Abstract
Cerebral ischemic injury may lead to a series of serious brain diseases, death or different degrees of disability. Hypoxia-inducible factor-1α (HIF-1α) is an oxygen-sensitive transcription factor, which mediates the adaptive metabolic response to hypoxia and serves a key role in cerebral ischemia. HIF-1α is the main molecule that responds to hypoxia. HIF-1α serves an important role in the development of cerebral ischemia by participating in numerous processes, including metabolism, proliferation and angiogenesis. The present review focuses on the endogenous protective mechanism of cerebral ischemia and elaborates on the role of HIF-1α in cerebral ischemia. In addition, it focuses on cerebral ischemia interventions that act on the HIF-1α target, including biological factors, non-coding RNA, hypoxic-ischemic preconditioning and drugs, and expands upon the measures to strengthen the endogenous compensatory response to support HIF-1α as a therapeutic target, thus providing novel suggestions for the treatment of cerebral ischemia.
Keywords: hypoxia-inducible factor-1α cerebral ischemia, endogenous protection, angiogenesis, neuroprotection, pretreatment
1. Introduction
In 2017, stroke was recorded as the second leading cause of death in people >60 years old worldwide and the leading cause of permanent disability (1,2). The condition has become a huge global health problem (3). Ischemic stroke is the most common type of stroke and the third leading cause of disability worldwide (4,5). Ischemic stroke is a pathological state of insufficient blood supply in specific parts of the brain, particularly in the middle cerebral artery, due to sudden rupture of cerebral vessels or local ischemia caused by cerebral artery thrombosis or embolism, resulting in an insufficient supply of nutrients, oxygen and glucose, energy imbalance, and finally, neuronal cell death (6–9). The pathogenesis of ischemic stroke is complex, involving numerous mechanisms, including oxidative stress, neuroinflammation, excitatory neurotoxicity, ion imbalance, energy metabolism and apoptosis (10–12). At present, recombinant tissue plasminogen activator is the only drug approved by the Food and Drug Administration for the treatment of acute ischemic stroke (13). Therefore, the search for alternative treatment strategies for ischemic stroke has attracted increasing attention. Endogenous protection is an important mechanism of protection and recovery after cerebral ischemia. The hypoxia-inducible factor-1α (HIF-1α) signaling pathway serves an important role in endogenous protection. HIF-1α regulates angiogenesis, neuroprotection, neurogenesis, migration of neuronal stem cells to the ischemic area and proliferation to functional neurons by regulating the transcription of downstream target genes (14). Strengthening the endogenous compensatory response may become an interesting potential treatment strategy in stroke.
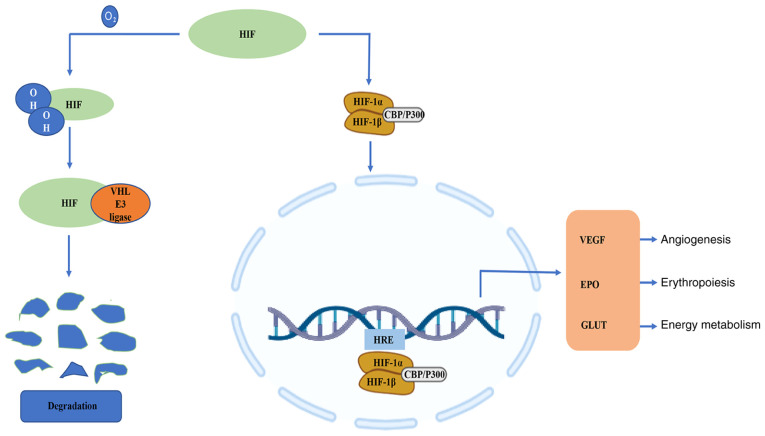
It is worth noting that the HIFs are a family of transcription factors involved in the hypoxia response and one of the key regulatory mechanisms of hypoxic stress at the cellular level (15,16). HIF-1 consists of an oxygen-regulated α subunit, HIF-1α, and a constitutively expressed β subunit, HIF-1β (17). Although mammals have a number of hypoxia adaptation mechanisms, including those that have a faster response time than the HIF-1α system, the unique degree of influence of the HIF system makes it a more important hypoxia response regulation mechanism (18). Under normoxic conditions, the proline and lysine residues on the oxygen-dependent degradation domain of HIF-1α are hydroxylated, and the modified HIF-1α interacts with the Von Hippel-Lindau E3 ubiquitin ligase complex via ubiquitin-proteasome pathway degradation (19). However, HIF-1α is stable under hypoxic conditions (20). With the assistance of co-activators, such as cyclic adenosine monophosphate response element binding protein and acetyltransferase, HIF-1α forms a heterodimer with HIF-1β (21), and then HIF-1α is transferred to the nucleus and combines with the target gene hypoxia response element (HRE) to induce the expression of downstream genes (Fig. 1). HIF-1α regulates the transcription of >100 genes (22); its target genes encode molecules involved in vasomotor control, angiogenesis, erythropoiesis, cell proliferation and energy metabolism, and complex physiological and pathological processes, such as cell death and inflammation (23–26). During cerebral ischemia, HIF-1α is expressed in the chronic hypoxic area around the infarct area (27). Therefore, HIF-1α may become a novel and valuable therapeutic target.
Figure 1.
Under normoxic conditions, the proline and lysine residues on the oxygen-dependent degradation domain of HIF-1α are hydroxylated, and modified HIF-1α interacts with the Von Hippel-Lindau E3 ubiquitin ligase complex via ubiquitin-proteasome pathway degradation. However, HIF-1α is stable under hypoxic conditions. With the help of co-activators, such as CBP and p300, HIF-1α forms a heterodimer with HIF-1β, and then HIF-1α is transferred to the nucleus and combines with the target gene HRE to induce downstream gene expression. HIF, hypoxia-inducible factor; HRE, hypoxia response element; CBP, cyclic adenosine monophosphate response element binding protein; p300, acetyltransferase; VEGF, vascular endothelial growth factor; EPO, erythropoietin; GLUT, glucose transporter type.
2. Literature screening method
The literature was searched using PubMed and ScienceDirect databases. With use of ‘pathogenesis of cerebral ischemia’, ‘HIF-1α’ and ‘cerebral ischemia and HIF-1α’ as search terms, 2,199, 1,550 and 42 relevant articles were retrieved, respectively. The resulting articles were then screened according to the clarity and specificity of the research objectives, and the date of publication (from December 2000 to present). A total of 132 articles were selected for assessment in the current study.
3. Role of HIF-1α in cerebral ischemia
The role of HIF-1α in cerebral ischemia is related to hypoxia. During cerebral ischemia, the oxygen supply is insufficient and partial oxygen pressure in the tissue decreases, leading to the activation of HIF-1α (28). HIF-1α is mainly induced in the penumbra of the cerebral ischemic region and serves an important role in angiogenesis, glucose metabolism and cell survival following an ischemic stroke (29–31).
