Abstract
Binge drinking is a widespread public health concern with limited effective treatment options. To better select pharmaceutical targets, it is imperative to expand our knowledge of the underlying neural mechanisms involved in binge drinking. Our previous experiments in C57BL/6J female mice found that increasing activity in the nucleus accumbens (NAc) core using excitatory Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) reduced binge-like drinking. These results differed from what has been found in males; however, it is unclear whether differences in experimental procedures or sex underlie these discrepancies. We matched the conditions used in our female study and asked whether bidirectional manipulation of NAc core activity has different effects on binge-like drinking in males. Male C57BL/6J mice were stereotaxically injected with AAV2 hSyn-HA hM3Dq (excitatory), -hM4Di (inhibitory), or -eGFP bilaterally into the NAc core. We tested the effects of altering NAc activity on binge-like ethanol intake using Drinking in the Dark (DID). During the first week, mice were pre-treated with vehicle to establish baseline ethanol intake. In week 2 mice were treated with 1mg/kg CNO prior to DID to determine the effects of DREADD-induced changes in NAc core activity on ethanol intake. Decreasing activity via CNO/hM4Di significantly decreased binge-like drinking in male mice relative to eGFP and hM4Di groups. We also measured intake of sucrose, quinine, and water after CNO treatment and found that increasing NAc core activity via CNO/hM3Dq increased quinine intake, and increased water intake over time. We did not observe significant differences in the GFP or hM4Di groups. This work suggests there exist apparent sex-related differences in NAc core contributions to binge-like alcohol drinking, thus demonstrating the need for inclusion of both sexes in future work.
Keywords: chemogenetics, nucleus accumbens, binge drinking, sex differences, DREADDs
Introduction:
Alcohol is the third leading preventable cause of death in the U.S. and its misuse costs approximately $249 billion annually (NIAAA 2019). The National Survey on Drug Use reported that in 2017 5.7% of adults in the U.S. had an alcohol use disorder (AUD; SAMHSA, 2017). Engaging in binge drinking (reaching a blood alcohol concentration of 0.08 g/dL) increases an individual’s risk of developing AUD and accounts for approximately three-quarters of the cost associated with alcohol misuse (NIAAA, 2004; Sacks et al., 2015). Understanding the brain regions and neural substrates that underlie this dangerous pattern of drinking is an important first step in learning about vulnerability factors for AUD. From an experimental perspective, the problem in dissecting the neurobiology of binge drinking has been the lack of a suitable animal model where consumption will rapidly lead to intoxicating blood ethanol concentrations (BECs). However, a model of binge-like drinking, Drinking in the Dark (DID), has been developed where C57BL6/J mice will routinely drink to intoxication (i.e. > 80 mg% BEC; Rhodes et al., 2005). The C57BL/6J strain represents a genetic model of risk for high alcohol consumption, as these mice drink high amounts of ethanol in acute and chronic DID, as well as in other models of alcohol drinking such as two-bottle choice and chronic intermittent ethanol vapor-induced escalation in drinking (Rhodes et al., 2007; Lopez et al., 2017; Yoneyama et al., 2008).
The nucleus accumbens (NAc) selects and integrates the most relevant information from limbic and cortical efferents to mediate behavior and has been implicated in all aspects of alcohol addiction (Cassataro et al., 2014; Ito and Hayen, 2011; Koob and Volkow 2010; Lovinger and Kash, 2015; Lovinger and Roberto, 2013; Luscher and Malenka, 2011; Pozhidayeva et al., 2020; Purohit et al., 2018; Siciliano et al., 2015; Zhou et al., 2007). Lesioning the NAc reduced relapse rates in patients with alcohol dependence (Wu et al., 2010). Similarly, lesions to the NAc core or shell reduced limited access drinking in mice (Cassataro et al., 2014; Dhaher et al., 2009). While lesion studies have promising results, the procedure is permanent and can have undesired effects on cognition (He et al., 2008). An alternative approach to studying the role of the NAc in AUDs is through chemogenetic manipulation of neurons using DREADDs (Designer Receptors Exclusively Activated by Designer Drugs; Alexander et al., 2009). A study by Cassataro et al. (2014) expressed excitatory (hm3Dq) or inhibitory (hM4Di) DREADDs in the NAc core of male C57BL/6J mice and tested the effect that clozapine-n-oxide (CNO, ligand specific to DREADDs) had on DID. They found that decreasing activity of the NAc core in male mice decreased ethanol consumption while not affecting intake of water.
