as mycophenolic acid exhibit antiviral properties in vitro (107). Similar to adults, children with chronic autoimmune liver disease on immunosuppressive maintenance medications do not appear to be at greater risk from COVID-19 (8,15,108–110). Similarly, children on chronic immunosuppression for other conditions such as inflammatory bowel diseases (111,112), rheumatologic (113,114), and renal disorders (115,116) do not exhibit increased mortality or risk of severe COVID-19 when compared with age-matched children without chronic conditions. An exception is represented by the autoimmune polyendocrine syndrome type 1 due to mutations in the AIRE gene, and possible rare cause of autoimmune hepatitis, in which preexisting anti-endogenous IFN-I antibodies can lead to severe pneumonia (117). Overall, maintenance immunosuppressive medications do not appear to predispose to more severe SARS-CoV2 infection in children with autoimmune liver disease or in LT recipients (109,118,119).
Statement:
7. Based on the limited available pediatric data and in line with guidelines for adults, routine reduction or withdrawal of established immunosuppressive therapy in children with autoimmune liver disease is not recommended. Reductions could be considered based on general principles for managing infections in immunosuppressed patients and to decrease the risk of superinfection.
(Agreement: 4/4/4/4/4/4/4/4/4)
The Course of Coronavirus Disease 2019 in Liver Transplant Recipients
A recent meta-analysis (120) of 2772 pooled adult solid organ transplant recipients, including 505 LT recipients, showed an incidence of lower respiratory tract infection of 79.7%, ICU admission rate of 29% and mortality of 18%, all of which are comparable to the general population (121). LT status did not significantly increase the risk of death in adults with SARS-CoV2 infection, although older age and presence of comorbidities did (99,100).
A study from the joint North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) and Society of Pediatric Liver Transplantation (SPLIT) SARS-CoV2 pediatric registry described 47 LT recipients under the age of 21 years and found that no patients required mechanical ventilation or died. From this cohort, almost 30% were asymptomatic and most commonly presented with respiratory symptoms (36%), fever (34%), and gastrointestinal (GI) symptoms (25%). Immunosuppression changes included reduction (28%), discontinuation of a second agent (15%) and no adjustments in 68% (8). While interpreting these data, the reader should consider the inherent bias of a registry design. The registry continues to grow, and since the initial publication there are now 180 LT children reported. Of these, 30 patients required non-ICU hospital admission (median = 5 days), and three patients required ICU level care with no requirement for vasoactive support or mechanical ventilation; three patients required supplemental oxygen, and one renal replacement therapy; no deaths were reported [unpublished data]. These data are in accordance with other case reports and case series, which report that children on post-transplant immunosuppressive regimens, similar to the general population of children, tend to experience mild disease (118,119,122–126); however, one case was reported of a three-year-old boy transplanted at the age of 18 months for biliary atresia who developed multiorgan failure after SARS-CoV2 infection and died on day 6 after hospital admission (127).
In summary, current evidence suggests that children after LT may not be at higher risk of severe complications of COVID-19 infection and that they present with similar symptoms and similar mild disease course as the general pediatric population. However, due to limited data and evolution of viral mutations, caution and further studies are needed.
Statement:
8. Based on current evidence that the degree of immunosuppression may not predict worse outcomes from SARS-CoV2 infection, standard immunosuppression should not be routinely reduced or withdrawn in children following LT with mild or moderate SARS-CoV2 infection.
(Agreement: 4/4/4/4/4/4/4/4/4)
Impact on Organ Donation and Clinical Practices
The COVID-19 pandemic initially resulted in a significant decrease in transplant activity. Data from the United States (US) showed an approximate 10% reduction in all solid organ pediatric transplantations in 2020 compared to 2019. A significant decrease in living donor LT (LDLT) compared to deceased donor donation has been reported in some centers, which likely reflects a reduction in elective procedures during the early phase of the pandemic (98). In Europe, only 10 of 18 (55%) pediatric transplant centers remained active in March of 2020, seven centers limited their activity to urgent cases and one center completely suspended their transplant program (122). Data from India showed a reduction in the number of referrals in mid-2020 and higher average PELD scores in LT candidates compared to 2019. Timely LDLT could not be performed in two children due to COVID-lockdown related delays and both died on the waiting list (123). Nevertheless, successful LT is feasible in the setting of a pandemic (128) and has been safely performed shortly after recovery from SARS-CoV2 infection (129).
There is not enough evidence to guide the acceptance of deceased donor grafts from donors actively infected with SARS-CoV2 or transplantation of recipients with ongoing SARS-CoV2 infection. Cases should be gauged on an individual basis balancing the urgency of the LT with the potential risk of disease transmission to the recipient and other care providers, as well as post-transplant complications.