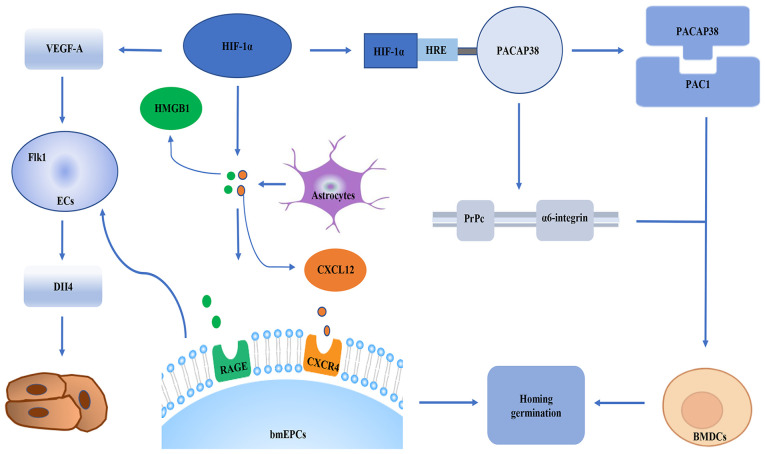
The formation of neovascularization promotes the nerve recovery of ischemic injury after cerebral ischemia (32). Angiogenesis is one of the most important modes of neovascularization, which depends on endothelial progenitor cells (EPCs) (32). HIF-1α is a transcription factor that regulates angiogenesis. It has been widely accepted that HIF-1α serves a role in regulating angiogenesis by regulating endothelial cells (ECs). For example, in the acute phase of ischemia, HIF-1α serves an important role in homing and germination of bone marrow-derived EPCs (bmEPC) in Sprague Dawley rat brain tissues. This effect is related to maintaining proper astrocyte responses in the ischemic brain. From a molecular perspective, the signals of the chemokine (C-X-C motif) ligand 12 (CXCL12)/chemokine C-X-C-motif receptor 4 (CXCR4) axis, high mobility group protein B1 (HMGB1) and vascular endothelial growth factor A (VEGF-A)/vascular endothelial growth factor receptor 2 (Flk1)-neuropilin-1 (Nrp1)/delta-like ligand-4 (Dll4) axis between astrocytes and bmEPCs are the basis for HIF-1α to regulate the homing and sprouting of bmEPCs (33). After a stroke, bmEPCs are mobilized from the bone marrow to the peripheral blood and recruited to the ischemic brain (34), and CXCL12 and HMGB1 expression is upregulated. CXCL12/CXCR4 and HMGB1 are important for the homing of EPCs (35,36). EPCs then attach to damaged blood vessels and repair them or migrate outward to start angiogenesis (37). Bud angiogenesis is another important mode of angiogenesis guided by apical cells (38,39). Apical cells are usually mobile and located at the top of vascular buds (38,39). Morphologically, apical cells are rich in filamentous pseudopodia, which contributes to the formation of vascular tubes (40). ECs serve as a bank for apical cells. The differentiation of ECs into apical cells is controlled by the VEGF-A/Flk1/Dll4 signaling pathway (38,39). Specifically, when Flk1-positive ECs are stimulated by VEGF-A, the levels of Dll4 are increased in these cells, and thus, these cells begin to differentiate into tip cells (38,39). In addition, Nrp1 is a co-receptor of Flk1 and is necessary for apical cell formation (41,42). The role of astrocytes in angiogenesis is that activated astrocytes recruit bmEPCs by secreting HMGB1 and CXCL12 (35). These two substances are ligands of advanced glycosylation end product-specific receptor and recombinant CXCR4, which are expressed on the cell membrane of EPCs (35,43). It has been revealed that inhibition of HIF-1α can reduce the expression levels of CXCR4 in bmEPCs, as well as reducing the expression levels of VEGF-A, VEGF-A/Flk-1, NRP1 and Dll4 in bmEPCs. These findings suggest that HIF-α may participate in the homing of bmEPCs via CXCL12/CXCR4 and HMGB1, and promotes the germination of bmEPCs via VEGF-A/Flk1-Nrp1/Dll4 (33). Additionally, knockdown of HIF-1α in vivo reduces the number of reactive astrocytes in the ischemic brain (33,44). Furthermore, a previous study (31) found that the number of reactive astrocytes increased in the brain of ischemic mice with insufficient prolyl hydroxylases (PHDs), and insufficient PHDs led to the stabilization of HIF-1α. This indicated that astrocytes serve a key role in the homing and germination of bmEPCs, and HIF-1α serves a direct role in the homing and germination of bmEPCs via regulation of the pathway between astrocytes and bmEPCs. Conversely, affecting the number of astrocytes has an indirect effect on the homing and germination of bmEPCs. In addition, HIF-1α induces bone marrow dendritic cell (BMDC) homing to ECs and regulates angiogenesis (45). In the molecular mechanism of BMDC transport, pituitary adenylate cyclase-activating peptide 38 (PACAP38) increases the expression levels of adhesion/migration-related proteins cellular prion protein (PrPc), α6-integrin, B1 integrin, focal adhesion kinase and CXCR4 (46–52), enhances the activities of MMP9 and MMP2 in BMDCs, and promotes the homing and migration of BMDCs. The PACAP38-pituitary adenylate cyclase-activating polypeptide type I receptor isoform 1 (PAC1) signal is an important part of the homing mechanism. During ischemia and hypoxia, HIF-1α upregulates PACAP38 by binding to the HRE on the PACAP38 promoter. The PACAP38 receptor PAC1 is widely expressed on BMDCs. PACAP38 binds to the receptor and promotes the homing of BMDCs to the ischemic area. In addition, PACAP38 upregulates the expression levels of α6-integrin and PrPc on the surface of BMDCs. This may stimulate the bone marrow mesenchymal cells to move to the blood vessels and increase their binding to laminin. Laminin is concentrated on the surface of the blood vessel (53,54). The interaction can enable BMDCs to integrate into the vascularized parenchymal area of the ischemic brain to promote tissue repair (Fig. 2) (53,54).
Figure 2.
HIF-1α may participate in the homing of bmEPCs via CXCL12/CXCR4 and HMGB1, and promotes the germination of bmEPCs via VEGF-A/Flk1/Dll4. HIF-1α upregulates PACAP38 by binding to the HRE on the PACAP38 promoter during ischemia and hypoxia. PACAP38 binds to the receptor PAC1 and promotes the homing of BMDCs to the ischemic region. In addition, PACAP38 upregulates the expression levels of α6-integrin and PrPc on the surface of BMDCs. ECs, endothelial cells; CXCL12, chemokine (C-X-C motif) ligand 12; CXCR4, chemokine C-X-C-motif receptor 4; HMGB1, high mobility group protein B1; RAGE, ligands of advanced glycosylation end product-specific receptor; VEGF-A, vascular endothelial growth factor A; Flk1, vascular endothelial growth factor receptor 2; Dll4, delta-like ligand-4; BMDCs, bone marrow dendritic cells; HIF-1α, hypoxia-inducible factor 1α; HRE, hypoxia response element; PACAP38, pituitary adenylate cyclase-activating peptide 38; PAC1, PACAP type 1 receptor; PrPc, cellular prion protein; bmEPCs, bone marrow-derived EPCs.
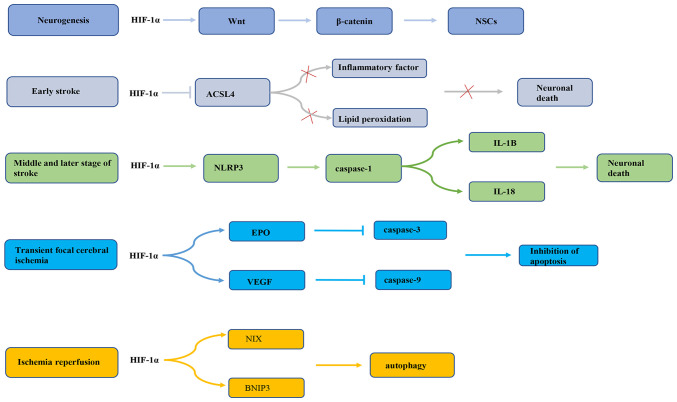
Previous research has revealed that HIF-1α serves a dual role in promoting survival or death of nerve cells during cerebral ischemia. First, endogenous neurogenesis is enhanced during cerebral ischemia and hypoxia (55), which may be related to the activation of endogenous neural stem cells (NSCs) during cerebral ischemia (56,57). Previous studies have demonstrated that both global and focal cerebral ischemia can increase the proliferation and neural differentiation of NSCs located in the subgranular area of the dentate gyrus, the anterior subventricular area and the posterior peripheral area of the ventricle adjacent to the hippocampus (58,59). HIF-1α, by increasing activation of the Wnt/β-catenin signaling pathway, stimulates NSC proliferation (60). The Wnt signaling pathway regulates the embryonic NSC pattern, cell fate determination and cell proliferation (61). Wnt signaling may regulate hippocampal neurogenesis in adult rats (62). In fact, Wnt3α mutant mice exhibit hippocampal hypoplasia due to a lack of proliferation (62). Wnt family members are expressed in hippocampal astrocytes, while hippocampal stem/progenitor cells express Wnt protein receptors and signal components (63). It has been reported that HIF-1α signaling is inhibited under oxygen-deprived conditions, which may reduce β-catenin nuclear translocation and cyclin D1 expression, delaying NSC proliferation (60). In addition, HIF-1α has different effects on the occurrence of apoptosis in different periods of cerebral ischemia (64,65). A previous study has demonstrated that HIF-1α may have a neuroprotective effect in the early stage of an ischemic stroke (66). HIF-1α is highly expressed in rat brain tissue in the early stage of ischemic stroke and may markedly reduce infarct cell apoptosis. This effect may be related to the inhibition of acyl CoA synthase long chain family member 4 (ACSL4) by HIF-1α (67). ACSL4 is an important metabolic isoenzyme of polyunsaturated fatty acids. ACSL4 promotes neuronal death by enhancing lipid peroxidation (a marker of iron drop disease). In addition, ACSL4 may promote the microglia mediated inflammatory response. HIF-1α inhibits ACSL4 expression, thereby reducing lipid peroxidation and inflammation, and exhibiting a neuroprotective effect on cerebral ischemia (67). On the contrary, a previous study conducted by Panchision (68) demonstrated that HIF-1α expression could promote neuronal apoptosis after long-term severe ischemia and hypoxia. HIF-1α may regulate the inflammatory response through the NLR family containing pyrin domain protein 3 (NLRP3) inflammasome complex, thereby promoting apoptosis and pyrophagocytic cell death after stroke (69). NLRP3 inflammatory bodies are the main mediators of the inflammatory response during ischemic stroke (70,71). Upregulation of NLRP3 inflammatory bodies activates pre-caspase-1 by cleavage (72), thus promoting the maturation of IL-1B and IL-18. HIF-1α regulates the NLRP3 inflammatory focal pathway, resulting in brain cell death (69). In addition, in the transient focal cerebral ischemia model, on the one hand, HIF-1α upregulates erythropoietin (EPO) expression, thereby inhibiting the expression of activated caspase-3 in neurons and inhibiting neuronal apoptosis to improve the recovery of nerve function (73). On the other hand, HIF-1α exerts a neuroprotective effect on transient focal cerebral ischemia by upregulating VEGF and downregulating caspase-9 (74). A previous study also found that the anti-apoptotic effect of HIF-1α gene therapy effectively reduced the neurological deficit score and brain edema at 24 and 72 h after reperfusion, and inhibited the pathological damage and apoptosis of nerve cells in a rat middle cerebral artery occlusion model (75). Additionally, a previous study reported that HIF-1α may improve brain damage after ischemia/reperfusion (I/R) via BCL2/adenovirus E1B interacting protein 3 (BNIP3) and Bcl-2 family proteins containing BH3 domain-dependent enhancement of autophagy cell survival (76). Taken together, these observations suggest that HIF-1α may induce cell death in severe and long-term ischemia, but that activation in mild ischemic stress could promote cell survival (Fig. 3). This may be related to different mechanisms being involved in the regulation of the response to ischemic stroke by HIF-1α. The role of HIF-1α in neuroprotection requires further study.
