Summary
Background
The coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 has killed millions of people worldwide. The current crisis has created an unprecedented demand for rapid test of SARS-CoV-2 infection.
Methods
Reverse transcription loop-mediated isothermal amplification (RT-LAMP) is a fast and convenient method to amplify and identify the transcripts of a targeted pathogen. However, the sensitivity and specificity of RT-LAMP were generally regarded as inferior when compared with the gold standard RT-qPCR. To address this issue, we combined bioinformatic and experimental analyses to improve the assay performance for COVID-19 diagnosis.
Findings
First, by experimental screening as well as high-throughput sequencing studies, we discovered new primer features that impacted LAMP sensitivity and specificity. These features were then used to build an improved bioinformatics algorithm to design LAMP primers targeting SARS-CoV-2. We further rigorously validated these new assays for their efficacy and specificity. We demonstrated that multiplexed RT-LAMP assay could directly detect as low as 1.5 copies/µL of SARS-CoV-2 particles in saliva, without the need of RNA isolation. We further tested this ultra-sensitive and specific RT-LAMP assay using saliva samples from COVID-19 patients. Clinical validation results indicated that the new RT-LAMP assay was comparable to standard RT-qPCR in overall assay sensitivity and specificity.
Interpretation
In summary, our new LAMP primer design algorithm along with the validated assays provide a fast and reliable method for the diagnosis of COVID-19 cases.
Funding
National Institutes of Health.
Keywords: COVID-19, SARS-CoV-2, RT-LAMP, Diagnostic assay
Introduction
The pandemic of COVID-19 has killed over 5.01 million people worldwide, including over 747,000 in the USA as of November 2, 2021. More than 247 million people have been infected worldwide. Each day, hundreds of thousands are added to the numbers (while this manuscript was being prepared). Rapid testing to identify the SARS-CoV-2 positive population followed by quarantine is important to curb the pandemic. Thus, the current crisis has created an unprecedented demand for rapid tests with high sensitivity and specificity for point-of-care diagnosis. Vaccine distribution has curbed the pandemic significantly, although it is also expected that the virus will be present in communities in the long term.1
To detect SARS-CoV-2, many research groups have developed new diagnostic assays based on various technology platforms. Among them, nucleic acid detection by reverse transcription quantitative PCR (RT-qPCR) is considered the gold standard for virus diagnosis. In addition, there are other alternative nucleic acid detection methods developed in recent years, providing fast diagnostic results.2 However, these newly emerged methods generally have inferior diagnostic performance compared with RT-qPCR.3 Among these methods, loop-mediated isothermal amplification (LAMP) was developed by Notomi T et al. in 2000.4 Since then, LAMP has been widely adopted for the detection of many pathogens, such as malaria,5 salmonella,6 influenza virus,7 dengue virus,8 Chikungunya virus,9 and Zika virus.10 Although multiple groups have attempted to apply the LAMP technique to SARS-CoV-2 detection,11, 12, 13 there are still major unresolved technical challenges preventing its application in clinical practice.
The advantage of LAMP assays mainly lies in their fast turnaround time and simplicity in assay setup. Specifically, LAMP does not require high-end instruments, making it widely accessible worldwide including developing countries. Moreover, LAMP works well even for a variety of unpurified samples, which is important for convenient and fast assay setup. Another major advantage is that LAMP readout can be colorimetric or turbidity changes, easily visualized and recorded by a phone camera. In addition, being performed under lower temperatures than PCR, LAMP can be easily combined with the RT reaction (i.e., RT-LAMP), directly detecting target RNA without a separate RT step. In this way, the total reaction time can be greatly shortened. Therefore, LAMP is a powerful tool for point-of-care diagnosis with wide applications.2 Despite these advantages, current LAMP assays are regarded as inferior to RT-qPCR in detection sensitivity and specificity.11,14
Compared with PCR, LAMP is still an emerging technology with major unresolved issues. It is a major challenge to design robust LAMP assays for pathogen detection. A typical LAMP assay requires six primers targeting eight regions of the target sequence. At present, few tools are available publicly for LAMP primer design, and available tools did not sufficiently consider the complexity of LAMP reactions. For example, one major issue is related to high-level unintended primer cross-reactivity, given that a set of six primers are included in a single LAMP reaction. This frequently leads to false-positive results as reported by many investigators.11,15, 16, 17, 18
In this study, we developed an improved bioinformatics algorithm to design LAMP primer sets targeting the nucleocapsid (N), membrane (M), and spike (S) genes of SARS-CoV-2. The performance of these primer sets was rigorously validated experimentally. A combination of the best primer sets could reliably detect as low as 1.5 copies/µL of SARS-CoV-2 particles in unpurified specimens (i.e., saliva) without the need of RNA isolation. This represents significant improvement over other reported LAMP assays.11, 12, 13 Finally, further testing using simulated saliva samples as well as patient saliva samples indicated that our new assays were as effective as standard RT-qPCR in SARS-CoV-2 detection. Importantly, our assays have unique advantages over RT-qPCR due to their fast and direct detection of viral RNA in saliva with colorimetric readout, making it ideal for inexpensive point-of-care diagnosis. Our new LAMP design method as well as experimentally validated assays provide a valuable resource to fight the pandemic of COVID-19.
Methods
Assay targets for SARS-CoV-2 diagnosis
The plasmid containing the N gene sequence of SARS-CoV-2 was purchased from Integrated DNA Technologies (IDT; 10006625, CoV-19 positive control). Synthetic SARS-CoV-2 genomic RNA [referred by the U.S. Food and Drug Administration (FDA)] was purchased from Twist Bioscience (102024, sequence based on GenBank accession MN908947.3). Heat-inactivated SARS-CoV-2 particles were acquired from the ATCC (VR-1986HK, 4.2 × 105 particles/µL).
LAMP and RT-LAMP assays
All DNA oligo primers were purchased from Sigma-Aldrich (desalt grade or as indicated). The N gene plasmid or SARS-CoV-2 RNA was diluted with DNase/RNase-free water or mixed with HeLa or SiHa cell total RNA (10 ng/µL). The LAMP or RT-LAMP reactions were assembled in a 96 or 384-well plate (Applied Biosystems). Each 10 µL reaction contained 5 µL of 2X WarmStart Colorimetric Master Mix (New England Biolabs or NEB, M1800), 0.5 µL of 20X SYBR Green I Nucleic Acid Gel Stain (10,000X, Life Technologies), 1 µL of primer set mix (final concentrations of 1.6 µM for FIP or BIP primer, 0.4 µM for FL or BL primer, and 0.2 µM for F3 or B3 primer), 0.5 µL of guanidine hydrochloride (840 mM), 0.5 µL of target RNA or DNA, and 2.5 µL of DNase/RNase-free water. The LAMP or RT-LAMP assay in 10 µL reaction volume was used for primer screening and initial saliva assay optimization. A larger reaction volume (20-120 µL) with the same reagent concentrations, assembled in either a 96-well plate (Life technology) or a 0.5-1.5 mL Eppendorf tube, was subsequently adopted for assay validation using simulated and clinical saliva samples. For each 20-120 µL reaction, 20-50 µL of mineral oil (Sigma-Aldrich), respectively, was included to overlay the reaction mixture before initiating the reaction. When combining two LAMP primer sets in a single reaction, the concentration of each FIP or BIP primer was adjusted to 1.0 µM, while the concentrations of other primers remained the same. All LAMP and RT-LAMP reactions were performed in a real-time PCR instrument (QuantStudio, Applied Biosystems) or in a heating block (Isotemp, Fisher Scientific) for 60-100 min at 65°C, with fluorescence signals acquired as fixed intervals when performed on a real-time PCR instrument. The colorimetric change of the reaction (i.e., positive samples turning yellow or orange from pink) was recorded by a phone camera both before and at the end of the reaction. The efficacy of LAMP or RT-LAMP amplification was evaluated by detection of fluorescence signals over a threshold readout when performed in a real-time PCR instrument (i.e., Time to Threshold, or TT). Optimal threshold was established using QuantStudio Design and Analysis Software (Applied Biosystems). Readings from 3-10 replicated reactions or as indicated were averaged and the results were presented as mean±SD.
RT-qPCR assays
E-Sarbeco, a widely used qPCR primer set targeting the E gene of SARS-CoV-211,19 and recommended by the CDC, was included in RT-qPCR reactions as standard diagnosis method for COVID-19 (Supplementary Table S8). RT reactions were performed with the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Each 10 µL of RT reaction included 1 µL of 10X RT buffer, 0.4 µL of 25X dNTP (100 mM), 1 µL of 10X random primers, 0.5 µL of reverse transcriptase, 1-5 µL of the RNA sample, and water to final volume of 10 µL. The RT reaction mixture was incubated at 25°C for 20 min followed by 37°C for 60 min, and finally heat inactivated at 85°C for 5 min. Real-time PCR was performed with Power SYBR Green Master Mix (Applied Biosystems). Each 14 µL reaction included 1 µL of diluted RT product, 7 µL of 2X Power SYBR Green Master Mix, 6 µL of the primer mix (final concentration at 250 nM for each E-Sarbeco primer). The real-time PCR running protocol was 10 min at 95°C, followed by 40 amplification cycles (95°C for 10 s and 60°C for 30 s).