The aforementioned lesion and DREADDs studies were performed only in males and this sex bias remains a barrier in the development of treatments for alcohol addiction. Reports of sex differences exist in alcohol-related behaviors in animal models and in health outcomes following AUD in humans, yet there are almost no studies that use NAc lesions or DREADDs in females (Erol & Karpyak, 2015; Pomrenze & Giovanetti, 2018; Rhodes et al., 2007; see discussion). We recently used DREADDs to examine the role of the NAc in binge-like drinking using female C57BL/6J mice and found effects opposite to those reported by Cassataro et al. (2014): increasing activity in the NAc using excitatory DREADDs decreased ethanol consumed during DID in females (Purohit et al., 2018). These results suggest that sex is an important biological variable in alcohol-related NAc circuitry. A commentary on our findings by Pomrenze & Giovanetti (2018) implicated the lack of NAc studies done in female mice as a limitation in understanding the discrepancies between Purohit et al. (2018) and Cassataro et al (2014); and, noted that differences in methodology was also a confound. Purohit et al. (2018) administered 1 mg/kg CNO systemically (i.p.) 30 minutes prior to a two-hour ethanol access period. In the study by Cassataro et al. (2014) mice had access to water containing CNO (estimated 10 mg/kg daily intake) prior to a four-hour access period. The purpose of the present study is to replicate the methodology of Purohit et al. (2018) in male mice in order to assess whether differences in methodology are driving the opposing effects of NAc core DREADDs on binge-like drinking seen in male and female mice. We hypothesize that sex, rather than methodology, is responsible for the differences and that inhibiting the NAc core will decrease ethanol consumption while exciting the NAc core will have no effect on ethanol intake in male mice.
Methods:
Animals:
Adult C57BL/6J male mice (Jackson Laboratory, Bar Harbor, Maine, USA) were used for all experiments. All mice were group housed on Bed-o’cobs® bedding (The Andersons, Inc., OH, USA) in a reverse 12:12 light/dark cycle (lights on at 0730 PST) with food (5LOD, PMI Nutrition International, MO, USA) and water access ad libitum unless otherwise specified. All procedures were approved by local Institutional Animal Care and Use Committees and were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Stereotaxic Surgeries:
Stereotaxic surgeries were performed similarly to Purohit et al. (2018). Mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) in saline (0.09% NaCl). Bilateral stereotaxic injections of 1 μl purified high titer AAV2-hSyn-HA-hM3D(Gq)-IRES-mCherry (hM3Dq; Addgene, cat# 50474-AAV2), AAV2-hSyn-HA-hM4D(Gi)-IRES-mCherry (hM4Di; Addgene, cat# 44362-AAV2), or AAV2-hSyn-EGFP (eGFP; Addgene, cat#50465-AAV2) were injected into the NAc core (from bregma: angle 10°, AP +1.34 mm, ML +/− 1.5, DV −4.0 and −4.5) using a 33 gauge Hamilton syringe (Hamilton, Reno, NV, USA). AAV titers >10E12 vg/mL produced by Addgene (Watertown, MA, USA) were used in this study. Virus was infused at a rate of 0.1 μl/minute for 10 minutes, and the needle was kept in place for an additional 5 minutes post-injection before being slowly withdrawn. Mice recovered for 2-3 weeks in their home cage to allow for full viral expression.
Drugs:
Mice received injections (i.p.) of either vehicle [1% DMSO (Hybri-Max, Sigma Life Sciences, MO, USA) in 0.09% NaCl (Baxter International, Il, USA)] or 1 mg/kg CNO (RTI International, NC, USA; in vehicle) in a volume of 10mL/kg (a 20g mouse received 0.2mL injection). This dose of CNO was selected for its ability to successfully stimulate DREADDs without being back-converted to physiologically relevant levels of clozapine (Jendryka et al., 2019; Purohit et al., 2018).
Ethanol (EtOH; Decon Laboratories, Inc., PA, USA) used for DID was diluted to 20% in tap water (v/v). Sucrose (Sigma Life Sciences, MO, USA) was dissolved in tap water to make 2.5% and 5% solutions (w/v). Quinine hemisulfate salt monohydrate (Sigma Life Science, MO, USA) was dissolved in tap water to reach concentrations of 0.03 mM and 0.06 mM.
NAc core DREADDs:
In order to determine whether increasing or decreasing NAc core activity could chronically alter binge-like ethanol drinking, we assayed the effects of intra-NAc DREADDs (excitatory=hM3Dq, inhibitory=hM4Di, or control=eGFP; n=11-15/group) on ethanol intake using a two-week DID paradigm, followed by serial testing of other tastants and water [described below (and in Purohit et al., 2018); Figure 1]. These DREADDs have been previously validated for their functionality (Purohit et al., 2018). During the first week of DID, mice were pretreated with vehicle (i.p.) to establish baseline levels of ethanol intake. During the second week, mice were treated with CNO and ethanol intake was measured in a two-hour access period. To test whether any effects that DREADDs had on drinking generalized to other tastants (sucrose and quinine) or water, two-hour intakes of each solution were measured for four days with CNO treatment.