Figure 3.
HIF-1α expression in ischemic stroke is controlled by different mechanisms. NSCs, neural stem cells; HIF-1α, hypoxia-inducible factor-1α; ACSL4, acyl CoA synthase long chain family member 4; NLRP3, NLR family containing pyrin domain protein 3; IL-18, interleukin 18; IL-1B, interleukin 1B; EPO, erythropoietin; VEGF, vascular endothelial growth factor; NIX, Bcl-2 family proteins containing BH3 domain; BNIP3, BCL2/adenovirus E1B interacting protein 3.
4. HIF-1α protects the brain via the regulation of endogenous substances
During cerebral ischemia, endogenous factors, such as neurotransmitters, amino acids and inorganic salts, serve a protective role against cerebral ischemia by regulating HIF-1α to influence the angiogenesis and neuroprotection of ischemic brain tissue (77–80). For example, choline may increase the levels of α7 nicotinic acetylcholine receptor, induce the expression of HIF-1α and VEGF, and promote the formation of cerebral arteries and cerebral cortex capillaries, thereby effectively reducing cerebral ischemic damage in permanent middle cerebral artery occlusion (MCAO) rats (77). Peroxynitrite promotes neurogenesis by activating HIF-1α and enhancing the Wnt/β-catenin signaling pathway (78). Arginine reduces the inflammatory response mediated by HIF-1α and protects against the death of ischemic neurons after I/R injury in rats (79). Glycine inhibits HIF-1α by inhibiting the upregulation of NF-κB/p65 after I/R injury, thereby inhibiting pro-inflammatory activity (80).
MicroRNAs (miRNAs/miRs) are a type of small endogenous non-coding single-stranded RNA that regulate protein expression by inducing mRNA degradation or interfering with translation. miRNAs have been found to be involved in the pathogenesis of stroke. To date, numerous miRNAs have been determined to be involved in the molecular process of the ischemic cascade (81,82). Several miRNAs, including miR-376b-5p, miR-433, miR-335, miRNA-210 and miR-155-5p, have also been demonstrated to regulate HIF-1α during cerebral ischemia. Notably, among these miRNAs, miRNA-210 is positively associated with HIF-1n expression. When miRNA-210 expression is upregulated, the gene and protein expression levels of HIF-1α and VEGF are increased, and their expression trends are consistent (83). Recently, it has been revealed that elevated levels of miRNA-210 increase neuronal cell apoptosis by activating the HIF-1α-VEGF signaling pathway. By contrast, downregulation of miRNA-210 expression markedly inhibits the gene and protein expression of HIF-1α and VEGF (83). The expression levels of miR-376b-5p, miR-433, miR-335 and miR-155-5p are negatively associated with the expression levels of HIF-1α (84–87). miR-376b-5p inhibits angiogenesis after cerebral ischemia via the HIF-1α-mediated VEGFA-Notch1 signaling pathway (84). Overexpression or downregulation of miR-433 alters the mRNA and protein levels of HIF-1α and its downstream genes, VEGF, glucose transporter 1 (GLUT1) and angiopoietin 2 (Angpt2) (85). In addition, miR-335, as a direct regulator of HIF-1α, serves different roles in different periods of cerebral ischemia, which may be related to the different effects of HIF-1α in different periods of cerebral ischemia. In the early stage of cerebral ischemia, miR-335 mimic may reduce the area of cerebral infarction, while the levels of HIF-1α protein are lower. This results in the decreased expression of downstream target genes of HIF-1α, including Angpt2, BNIP3, MMP9, plasminogen activator inhibitor-1 and VEGF-A. By contrast, in the middle and late stages of cerebral ischemia, the use of anti-miR-335 is beneficial. HIF-1α protein is upregulated and then its downstream gene expression levels increase (86). miR-335 regulates HIF-1α expression and also affects neurovascular permeability, cell death and the blood-brain barrier, resulting in a reduction in infarct volume (86). Furthermore, a recent study has demonstrated that miR-155-5p targets HIF-1α in NSCs (87). Inhibition of miR-155-5p may promote the viability of NSCs and inhibit cell apoptosis induced by oesophago-gastro-duodenoscopy (OGD). Additionally, after transplantation, NSCs inhibit miR-155-5p, and this also enhances the inhibition of inflammation and oxidative stress, which enhances the protection against cerebral infarction. Long non-coding RNAs (lncRNAs) are a relatively newly discovered class of non-coding RNA, ranging in length from ~200 nucleotides to several kilobases (88). lncRNAs are dynamically expressed in tissues, based on differentiation stages and cell type-specific patterns, and participate in numerous normal cellular processes. lncRNAs can compete with specific mRNAs for the same miRNA pool. The result is that the binding of miRNAs to target mRNAs is inhibited or reduced, and the function of miRNA post-transcriptional silencing is impaired (89,90). HIF-1α-AS2 is an antisense lncRNA derived from the natural antisense transcript of HIF-1α (91). A previous study has revealed that the expression levels of HIF-1α-AS2 are upregulated in hypoxic human umbilical vein ECs (HUVECs) (92). Upregulation of HIF-1α-AS2 leads to downregulation of miR-153-3p, which can reduce the post-transcriptional silencing of HIF-1α. MCAO reduces the level of miR-153-3p RNA in the infarct area and increases the protein levels of HIF-1α, VEGF-A and Notch1. This function of HIF-1α-AS2 promotes the activation of the HIF-1α/VEGFA/Notch1 cascade, thereby promoting the vitality, migration and tube formation of HUVECs (92).
5. Role of HIF-1α in cerebral ischemic preconditioning (IPC)
Preconditioning has a certain protective effect on cerebral ischemia. To date, several preconditioning methods have been used to induce ischemic tolerance (IT). Studies have revealed that during pretreatment, HIF-1α is activated to serve a neuroprotective role (93,94). Hypoxic preconditioning is a phenomenon in which mild hypoxia may induce a strong state of IT to resist the subsequent damage caused by severe hypoxia, which exists in a number of organs, especially the brain (95–98). Hypoxic preconditioning improves the survival rate of rats with cerebral ischemia, reduces neurological deficits, increases the object recognition and social recognition memory of the rat, and inhibits the inflammatory response caused by cerebral ischemia. These effects are regulated by HIF-1α (99). During hypoxia preconditioning, IT levels increase, which is related to the activation of HIF-1α and the expression of its target genes GLUT1, EPO, VEGFα, Bcl-2 and inducible nitric oxide synthase. Also during hypoxic preconditioning, olfactory mucosal mesenchymal stem cells activate HIF-1α in vitro to inhibit the pyrolysis and apoptosis of microglia after cerebral I/R injury (100). In addition to hypoxic preconditioning, IPC is also used as a means of cerebral ischemia protection. IPC is defined as a transient sublethal ischemic injury, which may mobilize protective mechanisms to improve neuronal damage following fatal ischemia (101). A previous study has revealed that IPC protects CA1 pyramidal neurons from non-IPC lethal ischemia by increasing HIF-1α expression in CA1 pyramidal neurons, thereby enhancing the expression of VEGF and the activation of NF-κB (102). The increase of HIF-1α may be related to the continuous increase of P2X7 receptors in astrocytes. Activation of P2X7 receptors leads to the increase of HIF-1α. This hypoxia-independent, but P2X7 receptor-dependent mechanism could induce persistent expression of HIF-1α in astrocytes, thereby effectively inducing IT and neuroprotection against ischemia (103). Studies have also demonstrated that remote IPC (RIPC) could improve the response of peripheral immune cells by regulating the upregulation of HIF-1α (104,105). This effect may be the protective effect of cerebral ischemia mediated by RIPC activation of the HIF-1α/AMP-activated protein kinase (AMPK)/70-kilodalton heat shock protein (HSP70) signaling pathway. Highly conserved HSPs, as molecular chaperones of abnormally folded proteins in cellular stress, are induced through the HIF-1α pathway during hypoxia, and the existing evidence also demonstrates that, in newborns, HSPs transform into the mature conformation, and HSP70 and HSP90 serve an important role in the post-translational process (106). The AMPK-histone deacetylase 5 signaling pathway promotes HIF-1α through the deacetylation of HSP70 in the cytoplasm (107,108). The nuclear accumulation of HIF-1α and the activation of HIF-1α function indicate that RIPC mediates ischemic protection through the interaction between AMPK, HIF-1α and HSP70 (109). In addition, exercise preconditioning is a special neuronal IPC, which may induce brain tolerance to ischemia, enhance neuroprotection and resist a series of brain damages caused by ischemia (110,111). Previous studies have demonstrated that exercise pretreatment 3 weeks before stroke improves the structural integrity of the brain microvascular structure in rats (112–114). This effect may be related to HIF-1α. The pre-IPC exercise induces cerebral IT mediated by neurons and astrocytes by increasing the expression levels of HIF-1α (115,116). In addition, HIF-1α triggers the expression of endothelin 1, increases the expression of B-type natriuretic peptide and has a neuroprotective effect (117).