HeLa and SiHa cell RNA
HeLa (ATCC Cat# CCL-2, RRID: CVCL_0030) and SiHa cells (ATCC Cat# HTB-35, RRID: CVCL_0032) were cultured at 37°C with 5% CO2 in DMEM media supplemented with 10% FBS. Mycoplasma testing was performed to confirm the absence of contamination. STR profiling was performed to confirm the genuineness of the cell lines. Total RNA was isolated using the mirVana kit (Life Technologies) according to the recommended protocol.
Processing of saliva samples
Patient saliva samples were previously collected for COVID-19 diagnosis at a UIC clinical lab. Patient inclusion criteria: adults of age 18 years or older; exclusion criteria: children. In addition, we also analyzed pooled saliva samples from healthy individuals (Innovative Research). Saliva samples (50-100 µL) were first diluted with 0.7X volume of TE buffer (10 mM Tris, 1 mM EDTA pH 8.0). Then, 1X RNAsecure (25X, ThermoFisher) and 50 units/mL protease K (800 units/ml, NEB) were added to make 1:1 dilution of the saliva sample. The reaction mixture was incubated at room temperature (20-25°C) for 10 min, 65°C for 10 min, and finally 100°C for 12 min to inactivate protease K. For spike-in experiments, heat-inactivated SAR-CoV-2 particles were added to the saliva before TE dilution. For patient saliva samples (n=20), they were first heated at 65°C for 30 min to inactivate the SARS-CoV-2 virus, and then further processed as described above. For each saliva sample, an aliquot (300 µL) was used for RNA isolation with the MagMAX Viral/Pathogen Nucleic Acid Isolation Kit (A42352, Applied Biosystems), followed by RT-qPCR.
Sequencing of LAMP products
DNA sequencing libraries were prepared by PCR amplification of LAMP or RT-LAMP products with modified universal primers for Illumina sequencing. The indexed primers contain the P5 or P7 sequence for Illumina sequencing at the 5’-end, a 9-bp index sequence in the middle, followed by an adaptor sequence and a 3’-end F2 or B2 sequence specific to the LAMP assay. The PCR reaction was set up by mixing 1 µL of diluted LAMP product (100-fold), 1 µL of primer mix (10 µM), 12.5 µL of 2X PCR master mix (Tag Plus Master Mix Red, Lamda Biotech), and 10.5 µL of water. The PCR was performed at 94°C for 10 s, followed by 35 cycles of amplification (94°C for 10 s and 60°C for 45 s), and then 72°C for 1 min. The PCR products were purified with Ampure beads (Agencourt) and then quantified with QuantiFluor (Promega). The library samples were sequenced on MiniSeq (Illumina). The sequencing reads were aligned to the SARS-CoV-2 genome to identify virus-specific sequences. In addition, BLAST was performed to identify any sequence match to the NCBI GenBank database.
Statistical analysis
Data were analyzed using GraphPad Prism software. Summary statistics on the TT values are presented as mean ± standard error of the mean.
Ethic statement
The study was approved by the Institutional Review Board of the University of Illinois Chicago (2021-0914).
Role of the funding source
This study was funded by the National Institutes of Health, and designed by study investigators.
The funder had no role in study design, data collection, data analysis, interpretation, or writing of this report.
Results
Optimization of the primer length to improve LAMP efficiency
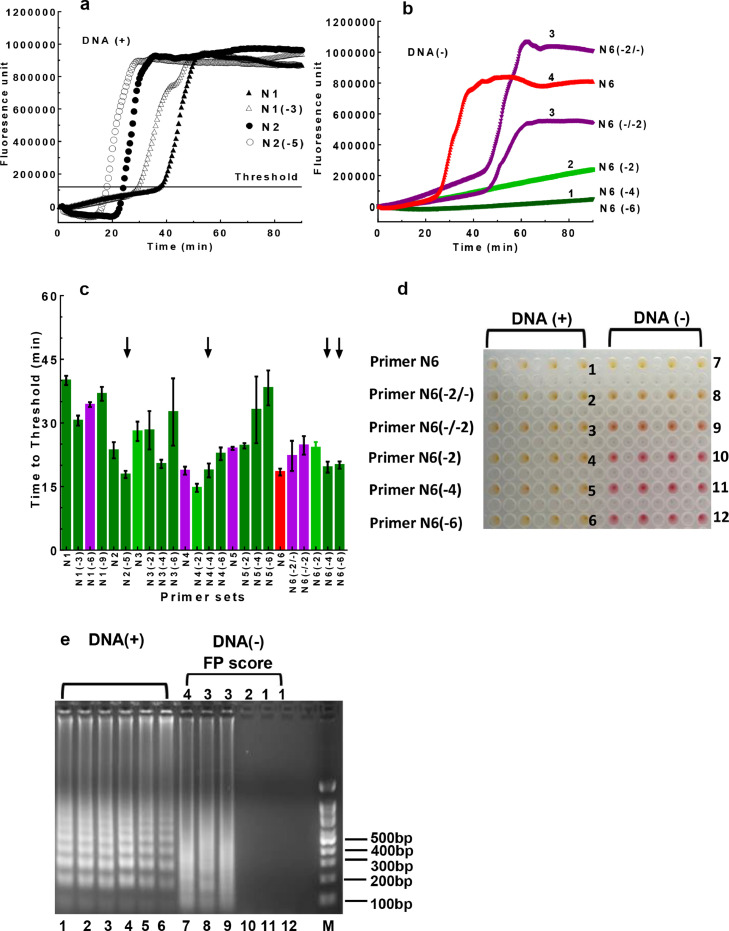
One major goal of our study was to improve the efficacy of LAMP assays for SARS-CoV-2 detection. During the LAMP reaction, the amplification stage is initiated with the formation of a dumbbell structure, in which F1/F1c (or B1/B1c) and F2 (or B2) form the stem and loop, respectively. As the first step, two long inner primers, FIP (F1c+F2) and BIP (B1c+B2) are annealed to the target to initiate the LAMP reaction. In general, long oligo primers have relatively poor annealing efficiency due to the potential formation of secondary structures. The poor annealing efficiency of long oligo primers has been extensively documented in numerous PCR studies.20 Thus, we reasoned that the efficacy of LAMP assays could be improved by shortening the FIP and BIP primers. To this end, we determined the optimal length of the stem region (F1c or B1c) within the primers. As shown in Table 1, we first designed N1(-3), and N2(-5) assays, which had shortened stems (as compared to standard design) to target the N gene of SARS-CoV-2. The shortening of the stems resulted in significantly improved TT values in comparison to that of original N1 and N2 assays (Fig. 1a). Thus, it was clear that standard LAMP design guidelines for the dumbbell structure, as recommended by previous studies,4,21 should be further optimized.
Table 1.
Effects of shortening the loop stem (F1c and B1c) on amplification efficiency and specificity.