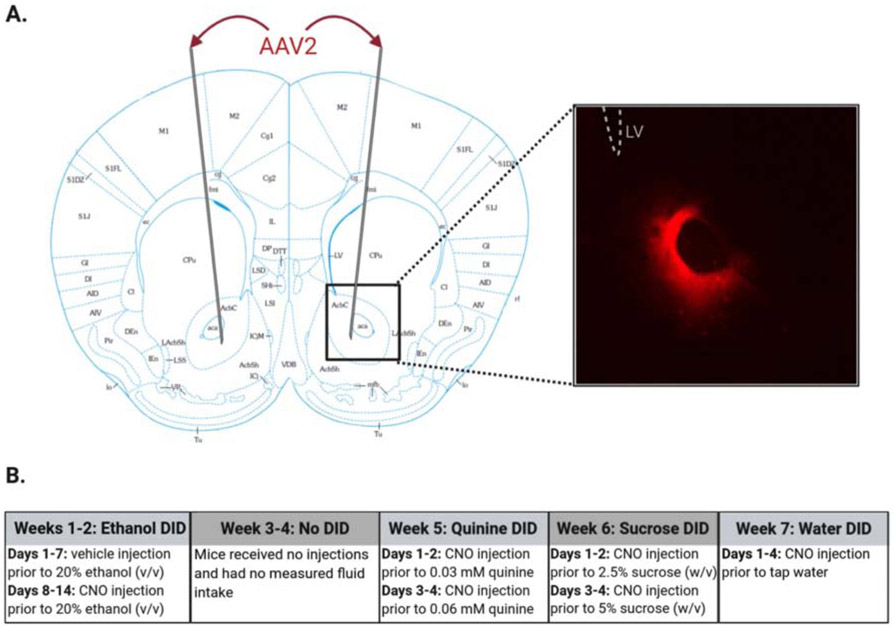
Figure 1. Determining the effects of chemogenetic manipulation of the NAc core on drinking in C57BL/6J male mice using Drinking in the Dark (DID) paradigm.
(A) Coronal section 1.34 mm anterior of Bregma (modified from Franklin & Paxinos, 2007) demonstrating target region for stereotaxic delivery of AAV2 hM3Dq, hM4Di, or GFP. Inset shows representative image (4x magnification) of viral expression in the NAc core. (B) Mice underwent a 7-week DID schedule with four distinct DID periods and a 2-week period of no testing (weeks 3-4). Mice received an injection (i.p.) of either vehicle or clozapine-n-oxide (CNO; 1 mg/kg) at zeitgeber time (ZT) 14.5 (30 minutes before DID). From ZT 15-17, mice had access to one of the following fluids: ethanol (20% v/v; weeks 1-2), quinine (0.03 or 0.06 mM; week 5), sucrose (2.5% or 5% w/v; week 6), or water (week 7).
Drinking in the Dark:
DID was carried out as in Purohit et al. (2018). Mice were habituated to individual housing and sipper bottles for one week prior to testing. Thirty minutes prior to DID, mice were injected (i.p.) with vehicle (0.1% DMSO in saline) or CNO (1 mg/kg). Each measured fluid was presented as the only available fluid for two hours starting three hours into the dark cycle [zeitgeber time (ZT)15-17, where ZT0 is lights on and ZT12 is lights off]. After two hours, measured fluids were removed and water (unmeasured) was replaced for the remainder of the day.
There were four periods of testing over 7 weeks (Figure 1b). The first period consisted of a 2-week long, 2-hour daily DID schedule designed to assess the effect of changing NAc core activity on binge-like ethanol drinking (20% v/v in tap water). Thirty minutes prior to DID each day, mice received vehicle injections for 7 consecutive days to establish a baseline intake and then received CNO injections for an additional 7 days. Mice were then left undisturbed for two weeks before beginning tastant testing (periods 2-4). The remaining periods of testing were included to assess if any change in ethanol extended to other tastants and/or water. Each of these three periods consisted of 4 consecutive days of CNO treatment (i.p.; 30 minutes prior to measured fluid access) accompanying the presentation of one of the following solutions (in order of presentation): quinine (0.03 mM and 0.06 mM; 2 days/concentration), sucrose (2.5% or 5.0 % w/v; 2 days/concentration), or tap water. In between each tastant period, mice were left undisturbed for 3 days.
Immunohistochemistry:
Immediately following the final day of water DID, mice were deeply anesthetized with 250 mg/kg ketamine and 25mg/kg xylazine, and transcardially perfused with PBS and 4% paraformaldehyde in PBS. The brains were extracted and allowed to post-fix in 4% paraformaldehyde for 24 hours and then placed in 30% glycerol in PBS for an additional 24 hours before being stored in PBS-0.01% sodium azide. Using a freezing microtome, 30 μm coronal brain sections were obtained and immunohistochemical staining was carried out using standard procedures. The antibodies used to analyze viral spread were chicken anti-GFP (AbCam, Cambridge, MA, USA; cat#13970) with goat anti-chicken 488 (Thermo Fisher Scientific, Waltham, MA, USA; cat#A11039) for control virus or rabbit anti-mCherry (AbCam, cat#ab167453) with goat anti-rabbit 594 (Thermo Fisher Scientific, cat#A11012) for DREADDs. Brain sections were mounted using Vectashield (Vector Labs, Burlingame, CA) with DAPI counterstaining and observed with an epifluorescence microscope with a 4x and 10x objective. Animals were excluded from the study if their viral spread was not localized to the NAc core. Exclusion by these criteria accounted for 3 mice from the hM3Dq group.