The COVID-19 pandemic has necessitated the advancement and increased use of telemedicine technologies to deliver pre- and post-transplant care. According to a US adult survey, telemedicine is now being used by programs for transplant evaluations (65%), waitlist management (58%) and post-transplant care (98%) (98). In Europe, outpatient visits of pediatric transplant patients were initially impacted in 17 of 18 centers (96%) and seven centers (40%) started or increased the use of telemedicine during the pandemic. Ongoing adjustments in telemedicine utilization and in-person visits are evolving with a likely continuation of telemedicine beyond the confines of the pandemic.
Statement:
9. The decision of whether to perform a deceased donor LT in a child from a SARS-CoV2 infected donor or into a SARS-CoV2 infected child should be made on a case-by-case basis, balancing the risk related to the underlying indication with the risk related to SARS-CoV2.
(Agreement: 4/4/4/4/4/4/4/3/3)
Treatment and Prognosis of Acute Severe Acute Respiratory Syndrome Coronavirus Type 2 Infection
Two phases have been recognized in the clinical course related to the pathogenesis of SARS-CoV2 infection. In the early course of the infection, the disease is driven by replication of the virus and antivirals have the greatest effect. Later, the disease is caused by the immune/inflammatory response to the virus and immunosuppressive/anti-inflammatory therapies are indicated. Table 2 summarizes the treatments available for children with COVID-19.
TABLE 2.
Treatment options for acute SARS-CoV2 infection and vaccines schedules
| Treatment | Summary of main indications |
| Supportive care (adequate intake of daily calories and of water, antipyretic drugs, oxygen/ventilatory support) | As for clinical practice |
| Remdesivir (children older 12 y of age; >40 kg)∗ Dose: 200 mg intravenous on day 1 and 100 mg/day for up to 5 consecutive days | Recommended for use in hospitalized patients who require supplemental oxygen not on mechanical ventilation |
| Steroids (dexamethasone) | Recommended in hospitalized children who require high-flow oxygen, noninvasive ventilation, invasive mechanical ventilation, or extracorporeal membrane oxygenation |
| Monoclonal antibodies (bamlanivimab plus etesevimab and casirivimab plus imdevimab) | No experience on the use in young children; emergency use for patients >12 y of age and > 40 kg at high risk for disease progression† |
| Vaccines‡ | Schedule |
| Pfizer-BioNTech (children older 12 y of age; US Food and Drug Administration and European Medicines Agency) | Two intramuscular injections given 3 wk (21 days) apart§ |
| Moderna (children older 12 y of age; European Medicines Agency) | Two intramuscular injections given 4 wk (28 days) apart† |
Limited data exist regarding Remdesivir use in children <12 y of age (see Chiotos K et al, J Pediatric Infect Dis Soc 2021;10:34–48).
The relevant risk categories are: obesity; chronic kidney disease; diabetes; sickle cell disease; immunosuppressive disease or immunosuppressive treatment; congenital or acquired heart disease; neurodevelopmental disorder; medical-related technology dependence; asthma, reactive airway disease, or other chronic respiratory disease. However, given the overall low rate of adverse infection outcomes, in children and adolescents the presence of this risk condition would not necessarily be sufficient to indicate use of such agents (see Wolf J, et at, J Pediatric Infect Dis Soc 2021;10:629–634.
As of September 2021.
An additional dose at least 28 days after the second dose could be considered in immunocompromised individuals, including organ transplant recipients (see 1) Center for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States, August 31, 2021. CDC: Atlanta; 2021; and (2) European Centre for Disease Prevention and Control. Interim public health considerations for the provision of additional COVID-19 vaccine doses, 1 September 2021. ECDC: Stockholm; 2021.
Supportive Care
Supportive care to liver patients with SARS-CoV2 infection includes ensuring an adequate intake of daily calories and water, administration of antipyretic drugs for a body temperature above 38.5°C and supplemental oxygen/ventilatory support when indicated.
Antivirals
Remdesivir is the only antiviral approved for use in adult and children >12 years of age and >40 kg for the treatment of SARS-CoV2 infection (200 mg IV on day 1 and 100 mg/day for up to 5 consecutive days) (130–132). It is recommended for use in hospitalized patients who require supplemental oxygen. No clear benefit has been demonstrated for those who require mechanical ventilation. For selected hospitalized children <12 years of age and <40 kg, the emergency use authorization remains in effect. Elevated liver biochemistries have been observed in patients treated with remdesivir (132). Aminotransferases and bilirubin should be checked before starting the drug and during treatment for both children with and without a pre-existing liver conditions.
Anti-Inflammatories
Steroids (dexamethasone) improve survival in hospitalized adults who require supplemental oxygen, with the greatest effect observed in patients who require mechanical ventilation.