| Primers | F1c 5’ | F1c | Length(n.t.) | Tm (°C) | B1c 5’ | B1c | Length(n.t.) | Tm (°C) | TT (min) | FP |
|---|---|---|---|---|---|---|---|---|---|---|
| N1 | ctctgctcccttctgcgtaga | 21 | 63.0 | tgatgctgctcttgctttgctg | 22 | 60.4 | 40.0±1.1 | 2 | ||
| N1(-3) | (-3) | tgctcccttctgcgtaga | 18 | 59.7 | (-4) | gctgctcttgctttgctg | 18 | 59.5 | 30.5±1.2 | 2 |
| N1(-6) | (-6) | tcccttctgcgtaga | 15 | 51.7 | (-7) | gctcttgctttgctg | 15 | 51.9 | 34.3±0.6 | 3 |
| N1(-9) | (-9) | cttctgcgtaga | 12 | 39.4 | (-10) | cttgctttgctg | 12 | 41.1 | 36.9±1.6 | 2 |
| N2 | attcaaggctccctcagttgc | 21 | 62.4 | atcacattggcacccgcaatc | 21 | 63.6 | 23.6±1.9 | 1 | ||
| N2(-5) | (-5) | aggctccctcagttgc | 16 | 57.7 | (-5) | attggcacccgcaatc | 16 | 57.1 | 17.9±0.8 | 1 |
| N3 | cagtattattgggtaaacctt | 21 | 52.5 | atggcaaggaagacct | 16 | 53.6 | 28.0±2.3 | 2 | ||
| N3(-2) | (-2) | gtattattgggtaaacctt | 19 | 48.8 | (-2) | ggcaaggaagacct | 14 | 49.8 | 28.3±4.5 | 1 |
| N3(-4) | (-4) | attattgggtaaacctt | 17 | 46.0 | (-4) | caaggaagacct | 12 | 38.8 | 20.3±1.0 | 1 |
| N3(-6) | (-6) | tattgggtaaacctt | 15 | 42.8 | (-6) | aggaagacct | 10 | 29.7 | 32.6±7.9 | 1 |
| N4 | ggtgccaatgtgatctt | 17 | 53.9 | ctgctaacaatgctgc | 16 | 52.4 | 18.7±0.9 | 3 | ||
| N4(-2) | (-2) | tgccaatgtgatctt | 15 | 47.9 | (-2) | gctaacaatgctgc | 14 | 47.7 | 14.7±1.0 | 2 |
| N4(-4) | (-4) | ccaatgtgatctt | 13 | 38.8 | (-4) | taacaatgctgc | 12 | 38.7 | 18.8±1.6 | 1 |
| N4(-6) | (-6) | aatgtgatctt | 11 | 27.5 | (-6) | acaatgctgc | 10 | 34.7 | 22.7±1.5 | 1 |
| N5 | ctcccttctgcgtaga | 16 | 53.6 | acgtagtcgcaacag | 15 | 52.3 | 24.0±0.1 | 3 | ||
| N5(-2) | (-2) | cccttctgcgtaga | 14 | 49.3 | (-2) | gtagtcgcaacag | 13 | 44.6 | 24.6±0.6 | 1 |
| N5(-4) | (-4) | cttctgcgtaga | 12 | 39.8 | (-4) | agtcgcaacag | 11 | 39.8 | 33.1±7.9 | 1 |
| N5(-6) | (-6) | tctgcgtaga | 10 | 32.2 | (-6) | tcgcaacag | 9 | 29.9 | 38.3±4.1 | 1 |
| N6 | ctgcctggagttgaat | 16 | 52.3 | cctgctagaatggctg | 16 | 53.1 | 18.4±0.8 | 4 | ||
| N6(-2/-) | (-2) | gcctggagttgaat | 14 | 47.3 | cctgctagaatggctg | 16 | 53.1 | 22.2±3.6 | 3 | |
| N6(-/-2) | ctgcctggagttgaat | 16 | 52.3 | (-2) | tgctagaatggctg | 14 | 47.1 | 24.7±2.2 | 3 | |
| N6(-2) | (-2) | gcctggagttgaat | 14 | 47.3 | (-2) | tgctagaatggctg | 14 | 47.1 | 24.1±2.0 | 2 |
| N6(-4) | (-4) | ctggagttgaat | 12 | 35.9 | (-4) | ctagaatggctg | 12 | 37.3 | 19.6±1.3 | 1 |
| N6(-6) | (-6) | ggagttgaat | 10 | 25.8 | (-6) | agaatggctg | 10 | 31.0 | 20.1±0.9 | 1 |
Note: LAMP assays (10 µL reaction) were performed as described in “Methods”. The DNA plasmid containing the N gene of SARS-CoV-2 was used as template (2000 cp/reaction, 3-10 replicates). In the control reaction (3-4 replicates), no template DNA was added. The TT values (Time to Threshold) were calculated from the replicated reactions and presented as mean±SD. The FP (false-positive) scores were calculated as described in Fig.1 and in the main text. For each primer set, an FP score was calculated for each control replicate, and the highest FP score from all replicates was selected and presented in the table.
Fig. 1.
Assessing the sensitivity and specificity of the LAMP assays. DNA plasmid containing the N gene of SARS-CoV-2 (2,000 cp/rxn) was used as template for LAMP amplification. a. Amplification plots of LAMP reactions. Each curve represents an average of 3 independent measurements. b. False-positive signals produced by the N6 and N6-derived assays. The LAMP assays were performed in the absence of the N gene template. The false-positive (FP) signals were evaluated using the FP score (score range 1-4). 1) Dark green: no or minimal fluorescence signals at endpoint; 2) light green: low but detectable signals at endpoint; 3) purple: signals detected during late stage of the reaction (45-90 min); 4) red: signals detected during early stage of the reaction (< 45 min). c.The TT values and FP scores of the assays targeting the N gene. The same color scheme in b was used to assess false-positive signals as determined from no-template control reactions. Primer sets N1 to N6 as well as their derived assays, which contained identical F2, B2, FL, BL, F3, and B3 primers but shortened F1c/B1c primers, were assessed. The arrows indicated selected assays with best overall performance in LAMP efficacy and specificity. d. Colorimetric changes of the reactions using the N6 and N6-derived primer sets. Left panel: with DNA template included; right panel: no DNA template. e. Gel electrophoresis analysis of the LAMP products. The same LAMP products as described in d were analyzed on DNA agarose gel. On-target products showed typical DNA ladders (lanes 1-6) while non-specific products with high FP scores showed DNA smears (lanes 7-9). rxn: reaction.
To comprehensively determine the optimal length of the stem region, we further designed twenty primer sets with variable F1c and B1c lengths (derived from the N1 to N6 assays). The testing results and the sequences of these primer sets are summarized in Table 1 and Supplementary Table S1, respectively. Both the length and melting temperature (Tm) of the stem region were important determinants of assay efficiency. While stem shortening helped improve TT values as compared to the standard design, excessive further shortening of the stem region was detrimental as a result of significantly lowered Tm value. Based on these 24 LAMP primer sets, observed optimal stem length was in the range of 12-17 bp with Tm >45°C.
Assessment of non-specific LAMP products
Besides amplification efficiency (as represented by TT), another major consideration of LAMP performance is the specificity of the assays. False-positive products are commonly observed in LAMP reactions even in the absence of nucleic acid targets, as reported by numerous studies.11,15, 16, 17, 18 For example, multiple LAMP assays derived from the N6 assay (Table 1) showed various levels of false-positive readout in the absence of the target template (Fig. 1b). Thus, it is crucial to design primer sets with low cross-reactivity to avoid false-positive amplification. To this end, we categorized potential false-positive (FP) reactions into four levels (FP scores 1-4) during primer screening, with representative examples listed in Fig. 1b: 1) FP score of 1, no or minimal fluorescence signals at the endpoint, as represented by N6(-4) and N6(-6) assays; 2) FP score of 2, low but detectable fluorescence signals at the endpoint, as represented by N6(-2); 3) FP score of 3, high fluorescence signals detected during the late stage of the reaction (45-90 min), as represented by N6(-2/-) and N6(-/-2); 4) FP score of 4, false amplification observed during the early stage of the reaction (<45 min), as represented by N6. By employing this color-coded scoring scheme, we were able to concurrently evaluate both the efficiency and specificity of the LAMP assays (Fig. 1c).
LAMP assays with various FP scores had distinct colorimetric readouts and electrophoresis patterns in the absence of target DNA (Fig. 1d & e). Specifically, for assays with an FP score of 1 or 2, no colorimetric change was observed (Fig. 1d, pink wells in the right section for N6(-2), N6(-4), and N6(-6) assays). In addition, no amplification product was observed by gel electrophoresis (Fig. 1e, lanes 10-12). For assays with a score of 3 or 4, non-specific products were observed by both colorimetric changes Fig. 1d, yellow or orange wells in the right section for N6, N6 (-2/-), and N6(-/-2)] and gel electrophoresis (Fig. 1e, lanes 7-9). Interestingly, the size distribution of non-specific products was distinctively different from that of on-target products (Fig. 1e lanes 1-6 vs. 7-9). To maximize assay specificity, in our study, only primer sets with an FP score of 1 were further evaluated with the goal of developing robust assays for SARS-CoV-2 diagnosis.
To understand the mechanism underlying the false-positive reactions, we further analyzed non-specific products from the N6 assay. The N6 primer set was prone to false-positive reaction, having an FP score of 4. Further analysis of the non-specific products by electrophoresis showed that false-positive reaction happened in an FIP/BIP dependent manner (Supplementary Fig. S1a). The false-positive reactions could occur even in the absence of FL, BL, F3, and B3 primers (lanes 5-8, FP 3-4), but not in the absence of FIP/BIP primers (lanes 9-12). Thus, it was clear that the FIP/BIP primers in the N6 assay were responsible for the false-positive products. We further characterized the false-positive products by high-throughput sequencing. To this end, PCR primers with F2 or B2 sequence at the 3’-end were used to amplify non-specific LAMP products from the N6 assay (Supplementary Table S2). Sequencing showed that both FIP and BIP primers were incorporated into the non-specific products, with new linker sequences (1-5 bp) inserted between FIP and BIP (Supplementary Fig. S1b). These results support a model that tandem linkage of FIP and BIP is responsible for the false-positive LAMP reactions (Supplementary Fig. S1c).