Statistics:
Data are presented as the mean +/− SEM. Data were analyzed using a two-way ANOVA with repeated measures (daily intake) and one-way ANOVA (difference score). All main effects and interactions are reported, with post-hoc testing using Tukey’s HSD test carried out and reported when appropriate. An alpha level of 0.05 was used to determine significance. Data analysis was performed and graphs were generated using GraphPad Prism 8. Figure 1 was generated using BioRender.
Results:
Modulation of NAc core activity influences binge-like drinking in a chronic DID paradigm
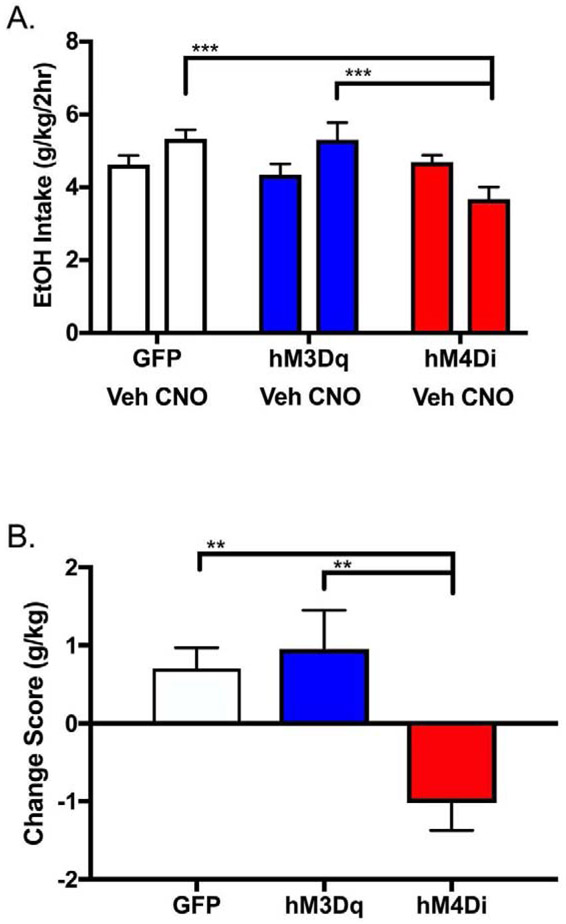
We determined the effects of CNO/DREADDs on binge-like ethanol drinking using a two-week DID paradigm in order to determine whether increasing or decreasing activity specifically in the NAc core sub-region could alter binge-like ethanol drinking in male mice. Average daily ethanol intake for each week (vehicle and CNO) is presented in Fig. 2a. Two-way ANOVA (viral group x treatment) identified a main effect of viral group (F(2, 38)=3.53, p=0.0392) and a viral group x treatment interaction (F(2,38)=8.97, p=0.0006), though no main effect of treatment (F(1,38)=0.98, p=0.33). Tukey’s post-hoc testing revealed that mice expressing GFP, hM3Dq, or hM4Di in the NAc core did not differ in baseline ethanol intakes (during vehicle injections in week 1; hM4Di vs hM3Dq p=0.70, hM4Di vs GFP p=0.98, hM3Dq vs GFP p=0.80); however, when treated with CNO in week 2, mice expressing hM4Di consumed less ethanol compared to those expressing hM3Dq (p=0.0009) and GFP (p=0.0002). We further pursued this interaction by calculating the difference score for each animal (intake during treatment with CNO – intake during treatment with vehicle) and then averaging these scores for each group (Fig. 2b). One-way ANOVA demonstrated an effect of viral group on drinking difference score (F(2,38)=8.96, p=0.0006). Tukey’s post-hoc testing revealed that mice in the hM4Di group drank less ethanol than those in the hM3Dq (p=0.0019) or GFP (p=0.0032) group when treated with CNO.
Figure 2. Bidirectional modulation of NAc core activity alters binge-like EtOH drinking in male C57BL/6J mice.
(A) CNO treatment resulted in differences in intake between male mice expressing hM4Di and hM3Dq (and hM4Di and GFP) in the NAc core (two-way ANOVA with Tukey’s HSD; ***p < 0.001). (B) EtOH intake presented as average individual difference scores (=intake during treatment with CNO – intake during treatment with vehicle) shows bidirectional modulation of binge-like drinking in mice expressing hM4Di as compared with hM3Dq (one-way ANOVA with Tukey’s HSD; **p < 0.01). n = 11-15/viral group.