Monoclonal Antibodies
The anti-SARS-CoV2 monoclonal antibodies bamlanivimab plus etesevimab (133) and casirivimab plus imdevimab (134) are available through emergency use authorizations for patients >12 years of age and > 40 kg who are at high risk for disease progression. Anti-SARS-CoV2 antibody-based therapies may have the greatest effect in the earliest stages of infection, before the host has mounted an effective immune response. There is no experience on the use of these drugs in children. The efficacy of these agents is challenged further by the emergence of viral mutations expressing different viral associated proteins.
Vaccines
The SARS-CoV2 viral spike glycoprotein has been used in COVID-19 vaccines through various delivery systems and vectors. As of September 2021, four COVID-19 vaccines were available for use (Pfizer-BioNTech (135), Moderna (136), AstraZeneca (137), Janssen-Johnson & Johnson (138)). The Pfizer-BioNTech mRNA vaccine has been authorized for ages <18 years (specifically >12 years) by FDA and EMA. EMA recently (July 2021) approved for the same age cohort the use of the Moderna mRNA vaccine. The landscape of the SARS-CoV2 vaccine clinical trials is constantly changing and several vaccines candidates are currently being evaluated in children. The Pfizer-BioNTech has been approved for children 12 years old and older, and it is now recruiting children between the age of 6 months to 11 years. The clinical trial of the Moderna vaccination included 3000 adolescents (aged 12–17 years), and the phase 2/3 clinical trial in children ages 6 months and 11 years was started. The phase 2 clinical trial for AstraZeneca in 300 children ages 6–17 years old was started in February 2021 but halted in April. The clinical trial of Johnson & Johnson vaccine in children is being strategized. In absence of specific evidence about safety and efficacy of SARS-CoV2 vaccines in young patients with chronic liver conditions, related recommendations are based on the high expected benefit, acceptability, and feasibility. Children and adolescents with compensated and decompensated cirrhosis, CLD (including NAFLD, non-alcoholic fatty liver disease), end stage liver disease awaiting transplantation, as well as LT recipients on immunosuppressive medications should be prioritized for early vaccine access because at risk for poorer outcomes from SARS-CoV2 infection, and to warrant routine access to healthcare. It is reasonable to vaccinate before transplantation or before starting immunosuppressive treatment for immune-mediated liver disease. In patients who are listed for LT, vaccination is indicated even if a vaccine with a two-dose schedule is used and LT is likely to occur before the second dose can be administered. Specifically, vaccination can provide protection after the first dose and should not delay deceased donor transplantation (135). It is difficult to predict the best time to administer the vaccine (either the first or the second dose) after LT. It has been suggested that adults receiving a LT can be vaccinated as early as six weeks posttransplant (139). Reducing immunosuppression to elicit the immune response is not recommended. Pre- or post-vaccination serological testing is not recommended outside specific immunogenicity studies (139). Recent data regarding antibody response rates show that a little over half of solid organ recipients develop anti-spike antibodies after vaccine schedule completion (140–142) but further studies are needed to determine clinical relevance and are ongoing.
Statements:
10. There are insufficient data to recommend for or against the use of specific antivirals or immunomodulatory agents for the treatment of COVID-19 in children with liver disease.
(Agreement: 4/4/4/4/4/4/3/3/3)
11. SARS-CoV2 vaccination should be recommended for all children 12–17 years of age with chronic liver disease, including autoimmune liver disease on immunosuppressive therapies, patients with cirrhosis, transplant recipients, those on the waiting list for LT and their caregivers. The same recommendation applies to younger children in which safety is currently being evaluated.
(Agreement: 4/4/4/4/4/4/4/4/3)
12. Future studies are needed to determine COVID-19 vaccine immunogenicity in children with chronic liver disease and LT recipients.
(Agreement: 4/4/4/4/4/4/4/4/4)
Therapeutic Approach to Multisystem Inflammatory Syndrome in Children
Treatments for MIS-C consist primarily of supportive care, antiplatelet and anticoagulation therapy and care directed against the underlying inflammatory process. Supportive measures are lifesaving and include fluid resuscitation, inotropic and respiratory support up to extracorporeal membranous oxygenation. Of note, therapies for MIS-C, including aspirin, enoxaparin and immunomodulatory therapies (intravenous immunoglobulins and steroids) can cause drug-induced liver injury. Anakinra and tocilizumab (recombinant human interleukin-1 and -6 receptors antagonists, respectively) may rarely cause clinically apparent liver injury and are more frequently associated with elevated serum aminotransferases (143).
Social Distancing and Infection Prevention
Preventive strategies are of paramount importance, particularly for children with chronic liver disease or LT recipients (144); however, the social behaviors recommended for personal safety and infection control do not differ from the general populations. The different gradations of social distancing, use of masks and face coverings, school openings, sport events and gatherings, and travelling are regulated by the local authorities based on epidemiological considerations.
Statement:
13. Children with CLD or LT recipients should follow similar social behavior to the general population regarding social distancing, mask-wearing and hand washing.