An improved bioinformatics algorithm for LAMP assay design
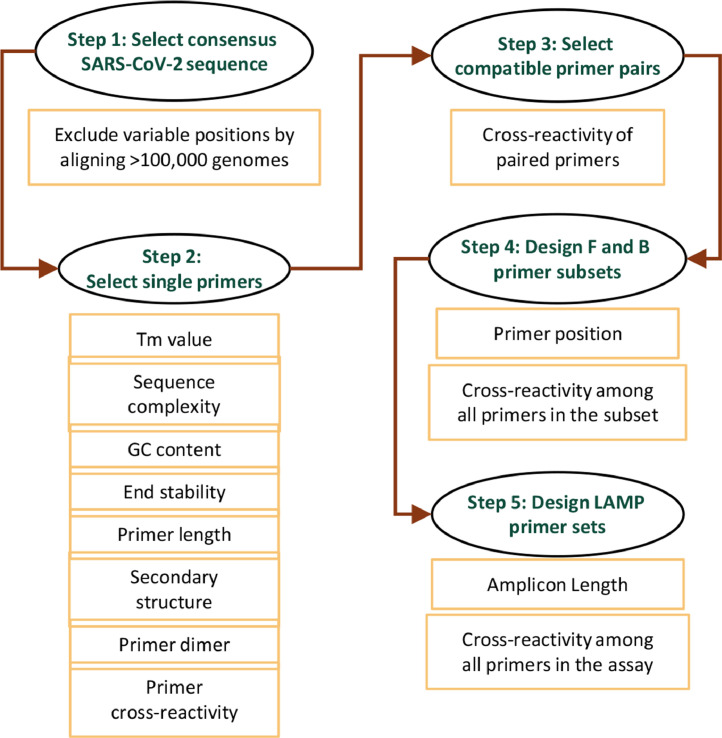
Based on the above experimental data for assay optimization, we developed an improved bioinformatics algorithm for designing LAMP primers for SARS-CoV-2 diagnosis. This algorithm incorporated many design features that were proven to be important for DNA amplification in our previous study.22, 23, 24 In addition, the new algorithm also included novel features that are unique for LAMP assays. The algorithm was implemented into a Perl bioinformatics tool running under Linux environment (available for download at GitHub). As summarized in Fig. 2, we first downloaded all 106,439 SARS-CoV-2 genome sequences submitted to the GISAID database (https://www.gisaid.org) as of September, 2020. These genome sequences were aligned to identify consensus viral genome sequence. In our assays, we excluded target regions that contain any variable nucleotide (<98% consensus).
Fig. 2.
Workflow of the LAMP primer design algorithm.
Next, single primer candidates were selected from the target region, and a primer candidate would be discarded if any one of the following criteria was not met. The Tm value of the primer was in the range of 60-64°C, except for F1c/B1c candidates (Tm >45°C). All Tm values were calculated using the Nearest Neighbor method.25,26 To avoid potential primer cross-reactivity due to low sequence complexity, a stretch of continuous same nucleotides was not allowed (4 C's, 4 G's, 5 A's, or 5 T's). To further exclude sequences of low complexity, the DUST program27 was employed, and any sequence identified by DUST was rejected. In addition, the GC content of the primer was in the range of 35-65% to ensure uniform priming. The 3’-end of the primer contributes most to non-specific primer extension, especially if the binding of these nucleotides is relatively stable.28 Thus, the ΔG value of five residues at the 3’-end was calculated, and a threshold value of -8 kcal/mol was used for sequence rejection. The design algorithm further assessed potential secondary structures, which could hinder primer annealing to the template, leading to reduced amplification efficiency; primer secondary structures could also result in non-specific LAMP products by initiating unintended mispriming events. In our design, we implemented multiple secondary structure filters for primer screening as we described previously.22, 23, 24 In addition, the Mfold program29 was employed to exclude primer candidates with a low ΔG value of predicted secondary structure.
After single primer candidates were selected, the design algorithm then chose compatible primer pairs that show no cross-reactivity based on sequence match.22 In particular, more stringent selection filters were applied to the end residues of paired primers to prevent the formation of primer heterodimers. Based on compatible primer pairs, the F and B primer subsets were selected, which were further assembled into a six-primer set for the LAMP assay. F1c and F2 were combined into the forward annealing primer (FIP); similarly, B1c and B2 were combined into the reverse annealing primer (BIP). Unintended primer cross-reactivity is a major challenge for DNA amplification, especially when multiple primers are included in the same reaction. To alleviate this concern, our algorithm includes filters to evaluate potential primer dimer formation or other mispriming events from all possible primer pairs among the six primers in the assay. Details of these cross-reaction filters were described previously.22, 23, 24 Moreover, restraints were placed on the relative positions of each primer in the assay for efficient LAMP amplification (Supplementary Fig. S2).
Experimental validation of the RT-LAMP assays for SARS-CoV-2 RNA detection
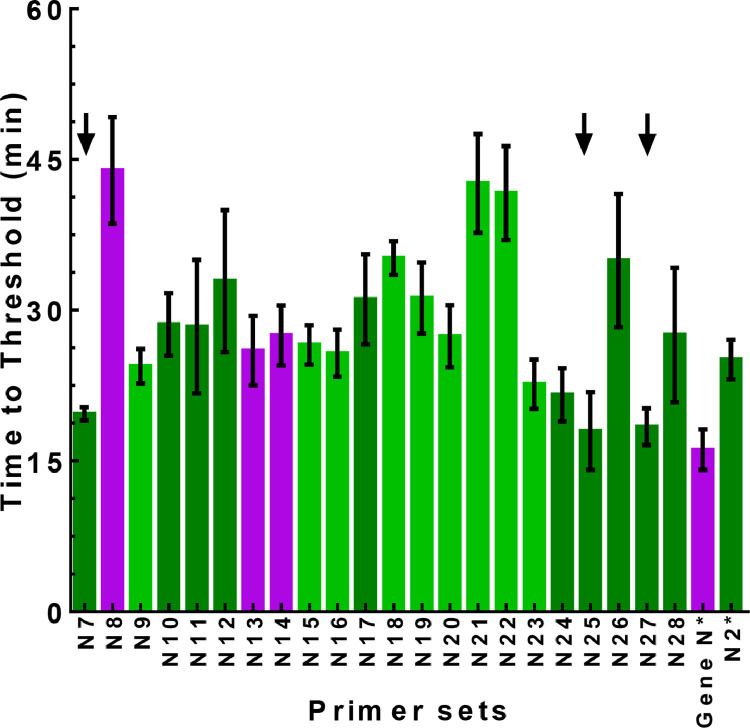
The algorithm described above was used to design LAMP primers for SARS-CoV-2 diagnosis. As summarized in Fig. 3 and Supplementary Table S1, we tested the performance of 22 newly designed primer sets (N7-N28), as evaluated by both the TT value and FP score, to target the N gene sequence in a DNA plasmid. Most of these assays (n=19) had low FP scores (<=2), indicating high specificity of the LAMP reaction. Moreover, the majority of the assays (n=14) were able to detect the target with high efficiency, as demonstrated by low TT values (<30 min). In particular, N7, N25, and N27 assays had the best overall performance, with both TT value less than 20 min and FP score of 1. Thus, these three assays were further evaluated for their diagnostic potential using the SARS-CoV-2 RNA genome as the target. We also evaluated the performance of two recently published RT-LAMP primer sets used in several publications.11,30, 31, 32 However, we found that one published primer set, Gene N30 (denoted as Gene N* in Fig. 3), had an FP score of 3, indicating a relatively high level of non-specific amplification. Another primer set, N2 (denoted as N2* in Fig. 3),30 had a higher TT value than N7, N5, and N27, indicating lower amplification efficiency.
Fig. 3.
LAMP validation of designed primer sets targeting the N gene of SARS-CoV-2. The plasmid containing the N gene of SARS-CoV-2 (2,000 cp/rxn) was used as template for the LAMP reactions. The same color scheme as descried in Fig. 1b was used to represent the FP score. The arrows indicated selected LAMP assays with best overall performance in efficacy and specificity. Two published primer sets, Gene N and N2, denoted as Gene N* and N2* in the figure, respectively, were also included.