Altering NAc core activity affects limited access quinine and water intake
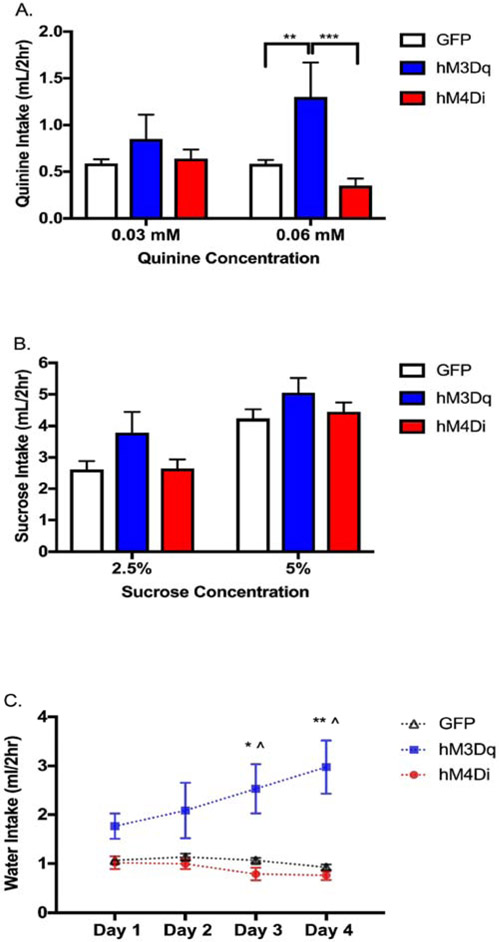
To determine whether the effects observed were generalizable to other fluids, we asked whether altering NAc activity changes quinine, sucrose, or water intake in the same cohort of mice. We used a modified version of DID, where instead of measuring ethanol intake, we measured intake of bitter quinine hemisulfate solutions (0.03 and 0.06 mM), sweet sucrose solutions (2.5% and 5% w/v), and tap water. Separate ANOVAs were carried out for each fluid. For quinine and sucrose, intake (for the two days each concentration was offered) was averaged for individual animals. Two-way ANOVA (viral group x concentration) of quinine intake revealed a main effect of viral group (F(2,36)=3.89, p=0.0294) and an interaction (F(2,36)=14.2, p<0.0001), but no main effect of concentration (F(1,36)=0.90, p=0.35). Tukey’s post-hoc further revealed that there was an increase in 0.06 mM quinine drinking for hM3Dq expressing mice as compared to hM4Di (p=0.0003) and GFP (p=0.0063) expressing mice (Figure 3a). Two-way ANOVA of sucrose intake (viral group x concentration) revealed a main effect of sucrose concentration (Figure 3b; F(1,36)=98.93, p<0.0001), but no main effect of viral group (F(2,36)=2.2, p=0.12) or interaction (F(2,36)=0.92, p=0.41) were observed. Two-way mixed factor ANOVA (viral group x day) of water intake revealed a main effect of viral group (F(2,34)=15, p<0.0001) and an interaction of viral group x day (F(6,102)=6.54, p<0.0001), but no main effect of day (F(1.95,66.25)=2.17, p=0.12). Tukey’s post-hoc revealed hM3Dq expressing mice drank more water than hM4Di or GFP expressing mice on days 3 (hM3Dq vs hM4Di p=0.0209, hM3Dq vs GFP p=0.0470) and 4 (Figure 3c; hM3Dq vs hM4Di p=0.0088, hM3Dq vs GFP p=0.0138).
Figure 3. Influence of bidirectional modulation of NAc core activity on water and tastants.
(A) CNO increased quinine intake in mice expressing hM3Dq in the NAc core as compared to hM4Di or GFP at the higher concentration of 0.06 mM, but not at 0.03 mM (two-way ANOVA with Tukey’s HSD; **p < 0.01, *** p < 0.001). (B) There was no effect of CNO/DREADDs on sucrose consumption at either concentration, but mice from all groups consumed more of the 5% sucrose concentration than the 2.5% concentration (two-way ANOVA wirh Tukey’s HSD; ****p<0.0001). (C) CNO increased water intake for mice expressing hM3Dq in the NAc core as compared to hM4Di or GFP expressing mice on days 3 and 4 (two-way mixed factor ANOVA with Tukey’s HSD; ^p<0.05 hM3Dq vs GFP; *p<0.05 hM3Dq vs hM4Di; **p<0.01 hM3Dq vs hM4Di). n = 11-15/viral group
Discussion
The NAc core’s central role in addiction circuitry makes it a region of interest for the development of potential AUD treatments. Here, we demonstrate that inhibition of NAc core activity using DREADDs decreases binge-like ethanol drinking in male C57BL/6J mice. CNO administered to mice expressing inhibitory DREADDs in the NAc core decreased binge-like ethanol drinking, whereas stimulation of excitatory DREADDs did not change binge-like drinking in male C57BL/6J mice. These findings agree with those of Cassataro et al. (2014) in which oral administration of CNO decreased ethanol consumption during a four-hour DID in male mice expressing hM4Di DREADDs.