6. Role of HIF-1α in the protective effects of natural compounds against cerebral ischemia
Platelet drugs and thrombolytic drugs are the only ischemic stroke drugs supported by strong clinical evidence (118). However, the application of these two treatment methods is often limited by the potential risk of cerebral hemorrhage and a narrow treatment time window. In general, after decades of practice and investigation, the number of effective interventions for ischemic stroke is limited. Therefore, it is necessary to study further feasible and effective treatment options. A number of studies (119–122) have demonstrated that certain natural compounds, chemical drugs and traditional Chinese medicine compounds may alleviate cerebral ischemic injury through HIF-1α. A previous study found that certain compounds and traditional Chinese medicines serve a role in angiogenesis and vascular protection through the HIF-1α/VEGF signaling pathway, thereby protecting against cerebral ischemic injury (123). In vivo and in vitro studies have demonstrated that catalpol directly activates the HIF-1α/VEGF signaling pathway in the brain and primary cerebral microvascular ECs of rats with cerebral ischemia, protects the vascular structure and promotes blood vessel generation (120). Astragaloside IV activates the HIF-1α/VEGF/Notch signaling pathway through miRNA-210 to promote angiogenesis (121). Fluoxetine induces a cascade of events leading to the upregulation of the expression of HIF-1α-Netrin/VEGF protein, promotes angiogenesis after ischemic stroke and improves long-term functional recovery after ischemic stroke (122,123). Racemic dl-3-n-butylphthalide treatment could also promote functional recovery after focal transient cerebral ischemia, and this recovery and dl-NBP may upregulate the expression of HIF-1α-VEGF and Notch-Dll4, and then affect the integrity of white matter, the number of capillaries and the expression of tight junction protein occludin (124). In addition, certain compounds serve a neuroprotective role by regulating HIF-1α. Berberine pretreatment enhances the accumulation of HIF-1α by activating PI3K/Akt and induces the production of sphingosine-1-phosphate (S1P) by promoting HIF-1α-mediated sphingosine kinase 2 (Sphk2) transcription and activation of Sphk2. S1P protects neuronal cells against hypoxia and ischemia by activating high-affinity G protein-coupled receptors, and serves an important protective role in tissue I/R injury (125). Methylene blue protects hippocampal-derived neuronal cells from OGD-reoxygenation damage by increasing the content of HIF-1α protein and activating the EPO signaling pathway (126). Salidroside induces the production of HIF-1α subunit and EPO via the PI3K/Akt signaling pathway, and exerts anti-inflammatory effects on cerebral ischemia and reperfusion (127). Certain drugs inhibit HIF-1α to exert neuroprotective effects, while others upregulate HIF-1α to produce the same effect. Curcumin exerts a neuroprotective effect by inhibiting the interaction between HIF-1α and autophagy in cerebral I/R injury (128). A recent study has reported that nateglinide stabilizes HIF-1 cerebral I/R injury by inhibiting STAT-3 phosphorylation and stops the expression of HIF-1α-dependent inflammation and mediators of apoptosis, namely phorbol-12-myristate-13-acetate-induced protein 1 and NF-κB (129). Different drugs have a neuroprotective effect through the opposite regulation of HIF-1α, which may be due to the different mechanisms of HIF-1α in the neuroprotective effect of cerebral ischemia. Different drugs affect different pathways by activating or inhibiting HIF-1α, and serve a role in cerebral ischemia. Angelica sinensis has a neuroprotective effect on astrocyte-mediated infarct expansion through HIF-1α-mediated angiogenesis, as well as HIF-1α-mediated anti-apoptotic effects. Angelica sinensis activates p38/MAPK/HIF-1α/VEGF-A/cAMP-response element binding protein/von Willebrand factor signaling to mediate angiogenesis (130). In addition, the anti-apoptotic effect of Angelica sinensis has been attributed to the activation of the HIF-1α/VEGF-A/p-Bad signaling pathway mediated by p38/MAPK (130). This activation could lead to Bad inactivation, maintain the integrity of the outer mitochondrial membrane and prevent cytochrome caspase-3-mediated apoptosis in the cortical ischemic penumbra, thereby exerting an anti-apoptotic effect (130). In addition, a previous study demonstrated that ligustilide can inhibit the upregulation of HIF-1α, VEGF and aquaporin 4 in an OGD-induced blood-brain barrier model and reduce the permeability of the OGD-induced blood-brain barrier in an in vitro model (131). Bu Yang Huan Wu decoction has a protective effect on the brain I/R injury in MCAO rats by inhibiting the activation of the HIF-1α/VEGF signaling pathway in the brain and stabilizing the β epithelial Na+ channel ion channel, suggesting that Bu Yang Huan Wu decoction may be used to treat acute brain injury during stroke (Table I) (132).
Table I.
Regulation of drugs affecting HIF-1α.
| Material | Impact on HIF-1α | Related pathway | Function | (Refs.) |
|---|---|---|---|---|
| Catalpol | Enhancement | HIF-1α/VEGF | Protects vascular structure, promotes angiogenesis | (120) |
| Astragaloside IV | Enhancement | HIF-1α/VEGF/Notch | Promotes angiogenesis | (121) |
| Fluoxetine | Enhancement | HIF-1α/Netrin/VEGF | Promotes angiogenesis | (122,123) |
| Dl-NBP | Enhancement | HIF-1α/VEGF, HIF-1α/Notch-Dll4 | Increases the number of microvessels and tight junction protein occludin | (124) |
| Berberine | Enhancement | HIF-1α/Sphk2/S1p | Protects neurons against hypoxia and ischemia | (125) |
| Methylene blue | Enhancement | HIF-1α/EPO | Protects hippocampal derived neurons | (126) |
| Salidroside | Enhancement | HIF-1α/PI3K/AKT, HIF-1α/EPO | Anti-inflammatory effect | (127) |
| Curcumin | Inhibition | To be studied | Anti-apoptosis, neuroprotection | (128) |
| Nateglinide | Inhibition | HIF-1α/NF-κB, TNF-β | Anti-inflammatory, neuroprotective | (129) |
| Angelica sinensis | Inhibition | HIF-1α/VEGF-A/CREB/vWF, HIF-1α/VEGF-A/P-Bad | Angiogenesis, anti-apoptosis, neuroprotection | (130) |
| Ligustilide | Inhibition | HIF/VEGF/AQP-4 | Reduces the permeability of blood-brain barrier | (131) |
| Bu Yang Huan Wu Decoction | Inhibition | HIF/VEGF | Maintains the integrity of blood-brain barrier | (132) |
Dl-NBP, dl-3-n-butylphthalide; HIF-1α, hypoxia-inducible factor-1α; VEGF, vascular endothelial growth factor; Sphk2, sphingosine kinase 2; S1p, sphingosine 1-phosphate; EPO, erythropoietin; PI3K, hosphatidylinositol 3-kinase; AKT, protein kinase B; NF-κB, nuclear factor κB; TNF-β, tumor necrosis factor β; MAPK, mitogen-activated protein kinase; VEGF-A, vascular endothelial growth factor-A; CREB, cAMP-response element binding protein; vWF, von Willebrand factor; AQP-4, aquaporin 4.
7. Concluding remarks and future perspectives
An increasing number of studies have demonstrated that HIF-1α serves a key role in cerebral ischemia. The present review describes the means by which HIF-1α is activated during cerebral ischemia and how it serves a protective role in cerebral ischemic tissues in terms of angiogenesis and neuroprotection. In terms of neuroprotection, since HIF-1α expression in ischemic stroke may be controlled by different mechanisms, HIF-1α has a dual effect. In addition, when cerebral ischemia occurs, endogenous regulatory factors directly or indirectly regulate HIF-1α, which may be the key mechanism of endogenous protection during cerebral ischemia. Recent research has also revealed that preconditioning has a positive therapeutic effect on cerebral ischemia and may become a novel clinical treatment for cerebral ischemia. Natural medicines and traditional Chinese medicines could be used to treat cerebral ischemia by regulating HIF-1α. HIF-1α is expected to become a novel target for the treatment of cerebral ischemic diseases, and identifying the effect of natural products on HIF-1α is also a future research direction.
Whether the signals and pathways initiated by HIF-1α in hypoxia (or hypoxic diseases) serve the same role in cerebral ischemia needs to be further confirmed. As HIF-1α regulates multiple downstream target genes, and the related pathways and mechanisms are complex, the current literature on the mechanism of HIF-1α in cerebral ischemia is not comprehensive and in-depth. Therefore, it is necessary to further study the role and mechanism of HIF-1α in the pathophysiology of cerebral ischemia.
Acknowledgements
Not applicable.