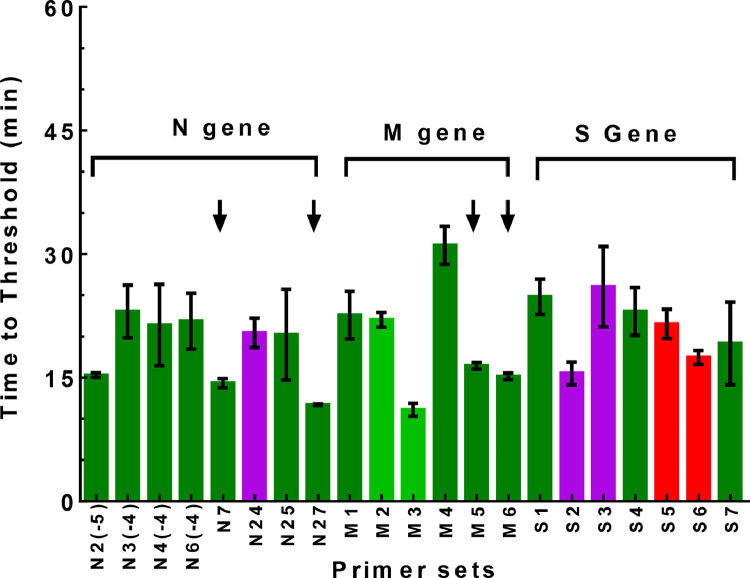
Besides primer sets for the N gene, we also designed and further tested six primer sets for the M gene and seven primer sets for the S gene, respectively, using SARS-CoV-2 RNA as the target (Fig. 4 and Supplementary Tables S3, S4 & S5). All the M gene assays were highly efficient at detecting SARS-CoV-2 RNA (TT value close to or below 30 min). Among them, M5 and M6 assays had the best overall performance (TT value close to or below 15 min with FP score of 1). Thus, these two assays were selected for further evaluation. As for the S gene assays, they had similar efficiency as compared to the M gene assays, but with elevated levels of non-specific amplification. Among them, S1, S4, and S7 assays had no observed non-specific products, with an FP score of 1. In the same way, we also tested selected N gene assays by RT-LAMP for viral RNA detection. Altogether, we identified four primer sets, N7, N27, M5, and M6, which had the best overall performance for both amplification efficiency and specificity (Fig. 4).
Fig. 4.
RT-LAMP validation of designed primer sets targeting the N, M, and S genes of SARS-CoV-2. SARS-CoV-2 RNA (2,000 cp/rxn) was used as template for the RT-LAMP reactions. The same color scheme as descried in Fig. 1b was used to represent the FP score. The arrows indicated selected RT-LAMP assays with best overall performance in efficacy and specificity.
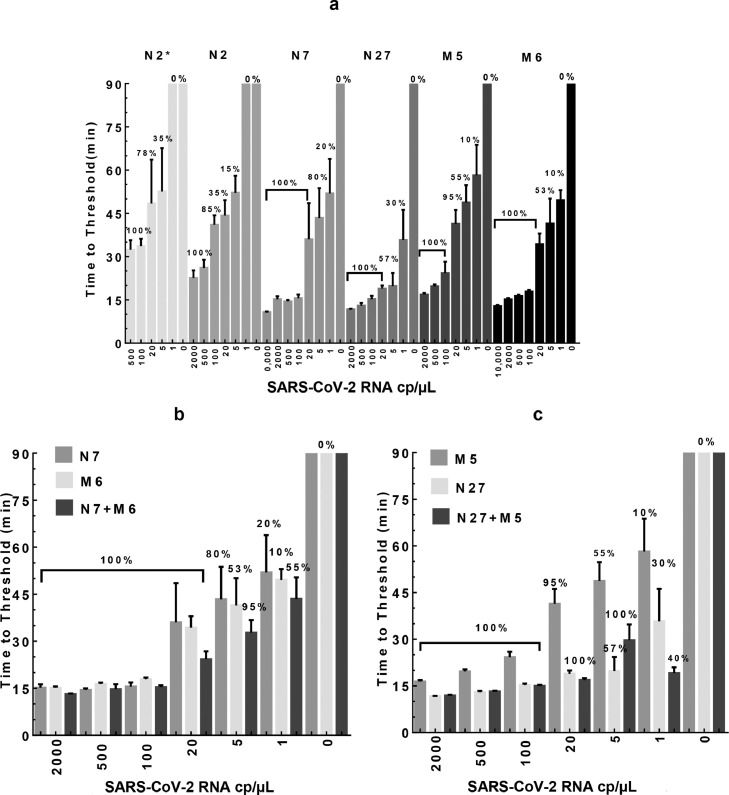
We further tested these four selected assays along with the published N2* assay in detection sensitivity by serial dilution of SARS-CoV-2 RNA. In addition, we also included the N2 primer set (shown in Fig. 1a), which was designed based on standard LAMP primer design principles (i.e., not including the new design features identified in this study). The RT-LAMP results (Fig. 5a) showed a dose-dependent increase in TT values (from 11 to 65 min) with decreasing amounts of viral RNA in the reactions (from 10,000 to 1 cp/µL, i.e.,100,000 cp/rxn to 10 cp/rxn). Except for N2, the other five assays could reliably detect 100 cp/µL (1,000 cp/rxn) of viral RNA in about 20-30 min or less (100% positivity). Interestingly, when the RNA amount was further diluted down to 20 cp/µL (200 cp/rxn), N7, N27, and M6 assays maintained a 100% positive detection rate with the TT of 19-36 min. Further, when 5 cp/µL (50 cp/rxn) of RNA was tested, N7 assay had a positive rate of 80% (average TT of 43 min); N27 had a rate of 40% (average TT of 19 min); M5 had a rate of 55% (average TT of 49 min); and M6 had a rate of 53% (average TT of 41 min). When only 1 cp/µL (10 cp/rxn) of RNA was added to the reaction (based on serial dilution), the positive rate of viral detection was much lower for all these assays, ranging from 0-20%.
Fig. 5.
Sensitivity of the RT-LAMP assays in detecting SARS-CoV-2 RNA. SARS-CoV-2 RNA was diluted in water and added to the reactions as template (0-10,000 cp/µL, i.e., 0-100,000 cp/rxn). The TT values were determined by averaging 10-20 independent measurements (n=10-15 for 20-2000 cp/µL; n=20 for 0-5 cp/µL) that came from 3-4 independent master mixes and presented as mean±SD. The percentage of positive reactions among all replicated reactions for each assay was presented (multiplexed assay results also summarized in Table 2). a. single primer sets, N2*, N2, N7, and N27 for the N gene, as well as M5 and M6 for the M gene. b. Multiplexed N7+M6 assay vs. N7 or M6 assay alone. c. Multiplexed N27+M5 assay vs. N27 or M5 assay alone.
Multiplexed RT-LAMP to further improve assay sensitivity
We next sought to determine whether RT-LAMP sensitivity could be further improved when assays were combined for simultaneous detection of multiple genes. Among the validated assays, N7/N27 and M5/M6 assays were designed to target the N gene and M gene, respectively. Thus, we established two multiplexed assays based on N7+M6 and N27+M5, respectively. Both assays had no detectable background (FP score of 1). Notably, as shown in Fig. 5 b & c, the efficiency and sensitivity of the multiplexed assays (as evaluated by TT and detection rate) were significantly improved over any single assay alone. Specifically, the N7/M6 assay could reliably detect 5 cp/µL (50 cp/rxn) of viral RNA in about 33 min (with a detection rate of 95% vs. 80% for N7 and 53% for M6, as shown in Fig. 5B and Table 2). Similarly, N27/M5 assay could detect 5 cp/µL (50 cp/rxn) of RNA in 30 min (detection rate of 100% vs. 55% for M5 and 57% for N27, Fig. 5C and Table 2). Most noticeably, with 1 cp/µL (10cp/rxn) of RNA (based on serial dilution), both multiplexed assays had significantly improved detection rate compared with the single assays (40-55% detection rates for the multiplexed assays vs. 10-30% for the single assays).
Table 2.