There were a few differences between the present study’s findings and those of Cassataro et al. (2014). For one, we tested the effects of DREADDs on ethanol intake in a two-hour drinking period. Cassataro et al. (2014) did not observe an effect at two hours of DID, but did observe an effect at four hours of DID. The difference in the length of access period needed to detect DREADD effects on drinking behavior could be a result of how CNO was administered (i.e., oral administration 20hr/day vs i.p. injection 30 minutes prior to DID). We also found that activating excitatory DREADDs in the NAC core increased quinine and water, but not sucrose, consumption relative to GFP and inhibitory DREADDs in male C57BL/6J mice. Cassataro et al. (2014) did not test quinine (or a comparable bitter tastant), but they did measure water and sucrose intake following CNO exposure and did not observe an effect of inhibiting NAc activity via CNO and hM4Di DREADDs. Notably, Cassataro et al. (2014) did not measure the effect of hM3Dq DREADDs on sucrose or water intake. Furthermore, they showed sucrose and water intake as a difference score between the presence and absence of CNO; however, we did not perform a similar analysis because we did not measure sucrose or water consumption in the absence of CNO. Despite these minor discrepancies, our results closely match those previously published and we conclude that inhibition of NAc core leads to a decrease in binge-like ethanol drinking in C57BL/6J male mice.
These data were collected in males, and are in contrast to our previous findings from multiple studies showing that increasing activity in the NAc core of female mice using DREADDs decreased their ethanol consumption (Pozhidayeva et al., 2020; Purohit et al., 2018). A limitation of this study is that the experiments in males and females were performed separately. That being said, the impact of separate experiments is likely small given that our findings in males are consistent with Cassataro et al. (2014) and that we have been able to replicate our findings in females in multiple experiments, at two different institutions, and in two different strains of mice (Pozhidayeva et al., 2020; Purohit et al., 2018). A brief table of these findings are presented in Supplemental Table 1 for comparison. Specifically, Differences in locomotor activity in response to DREADD activation could also be driving apparent sex-related differences in ethanol intake. While not directly measured here, a study by Zhu et al. (2016) analyzed the effects that DREADD manipulation of D1 and D2 neurons have on locomotion and reported that there were no differences between male and female mice. Altogether, the data from our lab indicate modulation of NAc core activity has apparent sex-related differences on binge-like drinking in male and female mice
One limitation of this study is that tastant and water intakes were not measured in the absence of CNO. Given that hM3Dq-treated mice only differ from GFP mice on the final two days of quinine or water treatment, it is possible that chronic CNO/hM3Dq treatment leads to generalized increases in fluid intake. If this is the case, however, we would expect to have seen a main effect of time and concentration for ethanol and sucrose, respectively, but did not observe this. Studies in male rats reveal that NAc neurons show variable responsiveness to taste stimuli. Notably, this previous work reported that NAc neurons were typically excited in response to intraoral infusion of quinine, whereas infusion of sucrose typically inhibited NAc neurons (Roitman et al., 2005). Therefore, we could hypothesize that increasing activity might increase intake of quinine, and decreasing activity might increase intake of sucrose in male mice. While this may explain our findings for the effects of chemogenetic stimulation of the NAc core on quinine intake, it does not explain the increased water intake seen with chemogenetic stimulation or the lack of an expected increase of sucrose intake with chemogenetic inhibition. Together, this suggests that the population of NAc core neurons targeted by chemogenetic stimulation (CNO/hM3Dq) in our study may be quinine and water, but not sucrose, responsive. Another limitation of this study is that we did not measure BECs and thus do not know if the mice drank to intoxication, and how DREADD/CNO treatment affected intoxication. Previous work suggests that the average intake of mice in this study could produce a BEC greater than 80mg%, and that even a small change in ethanol consumption can result in a large change in BEC (Rhodes et al., 2005, 2007).
Importantly, it is still unknown what mechanisms underlie the finding that inhibition of the NAc core in males and excitation in females both lead to a decrease in ethanol consumption. The NAc core contains D1 receptor- (Gs-coupled) and D2 receptor- (Gi/o-coupled) expressing GABAergic medium spiny neuron (MSN) subpopulations. It is currently unclear what role these two subpopulations of MSNs in the NAc core play in binge-like drinking, although dorsal striatal MSNs are thought to largely oppose each other in their role in addiction with direct disinhibition of the thalamus occurring via D1 receptors and indirect inhibition occurring via D2 receptors (Lobo & Nester, 2011). There are data supporting clear sex differences of D1-α subtype receptor expression patterns in the NAc, with male mice expressing more receptors per cell (Li et al., 2018). Thus, it is possible that a difference in relative ratios of D1- and D2- MSNs contributes to sex-differences in alcohol consumption. Interestingly, a recent meta-analysis of studies conducted in rats found that there is no basal or drug-induced difference in circulating dopamine levels between males and females (Egenrieder et al., 2019); hence, it is likely that we must look at the circuit level to understand our results.