Funding Statement
The present study was supported by the National Natural Science Foundation of China (grant no. 81973588) and the Joint Guidance Project of Provincial Natural Science Foundation (grant no. LH2020H094).
Availability of data and materials
Not applicable.
Authors' contributions
HH conceived and designed the review. QL retrieved the relevant literature and wrote the manuscript. PD reviewed and edited the manuscript. Data authentication is not applicable. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.WHO publishes definitive atlas on global heart disease and stroke epidemic. Indian J Med Sci. 2004;58:405–406. [PubMed] [Google Scholar]
- 2.Cramer SC, Wolf SL, Adams HP, Jr, Chen D, Dromerick AW, Dunning K, Ellerbe C, Grande A, Janis S, Lansberg MG, et al. Stroke recovery and rehabilitation research: Issues, opportunities, and the national institutes of health strokeNet. Stroke. 2017;48:813–819. doi: 10.1161/STROKEAHA.116.015501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2016 Causes of Death Collaborators, corp-author. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the global burden of disease study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, et al. Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jianrong S, Yanjun Z, Chen Y, Jianwen X. DUSP14 rescues cerebral ischemia/reperfusion (IR) injury by reducing inflammation and apoptosis via the activation of Nrf-2. Biochem Biophys Res Commun. 2019;509:713–721. doi: 10.1016/j.bbrc.2018.12.170. [DOI] [PubMed] [Google Scholar]
- 6.Kim JY, Kawabori M, Yenari MA. Innate inflammatory responses in stroke: Mechanisms and potential therapeutic targets. Curr Med Chem. 2014;21:2076–2097. doi: 10.2174/0929867321666131228205146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tobin MK, Bonds JA, Minshall RD, Pelligrino DA, Testai FD, Lazarov O. Neurogenesis and inflammation after ischemic stroke: What is known and where we go from here. J Cereb Blood Flow Metab. 2014;34:1573–1584. doi: 10.1038/jcbfm.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Sun X, Xie Y, Zan J, Tan W. Isosteviol sodium protects against permanent cerebral ischemia injury in mice via inhibition of NF-κB-mediated inflammatory and apoptotic responses. J Stroke Cerebrovasc Dis. 2017;26:2603–2614. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Ishrat T, Sayeed I, Atif F, Stein DG. Effects of progesterone administration on infarct volume and functional deficits following permanent focal cerebral ischemia in rats. Brain Res. 2009;1257:94–101. doi: 10.1016/j.brainres.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hazell AS. Excitotoxic mechanisms in stroke: An update of concepts and treatment strategies. Neurochem Int. 2007;50:941–953. doi: 10.1016/j.neuint.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 11.Rothman SM, Olney JW. Glutamate and the pathophysiology of hypoxic-ischemic brain damage. Ann Neurol. 1986;19:105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- 12.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 13.Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, Cohen G. Recombinant tissue plasminogen activator for acute ischaemic stroke: An updated systematic review and meta-analysis. Lancet. 2012;379:2364–2372. doi: 10.1016/S0140-6736(12)60738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev. 2012;92:967–1003. doi: 10.1152/physrev.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruschewski M, Foitzik T, Perez-Cantó A, Hübotter A, Buhr HJ. Changes of colonic mucosal microcirculation and histology in two colitis models: An experimental study using intravital microscopy and a new histological scoring system. Dig Dis Sci. 2001;46:2336–2343. doi: 10.1023/A:1012334727509. [DOI] [PubMed] [Google Scholar]
- 16.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447-5454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris AL. Hypoxia-a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 18.Wilkins SE, Abboud MI, Hancock RL, Schofield CJ. Targeting protein-protein interactions in the HIF system. ChemMedChem. 2016;11:773–786. doi: 10.1002/cmdc.201600012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pereira T, Zheng X, Poellinger L. Degradation of the hypoxia-inducible factor 1alpha: Where does it happen? Cell Cycle. 2006;5:2720–2722. doi: 10.4161/cc.5.23.3536. [DOI] [PubMed] [Google Scholar]
- 20.Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1)alpha: Its protein stability and biological functions. Exp Mol Med. 2004;36:1–12. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- 21.Rabie T, Marti HH. Brain protection by erythropoietin: A manifold task. Physiology (Bethesda) 2008;23:263–274. doi: 10.1152/physiol.00016.2008. [DOI] [PubMed] [Google Scholar]
- 22.Schito L, Semenza GL. Hypoxia-inducible factors: Master regulators of cancer progression. Trends Cancer. 2016;2:758–770. doi: 10.1016/j.trecan.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 24.Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci. 2004;5:437–448. doi: 10.1038/nrn1408. [DOI] [PubMed] [Google Scholar]
- 25.Ke XJ, Zhang JJ. Changes in HIF-1α, VEGF, NGF and BDNF levels in cerebrospinal fluid and their relationship with cognitive impairment in patients with cerebral infarction. J Huazhong Univ Sci Technolog Med Sci. 2013;33:433–437. doi: 10.1007/s11596-013-1137-4. [DOI] [PubMed] [Google Scholar]
- 26.Kuang S, Zheng J, Yang H, Li S, Duan S, Shen Y, Ji C, Gan J, Xu XW, Li J. Structure insight of GSDMD reveals the basis of GSDMD autoinhibition in cell pyroptosis. Proc Natl Acad Sci USA. 2017;114:10642–10647. doi: 10.1073/pnas.1708194114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chavez JC, LaManna JC. Activation of hypoxia-inducible factor-1 in the rat cerebral cortex after transient global ischemia: Potential role of insulin-like growth factor-1. J Neurosci. 2002;22:8922–8931. doi: 10.1523/JNEUROSCI.22-20-08922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masoud GN, Li W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5:378–389. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh N, Sharma G, Mishra V. Hypoxia inducible factor-1: Its potential role in cerebral ischemia. Cell Mol Neurobiol. 2012;32:491–507. doi: 10.1007/s10571-012-9803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu J, Jiang L, Zhu H, Zhang L, Wang T. Hypoxia-inducible factor-1α and erythropoietin expression in the hippocampus of neonatal rats following hypoxia-ischemia. J Nanosci Nanotechnol. 2014;14:5614–5619. doi: 10.1166/jnn.2014.8728. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Saliba P, Reischl S, Marti HH, Kunze R. Neuronal deficiency of HIF prolyl 4-hydroxylase 2 in mice improves ischemic stroke recovery in an HIF dependent manner. Neurobiol Dis. 2016;91:221–235. doi: 10.1016/j.nbd.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa H, Tajiri N, Shinozuka K, Vasconcellos J, Kaneko Y, Lee HJ, Mimura O, Dezawa M, Kim SU, Borlongan CV. Vasculogenesis in experimental stroke after human cerebral endothelial cell transplantation. Stroke. 2013;44:3473–3481. doi: 10.1161/STROKEAHA.113.001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Ran H, Xiao Y, Wang H, Chen Y, Chen W, Xu X. Knockdown of HIF-1α impairs post-ischemic vascular reconstruction in the brain via deficient homing and sprouting bmEPCs. Brain Pathol. 2018;28:860–874. doi: 10.1111/bpa.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borlongan CV, Glover LE, Tajiri N, Kaneko Y, Freeman TB. The great migration of bone marrow-derived stem cells toward the ischemic brain: Therapeutic implications for stroke and other neurological disorders. Prog Neurobiol. 2011;95:213–228. doi: 10.1016/j.pneurobio.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayakawa K, Pham LD, Katusic ZS, Arai K, Lo EH. Astrocytic high-mobility group box 1 promotes endothelial progenitor cell-mediated neurovascular remodeling during stroke recovery. Proc Natl Acad Sci USA. 2012;109:7505–7510. doi: 10.1073/pnas.1121146109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller JT, Bartley JH, Wimborne HJ, Walker AL, Hess DC, Hill WD, Carroll JE. The neuroblast and angioblast chemotaxic factor SDF-1 (CXCL12) expression is briefly up regulated by reactive astrocytes in brain following neonatal hypoxic-ischemic injury. BMC Neurosci. 2005;6:63. doi: 10.1186/1471-2202-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang ZG, Zhang L, Jiang Q, Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002;90:284–288. doi: 10.1161/hh0302.104460. [DOI] [PubMed] [Google Scholar]