Limit of Detection (LOD) analysis of the RT-LAMP assays.
| SARS-CoV-2 RNA with N7/M6 assay (10 µL reaction) | ||||||||
|---|---|---|---|---|---|---|---|---|
| In RT-LAMP reactions |
Diluted in water |
Mixed withHeLa RNA |
Mixed withpretreated saliva |
cp/µL in undiluted saliva | ||||
| RNA |
Positive/Replicates | Sensitivity | Positive/Replicates | Sensitivity | Positive/Replicates | Sensitivity | ||
| cp/µL | cp/rxn | |||||||
| 2,000 | 20,000 | 10/10 | 100% | 10/10 | 100% | 5/5 | 100% | 80,000 |
| 500 | 5,000 | 1010 | 100% | 10/10 | 100% | 5/5 | 100% | 20,000 |
| 100 | 1,000 | 10/10 | 100% | 10/10 | 100% | 5/5 | 100% | 4,000 |
| 20 | 200 | 20/20 | 100% | 20/20 | 100% | 30/30 | 100% | 800 |
| 5 | 50 | 19/20 | 95% | 20/20 | 100% | 24/30 | 80% | 200 |
| 1 | 10 | 11/20 | 55% | 16/20 | 80% | 15/30 | 50% | 40 |
| 0 | 0 | 0/20 | - | 0/20 | - | 0/30 | - | 0 |
| SARS-CoV-2 RNA with N27/M5 assay (10 µL reaction) | ||||||||
|---|---|---|---|---|---|---|---|---|
| RNA |
Diluted in water |
Mixed withHeLa RNA |
Mixed withpretreated saliva |
cp/µL in undiluted saliva | ||||
| cp/µL | cp/rxn | Positive/Replicates | Sensitivity | Positive/Replicates | Sensitivity | Positive/Replicates | Sensitivity | |
| 2,000 | 20,000 | 10/10 | 100% | 10/10 | 100% | 10/10 | 100% | 80,000 |
| 500 | 5,000 | 10/10 | 100% | 10/10 | 100% | 10/10 | 100% | 20,000 |
| 100 | 1,000 | 10/10 | 100% | 10/10 | 100% | 10/10 | 100% | 4,000 |
| 20 | 200 | 20/20 | 100% | 20/20 | 100% | 20/20 | 100% | 800 |
| 5 | 50 | 20/20 | 100% | 20/20 | 100% | 19/20 | 95% | 200 |
| 1 | 10 | 8/20 | 40% | 16/20 | 80% | 14/20 | 70% | 40 |
| 0 | 0 | 0/20 | - | 0/20 | - | 0/20 | - | 0 |
| Saliva spiked with SARS-CoV-2 particles (120 µL reaction) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Virus |
N27/M5 assay |
|||||||
| cp/µL | cp/rxn | Positive/Replicates | Sensitivity | cp/µL in undiluted saliva | ||||
| 0.11 | 13.5 | 22/22 | 100% | 1.5 | ||||
| 0.08 | 9 | 18/20 | 90% | 1.0 | ||||
| 0 | 0 | 0/26 | - | 0 | ||||
Note: the highlighted rows indicate threshold reactions to determine the LOD values of the respective assays. rxn, reaction.
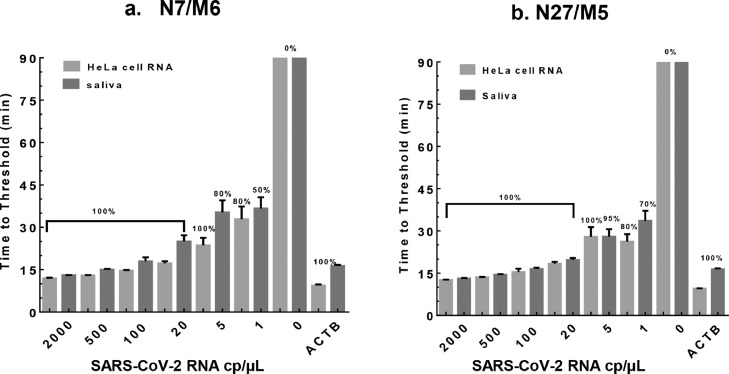
For clinical diagnosis, the human samples (e.g., saliva) to be tested contain transcripts of both virus and human origin. To evaluate potential assay cross-reaction to human RNA, we spiked in various amounts of SARS-CoV-2 genomic RNA into total RNA (10 ng/µL) from HeLa cells cells or SiHa cells or pretreated human saliva. Consistent with the data presented in Fig. 5 b & c, the RT-LAMP results showed that multiplexed N7/M6 and N27/M5 assays were efficient and sensitive at detecting as few as 5 cp/µL (50 cp/rxn) of viral RNA (100% detection rate with TT <30 min, Fig. 6 a & b) mixed with HeLa RNA. When only 1 cp/µL (10 cp/rxn) of viral RNA was tested, the detection rates of the multiplexed assays were ∼80% with the TT around 30 min. For pretreated saliva samples spiked with SARS-CoV-2 RNA, both assays showed similar but slightly decreased performance in sensitivity and efficiency compared with HeLa RNA (Fig. 6 a & b). Importantly, the reactions produced negative results (i.e., 0% detection rate) when viral RNA was omitted, indicating no cross-reactivity of the assays to HeLa/SiHa RNA or saliva RNA (data not shown for SiHa and Saliva RNA). As a positive control, we also performed RT-LAMP to target Beta-actin (ACTB) RNA in HeLa cells and human saliva (Supplementary Table S7)30, and the result indicated high efficacy of detection (TT ∼10 min for HeLa RNA and ∼16 min for saliva RNA, respectively).
Fig. 6.
The sensitivity and specificity of two multiplexed assays in detecting SARS-CoV-2 RNA mixed with HeLa RNA or human saliva. SARS-CoV-2 RNA (0-2,000 cp/µL) was mixed with HeLa cell RNA (10 ng/µL) or pretreated healthy human saliva. The TT values were determined by averaging 10-30 independent measurements and presented as mean±SD. Beta-actin (ACTB) RT-LAMP assay was used as positive control for human RNA detection. The numbers of replicates were: 100-2,000 cp/µL (i.e., 1,000-20,000 cp/rxn), n=5-10; 0-20 cp/µL (i.e.,0-200 cp/rxn), n=20-30. a. Validation of the N7/M6 assay. b. Validation of the N27/M5 assay.
Further optimization of the RT-LAMP assays for direct detection of SARS-CoV-2 in saliva
With the limit of detection (LOD, defined as ≥95% detection rate) at 5 cp/µL (50 cp/rxn) of viral RNA in the RT-LAMP reaction (Tables 2), the N7/M6 and N27/M5 assays were highly efficient and specific when SARS-CoV-2 RNA was diluted with water or HeLa RNA. However, the LODs were relatively low for SARS-CoV-2 detection in saliva samples mainly due to the limited amount (0.5 µL) of pretreated saliva being added to the RT-LAMP mixture as well as inhibitory effects of saliva components on the reactions (the LODs at 800 cp/µL and 200 cp/µL of viral RNA in undiluted saliva for N7/M6 and N27/M5 assays, respectively; Tables 2).
The N27/M5 assay showed generally better sensitivity in above-mentioned tests than the N7/M6 assay (based on observed positivity rate and amplification efficiency as shown in Table 2 and Figs. 5 & 6). To further improve the sensitivity for saliva testing, we increased the RT-LAMP reaction volume from 10 µL to 120 µL, such that more saliva could be added to the reaction. In addition, the amount of pretreated saliva being added to the reaction was optimized to 18 µL according to a recent publication 32 as well as our testing data. Moreover, we observed that HPLC purification of the FIP and BIP primers further boosted the efficiency of RT-LAMP (data not shown). With this new assay format, we evaluated the N27/M5 assay performance on SARS-CoV-2 detection based on either colorimetric (Supplementary Fig. S3a) or fluorescent readouts (Supplementary Fig. S3b). The reactions were completed within 50-60 min when tested on saliva samples spiked with 1 cp/µL SARS-CoV-2 (Supplementary Fig. S3b). The LOD of the 120 µL reaction was determined to be 0.11 cp/µL or 13.5 cp/rxn, as shown in Table 2 and supplementary Fig. S 3a & c. This was equivalent to an LOD of 1.5 cp/µL SARS-CoV-2 in undiluted saliva samples before being processed for RT-LAMP testing. In comparison, no colorimetric change or fluorescent signal was observed from any of the negative control reaction (with no spiked-in virus in the saliva). Moreover, the larger reaction volume (120 µL) also made it easier to observe colorimetric changes even by inexperienced users.
Evaluation of assay specificity using common human respiratory viruses
We further evaluated potential cross-reactivity of the N27/M5 assay using genomic RNA samples from five common respiratory viruses, including human coronavirus 229E (ATCC VR-740D), human coronavirus OC43 (ATCC VR-1558D), influenza A virus (H1N1, ATCC VR-1682D), human respiratory syncytial virus (ATCC VR-2455), and human rhinovirus 16 (ATCC VR-283DQ). The presence of viral RNA was confirmed by RT-qPCR (Ct values ranging between 14∼23) using primers specific to each virus genomic RNA (Supplementary Table S8). As shown in Supplementary Fig. S4, N27/M5 RT-LAMP showed no cross-reactivity with any of these virus genomic RNAs.