(Agreement: 4/4/4/4/4/4/4/4/3)
CONCLUSIONS
The present document aims at providing evidence-based guidance to health care providers about liver involvement in children with SARS-CoV2 infection and recommendations for the management of children with underlying liver disease and LT recipients. These populations are generally not at substantial risk of severe SARS-CoV2 infection, though critical attention is warranted for children who: present in ALF, have end-stage liver disease and are listed for transplantation as acute decompensation has been reported, or who are in the immediate post-transplant period with the highest degree off immunosuppressive burden. As COVID-19 vaccine distribution is underway, further studies are required to understand immunogenicity in LT recipients and children with CLD on immunosuppressive therapies. The resulting position statements in this document are primarily derived from expert review and summarize evidence from adults with cirrhosis or solid organ transplantation and the general pediatric population in addition to data from pediatric solid organ transplant recipients and children with CLD. Further studies are needed to better guide SARS-CoV2 therapeutics and management, inform timing of LT following SARS-CoV2 infection, understand COVID-19 vaccine immunogenicity and explore other special considerations in children with CLD or who have undergone pediatric LT.
Footnotes
The authors report no conflicts of interest.
Drs Emanuele Nicastro and Noelle H. Ebel contributed equally to this study; Mercedes Martinez, and Giuseppe Indolfi contributed equally to this study.
Disclaimer: ESPGHAN and SPLIT are not responsible for the practices of physicians and provide guidelines and position papers as indicators of best practice only. Diagnosis and treatment are at the discretion of physicians.
The authors have no funding to disclose.
REFERENCES
- 1.Jothimani D, Venugopal R, Abedin MF, et al. COVID-19 and the liver. J Hepatol 2020; 73:1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nardo AD, Schneeweiss-Gleixner M, Bakail M, et al. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int 2020; 41:20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pirola CJ, Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: putative mechanisms of liver involvement in COVID-19. Liver Int 2020; 40:2038–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloom PP, Meyerowitz EA, Reinus Z, et al. Liver biochemistries in hospitalized patients with COVID-19. Hepatology 2021; 73:890–900. [DOI] [PubMed] [Google Scholar]
- 5.Sonzogni A, Previtali G, Seghezzi M, et al. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int 2020; 40:2110–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moon AM, Webb GJ, Aloman C, et al. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: preliminary results from an international registry. J Hepatol 2020; 73:705–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anon. WHO Coronavirus (COVID-19) Dashboard. [Google Scholar]
- 8.Kehar M, Ebel NH, Ng VL, et al. SARS-CoV2 infection in children with liver transplant and native liver disease: an international observational registry study. J Pediatr Gastroenterol Nutr 2021; 72:807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. CDC. Multisystem Inflammatory Syndrome in Children (MIS-C). Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/mis-c/hcp/. Published February 11, 2020. Accessed March 7, 2021. [Google Scholar]
- 10. CDC. Cases, Data, and Surveillance: Risk for COVID-19 Infection, Hospitalization, and Death By Age Group. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html. Published February 11, 2020. Accessed March 7, 2021. [Google Scholar]
- 11.Tay MZ, Poh CM, Rénia L, et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020; 20:363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danziger-Isakov L, Blumberg EA, Manuel O, et al. Impact of COVID-19 in solid organ transplant recipients. Am J Transplant 2021; 21:925–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA 2020; 323:2427–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmermann P, Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child 2020; [Online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15.Nicastro E, Verdoni L, Bettini LR, et al. COVID-19 in immunosuppressed children. Front Pediatr 2021; 9:629240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paizis G, Tikellis C, Cooper ME, et al. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut 2005; 54:1790–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Q, Xie Q, Shi C-C, et al. Expression of angiotensin-converting enzyme 2 in CCL4-induced rat liver fibrosis. Int J Mol Med 2009; 23:717–723. [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Han Y, Nilsson-Payant BE, et al. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell 2020; 27:125.e7–136.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Liu S, Liu H, et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol 2020; 73:807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh K, Chen Y-C, Judy JT, et al. Network analysis and transcriptome profiling identify autophagic and mitochondrial dysfunctions in SARS-CoV-2 infection. Front Genet 2021; 12:599261.doi: 10.1101/2020.05.13.092536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhan K, Liao S, Li J, et al. Risk factors in patients with COVID-19 developing severe liver injury during ÿandomizedÿtion. Gut 2021; 70:628–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McConnell MJ, Kawaguchi N, Kondo R, et al. Liver injury in COVID-19 and IL-6 trans-signaling-induced endotheliopathy. J Hepatol 2021; 75:647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zampino R, Mele F, Florio LL, et al. Liver injury in remdesivir-treated COVID-19 patients. Hepatol Int 2020; 14:881–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muhović D, Bojović J, Bulatović A, et al. First case of drug-induced liver injury associated with the use of tocilizumab in a patient with COVID-19. Liver Int 2020; 40:1901–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Razai MS, Kankam HKN, Majeed A, et al. Mitigating ethnic disparities in COVID-19 and beyond. BMJ 2021; 372:m4921. [DOI] [PubMed] [Google Scholar]