- 38.Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol. 2010;22:617–625. doi: 10.1016/j.ceb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12:943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 40.Phng LK, Stanchi F, Gerhardt H. Filopodia are dispensable for endothelial tip cell guidance. Development. 2013;140:4031–4040. doi: 10.1242/dev.097352. [DOI] [PubMed] [Google Scholar]
- 41.Aspalter IM, Gordon E, Dubrac A, Ragab A, Narloch J, Vizán P, Geudens I, Collins RT, Franco CA, Abrahams CL, et al. Alk1 and Alk5 inhibition by Nrp1 controls vascular sprouting downstream of Notch. Nat Commun. 2015;6:7264. doi: 10.1038/ncomms8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fantin A, Vieira JM, Plein A, Denti L, Fruttiger M, Pollard JW, Ruhrberg C. NRP1 acts cell autonomously in endothelium to promote tip cell function during sprouting angiogenesis. Blood. 2013;121:2352–2362. doi: 10.1182/blood-2012-05-424713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Domanska UM, Kruizinga RC, Nagengast WB, Timmer-Bosscha H, Huls G, de Vries EG, Walenkamp AM. A review on CXCR4/CXCL12 axis in oncology: No place to hide. Eur J Cancer. 2013;49:219–230. doi: 10.1016/j.ejca.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Wang C, Lin G, Luan Y, Ding J, Li PC, Zhao Z, Qian C, Liu G, Ju S, Teng GJ. HIF-prolyl hydroxylase 2 silencing using siRNA delivered by MRI-visible nanoparticles improves therapy efficacy of transplanted EPCs for ischemic stroke. Biomaterials. 2019;197:229–243. doi: 10.1016/j.biomaterials.2018.05.053. [DOI] [PubMed] [Google Scholar]
- 45.Lin CH, Chiu L, Lee HT, Chiang CW, Liu SP, Hsu YH, Lin SZ, Hsu CY, Hsieh CH, Shyu WC. PACAP38/PAC1 signaling induces bone marrow-derived cells homing to ischemic brain. Stem Cells. 2015;33:1153–1172. doi: 10.1002/stem.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ Res. 2009;105:223–230. doi: 10.1161/CIRCRESAHA.109.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferrero E, Belloni D, Contini P, Foglieni C, Ferrero ME, Fabbri M, Poggi A, Zocchi MR. Transendothelial migration leads to protection from starvation-induced apoptosis in CD34+CD14+ circulating precursors: Evidence for PECAM-1 involvement through Akt/PKB activation. Blood. 2003;101:186–193. doi: 10.1182/blood-2002-03-0768. [DOI] [PubMed] [Google Scholar]
- 48.Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030–1041. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 49.Torzicky M, Viznerova P, Richter S, Strobl H, Scheinecker C, Foedinger D, Riedl E. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) and CD99 are critical in lymphatic transmigration of human dendritic cells. J Invest Dermatol. 2012;132:1149–1157. doi: 10.1038/jid.2011.420. [DOI] [PubMed] [Google Scholar]
- 50.de la Rosa G, Longo N, Rodríguez-Fernández JL, Puig-Kroger A, Pineda A, Corbí AL, Sánchez-Mateos P. Migration of human blood dendritic cells across endothelial cell monolayers: Adhesion molecules and chemokines involved in subset-specific transmigration. J Leukoc Biol. 2003;73:639–649. doi: 10.1189/jlb.1002516. [DOI] [PubMed] [Google Scholar]
- 51.Kaneider NC, Kaser A, Dunzendorfer S, Tilg H, Wiedermann CJ. Sphingosine kinase-dependent migration of immature dendritic cells in response to neurotoxic prion protein fragment. J Virol. 2003;77:5535–5539. doi: 10.1128/JVI.77.9.5535-5539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/S1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 53.Zhang CC, Steele AD, Lindquist S, Lodish HF. Prion protein is expressed on long-term repopulating hematopoietic stem cells and is important for their self-renewal. Proc Natl Acad Sci USA. 2006;103:2184–2189. doi: 10.1073/pnas.0510577103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gnecchi M, Melo LG. Bone marrow-derived mesenchymal stem cells: Isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol Biol. 2009;482:281–294. doi: 10.1007/978-1-59745-060-7_18. [DOI] [PubMed] [Google Scholar]
- 55.Miles DK, Kernie SG. Hypoxic-ischemic brain injury activates early hippocampal stem/progenitor cells to replace vulnerable neuroblasts. Hippocampus. 2008;18:793–806. doi: 10.1002/hipo.20439. [DOI] [PubMed] [Google Scholar]
- 56.Santilli G, Lamorte G, Carlessi L, Ferrari D, Rota Nodari L, Binda E, Delia D, Vescovi AL, De Filippis L. Mild hypoxia enhances proliferation and multipotency of human neural stem cells. PLoS One. 2010;5:e8575. doi: 10.1371/journal.pone.0008575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang P, Liu Y, Li J, Kang Q, Tian Y, Chen X, Shi Q, Song T. Cell proliferation in ependymal/subventricular zone and nNOS expression following focal cerebral ischemia in adult rats. Neurol Res. 2006;28:91–96. doi: 10.1179/016164106X91942. [DOI] [PubMed] [Google Scholar]
- 58.Bürgers HF, Schelshorn DW, Wagner W, Kuschinsky W, Maurer MH. Acute anoxia stimulates proliferation in adult neural stem cells from the rat brain. Exp Brain Res. 2008;188:33–43. doi: 10.1007/s00221-008-1336-6. [DOI] [PubMed] [Google Scholar]
- 59.Park KI, Hack MA, Ourednik J, Yandava B, Flax JD, Stieg PE, Gullans S, Jensen FE, Sidman RL, Ourednik V, Snyder EY. Acute injury directs the migration, proliferation, and differentiation of solid organ stem cells: Evidence from the effect of hypoxia-ischemia in the CNS on clonal ‘reporter’ neural stem cells. Exp Neurol. 2006;199:156–178. doi: 10.1016/j.expneurol.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Qi C, Zhang J, Chen X, Wan J, Wang J, Zhang P, Liu Y. Hypoxia stimulates neural stem cell proliferation by increasing HIF-1α expression and activating Wnt/β-catenin signaling. Cell Mol Biol (Noisy-le-grand) 2017;63:12–19. doi: 10.14715/cmb/2017.63.7.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ciani L, Salinas PC. WNTs in the vertebrate nervous system: From patterning to neuronal connectivity. Nat Rev Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- 62.Lee SM, Tole S, Grove E, McMahon AP. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127:457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- 63.Lie DC, Colamarino SA, Song HJ, Désiré L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 64.Cheng YL, Park JS, Manzanero S, Choi Y, Baik SH, Okun E, Gelderblom M, Fann DY, Magnus T, Launikonis BS, et al. Evidence that collaboration between HIF-1α and Notch-1 promotes neuronal cell death in ischemic stroke. Neurobiol Dis. 2014;62:286–295. doi: 10.1016/j.nbd.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Z, Zhao TZ, Zou YJ, Zhang JH, Feng H. Hypoxia induces autophagic cell death through hypoxia-inducible factor 1α in microglia. PLoS One. 2014;9:e96509. doi: 10.1371/journal.pone.0096509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun Y, He W, Geng L. Neuroprotective mechanism of HIF-1α overexpression in the early stage of acute cerebral infarction in rats. Exp Ther Med. 2016;12:391–395. doi: 10.3892/etm.2016.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cui Y, Zhang Y, Zhao X, Shao L, Liu G, Sun C, Xu R, Zhang Z. ACSL4 exacerbates ischemic stroke by promoting ferroptosis-induced brain injury and neuroinflammation. Brain Behav Immun. 2021;93:312–321. doi: 10.1016/j.bbi.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 68.Panchision DM. The role of oxygen in regulating neural stem cells in development and disease. J Cell Physiol. 2009;220:562–568. doi: 10.1002/jcp.21812. [DOI] [PubMed] [Google Scholar]
- 69.Jiang Q, Geng X, Warren J, Eugene Paul Cosky E, Kaura S, Stone C, Li F, Ding Y. Hypoxia inducible factor-1α (HIF-1α) mediates NLRP3 inflammasome-dependent-pyroptotic and apoptotic cell death following schemic stroke. Neuroscience. 2020;448:126–139. doi: 10.1016/j.neuroscience.2020.09.036. [DOI] [PubMed] [Google Scholar]
- 70.An P, Xie J, Qiu S, Liu Y, Wang J, Xiu X, Li L, Tang M. Hispidulin exhibits neuroprotective activities against cerebral ischemia reperfusion injury through suppressing NLRP3-mediated pyroptosis. Life Sci. 2019;232:116599. doi: 10.1016/j.lfs.2019.116599. [DOI] [PubMed] [Google Scholar]
- 71.Tang B, Tang WJ, Tang YH, Deng CQ. Astragaloside IV attenuates cerebral ischemia and reperfusion injury and reduces activation of NLRP3 inflammasome and NF-κB phosphorylation in rats following a transient middle cerebral artery occlusion. Sheng Li Xue Bao. 2019;71:424–430. (In Chinese) [PubMed] [Google Scholar]