Work by Woolley and colleagues highlights sex differences in the synaptic connectivity of the NAc (Forlano & Woolley, 2010; Wissman et al., 2011). Female rats have an increased number of excitatory synapses onto NAc core MSNs compared to males. MSNs in the female NAc also have increased distal dendritic spine density, increased proportion of large spines, and a prominent rostro-caudal gradient of spine synapse density. This work demonstrates clear differences in NAc core connectivity, supporting sex differences in the role that the NAc plays within reward-related circuitry. Another consideration not yet discussed is that the animals in this study are singly housed, which may differentially affect males and females and contribute to the differences between our findings here in males and in Purohit et al., 2018 and Pozhidayeva et al., 2020 in females. Additional possibilities exist for finding different effects of chemogenetic manipulation on drinking behaviors. For example, chemogenetic manipulation could be mimicking any number of G protein-coupled receptor signaling mechanisms. One example is corticotropin releasing factor signaling, which is known to underlie sex differences in stress disorders and contribute to appetitive and aversive behaviors via the NAc (Bangasser & Wiersielis, 2018; Lemos et al., 2012). Ongoing studies in our lab are using chemogenetics to map and manipulate regions with inputs onto the NAc core in order to uncover specific neural circuits involved in binge-like drinking in male and female mice. Knowing which projections are important for excessive drinking in males and females can inform points of intervention and lead to a better understanding of the development of AUD
Our current study confirms the viability of using chemogenetic approaches to alter binge-like drinking in mice and also supports the hypothesis that there exist differences in the role that the NAc core plays in drinking behavior between males and females. It is important to note that we only measured binge-like drinking behavior in this study, and that these sex specific effects of accumbal DREADDs may be reversed or absent in other behavioral paradigms modeling AUD – strengthening the case for using both females and males when studying drinking behaviors. One possible behavioral test of interest is conditioned taste aversion, given that we found sex-specific effects of DREADD activation on quinine intake and that the NAc is known to contribute to associative learning in the presence of taste stimuli Núñez-Jaramillo et al., 2012). Moving forward, it is important that we consider sex as a biological variable, as our work demonstrates apparent sex-related differences in how the NAc modulates binge-like ethanol drinking.
Supplementary Material
Highlights.
Chemogenetic inhibition of neurons in the nucleus accumbens core (NAc) neurons reduced binge-like ethanol intake in male mice, without altering intake of other fluids.
Although chemogenetic activation of NAc did not increase binge-like ethanol drinking in males, it did increase intake of quinine and water (but not sucrose) in male mice.
Findings in male mice are opposite of those of previously published female mice.
Acknowledgements
We thank the following individuals for their intellectual contributions and support: Mr. Bryan Jensen, Dr. Kolter Grigsby, Dr. Pamela Metten, and Dr. Antonia Savarese.
Funding: This work was supported by US Department of Veterans Affairs [Grant IK2 BX002488], National Institutes of Health [Grants AA010760, AA013519, AA020245, AA026997], Collins Medical Trust, and the Andrews Genomics Fund.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, and McNamara JO (2009) Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA and Wiersielis KR (2018) Sex differences in stress responses: a critical role for corticotropin-releasing factor. Hormones 17(1 ):5–13. [DOI] [PubMed] [Google Scholar]
- Cassataro D, Bergfeldt D, Malekian C, Van Snellenberg JX, Thanos PK, Fishell G, and Sjulson L (2014) Reverse pharmacogenetic modulation of the nucleus accumbens reduces ethanol consumption in a limited access paradigm. Neuropsychopharmacology 39(2):283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaher R, Finn DA, Oberbeck DL, Yoneyama N, Snelling CC, Wu W, and Hitzemann RJ (2009) Electrolytic lesions of the medial nucleus accumbens shell selectively decrease ethanol consumption without altering preference in a limited access procedure in C57BL/6J mice. Pharmacol Biochem Behav 92(2):335–342. [DOI] [PubMed] [Google Scholar]
- Egenrieder L, Mitricheva E, Spanagel R, and Noori HR (2019) No basal or drug-induced sex differences in striatal dopaminergic levels: a cluster and meta-analysis of rat microdialysis studies. J Neurochem 152(4):482–492 [DOI] [PubMed] [Google Scholar]
- Erol A and Karpyak VM (2015) Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug Alcohol Depend 156:1–13. [DOI] [PubMed] [Google Scholar]
- Forlano PM and Woolley CS (2010) Quantitative analysis of pre-and postsynaptic sex differences in the nucleus accumbens. J Comp Neurol 518(8):1330–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin K and Paxinos G (2007) The Mouse Brain In Stereotaxic Coordinates. 3rd edn. Amsterdam: Elsevier/Academic Press. [Google Scholar]
- He F, Guan H, Zhao Z, Miao X, Zhou Q, Li L, Huang D, Liu A, and Miao D (2008) Evaluation of short-term psychological functions in opiate addicts after ablating the nucleus accumbens via stereotactic surgery. Stereotact Funct Neurosurg 86(5):320–329. [DOI] [PubMed] [Google Scholar]