- 26. Anon. COVID-19 and children. UNICEF DATA. Available at: https://data.unicef.org/covid-19-and-children/. Accessed June 20, 2021. [Google Scholar]
- 27.Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19. J Am Acad Child Adolesc Psychiatry 2020; 59:1218.e3–1239.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czeisler MÉ, Lane RI, Petrosky E, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic – United States, June 24–30, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiotos K, Hayes M, Kimberlin DW, et al. Multicenter initial guidance on use of antivirals for children with coronavirus disease 2019/severe acute respiratory syndrome coronavirus 2. J Pediatric Infect Dis Soc 2020; 9:701–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venturini E, Montagnani C, Garazzino S, et al. Treatment of children with COVID-19: position paper of the Italian Society of Pediatric Infectious Disease. Ital J Pediatr 2020; 46:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anon. Clinical management of COVID-19. Available at: https://www.who.int/publications-detail-redirect/clinical-management-of-covid-19. Accessed February 7, 2021. [Google Scholar]
- 32. CDC. Healthcare Workers: Overview of Testing for SARS-CoV-2 (COVID-19). Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html. Published February 11, 2020. Accessed February 7, 2021. [Google Scholar]
- 33. Anon. Diagnostic testing and screening for SARS-CoV-2. European Centre for Disease Prevention and Control. Available at: https://www.ecdc.europa.eu/en/covid-19/latest-evidence/diagnostic-testing. Accessed February 7, 2021. [Google Scholar]
- 34.Palmas G, Moriondo M, Trapani S, et al. Nasal swab as preferred clinical specimen for COVID-19 testing in children. Pediatr Infect Dis J 2020; 39:e267–e270. [DOI] [PubMed] [Google Scholar]
- 35.Wong SCY, Tse H, Siu HK, et al. Posterior oropharyngeal saliva for the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020; 71:2939–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. CDC. Labs: CDC's Diagnostic Test for COVID-19 Only and Supplies. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/virus-requests.html. Published February 11, 2020. Accessed February 7, 2021. [Google Scholar]
- 37.Villaverde S, Domínguez-Rodríguez S, Sabrido G, et al. Diagnostic accuracy of the Panbio severe acute respiratory syndrome coronavirus 2 antigen rapid test compared with reverse-transcriptase polymerase chain reaction testing of nasopharyngeal samples in the pediatric population. J Pediatr 2021; 232:287.e4–289.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linares M, Pérez-Tanoira R, Carrero A, et al. Panbio antigen rapid test is reliable to diagnose SARS-CoV-2 infection in the first 7 days after the onset of symptoms. J Clin Virol 2020; 133:104659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albert E, Torres I, Bueno F, et al. Field evaluation of a rapid antigen test (PanbioTM COVID-19 Ag Rapid Test Device) for COVID-19 diagnosis in primary healthcare ÿandomi. Clin Microbiol Infect 2021; 27:472.e7–472.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pray IW, Ford L, Cole D, et al. Performance of an antigen-based test for asymptomatic and symptomatic SARS-CoV-2 testing at two university campuses—Wisconsin, September–October 2020. MMWR Morb Mortal Wkly Rep 2021; 69:1642–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. CDC. Interim Guidelines for COVID-19 Antibody Testing. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html. Published February 11, 2020. Accessed April 12, 2021. [Google Scholar]
- 42.Phipps MM, Barraza LH, LaSota ED, et al. Acute liver injury in COVID-19: prevalence and association with clinical outcomes in a large U.S. cohort. Hepatology 2020; 72:807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cantor A, Miller J, Zachariah P, et al. Acute hepatitis is a prominent presentation of the Multisystem Inflammatory Syndrome in Children: a single-center report. Hepatology 2020; 72:1522–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xin S, Xu J, Yu Y. Abnormal liver function tests of patients with coronavirus disease 2019 in mainland China: a systematic review and meta-analysis. J Gastrointestin Liver Dis 2020; 29:219–226. [DOI] [PubMed] [Google Scholar]
- 45.Effenberger M, Grander C, Grabherr F, et al. Systemic inflammation as fuel for acute liver injury in COVID-19. Dig Liver Dis 2021; 53:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mazza A, Di Giorgio A, Martelli L, et al. Patterns of presentation of SARS-CoV-2 infection in children. Experience at the Italian ÿandomize of the pandemic. Front Pediatr 2021; 9:629040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perez A, Kogan-Liberman D, Sheflin-Findling S, et al. Presentation of severe acute respiratory syndrome-coronavirus 2 infection as cholestatic jaundice in two healthy adolescents. J Pediatr 2020; 226:278–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carothers C, Birrer K, Vo M. Acetylcysteine for the treatment of suspected remdesivir-associated acute liver failure in COVID-19: a case series. Pharmacotherapy 2020; 40:1166–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feldstein LR, Tenforde MW, Friedman KG, et al. Characteristics and outcomes of US children and adolescents with Multisystem Inflammatory Syndrome in Children (MIS-C) compared with severe acute COVID-19. JAMA 2021; 