Performance comparison of the RT-LAMP assays using patient saliva samples
To evaluate the clinical utility of the RT-LAMP assays, we compared two alternative methods for SARS-CoV-2 diagnosis with saliva samples. 1) RT-LAMP directly using saliva; 2) RT-qPCR using RNA isolated from saliva. Currently, RT-qPCR is the most widely used method in the clinic for SARS-CoV-2 diagnosis mainly due to its high detection sensitivity and specificity. We evaluated a commonly used RT-qPCR assay, E-Sarbeco, which is recommended for COVID-19 diagnosis by the WHO and CDC.33 Our validation data indicated that the E-Sarbeco assay had an LOD of 0.5 cp/µL (i.e., 14 cp/rxn) of SARS-CoV-2 RNA, while producing no false positive signals from the negative control (Ct >40). This result is consistent with previously reported data on the E-Sarbeco assay and is also in the same LOD range with other RT-qPCR assays for COVID-19 diagnosis.3,19,34
We validated the N27/M5 RT-LAMP assay using twenty clinical saliva samples originally acquired for COVID-19 diagnosis. As reference control, the E-Sarbeco assay was also included for testing of saliva RNA. Out of the twenty cases, nine were tested positive (Fig. 7, patients #4, 22, 23, 27, 31, 1, 9, 10 and 26), and ten were tested negative (Fig. 7, patients #11, 12, 13,14, 17, 15, 18, 19, 20, and 8) by both the RT-LAMP and RT-qPCR assays. The viral concentrations in these positive samples varied greatly, as indicated by a broad range of Ct values (23.6-38.5) from RT-qPCR. Interestingly, one patient (#16) was tested negative by RT-qPCR but positive by the RT-LAMP. Electrophoresis analysis (not shown) and High-throughput sequencing of the resultant RT-LAMP products confirmed specific amplicon sequences from SARS-CoV-2 (Supplementary Table S6).
Fig. 7.
Validation of the RT-LAMP assays using clinical saliva samples containing the SARS-CoV-2 virus. RNA isolation, saliva treatment, RT-LAMP, and RT-qPCR were performed as described in Methods. RT-LAMP was performed on heat-inactivated saliva. An RT-qPCR assay was also included for performance comparison. Colorimetric results before and after the reactions (65°C for 1 h) were presented, with positive reactions determined by clearly visible color change from pink to orange or yellow. Each reaction was independently repeated 2-3 times with identical results as displayed here. *ND: not detected by RT-qPCR.
Discussion
RT-LAMP is an emerging technology for molecular diagnosis of human pathogens. Compared with RT-qPCR, the advantages of RT-LAMP assays are clear: 1) The reaction is fast; 2) the assay setup does not require any expensive instrument; and 3) the reaction is relatively insensitive to sample impurity, making it possible to directly test crude samples without RNA isolation. Thus, RT-LAMP with colorimetric readout is particularly attractive for point-of-care diagnosis of infectious pathogens. For example, multiple investigators have attempted to develop RT-LAMP assays for the diagnosis of COVID-19.32,35
Despite its potential as a powerful diagnostic tool, RT-LAMP has not been widely adopted in the clinical setting (in comparison of RT-qPCR). One major challenge is the design of robust LAMP primers. A LAMP assay includes a set of six primers, targeting eight regions of the nucleic acid sequence for amplification; thus, the primer design is complicated. Existing assay design methods often produce ineffective primer sets due to poor amplification efficiency or non-specific amplification. As a result, high-quality LAMP assays are commonly developed by trial and error based on experimental testing of many designed primer sets. Due to the major challenges in LAMP primer design, most LAMP studies for COVID-19 diagnosis adopted previously published primer sets that had suboptimal performance.
To address the aforementioned challenges, we developed an improved algorithm for LAMP primer design to target SARS-CoV-2. First, we optimized the length of F1c/B1c to significantly boost the amplification efficiency. F1c and B1c make up the loop stem of the dumbbell structure, which is formed at the beginning of the LAMP reaction and serves as template to initiate the amplification phase. Second, Next-generation sequencing demonstrated that false-positive reactions mainly resulted from unintended interactions between FIP and BIP, two longest primers that are required at much higher concentrations than other primers in the reaction. Thus, more stringent specificity filters should be applied to the design of FIP/BIP primers. Taking account of these LAMP-specific parameters as well as other common primer design criteria from our previous studies,22, 23, 24 we developed an improved bioinformatics algorithm for LAMP primer design. Our design method is highly effective at producing high-quality LAMP assays, as evidenced by the validation data for the primer sets targeting the N and M genes of SARS-CoV-2. However, for the S gene, it is still a challenge to design efficient primer sets, likely due to the higher GC content (58% for S vs.43% for M and 47% for N).
Saliva testing is an attractive method for COVID-19 diagnosis. 1) It is a non-invasive method for sample collection; 2) saliva can be collected by a non-medical care worker, thus reducing the risk of nosocomial transmission; and 3) saliva is relatively stable at a broad range of storage temperatures.36 Despite these advantages, there are also challenges facing COVID-19 saliva testing: 1) saliva has a broad range of virus loads (10-100,000 cp/µL);37 2) saliva contains many heterogeneous components, some of which are inhibitory to most diagnostic assays. As a result, RNA isolation is required for standard RT-qPCR methods for COVID-19 diagnosis with saliva samples.
The RT-LAMP assays we developed could directly detect the SARS-CoV-2 virus in saliva samples and were highly sensitive to cover a broad range of SARS-CoV-2 titers in patient saliva (10-100,000 cp/µL). Importantly, our RT-LAMP assays produced comparable diagnostic results to RT-qPCR when directly applied to patient saliva samples. Interestingly, SARS-CoV-2 loads in these saliva samples varied greatly based on the RT-qPCR results. As for very low virus loads, it could be a challenge to sensitively detect the virus, whether using RT-qPCR or RT-LAMP. However, this is not likely a major issue for COVID-19 screening; multiple studies showed that, when the virus load was below a certain level (<100-1,000 cp/µL in biofluid or Ct value >34), the patients are not likely to spread disease.38, 39, 40
Our RT-LAMP assays were fast as most reactions were completed within 30-50 min. Moreover, the assays could be easily adapted for point-of-care diagnosis with colorimetric readout under constant reaction temperature, or as a high-throughput screening assay in 96-well plate format. In term of recently emerged SARS-CoV-2 variants (Alpha, Beta, Delta, Gamma, Epsilon, Eta, and Kappa strains), most identified mutations are located in the S gene sequence,41,42 and thus our validated assays focusing on the N and M genes will not likely be compromised by these mutations. It is worth mentioning that SARS-CoV-2 is a relatively conserved RNA virus. Therefore, it is possible to design robust RT-LAMP assays to cover most variant strains. However, for highly mutated viruses, such as HIV-1, it could be a major challenge to design six LAMP primers to target conserved regions of the viral sequence. In that case, combining multiple designs into one multiplexed assay could be an option to alleviate this concern. Alternatively, a LAMP assay that is relatively tolerant to primer mismatch (e.g., inclusion of degenerate primers) could be designed. To this end, future experiments should be designed to determine the impact of primer mismatch on assay sensitivity and specificity, including both the number of nucleotide mismatches in one primer and the total number of mismatched primers.
In summary, we developed an improved algorithm for LAMP assay design, and further validated the assays targeting SARS-COV-2. The design algorithm as well as the validated highly specific and sensitive RT-LAMP assays provide powerful diagnostic tools to curb the pandemic of COVID-19.
Contributors
X.W. conceived and supervised the research. X.H. conducted the experiments. X.H. and G.T. performed the data analysis. N.I. helped on clinical validation of the methods. X.H., G.T., N.I., and X.W. interpreted the data and wrote the paper. The underlying data were verified by X.H., G.T., and X.W. All authors have read and approved the final version of the manuscript.
Declaration of Competing Interest
There are no conflicts of interest to declare.
Acknowledgments
Data sharing
Supplemental data are available at the journal's website. The sequencing data are available at NCBI GEO under accession GSE173086. The LAMP primer design tool is available for download at https://github.com/wang-lab/iPrimer.
Acknowledgments
This research was supported by grants from the National Institutes of Health (R01DE026471, R35GM141535, and R01GM089784 to X.W., R56HL149881 to X.H. and 3R01ES028615-06S1 to N.I.).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2021.103736.