- 72.Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li J, Tao T, Xu J, Liu Z, Zou Z, Jin M. HIF-1α attenuates neuronal apoptosis by upregulating EPO expression following cerebral ischemia-reperfusion injury in a rat MCAO model. Int J Mol Med. 2020;45:1027–1036. doi: 10.3892/ijmm.2020.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu T, Zhan L, Liang D, Hu J, Lu Z, Zhu X, Sun W, Liu L, Xu E. Hypoxia-inducible factor 1α mediates neuroprotection of hypoxic postconditioning against global cerebral ischemia. J Neuropathol Exp Neurol. 2014;73:975–986. doi: 10.1097/NEN.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 75.Yang ML, Tao T, Xu J, Liu Z, Xu D. Antiapoptotic effect of gene therapy with recombinant adenovirus vector containing hypoxia-inducible factor-1α after cerebral ischemia and reperfusion in rats. Chin Med J (Engl) 2017;130:1700–1706. doi: 10.4103/0366-6999.209909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guo Y. Role of HIF-1a in regulating autophagic cell survival during cerebral ischemia reperfusion in rats. Oncotarget. 2017;8:98482–98494. doi: 10.18632/oncotarget.21445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jin X, Wang RH, Wang H, Long CL, Wang H. Brain protection against ischemic stroke using choline as a new molecular bypass treatment. Acta Pharmacol Sin. 2015;36:1416–1425. doi: 10.1038/aps.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen X, Zhou B, Yan T, Wu H, Feng J, Chen H, Gao C, Peng T, Yang D, Shen J. Peroxynitrite enhances self-renewal, proliferation and neuronal differentiation of neural stem/progenitor cells through activating HIF-1α and Wnt/β-catenin signaling pathway. Free Radic Biol Med. 2018;117:158–167. doi: 10.1016/j.freeradbiomed.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 79.Chen SF, Pan MX, Tang JC, Cheng J, Zhao D, Zhang Y, Liao HB, Liu R, Zhuang Y, Zhang ZF, et al. Arginine is neuroprotective through suppressing HIF-1α/LDHA-mediated inflammatory response after cerebral ischemia/reperfusion injury. Mol Brain. 2020;13:63. doi: 10.1186/s13041-020-00601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu R, Liao XY, Pan MX, Tang JC, Chen SF, Zhang Y, Lu PX, Lu LJ, Zou YY, Qin XP, et al. Glycine exhibits neuroprotective effects in ischemic stroke in rats through the inhibition of M1 microglial polarization via the NF-κB p65/Hif-1α signaling pathway. J Immunol. 2019;202:1704–1714. doi: 10.4049/jimmunol.1801166. [DOI] [PubMed] [Google Scholar]
- 81.Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008;39:959–966. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y, Wang Y, Yang GY. MicroRNAs in cerebral ischemia. Stroke Res Treat. 2013;2013:276540. doi: 10.1155/2013/276540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun JJ, Zhang XY, Qin XD, Zhang J, Wang MX, Yang JB. miRNA-210 induces the apoptosis of neuronal cells of rats with cerebral ischemia through activating HIF-1α-VEGF pathway. Eur Rev Med Pharmacol Sci. 2019;23:2548–2554. doi: 10.26355/eurrev_201903_17403. [DOI] [PubMed] [Google Scholar]
- 84.Li LJ, Huang Q, Zhang N, Wang GB, Liu YH. miR-376b-5p regulates angiogenesis in cerebral ischemia. Mol Med Rep. 2014;10:527–535. doi: 10.3892/mmr.2014.2172. [DOI] [PubMed] [Google Scholar]
- 85.Zhang L, Zhang Y, Zhang X, Zhang Y, Jiang Y, Xiao X, Tan J, Yuan W, Liu Y. MicroRNA-433 inhibits the proliferation and migration of HUVECs and neurons by targeting hypoxia-inducible factor 1 alpha. J Mol Neurosci. 2017;61:135–143. doi: 10.1007/s12031-016-0853-1. [DOI] [PubMed] [Google Scholar]
- 86.Liu FJ, Kaur P, Karolina DS, Sepramaniam S, Armugam A, Wong PT, Jeyaseelan K. miR-335 regulates Hif-1α to reduce cell death in both mouse cell line and rat ischemic models. PLoS One. 2015;10:e0128432. doi: 10.1371/journal.pone.0128432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang D, Wang L, Bai L, Du Y, Liu L, Chen X. Effects of inhibition of miR-155-5p in neural stem cell subarachnoid transplant on rats with cerebral infarction. Hum Gene Ther Methods. 2019;30:184–193. doi: 10.1089/hgtb.2019.118. [DOI] [PubMed] [Google Scholar]
- 88.Zhang X, Li H, Burnett JC, Rossi JJ. The role of antisense long noncoding RNA in small RNA-triggered gene activation. RNA. 2014;20:1916–1928. doi: 10.1261/rna.043968.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.PLOS Genetics Staff, corp-author. Correction: fMiRNA-192 and miRNA-204 directly suppress lncRNA HOTTIP and interrupt GLS1-mediated glutaminolysis in hepatocellular carcinoma. PLoS Genet. 2016;12:e1005825. doi: 10.1371/journal.pgen.1005825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen ZH, Wang WT, Huang W, Fang K, Sun YM, Liu SR, Luo XQ, Chen YQ. The lncRNA HOTAIRM1 regulates the degradation of PML-RARA oncoprotein and myeloid cell differentiation by enhancing the autophagy pathway. Cell Death Differ. 2017;24:212–224. doi: 10.1038/cdd.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mineo M, Ricklefs F, Rooj AK, Lyons SM, Ivanov P, Ansari KI, Nakano I, Chiocca EA, Godlewski J, Bronisz A. The Long Non-coding RNA HIF1A-AS2 facilitates the maintenance of mesenchymal glioblastoma stem-like cells in hypoxic niches. Cell Rep. 2016;15:2500–2509. doi: 10.1016/j.celrep.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li L, Wang M, Mei Z, Cao W, Yang Y, Wang Y, Wen A. lncRNAs HIF1A-AS2 facilitates the up-regulation of HIF-1α by sponging to miR-153-3p, whereby promoting angiogenesis in HUVECs in hypoxia. Biomed Pharmacother. 2017;96:165–172. doi: 10.1016/j.biopha.2017.09.113. [DOI] [PubMed] [Google Scholar]
- 93.Fani L, Bos D, Mutlu U, Portegies MLP, Zonneveld HI, Koudstaal PJ, Vernooij MW, Ikram MA, Ikram MK. Global brain perfusion and the risk of transient ischemic attack and ischemic stroke: The rotterdam study. J Am Heart Assoc. 2019;8:e011565. doi: 10.1161/JAHA.118.011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsai MJ, Kuo YM, Tsai YH. Transient ischemic attack induced by melted solid lipid microparticles protects rat brains from permanent focal ischemia. Neuroscience. 2014;275:136–145. doi: 10.1016/j.neuroscience.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 95.Sprick JD, Mallet RT, Przyklenk K, Rickards CA. Ischaemic and hypoxic conditioning: Potential for protection of vital organs. Exp Physiol. 2019;104:278–294. doi: 10.1113/EP087122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rojas DR, Tegeder I, Kuner R, Agarwal N. Hypoxia-inducible factor 1α protects peripheral sensory neurons from diabetic peripheral neuropathy by suppressing accumulation of reactive oxygen species. J Mol Med. 2018;96:1395–1405. doi: 10.1007/s00109-018-1707-9. [DOI] [PubMed] [Google Scholar]
- 97.Rodríguez-Reynoso S, Leal-Cortés C, Portilla-de Buen E, López-De la Torre SP. Ischemic preconditioning preserves liver energy charge and function on hepatic ischemia/reperfusion injury in rats. Arch Med Res. 2018;49:373–380. doi: 10.1016/j.arcmed.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 98.Meng SS, Xu XP, Chang W, Lu ZH, Huang LL, Xu JY, Liu L, Qiu HB, Yang Y, Guo FM. LincRNA-p21 promotes mesenchymal stem cell migration capacity and survival through hypoxic preconditioning. Stem Cell Res Ther. 2018;9:280. doi: 10.1186/s13287-018-1031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang Y, Lu F, Zhuang L, Yang S, Kong Y, Tan W, Gong Z, Zhan S. Combined preconditioning with hypoxia and GYKI-52466 protects rats from cerebral ischemic injury by HIF-1α/eNOS pathway. Am J Transl Res. 2017;9:5308–5319. [PMC free article] [PubMed] [Google Scholar]
- 100.Huang Y, Tan F, Zhuo Y, Liu J, He J, Duan D, Lu M, Hu Z. Hypoxia-preconditioned olfactory mucosa mesenchymal stem cells abolish cerebral ischemia/reperfusion-induced pyroptosis and apoptotic death of microglial cells by activating HIF-1α. Aging (Albany NY) 2020;12:10931–10950. doi: 10.18632/aging.103307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.CIR.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 102.Lee JC, Tae HJ, Kim IH, Cho JH, Lee TK, Park JH, Ahn JH, Choi SY, Bai HC, Shin BN, et al. Roles of HIF-1α, VEGF, and NF-κB in ischemic preconditioning-mediated neuroprotection of hippocampal CA1 pyramidal neurons against a subsequent transient cerebral ischemia. Mol Neurobiol. 2017;54:6984–6998. doi: 10.1007/s12035-016-0219-2. [DOI] [PubMed] [Google Scholar]