325:1047–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheung EW, Zachariah P, Gorelik M, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA 2020; 324:294–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian ÿandomize of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020; 395:1771–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moraleda C, Serna-Pascual M, Soriano-Arandes A, et al. Multi-inflammatory syndrome in children related to SARS-CoV-2 in Spain. Clin Infect Dis 2020; 72:e397–e401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020; 395:1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang L, Tang K, Levin M, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis 2020; 20:e276–e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Anon. Multisystem inflammatory syndrome in children and adolescents with COVID-19. Available at: https://www.who.int/publications-detail-redirect/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. Accessed February 19, 2021. [Google Scholar]
- 56.Pere A, Cantor A, Rudolph B, et al. Liver involvement in children with SARS-COV-2 infection: two distinct clinical phenotypes caused by the same virus. Liver Int 2021; 41:2068–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ 2020; 369:m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pouletty M, Borocco C, Ouldali N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis 2020; 79:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.García-Salido A, de Carlos Vicente JC, Belda Hofheinz S, et al. Severe manifestations of SARS-CoV-2 in children and adolescents: from COVID-19 pneumonia to multisystem inflammatory syndrome: a multicentre study in pediatric intensive care units in Spain. Crit Care 2020; 24:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee PY, Day-Lewis M, Henderson LA, et al. Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J Clin Invest 2020; 130:5942–5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller J, Cantor A, Zachariah P, et al. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children that is related to coronavirus disease 2019: a single center experience of 44 cases. Gastroenterology 2020; 159:1571.e2–1574.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caro-Patón GdeL, de Azagra-Garde AM, García-Salido A, et al. Shock and myocardial injury in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection: what we know. Case series and review of the literature. J Intensive Care Med 2020; 36:392–403. [DOI] [PubMed] [Google Scholar]
- 63.Sethuraman U, Kannikeswaran N, Ang J, et al. Multisystem inflammatory syndrome in children associated with novel coronavirus SARS-CoV-2: presentations to a pediatric emergency department in Michigan. Am J Emerg Med 2021; 39:164–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boettler T, Newsome PN, Mondelli MU, et al. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep 2020; 2:100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim D, Adeniji N, Latt N, et al. Predictors of outcomes of COVID-19 in patients with chronic liver disease: US multi-center study. Clin Gastroenterol Hepatol 2020; 19:1469.e19–1479.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li C, Chen Q, Wang J, et al. Clinical characteristics of chronic liver disease with coronavirus disease 2019 (COVID-19): a cohort study in Wuhan, China. Aging (Albany NY) 2020; 12:15938–15945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang H, Xu J, Liang X, et al. Chronic liver disease independently associated with COVID-19 severity: evidence based on adjusted effect estimates. Hepatol Int 2021; 15:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lippi G, de Oliveira MHS, Henry BM. Chronic liver disease is not associated with severity or mortality in coronavirus disease 2019 (COVID-19): a pooled analysis. Eur J Gastroenterol Hepatol 2021; 33:114–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Di Giorgio A, Nicastro E, Arnaboldi S, et al. Health status of children with chronic liver disease during the SARS-CoV-2 outbreak: results from a multicentre study. Clin Res Hepatol Gastroenterol 2021; 45:101610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Di Giorgio A, Hartleif S, Warner S, et al. COVID-19 in children with liver disease. Front Pediatr 2021; 9:616381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liguoro I, Pilotto C, Bonanni M, et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr 2020; 179:1029–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu H, Chen S, Liu M, et al. Comorbid chronic diseases are strongly correlated with disease severity among COVID-19 patients: a systematic review and meta-analysis. Aging Dis 2020; 11:668–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh S, Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology 2020; 159:768.e3–771.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou Y-H, Zheng KI, Targher G, et al. Abnormal liver enzymes in children and infants with COVID-19: a narrative review of case-series studies. Pediatr Obes 2020; 15:e12723. [DOI] [PubMed] [Google Scholar]
- 75.Ji D, Qin E, Xu J, et al. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol 2020; 73:451–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hashemi N, Viveiros K, Redd WD, et al. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: a multicentre United States experience. Liver Int 2020; 40:2515–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis 2021; 103:246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fontana L, Eagon JC, Trujillo ME, et al. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007; 56:1010–1013. [DOI] [PubMed] [Google Scholar]
- 79.Gao F, Zheng KI, Wang X-B, et al. Obesity is a risk factor for greater COVID-19 severity. Diabetes Care 2020; 43:e72–e74. [DOI] [PubMed] [Google Scholar]