Appendix. Supplementary materials
References
- 1.Phillips N. The coronavirus is here to stay - here’s what that means. Nature. 2021;590(7846):382–384. doi: 10.1038/d41586-021-00396-2. [DOI] [PubMed] [Google Scholar]
- 2.James AS, Alawneh JI. COVID-19 Infection Diagnosis: Potential Impact of Isothermal Amplification Technology to Reduce Community Transmission of SARS-CoV-2. Diagnostics (Basel) 2020;10(6) doi: 10.3390/diagnostics10060399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esbin MN, Whitney ON, Chong S, Maurer A, Darzacq X, Tjian R. Overcoming the bottleneck to widespread testing: a rapid review of nucleic acid testing approaches for COVID-19 detection. RNA. 2020;26(7):771–783. doi: 10.1261/rna.076232.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Notomi T, Okayama H, Masubuchi H, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28(12):E63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poon LL, Wong BW, Ma EH, et al. Sensitive and inexpensive molecular test for falciparum malaria: detecting Plasmodium falciparum DNA directly from heat-treated blood by loop-mediated isothermal amplification. Clin Chem. 2006;52(2):303–306. doi: 10.1373/clinchem.2005.057901. [DOI] [PubMed] [Google Scholar]
- 6.Ohtsuka K, Yanagawa K, Takatori K, Hara-Kudo Y. Detection of Salmonella enterica in naturally contaminated liquid eggs by loop-mediated isothermal amplification, and characterization of Salmonella isolates. Appl Environ Microbiol. 2005;71(11):6730–6735. doi: 10.1128/AEM.71.11.6730-6735.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito M, Watanabe M, Nakagawa N, Ihara T, Okuno Y. Rapid detection and typing of influenza A and B by loop-mediated isothermal amplification: comparison with immunochromatography and virus isolation. J Virol Methods. 2006;135(2):272–275. doi: 10.1016/j.jviromet.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Jimena B, Bekaert M, Bakheit M, et al. Development and validation of four one-step real-time RT-LAMP assays for specific detection of each dengue virus serotype. PLoS Negl Trop Dis. 2018;12(5) doi: 10.1371/journal.pntd.0006381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Jimena B, Wehner S, Harold G, et al. Development of a single-tube one-step RT-LAMP assay to detect the Chikungunya virus genome. PLoS Negl Trop Dis. 2018;12(5) doi: 10.1371/journal.pntd.0006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kutsuna S, Saito S, Ohmagari N. Simultaneous diagnosis of dengue virus, Chikungunya virus, and Zika virus infection using a new point-of-care testing (POCT) system based on the loop-mediated isothermal amplification (LAMP) method. J Infect Chemother. 2020;26(12):1249–1253. doi: 10.1016/j.jiac.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Dao Thi VL, Herbst K, Boerner K, et al. A colorimetric RT-LAMP assay and LAMP-sequencing for detecting SARS-CoV-2 RNA in clinical samples. Sci Transl Med. 2020;12(556) doi: 10.1126/scitranslmed.abc7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan C, Cui J, Huang L, et al. Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay. Clin Microbiol Infect. 2020;26(6):773–779. doi: 10.1016/j.cmi.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang WE, Lim B, Hsu CC, et al. RT-LAMP for rapid diagnosis of coronavirus SARS-CoV-2. Microb Biotechnol. 2020;13(4):950–961. doi: 10.1111/1751-7915.13586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagura-Ikeda M, Imai K, Tabata S, et al. Clinical Evaluation of Self-Collected Saliva by Quantitative Reverse Transcription-PCR (RT-qPCR), Direct RT-qPCR, Reverse Transcription-Loop-Mediated Isothermal Amplification, and a Rapid Antigen Test To Diagnose COVID-19. J Clin Microbiol. 2020;58(9) doi: 10.1128/JCM.01438-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teng PH, Chen CL, Sung PF, Lee FC, Ou BR, Lee PY. Specific detection of reverse transcription-loop-mediated isothermal amplification amplicons for Taura syndrome virus by colorimetric dot-blot hybridization. J Virol Methods. 2007;146(1-2):317–326. doi: 10.1016/j.jviromet.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 16.Chou PH, Lin YC, Teng PH, Chen CL, Lee PY. Real-time target-specific detection of loop-mediated isothermal amplification for white spot syndrome virus using fluorescence energy transfer-based probes. J Virol Methods. 2011;173(1):67–74. doi: 10.1016/j.jviromet.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Inacio J, Flores O, Spencer-Martins I. Efficient identification of clinically relevant Candida yeast species by use of an assay combining panfungal loop-mediated isothermal DNA amplification with hybridization to species-specific oligonucleotide probes. J Clin Microbiol. 2008;46(2):713–720. doi: 10.1128/JCM.00514-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuboki N, Inoue N, Sakurai T, et al. Loop-mediated isothermal amplification for detection of African trypanosomes. J Clin Microbiol. 2003;41(12):5517–5524. doi: 10.1128/JCM.41.12.5517-5524.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogels CBF, Brito AF, Wyllie AL, et al. Analytical sensitivity and efficiency comparisons of SARS-CoV-2 RT-qPCR primer-probe sets. Nat Microbiol. 2020;5(10):1299–1305. doi: 10.1038/s41564-020-0761-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li K, Brownley A. Primer design for RT-PCR. Methods Mol Biol. 2010;630:271–299. doi: 10.1007/978-1-60761-629-0_18. [DOI] [PubMed] [Google Scholar]
- 21.Nagamine K, Hase T, Notomi T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol Cell Probes. 2002;16(3):223–229. doi: 10.1006/mcpr.2002.0415. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 2003;31(24):e154. doi: 10.1093/nar/gng154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X. A PCR-based platform for microRNA expression profiling studies. RNA. 2009;15(4):716–723. doi: 10.1261/rna.1460509. New York, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao G, Chernock RD, Gay HA, et al. A novel RT-PCR method for quantification of human papillomavirus transcripts in archived tissues and its application in oropharyngeal cancer prognosis. Int J Cancer. 2013;132(4):882–890. doi: 10.1002/ijc.27739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.SantaLucia J. A unified view of polymer, dumbbell, and oligonucleotide DNA nearest-neighbor thermodynamics. PNAS. 1998;95(4):1460–1465. doi: 10.1073/pnas.95.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugimoto N, Nakano S, Katoh M, et al. Thermodynamic parameters to predict stability of RNA/DNA hybrid duplexes. Biochemistry. 1995;34(35):11211–11216. doi: 10.1021/bi00035a029. [DOI] [PubMed] [Google Scholar]
- 27.Hancock JM, Armstrong JS. SIMPLE34: an improved and enhanced implementation for VAX and Sun computers of the SIMPLE algorithm for analysis of clustered repetitive motifs in nucleotide sequences. Comput Appl Biosci. 1994;10(1):67–70. doi: 10.1093/bioinformatics/10.1.67. [DOI] [PubMed] [Google Scholar]
- 28.Rychlik W. Priming efficiency in PCR. BioTechniques. 1995;18(1):84–86. 8-90. [PubMed] [Google Scholar]
- 29.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31(13):3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Ren G, Buss J, Barry AJ, Patton GC, Tanner NA. Enhancing colorimetric loop-mediated isothermal amplification speed and sensitivity with guanidine chloride. BioTechniques. 2020;69(3):178–185. doi: 10.2144/btn-2020-0078. [DOI] [PubMed] [Google Scholar]
- 31.Reynes B, Serra F, Palou A. Rapid visual detection of SARS-CoV-2 by colorimetric loop-mediated isothermal amplification. BioTechniques. 2021;70(4):218–225. doi: 10.2144/btn-2020-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lalli MA, Langmade JS, Chen X, et al. Rapid and Extraction-Free Detection of SARS-CoV-2 from Saliva by Colorimetric Reverse-Transcription Loop-Mediated Isothermal Amplification. Clin Chem. 2021;67(2):415–424. doi: 10.1093/clinchem/hvaa267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uhteg K, Jarrett J, Richards M, et al. Comparing the analytical performance of three SARS-CoV-2 molecular diagnostic assays. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bokelmann L, Nickel O, Maricic T, et al. Point-of-care bulk testing for SARS-CoV-2 by combining hybridization capture with improved colorimetric LAMP. Nat Commun. 2021;12(1):1467. doi: 10.1038/s41467-021-21627-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams E, Isles N, Chong B, et al. Detection of SARS-CoV-2 in saliva: implications for specimen transport and storage. J Med Microbiol. 2021;70(2) doi: 10.1099/jmm.0.001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu J, Guo J, Xu Y, Chen X. Viral dynamics of SARS-CoV-2 in saliva from infected patients. J Infect. 2020;81(3):e48–e50. doi: 10.1016/j.jinf.2020.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 39.La Scola B, Le Bideau M, Andreani J, et al. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur J Clin Microbiol Infect Dis. 2020;39(6):1059–1061. doi: 10.1007/s10096-020-03913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larremore DB, Wilder B, Lester E, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. 2021;7(1) doi: 10.1126/sciadv.abd5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ascoli CA. Could mutations of SARS-CoV-2 suppress diagnostic detection? Nat Biotechnol. 2021;39(3):274–275. doi: 10.1038/s41587-021-00845-3. [DOI] [PubMed] [Google Scholar]
- 42.Plante JA, Liu Y, Liu J, et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature. 2020 doi: 10.1038/s41586-020-2895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.