- Ito R and Hayen A (2011) Opposing roles of nucleus accumbens core and shell dopamine in the modulation of limbic information processing. J Neurosci 31(16):6001–6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendryka M, Palchaudhuri M, Ursu D, van der Venn B, Liss B, Katzel D, Niseen W, and Pekcec A (2019) Pharmacokinetic and pharmacodynamic actions of clozapine-n-oxide, clozapine, and compound 21 in DREADD-based chemogenetics in mice. Sci Rep 9:4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF and Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35(1 ):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, Van Bockstaele EJ, Chavkin C, and Phillips PE (2012). Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature 490(7420):402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Illenberger JM, McLaurin KA, Mactutus CF, and Booze RM (2018) Identification of Dopamine D1-Alpha Receptor Within Rodent Nucleus Accumbens by an Innovative RNA In Situ Detection Technology. J Vis Exp 133:e57444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK and Nestler EJ (2011) The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat 5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Miles MF, Williams RW, and Becker HC (2017) Variable effects of chronic intermittent ethanol exposure on ethanol drinking in a genetically diverse mouse cohort. Alcohol 58:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM and Kash TL (2015) Mechanisms of neuroplasticity and ethanol’s effects on plasticity in the striatum and bed nucleus of the stria terminalis. Alcohol Res 37:109. [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM and Roberto M (2010) Synaptic effects induced by alcohol, in Behavioral Neurobiology of Alcohol Addiction pp 31–86, Springer, Berlin. [Google Scholar]
- Lüscher C and Malenka RC (2011) Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69(4):650–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA). NIAAA Council Approves Definition of Binge Drinking. NIAAA Newsletter, No. 3, Winter 2004. Available at: pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA). Alcohol Facts and Statistics. December 2019. Available at: www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics.
- Núñnez-Jaramillo L, Rangel-Hernández JA, Burgueño B, and Miranda MI (2012) Activation of nucleus accumbens NMDA receptors differentially affects appetitive or aversive taste learning and memory. Front behav neurosci 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomrenze MB and Giovanetti SM (2018) DREADD to the Core. Commentary on Purohit et al. (2018): Pharmacogenetic Manipulation of the Nucleus Accumbens Alters Binge-Like Alcohol Drinking in Mice. Alcohol Clin Exp Res 42(9):1623–1626. [DOI] [PubMed] [Google Scholar]
- Pozhidayeva DYY, Farris SP, Goeke CM, Firsick EJ, Townsley KG, Guizzetti M, and Ozburn AR (2020) Chronic Chemogenetic Stimulation of the Nucleus Accumbens Produces Lasting Reductions in Binge Drinking and Ameliorates Alcohol-Related Morphological and Transcriptional Changes. Brain Sci 10(2):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Onishi KG, and Zucker I (2014) Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev 40:1–5. [DOI] [PubMed] [Google Scholar]
- Purohit K, Parekh PK, Kern J, Logan RW, Liu Z, Huang Y, McClung CA, Crabbe JC, and Ozburn AR (2018) Pharmacogenetic manipulation of the nucleus accumbens alters binge-like alcohol drinking in mice. Alcohol Clin Exper Res 42(5):879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, and Crabbe JC (2005) Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav 84:53–63. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T Jr, and Crabbe JC (2007) Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav 6:1–18. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM (2005) Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron 45:587–597. [DOI] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, and Brewer RD (2015) 2010 national and state costs of excessive alcohol consumption. Am J Prev Med 49(5):e73–e79. [DOI] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Carlson VCC, Helms CM, Lovinger DM, Grant KA, and Jones SR (2015) Voluntary ethanol intake predicts κ-opioid receptor supersensitivity and regionally distinct dopaminergic adaptations in macaques. J Neurosci 35(15): 5959–5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) (2017) National Survey on Drug Use and Health (NSDUH). Table 2.19B—Alcohol Use in Lifetime, Past Year, and Past Month Among Persons Aged 12 or Older, by Detailed Age Category: Percentages, 2016 and 2017. Available at: samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHFFR2017/NSDUHFFR2017.pdf [Google Scholar]
- Wissman AM, May RM, and Woolley CS (2012) Ultrastructural analysis of sex differences in nucleus accumbens synaptic connectivity. Brain Struct Funct 217(2): 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HM, Wang XL, Chang CW, Li N, Gao L, Geng N, Ma JH, Zhao W, and Gao GD (2010) Preliminary findings in ablating the nucleus accumbens using stereotactic surgery for alleviating psychological dependence on alcohol. Neurosci Lett 473(2):77–81. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, and Finn DA (2008) Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol 42(3):149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FC, Anthony B, Dunn KW, Lindquist WB, Xu ZC, and Deng P (2007) Chronic alcohol drinking alters neuronal dendritic spines in the brain reward center nucleus accumbens. Brain Res 1134:148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Ottenheimer D, and DiLeone RJ (2016) Activity of D1/2 receptor expressing neurons in the nucleus accumbens regulates running, locomotion, and food intake. Front Behav Neurosci 10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.