- 80.Korakas E, Ikonomidis I, Kousathana F, et al. Obesity and COVID-19: immune and metabolic derangement as a possible link to adverse clinical outcomes. Am J Physiol Endocrinol Metab 2020; 319:E105–E109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zachariah P, Johnson CL, Halabi KC, et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children's hospital in New York City, New York. JAMA Pediatr 2020; 174:e202430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr 2020; 174:868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Götzinger F, Santiago-García B, Noguera-Julián A, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health 2020; 4:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Henry BM, Benoit SW, de Oliveira MHS, et al. Laboratory abnormalities in children with mild and severe coronavirus disease 2019 (COVID-19): a pooled analysis and review. Clin Biochem 2020; 81:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patel KP, Patel PA, Vunnam RR, et al. Gastrointestinal, hepatobiliary, and pancreatic manifestations of COVID-19. J Clin Virol 2020; 128:104386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Redd WD, Zhou JC, Hathorn KE, et al. Prevalence and characteristics of gastrointestinal symptoms in patients with severe acute respiratory syndrome coronavirus 2 infection in the United States: a multicenter cohort study. Gastroenterology 2020; 159:765.e2–767.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 88.Xu L, Liu J, Lu M, et al. Liver injury during highly pathogenic human coronavirus infections. Liver Int 2020; 40:998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lenti MV, Borrelli de Andreis F, Pellegrino I, et al. Impact of COVID-19 on liver function: results from an internal medicine unit in Northern Italy. Intern Emerg Med 2020; 15:1399–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang C, Shi L, Wang F-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol 2020; 5:428–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Melquist S, Estepp K, Aleksandrovich Y, et al. COVID-19 presenting as fulminant hepatic failure: a case report. Medicine (Baltimore) 2020; 99:e22818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sarkar S, Rapista N, Jean L-G. Corona virus disease-19-induced acute liver failure leading to severe metabolic acidosis. Chest 2020; 158:A1002. [Google Scholar]
- 93.Gurala D, Al Moussawi H, Philipose J, et al. Acute liver failure in a COVID-19 patient without any preexisting liver disease. Cureus 2020; 12:e10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weber S, Mayerle J, Irlbeck M, et al. Severe liver failure during SARS-CoV-2 infection. Gut 2020; 69:1365–1367. [DOI] [PubMed] [Google Scholar]
- 95.Hammoudeh SM, Hammoudeh AM, Bhamidimarri PM, et al. Insight into molecular mechanisms underlying hepatic dysfunction in severe COVID-19 patients using systems biology. World J Gastroenterol 2021; 27:2850–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haji Esmaeil Memar E, Mamishi S, Sharifzadeh Ekbatani M, et al. Fulminant hepatic failure: a rare and devastating manifestation of Coronavirus disease 2019 in an 11-year-old boy. Arch Pediatr 2020; 27:502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yohanathan L, Corsini Campioli C, Mousa OY, et al. Liver transplantation for acute liver failure in a SARS-CoV-2PCR-positive patient. Am J Transplant 2021; 21:2890–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lobritto S, Danziger-Isakov L, Michaels MG, et al. Impact of COVID-19 pandemic on pediatrics and pediatric transplantation programs. Front Pediatr 2020; 8:612627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kates OS, Haydel BM, Florman SS, et al. COVID-19 in solid organ transplant: a multi-center cohort study. Clin Infect Dis 2020; [Online ahead of print]. [Google Scholar]
- 100.Webb GJ, Marjot T, Cook JA, et al. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol 2020; 5:1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Karruli A, Spiezia S, Boccia F, et al. Effect of immunosuppression maintenance in solid organ transplant recipients with COVID-19: systematic review and meta-analysis. Transpl Infect Dis 2021; 23:e13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Horby P, Lim WS, et al. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med 2021; 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fadel R, Morrison AR, Vahia A, et al. Early short-course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis 2020; 71:2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther 2020; 5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carbajo-Lozoya J, Müller MA, Kallies S, et al. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res 2012; 165:112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Belli LS, Fondevila C, Cortesi PA, et al. Protective role of tacrolimus, deleterious role of age and comorbidities in liver transplant recipients with Covid-19: results from the ELITA/ELTR multi-center European study. Gastroenterology 2020; 160:1151.e3–1163.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schoot TS, Kerckhoffs APM, Hilbrands LB, et al. Immunosuppressive drugs and COVID-19: a review. Front Pharmacol 2020; 11:1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marjot T, Buescher G, Sebode M, et al. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol 2021; 74:1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Di Giorgio A, Nicastro E, Speziani C, et al. Health status of patients with autoimmune liver disease during SARS-CoV-2 outbreak in northern Italy. J Hepatol 2020; 73:702–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rigamonti C, Cittone MG, De Benedittis C, et al. Rates of symptomatic SARS-CoV-2 infection in patients with autoimmune liver diseases in Northern Italy: a telemedicine study. Clin Gastroenterol Hepatol 2020; 18:2369.e1–2371.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Norsa L, Indriolo A, Sansotta N, et al. Uneventful course in patients with inflammatory bowel disease during the severe acute respiratory syndrome coronavirus 2 outbreak in Northern Italy. Gastroenterology 2020; 159:371–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mao R, Liang J, Shen J, et al. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol Hepatol 2020; 5:425–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Filocamo G, Minoia F, Carbogno S, et al. Absence of severe complications from SARS-CoV-2 infection in children with rheumatic diseases treated with biologic drugs. J Rheumatol 2020; 48:1343–1344. [DOI] [PubMed] [Google Scholar]