- 103.Hirayama Y, Koizumi S. Hypoxia-independent mechanisms of HIF-1α expression in astrocytes after ischemic preconditioning. Glia. 2017;65:523–530. doi: 10.1002/glia.23109. [DOI] [PubMed] [Google Scholar]
- 104.Yang J, Liu C, Du X, Liu M, Ji X, Du H, Zhao H. Hypoxia inducible factor 1α plays a key role in remote ischemia preconditioning against stroke by modulating inflammatory responses in rats. J Am Heart Assoc. 2018;7:e007589. doi: 10.1161/JAHA.117.007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu ZJ, Chen C, Li XR, Ran YY, Xu T, Zhang Y, Geng XK, Zhang Y, Du HS, Leak RK, et al. Remote ischemic preconditioning-mediated neuroprotection against stroke is associated with significant alterations in peripheral immune responses. CNS Neurosci Ther. 2016;22:43–52. doi: 10.1111/cns.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xia M, Ding Q, Zhang Z, Feng Q. Remote limb ischemic preconditioning protects rats against cerebral ischemia via HIF-1α/AMPK/HSP70 pathway. Cell Mol Neurobiol. 2017;37:1105–1114. doi: 10.1007/s10571-016-0444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fath DM, Kong X, Liang D, Lin Z, Chou A, Jiang Y, Fang J, Caro J, Sang N. Histone deacetylase inhibitors repress the transactivation potential of hypoxia-inducible factors independently of direct acetylation of HIF-alpha. J Biol Chem. 2006;281:13612–13619. doi: 10.1074/jbc.M600456200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liang D, Kong X, Sang N. Effects of histone deacetylase inhibitors on HIF-1. Cell Cycle. 2006;5:2430–2435. doi: 10.4161/cc.5.21.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen S, Yin C, Lao T, Liang D, He D, Wang C, Sang N. AMPK-HDAC5 pathway facilitates nuclear accumulation of HIF-1α and functional activation of HIF-1 by deacetylating Hsp70 in the cytosol. Cell Cycle. 2015;14:2520–2536. doi: 10.1080/15384101.2015.1055426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stetler RA, Leak RK, Gan Y, Li P, Zhang F, Hu X, Jing Z, Chen J, Zigmond MJ, Gao Y. Preconditioning provides neuroprotection in models of CNS disease: Paradigms and clinical significance. Prog Neurobiol. 2014;114:58–83. doi: 10.1016/j.pneurobio.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang F, Wu Y, Jia J. Exercise preconditioning and brain ischemic tolerance. Neuroscience. 2011;177:170–176. doi: 10.1016/j.neuroscience.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 112.Ding YH, Ding Y, Li J, Bessert DA, Rafols JA. Exercise pre-conditioning strengthens brain microvascular integrity in a rat stroke model. Neurol Res. 2006;28:184–189. doi: 10.1179/016164106X98053. [DOI] [PubMed] [Google Scholar]
- 113.Ding YH, Li J, Yao WX, Rafols JA, Clark JC, Ding Y. Exercise preconditioning upregulates cerebral integrins and enhances cerebrovascular integrity in ischemic rats. Acta Neuropathol. 2006;112:74–84. doi: 10.1007/s00401-006-0076-6. [DOI] [PubMed] [Google Scholar]
- 114.Kang KA, Seong H, Jin HB, Park J, Lee J, Jeon JY, Kim YJ. The effect of treadmill exercise on ischemic neuronal injury in the stroke animal model: Potentiation of cerebral vascular integrity. J Korean Acad Nurs. 2011;41:197–203. doi: 10.4040/jkan.2011.41.2.197. (In Korean) [DOI] [PubMed] [Google Scholar]
- 115.Otsuka S, Sakakima H, Terashi T, Takada S, Nakanishi K, Kikuchi K. Preconditioning exercise reduces brain damage and neuronal apoptosis through enhanced endogenous 14-3-3γ after focal brain ischemia in rats. Brain Struct Funct. 2019;224:727–738. doi: 10.1007/s00429-018-1800-4. [DOI] [PubMed] [Google Scholar]
- 116.Wang L, Deng W, Yuan Q, Yang H. Exercise preconditioning reduces ischemia reperfusion-induced focal cerebral infarct volume through up-regulating the expression of HIF-1α. Pak J Pharm Sci. 2015;28((Suppl 2)):S791–S798. [PubMed] [Google Scholar]
- 117.Wang H, Niu F, Fan W, Shi J, Zhang J, Li B. Modulating effects of preconditioning exercise in the expression of ET-1 and BNP via HIF-1α in ischemically injured brain. Metab Brain Dis. 2019;34:1299–1311. doi: 10.1007/s11011-019-00450-z. [DOI] [PubMed] [Google Scholar]
- 118.Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 119.Wang H, Xu X, Yin Y, Yu S, Ren H, Xue Q, Xu X. Catalpol protects vascular structure and promotes angiogenesis in cerebral ischemic rats by targeting HIF-1α/VEGF. Phytomedicine. 2020;78:153300. doi: 10.1016/j.phymed.2020.153300. [DOI] [PubMed] [Google Scholar]
- 120.Liang C, Ni GX, Shi XL, Jia L, Wang YL. Astragaloside IV regulates the HIF/VEGF/Notch signaling pathway through miRNA-210 to promote angiogenesis after ischemic stroke. Restor Neurol Neurosci. 2020;38:271–282. doi: 10.3233/RNN-201001. [DOI] [PubMed] [Google Scholar]
- 121.Hu Q, Liu L, Zhou L, Lu H, Wang J, Chen X, Wang Q. Effect of fluoxetine on HIF-1α-Netrin/VEGF cascade, angiogenesis and neuroprotection in a rat model of transient middle cerebral artery occlusion. Exp Neurol. 2020;329:113312. doi: 10.1016/j.expneurol.2020.113312. [DOI] [PubMed] [Google Scholar]
- 122.Wang J, Zhou X, Lu H, Song M, Zhao J, Wang Q. Fluoxetine induces vascular endothelial growth factor/Netrin over-expression via the mediation of hypoxia-inducible factor 1-alpha in SH-SY5Y cells. J Neurochem. 2016;136:1186–1195. doi: 10.1111/jnc.13521. [DOI] [PubMed] [Google Scholar]
- 123.Zou J, Fei Q, Xiao H, Wang H, Liu K, Liu M, Zhang H, Xiao X, Wang K, Wang N. VEGF-A promotes angiogenesis after acute myocardial infarction through increasing ROS production and enhancing ER stress-mediated autophagy. J Cell Physiol. 2019;234:17690–17703. doi: 10.1002/jcp.28395. [DOI] [PubMed] [Google Scholar]
- 124.Cheng X, Wang H, Liu C, Zhong S, Niu X, Zhang X, Qi R, Zhao S, Zhang X, Qu H, Zhao C. Dl-3-n-butylphthalide promotes remyelination process in cerebral white matter in rats subjected to ischemic stroke. Brain Res. 2019;1717:167–175. doi: 10.1016/j.brainres.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 125.Zhang Q, Bian H, Guo L, Zhu H. Berberine preconditioning protects neurons against ischemia via sphingosine-1-phosphate and hypoxia-inducible factor-1[Formula: See text] Am J Chin Med. 2016;44:927–941. doi: 10.1142/S0192415X16500518. [DOI] [PubMed] [Google Scholar]
- 126.Ryou MG, Choudhury GR, Li W, Winters A, Yuan F, Liu R, Yang SH. Methylene blue-induced neuronal protective mechanism against hypoxia-reoxygenation stress. Neuroscience. 2015;301:193–203. doi: 10.1016/j.neuroscience.2015.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wei Y, Hong H, Zhang X, Lai W, Wang Y, Chu K, Brown J, Hong G, Chen L. Salidroside inhibits inflammation through PI3K/Akt/HIF signaling after focal cerebral ischemia in rats. Inflammation. 2017;40:1297–1309. doi: 10.1007/s10753-017-0573-x. [DOI] [PubMed] [Google Scholar]
- 128.Hou Y, Wang J, Feng J. The neuroprotective effects of curcumin are associated with the regulation of the reciprocal function between autophagy and HIF-1α in cerebral ischemia-reperfusion injury. Drug Des Devel Ther. 2019;13:1135–1144. doi: 10.2147/DDDT.S194182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Saad MAE, Fahmy MIM, Al-Shorbagy M, Assaf N, Hegazy AAE, El-Yamany MF. Nateglinide exerts neuroprotective effects via downregulation of HIF-1α/TIM-3 inflammatory pathway and promotion of caveolin-1 expression in the rat's hippocampus subjected to focal cerebral ischemia/reperfusion injury. Inflammation. 2020;43:401–416. doi: 10.1007/s10753-019-01154-3. [DOI] [PubMed] [Google Scholar]
- 130.Cheng CY, Ho TY, Hsiang CY, Tang NY, Hsieh CL, Kao ST, Lee YC. Angelica sinensis exerts angiogenic and anti-apoptotic effects against cerebral ischemia-reperfusion injury by activating p38MAPK/HIF-1[Formula: See text]/VEGF-A signaling in rats. Am J Chin Med. 2017;45:1683–1708. doi: 10.1142/S0192415X17500914. [DOI] [PubMed] [Google Scholar]
- 131.Wu S, Wang N, Li J, Wang G, Seto SW, Chang D, Liang H. Ligustilide ameliorates the permeability of the blood-brain barrier model in vitro during oxygen-glucose deprivation injury through HIF/VEGF pathway. J Cardiovasc Pharmacol. 2019;73:316–325. doi: 10.1097/FJC.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 132.Chen ZZ, Gong X, Guo Q, Zhao H, Wang L. Bu Yang Huan Wu decoction prevents reperfusion injury following ischemic stroke in rats via inhibition of HIF-1α, VEGF and promotion β-ENaC expression. J Ethnopharmacol. 2019;228:70–81. doi: 10.1016/j.jep.2018.09.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.