- 114.Koker O, Demirkan FG, Kayaalp G, et al. Does immunosuppressive treatment entail an additional risk for children with rheumatic diseases? A survey-based study in the era of COVID-19. Rheumatol Int 2020; 40:1613–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Melgosa M, Madrid A, Alvárez O, et al. SARS-CoV-2 infection in Spanish children with chronic kidney pathologies. Pediatr Nephrol 2020; 35:1521–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mastrangelo A, Morello W, Vidal E, et al. Impact of COVID-19 pandemic in children with CKD or immunosuppression. Clin J Am Soc Nephrol 2020; 16:449–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bastard P, Orlova E, Sozaeva L, et al. Preexisting autoantibodies to type I IFNs underlie critical COVID-19 pneumonia in patients with APS-1. J Exp Med 2021; 218:e20210554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nicastro E, Di Giorgio A, Zambelli M, et al. Impact of the severe acute respiratory syndrome coronavirus 2 outbreak on pediatric liver transplant recipients in Lombardy, Northern Italy. Liver Transpl 2020; 26:1359–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.D’Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl 2020; 26:832–834. [DOI] [PubMed] [Google Scholar]
- 120.Raja MA, Mendoza MA, Villavicencio A, et al. COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis of current literature. Transplant Rev (Orlando) 2021; 35:100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Doná D, Torres Canizales J, Benetti E, et al. Pediatric transplantation in Europe during the COVID-19 pandemic: early impact on activity and healthcare. Clin Transplant 2020; 34:e14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Reddy MS, Menon J, Hakeem AR, et al. How can we reduce the impact of COVID-19 pandemic on timely access to liver transplantation in children? Hepatol Int 2021; 15:215–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Morand A, Roquelaure B, Colson P, et al. Child with liver transplant recovers from COVID-19 infection. A case report. Arch Pediatr 2020; 27:275–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Heinz N, Griesemer A, Kinney J, et al. A case of an Infant with SARS-CoV-2 hepatitis early after liver transplantation. Pediatr Transplant 2020; 24:e13778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Goss MB, Galván NTN, Ruan W, et al. The pediatric solid organ transplant experience with COVID-19: an initial multi-center, multi-organ case series. Pediatr Transplant 2020; 25:e13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nikoupour H, Kazemi K, Arasteh P, et al. Pediatric liver transplantation and COVID-19: a case report. BMC Surg 2020; 20:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Feldman AG, Adams MA, Wachs ME, et al. Successful non-directed living liver donor transplant for an infant with biliary atresia during the COVID-19 pandemic. Pediatr Transplant 2020; 24:e13816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Goss MB, Munoz FM, Ruan W, et al. Liver transplant in a recently COVID-19 positive child with hepatoblastoma. Pediatr Transplant 2020; 25:e13880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19—final report. N Engl J Med 2020; 383:1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med 2020; 383:1827–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Montastruc F, Thuriot S, Durrieu G. Hepatic disorders with the use of remdesivir for coronavirus. Clin Gastroenterol Hepatol 2020; 18:2835–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2021; 325:632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med 2021; 384:238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 2020; 383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four ÿandomized controlled trials in Brazil, South Africa, and the UK. Lancet 2021; 397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1-2a trial of Ad26.COV2.S COVID-19 vaccine. N Engl J Med 2021; 384:1824–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fix OK, Hameed B, Fontana RJ, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD Expert Panel Consensus Statement. Hepatology 2020; 72:287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Korth J, Jahn M, Dorsch O, et al. Impaired humoral response in renal transplant recipients to SARS-CoV-2 vaccination with BNT162b2 (Pfizer-BioNTech). Viruses 2021; 13:756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rabinowich L, Grupper A, Baruch R, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol 2021; 75:435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant 2021; 21:2719–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Henderson LA, Canna SW, Friedman KG, et al. American college of rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 2. Arthritis Rheumatol 2020; 73:e13–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. CDC. COVID-19 and Your Health: Social Distancing. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/social-distancing.html. Published February 11, 2020. Accessed March 3, 2021. [Google Scholar]